User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
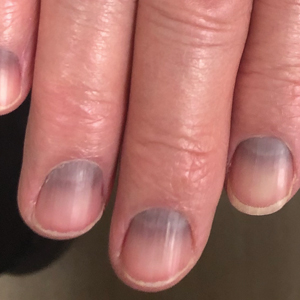
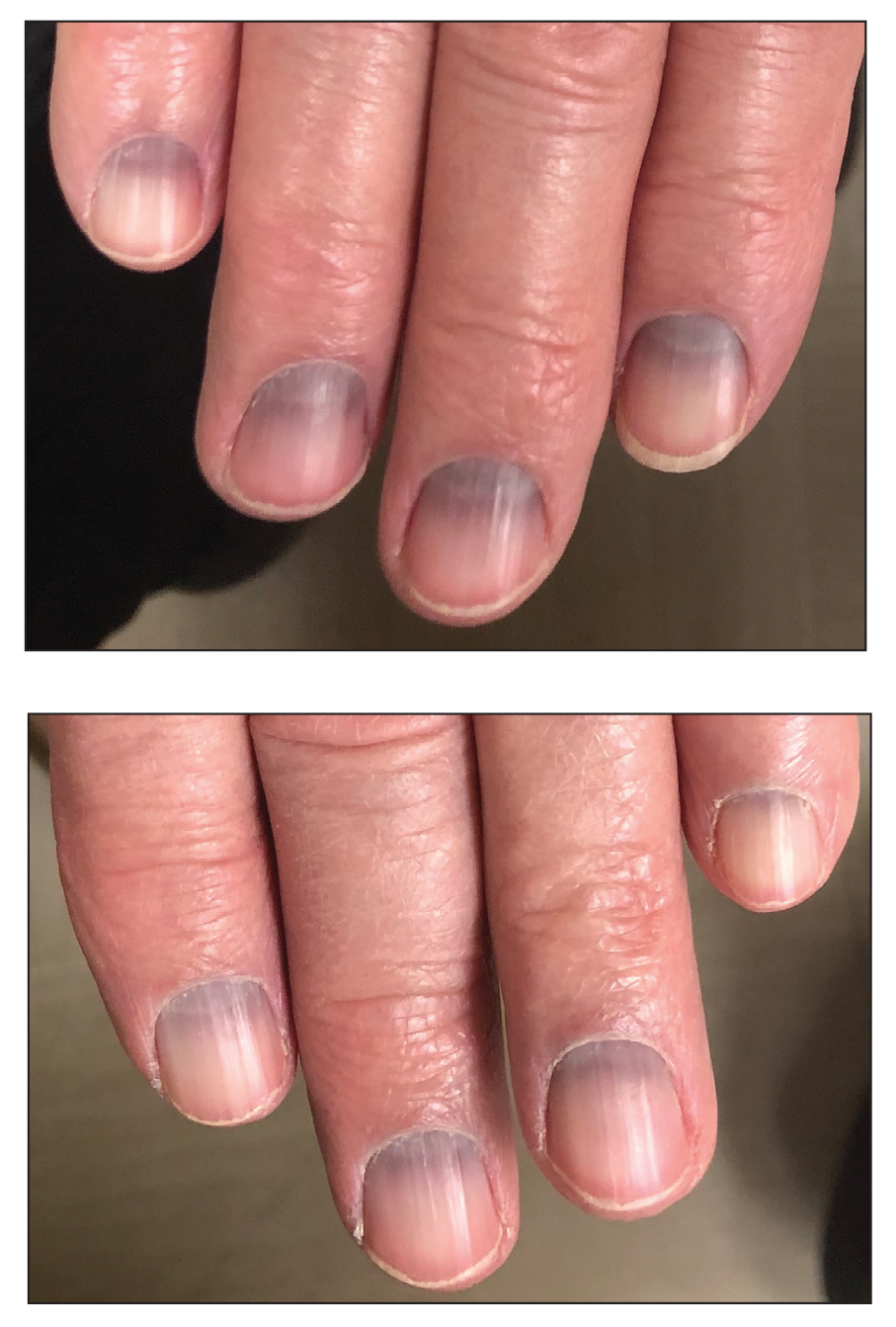
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
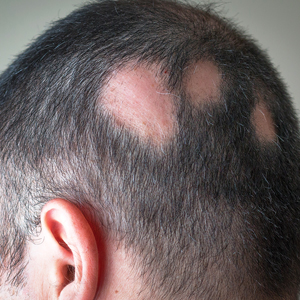
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
Superficial Vascular Anomaly of the Glabella Mimicking a Cutaneous Cyst
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
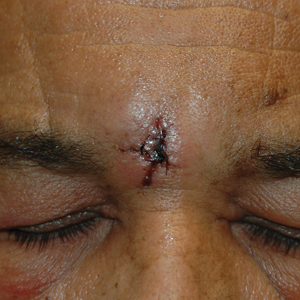
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.
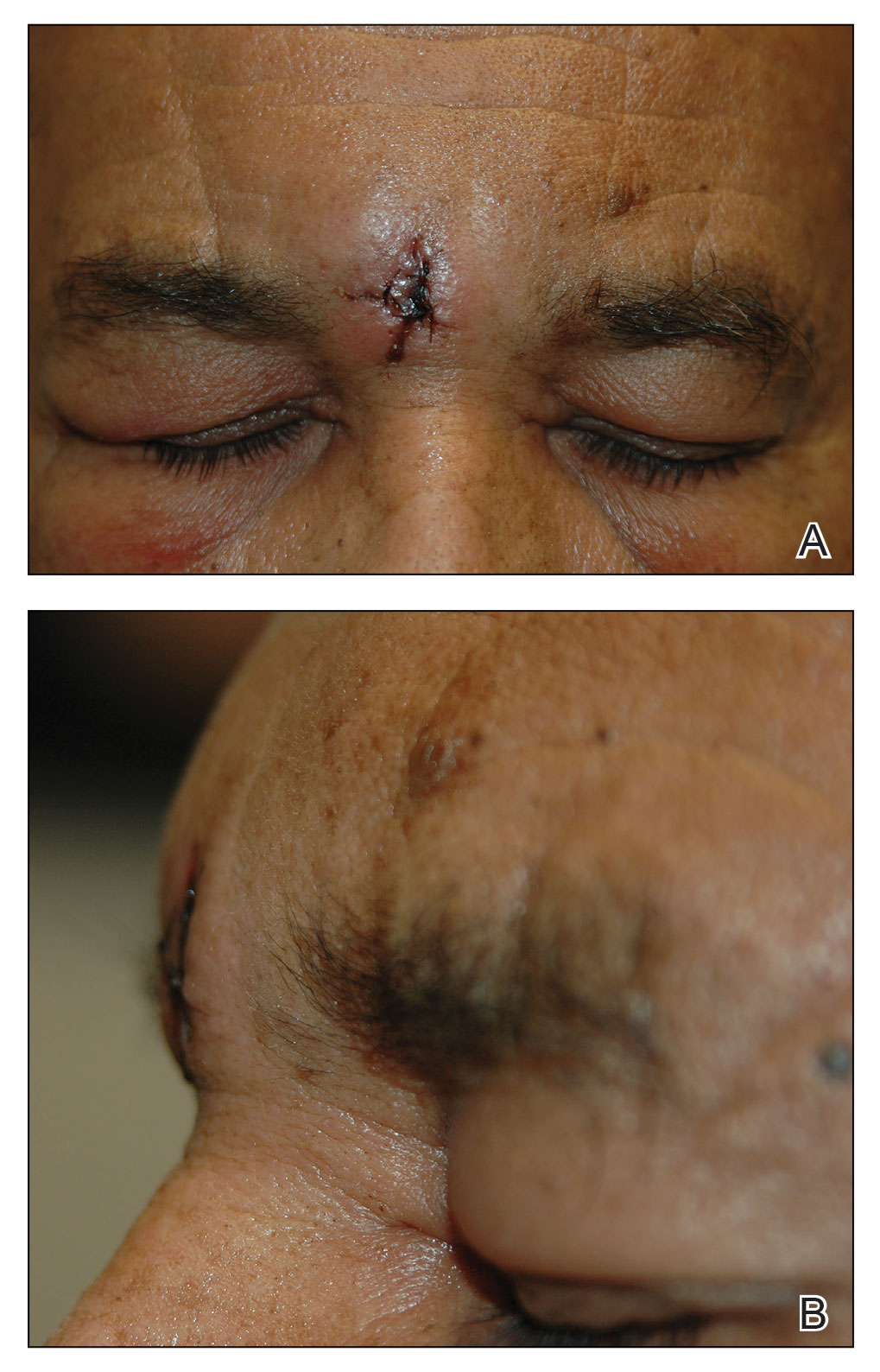
A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
Practice Points
- Vascular anomalies should be included in the differential diagnosis of soft cutaneous nodules, as management differs from cysts or lipomas.
- Preoperative evaluation for a cutaneous cyst excision on the head and neck should include ruling out findings of a vascular lesion through history, physical examination, and consideration of color Doppler ultrasonography in unclear cases.
- Surgical technique should involve sequential superficial incisions, descending at 1 to 2 mm per cut, until the suspected capsule is identified to minimize the risk for inadvertent injury to a cyst mimicker such as a vascular anomaly.
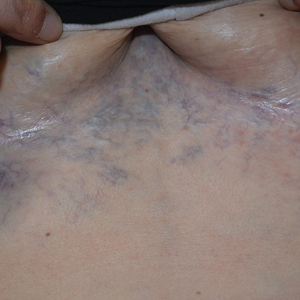
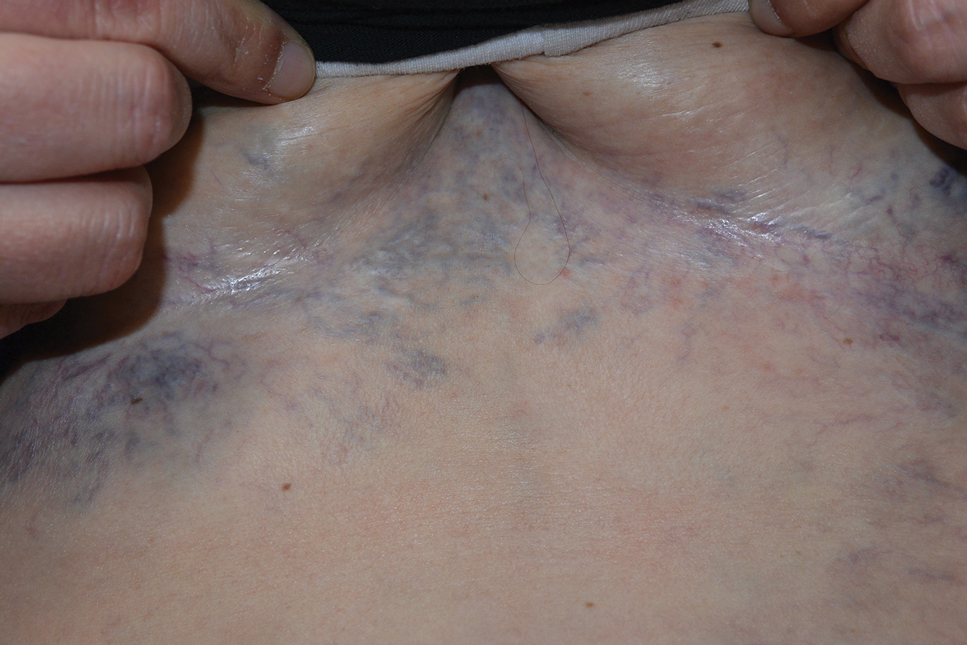
Ectatic Vessels on the Chest
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
The Diagnosis: Superior Vena Cava Syndrome
Computed tomography angiography of the chest confirmed a diagnosis of superior vena cava (SVC) syndrome due to external pressure of the indwelling catheter. Upon diagnosis, the left indwelling catheter was removed. Further testing to assess for a potential pulmonary embolism was negative. Resolution of the ectatic spider veins and patientreported intermittent facial swelling was achieved after catheter removal.
Superior vena cava syndrome occurs when the SVC is occluded due to extrinsic pressure or thrombosis. Although classically thought to be due to underlying bronchogenic carcinomas, all pathologies that cause compression of the SVC also can lead to vessel occlusion.1 Superior vena cava syndrome initially can be detected on physical examination. The most prominent skin finding includes diffusely dilated blood vessels on the central chest wall, which indicate the presence of collateral blood vessels.1 Imaging studies such as abdominal computed tomography can provide information on the etiology of the condition but are not required for diagnosis. Given the high correlation of SVC syndrome with underlying lung and mediastinal carcinomas, imaging was warranted in our patient. Imaging also can distinguish if the condition is due to external pressure or thrombosis.2 For SVC syndrome due to thrombosis, endovascular therapy is first-line management; however, mechanical thrombectomy may be preferred in patients with absolute contraindication to thrombolytic agents.3 In the setting of increased external pressure on the SVC, treatment includes the removal of the source of pressure.4
In a case series including 78 patients, ports and indwelling catheters accounted for 71% of benign SVC cases.5 Our patient’s SVC syndrome most likely was due to the indwelling catheter pressing on the SVC. The goal of treatment is to address the underlying cause—whether it be pressure or thrombosis. In the setting of increased external pressure, treatment includes removal of the source of pressure from the SVC.4
Other differential diagnoses to consider for newonset ectatic vessels on the chest wall include generalized essential telangiectasia, scleroderma, poikiloderma vasculare atrophicans, and caput medusae. Generalized essential telangiectasia is characterized by red or pink dilated capillary blood vessels in a branch or lacelike pattern predominantly on the lower limbs. The eruption primarily is asymptomatic, though tingling or numbness may be reported.6 The diagnosis can be made with a punch biopsy, with histopathology showing dilated vessels in the dermis.7
Scleroderma is a connective tissue fibrosis disorder with variable clinical presentations. The systemic sclerosis subset can be divided into localized systemic sclerosis and diffuse systemic sclerosis. Physical examination reveals cutaneous sclerosis in various areas of the body. Localized systemic sclerosis includes sclerosis of the fingers and face, while diffuse systemic sclerosis is notable for progression to the arms, legs, and trunk.8 In addition to sclerosis, diffuse telangiectases also can be observed. Systemic sclerosis is a clinical diagnosis based on physical examination and laboratory studies to identify antibodies such as antinuclear antibodies.
Poikiloderma vasculare atrophicans is a variant of cutaneous T-cell lymphoma. The initial presentation is characterized by plaques of hypopigmentation and hyperpigmentation with atrophy and telangiectases. The lesions may be asymptomatic or mildly pruritic and classically involve the trunk and flexural areas.9 The diagnosis is made with skin biopsy and immunohistochemical studies, with findings reflective of mycosis fungoides.
Caput medusae (palm tree sign) is a cardinal feature of portal hypertension characterized by grossly dilated and engorged periumbilical veins. To shunt blood from the portal venous system, cutaneous collateral veins between the umbilical veins and abdominal wall veins are used, resulting in the appearance of engorged veins in the anterior abdominal wall.10 The diagnosis can be made with abdominal ultrasonography showing the direction of blood flow through abdominal vessels.
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
- Drouin L, Pistorius MA, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases [in French]. Rev Med Interne. 2019;40:9-15.
- Richie E. Clinical pearl: diagnosing superior vena cava syndrome. Emergency Medicine News. 2017;39:22. doi:10.1097/01 .EEM.0000522220.37441.d2
- Azizi A, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020;13:2896-2910. doi:10.1016/j.jcin.2020.08.038
- Dumantepe M, Tarhan A, Ozler A. Successful treatment of central venous catheter induced superior vena cava syndrome with ultrasound accelerated catheter-directed thrombolysis. Catheter Cardiovasc Interv. 2013;81:E269-E273.
- Rice TW, Rodriguez RM, Light RW. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore) 2006;85:37-42. doi:10.1097/01.md.0000198474.99876.f0
- Long D, Marshman G. Generalized essential telangiectasia. Australas J Dermatol. 2004;45:67-69. doi:10.1111/j.1440-0960.2004.00033.x
- Braverman IM. Ultrastructure and organization of the cutaneous microvasculature in normal and pathologic states. J Invest Dermatol. 1989;93(2 suppl):2S-9S.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336. doi:10.1007 /s12016-017-8625-4
- Bloom B, Marchbein S, Fischer M, et al. Poikilodermatous mycosis fungoides. Dermatol Online J. 2012;18:4.
- Sharma B, Raina S. Caput medusae. Indian J Med Res. 2015;141:494. doi:10.4103/0971-5916.159322
A 32-year-old woman presented to vascular surgery for evaluation of spider veins of 2 years’ duration that originated on the breasts but later spread to include the central chest, inframammary folds, and back. She reported associated pain and discomfort as well as intermittent facial swelling and tachycardia but denied pruritus and bleeding. The patient had a history of a kidney transplant 6 months prior, Langerhans cell histiocytosis, and Sjögren syndrome with a left indwelling catheter. Her current medications included systemic immunosuppressive agents. Physical examination revealed blue-purple ectatic vessels on the inframammary folds and central chest extending to the back. Erythema on the face, neck, and arms was not appreciated. No palpable cervical, supraclavicular, or axillary lymph nodes were noted.
Fall Abstract Las Vegas Dermatology Seminar Compendium; Las Vegas, Nevada; November 2-4, 2023
Botanical Briefs: Neem Oil (Azadirachta indica)
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
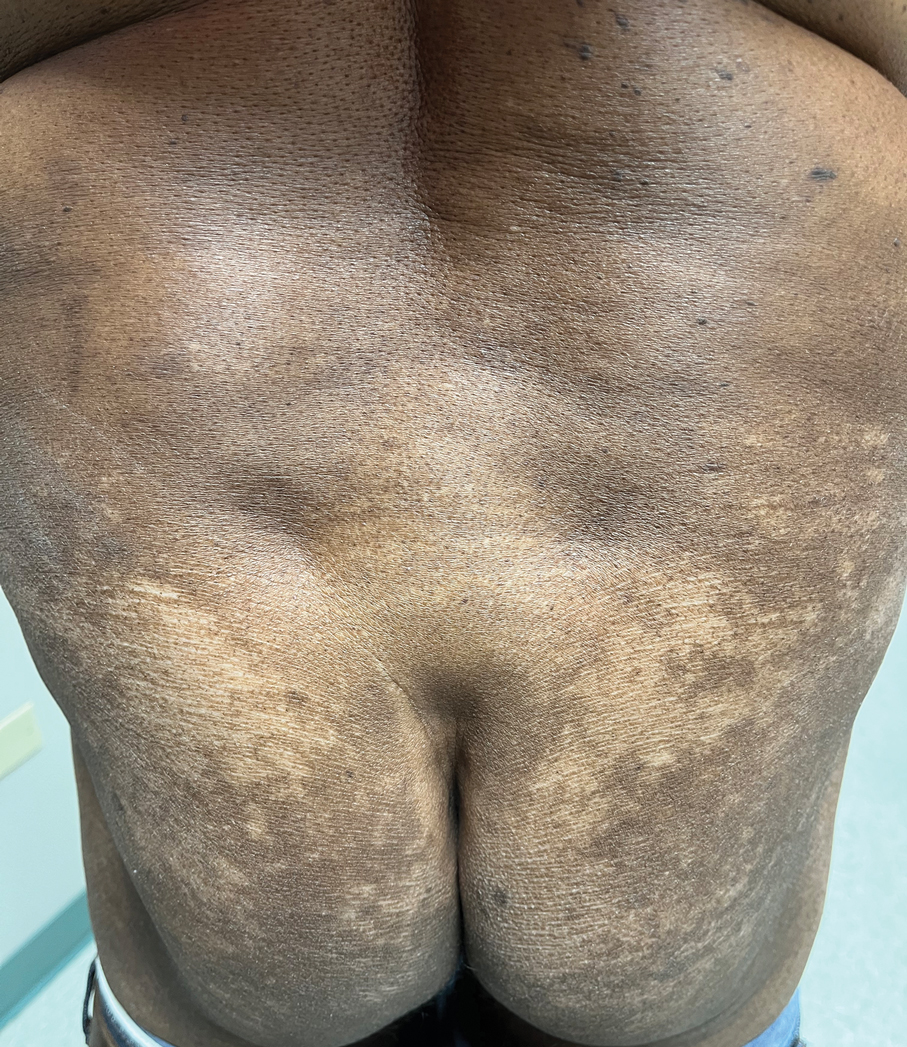
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.
Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Practice Points
- Neem is a traditional herb with various bioactivities, such as melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.
- Neem should be used with caution as a remedy because of its skin-lightening properties, which are attributed to melanogenesis-inhibitory activity via tyrosinase inhibition.
- Chemical leukoderma should be included in the differential diagnosis when a patient presents with a hypopigmented rash after topical use of neem products.
Analysis of Online Diet Recommendations for Vitiligo
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.
From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).
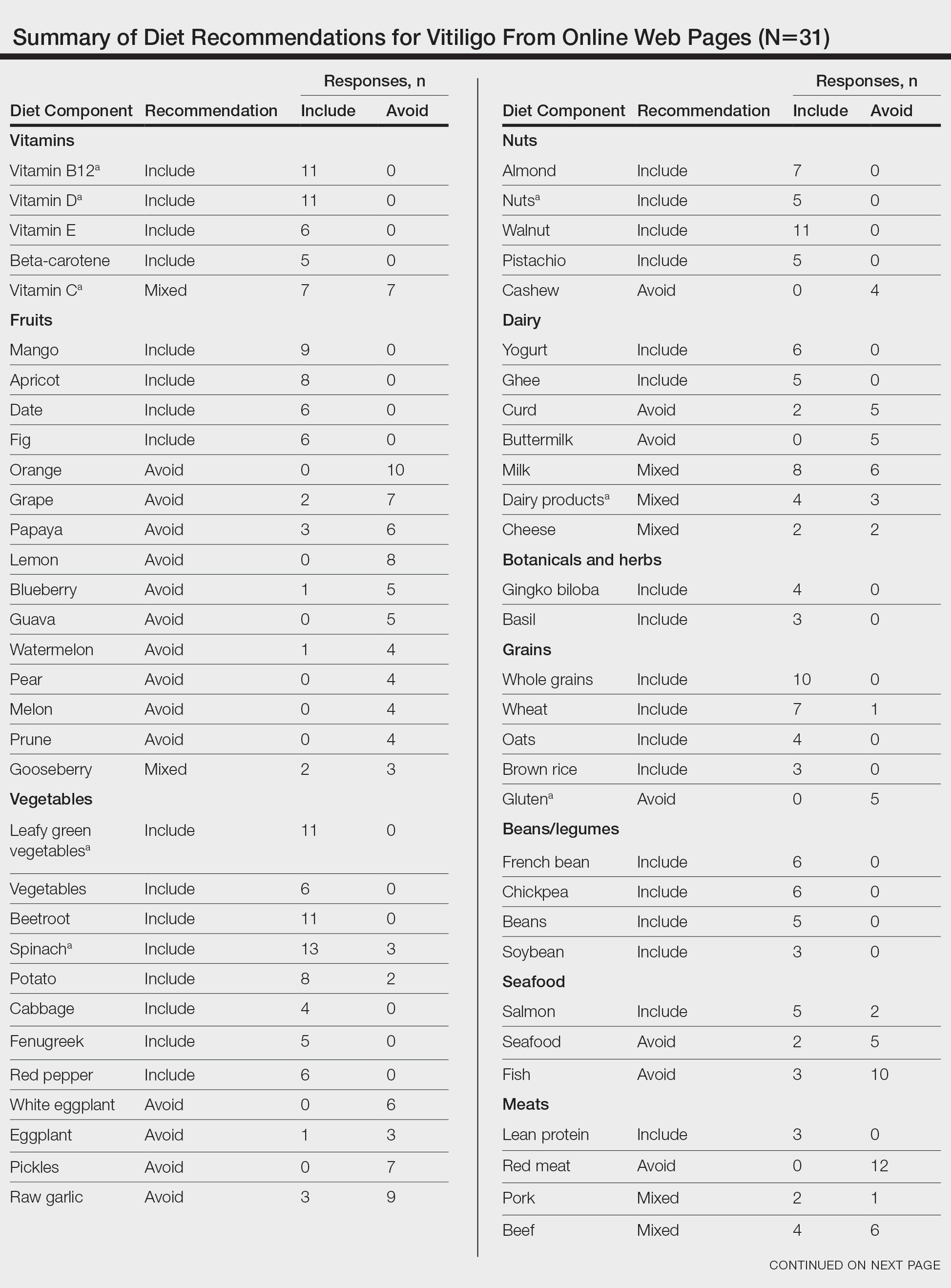
For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Practice Points
- There are numerous online dietary and supplement recommendations that claim to impact vitiligo but most are not authored by medical professionals or dermatologists.
- Scientific evidence supporting specific dietary and supplement recommendations for vitiligo is limited.
- Current preliminary data support the potential recommendation for dietary supplementation with vitamin D, vitamin B12, zinc, and omega-3 fatty acids.
Culprits of Medication-Induced Telogen Effluvium, Part 2
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
Medication-induced telogen effluvium (TE) is a nonscarring alopecia that typically is reversible. Appropriate management requires identification of the underlying trigger and cessation of potential culprit medications. In part 2 of this series, we review anticoagulant and antihypertensive medications as potential contributors to TE.
Anticoagulants
Anticoagulants target various parts of the coagulation cascade to prevent clot formation in patients with conditions that increase their risk for thromboembolic events. Common indications for initiating anticoagulant therapy include atrial fibrillation,1 venous thromboembolism,2 acute myocardial infarction,3 malignancy,4 and hypercoagulable states.5 Traditional anticoagulants include heparin and warfarin. Heparin is a glycosaminoglycan that exerts its anticoagulant effects through binding with antithrombin, greatly increasing its inactivation of thrombin and factor Xa of the coagulation cascade.6 Warfarin is a coumarin derivative that inhibits activation of vitamin K, subsequently limiting the function of vitamin K–dependent factors II, VII, IX, and X.7,8 Watras et al9 noted that heparin and warfarin were implicated in alopecia as their clinical use became widespread throughout the mid-20th century. Onset of alopecia following the use of heparin or warfarin was reported at 3 weeks to 3 months following medication initiation, with most cases clinically consistent with TE.9 Heparin and warfarin both have alopecia reported as a potential adverse effect in their structured product labeling documents.10,11
Heparin is further classified into unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH); the latter is a heterogeneous group of medications derived from chemical or enzymatic depolymerization of UFH.12 In contrast to UFH, LMWH exerts its anticoagulant effects through inactivation of factor Xa without the ability to bind thrombin.12 An animal study using anagen-induced mice demonstrated that intraperitoneal administration of heparin inhibited the development of anagen follicles, while in vitro studies showed that the addition of heparin inhibited mouse dermal papilla cell proliferation.13 Other animal and in vitro studies have examined the inhibitory effects of heparin on signaling pathways in tumor lymphangiogenesis, including the vascular endothelial growth factor C/vascular endothelial growth factor receptor 3 axis.14,15 Clinically, it has been demonstrated that heparin, especially LMWHs, may be associated with a survival benefit among certain cancer patients,16,17 with the impact of LMWHs attributed to antimitotic and antimetastatic effects of heparin on tumor growth.14 It is hypothesized that such antiangiogenic and antimitotic effects also are involved in the pathomechanisms of heparin-induced alopecia.18
More recently, the use of direct oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, and apixaban has increased due to their more favorable adverse-effect profile and minimal monitoring requirements. Bonaldo et al19 conducted an analysis of reports submitted to the World Health Organization’s VigiBase database of alopecia associated with DOACs until May 2, 2018. They found 1316 nonduplicate DOAC-induced cases of alopecia, with rivaroxaban as the most reported drug associated with alopecia development (58.8% [774/1316]). Only 4 cases demonstrated alopecia with DOAC rechallenge, suggesting onset of alopecia may have been unrelated to DOAC use or caused by a different trigger. Among 243 cases with a documented time to onset of alopecia, the median was 28 days, with an interquartile range of 63 days. Because TE most commonly occurs 3 to 4 months after the inciting event or medication trigger, there is little evidence to suggest DOACs as the cause of TE, and the observed cases of alopecia may be attributable to another preceding medical event and/or medication exposure.19 More studies are needed to examine the impact of anticoagulant medications on the hair cycle.
Antihypertensives
Hypertension is a modifiable risk factor for several cardiovascular diseases.20 According to the 2019 American College of Cardiology/American Heart Association Guideline on the Primary Prevention of Cardiovascular Disease,21 first-line medications include thiazide diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs).
Angiotensin-converting enzyme inhibitors exert their antihypertensive effects by reducing conversion of angiotensin I to angiotensin II, thereby limiting the downstream effects of vasoconstriction as well as sodium and water retention. Given the proven mortality benefit of ACE inhibition in patients with congestive heart failure, ACE inhibitors are used as first-line therapy in these patients.22,23 Alopecia associated with ACE inhibitors is rare and limited to case reports following their introduction and approval in 1981.24-28 In one case, a woman in her 60s with congestive heart failure initiated captopril with development of an erythematous pruritic rash on the extremities and diffuse scalp hair loss 2 months later; spontaneous hair growth resumed 1 month following captopril discontinuation.25 In this case, the hair loss may be secondary to the drug eruption rather than true medication-induced TE. Initiation of enalapril in a woman in her 30s with hypertension was associated with diffuse scalp alopecia 4 weeks later that resolved with cessation of the suspected culprit, enalapril; rechallenge with enalapril several months later reproduced the hair loss.27 Given limited reports of ACE inhibitor–associated hair loss relative to their pervasive use, a direct causal role between ACE inhibition and TE is unlikely, or it has not been rigorously identified. The structured product labeling for captopril includes alopecia in its list of adverse effects reported in approximately 0.5% to 2% of patients but did not appear at increased frequency compared to placebo or other treatments used in controlled trials.29 Alternative inciting causes of alopecia in patients prescribed ACE inhibitors may include use of other medications, hospitalization, or metabolic derangements related to their underlying cardiac disease.
Although not indicated as a primary treatment for hypertension, β-blockers have US Food and Drug Administration approval for the treatment of certain arrhythmias, hypertension, heart failure, myocardial infarction, hyperthyroidism, and other conditions.30β-Blockers are competitive antagonists of β-adrenergic receptors that limit the production of intracellular cyclic adenosine monophosphate, but the mechanism of β-blockers as antihypertensives is unclear.31 Evidence supporting the role of β-adrenergic antagonists in TE is limited to case reports. Widespread alopecia across the scalp and arms was noted in a man in his 30s several months after starting propranolol.32 Biopsy of an affected area of the scalp demonstrated an increased number of telogen follicles with no other abnormalities. Near-complete resolution of alopecia was seen 4 months following cessation of propranolol, which recurred within 4 weeks of rechallenge.
Minoxidil—Oral minoxidil originally was approved for use in patients with resistant hypertension, defined as blood pressure elevated above goal despite concurrent use of the maximum dose of 3 classes of antihypertensives.36 Unlike other antihypertensive medications, minoxidil appears to cause reversible hypertrichosis that affects nearly all patients using oral minoxidil for longer than 1 month.37 This common adverse effect was a desired outcome in patients affected by hair loss, and a topical formulation of minoxidil was approved for androgenetic alopecia in men and women in 1988 and 1991, respectively.38 Since its approval, topical minoxidil has been commonly prescribed in the treatment of several types of alopecia, though evidence of its efficacy in the treatment of TE is limited.39,40 Low-dose oral minoxidil also has been reported to aid hair growth in androgenetic alopecia and TE.41 Taken orally, minoxidil is converted by sulfotransferases in the liver to minoxidil sulfate, which causes opening of plasma membrane adenosine triphosphate–sensitive potassium channels.42-44 The subsequent membrane hyperpolarization reduces calcium ion influx, which also reduces cell excitability, and inhibits contraction in vascular smooth muscle cells, which results in the arteriolar vasodilatory and antihypertensive effects of minoxidil.43,45 The potassium channel–opening effects of minoxidil may underly its hair growth stimulatory action. Unrelated potassium channel openers such as diazoxide and pinacidil also cause hypertrichosis.46-48 An animal study showed that topical minoxidil, cromakalim (potassium channel opener), and P1075 (pinacidil analog) applied daily to the scalps of balding stump-tailed macaques led to significant increases in hair weight over a 20-week treatment period compared with the vehicle control group (P<.05 for minoxidil 100 mM and 250 mM, cromakalim 100 mM, and P1075 100 mM and 250 mM).50 For minoxidil, this effect on hair growth appears to be dose dependent, as cumulative hair weights for the study period were significantly greater in the 250-mM concentration compared with 100-mM minoxidil (P<.05).49 The potassium channel–opening activity of minoxidil may induce stimulation of microcirculation around hair follicles conducive to hair growth.50 Other proposed mechanisms for hair growth with minoxidil include effects on keratinocyte and fibroblast cell proliferation,51-53 collagen synthesis,52,54 and prostaglandin activity.44,55
Final Thoughts
Medication-induced TE is an undesired adverse effect of many commonly used medications, including retinoids, azole antifungals, mood stabilizers, anticoagulants, and antihypertensives. In part 156 of this 2-part series, we reviewed the existing literature on hair loss from retinoids, antifungals, and psychotropic medications. Herein, we focused on anticoagulant and antihypertensive medications as potential culprits of TE. Heparin and its derivatives have been associated with development of diffuse alopecia weeks to months after the start of treatment. Alopecia associated with ACE inhibitors and β-blockers has been described only in case reports, suggesting that they may be unlikely causes of TE. In contrast, minoxidil is an antihypertensive that can result in hypertrichosis and is used in the treatment of androgenetic alopecia. It should not be assumed that medications that share an indication or are part of the same medication class would similarly induce TE. The development of diffuse nonscarring alopecia should prompt suspicion for TE and thorough investigation of medications initiated 1 to 6 months prior to onset of clinically apparent alopecia. Suspected culprit medications should be carefully assessed for their likelihood of inducing TE.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
- Angiolillo DJ, Bhatt DL, Cannon CP, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583-596. doi:10.1161 /circulationaha.120.050438
- Kearon C, Kahn SR. Long-term treatment of venous thromboembolism. Blood. 2020;135:317-325. doi:10.1182/blood.2019002364
- Frishman WH, Ribner HS. Anticoagulation in myocardial infarction: modern approach to an old problem. Am J Cardiol. 1979;43:1207-1213. doi:10.1016/0002-9149(79)90155-3
- Khorana AA, Mackman N, Falanga A, et al. Cancer-associated venous thromboembolism. Nat Rev Dis Primers. 2022;8:11. doi:10.1038 /s41572-022-00336-y
- Umerah CO, Momodu, II. Anticoagulation. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK560651/
- Beurskens DMH, Huckriede JP, Schrijver R, et al. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120:1371-1383. doi:10.1055/s-0040-1715460
- Hirsh J, Dalen J, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 suppl):8S-21S. doi:10.1378/chest.119.1_suppl.8s
- Holbrook AM, Pereira JA, Labiris R, et al. Systematic overview of warfarin and its drug and food interactions. Arch Intern Med. 2005;165:1095-1106. doi:10.1001/archinte.165.10.1095
- Watras MM, Patel JP, Arya R. Traditional anticoagulants and hair loss: a role for direct oral anticoagulants? a review of the literature. Drugs Real World Outcomes. 2016;3:1-6. doi:10.1007/s40801-015-0056-z
- Heparin sodium. Product information. Hepalink USA Inc; January 2022. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c4c6bc1f-e0c7-fd0d-e053-2995a90abdef/spl-doc?hl=heparin
- Warfarin sodium. Product information. Bryant Ranch Prepack; April 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/c41b7c23-8053-428a-ac1d-8395e714c2f1/spl-doc?hl=alopecia%7Cwarfarin#section-6
- Hirsh J. Low-molecular-weight heparin. Circulation. 1998;98:1575-1582. doi:10.1161/01.CIR.98.15.1575
- Paus R. Hair growth inhibition by heparin in mice: a model system for studying the modulation of epithelial cell growth by glycosaminoglycans? Br J Dermatol. 1991;124:415-422. doi:10.1111/j.1365-2133.1991.tb00618.x
- Ma SN, Mao ZX, Wu Y, et al. The anti-cancer properties of heparin and its derivatives: a review and prospect. Cell Adh Migr. 2020;14:118-128. doi:10.1080/19336918.2020.1767489
- Choi JU, Chung SW, Al-Hilal TA, et al. A heparin conjugate, LHbisD4, inhibits lymphangiogenesis and attenuates lymph node metastasis by blocking VEGF-C signaling pathway. Biomaterials. 2017;139:56-66. doi:0.1016/j.biomaterials.2017.05.026
- Klerk CP, Smorenburg SM, Otten HM, et al. The effect of low molecular weight heparin on survival in patients with advanced malignancy. J Clin Oncol. 2005;23:2130-2135. doi:10.1200/jco.2005.03.134
- Altinbas M, Coskun HS, Er O, et al. A randomized clinical trial of combination chemotherapy with and without low-molecular-weight heparin in small cell lung cancer. J Thromb Haemost. 2004;2:1266-1271. doi:10.1111/j.1538-7836.2004.00871.x
- Weyand AC, Shavit JA. Agent specific effects of anticoagulant induced alopecia. Res Pract Thromb Haemost. 2017;1:90-92. doi:10.1002 /rth2.12001
- Bonaldo G, Vaccheri A, Motola D. Direct-acting oral anticoagulants and alopecia: the valuable support of postmarketing data. Br J Clin Pharmacol. 2020;86:1654-1660. doi:10.1111/bcp.14221
- Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285-292. doi:10.1161 /HYPERTENSIONAHA.119.14240
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:E596-E646. doi:10.1161/CIR.0000000000000678
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:E240-E327. doi:10.1161 /CIR.0b013e31829e8776
- Effects of enalapril on mortality in severe congestive heart failure. results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316:1429-1435. doi:10.1056 /nejm198706043162301
- Kataria V, Wang H, Wald JW, et al. Lisinopril-induced alopecia: a case report. J Pharm Pract. 2017;30:562-566. doi:10.1177/0897190016652554
- Motel PJ. Captopril and alopecia: a case report and review of known cutaneous reactions in captopril use. J Am Acad Dermatol. 1990;23:124-125. doi:10.1016/s0190-9622(08)81205-4
- Leaker B, Whitworth JA. Alopecia associated with captopril treatment. Aust N Z J Med. 1984;14:866. doi:10.1111/j.1445-5994.1984.tb03797.x
- Ahmad S. Enalapril and reversible alopecia. Arch Intern Med. 1991;151:404.
- Bicket DP. Using ACE inhibitors appropriately. Am Fam Physician. 2002;66:461-468.
- Captopril. Product information. Bryant Ranch Prepack; May 2023. Accessed December 11, 2023. https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/563737c5-4d63-4957-8022-e3bc3112dfac/spl-doc?hl=captopril
- Farzam K, Jan A. Beta blockers. StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK532906/
- Mason RP, Giles TD, Sowers JR. Evolving mechanisms of action of beta blockers: focus on nebivolol. J Cardiovasc Pharmacol. 2009; 54:123-128.
- Martin CM, Southwick EG, Maibach HI. Propranolol induced alopecia. Am Heart J. 1973;86:236-237. doi:10.1016/0002-8703(73)90250-0
- Graeber CW, Lapkin RA. Metoprolol and alopecia. Cutis. 1981; 28:633-634.
- Hilder RJ. Propranolol and alopecia. Cutis. 1979;24:63-64.
- Coreg. Prescribing information. Woodward Pharma Services LLC; 2023. Accessed December 11, 2023. https://www.accessdata.fda.gov/spl/data/34aa881a-3df4-460b-acad-fb9975ca3a06/34aa881a-3df4-460b-acad-fb9975ca3a06.xml
- Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:E53-E90. doi:10.1161/hyp.0000000000000084
- Campese VM. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257-278. doi:10.2165/00003495-198122040-00001
- Heymann WR. Coming full circle (almost): low dose oral minoxidil for alopecia. J Am Acad Dermatol. 2021;84:613-614. doi:10.1016/j .jaad.2020.12.053
- Yin S, Zhang B, Lin J, et al. Development of purification process for dual-function recombinant human heavy-chain ferritin by the investigation of genetic modification impact on conformation. Eng Life Sci. 2021;21:630-642. doi:10.1002/elsc.202000105
- Mysore V, Parthasaradhi A, Kharkar RD, et al. Expert consensus on the management of telogen effluvium in India. Int J Trichology. 2019;11:107-112.
- Gupta AK, Talukder M, Shemar A, et al. Low-dose oral minoxidil for alopecia: a comprehensive review [published online September 27, 2023]. Skin Appendage Disord. doi:10.1159/000531890
- Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988;245:751-760.
- Winquist RJ, Heaney LA, Wallace AA, et al. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989;248:149-56.
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j .1365-2133.2004.05785.x
- Alijotas-Reig J, García GV, Velthuis PJ, et al. Inflammatory immunemediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol. 2022;21:3181-3187. doi: 10.1111/jocd.15117
- Boskabadi SJ, Ramezaninejad S, Sohrab M, et al. Diazoxideinduced hypertrichosis in a neonate with transient hyperinsulinism. Clin Med Insights Case Rep. 2023;16:11795476231151330. doi:10.1177/11795476231151330
- Burton JL, Schutt WH, Caldwell IW. Hypertrichosis due to diazoxide. Br J Dermatol. 1975;93:707-711. doi:10.1111/j.1365-2133.1975.tb05123.x
- Goldberg MR. Clinical pharmacology of pinacidil, a prototype for drugs that affect potassium channels. J Cardiovasc Pharmacol. 1988;12 suppl 2:S41-S47. doi: 10.1097/00005344-198812002-00008
- Buhl AE, Waldon DJ, Conrad SJ, et al. Potassium channel conductance: a mechanism affecting hair growth both in vitro and in vivo. J Invest Dermatol. 1992;98:315-319. doi:10.1111/1523-1747.ep12499788
- Patel P, Nessel TA, Kumar DD. Minoxidil. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK482378/
- O’Keefe E, Payne RE Jr. Minoxidil: inhibition of proliferation of keratinocytes in vitro. J Invest Dermatol. 1991;97:534-536. doi:10.1111/1523-1747.ep12481560
- Murad S, Pinnell SR. Suppression of fibroblast proliferation and lysyl hydroxylase activity by minoxidil. J Biol Chem. 1987;262:11973-11978.
- Baden HP, Kubilus J. Effect of minoxidil on cultured keratinocytes. J Invest Dermatol. 1983;81:558-560. doi:10.1111/1523-1747.ep12523220
- Murad S, Walker LC, Tajima S, et al. Minimum structural requirements for minoxidil inhibition of lysyl hydroxylase in cultured fibroblasts. Arch Biochem Biophys. 1994;308:42-47. doi:10.1006/abbi.1994.1006
- Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37:867-874. doi:0.1016/0006-2952(88)90174-8
- Zhang D, LaSenna C, Shields BE. Culprits of medication-induced telogen effluvium, part 1. Cutis. 2023;112:267-271.
Practice Points
- Medications are a common culprit of telogen effluvium (TE), and medication-induced TE should be suspected in patients presenting with diffuse nonscarring alopecia who are taking systemic medication(s) such as heparin and its derivatives.
- Infection, illness, or hospitalization around the time of initiation of the suspected culprit medication may complicate identification of the inciting cause and may contribute to TE.
- Angiotensin-converting enzyme inhibitors and β-blockers are unlikely culprits of medication-induced TE, and the benefits of discontinuing a suspected culprit medication should be weighed carefully against the risks of medication cessation.
Pink Papules on the Cheek
The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7
A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9
Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12
Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7

A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9

Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12

Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.

The Diagnosis: Cutaneous Rosai-Dorfman Disease
Rosai-Dorfman disease is a rare benign non- Langerhans cell histiocytopathy that can manifest initially with lymph node involvement—classically, massive painless cervical lymphadenopathy.1 Cutaneous Rosai-Dorfman disease (CRDD) is a variant that can be associated with lymph node and internal involvement, but more than 80% of cases lack extracutaneous involvement.2,3 In cases with extracutaneous involvement, lymph node disease is most frequent.3 Cutaneous Rosai-Dorfman disease unassociated with extracutaneous disease is a benign self-limiting histiocytopathy that manifests as painless red-brown, yellow, or fleshcolored nodules, plaques, or papules that may become tender or ulcerated.4
Cutaneous Rosai-Dorfman disease represents a benign histiocytopathy of resident dendritic cell derivation.3 A characteristic immunohistochemical finding is S-100 positivity, which might suggest a Langerhans cell transdifferentiation phenotype, but other markers corroborative of a Langerhans cell phenotype—namely CD1a and langerin—will be negative. Biopsies typically show a mid to deep dermal histiocytic infiltration in a variably dense polymorphous inflammatory cell background comprised of a mixture of lymphocytes, plasma cells, and neutrophils.3 At times the extent of lymphocytic infiltration can be to a magnitude that resembles a lymphoma on histopathology. In our patient, lymphoma was excluded based on clinical presentation, as this patient lacked the typical symptoms of lymphadenopathy or B symptoms that come with B-cell lymphoma.5
The histiocytes in CRDD are characteristically large mononuclear cells exhibiting a low nuclear to cytoplasmic ratio reflective of the voluminous, nonvacuolated, watery cytoplasm. They have ill-defined cytoplasmic membranes resulting in a seemingly syncytial growth pattern. A hallmark of the histiocytes is emperipolesis characterized by intracytoplasmic localization of intact inflammatory cells including neutrophils, lymphocytes, and plasma cells.3
The differential diagnosis of CRDD includes Langerhans cell histiocytosis (LCH), indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. All of these conditions can be differentiated by their unique histopathologic and phenotypic characteristics.
Langerhans cell histiocytosis is a distinct clonal histiocytopathy that has a varied presentation ranging from cutaneous confined cases manifesting as a solitary lesion to one of disseminated cutaneous disease with the potential for multiorgan involvement. Regardless of the variant of LCH, the hallmark cell is one showing an eccentrically disposed, reniform nucleus with an open chromatin and abundant eosinophilic cytoplasm (Figure 1).6 Both LCH and CRDD stain positive for S-100. However, unlike the histiocytes in CRDD, those seen in LCH stain positive for CD1a and langerin and would not express factor XIIIA. Additionally, the neoplastic cells would not exhibit the same extent of CD68 positivity seen in CRDD.6 Treatment of LCH depends on the extent of disease, especially for the presence or absence of extracutaneous disease.7

A variant of LCH is indeterminate cell histiocytosis, which can be seen in neonates or adults. It represents a neoplastic proliferation of Langerhans cells that are devoid of Birbeck granules, reflective of an immature early phase of differentiation in the skin prior to the cells acquiring the Birbeck granule (as would be seen in neonates) or a later phase of differentiation after the mature Langerhans cell has encountered antigen and is en route to the lymph node (typically seen in adults).8 The phenotypic profile is identical to conventional LCH except the cells do not express langerin. Microscopically, the infiltrates are composed of Langerhans cells that are morphologically indistinguishable from classic LCH but without epidermotropism and exhibit a dominant localization in the dermis typically unassociated with other inflammatory cell elements (Figure 2).9

Xanthogranuloma is seen in young children (juvenile xanthogranuloma) as a solitary lesion, though a multifocal cutaneous variant and extracutaneous presentations have been described. Similar lesions can be seen in adults.10 These lesions are evolutionary in their morphology. In the inception of a juvenile xanthogranuloma, the lesions are highly cellular, and the histiocytes typically are poorly lipidized; there may be a dearth of other inflammatory cell elements. As the lesions mature, the histiocytes become lipidized, and characteristic Touton giant cells that exhibit a wreath of nuclei with peripheral lipidization may develop (Figure 3). In the later stages, there is considerable hyalinizing fibrosis, and the cells can acquire a spindled appearance. The absence of emperipolesis and the presence of notable lipidization are useful light microscopy features differentiating xanthogranuloma from CRDD.11 Treatment of xanthogranuloma can range from a conservative monitoring approach to an aggressive approach combining various antineoplastic therapies with immunosuppressive agents.12

Solitary and multicentric reticulohistiocytoma is another form of resident dendritic cell histiocytopathy that can resemble Rosai-Dorfman disease. It is a dermal histiocytic infiltrate accompanied by a polymorphous inflammatory cell infiltrate (Figure 4) and can show variable fibrosis.13 One of the hallmarks is the distinct amphophilic cytoplasms, possibly attributable to nuclear DNA released into the cytoplasm from effete nuclei.13 The solitary form is unassociated with systemic disease, whereas the multicentric variant can be a paraneoplastic syndrome in the setting of solid and hematologic malignancies.14 In addition, in the multicentric variant, similar lesions can affect any organ but there can be a proclivity to involve the hand and knee joints, leading to a crippling arthritis.15 We presented a case of CRDD, a benign resident dendritic cell histiocytopathy that can manifest as a cutaneous confined process in the skin where the clinical course is characteristically benign. It potentially can be confused with LCH, indeterminate cell histiocytosis, xanthogranuloma, and reticulohistiocytoma. For a solitary lesion, intralesional triamcinolone injection and excision are options. Multifocal cutaneous disease or CRDD with notable extracutaneous disease may require systemic treatment.16 In our patient, one intralesional triamcinolone injection was performed with notable resolution.

- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
- Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy: a newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63-70.
- Brenn T, Calonje E, Granter SR, et al. Cutaneous Rosai-Dorfman disease is a distinct clinical entity. Am J Dermatopathol. 2002;24:385.
- Ahmed A, Crowson N, Magro CM. A comprehensive assessment of cutaneous Rosai-Dorfman disease. Ann Diagn Pathol. 2019;40:166-173.
- Frater JL, Maddox JS, Obadiah JM, et al. Cutaneous Rosai-Dorfman disease: comprehensive review of cases reported in the medical literature since 1990 and presentation of an illustrative case. J Cutan Med Surg. 2006;10:281-290.
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941-952. Doi:10.1016/j.hoc.2008.07.002
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Board PPTE. Langerhans cell histiocytosis treatment (PDQ®). In: PDQ Cancer Information Summaries [Internet]. National Cancer Institute (US); 2009.
- Chu A, Eisinger M, Lee JS, et al. Immunoelectron microscopic identification of Langerhans cells using a new antigenic marker. J Invest Dermatol. 1982;78:177-180. doi:10.1111/1523-1747.ep12506352
- Berti E, Gianotti R, Alessi E. Unusual cutaneous histiocytosis expressing an intermediate immunophenotype between Langerhans’ cells and dermal macrophages. Arch Dermatol. 1988;124:1250-1253. doi:10.1001/archderm.1988.01670080062020
- Cypel TKS, Zuker RM. Juvenile xanthogranuloma: case report and review of the literature. Can J Plast Surg. 2008;16:175-177.
- Rodriguez J, Ackerman AB. Xanthogranuloma in adults. Arch Dermatol. 1976;112:43-44.
- Collie JS, Harper CD, Fillman EP. Juvenile xanthogranuloma. In: StatPearls [Internet]. StatPearls Publishing; 2022.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492. doi:10.1016/j. clindermatol.2006.07.010
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). An erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624. doi:10.2214/ajr.124.4.610
- Dalia S, Sagatys E, Sokol L, et al. Rosai-Dorfman disease: tumor biology, clinical features, pathology, and treatment. Cancer Control. 2014;21:322-327. doi:10.1177/107327481402100408
A 31-year-old woman presented with a slow-growing, tender, pruritic lesion on the right cheek of 4 to 5 months’ duration. She had been applying petroleum jelly and hydrocortisone cream 2.5% without any improvement. Physical examination revealed a 1×5-mm, pearly pink, erythematous, crusted papule with arborizing vessels surrounded by scattered pink papules with white dots within. No cervical lymphadenopathy was appreciated on physical examination, and the patient denied any other systemic symptoms. Shave and punch biopsies of the lesion were performed; stains for microorganisms were negative. The biopsy showed a dense reticular mixed inflammatory cell infiltrate comprised of a mixture of histiocytes (top), lymphocytes, neutrophils, and plasma cells that assumed a diffuse growth pattern within the dermis. The histiocytes exhibited abundant watery cytoplasms with ill-defined cytoplasmic membranes; intact leukocytes were found within the cytoplasms. The histiocytes demonstrated a unique phenotype characterized by S-100 (bottom) and CD68 positivity.
Androgenetic Alopecia: What Works?
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.
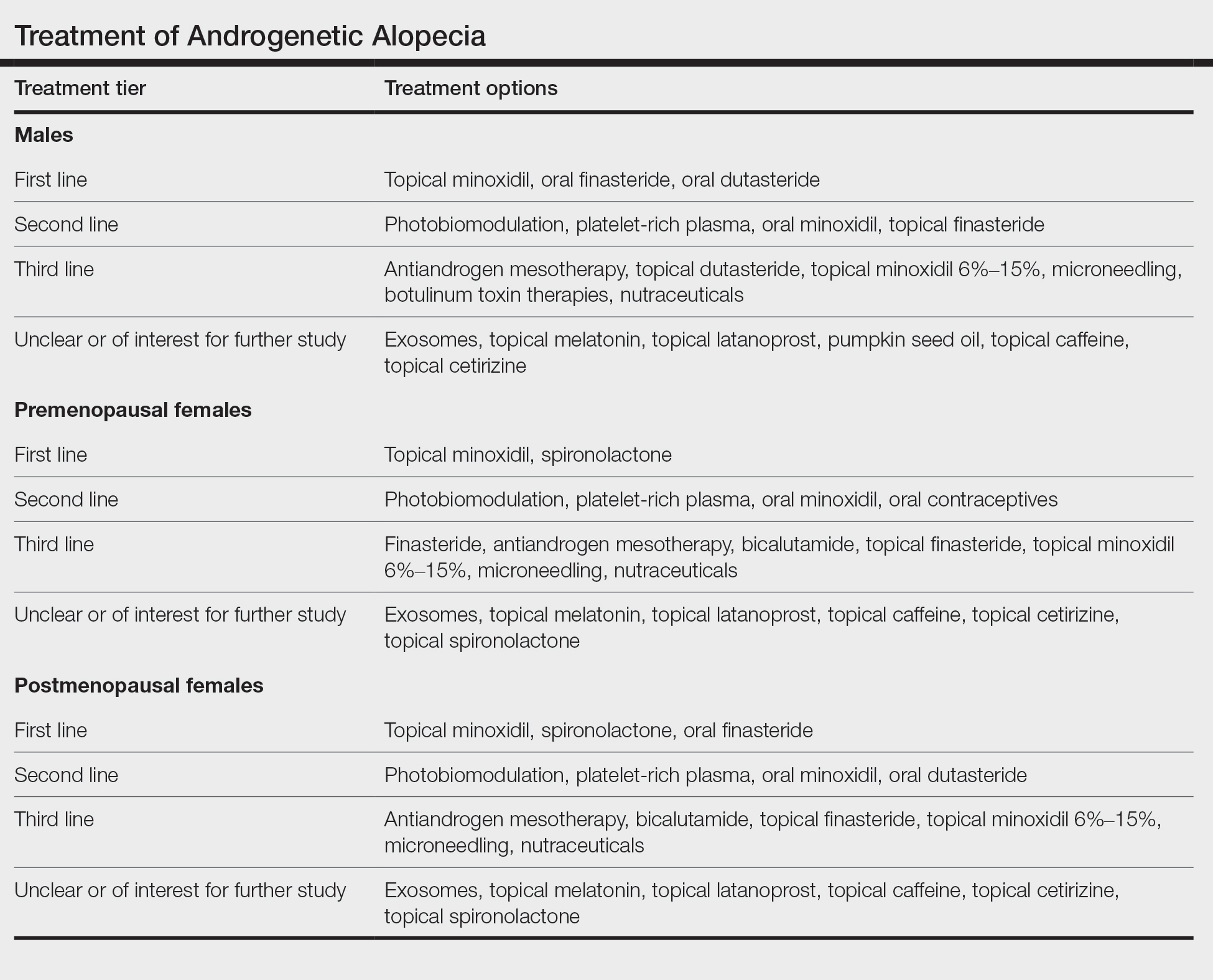
When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
When it comes to selecting medical treatments for androgenetic alopecia (AGA), patients and practitioners alike want to know, “What works?” The ideal AGA treatment is one that meets 4 criteria: highly effective, safe, affordable, and easy to use. To date, there is no known treatment for AGA that meets all these criteria. Some therapies are more effective than others, but there are no treatments at present that are able to completely and permanently reverse the condition. Some treatments are safer, some are less expensive, and some are easier to use than others. In the end, the treatment that the patient chooses is influenced not only by its known effectiveness but also by the value that the patient places on the other 3 categories—safety, affordability, and ease of use. Therefore, shared decision-making between patient and practitioner is central to the selection of specific AGA treatments.
Effectiveness: Some Treatments Work Better Than Others
Of the nearly 2 dozen medical treatments for AGA, some have been found to be more effective than others. Whether a given treatment should be considered a bona fide AGA therapy—and then whether to position it as a first-line, second-line, or third-line agent—depends on the answers to 3 fundamental questions:
- Does the treatment truly help patients with AGA?
- How effective is this treatment?
- How safe is it?
Does the Treatment Truly Help Patients?—Surprisingly, it is not always straightforward to confirm that a given treatment helps patients with AGA. Does oral finasteride help female AGA? Yes and no: Finasteride 1 mg is ineffective in the treatment of female AGA, but higher doses such as 2.5 or 5 mg likely have benefit.1,2 Does topical minoxidil help AGA? Yes and no: Minoxidil 5% is ineffective in the treatment of a male with Hamilton-Norwood stage VII AGA but often is helpful in earlier stages of the condition.
One of the best ways to determine if a treatment really helps AGA is to evaluate how it performs in the setting of a well-conducted, randomized, double-blind, placebo-controlled trial. These types of clinical trials have been performed for many known AGA treatments and give us some of the best evidence that a treatment truly works. The AGA treatments with the highest-quality evidence (level 1) are topical minoxidil, oral finasteride, and oral dutasteride for male AGA and topical minoxidil for female AGA.
How Effective Is This Treatment?—Patients are particularly interested to know whether a given treatment has the potential to notably restore hair density. It is one thing to know that use of the treatment might slightly improve hair density and another to know that it could potentially lead to dramatic improvement. In addition, patients want to know whether a specific treatment they are considering is more (or less) likely to improve their hair density compared to another treatment.
Advanced statistical methods such as the network meta-analysis are increasingly being used to understand how individual treatments from different studies compare. Two recent studies have provided us with powerful data on the relative efficacy of minoxidil and 5α-reductase inhibitors in the treatment of both male and female AGA.2,3 A 2022 network meta-analysis of male AGA ranked treatment efficacy from most to least effective: oral dutasteride 0.5 mg, oral finasteride 5 mg, oral minoxidil 5 mg, oral finasteride 1 mg, and topical minoxidil 5%.3 Similarly, a 2023 network meta-analysis of female AGA ranked treatment efficacy from most to least effective: oral 5 mg finasteride, minoxidil solution 5% twice daily, oral minoxidil 1 mg, and minoxidil foam 5% once daily.2 We are not yet able to rank all known treatments for AGA.
Things We Tend to Ignore: Quality of Data, Long-term Results, Nonresponders, and Study Populations—There are a few caveats for anyone treating AGA. First, the quality of published AGA studies is highly variable and many are of low quality. The highest-quality evidence (level 1) for male AGA comes from studies of minoxidil solution/foam 5% twice daily, oral finasteride 1 mg, and oral dutasteride 0.5 mg. For female AGA, the highest-quality evidence is for topical minoxidil—either 5% foam once daily or 2% solution twice daily. Lower-quality studies limit conclusions and the ability to properly compare treatments.
Second, long-term data are nonexistent for most of our AGA treatments. The exceptions include finasteride, dutasteride, and topical minoxidil, which have reasonably adequate long-term studies.4-6 However, most other treatments have been evaluated only through short-term studies. It is tempting to assume that results from a 24-week study can be used to infer how a patient might respond when using the same treatment over the course of many decades; however, making these assumptions would be unwise.
Third, most AGA treatments help improve hair density in only a proportion of patients who decide to use the given treatment. There usually is one subgroup of patients for whom the treatment does not seem to help much at all and one subgroup for whom the treatment halts further hair loss but does not regrow hair. For example, in the case of finasteride treatment of male AGA, approximately 10% of patients do not seem to respond to treatment at all, and another 50% seem to be able to halt further loss but never achieve hair regrowth.7 In an analysis of 12 studies with 3927 male patients, Mella et al8 showed that 5.6 patients needed to be treated short term and 3.4 patients needed to be treated long term for 1 patient to perceive an improvement in the hair. It is clear that many males who use finasteride will not see evidence of hair regrowth. This same general concept applies for all available treatments and is important to remember if a patient with AGA decides to start 2 new treatments simultaneously. Consider the 34-year-old man who starts oral minoxidil and platelet-rich plasma (PRP) for AGA. At his follow-up appointment 9 months later, the patient reports improved hair density and wants to know what contributed to the improvement: the oral minoxidil, the PRP, or both? Many practitioners would believe that both treatments likely provided some degree of benefit—but in reality, that represents a flaw in logic. If 2 hair loss treatments are started at exactly the same time, it is impossible to know the relative benefit of each treatment and whether one might not be helping at all. Combination therapies are still common in my practice and highly encouraged, but my personal preference is to stagger start dates whenever possible so I can determine each treatment’s contribution to the patient’s final outcome.
Finally, when evaluating what works for AGA, we need to define the specific patient subpopulation, as the available data are less robust for some patient groups than others. We have limited data in children and adolescents with AGA, as well as limited comparative data across different racial backgrounds, body mass indices, and underlying health issues. For example, data on the most effective strategies to treat female AGA in the setting of polycystic ovary syndrome, premature menopause, and other endocrine disorders are lacking.
Which Treatments Also Have Good Safety?—The treatment that a patient ultimately selects also depends on its actual or perceived safety. Patients have vastly different levels of risk tolerance. Some patients would much rather start a less effective treatment if they believe that the chances of experiencing treatment-related adverse effects would be lower. In general, topical and injectable treatments tend to have fewer adverse effects than oral therapies. Long-term safety data generally are lacking for many hair-loss therapies. A limited number of studies of topical minoxidil include data up to 5 years,4 and some studies of oral finasteride and oral dutasteride include patients who used these medications for up to 10 years.5,6
So Then, What Works?
The Table shows treatments for AGA and how I prioritize starting them in my own clinic. First-line treatment options often include those with level 1 evidence but also may include those with less-robust evidence plus a good history (over many years) of safety, affordability, ease of use, and effectiveness (eg, spironolactone and finasteride for female-pattern hair loss).
• Male AGA: I consider topical minoxidil, oral finasteride, and oral dutasteride as first-line agents, and low-level laser, PRP, oral minoxidil, and topical finasteride as second-line agents. Only topical minoxidil and oral finasteride are approved by the US Food and Drug Administration (FDA) for AGA in males; laser devices are FDA cleared.
• Premenopausal females with AGA: I use topical minoxidil and spironolactone as first-line agents. Low-level laser, PRP, oral minoxidil, and oral contraceptives are helpful second-line agents. Only topical minoxidil is FDA approved in women. I consider all treatments, with the exception of low-level laser, to be contraindicated in pregnancy.
• Postmenopausal females with AGA: I consider topical minoxidil, spironolactone, and oral finasteride as first-line agents. Low-level laser, PRP, oral minoxidil, and oral dutasteride are helpful second-line agents.

When choosing an initial treatment plan, I generally will start with one or more first-line options. I will then add or replace with remaining first-line options or a second-line option after 6 to 12 months depending on how well the patient responds to the first-line options. Patients who do not wish to use first-line options or have contraindications begin with second-line options. Third-line options are best reserved for patients who do not respond to or do not wish to use first- and second-line options.
Experts differ in opinion as to what constitutes a first-line treatment option and what constitutes a second- or third-line option. For example, some increasingly consider oral minoxidil to be a first-line option for AGA.9 In my opinion, the lack of high-quality comparative, randomized, controlled trials and long-term safety data keep oral minoxidil reserved as a respectable second-line option. Similarly, some experts reserve oral dutasteride as a second-line option for AGA.10 In my opinion, the data now are of the highest-quality evidence (level 1)9 to support placing oral dutasteride in the tier of first-line treatments.
Shared decision-making using an evidence-based approach is ultimately what connects patients with treatment plans that offer a good chance of helping to improve hair loss.
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
- Price VH, Roberts JL, Hordinsky M, et al. Lack of efficacy of finasteride in postmenopausal women with androgenetic alopecia. J Am Acad Dermatol. 2000;43(5 pt 1):768-776. doi:10.1067/mjd.2000.107953
- Gupta AK, Bamimore MA, Foley KA. Efficacy of non-surgical treatments for androgenetic alopecia in men and women: a systematic review with network meta-analyses, and an assessment of evidence quality. J Dermatolog Treat. 2022;33:62-72. doi:10.1080/09546634.2020.1749547
- Gupta AK, Wang T, Bamimore MA, et al. The relative effect of monotherapy with 5-alpha reductase inhibitors and minoxidil for female pattern hair loss: a network meta-analysis study [published online June 29, 2023]. J Cosmet Dermatol. doi:10.1111/jocd.15910
- Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22:64.
- Choi G-S, Sim W-Y, Kang H, et al. Long-term effectiveness and safety of dutasteride versus finasteride in patients with male androgenic alopecia in South Korea: a multicentre chart review study. Ann Dermatol. 2022;34:349-359. doi:10.5021/ad.22.027
- Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455-461.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578-89. doi:10.1016/s0190-9622(98)70007-6
- Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety offinasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146:1141-1150. doi:10.1001/archdermatol.2010.256
- Vañó-Galván S, Fernandez-Crehuet P, Garnacho G, et al; Spanish Trichology Research Group. Recommendations on the clinical management of androgenetic alopecia: a consensus statement from the Spanish Trichology Group of the Spanish Academy of Dermatology and Venererology (AEDV). Actas Dermosifiliogr. 2023 Oct 25:S0001-7310(23)00844-X. doi:10.1016/j.ad.2023.10.013. Online ahead of print.
- Kanti V, Messenger A, Dobos G, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Eur Acad Dermatol Venereol. 2018;32:11-22. doi: 10.1111/jdv.14624
The Many Uses of the Humble Alcohol Swab
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).
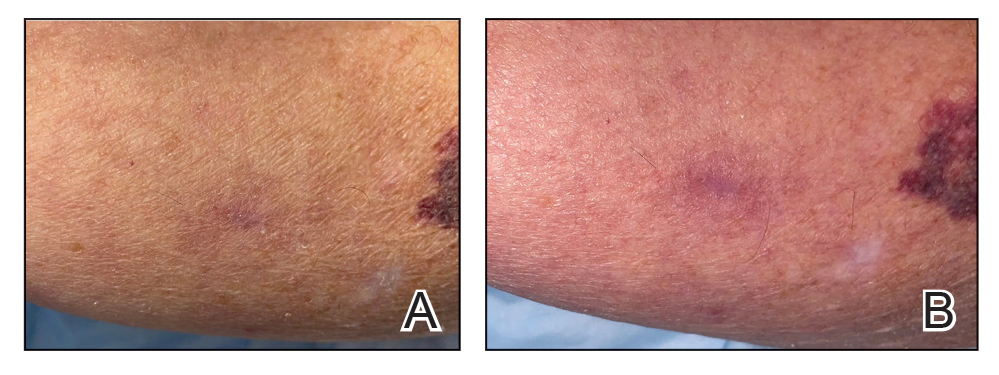
Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).

Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
Practice Gap
In light of inflation, rising costs of procedures, and decreased reimbursements,1 there is an increased need to identify and utilize inexpensive multitasking tools that can serve the dermatologic surgeon from preoperative to postoperative care. The 70% isopropyl alcohol swab may be the dermatologist’s most cost-effective and versatile surgical tool.
The Technique
When assessing a lesion, alcohol swabs can remove scale, crust, or residue from personal care products to help reveal primary morphology. They aid in the diagnosis of porokeratosis by highlighting the cornoid lamella when used following application of gentian violet.2 The alcohol swab also can lay down a liquid interface to facilitate contact dermoscopy and improve visualization while also reducing the transmission of pathogens by the dermatoscope.3 Rubbing an area with an alcohol swab can induce vasodilation of scar tissue, which also may help localize a prior biopsy or surgical site (Figure).

Before a surgical site is marked, an initial cleanse with an alcohol swab serves to both remove debris and provide antisepsis ahead of the procedure. Additionally, the swab may improve adherence of skin markers by clearing excess lipid from the skin surface. Assessing the amount of debris and oil removed in the process can help determine a patient’s baseline level of hygiene, which can aid postoperative wound care planning. In extreme cases, use of an alcohol swab may help diagnose dermatitis neglecta or terra firma-forme dermatosis by completely removing any pigmentation.4
After surgery, the alcohol swab can remove skin marker(s) and blood and prepare the site for the surgical dressing. There also is some evidence to suggest that cleansing the surgical site with an alcohol swab as part of routine postoperative wound care may decrease incidence of surgical-site infection.5 At follow-up, the swab can remove crust and clean the skin before suture removal. If infection is suspected, the swab can cleanse skin before a wound culture is obtained to remove skin commensals and flora on the outer surface of the wound.
Practice Implications
The 70% isopropyl alcohol swab can assist the dermatologist in numerous tasks related to everyday procedures. It is readily available in every clinic and costs only a few cents.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.
- Pollock JR, Chen JY, Dorius DA, et al. Decreasing physician Medicare reimbursement for dermatology services. J Am Acad Dermatol. 2022;86:1154-1156.
- Thomas CJ, Elston DM. Medical pearl: Gentian violet to highlight the cornoid lamella in disseminated superficial actinic porokeratosis.J Am Acad Dermatol. 2005;52(3 pt 1):513-514.
- Kelly SC, Purcell SM. Prevention of nosocomial infection during dermoscopy? Dermatol Surg. 2006;32:552-555.
- Blattner CM, Perry B, Snider K, et al. Clinical pearl: increasing utility of isopropyl alcohol for cutaneous dyschromia. Cutis. 2016;97:287;301.
- Vogt KN, Chadi S, Parry N, et al. Daily incision cleansing with alcohol reduces the rate of surgical site infections: a pilot study. Am Surg. 2015;81:1182-1186.