User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Are You Using the Correct Medication or a Look-Alike?
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Five years have passed since the member states of the World Health Organization (WHO) gathered at the 72nd World Health Assembly and decided that September 17 should be recognized as World Patient Safety Day, acknowledging it as a global health priority.
WHO data indicate the following findings related to medical safety:
- One in 10 patients is harmed while receiving healthcare, and 3 million die as a result.
- More than half of these incidents could be prevented.
- Indirect costs could amount to several billion US dollars annually.
Given the magnitude of preventable harm related to medication use, in 2017, the WHO launched the third Global Patient Safety Challenge: Medication Without Harm with the goal of reducing serious and preventable harm related to medication by 50%. In addition, considering the volume of medication packages prescribed in 2023 by physicians in Spain’s National Health System, it is necessary to understand the most common types of medication errors to provide an effective and efficient response.
According to Spain’s Institute for Safe Medication Practices (ISMP), the 10 types of medication errors detected in 2020 with the most serious consequences were the following:
- Errors due to omission or delay in medication.
- Administration of medication to the wrong patient.
- Errors related to allergies or known adverse effects of medications.
- Dosing errors in pediatric patients.
- Errors due to similarities in the labeling or packaging of marketed medications.
- Errors associated with the lack of use of smart infusion pumps.
- Errors due to accidental administration of neuromuscular blocking agents.
- Incorrect intravenous administration of oral liquid medications.
- Errors in medication reconciliation upon hospital admission and discharge.
- Errors due to patient misunderstandings regarding medication use.
I would like to focus on the fifth item, errors due to similarities in the labeling or packaging of marketed medications.
Medications with similar names or with similar labeling or packaging are known as “look alike–sound alike” medications. They are estimated to account for between 6.2% and 14.7% of all medication errors. Confusion can arise due to spelling and phonetic similarities.
As shown in bulletin no. 50 of the ISMP, difficulties in distinguishing different medications or different presentations of the same medication due to similar packaging and labeling have frequently been associated with reported incidents.
Most cases involve either medications marketed by the same laboratory with a design based on brand image or different medications marketed by different laboratories in screen-printed ampoules used in the same settings.
In 2020, the ISMP published 11 new cases of labeling or packaging that may promote errors on its website. It reported 49 incidents to the Spanish Agency for Medicines and Medical Devices.
Shortages caused by the COVID-19 pandemic have further contributed to these incidents, as healthcare facilities sometimes had to change the medications they usually acquired and purchase whatever was available, without being able to select products that would not be confused with existing medications in the facility.
The ISMP recommends the following general practices for healthcare institutions, professionals, and patients to prevent these errors:
- Develop short lists of easily confused medication names and distribute them among all healthcare professionals.
- Prioritize medication names by active ingredient instead of brand name.
- For similar names, highlight the differences in capital letters, eg, DOBUTamine, DOPamine.
- For similar active ingredients, use brand names.
- Avoid placing similar medications near each other.
- Prescribe all medications electronically to minimize the risk of selecting the wrong medication.
- Make manual prescriptions legible, with clearly written dosages and pharmaceutical forms.
- Encourage patients to actively participate in their treatment and consult a clinician if they have any questions about the medications they are receiving.
- Raise awareness among patients, family members, and caregivers about the issues caused by medication name confusion and inform them about how to avoid these errors.
- Instruct patients to focus on and always use the active ingredient name as an identifying element for the medications they are taking.
- Review treatments with patients to ensure they know the medications they are taking.
Julia María Ruiz Redondo is the regional nursing advisor inspector of Spanish Society of General and Family Physicians of Castilla-La Mancha (SEMG-CLM), coordinator of the National Working Group on Public Health in the SEMG, and director of the international public health master’s degree at TECH Technological University. This article is the result of an editorial collaboration between the SEMG and Univadis, which you can access here.
This story was translated from Univadis Spain, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Cancer Risk: Are Pesticides the New Smoking?
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Pesticides have transformed modern agriculture by boosting production yields and helping alleviate food insecurity amid rapid global population growth. However, from a public health perspective, exposure to pesticides has been linked to numerous harmful effects, including neurologic disorders like Parkinson’s disease, weakened immune function, and an increased risk for cancer.
thus offering a limited perspective.
A comprehensive assessment of how pesticide use affects cancer risk across a broader population has yet to be conducted.
A recent population-level study aimed to address this gap by evaluating cancer risks in the US population using a model that accounts for pesticide use and adjusts for various factors. The goal was to identify regional disparities in exposure and contribute to the development of public health policies that protect populations from potential harm.
Calculating Cancer Risk
Researchers developed a model using several data sources to estimate the additional cancer risk from agricultural pesticide use. Key data included:
- Pesticide use data from the US Geological Survey in 2019, which covered 69 agricultural pesticides across 3143 counties
- Cancer incidence rates per 100,000 people, which were collected between 2015 and 2019 by the National Institutes of Health and the Centers for Disease Control and Prevention; these data covered various cancers, including bladder, colorectal, leukemia, lung, non-Hodgkin lymphoma, and pancreatic cancers
- Covariates, including smoking prevalence, the Social Vulnerability Index, agricultural land use, and total US population in 2019
Pesticide use profile patterns were developed using latent class analysis, a statistical method used to identify homogeneous subgroups within a heterogeneous population. A generalized linear model then estimated how these pesticide use patterns and the covariates affected cancer incidence.
The model highlighted regions with the highest and lowest “additional” cancer risks linked to pesticide exposure, calculating the estimated increase in cancer cases per year that resulted from variations in agricultural pesticide use.
Midwest Most Affected
While this model doesn’t establish causality or assess individual risk, it reveals regional trends in the association between pesticide use patterns and cancer incidence from a population-based perspective.
The Midwest, known for its high corn production, emerged as the region most affected by pesticide use. Compared with regions with the lowest risk, the Midwest faced an additional 154,541 cancer cases annually across all types. For colorectal and pancreatic cancers, the yearly increases were 20,927 and 3835 cases, respectively. Similar trends were observed for leukemia and non-Hodgkin lymphoma.
Pesticides vs Smoking
The researchers also estimated the additional cancer risk related to smoking, using the same model. They found that pesticides contributed to a higher risk for cancer than smoking in several cases.
The most significant difference was observed with non-Hodgkin lymphoma, where pesticides were linked to 154.1% more cases than smoking. For all cancers combined, as well as bladder cancer and leukemia, the increases were moderate: 18.7%, 19.3%, and 21.0%, respectively.
This result highlights the importance of considering pesticide exposure alongside smoking when studying cancer risks.
Expanding Scope of Research
Some limitations of this study should be noted. Certain counties lacked complete data, and there was heterogeneity in the size and population of the counties studied. The research also did not account for seasonal and migrant workers, who are likely to be heavily exposed. In addition, the data used in the study were not independently validated, and they could not be used to assess individual risk.
The effect of pesticides on human health is a vast and critical field of research, often focusing on a limited range of pesticides or specific cancers. This study stands out by taking a broader, more holistic approach, aiming to highlight regional inequalities and identify less-studied pesticides that could be future research priorities.
Given the significant public health impact, the authors encouraged the authorities to share these findings with the most vulnerable communities to raise awareness.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Should There Be a Mandatory Retirement Age for Physicians?
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I’d like to pose a question: When should doctors retire? When, as practicing physicians or surgeons, do we become too old to deliver competent service?
You will be amazed to hear, those of you who have listened to my videos before — and although it is a matter of public knowledge — that I’m 68. I know it’s impossible to imagine, due to this youthful appearance, visage, and so on, but I am. I’ve been a cancer doctor for 40 years; therefore, I need to think a little about retirement.
There are two elements of this for me. I’m a university professor, and in Oxford we did vote, as a democracy of scholars, to have a mandatory retirement age around 68. This is so that we can bring new blood forward so that we can create the space to promote new professors, to bring youngsters in to make new ideas, and to get rid of us fusty old lot.
The other argument would be, of course, that we are wise, we’re experienced, we are world-weary, and we’re successful — otherwise, we wouldn’t have lasted as academics as long. Nevertheless, we voted to do that.
It’s possible to have a discussion with the university to extend this, and for those of us who are clinical academics, I have an honorary appointment as a consultant cancer physician in the hospital and my university professorial appointment, too.
I can extend it probably until I’m about 70. It feels like a nice, round number at which to retire — somewhat arbitrarily, one would admit. But does that feel right?
In the United States, more than 25% of the physician workforce is over the age of 65. There are many studies showing that there is a 20% cognitive decline for most individuals between the ages of 45 and 65.
Are we as capable as an elderly workforce as once we were? Clearly, it’s hardly individualistic. It depends on each of our own health status, where we started from, and so on, but are there any general rules that we can apply? I think these are starting to creep in around the sense of revalidation.
In the United Kingdom, we have a General Medical Council (GMC). I need to have a license to practice from the GMC and a sense of fitness to practice. I have annual appraisals within the hospital system, in which I explore delivery of care, how I’m doing as a mentor, am I reaching the milestones I’ve set in terms of academic achievements, and so on.
This is a peer-to-peer process. We have senior physicians — people like myself — who act as appraisers to support our colleagues and to maintain that sense of fitness to practice. Every 5 years, I’m revalidated by the GMC. They take account of the annual appraisals and a report made by the senior physician within my hospital network who’s a so-called designated person.
These two elements come together with patient feedback, with 360-degree feedback from colleagues, and so on. This is quite a firmly regulated system that I think works. Our mandatory retirement age of 65 has gone. That was phased out by the government. In fact, our NHS is making an effort to retain older elders in the workforce.
They see the benefits of mentorship, experience, leadership, and networks. At a time when the majority of NHS are actively seeking to retire when 65, the NHS is trying to retain and pull back those of us who have been around for that wee bit longer and who still feel committed to doing it.
I’d be really interested to see what you think. There’s variation from country to country. I know that, in Australia, they’re talking about annual appraisals of doctors over the age of 70. I’d be very interested to hear what you think is likely to happen in the United States.
I think our system works pretty well, as long as you’re within the NHS and hospital system. If you wanted to still practice, but practice privately, you would still have to find somebody who’d be prepared to conduct appraisals and so on outside of the NHS. It’s an interesting area.
For myself, I still feel competent. Patients seem to like me. That’s an objective assessment by this 360-degree thing in which patients reflected very positively, indeed, in my approach to the delivery of the care and so on, as did colleagues. I’m still publishing, I go to meetings, I cheer things, bits and bobs. I’d say I’m a wee bit unusual in terms of still having a strong academic profile in doing stuff.
It’s an interesting question. Richard Doll, one of the world’s great epidemiologists who, of course, was the dominant discoverer of the link between smoking and lung cancer, was attending seminars, sitting in the front row, and coming into university 3 days a week at age 90, continuing to be contributory with his extraordinarily sharp intellect and vast, vast experience.
When I think of experience, all young cancer doctors are now immunologists. When I was a young doctor, I was a clinical pharmacologist. There are many lessons and tricks that I learned which I do need to pass on to the younger generation of today. What do you think? Should there be a mandatory retirement age? How do we best measure, assess, and revalidate elderly physicians and surgeons? How can we continue to contribute to those who choose to do so? For the time being, as always, thanks for listening.
Dr. Kerr is professor, Nuffield Department of Clinical Laboratory Science, University of Oxford, and professor of cancer medicine, Oxford Cancer Centre, Oxford, United Kingdom. He has disclosed ties with Celleron Therapeutics, Oxford Cancer Biomarkers (Board of Directors); Afrox (charity; Trustee); GlaxoSmithKline and Bayer HealthCare Pharmaceuticals (Consultant), Genomic Health; Merck Serono, and Roche.
A version of this article appeared on Medscape.com.
Expert Warns of Problems with Large Language Models in Dermatology
HUNTINGTON BEACH, CALIF. — and alarmed.
Of the 134 respondents who completed the survey, 87 (65%) reported using LLMs in a clinical setting. Of those 87 respondents, 17 (20%) used LMMs daily, 28 (32%) weekly, 5 (6%) monthly, and 37 (43%) rarely. That represents “pretty significant usage,” Dr. Daneshjou, assistant professor of biomedical data science and dermatology at Stanford University, Palo Alto, California, said at the annual meeting of the Pacific Dermatologic Association.
Most of the respondents reported using LLMs for patient care (79%), followed by administrative tasks (74%), medical records (43%), and education (18%), “which can be problematic,” she said. “These models are not appropriate to use for patient care.”
When asked about their thoughts on the accuracy of LLMs, 58% of respondents deemed them to be “somewhat accurate” and 7% viewed them as “extremely accurate.”
The overall survey responses raise concern because LLMs “are not trained for accuracy; they are trained initially as a next-word predictor on large bodies of tech data,” Dr. Daneshjou said. “LLMs are already being implemented but have the potential to cause harm and bias, and I believe they will if we implement them the way things are rolling out right now. I don’t understand why we’re implementing something without any clinical trial or showing that it improves care before we throw untested technology into our healthcare system.”
Meanwhile, Epic and Microsoft are collaborating to bring AI technology to electronic health records, she said, and Epic is building more than 100 new AI features for physicians and patients. “I think it’s important for every physician and trainee to understand what is going on in the realm of AI,” said Dr. Daneshjou, who is an associate editor for the monthly journal NEJM AI. “Be involved in the conversation because we are the clinical experts, and a lot of people making decisions and building tools do not have the clinical expertise.”
To further illustrate her concerns, Dr. Daneshjou referenced a red teaming event she and her colleagues held with computer scientists, biomedical data scientists, engineers, and physicians across multiple specialties to identify issues related to safety, bias, factual errors, and/or security issues in GPT-3.5, GPT-4, and GPT-4 with internet. The goal was to mimic clinical health scenarios, ask the LLM to respond, and have the team members review the accuracy of LLM responses.
The participants found that nearly 20% of LLM responses were inappropriate. For example, in one task, an LLM was asked to calculate a RegiSCAR score for Drug Reaction With Eosinophilia and Systemic Symptoms for a patient, but the response included an incorrect score for eosinophilia. “That’s why these tools can be so dangerous because you’re reading along and everything seems right, but there might be something so minor that can impact patient care and you might miss it,” Dr. Daneshjou said.
On a related note, she advised against dermatologists uploading images into GPT-4 Vision, an LLM that can analyze images and provide textual responses to questions about them, and she recommends not using GPT-4 Vision for any diagnostic support. At this time, “GPT-4 Vision overcalls malignancies, and the specificity and sensitivity are not very good,” she explained.
Dr. Daneshjou disclosed that she has served as an adviser to MDalgorithms and Revea and has received consulting fees from Pfizer, L’Oréal, Frazier Healthcare Partners, and DWA and research funding from UCB.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIF. — and alarmed.
Of the 134 respondents who completed the survey, 87 (65%) reported using LLMs in a clinical setting. Of those 87 respondents, 17 (20%) used LMMs daily, 28 (32%) weekly, 5 (6%) monthly, and 37 (43%) rarely. That represents “pretty significant usage,” Dr. Daneshjou, assistant professor of biomedical data science and dermatology at Stanford University, Palo Alto, California, said at the annual meeting of the Pacific Dermatologic Association.
Most of the respondents reported using LLMs for patient care (79%), followed by administrative tasks (74%), medical records (43%), and education (18%), “which can be problematic,” she said. “These models are not appropriate to use for patient care.”
When asked about their thoughts on the accuracy of LLMs, 58% of respondents deemed them to be “somewhat accurate” and 7% viewed them as “extremely accurate.”
The overall survey responses raise concern because LLMs “are not trained for accuracy; they are trained initially as a next-word predictor on large bodies of tech data,” Dr. Daneshjou said. “LLMs are already being implemented but have the potential to cause harm and bias, and I believe they will if we implement them the way things are rolling out right now. I don’t understand why we’re implementing something without any clinical trial or showing that it improves care before we throw untested technology into our healthcare system.”
Meanwhile, Epic and Microsoft are collaborating to bring AI technology to electronic health records, she said, and Epic is building more than 100 new AI features for physicians and patients. “I think it’s important for every physician and trainee to understand what is going on in the realm of AI,” said Dr. Daneshjou, who is an associate editor for the monthly journal NEJM AI. “Be involved in the conversation because we are the clinical experts, and a lot of people making decisions and building tools do not have the clinical expertise.”
To further illustrate her concerns, Dr. Daneshjou referenced a red teaming event she and her colleagues held with computer scientists, biomedical data scientists, engineers, and physicians across multiple specialties to identify issues related to safety, bias, factual errors, and/or security issues in GPT-3.5, GPT-4, and GPT-4 with internet. The goal was to mimic clinical health scenarios, ask the LLM to respond, and have the team members review the accuracy of LLM responses.
The participants found that nearly 20% of LLM responses were inappropriate. For example, in one task, an LLM was asked to calculate a RegiSCAR score for Drug Reaction With Eosinophilia and Systemic Symptoms for a patient, but the response included an incorrect score for eosinophilia. “That’s why these tools can be so dangerous because you’re reading along and everything seems right, but there might be something so minor that can impact patient care and you might miss it,” Dr. Daneshjou said.
On a related note, she advised against dermatologists uploading images into GPT-4 Vision, an LLM that can analyze images and provide textual responses to questions about them, and she recommends not using GPT-4 Vision for any diagnostic support. At this time, “GPT-4 Vision overcalls malignancies, and the specificity and sensitivity are not very good,” she explained.
Dr. Daneshjou disclosed that she has served as an adviser to MDalgorithms and Revea and has received consulting fees from Pfizer, L’Oréal, Frazier Healthcare Partners, and DWA and research funding from UCB.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIF. — and alarmed.
Of the 134 respondents who completed the survey, 87 (65%) reported using LLMs in a clinical setting. Of those 87 respondents, 17 (20%) used LMMs daily, 28 (32%) weekly, 5 (6%) monthly, and 37 (43%) rarely. That represents “pretty significant usage,” Dr. Daneshjou, assistant professor of biomedical data science and dermatology at Stanford University, Palo Alto, California, said at the annual meeting of the Pacific Dermatologic Association.
Most of the respondents reported using LLMs for patient care (79%), followed by administrative tasks (74%), medical records (43%), and education (18%), “which can be problematic,” she said. “These models are not appropriate to use for patient care.”
When asked about their thoughts on the accuracy of LLMs, 58% of respondents deemed them to be “somewhat accurate” and 7% viewed them as “extremely accurate.”
The overall survey responses raise concern because LLMs “are not trained for accuracy; they are trained initially as a next-word predictor on large bodies of tech data,” Dr. Daneshjou said. “LLMs are already being implemented but have the potential to cause harm and bias, and I believe they will if we implement them the way things are rolling out right now. I don’t understand why we’re implementing something without any clinical trial or showing that it improves care before we throw untested technology into our healthcare system.”
Meanwhile, Epic and Microsoft are collaborating to bring AI technology to electronic health records, she said, and Epic is building more than 100 new AI features for physicians and patients. “I think it’s important for every physician and trainee to understand what is going on in the realm of AI,” said Dr. Daneshjou, who is an associate editor for the monthly journal NEJM AI. “Be involved in the conversation because we are the clinical experts, and a lot of people making decisions and building tools do not have the clinical expertise.”
To further illustrate her concerns, Dr. Daneshjou referenced a red teaming event she and her colleagues held with computer scientists, biomedical data scientists, engineers, and physicians across multiple specialties to identify issues related to safety, bias, factual errors, and/or security issues in GPT-3.5, GPT-4, and GPT-4 with internet. The goal was to mimic clinical health scenarios, ask the LLM to respond, and have the team members review the accuracy of LLM responses.
The participants found that nearly 20% of LLM responses were inappropriate. For example, in one task, an LLM was asked to calculate a RegiSCAR score for Drug Reaction With Eosinophilia and Systemic Symptoms for a patient, but the response included an incorrect score for eosinophilia. “That’s why these tools can be so dangerous because you’re reading along and everything seems right, but there might be something so minor that can impact patient care and you might miss it,” Dr. Daneshjou said.
On a related note, she advised against dermatologists uploading images into GPT-4 Vision, an LLM that can analyze images and provide textual responses to questions about them, and she recommends not using GPT-4 Vision for any diagnostic support. At this time, “GPT-4 Vision overcalls malignancies, and the specificity and sensitivity are not very good,” she explained.
Dr. Daneshjou disclosed that she has served as an adviser to MDalgorithms and Revea and has received consulting fees from Pfizer, L’Oréal, Frazier Healthcare Partners, and DWA and research funding from UCB.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Diabetes Drug Improved Symptoms in Small Study of Women With Central Centrifugal Cicatricial Alopecia
TOPLINE:
in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 up-regulated genes, which included up-regulated of 23 hair keratin–associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were down-regulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. Additionally, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 up-regulated genes, which included up-regulated of 23 hair keratin–associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were down-regulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. Additionally, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
in a retrospective case series.
METHODOLOGY:
- Researchers conducted a case series involving 12 Black women in their 30s, 40s, and 50s, with biopsy-confirmed, treatment-refractory CCCA, a chronic inflammatory hair disorder characterized by permanent hair loss, from the Johns Hopkins University alopecia clinic.
- Participants received CCCA treatment for at least 6 months and had stagnant or worsening symptoms before oral extended-release metformin (500 mg daily) was added to treatment. (Treatments included topical clobetasol, compounded minoxidil, and platelet-rich plasma injections.)
- Scalp biopsies were collected from four patients before and after metformin treatment to evaluate gene expression changes.
- Changes in clinical symptoms were assessed, including pruritus, inflammation, pain, scalp resistance, and hair regrowth, following initiation of metformin treatment.
TAKEAWAY:
- Metformin led to significant clinical improvement in eight patients, which included reductions in scalp pain, scalp resistance, pruritus, and inflammation. However, two patients experienced worsening symptoms.
- Six patients showed clinical evidence of hair regrowth after at least 6 months of metformin treatment with one experiencing hair loss again 3 months after discontinuing treatment.
- Transcriptomic analysis revealed 34 up-regulated genes, which included up-regulated of 23 hair keratin–associated proteins, and pathways related to keratinization, epidermis development, and the hair cycle. In addition, eight genes were down-regulated, with pathways that included those associated with extracellular matrix organization, collagen fibril organization, and collagen metabolism.
- Gene set variation analysis showed reduced expression of T helper 17 cell and epithelial-mesenchymal transition pathways and elevated adenosine monophosphate kinase signaling and keratin-associated proteins after treatment with metformin.
IN PRACTICE:
“Metformin’s ability to concomitantly target fibrosis and inflammation provides a plausible mechanism for its therapeutic effects in CCCA and other fibrosing alopecia disorders,” the authors concluded. But, they added, “larger prospective, placebo-controlled randomized clinical trials are needed to rigorously evaluate metformin’s efficacy and optimal dosing for treatment of cicatricial alopecias.”
SOURCE:
The study was led by Aaron Bao, Department of Dermatology, Johns Hopkins University School of Medicine, Baltimore, Maryland, and was published online on September 4 in JAMA Dermatology.
LIMITATIONS:
A small sample size, retrospective design, lack of a placebo control group, and the single-center setting limited the generalizability of the study findings. Additionally, the absence of a validated activity or severity scale for CCCA and the single posttreatment sampling limit the assessment and comparison of clinical symptoms and transcriptomic changes.
DISCLOSURES:
The study was supported by the American Academy of Dermatology. One author reported several ties with pharmaceutical companies, a pending patent, and authorship for the UpToDate section on CCCA.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Laser, Radiofrequency Therapies Offer Little Benefit for Genitourinary Syndrome of Menopause
CHICAGO — Use of CO2 lasers and similar “energy-based” treatments result in little to no benefit for genitourinary syndrome of menopause (GSM) symptoms, according to research presented at the The Menopause Society 2024 Annual Meeting in Chicago on September 12.
“There was a concern that menopausal women are being targeted for treatments that may not have a lot of benefit and might have significant harms,” Elisheva Danan, MD, MPH, a physician at the Minneapolis VA Health Care System and an assistant professor of medicine at the University of Minnesota Medical School in Minneapolis, told this news organization. While she was not surprised to find little evidence of benefit, “we were a little bit surprised that we also didn’t find significant evidence of harms.”
The study was unable to evaluate the potential for financial harms, but Dr. Danan noted that these therapies are often expensive and not typically covered by insurance. The treatments appear to be used primarily in private practice, she said, while “most academic clinicians were not familiar with these and do not use these lasers.”
The American Urological Association had requested the review, Dr. Danan said, “to inform clinical guidelines that they could put out for practitioners about treating genital urinary syndrome from menopause.” Yet the evidence available remains slim. “There’s a lot of outcomes that were not looked at by most of these [trials], or they were looked at in a way that we couldn’t separate out,” she said.
Kamalini Das, MD, a professor of ob.gyn. at the University of Minnesota who was not involved in the research, was surprised by the findings because studies to date have been variable, “but since this looks at multiple studies and they find no benefits, I would take these results as more significant than any of the small studies,” she told this news organization.
Dr. Das said she has patients who ask about using these therapies and have had them done. “So far, I’ve told them the jury is out on whether it will help or not, that there are some studies that say they’re beneficial and some studies that they’re not,” Dr. Das said.
But this new review changes what she will tell patients going forward, she said. “This is a good study because it consolidates lots of little studies, so I think I would use this to say, looking at all the studies together, this treatment is not beneficial.”
GSM occurs due to the body’s reduced production of estrogen and affects anywhere from 27% to 84% of postmenopausal women. It can involve a constellation of symptoms ranging from vaginal discomfort and irritation to painful urination or intercourse. Typical recommended treatments for GSM include systemic hormone therapy, localized hormonal treatments such as vaginal estrogen or dehydroepiandrosterone, nonhormonal creams and moisturizers, and the prescription drug ospemifene.
Most of these have been found effective, according to a recent systematic review Dr. Danan published in the Annals of Internal Medicine that this news organization covered. But recent years have also seen a rapid increase in interest and the availability of energy-based treatments for GSM, such as CO2 laser and radiofrequency interventions, particularly for those who cannot or do not want to use hormonal treatments. The idea behind these newer therapies is that they “heat tissue to cause a denaturation of collagen fibers and induce a wound-healing response,” with the aim of “enhancement of vaginal elasticity, restoration of premenopausal epithelial function, and symptom improvement,” the authors wrote.
Evidence has been scant and uneven for the safety and effectiveness of these treatments, and they have not been evaluated by the US Food and Drug Administration. The agency issued a warning in 2018 with remarks from then Commissioner Scott Gottlieb that the “products have serious risks and don’t have adequate evidence to support their use for these purposes.”
Much of the evidence has focused on CO2 lasers instead of other energy-based treatments, however, and a raft of new studies have been published on these interventions in the past 2 years. Dr. Danan and colleagues, therefore, assessed the most current state of the research with a systematic review of randomized controlled trials (RCTs) and prospective observational studies with control groups published through December 11, 2023.
Included studies needed to evaluate an energy-based treatment for at least 8 weeks in a minimum of 40 postmenopausal women (20 in each group) who had one or more GSM symptoms. The authors also included nonrandomized and uncontrolled studies with a follow-up of a year or more to assess possible adverse events. The studies also needed to assess at least one of eight core outcomes: Dyspareunia; vulvovaginal dryness; vulvovaginal discomfort/irritation; dysuria; change in most bothersome symptom; treatment satisfaction; adverse events; and distress, bother, or interference associated with genitourinary symptoms.
The authors identified 32 studies, including 16 RCTs, one quasi-RCT, and 15 nonrandomized studies. The researchers extracted and analyzed data from the 10 RCTs and one quasi-RCT that were rated as having low to moderate risk for bias.
Most of these studies assessed CO2 lasers alone, while three assessed erbium:yttrium-aluminum-garnet (Er:YAG) laser, and one looked at CO2 lasers vs radiofrequency treatments.
The average age of participants ranged from 56 to 64 years, and most trials were in the United States. Results showed that CO2 lasers led to little or no difference in dysuria, dyspareunia, or quality of life when compared with sham lasers. The CO2 laser therapy also showed little to no difference compared with vaginal estrogen creams for dyspareunia, dryness, discomfort/irritation, dysuria, or quality of life.
Most CO2 laser studies reported on most outcomes, but the Er:YAG studies tended to report only on quality of life and/or one or two other outcomes. The radiofrequency study only assessed dyspareunia and quality of life.
“Treatment effects on other outcomes and effects of Er:YAG laser or radiofrequency on any outcomes are very uncertain,” the authors reported. Few adverse events and no serious adverse events were reported based on 15 studies, including the additional non-RCTs that had follow-up for at least a year.
“There are case reports and other types of studies that have shown some bad outcomes using laser therapies, and we really wanted to be expansive and include anything, especially because this is such a new treatment and all these trials were in the last couple of years,” Dr. Danan said.
The review was limited by inconsistent or nonvalidated outcome reporting in the studies as well as small populations and short follow-up, typically less than 3 months.
The research was funded by the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute. Dr. Danan and Dr. Das had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO — Use of CO2 lasers and similar “energy-based” treatments result in little to no benefit for genitourinary syndrome of menopause (GSM) symptoms, according to research presented at the The Menopause Society 2024 Annual Meeting in Chicago on September 12.
“There was a concern that menopausal women are being targeted for treatments that may not have a lot of benefit and might have significant harms,” Elisheva Danan, MD, MPH, a physician at the Minneapolis VA Health Care System and an assistant professor of medicine at the University of Minnesota Medical School in Minneapolis, told this news organization. While she was not surprised to find little evidence of benefit, “we were a little bit surprised that we also didn’t find significant evidence of harms.”
The study was unable to evaluate the potential for financial harms, but Dr. Danan noted that these therapies are often expensive and not typically covered by insurance. The treatments appear to be used primarily in private practice, she said, while “most academic clinicians were not familiar with these and do not use these lasers.”
The American Urological Association had requested the review, Dr. Danan said, “to inform clinical guidelines that they could put out for practitioners about treating genital urinary syndrome from menopause.” Yet the evidence available remains slim. “There’s a lot of outcomes that were not looked at by most of these [trials], or they were looked at in a way that we couldn’t separate out,” she said.
Kamalini Das, MD, a professor of ob.gyn. at the University of Minnesota who was not involved in the research, was surprised by the findings because studies to date have been variable, “but since this looks at multiple studies and they find no benefits, I would take these results as more significant than any of the small studies,” she told this news organization.
Dr. Das said she has patients who ask about using these therapies and have had them done. “So far, I’ve told them the jury is out on whether it will help or not, that there are some studies that say they’re beneficial and some studies that they’re not,” Dr. Das said.
But this new review changes what she will tell patients going forward, she said. “This is a good study because it consolidates lots of little studies, so I think I would use this to say, looking at all the studies together, this treatment is not beneficial.”
GSM occurs due to the body’s reduced production of estrogen and affects anywhere from 27% to 84% of postmenopausal women. It can involve a constellation of symptoms ranging from vaginal discomfort and irritation to painful urination or intercourse. Typical recommended treatments for GSM include systemic hormone therapy, localized hormonal treatments such as vaginal estrogen or dehydroepiandrosterone, nonhormonal creams and moisturizers, and the prescription drug ospemifene.
Most of these have been found effective, according to a recent systematic review Dr. Danan published in the Annals of Internal Medicine that this news organization covered. But recent years have also seen a rapid increase in interest and the availability of energy-based treatments for GSM, such as CO2 laser and radiofrequency interventions, particularly for those who cannot or do not want to use hormonal treatments. The idea behind these newer therapies is that they “heat tissue to cause a denaturation of collagen fibers and induce a wound-healing response,” with the aim of “enhancement of vaginal elasticity, restoration of premenopausal epithelial function, and symptom improvement,” the authors wrote.
Evidence has been scant and uneven for the safety and effectiveness of these treatments, and they have not been evaluated by the US Food and Drug Administration. The agency issued a warning in 2018 with remarks from then Commissioner Scott Gottlieb that the “products have serious risks and don’t have adequate evidence to support their use for these purposes.”
Much of the evidence has focused on CO2 lasers instead of other energy-based treatments, however, and a raft of new studies have been published on these interventions in the past 2 years. Dr. Danan and colleagues, therefore, assessed the most current state of the research with a systematic review of randomized controlled trials (RCTs) and prospective observational studies with control groups published through December 11, 2023.
Included studies needed to evaluate an energy-based treatment for at least 8 weeks in a minimum of 40 postmenopausal women (20 in each group) who had one or more GSM symptoms. The authors also included nonrandomized and uncontrolled studies with a follow-up of a year or more to assess possible adverse events. The studies also needed to assess at least one of eight core outcomes: Dyspareunia; vulvovaginal dryness; vulvovaginal discomfort/irritation; dysuria; change in most bothersome symptom; treatment satisfaction; adverse events; and distress, bother, or interference associated with genitourinary symptoms.
The authors identified 32 studies, including 16 RCTs, one quasi-RCT, and 15 nonrandomized studies. The researchers extracted and analyzed data from the 10 RCTs and one quasi-RCT that were rated as having low to moderate risk for bias.
Most of these studies assessed CO2 lasers alone, while three assessed erbium:yttrium-aluminum-garnet (Er:YAG) laser, and one looked at CO2 lasers vs radiofrequency treatments.
The average age of participants ranged from 56 to 64 years, and most trials were in the United States. Results showed that CO2 lasers led to little or no difference in dysuria, dyspareunia, or quality of life when compared with sham lasers. The CO2 laser therapy also showed little to no difference compared with vaginal estrogen creams for dyspareunia, dryness, discomfort/irritation, dysuria, or quality of life.
Most CO2 laser studies reported on most outcomes, but the Er:YAG studies tended to report only on quality of life and/or one or two other outcomes. The radiofrequency study only assessed dyspareunia and quality of life.
“Treatment effects on other outcomes and effects of Er:YAG laser or radiofrequency on any outcomes are very uncertain,” the authors reported. Few adverse events and no serious adverse events were reported based on 15 studies, including the additional non-RCTs that had follow-up for at least a year.
“There are case reports and other types of studies that have shown some bad outcomes using laser therapies, and we really wanted to be expansive and include anything, especially because this is such a new treatment and all these trials were in the last couple of years,” Dr. Danan said.
The review was limited by inconsistent or nonvalidated outcome reporting in the studies as well as small populations and short follow-up, typically less than 3 months.
The research was funded by the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute. Dr. Danan and Dr. Das had no disclosures.
A version of this article first appeared on Medscape.com.
CHICAGO — Use of CO2 lasers and similar “energy-based” treatments result in little to no benefit for genitourinary syndrome of menopause (GSM) symptoms, according to research presented at the The Menopause Society 2024 Annual Meeting in Chicago on September 12.
“There was a concern that menopausal women are being targeted for treatments that may not have a lot of benefit and might have significant harms,” Elisheva Danan, MD, MPH, a physician at the Minneapolis VA Health Care System and an assistant professor of medicine at the University of Minnesota Medical School in Minneapolis, told this news organization. While she was not surprised to find little evidence of benefit, “we were a little bit surprised that we also didn’t find significant evidence of harms.”
The study was unable to evaluate the potential for financial harms, but Dr. Danan noted that these therapies are often expensive and not typically covered by insurance. The treatments appear to be used primarily in private practice, she said, while “most academic clinicians were not familiar with these and do not use these lasers.”
The American Urological Association had requested the review, Dr. Danan said, “to inform clinical guidelines that they could put out for practitioners about treating genital urinary syndrome from menopause.” Yet the evidence available remains slim. “There’s a lot of outcomes that were not looked at by most of these [trials], or they were looked at in a way that we couldn’t separate out,” she said.
Kamalini Das, MD, a professor of ob.gyn. at the University of Minnesota who was not involved in the research, was surprised by the findings because studies to date have been variable, “but since this looks at multiple studies and they find no benefits, I would take these results as more significant than any of the small studies,” she told this news organization.
Dr. Das said she has patients who ask about using these therapies and have had them done. “So far, I’ve told them the jury is out on whether it will help or not, that there are some studies that say they’re beneficial and some studies that they’re not,” Dr. Das said.
But this new review changes what she will tell patients going forward, she said. “This is a good study because it consolidates lots of little studies, so I think I would use this to say, looking at all the studies together, this treatment is not beneficial.”
GSM occurs due to the body’s reduced production of estrogen and affects anywhere from 27% to 84% of postmenopausal women. It can involve a constellation of symptoms ranging from vaginal discomfort and irritation to painful urination or intercourse. Typical recommended treatments for GSM include systemic hormone therapy, localized hormonal treatments such as vaginal estrogen or dehydroepiandrosterone, nonhormonal creams and moisturizers, and the prescription drug ospemifene.
Most of these have been found effective, according to a recent systematic review Dr. Danan published in the Annals of Internal Medicine that this news organization covered. But recent years have also seen a rapid increase in interest and the availability of energy-based treatments for GSM, such as CO2 laser and radiofrequency interventions, particularly for those who cannot or do not want to use hormonal treatments. The idea behind these newer therapies is that they “heat tissue to cause a denaturation of collagen fibers and induce a wound-healing response,” with the aim of “enhancement of vaginal elasticity, restoration of premenopausal epithelial function, and symptom improvement,” the authors wrote.
Evidence has been scant and uneven for the safety and effectiveness of these treatments, and they have not been evaluated by the US Food and Drug Administration. The agency issued a warning in 2018 with remarks from then Commissioner Scott Gottlieb that the “products have serious risks and don’t have adequate evidence to support their use for these purposes.”
Much of the evidence has focused on CO2 lasers instead of other energy-based treatments, however, and a raft of new studies have been published on these interventions in the past 2 years. Dr. Danan and colleagues, therefore, assessed the most current state of the research with a systematic review of randomized controlled trials (RCTs) and prospective observational studies with control groups published through December 11, 2023.
Included studies needed to evaluate an energy-based treatment for at least 8 weeks in a minimum of 40 postmenopausal women (20 in each group) who had one or more GSM symptoms. The authors also included nonrandomized and uncontrolled studies with a follow-up of a year or more to assess possible adverse events. The studies also needed to assess at least one of eight core outcomes: Dyspareunia; vulvovaginal dryness; vulvovaginal discomfort/irritation; dysuria; change in most bothersome symptom; treatment satisfaction; adverse events; and distress, bother, or interference associated with genitourinary symptoms.
The authors identified 32 studies, including 16 RCTs, one quasi-RCT, and 15 nonrandomized studies. The researchers extracted and analyzed data from the 10 RCTs and one quasi-RCT that were rated as having low to moderate risk for bias.
Most of these studies assessed CO2 lasers alone, while three assessed erbium:yttrium-aluminum-garnet (Er:YAG) laser, and one looked at CO2 lasers vs radiofrequency treatments.
The average age of participants ranged from 56 to 64 years, and most trials were in the United States. Results showed that CO2 lasers led to little or no difference in dysuria, dyspareunia, or quality of life when compared with sham lasers. The CO2 laser therapy also showed little to no difference compared with vaginal estrogen creams for dyspareunia, dryness, discomfort/irritation, dysuria, or quality of life.
Most CO2 laser studies reported on most outcomes, but the Er:YAG studies tended to report only on quality of life and/or one or two other outcomes. The radiofrequency study only assessed dyspareunia and quality of life.
“Treatment effects on other outcomes and effects of Er:YAG laser or radiofrequency on any outcomes are very uncertain,” the authors reported. Few adverse events and no serious adverse events were reported based on 15 studies, including the additional non-RCTs that had follow-up for at least a year.
“There are case reports and other types of studies that have shown some bad outcomes using laser therapies, and we really wanted to be expansive and include anything, especially because this is such a new treatment and all these trials were in the last couple of years,” Dr. Danan said.
The review was limited by inconsistent or nonvalidated outcome reporting in the studies as well as small populations and short follow-up, typically less than 3 months.
The research was funded by the Agency for Healthcare Research and Quality and Patient-Centered Outcomes Research Institute. Dr. Danan and Dr. Das had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE MENOPAUSE SOCIETY 2024
Study Helps Define Patient-Centered Definition of Atopic Dermatitis Flares
TOPLINE:
, which most agreed would help when communicating with their healthcare providers (HCPs).
METHODOLOGY:
- To develop a patient-centered definition of AD flare, researchers used a modified eDelphi method, which involved a focus group and survey to reach consensus on key aspects of an AD flare.
- The focus group included 26 US adults aged ≥ 18 years with AD who had experienced a flare within the past 12 months. The survey was conducted among 631 adults with AD to validate the identified concepts and assess their agreement with the consensus statements.
- Participants rated 98 statements on a scale from 1 to 9, with consensus defined as at least 70% rating a statement as 7-9 and less than 15% rating it as 1-3.
- In focus groups, participants identified six key concepts for a patient-centered definition of flare, including changes from baseline, mental and emotional consequences, and physical changes in skin.
TAKEAWAY:
- The focus group reached consensus on 15 statements, and survey participants reached consensus on 12 of those statements defining an AD flare, with the highest agreement on symptoms taking more attention than normal, worsening of physical symptoms associated with AD, and worsening of itching associated with AD.
- The statement “acute worsening of symptoms of AD” was ranked as the most important, while “a worsening of physical symptoms” was ranked the least important.
- Most participants (79.7%) reported that prior definitions of AD flare did not resonate with them.
- More than half (52.9%) agreed with their HCP on what constitutes an AD flare, and the majority (77.6%) indicated that a patient-centered definition would be useful for communication with their HCP and for self-management.
IN PRACTICE:
“In this consensus survey study, we identified statements that are critical to the definition of an AD flare from the patient perspective,” the authors wrote. These findings, they added, “may be useful in clinical practice to improve communication between patients and HCPs who may be using the term flare without a mutual understanding of its meaning” and “may also be applied to the development of outcome measures focused on AD flares, which is an important treatment outcome for people with AD.”
SOURCE:
The study was led by Aaron M. Drucker, MD, ScM, of the Division of Dermatology, Department of Medicine, University of Toronto, Ontario, Canada, and was published online September 11 in JAMA Dermatology.
LIMITATIONS:
Participants had higher-than-average knowledge about AD, and the study’s findings may not be generalizable to all people with AD. The study included a higher proportion of moderate to severe AD cases than the general population, which may introduce responder bias. The findings may not be applicable to children, caregivers, or individuals in other countries.
DISCLOSURES:
This work was supported by a grant to the National Eczema Association from Pfizer. Dr. Drucker disclosed received compensation from the British Journal of Dermatology, American Academy of Dermatology, and Canadian Dermatology Today, and consultant fees from the National Eczema Association and Canadian Agency for Drugs and Technologies in Health. Another author reported receiving personal fees from pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, which most agreed would help when communicating with their healthcare providers (HCPs).
METHODOLOGY:
- To develop a patient-centered definition of AD flare, researchers used a modified eDelphi method, which involved a focus group and survey to reach consensus on key aspects of an AD flare.
- The focus group included 26 US adults aged ≥ 18 years with AD who had experienced a flare within the past 12 months. The survey was conducted among 631 adults with AD to validate the identified concepts and assess their agreement with the consensus statements.
- Participants rated 98 statements on a scale from 1 to 9, with consensus defined as at least 70% rating a statement as 7-9 and less than 15% rating it as 1-3.
- In focus groups, participants identified six key concepts for a patient-centered definition of flare, including changes from baseline, mental and emotional consequences, and physical changes in skin.
TAKEAWAY:
- The focus group reached consensus on 15 statements, and survey participants reached consensus on 12 of those statements defining an AD flare, with the highest agreement on symptoms taking more attention than normal, worsening of physical symptoms associated with AD, and worsening of itching associated with AD.
- The statement “acute worsening of symptoms of AD” was ranked as the most important, while “a worsening of physical symptoms” was ranked the least important.
- Most participants (79.7%) reported that prior definitions of AD flare did not resonate with them.
- More than half (52.9%) agreed with their HCP on what constitutes an AD flare, and the majority (77.6%) indicated that a patient-centered definition would be useful for communication with their HCP and for self-management.
IN PRACTICE:
“In this consensus survey study, we identified statements that are critical to the definition of an AD flare from the patient perspective,” the authors wrote. These findings, they added, “may be useful in clinical practice to improve communication between patients and HCPs who may be using the term flare without a mutual understanding of its meaning” and “may also be applied to the development of outcome measures focused on AD flares, which is an important treatment outcome for people with AD.”
SOURCE:
The study was led by Aaron M. Drucker, MD, ScM, of the Division of Dermatology, Department of Medicine, University of Toronto, Ontario, Canada, and was published online September 11 in JAMA Dermatology.
LIMITATIONS:
Participants had higher-than-average knowledge about AD, and the study’s findings may not be generalizable to all people with AD. The study included a higher proportion of moderate to severe AD cases than the general population, which may introduce responder bias. The findings may not be applicable to children, caregivers, or individuals in other countries.
DISCLOSURES:
This work was supported by a grant to the National Eczema Association from Pfizer. Dr. Drucker disclosed received compensation from the British Journal of Dermatology, American Academy of Dermatology, and Canadian Dermatology Today, and consultant fees from the National Eczema Association and Canadian Agency for Drugs and Technologies in Health. Another author reported receiving personal fees from pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, which most agreed would help when communicating with their healthcare providers (HCPs).
METHODOLOGY:
- To develop a patient-centered definition of AD flare, researchers used a modified eDelphi method, which involved a focus group and survey to reach consensus on key aspects of an AD flare.
- The focus group included 26 US adults aged ≥ 18 years with AD who had experienced a flare within the past 12 months. The survey was conducted among 631 adults with AD to validate the identified concepts and assess their agreement with the consensus statements.
- Participants rated 98 statements on a scale from 1 to 9, with consensus defined as at least 70% rating a statement as 7-9 and less than 15% rating it as 1-3.
- In focus groups, participants identified six key concepts for a patient-centered definition of flare, including changes from baseline, mental and emotional consequences, and physical changes in skin.
TAKEAWAY:
- The focus group reached consensus on 15 statements, and survey participants reached consensus on 12 of those statements defining an AD flare, with the highest agreement on symptoms taking more attention than normal, worsening of physical symptoms associated with AD, and worsening of itching associated with AD.
- The statement “acute worsening of symptoms of AD” was ranked as the most important, while “a worsening of physical symptoms” was ranked the least important.
- Most participants (79.7%) reported that prior definitions of AD flare did not resonate with them.
- More than half (52.9%) agreed with their HCP on what constitutes an AD flare, and the majority (77.6%) indicated that a patient-centered definition would be useful for communication with their HCP and for self-management.
IN PRACTICE:
“In this consensus survey study, we identified statements that are critical to the definition of an AD flare from the patient perspective,” the authors wrote. These findings, they added, “may be useful in clinical practice to improve communication between patients and HCPs who may be using the term flare without a mutual understanding of its meaning” and “may also be applied to the development of outcome measures focused on AD flares, which is an important treatment outcome for people with AD.”
SOURCE:
The study was led by Aaron M. Drucker, MD, ScM, of the Division of Dermatology, Department of Medicine, University of Toronto, Ontario, Canada, and was published online September 11 in JAMA Dermatology.
LIMITATIONS:
Participants had higher-than-average knowledge about AD, and the study’s findings may not be generalizable to all people with AD. The study included a higher proportion of moderate to severe AD cases than the general population, which may introduce responder bias. The findings may not be applicable to children, caregivers, or individuals in other countries.
DISCLOSURES:
This work was supported by a grant to the National Eczema Association from Pfizer. Dr. Drucker disclosed received compensation from the British Journal of Dermatology, American Academy of Dermatology, and Canadian Dermatology Today, and consultant fees from the National Eczema Association and Canadian Agency for Drugs and Technologies in Health. Another author reported receiving personal fees from pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Imaging Tool Helps Identify Features of Nail Disorders
TOPLINE:
METHODOLOGY:
- The single-center, observational cross-sectional pilot study evaluated patients aged ≥ 7 years with newly diagnosed nail disorders between January 2022 and May 2023.
- A total of 128 patients (average age, 46.1 years; range, 8-84 years) with nail psoriasis, onychomycosis, idiopathic/traumatic onycholysis, brittle nail syndrome, nail lichen planus, retronychia, and other nail conditions and those with no nail findings (controls) were enrolled.
- Researchers performed nailfold capillaroscopy imaging and compared capillary features between patients with nail conditions and the controls.
TAKEAWAY:
- Patients with nail psoriasis had decreased capillary density and length (P < .001), more crossed and tortuous capillaries (P < .02), and increased abnormal capillary morphology (P = .03) compared with controls. Specific abnormalities, such as branching and meandering capillaries, were more common among those with nail psoriasis (both 26.5%).
- Patients with fingernail and toenail onychomycosis had a higher frequency of abnormal capillary morphology (P < .02), particularly meandering capillaries (75.0% for fingernails and 76.9% for toenails). However, other abnormalities were less frequently observed.
- Patients with nail lichen planus (P < .01), onychopapilloma (P = .01), and retronychia (P = .03) showed significantly shorter capillaries than controls. Retronychia was also associated with increased disorganized polymorphic capillaries (P = .02).
- Patients with brittle nail syndrome and eczema showed no significant differences compared with controls.
IN PRACTICE:
“Our findings highlight nailfold capillaroscopy as a potentially quick, cost-effective, and noninvasive imaging modality as an adjunct for diagnosis and treatment initiation for patients with onychodystrophies,” the authors wrote.
SOURCE:
This study was led by Jonathan K. Hwang, MD, Weill Cornell Medicine, New York City, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations included a small sample size for certain nail conditions and the single-center design, which limited generalizability. Additionally, the uneven surface, scaling, onycholysis, and thickening of toenails made some capillaroscopy images difficult to capture and interpret.
DISCLOSURES:
The study did not receive any funding. One author reported serving as a consultant for Eli Lilly, Ortho-Dermatologics, BelleTorus, and Moberg Pharma.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The single-center, observational cross-sectional pilot study evaluated patients aged ≥ 7 years with newly diagnosed nail disorders between January 2022 and May 2023.
- A total of 128 patients (average age, 46.1 years; range, 8-84 years) with nail psoriasis, onychomycosis, idiopathic/traumatic onycholysis, brittle nail syndrome, nail lichen planus, retronychia, and other nail conditions and those with no nail findings (controls) were enrolled.
- Researchers performed nailfold capillaroscopy imaging and compared capillary features between patients with nail conditions and the controls.
TAKEAWAY:
- Patients with nail psoriasis had decreased capillary density and length (P < .001), more crossed and tortuous capillaries (P < .02), and increased abnormal capillary morphology (P = .03) compared with controls. Specific abnormalities, such as branching and meandering capillaries, were more common among those with nail psoriasis (both 26.5%).
- Patients with fingernail and toenail onychomycosis had a higher frequency of abnormal capillary morphology (P < .02), particularly meandering capillaries (75.0% for fingernails and 76.9% for toenails). However, other abnormalities were less frequently observed.
- Patients with nail lichen planus (P < .01), onychopapilloma (P = .01), and retronychia (P = .03) showed significantly shorter capillaries than controls. Retronychia was also associated with increased disorganized polymorphic capillaries (P = .02).
- Patients with brittle nail syndrome and eczema showed no significant differences compared with controls.
IN PRACTICE:
“Our findings highlight nailfold capillaroscopy as a potentially quick, cost-effective, and noninvasive imaging modality as an adjunct for diagnosis and treatment initiation for patients with onychodystrophies,” the authors wrote.
SOURCE:
This study was led by Jonathan K. Hwang, MD, Weill Cornell Medicine, New York City, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations included a small sample size for certain nail conditions and the single-center design, which limited generalizability. Additionally, the uneven surface, scaling, onycholysis, and thickening of toenails made some capillaroscopy images difficult to capture and interpret.
DISCLOSURES:
The study did not receive any funding. One author reported serving as a consultant for Eli Lilly, Ortho-Dermatologics, BelleTorus, and Moberg Pharma.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- The single-center, observational cross-sectional pilot study evaluated patients aged ≥ 7 years with newly diagnosed nail disorders between January 2022 and May 2023.
- A total of 128 patients (average age, 46.1 years; range, 8-84 years) with nail psoriasis, onychomycosis, idiopathic/traumatic onycholysis, brittle nail syndrome, nail lichen planus, retronychia, and other nail conditions and those with no nail findings (controls) were enrolled.
- Researchers performed nailfold capillaroscopy imaging and compared capillary features between patients with nail conditions and the controls.
TAKEAWAY:
- Patients with nail psoriasis had decreased capillary density and length (P < .001), more crossed and tortuous capillaries (P < .02), and increased abnormal capillary morphology (P = .03) compared with controls. Specific abnormalities, such as branching and meandering capillaries, were more common among those with nail psoriasis (both 26.5%).
- Patients with fingernail and toenail onychomycosis had a higher frequency of abnormal capillary morphology (P < .02), particularly meandering capillaries (75.0% for fingernails and 76.9% for toenails). However, other abnormalities were less frequently observed.
- Patients with nail lichen planus (P < .01), onychopapilloma (P = .01), and retronychia (P = .03) showed significantly shorter capillaries than controls. Retronychia was also associated with increased disorganized polymorphic capillaries (P = .02).
- Patients with brittle nail syndrome and eczema showed no significant differences compared with controls.
IN PRACTICE:
“Our findings highlight nailfold capillaroscopy as a potentially quick, cost-effective, and noninvasive imaging modality as an adjunct for diagnosis and treatment initiation for patients with onychodystrophies,” the authors wrote.
SOURCE:
This study was led by Jonathan K. Hwang, MD, Weill Cornell Medicine, New York City, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations included a small sample size for certain nail conditions and the single-center design, which limited generalizability. Additionally, the uneven surface, scaling, onycholysis, and thickening of toenails made some capillaroscopy images difficult to capture and interpret.
DISCLOSURES:
The study did not receive any funding. One author reported serving as a consultant for Eli Lilly, Ortho-Dermatologics, BelleTorus, and Moberg Pharma.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
First Combined Face and Eye Transplant Performed
In a groundbreaking procedure, a team of surgeons from New York University Langone Health successfully performed the first combined face and eye transplant on a patient with extensive craniofacial tissue loss after an electrical accident.
The highly complex surgery lasted for 21 hours and involved more than 140 surgeons, nurses, and other healthcare professionals under the leadership of Eduardo D. Rodriguez. MD. It not only restored the patient’s facial features, but also integrated a functional eyeball, potentially setting a new standard for future treatments in similar cases.
The transplant took place in May 2023, and the case report was published on September 5 this year in JAMA.
The 46-year-old man lost a large part of his craniofacial tissue and his left eyeball. The approach was highly specialized. Advanced microsurgical techniques such as anastomoses of microscopic vessels and delicate suturing techniques were crucial for the transplant’s success.
Moreover, customized surgical devices, specific implants, and tissue manipulation tools were developed specifically for this case, thus ensuring the viability of the transplant and adequate perfusion of the transplanted ocular tissue.
The initial results are encouraging. Retinal arterial perfusion has been maintained, and retinal architecture has been preserved, as demonstrated by optical coherence tomography. Electroretinography confirmed retinal responses to light, suggesting that the transplanted eye may eventually contribute to the patient’s visual perception. These results are comparable to those of previous facial tissue transplants, but with the significant addition of ocular functionality, which is a notable advance.
“The successful revascularization of the transplanted eye achieved in this study may serve as a step toward the goal of globe transplant for restoration of vision,” wrote the authors.
The complexity of the combined transplant required a deep understanding of facial and ocular anatomy, as well as tissue preservation techniques. The surgical team reported significant challenges, including the need to align delicate anatomical structures and ensure immunological compatibility between the donor and recipient. Meticulous planning from donor selection to postoperative follow-up was considered essential to maximize the likelihood of success and minimize the risk for allograft rejection.
The patient will now be continuously monitored and receive treatment with immunosuppressants such as tacrolimus and prednisone, adjusted according to his response to the transplant. According to the researchers, further studies will be needed to assess the long-term functionality of the transplanted eye and its integration with the central nervous system.
Despite being the fifth facial transplant surgery performed under Dr. Rodriguez’s leadership, this is the first record of a whole-eye transplant. “The mere fact that we have successfully performed the first whole-eye transplant along with a face transplant is a tremendous achievement that many believed to be impossible,” the doctor said in a statement. “We have taken a giant step forward and paved the way for the next chapter in vision restoration.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In a groundbreaking procedure, a team of surgeons from New York University Langone Health successfully performed the first combined face and eye transplant on a patient with extensive craniofacial tissue loss after an electrical accident.
The highly complex surgery lasted for 21 hours and involved more than 140 surgeons, nurses, and other healthcare professionals under the leadership of Eduardo D. Rodriguez. MD. It not only restored the patient’s facial features, but also integrated a functional eyeball, potentially setting a new standard for future treatments in similar cases.
The transplant took place in May 2023, and the case report was published on September 5 this year in JAMA.
The 46-year-old man lost a large part of his craniofacial tissue and his left eyeball. The approach was highly specialized. Advanced microsurgical techniques such as anastomoses of microscopic vessels and delicate suturing techniques were crucial for the transplant’s success.
Moreover, customized surgical devices, specific implants, and tissue manipulation tools were developed specifically for this case, thus ensuring the viability of the transplant and adequate perfusion of the transplanted ocular tissue.
The initial results are encouraging. Retinal arterial perfusion has been maintained, and retinal architecture has been preserved, as demonstrated by optical coherence tomography. Electroretinography confirmed retinal responses to light, suggesting that the transplanted eye may eventually contribute to the patient’s visual perception. These results are comparable to those of previous facial tissue transplants, but with the significant addition of ocular functionality, which is a notable advance.
“The successful revascularization of the transplanted eye achieved in this study may serve as a step toward the goal of globe transplant for restoration of vision,” wrote the authors.
The complexity of the combined transplant required a deep understanding of facial and ocular anatomy, as well as tissue preservation techniques. The surgical team reported significant challenges, including the need to align delicate anatomical structures and ensure immunological compatibility between the donor and recipient. Meticulous planning from donor selection to postoperative follow-up was considered essential to maximize the likelihood of success and minimize the risk for allograft rejection.
The patient will now be continuously monitored and receive treatment with immunosuppressants such as tacrolimus and prednisone, adjusted according to his response to the transplant. According to the researchers, further studies will be needed to assess the long-term functionality of the transplanted eye and its integration with the central nervous system.
Despite being the fifth facial transplant surgery performed under Dr. Rodriguez’s leadership, this is the first record of a whole-eye transplant. “The mere fact that we have successfully performed the first whole-eye transplant along with a face transplant is a tremendous achievement that many believed to be impossible,” the doctor said in a statement. “We have taken a giant step forward and paved the way for the next chapter in vision restoration.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
In a groundbreaking procedure, a team of surgeons from New York University Langone Health successfully performed the first combined face and eye transplant on a patient with extensive craniofacial tissue loss after an electrical accident.
The highly complex surgery lasted for 21 hours and involved more than 140 surgeons, nurses, and other healthcare professionals under the leadership of Eduardo D. Rodriguez. MD. It not only restored the patient’s facial features, but also integrated a functional eyeball, potentially setting a new standard for future treatments in similar cases.
The transplant took place in May 2023, and the case report was published on September 5 this year in JAMA.
The 46-year-old man lost a large part of his craniofacial tissue and his left eyeball. The approach was highly specialized. Advanced microsurgical techniques such as anastomoses of microscopic vessels and delicate suturing techniques were crucial for the transplant’s success.
Moreover, customized surgical devices, specific implants, and tissue manipulation tools were developed specifically for this case, thus ensuring the viability of the transplant and adequate perfusion of the transplanted ocular tissue.
The initial results are encouraging. Retinal arterial perfusion has been maintained, and retinal architecture has been preserved, as demonstrated by optical coherence tomography. Electroretinography confirmed retinal responses to light, suggesting that the transplanted eye may eventually contribute to the patient’s visual perception. These results are comparable to those of previous facial tissue transplants, but with the significant addition of ocular functionality, which is a notable advance.
“The successful revascularization of the transplanted eye achieved in this study may serve as a step toward the goal of globe transplant for restoration of vision,” wrote the authors.
The complexity of the combined transplant required a deep understanding of facial and ocular anatomy, as well as tissue preservation techniques. The surgical team reported significant challenges, including the need to align delicate anatomical structures and ensure immunological compatibility between the donor and recipient. Meticulous planning from donor selection to postoperative follow-up was considered essential to maximize the likelihood of success and minimize the risk for allograft rejection.
The patient will now be continuously monitored and receive treatment with immunosuppressants such as tacrolimus and prednisone, adjusted according to his response to the transplant. According to the researchers, further studies will be needed to assess the long-term functionality of the transplanted eye and its integration with the central nervous system.
Despite being the fifth facial transplant surgery performed under Dr. Rodriguez’s leadership, this is the first record of a whole-eye transplant. “The mere fact that we have successfully performed the first whole-eye transplant along with a face transplant is a tremendous achievement that many believed to be impossible,” the doctor said in a statement. “We have taken a giant step forward and paved the way for the next chapter in vision restoration.”
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
‘Reform School’ for Pharmacy Benefit Managers: How Might Legislation Help Patients?
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).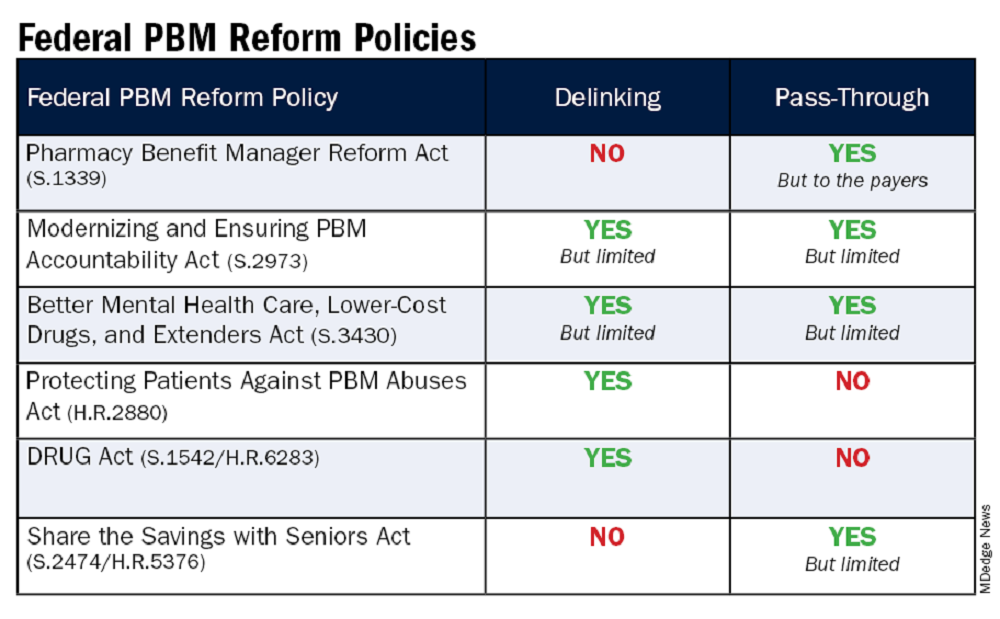
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.
The term “reform school” is a bit outdated. It used to refer to institutions where young offenders were sent instead of prison. Some argue that pharmacy benefit managers (PBMs) should bypass reform school and go straight to prison. “PBM reform” has become a ubiquitous term, encompassing any legislative or regulatory efforts aimed at curbing PBMs’ bad behavior. When discussing PBM reform, it’s crucial to understand the various segments of the healthcare system affected by PBMs. This complexity often makes it challenging to determine what these reform packages would actually achieve and who they would benefit.
Pharmacists have long been vocal critics of PBMs, and while their issues are extremely important, it is essential to remember that the ultimate victims of PBM misconduct, in terms of access to care, are patients. At some point, we will all be patients, making this issue universally relevant. It has been quite challenging to follow federal legislation on this topic as these packages attempt to address a number of bad behaviors by PBMs affecting a variety of victims. This discussion will examine those reforms that would directly improve patient’s access to available and affordable medications.
Policy Categories of PBM Reform
There are five policy categories of PBM reform legislation overall, including three that have the greatest potential to directly address patient needs. The first is patient access to medications (utilization management, copay assistance, prior authorization, etc.), followed by delinking drug list prices from PBM income and pass-through of price concessions from the manufacturer. The remaining two categories involve transparency and pharmacy-facing reform, both of which are very important. However, this discussion will revolve around the first three categories. It should be noted that many of the legislation packages addressing the categories of patient access, delinking, and pass-through also include transparency issues, particularly as they relate to pharmacy-facing issues.
Patient Access to Medications — Step Therapy Legislation
One of the major obstacles to patient access to medications is the use of PBM utilization management tools such as step therapy (“fail first”), prior authorizations, nonmedical switching, and formulary exclusions. These tools dictate when patients can obtain necessary medications and for how long patients who are stable on their current treatments can remain on them.
While many states have enacted step therapy reforms to prevent stable patients from being whip-sawed between medications that maximize PBM profits (often labeled as “savings”), these state protections apply only to state-regulated health plans. These include fully insured health plans and those offered through the Affordable Care Act’s Health Insurance Marketplace. It also includes state employees, state corrections, and, in some cases, state labor unions. State legislation does not extend to patients covered by employer self-insured health plans, called ERISA plans for the federal law that governs employee benefit plans, the Employee Retirement Income Security Act. These ERISA plans include nearly 35 million people nationwide.
This is where the Safe Step Act (S.652/H.R.2630) becomes crucial, as it allows employees to request exceptions to harmful fail-first protocols. The bill has gained significant momentum, having been reported out of the Senate HELP Committee and discussed in House markups. The Safe Step Act would mandate that an exception to a step therapy protocol must be granted if:
- The required treatment has been ineffective
- The treatment is expected to be ineffective, and delaying effective treatment would lead to irreversible consequences
- The treatment will cause or is likely to cause an adverse reaction
- The treatment is expected to prevent the individual from performing daily activities or occupational responsibilities
- The individual is stable on their current prescription drugs
- There are other circumstances as determined by the Employee Benefits Security Administration
This legislation is vital for ensuring that patients have timely access to the medications they need without unnecessary delays or disruptions.
Patient Access to Medications — Prior Authorizations
Another significant issue affecting patient access to medications is prior authorizations (PAs). According to an American Medical Association survey, nearly one in four physicians (24%) report that a PA has led to a serious adverse event for a patient in their care. In rheumatology, PAs often result in delays in care (even for those initially approved) and a significant increase in steroid usage. In particular, PAs in Medicare Advantage (MA) plans are harmful to Medicare beneficiaries.
The Improving Seniors’ Timely Access to Care Act (H.R.8702 / S.4532) aims to reform PAs used in MA plans, making the process more efficient and transparent to improve access to care for seniors. Unfortunately, it does not cover Part D drugs and may only cover Part B drugs depending on the MA plan’s benefit package. Here are the key provisions of the act:
- Electronic PA: Implementing real-time decisions for routinely approved items and services.
- Transparency: Requiring annual publication of PA information, such as the percentage of requests approved and the average response time.
- Quality and Timeliness Standards: The Centers for Medicare & Medicaid Services (CMS) will set standards for the quality and timeliness of PA determinations.
- Streamlining Approvals: Simplifying the approval process and reducing the time allowed for health plans to consider PA requests.
This bill passed the House in September 2022 but stalled in the Senate because of an unfavorable Congressional Budget Office score. CMS has since finalized portions of this bill via regulation, zeroing out the CBO score and increasing the chances of its passage.
Delinking Drug Prices from PBM Income and Pass-Through of Price Concessions
Affordability is a crucial aspect of accessibility, especially when it comes to medications. Over the years, we’ve learned that PBMs often favor placing the highest list price drugs on formularies because the rebates and various fees they receive from manufacturers are based on a percentage of the list price. In other words, the higher the medication’s price, the more money the PBM makes.
This practice is evident in both commercial and government formularies, where brand-name drugs are often preferred, while lower-priced generics are either excluded or placed on higher tiers. As a result, while major PBMs benefit from these rebates and fees, patients continue to pay their cost share based on the list price of the medication.
To improve the affordability of medications, a key aspect of PBM reform should be to disincentivize PBMs from selecting higher-priced medications and/or require the pass-through of manufacturer price concessions to patients.
Several major PBM reform bills are currently being considered that address either the delinking of price concessions from the list price of the drug or some form of pass-through of these concessions. These reforms are essential to ensure that patients can access affordable medications without being burdened by inflated costs.
The legislation includes the Pharmacy Benefit Manager Reform Act (S.1339); the Modernizing & Ensuring PBM Accountability Act (S.2973); the Better Mental Health Care, Lower Cost Drugs, and Extenders Act (S.3430); the Protecting Patients Against PBM Abuses Act (H.R. 2880); the DRUG Act (S.2474 / H.R.6283); and the Share the Savings with Seniors Act (S.2474 / H.R.5376).
As with all legislation, there are limitations and compromises in each of these. However, these bills are a good first step in addressing PBM remuneration (rebates and fees) based on the list price of the drug and/or passing through to the patient the benefit of manufacturer price concessions. By focusing on key areas like utilization management, delinking drug prices from PBM income, and allowing patients to directly benefit from manufacturer price concessions, we can work toward a more equitable and efficient healthcare system. Reigning in PBM bad behavior is a challenge, but the potential benefits for patient care and access make it a crucial fight worth pursuing.
Please help in efforts to improve patients’ access to available and affordable medications by contacting your representatives in Congress to impart to them the importance of passing legislation. The CSRO’s legislative map tool can help to inform you of the latest information on these and other bills and assist you in engaging with your representatives on them.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of Advocacy and Government Affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. She has no relevant conflicts of interest to disclose. You can reach her at rhnews@mdedge.com.



