User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
National Rapid Genome Testing Program Benefits NICU Care
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
FDA Clears Medical Grade Over-the-Counter Pulse Oximeter
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
Photoexposed Rash in an Older Adult
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
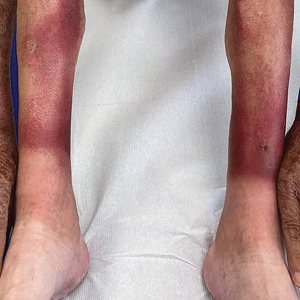
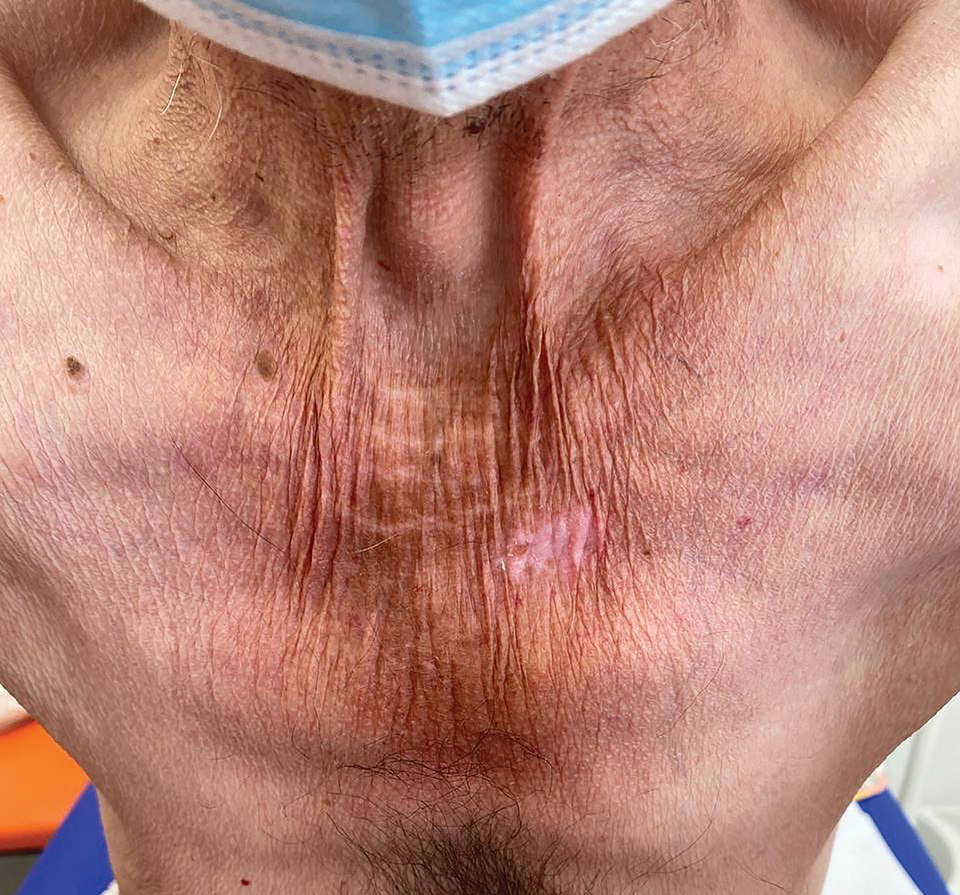
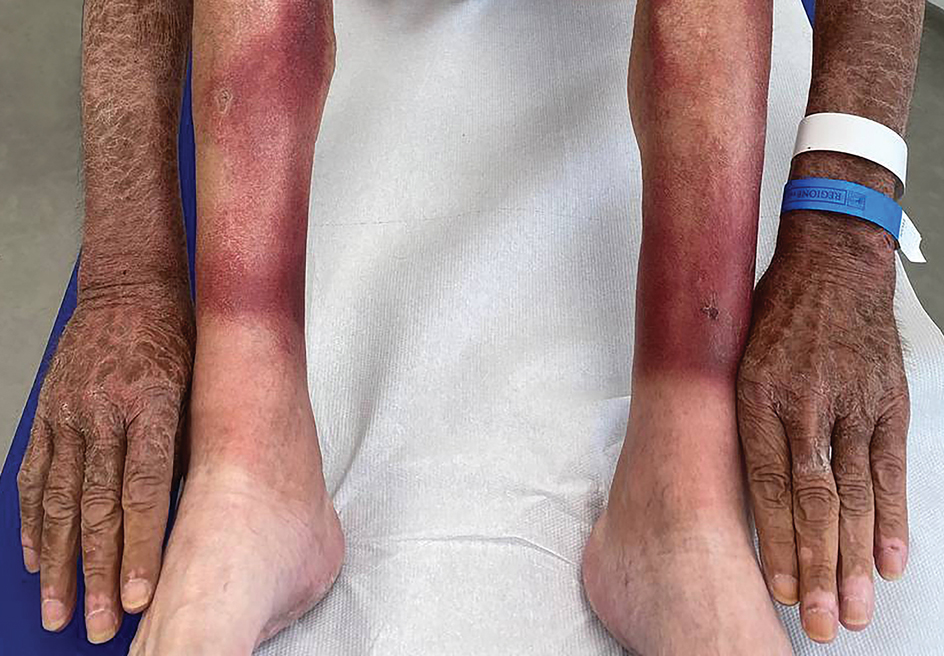
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3
The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
A 66-year-old man presented with an intermittent pruriginous symmetric rash on the dorsal aspects of the arms, legs, and upper chest of 4 months' duration. The patient’s hands, forearms, and neck were diffusely hyperpigmented, dry, cracked, and scaling with a ring of peripheral erythema. He also experienced recurrent photosensitivity reactions on the legs. His poor clinical condition including confusion and diarrhea hindered intake of a balanced diet. He also reported a history of excessive alcohol use. The patient’s vital signs were normal, and Doppler ultrasonography ruled out deep venous thrombosis of the lower legs. A complete blood cell count showed anemia with decreased hemoglobin levels (117 g/L [reference range, 140–180 g/L]) and increased mean corpuscular volume (107.1 fL [reference range, 80–100 fL]). Additionally, low serum levels of albumin, folate, and vitamin B12 were noted. The patient had been taking hydrochlorothiazide and salicylic acid for hypertension with no recent changes in his medication regimen.
Gout Increases the Risk for a Wide Range of Cardiovascular Diseases
People with gout are 58% more likely to develop cardiovascular disease (CVD), according to a new analysis. This increased risk was observed across 12 different cardiovascular conditions, including heart failure, arrhythmias, and valve diseases.
“These findings suggest that the organ damage associated with gout is likely to be much broader than originally thought,” Nathalie Conrad, PhD, senior author of the research and cardiovascular epidemiologist at KU Leuven, Leuven, Belgium, said in an email. This could be useful for future research on underlying biological mechanisms driving CVD risk in gout, she added.
While previous research has tied gout to increased cardiovascular risk, these studies “largely focused on coronary heart disease, stroke, and thromboembolic outcomes,” she explained, and have been smaller in size.
This new study included more than 862,000 individuals, which permitted researchers to investigate rarer CVD outcomes such as myocarditis and pericarditis.
For the study, researchers used electronic health records from the UK Clinical Practice Research Datalink, a primary care database that contains anonymized health data for about 22 million individuals. Using these data, they identified more than 152,600 individuals with gout. Patients included in the analysis were diagnosed between 2000 and 2017, younger than 80 years at diagnosis, and free of CVD for at least 12 months after their gout diagnosis.
Patients with gout were compared with nearly 710,000 controls, matched on demographic factors such as age, sex, and geographic region.
Researchers then investigated the incidence of 12 CVDs, including atherosclerotic diseases, degenerative and thromboembolic diseases, and arrythmias, between the two groups from January 1, 2000, to June 30, 2019.
The findings were published in the March 2024 issue of The Lancet Rheumatology. Overall, patients with gout were 58% more likely to develop any CVD than their matched comparators without gout. There was a higher disease incidence among patients with gout for each of the 12 conditions. This association was more pronounced in women (hazard ratio [HR], 1.88) than in men (HR, 1.49), and gout amplified the risk for CVD in younger individuals to a greater extent.
Individuals younger than 45 years with gout were more than twice as likely to develop CVD compared with similarly aged individuals without gout. For comparison, individuals aged 45-54 years with gout were 84% more likely to develop CVD, and individuals aged 55-64 years were 57% more likely to develop CVD than matched controls.
Conduction system disease had the highest incident risk (HR, 1.88), followed by heart failure and valve disease (HR, 1.85 for both).
Individuals with gout had higher rates of comorbidities than the controls, including hypertension, obesity, and dyslipidemia. Overall, CVD risk was slightly attenuated after adjustment for traditional CVD risk factors such as smoking, blood pressure, and body mass index but still significant: Patients with gout had a 31% higher risk for CVD than comparators.
This shows “that known CVD risk factors only explain part of the CVD risks seen in patients with gout,” Dr. Conrad said. Other factors such as inflammation and other disease activity factors could be at play, she explained, which would need to be explored in future research.
The study “shows the whole landscape” of CVD and gout, Michael H. Pillinger, MD, rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Grossman School of Medicine in New York City, said in an interview. He was not involved with the research.
“Every possible cardiovascular disease that they could think of was something that gout patients had more of than the non-gout patients,” he added. “I think this is going to be a paper that gets cited a lot, at minimum when describing the background of risk when we look at gout patients.”
The study had some limitations, including that researchers were unable to account for how medications such as nonsteroidal anti-inflammatory drugs, corticosteroids, colchicine, or allopurinol may have affected the association between gout and CVD.
“This is because analyses of nonrandomized treatment can be confounded by indication, wherein it is difficult to differentiate the effects of the treatment from underlying disease severity,” the authors wrote.
There was also a large amount of missing data on blood pressure, body mass index, smoking status, and other health information relevant to cardiovascular risk, so sensitivity analyses adjusting for these factors “should be interpreted with caution,” they added.
Dr. Pillinger also noted that the rates of comorbidities in the gout study population were lower than what have been found in US study populations. For example, about 40% of patients with gout in the analysis had hypertension, while other studies have suggested higher rates of 60%-70%, he said. However, it’s not clear if these differences could have affected outcomes. He added that these limitations do not “in any way weaken [the authors’] conclusion.”
The findings call for better strategies to reduce CVD risk in patients with gout, Dr. Conrad noted.
“Further improvements could come from better recognition and intervention on CVD risk factors (eg, through lifestyle changes or drug therapies where they are indicated), as well as proactive screening for heart disease in patients with gout, which could allow early diagnosis and interventions to delay more severe outcomes,” she added.
This study was funded by Research Foundation Flanders. Dr. Conrad was funded by a personal fellowship from the Research Foundation Flanders and a European Society of Cardiology research grant. She received royalties from Oxford University Innovation. Four of Dr. Conrad’s eight coauthors also reported financial relationships with pharmaceutical companies. Dr. Pillinger served as a consultant to Amgen, Federation Bio, Fortress Biotech, and Scilex, and he holds an investigator-initiated grant from Hikma.
A version of this article appeared on Medscape.com.
People with gout are 58% more likely to develop cardiovascular disease (CVD), according to a new analysis. This increased risk was observed across 12 different cardiovascular conditions, including heart failure, arrhythmias, and valve diseases.
“These findings suggest that the organ damage associated with gout is likely to be much broader than originally thought,” Nathalie Conrad, PhD, senior author of the research and cardiovascular epidemiologist at KU Leuven, Leuven, Belgium, said in an email. This could be useful for future research on underlying biological mechanisms driving CVD risk in gout, she added.
While previous research has tied gout to increased cardiovascular risk, these studies “largely focused on coronary heart disease, stroke, and thromboembolic outcomes,” she explained, and have been smaller in size.
This new study included more than 862,000 individuals, which permitted researchers to investigate rarer CVD outcomes such as myocarditis and pericarditis.
For the study, researchers used electronic health records from the UK Clinical Practice Research Datalink, a primary care database that contains anonymized health data for about 22 million individuals. Using these data, they identified more than 152,600 individuals with gout. Patients included in the analysis were diagnosed between 2000 and 2017, younger than 80 years at diagnosis, and free of CVD for at least 12 months after their gout diagnosis.
Patients with gout were compared with nearly 710,000 controls, matched on demographic factors such as age, sex, and geographic region.
Researchers then investigated the incidence of 12 CVDs, including atherosclerotic diseases, degenerative and thromboembolic diseases, and arrythmias, between the two groups from January 1, 2000, to June 30, 2019.
The findings were published in the March 2024 issue of The Lancet Rheumatology. Overall, patients with gout were 58% more likely to develop any CVD than their matched comparators without gout. There was a higher disease incidence among patients with gout for each of the 12 conditions. This association was more pronounced in women (hazard ratio [HR], 1.88) than in men (HR, 1.49), and gout amplified the risk for CVD in younger individuals to a greater extent.
Individuals younger than 45 years with gout were more than twice as likely to develop CVD compared with similarly aged individuals without gout. For comparison, individuals aged 45-54 years with gout were 84% more likely to develop CVD, and individuals aged 55-64 years were 57% more likely to develop CVD than matched controls.
Conduction system disease had the highest incident risk (HR, 1.88), followed by heart failure and valve disease (HR, 1.85 for both).
Individuals with gout had higher rates of comorbidities than the controls, including hypertension, obesity, and dyslipidemia. Overall, CVD risk was slightly attenuated after adjustment for traditional CVD risk factors such as smoking, blood pressure, and body mass index but still significant: Patients with gout had a 31% higher risk for CVD than comparators.
This shows “that known CVD risk factors only explain part of the CVD risks seen in patients with gout,” Dr. Conrad said. Other factors such as inflammation and other disease activity factors could be at play, she explained, which would need to be explored in future research.
The study “shows the whole landscape” of CVD and gout, Michael H. Pillinger, MD, rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Grossman School of Medicine in New York City, said in an interview. He was not involved with the research.
“Every possible cardiovascular disease that they could think of was something that gout patients had more of than the non-gout patients,” he added. “I think this is going to be a paper that gets cited a lot, at minimum when describing the background of risk when we look at gout patients.”
The study had some limitations, including that researchers were unable to account for how medications such as nonsteroidal anti-inflammatory drugs, corticosteroids, colchicine, or allopurinol may have affected the association between gout and CVD.
“This is because analyses of nonrandomized treatment can be confounded by indication, wherein it is difficult to differentiate the effects of the treatment from underlying disease severity,” the authors wrote.
There was also a large amount of missing data on blood pressure, body mass index, smoking status, and other health information relevant to cardiovascular risk, so sensitivity analyses adjusting for these factors “should be interpreted with caution,” they added.
Dr. Pillinger also noted that the rates of comorbidities in the gout study population were lower than what have been found in US study populations. For example, about 40% of patients with gout in the analysis had hypertension, while other studies have suggested higher rates of 60%-70%, he said. However, it’s not clear if these differences could have affected outcomes. He added that these limitations do not “in any way weaken [the authors’] conclusion.”
The findings call for better strategies to reduce CVD risk in patients with gout, Dr. Conrad noted.
“Further improvements could come from better recognition and intervention on CVD risk factors (eg, through lifestyle changes or drug therapies where they are indicated), as well as proactive screening for heart disease in patients with gout, which could allow early diagnosis and interventions to delay more severe outcomes,” she added.
This study was funded by Research Foundation Flanders. Dr. Conrad was funded by a personal fellowship from the Research Foundation Flanders and a European Society of Cardiology research grant. She received royalties from Oxford University Innovation. Four of Dr. Conrad’s eight coauthors also reported financial relationships with pharmaceutical companies. Dr. Pillinger served as a consultant to Amgen, Federation Bio, Fortress Biotech, and Scilex, and he holds an investigator-initiated grant from Hikma.
A version of this article appeared on Medscape.com.
People with gout are 58% more likely to develop cardiovascular disease (CVD), according to a new analysis. This increased risk was observed across 12 different cardiovascular conditions, including heart failure, arrhythmias, and valve diseases.
“These findings suggest that the organ damage associated with gout is likely to be much broader than originally thought,” Nathalie Conrad, PhD, senior author of the research and cardiovascular epidemiologist at KU Leuven, Leuven, Belgium, said in an email. This could be useful for future research on underlying biological mechanisms driving CVD risk in gout, she added.
While previous research has tied gout to increased cardiovascular risk, these studies “largely focused on coronary heart disease, stroke, and thromboembolic outcomes,” she explained, and have been smaller in size.
This new study included more than 862,000 individuals, which permitted researchers to investigate rarer CVD outcomes such as myocarditis and pericarditis.
For the study, researchers used electronic health records from the UK Clinical Practice Research Datalink, a primary care database that contains anonymized health data for about 22 million individuals. Using these data, they identified more than 152,600 individuals with gout. Patients included in the analysis were diagnosed between 2000 and 2017, younger than 80 years at diagnosis, and free of CVD for at least 12 months after their gout diagnosis.
Patients with gout were compared with nearly 710,000 controls, matched on demographic factors such as age, sex, and geographic region.
Researchers then investigated the incidence of 12 CVDs, including atherosclerotic diseases, degenerative and thromboembolic diseases, and arrythmias, between the two groups from January 1, 2000, to June 30, 2019.
The findings were published in the March 2024 issue of The Lancet Rheumatology. Overall, patients with gout were 58% more likely to develop any CVD than their matched comparators without gout. There was a higher disease incidence among patients with gout for each of the 12 conditions. This association was more pronounced in women (hazard ratio [HR], 1.88) than in men (HR, 1.49), and gout amplified the risk for CVD in younger individuals to a greater extent.
Individuals younger than 45 years with gout were more than twice as likely to develop CVD compared with similarly aged individuals without gout. For comparison, individuals aged 45-54 years with gout were 84% more likely to develop CVD, and individuals aged 55-64 years were 57% more likely to develop CVD than matched controls.
Conduction system disease had the highest incident risk (HR, 1.88), followed by heart failure and valve disease (HR, 1.85 for both).
Individuals with gout had higher rates of comorbidities than the controls, including hypertension, obesity, and dyslipidemia. Overall, CVD risk was slightly attenuated after adjustment for traditional CVD risk factors such as smoking, blood pressure, and body mass index but still significant: Patients with gout had a 31% higher risk for CVD than comparators.
This shows “that known CVD risk factors only explain part of the CVD risks seen in patients with gout,” Dr. Conrad said. Other factors such as inflammation and other disease activity factors could be at play, she explained, which would need to be explored in future research.
The study “shows the whole landscape” of CVD and gout, Michael H. Pillinger, MD, rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Grossman School of Medicine in New York City, said in an interview. He was not involved with the research.
“Every possible cardiovascular disease that they could think of was something that gout patients had more of than the non-gout patients,” he added. “I think this is going to be a paper that gets cited a lot, at minimum when describing the background of risk when we look at gout patients.”
The study had some limitations, including that researchers were unable to account for how medications such as nonsteroidal anti-inflammatory drugs, corticosteroids, colchicine, or allopurinol may have affected the association between gout and CVD.
“This is because analyses of nonrandomized treatment can be confounded by indication, wherein it is difficult to differentiate the effects of the treatment from underlying disease severity,” the authors wrote.
There was also a large amount of missing data on blood pressure, body mass index, smoking status, and other health information relevant to cardiovascular risk, so sensitivity analyses adjusting for these factors “should be interpreted with caution,” they added.
Dr. Pillinger also noted that the rates of comorbidities in the gout study population were lower than what have been found in US study populations. For example, about 40% of patients with gout in the analysis had hypertension, while other studies have suggested higher rates of 60%-70%, he said. However, it’s not clear if these differences could have affected outcomes. He added that these limitations do not “in any way weaken [the authors’] conclusion.”
The findings call for better strategies to reduce CVD risk in patients with gout, Dr. Conrad noted.
“Further improvements could come from better recognition and intervention on CVD risk factors (eg, through lifestyle changes or drug therapies where they are indicated), as well as proactive screening for heart disease in patients with gout, which could allow early diagnosis and interventions to delay more severe outcomes,” she added.
This study was funded by Research Foundation Flanders. Dr. Conrad was funded by a personal fellowship from the Research Foundation Flanders and a European Society of Cardiology research grant. She received royalties from Oxford University Innovation. Four of Dr. Conrad’s eight coauthors also reported financial relationships with pharmaceutical companies. Dr. Pillinger served as a consultant to Amgen, Federation Bio, Fortress Biotech, and Scilex, and he holds an investigator-initiated grant from Hikma.
A version of this article appeared on Medscape.com.
Is Stretching Now Underrated? Accumulating Research Says Yes
For many, stretching is the fitness equivalent of awkward small talk. It’s the opening act, the thing you tolerate because you know it will be over soon.
Others have challenged the practice, suggesting that stretching isn’t necessary at all. Some research has found that a preworkout stretch may even be disadvantageous, weakening muscles and hindering performance.
To put it plainly, no one seems terribly enthusiastic about touching their toes.
That’s why a 2020 study on exercise and mortality was such a head-scratcher. The study found that stretching was uniquely associated with a lower risk for all-cause mortality among American adults. That’s after controlling for participation in other types of exercise.
The finding seemed like a fluke, until a 2023 study found essentially the same thing.
That was slightly better than the risk reduction associated with high volumes of aerobic exercise and resistance training.
How can that be ? It turns out, stretching is linked to several health benefits that you might not expect.
The Surprising Benefits of Stretching
When we talk about stretching, we usually mean static stretching — getting into and holding a position that challenges a muscle, with the goal of improving range of motion around a joint.
It doesn’t need to be a big challenge. “Research shows you can get increases in flexibility by stretching to the initial point of discomfort,” said David Behm, PhD, an exercise scientist at Memorial University of Newfoundland in Canada who’s published dozens of studies on stretching over the past quarter-century.
That brings us to the first benefit.
Stretching Benefit #1: More Strength
At first glance, flexibility training and strength training have little in common. You lengthen muscles in the former and contract them in the latter.
But in both cases, Dr. Behm said, you’re applying tension to muscles and connective tissues. Tension activates proteins called integrins, which send and receive signals across cellular membranes. Those signals are the start of a cascade that leads to protein synthesis. That’s how muscles get bigger and stronger when you lift weights.
That mechanism could explain the small gains in muscle strength and size associated with static stretching, Dr. Behm said.
But can you really stretch your way to muscle growth? Theoretically, yes. But strength training is far more time-efficient, Dr. Behm says. Studies showing increases in muscle mass have typically stretched a single muscle (usually the calves, using a specialized device) for > 30 min/session, 6 d/wk for 6 weeks. And that’s for just one leg.
Still, stretching may be more accessible for some patients — research suggested that older and more sedentary people are most likely to benefit from stretching-induced gains in strength.
Stretching Benefit #2: Reduced Arterial Stiffness
“Most people don’t think about the cardiovascular benefits of stretching,” Dr. Behm said. There are some big ones.
If your body doesn’t move well, it’s not unreasonable to assume your blood doesn’t flow well. That is indeed the case: Poor flexibility is associated with arterial stiffness.
Stretching is associated not only with improved arterial function but also with reductions in resting heart rate and blood pressure and increased vasodilation.
Mobility improvements may have an indirect benefit on cardiovascular health as well.
“Studies show runners are more economical when they’re more flexible,” Dr. Behm said. If your movement is more efficient, you’ll probably do more of it. Doing more, in turn, would lead to improved fitness.
Stretching Benefit #3: Improved Performance
Research is equivocal on whether stretching improves athletic performance, said Joe Yoon, a sports massage therapist in Orlando, Florida, and author of Better Stretching.
“But I’ve always taken the approach that if you can improve your range of motion and get into positions” required for your sport, you’ll probably perform better, with less risk for injury, Mr. Yoon said.
It’s worth noting that some research over the past 30 years has linked pre-exercise static stretching with a loss of strength, power, and/or speed.
But consider this: In a 2016 review, Dr. Behm and his coauthors showed that performance reductions were most likely to occur in two situations:
When participants did extremely long stretches (duration, ≥ 60 sec per muscle).
When researchers tested the participants’ strength, power, or speed immediately after they stretched.
Avoiding those problems is easy, Dr. Behm said: Stretch each muscle for < 60 sec, and combine static stretches with more active warm-up exercises.
“Stretching can impair your performance but only if you do it wrong,” he said.
Stretching Benefit #4: Fewer Injuries
When you stretch, the point where you feel tension is where the muscle is most vulnerable. “That’s where injuries usually happen,” Dr. Behm said.
More flexibility in those areas allows your muscles to safely generate force at longer lengths. For an athlete, that means fewer injuries when they’re doing explosive movements or changing direction.
For nonathletes, flexibility reduces injuries by improving balance. Better balance reduces the risk of falling and helps mitigate the damage if you do take a tumble.
Help Your Patients Get the Benefits of Stretching
Stretching, like training for endurance or strength, can be as complex as you want to make it. But Mr. Yoon advocates a simpler approach.
“You see this flashy stuff online,” he said. “But if you see those trainers in real life or you book a session with them, they go right back to the basics.”
Ideally, Mr. Yoon said, a flexibility routine will work the entire body. But if that’s too big a stretch for your patient, he recommends starting with one or two stretches for the most problematic area.
For example, for a stiff back, try doing the puppy pose at least once a day, although twice is better. Hold the position for 30 seconds to 2 minutes, said Mr. Yoon. Even if you combine it with a dynamic movement like the cat-cow, the two exercises would take just a few minutes a day.
“There’s this misconception that you have to do a lot of it to be successful,” Mr. Yoon said.
Consistency is far more important than volume. Mr. Yoon recommends “a little bit every day — the minimum viable dose.”
As a bonus, stretching an area like your upper back will probably improve your shoulder mobility, Mr. Yoon said. Same with your lower body: Stretches for your hips, over time, should also benefit your knees and lower back.
And thanks to a phenomenon called nonlocal flexibility transfer, lower-body stretches should improve upper-body flexibility, at least temporarily. Shoulder stretches can also have an immediate effect on hip mobility.
“It’s all connected,” Mr. Yoon said, which brings us back to where we started.
If stretching can indeed reduce mortality risk, it’s probably because of interconnected pathways, rather than any single mechanism.
Most obviously, stretching improves flexibility, which makes movement easier, improves balance, and reduces the risk for falls and other types of injuries. It can also lead to small improvements in strength. Less obviously, stretching improves several aspects of cardiovascular function, including circulation.
“There seems to be a global effect in everything we do,” Dr. Behm said. “Whether you’re stretching or weight training, the message is sent throughout your body."
A version of this article appeared on Medscape.com.
For many, stretching is the fitness equivalent of awkward small talk. It’s the opening act, the thing you tolerate because you know it will be over soon.
Others have challenged the practice, suggesting that stretching isn’t necessary at all. Some research has found that a preworkout stretch may even be disadvantageous, weakening muscles and hindering performance.
To put it plainly, no one seems terribly enthusiastic about touching their toes.
That’s why a 2020 study on exercise and mortality was such a head-scratcher. The study found that stretching was uniquely associated with a lower risk for all-cause mortality among American adults. That’s after controlling for participation in other types of exercise.
The finding seemed like a fluke, until a 2023 study found essentially the same thing.
That was slightly better than the risk reduction associated with high volumes of aerobic exercise and resistance training.
How can that be ? It turns out, stretching is linked to several health benefits that you might not expect.
The Surprising Benefits of Stretching
When we talk about stretching, we usually mean static stretching — getting into and holding a position that challenges a muscle, with the goal of improving range of motion around a joint.
It doesn’t need to be a big challenge. “Research shows you can get increases in flexibility by stretching to the initial point of discomfort,” said David Behm, PhD, an exercise scientist at Memorial University of Newfoundland in Canada who’s published dozens of studies on stretching over the past quarter-century.
That brings us to the first benefit.
Stretching Benefit #1: More Strength
At first glance, flexibility training and strength training have little in common. You lengthen muscles in the former and contract them in the latter.
But in both cases, Dr. Behm said, you’re applying tension to muscles and connective tissues. Tension activates proteins called integrins, which send and receive signals across cellular membranes. Those signals are the start of a cascade that leads to protein synthesis. That’s how muscles get bigger and stronger when you lift weights.
That mechanism could explain the small gains in muscle strength and size associated with static stretching, Dr. Behm said.
But can you really stretch your way to muscle growth? Theoretically, yes. But strength training is far more time-efficient, Dr. Behm says. Studies showing increases in muscle mass have typically stretched a single muscle (usually the calves, using a specialized device) for > 30 min/session, 6 d/wk for 6 weeks. And that’s for just one leg.
Still, stretching may be more accessible for some patients — research suggested that older and more sedentary people are most likely to benefit from stretching-induced gains in strength.
Stretching Benefit #2: Reduced Arterial Stiffness
“Most people don’t think about the cardiovascular benefits of stretching,” Dr. Behm said. There are some big ones.
If your body doesn’t move well, it’s not unreasonable to assume your blood doesn’t flow well. That is indeed the case: Poor flexibility is associated with arterial stiffness.
Stretching is associated not only with improved arterial function but also with reductions in resting heart rate and blood pressure and increased vasodilation.
Mobility improvements may have an indirect benefit on cardiovascular health as well.
“Studies show runners are more economical when they’re more flexible,” Dr. Behm said. If your movement is more efficient, you’ll probably do more of it. Doing more, in turn, would lead to improved fitness.
Stretching Benefit #3: Improved Performance
Research is equivocal on whether stretching improves athletic performance, said Joe Yoon, a sports massage therapist in Orlando, Florida, and author of Better Stretching.
“But I’ve always taken the approach that if you can improve your range of motion and get into positions” required for your sport, you’ll probably perform better, with less risk for injury, Mr. Yoon said.
It’s worth noting that some research over the past 30 years has linked pre-exercise static stretching with a loss of strength, power, and/or speed.
But consider this: In a 2016 review, Dr. Behm and his coauthors showed that performance reductions were most likely to occur in two situations:
When participants did extremely long stretches (duration, ≥ 60 sec per muscle).
When researchers tested the participants’ strength, power, or speed immediately after they stretched.
Avoiding those problems is easy, Dr. Behm said: Stretch each muscle for < 60 sec, and combine static stretches with more active warm-up exercises.
“Stretching can impair your performance but only if you do it wrong,” he said.
Stretching Benefit #4: Fewer Injuries
When you stretch, the point where you feel tension is where the muscle is most vulnerable. “That’s where injuries usually happen,” Dr. Behm said.
More flexibility in those areas allows your muscles to safely generate force at longer lengths. For an athlete, that means fewer injuries when they’re doing explosive movements or changing direction.
For nonathletes, flexibility reduces injuries by improving balance. Better balance reduces the risk of falling and helps mitigate the damage if you do take a tumble.
Help Your Patients Get the Benefits of Stretching
Stretching, like training for endurance or strength, can be as complex as you want to make it. But Mr. Yoon advocates a simpler approach.
“You see this flashy stuff online,” he said. “But if you see those trainers in real life or you book a session with them, they go right back to the basics.”
Ideally, Mr. Yoon said, a flexibility routine will work the entire body. But if that’s too big a stretch for your patient, he recommends starting with one or two stretches for the most problematic area.
For example, for a stiff back, try doing the puppy pose at least once a day, although twice is better. Hold the position for 30 seconds to 2 minutes, said Mr. Yoon. Even if you combine it with a dynamic movement like the cat-cow, the two exercises would take just a few minutes a day.
“There’s this misconception that you have to do a lot of it to be successful,” Mr. Yoon said.
Consistency is far more important than volume. Mr. Yoon recommends “a little bit every day — the minimum viable dose.”
As a bonus, stretching an area like your upper back will probably improve your shoulder mobility, Mr. Yoon said. Same with your lower body: Stretches for your hips, over time, should also benefit your knees and lower back.
And thanks to a phenomenon called nonlocal flexibility transfer, lower-body stretches should improve upper-body flexibility, at least temporarily. Shoulder stretches can also have an immediate effect on hip mobility.
“It’s all connected,” Mr. Yoon said, which brings us back to where we started.
If stretching can indeed reduce mortality risk, it’s probably because of interconnected pathways, rather than any single mechanism.
Most obviously, stretching improves flexibility, which makes movement easier, improves balance, and reduces the risk for falls and other types of injuries. It can also lead to small improvements in strength. Less obviously, stretching improves several aspects of cardiovascular function, including circulation.
“There seems to be a global effect in everything we do,” Dr. Behm said. “Whether you’re stretching or weight training, the message is sent throughout your body."
A version of this article appeared on Medscape.com.
For many, stretching is the fitness equivalent of awkward small talk. It’s the opening act, the thing you tolerate because you know it will be over soon.
Others have challenged the practice, suggesting that stretching isn’t necessary at all. Some research has found that a preworkout stretch may even be disadvantageous, weakening muscles and hindering performance.
To put it plainly, no one seems terribly enthusiastic about touching their toes.
That’s why a 2020 study on exercise and mortality was such a head-scratcher. The study found that stretching was uniquely associated with a lower risk for all-cause mortality among American adults. That’s after controlling for participation in other types of exercise.
The finding seemed like a fluke, until a 2023 study found essentially the same thing.
That was slightly better than the risk reduction associated with high volumes of aerobic exercise and resistance training.
How can that be ? It turns out, stretching is linked to several health benefits that you might not expect.
The Surprising Benefits of Stretching
When we talk about stretching, we usually mean static stretching — getting into and holding a position that challenges a muscle, with the goal of improving range of motion around a joint.
It doesn’t need to be a big challenge. “Research shows you can get increases in flexibility by stretching to the initial point of discomfort,” said David Behm, PhD, an exercise scientist at Memorial University of Newfoundland in Canada who’s published dozens of studies on stretching over the past quarter-century.
That brings us to the first benefit.
Stretching Benefit #1: More Strength
At first glance, flexibility training and strength training have little in common. You lengthen muscles in the former and contract them in the latter.
But in both cases, Dr. Behm said, you’re applying tension to muscles and connective tissues. Tension activates proteins called integrins, which send and receive signals across cellular membranes. Those signals are the start of a cascade that leads to protein synthesis. That’s how muscles get bigger and stronger when you lift weights.
That mechanism could explain the small gains in muscle strength and size associated with static stretching, Dr. Behm said.
But can you really stretch your way to muscle growth? Theoretically, yes. But strength training is far more time-efficient, Dr. Behm says. Studies showing increases in muscle mass have typically stretched a single muscle (usually the calves, using a specialized device) for > 30 min/session, 6 d/wk for 6 weeks. And that’s for just one leg.
Still, stretching may be more accessible for some patients — research suggested that older and more sedentary people are most likely to benefit from stretching-induced gains in strength.
Stretching Benefit #2: Reduced Arterial Stiffness
“Most people don’t think about the cardiovascular benefits of stretching,” Dr. Behm said. There are some big ones.
If your body doesn’t move well, it’s not unreasonable to assume your blood doesn’t flow well. That is indeed the case: Poor flexibility is associated with arterial stiffness.
Stretching is associated not only with improved arterial function but also with reductions in resting heart rate and blood pressure and increased vasodilation.
Mobility improvements may have an indirect benefit on cardiovascular health as well.
“Studies show runners are more economical when they’re more flexible,” Dr. Behm said. If your movement is more efficient, you’ll probably do more of it. Doing more, in turn, would lead to improved fitness.
Stretching Benefit #3: Improved Performance
Research is equivocal on whether stretching improves athletic performance, said Joe Yoon, a sports massage therapist in Orlando, Florida, and author of Better Stretching.
“But I’ve always taken the approach that if you can improve your range of motion and get into positions” required for your sport, you’ll probably perform better, with less risk for injury, Mr. Yoon said.
It’s worth noting that some research over the past 30 years has linked pre-exercise static stretching with a loss of strength, power, and/or speed.
But consider this: In a 2016 review, Dr. Behm and his coauthors showed that performance reductions were most likely to occur in two situations:
When participants did extremely long stretches (duration, ≥ 60 sec per muscle).
When researchers tested the participants’ strength, power, or speed immediately after they stretched.
Avoiding those problems is easy, Dr. Behm said: Stretch each muscle for < 60 sec, and combine static stretches with more active warm-up exercises.
“Stretching can impair your performance but only if you do it wrong,” he said.
Stretching Benefit #4: Fewer Injuries
When you stretch, the point where you feel tension is where the muscle is most vulnerable. “That’s where injuries usually happen,” Dr. Behm said.
More flexibility in those areas allows your muscles to safely generate force at longer lengths. For an athlete, that means fewer injuries when they’re doing explosive movements or changing direction.
For nonathletes, flexibility reduces injuries by improving balance. Better balance reduces the risk of falling and helps mitigate the damage if you do take a tumble.
Help Your Patients Get the Benefits of Stretching
Stretching, like training for endurance or strength, can be as complex as you want to make it. But Mr. Yoon advocates a simpler approach.
“You see this flashy stuff online,” he said. “But if you see those trainers in real life or you book a session with them, they go right back to the basics.”
Ideally, Mr. Yoon said, a flexibility routine will work the entire body. But if that’s too big a stretch for your patient, he recommends starting with one or two stretches for the most problematic area.
For example, for a stiff back, try doing the puppy pose at least once a day, although twice is better. Hold the position for 30 seconds to 2 minutes, said Mr. Yoon. Even if you combine it with a dynamic movement like the cat-cow, the two exercises would take just a few minutes a day.
“There’s this misconception that you have to do a lot of it to be successful,” Mr. Yoon said.
Consistency is far more important than volume. Mr. Yoon recommends “a little bit every day — the minimum viable dose.”
As a bonus, stretching an area like your upper back will probably improve your shoulder mobility, Mr. Yoon said. Same with your lower body: Stretches for your hips, over time, should also benefit your knees and lower back.
And thanks to a phenomenon called nonlocal flexibility transfer, lower-body stretches should improve upper-body flexibility, at least temporarily. Shoulder stretches can also have an immediate effect on hip mobility.
“It’s all connected,” Mr. Yoon said, which brings us back to where we started.
If stretching can indeed reduce mortality risk, it’s probably because of interconnected pathways, rather than any single mechanism.
Most obviously, stretching improves flexibility, which makes movement easier, improves balance, and reduces the risk for falls and other types of injuries. It can also lead to small improvements in strength. Less obviously, stretching improves several aspects of cardiovascular function, including circulation.
“There seems to be a global effect in everything we do,” Dr. Behm said. “Whether you’re stretching or weight training, the message is sent throughout your body."
A version of this article appeared on Medscape.com.
FDA Approves 10th Humira Biosimilar, With Interchangeability
The US Food and Drug Administration has approved the first interchangeable, high-concentration, citrate-free adalimumab biosimilar, adalimumab-ryvk (Simlandi).
This is the 10th adalimumab biosimilar approved by the regulatory agency and the first biosimilar approval in the US market for the Icelandic pharmaceutical company Alvotech in partnership with Teva Pharmaceuticals.
“An interchangeable citrate-free, high-concentration biosimilar adalimumab has the potential to change the market dynamics in a rapidly evolving environment for biosimilars in the U.S.,” said Robert Wessman, chairman and CEO of Alvotech, in a company press release on February 23.
Adalimumab-ryvk was approved in the European Union in 2021 and in Australia and Canada in 2022.
Adalimumab-ryvk is indicated for adults with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, Crohn’s disease, plaque psoriasis, hidradenitis suppurativa, and noninfectious intermediate and posterior uveitis and panuveitis. In pediatric patients, it is indicated for polyarticular juvenile idiopathic arthritis in children 2 years of age and older and Crohn’s disease in children 6 years of age and older.
Adalimumab-ryvk is the third Humira biosimilar overall granted interchangeability status, which allows pharmacists (depending on state law) to substitute the biosimilar for the reference product without involving the prescribing clinician. Adalimumab-adbm (Cyltezo), manufactured by Boehringer Ingelheim, and adalimumab-afzb (Abrilada), manufactured by Pfizer, were previously granted interchangeability status; however, they both are interchangeable with the low-concentration formulation of Humira, which make up only an estimated 15% of Humira prescriptions, according to a report by Goodroot.
Adalimumab-ryvk will be launched “imminently” in the United States, according to the press release, but no specific dates were provided. It is also not yet known how the biosimilar will be priced compared with Humira. Other adalimumab biosimilars have launched with discounts from 5% to 85% of Humira’s list price.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration has approved the first interchangeable, high-concentration, citrate-free adalimumab biosimilar, adalimumab-ryvk (Simlandi).
This is the 10th adalimumab biosimilar approved by the regulatory agency and the first biosimilar approval in the US market for the Icelandic pharmaceutical company Alvotech in partnership with Teva Pharmaceuticals.
“An interchangeable citrate-free, high-concentration biosimilar adalimumab has the potential to change the market dynamics in a rapidly evolving environment for biosimilars in the U.S.,” said Robert Wessman, chairman and CEO of Alvotech, in a company press release on February 23.
Adalimumab-ryvk was approved in the European Union in 2021 and in Australia and Canada in 2022.
Adalimumab-ryvk is indicated for adults with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, Crohn’s disease, plaque psoriasis, hidradenitis suppurativa, and noninfectious intermediate and posterior uveitis and panuveitis. In pediatric patients, it is indicated for polyarticular juvenile idiopathic arthritis in children 2 years of age and older and Crohn’s disease in children 6 years of age and older.
Adalimumab-ryvk is the third Humira biosimilar overall granted interchangeability status, which allows pharmacists (depending on state law) to substitute the biosimilar for the reference product without involving the prescribing clinician. Adalimumab-adbm (Cyltezo), manufactured by Boehringer Ingelheim, and adalimumab-afzb (Abrilada), manufactured by Pfizer, were previously granted interchangeability status; however, they both are interchangeable with the low-concentration formulation of Humira, which make up only an estimated 15% of Humira prescriptions, according to a report by Goodroot.
Adalimumab-ryvk will be launched “imminently” in the United States, according to the press release, but no specific dates were provided. It is also not yet known how the biosimilar will be priced compared with Humira. Other adalimumab biosimilars have launched with discounts from 5% to 85% of Humira’s list price.
A version of this article appeared on Medscape.com.
The US Food and Drug Administration has approved the first interchangeable, high-concentration, citrate-free adalimumab biosimilar, adalimumab-ryvk (Simlandi).
This is the 10th adalimumab biosimilar approved by the regulatory agency and the first biosimilar approval in the US market for the Icelandic pharmaceutical company Alvotech in partnership with Teva Pharmaceuticals.
“An interchangeable citrate-free, high-concentration biosimilar adalimumab has the potential to change the market dynamics in a rapidly evolving environment for biosimilars in the U.S.,” said Robert Wessman, chairman and CEO of Alvotech, in a company press release on February 23.
Adalimumab-ryvk was approved in the European Union in 2021 and in Australia and Canada in 2022.
Adalimumab-ryvk is indicated for adults with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, Crohn’s disease, plaque psoriasis, hidradenitis suppurativa, and noninfectious intermediate and posterior uveitis and panuveitis. In pediatric patients, it is indicated for polyarticular juvenile idiopathic arthritis in children 2 years of age and older and Crohn’s disease in children 6 years of age and older.
Adalimumab-ryvk is the third Humira biosimilar overall granted interchangeability status, which allows pharmacists (depending on state law) to substitute the biosimilar for the reference product without involving the prescribing clinician. Adalimumab-adbm (Cyltezo), manufactured by Boehringer Ingelheim, and adalimumab-afzb (Abrilada), manufactured by Pfizer, were previously granted interchangeability status; however, they both are interchangeable with the low-concentration formulation of Humira, which make up only an estimated 15% of Humira prescriptions, according to a report by Goodroot.
Adalimumab-ryvk will be launched “imminently” in the United States, according to the press release, but no specific dates were provided. It is also not yet known how the biosimilar will be priced compared with Humira. Other adalimumab biosimilars have launched with discounts from 5% to 85% of Humira’s list price.
A version of this article appeared on Medscape.com.
Is Metformin a ‘Drug for All Diseases’?
In 2021 alone, clinicians wrote more than 91 million orders for the medication — up from 40 million 2004.
But is metformin just getting started? Emerging evidence suggests the drug may be effective for a much broader range of conditions beyond managing high blood glucose, including various cancers, obesity, liver disease, cardiovascular, neurodegenerative, and renal diseases. As the evidence for diverse uses accumulates, many trials have launched, with researchers looking to expand metformin’s indications and validate or explore new directions.
Metformin’s long history as a pharmaceutical includes an herbal ancestry, recognition in 1918 for its ability to lower blood glucose, being cast aside because of toxicity fears in the 1930s, rediscovery and synthesis in Europe in the 1940s, the first reported use for diabetes in 1957, and approval in the United States in 1994.
The drug has maintained its place as the preferred first-line treatment for type 2 diabetes since 2011, when it was first included in the World Health Organization’s essential medicines list.
“The focus hitherto has been primarily on its insulin sensitization effects,” Akshay Jain, MD, a clinical and research endocrinologist at TLC Diabetes and Endocrinology, in Surrey, British Columbia, Canada, told this news organization.
“The recent surge of renewed interest is in part related to its postulated effects on multiple other receptors,” he said. “In my mind, the metformin data on coronary artery disease reduction and cancer-protective effects have come farther along than other disease states.”
Cardiovascular Outcomes
Gregory G. Schwartz, MD, PhD, chief of the cardiology section at Rocky Mountain Regional VA Medical Center and professor of medicine at the University of Colorado School of Medicine in Aurora, is leading the VA-IMPACT trial. Despite metformin’s long history and widespread use, he said his study is the first placebo-controlled cardiovascular outcomes trial of the drug.
Launched in 2023, the study tests the hypothesis that metformin reduces the risk for death or nonfatal ischemic cardiovascular events in patients with prediabetes and established coronary, cerebrovascular, or peripheral artery disease, Dr. Schwartz said. The trial is being conducted at roughly 40 VA medical centers, with a planned enrollment of 7410 patients. The estimated completion date is March 2029.
“The principal mechanism of action of metformin is through activation of AMP [adenosine monophosphate]–activated protein kinase, a central pathway in metabolic regulation, cell protection, and survival,” Dr. Schwartz explained. “Experimental data have demonstrated attenuated development of atherosclerosis, reduced myocardial infarct size, improved endothelial function, and antiarrhythmic actions — none of those dependent on the presence of diabetes.”
Dr. Schwartz and his colleagues decided to test their hypothesis in people with prediabetes, rather than diabetes, to create a “true placebo-controlled comparison,” he said.
“If patients with type 2 diabetes had been chosen, there would be potential for confounding because a placebo group would require more treatment with other active antihyperglycemic medications to achieve the same degree of glycemic control as a metformin group,” Dr. Schwartz said.
“If proven efficacious in the VA-IMPACT trial, metformin could provide an inexpensive, generally safe, and well-tolerated approach to reduce cardiovascular morbidity and mortality in a large segment of the population,” Dr. Schwartz added. “Perhaps the old dog can learn some new tricks.”
Other recruiting trials looking at cardiovascular-related outcomes include Met-PEF, LIMIT, and Metformin as an Adjunctive Therapy to Catheter Ablation in Atrial Fibrillation.
Reducing Cancer Risks
Sai Yendamuri, MD, chair of the Department of Thoracic Surgery and director of the Thoracic Surgery Laboratory at Roswell Park Comprehensive Cancer Center in Buffalo, New York, is leading a phase 2 trial exploring whether metformin can prevent lung cancer in people with overweight or obesity who are at a high risk for the malignancy.
The study, which has accrued about 60% of its estimated enrollment, also will assess whether metformin can reprogram participants’ immune systems, with a view toward reducing the activity of regulatory T cells that are linked to development of tumors.
“In our preclinical and retrospective clinical data, we found that metformin had anticancer effects but only if the patients were overweight,” Dr. Yendamuri said. “In mice, we find that obesity increases regulatory T-cell function, which suppresses the immune system of the lungs. This effect is reversed by metformin.” The team is conducting the current study to examine if this happens in patients, as well. Results are expected next year.
Research is underway in other tumor types, including oral and endometrial, and brain cancers.
Preventing Alzheimer’s Disease
Cognitive function — or at least delaying its erosion — represents another front for metformin. José A. Luchsinger, MD, MPH, vice-chair for clinical and epidemiological research and director of the section on geriatrics, gerontology, and aging at Columbia University Irving Medical Center in New York City, is heading a phase 2/3 randomized controlled trial assessing the ability of the drug to prevent Alzheimer›s disease.
The study investigators hope to enroll 326 men and women aged 55-90 years with early and late mild cognitive impairment, overweight or obesity, and no diabetes.
“The hypothesis is that improving insulin and glucose levels can lead to lowering the risk of Alzheimer’s disease,” Dr. Luchsinger said. Recruitment should be complete by the end of 2024 and results are expected in late 2026.
Similar studies are underway in Europe and Asia.
Other areas of investigation, while tantalizing, are mostly in early stages, although bolstered by preclinical and mechanistic studies. The authors of a recent review on the potential mechanisms of action of metformin and existing evidence of the drug›s effectiveness — or lack thereof — in treating diseases other than diabetes, wrote: “Collectively, these data raise the question: Is metformin a drug for all diseases? It remains unclear as to whether all of these putative beneficial effects are secondary to its actions as an antihyperglycemic and insulin-sensitizing drug, or result from other cellular actions, including inhibition of mTOR (mammalian target for rapamycin), or direct antiviral actions.”
Off-Label Uses
Metformin currently is approved by the US Food and Drug Administration only for the treatment of type 2 diabetes, although it is also the only antidiabetic medication for prediabetes currently recommended by the American Diabetes Association.
Some studies currently are looking at its use in a variety of off-label indications, including obesity, gestational diabetes, weight gain from antipsychotics, and polycystic ovary syndrome.
For the most part, metformin is considered a safe drug, but it is not risk-free, Dr. Jain cautioned.
“Although it would certainly be helpful to see if this inexpensive medication that’s universally available can help in disease states, one shouldn’t overlook the potential risk of adverse effects, such as gastrointestinal, potential vitamin B12 deficiency, blunting of skeletal muscle development and the rare risk of lactic acidosis in those with kidney impairment,” he said.
“Similarly, with recent reports of the carcinogenic potential of certain formulations of long-acting metformin that contained NDMA [N-nitrosodimethylamine], it would be imperative that these kinks are removed before we incorporate metformin as the gift that keeps giving.”
Dr. Jain reported financial relationships with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk. Dr. Yendamuri disclosed serving on the scientific advisory board member of Karkinos Healthcare and research funding from Lumeda for the metformin study. Dr. Luchsinger reported receiving donated metformin and matching placebo from EMD Serono, a subsidiary of Merck, for the MAP study. Dr. Schwartz received research support from the US Department of Veterans Affairs as National Chair of the VA-IMPACT trial.
A version of this article appeared on Medscape.com.
In 2021 alone, clinicians wrote more than 91 million orders for the medication — up from 40 million 2004.
But is metformin just getting started? Emerging evidence suggests the drug may be effective for a much broader range of conditions beyond managing high blood glucose, including various cancers, obesity, liver disease, cardiovascular, neurodegenerative, and renal diseases. As the evidence for diverse uses accumulates, many trials have launched, with researchers looking to expand metformin’s indications and validate or explore new directions.
Metformin’s long history as a pharmaceutical includes an herbal ancestry, recognition in 1918 for its ability to lower blood glucose, being cast aside because of toxicity fears in the 1930s, rediscovery and synthesis in Europe in the 1940s, the first reported use for diabetes in 1957, and approval in the United States in 1994.
The drug has maintained its place as the preferred first-line treatment for type 2 diabetes since 2011, when it was first included in the World Health Organization’s essential medicines list.
“The focus hitherto has been primarily on its insulin sensitization effects,” Akshay Jain, MD, a clinical and research endocrinologist at TLC Diabetes and Endocrinology, in Surrey, British Columbia, Canada, told this news organization.
“The recent surge of renewed interest is in part related to its postulated effects on multiple other receptors,” he said. “In my mind, the metformin data on coronary artery disease reduction and cancer-protective effects have come farther along than other disease states.”
Cardiovascular Outcomes
Gregory G. Schwartz, MD, PhD, chief of the cardiology section at Rocky Mountain Regional VA Medical Center and professor of medicine at the University of Colorado School of Medicine in Aurora, is leading the VA-IMPACT trial. Despite metformin’s long history and widespread use, he said his study is the first placebo-controlled cardiovascular outcomes trial of the drug.
Launched in 2023, the study tests the hypothesis that metformin reduces the risk for death or nonfatal ischemic cardiovascular events in patients with prediabetes and established coronary, cerebrovascular, or peripheral artery disease, Dr. Schwartz said. The trial is being conducted at roughly 40 VA medical centers, with a planned enrollment of 7410 patients. The estimated completion date is March 2029.
“The principal mechanism of action of metformin is through activation of AMP [adenosine monophosphate]–activated protein kinase, a central pathway in metabolic regulation, cell protection, and survival,” Dr. Schwartz explained. “Experimental data have demonstrated attenuated development of atherosclerosis, reduced myocardial infarct size, improved endothelial function, and antiarrhythmic actions — none of those dependent on the presence of diabetes.”
Dr. Schwartz and his colleagues decided to test their hypothesis in people with prediabetes, rather than diabetes, to create a “true placebo-controlled comparison,” he said.
“If patients with type 2 diabetes had been chosen, there would be potential for confounding because a placebo group would require more treatment with other active antihyperglycemic medications to achieve the same degree of glycemic control as a metformin group,” Dr. Schwartz said.
“If proven efficacious in the VA-IMPACT trial, metformin could provide an inexpensive, generally safe, and well-tolerated approach to reduce cardiovascular morbidity and mortality in a large segment of the population,” Dr. Schwartz added. “Perhaps the old dog can learn some new tricks.”
Other recruiting trials looking at cardiovascular-related outcomes include Met-PEF, LIMIT, and Metformin as an Adjunctive Therapy to Catheter Ablation in Atrial Fibrillation.
Reducing Cancer Risks
Sai Yendamuri, MD, chair of the Department of Thoracic Surgery and director of the Thoracic Surgery Laboratory at Roswell Park Comprehensive Cancer Center in Buffalo, New York, is leading a phase 2 trial exploring whether metformin can prevent lung cancer in people with overweight or obesity who are at a high risk for the malignancy.
The study, which has accrued about 60% of its estimated enrollment, also will assess whether metformin can reprogram participants’ immune systems, with a view toward reducing the activity of regulatory T cells that are linked to development of tumors.
“In our preclinical and retrospective clinical data, we found that metformin had anticancer effects but only if the patients were overweight,” Dr. Yendamuri said. “In mice, we find that obesity increases regulatory T-cell function, which suppresses the immune system of the lungs. This effect is reversed by metformin.” The team is conducting the current study to examine if this happens in patients, as well. Results are expected next year.
Research is underway in other tumor types, including oral and endometrial, and brain cancers.
Preventing Alzheimer’s Disease
Cognitive function — or at least delaying its erosion — represents another front for metformin. José A. Luchsinger, MD, MPH, vice-chair for clinical and epidemiological research and director of the section on geriatrics, gerontology, and aging at Columbia University Irving Medical Center in New York City, is heading a phase 2/3 randomized controlled trial assessing the ability of the drug to prevent Alzheimer›s disease.
The study investigators hope to enroll 326 men and women aged 55-90 years with early and late mild cognitive impairment, overweight or obesity, and no diabetes.
“The hypothesis is that improving insulin and glucose levels can lead to lowering the risk of Alzheimer’s disease,” Dr. Luchsinger said. Recruitment should be complete by the end of 2024 and results are expected in late 2026.
Similar studies are underway in Europe and Asia.
Other areas of investigation, while tantalizing, are mostly in early stages, although bolstered by preclinical and mechanistic studies. The authors of a recent review on the potential mechanisms of action of metformin and existing evidence of the drug›s effectiveness — or lack thereof — in treating diseases other than diabetes, wrote: “Collectively, these data raise the question: Is metformin a drug for all diseases? It remains unclear as to whether all of these putative beneficial effects are secondary to its actions as an antihyperglycemic and insulin-sensitizing drug, or result from other cellular actions, including inhibition of mTOR (mammalian target for rapamycin), or direct antiviral actions.”
Off-Label Uses
Metformin currently is approved by the US Food and Drug Administration only for the treatment of type 2 diabetes, although it is also the only antidiabetic medication for prediabetes currently recommended by the American Diabetes Association.
Some studies currently are looking at its use in a variety of off-label indications, including obesity, gestational diabetes, weight gain from antipsychotics, and polycystic ovary syndrome.
For the most part, metformin is considered a safe drug, but it is not risk-free, Dr. Jain cautioned.
“Although it would certainly be helpful to see if this inexpensive medication that’s universally available can help in disease states, one shouldn’t overlook the potential risk of adverse effects, such as gastrointestinal, potential vitamin B12 deficiency, blunting of skeletal muscle development and the rare risk of lactic acidosis in those with kidney impairment,” he said.
“Similarly, with recent reports of the carcinogenic potential of certain formulations of long-acting metformin that contained NDMA [N-nitrosodimethylamine], it would be imperative that these kinks are removed before we incorporate metformin as the gift that keeps giving.”
Dr. Jain reported financial relationships with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk. Dr. Yendamuri disclosed serving on the scientific advisory board member of Karkinos Healthcare and research funding from Lumeda for the metformin study. Dr. Luchsinger reported receiving donated metformin and matching placebo from EMD Serono, a subsidiary of Merck, for the MAP study. Dr. Schwartz received research support from the US Department of Veterans Affairs as National Chair of the VA-IMPACT trial.
A version of this article appeared on Medscape.com.
In 2021 alone, clinicians wrote more than 91 million orders for the medication — up from 40 million 2004.
But is metformin just getting started? Emerging evidence suggests the drug may be effective for a much broader range of conditions beyond managing high blood glucose, including various cancers, obesity, liver disease, cardiovascular, neurodegenerative, and renal diseases. As the evidence for diverse uses accumulates, many trials have launched, with researchers looking to expand metformin’s indications and validate or explore new directions.
Metformin’s long history as a pharmaceutical includes an herbal ancestry, recognition in 1918 for its ability to lower blood glucose, being cast aside because of toxicity fears in the 1930s, rediscovery and synthesis in Europe in the 1940s, the first reported use for diabetes in 1957, and approval in the United States in 1994.
The drug has maintained its place as the preferred first-line treatment for type 2 diabetes since 2011, when it was first included in the World Health Organization’s essential medicines list.
“The focus hitherto has been primarily on its insulin sensitization effects,” Akshay Jain, MD, a clinical and research endocrinologist at TLC Diabetes and Endocrinology, in Surrey, British Columbia, Canada, told this news organization.
“The recent surge of renewed interest is in part related to its postulated effects on multiple other receptors,” he said. “In my mind, the metformin data on coronary artery disease reduction and cancer-protective effects have come farther along than other disease states.”
Cardiovascular Outcomes
Gregory G. Schwartz, MD, PhD, chief of the cardiology section at Rocky Mountain Regional VA Medical Center and professor of medicine at the University of Colorado School of Medicine in Aurora, is leading the VA-IMPACT trial. Despite metformin’s long history and widespread use, he said his study is the first placebo-controlled cardiovascular outcomes trial of the drug.
Launched in 2023, the study tests the hypothesis that metformin reduces the risk for death or nonfatal ischemic cardiovascular events in patients with prediabetes and established coronary, cerebrovascular, or peripheral artery disease, Dr. Schwartz said. The trial is being conducted at roughly 40 VA medical centers, with a planned enrollment of 7410 patients. The estimated completion date is March 2029.
“The principal mechanism of action of metformin is through activation of AMP [adenosine monophosphate]–activated protein kinase, a central pathway in metabolic regulation, cell protection, and survival,” Dr. Schwartz explained. “Experimental data have demonstrated attenuated development of atherosclerosis, reduced myocardial infarct size, improved endothelial function, and antiarrhythmic actions — none of those dependent on the presence of diabetes.”
Dr. Schwartz and his colleagues decided to test their hypothesis in people with prediabetes, rather than diabetes, to create a “true placebo-controlled comparison,” he said.
“If patients with type 2 diabetes had been chosen, there would be potential for confounding because a placebo group would require more treatment with other active antihyperglycemic medications to achieve the same degree of glycemic control as a metformin group,” Dr. Schwartz said.
“If proven efficacious in the VA-IMPACT trial, metformin could provide an inexpensive, generally safe, and well-tolerated approach to reduce cardiovascular morbidity and mortality in a large segment of the population,” Dr. Schwartz added. “Perhaps the old dog can learn some new tricks.”
Other recruiting trials looking at cardiovascular-related outcomes include Met-PEF, LIMIT, and Metformin as an Adjunctive Therapy to Catheter Ablation in Atrial Fibrillation.
Reducing Cancer Risks
Sai Yendamuri, MD, chair of the Department of Thoracic Surgery and director of the Thoracic Surgery Laboratory at Roswell Park Comprehensive Cancer Center in Buffalo, New York, is leading a phase 2 trial exploring whether metformin can prevent lung cancer in people with overweight or obesity who are at a high risk for the malignancy.
The study, which has accrued about 60% of its estimated enrollment, also will assess whether metformin can reprogram participants’ immune systems, with a view toward reducing the activity of regulatory T cells that are linked to development of tumors.
“In our preclinical and retrospective clinical data, we found that metformin had anticancer effects but only if the patients were overweight,” Dr. Yendamuri said. “In mice, we find that obesity increases regulatory T-cell function, which suppresses the immune system of the lungs. This effect is reversed by metformin.” The team is conducting the current study to examine if this happens in patients, as well. Results are expected next year.
Research is underway in other tumor types, including oral and endometrial, and brain cancers.
Preventing Alzheimer’s Disease
Cognitive function — or at least delaying its erosion — represents another front for metformin. José A. Luchsinger, MD, MPH, vice-chair for clinical and epidemiological research and director of the section on geriatrics, gerontology, and aging at Columbia University Irving Medical Center in New York City, is heading a phase 2/3 randomized controlled trial assessing the ability of the drug to prevent Alzheimer›s disease.
The study investigators hope to enroll 326 men and women aged 55-90 years with early and late mild cognitive impairment, overweight or obesity, and no diabetes.
“The hypothesis is that improving insulin and glucose levels can lead to lowering the risk of Alzheimer’s disease,” Dr. Luchsinger said. Recruitment should be complete by the end of 2024 and results are expected in late 2026.
Similar studies are underway in Europe and Asia.
Other areas of investigation, while tantalizing, are mostly in early stages, although bolstered by preclinical and mechanistic studies. The authors of a recent review on the potential mechanisms of action of metformin and existing evidence of the drug›s effectiveness — or lack thereof — in treating diseases other than diabetes, wrote: “Collectively, these data raise the question: Is metformin a drug for all diseases? It remains unclear as to whether all of these putative beneficial effects are secondary to its actions as an antihyperglycemic and insulin-sensitizing drug, or result from other cellular actions, including inhibition of mTOR (mammalian target for rapamycin), or direct antiviral actions.”
Off-Label Uses
Metformin currently is approved by the US Food and Drug Administration only for the treatment of type 2 diabetes, although it is also the only antidiabetic medication for prediabetes currently recommended by the American Diabetes Association.
Some studies currently are looking at its use in a variety of off-label indications, including obesity, gestational diabetes, weight gain from antipsychotics, and polycystic ovary syndrome.
For the most part, metformin is considered a safe drug, but it is not risk-free, Dr. Jain cautioned.
“Although it would certainly be helpful to see if this inexpensive medication that’s universally available can help in disease states, one shouldn’t overlook the potential risk of adverse effects, such as gastrointestinal, potential vitamin B12 deficiency, blunting of skeletal muscle development and the rare risk of lactic acidosis in those with kidney impairment,” he said.
“Similarly, with recent reports of the carcinogenic potential of certain formulations of long-acting metformin that contained NDMA [N-nitrosodimethylamine], it would be imperative that these kinks are removed before we incorporate metformin as the gift that keeps giving.”
Dr. Jain reported financial relationships with Abbott, Amgen, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, Medtronic, Merck, and Novo Nordisk. Dr. Yendamuri disclosed serving on the scientific advisory board member of Karkinos Healthcare and research funding from Lumeda for the metformin study. Dr. Luchsinger reported receiving donated metformin and matching placebo from EMD Serono, a subsidiary of Merck, for the MAP study. Dr. Schwartz received research support from the US Department of Veterans Affairs as National Chair of the VA-IMPACT trial.
A version of this article appeared on Medscape.com.
Does Bariatric Surgery Increase or Decrease Cancer Risk? It Depends.
Bariatric surgery appears to decrease the risk for some cancers, but it may increase the risk for others, particularly colorectal cancer (CRC), according to a synthesis of current evidence.
“There has been a recent burst of studies examining the association between bariatric surgery and the longitudinal risks of developing cancer,” corresponding author Zhi Ven Fong, MD, MPH, DrPH, surgical oncologist, Mayo Clinic Arizona, Phoenix, said in an interview. “However, there has not been a rigorous and critical analysis of the data published to date.”
In evaluating research showing an association between bariatric surgery and longitudinal cancer risk, the investigators found that the quality of the studies and their findings are “heterogeneous and might be susceptible to bias,” Dr. Fong said.
Bariatric surgery appears to have the strongest and most consistent association with the reduction of breast, ovarian, and endometrial cancer risk, first author Pei-Wen Lim, MD, MS, bariatric surgeon at Mayo Clinic Arizona, Phoenix, told this news organization. “However, there have been concerning signals from preclinical and epidemiological studies that bariatric surgery may be associated with a higher risk of developing colorectal cancers,” she added.
The authors cautioned against certain changes in clinical management.
“First, cancer surveillance frequency should not be altered after bariatric surgery because of any assumed reduction in longitudinal cancer risk, and surveillance strategy should mirror that of an average-risk individual,” they wrote. “Secondly, the indications for bariatric surgery should not be expanded for the purpose of cancer-risk mitigation.”
The review was published online in JAMA Surgery.
Protection Against Hormone-Related Cancers
The authors pointed to several studies that appear to support the association between bariatric surgery and decreased risk for hormone-related cancers.
Among them is an observational study of 6781 patients in Canada that showed a significant reduction in breast cancer risk at a median follow-up of 5 years in those who had bariatric surgery vs those who did not (P = .01).
The largest study to date on risk for hormone-related cancer after bariatric surgery was conducted using New York State data for 302,883 women.
It showed a lower rate of breast, endometrial, and ovarian cancers after bariatric surgery (hazard ratio [HR], 0.78; P < .001), with Roux-en-Y gastric bypass conferring the greatest benefit compared with laparoscopic sleeve gastrectomy (HR, 0.66; P = .006) and laparoscopic adjustable gastric banding (HR, 0.83; P = .006).
Beyond the shared mechanisms explaining obesity and cancer risk, a proposed explanation for the strong, consistent association between bariatric surgery and hormone-sensitive cancers is the role obesity-related changes in estrogen stimulation play in development of such cancers, the authors noted.
Association With GI Cancers
The association between bariatric surgery and development of esophageal, gastric, liver, and pancreas cancers is less clear. The data are heterogeneous, with studies showing either no association or decreased longitudinal incidence, the authors reported.
The data are also mixed when it comes to CRC. Epidemiological studies have demonstrated decreased longitudinal incidence of colon and rectal cancer after bariatric surgery; however, two studies have suggested an increased CRC risk after bariatric surgery, the authors noted.
A 15-year study from England that matched 8794 patients with obesity who underwent bariatric surgery with 8794 patients with obesity who did not have the surgery showed that gastric bypass (but not gastric banding or sleeve gastrectomy) was associated with a greater than twofold increased risk of developing colon and rectal cancer (odds ratio, 2.63).
These findings were corroborated in a Swedish cohort study with more than 10 years of follow-up data.
One potential explanation for the heterogeneous findings is that “present studies do not discriminate the sub-types of colon and rectal cancer, with bariatric surgery possibly increasing the incidence of colitis-associated cancers but not hereditary cancers,” the authors wrote.
“The mechanism by which gastric bypass may increase the risk of colorectal cancer is through changes in the gut’s microbiome. These changes in gut flora may triumph the protective effect of weight loss on the development of colorectal cancers,” Dr. Fong said.
Prospective studies are necessary to better delineate CRC risk after bariatric surgery, the authors wrote.
Benefits Outweigh Risk
“Ultimately, it has been proven that bariatric surgery saves lives by improving the metabolic profile of patients with obesity through reduction in cardiovascular risk factors such as hypertension, diabetes, and nonalcoholic fatty liver disease,” Dr. Lim said.
“If patients qualify for bariatric surgery on the basis of their BMI or comorbidities, they should pursue it for its metabolic benefits, but perhaps consider timely or closer-interval screening colonoscopies to monitor for potential colorectal cancer development,” Dr. Lim added.
When asked to comment on the review, Marina Kurian, MD, president, American Society for Metabolic and Bariatric Surgery, also pointed to the advantages of bariatric surgery in reducing major adverse cardiovascular events and improving hypertension, hyperlipidemia, and diabetes.
Bariatric surgery reduces many types of cancers, although the data specific to CRC risk with bariatric surgery are mixed, she added.
“The jury is still out,” said Dr. Kurian, clinical professor of surgery at NYU Langone Health in New York, who was not involved in the review. “There are papers and meta-analyses that show benefit even in colorectal cancer, but then there are a couple of papers out there that suggest a risk that seems to be specific to men.
“It could just be a numbers game, where we may not have enough data. We need more granular data that will help address these nuances and really determine what is the actual risk,” Dr. Kurian said. “But overall, for cancer, bariatric surgery is a win.”
This research had no specific funding. Dr. Fong and Dr. Lim had no relevant disclosures. Dr. Kurian disclosed relationships with Allergan, Allurion, CineMed, CSATS, Ezisurg Medical, Hernon, Johnson & Johnson, Medtronic, Novo, Stryker, and Vivus.
A version of this article appeared on Medscape.com.
Bariatric surgery appears to decrease the risk for some cancers, but it may increase the risk for others, particularly colorectal cancer (CRC), according to a synthesis of current evidence.
“There has been a recent burst of studies examining the association between bariatric surgery and the longitudinal risks of developing cancer,” corresponding author Zhi Ven Fong, MD, MPH, DrPH, surgical oncologist, Mayo Clinic Arizona, Phoenix, said in an interview. “However, there has not been a rigorous and critical analysis of the data published to date.”
In evaluating research showing an association between bariatric surgery and longitudinal cancer risk, the investigators found that the quality of the studies and their findings are “heterogeneous and might be susceptible to bias,” Dr. Fong said.
Bariatric surgery appears to have the strongest and most consistent association with the reduction of breast, ovarian, and endometrial cancer risk, first author Pei-Wen Lim, MD, MS, bariatric surgeon at Mayo Clinic Arizona, Phoenix, told this news organization. “However, there have been concerning signals from preclinical and epidemiological studies that bariatric surgery may be associated with a higher risk of developing colorectal cancers,” she added.
The authors cautioned against certain changes in clinical management.
“First, cancer surveillance frequency should not be altered after bariatric surgery because of any assumed reduction in longitudinal cancer risk, and surveillance strategy should mirror that of an average-risk individual,” they wrote. “Secondly, the indications for bariatric surgery should not be expanded for the purpose of cancer-risk mitigation.”
The review was published online in JAMA Surgery.
Protection Against Hormone-Related Cancers
The authors pointed to several studies that appear to support the association between bariatric surgery and decreased risk for hormone-related cancers.
Among them is an observational study of 6781 patients in Canada that showed a significant reduction in breast cancer risk at a median follow-up of 5 years in those who had bariatric surgery vs those who did not (P = .01).
The largest study to date on risk for hormone-related cancer after bariatric surgery was conducted using New York State data for 302,883 women.
It showed a lower rate of breast, endometrial, and ovarian cancers after bariatric surgery (hazard ratio [HR], 0.78; P < .001), with Roux-en-Y gastric bypass conferring the greatest benefit compared with laparoscopic sleeve gastrectomy (HR, 0.66; P = .006) and laparoscopic adjustable gastric banding (HR, 0.83; P = .006).
Beyond the shared mechanisms explaining obesity and cancer risk, a proposed explanation for the strong, consistent association between bariatric surgery and hormone-sensitive cancers is the role obesity-related changes in estrogen stimulation play in development of such cancers, the authors noted.
Association With GI Cancers
The association between bariatric surgery and development of esophageal, gastric, liver, and pancreas cancers is less clear. The data are heterogeneous, with studies showing either no association or decreased longitudinal incidence, the authors reported.
The data are also mixed when it comes to CRC. Epidemiological studies have demonstrated decreased longitudinal incidence of colon and rectal cancer after bariatric surgery; however, two studies have suggested an increased CRC risk after bariatric surgery, the authors noted.
A 15-year study from England that matched 8794 patients with obesity who underwent bariatric surgery with 8794 patients with obesity who did not have the surgery showed that gastric bypass (but not gastric banding or sleeve gastrectomy) was associated with a greater than twofold increased risk of developing colon and rectal cancer (odds ratio, 2.63).
These findings were corroborated in a Swedish cohort study with more than 10 years of follow-up data.
One potential explanation for the heterogeneous findings is that “present studies do not discriminate the sub-types of colon and rectal cancer, with bariatric surgery possibly increasing the incidence of colitis-associated cancers but not hereditary cancers,” the authors wrote.
“The mechanism by which gastric bypass may increase the risk of colorectal cancer is through changes in the gut’s microbiome. These changes in gut flora may triumph the protective effect of weight loss on the development of colorectal cancers,” Dr. Fong said.
Prospective studies are necessary to better delineate CRC risk after bariatric surgery, the authors wrote.
Benefits Outweigh Risk
“Ultimately, it has been proven that bariatric surgery saves lives by improving the metabolic profile of patients with obesity through reduction in cardiovascular risk factors such as hypertension, diabetes, and nonalcoholic fatty liver disease,” Dr. Lim said.
“If patients qualify for bariatric surgery on the basis of their BMI or comorbidities, they should pursue it for its metabolic benefits, but perhaps consider timely or closer-interval screening colonoscopies to monitor for potential colorectal cancer development,” Dr. Lim added.
When asked to comment on the review, Marina Kurian, MD, president, American Society for Metabolic and Bariatric Surgery, also pointed to the advantages of bariatric surgery in reducing major adverse cardiovascular events and improving hypertension, hyperlipidemia, and diabetes.
Bariatric surgery reduces many types of cancers, although the data specific to CRC risk with bariatric surgery are mixed, she added.
“The jury is still out,” said Dr. Kurian, clinical professor of surgery at NYU Langone Health in New York, who was not involved in the review. “There are papers and meta-analyses that show benefit even in colorectal cancer, but then there are a couple of papers out there that suggest a risk that seems to be specific to men.
“It could just be a numbers game, where we may not have enough data. We need more granular data that will help address these nuances and really determine what is the actual risk,” Dr. Kurian said. “But overall, for cancer, bariatric surgery is a win.”
This research had no specific funding. Dr. Fong and Dr. Lim had no relevant disclosures. Dr. Kurian disclosed relationships with Allergan, Allurion, CineMed, CSATS, Ezisurg Medical, Hernon, Johnson & Johnson, Medtronic, Novo, Stryker, and Vivus.
A version of this article appeared on Medscape.com.
Bariatric surgery appears to decrease the risk for some cancers, but it may increase the risk for others, particularly colorectal cancer (CRC), according to a synthesis of current evidence.
“There has been a recent burst of studies examining the association between bariatric surgery and the longitudinal risks of developing cancer,” corresponding author Zhi Ven Fong, MD, MPH, DrPH, surgical oncologist, Mayo Clinic Arizona, Phoenix, said in an interview. “However, there has not been a rigorous and critical analysis of the data published to date.”
In evaluating research showing an association between bariatric surgery and longitudinal cancer risk, the investigators found that the quality of the studies and their findings are “heterogeneous and might be susceptible to bias,” Dr. Fong said.
Bariatric surgery appears to have the strongest and most consistent association with the reduction of breast, ovarian, and endometrial cancer risk, first author Pei-Wen Lim, MD, MS, bariatric surgeon at Mayo Clinic Arizona, Phoenix, told this news organization. “However, there have been concerning signals from preclinical and epidemiological studies that bariatric surgery may be associated with a higher risk of developing colorectal cancers,” she added.
The authors cautioned against certain changes in clinical management.
“First, cancer surveillance frequency should not be altered after bariatric surgery because of any assumed reduction in longitudinal cancer risk, and surveillance strategy should mirror that of an average-risk individual,” they wrote. “Secondly, the indications for bariatric surgery should not be expanded for the purpose of cancer-risk mitigation.”
The review was published online in JAMA Surgery.
Protection Against Hormone-Related Cancers
The authors pointed to several studies that appear to support the association between bariatric surgery and decreased risk for hormone-related cancers.
Among them is an observational study of 6781 patients in Canada that showed a significant reduction in breast cancer risk at a median follow-up of 5 years in those who had bariatric surgery vs those who did not (P = .01).
The largest study to date on risk for hormone-related cancer after bariatric surgery was conducted using New York State data for 302,883 women.
It showed a lower rate of breast, endometrial, and ovarian cancers after bariatric surgery (hazard ratio [HR], 0.78; P < .001), with Roux-en-Y gastric bypass conferring the greatest benefit compared with laparoscopic sleeve gastrectomy (HR, 0.66; P = .006) and laparoscopic adjustable gastric banding (HR, 0.83; P = .006).
Beyond the shared mechanisms explaining obesity and cancer risk, a proposed explanation for the strong, consistent association between bariatric surgery and hormone-sensitive cancers is the role obesity-related changes in estrogen stimulation play in development of such cancers, the authors noted.
Association With GI Cancers
The association between bariatric surgery and development of esophageal, gastric, liver, and pancreas cancers is less clear. The data are heterogeneous, with studies showing either no association or decreased longitudinal incidence, the authors reported.
The data are also mixed when it comes to CRC. Epidemiological studies have demonstrated decreased longitudinal incidence of colon and rectal cancer after bariatric surgery; however, two studies have suggested an increased CRC risk after bariatric surgery, the authors noted.
A 15-year study from England that matched 8794 patients with obesity who underwent bariatric surgery with 8794 patients with obesity who did not have the surgery showed that gastric bypass (but not gastric banding or sleeve gastrectomy) was associated with a greater than twofold increased risk of developing colon and rectal cancer (odds ratio, 2.63).
These findings were corroborated in a Swedish cohort study with more than 10 years of follow-up data.
One potential explanation for the heterogeneous findings is that “present studies do not discriminate the sub-types of colon and rectal cancer, with bariatric surgery possibly increasing the incidence of colitis-associated cancers but not hereditary cancers,” the authors wrote.
“The mechanism by which gastric bypass may increase the risk of colorectal cancer is through changes in the gut’s microbiome. These changes in gut flora may triumph the protective effect of weight loss on the development of colorectal cancers,” Dr. Fong said.
Prospective studies are necessary to better delineate CRC risk after bariatric surgery, the authors wrote.
Benefits Outweigh Risk
“Ultimately, it has been proven that bariatric surgery saves lives by improving the metabolic profile of patients with obesity through reduction in cardiovascular risk factors such as hypertension, diabetes, and nonalcoholic fatty liver disease,” Dr. Lim said.
“If patients qualify for bariatric surgery on the basis of their BMI or comorbidities, they should pursue it for its metabolic benefits, but perhaps consider timely or closer-interval screening colonoscopies to monitor for potential colorectal cancer development,” Dr. Lim added.
When asked to comment on the review, Marina Kurian, MD, president, American Society for Metabolic and Bariatric Surgery, also pointed to the advantages of bariatric surgery in reducing major adverse cardiovascular events and improving hypertension, hyperlipidemia, and diabetes.
Bariatric surgery reduces many types of cancers, although the data specific to CRC risk with bariatric surgery are mixed, she added.
“The jury is still out,” said Dr. Kurian, clinical professor of surgery at NYU Langone Health in New York, who was not involved in the review. “There are papers and meta-analyses that show benefit even in colorectal cancer, but then there are a couple of papers out there that suggest a risk that seems to be specific to men.
“It could just be a numbers game, where we may not have enough data. We need more granular data that will help address these nuances and really determine what is the actual risk,” Dr. Kurian said. “But overall, for cancer, bariatric surgery is a win.”
This research had no specific funding. Dr. Fong and Dr. Lim had no relevant disclosures. Dr. Kurian disclosed relationships with Allergan, Allurion, CineMed, CSATS, Ezisurg Medical, Hernon, Johnson & Johnson, Medtronic, Novo, Stryker, and Vivus.
A version of this article appeared on Medscape.com.
Low-Glycemic Index Diet Benefits Mirror Fiber, Whole Grain
TOPLINE:
A diet with a low glycemic index (GI) had protective effects against diabetes and other chronic diseases similar to those of a diet high in fiber and whole grains.
METHODOLOGY:
- A 2019 Lancet report from the World Health Organization promoted fiber and whole grains to manage type 2 diabetes, cardiovascular disease, and cancer but rejected GI as a relevant dietary factor to prevent chronic diseases.
- This meta-analysis assessed the evidence of how GI and glycemic load are associated with four main outcomes and did the same for diets high in fiber and whole grain.
- Researchers identified 10 large prospective cohort studies (each including ≥ 100,000 participants) that assessed associations of GI, glycemic load, and fiber and whole grains with the outcomes of interest.
- The mean age was 56 years, and the mean follow-up duration was 12.6 years.
- The primary outcomes were incidence of type 2 diabetes, cardiovascular diseases and its components, diabetes-related cancers, and all-cause mortality.
TAKEAWAY:
- Compared with low-GI diets, high-GI diets were associated with an increased risk for:
- Type 2 diabetes (relative risk [RR], 1.27; P < .0001)
- Total cardiovascular disease (RR, 1.15; P < .0001)
- Diabetes-related cancers (RR, 1.05; P = .0001)
- All-cause mortality (RR, 1.08; P < .0001), statistically significant in women only.
- Foods with high glycemic load were associated with an increased risk for incident type 2 diabetes (RR, 1.15; P < .0001) and total cardiovascular disease (RR, 1.15; P < .0001) than foods with a low glycemic load.
- A diet high in fiber and whole grains reduced the risk for all four outcomes, with the association being similar to that observed for low-GI diet.
IN PRACTICE:
“These findings justify the combination of GI with fiber and whole grains in dietary recommendations to reduce the risk of diabetes and related chronic diseases,” the authors wrote.
SOURCE:
This study was led by David J.A. Jenkins, MD, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Ontario, Canada, and published online in The Lancet Diabetes & Endocrinology.
LIMITATIONS:
The lack of evaluation or absence of positive effects in some analyses may have led to a paucity of reported studies for some outcomes. Moreover, the findings for some outcomes may have had limited robustness because of a small difference in RR. Furthermore, only one or two cohorts were included to compare most disease outcomes related to GI with fiber and wholegrain exposure.
DISCLOSURES:
This study was funded by Banting and Best and the Karuna Foundation. The authors declared receiving research grants, payments, honoraria, and travel support from and having other ties with food and beverage growers, processors and manufacturers, as well as with foundations, chronic disease advocacy and research groups, professional societies, government organizations, and other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A diet with a low glycemic index (GI) had protective effects against diabetes and other chronic diseases similar to those of a diet high in fiber and whole grains.
METHODOLOGY:
- A 2019 Lancet report from the World Health Organization promoted fiber and whole grains to manage type 2 diabetes, cardiovascular disease, and cancer but rejected GI as a relevant dietary factor to prevent chronic diseases.
- This meta-analysis assessed the evidence of how GI and glycemic load are associated with four main outcomes and did the same for diets high in fiber and whole grain.
- Researchers identified 10 large prospective cohort studies (each including ≥ 100,000 participants) that assessed associations of GI, glycemic load, and fiber and whole grains with the outcomes of interest.
- The mean age was 56 years, and the mean follow-up duration was 12.6 years.
- The primary outcomes were incidence of type 2 diabetes, cardiovascular diseases and its components, diabetes-related cancers, and all-cause mortality.
TAKEAWAY:
- Compared with low-GI diets, high-GI diets were associated with an increased risk for:
- Type 2 diabetes (relative risk [RR], 1.27; P < .0001)
- Total cardiovascular disease (RR, 1.15; P < .0001)
- Diabetes-related cancers (RR, 1.05; P = .0001)
- All-cause mortality (RR, 1.08; P < .0001), statistically significant in women only.
- Foods with high glycemic load were associated with an increased risk for incident type 2 diabetes (RR, 1.15; P < .0001) and total cardiovascular disease (RR, 1.15; P < .0001) than foods with a low glycemic load.
- A diet high in fiber and whole grains reduced the risk for all four outcomes, with the association being similar to that observed for low-GI diet.
IN PRACTICE:
“These findings justify the combination of GI with fiber and whole grains in dietary recommendations to reduce the risk of diabetes and related chronic diseases,” the authors wrote.
SOURCE:
This study was led by David J.A. Jenkins, MD, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Ontario, Canada, and published online in The Lancet Diabetes & Endocrinology.
LIMITATIONS:
The lack of evaluation or absence of positive effects in some analyses may have led to a paucity of reported studies for some outcomes. Moreover, the findings for some outcomes may have had limited robustness because of a small difference in RR. Furthermore, only one or two cohorts were included to compare most disease outcomes related to GI with fiber and wholegrain exposure.
DISCLOSURES:
This study was funded by Banting and Best and the Karuna Foundation. The authors declared receiving research grants, payments, honoraria, and travel support from and having other ties with food and beverage growers, processors and manufacturers, as well as with foundations, chronic disease advocacy and research groups, professional societies, government organizations, and other sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A diet with a low glycemic index (GI) had protective effects against diabetes and other chronic diseases similar to those of a diet high in fiber and whole grains.
METHODOLOGY:
- A 2019 Lancet report from the World Health Organization promoted fiber and whole grains to manage type 2 diabetes, cardiovascular disease, and cancer but rejected GI as a relevant dietary factor to prevent chronic diseases.
- This meta-analysis assessed the evidence of how GI and glycemic load are associated with four main outcomes and did the same for diets high in fiber and whole grain.
- Researchers identified 10 large prospective cohort studies (each including ≥ 100,000 participants) that assessed associations of GI, glycemic load, and fiber and whole grains with the outcomes of interest.
- The mean age was 56 years, and the mean follow-up duration was 12.6 years.
- The primary outcomes were incidence of type 2 diabetes, cardiovascular diseases and its components, diabetes-related cancers, and all-cause mortality.
TAKEAWAY:
- Compared with low-GI diets, high-GI diets were associated with an increased risk for:
- Type 2 diabetes (relative risk [RR], 1.27; P < .0001)
- Total cardiovascular disease (RR, 1.15; P < .0001)
- Diabetes-related cancers (RR, 1.05; P = .0001)
- All-cause mortality (RR, 1.08; P < .0001), statistically significant in women only.
- Foods with high glycemic load were associated with an increased risk for incident type 2 diabetes (RR, 1.15; P < .0001) and total cardiovascular disease (RR, 1.15; P < .0001) than foods with a low glycemic load.
- A diet high in fiber and whole grains reduced the risk for all four outcomes, with the association being similar to that observed for low-GI diet.
IN PRACTICE:
“These findings justify the combination of GI with fiber and whole grains in dietary recommendations to reduce the risk of diabetes and related chronic diseases,” the authors wrote.
SOURCE:
This study was led by David J.A. Jenkins, MD, Department of Nutritional Sciences, Temerty Faculty of Medicine, University of Toronto, Ontario, Canada, and published online in The Lancet Diabetes & Endocrinology.
LIMITATIONS:
The lack of evaluation or absence of positive effects in some analyses may have led to a paucity of reported studies for some outcomes. Moreover, the findings for some outcomes may have had limited robustness because of a small difference in RR. Furthermore, only one or two cohorts were included to compare most disease outcomes related to GI with fiber and wholegrain exposure.
DISCLOSURES:
This study was funded by Banting and Best and the Karuna Foundation. The authors declared receiving research grants, payments, honoraria, and travel support from and having other ties with food and beverage growers, processors and manufacturers, as well as with foundations, chronic disease advocacy and research groups, professional societies, government organizations, and other sources.
A version of this article appeared on Medscape.com.
GLP-1s’ Next Target: Male Infertility?
The explosion of interest in glucagon-like peptide 1 receptor agonists (GLP-1 RAs), such as semaglutide and tirzepatide, has raised questions about what therapeutic effects this class of medication might have beyond their current indications for type 2 diabetes and obesity.
Recent clinical trials have recently identified benefits from GLP-1 agents for the heart, liver, and kidneys, but the current evidence base is murkier regarding how the drugs may affect male fertility.
For starters, overweight and obesity are strongly associated with male infertility in several overlapping ways. Obesity can disrupt hormones linked to fertility, increase the risk for defective sperm, adversely affect semen quality, and even make sexual intercourse more difficult due to obesity’s link to erectile dysfunction. As a result, GLP-1 RAs should at least in theory boost male fertility in men who take the drugs to lose weight.
But animal studies and a handful of small trials and observational data point to the potential for GLP-1 RAs to improve male fertility in other ways.
A recent narrative review on GLP-1 RAs and male reproductive health, published in the journal Medicina in December 2023, surveyed the potential of the drugs for male infertility and offered reason for optimism.
Hossein Sadeghi-Nejad, MD, director of urology at NYU Langone Health, New York, and a coauthor of the article, said that one reason he and his colleagues conducted their analysis was the known association between weight loss and an increase in testosterone.
“Most of the animal studies that are out there show that this class of drugs does affect testosterone levels,” Dr. Sadeghi-Nejad said; they wanted to better understand what other evidence showed about GLP-1 agonists and other fertility factors.
Link Between Obesity and Fertility
The recent paper first reviews the well-established link between obesity and poorer fertility outcomes.
“Certainly, obesity poses a significant societal problem with substantial impacts on both overall health and economic aspects,” senior author Ranjith Ramasamy, MD, associate professor of urology and director of the reproductive urology Fellowship program at the University of Miami’s Miller School of Medicine, told this news organization. “The escalating global obesity rates raise concerns, especially in the field of male infertility, where excessive body fat induces intrinsic hormonal changes leading to alterations, eventually, in semen parameters.”
The authors noted that obesity has been linked in the research to worse assisted reproductive technology (ART) outcomes and to subfecundity, taking more than 12 months to achieve pregnancy. They also referenced a systematic review that found men with obesity were more likely to have lower sperm counts and less viable sperm.
“From our standpoint, I think the key point was to raise awareness about the fact that obesity, because of the aromatization of testosterone to estradiol [from excess adipose tissue], will affect the hormonal axis and the availability of testosterone and, therefore, indirectly affects spermatogenesis,” Dr. Sadeghi-Nejad said.
Obesity is also linked to lower levels of inhibin B, which stimulates testosterone secretion in Sertoli cells, which, when combined with the proinflammatory state of obesity, “results in a less favorable environment for sperm production,” he said. Finally, the link between obesity and poorer sexual function further inhibits fertility potential, he added.
Until recently, the primary treatments for obesity in men experiencing fertility problems have been lifestyle modifications or surgical interventions. But the recent approval of GLP-1 RA drugs for obesity present an additional option depending on how these drugs affect other fertility parameters.
Direct or Indirect Effects?
Most of the available evidence on GLP-RAs and sperm parameters comes from preclinical research. One of the few clinical trials, published last year in the Journal of Clinical Medicine, investigated the effects of liraglutide in men with metabolic hypogonadism, a body mass index between (BMI) 30 and 40, and severe erectile dysfunction.
Among the 110 men enrolled in the study, only the 35 participants who said that they were not seeking fatherhood received liraglutide. After 4 months of treatment, these men had significantly improved semen concentration, motility, and morphology than did those wanting to conceive who received conventional fertility treatment. Erectile dysfunction was also more improved in the liraglutide group, according to the researchers.
Though this study demonstrated the potential for liraglutide to treat metabolic hypogonadism, the men in that group also had greater weight loss and BMI reduction than the other participants. The review cited several other studies — albeit small ones — in which weight loss was associated with improvements in sperm parameters, including one randomized controlled trial in which one group lost weight with liraglutide and the other with lifestyle modifications; both groups showed increases in the concentration and number of sperm.
One of the key questions requiring further research, then, is whether GLP-1 agents have direct effects on male fertility independent of a reduction in obesity. The randomized controlled trials comparing liraglutide and lifestyle modifications failed to find additional effects on semen in the men taking liraglutide; however, the study had only 56 participants, and results from liraglutide cannot be generalized to potential effects of semaglutide or tirzepatide, Dr. Sadeghi-Nejad said.
“Determining the relative contributions of weight loss versus direct drug actions on fertility outcomes remains challenging without robust data,” Dr. Ramasamy said. “While acknowledged that diet and physical activity positively impact fertility, confirming the synergistic role of GLP-1 receptor agonists requires evidence from well-designed randomized clinical trials.”
Rodent studies suggest that GLP-1 RAs may independently affect testicular function because GLP-1 receptors exist in Sertoli and Leydig cells of the testes. In one study, for example, obese mice who received the GLP-1 agonist exenatide for 8 weeks had “improved sperm motility, DNA integrity, and decreased expression of pro-inflammatory cytokines,” the authors of the review reported. But the precise mechanisms aren’t well understood.
“We know that there are GLP-1 receptors in the reproductive tract, but the extent of the downstream effect of stimulating those receptors, I don’t think we know well,” said John P. Lindsey II, MD, MEng, assistant professor of urology at University of California San Francisco Health.
Other hormonal effects of GLP-1 agonists, such as stimulating insulin production and better regulating blood glucose levels, are better understood, said Raevti Bole, MD, a urologist at Cleveland Clinic, in Ohio, but still other effects of the drugs may not yet be identified.
“I think the really big unknown is whether these types of drugs have effects that are not hormonal on male fertility and what those effects are, and how those affect sperm,” Dr. Bole said. “For example, we know that these drugs slow gastric emptying. Is it possible that slow gastric emptying affects some of the nutrients that you absorb, and that could affect fertility?” Similarly, she said, it’s not clear whether GLP-1 agonists would have any effects on the thyroid that could then affect fertility.
Effects on Offspring
Another open question about GLP-1 RAs and male fertility is their potential effects on the offspring, said Sriram Machineni, MBBS, associate professor of endocrinology at the Albert Einstein College of Medicine in New York City. The clinical trials involving the drugs for treating type 2 diabetes and obesity required both men and women to use contraception. If sperm contributing to a pregnancy are exposed to a GLP-1 agent, “we don’t know what the consequences could be,” Dr. Machineni said. “Just increasing the fertility of the man is not enough. We need to make sure it’s safe long-term for the fetus.”
Dr. Bole also pointed out the need for understanding potential effects in the fetus.
“We know that there are epigenetic changes that can happen to sperm that are influenced by the lifestyle and the physical health and environment of the parent,” Dr. Bole said. “So how could these drugs potentially affect those epigenetic changes that then potentially are passed on to the offspring? We don’t know that.”
An ideal source for that data would be a cohort registry of people who are taking the medication and then cause a pregnancy. “They have a registry for pregnant women,” Dr. Machineni said, “but we need something similar for men.”
Dr. Sadeghi-Nejad said that he and his coauthors are working on developing a registry for men who take GLP-1 RAs that would enable long-term tracking of multiple andrologic outcomes, including fertility and sexual dysfunction. Such a registry could theoretically be useful in tracking pregnancy and offspring outcomes as well.
Too Soon for Prescribing
Additional options for treating fertility in men with obesity would be welcome. Current treatments include the selective estrogen receptor modulator (SERM) clomiphene citrate and the aromatase inhibitor anastrozole. But these have their drawbacks, Dr. Sadeghi-Nejad pointed out; in the overweight population in particular, they “are not necessarily ideal,” he said.
“Although both are viable treatments for enhancing hormonal balance and semen parameters, clomiphene citrate has rare but documented side effects, including thromboembolism, gastrointestinal distress and occasional weight gain in men,” Dr. Sadeghi-Nejad and his colleagues wrote. “Furthermore, despite clomiphene citrate’s association with significant increases in sperm concentration, it is not universally effective, with a meta-analysis indicating a significant increase in sperm concentration in approximately 60% of men.”
For men who have obesity and oligospermia but normal levels of testosterone and estradiol, “conventional pharmaceutical approaches like clomiphene may not be suitable,” the authors wrote.
Still, GLP-1 RAs may have a role to play for this population.
“I think it is within the wheelhouse of a reproductive urologist to consider those types of medications,” Dr. Lindsey said. For example, for a patient who has overweight or obesity, “does it make sense to think about doing clomiphene therapy, which we often do for someone who has low testosterone, in conjunction [with a GLP-1 agonist]? Maybe there’s a kind of an additive effect of having both on board.”
Dr. Ramasamy similarly noted that GLP-1 agonists cannot replace SERMs but may work “synergistically” with them.
“Despite the established popularity of GLP-1 receptor agonists, there may be some reluctance among urologists and fertility specialists to prescribe them, with some others advocating for their use to enhance semen parameters,” Dr. Ramasamy said. “However, robust scientific evidence is still lacking, necessitating caution and a wait for more substantial data.”
Even if GLP-1 RAs prove to have therapeutic benefit for fertility, considerations such as availability and cost may affect prescribing.
“We do currently have safe and effective drugs that we use for male fertility, and those are generally nowhere near as expensive,” Dr. Bole said. “When we start talking about another drug that we can add, we have to think about the efficacy and the potential side effect but also, is this affordable for patients?”
Eventually, once more evidence become available, all of the urologists who spoke with this news organization said that they expect discussion about the possible therapeutic utility of GLP-1 agonists to make its way into clinical guidelines.
“Obesity is such a huge impediment for fertility in the modern environment,” Dr. Machineni said. “We will have to clarify the use of these agents, so I think this will be a part of the guidelines some point, but I think we need more information.”
The research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the American Cancer Society. The review authors and other quoted physicians reported no disclosures. Dr. Machineni has consulted for Novo Nordisk and Lilly and has conducted clinical trials with semaglutide and tirzepatide for those companies.
A version of this article appeared on Medscape.com.
The explosion of interest in glucagon-like peptide 1 receptor agonists (GLP-1 RAs), such as semaglutide and tirzepatide, has raised questions about what therapeutic effects this class of medication might have beyond their current indications for type 2 diabetes and obesity.
Recent clinical trials have recently identified benefits from GLP-1 agents for the heart, liver, and kidneys, but the current evidence base is murkier regarding how the drugs may affect male fertility.
For starters, overweight and obesity are strongly associated with male infertility in several overlapping ways. Obesity can disrupt hormones linked to fertility, increase the risk for defective sperm, adversely affect semen quality, and even make sexual intercourse more difficult due to obesity’s link to erectile dysfunction. As a result, GLP-1 RAs should at least in theory boost male fertility in men who take the drugs to lose weight.
But animal studies and a handful of small trials and observational data point to the potential for GLP-1 RAs to improve male fertility in other ways.
A recent narrative review on GLP-1 RAs and male reproductive health, published in the journal Medicina in December 2023, surveyed the potential of the drugs for male infertility and offered reason for optimism.
Hossein Sadeghi-Nejad, MD, director of urology at NYU Langone Health, New York, and a coauthor of the article, said that one reason he and his colleagues conducted their analysis was the known association between weight loss and an increase in testosterone.
“Most of the animal studies that are out there show that this class of drugs does affect testosterone levels,” Dr. Sadeghi-Nejad said; they wanted to better understand what other evidence showed about GLP-1 agonists and other fertility factors.
Link Between Obesity and Fertility
The recent paper first reviews the well-established link between obesity and poorer fertility outcomes.
“Certainly, obesity poses a significant societal problem with substantial impacts on both overall health and economic aspects,” senior author Ranjith Ramasamy, MD, associate professor of urology and director of the reproductive urology Fellowship program at the University of Miami’s Miller School of Medicine, told this news organization. “The escalating global obesity rates raise concerns, especially in the field of male infertility, where excessive body fat induces intrinsic hormonal changes leading to alterations, eventually, in semen parameters.”
The authors noted that obesity has been linked in the research to worse assisted reproductive technology (ART) outcomes and to subfecundity, taking more than 12 months to achieve pregnancy. They also referenced a systematic review that found men with obesity were more likely to have lower sperm counts and less viable sperm.
“From our standpoint, I think the key point was to raise awareness about the fact that obesity, because of the aromatization of testosterone to estradiol [from excess adipose tissue], will affect the hormonal axis and the availability of testosterone and, therefore, indirectly affects spermatogenesis,” Dr. Sadeghi-Nejad said.
Obesity is also linked to lower levels of inhibin B, which stimulates testosterone secretion in Sertoli cells, which, when combined with the proinflammatory state of obesity, “results in a less favorable environment for sperm production,” he said. Finally, the link between obesity and poorer sexual function further inhibits fertility potential, he added.
Until recently, the primary treatments for obesity in men experiencing fertility problems have been lifestyle modifications or surgical interventions. But the recent approval of GLP-1 RA drugs for obesity present an additional option depending on how these drugs affect other fertility parameters.
Direct or Indirect Effects?
Most of the available evidence on GLP-RAs and sperm parameters comes from preclinical research. One of the few clinical trials, published last year in the Journal of Clinical Medicine, investigated the effects of liraglutide in men with metabolic hypogonadism, a body mass index between (BMI) 30 and 40, and severe erectile dysfunction.
Among the 110 men enrolled in the study, only the 35 participants who said that they were not seeking fatherhood received liraglutide. After 4 months of treatment, these men had significantly improved semen concentration, motility, and morphology than did those wanting to conceive who received conventional fertility treatment. Erectile dysfunction was also more improved in the liraglutide group, according to the researchers.
Though this study demonstrated the potential for liraglutide to treat metabolic hypogonadism, the men in that group also had greater weight loss and BMI reduction than the other participants. The review cited several other studies — albeit small ones — in which weight loss was associated with improvements in sperm parameters, including one randomized controlled trial in which one group lost weight with liraglutide and the other with lifestyle modifications; both groups showed increases in the concentration and number of sperm.
One of the key questions requiring further research, then, is whether GLP-1 agents have direct effects on male fertility independent of a reduction in obesity. The randomized controlled trials comparing liraglutide and lifestyle modifications failed to find additional effects on semen in the men taking liraglutide; however, the study had only 56 participants, and results from liraglutide cannot be generalized to potential effects of semaglutide or tirzepatide, Dr. Sadeghi-Nejad said.
“Determining the relative contributions of weight loss versus direct drug actions on fertility outcomes remains challenging without robust data,” Dr. Ramasamy said. “While acknowledged that diet and physical activity positively impact fertility, confirming the synergistic role of GLP-1 receptor agonists requires evidence from well-designed randomized clinical trials.”
Rodent studies suggest that GLP-1 RAs may independently affect testicular function because GLP-1 receptors exist in Sertoli and Leydig cells of the testes. In one study, for example, obese mice who received the GLP-1 agonist exenatide for 8 weeks had “improved sperm motility, DNA integrity, and decreased expression of pro-inflammatory cytokines,” the authors of the review reported. But the precise mechanisms aren’t well understood.
“We know that there are GLP-1 receptors in the reproductive tract, but the extent of the downstream effect of stimulating those receptors, I don’t think we know well,” said John P. Lindsey II, MD, MEng, assistant professor of urology at University of California San Francisco Health.
Other hormonal effects of GLP-1 agonists, such as stimulating insulin production and better regulating blood glucose levels, are better understood, said Raevti Bole, MD, a urologist at Cleveland Clinic, in Ohio, but still other effects of the drugs may not yet be identified.
“I think the really big unknown is whether these types of drugs have effects that are not hormonal on male fertility and what those effects are, and how those affect sperm,” Dr. Bole said. “For example, we know that these drugs slow gastric emptying. Is it possible that slow gastric emptying affects some of the nutrients that you absorb, and that could affect fertility?” Similarly, she said, it’s not clear whether GLP-1 agonists would have any effects on the thyroid that could then affect fertility.
Effects on Offspring
Another open question about GLP-1 RAs and male fertility is their potential effects on the offspring, said Sriram Machineni, MBBS, associate professor of endocrinology at the Albert Einstein College of Medicine in New York City. The clinical trials involving the drugs for treating type 2 diabetes and obesity required both men and women to use contraception. If sperm contributing to a pregnancy are exposed to a GLP-1 agent, “we don’t know what the consequences could be,” Dr. Machineni said. “Just increasing the fertility of the man is not enough. We need to make sure it’s safe long-term for the fetus.”
Dr. Bole also pointed out the need for understanding potential effects in the fetus.
“We know that there are epigenetic changes that can happen to sperm that are influenced by the lifestyle and the physical health and environment of the parent,” Dr. Bole said. “So how could these drugs potentially affect those epigenetic changes that then potentially are passed on to the offspring? We don’t know that.”
An ideal source for that data would be a cohort registry of people who are taking the medication and then cause a pregnancy. “They have a registry for pregnant women,” Dr. Machineni said, “but we need something similar for men.”
Dr. Sadeghi-Nejad said that he and his coauthors are working on developing a registry for men who take GLP-1 RAs that would enable long-term tracking of multiple andrologic outcomes, including fertility and sexual dysfunction. Such a registry could theoretically be useful in tracking pregnancy and offspring outcomes as well.
Too Soon for Prescribing
Additional options for treating fertility in men with obesity would be welcome. Current treatments include the selective estrogen receptor modulator (SERM) clomiphene citrate and the aromatase inhibitor anastrozole. But these have their drawbacks, Dr. Sadeghi-Nejad pointed out; in the overweight population in particular, they “are not necessarily ideal,” he said.
“Although both are viable treatments for enhancing hormonal balance and semen parameters, clomiphene citrate has rare but documented side effects, including thromboembolism, gastrointestinal distress and occasional weight gain in men,” Dr. Sadeghi-Nejad and his colleagues wrote. “Furthermore, despite clomiphene citrate’s association with significant increases in sperm concentration, it is not universally effective, with a meta-analysis indicating a significant increase in sperm concentration in approximately 60% of men.”
For men who have obesity and oligospermia but normal levels of testosterone and estradiol, “conventional pharmaceutical approaches like clomiphene may not be suitable,” the authors wrote.
Still, GLP-1 RAs may have a role to play for this population.
“I think it is within the wheelhouse of a reproductive urologist to consider those types of medications,” Dr. Lindsey said. For example, for a patient who has overweight or obesity, “does it make sense to think about doing clomiphene therapy, which we often do for someone who has low testosterone, in conjunction [with a GLP-1 agonist]? Maybe there’s a kind of an additive effect of having both on board.”
Dr. Ramasamy similarly noted that GLP-1 agonists cannot replace SERMs but may work “synergistically” with them.
“Despite the established popularity of GLP-1 receptor agonists, there may be some reluctance among urologists and fertility specialists to prescribe them, with some others advocating for their use to enhance semen parameters,” Dr. Ramasamy said. “However, robust scientific evidence is still lacking, necessitating caution and a wait for more substantial data.”
Even if GLP-1 RAs prove to have therapeutic benefit for fertility, considerations such as availability and cost may affect prescribing.
“We do currently have safe and effective drugs that we use for male fertility, and those are generally nowhere near as expensive,” Dr. Bole said. “When we start talking about another drug that we can add, we have to think about the efficacy and the potential side effect but also, is this affordable for patients?”
Eventually, once more evidence become available, all of the urologists who spoke with this news organization said that they expect discussion about the possible therapeutic utility of GLP-1 agonists to make its way into clinical guidelines.
“Obesity is such a huge impediment for fertility in the modern environment,” Dr. Machineni said. “We will have to clarify the use of these agents, so I think this will be a part of the guidelines some point, but I think we need more information.”
The research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the American Cancer Society. The review authors and other quoted physicians reported no disclosures. Dr. Machineni has consulted for Novo Nordisk and Lilly and has conducted clinical trials with semaglutide and tirzepatide for those companies.
A version of this article appeared on Medscape.com.
The explosion of interest in glucagon-like peptide 1 receptor agonists (GLP-1 RAs), such as semaglutide and tirzepatide, has raised questions about what therapeutic effects this class of medication might have beyond their current indications for type 2 diabetes and obesity.
Recent clinical trials have recently identified benefits from GLP-1 agents for the heart, liver, and kidneys, but the current evidence base is murkier regarding how the drugs may affect male fertility.
For starters, overweight and obesity are strongly associated with male infertility in several overlapping ways. Obesity can disrupt hormones linked to fertility, increase the risk for defective sperm, adversely affect semen quality, and even make sexual intercourse more difficult due to obesity’s link to erectile dysfunction. As a result, GLP-1 RAs should at least in theory boost male fertility in men who take the drugs to lose weight.
But animal studies and a handful of small trials and observational data point to the potential for GLP-1 RAs to improve male fertility in other ways.
A recent narrative review on GLP-1 RAs and male reproductive health, published in the journal Medicina in December 2023, surveyed the potential of the drugs for male infertility and offered reason for optimism.
Hossein Sadeghi-Nejad, MD, director of urology at NYU Langone Health, New York, and a coauthor of the article, said that one reason he and his colleagues conducted their analysis was the known association between weight loss and an increase in testosterone.
“Most of the animal studies that are out there show that this class of drugs does affect testosterone levels,” Dr. Sadeghi-Nejad said; they wanted to better understand what other evidence showed about GLP-1 agonists and other fertility factors.
Link Between Obesity and Fertility
The recent paper first reviews the well-established link between obesity and poorer fertility outcomes.
“Certainly, obesity poses a significant societal problem with substantial impacts on both overall health and economic aspects,” senior author Ranjith Ramasamy, MD, associate professor of urology and director of the reproductive urology Fellowship program at the University of Miami’s Miller School of Medicine, told this news organization. “The escalating global obesity rates raise concerns, especially in the field of male infertility, where excessive body fat induces intrinsic hormonal changes leading to alterations, eventually, in semen parameters.”
The authors noted that obesity has been linked in the research to worse assisted reproductive technology (ART) outcomes and to subfecundity, taking more than 12 months to achieve pregnancy. They also referenced a systematic review that found men with obesity were more likely to have lower sperm counts and less viable sperm.
“From our standpoint, I think the key point was to raise awareness about the fact that obesity, because of the aromatization of testosterone to estradiol [from excess adipose tissue], will affect the hormonal axis and the availability of testosterone and, therefore, indirectly affects spermatogenesis,” Dr. Sadeghi-Nejad said.
Obesity is also linked to lower levels of inhibin B, which stimulates testosterone secretion in Sertoli cells, which, when combined with the proinflammatory state of obesity, “results in a less favorable environment for sperm production,” he said. Finally, the link between obesity and poorer sexual function further inhibits fertility potential, he added.
Until recently, the primary treatments for obesity in men experiencing fertility problems have been lifestyle modifications or surgical interventions. But the recent approval of GLP-1 RA drugs for obesity present an additional option depending on how these drugs affect other fertility parameters.
Direct or Indirect Effects?
Most of the available evidence on GLP-RAs and sperm parameters comes from preclinical research. One of the few clinical trials, published last year in the Journal of Clinical Medicine, investigated the effects of liraglutide in men with metabolic hypogonadism, a body mass index between (BMI) 30 and 40, and severe erectile dysfunction.
Among the 110 men enrolled in the study, only the 35 participants who said that they were not seeking fatherhood received liraglutide. After 4 months of treatment, these men had significantly improved semen concentration, motility, and morphology than did those wanting to conceive who received conventional fertility treatment. Erectile dysfunction was also more improved in the liraglutide group, according to the researchers.
Though this study demonstrated the potential for liraglutide to treat metabolic hypogonadism, the men in that group also had greater weight loss and BMI reduction than the other participants. The review cited several other studies — albeit small ones — in which weight loss was associated with improvements in sperm parameters, including one randomized controlled trial in which one group lost weight with liraglutide and the other with lifestyle modifications; both groups showed increases in the concentration and number of sperm.
One of the key questions requiring further research, then, is whether GLP-1 agents have direct effects on male fertility independent of a reduction in obesity. The randomized controlled trials comparing liraglutide and lifestyle modifications failed to find additional effects on semen in the men taking liraglutide; however, the study had only 56 participants, and results from liraglutide cannot be generalized to potential effects of semaglutide or tirzepatide, Dr. Sadeghi-Nejad said.
“Determining the relative contributions of weight loss versus direct drug actions on fertility outcomes remains challenging without robust data,” Dr. Ramasamy said. “While acknowledged that diet and physical activity positively impact fertility, confirming the synergistic role of GLP-1 receptor agonists requires evidence from well-designed randomized clinical trials.”
Rodent studies suggest that GLP-1 RAs may independently affect testicular function because GLP-1 receptors exist in Sertoli and Leydig cells of the testes. In one study, for example, obese mice who received the GLP-1 agonist exenatide for 8 weeks had “improved sperm motility, DNA integrity, and decreased expression of pro-inflammatory cytokines,” the authors of the review reported. But the precise mechanisms aren’t well understood.
“We know that there are GLP-1 receptors in the reproductive tract, but the extent of the downstream effect of stimulating those receptors, I don’t think we know well,” said John P. Lindsey II, MD, MEng, assistant professor of urology at University of California San Francisco Health.
Other hormonal effects of GLP-1 agonists, such as stimulating insulin production and better regulating blood glucose levels, are better understood, said Raevti Bole, MD, a urologist at Cleveland Clinic, in Ohio, but still other effects of the drugs may not yet be identified.
“I think the really big unknown is whether these types of drugs have effects that are not hormonal on male fertility and what those effects are, and how those affect sperm,” Dr. Bole said. “For example, we know that these drugs slow gastric emptying. Is it possible that slow gastric emptying affects some of the nutrients that you absorb, and that could affect fertility?” Similarly, she said, it’s not clear whether GLP-1 agonists would have any effects on the thyroid that could then affect fertility.
Effects on Offspring
Another open question about GLP-1 RAs and male fertility is their potential effects on the offspring, said Sriram Machineni, MBBS, associate professor of endocrinology at the Albert Einstein College of Medicine in New York City. The clinical trials involving the drugs for treating type 2 diabetes and obesity required both men and women to use contraception. If sperm contributing to a pregnancy are exposed to a GLP-1 agent, “we don’t know what the consequences could be,” Dr. Machineni said. “Just increasing the fertility of the man is not enough. We need to make sure it’s safe long-term for the fetus.”
Dr. Bole also pointed out the need for understanding potential effects in the fetus.
“We know that there are epigenetic changes that can happen to sperm that are influenced by the lifestyle and the physical health and environment of the parent,” Dr. Bole said. “So how could these drugs potentially affect those epigenetic changes that then potentially are passed on to the offspring? We don’t know that.”
An ideal source for that data would be a cohort registry of people who are taking the medication and then cause a pregnancy. “They have a registry for pregnant women,” Dr. Machineni said, “but we need something similar for men.”
Dr. Sadeghi-Nejad said that he and his coauthors are working on developing a registry for men who take GLP-1 RAs that would enable long-term tracking of multiple andrologic outcomes, including fertility and sexual dysfunction. Such a registry could theoretically be useful in tracking pregnancy and offspring outcomes as well.
Too Soon for Prescribing
Additional options for treating fertility in men with obesity would be welcome. Current treatments include the selective estrogen receptor modulator (SERM) clomiphene citrate and the aromatase inhibitor anastrozole. But these have their drawbacks, Dr. Sadeghi-Nejad pointed out; in the overweight population in particular, they “are not necessarily ideal,” he said.
“Although both are viable treatments for enhancing hormonal balance and semen parameters, clomiphene citrate has rare but documented side effects, including thromboembolism, gastrointestinal distress and occasional weight gain in men,” Dr. Sadeghi-Nejad and his colleagues wrote. “Furthermore, despite clomiphene citrate’s association with significant increases in sperm concentration, it is not universally effective, with a meta-analysis indicating a significant increase in sperm concentration in approximately 60% of men.”
For men who have obesity and oligospermia but normal levels of testosterone and estradiol, “conventional pharmaceutical approaches like clomiphene may not be suitable,” the authors wrote.
Still, GLP-1 RAs may have a role to play for this population.
“I think it is within the wheelhouse of a reproductive urologist to consider those types of medications,” Dr. Lindsey said. For example, for a patient who has overweight or obesity, “does it make sense to think about doing clomiphene therapy, which we often do for someone who has low testosterone, in conjunction [with a GLP-1 agonist]? Maybe there’s a kind of an additive effect of having both on board.”
Dr. Ramasamy similarly noted that GLP-1 agonists cannot replace SERMs but may work “synergistically” with them.
“Despite the established popularity of GLP-1 receptor agonists, there may be some reluctance among urologists and fertility specialists to prescribe them, with some others advocating for their use to enhance semen parameters,” Dr. Ramasamy said. “However, robust scientific evidence is still lacking, necessitating caution and a wait for more substantial data.”
Even if GLP-1 RAs prove to have therapeutic benefit for fertility, considerations such as availability and cost may affect prescribing.
“We do currently have safe and effective drugs that we use for male fertility, and those are generally nowhere near as expensive,” Dr. Bole said. “When we start talking about another drug that we can add, we have to think about the efficacy and the potential side effect but also, is this affordable for patients?”
Eventually, once more evidence become available, all of the urologists who spoke with this news organization said that they expect discussion about the possible therapeutic utility of GLP-1 agonists to make its way into clinical guidelines.
“Obesity is such a huge impediment for fertility in the modern environment,” Dr. Machineni said. “We will have to clarify the use of these agents, so I think this will be a part of the guidelines some point, but I think we need more information.”
The research was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the American Cancer Society. The review authors and other quoted physicians reported no disclosures. Dr. Machineni has consulted for Novo Nordisk and Lilly and has conducted clinical trials with semaglutide and tirzepatide for those companies.
A version of this article appeared on Medscape.com.