User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
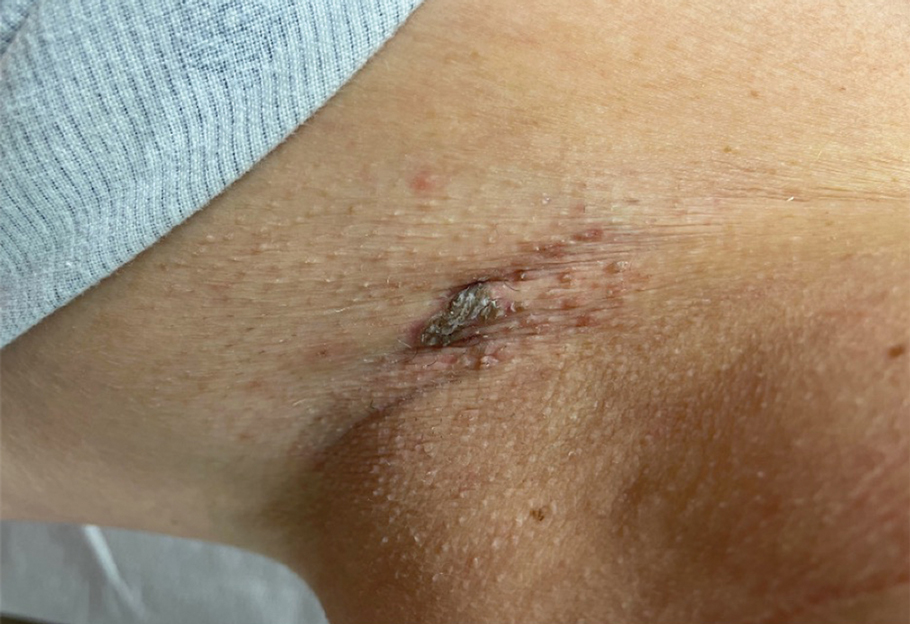
Brown Plaque in the Axilla Following Immobilization of the Arm
The Diagnosis: Granular Parakeratosis
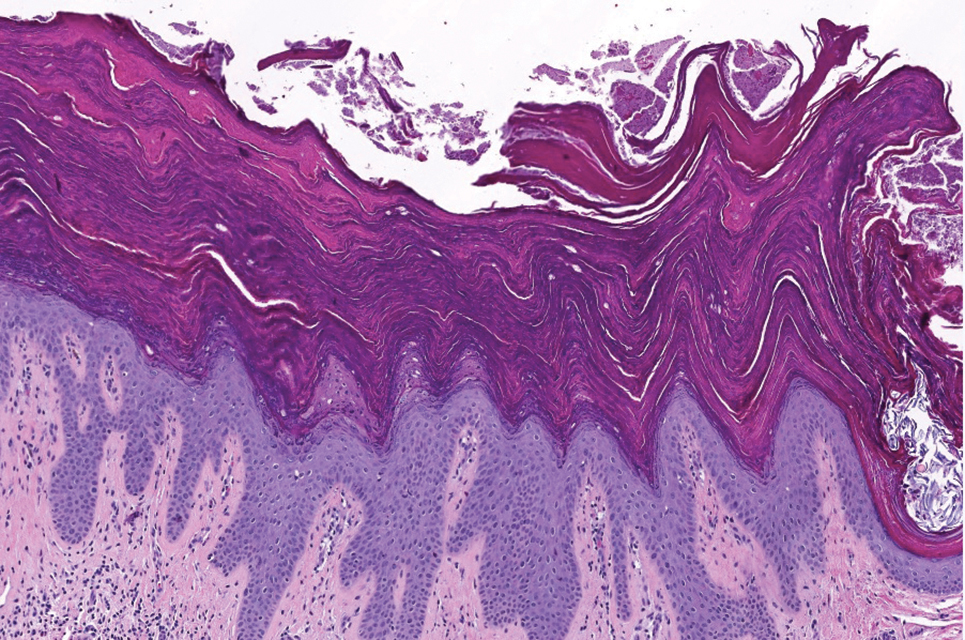
Histopathology demonstrated diffuse parakeratosis with retention of keratohyalin granules throughout the stratum corneum consistent with a diagnosis of granular parakeratosis (Figure), a rare benign cutaneous condition that is thought to occur due to a defect in epidermal differentiation. The lesion resolved without additional treatment.
The pathogenesis of granular parakeratosis is unclear, but a reactive process in which locoregional irritation or occlusion prompts increased cell turnover and prevention of profilaggrin breakdown has been proposed.1,2 The diagnosis is linked to various precipitating agents, most commonly topical products (eg, zinc oxide, antiperspirants) and products with benzalkonium chloride (eg, laundry rinses). These agents are thought to cause retention of keratohyalin granules in the stratum corneum during epidermal differentiation.1,2
Most affected patients are middle-aged women (mean age at diagnosis, 37.8 years).2 Patients present with eruptions of erythematous, brown, hyperkeratotic patches and papules that coalesce into plaques.1,2 These lesions can be pruritic and painful or asymptomatic. They often manifest bilaterally in intertriginous sites, most commonly the axillae, groin, or inguinal folds.1,2
Treatment involves identification and removal of potential triggers including changing antiperspirants, limiting use of irritating agents (eg, topical products with strong fragrances), and reducing heat and moisture in the affected areas. If the lesion persists, stepwise treatment can be initiated with topical agents (eg, corticosteroids, vitamin D analogues, retinoids, keratolytics, calcineurin inhibitors) followed by systemic medications (eg, antibiotics, isotretinoin, antifungals, dexamethasone) and procedures (eg, botulinum toxin injections, surgery, laser, cryotherapy).1,2
Unilateral granular parakeratosis, as seen in our patient, is an uncommon manifestation. Our case supports the theory that occlusion is a precipitating factor for this condition, given persistent axillary exposure to heat, sweat, and friction in the setting of limb immobilization.3
Granular parakeratosis is a challenge to diagnose due to clinical overlap with several other cutaneous conditions; histopathologic confirmation is required. Fox- Fordyce disease is a rare condition that is thought to result from keratin buildup or occlusion of apocrine or apoeccrine sweat ducts leading to duct rupture and surrounding inflammation.4 Common triggers include laser hair removal, hormonal changes, and living conditions that promote hot and humid environments.5 It can manifest similarly to granular parakeratosis, with eruptions of multiple red-violet papules that appear bilaterally in aprocine gland–rich areas, including the axillae and less commonly the genital, periareolar, thoracic, abdominal, and facial areas.4,5 However, most patients with Fox-Fordyce disease tend to be younger females (aged 13–35 years) with severely pruritic lesions,4,5 unlike our patient. In addition, histopathology shows hyperkeratosis, hair follicle plugging, and sweat gland and duct dilation.4
Seborrheic keratoses are common benign epidermal tumors caused by an overproliferation of immature keratinocytes.6,7 Similar to granular parakeratosis, they commonly manifest in older adults as hyperpigmented, well-demarcated, verrucous plaques with a hyperkeratotic surface.6 However, they are more common on the face, neck, trunk, and extremities, and they tend to be asymptomatic, differentiating them from granular parakerosis.6 Histopathology demonstrates a papillomatous epidermal surface, large capillaries in the dermal papillae, and intraepidermal and pseudohorn epidermal cysts.7
Inverse lichen planus, a variant of lichen planus, is a rare inflammatory condition that involves the lysis of basal keratinocytes by CD8+ lymphocytes.8 Similar to granular parakeratosis, lichen planus commonly affects middle-aged women (aged 30–60 years), and this particular variant manifests with asymptomatic or mildly pruritic, hyperpigmented patches and plaques in intertriginous areas. Although it also shows hyperkeratosis on histopathology, it can be differentiated from granular parakeratosis by the additional findings of epidermal hypergranulosis, sawtooth acanthosis of rete ridges, apoptotic keratinocytes in the dermoepidermal junction, and lymphocytic infiltrate in the upper dermis.8
Hailey-Hailey disease (also known as familial benign pemphigus) is a rare condition caused by an autosomaldominant mutation affecting intracellular calcium signaling that impairs keratinocyte adhesion.9 Similar to granular parakeratosis, it is most common in middle-aged adults (aged 30–40 years) and manifests as pruritic and burning lesions in symmetric intertriginous areas that also can be triggered by heat and sweating. However, patients present with recurrent blistering and vesicular lesions that may lead to erosions and secondary infections, which reduced clinical suspicion for this diagnosis in our patient. Histopathology shows suprabasilar and intraepidermal clefts, full-thickness acantholysis, protruding dermal papillae, and a perivascular lymphocytic infiltrate in the superficial dermis.9
- Ding CY, Liu H, Khachemoune A. Granular parakeratosis: a comprehensive review and a critical reappraisal. Am J Clin Dermatol. 2015;16:495-500. doi:10.1007/s40257-015-0148-2
- Ip KH, Li A. Clinical features, histology, and treatment outcomes of granular parakeratosis: a systematic review. Int J Dermatol. 2022;61:973-978. doi:10.1111/ijd.16107
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496. doi:10.1016/s0190-9622(98)70333-0
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48:453-455. doi:10.1067/mjd.2003.93
- Salloum A, Bouferraa Y, Bazzi N, et al. Pathophysiology, clinical findings, and management of Fox-Fordyce disease: a systematic review. J Cosmet Dermatol. 2022;21:482-500. doi:10.1111/jocd.14135
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217. doi:10.1159/000517070
- Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44:518-524. doi:10.1111/1346-8138.13657
- Weston G, Payette M. Update on lichen planus and its clinical variants [published online September 16, 2015]. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Ben Lagha I, Ashack K, Khachemoune A. Hailey-Hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol. 2020;21:49-68. doi:10.1007/s40257-019-00477-z
The Diagnosis: Granular Parakeratosis
Histopathology demonstrated diffuse parakeratosis with retention of keratohyalin granules throughout the stratum corneum consistent with a diagnosis of granular parakeratosis (Figure), a rare benign cutaneous condition that is thought to occur due to a defect in epidermal differentiation. The lesion resolved without additional treatment.

The pathogenesis of granular parakeratosis is unclear, but a reactive process in which locoregional irritation or occlusion prompts increased cell turnover and prevention of profilaggrin breakdown has been proposed.1,2 The diagnosis is linked to various precipitating agents, most commonly topical products (eg, zinc oxide, antiperspirants) and products with benzalkonium chloride (eg, laundry rinses). These agents are thought to cause retention of keratohyalin granules in the stratum corneum during epidermal differentiation.1,2
Most affected patients are middle-aged women (mean age at diagnosis, 37.8 years).2 Patients present with eruptions of erythematous, brown, hyperkeratotic patches and papules that coalesce into plaques.1,2 These lesions can be pruritic and painful or asymptomatic. They often manifest bilaterally in intertriginous sites, most commonly the axillae, groin, or inguinal folds.1,2
Treatment involves identification and removal of potential triggers including changing antiperspirants, limiting use of irritating agents (eg, topical products with strong fragrances), and reducing heat and moisture in the affected areas. If the lesion persists, stepwise treatment can be initiated with topical agents (eg, corticosteroids, vitamin D analogues, retinoids, keratolytics, calcineurin inhibitors) followed by systemic medications (eg, antibiotics, isotretinoin, antifungals, dexamethasone) and procedures (eg, botulinum toxin injections, surgery, laser, cryotherapy).1,2
Unilateral granular parakeratosis, as seen in our patient, is an uncommon manifestation. Our case supports the theory that occlusion is a precipitating factor for this condition, given persistent axillary exposure to heat, sweat, and friction in the setting of limb immobilization.3
Granular parakeratosis is a challenge to diagnose due to clinical overlap with several other cutaneous conditions; histopathologic confirmation is required. Fox- Fordyce disease is a rare condition that is thought to result from keratin buildup or occlusion of apocrine or apoeccrine sweat ducts leading to duct rupture and surrounding inflammation.4 Common triggers include laser hair removal, hormonal changes, and living conditions that promote hot and humid environments.5 It can manifest similarly to granular parakeratosis, with eruptions of multiple red-violet papules that appear bilaterally in aprocine gland–rich areas, including the axillae and less commonly the genital, periareolar, thoracic, abdominal, and facial areas.4,5 However, most patients with Fox-Fordyce disease tend to be younger females (aged 13–35 years) with severely pruritic lesions,4,5 unlike our patient. In addition, histopathology shows hyperkeratosis, hair follicle plugging, and sweat gland and duct dilation.4
Seborrheic keratoses are common benign epidermal tumors caused by an overproliferation of immature keratinocytes.6,7 Similar to granular parakeratosis, they commonly manifest in older adults as hyperpigmented, well-demarcated, verrucous plaques with a hyperkeratotic surface.6 However, they are more common on the face, neck, trunk, and extremities, and they tend to be asymptomatic, differentiating them from granular parakerosis.6 Histopathology demonstrates a papillomatous epidermal surface, large capillaries in the dermal papillae, and intraepidermal and pseudohorn epidermal cysts.7
Inverse lichen planus, a variant of lichen planus, is a rare inflammatory condition that involves the lysis of basal keratinocytes by CD8+ lymphocytes.8 Similar to granular parakeratosis, lichen planus commonly affects middle-aged women (aged 30–60 years), and this particular variant manifests with asymptomatic or mildly pruritic, hyperpigmented patches and plaques in intertriginous areas. Although it also shows hyperkeratosis on histopathology, it can be differentiated from granular parakeratosis by the additional findings of epidermal hypergranulosis, sawtooth acanthosis of rete ridges, apoptotic keratinocytes in the dermoepidermal junction, and lymphocytic infiltrate in the upper dermis.8
Hailey-Hailey disease (also known as familial benign pemphigus) is a rare condition caused by an autosomaldominant mutation affecting intracellular calcium signaling that impairs keratinocyte adhesion.9 Similar to granular parakeratosis, it is most common in middle-aged adults (aged 30–40 years) and manifests as pruritic and burning lesions in symmetric intertriginous areas that also can be triggered by heat and sweating. However, patients present with recurrent blistering and vesicular lesions that may lead to erosions and secondary infections, which reduced clinical suspicion for this diagnosis in our patient. Histopathology shows suprabasilar and intraepidermal clefts, full-thickness acantholysis, protruding dermal papillae, and a perivascular lymphocytic infiltrate in the superficial dermis.9
The Diagnosis: Granular Parakeratosis
Histopathology demonstrated diffuse parakeratosis with retention of keratohyalin granules throughout the stratum corneum consistent with a diagnosis of granular parakeratosis (Figure), a rare benign cutaneous condition that is thought to occur due to a defect in epidermal differentiation. The lesion resolved without additional treatment.

The pathogenesis of granular parakeratosis is unclear, but a reactive process in which locoregional irritation or occlusion prompts increased cell turnover and prevention of profilaggrin breakdown has been proposed.1,2 The diagnosis is linked to various precipitating agents, most commonly topical products (eg, zinc oxide, antiperspirants) and products with benzalkonium chloride (eg, laundry rinses). These agents are thought to cause retention of keratohyalin granules in the stratum corneum during epidermal differentiation.1,2
Most affected patients are middle-aged women (mean age at diagnosis, 37.8 years).2 Patients present with eruptions of erythematous, brown, hyperkeratotic patches and papules that coalesce into plaques.1,2 These lesions can be pruritic and painful or asymptomatic. They often manifest bilaterally in intertriginous sites, most commonly the axillae, groin, or inguinal folds.1,2
Treatment involves identification and removal of potential triggers including changing antiperspirants, limiting use of irritating agents (eg, topical products with strong fragrances), and reducing heat and moisture in the affected areas. If the lesion persists, stepwise treatment can be initiated with topical agents (eg, corticosteroids, vitamin D analogues, retinoids, keratolytics, calcineurin inhibitors) followed by systemic medications (eg, antibiotics, isotretinoin, antifungals, dexamethasone) and procedures (eg, botulinum toxin injections, surgery, laser, cryotherapy).1,2
Unilateral granular parakeratosis, as seen in our patient, is an uncommon manifestation. Our case supports the theory that occlusion is a precipitating factor for this condition, given persistent axillary exposure to heat, sweat, and friction in the setting of limb immobilization.3
Granular parakeratosis is a challenge to diagnose due to clinical overlap with several other cutaneous conditions; histopathologic confirmation is required. Fox- Fordyce disease is a rare condition that is thought to result from keratin buildup or occlusion of apocrine or apoeccrine sweat ducts leading to duct rupture and surrounding inflammation.4 Common triggers include laser hair removal, hormonal changes, and living conditions that promote hot and humid environments.5 It can manifest similarly to granular parakeratosis, with eruptions of multiple red-violet papules that appear bilaterally in aprocine gland–rich areas, including the axillae and less commonly the genital, periareolar, thoracic, abdominal, and facial areas.4,5 However, most patients with Fox-Fordyce disease tend to be younger females (aged 13–35 years) with severely pruritic lesions,4,5 unlike our patient. In addition, histopathology shows hyperkeratosis, hair follicle plugging, and sweat gland and duct dilation.4
Seborrheic keratoses are common benign epidermal tumors caused by an overproliferation of immature keratinocytes.6,7 Similar to granular parakeratosis, they commonly manifest in older adults as hyperpigmented, well-demarcated, verrucous plaques with a hyperkeratotic surface.6 However, they are more common on the face, neck, trunk, and extremities, and they tend to be asymptomatic, differentiating them from granular parakerosis.6 Histopathology demonstrates a papillomatous epidermal surface, large capillaries in the dermal papillae, and intraepidermal and pseudohorn epidermal cysts.7
Inverse lichen planus, a variant of lichen planus, is a rare inflammatory condition that involves the lysis of basal keratinocytes by CD8+ lymphocytes.8 Similar to granular parakeratosis, lichen planus commonly affects middle-aged women (aged 30–60 years), and this particular variant manifests with asymptomatic or mildly pruritic, hyperpigmented patches and plaques in intertriginous areas. Although it also shows hyperkeratosis on histopathology, it can be differentiated from granular parakeratosis by the additional findings of epidermal hypergranulosis, sawtooth acanthosis of rete ridges, apoptotic keratinocytes in the dermoepidermal junction, and lymphocytic infiltrate in the upper dermis.8
Hailey-Hailey disease (also known as familial benign pemphigus) is a rare condition caused by an autosomaldominant mutation affecting intracellular calcium signaling that impairs keratinocyte adhesion.9 Similar to granular parakeratosis, it is most common in middle-aged adults (aged 30–40 years) and manifests as pruritic and burning lesions in symmetric intertriginous areas that also can be triggered by heat and sweating. However, patients present with recurrent blistering and vesicular lesions that may lead to erosions and secondary infections, which reduced clinical suspicion for this diagnosis in our patient. Histopathology shows suprabasilar and intraepidermal clefts, full-thickness acantholysis, protruding dermal papillae, and a perivascular lymphocytic infiltrate in the superficial dermis.9
- Ding CY, Liu H, Khachemoune A. Granular parakeratosis: a comprehensive review and a critical reappraisal. Am J Clin Dermatol. 2015;16:495-500. doi:10.1007/s40257-015-0148-2
- Ip KH, Li A. Clinical features, histology, and treatment outcomes of granular parakeratosis: a systematic review. Int J Dermatol. 2022;61:973-978. doi:10.1111/ijd.16107
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496. doi:10.1016/s0190-9622(98)70333-0
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48:453-455. doi:10.1067/mjd.2003.93
- Salloum A, Bouferraa Y, Bazzi N, et al. Pathophysiology, clinical findings, and management of Fox-Fordyce disease: a systematic review. J Cosmet Dermatol. 2022;21:482-500. doi:10.1111/jocd.14135
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217. doi:10.1159/000517070
- Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44:518-524. doi:10.1111/1346-8138.13657
- Weston G, Payette M. Update on lichen planus and its clinical variants [published online September 16, 2015]. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Ben Lagha I, Ashack K, Khachemoune A. Hailey-Hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol. 2020;21:49-68. doi:10.1007/s40257-019-00477-z
- Ding CY, Liu H, Khachemoune A. Granular parakeratosis: a comprehensive review and a critical reappraisal. Am J Clin Dermatol. 2015;16:495-500. doi:10.1007/s40257-015-0148-2
- Ip KH, Li A. Clinical features, histology, and treatment outcomes of granular parakeratosis: a systematic review. Int J Dermatol. 2022;61:973-978. doi:10.1111/ijd.16107
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496. doi:10.1016/s0190-9622(98)70333-0
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48:453-455. doi:10.1067/mjd.2003.93
- Salloum A, Bouferraa Y, Bazzi N, et al. Pathophysiology, clinical findings, and management of Fox-Fordyce disease: a systematic review. J Cosmet Dermatol. 2022;21:482-500. doi:10.1111/jocd.14135
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217. doi:10.1159/000517070
- Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44:518-524. doi:10.1111/1346-8138.13657
- Weston G, Payette M. Update on lichen planus and its clinical variants [published online September 16, 2015]. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Ben Lagha I, Ashack K, Khachemoune A. Hailey-Hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol. 2020;21:49-68. doi:10.1007/s40257-019-00477-z
A 62-year-old woman presented to our clinic for evaluation of a brown plaque in the left axilla of 2 weeks’ duration. She had a history of a rotator cuff injury and adhesive capsulitis several months prior that required immobilization of the left arm in a shoulder orthosis for several months. After the sling was removed, she noticed the lesion and reported mild cutaneous pain. Physical examination revealed a 1.5-cm, verrucous, red-brown plaque in the left axillary vault. A shave biopsy of the plaque was performed.
Photoexposed Rash in an Older Adult
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
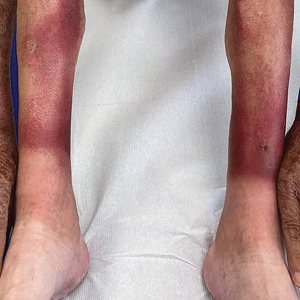
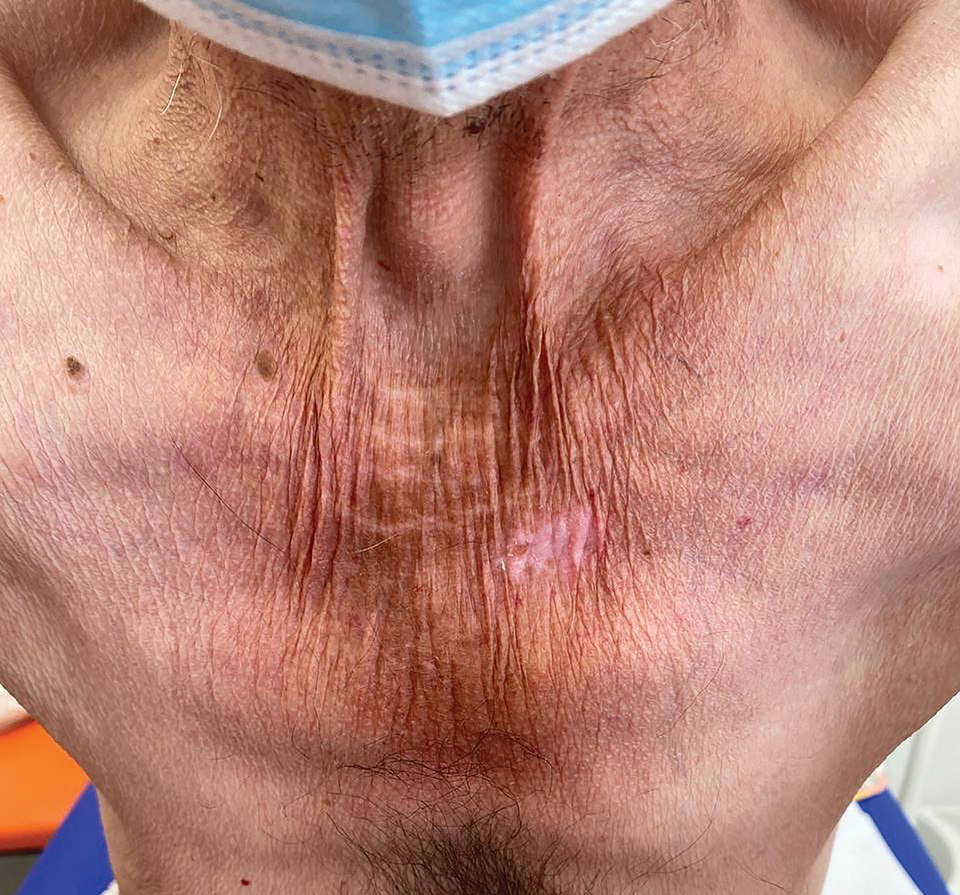
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3
The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
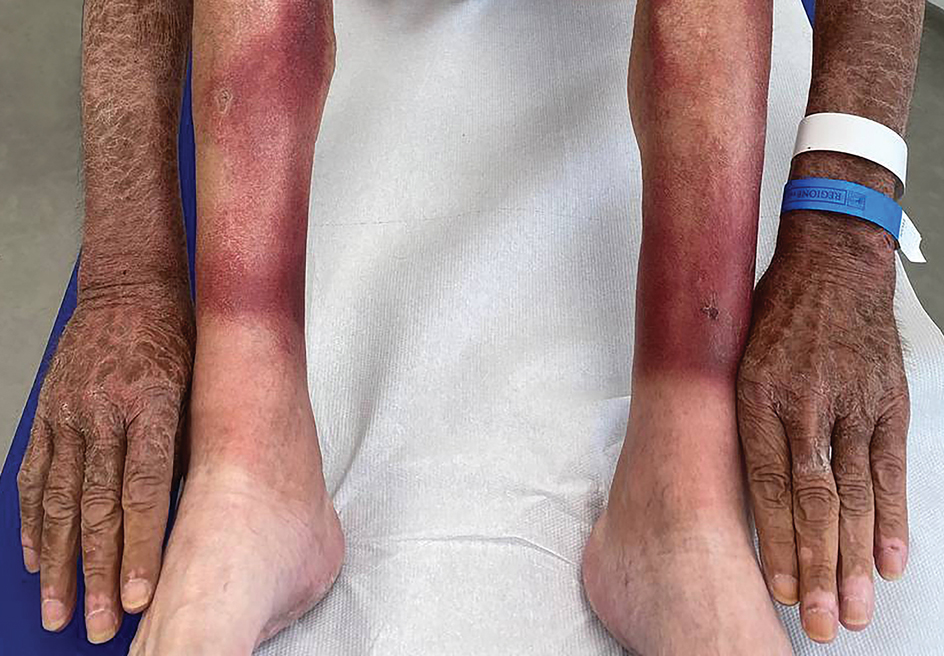
A 66-year-old man presented with an intermittent pruriginous symmetric rash on the dorsal aspects of the arms, legs, and upper chest of 4 months' duration. The patient’s hands, forearms, and neck were diffusely hyperpigmented, dry, cracked, and scaling with a ring of peripheral erythema. He also experienced recurrent photosensitivity reactions on the legs. His poor clinical condition including confusion and diarrhea hindered intake of a balanced diet. He also reported a history of excessive alcohol use. The patient’s vital signs were normal, and Doppler ultrasonography ruled out deep venous thrombosis of the lower legs. A complete blood cell count showed anemia with decreased hemoglobin levels (117 g/L [reference range, 140–180 g/L]) and increased mean corpuscular volume (107.1 fL [reference range, 80–100 fL]). Additionally, low serum levels of albumin, folate, and vitamin B12 were noted. The patient had been taking hydrochlorothiazide and salicylic acid for hypertension with no recent changes in his medication regimen.
Herpes Zoster and Varicella Encephalitis Following the Recombinant Zoster Vaccine
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
To the Editor:
Reported adverse effects following the recombinant zoster vaccine (RZV) include pyrexia, myalgia, and fatigue.1 We report the case of a patient who developed herpes zoster and subsequent varicella encephalitis within 8 days of receiving the second dose of the RZV.
A 75-year-old man presented to the emergency department with burning pain and pruritus involving the left hip and calf 2 days after receiving the second dose of the RZV. He had a history of chronic lymphocytic leukemia (CLL) and was being clinically monitored. He received the first dose of the RZV without complication 3 months prior. In the emergency department, he was diagnosed with “nerve pain,” given acetaminophen, and discharged home; however, he continued to have worsening pain 8 days later followed by a vesicular eruption that wrapped around the left leg and was concentrated on the inner thigh/groin area in a dermatomal distribution. His primary care physician diagnosed him with herpes zoster and prescribed valacyclovir 1000 mg every 8 hours for 7 days. Two days later, the patient developed weakness and confusion and returned to the emergency department. Upon admission, computed tomography and magnetic resonance imaging/magnetic resonance angiography of the brain was normal. A lumbar puncture confirmed varicella encephalitis via a polymerase chain reaction assay. He was treated with intravenous acyclovir and discharged to a rehabilitation facility. His course was further complicated by a subarachnoid hemorrhage and normal pressure hydrocephalus. He did not require a shunt but continues to have memory impairment, weakness, and cognitive impairment. He is steadily improving with rehabilitative services.
The RZV is an inactivated vaccine composed of the varicella-zoster virus (VZV) glycoprotein E antigen and an adjuvant, AS01B, that boosts both innate and adaptive immunity.2 It was approved by the US Food and Drug Administration in 2017 for prevention of herpes zoster in adults aged 50 years or older. It requires 2 separate injections administered 2 to 6 months apart. Its efficacy for the prevention of cutaneous herpes zoster and postherpetic neuralgia is 97% and 80% to 91%, respectively. It was developed to improve on the existing zoster vaccine live, which contains a live attenuated virus, with efficacy ranging from 38% to 70%.3
The Centers for Disease Control and Prevention initially recommended the RZV for immunocompetent individuals or those taking low-dose immunosuppressant medications as well those who have recovered from an immunocompromising illness. In immunocompetent patients, reported adverse effects include injection site pain and redness, headache, myalgia, fatigue, shivering, fever, and gastrointestinal tract symptoms; however, when the vaccine first came out, many of the studies excluded patients with CLL.4 Our patient’s herpes zoster and varicella encephalitis occurred following administration of the second dose of the RZV. Herpes zoster occurs from declining VZV-specific cell-mediated immunity. Given that the vaccine contains inactive virus, it is unlikely that our patient’s infection was the direct result of dissemination of the virus contained within the vaccine. The RZV specifically generates T-cell responses to the glycoprotein E subunit of VZV, which is thought to be responsible for the high levels of VZV-specific memory T cells with the RZV compared to the zoster vaccine live.5 However, this response does not occur until after the second dose of RZV. Although our patient already had 1 dose of RZV, it was unlikely that he had a substantial number of glycoprotein E and VZV-specific memory T cells to combat virus reactivation. Additionally, his CLL, though mild, may have resulted in an aberrant T-cell response in the presence of already low VZV-specific lymphocytes, allowing for reactivation and dissemination of the virus. Since then, there has been more of an emphasis on looking at the immunogenicity elicited by the vaccine in patients with CLL—both those who are treatment naive and those treated with Bruton tyrosine kinase inhibitors. Both groups of patients have demonstrated reduced immunogenicity in response to RZV, leaving the opportunity for viral reactivation in this patient population.6,7
The safety of the RZV has now been demonstrated in patients with CLL.7 However, even after RZV vaccination, patients with CLL are still at risk for herpes zoster reactivation and may have an aberrant response due to immune cell dysregulation. Our case demonstrates the need to increase monitoring of CLL patients for signs of viral reactivation and shift our focus to providing antiviral therapy quickly after symptom occurrence.
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
- Centers for Disease Control and Prevention. Shingles: about the vaccine. Updated January 24, 2022. Accessed February 7, 2024. https://www.cdc.gov/vaccines/vpd/shingles/hcp/shingrix/about-vaccine.html
- Dooling KL, Guo A, Patel M, et al. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108. doi:10.15585/mmwr.mm6703a5external icon
- Hunter P, Fryhofer SA, Szilagyi PG. Vaccination of adults in general medical practice. Mayo Clin Proc. 2020;95:169-183. doi:10.1016/j.mayocp.2019.02.024
- Dagnew AF, Ilhan O, Lee WS, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis [published correction appears in Lancet Infect Dis. 2020;20:E1]. Lancet Infect Dis. 2019;19:988-1000. doi:10.1016/S1473-3099(19)30163-X
- Levin MJ, Kroehl ME, Johnson MJ, et al. Th1 memory differentiates recombinant from live herpes zoster vaccines. J Clin Invest. 2018;128:4429-4440.
- Pleyer C, Laing KJ, Ali MA, et al. BTK inhibitors impair humoral and cellular responses to recombinant zoster vaccine in CLL. Blood Adv. 2022;6:1732-1740. doi:10.1182/bloodadvances.2021006574
- Pleyer C, Cohen J, Soto S, et al. Response to the Shingrix varicella zoster virus (VZV) vaccine in patients with chronic lymphocytic leukemia (CLL) that are treatment naive or treated with a Bruton’s tyrosine kinase inhibitor (BTK-I). Blood. 2019;134(suppl 1):3053. doi:10.1182/blood-2019-121675
Practice Points
- Patients with chronic lymphocytic leukemia (CLL) are at risk for herpes zoster reactivation even with vaccination due to a decreased immune response. These patients may have an aberrant response due to immune cell dysregulation.
- It is important to increase monitoring of CLL patients for signs of viral reactivation and shift the focus to providing antiviral therapy quickly if herpes zoster symptoms occur.
Rapidly Progressive Necrotizing Myositis Mimicking Pyoderma Gangrenosum
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
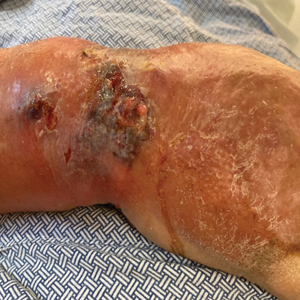
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.
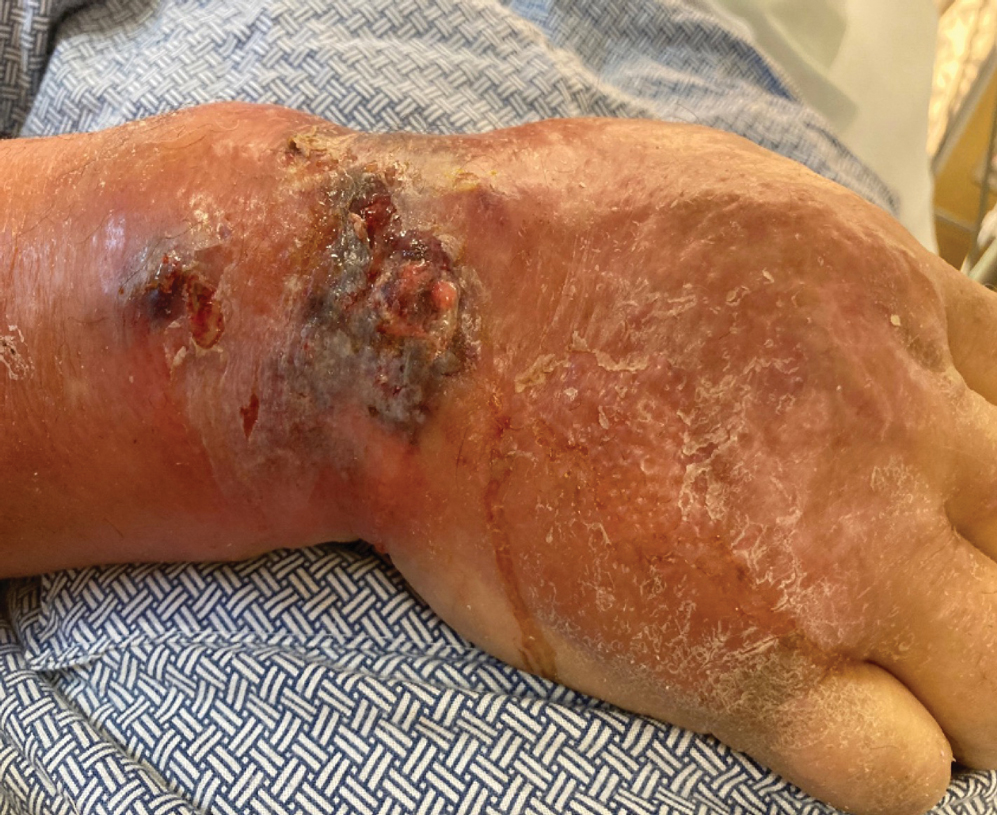
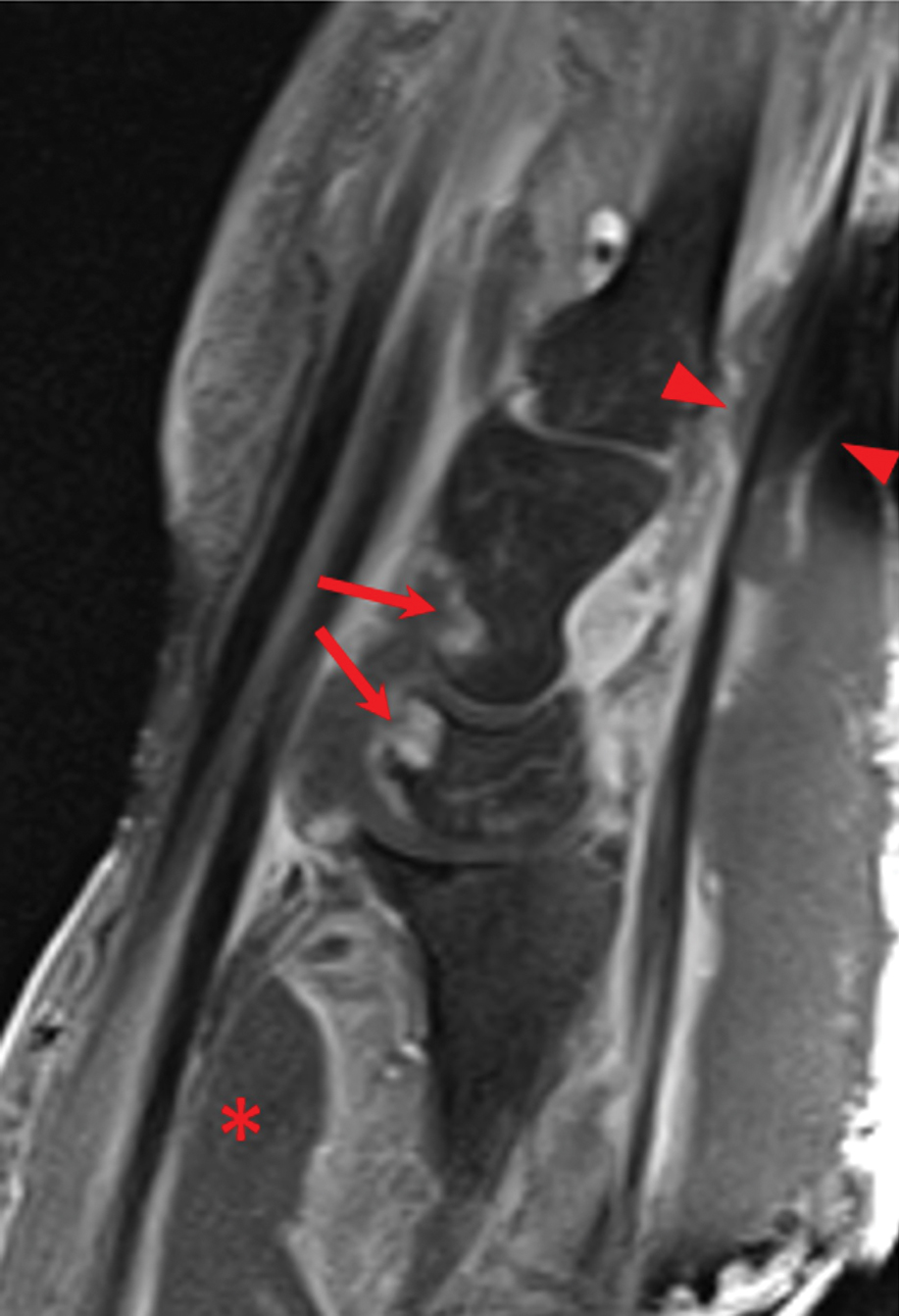
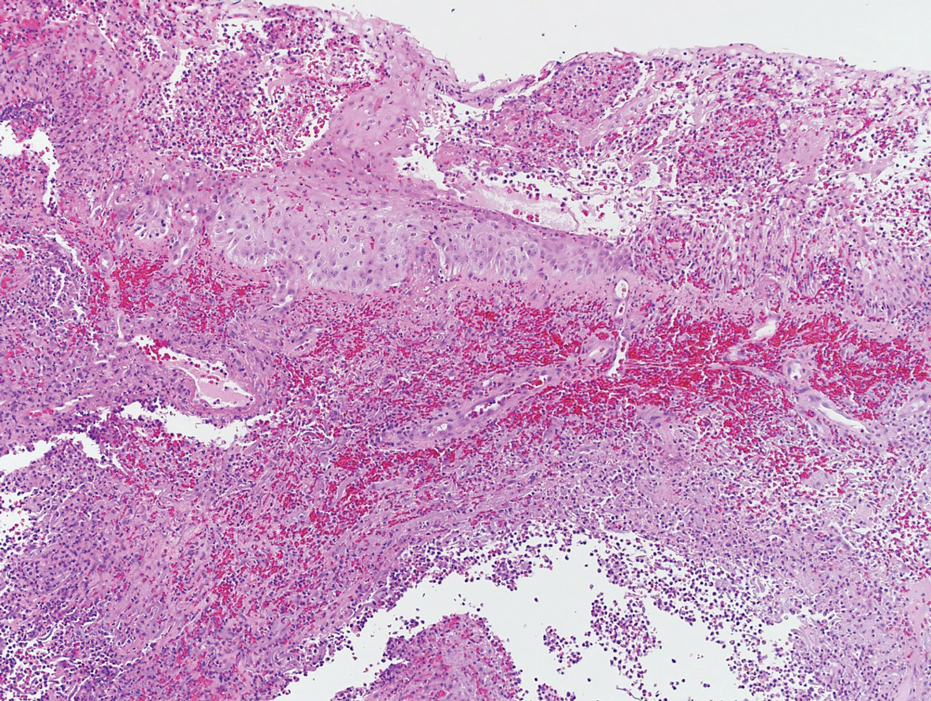
Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).
Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.
Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
Practice Points
- The accurate diagnosis of necrotizing myositis (NM) requires a multimodal approach with complete clinical, histological, and radiographic correlation.
- Necrotizing myositis can manifest as violaceous erythematous plaques, bullae, blisters, or vesicles with petechiae, marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane.
- The differential diagnosis of NM includes pyoderma gangrenosum.
Dermatologic Reactions Following COVID-19 Vaccination: A Case Series
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).
Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
Cutaneous reactions associated with the Pfizer-BioNTech COVID-19 vaccine have been reported worldwide since December 2020. Local injection site reactions (<1%) such as erythema, swelling, delayed local reactions (1%–10%), morbilliform rash, urticarial reactions, pityriasis rosea, Rowell syndrome, and lichen planus have been reported following the Pfizer-BioNTech COVID-19 vaccine.1 Cutaneous reactions reported in association with the Sinovac-Coronavac COVID-19 vaccine include swelling, redness, itching, discoloration, induration (1%–10%), urticaria, petechial rash, and exacerbation of psoriasis at the local injection site (<1%).2
We describe 7 patients from Turkey who presented with various dermatologic problems 5 to 28 days after COVID-19 vaccination, highlighting the possibility of early and late cutaneous reactions related to the vaccine (Table).

Case Reports
Patient 1—A 44-year-old woman was admitted to the dermatology clinic with painful lesions on the trunk of 3 days’ duration. Dermatologic examination revealed grouped erythematous vesicles showing dermatomal spread in the right thoracolumbar (dermatome T10) region. The patient reported that she had received 2 doses of the Sinovac-Coronavac vaccine (doses 1 and 2) and 2 doses of the BioNTech COVID-19 vaccine (doses 3 and 4); the rash had developed 28 days after she received the 4th dose. Her medical history was unremarkable. The lesions regressed after 1 week of treatment with oral valacyclovir 1000 mg 3 times daily, but she developed postherpetic neuralgia 1 week after starting treatment, which resolved after 8 weeks.
Patient 2—A 68-year-old woman presented to the dermatology clinic for evaluation of painful sores on the upper lip of 1 day’s duration. She had a history of rheumatoid arthritis, hypertension, and atopy and was currently taking prednisone and etanercept. Dermatologic examination revealed grouped vesicles on an erythematous base on the upper lip. A diagnosis of herpes labialis was made. The patient reported that she had received a third dose of the Sinovac-Coronavac vaccine 10 days prior to the appearance of the lesions. Her symptoms resolved completely within 2 weeks of treatment with topical acyclovir.
Patient 3—A 64-year-old woman was admitted to the hospital with pain, redness, and watery sores on and around the left eyelid of 2 days’ duration. Dermatologic evaluation revealed the erythematous surface of the left eyelid and periorbital area showed partial crusts, clustered vesicles, erythema, and edema. Additionally, the conjunctiva was purulent and erythematous. The patient’s medical history was notable for allergic asthma, hypertension, anxiety, and depression. For this reason, the patient was prescribed an angiotensin receptor blocker and a selective serotonin reuptake inhibitor. She noted that a similar rash had developed around the left eye 6 years prior that was diagnosed as herpes zoster (HZ). She also reported that she had received 2 doses of the Sinovac-Coronavac COVID-19 vaccine followed by 1 dose of the BioNTech COVID-19 vaccine, which she had received 2 weeks before the rash developed. The patient was treated at the eye clinic and was found to have ocular involvement. Ophthalmology was consulted and a diagnosis of herpes zoster ophthalmicus (HZO) was made. Systemic valacyclovir treatment was initiated, resulting in clinical improvement within 3 weeks.
Patient 4—A 75-year-old man was admitted to the hospital with chest and back pain and widespread muscle pain of several days’ duration. His medical history was remarkable for diabetes mellitus, hypertension, depression, and coronary artery bypass surgery. A medication history revealed treatment with a β-blocker, acetylsalicylic acid, a calcium channel blocker, a dipeptidyl peptidase 4 inhibitor, and a selective serotonin reuptake inhibitor. Dermatologic examination revealed grouped vesicles on an erythematous background in dermatome T5 on the right chest and back. A diagnosis of HZ was made. The patient reported that he had received 2 doses of the Sinovac-Coronavac vaccine followed by 1 dose of the Pfizer-BioNTech vaccine 2 weeks prior to the current presentation. He was treated with valacyclovir for 1 week, and his symptoms resolved entirely within 3 weeks.
Patient 5—A 50-year-old woman presented to the hospital for evaluation of painful sores on the back, chest, groin, and abdomen of 10 days’ duration. The lesions initially had developed 7 days after receiving the BioNTech COVID-19 vaccine; she previously had received 2 doses of the Sinovac-Coronavac vaccine. The patient had a history of untreated psoriasis. Dermatologic examination revealed grouped vesicles on an erythematous background in the T2–L2 dermatomes on the left side of the trunk. A diagnosis of HZ was made. The lesions resolved after 1 week of treatment with systemic valacyclovir; however, she subsequently developed postherpetic neuralgia, hypoesthesia, and postinflammatory hyperpigmentation in the affected regions.
Patient 6—A 37-year-old woman presented to the hospital with redness, swelling, and itching all over the body of 3 days’ duration. The patient noted that the rash would subside and reappear throughout the day. Her medical history was unremarkable, except for COVID-19 infection 6 months prior. She had received a second dose of the BioNTech vaccine 20 days prior to development of symptoms. Dermatologic examination revealed widespread erythematous urticarial plaques. A diagnosis of acute urticaria was made. The patient recovered completely after 1 week of treatment with a systemic steroid and 3 weeks of antihistamine treatment.
Patient 7—A 63-year-old woman presented to the hospital with widespread itching and rash that appeared 5 days after the first dose of the BioNTech COVID-19 vaccine. The patient reported that the rash resolved spontaneously within a few hours but then reappeared. Her medical history revealed that she was taking tamoxifen for breast cancer and that she previously had received 2 doses of the Sinovac-Coronavac vaccine. Dermatologic examination revealed erythematous urticarial plaques on the trunk and arms. A diagnosis of urticaria was made, and her symptoms resolved after 6 weeks of antihistamine treatment.
Comment
Skin lesions associated with COVID-19 infection have been reported worldwide3,4 as well as dermatologic reactions following COVID-19 vaccination. In one case from Turkey, HZ infection was reported in a 68-year-old man 5 days after he received a second dose of the COVID-19 vaccine.5 In another case, HZ infection developed in a 78-year-old man 5 days after COVID-19 vaccination.6 Numerous cases of HZ infection developing within 1 to 26 days of COVID-19 vaccination have been reported worldwide.7-9
In a study conducted in the United States, 40 skin reactions associated with the COVID-19 vaccine were investigated; of these cases, 87.5% (35/40) were reported as varicella-zoster virus, and 12.5% (5/40) were reported as herpes simplex reactivation; 54% (19/35) and 80% (4/5) of these cases, respectively, were associated with the Pfizer-BioNTech vaccine.10 The average age of patients who developed a skin reaction was 46 years, and 70% (28/40) were women. The time to onset of the reaction was 2 to 13 days after vaccination, and symptoms were reported to improve within 7 days on average.10
Another study from Spain examined 405 vaccine-related skin reactions, 40.2% of which were related to the Pfizer-BioNTech vaccine. Among them, 80.2% occurred in women; 13.8% of cases were diagnosed as varicella-zoster virus or HZ virus reactivation, and 14.6% were urticaria. Eighty reactions (21%) were classified as severe/very severe and 81% required treatment.11 One study reported 414 skin reactions from the COVID-19 vaccine from December 2020 to February 2021; of these cases, 83% occurred after the Moderna vaccine, which is not available in Turkey, and 17% occurred after the Pfizer-BioNTech vaccine.12A systematic review of 91 patients who developed HZ infection after COVID-19 vaccination reported that 10% (9/91) of cases were receiving immunosuppressive therapy and 13% (12/91) had an autoimmune disease.7 In our case series, it is known that at least 2 of the patients (patients 2 and 5), including 1 patient with rheumatoid arthritis (patient 2) who was on immunosuppressive treatment, had autoimmune disorders. However, reports in the literature indicate that most patients with autoimmune inflammatory rheumatic diseases remain stable after vaccination.13
Herpes zoster ophthalmicus is a rare form of HZ caused by involvement of the ophthalmic branch of the trigeminal nerve that manifests as vesicular lesions and retinitis, uveitis, keratitis, conjunctivitis, and pain on an erythematous background. Two cases of women who developed HZO infection after Pfizer-BioNTech vaccination were reported in the literature.14 Although patient 3 in our case series had a history of HZO 6 years prior, the possibility of the Pfizer-BioNTech vaccine triggering HZO should be taken into consideration.
Although cutaneous reactions after the Sinovac-Coronavac vaccine were observed in only 1 of 7 patients in our case series, skin reactions after Sinovac-Coronavac (an inactivated viral vaccine) have been reported in the literature. In one study, after a total of 35,229 injections, the incidence of cutaneous adverse events due to Sinovac-Coronavac was reported to be 0.94% and 0.70% after the first and second doses, respectively.15 Therefore, further study results are needed to directly attribute the reactions to COVID-19 vaccination.
Conclusion
Our case series highlights that clinicians should be vigilant in diagnosing cutaneous reactions following COVID-19 vaccination early to prevent potential complications. Early recognition of reactions is crucial, and the prognosis can be improved with appropriate treatment. Despite the potential dermatologic adverse effects of the COVID-19 vaccine, the most effective way to protect against serious COVID-19 infection is to continue to be vaccinated.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603-2615.
- Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192.
- Tan SW, Tam YC, Oh CC. Skin manifestations of COVID-19: a worldwide review. JAAD Int. 2021;2:119-133.
- Singh H, Kaur H, Singh K, et al. Cutaneous manifestations of COVID-19: a systematic review. advances in wound care. 2021;10:51-80.
- Aksu SB, Öztürk GZ. A rare case of shingles after COVID-19 vaccine: is it a possible adverse effect? clinical and experimental vaccine research. 2021;10:198-201.
- Bostan E, Yalici-Armagan B. Herpes zoster following inactivated COVID-19 vaccine: a coexistence or coincidence? J Cosmet Dermatol. 2021;20:1566-1567.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013. doi:10.3390/vaccines9091013
- Rodríguez-Jiménez P, Chicharro P, Cabrera LM, et al. Varicella-zoster virus reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: report of 5 cases. JAAD Case Rep. 2021;12:58-59. doi:10.1016/j.jdcr.2021.04.014
- Lee C, Cotter D, Basa J, et al. 20 Post-COVID-19 vaccine-related shingles cases seen at the Las Vegas Dermatology clinic and sent to us via social media. J Cosmet Dermatol. 2021;20:1960-1964.
- Fathy RA, McMahon DE, Lee C, et al. Varicella-zoster and herpes simplex virus reactivation post-COVID-19 vaccination: a review of 40 cases in an International Dermatology Registry. J Eur Acad Dermatol Venerol. 2022;36:E6-E9.
- Català A, Muñoz-Santos C, Galván-Casas C, et al. Cutaneous reactions after SARS-CoV-2 vaccination: a cross-sectional Spanish nationwide study of 405 cases. Br J Dermatol. 2022;186:142-152.
- McMahon DE, Amerson E, Rosenbach M, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85:46-55.
- Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330-1338.
- Bernardini N, Skroza N, Mambrin A, et al. Herpes zoster ophthalmicus in two women after Pfizer-BioNTech (BNT162b2) vaccine. J Med Virol. 2022;94:817-818.
- Rerknimitr P, Puaratanaarunkon T, Wongtada C, et al. Cutaneous adverse reactions from 35,229 doses of Sinovac and AstraZeneca COVID-19 vaccination: a prospective cohort study in healthcare workers. J Eur Acad Dermatol Venereol. 2022;36:E158-E161.
Practice Points
- Cutaneous reactions have been reported following COVID-19 vaccination.
- Herpes infections and urticarial reactions can be associated with COVID-19 vaccination, regardless of the delay in onset between the injection and symptom development.
How to Navigate Challenging Patient Encounters in Dermatology Residency
Dermatologists in training are exposed to many different clinical scenarios—from the quick 15-minute encounter to diagnose a case of atopic dermatitis to hours of digging through a medical record to identify a culprit medication in a hospitalized patient with a life-threatening cutaneous drug reaction. Amidst the day-to-day clinical work that we do, there inevitably are interactions we have with patients that are less than ideal. These challenging encounters—whether they be subtle microaggressions that unfortunately enter the workplace or blatant quarrels between providers and patients that leave both parties dissatisfied—are notable contributors to physician stress levels and can lead to burnout.1,2 However, there are positive lessons to be learned from these challenging patient encounters if we manage to withstand them. When we start to understand the factors contributing to difficult clinical encounters, we can begin to develop and apply effective communication tools to productively navigate these experiences.
Defining the Difficult Patient
In 2017, the Global Burden of Disease study revealed that skin disease is the fourth leading cause of nonfatal disease burden worldwide.3 Based on this statistic, it is easy to see how some patients may experience frustration associated with their condition and subsequently displace their discontent on the physician. In one study, nearly 1 of every 6 (16.7%) outpatient encounters was considered difficult by physicians.4 Family medicine physicians defined the difficult patient as one who is violent, demanding, aggressive, and rude.5 Others in primary care specialties have considered difficult patients to have characteristics that include mental health problems, more than 5 somatic symptoms, and abrasive personalities.4,6
Situational and Physician-Centered Factors in Difficult Patient Encounters
In our medical system, the narrative often is focused on the patient, for better or worse—the patient was difficult, thereby making the encounter difficult. However, it is important to remember that difficult encounters can be attributed to several different factors, including those related to the physician, the clinical situation, or both. For example, dermatology residents juggle their clinical duties; academic work including studying, teaching, and/or research; and systemic and personal pressures at all times, whether they are cognizant of it or not. For better or worse, by virtue of being human, residents bring these factors with them to each clinical encounter. The delicate balance of these components can have a considerable impact on our delivery of good health care. This is particularly relevant in dermatology, where residents are subject to limited time during visits, work culture among clinic staff that is out of our control, and prominent complex social issues (for those of us practicing in medically underserved areas). Poor communication skills, underlying bias toward specific health conditions, limited knowledge as a trainee, and our own personal stressors also may play large roles in perceiving a clinical encounter as difficult during dermatology residency.7
Strategies to Mitigate Difficult Encounters
As a resident, if you make a statement that sparks a negative response from the patient, acknowledge their negative emotion, try to offer help, or rephrase the original statement to quickly dispel the tension. Validating a patient’s emotions and helping them embrace uncertainty can go a long way in the therapeutic relationship, especially in dermatology where so many of our diseases are chronic and without a definite cure.8 Additionally, it is important to apply strategies to redirect and de-escalate the situation during emotionally charged conversations, such as active listening, validating and empathizing with emotions, exploring alternative solutions, and providing closure to the conversation. Consensus recommendations for managing challenging encounters established by the American Academy of Family Physicians in 2013 include setting boundaries or modifying schedules, as needed, to handle difficult encounters; employing empathetic listening skills and a nonjudgmental attitude to facilitate trust and adherence to treatment; and assessing for underlying psychological illnesses with referral for appropriate diagnosis and treatment. Finally, the CALMER method—catalyst for change, alter thoughts to change feelings, listen and then make a diagnosis, make an agreement, education and follow-up, reach out and discuss feelings—is another approach that may be useful.7 In dermatology, this approach may not only dissipate unwanted tension but also make progress toward a therapeutic relationship. We cannot control the patient’s behavior in a visit, but we need to keep in mind that we are in control of our own reactions to said behavior.9 After first acknowledging this, we can then guide patients to take steps toward overcoming the issue. Within the time restrictions of a dermatology clinic visit, residents may use this approach to quickly feel more in control of a distressing situation and remain calm to better care for the patient.
Final Thoughts
Difficult patient encounters are impossible to avoid in any field of medicine, and dermatology is no exception. It will only benefit residents to recognize the multiple factors impacting a challenging encounter now and learn or enhance conflict resolution and communication skills to navigate these dissatisfying and uncomfortable situations, as they are inevitable in our careers.
- Bodner S. Stress management in the difficult patient encounter. Dent Clin North Am. 2008;52:579-603, ix-xx. doi:10.1016/j.cden.2008.02.012
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi:10.1111/joim.12752
- Seth D, Cheldize K, Brown D, et al. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. 2017;6:204-210. doi:10.1007/s13671-017-0192-7
- An PGRabatin JSManwell LB, et al. Burden of difficult encounters in primary care: data from the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414. doi:10.1001/archinternmed.2008.549
- Steinmetz D, Tabenkin H. The ‘difficult patient’ as perceived by family physicians. Fam Pract. 2001;18:495-500. doi:10.1093/fampra/18.5.495
- Breuner CC, Moreno MA. Approaches to the difficult patient/parent encounter. Pediatrics. 2011;127:163-169. doi:10.1542/peds.2010-0072
- Cannarella Lorenzetti R, Jacques CH, Donovan C, et al. Managing difficult encounters: understanding physician, patient, and situational factors. Am Fam Physician. 2013;87:419-425.
- Bailey J, Martin SA, Bangs A. Managing difficult patient encounters. Am Fam Physician. 2023;108:494-500.
- Pomm HA, Shahady E, Pomm RM. The CALMER approach: teaching learners six steps to serenity when dealing with difficult patients. Fam Med. 2004;36:467-469.
Dermatologists in training are exposed to many different clinical scenarios—from the quick 15-minute encounter to diagnose a case of atopic dermatitis to hours of digging through a medical record to identify a culprit medication in a hospitalized patient with a life-threatening cutaneous drug reaction. Amidst the day-to-day clinical work that we do, there inevitably are interactions we have with patients that are less than ideal. These challenging encounters—whether they be subtle microaggressions that unfortunately enter the workplace or blatant quarrels between providers and patients that leave both parties dissatisfied—are notable contributors to physician stress levels and can lead to burnout.1,2 However, there are positive lessons to be learned from these challenging patient encounters if we manage to withstand them. When we start to understand the factors contributing to difficult clinical encounters, we can begin to develop and apply effective communication tools to productively navigate these experiences.
Defining the Difficult Patient
In 2017, the Global Burden of Disease study revealed that skin disease is the fourth leading cause of nonfatal disease burden worldwide.3 Based on this statistic, it is easy to see how some patients may experience frustration associated with their condition and subsequently displace their discontent on the physician. In one study, nearly 1 of every 6 (16.7%) outpatient encounters was considered difficult by physicians.4 Family medicine physicians defined the difficult patient as one who is violent, demanding, aggressive, and rude.5 Others in primary care specialties have considered difficult patients to have characteristics that include mental health problems, more than 5 somatic symptoms, and abrasive personalities.4,6
Situational and Physician-Centered Factors in Difficult Patient Encounters
In our medical system, the narrative often is focused on the patient, for better or worse—the patient was difficult, thereby making the encounter difficult. However, it is important to remember that difficult encounters can be attributed to several different factors, including those related to the physician, the clinical situation, or both. For example, dermatology residents juggle their clinical duties; academic work including studying, teaching, and/or research; and systemic and personal pressures at all times, whether they are cognizant of it or not. For better or worse, by virtue of being human, residents bring these factors with them to each clinical encounter. The delicate balance of these components can have a considerable impact on our delivery of good health care. This is particularly relevant in dermatology, where residents are subject to limited time during visits, work culture among clinic staff that is out of our control, and prominent complex social issues (for those of us practicing in medically underserved areas). Poor communication skills, underlying bias toward specific health conditions, limited knowledge as a trainee, and our own personal stressors also may play large roles in perceiving a clinical encounter as difficult during dermatology residency.7
Strategies to Mitigate Difficult Encounters
As a resident, if you make a statement that sparks a negative response from the patient, acknowledge their negative emotion, try to offer help, or rephrase the original statement to quickly dispel the tension. Validating a patient’s emotions and helping them embrace uncertainty can go a long way in the therapeutic relationship, especially in dermatology where so many of our diseases are chronic and without a definite cure.8 Additionally, it is important to apply strategies to redirect and de-escalate the situation during emotionally charged conversations, such as active listening, validating and empathizing with emotions, exploring alternative solutions, and providing closure to the conversation. Consensus recommendations for managing challenging encounters established by the American Academy of Family Physicians in 2013 include setting boundaries or modifying schedules, as needed, to handle difficult encounters; employing empathetic listening skills and a nonjudgmental attitude to facilitate trust and adherence to treatment; and assessing for underlying psychological illnesses with referral for appropriate diagnosis and treatment. Finally, the CALMER method—catalyst for change, alter thoughts to change feelings, listen and then make a diagnosis, make an agreement, education and follow-up, reach out and discuss feelings—is another approach that may be useful.7 In dermatology, this approach may not only dissipate unwanted tension but also make progress toward a therapeutic relationship. We cannot control the patient’s behavior in a visit, but we need to keep in mind that we are in control of our own reactions to said behavior.9 After first acknowledging this, we can then guide patients to take steps toward overcoming the issue. Within the time restrictions of a dermatology clinic visit, residents may use this approach to quickly feel more in control of a distressing situation and remain calm to better care for the patient.
Final Thoughts
Difficult patient encounters are impossible to avoid in any field of medicine, and dermatology is no exception. It will only benefit residents to recognize the multiple factors impacting a challenging encounter now and learn or enhance conflict resolution and communication skills to navigate these dissatisfying and uncomfortable situations, as they are inevitable in our careers.
Dermatologists in training are exposed to many different clinical scenarios—from the quick 15-minute encounter to diagnose a case of atopic dermatitis to hours of digging through a medical record to identify a culprit medication in a hospitalized patient with a life-threatening cutaneous drug reaction. Amidst the day-to-day clinical work that we do, there inevitably are interactions we have with patients that are less than ideal. These challenging encounters—whether they be subtle microaggressions that unfortunately enter the workplace or blatant quarrels between providers and patients that leave both parties dissatisfied—are notable contributors to physician stress levels and can lead to burnout.1,2 However, there are positive lessons to be learned from these challenging patient encounters if we manage to withstand them. When we start to understand the factors contributing to difficult clinical encounters, we can begin to develop and apply effective communication tools to productively navigate these experiences.
Defining the Difficult Patient
In 2017, the Global Burden of Disease study revealed that skin disease is the fourth leading cause of nonfatal disease burden worldwide.3 Based on this statistic, it is easy to see how some patients may experience frustration associated with their condition and subsequently displace their discontent on the physician. In one study, nearly 1 of every 6 (16.7%) outpatient encounters was considered difficult by physicians.4 Family medicine physicians defined the difficult patient as one who is violent, demanding, aggressive, and rude.5 Others in primary care specialties have considered difficult patients to have characteristics that include mental health problems, more than 5 somatic symptoms, and abrasive personalities.4,6
Situational and Physician-Centered Factors in Difficult Patient Encounters
In our medical system, the narrative often is focused on the patient, for better or worse—the patient was difficult, thereby making the encounter difficult. However, it is important to remember that difficult encounters can be attributed to several different factors, including those related to the physician, the clinical situation, or both. For example, dermatology residents juggle their clinical duties; academic work including studying, teaching, and/or research; and systemic and personal pressures at all times, whether they are cognizant of it or not. For better or worse, by virtue of being human, residents bring these factors with them to each clinical encounter. The delicate balance of these components can have a considerable impact on our delivery of good health care. This is particularly relevant in dermatology, where residents are subject to limited time during visits, work culture among clinic staff that is out of our control, and prominent complex social issues (for those of us practicing in medically underserved areas). Poor communication skills, underlying bias toward specific health conditions, limited knowledge as a trainee, and our own personal stressors also may play large roles in perceiving a clinical encounter as difficult during dermatology residency.7
Strategies to Mitigate Difficult Encounters
As a resident, if you make a statement that sparks a negative response from the patient, acknowledge their negative emotion, try to offer help, or rephrase the original statement to quickly dispel the tension. Validating a patient’s emotions and helping them embrace uncertainty can go a long way in the therapeutic relationship, especially in dermatology where so many of our diseases are chronic and without a definite cure.8 Additionally, it is important to apply strategies to redirect and de-escalate the situation during emotionally charged conversations, such as active listening, validating and empathizing with emotions, exploring alternative solutions, and providing closure to the conversation. Consensus recommendations for managing challenging encounters established by the American Academy of Family Physicians in 2013 include setting boundaries or modifying schedules, as needed, to handle difficult encounters; employing empathetic listening skills and a nonjudgmental attitude to facilitate trust and adherence to treatment; and assessing for underlying psychological illnesses with referral for appropriate diagnosis and treatment. Finally, the CALMER method—catalyst for change, alter thoughts to change feelings, listen and then make a diagnosis, make an agreement, education and follow-up, reach out and discuss feelings—is another approach that may be useful.7 In dermatology, this approach may not only dissipate unwanted tension but also make progress toward a therapeutic relationship. We cannot control the patient’s behavior in a visit, but we need to keep in mind that we are in control of our own reactions to said behavior.9 After first acknowledging this, we can then guide patients to take steps toward overcoming the issue. Within the time restrictions of a dermatology clinic visit, residents may use this approach to quickly feel more in control of a distressing situation and remain calm to better care for the patient.
Final Thoughts
Difficult patient encounters are impossible to avoid in any field of medicine, and dermatology is no exception. It will only benefit residents to recognize the multiple factors impacting a challenging encounter now and learn or enhance conflict resolution and communication skills to navigate these dissatisfying and uncomfortable situations, as they are inevitable in our careers.
- Bodner S. Stress management in the difficult patient encounter. Dent Clin North Am. 2008;52:579-603, ix-xx. doi:10.1016/j.cden.2008.02.012
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi:10.1111/joim.12752
- Seth D, Cheldize K, Brown D, et al. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. 2017;6:204-210. doi:10.1007/s13671-017-0192-7
- An PGRabatin JSManwell LB, et al. Burden of difficult encounters in primary care: data from the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414. doi:10.1001/archinternmed.2008.549
- Steinmetz D, Tabenkin H. The ‘difficult patient’ as perceived by family physicians. Fam Pract. 2001;18:495-500. doi:10.1093/fampra/18.5.495
- Breuner CC, Moreno MA. Approaches to the difficult patient/parent encounter. Pediatrics. 2011;127:163-169. doi:10.1542/peds.2010-0072
- Cannarella Lorenzetti R, Jacques CH, Donovan C, et al. Managing difficult encounters: understanding physician, patient, and situational factors. Am Fam Physician. 2013;87:419-425.
- Bailey J, Martin SA, Bangs A. Managing difficult patient encounters. Am Fam Physician. 2023;108:494-500.
- Pomm HA, Shahady E, Pomm RM. The CALMER approach: teaching learners six steps to serenity when dealing with difficult patients. Fam Med. 2004;36:467-469.
- Bodner S. Stress management in the difficult patient encounter. Dent Clin North Am. 2008;52:579-603, ix-xx. doi:10.1016/j.cden.2008.02.012
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi:10.1111/joim.12752
- Seth D, Cheldize K, Brown D, et al. Global burden of skin disease: inequities and innovations. Curr Dermatol Rep. 2017;6:204-210. doi:10.1007/s13671-017-0192-7
- An PGRabatin JSManwell LB, et al. Burden of difficult encounters in primary care: data from the minimizing error, maximizing outcomes study. Arch Intern Med. 2009;169:410-414. doi:10.1001/archinternmed.2008.549
- Steinmetz D, Tabenkin H. The ‘difficult patient’ as perceived by family physicians. Fam Pract. 2001;18:495-500. doi:10.1093/fampra/18.5.495
- Breuner CC, Moreno MA. Approaches to the difficult patient/parent encounter. Pediatrics. 2011;127:163-169. doi:10.1542/peds.2010-0072
- Cannarella Lorenzetti R, Jacques CH, Donovan C, et al. Managing difficult encounters: understanding physician, patient, and situational factors. Am Fam Physician. 2013;87:419-425.
- Bailey J, Martin SA, Bangs A. Managing difficult patient encounters. Am Fam Physician. 2023;108:494-500.
- Pomm HA, Shahady E, Pomm RM. The CALMER approach: teaching learners six steps to serenity when dealing with difficult patients. Fam Med. 2004;36:467-469.
RESIDENT PEARLS
- Challenging patient encounters are inevitable in our work as dermatology residents. Both physician- and patient-related factors can contribute.
- Setting boundaries, active listening, and addressing emotions during and after the visit can help to mitigate challenging encounters.
An Ethical Analysis of Treatment of an Active-Duty Service Member With Limited Follow-up
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
PRACTICE POINTS
- Dermatologic conditions are among the most common concerns reported by active-duty service members.
- The unique considerations of deployments are important for dermatologists to consider in the treatment of skin disease.
Nonepidemic Kaposi Sarcoma: A Case of a Rare Epidemiologic Subtype
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
To the Editor:
Kaposi sarcoma (KS) is a rare angioproliferative disorder associated with human herpesvirus 8 (HHV-8) infection.1 There are 4 main recognized epidemiologic forms of KS: classic, endemic, epidemic, and iatrogenic (Table). Nonepidemic KS is a recently described rare fifth type of KS that occurs in a subset of patients who do not fit the other classifications—HIV-negative patients without detectable cellular or humoral immune deficiency. This subset has been described as clinically similar to classic KS with limited disease but occurring in younger men.2,3 We describe a case of nonepidemic KS in a Middle Eastern heterosexual immunocompetent man.

A 30-year-old man presented for evaluation of a growth on the nose of 3 months’ duration. The patient reported being otherwise healthy and was not taking long-term medications. He denied a history of malignancy, organ transplant, or immunosuppressive therapy. He was born in Syria and lived in Thailand for several years prior to moving to the United States. HIV testing 6 months prior to presentation was negative. He denied fever, chills, lymphadenopathy, shortness of breath, hemoptysis, melena, hematochezia, and intravenous drug use.

Physical examination revealed a solitary shiny, 7-mm, pink-red papule on the nasal dorsum (Figure 1). No other skin or mucosal lesions were identified. There was no cervical, axillary, or inguinal lymphadenopathy. A laboratory workup consisting of serum immunoglobulins and serum protein electrophoresis was unremarkable. Tests for HIV-1 and HIV-2 as well as human T-lymphotropic virus 1 and 2 were negative. The CD4 and CD8 counts were within reference range. Histopathology of a shave biopsy revealed a dermal spindle cell proliferation arranged in short intersecting fascicles and admixed with plasma cells and occasional mitotic figures. Immunohistochemistry showed that the spindle cells stained positive for CD34, CD31, and HHV-8 (Figure 2). The lesion resolved after treatment with cryotherapy. Repeat HIV testing 3 months later was negative. No recurrence or new lesions were identified at 3-month follow-up.

Similar to the other subtypes of KS, the nonepidemic form is dependent on HHV-8 infection, which is more commonly transmitted via saliva and sexual contact.3,4 After infecting endothelial cells, HHV-8 is believed to activate the mammalian target of rapamycin and nuclear factor κB pathways, resulting in aberrant cellular differentiation and neoangiogenesis through upregulation of vascular endothelial growth factor and basic fibroblast growth factor.2,4 Similar to what is seen with other herpesviruses, HHV-8 infection typically is lifelong due to the virus’s ability to establish latency within human B cells and endothelial cells as well as undergo sporadic bouts of lytic reactivation during its life cycle.4
Nonepidemic KS resembles other variants clinically, manifesting as erythematous or violaceous, painless, nonblanchable macules, papules, and nodules.1 Early lesions often are asymptomatic and can manifest as pigmented macules or small papules that vary from pale pink to vivid purple. Nodules also can occur and be exophytic and ulcerated with bleeding.1 Secondary lymphoproliferative disorders including Castleman disease and lymphoma have been reported.2,5
In contrast to other types of KS in which pulmonary or gastrointestinal tract lesions can develop with hemoptysis or hematochezia, mucocutaneous and visceral lesions rarely are reported in nonepidemic KS.3 Lymphedema, a feature associated with endemic KS, is notably absent in nonepidemic KS.1,3
The differential diagnosis applicable to all KS subtypes includes other vascular lesions such as angiomatosis and angiosarcoma. Histopathologic analysis is critical to differentiate KS from these conditions; visual diagnosis alone has only an 80% positive predictive value for KS.4 The histopathologic presentation of KS is a vascular proliferation in the dermis accompanied by an increased number of vessels without an endothelial cell lining.4 Spindle cell proliferation also is a common feature and is considered to be the KS tumor cell. Immunostaining for HHV-8 antigen as well as for CD31 and CD34 can be used to confirm the diagnosis.4
The management and prognosis of KS depends on the epidemiologic subtype. Classic and nonepidemic KS generally are indolent with a good prognosis. Periodic follow-up is recommended because of an increased risk for secondary malignancy such as lymphoma. The treatment of epidemic KS is highly active antiretroviral therapy. Similarly, reduction of immunosuppression is warranted for iatrogenic KS. For all types, cutaneous lesions can be treated with local excision, cryosurgery, radiation, chemotherapy, intralesional vincristine, or a topical agent such as imiquimod or alitretinoin.6
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
- Hinojosa T, Lewis DJ, Liu M, et al. Nonepidemic Kaposi sarcoma: a recently proposed category. J Am Acad Dermatol. 2017;3:441-443. doi: 10.1016/j.jdcr.2017.04.012
- Heymann WR. Nonepidemic Kaposi sarcoma: the fifth dimension. Dermatology World Insights and Inquiries. Published October 16, 2019. Accessed January 30, 2024. https://www.aad.org/dw/dw-insights-and-inquiries/2019-archive/october/nonepidemic-kaposi-sarcoma
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542. doi: 10.1111/ijd.14080
- Cesarman E, Damania B, Krown SE, et al. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:9. doi:10.1038/s41572-019-0060-9
- Vecerek N, Truong A, Turner R, et al. Nonepidemic Kaposi’s sarcoma: an underrecognized subtype in HIV-negative patients. J Am Acad Dermatol. 2019;81(suppl 1):AB247. doi:10.1016/j.jaad.2019.09.1096
- Schneider JW, Dittmer DP. Diagnosis and treatment of Kaposi sarcoma. Am J Clin Dermatol. 2017;18:529-539. doi:10.1007/s40257-017-0270-4
Practice Points
- Nonepidemic Kaposi sarcoma (KS) is a recently described fifth subtype of the disease that typically occurs in younger men who are HIV-negative without detectable cellular or humoral immune deficiency.
- The cutaneous manifestations of nonepidemic KS are similar to those of classic KS, except that disease extent is limited and the prognosis is favorable in nonepidemic KS.
- Dermatologists should consider KS when a patient presents with clinically representative findings, even in the absence of typical risk factors such as immunosuppression.