User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Picosecond laser applications continue to expand
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
PHOENIX – Ever since PicoSure became the first picosecond laser cleared by the Food and Drug Administration for the treatment of unwanted tattoos and pigmented lesions in 2012, new uses for this technology continue to expand.
Now, These include PicoWay, PicoSure, Enlighten, PicoPlus, PiQo4, and Quanta Pico, among others.
“PicoWay technology has integrated nicely into my practice in Houston, the most ethnically diverse city in the country, with its ability to safely treat a number of various benign, congenital, and acquired epidermal and dermal pigmented lesions with ultrashort pulse duration and low thermal impact, which greatly reduces the risk of postinflammatory hyperpigmentation even in darker skin types,” Paul M. Friedman, MD, director of the Dermatology and Laser Surgery Center, Houston, said at the annual conference of the American Society for Laser Medicine and Surgery.
He emphasized the importance of therapeutic clinical endpoints, noting that with q-switched lasers, “you’re looking for immediate whitening, whereas with picosecond lasers, your endpoint is slight whitening or slight darkening depending on wavelength, indication, and skin type. The ability to fractionate picosecond pulses has also allowed us to utilize this technology for photoaging as well as acne scarring.”
The PicoWay system includes a 730-nm picosecond titanium sapphire handpiece, which is FDA cleared for treatment of benign pigmented lesions and blue and green tattoo removal. Dr. Friedman said that he has seen good clinical results using the handpiece for café-au-lait macules, particularly in skin of color.
In an abstract presented at the ASLMS meeting, he and his colleagues presented a retrospective review of 12 patients with café-au-lait macules with Fitzpatrick skin types III-VI who were treated with the PicoWay 730 nm handpiece between April 2021 and January 2023. Patients received a mean of 3.1 treatments at intervals that ranged from 5 to 40 weeks. Clinical photographs were graded by three board-certified dermatologists using a 5-point visual analogue scale.
Overall, patients were rated to have a mean improvement of 26%-50%. Two patients achieved 100% clearance after four to five treatment sessions. “Café-au-lait macules with smooth borders responded less well to laser treatment, confirming prior studies at our center,” he said. “We often educate parents that café-au-lait macules may recur over time, especially with repeated sun exposure.”
Treating melasma
Dr. Friedman’s go-to devices for melasma include the low-density, low-energy 1,927-nm fractional diode laser; the 1,064 nm picosecond Nd:YAG, the low-fluence 1,064 nm Q-switched Nd:YAG with a nanosecond pulse duration, and the 595-nm pulsed dye laser for lesions exhibiting underlying vascularity. He said that combining therapies that target pigment and vasculature may be ideal to prevent relapses. “Melasma is a multifactorial condition so by improving patient education and expectation alongside advances in laser treatment of melasma, we have ultimately improved our ability to treat this condition,” he said.
“We’re approaching it from all angles, with ultraviolet photography and spectrocolorimetry, behavioral modifications, topical skin-lightening agents, broad spectrum sunscreens with protection against visible light, and oral tranexamic acid in advanced cases. Then, we intervene with these energy-based modalities, and the bottom line is, less energy and density is more, with lengthened treatment intervals. In 2023, we’re better than we’ve ever been in terms of our ability to safely and effectively improve melasma.”
Novel lasers
Dr. Friedman also described the UltraClear, a novel ablative fractional 2,910-nm erbium-doped glass fiber laser that delivers a customized blend of ablation and coagulation based on the patient’s condition, skin type, and tolerability for down time. He provided an overview of the versatility of what he described as highly customizable technology for conditions such as photoaging and dyschromia in patients of various skin types, making it a very versatile platform in his practice.
The AVAVA MIRIA system is a “next generation” laser “where you’re able to use a focal point. Basically, you’re treating the skin from the inside out in a 3D manner and you’re able to focus intradermally up to 1 mm with high energy 1,064 nm or 1,550 nm,” he said. “It’s a unique conical geometry that spares the epidermis, combined with sapphire tip cooling and images the skin at the same time with the potential for personalized treatments of dyschromia and photoaging in all skin types. It’s truly remarkable where the technology is heading.”
Dr. Friedman disclosed that he has received consulting fees from Allergan, Galderma, Acclaro, Merz Aesthetics, Solta Medical, and Cytrellis. He has conducted contracted research for Sofwave and is a member of the speakers bureau for Solta Medical and Candela.
FROM ASLMS 2023
Five ways docs may qualify for discounts on medical malpractice premiums
Getting a better deal might simply mean taking advantage of incentives and discounts your insurer may already offer. These include claims-free, new-to-practice, and working part-time discounts.
However, if you decide to shop around, keep in mind that discounts are just one factor that can affect your premium price – insurers look at your specialty, location, and claims history.
One of the most common ways physicians can earn discounts is by participating in risk management programs. With this type of program, physicians evaluate elements of their practice and documentation practices and identify areas that might leave them at risk for a lawsuit. While they save money, physician risk management programs also are designed to reduce malpractice claims, which ultimately minimizes the potential for bigger financial losses, insurance experts say.
“It’s a win-win situation when liability insurers and physicians work together to minimize risk, and it’s a win for patients,” said Gary Price, MD, president of The Physicians Foundation.
Doctors in private practice or employed by small hospitals that are not self-insured can qualify for these discounts, said David Zetter, president of Zetter HealthCare Management Consultants.
“I do a lot of work with medical malpractice companies trying to find clients policies. All the carriers are transparent about what physicians have to do to lower their premiums. Physicians can receive the discounts if they follow through and meet the insurer’s requirements,” said Mr. Zetter.
State insurance departments regulate medical malpractice insurance, including the premium credits insurers offer. Most states cap discounts at 25%, but some go as high as 70%, according to The Doctors Company, a national physician-owned medical malpractice insurer.
Insurers typically offer doctors several ways to earn discounts. The size of the discount also can depend on whether a doctor is new to a practice, remains claims free, or takes risk management courses.
In addition to the premium discount, some online risk management classes and webinars are eligible for CME credits.
“The credits can add up and they can be used for recertification or relicensure,” said Susan Boisvert, senior patient safety risk manager at The Doctors Company.
Here are five ways you may qualify for discounts with your insurer.
1. Make use of discounts available to new doctors
Doctors can earn hefty discounts on their premiums when they are no longer interns or residents and start practicing medicine. The Doctors Company usually gives a 50% discount on member premiums the first year they’re in practice and a 25% discount credit in their second year. The discounts end after that.
Other insurance carriers offer similar discounts to doctors starting to practice medicine. The deepest one is offered in the first year (at least 50%) and a smaller one (20%-25%) the second year, according to medical malpractice brokers.
“The new-to-practice discount is based solely on when the physician left their formal training to begin their practice for the first time; it is not based on claim-free history,” explained Mr. Zetter.
This is a very common discount used by different insurer carriers, said Dr. Price. “New physicians don’t have the same amount of risk of a lawsuit when they’re starting out. It’s unlikely they will have a claim and most liability actions have a 2-year time limit from the date of injury to be filed.”
2. Take advantage of being claims free
If you’ve been claims free for at least a few years, you may be eligible for a large discount.
“Doctors without claims are a better risk. Once a doctor has one claim, they’re likely to have a second, which the research shows,” said Mr. Zetter.
The most common credit The Doctors Company offers is 3 years of being claim free – this earns doctors up to 25%, he said. Mr. Zetter explained that the criteria and size of The Doctors Company credit may depend on the state where physicians practice.
“We allowed insurance carriers that we acquired to continue with their own claim-free discount program such as Florida’s First Professionals Insurance Company we acquired in 2011,” he said.
Doctors with other medical malpractice insurers may also be eligible for a credit up to 25%. In some instances, they may have to be claims free for 5 or 10 years, say insurance experts.
It pays to shop around before purchasing insurance.
3. If you work part time, make sure your premium reflects that
Physicians who see patients part time can receive up to a 75% discount on their medical liability insurance premiums.
The discounts are based on the hours the physician works per week. The fewer hours worked, the larger the discount. This type of discount does not vary by specialty.
According to The Doctors Company, working 10 hours or less per week may entitle doctors to a 75% discount; working 11-20 hours per week may entitle them to a 50% discount, and working 21-30 hours per week may entitle them to a 25% discount. If you are in this situation, it pays to ask your insurer if there is a discount available to you.
4. Look into your professional medical society insurance company
“I would look at your state medical association [or] state specialty society and talk to your colleagues to learn what premiums they’re paying and about any discounts they’re getting,” advised Mr. Zetter.
Some state medical societies have formed their own liability companies and offer lower premiums to their members because “they’re organized and managed by doctors, which makes their premiums more competitive,” Dr. Price said.
Other state medical societies endorse specific insurance carriers and offer their members a 5% discount for enrolling with them.
5. Enroll in a risk management program
Most insurers offer online educational activities designed to improve patient safety and reduce the risk of a lawsuit. Physicians may be eligible for both premium discounts and CME credits.
Medical Liability Mutual Insurance Company, owned by Berkshire Hathaway, operates in New York and offers physicians a premium discount of up to 5%, CME credit, and maintenance of certification credit for successfully completing its risk management program every other year.
ProAssurance members nationwide can earn 5% in premium discounts if they complete a 2-hour video series called “Back to Basics: Loss Prevention and Navigating Everyday Risks: Using Data to Drive Change.”
They can earn one credit for completing each webinar on topics such as “Medication Management: Minimizing Errors and Improving Safety” and “Opioid Prescribing: Keeping Patients Safe.”
MagMutual offers its insured physicians 1 CME credit for completing their specialty’s risk assessment and courses, which may be applied toward their premium discounts.
The Doctors Company offers its members a 5% premium discount if they complete 4 CME credits. One of its most popular courses is “How To Get Rid of a Difficult Patient.”
“Busy residents like the shorter case studies worth one-quarter credit that they can complete in 15 minutes,” said Ms. Boisvert.
“This is a good bargain from the physician’s standpoint and the fact that risk management education is offered online makes it a lot easier than going to a seminar in person,” said Dr. Price.
A version of this article first appeared on Medscape.com.
Getting a better deal might simply mean taking advantage of incentives and discounts your insurer may already offer. These include claims-free, new-to-practice, and working part-time discounts.
However, if you decide to shop around, keep in mind that discounts are just one factor that can affect your premium price – insurers look at your specialty, location, and claims history.
One of the most common ways physicians can earn discounts is by participating in risk management programs. With this type of program, physicians evaluate elements of their practice and documentation practices and identify areas that might leave them at risk for a lawsuit. While they save money, physician risk management programs also are designed to reduce malpractice claims, which ultimately minimizes the potential for bigger financial losses, insurance experts say.
“It’s a win-win situation when liability insurers and physicians work together to minimize risk, and it’s a win for patients,” said Gary Price, MD, president of The Physicians Foundation.
Doctors in private practice or employed by small hospitals that are not self-insured can qualify for these discounts, said David Zetter, president of Zetter HealthCare Management Consultants.
“I do a lot of work with medical malpractice companies trying to find clients policies. All the carriers are transparent about what physicians have to do to lower their premiums. Physicians can receive the discounts if they follow through and meet the insurer’s requirements,” said Mr. Zetter.
State insurance departments regulate medical malpractice insurance, including the premium credits insurers offer. Most states cap discounts at 25%, but some go as high as 70%, according to The Doctors Company, a national physician-owned medical malpractice insurer.
Insurers typically offer doctors several ways to earn discounts. The size of the discount also can depend on whether a doctor is new to a practice, remains claims free, or takes risk management courses.
In addition to the premium discount, some online risk management classes and webinars are eligible for CME credits.
“The credits can add up and they can be used for recertification or relicensure,” said Susan Boisvert, senior patient safety risk manager at The Doctors Company.
Here are five ways you may qualify for discounts with your insurer.
1. Make use of discounts available to new doctors
Doctors can earn hefty discounts on their premiums when they are no longer interns or residents and start practicing medicine. The Doctors Company usually gives a 50% discount on member premiums the first year they’re in practice and a 25% discount credit in their second year. The discounts end after that.
Other insurance carriers offer similar discounts to doctors starting to practice medicine. The deepest one is offered in the first year (at least 50%) and a smaller one (20%-25%) the second year, according to medical malpractice brokers.
“The new-to-practice discount is based solely on when the physician left their formal training to begin their practice for the first time; it is not based on claim-free history,” explained Mr. Zetter.
This is a very common discount used by different insurer carriers, said Dr. Price. “New physicians don’t have the same amount of risk of a lawsuit when they’re starting out. It’s unlikely they will have a claim and most liability actions have a 2-year time limit from the date of injury to be filed.”
2. Take advantage of being claims free
If you’ve been claims free for at least a few years, you may be eligible for a large discount.
“Doctors without claims are a better risk. Once a doctor has one claim, they’re likely to have a second, which the research shows,” said Mr. Zetter.
The most common credit The Doctors Company offers is 3 years of being claim free – this earns doctors up to 25%, he said. Mr. Zetter explained that the criteria and size of The Doctors Company credit may depend on the state where physicians practice.
“We allowed insurance carriers that we acquired to continue with their own claim-free discount program such as Florida’s First Professionals Insurance Company we acquired in 2011,” he said.
Doctors with other medical malpractice insurers may also be eligible for a credit up to 25%. In some instances, they may have to be claims free for 5 or 10 years, say insurance experts.
It pays to shop around before purchasing insurance.
3. If you work part time, make sure your premium reflects that
Physicians who see patients part time can receive up to a 75% discount on their medical liability insurance premiums.
The discounts are based on the hours the physician works per week. The fewer hours worked, the larger the discount. This type of discount does not vary by specialty.
According to The Doctors Company, working 10 hours or less per week may entitle doctors to a 75% discount; working 11-20 hours per week may entitle them to a 50% discount, and working 21-30 hours per week may entitle them to a 25% discount. If you are in this situation, it pays to ask your insurer if there is a discount available to you.
4. Look into your professional medical society insurance company
“I would look at your state medical association [or] state specialty society and talk to your colleagues to learn what premiums they’re paying and about any discounts they’re getting,” advised Mr. Zetter.
Some state medical societies have formed their own liability companies and offer lower premiums to their members because “they’re organized and managed by doctors, which makes their premiums more competitive,” Dr. Price said.
Other state medical societies endorse specific insurance carriers and offer their members a 5% discount for enrolling with them.
5. Enroll in a risk management program
Most insurers offer online educational activities designed to improve patient safety and reduce the risk of a lawsuit. Physicians may be eligible for both premium discounts and CME credits.
Medical Liability Mutual Insurance Company, owned by Berkshire Hathaway, operates in New York and offers physicians a premium discount of up to 5%, CME credit, and maintenance of certification credit for successfully completing its risk management program every other year.
ProAssurance members nationwide can earn 5% in premium discounts if they complete a 2-hour video series called “Back to Basics: Loss Prevention and Navigating Everyday Risks: Using Data to Drive Change.”
They can earn one credit for completing each webinar on topics such as “Medication Management: Minimizing Errors and Improving Safety” and “Opioid Prescribing: Keeping Patients Safe.”
MagMutual offers its insured physicians 1 CME credit for completing their specialty’s risk assessment and courses, which may be applied toward their premium discounts.
The Doctors Company offers its members a 5% premium discount if they complete 4 CME credits. One of its most popular courses is “How To Get Rid of a Difficult Patient.”
“Busy residents like the shorter case studies worth one-quarter credit that they can complete in 15 minutes,” said Ms. Boisvert.
“This is a good bargain from the physician’s standpoint and the fact that risk management education is offered online makes it a lot easier than going to a seminar in person,” said Dr. Price.
A version of this article first appeared on Medscape.com.
Getting a better deal might simply mean taking advantage of incentives and discounts your insurer may already offer. These include claims-free, new-to-practice, and working part-time discounts.
However, if you decide to shop around, keep in mind that discounts are just one factor that can affect your premium price – insurers look at your specialty, location, and claims history.
One of the most common ways physicians can earn discounts is by participating in risk management programs. With this type of program, physicians evaluate elements of their practice and documentation practices and identify areas that might leave them at risk for a lawsuit. While they save money, physician risk management programs also are designed to reduce malpractice claims, which ultimately minimizes the potential for bigger financial losses, insurance experts say.
“It’s a win-win situation when liability insurers and physicians work together to minimize risk, and it’s a win for patients,” said Gary Price, MD, president of The Physicians Foundation.
Doctors in private practice or employed by small hospitals that are not self-insured can qualify for these discounts, said David Zetter, president of Zetter HealthCare Management Consultants.
“I do a lot of work with medical malpractice companies trying to find clients policies. All the carriers are transparent about what physicians have to do to lower their premiums. Physicians can receive the discounts if they follow through and meet the insurer’s requirements,” said Mr. Zetter.
State insurance departments regulate medical malpractice insurance, including the premium credits insurers offer. Most states cap discounts at 25%, but some go as high as 70%, according to The Doctors Company, a national physician-owned medical malpractice insurer.
Insurers typically offer doctors several ways to earn discounts. The size of the discount also can depend on whether a doctor is new to a practice, remains claims free, or takes risk management courses.
In addition to the premium discount, some online risk management classes and webinars are eligible for CME credits.
“The credits can add up and they can be used for recertification or relicensure,” said Susan Boisvert, senior patient safety risk manager at The Doctors Company.
Here are five ways you may qualify for discounts with your insurer.
1. Make use of discounts available to new doctors
Doctors can earn hefty discounts on their premiums when they are no longer interns or residents and start practicing medicine. The Doctors Company usually gives a 50% discount on member premiums the first year they’re in practice and a 25% discount credit in their second year. The discounts end after that.
Other insurance carriers offer similar discounts to doctors starting to practice medicine. The deepest one is offered in the first year (at least 50%) and a smaller one (20%-25%) the second year, according to medical malpractice brokers.
“The new-to-practice discount is based solely on when the physician left their formal training to begin their practice for the first time; it is not based on claim-free history,” explained Mr. Zetter.
This is a very common discount used by different insurer carriers, said Dr. Price. “New physicians don’t have the same amount of risk of a lawsuit when they’re starting out. It’s unlikely they will have a claim and most liability actions have a 2-year time limit from the date of injury to be filed.”
2. Take advantage of being claims free
If you’ve been claims free for at least a few years, you may be eligible for a large discount.
“Doctors without claims are a better risk. Once a doctor has one claim, they’re likely to have a second, which the research shows,” said Mr. Zetter.
The most common credit The Doctors Company offers is 3 years of being claim free – this earns doctors up to 25%, he said. Mr. Zetter explained that the criteria and size of The Doctors Company credit may depend on the state where physicians practice.
“We allowed insurance carriers that we acquired to continue with their own claim-free discount program such as Florida’s First Professionals Insurance Company we acquired in 2011,” he said.
Doctors with other medical malpractice insurers may also be eligible for a credit up to 25%. In some instances, they may have to be claims free for 5 or 10 years, say insurance experts.
It pays to shop around before purchasing insurance.
3. If you work part time, make sure your premium reflects that
Physicians who see patients part time can receive up to a 75% discount on their medical liability insurance premiums.
The discounts are based on the hours the physician works per week. The fewer hours worked, the larger the discount. This type of discount does not vary by specialty.
According to The Doctors Company, working 10 hours or less per week may entitle doctors to a 75% discount; working 11-20 hours per week may entitle them to a 50% discount, and working 21-30 hours per week may entitle them to a 25% discount. If you are in this situation, it pays to ask your insurer if there is a discount available to you.
4. Look into your professional medical society insurance company
“I would look at your state medical association [or] state specialty society and talk to your colleagues to learn what premiums they’re paying and about any discounts they’re getting,” advised Mr. Zetter.
Some state medical societies have formed their own liability companies and offer lower premiums to their members because “they’re organized and managed by doctors, which makes their premiums more competitive,” Dr. Price said.
Other state medical societies endorse specific insurance carriers and offer their members a 5% discount for enrolling with them.
5. Enroll in a risk management program
Most insurers offer online educational activities designed to improve patient safety and reduce the risk of a lawsuit. Physicians may be eligible for both premium discounts and CME credits.
Medical Liability Mutual Insurance Company, owned by Berkshire Hathaway, operates in New York and offers physicians a premium discount of up to 5%, CME credit, and maintenance of certification credit for successfully completing its risk management program every other year.
ProAssurance members nationwide can earn 5% in premium discounts if they complete a 2-hour video series called “Back to Basics: Loss Prevention and Navigating Everyday Risks: Using Data to Drive Change.”
They can earn one credit for completing each webinar on topics such as “Medication Management: Minimizing Errors and Improving Safety” and “Opioid Prescribing: Keeping Patients Safe.”
MagMutual offers its insured physicians 1 CME credit for completing their specialty’s risk assessment and courses, which may be applied toward their premium discounts.
The Doctors Company offers its members a 5% premium discount if they complete 4 CME credits. One of its most popular courses is “How To Get Rid of a Difficult Patient.”
“Busy residents like the shorter case studies worth one-quarter credit that they can complete in 15 minutes,” said Ms. Boisvert.
“This is a good bargain from the physician’s standpoint and the fact that risk management education is offered online makes it a lot easier than going to a seminar in person,” said Dr. Price.
A version of this article first appeared on Medscape.com.
Two phase 3 trials show benefits of dupilumab for prurigo nodularis
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
The results, which were published online in Nature Medicine, were the basis for the FDA approval of dupilumab (Dupixent) for adults with PN in September 2022, the first treatment approved for treating PN in the United States.
“These positive studies support the involvement of type 2 cytokines in driving PN disease pathogenesis and the targeting of the [interleukin]-4/IL-13 axis as a novel therapeutic paradigm for patients with PN,” wrote the researchers, who were led by principal investigator Gil Yosipovitch, MD, professor of dermatology at the University of Miami, Fla. Dupilumab, an IL-4 receptor alpha antagonist, blocks the shared receptor component (IL-4R alpha) for IL-4 and IL-13.
For the two phase 3 trials, which were called LIBERTY-PN PRIME and PRIME2 and were sponsored by Sanofi and Regeneron Pharmaceuticals, researchers randomized adults with PN with 20 or more nodules and severe itch uncontrolled with topical therapies 1:1 to 300 mg dupilumab or placebo subcutaneously every 2 weeks for 24 weeks. The primary endpoint was pruritus improvement, which was measured by the proportion of patients with a 4-point or greater reduction in Worst Itch Numeric Rating Scale (WI-NRS) from baseline at week 24 (PRIME) or week 12 (PRIME2). Key secondary endpoints included a reduction in the number of nodules to 5 or fewer at week 24.
PRIME and PRIME2 enrolled 151 and 160 patients, respectively. In PRIME, 60% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 24, compared with 18.4% of patients in the placebo arm (P < .001). In PRIME2, 37.2% of patients in the dupilumab arm achieved a 4-point or greater reduction in the WI-NRS at week 12, compared with 22% of patients in the placebo arm (P = .022).
The researchers also reported that, from an initial baseline of 20 to greater than 100 nodules, 32.0% of dupilumab-treated patients in PRIME and 25.6% in PRIME2 showed a reduction to 5 nodules or fewer, which corresponded to a response of “clear” or “almost clear” skin at week 12, compared with 11.8% and 12.2% of placebo-treated patients, respectively. This treatment effect on skin lesions continued to improve after week 12, with 48% of dupilumab-treated patients in PRIME and 44.9% in PRIME2 having five nodules or fewer at week 24, compared with 18.4% and 15.9% of placebo-treated patients, respectively. Safety was consistent with the known dupilumab safety profile.
“Validation is the first success of this paper,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study. “While both the safety and efficacy of dupilumab in these two phase 3 programs is the meat of the matter, nuanced highlights for me include the rigid nature of the exclusion criteria to ensure a study population that truly has PN as a stand-alone disease, rather than a secondary finding as we once believed to be the entire story. I think it’s important for us to recognize that it’s not one or the other, rather there is both ‘primary’ prurigo nodularis, and then there is secondary prurigo nodularis associated with something else [a wide range of underlying medical conditions], just like we divide primary and secondary hyperhidrosis.”
Dr. Yosipovitch reported having competing interests with several pharmaceutical companies, including Regeneron and Sanofi. Dr. Friedman disclosed that he is a consultant to and a speaker for Regeneron.
FROM NATURE MEDICINE
Boys may carry the weight, or overweight, of adults’ infertility
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Mohs surgery improves survival in early-stage Merkel cell carcinoma
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
SEATTLE – The use of
Compared with conventional wide local excision, survival was significantly improved among patients treated with Mohs, and a subgroup analysis showed that the survival benefit remained for patients with risk factors.
“At 10 years, overall survival was about 21% higher for those treated with Mohs surgery versus those treated with conventional surgery,” said lead author Shayan Cheraghlou, MD, a dermatology resident at the New York University School of Medicine. “On multivariable analysis, which controlled for tumor and patient factors, Mohs was associated with an over 40% reduction in the hazard for death.”
The findings were presented at the annual meeting of the American College of Mohs Surgery.
MCC is a rare, aggressive, neuroendocrine cutaneous malignancy that carries a high mortality rate. The estimated 5-year survival for patients with localized disease is about 50%, Dr. Cheraghlou noted. “That extrapolates to about 55% for T1 tumors and down to about 30% for T4 tumors.”
Although it’s considered to be a rare cancer, the incidence of MCC has been rapidly rising, and in fact it doubled during the period from the 1990s to the 2010s.
Most commonly treated with wide local excision with or without adjuvant radiation therapy, Mohs as monotherapy may offer an alternative treatment option for patients with MCC. It is generally accepted that the optimal treatment for tumors without regional lymph node involvement is surgical, but the data regarding the optimal surgical approach are mixed. Current National Comprehensive Cancer Network guidelines state that either Mohs surgery or wide local excision can be used.
“However, these guidelines do not indicate a preference for one modality over the other,” said Dr. Cheraghlou, “and present them as interchangeable treatment options.”
A growing body of literature supports Mohs surgery for many types of rare tumors, including MCC. For example, as previously reported at the 2021 ACMS meeting, one study found that Mohs surgery compared favorably with the standard treatment approach when it came to recurrence rates for patients with MCC. The 5-year disease-specific survival rate was 91.2% for patients with stage I disease and 68.6% for patients with stage IIa. These rates were comparable with rates for historical control patients who were treated with wide local excision, with or without radiation (81%-87% for stage I disease, and 63%-67% for stage II).
Study details
In the current study, Dr. Cheraghlou and colleagues sought to evaluate the association of the type of surgical approach with patient survival after excision of early-stage MCC. They conducted a retrospective cohort study using the National Cancer Database to identify cases of MCC with T1/T2 tumors. A total of 2,313 patients who were diagnosed from 2004 to 2018 with pathologically confirmed negative lymph node involvement and who were treated with Mohs surgery or wide lesion excision were included in the analysis.
“About 90% were T1 tumors, about 40% were located on the head and neck, and the vast majority – about 60% – were treated with wide local excision,” he explained. “Only about 5% received Mohs surgery for treatment of the primary tumor.”
But when the researchers assessed survival outcomes, they found that treatment with Mohs surgery was associated with significantly improved overall survival.
The unadjusted 3-, 5-, and 10-year survival rates for patients treated with Mohs was 87.4% (SE: 3.4%), 84.5% (SE: 3.9%), and 81.8% (SE: 4.6%), respectively, while for wide lesion excision, the rates were 86.1% (SE: 0.9%), 76.9% (SE: 1.2%), and 60.9% (SE: 2.0%), respectively.
For patients who underwent treatment with narrow margin excision, survival rates were similar as for those treated with wide lesion excision, with 3-, 5-, and 10-year survival rates of 84.8% (SE: 1.4%), 78.3% (SE: 1.7%), and 60.8% (SE: 3.6%), respectively.
On multivariable survival analysis, Mohs surgery was associated with significantly improved survival, compared with wide lesion excision (hazard ratio, 0.594; P = .038). This was also true after multivariable analysis for patients who had one or more NCCN risk factors, for whom improved survival was also seen with Mohs (HR, 0.530; P = .026).
The results did not differ after a sensitivity analysis that included T3 and T4 tumors.
Given that the use of Mohs was so infrequent, compared with standard surgery, the researchers investigated the factors that were associated with the use of Mohs. High-volume MCC centers were significantly more likely to utilize Mohs than wide lesion excision (odds ratio, 1.993; P < .001), compared with other facilities.
“This study has important implications going forward,” Dr. Cheraghlou concluded. “We think it’s important how few patients were treated with Mohs for Merkel cell, and it was slightly more likely to happen in a high-volume center.”
The reasoning for that may be that high-volume centers are more likely to have a surgeon trained to perform Mohs surgery for MCC. “Or perhaps they are more attuned to the benefits of this procedure,” he said. “We can’t tell that from our data, but its notable that it’s such a small proportion of patients – especially when we consider that it is associated with improved survival for the patients who receive it.”
He added that efforts to increase the utilization of Mohs may yield improved local control and overall survival for these patients. “And perhaps with more data, future versions of guidelines may indicate a preference for Mohs over conventional incisions.”
No changes to current practice
Asked to comment on the study, Anthony J. Olszanski, RPh, MD, associate professor, department of hematology/oncology, Fox Chase Cancer Center, Philadelphia, noted that while the results are intriguing, they must be interpreted with caution.
“This study was retrospective in nature, and unrecognized biases can influence results,” he said. “Additionally, given the relative rarity of Merkel cell carcinoma, the sample size is expectantly small.”
But importantly, Dr. Olszanski emphasized, Mohs may more often have been recommended for patients with lesions that appear less aggressive. “Many patients undergoing wide lesion excision may have been referred by Mohs surgeons secondary to features or characteristics of lesions which were worrisome,” he explained. “The results of this study do not opine on why Mohs would impact overall survival over wide lesion excision, a point worthy of consideration. Presently, both modalities can be considered for patients with T1/T2 MCC. The results of this study should not change current practice and would lend themselves to a more robust study.”
No external funding of the study was reported. Dr. Cheraghlou has disclosed no relevant financial relationships. Dr. Olszanski has received financial support from Merck and BMS for participated on advisory boards.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Study shifts burden of IgG4-related disease to women
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.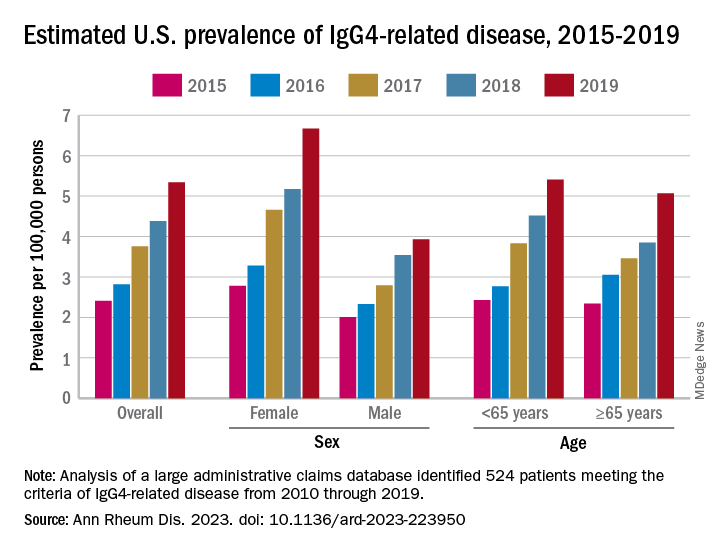
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
The incidence and prevalence of IgG4-related disease each rose considerably from 2015 to 2019 in the United States, and the risk of death in those with the immune-mediated condition is about 2.5 times higher than those who are not affected, based on an analysis of claims data from commercially insured adults.
The first population-based study of IgG4-RD incidence, prevalence, and mortality establishes “key benchmarks for informing the diagnosis and management of patients” with a condition “that causes fibrosing inflammatory lesions at nearly any anatomic site,” and wasn’t initially described until 2001, Zachary S. Wallace, MD, and associates said in Annals of the Rheumatic Diseases.
The increases in incidence and prevalence likely reflected increased disease awareness, they suggested. Overall U.S. incidence was 1.2 per 100,000 person-years for the 5-year period of 2015-2019, rising 86% from 0.78 per 100,000 person-years to 1.45 in 2018 before dropping to 1.39 in 2019. The change in prevalence was even greater, increasing 122% from 2.41 per 100,000 persons in 2015 to 5.34 per 100,000 in 2019, the investigators said.
Previous studies had indicated that the majority of patients with IgG4-RD were male, but the current study, using Optum’s Clinformatics Data Mart, which includes commercial health plan and Medicare Advantage members in all 50 states, showed that both incidence and prevalence (see graph) were higher among women, noted Dr. Wallace of Massachusetts General Hospital, Boston, and associates. They identified 524 patients (57.6% female) in the database who met the criteria for IgG4-RD from Jan. 1, 2010, to Dec. 31, 2019.
Incidence over the course of the study “was similar in patients identified as Asian or White but lower in those identified as Black or Hispanic,” they noted, adding that “the prevalence of IgG4-RD during this period reflected similar trends.” A jump in prevalence from 2018 to 2019, however, left White patients with a much higher rate (6.13 per 100,000 persons) than Asian patients (4.54 per 100,000), Black patients (3.42), and Hispanic patients (3.02).
For the mortality analysis, 516 patients with IgG4-RD were age-, sex-, and race-matched with 5,160 patients without IgG4-RD. Mortality was 3.42 and 1.46 per 100 person-years, respectively, over the 5.5 years of follow-up, so IgG4-RD was associated with a 2.5-fold higher risk of death. “The association of IgG4-RD with a higher risk of death was observed across the age spectrum and among both male and female patients,” the researchers said.
“Clinicians across specialties should be aware of IgG4-RD given the incidence, prevalence, and excess risk of death associated with this condition. ... Additional studies are urgently needed to define optimal management strategies to improve survival,” they wrote.
The study was supported by a grant to Massachusetts General Hospital from Sanofi, and Dr. Wallace received funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Rheumatology Research Foundation. He has received research support and consulting fees from several companies, and four coinvestigators are employees of Sanofi.
FROM ANNALS OF THE RHEUMATIC DISEASES
Axial spondyloarthritis versus axial psoriatic arthritis: Different entities?
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
Are there clinically significant differences between axial spondyloarthritis with psoriasis and psoriatic arthritis with axial symptoms? Does it matter?
It all depends on whom you ask, but right now the evidence seems to be tipping in favor of the “splitters” who cite evidence supporting their contention that axial spondyloarthritis (axSpA)/ankylosing spondylitis (AS) with psoriasis and psoriatic arthritis (PsA) with axial symptoms are distinct clinical entities that require more precise diagnosis and treatment.
“Lumpers,” in contrast, argue that they are different points on the same clinical spectrum.
The debate is not just of academic interest, but has real consequences for patients, say specialists on both sides of the aisle.
Overlapping features, different presentations
“Axial SpA and axPsA have overlapping features but also meaningful differences in genetics, clinical presentation, imaging, and immunophenotype. Efforts are underway to develop classification criteria for axPsA to aid research efforts as well as clinical diagnosis and management,” Philip J. Mease, MD, director of rheumatology research at Swedish Medical Center/Providence–St. Joseph Health in Seattle, and colleagues contend.
In an editorial published in the International Journal of Rheumatic Diseases, Dr. Mease and colleagues noted that, although HLA-B*27 is a genetic risk factor for both axPsA and axSpA, some HLA-B alleles are significantly associated with axPsA, whereas other alleles are associated with axSpA.
In addition, while genes in the interleukin-23 and IL-17 pathway are associated with increased risk for axSpA, genes in the IL-13 pathway have been identified as risk markers for axPsA, they noted.
Two cohorts better than one?
Dafna Gladman, MD, professor of medicine at the University of Toronto and senior scientist at the Schroeder Arthritis Institute at Toronto Western Hospital, and colleagues have a unique perspective on the similarities and differences between the disease entities.
Her group’s research uses data on cohorts of patients treated in two separate clinics at Toronto Western Hospital: one for patients with PsA, and one for patients with axial spondyloarthritis, including those with ankylosing spondylitis, nonradiographic axSpA, and spondylitis associated with inflammatory bowel disease.
“Our work has shown that there are differences, and one of the reasons that it’s now important is that the anti–IL-23 medications, both the IL-12/23 inhibitor ustekinumab [Stelara] and the IL-23 inhibitor guselkumab [Tremfya] work for psoriatic arthritis, whereas IL-23 did not work in ankylosing spondylitis, so that provided further impetus to look into the distinction between the two groups,” Dr. Gladman said in an interview.
Dr. Gladman and colleagues published a study in Rheumatology in which they compared clinical presentations and features of patients with AS with or without psoriasis with patients with axPsA.
They found that patients with AS with or without psoriasis tended to be younger, had a higher proportion of males to females, and were more likely to be positive for HLA-B*27. Patients with AS also had more back pain at presentation, worse axial disease activity scores, worse global assessments by physicians, and higher grades of sacroiliitis, and they were more likely to be taking biologic agents.
“What that showed, right off the top, that whether we’re looking at the total group or we’re looking specifically at those patients who have psoriasis or don’t have psoriasis, they are different from those with psoriatic arthritis with axial disease,” she said.
They concluded that “axPsA seems to be a distinct entity.”
Two clinics, same presentation
Because the aforementioned study included all patients with PsA with or without peripheral disease, the investigators decided to filter out some of the background noise and conduct a second study in which they compared patients who presented to the two clinics with the same presentation, either with spinal disease and psoriasis to the spondylitis clinic, or with psoriasis and isolated axial disease to the PsA clinic.
The results, published in Annals of the Rheumatic Diseases, showed that just 2.03% of patients with PsA had isolated axial disease, and an additional 29.38% had axial and peripheral disease.
In this study, “you can see that even in that group there are distinct differences. The patients that are labeled psoriatic spondylitis are different from those that are labeled ankylosing spondylitis with psoriasis,” Dr. Gladman said.
Isolated axial disease in patients with PsA was associated with HLA-B*27 positivity and lower Health Assessment Questionnaire scores. In addition, patients who were HLA-B*27 positive also had a nearly eightfold higher risk for developing peripheral disease over time.
Patients with isolated axial PsA were significantly more likely to be diagnosed at an older age (mean, 37.44 vs. 29.65 years), had higher Psoriasis Area Severity Index scores and a higher likelihood of having psoriatic nail lesions than patients with AS with isolated axial disease and psoriasis.
In contrast, patients with isolated axSpA with psoriasis were more likely to have inflammatory back pain, spinal pain, joint pain/swelling, and areas of localized tenderness, and they had greater severity of morning stiffness.
Dr. Gladman noted that, although AS and PsA are associated with the same gene that encodes for the IL-23 receptor, each condition is associated with a different single-nucleotide polymorphism.
Same disease, different flavors?
But as Mark Twain said, it is difference of opinion that makes horse races, and some specialists in rheumatology say that axSpA amd axPsA are just two sides of the same coin.
“There are always different schools of thought. I believe that they are not different diseases, but a spectrum of diseases,” said Shailendra Singh, MD, a rheumatologist at Unity Health Medical Center in Searcy, Ark., and past president of the Arkansas Rheumatology Association.
In an interview, Dr. Singh said that the spectrum ranges from diseases with primarily axial involvement, such as AS, to those with primarily peripheral involvement, such as reactive arthritis.
He pointed out that these conditions have overlapping symptoms, including enthesitis, dactylitis, and uveitis, and inflammatory arthritis.
Daniel Wendling, MD, PhD, from the Centre Hospitalier Régional Universitaire de Besançon (France), Université de Franche-Comté, and colleagues agreed.
“The criteria currently available for both SpA [ASAS (Assessment of Spondyloarthritis International Society) criteria] and PsA [CASPAR (Classification for Psoriatic Arthritis) criteria] are classification criteria, not diagnostic criteria. They are not very stringent and are not exclusive. Thus, the same patient can easily be classified simultaneously in both entities, making the distinction between axSpA with psoriasis and axPsA theoretical,” they wrote in an editorial published in Joint Bone Spine.
They cited as an example of the allegedly fuzzy criteria a prospective study conducted by the investigators in Bath, England, in which modified New York criteria for AS were met by 24% of patients with AS, and CASPAR criteria for PsA were met by an equal number of patients with AS.
Therapeutic implications
Dr. Wendling and colleagues acknowledge the differences cited in studies by Dr. Gladman, Dr. Mease, and others between patients with axPsA and those with axSpA, but argue that the differences are not that great and not so clear.
“It should also be emphasized that, although some differences between axPsA and axSpA reach statistical significance, they are mostly at the margin, with low odd ratios,” they wrote.
“It is also important to consider the variability in the definition of axPsA, sometimes simply ‘physician reported’ and elsewhere based on the modified New York radiographic criteria; the latter are only present late in the course of the disease, and this may induce bias,” they continued.
Dr. Singh agreed that, as noted by Dr. Gladman, some patients will respond to anti–IL-17, anti–IL-23, and anti–IL-12/23 agents, whereas others will have better responses with tumor necrosis factor (TNF) inhibitors, and still others, such as those with peripheral involvement in the hands and feet may fare better with nonbiologic disease-modifying antirheumatic drugs such as methotrexate.
Answers to come?
Dr. Gladman noted that the information available to date about the efficacy of IL-23 inhibition in axPsA is based on a post hoc analysis of the PSUMMIT 1 and 2 controlled trials in PsA, and is not definitive.
The randomized, controlled STAR trial, currently recruiting patients, is designed to see whether guselkumab can reduce axial symptoms and inflammation in patients with active axPsA.
“What I say is, there is a rationale for [anti–IL-23] to work in psoriatic arthritis, and not work in ankylosing spondylitis,” she said.
In contrast, IL-17 inhibitors, anti-TNF agents, and Janus kinase inhibitors show efficacy against both axPsA and AS. Rituximab is ineffective against PsA, but has shown efficacy against AS, especially in patients with neurologic complications from anti-TNF agents.
“There may be other medications that would work more specifically in axial psoriatic arthritis that don’t work in ankylosing spondylitis, but at least recognizing that there may be some differences, and that therefore a correct diagnosis should be obtained, might be important,” she said.
Ideally, the picture will become clearer with results from the ongoing Axial Involvement in Psoriatic Arthritis cohort, a joint project of ASAS and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis. The multinational, cross-sectional study is designed “to systematically evaluate clinical and imaging manifestations indicative of axial involvement in patients with PsA and to develop classification criteria and a unified nomenclature for axial involvement in PsA that would allow defining a homogeneous subgroup of patients for research.”
Stay tuned.
Dr. Gladman’s research is supported by a grant from the Krembil Foundation. Dr. Singh disclosed research support from various companies. Funding sources and conflict of interest disclosures from other works cited are contained in their respective references.
1,726-nm lasers poised to revolutionize acne treatment, expert predicts
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
PHOENIX – When Jeffrey Dover, MD, addressed audience members gathered for a session on cutting-edge technologies at the annual conference of the American Society for Laser Medicine and Surgery, he reflected on a conversation he had with R. Rox Anderson, MD, almost 40 years ago, about eventually finding a cure for acne.
“Despite the fact that we have over-the-counter therapies, prescription therapies, and all kinds of devices available to treat acne, there are still barriers to care that get in the way of treatment,” said Dr. Dover, director of SkinCare Physicians in Chestnut Hill, Mass. “If we had a device based on innovative light science that could meet the needs of the acne patient to get rid of these barriers, wouldn’t that be something wonderful?”
The answer to this question, he said, is now “yes,” because of advances in lasers that target sebaceous glands.
In a seminal paper published in 2012, Fernanda H. Sakamoto, MD, PhD, Dr. Anderson, and colleagues demonstrated the potential for a free electron laser to target sebaceous glands . Following several years of refinement, Dr. Dover said.
“With the 1,726-nm laser, there is some selective absorption in sebum in skin, which beats out absorption in the other chromophores,” he said. “But it’s not a big difference like it is, for example, for pulsed-dye lasers and vascular targets. ... This means that the therapeutic window is relatively small and protecting the rest of the epidermis and dermis is crucial to be able to target these lesions or the sebaceous gland without unnecessary damage. If we can protect the epidermis and heat just the sebaceous glands, we should be able to get Accutane-like results if we get durability [by] shrinking sebaceous glands.”
Effective cooling, whether contact cooling, bulk cooling, or air cooling, is crucial to success, he continued. “It’s got to be robust and highly specific to protect the skin, so you don’t end up with side effects that are worse than the disease.”
The AviClear laser delivers seven 3-mm spots, which takes into account the thermal relaxation times of the sebaceous glands. The algorithm delivers a treatment imprint at roughly 0.3 Hz and a 1.5-mm depth of penetration, and the device relies on contact cooling. In pivotal data submitted to the FDA, 104 individuals with moderate to severe acne received three treatments with the AviClear 1 month apart, with follow-up at 1, 3, 6, and 12 months post treatment. They had no other treatment regimens, and the primary endpoint was the percentage of patients who achieved a 50% reduction in inflammatory lesion count 3 months after the final treatment. The secondary endpoint was an Investigator’s Global Assessment (IGA) improvement of 2 or greater.
Dr. Dover, who helped design the study, said that, at 3 months, 80% of those treated achieved a 50% or greater reduction in inflammatory lesion count (P < .001). As for secondary endpoints, 36% of individuals were assessed as having clear or almost clear skin; 47% achieved a 2-point or greater improvement in IGA score, compared with baseline, and 87% achieved a 1-point or greater improvement in IGA score, compared with baseline. By 6 months, 88% of individuals achieved a 50% or greater reduction in inflammatory lesion count; this improved to 92% by 12 months (P < .001).
“All of these procedures were done with no topical anesthetic, no intralesional anesthetic, and they tolerated these quite well,” he said. “There was no down time that required medical intervention after the treatments. All posttreatment erythema and swelling resolved quickly,” and 75% of the patients were “very satisfied” with the treatments.
The Accure Laser System features a proprietary technology that precisely controls thermal gradient depth. “So instead of guessing whether you are delivering the correct amount of heat, it actually tells you,” said Dr. Dover, a past president of the ASLMS and the American Society for Dermatologic Surgery. “It correlates surface and at-depth temperatures, and there’s an infrared camera for real-time accurate temperature monitoring.” The device features highly controlled air cooling and a pulsing pattern that ensures treatment of sebaceous glands of all sizes and at all depths. The clinical end marker is peak epidermal temperature.
In a study supported by Accure, the manufacturer, researchers evaluated the efficacy of the Accure Laser System in 35 subjects with types I to VI skin, who received four monthly treatments 30-45 minutes each, and were followed 12, 26, 39, and 52 weeks following their last treatment. To date, data out to 52 weeks is available for 17 study participants. According to Dr. Dover, the researchers found 80% clearance at 12 weeks following the last treatment, with continued improvement at 52 weeks. One hundred percent of subjects responded. Side effects included erythema, edema, crusting, blisters, and inflammatory papules. “None of these were medically significant,” he said.
As dermatologists begin to incorporate the AviClear and Accure devices into their practices, Dr. Dover said that he is reminded of the conversation he had some 40 years ago with Dr. Anderson about finding a cure for acne, and he feels a bit awestruck. “These 1,726-nm lasers are effective for treating acne. I personally think they are going to revolutionize the way we treat at least some of our patients with acne. They may both be effective for treating facial acne scars. Time will tell. Further study of both scarring and acne are needed to fully categorize the benefit and to optimize treatments.”
To date no direct clinical comparisons have been made between the AviClear and Accure devices.
Dr. Dover reported that he is a consultant for Cutera, the manufacturer for AviClear. He also performs research for the company.
AT ASLMS 2023
Scarred med student inspired by dermatologist who treated her
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”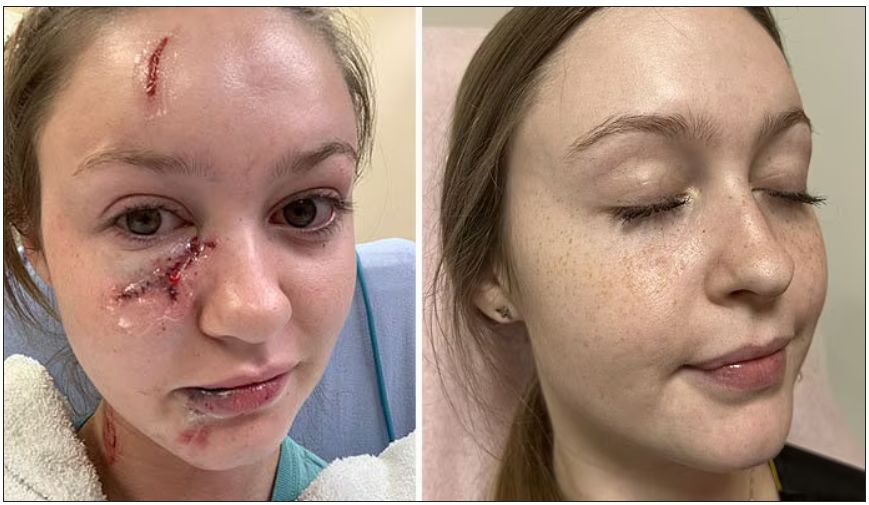
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
It’s not uncommon for a medical student to change specialty plans. For Jamie Harris, a second-year student at the University of Florida School of Medicine, Gainesville, that decision came as the result of a vicious dog and an empathetic doctor.
, a New York dermatologist whose approach involves early and aggressive treatment. After treating her, Dr. Bhanusali offered to have Ms. Harris shadow him.
She returned to school to shadow other dermatologists and to research the specialty before taking Dr. Bhanusali up on his offer. Ms. Harris sat in on procedures and meetings with patients and studied Dr. Bhanusali’s approach to the specialty. “I just fell in love with dermatology,” Ms. Harris told this news organization. “I knew that what I wanted for my own career was exactly how he runs his practice and how he treats patients.”
Life-changing injury
In 2020, Ms. Harris was a sophomore in the University of Florida’s medical honors program, an accelerated track that allows students to earn both a bachelor of science degree and a doctor of medicine degree in 7 years. She had finished studying at a friend’s apartment and was watching television when the rescue dog the friend adopted lunged at Ms. Harris, biting her on the face. “I was just cowering in the corner of the couch,” she recalls. “I didn’t go into fight-or-flight mode; I just went into hide mode.”
After receiving stitches in the emergency department, she visited several dermatologists and plastic surgeons for further treatment. There was scarring from her forehead to her chin, which was particularly severe on her upper cheek just under her eye. But because there was no infection or medical problems, the doctors turned her away. “They said, ‘OK, you look great.’ I did not look great,” she said.
Ms. Harris’ doctors advised her to wait a year before starting treatment for the scarring, a traditional approach. She was frustrated. “At the time, I was interested in becoming a pediatrician and thought, ‘No kid is going to want me as their doctor.’ ” But she accepted the medical advice – until her mother remembered a news story she’d seen.
Bridger Walker, a 6-year-old Wyoming boy, made headlines when he saved his younger sister from a dog that was attacking, but he was bitten multiple times as a result. Dr. Bhanusali treated the boy’s scarring.
Ms. Harris and her mother contacted the doctor, and after meeting via Zoom, Dr. Bhanusali agreed to treat her right away. He used lasers to resurface the skin, which created a suitable foundation for the scar cream, and he administered steroid injections to soften the scar tissue.
‘I see you’
Dr. Bhansali said he was impressed with the young student he treated. “There’s curiosity, and then there’s genuine passion. She has the latter,” he said in an interview. “Having gone through this, she will understand the value of research and keeping up with the literature and that just because something is being done a certain way today doesn’t mean it has to be that way tomorrow.”
Ms. Harris agrees that the experience will make her a better dermatologist. “One of the best parts about dermatology is that you can see your results in real time and really see what’s working and what’s not working. The potential for innovation is just amazing.”
But Ms. Harris believes she also gained empathy with dermatology patients. “I know exactly what it’s like to look in the mirror and not even recognize yourself, just have your eyes go straight to one thing and feel like the whole world is staring at you,” she said. “I’ll be able to reassure people that no matter what their concern is, whether it’s eczema or acne, whether it’s one pimple, I see you, and I know exactly how that feels.”
A version of this article first appeared on Medscape.com.
Contact allergens lurk in diabetes devices
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
in a presentation at the annual meeting of the American Contact Dermatitis Society.
Advanced technologies used for the management of diabetes fall into three main categories, said Dr. Chen, of the department of dermatology, Stanford University, Redwood City, Calif. Continuous glucose monitoring (CGM) devices, which are worn on the body, collect glucose measurements. Continuous subcutaneous insulin infusion (CSII) devices are attached to the body via an infusion set and are now available as tubing-free patch pumps that are attached directly to the skin via a catheter. Glucose-responsive insulin delivery systems combine the sensing and delivery features of the other two types of devices.
Once thought to be rare, reports of skin complications related to diabetes devices have been increasing in recent years, she said. Some reports suggest that at any given time, skin complications may affect as many as one quarter to one half of patients who use these devices, “so this is an important issue,” she emphasized. “Skin reactions are a major factor in device discontinuation, so we as clinicians need to be really proactive about treating these reactions.”
Risk factors for skin complications related to diabetes devices include sensitization to the adhesive used with the devices, as well as prolonged exposure to the device, Dr. Chen said. Younger age also appears to be a risk factor, as is a compromised skin barrier in the area where the device is used.
Unfortunately, obtaining details on the specific adhesives and the raw materials used in these devices, so as to customize patch testing, remains a challenge, she said. “Patch testing initially was often negative to commercially available allergens, even while patients were testing positive to pieces of device adhesive,” she noted.
Consider isobornyl acrylate
An article published in 2017 in Contact Dermatitis was “a major breakthrough” in that it identified isobornyl acrylate (IBOA) as an allergen in connection with the Freestyle Libre, a CGM device that was relatively new at the time. The finding was serendipitous, Dr. Chen said. A patient being treated for suspected allergic contact dermatitis in connection with use of a Freestyle Libre device was tested for IBOA accidentally, after the nurse administering the patch test thought that this was part of the standard acrylate series, she explained.
Subsequently, researchers identified 15 patients who had experienced reactions to the Freestyle Libre; 12 of 13 patients who were patch tested for IBOA tested positive. IBOA was found throughout the device, particularly where the top and bottom plastic components were connected, Dr. Chen said. This suggested that the IBOA was in the device housing and had diffused into the adhesive that attached the device to the skin.
An article published in 2018 in the Journal of Diabetes Science described three patients who developed severe allergic contact dermatitis from IBOA while using a CGM device, Dr. Chen said. The investigators confirmed that there were no reactions to the adhesive itself, again suggesting that IBOA had diffused into the adhesive from other parts of the device.
Although the authors were bound by a confidentiality agreement regarding the individual adhesive components, “the authors noted most of the acrylates in the adhesive were not present in commercially available acrylate series for patch testing,” she said.
IBOA, the ACDS’ Allergen of the Year in 2020, is common in sealants, glues, and adhesives, Dr. Chen said. Although IBOA had been reported infrequently as an allergen, it has now been identified as a “potential culprit” behind skin reactions in many diabetes devices, including CSII and CGM devices, she added.
In addition, N,N-dimethylacrylamide (DMAA) is an allergen that has been identified in several diabetes devices and often occurs with IBOA in medical-grade UV-cured adhesives, Dr. Chen noted. Other allergens identified in diabetes devices include colophony, which is present in many adhesives, as well as other acrylates and epoxy resin.
Diabetes devices are constantly evolving. IBOA is no longer found in Freestyle Libre devices. It is important that clinicians stay up to date with the medical literature and advocate for partnership with device manufacturers, she emphasized.
Patch testing
When diabetes devices are suspected as the source of allergic contact dermatitis, a minimum of a baseline series that contains colophony at a concentration of 20% in petrolatum should be carried out, Dr. Chen said. Commercialized patch test trays, which include plastics, glues, acrylates, epoxy resins/isocyanates, and colophony derivatives, should be ideal. “Personal-care products should be included if they are potentially relevant,” she added.
Dr. Chen shared tables published in Contact Dermatitis in 2021 with examples of screening test series. She said to consider including screening for other allergens more recently discovered in diabetes devices, including 2,2’-methylenebis(6-tert-butyl-4-methylphenol) monoacrylate (MBPA) 1.5% pet; dipropylene glycol diacrylate (DPGDA) 0.1% pet; and butylated hydroxytoluene (BHT) 2% pet.
Testing for monomethyl ether of hydroquinone should also be considered; this may be included in the test preparations for IBOA and DMAA.
Management strategies
For patients who experience skin reactions to their diabetes devices, consideration may given to relocating the device to another area of skin or changing sensors more frequently, according to Dr. Chen.
For some patients, the reaction can be managed with corticosteroid cream, ointment, solution, or nasal spray. Topical antibiotics or topical antihistamines can be helpful, as can barrier dressings, solutions, or sprays, she said. The best solution is to change to a device that does not have the culprit allergen, “but that is difficult, since we don’t know what is in these devices,” she added. Good alternatives include the Eversense CGM device or devices that have been demonstrated not to contain IBOA, such as the Freestyle Libre 2 or the newer version of the Omnipod, an insulin delivery system
Looking ahead, Dr. Chen said that “mandatory labeling is needed, as devices with the same name may have different compositions, depending on the date of manufacture.” Allergens relevant to people with diabetes are constantly evolving, and many are still unidentified, so clinicians and manufacturers need to work together to identify the culprit allergens and their sources, she said.
Dr. Chen has served as principal investigator or subinvestigator for Amgen, AbbVie, and Sanofi Regeneron and as a consultant for Purity Brands.
A version of this article first appeared on Medscape.com.
FROM ACDS 2023