User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Review supports continued mask-wearing in health care visits
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
A new study urges people to continue wearing protective masks in medical settings, even though the U.S. public health emergency declaration around COVID-19 has expired.
Masks continue to lower the risk of catching the virus during medical visits, according to the study, published in Annals of Internal Medicine. And there was not much difference between wearing surgical masks and N95 respirators in health care settings.
The researchers reviewed 3 randomized trials and 21 observational studies to compare the effectiveness of those and cloth masks in reducing COVID-19 transmission.
Tara N. Palmore, MD, of George Washington University, Washington, and David K. Henderson, MD, of the National Institutes of Health, Bethesda, Md., wrote in an opinion article accompanying the study.
“In our enthusiasm to return to the appearance and feeling of normalcy, and as institutions decide which mitigation strategies to discontinue, we strongly advocate not discarding this important lesson learned for the sake of our patients’ safety,” Dr. Palmore and Dr. Henderson wrote.
Surgical masks limit the spread of aerosols and droplets from people who have the flu, coronaviruses or other respiratory viruses, CNN reported. And while masks are not 100% effective, they substantially lower the amount of virus put into the air via coughing and talking.
The study said one reason people should wear masks to medical settings is because “health care personnel are notorious for coming to work while ill.” Transmission from patient to staff and staff to patient is still possible, but rare, when both are masked.
The review authors reported no conflicts of interest. Dr. Palmore has received grants from the NIH, Rigel, Gilead, and AbbVie, and Dr. Henderson is a past president of the Society for Healthcare Epidemiology of America.
A version of this article first appeared on WebMD.com.
FROM ANNALS OF INTERNAL MEDICINE
CDC warns of Mpox resurgence in summer of 2023
A resurgence of mpox this summer could be larger than last year’s caseload, the Centers for Disease Control and Prevention said in a warning to public health officials this week.
“The outbreak is not over,” the CDC alert stated, noting that springtime and summertime gatherings and festivals could lead to renewed virus spread. A new cluster of 13 cases is being investigated in Chicago, all among men, and four among people who recently traveled to New York City, New Orleans, or Mexico.
Mpox, formerly called monkeypox, is a virus that causes a rash and sometimes flulike symptoms. It is most often transmitted through sexual contact, but it can also be spread in nonsexual ways that involve contact with skin lesions or with saliva or upper respiratory secretions like snot or mucus, the CDC says. Most cases in the United States have been among gay or bisexual men, men who have sex with men, and transgender people.
Last year, the U.S. government declared mpox a public health emergency as cases peaked at 460 per day in August, infecting more than 30,000 people and killing 42 people. Public health officials worked to quickly distribute vaccinations to people at high risk for contracting the virus. The CDC says 23% of people most at risk of getting mpox have been vaccinated.
Vaccination does not necessarily prevent infection but can lessen the severity of symptoms. Nine of the men who were recently infected in Chicago were fully vaccinated.
“It’s important to remember that vaccines, while incredibly helpful, are not our only way to reduce the risk of contracting mpox,” Richard Silvera, MD, MPH, of the department of infectious diseases at Icahn School of Medicine at Mount Sinai, New York, told ABC News.
Other ways to reduce risk are “things like avoiding social and sexual contact if you have new skin lesions and asking your intimate contacts if they are experiencing symptoms or new skin changes,” Dr. Silvera said.
A version of this article first appeared on WebMD.com.
A resurgence of mpox this summer could be larger than last year’s caseload, the Centers for Disease Control and Prevention said in a warning to public health officials this week.
“The outbreak is not over,” the CDC alert stated, noting that springtime and summertime gatherings and festivals could lead to renewed virus spread. A new cluster of 13 cases is being investigated in Chicago, all among men, and four among people who recently traveled to New York City, New Orleans, or Mexico.
Mpox, formerly called monkeypox, is a virus that causes a rash and sometimes flulike symptoms. It is most often transmitted through sexual contact, but it can also be spread in nonsexual ways that involve contact with skin lesions or with saliva or upper respiratory secretions like snot or mucus, the CDC says. Most cases in the United States have been among gay or bisexual men, men who have sex with men, and transgender people.
Last year, the U.S. government declared mpox a public health emergency as cases peaked at 460 per day in August, infecting more than 30,000 people and killing 42 people. Public health officials worked to quickly distribute vaccinations to people at high risk for contracting the virus. The CDC says 23% of people most at risk of getting mpox have been vaccinated.
Vaccination does not necessarily prevent infection but can lessen the severity of symptoms. Nine of the men who were recently infected in Chicago were fully vaccinated.
“It’s important to remember that vaccines, while incredibly helpful, are not our only way to reduce the risk of contracting mpox,” Richard Silvera, MD, MPH, of the department of infectious diseases at Icahn School of Medicine at Mount Sinai, New York, told ABC News.
Other ways to reduce risk are “things like avoiding social and sexual contact if you have new skin lesions and asking your intimate contacts if they are experiencing symptoms or new skin changes,” Dr. Silvera said.
A version of this article first appeared on WebMD.com.
A resurgence of mpox this summer could be larger than last year’s caseload, the Centers for Disease Control and Prevention said in a warning to public health officials this week.
“The outbreak is not over,” the CDC alert stated, noting that springtime and summertime gatherings and festivals could lead to renewed virus spread. A new cluster of 13 cases is being investigated in Chicago, all among men, and four among people who recently traveled to New York City, New Orleans, or Mexico.
Mpox, formerly called monkeypox, is a virus that causes a rash and sometimes flulike symptoms. It is most often transmitted through sexual contact, but it can also be spread in nonsexual ways that involve contact with skin lesions or with saliva or upper respiratory secretions like snot or mucus, the CDC says. Most cases in the United States have been among gay or bisexual men, men who have sex with men, and transgender people.
Last year, the U.S. government declared mpox a public health emergency as cases peaked at 460 per day in August, infecting more than 30,000 people and killing 42 people. Public health officials worked to quickly distribute vaccinations to people at high risk for contracting the virus. The CDC says 23% of people most at risk of getting mpox have been vaccinated.
Vaccination does not necessarily prevent infection but can lessen the severity of symptoms. Nine of the men who were recently infected in Chicago were fully vaccinated.
“It’s important to remember that vaccines, while incredibly helpful, are not our only way to reduce the risk of contracting mpox,” Richard Silvera, MD, MPH, of the department of infectious diseases at Icahn School of Medicine at Mount Sinai, New York, told ABC News.
Other ways to reduce risk are “things like avoiding social and sexual contact if you have new skin lesions and asking your intimate contacts if they are experiencing symptoms or new skin changes,” Dr. Silvera said.
A version of this article first appeared on WebMD.com.
The antimicrobial peptide that even Pharma can love
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
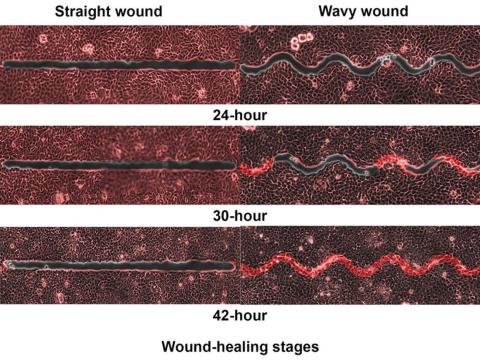
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Fastest peptide north, south, east, aaaaand west of the Pecos
Bacterial infections are supposed to be simple. You get infected, you get an antibiotic to treat it. Easy. Some bacteria, though, don’t play by the rules. Those antibiotics may kill 99.9% of germs, but what about the 0.1% that gets left behind? With their fallen comrades out of the way, the accidentally drug resistant species are free to inherit the Earth.
Antibiotic resistance is thus a major concern for the medical community. Naturally, anything that prevents doctors from successfully curing sick people is a priority. Unless you’re a major pharmaceutical company that has been loath to develop new drugs that can beat antibiotic-resistant bacteria. Blah blah, time and money, blah blah, long time between development and market application, blah blah, no profit. We all know the story with pharmaceutical companies.
Research from other sources has continued, however, and Brazilian scientists recently published research involving a peptide known as plantaricin 149. This peptide, derived from the bacterium Lactobacillus plantarum, has been known for nearly 30 years to have antibacterial properties. Pln149 in its natural state, though, is not particularly efficient at bacteria-killing. Fortunately, we have science and technology on our side.
The researchers synthesized 20 analogs of Pln149, of which Pln149-PEP20 had the best results. The elegantly named compound is less than half the size of the original peptide, less toxic, and far better at killing any and all drug-resistant bacteria the researchers threw at it. How much better? Pln149-PEP20 started killing bacteria less than an hour after being introduced in lab trials.
The research is just in its early days – just because something is less toxic doesn’t necessarily mean you want to go and help yourself to it – but we can only hope that those lovely pharmaceutical companies deign to look down upon us and actually develop a drug utilizing Pln149-PEP20 to, you know, actually help sick people, instead of trying to build monopolies or avoiding paying billions in taxes. Yeah, we couldn’t keep a straight face through that last sentence either.
Speed healing: The wavy wound gets the swirl
Did you know that wavy wounds heal faster than straight wounds? Well, we didn’t, but apparently quite a few people did, because somebody has been trying to figure out why wavy wounds heal faster than straight ones. Do the surgeons know about this? How about you dermatologists? Wavy over straight? We’re the media. We’re supposed to report this kind of stuff. Maybe hit us with a tweet next time you do something important, or push a TikTok our way, okay?
You could be more like the investigators at Nanyang Technological University in Singapore, who figured out the why and then released a statement about it.
They created synthetic wounds – some straight, some wavy – in micropatterned hydrogel substrates that mimicked human skin. Then they used an advanced optical technique known as particle image velocimetry to measure fluid flow and learn how cells moved to close the wound gaps.
The wavy wounds “induced more complex collective cell movements, such as a swirly, vortex-like motion,” according to the written statement from NTU Singapore. In the straight wounds, cell movements paralleled the wound front, “moving in straight lines like a marching band,” they pointed out, unlike some researchers who never call us unless they need money.
Complex epithelial cell movements are better, it turns out. Over an observation period of 64 hours the NTU team found that the healing efficiency of wavy gaps – measured by the area covered by the cells over time – is nearly five times faster than straight gaps.
The complex motion “enabled cells to quickly connect with similar cells on the opposite site of the wound edge, forming a bridge and closing the wavy wound gaps faster than straight gaps,” explained lead author Xu Hongmei, a doctoral student at NTU’s School of Mechanical and Aerospace Engineering, who seems to have time to toss out a tumblr or two to keep the press informed.
As for the rest of you, would it kill you to pick up a phone once in a while? Maybe let a journalist know that you’re still alive? We have feelings too, you know, and we worry.
A little Jekyll, a little Hyde, and a little shop of horrors
More “Little Shop of Horrors” references are coming, so be prepared.
We begin with Triphyophyllum peltatum. This woody vine is of great interest to medical and pharmaceutical researchers because its constituents have shown promise against pancreatic cancer and leukemia cells, among others, along with the pathogens that cause malaria and other diseases. There is another side, however. T. peltatum also has a tendency to turn into a realistic Audrey II when deprived.
No, of course they’re not craving human flesh, but it does become … carnivorous in its appetite.
T. peltatum, native to the West African tropics and not found in a New York florist shop, has the unique ability to change its diet and development based on the environmental circumstances. For some unknown reason, the leaves would develop adhesive traps in the form of sticky drops that capture insect prey. The plant is notoriously hard to grow, however, so no one could study the transformation under lab conditions. Until now.
A group of German scientists “exposed the plant to different stress factors, including deficiencies of various nutrients, and studied how it responded to each,” said Dr. Traud Winkelmann of Leibniz University Hannover. “Only in one case were we able to observe the formation of traps: in the case of a lack of phosphorus.”
Well, there you have it: phosphorus. We need it for healthy bones and teeth, which this plant doesn’t have to worry about, unlike its Tony Award–nominated counterpart. The investigators hope that their findings could lead to “future molecular analyses that will help understand the origins of carnivory,” but we’re guessing that a certain singing alien species will be left out of that research.
Expunging ‘penicillin allergy’: Your questions answered
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Last month, I described a 28-year-old patient with a history of injection drug use who presented with pain in his left forearm. His history showed that, within the past 2 years, he’d been seen for cutaneous infections multiple times as an outpatient and in the emergency department. His records indicated that he was diagnosed with a penicillin allergy as a child when he developed a rash after receiving amoxicillin. I believed the next course of action should be to test for a penicillin allergy with an oral amoxicillin challenge.
Thank you for your excellent questions regarding this case. Great to hear the enthusiasm for testing for penicillin allergy!
One question focused on the course of action in the case of a mild or moderate IgE-mediated reaction after a single dose test with amoxicillin. Treatment for these reactions should include an antihistamine. I would reserve intravenous antihistamines for more severe cases, which also require treatment with a course of corticosteroids. However, the risk for a moderate to severe reaction to amoxicillin on retesting is quite low.
Clinicians need to exercise caution in the use of systemic corticosteroids. These drugs can be lifesaving, but even short courses of corticosteroids are associated with potentially serious adverse events. In a review of adverse events associated with short-course systemic corticosteroids among children, the rate of vomiting was 5.4%; behavioral change, 4.7%; and sleep disturbance, 4.3%. One child died after contracting herpes zoster, more than one-third of children developed elevated blood pressure, and 81.1% had evidence of suppression of the hypothalamic-pituitary-adrenal axis.
Among adults, short courses of systemic corticosteroids are associated with acute increases in the risks for gastrointestinal bleeding and hypertension. Cumulative exposure to short courses of corticosteroids over time results in higher risks for obesity, type 2 diabetes, and osteoporosis.
Another question prompted by this young man’s case focused on the durability of IgE reactions against penicillin. The IgE response to penicillin does indeed wane over time; 80% of patients with a previous true penicillin allergy can tolerate the antibiotic after 10 years. Thus, about 95% of patients with a remote history of penicillin allergy are tolerant of penicillin, and testing can be performed using the algorithm described.
Clinicians should avoid applying current guidelines for the evaluation of patients with penicillin allergy to other common drug allergies. The overall prevalence of sulfonamide allergy is 3%-8%, and the vast majority of these reactions follow treatment with trimethoprim-sulfamethoxazole. Sulfa allergy is even more common among persons living with HIV infection. The natural history of sulfa allergy is not as well established as penicillin allergy. Allergy testing is encouraged in these cases. Graded oral challenge testing is best reserved for patients who are unlikely to have a true sulfa allergy based on their history.
A version of this article first appeared on Medscape.com.
Brepocitinib shows promise for psoriatic arthritis patients
The investigational drug brepocitinib showed superior symptom reduction in adults with moderate to severe active psoriatic arthritis (PsA), compared with placebo, by meeting primary and secondary endpoints of a phase 2b trial at 16 weeks, which persisted out to 1 year, according to data from 218 individuals.
Brepocitinib, a combination tyrosine kinase 2 and Janus kinase 1 inhibitor, is being studied for the treatment of several immunologic diseases including PsA, wrote Philip Mease, MD, of the Swedish Medical Center/Providence St. Joseph Health and the University of Washington, both in Seattle.
Previous studies in patients with PsA support the use of Janus kinase inhibitors and demonstrate the efficacy of tyrosine kinase 2 inhibitors, but more data are needed in patients with active PsA, the researchers noted.
In a study published in Arthritis & Rheumatology, the researchers randomized adults aged 18-75 years with moderate to severe PsA to once-daily oral doses of brepocitinib at 10 mg, 30 mg, or 60 mg, or a placebo for 16 weeks to assess safety, efficacy, and dose response. Placebo-treated patients were advanced to 30 mg or 60 mg of brepocitinib at week 16. Baseline demographics and disease characteristics were similar among the treatment groups. The mean Psoriatic Arthritis Disease Activity Score (PASDAS) and Disease Activity Index for PsA scores were 5.6 and 38.2, respectively, for the overall study population. Approximately two-thirds (64.7%) had a baseline Psoriasis Area and Severity Index (PASI) score greater than 0 and 3% or more of their body surface area affected by psoriasis.
The primary endpoint was the proportion of patients achieving 20% improvement in American College of Rheumatology response criteria (ACR 20) at week 16. Secondary endpoints included rates of patients meeting ACR 50 and ACR 70 response criteria, the proportion of patients achieving 75% and 90% improvement in PASI scores (PASI 70 and 90), as well as the rates of patients meeting Minimal Disease Activity (MDA) criteria at 16 and 52 weeks.
At week 16, ACR 20 response rates were significantly higher in the brepocitinib 30-mg and 60-mg groups, compared with the placebo group (66.7% and 74.6%, respectively, vs. 43.3%), but not for those who received brepocitinib 10 mg (64.5%).
Response rates for ACR 50, ACR 70, PASI 75, PASI 90, and MDA were similarly higher in the 30-mg and 60-mg brepocitinib groups, compared with placebo, and these responses persisted at week 52. Notably, significant differences in PASI 75 and PASI 90 were observed in patients taking 30 mg and 60 mg brepocitinib, compared with placebo, as early as weeks 4 and 8, respectively, the researchers said.
In addition, disease activity based on PASDAS improved significantly more from baseline to week 16 in all brepocitinib groups, compared with placebo.
The overall safety data were consistent with previous brepocitinib studies, and most of the adverse events were mild or moderate, the researchers said. A total of 12 participants (5.5%) experienced a total of 15 serious adverse events, including 6 infections with brepocitinib 30 mg or 60 mg. No major adverse cardiovascular events or deaths occurred during the study period.
The findings were limited by several factors, including the use of clinics in a limited geographic area (11 countries in Europe), small sample size, and mainly White population, the researchers noted. Other limitations included the large placebo effect and relatively short placebo-controlled period.
The study was supported by Pfizer. Dr. Mease disclosed relationships with Pfizer and other companies including AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Lilly, Novartis, Sun, and UCB. Many coauthors were employees of Pfizer, and others reported financial relationships with Pfizer and other pharmaceutical companies.
The investigational drug brepocitinib showed superior symptom reduction in adults with moderate to severe active psoriatic arthritis (PsA), compared with placebo, by meeting primary and secondary endpoints of a phase 2b trial at 16 weeks, which persisted out to 1 year, according to data from 218 individuals.
Brepocitinib, a combination tyrosine kinase 2 and Janus kinase 1 inhibitor, is being studied for the treatment of several immunologic diseases including PsA, wrote Philip Mease, MD, of the Swedish Medical Center/Providence St. Joseph Health and the University of Washington, both in Seattle.
Previous studies in patients with PsA support the use of Janus kinase inhibitors and demonstrate the efficacy of tyrosine kinase 2 inhibitors, but more data are needed in patients with active PsA, the researchers noted.
In a study published in Arthritis & Rheumatology, the researchers randomized adults aged 18-75 years with moderate to severe PsA to once-daily oral doses of brepocitinib at 10 mg, 30 mg, or 60 mg, or a placebo for 16 weeks to assess safety, efficacy, and dose response. Placebo-treated patients were advanced to 30 mg or 60 mg of brepocitinib at week 16. Baseline demographics and disease characteristics were similar among the treatment groups. The mean Psoriatic Arthritis Disease Activity Score (PASDAS) and Disease Activity Index for PsA scores were 5.6 and 38.2, respectively, for the overall study population. Approximately two-thirds (64.7%) had a baseline Psoriasis Area and Severity Index (PASI) score greater than 0 and 3% or more of their body surface area affected by psoriasis.
The primary endpoint was the proportion of patients achieving 20% improvement in American College of Rheumatology response criteria (ACR 20) at week 16. Secondary endpoints included rates of patients meeting ACR 50 and ACR 70 response criteria, the proportion of patients achieving 75% and 90% improvement in PASI scores (PASI 70 and 90), as well as the rates of patients meeting Minimal Disease Activity (MDA) criteria at 16 and 52 weeks.
At week 16, ACR 20 response rates were significantly higher in the brepocitinib 30-mg and 60-mg groups, compared with the placebo group (66.7% and 74.6%, respectively, vs. 43.3%), but not for those who received brepocitinib 10 mg (64.5%).
Response rates for ACR 50, ACR 70, PASI 75, PASI 90, and MDA were similarly higher in the 30-mg and 60-mg brepocitinib groups, compared with placebo, and these responses persisted at week 52. Notably, significant differences in PASI 75 and PASI 90 were observed in patients taking 30 mg and 60 mg brepocitinib, compared with placebo, as early as weeks 4 and 8, respectively, the researchers said.
In addition, disease activity based on PASDAS improved significantly more from baseline to week 16 in all brepocitinib groups, compared with placebo.
The overall safety data were consistent with previous brepocitinib studies, and most of the adverse events were mild or moderate, the researchers said. A total of 12 participants (5.5%) experienced a total of 15 serious adverse events, including 6 infections with brepocitinib 30 mg or 60 mg. No major adverse cardiovascular events or deaths occurred during the study period.
The findings were limited by several factors, including the use of clinics in a limited geographic area (11 countries in Europe), small sample size, and mainly White population, the researchers noted. Other limitations included the large placebo effect and relatively short placebo-controlled period.
The study was supported by Pfizer. Dr. Mease disclosed relationships with Pfizer and other companies including AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Lilly, Novartis, Sun, and UCB. Many coauthors were employees of Pfizer, and others reported financial relationships with Pfizer and other pharmaceutical companies.
The investigational drug brepocitinib showed superior symptom reduction in adults with moderate to severe active psoriatic arthritis (PsA), compared with placebo, by meeting primary and secondary endpoints of a phase 2b trial at 16 weeks, which persisted out to 1 year, according to data from 218 individuals.
Brepocitinib, a combination tyrosine kinase 2 and Janus kinase 1 inhibitor, is being studied for the treatment of several immunologic diseases including PsA, wrote Philip Mease, MD, of the Swedish Medical Center/Providence St. Joseph Health and the University of Washington, both in Seattle.
Previous studies in patients with PsA support the use of Janus kinase inhibitors and demonstrate the efficacy of tyrosine kinase 2 inhibitors, but more data are needed in patients with active PsA, the researchers noted.
In a study published in Arthritis & Rheumatology, the researchers randomized adults aged 18-75 years with moderate to severe PsA to once-daily oral doses of brepocitinib at 10 mg, 30 mg, or 60 mg, or a placebo for 16 weeks to assess safety, efficacy, and dose response. Placebo-treated patients were advanced to 30 mg or 60 mg of brepocitinib at week 16. Baseline demographics and disease characteristics were similar among the treatment groups. The mean Psoriatic Arthritis Disease Activity Score (PASDAS) and Disease Activity Index for PsA scores were 5.6 and 38.2, respectively, for the overall study population. Approximately two-thirds (64.7%) had a baseline Psoriasis Area and Severity Index (PASI) score greater than 0 and 3% or more of their body surface area affected by psoriasis.
The primary endpoint was the proportion of patients achieving 20% improvement in American College of Rheumatology response criteria (ACR 20) at week 16. Secondary endpoints included rates of patients meeting ACR 50 and ACR 70 response criteria, the proportion of patients achieving 75% and 90% improvement in PASI scores (PASI 70 and 90), as well as the rates of patients meeting Minimal Disease Activity (MDA) criteria at 16 and 52 weeks.
At week 16, ACR 20 response rates were significantly higher in the brepocitinib 30-mg and 60-mg groups, compared with the placebo group (66.7% and 74.6%, respectively, vs. 43.3%), but not for those who received brepocitinib 10 mg (64.5%).
Response rates for ACR 50, ACR 70, PASI 75, PASI 90, and MDA were similarly higher in the 30-mg and 60-mg brepocitinib groups, compared with placebo, and these responses persisted at week 52. Notably, significant differences in PASI 75 and PASI 90 were observed in patients taking 30 mg and 60 mg brepocitinib, compared with placebo, as early as weeks 4 and 8, respectively, the researchers said.
In addition, disease activity based on PASDAS improved significantly more from baseline to week 16 in all brepocitinib groups, compared with placebo.
The overall safety data were consistent with previous brepocitinib studies, and most of the adverse events were mild or moderate, the researchers said. A total of 12 participants (5.5%) experienced a total of 15 serious adverse events, including 6 infections with brepocitinib 30 mg or 60 mg. No major adverse cardiovascular events or deaths occurred during the study period.
The findings were limited by several factors, including the use of clinics in a limited geographic area (11 countries in Europe), small sample size, and mainly White population, the researchers noted. Other limitations included the large placebo effect and relatively short placebo-controlled period.
The study was supported by Pfizer. Dr. Mease disclosed relationships with Pfizer and other companies including AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Galapagos, Gilead, GlaxoSmithKline, Inmagene, Janssen, Lilly, Novartis, Sun, and UCB. Many coauthors were employees of Pfizer, and others reported financial relationships with Pfizer and other pharmaceutical companies.
FROM ARTHRITIS & RHEUMATOLOGY
Docs fervently hope federal ban on noncompete clauses goes through
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
Teledermatology follow-up after Mohs surgery gets a thumbs up from patients
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Foot ulcers red flag for eye disease in diabetes
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARVO 2023
Mohs surgery workforce continues to increase
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
CDC: Drug-resistant ringworm reported in New York
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.