User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
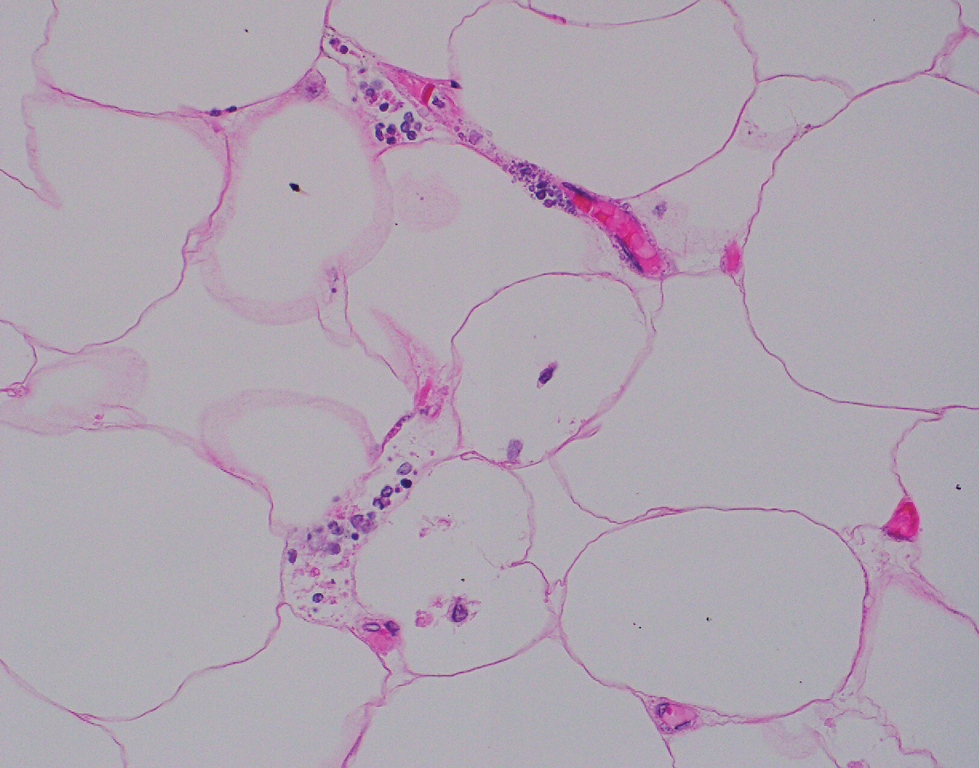
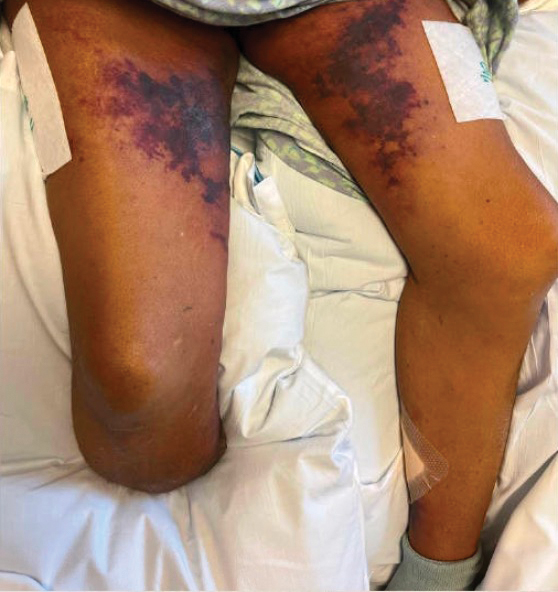
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
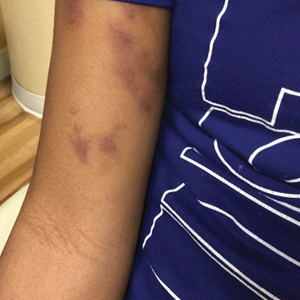
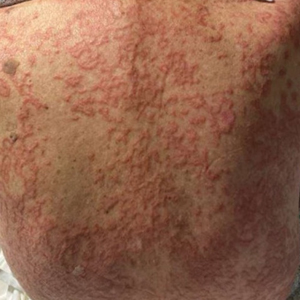
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.
Methotrexate-Induced Mucositis in a Patient With Angioimmunoblastic T-cell Lymphoma
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
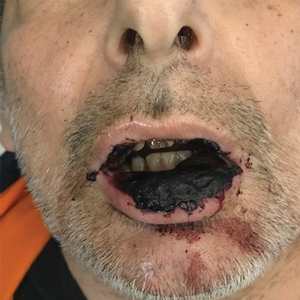
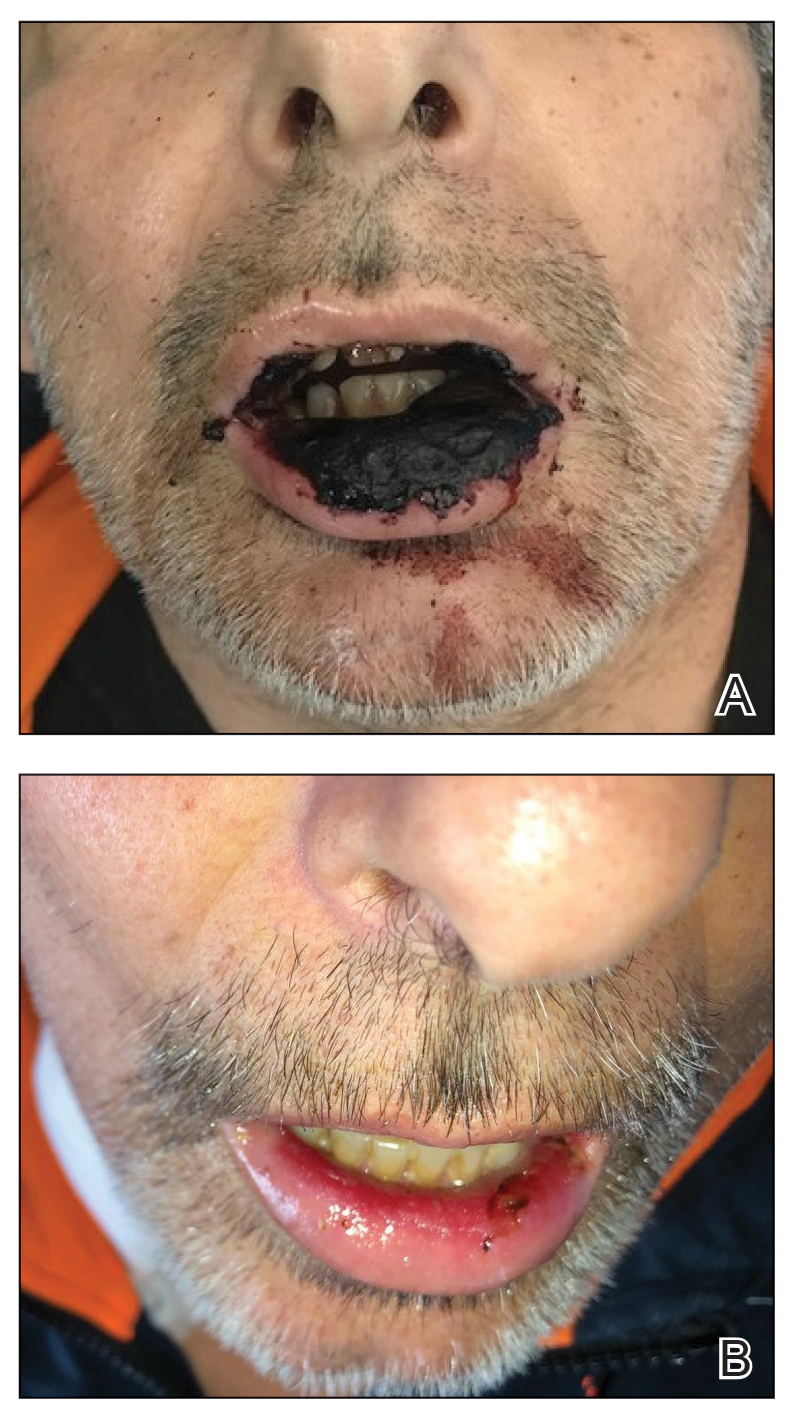
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.
Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.

Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
To the Editor:
Angioimmunoblastic T-cell lymphoma (AITL) is an uncommon peripheral T-cell lymphoma that accounts for 1% to 2% of all forms of non-Hodgkin lymphoma and usually affects middle-aged individuals.1 It primarily appears on the skin and mimics an inflammatory dermatosis, leading to diagnostic and therapeutic delays.2 No gold-standard treatment has been identified for AITL; the prognosis often remains poor, with a 5-year progression-free survival rate of approximately 25%.3 Because of the rarity of AITL and the unmet need of a standard-of-care treatment regimen, relapsing and remitting disease is common and continues to challenge clinicians.
Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed at a higher dosage (>500 mg/m2) to manage cancers, including refractory AITL.4 In blocking dihydrofolate reductase, MTX reduces the folate pool, with the possible adverse effect of bone marrow suppression. Another important toxic effect is acute kidney injury, which may be due to an overdose of MTX or a patient’s predisposition to chronic kidney failure.4
A 50-year-old man was admitted to our inpatient clinic for evaluation of acute oral and genital mucositis. He had a 5-year history of AITL. He was previously treated by hematology with 3 lines of chemotherapy for multiple supradiaphragmatic and subdiaphragmatic localizations of lymphoma, without success. Six days prior to the current presentation, the hematologist started high-dose (3.5 g/m2) intravenous MTX therapy. Five days later, the patient developed transfusion-resistant pancytopenia and fever (maximum body temperature, 102.7°F [39.3°C]).
Physical examination at the current presentation revealed massive necrosis of the lower lip (Figure, A) and partial necrosis of the upper lip. Severe purulent balanoposthitis, causing penile edema and phimosis, complicated the clinical condition. Analysis of a specimen from a cutaneous swab of the penis showed infection with Pseudomonas aeruginosa and Enterococcus faecalis. Considering the clinical presentation and time of onset of signs and symptoms, a diagnosis of acute MTX-induced mucositis was made.
Rescue therapy was started immediately, including high-dose intravenous leucovorin (120 mg 4 times daily), oral sulfamethoxazole-trimethoprim (800 mg/160 mg 3 times daily for 3 days per week), and oral levofloxacin (500 mg/d). After 4 days of treatment, the patient was afebrile. Mucositis of the lips had almost resolved (Figure, B), and balanoposthitis also improved after this rescue therapy. Methotrexate was not resumed because rituximab had been started.

Methotrexate-induced mucositis is a rare severe skin manifestation of MTX toxicity. Prolonged renal toxicity from MTX can predispose a patient to massive myelosuppression, multiorgan failure, and mucositis.5 Pancytopenia manifests during the first 10 days of treatment. Because accumulation of MTX is higher in mucosal epithelial cells than in bone marrow stem cells, mucositis usually occurs during the first 7 days of administration, prior to onset of pancytopenia.
Skin involvement usually manifests as oral and genital mucositis due to direct toxicity against epithelial cells, with a pattern of severe keratinocyte necrosis on histopathology, known as MTX-induced epidermal necrosis.6 The principal condition in the differential diagnosis is Stevens-Johnson syndrome—including its severe form, toxic epidermal necrolysis—characterized by widespread blistering and more extensive skin detachment caused by an immune-mediated cytotoxic T-cell drug-specific reaction.7
To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy. These tests should be repeated during treatment to monitor for MTX toxicity.
Leucovorin (folinic acid) counteracts MTX-induced epidermal necrosis by neutralizing the effect of MTX, including antitumoral effectiveness of the drug.8 For that reason, leucovorin cannot be started prophylactically.
The main challenges that we encountered in our patient's case were the rarity of reports of AITL in the literature and failure of 3 different lines of chemotherapy previously, which meant that MTX could not possibly be suspended because the drug represented the last therapeutic option. Our case confirms that timely clinical diagnosis and a rapid combined approach consisting of discontinuation of MTX and initiation of leucovorin rescue therapy represents an effective strategy to prevent further toxicity and to alleviate mucositis, even in patients with this rare subset of lymphoma.
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
- Swarup S, Kopel J, Thein, KZ, et al. Sequential complications of hypercalcemia, necrotizing granulomatous vasculitis, and aplastic anemia occurring in one patient with angioimmunoblastic T cell lymphoma. Am J Med Sci. 2021;361:375-382. doi:10.1016/j.amjms.2020.09.003
- Wang L, Lee HY, Koh HY, et al. Cutaneous presentation of angioimmunoblastic T-cell lymphoma: a harbinger of poor prognosis? Skinmed. 2016;14:469-471.
- Kameoka Y, Takahashi N, Itou S, et al. Analysis of clinical characteristics and prognostic factors for angioimmunoblastic T-cell lymphoma. Int J Hematol. 2015;101:536-542. doi:10.1007/s12185-015-1763-7
- Howard SC, McCormick J, Pui C-H, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471-1482. doi:10.1634/theoncologist.2015-0164
- Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949-959. doi:10.1200/JCO.2013.53.0808
- Yélamos O, Català A, Vilarrasa E, et al. Acute severe methotrexate toxicity in patients with psoriasis: a case series and discussion. Dermatology. 2014;229:306-309. doi:10.1159/000366501
- Delyon J, Ortonne N, Benayoun E, et al. Low-dose methotrexate-induced skin toxicity: keratinocyte dystrophy as a histologic marker.J Am Acad Dermatol. 2015;73:484-490. doi:10.1016/j.jaad.2015.06.015
- Chen T-J, Chung W-H, Chen C-B, et al. Methotrexate-induced epidermal necrosis: a case series of 24 patients. J Am Acad Dermatol. 2017;77:247-255.e2. doi:10.1016/j.jaad.2017.02.021
PRACTICE POINTS
- Methotrexate (MTX), a dihydrofolate reductase inhibitor used to treat many autoimmune diseases, is prescribed to manage cancers such as refractory angioimmunoblastic T-cell lymphoma.
- Dermatologists should be aware of the potential mucocutaneous adverse effects of high-dosage MTX.
- To prevent MTX toxicity, liver and renal function should be assessed and a complete blood cell count should be performed before starting therapy.
A Cross-sectional Analysis of Regional Trends in Medicare Reimbursement for Phototherapy Services From 2010 to 2023
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.
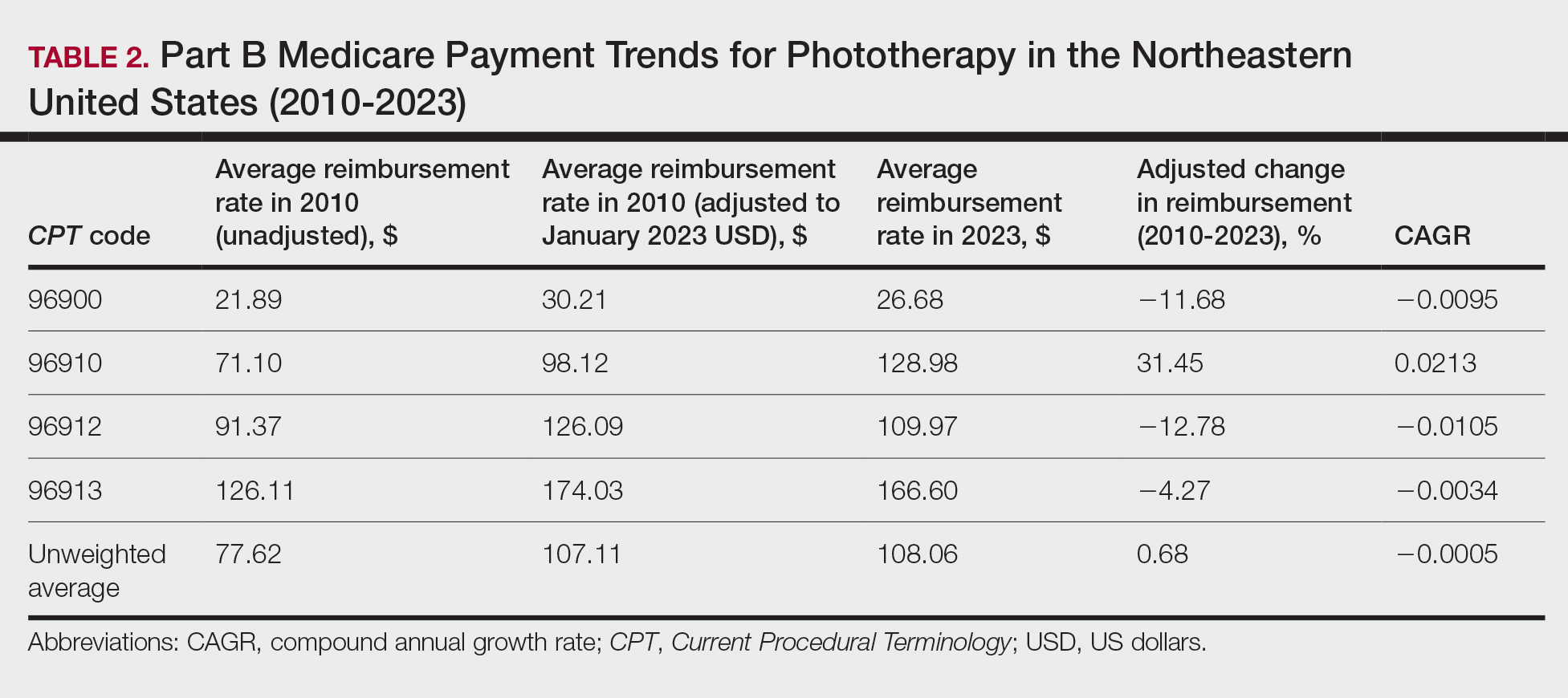
On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).
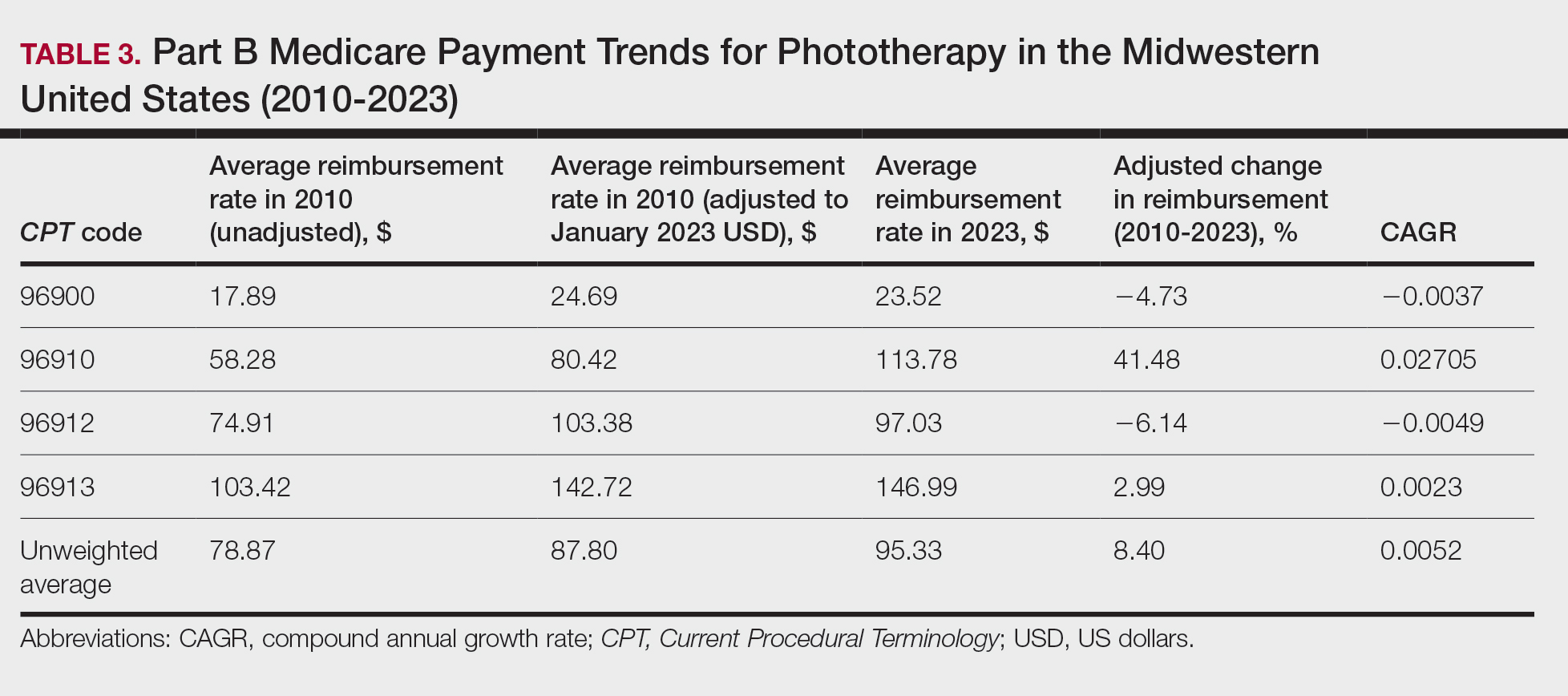
On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).
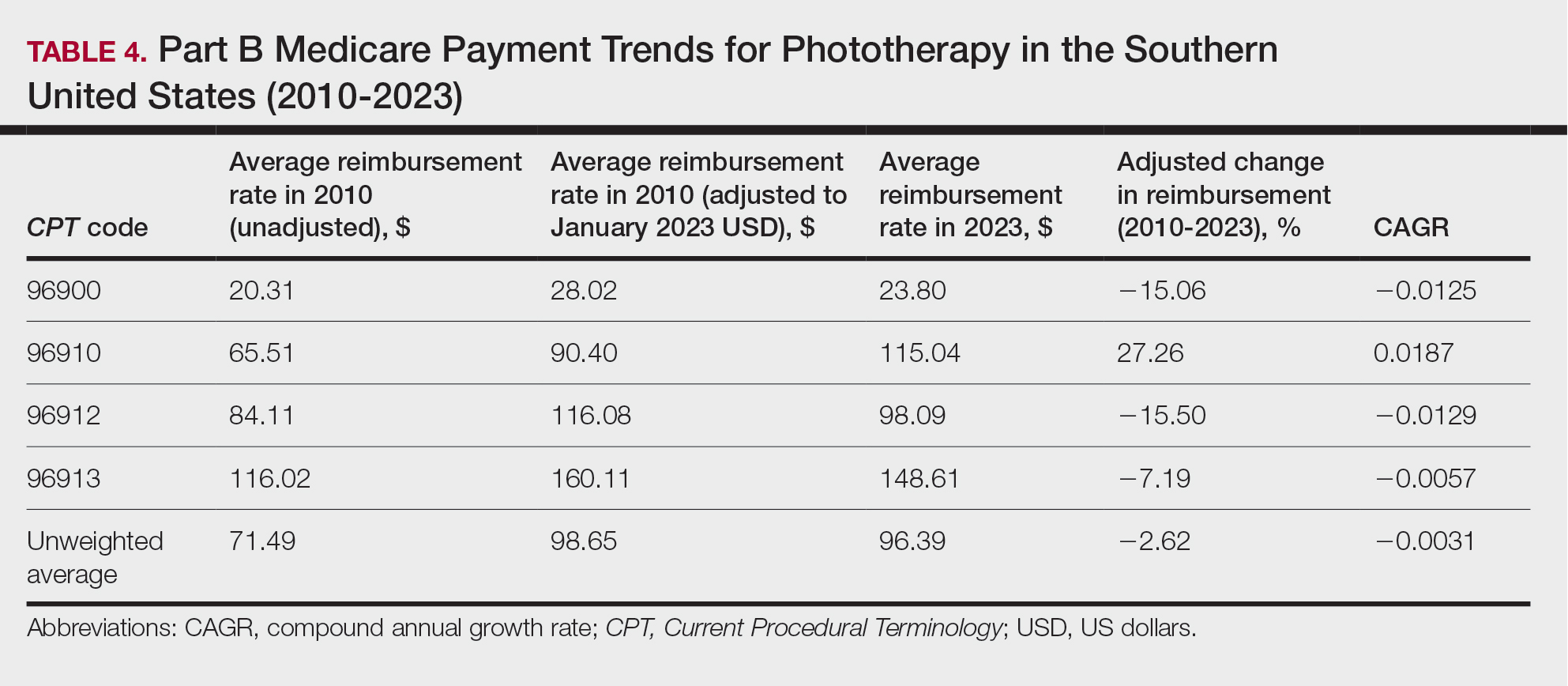
On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).
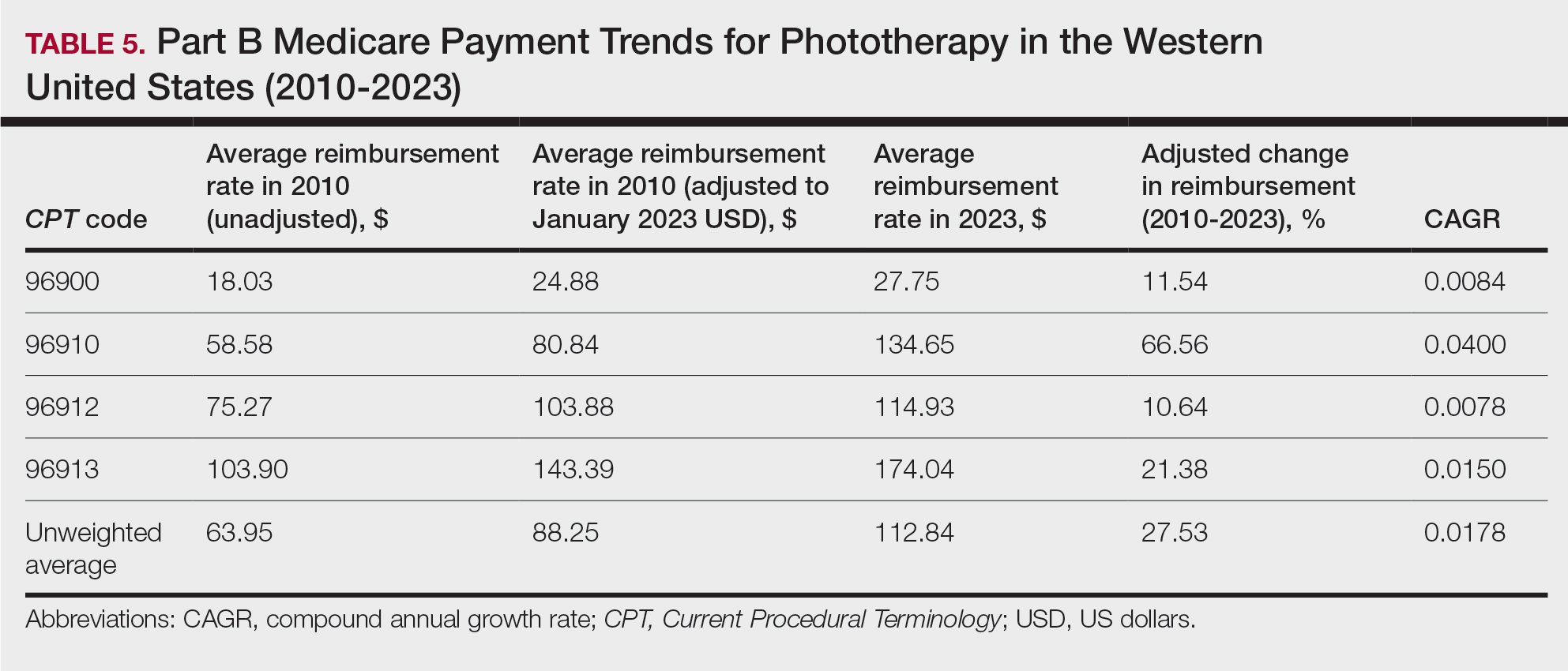
On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).
In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
To the Editor:
Phototherapy regularly is utilized in the outpatient setting to address various skin pathologies, including atopic dermatitis, psoriasis, pruritus, vitiligo, and mycosis fungoides.1,2 Phototherapy is broadly defined by the measured administration of nonionizing radiation within the UV range including wavelengths within the UVA (eg, psoralen sensitizer plus UVA-1) and UVB (eg, broadband UVB, narrowband UVB) spectrums.1,3 Generally, the mechanism of action is derived from effects on inflammatory components of cutaneous disorders and the induction of apoptosis, both precipitating numerous downstream events.4
From 2015 to 2018, there were more than 1.3 million outpatient phototherapy visits in the United States, with the most common procedural indications being dermatitis not otherwise specified, atopic dermatitis, and pruritus.5 From 2000 to 2015, the quantity of phototherapy services billed to Medicare trended upwards by an average of 5% per year, increasing from 334,670 in the year 2000 to 692,093 in 2015.6 Therefore, an illustration of associated costs would be beneficial. Additionally, because total cost and physician reimbursement fluctuate from year to year, studies demonstrating overall trends can inform both US policymakers and physicians. There is a paucity of research on geographical trends for procedural reimbursements in dermatology for phototherapy. Understanding geographic trends of reimbursement could duly serve to optimize dermatologist practice patterns involving access to viable and quality care for patients seeking treatment as well as draw health policymakers’ attention to striking adjustments in physician fees. Therefore, in this study we aimed to illustrate the most recent regional payment trends in phototherapy procedures for Medicare B patients.
We queried the Centers for Medicare & Medicaid Services Medicare Physician Fee Schedule (MPFS) database (https://www.cms.gov/medicare/payment/fee-schedules/physician/lookup-tool) for the years 2010 to 2023 for Current Procedural Terminology (CPT) codes common to phototherapy procedures: actinotherapy (96900); photochemotherapy by Goeckerman treatment or using petrolatum and UVB (96910); photochemotherapy using psoralen plus UVA (96912); and photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision (96913). Nonfacility prices for these procedures were analyzed. For 2010, due to midyear alterations to Medicare reimbursement (owed to bills HR 3962 and HR 4872), the mean price data of MPFS files 2010A and 2010B were used. All dollar values were converted to January 2023 US dollars using corresponding consumer price index inflation data. The Medicare Administrative Contractors were used to group state pricing information by region in accordance with established US Census Bureau subdivisions (https://www.census.gov/programs-surveys/economic-census/guidance-geographies/levels.html). Weighted percentage change in reimbursement rate was calculated using physician (MD or DO) utilization (procedure volume) data available in the 2020 Physician and Other Practitioners Public Use File (https://data.cms.gov/provider-summary-by-type-of-service/medicare-physician-other-practitioners/medicare-physician-other-practitioners-by-provider-and-service). All descriptive statistics and visualization were generated using R software (v4.2.2)(R Development Core Team).
Table 1 provides physician utilization data and the corresponding number of Part B beneficiaries for phototherapy procedures in 2020. There were 65,045 services of actinotherapy provided to a total of 6855 unique Part B beneficiaries, 173,979 services of photochemotherapy by Goeckerman treatment or using petrolatum and UVB provided to 13,122 unique Part B beneficiaries, 2524 services of photochemotherapy using psoralen plus UVA provided to a total of 357 unique Part B beneficiaries, and 37 services of photochemotherapy of severe dermatoses requiring a minimum of 4 hours of care under direct physician supervision provided to a total of 27 unique Part B beneficiaries.

On average (unweighted), phototherapy reimbursement rates in the North increased by 0.68% between 2010 and 2023 (Table 2). After weighting for 2020 physician utilization, the average change in reimbursement rate was +19.37%. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+31.45%)($98.12 to $128.98; compound annual growth rate [CAGR], +0.0213), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−12.76%)($126.09 to $109.97; CAGR, −0.0105). For CPT code 96900, the reported adjusted decrease in reimbursement was −11.68% ($30.21 to $26.68; CAGR, −0.0095), and for CPT code 96913, the reported adjusted decrease in reimbursement was −4.27% ($174.03 to $166.60; CAGR, −0.0034).

On average (unweighted), phototherapy reimbursement rates in the Midwest increased by 8.40% between 2010 and 2023 (Table 3). After weighting for 2020 physician utilization, the average change in reimbursement rate was +28.53%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+41.48%)($80.42 to $113.78; CAGR, +0.0270), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−6.14%)($103.28 to $97.03; CAGR, −0.0049). For CPT code 96900, the reported adjusted decrease in reimbursement was −4.73% ($24.69 to $23.52; CAGR, −0.0037), and for CPT code 96913, the reported adjusted increase in reimbursement was +2.99% ($142.72 to $146.99; CAGR, +0.0023).

On average (unweighted), phototherapy reimbursement rates in the South decreased by 2.62% between 2010 and 2023 (Table 4). After weighting for 2020 physician utilization, the average change in reimbursement rate was +15.41%. During this time period, CPT code 96910 reported the greatest adjusted change in reimbursement (+27.26%)($90.40 to $115.04 USD; CAGR, +0.0187), and CPT code 96912 reported the greatest adjusted decrease in reimbursement (−15.50%)($116.08 to $98.09; CAGR, −0.0129). For CPT code 96900, the reported adjusted decrease in reimbursement was −15.06% ($28.02 to $23.80; CAGR, −0.0125), and for CPT code 96913, the reported adjusted decrease in reimbursement was −7.19% ($160.11 to $148.61; CAGR, −0.0057).

On average (unweighted), phototherapy reimbursement rates in the West increased by 27.53% between 2010 and 2023 (Table 5). After weighting for 2020 physician utilization, the average change in reimbursement rate was +51.16%. Reimbursement for all analyzed procedures increased in the western United States. During this time period, CPT code 96910 reported the greatest adjusted increase in reimbursement (+66.56%)($80.84 to $134.65; CAGR, +0.0400), and CPT code 96912 reported the lowest adjusted increase in reimbursement (+10.64%)($103.88 to $114.93; CAGR, +0.0078). For CPT code 96900, the reported adjusted increase in reimbursement was 11.54% ($24.88 to $27.75; CAGR, +0.0084), and for CPT code 96913, the reported adjusted increase in reimbursement was 21.38% ($143.39 to $174.04; CAGR, +0.0150).

In this study evaluating geographical payment trends for phototherapy from 2010 to 2023, we demonstrated regional inconsistency in mean inflation-adjusted Medicare reimbursement rates. We found that all phototherapy procedures had increased reimbursement in the western United States, whereas all other regions reported cuts in reimbursement rates for at least half of the analyzed procedures. After adjusting for procedure utilization by physicians, weighted mean reimbursement for phototherapy increased in all US regions.
In a cross-sectional study that explored trends in the geographic distribution of dermatologists from 2012 to 2017, dermatologists in the northeastern and western United States were more likely to be located in higher-income zip codes, whereas dermatologists in the southern United States were more likely to be located in lower-income zip codes,7 suggesting that payment rate changes are not concordant with cost of living. Additionally, Lauck and colleagues8 observed that 75% of the top 20 most common procedures performed by dermatologists had decreased reimbursement (mean change, −10.8%) from 2011 to 2021. Other studies on Medicare reimbursement trends over the last 2 decades have reported major decreases within other specialties, suggesting that declining Medicare reimbursements are not unique to dermatology.9,10 It is critical to monitor these developments, as the Centers for Medicare & Medicaid Services emphasized health care policy changes aimed at increasing reimbursements for evaluation and management services with compensatory payment cuts in billing for procedural services.11
Mazmudar et al12 previously reported a mean reimbursement decrease of −6.6% for laser/phototherapy procedures between 2007 and 2021, but these data did not include the heavily utilized Goeckerman treatment. Changes in reimbursement pose major ramifications for dermatologists—for practice size, scope, and longevity—as rates influence changes in commercial insurance reimbursements.13 Medicare plays a major role in the US health care system as the second largest expenditure14; indeed, between 2000 and 2015, Part B billing volume for phototherapy procedures increased 5% annually. However, phototherapy remains inaccessible in many locations due to unequal regional distribution of phototherapy clinics.6 Moreover, home phototherapy units are not yet widely utilized because of safety and efficacy concerns, lack of physician oversight, and difficulty obtaining insurance coverage.15 Acknowledgment and consideration of these geographical trends may persuasively allow policymakers, hospitals, and physicians to facilitate cost-effective phototherapy reimbursements that ensure continued access to quality and sustainable dermatologic care in the United States that tailor to regional needs.
In sum, this analysis reveals regional trends in Part B physician reimbursement for phototherapy procedures, with all US regions reporting a mean increase in phototherapy reimbursement after adjusting for utilization, albeit to varying degrees. Mean reimbursement for photochemotherapy by Goeckerman treatment or using petrolatum and UVB increased most among phototherapy procedures. Mean reimbursement for both actinotherapy and photochemotherapy using psoralen plus UVA decreased in all regions except the western United States.
Limitations include the restriction to Part B MPFS and the reliance on single-year (2020) physician utilization data to compute weighted changes in average reimbursement across a multiyear range, effectively restricting sweeping conclusions. Still, this study puts forth actionable insights for dermatologists and policymakers alike to appreciate and consider.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
- Rathod DG, Muneer H, Masood S. Phototherapy. StatPearls. StatPearls Publishing; 2002.
- Branisteanu DE, Dirzu DS, Toader MP, et al. Phototherapy in dermatological maladies (Review). Exp Ther Med. 2022;23:259. doi:10.3892/etm.2022.11184
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407. doi:10.1016/j.abd.2021.03.001
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021;222:107784. doi:10.1016/j.pharmthera.2020.107784
- Oulee A, Javadi SS, Martin A, et al. Phototherapy trends in dermatology 2015-2018. J Dermatolog Treat. 2022;33:2545-2546. doi:10.1080/09546634.2021.2019660
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679. doi:10.1016/j.jaad.2018.03.018
- Benlagha I, Nguyen BM. Changes in dermatology practice characteristics in the United States from 2012 to 2017. JAAD Int. 2021;3:92-101. doi:10.1016/j.jdin.2021.03.005
- Lauck K, Nguyen QB, Hebert A. Trends in Medicare reimbursement within dermatology: 2011-2021. Skin. 2022;6:122-131. doi:10.25251/skin.6.2.5
- Smith JF, Moore ML, Pollock JR, et al. National and geographic trends in Medicare reimbursement rates for orthopedic shoulder and upper extremity surgery from 2000 to 2020. J Shoulder Elbow Surg. 2022;31:860-867. doi:10.1016/j.jse.2021.09.001
- Haglin JM, Eltorai AEM, Richter KR, et al. Medicare reimbursement for general surgery procedures: 2000 to 2018. Ann Surg. 2020;271:17-22. doi:10.1097/SLA.0000000000003289
- Fleishon HB. Evaluation and management coding initiative. J Am Coll Radiol. 2020;17:1539-1540. doi:10.1016/j.jacr.2020.09.057
- Mazmudar RS, Sheth A, Tripathi R, et al. Inflation-adjusted trends in Medicare reimbursement for common dermatologic procedures, 2007-2021. JAMA Dermatol. 2021;157:1355-1358. doi:10.1001/jamadermatol.2021.3453
- Clemens J, Gottlieb JD. In the shadow of a giant: Medicare’s influence on private physician payments. J Polit Econ. 2017;125:1-39. doi:10.1086/689772
- Ya J, Ezaldein HH, Scott JF. Trends in Medicare utilization by dermatologists, 2012-2015. JAMA Dermatol. 2019;155:471-474. doi:10.1001/jamadermatol.2018.4212
- Rajpara AN, O’Neill JL, Nolan BV, et al. Review of home phototherapy. Dermatol Online J. 2010;16:2.
Practice Points
- After weighting for procedure utilization, mean reimbursement for phototherapy increased across all US regions from 2010 to 2023 (mean change, +28.62%), yet with marked regional diversity.
- The southern United States reported the least growth in weighted mean reimbursement (+15.41%), and the western United States reported the greatest growth in weighted mean reimbursement (+51.16%).
- Region- and procedure-specific payment changes are especially valuable to dermatologists and policymakers alike, potentially reinvigorating payment reform discussions.
Psychogenic Purpura
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.
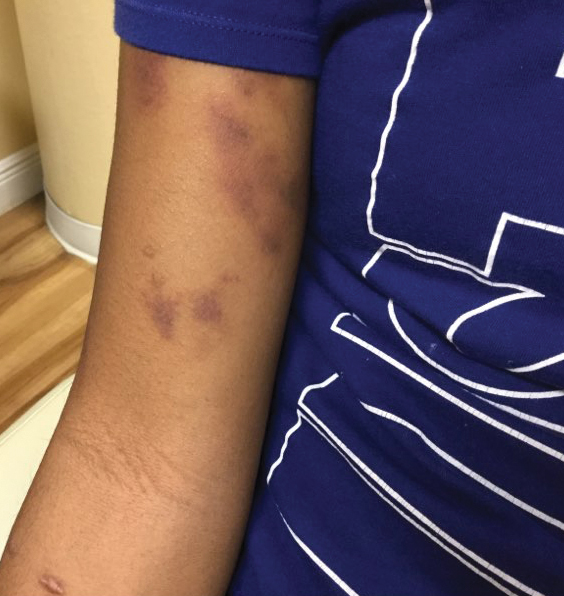
Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.
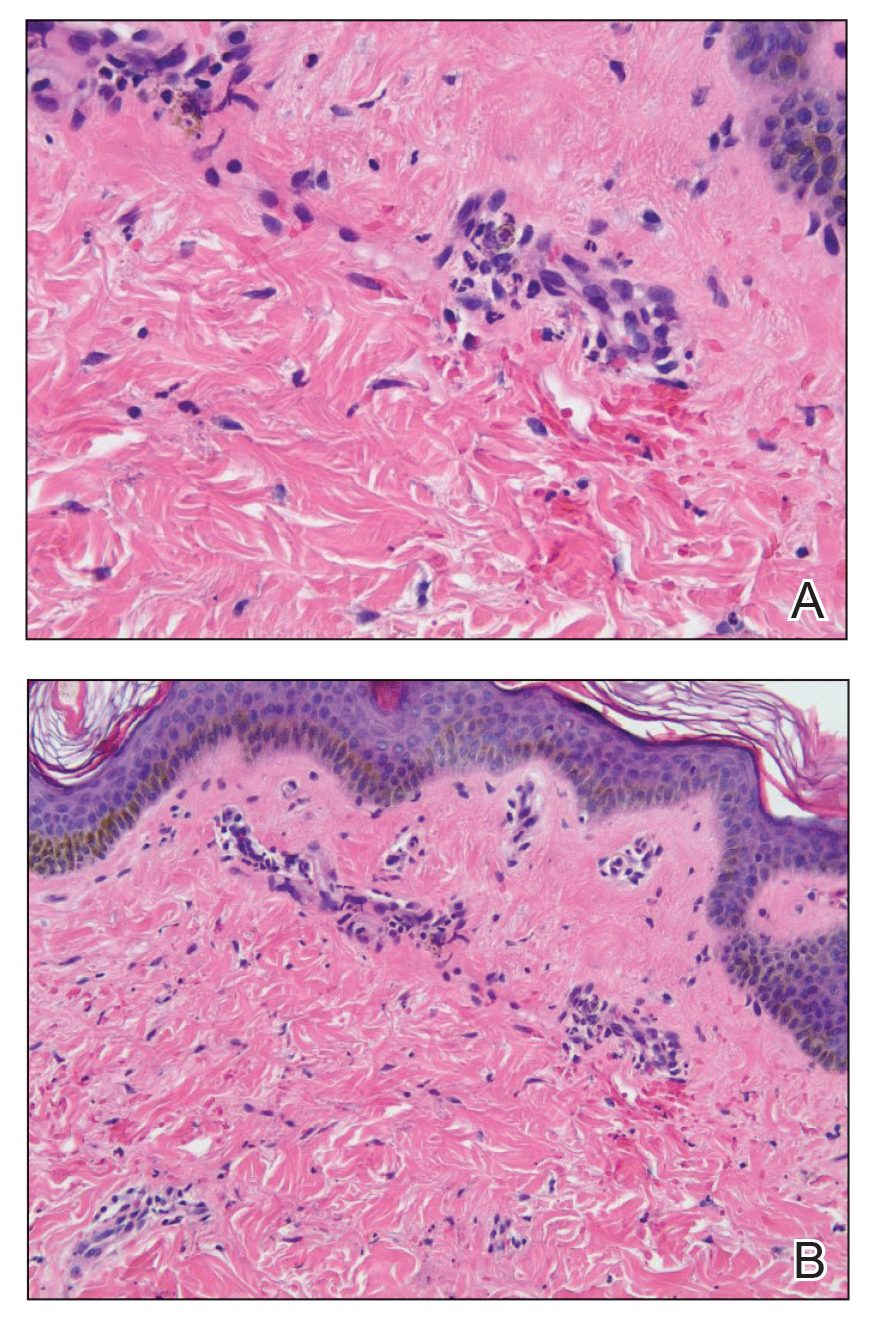
Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.

Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.

Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
To the Editor:
A 14-year-old Black adolescent girl presented with episodic, painful, edematous plaques that occurred symmetrically on the arms and legs of 5 years’ duration. The plaques evolved into hyperpigmented patches within 24 to 48 hours before eventually resolving. Fatigue, headache, arthralgias of the arms and legs, chest pain, abdominal pain, nausea, and vomiting variably accompanied these episodes.
Prior to visiting our clinic, the patient had been seen by numerous specialists. A review of her medical records revealed an initial diagnosis of Henoch-Schönlein purpura (HSP), then urticarial vasculitis. She had been treated with antihistamines, topical and systemic steroids, hydroxychloroquine, mycophenolate mofetil, dapsone, azathioprine, and gabapentin. All treatments were ineffectual. She underwent extensive diagnostic testing and imaging, which were normal or noncontributory, including type I allergy testing; multiple exhaustive batteries of hematologic testing; and computed tomography/magnetic resonance imaging/magnetic resonance angiography of the brain, chest, abdomen, and pelvic region. Biopsies from symptomatic segments of the gastrointestinal tract were normal.
Chronic treatment with systemic steroids over 9 months resulted in gastritis and an episode of hematemesis requiring emergent hospitalization. A lengthy multidisciplinary evaluation was conducted at the patient’s local community hospital; the team concluded that she had an urticarial-type rash with accompanying symptoms that did not have an autoimmune, rheumatologic, or inflammatory basis.
The patient’s medical history was remarkable for recent-onset panic attacks. Her family medical history was noncontributory. Physical examination revealed multiple violaceous hyperpigmented patches diffusely located on the proximal upper arms (Figure 1). There were no additional findings on physical examination.

Punch biopsies were performed on lesional areas of the arm. Histopathology indicated a mild superficial perivascular dermal mixed infiltrate and extravasated erythrocytes (Figure 2). Direct immunofluorescence (DIF) testing was negative for vasculitis. Immunohistochemical stains for CD117 and tryptase demonstrated a slight increase in the number of dermal mast cells; however, the increase was not sufficient to diagnose cutaneous mastocytosis, which was in the differential. We proposed a diagnosis of psychogenic purpura (PP)(also known as Gardner-Diamond syndrome). She was treated with gabapentin, a selective serotonin reuptake inhibitor, and cognitive therapy. Unfortunately, after starting therapy the patient was lost to follow-up.

Psychogenic purpura is a rare vasculopathy of unknown etiology that may be a special form of factitious disorder.1,2 In one study, PP occurred predominantly in females aged 15 to 66 years, with a median onset age of 33 years.3 A prodrome of localized itching, burning, and/or pain precedes the development of edematous plaques. The plaques evolve into painful ecchymoses within 1 to 2 days and resolve in 10 days or fewer without treatment. Lesions most commonly occur on the extremities but may occur anywhere on the body. The most common associated finding is an underlying depressive disorder. Episodes may be accompanied by headache, dizziness, fatigue, fever, arthralgia, nausea, vomiting, abdominal pain, menstrual irregularities, myalgia, and urologic conditions.
In 1955, Gardner and Diamond4 described the first cases of PP in 4 female patients at Peter Bent Brigham Hospital in Boston, Massachusetts. The investigators were able to replicate the painful ecchymoses with intradermal injection of the patient’s own erythrocytes into the skin. They proposed that the underlying pathogenesis involved autosensitization to erythrocyte stroma.4 Since then, others have suggested that the pathogenesis may include autosensitization to erythrocyte phosphatidylserine, tonus dysregulation of venous capillaries, abnormal endothelial fibrin synthesis, and capillary wall instability.5-7
Histopathology typically reveals superficial and deep perivascular inflammation with extravasated erythrocytes. Direct immunofluorescence is negative for vasculitis.8 Diagnostics and laboratory findings for underlying systemic illness are negative or noncontributory. Cutaneous injection of 1 mL of the patient’s own washed erythrocytes may result in the formation of the characteristic painful plaques within 24 hours; however, this test is limited by lack of standardization and low sensitivity.3
Psychogenic purpura may share clinical features with cutaneous small vessel vasculitis, such as HSP or urticarial vasculitis. Some of the findings that our patient was experiencing, including purpura, arthralgia, and abdominal pain, are associated with HSP. However, HSP typically is self-limiting and classically features palpable purpura distributed across the lower extremities and buttocks. Histopathology demonstrates the classic findings of leukocytoclastic vasculitis; DIF typically is positive for perivascular IgA and C3 deposition. Increased serum IgA may be present.9 Urticarial vasculitis appears as erythematous indurated wheals that favor a proximal extremity and truncal distribution. They characteristically last longer than 24 hours, are frequently associated with nonprodromal pain or burning, and resolve with hyperpigmentation. Arthralgia and gastrointestinal, renal, pulmonary, cardiac, and neurologic symptoms may be present, especially in patients with low complement levels.10 Skin biopsy demonstrates leukocytoclasia that must be accompanied by vessel wall necrosis. Fibrinoid deposition, erythrocyte extravasation, or perivascular inflammation may be present. In 70% of cases revealing perivascular immunoglobulin, C3, and fibrinogen deposition, DIF is positive. Serum C1q autoantibody may be associated with the hypocomplementemic form.10
The classic histopathologic findings in leukocytoclastic vasculitis include transmural neutrophilic infiltration of the walls of small vessels, fibrinoid necrosis of vessel walls, leukocytoclasia, extravasated erythrocytes, and signs of endothelial cell damage.9 A prior punch biopsy in this patient demonstrated rare neutrophilic nuclear debris within the vessel walls without fibrin deposition. Although the presence of nuclear debris and extravasated erythrocytes could be compatible with a manifestation of urticarial vasculitis, the lack of direct evidence of vessel wall necrosis combined with subsequent biopsies unequivocally ruled out cutaneous small vessel vasculitis in our patient.
Psychogenic purpura has been reported to occur frequently in the background of psycho-emotional distress. In 1989, Ratnoff11 noted that many of the patients he was treating at the University Hospitals of Cleveland, Ohio, had a depressive syndrome. A review of patients treated at the Mayo Clinic in Rochester, Minnesota, illustrated concomitant psychiatric illnesses in 41 of 76 (54%) patients treated for PP, most commonly depressive, personality, and anxiety disorders.3
There is no consensus on therapy for PP. Treatment is based on providing symptomatic relief and relieving underlying psychiatric distress. Block et al12 found the use of selective serotonin reuptake inhibitors, tricyclic antidepressants, and psychotherapy to be successful in improving symptoms and reducing lesions at follow-up visits.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
- Piette WW. Purpura: mechanisms and differential diagnosis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:376-389.
- Harth W, Taube KM, Gieler U. Factitious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Sridharan M, Ali U, Hook CC, et al. The Mayo Clinic experience with psychogenic purpura (Gardner-Diamond syndrome). Am J Med Sci. 2019;357:411‐420.
- Gardner FH, Diamond LK. Autoerythrocyte sensitization; a form of purpura producing painful bruising following autosensitization to red blood cells in certain women. Blood. 1955;10:675-690.
- Groch GS, Finch SC, Rogoway W, et al. Studies in the pathogenesis of autoerythrocyte sensitization syndrome. Blood. 1966;28:19-33.
- Strunecká A, Krpejsová L, Palecek J, et al. Transbilayer redistribution of phosphatidylserine in erythrocytes of a patient with autoerythrocyte sensitization syndrome (psychogenic purpura). Folia Haematol Int Mag Klin Morphol Blutforsch. 1990;117:829-841.
- Merlen JF. Ecchymotic patches of the fingers and Gardner-Diamond vascular purpura. Phlebologie. 1987;40:473-487.
- Ivanov OL, Lvov AN, Michenko AV, et al. Autoerythrocyte sensitization syndrome (Gardner-Diamond syndrome): review of the literature. J Eur Acad Dermatol Venereol. 2009;23:499-504.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Cerroni L. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Hamad A, Jithpratuck W, Krishnaswamy G. Urticarial vasculitis and associated disorders. Ann Allergy Asthma Immunol. 2017;118:394-398.
- Ratnoff OD. Psychogenic purpura (autoerythrocyte sensitization): an unsolved dilemma. Am J Med. 1989;87:16N-21N.
- Block ME, Sitenga JL, Lehrer M, et al. Gardner‐Diamond syndrome: a systematic review of treatment options for a rare psychodermatological disorder. Int J Dermatol. 2019;58:782-787.
PRACTICE POINTS
- Psychogenic purpura is a rare vasculopathy characterized by painful recurrent episodes of purpura. It is a diagnosis of exclusion that may manifest with signs similar to cutaneous small vessel vasculitis.
- Awareness of this condition could help prevent unnecessary diagnostics, medications, and adverse events.
What’s Eating You? Rhipicephalus Ticks Revisited
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
Characteristics
Rhipicephalus ticks belong to the Ixodidae family of hard-bodied ticks. They are large and teardrop shaped with an inornate scutum (hard dorsal plate) and relatively short mouthparts attached at a hexagonal basis capitulum (base of the head to which mouthparts are attached)(Figure).1 Widely spaced eyes and festoons also are present. The first pair of coxae—attachment base for the first pair of legs—are characteristically bifid; males have a pair of sclerotized adanal plates on the ventral surface adjacent to the anus as well as accessory adanal shields.2Rhipicephalus (formerly Boophilus) microplus (the so-called cattle tick) is a newly added species; it lacks posterior festoons, and the anal groove is absent.3

Almost all Rhipicephalus ticks, except for R microplus, are 3-host ticks in which a single blood meal is consumed from a vertebrate host at each active life stage—larva, nymph, and adult—to complete development.4,5 In contrast to most ixodid ticks, which are exophilic (living outside of human habitation), the Rhipicephalus sanguineus sensu lato species (the brown dog tick) is highly endophilic (adapted to indoor living) and often can be found hidden in cracks and crevices of walls in homes and peridomestic structures.6 It is predominately monotropic (all developmental stages feed on the same host species) and has a strong host preference for dogs, though it occasionally feeds on other hosts (eg, humans).7 Although most common in tropical and subtropical climates, they can be found anywhere there are dogs due to their ability to colonize indoor dwellings.8 In contrast, R microplus ticks have a predilection for cattle and livestock rather than humans, posing a notable concern to livestock worldwide. Infestation results in transmission of disease-causing pathogens, such as Babesia and Anaplasma species, which costs the cattle industry billions of dollars annually.9
Clinical Manifestations and Treatment
Tick bites usually manifest as intensely pruritic, erythematous papules at the site of tick attachment due to a local type IV hypersensitivity reaction to antigens in the tick’s saliva. This reaction can be long-lasting. In addition to pruritic papules following a bite, an attached tick can be mistaken for a skin neoplasm or nevus. Given that ticks are small, especially during the larval stage, dermoscopy may be helpful in making a diagnosis.10 Symptomatic relief usually can be achieved with topical antipruritics or oral antihistamines.
Of public health concern, brown dog ticks are important vectors of Rickettsia rickettsii (the causative organism of Rocky Mountain spotted fever [RMSF]) in the Western hemisphere, and Rickettsia conorii (the causative organism of Mediterranean spotted fever [MSF][also known as Boutonneuse fever]) in the Eastern hemisphere.11 Bites by ticks carrying rickettsial disease classically manifest with early symptoms of fever, headache, and myalgia, followed by a rash or by a localized eschar or tache noire (a black, necrotic, scabbed lesion) that represents direct endothelial invasion and vascular damage by Rickettsia.12 Rocky Mountain spotted fever and MSF are more prevalent during summer, likely due, in part, to the combination of increased outdoor activity and a higher rate of tick-questing (host-seeking) behavior in warmer climates.4,7
Rocky Mountain Spotted Fever—Dermacentor variabilis is the primary vector of RMSF in the southeastern United States; Dermacentor andersoni is the major vector of RMSF in Rocky Mountain states. Rhipicephalus sanguineus sensu lato is an important vector of RMSF in the southwestern United States, Mexico, and Central America.11,13
Early symptoms of RMSF are nonspecific and can include fever, headache, arthralgia, myalgia, and malaise. Gastrointestinal tract symptoms (eg, nausea, vomiting, anorexia) may occur; notable abdominal pain occurs in some patients, particularly children. A characteristic petechial rash occurs in as many as 90% of patients, typically at the third to fifth day of illness, and classically begins on the wrists and ankles, with progression to the palms and soles before spreading centripetally to the arms, legs, and trunk.14 An eschar at the inoculation site is uncommon in RMSF; when present, it is more suggestive of MSF.15
The classic triad of fever, headache, and rash is present in 3% of patients during the first 3 days after a tick bite and in 60% to 70% within 2 weeks.16 A rash often is absent when patients first seek medical attention and may not develop (absent in 9% to 12% of cases; so-called spotless RMSF). Therefore, absence of rash should not be a reason to withhold treatment.16 Empiric treatment with doxycycline should be started promptly for all suspected cases of RMSF because of the rapid progression of disease and an increased risk for morbidity and mortality with delayed diagnosis.
Patients do not become antibody positive until 7 to 10 days after symptoms begin; therefore, treatment should not be delayed while awaiting serologic test results. The case fatality rate in the United States is estimated to be 5% to 10% overall and as high as 40% to 50% among patients who are not treated until day 8 or 9 of illness.17
Cutaneous complications include skin necrosis and gangrene due to continuous tissue damage in severe cases.16 Severe infection also may manifest with signs of multiorgan system damage, including altered mental status, cerebral edema, meningismus, transient deafness, myocarditis, pulmonary hemorrhage and edema, conjunctivitis, retinal abnormalities, and acute renal failure.14,16 Risk factors for more severe illness include delayed treatment, age 40 years or older or younger than 10 years, and underlying medical conditions such as alcoholic liver disease and glucose-6-phosphate dehydrogenase deficiency. However, even some healthy young patients die of this disease.17
Mediterranean Spotted Fever—Rhipicephalus sanguineus sensu lato is the primary vector of MSF, which is prevalent in areas adjacent to the Mediterranean Sea, including southern Europe, Africa, and Central Asia; Sicily is the most highly affected region.18 Findings with MSF are nearly identical to those of RMSF, except that tache noire is more common, present in as many as 70% of cases at the site of the inoculating tick bite, and MSF typically follows a less severe clinical course.12 Similar to other rickettsial diseases, the pathogenesis of MSF involves direct injury to vascular endothelial cells, causing a vasculitis that is responsible for the clinical abnormalities observed.
Patients with severe MSF experience complications similar to severe RMSF, including neurologic manifestations and multiorgan damage.18 Risk factors include advanced age, immunocompromised state, cardiac disease, chronic alcoholism, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, respiratory insufficiency, and delayed treatment.18
Treatment—For all spotted fever group rickettsial infections, doxycycline is the treatment of choice for all patients, including children and pregnant women. Treatment should be started without delay; recommended dosages are 100 mg twice daily for children weighing more than 45 kg and adults, and 2.2 mg/kg twice daily for children weighing 45 kg or less.12
Rhipicephalus tick bites rarely can result in paralysis; however, Dermacentor ticks are responsible for most cases of tick-related paralysis in North America. Other pathogens proven or reputed to be transmitted by Rhipicephalus sanguineus sensu lato with zoonotic potential include but are not limited to Rickettsia massiliae, Coxiella burnetti, Anaplasma platys, Leishmania infantum, and Crimean-Congo hemorrhagic fever virus (Nairovirus).19
Environmental Treatment and Prevention
The most effective way to prevent tick-borne illness is avoidance of tick bites. Primary prevention methods include vector control, use of repellents (eg, N,N-diethyl-meta-toluamide [DEET]), picaridin, permethrin), avoidance of areas with a high tick burden, use of protective clothing, and detection and removal of ticks as soon as possible.
Environmental and veterinary controls also are important methods of tick-bite prevention. A veterinarian can recommend a variety of agents for dogs and cats that prevent attachment of ticks. Environmental controls include synthetic or natural product-based chemical acaricides and nonchemical methods, such as landscape management (eg, sealing cracks and crevices in homes and controlling tall grasses, weeds, and leaf debris) to minimize potential tick habitat.20 Secondary prevention includes antibiotics for prophylaxis or for treatment of tick-borne disease, when indicated.
Numerous tick repellents are available commercially; others are being studied. DEET, the most widely used topical repellent, has a broad spectrum of activity against many tick species.21 In addition, DEET has a well-known safety and toxicity profile, with rare adverse effects, and is safe for use in pregnant women and children older than 2 years. Alternative repellents, such as those containing picaridin, ethyl butylacetylaminopropionate (IR3535 [Merck]), oil of lemon eucalyptus, and 2-undecanone can be effective; some show efficacy comparable to that of DEET.22 Permethrin, a synthetic pyrethroid, is a highly efficacious tick repellent and insecticide, especially when used in conjunction with a topical repellent such as DEET. Unlike topically applied repellents, permethrin spray is applied to fabric (eg, clothing, shoes, bed nets, camping gear), not to skin.
Indiscriminate use of acaricides worldwide has led to increasing selection of acaricide resistance in Rhipicephalus tick species, which is especially true with the use of acaricides in controlling R microplus livestock infestations; several tick populations now show resistance to all major classes of these compounds.23-25 For that reason, there has been an increasing effort to develop new chemical and nonchemical approaches to tick control that are more environmentally sustainable and strategies to minimize development and progression of resistance such as rotation of acaricides; reducing the frequency of their application; use of pesticide mixtures, synergists, or both; and increasing use of nonacaricidal methods of control.26
Prompt removal of ticks is important for preventing the transmission of tick-borne disease. Proper removal involves rubbing the tick in a circular motion with a moist gauze pad or using fine-tipped tweezers to grasp the tick as close to the skin surface as possible and pulling upward with a steady pressure.17,27 It is important not to jerk, twist, squeeze, smash, or burn the tick, as this can result in insufficient removal of mouthparts or spread contaminated tick fluids to mucous membranes, increasing the risk for infection. Application of petroleum jelly or nail polish to aid in tick removal have not been shown to be effective and are not recommended.16,28
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173-185. doi:10.1016/j.vetpar.2007.12.030
- Madder M, Fourie JJ, Schetters TPM. Arachnida, Metastigmata, Ixodidae (except Ixodes holocyclus). In: Marchiondo AA, Cruthers LR, Fourie JJ, eds. Parasiticide Screening: In Vitro and In Vivo Tests With Relevant Parasite Rearing and Host Infection/Infestation Methods. Volume 1. Elsevier Academic Press; 2019:19-20.
- Burger TD, Shao R, Barker SC. Phylogenetic analysis of mitochondrial genome sequences indicates that the cattle tick, Rhipicephalus (Boophilus) microplus, contains a cryptic species. Mol Phylogenet Evol. 2014;76:241-253. doi:10.1016/j.ympev.2014.03.017
- Gray J, Dantas-Torres F, Estrada-Peña A, et al. Systematics and ecology of the brown dog tick, Rhipicephalus sanguineus. Ticks Tick Borne Dis. 2013;4:171-180. doi:10.1016/j.ttbdis.2012.12.003
- Tian Y, Lord CC, Kaufman PE. Brown dog tick, Rhipicephalus Sanguineus Latrielle (Arachnida: Acari: Ixodidae): EENY-221/IN378. EDIS. March 26, 2020. Accessed January 3, 2024. https://doi.org/10.32473/edis-in378-2020
- Saleh MN, Allen KE, Lineberry MW, et al. Ticks infesting dogs and cats in North America: biology, geographic distribution, and pathogen transmission. Vet Parasitol. 2021;294:109392. doi:10.1016/j.vetpar.2021.109392
- Dantas-Torres F. Biology and ecology of the brown dog tick, Rhipicephalus sanguineus. Parasit Vectors. 2010;3:26. doi:10.1186/1756-3305-3-26
- Dryden MW, Payne PA. Biology and control of ticks infesting dogs and cats in North America. Vet Ther. 2004;5:139-154.
- Nyangiwe N, Yawa M, Muchenje V. Driving forces for changes in geographic range of cattle ticks (Acari: Ixodidae) in Africa: a Review. S Afr J Anim Sci. 2018;48:829. doi:10.4314/sajas.v48i5.4
- Ramot Y, Zlotogorski A, Mumcuoglu KY. Brown dog tick (Rhipicephalus sanguineus) infestation of the penis detected by dermoscopy. Int J Dermatol. 2012;51:1402-1403. doi:10.1111/j.1365-4632.2010.04756.x
- Tucker NSG, Weeks ENI, Beati L, et al. Prevalence and distribution of pathogen infection and permethrin resistance in tropical and temperate populations of Rhipicephalus sanguineus s.l. collected worldwide. Med Vet Entomol. 2021;35:147-157. doi:10.1111/mve.12479
- McClain MT, Sexton DJ, Hall KK, eds. Other spotted fever group rickettsial infections. UpToDate. Updated October 10, 2022. Accessed January 3, 2024. https://www.uptodate.com/contents/other-spotted-fever-group-rickettsial-infections
- Ribeiro CM, Carvalho JLB, Bastos PAS, et al. Prevalence of Rickettsia rickettsii in ticks: systematic review and meta-analysis. Vector Borne Zoonotic Dis. 2021;21:557-565. doi:10.1089/vbz.2021.0004
- Pace EJ, O’Reilly M. Tickborne diseases: diagnosis and management. Am Fam Physician. 2020;101:530-540.
- Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2020.
- Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724-732. doi:10.1016/S1473-3099(07)70261-X
- Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2016;65:1-44. doi:10.15585/mmwr.rr6502a1
- Rossio R, Conalbi V, Castagna V, et al. Mediterranean spotted fever and hearing impairment: a rare complication. Int J Infect Dis. 2015;35:34-36. doi:10.1016/j.ijid.2015.04.005
- Dantas-Torres F, Otranto D. Further thoughts on the taxonomy and vector role of Rhipicephalus sanguineus group ticks. Vet Parasitol. 2015;208:9-13. doi:10.1016/j.vetpar.2014.12.014
- Eisen RJ, Kugeler KJ, Eisen L, et al. Tick-borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 2017;58:319-335. doi:10.1093/ilar/ilx005
- Nguyen QD, Vu MN, Hebert AA. Insect repellents: an updated review for the clinician. J Am Acad Dermatol. 2018;88:123-130. doi:10.1016/j.jaad.2018.10.053
- Pages F, Dautel H, Duvallet G, et al. Tick repellents for human use: prevention of tick bites and tick-borne diseases. Vector Borne Zoonotic Dis. 2014;14:85-93. doi:10.1089/vbz.2013.1410
- Rodriguez-Vivas RI, Alonso-Díaz MA, et al. Prevalence and potential risk factors for organophosphate and pyrethroid resistance in Boophilus microplus ticks on cattle ranches from the State of Yucatan, Mexico. Vet Parasitol. 2006;136:335-342. doi:10.1016/j.vetpar.2005.05.069
- Rodríguez-Vivas RI, Rodríguez-Arevalo F, Alonso-Díaz MA, et al. Prevalence and potential risk factors for amitraz resistance in Boophilus microplus ticks in cattle farms in the State of Yucatan, Mexico. Prev Vet Med. 2006;75:280-286. doi:10.1016/j.prevetmed.2006.04.001
- Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, et al. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165-169. doi:10.1016/j.vetpar.2009.10.021
- Rodriguez-Vivas RI, Jonsson NN, Bhushan C. Strategies for the control of Rhipicephalus microplus ticks in a world of conventional acaricide and macrocyclic lactone resistance. Parasitol Res.2018;117:3-29. doi:10.1007/s00436-017-5677-6
- Centers for Disease Control and Prevention. Tick removal. Updated May 13, 2022. Accessed January 3, 2024. https://www.cdc.gov/ticks/removing_a_tick.html
- Diaz JH. Chemical and plant-based insect repellents: efficacy, safety, and toxicity. Wilderness Environ Med. 2016;27:153-163. doi:10.1016/j.wem.2015.11.007
PRACTICE POINTS
- Rhipicephalus ticks are vectors of a variety of diseases, including the rickettsial diseases Rocky Mountain spotted fever and Mediterranean spotted fever.
- Presenting symptoms of a tick bite include intensely pruritic, erythematous papules and nodules at the site of tick attachment.
- If rickettsial disease is suspected, treatment with doxycycline should be initiated immediately; do not delay treatment to await results of confirmatory tests or because of the absence of a rash.
- Primary methods of prevention of tick-borne disease include repellents, protective clothing, vector control, and prompt removal of the tick.
Noduloplaque on the Forehead
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.
Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.
The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
The Diagnosis: Giant Apocrine Hidrocystoma
Histopathology of the noduloplaque revealed an unremarkable epidermis with multilocular cystic spaces centered in the dermis. The cysts had a double-lined epithelium with inner columnar to cuboidal cells and outer myoepithelial cells (bottom quiz image). Columnar cells showing decapitation secretion could be appreciated at places indicating apocrine secretion (Figure). A final diagnosis of apocrine hidrocystoma was made.

Hidrocystomas are rare, benign, cystic lesions derived either from apocrine or eccrine glands.1 Apocrine hidrocystoma usually manifests as asymptomatic, solitary, dome-shaped papules or nodules with a predilection for the head and neck region. Hidrocystomas can vary from flesh colored to blue, brown, or black. Pigmentation in hidrocystoma is seen in 5% to 80% of cases and is attributed to the Tyndall effect.1 The tumor usually is less than 20 mm in diameter; larger lesions are termed giant apocrine hidrocystoma.2 Apocrine hidrocystoma manifesting with multiple lesions and a size greater than 10 mm, as seen in our case, is uncommon.
Zaballos et al3 described dermoscopy of apocrine hidrocystoma in 22 patients. Hallmark dermoscopic findings were the presence of a homogeneous flesh-colored, yellowish, blue to pinkish-blue area involving the entire lesion with arborizing vessels and whitish structures.3 Similar dermoscopic findings were present in our patient. The homogeneous area histologically correlates to the multiloculated cysts located in the dermis. The exact reason for white structures is unknown; however, their visualization in apocrine hidrocystoma could be attributed to the alternation in collagen orientation secondary to the presence of large or multiple cysts in the dermis.
The presence of shiny white dots arranged in a square resembling a four-leaf clover (also known as white rosettes) was a unique dermoscopic finding in our patient. These rosettes can be appreciated only with polarized dermoscopy, and they have been described in actinic keratosis, seborrheic keratosis, squamous cell carcinoma, and basal cell carcinoma.4 The exact morphologic correlate of white rosettes is unknown but is postulated to be secondary to material inside adnexal openings in small rosettes and concentric perifollicular fibrosis in larger rosettes.4 In our patient, we believe the white rosettes can be attributed to the accumulated secretions in the dermal glands, which also were seen via histopathology. Dermoscopy also revealed increased peripheral, brown, networklike pigmentation, which was unique and could be secondary to the patient’s darker skin phenotype.
Differential diagnoses of apocrine hidrocystoma include both melanocytic and nonmelanocytic conditions such as epidermal cyst, nodular melanoma, nodular hidradenoma, syringoma, blue nevus, pilomatricoma, eccrine poroma, nodular Kaposi sarcoma, and venous lake.1 Histopathology showing large unilocular or multilocular dermal cysts with double lining comprising outer myoepithelial cells and inner columnar or cuboidal cell with decapitation secretion is paramount in confirming the diagnosis of apocrine hidrocystoma.
Dermoscopy can act as a valuable noninvasive modality in differentiating apocrine hidrocystoma from its melanocytic and nonmelanocytic differential diagnoses (Table).5-8 In our patient, the presence of a homogeneous pink to bluish area involving the entire lesion, linear branched vessels, and whitish structures on dermoscopy pointed to the diagnosis of apocrine hidrocystoma, which was further confirmed by characteristic histopathologic findings.

The treatment of apocrine hidrocystoma includes surgical excision for solitary lesions, with electrodesiccation and curettage, chemical cautery, and CO2 laser ablation employed for multiple lesions.1 Our patient was scheduled for CO2 laser ablation, considering the multiple lesions and size of the apocrine hidrocystoma but was subsequently lost to follow-up.
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp [published online August 15, 2020]. Dermatol Online J. 2020;26:13030/qt7rt3s4pp.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703. doi:10.1111/j.1365-4632.2005.02512.x
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Haspeslagh M, Noë M, De Wispelaere I, et al. Rosettes and other white shiny structures in polarized dermoscopy: histological correlate and optical explanation. J Eur Acad Dermatol Venereol. 2016;30:311-313. doi:10.1111/jdv.13080
- Suh KS, Kang DY, Park JB, et al. Usefulness of dermoscopy in the differential diagnosis of ruptured and unruptured epidermal cysts. Ann Dermatol. 2017;29:33-38. doi:10.5021/ad.2017.29.1.33
- Serrano P, Lallas A, Del Pozo LJ, et al. Dermoscopy of nodular hidradenoma, a great masquerader: a morphological study of 28 cases. Dermatology. 2016;232:78-82. doi:10.1159/000441218
- Russo T, Piccolo V, Lallas A, et al. Dermoscopy of malignant skin tumours: what’s new? Dermatology. 2017;233:64-73. doi:10.1159/000472253
- Zaballos P, Llambrich A, Puig S, et al. Dermoscopic findings of pilomatricomas. Dermatology. 2008;217:225-230. doi:10.1159 /000148248
A 21-year-old man presented with a raised lesion on the forehead that had started as a single papule 16 years prior and gradually increased in number and size. There were no associated symptoms and no history of seasonal variation in the size of the lesions. Physical examination revealed multiple erythematous to slightly bluish translucent papules that coalesced to form a 3×3-cm noduloplaque with cystic consistency on the right side of the forehead (top). Dermoscopic examination (middle) (polarized noncontact mode) revealed a homogeneous pink to bluish background, scattered linear vessels with branches (black arrows), multiple chrysalislike shiny white lines (blue arrows), and dots arranged in a 4-dot pattern (black circle) resembling a four-leaf clover. Increased peripheral, brown, networklike pigmentation (black stars) also was noted on dermoscopy. Histopathologic examination of the noduloplaque was performed (bottom).
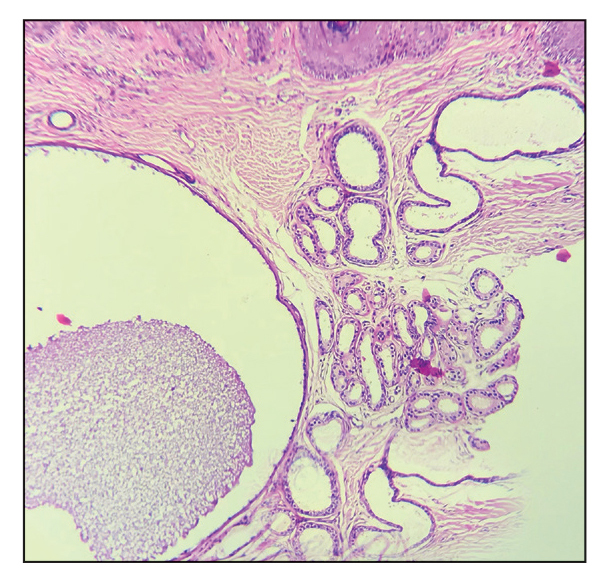
Nonblanching, Erythematous, Cerebriform Plaques on the Foot
The Diagnosis: Coral Dermatitis
At 3-week follow-up, the patient demonstrated remarkable improvement in the intensity and size of the erythematous cerebriform plaques following daily application of triamcinolone acetonide cream 0.1% (Figure). The lesion disappeared after several months and did not recur. The delayed presentation of symptoms with a history of incidental coral contact during snorkeling most likely represents the type IV hypersensitivity reaction seen in the diagnosis of coral dermatitis, an extraordinarily rare form of contact dermatitis.1 Not all coral trigger skin reactions. Species of coral that contain nematocysts in their tentacles (aptly named stinging capsules) are responsible for the sting preceding coral dermatitis, as the nematocysts eject a coiled filament in response to human tactile stimulation that injects toxins into the epidermis.2
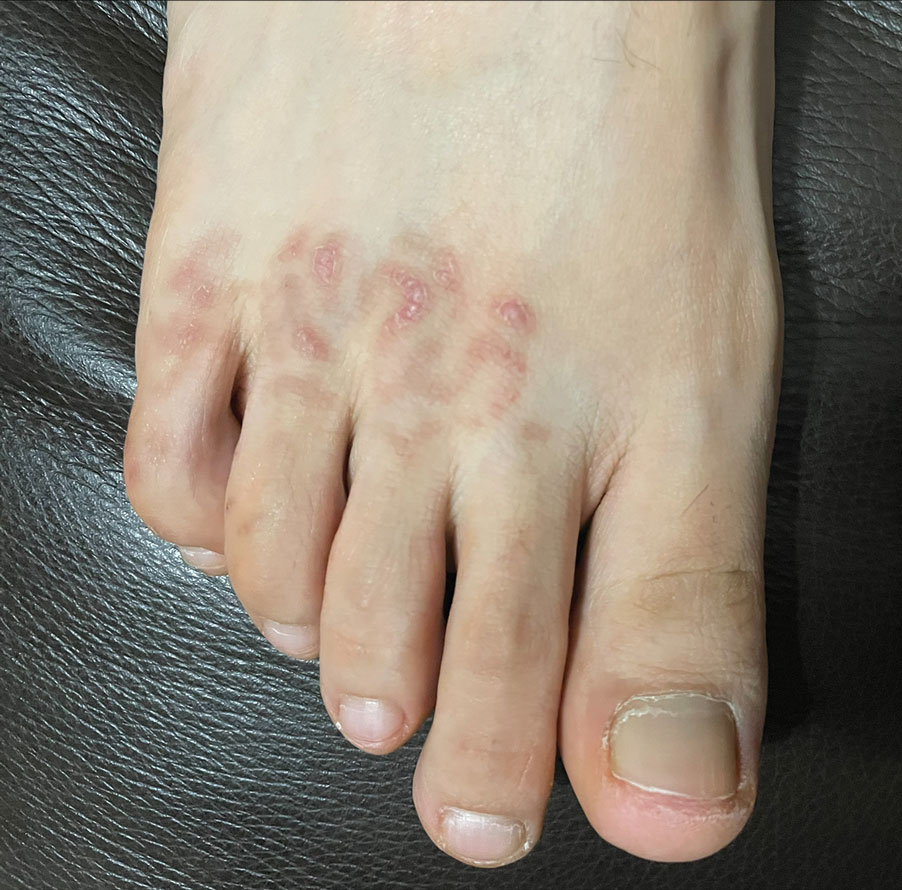
Acute, delayed, or chronic cutaneous changes follow envenomation. Acute responses arise immediately to a few hours after initial contact and are considered an irritant contact dermatitis.3 Local tissue histamine release and cascades of cytotoxic reactions often result in the characteristic urticarial or vesiculobullous plaques in addition to necrosis, piloerection, and localized lymphadenopathy.2-4 Although relatively uncommon, there may be rapid onset of systemic symptoms such as fever, malaise, hives, nausea, or emesis. Cardiopulmonary events, hepatotoxicity, renal failure, or anaphylaxis are rare.2 Histopathology of biopsy specimens reveals epidermal spongiosis with microvesicles and papillary dermal edema.1,5 In comparison, delayed reactions occur within days to weeks and exhibit epidermal parakeratosis, spongiosis, basal layer vacuolization, focal necrosis, lymphocyte exocytosis, and papillary dermal edema with extravasated erythrocytes.1,6 Clinically, it may present as linear rows of erythematous papules with burning and pruritus.6 Chronic reactions manifest after months as difficult-to-treat, persistent lichenoid dermatitis occasionally accompanied by granulomatous changes.1,2,4 Primary prevention measures after initial contact include an acetic acid rinse and cold compression to wash away residual nematocysts in the affected area.4,7,8 If a rash develops, topical steroids are the mainstay of treatment.3,8
In tandem with toxic nematocysts, the rigid calcified bodies of coral provide an additional self-defense mechanism against human contact.2,4 The irregular haphazard nature of coral may catch novice divers off guard and lead to laceration of a mispositioned limb, thereby increasing the risk for secondary infections due to the introduction of calcium carbonate and toxic mucinous deposits at the wound site, warranting antibiotic treatment.2,4,7 Because tropical locales are home to other natural dangers that inflict disease and mimic early signs of coral dermatitis, reaching an accurate diagnosis can be difficult, particularly for lower limb lesions. In summary, the diagnosis of coral dermatitis can be rendered based on morphology of the lesion and clinical context (exposure to corals and delayed symptoms) as well as response to topical steroids.
The differential diagnosis includes accidental trauma. Variations in impact force and patient skin integrity lead to a number of possible cutaneous manifestations seen in accidental trauma,9 which includes contusions resulting from burst capillaries underneath intact skin, abrasions due to the superficial epidermis scuffing away, and lacerations caused by enough force to rip and split the skin, leaving subcutaneous tissue between the intact tissue.9,10 Typically, the pattern of injury can provide hints to match what object or organism caused the wound.9 However, delayed response and worsening symptoms, as seen in coral dermatitis, would be unusual in accidental trauma unless it is complicated by secondary infection (infectious dermatitis), which does not respond to topical steroids and requires antibiotic treatment.
Another differential diagnosis includes cutaneous larva migrans, which infests domesticated and stray animals. For example, hookworm larvae propagate their eggs inside the intestines of their host before fecal-soil transmission in sandy locales.11 Unexpecting beachgoers travel barefoot on this contaminated soil, offering ample opportunity for the parasite to burrow into the upper dermis.11,12 The clinical presentation includes signs and symptoms of creeping eruption such as pruritic, linear, serpiginous tracks. Topical treatment with thiabendazole requires application 3 times daily for 15 days, which increases the risk for nonadherence, yet this therapy proves advantageous if a patient does not tolerate oral agents due to systemic adverse effects.11,12 Oral agents (eg, ivermectin, albendazole) offer improved adherence with a single dose11,13; the cure rate was higher with a single dose of ivermectin 12 mg vs a single dose of albendazole 400 mg.13 The current suggested treatment is ivermectin 200 μg/kg by mouth daily for 1 or 2 days.14
The incidence of seabather’s eruption (also known as chinkui dermatitis) is highest during the summer season and fluctuates between epidemic and nonepidemic years.15,16 It occurs sporadically worldwide mostly in tropical climates due to trapping of larvae spawn of sea animals such as crustaceans in swimwear. Initially, it presents as a pruritic and burning sensation after exiting the water, manifesting as a macular, papular, or maculopapular rash on areas covered by the swimsuit.15,16 The sensation is worse in areas that are tightly banded on the swimsuit, including the waistband and elastic straps.15 Commonly, the affected individual will seek relief via a shower, which intensifies the burning, especially if the swimsuit has not been removed. The contaminated swimwear should be immediately discarded, as the trapped sea larvae’s nematocysts activate with the pressure and friction of movement.15 Seabather’s eruption typically resolves spontaneously within a week, but symptom management can be achieved with topical steroids (triamcinolone 0.1% or clobetasol 0.05%).15,16 Unlike coral dermatitis, in seabather’s eruption the symptoms are immediate and the location of the eruption coincides with areas covered by the swimsuit.
- Ahn HS, Yoon SY, Park HJ, et al. A patient with delayed contact dermatitis to coral and she displayed superficial granuloma. Ann Dermatol. 2009;21:95-97. doi:10.5021/ad.2009.21.1.95
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-752. doi:10.1016/j.jaad.2009.01.046
- Salik J, Tang R. Images in clinical medicine. Coral dermatitis. N Engl J Med. 2015;373:E2. doi:10.1056/NEJMicm1412907
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131. doi:10.1249/JSR.0000000000000042
- Addy JH. Red sea coral contact dermatitis. Int J Dermatol. 1991; 30:271-273. doi:10.1111/j.1365-4362.1991.tb04636.x
- Miracco C, Lalinga AV, Sbano P, et al. Delayed skin reaction to Red Sea coral injury showing superficial granulomas and atypical CD30+ lymphocytes: report of a case. Br J Dermatol. 2001;145:849-851. doi:10.1046/j.1365-2133.2001.04454.x
- Ceponis PJ, Cable R, Weaver LK. Don’t kick the coral! Wilderness Environ Med. 2017;28:153-155. doi:10.1016/j.wem.2017.01.025
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dematoses. part 2-in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002. doi:10.1111/j.1365-4632.2010.04476.x
- Simon LV, Lopez RA, King KC. Blunt force trauma. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed January 12, 2034. https://www.ncbi.nlm.nih.gov/books/NBK470338/
- Gentile S, Kneubuehl BP, Barrera V, et al. Fracture energy threshold in parry injuries due to sharp and blunt force. Int J Legal Med. 2019;133:1429-1435.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814. doi:10.1086/313787
- Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588-591. doi:10.1001 /archderm.129.5.588
- Caumes E, Carriere J, Datry A, et al. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg. 1993;49:641-644. doi:10.4269 /ajtmh.1993.49.641
- Schuster A, Lesshafft H, Reichert F, et al. Hookworm-related cutaneous larva migrans in northern Brazil: resolution of clinical pathology after a single dose of ivermectin. Clin Infect Dis. 2013;57:1155-1157. doi:10.1093/cid/cit440
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Odagawa S, Watari T, Yoshida M. Chinkui dermatitis: the sea bather’s eruption. QJM. 2022;115:100-101. doi:10.1093/qjmed/hcab277
The Diagnosis: Coral Dermatitis
At 3-week follow-up, the patient demonstrated remarkable improvement in the intensity and size of the erythematous cerebriform plaques following daily application of triamcinolone acetonide cream 0.1% (Figure). The lesion disappeared after several months and did not recur. The delayed presentation of symptoms with a history of incidental coral contact during snorkeling most likely represents the type IV hypersensitivity reaction seen in the diagnosis of coral dermatitis, an extraordinarily rare form of contact dermatitis.1 Not all coral trigger skin reactions. Species of coral that contain nematocysts in their tentacles (aptly named stinging capsules) are responsible for the sting preceding coral dermatitis, as the nematocysts eject a coiled filament in response to human tactile stimulation that injects toxins into the epidermis.2

Acute, delayed, or chronic cutaneous changes follow envenomation. Acute responses arise immediately to a few hours after initial contact and are considered an irritant contact dermatitis.3 Local tissue histamine release and cascades of cytotoxic reactions often result in the characteristic urticarial or vesiculobullous plaques in addition to necrosis, piloerection, and localized lymphadenopathy.2-4 Although relatively uncommon, there may be rapid onset of systemic symptoms such as fever, malaise, hives, nausea, or emesis. Cardiopulmonary events, hepatotoxicity, renal failure, or anaphylaxis are rare.2 Histopathology of biopsy specimens reveals epidermal spongiosis with microvesicles and papillary dermal edema.1,5 In comparison, delayed reactions occur within days to weeks and exhibit epidermal parakeratosis, spongiosis, basal layer vacuolization, focal necrosis, lymphocyte exocytosis, and papillary dermal edema with extravasated erythrocytes.1,6 Clinically, it may present as linear rows of erythematous papules with burning and pruritus.6 Chronic reactions manifest after months as difficult-to-treat, persistent lichenoid dermatitis occasionally accompanied by granulomatous changes.1,2,4 Primary prevention measures after initial contact include an acetic acid rinse and cold compression to wash away residual nematocysts in the affected area.4,7,8 If a rash develops, topical steroids are the mainstay of treatment.3,8
In tandem with toxic nematocysts, the rigid calcified bodies of coral provide an additional self-defense mechanism against human contact.2,4 The irregular haphazard nature of coral may catch novice divers off guard and lead to laceration of a mispositioned limb, thereby increasing the risk for secondary infections due to the introduction of calcium carbonate and toxic mucinous deposits at the wound site, warranting antibiotic treatment.2,4,7 Because tropical locales are home to other natural dangers that inflict disease and mimic early signs of coral dermatitis, reaching an accurate diagnosis can be difficult, particularly for lower limb lesions. In summary, the diagnosis of coral dermatitis can be rendered based on morphology of the lesion and clinical context (exposure to corals and delayed symptoms) as well as response to topical steroids.
The differential diagnosis includes accidental trauma. Variations in impact force and patient skin integrity lead to a number of possible cutaneous manifestations seen in accidental trauma,9 which includes contusions resulting from burst capillaries underneath intact skin, abrasions due to the superficial epidermis scuffing away, and lacerations caused by enough force to rip and split the skin, leaving subcutaneous tissue between the intact tissue.9,10 Typically, the pattern of injury can provide hints to match what object or organism caused the wound.9 However, delayed response and worsening symptoms, as seen in coral dermatitis, would be unusual in accidental trauma unless it is complicated by secondary infection (infectious dermatitis), which does not respond to topical steroids and requires antibiotic treatment.
Another differential diagnosis includes cutaneous larva migrans, which infests domesticated and stray animals. For example, hookworm larvae propagate their eggs inside the intestines of their host before fecal-soil transmission in sandy locales.11 Unexpecting beachgoers travel barefoot on this contaminated soil, offering ample opportunity for the parasite to burrow into the upper dermis.11,12 The clinical presentation includes signs and symptoms of creeping eruption such as pruritic, linear, serpiginous tracks. Topical treatment with thiabendazole requires application 3 times daily for 15 days, which increases the risk for nonadherence, yet this therapy proves advantageous if a patient does not tolerate oral agents due to systemic adverse effects.11,12 Oral agents (eg, ivermectin, albendazole) offer improved adherence with a single dose11,13; the cure rate was higher with a single dose of ivermectin 12 mg vs a single dose of albendazole 400 mg.13 The current suggested treatment is ivermectin 200 μg/kg by mouth daily for 1 or 2 days.14
The incidence of seabather’s eruption (also known as chinkui dermatitis) is highest during the summer season and fluctuates between epidemic and nonepidemic years.15,16 It occurs sporadically worldwide mostly in tropical climates due to trapping of larvae spawn of sea animals such as crustaceans in swimwear. Initially, it presents as a pruritic and burning sensation after exiting the water, manifesting as a macular, papular, or maculopapular rash on areas covered by the swimsuit.15,16 The sensation is worse in areas that are tightly banded on the swimsuit, including the waistband and elastic straps.15 Commonly, the affected individual will seek relief via a shower, which intensifies the burning, especially if the swimsuit has not been removed. The contaminated swimwear should be immediately discarded, as the trapped sea larvae’s nematocysts activate with the pressure and friction of movement.15 Seabather’s eruption typically resolves spontaneously within a week, but symptom management can be achieved with topical steroids (triamcinolone 0.1% or clobetasol 0.05%).15,16 Unlike coral dermatitis, in seabather’s eruption the symptoms are immediate and the location of the eruption coincides with areas covered by the swimsuit.
The Diagnosis: Coral Dermatitis
At 3-week follow-up, the patient demonstrated remarkable improvement in the intensity and size of the erythematous cerebriform plaques following daily application of triamcinolone acetonide cream 0.1% (Figure). The lesion disappeared after several months and did not recur. The delayed presentation of symptoms with a history of incidental coral contact during snorkeling most likely represents the type IV hypersensitivity reaction seen in the diagnosis of coral dermatitis, an extraordinarily rare form of contact dermatitis.1 Not all coral trigger skin reactions. Species of coral that contain nematocysts in their tentacles (aptly named stinging capsules) are responsible for the sting preceding coral dermatitis, as the nematocysts eject a coiled filament in response to human tactile stimulation that injects toxins into the epidermis.2

Acute, delayed, or chronic cutaneous changes follow envenomation. Acute responses arise immediately to a few hours after initial contact and are considered an irritant contact dermatitis.3 Local tissue histamine release and cascades of cytotoxic reactions often result in the characteristic urticarial or vesiculobullous plaques in addition to necrosis, piloerection, and localized lymphadenopathy.2-4 Although relatively uncommon, there may be rapid onset of systemic symptoms such as fever, malaise, hives, nausea, or emesis. Cardiopulmonary events, hepatotoxicity, renal failure, or anaphylaxis are rare.2 Histopathology of biopsy specimens reveals epidermal spongiosis with microvesicles and papillary dermal edema.1,5 In comparison, delayed reactions occur within days to weeks and exhibit epidermal parakeratosis, spongiosis, basal layer vacuolization, focal necrosis, lymphocyte exocytosis, and papillary dermal edema with extravasated erythrocytes.1,6 Clinically, it may present as linear rows of erythematous papules with burning and pruritus.6 Chronic reactions manifest after months as difficult-to-treat, persistent lichenoid dermatitis occasionally accompanied by granulomatous changes.1,2,4 Primary prevention measures after initial contact include an acetic acid rinse and cold compression to wash away residual nematocysts in the affected area.4,7,8 If a rash develops, topical steroids are the mainstay of treatment.3,8
In tandem with toxic nematocysts, the rigid calcified bodies of coral provide an additional self-defense mechanism against human contact.2,4 The irregular haphazard nature of coral may catch novice divers off guard and lead to laceration of a mispositioned limb, thereby increasing the risk for secondary infections due to the introduction of calcium carbonate and toxic mucinous deposits at the wound site, warranting antibiotic treatment.2,4,7 Because tropical locales are home to other natural dangers that inflict disease and mimic early signs of coral dermatitis, reaching an accurate diagnosis can be difficult, particularly for lower limb lesions. In summary, the diagnosis of coral dermatitis can be rendered based on morphology of the lesion and clinical context (exposure to corals and delayed symptoms) as well as response to topical steroids.
The differential diagnosis includes accidental trauma. Variations in impact force and patient skin integrity lead to a number of possible cutaneous manifestations seen in accidental trauma,9 which includes contusions resulting from burst capillaries underneath intact skin, abrasions due to the superficial epidermis scuffing away, and lacerations caused by enough force to rip and split the skin, leaving subcutaneous tissue between the intact tissue.9,10 Typically, the pattern of injury can provide hints to match what object or organism caused the wound.9 However, delayed response and worsening symptoms, as seen in coral dermatitis, would be unusual in accidental trauma unless it is complicated by secondary infection (infectious dermatitis), which does not respond to topical steroids and requires antibiotic treatment.
Another differential diagnosis includes cutaneous larva migrans, which infests domesticated and stray animals. For example, hookworm larvae propagate their eggs inside the intestines of their host before fecal-soil transmission in sandy locales.11 Unexpecting beachgoers travel barefoot on this contaminated soil, offering ample opportunity for the parasite to burrow into the upper dermis.11,12 The clinical presentation includes signs and symptoms of creeping eruption such as pruritic, linear, serpiginous tracks. Topical treatment with thiabendazole requires application 3 times daily for 15 days, which increases the risk for nonadherence, yet this therapy proves advantageous if a patient does not tolerate oral agents due to systemic adverse effects.11,12 Oral agents (eg, ivermectin, albendazole) offer improved adherence with a single dose11,13; the cure rate was higher with a single dose of ivermectin 12 mg vs a single dose of albendazole 400 mg.13 The current suggested treatment is ivermectin 200 μg/kg by mouth daily for 1 or 2 days.14
The incidence of seabather’s eruption (also known as chinkui dermatitis) is highest during the summer season and fluctuates between epidemic and nonepidemic years.15,16 It occurs sporadically worldwide mostly in tropical climates due to trapping of larvae spawn of sea animals such as crustaceans in swimwear. Initially, it presents as a pruritic and burning sensation after exiting the water, manifesting as a macular, papular, or maculopapular rash on areas covered by the swimsuit.15,16 The sensation is worse in areas that are tightly banded on the swimsuit, including the waistband and elastic straps.15 Commonly, the affected individual will seek relief via a shower, which intensifies the burning, especially if the swimsuit has not been removed. The contaminated swimwear should be immediately discarded, as the trapped sea larvae’s nematocysts activate with the pressure and friction of movement.15 Seabather’s eruption typically resolves spontaneously within a week, but symptom management can be achieved with topical steroids (triamcinolone 0.1% or clobetasol 0.05%).15,16 Unlike coral dermatitis, in seabather’s eruption the symptoms are immediate and the location of the eruption coincides with areas covered by the swimsuit.
- Ahn HS, Yoon SY, Park HJ, et al. A patient with delayed contact dermatitis to coral and she displayed superficial granuloma. Ann Dermatol. 2009;21:95-97. doi:10.5021/ad.2009.21.1.95
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-752. doi:10.1016/j.jaad.2009.01.046
- Salik J, Tang R. Images in clinical medicine. Coral dermatitis. N Engl J Med. 2015;373:E2. doi:10.1056/NEJMicm1412907
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131. doi:10.1249/JSR.0000000000000042
- Addy JH. Red sea coral contact dermatitis. Int J Dermatol. 1991; 30:271-273. doi:10.1111/j.1365-4362.1991.tb04636.x
- Miracco C, Lalinga AV, Sbano P, et al. Delayed skin reaction to Red Sea coral injury showing superficial granulomas and atypical CD30+ lymphocytes: report of a case. Br J Dermatol. 2001;145:849-851. doi:10.1046/j.1365-2133.2001.04454.x
- Ceponis PJ, Cable R, Weaver LK. Don’t kick the coral! Wilderness Environ Med. 2017;28:153-155. doi:10.1016/j.wem.2017.01.025
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dematoses. part 2-in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002. doi:10.1111/j.1365-4632.2010.04476.x
- Simon LV, Lopez RA, King KC. Blunt force trauma. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed January 12, 2034. https://www.ncbi.nlm.nih.gov/books/NBK470338/
- Gentile S, Kneubuehl BP, Barrera V, et al. Fracture energy threshold in parry injuries due to sharp and blunt force. Int J Legal Med. 2019;133:1429-1435.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814. doi:10.1086/313787
- Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588-591. doi:10.1001 /archderm.129.5.588
- Caumes E, Carriere J, Datry A, et al. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg. 1993;49:641-644. doi:10.4269 /ajtmh.1993.49.641
- Schuster A, Lesshafft H, Reichert F, et al. Hookworm-related cutaneous larva migrans in northern Brazil: resolution of clinical pathology after a single dose of ivermectin. Clin Infect Dis. 2013;57:1155-1157. doi:10.1093/cid/cit440
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Odagawa S, Watari T, Yoshida M. Chinkui dermatitis: the sea bather’s eruption. QJM. 2022;115:100-101. doi:10.1093/qjmed/hcab277
- Ahn HS, Yoon SY, Park HJ, et al. A patient with delayed contact dermatitis to coral and she displayed superficial granuloma. Ann Dermatol. 2009;21:95-97. doi:10.5021/ad.2009.21.1.95
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-752. doi:10.1016/j.jaad.2009.01.046
- Salik J, Tang R. Images in clinical medicine. Coral dermatitis. N Engl J Med. 2015;373:E2. doi:10.1056/NEJMicm1412907
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131. doi:10.1249/JSR.0000000000000042
- Addy JH. Red sea coral contact dermatitis. Int J Dermatol. 1991; 30:271-273. doi:10.1111/j.1365-4362.1991.tb04636.x
- Miracco C, Lalinga AV, Sbano P, et al. Delayed skin reaction to Red Sea coral injury showing superficial granulomas and atypical CD30+ lymphocytes: report of a case. Br J Dermatol. 2001;145:849-851. doi:10.1046/j.1365-2133.2001.04454.x
- Ceponis PJ, Cable R, Weaver LK. Don’t kick the coral! Wilderness Environ Med. 2017;28:153-155. doi:10.1016/j.wem.2017.01.025
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dematoses. part 2-in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002. doi:10.1111/j.1365-4632.2010.04476.x
- Simon LV, Lopez RA, King KC. Blunt force trauma. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed January 12, 2034. https://www.ncbi.nlm.nih.gov/books/NBK470338/
- Gentile S, Kneubuehl BP, Barrera V, et al. Fracture energy threshold in parry injuries due to sharp and blunt force. Int J Legal Med. 2019;133:1429-1435.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814. doi:10.1086/313787
- Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical presentation and management of 60 cases presenting to a tropical disease unit. Arch Dermatol. 1993;129:588-591. doi:10.1001 /archderm.129.5.588
- Caumes E, Carriere J, Datry A, et al. A randomized trial of ivermectin versus albendazole for the treatment of cutaneous larva migrans. Am J Trop Med Hyg. 1993;49:641-644. doi:10.4269 /ajtmh.1993.49.641
- Schuster A, Lesshafft H, Reichert F, et al. Hookworm-related cutaneous larva migrans in northern Brazil: resolution of clinical pathology after a single dose of ivermectin. Clin Infect Dis. 2013;57:1155-1157. doi:10.1093/cid/cit440
- Freudenthal AR, Joseph PR. Seabather’s eruption. N Engl J Med. 1993;329:542-544. doi:10.1056/NEJM199308193290805
- Odagawa S, Watari T, Yoshida M. Chinkui dermatitis: the sea bather’s eruption. QJM. 2022;115:100-101. doi:10.1093/qjmed/hcab277
A 48-year-old otherwise healthy man presented with a tender lesion on the dorsal aspect of the right foot with dysesthesia and progressive pruritus that he originally noticed 9 days prior after snorkeling in the Caribbean. He recalled kicking what he assumed was a rock while swimming. Initially there was negligible discomfort; however, on day 7 the symptoms started to worsen and the lesion started to swell. Application of a gauze pad soaked in hydrogen peroxide 3% failed to alleviate symptoms. Physical examination revealed a 4-cm region of well-demarcated, nonblanching, erythematous plaques in a lattice pattern accompanied by edematous and bullous changes. Triamcinolone acetonide cream 0.1% was prescribed.
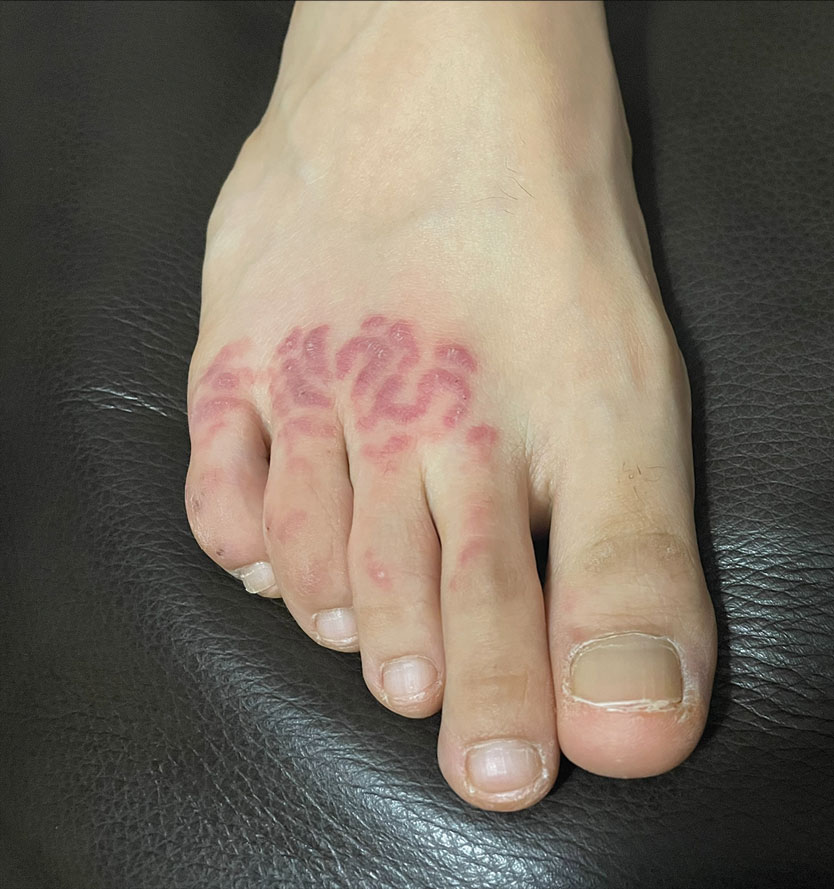
Annular Erythematous Plaques on the Back
The Diagnosis: Granuloma Annulare
The biopsies revealed palisading granulomatous dermatitis consistent with granuloma annulare (GA). This diagnosis was supported by the clinical presentation and histopathologic findings. Although the pathogenesis of GA is unclear, it is a benign, self-limiting condition. Primarily affected sites include the trunk and forearms. Generalized GA (or GA with ≥10 lesions) may warrant workup for malignancy, as it may represent a paraneoplastic process.1 Histopathology reveals granulomas comprising a dermal lymphohistiocytic infiltrate as well as central mucin and nuclear debris. There are a few histologic subtypes of GA, including palisading and interstitial, which refer to the distribution of the histiocytic infiltrate.2,3 This case—with palisading histiocytes lining the collection of necrobiosis and mucin (bottom quiz image)—features palisading GA. Notably, GA exhibits central rather than diffuse mucin.4
Erythema gyratum repens is a paraneoplastic arcuate erythema that manifests as erythematous figurate, gyrate, or annular plaques exhibiting a trailing scale. Clinically, erythema gyratum repens spreads rapidly—as quickly as 1 cm/d—and can be extensive (as in this case). Histopathology ruled out this diagnosis in our patient. Nonspecific findings of acanthosis, parakeratosis, and superficial spongiosis can be found in erythema gyratum repens. A superficial and deep perivascular lymphohistiocytic infiltrate may be seen in figurate erythemas (Figure 1).5 Unlike GA, this infiltrate does not form granulomas, is more superficial, and does not contain mucin.
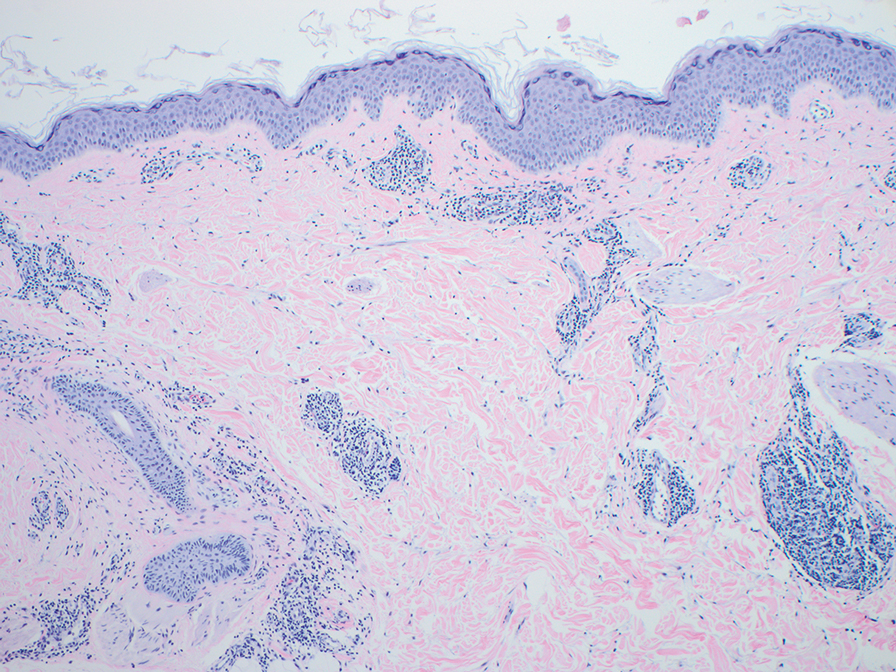
Histopathology also can help establish the diagnosis of leprosy and its specific subtype, as leprosy exists on a spectrum from tuberculoid to lepromatous, with a great deal of overlap in between.6 Lepromatous leprosy has many cutaneous clinical presentations but typically manifests as erythematous papules or nodules. It is multibacillary, and these mycobacteria form clumps known as globi that can be seen on Fite stain.7 In lepromatous leprosy, there is a characteristic dense lymphohistiocytic infiltrate (Figure 2) above which a Grenz zone can be seen.4,8 There are no well-formed granulomas in lepromatous leprosy, unlike in tuberculoid leprosy, which is paucibacillary and creates a granulomatous response surrounding nerves and adnexal structures.6
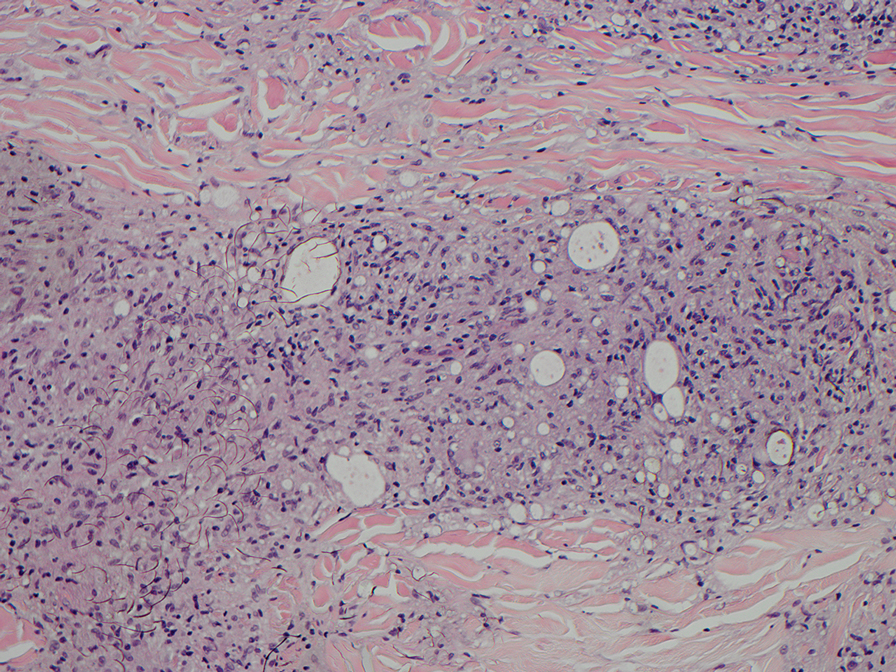
Mycosis fungoides (MF) is the most common cutaneous lymphoma. There are patch, plaque, and tumor stages of MF, each of which exhibits various histopathologic findings.9 In early patch-stage MF, lymphocytes have perinuclear clearing, and the degree of lymphocytic infiltrate is out of proportion to the spongiosis present. Epidermotropism and Pautrier microabscesses often are present in the epidermis (Figure 3). In the plaque stage, there is a denser lymphoid infiltrate in a lichenoid pattern with epidermotropism and Pautrier microabscesses. The tumor stage shows a dense dermal lymphoid infiltrate with more atypia and typically a lack of epidermotropism. Rarely, MF can exhibit a granulomatous variant in which epithelioid histiocytes collect to form granulomas along with atypical lymphocytes.10
The diagnosis of cutaneous sarcoidosis requires clinicopathologic corroboration. Histopathology demonstrates epithelioid histiocytes forming noncaseating granulomas with little to no lymphocytic infiltrate (Figure 4). There typically is no necrosis or necrobiosis as there is in GA. The diagnosis of sarcoidosis can be challenging histopathologically, and stains should be used to rule out infectious processes.4 Asteroid bodies— star-shaped eosinophilic inclusions within giant cells—may be present but are nonspecific for sarcoidosis.11 Schaumann bodies—inclusions of calcifications within giant cells—also may be present and can aid in diagnosis.12
- Kovich O, Burgin S. Generalized granuloma annulare [published online December 30, 2005]. Dermatol Online J. 2005;11:23.
- Al Ameer MA, Al-Natour SH, Alsahaf HAA, et al. Eruptive granuloma annulare in an elderly man with diabetes [published online January 14, 2022]. Cureus. 2022;14:E21242. doi:10.7759/cureus.21242
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, et al, eds. Dermatology. Mosby; 2003:1455.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2018.
- Gore M, Winters ME. Erythema gyratum repens: a rare paraneoplastic rash. West J Emerg Med. 2011;12:556-558. doi:10.5811/westjem.2010.11.2090
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1-14. doi:10.1016/j.jaad.2019.12.080
- Pedley JC, Harman DJ, Waudby H, et al. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198-204. doi:10.1136/jnnp.43.3.198
- Booth AV, Kovich OI. Lepromatous leprosy [published online January 27, 2007]. Dermatol Online J. 2007;13:9.
- Robson A. The pathology of cutaneous T-cell lymphoma. Oncology (Williston Park). 2007;21(2 suppl 1):9-12.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001/archdermatol.2008.46
- Azar HA, Lunardelli C. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am J Pathol. 1969;57:81-92.
- Sreeja C, Priyadarshini A, Premika, et al. Sarcoidosis—a review article. J Oral Maxillofac Pathol. 2022;26:242-253. doi:10.4103 /jomfp.jomfp_373_21
The Diagnosis: Granuloma Annulare
The biopsies revealed palisading granulomatous dermatitis consistent with granuloma annulare (GA). This diagnosis was supported by the clinical presentation and histopathologic findings. Although the pathogenesis of GA is unclear, it is a benign, self-limiting condition. Primarily affected sites include the trunk and forearms. Generalized GA (or GA with ≥10 lesions) may warrant workup for malignancy, as it may represent a paraneoplastic process.1 Histopathology reveals granulomas comprising a dermal lymphohistiocytic infiltrate as well as central mucin and nuclear debris. There are a few histologic subtypes of GA, including palisading and interstitial, which refer to the distribution of the histiocytic infiltrate.2,3 This case—with palisading histiocytes lining the collection of necrobiosis and mucin (bottom quiz image)—features palisading GA. Notably, GA exhibits central rather than diffuse mucin.4
Erythema gyratum repens is a paraneoplastic arcuate erythema that manifests as erythematous figurate, gyrate, or annular plaques exhibiting a trailing scale. Clinically, erythema gyratum repens spreads rapidly—as quickly as 1 cm/d—and can be extensive (as in this case). Histopathology ruled out this diagnosis in our patient. Nonspecific findings of acanthosis, parakeratosis, and superficial spongiosis can be found in erythema gyratum repens. A superficial and deep perivascular lymphohistiocytic infiltrate may be seen in figurate erythemas (Figure 1).5 Unlike GA, this infiltrate does not form granulomas, is more superficial, and does not contain mucin.

Histopathology also can help establish the diagnosis of leprosy and its specific subtype, as leprosy exists on a spectrum from tuberculoid to lepromatous, with a great deal of overlap in between.6 Lepromatous leprosy has many cutaneous clinical presentations but typically manifests as erythematous papules or nodules. It is multibacillary, and these mycobacteria form clumps known as globi that can be seen on Fite stain.7 In lepromatous leprosy, there is a characteristic dense lymphohistiocytic infiltrate (Figure 2) above which a Grenz zone can be seen.4,8 There are no well-formed granulomas in lepromatous leprosy, unlike in tuberculoid leprosy, which is paucibacillary and creates a granulomatous response surrounding nerves and adnexal structures.6

Mycosis fungoides (MF) is the most common cutaneous lymphoma. There are patch, plaque, and tumor stages of MF, each of which exhibits various histopathologic findings.9 In early patch-stage MF, lymphocytes have perinuclear clearing, and the degree of lymphocytic infiltrate is out of proportion to the spongiosis present. Epidermotropism and Pautrier microabscesses often are present in the epidermis (Figure 3). In the plaque stage, there is a denser lymphoid infiltrate in a lichenoid pattern with epidermotropism and Pautrier microabscesses. The tumor stage shows a dense dermal lymphoid infiltrate with more atypia and typically a lack of epidermotropism. Rarely, MF can exhibit a granulomatous variant in which epithelioid histiocytes collect to form granulomas along with atypical lymphocytes.10

The diagnosis of cutaneous sarcoidosis requires clinicopathologic corroboration. Histopathology demonstrates epithelioid histiocytes forming noncaseating granulomas with little to no lymphocytic infiltrate (Figure 4). There typically is no necrosis or necrobiosis as there is in GA. The diagnosis of sarcoidosis can be challenging histopathologically, and stains should be used to rule out infectious processes.4 Asteroid bodies— star-shaped eosinophilic inclusions within giant cells—may be present but are nonspecific for sarcoidosis.11 Schaumann bodies—inclusions of calcifications within giant cells—also may be present and can aid in diagnosis.12

The Diagnosis: Granuloma Annulare
The biopsies revealed palisading granulomatous dermatitis consistent with granuloma annulare (GA). This diagnosis was supported by the clinical presentation and histopathologic findings. Although the pathogenesis of GA is unclear, it is a benign, self-limiting condition. Primarily affected sites include the trunk and forearms. Generalized GA (or GA with ≥10 lesions) may warrant workup for malignancy, as it may represent a paraneoplastic process.1 Histopathology reveals granulomas comprising a dermal lymphohistiocytic infiltrate as well as central mucin and nuclear debris. There are a few histologic subtypes of GA, including palisading and interstitial, which refer to the distribution of the histiocytic infiltrate.2,3 This case—with palisading histiocytes lining the collection of necrobiosis and mucin (bottom quiz image)—features palisading GA. Notably, GA exhibits central rather than diffuse mucin.4
Erythema gyratum repens is a paraneoplastic arcuate erythema that manifests as erythematous figurate, gyrate, or annular plaques exhibiting a trailing scale. Clinically, erythema gyratum repens spreads rapidly—as quickly as 1 cm/d—and can be extensive (as in this case). Histopathology ruled out this diagnosis in our patient. Nonspecific findings of acanthosis, parakeratosis, and superficial spongiosis can be found in erythema gyratum repens. A superficial and deep perivascular lymphohistiocytic infiltrate may be seen in figurate erythemas (Figure 1).5 Unlike GA, this infiltrate does not form granulomas, is more superficial, and does not contain mucin.

Histopathology also can help establish the diagnosis of leprosy and its specific subtype, as leprosy exists on a spectrum from tuberculoid to lepromatous, with a great deal of overlap in between.6 Lepromatous leprosy has many cutaneous clinical presentations but typically manifests as erythematous papules or nodules. It is multibacillary, and these mycobacteria form clumps known as globi that can be seen on Fite stain.7 In lepromatous leprosy, there is a characteristic dense lymphohistiocytic infiltrate (Figure 2) above which a Grenz zone can be seen.4,8 There are no well-formed granulomas in lepromatous leprosy, unlike in tuberculoid leprosy, which is paucibacillary and creates a granulomatous response surrounding nerves and adnexal structures.6

Mycosis fungoides (MF) is the most common cutaneous lymphoma. There are patch, plaque, and tumor stages of MF, each of which exhibits various histopathologic findings.9 In early patch-stage MF, lymphocytes have perinuclear clearing, and the degree of lymphocytic infiltrate is out of proportion to the spongiosis present. Epidermotropism and Pautrier microabscesses often are present in the epidermis (Figure 3). In the plaque stage, there is a denser lymphoid infiltrate in a lichenoid pattern with epidermotropism and Pautrier microabscesses. The tumor stage shows a dense dermal lymphoid infiltrate with more atypia and typically a lack of epidermotropism. Rarely, MF can exhibit a granulomatous variant in which epithelioid histiocytes collect to form granulomas along with atypical lymphocytes.10

The diagnosis of cutaneous sarcoidosis requires clinicopathologic corroboration. Histopathology demonstrates epithelioid histiocytes forming noncaseating granulomas with little to no lymphocytic infiltrate (Figure 4). There typically is no necrosis or necrobiosis as there is in GA. The diagnosis of sarcoidosis can be challenging histopathologically, and stains should be used to rule out infectious processes.4 Asteroid bodies— star-shaped eosinophilic inclusions within giant cells—may be present but are nonspecific for sarcoidosis.11 Schaumann bodies—inclusions of calcifications within giant cells—also may be present and can aid in diagnosis.12

- Kovich O, Burgin S. Generalized granuloma annulare [published online December 30, 2005]. Dermatol Online J. 2005;11:23.
- Al Ameer MA, Al-Natour SH, Alsahaf HAA, et al. Eruptive granuloma annulare in an elderly man with diabetes [published online January 14, 2022]. Cureus. 2022;14:E21242. doi:10.7759/cureus.21242
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, et al, eds. Dermatology. Mosby; 2003:1455.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2018.
- Gore M, Winters ME. Erythema gyratum repens: a rare paraneoplastic rash. West J Emerg Med. 2011;12:556-558. doi:10.5811/westjem.2010.11.2090
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1-14. doi:10.1016/j.jaad.2019.12.080
- Pedley JC, Harman DJ, Waudby H, et al. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198-204. doi:10.1136/jnnp.43.3.198
- Booth AV, Kovich OI. Lepromatous leprosy [published online January 27, 2007]. Dermatol Online J. 2007;13:9.
- Robson A. The pathology of cutaneous T-cell lymphoma. Oncology (Williston Park). 2007;21(2 suppl 1):9-12.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001/archdermatol.2008.46
- Azar HA, Lunardelli C. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am J Pathol. 1969;57:81-92.
- Sreeja C, Priyadarshini A, Premika, et al. Sarcoidosis—a review article. J Oral Maxillofac Pathol. 2022;26:242-253. doi:10.4103 /jomfp.jomfp_373_21
- Kovich O, Burgin S. Generalized granuloma annulare [published online December 30, 2005]. Dermatol Online J. 2005;11:23.
- Al Ameer MA, Al-Natour SH, Alsahaf HAA, et al. Eruptive granuloma annulare in an elderly man with diabetes [published online January 14, 2022]. Cureus. 2022;14:E21242. doi:10.7759/cureus.21242
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, et al, eds. Dermatology. Mosby; 2003:1455.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 3rd ed. Elsevier; 2018.
- Gore M, Winters ME. Erythema gyratum repens: a rare paraneoplastic rash. West J Emerg Med. 2011;12:556-558. doi:10.5811/westjem.2010.11.2090
- Maymone MBC, Laughter M, Venkatesh S, et al. Leprosy: clinical aspects and diagnostic techniques. J Am Acad Dermatol. 2020;83:1-14. doi:10.1016/j.jaad.2019.12.080
- Pedley JC, Harman DJ, Waudby H, et al. Leprosy in peripheral nerves: histopathological findings in 119 untreated patients in Nepal. J Neurol Neurosurg Psychiatry. 1980;43:198-204. doi:10.1136/jnnp.43.3.198
- Booth AV, Kovich OI. Lepromatous leprosy [published online January 27, 2007]. Dermatol Online J. 2007;13:9.
- Robson A. The pathology of cutaneous T-cell lymphoma. Oncology (Williston Park). 2007;21(2 suppl 1):9-12.
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001/archdermatol.2008.46
- Azar HA, Lunardelli C. Collagen nature of asteroid bodies of giant cells in sarcoidosis. Am J Pathol. 1969;57:81-92.
- Sreeja C, Priyadarshini A, Premika, et al. Sarcoidosis—a review article. J Oral Maxillofac Pathol. 2022;26:242-253. doi:10.4103 /jomfp.jomfp_373_21
An 84-year-old man presented to the clinic for evaluation of a pruritic rash on the back of 6 months’ duration that spread to the neck and chest over the past 2 months and then to the abdomen and thighs more recently. His primary care provider prescribed a 1-week course of oral steroids and steroid cream. The oral medication did not help, but the cream alleviated the pruritus. He had a medical history of coronary artery disease, hypertension, and diabetes mellitus. He also had a rash on the forearms that had waxed and waned for many years but was not associated with pruritus. He had not sought medical care for the rash and had never treated it. Physical examination revealed pink to violaceous annular plaques with central clearing and raised borders that coalesced into larger plaques on the trunk (top). Dusky, scaly, pink plaques were present on the dorsal forearms. Three punch biopsies—2 from the upper back (bottom) and 1 from the left forearm—all demonstrated consistent findings.
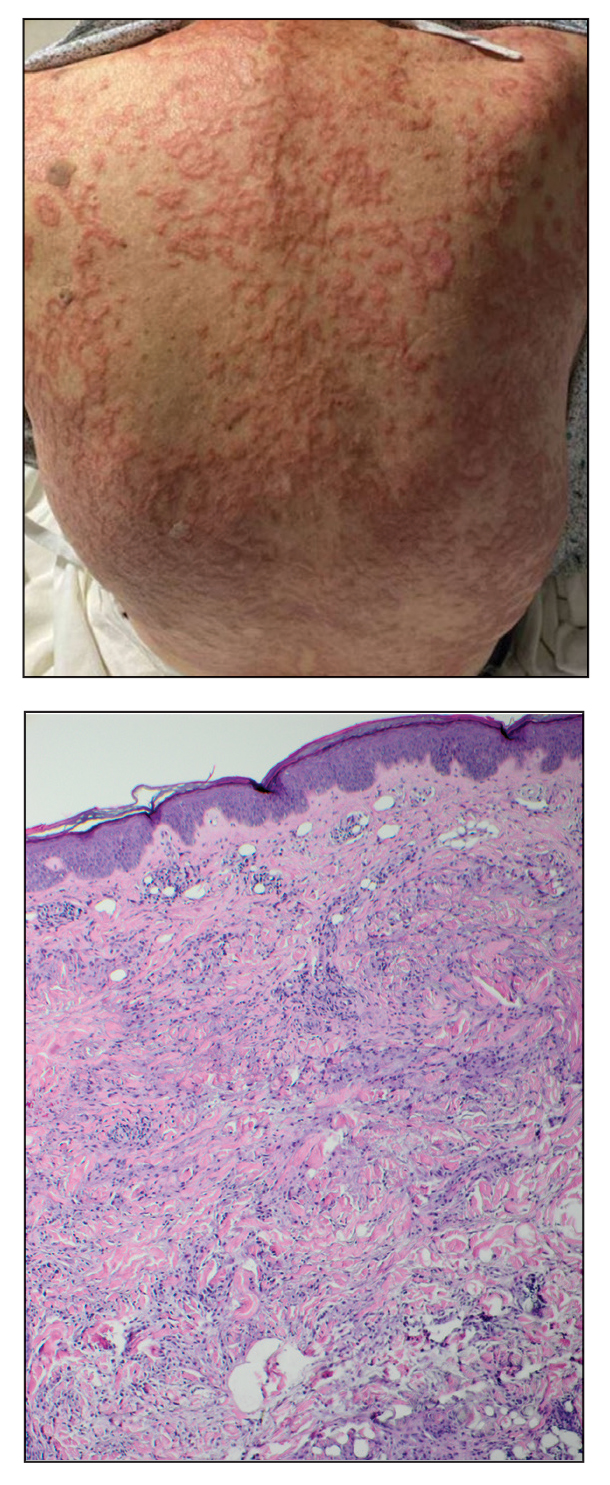
The Potential Benefits of Dietary Changes in Psoriasis Patients
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
Psoriasis is a chronic inflammatory skin disease for which several lifestyle factors—smoking, alcohol use, and psychological stress—are associated with higher incidence and more severe disease.1-3 Diet also has been implicated as a factor that can affect psoriasis,4 and many patients have shown interest in possible dietary interventions to help their disease.5
In 2018, the National Psoriasis Foundation (NPF) presented dietary recommendations for patients based on results from a systematic review. From the available literature, only dietary weight reduction with hypocaloric diets in overweight or obese patients could be strongly recommended, and it has been proven that obesity is associated with worse psoriasis severity.6 Other more recent studies have shown that dietary modifications such as intermittent fasting and the ketogenic diet also led to weight loss and improved psoriasis severity in overweight patients; however, it is difficult to discern if the improvement was due to weight loss alone or if the dietary patterns themselves played a role.7,8 The paucity of well-designed studies evaluating the effects of other dietary changes has prevented further guidelines from being written. We propose that dietary patterns such as the Mediterranean diet (MeD) and vegan/vegetarian diets—even without strong data showing benefits in skin disease—may help to decrease systemic inflammation, improve gut dysbiosis, and help decrease the risk for cardiometabolic comorbidities that are associated with psoriasis.
Mediterranean Diet
The MeD is based on the dietary tendencies of inhabitants from the regions surrounding the Mediterranean Sea and is centered around nutrient-rich foods such as vegetables, olive oil, and legumes while limiting meat and dairy.9 The NPF recommended considering a trial of the MeD based on low-quality evidence.6 Observational studies have indicated that psoriasis patients are less likely to adhere to the MeD, but those who do have less severe disease.8 However, a search of PubMed articles indexed for MEDLINE using the terms Mediterranean diet and psoriasis yielded no prospective interventional studies. Given the association of the MeD with less severe disease, it is important to understand which specific foods in the MeD could be beneficial. Intake of omega-3 fatty acids, such as those found in fatty fish, are important for modulation of systemic inflammation.7 High intake of polyphenols—found in fruits and vegetables, extra-virgin olive oil, and wine—also have been implicated in improving inflammatory diseases due to potent antioxidant and anti-inflammatory properties. Individually, fruits, vegetables, whole grains, and sea fish have been associated with lowering C-reactive protein levels, which also is indicative of the benefits of these foods on systemic inflammation.7
Vegan/Vegetarian Diets
Although fruits, vegetables, legumes, and whole grains are a substantial component of the MeD, there are limited data on vegetarian or purely vegan plant-based diets. An observational study from the NPF found that only 48.4% (15/31) of patients on the MeD vs 69.0% (20/29) on a vegan diet reported a favorable skin response.5 Two case reports also have shown beneficial results of a strict vegan diet for psoriasis and psoriatic arthritis, where whole-food plant-based diets also improved joint symptoms.10-12 As with any diet, those who pursue a plant-based diet should strive to consume a variety of foods to avoid nutrient deficiencies. A recent systematic meta-analysis of 141 studies evaluated nutrient status of vegan and vegetarian diets compared to pescovegetarians and those who consume meat. All dietary patterns showed varying degrees of low levels of different nutrients.13 Of note, the researchers found that vitamin B12, vitamin D, iron, zinc, iodine, calcium, and docosahexaenoic acid were lower in plant-based diets. In contrast, folate; vitamins B1, B6, C, and E; polyunsaturated fatty acids; α-linolenic acid; and magnesium intake were higher. Those who consumed meat were at risk for inadequate intake of fiber, polyunsaturated fatty acids, α-linolenic acid, folate, vitamin E, calcium, magnesium, and vitamin D, though vitamin D intake was higher than in vegans/vegetarians.13 The results of this meta-analysis indicated the importance of educating patients on what constitutes a well-rounded, micronutrient-rich diet or appropriate supplementation for any diet.
Effects on Gut Microbiome
Any changes in diet can lead to alterations in the gut microbiome, which may impact skin disease, as evidence indicates a bidirectional relationship between gut and skin health.10 A metagenomic analysis of the gut microbiota in patients with untreated plaque psoriasis revealed a signature dysbiosis for which the researchers developed a psoriasis microbiota index, suggesting the gut microbiota may play a role in psoriasis pathophysiology.14 Research shows that both the MeD and vegan/vegetarian diets, which are relatively rich in fiber and omega-3 fatty acids and low in saturated fat and animal protein compared to many diets, cause increases in dietary fiber–metabolizing bacteria that produce short-chain fatty acids. These short-chain fatty acids improve gut epithelial integrity and alleviate both gut and systemic inflammation.10
The changes to the gut microbiome induced by a high-fat diet also are concerning. In contrast to the MeD or vegan/vegetarian diets, consumption of a high-fat diet induces alterations in the composition of the gut microbiota that in turn increase the release of proinflammatory cytokines and promote higher intestinal permeability.10 Similarly, high sugar consumption promotes increased intestinal permeability and shifts the gut microbiota to organisms that can rapidly utilize simple carbohydrates at the expense of other beneficial organisms, reducing bacterial diversity.15 The Western diet, which is notable for both high fat and high sugar content, is sometimes referred to as a proinflammatory diet and has been shown to worsen psoriasiformlike lesions in mice.16 Importantly, most research indicates that high fat and high sugar consumption appear to be more prevalent in psoriasis patients,8 but the type of fat consumed in the diet matters. The Western diet includes abundant saturated fat found in meat, dairy products, palm and coconut oils, and processed foods, as well as omega-6 fatty acids that are found in meat, poultry, and eggs. Saturated fat has been shown to promote helper T cell (TH17) accumulation in the skin, and omega-6 fatty acids serve as precursors to various inflammatory lipid mediators.4 This distinction of sources of fat between the Western diet and MeD is important in understanding the diets’ different effects on psoriasis and overall health. As previously discussed, the high intake of omega-3 acids in the MeD is one of the ways it may exert its anti-inflammatory benefits.7
Next Steps in Advising Psoriasis Patients
A major limitation of the data for MeD and vegan/vegetarian diets is limited randomized controlled trials evaluating the impact of these diets on psoriasis. Thus, dietary recommendations for psoriasis are not as strong as for other diseases for which more conclusive data exist.8 Although the data on diet and psoriasis are not definitive, perhaps dermatologists should shift the question from “Does this diet definitely improve psoriasis?” to “Does this diet definitely improve my patient’s health as a whole and maybe also their psoriasis?” For instance, the MeD has been shown to reduce the risk for type 2 diabetes mellitus and cardiovascular disease as well as to slow cognitive decline.17 Vegan/vegetarian diets focusing on whole vs processed foods have been shown to be highly effective in combatting obesity, type 2 diabetes mellitus, coronary artery disease including severe atherosclerosis, and hypertension.18 Psoriasis patients are at increased risk for many of the ailments that the MeD and plant-based diets protect against, making these diets potentially even more impactful than for someone without psoriasis.19 Dietary recommendations should still be made in conjunction with continuing traditional therapies for psoriasis and in consultation with the patient’s primary care physician and/or dietitian; however, rather than waiting for more randomized controlled trials before making health-promoting recommendations, what would be the downside of starting now? At worst, the dietary change decreases their risk for several metabolic conditions, and at best they may even see an improvement in their psoriasis.
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case–control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Armstrong AW, Harskamp CT, Dhillon JS, et al. Psoriasis and smoking: a systematic review and meta‐analysis. Br J Dermatol. 2014;170:304-314. doi:10.1111/bjd.12670
- Zhu K, Zhu C, Fan Y. Alcohol consumption and psoriatic risk: a meta‐analysis of case–control studies. J Dermatol. 2012;39:770-773. doi:10.1111/j.1346-8138.2012.01577.x
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21:5405. doi:10.3390/ijms21155405
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther. 2017;7:227-242. doi:10.1007/s13555-017-0183-4
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a systematic review. JAMA Dermatol. 2018;154:934. doi:10.1001/jamadermatol.2018.1412
- Duchnik E, Kruk J, Tuchowska A, et al. The impact of diet and physical activity on psoriasis: a narrative review of the current evidence. Nutrients. 2023;15:840. doi:10.3390/nu15040840
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis Targets Ther. 2022;12:151-176. doi:10.2147/PTT.S328581
- Mazza E, Ferro Y, Pujia R, et al. Mediterranean diet in healthy aging. J Nutr Health Aging. 2021;25:1076-1083. doi:10.1007/s12603-021-1675-6
- Flores-Balderas X, Peña-Peña M, Rada KM, et al. Beneficial effects of plant-based diets on skin health and inflammatory skin diseases. Nutrients. 2023;15:2842. doi:10.3390/nu15132842
- Bonjour M, Gabriel S, Valencia A, et al. Challenging case in clinical practice: prolonged water-only fasting followed by an exclusively whole-plant-food diet in the management of severe plaque psoriasis. Integr Complement Ther. 2022;28:85-87. doi:10.1089/ict.2022.29010.mbo
- Lewandowska M, Dunbar K, Kassam S. Managing psoriatic arthritis with a whole food plant-based diet: a case study. Am J Lifestyle Med. 2021;15:402-406. doi:10.1177/1559827621993435
- Neufingerl N, Eilander A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: a systematic review. Nutrients. 2021;14:29. doi:10.3390/nu14010029
- Dei-Cas I, Giliberto F, Luce L, et al. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new psoriasis-microbiome index. Sci Rep. 2020;10:12754. doi:10.1038/s41598-020-69537-3
- Satokari R. High intake of sugar and the balance between pro- and anti-inflammatory gut bacteria. Nutrients. 2020;12:1348. doi:10.3390/nu12051348
- Shi Z, Wu X, Santos Rocha C, et al. Short-term Western diet intake promotes IL-23–mediated skin and joint inflammation accompanied by changes to the gut microbiota in mice. J Invest Dermatol. 2021;141:1780-1791. doi:10.1016/j.jid.2020.11.032
- Romagnolo DF, Selmin OI. Mediterranean diet and prevention of chronic diseases. Nutr Today. 2017;52:208-222. doi:10.1097/NT.0000000000000228
- Tuso PJ, Ismail MH, Ha BP, et al. Nutritional update for physicians: plant-based diets. Perm J. 2013;17:61-66. doi:10.7812/TPP/12-085
- Parisi R, Symmons DPM, Griffiths CEM, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
Practice Points
- Psoriasis is affected by lifestyle factors such as diet, which is an area of interest for many patients.
- Low-calorie diets are strongly recommended for overweight/obese patients with psoriasis to improve their disease.
- Changes in dietary patterns, such as adopting a Mediterranean diet or a plant-based diet, also have shown promise.
The Potential for Artificial Intelligence Tools in Residency Recruitment
According to Electronic Residency Application Service (ERAS) statistics, there were more than 1400 dermatology applicants in 2022, with an average of almost 560 applications received per program.1,2 With the goal to expand the diversity of board-certified dermatologists, there is increasing emphasis on the holistic review of applications, forgoing filtering by discrete metrics such as AOA (American Osteopathic Association) membership and US Medical Licensing Examination (USMLE) scores.3 According to the Association of American Medical Colleges, holistic review focuses on an individual applicant’s experience and unique attributes in addition to their academic achievements.4 Recent strategies to enhance the residency recruitment process have included the introduction of standardized letters of recommendation, preference signaling, and supplemental applications.5,6
Because it has become increasingly important to include applicant factors and achievements that extend beyond academics, the number of data points that are required for holistic review has expanded. If each application required 20 minutes to review, this would result in 166 total hours for complete holistic review of 500 applications. Tools that can facilitate holistic review of candidates and select applicants whose interests and career goals align with individual residency programs have the potential to optimize review. Artificial intelligence (AI) may aid in this process. This column highlights some of the published research on novel AI strategies that have the potential to impact dermatology residency recruitment.
Machine Learning to Screen Applicants
Artificial intelligence involves a machine-based system that can make decisions, predictions, and recommendations when provided a given set of human-defined objectives.7 Autonomous systems, machine learning (ML), and generative AI are examples of AI models.8 Machine learning has been explored to shorten and streamline the application review process and decrease bias. Because ML is a model in which the computer learns patterns based on large amounts of input data,9 it is possible that models could be developed and used in future cycles. Some studies found that applicants were discovered who traditionally would not have made it to the next stage of consideration based primarily on academic metrics.10,11 Burk-Rafel et al10 developed and validated an ML-based decision support tool for residency program directors to use for interview invitation decisions. The tool utilized 61 variables from ERAS data from more than 8000 applications in 3 prior application cycles at a single internal medicine residency program. An interview invitation was designated as the target outcome. Ultimately, the model would output a probability score for an interview invitation. The authors were able to tune the model to a 91% sensitivity and 85% specificity; for a pool of 2000 applicants and an invite rate of 15%, 1475 applicants would be screened out with a negative predictive value of 98% with maintenance of performance, even with removal of USMLE Step 1 examination scores. Their ML model was prospectively validated during an ongoing resident selection cycle, and when compared with human review, the AI model found an additional 20 applicants to invite for interviews. They concluded that this tool could potentially augment the human review process and reveal applicants who may have otherwise been overlooked.10
Rees and Ryder11 utilized another ML screening approach with the target outcome of ranked and matriculated compared with ranked applicants based on ERAS data using 72 unique variables for more than 5000 applicants. Their model was able to identify ranked candidates from the overall applicant pool with high accuracy; identification of ranked applicants that matriculated at the program was more modest but better than random probability.11Both the Burk-Rafel et al10 and Rees and Ryder11 models excluded some unstructured data components of the residency application, such as personal statements, medical student performance evaluation letters, transcripts, and letters of reference, that some may consider strongly in the holistic review process. Drum et al12 explored the value of extraction of this type of data. They created a program to extract “snippets” of text that pertained to values of successful residents for their internal medicine–pediatrics residency program that they previously validated via a modified Delphi method, which then were annotated by expert reviewers. Natural language processing was used to train an ML algorithm (MLA) to classify snippets into 11 value categories. Four values had more than 66% agreement with human annotation: academic strength; leadership; communication; and justice, equity, diversity, and inclusion. Although this MLA has not reached high enough levels of agreement for all the predetermined success values, the authors hope to generate a model that could produce a quantitative score to use as an initial screening tool to select applicants for interview.12 This type of analysis also could be incorporated into other MLAs for further refinement of the mentoring and application process.
Knapke et al13 evaluated the use of a natural language modeling platform to look for semantic patterns in medical school applications that could predict which students would be more likely to pursue family medicine residency, thus beginning the recruitment process even before residency application. This strategy could be particularly valuable for specialties for which there may be greater need in the workforce.
AI for Administrative Purposes
Artificial intelligence also has been used for nonapplication aspects of the residency recruitment process, such as interview scheduling. In the absence of coordinated interview release dates (as was implemented in dermatology starting in the 2020-2021 application cycle), a deluge of responses to schedule an interview comes flooding in as soon as invitations for interviewees are sent out, which can produce anxiety both for applicants and residency program staff as the schedule is sorted out and can create delays at both ends. Stephens et al14 utilized a computerized scheduling program for pediatric surgery fellowship applicants. It was used in 2016 to schedule 26 interviews, and it was found to reduce the average time to schedule an interview from 14.4 hours to 1.7 hours. It also reduced the number of email exchanges needed to finalize scheduling.14
Another aspect of residency recruitment that is amenable to AI is information gathering. Many would-be applicants turn to the internet and social media to learn about residency programs—their unique qualities, assets, and potential alignment of career goals.15 This exchange often is unidirectional, as the applicant clicks through the website searching for information. Yi et al16 explored the use of a chatbot, which mimics human conversation and exchange, on their institution’s pain fellowship website. Fellowship applicants could create specific prompts, such as “Show me faculty that trained at <applicant’s home program>,” and the chatbot would reply with the answer. The researchers sent a survey to all 258 applicants to the pain fellowship program that was completed by 48 applicants. Of these respondents, more than 70% (35/48) utilized the chatbot, and 84% (40/48) stated that they had found the information that was requested. The respondents overall found the chatbot to be a useful and positive experience.16
Specific Tools to Consider
There are some tools that are publicly available for programs and applicants to use that rely on AI.
In collaboration with ERAS and the Association of American Medical Colleges, Cortex powered by Thalamus (SJ MedConnect Inc)(https://thalamusgme.com/cortex-application-screening/) offers technology-assisted holistic review of residency and fellowship applications by utilizing natural language processing and optical character recognition to aggregate data from ERAS.
Tools also are being leveraged by applicants to help them find residency programs that fit their criteria, prepare for interviews, and complete portions of the application. Match A Resident (https://www.matcharesident.com/) is a resource for the international medical graduate community. As part of the service, the “Learn More with MARai” feature uses AI to generate information on residency programs to increase applicants’ confidence going into the interview process.17 Big Interview Medical (https://www.biginterviewmedical.com/ai-feedback), a paid interview preparation system developed by interview experts, utilizes AI to provide feedback to residents practicing for the interview process by measuring the amount of natural eye contact, language used, and pace of speech. A “Power Word” score is provided that incorporates aspects such as using filler words (“umm,” “uhh”). A Pace of Speech Tool provides rate of speaking feedback presuming that there is an ideal pace to decrease the impression that the applicant is nervous. Johnstone et al18 used ChatGPT (https://chat.openai.com/auth/login) to generate 2 personal statements for anesthesia residency applicants. Based on survey responses from 31 program directors, 22 rated the statements as good or excellent.18
Ethnical Concerns and Limitations of AI
The potential use of AI tools by residency applicants inevitably brings forth consideration of biases, ethics, and current limitations. These tools are highly dependent on the quality and quantity of data used for training and validation. Information considered valuable in the holistic review of applications includes unstructured data such as personal statements and letters of recommendation, and incorporating this information can be challenging in ML models, in contrast to discrete structured data such as grades, test scores, and awards. In addition, MLAs depend on large quantities of data to optimize performance.19 Depending on the size of the applicant pool and the amount of data available, this can present a limitation for smaller programs in developing ML tools for residency recruitment. Studies evaluating the use of AI in the residency application process often are from single institutions, and therefore generalizability is uncertain. The risk for latent bias—whereby a historical or pre-existing stereotype gets perpetuated through the system—must be considered, with the development of tools to detect and address this if found. Choosing which data to use to train the model can be tricky as well as choosing the outcome of interest. For these interventions to become more resilient, programs need to self-examine what defines their criteria for a successful match to their program to incorporate this data into their ML studies. The previously described models in this overview focused on outcomes such as whether an applicant was invited to interview, whether the applicant was ranked, and whether the applicant matriculated to their program.10,11 For supervised ML models that rely on outcomes to develop a prediction, continued research as to what outcomes represent resident success (eg, passing board certification examinations, correlation with clinical performance) would be important. There also is the possibility of applicants restructuring their applications to align with goals of an AI-assisted search and using AI to generate part or all of their application. The use of ChatGPT and other AI tools in the preparation of personal statements and curriculum vitae may provide benefits such as improved efficiency and grammar support.20 However, as use becomes more widespread, there is the potential increased similarity of personal statements and likely varied opinions on the use of such tools as writing aids.21,22 Continued efforts to develop guidance on generative AI use cases is ongoing; an example is the launch of VALID AI (https://validai.health/), a collaboration among health systems, health plans, and AI research organizations and nonprofits.23
Final Thoughts
Artificial intelligence tools may be a promising resource for residency and fellowship programs seeking to find meaningful ways to select applicants who are good matches for their training environment. Prioritizing the holistic review of applications has been promoted as a method to evaluate the applicant beyond their test scores and grades. The use of MLAs may streamline this review process, aid in scheduling interviews, and help discover trends in successful matriculants.
- Association of American Medical Colleges. ERAS® Statistics. Accessed January 16, 2024. https://www.aamc.org/data-reports/data/eras-statistics-data
- National Resident Matching Program, Data Release and ResearchCommittee: Results of the 2022 NRMP Program Director Survey. Accessed January 18, 2024. https://www.nrmp.org/wp-content/uploads/2022/09/PD-Survey-Report-2022_FINALrev.pdf
- Isaq NA, Bowers S, Chen ST. Taking a “step” toward diversity in dermatology: de-emphasizing USMLE Step 1 scores in residency applications. Int J Womens Dermatol. 2020;6:209-210. doi:10.1016/j.ijwd.2020.02.008
- Association of American Medical Colleges. Holistic review in medical school admissions. Accessed January 16, 2024. https://students-residents.aamc.org/choosing-medical-career/holistic-review-medical-school-admissions
- Association of American Medical Colleges. The MyERAS® application and program signaling for 2023-24. Accessed January 16, 2024. https://students-residents.aamc.org/applying-residencies-eras/myeras-application-and-program-signaling-2023-24
- Tavarez MM, Baghdassarian A, Bailey J, et al. A call to action for standardizing letters of recommendation. J Grad Med Educ. 2022;14:642-646. doi:10.4300/JGME-D-22-00131.1
- US Department of State. Artificial intelligence (AI). Accessed January 16, 2024. https://www.state.gov/artificial-intelligence/
- Stanford University Human-Centered Artificial Intelligence. Artificial intelligence definitions. Accessed January 16, 2024.https://hai.stanford.edu/sites/default/files/2023-03/AI-Key-Terms-Glossary-Definition.pdf
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. doi:10.1056/NEJMra1814259
- Burk-Rafel J, Reinstein I, Feng J, et al. Development and validation of a machine learning-based decision support tool for residency applicant screening and review. Acad Med. 2021;96(11S):S54-S61. doi:10.1097/ACM.0000000000004317
- Rees CA, Ryder HF. Machine learning for the prediction of ranked applicants and matriculants to an internal medicine residency program. Teach Learn Med. 2023;35:277-286. doi:10.1080/10401334.2022.2059664
- Drum B, Shi J, Peterson B, et al. Using natural language processing and machine learning to identify internal medicine-pediatrics residency values in applications. Acad Med. 2023;98:1278-1282. doi:10.1097/ACM.0000000000005352
- Knapke JM, Mount HR, McCabe E, et al. Early identification of family physicians using qualitative admissions data. Fam Med. 2023;55:245-252. doi:10.22454/FamMed.2023.596964
- Stephens CQ, Hamilton NA, Thompson AE, et al. Use of computerized interview scheduling program for pediatric surgery match applicants. J Pediatr Surg. 2017;52:1056-1059. doi:10.1016/j.jpedsurg.2017.03.033
- Nickles MA, Kulkarni V, Varghese JA, et al. Dermatology residency programs’ websites in the virtual era: a cross-sectional analysis. J Am Acad Dermatol. 2022;86:447-448. doi:10.1016/j.jaad.2021.09.064
- Yi PK, Ray ND, Segall N. A novel use of an artificially intelligent Chatbot and a live, synchronous virtual question-and answer session for fellowship recruitment. BMC Med Educ. 2023;23:152. doi:10.1186/s12909-022-03872-z
- Introducing “Learn More with MARai”—the key to understanding your target residency programs. Match A Resident website. Published September 23, 2023. Accessed January 16, 2024. https://blog.matcharesident.com/ai-powered-residency-insights/
- Johnstone RE, Neely G, Sizemore DC. Artificial intelligence softwarecan generate residency application personal statements that program directors find acceptable and difficult to distinguish from applicant compositions. J Clin Anesth. 2023;89:111185. doi:10.1016/j.jclinane.2023.111185
- Khalid N, Qayyum A, Bilal M, et al. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158:106848. doi:10.1016/j.compbiomed.2023.106848
- Birt J. How to optimize your resume for AI scanners (with tips). Indeed website. Updated December 30, 2022. Accessed January 16, 2024. https://www.indeed.com/career-advice/resumes-cover-letters/resume-ai
- Patel V, Deleonibus A, Wells MW, et al. Distinguishing authentic voices in the age of ChatGPT: comparing AI-generated and applicant-written personal statements for plastic surgery residency application. Ann Plast Surg. 2023;91:324-325. doi:10.1097/SAP.0000000000003653
- Woodfin MW. The personal statement in the age of artificial intelligence. Acad Med. 2023;98:869. doi:10.1097/ACM.0000000000005266
- Diaz N. UC Davis Health to lead new gen AI collaborative. Beckers Healthcare website. Published October 10, 2023. AccessedJanuary 16, 2024. https://www.beckershospitalreview.com/digital-health/uc-davis-health-to-lead-new-gen-ai-collaborative.html
According to Electronic Residency Application Service (ERAS) statistics, there were more than 1400 dermatology applicants in 2022, with an average of almost 560 applications received per program.1,2 With the goal to expand the diversity of board-certified dermatologists, there is increasing emphasis on the holistic review of applications, forgoing filtering by discrete metrics such as AOA (American Osteopathic Association) membership and US Medical Licensing Examination (USMLE) scores.3 According to the Association of American Medical Colleges, holistic review focuses on an individual applicant’s experience and unique attributes in addition to their academic achievements.4 Recent strategies to enhance the residency recruitment process have included the introduction of standardized letters of recommendation, preference signaling, and supplemental applications.5,6
Because it has become increasingly important to include applicant factors and achievements that extend beyond academics, the number of data points that are required for holistic review has expanded. If each application required 20 minutes to review, this would result in 166 total hours for complete holistic review of 500 applications. Tools that can facilitate holistic review of candidates and select applicants whose interests and career goals align with individual residency programs have the potential to optimize review. Artificial intelligence (AI) may aid in this process. This column highlights some of the published research on novel AI strategies that have the potential to impact dermatology residency recruitment.
Machine Learning to Screen Applicants
Artificial intelligence involves a machine-based system that can make decisions, predictions, and recommendations when provided a given set of human-defined objectives.7 Autonomous systems, machine learning (ML), and generative AI are examples of AI models.8 Machine learning has been explored to shorten and streamline the application review process and decrease bias. Because ML is a model in which the computer learns patterns based on large amounts of input data,9 it is possible that models could be developed and used in future cycles. Some studies found that applicants were discovered who traditionally would not have made it to the next stage of consideration based primarily on academic metrics.10,11 Burk-Rafel et al10 developed and validated an ML-based decision support tool for residency program directors to use for interview invitation decisions. The tool utilized 61 variables from ERAS data from more than 8000 applications in 3 prior application cycles at a single internal medicine residency program. An interview invitation was designated as the target outcome. Ultimately, the model would output a probability score for an interview invitation. The authors were able to tune the model to a 91% sensitivity and 85% specificity; for a pool of 2000 applicants and an invite rate of 15%, 1475 applicants would be screened out with a negative predictive value of 98% with maintenance of performance, even with removal of USMLE Step 1 examination scores. Their ML model was prospectively validated during an ongoing resident selection cycle, and when compared with human review, the AI model found an additional 20 applicants to invite for interviews. They concluded that this tool could potentially augment the human review process and reveal applicants who may have otherwise been overlooked.10
Rees and Ryder11 utilized another ML screening approach with the target outcome of ranked and matriculated compared with ranked applicants based on ERAS data using 72 unique variables for more than 5000 applicants. Their model was able to identify ranked candidates from the overall applicant pool with high accuracy; identification of ranked applicants that matriculated at the program was more modest but better than random probability.11Both the Burk-Rafel et al10 and Rees and Ryder11 models excluded some unstructured data components of the residency application, such as personal statements, medical student performance evaluation letters, transcripts, and letters of reference, that some may consider strongly in the holistic review process. Drum et al12 explored the value of extraction of this type of data. They created a program to extract “snippets” of text that pertained to values of successful residents for their internal medicine–pediatrics residency program that they previously validated via a modified Delphi method, which then were annotated by expert reviewers. Natural language processing was used to train an ML algorithm (MLA) to classify snippets into 11 value categories. Four values had more than 66% agreement with human annotation: academic strength; leadership; communication; and justice, equity, diversity, and inclusion. Although this MLA has not reached high enough levels of agreement for all the predetermined success values, the authors hope to generate a model that could produce a quantitative score to use as an initial screening tool to select applicants for interview.12 This type of analysis also could be incorporated into other MLAs for further refinement of the mentoring and application process.
Knapke et al13 evaluated the use of a natural language modeling platform to look for semantic patterns in medical school applications that could predict which students would be more likely to pursue family medicine residency, thus beginning the recruitment process even before residency application. This strategy could be particularly valuable for specialties for which there may be greater need in the workforce.
AI for Administrative Purposes
Artificial intelligence also has been used for nonapplication aspects of the residency recruitment process, such as interview scheduling. In the absence of coordinated interview release dates (as was implemented in dermatology starting in the 2020-2021 application cycle), a deluge of responses to schedule an interview comes flooding in as soon as invitations for interviewees are sent out, which can produce anxiety both for applicants and residency program staff as the schedule is sorted out and can create delays at both ends. Stephens et al14 utilized a computerized scheduling program for pediatric surgery fellowship applicants. It was used in 2016 to schedule 26 interviews, and it was found to reduce the average time to schedule an interview from 14.4 hours to 1.7 hours. It also reduced the number of email exchanges needed to finalize scheduling.14
Another aspect of residency recruitment that is amenable to AI is information gathering. Many would-be applicants turn to the internet and social media to learn about residency programs—their unique qualities, assets, and potential alignment of career goals.15 This exchange often is unidirectional, as the applicant clicks through the website searching for information. Yi et al16 explored the use of a chatbot, which mimics human conversation and exchange, on their institution’s pain fellowship website. Fellowship applicants could create specific prompts, such as “Show me faculty that trained at <applicant’s home program>,” and the chatbot would reply with the answer. The researchers sent a survey to all 258 applicants to the pain fellowship program that was completed by 48 applicants. Of these respondents, more than 70% (35/48) utilized the chatbot, and 84% (40/48) stated that they had found the information that was requested. The respondents overall found the chatbot to be a useful and positive experience.16
Specific Tools to Consider
There are some tools that are publicly available for programs and applicants to use that rely on AI.
In collaboration with ERAS and the Association of American Medical Colleges, Cortex powered by Thalamus (SJ MedConnect Inc)(https://thalamusgme.com/cortex-application-screening/) offers technology-assisted holistic review of residency and fellowship applications by utilizing natural language processing and optical character recognition to aggregate data from ERAS.
Tools also are being leveraged by applicants to help them find residency programs that fit their criteria, prepare for interviews, and complete portions of the application. Match A Resident (https://www.matcharesident.com/) is a resource for the international medical graduate community. As part of the service, the “Learn More with MARai” feature uses AI to generate information on residency programs to increase applicants’ confidence going into the interview process.17 Big Interview Medical (https://www.biginterviewmedical.com/ai-feedback), a paid interview preparation system developed by interview experts, utilizes AI to provide feedback to residents practicing for the interview process by measuring the amount of natural eye contact, language used, and pace of speech. A “Power Word” score is provided that incorporates aspects such as using filler words (“umm,” “uhh”). A Pace of Speech Tool provides rate of speaking feedback presuming that there is an ideal pace to decrease the impression that the applicant is nervous. Johnstone et al18 used ChatGPT (https://chat.openai.com/auth/login) to generate 2 personal statements for anesthesia residency applicants. Based on survey responses from 31 program directors, 22 rated the statements as good or excellent.18
Ethnical Concerns and Limitations of AI
The potential use of AI tools by residency applicants inevitably brings forth consideration of biases, ethics, and current limitations. These tools are highly dependent on the quality and quantity of data used for training and validation. Information considered valuable in the holistic review of applications includes unstructured data such as personal statements and letters of recommendation, and incorporating this information can be challenging in ML models, in contrast to discrete structured data such as grades, test scores, and awards. In addition, MLAs depend on large quantities of data to optimize performance.19 Depending on the size of the applicant pool and the amount of data available, this can present a limitation for smaller programs in developing ML tools for residency recruitment. Studies evaluating the use of AI in the residency application process often are from single institutions, and therefore generalizability is uncertain. The risk for latent bias—whereby a historical or pre-existing stereotype gets perpetuated through the system—must be considered, with the development of tools to detect and address this if found. Choosing which data to use to train the model can be tricky as well as choosing the outcome of interest. For these interventions to become more resilient, programs need to self-examine what defines their criteria for a successful match to their program to incorporate this data into their ML studies. The previously described models in this overview focused on outcomes such as whether an applicant was invited to interview, whether the applicant was ranked, and whether the applicant matriculated to their program.10,11 For supervised ML models that rely on outcomes to develop a prediction, continued research as to what outcomes represent resident success (eg, passing board certification examinations, correlation with clinical performance) would be important. There also is the possibility of applicants restructuring their applications to align with goals of an AI-assisted search and using AI to generate part or all of their application. The use of ChatGPT and other AI tools in the preparation of personal statements and curriculum vitae may provide benefits such as improved efficiency and grammar support.20 However, as use becomes more widespread, there is the potential increased similarity of personal statements and likely varied opinions on the use of such tools as writing aids.21,22 Continued efforts to develop guidance on generative AI use cases is ongoing; an example is the launch of VALID AI (https://validai.health/), a collaboration among health systems, health plans, and AI research organizations and nonprofits.23
Final Thoughts
Artificial intelligence tools may be a promising resource for residency and fellowship programs seeking to find meaningful ways to select applicants who are good matches for their training environment. Prioritizing the holistic review of applications has been promoted as a method to evaluate the applicant beyond their test scores and grades. The use of MLAs may streamline this review process, aid in scheduling interviews, and help discover trends in successful matriculants.
According to Electronic Residency Application Service (ERAS) statistics, there were more than 1400 dermatology applicants in 2022, with an average of almost 560 applications received per program.1,2 With the goal to expand the diversity of board-certified dermatologists, there is increasing emphasis on the holistic review of applications, forgoing filtering by discrete metrics such as AOA (American Osteopathic Association) membership and US Medical Licensing Examination (USMLE) scores.3 According to the Association of American Medical Colleges, holistic review focuses on an individual applicant’s experience and unique attributes in addition to their academic achievements.4 Recent strategies to enhance the residency recruitment process have included the introduction of standardized letters of recommendation, preference signaling, and supplemental applications.5,6
Because it has become increasingly important to include applicant factors and achievements that extend beyond academics, the number of data points that are required for holistic review has expanded. If each application required 20 minutes to review, this would result in 166 total hours for complete holistic review of 500 applications. Tools that can facilitate holistic review of candidates and select applicants whose interests and career goals align with individual residency programs have the potential to optimize review. Artificial intelligence (AI) may aid in this process. This column highlights some of the published research on novel AI strategies that have the potential to impact dermatology residency recruitment.
Machine Learning to Screen Applicants
Artificial intelligence involves a machine-based system that can make decisions, predictions, and recommendations when provided a given set of human-defined objectives.7 Autonomous systems, machine learning (ML), and generative AI are examples of AI models.8 Machine learning has been explored to shorten and streamline the application review process and decrease bias. Because ML is a model in which the computer learns patterns based on large amounts of input data,9 it is possible that models could be developed and used in future cycles. Some studies found that applicants were discovered who traditionally would not have made it to the next stage of consideration based primarily on academic metrics.10,11 Burk-Rafel et al10 developed and validated an ML-based decision support tool for residency program directors to use for interview invitation decisions. The tool utilized 61 variables from ERAS data from more than 8000 applications in 3 prior application cycles at a single internal medicine residency program. An interview invitation was designated as the target outcome. Ultimately, the model would output a probability score for an interview invitation. The authors were able to tune the model to a 91% sensitivity and 85% specificity; for a pool of 2000 applicants and an invite rate of 15%, 1475 applicants would be screened out with a negative predictive value of 98% with maintenance of performance, even with removal of USMLE Step 1 examination scores. Their ML model was prospectively validated during an ongoing resident selection cycle, and when compared with human review, the AI model found an additional 20 applicants to invite for interviews. They concluded that this tool could potentially augment the human review process and reveal applicants who may have otherwise been overlooked.10
Rees and Ryder11 utilized another ML screening approach with the target outcome of ranked and matriculated compared with ranked applicants based on ERAS data using 72 unique variables for more than 5000 applicants. Their model was able to identify ranked candidates from the overall applicant pool with high accuracy; identification of ranked applicants that matriculated at the program was more modest but better than random probability.11Both the Burk-Rafel et al10 and Rees and Ryder11 models excluded some unstructured data components of the residency application, such as personal statements, medical student performance evaluation letters, transcripts, and letters of reference, that some may consider strongly in the holistic review process. Drum et al12 explored the value of extraction of this type of data. They created a program to extract “snippets” of text that pertained to values of successful residents for their internal medicine–pediatrics residency program that they previously validated via a modified Delphi method, which then were annotated by expert reviewers. Natural language processing was used to train an ML algorithm (MLA) to classify snippets into 11 value categories. Four values had more than 66% agreement with human annotation: academic strength; leadership; communication; and justice, equity, diversity, and inclusion. Although this MLA has not reached high enough levels of agreement for all the predetermined success values, the authors hope to generate a model that could produce a quantitative score to use as an initial screening tool to select applicants for interview.12 This type of analysis also could be incorporated into other MLAs for further refinement of the mentoring and application process.
Knapke et al13 evaluated the use of a natural language modeling platform to look for semantic patterns in medical school applications that could predict which students would be more likely to pursue family medicine residency, thus beginning the recruitment process even before residency application. This strategy could be particularly valuable for specialties for which there may be greater need in the workforce.
AI for Administrative Purposes
Artificial intelligence also has been used for nonapplication aspects of the residency recruitment process, such as interview scheduling. In the absence of coordinated interview release dates (as was implemented in dermatology starting in the 2020-2021 application cycle), a deluge of responses to schedule an interview comes flooding in as soon as invitations for interviewees are sent out, which can produce anxiety both for applicants and residency program staff as the schedule is sorted out and can create delays at both ends. Stephens et al14 utilized a computerized scheduling program for pediatric surgery fellowship applicants. It was used in 2016 to schedule 26 interviews, and it was found to reduce the average time to schedule an interview from 14.4 hours to 1.7 hours. It also reduced the number of email exchanges needed to finalize scheduling.14
Another aspect of residency recruitment that is amenable to AI is information gathering. Many would-be applicants turn to the internet and social media to learn about residency programs—their unique qualities, assets, and potential alignment of career goals.15 This exchange often is unidirectional, as the applicant clicks through the website searching for information. Yi et al16 explored the use of a chatbot, which mimics human conversation and exchange, on their institution’s pain fellowship website. Fellowship applicants could create specific prompts, such as “Show me faculty that trained at <applicant’s home program>,” and the chatbot would reply with the answer. The researchers sent a survey to all 258 applicants to the pain fellowship program that was completed by 48 applicants. Of these respondents, more than 70% (35/48) utilized the chatbot, and 84% (40/48) stated that they had found the information that was requested. The respondents overall found the chatbot to be a useful and positive experience.16
Specific Tools to Consider
There are some tools that are publicly available for programs and applicants to use that rely on AI.
In collaboration with ERAS and the Association of American Medical Colleges, Cortex powered by Thalamus (SJ MedConnect Inc)(https://thalamusgme.com/cortex-application-screening/) offers technology-assisted holistic review of residency and fellowship applications by utilizing natural language processing and optical character recognition to aggregate data from ERAS.
Tools also are being leveraged by applicants to help them find residency programs that fit their criteria, prepare for interviews, and complete portions of the application. Match A Resident (https://www.matcharesident.com/) is a resource for the international medical graduate community. As part of the service, the “Learn More with MARai” feature uses AI to generate information on residency programs to increase applicants’ confidence going into the interview process.17 Big Interview Medical (https://www.biginterviewmedical.com/ai-feedback), a paid interview preparation system developed by interview experts, utilizes AI to provide feedback to residents practicing for the interview process by measuring the amount of natural eye contact, language used, and pace of speech. A “Power Word” score is provided that incorporates aspects such as using filler words (“umm,” “uhh”). A Pace of Speech Tool provides rate of speaking feedback presuming that there is an ideal pace to decrease the impression that the applicant is nervous. Johnstone et al18 used ChatGPT (https://chat.openai.com/auth/login) to generate 2 personal statements for anesthesia residency applicants. Based on survey responses from 31 program directors, 22 rated the statements as good or excellent.18
Ethnical Concerns and Limitations of AI
The potential use of AI tools by residency applicants inevitably brings forth consideration of biases, ethics, and current limitations. These tools are highly dependent on the quality and quantity of data used for training and validation. Information considered valuable in the holistic review of applications includes unstructured data such as personal statements and letters of recommendation, and incorporating this information can be challenging in ML models, in contrast to discrete structured data such as grades, test scores, and awards. In addition, MLAs depend on large quantities of data to optimize performance.19 Depending on the size of the applicant pool and the amount of data available, this can present a limitation for smaller programs in developing ML tools for residency recruitment. Studies evaluating the use of AI in the residency application process often are from single institutions, and therefore generalizability is uncertain. The risk for latent bias—whereby a historical or pre-existing stereotype gets perpetuated through the system—must be considered, with the development of tools to detect and address this if found. Choosing which data to use to train the model can be tricky as well as choosing the outcome of interest. For these interventions to become more resilient, programs need to self-examine what defines their criteria for a successful match to their program to incorporate this data into their ML studies. The previously described models in this overview focused on outcomes such as whether an applicant was invited to interview, whether the applicant was ranked, and whether the applicant matriculated to their program.10,11 For supervised ML models that rely on outcomes to develop a prediction, continued research as to what outcomes represent resident success (eg, passing board certification examinations, correlation with clinical performance) would be important. There also is the possibility of applicants restructuring their applications to align with goals of an AI-assisted search and using AI to generate part or all of their application. The use of ChatGPT and other AI tools in the preparation of personal statements and curriculum vitae may provide benefits such as improved efficiency and grammar support.20 However, as use becomes more widespread, there is the potential increased similarity of personal statements and likely varied opinions on the use of such tools as writing aids.21,22 Continued efforts to develop guidance on generative AI use cases is ongoing; an example is the launch of VALID AI (https://validai.health/), a collaboration among health systems, health plans, and AI research organizations and nonprofits.23
Final Thoughts
Artificial intelligence tools may be a promising resource for residency and fellowship programs seeking to find meaningful ways to select applicants who are good matches for their training environment. Prioritizing the holistic review of applications has been promoted as a method to evaluate the applicant beyond their test scores and grades. The use of MLAs may streamline this review process, aid in scheduling interviews, and help discover trends in successful matriculants.
- Association of American Medical Colleges. ERAS® Statistics. Accessed January 16, 2024. https://www.aamc.org/data-reports/data/eras-statistics-data
- National Resident Matching Program, Data Release and ResearchCommittee: Results of the 2022 NRMP Program Director Survey. Accessed January 18, 2024. https://www.nrmp.org/wp-content/uploads/2022/09/PD-Survey-Report-2022_FINALrev.pdf
- Isaq NA, Bowers S, Chen ST. Taking a “step” toward diversity in dermatology: de-emphasizing USMLE Step 1 scores in residency applications. Int J Womens Dermatol. 2020;6:209-210. doi:10.1016/j.ijwd.2020.02.008
- Association of American Medical Colleges. Holistic review in medical school admissions. Accessed January 16, 2024. https://students-residents.aamc.org/choosing-medical-career/holistic-review-medical-school-admissions
- Association of American Medical Colleges. The MyERAS® application and program signaling for 2023-24. Accessed January 16, 2024. https://students-residents.aamc.org/applying-residencies-eras/myeras-application-and-program-signaling-2023-24
- Tavarez MM, Baghdassarian A, Bailey J, et al. A call to action for standardizing letters of recommendation. J Grad Med Educ. 2022;14:642-646. doi:10.4300/JGME-D-22-00131.1
- US Department of State. Artificial intelligence (AI). Accessed January 16, 2024. https://www.state.gov/artificial-intelligence/
- Stanford University Human-Centered Artificial Intelligence. Artificial intelligence definitions. Accessed January 16, 2024.https://hai.stanford.edu/sites/default/files/2023-03/AI-Key-Terms-Glossary-Definition.pdf
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. doi:10.1056/NEJMra1814259
- Burk-Rafel J, Reinstein I, Feng J, et al. Development and validation of a machine learning-based decision support tool for residency applicant screening and review. Acad Med. 2021;96(11S):S54-S61. doi:10.1097/ACM.0000000000004317
- Rees CA, Ryder HF. Machine learning for the prediction of ranked applicants and matriculants to an internal medicine residency program. Teach Learn Med. 2023;35:277-286. doi:10.1080/10401334.2022.2059664
- Drum B, Shi J, Peterson B, et al. Using natural language processing and machine learning to identify internal medicine-pediatrics residency values in applications. Acad Med. 2023;98:1278-1282. doi:10.1097/ACM.0000000000005352
- Knapke JM, Mount HR, McCabe E, et al. Early identification of family physicians using qualitative admissions data. Fam Med. 2023;55:245-252. doi:10.22454/FamMed.2023.596964
- Stephens CQ, Hamilton NA, Thompson AE, et al. Use of computerized interview scheduling program for pediatric surgery match applicants. J Pediatr Surg. 2017;52:1056-1059. doi:10.1016/j.jpedsurg.2017.03.033
- Nickles MA, Kulkarni V, Varghese JA, et al. Dermatology residency programs’ websites in the virtual era: a cross-sectional analysis. J Am Acad Dermatol. 2022;86:447-448. doi:10.1016/j.jaad.2021.09.064
- Yi PK, Ray ND, Segall N. A novel use of an artificially intelligent Chatbot and a live, synchronous virtual question-and answer session for fellowship recruitment. BMC Med Educ. 2023;23:152. doi:10.1186/s12909-022-03872-z
- Introducing “Learn More with MARai”—the key to understanding your target residency programs. Match A Resident website. Published September 23, 2023. Accessed January 16, 2024. https://blog.matcharesident.com/ai-powered-residency-insights/
- Johnstone RE, Neely G, Sizemore DC. Artificial intelligence softwarecan generate residency application personal statements that program directors find acceptable and difficult to distinguish from applicant compositions. J Clin Anesth. 2023;89:111185. doi:10.1016/j.jclinane.2023.111185
- Khalid N, Qayyum A, Bilal M, et al. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158:106848. doi:10.1016/j.compbiomed.2023.106848
- Birt J. How to optimize your resume for AI scanners (with tips). Indeed website. Updated December 30, 2022. Accessed January 16, 2024. https://www.indeed.com/career-advice/resumes-cover-letters/resume-ai
- Patel V, Deleonibus A, Wells MW, et al. Distinguishing authentic voices in the age of ChatGPT: comparing AI-generated and applicant-written personal statements for plastic surgery residency application. Ann Plast Surg. 2023;91:324-325. doi:10.1097/SAP.0000000000003653
- Woodfin MW. The personal statement in the age of artificial intelligence. Acad Med. 2023;98:869. doi:10.1097/ACM.0000000000005266
- Diaz N. UC Davis Health to lead new gen AI collaborative. Beckers Healthcare website. Published October 10, 2023. AccessedJanuary 16, 2024. https://www.beckershospitalreview.com/digital-health/uc-davis-health-to-lead-new-gen-ai-collaborative.html
- Association of American Medical Colleges. ERAS® Statistics. Accessed January 16, 2024. https://www.aamc.org/data-reports/data/eras-statistics-data
- National Resident Matching Program, Data Release and ResearchCommittee: Results of the 2022 NRMP Program Director Survey. Accessed January 18, 2024. https://www.nrmp.org/wp-content/uploads/2022/09/PD-Survey-Report-2022_FINALrev.pdf
- Isaq NA, Bowers S, Chen ST. Taking a “step” toward diversity in dermatology: de-emphasizing USMLE Step 1 scores in residency applications. Int J Womens Dermatol. 2020;6:209-210. doi:10.1016/j.ijwd.2020.02.008
- Association of American Medical Colleges. Holistic review in medical school admissions. Accessed January 16, 2024. https://students-residents.aamc.org/choosing-medical-career/holistic-review-medical-school-admissions
- Association of American Medical Colleges. The MyERAS® application and program signaling for 2023-24. Accessed January 16, 2024. https://students-residents.aamc.org/applying-residencies-eras/myeras-application-and-program-signaling-2023-24
- Tavarez MM, Baghdassarian A, Bailey J, et al. A call to action for standardizing letters of recommendation. J Grad Med Educ. 2022;14:642-646. doi:10.4300/JGME-D-22-00131.1
- US Department of State. Artificial intelligence (AI). Accessed January 16, 2024. https://www.state.gov/artificial-intelligence/
- Stanford University Human-Centered Artificial Intelligence. Artificial intelligence definitions. Accessed January 16, 2024.https://hai.stanford.edu/sites/default/files/2023-03/AI-Key-Terms-Glossary-Definition.pdf
- Rajkomar A, Dean J, Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347-1358. doi:10.1056/NEJMra1814259
- Burk-Rafel J, Reinstein I, Feng J, et al. Development and validation of a machine learning-based decision support tool for residency applicant screening and review. Acad Med. 2021;96(11S):S54-S61. doi:10.1097/ACM.0000000000004317
- Rees CA, Ryder HF. Machine learning for the prediction of ranked applicants and matriculants to an internal medicine residency program. Teach Learn Med. 2023;35:277-286. doi:10.1080/10401334.2022.2059664
- Drum B, Shi J, Peterson B, et al. Using natural language processing and machine learning to identify internal medicine-pediatrics residency values in applications. Acad Med. 2023;98:1278-1282. doi:10.1097/ACM.0000000000005352
- Knapke JM, Mount HR, McCabe E, et al. Early identification of family physicians using qualitative admissions data. Fam Med. 2023;55:245-252. doi:10.22454/FamMed.2023.596964
- Stephens CQ, Hamilton NA, Thompson AE, et al. Use of computerized interview scheduling program for pediatric surgery match applicants. J Pediatr Surg. 2017;52:1056-1059. doi:10.1016/j.jpedsurg.2017.03.033
- Nickles MA, Kulkarni V, Varghese JA, et al. Dermatology residency programs’ websites in the virtual era: a cross-sectional analysis. J Am Acad Dermatol. 2022;86:447-448. doi:10.1016/j.jaad.2021.09.064
- Yi PK, Ray ND, Segall N. A novel use of an artificially intelligent Chatbot and a live, synchronous virtual question-and answer session for fellowship recruitment. BMC Med Educ. 2023;23:152. doi:10.1186/s12909-022-03872-z
- Introducing “Learn More with MARai”—the key to understanding your target residency programs. Match A Resident website. Published September 23, 2023. Accessed January 16, 2024. https://blog.matcharesident.com/ai-powered-residency-insights/
- Johnstone RE, Neely G, Sizemore DC. Artificial intelligence softwarecan generate residency application personal statements that program directors find acceptable and difficult to distinguish from applicant compositions. J Clin Anesth. 2023;89:111185. doi:10.1016/j.jclinane.2023.111185
- Khalid N, Qayyum A, Bilal M, et al. Privacy-preserving artificial intelligence in healthcare: techniques and applications. Comput Biol Med. 2023;158:106848. doi:10.1016/j.compbiomed.2023.106848
- Birt J. How to optimize your resume for AI scanners (with tips). Indeed website. Updated December 30, 2022. Accessed January 16, 2024. https://www.indeed.com/career-advice/resumes-cover-letters/resume-ai
- Patel V, Deleonibus A, Wells MW, et al. Distinguishing authentic voices in the age of ChatGPT: comparing AI-generated and applicant-written personal statements for plastic surgery residency application. Ann Plast Surg. 2023;91:324-325. doi:10.1097/SAP.0000000000003653
- Woodfin MW. The personal statement in the age of artificial intelligence. Acad Med. 2023;98:869. doi:10.1097/ACM.0000000000005266
- Diaz N. UC Davis Health to lead new gen AI collaborative. Beckers Healthcare website. Published October 10, 2023. AccessedJanuary 16, 2024. https://www.beckershospitalreview.com/digital-health/uc-davis-health-to-lead-new-gen-ai-collaborative.html
Practice Points
- Artificial intelligence solutions may increase the efficiency of the holistic review process and enhance the opportunity to find applicants who may have been overlooked by a traditional review process.
- Artificial intelligence support also may be utilized by applicants to aid in discovering training programs that fit their interests, practice interview strategies, and refine their written application.