User login
AVXS-101 may result in long-term motor improvements in SMA
CHARLOTTE, N.C. – AVXS-101, the Food and Drug Administration–approved therapy for spinal muscular atrophy (SMA), yields rapid, sustained improvements in CHOP INTEND scores, better survival, and motor function improvements at long-term follow-up, according to an analysis presented at the annual meeting of the Child Neurology Society. The results provide a clinical demonstration of continuous expression of the SMN protein, according to the investigators. In addition, AVXS-101 is associated with reduced health care utilization in treated infants, which could decrease costs, lessen the burden on patients and caregivers, and improve quality of life.
SMA1 is a progressive neurologic disease that causes loss of the lower motor neurons in the spinal cord and brainstem. Patients have increasing muscle weakness that leads to death or the need for permanent ventilation by age 2 years. The disease results from mutations in the SMN1 gene. AVXS-101 replaces the missing or nonfunctional SMN1 with a healthy copy of a human SMN gene.
AveXis, the company that developed the therapy, enrolled 12 patients with SMA1 in a phase 1/2a study between December 2014 and December 2015. All participants received one intravenous infusion of AVXS-101. Omar Dabbous, MD, vice president of global health economics, outcomes research, and real world evidence at AveXis in Bannockburn, Ill., and colleagues evaluated participants’ rates of event-free survival (i.e., absence of death or need for permanent ventilation), pulmonary or nutritional interventions, swallowing, hospitalization, and CHOP INTEND scores, as well as therapeutic safety at 2 years.
At study completion, all patients who had received a therapeutic dose had event-free survival. Seven participants did not need daily noninvasive ventilation. Eleven participants had stable or improved swallowing. All of the latter patients fed orally, and six fed exclusively by mouth. Eleven patients spoke.
Participants had a mean of 1.4 respiratory hospitalizations per year. Mean proportion of time participants spent hospitalized was 4.4%. Mean hospitalization rate per year was 2.1, and mean length of hospital stay was 6.7 days. In addition, participants’ CHOP INTEND scores increased from baseline by 9.8 points at 1 month and by 15.4 points at 3 months. Patients who received a therapeutic dose of AVXS-101 have maintained their motor milestones at long-term follow-up, which suggests that treatment effects persist over the long term. Adverse events included elevated serum aminotransferase levels, which were reduced by prednisolone.
Dr. Dabbous is an employee of AveXis, which developed AVXS-101.
SOURCE: Dabbous O et al. CNS 2019. Abstract 199.
CHARLOTTE, N.C. – AVXS-101, the Food and Drug Administration–approved therapy for spinal muscular atrophy (SMA), yields rapid, sustained improvements in CHOP INTEND scores, better survival, and motor function improvements at long-term follow-up, according to an analysis presented at the annual meeting of the Child Neurology Society. The results provide a clinical demonstration of continuous expression of the SMN protein, according to the investigators. In addition, AVXS-101 is associated with reduced health care utilization in treated infants, which could decrease costs, lessen the burden on patients and caregivers, and improve quality of life.
SMA1 is a progressive neurologic disease that causes loss of the lower motor neurons in the spinal cord and brainstem. Patients have increasing muscle weakness that leads to death or the need for permanent ventilation by age 2 years. The disease results from mutations in the SMN1 gene. AVXS-101 replaces the missing or nonfunctional SMN1 with a healthy copy of a human SMN gene.
AveXis, the company that developed the therapy, enrolled 12 patients with SMA1 in a phase 1/2a study between December 2014 and December 2015. All participants received one intravenous infusion of AVXS-101. Omar Dabbous, MD, vice president of global health economics, outcomes research, and real world evidence at AveXis in Bannockburn, Ill., and colleagues evaluated participants’ rates of event-free survival (i.e., absence of death or need for permanent ventilation), pulmonary or nutritional interventions, swallowing, hospitalization, and CHOP INTEND scores, as well as therapeutic safety at 2 years.
At study completion, all patients who had received a therapeutic dose had event-free survival. Seven participants did not need daily noninvasive ventilation. Eleven participants had stable or improved swallowing. All of the latter patients fed orally, and six fed exclusively by mouth. Eleven patients spoke.
Participants had a mean of 1.4 respiratory hospitalizations per year. Mean proportion of time participants spent hospitalized was 4.4%. Mean hospitalization rate per year was 2.1, and mean length of hospital stay was 6.7 days. In addition, participants’ CHOP INTEND scores increased from baseline by 9.8 points at 1 month and by 15.4 points at 3 months. Patients who received a therapeutic dose of AVXS-101 have maintained their motor milestones at long-term follow-up, which suggests that treatment effects persist over the long term. Adverse events included elevated serum aminotransferase levels, which were reduced by prednisolone.
Dr. Dabbous is an employee of AveXis, which developed AVXS-101.
SOURCE: Dabbous O et al. CNS 2019. Abstract 199.
CHARLOTTE, N.C. – AVXS-101, the Food and Drug Administration–approved therapy for spinal muscular atrophy (SMA), yields rapid, sustained improvements in CHOP INTEND scores, better survival, and motor function improvements at long-term follow-up, according to an analysis presented at the annual meeting of the Child Neurology Society. The results provide a clinical demonstration of continuous expression of the SMN protein, according to the investigators. In addition, AVXS-101 is associated with reduced health care utilization in treated infants, which could decrease costs, lessen the burden on patients and caregivers, and improve quality of life.
SMA1 is a progressive neurologic disease that causes loss of the lower motor neurons in the spinal cord and brainstem. Patients have increasing muscle weakness that leads to death or the need for permanent ventilation by age 2 years. The disease results from mutations in the SMN1 gene. AVXS-101 replaces the missing or nonfunctional SMN1 with a healthy copy of a human SMN gene.
AveXis, the company that developed the therapy, enrolled 12 patients with SMA1 in a phase 1/2a study between December 2014 and December 2015. All participants received one intravenous infusion of AVXS-101. Omar Dabbous, MD, vice president of global health economics, outcomes research, and real world evidence at AveXis in Bannockburn, Ill., and colleagues evaluated participants’ rates of event-free survival (i.e., absence of death or need for permanent ventilation), pulmonary or nutritional interventions, swallowing, hospitalization, and CHOP INTEND scores, as well as therapeutic safety at 2 years.
At study completion, all patients who had received a therapeutic dose had event-free survival. Seven participants did not need daily noninvasive ventilation. Eleven participants had stable or improved swallowing. All of the latter patients fed orally, and six fed exclusively by mouth. Eleven patients spoke.
Participants had a mean of 1.4 respiratory hospitalizations per year. Mean proportion of time participants spent hospitalized was 4.4%. Mean hospitalization rate per year was 2.1, and mean length of hospital stay was 6.7 days. In addition, participants’ CHOP INTEND scores increased from baseline by 9.8 points at 1 month and by 15.4 points at 3 months. Patients who received a therapeutic dose of AVXS-101 have maintained their motor milestones at long-term follow-up, which suggests that treatment effects persist over the long term. Adverse events included elevated serum aminotransferase levels, which were reduced by prednisolone.
Dr. Dabbous is an employee of AveXis, which developed AVXS-101.
SOURCE: Dabbous O et al. CNS 2019. Abstract 199.
REPORTING FROM CNS 2019
POTS heterogeneity requires individualized treatment
AUSTIN, TEX. – Postural orthostatic tachycardia syndrome (POTS) is not a single disorder, but rather includes multiple overlapping subtypes, according to Steven Vernino, MD, PhD, a professor of neurology at the University of Texas, Dallas.
“It’s pretty well established that there’s a heterogeneous spectrum of disorders that can present this way,” Dr. Vernino told attendees at the annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine. “Investigation is somewhat difficult because we have limited tools.”
In his overview of POTS, Dr. Vernino defined it as a chronic condition with an “inappropriate orthostatic increase in heart rate” and symptoms that persist for at least 6 months. The heart rate increase should be at least 30 beats per minute – or 40 bpm in those aged 12-19 years – within 5-10 minutes of quiet standing or an upright tilt, but the patient lacks orthostatic hypotension. Often, however, other symptoms continue even if the tachycardia is not always present.
These symptoms range widely, including fainting, shortness of breath, headaches, fatigue, fibromyalgia, dizziness, brain fog, chest tightens, sensitivity to light or sound, tingling, heat intolerance, and gastrointestinal problems. Pain is particularly common.
Though peak incidence occurs around age 14 years, the average age of patients with POTS is 30 years. Women comprise 86% of those with POTS and 93% of patients are white, though this last figure may result from multiple reporting biases. A quarter of patients are disabled to a degree similar to heart failure or chronic obstructive pulmonary disease, he said.
Prevalence estimates are all over the map, ranging in academic literature from “up to 1% of teens” to “millions of Americans,” Dr. Vernino said. A commonly accepted range puts the estimate at 500,000 to 3 million Americans, the number used by Dysautonomia International.
Key to treatment of POTS is assessing possible underlying causes and individualizing treatment based on likely contributing etiologies, such as hypovolemia, deconditioning, and autoimmunity, Dr. Vernino said.
Classifications and etiologies of POTS
With its various possible etiologies, “it’s our job as physicians to try to understand, if you can, what the underlying the etiology is and try to address that,” Dr. Vernino said. About 11% of patients have a family history of POTS, and some research has suggested genes that may be involved, including the one that encodes the norepinephrine transporter and alpha tryptase.
Patients with neuropathic POTS have a mild or partial peripheral autonomic neuropathy “that causes a problem with the vasomotor function so that when patients stand, they don’t have an adequate increase in vascular tone, blood pools in the feet and they develop relative hypovolemia, and the autonomic nervous system compensates with tachycardia,” he said. The Quantitative Sudomotor Axon Reflex Test may show distal sweating, and a skin biopsy can be done to assess intraepidermal nerve fiber density.
Hyperadrenergic POTS involves “the presence of a dramatic, excessive rise of norepinephrine” and can involve tremor, nausea, sweating, and headache when patients are upright, Dr. Vernino said.
“These are patients who appear, clinically and in laboratory testing, to have inappropriate sympathetic response to standing up,” he said, and they may have orthostatic hypertension along with an increased heart rate.
Other subtypes of POTS can overlap neuropathic and hyperadrenergic types, which can also overlap one another. About 30% of patients appear hypovolemic, with a 13%-17% volume deficit, even with copious intake of water and sodium, he said. Despite this deficit, renin levels are typically normal in these patients, and aldosterone levels may be paradoxically low. Reduced red blood cell mass may be present, too (Circulation. 2005 Apr 5;111[13]:1574-82).
“What causes that and how that’s related to the other features is a bit unclear, and then, either as a primary or as a secondary component of POTS, there can be cardiac deconditioning,” Dr. Vernino said, requiring quantitative ECG. “It’s unclear whether that deconditioning happens as a consequence of disability from POTS or as a primary part of it.”
Questions still exist regarding whether autoimmunity is one of the underpinnings of POTS, Dr. Vernino said. It’s associated with elevated inflammatory biomarker levels and systemic autoimmune disorders such as Sjögren’s syndrome, as well as with antiphospholipid antibodies.
“More recently there’s been evidence on specific autoantibodies that have been found in POTS patients, and we’re still working through what all that means,” he said. “The real question is whether these antibodies are the cause of POTS” versus an effect or an epiphenomenon.
These antibodies include some G protein–coupled receptor antibodies, such as adrenergic receptor autoantibodies, angiotensin II type 1 receptor antibodies, and muscarinic acetylcholine receptor M3 antibodies. Others include thyroid autoantibodies, ganglionic acetylcholine receptor antibodies, and IgG antibodies, as well as several dozen cardiac membrane proteins.
Comorbidities and risk factors
Although 41% of patients with POTS report some health event preceding onset of symptoms, it’s unclear which, if any, of these events may be related to the condition. The most common antecedent event is infection, reported by 41% of patients in the “Big POTS Survey” conducted by Dysautonomia International, Dr. Vernino said. Other antecedent events reported included surgery (12%), pregnancy (9%), an accident (6%), vaccination (6%), puberty (5%), concussion (4%), and emotional trauma (3%). Research has found associations with migraine, concussion, and infection.
Comorbidities are also common, reported by 84% of patients in the same survey. Migraine, vitamin D deficiency, and joint hypermobility (Ehlers-Danlos syndrome type 3) top the list of comorbidities, and various autoimmune conditions, particularly Sjögren’s syndrome, may co-occur with POTS. Other comorbidities include small fiber neuropathy, mast-cell activation syndrome, chronic fatigue, gastrointestinal problems, vasovagal syncope, and sleeping difficulties.
Joint hypermobility appears to be a “pretty strong risk factor for development” of POTS, Dr. Vernino said, and patients may even be involved in activities where that’s helpful, such as gymnastics. “You can make this diagnosis clinically – there isn’t a genetic test for joint hypermobility syndrome – and you usually don’t have the other features of Marfan syndrome,” he told attendees.
Other risk factors include low body mass, mitral valve prolapse, migraine, anxiety, irritable bowel syndrome, prolonged bed rest after an illness, and mast-cell activation syndrome.
Prognosis and treatment
POTS is very common but often still unrecognized, Dr. Vernino said, “because the symptoms are somewhat diverse and broad and vague.” Even providers who recognize POTS can become preoccupied with “the heart rate increase being the whole picture, but there are many other symptoms, and that leads to a significant impact on the quality of life of these patients.”
The course of POTS varies across patients. In about half of patients, symptoms persist but the severity improves, and one in five patients fully resolve. Severity only tends to worsen over time in about 3.5% of patients, and severity remains constant in 8.7% (J Pediatr. 2016 Jun;173:149-53. doi: 10.1016/j.jpeds.2016.02.035).
“It would probably be simpler if POTS was a single entity that had a single etiology that we could target,” Dr. Vernino said. But its heterogeneity means “we have to investigate patients individually and understand their particular situation, individualize their treatment, whether it be nonpharmacological or pharmacological, to their particular potential etiologies.”
Dr. Vernino has received research support from Genentech, Grifols, Athena/Quest, Biohaven Pharmaceutical, Dysautonomia International, and the Rex Griswold Foundation.
AUSTIN, TEX. – Postural orthostatic tachycardia syndrome (POTS) is not a single disorder, but rather includes multiple overlapping subtypes, according to Steven Vernino, MD, PhD, a professor of neurology at the University of Texas, Dallas.
“It’s pretty well established that there’s a heterogeneous spectrum of disorders that can present this way,” Dr. Vernino told attendees at the annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine. “Investigation is somewhat difficult because we have limited tools.”
In his overview of POTS, Dr. Vernino defined it as a chronic condition with an “inappropriate orthostatic increase in heart rate” and symptoms that persist for at least 6 months. The heart rate increase should be at least 30 beats per minute – or 40 bpm in those aged 12-19 years – within 5-10 minutes of quiet standing or an upright tilt, but the patient lacks orthostatic hypotension. Often, however, other symptoms continue even if the tachycardia is not always present.
These symptoms range widely, including fainting, shortness of breath, headaches, fatigue, fibromyalgia, dizziness, brain fog, chest tightens, sensitivity to light or sound, tingling, heat intolerance, and gastrointestinal problems. Pain is particularly common.
Though peak incidence occurs around age 14 years, the average age of patients with POTS is 30 years. Women comprise 86% of those with POTS and 93% of patients are white, though this last figure may result from multiple reporting biases. A quarter of patients are disabled to a degree similar to heart failure or chronic obstructive pulmonary disease, he said.
Prevalence estimates are all over the map, ranging in academic literature from “up to 1% of teens” to “millions of Americans,” Dr. Vernino said. A commonly accepted range puts the estimate at 500,000 to 3 million Americans, the number used by Dysautonomia International.
Key to treatment of POTS is assessing possible underlying causes and individualizing treatment based on likely contributing etiologies, such as hypovolemia, deconditioning, and autoimmunity, Dr. Vernino said.
Classifications and etiologies of POTS
With its various possible etiologies, “it’s our job as physicians to try to understand, if you can, what the underlying the etiology is and try to address that,” Dr. Vernino said. About 11% of patients have a family history of POTS, and some research has suggested genes that may be involved, including the one that encodes the norepinephrine transporter and alpha tryptase.
Patients with neuropathic POTS have a mild or partial peripheral autonomic neuropathy “that causes a problem with the vasomotor function so that when patients stand, they don’t have an adequate increase in vascular tone, blood pools in the feet and they develop relative hypovolemia, and the autonomic nervous system compensates with tachycardia,” he said. The Quantitative Sudomotor Axon Reflex Test may show distal sweating, and a skin biopsy can be done to assess intraepidermal nerve fiber density.
Hyperadrenergic POTS involves “the presence of a dramatic, excessive rise of norepinephrine” and can involve tremor, nausea, sweating, and headache when patients are upright, Dr. Vernino said.
“These are patients who appear, clinically and in laboratory testing, to have inappropriate sympathetic response to standing up,” he said, and they may have orthostatic hypertension along with an increased heart rate.
Other subtypes of POTS can overlap neuropathic and hyperadrenergic types, which can also overlap one another. About 30% of patients appear hypovolemic, with a 13%-17% volume deficit, even with copious intake of water and sodium, he said. Despite this deficit, renin levels are typically normal in these patients, and aldosterone levels may be paradoxically low. Reduced red blood cell mass may be present, too (Circulation. 2005 Apr 5;111[13]:1574-82).
“What causes that and how that’s related to the other features is a bit unclear, and then, either as a primary or as a secondary component of POTS, there can be cardiac deconditioning,” Dr. Vernino said, requiring quantitative ECG. “It’s unclear whether that deconditioning happens as a consequence of disability from POTS or as a primary part of it.”
Questions still exist regarding whether autoimmunity is one of the underpinnings of POTS, Dr. Vernino said. It’s associated with elevated inflammatory biomarker levels and systemic autoimmune disorders such as Sjögren’s syndrome, as well as with antiphospholipid antibodies.
“More recently there’s been evidence on specific autoantibodies that have been found in POTS patients, and we’re still working through what all that means,” he said. “The real question is whether these antibodies are the cause of POTS” versus an effect or an epiphenomenon.
These antibodies include some G protein–coupled receptor antibodies, such as adrenergic receptor autoantibodies, angiotensin II type 1 receptor antibodies, and muscarinic acetylcholine receptor M3 antibodies. Others include thyroid autoantibodies, ganglionic acetylcholine receptor antibodies, and IgG antibodies, as well as several dozen cardiac membrane proteins.
Comorbidities and risk factors
Although 41% of patients with POTS report some health event preceding onset of symptoms, it’s unclear which, if any, of these events may be related to the condition. The most common antecedent event is infection, reported by 41% of patients in the “Big POTS Survey” conducted by Dysautonomia International, Dr. Vernino said. Other antecedent events reported included surgery (12%), pregnancy (9%), an accident (6%), vaccination (6%), puberty (5%), concussion (4%), and emotional trauma (3%). Research has found associations with migraine, concussion, and infection.
Comorbidities are also common, reported by 84% of patients in the same survey. Migraine, vitamin D deficiency, and joint hypermobility (Ehlers-Danlos syndrome type 3) top the list of comorbidities, and various autoimmune conditions, particularly Sjögren’s syndrome, may co-occur with POTS. Other comorbidities include small fiber neuropathy, mast-cell activation syndrome, chronic fatigue, gastrointestinal problems, vasovagal syncope, and sleeping difficulties.
Joint hypermobility appears to be a “pretty strong risk factor for development” of POTS, Dr. Vernino said, and patients may even be involved in activities where that’s helpful, such as gymnastics. “You can make this diagnosis clinically – there isn’t a genetic test for joint hypermobility syndrome – and you usually don’t have the other features of Marfan syndrome,” he told attendees.
Other risk factors include low body mass, mitral valve prolapse, migraine, anxiety, irritable bowel syndrome, prolonged bed rest after an illness, and mast-cell activation syndrome.
Prognosis and treatment
POTS is very common but often still unrecognized, Dr. Vernino said, “because the symptoms are somewhat diverse and broad and vague.” Even providers who recognize POTS can become preoccupied with “the heart rate increase being the whole picture, but there are many other symptoms, and that leads to a significant impact on the quality of life of these patients.”
The course of POTS varies across patients. In about half of patients, symptoms persist but the severity improves, and one in five patients fully resolve. Severity only tends to worsen over time in about 3.5% of patients, and severity remains constant in 8.7% (J Pediatr. 2016 Jun;173:149-53. doi: 10.1016/j.jpeds.2016.02.035).
“It would probably be simpler if POTS was a single entity that had a single etiology that we could target,” Dr. Vernino said. But its heterogeneity means “we have to investigate patients individually and understand their particular situation, individualize their treatment, whether it be nonpharmacological or pharmacological, to their particular potential etiologies.”
Dr. Vernino has received research support from Genentech, Grifols, Athena/Quest, Biohaven Pharmaceutical, Dysautonomia International, and the Rex Griswold Foundation.
AUSTIN, TEX. – Postural orthostatic tachycardia syndrome (POTS) is not a single disorder, but rather includes multiple overlapping subtypes, according to Steven Vernino, MD, PhD, a professor of neurology at the University of Texas, Dallas.
“It’s pretty well established that there’s a heterogeneous spectrum of disorders that can present this way,” Dr. Vernino told attendees at the annual meeting of the American Association for Neuromuscular and Electrodiagnostic Medicine. “Investigation is somewhat difficult because we have limited tools.”
In his overview of POTS, Dr. Vernino defined it as a chronic condition with an “inappropriate orthostatic increase in heart rate” and symptoms that persist for at least 6 months. The heart rate increase should be at least 30 beats per minute – or 40 bpm in those aged 12-19 years – within 5-10 minutes of quiet standing or an upright tilt, but the patient lacks orthostatic hypotension. Often, however, other symptoms continue even if the tachycardia is not always present.
These symptoms range widely, including fainting, shortness of breath, headaches, fatigue, fibromyalgia, dizziness, brain fog, chest tightens, sensitivity to light or sound, tingling, heat intolerance, and gastrointestinal problems. Pain is particularly common.
Though peak incidence occurs around age 14 years, the average age of patients with POTS is 30 years. Women comprise 86% of those with POTS and 93% of patients are white, though this last figure may result from multiple reporting biases. A quarter of patients are disabled to a degree similar to heart failure or chronic obstructive pulmonary disease, he said.
Prevalence estimates are all over the map, ranging in academic literature from “up to 1% of teens” to “millions of Americans,” Dr. Vernino said. A commonly accepted range puts the estimate at 500,000 to 3 million Americans, the number used by Dysautonomia International.
Key to treatment of POTS is assessing possible underlying causes and individualizing treatment based on likely contributing etiologies, such as hypovolemia, deconditioning, and autoimmunity, Dr. Vernino said.
Classifications and etiologies of POTS
With its various possible etiologies, “it’s our job as physicians to try to understand, if you can, what the underlying the etiology is and try to address that,” Dr. Vernino said. About 11% of patients have a family history of POTS, and some research has suggested genes that may be involved, including the one that encodes the norepinephrine transporter and alpha tryptase.
Patients with neuropathic POTS have a mild or partial peripheral autonomic neuropathy “that causes a problem with the vasomotor function so that when patients stand, they don’t have an adequate increase in vascular tone, blood pools in the feet and they develop relative hypovolemia, and the autonomic nervous system compensates with tachycardia,” he said. The Quantitative Sudomotor Axon Reflex Test may show distal sweating, and a skin biopsy can be done to assess intraepidermal nerve fiber density.
Hyperadrenergic POTS involves “the presence of a dramatic, excessive rise of norepinephrine” and can involve tremor, nausea, sweating, and headache when patients are upright, Dr. Vernino said.
“These are patients who appear, clinically and in laboratory testing, to have inappropriate sympathetic response to standing up,” he said, and they may have orthostatic hypertension along with an increased heart rate.
Other subtypes of POTS can overlap neuropathic and hyperadrenergic types, which can also overlap one another. About 30% of patients appear hypovolemic, with a 13%-17% volume deficit, even with copious intake of water and sodium, he said. Despite this deficit, renin levels are typically normal in these patients, and aldosterone levels may be paradoxically low. Reduced red blood cell mass may be present, too (Circulation. 2005 Apr 5;111[13]:1574-82).
“What causes that and how that’s related to the other features is a bit unclear, and then, either as a primary or as a secondary component of POTS, there can be cardiac deconditioning,” Dr. Vernino said, requiring quantitative ECG. “It’s unclear whether that deconditioning happens as a consequence of disability from POTS or as a primary part of it.”
Questions still exist regarding whether autoimmunity is one of the underpinnings of POTS, Dr. Vernino said. It’s associated with elevated inflammatory biomarker levels and systemic autoimmune disorders such as Sjögren’s syndrome, as well as with antiphospholipid antibodies.
“More recently there’s been evidence on specific autoantibodies that have been found in POTS patients, and we’re still working through what all that means,” he said. “The real question is whether these antibodies are the cause of POTS” versus an effect or an epiphenomenon.
These antibodies include some G protein–coupled receptor antibodies, such as adrenergic receptor autoantibodies, angiotensin II type 1 receptor antibodies, and muscarinic acetylcholine receptor M3 antibodies. Others include thyroid autoantibodies, ganglionic acetylcholine receptor antibodies, and IgG antibodies, as well as several dozen cardiac membrane proteins.
Comorbidities and risk factors
Although 41% of patients with POTS report some health event preceding onset of symptoms, it’s unclear which, if any, of these events may be related to the condition. The most common antecedent event is infection, reported by 41% of patients in the “Big POTS Survey” conducted by Dysautonomia International, Dr. Vernino said. Other antecedent events reported included surgery (12%), pregnancy (9%), an accident (6%), vaccination (6%), puberty (5%), concussion (4%), and emotional trauma (3%). Research has found associations with migraine, concussion, and infection.
Comorbidities are also common, reported by 84% of patients in the same survey. Migraine, vitamin D deficiency, and joint hypermobility (Ehlers-Danlos syndrome type 3) top the list of comorbidities, and various autoimmune conditions, particularly Sjögren’s syndrome, may co-occur with POTS. Other comorbidities include small fiber neuropathy, mast-cell activation syndrome, chronic fatigue, gastrointestinal problems, vasovagal syncope, and sleeping difficulties.
Joint hypermobility appears to be a “pretty strong risk factor for development” of POTS, Dr. Vernino said, and patients may even be involved in activities where that’s helpful, such as gymnastics. “You can make this diagnosis clinically – there isn’t a genetic test for joint hypermobility syndrome – and you usually don’t have the other features of Marfan syndrome,” he told attendees.
Other risk factors include low body mass, mitral valve prolapse, migraine, anxiety, irritable bowel syndrome, prolonged bed rest after an illness, and mast-cell activation syndrome.
Prognosis and treatment
POTS is very common but often still unrecognized, Dr. Vernino said, “because the symptoms are somewhat diverse and broad and vague.” Even providers who recognize POTS can become preoccupied with “the heart rate increase being the whole picture, but there are many other symptoms, and that leads to a significant impact on the quality of life of these patients.”
The course of POTS varies across patients. In about half of patients, symptoms persist but the severity improves, and one in five patients fully resolve. Severity only tends to worsen over time in about 3.5% of patients, and severity remains constant in 8.7% (J Pediatr. 2016 Jun;173:149-53. doi: 10.1016/j.jpeds.2016.02.035).
“It would probably be simpler if POTS was a single entity that had a single etiology that we could target,” Dr. Vernino said. But its heterogeneity means “we have to investigate patients individually and understand their particular situation, individualize their treatment, whether it be nonpharmacological or pharmacological, to their particular potential etiologies.”
Dr. Vernino has received research support from Genentech, Grifols, Athena/Quest, Biohaven Pharmaceutical, Dysautonomia International, and the Rex Griswold Foundation.
EXPERT ANALYSIS FROM AANEM 2019
Female Runner, 47, with Inguinal Lump
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
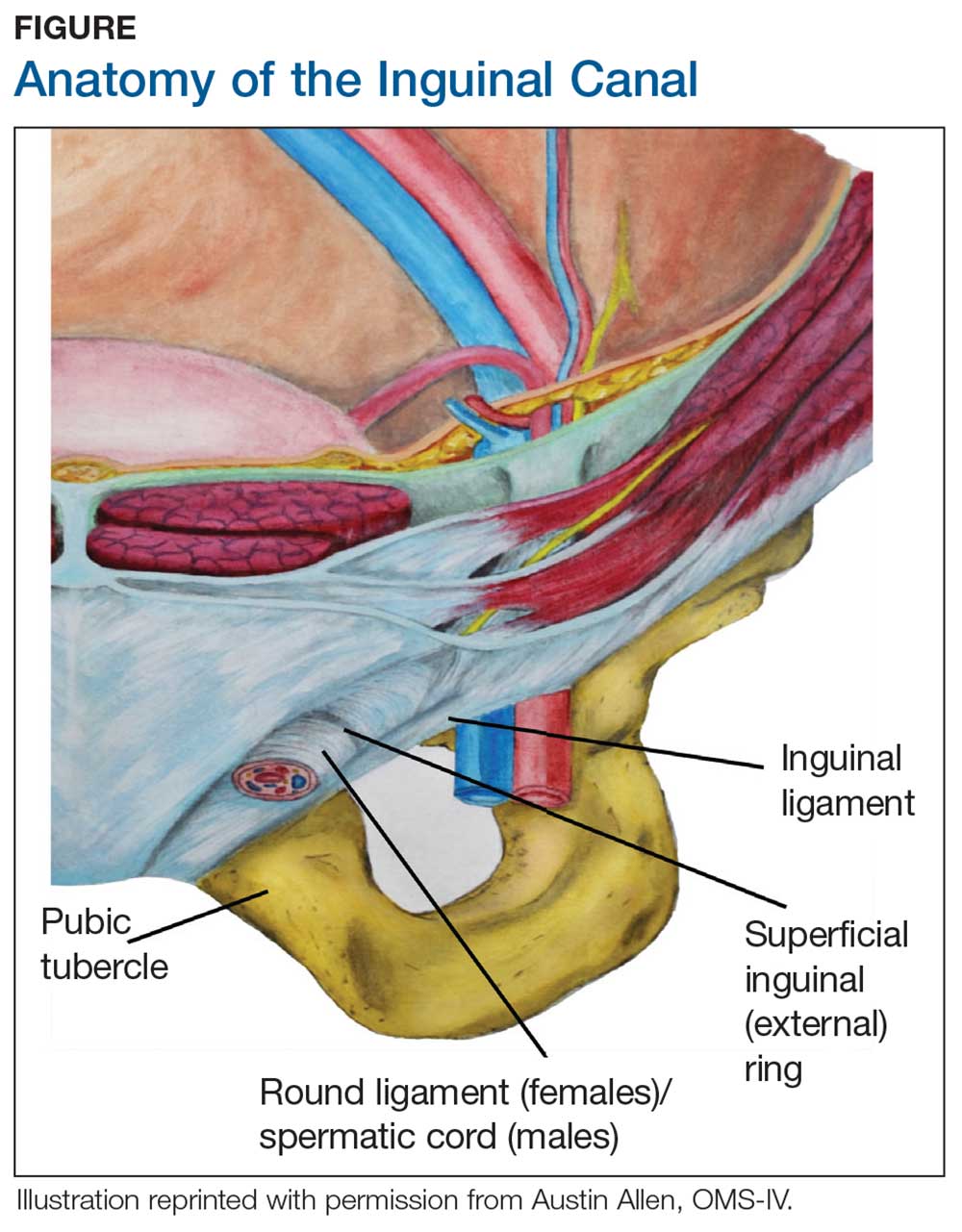
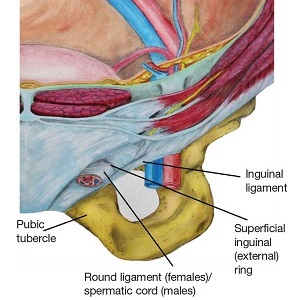
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
A 47-year-old woman was referred to the gynecology office by her primary care NP for surgical excision of an enlarging nodule on the right side of her mons pubis. Onset occurred about 6 months earlier. The patient reported that symptoms waxed and waned but had worsened progressively over the past 2 to 3 months, adding that the nodule hurt only occasionally. She noted that symptoms were exacerbated by exercise, specifically running. Further questioning prompted the observation that her symptoms were more noticeable at the time of menses.
The patient’s medical history was unremarkable, with no chronic conditions; her surgical history consisted of a wisdom tooth extraction. She had no known drug allergies. Her family history included cerebrovascular accident, hypertension, and arthritis. Reproductive history revealed that she was G1 P1, with a 38-week uncomplicated vaginal delivery. She experienced menarche at age 14, and her menses was regular at every 28 days. For the past 5 days, there had been no dysmenorrhea. The patient was married, exercised regularly, and did not use tobacco, alcohol, or illicit drugs.
On examination, the patient’s blood pressure was 123/73 mm Hg; heart rate, 77 beats/min; respiratory rate, 12 breaths/min; weight, 128 lb; height, 5 ft 7 in; O2 saturation, 99% on room air; and BMI, 20. The patient was alert and oriented to person, place, and time. She was thin, appeared physically fit, and exhibited no signs of distress. Her physical exam was unremarkable, apart from a firm, minimally tender, well-circumscribed, 3.5 × 3.5–cm nodule right of midline on the mons pubis.
The patient was scheduled for outpatient surgical excision of a benign skin lesion (excluding skin tags) of the genitalia, 3.1 to 3.5 cm (CPT code 11424). During this procedure, it became evident that this was not a lipoma. The lesion was exceptionally hard, and it was difficult to discern if it was incorporated into the rectus abdominis near the point of attachment to the pubic symphysis. The lesion was unintentionally disrupted, revealing black powdery material within the capsule. The tissue was sent for a fast, frozen section that showed “soft tissue with extensive involvement by endometriosis.” The pathology report noted “[m]any endometrial glands in a background of stromal tissue. Necrosis was not a feature. No evidence of atypia.” The patient’s postoperative diagnosis was endometriosis.
DISCUSSION
Endometriosis occurs when endometrial or “endometrial-like” tissue is displaced to sites other than within the uterus. It is most frequently found on tissues close to the uterus, such as the ovaries or pelvic peritoneum. Estrogen is the driving force that feeds the endometrium, causing it to proliferate, whether inside or outside the uterus. Given this dependence on hormones, endometriosis occurs most often during a woman’s fertile years, although it can occur after menopause. Endometriosis is common, affecting at least 10% of premenopausal women; moreover, it is identified as the cause in 70% of all female chronic pelvic pain cases.1-4
Endometriosis has certain identifiable features, such as chronic pain, dyspareunia, infertility, and menstrual and gastrointestinal symptoms. However, it is seldom diagnosed quickly; studies indicate that diagnosis can be delayed by 5 to 10 years after a patient has first sought treatment for symptoms.2,4 Multiple factors contribute to a lag in diagnosis: Presentation is not always straightforward. There are no definitive lab values or biomarkers. Symptoms vary from patient to patient, as do clinical skills from one diagnostician to another.1
Unlike pelvic endometriosis, inguinal endometriosis is not common; disease in this location encompasses only 0.3% to 0.6% of all diagnosed cases.3,5-7 Since the discovery of the first known case of round ligament endometriosis in 1896, there have been only 70 cases reported in the medical literature.6,7
If the more common form of endometriosis is frequently missed, this rarely seen variant presents an even greater diagnostic challenge. The typical presentation of inguinal endometriosis includes a firm nodule in the groin, accompanied by tenderness and swelling. A careful history will allude to pain that occurs cyclically with menses.
Cause
Among several theories about the etiology of endometriosis, the most popular has been retrograde menstruation.1,4,5 According to this hypothesis, the flow of menstrual blood moves backward through the fallopian tubes, spilling into the pelvic cavity and carrying endometrial tissue with it. One theory purports that endometrial tissue is transplanted from the uterus to other areas of the body via the bloodstream or the lymphatics, much like a metastatic disease.1,4 Another theory states that cells outside the uterus, which line the peritoneum, transform into endometrial cells through metaplasia.4,5 Endometrial tissue can also be transplanted iatrogenically during surgery—for example, when endometrial tissue is displaced during a cesarean delivery, resulting in implants above the fascia and below the subcutaneous layers. Several other hypotheses concern stem-cell involvement, hormonal factors, immune system dysfunction, and genetics.4,5 Currently, there are no definitive answers.
Location
During maturation, the parietal peritoneum develops a pouch called the processus vaginalis, which serves as a passageway for the gubernaculum to transport the round ligament running from the uterus, through the inguinal canal, and ending at the labia. After these structures reach their destination, in normal development, the processus vaginalis degenerates, closing the inguinal canal. Occasionally the processus vaginalis fails to close, allowing for a communication pathway between the peritoneal cavity and the inguinal canal. This leaves the canal vulnerable to the contents of the pelvic cavity, such as a hernia or hydrocele, and provides a clear path for endometriosis.5-7 The implant found in the case patient was at the point where the external ring lies, just above the right pubic tubercle (see Figure 1).
Endometriosis implants can occur anywhere along the round ligament in either the intrapelvic or extrapelvic segments. Implants have also been found in the wall of a hernia sac, the wall of a Nuck canal hydrocele, or even in the subcutaneous tissue surrounding the inguinal canal.3 Interestingly, inguinal endometriosis occurs more often in the right side (up to 94% of cases) than in the left side, as was the case with our patient.5-7 The reason for this predominance has not been established, although there are several theories, including one that suggests the left side is afforded protection by the sigmoid colon.5-7
Laboratory diagnosis
Imaging, such as ultrasound and MRI, offers some diagnostic benefit, although its usefulness is most often realized in the pelvis. Pelvic ultrasound can be used to identify ovarian endometriomas.1 MRI can help rule out, locate, or sometimes determine the degree of deep infiltrating endometriosis, which is an indispensable tool for surgical planning.5,7 Unfortunately, the diagnostic accuracy for extra-pelvic lesions is variable; neither modality is particularly useful in identifying superficial lesions, which comprises most cases.
Ultrasound of the groin can be employed to evaluate for hernia; if a hernia has been excluded, histologic confirmation can be obtained via fine-needle aspiration of nodule contents.5,7 One caveat is that these tests are helpful only if the clinician suspects the diagnosis and orders them. The definitive diagnostic test remains direct visualization, which requires laparoscopy.1,5
Differential diagnosis
Lipoma was a favored diagnosis in this case because of the palpable, well-circumscribed borders, nontender on exam; intermittent, minimal tenderness; and no evidence of erythema or color change. A second possibility was an enlarged lymph node, which was less likely due to the location, large size, and sudden onset without any accompanying symptoms of infection or chronic illness. Finally, an inguinal hernia was least likely, again because of well-defined borders, no history of a lump in the area, a nodule that was not reducible, only minimal tenderness, and no color changes on the skin.
Management
Definitive treatment for inguinal endometriosis entails complete surgical excision.5-7 The provider should be prepared to repair a defect after the excision; there is potential for a substantial defect that might require mesh. Additionally, a herniorrhaphy may be indicated if there is a coexisting hernia.5 The risk for recurrent disease in the inguinal canal after treatment is uncommon, unless the excision was not complete.3
There is an association between inguinal and pelvic endometriosis but not a direct correlation. Data on concomitant pelvic and inguinal endometriosis have been variable. In one case series of 9 patients diagnosed with inguinal endometriosis, none had a history of pelvic endometriosis, and only 1 was subsequently diagnosed with pelvic endometriosis.7 An increased association was noted for patients with implants found on the proximal segment of the round ligament.7 However, implants on the extrapelvic segment were not likely to represent pelvic disease but rather isolated lesions in the canal.7 For those with pelvic endometriosis, complications and recurrence are likely, resulting in the need for long-term treatment.
There is some debate in the literature whether to proceed with laparoscopy once inguinal endometriosis has been identified. Diagnostic laparoscopy to evaluate the pelvis is indicated for symptomatic patients or for cases in which an indirect inguinal hernia is suspected.5 Laparoscopy can offer the benefit of both a diagnostic tool and a mechanism for treatment. However, this is an invasive procedure that also incurs risks. The medical provider, in discussion with the patient, must weigh the risks against the benefits of an invasive procedure before determining how to proceed.
OUTCOME FOR THE CASE PATIENT
The lesion was excised completely. Since the patient had been entirely asymptomatic until age 47, and the risks of a potentially unnecessary surgery outweighed the theoretical benefits, the decision was made not to perform a diagnostic laparoscopy to investigate for pelvic endometriosis. The patient made a complete and uneventful recovery. No further treatment was initiated. She continues to be asymptomatic, denying any menstrual complaints, dyspareunia, or further problems with the groin.
CONCLUSION
This case describes a satellite lesion of endometrial tissue found in an unusual location, in a patient with no history, no risk factors, and no symptoms. The final diagnosis had been omitted from the differential—perhaps because the patient initially associated her symptoms with exercise and mentioned the correlation to her menstrual cycle as an afterthought. Fortunately, the correct diagnosis was made and the appropriate treatment provided.
There are numerous presentations of endometriosis; extrapelvic lesions can have very different, often vague, presentations when compared to the familiar symptoms of pelvic disease. Unfortunately, diagnosis is often delayed. Obscure presentations, in unusual sites, can further impede both speed and accuracy of diagnosis. To date, there are no lab tests or biomarkers to aid diagnosis; imaging studies are inconsistent. Until more accurate diagnostic tools become available, the diagnosis remains dependent on history taking, physical exam, and the clinical judgment of the provider. The astute clinician will recognize the catamenial pattern and consider endometriosis as part of the differential.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
1. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34-41.
2. Soliman AM, Fuldeore M, Snabes MC. Factors associated with time to endometriosis diagnosis in the United States. J Womens Health (Larchmt). 2017;26(7):788-797.
3. Niitsu H, Tsumura H, Kanehiro T, et al. Clinical characteristics and surgical treatment for inguinal endometriosis in young women of reproductive age. Dig Surg. 2019;36(2):166-172.
4. Mehedintu C, Plotogea MN, Ionescu S, Antonovici M. Endometriosis still a challenge. J Med Life. 2014;7(3):349-357.
5. Wolfhagen N, Simons NE, de Jong KH, et al. Inguinal endometriosis, a rare entity of which surgeons should be aware: clinical aspects and long-term follow-up of nine cases. Hernia. 2018;22(5):881-886.
6. Prabhu R, Krishna S, Shenoy R, Thangavelu S. Endometriosis of extra-pelvic round ligament, a diagnostic dilemma for physicians. BMJ Case Rep. 2013;2013.
7. Pandey D, Coondoo A, Shetty J, Mathew S. Jack in the box: inguinal endometriosis. BMJ Case Rep. 2015;2015.
Free HIV self-tests for at-risk groups can increase awareness, testing frequency
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
A new study has found that distributing HIV self-tests to at-risk groups such as men who have sex with men can increase testing frequency and uncover more previously undiagnosed infections.
“Based on these findings, HIV prevention programs might consider adding an HIV self-testing mail distribution component to their portfolio of HIV prevention services for high-risk populations,” wrote Robin J. MacGowan, MPH, of the Centers for Disease Control and Prevention and coauthors. The study was published in JAMA Internal Medicine.
To assess the potential benefits of expanded HIV self-testing, the CDC sponsored a 12-month randomized clinical trial called the Evaluation of Rapid HIV Self-testing Among MSM Project (eSTAMP). Participants were recruited via social media, music and dating websites; criteria included being aged at least 18 years, never having tested positive for HIV, and having engaged in anal sex with at least one man in the past year. The 2,665 participants were assigned to either the self-testing (ST) group (n = 1,325) or the control group (n = 1,340); the ST group received four self-tests in the mail with the option for more each quarter. All participants were asked to complete follow-up surveys every 3 months.
Of all participants, 1,991 (74.7%) initiated at least one follow-up survey. Participants in the ST group reported testing more frequently than those in the control group (an average of 5.3 tests vs. 1.5 tests; P less than .001). In addition, a much higher percentage of ST participants tested at least three times in 12 months (777 of 1014 [76.6%]), compared with controls (215 of 977 [22.0%]). A total of 36 participants tested newly positive for HIV during the study; over the first 3 months, 12 of the 14 infections were identified in the ST group (P less than .007). Over 12 months, 25 of the infections came from the ST group, compared with 11 in the control group (P = .02).
When HIV tests are free and convenient, members of high-risk populations will use them, wrote Julia M. Janssen, MD, of the University of California, San Francisco, and Mitchell H. Katz, MD, of New York City Health and Hospitals in an accompanying editorial (JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5442). But tests are not enough; the authors noted the role of primary care physicians in prescribing pre-exposure prophylaxis (PrEP) for at-risk patients as key in decreasing rates of new HIV diagnoses.
they added, “and are another way to accelerate the end of the epidemic.”
The study was funded by the CDC. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network. Dr. Katz reported receiving royalties for a chapter on HIV in Lange’s Current Medicine and Diagnostic Testing.
SOURCE: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Providing free HIV self-tests can lead to increased testing and more newly identified infections.
Major finding: About 77% of participants in the self-testing group tested three times or more in 12 months, compared with 22% of controls.
Study details: A 12-month longitudinal, two-group, randomized clinical trial of 2,665 men who have sex with men.
Disclosures: The study was funded by the Centers for Disease Control and Prevention. One author reported receiving grants and fees from the CDC and the National Institutes of Health, along with personal fees from Elsevier and the Ontario HIV Treatment Network.
Source: MacGowan RJ et al. JAMA Intern Med. 2019 Nov 18. doi: 10.1001/jamainternmed.2019.5222.
Opioid prescribing patterns mostly ‘unchanged’ with laparoscopy
SAN FRANCISCO – Opioid prescription is surprisingly high after laparoscopic colorectal surgery, and is higher at larger hospitals and in some regions of the United States, according to a new study.
“In theory, for management of pain, any opioids required should be lower after laparoscopic surgery, but opioids are still ubiquitous and prescribing patterns have largely been unchanged with laparoscopy,” Deborah S. Keller, MD, assistant professor of surgery at Columbia University, New York, said at the annual clinical congress of the American College of Surgeons.
Several studies show wide variation in opioid use and prescribing after laparoscopic colorectal surgery, Dr. Keller said. She also pointed out that available data are limited on inpatient opioid use and the causes of high opioid use.
The team analyzed records of 18,395 subjects from the Premier Inpatient Database, between Jan. 1, 2014, and Sept. 30, 2015. The mean age was 61 years, and 54% were female. The distribution of hospital-stay milligram morphine equivalents (MME) was 48 at the 25th percentile, 108 at the 50th, and 246 at the 75th percentile. Overall, 18% of patients were in the high use category.
Some factors were associated with high opioid use, including emergency surgery (odds ratio, 1.28; P = .0002), being aged 18-34 (OR, 5.8; P less than .0001), major severity of illness (OR, 4.2; P less than .0001), chronic obstructive pulmonary disease comorbidity (OR, 1.13; P =.0350), having Medicaid insurance (OR, 1.35; P less than .0001), and being treated in a rural hospital (OR, 1.44; P less than.0001).
Factors associated with lower opioid use included female sex (OR, 0.90; P = .0064), being treated in a facility with fewer than 500 beds (OR, 0.706-0.822, all statistically significant), being treated in the Midwest (OR, 0.62; P less than .0001) or the South (OR, 0.66; P less than .0001), and treatment by a surgeon with a lower surgical volume (fewer than 65 cases vs. 300; OR, 0.58; P = .0286).
, and one questioner at the session wondered about the validity of the 75% cutoff for high use, suggesting that it would be better to pick a value that was associated with a known increased risk of opioid dependence.
The findings could help inform future guidelines, Dr. Keller said. “On a local level, it can help optimize enhanced recovery protocols, (assist) providers to proactively recognize patients and scenarios at risk for high use, and create targeted education for younger patients, hospitals in specific geographic regions, and larger bedside hospitals so that they can follow best practices,” she added.
The finding that institutions with fewer beds were associated with lower chances of high opioid use was a surprise. “We’re looking into that,” she said.
Dr. Keller had no relevant financial disclosures.
SOURCE: Keller DS et al. Clinical Congress 2019, Abstract.
SAN FRANCISCO – Opioid prescription is surprisingly high after laparoscopic colorectal surgery, and is higher at larger hospitals and in some regions of the United States, according to a new study.
“In theory, for management of pain, any opioids required should be lower after laparoscopic surgery, but opioids are still ubiquitous and prescribing patterns have largely been unchanged with laparoscopy,” Deborah S. Keller, MD, assistant professor of surgery at Columbia University, New York, said at the annual clinical congress of the American College of Surgeons.
Several studies show wide variation in opioid use and prescribing after laparoscopic colorectal surgery, Dr. Keller said. She also pointed out that available data are limited on inpatient opioid use and the causes of high opioid use.
The team analyzed records of 18,395 subjects from the Premier Inpatient Database, between Jan. 1, 2014, and Sept. 30, 2015. The mean age was 61 years, and 54% were female. The distribution of hospital-stay milligram morphine equivalents (MME) was 48 at the 25th percentile, 108 at the 50th, and 246 at the 75th percentile. Overall, 18% of patients were in the high use category.
Some factors were associated with high opioid use, including emergency surgery (odds ratio, 1.28; P = .0002), being aged 18-34 (OR, 5.8; P less than .0001), major severity of illness (OR, 4.2; P less than .0001), chronic obstructive pulmonary disease comorbidity (OR, 1.13; P =.0350), having Medicaid insurance (OR, 1.35; P less than .0001), and being treated in a rural hospital (OR, 1.44; P less than.0001).
Factors associated with lower opioid use included female sex (OR, 0.90; P = .0064), being treated in a facility with fewer than 500 beds (OR, 0.706-0.822, all statistically significant), being treated in the Midwest (OR, 0.62; P less than .0001) or the South (OR, 0.66; P less than .0001), and treatment by a surgeon with a lower surgical volume (fewer than 65 cases vs. 300; OR, 0.58; P = .0286).
, and one questioner at the session wondered about the validity of the 75% cutoff for high use, suggesting that it would be better to pick a value that was associated with a known increased risk of opioid dependence.
The findings could help inform future guidelines, Dr. Keller said. “On a local level, it can help optimize enhanced recovery protocols, (assist) providers to proactively recognize patients and scenarios at risk for high use, and create targeted education for younger patients, hospitals in specific geographic regions, and larger bedside hospitals so that they can follow best practices,” she added.
The finding that institutions with fewer beds were associated with lower chances of high opioid use was a surprise. “We’re looking into that,” she said.
Dr. Keller had no relevant financial disclosures.
SOURCE: Keller DS et al. Clinical Congress 2019, Abstract.
SAN FRANCISCO – Opioid prescription is surprisingly high after laparoscopic colorectal surgery, and is higher at larger hospitals and in some regions of the United States, according to a new study.
“In theory, for management of pain, any opioids required should be lower after laparoscopic surgery, but opioids are still ubiquitous and prescribing patterns have largely been unchanged with laparoscopy,” Deborah S. Keller, MD, assistant professor of surgery at Columbia University, New York, said at the annual clinical congress of the American College of Surgeons.
Several studies show wide variation in opioid use and prescribing after laparoscopic colorectal surgery, Dr. Keller said. She also pointed out that available data are limited on inpatient opioid use and the causes of high opioid use.
The team analyzed records of 18,395 subjects from the Premier Inpatient Database, between Jan. 1, 2014, and Sept. 30, 2015. The mean age was 61 years, and 54% were female. The distribution of hospital-stay milligram morphine equivalents (MME) was 48 at the 25th percentile, 108 at the 50th, and 246 at the 75th percentile. Overall, 18% of patients were in the high use category.
Some factors were associated with high opioid use, including emergency surgery (odds ratio, 1.28; P = .0002), being aged 18-34 (OR, 5.8; P less than .0001), major severity of illness (OR, 4.2; P less than .0001), chronic obstructive pulmonary disease comorbidity (OR, 1.13; P =.0350), having Medicaid insurance (OR, 1.35; P less than .0001), and being treated in a rural hospital (OR, 1.44; P less than.0001).
Factors associated with lower opioid use included female sex (OR, 0.90; P = .0064), being treated in a facility with fewer than 500 beds (OR, 0.706-0.822, all statistically significant), being treated in the Midwest (OR, 0.62; P less than .0001) or the South (OR, 0.66; P less than .0001), and treatment by a surgeon with a lower surgical volume (fewer than 65 cases vs. 300; OR, 0.58; P = .0286).
, and one questioner at the session wondered about the validity of the 75% cutoff for high use, suggesting that it would be better to pick a value that was associated with a known increased risk of opioid dependence.
The findings could help inform future guidelines, Dr. Keller said. “On a local level, it can help optimize enhanced recovery protocols, (assist) providers to proactively recognize patients and scenarios at risk for high use, and create targeted education for younger patients, hospitals in specific geographic regions, and larger bedside hospitals so that they can follow best practices,” she added.
The finding that institutions with fewer beds were associated with lower chances of high opioid use was a surprise. “We’re looking into that,” she said.
Dr. Keller had no relevant financial disclosures.
SOURCE: Keller DS et al. Clinical Congress 2019, Abstract.
REPORTING FROM CLINICAL CONGRESS 2019
Dermatologists: Beware the ‘insulin ball’
LAS VEGAS – The patient, a 61-year-old man, came to see a dermatologist here about subcutaneous masses on his left arm, abdomen, and on both thighs.
It didn’t take long for Curt Samlaska, MD, of the University of Nevada, Reno, to link the masses to the patient’s daily regimen of seven insulin injections.
But diagnosing the condition required more than asking a few questions. At first, the man appeared to suffer from lipohypertrophy – a lump caused by an accumulation of fat at the site of insulin injections. But, Dr. Samlaska told colleagues, the patient had a different condition that’s barely been discussed in the dermatologic literature – insulin-derived amyloidosis, also known as “insulin ball.”
“It’s probably much more prevalent than we currently appreciate,” said Dr. Samlaska, who spoke in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Many cases are not [fully] evaluated and thought to be lipohypertrophy.”
Dr. Samlaska’s patient had suffered from diabetes since age 23 and tightly controls his blood sugar through seven daily injections. He injects short-acting insulin into his arms and abdomen, and long-acting insulin into his thighs.
The masses began appearing about 10 years ago, he told Dr. Samlaska, and he’s suffered more pain while injecting them over time. But the masses are easier to grasp during injections, and the patient’s body did not offer many other sites for injections.
, almost all in endocrinology journals. Ninety percent have a single lump, most commonly in the abdomen, and most have poor glycemic control, he said. (His patient is an outlier.)
Research suggests that insulin balls absorb about 34% of the insulin that’s injected, meaning that patients must inject more than usual to get the same effect. Be careful to advise patients about this, Dr. Samlaska said, because they might try alternative injection sites and get a sudden unexpected flood of insulin – potentially causing hypoglycemia.
He added that another drug – the HIV fusion inhibitor enfuvirtide – also has been linked to amyloidosis.
Pathology can offer insight into whether a mass is an insulin ball or a case of lipohypertrophy, he said. “They’re difficult to distinguish on clinical grounds,” he said, although lipohypertrophy masses are firmer, and they shrink when patients stop injecting insulin. Insulin balls do not.
The treatment for insulin balls is surgical excision, he said. “It’s very easy to do. With the extrusion technique, it comes out like a cheese, like a cyst.”
He said his patient was scheduled to soon undergo excision treatment.
Dr. Samlaska reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – The patient, a 61-year-old man, came to see a dermatologist here about subcutaneous masses on his left arm, abdomen, and on both thighs.
It didn’t take long for Curt Samlaska, MD, of the University of Nevada, Reno, to link the masses to the patient’s daily regimen of seven insulin injections.
But diagnosing the condition required more than asking a few questions. At first, the man appeared to suffer from lipohypertrophy – a lump caused by an accumulation of fat at the site of insulin injections. But, Dr. Samlaska told colleagues, the patient had a different condition that’s barely been discussed in the dermatologic literature – insulin-derived amyloidosis, also known as “insulin ball.”
“It’s probably much more prevalent than we currently appreciate,” said Dr. Samlaska, who spoke in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Many cases are not [fully] evaluated and thought to be lipohypertrophy.”
Dr. Samlaska’s patient had suffered from diabetes since age 23 and tightly controls his blood sugar through seven daily injections. He injects short-acting insulin into his arms and abdomen, and long-acting insulin into his thighs.
The masses began appearing about 10 years ago, he told Dr. Samlaska, and he’s suffered more pain while injecting them over time. But the masses are easier to grasp during injections, and the patient’s body did not offer many other sites for injections.
, almost all in endocrinology journals. Ninety percent have a single lump, most commonly in the abdomen, and most have poor glycemic control, he said. (His patient is an outlier.)
Research suggests that insulin balls absorb about 34% of the insulin that’s injected, meaning that patients must inject more than usual to get the same effect. Be careful to advise patients about this, Dr. Samlaska said, because they might try alternative injection sites and get a sudden unexpected flood of insulin – potentially causing hypoglycemia.
He added that another drug – the HIV fusion inhibitor enfuvirtide – also has been linked to amyloidosis.
Pathology can offer insight into whether a mass is an insulin ball or a case of lipohypertrophy, he said. “They’re difficult to distinguish on clinical grounds,” he said, although lipohypertrophy masses are firmer, and they shrink when patients stop injecting insulin. Insulin balls do not.
The treatment for insulin balls is surgical excision, he said. “It’s very easy to do. With the extrusion technique, it comes out like a cheese, like a cyst.”
He said his patient was scheduled to soon undergo excision treatment.
Dr. Samlaska reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
LAS VEGAS – The patient, a 61-year-old man, came to see a dermatologist here about subcutaneous masses on his left arm, abdomen, and on both thighs.
It didn’t take long for Curt Samlaska, MD, of the University of Nevada, Reno, to link the masses to the patient’s daily regimen of seven insulin injections.
But diagnosing the condition required more than asking a few questions. At first, the man appeared to suffer from lipohypertrophy – a lump caused by an accumulation of fat at the site of insulin injections. But, Dr. Samlaska told colleagues, the patient had a different condition that’s barely been discussed in the dermatologic literature – insulin-derived amyloidosis, also known as “insulin ball.”
“It’s probably much more prevalent than we currently appreciate,” said Dr. Samlaska, who spoke in a presentation at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “Many cases are not [fully] evaluated and thought to be lipohypertrophy.”
Dr. Samlaska’s patient had suffered from diabetes since age 23 and tightly controls his blood sugar through seven daily injections. He injects short-acting insulin into his arms and abdomen, and long-acting insulin into his thighs.
The masses began appearing about 10 years ago, he told Dr. Samlaska, and he’s suffered more pain while injecting them over time. But the masses are easier to grasp during injections, and the patient’s body did not offer many other sites for injections.
, almost all in endocrinology journals. Ninety percent have a single lump, most commonly in the abdomen, and most have poor glycemic control, he said. (His patient is an outlier.)
Research suggests that insulin balls absorb about 34% of the insulin that’s injected, meaning that patients must inject more than usual to get the same effect. Be careful to advise patients about this, Dr. Samlaska said, because they might try alternative injection sites and get a sudden unexpected flood of insulin – potentially causing hypoglycemia.
He added that another drug – the HIV fusion inhibitor enfuvirtide – also has been linked to amyloidosis.
Pathology can offer insight into whether a mass is an insulin ball or a case of lipohypertrophy, he said. “They’re difficult to distinguish on clinical grounds,” he said, although lipohypertrophy masses are firmer, and they shrink when patients stop injecting insulin. Insulin balls do not.
The treatment for insulin balls is surgical excision, he said. “It’s very easy to do. With the extrusion technique, it comes out like a cheese, like a cyst.”
He said his patient was scheduled to soon undergo excision treatment.
Dr. Samlaska reported no relevant disclosures. SDEF and this news organization are owned by the same parent company.
REPORTING FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
AAP calls for increased attention on unique health needs of adolescents
Adolescence is a critical period of development that brings with it unique health challenges, which has prompted the American Academy of Pediatrics to publish a policy statement addressing those issues.
“The importance of addressing the physical and mental health of adolescents has become more evident, with investigators in recent studies pointing to the fact that unmet health needs during adolescence and in the transition to adulthood predict not only poor health outcomes as adults but also lower quality of life in adulthood,” wrote lead authors Elizabeth M. Alderman, MD, and Cora Collette Breuner, MD, MPH, of the AAP’s Committee on Adolescence.
The first key health risk the authors highlighted was risky and risk-taking behaviors, pointing out that nearly three-quarters of adolescent deaths result from vehicle crashes, injuries from firearms, alcohol and illicit substances, homicide, or suicide. They also cited increased concern about the use of e-cigarettes among adolescents.
Recommendations exist on screening for and counseling on high-risk behaviors, but evidence showing that relatively few adolescents actually receive any kind of preventive counseling or discuss these health risks with pediatricians or primary care physicians suggests that improvement is needed.
“New screening codes for depression, substance use, and alcohol and tobacco use as well as brief intervention services may provide opportunities to receive payment for the services pediatricians are providing to adolescents,” wrote Dr. Alderman of the division of adolescent medicine in the department of pediatrics at Albert Einstein College of Medicine and the Children’s Hospital at Montefiore, New York, and Dr. Breuner of the division of adolescent medicine at the University of Washington and Seattle Children’s Hospital.
Thanks to technological advances in pediatric medical care, more adolescents are being identified with chronic medical conditions and developmental challenges. One survey suggested that as many as 31% of adolescents have one moderate to severe chronic health condition, such as asthma, cardiac disease, HIV, and developmental disabilities. Many, however, have unmet health needs that could affect their physical growth and development during adolescence.
, with evidence suggesting this group of adolescents is at risk of depression because of the isolation and discrimination they experience.
Similarly, the statement acknowledged the growing diversity of adolescent populations – for example, adolescents who identify as lesbian, gay, bisexual, or transgender – and the importance of delivering appropriate care to those populations.
“Sexual orientation and behaviors should be assessed by the pediatrician without making assumptions,” the authors wrote. “Adolescents should be allowed to apply and explain the labels they choose to use for sexuality and gender using open-ended questions.”
The authors drew attention to the greater mental health risks of adolescents, pointing out that about one in five adolescents have a diagnosable mental health disorder and one-quarter of adults with mood disorders had their first major depressive episode during adolescence. They also cited the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Survey of high school students, which showed that adolescents with a parent serving in the military are at increased risk of suicidal ideation.
In addition, Dr. Alderman and Dr. Breuner said, mental health problems experienced by adolescents often are comorbid with eating disorders. Formerly obese adolescents, male teenagers, and young people from lower socioeconomic groups are increasingly developing anorexia nervosa, bulimia nervosa, and other disordered eating.
The paper called for more financial, educational, and training support for pediatricians and other health care professionals to enable them to better meet the health and developmental needs of adolescents.
Dr. Alderman and Dr. Breuner declared having no conflicts of interest.
SOURCE: Alderman EM and Breuner CC. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3150 .
Adolescence is a critical period of development that brings with it unique health challenges, which has prompted the American Academy of Pediatrics to publish a policy statement addressing those issues.
“The importance of addressing the physical and mental health of adolescents has become more evident, with investigators in recent studies pointing to the fact that unmet health needs during adolescence and in the transition to adulthood predict not only poor health outcomes as adults but also lower quality of life in adulthood,” wrote lead authors Elizabeth M. Alderman, MD, and Cora Collette Breuner, MD, MPH, of the AAP’s Committee on Adolescence.
The first key health risk the authors highlighted was risky and risk-taking behaviors, pointing out that nearly three-quarters of adolescent deaths result from vehicle crashes, injuries from firearms, alcohol and illicit substances, homicide, or suicide. They also cited increased concern about the use of e-cigarettes among adolescents.
Recommendations exist on screening for and counseling on high-risk behaviors, but evidence showing that relatively few adolescents actually receive any kind of preventive counseling or discuss these health risks with pediatricians or primary care physicians suggests that improvement is needed.
“New screening codes for depression, substance use, and alcohol and tobacco use as well as brief intervention services may provide opportunities to receive payment for the services pediatricians are providing to adolescents,” wrote Dr. Alderman of the division of adolescent medicine in the department of pediatrics at Albert Einstein College of Medicine and the Children’s Hospital at Montefiore, New York, and Dr. Breuner of the division of adolescent medicine at the University of Washington and Seattle Children’s Hospital.
Thanks to technological advances in pediatric medical care, more adolescents are being identified with chronic medical conditions and developmental challenges. One survey suggested that as many as 31% of adolescents have one moderate to severe chronic health condition, such as asthma, cardiac disease, HIV, and developmental disabilities. Many, however, have unmet health needs that could affect their physical growth and development during adolescence.
, with evidence suggesting this group of adolescents is at risk of depression because of the isolation and discrimination they experience.
Similarly, the statement acknowledged the growing diversity of adolescent populations – for example, adolescents who identify as lesbian, gay, bisexual, or transgender – and the importance of delivering appropriate care to those populations.
“Sexual orientation and behaviors should be assessed by the pediatrician without making assumptions,” the authors wrote. “Adolescents should be allowed to apply and explain the labels they choose to use for sexuality and gender using open-ended questions.”
The authors drew attention to the greater mental health risks of adolescents, pointing out that about one in five adolescents have a diagnosable mental health disorder and one-quarter of adults with mood disorders had their first major depressive episode during adolescence. They also cited the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Survey of high school students, which showed that adolescents with a parent serving in the military are at increased risk of suicidal ideation.
In addition, Dr. Alderman and Dr. Breuner said, mental health problems experienced by adolescents often are comorbid with eating disorders. Formerly obese adolescents, male teenagers, and young people from lower socioeconomic groups are increasingly developing anorexia nervosa, bulimia nervosa, and other disordered eating.
The paper called for more financial, educational, and training support for pediatricians and other health care professionals to enable them to better meet the health and developmental needs of adolescents.
Dr. Alderman and Dr. Breuner declared having no conflicts of interest.
SOURCE: Alderman EM and Breuner CC. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3150 .
Adolescence is a critical period of development that brings with it unique health challenges, which has prompted the American Academy of Pediatrics to publish a policy statement addressing those issues.
“The importance of addressing the physical and mental health of adolescents has become more evident, with investigators in recent studies pointing to the fact that unmet health needs during adolescence and in the transition to adulthood predict not only poor health outcomes as adults but also lower quality of life in adulthood,” wrote lead authors Elizabeth M. Alderman, MD, and Cora Collette Breuner, MD, MPH, of the AAP’s Committee on Adolescence.
The first key health risk the authors highlighted was risky and risk-taking behaviors, pointing out that nearly three-quarters of adolescent deaths result from vehicle crashes, injuries from firearms, alcohol and illicit substances, homicide, or suicide. They also cited increased concern about the use of e-cigarettes among adolescents.
Recommendations exist on screening for and counseling on high-risk behaviors, but evidence showing that relatively few adolescents actually receive any kind of preventive counseling or discuss these health risks with pediatricians or primary care physicians suggests that improvement is needed.
“New screening codes for depression, substance use, and alcohol and tobacco use as well as brief intervention services may provide opportunities to receive payment for the services pediatricians are providing to adolescents,” wrote Dr. Alderman of the division of adolescent medicine in the department of pediatrics at Albert Einstein College of Medicine and the Children’s Hospital at Montefiore, New York, and Dr. Breuner of the division of adolescent medicine at the University of Washington and Seattle Children’s Hospital.
Thanks to technological advances in pediatric medical care, more adolescents are being identified with chronic medical conditions and developmental challenges. One survey suggested that as many as 31% of adolescents have one moderate to severe chronic health condition, such as asthma, cardiac disease, HIV, and developmental disabilities. Many, however, have unmet health needs that could affect their physical growth and development during adolescence.
, with evidence suggesting this group of adolescents is at risk of depression because of the isolation and discrimination they experience.
Similarly, the statement acknowledged the growing diversity of adolescent populations – for example, adolescents who identify as lesbian, gay, bisexual, or transgender – and the importance of delivering appropriate care to those populations.
“Sexual orientation and behaviors should be assessed by the pediatrician without making assumptions,” the authors wrote. “Adolescents should be allowed to apply and explain the labels they choose to use for sexuality and gender using open-ended questions.”
The authors drew attention to the greater mental health risks of adolescents, pointing out that about one in five adolescents have a diagnosable mental health disorder and one-quarter of adults with mood disorders had their first major depressive episode during adolescence. They also cited the Centers for Disease Control and Prevention’s 2017 Youth Risk Behavior Survey of high school students, which showed that adolescents with a parent serving in the military are at increased risk of suicidal ideation.
In addition, Dr. Alderman and Dr. Breuner said, mental health problems experienced by adolescents often are comorbid with eating disorders. Formerly obese adolescents, male teenagers, and young people from lower socioeconomic groups are increasingly developing anorexia nervosa, bulimia nervosa, and other disordered eating.
The paper called for more financial, educational, and training support for pediatricians and other health care professionals to enable them to better meet the health and developmental needs of adolescents.
Dr. Alderman and Dr. Breuner declared having no conflicts of interest.
SOURCE: Alderman EM and Breuner CC. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3150 .
FROM PEDIATRICS
Key clinical point: New screening codes for depression, substance use, and other intervention services may make it possible for pediatricians to receive payment for services.
Major finding: Adolescents might have particular health issues around risk-taking behaviors, mental health, and other issues.
Study details: Policy statement from the American Academy of Pediatrics.
Disclosures: No funding or conflicts of interest were declared.
Source: Alderman EM and Breuner CC. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3150.
AAP advises pediatricians to support emergency contraception for all teenagers
Educating pediatricians to inform their teenage patients about emergency contraception is an important step toward reducing adolescent pregnancy in the United States, according to a policy statement issued by the American Academy of Pediatrics.
“Improved use of contraception, not declines in sexual activity, has been the most significant contributor to the decline in pregnancy risk among U.S. teenagers over the past decade,” wrote Krishna K. Upadhya, MD, MPH, and colleagues on the AAP’s Committee on Adolescence.
; however, many pediatricians do not routinely counsel adolescents about emergency contraception, they noted.
In the statement published Nov. 18 in Pediatrics, the committee listed indications for emergency contraception as unprotected or underprotected intercourse for reasons including sexual assault, lack of contraception use, or ineffective contraception use. The committee recommended that pediatricians provide emergency contraception in the form of oral pills (levonorgestrel or ulipristal acetate) or copper IUDs to adolescents in immediate need of emergency contraception, and ideally, to make those products available in advance so teens have them on hand.
The committee recommended the use of combined contraceptive pills known as the Yuzpe method, if dedicated emergency contraceptive pills or IUDs are not available, and emphasized the possible impact of overweight and obesity on the effectiveness of emergency contraceptive pills.
The recommendations also include advising adolescents about proper use of emergency contraception, and the need for follow-up visits to address ongoing contraception and testing for sexually transmitted diseases. The committee noted that adolescents using emergency contraceptive pills must be counseled to abstain or use additional contraception (such as condoms) because of the delay in ovulation associated with these products.
The committee recommended that all adolescents receive counseling on emergency contraception as part of a general discussion on sexual health, regardless of current sexual activity or lack of it. “In addition, it is important that information about EC be included in all contraceptive and STI counseling for adolescents wherever these visits occur, including emergency departments, clinics, and hospitals,” and that pediatricians provide this information to teens with physical and cognitive disabilities and their parents as well, they wrote.
The committee concluded the recommendations by asking clinicians to advocate for free or inexpensive nonprescription access to emergency contraceptive pills for adolescents regardless of age and insurance status.
M. Susan Jay, MD, program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, Milwaukee, commented in an interview, “Forty years ago, as I completed my training, I don’t believe it would have been possible to contemplate the growth of adolescent health care, including reproductive health care that current pediatric practitioners are asked to provide to the adolescents under their care.”
“Today we are asked to be a resource from topics related to vaping and trafficking as well as psychosocial concerns from anxiety to eating disorders. This policy statement from the AAP addresses how best to approach and counsel both young women and young men as they traverse the issues of sexual engagement and responsibility. I have been privileged to work with pediatric residents who are far more sophisticated and knowledgeable than I have ever been, but they call and ask the very questions so adroitly presented in this policy statement. Most of my pediatric colleagues have had a limited adolescent medicine experience, and yet they are asked to care for youth in sensitive situations and want the tools necessary to provide the very best and safest care to their patients. Most of us will not be skilled in the placement of copper IUDs as outlined as an option for emergency contraception, but knowledge of the medications reviewed is of importance and relevant to everyday practice.
"This policy statement is a resource and educational update rolled into one, and Dr. Upadhya and her colleagues on the AAP’s Committee on Adolescence should be commended for assisting providers to offer the best and safest care,” said Dr. Jay, who was not involved in writing the AAP policy statement, and is a member of the Pediatric News Editorial Advisory Board who was asked to comment on the new policy statement.
The American College of Pediatricians (ACPeds), a conservative-leaning pediatric organization opposes the AAP’s recent opinion and the provision of emergency contraception to youth, Michelle Cretella, MD, executive director for the group, said in an interview. In its own position statement, ACPeds wrote that preprescribing EC to adolescent patients, or making them available without prescription, “carries significant medical risk and is counterproductive to the parent-adolescent and patient-physician relationships.”
“Increased access to [EC] does not result in lower pregnancy rates among adolescents and young adults,” said Dr. Cretella, a board-certified pediatrician who is not currently in practice. The ACPeds position statement cites a 2012 study that examined a Washington state program that allowed patients to access EC through pharmacies without a prescription. The analysis found the increased access to EC resulted in a statistically significant rise in gonorrhea for women and overall for both genders. The increased access to EC did not impact birth rates or abortion rates, according to the study (Economic Inquiry. 2013 Jul;51[3]:1682-95).
The ACPeds statement also notes a report by the Heritage Foundation that found sexually active teenagers were less likely to be happy and more likely to be depressed than were youth who were not having sex. The 2003 report, which examined responses from 6,500 adolescents through the 1996 National Longitudinal Survey of Adolescent Health, also found that sexually active teenagers were significantly more likely to attempt suicide, compared with teens who were not sexually active.
Dr. Upadhya disclosed having no financial conflicts.
SOURCE: Upadhya KK et al. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3149.
This article was updated on 11/19/19 and 12/16/19.
Despite declining teen birth rates, the United States has the highest rate of teen pregnancy among developed nations outside the former Soviet Bloc, according to the Guttmacher Institute. This high rate remains in part because of the significant barriers that prevent access to reproductive health services for adolescents. Teen pregnancy prevention remains an important adolescent health issue because of the high risk of poor health outcomes facing teen parents and their children. As advocates for children, pediatricians should educate, advocate for, and provide contraception to their patients. To this end, the AAP’s policy statement on emergency contraception (EC) provides practical guidance to increase access for EC for adolescents.
Simply put, EC provides contraception for “emergencies” such as unprotected sex, sexual assault, missed birth control pills, and condom failure. While EC is not meant to be the sole form of contraception used by adolescents, it is an important stop-gap measure – and the only one that can be used after sex. The “gold standard” for contraception in teens remains long-acting reversible contraception (LARC) methods such as the intrauterine device and the hormonal implant. These methods are recommended first line by the AAP because of their high efficacy.
Despite these recommendations, LARC use remains low, with only 6% of sexually active U.S. teens using these methods. While pediatricians should continue to encourage LARC methods, they should not neglect counseling on other contraceptive methods, including on EC.
In fact, studies demonstrate that pediatricians often omit counseling about EC, and most do not prescribe these medications routinely. Despite several available over-the-counter formulations, there still are significant barriers to teens in accessing these medications. In my practice, I have experienced teens who miss the opportunity to use this medication because of its cost and nonavailability when it is needed – from either inadequate stock at the pharmacy or from pharmacists’ conscientious objections. Ideally, counseling on EC should be part of the routine anticipatory guidance provided to all adolescents, and routine prescriptions should be given to adolescent women. When I prescribe EC to teens preventively, I tell them to fill the prescription and have it “on hand” at home in case it is ever needed, given the time-sensitive nature of most formulations. This policy also saliently addresses counseling for adolescent men – who often are overlooked in conversations about EC as they cannot use these methods. However, increasing their awareness and knowledge of this method can increase its use in their partners.
This policy provides excellent technical information on different formulations of EC, side effects, contraindications, and anticipatory guidance to give patients about the use of these medications. Additionally, it highlights the copper IUD – the often forgotten, but most effective form of EC that provides lasting pregnancy prevention. Overall, this policy provides great information to “demystify” EC and encourages pediatricians to engage in improving reproductive health access for adolescents.*
Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma Health Sciences Center, Oklahoma City. She was asked to comment on the AAP policy statement on emergency contraception. Dr. Curran is a member of the Pediatric News Editorial Advisory Board. Email her at pdnews@mdedge.com.
Despite declining teen birth rates, the United States has the highest rate of teen pregnancy among developed nations outside the former Soviet Bloc, according to the Guttmacher Institute. This high rate remains in part because of the significant barriers that prevent access to reproductive health services for adolescents. Teen pregnancy prevention remains an important adolescent health issue because of the high risk of poor health outcomes facing teen parents and their children. As advocates for children, pediatricians should educate, advocate for, and provide contraception to their patients. To this end, the AAP’s policy statement on emergency contraception (EC) provides practical guidance to increase access for EC for adolescents.
Simply put, EC provides contraception for “emergencies” such as unprotected sex, sexual assault, missed birth control pills, and condom failure. While EC is not meant to be the sole form of contraception used by adolescents, it is an important stop-gap measure – and the only one that can be used after sex. The “gold standard” for contraception in teens remains long-acting reversible contraception (LARC) methods such as the intrauterine device and the hormonal implant. These methods are recommended first line by the AAP because of their high efficacy.
Despite these recommendations, LARC use remains low, with only 6% of sexually active U.S. teens using these methods. While pediatricians should continue to encourage LARC methods, they should not neglect counseling on other contraceptive methods, including on EC.
In fact, studies demonstrate that pediatricians often omit counseling about EC, and most do not prescribe these medications routinely. Despite several available over-the-counter formulations, there still are significant barriers to teens in accessing these medications. In my practice, I have experienced teens who miss the opportunity to use this medication because of its cost and nonavailability when it is needed – from either inadequate stock at the pharmacy or from pharmacists’ conscientious objections. Ideally, counseling on EC should be part of the routine anticipatory guidance provided to all adolescents, and routine prescriptions should be given to adolescent women. When I prescribe EC to teens preventively, I tell them to fill the prescription and have it “on hand” at home in case it is ever needed, given the time-sensitive nature of most formulations. This policy also saliently addresses counseling for adolescent men – who often are overlooked in conversations about EC as they cannot use these methods. However, increasing their awareness and knowledge of this method can increase its use in their partners.
This policy provides excellent technical information on different formulations of EC, side effects, contraindications, and anticipatory guidance to give patients about the use of these medications. Additionally, it highlights the copper IUD – the often forgotten, but most effective form of EC that provides lasting pregnancy prevention. Overall, this policy provides great information to “demystify” EC and encourages pediatricians to engage in improving reproductive health access for adolescents.*
Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma Health Sciences Center, Oklahoma City. She was asked to comment on the AAP policy statement on emergency contraception. Dr. Curran is a member of the Pediatric News Editorial Advisory Board. Email her at pdnews@mdedge.com.
Despite declining teen birth rates, the United States has the highest rate of teen pregnancy among developed nations outside the former Soviet Bloc, according to the Guttmacher Institute. This high rate remains in part because of the significant barriers that prevent access to reproductive health services for adolescents. Teen pregnancy prevention remains an important adolescent health issue because of the high risk of poor health outcomes facing teen parents and their children. As advocates for children, pediatricians should educate, advocate for, and provide contraception to their patients. To this end, the AAP’s policy statement on emergency contraception (EC) provides practical guidance to increase access for EC for adolescents.
Simply put, EC provides contraception for “emergencies” such as unprotected sex, sexual assault, missed birth control pills, and condom failure. While EC is not meant to be the sole form of contraception used by adolescents, it is an important stop-gap measure – and the only one that can be used after sex. The “gold standard” for contraception in teens remains long-acting reversible contraception (LARC) methods such as the intrauterine device and the hormonal implant. These methods are recommended first line by the AAP because of their high efficacy.
Despite these recommendations, LARC use remains low, with only 6% of sexually active U.S. teens using these methods. While pediatricians should continue to encourage LARC methods, they should not neglect counseling on other contraceptive methods, including on EC.
In fact, studies demonstrate that pediatricians often omit counseling about EC, and most do not prescribe these medications routinely. Despite several available over-the-counter formulations, there still are significant barriers to teens in accessing these medications. In my practice, I have experienced teens who miss the opportunity to use this medication because of its cost and nonavailability when it is needed – from either inadequate stock at the pharmacy or from pharmacists’ conscientious objections. Ideally, counseling on EC should be part of the routine anticipatory guidance provided to all adolescents, and routine prescriptions should be given to adolescent women. When I prescribe EC to teens preventively, I tell them to fill the prescription and have it “on hand” at home in case it is ever needed, given the time-sensitive nature of most formulations. This policy also saliently addresses counseling for adolescent men – who often are overlooked in conversations about EC as they cannot use these methods. However, increasing their awareness and knowledge of this method can increase its use in their partners.
This policy provides excellent technical information on different formulations of EC, side effects, contraindications, and anticipatory guidance to give patients about the use of these medications. Additionally, it highlights the copper IUD – the often forgotten, but most effective form of EC that provides lasting pregnancy prevention. Overall, this policy provides great information to “demystify” EC and encourages pediatricians to engage in improving reproductive health access for adolescents.*
Kelly Curran, MD, specializes in adolescent medicine at the University of Oklahoma Health Sciences Center, Oklahoma City. She was asked to comment on the AAP policy statement on emergency contraception. Dr. Curran is a member of the Pediatric News Editorial Advisory Board. Email her at pdnews@mdedge.com.
Educating pediatricians to inform their teenage patients about emergency contraception is an important step toward reducing adolescent pregnancy in the United States, according to a policy statement issued by the American Academy of Pediatrics.
“Improved use of contraception, not declines in sexual activity, has been the most significant contributor to the decline in pregnancy risk among U.S. teenagers over the past decade,” wrote Krishna K. Upadhya, MD, MPH, and colleagues on the AAP’s Committee on Adolescence.
; however, many pediatricians do not routinely counsel adolescents about emergency contraception, they noted.
In the statement published Nov. 18 in Pediatrics, the committee listed indications for emergency contraception as unprotected or underprotected intercourse for reasons including sexual assault, lack of contraception use, or ineffective contraception use. The committee recommended that pediatricians provide emergency contraception in the form of oral pills (levonorgestrel or ulipristal acetate) or copper IUDs to adolescents in immediate need of emergency contraception, and ideally, to make those products available in advance so teens have them on hand.
The committee recommended the use of combined contraceptive pills known as the Yuzpe method, if dedicated emergency contraceptive pills or IUDs are not available, and emphasized the possible impact of overweight and obesity on the effectiveness of emergency contraceptive pills.
The recommendations also include advising adolescents about proper use of emergency contraception, and the need for follow-up visits to address ongoing contraception and testing for sexually transmitted diseases. The committee noted that adolescents using emergency contraceptive pills must be counseled to abstain or use additional contraception (such as condoms) because of the delay in ovulation associated with these products.
The committee recommended that all adolescents receive counseling on emergency contraception as part of a general discussion on sexual health, regardless of current sexual activity or lack of it. “In addition, it is important that information about EC be included in all contraceptive and STI counseling for adolescents wherever these visits occur, including emergency departments, clinics, and hospitals,” and that pediatricians provide this information to teens with physical and cognitive disabilities and their parents as well, they wrote.
The committee concluded the recommendations by asking clinicians to advocate for free or inexpensive nonprescription access to emergency contraceptive pills for adolescents regardless of age and insurance status.
M. Susan Jay, MD, program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, Milwaukee, commented in an interview, “Forty years ago, as I completed my training, I don’t believe it would have been possible to contemplate the growth of adolescent health care, including reproductive health care that current pediatric practitioners are asked to provide to the adolescents under their care.”
“Today we are asked to be a resource from topics related to vaping and trafficking as well as psychosocial concerns from anxiety to eating disorders. This policy statement from the AAP addresses how best to approach and counsel both young women and young men as they traverse the issues of sexual engagement and responsibility. I have been privileged to work with pediatric residents who are far more sophisticated and knowledgeable than I have ever been, but they call and ask the very questions so adroitly presented in this policy statement. Most of my pediatric colleagues have had a limited adolescent medicine experience, and yet they are asked to care for youth in sensitive situations and want the tools necessary to provide the very best and safest care to their patients. Most of us will not be skilled in the placement of copper IUDs as outlined as an option for emergency contraception, but knowledge of the medications reviewed is of importance and relevant to everyday practice.
"This policy statement is a resource and educational update rolled into one, and Dr. Upadhya and her colleagues on the AAP’s Committee on Adolescence should be commended for assisting providers to offer the best and safest care,” said Dr. Jay, who was not involved in writing the AAP policy statement, and is a member of the Pediatric News Editorial Advisory Board who was asked to comment on the new policy statement.
The American College of Pediatricians (ACPeds), a conservative-leaning pediatric organization opposes the AAP’s recent opinion and the provision of emergency contraception to youth, Michelle Cretella, MD, executive director for the group, said in an interview. In its own position statement, ACPeds wrote that preprescribing EC to adolescent patients, or making them available without prescription, “carries significant medical risk and is counterproductive to the parent-adolescent and patient-physician relationships.”
“Increased access to [EC] does not result in lower pregnancy rates among adolescents and young adults,” said Dr. Cretella, a board-certified pediatrician who is not currently in practice. The ACPeds position statement cites a 2012 study that examined a Washington state program that allowed patients to access EC through pharmacies without a prescription. The analysis found the increased access to EC resulted in a statistically significant rise in gonorrhea for women and overall for both genders. The increased access to EC did not impact birth rates or abortion rates, according to the study (Economic Inquiry. 2013 Jul;51[3]:1682-95).
The ACPeds statement also notes a report by the Heritage Foundation that found sexually active teenagers were less likely to be happy and more likely to be depressed than were youth who were not having sex. The 2003 report, which examined responses from 6,500 adolescents through the 1996 National Longitudinal Survey of Adolescent Health, also found that sexually active teenagers were significantly more likely to attempt suicide, compared with teens who were not sexually active.
Dr. Upadhya disclosed having no financial conflicts.
SOURCE: Upadhya KK et al. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3149.
This article was updated on 11/19/19 and 12/16/19.
Educating pediatricians to inform their teenage patients about emergency contraception is an important step toward reducing adolescent pregnancy in the United States, according to a policy statement issued by the American Academy of Pediatrics.
“Improved use of contraception, not declines in sexual activity, has been the most significant contributor to the decline in pregnancy risk among U.S. teenagers over the past decade,” wrote Krishna K. Upadhya, MD, MPH, and colleagues on the AAP’s Committee on Adolescence.
; however, many pediatricians do not routinely counsel adolescents about emergency contraception, they noted.
In the statement published Nov. 18 in Pediatrics, the committee listed indications for emergency contraception as unprotected or underprotected intercourse for reasons including sexual assault, lack of contraception use, or ineffective contraception use. The committee recommended that pediatricians provide emergency contraception in the form of oral pills (levonorgestrel or ulipristal acetate) or copper IUDs to adolescents in immediate need of emergency contraception, and ideally, to make those products available in advance so teens have them on hand.
The committee recommended the use of combined contraceptive pills known as the Yuzpe method, if dedicated emergency contraceptive pills or IUDs are not available, and emphasized the possible impact of overweight and obesity on the effectiveness of emergency contraceptive pills.
The recommendations also include advising adolescents about proper use of emergency contraception, and the need for follow-up visits to address ongoing contraception and testing for sexually transmitted diseases. The committee noted that adolescents using emergency contraceptive pills must be counseled to abstain or use additional contraception (such as condoms) because of the delay in ovulation associated with these products.
The committee recommended that all adolescents receive counseling on emergency contraception as part of a general discussion on sexual health, regardless of current sexual activity or lack of it. “In addition, it is important that information about EC be included in all contraceptive and STI counseling for adolescents wherever these visits occur, including emergency departments, clinics, and hospitals,” and that pediatricians provide this information to teens with physical and cognitive disabilities and their parents as well, they wrote.
The committee concluded the recommendations by asking clinicians to advocate for free or inexpensive nonprescription access to emergency contraceptive pills for adolescents regardless of age and insurance status.
M. Susan Jay, MD, program director of adolescent health and medicine at the Children’s Hospital of Wisconsin, Milwaukee, commented in an interview, “Forty years ago, as I completed my training, I don’t believe it would have been possible to contemplate the growth of adolescent health care, including reproductive health care that current pediatric practitioners are asked to provide to the adolescents under their care.”
“Today we are asked to be a resource from topics related to vaping and trafficking as well as psychosocial concerns from anxiety to eating disorders. This policy statement from the AAP addresses how best to approach and counsel both young women and young men as they traverse the issues of sexual engagement and responsibility. I have been privileged to work with pediatric residents who are far more sophisticated and knowledgeable than I have ever been, but they call and ask the very questions so adroitly presented in this policy statement. Most of my pediatric colleagues have had a limited adolescent medicine experience, and yet they are asked to care for youth in sensitive situations and want the tools necessary to provide the very best and safest care to their patients. Most of us will not be skilled in the placement of copper IUDs as outlined as an option for emergency contraception, but knowledge of the medications reviewed is of importance and relevant to everyday practice.
"This policy statement is a resource and educational update rolled into one, and Dr. Upadhya and her colleagues on the AAP’s Committee on Adolescence should be commended for assisting providers to offer the best and safest care,” said Dr. Jay, who was not involved in writing the AAP policy statement, and is a member of the Pediatric News Editorial Advisory Board who was asked to comment on the new policy statement.
The American College of Pediatricians (ACPeds), a conservative-leaning pediatric organization opposes the AAP’s recent opinion and the provision of emergency contraception to youth, Michelle Cretella, MD, executive director for the group, said in an interview. In its own position statement, ACPeds wrote that preprescribing EC to adolescent patients, or making them available without prescription, “carries significant medical risk and is counterproductive to the parent-adolescent and patient-physician relationships.”
“Increased access to [EC] does not result in lower pregnancy rates among adolescents and young adults,” said Dr. Cretella, a board-certified pediatrician who is not currently in practice. The ACPeds position statement cites a 2012 study that examined a Washington state program that allowed patients to access EC through pharmacies without a prescription. The analysis found the increased access to EC resulted in a statistically significant rise in gonorrhea for women and overall for both genders. The increased access to EC did not impact birth rates or abortion rates, according to the study (Economic Inquiry. 2013 Jul;51[3]:1682-95).
The ACPeds statement also notes a report by the Heritage Foundation that found sexually active teenagers were less likely to be happy and more likely to be depressed than were youth who were not having sex. The 2003 report, which examined responses from 6,500 adolescents through the 1996 National Longitudinal Survey of Adolescent Health, also found that sexually active teenagers were significantly more likely to attempt suicide, compared with teens who were not sexually active.
Dr. Upadhya disclosed having no financial conflicts.
SOURCE: Upadhya KK et al. Pediatrics. 2019 Nov 18. doi: 10.1542/peds.2019-3149.
This article was updated on 11/19/19 and 12/16/19.
FROM PEDIATRICS
Are you operating in the black when it comes to vaccine administration?
NEW ORLEANS – One way to make sure your practice providing immunizations is in the black is to calculate your “carrying costs” and apply them to the cost of your vaccines.
Another is to make sure that you join an effectively managed and effective group purchasing organization.
Those are two tips that Chip Hart shared with attendees at the annual meeting of the American Academy of Pediatrics.
said Mr. Hart, director of the Winooski, Vt.–based the Pediatric Solutions Consulting Group at the Physicians Computer Company. “Providing immunizations is the single most valuable thing that you do, by far. Yet you get ripped off by the payers all the time.”
Two documents from the AAP – “The business case for pricing vaccines” and “The business case for pricing immunization administration” – provide clear-cut guidance on the impact of vaccine delivery to your bottom line. Based on data from his company’s client base, Mr. Hart said that vaccines have grown from 13% of an average pediatric practice’s revenue in 2003 to 22% in 2018. “The AAP’s own research shows that you need to generate 17%-28% above what you paid for the vaccine in order just to break even,” he said. That’s to cover the administrative overhead required to purchase and store the product in an office-based refrigerator, and the staff time to administer it. Such “carrying costs” often are not factored into the analysis of many managing pediatricians.
“The unfortunate reality is, you are not paid for carrying costs related to the administration of vaccines, including your refrigerator, your sharps and waste management, claim denials, and especially every time you waste a vaccine,” Mr. Hart said. “None of those things are part of any fee schedule.”
How to determine your vaccine product overhead
There are two ways to go about determining your vaccine product overhead. The first is to perform an in-depth analysis of your costs, including time studies and cost accounting. For example, he said that if your hazardous waste costs are $3,500 per year and half of the material is composed of vaccine waste, that leaves $1,750. “If you divide that by the number of vaccines you did last year, it might come out to 13 cents per vaccine,” Mr. Hart said, “but these things add up.” On the administration side, he offered the example of a nurse who makes $45,000 per year and who devotes 10% of her time to vaccines in a practice that administers 13,000 vaccinations per year. In this case, $45,000 per year divided by 13,000 vaccines equals 35 cents than can be added to the cost of every vaccine.
“You can go into each one of these elements and figure out how much you need to clear in order to do all right,” he said.
Alternatively, you can use the research from the AAP to presume that you need to have a margin of 17%-28% on your product. “Use a figure like 20% or 25% – it’s likely as accurate as any analysis a busy private practice is capable of doing, and you can immediately determine if you are in the profitability ballpark,” Mr. Hart said. On the administration side of the equation, in 2009, researchers estimated that the total documented variable cost per injection, excluding vaccine cost, was $11.51 (Pediatrics. 2009 Dec;124 [Suppl 5]:S492-8). That figure is more like $14 or $15 per vaccine in today’s dollars, Mr. Hart estimated. “You can perform a time-motion study and determine all of your immunization administration costs or you can just simply pick an evidence-based figure like $14 and see how well you are doing,” he said.
On his company’s web site, he offers a free administrative analysis tool that clinicians can use to determine how they fare. The AAP also provides information about vaccine financing here.
How to make sure you are operating in the red
Mr. Hart advises practices operating in the red to review their vaccine delivery work flow “to look for leaks,” to use proper administrative codes, and to negotiate the price of vaccine product with payers. “The only payers that don’t negotiate are state Medicaid and Tricare,” he said. “Everyone else negotiates. You want to determine the methodology they use to calculate what they pay you for the vaccine product. Different payers have different rule sets.”
Another strategy to join a group purchasing organization (GPO), which can leverage volume purchasing to negotiate discounts on vaccines. “They’re like [the] Costco or Sam’s Club of vaccine purchasing, and in most cases they can save you about $10,000 per year,” Mr. Hart said. A list of GPOs from the AAP can be found here.
Implementing effective inventory management is also key. “Practices that have the discipline to maintain their inventories are inevitably the ones who are more profitable,” Mr. Hart said. “I’ve worked with too many practices where flu shots go missing. Staff take them home or bring in their friends after hours. You need inventory control, and you should be able to generate an inventory report out of your practice management system. You also should be able to generate a report out of your EHR.”
Mr. Hart reported having no relevant financial disclosures.
NEW ORLEANS – One way to make sure your practice providing immunizations is in the black is to calculate your “carrying costs” and apply them to the cost of your vaccines.
Another is to make sure that you join an effectively managed and effective group purchasing organization.
Those are two tips that Chip Hart shared with attendees at the annual meeting of the American Academy of Pediatrics.
said Mr. Hart, director of the Winooski, Vt.–based the Pediatric Solutions Consulting Group at the Physicians Computer Company. “Providing immunizations is the single most valuable thing that you do, by far. Yet you get ripped off by the payers all the time.”
Two documents from the AAP – “The business case for pricing vaccines” and “The business case for pricing immunization administration” – provide clear-cut guidance on the impact of vaccine delivery to your bottom line. Based on data from his company’s client base, Mr. Hart said that vaccines have grown from 13% of an average pediatric practice’s revenue in 2003 to 22% in 2018. “The AAP’s own research shows that you need to generate 17%-28% above what you paid for the vaccine in order just to break even,” he said. That’s to cover the administrative overhead required to purchase and store the product in an office-based refrigerator, and the staff time to administer it. Such “carrying costs” often are not factored into the analysis of many managing pediatricians.
“The unfortunate reality is, you are not paid for carrying costs related to the administration of vaccines, including your refrigerator, your sharps and waste management, claim denials, and especially every time you waste a vaccine,” Mr. Hart said. “None of those things are part of any fee schedule.”
How to determine your vaccine product overhead
There are two ways to go about determining your vaccine product overhead. The first is to perform an in-depth analysis of your costs, including time studies and cost accounting. For example, he said that if your hazardous waste costs are $3,500 per year and half of the material is composed of vaccine waste, that leaves $1,750. “If you divide that by the number of vaccines you did last year, it might come out to 13 cents per vaccine,” Mr. Hart said, “but these things add up.” On the administration side, he offered the example of a nurse who makes $45,000 per year and who devotes 10% of her time to vaccines in a practice that administers 13,000 vaccinations per year. In this case, $45,000 per year divided by 13,000 vaccines equals 35 cents than can be added to the cost of every vaccine.
“You can go into each one of these elements and figure out how much you need to clear in order to do all right,” he said.
Alternatively, you can use the research from the AAP to presume that you need to have a margin of 17%-28% on your product. “Use a figure like 20% or 25% – it’s likely as accurate as any analysis a busy private practice is capable of doing, and you can immediately determine if you are in the profitability ballpark,” Mr. Hart said. On the administration side of the equation, in 2009, researchers estimated that the total documented variable cost per injection, excluding vaccine cost, was $11.51 (Pediatrics. 2009 Dec;124 [Suppl 5]:S492-8). That figure is more like $14 or $15 per vaccine in today’s dollars, Mr. Hart estimated. “You can perform a time-motion study and determine all of your immunization administration costs or you can just simply pick an evidence-based figure like $14 and see how well you are doing,” he said.
On his company’s web site, he offers a free administrative analysis tool that clinicians can use to determine how they fare. The AAP also provides information about vaccine financing here.
How to make sure you are operating in the red
Mr. Hart advises practices operating in the red to review their vaccine delivery work flow “to look for leaks,” to use proper administrative codes, and to negotiate the price of vaccine product with payers. “The only payers that don’t negotiate are state Medicaid and Tricare,” he said. “Everyone else negotiates. You want to determine the methodology they use to calculate what they pay you for the vaccine product. Different payers have different rule sets.”
Another strategy to join a group purchasing organization (GPO), which can leverage volume purchasing to negotiate discounts on vaccines. “They’re like [the] Costco or Sam’s Club of vaccine purchasing, and in most cases they can save you about $10,000 per year,” Mr. Hart said. A list of GPOs from the AAP can be found here.
Implementing effective inventory management is also key. “Practices that have the discipline to maintain their inventories are inevitably the ones who are more profitable,” Mr. Hart said. “I’ve worked with too many practices where flu shots go missing. Staff take them home or bring in their friends after hours. You need inventory control, and you should be able to generate an inventory report out of your practice management system. You also should be able to generate a report out of your EHR.”
Mr. Hart reported having no relevant financial disclosures.
NEW ORLEANS – One way to make sure your practice providing immunizations is in the black is to calculate your “carrying costs” and apply them to the cost of your vaccines.
Another is to make sure that you join an effectively managed and effective group purchasing organization.
Those are two tips that Chip Hart shared with attendees at the annual meeting of the American Academy of Pediatrics.
said Mr. Hart, director of the Winooski, Vt.–based the Pediatric Solutions Consulting Group at the Physicians Computer Company. “Providing immunizations is the single most valuable thing that you do, by far. Yet you get ripped off by the payers all the time.”
Two documents from the AAP – “The business case for pricing vaccines” and “The business case for pricing immunization administration” – provide clear-cut guidance on the impact of vaccine delivery to your bottom line. Based on data from his company’s client base, Mr. Hart said that vaccines have grown from 13% of an average pediatric practice’s revenue in 2003 to 22% in 2018. “The AAP’s own research shows that you need to generate 17%-28% above what you paid for the vaccine in order just to break even,” he said. That’s to cover the administrative overhead required to purchase and store the product in an office-based refrigerator, and the staff time to administer it. Such “carrying costs” often are not factored into the analysis of many managing pediatricians.
“The unfortunate reality is, you are not paid for carrying costs related to the administration of vaccines, including your refrigerator, your sharps and waste management, claim denials, and especially every time you waste a vaccine,” Mr. Hart said. “None of those things are part of any fee schedule.”
How to determine your vaccine product overhead
There are two ways to go about determining your vaccine product overhead. The first is to perform an in-depth analysis of your costs, including time studies and cost accounting. For example, he said that if your hazardous waste costs are $3,500 per year and half of the material is composed of vaccine waste, that leaves $1,750. “If you divide that by the number of vaccines you did last year, it might come out to 13 cents per vaccine,” Mr. Hart said, “but these things add up.” On the administration side, he offered the example of a nurse who makes $45,000 per year and who devotes 10% of her time to vaccines in a practice that administers 13,000 vaccinations per year. In this case, $45,000 per year divided by 13,000 vaccines equals 35 cents than can be added to the cost of every vaccine.
“You can go into each one of these elements and figure out how much you need to clear in order to do all right,” he said.
Alternatively, you can use the research from the AAP to presume that you need to have a margin of 17%-28% on your product. “Use a figure like 20% or 25% – it’s likely as accurate as any analysis a busy private practice is capable of doing, and you can immediately determine if you are in the profitability ballpark,” Mr. Hart said. On the administration side of the equation, in 2009, researchers estimated that the total documented variable cost per injection, excluding vaccine cost, was $11.51 (Pediatrics. 2009 Dec;124 [Suppl 5]:S492-8). That figure is more like $14 or $15 per vaccine in today’s dollars, Mr. Hart estimated. “You can perform a time-motion study and determine all of your immunization administration costs or you can just simply pick an evidence-based figure like $14 and see how well you are doing,” he said.
On his company’s web site, he offers a free administrative analysis tool that clinicians can use to determine how they fare. The AAP also provides information about vaccine financing here.
How to make sure you are operating in the red
Mr. Hart advises practices operating in the red to review their vaccine delivery work flow “to look for leaks,” to use proper administrative codes, and to negotiate the price of vaccine product with payers. “The only payers that don’t negotiate are state Medicaid and Tricare,” he said. “Everyone else negotiates. You want to determine the methodology they use to calculate what they pay you for the vaccine product. Different payers have different rule sets.”
Another strategy to join a group purchasing organization (GPO), which can leverage volume purchasing to negotiate discounts on vaccines. “They’re like [the] Costco or Sam’s Club of vaccine purchasing, and in most cases they can save you about $10,000 per year,” Mr. Hart said. A list of GPOs from the AAP can be found here.
Implementing effective inventory management is also key. “Practices that have the discipline to maintain their inventories are inevitably the ones who are more profitable,” Mr. Hart said. “I’ve worked with too many practices where flu shots go missing. Staff take them home or bring in their friends after hours. You need inventory control, and you should be able to generate an inventory report out of your practice management system. You also should be able to generate a report out of your EHR.”
Mr. Hart reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Early use of ustekinumab levels could predict psoriasis outcomes
Early ustekinumab levels are significantly associated with a 75% reduction in Psoriasis Area and Severity Index scores from baseline, according to data from 491 adults with psoriasis.
“Evidence suggests that ustekinumab dosing is suboptimal in some patients,” because of factors including weight-based dosing and dosing intervals; therefore “individualized dose optimization and therapeutic drug monitoring (TDM) of ustekinumab may have clinical utility,” Teresa Tsakok, MRCP, of King’s College London, and colleagues wrote in JAMA Dermatology.
The researchers identified 491 adults with psoriasis who were part of the BSTOP (Biomarkers of Systemic Treatment Outcomes in Psoriasis) cohort study in the United Kingdom. Blood samples were collected during clinical reviews to assess ustekinumab levels, and primary treatment response was 75% reduction in Psoriasis Area and Severity Index (PASI 75) scores.
Ustekinumab levels measured from 1-12 weeks after the start of treatment were significantly associated with PASI 75 after 6 months (odds ratio, 1.38) after controlling for factors including baseline PASI scores, age, and ustekinumab dose. The association, however, did not hold for other PASI outcomes, including PASI 90 and PASI scores of 1.5 or less.
The participants had at least one serum sample collected at 0-56 weeks from the start of treatment and at least one PSAI score measured within the first year of treatment. The average baseline PASI score was 13.3, the average body mass index was 32 kg/m2, and 65% of the patients were male.
Antidrug antibodies were detected in 17 patients (3.5% of the study population), compared with a rate of 37.5% in patients from the same study cohort who were taking adalimumab, the researchers noted.
The study findings were limited by several factors, including a dropoff in patient numbers over the study period and the difficulty in accounting for the association between drug level and treatment response in a logistic regression model, the researchers said.
The results, however, suggest “that adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome with ustekinumab,” and that “future work should focus on pharmacokinetic-pharmacodynamic modeling of the whole time course of response to ustekinumab” as a key step toward personalized treatment regimens, they concluded.
The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center and the Psoriasis Association. Dr. Tsakok had no financial conflicts to disclose and was supported by an MRC Clinical Research Training Fellowship.
SOURCE: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
Early ustekinumab levels are significantly associated with a 75% reduction in Psoriasis Area and Severity Index scores from baseline, according to data from 491 adults with psoriasis.
“Evidence suggests that ustekinumab dosing is suboptimal in some patients,” because of factors including weight-based dosing and dosing intervals; therefore “individualized dose optimization and therapeutic drug monitoring (TDM) of ustekinumab may have clinical utility,” Teresa Tsakok, MRCP, of King’s College London, and colleagues wrote in JAMA Dermatology.
The researchers identified 491 adults with psoriasis who were part of the BSTOP (Biomarkers of Systemic Treatment Outcomes in Psoriasis) cohort study in the United Kingdom. Blood samples were collected during clinical reviews to assess ustekinumab levels, and primary treatment response was 75% reduction in Psoriasis Area and Severity Index (PASI 75) scores.
Ustekinumab levels measured from 1-12 weeks after the start of treatment were significantly associated with PASI 75 after 6 months (odds ratio, 1.38) after controlling for factors including baseline PASI scores, age, and ustekinumab dose. The association, however, did not hold for other PASI outcomes, including PASI 90 and PASI scores of 1.5 or less.
The participants had at least one serum sample collected at 0-56 weeks from the start of treatment and at least one PSAI score measured within the first year of treatment. The average baseline PASI score was 13.3, the average body mass index was 32 kg/m2, and 65% of the patients were male.
Antidrug antibodies were detected in 17 patients (3.5% of the study population), compared with a rate of 37.5% in patients from the same study cohort who were taking adalimumab, the researchers noted.
The study findings were limited by several factors, including a dropoff in patient numbers over the study period and the difficulty in accounting for the association between drug level and treatment response in a logistic regression model, the researchers said.
The results, however, suggest “that adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome with ustekinumab,” and that “future work should focus on pharmacokinetic-pharmacodynamic modeling of the whole time course of response to ustekinumab” as a key step toward personalized treatment regimens, they concluded.
The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center and the Psoriasis Association. Dr. Tsakok had no financial conflicts to disclose and was supported by an MRC Clinical Research Training Fellowship.
SOURCE: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
Early ustekinumab levels are significantly associated with a 75% reduction in Psoriasis Area and Severity Index scores from baseline, according to data from 491 adults with psoriasis.
“Evidence suggests that ustekinumab dosing is suboptimal in some patients,” because of factors including weight-based dosing and dosing intervals; therefore “individualized dose optimization and therapeutic drug monitoring (TDM) of ustekinumab may have clinical utility,” Teresa Tsakok, MRCP, of King’s College London, and colleagues wrote in JAMA Dermatology.
The researchers identified 491 adults with psoriasis who were part of the BSTOP (Biomarkers of Systemic Treatment Outcomes in Psoriasis) cohort study in the United Kingdom. Blood samples were collected during clinical reviews to assess ustekinumab levels, and primary treatment response was 75% reduction in Psoriasis Area and Severity Index (PASI 75) scores.
Ustekinumab levels measured from 1-12 weeks after the start of treatment were significantly associated with PASI 75 after 6 months (odds ratio, 1.38) after controlling for factors including baseline PASI scores, age, and ustekinumab dose. The association, however, did not hold for other PASI outcomes, including PASI 90 and PASI scores of 1.5 or less.
The participants had at least one serum sample collected at 0-56 weeks from the start of treatment and at least one PSAI score measured within the first year of treatment. The average baseline PASI score was 13.3, the average body mass index was 32 kg/m2, and 65% of the patients were male.
Antidrug antibodies were detected in 17 patients (3.5% of the study population), compared with a rate of 37.5% in patients from the same study cohort who were taking adalimumab, the researchers noted.
The study findings were limited by several factors, including a dropoff in patient numbers over the study period and the difficulty in accounting for the association between drug level and treatment response in a logistic regression model, the researchers said.
The results, however, suggest “that adequate drug exposure early in the treatment cycle may be particularly important in determining clinical outcome with ustekinumab,” and that “future work should focus on pharmacokinetic-pharmacodynamic modeling of the whole time course of response to ustekinumab” as a key step toward personalized treatment regimens, they concluded.
The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center and the Psoriasis Association. Dr. Tsakok had no financial conflicts to disclose and was supported by an MRC Clinical Research Training Fellowship.
SOURCE: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.
FROM JAMA DERMATOLOGY
Key clinical point: Measurement of ustekinumab levels early in treatment could help direct strategies and ensure correct dosage in psoriasis patients.
Major finding: Early serum ustekinumab levels 1-12 weeks after starting treatment were associated with a 75% reduction in PASI scores from baseline at 6 months.
Study details: The prospective, observational study involved 491 adults with psoriasis.
Disclosures: The study was supported by several entities, including the Medical Research Council (MRC), the National Institute for Health Research Biomedical Research Center, and the Psoriasis Association. Dr. Tsakok disclosed financial conflicts and was supported by an MRC Clinical Research Training Fellowship.
Source: Tsakok T et al. JAMA Dermatol. 2019 Sep 18. doi: 10.1001/jamadermatol.2019.1783.