User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Experts Expect New Human Cases of Avian Flu
With avian influenza spreading quickly around the globe, the virus has more opportunities to mutate and cause problems for people. By some calculations, H5N1 bird flu is still at least two mutations away from widespread human infections, but experts warn that new flu symptoms in individuals at high risk are likely to start turning up in health systems this summer.
“Some of this will not be obvious or at the forefront of our minds.”
Dr. Dugan is leading the team of CDC scientists that is working with partners from the US Department of Agriculture, the US Food and Drug Administration (FDA), and state and local health departments to track and respond to the H5N1 bird flu outbreak currently sweeping through the United States.
Since 2022, avian influenza A viruses have been detected in more than 9300 wild birds in 50 states and territories and in commercial and backyard flocks.
“It’s a bad situation,” said Florian Krammer, PhD, professor of vaccinology at the Icahn School of Medicine at Mount Sinai in New York. “Globally, we’ve seen tons of exposure in cities around the world and even in the birds here in New York City where I am.”
Birds shed the virus in their saliva, mucous, and feces, so people or other animals with close, unprotected contact with infected birds or their contaminated environments can be infected.
And for the first time in March 2024, H5N1 bird flu was reported in dairy cows. The US Department of Agriculture said that at last count, 101 dairy herds in 12 states had been infected, with several cases also found in dairy workers.
From Birds to Cattle and Farm Workers
The National Veterinary Services Laboratories confirmed the infections were highly pathogenic avian influenza H5N1 clade 2.3.4.4b of Eurasian lineage. Also known as the goose, Guangdong clade from China, phylogenetic analysis and epidemiology suggests a single introduction into cows followed by onward transmission.
“I was surprised when H5 was introduced to dairy cattle in this way,” Dr. Dugan said. “Influenza viruses are always surprising us and it reminds me to stay humble and keep an open mind when dealing with them.”
People rarely inhale or get sufficient virus in their eyes or mouth to get sick, Dr. Dugan said, but those in close contact with animals are still at risk for infection, which could lead to upper respiratory tract symptoms such as shortness of breath, cough, sore throat, or runny or stuffy nose.
Like with other viruses, people can also experience muscle or body aches, headache, fatigue, fever or, as was seen in farm workers, conjunctivitis.
But there are less-common symptoms too like diarrhea, nausea, and vomiting — and sometimes, even seizures.
The risk to the general public is still low, Dr. Dugan said, but authorities recommend that people working with animals wash their hands with soap and water and wear personal protective equipment that includes fluid-resistant coveralls, a waterproof apron, a safety-approved respirator, properly fitted goggles or face shield, a head or hair cover, gloves, and boots.
Dr. Dugan said that health care providers often don’t take a history of occupational exposures when a patient presents with flu. But with rising rates of bird flu in new animal hosts, “this will be an important next step.”
Asking Unusual Questions
This approach is not standardized on most electronic health records, so these are questions that clinicians will need to initiate themselves.
“Physicians should ask about work,” said Meghan Davis, PhD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “If it’s not already on the radar, asking about any direct contact with dairy cows, poultry, pigs, wild birds, or wild mammals is important.”
Dr. Davis says she’s worried about a new study tracking risk factors for farm-to-farm transmission because it shows that farms testing positive for avian influenza often have workers with a family member also employed on another farm. “This suggests that we might need to be on the lookout for possible transmission within families,” she said. Now, we have to ask “not just if the person with symptoms has contact with or works on a dairy farm, milk processing plant, or slaughterhouse, but also if any family member does.”
Dr. Davis said that it’s important to bear in mind when taking these histories that there may be younger workers on farms and in slaughter and processing facilities due to exemptions or illegal work.
What is important now is to get the situation under control this season in dairy cattle, Dr. Krammer said. “This will be easier to stop in cows than humans, so this is the time to stop moving dairy cattle and start vaccinating them.”
Spotting New Cases
Since April 2024, there have been three human cases of avian influenza after exposure to dairy cows reported. “And what we don’t want to see this summer is an unusual human cluster of influenza. It’s important we keep a close, watchful eye for this,” Dr. Krammer said.
“Influenza viruses do very interesting things and as we head into fall and winter flu season, we don’t want new human co-infections that could cause major problems for us,” he said.
If people become mixing vessels of a seasonal cocktail of multiple viruses, that could empower H5N1 to mutate again into something more dangerous, sparking a new pandemic.
“It wasn’t all that long ago that we were asking China difficult questions about the steps Chinese authorities took to protect human lives from SARS-CoV-2 in the COVID pandemic. Now, we must ask ourselves many of these questions,” Dr. Krammer said. “We are at a crucial crossroad where we will either elude a new pandemic or see one take off, risking 10 to 20 million lives.”
There is a precedent for safely evading more trouble, Dr. Krammer pointed out. Government agencies have already been working with the poultry industry for a couple of years now. “And here, we have successfully stopped H5N1 with new regulations and policies.”
But moving from poultry farms to cattle has not been an easy transition, Dr. Dugan said. Cattle farms have no experience with bird flu or tactics to contain it with regulations, and officials too are working in new, unfamiliar terrain.
“What we have now isn’t a science problem, it’s a policy issue, and it hasn’t always been clear who is in charge,” Dr. Krammer said.
“Agencies are working together at the state, federal, and global level,” said Dr. Dugan. “We are increasing our transparency and are working to share what we know, when we know it.”
The infrastructure built during the COVID pandemic has helped teams prepare for this new crisis, Dr. Dugan said. Year-round, layered monitoring has clinical labs reporting seasonal influenza and novel cases.
“Laboratories are ready to help with testing,” Dr. Dugan said.
Specimens should be collected as soon as possible from patients with flu symptoms. A nasopharyngeal swab is recommended with a nasal swab combined with an oropharyngeal swab. If a patient has conjunctivitis with or without respiratory symptoms, both a conjunctival swab and a nasopharyngeal swab should be collected.
People with severe respiratory disease should also have lower respiratory tract specimens collected.
Standard, contact, and airborne precautions are recommended for patients presenting for medical care who have illness consistent with influenza and recent exposure to birds or other animals.
Antiviral Drugs
There are four FDA-approved antivirals for influenza: Oseltamivir phosphate (available as a generic drug or by the trade name Tamiflu), zanamivir (Relenza), peramivir (Rapivab) , and baloxavir (Xofluza).
For people with suspected or confirmed avian influenza, treatment is recommended as soon as possible.
There are no clinical trials measuring the outcome of antivirals in people infected with avian influenza. However, data from animal models and human observational studies suggest a benefit.
“We can’t afford to wait this summer,” Dr. Krammer said. “We have an opportunity right now to stop this in cows before we risk infecting more people. I hope we do.”
A version of this article first appeared on Medscape.com.
With avian influenza spreading quickly around the globe, the virus has more opportunities to mutate and cause problems for people. By some calculations, H5N1 bird flu is still at least two mutations away from widespread human infections, but experts warn that new flu symptoms in individuals at high risk are likely to start turning up in health systems this summer.
“Some of this will not be obvious or at the forefront of our minds.”
Dr. Dugan is leading the team of CDC scientists that is working with partners from the US Department of Agriculture, the US Food and Drug Administration (FDA), and state and local health departments to track and respond to the H5N1 bird flu outbreak currently sweeping through the United States.
Since 2022, avian influenza A viruses have been detected in more than 9300 wild birds in 50 states and territories and in commercial and backyard flocks.
“It’s a bad situation,” said Florian Krammer, PhD, professor of vaccinology at the Icahn School of Medicine at Mount Sinai in New York. “Globally, we’ve seen tons of exposure in cities around the world and even in the birds here in New York City where I am.”
Birds shed the virus in their saliva, mucous, and feces, so people or other animals with close, unprotected contact with infected birds or their contaminated environments can be infected.
And for the first time in March 2024, H5N1 bird flu was reported in dairy cows. The US Department of Agriculture said that at last count, 101 dairy herds in 12 states had been infected, with several cases also found in dairy workers.
From Birds to Cattle and Farm Workers
The National Veterinary Services Laboratories confirmed the infections were highly pathogenic avian influenza H5N1 clade 2.3.4.4b of Eurasian lineage. Also known as the goose, Guangdong clade from China, phylogenetic analysis and epidemiology suggests a single introduction into cows followed by onward transmission.
“I was surprised when H5 was introduced to dairy cattle in this way,” Dr. Dugan said. “Influenza viruses are always surprising us and it reminds me to stay humble and keep an open mind when dealing with them.”
People rarely inhale or get sufficient virus in their eyes or mouth to get sick, Dr. Dugan said, but those in close contact with animals are still at risk for infection, which could lead to upper respiratory tract symptoms such as shortness of breath, cough, sore throat, or runny or stuffy nose.
Like with other viruses, people can also experience muscle or body aches, headache, fatigue, fever or, as was seen in farm workers, conjunctivitis.
But there are less-common symptoms too like diarrhea, nausea, and vomiting — and sometimes, even seizures.
The risk to the general public is still low, Dr. Dugan said, but authorities recommend that people working with animals wash their hands with soap and water and wear personal protective equipment that includes fluid-resistant coveralls, a waterproof apron, a safety-approved respirator, properly fitted goggles or face shield, a head or hair cover, gloves, and boots.
Dr. Dugan said that health care providers often don’t take a history of occupational exposures when a patient presents with flu. But with rising rates of bird flu in new animal hosts, “this will be an important next step.”
Asking Unusual Questions
This approach is not standardized on most electronic health records, so these are questions that clinicians will need to initiate themselves.
“Physicians should ask about work,” said Meghan Davis, PhD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “If it’s not already on the radar, asking about any direct contact with dairy cows, poultry, pigs, wild birds, or wild mammals is important.”
Dr. Davis says she’s worried about a new study tracking risk factors for farm-to-farm transmission because it shows that farms testing positive for avian influenza often have workers with a family member also employed on another farm. “This suggests that we might need to be on the lookout for possible transmission within families,” she said. Now, we have to ask “not just if the person with symptoms has contact with or works on a dairy farm, milk processing plant, or slaughterhouse, but also if any family member does.”
Dr. Davis said that it’s important to bear in mind when taking these histories that there may be younger workers on farms and in slaughter and processing facilities due to exemptions or illegal work.
What is important now is to get the situation under control this season in dairy cattle, Dr. Krammer said. “This will be easier to stop in cows than humans, so this is the time to stop moving dairy cattle and start vaccinating them.”
Spotting New Cases
Since April 2024, there have been three human cases of avian influenza after exposure to dairy cows reported. “And what we don’t want to see this summer is an unusual human cluster of influenza. It’s important we keep a close, watchful eye for this,” Dr. Krammer said.
“Influenza viruses do very interesting things and as we head into fall and winter flu season, we don’t want new human co-infections that could cause major problems for us,” he said.
If people become mixing vessels of a seasonal cocktail of multiple viruses, that could empower H5N1 to mutate again into something more dangerous, sparking a new pandemic.
“It wasn’t all that long ago that we were asking China difficult questions about the steps Chinese authorities took to protect human lives from SARS-CoV-2 in the COVID pandemic. Now, we must ask ourselves many of these questions,” Dr. Krammer said. “We are at a crucial crossroad where we will either elude a new pandemic or see one take off, risking 10 to 20 million lives.”
There is a precedent for safely evading more trouble, Dr. Krammer pointed out. Government agencies have already been working with the poultry industry for a couple of years now. “And here, we have successfully stopped H5N1 with new regulations and policies.”
But moving from poultry farms to cattle has not been an easy transition, Dr. Dugan said. Cattle farms have no experience with bird flu or tactics to contain it with regulations, and officials too are working in new, unfamiliar terrain.
“What we have now isn’t a science problem, it’s a policy issue, and it hasn’t always been clear who is in charge,” Dr. Krammer said.
“Agencies are working together at the state, federal, and global level,” said Dr. Dugan. “We are increasing our transparency and are working to share what we know, when we know it.”
The infrastructure built during the COVID pandemic has helped teams prepare for this new crisis, Dr. Dugan said. Year-round, layered monitoring has clinical labs reporting seasonal influenza and novel cases.
“Laboratories are ready to help with testing,” Dr. Dugan said.
Specimens should be collected as soon as possible from patients with flu symptoms. A nasopharyngeal swab is recommended with a nasal swab combined with an oropharyngeal swab. If a patient has conjunctivitis with or without respiratory symptoms, both a conjunctival swab and a nasopharyngeal swab should be collected.
People with severe respiratory disease should also have lower respiratory tract specimens collected.
Standard, contact, and airborne precautions are recommended for patients presenting for medical care who have illness consistent with influenza and recent exposure to birds or other animals.
Antiviral Drugs
There are four FDA-approved antivirals for influenza: Oseltamivir phosphate (available as a generic drug or by the trade name Tamiflu), zanamivir (Relenza), peramivir (Rapivab) , and baloxavir (Xofluza).
For people with suspected or confirmed avian influenza, treatment is recommended as soon as possible.
There are no clinical trials measuring the outcome of antivirals in people infected with avian influenza. However, data from animal models and human observational studies suggest a benefit.
“We can’t afford to wait this summer,” Dr. Krammer said. “We have an opportunity right now to stop this in cows before we risk infecting more people. I hope we do.”
A version of this article first appeared on Medscape.com.
With avian influenza spreading quickly around the globe, the virus has more opportunities to mutate and cause problems for people. By some calculations, H5N1 bird flu is still at least two mutations away from widespread human infections, but experts warn that new flu symptoms in individuals at high risk are likely to start turning up in health systems this summer.
“Some of this will not be obvious or at the forefront of our minds.”
Dr. Dugan is leading the team of CDC scientists that is working with partners from the US Department of Agriculture, the US Food and Drug Administration (FDA), and state and local health departments to track and respond to the H5N1 bird flu outbreak currently sweeping through the United States.
Since 2022, avian influenza A viruses have been detected in more than 9300 wild birds in 50 states and territories and in commercial and backyard flocks.
“It’s a bad situation,” said Florian Krammer, PhD, professor of vaccinology at the Icahn School of Medicine at Mount Sinai in New York. “Globally, we’ve seen tons of exposure in cities around the world and even in the birds here in New York City where I am.”
Birds shed the virus in their saliva, mucous, and feces, so people or other animals with close, unprotected contact with infected birds or their contaminated environments can be infected.
And for the first time in March 2024, H5N1 bird flu was reported in dairy cows. The US Department of Agriculture said that at last count, 101 dairy herds in 12 states had been infected, with several cases also found in dairy workers.
From Birds to Cattle and Farm Workers
The National Veterinary Services Laboratories confirmed the infections were highly pathogenic avian influenza H5N1 clade 2.3.4.4b of Eurasian lineage. Also known as the goose, Guangdong clade from China, phylogenetic analysis and epidemiology suggests a single introduction into cows followed by onward transmission.
“I was surprised when H5 was introduced to dairy cattle in this way,” Dr. Dugan said. “Influenza viruses are always surprising us and it reminds me to stay humble and keep an open mind when dealing with them.”
People rarely inhale or get sufficient virus in their eyes or mouth to get sick, Dr. Dugan said, but those in close contact with animals are still at risk for infection, which could lead to upper respiratory tract symptoms such as shortness of breath, cough, sore throat, or runny or stuffy nose.
Like with other viruses, people can also experience muscle or body aches, headache, fatigue, fever or, as was seen in farm workers, conjunctivitis.
But there are less-common symptoms too like diarrhea, nausea, and vomiting — and sometimes, even seizures.
The risk to the general public is still low, Dr. Dugan said, but authorities recommend that people working with animals wash their hands with soap and water and wear personal protective equipment that includes fluid-resistant coveralls, a waterproof apron, a safety-approved respirator, properly fitted goggles or face shield, a head or hair cover, gloves, and boots.
Dr. Dugan said that health care providers often don’t take a history of occupational exposures when a patient presents with flu. But with rising rates of bird flu in new animal hosts, “this will be an important next step.”
Asking Unusual Questions
This approach is not standardized on most electronic health records, so these are questions that clinicians will need to initiate themselves.
“Physicians should ask about work,” said Meghan Davis, PhD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “If it’s not already on the radar, asking about any direct contact with dairy cows, poultry, pigs, wild birds, or wild mammals is important.”
Dr. Davis says she’s worried about a new study tracking risk factors for farm-to-farm transmission because it shows that farms testing positive for avian influenza often have workers with a family member also employed on another farm. “This suggests that we might need to be on the lookout for possible transmission within families,” she said. Now, we have to ask “not just if the person with symptoms has contact with or works on a dairy farm, milk processing plant, or slaughterhouse, but also if any family member does.”
Dr. Davis said that it’s important to bear in mind when taking these histories that there may be younger workers on farms and in slaughter and processing facilities due to exemptions or illegal work.
What is important now is to get the situation under control this season in dairy cattle, Dr. Krammer said. “This will be easier to stop in cows than humans, so this is the time to stop moving dairy cattle and start vaccinating them.”
Spotting New Cases
Since April 2024, there have been three human cases of avian influenza after exposure to dairy cows reported. “And what we don’t want to see this summer is an unusual human cluster of influenza. It’s important we keep a close, watchful eye for this,” Dr. Krammer said.
“Influenza viruses do very interesting things and as we head into fall and winter flu season, we don’t want new human co-infections that could cause major problems for us,” he said.
If people become mixing vessels of a seasonal cocktail of multiple viruses, that could empower H5N1 to mutate again into something more dangerous, sparking a new pandemic.
“It wasn’t all that long ago that we were asking China difficult questions about the steps Chinese authorities took to protect human lives from SARS-CoV-2 in the COVID pandemic. Now, we must ask ourselves many of these questions,” Dr. Krammer said. “We are at a crucial crossroad where we will either elude a new pandemic or see one take off, risking 10 to 20 million lives.”
There is a precedent for safely evading more trouble, Dr. Krammer pointed out. Government agencies have already been working with the poultry industry for a couple of years now. “And here, we have successfully stopped H5N1 with new regulations and policies.”
But moving from poultry farms to cattle has not been an easy transition, Dr. Dugan said. Cattle farms have no experience with bird flu or tactics to contain it with regulations, and officials too are working in new, unfamiliar terrain.
“What we have now isn’t a science problem, it’s a policy issue, and it hasn’t always been clear who is in charge,” Dr. Krammer said.
“Agencies are working together at the state, federal, and global level,” said Dr. Dugan. “We are increasing our transparency and are working to share what we know, when we know it.”
The infrastructure built during the COVID pandemic has helped teams prepare for this new crisis, Dr. Dugan said. Year-round, layered monitoring has clinical labs reporting seasonal influenza and novel cases.
“Laboratories are ready to help with testing,” Dr. Dugan said.
Specimens should be collected as soon as possible from patients with flu symptoms. A nasopharyngeal swab is recommended with a nasal swab combined with an oropharyngeal swab. If a patient has conjunctivitis with or without respiratory symptoms, both a conjunctival swab and a nasopharyngeal swab should be collected.
People with severe respiratory disease should also have lower respiratory tract specimens collected.
Standard, contact, and airborne precautions are recommended for patients presenting for medical care who have illness consistent with influenza and recent exposure to birds or other animals.
Antiviral Drugs
There are four FDA-approved antivirals for influenza: Oseltamivir phosphate (available as a generic drug or by the trade name Tamiflu), zanamivir (Relenza), peramivir (Rapivab) , and baloxavir (Xofluza).
For people with suspected or confirmed avian influenza, treatment is recommended as soon as possible.
There are no clinical trials measuring the outcome of antivirals in people infected with avian influenza. However, data from animal models and human observational studies suggest a benefit.
“We can’t afford to wait this summer,” Dr. Krammer said. “We have an opportunity right now to stop this in cows before we risk infecting more people. I hope we do.”
A version of this article first appeared on Medscape.com.
Managing Heart Failure in Women: Key Differences and Clinical Tips
This transcript has been edited for clarity.
Hi. I’m Dr Eileen Hsich. I’m the medical director for heart transplantation at the Cleveland Clinic, and my specialty is sex differences in heart failure. I’m excited to talk to you about heart failure treatment in women, addressing the differences in managing heart failure in women as well as practical tips for clinicians. You think that I’m going to be starting off by telling you about the differences in how we’re going to manage the patients, but I’m not. The reason I’m not going to do that is because our national guidelines are not sex specific.
What I’m really going to discuss with you today are the data so that you can decide for yourself what we should do and whether there really are differences. As we begin, I always think about the prevalence of the disease. Currently, there are 6.7 million Americans with heart failure, and approximately 45% of them are women. Globally, our best research shows that there are over 56 million people living with heart failure, and half of them are women.
We also know that there are different underlying causes in women and men. For women, the four risk factors are hypertension, diabetes, atrial fibrillation (AFib), and left bundle branch block. I know you knew about hypertension. Diabetes may not have been right up there in your mind. You see many women with AFib, so I know that you were thinking about it. We’re going to come back to left bundle branch block; it really is very interesting.
For men, it is the risk for heart failure development after a myocardial infarction. Men are more likely to have an ischemic cardiomyopathy. It is also important to state that when women have heart failure, it is often with more preserved ejection fraction. We know that heart failure with preserved ejection fraction (HFpEF) is more common in women and heart failure with reduced ejection fraction (HFrEF) is more common in men.
Now we’re going to talk about the four pillars in medical management, and we’re going to start out with the easy medications that show no sex differences in benefit. The mineralocorticoid receptor antagonists (MRAs) show that there are no sex differences in regard to benefit. Women benefit as much as men, based on two of the largest studies, which were the RALES study, which studied heart failure that was ischemic and nonischemic, and then the EPHESUS study, which was specific to patients who had myocardial infarction. There was a mortality benefit in the women.
The next set of drugs that we’re going to mention are the sodium-glucose cotransporter 2 (SGLT2) inhibitors. The combined endpoint for women and men was a combined endpoint of death and heart failure hospitalization. No matter what the ejection fraction was, women benefited like men for this drug.
The third class of agents that I want to discuss is the beta-blockers, which are really very interesting because they’re so powerful. The studies for these drugs were stopped prematurely. When you take into consideration that women are underenrolled in clinical trials, remember that the studies for these drugs were stopped, so there weren’t that many women. The fact that we showed a mortality benefit is really important.
The first drug that we’re going to refer to is bisoprolol because CIBIS II was the first trial for this drug to demonstrate a mortality benefit in women and men. The second drug that I want to mention is metoprolol XL, which did not demonstrate a mortality benefit in the MERIT-HF study, but did demonstrate a benefit in reduced heart failure hospitalizations, which is also very important.
The third drug is carvedilol, which had been shown to reduce a combined endpoint of mortality and heart failure hospitalizations for patients with moderate symptoms. When I talk about these studies, they have anywhere from 250 to 1000 women enrolled, so these are relatively small studies and they still did demonstrate a benefit.
When we talk about angiotensin receptor–neprilysin inhibitors (ARNI), I think that’s when it gets a little complex. The data are not very clear because ARNI is a combination pill — sacubitril combined with valsartan. When you have an ideal control for a study and you want to know what your magic ingredient is, which is the sacubitril, you really want to compare valsartan with ARNI so that you can find out what your magic little ingredient is doing.
When we had the PARAGON-HF study, which was for HFpEF patients who had an ejection fraction greater than 45%, there was a benefit in the women and not in the men, and that really was in the women with the lower ejection fractions. That’s very interesting because the control was valsartan.
When we had the PARADIGM-HF study, that was more complex. The control was an angiotensin-converting enzyme (ACE) inhibitor, which is not an ideal control for women since, even in a meta-analysis that had over 1000 women, there has not been a proven benefit. The confidence intervals remain wide. Therefore, it’s not quite a fair comparison to randomize women to ARNI versus an ACE inhibitor. Comparing ARNI to valsartan would be better in order to determine the additional benefit of sacubitril since valsartan alone has already been shown, in the Val-HeFT study, to reduce heart failure hospitalizations in women — although not mortality. There was a benefit.
When you look at the PARADIGM-HF study, which was for HFrEF patients, and you see that there is a benefit in the women, where the combined endpoint was heart failure hospitalization and mortality, you then see that there’s a figure that shows what happens when we look at mortality alone. The benefit is not driven by mortality; it’s driven by heart failure hospitalizations for the women, for which valsartan already had been shown to do this. Therefore, I don’t know if sacubitril/valsartan is more powerful because we didn’t have the right control in studies. From my standpoint, the data really are not there. We can all have our own biased opinions.
When we talk about devices, that gets really interesting because it goes back to those risk factors. We’re going to start with implantable cardioverter defibrillators (ICDs). We have shown in many ICD trials that women and men had similar survival. There were very few women in these device trials. If you think the medical trials had only a few women, just imagine what the ICD trials had.
Santangeli and colleagues hypothesized that an ICD only saves you from sudden death. It doesn›t really save you from anything else. In heart failure, women do live longer than men. Is this device really saving you? They weren’t interested in all-cause mortality; they were interested in whether the device fired appropriately for ventricular tachycardia or ventricular fibrillation. They demonstrated in that meta-analysis that it was not very clear that women had the benefit. The rationale behind that comes from the MADIT studies that showed that men were more likely than women to have ventricular arrhythmias.
This is also true based on the Seattle Heart Failure Model. The derivation cohort had very few ICDs at that time, and women were less likely than men to have ventricular arrhythmias as the cause of death. It’s not that we shouldn’t put them in — I very strongly believe that we should — but we don’t have that data.
In fact, in the Santangeli and colleagues study, women were more likely to have inappropriate firing for AFib. Remember that we talked about how one of the risk factors for heart failure was AFib. Women are more likely to have AFib and the ICD firing for AFib and not ventricular arrhythmias. This may be dependent on the type of cardiomyopathy.
Next, we’re going to talk about biventricular pacemakers. Women tend to benefit more so that there is an improvement in symptoms and survival. What is fascinating is that left bundle branch block is a risk factor for the development of heart failure in women, which makes this next statement even more fascinating.
The FDA does their own analysis when they are reviewing devices and everything else, and they published one of them in JAMA Internal Medicine, taking three studies and seeing the benefit in women and men. They found that everybody benefits when the left bundle branch block has a QRS greater than 150 milliseconds. But with a QRS between 130 and 149 milliseconds, only the women benefited. That›s fascinating because that is a risk factor — the development of the left bundle branch block causing heart failure in women. It makes you wonder whether you are correcting something that actually was responsible for their heart failure.
In advanced heart failure, we have left ventricular assist devices (LVADs) and heart transplantation. For years, we couldn’t get LVADs small enough to fit in women. When they were larger, there were complications that were more common in women, such as stroke. With the newer devices — the HeartMate 3 is small, for instance — complications for everyone are very infrequent, and women and men benefit. I’m going to encourage clinicians to use them.
For heart transplantation, as I mentioned before, women tend to get HFpEF. I didn’t mention that they get heart failure when they’re older, for the most part. There are fewer women who are transplanted than men and eligible at younger ages. What we had for decades was that women were dying while they were on the waitlist for heart transplantation at a faster rate than men but living longer after transplantation. As LVADs became more appropriately sized for women, the complication rates went down; and we did see an improvement on the waitlist mortality rate before we changed the allocation system. But it really wasn’t until after we changed the allocation system in 2018 that we saw great success. Now, women have similar survival while on the waitlist. They’re transplanted at a faster rate despite the fact that they’re less likely to receive the temporary mechanical support, and they tend to still do very well.
We have some differences in therapy response. Thank you.
Dr. Hsich disclosed ties with Natera, DEFINE steering committee (no money), and MEDCAC (Medicare/Medicaid) committee. She received research grant from the National Institutes of Health.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Dr Eileen Hsich. I’m the medical director for heart transplantation at the Cleveland Clinic, and my specialty is sex differences in heart failure. I’m excited to talk to you about heart failure treatment in women, addressing the differences in managing heart failure in women as well as practical tips for clinicians. You think that I’m going to be starting off by telling you about the differences in how we’re going to manage the patients, but I’m not. The reason I’m not going to do that is because our national guidelines are not sex specific.
What I’m really going to discuss with you today are the data so that you can decide for yourself what we should do and whether there really are differences. As we begin, I always think about the prevalence of the disease. Currently, there are 6.7 million Americans with heart failure, and approximately 45% of them are women. Globally, our best research shows that there are over 56 million people living with heart failure, and half of them are women.
We also know that there are different underlying causes in women and men. For women, the four risk factors are hypertension, diabetes, atrial fibrillation (AFib), and left bundle branch block. I know you knew about hypertension. Diabetes may not have been right up there in your mind. You see many women with AFib, so I know that you were thinking about it. We’re going to come back to left bundle branch block; it really is very interesting.
For men, it is the risk for heart failure development after a myocardial infarction. Men are more likely to have an ischemic cardiomyopathy. It is also important to state that when women have heart failure, it is often with more preserved ejection fraction. We know that heart failure with preserved ejection fraction (HFpEF) is more common in women and heart failure with reduced ejection fraction (HFrEF) is more common in men.
Now we’re going to talk about the four pillars in medical management, and we’re going to start out with the easy medications that show no sex differences in benefit. The mineralocorticoid receptor antagonists (MRAs) show that there are no sex differences in regard to benefit. Women benefit as much as men, based on two of the largest studies, which were the RALES study, which studied heart failure that was ischemic and nonischemic, and then the EPHESUS study, which was specific to patients who had myocardial infarction. There was a mortality benefit in the women.
The next set of drugs that we’re going to mention are the sodium-glucose cotransporter 2 (SGLT2) inhibitors. The combined endpoint for women and men was a combined endpoint of death and heart failure hospitalization. No matter what the ejection fraction was, women benefited like men for this drug.
The third class of agents that I want to discuss is the beta-blockers, which are really very interesting because they’re so powerful. The studies for these drugs were stopped prematurely. When you take into consideration that women are underenrolled in clinical trials, remember that the studies for these drugs were stopped, so there weren’t that many women. The fact that we showed a mortality benefit is really important.
The first drug that we’re going to refer to is bisoprolol because CIBIS II was the first trial for this drug to demonstrate a mortality benefit in women and men. The second drug that I want to mention is metoprolol XL, which did not demonstrate a mortality benefit in the MERIT-HF study, but did demonstrate a benefit in reduced heart failure hospitalizations, which is also very important.
The third drug is carvedilol, which had been shown to reduce a combined endpoint of mortality and heart failure hospitalizations for patients with moderate symptoms. When I talk about these studies, they have anywhere from 250 to 1000 women enrolled, so these are relatively small studies and they still did demonstrate a benefit.
When we talk about angiotensin receptor–neprilysin inhibitors (ARNI), I think that’s when it gets a little complex. The data are not very clear because ARNI is a combination pill — sacubitril combined with valsartan. When you have an ideal control for a study and you want to know what your magic ingredient is, which is the sacubitril, you really want to compare valsartan with ARNI so that you can find out what your magic little ingredient is doing.
When we had the PARAGON-HF study, which was for HFpEF patients who had an ejection fraction greater than 45%, there was a benefit in the women and not in the men, and that really was in the women with the lower ejection fractions. That’s very interesting because the control was valsartan.
When we had the PARADIGM-HF study, that was more complex. The control was an angiotensin-converting enzyme (ACE) inhibitor, which is not an ideal control for women since, even in a meta-analysis that had over 1000 women, there has not been a proven benefit. The confidence intervals remain wide. Therefore, it’s not quite a fair comparison to randomize women to ARNI versus an ACE inhibitor. Comparing ARNI to valsartan would be better in order to determine the additional benefit of sacubitril since valsartan alone has already been shown, in the Val-HeFT study, to reduce heart failure hospitalizations in women — although not mortality. There was a benefit.
When you look at the PARADIGM-HF study, which was for HFrEF patients, and you see that there is a benefit in the women, where the combined endpoint was heart failure hospitalization and mortality, you then see that there’s a figure that shows what happens when we look at mortality alone. The benefit is not driven by mortality; it’s driven by heart failure hospitalizations for the women, for which valsartan already had been shown to do this. Therefore, I don’t know if sacubitril/valsartan is more powerful because we didn’t have the right control in studies. From my standpoint, the data really are not there. We can all have our own biased opinions.
When we talk about devices, that gets really interesting because it goes back to those risk factors. We’re going to start with implantable cardioverter defibrillators (ICDs). We have shown in many ICD trials that women and men had similar survival. There were very few women in these device trials. If you think the medical trials had only a few women, just imagine what the ICD trials had.
Santangeli and colleagues hypothesized that an ICD only saves you from sudden death. It doesn›t really save you from anything else. In heart failure, women do live longer than men. Is this device really saving you? They weren’t interested in all-cause mortality; they were interested in whether the device fired appropriately for ventricular tachycardia or ventricular fibrillation. They demonstrated in that meta-analysis that it was not very clear that women had the benefit. The rationale behind that comes from the MADIT studies that showed that men were more likely than women to have ventricular arrhythmias.
This is also true based on the Seattle Heart Failure Model. The derivation cohort had very few ICDs at that time, and women were less likely than men to have ventricular arrhythmias as the cause of death. It’s not that we shouldn’t put them in — I very strongly believe that we should — but we don’t have that data.
In fact, in the Santangeli and colleagues study, women were more likely to have inappropriate firing for AFib. Remember that we talked about how one of the risk factors for heart failure was AFib. Women are more likely to have AFib and the ICD firing for AFib and not ventricular arrhythmias. This may be dependent on the type of cardiomyopathy.
Next, we’re going to talk about biventricular pacemakers. Women tend to benefit more so that there is an improvement in symptoms and survival. What is fascinating is that left bundle branch block is a risk factor for the development of heart failure in women, which makes this next statement even more fascinating.
The FDA does their own analysis when they are reviewing devices and everything else, and they published one of them in JAMA Internal Medicine, taking three studies and seeing the benefit in women and men. They found that everybody benefits when the left bundle branch block has a QRS greater than 150 milliseconds. But with a QRS between 130 and 149 milliseconds, only the women benefited. That›s fascinating because that is a risk factor — the development of the left bundle branch block causing heart failure in women. It makes you wonder whether you are correcting something that actually was responsible for their heart failure.
In advanced heart failure, we have left ventricular assist devices (LVADs) and heart transplantation. For years, we couldn’t get LVADs small enough to fit in women. When they were larger, there were complications that were more common in women, such as stroke. With the newer devices — the HeartMate 3 is small, for instance — complications for everyone are very infrequent, and women and men benefit. I’m going to encourage clinicians to use them.
For heart transplantation, as I mentioned before, women tend to get HFpEF. I didn’t mention that they get heart failure when they’re older, for the most part. There are fewer women who are transplanted than men and eligible at younger ages. What we had for decades was that women were dying while they were on the waitlist for heart transplantation at a faster rate than men but living longer after transplantation. As LVADs became more appropriately sized for women, the complication rates went down; and we did see an improvement on the waitlist mortality rate before we changed the allocation system. But it really wasn’t until after we changed the allocation system in 2018 that we saw great success. Now, women have similar survival while on the waitlist. They’re transplanted at a faster rate despite the fact that they’re less likely to receive the temporary mechanical support, and they tend to still do very well.
We have some differences in therapy response. Thank you.
Dr. Hsich disclosed ties with Natera, DEFINE steering committee (no money), and MEDCAC (Medicare/Medicaid) committee. She received research grant from the National Institutes of Health.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Dr Eileen Hsich. I’m the medical director for heart transplantation at the Cleveland Clinic, and my specialty is sex differences in heart failure. I’m excited to talk to you about heart failure treatment in women, addressing the differences in managing heart failure in women as well as practical tips for clinicians. You think that I’m going to be starting off by telling you about the differences in how we’re going to manage the patients, but I’m not. The reason I’m not going to do that is because our national guidelines are not sex specific.
What I’m really going to discuss with you today are the data so that you can decide for yourself what we should do and whether there really are differences. As we begin, I always think about the prevalence of the disease. Currently, there are 6.7 million Americans with heart failure, and approximately 45% of them are women. Globally, our best research shows that there are over 56 million people living with heart failure, and half of them are women.
We also know that there are different underlying causes in women and men. For women, the four risk factors are hypertension, diabetes, atrial fibrillation (AFib), and left bundle branch block. I know you knew about hypertension. Diabetes may not have been right up there in your mind. You see many women with AFib, so I know that you were thinking about it. We’re going to come back to left bundle branch block; it really is very interesting.
For men, it is the risk for heart failure development after a myocardial infarction. Men are more likely to have an ischemic cardiomyopathy. It is also important to state that when women have heart failure, it is often with more preserved ejection fraction. We know that heart failure with preserved ejection fraction (HFpEF) is more common in women and heart failure with reduced ejection fraction (HFrEF) is more common in men.
Now we’re going to talk about the four pillars in medical management, and we’re going to start out with the easy medications that show no sex differences in benefit. The mineralocorticoid receptor antagonists (MRAs) show that there are no sex differences in regard to benefit. Women benefit as much as men, based on two of the largest studies, which were the RALES study, which studied heart failure that was ischemic and nonischemic, and then the EPHESUS study, which was specific to patients who had myocardial infarction. There was a mortality benefit in the women.
The next set of drugs that we’re going to mention are the sodium-glucose cotransporter 2 (SGLT2) inhibitors. The combined endpoint for women and men was a combined endpoint of death and heart failure hospitalization. No matter what the ejection fraction was, women benefited like men for this drug.
The third class of agents that I want to discuss is the beta-blockers, which are really very interesting because they’re so powerful. The studies for these drugs were stopped prematurely. When you take into consideration that women are underenrolled in clinical trials, remember that the studies for these drugs were stopped, so there weren’t that many women. The fact that we showed a mortality benefit is really important.
The first drug that we’re going to refer to is bisoprolol because CIBIS II was the first trial for this drug to demonstrate a mortality benefit in women and men. The second drug that I want to mention is metoprolol XL, which did not demonstrate a mortality benefit in the MERIT-HF study, but did demonstrate a benefit in reduced heart failure hospitalizations, which is also very important.
The third drug is carvedilol, which had been shown to reduce a combined endpoint of mortality and heart failure hospitalizations for patients with moderate symptoms. When I talk about these studies, they have anywhere from 250 to 1000 women enrolled, so these are relatively small studies and they still did demonstrate a benefit.
When we talk about angiotensin receptor–neprilysin inhibitors (ARNI), I think that’s when it gets a little complex. The data are not very clear because ARNI is a combination pill — sacubitril combined with valsartan. When you have an ideal control for a study and you want to know what your magic ingredient is, which is the sacubitril, you really want to compare valsartan with ARNI so that you can find out what your magic little ingredient is doing.
When we had the PARAGON-HF study, which was for HFpEF patients who had an ejection fraction greater than 45%, there was a benefit in the women and not in the men, and that really was in the women with the lower ejection fractions. That’s very interesting because the control was valsartan.
When we had the PARADIGM-HF study, that was more complex. The control was an angiotensin-converting enzyme (ACE) inhibitor, which is not an ideal control for women since, even in a meta-analysis that had over 1000 women, there has not been a proven benefit. The confidence intervals remain wide. Therefore, it’s not quite a fair comparison to randomize women to ARNI versus an ACE inhibitor. Comparing ARNI to valsartan would be better in order to determine the additional benefit of sacubitril since valsartan alone has already been shown, in the Val-HeFT study, to reduce heart failure hospitalizations in women — although not mortality. There was a benefit.
When you look at the PARADIGM-HF study, which was for HFrEF patients, and you see that there is a benefit in the women, where the combined endpoint was heart failure hospitalization and mortality, you then see that there’s a figure that shows what happens when we look at mortality alone. The benefit is not driven by mortality; it’s driven by heart failure hospitalizations for the women, for which valsartan already had been shown to do this. Therefore, I don’t know if sacubitril/valsartan is more powerful because we didn’t have the right control in studies. From my standpoint, the data really are not there. We can all have our own biased opinions.
When we talk about devices, that gets really interesting because it goes back to those risk factors. We’re going to start with implantable cardioverter defibrillators (ICDs). We have shown in many ICD trials that women and men had similar survival. There were very few women in these device trials. If you think the medical trials had only a few women, just imagine what the ICD trials had.
Santangeli and colleagues hypothesized that an ICD only saves you from sudden death. It doesn›t really save you from anything else. In heart failure, women do live longer than men. Is this device really saving you? They weren’t interested in all-cause mortality; they were interested in whether the device fired appropriately for ventricular tachycardia or ventricular fibrillation. They demonstrated in that meta-analysis that it was not very clear that women had the benefit. The rationale behind that comes from the MADIT studies that showed that men were more likely than women to have ventricular arrhythmias.
This is also true based on the Seattle Heart Failure Model. The derivation cohort had very few ICDs at that time, and women were less likely than men to have ventricular arrhythmias as the cause of death. It’s not that we shouldn’t put them in — I very strongly believe that we should — but we don’t have that data.
In fact, in the Santangeli and colleagues study, women were more likely to have inappropriate firing for AFib. Remember that we talked about how one of the risk factors for heart failure was AFib. Women are more likely to have AFib and the ICD firing for AFib and not ventricular arrhythmias. This may be dependent on the type of cardiomyopathy.
Next, we’re going to talk about biventricular pacemakers. Women tend to benefit more so that there is an improvement in symptoms and survival. What is fascinating is that left bundle branch block is a risk factor for the development of heart failure in women, which makes this next statement even more fascinating.
The FDA does their own analysis when they are reviewing devices and everything else, and they published one of them in JAMA Internal Medicine, taking three studies and seeing the benefit in women and men. They found that everybody benefits when the left bundle branch block has a QRS greater than 150 milliseconds. But with a QRS between 130 and 149 milliseconds, only the women benefited. That›s fascinating because that is a risk factor — the development of the left bundle branch block causing heart failure in women. It makes you wonder whether you are correcting something that actually was responsible for their heart failure.
In advanced heart failure, we have left ventricular assist devices (LVADs) and heart transplantation. For years, we couldn’t get LVADs small enough to fit in women. When they were larger, there were complications that were more common in women, such as stroke. With the newer devices — the HeartMate 3 is small, for instance — complications for everyone are very infrequent, and women and men benefit. I’m going to encourage clinicians to use them.
For heart transplantation, as I mentioned before, women tend to get HFpEF. I didn’t mention that they get heart failure when they’re older, for the most part. There are fewer women who are transplanted than men and eligible at younger ages. What we had for decades was that women were dying while they were on the waitlist for heart transplantation at a faster rate than men but living longer after transplantation. As LVADs became more appropriately sized for women, the complication rates went down; and we did see an improvement on the waitlist mortality rate before we changed the allocation system. But it really wasn’t until after we changed the allocation system in 2018 that we saw great success. Now, women have similar survival while on the waitlist. They’re transplanted at a faster rate despite the fact that they’re less likely to receive the temporary mechanical support, and they tend to still do very well.
We have some differences in therapy response. Thank you.
Dr. Hsich disclosed ties with Natera, DEFINE steering committee (no money), and MEDCAC (Medicare/Medicaid) committee. She received research grant from the National Institutes of Health.
A version of this article appeared on Medscape.com.
Mediterranean Diet Lowers Tachyarrhythmia in Paroxysmal AF
BOSTON — A Mediterranean diet with extra virgin olive oil (EVOO) significantly reduced the risk for tachyarrhythmia recurrence after atrial fibrillation (AF) ablation in patients with paroxysmal disease, but the diet had less of an impact on patients with persistent AF, a new study showed.
“An intervention with the Mediterranean diet with EVOO produced a nonsignificant reduction in any atrial tachycardia in a selected population after undergoing atrial fibrillation ablation, but this intervention produced a significant reduction in any atrial tachyarrhythmias in patients with paroxysmal AF,” said Maria Teresa Barrio-Lopez, MD, PhD, an electrophysiologist at University Hospital HM Monteprincipe in Madrid, Spain, who presented results from the PREDIMAR trial at the Heart Rhythm Society (HRS) 2024 annual meeting.
The PREDIMAR study enrolled 720 patients from the larger PREDIMED study, which showed that patients without AF at enrollment and who followed a Mediterranean diet enriched with EVOO had a 38% lower rate of incidental AF than control individuals.
PREDIMAR evaluated the impact of the diet on arrhythmia recurrence in patients after ablation. The patients were randomized in a 1:1 ratio to either the dietary intervention group or the control group.
PREDIMAR Study Results
However, among the 431 patients with paroxysmal AF, 25.2% in the diet group and 34.7% in the control group had no tachyarrhythmia recurrence, which translates into a 31% lower risk in the diet group.
In this study, the diet was rich in fish, nuts, fruits, and vegetables and was complemented with EVOO. Participants were also permitted moderate wine consumption.
The intervention involved dietitians who remotely followed patients and made periodic telephone calls to encourage them to stay on the diet. Participants had weight and body measurements taken at baseline and at 3, 6, 12, and 18 months and underwent an ECG at 6, 12, and 18 months. Labs were obtained at baseline and at 12 months. Participants were also given educational materials throughout the intervention.
Average scores, based on a scale of 0-13, excluding an item for wine intake, were 7.8 in the diet group and 7.2 in the control group.
Daily average alcohol intake was higher in the diet group than in the control group (9.8 vs 8.2 g), but “the weight of the patient during the study didn’t change in any group,” Dr. Barrio-Lopez reported.
Baseline characteristics were similar in the two groups. About 60% were taking antiarrhythmic drugs, and about 84% were taking anticoagulants.
‘A Tour de Force’
PREDIMAR was “really a tour de force,” Christine Albert, MD, MPH, chair of cardiology at the Smidt Heart Institute at the Cedars-Sinai Medical Center in Los Angeles, California, said during a commentary presented at HRS. “We talk about how we’re going to do these dietary interventions and weight loss and all the risk-factor reduction, and they pulled it off with 700 individuals and also did it in a way that was very novel.”
This is the first large-scale dietary intervention trial of patients with AF. However, Dr. Albert noted later in an interview, the Mediterranean diet poses potential challenges for some people with AF.
“The Mediterranean diet recommends that people drink wine, but then there’s clear evidence that abstinence from alcohol actually reduces recurrences of atrial fibrillation, so even though there are a lot of things about the Mediterranean diet that are probably healthy and good for atrial fibrillation, that aspect of it might be working against the patient,” she explained.
The finding that patients in the Mediterranean diet group experienced no significant weight loss could be counterintuitive when it comes to preventing AF. But “you could adapt the diet for AF,” Dr. Albert said. You could “leave out the wine and focus more on weight loss if the patient is obese because those are also the pillars of what we’ve learned for patients with atrial fibrillation.”
Making weight loss a key component of the study could be significant for the American population. “At least in the United States, that’s a huge part of the risk factors for atrial fibrillation after ablation,” she said.
The remote follow-up component of the PREDIMAR study is also intriguing. “I think what’s most exciting about what they did is, they showed they can do all these things remotely,” Dr. Albert added.
Dr. Barrio-Lopez had no relevant financial relationships. Dr. Albert disclosed relationships with Abbott, Roche Diagnostics, St. Jude Medical, Boston Scientific, Medtronic, and Element Science.
A version of this article appeared on Medscape.com.
BOSTON — A Mediterranean diet with extra virgin olive oil (EVOO) significantly reduced the risk for tachyarrhythmia recurrence after atrial fibrillation (AF) ablation in patients with paroxysmal disease, but the diet had less of an impact on patients with persistent AF, a new study showed.
“An intervention with the Mediterranean diet with EVOO produced a nonsignificant reduction in any atrial tachycardia in a selected population after undergoing atrial fibrillation ablation, but this intervention produced a significant reduction in any atrial tachyarrhythmias in patients with paroxysmal AF,” said Maria Teresa Barrio-Lopez, MD, PhD, an electrophysiologist at University Hospital HM Monteprincipe in Madrid, Spain, who presented results from the PREDIMAR trial at the Heart Rhythm Society (HRS) 2024 annual meeting.
The PREDIMAR study enrolled 720 patients from the larger PREDIMED study, which showed that patients without AF at enrollment and who followed a Mediterranean diet enriched with EVOO had a 38% lower rate of incidental AF than control individuals.
PREDIMAR evaluated the impact of the diet on arrhythmia recurrence in patients after ablation. The patients were randomized in a 1:1 ratio to either the dietary intervention group or the control group.
PREDIMAR Study Results
However, among the 431 patients with paroxysmal AF, 25.2% in the diet group and 34.7% in the control group had no tachyarrhythmia recurrence, which translates into a 31% lower risk in the diet group.
In this study, the diet was rich in fish, nuts, fruits, and vegetables and was complemented with EVOO. Participants were also permitted moderate wine consumption.
The intervention involved dietitians who remotely followed patients and made periodic telephone calls to encourage them to stay on the diet. Participants had weight and body measurements taken at baseline and at 3, 6, 12, and 18 months and underwent an ECG at 6, 12, and 18 months. Labs were obtained at baseline and at 12 months. Participants were also given educational materials throughout the intervention.
Average scores, based on a scale of 0-13, excluding an item for wine intake, were 7.8 in the diet group and 7.2 in the control group.
Daily average alcohol intake was higher in the diet group than in the control group (9.8 vs 8.2 g), but “the weight of the patient during the study didn’t change in any group,” Dr. Barrio-Lopez reported.
Baseline characteristics were similar in the two groups. About 60% were taking antiarrhythmic drugs, and about 84% were taking anticoagulants.
‘A Tour de Force’
PREDIMAR was “really a tour de force,” Christine Albert, MD, MPH, chair of cardiology at the Smidt Heart Institute at the Cedars-Sinai Medical Center in Los Angeles, California, said during a commentary presented at HRS. “We talk about how we’re going to do these dietary interventions and weight loss and all the risk-factor reduction, and they pulled it off with 700 individuals and also did it in a way that was very novel.”
This is the first large-scale dietary intervention trial of patients with AF. However, Dr. Albert noted later in an interview, the Mediterranean diet poses potential challenges for some people with AF.
“The Mediterranean diet recommends that people drink wine, but then there’s clear evidence that abstinence from alcohol actually reduces recurrences of atrial fibrillation, so even though there are a lot of things about the Mediterranean diet that are probably healthy and good for atrial fibrillation, that aspect of it might be working against the patient,” she explained.
The finding that patients in the Mediterranean diet group experienced no significant weight loss could be counterintuitive when it comes to preventing AF. But “you could adapt the diet for AF,” Dr. Albert said. You could “leave out the wine and focus more on weight loss if the patient is obese because those are also the pillars of what we’ve learned for patients with atrial fibrillation.”
Making weight loss a key component of the study could be significant for the American population. “At least in the United States, that’s a huge part of the risk factors for atrial fibrillation after ablation,” she said.
The remote follow-up component of the PREDIMAR study is also intriguing. “I think what’s most exciting about what they did is, they showed they can do all these things remotely,” Dr. Albert added.
Dr. Barrio-Lopez had no relevant financial relationships. Dr. Albert disclosed relationships with Abbott, Roche Diagnostics, St. Jude Medical, Boston Scientific, Medtronic, and Element Science.
A version of this article appeared on Medscape.com.
BOSTON — A Mediterranean diet with extra virgin olive oil (EVOO) significantly reduced the risk for tachyarrhythmia recurrence after atrial fibrillation (AF) ablation in patients with paroxysmal disease, but the diet had less of an impact on patients with persistent AF, a new study showed.
“An intervention with the Mediterranean diet with EVOO produced a nonsignificant reduction in any atrial tachycardia in a selected population after undergoing atrial fibrillation ablation, but this intervention produced a significant reduction in any atrial tachyarrhythmias in patients with paroxysmal AF,” said Maria Teresa Barrio-Lopez, MD, PhD, an electrophysiologist at University Hospital HM Monteprincipe in Madrid, Spain, who presented results from the PREDIMAR trial at the Heart Rhythm Society (HRS) 2024 annual meeting.
The PREDIMAR study enrolled 720 patients from the larger PREDIMED study, which showed that patients without AF at enrollment and who followed a Mediterranean diet enriched with EVOO had a 38% lower rate of incidental AF than control individuals.
PREDIMAR evaluated the impact of the diet on arrhythmia recurrence in patients after ablation. The patients were randomized in a 1:1 ratio to either the dietary intervention group or the control group.
PREDIMAR Study Results
However, among the 431 patients with paroxysmal AF, 25.2% in the diet group and 34.7% in the control group had no tachyarrhythmia recurrence, which translates into a 31% lower risk in the diet group.
In this study, the diet was rich in fish, nuts, fruits, and vegetables and was complemented with EVOO. Participants were also permitted moderate wine consumption.
The intervention involved dietitians who remotely followed patients and made periodic telephone calls to encourage them to stay on the diet. Participants had weight and body measurements taken at baseline and at 3, 6, 12, and 18 months and underwent an ECG at 6, 12, and 18 months. Labs were obtained at baseline and at 12 months. Participants were also given educational materials throughout the intervention.
Average scores, based on a scale of 0-13, excluding an item for wine intake, were 7.8 in the diet group and 7.2 in the control group.
Daily average alcohol intake was higher in the diet group than in the control group (9.8 vs 8.2 g), but “the weight of the patient during the study didn’t change in any group,” Dr. Barrio-Lopez reported.
Baseline characteristics were similar in the two groups. About 60% were taking antiarrhythmic drugs, and about 84% were taking anticoagulants.
‘A Tour de Force’
PREDIMAR was “really a tour de force,” Christine Albert, MD, MPH, chair of cardiology at the Smidt Heart Institute at the Cedars-Sinai Medical Center in Los Angeles, California, said during a commentary presented at HRS. “We talk about how we’re going to do these dietary interventions and weight loss and all the risk-factor reduction, and they pulled it off with 700 individuals and also did it in a way that was very novel.”
This is the first large-scale dietary intervention trial of patients with AF. However, Dr. Albert noted later in an interview, the Mediterranean diet poses potential challenges for some people with AF.
“The Mediterranean diet recommends that people drink wine, but then there’s clear evidence that abstinence from alcohol actually reduces recurrences of atrial fibrillation, so even though there are a lot of things about the Mediterranean diet that are probably healthy and good for atrial fibrillation, that aspect of it might be working against the patient,” she explained.
The finding that patients in the Mediterranean diet group experienced no significant weight loss could be counterintuitive when it comes to preventing AF. But “you could adapt the diet for AF,” Dr. Albert said. You could “leave out the wine and focus more on weight loss if the patient is obese because those are also the pillars of what we’ve learned for patients with atrial fibrillation.”
Making weight loss a key component of the study could be significant for the American population. “At least in the United States, that’s a huge part of the risk factors for atrial fibrillation after ablation,” she said.
The remote follow-up component of the PREDIMAR study is also intriguing. “I think what’s most exciting about what they did is, they showed they can do all these things remotely,” Dr. Albert added.
Dr. Barrio-Lopez had no relevant financial relationships. Dr. Albert disclosed relationships with Abbott, Roche Diagnostics, St. Jude Medical, Boston Scientific, Medtronic, and Element Science.
A version of this article appeared on Medscape.com.
FROM HRS 2024
Long COVID Can’t Be Solved Until We Decide What It Is
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
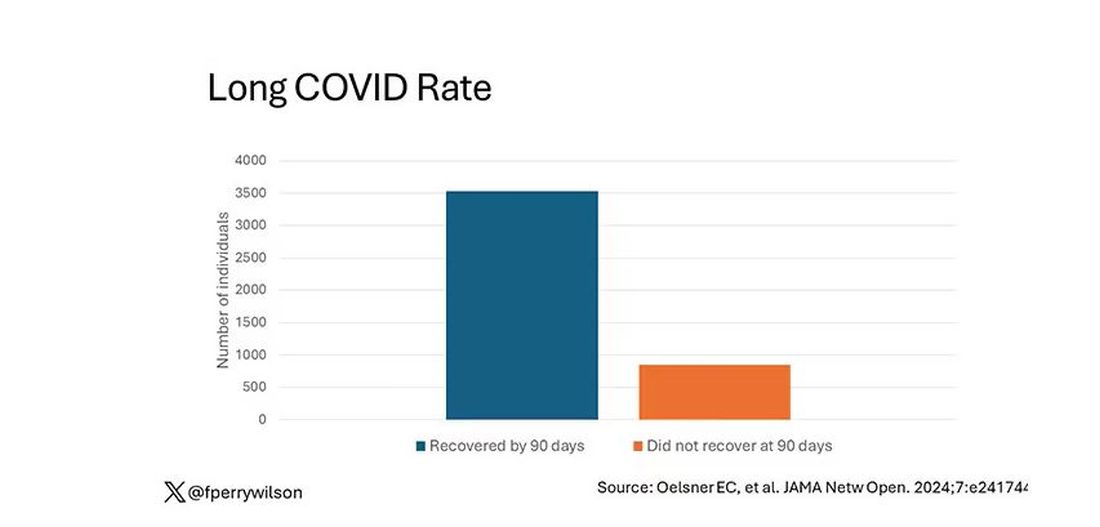
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
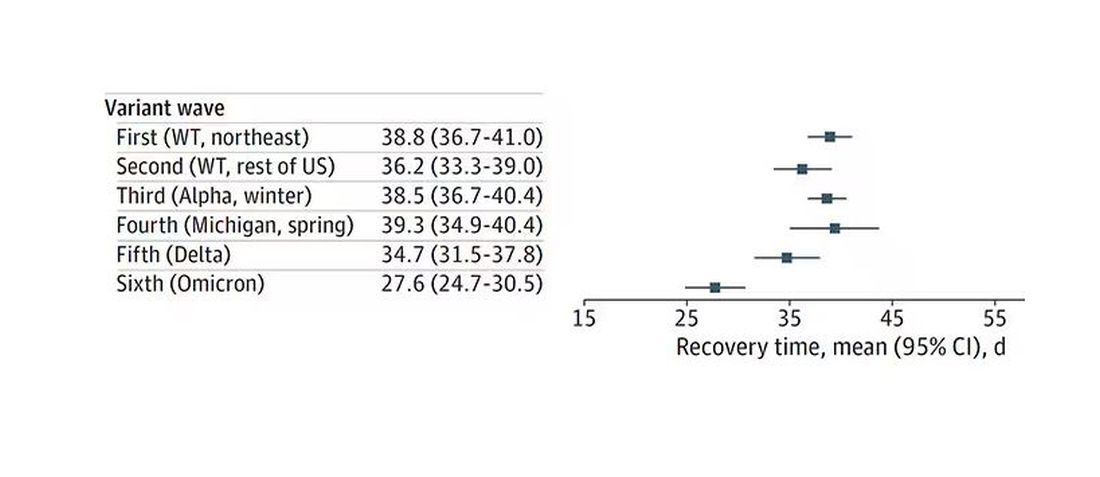
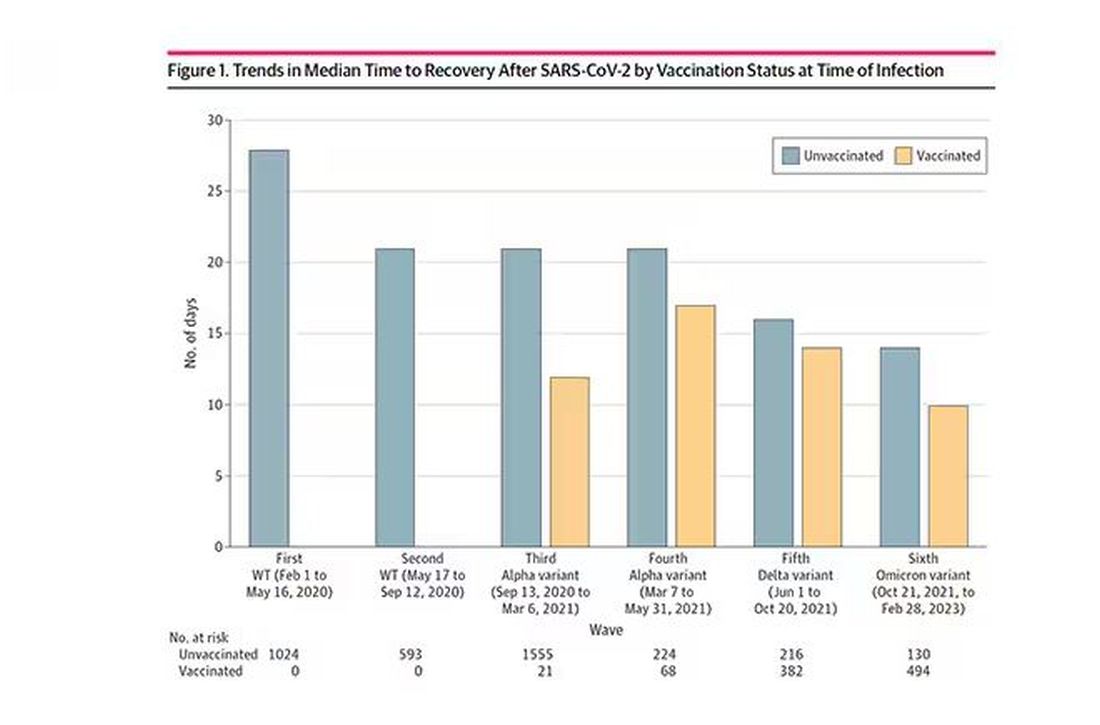
Recovery time was longer in the first waves of COVID than in the Omicron wave.
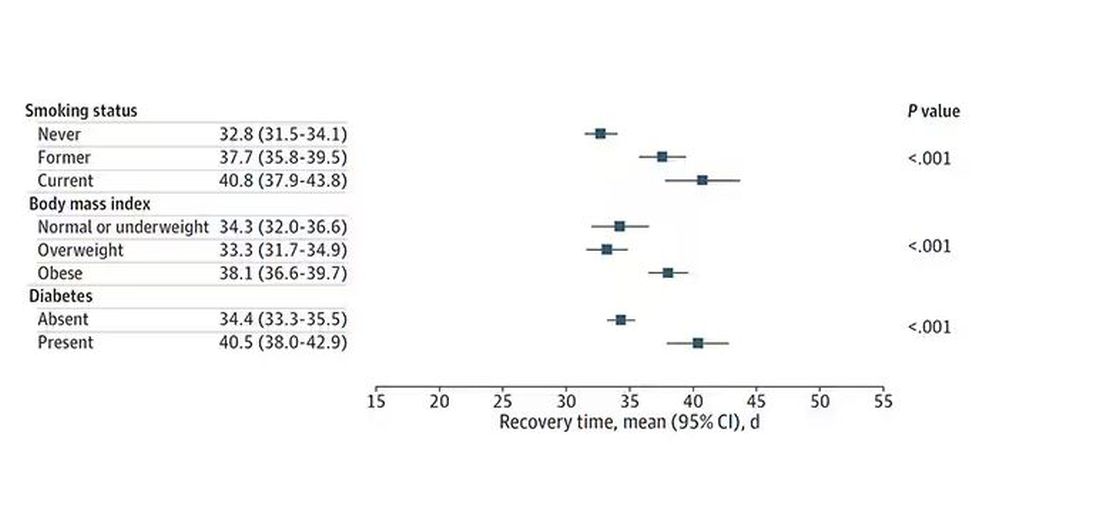
Recovery times were longer for smokers, those with diabetes, and those who were obese.
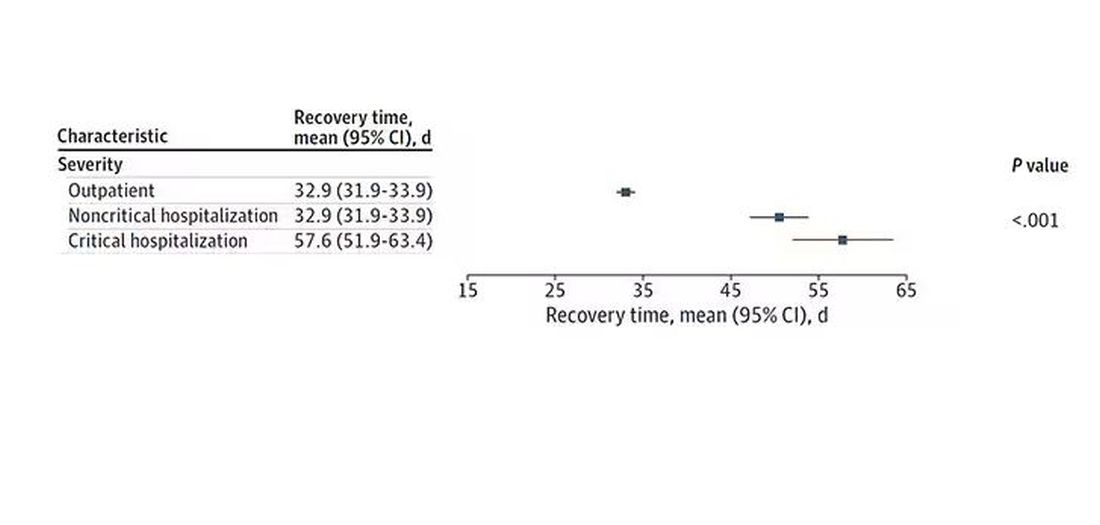
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here.
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
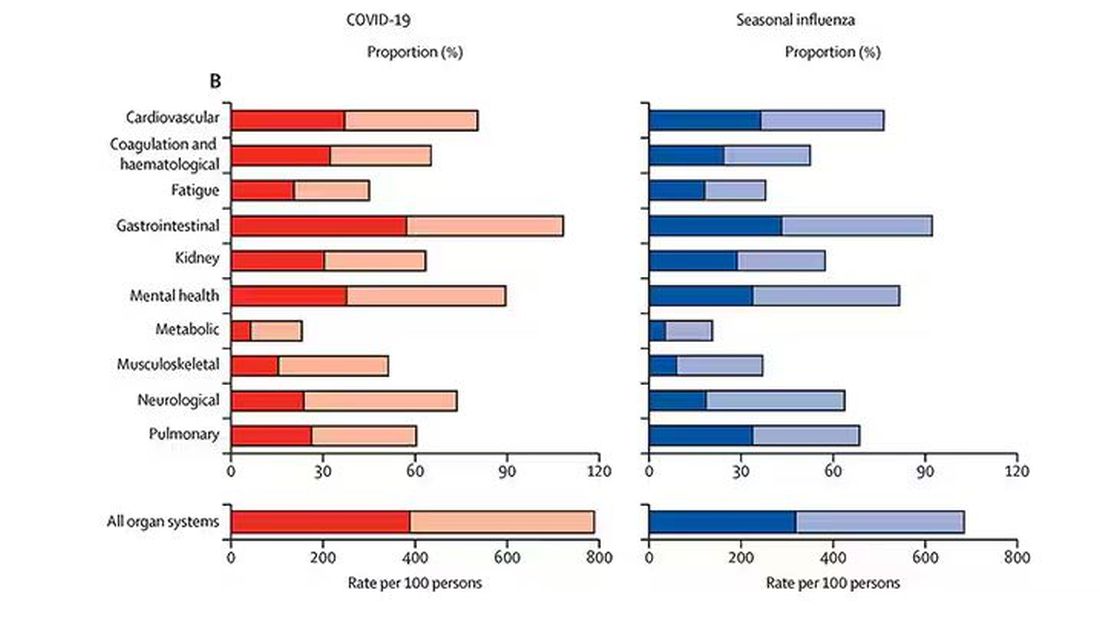
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here.
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to help people suffering from long COVID as much as anyone. But we have a real problem. In brief, we are being too inclusive. The first thing you learn, when you start studying the epidemiology of diseases, is that you need a good case definition. And our case definition for long COVID sucks. Just last week, the National Academies of Sciences, Engineering, and Medicine (NASEM) issued a definition of long COVID with the aim of “improving consistency, documentation, and treatment.” Good news, right? Here’s the definition: “Long COVID is an infection-associated chronic condition that occurs after SARS-CoV-2 infection and is present for at least 3 months as a continuous, relapsing and remitting, or progressive disease state that affects one or more organ systems.”
This is not helpful. The symptoms can be in any organ system, can be continuous or relapsing and remitting. Basically, if you’ve had COVID — and essentially all of us have by now — and you have any symptom, even one that comes and goes, 3 months after that, it’s long COVID. They don’t even specify that it has to be a new symptom.
And I have sort of a case study in this problem today, based on a paper getting a lot of press suggesting that one out of every five people has long COVID.
We are talking about this study, “Epidemiologic Features of Recovery From SARS-CoV-2 Infection,” appearing in JAMA Network Open this week. While I think the idea is important, the study really highlights why it can be so hard to study long COVID.
As part of efforts to understand long COVID, the National Institutes of Health (NIH) leveraged 14 of its ongoing cohort studies. The NIH has multiple longitudinal cohort studies that follow various groups of people over time. You may have heard of the REGARDS study, for example, which focuses on cardiovascular risks to people living in the southern United States. Or the ARIC study, which followed adults in four communities across the United States for the development of heart disease. All 14 of the cohorts in this study are long-running projects with ongoing data collection. So, it was not a huge lift to add some questions to the yearly surveys and studies the participants were already getting.
To wit: “Do you think that you have had COVID-19?” and “Would you say that you are completely recovered now?” Those who said they weren’t fully recovered were asked how long it had been since their infection, and anyone who answered with a duration > 90 days was considered to have long COVID.
So, we have self-report of infection, self-report of duration of symptoms, and self-report of recovery. This is fine, of course; individuals’ perceptions of their own health are meaningful. But the vagaries inherent in those perceptions are going to muddy the waters as we attempt to discover the true nature of the long COVID syndrome.
But let’s look at some results. Out of 4708 individuals studied, 842 (17.9%) had not recovered by 90 days.
This study included not only people hospitalized with COVID, as some prior long COVID studies did, but people self-diagnosed, tested at home, etc. This estimate is as reflective of the broader US population as we can get.
And there are some interesting trends here.
Recovery time was longer in the first waves of COVID than in the Omicron wave.
Recovery times were longer for smokers, those with diabetes, and those who were obese.
Recovery times were longer if the disease was more severe, in general. Though there is an unusual finding that women had longer recovery times despite their lower average severity of illness.
Vaccination was associated with shorter recovery times, as you can see here.
This is all quite interesting. It’s clear that people feel they are sick for a while after COVID. But we need to understand whether these symptoms are due to the lingering effects of a bad infection that knocks you down a peg, or to an ongoing syndrome — this thing we call long COVID — that has a physiologic basis and thus can be treated. And this study doesn’t help us much with that.
Not that this was the authors’ intention. This is a straight-up epidemiology study. But the problem is deeper than that. Let’s imagine that you want to really dig into this long COVID thing and get blood samples from people with it, ideally from controls with some other respiratory virus infection, and do all kinds of genetic and proteomic studies and stuff to really figure out what’s going on. Who do you enroll to be in the long COVID group? Do you enroll anyone who says they had COVID and still has some symptom more than 90 days after? You are going to find an awful lot of eligible people, and I guarantee that if there is a pathognomonic signature of long COVID, not all of them will have it.
And what about other respiratory viruses? This study in The Lancet Infectious Diseases compared long-term outcomes among hospitalized patients with COVID vs influenza. In general, the COVID outcomes are worse, but let’s not knock the concept of “long flu.” Across the board, roughly 50% of people report symptoms across any given organ system.
What this is all about is something called misclassification bias, a form of information bias that arises in a study where you label someone as diseased when they are not, or vice versa. If this happens at random, it’s bad; you’ve lost your ability to distinguish characteristics from the diseased and nondiseased population.
When it’s not random, it’s really bad. If we are more likely to misclassify women as having long COVID, for example, then it will appear that long COVID is more likely among women, or more likely among those with higher estrogen levels, or something. And that might simply be wrong.
I’m not saying that’s what happened here; this study does a really great job of what it set out to do, which was to describe the patterns of lingering symptoms after COVID. But we are not going to make progress toward understanding long COVID until we are less inclusive with our case definition. To paraphrase Syndrome from The Incredibles: If everyone has long COVID, then no one does.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA Approves New Pneumococcal Vaccine
A new vaccine to prevent invasive pneumococcal disease and pneumococcal pneumonia in adults has been approved by the Food and Drug Administration.
The injectable drug, Capvaxive (Pneumococcal 21-valent Conjugate Vaccine), protects against 22 serotypes that cause invasive pneumococcal disease in adults, the company said in a news release. These strains account for about 84% of invasive pneumococcal disease cases among adults aged 50 years or older and about 85% of these cases in adults aged 65 years or older.
The drug company said about 150,000 adults in the United States are hospitalized annually because of pneumococcal pneumonia.
“Many cases of adult disease are caused by serotypes not included in other approved pneumococcal conjugate vaccines,” Walter Orenstein, MD, a professor emeritus of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta, Georgia, and a member of Merck’s Scientific Advisory Committee, said in the release.
A draft agenda shows a Centers for Disease Control and Prevention (CDC) advisory panel will meet on June 27 to discuss the vaccine. If the committee votes to approve Capvaxive, the CDC director will decide whether to make it available across the country.
Testing showed that Capvaxive was well tolerated by people it was tested on, with the main reports being pain where they got the shot, fatigue, headaches, and muscle aches, Merck said.
The eight unique serotypes included in CAPVAXIVE will protect against invasive pneumococcal disease and pneumococcal pneumonia, not just pneumonia.
According to Reuters, Merck said Capvaxive has a wholesale acquisition price of $287 per dose, but most people will probably have access to it at no cost if the drug receives a routine CDC recommendation. Capvaxive’s main competition is expected to be Pfizer’s shot, Prevnar 20, which was approved in 2021 for use in adults aged 18 years or older, Reuters reported.
A version of this article appeared on Medscape.com.
A new vaccine to prevent invasive pneumococcal disease and pneumococcal pneumonia in adults has been approved by the Food and Drug Administration.
The injectable drug, Capvaxive (Pneumococcal 21-valent Conjugate Vaccine), protects against 22 serotypes that cause invasive pneumococcal disease in adults, the company said in a news release. These strains account for about 84% of invasive pneumococcal disease cases among adults aged 50 years or older and about 85% of these cases in adults aged 65 years or older.
The drug company said about 150,000 adults in the United States are hospitalized annually because of pneumococcal pneumonia.
“Many cases of adult disease are caused by serotypes not included in other approved pneumococcal conjugate vaccines,” Walter Orenstein, MD, a professor emeritus of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta, Georgia, and a member of Merck’s Scientific Advisory Committee, said in the release.
A draft agenda shows a Centers for Disease Control and Prevention (CDC) advisory panel will meet on June 27 to discuss the vaccine. If the committee votes to approve Capvaxive, the CDC director will decide whether to make it available across the country.
Testing showed that Capvaxive was well tolerated by people it was tested on, with the main reports being pain where they got the shot, fatigue, headaches, and muscle aches, Merck said.
The eight unique serotypes included in CAPVAXIVE will protect against invasive pneumococcal disease and pneumococcal pneumonia, not just pneumonia.
According to Reuters, Merck said Capvaxive has a wholesale acquisition price of $287 per dose, but most people will probably have access to it at no cost if the drug receives a routine CDC recommendation. Capvaxive’s main competition is expected to be Pfizer’s shot, Prevnar 20, which was approved in 2021 for use in adults aged 18 years or older, Reuters reported.
A version of this article appeared on Medscape.com.
A new vaccine to prevent invasive pneumococcal disease and pneumococcal pneumonia in adults has been approved by the Food and Drug Administration.
The injectable drug, Capvaxive (Pneumococcal 21-valent Conjugate Vaccine), protects against 22 serotypes that cause invasive pneumococcal disease in adults, the company said in a news release. These strains account for about 84% of invasive pneumococcal disease cases among adults aged 50 years or older and about 85% of these cases in adults aged 65 years or older.
The drug company said about 150,000 adults in the United States are hospitalized annually because of pneumococcal pneumonia.
“Many cases of adult disease are caused by serotypes not included in other approved pneumococcal conjugate vaccines,” Walter Orenstein, MD, a professor emeritus of medicine, epidemiology, global health, and pediatrics at Emory University, Atlanta, Georgia, and a member of Merck’s Scientific Advisory Committee, said in the release.
A draft agenda shows a Centers for Disease Control and Prevention (CDC) advisory panel will meet on June 27 to discuss the vaccine. If the committee votes to approve Capvaxive, the CDC director will decide whether to make it available across the country.
Testing showed that Capvaxive was well tolerated by people it was tested on, with the main reports being pain where they got the shot, fatigue, headaches, and muscle aches, Merck said.
The eight unique serotypes included in CAPVAXIVE will protect against invasive pneumococcal disease and pneumococcal pneumonia, not just pneumonia.
According to Reuters, Merck said Capvaxive has a wholesale acquisition price of $287 per dose, but most people will probably have access to it at no cost if the drug receives a routine CDC recommendation. Capvaxive’s main competition is expected to be Pfizer’s shot, Prevnar 20, which was approved in 2021 for use in adults aged 18 years or older, Reuters reported.
A version of this article appeared on Medscape.com.
Urgent Need for Better Care, New Policies to Lower Insomnia Burden
HOUSTON — A new analysis highlights the high burden of insomnia across the Americas, with about 17% of adults suffering from this chronic sleep disorder.
“Our findings underscore the urgent need for enhanced clinical care pathways and policy interventions to effectively diagnose and treat insomnia. It is crucial to foster greater awareness of the critical role that sleep plays in overall health,” lead investigator Adam Benjafield, PhD, vice president for medical affairs at ResMed, Sydney, Australia, said in an interview.
“Insomnia not only affects individuals’ health and quality of life but also has broader implications for public health systems. Developing comprehensive care strategies and promoting education about sleep health could significantly improve outcomes for individuals suffering from insomnia disorder,” Dr. Benjafield said.
The findings were presented at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies.
Underdiagnosed, Undertreated
Sleep disruptions contribute to various medical problems, including cognitive impairment, reduced immune function, metabolic imbalance, and exacerbation of psychiatric conditions. While the prevalence of insomnia in developed countries like the United States and Canada is known, there is limited epidemiologic evidence, and no reliable estimate for the disorder across the Americas — especially in low- and middle-income countries.
The researchers used published nation-specific data to estimate the prevalence of adult insomnia disorder across the 55 countries defined by the United Nations as comprising the Americas.
Based on the available data, the researchers estimated that about 123 million adults across the Americas have insomnia disorder (16.8%) — with greater prevalence in women (73 million, 19.5%) than in men (50 million, 14%).
The nations with the greatest burden of insomnia disorder are the United States (37 million), Brazil (29 million), and Mexico (16 million).
“While our study did not specifically investigate trends over time due to its scope, evidence from other research suggests that insomnia is becoming more prevalent over the long term. This growing trend highlights the increasing need for awareness and intervention in managing sleep health,” Dr. Benjafield said.
Insomnia is underdiagnosed and undertreated partly because of general lack of awareness about the importance of addressing sleep disorders and the fact that cognitive-behavioral therapy for insomnia (CBT-I), which is recommended as first-line treatment, is not widely accessible because of a shortage of trained CBT-I practitioners.
“Many individuals with insomnia struggle to find and receive this effective nonpharmacological treatment. Consequently, there is an overreliance on pharmaceutical solutions, which are ideally used for short-term management but are often extended due to the lack of alternatives. These medications can lead to dependency and other side effects,” Dr. Benjafield said.
Ask About Sleep
Insomnia symptoms are a “common presenting complaint in doctors’ offices in the United States. The percentages in this poster show that insomnia disorder has a similar, high percent prevalence across countries in the Americas,” Boris Gilyadov, MD, assistant professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“During preventive care visits and general physical exams, patients should be asked about the quality of their sleep. Patients may benefit from a referral to the sleep medicine clinic when appropriate,” said Dr. Gilyadov, who wasn’t involved in the study.
“CBT-I is the first-line treatment for chronic insomnia disorder and can be an effective treatment for most patients. An alternative to CBT-I, when it is not available, is digital CBT-I,” Dr. Gilyadov said.
“There are also behavioral therapies called BBT-I [brief behavioral treatment for insomnia] and ACT [acceptance and commitment therapy]. These are therapies that may be offered by psychologists who specialize in the treatment of chronic insomnia disorder,” Dr. Gilyadov noted.
The study was conducted in collaboration with medXcloud and funded by ResMed. Dr. Benjafield is an employee of ResMed. Dr. Gilyadov had no relevant disclosures.
A version of this article first appeared on Medscape.com.
HOUSTON — A new analysis highlights the high burden of insomnia across the Americas, with about 17% of adults suffering from this chronic sleep disorder.
“Our findings underscore the urgent need for enhanced clinical care pathways and policy interventions to effectively diagnose and treat insomnia. It is crucial to foster greater awareness of the critical role that sleep plays in overall health,” lead investigator Adam Benjafield, PhD, vice president for medical affairs at ResMed, Sydney, Australia, said in an interview.
“Insomnia not only affects individuals’ health and quality of life but also has broader implications for public health systems. Developing comprehensive care strategies and promoting education about sleep health could significantly improve outcomes for individuals suffering from insomnia disorder,” Dr. Benjafield said.
The findings were presented at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies.
Underdiagnosed, Undertreated
Sleep disruptions contribute to various medical problems, including cognitive impairment, reduced immune function, metabolic imbalance, and exacerbation of psychiatric conditions. While the prevalence of insomnia in developed countries like the United States and Canada is known, there is limited epidemiologic evidence, and no reliable estimate for the disorder across the Americas — especially in low- and middle-income countries.
The researchers used published nation-specific data to estimate the prevalence of adult insomnia disorder across the 55 countries defined by the United Nations as comprising the Americas.
Based on the available data, the researchers estimated that about 123 million adults across the Americas have insomnia disorder (16.8%) — with greater prevalence in women (73 million, 19.5%) than in men (50 million, 14%).
The nations with the greatest burden of insomnia disorder are the United States (37 million), Brazil (29 million), and Mexico (16 million).
“While our study did not specifically investigate trends over time due to its scope, evidence from other research suggests that insomnia is becoming more prevalent over the long term. This growing trend highlights the increasing need for awareness and intervention in managing sleep health,” Dr. Benjafield said.
Insomnia is underdiagnosed and undertreated partly because of general lack of awareness about the importance of addressing sleep disorders and the fact that cognitive-behavioral therapy for insomnia (CBT-I), which is recommended as first-line treatment, is not widely accessible because of a shortage of trained CBT-I practitioners.
“Many individuals with insomnia struggle to find and receive this effective nonpharmacological treatment. Consequently, there is an overreliance on pharmaceutical solutions, which are ideally used for short-term management but are often extended due to the lack of alternatives. These medications can lead to dependency and other side effects,” Dr. Benjafield said.
Ask About Sleep
Insomnia symptoms are a “common presenting complaint in doctors’ offices in the United States. The percentages in this poster show that insomnia disorder has a similar, high percent prevalence across countries in the Americas,” Boris Gilyadov, MD, assistant professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“During preventive care visits and general physical exams, patients should be asked about the quality of their sleep. Patients may benefit from a referral to the sleep medicine clinic when appropriate,” said Dr. Gilyadov, who wasn’t involved in the study.
“CBT-I is the first-line treatment for chronic insomnia disorder and can be an effective treatment for most patients. An alternative to CBT-I, when it is not available, is digital CBT-I,” Dr. Gilyadov said.
“There are also behavioral therapies called BBT-I [brief behavioral treatment for insomnia] and ACT [acceptance and commitment therapy]. These are therapies that may be offered by psychologists who specialize in the treatment of chronic insomnia disorder,” Dr. Gilyadov noted.
The study was conducted in collaboration with medXcloud and funded by ResMed. Dr. Benjafield is an employee of ResMed. Dr. Gilyadov had no relevant disclosures.
A version of this article first appeared on Medscape.com.
HOUSTON — A new analysis highlights the high burden of insomnia across the Americas, with about 17% of adults suffering from this chronic sleep disorder.
“Our findings underscore the urgent need for enhanced clinical care pathways and policy interventions to effectively diagnose and treat insomnia. It is crucial to foster greater awareness of the critical role that sleep plays in overall health,” lead investigator Adam Benjafield, PhD, vice president for medical affairs at ResMed, Sydney, Australia, said in an interview.
“Insomnia not only affects individuals’ health and quality of life but also has broader implications for public health systems. Developing comprehensive care strategies and promoting education about sleep health could significantly improve outcomes for individuals suffering from insomnia disorder,” Dr. Benjafield said.
The findings were presented at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies.
Underdiagnosed, Undertreated
Sleep disruptions contribute to various medical problems, including cognitive impairment, reduced immune function, metabolic imbalance, and exacerbation of psychiatric conditions. While the prevalence of insomnia in developed countries like the United States and Canada is known, there is limited epidemiologic evidence, and no reliable estimate for the disorder across the Americas — especially in low- and middle-income countries.
The researchers used published nation-specific data to estimate the prevalence of adult insomnia disorder across the 55 countries defined by the United Nations as comprising the Americas.
Based on the available data, the researchers estimated that about 123 million adults across the Americas have insomnia disorder (16.8%) — with greater prevalence in women (73 million, 19.5%) than in men (50 million, 14%).
The nations with the greatest burden of insomnia disorder are the United States (37 million), Brazil (29 million), and Mexico (16 million).
“While our study did not specifically investigate trends over time due to its scope, evidence from other research suggests that insomnia is becoming more prevalent over the long term. This growing trend highlights the increasing need for awareness and intervention in managing sleep health,” Dr. Benjafield said.
Insomnia is underdiagnosed and undertreated partly because of general lack of awareness about the importance of addressing sleep disorders and the fact that cognitive-behavioral therapy for insomnia (CBT-I), which is recommended as first-line treatment, is not widely accessible because of a shortage of trained CBT-I practitioners.
“Many individuals with insomnia struggle to find and receive this effective nonpharmacological treatment. Consequently, there is an overreliance on pharmaceutical solutions, which are ideally used for short-term management but are often extended due to the lack of alternatives. These medications can lead to dependency and other side effects,” Dr. Benjafield said.
Ask About Sleep
Insomnia symptoms are a “common presenting complaint in doctors’ offices in the United States. The percentages in this poster show that insomnia disorder has a similar, high percent prevalence across countries in the Americas,” Boris Gilyadov, MD, assistant professor of pulmonary, critical care, and sleep medicine at the Icahn School of Medicine at Mount Sinai in New York City, said in an interview.
“During preventive care visits and general physical exams, patients should be asked about the quality of their sleep. Patients may benefit from a referral to the sleep medicine clinic when appropriate,” said Dr. Gilyadov, who wasn’t involved in the study.
“CBT-I is the first-line treatment for chronic insomnia disorder and can be an effective treatment for most patients. An alternative to CBT-I, when it is not available, is digital CBT-I,” Dr. Gilyadov said.
“There are also behavioral therapies called BBT-I [brief behavioral treatment for insomnia] and ACT [acceptance and commitment therapy]. These are therapies that may be offered by psychologists who specialize in the treatment of chronic insomnia disorder,” Dr. Gilyadov noted.
The study was conducted in collaboration with medXcloud and funded by ResMed. Dr. Benjafield is an employee of ResMed. Dr. Gilyadov had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2024
Doctors Endorsing Products on X May Not Disclose Company Ties
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Lead author Aaron Mitchell, MD, MPH, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York City, told this news organization that he and his colleagues undertook the study in part to see whether physicians were adhering to professional and industry guidelines regarding marketing communications.
The team reviewed posts by physicians on X during 2022, looking for key words that might indicate that the posts were intended as endorsements of a product. The researchers then delved into the Centers for Medicare and Medicaid Services Open Payments database to see how many of those identified as having endorsed a product were paid by the manufacturers.
What Dr. Mitchell found concerned him, he said.
Overall, the researchers identified 28 physician endorsers who received a total of $1.4 million from sponsors in 2022. Among these, 26 physicians (93%) received payments from the product’s manufacturer, totaling $713,976, and 24 physicians (86%) accepted payments related to the endorsed drug or device, totaling $492,098.
While most did disclose that the posts were sponsored — by adding the word “sponsored” or using #sponsored — nine physicians did not.
Although 28 physician endorsers represent a “small fraction” of the overall number of physicians who use X, each endorsement was ultimately posted dozens, if not hundreds of times, said Dr. Mitchell. In fact, he said he saw the same particular endorsement post every time he opened his X app for months.
Overall, Dr. Mitchell noted that it’s less about the fact that the endorsements are occurring on social media and more that there are these paid endorsements taking place at all.
Among the physician specialties promoting a product, urologists and oncologists dominated. Almost one third were urologists, and 57% were oncologists — six medical oncologists, six radiation oncologists, and four gynecologic oncologists. Of the remaining three physicians, two were internists and one was a pulmonary and critical care medicine specialist.
The authors tracked posts from physicians and industry accounts. Many of the posts on industry accounts were physician testimonials, usually videos. Almost half — 8 of 17 — of those testimonials did not disclose that the doctor was being paid by the manufacturer. In another case, a physician did not disclose that they were paid to endorse a white paper.
Fifteen promotional posts were for a Boston Scientific product, followed by six for GlaxoSmithKline, two for Eisai, two for Exelixis, and one each for AstraZeneca, Novartis, and Pfizer.
In general, Dr. Mitchell said, industry guidelines suggest that manufacturer-paid speakers or consultants should have well-regarded expertise in the area they are being asked to weigh in on, but most physician endorsers in the study were not key opinion leaders or experts.
The authors examined the paid endorsers’ H-index — a measure of academic productivity provided by Scopus. Overall, 19 of the 28 physicians had an H-index below 20, which is considered less accomplished, and 14 had no published research related to the endorsed product.
Ten received payments from manufacturers for research purposes, and only one received research payments related to the endorsed product ($224,577).
“Physicians’ participation in industry marketing raises questions regarding professionalism and their responsibilities as patient advocates,” the JAMA authors wrote.
The study was supported by grants from the National Cancer Institute. Dr. Mitchell reported no relevant financial relationships. Coauthors Samer Al Hadidi, MD, reported receiving personal fees from Pfizer, Sanofi, and Janssen during the conduct of the study, and Timothy S. Anderson, MD, reported receiving grants from the National Institute on Aging, the American Heart Association, and the American College of Cardiology, and receiving consulting fees from the American Medical Student Association. Dr. Anderson is also an associate editor of JAMA Internal Medicine.
A version of this article appeared on Medscape.com.
Delays After Tests for Suspected Heart Failure ‘a Scandal’
LISBON, PORTUGAL — Few people with suspected heart failure and elevated N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels are receiving a diagnosis after a year, reported investigators, who say high rates of hospitalization are common.
Presenting here at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2024, researchers shared results from the REVOLUTION-HF study involving almost 8000 people who consulted outpatient primary and secondary care over a 5-year period.
The outcomes were even worse in patients with high NT-proBNP levels.
Patients with suspected heart failure are “waiting far too long to see a specialist, and that results in a delay to guideline-directed medical therapy, despite the fact that we’re perfectly happy to slap them all on diuretics,” said study presenter Lisa Anderson, MD, PhD, Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Research Institute, St George’s Hospital, University of London, England.
“We need to rethink our management of heart failure patients presenting in the community,” she said.
A big gap exists internationally between presentation with heart failure, an elevated NT-proBNP, and confirmatory specialist assessment, she explained.
“It’s a scandal that patients are coming to the GP with signs and symptoms of heart failure, they get tested for natriuretic peptides, and nothing happens,” said co-author Antoni Bayés-Genís, MD, PhD, Heart Institute director, Hospital Universitari Germans Trias i Pujol Catedràtic, Barcelona, Spain.
“These patients may receive an echo, or not, in the coming 12 months,” and “during these 12 months, there is a huge number of heart failure hospitalizations and deaths that could probably be prevented.”
Why the Reluctance to Diagnose?
Many issues get in the way of early diagnosis, Dr. Bayés-Genís said. “Inertia, comorbidities, ageism.”
A lot of patients with heart failure are elderly women with some degree of weight gain, he said. “And they come to the clinic with fatigue, so we tell them, ‘Well, that’s normal.”
But “it may not be normal,” he added. “This is a very important topic that we, as a society, need to address.”
There are several “misconceptions” about heart failure, said Ileana L. Piña, MD, MPH, the Robert Stein Chair for Quality and Safety, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, who was not involved in the study.
For example, “we’re all convinced that guideline-directed medical therapy works,” but the evidence is only for patients “with a diagnosis.” In addition, “millions of patients get tested” for heart failure, but they already have a “known diagnosis.”
“When we study these drugs, we’re studying them on patients with manifest disease,” who are only then randomized, Dr. Piña said. “But we seldom see them while they’re developing heart failure. And it’s a process; it doesn’t happen overnight.”
Patients initially often think they may have asthma, and so what follows is an extended period of “uncertainty” and “important time lost” before they finally undergo the assessments that show that they have heart failure, she said.
However, “uncertainty” often lands a patient “in the emergency room or with an unscheduled office visit, where NT-proBNP might get ordered and there’s a long lineup for an echo.”
There are several strengths of the current study, Dr. Piña said, including the fact that 50% of the study population were women, and they were older than a typical trial population. Nevertheless, the results were “eye-opening but not surprising” and, in the end, “disappointing.”
“I agree, we need a revolution, Dr. Anderson,” Dr. Piña said. “The revolution of paying attention to the NT-proBNP when you get it and it’s elevated” and then following through with echocardiography and starting “guideline-directed medical therapy early.”
The diagnosis of heart failure “relies on the presentation of patients with nonspecific signs and symptoms,” such as dyspnea and peripheral edema, “but initiation of guideline-directed medical therapy — life-saving treatment — has to wait until we have a formal echocardiography and specialist clinician assessment,” Dr. Anderson said.
The latest clinical consensus statement from the Heart Failure Association “proposes both rule-in and rule-out NT-proBNP levels for heart failure diagnosis, and obviously we all recognize that it’s important to treat patients as soon as they’re diagnosed,” she explained.
REVOLUTION-HF
To examine the risk profile for patients presenting to outpatient care with suspected heart failure, the researchers conducted REVOLUTION-HF, which leveraged nationwide Swedish linked data from general practices, specialists, pharmacies, hospitals, and cause of death registers.
“Really impressively, most of these NT-proBNP tests were coming back within a day,” Dr. Anderson said, “so a really, really good turnaround.”
Individuals were excluded if they had an inpatient admission, echocardiography, or heart failure diagnosis between presentation and the NT-proBNP measurement.
These people were then compared with those presenting to primary or secondary outpatient care for any reason and matched for age, sex, care level, and index year. Both groups were followed up for 1 year.
“Despite this really impressive, almost immediate NT-proBNP testing,” the waiting times to undergo echocardiography were “really disappointing,” Dr. Anderson said.
The median time to first registered echocardiography was 40 days, and only 29% of patients with suspected heart failure received a diagnosis within a year of the index presentation date, which she described as “inadequately slow.”
“And how does this translate to medical therapy?” she asked.
Heart Failure Drugs
After the index presentation, the rate of loop diuretic use quadrupled among individuals suspected of having heart failure, but there was a “muted response” when it came to the prescribing of beta-blockers and the other pillars of heart failure therapy, which Dr. Anderson called “very disappointing.”
For outcomes after the index presentation, the rate of hospitalization was much higher in the group with suspected heart failure than in the control group (16.1 vs 2.2 events per 100 person-years). And all-cause mortality occurred more often in the group with suspected heart failure than in the control group (10.3 vs 6.5 events per 100 person-years).
Among patients with NT-proBNP levels of 2000 ng/L, there was a “rapid” onset of hospitalization “within the first few days” of the index presentation, which was tracked by a more linear rise in all-cause deaths, Dr. Anderson reported.
In the United Kingdom, “we are very proud of our 2- and 6-week pathways,” which stipulate that suspected heart failure patients with NT-proBNP levels between 400 and 2000 ng/L are to have a specialist assessment and transthoracic echocardiography within 6 weeks; for those with levels > 2000 ng/L, that interval is accelerated to 2 weeks, she said.
The current results show that “2 weeks is too slow.” And looking at the rest of the cohort with lower NT-proBNP levels, “patients have already been admitted and died” by 6 weeks, she said.
When patients are stratified by age, “you get exactly what you would expect,” Dr. Anderson said. “The older patients are the most at risk” for both hospitalization and all-cause mortality.
A version of this article appeared on Medscape.com.
LISBON, PORTUGAL — Few people with suspected heart failure and elevated N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels are receiving a diagnosis after a year, reported investigators, who say high rates of hospitalization are common.
Presenting here at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2024, researchers shared results from the REVOLUTION-HF study involving almost 8000 people who consulted outpatient primary and secondary care over a 5-year period.
The outcomes were even worse in patients with high NT-proBNP levels.
Patients with suspected heart failure are “waiting far too long to see a specialist, and that results in a delay to guideline-directed medical therapy, despite the fact that we’re perfectly happy to slap them all on diuretics,” said study presenter Lisa Anderson, MD, PhD, Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Research Institute, St George’s Hospital, University of London, England.
“We need to rethink our management of heart failure patients presenting in the community,” she said.
A big gap exists internationally between presentation with heart failure, an elevated NT-proBNP, and confirmatory specialist assessment, she explained.
“It’s a scandal that patients are coming to the GP with signs and symptoms of heart failure, they get tested for natriuretic peptides, and nothing happens,” said co-author Antoni Bayés-Genís, MD, PhD, Heart Institute director, Hospital Universitari Germans Trias i Pujol Catedràtic, Barcelona, Spain.
“These patients may receive an echo, or not, in the coming 12 months,” and “during these 12 months, there is a huge number of heart failure hospitalizations and deaths that could probably be prevented.”
Why the Reluctance to Diagnose?
Many issues get in the way of early diagnosis, Dr. Bayés-Genís said. “Inertia, comorbidities, ageism.”
A lot of patients with heart failure are elderly women with some degree of weight gain, he said. “And they come to the clinic with fatigue, so we tell them, ‘Well, that’s normal.”
But “it may not be normal,” he added. “This is a very important topic that we, as a society, need to address.”
There are several “misconceptions” about heart failure, said Ileana L. Piña, MD, MPH, the Robert Stein Chair for Quality and Safety, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, who was not involved in the study.
For example, “we’re all convinced that guideline-directed medical therapy works,” but the evidence is only for patients “with a diagnosis.” In addition, “millions of patients get tested” for heart failure, but they already have a “known diagnosis.”
“When we study these drugs, we’re studying them on patients with manifest disease,” who are only then randomized, Dr. Piña said. “But we seldom see them while they’re developing heart failure. And it’s a process; it doesn’t happen overnight.”
Patients initially often think they may have asthma, and so what follows is an extended period of “uncertainty” and “important time lost” before they finally undergo the assessments that show that they have heart failure, she said.
However, “uncertainty” often lands a patient “in the emergency room or with an unscheduled office visit, where NT-proBNP might get ordered and there’s a long lineup for an echo.”
There are several strengths of the current study, Dr. Piña said, including the fact that 50% of the study population were women, and they were older than a typical trial population. Nevertheless, the results were “eye-opening but not surprising” and, in the end, “disappointing.”
“I agree, we need a revolution, Dr. Anderson,” Dr. Piña said. “The revolution of paying attention to the NT-proBNP when you get it and it’s elevated” and then following through with echocardiography and starting “guideline-directed medical therapy early.”
The diagnosis of heart failure “relies on the presentation of patients with nonspecific signs and symptoms,” such as dyspnea and peripheral edema, “but initiation of guideline-directed medical therapy — life-saving treatment — has to wait until we have a formal echocardiography and specialist clinician assessment,” Dr. Anderson said.
The latest clinical consensus statement from the Heart Failure Association “proposes both rule-in and rule-out NT-proBNP levels for heart failure diagnosis, and obviously we all recognize that it’s important to treat patients as soon as they’re diagnosed,” she explained.
REVOLUTION-HF
To examine the risk profile for patients presenting to outpatient care with suspected heart failure, the researchers conducted REVOLUTION-HF, which leveraged nationwide Swedish linked data from general practices, specialists, pharmacies, hospitals, and cause of death registers.
“Really impressively, most of these NT-proBNP tests were coming back within a day,” Dr. Anderson said, “so a really, really good turnaround.”
Individuals were excluded if they had an inpatient admission, echocardiography, or heart failure diagnosis between presentation and the NT-proBNP measurement.
These people were then compared with those presenting to primary or secondary outpatient care for any reason and matched for age, sex, care level, and index year. Both groups were followed up for 1 year.
“Despite this really impressive, almost immediate NT-proBNP testing,” the waiting times to undergo echocardiography were “really disappointing,” Dr. Anderson said.
The median time to first registered echocardiography was 40 days, and only 29% of patients with suspected heart failure received a diagnosis within a year of the index presentation date, which she described as “inadequately slow.”
“And how does this translate to medical therapy?” she asked.
Heart Failure Drugs
After the index presentation, the rate of loop diuretic use quadrupled among individuals suspected of having heart failure, but there was a “muted response” when it came to the prescribing of beta-blockers and the other pillars of heart failure therapy, which Dr. Anderson called “very disappointing.”
For outcomes after the index presentation, the rate of hospitalization was much higher in the group with suspected heart failure than in the control group (16.1 vs 2.2 events per 100 person-years). And all-cause mortality occurred more often in the group with suspected heart failure than in the control group (10.3 vs 6.5 events per 100 person-years).
Among patients with NT-proBNP levels of 2000 ng/L, there was a “rapid” onset of hospitalization “within the first few days” of the index presentation, which was tracked by a more linear rise in all-cause deaths, Dr. Anderson reported.
In the United Kingdom, “we are very proud of our 2- and 6-week pathways,” which stipulate that suspected heart failure patients with NT-proBNP levels between 400 and 2000 ng/L are to have a specialist assessment and transthoracic echocardiography within 6 weeks; for those with levels > 2000 ng/L, that interval is accelerated to 2 weeks, she said.
The current results show that “2 weeks is too slow.” And looking at the rest of the cohort with lower NT-proBNP levels, “patients have already been admitted and died” by 6 weeks, she said.
When patients are stratified by age, “you get exactly what you would expect,” Dr. Anderson said. “The older patients are the most at risk” for both hospitalization and all-cause mortality.
A version of this article appeared on Medscape.com.
LISBON, PORTUGAL — Few people with suspected heart failure and elevated N-terminal prohormone of brain natriuretic peptide (NT-proBNP) levels are receiving a diagnosis after a year, reported investigators, who say high rates of hospitalization are common.
Presenting here at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2024, researchers shared results from the REVOLUTION-HF study involving almost 8000 people who consulted outpatient primary and secondary care over a 5-year period.
The outcomes were even worse in patients with high NT-proBNP levels.
Patients with suspected heart failure are “waiting far too long to see a specialist, and that results in a delay to guideline-directed medical therapy, despite the fact that we’re perfectly happy to slap them all on diuretics,” said study presenter Lisa Anderson, MD, PhD, Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Research Institute, St George’s Hospital, University of London, England.
“We need to rethink our management of heart failure patients presenting in the community,” she said.
A big gap exists internationally between presentation with heart failure, an elevated NT-proBNP, and confirmatory specialist assessment, she explained.
“It’s a scandal that patients are coming to the GP with signs and symptoms of heart failure, they get tested for natriuretic peptides, and nothing happens,” said co-author Antoni Bayés-Genís, MD, PhD, Heart Institute director, Hospital Universitari Germans Trias i Pujol Catedràtic, Barcelona, Spain.
“These patients may receive an echo, or not, in the coming 12 months,” and “during these 12 months, there is a huge number of heart failure hospitalizations and deaths that could probably be prevented.”
Why the Reluctance to Diagnose?
Many issues get in the way of early diagnosis, Dr. Bayés-Genís said. “Inertia, comorbidities, ageism.”
A lot of patients with heart failure are elderly women with some degree of weight gain, he said. “And they come to the clinic with fatigue, so we tell them, ‘Well, that’s normal.”
But “it may not be normal,” he added. “This is a very important topic that we, as a society, need to address.”
There are several “misconceptions” about heart failure, said Ileana L. Piña, MD, MPH, the Robert Stein Chair for Quality and Safety, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, who was not involved in the study.
For example, “we’re all convinced that guideline-directed medical therapy works,” but the evidence is only for patients “with a diagnosis.” In addition, “millions of patients get tested” for heart failure, but they already have a “known diagnosis.”
“When we study these drugs, we’re studying them on patients with manifest disease,” who are only then randomized, Dr. Piña said. “But we seldom see them while they’re developing heart failure. And it’s a process; it doesn’t happen overnight.”
Patients initially often think they may have asthma, and so what follows is an extended period of “uncertainty” and “important time lost” before they finally undergo the assessments that show that they have heart failure, she said.
However, “uncertainty” often lands a patient “in the emergency room or with an unscheduled office visit, where NT-proBNP might get ordered and there’s a long lineup for an echo.”
There are several strengths of the current study, Dr. Piña said, including the fact that 50% of the study population were women, and they were older than a typical trial population. Nevertheless, the results were “eye-opening but not surprising” and, in the end, “disappointing.”
“I agree, we need a revolution, Dr. Anderson,” Dr. Piña said. “The revolution of paying attention to the NT-proBNP when you get it and it’s elevated” and then following through with echocardiography and starting “guideline-directed medical therapy early.”
The diagnosis of heart failure “relies on the presentation of patients with nonspecific signs and symptoms,” such as dyspnea and peripheral edema, “but initiation of guideline-directed medical therapy — life-saving treatment — has to wait until we have a formal echocardiography and specialist clinician assessment,” Dr. Anderson said.
The latest clinical consensus statement from the Heart Failure Association “proposes both rule-in and rule-out NT-proBNP levels for heart failure diagnosis, and obviously we all recognize that it’s important to treat patients as soon as they’re diagnosed,” she explained.
REVOLUTION-HF
To examine the risk profile for patients presenting to outpatient care with suspected heart failure, the researchers conducted REVOLUTION-HF, which leveraged nationwide Swedish linked data from general practices, specialists, pharmacies, hospitals, and cause of death registers.
“Really impressively, most of these NT-proBNP tests were coming back within a day,” Dr. Anderson said, “so a really, really good turnaround.”
Individuals were excluded if they had an inpatient admission, echocardiography, or heart failure diagnosis between presentation and the NT-proBNP measurement.
These people were then compared with those presenting to primary or secondary outpatient care for any reason and matched for age, sex, care level, and index year. Both groups were followed up for 1 year.
“Despite this really impressive, almost immediate NT-proBNP testing,” the waiting times to undergo echocardiography were “really disappointing,” Dr. Anderson said.
The median time to first registered echocardiography was 40 days, and only 29% of patients with suspected heart failure received a diagnosis within a year of the index presentation date, which she described as “inadequately slow.”
“And how does this translate to medical therapy?” she asked.
Heart Failure Drugs
After the index presentation, the rate of loop diuretic use quadrupled among individuals suspected of having heart failure, but there was a “muted response” when it came to the prescribing of beta-blockers and the other pillars of heart failure therapy, which Dr. Anderson called “very disappointing.”
For outcomes after the index presentation, the rate of hospitalization was much higher in the group with suspected heart failure than in the control group (16.1 vs 2.2 events per 100 person-years). And all-cause mortality occurred more often in the group with suspected heart failure than in the control group (10.3 vs 6.5 events per 100 person-years).
Among patients with NT-proBNP levels of 2000 ng/L, there was a “rapid” onset of hospitalization “within the first few days” of the index presentation, which was tracked by a more linear rise in all-cause deaths, Dr. Anderson reported.
In the United Kingdom, “we are very proud of our 2- and 6-week pathways,” which stipulate that suspected heart failure patients with NT-proBNP levels between 400 and 2000 ng/L are to have a specialist assessment and transthoracic echocardiography within 6 weeks; for those with levels > 2000 ng/L, that interval is accelerated to 2 weeks, she said.
The current results show that “2 weeks is too slow.” And looking at the rest of the cohort with lower NT-proBNP levels, “patients have already been admitted and died” by 6 weeks, she said.
When patients are stratified by age, “you get exactly what you would expect,” Dr. Anderson said. “The older patients are the most at risk” for both hospitalization and all-cause mortality.
A version of this article appeared on Medscape.com.
FROM HFA-ESC 2024
Surgeons Most Likely to Behave Unprofessionally: Study
Most doctors mind their manners. But surgeons are the most likely to be reported for unprofessional behavior, while physicians practicing in pediatric settings are the least likely, according to a recent study of more than 35,000 physicians.
The research, published on June 6 in JAMA Network Open, found that fewer than 10% of physicians were reported by their coworkers for at least one instance of unprofessional behavior, and only 1% showed a pattern of such reports.
Data were gathered from the Center for Patient and Professional Advocacy’s (CPPA’s) Coworker Observation Reporting System (CORS) program, a national collaborative in which 193 participating hospitals and practice sites file safety-event reports involving medical workers’ unprofessional behaviors. An algorithm that weights CORS reports based on recency and severity was used to analyze the data. The study was spearheaded by William O. Cooper, MD, MPH, director of the CPPA at Vanderbilt University Medical Center, Nashville, Tennessee.
The retrospective cohort study included deidentified data on credentialed physicians, not including residents or fellows, who practiced at a CORS site between 2018 and 2022.
Why Surgeons?
The authors speculated that the reason surgeons were reported for unprofessional behavior more often than their colleagues in nonsurgical specialties was because surgery is a more stressful environment than other specialties and requires more teamwork, resulting in more interactions during high-stakes events.
Daniel Katz, MD, professor and vice chair of education for the Department of Anesthesiology, Perioperative and Pain Medicine at the Icahn School of Medicine at Mount Sinai, New York City, added that part of the problem is that surgeons are expected to perform at very high levels all the time.
“When things that are outside the control of the surgeon don’t go well,” Dr. Katz said, “that can lead to increased frustration and negative emotions, which will then bring out these kinds of behaviors.”
Types of Unprofessional Behaviors
The most common out-of-bounds behaviors reported involved disrespectful communication or lack of professional responsibility. In one example, a physician called a coworker a “bossy cow” when the coworker reminded the physician of the need to do a timeout before beginning a bronchoscopy.
In another case involving professional responsibility, a coworker asked a physician if the team should wait for a disoriented patient’s spouse to arrive. The doctor’s response: “We’ll be here all night if we do that. If you won’t sign as a witness, I’ll get someone else who will.”
The least common reports involved unprofessionalism related to medical care or professional integrity. One cited a physician removing a Foley catheter without wearing gloves and having visible urine on his hands and not washing them before touching other things in the room. In a reported lapse of professional integrity, a physician billed at level five after spending only 4 minutes with a patient.
Impact of Unprofessional Behavior
Unprofessional behavior among physicians is more than just unpleasant. It can threaten the functioning of teams and increase patient complications. In addition, individuals who model unprofessional behaviors are associated with increased malpractice claims, the study’s authors wrote.
Dr. Katz agreed that unprofessional behavior is damaging to both patients and the profession as a whole.
However, this doesn’t happen because some doctors are bad, he said. Physicians today are working in a pressure cooker. The current healthcare environment, with its increased administrative burdens, lack of staffing, and other problems, has increased the overall level of stress and led to burnout among healthcare personnel.
“You have to fix the system to create a working environment that doesn’t cause somebody to explode,” Dr. Katz said.
The goal of the CORS program and this study, Dr. Cooper said, is to help physicians better weather these stresses.
Study Limitations
The authors noted some weaknesses in the study. Some unprofessional behavior may go unreported because of fear of retaliation or for other reasons victims or witnesses did not feel safe to report their colleagues. Also, reports were not evaluated to ensure the truth of the accusations. The records reviewed did not include the gender of the physician, though the researchers pointed out that previous studies have shown that women are less likely than men to receive CORS reports.
A version of this article appeared on Medscape.com.
Most doctors mind their manners. But surgeons are the most likely to be reported for unprofessional behavior, while physicians practicing in pediatric settings are the least likely, according to a recent study of more than 35,000 physicians.
The research, published on June 6 in JAMA Network Open, found that fewer than 10% of physicians were reported by their coworkers for at least one instance of unprofessional behavior, and only 1% showed a pattern of such reports.
Data were gathered from the Center for Patient and Professional Advocacy’s (CPPA’s) Coworker Observation Reporting System (CORS) program, a national collaborative in which 193 participating hospitals and practice sites file safety-event reports involving medical workers’ unprofessional behaviors. An algorithm that weights CORS reports based on recency and severity was used to analyze the data. The study was spearheaded by William O. Cooper, MD, MPH, director of the CPPA at Vanderbilt University Medical Center, Nashville, Tennessee.
The retrospective cohort study included deidentified data on credentialed physicians, not including residents or fellows, who practiced at a CORS site between 2018 and 2022.
Why Surgeons?
The authors speculated that the reason surgeons were reported for unprofessional behavior more often than their colleagues in nonsurgical specialties was because surgery is a more stressful environment than other specialties and requires more teamwork, resulting in more interactions during high-stakes events.
Daniel Katz, MD, professor and vice chair of education for the Department of Anesthesiology, Perioperative and Pain Medicine at the Icahn School of Medicine at Mount Sinai, New York City, added that part of the problem is that surgeons are expected to perform at very high levels all the time.
“When things that are outside the control of the surgeon don’t go well,” Dr. Katz said, “that can lead to increased frustration and negative emotions, which will then bring out these kinds of behaviors.”
Types of Unprofessional Behaviors
The most common out-of-bounds behaviors reported involved disrespectful communication or lack of professional responsibility. In one example, a physician called a coworker a “bossy cow” when the coworker reminded the physician of the need to do a timeout before beginning a bronchoscopy.
In another case involving professional responsibility, a coworker asked a physician if the team should wait for a disoriented patient’s spouse to arrive. The doctor’s response: “We’ll be here all night if we do that. If you won’t sign as a witness, I’ll get someone else who will.”
The least common reports involved unprofessionalism related to medical care or professional integrity. One cited a physician removing a Foley catheter without wearing gloves and having visible urine on his hands and not washing them before touching other things in the room. In a reported lapse of professional integrity, a physician billed at level five after spending only 4 minutes with a patient.
Impact of Unprofessional Behavior
Unprofessional behavior among physicians is more than just unpleasant. It can threaten the functioning of teams and increase patient complications. In addition, individuals who model unprofessional behaviors are associated with increased malpractice claims, the study’s authors wrote.
Dr. Katz agreed that unprofessional behavior is damaging to both patients and the profession as a whole.
However, this doesn’t happen because some doctors are bad, he said. Physicians today are working in a pressure cooker. The current healthcare environment, with its increased administrative burdens, lack of staffing, and other problems, has increased the overall level of stress and led to burnout among healthcare personnel.
“You have to fix the system to create a working environment that doesn’t cause somebody to explode,” Dr. Katz said.
The goal of the CORS program and this study, Dr. Cooper said, is to help physicians better weather these stresses.
Study Limitations
The authors noted some weaknesses in the study. Some unprofessional behavior may go unreported because of fear of retaliation or for other reasons victims or witnesses did not feel safe to report their colleagues. Also, reports were not evaluated to ensure the truth of the accusations. The records reviewed did not include the gender of the physician, though the researchers pointed out that previous studies have shown that women are less likely than men to receive CORS reports.
A version of this article appeared on Medscape.com.
Most doctors mind their manners. But surgeons are the most likely to be reported for unprofessional behavior, while physicians practicing in pediatric settings are the least likely, according to a recent study of more than 35,000 physicians.
The research, published on June 6 in JAMA Network Open, found that fewer than 10% of physicians were reported by their coworkers for at least one instance of unprofessional behavior, and only 1% showed a pattern of such reports.
Data were gathered from the Center for Patient and Professional Advocacy’s (CPPA’s) Coworker Observation Reporting System (CORS) program, a national collaborative in which 193 participating hospitals and practice sites file safety-event reports involving medical workers’ unprofessional behaviors. An algorithm that weights CORS reports based on recency and severity was used to analyze the data. The study was spearheaded by William O. Cooper, MD, MPH, director of the CPPA at Vanderbilt University Medical Center, Nashville, Tennessee.
The retrospective cohort study included deidentified data on credentialed physicians, not including residents or fellows, who practiced at a CORS site between 2018 and 2022.
Why Surgeons?
The authors speculated that the reason surgeons were reported for unprofessional behavior more often than their colleagues in nonsurgical specialties was because surgery is a more stressful environment than other specialties and requires more teamwork, resulting in more interactions during high-stakes events.
Daniel Katz, MD, professor and vice chair of education for the Department of Anesthesiology, Perioperative and Pain Medicine at the Icahn School of Medicine at Mount Sinai, New York City, added that part of the problem is that surgeons are expected to perform at very high levels all the time.
“When things that are outside the control of the surgeon don’t go well,” Dr. Katz said, “that can lead to increased frustration and negative emotions, which will then bring out these kinds of behaviors.”
Types of Unprofessional Behaviors
The most common out-of-bounds behaviors reported involved disrespectful communication or lack of professional responsibility. In one example, a physician called a coworker a “bossy cow” when the coworker reminded the physician of the need to do a timeout before beginning a bronchoscopy.
In another case involving professional responsibility, a coworker asked a physician if the team should wait for a disoriented patient’s spouse to arrive. The doctor’s response: “We’ll be here all night if we do that. If you won’t sign as a witness, I’ll get someone else who will.”
The least common reports involved unprofessionalism related to medical care or professional integrity. One cited a physician removing a Foley catheter without wearing gloves and having visible urine on his hands and not washing them before touching other things in the room. In a reported lapse of professional integrity, a physician billed at level five after spending only 4 minutes with a patient.
Impact of Unprofessional Behavior
Unprofessional behavior among physicians is more than just unpleasant. It can threaten the functioning of teams and increase patient complications. In addition, individuals who model unprofessional behaviors are associated with increased malpractice claims, the study’s authors wrote.
Dr. Katz agreed that unprofessional behavior is damaging to both patients and the profession as a whole.
However, this doesn’t happen because some doctors are bad, he said. Physicians today are working in a pressure cooker. The current healthcare environment, with its increased administrative burdens, lack of staffing, and other problems, has increased the overall level of stress and led to burnout among healthcare personnel.
“You have to fix the system to create a working environment that doesn’t cause somebody to explode,” Dr. Katz said.
The goal of the CORS program and this study, Dr. Cooper said, is to help physicians better weather these stresses.
Study Limitations
The authors noted some weaknesses in the study. Some unprofessional behavior may go unreported because of fear of retaliation or for other reasons victims or witnesses did not feel safe to report their colleagues. Also, reports were not evaluated to ensure the truth of the accusations. The records reviewed did not include the gender of the physician, though the researchers pointed out that previous studies have shown that women are less likely than men to receive CORS reports.
A version of this article appeared on Medscape.com.
Better Sleep Tied to Less Loneliness
HOUSTON — Sleep may have a role in driving down rates of loneliness, especially among younger adults.
A study of nearly 2300 participants showed that better sleep health is associated with significantly lower levels of loneliness across ages and that the association is particularly strong in younger individuals.
The US Surgeon General has identified loneliness as “a major public health concern, linked to high rates of negative physical and mental health outcomes,” lead researcher Joseph Dzierzewski, PhD, vice president for research and scientific affairs at the National Sleep Foundation, told this news organization.
“Loneliness is an urgent public health crisis, and there is a pressing need for providers to better understand and treat it,” Dr. Dzierzewski said in a statement.
“Better sleep health might be connected to lower feelings of loneliness by empowering people to engage in social activities, reducing feelings of negative emotions and increasing the likelihood that people interpret interactions in a positive way,” he added.
The findings were presented at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies and recently published in an online supplement of the journal Sleep.
Rested, Connected
An American Psychiatric Association poll conducted earlier this year showed 30% of US adults reported feelings of loneliness at least once a week over the past year, and 10% reported feeling lonely every day.
Younger people are more likely to report feeling lonely, with 30% of Americans, aged 18-34 years, feeling lonely every day or several times a week.
While there is growing research identifying a relationship between loneliness and poor sleep in different age groups, few studies have explored ties between social and emotional loneliness and sleep health across the adult lifespan.
In the current study led by Dr. Dzierzewski, 2297 adults (mean age, 44 years; 51% male) completed a validated sleep health questionnaire and loneliness scale.
Linear regression analyses were used to examine the direct associations between sleep health, age, and loneliness. Moderation analyses tested whether the link between sleep health and loneliness differed by age.
On average, the total sleep score was 7.7 (range, 0-12), with higher scores indicating better multidimensional sleep health, and total loneliness scale score was 8.9 (out of 11), indicating moderate levels of loneliness.
Better sleep health and younger age were associated with significantly lower loneliness total scores and social and emotional loneliness subscale scores (all P < .001).
Age significantly moderated the association between sleep health and total (P < .001) and emotional loneliness scores (P < .001) but did not moderate the association between sleep health and social loneliness (P = .034). Better sleep health was associated with lower loneliness across ages, and this association was stronger at younger ages.
“Why younger adults might experience more sleep-related benefits to loneliness than older adults is unknown and intriguing — certainly worth further investigation,” Dr. Dzierzewski said in a conference statement.
Untapped Avenue
Promoting sleep health may be an “untapped avenue” to support efforts and programs that aim to reduce loneliness and increase engagement in all age groups but especially in younger ages, the researchers noted.
Future research should consider monitoring sleep health in programs or interventions that address loneliness, they added.
“Healthcare providers should be aware of the important link between sleep health and loneliness as both sleep and social connections are essential to health and well-being. When sitting across from patients, asking about both sleep health and loneliness might yield important insights into avenues for health promotion,” said Dr. Dzierzewski.
Michael Breus, PhD, clinical psychologist and founder of SleepDoctor.com, who wasn’t involved in the study, is not surprised by the results.
It makes sense that better sleep would lead to less feelings of loneliness, he told this news organization.
Research has shown that when someone is not sleeping well, they “give others a sense of unhappiness, which socially deflects new encounters or even encounters with friends. So social awareness and social initiation would appear to both be affected by sleep quality, therefore potentially leading, at least in part, to loneliness,” he said.
Support for the study was provided by the National Institute on Aging. Dr. Dzierzewski and Dr. Breus had no relevant disclosures.
A version of this article first appeared on Medscape.com.
HOUSTON — Sleep may have a role in driving down rates of loneliness, especially among younger adults.
A study of nearly 2300 participants showed that better sleep health is associated with significantly lower levels of loneliness across ages and that the association is particularly strong in younger individuals.
The US Surgeon General has identified loneliness as “a major public health concern, linked to high rates of negative physical and mental health outcomes,” lead researcher Joseph Dzierzewski, PhD, vice president for research and scientific affairs at the National Sleep Foundation, told this news organization.
“Loneliness is an urgent public health crisis, and there is a pressing need for providers to better understand and treat it,” Dr. Dzierzewski said in a statement.
“Better sleep health might be connected to lower feelings of loneliness by empowering people to engage in social activities, reducing feelings of negative emotions and increasing the likelihood that people interpret interactions in a positive way,” he added.
The findings were presented at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies and recently published in an online supplement of the journal Sleep.
Rested, Connected
An American Psychiatric Association poll conducted earlier this year showed 30% of US adults reported feelings of loneliness at least once a week over the past year, and 10% reported feeling lonely every day.
Younger people are more likely to report feeling lonely, with 30% of Americans, aged 18-34 years, feeling lonely every day or several times a week.
While there is growing research identifying a relationship between loneliness and poor sleep in different age groups, few studies have explored ties between social and emotional loneliness and sleep health across the adult lifespan.
In the current study led by Dr. Dzierzewski, 2297 adults (mean age, 44 years; 51% male) completed a validated sleep health questionnaire and loneliness scale.
Linear regression analyses were used to examine the direct associations between sleep health, age, and loneliness. Moderation analyses tested whether the link between sleep health and loneliness differed by age.
On average, the total sleep score was 7.7 (range, 0-12), with higher scores indicating better multidimensional sleep health, and total loneliness scale score was 8.9 (out of 11), indicating moderate levels of loneliness.
Better sleep health and younger age were associated with significantly lower loneliness total scores and social and emotional loneliness subscale scores (all P < .001).
Age significantly moderated the association between sleep health and total (P < .001) and emotional loneliness scores (P < .001) but did not moderate the association between sleep health and social loneliness (P = .034). Better sleep health was associated with lower loneliness across ages, and this association was stronger at younger ages.
“Why younger adults might experience more sleep-related benefits to loneliness than older adults is unknown and intriguing — certainly worth further investigation,” Dr. Dzierzewski said in a conference statement.
Untapped Avenue
Promoting sleep health may be an “untapped avenue” to support efforts and programs that aim to reduce loneliness and increase engagement in all age groups but especially in younger ages, the researchers noted.
Future research should consider monitoring sleep health in programs or interventions that address loneliness, they added.
“Healthcare providers should be aware of the important link between sleep health and loneliness as both sleep and social connections are essential to health and well-being. When sitting across from patients, asking about both sleep health and loneliness might yield important insights into avenues for health promotion,” said Dr. Dzierzewski.
Michael Breus, PhD, clinical psychologist and founder of SleepDoctor.com, who wasn’t involved in the study, is not surprised by the results.
It makes sense that better sleep would lead to less feelings of loneliness, he told this news organization.
Research has shown that when someone is not sleeping well, they “give others a sense of unhappiness, which socially deflects new encounters or even encounters with friends. So social awareness and social initiation would appear to both be affected by sleep quality, therefore potentially leading, at least in part, to loneliness,” he said.
Support for the study was provided by the National Institute on Aging. Dr. Dzierzewski and Dr. Breus had no relevant disclosures.
A version of this article first appeared on Medscape.com.
HOUSTON — Sleep may have a role in driving down rates of loneliness, especially among younger adults.
A study of nearly 2300 participants showed that better sleep health is associated with significantly lower levels of loneliness across ages and that the association is particularly strong in younger individuals.
The US Surgeon General has identified loneliness as “a major public health concern, linked to high rates of negative physical and mental health outcomes,” lead researcher Joseph Dzierzewski, PhD, vice president for research and scientific affairs at the National Sleep Foundation, told this news organization.
“Loneliness is an urgent public health crisis, and there is a pressing need for providers to better understand and treat it,” Dr. Dzierzewski said in a statement.
“Better sleep health might be connected to lower feelings of loneliness by empowering people to engage in social activities, reducing feelings of negative emotions and increasing the likelihood that people interpret interactions in a positive way,” he added.
The findings were presented at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies and recently published in an online supplement of the journal Sleep.
Rested, Connected
An American Psychiatric Association poll conducted earlier this year showed 30% of US adults reported feelings of loneliness at least once a week over the past year, and 10% reported feeling lonely every day.
Younger people are more likely to report feeling lonely, with 30% of Americans, aged 18-34 years, feeling lonely every day or several times a week.
While there is growing research identifying a relationship between loneliness and poor sleep in different age groups, few studies have explored ties between social and emotional loneliness and sleep health across the adult lifespan.
In the current study led by Dr. Dzierzewski, 2297 adults (mean age, 44 years; 51% male) completed a validated sleep health questionnaire and loneliness scale.
Linear regression analyses were used to examine the direct associations between sleep health, age, and loneliness. Moderation analyses tested whether the link between sleep health and loneliness differed by age.
On average, the total sleep score was 7.7 (range, 0-12), with higher scores indicating better multidimensional sleep health, and total loneliness scale score was 8.9 (out of 11), indicating moderate levels of loneliness.
Better sleep health and younger age were associated with significantly lower loneliness total scores and social and emotional loneliness subscale scores (all P < .001).
Age significantly moderated the association between sleep health and total (P < .001) and emotional loneliness scores (P < .001) but did not moderate the association between sleep health and social loneliness (P = .034). Better sleep health was associated with lower loneliness across ages, and this association was stronger at younger ages.
“Why younger adults might experience more sleep-related benefits to loneliness than older adults is unknown and intriguing — certainly worth further investigation,” Dr. Dzierzewski said in a conference statement.
Untapped Avenue
Promoting sleep health may be an “untapped avenue” to support efforts and programs that aim to reduce loneliness and increase engagement in all age groups but especially in younger ages, the researchers noted.
Future research should consider monitoring sleep health in programs or interventions that address loneliness, they added.
“Healthcare providers should be aware of the important link between sleep health and loneliness as both sleep and social connections are essential to health and well-being. When sitting across from patients, asking about both sleep health and loneliness might yield important insights into avenues for health promotion,” said Dr. Dzierzewski.
Michael Breus, PhD, clinical psychologist and founder of SleepDoctor.com, who wasn’t involved in the study, is not surprised by the results.
It makes sense that better sleep would lead to less feelings of loneliness, he told this news organization.
Research has shown that when someone is not sleeping well, they “give others a sense of unhappiness, which socially deflects new encounters or even encounters with friends. So social awareness and social initiation would appear to both be affected by sleep quality, therefore potentially leading, at least in part, to loneliness,” he said.
Support for the study was provided by the National Institute on Aging. Dr. Dzierzewski and Dr. Breus had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SLEEP 2024