User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Evolve your website
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at dermnews@mdedge.com.
Men underrepresented in clinical trials of laser hair removal, review finds
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
Facial, hand, and foot dermatitis: Lebrikizumab and dupilumab show efficacy in new studies
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
AT RAD 2023
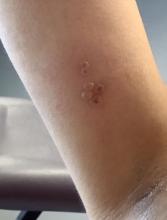
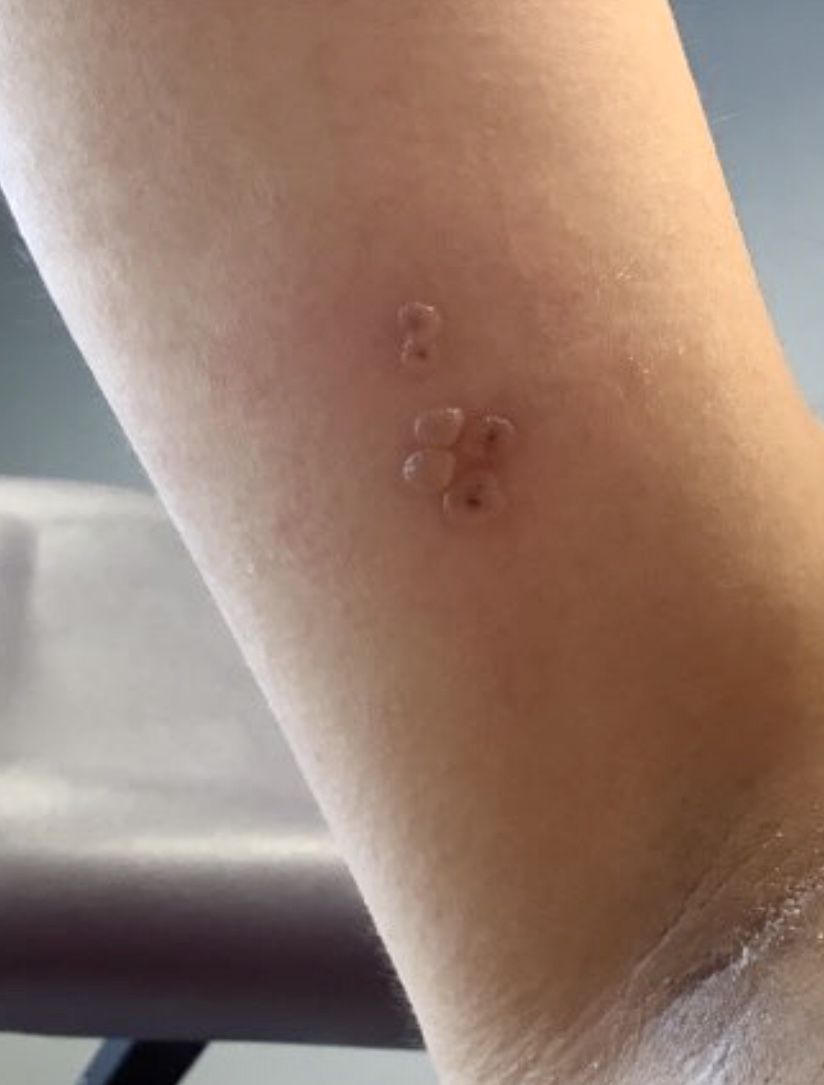
A healthy 36-year-old female presented with 4 days of itchy lesions on the right upper extremity
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Additionally, Orthopox DNA by PCR and Monkeypox (mpox) virus DNA by PCR were detected. Herpes simplex virus and bacterial viral cultures were negative. Valacyclovir was started at the time of presentation and the patient’s lesions resolved without sequelae.
Mpox is a zoonotic double-stranded DNA virus that is part of the Orthopoxvirus family, including the West African and Central African variants. This disease presents similarly to smallpox, so most mpox research was conducted around the time smallpox was eradicated. It was not until 1970, when the disease was isolated from a patient with suspected smallpox in the Democratic Republic of the Congo (DRC), that human mpox was considered a distinct disease. An epidemic outbreak in the United States occurred in 2003 related to infected prairie dogs, and travel-related outbreaks have been more recently reported up until May 2022, in which mpox was reported in nonendemic areas including North America, Europe, and Australia. Most cases in this outbreak occurred in men who have sex with men (MSM), but this is not always the case, and mpox is not necessarily considered a sexually transmitted infection. Mpox presents similarly to smallpox and VZV, so using laboratory tests is important in diagnosing and tracking this disease.
Although it is not easily transmitted, the disease can spread through bodily secretions both directly and indirectly. Mpox typically begins with a prodrome that includes fever, headache, myalgia, and fatigue. This is followed by lymphadenopathy that precedes and coincides with rash development. The lymph nodes are firm, tender, may be painful, and are a defining factor in presentation that differs from smallpox and varicella. The rash typically starts on the face, then presents on the body in a centrifugal distribution. However, cases related to sexual transmission present with anogenital lesions. The lesions are characterized by a progression from maculopapular to vesiculopustular, and can vary widely in quantity.
Notably, individuals are contagious from the onset of the prodrome until the lesions have scabbed over and fallen off. The eruptive nature of the later lesions poses a threat of secondary infection, and is often accompanied by a second febrile period that signifies deterioration of the patient’s condition. Other signs of secondary infection are variable and include pulmonary symptoms, vomiting, diarrhea, ocular infections, and in rare cases, encephalitis. These sequelae are more common in unvaccinated and immunocompromised individuals. Long-term complications of mpox include pitted scarring from cutaneous lesions with children being more susceptible to severe disease. The mortality rate for the disease is very low. (As of May 10, 2023, there have been 30,395 mpox cases reported in the United States, and 42 deaths, according to the Centers for Disease Control and Prevention.)
There are a variety of diagnostic tests that can aid in mpox identification, but they are most strongly supported when combined with clinical and epidemiological data. The best, least invasive method includes collection of lesion exudate or crust on a swab, and viral DNA is best preserved by keeping the specimen in a cool, dry, and dark environment. PCR is considered the standard, and electron microscopy and immunohistochemistry are valid tests, but all modalities require sophisticated technicians with the proper laboratory equipment. This is limiting because many cases present in underserved areas that lack the facilities for proper, real-time analysis. Antigen and antibody-based tests can be used, but cross-reactivity of other orthopoxviridae limits confirmation of mpox infection. Vaccination status, history and location must be considered.
Vaccination is the chief form of prevention for mpox, although it is not considered entirely protective. Smallpox vaccination provides protection, but widespread administration of the vaccine is no longer practiced, and an estimated 70% of the global population is no longer vaccinated. Vaccination is recommended for anyone at risk of exposure, but as this is a live, attenuated vaccine, the immune status of the patient is important to keep in mind. Tecovirimat and other antiviral medications including cidofovir and brincidofovir may be considered in severe cases.
This case is unique as our patient, who had no known risk factors for mpox, presented with mpox and VZV, simultaneously. Although clinical presentation and epidemiological patterns between these diseases differ, there have been a limited number of cases of coinfection reported in the literature, mainly in the DRC where mpox is endemic. Diagnosis must be made by separate laboratory tests and there are differences in presentation between independent and coinfection for these viruses. Notably, patients with mpox/VZV coinfection may be less likely to present with lesions on the face, thorax, arms, palms, and soles than those with only mpox but experience a higher lesion burden than those afflicted by only VZV. Coinfection may be related to reactivation of dormant VZV, or increased susceptibility to secondary infection when infected with one virus.
This case and photo were submitted by Lucas Shapiro, BS, of the Dr. Kiran C. Patel College of Osteopathic Medicine at Nova Southeastern University, Fort Lauderdale, Fla., and Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Macneil A et al. Clin Infect Dis. 2009 Jan 1;48(1):e6-8.
2. Di Gennaro F et al. Microorganisms. 2022 Aug 12;10(8):1633.
3. Hughes CM et al. Am J Trop Med Hyg. 2020 Dec 7;104(2):604-11.
Number of cancer survivors with functional limitations doubled in 20 years
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
Vishal Patel, BS, a student at the Dell Medical School at The University of Texas at Austin, and colleagues identified 51,258 cancer survivors from the National Health Interview Survey, representing a weighted population of approximately 178.8 million from 1999 to 2018.
Most survivors were women (60.2%) and were at least 65 years old (55.4%). In 1999, 3.6 million weighted survivors reported functional limitation. In 2018, the number increased to 8.2 million, a 2.25-fold increase.
The number of survivors who reported no limitations also increased, but not by as much. That group grew 1.34-fold during the study period.
For context, “the 70% prevalence of functional limitation among survivors in 2018 is nearly twice that of the general population,” the authors wrote.
Patients surveyed on function
Functional limitation was defined as “self-reported difficulty performing any of 12 routine physical or social activities without assistance.” Examples of the activities included difficulty sitting for more than 2 hours, difficulty participating in social activities or difficulty pushing or pulling an object the size of a living room chair.
Over the 2 decades analyzed, the adjusted prevalence of functional limitation was highest among survivors of pancreatic cancer (80.3%) and lung cancer (76.5%). Prevalence was lowest for survivors of melanoma (62.2%), breast (61.8%) and prostate (59.5%) cancers.
Not just a result of living longer
Mr. Patel told this publication that one assumption people might make when they read these results is that people are just living longer with cancer and losing functional ability accordingly.
“But, in fact, we found that the youngest [– those less than 65 years–] actually contributed to this trend more than the oldest people, which means it’s not just [happening], because people are getting older,” he said.
Hispanic and Black individuals had disproportionately higher increases in functional limitation; percentage point increases over the 2 decades were 19.5 for Black people, 25.1 for Hispanic people and 12.5 for White people. There may be a couple of reasons for that, Mr. Patel noted.
Those who are Black or Hispanic tend to have less access to cancer survivorship care for reasons including insurance status and historic health care inequities, he noted.
“The other potential reason is that they have had less access to cancer care historically. And if, 20 years ago Black and Hispanic individuals didn’t have access to some chemotherapies, and now they do, maybe it’s the increased access to care that’s causing these functional limitations. Because chemotherapy can sometimes be very toxic. It may be sort of a catch-up toxicity,” he said.
Quality of life beyond survivorship
Mr. Patel said the results seem to call for building on improved survival rates by tracking and improving function.
“It’s good to celebrate that there are more survivors. But now that we can keep people alive longer, maybe we can shift gears to improving their quality of life,” he said.
The more-than-doubling of functional limitations over 2 decades “is a very sobering trend,” he noted, while pointing out that the functional limitations applied to 8 million people in the United States – people whose needs are not being met.
There’s no sign of the trend stopping, he continued. “We saw no downward trend, only an upward trend.”
Increasingly, including functionality as an endpoint in cancer trials, in addition to improvements in mortality, is one place to start, he added.
“Our findings suggest an urgent need for care teams to understand and address function, for researchers to evaluate function as a core outcome in trials, and for health systems and policy makers to reimagine survivorship care, recognizing the burden of cancer and its treatment on physical, psychosocial, and cognitive function,” the authors wrote in their paper. Limitations of the study include the potential for recall bias, lack of cancer staging or treatment information, and the subjective perception of function.
A coauthor reported personal fees from Astellas, AstraZeneca, AAA, Blue Earth, Janssen, Lantheus, Myovant, Myriad Genetics, Novartis, Telix, and Sanofi, as well as grants from Pfizer and Bayer during the conduct of the study. No other disclosures were reported.
FROM JAMA ONCOLOGY
Cutaneous vasculitis curtails quality of life
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
, and its measurement with an organ-specific instrument may catch important disease outcomes better than a generic health-related quality of life index, according to survey responses from participants in the Vasculitis Patient-Powered Research Network (VPPRN).
Although cutaneous vasculitis often causes itching, pain, and ulceration, the impact of the disease on specific health-related quality of life (HRQOL) outcomes has not been systematically assessed, wrote Sarah Mann, MD, of the University of Pittsburgh, and colleagues.
In a study published in JAMA Dermatology, the researchers used the VPPRN to conduct an online survey of adults aged 18 years and older with cutaneous manifestations of vasculitis. The survey was conducted between January 2020 and August 2021.
The primary outcomes of HRQOL were determined using two validated measures. One measured skin-related HRQOL (the Effects of Skin Disease on Quality-of-Life Survey [Skindex-29]), and the other measured general health and well-being (36-Item Short Form Health Survey [SF-36]).
The final analysis included 190 survey responses. The mean age of the respondents was 50.5 years, 84.1% were female, and approximately two-thirds reported a duration of vasculitis of at least 5 years. Respondents’ vasculitides included cutaneous small-vessel vasculitis (14%), IgA vasculitis (6.5%), urticarial vasculitis (8.4%), granulomatosis with polyangiitis (17.6%), microscopic polyangiitis (10.3%), eosinophilic vasculitis (15%), polyarteritis nodosa (3.7%), and other vasculitis types (24.2%).
On the Skindex-29 domains, severely or very severely diminished HRQOL was reported by 77.6% of respondents for emotions, 78.5% for symptoms, 60.7% for functioning, and 75.7% for overall HRQOL.
On the SF-36, the HRQOL was below average on six of eight domains, and approximately half of the patients had summative physical component scores (56%) and mental component scores (52%) below 50.
The HRQOL outcomes of cutaneous vasculitis were worse on the Skindex-29 than the SF-36, the researchers noted. “This discordance may reflect the value of disease or organ-specific measures, which may be able to capture important outcomes of disease even when generic measures do not,” they said.
The study findings were limited by several factors, including the potential lack of generalizability to broader populations of vasculitis patients, the researchers noted. Other limitations included the underrepresentation of male patients and the lack of a disease-specific patient-reported outcome measure, they said.
In addition, “Because half of patients reported having disease which was in remission or mildly active, the study findings may underestimate the true role of active cutaneous vasculitis on HRQOL,” the researchers said.
More studies are needed to assess how HRQOL measures respond to disease treatment and control, the researchers wrote in their discussion. However, the results suggest that cutaneous vasculitis has a significant effect on patients’ perception of their health, as well as on their well-being and symptoms, they said.
The study was supported by the Patient-Centered Outcomes Research Institute and GlaxoSmithKline. Dr. Mann had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including GlaxoSmithKline.
FROM JAMA DERMATOLOGY
IVIG shows no impact on VTE risk in dermatomyositis patients
Use of intravenous immunoglobulin (IVIG) had no apparent effect on the risk of venous thromboembolism (VTE) in adults with dermatomyositis (DM), based on data from more than 400 individuals.
DM has been associated with an increased risk of VTE in previous studies, wrote Elizabeth T. Rotrosen, of Boston University and Brigham and Women’s Hospital, Boston, and colleagues. Although IVIG is often effective for DM patients with recalcitrant disease, it carries a boxed warning for increased thrombosis risk; however, the association between IVIG use and VTE risk in DM has not been well examined, the researchers said.
In a study published in JAMA Dermatology, the researchers identified 458 adults with DM based on the European Alliance of Associations for Reumatology/American College of Rheumatology criteria. The mean age of the participants was 51.8 years, 76% were female, and 82% were White. Of these, 178 were treated with IVIG and 280 were not. The mean duration of IVIG treatment was 32.9 months. The researchers used the chi square test to test for independence between binary variables, the Pearson chi square test to test for independence between categorical variables, and the unpaired t test to compare continuous variables in their statistical analysis.
A total of 23 patients experienced DM-associated VTEs; 6 in the IVIG group and 17 in the non-IVIG group (3.4% vs. 5.7%, P = .20), a nonsignificant difference. The patients in the IVIG group who experienced a DM-associated VTE all underwent IVIG treatment within 4 weeks before the event.
The most common risk factors for VTE in both the IVIG and non-IVIG groups were malignant neoplasm (66.7% and 58.8%, respectively), followed by immobilization (16.7% and 35.3%, respectively) and tobacco use (16.7% and 23.5%, respectively).
“Notably, 5 of the IVIG-treated patients with DM who experienced a VTE also had at least 1 additional underlying risk factor for VTE, including 4 with malignant neoplasm,” the researchers wrote.
A total of 76 patients had cancer-associated DM, including 12 treated with IVIG and 64 not treated with IVIG. Of these, 14 experienced a VTE (4 IVIG patients and 10 non-IVIG patients).
The study findings were limited by several factors, including the retrospective design and small number of VTEs. Prospective studies are needed for better assessment of the VTE risk in patients with DM treated with IVIG, the researchers noted. However, the study is the largest known to explore the association between IVIG use and VTE risk in patients with DM, they said, and the results suggest that clinicians may continue IVIG use in these patients with considerations of risks and benefits on an individual basis.
The study received no outside funding. Ms. Rotrosen had no financial conflicts to disclose. Two coauthors reported financial relationships with Pfizer unrelated to this study.
Use of intravenous immunoglobulin (IVIG) had no apparent effect on the risk of venous thromboembolism (VTE) in adults with dermatomyositis (DM), based on data from more than 400 individuals.
DM has been associated with an increased risk of VTE in previous studies, wrote Elizabeth T. Rotrosen, of Boston University and Brigham and Women’s Hospital, Boston, and colleagues. Although IVIG is often effective for DM patients with recalcitrant disease, it carries a boxed warning for increased thrombosis risk; however, the association between IVIG use and VTE risk in DM has not been well examined, the researchers said.
In a study published in JAMA Dermatology, the researchers identified 458 adults with DM based on the European Alliance of Associations for Reumatology/American College of Rheumatology criteria. The mean age of the participants was 51.8 years, 76% were female, and 82% were White. Of these, 178 were treated with IVIG and 280 were not. The mean duration of IVIG treatment was 32.9 months. The researchers used the chi square test to test for independence between binary variables, the Pearson chi square test to test for independence between categorical variables, and the unpaired t test to compare continuous variables in their statistical analysis.
A total of 23 patients experienced DM-associated VTEs; 6 in the IVIG group and 17 in the non-IVIG group (3.4% vs. 5.7%, P = .20), a nonsignificant difference. The patients in the IVIG group who experienced a DM-associated VTE all underwent IVIG treatment within 4 weeks before the event.
The most common risk factors for VTE in both the IVIG and non-IVIG groups were malignant neoplasm (66.7% and 58.8%, respectively), followed by immobilization (16.7% and 35.3%, respectively) and tobacco use (16.7% and 23.5%, respectively).
“Notably, 5 of the IVIG-treated patients with DM who experienced a VTE also had at least 1 additional underlying risk factor for VTE, including 4 with malignant neoplasm,” the researchers wrote.
A total of 76 patients had cancer-associated DM, including 12 treated with IVIG and 64 not treated with IVIG. Of these, 14 experienced a VTE (4 IVIG patients and 10 non-IVIG patients).
The study findings were limited by several factors, including the retrospective design and small number of VTEs. Prospective studies are needed for better assessment of the VTE risk in patients with DM treated with IVIG, the researchers noted. However, the study is the largest known to explore the association between IVIG use and VTE risk in patients with DM, they said, and the results suggest that clinicians may continue IVIG use in these patients with considerations of risks and benefits on an individual basis.
The study received no outside funding. Ms. Rotrosen had no financial conflicts to disclose. Two coauthors reported financial relationships with Pfizer unrelated to this study.
Use of intravenous immunoglobulin (IVIG) had no apparent effect on the risk of venous thromboembolism (VTE) in adults with dermatomyositis (DM), based on data from more than 400 individuals.
DM has been associated with an increased risk of VTE in previous studies, wrote Elizabeth T. Rotrosen, of Boston University and Brigham and Women’s Hospital, Boston, and colleagues. Although IVIG is often effective for DM patients with recalcitrant disease, it carries a boxed warning for increased thrombosis risk; however, the association between IVIG use and VTE risk in DM has not been well examined, the researchers said.
In a study published in JAMA Dermatology, the researchers identified 458 adults with DM based on the European Alliance of Associations for Reumatology/American College of Rheumatology criteria. The mean age of the participants was 51.8 years, 76% were female, and 82% were White. Of these, 178 were treated with IVIG and 280 were not. The mean duration of IVIG treatment was 32.9 months. The researchers used the chi square test to test for independence between binary variables, the Pearson chi square test to test for independence between categorical variables, and the unpaired t test to compare continuous variables in their statistical analysis.
A total of 23 patients experienced DM-associated VTEs; 6 in the IVIG group and 17 in the non-IVIG group (3.4% vs. 5.7%, P = .20), a nonsignificant difference. The patients in the IVIG group who experienced a DM-associated VTE all underwent IVIG treatment within 4 weeks before the event.
The most common risk factors for VTE in both the IVIG and non-IVIG groups were malignant neoplasm (66.7% and 58.8%, respectively), followed by immobilization (16.7% and 35.3%, respectively) and tobacco use (16.7% and 23.5%, respectively).
“Notably, 5 of the IVIG-treated patients with DM who experienced a VTE also had at least 1 additional underlying risk factor for VTE, including 4 with malignant neoplasm,” the researchers wrote.
A total of 76 patients had cancer-associated DM, including 12 treated with IVIG and 64 not treated with IVIG. Of these, 14 experienced a VTE (4 IVIG patients and 10 non-IVIG patients).
The study findings were limited by several factors, including the retrospective design and small number of VTEs. Prospective studies are needed for better assessment of the VTE risk in patients with DM treated with IVIG, the researchers noted. However, the study is the largest known to explore the association between IVIG use and VTE risk in patients with DM, they said, and the results suggest that clinicians may continue IVIG use in these patients with considerations of risks and benefits on an individual basis.
The study received no outside funding. Ms. Rotrosen had no financial conflicts to disclose. Two coauthors reported financial relationships with Pfizer unrelated to this study.
FROM JAMA DERMATOLOGY
Can this tool forecast peanut allergies?
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pediatricians may have a new aid to better predict peanut allergies among infants with atopic dermatitis.
Their study of the implementation of the scorecard was presented at the Pediatric Academic Societies annual meeting.
Infants with atopic dermatitis or eczema are six times more likely to have an egg allergy and eleven times more likely to have a peanut allergy at age 12 months than are infants without atopic dermatitis.
The scorecard reflects recent directives from the National Institute of Allergy and Infectious Diseases to help combat the public health problem.
“When the NIAID prevention of peanut allergy guidelines first came out, it asked pediatricians to serve as frontline practitioners in implementing them by identifying children at risk for peanut allergy and guiding families on what to do next,” said Waheeda Samady, MD, professor of pediatrics at Northwestern University, Chicago. “The impetus for the study was to further support pediatricians in this role.”
Although pediatricians are trained to identify and even treat mild to moderate cases of atopic dermatitis, little emphasis has gone to categorizing the condition on the basis of severity and to correlating peanut allergy risk.
The predictive scorecard captures 14 images from one infant of mixed race, two White infants, two Black infants, and two Hispanic infants.
To create the card, two in-house pediatric dermatologists assessed 58 images from 13 children and categorized images from 0 (no signs of atopic dermatitis) to 4 (severe signs of atopic dermatitis). After a first pass on categorization, the doctors agreed on 84% of images.
Of 189 pediatricians who used the card, fewer than half reported that they “sometimes,” “very often,” or “always” used the scorecard for atopic dermatitis evaluation. A little fewer than three-quarters reported that their ability to diagnose and categorize atopic dermatitis improved.
“Severity staging of atopic dermatitis is not something that the general pediatrician necessarily performs on a day-to-day basis,” said Kawaljit Brar, MD, professor of pediatrics in the division of allergy and immunology at Hassenfeld Children’s Hospital in New York.
Dr. Brar explained that children who are identified as being at high risk are often referred to specialists such as her, who then perform allergy screenings and can determine whether introduction of food at home is safe or whether office feedings supervised by an allergist are necessary. Researchers have found that early introduction to peanuts for children with moderate to severe atopic dermatitis could prevent peanut allergy.
“This represents a wonderful initiative to educate pediatricians so that they understand which patients require screening for peanut allergy and which patients don’t and can just get introduced to peanuts at home,” Dr. Brar said.
The atopic dermatitis scorecard reflects a growing recognition that varying skin tones show levels of severity incongruously.
“Many of us in clinical practice have recognized that our education has not always been inclusive of patients with varying skin tones,” Dr. Samady said. “When we looked for photos of patients with different skin tones, we simply could not find any that we thought were appropriate. So we decided to take some ourselves, and we’re currently continuing to take photos in order to improve the scorecard we currently have.”
The study was funded by the National Institute of Health and Food Allergy Research and Education. Dr. Samady and Dr. Brar reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PAS 2023
Medical students gain momentum in effort to ban legacy admissions
, which they say offer preferential treatment to applicants based on their association with donors or alumni.
While an estimated 25% of public colleges and universities still use legacy admissions, a growing list of top medical schools have moved away from the practice over the last decade, including Johns Hopkins University, Baltimore, and Tufts University, Medford, Mass.
Legacy admissions contradict schools’ more inclusive policies, Senila Yasmin, MPH, a second-year medical student at Tufts University, said in an interview. While Tufts maintains legacy admissions for its undergraduate applicants, the medical school stopped the practice in 2021, said Ms. Yasmin, a member of a student group that lobbied against the school’s legacy preferences.
Describing herself as a low-income, first-generation Muslim-Pakistani American, Ms. Yasmin wants to use her experience at Tufts to improve accessibility for students like herself.
As a member of the American Medical Association (AMA) Medical Student Section, she coauthored a resolution stating that legacy admissions go against the AMA’s strategic plan to advance racial justice and health equity. The Student Section passed the resolution in November, and in June, the AMA House of Delegates will vote on whether to adopt the policy.
Along with a Supreme Court decision that could strike down race-conscious college admissions, an AMA policy could convince medical schools to rethink legacy admissions and how to maintain diverse student bodies. In June, the court is expected to issue a decision in the Students for Fair Admissions lawsuit against Harvard University, Cambridge, Mass., and the University of North Carolina, Chapel Hill, which alleges that considering race in holistic admissions constitutes racial discrimination and violates the Equal Protection Clause.
Opponents of legacy admissions, like Ms. Yasmin, say it penalizes students from racial minorities and lower socioeconomic backgrounds, hampering a fair and equitable admissions process that attracts diverse medical school admissions.
Diversity of medical applicants
Diversity in medical schools continued to increase last year with more Black, Hispanic, and female students applying and enrolling, according to a recent report by the Association of American Medical Colleges (AAMC). However, universities often include nonacademic criteria in their admission assessments to improve educational access for underrepresented minorities.
Medical schools carefully consider each applicant’s background “to yield a diverse class of students,” Geoffrey Young, PhD, AAMC’s senior director of transforming the health care workforce, told this news organization.
Some schools, such as Morehouse School of Medicine, Atlanta, the University of Virginia School of Medicine, Charlottesville, and the University of Arizona College of Medicine, Tucson, perform a thorough review of candidates while offering admissions practices designed specifically for legacy applicants. The schools assert that legacy designation doesn’t factor into the student’s likelihood of acceptance.
The arrangement may show that schools want to commit to equity and fairness but have trouble moving away from entrenched traditions, two professors from Penn State College of Medicine, Hershey, Pa., who sit on separate medical admissions subcommittees, wrote last year in Bioethics Today.
Legislation may hasten legacies’ end
In December, Ms. Yasmin and a group of Massachusetts Medical Society student-members presented another resolution to the state medical society, which adopted it.
The society’s new policy opposes the use of legacy status in medical school admissions and supports mechanisms to eliminate its inclusion from the application process, Theodore Calianos II, MD, FACS, president of the Massachusetts Medical Society, said in an interview.
“Legacy preferences limit racial and socioeconomic diversity on campuses, so we asked, ‘What can we do so that everyone has equal access to medical education?’ It is exciting to see the students and young physicians – the future of medicine – become involved in policymaking.”
Proposed laws may also hasten the end of legacy admissions. Last year, the U.S. Senate began considering a bill prohibiting colleges receiving federal financial aid from giving preferential treatment to students based on their relations to donors or alumni. However, the bill allows the Department of Education to make exceptions for institutions serving historically underrepresented groups.
The New York State Senate and the New York State Assembly also are reviewing bills that ban legacy and early admissions policies at public and private universities. Connecticut announced similar legislation last year. Massachusetts legislators are considering two bills: one that would ban the practice at the state’s public universities and another that would require all schools using legacy status to pay a “public service fee” equal to a percentage of its endowment. Colleges with endowment assets exceeding $2 billion must pay at least $2 million, according to the bill’s text.
At schools like Harvard, whose endowment surpasses $50 billion, the option to pay the penalty will make the law moot, Michael Walls, DO, MPH, president of the American Medical Student Association (AMSA), said in an interview. “Smaller schools wouldn’t be able to afford the fine and are less likely to be doing [legacy admissions] anyway,” he said. “The schools that want to continue doing it could just pay the fine.”
Dr. Walls said AMSA supports race-conscious admissions processes and anything that increases fairness for medical school applicants. “Whatever [fair] means is up for interpretation, but it would be great to eliminate legacy admissions,” he said.
A version of this article originally appeared on Medscape.com.
, which they say offer preferential treatment to applicants based on their association with donors or alumni.
While an estimated 25% of public colleges and universities still use legacy admissions, a growing list of top medical schools have moved away from the practice over the last decade, including Johns Hopkins University, Baltimore, and Tufts University, Medford, Mass.
Legacy admissions contradict schools’ more inclusive policies, Senila Yasmin, MPH, a second-year medical student at Tufts University, said in an interview. While Tufts maintains legacy admissions for its undergraduate applicants, the medical school stopped the practice in 2021, said Ms. Yasmin, a member of a student group that lobbied against the school’s legacy preferences.
Describing herself as a low-income, first-generation Muslim-Pakistani American, Ms. Yasmin wants to use her experience at Tufts to improve accessibility for students like herself.
As a member of the American Medical Association (AMA) Medical Student Section, she coauthored a resolution stating that legacy admissions go against the AMA’s strategic plan to advance racial justice and health equity. The Student Section passed the resolution in November, and in June, the AMA House of Delegates will vote on whether to adopt the policy.
Along with a Supreme Court decision that could strike down race-conscious college admissions, an AMA policy could convince medical schools to rethink legacy admissions and how to maintain diverse student bodies. In June, the court is expected to issue a decision in the Students for Fair Admissions lawsuit against Harvard University, Cambridge, Mass., and the University of North Carolina, Chapel Hill, which alleges that considering race in holistic admissions constitutes racial discrimination and violates the Equal Protection Clause.
Opponents of legacy admissions, like Ms. Yasmin, say it penalizes students from racial minorities and lower socioeconomic backgrounds, hampering a fair and equitable admissions process that attracts diverse medical school admissions.
Diversity of medical applicants
Diversity in medical schools continued to increase last year with more Black, Hispanic, and female students applying and enrolling, according to a recent report by the Association of American Medical Colleges (AAMC). However, universities often include nonacademic criteria in their admission assessments to improve educational access for underrepresented minorities.
Medical schools carefully consider each applicant’s background “to yield a diverse class of students,” Geoffrey Young, PhD, AAMC’s senior director of transforming the health care workforce, told this news organization.
Some schools, such as Morehouse School of Medicine, Atlanta, the University of Virginia School of Medicine, Charlottesville, and the University of Arizona College of Medicine, Tucson, perform a thorough review of candidates while offering admissions practices designed specifically for legacy applicants. The schools assert that legacy designation doesn’t factor into the student’s likelihood of acceptance.
The arrangement may show that schools want to commit to equity and fairness but have trouble moving away from entrenched traditions, two professors from Penn State College of Medicine, Hershey, Pa., who sit on separate medical admissions subcommittees, wrote last year in Bioethics Today.
Legislation may hasten legacies’ end
In December, Ms. Yasmin and a group of Massachusetts Medical Society student-members presented another resolution to the state medical society, which adopted it.
The society’s new policy opposes the use of legacy status in medical school admissions and supports mechanisms to eliminate its inclusion from the application process, Theodore Calianos II, MD, FACS, president of the Massachusetts Medical Society, said in an interview.
“Legacy preferences limit racial and socioeconomic diversity on campuses, so we asked, ‘What can we do so that everyone has equal access to medical education?’ It is exciting to see the students and young physicians – the future of medicine – become involved in policymaking.”
Proposed laws may also hasten the end of legacy admissions. Last year, the U.S. Senate began considering a bill prohibiting colleges receiving federal financial aid from giving preferential treatment to students based on their relations to donors or alumni. However, the bill allows the Department of Education to make exceptions for institutions serving historically underrepresented groups.
The New York State Senate and the New York State Assembly also are reviewing bills that ban legacy and early admissions policies at public and private universities. Connecticut announced similar legislation last year. Massachusetts legislators are considering two bills: one that would ban the practice at the state’s public universities and another that would require all schools using legacy status to pay a “public service fee” equal to a percentage of its endowment. Colleges with endowment assets exceeding $2 billion must pay at least $2 million, according to the bill’s text.
At schools like Harvard, whose endowment surpasses $50 billion, the option to pay the penalty will make the law moot, Michael Walls, DO, MPH, president of the American Medical Student Association (AMSA), said in an interview. “Smaller schools wouldn’t be able to afford the fine and are less likely to be doing [legacy admissions] anyway,” he said. “The schools that want to continue doing it could just pay the fine.”
Dr. Walls said AMSA supports race-conscious admissions processes and anything that increases fairness for medical school applicants. “Whatever [fair] means is up for interpretation, but it would be great to eliminate legacy admissions,” he said.
A version of this article originally appeared on Medscape.com.
, which they say offer preferential treatment to applicants based on their association with donors or alumni.
While an estimated 25% of public colleges and universities still use legacy admissions, a growing list of top medical schools have moved away from the practice over the last decade, including Johns Hopkins University, Baltimore, and Tufts University, Medford, Mass.
Legacy admissions contradict schools’ more inclusive policies, Senila Yasmin, MPH, a second-year medical student at Tufts University, said in an interview. While Tufts maintains legacy admissions for its undergraduate applicants, the medical school stopped the practice in 2021, said Ms. Yasmin, a member of a student group that lobbied against the school’s legacy preferences.
Describing herself as a low-income, first-generation Muslim-Pakistani American, Ms. Yasmin wants to use her experience at Tufts to improve accessibility for students like herself.
As a member of the American Medical Association (AMA) Medical Student Section, she coauthored a resolution stating that legacy admissions go against the AMA’s strategic plan to advance racial justice and health equity. The Student Section passed the resolution in November, and in June, the AMA House of Delegates will vote on whether to adopt the policy.
Along with a Supreme Court decision that could strike down race-conscious college admissions, an AMA policy could convince medical schools to rethink legacy admissions and how to maintain diverse student bodies. In June, the court is expected to issue a decision in the Students for Fair Admissions lawsuit against Harvard University, Cambridge, Mass., and the University of North Carolina, Chapel Hill, which alleges that considering race in holistic admissions constitutes racial discrimination and violates the Equal Protection Clause.
Opponents of legacy admissions, like Ms. Yasmin, say it penalizes students from racial minorities and lower socioeconomic backgrounds, hampering a fair and equitable admissions process that attracts diverse medical school admissions.
Diversity of medical applicants
Diversity in medical schools continued to increase last year with more Black, Hispanic, and female students applying and enrolling, according to a recent report by the Association of American Medical Colleges (AAMC). However, universities often include nonacademic criteria in their admission assessments to improve educational access for underrepresented minorities.
Medical schools carefully consider each applicant’s background “to yield a diverse class of students,” Geoffrey Young, PhD, AAMC’s senior director of transforming the health care workforce, told this news organization.
Some schools, such as Morehouse School of Medicine, Atlanta, the University of Virginia School of Medicine, Charlottesville, and the University of Arizona College of Medicine, Tucson, perform a thorough review of candidates while offering admissions practices designed specifically for legacy applicants. The schools assert that legacy designation doesn’t factor into the student’s likelihood of acceptance.
The arrangement may show that schools want to commit to equity and fairness but have trouble moving away from entrenched traditions, two professors from Penn State College of Medicine, Hershey, Pa., who sit on separate medical admissions subcommittees, wrote last year in Bioethics Today.
Legislation may hasten legacies’ end
In December, Ms. Yasmin and a group of Massachusetts Medical Society student-members presented another resolution to the state medical society, which adopted it.
The society’s new policy opposes the use of legacy status in medical school admissions and supports mechanisms to eliminate its inclusion from the application process, Theodore Calianos II, MD, FACS, president of the Massachusetts Medical Society, said in an interview.
“Legacy preferences limit racial and socioeconomic diversity on campuses, so we asked, ‘What can we do so that everyone has equal access to medical education?’ It is exciting to see the students and young physicians – the future of medicine – become involved in policymaking.”
Proposed laws may also hasten the end of legacy admissions. Last year, the U.S. Senate began considering a bill prohibiting colleges receiving federal financial aid from giving preferential treatment to students based on their relations to donors or alumni. However, the bill allows the Department of Education to make exceptions for institutions serving historically underrepresented groups.
The New York State Senate and the New York State Assembly also are reviewing bills that ban legacy and early admissions policies at public and private universities. Connecticut announced similar legislation last year. Massachusetts legislators are considering two bills: one that would ban the practice at the state’s public universities and another that would require all schools using legacy status to pay a “public service fee” equal to a percentage of its endowment. Colleges with endowment assets exceeding $2 billion must pay at least $2 million, according to the bill’s text.
At schools like Harvard, whose endowment surpasses $50 billion, the option to pay the penalty will make the law moot, Michael Walls, DO, MPH, president of the American Medical Student Association (AMSA), said in an interview. “Smaller schools wouldn’t be able to afford the fine and are less likely to be doing [legacy admissions] anyway,” he said. “The schools that want to continue doing it could just pay the fine.”
Dr. Walls said AMSA supports race-conscious admissions processes and anything that increases fairness for medical school applicants. “Whatever [fair] means is up for interpretation, but it would be great to eliminate legacy admissions,” he said.
A version of this article originally appeared on Medscape.com.
Picosecond laser applications continue to expand
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
FROM ASLMS 2023