User login
Maskomania: Masks and COVID-19
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13
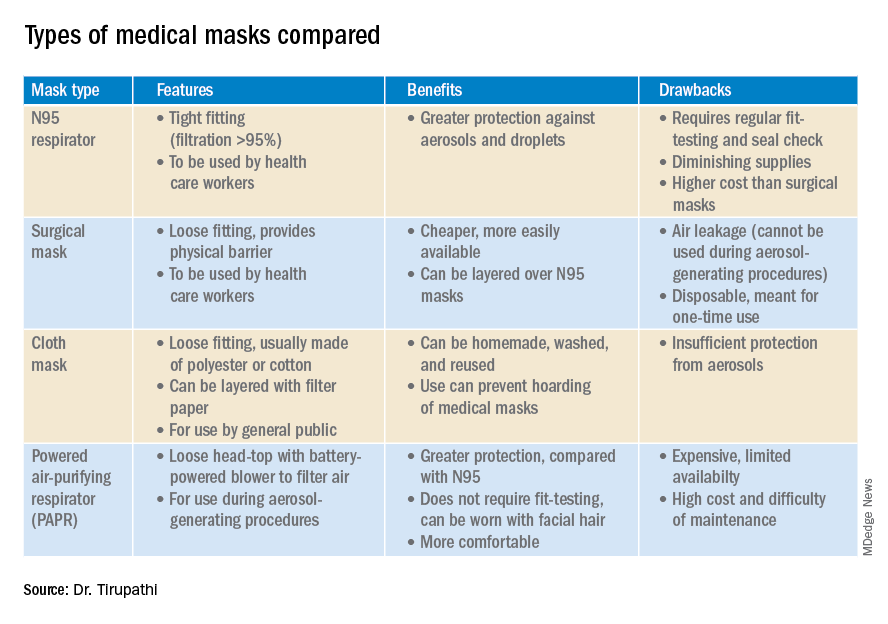
N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
A comprehensive review
A comprehensive review
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
On April 3, the Centers for Disease Control and Prevention issued an advisory that the general public wear cloth face masks when outside, particularly those residing in areas with significant severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) community transmission.1 Recent research reveals several factors related to the nature of the virus as well as the epidemiologic spread of the illness that may have led to this decision.
However, controversy still prevails whether this recommendation will alleviate or aggravate disease progression. With many hospitals across America lacking sufficient personal protective equipment (PPE) and scrambling for supplies, universal masking may create more chaos, especially with certain states imposing monetary fines on individuals spotted outdoors without a mask. With new information being discovered each day about COVID-19, it is more imperative than ever to update existing strategies and formulate more effective methods to flatten the curve.
Airborne vs. droplet transmission
According to a scientific brief released by the World Health Organization, there have been studies with mixed evidence and opinions regarding the presence of COVID-19 ribonucleic acid (RNA) in air samples.2 In medRxiv, Santarpia et al., from the University of Nebraska Medical Center, Omaha, detected viral RNA in samples taken from beneath a patient’s bed and from a window ledge, both areas in which neither the patient nor health care personnel had any direct contact. They also found that 66.7% of air samples taken from a hospital hallway carried virus-containing particles.3 It is worth noting that certain aerosol-generating procedures (AGP) may increase the likelihood of airborne dissemination. Whether airborne transmission is a major mode of COVID-19 spread in the community and routine clinical settings (with no aerosol-generating procedures) is still a debatable question without a definitive answer.
We should consider the epidemiology of COVID-19 thus far in the pandemic to determine if transmission patterns are more consistent with that of other common respiratory viral pathogens or more consistent with that of the agents we classically consider to be transmitted by the airborne route (measles, varicella zoster virus, and Mycobacterium tuberculosis). The attack rates in various settings (household, health care, and the public) as well as the expected number of secondary cases from a single infected individual in a susceptible population (R0) are more consistent with those of a droplet spread pathogen.
For measles, the R0 is 12-18, and the secondary household attack rates are ≥ 90%. In case of the varicella zoster virus, the R0 is ~10, and the secondary household attack rate is 85%. The R0 for pulmonary tuberculosis is up to 10 (per year) and the secondary household attack rate has been reported to be >50%. With COVID-19, the R0 appears to be around 2.5-3 and secondary household attack rates are ~ 10% from data available so far, similar to that of influenza viruses. This discrepancy suggests that droplet transmission may be more likely. The dichotomy of airborne versus droplet mode of spread may be better described as a continuum, as pointed out in a recent article in the JAMA. Infectious droplets form turbulent gas clouds allowing the virus particles to travel further and remain in the air longer.4 The necessary precautions for an airborne illness should be chosen over droplet precautions, especially when there is concern for an AGP.
Universal masking: Risks and benefits
The idea of universal masking has been debated extensively since the initial stages of the COVID-19 pandemic. According to public health authorities, significant exposure is defined as “face-to-face contact within 6 feet with a patient with symptomatic COVID-19” in the range of a few minutes up to 30 minutes.5 The researchers wrote in the New England Journal of Medicine that the chance of catching COVID-19 from a passing interaction in a public space is therefore minimal, and it may seem unnecessary to wear a mask at all times in public.
As reported in Science, randomized clinical studies performed on other viruses in the past have shown no added protection conferred by wearing a mask, though small sample sizes and noncompliance are limiting factors to their validity.6 On the contrary, mask wearing has been enforced in many parts of Asia, including Hong Kong and Singapore with promising results.5 Leung et al. stated in The Lancet that the lack of proof that masks are effective should not rule them as ineffective. Also, universal masking would reduce the stigma around symptomatic individuals covering their faces. It has become a cultural phenomenon in many southeast Asian countries and has been cited as one of the reasons for relatively successful containment in Singapore, South Korea, and Taiwan. The most important benefit of universal masking is protection attained by preventing spread from asymptomatic, mildly symptomatic, and presymptomatic carriers.7
In a study in the New England Journal of Medicine that estimated viral loads during various stages of COVID-19, researchers found that asymptomatic patients had similar viral loads to symptomatic patients, thereby suggesting high potential for transmission.8 Furthermore, numerous cases are being reported concerning the spread of illness from asymptomatic carriers.9-12 In an outbreak at a skilled nursing facility in Washington outlined in MMWR, 13 of 23 residents with positive test results were asymptomatic at the time of testing, and of those, 3 never developed any symptoms.12
Many hospitals are now embracing the policy of universal masking. A mask is a critical component of the personal protective equipment (PPE) clinicians need when caring for symptomatic patients with respiratory viral infections, in conjunction with a gown, gloves, and eye protection. Masking in this context is already part of routine operations in most hospitals. There are two scenarios in which there may be possible benefits. One scenario is the lower likelihood of transmission from asymptomatic and minimally symptomatic health care workers with COVID-19 to other providers and patients. The other less plausible benefit of universal masking among health care workers is that it may provide some protection in the possibility of caring for an unrecognized COVID-19 patient. However, universal masking should be coupled with other favorable practices like temperature checks and symptom screening on a daily basis to avail the maximum benefit from masking. Despite varied opinions on the outcomes of universal masking, this measure helps improve health care workers’ safety, psychological well-being, trust in their hospital, and decreases anxiety of acquiring the illness.
Efficacy of various types of masks
With the possibility of airborne transmission of the virus, are cloth masks as recommended by the CDC truly helpful in preventing infection? A study in the Journal of Medical Virology demonstrates 99.98%, 97.14%, and 95.15% efficacy for N95, surgical, and homemade masks, respectively, in blocking the avian influenza virus (comparable to coronavirus in size and physical characteristics). The homemade mask was created using one layer of polyester cloth and a four-layered kitchen filter paper.13

N95 masks (equivalent to FFP/P2 in European countries) are made of electrostatically charged polypropylene microfibers designed to filter particles measuring 100-300nm in diameter with 95% efficacy. A single SARS-CoV-2 molecule measures 125 nm approximately. N99 (FFP3) and N100 (P3) masks are also available, though not as widely used, with 99% and 99.7% efficacy respectively for the same size range. Though cloth masks are the clear-cut last resort for medical professionals, a few studies state no clinically proven difference in protection between surgical masks and N95 respirators.14,15 Even aerosolized droplets (< 5 mcm) were found to be blocked by surgical masks in a Nature Medicine study in which 4/10 subjects tested positive for coronavirus in exhaled breath samples without masks and 0/10 subjects with masks.16
On the contrary, an Annals of Internal Medicine study of four COVID-19 positive subjects that “neither surgical masks nor cloth masks effectively filtered SARS-CoV-2 during coughs of infected patients.” In fact, more contamination was found on the outer surface of the masks when compared to the inner surface, probably owing to the masks’ aerodynamic properties.17 Because of limitations present in the above-mentioned studies, further research is necessary to conclusively determine which types of masks are efficacious in preventing infection by the virus. In a scarcity of surgical masks and respirators for health care personnel, suboptimal masks can be of some use provided there is adherent use, minimal donning and doffing, and it is to be accompanied by adequate hand washing practices.14
In case of severe infections with high viral loads or patients undergoing aerosol-generating procedures, powered air-purifying respirators (PAPRs) also are advisable as they confer greater protection than N95 respirators, according to a study in the Annals of Work Exposures and Health. Despite being more comfortable for long-term use and accommodative of facial hair, their use is limited because of high cost and difficult maintenance.18 3-D printing also is being used to combat the current shortage of masks worldwide. However, a study from the International Journal of Oral & Maxillofacial Surgery reported that virologic testing for leakage between the two reusable components and contamination of the components themselves after one or multiple disinfection cycles is essential before application in real-life situations.19
Ongoing issues
WHO estimates a monthly requirement of nearly 90 million masks exclusively for health care workers to protect themselves against COVID-19.20 In spite of increasing the production rate by 40%, if the general public hoards masks and respirators, the results could be disastrous. Personal protective equipment is currently at 100 times the usual demand and 20 times the usual cost, with stocks backlogged by 4-6 months. The appropriate order of priority in distribution to health care professionals first, followed by those caring for infected patients is critical.20 In a survey conducted by the Association for Professionals in Infection Control and Epidemiology, results revealed that 48% of the U.S. health care facilities that responded were either out or nearly out of respirators as of March 25. 21
The gravest risk behind the universal masking policy is the likely depletion of medical resources.22 A possible solution to this issue could be to modify the policy to stagger the requirement based on the severity of community transmission in that area of residence. In the article appropriately titled “Rational use of face masks in the COVID-19 pandemic” published in The Lancet Respiratory Medicine, researchers described how the Chinese population was classified into moderate, low, and very-low risk of infection categories and advised to wear a surgical or disposable mask, disposable mask, and no mask respectively.23 This curbs widespread panic and eagerness by the general public to stock up on essential medical equipment when it may not even be necessary.
Reuse, extended use, and sterilization
Several studies have been conducted to identify the viability of the COVID-19 on various surfaces.24-25 The CDC and National Institute for Occupational Safety and Health (NIOSH) guidelines state that an N95 respirator can be used up to 8 hours with intermittent or continuous use, though this number is not fixed and heavily depends upon the extent of exposure, risk of contamination, and frequency of donning and doffing26,27. Though traditionally meant for single-time usage, after 8 hours, the mask can be decontaminated and reused. The CDC defines extended use as the “practice of wearing the same N95 respirator for repeated close-contact encounters with several patients, without removing the respirator between patient encounters.” Reuse is defined as “using the same N95 respirator for multiple encounters with patients but removing it (‘doffing’) after each encounter. The respirator is stored in between encounters to be put on again (‘donned’) prior to the next encounter with a patient.”
It has been established that extended use is more advisable than reuse given the lower risk of self-inoculation. Furthermore, health care professionals are urged to wear a cleanable face shield or disposable mask over the respirator to minimize contamination and practice diligent hand hygiene before and after handling the respirator. N95 respirators are to be discarded following aerosol-generating procedures or if they come in contact with blood, respiratory secretions, or bodily fluids. They should also be discarded in case of close contact with an infected patient or if they cause breathing difficulties to the wearer.27 This may not always be possible given the unprecedented shortage of PPE, hence decontamination techniques and repurposing are the need of the hour.
In Anesthesia & Analgesia, Naveen Nathan, MD, of Northwestern University, Chicago, recommends recycling four masks in a series, using one per day, keeping the mask in a dry, clean environment, and then repeating use of the first mask on the 5th day, the second on the 6th day, and so forth. This ensures clearance of the virus particles by the next use. Alternatively, respirators can be sterilized between uses by heating to 70º C (158º F) for 30 minutes. Liquid disinfectants such as alcohol and bleach as well as ultraviolet rays in sunlight tend to damage masks.28 Steam sterilization is the most commonly utilized technique in hospitals. Other methods, described by the N95/PPE Working Group, report include gamma irradiation at 20kGy (2MRad) for large-scale sterilization (though the facilities may not be widely available), vaporized hydrogen peroxide, ozone decontamination, ultraviolet germicidal irradiation, and ethylene oxide.29 Though a discussion on various considerations of decontamination techniques is out of the scope of this article, detailed guidelines have been published by the CDC30 and the COVID-19 Healthcare Coalition.30
Conclusion
A recent startling discovery reported on in Emerging Infectious Diseases suggests that the basic COVID-19 reproductive number (R0) is actually much higher than previously thought. Using expanded data, updated epidemiologic parameters, and the current outbreak dynamics in Wuhan, the team came to the conclusion that the R0 for the novel coronavirus is actually 5.7 (95% CI 3.8-8.9), compared with an initial estimate of 2.2-2.7.31 Concern for transmissibility demands heightened prevention strategies until more data evolves. The latest recommendation by the CDC regarding cloth masking in the public may help slow the progression of the pandemic. However, it is of paramount importance to keep in mind that masks alone are not enough to control the disease and must be coupled with other nonpharmacologic interventions such as social distancing, quarantining/isolation, and diligent hand hygiene.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Bharathidasan is a recent medical graduate from India with an interest in public health and community research; she plans to pursue residency training in the United States. Ms. Freshman is currently the regional director of infection prevention for WellSpan Health and has 35 years of experience in nursing. Dr. Palabindala is the medical director, utilization management and physician advisory services, at the University of Mississippi Medical Center, Jackson. He is an associate professor of medicine and academic hospitalist in the UMMC School of Medicine.
References
1. Centers for Disease Control and Prevention. Recommendation regarding the use of cloth face coverings.
2. World Health Organization. Modes of transmission of virus causing COVID-19 : implications for IPC precaution recommendations. Sci Br. 2020 Mar 29:1-3.
3. Santarpia JL et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. 2020 Mar 26. medRxiv. 2020;2020.03.23.20039446.
4. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: Potential implications for reducing transmission of COVID-19. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4756.
5. Klompas M et al. Universal masking in hospitals in the Covid-19 era. N Engl J Med. 2020 Apr 1. doi: 10.1056/NEJMp2006372.
6. Servick K. Would everyone wearing face masks help us slow the pandemic? Science 2020 Mar 28. doi: 10.1126/science.abb9371.
7. Leung CC et al. Mass masking in the COVID-19 epidemic: People need guidance. Lancet 2020 Mar 21;395(10228):945. doi: 10.1016/S0140-6736(20)30520-1.
8. Zou L et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020 Mar 19;382(12):1177-9.
9. Pan X et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020 Apr;20(4):410-1.
10. Bai Y et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 Feb 21;323(14):1406-7.
11. Wei WE et al. Presymptomatic transmission of SARS-CoV-2 – Singapore, Jan. 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020;69:411-5.
12. Kimball A et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility – King County, Washington, March 2020. 2020 Apr 3. MMWR Morb Mortal Wkly Rep 2020;69:377-81.
13. Ma Q-X et al. Potential utilities of mask wearing and instant hand hygiene for fighting SARS-CoV-2. J Med Virol. 2020 Mar 31;10.1002/jmv.25805. doi: 10.1002/jmv.25805.
14. Abd-Elsayed A et al. Utility of substandard face mask options for health care workers during the COVID-19 pandemic. Anesth Analg. 2020 Mar 31;10.1213/ANE.0000000000004841. doi: 10.1213/ANE.0000000000004841.
15. Long Y et al. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J Evid Based Med. 2020 Mar 13;10.1111/jebm.12381. doi: 10.1111/jebm.12381.
16. Leung NHL et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020 May;26(5):676-80.
17. Bae S et al. Effectiveness of surgical and cotton masks in blocking SARS-CoV-2: A controlled comparison in 4 patients. Ann Intern Med. 2020 Apr 6;M20-1342. doi: 10.7326/M20-1342.
18. Brosseau LM. Are powered air purifying respirators a solution for protecting healthcare workers from emerging aerosol-transmissible diseases? Ann Work Expo Health. 2020 Apr 30;64(4):339-41.
19. Swennen GRJ et al. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int J Oral Maxillofac Surg. 2020 May;49(5):673-7.
20. Mahase E. Coronavirus: Global stocks of protective gear are depleted, with demand at “100 times” normal level, WHO warns. BMJ. 2020 Feb 10;368:m543. doi: 10.1136/bmj.m543.
21. National survey shows dire shortages of PPE, hand sanitizer across the U.S. 2020 Mar 27. Association for Professionals in Infection Control and Epidemiology (APIC) press briefing.
22. Wu HL et al. Facemask shortage and the novel coronavirus disease (COVID-19) outbreak: Reflections on public health measures. EClinicalMedicine. 2020 Apr 3:100329. doi: 10.1016/j.eclinm.2020.100329.
23. Feng S et al. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020 May;8(5):434-6.
24. Chin AWH et al. Stability of SARS-CoV-2 in different environmental. The Lancet Microbe. 2020 May 1;5247(20):2004973. doi. org/10.1016/S2666-5247(20)30003-3.
25. van Doremalen N et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-7.
26. NIOSH – Workplace Safety and Health Topics: Recommended guidance for extended use and limited reuse of n95 filtering facepiece respirators in healthcare settings.
27. Centers for Disease Control and Prevention. COVID-19 decontamination and reuse of filtering facepiece respirators. 2020 Apr 15.
28. Nathan N. Waste not, want not: The re-usability of N95 masks. Anesth Analg. 2020 Mar 31.doi: 10.1213/ane.0000000000004843.
29. European Centre for Disease Prevention and Control technical report. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. 2020 Mar.
30. N95/PPE Working Group report. Evaluation of decontamination techniques for the reuse of N95 respirators. 2020 Apr 3;2:1-7.
31. Sanche Set al. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020 Jul. doi. org/10.3201/eid2607.200282.
COVID-19 and public health preparedness in the United States
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.
Background
On Dec. 31, 2019, the Chinese city of Wuhan reported an outbreak of pneumonia from an unknown cause. The outbreak was found to be linked to the Hunan seafood market because of a shared history of exposure by many patients. After a full-scale investigation, China’s Center for Disease Control activated a level 2 emergency response on Jan. 4, 2020. A novel coronavirus was officially identified as a causative pathogen for the outbreak.1
Coronavirus, first discovered in the 1960s, is a respiratory RNA virus, most commonly associated with the “common cold.” However, we have had two highly pathogenic forms of coronavirus that originated from animal reservoirs, leading to global epidemics. This includes SARS-CoV in 2002-2004 and MERS-CoV in 2012 with more than 10,000 combined cases. The primary host has been bats, but mammals like camels, cattle, cats, and palm civets have been intermediate hosts in previous epidemics.2
The International Committee on Taxonomy of Viruses named the 2019-nCoV officially as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes the coronavirus disease, COVID-19, on Feb. 11, 2020.3 Currently, the presentation includes fever, cough, trouble breathing, fatigue, and, rarely, watery diarrhea. More severe presentations include respiratory failure and death. Based on the incubation period of illness for Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) coronaviruses, as well as observational data from reports of travel-related COVID-19, CDC estimates that symptoms of COVID-19 occur within 2-14 days after exposure. Asymptomatic transmission is also documented in some cases.4
On Jan. 13, the first case of COVID-19 outside of China was identified in Thailand. On Jan. 21, the first case of COVID-19 was identified in the United States. On Jan. 23, Chinese authorities suspended travel in and out of Wuhan, followed by other cities in the Hubei Province, leading to a quarantine of 50 million people. By Jan. 30, the World Health Organization had identified COVID-19 as the highest level of an epidemic alert referred to as a PHEIC: Public Health Emergency of International Concern. On Feb. 2, the first death outside China from coronavirus was reported in the Philippines. As of March 4 there have been 95,000 confirmed cases and 3,246 deaths globally. Within China, there have been 80,200 cases with 2,981 deaths.5
Cases have now been diagnosed in increasing numbers in Italy, Japan, South Korea, Iran, and 76 countries. Of note, the fatalities were of patients already in critical condition, who were typically older (more than 60 years old, especially more than 80) and immunocompromised with comorbid conditions (cardiovascular disease, diabetes, chronic respiratory disease, cancer).6 To put this in perspective, since 2010, CDC reports 140,000-810,000 hospitalizations and 12,000-61,000 deaths from the influenza virus annually in the US.7
The current situation in the United States
In the United States, as of March 4, 2020, there are currently 152 confirmed cases in 16 states. The first U.S. case of coronavirus without any of the travel-related and exposure risk factors was identified on Feb. 27 in California, indicating the first instance of community spread.8 The first death was reported in Washington state on Feb. 28, after which the state’s governor declared a state of emergency.9 On March 1, Washington state health officials investigated an outbreak of coronavirus at a long-term nursing facility in which two people tested positive for the disease, heralding the probable first nosocomial transmission of the virus in the United States. Since then, there have been 10 deaths in Washington state related to the coronavirus.
Current interventions in the United States
The U.S. Centers for Disease Control and Prevention is leading a multiagency effort to combat the COVID-19 potential pandemic. A Feb. 24 report in Morbidity and Mortality Weekly Report revealed that 1,336 CDC staff members have been involved in the COVID-19 response.10 CDC staff members have been deployed to 39 locations in the United States and internationally. CDC staff members are working with state and local health departments and other public health authorities to assist with case identification, contact tracing, evaluation of persons under investigation (PUI) for COVID-19, and medical management of cases, as well as with research and academic institutions to understand the virulence, risk for transmission, and other characteristics of this novel virus. The CDC is also working with other agencies of the U.S. government including the U.S. Department of Defense, Department of Health & Human Services and the U.S. Department of State to safely evacuate U.S. citizens, residents, and their families from international locations with high incidence and transmission of COVID-19.
Specific real-time updated guidance has been developed and posted online for health care settings for patient management, infection control and prevention, laboratory testing, environmental cleaning, worker safety, and international travel. The CDC has developed communications materials in English and Spanish for communities and guidance for health care settings, public health, laboratories, schools, and businesses to prepare for a potential pandemic. Travel advisories to countries affected by the epidemic are regularly updated to inform travelers and clinicians about current health issues that need to be considered before travel.11 A level 3 travel notice (avoid all nonessential travel) for China has been in effect since Jan. 27, and on Feb. 29 this was upgraded to a level 4 travel notice.12 Airport screening has been implemented in the 11 U.S. international airports to which flights from China have been diverted, and a total of 46,016 air travelers had been screened by Feb. 23. Incoming passengers are screened for fever, cough, and shortness of breath.
Currently, the CDC has a comprehensive algorithm for further investigation of a PUI – fever, cough, shortness of breath, and a history of travel to areas with increased coronavirus circulation within 14 days of onset of symptoms, OR a close household contact of a confirmed case. When there is a PUI, the current protocol indicates health care providers should alert a local or state health department official. After the health department completes a case investigation, the CDC will help transport specimens (upper respiratory and lower respiratory specimens, and sometimes stool or urine) as soon as possible to the centralized lab for polymerase chain reaction (PCR) testing.13 CDC laboratories are currently using real-time reverse transcription–PCR (RT-PCR). The CDC is also developing a serologic test to assist with surveillance for SARS-CoV-2 circulation in the U.S. population. There is also a safe repository of viral isolates set up to help with sharing of isolates with academic institutions for research purposes.14
At hospitals and outpatient offices in the United States, we are preparing for potential cases by reminding frontline health care workers to routinely ask about travel history in addition to relevant symptoms. By eliciting the history early, they should be able to identify and isolate PUIs, appropriately minimizing exposure. Some facilities are displaying signage in waiting rooms to alert patients to provide relevant history, helping to improve triage. COVID-19 symptoms are like those of influenza (e.g., fever, cough, and shortness of breath), and the current outbreak is occurring during a time of year when respiratory illnesses from influenza and other viruses are highly prevalent. To prevent influenza and possible unnecessary evaluation for COVID-19, all persons aged 6 months and older are strongly encouraged to receive an annual influenza vaccine.
To decrease the risk for respiratory disease, persons can practice recommended preventive measures. Persons ill with symptoms of COVID-19 who have had contact with a person with COVID-19, or recent travel to countries with apparent community spread, should proactively communicate with their health care provider before showing up at the health care facility to help make arrangements to prevent possible transmission in the health care setting. In a medical emergency, they should inform emergency medical personnel about possible COVID-19 exposure. If found positive, the current recommendation is to place patients on airborne isolation. N95 masks are being recommended for health care professionals. Hospitals are reinforcing effective infection control procedures, updating pandemic preparedness protocols, and ensuring adequate supplies in the case of an enormous influx of patients.15
Challenges and opportunities
Many challenges present in the process of getting prepared for a potential outbreak. Personal protective equipment such as N-95 masks are in short supply, as they are in high demand in the general public.16 The CDC currently does not recommend that members of the general public use face masks, given low levels of circulation of SARS-CoV-2 currently in the United States. The CDC has developed several documents regarding infection control, hospital preparedness assessments, personal protective equipment (PPE) supply planning, clinical evaluation and management, and respirator conservation strategies.
The RT-PCR test developed by the CDC has had some setbacks, with recent testing kits showing “inconclusive results.” The testing was initially available only through the CDC lab in Atlanta, with a 48-hour turnaround. This led to potential delays in diagnosis and the timely isolation and treatment of infected patients. On March 3, the CDC broadened the guidelines for coronavirus testing, allowing clinicians to order a test for any patients who have symptoms of COVID-19 infection. The greatest need is for decentralized testing in local and state labs, as well as validated testing in local hospitals and commercial labs. The ability to develop and scale-up diagnostic abilities is critically important.
There is also concern about overwhelming hospitals with a strain on the availability of beds, ventilators, and airborne isolation rooms. The CDC is recommending leveraging telehealth tools to direct people to the right level of health care for their medical needs. Hospitalization should only be for the sickest patients.17
Funding for a pandemic response is of paramount importance. Proposed 2021 federal budget cuts include $2.9 billion in cuts to the National Institutes of Health, and $708 million in cuts to the CDC, which makes the situation look especially worrisome as we face a potentially severe pandemic. The Infectious Diseases Society of America identifies antimicrobial resistance, NIH research, global health security, global HIV epidemic, and CDC vaccine programs as five “deeply underfunded” areas in the federal budget.18
The NIH has recently begun the first randomized clinical trial, treating patients at the University of Nebraska with laboratory-confirmed SARS-CoV-2 with a broad-spectrum antiviral drug called remdesivir. Patients from the Diamond Princess Cruise ship are also participating in this clinical trial. This study will hopefully shed light on potential treatments for coronavirus to stop or alleviate the consequences in real time. Similar clinical trials are also occurring in China.19
Vaccine development is underway in many public and private research facilities, but it will take approximately 6-18 months before they will be available for use. In the absence of a vaccine or therapeutic, community mitigation measures are the primary method to respond to the widespread transmission, and supportive care is the current medical treatment. In the case of a pandemic, the mitigation measures might include school dismissals and social distancing in other settings, like suspension of mass gatherings, telework and remote-meeting options in workplaces.
Many respected medical journals in the United States have made access to SARS-CoV-2 articles and literature readily and freely available, which is a remarkable step. Multiple societies and journals have made information available in real time and have used media effectively (e.g., podcasts, e-learning) to disseminate information to the general public. Articles have been made available in other languages, including Chinese.
Conclusions
In summary, there have been 3,280 total deaths attributable to SARS-CoV-2 to date globally, mostly among geriatric patients with comorbidities. To provide some perspective on the statistics, influenza has killed almost 14,000 patients this season alone (much more than coronavirus). COVID-19 is undoubtedly a global public health threat. We in the U.S. health care system are taking swift public health actions, including isolation of patients and contacts to prevent secondary spread, but it is unclear if this is enough to stop an outbreak from becoming a pandemic.
The CDC is warning of significant social and economic disruption in the coming weeks, with more expected community spread and confirmed cases. It is challenging to prepare for a pandemic when the transmission dynamics are not clearly known, the duration of infectiousness is not well defined, and asymptomatic transmission is a possibility. It is time for the public to be informed from trusted sources and avoid unverified information, especially on social media which can lead to confusion and panic. The spread of COVID-19 infection in the United States is inevitable, and there must be sufficient, well-coordinated planning that can curtail the spread and reduce the impact.
Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson. Ms. Sathya Areti is a 3rd-year medical student at the Virginia Commonwealth University School of Medicine (class of 2021), planning to apply into Internal Medicine-Pediatrics. Dr. Swetha Areti is currently working as a hospitalist at Wellspan Chambersburg Hospital and is also a member of the Wellspan Pharmacy and Therapeutics committee.
References
1. Phelan AL et al. The novel coronavirus originating in Wuhan, China: Challenges for global health governance. JAMA. 2020;323(8):709-10. doi: 10.1001/jama.2020.1097.
2. del Rio C, Malani PN. 2019 Novel coronavirus – Important information for clinicians. JAMA. Published online Feb. 5, 2020. doi: 10.1001/jama.2020.1490.
3. Gorbalenya AE et al. Severe acute respiratory syndrome-related coronavirus: The species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. Published Jan. 1, 2020. doi: 10.1101/2020.02.07.937862.
4. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
5. Coronavirus disease 2019 (COVID-19). Situation Report – 40. Published Feb. 29, 2020.
6. Kaiyuan Sun, et al. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population level observational study, Feb. 20, 2020. Lancet Digital Health 2020. doi: 10.1016/S2589-7500(20)30026-1.
7. Rolfes MA et al. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12(1):132-7. doi: 10.1111/irv.12486.
8. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020;69:216-19. doi: 10.15585/mmwr.mm6908e1.
9. Jablon R, Baumann L. Washington governor declares state of emergency over virus. AP News. Published Feb. 29, 2020.
10. Jernigan DB, CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-219. doi: 10.15585/mmwr.mm6908e1.
11. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb 24, 2020.
12. Hines M. Coronavirus: Travel advisory for Italy, South Korea raised to level 4, ‘Do Not Travel’. USA Today. Published Feb. 29, 2020.
13. Information for health departments on reporting a person under investigation (PUI) or laboratory-confirmed case for COVID-19. Centers for Disease Control and Prevention. Published Feb. 24, 2020.
14. CDC Tests for COVID-19. Centers for Disease Control and Prevention. Published Feb. 25, 2020.
15. Jernigan DB. CDC COVID-19 response team. Update: Public health response to the coronavirus disease 2019 outbreak – United States, Feb. 24, 2020. MMWR Morbidity and Mortality Weekly Report 2020; 69:216-19. doi: 10.15585/mmwr.mm6908e1.
16. Gunia A. The global shortage of medical masks won’t be easing soon. Time. Published Feb. 27, 2020.
17. CDC in action: Preparing communities for potential spread of COVID-19. Centers for Disease Control and Prevention. Published Feb. 23, 2020.
18. Kadets L. White House budget cuts vital domestic and global public health programs. IDSA Home. Published 2020.
19. NIH clinical trial of remdesivir to treat COVID-19 begins. National Institutes of Health. Feb. 25, 2020.





