User login
Hong Kong, U.S., Israeli data illuminate COVID vaccine myocarditis
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.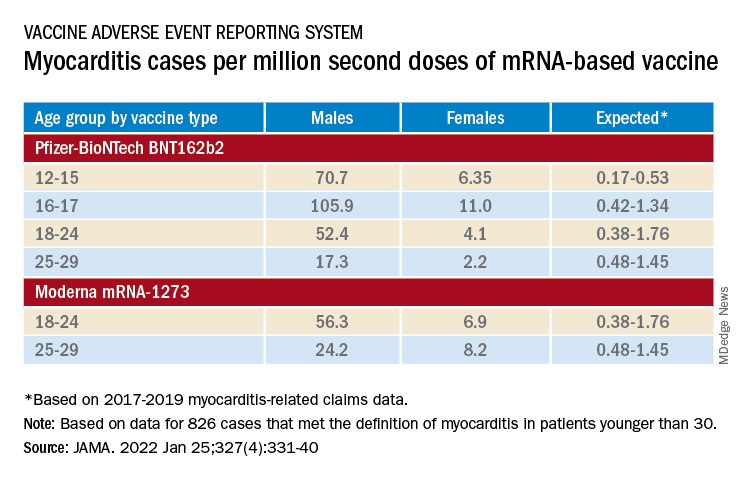
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Why some COVID-19 vaccines seem occasionally to cause a distinctive form of myocarditis, and why adolescent boys and young men appear most vulnerable, remain a mystery. But the entity’s prevalence, nuances of presentation, and likely clinical course have come into sharper view after recent additions to the literature.
Two new publications all but confirm that the rare cases of myocarditis closely following vaccination against SARS-CoV-2, primarily with one of the mRNA-based vaccines from Pfizer-BioNTech and Moderna, is a clinically different creature from myocarditis physicians were likely to see before the pandemic.
A third report unveils rates of hospitalization for myocarditis linked to Pfizer-BioNTech vaccination in the 12- to 15-year age group, based on active surveillance across Israel. Of note, the rates were lower than corresponding numbers among the country’s 16- to 19-year-olds published in late 2021 by the same authors.
No link with CoronaVac
A case-control study covering almost the entire population of Hong Kong from February to August 2021 confirms a slight but significant excess risk for myocarditis and, to a lesser degree, pericarditis, after injections of the Pfizer-BioNTech vaccine. As consistently reported from other studies, the risks were highest in adolescent and young adult males and after a second dose.
The study estimated an overall carditis incidence of 5.7 cases per million doses of Pfizer-BioNTech, for a risk 3.5 times that in the unvaccinated Hong Kong population. Carditis rates after a first dose were about 2.5 per million and 10 per million after a second dose.
Hong Kong launched its public SARS-CoV-2 immunization program in late February 2021 with the Chinese-made CoronaVac (Sinovac) inactivated-virus vaccine, and introduced the mRNA-based alternative several weeks later. By August 2021, the vaccines had reached about 3.3 million people in the region – 49% of the Hong Kong population at least 12 years of age.
In a novel finding, there were no excesses in carditis cases after CoronaVac vaccination. The difference between vaccines likely isn’t caused by chance, because three-fourths of the carditis-associated Pfizer-BioNTech injections arose within a week, whereas “71% of cases following the use of CoronaVac occurred more than 30 days after vaccination,” senior author Ian Chi Kei Wong, PhD, University of Hong Kong, said in an interview.
“This onset distribution for cases having received CoronaVac demonstrates that it is highly unlikely the carditis cases are related to the vaccine,” he said. And that “plausibly implies a specific underlying mechanism between vaccination and carditis that may only be applicable to mRNA vaccines.”
That inference is in line with case reports and other research, including large population-based studies from Israel and Denmark, although a recent study from the United Kingdom hinted at a potential excess myocarditis risk associated with the adenovirus-based AstraZeneca-Oxford vaccine.
The Hong Kong study identified 160 patients age 12 or older with a first diagnosis of carditis during February to August 2021, in electronic health records covering nearly the entire region.
“We used laboratory test results of troponin levels to further eliminate unlikely cases of carditis,” Dr. Wong said. The health records were linked to a “population-based vaccination record” maintained by the government’s department of health.
About 10 control patients from among all hospitalized patients without carditis were matched by age, sex, and admission date to each of the 160 carditis cases. About 83% of cases and 92% of the controls were unvaccinated.
Among those who received the Pfizer-BioNTech vaccine, representing 12.5% of cases and 4.2% of controls, the estimated carditis incidence was 0.57 per 100,000 doses. For those who received CoronaVac, representing 4.4% of cases and 3.9% of controls, it was 0.31 per 100,000 doses.
In adjusted analysis, the odds ratios for carditis among Pfizer-BioNTech vaccine recipients, compared with unvaccinated controls, were 3.57 (95% confidence interval, 1.93-6.60) overall, 4.68 (95% CI, 2.25-9.71) for males, 2.22 (95% CI, 0.57-8.69) for females, 2.41 (95% CI, 1.18-4.90) for ages 18 and older, and 13.8 (95% CI, 2.86-110.4) for ages 12-17
Myocarditis accounted for most of the excess cases, with an overall OR of 9.29 (95% CI, 3.94-21.9). The OR reached only 1.06 (95% CI, 0.35-3.22) for pericarditis alone.
The case-control study is noteworthy for its design, which contrasts with the many recent case series and passive or active surveillance studies, and even the more robust population-based studies of vaccine-related myocarditis, observed Dongngan Truong, MD, University of Utah and Primary Children’s Hospital, both in Salt Lake City, who wasn’t part of the study.
Among its strengths, she said in an interview, are its linkage of comprehensive hospital and vaccination data sets for two different vaccines; and that it corroborates other research suggesting there is “something in particular about mRNA vaccination that seems to be associated with the development of myocarditis.”
Active surveillance in Israel
In an October 2021 report based on an Israeli Ministry of Health database covering up to May 2021, rates of myocarditis arising within 21 days of a second Pfizer-BioNTech dose in 16- to 19-year-olds reached about 1 per 6,637 males and 1 per 99,853 females. Those numbers compared with 1 per 26,000 males and 1 per 218,000 females across all age groups.
Now authors led by Dror Mevorach, MD, Hadassah Medical Center, Jerusalem, have published corresponding numbers from the same data base for myocarditis associated with the same vaccine in males and females aged 12-15.
Their research covers 404,407 people in that age group who received a first dose of the mRNA-based vaccine and 326,463 who received the second dose from June to October, 2021. Only 18 cases of myocarditis were observed within 21 days of either dose.
The estimated rates for males were 0.56 cases per 100,000 after a first dose and 8.09 cases per 100,000 after a second dose.
For females, the estimates were 0 cases per 100,000 after a first dose and 0.69 cases per 100,000 after a second dose.
“The pattern observed, mainly following the second vaccination in males, suggests causality,” the group wrote.
Leveraging passive surveillance reports
Another new report adds a twist to updated numbers from the U.S. Vaccine Adverse Event Reporting System (VAERS).
Prevalences derived from the passive-surveillance data base, known for including case records of inconsistent quality or completeness, are considered especially prone to reporting bias, the authors acknowledged.
The current analysis, however, plunges deep into VAERS-reported cases of presumed SARS-CoV-2 vaccine-associated myocarditis to help clarify “more of the characteristics of the patients and some of the treatments and short-term outcomes,” Matthew E. Oster, MD, MPH, said in an interview.
Dr. Oster, from the Centers for Disease Control and Prevention and Emory University, Atlanta, is lead author on the study’s Jan. 25, 2022, publication in JAMA.
The group reviewed charts and interviewed involved clinicians to adjudicate and document presentations, therapies, and the clinical course of cases reported as SARS-CoV-2 vaccine–associated myocarditis from December 2020 to August 2021. Out of the nearly 2000 reports, which were limited to patients younger than 30, the group identified 1,626 likely cases of such myocarditis arising within 7 days of a second mRNA vaccine dose.
The confirmed cases consistently represented higher prevalences than expected compared with prepandemic myocarditis claims data for both sexes and across age groups spanning 12-29 years.
For example, rates were highest for adolescent males – about 106 and 71 cases per million second doses of the Pfizer-BioNTech vaccine in those aged 16-17 and 12-16, respectively, for example. They were lowest for women aged 25-29, at 2.23 cases per million second Pfizer-BioNTech doses; the highest rate among females was about 11 per million for the 16-17 age group.
The observed rates, Dr. Oster said, represent an update to VAERS numbers published June 2021 in Morbidity and Mortality Weekly Report covering cases through June 2021.
“Overall, the general risk of having myocarditis from the vaccines is still extremely low. Even in the highest risk groups, it is still extremely low, and still lower than the risk of having cardiac complications from COVID,” he noted.
How do patients fare clinically?
From their chart reviews and interviews with case clinicians, Dr. Oster said, “we started to learn quickly that this is really a different type of myocarditis.”
For example, its onset, typically within a few days of the potential immunologic cause, was more rapid than in viral myocarditis, and its symptoms resolved faster, the report notes. Clinical presentations tended to be less severe, treatments not as intensive, and outcomes not as serious, compared with “the kind of typical viral myocarditis that most of the providers were used to taking care of in the past,” he said. “The pattern for these cases was very consistent.”
The study covered VAERS reports of suspected myocarditis arising within a week of first dose of a mRNA-based vaccine from the United States launch of public vaccination in December 2020 to August 2021, the CDC-based group reported. By then, more than 192 million people in the country had received either the Pfizer-BioNTech (age 12 or older) or Moderna (age 18 or older) vaccines.
Of the 1,991 reports of myocarditis, including 391 also involving pericarditis, 1,626 met the study’s definition for myocarditis on adjudication; about 82% of the latter cases were in males.
Based on the investigators’ review of charts and clinician interviews connected with 826 cases that met their definition of myocarditis in patients younger than 30, 89% reported “chest pain, pressure, or discomfort” and 30% reported dyspnea or shortness of breath. Troponin levels were elevated in 98%, 72% of patients who underwent electrocardiography showed abnormalities, and 12% of those with echocardiography had left ventricular ejection fractions less than 50%.
About 96% were hospitalized, and presenting symptoms resolved by discharge in 87% of those with available data, the group noted. Among patients with data on in-hospital therapy, they wrote, NSAIDs were the most common therapy, in 87%.
‘Mild and self-limiting’
The case-control study from Hong Kong didn’t specifically examine patients’ treatment and clinical course, but it does portray their vaccine-associated myocarditis as contrasting with more familiar viral myocarditis.
Patients with “typical” myocarditis tend to be “overall much sicker than what we’re seeing with myocarditis following vaccination,” Dr. Truong agreed. None of the 20 patients with myocarditis after Pfizer-BioNTech vaccination in Hong Kong were admitted to the intensive care unit. That, she added, suggests none required extracorporeal membrane oxygenation or vasoactive support, often necessary in viral myocarditis. “And they had shorter hospital stays.”
In contrast, Dr. Wong noted, 14 of the study’s unvaccinated patients required ICU admission; 12 of them died during the follow-up period. None with vaccine-related carditis died during the study’s follow-up. “We also showed that cases following [Pfizer-BioNTech] vaccination were all mild and self-limiting.”
Dr. Truong largely agreed that SARS-CoV-2 vaccine myocarditis and most myocarditis seen before the pandemic can be viewed as distinct clinical entities, “at least in the short term. I think we do need to follow these patients to look at more long-term outcomes, because at this point I don’t think we know the long-term implications. But at least in the short term, it seems like these patients are different, are much less sick, and recover pretty quickly overall.”
Dr. Oster emphasized that the many and varied acute and long-term hazards from contracting COVID-19 far outweigh any risk for myocarditis from vaccination. But for individuals who were hit with myocarditis soon after their first mRNA vaccine dose, who have already established their susceptibility, he and his colleagues would recommend that they “consider alternatives and not get the vaccine again.”
Dr. Oster reported no relevant financial relationships. Dr. Wong and colleagues did not report any relevant disclosures. Dr. Truong has previously disclosed serving as a consultant to Pfizer.
A version of this article first appeared on Medscape.com.
Celebratory binge drinking a potential trigger for new-onset AFib
Emergency department visits for atrial fibrillation (AFib) appear to go up on days around some annual events in the United States that many people commemorate by consuming alcohol in excess – think Christmas, New Year’s Day, and Super Bowl Sunday.
The novel finding seemed especially true for people without a previous AFib diagnosis, suggesting that alcohol intake, and especially binge drinking, “may acutely enhance the risk” of new-onset AFib, propose researchers in their Jan. 12 report for the inaugural issue of Nature Cardiovascular Research.
Leveraging an international database of breathalyzer test results, the group saw jumps in alcohol intake across several days surrounding eight “recurrent, nationally recognized events,” which also included U.S. Independence Day and the FIFA World Cup.
They then compared the timing of those events to ED visits linked to acute alcohol ingestion and, separately, to ED visits coded for AFib in 10 years of data that cover all of California.
Collectively, the eight annual occasions for heavy alcohol use corresponded to spikes in both kinds of ED visit. Their relationship to AFib-related visits overall grew in strength when the analysis was restricted to new AFib diagnoses.
The researchers acknowledge the limitations of their observational study. Still, the findings represent “the first evidence that acute exposure to alcohol can lead to a given atrial fib episode in a short period of time, even among those without an established AFib diagnosis,” senior author Gregory M. Marcus, MD, MAS, University of California, San Francisco, told this news organization.
“The observation that this was detectable in the general population is a warning to those who drink heavily that any one episode of excessive alcohol consumption could land them in the ED with atrial fibrillation,” he said.
It’s “definitely speculation,” but such ED visits could represent an opportunity for individuals to link their new arrhythmia with a specific episode of excessive drinking, strengthening the message that the two are likely connected, Dr. Marcus observed. The experience could potentially inspire some to “reduce or eliminate” their alcohol intake in an effort to avoid future AFib.
The group obtained data from 2014 to 2016 on more than 1.2 million breath alcohol measurements from about 36,000 people in 59 countries, half residing in the United States, who used commercially available breathalyzer devices from one manufacturer (BACtrack).
The 8 days marking recurrent nationally recognized events, and the days before and after them, were associated with mean blood-alcohol concentrations in the top fifth percentile for the year.
The same eight occasions marked significant bumps in ED visits related to acute alcohol ingestion in records from the California Office of Statewide Health Planning and Development (OSHPD), which documented almost 1.2 million such visits from 2005 to 2015.
Collectively in adjusted analysis, the eight nationally recognized events, compared with other days of the year, accounted for 2,640 excess alcohol-related ED visits per 100,000 person-years across all of California (P < .001).
Separately, ED visits coded for a diagnosis of AFib concentrated significantly around those same 8 days, on which there was an excess of 719 such AFib-related visits per 100,000 person-years (P = .008).
The analysis was replicated after exclusion of OSHPD records from anyone with a previous AFib-related ED visit or hospitalization, or previous outpatient procedure related to AFib, such as ablation or cardioversion. It saw 1,757 excess ED visits per 100,000 person-years (P < .001) for what was considered new-onset AFib in association with the eight nationally recognized events, compared with the rest of the year.
The implication, that a bout of alcohol use leading to an ED visit can acutely raise the risk for a first episode of AFib, was subjected to a “negative control analysis” that focused on ED visits for supraventricular tachycardia. It showed no significant relationships with the eight nationally recognized events.
“We think that helps demonstrate that it’s not just more ED visits, more palpitations, or more heart-related visits per se” associated with acute alcohol use, Dr. Marcus said, “but that it’s something fairly specific to AFib.”
The authors declare no competing interests. Dr. Marcus has previously reported research with Medtronic, Eight Sleep, and Baylis; consulting for InCarda Therapeutics and Johnson & Johnson; and equity in InCarda Therapeutics as cofounder.
A version of this article first appeared on Medscape.com.
Emergency department visits for atrial fibrillation (AFib) appear to go up on days around some annual events in the United States that many people commemorate by consuming alcohol in excess – think Christmas, New Year’s Day, and Super Bowl Sunday.
The novel finding seemed especially true for people without a previous AFib diagnosis, suggesting that alcohol intake, and especially binge drinking, “may acutely enhance the risk” of new-onset AFib, propose researchers in their Jan. 12 report for the inaugural issue of Nature Cardiovascular Research.
Leveraging an international database of breathalyzer test results, the group saw jumps in alcohol intake across several days surrounding eight “recurrent, nationally recognized events,” which also included U.S. Independence Day and the FIFA World Cup.
They then compared the timing of those events to ED visits linked to acute alcohol ingestion and, separately, to ED visits coded for AFib in 10 years of data that cover all of California.
Collectively, the eight annual occasions for heavy alcohol use corresponded to spikes in both kinds of ED visit. Their relationship to AFib-related visits overall grew in strength when the analysis was restricted to new AFib diagnoses.
The researchers acknowledge the limitations of their observational study. Still, the findings represent “the first evidence that acute exposure to alcohol can lead to a given atrial fib episode in a short period of time, even among those without an established AFib diagnosis,” senior author Gregory M. Marcus, MD, MAS, University of California, San Francisco, told this news organization.
“The observation that this was detectable in the general population is a warning to those who drink heavily that any one episode of excessive alcohol consumption could land them in the ED with atrial fibrillation,” he said.
It’s “definitely speculation,” but such ED visits could represent an opportunity for individuals to link their new arrhythmia with a specific episode of excessive drinking, strengthening the message that the two are likely connected, Dr. Marcus observed. The experience could potentially inspire some to “reduce or eliminate” their alcohol intake in an effort to avoid future AFib.
The group obtained data from 2014 to 2016 on more than 1.2 million breath alcohol measurements from about 36,000 people in 59 countries, half residing in the United States, who used commercially available breathalyzer devices from one manufacturer (BACtrack).
The 8 days marking recurrent nationally recognized events, and the days before and after them, were associated with mean blood-alcohol concentrations in the top fifth percentile for the year.
The same eight occasions marked significant bumps in ED visits related to acute alcohol ingestion in records from the California Office of Statewide Health Planning and Development (OSHPD), which documented almost 1.2 million such visits from 2005 to 2015.
Collectively in adjusted analysis, the eight nationally recognized events, compared with other days of the year, accounted for 2,640 excess alcohol-related ED visits per 100,000 person-years across all of California (P < .001).
Separately, ED visits coded for a diagnosis of AFib concentrated significantly around those same 8 days, on which there was an excess of 719 such AFib-related visits per 100,000 person-years (P = .008).
The analysis was replicated after exclusion of OSHPD records from anyone with a previous AFib-related ED visit or hospitalization, or previous outpatient procedure related to AFib, such as ablation or cardioversion. It saw 1,757 excess ED visits per 100,000 person-years (P < .001) for what was considered new-onset AFib in association with the eight nationally recognized events, compared with the rest of the year.
The implication, that a bout of alcohol use leading to an ED visit can acutely raise the risk for a first episode of AFib, was subjected to a “negative control analysis” that focused on ED visits for supraventricular tachycardia. It showed no significant relationships with the eight nationally recognized events.
“We think that helps demonstrate that it’s not just more ED visits, more palpitations, or more heart-related visits per se” associated with acute alcohol use, Dr. Marcus said, “but that it’s something fairly specific to AFib.”
The authors declare no competing interests. Dr. Marcus has previously reported research with Medtronic, Eight Sleep, and Baylis; consulting for InCarda Therapeutics and Johnson & Johnson; and equity in InCarda Therapeutics as cofounder.
A version of this article first appeared on Medscape.com.
Emergency department visits for atrial fibrillation (AFib) appear to go up on days around some annual events in the United States that many people commemorate by consuming alcohol in excess – think Christmas, New Year’s Day, and Super Bowl Sunday.
The novel finding seemed especially true for people without a previous AFib diagnosis, suggesting that alcohol intake, and especially binge drinking, “may acutely enhance the risk” of new-onset AFib, propose researchers in their Jan. 12 report for the inaugural issue of Nature Cardiovascular Research.
Leveraging an international database of breathalyzer test results, the group saw jumps in alcohol intake across several days surrounding eight “recurrent, nationally recognized events,” which also included U.S. Independence Day and the FIFA World Cup.
They then compared the timing of those events to ED visits linked to acute alcohol ingestion and, separately, to ED visits coded for AFib in 10 years of data that cover all of California.
Collectively, the eight annual occasions for heavy alcohol use corresponded to spikes in both kinds of ED visit. Their relationship to AFib-related visits overall grew in strength when the analysis was restricted to new AFib diagnoses.
The researchers acknowledge the limitations of their observational study. Still, the findings represent “the first evidence that acute exposure to alcohol can lead to a given atrial fib episode in a short period of time, even among those without an established AFib diagnosis,” senior author Gregory M. Marcus, MD, MAS, University of California, San Francisco, told this news organization.
“The observation that this was detectable in the general population is a warning to those who drink heavily that any one episode of excessive alcohol consumption could land them in the ED with atrial fibrillation,” he said.
It’s “definitely speculation,” but such ED visits could represent an opportunity for individuals to link their new arrhythmia with a specific episode of excessive drinking, strengthening the message that the two are likely connected, Dr. Marcus observed. The experience could potentially inspire some to “reduce or eliminate” their alcohol intake in an effort to avoid future AFib.
The group obtained data from 2014 to 2016 on more than 1.2 million breath alcohol measurements from about 36,000 people in 59 countries, half residing in the United States, who used commercially available breathalyzer devices from one manufacturer (BACtrack).
The 8 days marking recurrent nationally recognized events, and the days before and after them, were associated with mean blood-alcohol concentrations in the top fifth percentile for the year.
The same eight occasions marked significant bumps in ED visits related to acute alcohol ingestion in records from the California Office of Statewide Health Planning and Development (OSHPD), which documented almost 1.2 million such visits from 2005 to 2015.
Collectively in adjusted analysis, the eight nationally recognized events, compared with other days of the year, accounted for 2,640 excess alcohol-related ED visits per 100,000 person-years across all of California (P < .001).
Separately, ED visits coded for a diagnosis of AFib concentrated significantly around those same 8 days, on which there was an excess of 719 such AFib-related visits per 100,000 person-years (P = .008).
The analysis was replicated after exclusion of OSHPD records from anyone with a previous AFib-related ED visit or hospitalization, or previous outpatient procedure related to AFib, such as ablation or cardioversion. It saw 1,757 excess ED visits per 100,000 person-years (P < .001) for what was considered new-onset AFib in association with the eight nationally recognized events, compared with the rest of the year.
The implication, that a bout of alcohol use leading to an ED visit can acutely raise the risk for a first episode of AFib, was subjected to a “negative control analysis” that focused on ED visits for supraventricular tachycardia. It showed no significant relationships with the eight nationally recognized events.
“We think that helps demonstrate that it’s not just more ED visits, more palpitations, or more heart-related visits per se” associated with acute alcohol use, Dr. Marcus said, “but that it’s something fairly specific to AFib.”
The authors declare no competing interests. Dr. Marcus has previously reported research with Medtronic, Eight Sleep, and Baylis; consulting for InCarda Therapeutics and Johnson & Johnson; and equity in InCarda Therapeutics as cofounder.
A version of this article first appeared on Medscape.com.
What does a pig-to-human heart transplant mean for medicine?
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Scientific achievements usually raise big new questions, and the remarkable surgery that took place on Jan. 7, when Maryland resident David Bennett was transplanted with a genetically modified heart from a pig, has been no different.
The 57-year-old with end-stage heart failure had been repeatedly turned down for a standard transplant and was judged a poor candidate for a ventricular assist device. Now his new heart is beating soundly and apparently accepted by his immune system as Mr. Bennett, his physicians at the University of Maryland where the procedure took place, and indeed the world set out on a journey with far more unknowns than knowns.
“I think even just a couple of years ago, people felt that xenotransplantation for the heart and other organs was still a long way off. And it seems like it’s started to move very quickly,” Larry A. Allen, MD, University of Colorado, Aurora, said in an interview.
Demand for donor hearts far outstrips supply, and despite advances in the development of ventricular assist pumps and artificial hearts, “there are still significant limitations to them in terms of clotting, stroke, and infection. We’ve seen the use of those devices plateau,” Dr. Allen said. “So, the concept of a nonhuman source of organs is exciting and very much in need, if people can get it to work.”
“I really credit the surgeons at the University of Maryland for courageous clinical work and a brilliant scientific innovation,” Clyde W. Yancy, MD, MSc, Northwestern University, Chicago, said in an interview. “But it’s always in the implementation that we have to hold our breath.” Heart xenotransplantation is an old idea that “has never before been successful,” he said. And standard heart transplantation has set a high bar, with a 1-year survival of about 90% and low 1-year risk for rejection. Whether the new procedure can meet that standard is unknown, as is its potential for complications, such as chronic rejection or cancers due to long-term immunosuppression. Those are “major questions requiring more time and careful follow-up.”
‘Still a nascent technology’
“This is an exciting and courageous step forward in heart transplantation, and kudos to the team at the University of Maryland,” said Mandeep R. Mehra, MD, Brigham and Woman’s Hospital, Boston. But “there are many challenges here.”
The procedure’s 10 gene modifications were reportedly aimed at preventing hyperacute rejection of the heart and its excessive growth after transplantation, and making the organ less immunogenic, Dr. Mehra said in an interview. But even if those goals are met, could the same changes potentially impede the heart’s adaptation to human physiology, such as during ambulation or stress?
That kind of adaptation may become important. For example, Dr. Mehra observed, normally a pig heart “provides flow in a four-footed configuration, and pig temperature is inherently higher than humans by several degrees, so it will be functioning in a relatively hypothermic environment.”
Transplantation remains the gold standard for patients with advanced heart failure despite modern medical and device therapy, Dr. Allen agreed. But “if we can raise pig hearts that provide the organ, and it can be implanted with a surgery that’s been done for 50 years, and rejection can be managed with gene editing and tailored immunosuppression, then it’s not hard to think about this very rapidly replacing a lot of what we do in the advanced heart failure and transplantation world.”
Certainly, it would be a major advance if the gene editing technique successfully improves the heart’s immunologic compatibility, Dr. Yancy noted. But do we have enough genomic knowledge to select gene deletions and insertions in the safest way for a successful outcome? “We have to appreciate that this is still a nascent technology, and we should be careful that there might be consequences that we haven’t anticipated.”
For example, he said, the xenotransplantation and gene-modifying techniques should be explored in a range of patients, including older and younger people, women and men, and people of different ethnicities and races.
“There may be some differences based on ancestry, based on gender, based on aging, that will influence the way in which these engineered donor hearts are experienced clinically,” Dr. Yancy said.
The xenotransplantation technique’s potential impact on health equity should also be considered, as it “almost assuredly will be a very expensive technology that will be utilized in a very select population,” he noted. “We need to have a really wide lens to think about all of the potential ramifications.”
‘This field needs to evolve’
Dr. Mehra also flagged the procedure’s potential cost should it become mainstream. Perhaps that would promote dialogue on how to primarily use it “after legitimately exhausting all available options, such as total artificial heart support.”
It might also teach the field to take greater advantage of the many donated hearts discarded as suboptimal. “The general usage rate for offered organs is around a third,” despite opportunities to expand use of those that are “less than perfect,” Dr. Mehra said. “I think that the field will grow with the community focusing on reduced discards of current available heart organs, and not necessarily grow because of the availability of ‘xeno-organs.’ ”
“This field needs to evolve because we’re actively transplanting patients today. But in my mind, the real future is to have such a sufficient understanding of the biology of left ventricular dysfunction that transplantation is a rare event,” Dr. Yancy proposed.
“I’m not certain that heart transplantation per se is the endgame. I think the avoidance of transplantation is the real endgame,” he said. “This may be controversial, but my vision of the future is not one where we have a supply of animals that we can use for transplantation. My vision of the future is that heart transplantation becomes obsolete.”
A version of this article first appeared on Medscape.com.
Pig heart successfully transplanted to man
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
A genetically modified pig heart has been successfully transplanted into a 57-year-old man who had no other treatment options but is “doing well” 3 days after the procedure, officials at the University of Maryland Medical Center (UMMC), Baltimore, announced Jan. 10.
“This organ transplant demonstrated for the first time that a genetically modified animal heart can function like a human heart without immediate rejection by the body,” they said.
Three genes associated with antibody-mediated rejection had been knocked out in the pig supplying the transplanted heart, and six human genes associated with immune acceptance of the organ had been inserted into the pig’s genome, notes a UMMC press release.
“Lastly, one additional gene in the pig was knocked out to prevent excessive growth of the pig heart tissue, which totaled 10 unique gene edits made in the donor pig,” the release states.
The patient, Maryland resident David Bennett, had required mechanical circulatory support to stay alive but was rejected for standard heart transplantation at UMMC and other centers. He was ineligible for an implanted ventricular assist device due to ventricular arrhythmias.
Mr. Bennett “is being carefully monitored over the next days and weeks to determine whether the transplant provides lifesaving benefits,” the announcement says.
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future,” notes a quote from Bartley P. Griffith, MD, the UMMC surgeon who performed the procedure.
The pig supplying the heart was provided to the center by Revivicor (Blacksburg, Virginia), a regenerative medicine company. An experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Massachusetts) was also used, in addition to standard immunosuppressants.
A version of this article first appeared on Medscape.com.
COVID-vaccine myocarditis: Rare, mild, and usually in young men
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
The risk of myocarditis after immunization with mRNA-based vaccines against SARS-CoV-2 raised concerns when it came to light in early 2021. But as report after report showed such cases to be rare and usually mild and self-limited, focus has turned to the “how and why.”
The mechanism linking the BNT162b2 (Pfizer-BioNTech) and especially mRNA-1273 (Moderna) vaccines to the occurrence of myocarditis is unclear for now, but one potential driver may be tied to a peculiarity that became apparent early: It occurs overwhelmingly in younger males, from 16 to perhaps 40 or 50 years of age. Excess risk has not been consistently seen among women, girls, and older men.
That observation has led to speculation that higher testosterone levels in adolescent boys and young men may somehow promote the adverse vaccine effect, whereas greater levels of estrogen among girls and women in the same age range may be cardioprotective.
Unlikely, brief, and ‘benign’
“Most of the myocarditis is benign, by which I mean that maybe the patients are admitted due to chest pain, but without reduction in ventricular function,” Enrico Ammirati, MD, PhD, a myocarditis expert at De Gasperis Cardio Center and Transplant Center, Niguarda Hospital, Milan, said in an interview.
In a Nov. 14 address on this topic at the annual scientific sessions of the American Heart Association, Dror Mevorach, MD, described the typical case presentation as “mild” and one that clears in fairly short order based on resolution of “clinical symptoms, inflammatory markers and troponin decline, EKG normalization, echo normalization, and a relatively short length of hospital stay.”
Dr. Mevorach, of Hadassah Hebrew University Medical Center, Jerusalem, subsequently published the findings in a report in the New England Journal of Medicine that described 136 confirmed myocarditis cases among more than 5 million people in Israel immunized with the Pfizer-BioNTech vaccine. Myocarditis was considered “mild” in 129 cases, or 95%.
And the risk is tiny, compared with myocarditis from infection by SARS-CoV-2, not to mention the possibility of nasty clinical COVID-19 complications such as pneumonia and pulmonary embolism, Dr. Mevorach observed.
Many other reports agree that the incidence is minimal, especially given the rewards of vaccination. In a separate NEJM publication in September 2021 – from Noam Barda, MD, Clalit (Israel) Research Institute, and colleagues on 1.7 million people in that country, about half unvaccinated and half given the Pfizer-BioNTech vaccine – there were an estimated 2.7 cases of myocarditis per 100,000 vaccinated persons. There were also 11 cases of myocarditis per 100,000 persons who were positive for SARS-CoV-2 infection.
And in a recent case series of vaccinated people aged 16 or older, the myocarditis rate after a first or second Pfizer-BioNTech or Moderna injection was estimated at 1 or fewer per 100,000. The corresponding estimate was 4 such cases per 100,000 after a positive SARS-CoV-2 test among the same population, notes a report published Dec.14, 2021, in Nature Medicine.
In general, “the risk of any kind of cardiac injury is vastly lower with a vaccine than it is with the actual viral infection,” Leslie T. Cooper Jr., MD, a myocarditis expert and clinical trialist at the Mayo Clinic, Jacksonville, Fla., said in an interview. With the mRNA-based vaccines, “we do not have any conceivable danger signal that would outweigh the benefit of vaccination.”
Males of a certain age
Evidence that such myocarditis predominates in young adult men and adolescent boys, especially following a second vaccine dose, is remarkably consistent.
The risk was elevated only among mRNA-based vaccine recipients who were younger than 40 in the recent Nature Medicine analysis. Among that group, estimates after a second dose numbered fewer than 1 case per 100,000 for Pfizer-BioNTech and 1.5 per 100,000 for Moderna.
In a third analysis from Israel – also in NEJM, from Guy Witberg, MD, Rabin Medical Center, Petah Tikva, and colleagues, based on 2.5 million people aged 16 and older with at least one Pfizer-BioNTech injection – 2.1 cases per 100,000 were estimated overall, but the number rose to 10.7 per 100,000 among those aged 16-29 years.
In Dr. Mevorach’s NEJM report, estimates after a second Pfizer-BioNTech vaccine dose were 1 per 26,000 males versus 1 in 218,000 females, compared with 1 myocarditis case in 10,857 persons among “the general unvaccinated population.”
Most recipients of a first vaccine dose were younger than 50, and 16- to 29-year-olds accounted for most who completed two doses, noted Dr. Mevorach. Younger males bore the brunt of any myocarditis: the estimated prevalence after a second dose among males aged 16-19 was 1 per 6,637, compared with 1 per 99,853 females in the same age range, the group reported.
In the BMJ report, based on about 5 million people 12 years of age or older in Denmark, the estimated rates of myocarditis or pericarditis associated with Moderna immunization were 2 per 100,000 among women but 6.3 per 100,000 for men. The incidence and sex difference was much lower among those getting the Pfizer-BioNTech vaccine: 1.3 per 100,000 and 1.5 per 100,000 in women and men, respectively.
Sex hormones may be key
The predominance of vaccine-associated myocarditis among adolescent and young adult males is probably more about the myocarditis itself than the vaccines, observed Biykem Bozkurt, MD, PhD, who has been studying COVID-related myocarditis at Baylor College of Medicine, Houston.
Male sex historically is associated in both epidemiologic studies and experimental models with a greater propensity for most any form of myocarditis, Dr. Bozkurt said in an interview. Given that males aged 16-19 or so appear to be at highest risk of myocarditis as a complication of SARS-CoV-2 vaccination, the mechanism may well be related to sex hormones.
“Therefore, testosterone is implicated as a player in their higher risk of inflammation and injury and lack of adaptive response in terms of healing, and in terms of prevention of injury,” Dr. Bozkurt said. For its part, estrogen inhibits proinflammatory processes and, in particular, “blunts cell-mediated immune responses.”
“We don’t know the mechanism, but a theory that attributes a protective role to estrogen, or a risk associated with testosterone, is reasonable. It makes sense, at least based on epidemiological data,” Dr. Ammirati agreed. Still, “we do not have any direct evidence in human beings.”
Sex-associated differences in experimental myocarditis have been reported in the journals for at least 70 years, but “the testosterone literature and the estrogen literature have not been evaluated in detail in vaccine-associated myocarditis,” Dr. Cooper said.
Most myocarditis in the laboratory is viral, Dr. Cooper observed, and “the links between testosterone, viruses, and inflammation have been pretty well worked out, I would say, if you’re a mouse. If you’re a human, I think it’s still a bit uncertain.”
Were it to apply in humans, greater testosterone levels might independently promote myocarditis, “and if estrogen is cardioprotective, it would be another mechanism,” Dr. Cooper said. “That would translate to slight male predominance in most kinds of myocarditis.”
In males, compared with females, “the heart can be more vulnerable to events such as arrhythmias or to immune-mediated phenomena. So, probably there is also higher vulnerability to myocarditis in men,” Dr. Ammirati noted.
Male predominance in vaccine-related myocarditis is provocative, so it’s worth considering whether testosterone is part of the mechanism as well as the possibility of estrogen cardioprotection, Dr. Ammirati said. But given limitations of the animal models, “we don’t really have robust data to support any part of that.”
Although myocarditis is in some way immune mediated, “and hormones can modulate the response,” the mechanism has to be more than just sex hormones, he said. “They probably cannot explain the specificity for the heart. It’s not a systemic response, it’s an organ-specific response.”
Modulation of immune responses
Details about the immune processes underlying mRNA-vaccine myocarditis, hormone modulated or not, have been elusive. The complication doesn’t resemble serum sickness, nor does it seem to be a reaction to infection by other cardiotropic viruses, such as coxsackie virus B, a cause of viral myocarditis, Dr. Bozkurt said. The latter had been a compelling possibility because such hypersensitivity to smallpox vaccination is well recognized.
“We don’t know the mechanism, that’s the short answer. But there are many hypotheses,” she said. One candidate widely proposed in the literature: autoantibodies driven by molecular mimicry between the SARS-CoV-2 spike protein targeted by the mRNA vaccines and a structurally similar myocardial protein, possibly alpha-myosin, noted Dr. Bozkurt and colleagues in a recent publication.
But elevations in specific “antiheart antibodies” have not been documented in recipients of the two mRNA-based vaccines, said Dr. Cooper. “So, I would say that – although molecular mimicry is a well-established mechanism of, for example, rheumatic carditis after a streptococcal A infection – that has not been demonstrated yet for COVID-19 mRNA vaccination–related myocarditis.”
“We probably won’t know, ever, with a huge level of certainty, the exact mechanisms,” Dr. Cooper added. There is no animal model for vaccine-induced myocarditis, and “We’re still talking very, very small numbers of patients. The vast majority of them recover,” and so don’t generally provide mechanistic clues.
Prospects for younger children
Vaccination against SARS-CoV-2 has now been authorized by the Centers for Disease Control and Prevention for kids as young as 5-11 years, using the Pfizer-BioNTech vaccine. Experience so far suggests the immunization is safe in that age group with negligible risk of myocarditis or other complications. But with prospects of possible authorization in children younger than 5, should myocarditis be a concern for them?
Probably not, if the complication is driven primarily by sex hormones, Dr. Cooper proposed. “One would predict that before puberty you would have a lower – much, much lower – rate of myocarditis in males than you would in the 16- to 19-year-old range, and that it would be roughly equal to females.” Dr. Ammirati and Dr. Bozkurt largely agreed.
It remains to be seen whether the vaccine-related myocarditis risk applies to children younger than 12, “but I doubt it. I think it’s going to be puberty-related,” Dr. Bozkurt said. Still, “I don’t want to hypothesize without data.”
A version of this article first appeared on Medscape.com.
FDA approves first-in-class inclisiran to lower LDL-C
The Food and Drug Administration has approved inclisiran (Leqvio) as an adjunct to statins for further reduction of LDL cholesterol levels, the drug’s developer, Novartis, announced on Dec. 22, 2021.
The first-in-class small interfering RNA (siRNA) agent is also novel among peer drug therapies for its administration by injection initially, at 3 months, and thereafter twice per year.
Inclisiran is indicated for use atop maximally tolerated statins in adults with clinical cardiovascular disease or in patients with heterozygous familial hypercholesterolemia, the company reported.
Such patients who received inclisiran, compared with placebo, in the ORION-9, ORION-10, and ORION-11 randomized trials on which the FDA approval was based showed LDL-C reductions exceeding 50% over 1-2 years.
The drug works by “silencing” RNA involved in synthesis of PCSK9, which has a role in controlling the number of LDL cholesterol cell-surface receptors, a unique mechanism of action among available treatments for dyslipidemia.
Novartis, the company said, “has obtained global rights to develop, manufacture, and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved inclisiran (Leqvio) as an adjunct to statins for further reduction of LDL cholesterol levels, the drug’s developer, Novartis, announced on Dec. 22, 2021.
The first-in-class small interfering RNA (siRNA) agent is also novel among peer drug therapies for its administration by injection initially, at 3 months, and thereafter twice per year.
Inclisiran is indicated for use atop maximally tolerated statins in adults with clinical cardiovascular disease or in patients with heterozygous familial hypercholesterolemia, the company reported.
Such patients who received inclisiran, compared with placebo, in the ORION-9, ORION-10, and ORION-11 randomized trials on which the FDA approval was based showed LDL-C reductions exceeding 50% over 1-2 years.
The drug works by “silencing” RNA involved in synthesis of PCSK9, which has a role in controlling the number of LDL cholesterol cell-surface receptors, a unique mechanism of action among available treatments for dyslipidemia.
Novartis, the company said, “has obtained global rights to develop, manufacture, and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved inclisiran (Leqvio) as an adjunct to statins for further reduction of LDL cholesterol levels, the drug’s developer, Novartis, announced on Dec. 22, 2021.
The first-in-class small interfering RNA (siRNA) agent is also novel among peer drug therapies for its administration by injection initially, at 3 months, and thereafter twice per year.
Inclisiran is indicated for use atop maximally tolerated statins in adults with clinical cardiovascular disease or in patients with heterozygous familial hypercholesterolemia, the company reported.
Such patients who received inclisiran, compared with placebo, in the ORION-9, ORION-10, and ORION-11 randomized trials on which the FDA approval was based showed LDL-C reductions exceeding 50% over 1-2 years.
The drug works by “silencing” RNA involved in synthesis of PCSK9, which has a role in controlling the number of LDL cholesterol cell-surface receptors, a unique mechanism of action among available treatments for dyslipidemia.
Novartis, the company said, “has obtained global rights to develop, manufacture, and commercialize Leqvio under a license and collaboration agreement with Alnylam Pharmaceuticals.”
A version of this article first appeared on Medscape.com.
AHA challenges diet doctor’s study alleging COVID vax risks
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
An abstract and poster presentation questioning the safety of mRNA-based COVID-19 vaccines, embraced by some and lambasted by others, has drawn an “expression of concern” from the American Heart Association, along with a bid for correction.
The abstract in question concludes that COVID vaccines “dramatically increase” levels of certain inflammatory biomarkers, and therefore, the 5-year risk of acute coronary syndromes (ACS), based on pre- and post-vaccination results of an obscure blood panel called the PULS Cardiac Test (GD Biosciences). The findings were presented at the AHA’s 2021 Scientific Sessionsas, an uncontrolled observational study of 566 patients in a preventive cardiology practice.
Some on social media have seized on the abstract as evidence of serious potential harm from the two available mRNA-based SARS-CoV-2 vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). But others contend that the study’s described design and findings are specious and its conclusions overstated.
They also point to the notoriety of its one listed author, Steven R. Gundry, MD, who promotes his diet books and supplements as well as fringe, highly criticized theories about diet and disease on several websites, including drgundry.com. Dr. Gundry has not responded to requests for an interview.
Dr. Gundry’s abstract from the AHA Scientific Sessions 2021, available on the meeting’s program planner, was marked with an “expression of concern” by the AHA that is to stand “until a suitable correction is published, to indicate that the abstract in its current version may not be reliable.”
The expression of concern statement, also published online Nov. 24 in Circulation, says “potential errors in the abstract” were brought to the attention of the meeting planners. “Specifically, there are several typographical errors, there is no data in the abstract regarding myocardial T-cell infiltration, there are no statistical analyses for significance provided, and the author is not clear that only anecdotal data was used.”
The biomarker elevations on which the abstract’s conclusions are based included hepatocyte growth factor, “which serves as a marker for chemotaxis of T-cells into epithelium and cardiac tissue,” it states.
“The expression of concern about the abstract will remain in place until a correction is accepted and published” in Circulation, AHA spokesperson Suzanne Grant told this news organization by email.
“The specific data needed will be up to the abstract author to determine and supply,” she said, noting that Dr. Gundry “has been in communication with the journal throughout this process.”
Submitting researchers “must always attest to the validity of the abstract,” Ms. Grant said. “Abstracts are then curated by independent review panels, blinded to the identities of the abstract authors, and are considered based on the potential to add to the diversity of scientific issues and views discussed at the meeting.”
Regarding the AHA’s system for vetting abstracts vying for acceptance to the scientific sessions, she said it is not primarily intended to “evaluate scientific validity” and that the organization is “currently reviewing its existing abstract submission processes.”
A recent Reuters report reviews the controversy and provides links to criticisms of the study on social media.
A version of this article first appeared on Medscape.com.
Could an oral PCSK9 inhibitor be on the horizon?
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
The investigational PCSK9 inhibitor that Merck showcased recently would be more than a “me-too” drug if it ultimately wins approval, despite competition from several approved agents that slash elevated cholesterol levels by targeting the same protein.
In fact, it would be something of a breakthrough. The new agent under study – now called MK-0616 – comes in pill form, in contrast to the three currently available PCSK9-lowering drugs that must be given in injections separated by weeks to months.
The drug faces an uncertain road to regulatory review and any approval, but MK-0616 at least seems to be starting out in the right direction.
In two phase 1 studies with a total of 100 participants, plasma PCSK9 levels plunged more than 90% after a single dose of the drug; and low-density-lipoprotein cholesterol (LDL-C) levels dropped about 65% when MK-0616 was given daily for 2 weeks on a background of statin therapy.
Moreover, “MK-0616 was generally well tolerated at up to and including single doses of 300 milligrams,” the maximum tested in the studies, Douglas G. Johns, PhD, reported at the virtual American Heart Association scientific sessions.
The collective results from the oral agent’s earliest human experience are “definitely encouraging” and support MK-0616 as a potential LDL-lowering agent that would be more convenient and arguably more accessible to patients compared to current injectable PCSK9 inhibitors, proposed Dr. Johns, clinical director of translational medicine for Merck in Kenilworth, N.J.
Available PCSK9-targeting agents include alirocumab (Praluent, Sanofi/Regeneron), Food and Drug Administration–approved in July 2015, and evolocumab (Repatha, Amgen), approved by the agency the following month. Both are monoclonal antibodies with neutralizing specificity for the PCSK9 protein; whereas the third such agent, inclisiran (Leqvio, Novartis) is a small-molecule interfering-RNA that suppresses PCSK9 synthesis. Inclisiran is approved in the European Union but its case to the FDA was turned down in 2020.
Dr. Johns said MK-0616 is a cyclic peptide that is “about one-hundredth the size of a monoclonal antibody, but we’re able to achieve monoclonal antibody-like potency and selectivity with this much smaller footprint.”
Added to statin therapy, the current PCSK9-targeting agents reduce LDL-C by an additional one-half or more, and the two antibody-based agents “also decrease atherosclerotic cardiovascular events. They are, however, expensive and not always available, requiring insurance or other approval,” observed Anne C. Goldberg, MD, as invited discussant after Dr. Johns’ presentation.
“They require every 2- to 4-week injections. They’re generally reserved for secondary prevention, and sometimes primary prevention as in familial hypercholesterolemia,” said Dr. Goldberg, of Washington University, St. Louis. Inclisiran, she noted, requires injections every 6 months and has yet to show its mettle in cardiovascular outcomes trials.
“Certainly, an oral form would be easier to use,” she said. “This would be particularly helpful in patients averse to injections,” especially, perhaps, in children. “Children with familial hypercholesterolemia could benefit with greater cholesterol lowering and might be better off with a pill than an injection.” That would be good reason to emphasize the enrollment of children in the drug’s upcoming clinical trials, Dr. Goldberg said.
But cost could potentially become restrictive for MK-0616 as well, should it ever be approved. “If it’s priced too high, then are you really going to see the increased use?” she posed. “Certainly, there’s a high bar for therapies that are add-on to statins in terms of cost effectiveness.”
In the first of the two trials, 60 predominantly White male participants aged 50 or younger were randomly assigned to receive a single dose of MK-0616, at different levels ranging from 10 mg to 300 mg, or placebo. They subsequently crossed over to a different group for a second round of dosing. Both times, three participants took the drug for every one who received placebo.
Participants who took the active drug, regardless of dosage, showed greater than 90% reductions in circulating PCSK9 levels compared to baseline. Six participants discontinued the study before its completion.
In the second trial, 40 White adults aged 65 or younger (mean, 58), including 13 women, with LDL-C of 60 mg/dL to 160 mg/dL (mean, 87 mg/dL) on statin therapy for at least 3 months were randomly assigned 3-to-1 to add-on MK-0616, either 10 mg or 20 mg daily, or placebo for 14 days.
LDL-C levels fell an average of about 65% over the 2 weeks among those taking the active drug; they declined less than 5% for those who took placebo.
There were no deaths or serious adverse events in either trial, Dr. Johns reported. On the other hand, pharmacokinetics studies showed that exposure to the drug fell by “about 50%-60%” when dosing was preceded by food intake within the previous 30 minutes. “However, if a meal is consumed 30 minutes after the dose, this food effect is much, much less prominent, almost negligible.”
These preliminary results show the drug is “orally bioavailable and exerts a clinically meaningful effect,” Dr. Johns said. “However, there’s definitely more to be done. And we are planning the next phase of clinical development, a phase 2 trial, sometime next year.”
The research was funded by Merck. Dr. Johns disclosed employment with and equity ownership in Merck, as did all the study’s coauthors. Dr. Goldberg disclosed holding research contracts through her institution with Regeneron/Sanofi-Aventis, Amarin, Amgen, Pfizer, IONIS/Akcea, Regeneron, Novartis, Arrowroot Pharmaceuticals, and the FH Foundation; and consulting for Novartis, Akcea, Regeneron, and Esperion.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Empagliflozin a winner in challenging arena of stabilized acute HF
The sodium-glucose transporter 2 inhibitors, relative newcomers among first-line agents for chronic heart failure (HF), could well attain the same go-to status in patients hospitalized with acute HF if the EMPULSE trial has anything to say about it.
Of the study’s 530 such patients, those started on daily empagliflozin (Jardiance) soon after they were stabilized, compared with a control group, were less likely to die or be rehospitalized for HF over the next 3 months.
Also, “we saw an improvement in quality of life, we saw a greater reduction in body weight, and we didn’t see any safety concerns in this very vulnerable and sick patient population,” Adriaan A. Voors, MD, University Medical Center Groningen (the Netherlands), said when presenting the trial at the American Heart Association scientific sessions.
Patients assigned to empagliflozin had a 36% greater likelihood of showing a benefit as reflected in the treatment’s win ratio when opposed by placebo, an emerging way to express outcomes in cardiovascular clinical trials. The SGLT2 inhibitor’s win ratio for the primary endpoint was 1.36 (95% confidence interval, 1.09-1.68, P = .0054), Dr. Voors reported. The outcome consisted of death, number of HF events, time to first HF event, and 90-day change in quality of life scores.
There is reluctance in practice to start patients that early after decompensation on drugs used in chronic HF, Dr. Voors said in an interview. Empagliflozin in the trial was initiated in the stabilized setting an average of 3 days after hospital admission, he said. The trial should reassure physicians that the drug “is not only safe to start early in hospital, but it’s also beneficial to start early in hospital.”
EMPULSE, combined with support from other recent trials, “should be clinical practice changing, with early in-hospital initiation of SGLT2 inhibitors in patients hospitalized with HF being the expectation, along with clear recognition that delaying SGLT2 inhibitor initiation may expose patients to unnecessary harms and delays in improved health status,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, told this news organization.
“For patients with HF, irrespective of ejection fraction, early in-hospital initiation of SGLT2 inhibitors – once stabilized and in the absence of contraindications – should be considered a new standard of care,” said Fonarow, who was not part of EMPULSE.
The trial also lends new weight to the strategy of “simultaneous or rapid-sequence initiation” of the so-called four pillars of guideline-directed medical therapy of HF with reduced ejection fraction in patients hospitalized with HFrEF, once they are stabilized, Dr. Fonarow said. The four-pronged approach, he noted, consists of sacubitril/valsartan (Entresto), a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor.
Indeed, the new findings “fill an important gap and are clearly practice changing,” agreed Nancy K. Sweitzer, MD, PhD, University of Arizona Sarver Heart Center, Tucson, as an invited discussant following Dr. Voors’ presentation. “Few therapies have been shown to impact the course of those hospitalized with acute decompensated heart failure.”
Of note in the trial, Dr. Sweitzer continued, patients were started on empagliflozin regardless of any drug therapy they might already be on for chronic HF. “Because patients in the EMPULSE trial could be enrolled with a new diagnosis of heart failure, they were, by definition, not all on chronic guideline-directed heart failure therapy. Nevertheless, such patients benefited equally from the study intervention,” she said.
“This is crucial, as it tells us these drugs have immediate and important effects and should not be withheld while other drug classes are initiated and optimized.”
EMPULSE entered patients hospitalized for acute HF, which could be de novo or a decompensation of chronic HF, without regard to ejection fraction or whether they had diabetes, and who were clinically stable after at least one dose of loop diuretics. Their ejection fractions averaged 35% and exceeded 40% in about one-third of the total cohort.
At 90 days in the win ratio analysis, the 265 patients assigned to empagliflozin 10 mg once daily were the “winners”; that is, they were more likely to show a clinical benefit about 54% of the time in paired match-ups of patient outcomes, compared with about 40% for the 265 in the control group. The match-ups were a tie 6.4% of the time.
The empagliflozin group also benefited significantly for the endpoint of death from any cause or first HF event, with a hazard ratio of 0.65 (95% CI, 0.43-0.99; P = .042). They also were less likely to experience acute renal failure (7.7% vs. 12.1% for the control group) or serious adverse events (32.3% vs. 43.6%), Dr. Voors reported.
Tempting as it might be, the findings can’t necessarily be generalized to other SGLT2 inhibitors without an evidence base. But as Dr. Voors observed, several ongoing trials are exploring dapagliflozin (Farxiga) in a similar clinical setting.
They include DICTATE-AHF in patients with diabetes admitted with acute HF, and DAPA ACT HF-TIMI 68, which is entering patients stabilized during hospitalization with acute decompensated HFrEF. The trials are scheduled for completion in 2022 and 2023, respectively.
EMPULSE was supported by the Boehringer Ingelheim–Eli Lilly Diabetes Alliance. Dr. Voors disclosed research support and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. Dr. Sweitzer disclosed honoraria from Acorda and Myokardia, and reported receiving research support from Novartis and Merck. Dr. Fonarow cited honoraria from Abbott, Amgen, Janssen, Medtronic, Bayer, Merck, AstraZeneca, Cytokinetics, and Novartis.
A version of this article first appeared on Medscape.com.
The sodium-glucose transporter 2 inhibitors, relative newcomers among first-line agents for chronic heart failure (HF), could well attain the same go-to status in patients hospitalized with acute HF if the EMPULSE trial has anything to say about it.
Of the study’s 530 such patients, those started on daily empagliflozin (Jardiance) soon after they were stabilized, compared with a control group, were less likely to die or be rehospitalized for HF over the next 3 months.
Also, “we saw an improvement in quality of life, we saw a greater reduction in body weight, and we didn’t see any safety concerns in this very vulnerable and sick patient population,” Adriaan A. Voors, MD, University Medical Center Groningen (the Netherlands), said when presenting the trial at the American Heart Association scientific sessions.
Patients assigned to empagliflozin had a 36% greater likelihood of showing a benefit as reflected in the treatment’s win ratio when opposed by placebo, an emerging way to express outcomes in cardiovascular clinical trials. The SGLT2 inhibitor’s win ratio for the primary endpoint was 1.36 (95% confidence interval, 1.09-1.68, P = .0054), Dr. Voors reported. The outcome consisted of death, number of HF events, time to first HF event, and 90-day change in quality of life scores.
There is reluctance in practice to start patients that early after decompensation on drugs used in chronic HF, Dr. Voors said in an interview. Empagliflozin in the trial was initiated in the stabilized setting an average of 3 days after hospital admission, he said. The trial should reassure physicians that the drug “is not only safe to start early in hospital, but it’s also beneficial to start early in hospital.”
EMPULSE, combined with support from other recent trials, “should be clinical practice changing, with early in-hospital initiation of SGLT2 inhibitors in patients hospitalized with HF being the expectation, along with clear recognition that delaying SGLT2 inhibitor initiation may expose patients to unnecessary harms and delays in improved health status,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, told this news organization.
“For patients with HF, irrespective of ejection fraction, early in-hospital initiation of SGLT2 inhibitors – once stabilized and in the absence of contraindications – should be considered a new standard of care,” said Fonarow, who was not part of EMPULSE.
The trial also lends new weight to the strategy of “simultaneous or rapid-sequence initiation” of the so-called four pillars of guideline-directed medical therapy of HF with reduced ejection fraction in patients hospitalized with HFrEF, once they are stabilized, Dr. Fonarow said. The four-pronged approach, he noted, consists of sacubitril/valsartan (Entresto), a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor.
Indeed, the new findings “fill an important gap and are clearly practice changing,” agreed Nancy K. Sweitzer, MD, PhD, University of Arizona Sarver Heart Center, Tucson, as an invited discussant following Dr. Voors’ presentation. “Few therapies have been shown to impact the course of those hospitalized with acute decompensated heart failure.”
Of note in the trial, Dr. Sweitzer continued, patients were started on empagliflozin regardless of any drug therapy they might already be on for chronic HF. “Because patients in the EMPULSE trial could be enrolled with a new diagnosis of heart failure, they were, by definition, not all on chronic guideline-directed heart failure therapy. Nevertheless, such patients benefited equally from the study intervention,” she said.
“This is crucial, as it tells us these drugs have immediate and important effects and should not be withheld while other drug classes are initiated and optimized.”
EMPULSE entered patients hospitalized for acute HF, which could be de novo or a decompensation of chronic HF, without regard to ejection fraction or whether they had diabetes, and who were clinically stable after at least one dose of loop diuretics. Their ejection fractions averaged 35% and exceeded 40% in about one-third of the total cohort.
At 90 days in the win ratio analysis, the 265 patients assigned to empagliflozin 10 mg once daily were the “winners”; that is, they were more likely to show a clinical benefit about 54% of the time in paired match-ups of patient outcomes, compared with about 40% for the 265 in the control group. The match-ups were a tie 6.4% of the time.
The empagliflozin group also benefited significantly for the endpoint of death from any cause or first HF event, with a hazard ratio of 0.65 (95% CI, 0.43-0.99; P = .042). They also were less likely to experience acute renal failure (7.7% vs. 12.1% for the control group) or serious adverse events (32.3% vs. 43.6%), Dr. Voors reported.
Tempting as it might be, the findings can’t necessarily be generalized to other SGLT2 inhibitors without an evidence base. But as Dr. Voors observed, several ongoing trials are exploring dapagliflozin (Farxiga) in a similar clinical setting.
They include DICTATE-AHF in patients with diabetes admitted with acute HF, and DAPA ACT HF-TIMI 68, which is entering patients stabilized during hospitalization with acute decompensated HFrEF. The trials are scheduled for completion in 2022 and 2023, respectively.
EMPULSE was supported by the Boehringer Ingelheim–Eli Lilly Diabetes Alliance. Dr. Voors disclosed research support and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. Dr. Sweitzer disclosed honoraria from Acorda and Myokardia, and reported receiving research support from Novartis and Merck. Dr. Fonarow cited honoraria from Abbott, Amgen, Janssen, Medtronic, Bayer, Merck, AstraZeneca, Cytokinetics, and Novartis.
A version of this article first appeared on Medscape.com.
The sodium-glucose transporter 2 inhibitors, relative newcomers among first-line agents for chronic heart failure (HF), could well attain the same go-to status in patients hospitalized with acute HF if the EMPULSE trial has anything to say about it.
Of the study’s 530 such patients, those started on daily empagliflozin (Jardiance) soon after they were stabilized, compared with a control group, were less likely to die or be rehospitalized for HF over the next 3 months.
Also, “we saw an improvement in quality of life, we saw a greater reduction in body weight, and we didn’t see any safety concerns in this very vulnerable and sick patient population,” Adriaan A. Voors, MD, University Medical Center Groningen (the Netherlands), said when presenting the trial at the American Heart Association scientific sessions.
Patients assigned to empagliflozin had a 36% greater likelihood of showing a benefit as reflected in the treatment’s win ratio when opposed by placebo, an emerging way to express outcomes in cardiovascular clinical trials. The SGLT2 inhibitor’s win ratio for the primary endpoint was 1.36 (95% confidence interval, 1.09-1.68, P = .0054), Dr. Voors reported. The outcome consisted of death, number of HF events, time to first HF event, and 90-day change in quality of life scores.
There is reluctance in practice to start patients that early after decompensation on drugs used in chronic HF, Dr. Voors said in an interview. Empagliflozin in the trial was initiated in the stabilized setting an average of 3 days after hospital admission, he said. The trial should reassure physicians that the drug “is not only safe to start early in hospital, but it’s also beneficial to start early in hospital.”
EMPULSE, combined with support from other recent trials, “should be clinical practice changing, with early in-hospital initiation of SGLT2 inhibitors in patients hospitalized with HF being the expectation, along with clear recognition that delaying SGLT2 inhibitor initiation may expose patients to unnecessary harms and delays in improved health status,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, told this news organization.
“For patients with HF, irrespective of ejection fraction, early in-hospital initiation of SGLT2 inhibitors – once stabilized and in the absence of contraindications – should be considered a new standard of care,” said Fonarow, who was not part of EMPULSE.
The trial also lends new weight to the strategy of “simultaneous or rapid-sequence initiation” of the so-called four pillars of guideline-directed medical therapy of HF with reduced ejection fraction in patients hospitalized with HFrEF, once they are stabilized, Dr. Fonarow said. The four-pronged approach, he noted, consists of sacubitril/valsartan (Entresto), a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and an SGLT2 inhibitor.
Indeed, the new findings “fill an important gap and are clearly practice changing,” agreed Nancy K. Sweitzer, MD, PhD, University of Arizona Sarver Heart Center, Tucson, as an invited discussant following Dr. Voors’ presentation. “Few therapies have been shown to impact the course of those hospitalized with acute decompensated heart failure.”
Of note in the trial, Dr. Sweitzer continued, patients were started on empagliflozin regardless of any drug therapy they might already be on for chronic HF. “Because patients in the EMPULSE trial could be enrolled with a new diagnosis of heart failure, they were, by definition, not all on chronic guideline-directed heart failure therapy. Nevertheless, such patients benefited equally from the study intervention,” she said.
“This is crucial, as it tells us these drugs have immediate and important effects and should not be withheld while other drug classes are initiated and optimized.”
EMPULSE entered patients hospitalized for acute HF, which could be de novo or a decompensation of chronic HF, without regard to ejection fraction or whether they had diabetes, and who were clinically stable after at least one dose of loop diuretics. Their ejection fractions averaged 35% and exceeded 40% in about one-third of the total cohort.
At 90 days in the win ratio analysis, the 265 patients assigned to empagliflozin 10 mg once daily were the “winners”; that is, they were more likely to show a clinical benefit about 54% of the time in paired match-ups of patient outcomes, compared with about 40% for the 265 in the control group. The match-ups were a tie 6.4% of the time.
The empagliflozin group also benefited significantly for the endpoint of death from any cause or first HF event, with a hazard ratio of 0.65 (95% CI, 0.43-0.99; P = .042). They also were less likely to experience acute renal failure (7.7% vs. 12.1% for the control group) or serious adverse events (32.3% vs. 43.6%), Dr. Voors reported.
Tempting as it might be, the findings can’t necessarily be generalized to other SGLT2 inhibitors without an evidence base. But as Dr. Voors observed, several ongoing trials are exploring dapagliflozin (Farxiga) in a similar clinical setting.
They include DICTATE-AHF in patients with diabetes admitted with acute HF, and DAPA ACT HF-TIMI 68, which is entering patients stabilized during hospitalization with acute decompensated HFrEF. The trials are scheduled for completion in 2022 and 2023, respectively.
EMPULSE was supported by the Boehringer Ingelheim–Eli Lilly Diabetes Alliance. Dr. Voors disclosed research support and consulting for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Merck, Myokardia, Novo Nordisk, Novartis, and Roche Diagnostics. Dr. Sweitzer disclosed honoraria from Acorda and Myokardia, and reported receiving research support from Novartis and Merck. Dr. Fonarow cited honoraria from Abbott, Amgen, Janssen, Medtronic, Bayer, Merck, AstraZeneca, Cytokinetics, and Novartis.
A version of this article first appeared on Medscape.com.
FROM AHA 2021
Alcoholic drinks stand out in novel trial exploring AFib triggers
People with paroxysmal atrial fibrillation who explored potential triggers of their arrhythmia, and used them to make lifestyle changes, went on to show a 40% decline in subjectively experienced bouts of AFib in a randomized trial with an unusual design.
But the study didn’t provide evidence that the drop in self-reported AFib necessarily improved their quality of life, its primary endpoint. Nor was there any apparent relationship between potential triggers and AFib episodes detected less subjectively using a handheld electrocardiography monitor.
Although the study – called I-STOP-AFib – has limitations, its results jibe with alcohol intake’s increasingly appreciated status as a potential AFib trigger. It was alone among many possible triggers tested in showing a consistent association with self-reported AFib.
As a result, the study offers no support for such a link between the arrhythmia and caffeine intake, sleep deprivation, dehydration, exercise, or other conditions sometimes perceived as triggers, observed principal investigator Gregory M. Marcus, MD, MAS, University of California, San Francisco, when presenting results at the American Heart Association scientific sessions. He is also lead author on the study’s simultaneous publication in JAMA Cardiology.
The I-STOP-AFib trial was unusual in part for its virtual design, in which participants followed instructions and tracked AFib episodes – both perceived and detected by the handheld ECG device – through a smartphone application. It also featured an N-of-1 randomized comparisons of different weeks in which individuals were or were not exposed to their self-selected trigger.
Such patients following their own weekly personalized randomization were compared to an entirely separate randomized control arm of the trial, in which patients simply tracked any ECG-monitored and self-perceived AFib episodes.
Current use in patients
Although wearable and smartphone-based ECG recorders are increasingly popular for AFib screening, Dr. Marcus said the devices may be especially helpful for validating whether a person’s symptoms are actually caused by AFib.
“I have actually suggested to some of my patients that they run some of these experiments,” he said at a media briefing on I-STOP-AFib before his main presentation of the trial. The demonstration might help patients recognize that some perceived triggers actually do not induce AFib.
Allowing patients to determine on their own whether a substance indeed triggers their AFib “is an efficient use of these devices,” Dr. Marcus said. Such N-of-1 exploration of possible triggers “might help free patients up to enjoy substances – caffeine or coffee is one example – that they otherwise might not, and may help actually reassure them that certain exposures –like certain exercises, which can also be beneficial – might actually not be harmful.”
Dr. Marcus and the other authors on the report noted – as he did at the AHA sessions – that the study has several limitations, such as the subjectivity of self-reported AFib, dropouts from the trial that shrank the randomization arms, and a population that may not be very representative.
There is also the potential for detection bias in the group assigned to track their selected triggers, as Dr. Marcus and some observers have noted.
It follows that conscious avoidance of a potential AFib trigger might well lead to a reduction in AFib subjectively identified by symptoms, proposed David Conen, MD, MPH, Population Health Research Institute, McMaster University, Hamilton, Ont. But perhaps there would have been no reduction in AFib had it been objectively documented with the handheld ECG device, he said in an interview.
“If I were to redesign the study,” he said, “I think the primary endpoint should be confirmed atrial fibrillation, because we would have to show first that the specific trigger actually reduced objective AFib events before we then try to address the question whether reducing that trigger improves quality of life.”
Unrepresentative sample
The trial entered 446 overwhelmingly White and college-educated adults known to have symptomatic paroxysmal AFib who were “interested in testing a presumed AFib trigger they could readily introduce or withhold” and who owned a smartphone; the average age was 58 years, and 58% were men. The cohort was randomly assigned to the trigger-testing group or the control group, charged only with tracking their AFib.
Of the total, 320, or about 72%, completed the study; those who did not were mostly from the trigger-testing arm, leaving 136 in that group versus 184 patients in the control group.
Potential triggers that participants selected for tracking included, foremost, caffeine, alcohol, reduced sleep, and exercise, followed by lying on the left side, dehydration, large meals, and cold food or drink, the report noted.
Patients in the control group used the smartphone app and handheld ECG monitor (KardiaMobile, AliveCor) to document the duration and severity of AFib episodes daily and received data summary reports through the app weekly for 10 weeks. They then had the option to follow the trigger-testing protocol at least once.
Those in the trigger-testing group conducted their N-of-1 trials by exposing themselves to their chosen potential trigger during 3 separate weeks and avoiding the trigger during 3 other weeks, alternating each of the 6 weeks of trigger exposure or avoidance. They were instructed through the app to start the 6-week sequence with one or the other strategy randomly and to regularly track their AFib.
At the end of 6 weeks, each participant in the trigger-testing group had the opportunity to review their data for any potential trigger-AFib associations. They were then to use the next 4 weeks to enact lifestyle changes based on what they learned – as described in the report and on clinicaltrials.gov. They had the option of repeating the entire N-of-1 sequence at least one more time.
Participants in both the trigger-tracking and control arms were tested at baseline and at 10 weeks using the validated Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire.
AFEQT scores didn’t change significantly over the 10 weeks in either arm, nor were they significantly different in one arm, compared with the other.
On the other hand, patients in the trigger-tracking arm reported significantly fewer daily AFib episodes during the final 4-week period of lifestyle changes based on their N-of-1 trial results, compared to the monitoring-only control group’s final 4 weeks.
The adjusted relative risk in the trigger-tracking arm was 0.60 (95% confidence interval, 0.43-0.83; P < .001), the difference driven by patients who selected alcohol, dehydration, or exercise for their trigger, Dr. Marcus reported.
Only alcohol intake emerged consistently as a significant predictor of risk for self-reported AFib episodes in a series of meta-analyses conducted using all of the individual N-of-1 trials that provided per-protocol data. The odds ratio was 1.77 (95% CI, 1.20-2.69).
I-STOP-AFib explored an important subject “that has been understudied,” Dr. Conen said. “The trial has some limitations that the authors address themselves, but hopefully it opens the path to future studies that can build upon this experience.”
Dr. Marcus reported receiving personal fees and equity interest from InCarda Therapeutics; personal fees from Johnson & Johnson; and grants from Baylis Medical, Medtronic, the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the California Tobacco-Related Disease Research Program.
A version of this article first appeared on Medscape.com.
People with paroxysmal atrial fibrillation who explored potential triggers of their arrhythmia, and used them to make lifestyle changes, went on to show a 40% decline in subjectively experienced bouts of AFib in a randomized trial with an unusual design.
But the study didn’t provide evidence that the drop in self-reported AFib necessarily improved their quality of life, its primary endpoint. Nor was there any apparent relationship between potential triggers and AFib episodes detected less subjectively using a handheld electrocardiography monitor.
Although the study – called I-STOP-AFib – has limitations, its results jibe with alcohol intake’s increasingly appreciated status as a potential AFib trigger. It was alone among many possible triggers tested in showing a consistent association with self-reported AFib.
As a result, the study offers no support for such a link between the arrhythmia and caffeine intake, sleep deprivation, dehydration, exercise, or other conditions sometimes perceived as triggers, observed principal investigator Gregory M. Marcus, MD, MAS, University of California, San Francisco, when presenting results at the American Heart Association scientific sessions. He is also lead author on the study’s simultaneous publication in JAMA Cardiology.
The I-STOP-AFib trial was unusual in part for its virtual design, in which participants followed instructions and tracked AFib episodes – both perceived and detected by the handheld ECG device – through a smartphone application. It also featured an N-of-1 randomized comparisons of different weeks in which individuals were or were not exposed to their self-selected trigger.
Such patients following their own weekly personalized randomization were compared to an entirely separate randomized control arm of the trial, in which patients simply tracked any ECG-monitored and self-perceived AFib episodes.
Current use in patients
Although wearable and smartphone-based ECG recorders are increasingly popular for AFib screening, Dr. Marcus said the devices may be especially helpful for validating whether a person’s symptoms are actually caused by AFib.
“I have actually suggested to some of my patients that they run some of these experiments,” he said at a media briefing on I-STOP-AFib before his main presentation of the trial. The demonstration might help patients recognize that some perceived triggers actually do not induce AFib.
Allowing patients to determine on their own whether a substance indeed triggers their AFib “is an efficient use of these devices,” Dr. Marcus said. Such N-of-1 exploration of possible triggers “might help free patients up to enjoy substances – caffeine or coffee is one example – that they otherwise might not, and may help actually reassure them that certain exposures –like certain exercises, which can also be beneficial – might actually not be harmful.”
Dr. Marcus and the other authors on the report noted – as he did at the AHA sessions – that the study has several limitations, such as the subjectivity of self-reported AFib, dropouts from the trial that shrank the randomization arms, and a population that may not be very representative.
There is also the potential for detection bias in the group assigned to track their selected triggers, as Dr. Marcus and some observers have noted.
It follows that conscious avoidance of a potential AFib trigger might well lead to a reduction in AFib subjectively identified by symptoms, proposed David Conen, MD, MPH, Population Health Research Institute, McMaster University, Hamilton, Ont. But perhaps there would have been no reduction in AFib had it been objectively documented with the handheld ECG device, he said in an interview.
“If I were to redesign the study,” he said, “I think the primary endpoint should be confirmed atrial fibrillation, because we would have to show first that the specific trigger actually reduced objective AFib events before we then try to address the question whether reducing that trigger improves quality of life.”
Unrepresentative sample
The trial entered 446 overwhelmingly White and college-educated adults known to have symptomatic paroxysmal AFib who were “interested in testing a presumed AFib trigger they could readily introduce or withhold” and who owned a smartphone; the average age was 58 years, and 58% were men. The cohort was randomly assigned to the trigger-testing group or the control group, charged only with tracking their AFib.
Of the total, 320, or about 72%, completed the study; those who did not were mostly from the trigger-testing arm, leaving 136 in that group versus 184 patients in the control group.
Potential triggers that participants selected for tracking included, foremost, caffeine, alcohol, reduced sleep, and exercise, followed by lying on the left side, dehydration, large meals, and cold food or drink, the report noted.
Patients in the control group used the smartphone app and handheld ECG monitor (KardiaMobile, AliveCor) to document the duration and severity of AFib episodes daily and received data summary reports through the app weekly for 10 weeks. They then had the option to follow the trigger-testing protocol at least once.
Those in the trigger-testing group conducted their N-of-1 trials by exposing themselves to their chosen potential trigger during 3 separate weeks and avoiding the trigger during 3 other weeks, alternating each of the 6 weeks of trigger exposure or avoidance. They were instructed through the app to start the 6-week sequence with one or the other strategy randomly and to regularly track their AFib.
At the end of 6 weeks, each participant in the trigger-testing group had the opportunity to review their data for any potential trigger-AFib associations. They were then to use the next 4 weeks to enact lifestyle changes based on what they learned – as described in the report and on clinicaltrials.gov. They had the option of repeating the entire N-of-1 sequence at least one more time.
Participants in both the trigger-tracking and control arms were tested at baseline and at 10 weeks using the validated Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire.
AFEQT scores didn’t change significantly over the 10 weeks in either arm, nor were they significantly different in one arm, compared with the other.
On the other hand, patients in the trigger-tracking arm reported significantly fewer daily AFib episodes during the final 4-week period of lifestyle changes based on their N-of-1 trial results, compared to the monitoring-only control group’s final 4 weeks.
The adjusted relative risk in the trigger-tracking arm was 0.60 (95% confidence interval, 0.43-0.83; P < .001), the difference driven by patients who selected alcohol, dehydration, or exercise for their trigger, Dr. Marcus reported.
Only alcohol intake emerged consistently as a significant predictor of risk for self-reported AFib episodes in a series of meta-analyses conducted using all of the individual N-of-1 trials that provided per-protocol data. The odds ratio was 1.77 (95% CI, 1.20-2.69).
I-STOP-AFib explored an important subject “that has been understudied,” Dr. Conen said. “The trial has some limitations that the authors address themselves, but hopefully it opens the path to future studies that can build upon this experience.”
Dr. Marcus reported receiving personal fees and equity interest from InCarda Therapeutics; personal fees from Johnson & Johnson; and grants from Baylis Medical, Medtronic, the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the California Tobacco-Related Disease Research Program.
A version of this article first appeared on Medscape.com.
People with paroxysmal atrial fibrillation who explored potential triggers of their arrhythmia, and used them to make lifestyle changes, went on to show a 40% decline in subjectively experienced bouts of AFib in a randomized trial with an unusual design.
But the study didn’t provide evidence that the drop in self-reported AFib necessarily improved their quality of life, its primary endpoint. Nor was there any apparent relationship between potential triggers and AFib episodes detected less subjectively using a handheld electrocardiography monitor.
Although the study – called I-STOP-AFib – has limitations, its results jibe with alcohol intake’s increasingly appreciated status as a potential AFib trigger. It was alone among many possible triggers tested in showing a consistent association with self-reported AFib.
As a result, the study offers no support for such a link between the arrhythmia and caffeine intake, sleep deprivation, dehydration, exercise, or other conditions sometimes perceived as triggers, observed principal investigator Gregory M. Marcus, MD, MAS, University of California, San Francisco, when presenting results at the American Heart Association scientific sessions. He is also lead author on the study’s simultaneous publication in JAMA Cardiology.
The I-STOP-AFib trial was unusual in part for its virtual design, in which participants followed instructions and tracked AFib episodes – both perceived and detected by the handheld ECG device – through a smartphone application. It also featured an N-of-1 randomized comparisons of different weeks in which individuals were or were not exposed to their self-selected trigger.
Such patients following their own weekly personalized randomization were compared to an entirely separate randomized control arm of the trial, in which patients simply tracked any ECG-monitored and self-perceived AFib episodes.
Current use in patients
Although wearable and smartphone-based ECG recorders are increasingly popular for AFib screening, Dr. Marcus said the devices may be especially helpful for validating whether a person’s symptoms are actually caused by AFib.
“I have actually suggested to some of my patients that they run some of these experiments,” he said at a media briefing on I-STOP-AFib before his main presentation of the trial. The demonstration might help patients recognize that some perceived triggers actually do not induce AFib.
Allowing patients to determine on their own whether a substance indeed triggers their AFib “is an efficient use of these devices,” Dr. Marcus said. Such N-of-1 exploration of possible triggers “might help free patients up to enjoy substances – caffeine or coffee is one example – that they otherwise might not, and may help actually reassure them that certain exposures –like certain exercises, which can also be beneficial – might actually not be harmful.”
Dr. Marcus and the other authors on the report noted – as he did at the AHA sessions – that the study has several limitations, such as the subjectivity of self-reported AFib, dropouts from the trial that shrank the randomization arms, and a population that may not be very representative.
There is also the potential for detection bias in the group assigned to track their selected triggers, as Dr. Marcus and some observers have noted.
It follows that conscious avoidance of a potential AFib trigger might well lead to a reduction in AFib subjectively identified by symptoms, proposed David Conen, MD, MPH, Population Health Research Institute, McMaster University, Hamilton, Ont. But perhaps there would have been no reduction in AFib had it been objectively documented with the handheld ECG device, he said in an interview.
“If I were to redesign the study,” he said, “I think the primary endpoint should be confirmed atrial fibrillation, because we would have to show first that the specific trigger actually reduced objective AFib events before we then try to address the question whether reducing that trigger improves quality of life.”
Unrepresentative sample
The trial entered 446 overwhelmingly White and college-educated adults known to have symptomatic paroxysmal AFib who were “interested in testing a presumed AFib trigger they could readily introduce or withhold” and who owned a smartphone; the average age was 58 years, and 58% were men. The cohort was randomly assigned to the trigger-testing group or the control group, charged only with tracking their AFib.
Of the total, 320, or about 72%, completed the study; those who did not were mostly from the trigger-testing arm, leaving 136 in that group versus 184 patients in the control group.
Potential triggers that participants selected for tracking included, foremost, caffeine, alcohol, reduced sleep, and exercise, followed by lying on the left side, dehydration, large meals, and cold food or drink, the report noted.
Patients in the control group used the smartphone app and handheld ECG monitor (KardiaMobile, AliveCor) to document the duration and severity of AFib episodes daily and received data summary reports through the app weekly for 10 weeks. They then had the option to follow the trigger-testing protocol at least once.
Those in the trigger-testing group conducted their N-of-1 trials by exposing themselves to their chosen potential trigger during 3 separate weeks and avoiding the trigger during 3 other weeks, alternating each of the 6 weeks of trigger exposure or avoidance. They were instructed through the app to start the 6-week sequence with one or the other strategy randomly and to regularly track their AFib.
At the end of 6 weeks, each participant in the trigger-testing group had the opportunity to review their data for any potential trigger-AFib associations. They were then to use the next 4 weeks to enact lifestyle changes based on what they learned – as described in the report and on clinicaltrials.gov. They had the option of repeating the entire N-of-1 sequence at least one more time.
Participants in both the trigger-tracking and control arms were tested at baseline and at 10 weeks using the validated Atrial Fibrillation Effect on Quality-of-Life (AFEQT) questionnaire.
AFEQT scores didn’t change significantly over the 10 weeks in either arm, nor were they significantly different in one arm, compared with the other.
On the other hand, patients in the trigger-tracking arm reported significantly fewer daily AFib episodes during the final 4-week period of lifestyle changes based on their N-of-1 trial results, compared to the monitoring-only control group’s final 4 weeks.
The adjusted relative risk in the trigger-tracking arm was 0.60 (95% confidence interval, 0.43-0.83; P < .001), the difference driven by patients who selected alcohol, dehydration, or exercise for their trigger, Dr. Marcus reported.
Only alcohol intake emerged consistently as a significant predictor of risk for self-reported AFib episodes in a series of meta-analyses conducted using all of the individual N-of-1 trials that provided per-protocol data. The odds ratio was 1.77 (95% CI, 1.20-2.69).
I-STOP-AFib explored an important subject “that has been understudied,” Dr. Conen said. “The trial has some limitations that the authors address themselves, but hopefully it opens the path to future studies that can build upon this experience.”
Dr. Marcus reported receiving personal fees and equity interest from InCarda Therapeutics; personal fees from Johnson & Johnson; and grants from Baylis Medical, Medtronic, the National Institutes of Health, the Patient-Centered Outcomes Research Institute, and the California Tobacco-Related Disease Research Program.
A version of this article first appeared on Medscape.com.