User login
Update on Dermatology Reimbursement in 2024
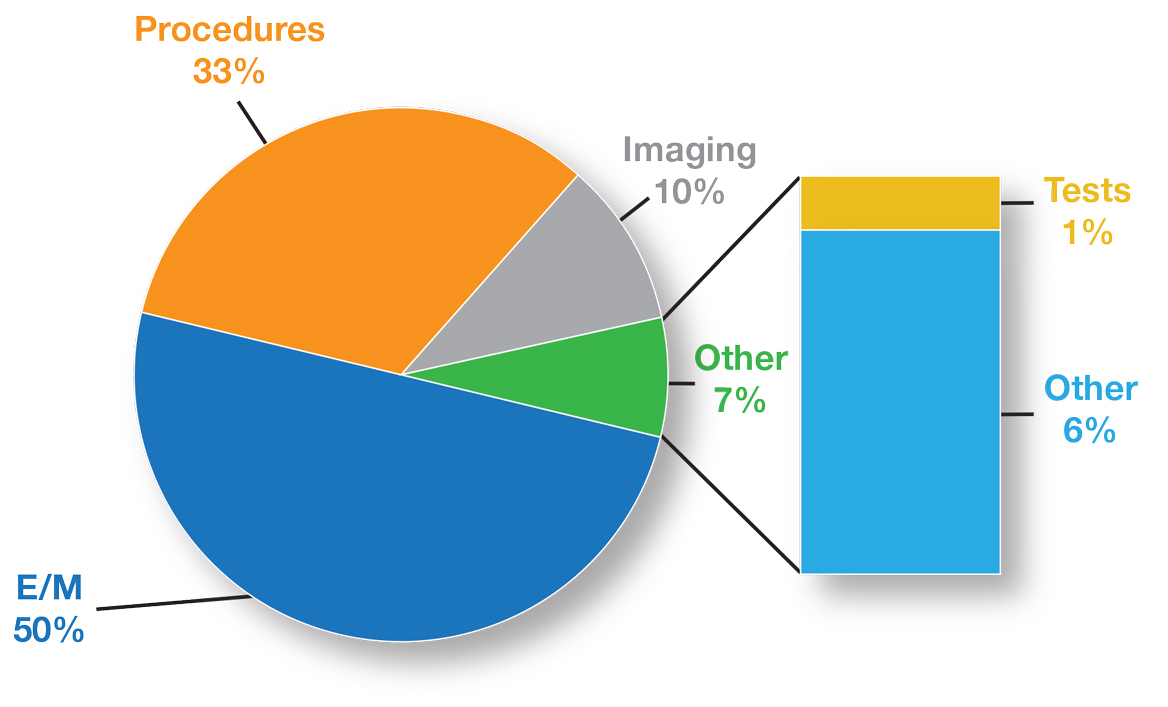
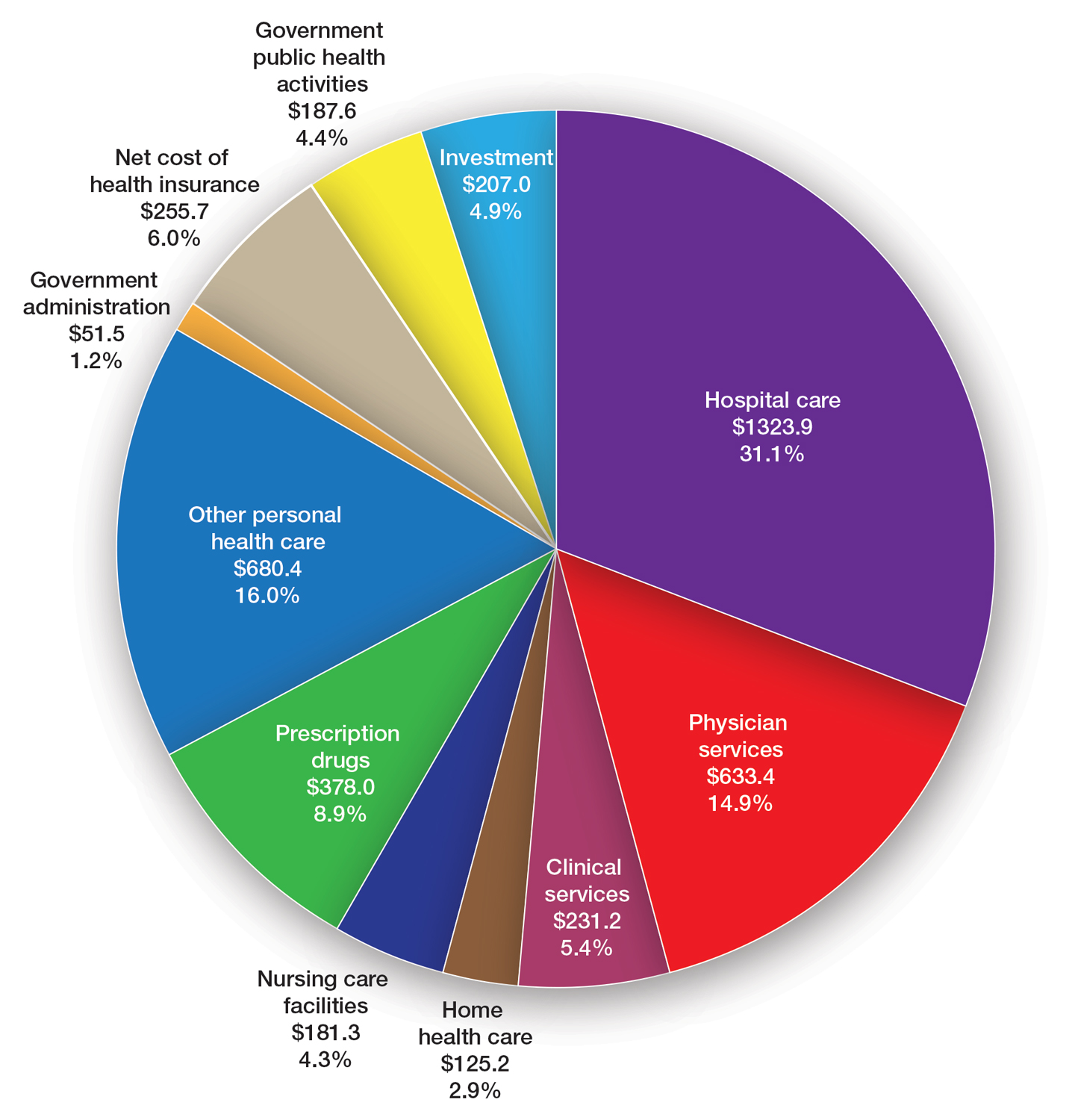
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4
The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
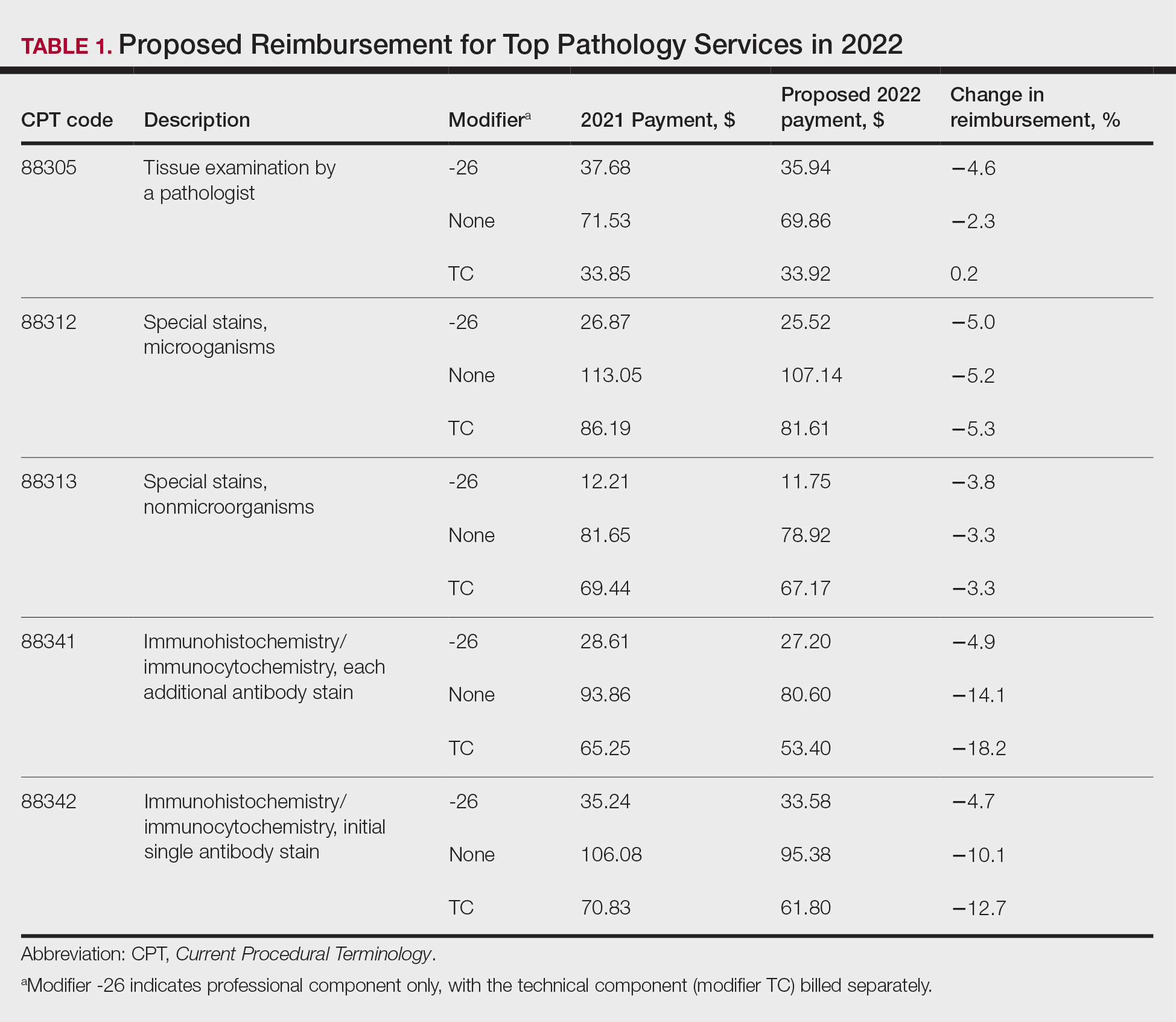
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee (privatepayer@aad.org) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee (privatepayer@aad.org) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee (privatepayer@aad.org) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
PRACTICE POINTS
- The proposed 2024 Medicare physician fee schedule published by the Centers for Medicare & Medicaid Services in July 2023 will negatively impact dermatology practices.
- The final regulations are expected in November 2023.
Advocacy Update: Ringing in 2023
New Year, New Codes: A Win-Win for Digital Pathology
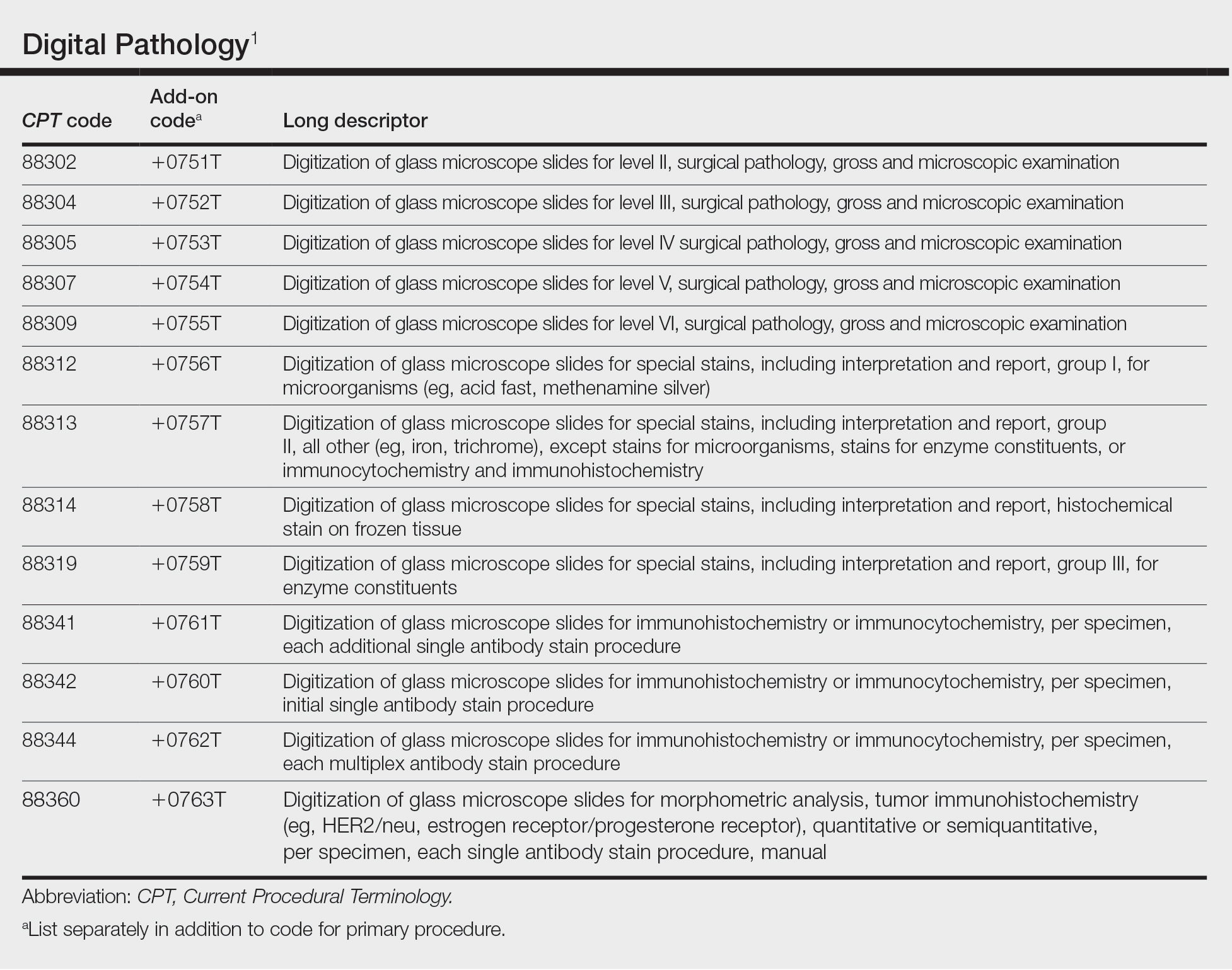
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.
Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
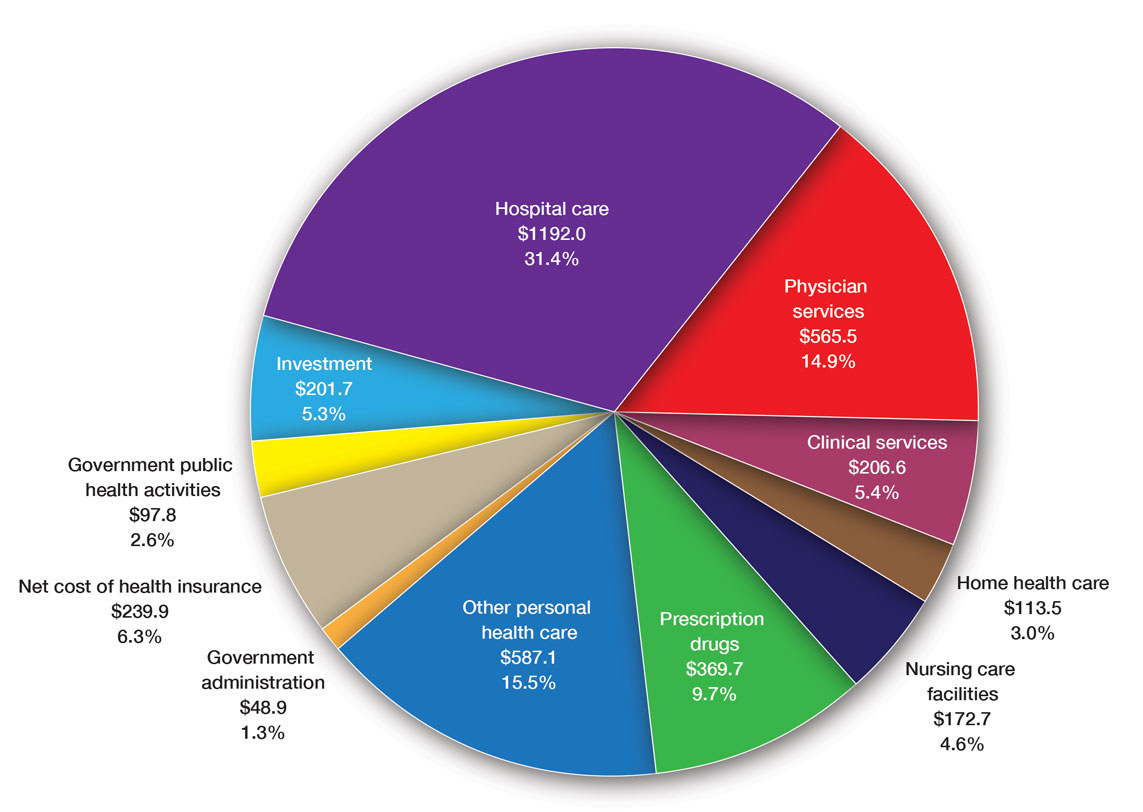
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2
Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
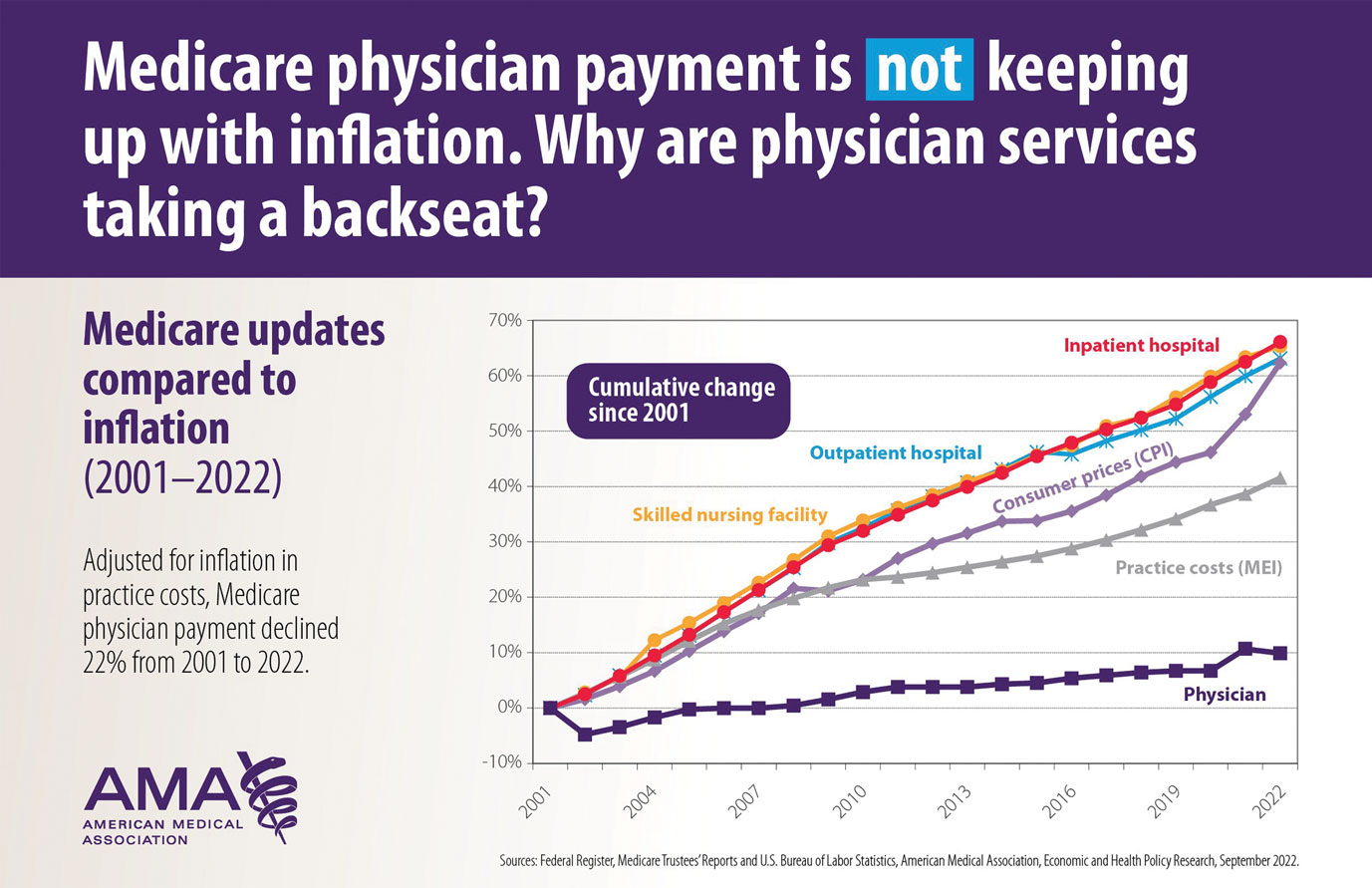
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5
The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.
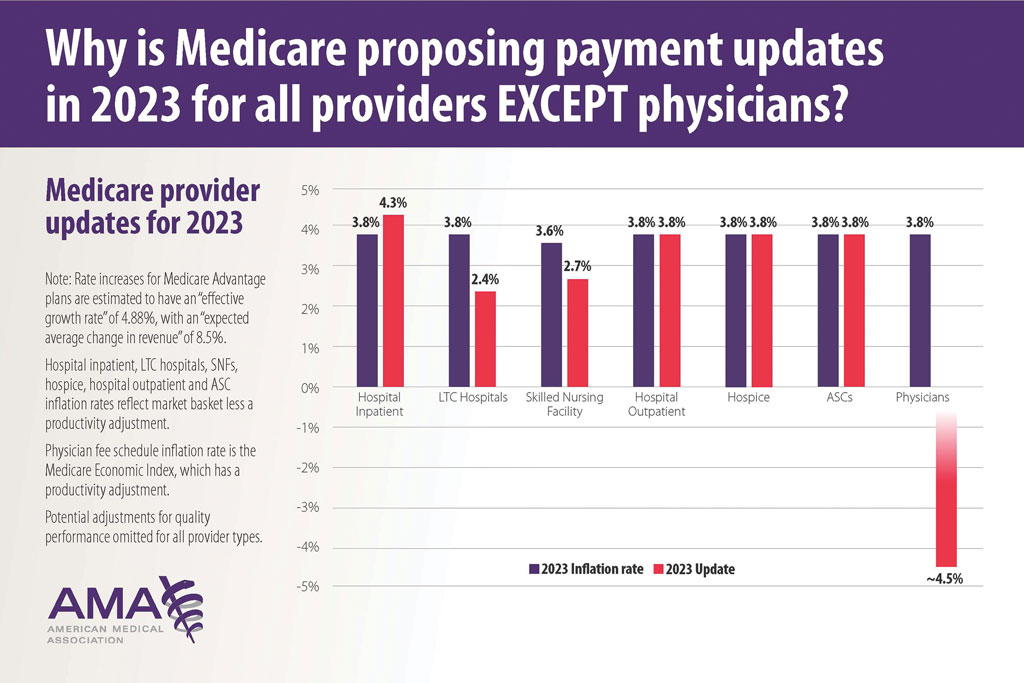
In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
New Year, New Codes: A Win-Win for Digital Pathology
In July 2022, the American Medical Association CPT (Current Procedural Terminology) Editorial Panel released 13 new digital pathology add-on Category III codes for 2023 that the College of American Pathologists successfully advocated for inclusion.1 These codes are for reporting additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis (Table). They go into effect on January 1, 2023.

Although there is no additional compensation with the new Category III codes, dermatopathology laboratories will be able to report when they have made a diagnosis using digital pathology. The new CPT codes will provide payers with data they need to directly understand the utilization and increased value of digital pathology, which will bring dermatopathology laboratories one step closer to receiving additional reimbursement for digital interpretation.
The adoption of digital pathology has been accelerating in the United States but still lags behind many European countries where reimbursement for digital pathology has been established for many years. Many of the barriers to digital pathology have improved—cloud storage is more affordable, scanners have a higher throughput, digital pathology platforms have improved, and the US Food and Drug Administration has granted approvals for digital pathology. Digital pathology allows for more efficient workflow, which results in increased productivity and a reduction in turnaround times. It also allows for a wide spectrum of clinical applications and more innovation as well as research and educational applications.
The new Category III codes cannot be reported solely for archival purposes (eg, after the Category I service has already been performed and reported), solely for educational purposes (eg, when services are not used for individual patient reporting), solely for developing a database for training or validation of artificial intelligence algorithms, and solely for clinical conference presentations (eg, tumor board interdisciplinary conferences).
The new codes are a major victory for the adoption and future compensation for digital pathology.
New Year, New Cuts: Proposed 2023 Medicare Policy and Payment Changes for Dermatologists
The United States Spent $3.8 Trillion on Health Care in 2019: Where Did It Go?—In 2019, approximately $3.8 trillion was spent on health care in the United States (Figure 1). Physician services accounted for approximately 15% of total health care spending.2

Medicare Payments for Physician Services—Medicare payments for physician services are determined by a relative value unit (RVU) multiplied by a conversion factor (CF). Relative value units were set up in 1992 by what is now the Centers for Medicare & Medicaid Services, and they calculated the time it took a physician to complete a task or RVU and multiplied it by $32.00 (CF).3
Thirty years later—in 2022—the CF is $34.61. If the CF had increased with inflation, it would be $59.00. If the Proposed Rule is adopted, the 2023 fee schedule payment formula would decrease by 4.4% (to $33.08) relative to that of the 2022 fee schedule ($34.61), which is a decrease of 8.2% since 2019 ($36.04). This decrease is due to expiration of the 3% increase to Medicare fee schedule payments for 2022 required by the Protecting Medicare and American Farmers from Sequester Cuts Act and the required budget neutrality adjustment required by changes in RVUs. Medicare physician payment has declined 22% from 2001 to 2022 (Figure 2).4,5

The adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index; (2) expenditure target “performance adjustment”; and (3) miscellaneous adjustments, including those for “budget neutrality” required by law.
Medicare Physician Payments Compared With Other Provider Types and Inflation—The proposed Medicare physician payment policy is unsustainable for outpatient dermatologists. Practice overhead has increased markedly since 1992. Other service providers, such as those in skilled nursing facilities and hospitals (Figure 3), have received favorable payment increases compared with practice cost inflation and the Consumer Price Index.3-6 Flat reimbursement affects all physicians who accept insurance, as even private insurers base their reimbursement on Medicare.

In addition, there are other issues resulting in decreased physician payments when evaluation and management services are reported with same-day procedures using modifier −25 as well as preserving or finding alternative strategies for 10- and 90-day global period payments for medical procedures. When Medicare cuts physician payments, dermatologists find it difficult to own and operate their own practices, resulting in health market consolidation, limited competition, increased health care costs, limited patient access to care, and decreased quality of health care.
Medicare Payment Reform—Medicare payment reform is necessary to stop annual payment cuts and create a stable predictable payment system that ensures patient access to quality, value-based care. Medicare physician payment reform needs to happen at a national level. The American Academy of Dermatology Association (AADA) is working with the House of Medicine and the medical specialty community to develop specific proposals, such as “Characteristics of a Rational Medicare Physician Payment System,” to reform Medicare’s payment system.7 Advocacy groups, including the AADA, have been working to mitigate the proposed 2023 cuts by engaging with Congress and urging them to act before these changes go into effect on January 1, 2023.
Make Advocacy Your New Year’s Resolution: AADA’s Top Advocacy Priorities
The AADA’s top priority is Medicare payment policies.3 In addition, the AADA is working on drug access and cost by cutting the bureaucratic red tape caused by prior authorization (PA) and step therapy policies. The AADA collaborates with manufacturers, the health care community, policymakers, private payers, pharmacists, pharmacy benefit managers, and patients to minimize and/or eliminate barriers that patients face in accessing needed medications. Specifically, the AADA advocates for legislation that limits obstacles associated with health insurance step therapy requirements, streamlines PA, and prohibits mid-year formulary changes.8
Step therapy requires that patients first try a medication specified by the insurance company; the therapy must fail before the patient is placed on the medication originally prescribed by the provider. Regarding PA, the AADA tries to ensure that determinations are standardized, requires the speed of determinations to be quantified and minimized, and ensures that PA and appeals policies do not unduly burden physicians or patients in accessing optimal drug therapy.8
Another advocacy priority is telehealth. The AADA is advocating for legislation on expansion of telehealth in underserved areas and modifications to state licensure requirements, liability issues, and reimbursement for store-and-forward technology. The AADA is involved in protecting scope of practice, truth in advertising, and access to specialty care, as well as monitoring legislation and regulation concerning the potential environmental impact of sunscreen ingredients, indoor tanning restrictions, and skin cancer prevention.8
Advocacy Matters and Makes a Difference—It is important to learn about and support advocacy priorities and efforts and join forces to protect your practice. The AADA advocacy priorities are to protect the value of dermatology services, mobilize dermatologists for political action, ensure dermatologists can participate in new payment models, and strengthen the profession.9 Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves. All of us are in it together, and a collaborative collective voice can make a difference. Take action, join the AADA, and contact Congress today to stop Medicare payment cuts (https://takeaction.aad.org/).
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
- Kaplan KJ. AMA announces new add-on digital pathology codes—no reimbursement (yet). July 18, 2022. Accessed October 19, 2022. https://tissuepathology.com/2022/07/18/ama-announces-new-add-on-digital-pathology-codes-no-reimbursement-yet/
- Centers for Medicare & Medicaid Services. National Health Expenditure Data: NHE fact sheet. Published April 2020. Accessed November 21, 2022. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Houghton V. Ask the expert (Dr. Mark Kaufmann): fighting for fair Medicare reimbursement. Dermatology World. October 2022. Accessed November 21, 2022. https://digitaleditions.walsworth.com/article/Advocacy+News/4355162/763056/article.html
- Federal Register, Medicare Trustees’ Reports and U.S. Bureau of Labor Statistics, AMA, Economic and Health Policy Research. September 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Current Medicare payment system on unsustainable path: contact Congress. September 30, 2022. Accessed November 21, 2022. https://www.ama-assn.org/practice-management/medicare-medicaid/current-medicare-payment-system-unsustainable-path-contact
- U.S. Bureau of Labor Statistics, American Medical Association, Economic and Health Policy Research, February 2022. Accessed November 21, 2022. https://www.ama-assn.org/system/files/key-measures-medicare-economic-index-chart.pdf
- American Medical Association. Characteristics of a rational Medicare payment system. Accessed November 22, 2022. https://www.ama-assn.org/system/files/characteristics-rational-medicare-payment-principles-signatories.pdf
- Ensuring patient access to effective and affordable treatments remains a top priority for the AAD. Dermatology Practice Management. June 2020. Accessed November 21, 2022. https://dermatologypracticemanagement.com/issues/2020/june-2020-vol-1-no-1/11-supporting-access-to-treatment-exceptional-customer-experience-innovation-and-growth-a-conversation-with-sumner-madden
- Marteja L. Advocacy: when, where, and how for dermatologists. The Dermatologist. September 2021. Accessed November 21, 2022. https://www.hmpgloballearningnetwork.com/site/thederm/cover-story/advocacy-when-where-and-how-dermatologists
Practice Points
- New digital pathology codes proposed by the American Medical Association can be used starting January 1, 2023.
- A proposed 2023 fee schedule negatively impacting dermatology practices was published by the Centers for Medicare & Medicaid Services in July 2022.
- Advocacy involvement provides a collaborative collective voice for our specialty to help our patients improve their care.
Advocacy Update: Is Your Practice Equipped to Handle Looming Changes in Dermatopathology?
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
The proposed 2022 Medicare physician fee schedule and quality payment program (QPP) regulations were released on July 13, 2021.1 Final regulations are expected to be released on or around November 1, 2021, but they may be delayed. Multiple national medical organizations, including the College of American Pathologists (CAP), the American Society of Dermatopathology, the American Academy of Dermatology Association (AADA), and the American Medical Association (AMA) Physicians’ Grassroots Network all work together to engage with the Centers for Medicare & Medicaid Services (CMS) to influence these regulations. Stated advocacy priorities include protecting the value of dermatopathology services, mobilizing dermatopathologists for political action, ensuring dermatopathologists can participate in new payment models, strengthening the profession with advocacy on a state level, and conducting socioeconomic research. Is your practice aware and prepared to handle the changes coming in 2022?
The recent revisions and revaluations of the outpatient evaluation and management (E/M) codes2 resulted in a considerable redistribution of Medicare dollars in 2021, negatively impacting dermatopathologists and other specialties and services due to budget neutrality required by law (Figure). Important steps were taken to mitigate the 2021 Medicare cuts for all non–office-based dermatopathology services (eg, pathology, surgical services, emergency department).1,3 Direct engagement by the CAP, American Society of Dermatopathology, and AADA, along with the AMA Physicians’ Grassroots Network resulted in legislative action on December 27, 2020, which directed Medicare to make a 3.75% positive adjustment to the 2021 physician payments. Additionally, the CMS updated the 2021 physician conversion factor to $34.8931, a 3.3% reduction from the 2020 conversion factor rather than $32.41, or a 10.20% decrease. The 2% payment adjustment (sequestration) through December 21, 2021, also was suspended, and Congress and the Biden administration mandated delayed implementation of the inherent complexity add-on code for E/M services (G2211) until 2024.1,3
Threat of Medicare Cuts in 2022
Based on dermatopathology utilization data, the overall impact on reimbursement for 2022 represents an approximately 5% decrease from 2021 dermatopathology payments (Table 1).1,4 This represents a 3.75% cut from revaluation of E/M services, and a 1% cut due to changes in practice expense pricing. The estimated change in reimbursement for independent laboratories is a 6% decrease. Advocacy groups have been working to mitigate the 2022 cuts by engaging with Congress and urging them to act before these changes go into effect next year. Keep in mind that approximately half of all pathology Current Procedural Terminology (CPT) codes have been targeted for evaluation by the CMS since 2006.1,4
The current clinical pathology consultation services (CPT codes 80500 and 80502) previously were identified as potentially misvalued for review by the AMA Relative Value Scale Update Committee’s (RUC’s) relativity assessment workgroup.4 Consequently, the CAP worked with the AMA’s CPT Editorial Panel to delete codes 80500 and 80502, as well as to modernize and create the 4 new clinical pathology consultation codes: 80XX0, 80XX1, 80XX2, and 80XX3. Then the CAP worked with the RUC to develop physician work and practice expense values for the new clinical pathology consultation codes. Once the fee schedule is finalized, pathologists can begin using the new codes to bill these services in 2022 (Table 2).4
According to CPT, clinical pathology consultation services may be reported when the following criteria have been met: (1) the pathologist renders a clinical pathology consultation at the request of a physician or qualified health care professional at the same or another institution; (2) the pathology clinical consultation request relating to pathology and laboratory findings or other relevant clinical or diagnostic information requiring additional medical interpretative judgment is made; and (3) these codes are not reported in conjunction with codes 88321, 88323, and 88325.4
Proposed 2022 Medicare QPP Requirements
On July 13, 2021, the CMS also published its proposed 2022 QPP proposals that will take effect next year.4 According to the proposed regulation, nearly all dermatopathologists will be required to participate in Medicare’s QPP, either through advanced alternative payment models (APMs) or the Merit-based Incentive Payment System (MIPS). The CAP has long advocated for reducing MIPS reporting burdens for dermatopathologists. In this regulation, the CMS is proposing key program changes that move the program forward but also introduce additional complexities; for example, the CMS will move forward with a new participation pathway called MIPS Value Pathways (MVPs). The CMS proposed 7 specific MVPs that align with certain clinical topics; however, it will not implement these MVPs until the 2023 MIPS performance period.
In 2022, dermatopathologists who are eligible for MIPS will have to take action to avoid penalties that reduce future Medicare Part B payments for their services. Performance in MIPS in 2022 affects Medicare Part B payments in 2024 by an increase of 9% to a decrease of 9%.
In its proposed 2022 QPP regulations, the CMS proposed an increase of the performance threshold from 60 MIPS points to 75 MIPS points. It also proposed an increase of the exceptional Performance Threshold from 85 MIPS points to 89 MIPS points.
The CMS also proposed notable scoring changes for quality measures, including removing the 3-point floor for measures that can be scored against a benchmark. These measures would receive 1 to 10 points. Measures without a benchmark or that do not meet case requirements would earn 0 points, with an exception for small practices. The CMS also proposed removing bonus points for reporting additional outcomes and high-priority measures beyond the 1 that is required, as well as establishing a 5-point floor for the first 2 performance periods for new measures, which is in line with the CAP’s advocacy.
The Pathology Specialty Measure Set will remain the same as the 2021 set containing 6 quality measures, including the AADA-stewarded quality measure #440 (skin cancer: biopsy reporting time—pathologist to clinician). Although the CAP recognizes the importance of prompt turnaround of biopsy reports, it also is working with the CMS and the AADA to mitigate the operational challenges dermatopathologists encounter when using this measure.
Due to advocacy from the CAP, the CMS included a CAP-proposed improvement activity on implementation of a laboratory preparedness plan to support continued or expanded patient care during the COVID-19 pandemic or another public health emergency. This plan should address how the laboratory would maintain or expand access to improve beneficiary health outcomes and reduce health care disparities.
The CAP has actively worked with the CMS to demonstrate the need for more appropriate and alternative measures and improvement activities so that pathologists can more fully participate in MIPS.
Alternative Payment Models—For those dermatopathologists who practice in an APM, the proposed 2022 QPP makes minimal changes to the advanced APM track while adding transition time for accountable care organizations in the Medicare Shared Savings Program to report on certain quality measures and increasing flexibility related to the program’s quality performance standard.
Cures Act 2021: To Do No Harm
The 21st Century Cures Act (Cures Act) was signed into federal law in 2016. The Office of the National Coordinator for Health Information Technology (ONC) laid the groundwork for patients to have easier access to and control of their health information.5 The ONC’s final rule, which went into effect on April 5, 2021, requires that all providers make their office notes, laboratory results, and other diagnostic reports (including dermatopathology reports) available to patients as soon as the physician’s office receives an electronic copy. Penalty for noncompliance has not been determined.
There are information-blocking exceptions, but delaying access to a patient’s report so that a provider can review the result before the patient receives it is not considered an exception.6 The exceptions are situational and must be evaluated by the referring clinician or their employer. Documentation of the exception is critical. The specific facts and circumstances associated with your decision to use an exception will be important to include in your documentation. Information blocking necessary to prevent “harm” to a patient or another person requires a reasonable belief that the practice will substantially reduce the risk of harm.6
The AMA passed a resolution in June 2021 calling for changes to this rule to allow for a delay of pathology results, advocating to the Office for Civil Rights to revise the harm exception to include psychological distress.6 In August 2021, the AADA met with senior officials at the ONC also asking to revise its definition of harm, sharing examples of emotional strain that resulted from receiving results without clinical context.7 California enacted a law requiring a delay before a patient receives the result of a malignant diagnosis, giving the clinician time to contact the patient before they see their report.8
The Cures Act requirements are about patients accessing their health care information. Always consider what is best for the patient and ensure that your policies and procedures reflect this.5
Final Thoughts
It is important to learn and support advocacy priorities and efforts and to join forces to protect your practice. Physician advocacy is no longer an elective pursuit. We need to be involved and engaged through our medical societies to help patients, communities, and ourselves.
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2022 Medicare Physician Fee Schedule Proposed Rule. Published July 13, 2021. Accessed October 22, 2021. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2022-medicare-physician-fee-schedule-proposed-rule
- Healthcare spending and the Medicare program. Medicare Payment Advisory Commission; July 2020. Accessed October 25, 2021.http://www.medpac.gov/docs/default-source/data-book/july2020_databook_entirereport_sec.pdf
- Frieden J. 2021 Medicare fee schedule includes 10.2% cut in conversion factor. MedPage Today website. Published December 2, 2020. Accessed October 22, 2021. https://www.medpagetoday.com/practicemanagement/reimbursement/89970
- Advocacy. College of American Pathologists website. Accessed October 13, 2021. https://www.cap.org/advocacy
- ONC’s Cures Act Final Rule. The Office of the National Coordinator for Health Information Technology website. Accessed October 13, 2021. https://www.healthit.gov/curesrule/
- Nelson H. Delegates call AMA to advocate for provider info-blocking flexibility. Published June 18, 2021. Accessed October 13, 2021. https://ehrintelligence.com/news/delegates-call-ama-to-advocate-for-provider-info-blocking-flexibility
- Rosamilia LL. Immediate Pathology report release to patients—is the 21st Century Cures Act worse than the disease? American Academy of Dermatology website. Published August 25, 2021. Accessed October 22, 2021. https://www.aad.org/dw/dw-insights-and-inquiries/archive/2021/cures-act-immediate-pathology-report-release-to-patients
- Purington K, Alfreds ST, Pritts J, et al; The National Academy for State Health Policy. Electronic release of clinical laboratory results: a review of state and federal policy. Published January 2010. Accessed October 13, 2021. https://www.nashp.org/wp-content/uploads/2010/02/ElectronicLabResultsExchangePolicy.pdf
Practice Points
- A proposed 2022 fee schedule negatively impacting dermatopathology practices has been published by the Centers for Medicare & Medicaid Services (CMS) in July 2021.
- New pathology consultation codes with new payment rates proposed by CMS can be used starting January 1, 2022.
- The 21st Century Cures Act Final Rule has information blocking provisions.
Oral Bowenoid Papulosis
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.
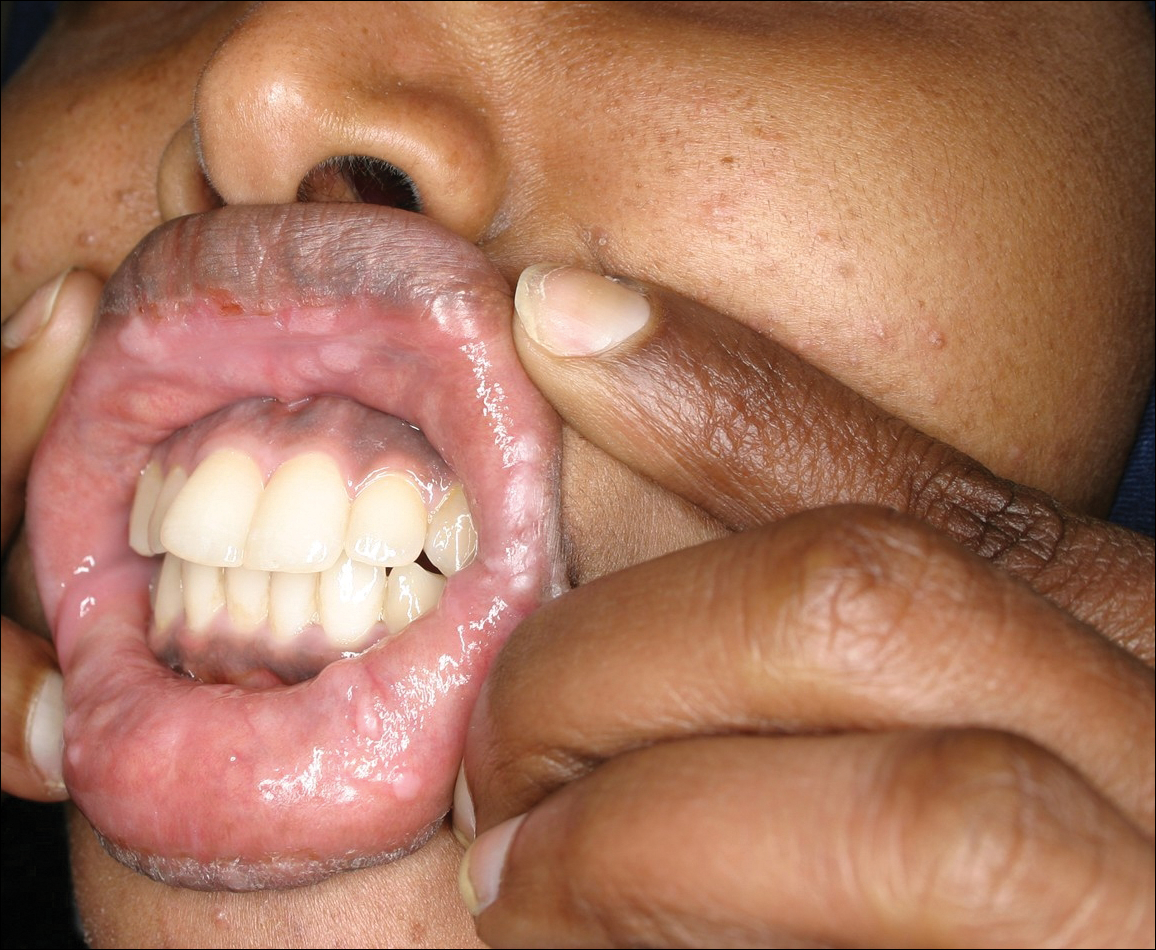
Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.
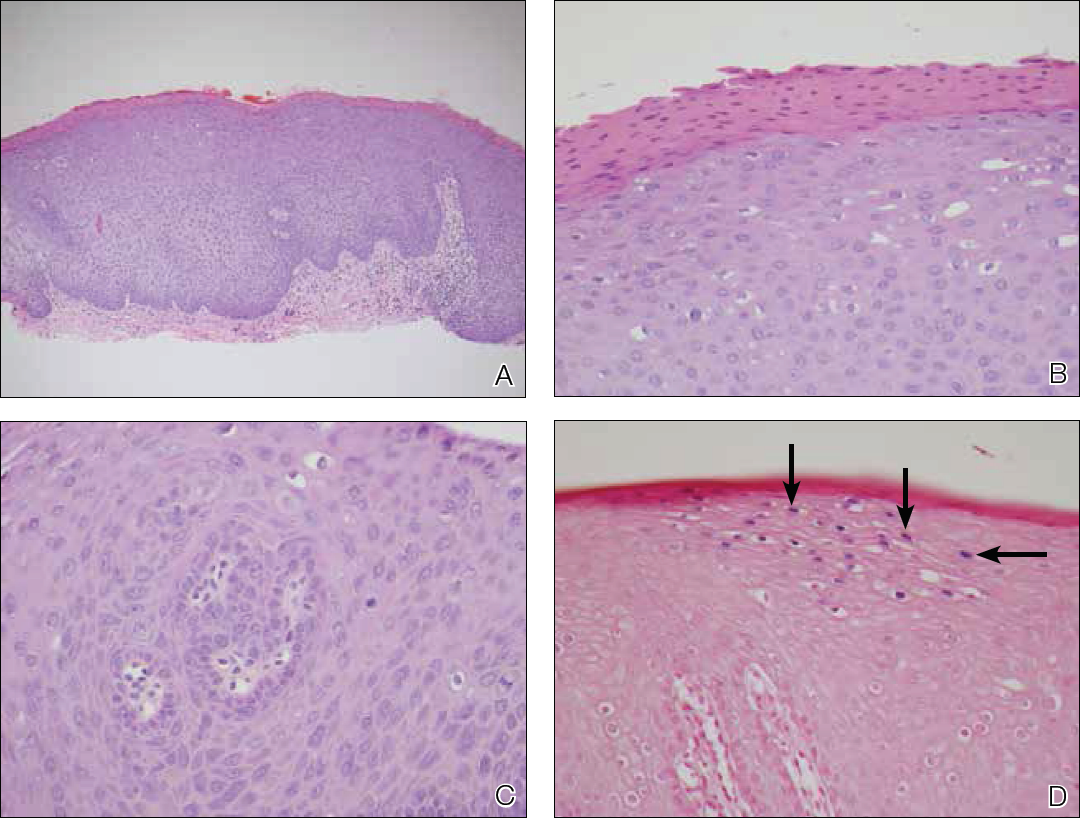
The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.

Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.

The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
To the Editor:
A 22-year-old Somali woman presented to our institution with oral lesions of 2 years’ duration. The lesions started as small papules in the corners of the mouth that gradually continued to spread to the mucosal lips and gums. The lesions did not drain any material. The patient reported that they were not painful and had not regressed. She was concerned about the cosmetic appearance of the lesions. The patient believed the lesions had developed from working in a chicken factory and was concerned that they appeared possibly due to contact with a substance in the factory. Additionally, she noted that her voice had become hoarse. She was otherwise healthy and denied any sexual contact or ever having a blood transfusion.
Physical examination revealed 10 to 15 flesh-colored papules measuring 2 to 3 mm in diameter on the vermilion, mucosal surfaces of the lips, and upper and lower gingivae (Figure 1). No lesions were seen on the hard and soft palate, tongue, buccal mucosa, or posterior pharynx.

Skin biopsy of the left lower mucosal lip revealed parakeratosis, acanthosis, superficial koilocytes, and atypical keratinocytes with frequent mitoses (Figures 2A–2C). In situ hybridization testing for human papillomavirus (HPV) was negative for low-risk types 6 and 11 but positive for high-risk types 16 and 18 (Figure 2D). Laboratory investigations including complete blood cell count, electrolyte panel, and liver function studies were normal, and serum was negative for syphilis and human immunodeficiency virus antibodies.

The combined clinical and histologic findings were diagnostic of oral bowenoid papulosis. Gynecologic evaluation showed that the patient had undergone female circumcision, and she had a normal Papanicolaou test. The patient was referred to both the ear, nose, and throat clinic as well as the dermatologic surgery department to discuss treatment options, but she was lost to follow-up.
Bowenoid papulosis is triggered by HPV infection and manifests clinically as solitary or multiple verrucous papules and plaques that are usually located on the genitalia.1 Only a few cases of bowenoid papulosis have been reported in the oral cavity.1-5 Because this disease is sexually transmitted, the mean age of onset of bowenoid papulosis is 31 years.2 There is a small risk (2%–3%) of developing invasive carcinoma in bowenoid papulosis.1-3,6 Most lesions are associated with HPV type 16; however, bowenoid papulosis also has been associated with HPV types 18, 31, 32, 35, and 39.2
Some investigators consider bowenoid papulosis and Bowen disease (a type of squamous cell carcinoma [SCC] in situ) to be histologically identical1,6; however, some histologic differences have been reported.1-3,6 Bowenoid papulosis has more dilated and tortuous dermal capillaries and less atypia and dyskeratosis than Bowen disease.1,6 In contrast to bowenoid papulosis, Bowen disease is characterized clinically as well-defined scaly plaques on sun-exposed areas of the skin in older adults. Invasive SCC can be seen in 5% of skin lesions and 30% of penile lesions associated with Bowen disease.2 Risk factors for Bowen disease include sun exposure; arsenic poisoning; and infection with HPV types 2, 16, 18, 31, 33, 52, and 67.1,6
Oral bowenoid papulosis is rare. A PubMed search of articles indexed for MEDLINE using the term oral bowenoid papulosis yielded 7 additional cases, which are summarized in the Table. In 1987 Lookingbill et al2 described one of the first reported cases of oral disease in a 33-year-old immunosuppressed man receiving prednisone therapy for systemic lupus erythematosus who had both mouth and genital lesions. All lesions were positive for HPV type 16. The patient subsequently developed SCC of the tongue.2

The risk for progression of oral bowenoid papulosis to invasive SCC is not known. Our search yielded only 1 case of this occurrence.2
Two of 3 cases of solitary lip lesions in oral bowenoid papulosis were treated with surgical excision.1 Other treatment options include CO2 laser therapy, cryotherapy, 5-fluorouracil, bleomycin, intralesional interferon alfa, and imiquimod.1-3,5,6
Our case represents a rare report of oral bowenoid papulosis. Recognition of this unusual presentation is important for the diagnosis and management of this disease.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
- Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathogenetic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466-473.
- Lookingbill DP, Kreider JW, Howett MK, et al. Human papillomavirus type 16 in bowenoid papulosis, intraoral papillomas, and squamous cell carcinoma of the tongue. Arch Dermatol. 1987;123:363-368.
- Kratochvil FJ, Cioffi GA, Auclair PL, et al. Virus-associated dysplasia (bowenoid papulosis?) of the oral cavity. Oral Surg Oral Med Oral Pathol. 1989;68:312-316.
- Degener AM, Latino L, Pierangeli A, et al. Human papilloma virus-32-positive extragenital bowenoid papulosis in a HIV patient with typical genital bowenoid papulosis localization. Sex Transm Dis. 2004;31:619-622.
- Rinaggio J, Glick M, Lambert WC. Oral bowenoid papulosis in an HIV-positive male [published online October 14, 2005]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:328-332.
- Regezi JA, Dekker NP, Ramos DM, et al. Proliferation and invasion factors in HIV-associated dysplastic and nondysplastic oral warts and in oral squamous cell carcinoma: an immunohistochemical and RT-PCR evaluation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:724-731.
Practice Points
- Bowenoid papulosis is triggered by human papillomavirus infection and manifests clinically as solitary or multiple verrucous papules and plaques that usually are located on the genitalia.
- Oral bowenoid papulosis is rare, and recognition of this unusual presentation is important for the diagnosis and management of this disease.
Co-occurrence of Steatocystoma Multiplex, Eruptive Vellus Hair Cysts, and Trichofolliculomas
An association between steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) has been recognized. They are related conditions representing nevoid malformations of the pilosebaceous junctions1-10 that have similar clinical features but distinctive histologic features. Both conditions most commonly involve the anterior aspect of the chest. Six cases of a rare facial variant of SCM have been reported,11-16 3 involving lesions limited to the forehead.13-15 Two patients with a rare facial variant of EVHC also have been reported.17 The development of separate lesions of SCM and EVHC on the trunk can uncommonly occur.5,6,10 One case of SCM and EVHC on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. The simultaneous occurrence of multiple trichoblastomas, trichoepitheliomas, and SCM on the face and trunk has been reported in 1 case.18 Milia, SCM, and EVHC on the face and trunk have been reported in 1 family.4 A report of facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus also has occurred in 1 patient.19 Here, we report the simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead.
Case Report
A 37-year-old man had an increasing number of flesh-colored to yellow papules on the forehead that had been present since puberty. Although the lesions were asymptomatic, some had recently become tender, which led him to seek medical care. There was no history of trauma, burns, irradiation, or application of topical agents to the area or use of eyeglasses or goggles. The patient’s father had similar lesions limited to the forehead, which developed during adolescence.
On evaluation at our clinic, skin examination revealed 16 discrete, 0.3- to 1-cm, flesh-colored, yellow to blue, mobile, smooth papules, as well as flesh-colored papules with a central black punctum, on the forehead (Figure 1). Similar lesions were not present on the rest of the face; around the ears; or on the scalp, neck, chest, back, abdomen, genitalia, buttocks, palms, soles, axillae, arms, or legs. There were no nail abnormalities.
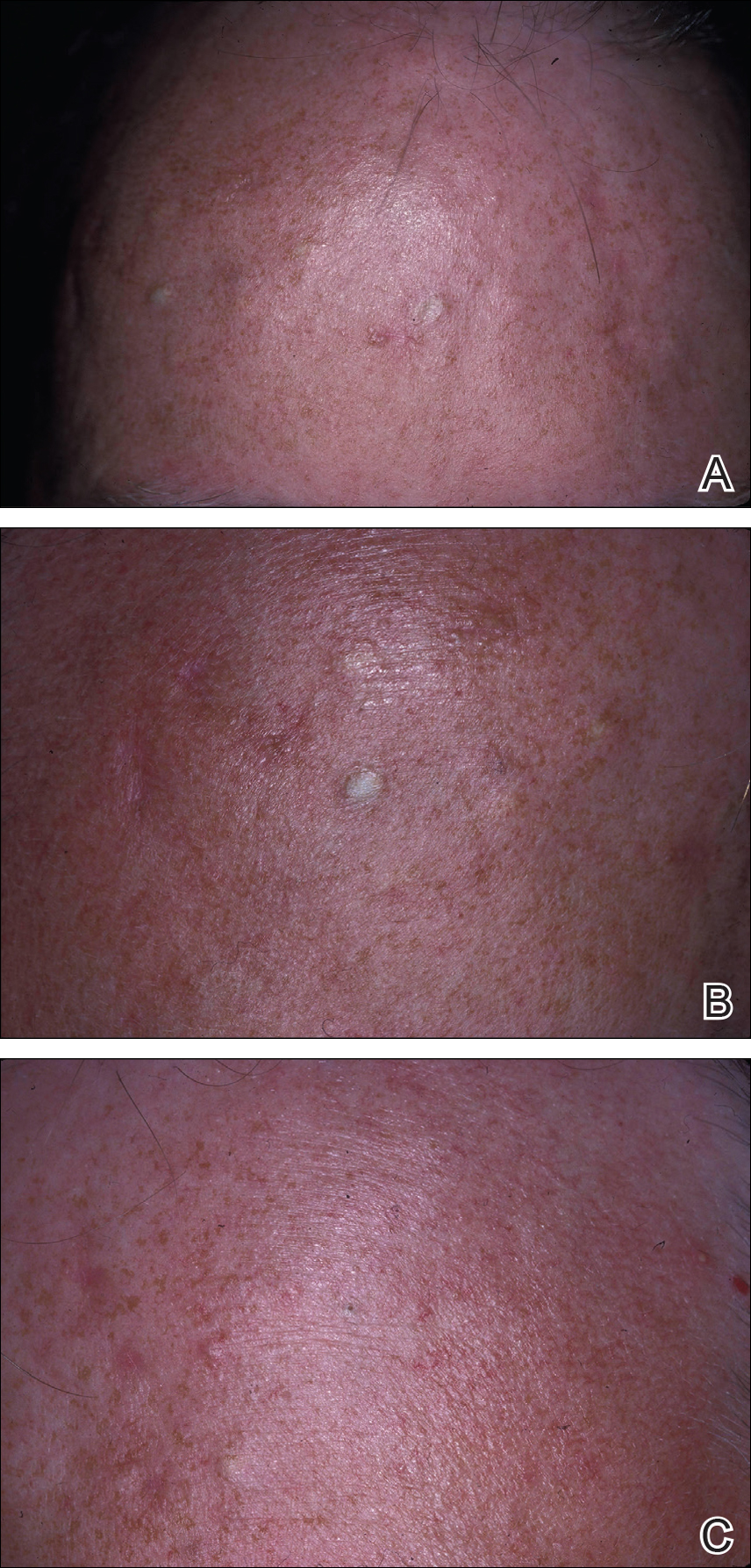
Multiple 3-, 4-, and 6-mm punch and excisional biopsies were performed to remove all 16 lesions on the forehead. Histologic examination revealed a collapsed cystic structure in the mid dermis in 10 lesions. The cysts were lined with a squamous epithelium without a granular layer but with an eosinophilic corrugated lining, and the cyst cavity contained scant homogeneous eosinophilic secretion. Mature sebaceous glands were adjacent to the outer portion of the cyst wall. These histologic findings were consistent with SCM (Figure 2).
In 3 lesions, histologic examination revealed a cystic structure lined by a few layers of stratified squamous epithelium in the mid dermis. The cyst cavity contained numerous small vellus hairs and laminated keratin. These histologic findings were consistent with EVHC (Figure 3).
In the other 3 lesions, histologic examination revealed a dilated central cystic cavity filled with laminated keratin in the mid dermis. Multiple small follicles arose from the cysts and showed differentiation toward germinative epithelium. The surrounding stroma was fibrotic and contained a patchy lymphocytic infiltrate. These histologic findings were consistent with trichofolliculomas (Figure 4).
Comment
Characteristics of SCM
Steatocystoma multiplex is an uncommon condition characterized by the formation of asymptomatic, 0.2- to 2-cm, yellow to flesh-colored, soft, mobile papules or nodules on the trunk, extremities, axillae, genitalia, and/or chest. The lesions contain a clear or opaque, oily, milky or yellow, odorless fluid and most commonly are located on the anterior aspect of the chest. The face is not a commonly involved site in this condition. Six cases of a rare facial variant of SCM have been reported,11-16 with lesions limited to the forehead in 3 cases.13-15
In 1937, Mount20 credited Bozellini for describing the first case, though 3 cases reported in the late 1800s probably were SCM.21 In 1899, Pringle22 coined the term steatocystoma multiplex for this condition. It can be sporadic or have an autosomal-dominant inheritance pattern. Steatocystoma multiplex can occur at any age, though lesions develop most frequently in adolescence or young adulthood. There is no sex predilection.
Steatocystoma multiplex with pachyonychia congenita has been reported in a familial case.23 Other findings reported in patients with SCM include ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf and hypertrophic lichen planus, hidradenitis suppurativa, hypotrichosis, multiple keratoacanthomas, and rheumatoid arthritis.12,24-26
Steatocystoma multiplex is a cyst lined by stratified squamous epithelium without a granular layer but with a thick eosinophilic cuticle. Mature sebaceous lobules are closely associated with the cyst wall. Steatocystoma multiplex arises from the sebaceous duct because the lining of the lumen is composed of undulating eosinophilic cuticle.
Characteristics of EVHCs
Eruptive vellus hair cysts, which were first described by Esterly et al,27 can occur at any age but develop most frequently in adolescents or young adults. Sometimes the lesions are congenital or appear in childhood. There is no sex predilection. They can be sporadic or have an autosomal-dominant inheritance pattern.
Eruptive vellus hair cysts are asymptomatic, 1- to 2-mm, smooth, crusted, or umbilicated papules on the chest or arms and legs. Eruptive vellus hair cysts most commonly involve the anterior aspect of the chest. The lesions are flesh-colored to yellow, though they have a slate gray color in darker-skinned individuals. A rare facial variant has been reported in 2 patients of Asian descent.17
Eruptive vellus hair cysts are small cystic structures lined by a stratified squamous epithelium with a granular layer. The cyst cavity contains numerous small vellus hair shafts and laminated keratin. Eruptive vellus hair cysts originate from the infundibulum or less frequently the isthmus or infundibular-isthmic junction of the hair follicle.
Characteristics of Trichofolliculomas
Trichofolliculomas are solitary, 3- to 5-mm, flesh-colored papules that occur on the face. They are highly differentiated, benign, neoplastic proliferations of an actively trichogenic epithelium, with structural components reflecting all portions of the pilosebaceous unit. Trichofolliculomas consist of a central dilated primary follicle contiguous with the surface epidermis embedded in a fibrous stroma. Multiple small secondary follicles with varying degrees of follicular differentiation arise from the primary follicle.
Co-occurrence of Lesions
An association between SCM and EVHC has been recognized.5-10 Steatocystoma multiplex and EVHC have similar clinical features but distinctive histologic features. They also have a similar age of onset, location/appearance of lesions, and mode of inheritance. Steatocystoma multiplex and EVHC can be distinguished by immunohistochemical techniques: SCM shows expression of keratin 10 and keratin 17, whereas EVHCs express only keratin 17.28
Steatocystoma multiplex and EVHC have only rarely been reported to occur together on the trunk. One case of SCM and EVHC occurring on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. Milia, SCM, and EVHC on the face and trunk have been reported in 1 family,4 and facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus was reported in 1 patient.19 Although trichofolliculomas have not been reported to occur with SCM or EVHC, 2 related follicular neoplasms—trichoepitheliomas and trichoblastomas—have been reported to occur in association with SCM on the face and chest and around the ears in 1 case.18
Differential Diagnosis
The clinical differential diagnosis includes multiple epidermoid cysts, dermoid cysts, Gardner syndrome, sebaceous adenomas, Muir-Torre syndrome, syringomas, milia, leiomyomas, lipomas, acneiform folliculitis, multiple familial and nonfamilial trichoepitheliomas, cylindromas, and angiofibromas.3,29
Conclusion
Our patient represents a rare case of simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead. The patient had multiple neoplasms involving differentiation toward various regions of the pilosebaceous unit. This case gives further support to the hypothesis that these benign follicular neoplasms are closely related but are distinct conditions within the spectrum of the same disease process. They represent nevoid malformations of the pilosebaceous unit that can be sporadic or inherited in an autosomal-dominant pattern. Pure types of these lesions may represent one end of the spectrum, but in some patients, there are overlapping features or hybrids of each condition. Several biopsies from patients with multiple lesions should be performed to establish an accurate diagnosis.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19:570-571.
- Sanchez Yus E, Requena L. Eruptive vellus hair cyst and steatocystoma multiplex. Am J Dermatopathol. 1990;12:536-537.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Kiene P, Hauschild A, Christophers E. Eruptive vellus hair cysts and steatocystoma multiplex: variants of one entity? Br J Dermatol. 1996;134:365-367.
- Hurlimann AF, Panizzon RG, Burg G. Eruptive vellus hair cyst and steatocystoma multiplex: hybrid cysts. Dermatology. 1996;192:64-66.
- Sexton M, Murdock DK. Eruptive vellus hair cysts: a follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1989;11:364-368.
- Sanchez-Yus E, Aguilar-Martinez A, Cristobal-Gil MC, et al. Eruptive vellus hair cyst and steatocystoma multiplex: two related conditions? J Cutan Pathol. 1988;15:40-42.
- Ahn SK, Chung J, Lee WS, et al. Hybrid cysts showing alternate combination of eruptive vellus hair cyst, steatocystoma multiplex, and epidermoid cyst, and an association among the three conditions. Am J Dermatopathol. 1996;18:645-649.
- Ahn SK, Hwang SM, Lee SH, et al. Steatocystoma multiplex localized only in the face. Int J Dermatol. 1997;36:372-373.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Hansen KK, Troy JL, Fairley JA. Multiple papules of the scalp and forehead. steatocystoma multiplex (facial papular variant). Arch Dermatol. 1995;131:835-838.
- Nishimura M, Kohda H, Urabe A. Steatocystoma multiplex: a facial popular variant. Arch Dermatol. 1986;122:205-207.
- Requena L, Martin L, Renedo G, et al. A facial variant of steatocystoma multiplex. Cutis. 1993;51:449-452.
- Holmes R, Black MM. Steatocystoma multiplex with unusually prominent cysts on the face. Br J Dermatol. 1980;102:711-713.
- Kumakiri M, Takashima I, Iju M, et al. Eruptive vellus hair cysts: a facial variant. J Am Acad Dermatol. 1982;7:461-467.
- Gianotti R, Cavicchini S, Alessi E. Simultaneous occurrence of multiple trichoblastomas and steatocystoma multiplex. Am J Dermatopathol. 1997;19:294-298.
- Sardana K, Sharma RC, Jain A, et al. Facial steatocystoma multiplex associated with pilar cyst and bilateral preauricular sinus. J Dermatol. 2002;29:157-159.
- Mount LB. Steatocystoma multiplex. Arch Dermatol Syphilol. 1937;36:31-39.
- Dubreuilh W, Auche B. Kystes grassieux sudoripares. Arch Clin de Bordeaux. 1896;5:387-391.
- Pringle JJ. A case of peculiar multiple sebaceous cysts (steatocystoma multiplex). Br J Dermatol. 1899;11:381-88.
- Vineyard WR, Scott RA. Steatocystoma multiplex with pachyonychia congenital: eight cases in four generations. Arch Dermatol. 1961;84:824-827.
- Contreras MA, Costello MJ. Steatocystoma multiplex with embryonal hair formation: case presentation and consideration of pathogenesis. AMA Arch Derm. 1957;76:720-725.
- Sohn D, Chin TC, Fellner MJ. Multiple keratoacanthomas associated with steatocystoma multiplex and rheumatoid arthritis: a case report. Arch Dermatol. 1980;116:913-915.
- Verbov J. Acrokeratosis verruciformis of Hopf with steatocystoma multiplex and hypertrophic lichen planus. Br J Dermatol. 1972;86:91-94.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Tomkova H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Feinstein A, Trau H, Movshovitz M, et al. Steatocystoma multiplex. Cutis. 1983;31:425-427.
An association between steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) has been recognized. They are related conditions representing nevoid malformations of the pilosebaceous junctions1-10 that have similar clinical features but distinctive histologic features. Both conditions most commonly involve the anterior aspect of the chest. Six cases of a rare facial variant of SCM have been reported,11-16 3 involving lesions limited to the forehead.13-15 Two patients with a rare facial variant of EVHC also have been reported.17 The development of separate lesions of SCM and EVHC on the trunk can uncommonly occur.5,6,10 One case of SCM and EVHC on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. The simultaneous occurrence of multiple trichoblastomas, trichoepitheliomas, and SCM on the face and trunk has been reported in 1 case.18 Milia, SCM, and EVHC on the face and trunk have been reported in 1 family.4 A report of facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus also has occurred in 1 patient.19 Here, we report the simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead.
Case Report
A 37-year-old man had an increasing number of flesh-colored to yellow papules on the forehead that had been present since puberty. Although the lesions were asymptomatic, some had recently become tender, which led him to seek medical care. There was no history of trauma, burns, irradiation, or application of topical agents to the area or use of eyeglasses or goggles. The patient’s father had similar lesions limited to the forehead, which developed during adolescence.
On evaluation at our clinic, skin examination revealed 16 discrete, 0.3- to 1-cm, flesh-colored, yellow to blue, mobile, smooth papules, as well as flesh-colored papules with a central black punctum, on the forehead (Figure 1). Similar lesions were not present on the rest of the face; around the ears; or on the scalp, neck, chest, back, abdomen, genitalia, buttocks, palms, soles, axillae, arms, or legs. There were no nail abnormalities.

Multiple 3-, 4-, and 6-mm punch and excisional biopsies were performed to remove all 16 lesions on the forehead. Histologic examination revealed a collapsed cystic structure in the mid dermis in 10 lesions. The cysts were lined with a squamous epithelium without a granular layer but with an eosinophilic corrugated lining, and the cyst cavity contained scant homogeneous eosinophilic secretion. Mature sebaceous glands were adjacent to the outer portion of the cyst wall. These histologic findings were consistent with SCM (Figure 2).

In 3 lesions, histologic examination revealed a cystic structure lined by a few layers of stratified squamous epithelium in the mid dermis. The cyst cavity contained numerous small vellus hairs and laminated keratin. These histologic findings were consistent with EVHC (Figure 3).

In the other 3 lesions, histologic examination revealed a dilated central cystic cavity filled with laminated keratin in the mid dermis. Multiple small follicles arose from the cysts and showed differentiation toward germinative epithelium. The surrounding stroma was fibrotic and contained a patchy lymphocytic infiltrate. These histologic findings were consistent with trichofolliculomas (Figure 4).

Comment
Characteristics of SCM
Steatocystoma multiplex is an uncommon condition characterized by the formation of asymptomatic, 0.2- to 2-cm, yellow to flesh-colored, soft, mobile papules or nodules on the trunk, extremities, axillae, genitalia, and/or chest. The lesions contain a clear or opaque, oily, milky or yellow, odorless fluid and most commonly are located on the anterior aspect of the chest. The face is not a commonly involved site in this condition. Six cases of a rare facial variant of SCM have been reported,11-16 with lesions limited to the forehead in 3 cases.13-15
In 1937, Mount20 credited Bozellini for describing the first case, though 3 cases reported in the late 1800s probably were SCM.21 In 1899, Pringle22 coined the term steatocystoma multiplex for this condition. It can be sporadic or have an autosomal-dominant inheritance pattern. Steatocystoma multiplex can occur at any age, though lesions develop most frequently in adolescence or young adulthood. There is no sex predilection.
Steatocystoma multiplex with pachyonychia congenita has been reported in a familial case.23 Other findings reported in patients with SCM include ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf and hypertrophic lichen planus, hidradenitis suppurativa, hypotrichosis, multiple keratoacanthomas, and rheumatoid arthritis.12,24-26
Steatocystoma multiplex is a cyst lined by stratified squamous epithelium without a granular layer but with a thick eosinophilic cuticle. Mature sebaceous lobules are closely associated with the cyst wall. Steatocystoma multiplex arises from the sebaceous duct because the lining of the lumen is composed of undulating eosinophilic cuticle.
Characteristics of EVHCs
Eruptive vellus hair cysts, which were first described by Esterly et al,27 can occur at any age but develop most frequently in adolescents or young adults. Sometimes the lesions are congenital or appear in childhood. There is no sex predilection. They can be sporadic or have an autosomal-dominant inheritance pattern.
Eruptive vellus hair cysts are asymptomatic, 1- to 2-mm, smooth, crusted, or umbilicated papules on the chest or arms and legs. Eruptive vellus hair cysts most commonly involve the anterior aspect of the chest. The lesions are flesh-colored to yellow, though they have a slate gray color in darker-skinned individuals. A rare facial variant has been reported in 2 patients of Asian descent.17
Eruptive vellus hair cysts are small cystic structures lined by a stratified squamous epithelium with a granular layer. The cyst cavity contains numerous small vellus hair shafts and laminated keratin. Eruptive vellus hair cysts originate from the infundibulum or less frequently the isthmus or infundibular-isthmic junction of the hair follicle.
Characteristics of Trichofolliculomas
Trichofolliculomas are solitary, 3- to 5-mm, flesh-colored papules that occur on the face. They are highly differentiated, benign, neoplastic proliferations of an actively trichogenic epithelium, with structural components reflecting all portions of the pilosebaceous unit. Trichofolliculomas consist of a central dilated primary follicle contiguous with the surface epidermis embedded in a fibrous stroma. Multiple small secondary follicles with varying degrees of follicular differentiation arise from the primary follicle.
Co-occurrence of Lesions
An association between SCM and EVHC has been recognized.5-10 Steatocystoma multiplex and EVHC have similar clinical features but distinctive histologic features. They also have a similar age of onset, location/appearance of lesions, and mode of inheritance. Steatocystoma multiplex and EVHC can be distinguished by immunohistochemical techniques: SCM shows expression of keratin 10 and keratin 17, whereas EVHCs express only keratin 17.28
Steatocystoma multiplex and EVHC have only rarely been reported to occur together on the trunk. One case of SCM and EVHC occurring on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. Milia, SCM, and EVHC on the face and trunk have been reported in 1 family,4 and facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus was reported in 1 patient.19 Although trichofolliculomas have not been reported to occur with SCM or EVHC, 2 related follicular neoplasms—trichoepitheliomas and trichoblastomas—have been reported to occur in association with SCM on the face and chest and around the ears in 1 case.18
Differential Diagnosis
The clinical differential diagnosis includes multiple epidermoid cysts, dermoid cysts, Gardner syndrome, sebaceous adenomas, Muir-Torre syndrome, syringomas, milia, leiomyomas, lipomas, acneiform folliculitis, multiple familial and nonfamilial trichoepitheliomas, cylindromas, and angiofibromas.3,29
Conclusion
Our patient represents a rare case of simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead. The patient had multiple neoplasms involving differentiation toward various regions of the pilosebaceous unit. This case gives further support to the hypothesis that these benign follicular neoplasms are closely related but are distinct conditions within the spectrum of the same disease process. They represent nevoid malformations of the pilosebaceous unit that can be sporadic or inherited in an autosomal-dominant pattern. Pure types of these lesions may represent one end of the spectrum, but in some patients, there are overlapping features or hybrids of each condition. Several biopsies from patients with multiple lesions should be performed to establish an accurate diagnosis.
An association between steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) has been recognized. They are related conditions representing nevoid malformations of the pilosebaceous junctions1-10 that have similar clinical features but distinctive histologic features. Both conditions most commonly involve the anterior aspect of the chest. Six cases of a rare facial variant of SCM have been reported,11-16 3 involving lesions limited to the forehead.13-15 Two patients with a rare facial variant of EVHC also have been reported.17 The development of separate lesions of SCM and EVHC on the trunk can uncommonly occur.5,6,10 One case of SCM and EVHC on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. The simultaneous occurrence of multiple trichoblastomas, trichoepitheliomas, and SCM on the face and trunk has been reported in 1 case.18 Milia, SCM, and EVHC on the face and trunk have been reported in 1 family.4 A report of facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus also has occurred in 1 patient.19 Here, we report the simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead.
Case Report
A 37-year-old man had an increasing number of flesh-colored to yellow papules on the forehead that had been present since puberty. Although the lesions were asymptomatic, some had recently become tender, which led him to seek medical care. There was no history of trauma, burns, irradiation, or application of topical agents to the area or use of eyeglasses or goggles. The patient’s father had similar lesions limited to the forehead, which developed during adolescence.
On evaluation at our clinic, skin examination revealed 16 discrete, 0.3- to 1-cm, flesh-colored, yellow to blue, mobile, smooth papules, as well as flesh-colored papules with a central black punctum, on the forehead (Figure 1). Similar lesions were not present on the rest of the face; around the ears; or on the scalp, neck, chest, back, abdomen, genitalia, buttocks, palms, soles, axillae, arms, or legs. There were no nail abnormalities.

Multiple 3-, 4-, and 6-mm punch and excisional biopsies were performed to remove all 16 lesions on the forehead. Histologic examination revealed a collapsed cystic structure in the mid dermis in 10 lesions. The cysts were lined with a squamous epithelium without a granular layer but with an eosinophilic corrugated lining, and the cyst cavity contained scant homogeneous eosinophilic secretion. Mature sebaceous glands were adjacent to the outer portion of the cyst wall. These histologic findings were consistent with SCM (Figure 2).

In 3 lesions, histologic examination revealed a cystic structure lined by a few layers of stratified squamous epithelium in the mid dermis. The cyst cavity contained numerous small vellus hairs and laminated keratin. These histologic findings were consistent with EVHC (Figure 3).

In the other 3 lesions, histologic examination revealed a dilated central cystic cavity filled with laminated keratin in the mid dermis. Multiple small follicles arose from the cysts and showed differentiation toward germinative epithelium. The surrounding stroma was fibrotic and contained a patchy lymphocytic infiltrate. These histologic findings were consistent with trichofolliculomas (Figure 4).

Comment
Characteristics of SCM
Steatocystoma multiplex is an uncommon condition characterized by the formation of asymptomatic, 0.2- to 2-cm, yellow to flesh-colored, soft, mobile papules or nodules on the trunk, extremities, axillae, genitalia, and/or chest. The lesions contain a clear or opaque, oily, milky or yellow, odorless fluid and most commonly are located on the anterior aspect of the chest. The face is not a commonly involved site in this condition. Six cases of a rare facial variant of SCM have been reported,11-16 with lesions limited to the forehead in 3 cases.13-15
In 1937, Mount20 credited Bozellini for describing the first case, though 3 cases reported in the late 1800s probably were SCM.21 In 1899, Pringle22 coined the term steatocystoma multiplex for this condition. It can be sporadic or have an autosomal-dominant inheritance pattern. Steatocystoma multiplex can occur at any age, though lesions develop most frequently in adolescence or young adulthood. There is no sex predilection.
Steatocystoma multiplex with pachyonychia congenita has been reported in a familial case.23 Other findings reported in patients with SCM include ichthyosis, koilonychia, acrokeratosis verruciformis of Hopf and hypertrophic lichen planus, hidradenitis suppurativa, hypotrichosis, multiple keratoacanthomas, and rheumatoid arthritis.12,24-26
Steatocystoma multiplex is a cyst lined by stratified squamous epithelium without a granular layer but with a thick eosinophilic cuticle. Mature sebaceous lobules are closely associated with the cyst wall. Steatocystoma multiplex arises from the sebaceous duct because the lining of the lumen is composed of undulating eosinophilic cuticle.
Characteristics of EVHCs
Eruptive vellus hair cysts, which were first described by Esterly et al,27 can occur at any age but develop most frequently in adolescents or young adults. Sometimes the lesions are congenital or appear in childhood. There is no sex predilection. They can be sporadic or have an autosomal-dominant inheritance pattern.
Eruptive vellus hair cysts are asymptomatic, 1- to 2-mm, smooth, crusted, or umbilicated papules on the chest or arms and legs. Eruptive vellus hair cysts most commonly involve the anterior aspect of the chest. The lesions are flesh-colored to yellow, though they have a slate gray color in darker-skinned individuals. A rare facial variant has been reported in 2 patients of Asian descent.17
Eruptive vellus hair cysts are small cystic structures lined by a stratified squamous epithelium with a granular layer. The cyst cavity contains numerous small vellus hair shafts and laminated keratin. Eruptive vellus hair cysts originate from the infundibulum or less frequently the isthmus or infundibular-isthmic junction of the hair follicle.
Characteristics of Trichofolliculomas
Trichofolliculomas are solitary, 3- to 5-mm, flesh-colored papules that occur on the face. They are highly differentiated, benign, neoplastic proliferations of an actively trichogenic epithelium, with structural components reflecting all portions of the pilosebaceous unit. Trichofolliculomas consist of a central dilated primary follicle contiguous with the surface epidermis embedded in a fibrous stroma. Multiple small secondary follicles with varying degrees of follicular differentiation arise from the primary follicle.
Co-occurrence of Lesions
An association between SCM and EVHC has been recognized.5-10 Steatocystoma multiplex and EVHC have similar clinical features but distinctive histologic features. They also have a similar age of onset, location/appearance of lesions, and mode of inheritance. Steatocystoma multiplex and EVHC can be distinguished by immunohistochemical techniques: SCM shows expression of keratin 10 and keratin 17, whereas EVHCs express only keratin 17.28
Steatocystoma multiplex and EVHC have only rarely been reported to occur together on the trunk. One case of SCM and EVHC occurring on the forehead has been described.3 Other types of benign follicular neoplasms simultaneously developing in association with SCM or EVHC also are rare. Milia, SCM, and EVHC on the face and trunk have been reported in 1 family,4 and facial steatocystoma associated with a pilar cyst and bilateral preauricular sinus was reported in 1 patient.19 Although trichofolliculomas have not been reported to occur with SCM or EVHC, 2 related follicular neoplasms—trichoepitheliomas and trichoblastomas—have been reported to occur in association with SCM on the face and chest and around the ears in 1 case.18
Differential Diagnosis
The clinical differential diagnosis includes multiple epidermoid cysts, dermoid cysts, Gardner syndrome, sebaceous adenomas, Muir-Torre syndrome, syringomas, milia, leiomyomas, lipomas, acneiform folliculitis, multiple familial and nonfamilial trichoepitheliomas, cylindromas, and angiofibromas.3,29
Conclusion
Our patient represents a rare case of simultaneous occurrence of SCM, EVHC, and trichofolliculomas localized to the forehead. The patient had multiple neoplasms involving differentiation toward various regions of the pilosebaceous unit. This case gives further support to the hypothesis that these benign follicular neoplasms are closely related but are distinct conditions within the spectrum of the same disease process. They represent nevoid malformations of the pilosebaceous unit that can be sporadic or inherited in an autosomal-dominant pattern. Pure types of these lesions may represent one end of the spectrum, but in some patients, there are overlapping features or hybrids of each condition. Several biopsies from patients with multiple lesions should be performed to establish an accurate diagnosis.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19:570-571.
- Sanchez Yus E, Requena L. Eruptive vellus hair cyst and steatocystoma multiplex. Am J Dermatopathol. 1990;12:536-537.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Kiene P, Hauschild A, Christophers E. Eruptive vellus hair cysts and steatocystoma multiplex: variants of one entity? Br J Dermatol. 1996;134:365-367.
- Hurlimann AF, Panizzon RG, Burg G. Eruptive vellus hair cyst and steatocystoma multiplex: hybrid cysts. Dermatology. 1996;192:64-66.
- Sexton M, Murdock DK. Eruptive vellus hair cysts: a follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1989;11:364-368.
- Sanchez-Yus E, Aguilar-Martinez A, Cristobal-Gil MC, et al. Eruptive vellus hair cyst and steatocystoma multiplex: two related conditions? J Cutan Pathol. 1988;15:40-42.
- Ahn SK, Chung J, Lee WS, et al. Hybrid cysts showing alternate combination of eruptive vellus hair cyst, steatocystoma multiplex, and epidermoid cyst, and an association among the three conditions. Am J Dermatopathol. 1996;18:645-649.
- Ahn SK, Hwang SM, Lee SH, et al. Steatocystoma multiplex localized only in the face. Int J Dermatol. 1997;36:372-373.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Hansen KK, Troy JL, Fairley JA. Multiple papules of the scalp and forehead. steatocystoma multiplex (facial papular variant). Arch Dermatol. 1995;131:835-838.
- Nishimura M, Kohda H, Urabe A. Steatocystoma multiplex: a facial popular variant. Arch Dermatol. 1986;122:205-207.
- Requena L, Martin L, Renedo G, et al. A facial variant of steatocystoma multiplex. Cutis. 1993;51:449-452.
- Holmes R, Black MM. Steatocystoma multiplex with unusually prominent cysts on the face. Br J Dermatol. 1980;102:711-713.
- Kumakiri M, Takashima I, Iju M, et al. Eruptive vellus hair cysts: a facial variant. J Am Acad Dermatol. 1982;7:461-467.
- Gianotti R, Cavicchini S, Alessi E. Simultaneous occurrence of multiple trichoblastomas and steatocystoma multiplex. Am J Dermatopathol. 1997;19:294-298.
- Sardana K, Sharma RC, Jain A, et al. Facial steatocystoma multiplex associated with pilar cyst and bilateral preauricular sinus. J Dermatol. 2002;29:157-159.
- Mount LB. Steatocystoma multiplex. Arch Dermatol Syphilol. 1937;36:31-39.
- Dubreuilh W, Auche B. Kystes grassieux sudoripares. Arch Clin de Bordeaux. 1896;5:387-391.
- Pringle JJ. A case of peculiar multiple sebaceous cysts (steatocystoma multiplex). Br J Dermatol. 1899;11:381-88.
- Vineyard WR, Scott RA. Steatocystoma multiplex with pachyonychia congenital: eight cases in four generations. Arch Dermatol. 1961;84:824-827.
- Contreras MA, Costello MJ. Steatocystoma multiplex with embryonal hair formation: case presentation and consideration of pathogenesis. AMA Arch Derm. 1957;76:720-725.
- Sohn D, Chin TC, Fellner MJ. Multiple keratoacanthomas associated with steatocystoma multiplex and rheumatoid arthritis: a case report. Arch Dermatol. 1980;116:913-915.
- Verbov J. Acrokeratosis verruciformis of Hopf with steatocystoma multiplex and hypertrophic lichen planus. Br J Dermatol. 1972;86:91-94.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Tomkova H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Feinstein A, Trau H, Movshovitz M, et al. Steatocystoma multiplex. Cutis. 1983;31:425-427.
- Cho S, Chang SE, Choi JH, et al. Clinical and histologic features of 64 cases of steatocystoma multiplex. J Dermatol. 2002;29:152-156.
- Ogawa Y, Nogita T, Kawashima M. The coexistence of eruptive vellus hair cysts and steatocystoma multiplex. J Dermatol. 1992;19:570-571.
- Sanchez Yus E, Requena L. Eruptive vellus hair cyst and steatocystoma multiplex. Am J Dermatopathol. 1990;12:536-537.
- Patrizi A, Neri I, Guerrini V, et al. Persistent milia, steatocystoma multiplex and eruptive vellus hair cysts: variable expression of multiple pilosebaceous cysts within an affected family. Dermatology. 1998;196:392-396.
- Ohtake N, Kubota Y, Takayama O, et al. Relationship between steatocystoma multiplex and eruptive vellus hair cysts. J Am Acad Dermatol. 1992;26(5, pt 2):876-878.
- Kiene P, Hauschild A, Christophers E. Eruptive vellus hair cysts and steatocystoma multiplex: variants of one entity? Br J Dermatol. 1996;134:365-367.
- Hurlimann AF, Panizzon RG, Burg G. Eruptive vellus hair cyst and steatocystoma multiplex: hybrid cysts. Dermatology. 1996;192:64-66.
- Sexton M, Murdock DK. Eruptive vellus hair cysts: a follicular cyst of the sebaceous duct (sometimes). Am J Dermatopathol. 1989;11:364-368.
- Sanchez-Yus E, Aguilar-Martinez A, Cristobal-Gil MC, et al. Eruptive vellus hair cyst and steatocystoma multiplex: two related conditions? J Cutan Pathol. 1988;15:40-42.
- Ahn SK, Chung J, Lee WS, et al. Hybrid cysts showing alternate combination of eruptive vellus hair cyst, steatocystoma multiplex, and epidermoid cyst, and an association among the three conditions. Am J Dermatopathol. 1996;18:645-649.
- Ahn SK, Hwang SM, Lee SH, et al. Steatocystoma multiplex localized only in the face. Int J Dermatol. 1997;36:372-373.
- Cole LA. Steatocystoma multiplex. Arch Dermatol. 1976;112:1437-1439.
- Hansen KK, Troy JL, Fairley JA. Multiple papules of the scalp and forehead. steatocystoma multiplex (facial papular variant). Arch Dermatol. 1995;131:835-838.
- Nishimura M, Kohda H, Urabe A. Steatocystoma multiplex: a facial popular variant. Arch Dermatol. 1986;122:205-207.
- Requena L, Martin L, Renedo G, et al. A facial variant of steatocystoma multiplex. Cutis. 1993;51:449-452.
- Holmes R, Black MM. Steatocystoma multiplex with unusually prominent cysts on the face. Br J Dermatol. 1980;102:711-713.
- Kumakiri M, Takashima I, Iju M, et al. Eruptive vellus hair cysts: a facial variant. J Am Acad Dermatol. 1982;7:461-467.
- Gianotti R, Cavicchini S, Alessi E. Simultaneous occurrence of multiple trichoblastomas and steatocystoma multiplex. Am J Dermatopathol. 1997;19:294-298.
- Sardana K, Sharma RC, Jain A, et al. Facial steatocystoma multiplex associated with pilar cyst and bilateral preauricular sinus. J Dermatol. 2002;29:157-159.
- Mount LB. Steatocystoma multiplex. Arch Dermatol Syphilol. 1937;36:31-39.
- Dubreuilh W, Auche B. Kystes grassieux sudoripares. Arch Clin de Bordeaux. 1896;5:387-391.
- Pringle JJ. A case of peculiar multiple sebaceous cysts (steatocystoma multiplex). Br J Dermatol. 1899;11:381-88.
- Vineyard WR, Scott RA. Steatocystoma multiplex with pachyonychia congenital: eight cases in four generations. Arch Dermatol. 1961;84:824-827.
- Contreras MA, Costello MJ. Steatocystoma multiplex with embryonal hair formation: case presentation and consideration of pathogenesis. AMA Arch Derm. 1957;76:720-725.
- Sohn D, Chin TC, Fellner MJ. Multiple keratoacanthomas associated with steatocystoma multiplex and rheumatoid arthritis: a case report. Arch Dermatol. 1980;116:913-915.
- Verbov J. Acrokeratosis verruciformis of Hopf with steatocystoma multiplex and hypertrophic lichen planus. Br J Dermatol. 1972;86:91-94.
- Esterly NB, Fretzin DF, Pinkus H. Eruptive vellus hair cysts. Arch Dermatol. 1977;113:500-503.
- Tomkova H, Fujimoto W, Arata J. Expression of keratins (K10 and K17) in steatocystoma multiplex, eruptive vellus hair cysts, and epidermoid and trichilemmal cysts. Am J Dermatopathol. 1997;19:250-253.
- Feinstein A, Trau H, Movshovitz M, et al. Steatocystoma multiplex. Cutis. 1983;31:425-427.
Practice Points
- Steatocystoma multiplex (SCM) and eruptive vellus hair cysts (EVHCs) have similar clinical features but distinctive histologic features.
- Milia, pilar cyst, trichoepitheliomas, and trichoblastomas simultaneously developing in association with SCM or EVHC on the face are rare.
- This case supports the hypothesis that these benign follicular neoplasms are related but distinct nevoid malformations of the pilosebaceous unit within the same disease spectrum.
Evaluating the Clinical and Demographic Features of Extrafacial Granuloma Faciale
Granuloma faciale (GF) is a chronic benign leukocytoclastic vasculitis that can be difficult to treat. It is characterized by single or multiple, soft, well-circumscribed papules, plaques, or nodules ranging in color from red, violet, or yellow to brown that may darken with sun exposure.1 Lesions usually are smooth with follicular orifices that are accentuated, thus producing a peau d’orange appearance. Lesions generally are slow to develop and asymptomatic, though some patients report pruritus or burning.2,3 Diagnosis of GF is based on the presence of distinct histologic features. The epidermis usually is spared, with a prominent grenz zone of normal collagen separating the epidermis from a dense infiltrate of neutrophils, lymphocytes, and eosinophils. This mixed inflammatory infiltrate is seen mainly in the superficial dermis but occasionally spreads to the lower dermis and subcutaneous tissues.4
As the name implies, GF usually is confined to the face but occasionally involves extrafacial sites.5-15 The clinical characteristics of these rare extrafacial lesions are not well understood. The purpose of this study was to identify the clinical and demographic features of extrafacial GF in patients treated at Mayo Clinic (Rochester, Minnesota) during a 54-year period.
Methods
This study was approved by the Mayo institutional review board. We searched the Mayo Clinic Rochester dermatology database for all patients with a diagnosis of GF from 1959 through 2013. All histopathology slides were reviewed by a board-certified dermatologist (A.G.B.) and dermatopathologist (A.G.B.) before inclusion in this study. Histologic criteria for diagnosis of GF included the presence of a mixed inflammatory infiltrate of neutrophils, eosinophils, lymphocytes, and histiocytes in the superficial or deep dermis; a prominent grenz zone separating the uninvolved epidermis; and the presence of vascular damage, as seen by fibrin deposition in dermal blood vessels.
Medical records were reviewed for patient demographics and for history pertinent to the diagnosis of GF, including sites involved, appearance, histopathology reports, symptoms, treatments, and outcomes.
Literature Search Strategy
A computerized Ovid MEDLINE database search was undertaken to identify English-language articles concerning GF in humans using the search terms granuloma faciale with extrafacial or disseminated. To ensure that no articles were overlooked, we conducted another search for English-language articles in the Embase database (1946-2013) using the terms granuloma faciale and extrafacial or disseminated.
Statistical Analysis
Descriptive clinical and histopathologic data were summarized using means, medians, and ranges or proportions as appropriate; statistical analysis was performed using SAS software (JMP package).
Results
Ninety-six patients with a diagnosis of GF were identified, and 12 (13%) had a diagnosis of extrafacial GF. Of them, 2 patients had a diagnosis of extrafacial GF supported only by histopathology slides without accompanying clinical records and therefore were excluded from the study. Thus, 10 cases of extrafacial GF were identified from our search and were included in the study group. Clinical data for these patients are summarized in Table 1. The mean age was 58.7 years (range, 26–87 years). Six (60%) patients were male, and all patients were white. Seven patients (70%) had facial GF in addition to extrafacial GF. Six patients reported no symptoms (60%), and 4 (40%) reported pruritus, discomfort, or both associated with their GF lesions.

Extrafacial GF was diagnosed in the following anatomic locations: scalp (n=3 [30%]), posterior auricular area (n=3 [30%]), mid upper back (n=1 [10%]), right shoulder (n=1 [10%]), both ears (n=1 [10%]), right elbow (n=1 [10%]), and left infra-auricular area (n=1 [10%]). Only 1 (10%) patient had multiple extrafacial sites identified.
The lesions were characterized clinically as violet, red, and yellow to brown smooth papules, plaques, and nodules (Figure 1). Biopsies from these lesions showed a subepidermal and adnexal grenz zone; a polymorphous perivascular and periadnexal dermal infiltrate composed of neutrophils, eosinophils, lymphocytes, histiocytes, and plasma cells; and a mild subtle leukocytoclastic vasculitis with subtle mild vascular necrosis (Figure 2).
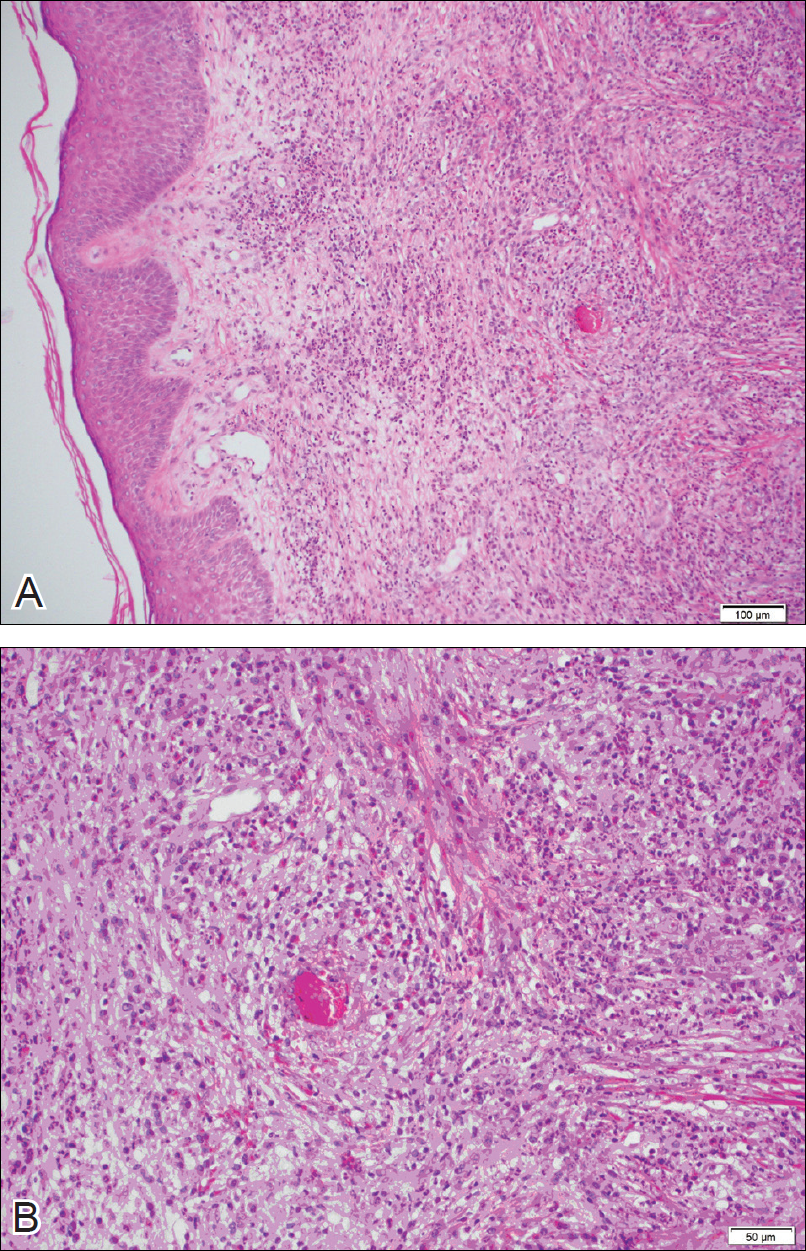
For the 9 patients who elected to undergo GF treatment, the average number of treatments attempted was 2.8 (range, 1–5). The most common method of treatment was a combination of intralesional and topical corticosteroids (n=5 [50%]). Other methods included surgery (n=3 [30%]), dapsone (n=2 [20%]), radiation therapy (n=2 [20%]), cryosurgery (n=1 [10%]), nitrogen mustard (n=1 [10%]), liquid nitrogen (n=1 [10%]), and tar shampoo and fluocinolone acetonide solution 0.01% (n=1 [10%]).
Treatment outcomes were available for 8 of 9 treated patients. Three patients (patients 7, 8, and 10) had long-term successful resolution of their lesions. Patient 7 had an extrafacial lesion that was successfully treated with intralesional and topical corticosteroids, but the facial lesions recurred. The extrafacial GF lesion in patient 8 was found adjacent to a squamous cell carcinoma and was removed with a wide surgical excision that included both lesions. Patient 10 was successfully treated with a combination of liquid nitrogen and topical corticosteroid. Patients 2 and 4 were well controlled while on dapsone; however, once the treatment was discontinued, primarily due to adverse effects, the lesions returned.
Literature Search
Our search of the English-language literature identified 20 patients with extrafacial GF (Table 2). Fifteen (75%) patients were male, which was similar to our study (6/10 [60%]). Our patient population was slightly older with a mean age of 58.7 years compared to a median age of 54 years among those identified in the literature. Additionally, 3 (30%) patients in our study had no facial lesions, as seen in classic GF, which is comparable to 8 (40%) patients identified in the literature.
Comment
Extrafacial GF primarily affects white individuals and is more prevalent in men, as demonstrated in our study. Extrafacial GF was most often found in association with facial lesions, with only 3 patients having exclusively extrafacial sites.
Data from the current study indicate that diverse modalities were used to treat extrafacial GF with variable outcomes (chronic recurrence to complete resolution). The most common first-line treatment, intralesional corticosteroid injection, was used in 5 (50%) patients but resulted in only 1 (10%) successful resolution. Other methods frequently used in our study and prior studies were surgical excision, cryotherapy, electrosurgery, and dermabrasion.1,20 These treatments do not appear to be uniformly definitive, and the ablative methods may result in scarring.1 Different laser treatments are emerging for the management of GF lesions. Prior reports of treating facial GF with argon and CO2 lasers have indicated minimized residual scarring and pigmentation.21-23 The use of pulsed dye lasers has resulted in complete clearance of facial GF lesions, without recurrence on long-term follow-up.20,24-26
The latest investigations of immunomodulatory drugs indicate these agents are promising for the management of facial GF. Eetam et al27 reported the successful use of topical tacrolimus to treat facial GF. The relatively low cost and ease of use make these topical medications a competitive alternative to currently available surgical and laser methods. The appearance of all of these novel therapeutic modalities creates the necessity for a randomized trial to establish their efficacy on extrafacial GF lesions.
The wide array of treatments reflects the recalcitrant nature of extrafacial GF lesions. Further insight into the etiology of these lesions is needed to understand their tendency to recur. The important contribution of our study is the observed
Conclusion
The findings from this study and the cases reviewed in the literature provide a unique contribution to the understanding of the clinical and demographic characteristics of extrafacial GF. The rarity of this condition is the single most important constraint of our study, reflected in the emblematic limitations of a retrospective analysis in a select group of patients. The results of analysis of data from our patients were similar to the findings reported in the English-language medical literature. Serious consideration should be given to the development of a national registry for patients with GF. A database containing the clinicopathologic features, treatments, and outcomes for patients with both facial and extrafacial manifestations of GF may be invaluable in evaluating various treatment options and increasing understanding of the etiology and epidemiology of the disease.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551.
- Guill MA, Aton JK. Facial granuloma responsive to dapsone therapy. Arch Dermatol. 1982;118:332-335.
- Ryan TJ. Cutaneous vasculitis. In: Champion RH, Burton JL, Burns DA, et al, eds. Rook/Wilkins/Ebling Textbook of Dermatology. 7th ed. Malden, MA: Blackwell Science; 2004.
- Castano E, Segurado A, Iglesias L, et al. Granuloma faciale entirely in an extrafacial location. Br J Dermatol. 1997;136:978-979.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Cecchi R, Paoli S, Giomi A. Granuloma faciale with extrafacial lesions. Eur J Dermatol. 2002;12:438.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;14:360-362.
- Kavanagh GM, McLaren KM, Hunter JA. Extensive extrafacial granuloma faciale of the scalp. Br J Dermatol. 1996;134:595-596.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Okun MR, Bauman L, Minor D. Granuloma faciale with lesions on the face and hand. Arch Dermatol. 1965;92:78-80.
- Roustan G, Sanchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- Rusin LJ, Dubin HV, Taylor WB. Disseminated granuloma faciale. Arch Dermatol. 1976;112:1575-1577.
- Sears JK, Gitter DG, Stone MS. Extrafacial granuloma faciale. Arch Dermatol. 1991;127:742-743.
- Zargari O. Disseminated granuloma faciale. Int J Dermatol. 2004;43:210-212.
- Lever WF, Lane CG, Downing JG, et al. Eosinophilic granuloma of the skin: report of three cases. Arch Derm Syphilol. 1948;58:430-438.
- Pedace FJ, Perry HO. Granuloma faciale: a clinical and histopathologic review. Arch Dermatol. 1966;94:387-395.
- Frost FA, Heenan PJ. Facial granuloma. Australas J Dermatol. 1984;25:121-124.
Konohana A. Extrafacial granuloma faciale. J Dermatol. 1994;21:680-682.- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
- Apfelberg DB, Druker D, Maser MR, et al. Granuloma faciale: treatment with the argon laser. Arch Dermatol. 1983;119:573-576.
- Apfelberg DB, Maser MR, Lash H, et al. Expanded role of the argon laser in plastic surgery. J Dermatol Surg Oncol. 1983;9:145-151.
- Wheeland RG, Ashley JR, Smith DA, et al. Carbon dioxide laser treatment of granuloma faciale. J Dermatol Surg Oncol. 1984;10:730-733.
- Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
- Chatrath V, Rohrer TE. Granuloma faciale successfully treated with long-pulsed tunable dye laser. Dermatol Surg. 2002;28:527-529.
- Elston DM. Treatment of granuloma faciale with the pulsed dye laser. Cutis. 2000;65:97-98.
- Eetam I, Ertekin B, Unal I, et al. Granuloma faciale: is it a new indication for pimecrolimus? a case report. J Dermatolog Treat. 2006;17:238-240.
- Johnson WC, Higdon RS, Helwig EB. Granuloma faciale. AMA Arch Derm. 1959;79:42-52.
Granuloma faciale (GF) is a chronic benign leukocytoclastic vasculitis that can be difficult to treat. It is characterized by single or multiple, soft, well-circumscribed papules, plaques, or nodules ranging in color from red, violet, or yellow to brown that may darken with sun exposure.1 Lesions usually are smooth with follicular orifices that are accentuated, thus producing a peau d’orange appearance. Lesions generally are slow to develop and asymptomatic, though some patients report pruritus or burning.2,3 Diagnosis of GF is based on the presence of distinct histologic features. The epidermis usually is spared, with a prominent grenz zone of normal collagen separating the epidermis from a dense infiltrate of neutrophils, lymphocytes, and eosinophils. This mixed inflammatory infiltrate is seen mainly in the superficial dermis but occasionally spreads to the lower dermis and subcutaneous tissues.4
As the name implies, GF usually is confined to the face but occasionally involves extrafacial sites.5-15 The clinical characteristics of these rare extrafacial lesions are not well understood. The purpose of this study was to identify the clinical and demographic features of extrafacial GF in patients treated at Mayo Clinic (Rochester, Minnesota) during a 54-year period.
Methods
This study was approved by the Mayo institutional review board. We searched the Mayo Clinic Rochester dermatology database for all patients with a diagnosis of GF from 1959 through 2013. All histopathology slides were reviewed by a board-certified dermatologist (A.G.B.) and dermatopathologist (A.G.B.) before inclusion in this study. Histologic criteria for diagnosis of GF included the presence of a mixed inflammatory infiltrate of neutrophils, eosinophils, lymphocytes, and histiocytes in the superficial or deep dermis; a prominent grenz zone separating the uninvolved epidermis; and the presence of vascular damage, as seen by fibrin deposition in dermal blood vessels.
Medical records were reviewed for patient demographics and for history pertinent to the diagnosis of GF, including sites involved, appearance, histopathology reports, symptoms, treatments, and outcomes.
Literature Search Strategy
A computerized Ovid MEDLINE database search was undertaken to identify English-language articles concerning GF in humans using the search terms granuloma faciale with extrafacial or disseminated. To ensure that no articles were overlooked, we conducted another search for English-language articles in the Embase database (1946-2013) using the terms granuloma faciale and extrafacial or disseminated.
Statistical Analysis
Descriptive clinical and histopathologic data were summarized using means, medians, and ranges or proportions as appropriate; statistical analysis was performed using SAS software (JMP package).
Results
Ninety-six patients with a diagnosis of GF were identified, and 12 (13%) had a diagnosis of extrafacial GF. Of them, 2 patients had a diagnosis of extrafacial GF supported only by histopathology slides without accompanying clinical records and therefore were excluded from the study. Thus, 10 cases of extrafacial GF were identified from our search and were included in the study group. Clinical data for these patients are summarized in Table 1. The mean age was 58.7 years (range, 26–87 years). Six (60%) patients were male, and all patients were white. Seven patients (70%) had facial GF in addition to extrafacial GF. Six patients reported no symptoms (60%), and 4 (40%) reported pruritus, discomfort, or both associated with their GF lesions.

Extrafacial GF was diagnosed in the following anatomic locations: scalp (n=3 [30%]), posterior auricular area (n=3 [30%]), mid upper back (n=1 [10%]), right shoulder (n=1 [10%]), both ears (n=1 [10%]), right elbow (n=1 [10%]), and left infra-auricular area (n=1 [10%]). Only 1 (10%) patient had multiple extrafacial sites identified.
The lesions were characterized clinically as violet, red, and yellow to brown smooth papules, plaques, and nodules (Figure 1). Biopsies from these lesions showed a subepidermal and adnexal grenz zone; a polymorphous perivascular and periadnexal dermal infiltrate composed of neutrophils, eosinophils, lymphocytes, histiocytes, and plasma cells; and a mild subtle leukocytoclastic vasculitis with subtle mild vascular necrosis (Figure 2).


For the 9 patients who elected to undergo GF treatment, the average number of treatments attempted was 2.8 (range, 1–5). The most common method of treatment was a combination of intralesional and topical corticosteroids (n=5 [50%]). Other methods included surgery (n=3 [30%]), dapsone (n=2 [20%]), radiation therapy (n=2 [20%]), cryosurgery (n=1 [10%]), nitrogen mustard (n=1 [10%]), liquid nitrogen (n=1 [10%]), and tar shampoo and fluocinolone acetonide solution 0.01% (n=1 [10%]).
Treatment outcomes were available for 8 of 9 treated patients. Three patients (patients 7, 8, and 10) had long-term successful resolution of their lesions. Patient 7 had an extrafacial lesion that was successfully treated with intralesional and topical corticosteroids, but the facial lesions recurred. The extrafacial GF lesion in patient 8 was found adjacent to a squamous cell carcinoma and was removed with a wide surgical excision that included both lesions. Patient 10 was successfully treated with a combination of liquid nitrogen and topical corticosteroid. Patients 2 and 4 were well controlled while on dapsone; however, once the treatment was discontinued, primarily due to adverse effects, the lesions returned.
Literature Search
Our search of the English-language literature identified 20 patients with extrafacial GF (Table 2). Fifteen (75%) patients were male, which was similar to our study (6/10 [60%]). Our patient population was slightly older with a mean age of 58.7 years compared to a median age of 54 years among those identified in the literature. Additionally, 3 (30%) patients in our study had no facial lesions, as seen in classic GF, which is comparable to 8 (40%) patients identified in the literature.

Comment
Extrafacial GF primarily affects white individuals and is more prevalent in men, as demonstrated in our study. Extrafacial GF was most often found in association with facial lesions, with only 3 patients having exclusively extrafacial sites.
Data from the current study indicate that diverse modalities were used to treat extrafacial GF with variable outcomes (chronic recurrence to complete resolution). The most common first-line treatment, intralesional corticosteroid injection, was used in 5 (50%) patients but resulted in only 1 (10%) successful resolution. Other methods frequently used in our study and prior studies were surgical excision, cryotherapy, electrosurgery, and dermabrasion.1,20 These treatments do not appear to be uniformly definitive, and the ablative methods may result in scarring.1 Different laser treatments are emerging for the management of GF lesions. Prior reports of treating facial GF with argon and CO2 lasers have indicated minimized residual scarring and pigmentation.21-23 The use of pulsed dye lasers has resulted in complete clearance of facial GF lesions, without recurrence on long-term follow-up.20,24-26
The latest investigations of immunomodulatory drugs indicate these agents are promising for the management of facial GF. Eetam et al27 reported the successful use of topical tacrolimus to treat facial GF. The relatively low cost and ease of use make these topical medications a competitive alternative to currently available surgical and laser methods. The appearance of all of these novel therapeutic modalities creates the necessity for a randomized trial to establish their efficacy on extrafacial GF lesions.
The wide array of treatments reflects the recalcitrant nature of extrafacial GF lesions. Further insight into the etiology of these lesions is needed to understand their tendency to recur. The important contribution of our study is the observed
Conclusion
The findings from this study and the cases reviewed in the literature provide a unique contribution to the understanding of the clinical and demographic characteristics of extrafacial GF. The rarity of this condition is the single most important constraint of our study, reflected in the emblematic limitations of a retrospective analysis in a select group of patients. The results of analysis of data from our patients were similar to the findings reported in the English-language medical literature. Serious consideration should be given to the development of a national registry for patients with GF. A database containing the clinicopathologic features, treatments, and outcomes for patients with both facial and extrafacial manifestations of GF may be invaluable in evaluating various treatment options and increasing understanding of the etiology and epidemiology of the disease.
Granuloma faciale (GF) is a chronic benign leukocytoclastic vasculitis that can be difficult to treat. It is characterized by single or multiple, soft, well-circumscribed papules, plaques, or nodules ranging in color from red, violet, or yellow to brown that may darken with sun exposure.1 Lesions usually are smooth with follicular orifices that are accentuated, thus producing a peau d’orange appearance. Lesions generally are slow to develop and asymptomatic, though some patients report pruritus or burning.2,3 Diagnosis of GF is based on the presence of distinct histologic features. The epidermis usually is spared, with a prominent grenz zone of normal collagen separating the epidermis from a dense infiltrate of neutrophils, lymphocytes, and eosinophils. This mixed inflammatory infiltrate is seen mainly in the superficial dermis but occasionally spreads to the lower dermis and subcutaneous tissues.4
As the name implies, GF usually is confined to the face but occasionally involves extrafacial sites.5-15 The clinical characteristics of these rare extrafacial lesions are not well understood. The purpose of this study was to identify the clinical and demographic features of extrafacial GF in patients treated at Mayo Clinic (Rochester, Minnesota) during a 54-year period.
Methods
This study was approved by the Mayo institutional review board. We searched the Mayo Clinic Rochester dermatology database for all patients with a diagnosis of GF from 1959 through 2013. All histopathology slides were reviewed by a board-certified dermatologist (A.G.B.) and dermatopathologist (A.G.B.) before inclusion in this study. Histologic criteria for diagnosis of GF included the presence of a mixed inflammatory infiltrate of neutrophils, eosinophils, lymphocytes, and histiocytes in the superficial or deep dermis; a prominent grenz zone separating the uninvolved epidermis; and the presence of vascular damage, as seen by fibrin deposition in dermal blood vessels.
Medical records were reviewed for patient demographics and for history pertinent to the diagnosis of GF, including sites involved, appearance, histopathology reports, symptoms, treatments, and outcomes.
Literature Search Strategy
A computerized Ovid MEDLINE database search was undertaken to identify English-language articles concerning GF in humans using the search terms granuloma faciale with extrafacial or disseminated. To ensure that no articles were overlooked, we conducted another search for English-language articles in the Embase database (1946-2013) using the terms granuloma faciale and extrafacial or disseminated.
Statistical Analysis
Descriptive clinical and histopathologic data were summarized using means, medians, and ranges or proportions as appropriate; statistical analysis was performed using SAS software (JMP package).
Results
Ninety-six patients with a diagnosis of GF were identified, and 12 (13%) had a diagnosis of extrafacial GF. Of them, 2 patients had a diagnosis of extrafacial GF supported only by histopathology slides without accompanying clinical records and therefore were excluded from the study. Thus, 10 cases of extrafacial GF were identified from our search and were included in the study group. Clinical data for these patients are summarized in Table 1. The mean age was 58.7 years (range, 26–87 years). Six (60%) patients were male, and all patients were white. Seven patients (70%) had facial GF in addition to extrafacial GF. Six patients reported no symptoms (60%), and 4 (40%) reported pruritus, discomfort, or both associated with their GF lesions.

Extrafacial GF was diagnosed in the following anatomic locations: scalp (n=3 [30%]), posterior auricular area (n=3 [30%]), mid upper back (n=1 [10%]), right shoulder (n=1 [10%]), both ears (n=1 [10%]), right elbow (n=1 [10%]), and left infra-auricular area (n=1 [10%]). Only 1 (10%) patient had multiple extrafacial sites identified.
The lesions were characterized clinically as violet, red, and yellow to brown smooth papules, plaques, and nodules (Figure 1). Biopsies from these lesions showed a subepidermal and adnexal grenz zone; a polymorphous perivascular and periadnexal dermal infiltrate composed of neutrophils, eosinophils, lymphocytes, histiocytes, and plasma cells; and a mild subtle leukocytoclastic vasculitis with subtle mild vascular necrosis (Figure 2).


For the 9 patients who elected to undergo GF treatment, the average number of treatments attempted was 2.8 (range, 1–5). The most common method of treatment was a combination of intralesional and topical corticosteroids (n=5 [50%]). Other methods included surgery (n=3 [30%]), dapsone (n=2 [20%]), radiation therapy (n=2 [20%]), cryosurgery (n=1 [10%]), nitrogen mustard (n=1 [10%]), liquid nitrogen (n=1 [10%]), and tar shampoo and fluocinolone acetonide solution 0.01% (n=1 [10%]).
Treatment outcomes were available for 8 of 9 treated patients. Three patients (patients 7, 8, and 10) had long-term successful resolution of their lesions. Patient 7 had an extrafacial lesion that was successfully treated with intralesional and topical corticosteroids, but the facial lesions recurred. The extrafacial GF lesion in patient 8 was found adjacent to a squamous cell carcinoma and was removed with a wide surgical excision that included both lesions. Patient 10 was successfully treated with a combination of liquid nitrogen and topical corticosteroid. Patients 2 and 4 were well controlled while on dapsone; however, once the treatment was discontinued, primarily due to adverse effects, the lesions returned.
Literature Search
Our search of the English-language literature identified 20 patients with extrafacial GF (Table 2). Fifteen (75%) patients were male, which was similar to our study (6/10 [60%]). Our patient population was slightly older with a mean age of 58.7 years compared to a median age of 54 years among those identified in the literature. Additionally, 3 (30%) patients in our study had no facial lesions, as seen in classic GF, which is comparable to 8 (40%) patients identified in the literature.

Comment
Extrafacial GF primarily affects white individuals and is more prevalent in men, as demonstrated in our study. Extrafacial GF was most often found in association with facial lesions, with only 3 patients having exclusively extrafacial sites.
Data from the current study indicate that diverse modalities were used to treat extrafacial GF with variable outcomes (chronic recurrence to complete resolution). The most common first-line treatment, intralesional corticosteroid injection, was used in 5 (50%) patients but resulted in only 1 (10%) successful resolution. Other methods frequently used in our study and prior studies were surgical excision, cryotherapy, electrosurgery, and dermabrasion.1,20 These treatments do not appear to be uniformly definitive, and the ablative methods may result in scarring.1 Different laser treatments are emerging for the management of GF lesions. Prior reports of treating facial GF with argon and CO2 lasers have indicated minimized residual scarring and pigmentation.21-23 The use of pulsed dye lasers has resulted in complete clearance of facial GF lesions, without recurrence on long-term follow-up.20,24-26
The latest investigations of immunomodulatory drugs indicate these agents are promising for the management of facial GF. Eetam et al27 reported the successful use of topical tacrolimus to treat facial GF. The relatively low cost and ease of use make these topical medications a competitive alternative to currently available surgical and laser methods. The appearance of all of these novel therapeutic modalities creates the necessity for a randomized trial to establish their efficacy on extrafacial GF lesions.
The wide array of treatments reflects the recalcitrant nature of extrafacial GF lesions. Further insight into the etiology of these lesions is needed to understand their tendency to recur. The important contribution of our study is the observed
Conclusion
The findings from this study and the cases reviewed in the literature provide a unique contribution to the understanding of the clinical and demographic characteristics of extrafacial GF. The rarity of this condition is the single most important constraint of our study, reflected in the emblematic limitations of a retrospective analysis in a select group of patients. The results of analysis of data from our patients were similar to the findings reported in the English-language medical literature. Serious consideration should be given to the development of a national registry for patients with GF. A database containing the clinicopathologic features, treatments, and outcomes for patients with both facial and extrafacial manifestations of GF may be invaluable in evaluating various treatment options and increasing understanding of the etiology and epidemiology of the disease.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551.
- Guill MA, Aton JK. Facial granuloma responsive to dapsone therapy. Arch Dermatol. 1982;118:332-335.
- Ryan TJ. Cutaneous vasculitis. In: Champion RH, Burton JL, Burns DA, et al, eds. Rook/Wilkins/Ebling Textbook of Dermatology. 7th ed. Malden, MA: Blackwell Science; 2004.
- Castano E, Segurado A, Iglesias L, et al. Granuloma faciale entirely in an extrafacial location. Br J Dermatol. 1997;136:978-979.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Cecchi R, Paoli S, Giomi A. Granuloma faciale with extrafacial lesions. Eur J Dermatol. 2002;12:438.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;14:360-362.
- Kavanagh GM, McLaren KM, Hunter JA. Extensive extrafacial granuloma faciale of the scalp. Br J Dermatol. 1996;134:595-596.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Okun MR, Bauman L, Minor D. Granuloma faciale with lesions on the face and hand. Arch Dermatol. 1965;92:78-80.
- Roustan G, Sanchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- Rusin LJ, Dubin HV, Taylor WB. Disseminated granuloma faciale. Arch Dermatol. 1976;112:1575-1577.
- Sears JK, Gitter DG, Stone MS. Extrafacial granuloma faciale. Arch Dermatol. 1991;127:742-743.
- Zargari O. Disseminated granuloma faciale. Int J Dermatol. 2004;43:210-212.
- Lever WF, Lane CG, Downing JG, et al. Eosinophilic granuloma of the skin: report of three cases. Arch Derm Syphilol. 1948;58:430-438.
- Pedace FJ, Perry HO. Granuloma faciale: a clinical and histopathologic review. Arch Dermatol. 1966;94:387-395.
- Frost FA, Heenan PJ. Facial granuloma. Australas J Dermatol. 1984;25:121-124.
Konohana A. Extrafacial granuloma faciale. J Dermatol. 1994;21:680-682.- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
- Apfelberg DB, Druker D, Maser MR, et al. Granuloma faciale: treatment with the argon laser. Arch Dermatol. 1983;119:573-576.
- Apfelberg DB, Maser MR, Lash H, et al. Expanded role of the argon laser in plastic surgery. J Dermatol Surg Oncol. 1983;9:145-151.
- Wheeland RG, Ashley JR, Smith DA, et al. Carbon dioxide laser treatment of granuloma faciale. J Dermatol Surg Oncol. 1984;10:730-733.
- Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
- Chatrath V, Rohrer TE. Granuloma faciale successfully treated with long-pulsed tunable dye laser. Dermatol Surg. 2002;28:527-529.
- Elston DM. Treatment of granuloma faciale with the pulsed dye laser. Cutis. 2000;65:97-98.
- Eetam I, Ertekin B, Unal I, et al. Granuloma faciale: is it a new indication for pimecrolimus? a case report. J Dermatolog Treat. 2006;17:238-240.
- Johnson WC, Higdon RS, Helwig EB. Granuloma faciale. AMA Arch Derm. 1959;79:42-52.
- Radin DA, Mehregan DR. Granuloma faciale: distribution of the lesions and review of the literature. Cutis. 2003;72:213-219.
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551.
- Guill MA, Aton JK. Facial granuloma responsive to dapsone therapy. Arch Dermatol. 1982;118:332-335.
- Ryan TJ. Cutaneous vasculitis. In: Champion RH, Burton JL, Burns DA, et al, eds. Rook/Wilkins/Ebling Textbook of Dermatology. 7th ed. Malden, MA: Blackwell Science; 2004.
- Castano E, Segurado A, Iglesias L, et al. Granuloma faciale entirely in an extrafacial location. Br J Dermatol. 1997;136:978-979.
- Castellano-Howard L, Fairbee SI, Hogan DJ, et al. Extrafacial granuloma faciale: report of a case and response to treatment. Cutis. 2001;67:413-415.
- Cecchi R, Paoli S, Giomi A. Granuloma faciale with extrafacial lesions. Eur J Dermatol. 2002;12:438.
- Inanir I, Alvur Y. Granuloma faciale with extrafacial lesions. Br J Dermatol. 2001;14:360-362.
- Kavanagh GM, McLaren KM, Hunter JA. Extensive extrafacial granuloma faciale of the scalp. Br J Dermatol. 1996;134:595-596.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Okun MR, Bauman L, Minor D. Granuloma faciale with lesions on the face and hand. Arch Dermatol. 1965;92:78-80.
- Roustan G, Sanchez Yus E, Salas C, et al. Granuloma faciale with extrafacial lesions. Dermatology. 1999;198:79-82.
- Rusin LJ, Dubin HV, Taylor WB. Disseminated granuloma faciale. Arch Dermatol. 1976;112:1575-1577.
- Sears JK, Gitter DG, Stone MS. Extrafacial granuloma faciale. Arch Dermatol. 1991;127:742-743.
- Zargari O. Disseminated granuloma faciale. Int J Dermatol. 2004;43:210-212.
- Lever WF, Lane CG, Downing JG, et al. Eosinophilic granuloma of the skin: report of three cases. Arch Derm Syphilol. 1948;58:430-438.
- Pedace FJ, Perry HO. Granuloma faciale: a clinical and histopathologic review. Arch Dermatol. 1966;94:387-395.
- Frost FA, Heenan PJ. Facial granuloma. Australas J Dermatol. 1984;25:121-124.
Konohana A. Extrafacial granuloma faciale. J Dermatol. 1994;21:680-682.- Ludwig E, Allam JP, Bieber T, et al. New treatment modalities for granuloma faciale. Br J Dermatol. 2003;149:634-637.
- Apfelberg DB, Druker D, Maser MR, et al. Granuloma faciale: treatment with the argon laser. Arch Dermatol. 1983;119:573-576.
- Apfelberg DB, Maser MR, Lash H, et al. Expanded role of the argon laser in plastic surgery. J Dermatol Surg Oncol. 1983;9:145-151.
- Wheeland RG, Ashley JR, Smith DA, et al. Carbon dioxide laser treatment of granuloma faciale. J Dermatol Surg Oncol. 1984;10:730-733.
- Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
- Chatrath V, Rohrer TE. Granuloma faciale successfully treated with long-pulsed tunable dye laser. Dermatol Surg. 2002;28:527-529.
- Elston DM. Treatment of granuloma faciale with the pulsed dye laser. Cutis. 2000;65:97-98.
- Eetam I, Ertekin B, Unal I, et al. Granuloma faciale: is it a new indication for pimecrolimus? a case report. J Dermatolog Treat. 2006;17:238-240.
- Johnson WC, Higdon RS, Helwig EB. Granuloma faciale. AMA Arch Derm. 1959;79:42-52.
Practice Points
- Extrafacial lesions are rare in granuloma faciale (GF).
- Extrafacial GF should be included in the differential diagnosis of well-demarcated plaques and nodules found on the trunk or extremities.
- Diagnosis of extrafacial GF is based on the presence of distinct histologic features identical to GF.
- Granuloma faciale is a chronic benign leukocytoclastic vasculitis that can be difficult to treat.
A Case of Pruritis Rash
History of Present Illness
A55-year-old male presented with a one and one-half week history of a sore throat, shortness of breath on exertion, ankle edema, and arthralgias that began in the ankles and subsequently spread to involve the elbows and wrists.
He had also developed a pruritic eruption involving the lower extremities, which consisted of erythematous palpable purpuric lesions and patches with superficial and central necrosis and ulceration, as well as a large 4-cm bulla of the right lateral ankle. (See Figures 1 and 2, below.) Other skin findings included petechiae of the palms and multiple ulcerations of the hard palate. Laboratory evaluation demonstrated c-ANCA antibody positivity (1:512), a proteinase 3 antibody level of greater than 100 U/ml, and a creatinine of 1.0mg/dl. TH
What is the most appropriate treatment for this condition?
- Prednisone;
- Azathioprine;
- Cyclophosphamide;
- Prednisone combined with cyclophosphamide; or
- Vancomycin combined with rifampin
Discussion
The answer is D: Wegener’s granulomatosis (WG) is a chronic granulomatous inflammatory response of unknown etiology that usually presents with the classic triad of systemic vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tracts, and glomerulonephritis. The generalized or classic form of WG can progress rapidly to cause irreversible organ dysfunction and death. Although the pathogenesis remains unknown, it is felt that WG may result from an exaggerated cell-mediated response to an unknown antigen.1
The average age of onset for WG is 45.2 years, with 63.5% of patients male and 91% Caucasian.2 A WG diagnosis can be very difficult, and elements of the classic triad may not all be present initially. Pulmonary infiltrates or nodules are seen via chest X-ray or CT scan in just less than half of patients as an early manifestation of WG.
Occasionally WG presents with skin lesions (13%) or oral ulcers (6%), however, 40% of patients eventually develop skin involvement consisting of painful subcutaneous nodules, papules, vesicles or bullae, petechiae, palpable purpura, and pyoderma gangrenosum-like lesions.3 Histologic evaluation of these skin lesions reveals non-specific perivascular lymphocytic inflammation, leukocytoclastic vasculitis-like changes, palisading granulomas, and granulomatous vasculitis; however, it is rare to see granulomatous vasculitis or palisading necrotizing granulomas in skin specimens.4,5
WG can also affect the eyes, heart, respiratory system, nervous system, kidneys, and joints.6 The upper respiratory tract is involved in the majority of patients, and symptoms reflecting otitis, epistaxis, rhinorrhea, or sinusitis are common and may be the first manifestation of disease. When mucosal necrotizing granulomas occur, they can result in the typical saddle nose deformity seen in patients with WG. Lower respiratory tract involvement is also common and can present with cough, dyspnea, chest pain, and hemoptysis.1
Patients with WG usually have a positive c-ANCA, however this is not specific for WG and may also indicate Churg-Strauss Syndrome and microscopic polyarteritis. The median survival of patients with untreated WG is five months, and corticosteroids used alone do not change this median survival. When corticosteroids are combined with cytotoxic agents, such as cyclophosphamide, the prognosis significantly improves in greater than 90% of patients, with a 75% remission rate, and an 87% survival of patients followed from six months to 24 years.3 TH
References
- Hannon CW, Swerlick RA. Vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Vol 1. New York: Elsevier Limited; 2003: 393-395.
- Cotch MF, Hoffman GS, Yerg DE, et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996 Jan;39(1):87-92.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. [see comments]. Ann Int Med. 1992;116:488-498.
- Hu CH, O’Loughlin S, Winkelmann RK. Cutaneous manifestations of Wegener granulomatosis. Arch Dermatol. 1997;113(2):175-182.
- Lie JT. Wegener’s granulomatosis: histological documentation of common and uncommon manifestations in 216 patients. Vasa. 1997;26:261-270.
- Yi ES, Colby TV. Wegener’s granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34-46.
History of Present Illness
A55-year-old male presented with a one and one-half week history of a sore throat, shortness of breath on exertion, ankle edema, and arthralgias that began in the ankles and subsequently spread to involve the elbows and wrists.
He had also developed a pruritic eruption involving the lower extremities, which consisted of erythematous palpable purpuric lesions and patches with superficial and central necrosis and ulceration, as well as a large 4-cm bulla of the right lateral ankle. (See Figures 1 and 2, below.) Other skin findings included petechiae of the palms and multiple ulcerations of the hard palate. Laboratory evaluation demonstrated c-ANCA antibody positivity (1:512), a proteinase 3 antibody level of greater than 100 U/ml, and a creatinine of 1.0mg/dl. TH
What is the most appropriate treatment for this condition?
- Prednisone;
- Azathioprine;
- Cyclophosphamide;
- Prednisone combined with cyclophosphamide; or
- Vancomycin combined with rifampin
Discussion
The answer is D: Wegener’s granulomatosis (WG) is a chronic granulomatous inflammatory response of unknown etiology that usually presents with the classic triad of systemic vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tracts, and glomerulonephritis. The generalized or classic form of WG can progress rapidly to cause irreversible organ dysfunction and death. Although the pathogenesis remains unknown, it is felt that WG may result from an exaggerated cell-mediated response to an unknown antigen.1
The average age of onset for WG is 45.2 years, with 63.5% of patients male and 91% Caucasian.2 A WG diagnosis can be very difficult, and elements of the classic triad may not all be present initially. Pulmonary infiltrates or nodules are seen via chest X-ray or CT scan in just less than half of patients as an early manifestation of WG.
Occasionally WG presents with skin lesions (13%) or oral ulcers (6%), however, 40% of patients eventually develop skin involvement consisting of painful subcutaneous nodules, papules, vesicles or bullae, petechiae, palpable purpura, and pyoderma gangrenosum-like lesions.3 Histologic evaluation of these skin lesions reveals non-specific perivascular lymphocytic inflammation, leukocytoclastic vasculitis-like changes, palisading granulomas, and granulomatous vasculitis; however, it is rare to see granulomatous vasculitis or palisading necrotizing granulomas in skin specimens.4,5
WG can also affect the eyes, heart, respiratory system, nervous system, kidneys, and joints.6 The upper respiratory tract is involved in the majority of patients, and symptoms reflecting otitis, epistaxis, rhinorrhea, or sinusitis are common and may be the first manifestation of disease. When mucosal necrotizing granulomas occur, they can result in the typical saddle nose deformity seen in patients with WG. Lower respiratory tract involvement is also common and can present with cough, dyspnea, chest pain, and hemoptysis.1
Patients with WG usually have a positive c-ANCA, however this is not specific for WG and may also indicate Churg-Strauss Syndrome and microscopic polyarteritis. The median survival of patients with untreated WG is five months, and corticosteroids used alone do not change this median survival. When corticosteroids are combined with cytotoxic agents, such as cyclophosphamide, the prognosis significantly improves in greater than 90% of patients, with a 75% remission rate, and an 87% survival of patients followed from six months to 24 years.3 TH
References
- Hannon CW, Swerlick RA. Vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Vol 1. New York: Elsevier Limited; 2003: 393-395.
- Cotch MF, Hoffman GS, Yerg DE, et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996 Jan;39(1):87-92.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. [see comments]. Ann Int Med. 1992;116:488-498.
- Hu CH, O’Loughlin S, Winkelmann RK. Cutaneous manifestations of Wegener granulomatosis. Arch Dermatol. 1997;113(2):175-182.
- Lie JT. Wegener’s granulomatosis: histological documentation of common and uncommon manifestations in 216 patients. Vasa. 1997;26:261-270.
- Yi ES, Colby TV. Wegener’s granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34-46.
History of Present Illness
A55-year-old male presented with a one and one-half week history of a sore throat, shortness of breath on exertion, ankle edema, and arthralgias that began in the ankles and subsequently spread to involve the elbows and wrists.
He had also developed a pruritic eruption involving the lower extremities, which consisted of erythematous palpable purpuric lesions and patches with superficial and central necrosis and ulceration, as well as a large 4-cm bulla of the right lateral ankle. (See Figures 1 and 2, below.) Other skin findings included petechiae of the palms and multiple ulcerations of the hard palate. Laboratory evaluation demonstrated c-ANCA antibody positivity (1:512), a proteinase 3 antibody level of greater than 100 U/ml, and a creatinine of 1.0mg/dl. TH
What is the most appropriate treatment for this condition?
- Prednisone;
- Azathioprine;
- Cyclophosphamide;
- Prednisone combined with cyclophosphamide; or
- Vancomycin combined with rifampin
Discussion
The answer is D: Wegener’s granulomatosis (WG) is a chronic granulomatous inflammatory response of unknown etiology that usually presents with the classic triad of systemic vasculitis, necrotizing granulomatous inflammation of the upper and lower respiratory tracts, and glomerulonephritis. The generalized or classic form of WG can progress rapidly to cause irreversible organ dysfunction and death. Although the pathogenesis remains unknown, it is felt that WG may result from an exaggerated cell-mediated response to an unknown antigen.1
The average age of onset for WG is 45.2 years, with 63.5% of patients male and 91% Caucasian.2 A WG diagnosis can be very difficult, and elements of the classic triad may not all be present initially. Pulmonary infiltrates or nodules are seen via chest X-ray or CT scan in just less than half of patients as an early manifestation of WG.
Occasionally WG presents with skin lesions (13%) or oral ulcers (6%), however, 40% of patients eventually develop skin involvement consisting of painful subcutaneous nodules, papules, vesicles or bullae, petechiae, palpable purpura, and pyoderma gangrenosum-like lesions.3 Histologic evaluation of these skin lesions reveals non-specific perivascular lymphocytic inflammation, leukocytoclastic vasculitis-like changes, palisading granulomas, and granulomatous vasculitis; however, it is rare to see granulomatous vasculitis or palisading necrotizing granulomas in skin specimens.4,5
WG can also affect the eyes, heart, respiratory system, nervous system, kidneys, and joints.6 The upper respiratory tract is involved in the majority of patients, and symptoms reflecting otitis, epistaxis, rhinorrhea, or sinusitis are common and may be the first manifestation of disease. When mucosal necrotizing granulomas occur, they can result in the typical saddle nose deformity seen in patients with WG. Lower respiratory tract involvement is also common and can present with cough, dyspnea, chest pain, and hemoptysis.1
Patients with WG usually have a positive c-ANCA, however this is not specific for WG and may also indicate Churg-Strauss Syndrome and microscopic polyarteritis. The median survival of patients with untreated WG is five months, and corticosteroids used alone do not change this median survival. When corticosteroids are combined with cytotoxic agents, such as cyclophosphamide, the prognosis significantly improves in greater than 90% of patients, with a 75% remission rate, and an 87% survival of patients followed from six months to 24 years.3 TH
References
- Hannon CW, Swerlick RA. Vasculitis. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. Vol 1. New York: Elsevier Limited; 2003: 393-395.
- Cotch MF, Hoffman GS, Yerg DE, et al. The epidemiology of Wegener’s granulomatosis. Estimates of the five-year period prevalence, annual mortality, and geographic disease distribution from population-based data sources. Arthritis Rheum. 1996 Jan;39(1):87-92.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. [see comments]. Ann Int Med. 1992;116:488-498.
- Hu CH, O’Loughlin S, Winkelmann RK. Cutaneous manifestations of Wegener granulomatosis. Arch Dermatol. 1997;113(2):175-182.
- Lie JT. Wegener’s granulomatosis: histological documentation of common and uncommon manifestations in 216 patients. Vasa. 1997;26:261-270.
- Yi ES, Colby TV. Wegener’s granulomatosis. Semin Diagn Pathol. 2001 Feb;18(1):34-46.