User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
PRAME Expression in Melanocytic Proliferations in Special Sites
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
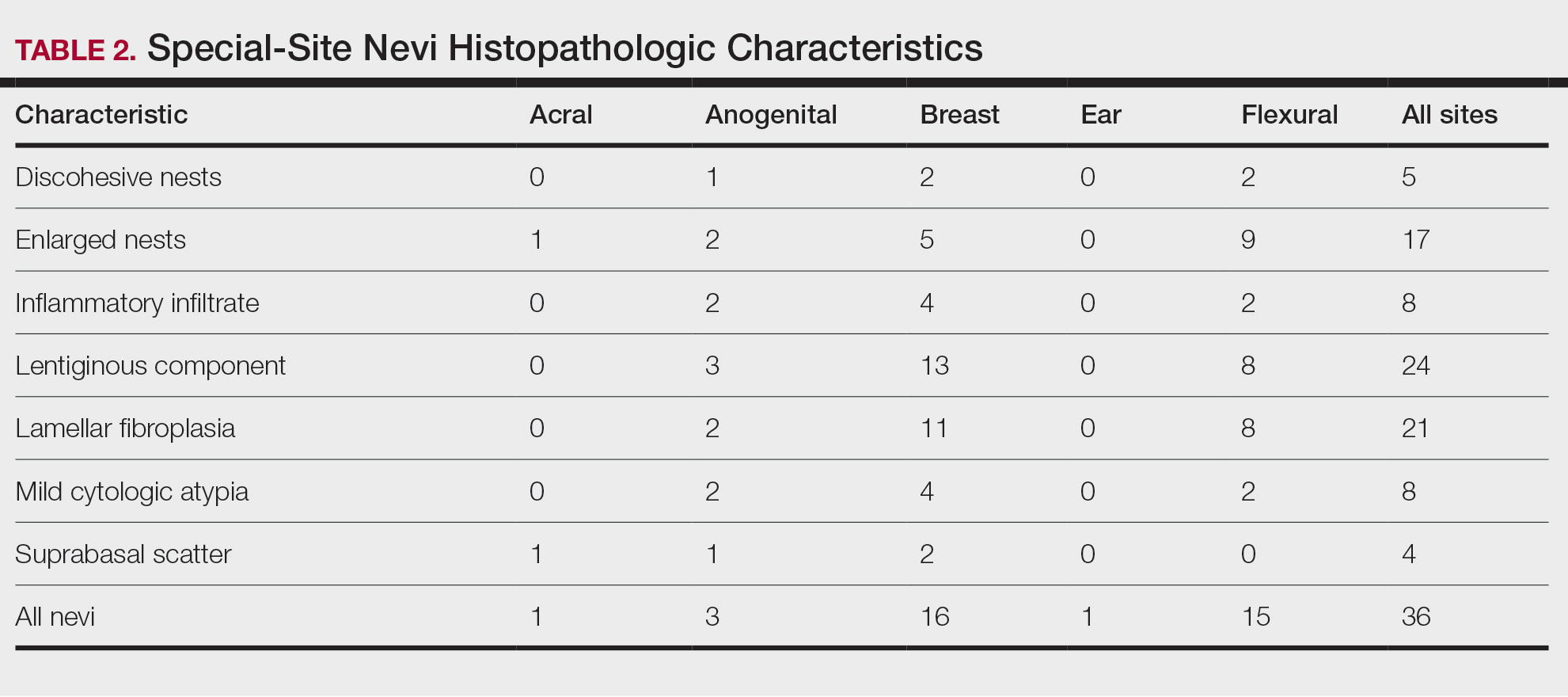
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.
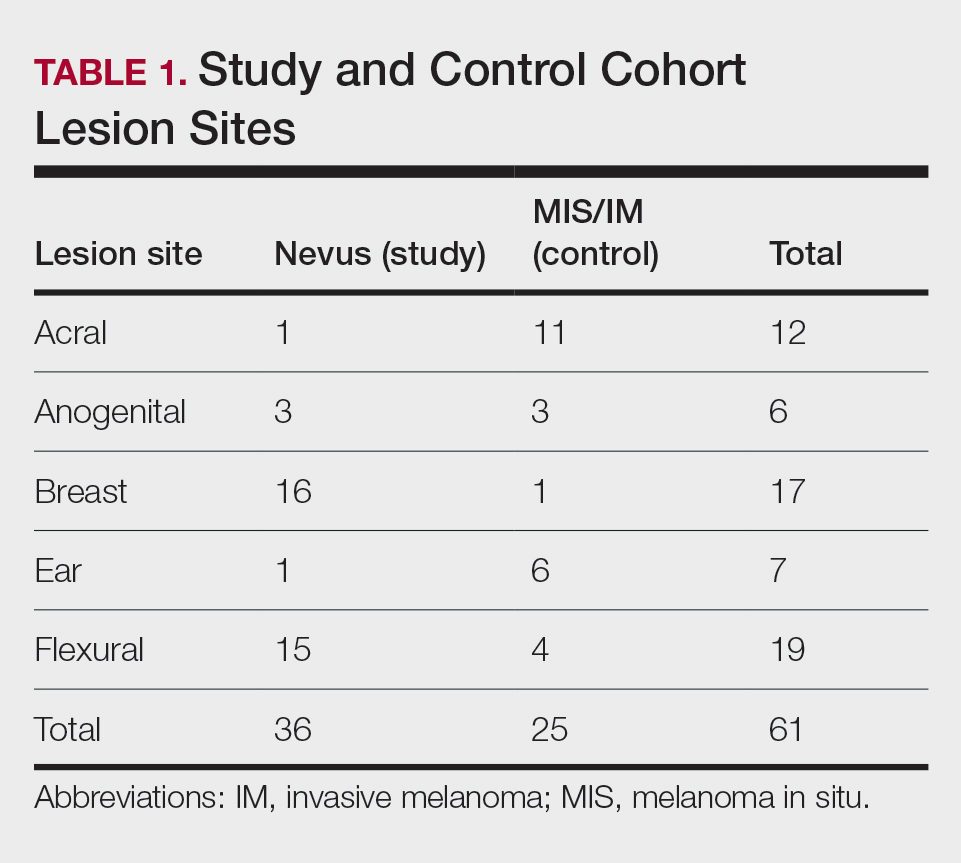
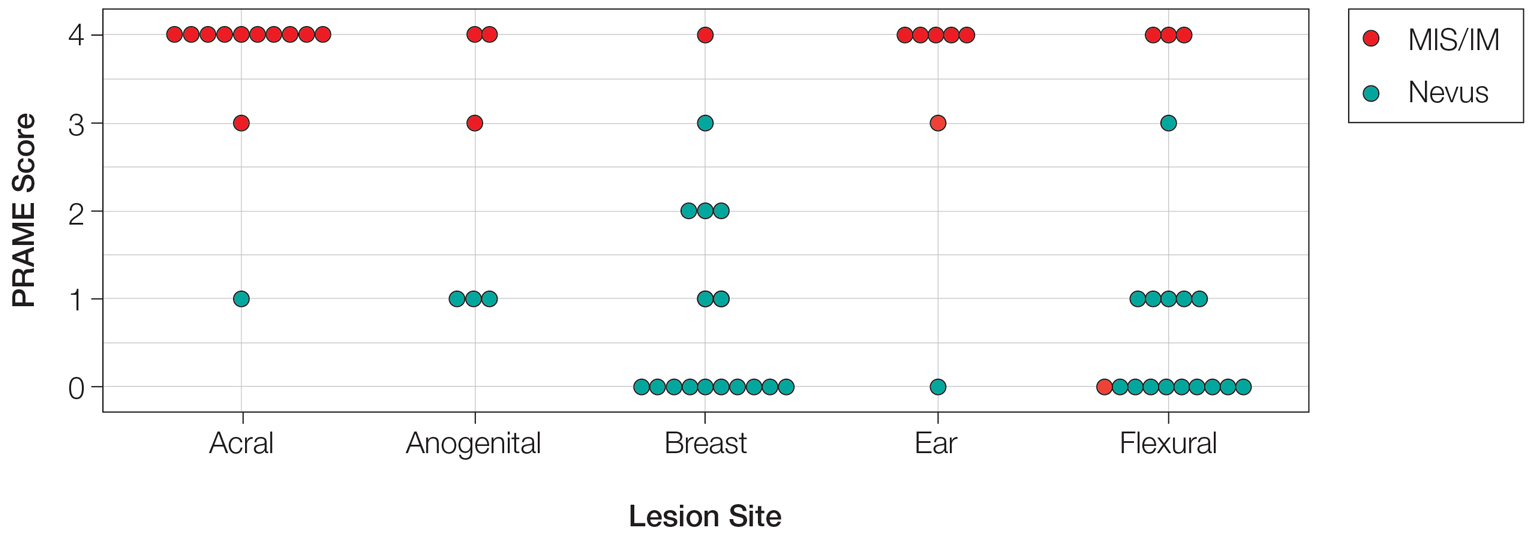
Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).
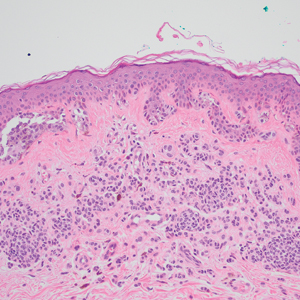
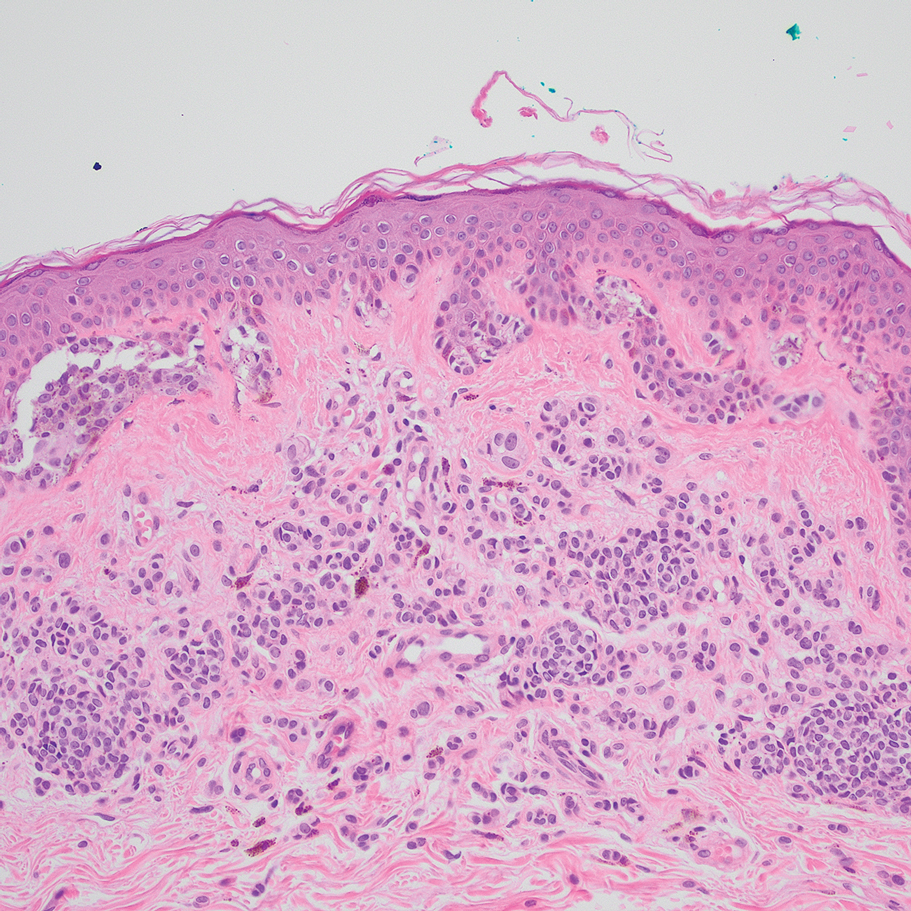
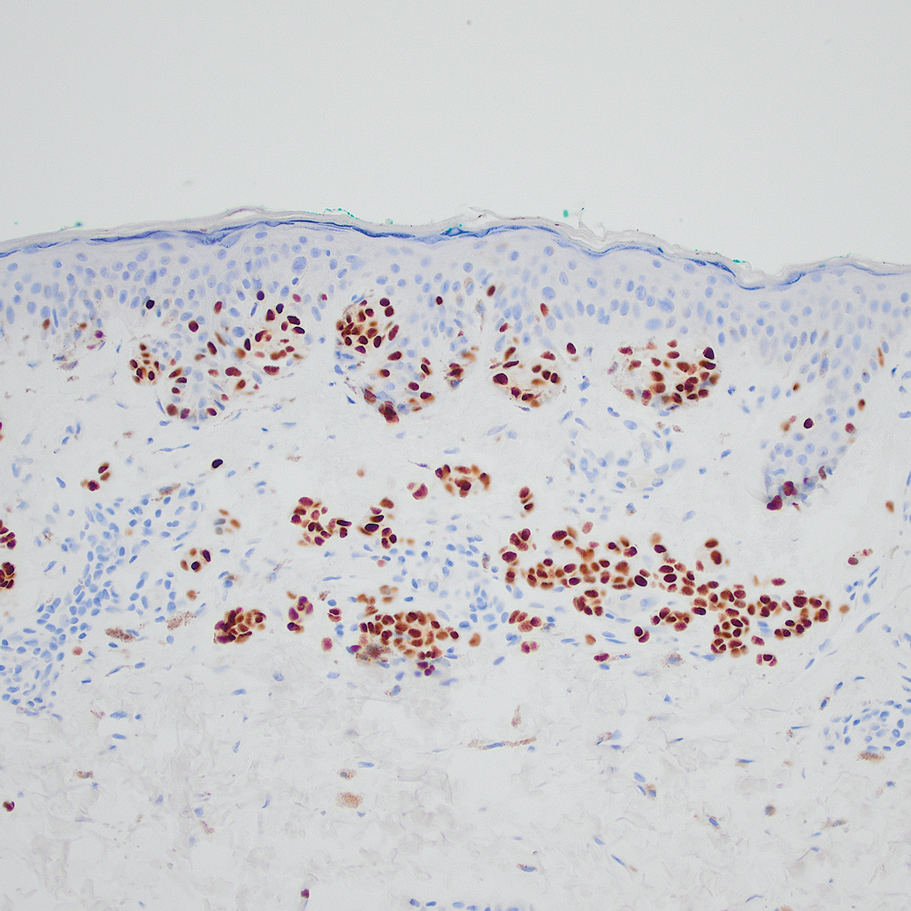
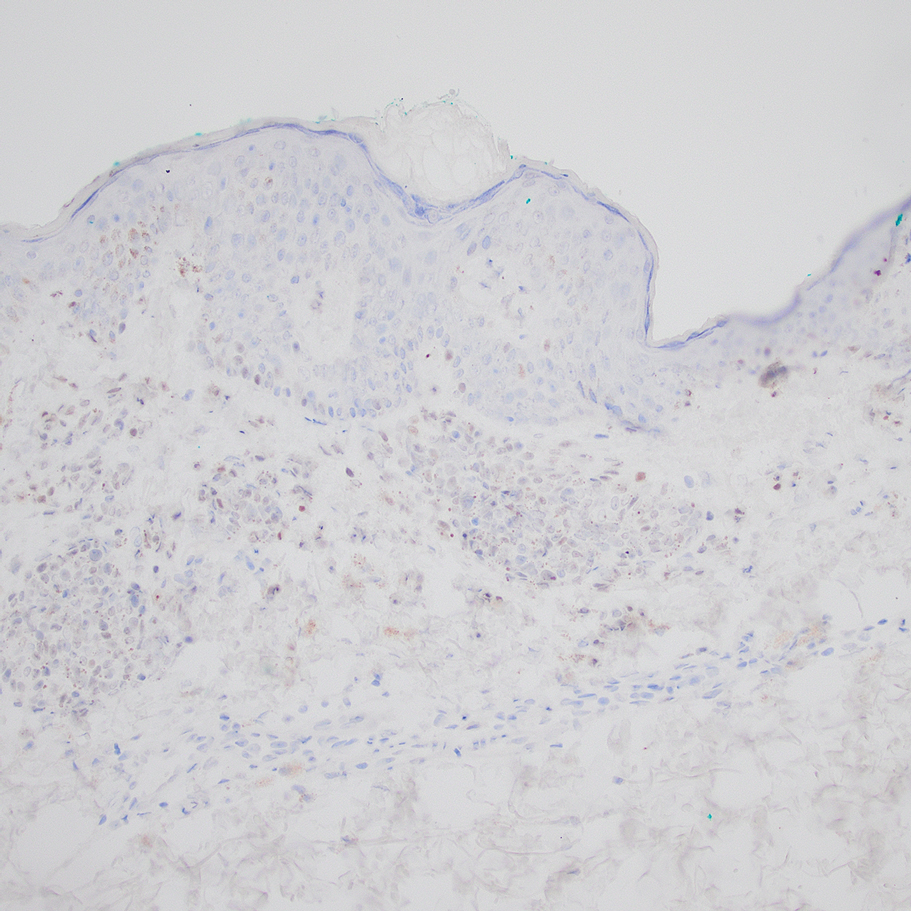
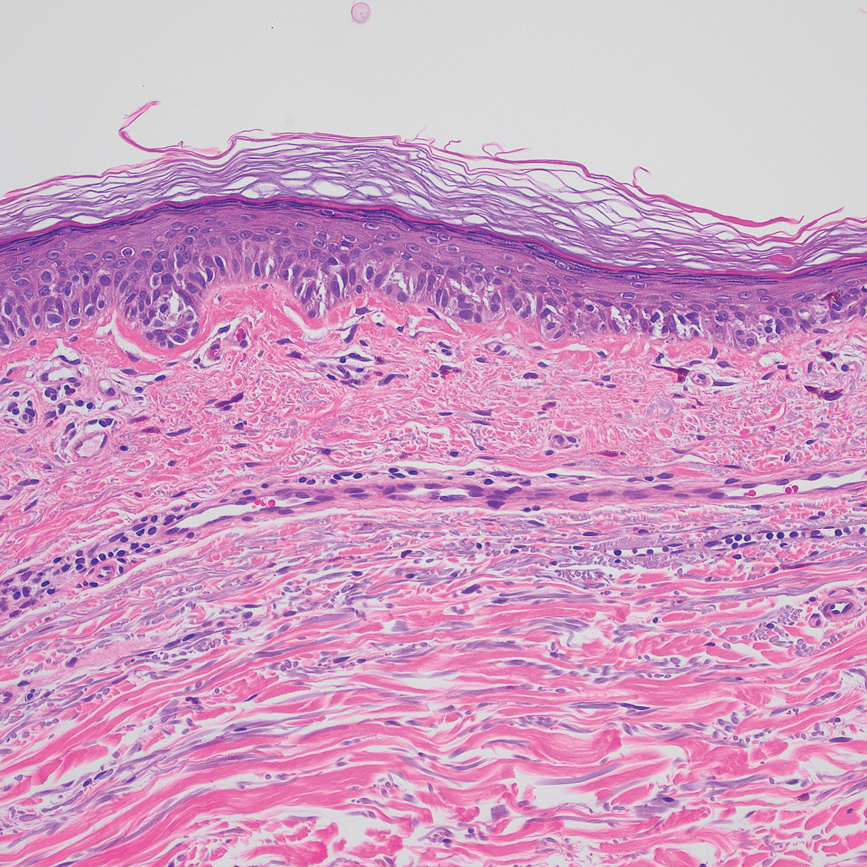
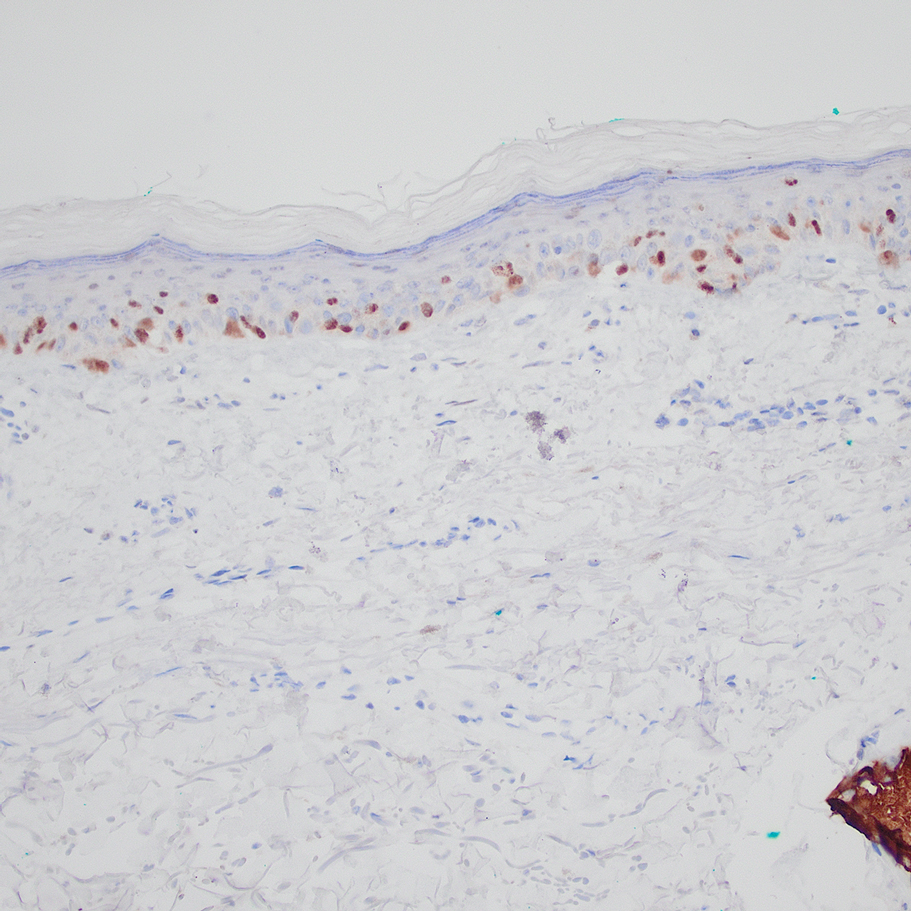
Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.
Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.
Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.
Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.
Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
The assessment and diagnosis of melanocytic lesions can present a formidable challenge to even a seasoned pathologist, which is especially true when dealing with the subset of nevi occurring at special sites—where baseline variations inherent to particular locations on the body can preclude the use of features routinely used to diagnose malignancy elsewhere. These so-called special-site nevi previously have been described in the literature along with suggested criteria for differentiating malignant lesions from their benign counterparts.1 Locations generally considered to be special sites include the acral skin, anogenital region, breast, ear, and flexural regions.1,2
When evaluating non–special-site melanocytic lesions, general characteristics associated with a malignant diagnosis include confluence or pagetoid spread of melanocytes, nuclear pleomorphism, cytologic atypia, and irregular architecture3; however, these features can be compatible with a benign diagnosis in special-site nevi depending on their extent and the site in question. Although they can be atypical, special-site nevi tend to have the bulk of their architectural distortion and cytologic atypia in the center of the lesion as opposed to the edges.1 If a given lesion is from a special site but lacks this reassuring feature, special care should be taken to rule out malignancy.
Preferentially expressed antigen in melanoma (PRAME) is an antigen first identified in tumor-reactive T-cell populations in patients with malignant melanoma. It is the product of an oncogene that frequently is overexpressed in melanomas, lung squamous cell carcinomas, sarcomas, and acute leukemias.4 It functions as an antagonist of the retinoic acid signaling pathway, which normally serves to induce further cell differentiation, senescence, or apoptosis.5 PRAME inhibits retinoid signaling by forming a complex with both the ligand-bound retinoic acid holoreceptor and the polycomb protein EZH2, which blocks retinoid-dependent gene expression by encouraging chromatin condensation at the RARβ promoter site5; therefore, expressing PRAME allows lesional cells a substantial growth advantage.
PRAME expression has been extensively characterized in non–special-site nevi and has filled the need for a rather specific marker of melanoma.6-10 Although PRAME has been studied in acral nevi,11 the expression pattern in nevi of special sites has yet to be elucidated. Herein, we present a dataset characterizing PRAME expression in these challenging lesions.
Methods
We performed a retrospective case review at the University of Virginia (Charlottesville, Virginia) and collected a panel of 36 special-site nevi that previously were diagnosed as benign by a trained dermatopathologist from January 2020 through December 2022. Special-site nevi were identified using a natural language filter for the following terms: acral, palm, sole, ear, auricular, lip, axilla, armpit, breast, groin, labia, vulva, umbilicus, and penis. This study was approved by the University of Virginia institutional review board.
The original hematoxylin and eosin slides used for primary diagnosis were re-examined to verify the prior diagnosis of benign nevus at a special site. We performed a detailed microscopic examination of all benign nevi in our cohort to determine the frequency of various characteristics at each special site. Sections were prepared from the formalin-fixed and paraffin-embedded tissue blocks and stained with a commercial PRAME antibody (#219650 [Abcam] at a 1:50 dilution) and counterstain. A trained dermatopathologist (S.S.R.) examined the stained sections and recorded the percentage of tumor cells with nuclear PRAME staining. We reported our results using previously established criteria for scoring PRAME immunohistochemistry7: 0 for no expression, 1+ for 1% to 25% expression, 2+ for 26% to 50% expression, 3+ for 51% to 75% expression, and 4+ for diffuse or 76% to 100% expression. Only strong clonal expression within a population of cells was graded.
Data handling and statistical testing were performed using the R Project for Statistical Computing (https://www.r-project.org/). Significance testing was performed using the Fisher exact test. Plot construction was performed using ggplot2 (https://ggplot2.tidyverse.org/).
Results
Our study cohort included 36 special-site nevi, and the control cohort comprised 25 melanoma in situ (MIS) or invasive melanoma (IM) lesions occurring at special sites. Table 1 provides a breakdown of the study and control cohorts by lesion site. Table 2 details the results of our microscopic examination, describing frequency of various characteristics of special-site nevi stratified by site.

Of the 36 special-site nevi in our cohort, 20 (56%) had no staining (0) for PRAME, 11 (31%) demonstrated 1+ PRAME expression, 3 (8%) demonstrated 2+ PRAME expression, and 2 (6%) demonstrated 3+ PRAME expression. No nevi showed 4+ expression. In the control cohort, 24 of 25 (96%) MIS and IM showed 3+ or 4+ expression, with 21 (84%) demonstrating diffuse/4+ expression. One control case (4%) demonstrated 0 PRAME expression. These data are summarized in Table 3 and Figure 1. There is a significant difference in diffuse (4+) PRAME expression between special-site nevi and MIS/IM occurring at special sites (P=1.039×10-12).


Based on our cohort, a positivity threshold of 3+ for PRAME expression for the diagnosis of melanoma in a special-site lesion would have a sensitivity of 96% and a specificity of 94%, while a positivity threshold of 4+ for PRAME expression would have a sensitivity of 84% and a specificity of 100%. Figures 2 through 4 show photomicrographs of a special-site nevus of the breast, which appropriately does not stain for PRAME; Figures 5 and 6 show an MIS at a special site that appropriately stains for PRAME.

Comment
The distinction between benign and malignant pigmented lesions at special sites presents a fair challenge for pathologists due to the larger degree of leniency for architectural distortion and cytologic atypia in benign lesions at these sites. The presence of architectural distortion or cytologic atypia at the lesion’s edge makes rendering a benign diagnosis especially difficult, and the need for a validated immunohistochemical stain is apparent. In our cohort, strong clonal PRAME expression provided a reliable immunohistochemical marker, allowing for the distinction of malignant lesions from benign nevi at special sites. Diffuse faint PRAME expression was present in several benign nevi within our cohort, and these lesions were considered negative (0) in our analysis.

Given the described test characteristics, we support the implementation of PRAME immunohistochemistry with a positivity threshold of 4+ expression as an ancillary test supporting the diagnosis of IM or MIS in special sites, which would allow clinicians to leverage the high specificity of 4+ PRAME expression to distinguish an IM or MIS from a benign nevus occurring at a special site. We do not recommend the use of 4+ PRAME expression as a screening test for melanoma or MIS among special-site nevi due to its comparatively low sensitivity; however, no one marker is always reliable, and we recommend continued clinicopathologic correlation for all cases.

Although our case series included nevi and MIS/IM from all special sites, we were limited in the number of acrogenital and ear nevi included due to a relative paucity of biopsied benign nevi from these locations at the University of Virginia. Additionally, although the magnitude of the difference in PRAME expression between the study and control groups is sufficient to demonstrate statistical significance, the overall strength of our argument would be increased with a larger study group. We were limited by the number of cases available at our institution, which did not utilize PRAME during the initial diagnosis of the case; including these cases in the study group would have undermined the integrity of our argument because the differentiation of benign vs malignant initially was made using PRAME immunohistochemistry.

Conclusion
Due to their atypical features, special-site nevi can be challenging to assess. In this study, we showed that PRAME expression can be a reliable marker to distinguish benign from malignant lesions. Our results showed that 100% of benign special-site nevi demonstrated 3+ expression or less, with 56% (20/36) demonstrating no expression at all. The presence of diffuse PRAME expression (4+ PRAME staining) appears to be a specific indicator of a malignant lesion, but results should always be interpreted with respect to the patient’s clinical history and the lesion’s histomorphologic features. Further study of a larger sample size would allow refinement of the sensitivity and specificity of diffuse PRAME expression in the determination of malignancy for special-site lesions.


Acknowledgment—The authors thank the pathologistsat the University of Virginia Biorepository and Tissue Research Facility (Charlottesville, Virginia) for their skill and expertise in performing immunohistochemical staining for this study.
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
- VandenBoom T, Gerami P. Melanocytic nevi of special sites. In: Pathology of Melanocytic Tumors. Elsevier; 2019:90-100. doi:10.1016/B978-0-323-37457-6.00007-9
- Hosler GA, Moresi JM, Barrett TL. Nevi with site-related atypia: a review of melanocytic nevi with atypical histologic features based on anatomic site. J Cutan Pathol. 2008;35:889-898. doi:10.1111/j.1600-0560.2008.01041.x.
- Brenn T. Melanocytic lesions—staying out of trouble. Ann Diagn Pathol. 2018;37:91-102. doi:10.1016/j.anndiagpath.2018.09.010
- Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199-208. doi:10.1016/s1074-7613(00)80426-4
- Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835-847. doi:10.1016/j.cell.2005.07.003
- Alomari AK, Tharp AW, Umphress B, et al. The utility of PRAME immunohistochemistry in the evaluation of challenging melanocytic tumors. J Cutan Pathol. 2021;48:1115-1123. doi:10.1111/cup.14000
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465. doi:10.1097/PAS.0000000000001134
- Gill P, Prieto VG, Austin MT, et al. Diagnostic utility of PRAME in distinguishing proliferative nodules from melanoma in giant congenital melanocytic nevi. J Cutan Pathol. 2021;48:1410-1415. doi:10.1111/cup.14091
- Googe PB, Flanigan KL, Miedema JR. Preferentially expressed antigen in melanoma immunostaining in a series of melanocytic neoplasms. Am J Dermatopathol. 2021;43):794-800. doi:10.1097/DAD.0000000000001885
- Raghavan SS, Wang JY, Kwok S, et al. PRAME expression in melanocytic proliferations with intermediate histopathologic or spitzoid features. J Cutan Pathol. 2020;47:1123-1131. doi:10.1111/cup.13818
- McBride JD, McAfee JL, Piliang M, et al. Preferentially expressed antigen in melanoma and p16 expression in acral melanocytic neoplasms. J Cutan Pathol. 2022;49:220-230. doi:10.1111/cup.14130
Practice Points
- Special-site nevi are benign melanocytic proliferations at special anatomic sites. Although cytologic atypia and architectural distortion may be present, they are centrally located and should not be present at the borders of the lesion.
- Strong expression of the preferentially expressed antigen in melanoma (PRAME) via immunohistochemistry provides a reliable indicator for benignity in differentiating a special-site nevus from a malignant melanoma occurring at a special site.
Axillary Contact Dermatitis: An Update on Potential Allergens and Management
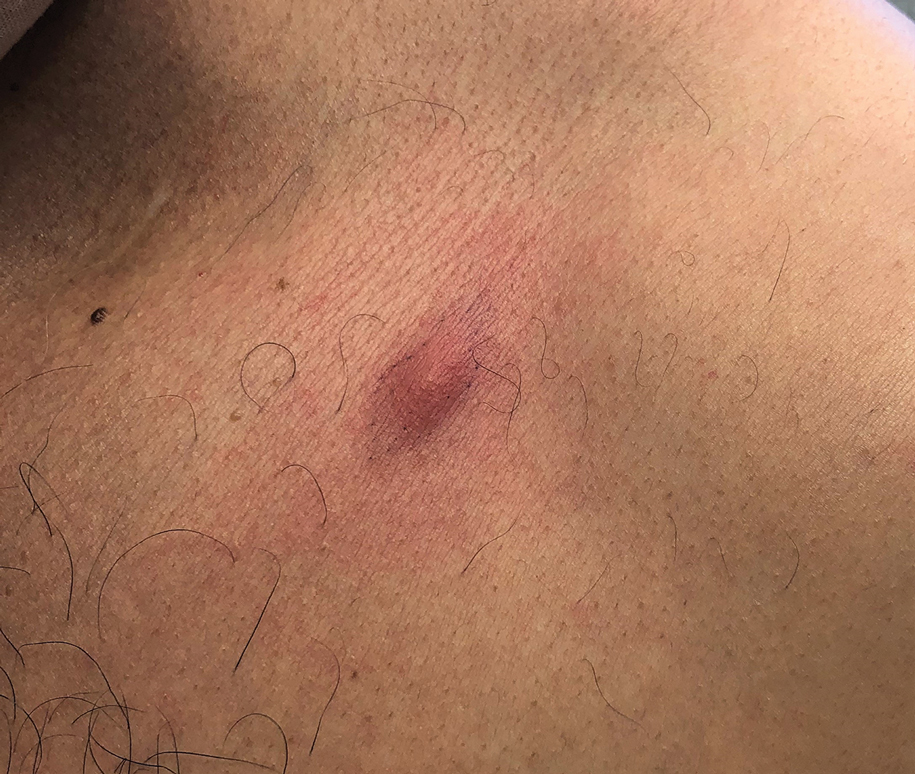
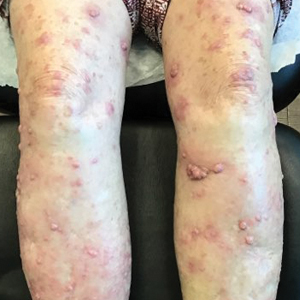
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
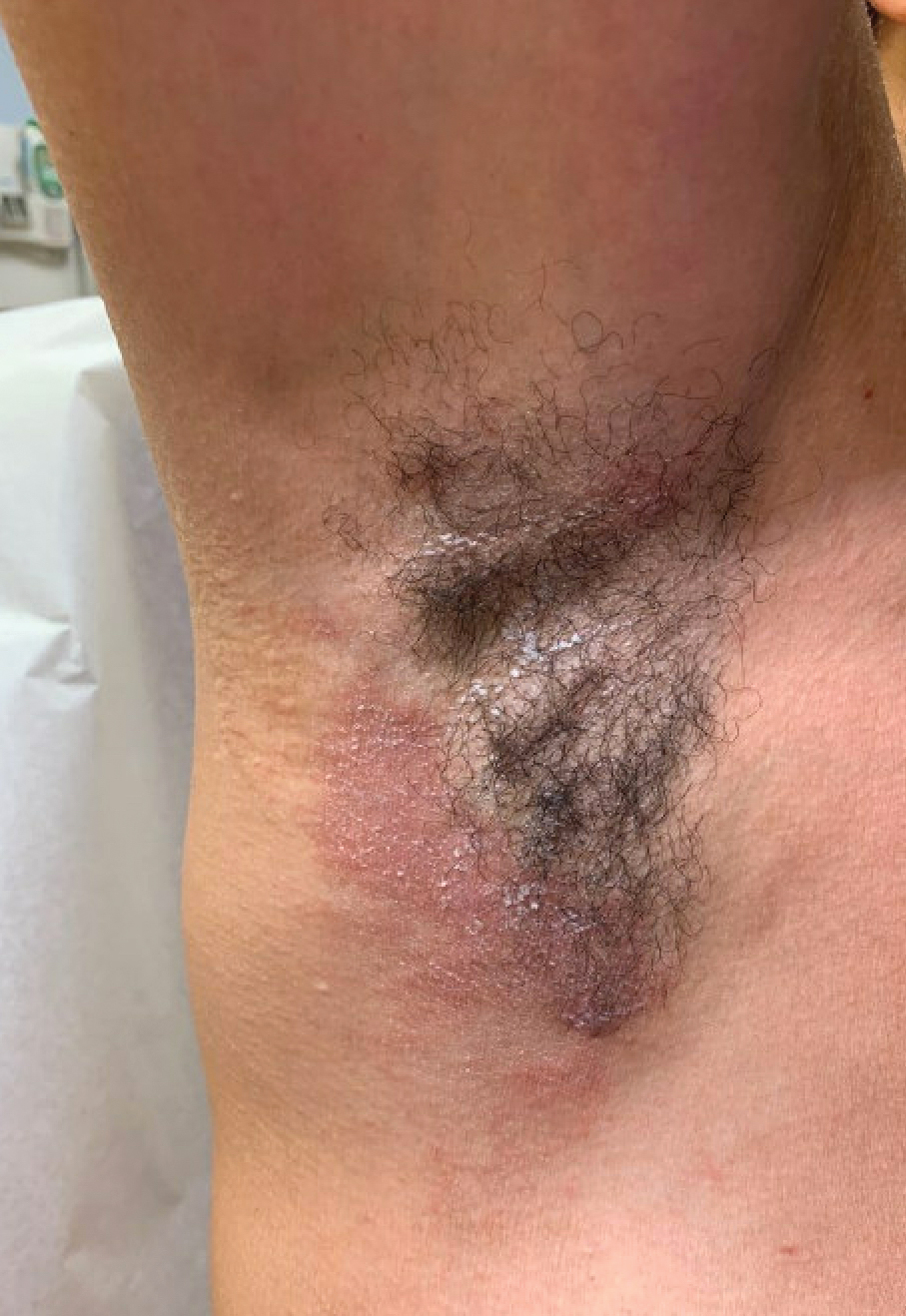
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34
Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58
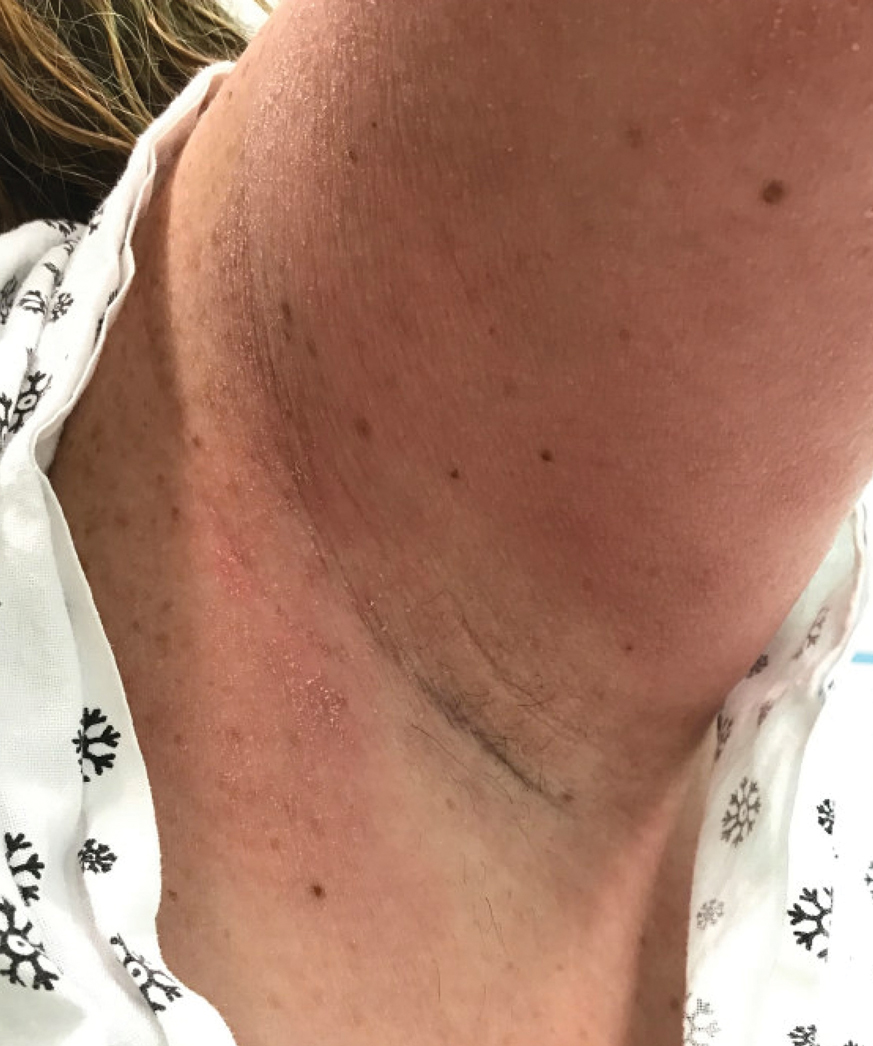
Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65
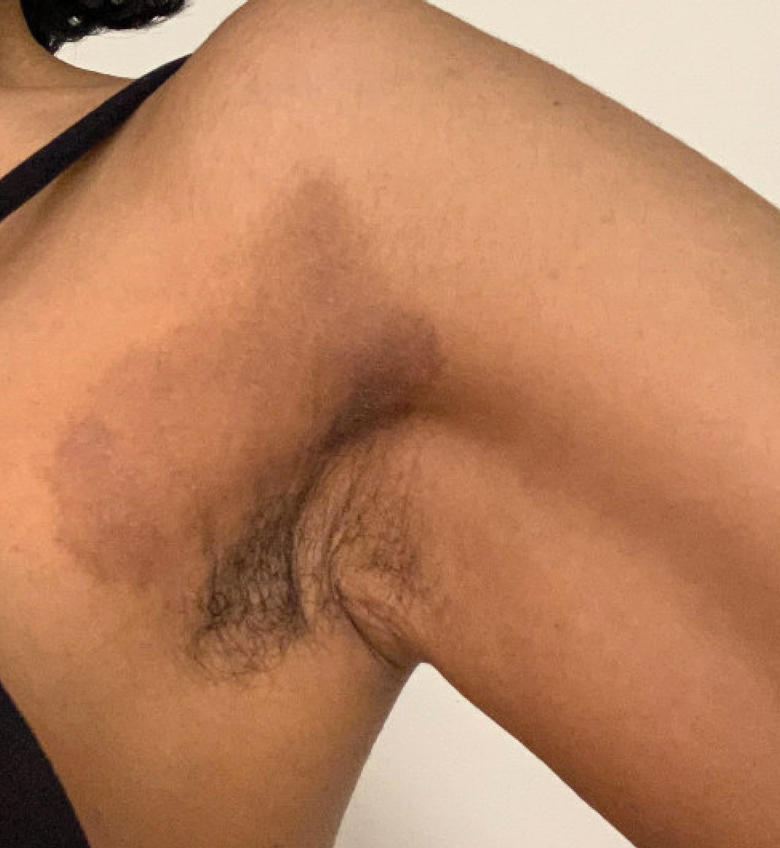
Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
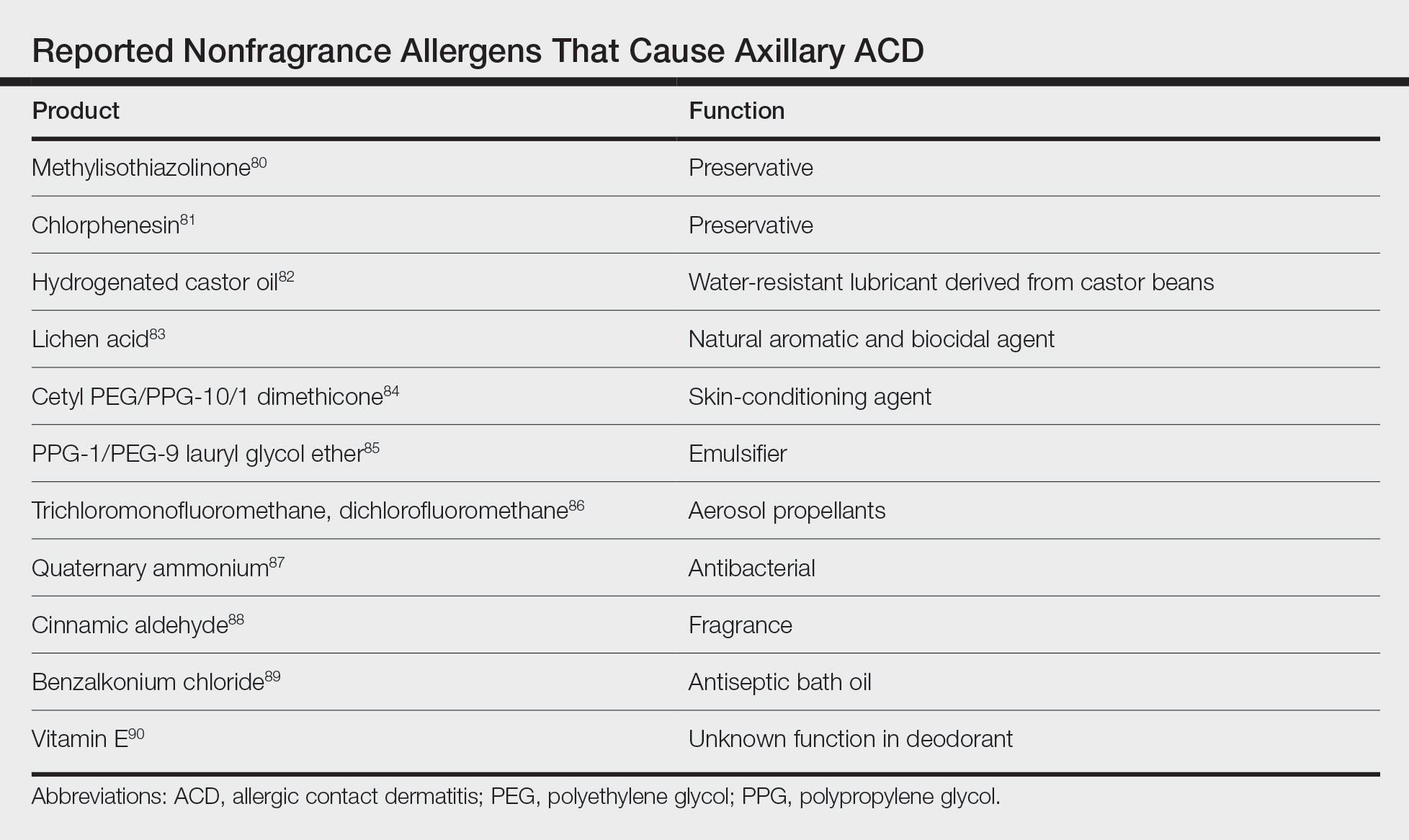
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92
Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
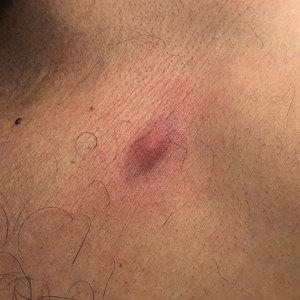
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
Approximately 20% of the general population has a contact allergy.1 Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction mediated by T lymphocytes.2 Axillary ACD presentation is variable but typically includes an eczematous eruption with erythematous scaly patches or plaques. Common products in contact with the axillae include deodorants, antiperspirants, razors, bodywash, and clothing.
Axillary skin is distinct from skin elsewhere on the body due to both anatomical characteristics and unique human self-care practices. Axillary skin has reduced barrier function, faster stratum corneum turnover, and altered lipid levels.3-5 Moreover, the axillae often are subject to shaving or other hair removal practices that alter the local environment, as layers of stratum corneum and hair are mechanically removed, which causes irritation and predisposes the skin to enhanced sensitivity to topical exposures.6,7 With the abundance of apocrine and eccrine glands, the axillae are prone to sweat, which also can accentuate contact allergy.2,3 Other factors, such as occlusion and friction, contribute to axillary contact allergy.8,9
Patch testing is the gold standard for the diagnosis of ACD and aids in identification of culprit allergens. A thorough patient history and examination of the rash distribution may provide further clues; for example, dermatitis due to a deodorant typically affects the vault, whereas textile dye dermatitis tends to spare the vault.10,11 Baseline-limited patch testing detects up to two-thirds of clinically relevant allergens.12 Therefore, patients may require subsequent testing with supplemental allergens.
The differential diagnosis for axillary lesions is broad—including inflammatory diseases such as irritant contact dermatitis and hidradenitis suppurativa, genetic disorders such as Hailey-Hailey disease, and infectious causes such as erythrasma—but may be narrowed with a thorough physical examination and patient history, histopathology, bedside diagnostic techniques (eg, scrapings and Wood lamp examination), and patch testing. Systemic contact dermatitis (SCD) or symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) also may be suspected in cases of intertriginous dermatoses.
We review the potential allergens in products used on the axillae as well as the management of axillary ACD. We also discuss axillary dermatitis as a manifestation of SCD and SDRIFE.
Top Allergens in Products Used on the Axillae
Fragrance—A 1982 North American Contact Dermatitis Group study on cosmetic products identified fragrances as the most common cause of ACD,13 and this trend continues to hold true with more recent data.14 The incidence of fragrance allergy may be increasing, with positive patch tests to a fragrance chemical in 10% of patch test clinic populations.15 Fragrances are a ubiquitous ingredient in deodorants and antiperspirants, which are important sources implicated in the development and elicitation of fragrance ACD.16 One study found that fragrance was present in 97 of 107 (90%) deodorants available at Walgreens pharmacies.11
In a study of patients with a history of an axillary rash caused by a deodorant spray, Johansen et al17 reported that the likelihood of fragrance allergy is increased by a factor of 2.4. This risk of developing a fragrance allergy may be exacerbated in those who shave; Edman18 reported that the odds ratio of developing a fragrance allergy among men who shave their beards was 2.9. Although there are no specific data on the effects of shaving on ACD, shaving in general can induce localized irritation and increase percutaneous absorption.19
The individual fragrance components in deodorants most likely to cause ACD include hydroxycitronellal, eugenol, and geraniol—all constituent ingredients in patch test formulations of fragrance mixture I.11,20 Other common fragrance allergens associated with ACD include hydroxymethylpentylcyclohexenecarboxaldehyde, farnesol, and balsam of Peru.21-27 Hydroperoxides of limonene and linalool, common fragrances in detergents and personal care products, are increasingly recognized as contact allergens and have been reported to cause axillary ACD from deodorants.28-30
Dermatitis involving the bilateral axillary vaults wherever deodorant or antiperspirant was directly applied is the most common presentation of ACD due to fragrance (Figure 1).17 An eczematous eruption is common, though scale may be less apparent than in nonflexural regions. Axillary ACD secondary to fragrances also may result from use of fragranced laundry detergents, fabric softeners, soaps, and perfumes, and may spare the vaults.10,29,31,32 Less common presentations of axillary ACD due to fragrance include pigmented dermatoses; for example, ACD from an antiperspirant containing hydroperoxide of limonene presented as hyperpigmented patches with minimal erythema and scaling in the edges of the axillary folds.33,34

Diagnosis of a fragrance ACD typically is made with a standard patch test series including fragrance mixture I and balsam of Peru, which may detect 75% and 50% of fragrance sensitivities, respectively.35 Patch testing may be followed with a repeated open application test of the product in question.36 Additionally, it may be appropriate to test for other fragrance allergens including balsam of Tolu, fragrance mixture II, lichen acid mix, and hydroxyperoxides of linalool and limonene (among other botanicals) if standard patch testing is negative and suspicion of fragrance ACD remains elevated.11
Propylene Glycol—Propylene glycol (PG)—a versatile substance that functions as a solvent, humectant, emulsifier, stabilizer, and antimicrobial—is the second most common contact allergen present in deodorants.11 It is prevalent in both personal care and household products, including deodorants, cosmetics, foods, toothpaste, cleaning agents, and detergents.11,37 Propylene glycol is both an allergen and an irritant. Among deodorants/antiperspirants, PG is both a common irritant and allergen, as its concentration may be particularly high (as much as 73%).38 One commonly reported example of PG contact dermatitis is from the topical medicament minoxidil.39,40
Patch testing data have demonstrated a positivity rate for PG ranging between 0.1% to 3.8%. The variability in these findings likely is due to differences in the tested concentrations of PG, as higher concentrations sometimes required to elicit an allergic reaction also may create a stronger irritation effect.41 Propylene glycol irritancy and the occlusive nature of the axillae may enhance sensitization to other allergens, as demonstrated by Agren-Jonsson and Magnusson,42 who reported sensitization to propantheline bromide and trichlorocarbanilide in patients who used a lotion with 90% PG. Many PG-containing products beyond deodorants/antiperspirants may be applied to the axillae, including steroid creams, lotions, shaving creams, and bodywashes.38,43
The diagnosis of PG allergy via patch testing is challenging and at times controversial given its irritant nature. False-positive irritant reactions have been documented, characterized by a weak reaction at 48 hours that is absent by 96 hours (decrescendo reaction). A reaction may not appear until 96 hours (crescendo reaction), which typically indicates a true contact allergy but in the case of PG also may be the substance acting as a “late irritant.”44 Fast (<24 hours) and well-demarcated reactions suggest irritation.45 Regardless, reactions to PG on patch testing, even those regarded as weak, may be considered relevant in consideration of the clinical context.37
Aluminum—Aluminum is the active ingredient in most antiperspirants, typically in the form of aluminum chloride, aluminum chlorohydrate, aluminum zirconium trichlorohydrex gly, or aluminum zirconium tetrachlorohydrex gly.46 Aluminum mechanically obstructs the eccrine glands to reduce sweat.47 Although aluminum is an uncommon allergen, a possible presentation of aluminum allergy is axillary vault dermatitis secondary to antiperspirant use.46 Another potential manifestation is a ringlike reaction to the Finn Chambers (SmartPractice) used in patch testing.46 In one case of aluminum-induced axillary dermatitis, a 28-year-old woman presented with eczema of the axillae, and subsequent patch testing revealed an allergy to aluminum chloride. The rash resolved upon cessation of use of an aluminum-containing deodorant.48
Aluminum has been reported to cause granulomatous dermatitis in the axillae. This reaction typically presents as red-brown, pruritic papules limited to the area in which deodorant was applied, with histopathology revealing epithelioid granulomas.49-51
Alum deodorants—considered a natural alternative—contain aluminum bound to potassium or ammonium in the form of a crystal or powder. Alum crystal deodorants have been reported to cause both a typical erythematous pruritic dermatitis as well as a granulomatous dermatitis with red-brown papules.52,53 The granulomatous dermatitis caused by either form of aluminum resolves with avoidance and use of topical steroids or topical tacrolimus.49,50,52,53
The diagnosis of aluminum ACD via patch testing may be identified with empty Finn Chambers, which are metallic aluminum, or with patch placement of aluminum chloride hexahydrate, though the former is only positive in patients with a strong allergy.54,55 In 2022, aluminum was named Allergen of the Year by the American Contact Dermatitis Society, with recommendations to conduct patch testing with aluminum chloride hexahydrate 10% rather than the traditional 2% to increase diagnostic yield.55 Additionally, it is recommended that aluminum be included in baseline patch testing for children due to the high prevalence of aluminum allergy in children and early exposure via childhood vaccines.54-56 In patients with aluminum allergy, providers may suggest purchasing aluminum-free deodorants or provide recipes for homemade deodorant that includes ingredients such as arrowroot powder, cornstarch, and diatomaceous earth.46
Nickel—Nickel is the most commonly identified contact allergen on patch testing yet an infrequent cause of axillary dermatitis. A case report from 2014 described axillary dermatitis in a woman that worsened during a positive patch test to nickel. Improvement was noted when the patient switched to titanium shaving razors.57 Nickel allergy also may present in the form of SCD. In one report, a woman developed dermatitis of the flexural areas, including the axillae, 3 months after undergoing a sterilization procedure in which nickel-containing tubal implants were placed.58 Patch testing revealed a positive reaction to nickel. The patient experienced complete resolution of the steroid-resistant dermatitis following removal of the implants via salpingectomy.58

Textile Dye—In contrast to dermatitis caused by deodorants/antiperspirants, contact allergy to textile dyes presents as dermatitis involving the axillary borders but sparing the axillary vaults (Figures 2 and 3).10 Other potential presentations of textile dye dermatitis include erythema multiforme–like eruptions and erythematous wheal–type reactions.59 Textile dyes are classified as disperse vs nondisperse, with the majority of contact dermatoses caused by disperse dyes, specifically Disperse Orange 1, blue 106, and blue 124.60-62 Ryberg et al61 found that the axilla is one of the more common locations to be affected by textile dye allergy, particularly in women, which was further supported by Seidenari et al,63 who found that skin folds were affected in 27% of study participants allergic to textile dyes (N=437), a finding that is likely due to friction, sweat, and occlusion.62 In one case report of a patient with dermatitis caused by reactive dyes, the garment required 3 washes before the patient experienced resolution of dermatitis.64 For patients with textile dye dermatitis, mitigation strategies include washing clothing before wearing, especially for darkly dyed items; avoiding tight clothing; wearing garments made of cotton, wool, silk, or linen; and choosing light-colored garments.9,64,65

Axillary Dermatitis as a Manifestation of SCD and SDRIFE
Systemic contact dermatitis occurs when an individual who was previously sensitized to a particular allergen develops ACD of the skin with systemic exposure to that allergen or immunochemically related allergens. Exposure may occur via ingestion, inhalation, intravenous, intramuscular, and transepidermal routes.66 Systemic contact dermatitis manifests in a variety of ways, including focal flares at sites of prior contact dermatitis (recall reaction), vesicular hand dermatitis, intertriginous eruptions including axillary dermatitis, and generalized eruptions.67
Systemic contact dermatitis rarely involves systemic symptoms, and onset typically is within days of exposure. The 3 most common groups of allergens causing SCD are metals, medications, and plants and herbals.68 These allergens have all been reported to cause axillary dermatitis via SCD.58,69,70 Foods containing balsam of Peru that may lead to SCD include citrus, chocolate, tomato, and certain alcohols.70,71 Patients with a positive patch test to balsam of Peru may experience improvement of their dermatitis after reduction of balsam of Peru–rich foods from their diet.70 Metals implicated in SCD include mercury, nickel, and gold.72-74 Finally, PG ingestion also has been implicated in cases of SCD.37
Symmetrical drug-related intertriginous and flexural exanthema is another condition that presents as intertriginous dermatitis and differs from SCD in that the eruption does not require presensitization; there may be no known prior exposure to the agent causing dermatitis. Historically, SDRIFE was described as baboon syndrome because of its frequent involvement of the buttocks with diffuse, well-demarcated, erythematous dermatitis resembling that of a baboon. This term is no longer used due to its insensitive nature and incomplete depiction of SDRIFE, which can affect body sites other than the buttocks.68,75,76 Specific criteria to make this diagnosis include sharply demarcated and/or V-shaped erythema of the gluteal/perianal area, involvement of at least 1 other intertriginous or flexural region, symmetry of affected areas, and an absence of systemic symptoms.76 There also may be papules, pustules, and vesicles present in affected areas. Symmetrical drug-related intertriginous and flexural exanthema most often is caused by β-lactam antibiotics, but other associated drugs include chemotherapeutic agents, such as mitomycin C.76
Histopathology of both SCD and SDRIFE is variable and typically nonspecific, often revealing epidermal spongiosis and a perivascular mononuclear cell infiltrate with occasional neutrophils and eosinophils.76 A case of SCD to mercury presenting as intertriginous dermatitis demonstrated a leukocytoclastic vasculitis pattern on biopsy.77
Systemic contact dermatitis is diagnosed via a patch test, while SDRIFE typically has a negative patch test result and requires oral rechallenge testing, which reproduces the rash within hours.78,79
Additional Allergens Causing Axillary ACD
Although fragrance is the most common allergen in deodorants, other ingredients have been shown to cause axillary ACD (Table).80-90 In addition to these ingredients, allergens not previously mentioned that may be present in deodorants include lanolin, essential oils, and parabens.11 Methylisothiazolinone in laundry detergent also has been found to instigate ACD.91 Fragrances and preservatives in laundry detergents also may contribute to dermatitis.92

Other products that have caused axillary contact dermatitis include topical exposure to medicaments including clindamycin,93 ethylenediamine in nystatin cream,94 methylprednisolone acetate95 and dipropylene glycol in a hydrocortisone lotion,96 wood dusts from tropical hardwoods,97 and tobacco.98
Management of ACD
The most effective strategy in the management of patients with contact dermatitis is avoidance of the offending agent. Additionally, clinicians may recommend the use of topical steroids and/or calcineurin inhibitors to hasten resolution.2
For patients with contact dermatitis, a clinician may recommend product substitutions with few potential allergens to use prior to patch testing. Patients with a fragrance allergy should look for products specifically labeled as “fragrance free” rather than “hypoallergenic” or “unscented,” as the latter two may still contain minimal amounts of fragrance.35 Patients should be educated on the functions of the allergens to which they are allergic so they may adequately avoid potential sources of contact.99 For suspected textile dye dermatitis, instructing patients to wash clothing before wearing and to avoid synthetic fabrics, dark dyes, and tightly fitted clothing may help.9,64,65
Differential Diagnosis
The differential diagnosis for axillary lesions is broad, including infectious, inflammatory, and autoimmune etiologies. Irritant contact dermatitis (ICD) presents similar to ACD, though it is more immediate in onsetand typically demonstrates symptoms of burning and stinging rather than pruritus. Although histopathology is not reliable in differentiating ICD and ACD, it has been shown that focal parakeratosis is associated with ACD, whereas necrotic epidermal keratinocytes are found in ICD.100
Intertrigo presents as large, erythematous, opposing patches or plaques confined to inguinal, submammary, axillary, and/or abdominal folds. Findings of beefy red erythema and peripheral satellite pustules may implicate presence of Candida, which can be identified with potassium hydroxide preparations.
Inverse psoriasis presents as sharply demarcated, erythematous, moist, smooth plaques or patches with minimal scale. The most common area of involvement is the inguinal folds, followed by the axillae, inframammary folds, perianal area, umbilicus, and retroauricular areas. Involvement of the elbows and knees or a positive family history of psoriasis may be useful knowledge in establishing the diagnosis. A biopsy may show dermal eosinophils, epidermal spongiosis, and focal serum in the scale, in addition to features of typical psoriasis plaques.101
Seborrheic dermatitis typically is an erythematous eruption, often with yellowish greasy scale. Simultaneous involvement of the face and scalp may be noted. Although typically a clinical diagnosis, biopsy demonstrates shoulder parakeratosis with follicular plugging and lymphocytic exocytosis.
Hailey-Hailey disease (also called benign familial pemphigus) is an autosomal-dominant genetic condition presenting as moist, malodorous, painful, vegetative plaques, patches, or scaly pustules in flexural areas, frequently with flaccid blisters. Lesions are provoked by traumatic stimuli. Onset occurs in the second to fourth decades and may improve with age. The diagnosis is confirmed by biopsy, which demonstrates acantholysis of the epidermis. The moist superficial patches of Hailey-Hailey disease help distinguish it from comparably drier Darier disease, the other acantholytic disease of the axillae.
Granular parakeratosis (also called hyperkeratotic flexural erythema) is an uncommon dermatosis most often observed in middle-aged women. It presents as red-brown keratotic papules coalescing into plaques, often with overlying scale in intertriginous areas. This disorder may be related to exposure to aluminum, a key component of antiperspirants.102 Diagnosis with a skin biopsy demonstrates granular parakeratosis.
Infections most commonly include erythrasma, tinea, and candidiasis. Erythrasma caused by Corynebacterium minutissimum may present in the axillae and/or groin with sharply demarcated, red-brown patches. Wood lamp examination reveals coral red fluorescence. Tinea corporis, a dermatophyte infection, may present as scaly erythematous plaques with advancing borders and central clearing. Fungal cultures and potassium hydroxide preparations are useful to confirm the diagnosis.
Pseudofolliculitis barbae most often is thought of as a condition affecting the beard in Black men, but it also may present in individuals of all races who shave the axillary and inguinal regions. Typical features include pruritic inflammatory papules and pustules with surrounding erythema and hyperpigmentation.
Fox-Fordyce disease is a disorder of the apocrine sweat glands that presents as several flesh-colored, perifollicular, monomorphic papules in the axillae. It typically is a disease of young females and also can involve the areola and vulva. Histopathology may show hyperkeratosis, irregular acanthosis, and dilated sweat glands.
Hidradenitis suppurativa is a chronic inflammatory condition that presents with multiple cysts; nodules; abscesses; sinus tract formation; and suppuration of the axillary, anogenital, and sometimes inframammary areas, typically at the onset of puberty. The diagnosis is best supported by history and physical examination, which may be notable for recurrent abscesses, draining tracts, double comedones, and ropelike scarring.
Extramammary Paget disease is a rare malignancy affecting apocrine gland–bearing areas, including axillary and genital regions. It most commonly presents as a unilateral or asymmetric, scaly, erythematous plaque. Histopathology demonstrates Paget cells with abundant clear cytoplasm and pleomorphic nuclei, typically grouped in the lower portion of the epidermis.
Final Thoughts
Axillary dermatoses often can be challenging to diagnose given the range of pathologies that can present in intertriginous areas. Allergic contact dermatitis is a common culprit due to unique anatomical considerations and self-care practices, including shaving/hair removal; use of deodorants, antiperspirants, bodywashes, and clothing; and frictional and moisture influences. The most likely offender among contact allergens is fragrance, but other possibilities to consider include PG, preservatives, aluminum, nickel, and textile dyes. Albeit less common, systemic exposure to allergens may result in SCD and SDRIFE with a rash in intertriginous zones, including the axillae. Additionally, other infectious, inflammatory, and autoimmune etiologies should be considered and ruled out.
Patch testing is the most reliable method to diagnose suspected ACD. Once confirmed, management includes the use of topical steroids and avoidance of the causative agent. Additionally, patients should be informed of the American Contact Dermatitis Society Contact Allergen Management Program (https://www.contactderm.org/patient-support/camp-access), which provides patients with useful information on products that are safe to use based on their patch testing results.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Brar KK. A review of contact dermatitis. Ann Allergy Asthma Immunol. 2021;126:32-39.
- Evans RL, Marriott RE, Harker M. Axillary skin: biology and care. Int J Cosmet Sci. 2012;34:389-395.
- Watkinson A, Lee RS, Moore AE, et al. Is the axilla a distinct skin phenotype? Int J Cosmet Sci. 2007;29:60.
- Wu JQ, Kilpatrick-Liverman L. Characterizing the composition of underarm and forearm skin using confocal raman spectroscopy. Int J Cosmet Sci. 2011;33:257-262.
- Marti VP, Lee RS, Moore AE, et al. Effect of shaving on axillary stratum corneum. Int J Cosmet Sci. 2003;25:193-198.
- Turner GA, Moore AE, Marti VPJ, et al. Impact of shaving and anti-perspirant use on the axillary vault. Int J Cosmet Sci. 2007;29:31-38.
- Zhai H, Maibach HI. Skin occlusion and irritant and allergic contact dermatitis: an overview. Contact Dermatitis. 2001;44:201-206.
- Lazarov A. Textile dermatitis in patients with contact sensitization in Israel: a 4-year prospective study. J Eur Acad Dermatol Venereol. 2004;18:531-537.
- Nelson JL, Mowad CM. Allergic contact dermatitis: patch testing beyond the TRUE Test. J Clin Aesthet Dermatol. 2010;3:36-41.
- Zirwas MJ, Moennich J. Antiperspirant and deodorant allergy: diagnosis and management. J Clin Aesthet Dermatol. 2008;1:38-43.
- DeKoven JG, Warshaw EM, Reeder MJ, et al. North American Contact Dermatitis Group Patch Test Results: 2019-2020. Dermatitis. 2023;34:90-104.
- Eiermann HJ, Larsen W, Maibach HI, et al. Prospective study of cosmetic reactions: 1977-1980. North American Contact Dermatitis Group. J Am Acad Dermatol. 1982;6:909-917.
- González-Muñoz P, Conde-Salazar L, Vañó-Galván S. Allergic contact dermatitis caused by cosmetic products. Actas Dermosifiliogr. 2014;105:822-832.
- Gerberick GF, Robinson MK, Felter SP, et al. Understanding fragrance allergy using an exposure-based risk assessment approach. Contact Dermatitis. 2001;45:333-340.
- Heisterberg MV, Menne T, Andersen KE, et al. Deodorants are the leading cause of allergic contact dermatitis to fragrance ingredients. Contact Dermatitis. 2011;64:258-264.
- Johansen JD, Andersen TF, Kjoller M, et al. Identification of risk products for fragrance contact allergy: a case-referent study based on patients’ histories. Am J Contact Dermat. 1998;9:80-86.
- Edman B. The influence of shaving method on perfume allergy. Contact Dermatitis. 1994;31:291-292.
- Hamza M, Tohid H, Maibach H. Shaving effects on percutaneous penetration: clinical implications. Cutan Ocul Toxicol. 2015;34:335-343.
- Geier J, Uter W, Lessmann H, et al. Fragrance mix I and II: results of breakdown tests. Flavour Fragr J. 2015;30:264-274.
- Handley J, Burrows D. Allergic contact dermatitis from the synthetic fragrances Lyral and acetyl cedrene in separate underarm deodorant preparations. Contact Dermatitis. 1994;31:288-290.
- Hendriks SA, Bousema MT, van Ginkel CJ. Allergic contact dermatitis from the fragrance ingredient Lyral in underarm deodorant. Contact Dermatitis. 1999;41:119.
- Jacob SE. Allergic contact dermatitis from lyral in an aerosol deodorant. Dermatitis. 2008;19:216-217.
- Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010;29:278-287.
- Goossens A, Merckx L. Allergic contact dermatitis from farnesol in a deodorant. Contact Dermatitis. 1997;37:179-180.
- Schnuch A, Uter W, Geier J, et al. Contact allergy to farnesol in 2021 consecutively patch tested patients. Results of the IVDK. Contact Dermatitis. 2004;50:117-121.
- Uter W, Geier J, Schnuch A, et al. Patch test results with patients’ own perfumes, deodorants and shaving lotions: results of the IVDK 1998–2002. J Eur Acad Dermatol Venereol. 2007;21:374-379.
- Dittmar D, Schuttelaar MLA. Contact sensitization to hydroperoxides of limonene and linalool: results of consecutive patch testing and clinical relevance. Contact Dermatitis. 2019;80:101-109.
- Yazar K, Johnsson S, Lind M-L, et al. Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis. 2011;64:265-272.
- Isaksson M, Karlberg A-T, Nilsson U. Allergic contact dermatitis caused by oxidized linalool in a deodorant. Contact Dermatitis. 2019;81:213-214.
- Chen J, Yi Z, Sun R, et al. Analysis of fragrance allergens in personal care products, toys, and water samples: a review. J AOAC Int. 2022;105:396-412.
- Larsen WG. Perfume dermatitis. J Am Acad Dermatol. 1985;12:1-9.
- Pincelli C, Magni R, Motolese A. Pigmented contact dermatitis from deodorant. Contact Dermatitis. 1993;28:305-306.
- Kwong HL, Lim SPR. Pigmented contact dermatitis in the axillae caused by hydroperoxides of limonene. JAAD Case Reports. 2020;6:476-478.
- Marks J, Anderson B, DeLeo V. Contact and Occupational Dermatology. 4th ed. Jaypee; 2016.
- Johansen JD. Fragrance contact allergy: a clinical review. Am J Clin Dermatol. 2003;4:789-798.
- McGowan MA, Scheman A, Jacob SE. Propylene glycol in contact dermatitis: a systematic review. Dermatitis. 2018;29:6-12.
- Fiume MM, Bergfeld WF, Belsito DV, et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int J Toxicol. 2012;31(5 suppl):245S-260S.
- Farrar CW, Bell HK, King CM. Allergic contact dermatitis from propylene glycol in Efudix cream. Contact Dermatitis. 2003;48:345.
- Friedman ES, Friedman PM, Cohen DE, et al. Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. 2002;46:309-312.
- Lessmann H, Schnuch A, Geier J, et al. Skin-sensitizing and irritant properties of propylene glycol. Contact Dermatitis. 2005;53:247-259.
- Agren-Jonsson S, Magnusson B. Sensitization to propantheline bromide, trichlorocarbanilide and propylene glycol in an antiperspirant. Contact Dermatitis. 1976;2:79-80.
- Catanzaro JM, Smith JG Jr. Propylene glycol dermatitis. J Am Acad Dermatol. 1991;24:90-95.
- Jacob SE, Scheman A, McGowan MA. Propylene glycol. Dermatitis. 2018;29:3-5.
- Carlson S, Gipson K, Nedorost S. Relevance of doubtful (“equivocal”) late patch-test readings. Dermatitis. 2010;21:102-108.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Benohanian A. Antiperspirants and deodorants. Clin Dermatol. 2001;19:398-405.
- Garg S, Loghdey S, Gawkrodger DJ. Allergic contact dermatitis from aluminum in deodorants. Contact Dermatitis. 2010;62:57-58.
- Montemarano AD, Sau P, Johnson FB, et al. Cutaneous granulomas caused by an aluminum-zirconium complex: an ingredient of antiperspirants. J Am Acad Dermatol. 1997;37:496-498.
- Rubin L, Slepyan AH, Weber LF, et al. Granulomas of the axillae caused by deodorants. JAMA. 1956;162:953-955.
- Williams S, Freemont AJ. Aerosol antiperspirants and axillary granulomata. Br Med J (Clin Res Ed). 1984;288:1651-1652.
- Gallego H, Lewis EJ, Crutchfield CE 3rd. Crystal deodorant dermatitis: irritant dermatitis to alum-containing deodorant. Cutis. 1999;64:65-66.
- Leventhal JS, Farhadian JA, Miller KE, et al. Crystal deodorant-induced axillary granulomatous dermatitis. Int J Dermatol. 2014;53:e59-e60.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;3:31-35.
- Bruze M, Netterlid E, Siemund I. Aluminum-allergen of the year 2022. Dermatitis. 2022;33:10-15.
- Goiset A, Darrigade A-S, Labrèze C, et al. Aluminum sensitization in a French paediatric patch test population. Contact Dermatitis. 2018;79:382-383.
- Admani S, Matiz C, Jacob SE. Nickel allergy—a potential cause of razor dermatitis. Pediatr Dermatol. 2014;31:392-393.
- Bibas N, Lassere J, Paul C, et al. Nickel-induced systemic contact dermatitis and intratubal implants: the baboon syndrome revisited. Dermatitis. 2013;24:35-36.
- Seidenari S, Manzini BM, Ddanese P. Contact sensitization to textile dyes: description of 100 subjects. Contact Dermatitis. 1991;24:253-258.
- Hatch KL, Maibach HI. Textile dye allergic contact dermatitis prevalence. Contact Dermatitis. 2000;42:187-195.
- Ryberg K, Isaksson M, Gruvberger B, et al. Contact allergy to textile dyes in southern Sweden. Contact Dermatitis. 2006;54:313-321.
- Pratt M, Taraska V. Disperse blue dyes 106 and 124 are common causes of textile dermatitis and should serve as screening allergens for this condition. Dermatitis. 2000;11:30-41.
- Seidenari S, Giusti F, Massone F, et al. Sensitization to disperse dyes in a patch test population over a five-year period. Am J Contact Dermat. 2002;13:101-107.
- Moreau L, Goossens A. Allergic contact dermatitis associated with reactive dyes in a dark garment: a case report. Contact Dermatitis. 2005;53:150-154.
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce dermatitis. Curr Treat Options Allergy. 2019;6:103-111.
- Jacob SE, Zapolanski T. Systemic contact dermatitis. Dermatitis. 2008;19:9-15.
- Hindsén M, Bruze M, Christensen OB. Flare-up reactions after oral challenge with nickel in relation to challenge dose and intensity and time of previous patch test reactions. J Am Acad Dermatol. 2001;44:616-623.
- Winnicki M, Shear NH. A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions. Am J Clin Dermatol. 2011;12:171-180.
- Kalita BJ, Das S, Dutta B. Itraconazole-induced symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): a rare occurrence. Int J Dermatol. 2020;59:e419-e421.
- Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
- Ramachandran V, Cline A, Summey B, et al. Systemic contact dermatitis related to alcoholic beverage consumption. Dermatol Online J. 2019;25:13030/qt3zg853qv.
- Moreno-Ramírez D, García-Bravo B, Pichardo AR, et al. Baboon syndrome in childhood: easy to avoid, easy to diagnose, but the problem continues. Pediatr Dermatol. 2004;21:250-253.
- Dou X, Liu L-L, Zhu X-J. Nickel-elicited systemic contact dermatitis. Contact Dermatitis. 2003;48:126-129.
- Möller H, Ohlsson K, Linder C, et al. The flare-up reactions after systemic provocation in contact allergy to nickel and gold. Contact Dermatitis. 1999;40:200-204.
- Andersen KE, Hjorth N, Menné T. The baboon syndrome: systemically-induced allergic contact dermatitis. Contact Dermatitis. 1984;10:97-100.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Tan MG, Pratt MD, Burns BF, et al. Baboon syndrome from mercury showing leukocytoclastic vasculitis on biopsy. Contact Dermatitis. 2020;83:415-417.
- Handisurya A, Stingl G, Wöhrl S. SDRIFE (baboon syndrome) induced by penicillin. Clin Exp Dermatol. 2009;34:355-357.
- Akay BN, Sanli H. Symmetrical drug-related intertriginous and flexural exanthem due to oral risperidone. Pediatr Dermatol. 2009;26:214-216.
- Amaro C, Santos R, Cardoso J. Contact allergy to methylisothiazolinone in a deodorant. Contact Dermatitis. 2011;64:298-299.
- Goh CL. Dermatitis from chlorphenesin in a deodorant. Contact Dermatitis. 1987;16:287.
- Taghipour K, Tatnall F, Orton D. Allergic axillary dermatitis due to hydrogenated castor oil in a deodorant. Contact Dermatitis. 2008;58:168-169.
- Sheu M, Simpson EL, Law S V, et al. Allergic contact dermatitis from a natural deodorant: a report of 4 cases associated with lichen acid mix allergy. J Am Acad Dermatol. 2006;55:332-337.
- Pastor-Nieto M-A, Gatica-Ortega M-E, Alcántara-Nicolás F-D-A, et al. Allergic contact dermatitis resulting from cetyl PEG/PPG-10/1 dimethicone in a deodorant cream. Contact Dermatitis. 2018;78:236-239.
- Corazza M, Lombardi AR, Virgili A. Non-eczematous urticarioid allergic contact dermatitis due to Eumulgin L in a deodorant. Contact Dermatitis. 1997;36:159-160.
- van Ketel WG. Allergic contact dermatitis from propellants in deodorant sprays in combination with allergy to ethyl chloride. Contact Dermatitis. 1976;2:115-119.
- Shmunes E, Levy EJ. Quaternary ammonium compound contact dermatitis from a deodorant. Arch Dermatol. 1972;105:91-93.
- Bruze M, Johansen JD, Andersen KE, et al. Deodorants: an experimental provocation study with cinnamic aldehyde. J Am Acad Dermatol. 2003;48:194-200.
- Hann S, Hughes TM, Stone NM. Flexural allergic contact dermatitis to benzalkonium chloride in antiseptic bath oil. Br J Dermatol. 2007;157:795-798.
- Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108:579-580.
- Cotton CH, Duah CG, Matiz C. Allergic contact dermatitis due to methylisothiazolinone in a young girl’s laundry detergent. Pediatr Dermatol. 2017;34:486-487.
- Magnano M, Silvani S, Vincenzi C, et al. Contact allergens and irritants in household washing and cleaning products. Contact Dermatitis. 2009;61:337-341.
- Voller LM, Kullberg SA, Warshaw EM. Axillary allergic contact dermatitis to topical clindamycin. Contact Dermatitis. 2020;82:313-314.
- Iammatteo M, Akenroye A, Jariwala S, et al. Severe contact dermatitis due to ethylenediamine dihydrochloride in nystatin cream. J Allergy Clin Immunol Pract. 2017;5:1448-1450.
- Coskey RJ, Bryan HG. Contact dermatitis due to methylprednisolone. JAMA. 1967;199:136.
- Peterson MY, Han J, Warshaw EM. Allergic contact dermatitis from dipropylene glycol in hydrocortisone lotion. Contact Dermatitis. 2022;87:112-114.
- Ferreira O, Cruz MJ, Mota A, et al. Erythema multiforme-like lesions revealing allergic contact dermatitis to exotic woods. Cutan Ocul Toxicol. 2012;31:61-63.
- Abraham NF, Feldman SR, Vallejos Q, et al. Contact dermatitis in tobacco farmworkers. Contact Dermatitis. 2007;57:40-43.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Frings VG, Böer-Auer A, Breuer K. Histomorphology and immunophenotype of eczematous skin lesions revisited-skinbiopsies are not reliable in differentiating allergic contact dermatitis, irritant contact dermatitis, and atopic dermatitis. Am J Dermatopathol. 2018;40:7-16.
- Knabel M, Mudaliar K. Histopathologic features of inverse psoriasis. J Cutan Pathol. 2022;49:246-251.
- Fujii M, Kishibe M, Honma M, et al. Aluminum chloride-induced apoptosis leads to keratinization arrest and granular parakeratosis. Am J Dermatopathol. 2020;42:756-761.
Practice Points
- The differential diagnosis of axillary dermatitis is broad. Contact dermatitis—both irritant and allergic—represents common etiologies.
- Understanding the clinical features and range of potential sources in axillary contact dermatitis allows for efficient recognition and elimination of causative exposure.
- For cases of suspected allergic contact dermatitis, patch testing and subsequent allergen avoidance are paramount in the management of axillary eruptions.
Squamous Cell Carcinoma Arising in Chronic Inflammatory Dermatoses
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10
Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28
In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
As many as one-quarter of human cancers are related to chronic inflammation, chronic infection, or both.1 Extrinsic inflammation leads to generation of proinflammatory cytokines that in turn recruit other inflammatory cells, which is thought to generate a positive amplification loop.2 Intrinsic stimuli from proto-oncogenes and mutations in tumor suppressor genes lead to transformed cancer cells that also secrete proinflammatory cytokines, thus propagating the cycle.
Numerous factors have been observed in association with tumor growth, progression, invasion, and metastasis.3 One factor for the development of squamous cell carcinoma (SCC) may be chronic inflammatory dermatoses. To date, reviews of chronic inflammation–associated malignancy have focused on solid organ cancers. We sought to provide an up-to-date review of SCC arising within chronic dermatoses, with an emphasis on the anatomic location of dermatoses involved in the transformation of cancer cells, the lag time from onset of dermatosis to diagnosis of SCC, and the distinctive mechanisms thought to be involved in the tumorigenesis in particular dermatoses.
Discoid Lupus Erythematosus
Discoid lupus erythematosus (DLE) is a chronic cutaneous lupus erythematosus variant with a female to male predominance of 3:1,4 and DLE lesions are prone to malignant transformation. Retrospective cohort studies have attempted to characterize who is at risk for SCC and how SCCs behave depending on their location. Cohorts from China,5 India,6 and Japan7 have noted a higher rate of SCC within DLE lesions in men (female to male ratios of 1:2.2, 1:1.6, and 1:2, respectively) and shorter lag times for SCC onset within DLE lesions of the lips (13, 5, and 10 years, respectively) compared to SCC arising in DLE elsewhere (19.2, 11.2, and 26 years, respectively). Studies have noted that DLE lesions of the lips may be prone to more rapid SCC tumorigenesis compared to DLE on cutaneous sites. One study reported SCC in DLE recurrence, metastasis, and death rates of 29%, 16.1%, and 19.4%, respectively,5 which exceeds reported rates in non-DLE SCCs (20%, 0.5% to 6%, and 1%, respectively).5,8
Because SCC arising within DLE is most common on the lips (Figure 1), it has been hypothesized that the high rate of transformation of DLE lesions on the lips may be due to constant exposure to irritation and tobacco, which may accelerate carcinogenesis.5 It also has been hypothesized that atrophic discoid lesions have lost sun protection and are more prone to mutagenic UV radiation,9 as SCCs arising in DLE lesions virtually always display prominent solar elastosis6; however, SCC has been observed to arise in non–sun-exposed DLE lesions in both White and Black patients.10

Additionally, use of immunosuppressant medications may accelerate the emergence of malignancy or more aggressive forms of malignancy; however, patients with autoimmune disease have a greater risk for malignancy at baseline,11 thus making it difficult to determine the excess risk from medications. There also may be a role for human papillomavirus (HPV) accelerating SCC development in DLE lesions, as demonstrated in a case of SCC arising in DLE lesions of the ears, with viral staining evident within the tumors.12 However, testing for HPV is not routinely performed in these cases.
Dermatologists need to be aware of the relatively rapid tumorigenesis and aggressive behavior of transformation and aggression seen with SCC arising within orolabial DLE lesions compared to cutaneous lesions, especially those on the lips.
Lichen Planus
Although patients with typical cutaneous lichen planus lesions do not have an increased risk for SCC,13 variants of lichen planus may predispose patients to SCC.
Oral Lichen Planus—Oral lichen planus (OLP) lesions are prone to malignant transformation. A systematic review of 16 studies evaluating the risk for OLP-associated SCC revealed an overall transformation rate of 1.09%, with a mean lag time of 4.3 years,14 compared to a reference rate of 0.2% for oral SCC.15 A meta-analysis of 19,676 patients with OLP and other oral lichenoid lesions revealed an oral SCC rate of 1.1%, with higher rates of transformation seen in cigarette smokers, alcoholics, and patients with hepatitis C virus infection.16 The ulcerative subtype of OLP appears to present a greater risk for malignant transformation.15 Dermatologists also should be cognizant that treatments for OLP such as topical calcineurin inhibitors may support the development of malignancy within inflammatory lesions.17
Hypertrophic Lichen Planus—The hypertrophic variant of lichen planus (HLP) also is prone to malignant transformation. A 1991 epidemiologic study from Sweden of malignancy arising in lichen planus revealed a disproportionate number of cases arising in verrucous or hypertrophic lesions, with a mean of 12.2 years from onset of the dermatosis to malignancy diagnosis.13 A subsequent 2015 retrospective study of 38 patients revealed that SCC had a propensity for the lower limb, favoring the pretibial region and the calf over the foot and the ankle with a reported lag time of 11 years.18
Although metastatic SCC arising in HLP is rare, 2 cases have been reported. A 24-year-old woman presented with an HLP plaque on the lower leg that developed during childhood and rapidly enlarged 2 months prior to presentation; she eventually died from metastatic disease.19 In another case, a 34-year-old man presented with an HLP lesion of approximately 10 years’ duration. A well-differentiated SCC was excised, and he developed lymph node metastases 5 months later.20
It is important to note that HLP on the legs often is misdiagnosed as SCC, as pseudoepitheliomatous hyperplasia and squamous metaplasia can be difficult to differentiate clinically and histologically.21,22 In the case of multiple eruptive SCCs of the lower leg, clinical correlation is essential to avoid unnecessary and ineffective surgical treatment.
Patients with HLP may exhibit Wickham striae, follicular accentuation, and mucocutaneous lichen planus at other sites, or a correlative initiation of possible culprit medications.23 Because true SCC arising within HLP is relatively rare, its malignant potential is not as clear as those arising within DLE; however, the lower limb appears to be the most common location for SCC within HLP.Nail Lichen Planus—Squamous cell carcinoma arising in nail lichen planus is rare. A report of 2 patients were diagnosed with lichen planus approximately 15 years prior to diagnosis of ungual SCC.24 Given the rarity of this presentation, it is difficult to ascertain the approximate lag time and other risk factors. Furthermore, the role of HPV in these cases was not ruled out. Oncogenic HPV strains have been reported in patients with periungual SCC.25,26
Lichen Sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatosis that favors the anogenital area in a female to male ratio of 10:1.27 It is considered a premalignant condition for SCC tumorigenesis and may be a strong predictor of vulvar SCC (Figure 2), as 62% of vulvar SCC cases (N=78) may have adjacent LS.28

In a Dutch cohort of 3038 women with LS, 2.6% of patients developed vulvar SCC at a median of 3.3 years after LS diagnosis.29 Other studies have estimated a lag time of 4 years until SCC presentation.30 An Italian cohort of 976 women similarly observed that 2.7% of patients developed premalignancy or SCC.31 It was previously estimated that 3% to 5% of patients with LS developed SCC; however, prior studies may have included cases of vulvar intraepithelial neoplasia with low risk for invasive SCC, which might have overestimated true risk of SCC.32 Another confounding factor for elucidating SCC on a background of LS may be the presence of HPV.33 Extragenital LS does not appear to have similar potential for malignant transformation.34
In a prospective Australian cohort of 507 women with LS (mean age, 55.4 years), remission was induced with potent topical corticosteroids.35 Patients who were adherent to a topical regimen did not develop SCC during follow-up. Those who were nonadherent or partially adherent had a 4.7% risk for SCC.35 In a similar prospective study of 83 women in France, the SCC rate was 9.6% in lesions that were untreated or irregularly treated.36 These studies provide essential evidence that appropriately treating LS can prevent SCC at a later date, though longer-term data are lacking.
The rate of SCC arising in male genital LS may approach 8.4%,37 with a lag time of 17 years from onset of LS to SCC diagnosis.38 Although circumcision often is considered curative for male genital LS, patients have been observed to develop penile SCC at least 5 years after circumcision.39 Male penile SCC in a background of LS may not necessarily be HPV associated.40
Marjolin Ulcer
Chronic ulcers or scars, typically postburn scars, may undergo malignant transformation, with SCC being the most common carcinoma.41 Squamous cell carcinoma in the context of a chronic ulcer or wound is known as a Marjolin ulcer (MU). Up to 2% of burn scars have been observed to undergo malignant transformation.42 Marjolin ulcers tend to behave aggressively once they form, and it has been proposed that removal of scar tissue may be a preventive therapeutic strategy.43 Cohort studies of MU on the lower extremities have observed lag times of 26.444 and 37.945 years, with both studies also noting relatively high rates of local recurrence.
The pathogenesis of MU appears to be multifactorial. Chronic inflammation and scar formation have been implicated. Chronic inflammation and irritation of lesions at natural creases are thought to increase mitotic activity,41 and local accumulation of toxin may promote mutagenesis.46 Scar formation may create a locally immunoprivileged site, allowing for developing tumors to evade the immune system47 and become even more aggressive as the tumor accumulates.48 Scar formation also may prevent the ability of immune cells to penetrate the tumor microenvironment and access lymphatic channels.49
Hidradenitis Suppurativa
As many as 3.2% of patients with chronic hidradenitis suppurativa (HS) experience malignant transformation to SCC.50 Early HS displays subclinical lymphedema in affected sites, which can progress to chronic fibrosis, stasis, and accumulation of protein-rich fluid.51 Stasis changes have been associated with altered local inflammatory proteins, such as toll-like receptors, β-defensins, and interleukins.52
A retrospective cohort study of 12 patients revealed a lag time of 28.5 years from HS diagnosis to the manifestation of malignancy.53 After local excision, 7 patients developed recurrence, with 100% mortality. Squamous cell carcinomas were well differentiated and moderately differentiated.53 A 2017 literature review of 62 case reports calculated a mean lag time of 27 years. Despite 85% of SCCs being well differentiated and moderately differentiated, nearly half of patients died within 2 years.54 As seen in other inflammatory conditions, HPV can complicate perineal HS and promote SCC tumorigenesis.55
Squamous cell carcinomas arising within HS lesions are more prevalent in males (6.75:1 ratio),54,56 despite HS being more prevalent in females (2:1 ratio).57 Similar to DLE, SCCs arising in HS are aggressive and are seen more in males, despite both conditions being female predominant. Incidence and mortality rates for primary cutaneous SCC are higher for men vs women58; however, the discordance in aggressive behavior seen more commonly in SCC arising from HS or DLE in male patients has yet to be explained.
Necrobiosis Lipoidica Diabeticorum
Malignancy arising within necrobiosis lipoidica diabeticorum (NLD) is rare. A review of 14 published cases noted that 13 were SCC and 1 was leiomyosarcoma.59 The lag time was 21.5 years; 31% of cases (N=14) presented with regional lymph node metastasis. Although chronic ulceration is a risk factor for SCC and occurs in as many as one-third of NLD cases, its correlation with ulceration and malignant transformation has not been characterized.
Epidermolysis Bullosa
Recessive dystrophic epidermolysis bullosa (RDEB) is a noninflammatory inherited blistering disease, and patients have an inherently high risk for aggressive SCC.60 Other forms of epidermolysis bullosa can lead to SCC, but the rarer RDEB accounts for 69% of SCC cases, with a median age of 36 years at presentation.61 Although SCCs tend to be well differentiated in RDEB (73.9%),61 they also exhibit highly aggressive behavior.62 In the most severe variant—RDEB-generalized severe—the cumulative risk for SCC-related death in an Australian population was 84.4% at 34 years of age.63
As RDEB is an inherited disorder with potential for malignancy at a young age, the pathogenesis is plausibly different from the previously discussed inflammatory dermatoses. This disease is characterized by a mutation in the collagen VII gene, leading to loss of anchoring fibrils and a basement membrane zone split.64 There also can be inherent fibroblast alterations; RDEB fibroblasts create an environment for tumor growth by supporting malignant-cell adhesion and invasion.65 Mutations in p53,66 local alterations in transforming growth factor β activity,67 and downstream matrix metalloproteinase activity68 have been implicated.
Additionally, keratinocytes may retain the N-terminal noncollagenous (NC1) domain of truncated collagen VII while losing the anchoring NC2 domain in mutated collagen VII RDEB, thereby supporting anchorless keratinocyte survival and higher metastatic potential.69 Retention of this truncated NC1 domain has shown conversion of RDEB keratinocytes to tumor in a xenotransplant mouse model.70 A high level of type VII collagen itself may inherently be protumorigenic for keratinocytes.71
There does not appear to be evidence for HPV involvement in RDEB-associated SCC.72 Squamous cell carcinoma development in RDEB appears to be multifactorial,73 but validated tumor models are lacking. Other than conventional oncologic therapy, future directions in the management of RDEB may include gene-, protein- and cell-targeted therapies.73
Conclusion
Squamous cell carcinomas are known to arise within chronic cutaneous inflammatory dermatoses. Tumorigenesis peaks relatively early in new orolabial DLE, LS, and OLP cases, and can occur over many decades in cutaneous DLE, HLP, HS, NLD, and chronic wounds or scars, summarized in the Table. Frequent SCCs are observed in high-risk subtypes of epidermolysis bullosa. Dermatologists must examine areas affected by these diseases at regular intervals, being mindful of the possibility of SCC development. Furthermore, dermatologists should adopt a lower threshold to biopsy suspicious lesions, especially those that develop within relatively new orolabial DLE, chronic HS, or chronic wound cases, as SCC in these settings is particularly aggressive and displays mortality and metastasis rates that exceed those of common cutaneous SCC.
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. doi:10.1002/ijc.23173
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436-444. doi:10.1038/nature07205
- Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. doi:10.3389/fimmu.2011.00098
- Tebbe B. Clinical course and prognosis of cutaneous lupus erythematosus. Clin Dermatol. 2004;22:121-124. doi:10.1016/j.clindermatol.2003.12.018
- Tao J, Zhang X, Guo N, et al. Squamous cell carcinoma complicating discoid lupus erythematosus in Chinese patients: review of the literature, 1964-2010. J Am Acad Dermatol. 2012;66:695-696. doi:10.1016 /j.jaad.2011.09.033
- Fernandes MS, Girisha BS, Viswanathan N, et al. Discoid lupus erythematosus with squamous cell carcinoma: a case report and review of the literature in Indian patients. Lupus. 2015;24:1562-1566. doi:10.1177/0961203315599245
- Makita E, Akasaka E, Sakuraba Y, et al. Squamous cell carcinoma on the lip arising from discoid lupus erythematosus: a case report and review of Japanese patients. Eur J Dermatol. 2016;26:395-396. doi:10.1684/ejd.2016.2780
- Clayman GL, Lee JJ, Holsinger FC, et al. Mortality risk from squamous cell skin cancer. J Clin Oncol. 2005;23:759-765. doi:10.1200/JCO.2005.02.155
- Arvanitidou I-E, Nikitakis NG, Georgaki M, et al. Multiple primary squamous cell carcinomas of the lower lip and tongue arising in discoid lupus erythematosus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:e22-e30. doi:10.1016/j.oooo.2017.08.012
- Alsanafi S, Werth VP. Squamous cell carcinomas arising in discoid lupus erythematosus scars: unusual occurrence in an African-American and in a sun-protected area. J Clin Rheumatol. 2011;17:35-36. doi:10.1097/RHU.0b013e3182051928
- Goobie GC, Bernatsky S, Ramsey-Goldman R, et al. Malignancies in systemic lupus erythematosus: a 2015 update. Curr Opin Rheumatol. 2015;27:454-460. doi:10.1097/BOR.0000000000000202
- Simpson JK, Medina-Flores R, Deng J-S. Squamous cell carcinoma arising in discoid lupus erythematosus lesions of the ears infected with human papillomavirus. Cutis. 2010;86:195-198.
- Sigurgeirsson B, B. Lichen planus and malignancy. an epidemiologic study of 2071 patients and a review of the literature. Arch Dermatol. 1991;127:1684-1688. doi:10.1001/archderm.127.11.1684
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J Am Dent Assoc. 2014;145:45-56. doi:10.14219/jada.2013.10
- Laniosz V, Torgerson RR, Ramos-Rodriguez AJ, et al. Incidence of squamous cell carcinoma in oral lichen planus: a 25-year population-based study. Int J Dermatol. 2019;58:296-301. doi:10.1111/ijd.14215
- Aghbari SMH, Abushouk AI, Attia A, et al. Malignant transformation of oral lichen planus and oral lichenoid lesions: a meta-analysis of 20095 patient data. Oral Oncol. 2017;68:92-102. doi:10.1016/j.oraloncology.2017.03.012
- Morita M, Asoda S, Tsunoda K, et al. The onset risk of carcinoma in patients continuing tacrolimus topical treatment for oral lichen planus: a case report. Odontology. 2017;105:262-266. doi:10.1007/s10266-016-0255-4
- Knackstedt TJ, Collins LK, Li Z, et al. Squamous cell carcinoma arising in hypertrophic lichen planus: a review and analysis of 38 cases. Dermatol Surg. 2015;41:1411-1418. doi:10.1097/DSS.0000000000000565
- Tong LX, Weinstock MJ, Drews R, et al. Widely metastatic squamous cell carcinoma originating from malignant transformation of hypertrophic lichen planus in a 24-year-old woman: case report and review of the literature. Pediatr Dermatol. 2015;32:e98-e101. doi:10.1111/pde.12549
- Ardabili M, Gambichler T, Rotterdam S, et al. Metastatic cutaneous squamous cell carcinoma arising from a previous area of chronic hypertrophic lichen planus. Dermatol Online J. 2003;9:10.
- Bowen AR, Burt L, Boucher K, et al. Use of proliferation rate, p53 staining and perforating elastic fibers in distinguishing keratoacanthoma from hypertrophic lichen planus: a pilot study. J Cutan Pathol. 2012;39:243-250. doi:10.1111/j.1600-0560.2011.01834.x
- Totonchy MB, Leventhal JS, Ko CJ, et al. Hypertrophic lichen planus and well-differentiated squamous cell carcinoma: a diagnostic conundrum. Dermatol Surg. 2018;44:1466-1470. doi:10.1097/DSS.0000000000001465
- Levandoski KA, Nazarian RM, Asgari MM. Hypertrophic lichen planus mimicking squamous cell carcinoma: the importance of clinicopathologic correlation. JAAD Case Rep. 2017;3:151-154. doi: 10.1016/j.jdcr.2017.01.020
- Okiyama N, Satoh T, Yokozeki H, et al. Squamous cell carcinoma arising from lichen planus of nail matrix and nail bed. J Am Acad Dermatol. 2005;53:908-909. doi:10.1016/j.jaad.2005.04.052
- Riddel C, Rashid R, Thomas V. Ungual and periungual human papillomavirus-associated squamous cell carcinoma: a review. J Am Acad Dermatol. 2011;64:1147-1153. doi:10.1016/j.jaad.2010.02.057
- Shimizu A, Kuriyama Y, Hasegawa M, et al. Nail squamous cell carcinoma: a hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81:1358-1370. doi:10.1016/j.jaad.2019.03.070
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. J Am Acad Dermatol. 1995;32:393-416. doi:10.1016/0190-9622(95)90060-8
- Leibowitch M, Neill S, Pelisse M, et al. The epithelial changes associated with squamous cell carcinoma of the vulva: a review of the clinical, histological and viral findings in 78 women. Br J Obstet Gynaecol. 1990;97:1135-1139. doi:10.1111/j.1471-0528.1990.tb02502.x
- Bleeker MCG, Visser PJ, Overbeek LIH, et al. Lichen sclerosus: incidence and risk of vulvar squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2016;25:1224-1230. doi:10.1158/1055-9965.EPI-16-0019
- Carlson JA, Ambros R, Malfetano J, et al. Vulvar lichen sclerosus and squamous cell carcinoma: a cohort, case control, and investigational study with historical perspective; implications for chronic inflammation and sclerosis in the development of neoplasia. Hum Pathol. 1998;29:932-948. doi:10.1016/s0046-8177(98)90198-8
- Micheletti L, Preti M, Radici G, et al. Vulvar lichen sclerosus and neoplastic transformation: a retrospective study of 976 cases. J Low Genit Tract Dis. 2016;20:180-183. doi:10.1097/LGT.0000000000000186
- Cooper SM, Madnani N, Margesson L. Reduced risk of squamous cell carcinoma with adequate treatment of vulvar lichen sclerosus. JAMA Dermatol. 2015;151:1059-1060. doi:10.1001/jamadermatol.2015.0644
- Rakislova N, Alemany L, Clavero O, et al; VVAP Study Group. Differentiated vulvar intraepithelial neoplasia-like and lichen sclerosus-like lesions in HPV-associated squamous cell carcinomas of the vulva. Am J Surg Pathol. 2018;42:828-835. doi:10.1097/PAS.0000000000001047
- Val I, Almeida G. An overview of lichen sclerosus. Clin Obstet Gynecol. 2005;48:808-817. doi:10.1097/01.grf.0000179635.64663.3d
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151:1061-1067. doi:10.1001/jamadermatol.2015.0643
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, et al. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709-712. doi:10.1001/archderm.140.6.709
- Minhas S, Manseck A, Watya S, et al. Penile cancer—prevention and premalignant conditions. Urology. 2010;76(2 suppl 1):S24-S35. doi:10.1016/j.urology.2010.04.007
- Nasca MR, Innocenzi D, Micali G. Penile cancer among patients with genital lichen sclerosus. J Am Acad Dermatol. 1999;41:911-914. doi:10.1016/s0190-9622(99)70245-8
- Philippou P, Shabbir M, Ralph DJ, et al. Genital lichen sclerosus/balanitis xerotica obliterans in men with penile carcinoma: a critical analysis. BJU Int. 2013;111:970-976. doi:10.1111/j.1464-410X.2012.11773.x
- Velazquez EF, Cubilla AL. Lichen sclerosus in 68 patients with squamous cell carcinoma of the penis: frequent atypias and correlation with special carcinoma variants suggests a precancerous role. Am J Surg Pathol. 2003;27:1448-1453. doi:10.1097/00000478-200311000-00007
- Pekarek B, Buck S, Osher L. A comprehensive review on Marjolin’s ulcers: diagnosis and treatment. J Am Col Certif Wound Spec. 2011;3:60-64. doi:10.1016/j.jcws.2012.04.001
- Aydogdu E, Yildirim S, Akoz T. Is surgery an effective and adequate treatment in advanced Marjolin’s ulcer? Burns. 2005;31:421-431. doi:10.1016/j.burns.2005.02.008
- Xiao H, Deng K, Liu R, et al. A review of 31 cases of Marjolin’s ulcer on scalp: is it necessary to preventively remove the scar? Int Wound J. 2019;16:479-485. doi:10.1111/iwj.13058
- Chaturvedi G, Gupta AK, Das S, et al. Marjolin ulcer: an observational epidemiological study from a tertiary care centre in India. Ann Plast Surg. 2019;83:518-522. doi:10.1097/SAP.0000000000001995
- Karasoy Yesilada A, Zeynep Sevim K, D, et al. Marjolin ulcer: clinical experience with 34 patients over 15 years. J Cutan Med Surg. 2013;17:404-409. doi:10.2310/7750.2013.13016
- D, Przybek-Mita J, B, et al. Marjolin’s ulcer in chronic wounds - review of available literature. Contemp Oncol (Pozn). 2017;21:197-202. doi:10.5114/wo.2017.70109
- Visuthikosol V, Boonpucknavig V, Nitiyanant P. Squamous carcinoma in scars: clinicopathological correlations. Ann Plast Surg. 1986;16:42-48. doi:10.1097/00000637-198601000-00004
- Bostwick J 3rd, Pendergrast WJ Jr, Vasconez LO. Marjolin’s ulcer: an immunologically privileged tumor? Plast Reconstr Surg. 1976;57:66-69.
- Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin’s ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184-191. doi:10.1097/PRS.0b013e3181904d86
- Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. Am Surg. 2008;74:1177-1181.
- Fabbrocini G, Ruocco E, De Vita V, et al. Squamous cell carcinoma arising in long-standing hidradenitis suppurativa: an overlooked facet of the immunocompromised district. Clin Dermatol. 2017;35:225-227. doi:10.1016/j.clindermatol.2016.10.019
- Baroni A, Buommino E, Piccolo V, et al. Alterations of skin innate immunity in lymphedematous limbs: correlations with opportunistic diseases. Clin Dermatol. 2014;32:592-598. doi:10.1016/j.clindermatol.2014.04.006
- Kohorst JJ, Shah KK, Hallemeier CL, et al. Squamous cell carcinoma in perineal, perianal, and gluteal hidradenitis suppurativa: experience in 12 patients. Dermatol Surg. 2019;45:519-526. doi:10.1097/DSS.0000000000001713
- Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore). 2017;96:e5857. doi:10.1097/MD.0000000000005857
- Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology. 2010;220:147-153. doi:10.1159/000269836
- Makris G-M, Poulakaki N, Papanota A-M, et al. Vulvar, perianal and perineal cancer after hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg. 2017;43:107-115. doi:10.1097/DSS.0000000000000944
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;68:412-419. doi:10.1016/j.jaad.2012.07.027
- Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48:2046-2053. doi:10.1016/j.ejca.2012.01.003
- Uva L, Freitas J, Soares de Almeida L, et al. Squamous cell carcinoma arising in ulcerated necrobiosis lipoidica diabeticorum. Int Wound J. 2015;12:741-743. doi:10.1111/iwj.12206
- McGrath JA, Schofield OM, Mayou BJ, et al. Epidermolysis bullosa complicated by squamous cell carcinoma: report of 10 cases. J Cutan Pathol. 1992;19:116-123. doi:10.1111/j.1600-0560.1992.tb01352.x
- H, Chiaverini C, Sbidian E, et al. Inherited epidermolysis bullosa and squamous cell carcinoma: a systematic review of 117 cases. Orphanet J Rare Dis. 2016;11:117. doi:10.1186/s13023-016-0489-9.
- Fine J-D. Inherited epidermolysis bullosa: past, present, and future. Ann N Y Acad Sci. 2010;1194:213-222. doi:10.1111/j.1749-6632.2010.05463.x
- Kim M, Li M, Intong-Wheeler LRA, et al. Epidemiology and outcome of squamous cell carcinoma in epidermolysis bullosa in Australia and New Zealand. Acta Derm Venereol. 2018;98:70-76. doi:10.2340/00015555-2781
- Bruckner-Tuderman L, Mitsuhashi Y, Schnyder UW, et al. Anchoring fibrils and type VII collagen are absent from skin in severe recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1989;93:3-9. doi:10.1111/1523-1747.ep12277331
- Ng Y-Z, Pourreyron C, Salas-Alanis JC, et al. Fibroblast-derived dermal matrix drives development of aggressive cutaneous squamous cell carcinoma in patients with recessive dystrophic epidermolysis bullosa. Cancer Res. 2012;72:3522-3534. doi:10.1158/0008-5472.CAN-11-2996
- Arbiser JL, Fan C-Y, Su X, et al. Involvement of p53 and p16 tumor suppressor genes in recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2004;123:788-790. doi:10.1111/j.0022-202X.2004.23418.x
- Knaup J, Gruber C, Krammer B, et al. TGFbeta-signaling in squamous cell carcinoma occurring in recessive dystrophic epidermolysis bullosa. Anal Cell Pathol (Amst). 2011;34:339-353. doi:10.3233/ACP-2011-0039
- Kivisaari AK, Kallajoki M, Mirtti T, et al. Transformation-specific matrix metalloproteinases (MMP)-7 and MMP-13 are expressed by tumour cells in epidermolysis bullosa-associated squamous cell carcinomas. Br J Dermatol. 2008;158:778-785. doi:10.1111/j.1365-2133.2008.08466.x
- Rodeck U, Fertala A, Uitto J. Anchorless keratinocyte survival: an emerging pathogenic mechanism for squamous cell carcinoma in recessive dystrophic epidermolysis bullosa. Exp Dermatol. 2007;16:465-467. doi:10.1111/j.1600-0625.2007.00563.x
- Ortiz-Urda S, Garcia J, Green CL, et al. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773-1776. doi:10.1126/science.1106209
- Pourreyron C, Chen M, McGrath JA, et al. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. doi:10.1111/bjd.12715
- Purdie KJ, Pourreyron C, Fassihi H, et al. No evidence that human papillomavirus is responsible for the aggressive nature of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. J Invest Dermatol. 2010;130:2853-2855. doi:10.1038/jid.2010.243
- South AP, O’Toole EA. Understanding the pathogenesis of recessive dystrophic epidermolysis bullosa squamous cell carcinoma. Dermatol Clin. 2010;28:171-178. doi:10.1016/j.det.2009.10.023
PRACTICE POINTS
- Squamous cell carcinoma can develop within chronic inflammatory dermatoses.
- Orolabial discoid lupus erythematosus (DLE), oral lichen planus, and lichen sclerosus can lead to relatively rapid tumorigenesis. Squamous cell carcinoma arising in cutaneous DLE, hidradenitis suppurativa (HS), necrobiosis lipoidica, chronic wounds, and hypertrophic lichen planus tends to appear after decades of inflammation.
- Be especially mindful of new orolabial DLE cases and chronic cases of HS and Marjolin ulcer because malignancies in these settings are particularly aggressive.
Asymptomatic Violaceous Plaques on the Face and Back
The Diagnosis: Cutaneous Sarcoidosis
A biopsy of a plaque on the back confirmed cutaneous sarcoidosis (CS). A chest radiograph demonstrated hilar nodes, and a referral was placed for comanagement with a pulmonologist. Histopathology was critical in making the diagnosis, with well-circumscribed noncaseating granulomas present in the dermis. The granulomas in CS often are described as naked, as there are minimal lymphocytes present and plasma cells normally are absent.1 Because the lungs are the most common site of involvement, a chest radiograph is necessary to examine for systemic sarcoidosis. Laboratory workup is used to evaluate for lymphopenia, hypercalcemia, elevated blood sedimentation rate, and elevated angiotensin- converting enzyme levels, which are common in systemic sarcoidosis.1
Sarcoidosis is a multisystemic granulomatous disorder with an unknown etiology. It is believed to develop in genetically predisposed individuals as a reaction to unidentified antigens in the environment.1 Helper T cells (TH1) respond to these environmental antigens in those who are susceptible, which leads to the disease process, but paradoxically, even with the elevation of cellular immune activity at the sites of the granulomatous inflammation, the peripheral immune response in these patients is suppressed as shown by lymphopenia.2
Cutaneous sarcoidosis is found in approximately one-third of patients with systemic sarcoidosis but can occur without systemic involvement.1,2 Sarcoidosis is reported worldwide and affects patients of all races and ethnicities, ages, and sexes but does have a higher prevalence among Black individuals in the United States, patients younger than 40 years (peak incidence, 20–29 years of age), and females.2 In 80% of patients, CS occurs before systemic sarcoidosis develops, or they may develop simultaneously.1
Cutaneous sarcoidosis has a wide range of clinical presentations that are classified as specific and nonspecific. Specific lesions in CS contain noncaseating granulomas while nonspecific lesions in CS appear as reactive processes.2 The most common specific presentation of CS includes papules that are brown in pigmentation in lighter skin tones and red to violaceous in darker skin tones (Figure). The most common nonspecific skin manifestation is erythema nodosum, which represents a hypersensitivity reaction. Cutaneous sarcoidosis can appear as hypopigmented or hyperpigmented patches or plaques.1
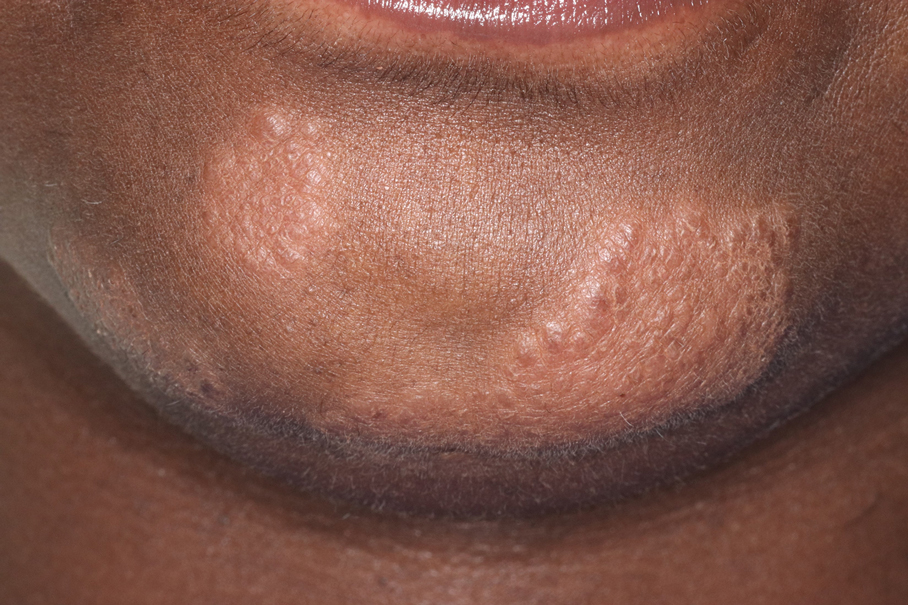
Treatments for CS vary based on the individual.1 For milder and more localized cases, topical or intralesional steroids may be used. If systemic sarcoidosis is suspected or if there is diffuse involvement of the skin, systemic steroids, antimalarials (eg, hydroxychloroquine), low-dose methotrexate, minocycline, allopurinol, azathioprine, isotretinoin, tumor necrosis factor α inhibitors, or psoralen plus long-wave UVA radiation may be used. If systemic sarcoidosis is present, referral to a pulmonologist is recommended for co-management.1
Cutaneous sarcoidosis is known as the “great imitator,” and there are multiple diseases to consider in the differential that are distinguished by the physical findings.1 In our case of a middle-aged Black woman with indurated plaques, a few diagnoses to consider were psoriasis, discoid lupus erythematosus (DLE), mycosis fungoides (MF), and tinea infection.
Psoriasis is a common disease, and 90% of patients have chronic plaquelike disease with well-demarcated erythematous plaques that have a silver-gray scale and a positive Auspitz sign (also known as pinpoint bleeding).3 Plaques often are distributed on the trunk, limb extensors, and scalp, along with nail changes. Some patients also have joint pain, indicating psoriatic arthritis. The etiology of psoriasis is unknown, but it develops due to unrestrained keratinocyte proliferation and defective differentiation, which leads to histopathology showing regular acanthosis and papillary dermal ectasia with rouleaux. Mild cases typically are treated with topical steroids or vitamin D, while more severe cases are treated with methotrexate, cyclosporine, retinoids, or biologics.3
Discoid lupus erythematosus occurs 4 times more often in Black patients than in White patients. Clinically, DLE begins as well-defined, erythematous, scaly patches that expand with hyperpigmentation at the periphery and leave an atrophic, scarred, hypopigmented center.4 It typically is localized to the head and neck, but in cases where it disseminates elsewhere on the body, the risk for systemic lupus erythematosus increases from 1.2% to 28%.5 Histopathology of DLE shows vacuolar degeneration of the basal cell layer in the epidermis along with patchy lymphocytic infiltrate in the dermis. Treatments range from topical steroids for mild cases to antimalarial agents, retinoids, anti-inflammatory drugs, and calcineurin inhibitors for more severe cases.4
Although there are multiple types of cutaneous T-cell lymphoma, the most common is MF, which traditionally is nonaggressive. The typical patient with MF is older than 60 years and presents with indolent, ongoing, flat to minimally indurated patches or plaques that have cigarette paper scale. As MF progresses, some plaques grow into tumors and can become more aggressive. Histologically, MF changes based on its clinical stage, with the initial phase showing epidermotropic atypical lymphocytes and later phases showing less epitheliotropic, larger, atypical lymphocytes. The treatment algorithm varies depending on cutaneous T-cell lymphoma staging.6
Tinea infections are caused by dermatophytes. In prepubertal children, they predominantly appear as tinea corporis (on the body) or tinea capitis (on the scalp), but in adults they appear as tinea cruris (on the groin), tinea pedis (on the feet), or tinea unguium (on the nails).7 Tinea infections classically are known to appear as an annular patch with an active erythematous scaling border and central clearing. The patches can be pruritic. Potassium hydroxide preparation of a skin scraping is a quick test to use in the office; if the results are inconclusive, a culture may be required. Treatment depends on the location of the infection but typically involves either topical or oral antifungal agents.7
- Tchernev G, Cardoso JC, Chokoeva AA, et al. The “mystery” of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. doi:10.1177/039463201402700302
- Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747-755. doi:10.1111/j.1468-3083.2009.03517.x
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment [published online March 23, 2019]. Int J Mol Sci. 2019;20:1475. doi:10.3390/ijms20061475
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Bhat MR, Hulmani M, Dandakeri S, et al. Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol. 2012;57:158-161. doi:10.4103/0019-5154.94298
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. 2017; 37:527-546. doi:10.1016/j.cll.2017.06.006
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
The Diagnosis: Cutaneous Sarcoidosis
A biopsy of a plaque on the back confirmed cutaneous sarcoidosis (CS). A chest radiograph demonstrated hilar nodes, and a referral was placed for comanagement with a pulmonologist. Histopathology was critical in making the diagnosis, with well-circumscribed noncaseating granulomas present in the dermis. The granulomas in CS often are described as naked, as there are minimal lymphocytes present and plasma cells normally are absent.1 Because the lungs are the most common site of involvement, a chest radiograph is necessary to examine for systemic sarcoidosis. Laboratory workup is used to evaluate for lymphopenia, hypercalcemia, elevated blood sedimentation rate, and elevated angiotensin- converting enzyme levels, which are common in systemic sarcoidosis.1
Sarcoidosis is a multisystemic granulomatous disorder with an unknown etiology. It is believed to develop in genetically predisposed individuals as a reaction to unidentified antigens in the environment.1 Helper T cells (TH1) respond to these environmental antigens in those who are susceptible, which leads to the disease process, but paradoxically, even with the elevation of cellular immune activity at the sites of the granulomatous inflammation, the peripheral immune response in these patients is suppressed as shown by lymphopenia.2
Cutaneous sarcoidosis is found in approximately one-third of patients with systemic sarcoidosis but can occur without systemic involvement.1,2 Sarcoidosis is reported worldwide and affects patients of all races and ethnicities, ages, and sexes but does have a higher prevalence among Black individuals in the United States, patients younger than 40 years (peak incidence, 20–29 years of age), and females.2 In 80% of patients, CS occurs before systemic sarcoidosis develops, or they may develop simultaneously.1
Cutaneous sarcoidosis has a wide range of clinical presentations that are classified as specific and nonspecific. Specific lesions in CS contain noncaseating granulomas while nonspecific lesions in CS appear as reactive processes.2 The most common specific presentation of CS includes papules that are brown in pigmentation in lighter skin tones and red to violaceous in darker skin tones (Figure). The most common nonspecific skin manifestation is erythema nodosum, which represents a hypersensitivity reaction. Cutaneous sarcoidosis can appear as hypopigmented or hyperpigmented patches or plaques.1

Treatments for CS vary based on the individual.1 For milder and more localized cases, topical or intralesional steroids may be used. If systemic sarcoidosis is suspected or if there is diffuse involvement of the skin, systemic steroids, antimalarials (eg, hydroxychloroquine), low-dose methotrexate, minocycline, allopurinol, azathioprine, isotretinoin, tumor necrosis factor α inhibitors, or psoralen plus long-wave UVA radiation may be used. If systemic sarcoidosis is present, referral to a pulmonologist is recommended for co-management.1
Cutaneous sarcoidosis is known as the “great imitator,” and there are multiple diseases to consider in the differential that are distinguished by the physical findings.1 In our case of a middle-aged Black woman with indurated plaques, a few diagnoses to consider were psoriasis, discoid lupus erythematosus (DLE), mycosis fungoides (MF), and tinea infection.
Psoriasis is a common disease, and 90% of patients have chronic plaquelike disease with well-demarcated erythematous plaques that have a silver-gray scale and a positive Auspitz sign (also known as pinpoint bleeding).3 Plaques often are distributed on the trunk, limb extensors, and scalp, along with nail changes. Some patients also have joint pain, indicating psoriatic arthritis. The etiology of psoriasis is unknown, but it develops due to unrestrained keratinocyte proliferation and defective differentiation, which leads to histopathology showing regular acanthosis and papillary dermal ectasia with rouleaux. Mild cases typically are treated with topical steroids or vitamin D, while more severe cases are treated with methotrexate, cyclosporine, retinoids, or biologics.3
Discoid lupus erythematosus occurs 4 times more often in Black patients than in White patients. Clinically, DLE begins as well-defined, erythematous, scaly patches that expand with hyperpigmentation at the periphery and leave an atrophic, scarred, hypopigmented center.4 It typically is localized to the head and neck, but in cases where it disseminates elsewhere on the body, the risk for systemic lupus erythematosus increases from 1.2% to 28%.5 Histopathology of DLE shows vacuolar degeneration of the basal cell layer in the epidermis along with patchy lymphocytic infiltrate in the dermis. Treatments range from topical steroids for mild cases to antimalarial agents, retinoids, anti-inflammatory drugs, and calcineurin inhibitors for more severe cases.4
Although there are multiple types of cutaneous T-cell lymphoma, the most common is MF, which traditionally is nonaggressive. The typical patient with MF is older than 60 years and presents with indolent, ongoing, flat to minimally indurated patches or plaques that have cigarette paper scale. As MF progresses, some plaques grow into tumors and can become more aggressive. Histologically, MF changes based on its clinical stage, with the initial phase showing epidermotropic atypical lymphocytes and later phases showing less epitheliotropic, larger, atypical lymphocytes. The treatment algorithm varies depending on cutaneous T-cell lymphoma staging.6
Tinea infections are caused by dermatophytes. In prepubertal children, they predominantly appear as tinea corporis (on the body) or tinea capitis (on the scalp), but in adults they appear as tinea cruris (on the groin), tinea pedis (on the feet), or tinea unguium (on the nails).7 Tinea infections classically are known to appear as an annular patch with an active erythematous scaling border and central clearing. The patches can be pruritic. Potassium hydroxide preparation of a skin scraping is a quick test to use in the office; if the results are inconclusive, a culture may be required. Treatment depends on the location of the infection but typically involves either topical or oral antifungal agents.7
The Diagnosis: Cutaneous Sarcoidosis
A biopsy of a plaque on the back confirmed cutaneous sarcoidosis (CS). A chest radiograph demonstrated hilar nodes, and a referral was placed for comanagement with a pulmonologist. Histopathology was critical in making the diagnosis, with well-circumscribed noncaseating granulomas present in the dermis. The granulomas in CS often are described as naked, as there are minimal lymphocytes present and plasma cells normally are absent.1 Because the lungs are the most common site of involvement, a chest radiograph is necessary to examine for systemic sarcoidosis. Laboratory workup is used to evaluate for lymphopenia, hypercalcemia, elevated blood sedimentation rate, and elevated angiotensin- converting enzyme levels, which are common in systemic sarcoidosis.1
Sarcoidosis is a multisystemic granulomatous disorder with an unknown etiology. It is believed to develop in genetically predisposed individuals as a reaction to unidentified antigens in the environment.1 Helper T cells (TH1) respond to these environmental antigens in those who are susceptible, which leads to the disease process, but paradoxically, even with the elevation of cellular immune activity at the sites of the granulomatous inflammation, the peripheral immune response in these patients is suppressed as shown by lymphopenia.2
Cutaneous sarcoidosis is found in approximately one-third of patients with systemic sarcoidosis but can occur without systemic involvement.1,2 Sarcoidosis is reported worldwide and affects patients of all races and ethnicities, ages, and sexes but does have a higher prevalence among Black individuals in the United States, patients younger than 40 years (peak incidence, 20–29 years of age), and females.2 In 80% of patients, CS occurs before systemic sarcoidosis develops, or they may develop simultaneously.1
Cutaneous sarcoidosis has a wide range of clinical presentations that are classified as specific and nonspecific. Specific lesions in CS contain noncaseating granulomas while nonspecific lesions in CS appear as reactive processes.2 The most common specific presentation of CS includes papules that are brown in pigmentation in lighter skin tones and red to violaceous in darker skin tones (Figure). The most common nonspecific skin manifestation is erythema nodosum, which represents a hypersensitivity reaction. Cutaneous sarcoidosis can appear as hypopigmented or hyperpigmented patches or plaques.1

Treatments for CS vary based on the individual.1 For milder and more localized cases, topical or intralesional steroids may be used. If systemic sarcoidosis is suspected or if there is diffuse involvement of the skin, systemic steroids, antimalarials (eg, hydroxychloroquine), low-dose methotrexate, minocycline, allopurinol, azathioprine, isotretinoin, tumor necrosis factor α inhibitors, or psoralen plus long-wave UVA radiation may be used. If systemic sarcoidosis is present, referral to a pulmonologist is recommended for co-management.1
Cutaneous sarcoidosis is known as the “great imitator,” and there are multiple diseases to consider in the differential that are distinguished by the physical findings.1 In our case of a middle-aged Black woman with indurated plaques, a few diagnoses to consider were psoriasis, discoid lupus erythematosus (DLE), mycosis fungoides (MF), and tinea infection.
Psoriasis is a common disease, and 90% of patients have chronic plaquelike disease with well-demarcated erythematous plaques that have a silver-gray scale and a positive Auspitz sign (also known as pinpoint bleeding).3 Plaques often are distributed on the trunk, limb extensors, and scalp, along with nail changes. Some patients also have joint pain, indicating psoriatic arthritis. The etiology of psoriasis is unknown, but it develops due to unrestrained keratinocyte proliferation and defective differentiation, which leads to histopathology showing regular acanthosis and papillary dermal ectasia with rouleaux. Mild cases typically are treated with topical steroids or vitamin D, while more severe cases are treated with methotrexate, cyclosporine, retinoids, or biologics.3
Discoid lupus erythematosus occurs 4 times more often in Black patients than in White patients. Clinically, DLE begins as well-defined, erythematous, scaly patches that expand with hyperpigmentation at the periphery and leave an atrophic, scarred, hypopigmented center.4 It typically is localized to the head and neck, but in cases where it disseminates elsewhere on the body, the risk for systemic lupus erythematosus increases from 1.2% to 28%.5 Histopathology of DLE shows vacuolar degeneration of the basal cell layer in the epidermis along with patchy lymphocytic infiltrate in the dermis. Treatments range from topical steroids for mild cases to antimalarial agents, retinoids, anti-inflammatory drugs, and calcineurin inhibitors for more severe cases.4
Although there are multiple types of cutaneous T-cell lymphoma, the most common is MF, which traditionally is nonaggressive. The typical patient with MF is older than 60 years and presents with indolent, ongoing, flat to minimally indurated patches or plaques that have cigarette paper scale. As MF progresses, some plaques grow into tumors and can become more aggressive. Histologically, MF changes based on its clinical stage, with the initial phase showing epidermotropic atypical lymphocytes and later phases showing less epitheliotropic, larger, atypical lymphocytes. The treatment algorithm varies depending on cutaneous T-cell lymphoma staging.6
Tinea infections are caused by dermatophytes. In prepubertal children, they predominantly appear as tinea corporis (on the body) or tinea capitis (on the scalp), but in adults they appear as tinea cruris (on the groin), tinea pedis (on the feet), or tinea unguium (on the nails).7 Tinea infections classically are known to appear as an annular patch with an active erythematous scaling border and central clearing. The patches can be pruritic. Potassium hydroxide preparation of a skin scraping is a quick test to use in the office; if the results are inconclusive, a culture may be required. Treatment depends on the location of the infection but typically involves either topical or oral antifungal agents.7
- Tchernev G, Cardoso JC, Chokoeva AA, et al. The “mystery” of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. doi:10.1177/039463201402700302
- Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747-755. doi:10.1111/j.1468-3083.2009.03517.x
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment [published online March 23, 2019]. Int J Mol Sci. 2019;20:1475. doi:10.3390/ijms20061475
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Bhat MR, Hulmani M, Dandakeri S, et al. Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol. 2012;57:158-161. doi:10.4103/0019-5154.94298
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. 2017; 37:527-546. doi:10.1016/j.cll.2017.06.006
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
- Tchernev G, Cardoso JC, Chokoeva AA, et al. The “mystery” of cutaneous sarcoidosis: facts and controversies. Int J Immunopathol Pharmacol. 2014;27:321-330. doi:10.1177/039463201402700302
- Ali MM, Atwan AA, Gonzalez ML. Cutaneous sarcoidosis: updates in the pathogenesis. J Eur Acad Dermatol Venereol. 2010;24:747-755. doi:10.1111/j.1468-3083.2009.03517.x
- Rendon A, Schäkel K. Psoriasis pathogenesis and treatment [published online March 23, 2019]. Int J Mol Sci. 2019;20:1475. doi:10.3390/ijms20061475
- McDaniel B, Sukumaran S, Koritala T, et al. Discoid lupus erythematosus. StatPearls [Internet]. StatPearls Publishing; 2023. Accessed December 11, 2023. https://www.ncbi.nlm.nih.gov/books/NBK493145/
- Bhat MR, Hulmani M, Dandakeri S, et al. Disseminated discoid lupus erythematosus leading to squamous cell carcinoma. Indian J Dermatol. 2012;57:158-161. doi:10.4103/0019-5154.94298
- Pulitzer M. Cutaneous T-cell Lymphoma. Clin Lab Med. 2017; 37:527-546. doi:10.1016/j.cll.2017.06.006
- Ely JW, Rosenfeld S, Seabury Stone M. Diagnosis and management of tinea infections. Am Fam Physician. 2014;90:702-710.
A 35-year-old Black woman presented to dermatology as a new patient for evaluation of an asymptomatic rash that had enlarged and spread to involve both the face and back over the last 4 months. She had not tried any treatments. She had no notable medical history and was uncertain of her family history. Physical examination showed indurated, flesh-colored to violaceous plaques around the alar-facial groove (top), nasal tip, chin, and back (bottom). The mucosae and nails were not involved.
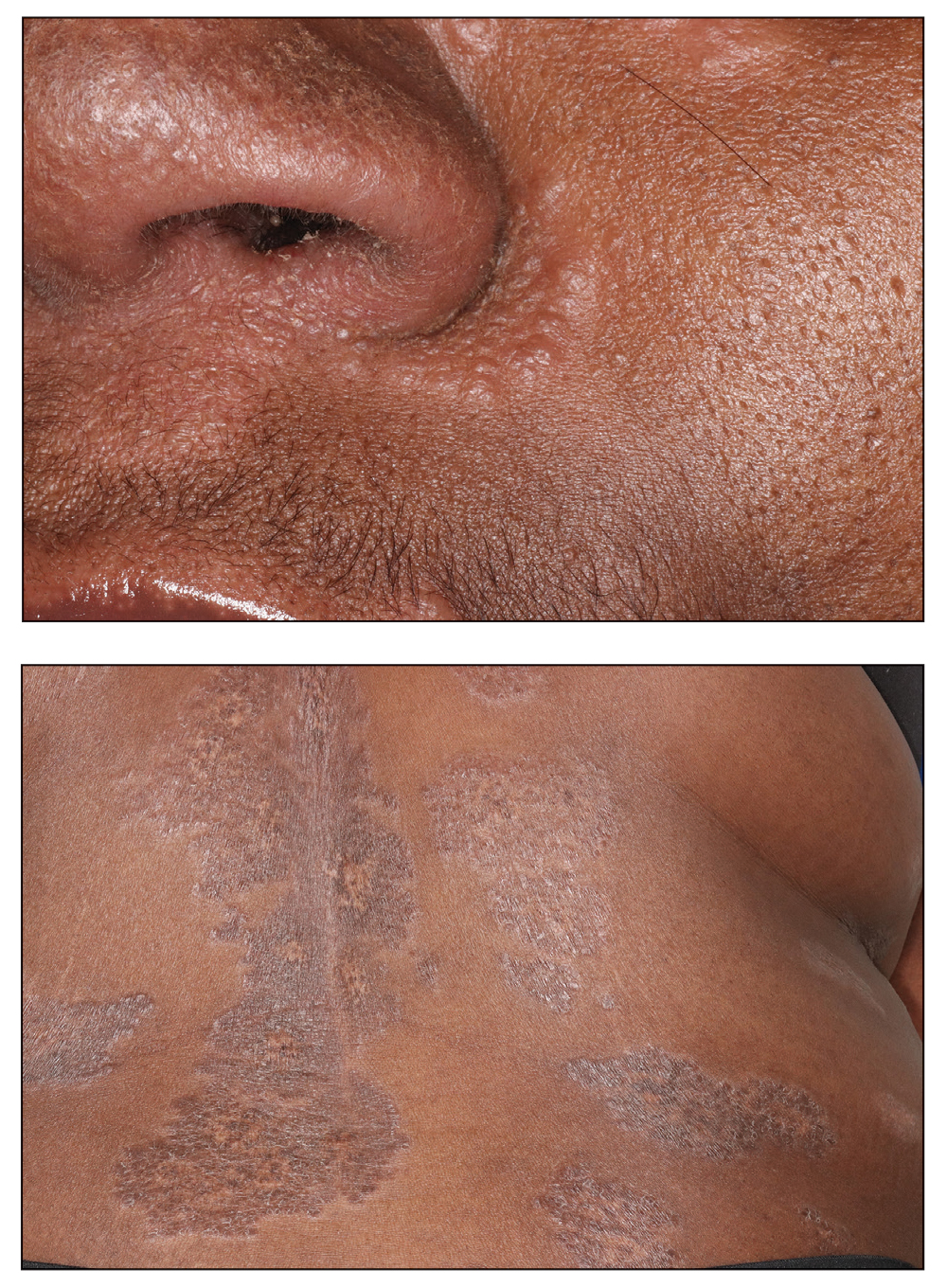
Botanical Briefs: Contact Dermatitis Induced by Western Poison Ivy (Toxicodendron rydbergii)
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
Clinical Importance
Western poison ivy (Toxicodendron rydbergii) is responsible for many of the cases of Toxicodendron contact dermatitis (TCD) reported in the western and northern United States. Toxicodendron plants cause more cases of allergic contact dermatitis (ACD) in North America than any other allergen1; 9 million Americans present to physician offices and 1.6 million present to emergency departments annually for ACD, emphasizing the notable medical burden of this condition.2,3 Exposure to urushiol, a plant resin containing potent allergens, precipitates this form of ACD.
An estimated 50% to 75% of adults in the United States demonstrate clinical sensitivity and exhibit ACD following contact with T rydbergii.4 Campers, hikers, firefighters, and forest workers often risk increased exposure through physical contact or aerosolized allergens in smoke. According to the Centers for Disease Control and Prevention, the incidence of visits to US emergency departments for TCD nearly doubled from 2002 to 2012,5 which may be explained by atmospheric CO2 levels that both promote increased growth of Toxicodendron species and augment their toxicity.6
Cutaneous Manifestations
The clinical presentation of T rydbergii contact dermatitis is similar to other allergenic members of the Toxicodendron genus. Patients sensitive to urushiol typically develop a pruritic erythematous rash within 1 to 2 days of exposure (range, 5 hours to 15 days).7 Erythematous and edematous streaks initially manifest on the extremities and often progress to bullae and oozing papulovesicles. In early disease, patients also may display black lesions on or near the rash8 (so-called black-dot dermatitis) caused by oxidized urushiol deposited on the skin—an uncommon yet classic presentation of TCD. Generally, symptoms resolve without complications and with few sequalae, though hyperpigmentation or a secondary infection can develop on or near affected areas.9,10
Taxonomy
The Toxicodendron genus belongs to the Anacardiaceae family, which includes pistachios, mangos, and cashews, and causes more cases of ACD than every other plant combined.4 (Shelled pistachios and cashews do not possess cross-reacting allergens and should not worry consumers; mango skin does contain urushiol.)
Toxicodendron (formerly part of the Rhus genus) includes several species of poison oak, poison ivy, and poison sumac and can be found in shrubs (T rydbergii and Toxicodendron diversilobum), vines (Toxicodendron radicans and Toxicodendron pubescens), and trees (Toxicodendron vernix). In addition, Toxicodendron taxa can hybridize with other taxa in close geographic proximity to form morphologic intermediates. Some individual plants have features of multiple species.11
Etymology
The common name of T rydbergii—western poison ivy—misleads the public; the plant contains no poison that can cause death and does not grow as ivy by wrapping around trees, as T radicans and English ivy (Hedera helix) do. Its formal genus, Toxicodendron, means “poison tree” in Greek and was given its generic name by the English botanist Phillip Miller in 1768,12 which caused the renaming of Rhus rydbergii as T rydbergii. The species name honors Per Axel Rydberg, a 19th and 20th century Swedish-American botanist.
Distribution
Toxicodendron rydbergii grows in California and other states in the western half of the United States as well as the states bordering Canada and Mexico. In Canada, it reigns as the most dominant form of poison ivy.13 Hikers and campers find T rydbergii in a variety of areas, including roadsides, river bottoms, sandy shores, talus slopes, precipices, and floodplains.11 This taxon grows under a variety of conditions and in distinct regions, and it thrives in both full sun or shade.
Identifying Features
Toxicodendron rydbergii turns red earlier than most plants; early red summer leaves should serve as a warning sign to hikers from a distance (Figure 1). It displays trifoliate ovate leaves (ie, each leaf contains 3 leaflets) on a dwarf nonclimbing shrub (Figure 2). Although the plant shares common features with its cousin T radicans (eastern poison ivy), T rydbergii is easily distinguished by its thicker stems, absence of aerial rootlets (abundant in T radicans), and short (approximately 1 meter) height.4

Curly hairs occupy the underside of T rydbergii leaflets and along the midrib; leaflet margins appear lobed or rounded. Lenticels appear as small holes in the bark that turn gray in the cold and become brighter come spring.13

The plant bears glabrous long petioles (leaf stems) and densely grouped clusters of yellow flowers. In autumn, the globose fruit—formed in clusters between each twig and leaf petiole (known as an axillary position)—change from yellow-green to tan (Figure 3). When urushiol exudes from damaged leaflets or other plant parts, it oxidizes on exposure to air and creates hardened black deposits on the plant. Even when grown in garden pots, T rydbergii maintains its distinguishing features.11

Dermatitis-Inducing Plant Parts
All parts of T rydbergii including leaves, stems, roots, and fruit contain the allergenic sap throughout the year.14 A person must damage or bruise the plant for urushiol to be released and produce its allergenic effects; softly brushing against undamaged plants typically does not induce dermatitis.4
Pathophysiology of Urushiol
Urushiol, a pale yellow, oily mixture of organic compounds conserved throughout all Toxicodendron species, contains highly allergenic alkyl catechols. These catechols possess hydroxyl groups at positions 1 and 2 on a benzene ring; the hydrocarbon side chain of poison ivies (typically 15–carbon atoms long) attaches at position 3.15 The catechols and the aliphatic side chain contribute to the plant’s antigenic and dermatitis-inducing properties.16
The high lipophilicity of urushiol allows for rapid and unforgiving absorption into the skin, notwithstanding attempts to wash it off. Upon direct contact, catechols of urushiol penetrate the epidermis and become oxidized to quinone intermediates that bind to antigen-presenting cells in the epidermis and dermis. Epidermal Langerhans cells and dermal macrophages internalize and present the antigen to CD4+ T cells in nearby lymph nodes. This sequence results in production of inflammatory mediators, clonal expansion of T-effector and T-memory cells specific to the allergenic catechols, and an ensuing cytotoxic response against epidermal cells and the dermal vasculature. Keratinocytes and monocytes mediate the inflammatory response by releasing other cytokines.4,17
Sensitization to urushiol generally occurs at 8 to 14 years of age; therefore, infants have lower susceptibility to dermatitis upon contact with T rydbergii.18 Most animals do not experience sensitization upon contact; in fact, birds and forest animals consume the urushiol-rich fruit of T rydbergii without harm.3
Prevention and Treatment
Toxicodendron dermatitis typically lasts 1 to 3 weeks but can remain for as long as 6 weeks without treatment.19 Recognition and physical avoidance of the plant provides the most promising preventive strategy. Immediate rinsing with soap and water can prevent TCD by breaking down urushiol and its allergenic components; however, this is an option for only a short time, as the skin absorbs 50% of urushiol within 10 minutes after contact.20 Nevertheless, patients must seize the earliest opportunity to wash off the affected area and remove any residual urushiol. Patients must be cautious when removing and washing clothing to prevent further contact.
Most health care providers treat TCD with a corticosteroid to reduce inflammation and intense pruritus. A high-potency topical corticosteroid (eg, clobetasol) may prove effective in providing early therapeutic relief in mild disease.21 A short course of a systemic steroid quickly and effectively quenches intense itching and should not be limited to what the clinician considers severe disease. Do not underestimate the patient’s symptoms with this eruption.
Prednisone dosing begins at 1 mg/kg daily and is then tapered slowly over 2 weeks (no shorter a time) for an optimal treatment course of 15 days.22 Prescribing an inadequate dosage and course of a corticosteroid leaves the patient susceptible to rebound dermatitis—and loss of trust in their provider.
Intramuscular injection of the long-acting corticosteroid triamcinolone acetonide with rapid-onset betamethasone provides rapid relief and fewer adverse effects than an oral corticosteroid.22 Despite the long-standing use of sedating oral antihistamines by clinicians, these drugs provide no benefit for pruritus or sleep because the histamine does not cause the itching of TCD, and antihistamines disrupt normal sleep architecture.23-25
Patients can consider several over-the-counter products that have varying degrees of efficacy.4,26 The few products for which prospective studies support their use include Tecnu (Tec Laboraties Inc), Zanfel (RhusTox), and the well-known soaps Dial (Henkel Corporation) and Goop (Critzas Industries, Inc).27,28
Aside from treating the direct effects of TCD, clinicians also must take note of any look for signs of secondary infection and occasionally should consider supplementing treatment with an antibiotic.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed December 23, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557866/
- The Lewin Group. The Burden of Skin Diseases 2005. Society for Investigative Dermatology and American Academy of Dermatology Association; 2005:37-40. Accessed December 26, 2023. https://www.lewin.com/content/dam/Lewin/Resources/Site_Sections/Publications/april2005skindisease.pdf
- Monroe J. Toxicodendron contact dermatitis: a case report and brief review. J Clin Aesthet Dermatol. 2020;13(9 Suppl 1):S29-S34.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128. doi:10.1580/pr31-05.1
- Fretwell S. Poison ivy cases on the rise. The State. Updated May 15,2017. Accessed December 26, 2023. https://www.thestate.com/news/local/article150403932.html
- Mohan JE, Ziska LH, Schlesinger WH, et al. Biomass and toxicity responses of poison ivy (Toxicodendron radicans) to elevated atmospheric CO2. Proc Natl Acad Sci U S A. 2006;103:9086-9089. doi:10.1073/pnas.0602392103
- Williams JV, Light J, Marks JG Jr. Individual variations in allergic contact dermatitis from urushiol. Arch Dermatol. 1999;135:1002-1003. doi:10.1001/archderm.135.8.1002
- Kurlan JG, Lucky AW. Black spot poison ivy: a report of 5 cases and a review of the literature. J Am Acad Dermatol. 2001;45:246-249. doi:10.1067/mjd.2001.114295
- Fisher AA. Poison ivy/oak/sumac. part II: specific features. Cutis. 1996;58:22-24.
- Brook I, Frazier EH, Yeager JK. Microbiology of infected poison ivy dermatitis. Br J Dermatol. 2000;142:943-946. doi:10.1046/j.1365-2133.2000.03475.x
- Gillis WT. The systematics and ecology of poison-ivy and the poison-oaks (Toxicodendron, Anacardiaceae). Rhodora. 1971;73:370-443.
- Reveal JL. Typification of six Philip Miller names of temperate North American Toxicodendron (Anacardiaceae) with proposals (999-1000) to reject T. crenatum and T. volubile. TAXON. 1991;40:333-335. doi:10.2307/1222994
- Guin JD, Gillis WT, Beaman JH. Recognizing the Toxicodendrons (poison ivy, poison oak, and poison sumac). J Am Acad Dermatol. 1981;4:99-114. doi:10.1016/s0190-9622(81)70014-8
- Lee NP, Arriola ER. Poison ivy, oak, and sumac dermatitis. West J Med. 1999;171:354-355.
- Marks JG Jr, Anderson BE, DeLeo VA, eds. Contact and Occupational Dermatology. Jaypee Brothers Medical Publishers Ltd; 2016.
- Dawson CR. The chemistry of poison ivy. Trans N Y Acad Sci. 1956;18:427-443. doi:10.1111/j.2164-0947.1956.tb00465.x
- Kalish RS. Recent developments in the pathogenesis of allergic contact dermatitis. Arch Dermatol. 1991;127:1558-1563.
- Fisher AA, Mitchell J. Toxicodendron plants and spices. In: Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. 4th ed. Williams & Wilkins; 1995:461-523.
- Labib A, Yosipovitch G. Itchy Toxicodendron plant dermatitis. Allergies. 2022;2:16-22. doi:10.3390/allergies2010002
- Fisher AA. Poison ivy/oak dermatitis part I: prevention—soap and water, topical barriers, hyposensitization. Cutis. 1996;57:384-386.
- Kim Y, Flamm A, ElSohly MA, et al. Poison ivy, oak, and sumac dermatitis: what is known and what is new? 2019;30:183-190. doi:10.1097/DER.0000000000000472
- Prok L, McGovern T. Poison ivy (Toxicodendron) dermatitis. UpToDate. Updated October 16, 2023. Accessed December 26, 2023. https://www.uptodate.com/contents/poison-ivy-toxicodendron-dermatitis
- Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522-1525. doi:10.1001/archderm.135.12.1522
- He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol. 2018;79:92-96. doi:10.1016/j.jaad.2017.12.077
- van Zuuren EJ, Apfelbacher CJ, Fedorowicz Z, et al. No high level evidence to support the use of oral H1 antihistamines as monotherapy for eczema: a summary of a Cochrane systematic review. Syst Rev. 2014;3:25. doi:10.1186/2046-4053-3-25
- Neill BC, Neill JA, Brauker J, et al. Postexposure prevention of Toxicodendron dermatitis by early forceful unidirectional washing with liquid dishwashing soap. J Am Acad Dermatol. 2019;81:E25. doi:10.1016/j.jaad.2017.12.081
- Stibich AS, Yagan M, Sharma V, et al. Cost-effective post-exposure prevention of poison ivy dermatitis. Int J Dermatol. 2000;39:515-518. doi:10.1046/j.1365-4362.2000.00003.x
- Davila A, Laurora M, Fulton J, et al. A new topical agent, Zanfel, ameliorates urushiol-induced Toxicodendron allergic contact dermatitis [abstract]. Ann Emerg Med. 2003;42:S98.
PRACTICE POINTS
- Western poison ivy (Toxicodendron rydbergii) accounts for many of the cases of Toxicodendron contact dermatitis (TCD) in the western and northern United States. Individuals in these regions should be educated on how to identify T rydbergii to avoid TCD.
- Dermatologists should include TCD in the differential diagnosis when a patient presents with an erythematous pruritic rash in a linear pattern with sharp borders.
- Most patients who experience intense itching and pain from TCD benefit greatly from prompt treatment with an oral or intramuscular corticosteroid. Topical steroids rarely provide relief; oral antihistamines provide no benefit.
Multiple New-Onset Pyogenic Granulomas During Treatment With Paclitaxel and Ramucirumab
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).
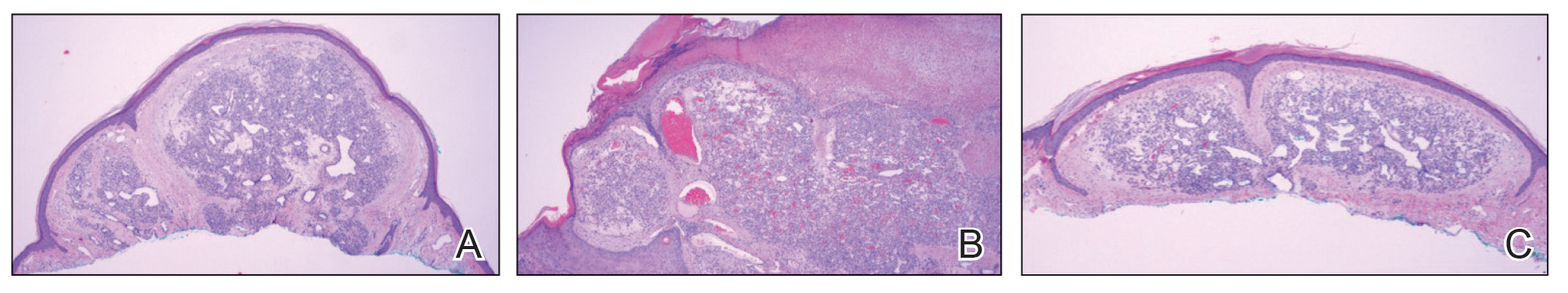
The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
To the Editor:
Pyogenic granuloma (PG) is a benign vascular tumor that clinically is characterized as a small eruptive friable papule.1 Lesions typically are solitary and most commonly occur in children but also are associated with pregnancy; trauma to the skin or mucosa; and use of certain medications such as isotretinoin, capecitabine, vemurafenib, or indinavir.1 Numerous antineoplastic medications have been associated with the development of solitary PGs, including the taxane mitotic inhibitor paclitaxel (PTX) and the vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody ramucirumab.2 We report a case of multiple PGs in a patient undergoing treatment with PTX and ramucirumab.

A 59-year-old woman presented to the dermatology clinic with red, itchy, bleeding skin lesions on the breast, superior chest, left cheek, and forearm of 1 month’s duration. She denied any preceding trauma to the areas. Her medical history was notable for gastroesophageal junction adenocarcinoma diagnosed more than 2 years prior to presentation. Her original treatment regimen included nivolumab, which was discontinued for unknown reasons 5 months prior to presentation, and she was started on combination therapy with PTX and ramucirumab at that time. She noted the formation of small red papules 2 months after the initiation of PTX-ramucirumab combination therapy, which grew larger over the course of the next month. Physical examination revealed 5 friable hemorrhagic papules and nodules ranging in size from 3 to 10 mm on the chest, cheek, and forearm consistent with PGs (Figure 1). Several scattered cherry angiomas were noted on the scalp and torso, but the patient reported these were not new. Biopsies of the PGs demonstrated lobular aggregates of small-caliber vessels set in an edematous inflamed stroma and partially enclosed by small collarettes of adnexal epithelium, confirming the clinical diagnosis of multiple PGs (Figure 2).

The first case of PTX-associated PG was reported in 2012.3 Based on a PubMed search of articles indexed for MEDLINE using the terms pyogenic granuloma, lobular capillary hemangioma, paclitaxel, taxane, and ramucirumab, there have been 9 cases of solitary PG development in the setting of PTX alone or in combination with ramucirumab since 2019 (Table).3-8 Pyogenic granulomas reported in patients who were treated exclusively with PTX were subungual, while the cases resulting from combined therapy were present on the scalp, face, oral mucosa, and surfaces of the hands sparing the nails. Ibe et al6 reported PG in a patient who received ramucirumab therapy without PTX but in combination with another taxane, docetaxel, which itself has been reported to cause subungual PG when used alone.9 Our case of the simultaneous development of multiple PGs in the setting of combined PTX and ramucirumab therapy added to the cutaneous distributions for which therapy-induced PGs have been observed (Table).

The development of PG, a vascular tumor, during treatment with the VEGFR2 inhibitor ramucirumab—whose mechanism of action is to inhibit angioneogenesis—is inherently paradoxical. In 2015, a rapidly expanding angioma with a mutation in the kinase domain receptor gene, KDR, that encodes VEGFR2 was identified in a patient undergoing ramucirumab therapy. The authors suggested that KDR mutation resulted in paradoxical activation of VEGFR2 in the setting of ramucirumab therapy.10 Since then, ramucirumab and PTX were suggested to have a synergistic effect in vascular proliferation,5 though an exact mechanism has not been proposed. Other authors have identified increased expression of VEGFR2 in biopsy specimens of PG during combined ramucirumab and taxane therapy.6 Although genetic studies have not been used to evaluate for the presence of KDR mutations specifically in our patient population, it is possible that patients who develop PG and other vascular tumors during combined taxane and ramucirumab therapy have a mutation that makes them more susceptible to VEGFR2 upregulation. UV exposure may have a role in the formation of PG in patients on combined ramucirumab and taxane therapy7; however, our patient’s lesions were distributed on both sun-exposed and unexposed areas. Although potential clinical implications have not yet been thoroughly investigated, following long-term outcomes for these patients may provide important information on the efficacy of the antineoplastic regimen in the subset of patients who develop cutaneous vascular tumors during antiangiogenic treatment.
Combination therapy with PTX and ramucirumab has been associated with the paradoxical development of cutaneous vascular tumors. We report a case of multiple new-onset PGs in a patient undergoing this treatment regimen.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
- Elston D, Neuhaus I, James WD, et al. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Pierson JC. Pyogenic granuloma (lobular capillary hemangioma) clinical presentation. Medscape. Updated February 21, 2020. Accessed December 26, 2023. https://emedicine.medscape.com/article/1084701-clinical#showall
- Paul LJ, Cohen PR. Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262-268.
- Alessandrini A, Starace M, Cerè G, et al. Management and outcome of taxane-induced nail side effects: experience of 79 patients from a single centre. Skin Appendage Disord. 2019;5:276-282.
- Watanabe R, Nakano E, Kawazoe A, et al. Four cases of paradoxical cephalocervical pyogenic granuloma during treatment with paclitaxel and ramucirumab. J Dermatol. 2019;46:E178-E180.
- Ibe T, Hamamoto Y, Takabatake M, et al. Development of pyogenic granuloma with strong vascular endothelial growth factor receptor-2 expression during ramucirumab treatment. BMJ Case Rep. 2019;12:E231464.
- Choi YH, Byun HJ, Lee JH, et al. Multiple cherry angiomas and pyogenic granuloma in a patient treated with ramucirumab and paclitaxel. Indian J Dermatol Venereol Leprol. 2020;86:199-202.
- Aragaki T, Tomomatsu N, Michi Y, et al. Ramucirumab-related oral pyogenic granuloma: a report of two cases [published online March 8, 2021]. Intern Med. 2021;60:2601-2605. doi:10.2169/internalmedicine.6650-20
- Devillers C, Vanhooteghem O, Henrijean A, et al. Subungual pyogenic granuloma secondary to docetaxel therapy. Clin Exp Dermatol. 2009;34:251-252.
- Lim YH, Odell ID, Ko CJ, et al. Somatic p.T771R KDR (VEGFR2) mutation arising in a sporadic angioma during ramucirumab therapy. JAMA Dermatol. 2015;151:1240-1243.
Practice Points
- Pyogenic granulomas (PGs) are benign vascular tumors that clinically are characterized as small, eruptive, friable papules.
- Ramucirumab is a monoclonal antibody against vascular endothelial growth factor receptor 2.
- Some patients experience paradoxical formation of vascular tumors such as PGs when treated with combination therapy with ramucirumab and a taxane such as paclitaxel.
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
Migratory Nodules in a Traveler
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.
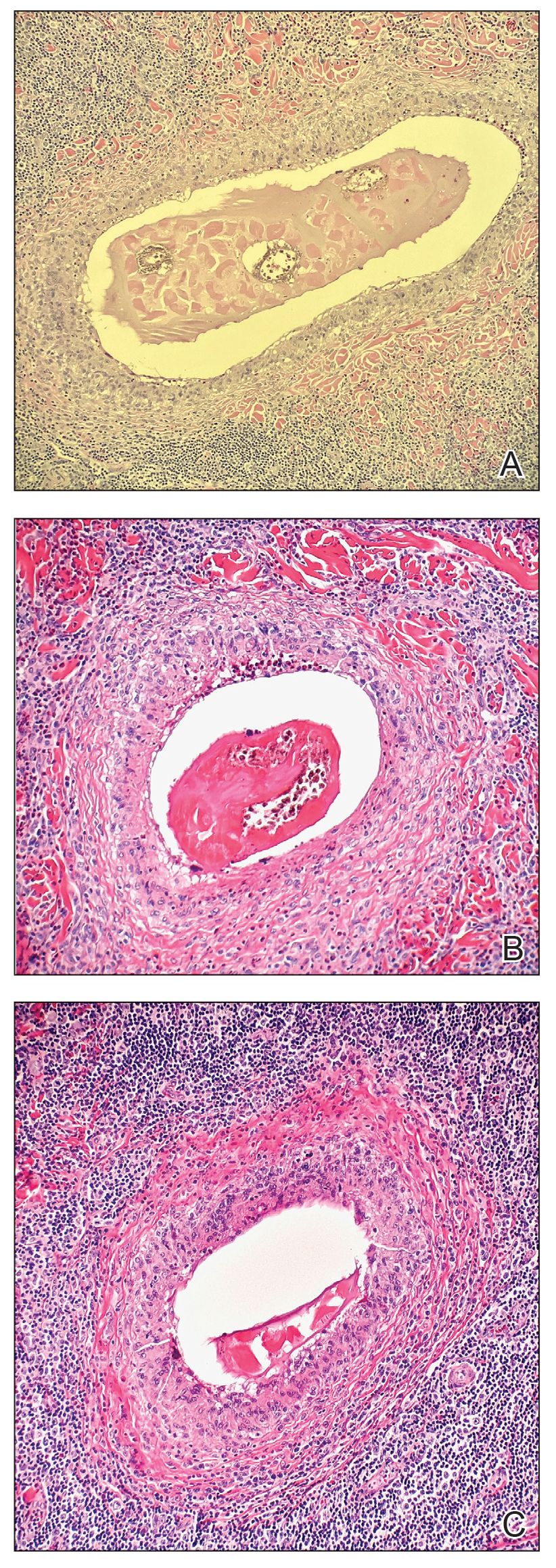
Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.

Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
The Diagnosis: Gnathostomiasis
The biopsy demonstrated a dense, eosinophilic, granulomatous infiltrate surrounding sections of a parasite with skeletal muscle bundles and intestines containing a brush border and luminal debris (Figure), which was consistent with a diagnosis of gnathostomiasis. Upon further questioning, he revealed that while in Peru he frequently consumed ceviche, which is a dish typically made from fresh raw fish cured in lemon or lime juice. He subsequently was treated with oral ivermectin 0.2 mg/kg once daily for 2 days with no evidence of recurrence 12 months later.

Cutaneous gnathostomiasis is the most common manifestation of infection caused by the third-stage larvae of the genus Gnathostoma. The nematode is endemic to tropical and subtropical regions of Japan and Southeast Asia, particularly Thailand. The disease has been increasingly observed in Central and South America. Humans can become infected through ingestion of undercooked meats, particularly freshwater fish but also poultry, snakes, or frogs. Few cases have been reported in North America and Europe presumably due to more stringent regulations governing the sourcing and storage of fish for consumption.1-3 Restaurants in endemic regions also may use cheaper local freshwater or brackish fish compared to restaurants in the West, which use more expensive saltwater fish that do not harbor Gnathostoma species.1 There is a false belief among restauranteurs and consumers that the larvae can be reliably killed by marinating meat in citrus juice or with concurrent consumption of alcohol or hot spices.2 Adequately cooking or freezing meat to 20 °C for 3 to 5 days are the only effective ways to ensure that the larvae are killed.1-3
The parasite requires its natural definitive hosts—fish-eating mammals such as pigs, cats, and dogs—to complete its life cycle and reproduce. Humans are accidental hosts in whom the parasite fails to reach sexual maturity.1-3 Consequently, symptoms commonly are due to the migration of only 1 larva, but occasionally infection with 2 or more has been observed.1,4
Human infection initially may result in malaise, fever, anorexia, abdominal pain, nausea, vomiting, and diarrhea as the parasite migrates through the stomach, intestines, and liver. After 2 to 4 weeks, larvae may reach the skin where they most commonly create ill-defined, erythematous, indurated, round or oval plaques or nodules described as nodular migratory panniculitis. These lesions tend to develop on the trunk or arms and correspond to the location of the migrating worm.1,3,5 The larvae have been observed to migrate at 1 cm/h.6 Symptoms often wax and wane, with individual nodules lasting approximately 1 to 2 weeks. Uniquely, larval migration can result in a trail of subcutaneous hemorrhage that is considered pathognomonic and helps to differentiate gnathostomiasis from other forms of parasitosis such as strongyloidiasis and sparganosis.1,3 Larvae are highly motile and invasive, and they are capable of producing a wide range of symptoms affecting virtually any part of the body.1,2 Depending on the anatomic location of the migrating worm, infection also may result in neurologic, gastrointestinal, pulmonary, or ocular symptoms.1-3,7 Eosinophilia is common but can subside in the chronic stage, as seen in our patient.1
The classic triad of intermittent migratory nodules, eosinophilia, and a history of travel to Southeast Asia or another endemic region should raise suspicion for gnathostomiasis.1-3,5,7 Unfortunately, confirmatory testing such as Gnathostoma serology is not readily available in the United States, and available serologic tests demonstrate frequent false positives and incomplete crossreactivity.1,2,8 Accordingly, the diagnosis most commonly is solidified by combining cardinal clinical features with histologic findings of a dense eosinophilic inflammatory infiltrate involving the dermis and hypodermis.2,5 In one study, the larva itself was only found in 12 of 66 (18%) skin biopsy specimens from patients with gnathostomiasis.5 If the larva is detected within the sections, it ranges from 2.5 to 12.5 mm in length and 0.4 to 1.2 mm in width and can exhibit cuticular spines, intestinal cells, and characteristic large lateral chords.1,5
The treatment of choice is surgical removal of the worm. Oral albendazole (400–800 mg/d for 21 days) also is considered a first-line treatment and results in clinical cure in approximately 90% of cases. Two doses of oral ivermectin (0.2 mg/kg) spaced 24 to 48 hours apart is an acceptable alternative with comparable efficacy.1-3 Care should be taken if involvement of the central nervous system is suspected, as antihelminthic treatment theoretically could be deleterious due to an inflammatory response to the dying larvae.1,2,9
In the differential diagnosis, loiasis can resemble gnathostomiasis, but the former is endemic to Africa.3 Cutaneous larva migrans most frequently is caused by hookworms from the genus Ancylostoma, which classically leads to superficial serpiginous linear plaques that migrate at a rate of several millimeters per day. However, the larvae are believed to lack the collagenase enzyme required to penetrate the epidermal basement membrane and thus are not capable of producing deep-seated nodules or visceral symptoms.3Strongyloidiasis (larva currens) generally exhibits a more linear morphology, and infection would result in positive Strongyloides serology.7 Erythema nodosum is a septal panniculitis that can be triggered by infection, pregnancy, medications, connective tissue diseases, inflammatory conditions, and underlying malignancy.10
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484-492.
- Liu GH, Sun MM, Elsheikha HM, et al. Human gnathostomiasis: a neglected food-borne zoonosis. Parasit Vectors. 2020;13:616.
- Tyring SK. Gnathostomiasis. In: Tyring SK, Lupi O, Hengge UR, eds. Tropical Dermatology. 2nd ed. Elsevier; 2017:77-78.
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33-50.
- Magaña M, Messina M, Bustamante F, et al. Gnathostomiasis: clinicopathologic study. Am J Dermatopathol. 2004;26:91-95.
- Chandenier J, Husson J, Canaple S, et al. Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154-E157.
- Hamilton WL, Agranoff D. Imported gnathostomiasis manifesting as cutaneous larva migrans and Löffler’s syndrome. BMJ Case Rep. 2018;2018:bcr2017223132.
- Neumayr A, Ollague J, Bravo F, et al. Cross-reactivity pattern of Asian and American human gnathostomiasis in western blot assays using crude antigens prepared from Gnathostoma spinigerum and Gnathostoma binucleatum third-stage larvae. Am J Trop Med Hyg. 2016;95:413-416.
- Kraivichian K, Nuchprayoon S, Sitichalernchai P, et al. Treatment of cutaneous gnathostomiasis with ivermectin. Am J Trop Med Hyg. 2004;71:623-628.
- Pérez-Garza DM, Chavez-Alvarez S, Ocampo-Candiani J, et al. Erythema nodosum: a practical approach and diagnostic algorithm. Am J Clin Dermatol. 2021;22:367-378.
A 41-year-old man presented to a dermatology clinic in the United States with a migratory subcutaneous nodule overlying the left upper chest that initially developed 12 months prior and continued to migrate along the trunk and proximal aspect of the arms. The patient had spent the last 3 years residing in Peru. He never observed more than 1 nodule at a time and denied associated fever, headache, visual changes, chest pain, cough, abdominal pain, and diarrhea. Laboratory studies including a blood eosinophil count and serum Strongyloides immunoglobulins were within reference range. An excisional biopsy was performed.