User login
Plasma biomarker distinguishes ARDS, acute heart failure
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Plasma levels of an interleukin-33 receptor that’s involved in inflammation regulation appeared able to discriminate between acute respiratory distress syndrome and acute decompensated heart failure in an analysis with 72 patients.
In a second study, high plasma levels of the same interleukin-33 receptor, known as soluble suppressor of tumorgenicity 2 (sST2), identified acute respiratory distress syndrome (ARDS) patients who were sicker and more responsive to conservative fluid management, Sean D. Levy, MD, said at an international conference of the American Thoracic Society.
In order to assess the ability of sST2 to reliably distinguish patients with ARDS from those with acute decompensated heart failure, he and his associates selected 72 patients seen at the Massachusetts General Hospital in Boston with an initial diagnosis of acute decompensated heart failure accompanied by bilateral lung infiltrates and acute hypoxemia respiratory failure requiring endotracheal intubation and mechanical ventilation. The investigators measured the sST2 level in a plasma specimen from each patient. In addition, after each patient either left the hospital or died, their case underwent review by two critical care physicians who retrospectively rediagnosed the patients as either having ARDS or acute decompensated heart failure. This divided the cohort into 30 patients with ARDS and 42 with true acute heart failure. The two subgroups matched up fairly closely for most clinical measures and comorbidities, but APACHE III (Acute Physiology and Chronic Health Evaluation III) scores averaged significantly higher in the ARDS patients.
The plasma levels of sST2 showed a dramatic split between the two subgroups. The 30 patients retrospectively diagnosed with ARDS had an average level of 386 ng/mL with an interquartile range of 318-611 ng/mL. The 42 acute decompensated heart failure patients averaged a sST2 level of 148 ng/mL, with an interquartile range of 84-225 ng/mL. The area under the receiver operator curve for discriminating between ARDS and acute heart failure using a cutpoint of 271 mg/mL was 0.86, showing “good” discrimination, Dr. Levy said. This cutpoint had a sensitivity of 83% and specificity of 88% for correctly distinguishing between ARDS and acute heart failure.
In a second analysis, Dr. Levy and his associates looked at the ability of sST2 levels to separate out patients with acute lung injury who had a more robust response to either the conservative or liberal fluid-management strategies tested in the Fluid and Catheter Treatment Trial (FACTT), run by the National Heart, Lung, and Blood Institute’s ARDS Clinical Trials Network. The primary outcome of FACTT was death from any cause 60 days after entry, and this showed no significant difference between conservative (restricted fluids and increased urine output) and liberal (the reverse) fluid management strategies in acute lung injury patients (N Engl J Med. 2006 Jun 15;354[14]:2564-75). From among the 1,001 patients enrolled in FACTT, 826 had specimens available for measuring sST2 (Crit Care Med. 2013 Nov;41[11]:2521-31),
The researchers applied the sST2 cut point they derived in the first analysis to the FACTT cohort and identified 133 (16%) patients with a low sST2 level and 693 (84%) with a high level. The patients with high sST2 were sicker, with significantly higher APACHE III scores, worse acidemia, and worse renal function.
Patients with high sST2 levels had a significant increase in ventilator-free days on conservative fluid management, compared with liberal management, while the two management strategies produced virtually identical results in the patients with low levels of sST2. Patients with high sST2 also had a significantly quicker time to extubation on a conservative strategy compared with the liberal strategy, and again this correlation did not exist among patients with low sST2. However, as in the overall trial a conservative strategy had no discernible impact on 60-day mortality, compared with the liberal strategy, even in the subgroup with high sST2.
Dr. Levy had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: An sST2 cutpoint of 271 ng/mL discriminated between ARDS and acute heart failure with 83% sensitivity and 88% specificity.
Data source: Review of 72 patients admitted for acute decompensated heart failure at one U.S. center.
Disclosures: Dr. Levy had no disclosures.
Do sleep interventions prevent atrial fibrillation?
WASHINGTON – If patients have sleep disordered breathing with obstructive sleep apnea, will its treatment have cardiovascular disease benefits, especially in terms of the incidence or severity of atrial fibrillation?
Observational evidence suggests that apnea interventions may help these patients, but no clear case yet exists to prove that a breathing intervention works, experts say, and, as a result, U.S. practice is mixed when it comes to using treatment for obstructive sleep apnea (OSA), specifically continuous positive airway pressure (CPAP), to prevent or treat atrial fibrillation.
“Only a very small number of patients with atrial fibrillation undergo a sleep study,” he said in an interview. “Before I’d send my mother for atrial fibrillation ablation, I would first look for sleep disordered breathing [SDB],” but this generally isn’t happening routinely. Patients with other types of cardiovascular disease who could potentially benefit from sleep disordered breathing diagnosis and treatment are those with hypertension, especially patients who don’t fully respond to three or more antihypertensive drugs and patients with heart failure with preserved ejection fraction, he added.
Dr. Oldenburg also echoed Dr. Mehra in saying that the evidence supporting this approach for managing atrial fibrillation is less than conclusive.
“We need more precise phenotyping of patients” to better focus on patients with cardiovascular disease and sleep disordered breathing who clearly benefit from CPAP intervention, he said.
Results from the Sleep Apnea Cardiovascular Endpoints (SAVE) trial, reported in September 2016, especially tarnished the notion that treating sleep disordered breathing in patients with various cardiovascular diseases can help avoid future cardiovascular events. The multicenter trial enrolled 2,717 adults with moderate to severe obstructive sleep apnea and cardiovascular disease to receive either CPAP plus optimal routine care or optimal routine care only. After an average follow-up of close to 4 years, the patients treated with CPAP showed no benefit in terms of reduced cardiovascular events (N Engl J Med. 2016 Sept 8;375[10]:919-31).
An editorial that ran with this report suggested that the neutral outcome may have occurred because the average nightly duration of CPAP that patients in the trial self administered was just over 3 hours, arguably an inadequate dose. Other possible reasons for the lack of benefit include the time during their sleep cycle when patients administered CPAP (at the start of sleep rather than later) and that CPAP may have a reduced ability to avert new cardiovascular events in patients with established cardiovascular disease (N Engl J Med. 2016 Sept 8;375[8]:994-6).
Regardless of the reasons, the SAVE results, coupled with the neutral results and suggestion of harm from using adaptive servo-ventilation in patients with heart failure with reduced ejection fraction and central sleep apnea in the SERVE-HF trial (N Engl J Med. 2015 Sept 17;373[12]:1095-105), have thrust the management of SDB in patients with cardiovascular disease back to the point where SDB interventions have no well-proven indications for cardiovascular disease patients.
“With the SERVE-HF and SAVE trials not showing benefit, we now have equipoise” for using or not using SDB interventions in these patients, Dr. Mehra said. “It’s not clear that treating OSA improves outcomes. That allows us to randomize patients to a control placebo arm” in future trials.
An important issue in the failure to clearly establish a role for treating OSA in patients with atrial fibrillation or other cardiovascular diseases may have been over reliance on the apnea-hypopnea index (AHI) as the arbiter of OSA severity, Dr. Oldenburg said. “Maybe there are parameters to look at aside from AHI, perhaps hypoxemia burden or desaturation time. AHI is not the whole truth; we need to look at other parameters. AHI may not be the correct metric to look at in patients with various cardiovascular diseases.”
Her analysis also showed that patients with at least 10 minutes of sleep time with an oxygen saturation rate of 90% or less had a 64% increased rate of later atrial fibrillation hospitalizations, compared with those with fewer than 10 minutes spent in this state. Nearly a quarter of the patients studied fell into this category.
“Nocturnal oxygen desaturation may be stronger than AHI for predicting atrial fibrillation development,” Dr. Kendzerska concluded. “The severity of OSA-related intermittent hypoxia may be more important than sleep fragmentation in the development of atrial fibrillation. These findings support a relationship between OSA, chronic nocturnal hypoxemia, and new onset atrial fibrillation.”
However, using oxygen desaturation instead of AHI to gauge the severity of OSA won’t solve all the challenges that sleep researchers currently face in trying to determine the efficacy of breathing interventions to prevent or treat cardiovascular disease. In the neutral SAVE trial, researchers used nocturnal oxygen saturation levels to select patients with clinically meaningful OSA.
Dr. Mehra and Dr. Kendzerska had no disclosures. Dr. Oldenburg has received consultant fees, honoraria, and/or research support from ResMed, Respicardia, and Weinmann.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
This article was updated on 7/10/17.
WASHINGTON – If patients have sleep disordered breathing with obstructive sleep apnea, will its treatment have cardiovascular disease benefits, especially in terms of the incidence or severity of atrial fibrillation?
Observational evidence suggests that apnea interventions may help these patients, but no clear case yet exists to prove that a breathing intervention works, experts say, and, as a result, U.S. practice is mixed when it comes to using treatment for obstructive sleep apnea (OSA), specifically continuous positive airway pressure (CPAP), to prevent or treat atrial fibrillation.
“Only a very small number of patients with atrial fibrillation undergo a sleep study,” he said in an interview. “Before I’d send my mother for atrial fibrillation ablation, I would first look for sleep disordered breathing [SDB],” but this generally isn’t happening routinely. Patients with other types of cardiovascular disease who could potentially benefit from sleep disordered breathing diagnosis and treatment are those with hypertension, especially patients who don’t fully respond to three or more antihypertensive drugs and patients with heart failure with preserved ejection fraction, he added.
Dr. Oldenburg also echoed Dr. Mehra in saying that the evidence supporting this approach for managing atrial fibrillation is less than conclusive.
“We need more precise phenotyping of patients” to better focus on patients with cardiovascular disease and sleep disordered breathing who clearly benefit from CPAP intervention, he said.
Results from the Sleep Apnea Cardiovascular Endpoints (SAVE) trial, reported in September 2016, especially tarnished the notion that treating sleep disordered breathing in patients with various cardiovascular diseases can help avoid future cardiovascular events. The multicenter trial enrolled 2,717 adults with moderate to severe obstructive sleep apnea and cardiovascular disease to receive either CPAP plus optimal routine care or optimal routine care only. After an average follow-up of close to 4 years, the patients treated with CPAP showed no benefit in terms of reduced cardiovascular events (N Engl J Med. 2016 Sept 8;375[10]:919-31).
An editorial that ran with this report suggested that the neutral outcome may have occurred because the average nightly duration of CPAP that patients in the trial self administered was just over 3 hours, arguably an inadequate dose. Other possible reasons for the lack of benefit include the time during their sleep cycle when patients administered CPAP (at the start of sleep rather than later) and that CPAP may have a reduced ability to avert new cardiovascular events in patients with established cardiovascular disease (N Engl J Med. 2016 Sept 8;375[8]:994-6).
Regardless of the reasons, the SAVE results, coupled with the neutral results and suggestion of harm from using adaptive servo-ventilation in patients with heart failure with reduced ejection fraction and central sleep apnea in the SERVE-HF trial (N Engl J Med. 2015 Sept 17;373[12]:1095-105), have thrust the management of SDB in patients with cardiovascular disease back to the point where SDB interventions have no well-proven indications for cardiovascular disease patients.
“With the SERVE-HF and SAVE trials not showing benefit, we now have equipoise” for using or not using SDB interventions in these patients, Dr. Mehra said. “It’s not clear that treating OSA improves outcomes. That allows us to randomize patients to a control placebo arm” in future trials.
An important issue in the failure to clearly establish a role for treating OSA in patients with atrial fibrillation or other cardiovascular diseases may have been over reliance on the apnea-hypopnea index (AHI) as the arbiter of OSA severity, Dr. Oldenburg said. “Maybe there are parameters to look at aside from AHI, perhaps hypoxemia burden or desaturation time. AHI is not the whole truth; we need to look at other parameters. AHI may not be the correct metric to look at in patients with various cardiovascular diseases.”
Her analysis also showed that patients with at least 10 minutes of sleep time with an oxygen saturation rate of 90% or less had a 64% increased rate of later atrial fibrillation hospitalizations, compared with those with fewer than 10 minutes spent in this state. Nearly a quarter of the patients studied fell into this category.
“Nocturnal oxygen desaturation may be stronger than AHI for predicting atrial fibrillation development,” Dr. Kendzerska concluded. “The severity of OSA-related intermittent hypoxia may be more important than sleep fragmentation in the development of atrial fibrillation. These findings support a relationship between OSA, chronic nocturnal hypoxemia, and new onset atrial fibrillation.”
However, using oxygen desaturation instead of AHI to gauge the severity of OSA won’t solve all the challenges that sleep researchers currently face in trying to determine the efficacy of breathing interventions to prevent or treat cardiovascular disease. In the neutral SAVE trial, researchers used nocturnal oxygen saturation levels to select patients with clinically meaningful OSA.
Dr. Mehra and Dr. Kendzerska had no disclosures. Dr. Oldenburg has received consultant fees, honoraria, and/or research support from ResMed, Respicardia, and Weinmann.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
This article was updated on 7/10/17.
WASHINGTON – If patients have sleep disordered breathing with obstructive sleep apnea, will its treatment have cardiovascular disease benefits, especially in terms of the incidence or severity of atrial fibrillation?
Observational evidence suggests that apnea interventions may help these patients, but no clear case yet exists to prove that a breathing intervention works, experts say, and, as a result, U.S. practice is mixed when it comes to using treatment for obstructive sleep apnea (OSA), specifically continuous positive airway pressure (CPAP), to prevent or treat atrial fibrillation.
“Only a very small number of patients with atrial fibrillation undergo a sleep study,” he said in an interview. “Before I’d send my mother for atrial fibrillation ablation, I would first look for sleep disordered breathing [SDB],” but this generally isn’t happening routinely. Patients with other types of cardiovascular disease who could potentially benefit from sleep disordered breathing diagnosis and treatment are those with hypertension, especially patients who don’t fully respond to three or more antihypertensive drugs and patients with heart failure with preserved ejection fraction, he added.
Dr. Oldenburg also echoed Dr. Mehra in saying that the evidence supporting this approach for managing atrial fibrillation is less than conclusive.
“We need more precise phenotyping of patients” to better focus on patients with cardiovascular disease and sleep disordered breathing who clearly benefit from CPAP intervention, he said.
Results from the Sleep Apnea Cardiovascular Endpoints (SAVE) trial, reported in September 2016, especially tarnished the notion that treating sleep disordered breathing in patients with various cardiovascular diseases can help avoid future cardiovascular events. The multicenter trial enrolled 2,717 adults with moderate to severe obstructive sleep apnea and cardiovascular disease to receive either CPAP plus optimal routine care or optimal routine care only. After an average follow-up of close to 4 years, the patients treated with CPAP showed no benefit in terms of reduced cardiovascular events (N Engl J Med. 2016 Sept 8;375[10]:919-31).
An editorial that ran with this report suggested that the neutral outcome may have occurred because the average nightly duration of CPAP that patients in the trial self administered was just over 3 hours, arguably an inadequate dose. Other possible reasons for the lack of benefit include the time during their sleep cycle when patients administered CPAP (at the start of sleep rather than later) and that CPAP may have a reduced ability to avert new cardiovascular events in patients with established cardiovascular disease (N Engl J Med. 2016 Sept 8;375[8]:994-6).
Regardless of the reasons, the SAVE results, coupled with the neutral results and suggestion of harm from using adaptive servo-ventilation in patients with heart failure with reduced ejection fraction and central sleep apnea in the SERVE-HF trial (N Engl J Med. 2015 Sept 17;373[12]:1095-105), have thrust the management of SDB in patients with cardiovascular disease back to the point where SDB interventions have no well-proven indications for cardiovascular disease patients.
“With the SERVE-HF and SAVE trials not showing benefit, we now have equipoise” for using or not using SDB interventions in these patients, Dr. Mehra said. “It’s not clear that treating OSA improves outcomes. That allows us to randomize patients to a control placebo arm” in future trials.
An important issue in the failure to clearly establish a role for treating OSA in patients with atrial fibrillation or other cardiovascular diseases may have been over reliance on the apnea-hypopnea index (AHI) as the arbiter of OSA severity, Dr. Oldenburg said. “Maybe there are parameters to look at aside from AHI, perhaps hypoxemia burden or desaturation time. AHI is not the whole truth; we need to look at other parameters. AHI may not be the correct metric to look at in patients with various cardiovascular diseases.”
Her analysis also showed that patients with at least 10 minutes of sleep time with an oxygen saturation rate of 90% or less had a 64% increased rate of later atrial fibrillation hospitalizations, compared with those with fewer than 10 minutes spent in this state. Nearly a quarter of the patients studied fell into this category.
“Nocturnal oxygen desaturation may be stronger than AHI for predicting atrial fibrillation development,” Dr. Kendzerska concluded. “The severity of OSA-related intermittent hypoxia may be more important than sleep fragmentation in the development of atrial fibrillation. These findings support a relationship between OSA, chronic nocturnal hypoxemia, and new onset atrial fibrillation.”
However, using oxygen desaturation instead of AHI to gauge the severity of OSA won’t solve all the challenges that sleep researchers currently face in trying to determine the efficacy of breathing interventions to prevent or treat cardiovascular disease. In the neutral SAVE trial, researchers used nocturnal oxygen saturation levels to select patients with clinically meaningful OSA.
Dr. Mehra and Dr. Kendzerska had no disclosures. Dr. Oldenburg has received consultant fees, honoraria, and/or research support from ResMed, Respicardia, and Weinmann.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
This article was updated on 7/10/17.
EXPERT ANALYSIS FROM ATS 2017
Frequent bronchiectasis exacerbations linked to higher mortality
WASHINGTON – Bronchiectasis patients with three or more exacerbations per year had twice the mortality during 5-year follow-up as patients with no recent exacerbations, in a prospective registry of nearly 2,600 European bronchiectasis patients.
A multivariate analysis showed this statistically significant doubled death rate after adjustment for baseline demographic and clinical differences between patients with no exacerbations during the year before they entered the registry, James D. Chalmers, MD, said at an international conference of the American Thoracic Society.
This 37% prevalence contrasted with a 19% U.S. prevalence of bronchiectasis patients having two or more exacerbations per year among 2,114 patients enrolled in a 13-center U.S. registry that was reported during the same session by Timothy R. Aksamit, MD, a pulmonologist at the Mayo Clinic in Rochester, Minn. During his talk, Dr. Aksamit suggested that the European cohort might include more patients with asthma, chronic obstructive pulmonary disease, or other complications in addition to bronchiectasis. Dr. Aksamit contended that the U.S. registry tried to exclusively enroll patients with bronchiectasis and no other disorder, possibly explaining the prevalence difference between Europe and the United States.
The European registry included patients with bronchiectasis seen in 10 centers in seven European countries and Israel. They averaged 67 years of age. While more than a third had a history of at least three exacerbations a year, one-quarter had no exacerbations during the year before they entered the study.
The U.S. registry reported by Dr. Aksamit had 2-year follow-up data for 1,049 of the enrolled patients, a subgroup that closely matched the entire population initially enrolled. The 2-year follow-up showed an overall average exacerbation rate of 0.75 episodes per year, but this was driven largely by the subgroup of patients who entered the registry with a history of two or more exacerbations per year, who then averaged about 2.6 exacerbations during follow-up. In contrast, patients who entered the registry with a history of fewer than two exacerbations per year averaged fewer than a third of an exacerbation per year during follow-up, Dr. Aksamit reported.
The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Bronchiectasis patients with three or more exacerbations per year had twice the mortality during 5-year follow-up as patients with no recent exacerbations, in a prospective registry of nearly 2,600 European bronchiectasis patients.
A multivariate analysis showed this statistically significant doubled death rate after adjustment for baseline demographic and clinical differences between patients with no exacerbations during the year before they entered the registry, James D. Chalmers, MD, said at an international conference of the American Thoracic Society.
This 37% prevalence contrasted with a 19% U.S. prevalence of bronchiectasis patients having two or more exacerbations per year among 2,114 patients enrolled in a 13-center U.S. registry that was reported during the same session by Timothy R. Aksamit, MD, a pulmonologist at the Mayo Clinic in Rochester, Minn. During his talk, Dr. Aksamit suggested that the European cohort might include more patients with asthma, chronic obstructive pulmonary disease, or other complications in addition to bronchiectasis. Dr. Aksamit contended that the U.S. registry tried to exclusively enroll patients with bronchiectasis and no other disorder, possibly explaining the prevalence difference between Europe and the United States.
The European registry included patients with bronchiectasis seen in 10 centers in seven European countries and Israel. They averaged 67 years of age. While more than a third had a history of at least three exacerbations a year, one-quarter had no exacerbations during the year before they entered the study.
The U.S. registry reported by Dr. Aksamit had 2-year follow-up data for 1,049 of the enrolled patients, a subgroup that closely matched the entire population initially enrolled. The 2-year follow-up showed an overall average exacerbation rate of 0.75 episodes per year, but this was driven largely by the subgroup of patients who entered the registry with a history of two or more exacerbations per year, who then averaged about 2.6 exacerbations during follow-up. In contrast, patients who entered the registry with a history of fewer than two exacerbations per year averaged fewer than a third of an exacerbation per year during follow-up, Dr. Aksamit reported.
The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Bronchiectasis patients with three or more exacerbations per year had twice the mortality during 5-year follow-up as patients with no recent exacerbations, in a prospective registry of nearly 2,600 European bronchiectasis patients.
A multivariate analysis showed this statistically significant doubled death rate after adjustment for baseline demographic and clinical differences between patients with no exacerbations during the year before they entered the registry, James D. Chalmers, MD, said at an international conference of the American Thoracic Society.
This 37% prevalence contrasted with a 19% U.S. prevalence of bronchiectasis patients having two or more exacerbations per year among 2,114 patients enrolled in a 13-center U.S. registry that was reported during the same session by Timothy R. Aksamit, MD, a pulmonologist at the Mayo Clinic in Rochester, Minn. During his talk, Dr. Aksamit suggested that the European cohort might include more patients with asthma, chronic obstructive pulmonary disease, or other complications in addition to bronchiectasis. Dr. Aksamit contended that the U.S. registry tried to exclusively enroll patients with bronchiectasis and no other disorder, possibly explaining the prevalence difference between Europe and the United States.
The European registry included patients with bronchiectasis seen in 10 centers in seven European countries and Israel. They averaged 67 years of age. While more than a third had a history of at least three exacerbations a year, one-quarter had no exacerbations during the year before they entered the study.
The U.S. registry reported by Dr. Aksamit had 2-year follow-up data for 1,049 of the enrolled patients, a subgroup that closely matched the entire population initially enrolled. The 2-year follow-up showed an overall average exacerbation rate of 0.75 episodes per year, but this was driven largely by the subgroup of patients who entered the registry with a history of two or more exacerbations per year, who then averaged about 2.6 exacerbations during follow-up. In contrast, patients who entered the registry with a history of fewer than two exacerbations per year averaged fewer than a third of an exacerbation per year during follow-up, Dr. Aksamit reported.
The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: Patients with frequent exacerbations had twice the mortality rate, compared with bronchiectasis patients with no recent exacerbations.
Data source: A registry of 2,596 bronchiectasis patients from 10 centers in Europe and Israel.
Disclosures: The European bronchiectasis registry was partially funded by Bayer. Dr. Chalmers has been a consultant to Bayer and to AstraZeneca, Basilea, Grifols, Napp, and Raptor and has received research funding from Aradigm, AstraZeneca, Bayer, GlaxoSmithKline, and Pfizer. Dr. Aksamit had no disclosures.
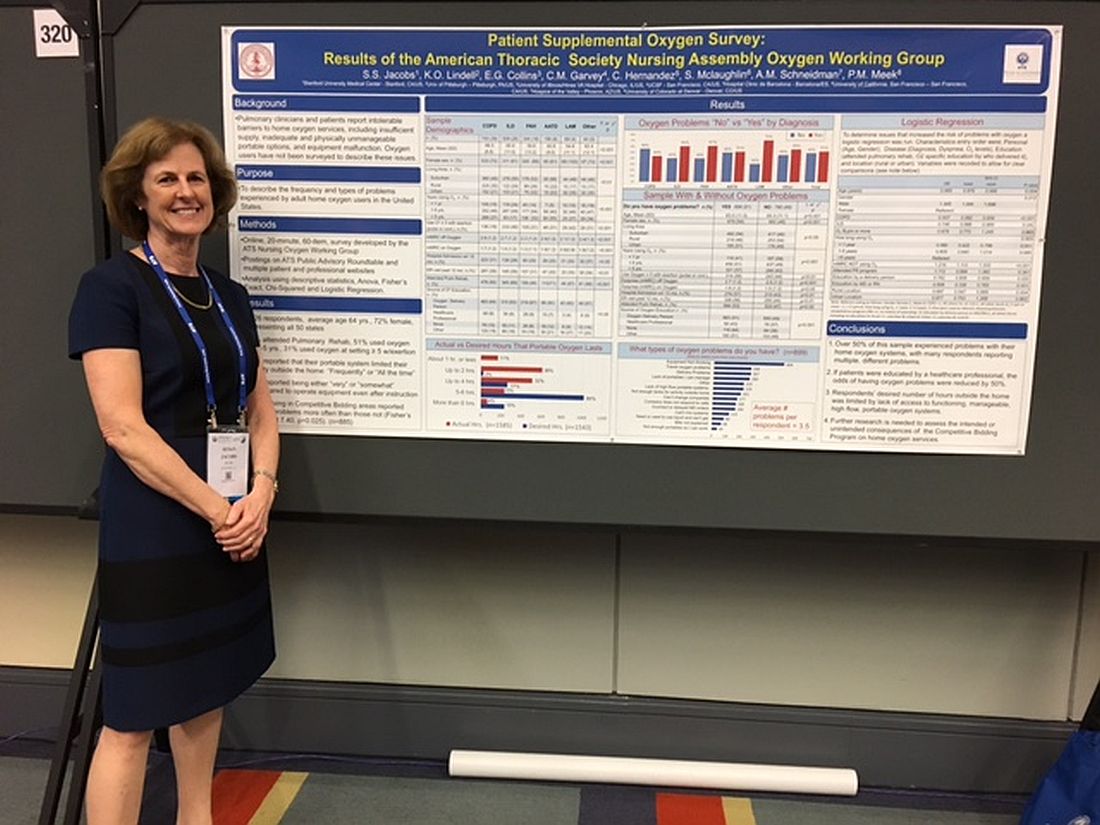
Patients report issues with home O2
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
WASHINGTON – Patient education in the use of home oxygen halves the number of system use issues reported by patients, based on results of a survey of nearly 2,000 patients.
Pulmonary clinicians and patients report “intolerable barriers to home oxygen services,” lead researcher Susan S. Jacobs, RN, MS, said in a poster session at an international conference of the American Thoracic Society. These barriers include insufficient oxygen supply, inadequate and physically unmanageable portable options, and equipment malfunction.
“We’ve demonstrated that, if the patients are educated by a health care professional, the problems with oxygen go down, Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University, said in an interview. “While physicians can provide oxygen for their patients, the patient oxygen education will most likely lie with the nurses and respiratory therapists.”
Of patients who responded to the survey question "Do you have oxygen problems?" 51% (899) said yes*. On average, these patients said they had experienced 3.5 types of problems with their systems.
Patients who were educated by a health care professional reported fewer problems and were more likely to report having no problems with their oxygen system. Of the patients who received oxygen therapy instruction from a health care professional, 76 (57%) did not report having any issues with their system. In contrast, of the patients who received no instruction, 116 (64%) said they had problems with their oxygen.
Most survey participants (1,113 patients) received oxygen therapy instruction from an oxygen delivery person instead of a health care professional. This group’s opinions about their oxygen systems were split, with 51% (563 patients) experiencing issues with their systems. The other 49% reported no problems.
Survey participants most frequently complained that their equipment was not working; 499 selected this response to the question, “What types of oxygen problems do you have?”
Many patients also reported being unable to spend as much time out of their homes as they wanted. This limitation resulted from their lack of access to functioning, manageable, high flow, portable oxygen systems, according to the researchers. Further, 43% of patients reported that their portable system limited their activity outside the home frequently or all of the time.
“Most of the reported problems were related to respondents not having portable systems that let them be out of their house for more than 2 to 4 hours or [to systems that] were too heavy for the patients to lift up and down their stairs and out of their cars, and they had problems operating them,” said Ms. Jacobs, who is a nurse coordinator in the division of pulmonary and critical care medicine at Stanford (Calif.) University.
The survey respondents also reported experiencing delivery problems, not being able to change the company providing them with oxygen, receiving incorrect or delayed orders from a physician, or being unable to get liquid oxygen. These responses were provided by 267, 177, 166, and 68 patients, respectively.
“There is a lot of confusion for the physicians as well as the nurses about what types of systems the patients can use [and] the pros and cons of each system. There’s lots of confusion and time spent about getting the initial orders right, getting them set up with a supplier, and ensuring the patient gets the equipment that was ordered. There is a lot of back and forth, which results in a delay to the patient, and the patients are upset because they are waiting for their oxygen supply,” she explained. “So, I think that physicians are very much wanting clarification to streamline the process and identify what patient systems are appropriate, which are high flow, [and] what their patients’ needs are to help physicians spend less time on this and help the patients get their oxygen set up in a timely manner.”
The study participants came from all 50 states and were 64 years of age on average and mostly women. A high percentage (39%) of the sample had chronic obstructive pulmonary disease, while 26% had interstitial lung diseases, 18% had pulmonary arterial hypertension, 8% had alpha-1 antitrypsin deficiency, and 4% had lymphangioleiomyomatosis.
Ms. Jacobs noted that she thought patients would benefit from greater physician knowledge of their prescribing options.
“A physician can dictate exactly what system they want. ... You can try to give [patients] a lighter system, a backpack, a smaller tank, more tanks per week, depending on their lifestyle and their needs. But physicians, a lot of times, like all of us and our patients, [are] not aware of all these choices,” she said, during the interview.
An online resource providing all of the pros and cons of the different types of portable oxygen systems that would be appropriate for physicians, nurses, and patients, as well as an examination of the quality standards of the oxygen suppliers, are needed, she noted
Ms. Jacobs reported no financial disclosures.
*This article was corrected June 16, 2017
AT ATS 2017
Key clinical point:
Major finding: Patients reported experiencing an average of 3.5 types of problems with their home oxygen systems.
Data source: An analysis of 1,926 home-oxygen users’ responses to an online, 60-question survey.
Disclosures: Ms. Jacobs reported no financial disclosures.
Angiotensin II may improve vasopressors’ efficacy
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – Adding angiotensin II to available vasopressor therapies correlated with significantly improved arterial pressure in patients with catecholamine-resistant vasodilatory shock and less adverse effects, according to a study presented at the recent international conference of the American Thoracic Society.
In a double blind, controlled, phase III study, 70% of 163 patients given angiotensin II reached arterial pressure of at least 75 mm HG or improved by at least 10 mm Hg three hours later, compared with 23.4% of the 158 patients given a placebo (P less than .001).
Those in the angiotensin II group also saw a mean pressure increase of 12.5 mm Hg in the first 3 hours after initiating treatment, compared with 2.9 mm Hg in the placebo group (P less than .001), according to Ashish Khanna, MD, FCCP, of the Cleveland Clinic, and his fellow researchers (N Engl J Med. 2017 May 21. doi: 10.1056/NEJMoa1704154).
Current vasopressor therapies for vasodilatory patients are associated with dangerous side effects and a 30-day mortality rate of more than 50%, which is a major concern for patients who do not have many options to begin with, the researchers noted.
“Treatment options for patients with catecholamine-resistant vasodilatory shock are limited, and the treatments that are available are often associated with side effects,” said Dr. Khanna and his colleagues.
The researchers added the naturally occurring peptide hormone angiotensin II to vasodilatory patients’ treatment regimen in order to “more closely [mimic] natural physiologic responses to shock, which include increased secretion of catecholamines, vasopressin, and RAAS hormones.”
To test the efficacy of angiotensin II, researchers gathered patients with a median age of 64 years and a mean arterial pressure of 66.3 mm Hg.
Sepsis was the predominant cause of shock for 80.7% of the study’s participants.
Patients were injected with either 20 ng/kg of body weight per minute of angiotensin II or an equivalent dose of a placebo until mean arterial pressure reached 75 mm Hg. After 3 hours and 15 minutes of treatment, the dosages were adjusted to keep pressure between 65 and 75 mm Hg for the next 48 hours.
Among patients in the angiotensin II group, 67% of patients were able to decrease angiotensin II and vasopressor doses within 30 minutes of injection, according to researchers.
When researchers measured improvement using the cardiovascular Sequential Organ Failure Assessment, patients in the angiotensin II group saw an average decrease of 1.75 points, compared with 1.28 points in patients in the placebo group (P = .01) 48 hours after treatment.
The Sequential Organ Failure Assessment is scaled from 0-4, with higher scores indicating more severe organ failure.
When measuring for adverse affects, serious effects occurred in 60.7% of the angiotensin II patients, compared with in 67.1% of those in the placebo group.
At the 28-day mark, 75 angiotensin II patients (46.0%) died, compared with 85 patients (53.8%) of the placebo group.
This study was limited by the small sample size, “so the possibility of clinically important side effects attributable to angiotensin II therapy cannot be exuded,” the researches warned.
Also, the follow-up timeline of 28 days, may not have given researchers enough time to uncover the full extent of positive and negative long-term effects associated with angiotensin II.
This study was supported by La Jolla Pharmaceutical, from which multiple researchers reported receiving financial support in the form of personal fees and grants. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
At ATS 2017
Key clinical point:
Major finding: In the first 3 hours, patients taking angiotensin II improved arterial pressure by an average of 12.5 mm Hg, compared with 2.9 mm Hg in patients taking the placebo (P less than .001)
Data source: Double blind, randomized, control trial of 321 patients with catecholamine-resistant vasodilatory shock collected from 75 intensive care units globally during May 2015-January 2017.
Disclosures: Multiple investigators reported receiving support from La Jolla Pharmaceutical and similar companies in the form of grants and/or personal fees. Two of the researchers reported having patents related to administering angiotensin II and additional patents pending.
Patients’ surrogate decision-makers face stress
WASHINGTON – Making health care–related decisions for relatives in the ICU heightens an individual’s risk for psychological stress, a new study showed.
While 90% of patients admitted to the ICU lack decision capacity, only 20%-29% of the U.S. population has written advanced directives. Therefore, most often, family members are called upon to make decisions for ICU patients’ care.
Sean Cohen, MD, of the University of Washington presented the study’s results in a mini symposium at an international conference of the American Thoracic Society in May.
In this cohort study, 265 patients with acute respiratory distress syndrome and 162 of these patients’ family members were surveyed 3 months after each patient’s discharge. Associations between the family members’ roles and psychological symptoms were examined with regression models. These models were adjusted for family characteristics, which included sex, age, education, and legal next-of-kin status. Results showed that the patients’ family members were on average 52 years of age and that 94% were white, 73% were female, and 64% were legal next-of-kin, mostly spouses or children of the patients.
“I think looking more at identification of the decision-making role and attempting to go toward the surrogates’ preferred role actually mitigates some of these psychological symptoms,” concluded Dr. Cohen.
This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other disclosures.
WASHINGTON – Making health care–related decisions for relatives in the ICU heightens an individual’s risk for psychological stress, a new study showed.
While 90% of patients admitted to the ICU lack decision capacity, only 20%-29% of the U.S. population has written advanced directives. Therefore, most often, family members are called upon to make decisions for ICU patients’ care.
Sean Cohen, MD, of the University of Washington presented the study’s results in a mini symposium at an international conference of the American Thoracic Society in May.
In this cohort study, 265 patients with acute respiratory distress syndrome and 162 of these patients’ family members were surveyed 3 months after each patient’s discharge. Associations between the family members’ roles and psychological symptoms were examined with regression models. These models were adjusted for family characteristics, which included sex, age, education, and legal next-of-kin status. Results showed that the patients’ family members were on average 52 years of age and that 94% were white, 73% were female, and 64% were legal next-of-kin, mostly spouses or children of the patients.
“I think looking more at identification of the decision-making role and attempting to go toward the surrogates’ preferred role actually mitigates some of these psychological symptoms,” concluded Dr. Cohen.
This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other disclosures.
WASHINGTON – Making health care–related decisions for relatives in the ICU heightens an individual’s risk for psychological stress, a new study showed.
While 90% of patients admitted to the ICU lack decision capacity, only 20%-29% of the U.S. population has written advanced directives. Therefore, most often, family members are called upon to make decisions for ICU patients’ care.
Sean Cohen, MD, of the University of Washington presented the study’s results in a mini symposium at an international conference of the American Thoracic Society in May.
In this cohort study, 265 patients with acute respiratory distress syndrome and 162 of these patients’ family members were surveyed 3 months after each patient’s discharge. Associations between the family members’ roles and psychological symptoms were examined with regression models. These models were adjusted for family characteristics, which included sex, age, education, and legal next-of-kin status. Results showed that the patients’ family members were on average 52 years of age and that 94% were white, 73% were female, and 64% were legal next-of-kin, mostly spouses or children of the patients.
“I think looking more at identification of the decision-making role and attempting to go toward the surrogates’ preferred role actually mitigates some of these psychological symptoms,” concluded Dr. Cohen.
This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other disclosures.
AT ATS 2017
Key clinical point:
Major finding: Patients’ family members’ active involvement in the health care decision-making process are more likely to experience symptoms of PTSD (OR, 4.43), depression (OR, 1.80), and anxiety (OR, 1.11; P less .05 for all).
Data source: Cohort study of 265 patients with ARDS and 162 family members of the patients.
Disclosures: This study was sponsored by the Cambia Palliative Care Center of Excellence at the University of Washington in Seattle. Funding was provided by the National Institutes of Health and the American Lung Association. The authors reported no other financial disclosures.
Lung cancer linked to suicide
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – U.S. patients diagnosed with lung cancer have had the highest suicide rates among patients diagnosed with any of the other most common, non-skin cancers, and they also had a substantially higher suicide risk, compared with the general U.S. adult population, based on U.S. national data collected during 1973-2013.
Although U.S. lung cancer patients showed a “steep” decline in suicide rates starting in about 1985 that then accelerated beginning in the mid-1990s, as recently as 2010-2013 the rate was roughly twice as high in lung cancer patients when compared with the general U.S. adult population. The rate of lung cancer patients taking their lives was also significantly above the suicide rates among patients with breast, colorectal, or prostate cancer, Mohamed Rahouma, MD, reported at an international conference of the American Thoracic Society.
However, he also stressed that identification of lung cancer patients at especially high suicide risk was important to allow “proper psychological assessment, support, and counseling to reduce [suicide] rates.”
Lung cancer patients with the highest rates included men, widowed individuals, septuagenarians, and Asians, his analysis showed. Standardized mortality ratios (SMRs) for suicide of these highest-risk subgroups were near or exceeding 10 times fold higher than the suicide rates of comparable demographic groups among the general U.S. adult population, according to Dr. Rahouma and his associates.
The overall SMR for all lung cancer patients during the entire four decades studied, compared with the overall U.S. adult population, was 4. Even during the period 2005-2013, when suicide among lung cancer patients had fallen to its lowest level, the SMR for this group was still more than 2.
The investigators used data collected by the U.S. Surveillance Epidemiology and End Results (SEER) Program cancer database maintained by the National Cancer Institute. For suicide rates among the general U.S. population they used data from the National Vital Statistics Reports produced by the Centers for Disease Control and Prevention. The SEER database included entries for more than 3.6 million U.S. cancer patients during 1973-2013, of whom 6,661 patents had committed suicide, an overall SMR of 1.6.
When the researchers drilled down the SMRs for individual cancer types they found that while the SMR for lung cancer patients throughout the period studied was just above 4, the SMRs for breast and colorectal cancer patients were both 1.4, and 1.2 for patients with prostate cancer. This analysis adjusted for patients’ age, sex, race, and year of diagnosis, Dr. Rahouma reported.
The time from diagnosis to suicide was also strikingly quicker among lung cancer patients, at an average of 8 months, compared with average delays from diagnosis to suicide of 40-60 months for patients with breast, colorectal, or prostate cancer. Dr. Rahouma’s time-trend analysis showed that the SMRs for these three other cancer types held more or less steady within the range of 1-2 throughout the 4 decades examined, and by 2010-2013 the three SMRs all were at or just above 1. Lung cancer was the only malignancy in this group that showed a wide range in SMR over time, with the peak some 30-40 years ago.
Among the lung cancer patient subgroups that showed the highest SMRs for suicide during the entire period studied, men had a SMR of 9, Asians had a SMR of nearly 14, those with a deceased spouse had a SMR for suicide of almost 12, and septuagenarians had a SMR of 12, said Dr. Rahouma. The impact of these risk factors was greatest during the first 8 months following lung cancer diagnosis. After 8 months, the strength of the risk factors diminished, with the SMRs within each risk category dropping by roughly half.
The highest-risk subgroups that the analysis identified should especially be referred for psychiatric support, Dr. Rahouma concluded. “These data will change our practice” at Cornell, he predicted.
Dr. Rahouma had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: During 1973-2013, suicide among U.S. lung cancer patients was four times higher than the general adult U.S. population.
Data source: Statistics on more than 3.6 million U.S. cancer patients in the SEER Program.
Disclosures: Dr. Rahouma had no disclosures.
Noninvasive therapy cut COPD readmissions
WASHINGTON – The addition of noninvasive ventilation to home oxygen therapy regimens correlated with increased time to readmission or death among patients with exacerbated chronic obstructive pulmonary diseases (COPD), according to a study presented at an international conference of the American Thoracic Society.
Among 116 patients observed with COPD, the 57 patients given home oxygen and noninvasive ventilation reported an average time to readmission of 4.3 months, compared with 1.4 months among the 59 patients given only home oxygen, according to Patrick B. Murphy, PhD, of St. Thomas’ Hospital, London (JAMA. 2017 May 21. doi: 10.1001/jama.2017.4451), who presented this research on the same day it was published in JAMA.
Dr. Murphy said the findings are encouraging for patients with COPD suffering from exacerbations from the disease.
“Patients with established chronic respiratory failure secondary to COPD have poor outcomes with limited treatment options available,” the investigators noted. “The results of the current trial are reassuring, suggesting that home noninvasive ventilation added to home oxygen therapy in this population improved the overall clinical outcome without adding to the health burden of the patient.”
In this 12-month, phase III, multicenter, randomized clinical trial, the average age of the patients was 67 years, and the average body mass index was 21.6 mg/k2. The patients had an average partial pressure of carbon dioxide (PaCo2) level of 59, indicating persistent hypercapnia.
The investigators gave those in the intervention group one of three noninvasive home ventilators – nasal, oronasal, or total face mask – to use for a minimum of 6 hours nightly. Patients in both groups received 15 hours of oxygen therapy daily.
Doctors gathered data from patients after 6 weeks, 3 months, 6 months, and 12 months.
After 12 months, risk of readmission or death in the intervention group was 63.4%, while those in the oxygen-only group reported a risk of 80.4%. Despite a 17% risk reduction, a similar number of patients died during the experiment in both groups: five in the noninvasive intervention group and four in the control group, according to the investigators.
At the end of the trial, 16 patients (28%) in the intervention group and 19 (32%) in the control group died.
Dr. Murphy and his peers asserted that these deaths do not take away from the success of the treatment, as the focus of the study was to find a way to reduce readmissions, not necessarily mortality.
“The driver of the clinical improvement in the home oxygen therapy plus home noninvasive ventilation group was readmission avoidance with no significant difference in mortality,” they wrote. “This study has major clinical relevance because readmission avoidance is beneficial to the patient in terms of preservation of lung function and health-related quality of life, as well as providing a direct and indirect cost saving.”
The study was limited by the lack of a double-blind design; however, investigators said that a sham device may have made patients’ respiratory failure worse.
Some patients in the control group were later given ventilation treatment for safety reasons. Eighteen patients were given noninvasive ventilation, although reportedly after the primary endpoint was reached.
Philips Respironics, ResMed, the ResMed Foundation, and the Guy’s and St. Thomas’ Charity funded the study. Dr. Murphy and his coinvestigators reported receiving some manner of financial support from ResMed, Philips Respironics, and B&D Electromedical.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Vera A. De Palo, MD, FCCP, MBA, comments: A goal for any patient with a chronic disease is the best possible quality of life. Increasing hospital-free and exacerbation-free days helps to improve that quality of life. The authors report that the addition of noninvasive ventilation therapy increased the time to readmission due to COPD exacerbation. This adds another tool to the armamentarium to help improve outcomes for our COPD patients.
Vera A. De Palo, MD, FCCP, MBA, comments: A goal for any patient with a chronic disease is the best possible quality of life. Increasing hospital-free and exacerbation-free days helps to improve that quality of life. The authors report that the addition of noninvasive ventilation therapy increased the time to readmission due to COPD exacerbation. This adds another tool to the armamentarium to help improve outcomes for our COPD patients.
Vera A. De Palo, MD, FCCP, MBA, comments: A goal for any patient with a chronic disease is the best possible quality of life. Increasing hospital-free and exacerbation-free days helps to improve that quality of life. The authors report that the addition of noninvasive ventilation therapy increased the time to readmission due to COPD exacerbation. This adds another tool to the armamentarium to help improve outcomes for our COPD patients.
WASHINGTON – The addition of noninvasive ventilation to home oxygen therapy regimens correlated with increased time to readmission or death among patients with exacerbated chronic obstructive pulmonary diseases (COPD), according to a study presented at an international conference of the American Thoracic Society.
Among 116 patients observed with COPD, the 57 patients given home oxygen and noninvasive ventilation reported an average time to readmission of 4.3 months, compared with 1.4 months among the 59 patients given only home oxygen, according to Patrick B. Murphy, PhD, of St. Thomas’ Hospital, London (JAMA. 2017 May 21. doi: 10.1001/jama.2017.4451), who presented this research on the same day it was published in JAMA.
Dr. Murphy said the findings are encouraging for patients with COPD suffering from exacerbations from the disease.
“Patients with established chronic respiratory failure secondary to COPD have poor outcomes with limited treatment options available,” the investigators noted. “The results of the current trial are reassuring, suggesting that home noninvasive ventilation added to home oxygen therapy in this population improved the overall clinical outcome without adding to the health burden of the patient.”
In this 12-month, phase III, multicenter, randomized clinical trial, the average age of the patients was 67 years, and the average body mass index was 21.6 mg/k2. The patients had an average partial pressure of carbon dioxide (PaCo2) level of 59, indicating persistent hypercapnia.
The investigators gave those in the intervention group one of three noninvasive home ventilators – nasal, oronasal, or total face mask – to use for a minimum of 6 hours nightly. Patients in both groups received 15 hours of oxygen therapy daily.
Doctors gathered data from patients after 6 weeks, 3 months, 6 months, and 12 months.
After 12 months, risk of readmission or death in the intervention group was 63.4%, while those in the oxygen-only group reported a risk of 80.4%. Despite a 17% risk reduction, a similar number of patients died during the experiment in both groups: five in the noninvasive intervention group and four in the control group, according to the investigators.
At the end of the trial, 16 patients (28%) in the intervention group and 19 (32%) in the control group died.
Dr. Murphy and his peers asserted that these deaths do not take away from the success of the treatment, as the focus of the study was to find a way to reduce readmissions, not necessarily mortality.
“The driver of the clinical improvement in the home oxygen therapy plus home noninvasive ventilation group was readmission avoidance with no significant difference in mortality,” they wrote. “This study has major clinical relevance because readmission avoidance is beneficial to the patient in terms of preservation of lung function and health-related quality of life, as well as providing a direct and indirect cost saving.”
The study was limited by the lack of a double-blind design; however, investigators said that a sham device may have made patients’ respiratory failure worse.
Some patients in the control group were later given ventilation treatment for safety reasons. Eighteen patients were given noninvasive ventilation, although reportedly after the primary endpoint was reached.
Philips Respironics, ResMed, the ResMed Foundation, and the Guy’s and St. Thomas’ Charity funded the study. Dr. Murphy and his coinvestigators reported receiving some manner of financial support from ResMed, Philips Respironics, and B&D Electromedical.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
WASHINGTON – The addition of noninvasive ventilation to home oxygen therapy regimens correlated with increased time to readmission or death among patients with exacerbated chronic obstructive pulmonary diseases (COPD), according to a study presented at an international conference of the American Thoracic Society.
Among 116 patients observed with COPD, the 57 patients given home oxygen and noninvasive ventilation reported an average time to readmission of 4.3 months, compared with 1.4 months among the 59 patients given only home oxygen, according to Patrick B. Murphy, PhD, of St. Thomas’ Hospital, London (JAMA. 2017 May 21. doi: 10.1001/jama.2017.4451), who presented this research on the same day it was published in JAMA.
Dr. Murphy said the findings are encouraging for patients with COPD suffering from exacerbations from the disease.
“Patients with established chronic respiratory failure secondary to COPD have poor outcomes with limited treatment options available,” the investigators noted. “The results of the current trial are reassuring, suggesting that home noninvasive ventilation added to home oxygen therapy in this population improved the overall clinical outcome without adding to the health burden of the patient.”
In this 12-month, phase III, multicenter, randomized clinical trial, the average age of the patients was 67 years, and the average body mass index was 21.6 mg/k2. The patients had an average partial pressure of carbon dioxide (PaCo2) level of 59, indicating persistent hypercapnia.
The investigators gave those in the intervention group one of three noninvasive home ventilators – nasal, oronasal, or total face mask – to use for a minimum of 6 hours nightly. Patients in both groups received 15 hours of oxygen therapy daily.
Doctors gathered data from patients after 6 weeks, 3 months, 6 months, and 12 months.
After 12 months, risk of readmission or death in the intervention group was 63.4%, while those in the oxygen-only group reported a risk of 80.4%. Despite a 17% risk reduction, a similar number of patients died during the experiment in both groups: five in the noninvasive intervention group and four in the control group, according to the investigators.
At the end of the trial, 16 patients (28%) in the intervention group and 19 (32%) in the control group died.
Dr. Murphy and his peers asserted that these deaths do not take away from the success of the treatment, as the focus of the study was to find a way to reduce readmissions, not necessarily mortality.
“The driver of the clinical improvement in the home oxygen therapy plus home noninvasive ventilation group was readmission avoidance with no significant difference in mortality,” they wrote. “This study has major clinical relevance because readmission avoidance is beneficial to the patient in terms of preservation of lung function and health-related quality of life, as well as providing a direct and indirect cost saving.”
The study was limited by the lack of a double-blind design; however, investigators said that a sham device may have made patients’ respiratory failure worse.
Some patients in the control group were later given ventilation treatment for safety reasons. Eighteen patients were given noninvasive ventilation, although reportedly after the primary endpoint was reached.
Philips Respironics, ResMed, the ResMed Foundation, and the Guy’s and St. Thomas’ Charity funded the study. Dr. Murphy and his coinvestigators reported receiving some manner of financial support from ResMed, Philips Respironics, and B&D Electromedical.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM ATS 2017
Key clinical point:
Major finding: The average time until readmission or death was 4.3 months for patients using both oxygen therapy and ventilation, compared with an average of 1.4 months for patients using only oxygen therapy.
Data source: Phase III, multicenter, randomized clinical trial of 116 COPD patients gathered from 13 U.K. medical centers between February, 2010, and April, 2015.
Disclosures: Philips Respironics, ResMed, the ResMed Foundation, and the Guy’s and St. Thomas’ Charity funded the study. Dr. Patrick B. Murphy and his coinvestigators reported receiving some manner of financial support from ResMed, Philips Respironics, and B&D Electromedical.
New chest x-ray assessment reflects ARDS severity
WASHINGTON – A new way to semiquantitatively score chest x-rays that takes into account lung density and consolidation may be a useful adjunct to current methods for assessing severity of acute respiratory distress syndrome.
The score, know as the Radiographic Assessment of Lung Edema (RALE) score, showed good correlations with lung edema, the severity of acute respiratory distress syndrome (ARDS), and response to fluid management resulting in reduced pulmonary edema, Melissa A. Warren, MD, said at an international conference of the American Thoracic Society.
The RALE score that Dr. Warren and her associates devised rates a patient’s chest x-ray for two parameters: consolidation, which is based on the extent of alveolar opacity in each of the four lung quadrants (left upper, left lower, right upper, and right lower), and a density score that is based on the density of alveolar opacity in each quadrant.
The consolidation score for each quadrant is rated on a 0-4 scale with 0 corresponding to no opacity, 1 for 1%-24% opacity, 2 for 25%-49% opacity, 3 for 50%-75% opacity, and 4 for more than 75%. The density score is rated on a scale of 1-3 with 1 for hazy opacity, 2 for moderate opacity, and 3 for dense opacity. The score for each quadrant is obtained by multiplying the extent score by the density score. A patient’s total RALE score sums the scores from all four quadrants.
The researchers ran three tests of the clinical relevance of this scoring system. First, they used it to score chest x-rays of 72 preprocurement lungs donated for transplant but unable to be used for that purpose, and compared the scores with the extent of lung edema measured by the actual weight of each explanted lung. This showed high correlation between the scores and the amount of edema, Dr. Warren reported.
Next they assessed the RALE score as a marker of ARDS by retrospectively calculating the scores of 174 patients with baseline chest x-rays enrolled in the Fluids and Catheters Treatment Trial (FACTT) (N Engl J Med. 2006;354[24]:2564-75). This analysis showed that patients with the highest RALE scores had significantly worse survival during 90-day follow-up, compared with the patients with the lowest scores.
Finally, the researchers assessed how the RALE score changed in response to either the liberal or conservative fluid management approaches tested in FACTT. This showed that at baseline the average RALE scores were similar among 92 patients randomized to the liberal fluid management treatment arm and 82 patients assigned to the conservative fluid management arm. But after 3 days of treatment, patients in the conservative arm showed a roughly one third reduction in their average RALE score, while patients in the liberal fluid arm showed virtually no change in their score.
“A conservative fluid management strategy favorably impacted the RALE score, reflecting a decrease in pulmonary edema,” Dr. Warren concluded.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
Eric Gartman, MD, FCCP, comments: The results obtained from the use of this scoring system could be important in the prognostication of patients with ARDS, although it is unclear how the score would be used to alter clinical decision making. Further, issues may arise in its implementation given the somewhat subjective nature of the scoring (e.g., hazy vs. moderate vs. dense opacity), changing factors in the ICU that may affect the lung density on x-ray (e.g., different levels of positive end-expiratory pressure), and the variable quality of chest x-rays in the ICU.
Eric Gartman, MD, FCCP, comments: The results obtained from the use of this scoring system could be important in the prognostication of patients with ARDS, although it is unclear how the score would be used to alter clinical decision making. Further, issues may arise in its implementation given the somewhat subjective nature of the scoring (e.g., hazy vs. moderate vs. dense opacity), changing factors in the ICU that may affect the lung density on x-ray (e.g., different levels of positive end-expiratory pressure), and the variable quality of chest x-rays in the ICU.
Eric Gartman, MD, FCCP, comments: The results obtained from the use of this scoring system could be important in the prognostication of patients with ARDS, although it is unclear how the score would be used to alter clinical decision making. Further, issues may arise in its implementation given the somewhat subjective nature of the scoring (e.g., hazy vs. moderate vs. dense opacity), changing factors in the ICU that may affect the lung density on x-ray (e.g., different levels of positive end-expiratory pressure), and the variable quality of chest x-rays in the ICU.
WASHINGTON – A new way to semiquantitatively score chest x-rays that takes into account lung density and consolidation may be a useful adjunct to current methods for assessing severity of acute respiratory distress syndrome.
The score, know as the Radiographic Assessment of Lung Edema (RALE) score, showed good correlations with lung edema, the severity of acute respiratory distress syndrome (ARDS), and response to fluid management resulting in reduced pulmonary edema, Melissa A. Warren, MD, said at an international conference of the American Thoracic Society.
The RALE score that Dr. Warren and her associates devised rates a patient’s chest x-ray for two parameters: consolidation, which is based on the extent of alveolar opacity in each of the four lung quadrants (left upper, left lower, right upper, and right lower), and a density score that is based on the density of alveolar opacity in each quadrant.
The consolidation score for each quadrant is rated on a 0-4 scale with 0 corresponding to no opacity, 1 for 1%-24% opacity, 2 for 25%-49% opacity, 3 for 50%-75% opacity, and 4 for more than 75%. The density score is rated on a scale of 1-3 with 1 for hazy opacity, 2 for moderate opacity, and 3 for dense opacity. The score for each quadrant is obtained by multiplying the extent score by the density score. A patient’s total RALE score sums the scores from all four quadrants.
The researchers ran three tests of the clinical relevance of this scoring system. First, they used it to score chest x-rays of 72 preprocurement lungs donated for transplant but unable to be used for that purpose, and compared the scores with the extent of lung edema measured by the actual weight of each explanted lung. This showed high correlation between the scores and the amount of edema, Dr. Warren reported.
Next they assessed the RALE score as a marker of ARDS by retrospectively calculating the scores of 174 patients with baseline chest x-rays enrolled in the Fluids and Catheters Treatment Trial (FACTT) (N Engl J Med. 2006;354[24]:2564-75). This analysis showed that patients with the highest RALE scores had significantly worse survival during 90-day follow-up, compared with the patients with the lowest scores.
Finally, the researchers assessed how the RALE score changed in response to either the liberal or conservative fluid management approaches tested in FACTT. This showed that at baseline the average RALE scores were similar among 92 patients randomized to the liberal fluid management treatment arm and 82 patients assigned to the conservative fluid management arm. But after 3 days of treatment, patients in the conservative arm showed a roughly one third reduction in their average RALE score, while patients in the liberal fluid arm showed virtually no change in their score.
“A conservative fluid management strategy favorably impacted the RALE score, reflecting a decrease in pulmonary edema,” Dr. Warren concluded.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – A new way to semiquantitatively score chest x-rays that takes into account lung density and consolidation may be a useful adjunct to current methods for assessing severity of acute respiratory distress syndrome.
The score, know as the Radiographic Assessment of Lung Edema (RALE) score, showed good correlations with lung edema, the severity of acute respiratory distress syndrome (ARDS), and response to fluid management resulting in reduced pulmonary edema, Melissa A. Warren, MD, said at an international conference of the American Thoracic Society.
The RALE score that Dr. Warren and her associates devised rates a patient’s chest x-ray for two parameters: consolidation, which is based on the extent of alveolar opacity in each of the four lung quadrants (left upper, left lower, right upper, and right lower), and a density score that is based on the density of alveolar opacity in each quadrant.
The consolidation score for each quadrant is rated on a 0-4 scale with 0 corresponding to no opacity, 1 for 1%-24% opacity, 2 for 25%-49% opacity, 3 for 50%-75% opacity, and 4 for more than 75%. The density score is rated on a scale of 1-3 with 1 for hazy opacity, 2 for moderate opacity, and 3 for dense opacity. The score for each quadrant is obtained by multiplying the extent score by the density score. A patient’s total RALE score sums the scores from all four quadrants.
The researchers ran three tests of the clinical relevance of this scoring system. First, they used it to score chest x-rays of 72 preprocurement lungs donated for transplant but unable to be used for that purpose, and compared the scores with the extent of lung edema measured by the actual weight of each explanted lung. This showed high correlation between the scores and the amount of edema, Dr. Warren reported.
Next they assessed the RALE score as a marker of ARDS by retrospectively calculating the scores of 174 patients with baseline chest x-rays enrolled in the Fluids and Catheters Treatment Trial (FACTT) (N Engl J Med. 2006;354[24]:2564-75). This analysis showed that patients with the highest RALE scores had significantly worse survival during 90-day follow-up, compared with the patients with the lowest scores.
Finally, the researchers assessed how the RALE score changed in response to either the liberal or conservative fluid management approaches tested in FACTT. This showed that at baseline the average RALE scores were similar among 92 patients randomized to the liberal fluid management treatment arm and 82 patients assigned to the conservative fluid management arm. But after 3 days of treatment, patients in the conservative arm showed a roughly one third reduction in their average RALE score, while patients in the liberal fluid arm showed virtually no change in their score.
“A conservative fluid management strategy favorably impacted the RALE score, reflecting a decrease in pulmonary edema,” Dr. Warren concluded.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: The RALE score correlated with lung edema and ARDS severity, and changed in response to conservative fluid management.
Data source: A subgroup of 174 patients with ARDS enrolled in the FACTT trial, and also 72 donor lungs not available for transplantation.
Disclosures: Dr. Warren had no disclosures.
VIDEO: Hyperinflammatory ARDS responds to simvastatin
WASHINGTON – Acute respiratory distress syndrome (ARDS) appears to exist in at least two major forms, and one of these, the hyperinflammatory form, seemed responsive to simvastatin in a post-hoc analysis of trial data.
The other version of ARDs is a hypoinflammatory form, which occurred in 70% of ARDS patients in most of the analyses that have been done.
Researchers classified the 540 ARDS patients enrolled in a 2014 study of simvastatin as either hyperinflammatory or hypoinflammatory. Separating out the hyperinflammatory patients created a subclass that responded to simvastatin, with a 13% absolute reduction in mortality during follow-up, compared with no response among patients in the hypoinflammatory group, Carolyn S. Calfee, MD, said at an international conference of the American Thoracic Society.
“Hyperinflammatory patients treated with simvastatin may have improved outcomes, compared with hypoinflammatory* patients treated with placebo,” said Dr. Calfee, a pulmonologist at the University of California, San Francisco.
The finding raises the possibility that simvastatin, as well as other statins, may be an effective treatment for selected patients with ARDS, but proving this requires new prospective, randomized trials in hyperinflammatory patients, Dr. Calfee said in a video interview.
Currently, the tests Dr. Calfee uses to distinguish hyperinflammatory and hypoinflammatory ARDS patients take about 6-8 hours to complete. A “point of care test to stratify patients in real time,” is needed to further study the various forms of ARDs, Dr. Calfee noted. A critical next step would be the development of a “practical, rapid, bedside assay” to ease identification of hyperinflammatory ARDS patients. “The work we’ve done prior seems to indicate that we are going to definitely need to measure biomarkers in order to identity these subgroups,” she noted.
Hypoinflammatory patients also merit study, she added. Although hyperinflammatory patients have significant worse mortality rates, the hypoinflammatory subclass includes about 70% of ARDS patients, “so we need to better understand how to potentially treat this group.”
Dr. Calfee and her associates first reported finding the two ARDS subclasses, what they also call subphenotypes or endotypes, in two separate cohorts of ARDS patients in a 2014 report (Lancet Resp Med. 2014 Aug;2[8]:611-20). Then, they confirmed the finding in a third ARDS cohort in a 2017 report (Amer J Resp Crit Care Med. 2017 Feb 1;195[3]:331-8). These reports have documented other characteristics of the hyperinflammatory ARDS subclass: hypotension, metabolic acidosis, more frequent treatment with vasopressors, and a higher prevalence of sepsis and shock. Concurrent with the 2017 report, an editorial hailed the finding as “the dawn of personalized medicine for ARDS” (Amer J resp Crit Care Med. 2017 Feb 1;195[3]: 280-1).
To build on this, Dr. Calfee and her associates applied their method for identifying ARDS subclasses to a different cohort of 540 patients enrolled in the The HARP (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction)–2 study, a multicenter UK and Irish study designed to test the efficacy of daily simvastatin treatment in a heterogeneous group of ARDS patients. A 2014 report of the study’s primary results showed no significant effect from simvastatin for increasing the number of ventilator-free days nor did the drug improve any other measured efficacy endpoints (New Engl J Med. 2014 Oct 30;371[18]:1695-703).
Applying a statistical analysis called “latent class analysis,” which is designed to recognize subclass groupings that might not be readily apparent, Dr. Calfee and her team first confirmed that, in this fourth cohort, the ARDS patients again split into a hyperinflammatory subclass, in this case including 188 (35%) of the cohort, and a hypoinflammatory subclass with 352 (65%) patients. The next step was to see what impact simvastatin treatment had in each of the two patient subclasses. They focused the analysis on a secondary outcome in HARP-2, 28-day survival.
They found that simvastatin produced no significant difference in 28-day survival, compared with placebo among the hypoinflammatory patients, but, in the hyperinflammatory subclass, 28-day survival was 68% for patients on simvastatin and 55% for those on placebo, a statistically significant difference, Dr. Calfee reported (Am J Resp Crit Care Med. 2017;195:A6749).
“I’m excited that we are seeing, for the first time, a different response to pharmacotherapy” after dividing ARDS patients into these two subclasses, she said. But, the work remains in an early stage, she cautioned. “We need to test treatments [like statins] prospectively.” The new finding for simvastatin “is not the same as showing benefit in a prospective, randomized trial.”
In the meantime, Dr. Calfee plans to apply the same analytic approach to data collected in another failed statin trial in ARDS patients, the SAILS trial. That study failed to show benefit from rosuvastatin treatment in an unselected population of patients with sepsis-associated ARDS (New Engl J Med. 2014 June 5;370[23]:2191-200).
Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
*An earlier version of this article misquoted Dr. Calfee.
WASHINGTON – Acute respiratory distress syndrome (ARDS) appears to exist in at least two major forms, and one of these, the hyperinflammatory form, seemed responsive to simvastatin in a post-hoc analysis of trial data.
The other version of ARDs is a hypoinflammatory form, which occurred in 70% of ARDS patients in most of the analyses that have been done.
Researchers classified the 540 ARDS patients enrolled in a 2014 study of simvastatin as either hyperinflammatory or hypoinflammatory. Separating out the hyperinflammatory patients created a subclass that responded to simvastatin, with a 13% absolute reduction in mortality during follow-up, compared with no response among patients in the hypoinflammatory group, Carolyn S. Calfee, MD, said at an international conference of the American Thoracic Society.
“Hyperinflammatory patients treated with simvastatin may have improved outcomes, compared with hypoinflammatory* patients treated with placebo,” said Dr. Calfee, a pulmonologist at the University of California, San Francisco.
The finding raises the possibility that simvastatin, as well as other statins, may be an effective treatment for selected patients with ARDS, but proving this requires new prospective, randomized trials in hyperinflammatory patients, Dr. Calfee said in a video interview.
Currently, the tests Dr. Calfee uses to distinguish hyperinflammatory and hypoinflammatory ARDS patients take about 6-8 hours to complete. A “point of care test to stratify patients in real time,” is needed to further study the various forms of ARDs, Dr. Calfee noted. A critical next step would be the development of a “practical, rapid, bedside assay” to ease identification of hyperinflammatory ARDS patients. “The work we’ve done prior seems to indicate that we are going to definitely need to measure biomarkers in order to identity these subgroups,” she noted.
Hypoinflammatory patients also merit study, she added. Although hyperinflammatory patients have significant worse mortality rates, the hypoinflammatory subclass includes about 70% of ARDS patients, “so we need to better understand how to potentially treat this group.”
Dr. Calfee and her associates first reported finding the two ARDS subclasses, what they also call subphenotypes or endotypes, in two separate cohorts of ARDS patients in a 2014 report (Lancet Resp Med. 2014 Aug;2[8]:611-20). Then, they confirmed the finding in a third ARDS cohort in a 2017 report (Amer J Resp Crit Care Med. 2017 Feb 1;195[3]:331-8). These reports have documented other characteristics of the hyperinflammatory ARDS subclass: hypotension, metabolic acidosis, more frequent treatment with vasopressors, and a higher prevalence of sepsis and shock. Concurrent with the 2017 report, an editorial hailed the finding as “the dawn of personalized medicine for ARDS” (Amer J resp Crit Care Med. 2017 Feb 1;195[3]: 280-1).
To build on this, Dr. Calfee and her associates applied their method for identifying ARDS subclasses to a different cohort of 540 patients enrolled in the The HARP (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction)–2 study, a multicenter UK and Irish study designed to test the efficacy of daily simvastatin treatment in a heterogeneous group of ARDS patients. A 2014 report of the study’s primary results showed no significant effect from simvastatin for increasing the number of ventilator-free days nor did the drug improve any other measured efficacy endpoints (New Engl J Med. 2014 Oct 30;371[18]:1695-703).
Applying a statistical analysis called “latent class analysis,” which is designed to recognize subclass groupings that might not be readily apparent, Dr. Calfee and her team first confirmed that, in this fourth cohort, the ARDS patients again split into a hyperinflammatory subclass, in this case including 188 (35%) of the cohort, and a hypoinflammatory subclass with 352 (65%) patients. The next step was to see what impact simvastatin treatment had in each of the two patient subclasses. They focused the analysis on a secondary outcome in HARP-2, 28-day survival.
They found that simvastatin produced no significant difference in 28-day survival, compared with placebo among the hypoinflammatory patients, but, in the hyperinflammatory subclass, 28-day survival was 68% for patients on simvastatin and 55% for those on placebo, a statistically significant difference, Dr. Calfee reported (Am J Resp Crit Care Med. 2017;195:A6749).
“I’m excited that we are seeing, for the first time, a different response to pharmacotherapy” after dividing ARDS patients into these two subclasses, she said. But, the work remains in an early stage, she cautioned. “We need to test treatments [like statins] prospectively.” The new finding for simvastatin “is not the same as showing benefit in a prospective, randomized trial.”
In the meantime, Dr. Calfee plans to apply the same analytic approach to data collected in another failed statin trial in ARDS patients, the SAILS trial. That study failed to show benefit from rosuvastatin treatment in an unselected population of patients with sepsis-associated ARDS (New Engl J Med. 2014 June 5;370[23]:2191-200).
Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
*An earlier version of this article misquoted Dr. Calfee.
WASHINGTON – Acute respiratory distress syndrome (ARDS) appears to exist in at least two major forms, and one of these, the hyperinflammatory form, seemed responsive to simvastatin in a post-hoc analysis of trial data.
The other version of ARDs is a hypoinflammatory form, which occurred in 70% of ARDS patients in most of the analyses that have been done.
Researchers classified the 540 ARDS patients enrolled in a 2014 study of simvastatin as either hyperinflammatory or hypoinflammatory. Separating out the hyperinflammatory patients created a subclass that responded to simvastatin, with a 13% absolute reduction in mortality during follow-up, compared with no response among patients in the hypoinflammatory group, Carolyn S. Calfee, MD, said at an international conference of the American Thoracic Society.
“Hyperinflammatory patients treated with simvastatin may have improved outcomes, compared with hypoinflammatory* patients treated with placebo,” said Dr. Calfee, a pulmonologist at the University of California, San Francisco.
The finding raises the possibility that simvastatin, as well as other statins, may be an effective treatment for selected patients with ARDS, but proving this requires new prospective, randomized trials in hyperinflammatory patients, Dr. Calfee said in a video interview.
Currently, the tests Dr. Calfee uses to distinguish hyperinflammatory and hypoinflammatory ARDS patients take about 6-8 hours to complete. A “point of care test to stratify patients in real time,” is needed to further study the various forms of ARDs, Dr. Calfee noted. A critical next step would be the development of a “practical, rapid, bedside assay” to ease identification of hyperinflammatory ARDS patients. “The work we’ve done prior seems to indicate that we are going to definitely need to measure biomarkers in order to identity these subgroups,” she noted.
Hypoinflammatory patients also merit study, she added. Although hyperinflammatory patients have significant worse mortality rates, the hypoinflammatory subclass includes about 70% of ARDS patients, “so we need to better understand how to potentially treat this group.”
Dr. Calfee and her associates first reported finding the two ARDS subclasses, what they also call subphenotypes or endotypes, in two separate cohorts of ARDS patients in a 2014 report (Lancet Resp Med. 2014 Aug;2[8]:611-20). Then, they confirmed the finding in a third ARDS cohort in a 2017 report (Amer J Resp Crit Care Med. 2017 Feb 1;195[3]:331-8). These reports have documented other characteristics of the hyperinflammatory ARDS subclass: hypotension, metabolic acidosis, more frequent treatment with vasopressors, and a higher prevalence of sepsis and shock. Concurrent with the 2017 report, an editorial hailed the finding as “the dawn of personalized medicine for ARDS” (Amer J resp Crit Care Med. 2017 Feb 1;195[3]: 280-1).
To build on this, Dr. Calfee and her associates applied their method for identifying ARDS subclasses to a different cohort of 540 patients enrolled in the The HARP (Hydroxymethylglutaryl-CoA Reductase Inhibition with Simvastatin in Acute Lung Injury to Reduce Pulmonary Dysfunction)–2 study, a multicenter UK and Irish study designed to test the efficacy of daily simvastatin treatment in a heterogeneous group of ARDS patients. A 2014 report of the study’s primary results showed no significant effect from simvastatin for increasing the number of ventilator-free days nor did the drug improve any other measured efficacy endpoints (New Engl J Med. 2014 Oct 30;371[18]:1695-703).
Applying a statistical analysis called “latent class analysis,” which is designed to recognize subclass groupings that might not be readily apparent, Dr. Calfee and her team first confirmed that, in this fourth cohort, the ARDS patients again split into a hyperinflammatory subclass, in this case including 188 (35%) of the cohort, and a hypoinflammatory subclass with 352 (65%) patients. The next step was to see what impact simvastatin treatment had in each of the two patient subclasses. They focused the analysis on a secondary outcome in HARP-2, 28-day survival.
They found that simvastatin produced no significant difference in 28-day survival, compared with placebo among the hypoinflammatory patients, but, in the hyperinflammatory subclass, 28-day survival was 68% for patients on simvastatin and 55% for those on placebo, a statistically significant difference, Dr. Calfee reported (Am J Resp Crit Care Med. 2017;195:A6749).
“I’m excited that we are seeing, for the first time, a different response to pharmacotherapy” after dividing ARDS patients into these two subclasses, she said. But, the work remains in an early stage, she cautioned. “We need to test treatments [like statins] prospectively.” The new finding for simvastatin “is not the same as showing benefit in a prospective, randomized trial.”
In the meantime, Dr. Calfee plans to apply the same analytic approach to data collected in another failed statin trial in ARDS patients, the SAILS trial. That study failed to show benefit from rosuvastatin treatment in an unselected population of patients with sepsis-associated ARDS (New Engl J Med. 2014 June 5;370[23]:2191-200).
Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
*An earlier version of this article misquoted Dr. Calfee.
AT ATS 2017
Key clinical point:
Major finding: Among hyperinflammatory ARDS patients, 28-day survival was 68% with simvastatin and 55% with placebo, a statistically significant difference.
Data source: A post-hoc analysis of a multicenter randomized trial of 540 patients.
Disclosures: Dr. Calfee is a consultant to Bayer, Boehringer Ingelheim, and GlaxoSmithKline. She received research funding from GlaxoSmithKline.