User login
So Much More than Bald and Bloated
A 44-year-old previously healthy semiprofessional male athlete presented with five days of nausea, vomiting, and abdominal pain. He had also experienced several months of decreased energy and new episodes of constipation three weeks prior to presentation.
At this point, we do not have sufficient information to completely determine the cause of his abdominal symptoms. Common causes of abdominal pain and vomiting in adults of his age group include peptic ulcer disease, pancreatic or hepatobiliary track disorders, small or large bowel processes, appendicitis, or even renal pathology. Further characterization may be possible by describing the location and quality of pain and factors that might relieve or exacerbate his pain. Despite the ambiguity, multiple clues might allow us to narrow the broad differential diagnosis of abdominal pain. In a previously healthy, vigorous, middle-aged man with subacute abdominal pain associated with constipation, the differential diagnosis should include disease states that may cause a bowel obstruction; these states include inflammatory bowel disease (IBD), gastrointestinal malignancy, or peptic ulcer disease. Mechanical obstruction due to volvulus or intussusception would be less likely in his age group. Given his history of several months of fatigue and several weeks of constipation, he should be evaluated for metabolic causes of abdominal pain and constipation, such as hypothyroidism or hypercalcemia. In addition to basic laboratory and imaging studies, obtaining additional history regarding prior abdominal surgeries, medication use, alcohol intake, and family and travel history will be the key in directing the evaluation.
Six months prior to admission, the patient began to feel more fatigue and exercise intolerance, reduced sweating, increased cold intolerance, and increased presyncopal episodes. He was diagnosed with hypothyroidism (TSH 6.69 μIU/mL; free T4 not done) and initiated on levothyroxine. One month prior to presentation, he developed constipation, loss of taste, reduced appetite, and weight loss of 30 pounds. He developed blurry vision and photophobia. He also complained of erectile dysfunction, urinary hesitancy and straining, which were diagnosed as benign prostatic hypertrophy.
Given the addition of numerous historical features in a previously healthy man, it is important to strive for a parsimonious diagnosis to unify his seemingly disparate features. His fatigue, constipation, and cold intolerance are consistent with his diagnosis of hypothyroidism but are nonspecific. Whether the degree of hypothyroidism caused his symptoms or signs is doubtful. The constellation of symptoms and signs are more likely to be representative of a nonthyroidal illness. His abdominal pain, unexplained weight loss, and presyncopal episodes should raise consideration of adrenal insufficiency. The combination of hypothyroidism and adrenal insufficiency suggest the possibility of an autoimmune polyendocrine syndrome or other pituitary pathology. In this case, history of headache, dysgeusia, and visual disturbances might support the diagnosis of pituitary adenoma. A cosyntropin stimulation test could establish the diagnosis of adrenal insufficiency. A low ACTH level would establish a diagnosis of pituitary or hypothalamic hypofunction. If pituitary hypofunction is documented, then a brain MRI would be needed to confirm the diagnosis of pituitary adenoma.
His newly reported erectile dysfunction suggests the possibility of a psychiatric, neurologic, hormonal, or vascular process and should be explored further. Sexual dysfunction is also associated with adrenal insufficiency and hypopituitarism. However, the presence of suspected prostatic hypertrophy in a male competitive athlete in his forties also raises the question of exogenous androgen use.
His past medical history was notable for a two-year history of alopecia totalis, seasonal allergies, asthma, and a repaired congenital aortic web with known aortic insufficiency. He was married with two children, worked an office job, and had no history of injection drug use, blood transfusions, or multiple sexual partners. His family history was notable for hypothyroidism and asthma in several family members in addition to Crohn disease, celiac disease, diabetes, cardiovascular disease, and cancers of the breast and lung.
His past medical, surgical, and family history supports a diagnosis of autoimmune disease. Although there is a personal and family history of atopic disorders, including allergic rhinitis and asthma, no association is found between atopy and autoimmunity. His family history of hypothyroidism, Crohn disease, and diabetes suggests a familial autoimmune genetic predisposition. His history of alopecia totalis in the setting of hypothyroidism and possible autoimmune adrenal insufficiency or autoimmune hypophysitis raises suspicion for the previously suggested diagnosis of polyglandular autoimmune syndrome, also known as autoimmune polyendocrine syndrome. Type I polyglandular autoimmune syndrome is associated with hypoparathyroidism and mucocutaneous candidiasis. In the absence of these symptoms, the patient more likely has type II polyglandular autoimmune syndrome. Type II syndrome is more prevalent and can occur in the setting of other nonendocrine autoimmune disorders, such as vitiligo, myasthenia gravis, or rheumatoid arthritis. Adrenal insufficiency can be the initial and most prominent manifestation of type II syndrome.
On physical exam, he was afebrile, with a heart rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and normal oxygen saturation. His supine blood pressure and heart rate were 116/72 mm Hg and 66 beats per minute, respectively, and his standing blood pressure and heart rates were 80/48 mm Hg and 68 beats per minute respectively. He was thin, had diffuse scalp and body alopecia, and was ill-appearing with dry skin and dry mucous membranes. No evidence of Osler nodes, Janeway lesions, or splinter hemorrhages were found on cutaneous examination. No Roth spots or conjunctival hemorrhages were noted on ophthalmologic examination. He had both a 3/6 crescendo–decrescendo systolic murmur best heard at the right clavicle and radiated to the carotids and a 3/6 early diastolic decrescendo murmur best heard at the left sternal border. His abdomen was slightly protuberant, with reduced bowel sounds, hyperresonant to tympanitic on percussion, and a diffusely, moderately tender without peritoneal signs. Neurologic examination revealed 8 mm pupils with minimal response to light and accommodation. The remaining portions of his cranial nerve and complete neurologic examination were normal.
The presence of postural hypotension supports the previous suspicion of adrenal insufficiency, and the possibility of a pituitary or hypothalamic process remains. However, his dilated and minimally responsive pupils and potentially adynamic bowel are inconsistent with these diagnoses. Mydriasis and adynamic bowel in combination with orthostatic hypotension, dysgeusia, urinary retention, and erectile dysfunction are strongly suggestive of an autonomic process. Endocarditis is worth considering given his multisystem involvement, subacute decline, and known valve pathology. The absence of fever or stigmata of endocarditis make it difficult to explain his clinical syndrome. An echocardiogram would be reasonable for further assessment. At this point, it is prudent to explore his adrenal and pituitary function; if unrevealing, embark on an evaluation of his autonomic dysfunction.
Initial laboratory investigations were notable for mild normocytic anemia and hypoalbuminemia. His cosyntropin stimulation test was normal at 60 minutes. An abdominal CT scan demonstrated marked dilation in the small bowel loops (6 cm in caliber) with associated small bowel wall thickening and hyperemia. The echocardiogram was unrevealing and only confirmed the ongoing, progression of his known valve pathology without evidence of vegetation.
The above testing rules out primary adrenal insufficiency, but an appropriate response to the cosyntropin stimulation test does not rule out secondary, or pituitary, adrenal insufficiency. The echocardiogram and lack of other features make infective endocarditis unlikely. Thus, as mentioned, it is important now to commence a complete work-up of his probable dysautonomia to explain the majority of his features. Additionally, his hypothyroidism (if more than sick euthyroid syndrome), family history of autoimmune processes, and alopecia totalis all suggest the possibility of an immune-related syndrome. His CT scan revealed some thickened hyperemic bowel, which could suggest an IBD, such as Crohn disease; however, the absence of other signs, such as fever, diarrhea, or bloody stools, argues against this diagnosis. A syndrome that could unify his presentation is autoimmune autonomic ganglionopathy (AAG), a rare genetic autonomic system disorder that presents with pandysautonomia. The spectrum of autoimmunity was considered early in this case, but the differential diagnosis included more common conditions, such as adrenal insufficiency. Similarly, IBD remains a consideration. The serologic studies for IBD can be useful but they lack definitive diagnostic accuracy. Given that treatment for AAG differs from that for IBD, additional information will help guide the therapeutic approach. Anti-α3gnAChR antibodies, which are associated with AAG, should be checked.
His history of presyncope, anhidrosis, urinary retention, and ileus raised suspicion for pandysautonomia, as characterized by signs of sympathetic and parasympathetic dysfunction. The suspicion for pandysautonomia was confirmed via specialized autonomic testing, which included reduced heart rate variation on Valsalva and deep breathing maneuvers, orthostatic hypotension consistent autonomic insufficiency on Tilt table testing, and reduced sweat response to acetylcholine application (QSART test). The patient underwent further diagnostic serologic testing to differentiate causes of autonomic failure (Table 1). His personal and family history of autoimmunity led to the working diagnosis of AAG. Ultimate testing revealed high titers of autoantibodies, specifically anti-α3gnAChR (3.29 nmol/L, normal <0.02 nmol/L), directed against the ganglionic nicotinic acetylcholine receptor. This finding strongly supported the diagnosis of AAG.1,4-7
He was initially treated empirically with intravenous immunoglobulin (IVIG) with minimal improvement. He received additional immunomodulating therapies including methylprednisolone, plasmapheresis, and rituximab but did not tolerate a trial of mycophenolate. Six weeks after therapy initiation, his antibody titers decreased to 0.89 nmol/L with associated clinical improvement. Ultimately, he was discharged from the hospital on day 73 with a feeding tube and supplemental total parenteral nutrition. Four months postdischarge, he had returned to his prediagnosis weight, had eased back into his prior activities, and was off supplemental nutrition. Over a year later, he completed a 10-month prednisone taper and continued to receive monthly IVIG infusion
DISCUSSION
The clinical approach to dysautonomia is based on different etiologies: (1) those associated with neurodegenerative disorders; (2) those associated with peripheral neuropathies, and (3) isolated autonomic failure.2 Thus, clinical history and physical examination can assist greatly in guiding the evaluation of patients. Neurodegenerative disorders (such as Parkinson disease), combined disorders (such as multiple-system atrophy), and acquired or familial processes were considered. Our patient had neither a personal or family history nor physical examination supporting a neurodegenerative disorder. Disorders of the peripheral nerves were considered and can broadly be categorized as chronic sensorimotor neuropathies, sensory ganglionopathies, distal painful neuropathies, and acute or subacute motor polyradiculopathies. During evaluation, no historical, physical examination, or laboratory findings supported diabetes, amyloidosis, heavy metals, Sjögren syndrome, paraneoplastic neuropathy, sodium channel disorders, infectious etiologies, or porphyria (Table 1). Thus, in the absence of supportive evidence for primary neurodegenerative disorders or peripheral neuropathies, his syndrome appeared most compatible with an isolated autonomic failure syndrome. The principal differential for this syndrome is pure autonomic failure versus an immune-mediated autonomic disorder, including paraneoplastic autoimmune neuropathy and AAG. The diagnosis of pure autonomic failure is made after there is no clear unifying syndrome after more than five years of investigation. After exploration, no evidence of malignancy was discovered on body cross sectional imaging, PET scanning, bone marrow biopsy, colonoscopy, or laboratory testing. Thus, positive serologic testing in the absence of an underlying malignancy suggests a diagnosis of AAG.
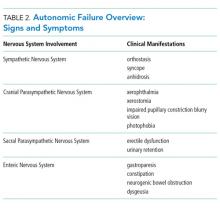
AAG was first described in 1969 and is a rare, acquired disorder characterized by combined failure of the parasympathetic, sympathetic, and enteric nervous systems. This disorder typically presents in young-to-middle aged patients but has been described in all age groups. It is more commonly seen in patients with coexistent autoimmune diseases and/or a history of familial autoimmunity. The onset of clinical AAG may be subacute (less than three months) or insidious (more than three months). Patients present with signs or symptoms of pandysautonomia, such as severe orthostatic hypotension, syncope, constipation and gastrointestinal dysmotility, urinary retention, fixed and dilated pupils, and dry mouth and eyes (Table 2). Up to 40% of patients with AAG may also have significant cognitive impairment.3,4 Diagnosis relies on a combination of typical clinical features as discussed above and the exclusion of other diagnostic considerations. Diagnosis of AAG is aided by the presence of autoantibodies to ganglionic nicotinic acetylcholine receptors (gnAChR), particularly antiganglionic acetylcholine receptor α3 (anti-α3gAChR).1 Anti-gnAChR antibodies are only present in about half of patients with AAG. Antibody titers are highest in subacute AAG (40%-50%)3 compared with chronic AAG (30%-40%) or paraneoplastic AAG (10%-20%).5 Anti-gnAChR antibodies are not specific to AAG and have been identified in low levels in up to 20% of patients with thymomas, postural orthostatic tachycardia syndrome, chronic idiopathic anhidrosis, idiopathic gastrointestinal dysmotility, Lambert–Eaton syndrome, and myasthenia gravis without thymoma.1,5-7 These associations raise the question of shared pathology and perhaps a syndrome overlap. Individuals with seropositive AAG may also have other paraneoplastic antibodies, making it clinically indistinguishable from paraneoplastic autonomic neuropathy.5,8 Although the autoantibody lacks sensitivity and is imperfectly specific, its presence supports a diagnosis of AAG. Anti-gnAChR antibodies have been shown to be pathological in rabbit and mouse models.4 In patients with AAG, higher autoantibody titers correlate with increased disease severity.1,6,7 A decrease in autoantibody titers correlates with decreased disease severity.6 Case report series also described a distinct entity of seronegative AAG.2,3 Maintaining a high clinical suspicion for AAG even with negative antibodies is important.
Given the rarity of the disease, no standard therapeutic regimens are available. About one-third of individuals improve on their own, while other individuals require extensive immunomodulation and symptom management. Case series and observational trials currently make up the vast array of treatment data. Therapies include glucocorticoids, plasmapheresis, IVIG, and other immunosuppressive agents, such as rituximab.9-12 Patients with and without identified anti-gnAChRs antibodies may respond to therapy.12 The overall long-term prognosis of the disease is poorly characterized.9,10,13
Despite the rarity of the syndrome discussed, this case represents how diagnostic reasoning strategies, such as law of parsimony, shift how the case is framed. For example, a middle-aged man with several new, distinctly unrelated diagnoses versus a middle-aged man with signs and symptoms of autonomic failure alters the subsequent clinical reasoning and diagnostic approach. Many diseases, both common and rare, are associated with dysautonomia. Therefore, clinicians should have an approach to autonomic failure
TEACHING POINTS
- Recognize the following signs and symptoms suggesting a dysautonomic syndrome: orthostasis, syncope, anhidrosis, xerophthalmia, xerostomia, impaired pupillary constriction, blurry vision, photophobia, erectile dysfunction, urinary retention, gastroparesis, constipation, neurogenic bowel obstruction, and dysgeusia.
- Recognize the clinical features, diagnostic approach, and management of autoimmune autonomic ganglionopathy.
- When faced with a complex clinical presentation, early application of the “law of parsimony” may help identify a unifying syndrome.
Acknowledgments
The authors wish to thank our Blinded Expert, Anthony Montanaro, MD, for his expertise and guidance during this process.
Disclosures
There are no known conflicts of interest.
1. Gibbons C, Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146(1-2):8-12. doi: 10.1016/j.autneu.2008.11.013. PubMed
2. Golden E, Bryarly M, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28(1):115-123. doi: 10.1007/s10286-017-0493-8. PubMed
3. Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61(1):44-48. doi: 10.1001/archneur.61.1.44. PubMed
4. Vernino S, Ermilov L, Sha L, Szurszewski J, Low P, Lennon V. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. doi: 10.1523/JNEUROSCI.1485-04.2004. PubMed
5. Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146(1-2):3-7. doi: 10.1016/j.autneu.2008.09.005. PubMed
6. Vernino S, Low P, Fealey R, Stewart J, Farrugia G, Lennon V. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. doi: 10.1056/NEJM200009213431204. PubMed
7. Gibbons C, Vernino S, Freeman R. Autoimmune autonomic ganglionopathy – Symptom antibody correlations. Auton Neurosci. 2015;192:130. doi: 10.1016/j.autneu.2015.07.241 .
8. Benarroch E. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014;10(7):396-407. doi: 10.1038/nrneurol.2014.88. PubMed
9. Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. 2009;146(1-2):29-32. doi: 10.1016/j.autneu.2008.10.016. PubMed
10. Gibbons C, Centi J, Vernino S. Autoimmune autonomic ganglionoapthy with reversible cognitive impairment. Arch Neurol. 2012;69(4):461-466. doi: 10.1001/archneurol.2011.2372. PubMed
11. Boydston E, Muppidi S, Vernino S. Long-term outcomes in autoimmune autonomic ganglionopathy (P05.210). Neurology. 2012;78(1):P05.210. doi: 10.1212/WNL.78.1_MeetingAbstracts.P05.210.
12. Gehrking T, Sletten D, Fealey R, Low P, Singer W. 11-year follow-up of a case of autoimmune autonomic ganglionopathy (P03.024). Neurology. 2013;80(7):P03.024.
13. Imrich R, Vernino S, Eldadah BA, Holmes C, Goldstein DS. Autoimmune autonomic ganglionopathy: treatment by plasma exchanges and rituximab. Clin Auton Res. 2009;19(4):259-262. doi: 10.1007/s10286-009-0012-7. PubMed
14. Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72(23):2002-8. doi: 10.1212/WNL.0b013e3181a92b52. PubMed
15. Hayashi M, Ishii Y. A Japanese case of autoimmune autonomic ganglionopathy (AAG) and a review of AAG cases in Japan. Auton Neurosci. 2009;146(1-2):26-8. doi: 10.1016/j.autneu.2008.12.013. PubMed
16. Baker, A. Simplicity. In: Baker A, Zalta E, eds. The Stanford Encyclopedia of Philosophy. Winter 2016 Edition. https://plato.stanford.edu/archives/win2016/entries/simplicity/. Accessed October 26, 2017.
A 44-year-old previously healthy semiprofessional male athlete presented with five days of nausea, vomiting, and abdominal pain. He had also experienced several months of decreased energy and new episodes of constipation three weeks prior to presentation.
At this point, we do not have sufficient information to completely determine the cause of his abdominal symptoms. Common causes of abdominal pain and vomiting in adults of his age group include peptic ulcer disease, pancreatic or hepatobiliary track disorders, small or large bowel processes, appendicitis, or even renal pathology. Further characterization may be possible by describing the location and quality of pain and factors that might relieve or exacerbate his pain. Despite the ambiguity, multiple clues might allow us to narrow the broad differential diagnosis of abdominal pain. In a previously healthy, vigorous, middle-aged man with subacute abdominal pain associated with constipation, the differential diagnosis should include disease states that may cause a bowel obstruction; these states include inflammatory bowel disease (IBD), gastrointestinal malignancy, or peptic ulcer disease. Mechanical obstruction due to volvulus or intussusception would be less likely in his age group. Given his history of several months of fatigue and several weeks of constipation, he should be evaluated for metabolic causes of abdominal pain and constipation, such as hypothyroidism or hypercalcemia. In addition to basic laboratory and imaging studies, obtaining additional history regarding prior abdominal surgeries, medication use, alcohol intake, and family and travel history will be the key in directing the evaluation.
Six months prior to admission, the patient began to feel more fatigue and exercise intolerance, reduced sweating, increased cold intolerance, and increased presyncopal episodes. He was diagnosed with hypothyroidism (TSH 6.69 μIU/mL; free T4 not done) and initiated on levothyroxine. One month prior to presentation, he developed constipation, loss of taste, reduced appetite, and weight loss of 30 pounds. He developed blurry vision and photophobia. He also complained of erectile dysfunction, urinary hesitancy and straining, which were diagnosed as benign prostatic hypertrophy.
Given the addition of numerous historical features in a previously healthy man, it is important to strive for a parsimonious diagnosis to unify his seemingly disparate features. His fatigue, constipation, and cold intolerance are consistent with his diagnosis of hypothyroidism but are nonspecific. Whether the degree of hypothyroidism caused his symptoms or signs is doubtful. The constellation of symptoms and signs are more likely to be representative of a nonthyroidal illness. His abdominal pain, unexplained weight loss, and presyncopal episodes should raise consideration of adrenal insufficiency. The combination of hypothyroidism and adrenal insufficiency suggest the possibility of an autoimmune polyendocrine syndrome or other pituitary pathology. In this case, history of headache, dysgeusia, and visual disturbances might support the diagnosis of pituitary adenoma. A cosyntropin stimulation test could establish the diagnosis of adrenal insufficiency. A low ACTH level would establish a diagnosis of pituitary or hypothalamic hypofunction. If pituitary hypofunction is documented, then a brain MRI would be needed to confirm the diagnosis of pituitary adenoma.
His newly reported erectile dysfunction suggests the possibility of a psychiatric, neurologic, hormonal, or vascular process and should be explored further. Sexual dysfunction is also associated with adrenal insufficiency and hypopituitarism. However, the presence of suspected prostatic hypertrophy in a male competitive athlete in his forties also raises the question of exogenous androgen use.
His past medical history was notable for a two-year history of alopecia totalis, seasonal allergies, asthma, and a repaired congenital aortic web with known aortic insufficiency. He was married with two children, worked an office job, and had no history of injection drug use, blood transfusions, or multiple sexual partners. His family history was notable for hypothyroidism and asthma in several family members in addition to Crohn disease, celiac disease, diabetes, cardiovascular disease, and cancers of the breast and lung.
His past medical, surgical, and family history supports a diagnosis of autoimmune disease. Although there is a personal and family history of atopic disorders, including allergic rhinitis and asthma, no association is found between atopy and autoimmunity. His family history of hypothyroidism, Crohn disease, and diabetes suggests a familial autoimmune genetic predisposition. His history of alopecia totalis in the setting of hypothyroidism and possible autoimmune adrenal insufficiency or autoimmune hypophysitis raises suspicion for the previously suggested diagnosis of polyglandular autoimmune syndrome, also known as autoimmune polyendocrine syndrome. Type I polyglandular autoimmune syndrome is associated with hypoparathyroidism and mucocutaneous candidiasis. In the absence of these symptoms, the patient more likely has type II polyglandular autoimmune syndrome. Type II syndrome is more prevalent and can occur in the setting of other nonendocrine autoimmune disorders, such as vitiligo, myasthenia gravis, or rheumatoid arthritis. Adrenal insufficiency can be the initial and most prominent manifestation of type II syndrome.
On physical exam, he was afebrile, with a heart rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and normal oxygen saturation. His supine blood pressure and heart rate were 116/72 mm Hg and 66 beats per minute, respectively, and his standing blood pressure and heart rates were 80/48 mm Hg and 68 beats per minute respectively. He was thin, had diffuse scalp and body alopecia, and was ill-appearing with dry skin and dry mucous membranes. No evidence of Osler nodes, Janeway lesions, or splinter hemorrhages were found on cutaneous examination. No Roth spots or conjunctival hemorrhages were noted on ophthalmologic examination. He had both a 3/6 crescendo–decrescendo systolic murmur best heard at the right clavicle and radiated to the carotids and a 3/6 early diastolic decrescendo murmur best heard at the left sternal border. His abdomen was slightly protuberant, with reduced bowel sounds, hyperresonant to tympanitic on percussion, and a diffusely, moderately tender without peritoneal signs. Neurologic examination revealed 8 mm pupils with minimal response to light and accommodation. The remaining portions of his cranial nerve and complete neurologic examination were normal.
The presence of postural hypotension supports the previous suspicion of adrenal insufficiency, and the possibility of a pituitary or hypothalamic process remains. However, his dilated and minimally responsive pupils and potentially adynamic bowel are inconsistent with these diagnoses. Mydriasis and adynamic bowel in combination with orthostatic hypotension, dysgeusia, urinary retention, and erectile dysfunction are strongly suggestive of an autonomic process. Endocarditis is worth considering given his multisystem involvement, subacute decline, and known valve pathology. The absence of fever or stigmata of endocarditis make it difficult to explain his clinical syndrome. An echocardiogram would be reasonable for further assessment. At this point, it is prudent to explore his adrenal and pituitary function; if unrevealing, embark on an evaluation of his autonomic dysfunction.
Initial laboratory investigations were notable for mild normocytic anemia and hypoalbuminemia. His cosyntropin stimulation test was normal at 60 minutes. An abdominal CT scan demonstrated marked dilation in the small bowel loops (6 cm in caliber) with associated small bowel wall thickening and hyperemia. The echocardiogram was unrevealing and only confirmed the ongoing, progression of his known valve pathology without evidence of vegetation.
The above testing rules out primary adrenal insufficiency, but an appropriate response to the cosyntropin stimulation test does not rule out secondary, or pituitary, adrenal insufficiency. The echocardiogram and lack of other features make infective endocarditis unlikely. Thus, as mentioned, it is important now to commence a complete work-up of his probable dysautonomia to explain the majority of his features. Additionally, his hypothyroidism (if more than sick euthyroid syndrome), family history of autoimmune processes, and alopecia totalis all suggest the possibility of an immune-related syndrome. His CT scan revealed some thickened hyperemic bowel, which could suggest an IBD, such as Crohn disease; however, the absence of other signs, such as fever, diarrhea, or bloody stools, argues against this diagnosis. A syndrome that could unify his presentation is autoimmune autonomic ganglionopathy (AAG), a rare genetic autonomic system disorder that presents with pandysautonomia. The spectrum of autoimmunity was considered early in this case, but the differential diagnosis included more common conditions, such as adrenal insufficiency. Similarly, IBD remains a consideration. The serologic studies for IBD can be useful but they lack definitive diagnostic accuracy. Given that treatment for AAG differs from that for IBD, additional information will help guide the therapeutic approach. Anti-α3gnAChR antibodies, which are associated with AAG, should be checked.
His history of presyncope, anhidrosis, urinary retention, and ileus raised suspicion for pandysautonomia, as characterized by signs of sympathetic and parasympathetic dysfunction. The suspicion for pandysautonomia was confirmed via specialized autonomic testing, which included reduced heart rate variation on Valsalva and deep breathing maneuvers, orthostatic hypotension consistent autonomic insufficiency on Tilt table testing, and reduced sweat response to acetylcholine application (QSART test). The patient underwent further diagnostic serologic testing to differentiate causes of autonomic failure (Table 1). His personal and family history of autoimmunity led to the working diagnosis of AAG. Ultimate testing revealed high titers of autoantibodies, specifically anti-α3gnAChR (3.29 nmol/L, normal <0.02 nmol/L), directed against the ganglionic nicotinic acetylcholine receptor. This finding strongly supported the diagnosis of AAG.1,4-7
He was initially treated empirically with intravenous immunoglobulin (IVIG) with minimal improvement. He received additional immunomodulating therapies including methylprednisolone, plasmapheresis, and rituximab but did not tolerate a trial of mycophenolate. Six weeks after therapy initiation, his antibody titers decreased to 0.89 nmol/L with associated clinical improvement. Ultimately, he was discharged from the hospital on day 73 with a feeding tube and supplemental total parenteral nutrition. Four months postdischarge, he had returned to his prediagnosis weight, had eased back into his prior activities, and was off supplemental nutrition. Over a year later, he completed a 10-month prednisone taper and continued to receive monthly IVIG infusion
DISCUSSION
The clinical approach to dysautonomia is based on different etiologies: (1) those associated with neurodegenerative disorders; (2) those associated with peripheral neuropathies, and (3) isolated autonomic failure.2 Thus, clinical history and physical examination can assist greatly in guiding the evaluation of patients. Neurodegenerative disorders (such as Parkinson disease), combined disorders (such as multiple-system atrophy), and acquired or familial processes were considered. Our patient had neither a personal or family history nor physical examination supporting a neurodegenerative disorder. Disorders of the peripheral nerves were considered and can broadly be categorized as chronic sensorimotor neuropathies, sensory ganglionopathies, distal painful neuropathies, and acute or subacute motor polyradiculopathies. During evaluation, no historical, physical examination, or laboratory findings supported diabetes, amyloidosis, heavy metals, Sjögren syndrome, paraneoplastic neuropathy, sodium channel disorders, infectious etiologies, or porphyria (Table 1). Thus, in the absence of supportive evidence for primary neurodegenerative disorders or peripheral neuropathies, his syndrome appeared most compatible with an isolated autonomic failure syndrome. The principal differential for this syndrome is pure autonomic failure versus an immune-mediated autonomic disorder, including paraneoplastic autoimmune neuropathy and AAG. The diagnosis of pure autonomic failure is made after there is no clear unifying syndrome after more than five years of investigation. After exploration, no evidence of malignancy was discovered on body cross sectional imaging, PET scanning, bone marrow biopsy, colonoscopy, or laboratory testing. Thus, positive serologic testing in the absence of an underlying malignancy suggests a diagnosis of AAG.
AAG was first described in 1969 and is a rare, acquired disorder characterized by combined failure of the parasympathetic, sympathetic, and enteric nervous systems. This disorder typically presents in young-to-middle aged patients but has been described in all age groups. It is more commonly seen in patients with coexistent autoimmune diseases and/or a history of familial autoimmunity. The onset of clinical AAG may be subacute (less than three months) or insidious (more than three months). Patients present with signs or symptoms of pandysautonomia, such as severe orthostatic hypotension, syncope, constipation and gastrointestinal dysmotility, urinary retention, fixed and dilated pupils, and dry mouth and eyes (Table 2). Up to 40% of patients with AAG may also have significant cognitive impairment.3,4 Diagnosis relies on a combination of typical clinical features as discussed above and the exclusion of other diagnostic considerations. Diagnosis of AAG is aided by the presence of autoantibodies to ganglionic nicotinic acetylcholine receptors (gnAChR), particularly antiganglionic acetylcholine receptor α3 (anti-α3gAChR).1 Anti-gnAChR antibodies are only present in about half of patients with AAG. Antibody titers are highest in subacute AAG (40%-50%)3 compared with chronic AAG (30%-40%) or paraneoplastic AAG (10%-20%).5 Anti-gnAChR antibodies are not specific to AAG and have been identified in low levels in up to 20% of patients with thymomas, postural orthostatic tachycardia syndrome, chronic idiopathic anhidrosis, idiopathic gastrointestinal dysmotility, Lambert–Eaton syndrome, and myasthenia gravis without thymoma.1,5-7 These associations raise the question of shared pathology and perhaps a syndrome overlap. Individuals with seropositive AAG may also have other paraneoplastic antibodies, making it clinically indistinguishable from paraneoplastic autonomic neuropathy.5,8 Although the autoantibody lacks sensitivity and is imperfectly specific, its presence supports a diagnosis of AAG. Anti-gnAChR antibodies have been shown to be pathological in rabbit and mouse models.4 In patients with AAG, higher autoantibody titers correlate with increased disease severity.1,6,7 A decrease in autoantibody titers correlates with decreased disease severity.6 Case report series also described a distinct entity of seronegative AAG.2,3 Maintaining a high clinical suspicion for AAG even with negative antibodies is important.
Given the rarity of the disease, no standard therapeutic regimens are available. About one-third of individuals improve on their own, while other individuals require extensive immunomodulation and symptom management. Case series and observational trials currently make up the vast array of treatment data. Therapies include glucocorticoids, plasmapheresis, IVIG, and other immunosuppressive agents, such as rituximab.9-12 Patients with and without identified anti-gnAChRs antibodies may respond to therapy.12 The overall long-term prognosis of the disease is poorly characterized.9,10,13
Despite the rarity of the syndrome discussed, this case represents how diagnostic reasoning strategies, such as law of parsimony, shift how the case is framed. For example, a middle-aged man with several new, distinctly unrelated diagnoses versus a middle-aged man with signs and symptoms of autonomic failure alters the subsequent clinical reasoning and diagnostic approach. Many diseases, both common and rare, are associated with dysautonomia. Therefore, clinicians should have an approach to autonomic failure
TEACHING POINTS
- Recognize the following signs and symptoms suggesting a dysautonomic syndrome: orthostasis, syncope, anhidrosis, xerophthalmia, xerostomia, impaired pupillary constriction, blurry vision, photophobia, erectile dysfunction, urinary retention, gastroparesis, constipation, neurogenic bowel obstruction, and dysgeusia.
- Recognize the clinical features, diagnostic approach, and management of autoimmune autonomic ganglionopathy.
- When faced with a complex clinical presentation, early application of the “law of parsimony” may help identify a unifying syndrome.
Acknowledgments
The authors wish to thank our Blinded Expert, Anthony Montanaro, MD, for his expertise and guidance during this process.
Disclosures
There are no known conflicts of interest.
A 44-year-old previously healthy semiprofessional male athlete presented with five days of nausea, vomiting, and abdominal pain. He had also experienced several months of decreased energy and new episodes of constipation three weeks prior to presentation.
At this point, we do not have sufficient information to completely determine the cause of his abdominal symptoms. Common causes of abdominal pain and vomiting in adults of his age group include peptic ulcer disease, pancreatic or hepatobiliary track disorders, small or large bowel processes, appendicitis, or even renal pathology. Further characterization may be possible by describing the location and quality of pain and factors that might relieve or exacerbate his pain. Despite the ambiguity, multiple clues might allow us to narrow the broad differential diagnosis of abdominal pain. In a previously healthy, vigorous, middle-aged man with subacute abdominal pain associated with constipation, the differential diagnosis should include disease states that may cause a bowel obstruction; these states include inflammatory bowel disease (IBD), gastrointestinal malignancy, or peptic ulcer disease. Mechanical obstruction due to volvulus or intussusception would be less likely in his age group. Given his history of several months of fatigue and several weeks of constipation, he should be evaluated for metabolic causes of abdominal pain and constipation, such as hypothyroidism or hypercalcemia. In addition to basic laboratory and imaging studies, obtaining additional history regarding prior abdominal surgeries, medication use, alcohol intake, and family and travel history will be the key in directing the evaluation.
Six months prior to admission, the patient began to feel more fatigue and exercise intolerance, reduced sweating, increased cold intolerance, and increased presyncopal episodes. He was diagnosed with hypothyroidism (TSH 6.69 μIU/mL; free T4 not done) and initiated on levothyroxine. One month prior to presentation, he developed constipation, loss of taste, reduced appetite, and weight loss of 30 pounds. He developed blurry vision and photophobia. He also complained of erectile dysfunction, urinary hesitancy and straining, which were diagnosed as benign prostatic hypertrophy.
Given the addition of numerous historical features in a previously healthy man, it is important to strive for a parsimonious diagnosis to unify his seemingly disparate features. His fatigue, constipation, and cold intolerance are consistent with his diagnosis of hypothyroidism but are nonspecific. Whether the degree of hypothyroidism caused his symptoms or signs is doubtful. The constellation of symptoms and signs are more likely to be representative of a nonthyroidal illness. His abdominal pain, unexplained weight loss, and presyncopal episodes should raise consideration of adrenal insufficiency. The combination of hypothyroidism and adrenal insufficiency suggest the possibility of an autoimmune polyendocrine syndrome or other pituitary pathology. In this case, history of headache, dysgeusia, and visual disturbances might support the diagnosis of pituitary adenoma. A cosyntropin stimulation test could establish the diagnosis of adrenal insufficiency. A low ACTH level would establish a diagnosis of pituitary or hypothalamic hypofunction. If pituitary hypofunction is documented, then a brain MRI would be needed to confirm the diagnosis of pituitary adenoma.
His newly reported erectile dysfunction suggests the possibility of a psychiatric, neurologic, hormonal, or vascular process and should be explored further. Sexual dysfunction is also associated with adrenal insufficiency and hypopituitarism. However, the presence of suspected prostatic hypertrophy in a male competitive athlete in his forties also raises the question of exogenous androgen use.
His past medical history was notable for a two-year history of alopecia totalis, seasonal allergies, asthma, and a repaired congenital aortic web with known aortic insufficiency. He was married with two children, worked an office job, and had no history of injection drug use, blood transfusions, or multiple sexual partners. His family history was notable for hypothyroidism and asthma in several family members in addition to Crohn disease, celiac disease, diabetes, cardiovascular disease, and cancers of the breast and lung.
His past medical, surgical, and family history supports a diagnosis of autoimmune disease. Although there is a personal and family history of atopic disorders, including allergic rhinitis and asthma, no association is found between atopy and autoimmunity. His family history of hypothyroidism, Crohn disease, and diabetes suggests a familial autoimmune genetic predisposition. His history of alopecia totalis in the setting of hypothyroidism and possible autoimmune adrenal insufficiency or autoimmune hypophysitis raises suspicion for the previously suggested diagnosis of polyglandular autoimmune syndrome, also known as autoimmune polyendocrine syndrome. Type I polyglandular autoimmune syndrome is associated with hypoparathyroidism and mucocutaneous candidiasis. In the absence of these symptoms, the patient more likely has type II polyglandular autoimmune syndrome. Type II syndrome is more prevalent and can occur in the setting of other nonendocrine autoimmune disorders, such as vitiligo, myasthenia gravis, or rheumatoid arthritis. Adrenal insufficiency can be the initial and most prominent manifestation of type II syndrome.
On physical exam, he was afebrile, with a heart rate of 68 beats per minute, respiratory rate of 16 breaths per minute, and normal oxygen saturation. His supine blood pressure and heart rate were 116/72 mm Hg and 66 beats per minute, respectively, and his standing blood pressure and heart rates were 80/48 mm Hg and 68 beats per minute respectively. He was thin, had diffuse scalp and body alopecia, and was ill-appearing with dry skin and dry mucous membranes. No evidence of Osler nodes, Janeway lesions, or splinter hemorrhages were found on cutaneous examination. No Roth spots or conjunctival hemorrhages were noted on ophthalmologic examination. He had both a 3/6 crescendo–decrescendo systolic murmur best heard at the right clavicle and radiated to the carotids and a 3/6 early diastolic decrescendo murmur best heard at the left sternal border. His abdomen was slightly protuberant, with reduced bowel sounds, hyperresonant to tympanitic on percussion, and a diffusely, moderately tender without peritoneal signs. Neurologic examination revealed 8 mm pupils with minimal response to light and accommodation. The remaining portions of his cranial nerve and complete neurologic examination were normal.
The presence of postural hypotension supports the previous suspicion of adrenal insufficiency, and the possibility of a pituitary or hypothalamic process remains. However, his dilated and minimally responsive pupils and potentially adynamic bowel are inconsistent with these diagnoses. Mydriasis and adynamic bowel in combination with orthostatic hypotension, dysgeusia, urinary retention, and erectile dysfunction are strongly suggestive of an autonomic process. Endocarditis is worth considering given his multisystem involvement, subacute decline, and known valve pathology. The absence of fever or stigmata of endocarditis make it difficult to explain his clinical syndrome. An echocardiogram would be reasonable for further assessment. At this point, it is prudent to explore his adrenal and pituitary function; if unrevealing, embark on an evaluation of his autonomic dysfunction.
Initial laboratory investigations were notable for mild normocytic anemia and hypoalbuminemia. His cosyntropin stimulation test was normal at 60 minutes. An abdominal CT scan demonstrated marked dilation in the small bowel loops (6 cm in caliber) with associated small bowel wall thickening and hyperemia. The echocardiogram was unrevealing and only confirmed the ongoing, progression of his known valve pathology without evidence of vegetation.
The above testing rules out primary adrenal insufficiency, but an appropriate response to the cosyntropin stimulation test does not rule out secondary, or pituitary, adrenal insufficiency. The echocardiogram and lack of other features make infective endocarditis unlikely. Thus, as mentioned, it is important now to commence a complete work-up of his probable dysautonomia to explain the majority of his features. Additionally, his hypothyroidism (if more than sick euthyroid syndrome), family history of autoimmune processes, and alopecia totalis all suggest the possibility of an immune-related syndrome. His CT scan revealed some thickened hyperemic bowel, which could suggest an IBD, such as Crohn disease; however, the absence of other signs, such as fever, diarrhea, or bloody stools, argues against this diagnosis. A syndrome that could unify his presentation is autoimmune autonomic ganglionopathy (AAG), a rare genetic autonomic system disorder that presents with pandysautonomia. The spectrum of autoimmunity was considered early in this case, but the differential diagnosis included more common conditions, such as adrenal insufficiency. Similarly, IBD remains a consideration. The serologic studies for IBD can be useful but they lack definitive diagnostic accuracy. Given that treatment for AAG differs from that for IBD, additional information will help guide the therapeutic approach. Anti-α3gnAChR antibodies, which are associated with AAG, should be checked.
His history of presyncope, anhidrosis, urinary retention, and ileus raised suspicion for pandysautonomia, as characterized by signs of sympathetic and parasympathetic dysfunction. The suspicion for pandysautonomia was confirmed via specialized autonomic testing, which included reduced heart rate variation on Valsalva and deep breathing maneuvers, orthostatic hypotension consistent autonomic insufficiency on Tilt table testing, and reduced sweat response to acetylcholine application (QSART test). The patient underwent further diagnostic serologic testing to differentiate causes of autonomic failure (Table 1). His personal and family history of autoimmunity led to the working diagnosis of AAG. Ultimate testing revealed high titers of autoantibodies, specifically anti-α3gnAChR (3.29 nmol/L, normal <0.02 nmol/L), directed against the ganglionic nicotinic acetylcholine receptor. This finding strongly supported the diagnosis of AAG.1,4-7
He was initially treated empirically with intravenous immunoglobulin (IVIG) with minimal improvement. He received additional immunomodulating therapies including methylprednisolone, plasmapheresis, and rituximab but did not tolerate a trial of mycophenolate. Six weeks after therapy initiation, his antibody titers decreased to 0.89 nmol/L with associated clinical improvement. Ultimately, he was discharged from the hospital on day 73 with a feeding tube and supplemental total parenteral nutrition. Four months postdischarge, he had returned to his prediagnosis weight, had eased back into his prior activities, and was off supplemental nutrition. Over a year later, he completed a 10-month prednisone taper and continued to receive monthly IVIG infusion
DISCUSSION
The clinical approach to dysautonomia is based on different etiologies: (1) those associated with neurodegenerative disorders; (2) those associated with peripheral neuropathies, and (3) isolated autonomic failure.2 Thus, clinical history and physical examination can assist greatly in guiding the evaluation of patients. Neurodegenerative disorders (such as Parkinson disease), combined disorders (such as multiple-system atrophy), and acquired or familial processes were considered. Our patient had neither a personal or family history nor physical examination supporting a neurodegenerative disorder. Disorders of the peripheral nerves were considered and can broadly be categorized as chronic sensorimotor neuropathies, sensory ganglionopathies, distal painful neuropathies, and acute or subacute motor polyradiculopathies. During evaluation, no historical, physical examination, or laboratory findings supported diabetes, amyloidosis, heavy metals, Sjögren syndrome, paraneoplastic neuropathy, sodium channel disorders, infectious etiologies, or porphyria (Table 1). Thus, in the absence of supportive evidence for primary neurodegenerative disorders or peripheral neuropathies, his syndrome appeared most compatible with an isolated autonomic failure syndrome. The principal differential for this syndrome is pure autonomic failure versus an immune-mediated autonomic disorder, including paraneoplastic autoimmune neuropathy and AAG. The diagnosis of pure autonomic failure is made after there is no clear unifying syndrome after more than five years of investigation. After exploration, no evidence of malignancy was discovered on body cross sectional imaging, PET scanning, bone marrow biopsy, colonoscopy, or laboratory testing. Thus, positive serologic testing in the absence of an underlying malignancy suggests a diagnosis of AAG.
AAG was first described in 1969 and is a rare, acquired disorder characterized by combined failure of the parasympathetic, sympathetic, and enteric nervous systems. This disorder typically presents in young-to-middle aged patients but has been described in all age groups. It is more commonly seen in patients with coexistent autoimmune diseases and/or a history of familial autoimmunity. The onset of clinical AAG may be subacute (less than three months) or insidious (more than three months). Patients present with signs or symptoms of pandysautonomia, such as severe orthostatic hypotension, syncope, constipation and gastrointestinal dysmotility, urinary retention, fixed and dilated pupils, and dry mouth and eyes (Table 2). Up to 40% of patients with AAG may also have significant cognitive impairment.3,4 Diagnosis relies on a combination of typical clinical features as discussed above and the exclusion of other diagnostic considerations. Diagnosis of AAG is aided by the presence of autoantibodies to ganglionic nicotinic acetylcholine receptors (gnAChR), particularly antiganglionic acetylcholine receptor α3 (anti-α3gAChR).1 Anti-gnAChR antibodies are only present in about half of patients with AAG. Antibody titers are highest in subacute AAG (40%-50%)3 compared with chronic AAG (30%-40%) or paraneoplastic AAG (10%-20%).5 Anti-gnAChR antibodies are not specific to AAG and have been identified in low levels in up to 20% of patients with thymomas, postural orthostatic tachycardia syndrome, chronic idiopathic anhidrosis, idiopathic gastrointestinal dysmotility, Lambert–Eaton syndrome, and myasthenia gravis without thymoma.1,5-7 These associations raise the question of shared pathology and perhaps a syndrome overlap. Individuals with seropositive AAG may also have other paraneoplastic antibodies, making it clinically indistinguishable from paraneoplastic autonomic neuropathy.5,8 Although the autoantibody lacks sensitivity and is imperfectly specific, its presence supports a diagnosis of AAG. Anti-gnAChR antibodies have been shown to be pathological in rabbit and mouse models.4 In patients with AAG, higher autoantibody titers correlate with increased disease severity.1,6,7 A decrease in autoantibody titers correlates with decreased disease severity.6 Case report series also described a distinct entity of seronegative AAG.2,3 Maintaining a high clinical suspicion for AAG even with negative antibodies is important.
Given the rarity of the disease, no standard therapeutic regimens are available. About one-third of individuals improve on their own, while other individuals require extensive immunomodulation and symptom management. Case series and observational trials currently make up the vast array of treatment data. Therapies include glucocorticoids, plasmapheresis, IVIG, and other immunosuppressive agents, such as rituximab.9-12 Patients with and without identified anti-gnAChRs antibodies may respond to therapy.12 The overall long-term prognosis of the disease is poorly characterized.9,10,13
Despite the rarity of the syndrome discussed, this case represents how diagnostic reasoning strategies, such as law of parsimony, shift how the case is framed. For example, a middle-aged man with several new, distinctly unrelated diagnoses versus a middle-aged man with signs and symptoms of autonomic failure alters the subsequent clinical reasoning and diagnostic approach. Many diseases, both common and rare, are associated with dysautonomia. Therefore, clinicians should have an approach to autonomic failure
TEACHING POINTS
- Recognize the following signs and symptoms suggesting a dysautonomic syndrome: orthostasis, syncope, anhidrosis, xerophthalmia, xerostomia, impaired pupillary constriction, blurry vision, photophobia, erectile dysfunction, urinary retention, gastroparesis, constipation, neurogenic bowel obstruction, and dysgeusia.
- Recognize the clinical features, diagnostic approach, and management of autoimmune autonomic ganglionopathy.
- When faced with a complex clinical presentation, early application of the “law of parsimony” may help identify a unifying syndrome.
Acknowledgments
The authors wish to thank our Blinded Expert, Anthony Montanaro, MD, for his expertise and guidance during this process.
Disclosures
There are no known conflicts of interest.
1. Gibbons C, Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146(1-2):8-12. doi: 10.1016/j.autneu.2008.11.013. PubMed
2. Golden E, Bryarly M, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28(1):115-123. doi: 10.1007/s10286-017-0493-8. PubMed
3. Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61(1):44-48. doi: 10.1001/archneur.61.1.44. PubMed
4. Vernino S, Ermilov L, Sha L, Szurszewski J, Low P, Lennon V. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. doi: 10.1523/JNEUROSCI.1485-04.2004. PubMed
5. Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146(1-2):3-7. doi: 10.1016/j.autneu.2008.09.005. PubMed
6. Vernino S, Low P, Fealey R, Stewart J, Farrugia G, Lennon V. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. doi: 10.1056/NEJM200009213431204. PubMed
7. Gibbons C, Vernino S, Freeman R. Autoimmune autonomic ganglionopathy – Symptom antibody correlations. Auton Neurosci. 2015;192:130. doi: 10.1016/j.autneu.2015.07.241 .
8. Benarroch E. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014;10(7):396-407. doi: 10.1038/nrneurol.2014.88. PubMed
9. Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. 2009;146(1-2):29-32. doi: 10.1016/j.autneu.2008.10.016. PubMed
10. Gibbons C, Centi J, Vernino S. Autoimmune autonomic ganglionoapthy with reversible cognitive impairment. Arch Neurol. 2012;69(4):461-466. doi: 10.1001/archneurol.2011.2372. PubMed
11. Boydston E, Muppidi S, Vernino S. Long-term outcomes in autoimmune autonomic ganglionopathy (P05.210). Neurology. 2012;78(1):P05.210. doi: 10.1212/WNL.78.1_MeetingAbstracts.P05.210.
12. Gehrking T, Sletten D, Fealey R, Low P, Singer W. 11-year follow-up of a case of autoimmune autonomic ganglionopathy (P03.024). Neurology. 2013;80(7):P03.024.
13. Imrich R, Vernino S, Eldadah BA, Holmes C, Goldstein DS. Autoimmune autonomic ganglionopathy: treatment by plasma exchanges and rituximab. Clin Auton Res. 2009;19(4):259-262. doi: 10.1007/s10286-009-0012-7. PubMed
14. Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72(23):2002-8. doi: 10.1212/WNL.0b013e3181a92b52. PubMed
15. Hayashi M, Ishii Y. A Japanese case of autoimmune autonomic ganglionopathy (AAG) and a review of AAG cases in Japan. Auton Neurosci. 2009;146(1-2):26-8. doi: 10.1016/j.autneu.2008.12.013. PubMed
16. Baker, A. Simplicity. In: Baker A, Zalta E, eds. The Stanford Encyclopedia of Philosophy. Winter 2016 Edition. https://plato.stanford.edu/archives/win2016/entries/simplicity/. Accessed October 26, 2017.
1. Gibbons C, Freeman R. Antibody titers predict clinical features of autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146(1-2):8-12. doi: 10.1016/j.autneu.2008.11.013. PubMed
2. Golden E, Bryarly M, Vernino S. Seronegative autoimmune autonomic neuropathy: a distinct clinical entity. Clin Auton Res. 2018;28(1):115-123. doi: 10.1007/s10286-017-0493-8. PubMed
3. Sandroni P, Vernino S, Klein CM, et al. Idiopathic autonomic neuropathy: comparison of cases seropositive and seronegative for ganglionic acetylcholine receptor antibody. Arch Neurol. 2004;61(1):44-48. doi: 10.1001/archneur.61.1.44. PubMed
4. Vernino S, Ermilov L, Sha L, Szurszewski J, Low P, Lennon V. Passive transfer of autoimmune autonomic neuropathy to mice. J Neurosci. 2004;24(32):7037-7042. doi: 10.1523/JNEUROSCI.1485-04.2004. PubMed
5. Vernino S, Hopkins S, Wang Z. Autonomic ganglia, acetylcholine receptor antibodies, and autoimmune ganglionopathy. Auton Neurosci. 2009;146(1-2):3-7. doi: 10.1016/j.autneu.2008.09.005. PubMed
6. Vernino S, Low P, Fealey R, Stewart J, Farrugia G, Lennon V. Autoantibodies to ganglionic acetylcholine receptors in autoimmune autonomic neuropathies. N Engl J Med. 2000;343(12):847-855. doi: 10.1056/NEJM200009213431204. PubMed
7. Gibbons C, Vernino S, Freeman R. Autoimmune autonomic ganglionopathy – Symptom antibody correlations. Auton Neurosci. 2015;192:130. doi: 10.1016/j.autneu.2015.07.241 .
8. Benarroch E. The clinical approach to autonomic failure in neurological disorders. Nat Rev Neurol. 2014;10(7):396-407. doi: 10.1038/nrneurol.2014.88. PubMed
9. Baker SK, Morillo C, Vernino S. Autoimmune autonomic ganglionopathy with late-onset encephalopathy. Auton Neurosci. 2009;146(1-2):29-32. doi: 10.1016/j.autneu.2008.10.016. PubMed
10. Gibbons C, Centi J, Vernino S. Autoimmune autonomic ganglionoapthy with reversible cognitive impairment. Arch Neurol. 2012;69(4):461-466. doi: 10.1001/archneurol.2011.2372. PubMed
11. Boydston E, Muppidi S, Vernino S. Long-term outcomes in autoimmune autonomic ganglionopathy (P05.210). Neurology. 2012;78(1):P05.210. doi: 10.1212/WNL.78.1_MeetingAbstracts.P05.210.
12. Gehrking T, Sletten D, Fealey R, Low P, Singer W. 11-year follow-up of a case of autoimmune autonomic ganglionopathy (P03.024). Neurology. 2013;80(7):P03.024.
13. Imrich R, Vernino S, Eldadah BA, Holmes C, Goldstein DS. Autoimmune autonomic ganglionopathy: treatment by plasma exchanges and rituximab. Clin Auton Res. 2009;19(4):259-262. doi: 10.1007/s10286-009-0012-7. PubMed
14. Iodice V, Kimpinski K, Vernino S, Sandroni P, Fealey RD, Low PA. Efficacy of immunotherapy in seropositive and seronegative putative autoimmune autonomic ganglionopathy. Neurology. 2009;72(23):2002-8. doi: 10.1212/WNL.0b013e3181a92b52. PubMed
15. Hayashi M, Ishii Y. A Japanese case of autoimmune autonomic ganglionopathy (AAG) and a review of AAG cases in Japan. Auton Neurosci. 2009;146(1-2):26-8. doi: 10.1016/j.autneu.2008.12.013. PubMed
16. Baker, A. Simplicity. In: Baker A, Zalta E, eds. The Stanford Encyclopedia of Philosophy. Winter 2016 Edition. https://plato.stanford.edu/archives/win2016/entries/simplicity/. Accessed October 26, 2017.
© 2018 Society of Hospital Medicine
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Chance Favors the Prepared Mind
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A previously healthy 18‐year‐old woman living in the Pacific Northwest was brought in by her parents to a local hospital with a 4‐day history of acting crazy. Two weeks prior to presentation, she complained of a new‐onset severe headache, diaphoresis, and chills. Four days prior to presentation, she became progressively more impulsive, which ultimately included jumping out of a moving vehicle and running away from home. She experienced unexplained emotional outbursts and was unable to identify familiar relatives or common objects. Additionally, she began having hyperventilation spells and auditory hallucinations.
In an adolescent presenting with erratic behavior, one should consider the possibility of substance abuse or a psychiatric disease such as bipolar disorder with manic features, psychotic manifestations of severe depression, or early schizophrenia. However, it is important to first rule out non‐psychiatric disease, with a diagnostic approach dependent on her human immunodeficiency virus (HIV) status. The presence of headache, diaphoresis, and chills raises concern for an infectious or noninfectious inflammatory central nervous system process. In addition to the effects of illicit drugs such as cocaine or methamphetamine, this presentation may be consistent with a medication‐ or herbal‐induced anticholinergic syndrome, which may present with confusion, ataxia, coma, and cardiopulmonary failure. Since this case originates in the Northwest, one should be aware of the regional outbreak of Cryptococcus gattii in immunocompetent hosts, and that local hallucinogenic plants, such as jimson weed or mushrooms (Amanita muscaria) can cause anticholinergic syndromes. At this point, the differential diagnosis is broad, and evaluation should focus on potentially reversible life‐threatening conditions; in particular, herpes encephalitis. In addition to a detailed history, examination, and routine laboratory studies including HIV serology, I would obtain a drug screen, and order a computed tomography (CT) scan of the brain before performing a lumbar puncture. I would also order a magnetic resonance imaging (MRI) study to evaluate for meningeal or cerebral enhancement suggestive of encephalitis.
The patient had no past medical, psychiatric, or surgical history and took no medications. She lived with her parents who thought she neither used illicit drugs or alcohol, nor was sexually active. She had recently graduated high school and was planning to attend college. Her family history was notable for a mother with bipolar and seizure disorders, and 2 healthy younger siblings. Her family had a healthy cat and dog, and reported a large number of bats living nearby. She had never traveled outside the western United States. The patient presented in late spring, but there was no obvious history of mosquito bites. Her last menstrual period was 4 months prior to presentation. Full review of systems was otherwise negative.
The family history of mood disorder supports continued consideration of bipolar disorder with psychotic manifestations. However, infectious or inflammatory processes remain highest on the differential at this point. The duration of symptoms makes common bacterial meningitis etiologies (Streptococcus, Neisseria, Haemophilus, Listeria) less likely, but would be consistent with herpes simplex encephalitis or lupus cerebritis. Additional infectious considerations would include other viral (eg, varicella zoster virus, Epstein‐Barr virus, enteroviruses, and the arthropod‐borne encephalitides) or unusual bacterial encephalitic syndromes. Although the health status of pets is rarely helpful, dogs can carry ticks that harbor Borrelia burgdorferi (the agent of Lyme disease), which may present with central nervous system (CNS) manifestations. Other conditions associated with pets (such as leptospirosis or cat scratch disease) seem unlikely. The exposure to bats raises the possibility of rabies infection. If she is HIV‐positive, one would need to consider the possibility of opportunistic infections such as cytomegalovirus (CMV), Cryptococcus, cerebral toxoplasmosis, and progressive multifocal leukoencephalopathy (PML) caused by JC virus reaction. Finally, regardless of history, given the patient's amenorrhea, we must perform a pregnancy test.
The patient's temperature was 97.3F, heart rate 129 beats per minute, respiratory rate 19 breaths per minute, and her blood pressure 144/97 mmHg. She was an obese, well‐developed young woman, who was drowsy but arousable, with marked speech latency. Her cranium and oropharynx were normal, and her neck was supple. Aside from tachycardia, her cardiopulmonary, musculoskeletal, and skin exams were normal. Her abdomen was obese and soft, without masses or organomegaly. A pelvic examination was not performed. On neurologic exam, her strength was symmetrically diminished throughout (3+/5). Otherwise, she was oriented to person and general location, but not to day of week, month, or year. Her cranial nerves, sensation, deep tendon reflexes, and muscle tone were normal. A cerebellar examination, plantar response, and gait test were not performed. A brain MRI revealed only a small subarachnoid cyst and possible subtle enhancement of temporal lobes. Initial laboratory studies demonstrated: white blood cell count 14,000/mm3 (72% neutrophils, 17% lymphocytes, 9% monocytes, 2% eosinophils); hemoglobin 14.0 g/dL (mean corpuscular volume 87.4 fL); platelet count 417,000/mm3. Serum electrolytes, liver function tests, coagulation studies, thyroid stimulating hormone, serum ammonia, and urinalysis were normal. Her serum pregnancy test and urine toxicology screen were negative. A room air arterial blood gas revealed a pH of 7.49, PaCO2 32 mmHg, PaO2 89 mmHg; and a bicarbonate 24 mmol/L. Cerebrospinal fluid demonstrated: red cell count of 2/mm3; white cell count 17/mm3 (88% lymphocytes, 3% neutrophils, 9% monocytes); protein 19 mg/dL (normal 1555 mg/dL); and glucose of 79 mg/dL (normal 4080 mg/dL). Gram stain, fungal and bacterial cultures, and HIV serology were negative, and herpes simplex virus was not detected via polymerase chain reaction (PCR).
The tachycardia, respiratory alkalosis, and leukocytosis continue to suggest an infection or inflammatory state. Her neurological deterioration without focal findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis with normal glucose and protein, and temporal lobe enhancement on MRI strongly suggest a meningoencephalitis. This would be an unusual presentation for most bacterial pathogens, but Mycobacterium, Rickettsia, Listeria, Mycoplasma, and Bartonella may rarely mimic encephalitis. Autoimmune encephalitis secondary to lupus, vasculitis, or other autoimmune disorder remains possible, but at this point an infectious encephalitis, particularly herpes encephalitis, is my highest concern. West Nile virus must be considered, but usually produces a severe illness only in immunocompromised or elderly patients. Additionally, despite the rarity of rabies, the patient's exposure to bats and the rapid clinical deterioration, suggest this possibility. In addition to routine bacterial and viral analyses (eg, enteroviral panel), samples should be sent for rabies PCR and antibody testing, West Nile virus, Lyme disease, syphilis, and mycobacterial and fungal pathogens, such as the aforementioned Cryptococcus gattii. Finally, given her presenting syndrome and MRI, immediate treatment with acyclovir and antibiotics is indicated.
The patient was treated for presumed meningoencephalitis with acyclovir and ceftriaxone, but over the following several days became unresponsive to all stimuli and developed repetitive thrusting movements of her mouth, tongue, and jaw. On hospital day 10, with concern for seizures, pentobarbital coma was induced, and the patient was intubated and transferred to our facility. On arrival, her physical examination was essentially unchanged aside from being in a medical coma. Hematology, chemistries, and thyroid‐stimulating hormone (TSH) were again unremarkable with the exception of an elevated creatine kinase (414 U/L) and a new anemia (hemoglobin 8.9 g/dL; mean corpuscular volume 87.6 fL) without evidence of iron deficiency or hemolysis. Blood and urine cultures were negative. Repeat cerebrospinal fluid analysis was essentially unchanged, revealing a red cell count of 1/mm3; white cell count 20/mm3 (86% lymphocytes, 2% neutrophils, 12% monocytes); protein 14 mg/dL; glucose 63 mg/dL, and negative Gram stain. Continuous electroencephalography revealed diffuse generalized slowing, but no seizure activity. An extensive evaluation for viral, bacterial, autoimmune, and paraneoplastic disorders was negative, including tests for anti‐acetylcholine (ACh) receptor binding antibody, anti‐striated muscle antibody, anti‐N‐type calcium channel antibody, anti‐P/Q‐type calcium channel antibodies, anto‐cancer associated retinopathy (CAR) antibody (also known as anti‐recoverin antibody), and anti‐collapsin respons mediator protein (CRMP‐5). Without confirmatory results and continued deterioration, she was empirically treated with methylprednisolone for presumed autoimmune encephalitis from hospital days 16 to 21. The patient remained unresponsive and ventilator‐dependent, despite removal of all sedation. She experienced intermittent fevers as high as 40.5C, remained tachycardic, hypertensive, and exhibited orofacial dyskinesias and jaw clenching, ultimately requiring botulinum toxin injections to prevent tongue biting. Given the lack of improvement despite attempted therapies, a working diagnosis of viral encephalitis with lasting neuropsychiatric sequelae was made. A tracheostomy and percutaneous gastrostomy tube were placed, and a long‐term ventilator care facility was identified.
I continue to wonder if this may be an autoimmune encephalitis, and am concerned about her unexplained fevers. Neuroleptic malignant syndrome secondary to misuse of her parents' medications should be considered in light of the elevated creatine kinase, although the severity and duration of the syndrome seem more profound than I would anticipate. Tetanus could present with jaw dystonia, but the rest of the case does not seem to fit. At this point, considering the patient's young age and poor prognosis without identified etiology, prior to discharge I would argue for a brain biopsy looking for evidence of rabies, or other infectious or autoimmune etiologies of the patient's progressive neurologic deterioration.
On hospital day 25, due to the persistent fevers with concern for occult abscess, an abdominopelvic CT was obtained, which identified a complex 11.8 cm 9.0 cm adnexal mass consistent with a teratoma (Figure 1).

Given the size of the mass, it is surprising that the patient did not report abdominal symptoms and that the physicians were unable to palpate it on examination. The differential diagnosis of a complex adnexal mass in an adolescent should include an ectopic pregnancy, ovarian cysts, tubo‐ovarian abscess, rarely an ovarian carcinoma or leiomyosarcoma, and a teratoma or dermoid tumor. While I mentioned the possibility of a malignancy at the outset, I did not further consider it. Common neoplasms encountered in adolescent patients include lymphoma and leukemia, germ cell tumors (including teratomas), central nervous system tumors and sarcomas, many of which have been reported to cause paraneoplastic disorders. At this point, I now think her presumed teratoma is associated with a paraneoplastic syndrome resulting in her presentation of limbic encephalitis.
A literature search was performed by the managing clinicians who rapidly identified the association between teratoma and limbic encephalitis. The patient was initially treated with intravenous immune globulin (IVIG), with transient improvement in her mental status. Serology returned positive for the anti‐N‐methyl‐D‐aspartate receptor antibody, confirming the diagnosis of anti‐N‐methyl‐D‐aspartate receptor encephalitis. On hospital day 36, her mass was resected (Figure 2). Pathology was consistent with a mature teratoma. Postoperatively, the patient improved daily, and was discharged on hospital day 43 with a near complete neurologic recovery. Four months following discharge, the patient had enrolled full time in college.
COMMENTARY
The N‐methyl‐D‐aspartate receptor (NMDAR) is an important regulator of synaptic transmission and memory within the CNS. Our patient's case illustrates the increasingly recognized syndrome of anti‐NMDAR encephalitis. NMDAR hypofunction is hypothesized to result in the cognitive and behavioral abnormalities of schizophrenia, and direct antagonism of the NMDAR by drugs such as phencyclidine (PCP) and ketamine results in symptoms such as psychosis, hallucinations, delusions, agitation, and dissociative amnesia.14 This constellation of symptoms is very similar to some of the initial neuropsychiatric symptoms observed in patients with anti‐NMDAR encephalitis.
Anti‐NMDAR encephalitis was first described in 2005 as a paraneoplastic limbic encephalitis associated with ovarian teratoma.5, 6 Characterized by the subacute onset (days to weeks) of short‐term memory loss, psychiatric symptoms, and sleep disturbances, limbic encephalitis is an inflammatory process caused by autoantibodies against intracellular or extracellar antigens in the limbic system and other brain structures. Limbic encephalitides associated with antibodies to intracellular antigens (such as Hu, Ma2, CV2/CRMP5, and Amphiphysin) are more often associated with malignancies, have worse outcomes (permanent neuropsychiatric sequelae and death), and are less responsive to immune therapy. Conversely, it appears that both the paraneoplastic and non‐paraneoplastic variants of limbic encephalitis associated with antibodies against cell membrane antigens (such as NMDAR and Voltage Gated Potassium Channels) respond more favorably to therapy.7
As with limbic encephalitis in general, anti‐NMDAR encephalitis can be non‐paraneoplastic as well as paraneoplastic in etiology. In a recently published series of 44 consecutive patients with anti‐NMDAR encephalitis, tumors were present in only 9 cases (8 teratomas).8 When associated with a teratoma, it has been postulated that anti‐NMDAR antibodies develop and cross the bloodbrain barrier to target central nervous system NMDA receptors. This process results in down‐regulation of the neuronal surface NMDAR which then causes the psychiatric and behavioral changes described.6 The mechanism by which these antibodies traverse the bloodbrain barrier is not completely understood, but likely requires some disruption of the barrier in order to trigger anti‐NMDAR encephalitis.8, 9 Non‐paraneoplastic cases evidently involve other unknown stimuli for NMDAR antibody synthesisone report has suggested that subunits of the NMDAR are expressed by normal ovarian tissue, something which may explain the female predilection even in the cohort unaffected by teratomas.10
Most patients with anti‐NMDAR encephalitis are female and young (median age 23 years), although men and children are also affected.8, 9, 11 While the exact incidence of anti‐NMDAR encephalitis is still unknown, the increasing number of case reports suggests that it may be more frequent than any other type of paraneoplastic encephalitis.12 The majority of patients with anti‐NMDAR encephalitis experience an antecedent infectious prodrome (eg, diarrheal illness or upper respiratory infection [URI]), followed 1020 days later by progressive neuropsychiatric and behavioral symptoms which include confusion, memory deficits, impaired responsiveness, seizures, central hypoventilation, and signs of autonomic instability (tachycardia, tachypnea, diaphoresis, cardiac dysrhythmia, blood pressure instability, and dysthermia). At this stage, patients may also manifest a unique constellation of choreoathetoid orofacial and limb movements such as lip licking, chewing, sustained jaw clenching, jaw opening dystonias, ocular deviation and disconjugation, grimacing, myoclonus, and bizarre arm movements. Due to cardiovascular complications and ventilator requirements, most patients require intensive care unit (ICU) level care. 8, 9, 11 As in our discussant's evaluation, other disorders to include in the differential diagnosis for this presentation includes paraneoplastic or autoimmune causes of limbic encephalitis, toxins, heavy metals, and viral causes of encephalitis; in particular, herpes simplex virus (HSV).7
The CNS imaging findings in this condition include brain MRI abnormalities in about 30%55% of patients, which can include increased signal on fluid‐attenuated inversion recovery (FLAIR) or T2 sequences of the cerebral cortex, overlying meninges, or basal ganglia. Abnormalities in the temporal lobes, corpus callosum, and brainstem have also been described. As in our patient, CSF lymphocytic pleocytosis has also been noted.6, 8, 9
Although many cases of limbic encephalitis portend a poor prognosis with permanent neuropsychiatric sequelae and death, anti‐NMDAR can be very responsive to treatment; particularly if diagnosed early. Successful treatment of anti‐NMDAR encephalitis involves immunotherapy and, preferably, early surgical resection of any tumor. 6, 8, 9 Non‐paraneoplastic cases appear to require more aggressive and prolonged immunotherapies to avoid relapse. In both groups, a trend towards improved outcome has been noted in patients treated early in disease course (<40 days from symptom onset).8 There are no established guidelines for the treatment of anti‐NMDAR encephalitis, and no randomized controlled trials have evaluated anti‐NMDAR encephalitis treatment. Observational studies of immune‐modulating therapies have shown efficacy with high‐dose steroids and the addition of plasma exchange and/or intravenous immune globulin. Rituximab and cyclophosphamide can be considered if patients fail to improve on other immunotherapies.9 Data from case series seem to suggest a lower risk of relapse in patients treated with immunotherapy.13
Exploration of this patient's persistent high fevers ultimately led to the serendipitous diagnosis of the increasingly recognized syndrome of anti‐NMDAR encephalitis, although in retrospect nearly all of the features of her presentation fit well with this condition. Thus, it was only by a chance finding on her abdominal CT scan that this patient was ultimately diagnosed with a treatable, noninfectious encephalitis associated with an ovarian teratoma. This case reinforces the importance of thorough patient evaluations and being prepared to draw meaningful conclusions from unexpected findings. Given how close this patient was to being discharged to a long‐term care facility, we found this case a fascinating yet sobering reminder to guard against prematurely concluding a syndrome to be untreatable.
KEY TEACHING POINTS
-
Anti‐NMDAR encephalitis is an increasingly recognized cause of autoimmune limbic encephalitis, and thus should be considered in patients with new‐onset psychiatric symptoms accompanied by seizures, autonomic instability, hypoventilation, or dyskinesias.
-
A thorough history, examination, and evaluation of data is critical to make an early diagnosis of anti‐NMDAR encephalitis, because, unlike other forms of limbic encephalitis, this condition may be very responsive to early initiation of treatment.
- , . N‐methyl‐D‐aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49.
- , . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308.
- , , . NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
- , , , et al. Ketamine‐induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118.
- , , , , , . Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604.
- , , , et al. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
- , . Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271.
- , , , et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667.
- , . NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304.
- , , , et al. Expression of various glutamate receptors including N‐methyl‐D‐aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti‐NMDAR encephalitis. Intern Med. 2010;49:2167–2173.
- , , , et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1074–1075.
- , , , , . Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
- , , , et al. Analysis of relapses in anti‐NMDAR encephalitis. Neurology. 2011;77:996–999.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A previously healthy 18‐year‐old woman living in the Pacific Northwest was brought in by her parents to a local hospital with a 4‐day history of acting crazy. Two weeks prior to presentation, she complained of a new‐onset severe headache, diaphoresis, and chills. Four days prior to presentation, she became progressively more impulsive, which ultimately included jumping out of a moving vehicle and running away from home. She experienced unexplained emotional outbursts and was unable to identify familiar relatives or common objects. Additionally, she began having hyperventilation spells and auditory hallucinations.
In an adolescent presenting with erratic behavior, one should consider the possibility of substance abuse or a psychiatric disease such as bipolar disorder with manic features, psychotic manifestations of severe depression, or early schizophrenia. However, it is important to first rule out non‐psychiatric disease, with a diagnostic approach dependent on her human immunodeficiency virus (HIV) status. The presence of headache, diaphoresis, and chills raises concern for an infectious or noninfectious inflammatory central nervous system process. In addition to the effects of illicit drugs such as cocaine or methamphetamine, this presentation may be consistent with a medication‐ or herbal‐induced anticholinergic syndrome, which may present with confusion, ataxia, coma, and cardiopulmonary failure. Since this case originates in the Northwest, one should be aware of the regional outbreak of Cryptococcus gattii in immunocompetent hosts, and that local hallucinogenic plants, such as jimson weed or mushrooms (Amanita muscaria) can cause anticholinergic syndromes. At this point, the differential diagnosis is broad, and evaluation should focus on potentially reversible life‐threatening conditions; in particular, herpes encephalitis. In addition to a detailed history, examination, and routine laboratory studies including HIV serology, I would obtain a drug screen, and order a computed tomography (CT) scan of the brain before performing a lumbar puncture. I would also order a magnetic resonance imaging (MRI) study to evaluate for meningeal or cerebral enhancement suggestive of encephalitis.
The patient had no past medical, psychiatric, or surgical history and took no medications. She lived with her parents who thought she neither used illicit drugs or alcohol, nor was sexually active. She had recently graduated high school and was planning to attend college. Her family history was notable for a mother with bipolar and seizure disorders, and 2 healthy younger siblings. Her family had a healthy cat and dog, and reported a large number of bats living nearby. She had never traveled outside the western United States. The patient presented in late spring, but there was no obvious history of mosquito bites. Her last menstrual period was 4 months prior to presentation. Full review of systems was otherwise negative.
The family history of mood disorder supports continued consideration of bipolar disorder with psychotic manifestations. However, infectious or inflammatory processes remain highest on the differential at this point. The duration of symptoms makes common bacterial meningitis etiologies (Streptococcus, Neisseria, Haemophilus, Listeria) less likely, but would be consistent with herpes simplex encephalitis or lupus cerebritis. Additional infectious considerations would include other viral (eg, varicella zoster virus, Epstein‐Barr virus, enteroviruses, and the arthropod‐borne encephalitides) or unusual bacterial encephalitic syndromes. Although the health status of pets is rarely helpful, dogs can carry ticks that harbor Borrelia burgdorferi (the agent of Lyme disease), which may present with central nervous system (CNS) manifestations. Other conditions associated with pets (such as leptospirosis or cat scratch disease) seem unlikely. The exposure to bats raises the possibility of rabies infection. If she is HIV‐positive, one would need to consider the possibility of opportunistic infections such as cytomegalovirus (CMV), Cryptococcus, cerebral toxoplasmosis, and progressive multifocal leukoencephalopathy (PML) caused by JC virus reaction. Finally, regardless of history, given the patient's amenorrhea, we must perform a pregnancy test.
The patient's temperature was 97.3F, heart rate 129 beats per minute, respiratory rate 19 breaths per minute, and her blood pressure 144/97 mmHg. She was an obese, well‐developed young woman, who was drowsy but arousable, with marked speech latency. Her cranium and oropharynx were normal, and her neck was supple. Aside from tachycardia, her cardiopulmonary, musculoskeletal, and skin exams were normal. Her abdomen was obese and soft, without masses or organomegaly. A pelvic examination was not performed. On neurologic exam, her strength was symmetrically diminished throughout (3+/5). Otherwise, she was oriented to person and general location, but not to day of week, month, or year. Her cranial nerves, sensation, deep tendon reflexes, and muscle tone were normal. A cerebellar examination, plantar response, and gait test were not performed. A brain MRI revealed only a small subarachnoid cyst and possible subtle enhancement of temporal lobes. Initial laboratory studies demonstrated: white blood cell count 14,000/mm3 (72% neutrophils, 17% lymphocytes, 9% monocytes, 2% eosinophils); hemoglobin 14.0 g/dL (mean corpuscular volume 87.4 fL); platelet count 417,000/mm3. Serum electrolytes, liver function tests, coagulation studies, thyroid stimulating hormone, serum ammonia, and urinalysis were normal. Her serum pregnancy test and urine toxicology screen were negative. A room air arterial blood gas revealed a pH of 7.49, PaCO2 32 mmHg, PaO2 89 mmHg; and a bicarbonate 24 mmol/L. Cerebrospinal fluid demonstrated: red cell count of 2/mm3; white cell count 17/mm3 (88% lymphocytes, 3% neutrophils, 9% monocytes); protein 19 mg/dL (normal 1555 mg/dL); and glucose of 79 mg/dL (normal 4080 mg/dL). Gram stain, fungal and bacterial cultures, and HIV serology were negative, and herpes simplex virus was not detected via polymerase chain reaction (PCR).
The tachycardia, respiratory alkalosis, and leukocytosis continue to suggest an infection or inflammatory state. Her neurological deterioration without focal findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis with normal glucose and protein, and temporal lobe enhancement on MRI strongly suggest a meningoencephalitis. This would be an unusual presentation for most bacterial pathogens, but Mycobacterium, Rickettsia, Listeria, Mycoplasma, and Bartonella may rarely mimic encephalitis. Autoimmune encephalitis secondary to lupus, vasculitis, or other autoimmune disorder remains possible, but at this point an infectious encephalitis, particularly herpes encephalitis, is my highest concern. West Nile virus must be considered, but usually produces a severe illness only in immunocompromised or elderly patients. Additionally, despite the rarity of rabies, the patient's exposure to bats and the rapid clinical deterioration, suggest this possibility. In addition to routine bacterial and viral analyses (eg, enteroviral panel), samples should be sent for rabies PCR and antibody testing, West Nile virus, Lyme disease, syphilis, and mycobacterial and fungal pathogens, such as the aforementioned Cryptococcus gattii. Finally, given her presenting syndrome and MRI, immediate treatment with acyclovir and antibiotics is indicated.
The patient was treated for presumed meningoencephalitis with acyclovir and ceftriaxone, but over the following several days became unresponsive to all stimuli and developed repetitive thrusting movements of her mouth, tongue, and jaw. On hospital day 10, with concern for seizures, pentobarbital coma was induced, and the patient was intubated and transferred to our facility. On arrival, her physical examination was essentially unchanged aside from being in a medical coma. Hematology, chemistries, and thyroid‐stimulating hormone (TSH) were again unremarkable with the exception of an elevated creatine kinase (414 U/L) and a new anemia (hemoglobin 8.9 g/dL; mean corpuscular volume 87.6 fL) without evidence of iron deficiency or hemolysis. Blood and urine cultures were negative. Repeat cerebrospinal fluid analysis was essentially unchanged, revealing a red cell count of 1/mm3; white cell count 20/mm3 (86% lymphocytes, 2% neutrophils, 12% monocytes); protein 14 mg/dL; glucose 63 mg/dL, and negative Gram stain. Continuous electroencephalography revealed diffuse generalized slowing, but no seizure activity. An extensive evaluation for viral, bacterial, autoimmune, and paraneoplastic disorders was negative, including tests for anti‐acetylcholine (ACh) receptor binding antibody, anti‐striated muscle antibody, anti‐N‐type calcium channel antibody, anti‐P/Q‐type calcium channel antibodies, anto‐cancer associated retinopathy (CAR) antibody (also known as anti‐recoverin antibody), and anti‐collapsin respons mediator protein (CRMP‐5). Without confirmatory results and continued deterioration, she was empirically treated with methylprednisolone for presumed autoimmune encephalitis from hospital days 16 to 21. The patient remained unresponsive and ventilator‐dependent, despite removal of all sedation. She experienced intermittent fevers as high as 40.5C, remained tachycardic, hypertensive, and exhibited orofacial dyskinesias and jaw clenching, ultimately requiring botulinum toxin injections to prevent tongue biting. Given the lack of improvement despite attempted therapies, a working diagnosis of viral encephalitis with lasting neuropsychiatric sequelae was made. A tracheostomy and percutaneous gastrostomy tube were placed, and a long‐term ventilator care facility was identified.
I continue to wonder if this may be an autoimmune encephalitis, and am concerned about her unexplained fevers. Neuroleptic malignant syndrome secondary to misuse of her parents' medications should be considered in light of the elevated creatine kinase, although the severity and duration of the syndrome seem more profound than I would anticipate. Tetanus could present with jaw dystonia, but the rest of the case does not seem to fit. At this point, considering the patient's young age and poor prognosis without identified etiology, prior to discharge I would argue for a brain biopsy looking for evidence of rabies, or other infectious or autoimmune etiologies of the patient's progressive neurologic deterioration.
On hospital day 25, due to the persistent fevers with concern for occult abscess, an abdominopelvic CT was obtained, which identified a complex 11.8 cm 9.0 cm adnexal mass consistent with a teratoma (Figure 1).

Given the size of the mass, it is surprising that the patient did not report abdominal symptoms and that the physicians were unable to palpate it on examination. The differential diagnosis of a complex adnexal mass in an adolescent should include an ectopic pregnancy, ovarian cysts, tubo‐ovarian abscess, rarely an ovarian carcinoma or leiomyosarcoma, and a teratoma or dermoid tumor. While I mentioned the possibility of a malignancy at the outset, I did not further consider it. Common neoplasms encountered in adolescent patients include lymphoma and leukemia, germ cell tumors (including teratomas), central nervous system tumors and sarcomas, many of which have been reported to cause paraneoplastic disorders. At this point, I now think her presumed teratoma is associated with a paraneoplastic syndrome resulting in her presentation of limbic encephalitis.
A literature search was performed by the managing clinicians who rapidly identified the association between teratoma and limbic encephalitis. The patient was initially treated with intravenous immune globulin (IVIG), with transient improvement in her mental status. Serology returned positive for the anti‐N‐methyl‐D‐aspartate receptor antibody, confirming the diagnosis of anti‐N‐methyl‐D‐aspartate receptor encephalitis. On hospital day 36, her mass was resected (Figure 2). Pathology was consistent with a mature teratoma. Postoperatively, the patient improved daily, and was discharged on hospital day 43 with a near complete neurologic recovery. Four months following discharge, the patient had enrolled full time in college.

COMMENTARY
The N‐methyl‐D‐aspartate receptor (NMDAR) is an important regulator of synaptic transmission and memory within the CNS. Our patient's case illustrates the increasingly recognized syndrome of anti‐NMDAR encephalitis. NMDAR hypofunction is hypothesized to result in the cognitive and behavioral abnormalities of schizophrenia, and direct antagonism of the NMDAR by drugs such as phencyclidine (PCP) and ketamine results in symptoms such as psychosis, hallucinations, delusions, agitation, and dissociative amnesia.14 This constellation of symptoms is very similar to some of the initial neuropsychiatric symptoms observed in patients with anti‐NMDAR encephalitis.
Anti‐NMDAR encephalitis was first described in 2005 as a paraneoplastic limbic encephalitis associated with ovarian teratoma.5, 6 Characterized by the subacute onset (days to weeks) of short‐term memory loss, psychiatric symptoms, and sleep disturbances, limbic encephalitis is an inflammatory process caused by autoantibodies against intracellular or extracellar antigens in the limbic system and other brain structures. Limbic encephalitides associated with antibodies to intracellular antigens (such as Hu, Ma2, CV2/CRMP5, and Amphiphysin) are more often associated with malignancies, have worse outcomes (permanent neuropsychiatric sequelae and death), and are less responsive to immune therapy. Conversely, it appears that both the paraneoplastic and non‐paraneoplastic variants of limbic encephalitis associated with antibodies against cell membrane antigens (such as NMDAR and Voltage Gated Potassium Channels) respond more favorably to therapy.7
As with limbic encephalitis in general, anti‐NMDAR encephalitis can be non‐paraneoplastic as well as paraneoplastic in etiology. In a recently published series of 44 consecutive patients with anti‐NMDAR encephalitis, tumors were present in only 9 cases (8 teratomas).8 When associated with a teratoma, it has been postulated that anti‐NMDAR antibodies develop and cross the bloodbrain barrier to target central nervous system NMDA receptors. This process results in down‐regulation of the neuronal surface NMDAR which then causes the psychiatric and behavioral changes described.6 The mechanism by which these antibodies traverse the bloodbrain barrier is not completely understood, but likely requires some disruption of the barrier in order to trigger anti‐NMDAR encephalitis.8, 9 Non‐paraneoplastic cases evidently involve other unknown stimuli for NMDAR antibody synthesisone report has suggested that subunits of the NMDAR are expressed by normal ovarian tissue, something which may explain the female predilection even in the cohort unaffected by teratomas.10
Most patients with anti‐NMDAR encephalitis are female and young (median age 23 years), although men and children are also affected.8, 9, 11 While the exact incidence of anti‐NMDAR encephalitis is still unknown, the increasing number of case reports suggests that it may be more frequent than any other type of paraneoplastic encephalitis.12 The majority of patients with anti‐NMDAR encephalitis experience an antecedent infectious prodrome (eg, diarrheal illness or upper respiratory infection [URI]), followed 1020 days later by progressive neuropsychiatric and behavioral symptoms which include confusion, memory deficits, impaired responsiveness, seizures, central hypoventilation, and signs of autonomic instability (tachycardia, tachypnea, diaphoresis, cardiac dysrhythmia, blood pressure instability, and dysthermia). At this stage, patients may also manifest a unique constellation of choreoathetoid orofacial and limb movements such as lip licking, chewing, sustained jaw clenching, jaw opening dystonias, ocular deviation and disconjugation, grimacing, myoclonus, and bizarre arm movements. Due to cardiovascular complications and ventilator requirements, most patients require intensive care unit (ICU) level care. 8, 9, 11 As in our discussant's evaluation, other disorders to include in the differential diagnosis for this presentation includes paraneoplastic or autoimmune causes of limbic encephalitis, toxins, heavy metals, and viral causes of encephalitis; in particular, herpes simplex virus (HSV).7
The CNS imaging findings in this condition include brain MRI abnormalities in about 30%55% of patients, which can include increased signal on fluid‐attenuated inversion recovery (FLAIR) or T2 sequences of the cerebral cortex, overlying meninges, or basal ganglia. Abnormalities in the temporal lobes, corpus callosum, and brainstem have also been described. As in our patient, CSF lymphocytic pleocytosis has also been noted.6, 8, 9
Although many cases of limbic encephalitis portend a poor prognosis with permanent neuropsychiatric sequelae and death, anti‐NMDAR can be very responsive to treatment; particularly if diagnosed early. Successful treatment of anti‐NMDAR encephalitis involves immunotherapy and, preferably, early surgical resection of any tumor. 6, 8, 9 Non‐paraneoplastic cases appear to require more aggressive and prolonged immunotherapies to avoid relapse. In both groups, a trend towards improved outcome has been noted in patients treated early in disease course (<40 days from symptom onset).8 There are no established guidelines for the treatment of anti‐NMDAR encephalitis, and no randomized controlled trials have evaluated anti‐NMDAR encephalitis treatment. Observational studies of immune‐modulating therapies have shown efficacy with high‐dose steroids and the addition of plasma exchange and/or intravenous immune globulin. Rituximab and cyclophosphamide can be considered if patients fail to improve on other immunotherapies.9 Data from case series seem to suggest a lower risk of relapse in patients treated with immunotherapy.13
Exploration of this patient's persistent high fevers ultimately led to the serendipitous diagnosis of the increasingly recognized syndrome of anti‐NMDAR encephalitis, although in retrospect nearly all of the features of her presentation fit well with this condition. Thus, it was only by a chance finding on her abdominal CT scan that this patient was ultimately diagnosed with a treatable, noninfectious encephalitis associated with an ovarian teratoma. This case reinforces the importance of thorough patient evaluations and being prepared to draw meaningful conclusions from unexpected findings. Given how close this patient was to being discharged to a long‐term care facility, we found this case a fascinating yet sobering reminder to guard against prematurely concluding a syndrome to be untreatable.
KEY TEACHING POINTS
-
Anti‐NMDAR encephalitis is an increasingly recognized cause of autoimmune limbic encephalitis, and thus should be considered in patients with new‐onset psychiatric symptoms accompanied by seizures, autonomic instability, hypoventilation, or dyskinesias.
-
A thorough history, examination, and evaluation of data is critical to make an early diagnosis of anti‐NMDAR encephalitis, because, unlike other forms of limbic encephalitis, this condition may be very responsive to early initiation of treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A previously healthy 18‐year‐old woman living in the Pacific Northwest was brought in by her parents to a local hospital with a 4‐day history of acting crazy. Two weeks prior to presentation, she complained of a new‐onset severe headache, diaphoresis, and chills. Four days prior to presentation, she became progressively more impulsive, which ultimately included jumping out of a moving vehicle and running away from home. She experienced unexplained emotional outbursts and was unable to identify familiar relatives or common objects. Additionally, she began having hyperventilation spells and auditory hallucinations.
In an adolescent presenting with erratic behavior, one should consider the possibility of substance abuse or a psychiatric disease such as bipolar disorder with manic features, psychotic manifestations of severe depression, or early schizophrenia. However, it is important to first rule out non‐psychiatric disease, with a diagnostic approach dependent on her human immunodeficiency virus (HIV) status. The presence of headache, diaphoresis, and chills raises concern for an infectious or noninfectious inflammatory central nervous system process. In addition to the effects of illicit drugs such as cocaine or methamphetamine, this presentation may be consistent with a medication‐ or herbal‐induced anticholinergic syndrome, which may present with confusion, ataxia, coma, and cardiopulmonary failure. Since this case originates in the Northwest, one should be aware of the regional outbreak of Cryptococcus gattii in immunocompetent hosts, and that local hallucinogenic plants, such as jimson weed or mushrooms (Amanita muscaria) can cause anticholinergic syndromes. At this point, the differential diagnosis is broad, and evaluation should focus on potentially reversible life‐threatening conditions; in particular, herpes encephalitis. In addition to a detailed history, examination, and routine laboratory studies including HIV serology, I would obtain a drug screen, and order a computed tomography (CT) scan of the brain before performing a lumbar puncture. I would also order a magnetic resonance imaging (MRI) study to evaluate for meningeal or cerebral enhancement suggestive of encephalitis.
The patient had no past medical, psychiatric, or surgical history and took no medications. She lived with her parents who thought she neither used illicit drugs or alcohol, nor was sexually active. She had recently graduated high school and was planning to attend college. Her family history was notable for a mother with bipolar and seizure disorders, and 2 healthy younger siblings. Her family had a healthy cat and dog, and reported a large number of bats living nearby. She had never traveled outside the western United States. The patient presented in late spring, but there was no obvious history of mosquito bites. Her last menstrual period was 4 months prior to presentation. Full review of systems was otherwise negative.
The family history of mood disorder supports continued consideration of bipolar disorder with psychotic manifestations. However, infectious or inflammatory processes remain highest on the differential at this point. The duration of symptoms makes common bacterial meningitis etiologies (Streptococcus, Neisseria, Haemophilus, Listeria) less likely, but would be consistent with herpes simplex encephalitis or lupus cerebritis. Additional infectious considerations would include other viral (eg, varicella zoster virus, Epstein‐Barr virus, enteroviruses, and the arthropod‐borne encephalitides) or unusual bacterial encephalitic syndromes. Although the health status of pets is rarely helpful, dogs can carry ticks that harbor Borrelia burgdorferi (the agent of Lyme disease), which may present with central nervous system (CNS) manifestations. Other conditions associated with pets (such as leptospirosis or cat scratch disease) seem unlikely. The exposure to bats raises the possibility of rabies infection. If she is HIV‐positive, one would need to consider the possibility of opportunistic infections such as cytomegalovirus (CMV), Cryptococcus, cerebral toxoplasmosis, and progressive multifocal leukoencephalopathy (PML) caused by JC virus reaction. Finally, regardless of history, given the patient's amenorrhea, we must perform a pregnancy test.
The patient's temperature was 97.3F, heart rate 129 beats per minute, respiratory rate 19 breaths per minute, and her blood pressure 144/97 mmHg. She was an obese, well‐developed young woman, who was drowsy but arousable, with marked speech latency. Her cranium and oropharynx were normal, and her neck was supple. Aside from tachycardia, her cardiopulmonary, musculoskeletal, and skin exams were normal. Her abdomen was obese and soft, without masses or organomegaly. A pelvic examination was not performed. On neurologic exam, her strength was symmetrically diminished throughout (3+/5). Otherwise, she was oriented to person and general location, but not to day of week, month, or year. Her cranial nerves, sensation, deep tendon reflexes, and muscle tone were normal. A cerebellar examination, plantar response, and gait test were not performed. A brain MRI revealed only a small subarachnoid cyst and possible subtle enhancement of temporal lobes. Initial laboratory studies demonstrated: white blood cell count 14,000/mm3 (72% neutrophils, 17% lymphocytes, 9% monocytes, 2% eosinophils); hemoglobin 14.0 g/dL (mean corpuscular volume 87.4 fL); platelet count 417,000/mm3. Serum electrolytes, liver function tests, coagulation studies, thyroid stimulating hormone, serum ammonia, and urinalysis were normal. Her serum pregnancy test and urine toxicology screen were negative. A room air arterial blood gas revealed a pH of 7.49, PaCO2 32 mmHg, PaO2 89 mmHg; and a bicarbonate 24 mmol/L. Cerebrospinal fluid demonstrated: red cell count of 2/mm3; white cell count 17/mm3 (88% lymphocytes, 3% neutrophils, 9% monocytes); protein 19 mg/dL (normal 1555 mg/dL); and glucose of 79 mg/dL (normal 4080 mg/dL). Gram stain, fungal and bacterial cultures, and HIV serology were negative, and herpes simplex virus was not detected via polymerase chain reaction (PCR).
The tachycardia, respiratory alkalosis, and leukocytosis continue to suggest an infection or inflammatory state. Her neurological deterioration without focal findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis with normal glucose and protein, and temporal lobe enhancement on MRI strongly suggest a meningoencephalitis. This would be an unusual presentation for most bacterial pathogens, but Mycobacterium, Rickettsia, Listeria, Mycoplasma, and Bartonella may rarely mimic encephalitis. Autoimmune encephalitis secondary to lupus, vasculitis, or other autoimmune disorder remains possible, but at this point an infectious encephalitis, particularly herpes encephalitis, is my highest concern. West Nile virus must be considered, but usually produces a severe illness only in immunocompromised or elderly patients. Additionally, despite the rarity of rabies, the patient's exposure to bats and the rapid clinical deterioration, suggest this possibility. In addition to routine bacterial and viral analyses (eg, enteroviral panel), samples should be sent for rabies PCR and antibody testing, West Nile virus, Lyme disease, syphilis, and mycobacterial and fungal pathogens, such as the aforementioned Cryptococcus gattii. Finally, given her presenting syndrome and MRI, immediate treatment with acyclovir and antibiotics is indicated.
The patient was treated for presumed meningoencephalitis with acyclovir and ceftriaxone, but over the following several days became unresponsive to all stimuli and developed repetitive thrusting movements of her mouth, tongue, and jaw. On hospital day 10, with concern for seizures, pentobarbital coma was induced, and the patient was intubated and transferred to our facility. On arrival, her physical examination was essentially unchanged aside from being in a medical coma. Hematology, chemistries, and thyroid‐stimulating hormone (TSH) were again unremarkable with the exception of an elevated creatine kinase (414 U/L) and a new anemia (hemoglobin 8.9 g/dL; mean corpuscular volume 87.6 fL) without evidence of iron deficiency or hemolysis. Blood and urine cultures were negative. Repeat cerebrospinal fluid analysis was essentially unchanged, revealing a red cell count of 1/mm3; white cell count 20/mm3 (86% lymphocytes, 2% neutrophils, 12% monocytes); protein 14 mg/dL; glucose 63 mg/dL, and negative Gram stain. Continuous electroencephalography revealed diffuse generalized slowing, but no seizure activity. An extensive evaluation for viral, bacterial, autoimmune, and paraneoplastic disorders was negative, including tests for anti‐acetylcholine (ACh) receptor binding antibody, anti‐striated muscle antibody, anti‐N‐type calcium channel antibody, anti‐P/Q‐type calcium channel antibodies, anto‐cancer associated retinopathy (CAR) antibody (also known as anti‐recoverin antibody), and anti‐collapsin respons mediator protein (CRMP‐5). Without confirmatory results and continued deterioration, she was empirically treated with methylprednisolone for presumed autoimmune encephalitis from hospital days 16 to 21. The patient remained unresponsive and ventilator‐dependent, despite removal of all sedation. She experienced intermittent fevers as high as 40.5C, remained tachycardic, hypertensive, and exhibited orofacial dyskinesias and jaw clenching, ultimately requiring botulinum toxin injections to prevent tongue biting. Given the lack of improvement despite attempted therapies, a working diagnosis of viral encephalitis with lasting neuropsychiatric sequelae was made. A tracheostomy and percutaneous gastrostomy tube were placed, and a long‐term ventilator care facility was identified.
I continue to wonder if this may be an autoimmune encephalitis, and am concerned about her unexplained fevers. Neuroleptic malignant syndrome secondary to misuse of her parents' medications should be considered in light of the elevated creatine kinase, although the severity and duration of the syndrome seem more profound than I would anticipate. Tetanus could present with jaw dystonia, but the rest of the case does not seem to fit. At this point, considering the patient's young age and poor prognosis without identified etiology, prior to discharge I would argue for a brain biopsy looking for evidence of rabies, or other infectious or autoimmune etiologies of the patient's progressive neurologic deterioration.
On hospital day 25, due to the persistent fevers with concern for occult abscess, an abdominopelvic CT was obtained, which identified a complex 11.8 cm 9.0 cm adnexal mass consistent with a teratoma (Figure 1).

Given the size of the mass, it is surprising that the patient did not report abdominal symptoms and that the physicians were unable to palpate it on examination. The differential diagnosis of a complex adnexal mass in an adolescent should include an ectopic pregnancy, ovarian cysts, tubo‐ovarian abscess, rarely an ovarian carcinoma or leiomyosarcoma, and a teratoma or dermoid tumor. While I mentioned the possibility of a malignancy at the outset, I did not further consider it. Common neoplasms encountered in adolescent patients include lymphoma and leukemia, germ cell tumors (including teratomas), central nervous system tumors and sarcomas, many of which have been reported to cause paraneoplastic disorders. At this point, I now think her presumed teratoma is associated with a paraneoplastic syndrome resulting in her presentation of limbic encephalitis.
A literature search was performed by the managing clinicians who rapidly identified the association between teratoma and limbic encephalitis. The patient was initially treated with intravenous immune globulin (IVIG), with transient improvement in her mental status. Serology returned positive for the anti‐N‐methyl‐D‐aspartate receptor antibody, confirming the diagnosis of anti‐N‐methyl‐D‐aspartate receptor encephalitis. On hospital day 36, her mass was resected (Figure 2). Pathology was consistent with a mature teratoma. Postoperatively, the patient improved daily, and was discharged on hospital day 43 with a near complete neurologic recovery. Four months following discharge, the patient had enrolled full time in college.

COMMENTARY
The N‐methyl‐D‐aspartate receptor (NMDAR) is an important regulator of synaptic transmission and memory within the CNS. Our patient's case illustrates the increasingly recognized syndrome of anti‐NMDAR encephalitis. NMDAR hypofunction is hypothesized to result in the cognitive and behavioral abnormalities of schizophrenia, and direct antagonism of the NMDAR by drugs such as phencyclidine (PCP) and ketamine results in symptoms such as psychosis, hallucinations, delusions, agitation, and dissociative amnesia.14 This constellation of symptoms is very similar to some of the initial neuropsychiatric symptoms observed in patients with anti‐NMDAR encephalitis.
Anti‐NMDAR encephalitis was first described in 2005 as a paraneoplastic limbic encephalitis associated with ovarian teratoma.5, 6 Characterized by the subacute onset (days to weeks) of short‐term memory loss, psychiatric symptoms, and sleep disturbances, limbic encephalitis is an inflammatory process caused by autoantibodies against intracellular or extracellar antigens in the limbic system and other brain structures. Limbic encephalitides associated with antibodies to intracellular antigens (such as Hu, Ma2, CV2/CRMP5, and Amphiphysin) are more often associated with malignancies, have worse outcomes (permanent neuropsychiatric sequelae and death), and are less responsive to immune therapy. Conversely, it appears that both the paraneoplastic and non‐paraneoplastic variants of limbic encephalitis associated with antibodies against cell membrane antigens (such as NMDAR and Voltage Gated Potassium Channels) respond more favorably to therapy.7
As with limbic encephalitis in general, anti‐NMDAR encephalitis can be non‐paraneoplastic as well as paraneoplastic in etiology. In a recently published series of 44 consecutive patients with anti‐NMDAR encephalitis, tumors were present in only 9 cases (8 teratomas).8 When associated with a teratoma, it has been postulated that anti‐NMDAR antibodies develop and cross the bloodbrain barrier to target central nervous system NMDA receptors. This process results in down‐regulation of the neuronal surface NMDAR which then causes the psychiatric and behavioral changes described.6 The mechanism by which these antibodies traverse the bloodbrain barrier is not completely understood, but likely requires some disruption of the barrier in order to trigger anti‐NMDAR encephalitis.8, 9 Non‐paraneoplastic cases evidently involve other unknown stimuli for NMDAR antibody synthesisone report has suggested that subunits of the NMDAR are expressed by normal ovarian tissue, something which may explain the female predilection even in the cohort unaffected by teratomas.10
Most patients with anti‐NMDAR encephalitis are female and young (median age 23 years), although men and children are also affected.8, 9, 11 While the exact incidence of anti‐NMDAR encephalitis is still unknown, the increasing number of case reports suggests that it may be more frequent than any other type of paraneoplastic encephalitis.12 The majority of patients with anti‐NMDAR encephalitis experience an antecedent infectious prodrome (eg, diarrheal illness or upper respiratory infection [URI]), followed 1020 days later by progressive neuropsychiatric and behavioral symptoms which include confusion, memory deficits, impaired responsiveness, seizures, central hypoventilation, and signs of autonomic instability (tachycardia, tachypnea, diaphoresis, cardiac dysrhythmia, blood pressure instability, and dysthermia). At this stage, patients may also manifest a unique constellation of choreoathetoid orofacial and limb movements such as lip licking, chewing, sustained jaw clenching, jaw opening dystonias, ocular deviation and disconjugation, grimacing, myoclonus, and bizarre arm movements. Due to cardiovascular complications and ventilator requirements, most patients require intensive care unit (ICU) level care. 8, 9, 11 As in our discussant's evaluation, other disorders to include in the differential diagnosis for this presentation includes paraneoplastic or autoimmune causes of limbic encephalitis, toxins, heavy metals, and viral causes of encephalitis; in particular, herpes simplex virus (HSV).7
The CNS imaging findings in this condition include brain MRI abnormalities in about 30%55% of patients, which can include increased signal on fluid‐attenuated inversion recovery (FLAIR) or T2 sequences of the cerebral cortex, overlying meninges, or basal ganglia. Abnormalities in the temporal lobes, corpus callosum, and brainstem have also been described. As in our patient, CSF lymphocytic pleocytosis has also been noted.6, 8, 9
Although many cases of limbic encephalitis portend a poor prognosis with permanent neuropsychiatric sequelae and death, anti‐NMDAR can be very responsive to treatment; particularly if diagnosed early. Successful treatment of anti‐NMDAR encephalitis involves immunotherapy and, preferably, early surgical resection of any tumor. 6, 8, 9 Non‐paraneoplastic cases appear to require more aggressive and prolonged immunotherapies to avoid relapse. In both groups, a trend towards improved outcome has been noted in patients treated early in disease course (<40 days from symptom onset).8 There are no established guidelines for the treatment of anti‐NMDAR encephalitis, and no randomized controlled trials have evaluated anti‐NMDAR encephalitis treatment. Observational studies of immune‐modulating therapies have shown efficacy with high‐dose steroids and the addition of plasma exchange and/or intravenous immune globulin. Rituximab and cyclophosphamide can be considered if patients fail to improve on other immunotherapies.9 Data from case series seem to suggest a lower risk of relapse in patients treated with immunotherapy.13
Exploration of this patient's persistent high fevers ultimately led to the serendipitous diagnosis of the increasingly recognized syndrome of anti‐NMDAR encephalitis, although in retrospect nearly all of the features of her presentation fit well with this condition. Thus, it was only by a chance finding on her abdominal CT scan that this patient was ultimately diagnosed with a treatable, noninfectious encephalitis associated with an ovarian teratoma. This case reinforces the importance of thorough patient evaluations and being prepared to draw meaningful conclusions from unexpected findings. Given how close this patient was to being discharged to a long‐term care facility, we found this case a fascinating yet sobering reminder to guard against prematurely concluding a syndrome to be untreatable.
KEY TEACHING POINTS
-
Anti‐NMDAR encephalitis is an increasingly recognized cause of autoimmune limbic encephalitis, and thus should be considered in patients with new‐onset psychiatric symptoms accompanied by seizures, autonomic instability, hypoventilation, or dyskinesias.
-
A thorough history, examination, and evaluation of data is critical to make an early diagnosis of anti‐NMDAR encephalitis, because, unlike other forms of limbic encephalitis, this condition may be very responsive to early initiation of treatment.
- , . N‐methyl‐D‐aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49.
- , . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308.
- , , . NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
- , , , et al. Ketamine‐induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118.
- , , , , , . Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604.
- , , , et al. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
- , . Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271.
- , , , et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667.
- , . NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304.
- , , , et al. Expression of various glutamate receptors including N‐methyl‐D‐aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti‐NMDAR encephalitis. Intern Med. 2010;49:2167–2173.
- , , , et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1074–1075.
- , , , , . Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
- , , , et al. Analysis of relapses in anti‐NMDAR encephalitis. Neurology. 2011;77:996–999.
- , . N‐methyl‐D‐aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49.
- , . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308.
- , , . NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
- , , , et al. Ketamine‐induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118.
- , , , , , . Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604.
- , , , et al. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
- , . Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271.
- , , , et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667.
- , . NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304.
- , , , et al. Expression of various glutamate receptors including N‐methyl‐D‐aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti‐NMDAR encephalitis. Intern Med. 2010;49:2167–2173.
- , , , et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1074–1075.
- , , , , . Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
- , , , et al. Analysis of relapses in anti‐NMDAR encephalitis. Neurology. 2011;77:996–999.
In the Literature: January 2010
In This Edition
Literature at a Glance
A guide to this month’s studies
- Resident duty hours and ICU patient outcomes
- Effect of ER boarding on patient outcomes
- ED voicemail signout of patients
- Adequacy of patient signouts
- D-dimer use in patients with low suspicion of pulmonary embolism
- Patient involvement in medication reconciliation
- Effects of on-screen reminders on outcomes
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Resident duty hours and ICU patient outcomes
- Effect of ER boarding on patient outcomes
- ED voicemail signout of patients
- Adequacy of patient signouts
- D-dimer use in patients with low suspicion of pulmonary embolism
- Patient involvement in medication reconciliation
- Effects of on-screen reminders on outcomes
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH
In This Edition
Literature at a Glance
A guide to this month’s studies
- Resident duty hours and ICU patient outcomes
- Effect of ER boarding on patient outcomes
- ED voicemail signout of patients
- Adequacy of patient signouts
- D-dimer use in patients with low suspicion of pulmonary embolism
- Patient involvement in medication reconciliation
- Effects of on-screen reminders on outcomes
Decreased ICU Duty Hours Does Not Affect Patient Mortality
Clinical question: Does the reduction in work hours for residents affect mortality in medical and surgical ICUs?
Background: A reduction in work hours for residents was enforced in July 2003. Several prior studies using administrative or claims data did not show an association of the reduced work hours for residents with mortality in teaching hospitals when compared with nonteaching hospitals.
Study design: Observational retrospective registry cohort.
Setting: Twelve academic, 12 community, and 16 nonteaching hospitals in the U.S.
Synopsis: Data from 230,151 patients were extracted as post-hoc analysis from a voluntary clinical registry that uses a well-validated severity-of-illness scoring system. The exposure was defined as date of admission to ICU within two years before and after the reform. Hospitals were categorized as academic, community with residents, or nonteaching. Sophisticated statistical analyses were performed, including interaction terms for teaching status and time. To test the effect the reduced work hours had on mortality, the mortality trends of academic hospitals and community hospitals with residents were compared with the baseline trend of nonteaching hospitals. After risk adjustments, all hospitals had improved in-hospital and ICU mortality after the reform. None of the statistical improvements were significantly different.
Study limitations include the selection bias, as only highly motivated hospitals participating in the registry were included, and misclassification bias, as not all hospitals implemented the reform at the same time. Nevertheless, this study supports the consistent literature on the topic and adds a more robust assessment of severity of illness.
Bottom line: The restriction on resident duty hours does not appear to affect patient mortality.
Citation: Prasad M, Iwashyna TJ, Christie JD, et al. Effect of work-hours regulations on intensive care unit mortality in United States teaching hospitals. Crit Care Med. 2009;37(9):2564-2569.
Emergency Department “Boarding” Results in Undesirable Events
Clinical question: What is the frequency and nature of undesirable events experienced by patients who “board” in the ED?
Background: Hospital crowding results in patients spending extended amounts of time—also known as “boarding”—in the ED as they wait for an inpatient bed. Prior studies have shown that longer ED boarding times are associated with adverse outcomes. Few studies have examined the nature and frequency of undesirable events that patients experience while boarding.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: In this pilot study, authors reviewed the charts of patients who were treated in the ED and subsequently admitted to the hospital on three different days during the study period (n=151). More than a quarter (27.8%) of patients experienced an undesirable event, such as missing a scheduled medication, while they were boarding. Older patients, those with comorbid illnesses, and those who endured prolonged boarding times (greater than six hours) were more likely to experience an undesirable event. In addition, 3.3% of patients experienced such adverse events as suboptimal blood pressure control, hypotension, hypoxia, or arrhythmia.
This study was performed at a single center and lacks a comparison group (i.e., nonboarded patients). It is intended to serve as an exploratory study for future analysis of adverse events in boarded patients.
Bottom line: Undesirable events are common among boarded patients, although it is unknown whether they are more common than in nonboarded patients.
Citation: Liu SW, Thomas SH, Gordon JA, Hamedani AG, Weissman JS. A pilot study examining undesirable events among emergency-department boarded patients awaiting inpatient beds. Ann Emerg Med. 2009;54(3):381-385.
Emergency Department Signout via Voicemail Yields Mixed Reviews
Clinical question: How does traditional, oral signout from emergency providers to inpatient medicine physicians compare to dictated, voicemail signout?
Background: Communication failures contribute to errors in care transition from ED to inpatient medicine units. Signout between ED providers and internal medicine (IM) physicians is typically oral (“synchronous communication”). It is not known how dictated signout to a voicemail system (“asynchronous communication”) affects the quality and safety of handoff communications.
Study design: Prospective, pre-post analysis.
Setting: A 944-bed urban academic medical center in Connecticut.
Synopsis: Surveys were administered to all IM and ED providers before and after the implementation of a voicemail signout system. In the new system, ED providers dictated signout for stable patients, rather than giving traditional synchronous telephone signout. It was the responsibility of the admitting IM physician to listen to the voicemail after receiving a text notification that a patient was being admitted.
ED providers recorded signouts in 89.5% of medicine admissions. However, voicemails were accessed only 58.5% of the time by receiving physicians. All ED providers and 56% of IM physicians believed signout was easier following the voicemail intervention. Overall, ED providers gave the quality, content, and accuracy of their signout communication higher ratings than IM physicians did; 69% of all providers felt the interaction among participants was worse following the intervention. There was no change in the rate of perceived adverse events or ICU transfers within 24 hours after admission.
This intervention was a QI initiative at a single center. Mixed results and small sample size limit generalizability of the study.
Bottom line: Asynchronous signout by voicemail increased efficiency, particularly among ED providers but decreased perceived quality of interaction between medical providers without obviously affecting patient safety.
Citation: Horwitz LI, Parwani V, Shah NR, et al. Evaluation of an asynchronous physician voicemail sign-out for emergency department admissions. Ann Emerg Med. 2009;54:368-378.
Patient Signout Is Not Uniformly Comprehensive and Often Lacks Critical Information
Clinical question: Do signouts vary in the quality and quantity of information, and what are the various factors affecting signout quality?
Background: Miscommunication during transfers of responsibility for hospitalized patients is common and can result in harm. Recommendations for safe and effective handoffs emphasize key content, clear communication, senior staff supervision, and adequate time for questions. Still, little is known about adherence to these recommendations in clinical practice.
Study design: Prospective, observational cohort.
Setting: Medical unit of an acute-care teaching hospital.
Synopsis: Oral signouts were audiotaped among IM house staff teams and the accompanying written signouts were collected for review of content. Signout sessions (n=88) included eight IM teams at one hospital and contained 503 patient signouts.
The median signout duration was 35 seconds (IQR 19-62) per patient. Key clinical information was present in just 62% of combined written or oral signouts. Most signouts included no questions from the recipient. Factors associated with higher rate of content inclusion included: familiarity with the patient, sense of responsibility (primary team vs. covering team), only one signout per day (as compared to sequential signout), presence of a senior resident, and comprehensive, written signouts.
Study limitations include the Hawthorne effect, as several participants mentioned that the presence of audiotape led to more comprehensive signouts than are typical. Also, the signout quality assessment in this study has not been validated with patient-safety outcomes.
Bottom line: Signouts among internal-medicine residents at this one hospital showed variability in terms of quantitative and qualitative information and often missed crucial information about patient care.
Citation: Horwitz LI, Moin T, Krumholz HM, Wang L, Bradley EH. What are covering doctors told about their patients? Analysis of sign-out among internal medicine house staff. Qual Saf Health Care. 2009;18(4):248-255.
Negative D-Dimer Test Can Safely Exclude Pulmonary Embolism in Patients at Low To Intermediate Clinical Risk
Clinical question: In patients with symptoms consistent with pulmonary embolism (PE), can evaluation with a clinical risk assessment tool and D-dimer assay identify patients who do not require CT angiography to exclude PE?
Background: D-dimer is a highly sensitive but nonspecific marker of VTE, and studies suggest that VTE can be ruled out without further imaging in patients with low clinical probability of disease and a negative D-dimer test. Nevertheless, this practice has not been adopted uniformly, and CT angiography (CTA) overuse continues.
Study design: Prospective registry cohort.
Setting: A 550-bed community teaching hospital in Chicago.
Synopsis: Consecutive patients presenting to the ED with symptoms suggestive of PE were evaluated with 1) revised Geneva score; 2) D-dimer assay; and 3) CTA. Among the 627 patients who underwent all three components of the evaluation, 44.8% were identified as low probability for PE by revised Geneva score, 52.6% as intermediate probability, and 2.6% as high probability. The overall prevalence of PE (using CTA as the gold standard) was very low (4.5%); just 2.1% of low-risk, 5.2% of intermediate-risk, and 31.2% of high-risk patients were ultimately found to have PE on CTA.
Using a cutoff of 1.2 mg/L, the D-dimer assay accurately detected all low- to intermediate-probability patients with PE (sensitivity and negative predictive value of 100%). One patient in the high probability group did have a PE, even though the patient had a D-dimer value <1.2 mg/L (sensitivity and NPV both 80%). Had diagnostic testing stopped after a negative D-dimer result in the low- to intermediate-probability patients, 172 CTAs (27%) would have been avoided.
Bottom line: In a low-prevalence cohort, no pulmonary emboli were identified by CTA in any patient with a low to intermediate clinical risk assessment and a negative quantitative D-dimer assay result.
Citation: Gupta RT, Kakarla RK, Kirshenbaum KJ, Tapson VF. D-dimers and efficacy of clinical risk estimation algorithms: sensitivity in evaluation of acute pulmonary embolism. AJR Am J Roentgenol. 2009;193(2):425-430.
Patient Participation in Medication Reconciliation at Discharge Helps Detect Prescribing Discrepancies
Clinical question: Does the inclusion of a medication adherence counseling session during a hospital discharge reconciliation process reduce discrepancies in the final medication regimen?
Background: Inadvertent medication prescribing errors are an important cause of preventable adverse drug events and commonly occur at transitions of care. Although medication reconciliation processes can identify errors, the best strategies for implementation remain unclear.
Study design: Prospective, observational cohort.
Setting: A 550-bed teaching hospital in the Netherlands.
Synopsis: Of 437 patients admitted to a pulmonary ward and screened for eligibility, 267 were included in the analysis. A pharmacy specialist reviewed all available community prescription records, inpatient documentation, and discharge medication lists in an effort to identify discrepancies. Potential errors were discussed with the prescriber. Then, the pharmacy specialist interviewed the patient and provided additional counseling. Any new discrepancies were discussed with the prescriber. All questions raised by the pharmacist were recorded, as were all subsequent prescriber interventions.
The primary outcome measure was the number of interventions made as a result of pharmacy review. A total of 940 questions were asked. At least one intervention was recorded for 87% of patients before counseling (mean 2.7 interventions/patient) and for 97% of patients after (mean 5.3 interventions/patient). Discrepancies were addressed for 63.7% of patients before counseling and 72.5% after. Pharmacotherapy was optimized for 67.2% of patients before counseling and 76.3% after.
Bottom line: Patient engagement in the medication reconciliation process incrementally improves the quality of the history and helps identify clinically meaningful discrepancies at the time of hospital discharge.
Citation: Karapinar-Carkit F, Borgsteede S, Zoer J, Smit HJ, Egberts AC, van den Bemt P. Effect of medication reconciliation with and without patient counseling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43(6):1001-1010.
Computer-Based Reminders Have Small to Modest Effect on Care Processes
Clinical question: Do on-screen, computer-based clinical reminders improve adherence to target processes of care or clinical outcomes?
Background: Gaps between practice guidelines and routine care are caused, in part, by the inability of clinicians to access or recall information at the point of care. Although automated reminder systems offer the promise of “just in time” recommendations, studies of electronic reminders have demonstrated mixed results.
Study design: Literature review and meta-analysis.
Setting: Multiple databases and information repositories, including MEDLINE, EMBASE, and CINAHL.
Synopsis: The authors conducted a literature search to identify randomized and quasi-randomized controlled trials measuring the effect of computer-based reminders on process measures or clinical outcomes. To avoid statistical challenges inherent in unit-of-analysis errors, the authors reported median improvement in process adherence or median change in clinical endpoints.
Out of a pool of 2,036 citations, 28 studies detailing 32 comparative analyses were included. Across the 28 studies, reminders resulted in a median improvement in target process adherence of 4.2% (3.3% for prescribing behavior, 2.8% for test ordering). Eight comparisons reported dichotomous clinical endpoints and collectively showed a median absolute improvement of 2.5%.
The greatest contribution to measured treatment effects came from large academic centers with well-established electronic health records and robust informatics departments. No characteristics of the reminder system or the clinical context were associated with the magnitude of impact. A potential limitation in reporting median effects across studies is that all studies were given equal weight.
Bottom line: Electronic reminders appear to have a small, positive effect on clinician adherence to recommended processes, although it is uncertain what contextual or design features are responsible for the greatest treatment effect.
Citation: Shojania K, Jennings A, Mayhew A, Ramsay CR, Eccles MP, Grimshaw J. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009(3):CD001096. TH