User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Navigating Psoriasis Treatment Innovations
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
Psoriasis is a chronic autoimmune skin condition that affects approximately 2% to 4% of the US population and notably impacts overall quality of life.1,2 There is no cure for this long-lasting condition. Fortunately, recent developments in research have led to more targeted therapies, paving the way for a more promising transformative landscape of psoriasis management. Herein, we explore the most up-to-date advancements and developments in the realm of psoriasis care.
Emerging Systemic Therapies
Biologics are cutting-edge treatments available for moderate to severe plaque psoriasis, as IL-17A, IL-23, and tumor necrosis factor α (TNF-α) have been recognized as key targets.3
IL-17—Bimekizumab is a unique monoclonal antibody that inhibits the activity of both IL-17A and IL-17F cytokines.3 This treatment was approved by the US Food and Drug Administration (FDA) in October 2023 for patients with moderate to severe plaque psoriasis who are candidates for systemic therapy or phototherapy.4
Bimekizumab outperformed ustekinumab in the BE VIVID phase 3 trial, with 273 of 321 patients (85%) receiving bimekizumab vs 81 of 163 patients (50%) receiving ustekinumab experiencing at least 90% improvement in psoriasis area and severity index (PASI) score at week 16.4 In a 2020 observational study (PSO-BIO-REAL), the efficacy rate of skin clearance after 6 months of treatment with biologics was only 25% (1/4).5 Aside from moderate to severe plaque psoriasis, bimekizumab demonstrated notable improvement in patients with psoriatic arthritis who had inadequate response or intolerance to TNF-α inhibitors compared to a placebo group in the BE COMPLETE phase 3 trial.6
IL-23—Guselkumab, risankizumab, and tildrakizumab are injectable therapies approved by the FDA in 2017 for moderate to severe plaque psoriasis.3 They inhibit IL-23 signaling by targeting the p19 subunit in addition to sparing IL-12.3,7
A novel oral therapeutic peptide, JNJ-2113—the first oral IL-23 receptor antagonist peptide that blocks IL-23 signaling—has been developed, offering a new way to treat moderate to severe plaque psoriasis. Trial results from a phase 2 study (FRONTIER1) have supported JNJ-2113’s advancement into phase 3.7,8 Patients who received JNJ-2113 successfully achieved PASI75 in addition to surpassing PASI90 and PASI100 at greater proportions compared to placebo at week 16.7
The promising early results of JNJ-2113 provide patients with greater flexibility and convenience for treatment options to address the manifestations of psoriasis. Although a considerable number of patients with moderate to severe plaque psoriasis qualify for advanced therapies, a substantial proportion remain untreated. Introducing an oral route of medication administration may help overcome barriers to therapy access due to a greater preference for pills over injections.9
TNF-α Inhibitors—Adalimumab is a TNF-α inhibitor that is used to treat moderate to severe chronic plaque psoriasis in adults who are candidates for systemic phototherapy.1,10 However, one of the main barriers to initiating treatment has been cost. Biosimilars contribute to market competition, thus allowing the possibility of lower drug prices.10
There are 9 FDA-approved biosimilar products for adalimumab, with 2 having interchangeable designation. The first interchangeable biosimilar to enter the US market, adalimumab-adbm, became available in July 2023. In October 2023, adalimumab-afzb was granted interchangeable designation,11 which enables pharmacists to swiftly substitute brand products for lower-cost biosimilars, providing patients with equally safe and effective alternatives without the delay of involving the prescribing clinician.12 Pricing information indicates an initial 5% discount, which may later increase to 60%, from brand name adalimumab. Hopefully, reduced drug costs due to market competition will allow more patients to overcome barriers to therapy access.
IL-12/IL-23—Ustekinumab is a monoclonal antibody that targets IL-12 and IL-23. The FDA recently approved ustekinumab-auub as the first interchangeable ustekinumab biosimilar for the treatment of various inflammatory diseases, including moderate to severe plaque psoriasis and psoriatic arthritis.12,13 The approval of ustekinumab-auub expands therapeutic options for the treatment of diverse inflammatory diseases. As the first interchangeable biosimilar in its category, this development underscores the importance of biosimilars in providing effective and accessible treatment.12,14
Topical Innovations
In October 2023, the FDA approved an expanded indication for roflumilast cream 0.3% to treat children as young as 6 years for plaque psoriasis, even for use in intertriginous areas,15 which is a milestone given the lack of treatment options for the pediatric population because topical steroids, the most common treatment option for plaque psoriasis, can have safety concerns related to long-term use. With the advent of this steroid-free topical agent, pediatric patients have a safe and well-tolerated option for managing plaque psoriasis.16 This promising effort will now expand to trials in children as young as 2 years to test efficacy.16
Engel et al17 proposed a new algorithmic approach to the topical management of psoriasis with roflumilast cream and tapinarof cream as first-line treatments for mild disease due to their novelty in treating intertriginous areas, whereas traditional topical steroids in these areas would be inapt.17 The latest indication for roflumilast cream suggests that this proposed recommendation could be a promising and convenient enhancement to psoriasis management, potentially outperforming traditional topical corticosteroids.15,17
Final Thoughts
Innovative targeted therapies ranging from new biologic agents to broader applications of topical treatments hold the potential to transform conventional psoriasis management with greater efficacy and safety, which can help create a more effective and personalized approach with greater patient satisfaction, ultimately enhancing overall quality of life. The choice of treatment is dependent not only on the severity of the disease but also on accessibility considerations such as cost. Overall, these innovative therapies add substantial value to the treatment armamentarium for psoriasis.
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
- Li C, Sunhe Y, Zhou H, Dong W. Efficacy and safety evaluations of adalimumab biosimilars in the treatment of psoriasis. J Dermatolog Treat. 2023;34:2249145. doi:10.1080/09546634.2023.2249145
- Liu J, Thatiparthi A, Martin A, et al. Association between psoriasis and thyroid dysfunction among US adults in the 2009-2014 National Health and Nutrition Examination Survey [published online Mary 17, 2021]. J Am Acad Dermatol. 2022;86:897-899. doi:10.1016/j.jaad.2021.03.030
- Lee EB, Amin M, Bhutani T, et al. Emerging therapies in psoriasis: a systematic review. Cutis. 2018;101(3S):5-9.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo-controlled phase 3 trial. Lancet. 2021;397:487-498. doi:10.1016/S0140-6736(21)00125-2
- Seneschal J, Lacour JP, Bewley A, et al. A multinational, prospective, observational study to estimate complete skin clearance in patients with moderate-to-severe plaque PSOriasis treated with BIOlogics in a REAL world setting (PSO-BIO-REAL) [published online June 8, 2020]. J Eur Acad Dermatol Venereol. 2020;34:2566-2573. doi:10.1111/jdv.16568
- Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-α inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE)[published online December 6, 2022]. Lancet. 2023;401:38-48. doi:10.1016/S0140-6736(22)02303-0
- Janssen announces positive topline results for JNJ-2113—a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News release. Janssen Pharmaceutical Companies; July 4, 2023.
- Bissonnette R, Pinter A, Ferris L, et al. A Phase 2, randomized, placebo-controlled, dose-ranging study of oral JNJ-77242113 for the treatment of moderate-to-severe plaque psoriasis: FRONTIER 1. Abstract presented at: World Congress of Dermatology, July 3-8, 2023; Singapore.
- Xu Y, Sudharshan L, Hsu MA, et al. Patient preferences associated with therapies for psoriatic arthritis: a conjoint analysis. Am Health Drug Benefits. 2018;11:408-417.
- Maurelli M, Girolomoni G, Gisondi P. Cost per responder of adalimumab biosimilars versus methotrexate in patients with psoriasis: a real-life experience. J Dermatolog Treat. 2023;34:2218504. doi:10.1080/09546634.2023.2218504
- Food and Drug Administration/Center for Drug Evaluation and Research. Expiration of first interchangeable exclusivity (“FIE”) when section 351(l)(6) litigation ends prior to the submission of an application for interchangeability [memorandum]. Published October 3, 2023. Accessed January 18, 2024. https://www.fda.gov/media/173749/download
- US Food & Drug Administration. Biosimilar and interchangeable biologics: more treatment choices. Accessed January 18, 2024. https://www.fda.gov/consumers/consumer-updates/biosimilar-and-interchangeable-biologics-more-treatment-choices
- Chow V, Mytych DT, Das S, et al. Pharmacokinetic similarity of ABP 654, an ustekinumab biosimilar candidate: results from a randomized, double-blind study in healthy subjects [published online July 7, 2023]. Clin Pharmacol Drug Dev. 2023;12:863-873. doi:10.1002/cpdd.1301
- Wezlana (ustekinumab-auub) [prescribing information]. Published October 2023. Accessed January 18, 2024. www.accessdata.fda.gov/drugsatfda_docs/label/2023/761285s000,761331s000lbl.pdf
- ZORYVE (roflumilast) topical cream [prescribing information]. Westlake Village, CA: Arcutis Biotherapeutics. Revised October 2023. Accessed January 18, 2024. https://www.arcutis.com/wp-content/uploads/USPI-roflumilast-cream.pdf
- Lie E, Choi M, Wang SP, et al. Topical management of pediatric psoriasis: a review of new developments and existing therapies. Paediatr Drugs. 2024;26:9-18. doi:10.1007/s40272-023-00592-9
- Engel PV, Smith B, Javadi SS, et al. It is time to consider anew topical algorithm for psoriasis. J Am Acad Dermatol. 2023:S0190-9622(23)02906-7. doi:10.1016/j.jaad.2023.07.1048
Skin Cancer Screening: The Paradox of Melanoma and Improved All-Cause Mortality
In April 2023, the US Preventive Services Task Force (USPSTF) issued a controversial recommendation that the current evidence is insufficient to assess the benefits vs harms of visual skin examination by clinicians for skin cancer screening in adolescents and adults who do not have signs or symptoms of skin cancer.1,2 This recommendation by the USPSTF has not changed in a quarter century,3 but a recent study described an interesting paradox that should trigger wide evaluation and debate.
Patel et al4 analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program from January 2000 to December 2018 to identify adults with a diagnosis of first primary melanoma in situ (MIS). Overall mortality was then determined through the National Vital Statistics System, which provides cause-of-death information for all deaths in the United States. The authors found 137,872 patients who had 1—and only 1—MIS discovered over the observation period. These patients predominantly were White (96.7%), and the mean (SD) age at diagnosis was 61.9 (16.5) years. During 910,308 total person-years of follow-up (mean [SD], 6.6 [5.1] years), 893 (0.6%) patients died of melanoma and 17,327 (12.6%) died of any cause. The 15-year melanoma-specific standardized mortality rate (SMR) was 1.89 (95% CI, 1.77-2.02), yet the 15-year overall survival relative to matched population controls was 112.4% (95% CI, 112.0%-112.8%), thus all-cause SMR was significantly lower at 0.68 (95% CI, 0.67-0.7). Although MIS was associated with a small increase in cohort melanoma mortality, overall mortality was actually lower than in the general population.4
Patel et al4 did a further broader search that included an additional 18,379 patients who also experienced a second primary melanoma, of which 6751 (36.7%) were invasive and 11,628 (63.3%) were in situ, with a melanoma-specific survival of 98.2% (95% CI, 97.6%-98.5%). Yet relative all-cause survival was significantly higher at 126.7% (95% CI, 125.5%-128.0%). Even among patients in whom a second primary melanoma was invasive, melanoma-specific survival was reduced to 91.1% (95% CI, 90.0%-92.1%), but relative all-cause survival was 116.7% (95% CI, 115%-118.4%). These data in the overall cohort of 155,251 patients showed a discordance between melanoma mortality, which was 4.27-times higher than in the general population (SMR, 4.27; 95% CI, 4.07-4.48), and a lower risk for death from all causes that was approximately 27% lower than in the general population (SMR, 0.73; 95% CI, 0.72-0.74). The authors showed that their findings were not associated with socioeconomic status.4
The analysis by Patel et al4 is now the second study in the literature to show this discordant melanoma survival pattern. In an earlier Australian study of 2452 melanoma patients, Watts et al5 reported that melanoma detection during routine skin checks was associated with a 25% lower all-cause mortality (hazard ratio, 0.75; 95% CI, 0.63-0.90) but not melanoma-specific mortality after multivariable adjustment for a variety of factors including socioeconomic status.These analyses by 2 different groups of investigators have broad implications. Both groups suggested that the improved life span in melanoma patients may be due to health-seeking behavior, which has been defined as “any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.”6
Once treated for melanoma, it is clear that patients are likely to return at regular intervals for thorough full-body skin examinations, but this activity alone could not be responsible for improved all-cause mortality in the face of increased melanoma-specific mortality. It seems the authors are implying a broader concept of good health behavior, originally defined by MacKian7 as encompassing “activities undertaken to maintain good health, to prevent ill health, as well as dealing with any departure from a good state of health,” such as overt behavioral patterns, actions, and habits with the goal of maintenance, restoration, and improvement of one’s health. A variety of behaviors fall within such a definition including smoking cessation, decreased alcohol use, good diet, more physical activity, safe sexual behavior, scheduling physician visits, medication adherence, vaccination, and yes—screening examinations for health problems.8
The concept that individuals who are diagnosed with melanoma fall into a pattern of good health behavior is an interesting hypothesis that must remain speculative until the multiple aspects of good health behavior are rigorously studied. This concept coexists with the hypothesis of melanoma “overdiagnosis”—the idea that many melanomas are detected that will never lead to death.9 Both concepts deserve further analysis. Unquestionably, a randomized controlled trial could never recruit patients willing to undergo long-term untreated observation of their melanomas to test the hypothesis that their melanoma diagnosis would eventually lead to death. Furthermore, Patel et al4 do suggest that even MIS carries a small but measurable increased risk for death from the disease, which is not particularly supportive of the overdiagnosis hypothesis; however, analysis of the concept that improved individual health behavior is at least in part responsible for the first discovery of melanomas is certainly approachable. Here is the key question: Did the melanoma diagnosis trigger a sudden change in multiple aspects of health behavior that led to significant all-cause mortality benefits? The average age of the population studied by Patel et al4 was approximately 62 years. One wonders whether the consequences of a lifetime of established health behavior patterns can be rapidly modified—certainly possible but again remains to be proven by further studies.
Conversely, the alternative hypothesis is that discovery of MIS was the result of active pursuit of self-examination and screening procedures as part of individually ingrained good health behavior over a lifetime. Goodwin et al10 carried out a study in a sample of the Medicare population aged 69 to 90 years looking at men who had prostate cancer screening via prostate-specific antigen measurement and women who had undergone mammography in older age, compared to the contrast population who had not had these screening procedures. They tracked date of death in Medicare enrollment files. They identified 543,970 women and 362,753 men who were aged 69 to 90 years as of January 1, 2003. Patients were stratified by life expectancy based on age and comorbidity. Within each stratum, the patients with cancer screening had higher actual median survival than those who were not screened, with differences ranging from 1.7 to 2.1 years for women and 0.9 to 1.1 years for men.10 These results were not the result of lower prostate or breast cancer mortality. Rather, one surmises that other health factors yielded lower mortality in the screened cohorts.
A full-body skin examination is a time-consuming process. Patients who come to their physician for a routine annual physical don’t expect a skin examination and very few physicians have the time for a long detailed full-body skin examination. When the patient presents to a dermatologist for an examination, it often is because they have real concerns; for example, they may have had a family member who died of skin cancer, or the patient themself may have noticed a worrisome lesion. Patients, not physicians, are the drivers of skin cancer screening, a fact that often is dismissed by those who are not necessarily supportive of the practice.
In light of the findings of Patel et al,4 it is essential that the USPSTF reviews be reanalyzed to compare skin cancer–specific mortality, all-cause mortality, and lifespan in individuals who pursue skin cancer screening; the reanalysis also should not be exclusively limited to survival. With the advent of the immune checkpoint inhibitors, patients with metastatic melanoma are living much longer.11 The burden of living with metastatic cancer must be characterized and measured to have a complete picture and a valid analysis.
After the release of the USPSTF recommendation, there have been calls for large-scale studies to prove the benefits of skin cancer screening.12 Such studies may be valuable; however, if the hypothesis that overall healthy behavior as the major outcome determinant is substantiated, it may prove quite challenging to perform tests of association with specific interventions. It has been shown that skin cancer screening does lead to discovery of more melanomas,13 yet in light of the paradox described by Patel et al,4 it also is likely that causes of death other than melanoma impact overall mortality. Patients who pursue skin examinations may engage in multiple different health activities that are beneficial in the long term, making it difficult to analyze the specific benefit of skin cancer screening in isolation. It may prove difficult to ask patients to omit selected aspects of healthy behavior to try to prove the point. At this time, there is much more work to be done prior to offering opinions on the importance of skin cancer examination in isolation to improve overall health care. In the meantime, dermatologists owe it to our patients to continue to diligently pursue thorough and detailed skin examinations.
- US Preventive Services Task Force; Mangione CM, Barry MJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295.
- Henrikson NB, Ivlev I, Blasi PR, et al. Skin cancer screening: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329:1296-1307.
- US Preventive Services Task Force Guide to Clinical Preventive Services. 2nd ed. Agency for Healthcare Research and Quality; 1996.
- Patel VR, Roberson ML, Pignone MP, et al. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;169:703-710.
- Watts CG, McLoughlin K, Goumas C, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157:1425-1436.
- Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351-773.
- MacKian S. A review of health seeking behaviour: problems and prospects. health systems development programme. University of Manchester; 2003. Accessed January 19, 2024. https://assets.publishing.service.gov.uk/media/57a08d1de5274a27b200163d/05-03_health_seeking_behaviour.pdf
- Conner M, Norman P. Health behaviour: current issues and challenges. Psychol Health. 2017;32:895-906.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613.
- Goodwin JS, Sheffield K, Li S, et al. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;11:1308-1314.
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267.
- Adamson AS. The USPSTF statement on skin cancer screening—not a disappointment but an opportunity. JAMA Dermatol. 2023;159:579-581. doi:10.1001/jamadermatol.2023.0706
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
In April 2023, the US Preventive Services Task Force (USPSTF) issued a controversial recommendation that the current evidence is insufficient to assess the benefits vs harms of visual skin examination by clinicians for skin cancer screening in adolescents and adults who do not have signs or symptoms of skin cancer.1,2 This recommendation by the USPSTF has not changed in a quarter century,3 but a recent study described an interesting paradox that should trigger wide evaluation and debate.
Patel et al4 analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program from January 2000 to December 2018 to identify adults with a diagnosis of first primary melanoma in situ (MIS). Overall mortality was then determined through the National Vital Statistics System, which provides cause-of-death information for all deaths in the United States. The authors found 137,872 patients who had 1—and only 1—MIS discovered over the observation period. These patients predominantly were White (96.7%), and the mean (SD) age at diagnosis was 61.9 (16.5) years. During 910,308 total person-years of follow-up (mean [SD], 6.6 [5.1] years), 893 (0.6%) patients died of melanoma and 17,327 (12.6%) died of any cause. The 15-year melanoma-specific standardized mortality rate (SMR) was 1.89 (95% CI, 1.77-2.02), yet the 15-year overall survival relative to matched population controls was 112.4% (95% CI, 112.0%-112.8%), thus all-cause SMR was significantly lower at 0.68 (95% CI, 0.67-0.7). Although MIS was associated with a small increase in cohort melanoma mortality, overall mortality was actually lower than in the general population.4
Patel et al4 did a further broader search that included an additional 18,379 patients who also experienced a second primary melanoma, of which 6751 (36.7%) were invasive and 11,628 (63.3%) were in situ, with a melanoma-specific survival of 98.2% (95% CI, 97.6%-98.5%). Yet relative all-cause survival was significantly higher at 126.7% (95% CI, 125.5%-128.0%). Even among patients in whom a second primary melanoma was invasive, melanoma-specific survival was reduced to 91.1% (95% CI, 90.0%-92.1%), but relative all-cause survival was 116.7% (95% CI, 115%-118.4%). These data in the overall cohort of 155,251 patients showed a discordance between melanoma mortality, which was 4.27-times higher than in the general population (SMR, 4.27; 95% CI, 4.07-4.48), and a lower risk for death from all causes that was approximately 27% lower than in the general population (SMR, 0.73; 95% CI, 0.72-0.74). The authors showed that their findings were not associated with socioeconomic status.4
The analysis by Patel et al4 is now the second study in the literature to show this discordant melanoma survival pattern. In an earlier Australian study of 2452 melanoma patients, Watts et al5 reported that melanoma detection during routine skin checks was associated with a 25% lower all-cause mortality (hazard ratio, 0.75; 95% CI, 0.63-0.90) but not melanoma-specific mortality after multivariable adjustment for a variety of factors including socioeconomic status.These analyses by 2 different groups of investigators have broad implications. Both groups suggested that the improved life span in melanoma patients may be due to health-seeking behavior, which has been defined as “any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.”6
Once treated for melanoma, it is clear that patients are likely to return at regular intervals for thorough full-body skin examinations, but this activity alone could not be responsible for improved all-cause mortality in the face of increased melanoma-specific mortality. It seems the authors are implying a broader concept of good health behavior, originally defined by MacKian7 as encompassing “activities undertaken to maintain good health, to prevent ill health, as well as dealing with any departure from a good state of health,” such as overt behavioral patterns, actions, and habits with the goal of maintenance, restoration, and improvement of one’s health. A variety of behaviors fall within such a definition including smoking cessation, decreased alcohol use, good diet, more physical activity, safe sexual behavior, scheduling physician visits, medication adherence, vaccination, and yes—screening examinations for health problems.8
The concept that individuals who are diagnosed with melanoma fall into a pattern of good health behavior is an interesting hypothesis that must remain speculative until the multiple aspects of good health behavior are rigorously studied. This concept coexists with the hypothesis of melanoma “overdiagnosis”—the idea that many melanomas are detected that will never lead to death.9 Both concepts deserve further analysis. Unquestionably, a randomized controlled trial could never recruit patients willing to undergo long-term untreated observation of their melanomas to test the hypothesis that their melanoma diagnosis would eventually lead to death. Furthermore, Patel et al4 do suggest that even MIS carries a small but measurable increased risk for death from the disease, which is not particularly supportive of the overdiagnosis hypothesis; however, analysis of the concept that improved individual health behavior is at least in part responsible for the first discovery of melanomas is certainly approachable. Here is the key question: Did the melanoma diagnosis trigger a sudden change in multiple aspects of health behavior that led to significant all-cause mortality benefits? The average age of the population studied by Patel et al4 was approximately 62 years. One wonders whether the consequences of a lifetime of established health behavior patterns can be rapidly modified—certainly possible but again remains to be proven by further studies.
Conversely, the alternative hypothesis is that discovery of MIS was the result of active pursuit of self-examination and screening procedures as part of individually ingrained good health behavior over a lifetime. Goodwin et al10 carried out a study in a sample of the Medicare population aged 69 to 90 years looking at men who had prostate cancer screening via prostate-specific antigen measurement and women who had undergone mammography in older age, compared to the contrast population who had not had these screening procedures. They tracked date of death in Medicare enrollment files. They identified 543,970 women and 362,753 men who were aged 69 to 90 years as of January 1, 2003. Patients were stratified by life expectancy based on age and comorbidity. Within each stratum, the patients with cancer screening had higher actual median survival than those who were not screened, with differences ranging from 1.7 to 2.1 years for women and 0.9 to 1.1 years for men.10 These results were not the result of lower prostate or breast cancer mortality. Rather, one surmises that other health factors yielded lower mortality in the screened cohorts.
A full-body skin examination is a time-consuming process. Patients who come to their physician for a routine annual physical don’t expect a skin examination and very few physicians have the time for a long detailed full-body skin examination. When the patient presents to a dermatologist for an examination, it often is because they have real concerns; for example, they may have had a family member who died of skin cancer, or the patient themself may have noticed a worrisome lesion. Patients, not physicians, are the drivers of skin cancer screening, a fact that often is dismissed by those who are not necessarily supportive of the practice.
In light of the findings of Patel et al,4 it is essential that the USPSTF reviews be reanalyzed to compare skin cancer–specific mortality, all-cause mortality, and lifespan in individuals who pursue skin cancer screening; the reanalysis also should not be exclusively limited to survival. With the advent of the immune checkpoint inhibitors, patients with metastatic melanoma are living much longer.11 The burden of living with metastatic cancer must be characterized and measured to have a complete picture and a valid analysis.
After the release of the USPSTF recommendation, there have been calls for large-scale studies to prove the benefits of skin cancer screening.12 Such studies may be valuable; however, if the hypothesis that overall healthy behavior as the major outcome determinant is substantiated, it may prove quite challenging to perform tests of association with specific interventions. It has been shown that skin cancer screening does lead to discovery of more melanomas,13 yet in light of the paradox described by Patel et al,4 it also is likely that causes of death other than melanoma impact overall mortality. Patients who pursue skin examinations may engage in multiple different health activities that are beneficial in the long term, making it difficult to analyze the specific benefit of skin cancer screening in isolation. It may prove difficult to ask patients to omit selected aspects of healthy behavior to try to prove the point. At this time, there is much more work to be done prior to offering opinions on the importance of skin cancer examination in isolation to improve overall health care. In the meantime, dermatologists owe it to our patients to continue to diligently pursue thorough and detailed skin examinations.
In April 2023, the US Preventive Services Task Force (USPSTF) issued a controversial recommendation that the current evidence is insufficient to assess the benefits vs harms of visual skin examination by clinicians for skin cancer screening in adolescents and adults who do not have signs or symptoms of skin cancer.1,2 This recommendation by the USPSTF has not changed in a quarter century,3 but a recent study described an interesting paradox that should trigger wide evaluation and debate.
Patel et al4 analyzed data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program from January 2000 to December 2018 to identify adults with a diagnosis of first primary melanoma in situ (MIS). Overall mortality was then determined through the National Vital Statistics System, which provides cause-of-death information for all deaths in the United States. The authors found 137,872 patients who had 1—and only 1—MIS discovered over the observation period. These patients predominantly were White (96.7%), and the mean (SD) age at diagnosis was 61.9 (16.5) years. During 910,308 total person-years of follow-up (mean [SD], 6.6 [5.1] years), 893 (0.6%) patients died of melanoma and 17,327 (12.6%) died of any cause. The 15-year melanoma-specific standardized mortality rate (SMR) was 1.89 (95% CI, 1.77-2.02), yet the 15-year overall survival relative to matched population controls was 112.4% (95% CI, 112.0%-112.8%), thus all-cause SMR was significantly lower at 0.68 (95% CI, 0.67-0.7). Although MIS was associated with a small increase in cohort melanoma mortality, overall mortality was actually lower than in the general population.4
Patel et al4 did a further broader search that included an additional 18,379 patients who also experienced a second primary melanoma, of which 6751 (36.7%) were invasive and 11,628 (63.3%) were in situ, with a melanoma-specific survival of 98.2% (95% CI, 97.6%-98.5%). Yet relative all-cause survival was significantly higher at 126.7% (95% CI, 125.5%-128.0%). Even among patients in whom a second primary melanoma was invasive, melanoma-specific survival was reduced to 91.1% (95% CI, 90.0%-92.1%), but relative all-cause survival was 116.7% (95% CI, 115%-118.4%). These data in the overall cohort of 155,251 patients showed a discordance between melanoma mortality, which was 4.27-times higher than in the general population (SMR, 4.27; 95% CI, 4.07-4.48), and a lower risk for death from all causes that was approximately 27% lower than in the general population (SMR, 0.73; 95% CI, 0.72-0.74). The authors showed that their findings were not associated with socioeconomic status.4
The analysis by Patel et al4 is now the second study in the literature to show this discordant melanoma survival pattern. In an earlier Australian study of 2452 melanoma patients, Watts et al5 reported that melanoma detection during routine skin checks was associated with a 25% lower all-cause mortality (hazard ratio, 0.75; 95% CI, 0.63-0.90) but not melanoma-specific mortality after multivariable adjustment for a variety of factors including socioeconomic status.These analyses by 2 different groups of investigators have broad implications. Both groups suggested that the improved life span in melanoma patients may be due to health-seeking behavior, which has been defined as “any action undertaken by individuals who perceive themselves to have a health problem or to be ill for the purpose of finding an appropriate remedy.”6
Once treated for melanoma, it is clear that patients are likely to return at regular intervals for thorough full-body skin examinations, but this activity alone could not be responsible for improved all-cause mortality in the face of increased melanoma-specific mortality. It seems the authors are implying a broader concept of good health behavior, originally defined by MacKian7 as encompassing “activities undertaken to maintain good health, to prevent ill health, as well as dealing with any departure from a good state of health,” such as overt behavioral patterns, actions, and habits with the goal of maintenance, restoration, and improvement of one’s health. A variety of behaviors fall within such a definition including smoking cessation, decreased alcohol use, good diet, more physical activity, safe sexual behavior, scheduling physician visits, medication adherence, vaccination, and yes—screening examinations for health problems.8
The concept that individuals who are diagnosed with melanoma fall into a pattern of good health behavior is an interesting hypothesis that must remain speculative until the multiple aspects of good health behavior are rigorously studied. This concept coexists with the hypothesis of melanoma “overdiagnosis”—the idea that many melanomas are detected that will never lead to death.9 Both concepts deserve further analysis. Unquestionably, a randomized controlled trial could never recruit patients willing to undergo long-term untreated observation of their melanomas to test the hypothesis that their melanoma diagnosis would eventually lead to death. Furthermore, Patel et al4 do suggest that even MIS carries a small but measurable increased risk for death from the disease, which is not particularly supportive of the overdiagnosis hypothesis; however, analysis of the concept that improved individual health behavior is at least in part responsible for the first discovery of melanomas is certainly approachable. Here is the key question: Did the melanoma diagnosis trigger a sudden change in multiple aspects of health behavior that led to significant all-cause mortality benefits? The average age of the population studied by Patel et al4 was approximately 62 years. One wonders whether the consequences of a lifetime of established health behavior patterns can be rapidly modified—certainly possible but again remains to be proven by further studies.
Conversely, the alternative hypothesis is that discovery of MIS was the result of active pursuit of self-examination and screening procedures as part of individually ingrained good health behavior over a lifetime. Goodwin et al10 carried out a study in a sample of the Medicare population aged 69 to 90 years looking at men who had prostate cancer screening via prostate-specific antigen measurement and women who had undergone mammography in older age, compared to the contrast population who had not had these screening procedures. They tracked date of death in Medicare enrollment files. They identified 543,970 women and 362,753 men who were aged 69 to 90 years as of January 1, 2003. Patients were stratified by life expectancy based on age and comorbidity. Within each stratum, the patients with cancer screening had higher actual median survival than those who were not screened, with differences ranging from 1.7 to 2.1 years for women and 0.9 to 1.1 years for men.10 These results were not the result of lower prostate or breast cancer mortality. Rather, one surmises that other health factors yielded lower mortality in the screened cohorts.
A full-body skin examination is a time-consuming process. Patients who come to their physician for a routine annual physical don’t expect a skin examination and very few physicians have the time for a long detailed full-body skin examination. When the patient presents to a dermatologist for an examination, it often is because they have real concerns; for example, they may have had a family member who died of skin cancer, or the patient themself may have noticed a worrisome lesion. Patients, not physicians, are the drivers of skin cancer screening, a fact that often is dismissed by those who are not necessarily supportive of the practice.
In light of the findings of Patel et al,4 it is essential that the USPSTF reviews be reanalyzed to compare skin cancer–specific mortality, all-cause mortality, and lifespan in individuals who pursue skin cancer screening; the reanalysis also should not be exclusively limited to survival. With the advent of the immune checkpoint inhibitors, patients with metastatic melanoma are living much longer.11 The burden of living with metastatic cancer must be characterized and measured to have a complete picture and a valid analysis.
After the release of the USPSTF recommendation, there have been calls for large-scale studies to prove the benefits of skin cancer screening.12 Such studies may be valuable; however, if the hypothesis that overall healthy behavior as the major outcome determinant is substantiated, it may prove quite challenging to perform tests of association with specific interventions. It has been shown that skin cancer screening does lead to discovery of more melanomas,13 yet in light of the paradox described by Patel et al,4 it also is likely that causes of death other than melanoma impact overall mortality. Patients who pursue skin examinations may engage in multiple different health activities that are beneficial in the long term, making it difficult to analyze the specific benefit of skin cancer screening in isolation. It may prove difficult to ask patients to omit selected aspects of healthy behavior to try to prove the point. At this time, there is much more work to be done prior to offering opinions on the importance of skin cancer examination in isolation to improve overall health care. In the meantime, dermatologists owe it to our patients to continue to diligently pursue thorough and detailed skin examinations.
- US Preventive Services Task Force; Mangione CM, Barry MJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295.
- Henrikson NB, Ivlev I, Blasi PR, et al. Skin cancer screening: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329:1296-1307.
- US Preventive Services Task Force Guide to Clinical Preventive Services. 2nd ed. Agency for Healthcare Research and Quality; 1996.
- Patel VR, Roberson ML, Pignone MP, et al. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;169:703-710.
- Watts CG, McLoughlin K, Goumas C, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157:1425-1436.
- Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351-773.
- MacKian S. A review of health seeking behaviour: problems and prospects. health systems development programme. University of Manchester; 2003. Accessed January 19, 2024. https://assets.publishing.service.gov.uk/media/57a08d1de5274a27b200163d/05-03_health_seeking_behaviour.pdf
- Conner M, Norman P. Health behaviour: current issues and challenges. Psychol Health. 2017;32:895-906.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613.
- Goodwin JS, Sheffield K, Li S, et al. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;11:1308-1314.
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267.
- Adamson AS. The USPSTF statement on skin cancer screening—not a disappointment but an opportunity. JAMA Dermatol. 2023;159:579-581. doi:10.1001/jamadermatol.2023.0706
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- US Preventive Services Task Force; Mangione CM, Barry MJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295.
- Henrikson NB, Ivlev I, Blasi PR, et al. Skin cancer screening: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2023;329:1296-1307.
- US Preventive Services Task Force Guide to Clinical Preventive Services. 2nd ed. Agency for Healthcare Research and Quality; 1996.
- Patel VR, Roberson ML, Pignone MP, et al. Risk of mortality after a diagnosis of melanoma in situ. JAMA Dermatol. 2023;169:703-710.
- Watts CG, McLoughlin K, Goumas C, et al. Association between melanoma detected during routine skin checks and mortality. JAMA Dermatol. 2021;157:1425-1436.
- Chrisman NJ. The health seeking process: an approach to the natural history of illness. Cult Med Psychiatry. 1977;1:351-773.
- MacKian S. A review of health seeking behaviour: problems and prospects. health systems development programme. University of Manchester; 2003. Accessed January 19, 2024. https://assets.publishing.service.gov.uk/media/57a08d1de5274a27b200163d/05-03_health_seeking_behaviour.pdf
- Conner M, Norman P. Health behaviour: current issues and challenges. Psychol Health. 2017;32:895-906.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613.
- Goodwin JS, Sheffield K, Li S, et al. Receipt of cancer screening is a predictor of life expectancy. J Gen Intern Med. 2016;11:1308-1314.
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254-267.
- Adamson AS. The USPSTF statement on skin cancer screening—not a disappointment but an opportunity. JAMA Dermatol. 2023;159:579-581. doi:10.1001/jamadermatol.2023.0706
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
Practice Points
- Screening for skin cancer often is performed at the patient’s request.
- Patients who want full-body skin examinations may exhibit other health-promoting behaviors.
- Studies claiming “overdiagnosis” of skin cancer have not previously evaluated all-cause mortality.
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17

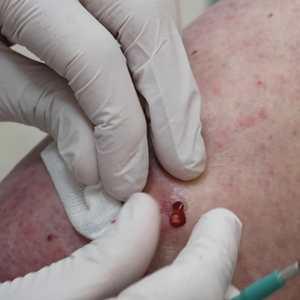
Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Practice Points
- As the ketogenic diet gains in popularity, dermatologists may inform patients that there is emerging evidence supporting the idea that low-glycemic diets may contribute to improvement in inflammatory skin conditions.
- Dermatologists may educate patients about the potential benefits of a low-glycemic diet as a supplementary treatment for acne based on existing evidence.
- Current evidence is insufficient to endorse a ketogenic diet as superior to other dietary approaches in treating inflammatory skin conditions.
Punch Biopsy Extraction With Fingers
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.
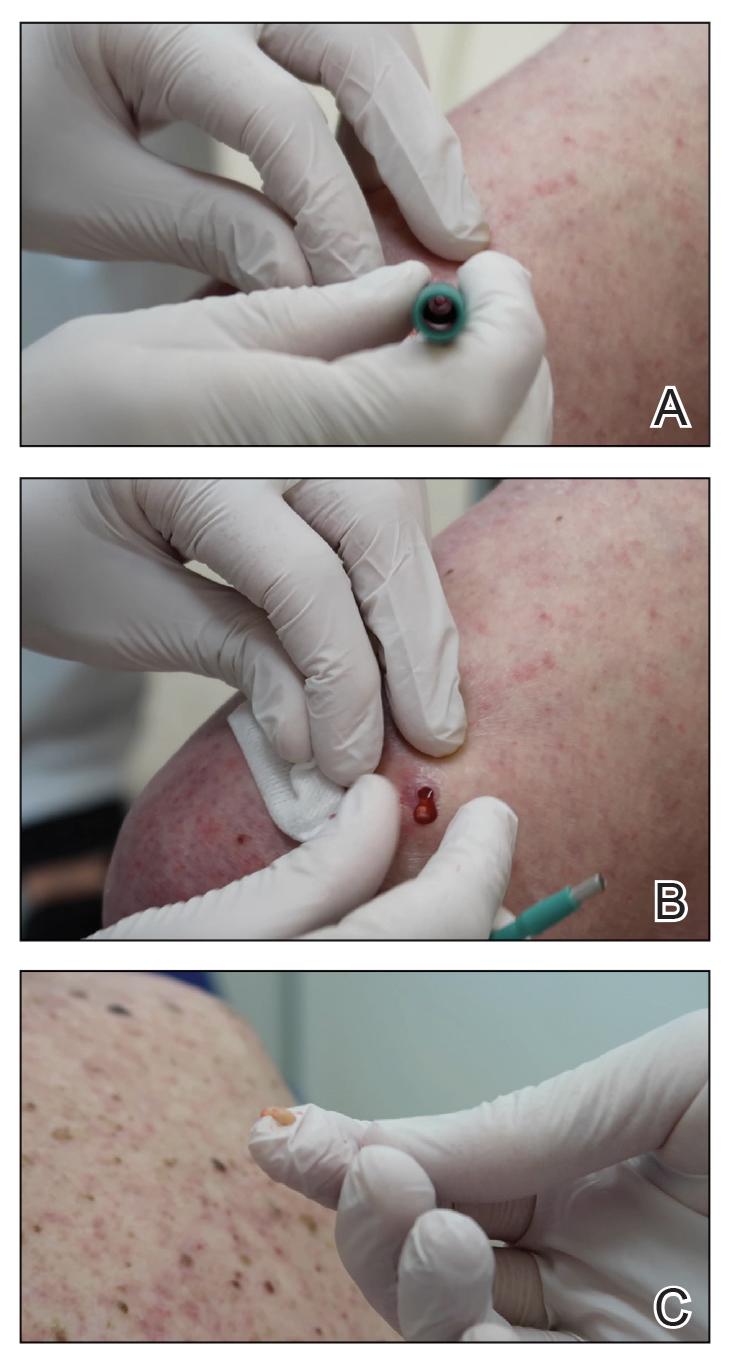
Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.

Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
Practice Gap
Punch biopsies are utilized frequently by dermatologists to aid in the diagnosis of various skin diseases.1 When performing a punch biopsy, dermatologists are taught to use either forceps or skin hooks in addition to scissors to extract the tissue from the skin.2 However, the use of these sterile instruments for a simple biopsy adds extra costs to the procedure. Herein, a cheaper and often faster method of obtaining the specimen from the patient is described.
The Technique
A 3- or 4-mm disposable punch biopsy tool is employed for this method. After locally anesthetizing the skin, the skin is punched to a subcutaneous depth utilizing the full length of the blade and a little extra pressure is applied downward while stretching the skin around (Figure, A). This may be helpful to dislodge the punch specimen from the surrounding skin. The specimen now can be easily removed by gently grasping it with the thumb and index finger (Figure, B and C). It then can be transferred immediately to the formalin container.

Practice Implications
This technique saves time as well as financial and environmental costs associated with the use of sterile instruments. An additional advantage to this simple method is avoiding specimen crush injuries, which are common when using forceps. This solution works in most cases but may not be suitable for certain special anatomic locations such as the scalp, nose, and ears.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
- Gronbeck C, Feng H. Volume and distribution of skin biopsies performed by dermatologists and other health care providers in the Medicare population in 2019. J Am Acad Dermatol. 2022;87:675-678.
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology. Elsevier; 2018.
Expanding the Psoriasis Framework: Immunopathogenesis and Treatment Updates
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.
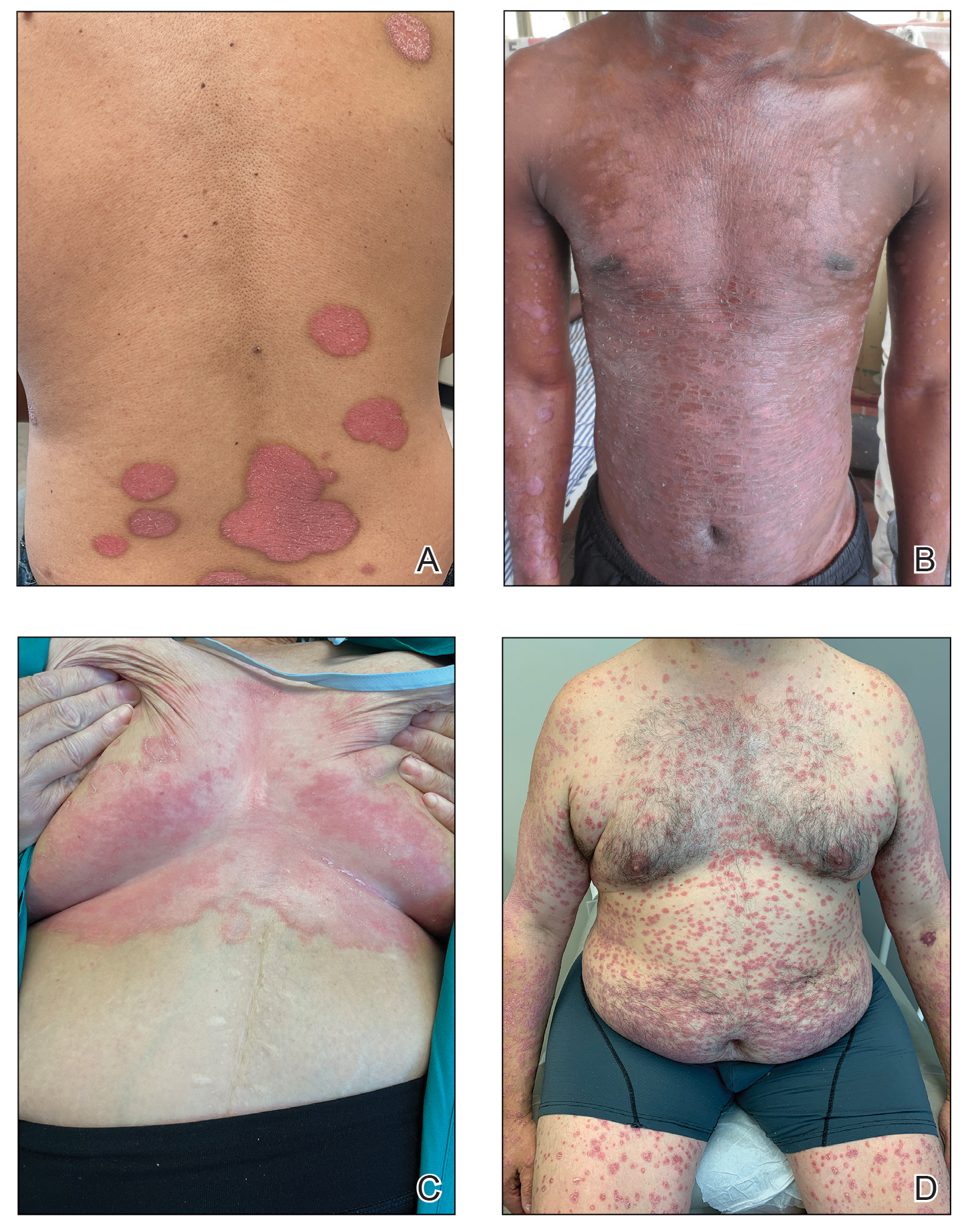
A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.
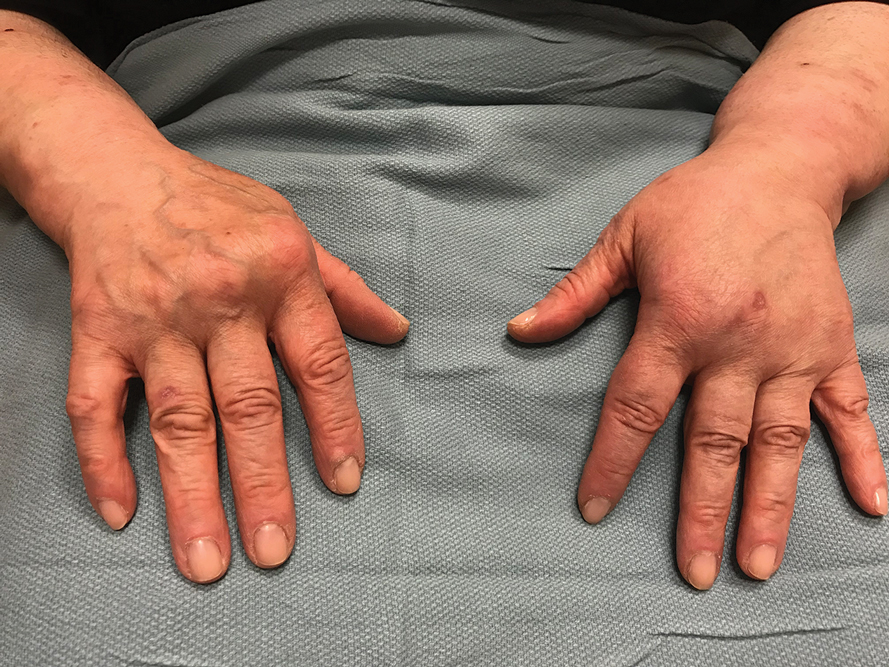
The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).
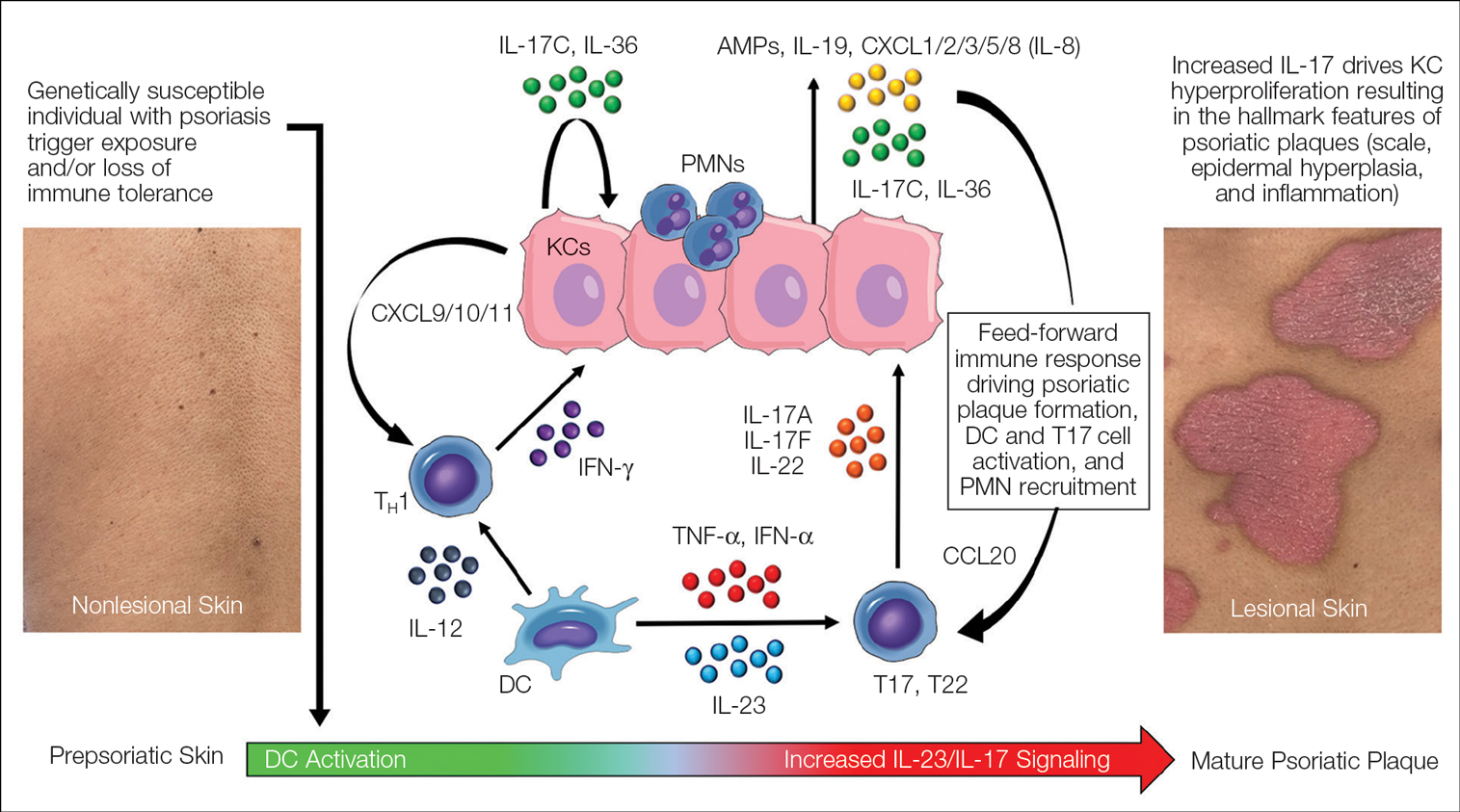
Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.
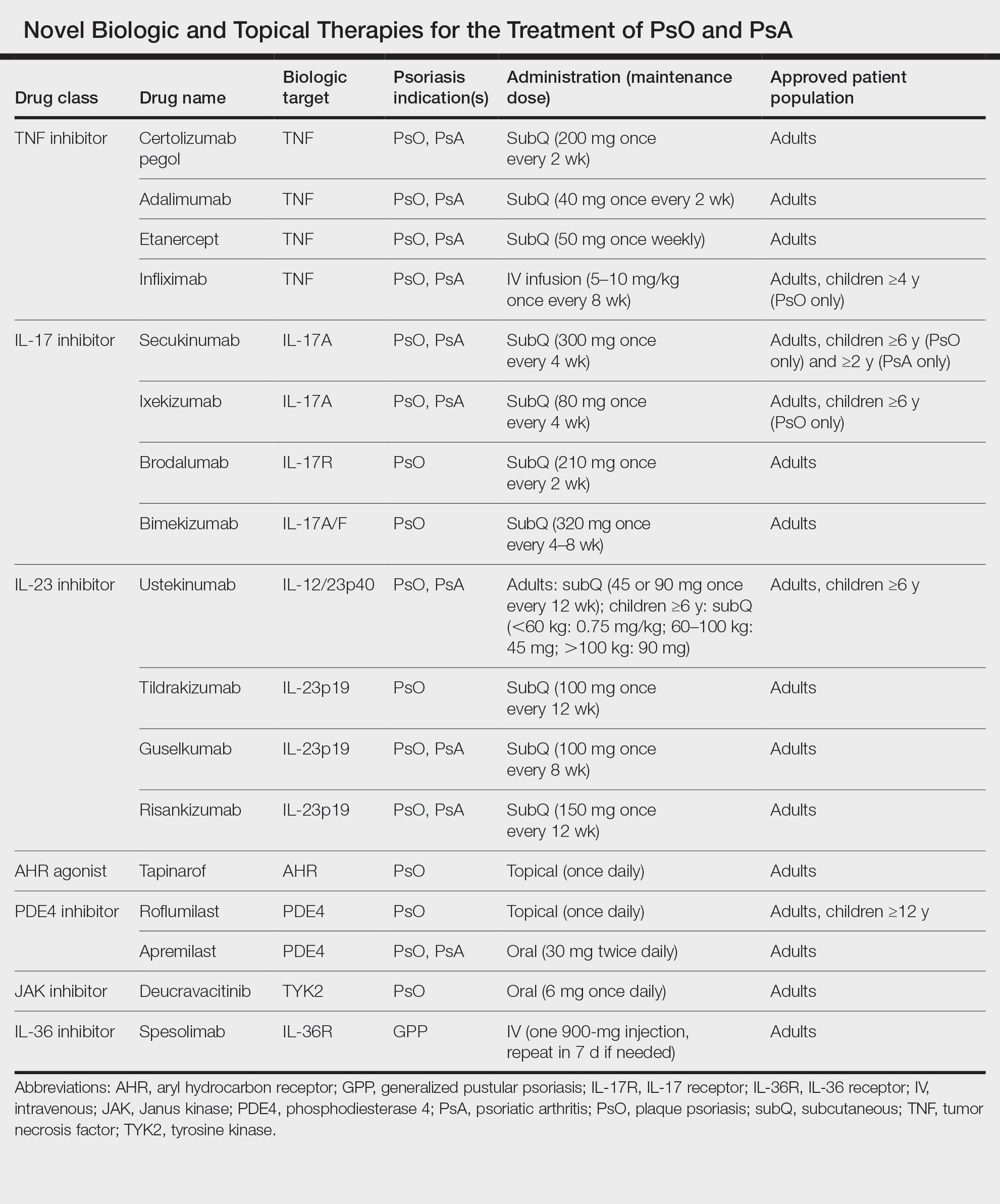
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

Psoriasis is a chronic inflammatory disease that affects approximately 3% of the US population.1 Plaque psoriasis comprises 80% to 90% of cases, while pustular, erythrodermic, guttate, inverse, and palmoplantar disease are less common variants (Figure 1). Psoriatic skin manifestations range from localized to widespread or generalized disease with recurrent flares. Body surface area or psoriasis area and severity index (PASI) measurements primarily focus on skin manifestations and are important for evaluating disease activity and response to treatment, but they have inherent limitations: they do not capture extracutaneous disease activity, systemic inflammation, comorbid conditions, quality of life impact, or the economic burden of psoriasis.

A common manifestation of psoriasis is psoriatic arthritis (PsA), which can involve the nails, joints, ligaments, or tendons in 30% to 41% of affected individuals (Figure 2).2,3 A growing number of psoriasis-associated comorbidities also have been reported including metabolic syndrome4; hyperlipidemia5; cardiovascular disease6; stroke7; hypertension8; obesity9; sleep disorders10; malignancy11; infections12; inflammatory bowel disease13; and mental health disorders such as depression,14 anxiety,15 and suicidal ideation.15 Psoriatic disease also interferes with daily life activities and a patient’s overall quality of life, including interpersonal relationships, intimacy, employment, and work productivity.16 Finally, the total estimated cost of psoriasis-related health care is more than $35 billion annually,17 representing a substantial economic burden to our health care system and individual patients.

The overall burden of psoriatic disease has declined markedly in the last 2 decades due to revolutionary advances in our understanding of the immunopathogenesis of psoriasis and the subsequent development of improved therapies that predominantly interrupt IL-23/IL-17 cytokine signaling; however, critical knowledge and treatment gaps persist, underscoring the importance of ongoing clinical and research efforts in psoriatic disease. We review the working immune model of psoriasis, summarize related immune discoveries, and highlight recent therapeutic innovations that are shaping psoriatic disease management.
Current Immune Model of Psoriatic Disease
Psoriasis is an autoinflammatory T cell–mediated disease with negligible contributions from the humoral immune response. Early clinical observations reported increased inflammatory infiltrates in psoriatic skin lesions primarily consisting of both CD4+ and CD8+ T-cell populations.18,19 Additionally, patients treated with broad-acting, systemic immunosuppressive medications (eg, cyclosporine, oral corticosteroids) experienced improvement of psoriatic lesions and normalization of the immune infiltrates observed in skin biopsy specimens.20,21 These early clinical findings led to more sophisticated experimentation in xenotransplant models of psoriasis,22,23 which explored the clinical efficacy of several less immunosuppressive (eg, methotrexate, anti–tumor necrosis factor [TNF] biologics)24 or T cell–specific agents (eg, alefacept, abatacept, efalizumab).25-27 The results of these translational studies provided indisputable evidence for the role of the dysregulated immune response as the primary pathogenic process driving plaque formation; they also led to a paradigm shift in how the immunopathogenesis of psoriatic disease was viewed and paved the way for the identification and targeting of other specific proinflammatory signals produced by activated dendritic cell (DC) and T-lymphocyte populations. Among the psoriasis-associated cytokines subsequently identified and studied, elevated IL-23 and IL-17 cytokine levels in psoriatic skin were most closely associated with disease activity, and rapid normalization of IL-23/IL-17 signaling in response to effective oral or injectable antipsoriatic treatments was the hallmark of skin clearance.28 The predominant role of IL-23/IL-17 signaling in the development and maintenance of psoriatic disease is the central feature of all working immune models for this disease (Figure 3).

Psoriasis-Associated Genetic and Environmental Risk Factors
The exact sequence of events that lead to the initiation and formation of plaque psoriasis in susceptible individuals is still poorly understood; however, several important risk factors and key immune events have been identified. First, decades of genetic research have reported more than 80 known psoriasis-associated susceptibility loci,29 which explains approximately 50% of psoriasis heritability. The major genetic determinant of psoriasis, HLA-C*06:02 (formerly HLA-Cw6), resides in the major histocompatibility complex class I region on chromosome 6p21.3 (psoriasis susceptibility gene 1, PSORS1) and is most strongly associated with psoriatic disease.30 Less common psoriasis-associated susceptibility genes also are known to directly or indirectly impact innate and adaptive immune functions that contribute to the pathogenesis of psoriasis.
Second, several nongenetic environmental risk factors for psoriasis have been reported across diverse patient populations, including skin trauma/injury, infections, alcohol/tobacco use, obesity, medication exposure (eg, lithium, antimalarials, beta-blockers), and stress.31 These genetic and/or environmental risk factors can trigger the onset of psoriatic disease at any stage of life, though most patients develop disease in early adulthood or later (age range, 50–60 years). Some patients never develop psoriasis despite exposure to environmental risk factors and/or a genetic makeup that is similar to affected first-degree relatives, which requires further study.
Prepsoriatic Skin and Initiation of Plaque Development
In response to environmental stimuli and/or other triggers of the immune system, DC and resident IL-17–producing T-cell (T17) populations become activated in predisposed individuals. Dendritic cell activation leads to the upregulation and increase of several proinflammatory cytokines, including TNF, interferon (IFN) α, IFN-γ, IL-12, and IL-23. Tumor necrosis factor and IL-23 play a vital role in psoriasis by helping to regulate the polarization and expansion of T22 and T17 cells in the skin, whereas IL-12 promotes a corresponding type 1 inflammatory response.32 Increased IL-17 and IL-22 result in alteration of the terminal differentiation and proliferative potential of epidermal keratinocytes, leading to the early clinical hallmarks of psoriatic plaques. The potential contribution of overexpressed psoriasis-related autoantigens, such as LL-37/cathelicidin, ADAMTSL5, and PLA2G4D,33 in the initiation of psoriatic plaques has been suggested but is poorly characterized.34 Whether these specific autoantigens or others presented by HLA-C variants found on antigen-presenting cells are required for the breakdown of immune tolerance and psoriatic disease initiation is highly relevant but requires further investigation and validation.
Feed-Forward Inflammation, Mature Psoriatic Plaques, and Resident Memory T Cells
In response to the upstream production of IL-23 by dermal DCs, high levels of IL-17 cytokines can be found in mature psoriatic plaques. The IL-17 family consists of 6 dimeric cytokines (IL-17A through IL-17F) that provide innate cutaneous protection against bacterial, viral, and fungal infectious agents, such as Candida albicans. Unlike other IL-17 isoforms, IL-17A and IL-17F share the same receptor complex and have the highest structural homology of any pair (approximately 50% similar).35 The relative expression of IL-17F is higher than IL-17A in psoriasis,36 though IL-17A has been considered as the predominant IL-17 cytokine found in psoriatic skin lesions due to its higher potency.
Binding of IL-17A/F with the IL-17 receptor (IL-17R) on keratinocytes contributes to the development of psoriatic plaques by inducing epidermal hyperplasia via activation of CCAAT/enhancer-binding proteins β and δ, nuclear factor κB, and signal transducer and activator of transcription 1 gene (STAT1).37,38 This also increases the expression of other keratinocyte-derived proteins (eg, human β-defensins, S-100 proteins, LL-37, other antimicrobial peptides, IL-19, IL-36, IL-17C) that act as reinforcing proinflammatory signals or chemotactic factors (eg, chemokine [C-C motif] ligand 20 [CCL20], chemokine [C-C motif] ligand 1/2/3/5 [CXCL1/2/3/5], CXCL8, IL-8) that facilitate the recruitment of additional immune cells to the skin including polymorphonuclear neutrophils (PMNs), macrophages, and DCs.39-41 Routine immunohistochemical staining for these keratinocyte-derived proteins reveals a striking epidermal gene expression gradient wherein levels of IL-17–induced proteins are most highly expressed in the uppermost layers of keratinocytes and facilitate the recruitment of immune cells into the epidermis. Activated T17 cells also stimulate the production of keratinocyte-derived chemokines (eg, CXCL9/10/11), which recruit type 1 inflammatory T-cell populations into developing psoriatic plaques.42,43 Finally, TNF, IL-36, and IL-17C cytokines act synergistically with IL-17A/F to amplify the proinflammatory effects of IL-17 signaling and further stimulate their production from T17 cell populations.40 This inflammatory circuit in the skin creates and supports a self-amplifying or positive feedback loop between the skin and immune system that commonly is referred to as feed-forward inflammation (Figure 3).34 The feed-forward inflammatory loop in psoriasis—predominantly driven by increased IL-23/IL-17 signaling—best characterizes the mature psoriatic plaque.
Several findings suggest that the influx of persistent, long-lived resident memory T cells (Trms) may contribute to the mature psoriatic plaque. It is believed that CD8+CD103+CD49a− Trm cell populations may be responsible for the sharply demarcated borders of untreated psoriasis plaques or their recurrence at specific body sites such as the scalp, buttocks, extremity extensor surfaces, umbilicus, or acral skin following specific stimuli or trauma (Koebner phenomenon or isomorphic response).44,45 It is not known if repeated stimuli or trauma induce disease formation via the activation of Trm cell populations; further study in large patient cohorts is needed, but this remains an intriguing area of study for durable treatment responses and potential cures for psoriasis.
Recent Discoveries in Psoriatic Disease
Remarkable treatment outcomes for psoriasis have been achieved with multiple selective IL-17 and IL-23 inhibitors (eTable). As demonstrated in several pivotal phase 3 clinical trials for members of these classes of medications, the majority of treated psoriasis patients achieved PASI90 clearance.46 Due to their more favorable dosing schedule (ie, fewer injections) and ability to induce a durable remissionlike treatment response, IL-23 inhibitors have become the preferred treatment class for cutaneous disease, while IL-17 inhibitors may be preferred when treating patients with both plaque psoriasis and PsA.47,48 Nevertheless, the complexity of this disease is punctuated by treated patients who do not adequately respond to selective IL-23/IL-17 blockade.49 Recent and emerging treatments may shed light on these recalcitrant cases and will add to the rapidly growing arsenal of available psoriasis therapies.
The Role of IL-17F in Psoriasis and Other Inflammatory Skin Diseases
Dysregulation of IL-17A and IL-17F is associated with several chronic inflammatory conditions, such as psoriasis and PsA.35,50 Both cytokines, either as homodimers or heterodimers, can selectively bind to the heterodimeric IL-17R formed by the IL-17RA and IL-17RC subunits.35 IL-17F and IL-17C also can synergize with TNF and other cytokines to promote and support the self-sustaining inflammatory circuits in mature psoriatic plaques, though their inflammatory effects in the skin are more limited than IL-17A.51,52 Therefore, incomplete blockade of IL-17 signaling (ie, unopposed IL-17F and IL-17C) represents a potential mechanism to explain the persistence of psoriasis in patients treated with selective IL-17A inhibitors. This hypothesis is supported by reports of psoriasis patients who have inadequate clinical responses to selective IL-17A inhibition but subsequently improve with IL-17R blockade, which results in disruption of IL-17A as well as IL-17C/E/F cytokine signaling. This formed the basis for further study into the specific role of IL-17F in psoriatic disease and any potential therapeutic benefits associated with its inhibition.
Recently approved in the European Union, Canada, Australia, Japan, the United Kingdom, and the United States for moderate to severe psoriasis, bimekizumab is a novel humanized IgG antibody that selectively inhibits both IL-17A and IL-17F cytokines.53 Specifically, bimekizumab simultaneously prevents binding of IL-17A/A, IL-17A/F, and IL-17F/F dimers with the IL-17R. Compared to other IL-17 and IL-23 biologic therapies, bimekizumab (320 mg) achieved relatively higher response rates for PASI75, PASI90, and PASI100.49 Neutralization of IL-17A and IL-17F by bimekizumab also resulted in more complete suppression of cytokine responses and PMN chemotaxis than either cytokine alone in treated PsA patients,54 which is notable because of the incremental benefits of recent IL-23 and IL-17 inhibitors on inflammatory arthritis symptoms in contrast to the substantial improvements observed for cutaneous disease with those same agents.
The primary disadvantage of bimekizumab and its more complete blockade of the IL-17 signaling pathway is that treated patients have a substantially increased risk for oral candidiasis (>10%).55 However, the precise link between candidiasis and IL-17 blockade is not yet fully understood because other targeted agents that also broadly suppress IL-17 signaling (ie, IL-17R, IL-23 inhibitors) are associated with much lower rates of candidiasis.56-58 Bimekizumab also is being investigated as a novel therapy for hidradenitis suppurativa and will provide important reference information regarding the role for bispecific biologic agents in the treatment of chronic inflammatory skin diseases.59
IL-36 Signaling and Generalized Pustular Psoriasis
Recent genetic and clinical studies have expanded our understanding of the role of IL-36 signaling in the immunopathogenesis of pustular psoriasis variants. Generalized pustular psoriasis (GPP) is a rare distinct psoriasis subtype characterized by the recurrent development of widespread erythema, superficial sterile pustules, and desquamation. Systemic symptoms such as fever, malaise, itching, and skin pain accompany acute GPP flares.60 Generalized pustular psoriasis is more common in female patients (in contrast with plaque psoriasis), and acute flares may be caused by multiple stimuli including infections, hypocalcemia, initiation or discontinuation of medications (eg, oral corticosteroids), pregnancy, or stress.61,62 Flares of GPP often require emergency or in-patient care, as untreated symptoms increase the risk for severe health complications such as secondary infections, sepsis, or multisystem organ failure.63 The prevalence of GPP is estimated to be approximately 1 in 10,000 individuals in the United States,64-67 with mortality rates ranging from 0 to 3.3 deaths per 100 patient-years.67
In contrast to plaque psoriasis, aberrant IL-36 signaling is the predominant driver of GPP. IL-36 is a member of the IL-1 cytokine family that includes three IL-36 agonists (IL-36α, IL-36β, IL-36γ) and 1 endogenous antagonist (IL-36Ra, encoded by IL36RN).68 The immunopathogenesis of GPP involves dysregulation of the IL-36–chemokine–PMN axis, resulting in unopposed IL-36 signaling and the subsequent recruitment and influx of PMNs into the epidermis. IL36RN mutations are strongly associated with GPP and result in impaired function of the IL-36Ra protein, leading to unopposed IL-36 signaling.69 However, approximately two-thirds of GPP patients lack identifiable gene mutations, suggesting other immune mechanisms or triggers causing upregulated IL-36 signaling.70 In response to these triggers, increased IL-36 cytokines released by keratinocytes bind to the IL-36R, resulting in substantial keratinocyte hyperproliferation, increased IL-36 levels, and the expression of hundreds of additional inflammatory signals (eg, IL-17C, antimicrobial peptides, TNF, IL-6).71 Increased IL-36 levels also drive the production of PMN chemotactic proteins (eg, CXCL1/2/3/5/6/8 and CXCR1/2) and act synergistically with IL-17 cytokines to create an autoamplifying circuit that is analogous to the feed-forward inflammatory loop in plaque psoriasis.72 Biopsies of involved GPP skin reveal increased expression of IL-36 in the uppermost layers of the epidermis, which creates a gene expression gradient that acts as a strong attractant for PMNs and forms the basis for the hallmark pustular lesions observed in GPP patients.
Until recently, treatment strategies for GPP involved the off-label use of topical, oral, or biologic therapies approved for plaque psoriasis, which often was associated with variable or incomplete disease control. In September 2022, the US Food and Drug Administration (FDA) approved intravenous spesolimab as a first-in-class humanized monoclonal IgG1 antibody for the treatment of GPP flares in adults. Spesolimab binds to IL-36R and prevents its activation by its endogenous agonists. A phase 2, randomized, 12-week clinical trial (Effisayil-1) evaluated the efficacy and safety of a single 900-mg intravenous dose of spesolimab followed by an optional second dose 1 week later for inadequate treatment responses in 53 enrolled GPP patients (2:1 treatment to placebo randomization).73 Remarkably, more than half (19/35 [54%]) of GPP patients experienced complete resolution of pustules (GPP physician global assessment subscore of 0 [range, 0–4]) and showed sustained efficacy out to week 12 after just 1 or 2 doses of spesolimab. Overall, the safety profile of spesolimab was good; asthenia, fatigue, nausea, vomiting, headache, pruritus, infusion-related reaction and symptoms, and mild infections (eg, urinary tract infection) were the most common adverse events reported.73
Imsidolimab, a high-affinity humanized IgG4 monoclonal antibody that binds and blocks activation of IL-36R, also has completed phase 2 testing,74 with phase 3 study results expected in early 2024. The rapid onset of action and overall safety of imsidolimab was in line with and similar to spesolimab. Future approval of imsidolimab would add to the limited treatment options available for GPP and has the additional convenience of being administered to patients subcutaneously. Overall, the development of selective IL-36R inhibitors offers a much-needed therapeutic option for GPP and illustrates the importance of translational research.
Role of Tyrosine Kinase in Psoriatic Disease
The Janus kinase (JAK) enzyme family consists of 4 enzymes—tyrosine kinase 2 (TYK2), JAK1, JAK2, and JAK3—that function as intracellular transduction signals that mediate the biologic response of most extracellular cytokines and growth factors.75 Critical psoriasis-related cytokines are dependent on intact JAK-STAT signaling, including IL-23, IL-12, and type I IFNs. In 2010, a genome-wide association identified TYK2 as a psoriasis susceptibility locus,76 and loss-of-function TYK2 mutations confer a reduced risk for psoriasis.77 Unlike other JAK isoforms, TYK2 mediates biologic functions that are highly restricted to the immune responses associated with IL-23, IL-12, and type I IFN signaling.78,79 For these reasons, blockade of TYK2 signaling is an attractive therapeutic target for the potential treatment of psoriatic disease.
In September 2022, the FDA approved deucravacitinib as a first-in-class, oral, selective TYK2 inhibitor for the treatment of adult patients with moderate to severe plaque psoriasis. It was the first FDA approval of an oral small-molecule treatment for plaque psoriasis in nearly a decade. Deucravacitinib inhibits TYK2 signaling via selective binding of its unique regulatory domain, resulting in a conformational (allosteric) change that interferes with its active domain.80 This novel mechanism of action limits the unwanted blockade of other broad biologic processes mediated by JAK1/2/3. Of note, the FDA did not issue any boxed warnings for deucravacitinib as it did for other FDA-approved JAK inhibitors.
In a head-to-head, 52-week, double-blind, prospective, randomized, phase 3 study, deucravacitinib showed clear superiority over apremilast for PASI75 at week 16 (53.0% [271/511] vs 39.8% [101/254]) and week 24 (58.7% [296/504] vs 37.8% [96/254]).81 Clinical responses were sustained through week 52 and showed efficacy for difficult-to-treat areas such as the scalp, acral sites, and nails. Other advantages of deucravacitinib include once-daily dosing with no need for dose titration or adjustments for renal insufficiency as well as the absence of statistically significant differences in gastrointestinal tract symptoms compared to placebo. The most common adverse effects included nasopharyngitis, upper respiratory tract infections, headache, diarrhea, and herpes infections.81 The potential benefit of deucravacitinib for PsA and psoriasis comorbidities remains to be seen, but it is promising due to its simultaneous disruption of multiple psoriasis-related cytokine networks. Several other TYK2 inhibitors are being developed for psoriatic disease and related inflammatory conditions, underscoring the promise of targeting this intracellular pathway.
Aryl Hydrocarbon Receptor Agonism
Topical steroids are the mainstay treatment option for localized or limited plaque psoriasis due to their potent immunosuppressive effect on the skin and relatively low cost. Combined with vitamin D analogs, topical steroids result in marked improvements in disease severity and improved tolerability.82 However, chronic use of topical steroids is limited by the need for twice-daily application, resulting in poor treatment compliance; loss of efficacy over time; risk for steroid-induced skin atrophy on special body sites; and patient concerns of potential systemic effects. The discovery of novel drug targets amenable to topical inhibition is needed.
Dysregulated aryl hydrocarbon receptor (AHR) levels have been reported in atopic dermatitis and psoriasis.83 Aryl hydrocarbon receptors are ubiquitously expressed in many cell types and play an integral role in immune homeostasis within the skin, skin barrier function, protection against oxidative stressors, and regulation of proliferating melanocytes and keratinocytes.84,85 They are widely expressed in multiple immune cell types (eg, antigen-presenting cells, T lymphocytes, fibroblasts) and modulate the differentiation of T17 and T22 cells as well as their balance with regulatory T-cell populations.86 In keratinocytes, AHR helps to regulate terminal differentiation, enhance skin barrier integrity via AHR-dependent filaggrin (FLG) expression, and prevent transepidermal water loss.87,88 The mechanisms by which AHR ligands lead to the upregulation or downregulation of specific genes is intricate and highly context dependent, such as the specific ligand and cell type involved. In preclinical studies, AHR-deficient mice develop psoriasiform skin inflammation, increased IL-17 and IL-22 expression, and abnormal skin barrier function.89 Keratinocytes treated with AHR ligands in vitro modulated psoriasis-associated inflammatory cytokines, such as IL-6, IL-8, and type I and II IFNs.89,90 The use of coal tar, one of the earliest historical treatments for psoriasis, is thought to activate AHRs in the skin via organic compound mixtures containing polyaromatic hydrocarbons that help normalize the proinflammatory environment in psoriatic skin.91
In June 2022, the FDA approved tapinarof as a first-in-class, topical, nonsteroidal AHR agonist for the treatment of plaque psoriasis in adults. Although the exact mechanism of action for tapinarof has not been fully elucidated, early studies suggest that its primary function is the activation of AHR, leading to reduced T-cell expansion and T17 cell differentiation. In the imiquimod mouse model, cytokine expression of IL-17A, IL-17F, IL-19, IL-22, IL-23A, and IL-lβ in psoriasiform skin lesions were downregulated following tapinarof treatment.92 In humans, tapinarof treatment is associated with a remittive effect, in which the average time for tapinarof-treated psoriasis lesions to remain clear was approximately 4 months.93 Preliminary research investigating the mechanism by which tapinarof induces this remittive effect is ongoing and may involve the reduced activation and influx of T17 and Trm populations into the skin.94 However, these preclinical studies were performed on healthy dermatome-derived skin tissue cultured in T17-skewing conditions and needs to be replicated in larger samples sizes using human-derived psoriatic tissue. Alternatively, a strong inhibitory effect on IL-23 cytokine signaling may, in part, explain the remittive effect of tapinarof, as an analogous response is observed in patients who start and discontinue treatment with selective IL-23 antagonists. Regardless, the once-daily dosing of tapinarof and sustained treatment response is appealing to psoriasis patients. Tapinarof generally is well tolerated with mild folliculitis (>20% of patients) and contact dermatitis (5% of patients) reported as the most common skin-related adverse events.
New Roles for Phosphodiesterase 4 Inhibition
Phosphodiesterases (PDEs) are enzymes that hydrolyze cyclic nucleotides (eg, cyclic adenosine monophosphate) to regulate intracellular secondary messengers involved in the inflammatory response. One of several enzymes in the PDE family, PDE4, has been shown to have greater activity in psoriatic skin compared to healthy skin.95 Phosphodiesterase inhibitors decrease the degradation of cyclic adenosine monophosphate, which triggers protein kinase A to downregulate proinflammatory (eg, TNF-α, IL-6, IL-17, IL-12, IL-23) cytokines and increased expression of anti-inflammatory signals such as IL-10.96,97 Apremilast, the first oral PDE4 inhibitor approved by the FDA for psoriasis, offered a safe alternative to traditional oral immunosuppressive agents that had extensive risks and potential end-organ adverse effects. Unfortunately, apremilast demonstrated modest efficacy for psoriatic disease (better efficacy in the skin vs joint manifestations) and was supplanted easily by next-generation targeted biologic agents that were more efficacious and lacked the troublesome gastrointestinal tract adverse effects of PDE4 inhibition.98
Crisaborole became the first topical PDE4 inhibitor approved in the United States in December 2016 for twice-daily treatment of atopic dermatitis. Although phase 2 trial results were reported in psoriasis, this indication was never pursued, presumably due to similar improvements in primary outcome measures at week 12, compared to placebo (ClinicalTrials.gov Identifier NCT01300052).
In July 2022, the first topical PDE4 inhibitor indicated for plaque psoriasis was approved by the FDA—roflumilast cream 0.3% for once-daily use in individuals 12 years and older. Roflumilast was found to be clinically efficacious as early as 2 weeks after its use in an early-phase clinical trial.99 In 2 phase 3 clinical trials (DERMIS-1 and DERMIS-2), roflumilast significantly increased the proportion of patients achieving PASI75 at week 8 compared to vehicle (39%–41.6% vs 5.3%–7.6%, respectively)(P<.001).100 Overall, this nonsteroidal topical therapy was found to be well tolerated, with infrequent reports of application site pain or irritation as adverse events. Similar to tapinarof, patients can apply roflumilast on all body surface areas including the face, external genitalia, and other intertriginous areas.100 Importantly, the broad immune impact of PDE4 inhibition suggests that topical roflumilast likely will be an effective treatment for several additional inflammatory conditions, including seborrheic dermatitis and atopic dermatitis, which would expand the clinical utility of this specific medication.
Conclusion
In the last 2 decades, we have witnessed a translational revolution in our understanding of the underlying genetics and immunology of psoriatic disease. Psoriasis is widely considered one of the best-managed inflammatory conditions in all of medicine due to the development and availability of highly targeted, effective topical and systemic therapies that predominantly disrupt IL-23/IL-17 cytokine signaling in affected tissues. However, future clinical studies and laboratory research are necessary to elucidate the precise cause of psoriasis as well as the underlying genetic and immune signaling pathways driving less common clinical variants and recalcitrant disease.

- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Ogdie A, Langan S, Love T, et al. Prevalence and treatment patterns of psoriatic arthritis in the UK. Rheumatology (Oxford). 2013;52:568-575. doi:10.1093/rheumatology/kes324
- Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologistdiagnosed psoriatic arthritis in patients with psoriasis in European/ North American dermatology clinics. J Am Acad Dermatol. 2013;69:729- 735. doi:10.1016/j.jaad.2013.07.023
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132(3 pt 1):556-562. doi:10.1038/jid.2011.365
- Wu S, Li WQ, Han J, et al. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66:304-310. doi:10.1002/art.38227
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and hypertension: a systematic review and meta-analysis of observational studies. J Hypertens. 2013;31:433-442; discussion 442-443. doi:10.1097/HJH.0b013e32835bcce1
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418. doi:10.1038 /jid.2009.112
- Armstrong AW, Lin SW, Chambers CJ, et al. Psoriasis and hypertension severity: results from a case-control study. PLoS One. 2011;6:E18227. doi:10.1371/journal.pone.0018227
- Naldi L, Chatenoud L, Linder D, et al. Cigarette smoking, body mass index, and stressful life events as risk factors for psoriasis: results from an Italian case-control study. J Invest Dermatol. 2005;125:61-67. doi:10.1111/j.0022-202X.2005.23681.x
- Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285-289. doi:10.1016/j.sleep.2011.07.018
- Vaengebjerg S, Skov L, Egeberg A, et al. Prevalence, incidence, and risk of cancer in patients with psoriasis and psoriatic arthritis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156:421-429. doi:10.1001/jamadermatol.2020.0024
- Loft N, Skov L, Richardson C, et al. A nationwide population-based cohort study of the incidence of severe and rare infections among adults with psoriasis in Denmark. Br J Dermatol. 2022;187:353-363. doi:10.1111/bjd.21595
- Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417-1423. doi:10.1001/jamadermatol.2018.3631
- Dowlatshahi EA, Wakkee M, Arends LR, et al. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis. J Invest Dermatol. 2014;134:1542-1551. doi:10.1038/jid.2013.508
- Kurd SK, Troxel AB, Crits-Christoph P, et al. The risk of depression, anxiety, and suicidality in patients with psoriasis: a populationbased cohort study. Arch Dermatol. 2010;146:891-895. doi:10.1001 /archdermatol.2010.186
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113. doi:10.1016/j.jaad.2018.11.058
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States. J Am Acad Dermatol. 2015;72:961-967. doi:10.1016/j.jaad.2015.02.1099
- Bos JD, Hagenaars C, Das PK, et al. Predominance of “memory” T cells (CD4+, CDw29+) over “naive” T cells (CD4+, CD45R+) in both normal and diseased human skin. Arch Dermatol Res. 1989;281:24-30. doi:10.1007/BF00424268
- Bos JD, Hulsebosch HJ, Krieg SR, et al. Immunocompetent cells in psoriasis. in situ immunophenotyping by monoclonal antibodies. Arch Dermatol Res. 1983;275:181-189. doi:10.1007/BF00510050
- Griffiths CE, Powles AV, Leonard JN, et al. Clearance of psoriasis with low dose cyclosporin. Br Med J (Clin Res Ed). 1986;293:731-732. doi:10.1136/bmj.293.6549.731
- Ellis CN, Gorsulowsky DC, Hamilton TA, et al. Cyclosporine improves psoriasis in a double-blind study. JAMA. 1986;256:3110-3116.
- Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233-240. doi:10.1084/jem.20041257
- Ferber IA, Brocke S, Taylor-Edwards C, et al. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol. 1996;156:5-7.
- Bharadwaj R, Lusi CF, Mashayekh S, et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity. 2023;56:998-1012. doi:10.1016/j. immuni.2023.04.001
- Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3:411-420.
- Pariser DM, Gordon KB, Papp KA, et al. Clinical efficacy of efalizumab in patients with chronic plaque psoriasis: results from three randomized placebo-controlled phase III trials: part I. J Cutan Med Surg. 2005; 9:303-312. doi:10.1007/s10227-005-0116-1
- Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis. 2017;76:1550-1558. doi:10.1136/annrheumdis-2016-210724
- Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol. 2018;201:1605-1613. doi:10.4049/jimmunol.1800013
- Ogawa K, Okada Y. The current landscape of psoriasis genetics in 2020. J Dermatol Sci. 2020;99:2-8. doi:10.1016/j.jdermsci.2020.05.008
- Arakawa A, Siewert K, Stohr J, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212:2203-2212. doi:10.1084/jem.20151093
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263-271. doi:10.1016/S0140-6736(07)61128-3
- Wang CQF, Akalu YT, Suarez-Farinas M, et al. IL-17 and TNF synergistically modulate cytokine expression while suppressing melanogenesis: potential relevance to psoriasis. J Invest Dermatol. 2013;133:2741-2752. doi:10.1038/jid.2013.237
- Cheung KL, Jarrett R, Subramaniam S, et la. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213:2399-2412. doi:10.1084/jem.20160258
- Hawkes JE, Gonzalez JA, Krueger JG. Autoimmunity in psoriasis: evidence for specific autoantigens. Curr Dermatol Rep. 2017;6:104-112. doi:10.1007/s13671-017-0177-6
- Johansen C, Usher PA, Kjellerup RB, et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319-324. doi:10.1111/j.1365-2133 .2008.08902.x
- Kolbinger F, Loesche C, Valentin MA, et al. beta-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139:923-932. doi:10.1016/j .jaci.2016.06.038
- Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559-2567. doi:10.1074/jbc.M308809200
- Shen F, Hu Z, Goswami J, et al. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J Biol Chem. 2006;281:24138-24148. doi:10.1074/jbc.M604597200
- Harper EG, Guo C, Rizzo H, et al. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol. 2009;129:2175-2183. doi:10.1038/jid.2009.65
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M, et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol. 2011;131:677-687. doi:10.1038/jid.2010.340
- Homey B, Dieu-Nosjean MC, Wiesenborn A, et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J Immunol. 2000;164:6621-6632. doi:10.4049 /jimmunol.164.12.6621
- Stephen-Victor E, Fickenscher H, Bayry J. IL-26: an emerging proinflammatory member of the IL-10 Cytokine family with multifaceted actions in antiviral, antimicrobial, and autoimmune responses. PLoS Pathog. 2016;12:E1005624. doi:10.1371/journal.ppat.1005624
- Wolk K, Witte K, Witte E, et al. IL-29 is produced by T(H)17 cells and mediates the cutaneous antiviral competence in psoriasis [published online September 25, 2013]. Sci Transl Med. doi:10.1126 /scitranslmed.3006245
- Kasprowicz-Furmanczyk M, Czerwinska J, Placek W, et al. Assessment of the tissue resident memory cells in lesional skin of patients with psoriasis and in healthy skin of healthy volunteers. Int J Environ Res Public Health. 2021;18:11251. doi:10.3390/ijerph182111251
- Cheuk S, Schlums H, Gallais Serezal I, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287-300. doi:10.1016/j.immuni.2017.01.009
- Sawyer LM, Malottki K, Sabry-Grant C, et al. Assessing the relative efficacy of interleukin-17 and interleukin-23 targeted treatments for moderate-to-severe plaque psoriasis: a systematic review and network meta-analysis of PASI response. PLoS One. 2019;14:E0220868. doi:10.1371/journal.pone.0220868
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060-1071. doi:10.1002/art.39573
- Wang EA, Suzuki E, Maverakis E, et al. Targeting IL-17 in psoriatic arthritis. Eur J Rheumatol. 2017;4:272-277. doi:10.5152/eurjrheum.2017.17037
- Armstrong A, Fahrbach K, Leonardi C, et al. Efficacy of bimekizumab and other biologics in moderate to severe plaque psoriasis: a systematic literature review and a network meta-analysis. Dermatol Ther (Heidelb). 2022;12:1777-1792. doi:10.1007/s13555-022-00760-8
- van Baarsen LG, Lebre MC, van der Coelen D, et al. Heterogeneous expression pattern of interleukin 17A (IL-17A), IL-17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti-IL-17 therapy? Arthritis Res Ther. 2014;16:426. doi:10.1186/s13075-014-0426-z
- Hot A, Zrioual S, Toh ML, et al. IL-17A- versus IL-17F-induced intracellular signal transduction pathways and modulation by IL-17RA and IL-17RC RNA interference in rheumatoid synoviocytes. Ann Rheum Dis. 2011;70:341-348. doi:10.1136/ard.2010.132233
- Adams R, Maroof A, Baker T, et al. Bimekizumab, a novel humanized IgG1 antibody that neutralizes both IL-17A and IL-17F. Front Immunol. 2020;11:1894. doi:10.3389/fimmu.2020.01894
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397:475-486. doi:10.1016/S0140-6736(21)00126-4
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532. doi:10.1136 /annrheumdis-2017-212127
- Gordon KB, Langley RG, Warren RB, et al. Bimekizumab safety in patients with moderate to severe plaque psoriasis: pooled results from phase 2 and phase 3 randomized clinical trials. JAMA Dermatol. 2022;158:735-744. doi:10.1001/jamadermatol.2022.1185
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385:142-152. doi:10.1056/NEJMoa2102383
- Reich K, Iversen L, Puig L, et al. Long-term efficacy and safety of brodalumab in moderate-to-severe plaque psoriasis: a post hoc pooled analysis of AMAGINE-2 and -3. J Eur Acad Dermatol Venereol. 2022;36:1275-1283. doi:10.1111/jdv.18068
- Papp KA, Blauvelt A, Puig L, et al. Long-term safety and efficacy of risankizumab for the treatment of moderate-to-severe plaque psoriasis: interim analysis of the LIMMitless open-label extension trial up to 5 years of follow-up. J Am Acad Dermatol. 2023;89:1149-1158. doi: 10.1016/j.jaad.2023.07.1024
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, doubleblind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157:1279-1288. doi:10.1001/jamadermatol.2021.2905
- Choon SE, Lai NM, Mohammad NA, et al. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676-684. doi:10.1111/ijd.12070
- Zheng M, Jullien D, Eyerich K. The prevalence and disease characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):5-12. doi:10.1007/s40257-021-00664-x
- Fujita H, Gooderham M, Romiti R. Diagnosis of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23(suppl 1):31-38. doi:10.1007/s40257-021-00652-1
- Choon SE, Navarini AA, Pinter A. Clinical course and characteristics of generalized pustular psoriasis. Am J Clin Dermatol. 2022;23 (suppl 1):21-29. doi:10.1007/s40257-021-00654-z
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16:669-673.
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76:68-71. doi:10.2340/00015555766871
- Lee JY, Kang S, Park JS, et al. Prevalence of psoriasis in Korea: A population-based epidemiological study using the Korean National Health Insurance database. Ann Dermatol. 2017;29:761-767. doi:10.5021 /ad.2017.29.6.761
- Prinz JC, Choon SE, Griffiths CEM, et al. Prevalence, comorbidities and mortality of generalized pustular psoriasis: a literature review. J Eur Acad Dermatol Venereol. 2023;37:256-273. doi:10.1111/jdv.18720
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120. doi:10.1016/j.jaci.2016.08.056
- Rajan N, Sinclair N, Nakai H, et al. A tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. 2016;174:417-420. doi:10.1111/bjd.14003
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37-42. doi:10.2147/PTT.S181808
- Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). 2022;12:315-328. doi:10.1007 /s13555-021-00677-8
- Akiyama M, Takeichi T, McGrath JA, et al. Autoinflammatory keratinization diseases: an emerging concept encompassing various inflammatory keratinization disorders of the skin. J Dermatol Sci. 2018;90:105-111. doi:10.1016/j.jdermsci.2018.01.012
- Bachelez H, Choon SE, Marrakchi S, et al. Trial of spesolimab for generalized pustular psoriasis. N Engl J Med. 2021;385:2431-2440. doi:10.1056/NEJMoa2111563
- Warren RB, Reich A, Kaszuba A, et al. Imsidolimab, an anti-IL-36 receptor monoclonal antibody for the treatment of generalised pustular psoriasis: results from the phase 2 GALLOP trial. Br J Dermatol. 2023;189:161-169. doi:10.1093/bjd/ljad083
- Villarino AV, Kanno Y, O’Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol. 2017; 18:374-384. doi:10.1038/ni.3691
- Genetic Analysis of Psoriasis Consortium & the Wellcome Trust Case Control Consortium 2; Strange A, Capon F, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nat Genet. 2010;42:985-990. doi:10.1038/ng.694
- Enerback C, Sandin C, Lambert S, et al. The psoriasis-protective TYK2 I684S variant impairs IL-12 stimulated pSTAT4 response in skin-homing CD4+ and CD8+ memory T-cells. Sci Rep. 2018;8:7043. doi:10.1038/s41598-018-25282-2
- Shimoda K, Kato K, Aoki K, et al. Tyk2 plays a restricted role in IFN alpha signaling, although it is required for IL-12-mediated T cell function. Immunity. 2000;13:561-571. doi:10.1016/s1074-7613(00)00055-8
- Karaghiosoff M, Neubauer H, Lassnig C, et al. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549-560. doi:10.1016/s1074-7613(00)00054-6
- Burke JR, Cheng L, Gillooly KM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain [published online July 24, 2019]. Sci Transl Med. doi:10.1126/scitranslmed.aaw1736
- Strober B, Thaci D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 program for evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Stein Gold L, Lebwohl M, Menter A, et al. Aerosol foam formulation of fixed combination calcipotriene plus betamethasone dipropionate is highly efficacious in patients with psoriasis vulgaris: pooled data from three randomized controlled studies. J Drugs Dermatol. 2016;15:951-957.
- Beranek M, Fiala Z, Kremlacek J, et al. Serum levels of aryl hydrocarbon receptor, cytochromes p450 1a1 and 1b1 in patients with exacerbated psoriasis vulgaris. Folia Biol (Praha). 2018;64:97-102.
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67:259- 279. doi:10.1124/pr.114.009001
- Furue M, Uchi H, Mitoma C, et al. Antioxidants for healthy skin: the emerging role of aryl hydrocarbon receptors and nuclear factorerythroid 2-related factor-2. Nutrients. 2017;9:223. doi:10.3390/nu9030223
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371:1675-1684. doi:10.1016/S0140-6736(08)60726-6
- Sutter CH, Olesen KM, Bhuju J, et al. AHR regulates metabolic reprogramming to promote SIRT1-dependent keratinocyte differentiation. J Invest Dermatol. 2019;139:818-826. doi:10.1016/j.jid.2018.10.019
- Haas K, Weighardt H, Deenen R, et al. Aryl hydrocarbon receptor in keratinocytes is essential for murine skin barrier integrity. J Invest Dermatol. 2016;136:2260-2269. doi:10.1016/j.jid.2016.06.627
- Di Meglio P, Duarte JH, Ahlfors H, et al. Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity. 2014;40:989-1001. doi:10.1016/j.immuni.2014.04.019
- Kim HO, Kim JH, Chung BY, et al. Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases. Exp Dermatol. 2014;23:278-281. doi:10.1111/exd.12350
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013;123:917-927. doi:10.1172/JCI65642
- Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof is a natural AHR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110-2119. doi:10.1016/j.jid.2017.05.004
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Mooney N, Teague JE, Gehad AE, et al. Tapinarof inhibits the formation, cytokine production, and persistence of resident memory T cells in vitro. SKIN J Cutan Med. 2023;7:S194. doi:10.25251/skin.7.supp.194
- Schafer PH, Truzzi F, Parton A, et al. Phosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal. 2016;28:753-763. doi:10.1016/j.cellsig.2016.01.007
- Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol. 2018;9:1048. doi:10.3389/ fphar.2018.01048
- Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159:842-855. doi:10.1111/j.1476-5381.2009.00559.x
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49. doi:10.1016/j .jaad.2015.03.049
- Papp KA, Gooderham M, Droege M, et al. Roflumilast cream improves signs and symptoms of plaque psoriasis: results from a phase 1/2a randomized, controlled study. J Drugs Dermatol. 2020;19:734-740. doi:10.36849/JDD.2020.5370
- Lebwohl MG, Kircik LH, Moore AY, et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328:1073-1084. doi:10.1001/jama.2022.15632
Practice Points
- Psoriasis is a chronic inflammatory condition characterized by systemic inflammation and dysregulated IL-23/IL-17 signaling.
- Modern discoveries highlight the role of additional immune signals in psoriatic disease such as IL-17C, IL-17F, IL-36, and tyrosine kinase 2, which also contribute to disease development.
- Novel systemic, oral, and topical therapies have become available and add to the rapidly growing armamentarium of safe and effective treatments for psoriatic disease.
Diffusely Scattered Macules Following Radiation Therapy
The Diagnosis: Cutaneous Mastocytosis
A shave skin biopsy from the right lateral breast and a punch skin biopsy from the right thigh showed similar histopathology. There were dermal predominantly perivascular aggregates of cells demonstrating basophilic granular cytoplasm and round to oval nuclei (Figure, A and B). These cells were highlighted by CD117 immunohistochemical stain (Figure, C), consistent with mastocytes. Additionally, occasional lymphocytes and rare eosinophils were noted. These histopathologic findings confirmed the diagnosis of cutaneous mastocytosis (CM). The patient’s complete blood cell count was within reference range, but serum tryptase was elevated at 15.7 μg/L (reference range, <11.0 μg/L), which prompted a bone marrow biopsy to rule out systemic mastocytosis (SM). The result showed normocellular bone marrow with no evidence of dysplasia or increased blasts, granuloma, lymphoproliferative disorder, or malignancy. Fluorescence in situ hybridization for PDGFRA (platelet-derived growth factor receptor alpha) and KIT mutation was negative. Because CM developed predominantly on the right breast where the patient previously had received radiation therapy, we concluded that this reaction was triggered by exposure to ionizing radiation.
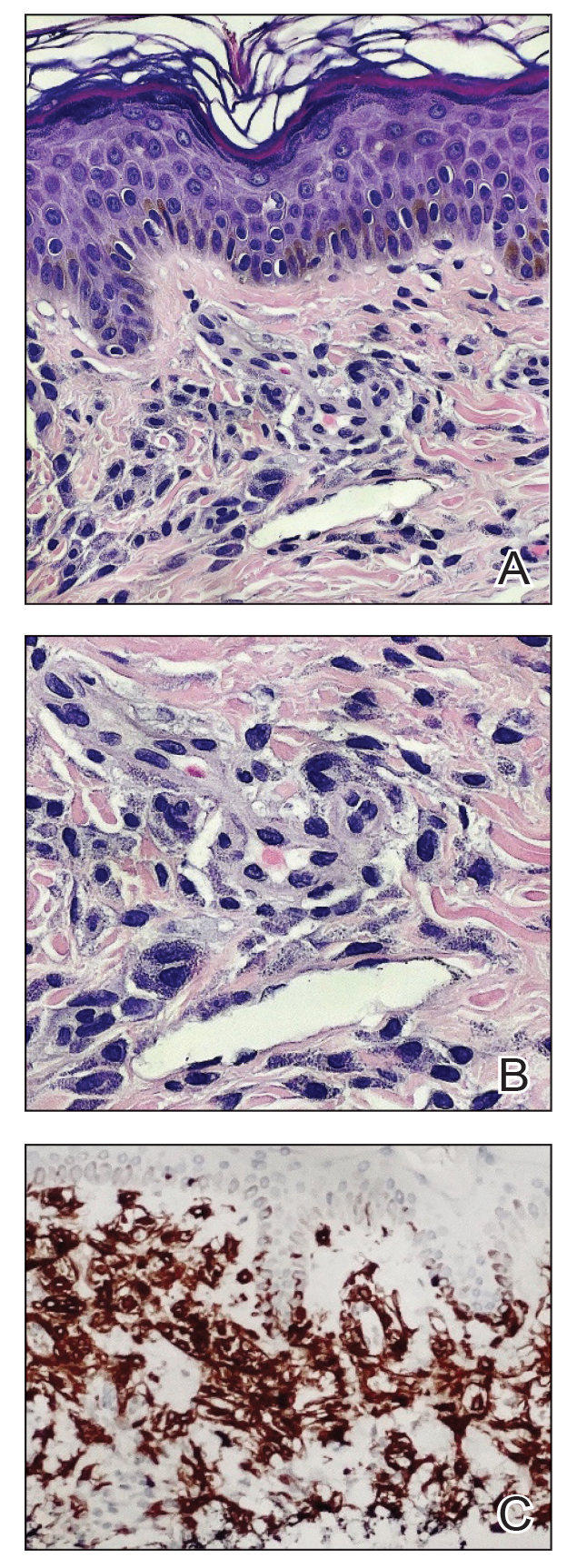
Mastocytosis can be divided into 2 groups: CM and SM.1 The histologic differential diagnosis of CM includes solitary mastocytoma, urticaria pigmentosa, telangiectasia macularis eruptiva perstans, and diffuse mastocytosis.2 Clinicopathologic correlation is of crucial importance to render the final diagnosis in these disorders. Immunohistochemically, mast cells express CD177, CD5, CD68, tryptase, and chymase. Unlike normal mast cells, neoplastic cells express CD2 and/or CD25; CD25 is commonly expressed in cutaneous involvement by SM.2
Macdonald and Feiwel3 reported the first case of CM following ionizing radiation. Cutaneous mastocytosis is most common in female patients and presents with redbrown macules originating at the site of radiation therapy. Prior literature suggests that radiation-associated CM has a predilection for White patients4; however, our patient was Hispanic. It also is important to note that the presentation of this rash may differ in individuals with skin of color. In one case it spread beyond the radiation site.2 Systemic mast call–mediated symptoms can occur in both CM and SM. The macules manifest as blanching with pressure.5 Typically these macules also are asymptomatic, though a positive Darier sign has been reported.6,7 The interval between radiotherapy and CM has ranged from 3 to 24 months.2
Patients with CM should have a serum tryptase evaluation along with a complete blood cell count, serum biochemistry, and liver function tests. Elevated serum tryptase has a high positive predictive value for SM and should prompt a bone marrow biopsy. Our patient’s bone marrow biopsy results failed to establish SM; however, her serum tryptase levels will be carefully monitored going forward. At the time of publication, the skin macules were still persistent but not worsening or symptomatic.
Treatment is focused on symptomatic relief of cutaneous symptoms, if present; avoiding triggers of mast cell degranulation; and implementing the use of oral antihistamines and leukotriene antagonists as needed. Because our patient was completely asymptomatic, we did not recommend any topical or oral treatment. However, we do counsel patients on avoiding triggers of mast cell degranulation including nonsteroidal anti-inflammatory drugs, morphine and codeine derivatives, alcohol, certain anesthetics, and anticholinergic medications.8
Additional diagnoses were ruled out for the following reasons: Although lichen planus pigmentosus presents with ill-defined, oval, gray-brown macules, histopathology shows a bandlike lymphocytic infiltrate at the dermoepidermal junction. Solar lentiginosis is characterized by grouped tan macules in a sun-exposed distribution. A fixed drug eruption is a delayed hypersensitivity reaction, usually to an ingested medication, characterized by violaceous or hyperpigmented patches, with histopathology showing interface dermatitis with a lymphoeosinophilic infiltrate. Eruptive seborrheic keratoses can result from sunburn or dermatitis but does not show mastocytes on histopathology.8
In conclusion, dermatologists should be reminded of the rare possibility of CM when evaluating an atypical eruption in a prior radiation field.
- Landy RE, Stross WC, May JM, et al. Idiopathic mast cell activation syndrome and radiation therapy: a case study, literature review, and discussion of mast cell disorders and radiotherapy [published online December 9, 2019]. Radiat Oncol. 2019;14:222. doi:10.1186 /s13014-019-1434-6
- Easwaralingam N, Wu Y, Cheung D, et al. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis—a case report and literature review. J Surg Case Rep. 2018;2018:1-3. doi:10.1093/jscr/rjy317
- Macdonald A, Feiwel M. Cutaneous mastocytosis: an unusual radiation dermatitis. Proc R Soc Med. 1971;64:29-30.
- Kirshenbaum AS, Abuhay H, Bolan H, et al. Maculopapular cutaneous mastocytosis in a diverse population. J Allergy Clin Immunol Pract. 2019;7:2845-2847. doi:10.1016/j.jaip.2019.04.003
- Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. 2008;34:111-112. doi:10.1111 /j.1365-2230.2008.02931.x
- Comte C, Bessis D, Dereure O, et al. Urticaria pigmentosa localized on radiation field. Eur J Dermatol. 2003;13:408-409.
- Davidson SJ, Coates D. Cutaneous mastocytosis extending beyond a radiotherapy site: a form of radiodermatitis or a neoplastic phenomenon? Australas J Dermatol. 2012;54:E85-E87. doi:10.1111 /j.1440-0960.2012.00961.x
- Bolognia J, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2022.
The Diagnosis: Cutaneous Mastocytosis
A shave skin biopsy from the right lateral breast and a punch skin biopsy from the right thigh showed similar histopathology. There were dermal predominantly perivascular aggregates of cells demonstrating basophilic granular cytoplasm and round to oval nuclei (Figure, A and B). These cells were highlighted by CD117 immunohistochemical stain (Figure, C), consistent with mastocytes. Additionally, occasional lymphocytes and rare eosinophils were noted. These histopathologic findings confirmed the diagnosis of cutaneous mastocytosis (CM). The patient’s complete blood cell count was within reference range, but serum tryptase was elevated at 15.7 μg/L (reference range, <11.0 μg/L), which prompted a bone marrow biopsy to rule out systemic mastocytosis (SM). The result showed normocellular bone marrow with no evidence of dysplasia or increased blasts, granuloma, lymphoproliferative disorder, or malignancy. Fluorescence in situ hybridization for PDGFRA (platelet-derived growth factor receptor alpha) and KIT mutation was negative. Because CM developed predominantly on the right breast where the patient previously had received radiation therapy, we concluded that this reaction was triggered by exposure to ionizing radiation.

Mastocytosis can be divided into 2 groups: CM and SM.1 The histologic differential diagnosis of CM includes solitary mastocytoma, urticaria pigmentosa, telangiectasia macularis eruptiva perstans, and diffuse mastocytosis.2 Clinicopathologic correlation is of crucial importance to render the final diagnosis in these disorders. Immunohistochemically, mast cells express CD177, CD5, CD68, tryptase, and chymase. Unlike normal mast cells, neoplastic cells express CD2 and/or CD25; CD25 is commonly expressed in cutaneous involvement by SM.2
Macdonald and Feiwel3 reported the first case of CM following ionizing radiation. Cutaneous mastocytosis is most common in female patients and presents with redbrown macules originating at the site of radiation therapy. Prior literature suggests that radiation-associated CM has a predilection for White patients4; however, our patient was Hispanic. It also is important to note that the presentation of this rash may differ in individuals with skin of color. In one case it spread beyond the radiation site.2 Systemic mast call–mediated symptoms can occur in both CM and SM. The macules manifest as blanching with pressure.5 Typically these macules also are asymptomatic, though a positive Darier sign has been reported.6,7 The interval between radiotherapy and CM has ranged from 3 to 24 months.2
Patients with CM should have a serum tryptase evaluation along with a complete blood cell count, serum biochemistry, and liver function tests. Elevated serum tryptase has a high positive predictive value for SM and should prompt a bone marrow biopsy. Our patient’s bone marrow biopsy results failed to establish SM; however, her serum tryptase levels will be carefully monitored going forward. At the time of publication, the skin macules were still persistent but not worsening or symptomatic.
Treatment is focused on symptomatic relief of cutaneous symptoms, if present; avoiding triggers of mast cell degranulation; and implementing the use of oral antihistamines and leukotriene antagonists as needed. Because our patient was completely asymptomatic, we did not recommend any topical or oral treatment. However, we do counsel patients on avoiding triggers of mast cell degranulation including nonsteroidal anti-inflammatory drugs, morphine and codeine derivatives, alcohol, certain anesthetics, and anticholinergic medications.8
Additional diagnoses were ruled out for the following reasons: Although lichen planus pigmentosus presents with ill-defined, oval, gray-brown macules, histopathology shows a bandlike lymphocytic infiltrate at the dermoepidermal junction. Solar lentiginosis is characterized by grouped tan macules in a sun-exposed distribution. A fixed drug eruption is a delayed hypersensitivity reaction, usually to an ingested medication, characterized by violaceous or hyperpigmented patches, with histopathology showing interface dermatitis with a lymphoeosinophilic infiltrate. Eruptive seborrheic keratoses can result from sunburn or dermatitis but does not show mastocytes on histopathology.8
In conclusion, dermatologists should be reminded of the rare possibility of CM when evaluating an atypical eruption in a prior radiation field.
The Diagnosis: Cutaneous Mastocytosis
A shave skin biopsy from the right lateral breast and a punch skin biopsy from the right thigh showed similar histopathology. There were dermal predominantly perivascular aggregates of cells demonstrating basophilic granular cytoplasm and round to oval nuclei (Figure, A and B). These cells were highlighted by CD117 immunohistochemical stain (Figure, C), consistent with mastocytes. Additionally, occasional lymphocytes and rare eosinophils were noted. These histopathologic findings confirmed the diagnosis of cutaneous mastocytosis (CM). The patient’s complete blood cell count was within reference range, but serum tryptase was elevated at 15.7 μg/L (reference range, <11.0 μg/L), which prompted a bone marrow biopsy to rule out systemic mastocytosis (SM). The result showed normocellular bone marrow with no evidence of dysplasia or increased blasts, granuloma, lymphoproliferative disorder, or malignancy. Fluorescence in situ hybridization for PDGFRA (platelet-derived growth factor receptor alpha) and KIT mutation was negative. Because CM developed predominantly on the right breast where the patient previously had received radiation therapy, we concluded that this reaction was triggered by exposure to ionizing radiation.

Mastocytosis can be divided into 2 groups: CM and SM.1 The histologic differential diagnosis of CM includes solitary mastocytoma, urticaria pigmentosa, telangiectasia macularis eruptiva perstans, and diffuse mastocytosis.2 Clinicopathologic correlation is of crucial importance to render the final diagnosis in these disorders. Immunohistochemically, mast cells express CD177, CD5, CD68, tryptase, and chymase. Unlike normal mast cells, neoplastic cells express CD2 and/or CD25; CD25 is commonly expressed in cutaneous involvement by SM.2
Macdonald and Feiwel3 reported the first case of CM following ionizing radiation. Cutaneous mastocytosis is most common in female patients and presents with redbrown macules originating at the site of radiation therapy. Prior literature suggests that radiation-associated CM has a predilection for White patients4; however, our patient was Hispanic. It also is important to note that the presentation of this rash may differ in individuals with skin of color. In one case it spread beyond the radiation site.2 Systemic mast call–mediated symptoms can occur in both CM and SM. The macules manifest as blanching with pressure.5 Typically these macules also are asymptomatic, though a positive Darier sign has been reported.6,7 The interval between radiotherapy and CM has ranged from 3 to 24 months.2
Patients with CM should have a serum tryptase evaluation along with a complete blood cell count, serum biochemistry, and liver function tests. Elevated serum tryptase has a high positive predictive value for SM and should prompt a bone marrow biopsy. Our patient’s bone marrow biopsy results failed to establish SM; however, her serum tryptase levels will be carefully monitored going forward. At the time of publication, the skin macules were still persistent but not worsening or symptomatic.
Treatment is focused on symptomatic relief of cutaneous symptoms, if present; avoiding triggers of mast cell degranulation; and implementing the use of oral antihistamines and leukotriene antagonists as needed. Because our patient was completely asymptomatic, we did not recommend any topical or oral treatment. However, we do counsel patients on avoiding triggers of mast cell degranulation including nonsteroidal anti-inflammatory drugs, morphine and codeine derivatives, alcohol, certain anesthetics, and anticholinergic medications.8
Additional diagnoses were ruled out for the following reasons: Although lichen planus pigmentosus presents with ill-defined, oval, gray-brown macules, histopathology shows a bandlike lymphocytic infiltrate at the dermoepidermal junction. Solar lentiginosis is characterized by grouped tan macules in a sun-exposed distribution. A fixed drug eruption is a delayed hypersensitivity reaction, usually to an ingested medication, characterized by violaceous or hyperpigmented patches, with histopathology showing interface dermatitis with a lymphoeosinophilic infiltrate. Eruptive seborrheic keratoses can result from sunburn or dermatitis but does not show mastocytes on histopathology.8
In conclusion, dermatologists should be reminded of the rare possibility of CM when evaluating an atypical eruption in a prior radiation field.
- Landy RE, Stross WC, May JM, et al. Idiopathic mast cell activation syndrome and radiation therapy: a case study, literature review, and discussion of mast cell disorders and radiotherapy [published online December 9, 2019]. Radiat Oncol. 2019;14:222. doi:10.1186 /s13014-019-1434-6
- Easwaralingam N, Wu Y, Cheung D, et al. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis—a case report and literature review. J Surg Case Rep. 2018;2018:1-3. doi:10.1093/jscr/rjy317
- Macdonald A, Feiwel M. Cutaneous mastocytosis: an unusual radiation dermatitis. Proc R Soc Med. 1971;64:29-30.
- Kirshenbaum AS, Abuhay H, Bolan H, et al. Maculopapular cutaneous mastocytosis in a diverse population. J Allergy Clin Immunol Pract. 2019;7:2845-2847. doi:10.1016/j.jaip.2019.04.003
- Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. 2008;34:111-112. doi:10.1111 /j.1365-2230.2008.02931.x
- Comte C, Bessis D, Dereure O, et al. Urticaria pigmentosa localized on radiation field. Eur J Dermatol. 2003;13:408-409.
- Davidson SJ, Coates D. Cutaneous mastocytosis extending beyond a radiotherapy site: a form of radiodermatitis or a neoplastic phenomenon? Australas J Dermatol. 2012;54:E85-E87. doi:10.1111 /j.1440-0960.2012.00961.x
- Bolognia J, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Landy RE, Stross WC, May JM, et al. Idiopathic mast cell activation syndrome and radiation therapy: a case study, literature review, and discussion of mast cell disorders and radiotherapy [published online December 9, 2019]. Radiat Oncol. 2019;14:222. doi:10.1186 /s13014-019-1434-6
- Easwaralingam N, Wu Y, Cheung D, et al. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis—a case report and literature review. J Surg Case Rep. 2018;2018:1-3. doi:10.1093/jscr/rjy317
- Macdonald A, Feiwel M. Cutaneous mastocytosis: an unusual radiation dermatitis. Proc R Soc Med. 1971;64:29-30.
- Kirshenbaum AS, Abuhay H, Bolan H, et al. Maculopapular cutaneous mastocytosis in a diverse population. J Allergy Clin Immunol Pract. 2019;7:2845-2847. doi:10.1016/j.jaip.2019.04.003
- Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. 2008;34:111-112. doi:10.1111 /j.1365-2230.2008.02931.x
- Comte C, Bessis D, Dereure O, et al. Urticaria pigmentosa localized on radiation field. Eur J Dermatol. 2003;13:408-409.
- Davidson SJ, Coates D. Cutaneous mastocytosis extending beyond a radiotherapy site: a form of radiodermatitis or a neoplastic phenomenon? Australas J Dermatol. 2012;54:E85-E87. doi:10.1111 /j.1440-0960.2012.00961.x
- Bolognia J, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2022.
A 41-year-old woman was referred to dermatology by her radiation oncologist for evaluation of a rash on the right breast at the site of prior radiation therapy of 4 to 6 weeks’ duration. Approximately 2 years prior, the patient was diagnosed with triple-negative invasive ductal carcinoma of the right breast. She was treated with neoadjuvant chemotherapy, bilateral simple mastectomies, and 28 doses of adjuvant radiation therapy. Thirteen months after completing radiation therapy, the patient noted the onset of asymptomatic freckles on the right breast that had appeared over weeks and seemed to be multiplying. Physical examination at the time of dermatology consultation revealed multiple diffusely scattered, brownishred, 3- to 5-mm macules concentrated on the right breast but also involving the right supraclavicular and right axillary areas, abdomen, and thighs.
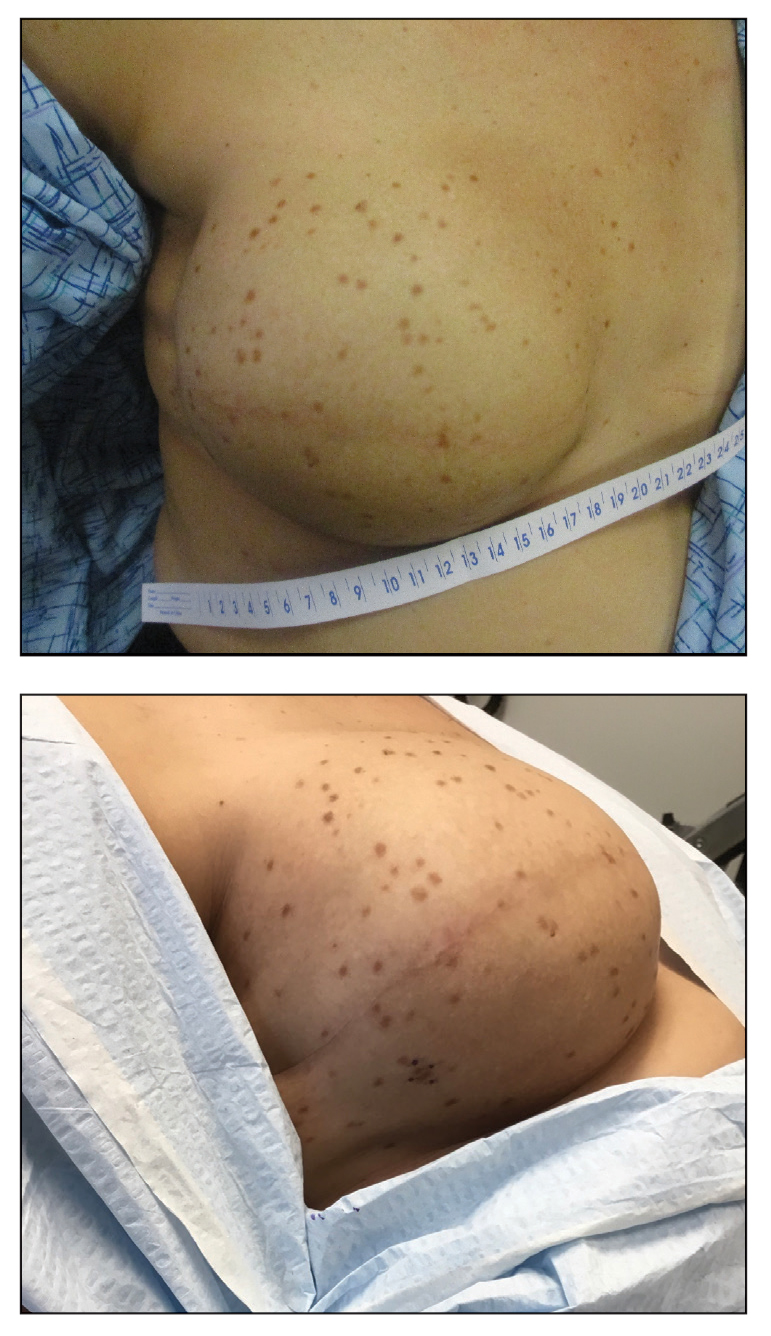
Association Between LDL-C and Androgenetic Alopecia Among Female Patients in a Specialty Alopecia Clinic
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).
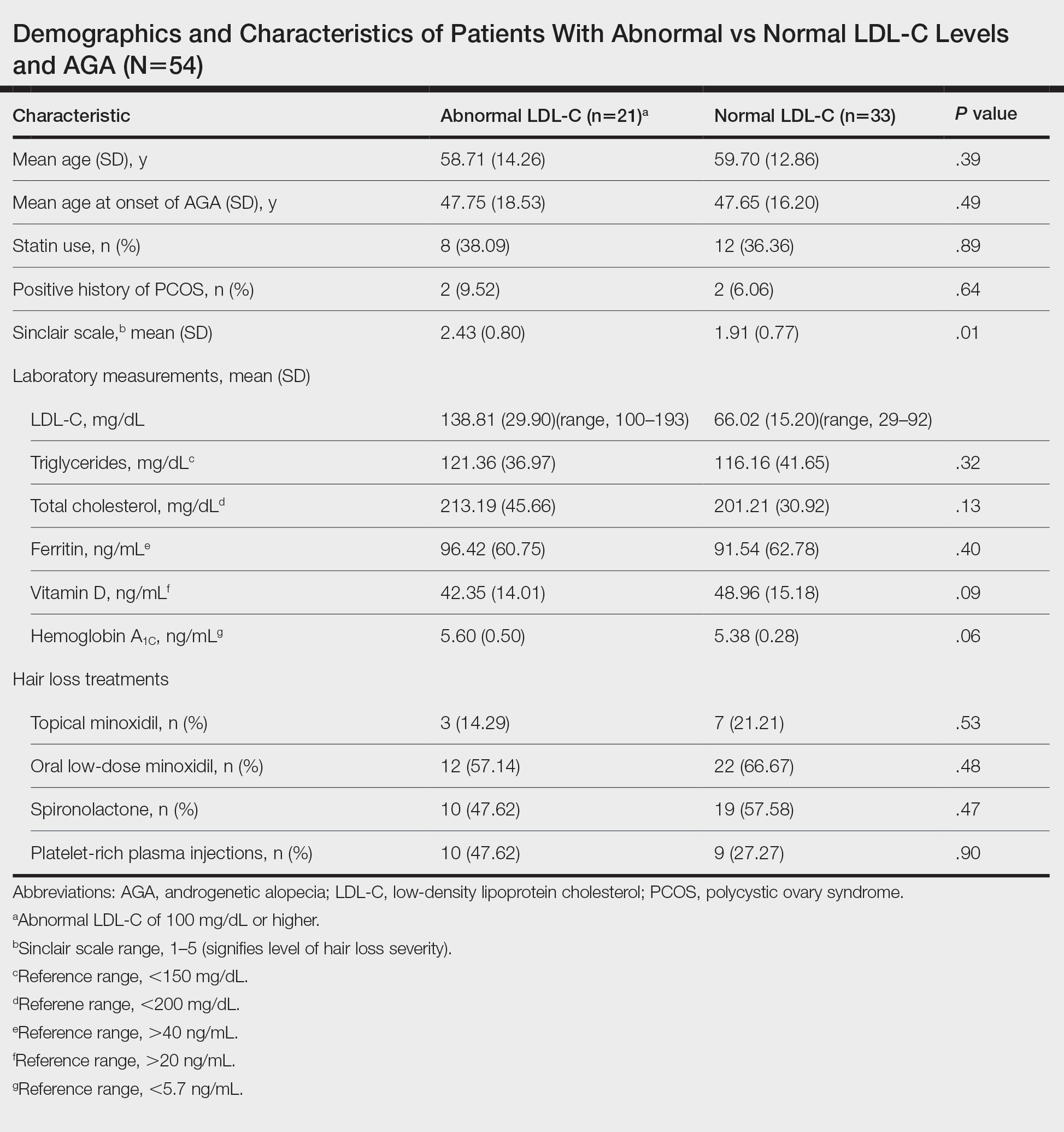
We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
To the Editor:
Female pattern hair loss (FPHL), or androgenetic alopecia (AGA), is the most common form of alopecia worldwide and is characterized by a reduction of hair follicles spent in the anagen phase of growth as well as progressive terminal hair loss.1 It is caused by an excessive response to androgens and leads to the characteristic distribution of hair loss in both sexes. Studies have shown a notable association between AGA and markers of metabolic syndrome such as dyslipidemia, insulin resistance, and obesity in age- and sex-matched controls.2,3 However, research describing the relationship between AGA severity and these markers is scarce.
To understand the relationship between FPHL severity and abnormal cholesterol levels, we performed a retrospective chart review of patients diagnosed with FPHL at a specialty alopecia clinic from June 2022 to December 2022. Patient age and age at onset of FPHL were collected. The severity of FPHL was measured using the Sinclair scale (score range, 1–5) and unidentifiable patient photographs. Laboratory values were collected; abnormal cholesterol was defined by the American Heart Association as having a low-density lipoprotein cholesterol (LDL-C) level of 100 mg/dL or higher.4 Finally, data on medication use were noted to understand patient treatment status (Table).

We identified 54 female patients with FPHL with an average age of 59 years (range, 34–80 years). Thirty-three females (61.11%) had a normal LDL-C level and 21 (38.89%) had an abnormal level. The mean (SD) LDL-C level was 66.02 (15.20) mg/dL (range, 29–92 mg/dL) in the group with normal levels and 138.81 (29.90) mg/dL (range, 100–193 mg/dL) in the group with abnormal levels. Patients with abnormal LDL-C had significantly higher Sinclair scale scores compared to those with normal levels (2.43 vs 1.91; P=.01). There were no significant differences in patient age (58.71 vs 59.70 years; P=.39), age at onset of AGA (47.75 vs 47.65 years; P=.49), history of polycystic ovary syndrome (9.52% vs 6.06%; P=.64), or statin use (38.09% vs 36.36%; P=.89) between patients with abnormal and normal LDL-C levels, respectively. There also were no significant differences in ferritin (96.42 vs 91.54 ng/mL; P=.40), vitamin D (42.35 vs 48.96 ng/mL; P=.09), or hemoglobin A1c levels (5.60 ng/mL vs 5.38 ng/mL; P=.06)—variables that could have confounded this relationship. Triglycerides were within reference range in both groups (121.36 vs 116.16 mg/dL; P=.32), while total cholesterol was mildly elevated in both groups but not significantly different (213.19 vs 201.21 mg/dL; P=.13). Use of hair loss treatments such as topical minoxidil (14.29% vs 21.21%; P=.53), oral low-dose minoxidil (57.14% vs 66.67%; P=.48), oral spironolactone (47.62% vs 57.58%; P=.47), and platelet-rich plasma injections (47.62% vs 27.27%; P=.90) were not significantly different across both groups.
The data suggest a significant (P<.05) association between abnormal LDL-C and hair loss severity in FPHL patients. Our study was limited by its small sample size and lack of causality; however, it coincides with and reiterates the findings established in the literature. The mechanism of the association between hyperlipidemia and AGA is not well understood but is thought to stem from the homology between cholesterol and androgens. Increased cholesterol release from dermal adipocytes and subsequent absorption into hair follicle cell populations may increase hair follicle steroidogenesis, thereby accelerating the anagen-catagen transition and inducing AGA. Alternatively, impaired cholesterol homeostasis may disrupt normal hair follicle cycling by interrupting signaling pathways in follicle proliferation and differentiation.5 Adequate control and monitoring of LDL-C levels may be important, particularly in patients with more severe FPHL.
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
- Herskovitz I, Tosti A. Female pattern hair loss. Int J Endocrinol Metab. 2013;11:E9860. doi:10.5812/ijem.9860
- El Sayed MH, Abdallah MA, Aly DG, et al. Association of metabolic syndrome with female pattern hair loss in women: a case-control study. Int J Dermatol. 2016;55:1131-1137. doi:10.1111/ijd.13303
- Kim MW, Shin IS, Yoon HS, et al. Lipid profile in patients with androgenetic alopecia: a meta-analysis. J Eur Acad Dermatol Venereol. 2017;31:942-951. doi:10.1111/jdv.14000
- Birtcher KK, Ballantyne CM. Cardiology patient page. measurement of cholesterol: a patient perspective. Circulation. 2004;110:E296-E297. doi:10.1161/01.CIR.0000141564.89465.4E
- Palmer MA, Blakeborough L, Harries M, et al. Cholesterol homeostasis: links to hair follicle biology and hair disorders. Exp Dermatol. 2020;29:299-311. doi:10.1111/exd.13993
Practice Points
- Associations have been shown between hair loss and markers of bad health such as insulin resistance and high cholesterol. Research has not yet shown the relationship between hair loss severity and these markers, particularly cholesterol.
Acne and Pregnancy: A Clinical Review and Practice Pearls
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.
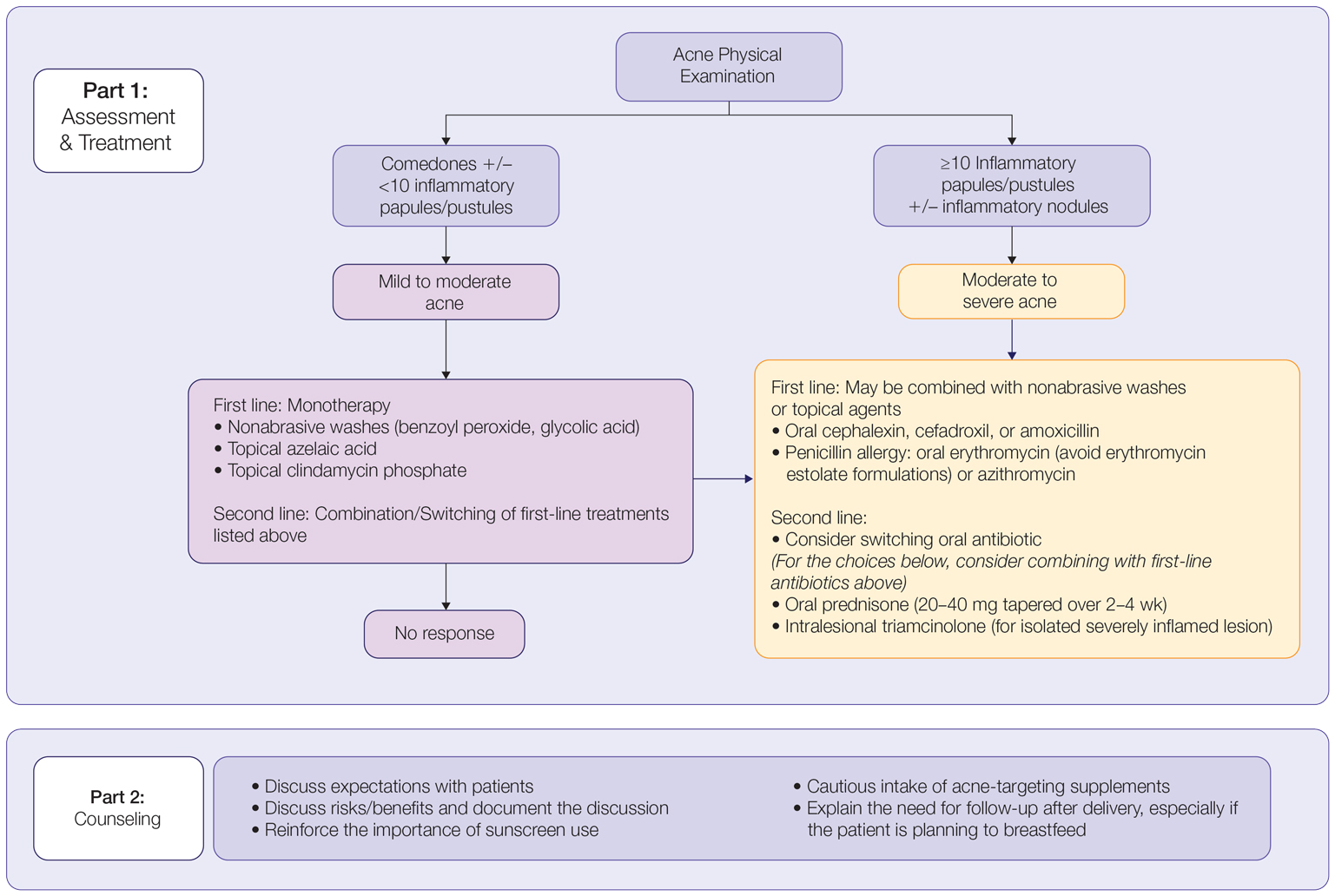
In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
Acne vulgaris, or acne, is a highly common inflammatory skin disorder affecting up to 85% of the population, and it constitutes the most commonly presenting chief concern in routine dermatology practice.1 Older teenagers and young adults are most often affected by acne.2 Although acne generally is more common in males, adult-onset acne occurs more frequently in women.2,3 Black and Hispanic women are at higher risk for acne compared to those of Asian, White, or Continental Indian descent.4 As such, acne is a common concern in all women of childbearing age.
Concerns for maternal and fetal safety are important therapeutic considerations, especially because hormonal and physiologic changes in pregnancy can lead to onset of inflammatory acne lesions, particularly during the second and third trimesters.5 Female patients younger than 25 years; with a higher body mass index, prior irregular menstruation, or polycystic ovary syndrome; or those experiencing their first pregnancy are thought to be more commonly affected.5-7 In fact, acne affects up to 43% of pregnant women, and lesions typically extend beyond the face to involve the trunk.6,8-10 Importantly, one-third of women with a history of acne experience symptom relapse after disease-free periods, while two-thirds of those with ongoing disease experience symptom deterioration during pregnancy.10 Although acne is not a life-threatening condition, it has a well-documented, detrimental impact on social, emotional, and psychological well-being, namely self-perception, social interactions, quality-of-life scores, depression, and anxiety.11
Therefore, safe and effective treatment of pregnant women is of paramount importance. Because pregnant women are not included in clinical trials, there is a paucity of medication safety data, further augmented by inefficient access to available information. The US Food and Drug Administration (FDA) pregnancy safety categories were updated in 2015, letting go of the traditional A, B, C, D, and X categories.12 The Table reviews the current pregnancy classification system. In this narrative review, we summarize the most recent available data and recommendations on the safety and efficacy of acne treatment during pregnancy.

Topical Treatments for Acne
Benzoyl Peroxide—Benzoyl peroxide commonly is used as first-line therapy alone or in combination with other agents for the treatment of mild to moderate acne.13 It is safe for use during pregnancy.14 Although the medication is systemically absorbed, it undergoes complete metabolism to benzoic acid, a commonly used food additive.15,16 Benzoic acid has low bioavailability, as it gets rapidly metabolized by the kidneys; therefore, benzoyl peroxide is unlikely to reach clinically significant levels in the maternal circulation and consequently the fetal circulation. Additionally, it has a low risk for causing congenital malformations.17
Salicylic Acid—For mild to moderate acne, salicylic acid is a second-line agent that likely is safe for use by pregnant women at low concentrations and over limited body surface areas.14,18,19 There is minimal systemic absorption of the drug.20 Additionally, aspirin, which is broken down in the body into salicylic acid, is used in low doses for the treatment of pre-eclampsia during pregnancy.21
Dapsone—The use of dapsone gel 5% as a second-line agent has shown efficacy for mild to moderate acne.22 The oral formulation, commonly used for malaria and leprosy prophylaxis, has failed to show associated fetal toxicity or congenital anomalies.23,24 It also has been used as a first-line treatment for dermatitis herpetiformis in pregnancy.25 Although the medication likely is safe, it is better to minimize its use during the third trimester to reduce the theoretical risk for hyperbilirubinemia in the neonate.17,26-29
Azelaic Acid—Azelaic acid effectively targets noninflammatory and inflammatory acne and generally is well tolerated, harboring a good safety profile.30 Topical 20% azelaic acid has localized antibacterial and comedolytic effects and is safe for use during pregnancy.31,32
Glycolic Acid—Limited data exist on the safety of glycolic acid during pregnancy. In vitro studies have shown up to 27% systemic absorption depending on pH, concentration, and duration of application.33 Animal reproductive studies involving rats have shown fetal multisystem malformations and developmental abnormalities with oral administration of glycolic acid at doses far exceeding those used in humans.34 Although no human reproductive studies exist, topical glycolic acid is unlikely to reach the developing fetus in notable amounts, and the medication is likely safe for use.17,35
Clindamycin—Topical clindamycin phosphate is an effective and well-tolerated agent for the treatment of mild to moderate acne.36 Its systemic absorption is minimal, and it is considered safe for use during all trimesters of pregnancy.14,17,26,27,35,37
Erythromycin—Topical erythromycin is another commonly prescribed topical antibiotic used to target mild to moderate acne. However, its use recently has been associated with a decrease in efficacy secondary to the rise of antibacterial resistance in the community.38-40 Nevertheless, it remains a safe treatment for use during all trimesters of pregnancy.14,17,26,27,35,37
Topical Retinoids—Vitamin A derivatives (also known as retinoids) are the mainstay for the treatment of mild to moderate acne. Limited data exist regarding pregnancy outcomes after in utero exposure.41 A rare case report suggested topical tretinoin has been associated with fetal otocerebral anomalies.42 For tazarotene, teratogenic effects were seen in animal reproductive studies at doses exceeding maximum recommended human doses.41,43 However, a large meta-analysis failed to find a clear risk for increased congenital malformations, spontaneous abortions, stillbirth, elective termination of pregnancy, low birthweight, or prematurity following first-trimester exposure to topical retinoids.44 As the level of exposure that could lead to teratogenicity in humans is unknown, avoidance of both tretinoin and tazarotene is recommended in pregnant women.41,45 Nevertheless, women inadvertently exposed should be reassured.44
Conversely, adapalene has been associated with 1 case of anophthalmia and agenesis of the optic chiasma in a fetus following exposure until 13 weeks’ gestation.46 However, a large, open-label trial prior to the patient transitioning from adapalene to over-the-counter treatment showed that the drug harbors a large and reassuring margin of safety and no risk for teratogenicity in a maximal usage trial and Pregnancy Safety Review.47 Therefore, adapalene gel 0.1% is a safe and effective medication for the treatment of acne in a nonprescription environment and does not pose harm to the fetus.
Clascoterone—Clascoterone is a novel topical antiandrogenic drug approved for the treatment of hormonal and inflammatory moderate to severe acne.48-51 Human reproductive data are limited to 1 case of pregnancy that occurred during phase 3 trial investigations, and no adverse outcomes were reported.51 Minimal systemic absorption follows topical use.52 Nonetheless, dose-independent malformations were reported in animal reproductive studies.53 As such, it remains better to avoid the use of clascoterone during pregnancy pending further safety data.
Minocycline Foam—Minocycline foam 4% is approved to treat inflammatory lesions of nonnodular moderate to severe acne in patients 9 years and older.54 Systemic absorption is minimal, and the drug has limited bioavailability with minimal systemic accumulation in the patient’s serum.55 Given this information, it is unlikely that topical minocycline will reach notable levels in the fetal serum or harbor teratogenic effects, as seen with the oral formulation.56 However, it may be best to avoid its use during the second and third trimesters given the potential risk for tooth discoloration in the fetus.57,58
Systemic Treatments for Acne
Isotretinoin—Isotretinoin is the most effective treatment for moderate to severe acne with a well-documented potential for long-term clearance.59 Its use during pregnancy is absolutely contraindicated, as the medication is a well-known teratogen. Associated congenital malformations include numerous craniofacial defects, cardiovascular and neurologic malformations, or thymic disorders that are estimated to affect 20% to 35% of infants exposed in utero.60 Furthermore, strict contraception use during treatment is mandated for patients who can become pregnant. It is recommended to wait at least 1 month and 1 menstrual cycle after medication discontinuation before attempting to conceive.17 Pregnancy termination is recommended if conception occurs during treatment with isotretinoin.
Spironolactone—Spironolactone is an androgen-receptor antagonist commonly prescribed off label for mild to severe acne in females.61,62 Spironolactone promotes the feminization of male fetuses and should be avoided in pregnancy.63
Doxycycline/Minocycline—Tetracyclines are the most commonly prescribed oral antibiotics for moderate to severe acne.64 Although highly effective at treating acne, tetracyclines generally should be avoided in pregnancy. First-trimester use of doxycycline is not absolutely contraindicated but should be reserved for severe illness and not employed for the treatment of acne. However, accidental exposure to doxycycline has not been associated with congenital malformations.65 Nevertheless, after the 15th week of gestation, permanent tooth discoloration and bone growth inhibition in the fetus are serious and well-documented risks.14,17 Additional adverse events following in utero exposure include infantile inguinal hernia, hypospadias, and limb hypoplasia.63
Sarecycline—Sarecycline is a novel tetracycline-class antibiotic for the treatment of moderate to severe inflammatory acne. It has a narrower spectrum of activity compared to its counterparts within its class, which translates to an improved safety profile, namely when it comes to gastrointestinal tract microbiome disruption and potentially decreased likelihood of developing bacterial resistance.66 Data on human reproductive studies are limited, but it is advisable to avoid sarecycline in pregnancy, as it may cause adverse developmental effects in the fetus, such as reduced bone growth, in addition to the well-known tetracycline-associated risk for permanent discoloration of the teeth if used during the second and third trimesters.67,68
Erythromycin—Oral erythromycin targets moderate to severe inflammatory acne and is considered safe for use during pregnancy.69,70 There has been 1 study reporting an increased risk for atrial and ventricular septal defects (1.8%) and pyloric stenosis (0.2%), but these risks are still uncertain, and erythromycin is considered compatible with pregnancy.71 However, erythromycin estolate formulations should be avoided given the associated 10% to 15% risk for reversible cholestatic liver injury.72 Erythromycin base or erythromycin ethylsuccinate formulations should be favored.
Systemic Steroids—Prednisone is indicated for severe acne with scarring and should only be used during pregnancy after clearance from the patient’s obstetrician. Doses of 0.5 mg/kg or less should be prescribed in combination with systemic antibiotics as well as agents for bone and gastrointestinal tract prophylaxis.29
Zinc—The exact mechanism by which zinc exerts its effects to improve acne remains largely obscure. It has been found effective against inflammatory lesions of mild to moderate acne.73 Generally recommended dosages range from 30 to 200 mg/d but may be associated with gastrointestinal tract disturbances. Dosages of 75 mg/d have shown no harm to the fetus.74 When taking this supplement, patients should not exceed the recommended doses given the risk for hypocupremia associated with high-dose zinc supplementation.
Light-Based Therapies
Phototherapy—Narrowband UVB phototherapy is effective for the treatment of mild to moderate acne.75 It has been proven to be a safe treatment option during pregnancy, but its use has been associated with decreased folic acid levels.76-79 Therefore, in addition to attaining baseline folic acid serum levels, supplementation with folic acid prior to treatment, as per routine prenatal guidelines, should be sought.80
AviClear—The AviClear (Cutera) laser is the first device cleared by the FDA for mild to severe acne in March 2022.81 The FDA clearance for the Accure (Accure Acne Inc) laser, also targeting mild to severe acne, followed soon after (November 2022). Both lasers harbor a wavelength of 1726 nm and target sebaceous glands with electrothermolysis.82,83 Further research and long-term safety data are required before using them in pregnancy.
Other Therapies
Cosmetic Peels—Glycolic acid peels induce epidermolysis and desquamation.84 Although data on use during pregnancy are limited, these peels have limited dermal penetration and are considered safe for use in pregnancy.33,85,86 Similarly, keratolytic lactic acid peels harbor limited dermal penetration and can be safely used in pregnant women.87-89 Salicylic acid peels also work through epidermolysis and desquamation84; however, they tend to penetrate deeper into the skin, reaching down to the basal layer, if large areas are treated or when applied under occlusion.86,90 Although their use is not contraindicated in pregnancy, they should be limited to small areas of coverage.91
Intralesional Triamcinolone—Acne cysts and inflammatory papules can be treated with intralesional triamcinolone injections to relieve acute symptoms such as pain.92 Low doses at concentrations of 2.5 mg/mL are considered compatible with pregnancy when indicated.29
Approaching the Patient Clinical Encounter
In patients seeking treatment prior to conception, a few recommendations can be made to minimize the risk for acne recurrence or flares during pregnancy. For instance, because data show an association between increased acne severity in those with a higher body mass index and in pregnancy, weight loss may be recommended prior to pregnancy to help mitigate symptoms after conception.7 The Figure summarizes our recommendations for approaching and treating acne in pregnancy.

In all patients, grading the severity of the patient’s acne as mild, moderate, or severe is the first step. The presence of scarring is an additional consideration during the physical examination and should be documented. A careful discussion of treatment expectations and prognosis should be the focus before treatment initiation. Meticulous documentation of the physical examination and discussion with the patient should be prioritized.
To minimize toxicity and risks to the developing fetus, monotherapy is favored. Topical therapy should be considered first line. Safe regimens include mild nonabrasive washes, such as those containing benzoyl peroxide or glycolic acid, or topical azelaic acid or clindamycin phosphate for mild to moderate acne. More severe cases warrant the consideration of systemic medications as second line, as more severe acne is better treated with oral antibiotics such as the macrolides erythromycin or clindamycin or systemic corticosteroids when concern exists for severe scarring. The additional use of physical sunscreen also is recommended.
An important topic to address during the clinical encounter is cautious intake of oral supplements for acne during pregnancy, as they may contain harmful and teratogenic ingredients. A recent search focusing on acne supplements available online between March and May 2020 uncovered 49 different supplements, 26 (53%) of which contained vitamin A.93 Importantly, 3 (6%) of these 49 supplements were likely teratogenic, 4 (8%) contained vitamin A doses exceeding the recommended daily nutritional intake level, and 15 (31%) harbored an unknown teratogenic risk. Furthermore, among the 6 (12%) supplements with vitamin A levels exceeding 10,000 IU, 2 lacked any mention of pregnancy warning, including the supplement with the highest vitamin A dose found in this study.93 Because dietary supplements are not subject to the same stringent regulations by the FDA as drugs, inadvertent use by unaware patients ought to be prevented by careful counseling and education.
Finally, patients should be counseled to seek care following delivery for potentially updated medication management of acne, especially if they are breastfeeding. Co-management with a pediatrician may be indicated during lactation, particularly when newborns are born preterm or with other health conditions that may warrant additional caution with the use of certain agents.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Fisk WA, Lev-Tov HA, Sivamani RK. Epidemiology and management of acne in adult women. Curr Dermatol Rep. 2014;3:29-39.
- Perkins A, Cheng C, Hillebrand G, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
- Yang CC, Huang YT, Yu CH, et al. Inflammatory facial acne during uncomplicated pregnancy and post‐partum in adult women: a preliminary hospital‐based prospective observational study of 35 cases from Taiwan. J Eur Acad Dermatol Venereol. 2016;30:1787-1789.
- Dréno B, Blouin E, Moyse D, et al. Acne in pregnant women: a French survey. Acta Derm Venereol. 2014;94:82-83.
- Kutlu Ö, Karadag˘ AS, Ünal E, et al. Acne in pregnancy: a prospective multicenter, cross‐sectional study of 295 patients in Turkey. Int J Dermatol. 2020;59:1098-1105.
- Hoefel IDR, Weber MB, Manzoni APD, et al. Striae gravidarum, acne, facial spots, and hair disorders: risk factors in a study with 1284 puerperal patients. J Pregnancy. 2020;2020:8036109.
- Ayanlowo OO, Otrofanowei E, Shorunmu TO, et al. Pregnancy dermatoses: a study of patients attending the antenatal clinic at two tertiary care centers in south west Nigeria. PAMJ Clin Med. 2020;3.
- Bechstein S, Ochsendorf F. Acne and rosacea in pregnancy. Hautarzt. 2017;68:111-119.
- Habeshian KA, Cohen BA. Current issues in the treatment of acne vulgaris. Pediatrics. 2020;145(suppl 2):S225-S230.
- Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (21 CFR 201). Fed Regist. 2014;79:72064-72103.
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opin Pharmacother. 2009;10:2555-2562.
- Murase JE, Heller MM, Butler DC. Safety of dermatologic medications in pregnancy and lactation: part I. Pregnancy. J Am Acad Dermatol. 2014;70:401.e1-401.e14; quiz 415.
- Wolverton SE. Systemic corticosteroids. Comprehensive Dermatol Drug Ther. 2012;3:143-168.
- Kirtschig G, Schaefer C. Dermatological medications and local therapeutics. In: Schaefer C, Peters P, Miller RK, eds. Drugs During Pregnancy and Lactation. 3rd edition. Elsevier; 2015:467-492.
- Pugashetti R, Shinkai K. Treatment of acne vulgaris in pregnant patients. Dermatol Ther. 2013;26:302-311.
- Touitou E, Godin B, Shumilov M, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. J Eur Acad Dermatol Venereol. 2008;22:629-631.
- Schaefer C, Peters PW, Miller RK, eds. Drugs During Pregnancy and Lactation: Treatment Options and Risk Assessment. 2nd ed. Academic Press; 2014.
- Birmingham B, Greene D, Rhodes C. Systemic absorption of topical salicylic acid. Int J Dermatol. 1979;18:228-231.
- Trivedi NA. A meta-analysis of low-dose aspirin for prevention of preeclampsia. J Postgrad Med. 2011;57:91-95.
- Lucky AW, Maloney JM, Roberts J, et al. Dapsone gel 5% for the treatment of acne vulgaris: safety and efficacy of long-term (1 year) treatment. J Drugs Dermatol. 2007;6:981-987.
- Nosten F, McGready R, d’Alessandro U, et al. Antimalarial drugs in pregnancy: a review. Curr Drug Saf. 2006;1:1-15.
- Brabin BJ, Eggelte TA, Parise M, et al. Dapsone therapy for malaria during pregnancy: maternal and fetal outcomes. Drug Saf. 2004;27:633-648.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Meredith FM, Ormerod AD. The management of acne vulgaris in pregnancy. Am J Clin Dermatol. 2013;14:351-358.
- Kong Y, Tey H. Treatment of acne vulgaris during pregnancy and lactation. Drugs. 2013;73:779-787.
- Leachman SA, Reed BR. The use of dermatologic drugs in pregnancy and lactation. Dermatol Clin. 2006;24:167-197.
- Ly S, Kamal K, Manjaly P, et al. Treatment of acne vulgaris during pregnancy and lactation: a narrative review. Dermatol Ther. 2023;13:115-130.
- Webster G. Combination azelaic acid therapy for acne vulgaris. J Am Acad Dermatol. 2000;43:S47-S50.
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clin Exp Dermatol. 2012;37(suppl 1):1-6.
- Graupe K, Cunliffe W, Gollnick H, et al. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996;57(1 suppl):20-35.
- Bozzo P, Chua-Gocheco A, Einarson A. Safety of skin care products during pregnancy. Can Fam Physician. 2011;57:665-667.
- Munley SM, Kennedy GL, Hurtt ME. Developmental toxicity study of glycolic acid in rats. Drug Chem Toxicol. 1999;22:569-582.
- Chien AL, Qi J, Rainer B, et al. Treatment of acne in pregnancy. J Am Board Fam Med. 2016;29:254-262.
- Stuart B, Maund E, Wilcox C, et al. Topical preparations for the treatment of mild‐to‐moderate acne vulgaris: systematic review and network meta‐analysis. Br J Dermatol. 2021;185:512-525.
- van Hoogdalem EJ, Baven TL, Spiegel‐Melsen I, et al. Transdermal absorption of clindamycin and tretinoin from topically applied anti‐acne formulations in man. Biopharm Drug Dispos. 1998;19:563-569.
- Austin BA, Fleischer AB Jr. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J Dermatolog Treat. 2017;28:145-148.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J. Dermatol. 1989;121:51-57.
- Alkhawaja E, Hammadi S, Abdelmalek M, et al. Antibiotic resistant Cutibacterium acnes among acne patients in Jordan: a cross sectional study. BMC Dermatol. 2020;20:1-9.
- Han G, Wu JJ, Del Rosso JQ. Use of topical tazarotene for the treatment of acne vulgaris in pregnancy: a literature review. J Clin Aesthet Dermatol. 2020;13:E59-E65.
- Selcen D, Seidman S, Nigro MA. Otocerebral anomalies associated with topical tretinoin use. Brain Dev. 2000;22:218-220.
- Moretz D. Drug Class Update with New Drug Evaluations: Topical Products for Inflammatory Skin Conditions. Oregon State University Drug Use & Research Management Program; December 2022. Accessed January 8, 2024. https://www.orpdl.org/durm/meetings/meetingdocs/2022_12_01/archives/2022_12_01_Inflammatory_Skin_Dz_ClassUpdate.pdf
- Kaplan YC, Ozsarfati J, Etwel F, et al. Pregnancy outcomes following first‐trimester exposure to topical retinoids: a systematic review and meta‐analysis. Br J Dermatol. 2015;173:1132-1141.
- Menter A. Pharmacokinetics and safety of tazarotene. J Am Acad Dermatol. 2000;43(2, pt 3):S31-S35.
- Autret E, Berjot M, Jonville-Béra A-P, et al. Anophthalmia and agenesis of optic chiasma associated with adapalene gel in early pregnancy. Lancet. 1997;350:339.
- Weiss J, Mallavalli S, Meckfessel M, et al. Safe use of adapalene 0.1% gel in a non-prescription environment. J Drugs Dermatol. 2021;20:1330-1335.
- Alessandro Mazzetti M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18:570-575.
- Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83:477-485.
- Trifu V, Tiplica GS, Naumescu E, et al. Cortexolone 17α‐propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. a pilot randomized, double‐blind comparative study vs. placebo and tretinoin 0.05% cream. Br J Dermatol. 2011;165:177-183.
- Hebert A, Thiboutot D, Gold LS, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630.
- Alkhodaidi ST, Al Hawsawi KA, Alkhudaidi IT, et al. Efficacy and safety of topical clascoterone cream for treatment of acne vulgaris: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Dermatol Ther. 2021;34:e14609.
- Clasoterone. Package insert. Cassiopea Inc; 2020.
- Paik J. Topical minocycline foam 4%: a review in acne vulgaris. Am J Clin Dermatol. 2020;21:449-456.
- Jones TM, Ellman H. Pharmacokinetic comparison of once-daily topical minocycline foam 4% vs oral minocycline for moderate-to-severe acne. J Drugs Dermatol. 2017;16:1022-1028.
- Minocycline hydrochloride extended-release tablets. Package insert. JG Pharma; July 2020. Accessed January 8, 2024. https://www.jgpharmainc.com/assets/pdf/minocycline-hydrochloride.pdf
- Dinnendahl V, Fricke U (eds). Arzneistoff-Profile: Basisinformation über arzneiliche Wirkstoffe. Govi Pharmazeutischer Verlag; 2010.
- Martins AM, Marto JM, Johnson JL, et al. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10:757.
- Landis MN. Optimizing isotretinoin treatment of acne: update on current recommendations for monitoring, dosing, safety, adverse effects, compliance, and outcomes. Am J Clin Dermatol. 2020;21:411-419.
- Draghici C-C, Miulescu R-G, Petca R-C, et al. Teratogenic effect of isotretinoin in both fertile females and males. Exp Ther Med. 2021;21:1-5.
- Barker RA, Wilcox C, Layton AM. Oral spironolactone for acne vulgaris in adult females: an update of the literature. Am J Clin Dermatol. 2020;21:303-305.
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins; 2012.
- Patel DJ, Bhatia N. Oral antibiotics for acne. Am J Clin Dermatol. 2021;22:193-204.
- Jick H, Holmes LB, Hunter JR, et al. First-trimester drug use and congenital disorders. JAMA. 1981;246:343-346.
- Valente Duarte de Sousa IC. An overview of sarecycline for the treatment of moderate-to-severe acne vulgaris. Exp Opin Pharmacother. 2021;22:145-154.
- Hussar DA, Chahine EB. Omadacycline tosylate, sarecycline hydrochloride, rifamycin sodium, and moxidectin. J Am Pharm Assoc. 2019;59:756-760.
- Haidari W, Bruinsma R, Cardenas-de la Garza JA, et al. Sarecycline review. Ann Pharmacother. 2020;54:164-170.
- Feldman S, Careccia RE, Barham KL, et al. Diagnosis and treatment of acne. Am Fam Physician. 2004;69:2123-2130.
- Gammon WR, Meyer C, Lantis S, et al. Comparative efficacy of oral erythromycin versus oral tetracycline in the treatment of acne vulgaris: a double-blind study. J Am Acad Dermatol. 1986;14:183-186.
- Källén BA, Olausson PO, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol. 2005;20:209-214.
- McCormack WM, George H, Donner A, et al. Hepatotoxicity of erythromycin estolate during pregnancy. Antimicrob Agents Chemother. 1977;12:630-635.
- Cervantes J, Eber AE, Perper M, et al. The role of zinc in the treatment of acne: a review of the literature. Dermatolog Ther. 2018;31:e12576.
- Dréno B, Blouin E. Acne, pregnant women and zinc salts: a literature review [in French]. Ann Dermatol Venereol. 2008;135:27-33.
- Eid MM, Saleh MS, Allam NM, et al. Narrow band ultraviolet B versus red light-emitting diodes in the treatment of facial acne vulgaris: a randomized controlled trial. Photobiomodul Photomed Laser Surg. 2021;39:418-424.
- Zeichner JA. Narrowband UV-B phototherapy for the treatment of acne vulgaris during pregnancy. Arch Dermatol. 2011;147:537-539.
- El-Saie LT, Rabie AR, Kamel MI, et al. Effect of narrowband ultraviolet B phototherapy on serum folic acid levels in patients with psoriasis. Lasers Med Sci. 2011;26:481-485.
- Park KK, Murase JE. Narrowband UV-B phototherapy during pregnancy and folic acid depletion. Arch Dermatol. 2012;148:132-133.
- Jablonski NG. A possible link between neural tube defects and ultraviolet light exposure. Med Hypotheses. 1999;52:581-582.
- Zhang M, Goyert G, Lim HW. Folate and phototherapy: what should we inform our patients? J Am Acad Dermatol. 2017;77:958-964.
- AviClear. Cutera website. Accessed January 8, 2024. https://www.cutera.com/solutions/aviclear/
- Wu X, Yang Y, Wang Y, et al. Treatment of refractory acne using selective sebaceous gland electro-thermolysis combined with non-thermal plasma. J Cosmet Laser Ther. 2021;23:188-194.
- Ahn GR, Kim JM, Park SJ, et al. Selective sebaceous gland electrothermolysis using a single microneedle radiofrequency device for acne patients: a prospective randomized controlled study. Lasers Surg Med. 2020;52:396-401.
- Fabbrocini G, De Padova MP, Tosti A. Chemical peels: what’s new and what isn’t new but still works well. Facial Plast Surg. 2009;25:329-336.
- Andersen FA. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and TEA-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. Int J Toxicol. 1998;17(1_suppl):1-241.
- Lee KC, Korgavkar K, Dufresne RG Jr, et al. Safety of cosmetic dermatologic procedures during pregnancy. Dermatol Surg. 2013;39:1573-1586.
- James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63:49-57.
- Zhou W-S, Xu L, Xie S-H, et al. Decreased birth weight in relation to maternal urinary trichloroacetic acid levels. Sci Total Environ. 2012;416:105-110.
- Schwartz DB, Greenberg MD, Daoud Y, et al. Genital condylomas in pregnancy: use of trichloroacetic acid and laser therapy. Am J Obstet Gynecol. 1988;158:1407-1416.
- Starkman SJ, Mangat DS. Chemical peel (deep, medium, light). Facial Plast Surg Clin North Am. 2020;28:45-57.
- Trivedi M, Kroumpouzos G, Murase J. A review of the safety of cosmetic procedures during pregnancy and lactation. Int J Womens Dermatol. 2017;3:6-10.
- Gallagher T, Taliercio M, Nia JK, et al. Dermatologist use of intralesional triamcinolone in the treatment of acne. J Clin Aesthet Dermatol. 2020;13:41-43.
- Zamil DH, Burns EK, Perez-Sanchez A, et al. Risk of birth defects from vitamin A “acne supplements” sold online. Dermatol Pract Concept. 2021;11:e2021075.
Practice Points
- The management of acne in pregnancy requires careful consideration of therapeutic choices to guarantee the safety of both the mother and the developing fetus.
- The use of topicals should be observed as first-line therapy, but consideration for systemic therapy in cases of treatment failure or more severe disease is warranted.
- Discussion of patient expectations and involving them in decision-making for therapeutic choice is crucial.
Analysis of Nail Excision Practice Patterns in the Medicare Provider Utilization and Payment Database 2012-2017
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.
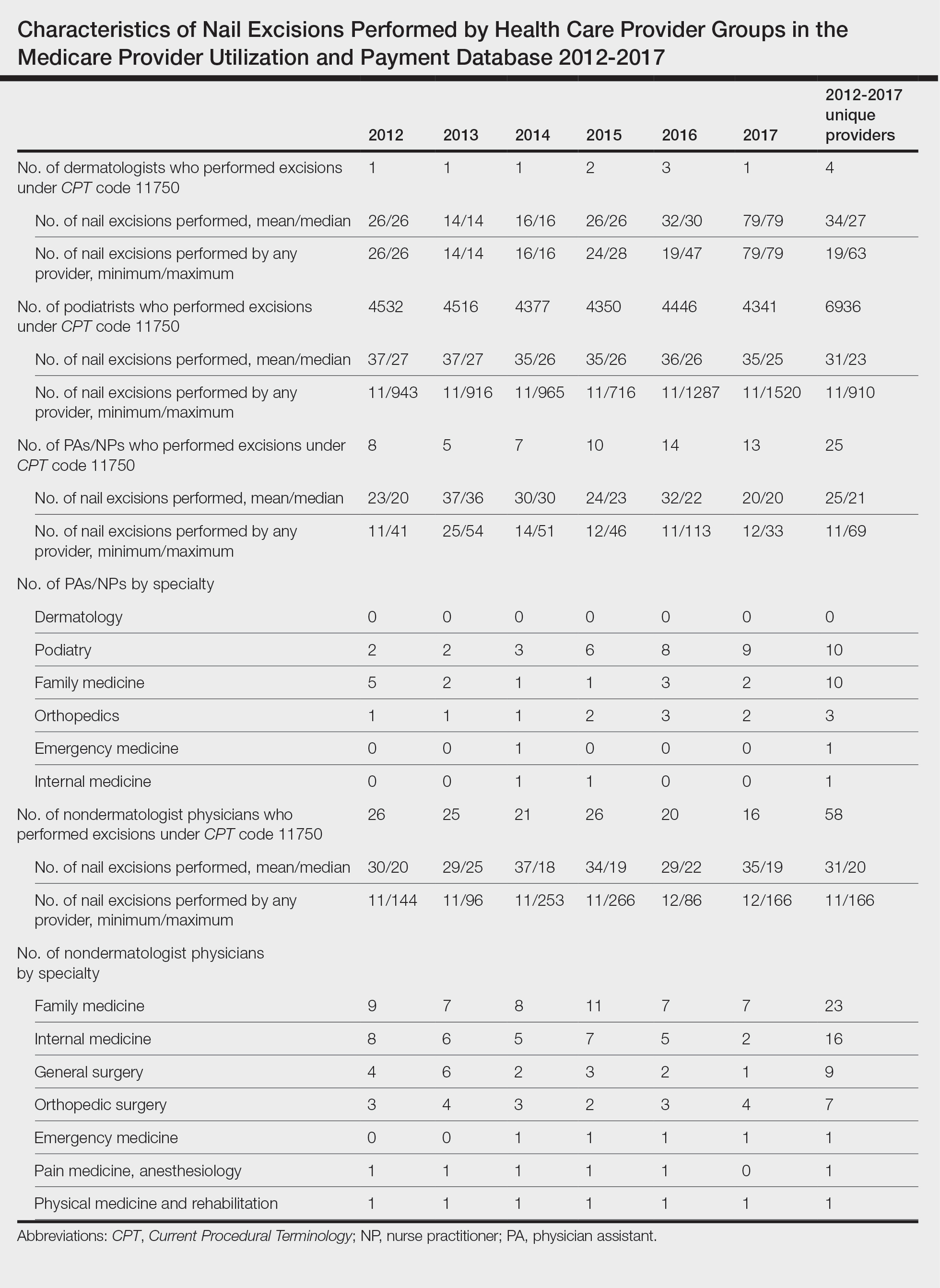
A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).
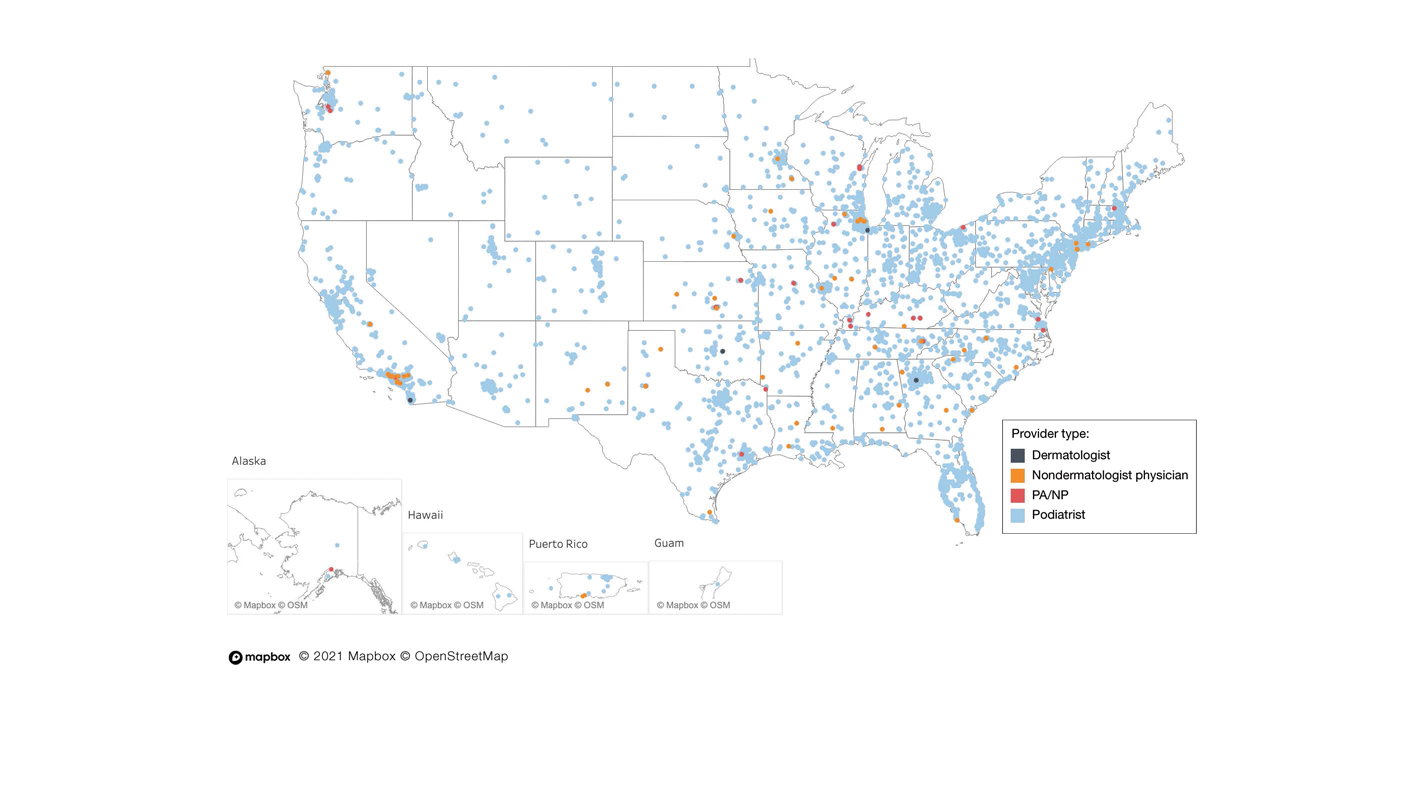
Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
Practice Points
- Dermatologists are considered nail experts but perform nail excisions less frequently than their podiatric counterparts and physicians in other specialties.
- Aspects of podiatric surgical training should be incorporated into dermatology residency to increase competency and comfort of dermatologists in nail excision procedures.
- Dermatologists may not be perceived as nail experts by the public, indicating a need for increased community education on the role of dermatologists in treating nail disease.