User login
Physician convicted in buprenorphine scheme faces up to 20 years in prison
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
A West Virginia physician faces up to 20 years in prison in the wake of his conviction by a federal jury for illegally distributing buprenorphine.
The jury convicted Sriramloo Kesari, MD, 78, of Charleston, for distributing buprenorphine outside the scope of medical practice, according to a U.S. Department of Justice statement.
Investigators from the Drug Enforcement Administration presented evidence at the trial that Dr. Kesari, a general practitioner, operated a cash-only business selling buprenorphine prescriptions.
Federal prosecutors said that the physician signed prescriptions, which were then distributed by an employee in exchange for cash. Dr. Kesari was often absent, at times physically located in California, according to the federal government.
Prosecutors indicted the West Virginia physician in September 2019 as part of an “opioid strikeforce takedown” in Ohio, Virginia, and West Virginia that resulted in charges against 13 individuals, including 11 physicians.
Dr. Kesari’s attorneys filed motions during the course of the lengthy case showing that psychiatric and neurological exams indicated that the physician was cognitively impaired.
Based on that evidence and the federal indictment, the West Virginia Board of Medicine suspended Dr. Kesari’s license in February 2020, stating that he is not “mentally and/or physically fit to practice medicine and surgery with reasonable skill and safety.”
Dr. Kesari was first licensed in West Virginia in 1979. In 1987, the Board of Medicine placed Dr. Kesari on a 3-year probation because of his failure to keep records for patients for whom he was prescribing controlled substances.
However, within a few months, the Board changed the probation order to allow Dr. Kesari to write prescriptions for schedule II and III substances in the Boone Hospital emergency room where he continued to work.
The physician had no other disciplinary actions until his license suspension, but the Board lists settlement of four malpractice cases and the dismissal of a fifth between 1986 and 2001.
A version of this article first appeared on Medscape.com.
Study finds little impact of private equity on dermatology practices
A new
The authors reported that – with an average of five quarters postacquisition – there was no statistically significant differential between investor-owned and non–investor-owned practices “in total spending, overall use of dermatology procedures per patient, or specific high-volume and profitable procedures.”
Essentially, the study findings were equivocal, reported Robert Tyler Braun, PhD and his colleagues at Weill Cornell Medicine, New York. “The results provide mixed support for both proponents and opponents of private equity acquisitions,” they wrote in the study, which was published in Health Affairs.
But two dermatologists not involved with the study said the analysis has significant limitations, including a lack of pathology data, a lack of Medicare data, and a lack of insight into how advanced practice clinicians, such as nurse practitioners and physician assistants, were used by the private equity (PE)–owned practices. The study was not able to track “incident to billing.”
Leaving out Medicare data is a “huge oversight,” Joseph K. Francis, MD, a Mohs surgeon at the University of Florida, Gainesville, said in an interview. “The study is fundamentally flawed.”
“With all of these limitations, it’s difficult to draw meaningful conclusions,” agreed Clifford Perlis, MD, Mbe, of Keystone Dermatology in King of Prussia, Pa.
Both Dr. Francis and Dr. Perlis also questioned the influence of one of the study’s primary sponsors, the Physicians Foundation, formed out of the settlement of a class action lawsuit against third-party payers.
In addition, Dr. Francis and Dr. Perlis said they thought the study did not follow the PE-owned practices for a long enough period of time after acquisition to detect any differences, and that the dataset – looking at practice acquisitions from 2012 to 2017 – was too old to paint a reliable picture of the current state of PE-owned practices. Acquisitions have accelerated since 2017.
In March 2021, Harvard researchers reported in JAMA Health Forum that PE purchases in health care peaked in the first quarter of 2018 and surged to almost as high a level in the fourth quarter of 2020, with 153 deals announced in the second half of the year. Of the 153 acquisitions, 98, or 64%, were for physician practices or other health care services.
Dr. Braun said his study focused on 2012-2017 because it was an available data set. And, he defended the snapshot, saying that he and his colleagues had as much as 4 years of postacquisition data for the practices that were purchased in 2013. He acknowledged there were less data on practices purchased from 2014 to 2016.
“It is possible that our results would change with a longer postacquisition period,” Dr. Braun said in an interview. But, he said there was no way to predict whether that change “would look better or worse for private equity.”
Modest price increases
The authors analyzed data from the Health Care Cost Institute, which aggregates claims for some 50 million individuals insured by Aetna, Humana, and United Healthcare. The data do not include Medicare claims.
They examined dermatologists in practices bought between 2013 and 2016 and compared them to dermatologists who were in practices not owned by private equity. Each dermatologist had to have at least 2 years of data, and the authors compared preacquisition with postacquisition data for those in PE-owned practices.
They identified 64 practices – with 246 dermatologists – bought by private investors. Preacquisition, PE practices were larger than non-PE practices, with 4.2 clinicians (including advanced practitioners) per practice, compared with 1.7 in non–investor-owned practices.
The authors looked at volume and prices for routine office visits (CPT code 99213), biopsies (11101), excisions (11602), destruction of first lesion (cryotherapy; 17000), and Mohs micrographic surgery (17311).
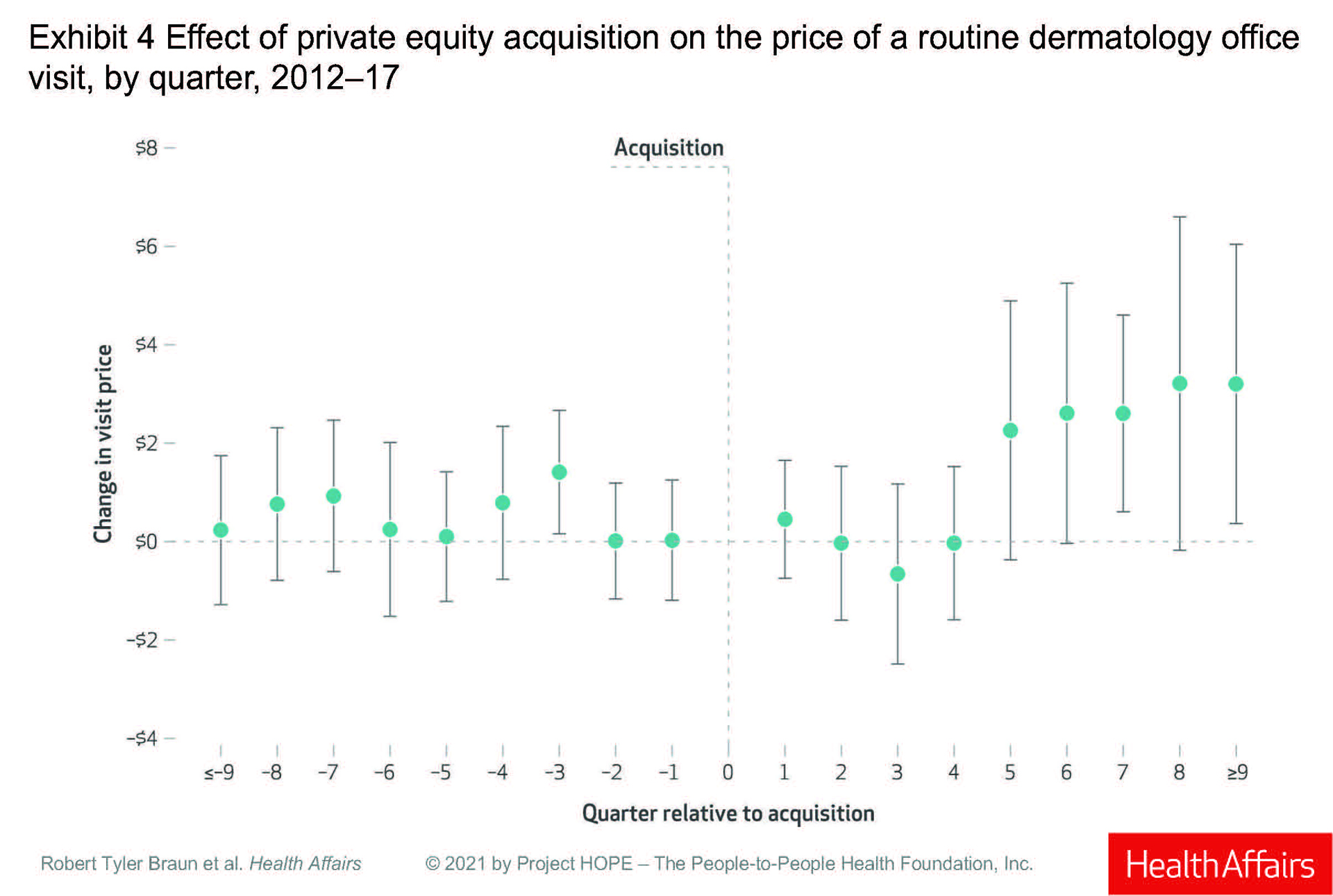
Prices for a routine office visit rose nominally in the first quarter after acquisition (under $1), stayed at 0 in the second quarter, decreased in the third quarter, and was 0 again in the fourth quarter. It was not until the fifth quarter post acquisition that prices rose, increasing by 3% ($2.26), and then rising 5% ($3.20) in the ninth quarter.
Dr. Braun said the price increases make sense because practices get “rolled up” into larger platforms, theoretically giving them more negotiating leverage with insurers. And he said the paper’s results “are consistent with physician practice consolidation research – mainly hospitals acquiring practices – that prices increase after acquisition.” He acknowledged that the dermatology paper found “more modest effects,” than other studies.
Dr. Francis, however, said the increases are basically “pocket change,” and that they reflect a failed promise from private investors that clinicians in PE-owned practices will be paid more. The small differences in pay may also mean that insurers are likely not acquiescing to the theoretical leverage of larger dermatology entities.
PE-owned dermatologists saw about 5% more patients per quarter initially, rising to 17% more per quarter by eight quarters after purchase, according to the study.
The study reported a significant increase in Mohs surgery and cryotherapy in the first quarter post purchase, and a significant increase in biopsies after eight quarters. But Dr. Braun and colleagues concluded that total spending per patient did not change significantly after acquisition. “That says that maybe physicians aren’t changing their behaviors that much,” Dr. Braun said in an interview.
Dr. Perlis disagreed, noting that practices rarely change quickly. “Anecdotally, most groups that are taken over, nothing changes initially,” while the new owners are getting a feel for the practice.
He also said the paper erred in not addressing quality of and access to care. “Quality and patient satisfaction and access are also other important factors that need to be examined.”
Not benign players
Both Dr. Perlis and Dr. Francis said the study may have been improved by having a dermatologist as a coauthor. Dr. Braun countered that he and his colleagues consulted several dermatologists during the course of the study, and that they also conducted 30 interviews with proponents and opponents of PE ownership.
The authors warned of what they viewed as some disturbing trends in PE-owned practices, including what Dr. Braun called “stealth” consolidation – investors making small purchases that fall outside of federal regulation, and then amassing them into large entities.
He also commented that it was “alarming” that PE-owned practices were hiring a larger number of advanced practitioners. The authors also expressed concern about leveraged buyouts, in which investors require a practice to carry high debt loads that can eventually drive it into bankruptcy.
“These are not benign players,” said Dr. Francis. He noted that it took “an act of Congress to stop surprise billing,” a tactic employed by PE-owned health care entities. “Policy makers should be looking at what’s best for patients, especially Medicare and vulnerable patients.”
Dr. Perlis also has qualms about PE-owned practices. “The money to support returns to investors has to come from somewhere and that creates an inherent tension between patient care and optimizing revenue for investors,” he said. “It’s a pretty head-on conflict.”
A new
The authors reported that – with an average of five quarters postacquisition – there was no statistically significant differential between investor-owned and non–investor-owned practices “in total spending, overall use of dermatology procedures per patient, or specific high-volume and profitable procedures.”
Essentially, the study findings were equivocal, reported Robert Tyler Braun, PhD and his colleagues at Weill Cornell Medicine, New York. “The results provide mixed support for both proponents and opponents of private equity acquisitions,” they wrote in the study, which was published in Health Affairs.
But two dermatologists not involved with the study said the analysis has significant limitations, including a lack of pathology data, a lack of Medicare data, and a lack of insight into how advanced practice clinicians, such as nurse practitioners and physician assistants, were used by the private equity (PE)–owned practices. The study was not able to track “incident to billing.”
Leaving out Medicare data is a “huge oversight,” Joseph K. Francis, MD, a Mohs surgeon at the University of Florida, Gainesville, said in an interview. “The study is fundamentally flawed.”
“With all of these limitations, it’s difficult to draw meaningful conclusions,” agreed Clifford Perlis, MD, Mbe, of Keystone Dermatology in King of Prussia, Pa.
Both Dr. Francis and Dr. Perlis also questioned the influence of one of the study’s primary sponsors, the Physicians Foundation, formed out of the settlement of a class action lawsuit against third-party payers.
In addition, Dr. Francis and Dr. Perlis said they thought the study did not follow the PE-owned practices for a long enough period of time after acquisition to detect any differences, and that the dataset – looking at practice acquisitions from 2012 to 2017 – was too old to paint a reliable picture of the current state of PE-owned practices. Acquisitions have accelerated since 2017.
In March 2021, Harvard researchers reported in JAMA Health Forum that PE purchases in health care peaked in the first quarter of 2018 and surged to almost as high a level in the fourth quarter of 2020, with 153 deals announced in the second half of the year. Of the 153 acquisitions, 98, or 64%, were for physician practices or other health care services.
Dr. Braun said his study focused on 2012-2017 because it was an available data set. And, he defended the snapshot, saying that he and his colleagues had as much as 4 years of postacquisition data for the practices that were purchased in 2013. He acknowledged there were less data on practices purchased from 2014 to 2016.
“It is possible that our results would change with a longer postacquisition period,” Dr. Braun said in an interview. But, he said there was no way to predict whether that change “would look better or worse for private equity.”
Modest price increases
The authors analyzed data from the Health Care Cost Institute, which aggregates claims for some 50 million individuals insured by Aetna, Humana, and United Healthcare. The data do not include Medicare claims.

They examined dermatologists in practices bought between 2013 and 2016 and compared them to dermatologists who were in practices not owned by private equity. Each dermatologist had to have at least 2 years of data, and the authors compared preacquisition with postacquisition data for those in PE-owned practices.
They identified 64 practices – with 246 dermatologists – bought by private investors. Preacquisition, PE practices were larger than non-PE practices, with 4.2 clinicians (including advanced practitioners) per practice, compared with 1.7 in non–investor-owned practices.
The authors looked at volume and prices for routine office visits (CPT code 99213), biopsies (11101), excisions (11602), destruction of first lesion (cryotherapy; 17000), and Mohs micrographic surgery (17311).
Prices for a routine office visit rose nominally in the first quarter after acquisition (under $1), stayed at 0 in the second quarter, decreased in the third quarter, and was 0 again in the fourth quarter. It was not until the fifth quarter post acquisition that prices rose, increasing by 3% ($2.26), and then rising 5% ($3.20) in the ninth quarter.
Dr. Braun said the price increases make sense because practices get “rolled up” into larger platforms, theoretically giving them more negotiating leverage with insurers. And he said the paper’s results “are consistent with physician practice consolidation research – mainly hospitals acquiring practices – that prices increase after acquisition.” He acknowledged that the dermatology paper found “more modest effects,” than other studies.
Dr. Francis, however, said the increases are basically “pocket change,” and that they reflect a failed promise from private investors that clinicians in PE-owned practices will be paid more. The small differences in pay may also mean that insurers are likely not acquiescing to the theoretical leverage of larger dermatology entities.
PE-owned dermatologists saw about 5% more patients per quarter initially, rising to 17% more per quarter by eight quarters after purchase, according to the study.
The study reported a significant increase in Mohs surgery and cryotherapy in the first quarter post purchase, and a significant increase in biopsies after eight quarters. But Dr. Braun and colleagues concluded that total spending per patient did not change significantly after acquisition. “That says that maybe physicians aren’t changing their behaviors that much,” Dr. Braun said in an interview.
Dr. Perlis disagreed, noting that practices rarely change quickly. “Anecdotally, most groups that are taken over, nothing changes initially,” while the new owners are getting a feel for the practice.
He also said the paper erred in not addressing quality of and access to care. “Quality and patient satisfaction and access are also other important factors that need to be examined.”
Not benign players
Both Dr. Perlis and Dr. Francis said the study may have been improved by having a dermatologist as a coauthor. Dr. Braun countered that he and his colleagues consulted several dermatologists during the course of the study, and that they also conducted 30 interviews with proponents and opponents of PE ownership.
The authors warned of what they viewed as some disturbing trends in PE-owned practices, including what Dr. Braun called “stealth” consolidation – investors making small purchases that fall outside of federal regulation, and then amassing them into large entities.
He also commented that it was “alarming” that PE-owned practices were hiring a larger number of advanced practitioners. The authors also expressed concern about leveraged buyouts, in which investors require a practice to carry high debt loads that can eventually drive it into bankruptcy.
“These are not benign players,” said Dr. Francis. He noted that it took “an act of Congress to stop surprise billing,” a tactic employed by PE-owned health care entities. “Policy makers should be looking at what’s best for patients, especially Medicare and vulnerable patients.”
Dr. Perlis also has qualms about PE-owned practices. “The money to support returns to investors has to come from somewhere and that creates an inherent tension between patient care and optimizing revenue for investors,” he said. “It’s a pretty head-on conflict.”
A new
The authors reported that – with an average of five quarters postacquisition – there was no statistically significant differential between investor-owned and non–investor-owned practices “in total spending, overall use of dermatology procedures per patient, or specific high-volume and profitable procedures.”
Essentially, the study findings were equivocal, reported Robert Tyler Braun, PhD and his colleagues at Weill Cornell Medicine, New York. “The results provide mixed support for both proponents and opponents of private equity acquisitions,” they wrote in the study, which was published in Health Affairs.
But two dermatologists not involved with the study said the analysis has significant limitations, including a lack of pathology data, a lack of Medicare data, and a lack of insight into how advanced practice clinicians, such as nurse practitioners and physician assistants, were used by the private equity (PE)–owned practices. The study was not able to track “incident to billing.”
Leaving out Medicare data is a “huge oversight,” Joseph K. Francis, MD, a Mohs surgeon at the University of Florida, Gainesville, said in an interview. “The study is fundamentally flawed.”
“With all of these limitations, it’s difficult to draw meaningful conclusions,” agreed Clifford Perlis, MD, Mbe, of Keystone Dermatology in King of Prussia, Pa.
Both Dr. Francis and Dr. Perlis also questioned the influence of one of the study’s primary sponsors, the Physicians Foundation, formed out of the settlement of a class action lawsuit against third-party payers.
In addition, Dr. Francis and Dr. Perlis said they thought the study did not follow the PE-owned practices for a long enough period of time after acquisition to detect any differences, and that the dataset – looking at practice acquisitions from 2012 to 2017 – was too old to paint a reliable picture of the current state of PE-owned practices. Acquisitions have accelerated since 2017.
In March 2021, Harvard researchers reported in JAMA Health Forum that PE purchases in health care peaked in the first quarter of 2018 and surged to almost as high a level in the fourth quarter of 2020, with 153 deals announced in the second half of the year. Of the 153 acquisitions, 98, or 64%, were for physician practices or other health care services.
Dr. Braun said his study focused on 2012-2017 because it was an available data set. And, he defended the snapshot, saying that he and his colleagues had as much as 4 years of postacquisition data for the practices that were purchased in 2013. He acknowledged there were less data on practices purchased from 2014 to 2016.
“It is possible that our results would change with a longer postacquisition period,” Dr. Braun said in an interview. But, he said there was no way to predict whether that change “would look better or worse for private equity.”
Modest price increases
The authors analyzed data from the Health Care Cost Institute, which aggregates claims for some 50 million individuals insured by Aetna, Humana, and United Healthcare. The data do not include Medicare claims.

They examined dermatologists in practices bought between 2013 and 2016 and compared them to dermatologists who were in practices not owned by private equity. Each dermatologist had to have at least 2 years of data, and the authors compared preacquisition with postacquisition data for those in PE-owned practices.
They identified 64 practices – with 246 dermatologists – bought by private investors. Preacquisition, PE practices were larger than non-PE practices, with 4.2 clinicians (including advanced practitioners) per practice, compared with 1.7 in non–investor-owned practices.
The authors looked at volume and prices for routine office visits (CPT code 99213), biopsies (11101), excisions (11602), destruction of first lesion (cryotherapy; 17000), and Mohs micrographic surgery (17311).
Prices for a routine office visit rose nominally in the first quarter after acquisition (under $1), stayed at 0 in the second quarter, decreased in the third quarter, and was 0 again in the fourth quarter. It was not until the fifth quarter post acquisition that prices rose, increasing by 3% ($2.26), and then rising 5% ($3.20) in the ninth quarter.
Dr. Braun said the price increases make sense because practices get “rolled up” into larger platforms, theoretically giving them more negotiating leverage with insurers. And he said the paper’s results “are consistent with physician practice consolidation research – mainly hospitals acquiring practices – that prices increase after acquisition.” He acknowledged that the dermatology paper found “more modest effects,” than other studies.
Dr. Francis, however, said the increases are basically “pocket change,” and that they reflect a failed promise from private investors that clinicians in PE-owned practices will be paid more. The small differences in pay may also mean that insurers are likely not acquiescing to the theoretical leverage of larger dermatology entities.
PE-owned dermatologists saw about 5% more patients per quarter initially, rising to 17% more per quarter by eight quarters after purchase, according to the study.
The study reported a significant increase in Mohs surgery and cryotherapy in the first quarter post purchase, and a significant increase in biopsies after eight quarters. But Dr. Braun and colleagues concluded that total spending per patient did not change significantly after acquisition. “That says that maybe physicians aren’t changing their behaviors that much,” Dr. Braun said in an interview.
Dr. Perlis disagreed, noting that practices rarely change quickly. “Anecdotally, most groups that are taken over, nothing changes initially,” while the new owners are getting a feel for the practice.
He also said the paper erred in not addressing quality of and access to care. “Quality and patient satisfaction and access are also other important factors that need to be examined.”
Not benign players
Both Dr. Perlis and Dr. Francis said the study may have been improved by having a dermatologist as a coauthor. Dr. Braun countered that he and his colleagues consulted several dermatologists during the course of the study, and that they also conducted 30 interviews with proponents and opponents of PE ownership.
The authors warned of what they viewed as some disturbing trends in PE-owned practices, including what Dr. Braun called “stealth” consolidation – investors making small purchases that fall outside of federal regulation, and then amassing them into large entities.
He also commented that it was “alarming” that PE-owned practices were hiring a larger number of advanced practitioners. The authors also expressed concern about leveraged buyouts, in which investors require a practice to carry high debt loads that can eventually drive it into bankruptcy.
“These are not benign players,” said Dr. Francis. He noted that it took “an act of Congress to stop surprise billing,” a tactic employed by PE-owned health care entities. “Policy makers should be looking at what’s best for patients, especially Medicare and vulnerable patients.”
Dr. Perlis also has qualms about PE-owned practices. “The money to support returns to investors has to come from somewhere and that creates an inherent tension between patient care and optimizing revenue for investors,” he said. “It’s a pretty head-on conflict.”
FROM HEALTH AFFAIRS
APA, AMA, others move to stop insurer from overturning mental health claims ruling
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
The American Psychiatric Association has joined with the American Medical Association and other medical societies to oppose United Behavioral Health’s (UBH) request that a court throw out a ruling that found the insurer unfairly denied tens of thousands of claims for mental health and substance use disorder services.
Wit v. United Behavioral Health, in litigation since 2014, is being closely watched by clinicians, patients, providers, and attorneys.
Reena Kapoor, MD, chair of the APA’s Committee on Judicial Action, said in an interview that the APA is hopeful that “whatever the court says about UBH should be applicable to all insurance companies that are providing employer-sponsored health benefits.”
In a friend of the court (amicus curiae) brief, the APA, AMA, the California Medical Association, Southern California Psychiatric Society, Northern California Psychiatric Society, Orange County Psychiatric Society, Central California Psychiatric Society, and San Diego Psychiatric Society argue that “despite the availability of professionally developed, evidence-based guidelines embodying generally accepted standards of care for mental health and substance use disorders, managed care organizations commonly base coverage decisions on internally developed ‘level of care guidelines’ that are inappropriately restrictive.”
The guidelines “may lead to denial of coverage for treatment that is recommended by a patient’s physician and even cut off coverage when treatment is already being delivered,” said the groups.
The U.S. Department of Labor also filed a brief in support of the plaintiffs who are suing UBH. Those individuals suffered injury when they were denied coverage, said the federal agency, which regulates employer-sponsored insurance plans.
California Attorney General Rob Bonta also made an amicus filing supporting the plaintiffs.
“When insurers limit access to this critical care, they leave Californians who need it feeling as if they have no other option than to try to cope alone,” said Mr. Bonta in a statement.
‘Discrimination must end’
Mr. Bonta said he agreed with a 2019 ruling by the U.S. District Court for the Northern District of California that UBH had violated its fiduciary duties by wrongfully using its internally developed coverage determination guidelines and level of care guidelines to deny care.
The court also found that UBH’s medically necessary criteria meant that only “acute” episodes would be covered. Instead, said the court last November, chronic and comorbid conditions should always be treated, according to Maureen Gammon and Kathleen Rosenow of Willis Towers Watson, a risk advisor.
In November, the same Northern California District Court ruled on the remedies it would require of United, including that the insurer reprocess more than 67,000 claims. UBH was also barred indefinitely from using any of its guidelines to make coverage determinations. Instead, it was ordered to make determinations “consistent with generally accepted standards of care,” and consistent with state laws.
The District Court denied a request by UBH to put a hold on the claims reprocessing until it appealed the overall case. But the Ninth Circuit Court of Appeals in February granted that request.
Then, in March, United appealed the District Court’s overall ruling, claiming that the plaintiffs had not proven harm.
The U.S. Chamber of Commerce has filed a brief in support of United, agreeing with its arguments.
However, the APA and other clinician groups said there is no question of harm.
“Failure to provide appropriate levels of care for treatment of mental illness and substance use disorders leads to relapse, overdose, transmission of infectious diseases, and death,” said APA CEO and Medical Director Saul Levin, MD, MPA, in a statement.
APA President Vivian Pender, MD, said guidelines that “are overly focused on stabilizing acute symptoms of mental health and substance use disorders” are not treating the underlying disease. “When the injury is physical, insurers treat the underlying disease and not just the symptoms. Discrimination against patients with mental illness must end,” she said.
No court has ever recognized the type of claims reprocessing ordered by the District Court judge, said attorneys Nathaniel Cohen and Joseph Laska of Manatt, Phelps & Phillips, in an analysis of the case.
Mr. Cohen and Mr. Laska write. “Practitioners, health plans, and health insurers would be wise to track UBH’s long-awaited appeal to the Ninth Circuit.”
This article first appeared on Medscape.com.
HHS to inject billions into mental health, substance use disorders
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
The U.S. Department of Health and Human Services will inject billions of dollars into programs designed to address mental health and substance use disorders, including $3 billion released to states as of May 18, said federal officials.
The American Rescue Plan, a COVID-relief package signed into law in March, contained the money, which will be divided equally between the Community Mental Health Services Block Grant Program and the Substance Abuse Prevention and Treatment Block Grant Program, said Tom Coderre, Acting Assistant Secretary for Mental Health and Substance Use, in a call with reporters.
The award amounts will vary by state.
The mental health program helps states and territories provide services for children with serious emotional issues and adults with serious mental illness.
The substance use program provides money to plan, implement, and evaluate prevention, intervention, treatment, and recovery services.
, which fueled an increase in anxiety, depression, and overdose, said Assistant Secretary for Health Rachel Levine, MD, on the call.
“We know multiple stressors during the pandemic – isolation, sickness, grief, job loss, food instability, and loss of routines – have devastated many Americans and presented the unprecedented behavioral health challenges across the nation,” said Dr. Levine.
The HHS also announced that it is re-establishing a Behavioral Health Coordinating Council (BHCC). Dr. Levine and Mr. Coderre will serve as cochairs of the Council, which will coordinate action-oriented approaches to addressing the HHS’s behavioral health efforts.
However, in 2014, the U.S. Government Accountability Office criticized the BHCC for only focusing on the HHS, and noted the lack of coordination across the federal government’s various efforts to address mental health.
‘A huge step forward’
The American Psychiatric Association welcomed the new money and the return of the council.
“In the wake of the pandemic an unprecedented, and as of yet untold, number of Americans are faced with mental health and substance use disorders, particularly in communities impacted by structural racism,” said APA President Vivian Pender, MD, in a statement. “With the creation of this Council and this investment in mental health, the administration is taking a huge step forward.”
APA CEO and Medical Director Saul Levin, MD, MPA, added: “This Council has great potential to ease the challenges we face as we begin to recover from the pandemic’s impact on our society, and [the] APA looks forward to assisting in their efforts.”
HHS Secretary Xavier Becerra noted in a statement that the COVID-19 pandemic “has made clear the need to invest resources in our nation’s mental health and address the inequities that still exist around behavioral health care.” He added, “This national problem calls for department-wide coordination to address the issue.”
Dr. Levine said the Council “will assure the right prioritization and guidelines are in place to provide pathways to prevention, intervention, treatment, and recovery services.”
A version of this article first appeared on Medscape.com.
HHS prohibits discrimination against LGBTQ patients: Action reverses Trump-era policy
The Biden administration is reversing a Trump-era policy that allowed health care providers to bar services to lesbian, gay, bisexual, transgender, or queer (LGBTQ) patients.
The U.S. Department of Health and Human Services gave notice on Monday that it would interpret the Affordable Care Act’s Section 1557 – which bars discrimination on the basis of sex – to include discrimination on the basis of sexual orientation or gender identity. The department said its position is consistent with a June 2020 U.S. Supreme Court ruling in Bostock v. Clayton County, GA. The ruling determined that the Civil Rights Act’s prohibition of employment discrimination on the basis of sex includes sexual orientation and gender identity.
“The mission of our Department is to enhance the health and well-being of all Americans, no matter their gender identity or sexual orientation,” said HHS Assistant Secretary for Health Rachel Levine, MD, in a statement released Monday.
“All people need access to health care services to fix a broken bone, protect their heart health, and screen for cancer risk,” she said. “No one should be discriminated against when seeking medical services because of who they are.”
Many physician organizations applauded the decision.
“The Biden administration did the right thing by terminating a short-lived effort to allow discrimination based on gender or sexual orientation when seeking health care,” said Susan R. Bailey, MD, president of the American Medical Association, in a statement.
When, in 2019, the Trump administration proposed to allow providers to deny care to LGBTQ people, the AMA said in a letter to the HHS that its interpretation “was contrary to the intent and the plain language of the law.”
Now, said Bailey, the AMA welcomes the Biden administration’s interpretation. It “is a victory for health equity and ends a dismal chapter in which a federal agency sought to remove civil rights protections,” she said.
An alliance of patient groups – including the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Epilepsy Foundation, the National Multiple Sclerosis Society, and the National Organization for Rare Disorders – also applauded the new policy. “This community already faces significant health disparities,” the groups noted in a statement. People with chronic illness such as HIV and cancer “need to be able to access care quickly and without fear of discrimination,” they said.
The groups had filed a friend of the court brief in a case against the Trump administration rule.
“We welcome this positive step to ensure access is preserved without hindrance, as intended by the health care law,” they said.
Twenty-two states and Washington, D.C. – led by former California Attorney General Xavier Becerra, who is now HHS secretary – sued the Trump administration in July 2020, aiming to overturn the rule.
Chase Strangio, deputy director for Trans Justice with the American Civil Liberties Union LGBTQ & HIV Project, noted that the HHS announcement was crucial in the face of efforts in multiple states to bar health care for transgender youth. “The Biden administration has affirmed what courts have said for decades: Discrimination against LGBTQ people is against the law. It also affirms what transgender people have long said: Gender-affirming care is life-saving care,” he said in a statement.
Lambda Legal, which led another lawsuit against the Trump administration rule, said it welcomed the HHS action but noted in a statement by the organization’s senior attorney, Omar Gonzalez-Pagan, that it “does not address significant aspects of the Trump-era rule that we and others have challenged in court.”
The Trump rule also “limited the remedies available to people who face health disparities, limited access to health care for people with Limited English Proficiency, unlawfully incorporated religious exemptions, and dramatically reduced the number of health care entities and insurance subject to the rule, all of which today’s action does not address,” said Gonzalez-Pagan.
“We encourage Secretary Xavier Becerra and the Biden administration to take additional steps to ensure that all LGBTQ people are completely covered wherever and whenever they may encounter discrimination during some of the most delicate and precarious moments of their lives: When seeking health care,” he said.
A version of this article first appeared on Medscape.com.
The Biden administration is reversing a Trump-era policy that allowed health care providers to bar services to lesbian, gay, bisexual, transgender, or queer (LGBTQ) patients.
The U.S. Department of Health and Human Services gave notice on Monday that it would interpret the Affordable Care Act’s Section 1557 – which bars discrimination on the basis of sex – to include discrimination on the basis of sexual orientation or gender identity. The department said its position is consistent with a June 2020 U.S. Supreme Court ruling in Bostock v. Clayton County, GA. The ruling determined that the Civil Rights Act’s prohibition of employment discrimination on the basis of sex includes sexual orientation and gender identity.
“The mission of our Department is to enhance the health and well-being of all Americans, no matter their gender identity or sexual orientation,” said HHS Assistant Secretary for Health Rachel Levine, MD, in a statement released Monday.
“All people need access to health care services to fix a broken bone, protect their heart health, and screen for cancer risk,” she said. “No one should be discriminated against when seeking medical services because of who they are.”
Many physician organizations applauded the decision.
“The Biden administration did the right thing by terminating a short-lived effort to allow discrimination based on gender or sexual orientation when seeking health care,” said Susan R. Bailey, MD, president of the American Medical Association, in a statement.
When, in 2019, the Trump administration proposed to allow providers to deny care to LGBTQ people, the AMA said in a letter to the HHS that its interpretation “was contrary to the intent and the plain language of the law.”
Now, said Bailey, the AMA welcomes the Biden administration’s interpretation. It “is a victory for health equity and ends a dismal chapter in which a federal agency sought to remove civil rights protections,” she said.
An alliance of patient groups – including the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Epilepsy Foundation, the National Multiple Sclerosis Society, and the National Organization for Rare Disorders – also applauded the new policy. “This community already faces significant health disparities,” the groups noted in a statement. People with chronic illness such as HIV and cancer “need to be able to access care quickly and without fear of discrimination,” they said.
The groups had filed a friend of the court brief in a case against the Trump administration rule.
“We welcome this positive step to ensure access is preserved without hindrance, as intended by the health care law,” they said.
Twenty-two states and Washington, D.C. – led by former California Attorney General Xavier Becerra, who is now HHS secretary – sued the Trump administration in July 2020, aiming to overturn the rule.
Chase Strangio, deputy director for Trans Justice with the American Civil Liberties Union LGBTQ & HIV Project, noted that the HHS announcement was crucial in the face of efforts in multiple states to bar health care for transgender youth. “The Biden administration has affirmed what courts have said for decades: Discrimination against LGBTQ people is against the law. It also affirms what transgender people have long said: Gender-affirming care is life-saving care,” he said in a statement.
Lambda Legal, which led another lawsuit against the Trump administration rule, said it welcomed the HHS action but noted in a statement by the organization’s senior attorney, Omar Gonzalez-Pagan, that it “does not address significant aspects of the Trump-era rule that we and others have challenged in court.”
The Trump rule also “limited the remedies available to people who face health disparities, limited access to health care for people with Limited English Proficiency, unlawfully incorporated religious exemptions, and dramatically reduced the number of health care entities and insurance subject to the rule, all of which today’s action does not address,” said Gonzalez-Pagan.
“We encourage Secretary Xavier Becerra and the Biden administration to take additional steps to ensure that all LGBTQ people are completely covered wherever and whenever they may encounter discrimination during some of the most delicate and precarious moments of their lives: When seeking health care,” he said.
A version of this article first appeared on Medscape.com.
The Biden administration is reversing a Trump-era policy that allowed health care providers to bar services to lesbian, gay, bisexual, transgender, or queer (LGBTQ) patients.
The U.S. Department of Health and Human Services gave notice on Monday that it would interpret the Affordable Care Act’s Section 1557 – which bars discrimination on the basis of sex – to include discrimination on the basis of sexual orientation or gender identity. The department said its position is consistent with a June 2020 U.S. Supreme Court ruling in Bostock v. Clayton County, GA. The ruling determined that the Civil Rights Act’s prohibition of employment discrimination on the basis of sex includes sexual orientation and gender identity.
“The mission of our Department is to enhance the health and well-being of all Americans, no matter their gender identity or sexual orientation,” said HHS Assistant Secretary for Health Rachel Levine, MD, in a statement released Monday.
“All people need access to health care services to fix a broken bone, protect their heart health, and screen for cancer risk,” she said. “No one should be discriminated against when seeking medical services because of who they are.”
Many physician organizations applauded the decision.
“The Biden administration did the right thing by terminating a short-lived effort to allow discrimination based on gender or sexual orientation when seeking health care,” said Susan R. Bailey, MD, president of the American Medical Association, in a statement.
When, in 2019, the Trump administration proposed to allow providers to deny care to LGBTQ people, the AMA said in a letter to the HHS that its interpretation “was contrary to the intent and the plain language of the law.”
Now, said Bailey, the AMA welcomes the Biden administration’s interpretation. It “is a victory for health equity and ends a dismal chapter in which a federal agency sought to remove civil rights protections,” she said.
An alliance of patient groups – including the American Cancer Society, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association, the Epilepsy Foundation, the National Multiple Sclerosis Society, and the National Organization for Rare Disorders – also applauded the new policy. “This community already faces significant health disparities,” the groups noted in a statement. People with chronic illness such as HIV and cancer “need to be able to access care quickly and without fear of discrimination,” they said.
The groups had filed a friend of the court brief in a case against the Trump administration rule.
“We welcome this positive step to ensure access is preserved without hindrance, as intended by the health care law,” they said.
Twenty-two states and Washington, D.C. – led by former California Attorney General Xavier Becerra, who is now HHS secretary – sued the Trump administration in July 2020, aiming to overturn the rule.
Chase Strangio, deputy director for Trans Justice with the American Civil Liberties Union LGBTQ & HIV Project, noted that the HHS announcement was crucial in the face of efforts in multiple states to bar health care for transgender youth. “The Biden administration has affirmed what courts have said for decades: Discrimination against LGBTQ people is against the law. It also affirms what transgender people have long said: Gender-affirming care is life-saving care,” he said in a statement.
Lambda Legal, which led another lawsuit against the Trump administration rule, said it welcomed the HHS action but noted in a statement by the organization’s senior attorney, Omar Gonzalez-Pagan, that it “does not address significant aspects of the Trump-era rule that we and others have challenged in court.”
The Trump rule also “limited the remedies available to people who face health disparities, limited access to health care for people with Limited English Proficiency, unlawfully incorporated religious exemptions, and dramatically reduced the number of health care entities and insurance subject to the rule, all of which today’s action does not address,” said Gonzalez-Pagan.
“We encourage Secretary Xavier Becerra and the Biden administration to take additional steps to ensure that all LGBTQ people are completely covered wherever and whenever they may encounter discrimination during some of the most delicate and precarious moments of their lives: When seeking health care,” he said.
A version of this article first appeared on Medscape.com.
Small clinics, practices key to COVID-19 vaccine success: State officials
Primary care physicians and providers in small offices and clinics are going to be key to ensuring that the remaining half of the nation receives a COVID-19 vaccination, state health officials said Wednesday, and the federal government will soon start shipping smaller packages of the Pfizer/BioNTech vaccine that can be more readily used by individual doctors.
According to the Centers for Disease Control and Prevention, as of April 21, more than 215 million doses have been administered. About 40% – 134 million Americans – have had at least one dose of a vaccine.
Among those who still haven’t received a shot are people who don’t have the time, may be homebound, or who have questions about the vaccine, or might say they will never be vaccinated, said Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials and director of the Maine Center for Disease Control and Prevention, on a call with reporters.
Especially for those who fall into the “not-ever” category, state officials “are working to find trusted messengers like doctors” who can connect with these individuals and give them information, he said.
Primary care physicians’ offices and other small practice settings are “where we are most likely to reach many of the remaining 50%,” Steven Stack, MD, MBA, FACEP, commissioner of the Kentucky Department for Public Health, said on the briefing.
State officials also “need to support all people to consult their personal physicians in whom they have confidence and trust to be informed of the benefits of COVID vaccination and the safety of this vaccination,” he said, adding that “this is the way we put this pandemic in the rearview mirror and move on with our lives.”
Dr. Stack said the federal government is starting by working with Pfizer to slim down its packages from 1,170 doses to 450 doses. That should happen before June, said Dr. Stack, adding that state health officials will be able to distribute the smaller packages “more widely and to smaller settings.”
Ideally, packaging for all vaccines will get down to single-dose, pre-filled syringes, he said. But that is a “journey” that the federal government has just begun, said Dr. Stack.
The White House had not responded to a request from this news organization for comment by press time.
Having vaccines onsite in a physician’s office is important, Dr. Stack said, adding that doctors “need to reach people in their persuadable moment.”
Bringing pediatricians on board
Illinois state health officials have begun a process that will let pediatricians have weekly vaccination clinics and also have vaccine on hand to meet patients in the moment, said Ngozi Ezike, MD, director of the Illinois Department of Public Health, on the briefing.
She said the distribution can start even before the Pfizer vaccine is shipped in smaller packages – and as soon as the Food and Drug Administration authorizes the vaccine for adolescents. Pfizer applied for emergency use approval for children aged 12-15 on April 9.
Local health departments will store the vaccine in their ultra-cold freezers. Pediatricians will identify how many people they hope to vaccinate each week and receive the doses on Monday, with the understanding that they must use the vaccine within 5 days, said Dr. Ezike.
The aim is to support vaccination clinics but also to ensure doctors have “doses on hand,” so that a parent or adolescent could opt for vaccination during a visit.
Although estimating the number of doses required will be difficult and likely involve some waste, Dr. Ezike said it’s important to be able to offer a vaccine in the office instead of having to refer someone elsewhere.
A version of this article first appeared on Medscape.com.
Primary care physicians and providers in small offices and clinics are going to be key to ensuring that the remaining half of the nation receives a COVID-19 vaccination, state health officials said Wednesday, and the federal government will soon start shipping smaller packages of the Pfizer/BioNTech vaccine that can be more readily used by individual doctors.
According to the Centers for Disease Control and Prevention, as of April 21, more than 215 million doses have been administered. About 40% – 134 million Americans – have had at least one dose of a vaccine.
Among those who still haven’t received a shot are people who don’t have the time, may be homebound, or who have questions about the vaccine, or might say they will never be vaccinated, said Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials and director of the Maine Center for Disease Control and Prevention, on a call with reporters.
Especially for those who fall into the “not-ever” category, state officials “are working to find trusted messengers like doctors” who can connect with these individuals and give them information, he said.
Primary care physicians’ offices and other small practice settings are “where we are most likely to reach many of the remaining 50%,” Steven Stack, MD, MBA, FACEP, commissioner of the Kentucky Department for Public Health, said on the briefing.
State officials also “need to support all people to consult their personal physicians in whom they have confidence and trust to be informed of the benefits of COVID vaccination and the safety of this vaccination,” he said, adding that “this is the way we put this pandemic in the rearview mirror and move on with our lives.”
Dr. Stack said the federal government is starting by working with Pfizer to slim down its packages from 1,170 doses to 450 doses. That should happen before June, said Dr. Stack, adding that state health officials will be able to distribute the smaller packages “more widely and to smaller settings.”
Ideally, packaging for all vaccines will get down to single-dose, pre-filled syringes, he said. But that is a “journey” that the federal government has just begun, said Dr. Stack.
The White House had not responded to a request from this news organization for comment by press time.
Having vaccines onsite in a physician’s office is important, Dr. Stack said, adding that doctors “need to reach people in their persuadable moment.”
Bringing pediatricians on board
Illinois state health officials have begun a process that will let pediatricians have weekly vaccination clinics and also have vaccine on hand to meet patients in the moment, said Ngozi Ezike, MD, director of the Illinois Department of Public Health, on the briefing.
She said the distribution can start even before the Pfizer vaccine is shipped in smaller packages – and as soon as the Food and Drug Administration authorizes the vaccine for adolescents. Pfizer applied for emergency use approval for children aged 12-15 on April 9.
Local health departments will store the vaccine in their ultra-cold freezers. Pediatricians will identify how many people they hope to vaccinate each week and receive the doses on Monday, with the understanding that they must use the vaccine within 5 days, said Dr. Ezike.
The aim is to support vaccination clinics but also to ensure doctors have “doses on hand,” so that a parent or adolescent could opt for vaccination during a visit.
Although estimating the number of doses required will be difficult and likely involve some waste, Dr. Ezike said it’s important to be able to offer a vaccine in the office instead of having to refer someone elsewhere.
A version of this article first appeared on Medscape.com.
Primary care physicians and providers in small offices and clinics are going to be key to ensuring that the remaining half of the nation receives a COVID-19 vaccination, state health officials said Wednesday, and the federal government will soon start shipping smaller packages of the Pfizer/BioNTech vaccine that can be more readily used by individual doctors.
According to the Centers for Disease Control and Prevention, as of April 21, more than 215 million doses have been administered. About 40% – 134 million Americans – have had at least one dose of a vaccine.
Among those who still haven’t received a shot are people who don’t have the time, may be homebound, or who have questions about the vaccine, or might say they will never be vaccinated, said Nirav Shah, MD, JD, president of the Association of State and Territorial Health Officials and director of the Maine Center for Disease Control and Prevention, on a call with reporters.
Especially for those who fall into the “not-ever” category, state officials “are working to find trusted messengers like doctors” who can connect with these individuals and give them information, he said.
Primary care physicians’ offices and other small practice settings are “where we are most likely to reach many of the remaining 50%,” Steven Stack, MD, MBA, FACEP, commissioner of the Kentucky Department for Public Health, said on the briefing.
State officials also “need to support all people to consult their personal physicians in whom they have confidence and trust to be informed of the benefits of COVID vaccination and the safety of this vaccination,” he said, adding that “this is the way we put this pandemic in the rearview mirror and move on with our lives.”
Dr. Stack said the federal government is starting by working with Pfizer to slim down its packages from 1,170 doses to 450 doses. That should happen before June, said Dr. Stack, adding that state health officials will be able to distribute the smaller packages “more widely and to smaller settings.”
Ideally, packaging for all vaccines will get down to single-dose, pre-filled syringes, he said. But that is a “journey” that the federal government has just begun, said Dr. Stack.
The White House had not responded to a request from this news organization for comment by press time.
Having vaccines onsite in a physician’s office is important, Dr. Stack said, adding that doctors “need to reach people in their persuadable moment.”
Bringing pediatricians on board
Illinois state health officials have begun a process that will let pediatricians have weekly vaccination clinics and also have vaccine on hand to meet patients in the moment, said Ngozi Ezike, MD, director of the Illinois Department of Public Health, on the briefing.
She said the distribution can start even before the Pfizer vaccine is shipped in smaller packages – and as soon as the Food and Drug Administration authorizes the vaccine for adolescents. Pfizer applied for emergency use approval for children aged 12-15 on April 9.
Local health departments will store the vaccine in their ultra-cold freezers. Pediatricians will identify how many people they hope to vaccinate each week and receive the doses on Monday, with the understanding that they must use the vaccine within 5 days, said Dr. Ezike.
The aim is to support vaccination clinics but also to ensure doctors have “doses on hand,” so that a parent or adolescent could opt for vaccination during a visit.
Although estimating the number of doses required will be difficult and likely involve some waste, Dr. Ezike said it’s important to be able to offer a vaccine in the office instead of having to refer someone elsewhere.
A version of this article first appeared on Medscape.com.
Percentage of doctors who are Black barely changed in 120 years
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study.
In 1900, 1.3% of physicians were Black. In 1940, 2.8% of physicians were Black, and by 2018 – when almost 13% of the population was Black – 5.4% of doctors were Black, reports Dan Ly, MD, PhD, MPP, an assistant professor of medicine at the University of California, Los Angeles, in a study published online April 19, 2021, in the Journal of General Internal Medicine.
The proportion of male Black physicians was 2.7% in 1940 and 2.6% in 2018.
Dr. Ly also found a significant wage gap. The median income earned by White doctors was $50,000 more than the median income of Black physicians in 2018. Dr. Ly based his findings on the U.S. Census Decennial Census long form, accessed via IPUMS, a free database funded by the National Institutes of Health and other organizations.
“If we care about the health of the population, particularly the health of Black patients, we should care about how small the proportion of our physicians who are Black is and the extremely slow progress we have made as a medical system in increasing that proportion,” Dr. Ly said in an interview.
Dr. Ly said he took on this research in part because previous studies have shown that Black patients are more likely to seek preventive care from Black doctors. Thus, increasing the numbers of Black physicians could narrow gaps in life expectancy between Whites and Blacks.
He also wanted to see whether progress had been made as a result of various medical organizations and the Association of American Medical Colleges undertaking initiatives to increase workforce diversity. There has been “very, very little” progress, he said.
Norma Poll-Hunter, PhD, the AAMC’s senior director of workforce diversity, said Dr. Ly’s report “was not surprising at all.”
The AAMC reported in 2014 that the number of Black men who apply to and matriculate into medical schools has been declining since 1978. That year, there were 1,410 Black male applicants and 542 Black enrollees. In 2014, there were 1,337 applicants and 515 enrollees.
Since 2014, Black male enrollment has increased slightly, rising from 2.4% in the 2014-2015 school year to 2.9% in the 2019-2020 year, the AAMC reported last year.
In addition, among other historically underrepresented minorities, “we really have seen very small progress” despite the increase in the number of medical schools, Dr. Poll-Hunter said in an interview.
The AAMC and the National Medical Association consider the lack of Black male applicants and matriculants to be a national crisis. The two groups started an alliance in 2020 aimed at finding ways to amplify and support Black men’s interest in medicine and the biomedical sciences and to “develop systems-based solutions to address exclusionary practices that create barriers for Black men and prevent them from having equitable opportunities to successfully enroll in medical school.”
Solutions include requiring medical school admissions committees and application screeners to undergo implicit bias awareness and mitigation training, adopting holistic admissions reviews, and incentivizing institutions of higher learning to partner with Black communities in urban and rural school systems to establish K-12 health sciences academies, said NMA President Leon McDougle, MD, MPH.
“There are the systems factors, and racism is a big one that we have to tackle,” said Dr. Poll-Hunter.
Diversity isn’t just about numbers, said Dr. McDougle, a professor of family medicine and associate dean for diversity and inclusion at Ohio State University, Columbus. “We know that medical school graduates who are African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native are more likely to serve those communities as practicing physicians.
“The COVID-19 pandemic highlighted the urgent need for more African American or Black, Hispanic or Latinx, or American Indian or Alaskan Native physicians,” he said. “Inadequate access to culturally competent care has exacerbated existing health disparities, resulting in death and hospitalization rates up to three to four times the rates of European American or White people.”
Dr. Poll-Hunter also said that studies have shown that diversity in the classroom creates a more enriched learning environment and increases civic mindedness and cognitive complexity, “as well as helps us understand people who are different than ourselves.”
The diversity goal “is not about quotas, it’s about excellence,” she said. “We know that there’s talent that exists, and we want to make sure that everyone has an opportunity to be successful.”
Dr. Ly has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HHS proposes overturning Title X ‘gag’ rule
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
The Department of Health & Human Services has proposed overturning rules issued during the Trump administration that effectively prohibit clinicians at Title X–funded health clinics from discussing abortion or referring patients for abortions.
HHS proposed the overhaul of the Title X regulations on April 14. The previous administration’s 2019 rules “have undermined the public health of the population the program is meant to serve,” HHS said in the introduction to its proposal.
Medical organizations and reproductive health specialists lauded the move.
“Clinicians providing care to patients must be empowered to share the full spectrum of accurate medical information necessary to ensure that their patients are able to make timely, fully informed medical decisions,” Maureen G. Phipps, MD, MPH, CEO of the American College of Obstetricians and Gynecologists, said in a statement. “This means transparent, respectful, evidence-based conversations about contraception and abortion care. The proposed rule will ensure that those conversations can once again happen without restrictions, interference, or threat of financial loss.”
“Providers of comprehensive reproductive health care, including abortion care, base their relationships with their patients on trust,” Physicians for Reproductive Health President and CEO Jamila Perritt, MD, said in a statement. “The Title X gag rule went against everything we knew as providers of ethical, evidence-based health care by forcing providers at Title X funded clinics to withhold information that their patients needed and requested.”
HHS said that, since 2019, more than 1,000 Title X–funded service sites (25% of the total) have withdrawn from the program. Currently, Title X services – which include family planning, STI testing, cancer screening, and HIV testing and treatment – are not available in six states and are only available on a limited basis in six additional states. Planned Parenthood fully withdrew from Title X.
HHS said that tens of thousands fewer birth control implant procedures have been performed and that hundreds of thousands fewer Pap tests and a half-million or more fewer tests for chlamydia and gonorrhea have been conducted. In addition, the reduction in services may have led to up to 181,477 unintended pregnancies, HHS said.
The closure of sites and decreased availability of services have also exacerbated health inequities, according to the department.
The loss of services “has been especially felt by those already facing disproportionate barriers to accessing care, including the Black, Latinx and Indigenous communities that have also suffered the most harm during the COVID-19 pandemic,” agreed Dr. Phipps.
The new regulation proposes to “ensure access to equitable, affordable, client-centered, quality family-planning services for all clients, especially for low-income clients,” HHS said.
The proposed change in the rules “brings us one step closer to restoring access to necessary care for millions of low-income and uninsured patients who depend on Title X for family planning services,” American Medical Association President Susan R. Bailey, MD, said in a statement. “We are pleased that the Biden administration shares our commitment to undoing this dangerous and discriminatory ‘gag rule,’ and look forward to its elimination through any means necessary to achieve the best outcome for patients and physicians – improving the health of our nation.”
Planned Parenthood also applauded the move, and the HIV Medicine Association thanked the Biden administration for its proposal, which it called “a major step to improve #HealthEquity for all people in this country,” in a tweet.
March for Life, an antiabortion group, however, said it strongly opposed the HHS proposal. The rules “appear specifically designed to bring America’s largest abortion provider, Planned Parenthood, back into the taxpayer-funded program and keep prolife organizations out,” said the group in a tweet.
“Abortion is neither health care nor family planning, and the Title X program should not be funding it,” said the group.
The Title X program does not pay for abortions, however.
The Trump administration rules prohibit abortion referrals and impose counseling standards for pregnant patients and what the Guttmacher Institute called “unnecessary and stringent requirements for the physical and financial separation of Title X–funded activities from a range of abortion-related activities.”
The new rules would reestablish regulations from 2000, with some new additions. For instance, the program will “formally integrate elements of quality family-planning services guidelines developed by [Centers for Disease Control and Prevention] and Office of Population Affairs,” tweeted Alina Salganicoff, director of women’s health policy at the Kaiser Family Foundation. “That means that higher standards for providing family planning will be required,” she tweeted. In addition, sites that offer natural family planning and abstinence “will only be able to participate if they offer referrals to other providers that offer clients access to the contraceptive of their choice.”
The proposed rules are open for public comment for 30 days. They could be made final by the fall. The Kaiser Family Foundation reports that many sites could be ready to return to the program by then, especially since the recently passed coronavirus relief package, the American Rescue Plan, included a $50 million supplemental appropriation for Title X.
The 2019 rules remain in effect in the meantime, although the U.S. Supreme Court agreed in February to hear a challenge mounted by 21 states, the city of Baltimore, and organizations that included the AMA and Planned Parenthood. Those plaintiffs have requested that the case be dismissed, but it currently remains on the docket.
Not all medical providers are likely to support the new rules if they go into effect. The American Association of Pro-Life Obstetricians and Gynecologists, the Christian Medical and Dental Associations, and the Catholic Medical Association filed motions in the Supreme Court case to defend the Trump regulations.
A version of this article first appeared on Medscape.com.
Feds let Illinois extend postpartum Medicaid coverage: HHS encourages other states to follow suit
The federal government has approved a request by Illinois to extend postpartum Medicaid coverage to a full year from the current standard of 60 days.
Health & Human Services Secretary Xavier Becerra announced the approval at a press briefing on April 12, noting that it was occurring during Black Maternal Health Week. The coverage extension is aimed at decreasing maternal morbidity and mortality, particularly among women of color.
Black women are two times more likely to die from a pregnancy-related cause than White women, according to HHS. Mr. Becerra noted that, in the United States, 52% of pregnancy-related deaths take place up to 1 year post partum, and that in Illinois the figure is 80%.
“The continuity of coverage available through this action will help new mothers manage chronic conditions like hypertension and diabetes, and it will provide access to behavioral health and other mental health care services,” he said.
Continuing Medicaid coverage for new mothers has been backed by the American Medical Association, is a priority of the American College of Obstetricians and Gynecologists, and has been promoted by Republicans and Democrats in Congress and state legislatures.
Illinois is the first state to seek and win approval to extend its Medicaid coverage from the current 60-days postbirth requirement. The program was granted through an existing section 1115 waiver program. It begins today and is authorized through Dec. 31, 2025. The state estimates that some 2,500 women with incomes up to 208% of the federal poverty level will receive the year of continuous Medicaid coverage. Illinois will evaluate whether the extension improves women’s health and if it benefits the Medicaid program overall.
However, the recently passed coronavirus rescue package creates a new process that lets states more easily expand postpartum coverage, but they must act by April 2022. Mr. Becerra said the federal government is encouraging more states to follow Illinois’ lead in extending postpartum eligibility by taking advantage of the new process.
States won’t get extra money – they will receive the regular per capita–based federal match if they extend Medicaid coverage through this pathway. Even so, Mr. Becerra said there has been much interest.
“I hope that we begin to see states not only express interest but actually submit their proposals on how they would do this,” Mr. Becerra said.
Medicaid has become one of the key providers of maternal health care in the United States, as it covers 4 in 10 births, according to the Kaiser Family Foundation. But postpartum coverage after the 60-day federal requirement is a patchwork. In 38 states (plus Washington, D.C.) that have expanded Medicaid under the Affordable Care Act, mothers who earn up to 138% of the federal poverty level can continue on Medicaid; for those who earn more than that, they can get coverage through the ACA.
In the 12 states that did not expand Medicaid, new mothers have to seek Medicaid coverage after 60 days as parents, and the income limits are strict. In Texas, for example, a married mother with a newborn loses Medicaid coverage 2 months after giving birth if she and her partner have an annual income above $3,733, reports the Kaiser Family Foundation.
Coverage disruptions are harmful to mothers, said Mr. Becerra. HHS data shows that more than half of pregnant women in Medicaid experienced a coverage gap in the first 6 months postpartum and that disruptions in coverage often lead to delayed care and less preventive care.
Mr. Becerra also announced that the Health Resources and Services Administration will make $12 million available over 4 years for the Rural Maternity and Obstetrics Management Strategies program. Applicants for the new funds will be required to focus on populations that have historically suffered from poorer health outcomes, health disparities, and other inequities.
A version of this article first appeared on Medscape.com.
The federal government has approved a request by Illinois to extend postpartum Medicaid coverage to a full year from the current standard of 60 days.
Health & Human Services Secretary Xavier Becerra announced the approval at a press briefing on April 12, noting that it was occurring during Black Maternal Health Week. The coverage extension is aimed at decreasing maternal morbidity and mortality, particularly among women of color.
Black women are two times more likely to die from a pregnancy-related cause than White women, according to HHS. Mr. Becerra noted that, in the United States, 52% of pregnancy-related deaths take place up to 1 year post partum, and that in Illinois the figure is 80%.
“The continuity of coverage available through this action will help new mothers manage chronic conditions like hypertension and diabetes, and it will provide access to behavioral health and other mental health care services,” he said.
Continuing Medicaid coverage for new mothers has been backed by the American Medical Association, is a priority of the American College of Obstetricians and Gynecologists, and has been promoted by Republicans and Democrats in Congress and state legislatures.
Illinois is the first state to seek and win approval to extend its Medicaid coverage from the current 60-days postbirth requirement. The program was granted through an existing section 1115 waiver program. It begins today and is authorized through Dec. 31, 2025. The state estimates that some 2,500 women with incomes up to 208% of the federal poverty level will receive the year of continuous Medicaid coverage. Illinois will evaluate whether the extension improves women’s health and if it benefits the Medicaid program overall.
However, the recently passed coronavirus rescue package creates a new process that lets states more easily expand postpartum coverage, but they must act by April 2022. Mr. Becerra said the federal government is encouraging more states to follow Illinois’ lead in extending postpartum eligibility by taking advantage of the new process.
States won’t get extra money – they will receive the regular per capita–based federal match if they extend Medicaid coverage through this pathway. Even so, Mr. Becerra said there has been much interest.
“I hope that we begin to see states not only express interest but actually submit their proposals on how they would do this,” Mr. Becerra said.
Medicaid has become one of the key providers of maternal health care in the United States, as it covers 4 in 10 births, according to the Kaiser Family Foundation. But postpartum coverage after the 60-day federal requirement is a patchwork. In 38 states (plus Washington, D.C.) that have expanded Medicaid under the Affordable Care Act, mothers who earn up to 138% of the federal poverty level can continue on Medicaid; for those who earn more than that, they can get coverage through the ACA.
In the 12 states that did not expand Medicaid, new mothers have to seek Medicaid coverage after 60 days as parents, and the income limits are strict. In Texas, for example, a married mother with a newborn loses Medicaid coverage 2 months after giving birth if she and her partner have an annual income above $3,733, reports the Kaiser Family Foundation.
Coverage disruptions are harmful to mothers, said Mr. Becerra. HHS data shows that more than half of pregnant women in Medicaid experienced a coverage gap in the first 6 months postpartum and that disruptions in coverage often lead to delayed care and less preventive care.
Mr. Becerra also announced that the Health Resources and Services Administration will make $12 million available over 4 years for the Rural Maternity and Obstetrics Management Strategies program. Applicants for the new funds will be required to focus on populations that have historically suffered from poorer health outcomes, health disparities, and other inequities.
A version of this article first appeared on Medscape.com.
The federal government has approved a request by Illinois to extend postpartum Medicaid coverage to a full year from the current standard of 60 days.
Health & Human Services Secretary Xavier Becerra announced the approval at a press briefing on April 12, noting that it was occurring during Black Maternal Health Week. The coverage extension is aimed at decreasing maternal morbidity and mortality, particularly among women of color.
Black women are two times more likely to die from a pregnancy-related cause than White women, according to HHS. Mr. Becerra noted that, in the United States, 52% of pregnancy-related deaths take place up to 1 year post partum, and that in Illinois the figure is 80%.
“The continuity of coverage available through this action will help new mothers manage chronic conditions like hypertension and diabetes, and it will provide access to behavioral health and other mental health care services,” he said.
Continuing Medicaid coverage for new mothers has been backed by the American Medical Association, is a priority of the American College of Obstetricians and Gynecologists, and has been promoted by Republicans and Democrats in Congress and state legislatures.
Illinois is the first state to seek and win approval to extend its Medicaid coverage from the current 60-days postbirth requirement. The program was granted through an existing section 1115 waiver program. It begins today and is authorized through Dec. 31, 2025. The state estimates that some 2,500 women with incomes up to 208% of the federal poverty level will receive the year of continuous Medicaid coverage. Illinois will evaluate whether the extension improves women’s health and if it benefits the Medicaid program overall.
However, the recently passed coronavirus rescue package creates a new process that lets states more easily expand postpartum coverage, but they must act by April 2022. Mr. Becerra said the federal government is encouraging more states to follow Illinois’ lead in extending postpartum eligibility by taking advantage of the new process.
States won’t get extra money – they will receive the regular per capita–based federal match if they extend Medicaid coverage through this pathway. Even so, Mr. Becerra said there has been much interest.
“I hope that we begin to see states not only express interest but actually submit their proposals on how they would do this,” Mr. Becerra said.
Medicaid has become one of the key providers of maternal health care in the United States, as it covers 4 in 10 births, according to the Kaiser Family Foundation. But postpartum coverage after the 60-day federal requirement is a patchwork. In 38 states (plus Washington, D.C.) that have expanded Medicaid under the Affordable Care Act, mothers who earn up to 138% of the federal poverty level can continue on Medicaid; for those who earn more than that, they can get coverage through the ACA.
In the 12 states that did not expand Medicaid, new mothers have to seek Medicaid coverage after 60 days as parents, and the income limits are strict. In Texas, for example, a married mother with a newborn loses Medicaid coverage 2 months after giving birth if she and her partner have an annual income above $3,733, reports the Kaiser Family Foundation.
Coverage disruptions are harmful to mothers, said Mr. Becerra. HHS data shows that more than half of pregnant women in Medicaid experienced a coverage gap in the first 6 months postpartum and that disruptions in coverage often lead to delayed care and less preventive care.
Mr. Becerra also announced that the Health Resources and Services Administration will make $12 million available over 4 years for the Rural Maternity and Obstetrics Management Strategies program. Applicants for the new funds will be required to focus on populations that have historically suffered from poorer health outcomes, health disparities, and other inequities.
A version of this article first appeared on Medscape.com.
Detroit cardiologists prevail in retaliation suit against Tenet
After losing at arbitration, as well as in federal court and partially on appeal, Tenet Healthcare is refusing to comment on whether it will continue to battle two Detroit-area cardiologists whom the hospital corporation fired from leadership positions in 2018.
The cardiologists were awarded $10.6 million from an arbitrator, who found that Detroit Medical Center (DMC) and its parent, Tenet, retaliated against Amir Kaki, MD, and Mahir Elder, MD, when the doctors repeatedly reported concerns about patient safety and potential fraud.
The award was made public when it was upheld in federal court in February 2021 and was partially upheld on appeal days later by the Sixth Circuit Court of Appeals.
The Sixth Circuit Court of Appeals denied Tenet’s motion to bar Dr. Kaki and Dr. Elder from returning to work with full privileges but said it would continue to consider the overall appeal. Tenet argued that it needed to keep the cardiologists out of DMC because of “behavioral issues.”
Those allegations are “complete nonsense,” said the cardiologists’ attorney, Deborah Gordon, of Bloomfield Hills, Mich. The alleged problems regarding Dr. Kaki and Dr. Elder were examined by an arbitrator, who “found that all of those complaints were unsubstantiated,” Ms. Gordon said in an interview.
In her final ruling, arbitrator Mary Beth Kelly wrote, “Both Kaki and Elder testified credibly regarding the humiliation, the emotional distress and the reputational damage they have suffered to their national reputations.”
A spokesperson for Tenet and DMC said the organizations had no further comment.
Ms. Gordon said she believes it’s unlikely Tenet will prevail in the Sixth Circuit Court of Appeals, noting that the court already had examined the merits of the case to determine whether Dr. Kaki and Dr. Elder could go back to work. In the court’s opinion, shared in an interview, nothing substantive in Tenet’s appeal prevented the doctors from returning to the hospital, she said.
As of now, both cardiologists have 1 year of privileges at the DMC-affiliated hospitals. Only Dr. Kaki has returned to work, said Ms. Gordon. Neither is speaking to the media, she said.
From respected to reviled
Both Dr. Kaki and Dr. Elder were respected at DMC, according to court filings.
Dr. Kaki was recruited from Weill Cornell Medical College by a Detroit mayor because of his expertise in interventional cardiology. He had staff privileges at DMC beginning in 2012 and was a clinical associate professor and assistant program director of the interventional cardiology fellowship program at Wayne State University in Detroit. He became director of the cardiac catheterization services unit at the new DMC Heart Hospital at Harper-Hutzel Hospital in Detroit in 2014, and 4 years later was appointed director of the facility’s anticoagulation clinic. Dr. Kaki was nominated for and completed Tenet’s Leadership Academy.
Dr. Elder was a clinical professor and assistant fellowship director at Wayne State and was a clinical professor of medicine at Michigan State University. Beginning in 2008, he held directorships at DMC’s cardiac care unit, ambulatory services program, cardiac CT angiogram program, PERT program, and carotid stenting program. Dr. Elder was voted Teacher of the Year for 10 consecutive years by DMC cardiology fellows.
The two doctors aimed high when it came to quality of care and ethics, according to legal filings. Over the years, Dr. Kaki and Dr. Elder repeatedly reported what they considered to be egregious violations of patient safety and of Medicare and Medicaid fraud laws. The clinicians complained about unsterile surgical instruments and the removal of a stat laboratory from the cardiac catheterization unit, noting that the removal would cause delays that would endanger lives.
At peer review meetings, as well as with administrators, they flagged colleagues who they said were performing unnecessary or dangerous procedures solely to generate revenue. At least one doctor falsified records of such a procedure after a patient died, alleged Dr. Kaki and Dr. Elder.
Tenet hired outside attorneys in the fall of 2018, telling Dr. Kaki and Dr. Elder that the legal team would investigate their complaints. However, the investigation was a sham: Filings allege that the investigation was used instead to build a case against Dr. Kaki and Dr. Elder and that Tenet leadership used the inquiry to pressure the cardiologists to resign.
They refused, and in October 2018, they were fired from their leadership positions. DMC and Tenet then held a press conference in which they said that Dr. Kaki and Dr. Elder had been dismissed for “violations” of the “Tenet Standards of Conduct.”
Cardiologists push back
Dr. Kaki and Dr. Elder, however, were not willing to just walk away. They sought reinstatement through an internal DMC appeals panel of their peers. The clinicians who participated on that panel ruled that neither firing was justified.
But DMC’s governing board voted in April 2020 to deny privileges to both cardiologists.
Tenet continued a campaign of retaliation, according to legal filings, by not paying the clinicians for being on call, by removing them from peer review committees, and by prohibiting them from teaching or giving lectures. DMC refused to give Dr. Kaki his personnel record, stating that he was never an employee when he was in the leadership position. Dr. Kaki sued, and a Wayne County Circuit Court judge granted his motion to get his file. DMC and Tenet appealed that ruling but lost.
Eventually, Ms. Gordon sued DMC and Tenet in federal court, alleging the hospital retaliated against the cardiologists, interfered with their ability to earn a living by disparaging them, refused to renew their privileges in 2019, and committed violations under multiple federal and state statutes, including the False Claims Act and the Fair Labor Standards Act.
Tenet successfully argued that the case should go to arbitration.
Arbitrator Mary Beth Kelly, though, ruled in December 2020 that the vast majority of the complaints compiled against the two physicians in the external investigation were not verified or supported and that Tenet and DMC had retaliated against Dr. Kaki and Dr. Elder.
For that harm, Ms. Kelly awarded each clinician $1 million, according to the final ruling shared in an interview.
In addition, she awarded Dr. Kaki $2.3 million in back pay and 2 years of front pay (slightly more than $1 million). She awarded Dr. Elder $2.3 million in back pay and $2.1 million in front pay for 4 years, noting that “his strong association with DMC may make it more difficult for him to successfully transition into the situation he enjoyed prior to termination and nonrenewal.”
The clinicians also were awarded legal fees of $623,816 and court costs of $110,673.
“Wholesale retrial”
To secure the award, Ms. Gordon had to seek a ruling from the U.S. District Court for Eastern Michigan. Tenet asked that court to overturn the arbitrator’s award and to keep it sealed from public view.
In his February ruling, Judge Arthur J. Tarnow wrote that Tenet and DMC “not only attempt to relitigate the legal issues, but also endeavor to introduce a factual counternarrative unmoored from the findings of the Arbitrator and including evidence which the Arbitrator specifically found inadmissible.
“By seeking a wholesale retrial of their case after forcing plaintiffs to arbitrate in the first place,” Tenet and DMC basically ignored the goal of arbitration, which is to relieve judicial congestion and provide a faster and cheaper alternative to litigation, he wrote.
Judge Tarnow also warned Tenet and DMC against taking too long to reinstate privileges for Dr. Kaki and Dr. Elder. If they “continue to delay the restoration of plaintiffs’ privileges in the hopes of a different result on appeal, they will be in violation of this Order,” said the judge.
Tenet, however, tried one more avenue to block the cardiologists from getting privileges, appealing to the Sixth Circuit, which again ordered the company to grant the 1-year privileges.
A version of this article first appeared on Medscape.com.
After losing at arbitration, as well as in federal court and partially on appeal, Tenet Healthcare is refusing to comment on whether it will continue to battle two Detroit-area cardiologists whom the hospital corporation fired from leadership positions in 2018.
The cardiologists were awarded $10.6 million from an arbitrator, who found that Detroit Medical Center (DMC) and its parent, Tenet, retaliated against Amir Kaki, MD, and Mahir Elder, MD, when the doctors repeatedly reported concerns about patient safety and potential fraud.
The award was made public when it was upheld in federal court in February 2021 and was partially upheld on appeal days later by the Sixth Circuit Court of Appeals.
The Sixth Circuit Court of Appeals denied Tenet’s motion to bar Dr. Kaki and Dr. Elder from returning to work with full privileges but said it would continue to consider the overall appeal. Tenet argued that it needed to keep the cardiologists out of DMC because of “behavioral issues.”
Those allegations are “complete nonsense,” said the cardiologists’ attorney, Deborah Gordon, of Bloomfield Hills, Mich. The alleged problems regarding Dr. Kaki and Dr. Elder were examined by an arbitrator, who “found that all of those complaints were unsubstantiated,” Ms. Gordon said in an interview.
In her final ruling, arbitrator Mary Beth Kelly wrote, “Both Kaki and Elder testified credibly regarding the humiliation, the emotional distress and the reputational damage they have suffered to their national reputations.”
A spokesperson for Tenet and DMC said the organizations had no further comment.
Ms. Gordon said she believes it’s unlikely Tenet will prevail in the Sixth Circuit Court of Appeals, noting that the court already had examined the merits of the case to determine whether Dr. Kaki and Dr. Elder could go back to work. In the court’s opinion, shared in an interview, nothing substantive in Tenet’s appeal prevented the doctors from returning to the hospital, she said.
As of now, both cardiologists have 1 year of privileges at the DMC-affiliated hospitals. Only Dr. Kaki has returned to work, said Ms. Gordon. Neither is speaking to the media, she said.
From respected to reviled
Both Dr. Kaki and Dr. Elder were respected at DMC, according to court filings.
Dr. Kaki was recruited from Weill Cornell Medical College by a Detroit mayor because of his expertise in interventional cardiology. He had staff privileges at DMC beginning in 2012 and was a clinical associate professor and assistant program director of the interventional cardiology fellowship program at Wayne State University in Detroit. He became director of the cardiac catheterization services unit at the new DMC Heart Hospital at Harper-Hutzel Hospital in Detroit in 2014, and 4 years later was appointed director of the facility’s anticoagulation clinic. Dr. Kaki was nominated for and completed Tenet’s Leadership Academy.
Dr. Elder was a clinical professor and assistant fellowship director at Wayne State and was a clinical professor of medicine at Michigan State University. Beginning in 2008, he held directorships at DMC’s cardiac care unit, ambulatory services program, cardiac CT angiogram program, PERT program, and carotid stenting program. Dr. Elder was voted Teacher of the Year for 10 consecutive years by DMC cardiology fellows.
The two doctors aimed high when it came to quality of care and ethics, according to legal filings. Over the years, Dr. Kaki and Dr. Elder repeatedly reported what they considered to be egregious violations of patient safety and of Medicare and Medicaid fraud laws. The clinicians complained about unsterile surgical instruments and the removal of a stat laboratory from the cardiac catheterization unit, noting that the removal would cause delays that would endanger lives.
At peer review meetings, as well as with administrators, they flagged colleagues who they said were performing unnecessary or dangerous procedures solely to generate revenue. At least one doctor falsified records of such a procedure after a patient died, alleged Dr. Kaki and Dr. Elder.
Tenet hired outside attorneys in the fall of 2018, telling Dr. Kaki and Dr. Elder that the legal team would investigate their complaints. However, the investigation was a sham: Filings allege that the investigation was used instead to build a case against Dr. Kaki and Dr. Elder and that Tenet leadership used the inquiry to pressure the cardiologists to resign.
They refused, and in October 2018, they were fired from their leadership positions. DMC and Tenet then held a press conference in which they said that Dr. Kaki and Dr. Elder had been dismissed for “violations” of the “Tenet Standards of Conduct.”
Cardiologists push back
Dr. Kaki and Dr. Elder, however, were not willing to just walk away. They sought reinstatement through an internal DMC appeals panel of their peers. The clinicians who participated on that panel ruled that neither firing was justified.
But DMC’s governing board voted in April 2020 to deny privileges to both cardiologists.
Tenet continued a campaign of retaliation, according to legal filings, by not paying the clinicians for being on call, by removing them from peer review committees, and by prohibiting them from teaching or giving lectures. DMC refused to give Dr. Kaki his personnel record, stating that he was never an employee when he was in the leadership position. Dr. Kaki sued, and a Wayne County Circuit Court judge granted his motion to get his file. DMC and Tenet appealed that ruling but lost.
Eventually, Ms. Gordon sued DMC and Tenet in federal court, alleging the hospital retaliated against the cardiologists, interfered with their ability to earn a living by disparaging them, refused to renew their privileges in 2019, and committed violations under multiple federal and state statutes, including the False Claims Act and the Fair Labor Standards Act.
Tenet successfully argued that the case should go to arbitration.
Arbitrator Mary Beth Kelly, though, ruled in December 2020 that the vast majority of the complaints compiled against the two physicians in the external investigation were not verified or supported and that Tenet and DMC had retaliated against Dr. Kaki and Dr. Elder.
For that harm, Ms. Kelly awarded each clinician $1 million, according to the final ruling shared in an interview.
In addition, she awarded Dr. Kaki $2.3 million in back pay and 2 years of front pay (slightly more than $1 million). She awarded Dr. Elder $2.3 million in back pay and $2.1 million in front pay for 4 years, noting that “his strong association with DMC may make it more difficult for him to successfully transition into the situation he enjoyed prior to termination and nonrenewal.”
The clinicians also were awarded legal fees of $623,816 and court costs of $110,673.
“Wholesale retrial”
To secure the award, Ms. Gordon had to seek a ruling from the U.S. District Court for Eastern Michigan. Tenet asked that court to overturn the arbitrator’s award and to keep it sealed from public view.
In his February ruling, Judge Arthur J. Tarnow wrote that Tenet and DMC “not only attempt to relitigate the legal issues, but also endeavor to introduce a factual counternarrative unmoored from the findings of the Arbitrator and including evidence which the Arbitrator specifically found inadmissible.
“By seeking a wholesale retrial of their case after forcing plaintiffs to arbitrate in the first place,” Tenet and DMC basically ignored the goal of arbitration, which is to relieve judicial congestion and provide a faster and cheaper alternative to litigation, he wrote.
Judge Tarnow also warned Tenet and DMC against taking too long to reinstate privileges for Dr. Kaki and Dr. Elder. If they “continue to delay the restoration of plaintiffs’ privileges in the hopes of a different result on appeal, they will be in violation of this Order,” said the judge.
Tenet, however, tried one more avenue to block the cardiologists from getting privileges, appealing to the Sixth Circuit, which again ordered the company to grant the 1-year privileges.
A version of this article first appeared on Medscape.com.
After losing at arbitration, as well as in federal court and partially on appeal, Tenet Healthcare is refusing to comment on whether it will continue to battle two Detroit-area cardiologists whom the hospital corporation fired from leadership positions in 2018.
The cardiologists were awarded $10.6 million from an arbitrator, who found that Detroit Medical Center (DMC) and its parent, Tenet, retaliated against Amir Kaki, MD, and Mahir Elder, MD, when the doctors repeatedly reported concerns about patient safety and potential fraud.
The award was made public when it was upheld in federal court in February 2021 and was partially upheld on appeal days later by the Sixth Circuit Court of Appeals.
The Sixth Circuit Court of Appeals denied Tenet’s motion to bar Dr. Kaki and Dr. Elder from returning to work with full privileges but said it would continue to consider the overall appeal. Tenet argued that it needed to keep the cardiologists out of DMC because of “behavioral issues.”
Those allegations are “complete nonsense,” said the cardiologists’ attorney, Deborah Gordon, of Bloomfield Hills, Mich. The alleged problems regarding Dr. Kaki and Dr. Elder were examined by an arbitrator, who “found that all of those complaints were unsubstantiated,” Ms. Gordon said in an interview.
In her final ruling, arbitrator Mary Beth Kelly wrote, “Both Kaki and Elder testified credibly regarding the humiliation, the emotional distress and the reputational damage they have suffered to their national reputations.”
A spokesperson for Tenet and DMC said the organizations had no further comment.
Ms. Gordon said she believes it’s unlikely Tenet will prevail in the Sixth Circuit Court of Appeals, noting that the court already had examined the merits of the case to determine whether Dr. Kaki and Dr. Elder could go back to work. In the court’s opinion, shared in an interview, nothing substantive in Tenet’s appeal prevented the doctors from returning to the hospital, she said.
As of now, both cardiologists have 1 year of privileges at the DMC-affiliated hospitals. Only Dr. Kaki has returned to work, said Ms. Gordon. Neither is speaking to the media, she said.
From respected to reviled
Both Dr. Kaki and Dr. Elder were respected at DMC, according to court filings.
Dr. Kaki was recruited from Weill Cornell Medical College by a Detroit mayor because of his expertise in interventional cardiology. He had staff privileges at DMC beginning in 2012 and was a clinical associate professor and assistant program director of the interventional cardiology fellowship program at Wayne State University in Detroit. He became director of the cardiac catheterization services unit at the new DMC Heart Hospital at Harper-Hutzel Hospital in Detroit in 2014, and 4 years later was appointed director of the facility’s anticoagulation clinic. Dr. Kaki was nominated for and completed Tenet’s Leadership Academy.
Dr. Elder was a clinical professor and assistant fellowship director at Wayne State and was a clinical professor of medicine at Michigan State University. Beginning in 2008, he held directorships at DMC’s cardiac care unit, ambulatory services program, cardiac CT angiogram program, PERT program, and carotid stenting program. Dr. Elder was voted Teacher of the Year for 10 consecutive years by DMC cardiology fellows.
The two doctors aimed high when it came to quality of care and ethics, according to legal filings. Over the years, Dr. Kaki and Dr. Elder repeatedly reported what they considered to be egregious violations of patient safety and of Medicare and Medicaid fraud laws. The clinicians complained about unsterile surgical instruments and the removal of a stat laboratory from the cardiac catheterization unit, noting that the removal would cause delays that would endanger lives.
At peer review meetings, as well as with administrators, they flagged colleagues who they said were performing unnecessary or dangerous procedures solely to generate revenue. At least one doctor falsified records of such a procedure after a patient died, alleged Dr. Kaki and Dr. Elder.
Tenet hired outside attorneys in the fall of 2018, telling Dr. Kaki and Dr. Elder that the legal team would investigate their complaints. However, the investigation was a sham: Filings allege that the investigation was used instead to build a case against Dr. Kaki and Dr. Elder and that Tenet leadership used the inquiry to pressure the cardiologists to resign.
They refused, and in October 2018, they were fired from their leadership positions. DMC and Tenet then held a press conference in which they said that Dr. Kaki and Dr. Elder had been dismissed for “violations” of the “Tenet Standards of Conduct.”
Cardiologists push back
Dr. Kaki and Dr. Elder, however, were not willing to just walk away. They sought reinstatement through an internal DMC appeals panel of their peers. The clinicians who participated on that panel ruled that neither firing was justified.
But DMC’s governing board voted in April 2020 to deny privileges to both cardiologists.
Tenet continued a campaign of retaliation, according to legal filings, by not paying the clinicians for being on call, by removing them from peer review committees, and by prohibiting them from teaching or giving lectures. DMC refused to give Dr. Kaki his personnel record, stating that he was never an employee when he was in the leadership position. Dr. Kaki sued, and a Wayne County Circuit Court judge granted his motion to get his file. DMC and Tenet appealed that ruling but lost.
Eventually, Ms. Gordon sued DMC and Tenet in federal court, alleging the hospital retaliated against the cardiologists, interfered with their ability to earn a living by disparaging them, refused to renew their privileges in 2019, and committed violations under multiple federal and state statutes, including the False Claims Act and the Fair Labor Standards Act.
Tenet successfully argued that the case should go to arbitration.
Arbitrator Mary Beth Kelly, though, ruled in December 2020 that the vast majority of the complaints compiled against the two physicians in the external investigation were not verified or supported and that Tenet and DMC had retaliated against Dr. Kaki and Dr. Elder.
For that harm, Ms. Kelly awarded each clinician $1 million, according to the final ruling shared in an interview.
In addition, she awarded Dr. Kaki $2.3 million in back pay and 2 years of front pay (slightly more than $1 million). She awarded Dr. Elder $2.3 million in back pay and $2.1 million in front pay for 4 years, noting that “his strong association with DMC may make it more difficult for him to successfully transition into the situation he enjoyed prior to termination and nonrenewal.”
The clinicians also were awarded legal fees of $623,816 and court costs of $110,673.
“Wholesale retrial”
To secure the award, Ms. Gordon had to seek a ruling from the U.S. District Court for Eastern Michigan. Tenet asked that court to overturn the arbitrator’s award and to keep it sealed from public view.
In his February ruling, Judge Arthur J. Tarnow wrote that Tenet and DMC “not only attempt to relitigate the legal issues, but also endeavor to introduce a factual counternarrative unmoored from the findings of the Arbitrator and including evidence which the Arbitrator specifically found inadmissible.
“By seeking a wholesale retrial of their case after forcing plaintiffs to arbitrate in the first place,” Tenet and DMC basically ignored the goal of arbitration, which is to relieve judicial congestion and provide a faster and cheaper alternative to litigation, he wrote.
Judge Tarnow also warned Tenet and DMC against taking too long to reinstate privileges for Dr. Kaki and Dr. Elder. If they “continue to delay the restoration of plaintiffs’ privileges in the hopes of a different result on appeal, they will be in violation of this Order,” said the judge.
Tenet, however, tried one more avenue to block the cardiologists from getting privileges, appealing to the Sixth Circuit, which again ordered the company to grant the 1-year privileges.
A version of this article first appeared on Medscape.com.









