User login
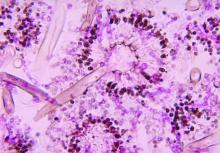
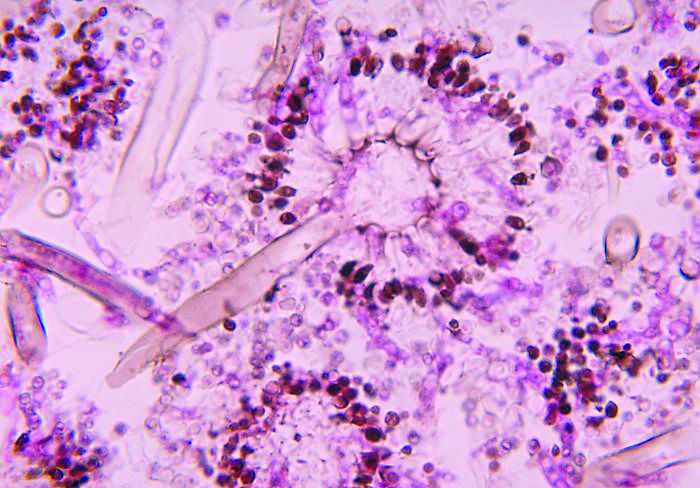
Severe influenza increases risk of invasive pulmonary aspergillosis in the ICU
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
Severe influenza is an independent risk factor for invasive pulmonary aspergillosis with an accompanying increased mortality in the ICU, according to a multicenter retrospective cohort study at seven tertiary centers in Belgium and the Netherlands.
Data was collected from criteria-meeting adult patients admitted to the ICU for more than 24 hours with acute respiratory failure during the 2009-2016 influenza seasons. The included cohort of 432 patients was composed of 56% men and had a median age of 59 years; all participants were diagnosed as having severe type A or type B influenza infection according to positive airway RT-PCR results.
The full cohort was subcategorized into 117 immunocompromised and 315 as nonimmunocompromised individuals using criteria established by the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study group (EORTC/MSG) . To assess influenza as an independent variable in the development of invasive pulmonary aspergillosis, the 315 nonimmunocompromised influenza positive individuals were compared to an influenza-negative control group of 315 nonimmunocompromised patients admitted to the ICU that presented similar respiratory insufficiency symptoms with community-acquired pneumonia.
Determination of other independent risk factors for incidence of invasive pulmonary aspergillosis was achieved by multivariate analysis of factors such as sex, diabetes status, prednisone use, age, and acute physiology and chronic health evaluation (APACHE) II score. The mean APACHE II score was 22, with the majority of patients requiring intubation for mechanical ventilation for a median duration of 11 days.
Influenza is not considered a host factor for invasive pulmonary aspergillosis and will often miss being diagnosed when using strict interpretation of the current EORTC/MSG or AspICU algorithm criteria, according to the researchers. Consequently for patients with influenza and the noninfluenza control group with community-acquired pneumonia, the definition of invasive pulmonary aspergillosis was modified from the AspICU algorithm. Stringent mycological criteria, including bronchoaveolar lavage (BAL) culture, a positive Aspergillus culture, positive galactomannan test, and/or positive serum galactomannan tests, provided supporting diagnostics for an invasive pulmonary aspergillosis determination.
At a median of 3 days following admission to the ICU, a diagnosis of invasive pulmonary aspergillosis was determined for 19% of the 432 influenza patients. Similar incident percentages of invasive pulmonary aspergillosis occurring for type A and type B, 71/355 (20%) and 12/77 (16%) patients respectively, showed that there was no clear association of the disease development with influenza subtypes that occurred during different annual seasons.
AspICU or EORTC/MSG criteria characterized only 43% and 58% of cases as proven or possible aspergillosis, respectively. On the other hand, stringent mycological tests yielded better invasive pulmonary aspergillosis classification, with 63% of BAL cultures being positive for Aspergillus, 88% of BAL galactomannan tests being positive, and 65% of serum galactomannan tests being positive in the 81/83 patients tested.
The study found that, for influenza patients, being immunocompromised more than doubled the incidence of invasive pulmonary aspergillosis, at 32% versus the 14% of those patients who were nonimmunocompromised. In contrast only 5% in the control group developed invasive pulmonary aspergillosis.
Influenza patients who developed invasive pulmonary aspergillosis in the ICU tended to have their stays significantly lengthened from 9 days (interquartile range, 5-20 days) for those without it to 19 days (IQR, 12-38 days) for those infected (P less than .0001). Likewise, 90-day mortality significantly rose from 28% for those influenza patients without invasive pulmonary aspergillosis to 51% for those with it (P = .0001).
The authors concluded that influenza was “independently associated with invasive pulmonary aspergillosis (adjusted odds ratio, 5.19; P less than.0001) along with a higher APACHE II score, male sex, and use of corticosteroids.”
Furthermore, as influenza appears to be an independent risk factor for invasive pulmonary aspergillosis and its associated high mortality, the authors suggested that “future studies should assess whether a faster diagnosis or antifungal prophylaxis could improve the outcome of influenza-associated aspergillosis.”
The authors reported that they had no conflicts of interest.
SOURCE: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1
FROM THE LANCET RESPIRATORY MEDICINE
Key clinical point: ICU admission for severe influenza as significant a risk factor should be included in the existing diagnostic criteria for predicting incidence of invasive pulmonary aspergillosis.
Major finding: Influenza is an independent risk factor associated with invasive pulmonary aspergillosis, with 90-day mortality rising from 28% to 51% when this fungal infection occurs.
Study details: Multicenter retrospective study of 432 adult patients with confirmed severe influenza admitted to the ICU with acute respiratory failure.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Schauwvlieghe AFAD et al. Lancet Respir Med. 2018 Jul 31. doi: 10.1016/S2213-2600(18)30274-1.
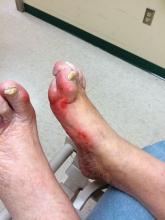
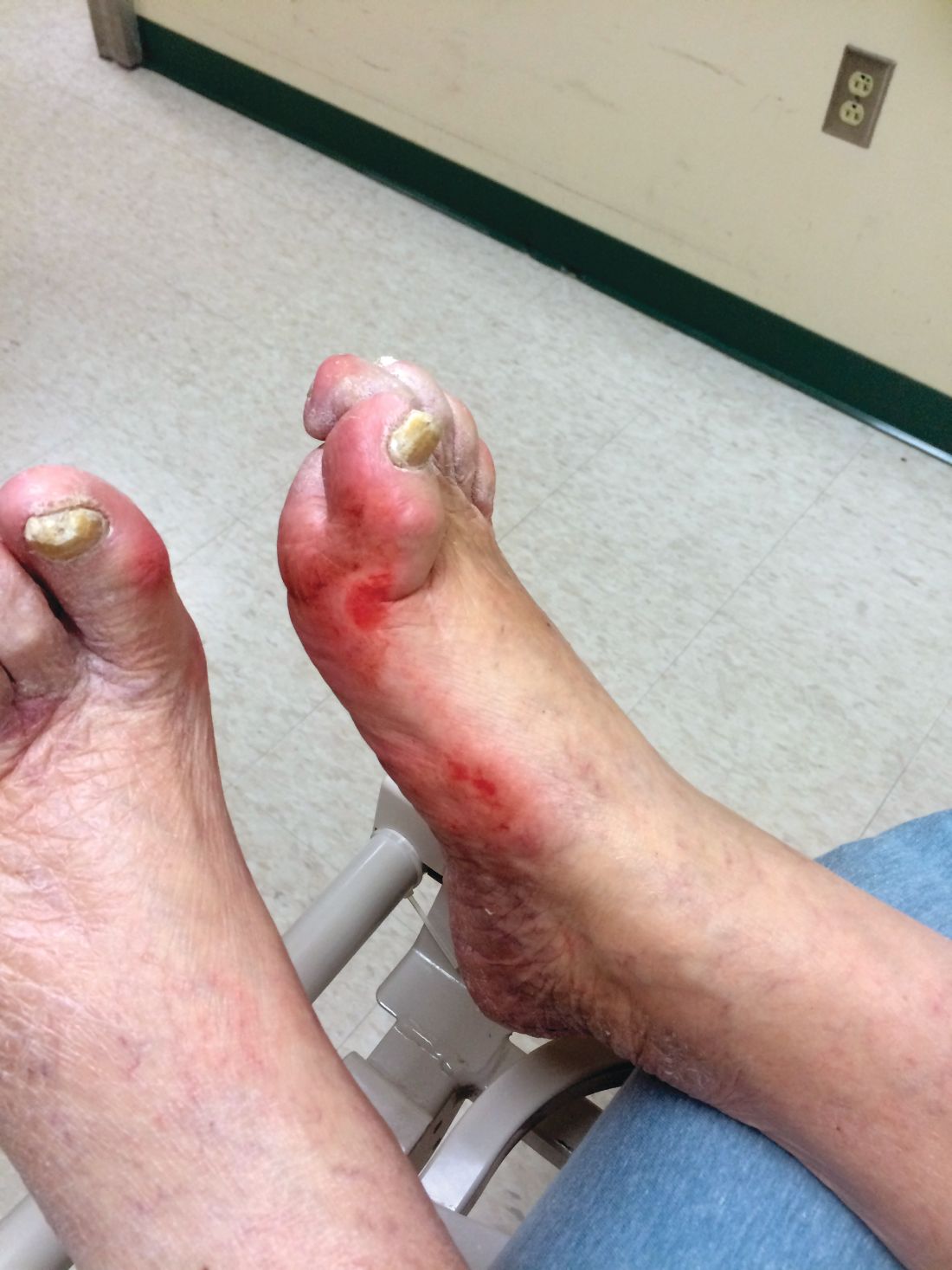
Diabetic foot ulcer healing is predictable by WIfI stage scores
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Diabetic foot ulcer healing is predictable with the Wound, Ischemia, and foot Infection (WIfI) classification system when used alone or with multivariable risk-adjustment analysis, according to a study published in the Journal of Vascular Surgery.
The research was conducted by Caitlin W. Hicks, MD, of Johns Hopkins University, Baltimore, and her colleagues as a retrospective study using prospective database information from enrolled type 1 and type 2 medication-dependent diabetic patients presenting to the multidisciplinary diabetic limb preservation service at Johns Hopkins Hospital from June 2012 to July 2017. The cohort of 310 patients with diabetic foot ulcer (DFU) in the study had a median age of 59 years and was composed of 60.3% men, with 60.0% of patients being black.
Infectious disease, plastic surgery, and orthopedic foot and ankle consultations were provided as needed. Individuals with evidence of peripheral artery disease (PAD) were provided lower extremity revascularization as determined to be appropriate by the primary vascular surgeon.
The 709 presented DFUs were assessed by x-ray imaging and follow-up MRI as needed. Wounds were debrided to clean margins and antibiotic treatments were administered as appropriate. At each visit the primary team assessed and assigned each wound a WIfI classified stage of 1-4 according to the calculation based on previously accepted Society of Vascular Surgery definitions, with PAD considered separately in final multivariable model analysis.
The association between WIfI stage and wound characteristics and healing was tested by univariable analysis. Multivariable Cox proportional hazards models that included sociodemographic, comorbidity, and wound characteristics were subsequently created to test WIfI stage as an independent predictor for wound healing after adjusting for those variables. Differences between models were related to wound location.
Most of the treated wounds occurred on toes, with the least common wound location being the leg/ankle. Of the 709 treated wounds, 32.4% (n = 230) were WIfI stage 1, 19.9% (n = 141) were stage 2, 25.2% (n = 179) were stage 3, and 22.4% (n = 329) were stage 4.
Differences between the stages included larger increases in mean wound area size, wound depth, and mean time from wound onset to initial assessment as WIfI stages increased from 1 to 4.
Healed wounds were defined as “maintained complete epithelialization with the restoration of sustained functional and anatomic continuity for 6 weeks after complete healing.”
The researchers found that wound healing time significantly increased with increasing WIfI stage, with a mean wound healing time of 96.9 days for WIfI stage 1 wounds, increasing to 195.1 days for WIfI stage 4 wounds (P less than .001). The authors found a likelihood of 94.1% for stage 1 wounds to be healed at 1 year, decreasing to a low of 67.4% for stage 4 wounds (P less than .001).
In univariable and risk-adjusting multivariable analysis, WIfI stage had an independent negative association with wound healing. With inclusion of risk adjustment, the probability of wound healing at 1 year was significantly lowered for stage 4 wounds, compared with stage 1 wounds (hazard ratio, 0.44). The three most prominent independently associated factors associated with poorer wound healing results include concomitant PAD (HR, 0.73), increasing wound area (HR, 0.99 per 1 cm2 area increase), and longer time from wound onset to initial assessment (HR, 0.97 per month). The strongest predictors for poor wound healing were increasing wound area (z score, –3.14), WIfI stage 3 (z score, –3.11), and WIfI stage 4 (z score, –5.40).
In this expanded study of previous work, the authors stated that they were the first to provide validating evidence for use of the WIfI classification system in giving “wound healing prognoses regardless of patient risk factors, comorbidities, and wound location.” Their findings also demonstrated that this classification system has broader applications than its original purpose to provide prognostic information and risk expectations for major amputation for patients presenting with foot wounds, Dr. Hicks and her colleagues concluded.
The authors reported no conflicts of interest.
SOURCE: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: The Wound, Ischemia, and foot Infection (WIfI) classification of diabetic foot ulcers provides a predictable primary outcome for wound healing at 1 year.
Major finding: Wound healing probability at 1 year was 94.1% for WIfI stage 1 wounds and 67.4% for stage 4 wounds.
Study details: A single-location, multidisciplinary-setting, retrospective study of 709 WIfI stage 1-4 wounds presented by 310 diabetic foot ulcer patients.
Disclosures: The authors reported no conflicts of interest.
Source: Hicks CW et al. J Vasc Surg. 2018 Apr 2. doi: 10.1016/j.jvs.2017.12.079.
Ruptured abdominal aortic aneurysm repair: Preop measures that predict death
Four preoperative variables – age over 76 years, creatinine concentration greater than 2.0 mg/dL, pH less than 7.2, and lowest ever systolic blood pressure less than 70 mm Hg – predicted 30-day mortality following repair of ruptured abdominal aortic aneurysms (rAAAs), in a retrospective study of 303 patients treated at Harborview Medical Center at the University of Washington, Seattle.
Brandon T. Garland, MD, and his colleagues at Harborview, reviewed the data set of patients, noting 50% were aged older than 76 years and 80% were male. Many patients had typical vascular risk factors: 65% had hypertension, 39% had coronary artery disease, and 22% had chronic obstructive vascular disease. Patients who were treated for rAAA after 2007 and had preoperative computed tomography scans were assessed for endovascular aneurysm repair (rEVAR) based on infrarenal neck length and diameter and access vessel size. Noneligible patients and all patients treated prior to 2007 had open repair (rOR) surgery.
A primary screen of selected preoperative variables included age, hematocrit, systolic blood pressure values, use of cardiopulmonary resuscitation, pH, international normalized ratio, creatinine concentration, temperature, partial thromboplastin time, weight, history of coronary artery disease, and loss of consciousness at any time.
The four statistically significant associations were age over 76 years (odds ratio, 2.11; P less than 0.11), creatinine concentration over 2.0 mg/dL (OR, 3.66; P less than .001), pH less than 7.2 (OR 2.58; P less than .009) and lowest ever systolic blood pressure less than 70 mm Hg (OR, 2.70; P less than .002) Each of the four predictive preoperative rAAA variables was assigned a value of 1 point. Individualized scores are simply calculated by totaling the number of preoperative risk predictors.
Of the original 303 patients, 154 were alive at 30 days following rAAA repair, and there was a significant benefit from using rEVAR. Overall, patients with 1-, 2-, 3-, and 4-point mortality scores had 30-day mortality risks of 22%, 69%, 80%, and 100%, respectively. rEVAR mortalities dropped to 7% for a 1-point score and to 70% for a 3-point score. There were no 30-day survivors with 4-point risk scores regardless of whether they had rEVAR or rOR procedures.
The predictive risk scores for rAAA mortality outcomes provide helpful guides for patient care recommendations, and can be used to supplement the rOR-validated Glasgow Aneurysm Score, Hardman index, and Vascular Study Group of New England risk-predicting algorithms to “aid in clinical decision-making in the endovascular era,” the researchers wrote. The scores also add “prognostic information to the decision to transfer patients to tertiary care centers and aid in preoperative discussions with patients and their families.”
The authors reported that they had no conflicts of interest.
SOURCE: Garland BT et al. J Vasc Surg. 2018 May 9. doi: 10.1016/j.jvs.2017.12.075.
Four preoperative variables – age over 76 years, creatinine concentration greater than 2.0 mg/dL, pH less than 7.2, and lowest ever systolic blood pressure less than 70 mm Hg – predicted 30-day mortality following repair of ruptured abdominal aortic aneurysms (rAAAs), in a retrospective study of 303 patients treated at Harborview Medical Center at the University of Washington, Seattle.
Brandon T. Garland, MD, and his colleagues at Harborview, reviewed the data set of patients, noting 50% were aged older than 76 years and 80% were male. Many patients had typical vascular risk factors: 65% had hypertension, 39% had coronary artery disease, and 22% had chronic obstructive vascular disease. Patients who were treated for rAAA after 2007 and had preoperative computed tomography scans were assessed for endovascular aneurysm repair (rEVAR) based on infrarenal neck length and diameter and access vessel size. Noneligible patients and all patients treated prior to 2007 had open repair (rOR) surgery.
A primary screen of selected preoperative variables included age, hematocrit, systolic blood pressure values, use of cardiopulmonary resuscitation, pH, international normalized ratio, creatinine concentration, temperature, partial thromboplastin time, weight, history of coronary artery disease, and loss of consciousness at any time.
The four statistically significant associations were age over 76 years (odds ratio, 2.11; P less than 0.11), creatinine concentration over 2.0 mg/dL (OR, 3.66; P less than .001), pH less than 7.2 (OR 2.58; P less than .009) and lowest ever systolic blood pressure less than 70 mm Hg (OR, 2.70; P less than .002) Each of the four predictive preoperative rAAA variables was assigned a value of 1 point. Individualized scores are simply calculated by totaling the number of preoperative risk predictors.
Of the original 303 patients, 154 were alive at 30 days following rAAA repair, and there was a significant benefit from using rEVAR. Overall, patients with 1-, 2-, 3-, and 4-point mortality scores had 30-day mortality risks of 22%, 69%, 80%, and 100%, respectively. rEVAR mortalities dropped to 7% for a 1-point score and to 70% for a 3-point score. There were no 30-day survivors with 4-point risk scores regardless of whether they had rEVAR or rOR procedures.
The predictive risk scores for rAAA mortality outcomes provide helpful guides for patient care recommendations, and can be used to supplement the rOR-validated Glasgow Aneurysm Score, Hardman index, and Vascular Study Group of New England risk-predicting algorithms to “aid in clinical decision-making in the endovascular era,” the researchers wrote. The scores also add “prognostic information to the decision to transfer patients to tertiary care centers and aid in preoperative discussions with patients and their families.”
The authors reported that they had no conflicts of interest.
SOURCE: Garland BT et al. J Vasc Surg. 2018 May 9. doi: 10.1016/j.jvs.2017.12.075.
Four preoperative variables – age over 76 years, creatinine concentration greater than 2.0 mg/dL, pH less than 7.2, and lowest ever systolic blood pressure less than 70 mm Hg – predicted 30-day mortality following repair of ruptured abdominal aortic aneurysms (rAAAs), in a retrospective study of 303 patients treated at Harborview Medical Center at the University of Washington, Seattle.
Brandon T. Garland, MD, and his colleagues at Harborview, reviewed the data set of patients, noting 50% were aged older than 76 years and 80% were male. Many patients had typical vascular risk factors: 65% had hypertension, 39% had coronary artery disease, and 22% had chronic obstructive vascular disease. Patients who were treated for rAAA after 2007 and had preoperative computed tomography scans were assessed for endovascular aneurysm repair (rEVAR) based on infrarenal neck length and diameter and access vessel size. Noneligible patients and all patients treated prior to 2007 had open repair (rOR) surgery.
A primary screen of selected preoperative variables included age, hematocrit, systolic blood pressure values, use of cardiopulmonary resuscitation, pH, international normalized ratio, creatinine concentration, temperature, partial thromboplastin time, weight, history of coronary artery disease, and loss of consciousness at any time.
The four statistically significant associations were age over 76 years (odds ratio, 2.11; P less than 0.11), creatinine concentration over 2.0 mg/dL (OR, 3.66; P less than .001), pH less than 7.2 (OR 2.58; P less than .009) and lowest ever systolic blood pressure less than 70 mm Hg (OR, 2.70; P less than .002) Each of the four predictive preoperative rAAA variables was assigned a value of 1 point. Individualized scores are simply calculated by totaling the number of preoperative risk predictors.
Of the original 303 patients, 154 were alive at 30 days following rAAA repair, and there was a significant benefit from using rEVAR. Overall, patients with 1-, 2-, 3-, and 4-point mortality scores had 30-day mortality risks of 22%, 69%, 80%, and 100%, respectively. rEVAR mortalities dropped to 7% for a 1-point score and to 70% for a 3-point score. There were no 30-day survivors with 4-point risk scores regardless of whether they had rEVAR or rOR procedures.
The predictive risk scores for rAAA mortality outcomes provide helpful guides for patient care recommendations, and can be used to supplement the rOR-validated Glasgow Aneurysm Score, Hardman index, and Vascular Study Group of New England risk-predicting algorithms to “aid in clinical decision-making in the endovascular era,” the researchers wrote. The scores also add “prognostic information to the decision to transfer patients to tertiary care centers and aid in preoperative discussions with patients and their families.”
The authors reported that they had no conflicts of interest.
SOURCE: Garland BT et al. J Vasc Surg. 2018 May 9. doi: 10.1016/j.jvs.2017.12.075.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: Age, creatinine concentration, pH, and systolic blood pressure measures can be used to determine a 30-day mortality risk score for patients undergoing repair of ruptured abdominal aortic aneurysms (rAAAs).
Major finding: The Harborview Medical Center risk scores range from 0 to 4 points with 1-, 2-, and 3-point scores corresponding respectively to 22%, 69%, and 80% risks of 30-day mortality following rAAA repair.
Study details: A single-location retrospective study of 303 patients presenting with ruptured rAAAs.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Garland BT et al. J Vasc Surg. 2018 May 9. doi: 10.1016/j.jvs.2017.12.075.
Comorbidity occurs earlier and more commonly with HIV infection
The research was conducted as a cross-sectional analysis using medical chart data from a total of 416 patients treated at the Hospital de Clínicas de Porto Alegre, a tertiary referral hospital in south Brazil, Rafael Aguilar Maciel, MD of the Universidade Federal do Rio Grande do Sul, Porto Alegre, and his associates reported in the International Journal of Infectious Diseases.
The randomly selected participants were 208 well-controlled persons living with HIV (PLWH) – outpatients identified from the South Brazilian HIV Cohort unit – who were individually matched by age, sex, and ethnicity to HIV-negative control counterparts from the primary practice unit of the same hospital. The study group (median age, 57 years) consisted primarily of individuals who were white in origin (79%); 44.2% were women. Nearly all (98.1%) PLWH participants were on highly active antiretroviral therapy (HAART), with 88% having an undetectable viral load. A higher tendency for alcohol use (16% vs. 6%) and a lower mean body mass index (26 kg/m2 vs. 26-30 kg/m2) also were noted for the PLWH group, compared with the HIV-negative control group.
Individuals with multimorbidity had at least two chronic comorbid diseases including hypertension, diabetes mellitus, chronic kidney disease, bone disease, hepatic disease, or cardiovascular disease at the time of the study. Neoplastic disease also was included if reported previously or as a current condition. Results from the Poisson regression analysis used to identify multimorbidity-associated factors and potential confounders were stratified by age category (50-55, 56-60, 61-65, and older than 65).
Individuals with HIV had a significantly higher prevalence of multimorbidity than did HIV-negative controls (63% vs. 43%; P less than .001). Renal, hepatic, and bone diseases were the main sources of this difference. The overall median number of comorbidities of the PLWH individuals was two, which was double the median number observed in the HIV-negative controls. Furthermore, examination of the age-stratified data showed that the PLWH patients in the 50-60 age group had almost twice the burden of disease as the corresponding HIV-negative individuals. Similarity in prevalence of comorbidities was apparent when comparing the youngest PLWH age group (50-55) with their HIV-negative counterparts who were at least 10 years older, the investigators said.
Regression analysis failed to reveal a specific covariate risk factor associated with a higher prevalence of multimorbidity between the HIV and control groups. However, univariate analysis showed significant associations with age (15% per 5 years over 50 years old; P less than .001) and being HIV positive (prevalence ratio, 1.47; P less than .001). Further investigation found that duration of HIV infection and cART exposure time were significantly associated with multimorbidity, resulting in an adjusted model of age (P = .015), duration of HIV infection (P = .027), and time on cART (P = .015), they said.
Of the considered comorbidities, it was noted that between groups there was no significant difference in cardiovascular disease, which may be due to better awareness and care of this risk in HIV-positive patients. In contrast, there was an increased prevalence of HIV-associated bone and kidney disease consistent with the proposed mechanisms of long-term chronic HIV-induced inflammation and antiretroviral toxicities that have previously been implicated as risk factors for excess multimorbidity in aging HIV populations.
This study was designed to investigate the burden of noninfectious comorbidities associated with HIV and, by conducting it in southern Brazil, explore “the risk factors for the occurrence of multimorbidity in HIV-positive individuals in the developing world,” Dr. Maciel and his associates wrote.
The 63% prevalence of multimorbidity associated with HIV was high compared with other reports that ranged from 7% to 29%. This may in part be associated with the Brazilian socioeconomic status in comparison with high-income countries. The researchers also showed that PLWH individuals would develop similar age-related comorbidities as HIV-negative controls but 10 years earlier. Based on this and the high multimorbidity occurrence they had reported in this study, the investigators said that health care providers “must be ready to face the emerging epidemic of multimorbidity affecting people living with HIV in the developing world.”
The authors reported that they had no conflicts of interest.
SOURCE: Maciel RA et al. Int J Infect Dis. 2018 May;70:30-5. doi: 10.1016/j.ijid.2018.02.009.
The research was conducted as a cross-sectional analysis using medical chart data from a total of 416 patients treated at the Hospital de Clínicas de Porto Alegre, a tertiary referral hospital in south Brazil, Rafael Aguilar Maciel, MD of the Universidade Federal do Rio Grande do Sul, Porto Alegre, and his associates reported in the International Journal of Infectious Diseases.
The randomly selected participants were 208 well-controlled persons living with HIV (PLWH) – outpatients identified from the South Brazilian HIV Cohort unit – who were individually matched by age, sex, and ethnicity to HIV-negative control counterparts from the primary practice unit of the same hospital. The study group (median age, 57 years) consisted primarily of individuals who were white in origin (79%); 44.2% were women. Nearly all (98.1%) PLWH participants were on highly active antiretroviral therapy (HAART), with 88% having an undetectable viral load. A higher tendency for alcohol use (16% vs. 6%) and a lower mean body mass index (26 kg/m2 vs. 26-30 kg/m2) also were noted for the PLWH group, compared with the HIV-negative control group.
Individuals with multimorbidity had at least two chronic comorbid diseases including hypertension, diabetes mellitus, chronic kidney disease, bone disease, hepatic disease, or cardiovascular disease at the time of the study. Neoplastic disease also was included if reported previously or as a current condition. Results from the Poisson regression analysis used to identify multimorbidity-associated factors and potential confounders were stratified by age category (50-55, 56-60, 61-65, and older than 65).
Individuals with HIV had a significantly higher prevalence of multimorbidity than did HIV-negative controls (63% vs. 43%; P less than .001). Renal, hepatic, and bone diseases were the main sources of this difference. The overall median number of comorbidities of the PLWH individuals was two, which was double the median number observed in the HIV-negative controls. Furthermore, examination of the age-stratified data showed that the PLWH patients in the 50-60 age group had almost twice the burden of disease as the corresponding HIV-negative individuals. Similarity in prevalence of comorbidities was apparent when comparing the youngest PLWH age group (50-55) with their HIV-negative counterparts who were at least 10 years older, the investigators said.
Regression analysis failed to reveal a specific covariate risk factor associated with a higher prevalence of multimorbidity between the HIV and control groups. However, univariate analysis showed significant associations with age (15% per 5 years over 50 years old; P less than .001) and being HIV positive (prevalence ratio, 1.47; P less than .001). Further investigation found that duration of HIV infection and cART exposure time were significantly associated with multimorbidity, resulting in an adjusted model of age (P = .015), duration of HIV infection (P = .027), and time on cART (P = .015), they said.
Of the considered comorbidities, it was noted that between groups there was no significant difference in cardiovascular disease, which may be due to better awareness and care of this risk in HIV-positive patients. In contrast, there was an increased prevalence of HIV-associated bone and kidney disease consistent with the proposed mechanisms of long-term chronic HIV-induced inflammation and antiretroviral toxicities that have previously been implicated as risk factors for excess multimorbidity in aging HIV populations.
This study was designed to investigate the burden of noninfectious comorbidities associated with HIV and, by conducting it in southern Brazil, explore “the risk factors for the occurrence of multimorbidity in HIV-positive individuals in the developing world,” Dr. Maciel and his associates wrote.
The 63% prevalence of multimorbidity associated with HIV was high compared with other reports that ranged from 7% to 29%. This may in part be associated with the Brazilian socioeconomic status in comparison with high-income countries. The researchers also showed that PLWH individuals would develop similar age-related comorbidities as HIV-negative controls but 10 years earlier. Based on this and the high multimorbidity occurrence they had reported in this study, the investigators said that health care providers “must be ready to face the emerging epidemic of multimorbidity affecting people living with HIV in the developing world.”
The authors reported that they had no conflicts of interest.
SOURCE: Maciel RA et al. Int J Infect Dis. 2018 May;70:30-5. doi: 10.1016/j.ijid.2018.02.009.
The research was conducted as a cross-sectional analysis using medical chart data from a total of 416 patients treated at the Hospital de Clínicas de Porto Alegre, a tertiary referral hospital in south Brazil, Rafael Aguilar Maciel, MD of the Universidade Federal do Rio Grande do Sul, Porto Alegre, and his associates reported in the International Journal of Infectious Diseases.
The randomly selected participants were 208 well-controlled persons living with HIV (PLWH) – outpatients identified from the South Brazilian HIV Cohort unit – who were individually matched by age, sex, and ethnicity to HIV-negative control counterparts from the primary practice unit of the same hospital. The study group (median age, 57 years) consisted primarily of individuals who were white in origin (79%); 44.2% were women. Nearly all (98.1%) PLWH participants were on highly active antiretroviral therapy (HAART), with 88% having an undetectable viral load. A higher tendency for alcohol use (16% vs. 6%) and a lower mean body mass index (26 kg/m2 vs. 26-30 kg/m2) also were noted for the PLWH group, compared with the HIV-negative control group.
Individuals with multimorbidity had at least two chronic comorbid diseases including hypertension, diabetes mellitus, chronic kidney disease, bone disease, hepatic disease, or cardiovascular disease at the time of the study. Neoplastic disease also was included if reported previously or as a current condition. Results from the Poisson regression analysis used to identify multimorbidity-associated factors and potential confounders were stratified by age category (50-55, 56-60, 61-65, and older than 65).
Individuals with HIV had a significantly higher prevalence of multimorbidity than did HIV-negative controls (63% vs. 43%; P less than .001). Renal, hepatic, and bone diseases were the main sources of this difference. The overall median number of comorbidities of the PLWH individuals was two, which was double the median number observed in the HIV-negative controls. Furthermore, examination of the age-stratified data showed that the PLWH patients in the 50-60 age group had almost twice the burden of disease as the corresponding HIV-negative individuals. Similarity in prevalence of comorbidities was apparent when comparing the youngest PLWH age group (50-55) with their HIV-negative counterparts who were at least 10 years older, the investigators said.
Regression analysis failed to reveal a specific covariate risk factor associated with a higher prevalence of multimorbidity between the HIV and control groups. However, univariate analysis showed significant associations with age (15% per 5 years over 50 years old; P less than .001) and being HIV positive (prevalence ratio, 1.47; P less than .001). Further investigation found that duration of HIV infection and cART exposure time were significantly associated with multimorbidity, resulting in an adjusted model of age (P = .015), duration of HIV infection (P = .027), and time on cART (P = .015), they said.
Of the considered comorbidities, it was noted that between groups there was no significant difference in cardiovascular disease, which may be due to better awareness and care of this risk in HIV-positive patients. In contrast, there was an increased prevalence of HIV-associated bone and kidney disease consistent with the proposed mechanisms of long-term chronic HIV-induced inflammation and antiretroviral toxicities that have previously been implicated as risk factors for excess multimorbidity in aging HIV populations.
This study was designed to investigate the burden of noninfectious comorbidities associated with HIV and, by conducting it in southern Brazil, explore “the risk factors for the occurrence of multimorbidity in HIV-positive individuals in the developing world,” Dr. Maciel and his associates wrote.
The 63% prevalence of multimorbidity associated with HIV was high compared with other reports that ranged from 7% to 29%. This may in part be associated with the Brazilian socioeconomic status in comparison with high-income countries. The researchers also showed that PLWH individuals would develop similar age-related comorbidities as HIV-negative controls but 10 years earlier. Based on this and the high multimorbidity occurrence they had reported in this study, the investigators said that health care providers “must be ready to face the emerging epidemic of multimorbidity affecting people living with HIV in the developing world.”
The authors reported that they had no conflicts of interest.
SOURCE: Maciel RA et al. Int J Infect Dis. 2018 May;70:30-5. doi: 10.1016/j.ijid.2018.02.009.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Key clinical point: Toxicity of, and time on, combined antiretroviral therapy (cART) are implicated in contributing to higher comorbidity occurring in an aging HIV population.
Major finding: Compared with HIV-negative controls, individuals at least 50 years old with well-managed HIV infection had a higher frequency (63% vs. 43%) of multimorbidity and a median of two comorbidities to one for controls.
Study details: A cross-sectional study conducted in Brazil from Jan. 1 to June 30, 2016, with 208 HIV-positive and 208 HIV-negative patients matched by age, sex, and ethnicity.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Maciel RA et al. Int J Infect Dis. 2018 May;70:30-5.
PVT after sleeve gastrectomy treatable with anticoagulants
can be effectively treated with extended postoperative anticoagulation therapy, findings from a large-scale, retrospective study indicate.
The research was conducted using data from medical records of created by physicians from five Australian bariatric centers, reported Stephanie Bee Ming Tan, MBBS, of the Gold Coast University Hospital, Queensland, Australia, and her associates in the journal Surgery for Obesity and Related Diseases. Following elective laparoscopic sleeve gastrectomy (LSG), a total of 18 (0.3%) of the 5,951 obese patients were diagnosed with portomesenteric vein thrombosis (PVT). The PVT-affected population was a mean age of 44 years and 61% were women. All of these patients had at least one venous thrombosis systematic predisposition factor such as morbid obesity (50%), smoking (50%), or a personal or family history of a clotting disorder (39%).
All study patients were given thromboprophylaxis of low-molecular-weight heparin (LMWH) or unfractionated heparin plus mechanical thromboprophylaxis during admission for LSG and at discharge when surgeons identified them as high risk.
PVT following LSG can be difficult to diagnose because presenting symptoms tend to be nonspecific. Within an average of 13 days following surgery, 77% of patients diagnosed with PVT reported abdominal pain, 33% reported nausea and vomiting, and also reported less common symptoms that included shoulder tip pain, problems in tolerating fluids, constipation, and diarrhea. Final diagnosis of PVT was determined with independent or a combination of CT and duplex ultrasound.
Complications from PVT can have serious consequences, including abdominal swelling from fluid accumulation, enlarged esophageal veins, terminal esophageal bleeding, and bowel infarction. As with admission thromboprophylaxis treatments, patients diagnosed with PVT received varied anticoagulation treatments with most, in equal numbers, receiving either LMWH or a heparin infusion, and the remaining 12% receiving anticoagulation with rivaroxaban and warfarin. Adjustments were made following initial treatments such that 37% and 66% of patients continued with longer-term therapy on LMWH or warfarin, respectively. Treatments generally lasted 3-6 months with only 11% continuing on warfarin because of a history of clotting disorder. The anticoagulation treatments were successful with the majority (94%) of patients with only one patient requiring surgical intervention.
Follow-up with the patients who had a PVT diagnosis of more than 6 months (with an average of 10 months) showed the overall success of the post-LSG anticoagulation and surgical therapies, without any mortalities.
The authors summarized earlier theories about confounding health conditions that may contribute to the development of PVT and the risks for PVT linked to laparoscopic surgery. In this retrospective study, they noted that PVT incidence following LSG was low at 0.3% but was still higher than with two other bariatric operative methods and suggested intraoperative and postoperative factors that could contribute to this difference. Because of the nonspecific early symptoms and the difficulty of diagnosing PVT, the investigators recommended that physicians be vigilant for this postoperative complication in LSG patients, and use “cross-sectional imagining with CT of the abdomen” for diagnosis. Furthermore, with diagnosed PVT “anticoagulation for 3 to 6 months with a target international normalized ratio of 2:3 is recommended unless the patient has additional risk factors and [is] therefore indicated for longer treatment.”
The authors reported that they had no conflicts of interest.
SOURCE: Tan SBM et al. Surg Obes Relat Dis. 2018 Mar;14:271-6.
can be effectively treated with extended postoperative anticoagulation therapy, findings from a large-scale, retrospective study indicate.
The research was conducted using data from medical records of created by physicians from five Australian bariatric centers, reported Stephanie Bee Ming Tan, MBBS, of the Gold Coast University Hospital, Queensland, Australia, and her associates in the journal Surgery for Obesity and Related Diseases. Following elective laparoscopic sleeve gastrectomy (LSG), a total of 18 (0.3%) of the 5,951 obese patients were diagnosed with portomesenteric vein thrombosis (PVT). The PVT-affected population was a mean age of 44 years and 61% were women. All of these patients had at least one venous thrombosis systematic predisposition factor such as morbid obesity (50%), smoking (50%), or a personal or family history of a clotting disorder (39%).
All study patients were given thromboprophylaxis of low-molecular-weight heparin (LMWH) or unfractionated heparin plus mechanical thromboprophylaxis during admission for LSG and at discharge when surgeons identified them as high risk.
PVT following LSG can be difficult to diagnose because presenting symptoms tend to be nonspecific. Within an average of 13 days following surgery, 77% of patients diagnosed with PVT reported abdominal pain, 33% reported nausea and vomiting, and also reported less common symptoms that included shoulder tip pain, problems in tolerating fluids, constipation, and diarrhea. Final diagnosis of PVT was determined with independent or a combination of CT and duplex ultrasound.
Complications from PVT can have serious consequences, including abdominal swelling from fluid accumulation, enlarged esophageal veins, terminal esophageal bleeding, and bowel infarction. As with admission thromboprophylaxis treatments, patients diagnosed with PVT received varied anticoagulation treatments with most, in equal numbers, receiving either LMWH or a heparin infusion, and the remaining 12% receiving anticoagulation with rivaroxaban and warfarin. Adjustments were made following initial treatments such that 37% and 66% of patients continued with longer-term therapy on LMWH or warfarin, respectively. Treatments generally lasted 3-6 months with only 11% continuing on warfarin because of a history of clotting disorder. The anticoagulation treatments were successful with the majority (94%) of patients with only one patient requiring surgical intervention.
Follow-up with the patients who had a PVT diagnosis of more than 6 months (with an average of 10 months) showed the overall success of the post-LSG anticoagulation and surgical therapies, without any mortalities.
The authors summarized earlier theories about confounding health conditions that may contribute to the development of PVT and the risks for PVT linked to laparoscopic surgery. In this retrospective study, they noted that PVT incidence following LSG was low at 0.3% but was still higher than with two other bariatric operative methods and suggested intraoperative and postoperative factors that could contribute to this difference. Because of the nonspecific early symptoms and the difficulty of diagnosing PVT, the investigators recommended that physicians be vigilant for this postoperative complication in LSG patients, and use “cross-sectional imagining with CT of the abdomen” for diagnosis. Furthermore, with diagnosed PVT “anticoagulation for 3 to 6 months with a target international normalized ratio of 2:3 is recommended unless the patient has additional risk factors and [is] therefore indicated for longer treatment.”
The authors reported that they had no conflicts of interest.
SOURCE: Tan SBM et al. Surg Obes Relat Dis. 2018 Mar;14:271-6.
can be effectively treated with extended postoperative anticoagulation therapy, findings from a large-scale, retrospective study indicate.
The research was conducted using data from medical records of created by physicians from five Australian bariatric centers, reported Stephanie Bee Ming Tan, MBBS, of the Gold Coast University Hospital, Queensland, Australia, and her associates in the journal Surgery for Obesity and Related Diseases. Following elective laparoscopic sleeve gastrectomy (LSG), a total of 18 (0.3%) of the 5,951 obese patients were diagnosed with portomesenteric vein thrombosis (PVT). The PVT-affected population was a mean age of 44 years and 61% were women. All of these patients had at least one venous thrombosis systematic predisposition factor such as morbid obesity (50%), smoking (50%), or a personal or family history of a clotting disorder (39%).
All study patients were given thromboprophylaxis of low-molecular-weight heparin (LMWH) or unfractionated heparin plus mechanical thromboprophylaxis during admission for LSG and at discharge when surgeons identified them as high risk.
PVT following LSG can be difficult to diagnose because presenting symptoms tend to be nonspecific. Within an average of 13 days following surgery, 77% of patients diagnosed with PVT reported abdominal pain, 33% reported nausea and vomiting, and also reported less common symptoms that included shoulder tip pain, problems in tolerating fluids, constipation, and diarrhea. Final diagnosis of PVT was determined with independent or a combination of CT and duplex ultrasound.
Complications from PVT can have serious consequences, including abdominal swelling from fluid accumulation, enlarged esophageal veins, terminal esophageal bleeding, and bowel infarction. As with admission thromboprophylaxis treatments, patients diagnosed with PVT received varied anticoagulation treatments with most, in equal numbers, receiving either LMWH or a heparin infusion, and the remaining 12% receiving anticoagulation with rivaroxaban and warfarin. Adjustments were made following initial treatments such that 37% and 66% of patients continued with longer-term therapy on LMWH or warfarin, respectively. Treatments generally lasted 3-6 months with only 11% continuing on warfarin because of a history of clotting disorder. The anticoagulation treatments were successful with the majority (94%) of patients with only one patient requiring surgical intervention.
Follow-up with the patients who had a PVT diagnosis of more than 6 months (with an average of 10 months) showed the overall success of the post-LSG anticoagulation and surgical therapies, without any mortalities.
The authors summarized earlier theories about confounding health conditions that may contribute to the development of PVT and the risks for PVT linked to laparoscopic surgery. In this retrospective study, they noted that PVT incidence following LSG was low at 0.3% but was still higher than with two other bariatric operative methods and suggested intraoperative and postoperative factors that could contribute to this difference. Because of the nonspecific early symptoms and the difficulty of diagnosing PVT, the investigators recommended that physicians be vigilant for this postoperative complication in LSG patients, and use “cross-sectional imagining with CT of the abdomen” for diagnosis. Furthermore, with diagnosed PVT “anticoagulation for 3 to 6 months with a target international normalized ratio of 2:3 is recommended unless the patient has additional risk factors and [is] therefore indicated for longer treatment.”
The authors reported that they had no conflicts of interest.
SOURCE: Tan SBM et al. Surg Obes Relat Dis. 2018 Mar;14:271-6.
FROM SURGERY FOR OBESITY AND RELATED DISEASES
Key clinical point: Anticoagulation treatments effectively managed most portomesenteric vein thrombosis cases following laparoscopic sleeve gastrectomy.
Major finding: PVT is rare (0.3%) but occurs more frequently with laparoscopic sleeve gastrectomy, compared with other bariatric surgery procedures.
Study details: A multicenter, retrospective study conducted in Australia from 2007 to 2016 with 5,951 adult obese patients who received elective laparoscopic sleeve gastrectomy.
Disclosures: The authors reported no conflicts of interest.
Source: Tan SBM et al. Surg Obes Relat Dis. Mar 2018;14:271-6.
Colorectal cancer risk stratification enhanced by combining family history and genetic risk scores
Stratification of colorectal cancer (CRC) risk was enhanced by joint consideration of the independent family history and genetic risk score predictors, according to an ongoing population-based, case-control study of patients recruited during 2003-2010.
The research was conducted using data from DACHS (Colorectal Cancer: Chances for Prevention Through Screening), an ongoing population-based, case-control study in Germany, reported Korbinian Weigl, PhD, and his colleagues in the journal Clinical Epidemiology (doi: 10.2147/CLEP.S145636). They included 2,363 eligible CRC patients who were identified by 22 participating hospitals and frequency matched with respect to sex, age, and residential location to 2,198 randomly selected controls that had genome-wide association studies data. The population consisted of 40% women, and the median ages for cases and controls were 69 and 70 years, respectively.
Genetic risk score was calculated by genotyping 53 single-nucleotide polymorphisms reported in published literature to be associated with higher CRC risk for individuals of European descent. Seven genetic risk score groups – very low, low, low-medium, medium, medium-high, high, and very high – were established according to categories generated on the basis of weighted risk allele distribution among controls. Family history referred to CRC in first-degree and second-degree relatives. Selected potential confounders included age, sex, body mass index, education, hormone replacement therapy in women, smoking, and colonoscopy history. Odds ratios with 95% confidence intervals were estimated by multiple logistic regression models that included adjustment for potential confounders. Statistical calculations examined individual and joint family history and genetic risk score associations with risk for CRC and the effect of potential confounding factors.
At least one colonoscopy was performed on over half the individuals in the control group, while a significantly lower number (P less than .0001) were performed on case individuals (22.1%). Family history of CRC in first-degree relatives was reported by 316 case participants (13.4%) and 214 controls (9.7%; P less than .0001). The calculated genetic risk score ranged from 20 to 48, with a substantially higher proportion of cases in the higher deciles.
Investigators compared the risk for CRC in the top decile with that in the lowest and found an increased risk of 2.9-fold (OR, 2.94) based on genetic risk analysis adjusted for sex and age and an increased risk of 3.0-fold (OR, 3.0) when all other covariates except family history were included. Comparing results against analysis with the 27 single-nucleotide polymorphisms that had been used in previous studies indicated a sizable improvement in genetic risk stratification as a result of increasing the number of single-nucleotide polymorphisms (P value for increase in c statistic = .003) included in the analysis.
Risk associated with having a family history of CRC in a first-degree relative was 1.5-fold (OR, 1.47) higher in an age- and sex-adjusted analysis. Risk prediction increased to an OR of 1.86 when calculations were adjusted with covariates, especially with previous colonoscopies. Using genetic risk scoring as a calculation adjustment only slightly changed the result (OR, 1.83). A similar trend, but with lower-magnitude associations, was observed with family history of CRC in second-degree relatives.
A dose-response association between the number of risk alleles and CRC risk determined by a logistic regression model revealed a curvilinear relationship between genetic risk score and CRC risk. At higher genetic risk score levels, the increase in CRC risk was particularly strong. The dose-response association indicated an independent relationship between family history and CRC such that individuals with first-degree relatives with CRC will reach the same risk level with a lower genetic risk score as those with a higher genetic risk score but no first-degree relatives with CRC.
Joint risk stratification that combined family history and genetic risk scores was compared with risks determined by each predictor. As the genetic risk score increased there was an observed increased risk for individuals with first-degree relatives, second-degree relatives, or without family history. Considering only genetic risk score, the increase in risk from the lowest to highest decile was 2.8-fold. In contrast, the increased risk from the lowest to highest decile was 6.14-fold when stratification included both genetic risk score and considering family history in first-degree relatives, thus demonstrating the enhancing effect of combining the independent relationship of these two predictors.
The investigators concluded from their results that, by combining the genetic risk scores with family history and other easy-to-collect risk factor information,
The authors reported that they had no conflicts of interest.
SOURCE: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
Stratification of colorectal cancer (CRC) risk was enhanced by joint consideration of the independent family history and genetic risk score predictors, according to an ongoing population-based, case-control study of patients recruited during 2003-2010.
The research was conducted using data from DACHS (Colorectal Cancer: Chances for Prevention Through Screening), an ongoing population-based, case-control study in Germany, reported Korbinian Weigl, PhD, and his colleagues in the journal Clinical Epidemiology (doi: 10.2147/CLEP.S145636). They included 2,363 eligible CRC patients who were identified by 22 participating hospitals and frequency matched with respect to sex, age, and residential location to 2,198 randomly selected controls that had genome-wide association studies data. The population consisted of 40% women, and the median ages for cases and controls were 69 and 70 years, respectively.
Genetic risk score was calculated by genotyping 53 single-nucleotide polymorphisms reported in published literature to be associated with higher CRC risk for individuals of European descent. Seven genetic risk score groups – very low, low, low-medium, medium, medium-high, high, and very high – were established according to categories generated on the basis of weighted risk allele distribution among controls. Family history referred to CRC in first-degree and second-degree relatives. Selected potential confounders included age, sex, body mass index, education, hormone replacement therapy in women, smoking, and colonoscopy history. Odds ratios with 95% confidence intervals were estimated by multiple logistic regression models that included adjustment for potential confounders. Statistical calculations examined individual and joint family history and genetic risk score associations with risk for CRC and the effect of potential confounding factors.
At least one colonoscopy was performed on over half the individuals in the control group, while a significantly lower number (P less than .0001) were performed on case individuals (22.1%). Family history of CRC in first-degree relatives was reported by 316 case participants (13.4%) and 214 controls (9.7%; P less than .0001). The calculated genetic risk score ranged from 20 to 48, with a substantially higher proportion of cases in the higher deciles.
Investigators compared the risk for CRC in the top decile with that in the lowest and found an increased risk of 2.9-fold (OR, 2.94) based on genetic risk analysis adjusted for sex and age and an increased risk of 3.0-fold (OR, 3.0) when all other covariates except family history were included. Comparing results against analysis with the 27 single-nucleotide polymorphisms that had been used in previous studies indicated a sizable improvement in genetic risk stratification as a result of increasing the number of single-nucleotide polymorphisms (P value for increase in c statistic = .003) included in the analysis.
Risk associated with having a family history of CRC in a first-degree relative was 1.5-fold (OR, 1.47) higher in an age- and sex-adjusted analysis. Risk prediction increased to an OR of 1.86 when calculations were adjusted with covariates, especially with previous colonoscopies. Using genetic risk scoring as a calculation adjustment only slightly changed the result (OR, 1.83). A similar trend, but with lower-magnitude associations, was observed with family history of CRC in second-degree relatives.
A dose-response association between the number of risk alleles and CRC risk determined by a logistic regression model revealed a curvilinear relationship between genetic risk score and CRC risk. At higher genetic risk score levels, the increase in CRC risk was particularly strong. The dose-response association indicated an independent relationship between family history and CRC such that individuals with first-degree relatives with CRC will reach the same risk level with a lower genetic risk score as those with a higher genetic risk score but no first-degree relatives with CRC.
Joint risk stratification that combined family history and genetic risk scores was compared with risks determined by each predictor. As the genetic risk score increased there was an observed increased risk for individuals with first-degree relatives, second-degree relatives, or without family history. Considering only genetic risk score, the increase in risk from the lowest to highest decile was 2.8-fold. In contrast, the increased risk from the lowest to highest decile was 6.14-fold when stratification included both genetic risk score and considering family history in first-degree relatives, thus demonstrating the enhancing effect of combining the independent relationship of these two predictors.
The investigators concluded from their results that, by combining the genetic risk scores with family history and other easy-to-collect risk factor information,
The authors reported that they had no conflicts of interest.
SOURCE: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
Stratification of colorectal cancer (CRC) risk was enhanced by joint consideration of the independent family history and genetic risk score predictors, according to an ongoing population-based, case-control study of patients recruited during 2003-2010.
The research was conducted using data from DACHS (Colorectal Cancer: Chances for Prevention Through Screening), an ongoing population-based, case-control study in Germany, reported Korbinian Weigl, PhD, and his colleagues in the journal Clinical Epidemiology (doi: 10.2147/CLEP.S145636). They included 2,363 eligible CRC patients who were identified by 22 participating hospitals and frequency matched with respect to sex, age, and residential location to 2,198 randomly selected controls that had genome-wide association studies data. The population consisted of 40% women, and the median ages for cases and controls were 69 and 70 years, respectively.
Genetic risk score was calculated by genotyping 53 single-nucleotide polymorphisms reported in published literature to be associated with higher CRC risk for individuals of European descent. Seven genetic risk score groups – very low, low, low-medium, medium, medium-high, high, and very high – were established according to categories generated on the basis of weighted risk allele distribution among controls. Family history referred to CRC in first-degree and second-degree relatives. Selected potential confounders included age, sex, body mass index, education, hormone replacement therapy in women, smoking, and colonoscopy history. Odds ratios with 95% confidence intervals were estimated by multiple logistic regression models that included adjustment for potential confounders. Statistical calculations examined individual and joint family history and genetic risk score associations with risk for CRC and the effect of potential confounding factors.
At least one colonoscopy was performed on over half the individuals in the control group, while a significantly lower number (P less than .0001) were performed on case individuals (22.1%). Family history of CRC in first-degree relatives was reported by 316 case participants (13.4%) and 214 controls (9.7%; P less than .0001). The calculated genetic risk score ranged from 20 to 48, with a substantially higher proportion of cases in the higher deciles.
Investigators compared the risk for CRC in the top decile with that in the lowest and found an increased risk of 2.9-fold (OR, 2.94) based on genetic risk analysis adjusted for sex and age and an increased risk of 3.0-fold (OR, 3.0) when all other covariates except family history were included. Comparing results against analysis with the 27 single-nucleotide polymorphisms that had been used in previous studies indicated a sizable improvement in genetic risk stratification as a result of increasing the number of single-nucleotide polymorphisms (P value for increase in c statistic = .003) included in the analysis.
Risk associated with having a family history of CRC in a first-degree relative was 1.5-fold (OR, 1.47) higher in an age- and sex-adjusted analysis. Risk prediction increased to an OR of 1.86 when calculations were adjusted with covariates, especially with previous colonoscopies. Using genetic risk scoring as a calculation adjustment only slightly changed the result (OR, 1.83). A similar trend, but with lower-magnitude associations, was observed with family history of CRC in second-degree relatives.
A dose-response association between the number of risk alleles and CRC risk determined by a logistic regression model revealed a curvilinear relationship between genetic risk score and CRC risk. At higher genetic risk score levels, the increase in CRC risk was particularly strong. The dose-response association indicated an independent relationship between family history and CRC such that individuals with first-degree relatives with CRC will reach the same risk level with a lower genetic risk score as those with a higher genetic risk score but no first-degree relatives with CRC.
Joint risk stratification that combined family history and genetic risk scores was compared with risks determined by each predictor. As the genetic risk score increased there was an observed increased risk for individuals with first-degree relatives, second-degree relatives, or without family history. Considering only genetic risk score, the increase in risk from the lowest to highest decile was 2.8-fold. In contrast, the increased risk from the lowest to highest decile was 6.14-fold when stratification included both genetic risk score and considering family history in first-degree relatives, thus demonstrating the enhancing effect of combining the independent relationship of these two predictors.
The investigators concluded from their results that, by combining the genetic risk scores with family history and other easy-to-collect risk factor information,
The authors reported that they had no conflicts of interest.
SOURCE: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
FROM CLINICAL EPIDEMIOLOGY
Key clinical point: Jointly using family history and genetic risk scores enhances their independent considerations for CRC risk stratifications.
Major finding: CRC in the highest decile compared with lowest decile was associated with 3.0-fold and 6.1-fold increased risks when considering genetic risk scores independently and jointly with family history, respectively.
Study details: Ongoing population-based study involving 22 hospitals in Germany with 2,363 CRC cases compared to 2,198 controls.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Weigl K et al. Clin Epidemiol. 2018;10:143-52.
Large-vessel vasculitis: More severe in HIV-infected patients
Large-vessel vasculitis (LVV) associated with HIV-infected patients occurs more frequently in men and is significantly more severe, compared with the disease in their noninfected counterparts, according to a retrospective analysis of medical records during 2000-2015.
The study was conducted at Pitié-Salpêtrière Hospital in Paris to investigate the occurrence, characteristics, and treatment outcomes of LVV of patients infected with HIV, reported in the Journal of Vascular Surgery. Yasmina Ferfar, MD, and her colleagues diagnosed LVV using available imaging (computed tomography, positron emission tomography, and magnetic resonance angiography), histology, and Ishikawa or American College of Rheumatology (ACR) criteria of Takayasu arteritis (TA). All 93 patients selected for the study had LVV.
Vascular lesions were located in three areas; aorta (n = 7), supra-aortic trunks (7), and in digestive arteries (3). The Ishikawa or ACR criteria for TA diagnosis were met by 7 of the 11 (64%) patients.
Highly active antiretroviral therapy was prescribed for six patients at the time of vasculitis diagnosis and for four after diagnosis; HAART was delayed for the remaining patient whose HIV infection was under control.
Steroids, immunosuppressive, statins, and antiplatelet agents were provided in custom combinations to nine patients. Open surgical or endovascular repair was performed on 8 (73%) of the 11 patients with a mean follow-up at 95 months. As recorded at the last follow-up, 6 of the 11 patients had obtained stabilization of the vasculitis, improvement of vascular lesions had occurred in 4 of the 11 patients, and 1 patient had died from complications related to vascular surgery.
An age-matched control test comparison was performed between HIV-infected patients with large-vessel vasculitis and the HIV-negative patients with Takayasu arteritis that were identified in this study. The 82 patients in the LVV HIV-negative group had a median age of 42 years and 72 (88%) were women. Inflammatory syndrome was reported for 30 patients. Glucocorticosteroid treatments were given to 70 patients and immunosuppressive treatments were given to 57 patients.
Comparing LVV between the HIV-infected and their HIV-negative counterparts showed that vascular disease was significantly more severe for HIV-infected patients, according to the Ishikawa score (P = .017) and there was significantly greater use of vascular procedures (P = .047). LVV in association with HIV-infection also occurred more frequently with men (P = .014).
Dr. Ferfar and colleagues suggested that “HIV infection might be an accelerant for vasculitis,” based upon the increased severity of the disease they noted. They also indicated that glucocorticosteroids and immunosuppressive treatments were less often prescribed in HIV-positive patients (P = .001).
The effectiveness of steroids and immunosuppressant drugs seen with 7 of the 11 HIV-positive patients that met the Ishikawa or ACR criteria of TA suggests an overlap between HIV-associated LVV and TA, according to the researchers. Therefore, “HIV-infected patients with LVV should be treated with corticosteroids to avoid complications. Studies with a greater number of HIV-infected patients with LVV are required to confirm this hypothesis”.
The authors reported that they had no conflicts of interest.
SOURCE: Ferfar Y. et al. J Vasc Surg. 2018 Dec 11. doi. org/10.1016/j.jvs.2017.08.099.
Large-vessel vasculitis (LVV) associated with HIV-infected patients occurs more frequently in men and is significantly more severe, compared with the disease in their noninfected counterparts, according to a retrospective analysis of medical records during 2000-2015.
The study was conducted at Pitié-Salpêtrière Hospital in Paris to investigate the occurrence, characteristics, and treatment outcomes of LVV of patients infected with HIV, reported in the Journal of Vascular Surgery. Yasmina Ferfar, MD, and her colleagues diagnosed LVV using available imaging (computed tomography, positron emission tomography, and magnetic resonance angiography), histology, and Ishikawa or American College of Rheumatology (ACR) criteria of Takayasu arteritis (TA). All 93 patients selected for the study had LVV.
Vascular lesions were located in three areas; aorta (n = 7), supra-aortic trunks (7), and in digestive arteries (3). The Ishikawa or ACR criteria for TA diagnosis were met by 7 of the 11 (64%) patients.
Highly active antiretroviral therapy was prescribed for six patients at the time of vasculitis diagnosis and for four after diagnosis; HAART was delayed for the remaining patient whose HIV infection was under control.
Steroids, immunosuppressive, statins, and antiplatelet agents were provided in custom combinations to nine patients. Open surgical or endovascular repair was performed on 8 (73%) of the 11 patients with a mean follow-up at 95 months. As recorded at the last follow-up, 6 of the 11 patients had obtained stabilization of the vasculitis, improvement of vascular lesions had occurred in 4 of the 11 patients, and 1 patient had died from complications related to vascular surgery.
An age-matched control test comparison was performed between HIV-infected patients with large-vessel vasculitis and the HIV-negative patients with Takayasu arteritis that were identified in this study. The 82 patients in the LVV HIV-negative group had a median age of 42 years and 72 (88%) were women. Inflammatory syndrome was reported for 30 patients. Glucocorticosteroid treatments were given to 70 patients and immunosuppressive treatments were given to 57 patients.
Comparing LVV between the HIV-infected and their HIV-negative counterparts showed that vascular disease was significantly more severe for HIV-infected patients, according to the Ishikawa score (P = .017) and there was significantly greater use of vascular procedures (P = .047). LVV in association with HIV-infection also occurred more frequently with men (P = .014).
Dr. Ferfar and colleagues suggested that “HIV infection might be an accelerant for vasculitis,” based upon the increased severity of the disease they noted. They also indicated that glucocorticosteroids and immunosuppressive treatments were less often prescribed in HIV-positive patients (P = .001).
The effectiveness of steroids and immunosuppressant drugs seen with 7 of the 11 HIV-positive patients that met the Ishikawa or ACR criteria of TA suggests an overlap between HIV-associated LVV and TA, according to the researchers. Therefore, “HIV-infected patients with LVV should be treated with corticosteroids to avoid complications. Studies with a greater number of HIV-infected patients with LVV are required to confirm this hypothesis”.
The authors reported that they had no conflicts of interest.
SOURCE: Ferfar Y. et al. J Vasc Surg. 2018 Dec 11. doi. org/10.1016/j.jvs.2017.08.099.
Large-vessel vasculitis (LVV) associated with HIV-infected patients occurs more frequently in men and is significantly more severe, compared with the disease in their noninfected counterparts, according to a retrospective analysis of medical records during 2000-2015.
The study was conducted at Pitié-Salpêtrière Hospital in Paris to investigate the occurrence, characteristics, and treatment outcomes of LVV of patients infected with HIV, reported in the Journal of Vascular Surgery. Yasmina Ferfar, MD, and her colleagues diagnosed LVV using available imaging (computed tomography, positron emission tomography, and magnetic resonance angiography), histology, and Ishikawa or American College of Rheumatology (ACR) criteria of Takayasu arteritis (TA). All 93 patients selected for the study had LVV.
Vascular lesions were located in three areas; aorta (n = 7), supra-aortic trunks (7), and in digestive arteries (3). The Ishikawa or ACR criteria for TA diagnosis were met by 7 of the 11 (64%) patients.
Highly active antiretroviral therapy was prescribed for six patients at the time of vasculitis diagnosis and for four after diagnosis; HAART was delayed for the remaining patient whose HIV infection was under control.
Steroids, immunosuppressive, statins, and antiplatelet agents were provided in custom combinations to nine patients. Open surgical or endovascular repair was performed on 8 (73%) of the 11 patients with a mean follow-up at 95 months. As recorded at the last follow-up, 6 of the 11 patients had obtained stabilization of the vasculitis, improvement of vascular lesions had occurred in 4 of the 11 patients, and 1 patient had died from complications related to vascular surgery.
An age-matched control test comparison was performed between HIV-infected patients with large-vessel vasculitis and the HIV-negative patients with Takayasu arteritis that were identified in this study. The 82 patients in the LVV HIV-negative group had a median age of 42 years and 72 (88%) were women. Inflammatory syndrome was reported for 30 patients. Glucocorticosteroid treatments were given to 70 patients and immunosuppressive treatments were given to 57 patients.
Comparing LVV between the HIV-infected and their HIV-negative counterparts showed that vascular disease was significantly more severe for HIV-infected patients, according to the Ishikawa score (P = .017) and there was significantly greater use of vascular procedures (P = .047). LVV in association with HIV-infection also occurred more frequently with men (P = .014).
Dr. Ferfar and colleagues suggested that “HIV infection might be an accelerant for vasculitis,” based upon the increased severity of the disease they noted. They also indicated that glucocorticosteroids and immunosuppressive treatments were less often prescribed in HIV-positive patients (P = .001).
The effectiveness of steroids and immunosuppressant drugs seen with 7 of the 11 HIV-positive patients that met the Ishikawa or ACR criteria of TA suggests an overlap between HIV-associated LVV and TA, according to the researchers. Therefore, “HIV-infected patients with LVV should be treated with corticosteroids to avoid complications. Studies with a greater number of HIV-infected patients with LVV are required to confirm this hypothesis”.
The authors reported that they had no conflicts of interest.
SOURCE: Ferfar Y. et al. J Vasc Surg. 2018 Dec 11. doi. org/10.1016/j.jvs.2017.08.099.
FROM THE JOURNAL OF VASCULAR SURGERY
Key clinical point: Corticosteroid treatments may reduce severity and avoid some complications of LVV in HIV-infected patients.
Major finding: LVV is significantly more severe in HIV-infected patients and is associated more frequently with men.
Study details: A Pitié-Salpêtrière Hospital retrospective study of 11 HIV-infected patients with LVV, compared with 82 uninfected age-matched patients with LVV.
Disclosures: The authors reported that they had no conflicts of interest.
Source: Ferfar Y et al. J Vasc Surg. 2018. doi. org/10.1016/j.jvs.2017.08.099.
Preop physiotherapy training reduces risk of postop pulmonary complications
A single 30-minute coaching session with a physiotherapist within 6 weeks of major upper abdominal surgery significantly reduced postoperative pulmonary complications (PPC), according to the results of a prospective trial.
Ianthe Boden and her colleagues recruited 441 eligible adults scheduled for elective major upper abdominal surgery to participate in the prospective, multicenter, double-blinded, controlled superiority study to assess whether PPC outcomes were affected by preoperative physiotherapy. Consecutive participants were obtained from outpatient preadmission assessment clinics during June 2013 to August 2015; they were assigned randomly in a 1:1 ratio to the control (219) or intervention (222) groups. The median patient age was 68 years for the control and 63 for the intervention group, and each group was composed of 31% women.
Immediately after receiving the booklets, however, participants in the intervention group were also given an added 30-minute education and training session by preoperative physiotherapists. This instruction covered factors contributing to PPC occurrence, strategies to help prevention it, and three coached repetitions of breathing exercises. Emphasis was placed on initiating prescribed breathing exercises upon regaining postoperative consciousness and continuing them every hour until the patients were fully ambulatory.
The primary outcome was evaluated by masked assessors using the Melbourne group score criteria to determine PPC incidence within 14 postoperative days or by the time of hospital discharge, whichever was sooner. Nine participants, 4 from the intervention and 5 from the control group, withdrew from the study. Of the total remaining 432 participants, 85 (20%) had a documented PPC incident, including hospital acquired pneumonia, within the specified postoperative time frame, as reported in the BMJ.
Results showed that the physiotherapy group had significantly fewer PPC occurrences (27/218, 12%) than did the control group (58/214, 27%). The calculated absolute risk reduction was 15% (P less than .001). Adjustment for three of the prespecified covariates (age, respiratory comorbidity, and surgical procedure) showed PPC incidence remained halved (hazard ratio, 0.48; P = .001) for the intervention group with a number needed to treat of 7 (95% confidence interval, 5-14).
Secondary outcomes included incidence of hospital acquired pneumonia, hospital utilization, mobility, patient reported complications at 6 weeks, and mortality rates in hospital, at 6 weeks, and at 12 months. For secondary outcomes in the adjusted analysis, incidences of pneumonia were halved in the physiotherapy intervention group with a number needed to treat of 9 (95% CI, 6-21). No significant differences in secondary outcomes were detected between the control and treatment groups.
Sensitivity analysis that removed participants who had lower abdominal and laparoscopic surgery strengthened both primary and secondary outcome results to favor the preoperative physiotherapy intervention for reducing PPC. The researchers found that, in an adjusted analysis of subgroup effects, there was a gradient in reduction of PPCs according to surgical category.
Shorter lengths hospital stay and lower all-cause 12-month mortality were also associated with more experienced physiotherapists providing the preoperative education and training.
Ms. Boden and her colleagues proposed that the timing for patients to begin breathing exercises after major open upper abdominal surgery could be critical in reducing PPC incidence. Initiating breathing exercises within the first 24 hours after surgery – in contrast to the common practice of waiting 1-2 days to begin postoperative physiotherapy – could prevent general anesthesia-associated mild atelectasis from developing into severe atelectasis and PPCs.
The researchers concluded that “in a general population of patients listed for elective upper abdominal surgery, a 30-minute preoperative physiotherapy session provided within existing hospital multidisciplinary preadmission clinics halves the incidence of PPCs and specifically hospital acquired pneumonia. Further research is required to investigate benefits to mortality and length of stay.”
The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
SOURCE: Boden I et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
A single 30-minute coaching session with a physiotherapist within 6 weeks of major upper abdominal surgery significantly reduced postoperative pulmonary complications (PPC), according to the results of a prospective trial.
Ianthe Boden and her colleagues recruited 441 eligible adults scheduled for elective major upper abdominal surgery to participate in the prospective, multicenter, double-blinded, controlled superiority study to assess whether PPC outcomes were affected by preoperative physiotherapy. Consecutive participants were obtained from outpatient preadmission assessment clinics during June 2013 to August 2015; they were assigned randomly in a 1:1 ratio to the control (219) or intervention (222) groups. The median patient age was 68 years for the control and 63 for the intervention group, and each group was composed of 31% women.
Immediately after receiving the booklets, however, participants in the intervention group were also given an added 30-minute education and training session by preoperative physiotherapists. This instruction covered factors contributing to PPC occurrence, strategies to help prevention it, and three coached repetitions of breathing exercises. Emphasis was placed on initiating prescribed breathing exercises upon regaining postoperative consciousness and continuing them every hour until the patients were fully ambulatory.
The primary outcome was evaluated by masked assessors using the Melbourne group score criteria to determine PPC incidence within 14 postoperative days or by the time of hospital discharge, whichever was sooner. Nine participants, 4 from the intervention and 5 from the control group, withdrew from the study. Of the total remaining 432 participants, 85 (20%) had a documented PPC incident, including hospital acquired pneumonia, within the specified postoperative time frame, as reported in the BMJ.
Results showed that the physiotherapy group had significantly fewer PPC occurrences (27/218, 12%) than did the control group (58/214, 27%). The calculated absolute risk reduction was 15% (P less than .001). Adjustment for three of the prespecified covariates (age, respiratory comorbidity, and surgical procedure) showed PPC incidence remained halved (hazard ratio, 0.48; P = .001) for the intervention group with a number needed to treat of 7 (95% confidence interval, 5-14).
Secondary outcomes included incidence of hospital acquired pneumonia, hospital utilization, mobility, patient reported complications at 6 weeks, and mortality rates in hospital, at 6 weeks, and at 12 months. For secondary outcomes in the adjusted analysis, incidences of pneumonia were halved in the physiotherapy intervention group with a number needed to treat of 9 (95% CI, 6-21). No significant differences in secondary outcomes were detected between the control and treatment groups.
Sensitivity analysis that removed participants who had lower abdominal and laparoscopic surgery strengthened both primary and secondary outcome results to favor the preoperative physiotherapy intervention for reducing PPC. The researchers found that, in an adjusted analysis of subgroup effects, there was a gradient in reduction of PPCs according to surgical category.
Shorter lengths hospital stay and lower all-cause 12-month mortality were also associated with more experienced physiotherapists providing the preoperative education and training.
Ms. Boden and her colleagues proposed that the timing for patients to begin breathing exercises after major open upper abdominal surgery could be critical in reducing PPC incidence. Initiating breathing exercises within the first 24 hours after surgery – in contrast to the common practice of waiting 1-2 days to begin postoperative physiotherapy – could prevent general anesthesia-associated mild atelectasis from developing into severe atelectasis and PPCs.
The researchers concluded that “in a general population of patients listed for elective upper abdominal surgery, a 30-minute preoperative physiotherapy session provided within existing hospital multidisciplinary preadmission clinics halves the incidence of PPCs and specifically hospital acquired pneumonia. Further research is required to investigate benefits to mortality and length of stay.”
The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
SOURCE: Boden I et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
A single 30-minute coaching session with a physiotherapist within 6 weeks of major upper abdominal surgery significantly reduced postoperative pulmonary complications (PPC), according to the results of a prospective trial.
Ianthe Boden and her colleagues recruited 441 eligible adults scheduled for elective major upper abdominal surgery to participate in the prospective, multicenter, double-blinded, controlled superiority study to assess whether PPC outcomes were affected by preoperative physiotherapy. Consecutive participants were obtained from outpatient preadmission assessment clinics during June 2013 to August 2015; they were assigned randomly in a 1:1 ratio to the control (219) or intervention (222) groups. The median patient age was 68 years for the control and 63 for the intervention group, and each group was composed of 31% women.
Immediately after receiving the booklets, however, participants in the intervention group were also given an added 30-minute education and training session by preoperative physiotherapists. This instruction covered factors contributing to PPC occurrence, strategies to help prevention it, and three coached repetitions of breathing exercises. Emphasis was placed on initiating prescribed breathing exercises upon regaining postoperative consciousness and continuing them every hour until the patients were fully ambulatory.
The primary outcome was evaluated by masked assessors using the Melbourne group score criteria to determine PPC incidence within 14 postoperative days or by the time of hospital discharge, whichever was sooner. Nine participants, 4 from the intervention and 5 from the control group, withdrew from the study. Of the total remaining 432 participants, 85 (20%) had a documented PPC incident, including hospital acquired pneumonia, within the specified postoperative time frame, as reported in the BMJ.
Results showed that the physiotherapy group had significantly fewer PPC occurrences (27/218, 12%) than did the control group (58/214, 27%). The calculated absolute risk reduction was 15% (P less than .001). Adjustment for three of the prespecified covariates (age, respiratory comorbidity, and surgical procedure) showed PPC incidence remained halved (hazard ratio, 0.48; P = .001) for the intervention group with a number needed to treat of 7 (95% confidence interval, 5-14).
Secondary outcomes included incidence of hospital acquired pneumonia, hospital utilization, mobility, patient reported complications at 6 weeks, and mortality rates in hospital, at 6 weeks, and at 12 months. For secondary outcomes in the adjusted analysis, incidences of pneumonia were halved in the physiotherapy intervention group with a number needed to treat of 9 (95% CI, 6-21). No significant differences in secondary outcomes were detected between the control and treatment groups.
Sensitivity analysis that removed participants who had lower abdominal and laparoscopic surgery strengthened both primary and secondary outcome results to favor the preoperative physiotherapy intervention for reducing PPC. The researchers found that, in an adjusted analysis of subgroup effects, there was a gradient in reduction of PPCs according to surgical category.
Shorter lengths hospital stay and lower all-cause 12-month mortality were also associated with more experienced physiotherapists providing the preoperative education and training.
Ms. Boden and her colleagues proposed that the timing for patients to begin breathing exercises after major open upper abdominal surgery could be critical in reducing PPC incidence. Initiating breathing exercises within the first 24 hours after surgery – in contrast to the common practice of waiting 1-2 days to begin postoperative physiotherapy – could prevent general anesthesia-associated mild atelectasis from developing into severe atelectasis and PPCs.
The researchers concluded that “in a general population of patients listed for elective upper abdominal surgery, a 30-minute preoperative physiotherapy session provided within existing hospital multidisciplinary preadmission clinics halves the incidence of PPCs and specifically hospital acquired pneumonia. Further research is required to investigate benefits to mortality and length of stay.”
The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
SOURCE: Boden I et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
FROM THE BMJ
Key clinical point: Reduction in PPC incidences corresponded to physiotherapists providing preoperative education and coaching intervention.
Major finding: Compared with the control group, Absolute risk was reduced by 15%, and seven was determined as number needed to treat.
Study details: Prospective, blinded study of 441 adult participants randomly assigned in a 1:1 ratio, comparing PPC outcomes associated with preop practices for upper abdominal surgeries.
Disclosures: The authors reported that they received grants from the Clifford Craig Foundation; the University of Tasmania (Hobart), Australia; and the Waitemata District Health Board in Auckland, New Zealand.
Source: Boden I. et al. BMJ. 2018. doi: 10.1136/bmj.j5916.
Are two drugs as good as three in maintaining HIV suppression?
Phase 3 clinical results show an oral, two-drug antiretroviral therapy (ART) of dolutegravir and rilpivirine to be an effective and safe alternative to a triple-drug current antiretroviral regimen (CAR) for maintaining virologic suppression of HIV-1 in adults.
Josep M Llibre, MD, from the department of infectious diseases at the Pujol University Hospital Germans Trias in Barcelona and his colleagues conducted a phase 3, randomized, parallel-group (SWORD-1 and SWORD-2), multicenter, noninferiority investigation. The pooled, open-label study reported on a total of 1,028 individuals, median age 43 years, who met a base criteria of stable viral suppression (viral load fewer than 50 copies/mL) by a first or second CAR regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug (nonnucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors, or a protease inhibitor) for a minimum of 6 months at time of screening. The overall population was 80% white, with women making up 22% of the pooled populations. Random assignment with stratification according to class, age, and planned substudy participation resulted in 512 participants continuing their CAR regimen and an intention-to-treat population of 516 participants switching to the oral, once-daily, two-drug dolutegravir/rilpivirine regimen (dolutegravir 50 mg plus rilpivirine 25 mg).
Analysis of pooled SWORD1 and SWORD2 trials at the primary 48-week endpoint showed 95% of the participants for both the intent-to-treat, two-drug dolutegravir/rilpivirine therapy and the population maintaining their standard triple-drug CAR therapy continued to have successful viral suppression as evidenced by viral loads of fewer than 50 copies/mL. This result together with an adjusted treatment difference of –2.2% (95% confidence interval, –3.0 to 2.5) supports a noninferiority with a predefined margin of –8% of the dolutegravir/rilpivirine therapy to CAR for viral suppression as reported in the Lancet.
Reports of at least one adverse event by week 48 were slightly higher for the dolutegravir/rilpivirine therapy group (77%) when compared with those in the CAR group (71%). The most commonly experienced adverse effects were nasopharyngitis (10% each for dolutegravir/rilpivirine and CAR) and headache (8% for dolutegravir/rilpivirine vs. 5% for CAR). However, adverse effects were associated with higher withdrawals from the dolutegravir/rilpivirine therapy group, compared with the CAR group (3% vs. less than 1%, respectively) by the primary 48-week endpoint.
“Once-daily oral dolutegravir/rilpivirine therapy would be the first oral two-drug regimen that provides patients with an alternative to guideline-preferred triple-drug regimens, avoids major NRTI [nucleoside reverse transcriptase inhibitor] toxicities, has limited potential for drug-drug interactions, and does not increase lipid concentrations or inflammatory biomarkers,” according to Dr. Llibre and his colleagues.
The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
In an accompanying commentary (Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736[18]30008-4), Mark A Boyd, MD, and David A Cooper, MD, of the University of Adelaide, Australia, wrote that studies done during the first 10 years of antiretroviral therapy led to the conclusion that triple therapy was the minimum required to fully maintain suppression of HIV replication. The results of this study and others make a convincing case for using dual therapy instead. “The potential pipeline for a variety of effective dual-therapy options appears rich,” they concluded.
Dr. Boyd reported receiving personal fees from Janssen-Cilag and ViiV Healthcare, while Dr. Cooper had no disclosures.
In an accompanying commentary (Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736[18]30008-4), Mark A Boyd, MD, and David A Cooper, MD, of the University of Adelaide, Australia, wrote that studies done during the first 10 years of antiretroviral therapy led to the conclusion that triple therapy was the minimum required to fully maintain suppression of HIV replication. The results of this study and others make a convincing case for using dual therapy instead. “The potential pipeline for a variety of effective dual-therapy options appears rich,” they concluded.
Dr. Boyd reported receiving personal fees from Janssen-Cilag and ViiV Healthcare, while Dr. Cooper had no disclosures.
In an accompanying commentary (Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736[18]30008-4), Mark A Boyd, MD, and David A Cooper, MD, of the University of Adelaide, Australia, wrote that studies done during the first 10 years of antiretroviral therapy led to the conclusion that triple therapy was the minimum required to fully maintain suppression of HIV replication. The results of this study and others make a convincing case for using dual therapy instead. “The potential pipeline for a variety of effective dual-therapy options appears rich,” they concluded.
Dr. Boyd reported receiving personal fees from Janssen-Cilag and ViiV Healthcare, while Dr. Cooper had no disclosures.
Phase 3 clinical results show an oral, two-drug antiretroviral therapy (ART) of dolutegravir and rilpivirine to be an effective and safe alternative to a triple-drug current antiretroviral regimen (CAR) for maintaining virologic suppression of HIV-1 in adults.
Josep M Llibre, MD, from the department of infectious diseases at the Pujol University Hospital Germans Trias in Barcelona and his colleagues conducted a phase 3, randomized, parallel-group (SWORD-1 and SWORD-2), multicenter, noninferiority investigation. The pooled, open-label study reported on a total of 1,028 individuals, median age 43 years, who met a base criteria of stable viral suppression (viral load fewer than 50 copies/mL) by a first or second CAR regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug (nonnucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors, or a protease inhibitor) for a minimum of 6 months at time of screening. The overall population was 80% white, with women making up 22% of the pooled populations. Random assignment with stratification according to class, age, and planned substudy participation resulted in 512 participants continuing their CAR regimen and an intention-to-treat population of 516 participants switching to the oral, once-daily, two-drug dolutegravir/rilpivirine regimen (dolutegravir 50 mg plus rilpivirine 25 mg).
Analysis of pooled SWORD1 and SWORD2 trials at the primary 48-week endpoint showed 95% of the participants for both the intent-to-treat, two-drug dolutegravir/rilpivirine therapy and the population maintaining their standard triple-drug CAR therapy continued to have successful viral suppression as evidenced by viral loads of fewer than 50 copies/mL. This result together with an adjusted treatment difference of –2.2% (95% confidence interval, –3.0 to 2.5) supports a noninferiority with a predefined margin of –8% of the dolutegravir/rilpivirine therapy to CAR for viral suppression as reported in the Lancet.
Reports of at least one adverse event by week 48 were slightly higher for the dolutegravir/rilpivirine therapy group (77%) when compared with those in the CAR group (71%). The most commonly experienced adverse effects were nasopharyngitis (10% each for dolutegravir/rilpivirine and CAR) and headache (8% for dolutegravir/rilpivirine vs. 5% for CAR). However, adverse effects were associated with higher withdrawals from the dolutegravir/rilpivirine therapy group, compared with the CAR group (3% vs. less than 1%, respectively) by the primary 48-week endpoint.
“Once-daily oral dolutegravir/rilpivirine therapy would be the first oral two-drug regimen that provides patients with an alternative to guideline-preferred triple-drug regimens, avoids major NRTI [nucleoside reverse transcriptase inhibitor] toxicities, has limited potential for drug-drug interactions, and does not increase lipid concentrations or inflammatory biomarkers,” according to Dr. Llibre and his colleagues.
The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
Phase 3 clinical results show an oral, two-drug antiretroviral therapy (ART) of dolutegravir and rilpivirine to be an effective and safe alternative to a triple-drug current antiretroviral regimen (CAR) for maintaining virologic suppression of HIV-1 in adults.
Josep M Llibre, MD, from the department of infectious diseases at the Pujol University Hospital Germans Trias in Barcelona and his colleagues conducted a phase 3, randomized, parallel-group (SWORD-1 and SWORD-2), multicenter, noninferiority investigation. The pooled, open-label study reported on a total of 1,028 individuals, median age 43 years, who met a base criteria of stable viral suppression (viral load fewer than 50 copies/mL) by a first or second CAR regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a third drug (nonnucleoside reverse transcriptase inhibitors, integrase strand transfer inhibitors, or a protease inhibitor) for a minimum of 6 months at time of screening. The overall population was 80% white, with women making up 22% of the pooled populations. Random assignment with stratification according to class, age, and planned substudy participation resulted in 512 participants continuing their CAR regimen and an intention-to-treat population of 516 participants switching to the oral, once-daily, two-drug dolutegravir/rilpivirine regimen (dolutegravir 50 mg plus rilpivirine 25 mg).
Analysis of pooled SWORD1 and SWORD2 trials at the primary 48-week endpoint showed 95% of the participants for both the intent-to-treat, two-drug dolutegravir/rilpivirine therapy and the population maintaining their standard triple-drug CAR therapy continued to have successful viral suppression as evidenced by viral loads of fewer than 50 copies/mL. This result together with an adjusted treatment difference of –2.2% (95% confidence interval, –3.0 to 2.5) supports a noninferiority with a predefined margin of –8% of the dolutegravir/rilpivirine therapy to CAR for viral suppression as reported in the Lancet.
Reports of at least one adverse event by week 48 were slightly higher for the dolutegravir/rilpivirine therapy group (77%) when compared with those in the CAR group (71%). The most commonly experienced adverse effects were nasopharyngitis (10% each for dolutegravir/rilpivirine and CAR) and headache (8% for dolutegravir/rilpivirine vs. 5% for CAR). However, adverse effects were associated with higher withdrawals from the dolutegravir/rilpivirine therapy group, compared with the CAR group (3% vs. less than 1%, respectively) by the primary 48-week endpoint.
“Once-daily oral dolutegravir/rilpivirine therapy would be the first oral two-drug regimen that provides patients with an alternative to guideline-preferred triple-drug regimens, avoids major NRTI [nucleoside reverse transcriptase inhibitor] toxicities, has limited potential for drug-drug interactions, and does not increase lipid concentrations or inflammatory biomarkers,” according to Dr. Llibre and his colleagues.
The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
FROM THE LANCET
Key clinical point: No difference was seen in maintaining virologic suppression of HIV-1 in adults switching from a traditional triple-drug regimen to a dolutegravir/rilpivirine dual-drug antiretroviral therapy.
Major finding: Of the participants in both the dual- and triple-drug groups, 95% maintained suppression to a viral load of fewer than 50 copies/mL.Study details: Two identical parallel-group studies (SWORD1 and SWORD2) of 1,024 adults participants randomly assigned in a 1:1 ratio comparing two-drug dolutegravir/rilpivirine with the active-control current triple-drug group.
Disclosures: The authors disclosed associations with financial and regulatory sponsor ViiV Healthcare and partner participation by Janssen Pharmaceutica NV in the development of the dolutegravir/rilpivirine two-drug regimen.
Source: Llibre JM et al. Lancet. 2018 Jan 10. doi: 10.1016/S0140-6736(17)33095-7.
Stenting improved outcomes for treating chronic IVC obstruction
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point:
Major finding: Most patients showed a significant improvement from the baseline score of 8.5 score down to 7.0 (P = .007).
Data source: A retrospective analysis of 20 patients referred to the Norwegian National Unit for Reconstructive Deep Venous Surgery for stenting.
Disclosures: The authors reported they had no conflict of interest.