User login
Inmate Falls From Top Bunk
ANSWER
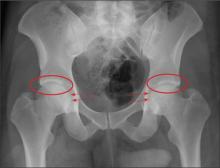
The radiograph demonstrates no acute osseous injury, such as fracture or dislocation. Of interest and note is increased sclerosis within both femoral heads, more so on the left versus the right side. Given the patient’s young age, such findings could be related to early avascular necrosis. His clinical symptoms certainly correlate. MRI or bone scan, as well as orthopedic evaluation, is warranted in such a case.
Fortunately, subsequent MRI of both hips did not show any avascular necrosis but rather osteoarthritic changes. The MRI of his spinal column was negative as well.
ANSWER
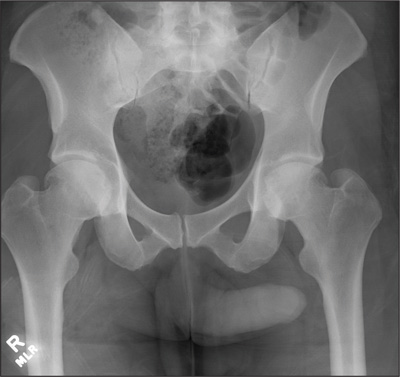
The radiograph demonstrates no acute osseous injury, such as fracture or dislocation. Of interest and note is increased sclerosis within both femoral heads, more so on the left versus the right side. Given the patient’s young age, such findings could be related to early avascular necrosis. His clinical symptoms certainly correlate. MRI or bone scan, as well as orthopedic evaluation, is warranted in such a case.
Fortunately, subsequent MRI of both hips did not show any avascular necrosis but rather osteoarthritic changes. The MRI of his spinal column was negative as well.
ANSWER
The radiograph demonstrates no acute osseous injury, such as fracture or dislocation. Of interest and note is increased sclerosis within both femoral heads, more so on the left versus the right side. Given the patient’s young age, such findings could be related to early avascular necrosis. His clinical symptoms certainly correlate. MRI or bone scan, as well as orthopedic evaluation, is warranted in such a case.
Fortunately, subsequent MRI of both hips did not show any avascular necrosis but rather osteoarthritic changes. The MRI of his spinal column was negative as well.
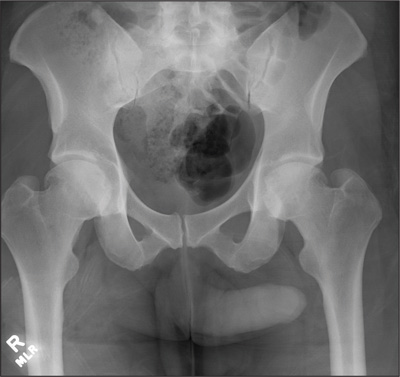
A 30-year-old man is transferred to your facility for evaluation of reported paraplegia after a fall. The patient is an inmate at a local prison. He states he was sleeping on the top bunk when he rolled over and fell off the bed, landing flat on his back on the concrete floor. He immediately started having severe back and hip pain and noticed that he could not move his legs. His primary complaint is severe bilateral hip pain. He was initially evaluated at an outside hospital, where CT of his head, cervical spine, and lumbar spine was negative for any acute pathology. He was sent to your facility for an MRI to rule out contusion or acute herniated disc. The patient denies any significant medical history, including back trauma. Currently, he reports no bowel/bladder issues or saddle anesthesia. On initial exam, he is awake, alert, and oriented, with normal vital signs. Musculoskeletal exam demonstrates a moderate amount of paraspinous tenderness and bilateral hip/pelvis tenderness. There is no instability detected, nor any leg shortening or rotation. He does have bilateral weakness in both lower extremities on the magnitude of 3-/5, although his exam seems limited due to the severity of his hip pain. Sensation is completely intact in both lower extremities. While the patient is awaiting his MRI, you order a portable pelvis radiograph, since none was performed at the outside facility. What is your impression?
Healthy and Active, but Getting Fatigued
ANSWER
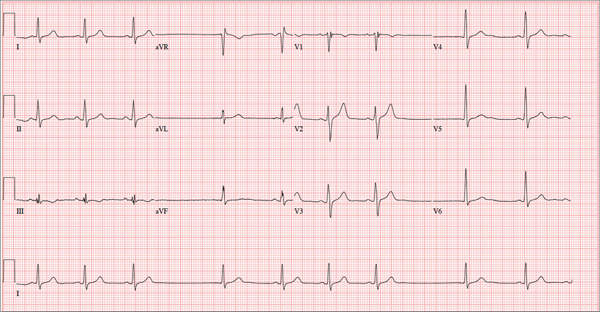
The correct interpretation of this ECG includes sinus bradycardia with marked sinus arrhythmia and junctional escape beats with sinus arrest. An intraventricular conduction defect is also present.
Sinus bradycardia is indicated by the normal PQRST complexes at a rate of less than 60 beats/min. A marked sinus arrhythmia is evidenced by more than one pause (between third and fourth beats and seventh and eighth beats on the lead I rhythm strip) on the ECG.
Sinus arrest occurs when the sinus node fails to conduct (absence of P wave during the interval of the pause). A normal QRS complex without a preceding P wave indicates a junctional escape beat. Finally, an intraventricular conduction defect is documented by a QRS duration ≥ 110 ms in the absence of a right or left bundle branch block.
ANSWER
The correct interpretation of this ECG includes sinus bradycardia with marked sinus arrhythmia and junctional escape beats with sinus arrest. An intraventricular conduction defect is also present.
Sinus bradycardia is indicated by the normal PQRST complexes at a rate of less than 60 beats/min. A marked sinus arrhythmia is evidenced by more than one pause (between third and fourth beats and seventh and eighth beats on the lead I rhythm strip) on the ECG.
Sinus arrest occurs when the sinus node fails to conduct (absence of P wave during the interval of the pause). A normal QRS complex without a preceding P wave indicates a junctional escape beat. Finally, an intraventricular conduction defect is documented by a QRS duration ≥ 110 ms in the absence of a right or left bundle branch block.
ANSWER
The correct interpretation of this ECG includes sinus bradycardia with marked sinus arrhythmia and junctional escape beats with sinus arrest. An intraventricular conduction defect is also present.
Sinus bradycardia is indicated by the normal PQRST complexes at a rate of less than 60 beats/min. A marked sinus arrhythmia is evidenced by more than one pause (between third and fourth beats and seventh and eighth beats on the lead I rhythm strip) on the ECG.
Sinus arrest occurs when the sinus node fails to conduct (absence of P wave during the interval of the pause). A normal QRS complex without a preceding P wave indicates a junctional escape beat. Finally, an intraventricular conduction defect is documented by a QRS duration ≥ 110 ms in the absence of a right or left bundle branch block.
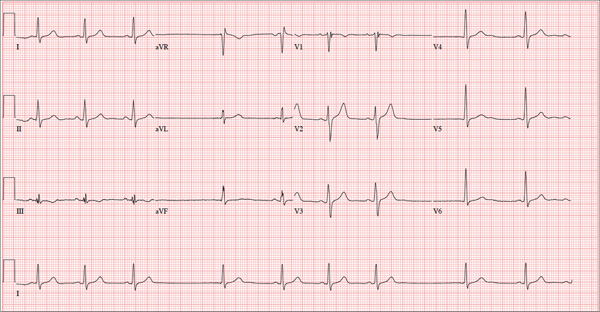
A 68-year-old retired high school teacher became fatigued while doing yardwork. After sitting down to rest, he noticed that his heart seemed to be skipping beats. He asked his daughter, a pediatric nurse, to come over and check his pulse. She confirmed his suspicion and recommended he go to the emergency department. The patient refused but made an appointment to see his primary care provider. Since you are covering for his usual provider (who is on maternity leave), the patient presents to you. Review of his chart indicates that he has been healthy and active his entire life and has never had any cardiac issues. He does not have hypertension, diabetes, hypothyroidism, or pulmonary problems. His history includes GERD, kidney stones, hyperlipidemia, and a fractured left clavicle. All immunizations and tetanus booster are current. The patient denies any history of chest pain, dyspnea, syncope, near-syncope, palpitations, or other heart rhythm issues (eg, tachycardia, bradycardia, or atrial fibrillation). His last ECG, performed three years ago during a routine visit, showed normal sinus rhythm with normal intervals and no evidence of chamber enlargement; hypertrophy; arrhythmia; P, QRS, or QT interval abnormalities; or blocks. His current medications include esomeprazole magnesium, simvastatin, niacin, and aspirin. He denies illicit or homeopathic drug use and has no known drug allergies. He is a widower who does not drink alcohol or smoke cigarettes. Vital signs include a blood pressure of 108/58 mm Hg; pulse, 60 beats/min with occasional pauses; respiratory rate, 14 breaths/min-1; O2 saturation, 98% on room air; and temperature, 98.9°F. His weight is 169 lb and his height, 74 in. Physical exam reveals a tall, thin, healthy-appearing male in no distress. The HEENT exam is remarkable only for corrective lenses. There is no thyromegaly, jugular venous distention, or lymphadenopathy. The lungs are clear in all fields. The cardiac exam reveals a regular rhythm with occasional pauses and no evidence of murmurs, rubs, or extra heart sounds. The abdomen is soft and nontender, without evidence of organomegaly or masses. The peripheral pulses are 2+ bilaterally in all extremities, and the neurologic exam is intact. An ECG is performed, which reveals a ventricular rate of 55 beats/min; PR interval, 146 ms; QRS duration, 122 ms; QT/QTc interval, 424/405 ms; P axis, 60°; R axis, 38°; and T axis, 29°. What is your interpretation of this ECG?
The Value of Certainty in Diagnosis
ANSWER
For a number of reasons (discussed more fully below), the correct answer is to follow up with the pathologist (choice “c”); the biopsying provider, who is the only person to have seen the lesion, is responsible for resolving any discordance between the report and the clinical presentation/appearance.
Simply accepting the report as fact and notifying the patient of the result (choice “a”) is unacceptable. Removing more tissue from the base of the site (choice “b”) is not likely to provide any useful clinical information. Watching the site for change (choice “d”) ignores the possibility that the original lesion has already spread.
DISCUSSION
Skin tags, also known as fibroepithelioma or acrochorda, are extremely common, benign lesions encountered daily by almost all medical providers. Melanoma in tag form is decidedly unusual, but far from unknown. Around 80% of melanomas are essentially flat (macular), and about 10% are nodular. The rest, from a morphologic standpoint, are all over the map. They can be red, blue, and even white. Contrary to popular misconception, they rarely itch, and you probably wouldn’t want to depend on your dog to alert you to their presence.
My point? Although we conceive of melanomas as looking a certain way (a useful and necessary view), the reality is that their morphologic presentations are astonishingly diverse. They include pedunculated tags.
This means that unless we have a very good reason to do otherwise, we should send almost every skin lesion we remove for pathologic examination. Simple, small tags, warts, and the like can be safely discarded. But anything of substance, or anything that appears to be the least bit odd, must be submitted to pathology.
Furthermore, the pathology reports must be carefully read and the results connected to the particular lesion. This case illustrates that necessity nicely. With its black tip, this lesion was more than a little worrisome. When no mention was made of the pigmentary changes, a call to the pathologist was in order.
In this case, the pathologist was more than happy to order new and deeper cuts to be made in the specimen. Within two days, he issued a new report, which showed benign nevoid changes that explained the dark pigment and failed to show any atypia. Then, and only then, were we able to give the results to the patient.
This principle can be extrapolated to results from other types of tests. They are not to be accepted blindly by the ordering provider, who is in the unique position of having seen the patient.
ANSWER
For a number of reasons (discussed more fully below), the correct answer is to follow up with the pathologist (choice “c”); the biopsying provider, who is the only person to have seen the lesion, is responsible for resolving any discordance between the report and the clinical presentation/appearance.
Simply accepting the report as fact and notifying the patient of the result (choice “a”) is unacceptable. Removing more tissue from the base of the site (choice “b”) is not likely to provide any useful clinical information. Watching the site for change (choice “d”) ignores the possibility that the original lesion has already spread.
DISCUSSION
Skin tags, also known as fibroepithelioma or acrochorda, are extremely common, benign lesions encountered daily by almost all medical providers. Melanoma in tag form is decidedly unusual, but far from unknown. Around 80% of melanomas are essentially flat (macular), and about 10% are nodular. The rest, from a morphologic standpoint, are all over the map. They can be red, blue, and even white. Contrary to popular misconception, they rarely itch, and you probably wouldn’t want to depend on your dog to alert you to their presence.
My point? Although we conceive of melanomas as looking a certain way (a useful and necessary view), the reality is that their morphologic presentations are astonishingly diverse. They include pedunculated tags.
This means that unless we have a very good reason to do otherwise, we should send almost every skin lesion we remove for pathologic examination. Simple, small tags, warts, and the like can be safely discarded. But anything of substance, or anything that appears to be the least bit odd, must be submitted to pathology.
Furthermore, the pathology reports must be carefully read and the results connected to the particular lesion. This case illustrates that necessity nicely. With its black tip, this lesion was more than a little worrisome. When no mention was made of the pigmentary changes, a call to the pathologist was in order.
In this case, the pathologist was more than happy to order new and deeper cuts to be made in the specimen. Within two days, he issued a new report, which showed benign nevoid changes that explained the dark pigment and failed to show any atypia. Then, and only then, were we able to give the results to the patient.
This principle can be extrapolated to results from other types of tests. They are not to be accepted blindly by the ordering provider, who is in the unique position of having seen the patient.
ANSWER
For a number of reasons (discussed more fully below), the correct answer is to follow up with the pathologist (choice “c”); the biopsying provider, who is the only person to have seen the lesion, is responsible for resolving any discordance between the report and the clinical presentation/appearance.
Simply accepting the report as fact and notifying the patient of the result (choice “a”) is unacceptable. Removing more tissue from the base of the site (choice “b”) is not likely to provide any useful clinical information. Watching the site for change (choice “d”) ignores the possibility that the original lesion has already spread.
DISCUSSION
Skin tags, also known as fibroepithelioma or acrochorda, are extremely common, benign lesions encountered daily by almost all medical providers. Melanoma in tag form is decidedly unusual, but far from unknown. Around 80% of melanomas are essentially flat (macular), and about 10% are nodular. The rest, from a morphologic standpoint, are all over the map. They can be red, blue, and even white. Contrary to popular misconception, they rarely itch, and you probably wouldn’t want to depend on your dog to alert you to their presence.
My point? Although we conceive of melanomas as looking a certain way (a useful and necessary view), the reality is that their morphologic presentations are astonishingly diverse. They include pedunculated tags.
This means that unless we have a very good reason to do otherwise, we should send almost every skin lesion we remove for pathologic examination. Simple, small tags, warts, and the like can be safely discarded. But anything of substance, or anything that appears to be the least bit odd, must be submitted to pathology.
Furthermore, the pathology reports must be carefully read and the results connected to the particular lesion. This case illustrates that necessity nicely. With its black tip, this lesion was more than a little worrisome. When no mention was made of the pigmentary changes, a call to the pathologist was in order.
In this case, the pathologist was more than happy to order new and deeper cuts to be made in the specimen. Within two days, he issued a new report, which showed benign nevoid changes that explained the dark pigment and failed to show any atypia. Then, and only then, were we able to give the results to the patient.
This principle can be extrapolated to results from other types of tests. They are not to be accepted blindly by the ordering provider, who is in the unique position of having seen the patient.

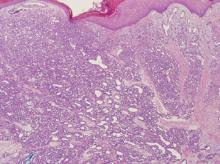
A 48-year-old woman self-refers to dermatology for evaluation of several relatively minor skin problems. One of them is a taglike lesion on the skin of her low back. Present for years, it has begun to bother her a bit; it rubs against her clothes and is occasionally traumatized enough to bleed. The patient isn’t worried about it but does want it removed. Her history is unremarkable, with no personal or family history of skin cancer. She is fair and tolerates the sun poorly, but for that reason she has limited her sun exposure throughout her life. The lesion is a 5 x 6–mm taglike nodule located in the midline of her low back. At first glance, it appears to be traumatized. But on closer inspection, the distal half of the lesion is simply black, with indistinct margins. On palpation, the lesion is firmer than most tags but nontender. A few drops of lidocaine with epinephrine are injected into the base of the lesion, which is then saucerized. Minor bleeding is easily controlled by electrocautery, and the lesion is submitted to pathology. The resultant report shows a simple benign tag. No explanation for the darker portion of the lesion is given.
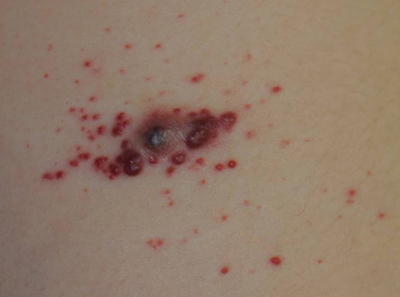
Erythematous Friable Papules on the Flank
The Diagnosis: Recurrent Pyogenic Granuloma With Satellite Papules
Pyogenic granulomas, also known as lobular capillary hemangiomas, are common benign, friable, rapidly growing, erythematous and exophytic papules with a surrounding collarette of scale. Due to the friable nature of these lesions, they are often hemorrhagic and ulcerative on presentation; however, disseminated pyogenic granulomas frequently are more sessile in appearance with a smooth surface. The pathogenesis of these lesions is largely unknown, but they exhibit a number of clinical features suggestive of reactive neovascularization.1
Primary pyogenic granulomas occur most often on sites with high vascularity (eg, gingiva, lips, fingers, face, tongue), but no site on the skin is exempt.2,3 They are found most commonly in children and young adults after minor trauma and also are associated with pregnancy with a higher frequency of occurrence on the gingiva in that particular setting.2
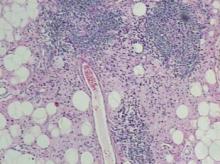
The differential diagnosis of pyogenic granuloma includes warts or inflamed intradermal nevi as well as bacillary angiomatosis and Kaposi sarcoma in an immunosuppressed patient. Most importantly, pyogenic granulomas can be confused with amelanotic melanoma; therefore, biopsy to rule out this condition is mandatory. On histopathology, pyogenic granuloma is characterized by a proliferation of capillaries arranged in a lobular pattern separated by a pale stroma (Figure).
Pyogenic granulomas usually are solitary lesions; however, recurrence after treatment is common. These benign growths have been reported to arise from certain systemic agents including epidermal growth factor receptor inhibitors, systemic retinoids, and indinavir.1 Disseminated pyogenic granulomas are rare, but cases in children and adults have been reported, many associated with prior trauma. In addition, congenital disseminated pyogenic granulomas have been described, which could be easily confused with infantile hemangiomatosis; however, the former are GLUT-1 (glucose transporter type 1) negative.4
Although recurrence of pyogenic granulomas is common, recurrence with satellite lesions is relatively rare. In documented cases, such as the Blickenstaff et al2 report, recurrences occurred most commonly on the trunk, especially in the scapular area in adolescents and young adults. They often occur after prior irritation or treatment of the primary lesion but also may spontaneously develop. After initial therapy, satellites may arise either with or without recurrence of the primary lesion. These satellite lesions usually are smaller than the original lesion, smooth, bright red, are not as friable, and lack a collarette.2,3
Recurrence of pyogenic granulomas with satellite lesions has been reported to occur in association with almost all forms of treatment, including excision with cautery and CO2 laser. These recurrences have been successfully treated with simple excision, cryotherapy, diathermy, and curettage; however, some physicians recommend a conservative approach to therapy, as a majority of these lesions tend to spontaneously resolve after 6 to 12 months.2,3,5
Ezzell et al6 and others have reported successful treatment of pyogenic granulomas with imiquimod cream 5%. Imiquimod is thought to trigger resolution of pyogenic granulomas due to its antitumor and immunoregulatory effects. As a topical agent, imiquimod would offer patients a safe and noninvasive therapy that would be especially beneficial for larger lesions with satellites, similar to the papules described in our patient.6,7
After a repeat biopsy in our patient confirmed pyogenic granuloma, she was started on imiquimod cream 5% applied nightly, which continued for 6 weeks. Follow-up after 6 weeks revealed no change in lesion size and the family opted to discontinue treatment at that time because of a lack of response. Although a continued conservative approach was recommended by our facility, the patient and guardians opted for surgical excision and were referred to a local pediatric surgeon for excision under general anesthesia.
1. North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1771-1794.
2. Blickenstaff RD, Roenigk RK, Peters MS, et al. Recurrent pyogenic granuloma with satellitosis. J Am Acad Dermatol. 1989;21:1241-1244.
3. Allen R, Rodman O. Pyogenic granuloma recurrent with satellite lesions. J Dermatol Surg Oncol. 1979;5:490-492.
4. Browning JC, Eldin KW, Kozakewich HP, et al. Congenital disseminated pyogenic granuloma. Pediatr Dermatol. 2009;26:323-327.
5. Fisher I. Recurrent multiple pyogenic granuloma with multiple satellites. features of Masson’s hemangioma. Cutis. 1977;20:201-205.
6. Ezzell TI, Fromowitz JS, Ramos-Caro FA. Recurrent pyogenic granuloma treated with topical imiquimod. J Am Acad Dermatol. 2006;54(suppl 5):S244-S245.
7. Tritton SM, Smith S, Wong LC, et al. Pyogenic granuloma in ten children treated with topical imiquimod. Pediatr Dermatol. 2009;26:269-272.
The Diagnosis: Recurrent Pyogenic Granuloma With Satellite Papules
Pyogenic granulomas, also known as lobular capillary hemangiomas, are common benign, friable, rapidly growing, erythematous and exophytic papules with a surrounding collarette of scale. Due to the friable nature of these lesions, they are often hemorrhagic and ulcerative on presentation; however, disseminated pyogenic granulomas frequently are more sessile in appearance with a smooth surface. The pathogenesis of these lesions is largely unknown, but they exhibit a number of clinical features suggestive of reactive neovascularization.1
Primary pyogenic granulomas occur most often on sites with high vascularity (eg, gingiva, lips, fingers, face, tongue), but no site on the skin is exempt.2,3 They are found most commonly in children and young adults after minor trauma and also are associated with pregnancy with a higher frequency of occurrence on the gingiva in that particular setting.2
The differential diagnosis of pyogenic granuloma includes warts or inflamed intradermal nevi as well as bacillary angiomatosis and Kaposi sarcoma in an immunosuppressed patient. Most importantly, pyogenic granulomas can be confused with amelanotic melanoma; therefore, biopsy to rule out this condition is mandatory. On histopathology, pyogenic granuloma is characterized by a proliferation of capillaries arranged in a lobular pattern separated by a pale stroma (Figure).
Pyogenic granulomas usually are solitary lesions; however, recurrence after treatment is common. These benign growths have been reported to arise from certain systemic agents including epidermal growth factor receptor inhibitors, systemic retinoids, and indinavir.1 Disseminated pyogenic granulomas are rare, but cases in children and adults have been reported, many associated with prior trauma. In addition, congenital disseminated pyogenic granulomas have been described, which could be easily confused with infantile hemangiomatosis; however, the former are GLUT-1 (glucose transporter type 1) negative.4
Although recurrence of pyogenic granulomas is common, recurrence with satellite lesions is relatively rare. In documented cases, such as the Blickenstaff et al2 report, recurrences occurred most commonly on the trunk, especially in the scapular area in adolescents and young adults. They often occur after prior irritation or treatment of the primary lesion but also may spontaneously develop. After initial therapy, satellites may arise either with or without recurrence of the primary lesion. These satellite lesions usually are smaller than the original lesion, smooth, bright red, are not as friable, and lack a collarette.2,3
Recurrence of pyogenic granulomas with satellite lesions has been reported to occur in association with almost all forms of treatment, including excision with cautery and CO2 laser. These recurrences have been successfully treated with simple excision, cryotherapy, diathermy, and curettage; however, some physicians recommend a conservative approach to therapy, as a majority of these lesions tend to spontaneously resolve after 6 to 12 months.2,3,5
Ezzell et al6 and others have reported successful treatment of pyogenic granulomas with imiquimod cream 5%. Imiquimod is thought to trigger resolution of pyogenic granulomas due to its antitumor and immunoregulatory effects. As a topical agent, imiquimod would offer patients a safe and noninvasive therapy that would be especially beneficial for larger lesions with satellites, similar to the papules described in our patient.6,7
After a repeat biopsy in our patient confirmed pyogenic granuloma, she was started on imiquimod cream 5% applied nightly, which continued for 6 weeks. Follow-up after 6 weeks revealed no change in lesion size and the family opted to discontinue treatment at that time because of a lack of response. Although a continued conservative approach was recommended by our facility, the patient and guardians opted for surgical excision and were referred to a local pediatric surgeon for excision under general anesthesia.
The Diagnosis: Recurrent Pyogenic Granuloma With Satellite Papules
Pyogenic granulomas, also known as lobular capillary hemangiomas, are common benign, friable, rapidly growing, erythematous and exophytic papules with a surrounding collarette of scale. Due to the friable nature of these lesions, they are often hemorrhagic and ulcerative on presentation; however, disseminated pyogenic granulomas frequently are more sessile in appearance with a smooth surface. The pathogenesis of these lesions is largely unknown, but they exhibit a number of clinical features suggestive of reactive neovascularization.1
Primary pyogenic granulomas occur most often on sites with high vascularity (eg, gingiva, lips, fingers, face, tongue), but no site on the skin is exempt.2,3 They are found most commonly in children and young adults after minor trauma and also are associated with pregnancy with a higher frequency of occurrence on the gingiva in that particular setting.2
The differential diagnosis of pyogenic granuloma includes warts or inflamed intradermal nevi as well as bacillary angiomatosis and Kaposi sarcoma in an immunosuppressed patient. Most importantly, pyogenic granulomas can be confused with amelanotic melanoma; therefore, biopsy to rule out this condition is mandatory. On histopathology, pyogenic granuloma is characterized by a proliferation of capillaries arranged in a lobular pattern separated by a pale stroma (Figure).
Pyogenic granulomas usually are solitary lesions; however, recurrence after treatment is common. These benign growths have been reported to arise from certain systemic agents including epidermal growth factor receptor inhibitors, systemic retinoids, and indinavir.1 Disseminated pyogenic granulomas are rare, but cases in children and adults have been reported, many associated with prior trauma. In addition, congenital disseminated pyogenic granulomas have been described, which could be easily confused with infantile hemangiomatosis; however, the former are GLUT-1 (glucose transporter type 1) negative.4
Although recurrence of pyogenic granulomas is common, recurrence with satellite lesions is relatively rare. In documented cases, such as the Blickenstaff et al2 report, recurrences occurred most commonly on the trunk, especially in the scapular area in adolescents and young adults. They often occur after prior irritation or treatment of the primary lesion but also may spontaneously develop. After initial therapy, satellites may arise either with or without recurrence of the primary lesion. These satellite lesions usually are smaller than the original lesion, smooth, bright red, are not as friable, and lack a collarette.2,3
Recurrence of pyogenic granulomas with satellite lesions has been reported to occur in association with almost all forms of treatment, including excision with cautery and CO2 laser. These recurrences have been successfully treated with simple excision, cryotherapy, diathermy, and curettage; however, some physicians recommend a conservative approach to therapy, as a majority of these lesions tend to spontaneously resolve after 6 to 12 months.2,3,5
Ezzell et al6 and others have reported successful treatment of pyogenic granulomas with imiquimod cream 5%. Imiquimod is thought to trigger resolution of pyogenic granulomas due to its antitumor and immunoregulatory effects. As a topical agent, imiquimod would offer patients a safe and noninvasive therapy that would be especially beneficial for larger lesions with satellites, similar to the papules described in our patient.6,7
After a repeat biopsy in our patient confirmed pyogenic granuloma, she was started on imiquimod cream 5% applied nightly, which continued for 6 weeks. Follow-up after 6 weeks revealed no change in lesion size and the family opted to discontinue treatment at that time because of a lack of response. Although a continued conservative approach was recommended by our facility, the patient and guardians opted for surgical excision and were referred to a local pediatric surgeon for excision under general anesthesia.
1. North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1771-1794.
2. Blickenstaff RD, Roenigk RK, Peters MS, et al. Recurrent pyogenic granuloma with satellitosis. J Am Acad Dermatol. 1989;21:1241-1244.
3. Allen R, Rodman O. Pyogenic granuloma recurrent with satellite lesions. J Dermatol Surg Oncol. 1979;5:490-492.
4. Browning JC, Eldin KW, Kozakewich HP, et al. Congenital disseminated pyogenic granuloma. Pediatr Dermatol. 2009;26:323-327.
5. Fisher I. Recurrent multiple pyogenic granuloma with multiple satellites. features of Masson’s hemangioma. Cutis. 1977;20:201-205.
6. Ezzell TI, Fromowitz JS, Ramos-Caro FA. Recurrent pyogenic granuloma treated with topical imiquimod. J Am Acad Dermatol. 2006;54(suppl 5):S244-S245.
7. Tritton SM, Smith S, Wong LC, et al. Pyogenic granuloma in ten children treated with topical imiquimod. Pediatr Dermatol. 2009;26:269-272.
1. North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1771-1794.
2. Blickenstaff RD, Roenigk RK, Peters MS, et al. Recurrent pyogenic granuloma with satellitosis. J Am Acad Dermatol. 1989;21:1241-1244.
3. Allen R, Rodman O. Pyogenic granuloma recurrent with satellite lesions. J Dermatol Surg Oncol. 1979;5:490-492.
4. Browning JC, Eldin KW, Kozakewich HP, et al. Congenital disseminated pyogenic granuloma. Pediatr Dermatol. 2009;26:323-327.
5. Fisher I. Recurrent multiple pyogenic granuloma with multiple satellites. features of Masson’s hemangioma. Cutis. 1977;20:201-205.
6. Ezzell TI, Fromowitz JS, Ramos-Caro FA. Recurrent pyogenic granuloma treated with topical imiquimod. J Am Acad Dermatol. 2006;54(suppl 5):S244-S245.
7. Tritton SM, Smith S, Wong LC, et al. Pyogenic granuloma in ten children treated with topical imiquimod. Pediatr Dermatol. 2009;26:269-272.
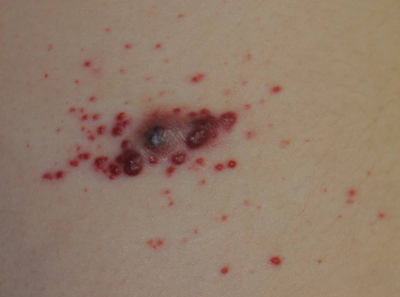
An 11-year-old girl presented to our clinic with a small erythematous friable papule on the right side of the flank. She reported that the papule bled easily and was mildly tender to palpation. A shave biopsy with electrocautery to the base of the lesion was performed. The patient returned 1 month later with recurrence of the papule and was treated with a 595-nm pulsed dye laser during her clinic visit. She returned 3 weeks later with persistence of the same papule and also with several new smaller but similar-appearing papules.
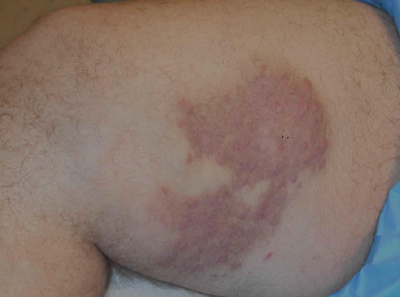
Indurated Thigh Plaque With Associated Lymphadenopathy
The Diagnosis: Rosai-Dorfman Disease
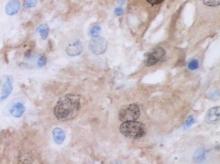
Punch biopsies of the mass from the right thigh were obtained. Hematoxylin and eosin staining revealed a reactive fibroinflammatory process of the dermis and subcutaneous tissue with fibrosis and prominent lymphoplasmacytic and histiocytic infiltrate with emperipolesis (Figure 1). The histiocytes stained positive for S-100 (Figure 2) and CD68. Markers for CD34, smooth muscle actin, and keratin were negative, as were acid-fast bacteria (Ziehl-Neelsen) and fungal (Gomori methenamine-silver) stains. The morphologic and immunohistochemical features of the biopsy specimens were consistent with Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy.
Rosai-Dorfman disease is a benign histiocytic proliferative disorder that usually involves the lymph nodes but may involve any organ system including the skin and soft tissue.1,2 Systemic RDD usually presents in children or young adults as massive cervical lymphadenopathy, often with fever, polyclonal hyperglobulinemia, mild anemia, elevated erythrocyte sedimentation rate, and occasionally neutropenia.2 Rosai-Dorfman disease was first described as a distinct clinicopathologic entity in 1969.3
Extranodal involvement occurs in approximately 40% of patients, with cutaneous disease in approximately 10% of cases. Other sites that may be affected include the eyelid and orbit, salivary glands, lungs, central nervous system, and bone.2 Purely cutaneous RDD is rare and has a variable skin distribution. Compared to systemic RDD, cutaneous disease affects older patients with a predilection for females and white individuals.4
Histologic examination of cutaneous RDD reveals a dense dermal infiltrate of foamy histiocytes with scattered lymphocytes, plasma cells, and neutrophils.2 Emperipolesis, consisting of intact lymphocytes and less commonly plasma cells in the cytoplasm of the histiocytes, is a characteristic feature of cutaneous RDD. Occasional findings include fibrosis, lymphoid aggregates, foamy histiocytes inside of dilated lymphatics, thick-walled venules with surrounding plasma cells, and multinucleate histiocytes.2 Immunohistochemistry is helpful in diagnosing RDD, as the histiocytes stain strongly positive for S-100, occasionally positive for CD68, and negative for CD1a.5
The pathogenesis of RDD remains unknown. Some cases of RDD have had positive serologies for human herpesvirus 6 and Epstein-Barr virus, though an infectious origin has not been proven.5 Clonality studies have shown the cellular infiltrate to be polyclonal, supporting a reactive rather than neoplastic process.6 The disease course of RDD varies from self-limited to protracted with remissions and exacerbations. Many cutaneous RDD lesions spontaneously heal and only require treatment when disseminated, destructive, or causing physical compromise.2 Treatment modalities for cutaneous RDD have exhibited varying degrees of success and include surgical excision; thalidomide; isotretinoin; radiotherapy; alkylating agents; and oral, topical, and intralesional steroids.2
The mass in our patient was surgically excised, though surgical margins remained positive. He was followed by the hematology-oncology service and later reported the development of 2 additional masses in the right leg with intermittent tenderness. Magnetic resonance imaging of the head was negative for intracranial abnormalities, though it did demonstrate prominent nasopharyngeal soft tissue and sinus disease. A complete skeletal radiograph survey and whole-body technetium bone scan did not show evidence of bone involvement. Chest computed tomography was negative for mass lesions. The patient deferred a bone marrow biopsy and was reportedly doing well at follow-up without further treatment.
1. Rubenstein MA, Farnsworth NN, Pielop JA, et al. Cutaneous Rosai-Dorfman disease. Dermatol Online J. 2006;12:8.
2. Goodman WT, Barrett TL. Histiocytoses. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. 2nd ed. Spain: Mosby Elsevier; 2008:1395-1410.
3. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
4. Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
5. Wartman DG, Perry A, Werchniak AE. Multiple nodules and plaques on the face and trunk. cutaneous Rosai Dorfman disease (RDD). Arch Dermatol. 2006;142:1501-1506.
6. Paulli M, Bergamaschi G, Tonon L. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Br J Haematol. 1995;91:415-418.
The Diagnosis: Rosai-Dorfman Disease
Punch biopsies of the mass from the right thigh were obtained. Hematoxylin and eosin staining revealed a reactive fibroinflammatory process of the dermis and subcutaneous tissue with fibrosis and prominent lymphoplasmacytic and histiocytic infiltrate with emperipolesis (Figure 1). The histiocytes stained positive for S-100 (Figure 2) and CD68. Markers for CD34, smooth muscle actin, and keratin were negative, as were acid-fast bacteria (Ziehl-Neelsen) and fungal (Gomori methenamine-silver) stains. The morphologic and immunohistochemical features of the biopsy specimens were consistent with Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy.
Rosai-Dorfman disease is a benign histiocytic proliferative disorder that usually involves the lymph nodes but may involve any organ system including the skin and soft tissue.1,2 Systemic RDD usually presents in children or young adults as massive cervical lymphadenopathy, often with fever, polyclonal hyperglobulinemia, mild anemia, elevated erythrocyte sedimentation rate, and occasionally neutropenia.2 Rosai-Dorfman disease was first described as a distinct clinicopathologic entity in 1969.3
Extranodal involvement occurs in approximately 40% of patients, with cutaneous disease in approximately 10% of cases. Other sites that may be affected include the eyelid and orbit, salivary glands, lungs, central nervous system, and bone.2 Purely cutaneous RDD is rare and has a variable skin distribution. Compared to systemic RDD, cutaneous disease affects older patients with a predilection for females and white individuals.4
Histologic examination of cutaneous RDD reveals a dense dermal infiltrate of foamy histiocytes with scattered lymphocytes, plasma cells, and neutrophils.2 Emperipolesis, consisting of intact lymphocytes and less commonly plasma cells in the cytoplasm of the histiocytes, is a characteristic feature of cutaneous RDD. Occasional findings include fibrosis, lymphoid aggregates, foamy histiocytes inside of dilated lymphatics, thick-walled venules with surrounding plasma cells, and multinucleate histiocytes.2 Immunohistochemistry is helpful in diagnosing RDD, as the histiocytes stain strongly positive for S-100, occasionally positive for CD68, and negative for CD1a.5
The pathogenesis of RDD remains unknown. Some cases of RDD have had positive serologies for human herpesvirus 6 and Epstein-Barr virus, though an infectious origin has not been proven.5 Clonality studies have shown the cellular infiltrate to be polyclonal, supporting a reactive rather than neoplastic process.6 The disease course of RDD varies from self-limited to protracted with remissions and exacerbations. Many cutaneous RDD lesions spontaneously heal and only require treatment when disseminated, destructive, or causing physical compromise.2 Treatment modalities for cutaneous RDD have exhibited varying degrees of success and include surgical excision; thalidomide; isotretinoin; radiotherapy; alkylating agents; and oral, topical, and intralesional steroids.2
The mass in our patient was surgically excised, though surgical margins remained positive. He was followed by the hematology-oncology service and later reported the development of 2 additional masses in the right leg with intermittent tenderness. Magnetic resonance imaging of the head was negative for intracranial abnormalities, though it did demonstrate prominent nasopharyngeal soft tissue and sinus disease. A complete skeletal radiograph survey and whole-body technetium bone scan did not show evidence of bone involvement. Chest computed tomography was negative for mass lesions. The patient deferred a bone marrow biopsy and was reportedly doing well at follow-up without further treatment.
The Diagnosis: Rosai-Dorfman Disease
Punch biopsies of the mass from the right thigh were obtained. Hematoxylin and eosin staining revealed a reactive fibroinflammatory process of the dermis and subcutaneous tissue with fibrosis and prominent lymphoplasmacytic and histiocytic infiltrate with emperipolesis (Figure 1). The histiocytes stained positive for S-100 (Figure 2) and CD68. Markers for CD34, smooth muscle actin, and keratin were negative, as were acid-fast bacteria (Ziehl-Neelsen) and fungal (Gomori methenamine-silver) stains. The morphologic and immunohistochemical features of the biopsy specimens were consistent with Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy.
Rosai-Dorfman disease is a benign histiocytic proliferative disorder that usually involves the lymph nodes but may involve any organ system including the skin and soft tissue.1,2 Systemic RDD usually presents in children or young adults as massive cervical lymphadenopathy, often with fever, polyclonal hyperglobulinemia, mild anemia, elevated erythrocyte sedimentation rate, and occasionally neutropenia.2 Rosai-Dorfman disease was first described as a distinct clinicopathologic entity in 1969.3
Extranodal involvement occurs in approximately 40% of patients, with cutaneous disease in approximately 10% of cases. Other sites that may be affected include the eyelid and orbit, salivary glands, lungs, central nervous system, and bone.2 Purely cutaneous RDD is rare and has a variable skin distribution. Compared to systemic RDD, cutaneous disease affects older patients with a predilection for females and white individuals.4
Histologic examination of cutaneous RDD reveals a dense dermal infiltrate of foamy histiocytes with scattered lymphocytes, plasma cells, and neutrophils.2 Emperipolesis, consisting of intact lymphocytes and less commonly plasma cells in the cytoplasm of the histiocytes, is a characteristic feature of cutaneous RDD. Occasional findings include fibrosis, lymphoid aggregates, foamy histiocytes inside of dilated lymphatics, thick-walled venules with surrounding plasma cells, and multinucleate histiocytes.2 Immunohistochemistry is helpful in diagnosing RDD, as the histiocytes stain strongly positive for S-100, occasionally positive for CD68, and negative for CD1a.5
The pathogenesis of RDD remains unknown. Some cases of RDD have had positive serologies for human herpesvirus 6 and Epstein-Barr virus, though an infectious origin has not been proven.5 Clonality studies have shown the cellular infiltrate to be polyclonal, supporting a reactive rather than neoplastic process.6 The disease course of RDD varies from self-limited to protracted with remissions and exacerbations. Many cutaneous RDD lesions spontaneously heal and only require treatment when disseminated, destructive, or causing physical compromise.2 Treatment modalities for cutaneous RDD have exhibited varying degrees of success and include surgical excision; thalidomide; isotretinoin; radiotherapy; alkylating agents; and oral, topical, and intralesional steroids.2
The mass in our patient was surgically excised, though surgical margins remained positive. He was followed by the hematology-oncology service and later reported the development of 2 additional masses in the right leg with intermittent tenderness. Magnetic resonance imaging of the head was negative for intracranial abnormalities, though it did demonstrate prominent nasopharyngeal soft tissue and sinus disease. A complete skeletal radiograph survey and whole-body technetium bone scan did not show evidence of bone involvement. Chest computed tomography was negative for mass lesions. The patient deferred a bone marrow biopsy and was reportedly doing well at follow-up without further treatment.
1. Rubenstein MA, Farnsworth NN, Pielop JA, et al. Cutaneous Rosai-Dorfman disease. Dermatol Online J. 2006;12:8.
2. Goodman WT, Barrett TL. Histiocytoses. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. 2nd ed. Spain: Mosby Elsevier; 2008:1395-1410.
3. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
4. Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
5. Wartman DG, Perry A, Werchniak AE. Multiple nodules and plaques on the face and trunk. cutaneous Rosai Dorfman disease (RDD). Arch Dermatol. 2006;142:1501-1506.
6. Paulli M, Bergamaschi G, Tonon L. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Br J Haematol. 1995;91:415-418.
1. Rubenstein MA, Farnsworth NN, Pielop JA, et al. Cutaneous Rosai-Dorfman disease. Dermatol Online J. 2006;12:8.
2. Goodman WT, Barrett TL. Histiocytoses. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. 2nd ed. Spain: Mosby Elsevier; 2008:1395-1410.
3. Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
4. Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
5. Wartman DG, Perry A, Werchniak AE. Multiple nodules and plaques on the face and trunk. cutaneous Rosai Dorfman disease (RDD). Arch Dermatol. 2006;142:1501-1506.
6. Paulli M, Bergamaschi G, Tonon L. Evidence for a polyclonal nature of the cell infiltrate in sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease). Br J Haematol. 1995;91:415-418.
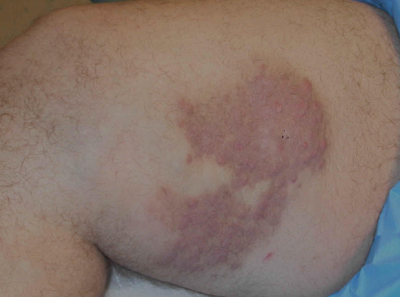
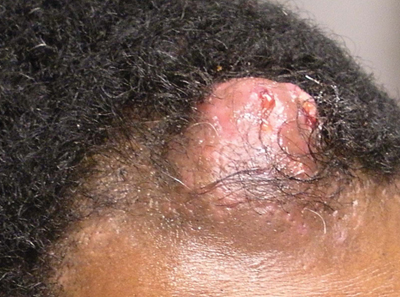
A 38-year-old white man with a history of lower extremity varicose veins was referred for evaluation of a plaque on the medial aspect of the right thigh and right inguinal lymphadenopathy. The patient reported having the lesion for approximately 1 year; over time it had become progressively more indurated. He denied pain, discomfort, and pruritus at the site, as well as any systemic symptoms. The patient had no known allergies, was not taking any medications, smoked 1 pack of tobacco daily for 20 years, drank alcohol socially, and was not sexually active. His family history was noncontributory. Prior to the dermatology consultation, a computed tomography scan of the right leg, abdomen, and pelvis was obtained and demonstrated a subcutaneous mass in the distal third of the medial aspect of the right thigh as well as pericaval and right inguinal lymphadenopathy. Physical examination revealed a violaceous, indurated, nodular plaque on the medial aspect of the right thigh measuring approximately 10×5 cm. Multiple small, nontender, mobile lymph nodes were palpated on the right side of the inguinal region. The remainder of the physical examination was unremarkable.
Elderly Woman Takes a Fall
ANSWER
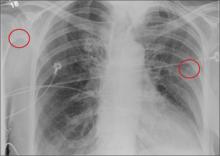
The radiograph shows the lungs overall to be clear. There are some slight increased markings, perhaps suggestive of mild congestion, but no infiltrate or consolidation.
Of note is a small nodule within the middle portion of the left upper lobe that requires monitoring and further workup. Also, although it is incompletely imaged, there appears to be a fracture of the right humeral neck.
Additional imaging confirmed the fracture. Orthopedic consultation was obtained.
ANSWER
The radiograph shows the lungs overall to be clear. There are some slight increased markings, perhaps suggestive of mild congestion, but no infiltrate or consolidation.
Of note is a small nodule within the middle portion of the left upper lobe that requires monitoring and further workup. Also, although it is incompletely imaged, there appears to be a fracture of the right humeral neck.
Additional imaging confirmed the fracture. Orthopedic consultation was obtained.
ANSWER
The radiograph shows the lungs overall to be clear. There are some slight increased markings, perhaps suggestive of mild congestion, but no infiltrate or consolidation.
Of note is a small nodule within the middle portion of the left upper lobe that requires monitoring and further workup. Also, although it is incompletely imaged, there appears to be a fracture of the right humeral neck.
Additional imaging confirmed the fracture. Orthopedic consultation was obtained.
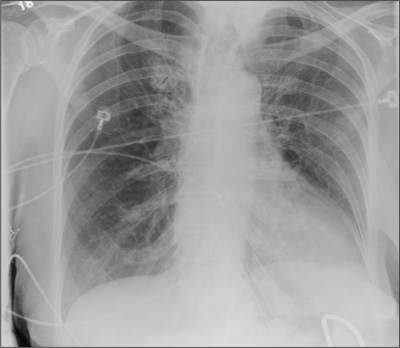
A 90-year-old woman is complaining of pain on the left side of her face, her chest wall, and right shoulder. Her family reports that she has fallen multiple times recently. On one occasion, there was brief loss of consciousness. History is significant for hypertension and osteoarthritis. Initial examination indicates she is awake, alert, oriented, and in no obvious distress. Vital signs are stable, and breath sounds are clear. There is tenderness on the left side of her face and decreased range of motion in her right shoulder, as well as localized tenderness. The hospitalist ordered a chest radiograph when the patient was admitted. What is your impression?
Too Tired to Stop and Smell the Roses
ANSWER
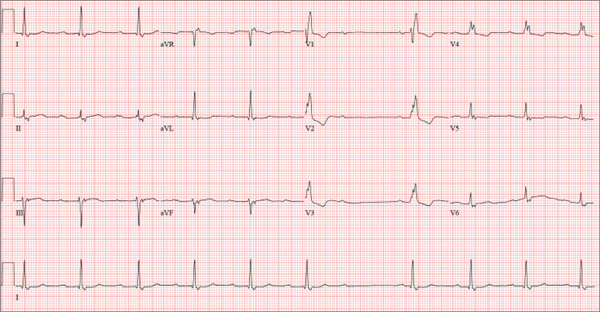
Findings on this ECG include sinus rhythm at a rate of 60 beats/min, evidence of a second-degree atrioventricular (AV) block (Mobitz I), and a right bundle branch block (RBBB).
To understand the rhythm, it is best to focus on the rhythm strip, particularly lead I at the bottom of the ECG. If you measure the P-to-P interval, you will notice that it is consistent and constant at a rate of 60 beats/min, regardless of the QRS complex. If you look at the PR interval from the second to the sixth QRS complex, you will notice that it is regular until the QRS is dropped after the P wave that follows the sixth QRS complex. Following the pause, the PR interval on the seventh, eighth, and ninth QRS complexes gradually prolongs. Although this is not a classic example of Mobitz I block, it is indicative of an AV node with a conduction abnormality.
Subsequent rhythm strips documented multiple blocked PR intervals that corresponded to the patient’s dizziness. The RBBB is evident by the RSR’ pattern seen in lead V1 with a QRS duration ≥ 120 ms.
ANSWER
Findings on this ECG include sinus rhythm at a rate of 60 beats/min, evidence of a second-degree atrioventricular (AV) block (Mobitz I), and a right bundle branch block (RBBB).
To understand the rhythm, it is best to focus on the rhythm strip, particularly lead I at the bottom of the ECG. If you measure the P-to-P interval, you will notice that it is consistent and constant at a rate of 60 beats/min, regardless of the QRS complex. If you look at the PR interval from the second to the sixth QRS complex, you will notice that it is regular until the QRS is dropped after the P wave that follows the sixth QRS complex. Following the pause, the PR interval on the seventh, eighth, and ninth QRS complexes gradually prolongs. Although this is not a classic example of Mobitz I block, it is indicative of an AV node with a conduction abnormality.
Subsequent rhythm strips documented multiple blocked PR intervals that corresponded to the patient’s dizziness. The RBBB is evident by the RSR’ pattern seen in lead V1 with a QRS duration ≥ 120 ms.
ANSWER
Findings on this ECG include sinus rhythm at a rate of 60 beats/min, evidence of a second-degree atrioventricular (AV) block (Mobitz I), and a right bundle branch block (RBBB).
To understand the rhythm, it is best to focus on the rhythm strip, particularly lead I at the bottom of the ECG. If you measure the P-to-P interval, you will notice that it is consistent and constant at a rate of 60 beats/min, regardless of the QRS complex. If you look at the PR interval from the second to the sixth QRS complex, you will notice that it is regular until the QRS is dropped after the P wave that follows the sixth QRS complex. Following the pause, the PR interval on the seventh, eighth, and ninth QRS complexes gradually prolongs. Although this is not a classic example of Mobitz I block, it is indicative of an AV node with a conduction abnormality.
Subsequent rhythm strips documented multiple blocked PR intervals that corresponded to the patient’s dizziness. The RBBB is evident by the RSR’ pattern seen in lead V1 with a QRS duration ≥ 120 ms.
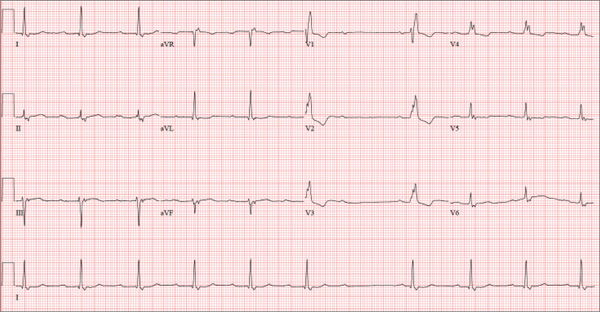
A 74-year-old man lives alone in his home and cares for a large garden of which he is very proud. Recently, his granddaughter noticed that the garden had not been tended to. When asked, her grandfather told her he had been too tired to take care of it. Further questioning revealed that he had experienced frequent dizzy spells and had passed out in his garden about two weeks ago. Since then, he has been reluctant to go outside. Concerned, his granddaughter brings him to your urgent care clinic for evaluation. During the history, you learn that the patient has not seen a clinician in more than 10 years because he “doesn’t like to be a bother.” He has a long-standing diagnosis of hypertension that is untreated because he doesn’t like to take pills. Eliciting information is difficult, but his granddaughter reports that he had a cholecystectomy in the distant past; she cannot recall any other problems. The patient currently takes no medications; he is allergic to penicillin, which produces a true anaphylactic response. He has a remote history of smoking, but he stopped after his wife died of lung cancer 12 years ago. He drinks two or three cans of beer per week and does not use recreational drugs or herbal medicines. He had one son, who died in an automobile accident five years ago; his daughter-in-law visits infrequently and his granddaughter frequently. He has no living siblings. Review of systems is remarkable for knee and hip pain and stiffness from osteoarthritis, as well as occasional constipation. He denies palpitations, irregular or rapid heartbeats, shortness of breath, and lower extremity swelling. Aside from his dizzy spells, he claims to be “healthy as a horse.” Physical exam reveals a blood pressure of 192/102 mm Hg; pulse, 60 beats/min and irregular; respiratory rate, 18 breaths/min; and temperature, 98.1°F. His height is 66 in and his weight, 164 lb. The patient wears corrective lenses, and arcus senilis is present. There are multiple teeth missing, but those that remain are in good repair. There is no thyromegaly, and a soft bruit is present over the left carotid artery. The patient is somewhat barrel chested, and all breath sounds are clear. There is a harsh, early systolic murmur best heard at the left upper sternal border and no extra heart sounds or rubs. The abdomen is scaphoid and soft, and surprisingly, despite the history of a cholecystectomy, there is no abdominal scar. The extremities are consistent with signs of longstanding osteoarthritis. Peripheral pulses are strong bilaterally, and the neurologic exam is grossly intact. You order a chemistry panel, complete blood count, thyroid function studies, liver function studies, and an ECG. While the laboratory data are still pending, you receive the results of the ECG, which show a ventricular rate of 56 beats/min; PR interval, not measurable; QRS duration, 144 ms; QT/QTc interval, 438/422 ms; P axis, 47°; R axis, –24°; and T axis, 55°. What is your interpretation of this ECG—and have you found a reason for his dizziness?
Relatively Asymptomatic, but Still Problematic
ANSWER
The correct answer is seborrheic dermatitis (choice “d”), a common cause of penile rashes that typically manifests initially as chronic dandruff or in some other form on the head or neck.
Herpes simplex (choice “a”) is certainly common, but it likely would have presented with grouped vesicles on an erythematous base. Furthermore, each episode would have been limited to about two weeks, and the eruption would have produced noticeable symptoms and responded to the valacyclovir.
Yeast infection (choice “b”), while often diagnosed, is in reality unusual, especially in the circumcised and otherwise healthy male. Nystatin, although far from the ideal treatment, should have had some effect.
Fixed drug eruption (FDE; choice “c”) could have been a suspect, had there been a drug to blame. FDE usually presents as a brownish red, shiny round macule that appears and reappears in the same area with repeated exposure to the same drug. The penile shaft is a favorite area for it. Drugs known to trigger FDE include NSAIDs, sulfa, tetracycline, penicillin, pseudoephedrine, and aspirin.
DISCUSSION
Seborrheic dermatitis (SD), also known as seborrhea, is an extremely common chronic papulosquamous disorder patterned on the sebum-rich areas of the scalp, face, and trunk. Although not directly caused by the highly lipophilic commensal yeast Malassezia furfur, it does appear to be related to increases in the number of those organisms, as well as to immunologic abnormalities and increased production of sebum. It can range from a mild scaly rash to whole-body erythroderma and can affect an astonishing range of areas, including the genitals.
SD almost always manifests with dandruff (or “cradle cap” in the infant), followed by faint scaling in and around the ears or on the face (eg, nasolabial folds, brows, and glabella), mid chest, axillae, periumbilical region, and genitals. Below the head and neck, SD often mystifies the nondermatology provider, who tends to call it “fungal infection” or, when it’s seen in moist intertriginous skin, “yeast infection.”
SD, especially in this case, represents the perfect example of the need to “look elsewhere” for clues when confronted with a mysterious rash. Patients can certainly have more than one dermatologic diagnosis at a time, but a single explanation is considerably more likely and should therefore be sought. In this case, corroboration for the diagnosis of SD was readily found by looking for it in its known locations.
SD can take on different looks, including a distinctly annular morphology, especially in patients with darker skin. It can occasionally be severe in patients with Parkinson’s disease, multiple sclerosis, or a history of stroke. This case mirrors my experience in that I see increased stress as a major precipitating factor in the worsening of pre-existing SD.
In addition to the items already mentioned, the differential for penile rashes includes lichen planus. However, the lesions of lichen planus tend to have a distinctly purple appearance and well-defined margins, and on the penis, they tend to spill over onto the penile corona and glans.
TREATMENT/PROGNOSIS
In this case, treatment comprised a combination of oxiconazole lotion and 2.5% hydrocortisone cream. Many other combinations have been used successfully, including pimecrolimus or tacrolimus combined with ketoconazole cream.
Whatever is used, a cure will not be forthcoming, since the condition is almost always chronic. The main value of an accurate diagnosis in such a case lies in easing the patient’s mind regarding the terrible diseases he doesn’t have.
ANSWER
The correct answer is seborrheic dermatitis (choice “d”), a common cause of penile rashes that typically manifests initially as chronic dandruff or in some other form on the head or neck.
Herpes simplex (choice “a”) is certainly common, but it likely would have presented with grouped vesicles on an erythematous base. Furthermore, each episode would have been limited to about two weeks, and the eruption would have produced noticeable symptoms and responded to the valacyclovir.
Yeast infection (choice “b”), while often diagnosed, is in reality unusual, especially in the circumcised and otherwise healthy male. Nystatin, although far from the ideal treatment, should have had some effect.
Fixed drug eruption (FDE; choice “c”) could have been a suspect, had there been a drug to blame. FDE usually presents as a brownish red, shiny round macule that appears and reappears in the same area with repeated exposure to the same drug. The penile shaft is a favorite area for it. Drugs known to trigger FDE include NSAIDs, sulfa, tetracycline, penicillin, pseudoephedrine, and aspirin.
DISCUSSION
Seborrheic dermatitis (SD), also known as seborrhea, is an extremely common chronic papulosquamous disorder patterned on the sebum-rich areas of the scalp, face, and trunk. Although not directly caused by the highly lipophilic commensal yeast Malassezia furfur, it does appear to be related to increases in the number of those organisms, as well as to immunologic abnormalities and increased production of sebum. It can range from a mild scaly rash to whole-body erythroderma and can affect an astonishing range of areas, including the genitals.
SD almost always manifests with dandruff (or “cradle cap” in the infant), followed by faint scaling in and around the ears or on the face (eg, nasolabial folds, brows, and glabella), mid chest, axillae, periumbilical region, and genitals. Below the head and neck, SD often mystifies the nondermatology provider, who tends to call it “fungal infection” or, when it’s seen in moist intertriginous skin, “yeast infection.”
SD, especially in this case, represents the perfect example of the need to “look elsewhere” for clues when confronted with a mysterious rash. Patients can certainly have more than one dermatologic diagnosis at a time, but a single explanation is considerably more likely and should therefore be sought. In this case, corroboration for the diagnosis of SD was readily found by looking for it in its known locations.
SD can take on different looks, including a distinctly annular morphology, especially in patients with darker skin. It can occasionally be severe in patients with Parkinson’s disease, multiple sclerosis, or a history of stroke. This case mirrors my experience in that I see increased stress as a major precipitating factor in the worsening of pre-existing SD.
In addition to the items already mentioned, the differential for penile rashes includes lichen planus. However, the lesions of lichen planus tend to have a distinctly purple appearance and well-defined margins, and on the penis, they tend to spill over onto the penile corona and glans.
TREATMENT/PROGNOSIS
In this case, treatment comprised a combination of oxiconazole lotion and 2.5% hydrocortisone cream. Many other combinations have been used successfully, including pimecrolimus or tacrolimus combined with ketoconazole cream.
Whatever is used, a cure will not be forthcoming, since the condition is almost always chronic. The main value of an accurate diagnosis in such a case lies in easing the patient’s mind regarding the terrible diseases he doesn’t have.
ANSWER
The correct answer is seborrheic dermatitis (choice “d”), a common cause of penile rashes that typically manifests initially as chronic dandruff or in some other form on the head or neck.
Herpes simplex (choice “a”) is certainly common, but it likely would have presented with grouped vesicles on an erythematous base. Furthermore, each episode would have been limited to about two weeks, and the eruption would have produced noticeable symptoms and responded to the valacyclovir.
Yeast infection (choice “b”), while often diagnosed, is in reality unusual, especially in the circumcised and otherwise healthy male. Nystatin, although far from the ideal treatment, should have had some effect.
Fixed drug eruption (FDE; choice “c”) could have been a suspect, had there been a drug to blame. FDE usually presents as a brownish red, shiny round macule that appears and reappears in the same area with repeated exposure to the same drug. The penile shaft is a favorite area for it. Drugs known to trigger FDE include NSAIDs, sulfa, tetracycline, penicillin, pseudoephedrine, and aspirin.
DISCUSSION
Seborrheic dermatitis (SD), also known as seborrhea, is an extremely common chronic papulosquamous disorder patterned on the sebum-rich areas of the scalp, face, and trunk. Although not directly caused by the highly lipophilic commensal yeast Malassezia furfur, it does appear to be related to increases in the number of those organisms, as well as to immunologic abnormalities and increased production of sebum. It can range from a mild scaly rash to whole-body erythroderma and can affect an astonishing range of areas, including the genitals.
SD almost always manifests with dandruff (or “cradle cap” in the infant), followed by faint scaling in and around the ears or on the face (eg, nasolabial folds, brows, and glabella), mid chest, axillae, periumbilical region, and genitals. Below the head and neck, SD often mystifies the nondermatology provider, who tends to call it “fungal infection” or, when it’s seen in moist intertriginous skin, “yeast infection.”
SD, especially in this case, represents the perfect example of the need to “look elsewhere” for clues when confronted with a mysterious rash. Patients can certainly have more than one dermatologic diagnosis at a time, but a single explanation is considerably more likely and should therefore be sought. In this case, corroboration for the diagnosis of SD was readily found by looking for it in its known locations.
SD can take on different looks, including a distinctly annular morphology, especially in patients with darker skin. It can occasionally be severe in patients with Parkinson’s disease, multiple sclerosis, or a history of stroke. This case mirrors my experience in that I see increased stress as a major precipitating factor in the worsening of pre-existing SD.
In addition to the items already mentioned, the differential for penile rashes includes lichen planus. However, the lesions of lichen planus tend to have a distinctly purple appearance and well-defined margins, and on the penis, they tend to spill over onto the penile corona and glans.
TREATMENT/PROGNOSIS
In this case, treatment comprised a combination of oxiconazole lotion and 2.5% hydrocortisone cream. Many other combinations have been used successfully, including pimecrolimus or tacrolimus combined with ketoconazole cream.
Whatever is used, a cure will not be forthcoming, since the condition is almost always chronic. The main value of an accurate diagnosis in such a case lies in easing the patient’s mind regarding the terrible diseases he doesn’t have.

A 31-year-old man is referred to dermatology for evaluation of a penile rash that has repeatedly manifested and resolved over a period of months. Relatively asymptomatic, the eruption has persisted despite a two-week course of valacyclovir 500 mg bid, followed by a month-long course of topical nystatin cream tid. The patient says he has been in otherwise good health. However, he reports being under a great deal of stress, as his job and his marriage ended within the space of a few weeks. He denies any sexual exposure outside his marriage. Other than those already mentioned, the patient has taken no medications, prescription or OTC. The problem area is obvious: a bright pink papulosquamous patch on the distal right shaft of his circumcised penis. This round lesion, which measures more than 3 cm in diameter, has a shiny appearance and slightly irregular margins. No other areas of involvement are noted in the genital area. However, there is a similar scaly pink rash behind both of the patient’s ears, as well as patches of dandruff in the scalp, especially over and behind the ears. A similar rash is seen in the patient’s umbilicus and surrounding area.
Thickened Velvety Plaques in a 75-Year-Old Woman
The Diagnosis: Tripe Palms and Acanthosis Nigricans
A shave biopsy specimen from the left palm showed slight epidermal hyperplasia with substantial papillomatosis and compact orthokeratosis. The complete blood cell count, thyrotropin level, uric acid level, liver function tests, mammogram, Papanicolaou test, and chest radiograph were unremarkable. A basic metabolic panel showed mildly elevated blood glucose at 111 mg/dL (reference range, 70-99 mg/dL) and hemoglobin A1c at 6.3% (reference range, <6.0%). Full-body computed tomography, endoscopy, and colonoscopy initially were normal. One year later after presenting with tripe palms, the patient had a bowel obstruction secondary to omental carcinomatosis from a primary ovarian tumor.
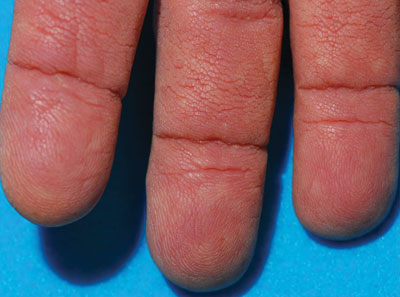
The term tripe in tripe palms refers to the resemblance to the edible lining of the bovine foregut. It originated in 1963 from a patient's own description of the rugose velvety texture of the palms.1 In the literature, tripe palms also is called acanthosis palmaris, acanthosis nigricans of the palms, palmar hyperkeratosis, palmar keratoderma, and pachydermatoglyphy. It is a rare cutaneous finding. Tripe palms is associated with other cutaneous paraneoplastic syndromes such as malignant acanthosis nigricans (72% of cases) and Leser-Trélat sign (10% of cases). It affects more men than women (63% vs 37%) and is almost exclusively seen in adults (median age, 62 years).1
The clinical appearance of tripe palms includes hypertrophy of the palms and often the soles with papillations producing a velvety or honeycomb appearance. In addition, the dermatoglyphics are pronounced. The histologic findings typically show hyperkeratosis and acanthosis. Other features that can be seen include dermal mucinosis and increased mast cells in the dermis. To differentiate tripe palms from other keratodermas, substantial papillations can be seen with less diffuse hyperkeratosis.1
Tripe palms has been associated with an underlying malignancy in more than 90% of published cases. In two-thirds of cases, tripe palms either appears before or concurrent with the diagnosis of cancer.2 It is rarely reported as an idiopathic finding or associated with nonneoplastic disorders. Malignancies most commonly associated are adenocarcinomas, especially of the stomach (27%) and lungs (22%). Other neoplasms, such as in our patient, include those of the genitourinary tract and breast. In a patient with tripe palms in the absence of acanthosis nigricans, the most common neoplasm is of the lung, especially when clubbing of the nails also is present.2 Thus, after a diagnosis of tripe palms is established, a thorough investigation for an underlying malignancy is the next most important step to direct specific therapy.
1. Cohen PR, Grossman ME, Silvers DN, et al. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
2. Moore RL, Devere TS. Epidermal manifestations of internal malignancy. Dermatol Clin. 2008;26:17-29.
The Diagnosis: Tripe Palms and Acanthosis Nigricans
A shave biopsy specimen from the left palm showed slight epidermal hyperplasia with substantial papillomatosis and compact orthokeratosis. The complete blood cell count, thyrotropin level, uric acid level, liver function tests, mammogram, Papanicolaou test, and chest radiograph were unremarkable. A basic metabolic panel showed mildly elevated blood glucose at 111 mg/dL (reference range, 70-99 mg/dL) and hemoglobin A1c at 6.3% (reference range, <6.0%). Full-body computed tomography, endoscopy, and colonoscopy initially were normal. One year later after presenting with tripe palms, the patient had a bowel obstruction secondary to omental carcinomatosis from a primary ovarian tumor.
The term tripe in tripe palms refers to the resemblance to the edible lining of the bovine foregut. It originated in 1963 from a patient's own description of the rugose velvety texture of the palms.1 In the literature, tripe palms also is called acanthosis palmaris, acanthosis nigricans of the palms, palmar hyperkeratosis, palmar keratoderma, and pachydermatoglyphy. It is a rare cutaneous finding. Tripe palms is associated with other cutaneous paraneoplastic syndromes such as malignant acanthosis nigricans (72% of cases) and Leser-Trélat sign (10% of cases). It affects more men than women (63% vs 37%) and is almost exclusively seen in adults (median age, 62 years).1
The clinical appearance of tripe palms includes hypertrophy of the palms and often the soles with papillations producing a velvety or honeycomb appearance. In addition, the dermatoglyphics are pronounced. The histologic findings typically show hyperkeratosis and acanthosis. Other features that can be seen include dermal mucinosis and increased mast cells in the dermis. To differentiate tripe palms from other keratodermas, substantial papillations can be seen with less diffuse hyperkeratosis.1
Tripe palms has been associated with an underlying malignancy in more than 90% of published cases. In two-thirds of cases, tripe palms either appears before or concurrent with the diagnosis of cancer.2 It is rarely reported as an idiopathic finding or associated with nonneoplastic disorders. Malignancies most commonly associated are adenocarcinomas, especially of the stomach (27%) and lungs (22%). Other neoplasms, such as in our patient, include those of the genitourinary tract and breast. In a patient with tripe palms in the absence of acanthosis nigricans, the most common neoplasm is of the lung, especially when clubbing of the nails also is present.2 Thus, after a diagnosis of tripe palms is established, a thorough investigation for an underlying malignancy is the next most important step to direct specific therapy.
The Diagnosis: Tripe Palms and Acanthosis Nigricans
A shave biopsy specimen from the left palm showed slight epidermal hyperplasia with substantial papillomatosis and compact orthokeratosis. The complete blood cell count, thyrotropin level, uric acid level, liver function tests, mammogram, Papanicolaou test, and chest radiograph were unremarkable. A basic metabolic panel showed mildly elevated blood glucose at 111 mg/dL (reference range, 70-99 mg/dL) and hemoglobin A1c at 6.3% (reference range, <6.0%). Full-body computed tomography, endoscopy, and colonoscopy initially were normal. One year later after presenting with tripe palms, the patient had a bowel obstruction secondary to omental carcinomatosis from a primary ovarian tumor.
The term tripe in tripe palms refers to the resemblance to the edible lining of the bovine foregut. It originated in 1963 from a patient's own description of the rugose velvety texture of the palms.1 In the literature, tripe palms also is called acanthosis palmaris, acanthosis nigricans of the palms, palmar hyperkeratosis, palmar keratoderma, and pachydermatoglyphy. It is a rare cutaneous finding. Tripe palms is associated with other cutaneous paraneoplastic syndromes such as malignant acanthosis nigricans (72% of cases) and Leser-Trélat sign (10% of cases). It affects more men than women (63% vs 37%) and is almost exclusively seen in adults (median age, 62 years).1
The clinical appearance of tripe palms includes hypertrophy of the palms and often the soles with papillations producing a velvety or honeycomb appearance. In addition, the dermatoglyphics are pronounced. The histologic findings typically show hyperkeratosis and acanthosis. Other features that can be seen include dermal mucinosis and increased mast cells in the dermis. To differentiate tripe palms from other keratodermas, substantial papillations can be seen with less diffuse hyperkeratosis.1
Tripe palms has been associated with an underlying malignancy in more than 90% of published cases. In two-thirds of cases, tripe palms either appears before or concurrent with the diagnosis of cancer.2 It is rarely reported as an idiopathic finding or associated with nonneoplastic disorders. Malignancies most commonly associated are adenocarcinomas, especially of the stomach (27%) and lungs (22%). Other neoplasms, such as in our patient, include those of the genitourinary tract and breast. In a patient with tripe palms in the absence of acanthosis nigricans, the most common neoplasm is of the lung, especially when clubbing of the nails also is present.2 Thus, after a diagnosis of tripe palms is established, a thorough investigation for an underlying malignancy is the next most important step to direct specific therapy.
1. Cohen PR, Grossman ME, Silvers DN, et al. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
2. Moore RL, Devere TS. Epidermal manifestations of internal malignancy. Dermatol Clin. 2008;26:17-29.
1. Cohen PR, Grossman ME, Silvers DN, et al. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
2. Moore RL, Devere TS. Epidermal manifestations of internal malignancy. Dermatol Clin. 2008;26:17-29.
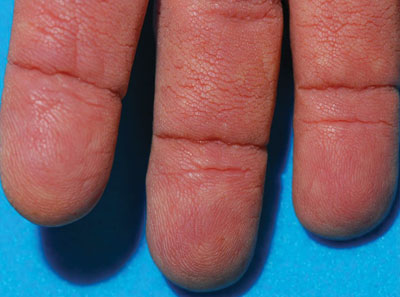
A 75-year-old woman presented with progressive, velvety, thick skin involving the bilateral axillae, inner thighs, palms, and buccal mucosa. She also reported weight loss of approximately 25 pounds over the last 12 months. Her medical history was notable for metabolic syndrome, allergic rhinitis, and colon polyps. She denied a family history of malignancy. On physical examination, she was a healthy-appearing overweight woman. The palmar surface of the bilateral hands was thickened and velvety with exaggerated dermatoglyphics. She had similarly thickened, velvety, gray-brown plaques on the bilateral axillae and proximal aspects of the inner thighs. The buccal mucosa had a thickened rugose texture.
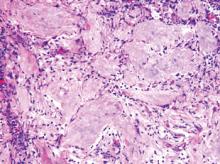
Solitary Nodular Lesion on the Scalp
The Diagnosis: Pilomatricoma
Pilomatricoma, first described by Malherbe and Chenantais1 in 1880, is a benign appendageal tumor derived from hair follicle matrix cells. It classically manifests as a solitary, asymptomatic, firm dermal nodule with a normal overlying epidermis. Less common morphologic variants include perforating, lymphagiectatic, keratoacanthomalike, pigmented, and anetodermalike surface changes.2 Inflammation and erosion through the skin surface are observed in the rare perforating variant, as seen in our patient. The average size is 1 cm, and it rarely exceeds 3 cm in diameter.3 The tumors predominantly occur on the head, neck, and upper extremities, with only 9.5% on the scalp.2 It may occur at any age, though it has a bimodal distribution with peaks in childhood and in adults older than 60 years. A slight preponderance in females has been observed with a female to male ratio of 1.5 to 1.2 Although our patient is black, most reported cases have occurred in individuals of European descent. Because cases of pilomatricoma are not systematically reported, it is uncertain if this finding represents a publication bias or if race is an actual risk factor. Multiple pilomatricomas and familial cases have been described in association with myotonic dystrophy, Turner syndrome, Gardner syndrome, Rubinstein-Taybi syndrome, polyfactorial coagulopathy, trisomy 9, xeroderma pigmentosum, and basal cell nevus syndrome.2,4
It has been shown that the proliferating cells of pilomatricomas stain with antibodies directed against Lef1 (lymphoid enhancer binding factor 1), a marker from hair matrix cells, providing biochemical evidence for the morphologic appearance of these neoplasms.5 Pilomatricomas have been associated with B-cell/chronic lymphocytic leukemia lymphoma 2 gene, BCL2, expression, a proto-oncogene that suppresses apoptosis in benign and malignant neoplasms, which may contribute to the pathogenesis of these tumors.6 Pilomatricomas also have been associated with β-catenin mutation, expression of Bmp2 (bone morphogenetic protein 2), and human hair keratin basic 1.7-9
Definitive diagnosis is obtained through biopsy, looking for characteristic histopathologic findings. The lesion usually is found in the lower dermis and subcutaneous fat. However, in the perforating variant, the lesion is more superficial, located in the papillary and mid dermis, as seen in our patient.10
Pilomatricomas are sharply demarcated, often surrounded by a connective-tissue capsule. Histopathologic analysis reveals islands of epithelial cells comprised of 3 subtypes: basophilic cells with scant cytoplasm, shadow cells with a central pallor (Figure), and transitional cells between the former 2 cellular types.11 The number of basophilic and transitional cells is inversely related to the number of shadow cells. In older lesions, the shadow cells predominate, while the basophilic cells are few in number or absent. Calcium deposits are seen in 80% of lesions with von Kossa staining.12
Transformation into malignancy, known as pilomatrical carcinoma, is rare. These malignant neoplasms are characterized by aggressive biologic behavior such as recurrence, diffuse spread, or metastasis, or by cytologic abnormalities such as poor cellular organization, squamous differentiation, and conspicuous mitotic activity.13 The recent growth of the long-standing lesion in our patient might be interpreted as a sign of malignant transformation. However, this observation may be related to the intense inflammatory reaction supported by the histopathology.
Pilomatricomas are not associated with mortality. Pilomatrical carcinomas are uncommon but are locally invasive and can cause visceral metastases and death. Spontaneous regression has never been observed and medical treatment is ineffective. The treatment of choice is incision and curettage or surgical excision.14 Although recurrence has only been reported in 2.6% of cases from a large case series (N=228), patients should be monitored after surgical excision.12
1. Malherbe A, Chenantais J. Note sur l'epithelioma calcifie des glandes sebacees. Prog Med. 1880;8:826-828.
2. Julian CG, Bowers PW. A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998;39(2, pt 1):191-195.
3. Lozzi GP, Soyer HP, Fruehauf J, et al. Giant pilomatricoma. Am J Dermatopathol. 2007;29:286-289.
4. Hubbard VG, Whittaker SJ. Multiple familial pilomatricomas: an unusual case. J Cutan Pathol. 2004;31:281-283.
5. Kizawa K, Toyoda M, Ito M, et al. Aberrantly differentiated cells in benign pilomatrixoma reflect the normal hair follicle: immunohistochemical analysis of Ca-binding S100A2, S100A3 and S100A6 proteins. Br J Dermatol. 2005;152:314-320.
6. Farrier S, Morgan M. bcl-2 expression in pilomatricoma. Am J Dermatopathol. 1997;19:254-257.
7. Park SW, Suh KS, Wang HY, et al. Beta-catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol. 2001;145:624-629.
8. Kurokawa I, Kusumoto K, Bessho K, et al. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
9. Cribier B, Asch PH, Regnier C, et al. Expression of human hair keratin basic 1 in pilomatrixoma: a study of 128 cases. Br J Dermatol. 1999;140:600-604.
10. Bayle P, Bazex J, Lamant L, et al. Multiple perforating and non perforating pilomatricomas in a patient with Churg-Strauss syndrome and Rubinstein-Taybi syndrome. J Eur Acad Dermatol Venereol. 2004;18:607-610.
11. Elder D, Elenitsas R, Ragsdale BD. Pilomatricoma. In: Elder D, Elenitsas R, Jaworsky C, et al, eds. Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott-Raven; 1997:757-759.
12. Forbis R Jr, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606-618.
13. Wood MG, Parhizgar B, Beerman H. Malignant pilomatricoma. Arch Dermatol. 1984;120:770-773.
14. Thomas RW, Perkins JA, Ruegemer JL, et al. Surgical excision of pilomatrixoma of the head and neck: a retrospective review of 26 cases. Ear Nose Throat J. 1999;78:541, 544-546, 548.
The Diagnosis: Pilomatricoma
Pilomatricoma, first described by Malherbe and Chenantais1 in 1880, is a benign appendageal tumor derived from hair follicle matrix cells. It classically manifests as a solitary, asymptomatic, firm dermal nodule with a normal overlying epidermis. Less common morphologic variants include perforating, lymphagiectatic, keratoacanthomalike, pigmented, and anetodermalike surface changes.2 Inflammation and erosion through the skin surface are observed in the rare perforating variant, as seen in our patient. The average size is 1 cm, and it rarely exceeds 3 cm in diameter.3 The tumors predominantly occur on the head, neck, and upper extremities, with only 9.5% on the scalp.2 It may occur at any age, though it has a bimodal distribution with peaks in childhood and in adults older than 60 years. A slight preponderance in females has been observed with a female to male ratio of 1.5 to 1.2 Although our patient is black, most reported cases have occurred in individuals of European descent. Because cases of pilomatricoma are not systematically reported, it is uncertain if this finding represents a publication bias or if race is an actual risk factor. Multiple pilomatricomas and familial cases have been described in association with myotonic dystrophy, Turner syndrome, Gardner syndrome, Rubinstein-Taybi syndrome, polyfactorial coagulopathy, trisomy 9, xeroderma pigmentosum, and basal cell nevus syndrome.2,4
It has been shown that the proliferating cells of pilomatricomas stain with antibodies directed against Lef1 (lymphoid enhancer binding factor 1), a marker from hair matrix cells, providing biochemical evidence for the morphologic appearance of these neoplasms.5 Pilomatricomas have been associated with B-cell/chronic lymphocytic leukemia lymphoma 2 gene, BCL2, expression, a proto-oncogene that suppresses apoptosis in benign and malignant neoplasms, which may contribute to the pathogenesis of these tumors.6 Pilomatricomas also have been associated with β-catenin mutation, expression of Bmp2 (bone morphogenetic protein 2), and human hair keratin basic 1.7-9
Definitive diagnosis is obtained through biopsy, looking for characteristic histopathologic findings. The lesion usually is found in the lower dermis and subcutaneous fat. However, in the perforating variant, the lesion is more superficial, located in the papillary and mid dermis, as seen in our patient.10
Pilomatricomas are sharply demarcated, often surrounded by a connective-tissue capsule. Histopathologic analysis reveals islands of epithelial cells comprised of 3 subtypes: basophilic cells with scant cytoplasm, shadow cells with a central pallor (Figure), and transitional cells between the former 2 cellular types.11 The number of basophilic and transitional cells is inversely related to the number of shadow cells. In older lesions, the shadow cells predominate, while the basophilic cells are few in number or absent. Calcium deposits are seen in 80% of lesions with von Kossa staining.12
Transformation into malignancy, known as pilomatrical carcinoma, is rare. These malignant neoplasms are characterized by aggressive biologic behavior such as recurrence, diffuse spread, or metastasis, or by cytologic abnormalities such as poor cellular organization, squamous differentiation, and conspicuous mitotic activity.13 The recent growth of the long-standing lesion in our patient might be interpreted as a sign of malignant transformation. However, this observation may be related to the intense inflammatory reaction supported by the histopathology.
Pilomatricomas are not associated with mortality. Pilomatrical carcinomas are uncommon but are locally invasive and can cause visceral metastases and death. Spontaneous regression has never been observed and medical treatment is ineffective. The treatment of choice is incision and curettage or surgical excision.14 Although recurrence has only been reported in 2.6% of cases from a large case series (N=228), patients should be monitored after surgical excision.12
The Diagnosis: Pilomatricoma
Pilomatricoma, first described by Malherbe and Chenantais1 in 1880, is a benign appendageal tumor derived from hair follicle matrix cells. It classically manifests as a solitary, asymptomatic, firm dermal nodule with a normal overlying epidermis. Less common morphologic variants include perforating, lymphagiectatic, keratoacanthomalike, pigmented, and anetodermalike surface changes.2 Inflammation and erosion through the skin surface are observed in the rare perforating variant, as seen in our patient. The average size is 1 cm, and it rarely exceeds 3 cm in diameter.3 The tumors predominantly occur on the head, neck, and upper extremities, with only 9.5% on the scalp.2 It may occur at any age, though it has a bimodal distribution with peaks in childhood and in adults older than 60 years. A slight preponderance in females has been observed with a female to male ratio of 1.5 to 1.2 Although our patient is black, most reported cases have occurred in individuals of European descent. Because cases of pilomatricoma are not systematically reported, it is uncertain if this finding represents a publication bias or if race is an actual risk factor. Multiple pilomatricomas and familial cases have been described in association with myotonic dystrophy, Turner syndrome, Gardner syndrome, Rubinstein-Taybi syndrome, polyfactorial coagulopathy, trisomy 9, xeroderma pigmentosum, and basal cell nevus syndrome.2,4
It has been shown that the proliferating cells of pilomatricomas stain with antibodies directed against Lef1 (lymphoid enhancer binding factor 1), a marker from hair matrix cells, providing biochemical evidence for the morphologic appearance of these neoplasms.5 Pilomatricomas have been associated with B-cell/chronic lymphocytic leukemia lymphoma 2 gene, BCL2, expression, a proto-oncogene that suppresses apoptosis in benign and malignant neoplasms, which may contribute to the pathogenesis of these tumors.6 Pilomatricomas also have been associated with β-catenin mutation, expression of Bmp2 (bone morphogenetic protein 2), and human hair keratin basic 1.7-9
Definitive diagnosis is obtained through biopsy, looking for characteristic histopathologic findings. The lesion usually is found in the lower dermis and subcutaneous fat. However, in the perforating variant, the lesion is more superficial, located in the papillary and mid dermis, as seen in our patient.10
Pilomatricomas are sharply demarcated, often surrounded by a connective-tissue capsule. Histopathologic analysis reveals islands of epithelial cells comprised of 3 subtypes: basophilic cells with scant cytoplasm, shadow cells with a central pallor (Figure), and transitional cells between the former 2 cellular types.11 The number of basophilic and transitional cells is inversely related to the number of shadow cells. In older lesions, the shadow cells predominate, while the basophilic cells are few in number or absent. Calcium deposits are seen in 80% of lesions with von Kossa staining.12
Transformation into malignancy, known as pilomatrical carcinoma, is rare. These malignant neoplasms are characterized by aggressive biologic behavior such as recurrence, diffuse spread, or metastasis, or by cytologic abnormalities such as poor cellular organization, squamous differentiation, and conspicuous mitotic activity.13 The recent growth of the long-standing lesion in our patient might be interpreted as a sign of malignant transformation. However, this observation may be related to the intense inflammatory reaction supported by the histopathology.
Pilomatricomas are not associated with mortality. Pilomatrical carcinomas are uncommon but are locally invasive and can cause visceral metastases and death. Spontaneous regression has never been observed and medical treatment is ineffective. The treatment of choice is incision and curettage or surgical excision.14 Although recurrence has only been reported in 2.6% of cases from a large case series (N=228), patients should be monitored after surgical excision.12
1. Malherbe A, Chenantais J. Note sur l'epithelioma calcifie des glandes sebacees. Prog Med. 1880;8:826-828.
2. Julian CG, Bowers PW. A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998;39(2, pt 1):191-195.
3. Lozzi GP, Soyer HP, Fruehauf J, et al. Giant pilomatricoma. Am J Dermatopathol. 2007;29:286-289.
4. Hubbard VG, Whittaker SJ. Multiple familial pilomatricomas: an unusual case. J Cutan Pathol. 2004;31:281-283.
5. Kizawa K, Toyoda M, Ito M, et al. Aberrantly differentiated cells in benign pilomatrixoma reflect the normal hair follicle: immunohistochemical analysis of Ca-binding S100A2, S100A3 and S100A6 proteins. Br J Dermatol. 2005;152:314-320.
6. Farrier S, Morgan M. bcl-2 expression in pilomatricoma. Am J Dermatopathol. 1997;19:254-257.
7. Park SW, Suh KS, Wang HY, et al. Beta-catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol. 2001;145:624-629.
8. Kurokawa I, Kusumoto K, Bessho K, et al. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
9. Cribier B, Asch PH, Regnier C, et al. Expression of human hair keratin basic 1 in pilomatrixoma: a study of 128 cases. Br J Dermatol. 1999;140:600-604.
10. Bayle P, Bazex J, Lamant L, et al. Multiple perforating and non perforating pilomatricomas in a patient with Churg-Strauss syndrome and Rubinstein-Taybi syndrome. J Eur Acad Dermatol Venereol. 2004;18:607-610.
11. Elder D, Elenitsas R, Ragsdale BD. Pilomatricoma. In: Elder D, Elenitsas R, Jaworsky C, et al, eds. Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott-Raven; 1997:757-759.
12. Forbis R Jr, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606-618.
13. Wood MG, Parhizgar B, Beerman H. Malignant pilomatricoma. Arch Dermatol. 1984;120:770-773.
14. Thomas RW, Perkins JA, Ruegemer JL, et al. Surgical excision of pilomatrixoma of the head and neck: a retrospective review of 26 cases. Ear Nose Throat J. 1999;78:541, 544-546, 548.
1. Malherbe A, Chenantais J. Note sur l'epithelioma calcifie des glandes sebacees. Prog Med. 1880;8:826-828.
2. Julian CG, Bowers PW. A clinical review of 209 pilomatricomas. J Am Acad Dermatol. 1998;39(2, pt 1):191-195.
3. Lozzi GP, Soyer HP, Fruehauf J, et al. Giant pilomatricoma. Am J Dermatopathol. 2007;29:286-289.
4. Hubbard VG, Whittaker SJ. Multiple familial pilomatricomas: an unusual case. J Cutan Pathol. 2004;31:281-283.
5. Kizawa K, Toyoda M, Ito M, et al. Aberrantly differentiated cells in benign pilomatrixoma reflect the normal hair follicle: immunohistochemical analysis of Ca-binding S100A2, S100A3 and S100A6 proteins. Br J Dermatol. 2005;152:314-320.
6. Farrier S, Morgan M. bcl-2 expression in pilomatricoma. Am J Dermatopathol. 1997;19:254-257.
7. Park SW, Suh KS, Wang HY, et al. Beta-catenin expression in the transitional cell zone of pilomatricoma. Br J Dermatol. 2001;145:624-629.
8. Kurokawa I, Kusumoto K, Bessho K, et al. Immunohistochemical expression of bone morphogenetic protein-2 in pilomatricoma. Br J Dermatol. 2000;143:754-758.
9. Cribier B, Asch PH, Regnier C, et al. Expression of human hair keratin basic 1 in pilomatrixoma: a study of 128 cases. Br J Dermatol. 1999;140:600-604.
10. Bayle P, Bazex J, Lamant L, et al. Multiple perforating and non perforating pilomatricomas in a patient with Churg-Strauss syndrome and Rubinstein-Taybi syndrome. J Eur Acad Dermatol Venereol. 2004;18:607-610.
11. Elder D, Elenitsas R, Ragsdale BD. Pilomatricoma. In: Elder D, Elenitsas R, Jaworsky C, et al, eds. Histopathology of the Skin. 8th ed. Philadelphia, PA: Lippincott-Raven; 1997:757-759.
12. Forbis R Jr, Helwig EB. Pilomatrixoma (calcifying epithelioma). Arch Dermatol. 1961;83:606-618.
13. Wood MG, Parhizgar B, Beerman H. Malignant pilomatricoma. Arch Dermatol. 1984;120:770-773.
14. Thomas RW, Perkins JA, Ruegemer JL, et al. Surgical excision of pilomatrixoma of the head and neck: a retrospective review of 26 cases. Ear Nose Throat J. 1999;78:541, 544-546, 548.
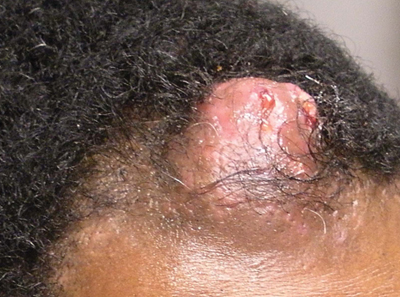
An otherwise healthy 40-year-old man presented for examination of a solitary nodular lesion on the frontal aspect of the scalp of 1 year’s duration. The lesion had rapidly increased in size in the 2 weeks prior to presentation. He presented to the emergency department after he noted pain and drainage from the lesion. Biopsy of the lesion revealed islands of pale eosinophilic shadow cells with an intense dermal infiltrate consisting of lymphocytes, histiocytes, plasma cells, and neutrophils.