User login
Inpatient care declining among family physicians
and by 2017, only one of four FPs was practicing hospital medicine, according to the American Academy of Family Physicians.
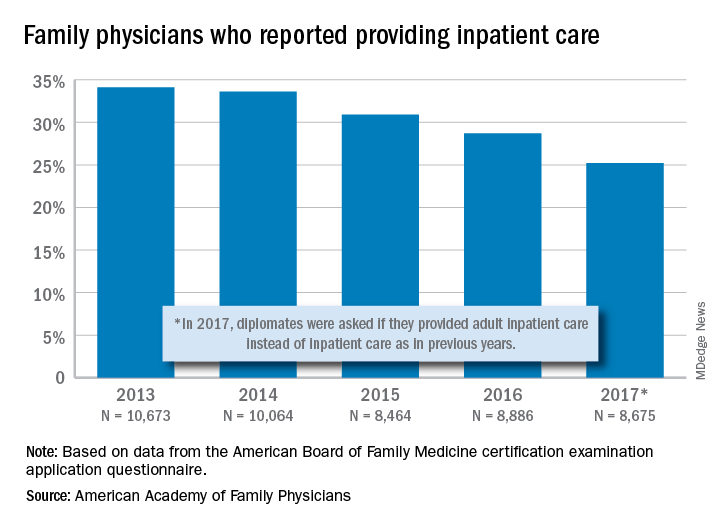
The share of family physicians who provided hospital care went from 34.1% in 2013 to 25.2% in 2017, for a relative decrease of 26% that left only a quarter of FPs seeing inpatients, based on data from the annual American Board of Family Medicine certification exam application questionnaire. For the 5-year period, 46,762 individuals were included in the study sample of FPs in direct patient care.
“As observed in other domains (prenatal care, home visits, nursing home care, and obstetric care), this study adds to the evidence demonstrating contracting scope of practice among FPs,” Anuradha Jetty, MPH, of the AAFP’s Robert Graham Center in Washington, D.C., and associates said in a recent Policy Brief published in the Journal of the American Board of Family Medicine.
Much of that contraction is occurring among new family physicians who can’t “find positions that allow them to use all their expertise,” the investigators said in a separate statement. The AAFP had previously reported that about 40% of family physicians had full hospital privileges in 2018, compared with 56% in 2012.
Many new FPs now work in large multispecialty practices or hospital systems, and “[some] of these employers dictate scope of practice, limiting family physicians to coordinating outpatient care and relying on subspecialists or hospitalists to provide inpatient care,” they noted.
and by 2017, only one of four FPs was practicing hospital medicine, according to the American Academy of Family Physicians.

The share of family physicians who provided hospital care went from 34.1% in 2013 to 25.2% in 2017, for a relative decrease of 26% that left only a quarter of FPs seeing inpatients, based on data from the annual American Board of Family Medicine certification exam application questionnaire. For the 5-year period, 46,762 individuals were included in the study sample of FPs in direct patient care.
“As observed in other domains (prenatal care, home visits, nursing home care, and obstetric care), this study adds to the evidence demonstrating contracting scope of practice among FPs,” Anuradha Jetty, MPH, of the AAFP’s Robert Graham Center in Washington, D.C., and associates said in a recent Policy Brief published in the Journal of the American Board of Family Medicine.
Much of that contraction is occurring among new family physicians who can’t “find positions that allow them to use all their expertise,” the investigators said in a separate statement. The AAFP had previously reported that about 40% of family physicians had full hospital privileges in 2018, compared with 56% in 2012.
Many new FPs now work in large multispecialty practices or hospital systems, and “[some] of these employers dictate scope of practice, limiting family physicians to coordinating outpatient care and relying on subspecialists or hospitalists to provide inpatient care,” they noted.
and by 2017, only one of four FPs was practicing hospital medicine, according to the American Academy of Family Physicians.

The share of family physicians who provided hospital care went from 34.1% in 2013 to 25.2% in 2017, for a relative decrease of 26% that left only a quarter of FPs seeing inpatients, based on data from the annual American Board of Family Medicine certification exam application questionnaire. For the 5-year period, 46,762 individuals were included in the study sample of FPs in direct patient care.
“As observed in other domains (prenatal care, home visits, nursing home care, and obstetric care), this study adds to the evidence demonstrating contracting scope of practice among FPs,” Anuradha Jetty, MPH, of the AAFP’s Robert Graham Center in Washington, D.C., and associates said in a recent Policy Brief published in the Journal of the American Board of Family Medicine.
Much of that contraction is occurring among new family physicians who can’t “find positions that allow them to use all their expertise,” the investigators said in a separate statement. The AAFP had previously reported that about 40% of family physicians had full hospital privileges in 2018, compared with 56% in 2012.
Many new FPs now work in large multispecialty practices or hospital systems, and “[some] of these employers dictate scope of practice, limiting family physicians to coordinating outpatient care and relying on subspecialists or hospitalists to provide inpatient care,” they noted.
How to bring telemedicine to your GI practice
CHICAGO – Is your practice ready for telemedicine – and should you dive in?
Once you and your practice managers work through regulatory, legal, and technical details, said Theresa Lee, MD, a gastroenterologist in private practice in Lone Tree, Colorado, speaking at the 2019 AGA Partners in Value meeting.
The general field of telehealth – in which images might be shared or patients might message their care team for medication refills – is a broad term, said Dr. Lee. She explained that telemedicine is narrowly defined for Medicare and Medicaid reimbursement purposes as “two-way, real-time interactive communication between the patient and the physician or practitioner at [a] distant site ... that includes, at a minimum, audio and video equipment.” This is the video visit that many people think of when they imagine telemedicine, she said.
There’s increasing acceptance of telehealth services, said Dr. Lee, with a recent online poll showing that two-thirds of those surveyed would be willing to use telehealth; this would translate to about 24 million Americans who would be potential telehealth patients. And a 2019 survey of internal medicine physicians showed that more than half are working in practices in which telehealth is used in some capacity. Both patients and clinicians can benefit in a telehealth relationship, said Dr. Lee. The lack of physical travel and the potential for access after normal clinic hours can be a real boon for patients; “So how does this help us? How does it improve practice and make our lives easier?” she asked. Telehealth services can lead to improved efficiency, patient satisfaction and retention, and the ability to stand out in a market, especially if a practice can initiate telehealth services now, during the rapid growth and adoption phase for this newer technology.
“You want to make sure you really understand what some of the legal issues are surrounding telehealth and telemedicine,” said Dr. Lee, to ensure compliance with state and federal laws. There can be barriers to practicing across state lines; some states require an initial in-person visit, or the signing of a consent form, before initiating telemedicine; others may limit controlled substance prescribing via telemedicine.
And the mode of communication matters, said Dr. Lee: “Why can’t we just use Facetime to call our patients? The first thing to think about is privacy, and unauthorized access to data,” so it’s critical to do your research and use fully HIPAA-compliant communications technology.
Technology – and pricing plans – can vary widely, she added. “There’s some benefit to including technology that integrates with other clinical programs;” the platform Dr. Lee’s group chose communicates with their EHR for such functions as scheduling.
Pricing models can vary; a common scheme charges a per-user monthly fee, though blanket-fee plans also exist. Some telemedicine platforms use a hybrid pricing model that charges a flat fee up to a certain number of users and then adds a per-user fee after that.
Best practices to manage liability include continuing to maintain high standards of compliance after attorney consultation and notifying your practice’s malpractice insurance carrier, said Dr. Lee.
Reimbursement is on the upswing, as insurers see the benefits of telemedicine, and employers see their patients needing less time off work for appointments, and there are fewer emergency department visits for after-hours problems. Medicaid reimbursement is fairly straightforward, but Medicare is more restrictive and requires the beneficiary to be in a rural originating site.
Coding for a telemedicine visit is strictly based on face-to-face time spent in video conference, said Dr. Lee, at levels on par with time-based coding for office visits. “But you’re not including that time you spend doing chart review and not including the time you spend coordinating care.”
Dr. Lee’s own experience with telemedicine began in late 2016, when the 22-physician general gastroenterology group looked into it as a way to increase growth.
During the first half of the next year, the gastroenterology group’s administrative leaders and an engaged physician proponent vetted a number of telemedicine companies, and the group tried the leading candidates’ technologies.
By mid-2017, the comprehensive gastroenterology group, which also employs six advanced-practice clinicians, was piloting video visits with a group of four physicians. “One of those physicians was actually one of my partners who had sustained an Achilles tendon injury, so wasn’t really coming to the office post surgery. He was starting to use this at home, to do video visits, and everything went pretty smoothly with that,” said Dr. Lee.
When this trial was successful, the group went all in, with on-boarding of clinicians accomplished by the end of the year, site visits and 1:1 training provided by the telemedicine platform providers.
The practice is seeing video visits continue to grow in popularity, among both patients and clinicians, said Dr. Lee. She shared some tips and lessons learned from her practice.
There’s currently no formal protocol that selects patients for participation in the telemedicine program at Dr. Lee’s clinic. Providers may offer video visits to patients, and triage nurses also can suggest that patients ask their provider about them; flyers in waiting rooms and exam rooms encourage patients to ask about the possibility.
The practice maintains a telehealth committee that includes the practice’s president and administrator, about three core physicians who are strong telehealth champions, and additional physicians who are high telehealth users. The committee also folds in the office and information technology managers to make sure issues of workflow, billing, and technology are addressed.
Some practical considerations can pose challenges to a successful telemedicine program, said Dr. Lee. Connectivity problems on the patient end are fairly frequent, and no-shows also can be a problem. On the clinic side, not all clinicians have embraced video visits. For these low users, telemedicine may not represent a good value proposition. However, she said, they are seeing more and more clinicians come on board with video visits as word gets out of the generally positive experiences others are having.
Dr. Lee suggested several ways to up telemedicine utilization and make it work within your practice. “Identify which patient would benefit most,” she said – this might be patients with inflammatory bowel disease who mostly need medication management, or patients with limited mobility or who live far away. Staff can also help a patient get a same-day visit by scheduling a video visit with an available clinician. By mentioning video visits as an option for uncomplicated issues or a way to get a rapid read on a new concern, clinicians can get patients thinking about telemedicine as an appealing option.
In some clinics, exam room space can limit clinician productivity, and scheduling a block of video visits when space is tight can be a great solution. Clinicians can optimize their schedules if they incorporate video visits, said Dr. Lee, citing the example of a physician assistant in her practice who stacks video visits in the evening hours, so she’s able to be with her preschool-aged children during the day. After-hours video visits have been popular among patients too, said Dr. Lee, so the scheduling flexibility may help with both patient and provider retention, and be a practice differentiator.
“There’s great potential for value through improved patient satisfaction, provider efficiency, improved health care outcomes, and cost efficiency,” she said.
Dr. Lee reported that she had no relevant disclosures.
AGA has partnered with SupportedPatientTM, a HIPAA-secure telemedicine platform. It allows you to expand your practice and connect your patients with additional specialists. Learn more at https://www.gastro.org/practice-guidance/practice-updates/supportedpatient .
CHICAGO – Is your practice ready for telemedicine – and should you dive in?
Once you and your practice managers work through regulatory, legal, and technical details, said Theresa Lee, MD, a gastroenterologist in private practice in Lone Tree, Colorado, speaking at the 2019 AGA Partners in Value meeting.
The general field of telehealth – in which images might be shared or patients might message their care team for medication refills – is a broad term, said Dr. Lee. She explained that telemedicine is narrowly defined for Medicare and Medicaid reimbursement purposes as “two-way, real-time interactive communication between the patient and the physician or practitioner at [a] distant site ... that includes, at a minimum, audio and video equipment.” This is the video visit that many people think of when they imagine telemedicine, she said.
There’s increasing acceptance of telehealth services, said Dr. Lee, with a recent online poll showing that two-thirds of those surveyed would be willing to use telehealth; this would translate to about 24 million Americans who would be potential telehealth patients. And a 2019 survey of internal medicine physicians showed that more than half are working in practices in which telehealth is used in some capacity. Both patients and clinicians can benefit in a telehealth relationship, said Dr. Lee. The lack of physical travel and the potential for access after normal clinic hours can be a real boon for patients; “So how does this help us? How does it improve practice and make our lives easier?” she asked. Telehealth services can lead to improved efficiency, patient satisfaction and retention, and the ability to stand out in a market, especially if a practice can initiate telehealth services now, during the rapid growth and adoption phase for this newer technology.
“You want to make sure you really understand what some of the legal issues are surrounding telehealth and telemedicine,” said Dr. Lee, to ensure compliance with state and federal laws. There can be barriers to practicing across state lines; some states require an initial in-person visit, or the signing of a consent form, before initiating telemedicine; others may limit controlled substance prescribing via telemedicine.
And the mode of communication matters, said Dr. Lee: “Why can’t we just use Facetime to call our patients? The first thing to think about is privacy, and unauthorized access to data,” so it’s critical to do your research and use fully HIPAA-compliant communications technology.
Technology – and pricing plans – can vary widely, she added. “There’s some benefit to including technology that integrates with other clinical programs;” the platform Dr. Lee’s group chose communicates with their EHR for such functions as scheduling.
Pricing models can vary; a common scheme charges a per-user monthly fee, though blanket-fee plans also exist. Some telemedicine platforms use a hybrid pricing model that charges a flat fee up to a certain number of users and then adds a per-user fee after that.
Best practices to manage liability include continuing to maintain high standards of compliance after attorney consultation and notifying your practice’s malpractice insurance carrier, said Dr. Lee.
Reimbursement is on the upswing, as insurers see the benefits of telemedicine, and employers see their patients needing less time off work for appointments, and there are fewer emergency department visits for after-hours problems. Medicaid reimbursement is fairly straightforward, but Medicare is more restrictive and requires the beneficiary to be in a rural originating site.
Coding for a telemedicine visit is strictly based on face-to-face time spent in video conference, said Dr. Lee, at levels on par with time-based coding for office visits. “But you’re not including that time you spend doing chart review and not including the time you spend coordinating care.”
Dr. Lee’s own experience with telemedicine began in late 2016, when the 22-physician general gastroenterology group looked into it as a way to increase growth.
During the first half of the next year, the gastroenterology group’s administrative leaders and an engaged physician proponent vetted a number of telemedicine companies, and the group tried the leading candidates’ technologies.
By mid-2017, the comprehensive gastroenterology group, which also employs six advanced-practice clinicians, was piloting video visits with a group of four physicians. “One of those physicians was actually one of my partners who had sustained an Achilles tendon injury, so wasn’t really coming to the office post surgery. He was starting to use this at home, to do video visits, and everything went pretty smoothly with that,” said Dr. Lee.
When this trial was successful, the group went all in, with on-boarding of clinicians accomplished by the end of the year, site visits and 1:1 training provided by the telemedicine platform providers.
The practice is seeing video visits continue to grow in popularity, among both patients and clinicians, said Dr. Lee. She shared some tips and lessons learned from her practice.
There’s currently no formal protocol that selects patients for participation in the telemedicine program at Dr. Lee’s clinic. Providers may offer video visits to patients, and triage nurses also can suggest that patients ask their provider about them; flyers in waiting rooms and exam rooms encourage patients to ask about the possibility.
The practice maintains a telehealth committee that includes the practice’s president and administrator, about three core physicians who are strong telehealth champions, and additional physicians who are high telehealth users. The committee also folds in the office and information technology managers to make sure issues of workflow, billing, and technology are addressed.
Some practical considerations can pose challenges to a successful telemedicine program, said Dr. Lee. Connectivity problems on the patient end are fairly frequent, and no-shows also can be a problem. On the clinic side, not all clinicians have embraced video visits. For these low users, telemedicine may not represent a good value proposition. However, she said, they are seeing more and more clinicians come on board with video visits as word gets out of the generally positive experiences others are having.
Dr. Lee suggested several ways to up telemedicine utilization and make it work within your practice. “Identify which patient would benefit most,” she said – this might be patients with inflammatory bowel disease who mostly need medication management, or patients with limited mobility or who live far away. Staff can also help a patient get a same-day visit by scheduling a video visit with an available clinician. By mentioning video visits as an option for uncomplicated issues or a way to get a rapid read on a new concern, clinicians can get patients thinking about telemedicine as an appealing option.
In some clinics, exam room space can limit clinician productivity, and scheduling a block of video visits when space is tight can be a great solution. Clinicians can optimize their schedules if they incorporate video visits, said Dr. Lee, citing the example of a physician assistant in her practice who stacks video visits in the evening hours, so she’s able to be with her preschool-aged children during the day. After-hours video visits have been popular among patients too, said Dr. Lee, so the scheduling flexibility may help with both patient and provider retention, and be a practice differentiator.
“There’s great potential for value through improved patient satisfaction, provider efficiency, improved health care outcomes, and cost efficiency,” she said.
Dr. Lee reported that she had no relevant disclosures.
AGA has partnered with SupportedPatientTM, a HIPAA-secure telemedicine platform. It allows you to expand your practice and connect your patients with additional specialists. Learn more at https://www.gastro.org/practice-guidance/practice-updates/supportedpatient .
CHICAGO – Is your practice ready for telemedicine – and should you dive in?
Once you and your practice managers work through regulatory, legal, and technical details, said Theresa Lee, MD, a gastroenterologist in private practice in Lone Tree, Colorado, speaking at the 2019 AGA Partners in Value meeting.
The general field of telehealth – in which images might be shared or patients might message their care team for medication refills – is a broad term, said Dr. Lee. She explained that telemedicine is narrowly defined for Medicare and Medicaid reimbursement purposes as “two-way, real-time interactive communication between the patient and the physician or practitioner at [a] distant site ... that includes, at a minimum, audio and video equipment.” This is the video visit that many people think of when they imagine telemedicine, she said.
There’s increasing acceptance of telehealth services, said Dr. Lee, with a recent online poll showing that two-thirds of those surveyed would be willing to use telehealth; this would translate to about 24 million Americans who would be potential telehealth patients. And a 2019 survey of internal medicine physicians showed that more than half are working in practices in which telehealth is used in some capacity. Both patients and clinicians can benefit in a telehealth relationship, said Dr. Lee. The lack of physical travel and the potential for access after normal clinic hours can be a real boon for patients; “So how does this help us? How does it improve practice and make our lives easier?” she asked. Telehealth services can lead to improved efficiency, patient satisfaction and retention, and the ability to stand out in a market, especially if a practice can initiate telehealth services now, during the rapid growth and adoption phase for this newer technology.
“You want to make sure you really understand what some of the legal issues are surrounding telehealth and telemedicine,” said Dr. Lee, to ensure compliance with state and federal laws. There can be barriers to practicing across state lines; some states require an initial in-person visit, or the signing of a consent form, before initiating telemedicine; others may limit controlled substance prescribing via telemedicine.
And the mode of communication matters, said Dr. Lee: “Why can’t we just use Facetime to call our patients? The first thing to think about is privacy, and unauthorized access to data,” so it’s critical to do your research and use fully HIPAA-compliant communications technology.
Technology – and pricing plans – can vary widely, she added. “There’s some benefit to including technology that integrates with other clinical programs;” the platform Dr. Lee’s group chose communicates with their EHR for such functions as scheduling.
Pricing models can vary; a common scheme charges a per-user monthly fee, though blanket-fee plans also exist. Some telemedicine platforms use a hybrid pricing model that charges a flat fee up to a certain number of users and then adds a per-user fee after that.
Best practices to manage liability include continuing to maintain high standards of compliance after attorney consultation and notifying your practice’s malpractice insurance carrier, said Dr. Lee.
Reimbursement is on the upswing, as insurers see the benefits of telemedicine, and employers see their patients needing less time off work for appointments, and there are fewer emergency department visits for after-hours problems. Medicaid reimbursement is fairly straightforward, but Medicare is more restrictive and requires the beneficiary to be in a rural originating site.
Coding for a telemedicine visit is strictly based on face-to-face time spent in video conference, said Dr. Lee, at levels on par with time-based coding for office visits. “But you’re not including that time you spend doing chart review and not including the time you spend coordinating care.”
Dr. Lee’s own experience with telemedicine began in late 2016, when the 22-physician general gastroenterology group looked into it as a way to increase growth.
During the first half of the next year, the gastroenterology group’s administrative leaders and an engaged physician proponent vetted a number of telemedicine companies, and the group tried the leading candidates’ technologies.
By mid-2017, the comprehensive gastroenterology group, which also employs six advanced-practice clinicians, was piloting video visits with a group of four physicians. “One of those physicians was actually one of my partners who had sustained an Achilles tendon injury, so wasn’t really coming to the office post surgery. He was starting to use this at home, to do video visits, and everything went pretty smoothly with that,” said Dr. Lee.
When this trial was successful, the group went all in, with on-boarding of clinicians accomplished by the end of the year, site visits and 1:1 training provided by the telemedicine platform providers.
The practice is seeing video visits continue to grow in popularity, among both patients and clinicians, said Dr. Lee. She shared some tips and lessons learned from her practice.
There’s currently no formal protocol that selects patients for participation in the telemedicine program at Dr. Lee’s clinic. Providers may offer video visits to patients, and triage nurses also can suggest that patients ask their provider about them; flyers in waiting rooms and exam rooms encourage patients to ask about the possibility.
The practice maintains a telehealth committee that includes the practice’s president and administrator, about three core physicians who are strong telehealth champions, and additional physicians who are high telehealth users. The committee also folds in the office and information technology managers to make sure issues of workflow, billing, and technology are addressed.
Some practical considerations can pose challenges to a successful telemedicine program, said Dr. Lee. Connectivity problems on the patient end are fairly frequent, and no-shows also can be a problem. On the clinic side, not all clinicians have embraced video visits. For these low users, telemedicine may not represent a good value proposition. However, she said, they are seeing more and more clinicians come on board with video visits as word gets out of the generally positive experiences others are having.
Dr. Lee suggested several ways to up telemedicine utilization and make it work within your practice. “Identify which patient would benefit most,” she said – this might be patients with inflammatory bowel disease who mostly need medication management, or patients with limited mobility or who live far away. Staff can also help a patient get a same-day visit by scheduling a video visit with an available clinician. By mentioning video visits as an option for uncomplicated issues or a way to get a rapid read on a new concern, clinicians can get patients thinking about telemedicine as an appealing option.
In some clinics, exam room space can limit clinician productivity, and scheduling a block of video visits when space is tight can be a great solution. Clinicians can optimize their schedules if they incorporate video visits, said Dr. Lee, citing the example of a physician assistant in her practice who stacks video visits in the evening hours, so she’s able to be with her preschool-aged children during the day. After-hours video visits have been popular among patients too, said Dr. Lee, so the scheduling flexibility may help with both patient and provider retention, and be a practice differentiator.
“There’s great potential for value through improved patient satisfaction, provider efficiency, improved health care outcomes, and cost efficiency,” she said.
Dr. Lee reported that she had no relevant disclosures.
AGA has partnered with SupportedPatientTM, a HIPAA-secure telemedicine platform. It allows you to expand your practice and connect your patients with additional specialists. Learn more at https://www.gastro.org/practice-guidance/practice-updates/supportedpatient .
EXPERT ANALYSIS FROM AGA PARTNERS IN VALUE MEETING
Will TP53-mutated AML respond to immunotherapy?
NATIONAL HARBOR, MD. – New research has shown increased immune infiltration in patients with TP53-mutated acute myeloid leukemia (AML).
Patients with TP53-mutated AML had higher levels of T-cell infiltration, immune checkpoint molecules, and interferon (IFN)–gamma signaling than patients with wild-type TP53.
These findings may indicate that patients with TP53-mutated AML will respond to T-cell targeting immunotherapies, but more investigation is needed, according to Sergio Rutella, MD, PhD, of Nottingham (England) Trent University.
Dr. Rutella described the findings at the annual meeting of the Society for Immunotherapy of Cancer.
He and his colleagues recently identified subgroups of AML, called “immune infiltrated” and “immune depleted,” that can predict chemotherapy resistance and response to flotetuzumab (ASH 2019, Abstract 460). However, the team has not determined the genetic drivers of immune infiltration in AML.*
With the current study, Dr. Rutella and his colleagues wanted to determine if TP53 mutations are associated with the AML immune milieu and see if TP53-mutated patients might benefit from immunotherapy.
Discovery cohort
The researchers first analyzed 147 patients with non-promyelocytic AML from the Cancer Genome Atlas. In total, 9% of these patients (n = 13) had TP53-mutated AML. The researchers assessed how 45 immune gene and biological activity signatures correlated with prognostic molecular lesions (TP53 mutations, FLT3-ITD, etc.) and clinical outcomes in this cohort.
The data showed that immune subtypes were associated with overall survival (OS). The median OS was 11.8 months in patients with immune-infiltrated AML, 16.4 months in patients with intermediate AML, and 25.8 months in patients with immune-depleted AML.
The inflammatory chemokine score (P = .011), IDO1 score (P = .027), IFN-gamma score (P = .036), and B7H3 score (P = .045) were all significantly associated with OS. In fact, these factors were all better predictors of OS than cytogenetic risk score (P = .049).
The IFN-gamma score, inflammatory chemokine score, and lymphoid score were all significantly higher in TP53-mutated patients than in patients with RUNX1 mutations, NPM1 mutations, FLT3-ITD (with or without NPM1 mutations), and TET2/DNMT3A/ASXL1 mutations (P values ranging from less than .0001 to .05).
Likewise, the tumor inflammation signature score was significantly higher among TP53-mutated patients than among patients with NPM1 mutations, FLT3-ITD (with or without NPM1 mutations), and TET2/DNMT3A/ASXL1 mutations (P values ranging from less than .0001 to .01).
Validation cohort and bone marrow samples
The researchers also looked at data from a validation cohort, which consisted of 140 patients with non-promyelocytic AML in the Beat AML Master Trial. Twelve percent of these patients (n = 17) had TP53 mutations.
Data in this cohort showed that CD3G messenger RNA (mRNA) was significantly higher in TP53-mutated AML than in TP53-wild-type AML (P = .04). The same was true for CD8A mRNA (P = .0002) and GZMB mRNA (P = .0005).
Likewise, IFN-gamma mRNA (P = .0052), IFIT2 mRNA (P = .0064), and IFIT3 mRNA (P = .003) were all significantly higher in patients with TP53-mutated AML.
Lastly, the researchers analyzed gene expression profiles of bone marrow samples from patients with AML, 36 with mutated TP53 and 24 with wild-type TP53.
The team found that IFN-gamma–induced genes (IFNG and IRF1), markers of T-cell infiltration (CD8A and CD3G) and senescence (EOMES, KLRD1, and HRAS), immune checkpoint molecules (IDO1, LAG3, PDL1, and VISTA), effector function molecules (GZMB, GZMK, and GZMM), and proinflammatory cytokines (IL17A and TNF) were all significantly overexpressed in TP53-mutated AML.
Among the top overexpressed genes in TP53-mutated AML were genes associated with IFN signaling and inflammation pathways – IL-33, IL-6, IFN-gamma, OASL, RIPK2, TNFAIP3, CSF1, and PTGER4. The IL-17 and TNF signaling pathways were the most enriched pathways in TP53-mutated AML.
“Our analysis of primary bone marrow samples showed that TP53-mutated samples are enriched in IL-17, TNF, and IFN signaling molecules, and show higher levels of T-cell infiltrations and immune checkpoints relative to their wild-type counterparts,” Dr. Rutella said.
“The in silico analysis indicated that TP53-mutated cases will show higher levels of T-cell infiltration, immune checkpoints, and IFN-gamma signaling, compared with AML subgroups without risk-defining molecular lesions,” he added. “This is speculative. Whether TP53-mutated AML can be amenable to respond to T-cell targeting immunotherapies is still to be determined.”
Dr. Rutella reported research support from NanoString Technologies, MacroGenics, and Kura Oncology.
SOURCE: Rutella S et al. SITC 2019. Abstract O3.
*This article was updated on 11/19/2019.
NATIONAL HARBOR, MD. – New research has shown increased immune infiltration in patients with TP53-mutated acute myeloid leukemia (AML).
Patients with TP53-mutated AML had higher levels of T-cell infiltration, immune checkpoint molecules, and interferon (IFN)–gamma signaling than patients with wild-type TP53.
These findings may indicate that patients with TP53-mutated AML will respond to T-cell targeting immunotherapies, but more investigation is needed, according to Sergio Rutella, MD, PhD, of Nottingham (England) Trent University.
Dr. Rutella described the findings at the annual meeting of the Society for Immunotherapy of Cancer.
He and his colleagues recently identified subgroups of AML, called “immune infiltrated” and “immune depleted,” that can predict chemotherapy resistance and response to flotetuzumab (ASH 2019, Abstract 460). However, the team has not determined the genetic drivers of immune infiltration in AML.*
With the current study, Dr. Rutella and his colleagues wanted to determine if TP53 mutations are associated with the AML immune milieu and see if TP53-mutated patients might benefit from immunotherapy.
Discovery cohort
The researchers first analyzed 147 patients with non-promyelocytic AML from the Cancer Genome Atlas. In total, 9% of these patients (n = 13) had TP53-mutated AML. The researchers assessed how 45 immune gene and biological activity signatures correlated with prognostic molecular lesions (TP53 mutations, FLT3-ITD, etc.) and clinical outcomes in this cohort.
The data showed that immune subtypes were associated with overall survival (OS). The median OS was 11.8 months in patients with immune-infiltrated AML, 16.4 months in patients with intermediate AML, and 25.8 months in patients with immune-depleted AML.
The inflammatory chemokine score (P = .011), IDO1 score (P = .027), IFN-gamma score (P = .036), and B7H3 score (P = .045) were all significantly associated with OS. In fact, these factors were all better predictors of OS than cytogenetic risk score (P = .049).
The IFN-gamma score, inflammatory chemokine score, and lymphoid score were all significantly higher in TP53-mutated patients than in patients with RUNX1 mutations, NPM1 mutations, FLT3-ITD (with or without NPM1 mutations), and TET2/DNMT3A/ASXL1 mutations (P values ranging from less than .0001 to .05).
Likewise, the tumor inflammation signature score was significantly higher among TP53-mutated patients than among patients with NPM1 mutations, FLT3-ITD (with or without NPM1 mutations), and TET2/DNMT3A/ASXL1 mutations (P values ranging from less than .0001 to .01).
Validation cohort and bone marrow samples
The researchers also looked at data from a validation cohort, which consisted of 140 patients with non-promyelocytic AML in the Beat AML Master Trial. Twelve percent of these patients (n = 17) had TP53 mutations.
Data in this cohort showed that CD3G messenger RNA (mRNA) was significantly higher in TP53-mutated AML than in TP53-wild-type AML (P = .04). The same was true for CD8A mRNA (P = .0002) and GZMB mRNA (P = .0005).
Likewise, IFN-gamma mRNA (P = .0052), IFIT2 mRNA (P = .0064), and IFIT3 mRNA (P = .003) were all significantly higher in patients with TP53-mutated AML.
Lastly, the researchers analyzed gene expression profiles of bone marrow samples from patients with AML, 36 with mutated TP53 and 24 with wild-type TP53.
The team found that IFN-gamma–induced genes (IFNG and IRF1), markers of T-cell infiltration (CD8A and CD3G) and senescence (EOMES, KLRD1, and HRAS), immune checkpoint molecules (IDO1, LAG3, PDL1, and VISTA), effector function molecules (GZMB, GZMK, and GZMM), and proinflammatory cytokines (IL17A and TNF) were all significantly overexpressed in TP53-mutated AML.
Among the top overexpressed genes in TP53-mutated AML were genes associated with IFN signaling and inflammation pathways – IL-33, IL-6, IFN-gamma, OASL, RIPK2, TNFAIP3, CSF1, and PTGER4. The IL-17 and TNF signaling pathways were the most enriched pathways in TP53-mutated AML.
“Our analysis of primary bone marrow samples showed that TP53-mutated samples are enriched in IL-17, TNF, and IFN signaling molecules, and show higher levels of T-cell infiltrations and immune checkpoints relative to their wild-type counterparts,” Dr. Rutella said.
“The in silico analysis indicated that TP53-mutated cases will show higher levels of T-cell infiltration, immune checkpoints, and IFN-gamma signaling, compared with AML subgroups without risk-defining molecular lesions,” he added. “This is speculative. Whether TP53-mutated AML can be amenable to respond to T-cell targeting immunotherapies is still to be determined.”
Dr. Rutella reported research support from NanoString Technologies, MacroGenics, and Kura Oncology.
SOURCE: Rutella S et al. SITC 2019. Abstract O3.
*This article was updated on 11/19/2019.
NATIONAL HARBOR, MD. – New research has shown increased immune infiltration in patients with TP53-mutated acute myeloid leukemia (AML).
Patients with TP53-mutated AML had higher levels of T-cell infiltration, immune checkpoint molecules, and interferon (IFN)–gamma signaling than patients with wild-type TP53.
These findings may indicate that patients with TP53-mutated AML will respond to T-cell targeting immunotherapies, but more investigation is needed, according to Sergio Rutella, MD, PhD, of Nottingham (England) Trent University.
Dr. Rutella described the findings at the annual meeting of the Society for Immunotherapy of Cancer.
He and his colleagues recently identified subgroups of AML, called “immune infiltrated” and “immune depleted,” that can predict chemotherapy resistance and response to flotetuzumab (ASH 2019, Abstract 460). However, the team has not determined the genetic drivers of immune infiltration in AML.*
With the current study, Dr. Rutella and his colleagues wanted to determine if TP53 mutations are associated with the AML immune milieu and see if TP53-mutated patients might benefit from immunotherapy.
Discovery cohort
The researchers first analyzed 147 patients with non-promyelocytic AML from the Cancer Genome Atlas. In total, 9% of these patients (n = 13) had TP53-mutated AML. The researchers assessed how 45 immune gene and biological activity signatures correlated with prognostic molecular lesions (TP53 mutations, FLT3-ITD, etc.) and clinical outcomes in this cohort.
The data showed that immune subtypes were associated with overall survival (OS). The median OS was 11.8 months in patients with immune-infiltrated AML, 16.4 months in patients with intermediate AML, and 25.8 months in patients with immune-depleted AML.
The inflammatory chemokine score (P = .011), IDO1 score (P = .027), IFN-gamma score (P = .036), and B7H3 score (P = .045) were all significantly associated with OS. In fact, these factors were all better predictors of OS than cytogenetic risk score (P = .049).
The IFN-gamma score, inflammatory chemokine score, and lymphoid score were all significantly higher in TP53-mutated patients than in patients with RUNX1 mutations, NPM1 mutations, FLT3-ITD (with or without NPM1 mutations), and TET2/DNMT3A/ASXL1 mutations (P values ranging from less than .0001 to .05).
Likewise, the tumor inflammation signature score was significantly higher among TP53-mutated patients than among patients with NPM1 mutations, FLT3-ITD (with or without NPM1 mutations), and TET2/DNMT3A/ASXL1 mutations (P values ranging from less than .0001 to .01).
Validation cohort and bone marrow samples
The researchers also looked at data from a validation cohort, which consisted of 140 patients with non-promyelocytic AML in the Beat AML Master Trial. Twelve percent of these patients (n = 17) had TP53 mutations.
Data in this cohort showed that CD3G messenger RNA (mRNA) was significantly higher in TP53-mutated AML than in TP53-wild-type AML (P = .04). The same was true for CD8A mRNA (P = .0002) and GZMB mRNA (P = .0005).
Likewise, IFN-gamma mRNA (P = .0052), IFIT2 mRNA (P = .0064), and IFIT3 mRNA (P = .003) were all significantly higher in patients with TP53-mutated AML.
Lastly, the researchers analyzed gene expression profiles of bone marrow samples from patients with AML, 36 with mutated TP53 and 24 with wild-type TP53.
The team found that IFN-gamma–induced genes (IFNG and IRF1), markers of T-cell infiltration (CD8A and CD3G) and senescence (EOMES, KLRD1, and HRAS), immune checkpoint molecules (IDO1, LAG3, PDL1, and VISTA), effector function molecules (GZMB, GZMK, and GZMM), and proinflammatory cytokines (IL17A and TNF) were all significantly overexpressed in TP53-mutated AML.
Among the top overexpressed genes in TP53-mutated AML were genes associated with IFN signaling and inflammation pathways – IL-33, IL-6, IFN-gamma, OASL, RIPK2, TNFAIP3, CSF1, and PTGER4. The IL-17 and TNF signaling pathways were the most enriched pathways in TP53-mutated AML.
“Our analysis of primary bone marrow samples showed that TP53-mutated samples are enriched in IL-17, TNF, and IFN signaling molecules, and show higher levels of T-cell infiltrations and immune checkpoints relative to their wild-type counterparts,” Dr. Rutella said.
“The in silico analysis indicated that TP53-mutated cases will show higher levels of T-cell infiltration, immune checkpoints, and IFN-gamma signaling, compared with AML subgroups without risk-defining molecular lesions,” he added. “This is speculative. Whether TP53-mutated AML can be amenable to respond to T-cell targeting immunotherapies is still to be determined.”
Dr. Rutella reported research support from NanoString Technologies, MacroGenics, and Kura Oncology.
SOURCE: Rutella S et al. SITC 2019. Abstract O3.
*This article was updated on 11/19/2019.
REPORTING FROM SITC 2019
CAR T-cell ‘cocktail’ may overcome antigen escape relapse
A chimeric antigen receptor (CAR) T-cell “cocktail” targeting both CD19 and CD22 could improve outcomes for patients with refractory or relapsed B-cell malignancies, according to investigators.
This dual approach, which appeared safe and effective, may be able to overcome antigen escape relapse, reported Na Wang, MD, of Huazhong University of Science and Technology in China, and colleagues.
The investigators tested this method in an open-label, single-arm pilot study involving 89 patients with refractory/relapsed B cell malignancies. Of these, 51 patients had B-cell acute lymphoblastic leukemia (B-ALL), while the remaining 38 had non-Hodgkin lymphoma (NHL). All patients had dual expression of CD19 and CD22 on malignant B cells, good performance status, and “essentially” normal organ function, the investigators reported in Blood.
Following lymphodepletion, patients were infused with CAR19 and CAR22 T cells, then evaluated for responses with imaging or bone marrow aspiration on a monthly basis for 6 months, then every 3 months thereafter.
After 30 days, most patients with ALL (96%) achieved a minimal residual disease-negative complete response or complete response with incomplete count recovery. After a median follow-up of 16.7 months, almost half of these responders relapsed (49%), median progression-free survival was 13.6 months, and overall survival was 31 months.
With a minimum follow-up of 3 months, half of the patients with NHL (50%) achieved complete responses, with the caveat that two patients who died of septic shock and severe cytokine release syndrome were excluded from this efficacy analysis. After a median follow-up of 14.4 months, in the NHL group, median progression-free survival was 9.9 months and overall survival was 18 months.
Across disease types, almost all patients (95.5%) experienced cytokine release syndrome, with more than three-quarters (77.6%) categorized as grade 1 or 2. CAR T cell-related encephalopathy syndrome (CRES) occurred in 13.5% of patients; most were low grade, apart from one case that was grade 4. In total, 12 patients died due to adverse events.
“The severe [adverse events] were mostly cytopenias and the most frequent fatal [adverse event] was lung infection, which was attributable in part to the high disease burden and heavy pretreatment of the enrolled patients,” the investigators wrote. “Nearly all the high-grade CRS and CRES were reversible and occurred in similar incidences as previously reported. Thus, the sequential infusion of CAR19/22 T-cell “cocktail” was an efficient and well-tolerated approach to circumvent antigen loss of CD19 or CD22.”
The investigators reported having no conflicts of interest.
SOURCE: Wang N et al. 2019 Oct 29. doi: 10.1182/blood.2019000017.
A chimeric antigen receptor (CAR) T-cell “cocktail” targeting both CD19 and CD22 could improve outcomes for patients with refractory or relapsed B-cell malignancies, according to investigators.
This dual approach, which appeared safe and effective, may be able to overcome antigen escape relapse, reported Na Wang, MD, of Huazhong University of Science and Technology in China, and colleagues.
The investigators tested this method in an open-label, single-arm pilot study involving 89 patients with refractory/relapsed B cell malignancies. Of these, 51 patients had B-cell acute lymphoblastic leukemia (B-ALL), while the remaining 38 had non-Hodgkin lymphoma (NHL). All patients had dual expression of CD19 and CD22 on malignant B cells, good performance status, and “essentially” normal organ function, the investigators reported in Blood.
Following lymphodepletion, patients were infused with CAR19 and CAR22 T cells, then evaluated for responses with imaging or bone marrow aspiration on a monthly basis for 6 months, then every 3 months thereafter.
After 30 days, most patients with ALL (96%) achieved a minimal residual disease-negative complete response or complete response with incomplete count recovery. After a median follow-up of 16.7 months, almost half of these responders relapsed (49%), median progression-free survival was 13.6 months, and overall survival was 31 months.
With a minimum follow-up of 3 months, half of the patients with NHL (50%) achieved complete responses, with the caveat that two patients who died of septic shock and severe cytokine release syndrome were excluded from this efficacy analysis. After a median follow-up of 14.4 months, in the NHL group, median progression-free survival was 9.9 months and overall survival was 18 months.
Across disease types, almost all patients (95.5%) experienced cytokine release syndrome, with more than three-quarters (77.6%) categorized as grade 1 or 2. CAR T cell-related encephalopathy syndrome (CRES) occurred in 13.5% of patients; most were low grade, apart from one case that was grade 4. In total, 12 patients died due to adverse events.
“The severe [adverse events] were mostly cytopenias and the most frequent fatal [adverse event] was lung infection, which was attributable in part to the high disease burden and heavy pretreatment of the enrolled patients,” the investigators wrote. “Nearly all the high-grade CRS and CRES were reversible and occurred in similar incidences as previously reported. Thus, the sequential infusion of CAR19/22 T-cell “cocktail” was an efficient and well-tolerated approach to circumvent antigen loss of CD19 or CD22.”
The investigators reported having no conflicts of interest.
SOURCE: Wang N et al. 2019 Oct 29. doi: 10.1182/blood.2019000017.
A chimeric antigen receptor (CAR) T-cell “cocktail” targeting both CD19 and CD22 could improve outcomes for patients with refractory or relapsed B-cell malignancies, according to investigators.
This dual approach, which appeared safe and effective, may be able to overcome antigen escape relapse, reported Na Wang, MD, of Huazhong University of Science and Technology in China, and colleagues.
The investigators tested this method in an open-label, single-arm pilot study involving 89 patients with refractory/relapsed B cell malignancies. Of these, 51 patients had B-cell acute lymphoblastic leukemia (B-ALL), while the remaining 38 had non-Hodgkin lymphoma (NHL). All patients had dual expression of CD19 and CD22 on malignant B cells, good performance status, and “essentially” normal organ function, the investigators reported in Blood.
Following lymphodepletion, patients were infused with CAR19 and CAR22 T cells, then evaluated for responses with imaging or bone marrow aspiration on a monthly basis for 6 months, then every 3 months thereafter.
After 30 days, most patients with ALL (96%) achieved a minimal residual disease-negative complete response or complete response with incomplete count recovery. After a median follow-up of 16.7 months, almost half of these responders relapsed (49%), median progression-free survival was 13.6 months, and overall survival was 31 months.
With a minimum follow-up of 3 months, half of the patients with NHL (50%) achieved complete responses, with the caveat that two patients who died of septic shock and severe cytokine release syndrome were excluded from this efficacy analysis. After a median follow-up of 14.4 months, in the NHL group, median progression-free survival was 9.9 months and overall survival was 18 months.
Across disease types, almost all patients (95.5%) experienced cytokine release syndrome, with more than three-quarters (77.6%) categorized as grade 1 or 2. CAR T cell-related encephalopathy syndrome (CRES) occurred in 13.5% of patients; most were low grade, apart from one case that was grade 4. In total, 12 patients died due to adverse events.
“The severe [adverse events] were mostly cytopenias and the most frequent fatal [adverse event] was lung infection, which was attributable in part to the high disease burden and heavy pretreatment of the enrolled patients,” the investigators wrote. “Nearly all the high-grade CRS and CRES were reversible and occurred in similar incidences as previously reported. Thus, the sequential infusion of CAR19/22 T-cell “cocktail” was an efficient and well-tolerated approach to circumvent antigen loss of CD19 or CD22.”
The investigators reported having no conflicts of interest.
SOURCE: Wang N et al. 2019 Oct 29. doi: 10.1182/blood.2019000017.
FROM BLOOD
CHEST 2020 Honor Lectures and Award Nominations
Each year, CHEST honors physicians and others who are making significant or meritorious contributions to chest medicine. All honorees are recognized for advancing work in specific areas of chest medicine, mentorship, and training, furthering the work of CHEST, and more.
If you believe you have a colleague who should be recognized for their distinguished work, please submit a nomination. Those selected for an annual award and honor lecture will be featured at CHEST 2020 in Chicago.
Deadline: Monday, January 6, 2020
Questions? Please contact Emily Petraglia, Manager, Volunteer Engagement (epetraglia@chestnet.org).
The following awards are now open for nominations:
Annual Awards
College Medalist Award
Distinguished Service AwardMaster FCCP
Honor and Memorial Lectures
Edward C. Rosenow III, MD, Master FCCP/Master Teacher Endowed Honor Lecture.
Roger C. Bone Memorial Lecture in Critical CareMurray Kornfeld Memorial Founders Award
Distinguished Scientist Honor Lecture in Cardiopulmonary PhysiologyPasquale Ciaglia Memorial Lecture in Interventional MedicineMargaret Pfrommer Endowed Memorial Lecture in Home-Based Mechanical VentilationThomas L. Petty, MD, Master FCCP Endowed Memorial Lecture
Educator Awards
Early Career Clinician Educator
Master Clinician Educator
Each year, CHEST honors physicians and others who are making significant or meritorious contributions to chest medicine. All honorees are recognized for advancing work in specific areas of chest medicine, mentorship, and training, furthering the work of CHEST, and more.
If you believe you have a colleague who should be recognized for their distinguished work, please submit a nomination. Those selected for an annual award and honor lecture will be featured at CHEST 2020 in Chicago.
Deadline: Monday, January 6, 2020
Questions? Please contact Emily Petraglia, Manager, Volunteer Engagement (epetraglia@chestnet.org).
The following awards are now open for nominations:
Annual Awards
College Medalist Award
Distinguished Service AwardMaster FCCP
Honor and Memorial Lectures
Edward C. Rosenow III, MD, Master FCCP/Master Teacher Endowed Honor Lecture.
Roger C. Bone Memorial Lecture in Critical CareMurray Kornfeld Memorial Founders Award
Distinguished Scientist Honor Lecture in Cardiopulmonary PhysiologyPasquale Ciaglia Memorial Lecture in Interventional MedicineMargaret Pfrommer Endowed Memorial Lecture in Home-Based Mechanical VentilationThomas L. Petty, MD, Master FCCP Endowed Memorial Lecture
Educator Awards
Early Career Clinician Educator
Master Clinician Educator
Each year, CHEST honors physicians and others who are making significant or meritorious contributions to chest medicine. All honorees are recognized for advancing work in specific areas of chest medicine, mentorship, and training, furthering the work of CHEST, and more.
If you believe you have a colleague who should be recognized for their distinguished work, please submit a nomination. Those selected for an annual award and honor lecture will be featured at CHEST 2020 in Chicago.
Deadline: Monday, January 6, 2020
Questions? Please contact Emily Petraglia, Manager, Volunteer Engagement (epetraglia@chestnet.org).
The following awards are now open for nominations:
Annual Awards
College Medalist Award
Distinguished Service AwardMaster FCCP
Honor and Memorial Lectures
Edward C. Rosenow III, MD, Master FCCP/Master Teacher Endowed Honor Lecture.
Roger C. Bone Memorial Lecture in Critical CareMurray Kornfeld Memorial Founders Award
Distinguished Scientist Honor Lecture in Cardiopulmonary PhysiologyPasquale Ciaglia Memorial Lecture in Interventional MedicineMargaret Pfrommer Endowed Memorial Lecture in Home-Based Mechanical VentilationThomas L. Petty, MD, Master FCCP Endowed Memorial Lecture
Educator Awards
Early Career Clinician Educator
Master Clinician Educator
The TWILIGHT of aspirin post-PCI for ACS?
PHILADELPHIA – Downshifting to ticagrelor monotherapy after just 3 months of dual antiplatelet therapy is a winning strategy in high-risk patients who’ve undergone PCI for non-ST-elevation acute coronary syndrome, Usman Baber, MD, reported at the American Heart Association scientific sessions.
He presented a prespecified subgroup analysis of the previously reported TWILIGHT study that was restricted to the 4,614 participants with non-ST-elevation ACS who underwent PCI, completed 3 months of dual antiplatelet therapy (DAPT) with ticagrelor and aspirin, and were then randomized double-blind to an additional 12 months on the same regimen or to ticagrelor plus placebo.
The key finding: After a year on ticagrelor monotherapy, the risk of clinically significant or major bleeding was reduced by 53%, compared with the DAPT group, and with no increased risk of ischemic major adverse cardiovascular events, said Dr. Baber, a cardiologist at the Icahn School of Medicine at Mount Sinai, New York.
This secondary analysis of the TWILIGHT study was carried out because none of the several prior studies of short-term DAPT followed by an aspirin-free strategy after PCI was double-blind. Nor did any include patients with non-ST-elevation ACS, he explained.
The TWILIGHT substudy included 2,494 participants with unstable angina and 2,120 with non-ST-elevation MI. Roughly two-thirds had four or more high-risk clinical or angiographic features, such as diabetes, chronic kidney disease, multivessel CAD, or left main lesions.
The primary study endpoint at month 15 – the rate of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events – was 7.6% with ticagrelor plus aspirin, compared with 3.6% with ticagrelor plus placebo, for a highly significant 53% relative risk reduction in favor of ticagrelor monotherapy. The key secondary endpoint, a composite of all-cause mortality, MI, or stroke, occurred in roughly 4.4% of patients in each study arm.
Of note, ticagrelor monotherapy after 3 months of DAPT was associated with a similar 50%-60% reduction in the risk of BARC 2, 3, or 5 bleeding regardless of whether patients had 1-3, 4 or 5, or 6-9 prespecified high-risk clinical and angiographic features. Nor was the impact of ticagrelor monotherapy on ischemic events impacted by risk factor burden.
Discussant Michelle L. O’Donoghue, MD, observed that while the current practice of most cardiologists in patients undergoing stenting in the setting of ACS is 12 months of DAPT followed by discontinuation of the P2Y12 inhibitor and indefinite continuation of aspirin, mounting evidence suggests there’s a better approach.
Indeed, the new TWILIGHT findings in patients with non-ST-elevation ACS dovetail nicely with the results of three other recent studies of discontinuing aspirin after 1-3 months versus continuing DAPT with ticagrelor or another P2Y12 inhibitor plus aspirin. These studies, GLOBAL LEADERS (Lancet. 2018 Sep 15;392[10151]:940-9); SMART CHOICE (JAMA. 2019 Jun 25;321[24]:2428-37); and STOPDAPT-2 (JAMA. 2019 Jun 25;321[24]:2414-27) included patients undergoing PCI either for stable coronary disease or for ST-elevation MI, but not for non-ST-elevation ACS.
Dr. O’Donoghue, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, Boston, conducted a meta-analysis including the TWILIGHT ACS trial and the other three studies. In a total population of 29,205 patients, a strategy of dropping aspirin while continuing a P2Y12 inhibitor after 1-3 months of DAPT was associated with a 40% relative risk reduction in major bleeding events when compared with continued DAPT, with no indication of an increased risk of major adverse cardiovascular events. When she looked specifically at the nearly 14,000 post-ACS patients in the studies, the same consistency with respect to outcomes held true: an overall 51% reduction in bleeding, and – if anything – a favorable trend involving an 11% reduction in the risk of major adverse cardiovascular events, although this difference didn’t reach statistical significance.
“I believe that discontinuation of aspirin markedly reduces bleeding when stopped 1-3 months post PCI for patients initially started on DAPT,” Dr. O’Donoghue declared. “The evidence to date does not indicate that stopping aspirin leads to any increase in the risk of major adverse cardiovascular events. And these findings now extend to patients with ACS, including those with high-risk clinical and angiographic features.”
The important remaining questions, she added, include the best-choice P2Y12 inhibitor for early monotherapy post-PCI, whether the medication should be continued indefinitely past the 12-month mark, and whether aspirin might be safely discontinued even earlier than at 1-3 months.
“If you are thinking about establishing a clopidogrel monotherapy, you need to keep in mind that there exists significant interpatient variability in terms of pharmacodynamic response,” she noted, adding that platelet function testing or genotyping to identify clopidogrel resistance is worth considering in such patients.
The primary results of the full TWILIGHT study, which included 7,119 randomized patients, have been published (N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419).
The TWILIGHT study was sponsored by AstraZeneca. Dr. Baber reported receiving honoraria from that company as well as Boston Scientific.
Dr. O’Donoghue reported receiving institutional research support from a handful of pharmaceutical companies.
SOURCE: Baber U. AHA late breaker.
PHILADELPHIA – Downshifting to ticagrelor monotherapy after just 3 months of dual antiplatelet therapy is a winning strategy in high-risk patients who’ve undergone PCI for non-ST-elevation acute coronary syndrome, Usman Baber, MD, reported at the American Heart Association scientific sessions.
He presented a prespecified subgroup analysis of the previously reported TWILIGHT study that was restricted to the 4,614 participants with non-ST-elevation ACS who underwent PCI, completed 3 months of dual antiplatelet therapy (DAPT) with ticagrelor and aspirin, and were then randomized double-blind to an additional 12 months on the same regimen or to ticagrelor plus placebo.
The key finding: After a year on ticagrelor monotherapy, the risk of clinically significant or major bleeding was reduced by 53%, compared with the DAPT group, and with no increased risk of ischemic major adverse cardiovascular events, said Dr. Baber, a cardiologist at the Icahn School of Medicine at Mount Sinai, New York.
This secondary analysis of the TWILIGHT study was carried out because none of the several prior studies of short-term DAPT followed by an aspirin-free strategy after PCI was double-blind. Nor did any include patients with non-ST-elevation ACS, he explained.
The TWILIGHT substudy included 2,494 participants with unstable angina and 2,120 with non-ST-elevation MI. Roughly two-thirds had four or more high-risk clinical or angiographic features, such as diabetes, chronic kidney disease, multivessel CAD, or left main lesions.
The primary study endpoint at month 15 – the rate of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events – was 7.6% with ticagrelor plus aspirin, compared with 3.6% with ticagrelor plus placebo, for a highly significant 53% relative risk reduction in favor of ticagrelor monotherapy. The key secondary endpoint, a composite of all-cause mortality, MI, or stroke, occurred in roughly 4.4% of patients in each study arm.
Of note, ticagrelor monotherapy after 3 months of DAPT was associated with a similar 50%-60% reduction in the risk of BARC 2, 3, or 5 bleeding regardless of whether patients had 1-3, 4 or 5, or 6-9 prespecified high-risk clinical and angiographic features. Nor was the impact of ticagrelor monotherapy on ischemic events impacted by risk factor burden.
Discussant Michelle L. O’Donoghue, MD, observed that while the current practice of most cardiologists in patients undergoing stenting in the setting of ACS is 12 months of DAPT followed by discontinuation of the P2Y12 inhibitor and indefinite continuation of aspirin, mounting evidence suggests there’s a better approach.
Indeed, the new TWILIGHT findings in patients with non-ST-elevation ACS dovetail nicely with the results of three other recent studies of discontinuing aspirin after 1-3 months versus continuing DAPT with ticagrelor or another P2Y12 inhibitor plus aspirin. These studies, GLOBAL LEADERS (Lancet. 2018 Sep 15;392[10151]:940-9); SMART CHOICE (JAMA. 2019 Jun 25;321[24]:2428-37); and STOPDAPT-2 (JAMA. 2019 Jun 25;321[24]:2414-27) included patients undergoing PCI either for stable coronary disease or for ST-elevation MI, but not for non-ST-elevation ACS.
Dr. O’Donoghue, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, Boston, conducted a meta-analysis including the TWILIGHT ACS trial and the other three studies. In a total population of 29,205 patients, a strategy of dropping aspirin while continuing a P2Y12 inhibitor after 1-3 months of DAPT was associated with a 40% relative risk reduction in major bleeding events when compared with continued DAPT, with no indication of an increased risk of major adverse cardiovascular events. When she looked specifically at the nearly 14,000 post-ACS patients in the studies, the same consistency with respect to outcomes held true: an overall 51% reduction in bleeding, and – if anything – a favorable trend involving an 11% reduction in the risk of major adverse cardiovascular events, although this difference didn’t reach statistical significance.
“I believe that discontinuation of aspirin markedly reduces bleeding when stopped 1-3 months post PCI for patients initially started on DAPT,” Dr. O’Donoghue declared. “The evidence to date does not indicate that stopping aspirin leads to any increase in the risk of major adverse cardiovascular events. And these findings now extend to patients with ACS, including those with high-risk clinical and angiographic features.”
The important remaining questions, she added, include the best-choice P2Y12 inhibitor for early monotherapy post-PCI, whether the medication should be continued indefinitely past the 12-month mark, and whether aspirin might be safely discontinued even earlier than at 1-3 months.
“If you are thinking about establishing a clopidogrel monotherapy, you need to keep in mind that there exists significant interpatient variability in terms of pharmacodynamic response,” she noted, adding that platelet function testing or genotyping to identify clopidogrel resistance is worth considering in such patients.
The primary results of the full TWILIGHT study, which included 7,119 randomized patients, have been published (N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419).
The TWILIGHT study was sponsored by AstraZeneca. Dr. Baber reported receiving honoraria from that company as well as Boston Scientific.
Dr. O’Donoghue reported receiving institutional research support from a handful of pharmaceutical companies.
SOURCE: Baber U. AHA late breaker.
PHILADELPHIA – Downshifting to ticagrelor monotherapy after just 3 months of dual antiplatelet therapy is a winning strategy in high-risk patients who’ve undergone PCI for non-ST-elevation acute coronary syndrome, Usman Baber, MD, reported at the American Heart Association scientific sessions.
He presented a prespecified subgroup analysis of the previously reported TWILIGHT study that was restricted to the 4,614 participants with non-ST-elevation ACS who underwent PCI, completed 3 months of dual antiplatelet therapy (DAPT) with ticagrelor and aspirin, and were then randomized double-blind to an additional 12 months on the same regimen or to ticagrelor plus placebo.
The key finding: After a year on ticagrelor monotherapy, the risk of clinically significant or major bleeding was reduced by 53%, compared with the DAPT group, and with no increased risk of ischemic major adverse cardiovascular events, said Dr. Baber, a cardiologist at the Icahn School of Medicine at Mount Sinai, New York.
This secondary analysis of the TWILIGHT study was carried out because none of the several prior studies of short-term DAPT followed by an aspirin-free strategy after PCI was double-blind. Nor did any include patients with non-ST-elevation ACS, he explained.
The TWILIGHT substudy included 2,494 participants with unstable angina and 2,120 with non-ST-elevation MI. Roughly two-thirds had four or more high-risk clinical or angiographic features, such as diabetes, chronic kidney disease, multivessel CAD, or left main lesions.
The primary study endpoint at month 15 – the rate of Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding events – was 7.6% with ticagrelor plus aspirin, compared with 3.6% with ticagrelor plus placebo, for a highly significant 53% relative risk reduction in favor of ticagrelor monotherapy. The key secondary endpoint, a composite of all-cause mortality, MI, or stroke, occurred in roughly 4.4% of patients in each study arm.
Of note, ticagrelor monotherapy after 3 months of DAPT was associated with a similar 50%-60% reduction in the risk of BARC 2, 3, or 5 bleeding regardless of whether patients had 1-3, 4 or 5, or 6-9 prespecified high-risk clinical and angiographic features. Nor was the impact of ticagrelor monotherapy on ischemic events impacted by risk factor burden.
Discussant Michelle L. O’Donoghue, MD, observed that while the current practice of most cardiologists in patients undergoing stenting in the setting of ACS is 12 months of DAPT followed by discontinuation of the P2Y12 inhibitor and indefinite continuation of aspirin, mounting evidence suggests there’s a better approach.
Indeed, the new TWILIGHT findings in patients with non-ST-elevation ACS dovetail nicely with the results of three other recent studies of discontinuing aspirin after 1-3 months versus continuing DAPT with ticagrelor or another P2Y12 inhibitor plus aspirin. These studies, GLOBAL LEADERS (Lancet. 2018 Sep 15;392[10151]:940-9); SMART CHOICE (JAMA. 2019 Jun 25;321[24]:2428-37); and STOPDAPT-2 (JAMA. 2019 Jun 25;321[24]:2414-27) included patients undergoing PCI either for stable coronary disease or for ST-elevation MI, but not for non-ST-elevation ACS.
Dr. O’Donoghue, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School, Boston, conducted a meta-analysis including the TWILIGHT ACS trial and the other three studies. In a total population of 29,205 patients, a strategy of dropping aspirin while continuing a P2Y12 inhibitor after 1-3 months of DAPT was associated with a 40% relative risk reduction in major bleeding events when compared with continued DAPT, with no indication of an increased risk of major adverse cardiovascular events. When she looked specifically at the nearly 14,000 post-ACS patients in the studies, the same consistency with respect to outcomes held true: an overall 51% reduction in bleeding, and – if anything – a favorable trend involving an 11% reduction in the risk of major adverse cardiovascular events, although this difference didn’t reach statistical significance.
“I believe that discontinuation of aspirin markedly reduces bleeding when stopped 1-3 months post PCI for patients initially started on DAPT,” Dr. O’Donoghue declared. “The evidence to date does not indicate that stopping aspirin leads to any increase in the risk of major adverse cardiovascular events. And these findings now extend to patients with ACS, including those with high-risk clinical and angiographic features.”
The important remaining questions, she added, include the best-choice P2Y12 inhibitor for early monotherapy post-PCI, whether the medication should be continued indefinitely past the 12-month mark, and whether aspirin might be safely discontinued even earlier than at 1-3 months.
“If you are thinking about establishing a clopidogrel monotherapy, you need to keep in mind that there exists significant interpatient variability in terms of pharmacodynamic response,” she noted, adding that platelet function testing or genotyping to identify clopidogrel resistance is worth considering in such patients.
The primary results of the full TWILIGHT study, which included 7,119 randomized patients, have been published (N Engl J Med. 2019 Sep 26. doi: 10.1056/NEJMoa1908419).
The TWILIGHT study was sponsored by AstraZeneca. Dr. Baber reported receiving honoraria from that company as well as Boston Scientific.
Dr. O’Donoghue reported receiving institutional research support from a handful of pharmaceutical companies.
SOURCE: Baber U. AHA late breaker.
REPORTING FROM AHA 2019
Rand analysis of proposed Medicare buy-in uncovers surprising findings
Early buy-in by people aged 50-64 might raise insurance premiums
Allowing people aged 50-64 to buy into Medicare might result in higher premiums for people who purchase their health insurance on the individual market, a finding that runs counter to many people’s expectations, said the authors of a newly released report.
In the report, Christine Eibner, PhD, of the RAND Corporation and colleagues estimated that the premium for so-called bronze market plans might increase by 2%-10%, depending on the design of a Medicare buy-in program. (The bronze plans are ones with fewer benefits sold on exchanges created through the implementation of the Affordable Care Act of 2010 [ACA].)
A perception has been that younger adults have been in effect subsidizing the cost of older ones in the marketplace plans. Instead, it appears that younger adults who enroll in the individual market tend to be relatively unhealthy and thus expensive to cover.
“When older adults leave the market, insurers are left with a smaller pool of younger, less healthy, and relatively expensive people given their age, leading to higher premiums,” Dr. Eibner and colleagues said in the report.
This result was unexpected, as there has been discussion of using a Medicare buy-in to reduce the cost of premiums for others in the marketplace, Dr. Eibner and colleagues said. But this result is consistent with other recent findings, including research presented by the consulting firm Milliman at a Society of Actuaries meeting in June. The Blue Cross Blue Shield Association estimates that losing a large group of customers could raise premiums by about 10% for the remaining pool of insured people, the New York Times has reported.
That might make it attractive to middle-aged Americans. Dr. Eibner and colleagues estimated the annual premium for a Medicare buy-in at $9,747 in 2022. For a 50-year-old, a bronze-level ACA plan would cost $9,208 and a gold-level one, $12,277. For a 60-year-old, the annual premium for a bronze-level plan might be $13,512 and $18,016 for a gold-level plan.
Total out-of-pocket health spending, including premiums, would fall, on average, by 16%-35% for those who moved from ACA-compliant individual market coverage to a buy-in. The lower spending reflects that buy-in enrollees would have access to Medicare payment rates, which are substantially lower than private rates, and lower administrative costs.
There may be growing interest in allowing people aged 50-64 to buy into Medicare if enthusiasm wanes for bids to create a giant national health program, Dr. Eibner said in an interview.
“If there is concern that Medicare for all is going too far, I think this option is something that could become more prominent,” she said.
Many Democratic lawmakers already are focused on a Medicare buy-in approach. Sen. Debbie Stabenow (D-Mich.) has 20 Democratic cosponsors for her bill, which would allow for a Medicare buy-in at age 50. Sen. Bernie Sanders (I-Vt.) has 14 Democratic cosponsors for the current version of his well-known “Medicare-for-all” bill. That’s two fewer than he had for the Medicare-for-all bill he offered in the 115th session of Congress (Jan. 3, 2017–Jan. 3, 2019).
Among the supporters of Sen. Stabenow’s bill are several 2020 presidential contenders: Sen. Cory Booker (D-N.J.), Sen. Amy Klobuchar (D-Minn.), and Sen. Kamala Harris (D-Calif). As of Saturday, Sen. Elizabeth Warren (D-Mass.) backed Sanders’ bill, but not Stabenow’s. But Sen. Warren also has spoken recently of a Medicare expansion for people at age 50 as a step on the path toward universal coverage. Former Vice President Joseph R. Biden and Mayor Pete Buttigieg of South Bend, Ind., have said they would like to offer Americans the option to buy into Medicare or a public plan.
The idea of lowering the Medicare age has been considered for many years by Democrats. It was seen as a way to help older Americans afford medical care before the enactment of the ACA. Before that law took effect, consumers were not guaranteed access to a health plan, causing many older Americans to go without coverage.
But Dr. Eibner and colleagues found that a Medicare buy-in would have little to no effect on total health insurance enrollment. A Medicare buy-in might increase enrollment by 400,000 to 1.6 million for those over age 50, while decreasing enrollment by 100,000 to 800,000 for those under age 50 because of rising premiums.
“It’s not doing a lot to get people covered,” Dr. Eibner said in the interview.
In the report, Dr. Eibner and colleagues estimated that between 2.8 million and 7.0 million people would choose to enroll, depending on the approach used to design a Medicare buy-in. They considered numerous potential options for the design of a Medicare expansion, including various levels of federal subsidy for people using the buy-in. Dr. Eibner and colleagues also considered whether insurers would respond by trying to selectively market to healthier individuals, increasing their chance of enrolling.
The envisioned Medicare buy-in would have no effect on the Medicare Trust Fund, which pools money available through previously collected dedicated taxes, Dr. Eibner and colleagues said. In creating their model, they drew upon data from the Survey of Income and Program Participation, the Medical Expenditure Panel Survey, and the Kaiser Family Foundation and Health Research and Educational Trust Employer Health Benefits Survey.
Dr. Eibner and colleagues noted “several important limitations” for their work. It does not look at how the buy-in might affect clinicians and hospitals. Lower Medicare payment rates might cause some physicians to turn away patients covered by a Medicare buy-in.
“On the other hand, even if some providers decided not to participate in the buy-in, the buy-in might offer enrollees a broader network than what is available on the individual market,” Dr. Eibner and colleagues wrote. “Furthermore, it is not clear that providers would be legally allowed to opt out of the buy-in while still accepting payment for current Medicare beneficiaries.”
The Rand report was developed with the support of funding by the AARP.
SOURCE: Eibner C et al. RAND Corporation. Research Report. 2019. doi: 10.7249/RR4246.
Early buy-in by people aged 50-64 might raise insurance premiums
Early buy-in by people aged 50-64 might raise insurance premiums
Allowing people aged 50-64 to buy into Medicare might result in higher premiums for people who purchase their health insurance on the individual market, a finding that runs counter to many people’s expectations, said the authors of a newly released report.
In the report, Christine Eibner, PhD, of the RAND Corporation and colleagues estimated that the premium for so-called bronze market plans might increase by 2%-10%, depending on the design of a Medicare buy-in program. (The bronze plans are ones with fewer benefits sold on exchanges created through the implementation of the Affordable Care Act of 2010 [ACA].)
A perception has been that younger adults have been in effect subsidizing the cost of older ones in the marketplace plans. Instead, it appears that younger adults who enroll in the individual market tend to be relatively unhealthy and thus expensive to cover.
“When older adults leave the market, insurers are left with a smaller pool of younger, less healthy, and relatively expensive people given their age, leading to higher premiums,” Dr. Eibner and colleagues said in the report.
This result was unexpected, as there has been discussion of using a Medicare buy-in to reduce the cost of premiums for others in the marketplace, Dr. Eibner and colleagues said. But this result is consistent with other recent findings, including research presented by the consulting firm Milliman at a Society of Actuaries meeting in June. The Blue Cross Blue Shield Association estimates that losing a large group of customers could raise premiums by about 10% for the remaining pool of insured people, the New York Times has reported.
That might make it attractive to middle-aged Americans. Dr. Eibner and colleagues estimated the annual premium for a Medicare buy-in at $9,747 in 2022. For a 50-year-old, a bronze-level ACA plan would cost $9,208 and a gold-level one, $12,277. For a 60-year-old, the annual premium for a bronze-level plan might be $13,512 and $18,016 for a gold-level plan.
Total out-of-pocket health spending, including premiums, would fall, on average, by 16%-35% for those who moved from ACA-compliant individual market coverage to a buy-in. The lower spending reflects that buy-in enrollees would have access to Medicare payment rates, which are substantially lower than private rates, and lower administrative costs.
There may be growing interest in allowing people aged 50-64 to buy into Medicare if enthusiasm wanes for bids to create a giant national health program, Dr. Eibner said in an interview.
“If there is concern that Medicare for all is going too far, I think this option is something that could become more prominent,” she said.
Many Democratic lawmakers already are focused on a Medicare buy-in approach. Sen. Debbie Stabenow (D-Mich.) has 20 Democratic cosponsors for her bill, which would allow for a Medicare buy-in at age 50. Sen. Bernie Sanders (I-Vt.) has 14 Democratic cosponsors for the current version of his well-known “Medicare-for-all” bill. That’s two fewer than he had for the Medicare-for-all bill he offered in the 115th session of Congress (Jan. 3, 2017–Jan. 3, 2019).
Among the supporters of Sen. Stabenow’s bill are several 2020 presidential contenders: Sen. Cory Booker (D-N.J.), Sen. Amy Klobuchar (D-Minn.), and Sen. Kamala Harris (D-Calif). As of Saturday, Sen. Elizabeth Warren (D-Mass.) backed Sanders’ bill, but not Stabenow’s. But Sen. Warren also has spoken recently of a Medicare expansion for people at age 50 as a step on the path toward universal coverage. Former Vice President Joseph R. Biden and Mayor Pete Buttigieg of South Bend, Ind., have said they would like to offer Americans the option to buy into Medicare or a public plan.
The idea of lowering the Medicare age has been considered for many years by Democrats. It was seen as a way to help older Americans afford medical care before the enactment of the ACA. Before that law took effect, consumers were not guaranteed access to a health plan, causing many older Americans to go without coverage.
But Dr. Eibner and colleagues found that a Medicare buy-in would have little to no effect on total health insurance enrollment. A Medicare buy-in might increase enrollment by 400,000 to 1.6 million for those over age 50, while decreasing enrollment by 100,000 to 800,000 for those under age 50 because of rising premiums.
“It’s not doing a lot to get people covered,” Dr. Eibner said in the interview.
In the report, Dr. Eibner and colleagues estimated that between 2.8 million and 7.0 million people would choose to enroll, depending on the approach used to design a Medicare buy-in. They considered numerous potential options for the design of a Medicare expansion, including various levels of federal subsidy for people using the buy-in. Dr. Eibner and colleagues also considered whether insurers would respond by trying to selectively market to healthier individuals, increasing their chance of enrolling.
The envisioned Medicare buy-in would have no effect on the Medicare Trust Fund, which pools money available through previously collected dedicated taxes, Dr. Eibner and colleagues said. In creating their model, they drew upon data from the Survey of Income and Program Participation, the Medical Expenditure Panel Survey, and the Kaiser Family Foundation and Health Research and Educational Trust Employer Health Benefits Survey.
Dr. Eibner and colleagues noted “several important limitations” for their work. It does not look at how the buy-in might affect clinicians and hospitals. Lower Medicare payment rates might cause some physicians to turn away patients covered by a Medicare buy-in.
“On the other hand, even if some providers decided not to participate in the buy-in, the buy-in might offer enrollees a broader network than what is available on the individual market,” Dr. Eibner and colleagues wrote. “Furthermore, it is not clear that providers would be legally allowed to opt out of the buy-in while still accepting payment for current Medicare beneficiaries.”
The Rand report was developed with the support of funding by the AARP.
SOURCE: Eibner C et al. RAND Corporation. Research Report. 2019. doi: 10.7249/RR4246.
Allowing people aged 50-64 to buy into Medicare might result in higher premiums for people who purchase their health insurance on the individual market, a finding that runs counter to many people’s expectations, said the authors of a newly released report.
In the report, Christine Eibner, PhD, of the RAND Corporation and colleagues estimated that the premium for so-called bronze market plans might increase by 2%-10%, depending on the design of a Medicare buy-in program. (The bronze plans are ones with fewer benefits sold on exchanges created through the implementation of the Affordable Care Act of 2010 [ACA].)
A perception has been that younger adults have been in effect subsidizing the cost of older ones in the marketplace plans. Instead, it appears that younger adults who enroll in the individual market tend to be relatively unhealthy and thus expensive to cover.
“When older adults leave the market, insurers are left with a smaller pool of younger, less healthy, and relatively expensive people given their age, leading to higher premiums,” Dr. Eibner and colleagues said in the report.
This result was unexpected, as there has been discussion of using a Medicare buy-in to reduce the cost of premiums for others in the marketplace, Dr. Eibner and colleagues said. But this result is consistent with other recent findings, including research presented by the consulting firm Milliman at a Society of Actuaries meeting in June. The Blue Cross Blue Shield Association estimates that losing a large group of customers could raise premiums by about 10% for the remaining pool of insured people, the New York Times has reported.
That might make it attractive to middle-aged Americans. Dr. Eibner and colleagues estimated the annual premium for a Medicare buy-in at $9,747 in 2022. For a 50-year-old, a bronze-level ACA plan would cost $9,208 and a gold-level one, $12,277. For a 60-year-old, the annual premium for a bronze-level plan might be $13,512 and $18,016 for a gold-level plan.
Total out-of-pocket health spending, including premiums, would fall, on average, by 16%-35% for those who moved from ACA-compliant individual market coverage to a buy-in. The lower spending reflects that buy-in enrollees would have access to Medicare payment rates, which are substantially lower than private rates, and lower administrative costs.
There may be growing interest in allowing people aged 50-64 to buy into Medicare if enthusiasm wanes for bids to create a giant national health program, Dr. Eibner said in an interview.
“If there is concern that Medicare for all is going too far, I think this option is something that could become more prominent,” she said.
Many Democratic lawmakers already are focused on a Medicare buy-in approach. Sen. Debbie Stabenow (D-Mich.) has 20 Democratic cosponsors for her bill, which would allow for a Medicare buy-in at age 50. Sen. Bernie Sanders (I-Vt.) has 14 Democratic cosponsors for the current version of his well-known “Medicare-for-all” bill. That’s two fewer than he had for the Medicare-for-all bill he offered in the 115th session of Congress (Jan. 3, 2017–Jan. 3, 2019).
Among the supporters of Sen. Stabenow’s bill are several 2020 presidential contenders: Sen. Cory Booker (D-N.J.), Sen. Amy Klobuchar (D-Minn.), and Sen. Kamala Harris (D-Calif). As of Saturday, Sen. Elizabeth Warren (D-Mass.) backed Sanders’ bill, but not Stabenow’s. But Sen. Warren also has spoken recently of a Medicare expansion for people at age 50 as a step on the path toward universal coverage. Former Vice President Joseph R. Biden and Mayor Pete Buttigieg of South Bend, Ind., have said they would like to offer Americans the option to buy into Medicare or a public plan.
The idea of lowering the Medicare age has been considered for many years by Democrats. It was seen as a way to help older Americans afford medical care before the enactment of the ACA. Before that law took effect, consumers were not guaranteed access to a health plan, causing many older Americans to go without coverage.
But Dr. Eibner and colleagues found that a Medicare buy-in would have little to no effect on total health insurance enrollment. A Medicare buy-in might increase enrollment by 400,000 to 1.6 million for those over age 50, while decreasing enrollment by 100,000 to 800,000 for those under age 50 because of rising premiums.
“It’s not doing a lot to get people covered,” Dr. Eibner said in the interview.
In the report, Dr. Eibner and colleagues estimated that between 2.8 million and 7.0 million people would choose to enroll, depending on the approach used to design a Medicare buy-in. They considered numerous potential options for the design of a Medicare expansion, including various levels of federal subsidy for people using the buy-in. Dr. Eibner and colleagues also considered whether insurers would respond by trying to selectively market to healthier individuals, increasing their chance of enrolling.
The envisioned Medicare buy-in would have no effect on the Medicare Trust Fund, which pools money available through previously collected dedicated taxes, Dr. Eibner and colleagues said. In creating their model, they drew upon data from the Survey of Income and Program Participation, the Medical Expenditure Panel Survey, and the Kaiser Family Foundation and Health Research and Educational Trust Employer Health Benefits Survey.
Dr. Eibner and colleagues noted “several important limitations” for their work. It does not look at how the buy-in might affect clinicians and hospitals. Lower Medicare payment rates might cause some physicians to turn away patients covered by a Medicare buy-in.
“On the other hand, even if some providers decided not to participate in the buy-in, the buy-in might offer enrollees a broader network than what is available on the individual market,” Dr. Eibner and colleagues wrote. “Furthermore, it is not clear that providers would be legally allowed to opt out of the buy-in while still accepting payment for current Medicare beneficiaries.”
The Rand report was developed with the support of funding by the AARP.
SOURCE: Eibner C et al. RAND Corporation. Research Report. 2019. doi: 10.7249/RR4246.
In recurrent ovarian cancer, secondary surgery does not extend survival
Phase 3 findings ‘call into question’ merits of surgical cytoreduction
Secondary surgical cytoreduction was feasible but did not extend overall survival among women with platinum-sensitive, recurrent ovarian cancer in a prospective, randomized, phase 3 clinical trial, investigators report.
Women who received platinum-based chemotherapy plus surgery had a median overall survival of about 51 months, compared with 64.7 months for women who received platinum-based chemotherapy and no surgery, according to the results of the Gynecologic Oncology Group (GOG)-0213 study, a multicenter, open-label, randomized, phase 3 trial.
These findings “call into question” the merits of surgical cytoreduction, said the authors, led by Robert L. Coleman, MD, of the department of gynecologic oncology and reproductive medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
Specifically, the shorter overall survival in the surgery group vs. no-surgery group emphasizes the “importance of formally assessing the value of the procedure in clinical care,” said Dr. Coleman and coauthors in the report on GOG-0213. The study was published in the New England Journal of Medicine.
Clinical practice guidelines from the National Comprehensive Cancer Network currently cite secondary cytoreduction as an option for treatment of patients who experience a treatment-free interval of at least 6 months after a complete remission achieved on prior chemotherapy, the GOG-0213 investigators wrote.
Beyond GOG-0213, there are several other randomized trials underway in this setting, including DESKTOP III, a multicenter study comparing the efficacy of chemotherapy alone to chemotherapy plus additional tumor debulking surgery in women with recurrent platinum-sensitive ovarian cancer.
wrote Dr. Coleman and colleagues.
The GOG-0213 study, conducted in 67 centers, 65 of which were in the United States, had both a chemotherapy objective and a surgical objective in patients with platinum-sensitive recurrent ovarian cancer, investigators said.
Results of the chemotherapy objective, published in 2017 in Lancet Oncology, indicated that bevacizumab added to standard chemotherapy, followed by maintenance bevacizumab until progression, improved median overall survival.
The more recently reported results focused on 485 women of who 245 were randomized to receive chemotherapy alone. While 240 were randomized to receive cytoreduction prior to chemotherapy, 15 declined surgery, leaving 225 eligible patients (94%).
The adjusted hazard ratio for death was 1.29 (95% confidence interval, 0.97-1.72; P = 0.08) for surgery, compared with no surgery, which translated into median overall survival times of 50.6 months in the surgery arm and 64.7 months in the no-surgery arm, Dr. Coleman and coauthors reported.
However, 30-day morbidity and mortality were low, at 9% (20 patients) and 0.4% (1 patient), and just 4% of cases (8 patients) were aborted, they added.
Quality of life significantly declined right after secondary cytoreduction, although after recovery no significant differences were found between groups, according to the investigators.
Taken together, those findings “did not indicate that surgery plus chemotherapy was superior to chemotherapy alone,” investigators concluded.
However, several factors in GOG-0213, including longer-than-expected survival times and substantial platinum sensitivity among women in the trial, could have diluted an independent surgical effect, they said.
Dr. Coleman reported disclosures related to several pharmaceutical companies, including Agenus, AstraZeneca, Clovis, GamaMabs, Genmab, Janssen, Medivation, Merck, Regeneron, Roche/Genentech, OncoQuest, and Tesaro.
SOURCE: Coleman RL et al. N Engl J Med. 2019;381:1929-39.
Phase 3 findings ‘call into question’ merits of surgical cytoreduction
Phase 3 findings ‘call into question’ merits of surgical cytoreduction
Secondary surgical cytoreduction was feasible but did not extend overall survival among women with platinum-sensitive, recurrent ovarian cancer in a prospective, randomized, phase 3 clinical trial, investigators report.
Women who received platinum-based chemotherapy plus surgery had a median overall survival of about 51 months, compared with 64.7 months for women who received platinum-based chemotherapy and no surgery, according to the results of the Gynecologic Oncology Group (GOG)-0213 study, a multicenter, open-label, randomized, phase 3 trial.
These findings “call into question” the merits of surgical cytoreduction, said the authors, led by Robert L. Coleman, MD, of the department of gynecologic oncology and reproductive medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
Specifically, the shorter overall survival in the surgery group vs. no-surgery group emphasizes the “importance of formally assessing the value of the procedure in clinical care,” said Dr. Coleman and coauthors in the report on GOG-0213. The study was published in the New England Journal of Medicine.
Clinical practice guidelines from the National Comprehensive Cancer Network currently cite secondary cytoreduction as an option for treatment of patients who experience a treatment-free interval of at least 6 months after a complete remission achieved on prior chemotherapy, the GOG-0213 investigators wrote.
Beyond GOG-0213, there are several other randomized trials underway in this setting, including DESKTOP III, a multicenter study comparing the efficacy of chemotherapy alone to chemotherapy plus additional tumor debulking surgery in women with recurrent platinum-sensitive ovarian cancer.
wrote Dr. Coleman and colleagues.
The GOG-0213 study, conducted in 67 centers, 65 of which were in the United States, had both a chemotherapy objective and a surgical objective in patients with platinum-sensitive recurrent ovarian cancer, investigators said.
Results of the chemotherapy objective, published in 2017 in Lancet Oncology, indicated that bevacizumab added to standard chemotherapy, followed by maintenance bevacizumab until progression, improved median overall survival.
The more recently reported results focused on 485 women of who 245 were randomized to receive chemotherapy alone. While 240 were randomized to receive cytoreduction prior to chemotherapy, 15 declined surgery, leaving 225 eligible patients (94%).
The adjusted hazard ratio for death was 1.29 (95% confidence interval, 0.97-1.72; P = 0.08) for surgery, compared with no surgery, which translated into median overall survival times of 50.6 months in the surgery arm and 64.7 months in the no-surgery arm, Dr. Coleman and coauthors reported.
However, 30-day morbidity and mortality were low, at 9% (20 patients) and 0.4% (1 patient), and just 4% of cases (8 patients) were aborted, they added.
Quality of life significantly declined right after secondary cytoreduction, although after recovery no significant differences were found between groups, according to the investigators.
Taken together, those findings “did not indicate that surgery plus chemotherapy was superior to chemotherapy alone,” investigators concluded.
However, several factors in GOG-0213, including longer-than-expected survival times and substantial platinum sensitivity among women in the trial, could have diluted an independent surgical effect, they said.
Dr. Coleman reported disclosures related to several pharmaceutical companies, including Agenus, AstraZeneca, Clovis, GamaMabs, Genmab, Janssen, Medivation, Merck, Regeneron, Roche/Genentech, OncoQuest, and Tesaro.
SOURCE: Coleman RL et al. N Engl J Med. 2019;381:1929-39.
Secondary surgical cytoreduction was feasible but did not extend overall survival among women with platinum-sensitive, recurrent ovarian cancer in a prospective, randomized, phase 3 clinical trial, investigators report.
Women who received platinum-based chemotherapy plus surgery had a median overall survival of about 51 months, compared with 64.7 months for women who received platinum-based chemotherapy and no surgery, according to the results of the Gynecologic Oncology Group (GOG)-0213 study, a multicenter, open-label, randomized, phase 3 trial.
These findings “call into question” the merits of surgical cytoreduction, said the authors, led by Robert L. Coleman, MD, of the department of gynecologic oncology and reproductive medicine at the University of Texas M.D. Anderson Cancer Center, Houston.
Specifically, the shorter overall survival in the surgery group vs. no-surgery group emphasizes the “importance of formally assessing the value of the procedure in clinical care,” said Dr. Coleman and coauthors in the report on GOG-0213. The study was published in the New England Journal of Medicine.
Clinical practice guidelines from the National Comprehensive Cancer Network currently cite secondary cytoreduction as an option for treatment of patients who experience a treatment-free interval of at least 6 months after a complete remission achieved on prior chemotherapy, the GOG-0213 investigators wrote.
Beyond GOG-0213, there are several other randomized trials underway in this setting, including DESKTOP III, a multicenter study comparing the efficacy of chemotherapy alone to chemotherapy plus additional tumor debulking surgery in women with recurrent platinum-sensitive ovarian cancer.
wrote Dr. Coleman and colleagues.
The GOG-0213 study, conducted in 67 centers, 65 of which were in the United States, had both a chemotherapy objective and a surgical objective in patients with platinum-sensitive recurrent ovarian cancer, investigators said.
Results of the chemotherapy objective, published in 2017 in Lancet Oncology, indicated that bevacizumab added to standard chemotherapy, followed by maintenance bevacizumab until progression, improved median overall survival.
The more recently reported results focused on 485 women of who 245 were randomized to receive chemotherapy alone. While 240 were randomized to receive cytoreduction prior to chemotherapy, 15 declined surgery, leaving 225 eligible patients (94%).
The adjusted hazard ratio for death was 1.29 (95% confidence interval, 0.97-1.72; P = 0.08) for surgery, compared with no surgery, which translated into median overall survival times of 50.6 months in the surgery arm and 64.7 months in the no-surgery arm, Dr. Coleman and coauthors reported.
However, 30-day morbidity and mortality were low, at 9% (20 patients) and 0.4% (1 patient), and just 4% of cases (8 patients) were aborted, they added.
Quality of life significantly declined right after secondary cytoreduction, although after recovery no significant differences were found between groups, according to the investigators.
Taken together, those findings “did not indicate that surgery plus chemotherapy was superior to chemotherapy alone,” investigators concluded.
However, several factors in GOG-0213, including longer-than-expected survival times and substantial platinum sensitivity among women in the trial, could have diluted an independent surgical effect, they said.
Dr. Coleman reported disclosures related to several pharmaceutical companies, including Agenus, AstraZeneca, Clovis, GamaMabs, Genmab, Janssen, Medivation, Merck, Regeneron, Roche/Genentech, OncoQuest, and Tesaro.
SOURCE: Coleman RL et al. N Engl J Med. 2019;381:1929-39.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Children may develop prolonged headache after concussion
CHARLOTTE, N.C. – , according to research presented at the annual meeting of the Child Neurology Society. The headache may be migraine, chronic daily headache, tension-type headache, or a combination of these headaches.
“We strongly recommend that individuals who develop persistent headache after a concussion be evaluated and treated by a neurologist with experience in administering treatment for headache,” said Marcus Barissi, Weller Scholar at the Cleveland Clinic, and colleagues. “Using this approach, we hope that their prolonged headaches will be lessened.”
Few studies have examined prolonged pediatric postconcussion headache
The Centers for Disease Control and Prevention estimates that between 1.6 million and 3.8 million concussions occur annually during athletic and recreational activities in the United States. About 90% of concussions affect children or adolescents. The symptom most often reported after concussion is headache.
Few studies have focused on new persistent postconcussion headache (NPPCH) in children. Mr. Barissi and colleagues did not find any previous study that had examined prolonged headache following concussion in patients without prior chronic headache. They sought to ascertain the prognosis of patients with NPPCH and no history of prior headache, to describe this clinical entity, and to identify beneficial treatment methods.
The investigators retrospectively reviewed charts for approximately 2,000 patients who presented to the Cleveland Clinic pediatric neurology department between June 2017 and August 2018 for headaches. They identified 259 patients who received a diagnosis of concussion, 69 (27%) of whom had headaches for longer than 2 months after injury.
Mr. Barissi and colleagues emailed these patients, and 33 (48%) of them agreed to complete a questionnaire and participate in a 10-minute phone interview. Thirty-one patients (43%) could not be contacted, and eight (11%) declined to participate. All participants confirmed that they had not had consistent headache before the concussion and that chronic headache had arisen after concussion. To determine participants’ medical outcomes, the researchers compared participants’ initial assessment data with posttreatment data collected during the interview process.
Healthy behaviors increased after concussion
Of the 69 eligible participants, 38 (55%) were female. The population’s median age was 17. Twenty-eight (85%) of the 33 patients who completed the questionnaire considered the information and treatment that they had received to be beneficial. Twenty-five (78%) patients continued to have headache after several months, despite treatment.
Participants had withstood a mean of 1.72 concussions, and the mean age at first injury was 12.49 years. The most common cause of injury was a fall for males (36%) and an automobile accident for females (18%).
Forty-eight patients (70%) reported having two types of headache. Fifty-two patients (75%) had migraines, and 65 (94%) had chronic daily headache or tension-type headache. Forty-eight (70%) participants had a family history of headache.
In all, 64 patients (93%) had used a headache medication. The most common headache medications used were amitriptyline, topiramate, and cyproheptadine. Few patients were still taking these medications at several months after evaluation. The most common nonprescription medications used were Migravent (i.e., magnesium, riboflavin, coenzyme Q10, and butterbur), ondansetron, and melatonin. Furthermore, 61 patients (88%) participated in nonmedicinal therapy such as physical therapy, chiropractic therapy, and acupuncture.
After evaluation, patients engaged in several healthy behaviors (e.g., adequate exercise, proper use of over-the-counter medications, and drinking sufficient water) more frequently, but did not get adequate sleep. Sixty-five participants (94%) had undergone CT or MRI imaging, but the results did not improve understanding of headache etiology or treatment. Many patients missed several days of school, but average attendance improved after months of treatment.
Long-term outcomes
Thirty-one survey respondents (94%) reported that their emotional, cognitive, sleep, and somatic postconcussion symptoms had resolved. Nevertheless, a majority of participants still had headache. “The persistence of postconcussion symptoms is uncommon, but lasting headache is not,” said the researchers. “If patients are not properly educated, conditions may deteriorate, extending the duration of disability.” A longer study with a larger sample size could provide valuable information, said the researchers. Future work should examine objectively the efficacy of various medications used to treat NPPCH and determine the best methods of treatment for this syndrome, which “can cause prolonged pain, suffering, and lack of function,” they concluded.
The investigators did not report any study funding or disclosures.
SOURCE: Barissi M et al. CNS 2019, Abstract 95.
CHARLOTTE, N.C. – , according to research presented at the annual meeting of the Child Neurology Society. The headache may be migraine, chronic daily headache, tension-type headache, or a combination of these headaches.
“We strongly recommend that individuals who develop persistent headache after a concussion be evaluated and treated by a neurologist with experience in administering treatment for headache,” said Marcus Barissi, Weller Scholar at the Cleveland Clinic, and colleagues. “Using this approach, we hope that their prolonged headaches will be lessened.”
Few studies have examined prolonged pediatric postconcussion headache
The Centers for Disease Control and Prevention estimates that between 1.6 million and 3.8 million concussions occur annually during athletic and recreational activities in the United States. About 90% of concussions affect children or adolescents. The symptom most often reported after concussion is headache.
Few studies have focused on new persistent postconcussion headache (NPPCH) in children. Mr. Barissi and colleagues did not find any previous study that had examined prolonged headache following concussion in patients without prior chronic headache. They sought to ascertain the prognosis of patients with NPPCH and no history of prior headache, to describe this clinical entity, and to identify beneficial treatment methods.
The investigators retrospectively reviewed charts for approximately 2,000 patients who presented to the Cleveland Clinic pediatric neurology department between June 2017 and August 2018 for headaches. They identified 259 patients who received a diagnosis of concussion, 69 (27%) of whom had headaches for longer than 2 months after injury.
Mr. Barissi and colleagues emailed these patients, and 33 (48%) of them agreed to complete a questionnaire and participate in a 10-minute phone interview. Thirty-one patients (43%) could not be contacted, and eight (11%) declined to participate. All participants confirmed that they had not had consistent headache before the concussion and that chronic headache had arisen after concussion. To determine participants’ medical outcomes, the researchers compared participants’ initial assessment data with posttreatment data collected during the interview process.
Healthy behaviors increased after concussion
Of the 69 eligible participants, 38 (55%) were female. The population’s median age was 17. Twenty-eight (85%) of the 33 patients who completed the questionnaire considered the information and treatment that they had received to be beneficial. Twenty-five (78%) patients continued to have headache after several months, despite treatment.
Participants had withstood a mean of 1.72 concussions, and the mean age at first injury was 12.49 years. The most common cause of injury was a fall for males (36%) and an automobile accident for females (18%).
Forty-eight patients (70%) reported having two types of headache. Fifty-two patients (75%) had migraines, and 65 (94%) had chronic daily headache or tension-type headache. Forty-eight (70%) participants had a family history of headache.
In all, 64 patients (93%) had used a headache medication. The most common headache medications used were amitriptyline, topiramate, and cyproheptadine. Few patients were still taking these medications at several months after evaluation. The most common nonprescription medications used were Migravent (i.e., magnesium, riboflavin, coenzyme Q10, and butterbur), ondansetron, and melatonin. Furthermore, 61 patients (88%) participated in nonmedicinal therapy such as physical therapy, chiropractic therapy, and acupuncture.
After evaluation, patients engaged in several healthy behaviors (e.g., adequate exercise, proper use of over-the-counter medications, and drinking sufficient water) more frequently, but did not get adequate sleep. Sixty-five participants (94%) had undergone CT or MRI imaging, but the results did not improve understanding of headache etiology or treatment. Many patients missed several days of school, but average attendance improved after months of treatment.
Long-term outcomes
Thirty-one survey respondents (94%) reported that their emotional, cognitive, sleep, and somatic postconcussion symptoms had resolved. Nevertheless, a majority of participants still had headache. “The persistence of postconcussion symptoms is uncommon, but lasting headache is not,” said the researchers. “If patients are not properly educated, conditions may deteriorate, extending the duration of disability.” A longer study with a larger sample size could provide valuable information, said the researchers. Future work should examine objectively the efficacy of various medications used to treat NPPCH and determine the best methods of treatment for this syndrome, which “can cause prolonged pain, suffering, and lack of function,” they concluded.
The investigators did not report any study funding or disclosures.
SOURCE: Barissi M et al. CNS 2019, Abstract 95.
CHARLOTTE, N.C. – , according to research presented at the annual meeting of the Child Neurology Society. The headache may be migraine, chronic daily headache, tension-type headache, or a combination of these headaches.
“We strongly recommend that individuals who develop persistent headache after a concussion be evaluated and treated by a neurologist with experience in administering treatment for headache,” said Marcus Barissi, Weller Scholar at the Cleveland Clinic, and colleagues. “Using this approach, we hope that their prolonged headaches will be lessened.”
Few studies have examined prolonged pediatric postconcussion headache
The Centers for Disease Control and Prevention estimates that between 1.6 million and 3.8 million concussions occur annually during athletic and recreational activities in the United States. About 90% of concussions affect children or adolescents. The symptom most often reported after concussion is headache.
Few studies have focused on new persistent postconcussion headache (NPPCH) in children. Mr. Barissi and colleagues did not find any previous study that had examined prolonged headache following concussion in patients without prior chronic headache. They sought to ascertain the prognosis of patients with NPPCH and no history of prior headache, to describe this clinical entity, and to identify beneficial treatment methods.
The investigators retrospectively reviewed charts for approximately 2,000 patients who presented to the Cleveland Clinic pediatric neurology department between June 2017 and August 2018 for headaches. They identified 259 patients who received a diagnosis of concussion, 69 (27%) of whom had headaches for longer than 2 months after injury.
Mr. Barissi and colleagues emailed these patients, and 33 (48%) of them agreed to complete a questionnaire and participate in a 10-minute phone interview. Thirty-one patients (43%) could not be contacted, and eight (11%) declined to participate. All participants confirmed that they had not had consistent headache before the concussion and that chronic headache had arisen after concussion. To determine participants’ medical outcomes, the researchers compared participants’ initial assessment data with posttreatment data collected during the interview process.
Healthy behaviors increased after concussion
Of the 69 eligible participants, 38 (55%) were female. The population’s median age was 17. Twenty-eight (85%) of the 33 patients who completed the questionnaire considered the information and treatment that they had received to be beneficial. Twenty-five (78%) patients continued to have headache after several months, despite treatment.
Participants had withstood a mean of 1.72 concussions, and the mean age at first injury was 12.49 years. The most common cause of injury was a fall for males (36%) and an automobile accident for females (18%).
Forty-eight patients (70%) reported having two types of headache. Fifty-two patients (75%) had migraines, and 65 (94%) had chronic daily headache or tension-type headache. Forty-eight (70%) participants had a family history of headache.
In all, 64 patients (93%) had used a headache medication. The most common headache medications used were amitriptyline, topiramate, and cyproheptadine. Few patients were still taking these medications at several months after evaluation. The most common nonprescription medications used were Migravent (i.e., magnesium, riboflavin, coenzyme Q10, and butterbur), ondansetron, and melatonin. Furthermore, 61 patients (88%) participated in nonmedicinal therapy such as physical therapy, chiropractic therapy, and acupuncture.
After evaluation, patients engaged in several healthy behaviors (e.g., adequate exercise, proper use of over-the-counter medications, and drinking sufficient water) more frequently, but did not get adequate sleep. Sixty-five participants (94%) had undergone CT or MRI imaging, but the results did not improve understanding of headache etiology or treatment. Many patients missed several days of school, but average attendance improved after months of treatment.
Long-term outcomes
Thirty-one survey respondents (94%) reported that their emotional, cognitive, sleep, and somatic postconcussion symptoms had resolved. Nevertheless, a majority of participants still had headache. “The persistence of postconcussion symptoms is uncommon, but lasting headache is not,” said the researchers. “If patients are not properly educated, conditions may deteriorate, extending the duration of disability.” A longer study with a larger sample size could provide valuable information, said the researchers. Future work should examine objectively the efficacy of various medications used to treat NPPCH and determine the best methods of treatment for this syndrome, which “can cause prolonged pain, suffering, and lack of function,” they concluded.
The investigators did not report any study funding or disclosures.
SOURCE: Barissi M et al. CNS 2019, Abstract 95.
REPORTING FROM CNS 2019
Early postmenopausal risk management leads to ‘optimum health’ for women
PHILADELPHIA – Postmenopausal women are at risk of numerous medical conditions after the onset of menopause, but many ob.gyns feel uncomfortable treating those patients, according to a keynote speaker at the annual meeting of the American Society for Reproductive Medicine.
The population of women entering menopause continues to rise, and they are at risk for developing chronic diseases about a decade after the onset of menopause, said Rogerio A. Lobo, MD, professor of obstetrics and gynecology at Columbia University, New York.
“For me, from a primary care perspective, there’s a major opportunity for all of us providers at the onset of menopause to identify risks and initiate preventive strategies,” he said.
These newly menopausal women are at risk for diseases across multiple specialty areas, which include obesity, metabolic syndrome, diabetes, cardiovascular disease, osteoporosis, chronic arthritis, dementia, cognitive decline, depression, and cancer. “My focus along these lines is really longevity, reduction in mortality as well as quality of life,” said Dr. Lobo.
Understanding of the benefits of estrogen therapy for postmenopausal women began with work in two studies in the 1990s. One paper by Meir J. Stampfer, MD, and associates on 15 studies examining the effects of hormone therapy on coronary heart disease (CHD) found that the relative risk of estrogen therapy on the disease was 0.50 (95% confidence interval, 0.43-0.56) after adjusting for only prospective and angiographic studies (Prev Med. 1991;20[1]:47-63).
A second paper by Deborah Grady, MD, MPH, and associates found that hormone therapy with estrogen plus progestin decreased the risk of CHD and hip fracture in women but increased the risk of endometrial and breast cancer, and carried a recommendation for using estrogen plus progestin for women who have received a hysterectomy or who are at high risk for CHD (Ann Intern Med. 1992 Dec 15;117[12]:1016-37).
In the early 2000s, data from the Women’s Health Initiative (WHI) began to show a different story: Therapy with estrogen plus progestin was shown to carry risks of early harm in postmenopausal women, and one study by JoAnn E. Manson, MD, DrPH, and associates had a hazard ratio of 1.24 (nominal 95% confidence interval, 1.00-1.54) for CHD in postmenopausal women aged 50-79 years receiving the combined therapy (N Engl J Med. 2003;349:523-34).
The confidence intervals were later adjusted so the association was not significant, but the results led to conclusions that hormone therapy was harmful to women and increased risk of breast cancer, declining cognition, and dementia, as well as cardiovascular diseases such as coronary disease, stroke and thrombosis.
“That was the dogma for many people to this day, but it was clearly a rush to judgment,” said Dr. Lobo. “More harm than good was done for the field.”
The contradictory findings from the WHI and other studies may be explained by the timing of hormone therapy, Dr. Lobo explained. In the ELITE trial, 643 postmenopausal women, stratified into early-postmenopausal (less than 6 years) and late-postmenopausal (equal to or greater than 10 years) groups, received 1 mg of daily oral 17-beta-estradiol with 45 mg of progesterone vaginal gel or placebo. Researchers found that it was beneficial for preventing the progression of subclinical atherosclerosis when therapy was initiated in early but not in late menopause (N Eng J Med. 2016; 374:1221-31).
Estrogen also has benefits for the brain, and might help improve rates of cognitive decline and Alzheimer’s disease in postmenopausal women, Dr. Lobo said. Of the 1,768 women in the Cache County Study who described their use of hormone therapy after menopause, 176 women developed Alzheimer’s; however, use of hormone therapy within 5 years of menopause was associated with a 30% reduced risk of Alzheimer’s (95% confidence interval, 0.49-0.99) and had better benefits for long-term use up to 10 years. But this effect was not present in women who started hormone therapy 5 years or more after onset of menopause (Neurology. 2012 Oct 30. doi: 10.1212/WNL.0b013e318271f823).
Clinicians also should look at the risk of hormone therapy in terms of absolute real risk rather than relative risk. “In WHI, even though many of these events were not statistically significant, even if they assumed they were, the absolute numbers were 7-8 events per 10,000 women per year,” he said. “Those, according to WHI, are rare events if they’re even true.”
“For breast cancer, which is a big concern a lot of women have, endogenous risk factors are much higher than what hormones do,” he added.
Yet clinicians continue to act on data from the WHI, Dr. Lobo noted. In fact, many ob.gyns. report that they are uncomfortable treating women with symptoms associated with menopause.
“Three out of four women who seek help for symptoms don’t receive it. The practice of menopause has largely disappeared from for many, many practices.”
The American Society for Reproductive Medicine used to have a “menopause day,” but the society no longer offers a track for menopause, Dr. Lobo said. One solution aimed at addressing the absence of training might be a menopause curriculum for ob.gyn. residents to help them initiate prevention strategies for postmenopausal women and have the confidence to manage this patient population. Dr. Lobo cited one study from Johns Hopkins where ob.gyn. residents underwent a 2-year menopause medicine curriculum and scored significantly higher on posttest scores after completing the program (78.7% vs. 57.3%; P less than .05). After the curriculum, 85.7% also reported they were more comfortable treating patients with menopause (Menopause. 2016 Mar. 23[3]:275-9).
Work also needs to be done on the front of understanding which hormone therapies are most effective for postmenopausal women. While there is currently no one hormone therapy to specifically recommend, in the future, pharmacogenetics and genetic or molecular risk analyses will play a role in knowing which products to prescribe. “It can be done, to be able to have a clear path for longevity and improved quality of life,” Dr. Lobo said.
Dr. Lobo reported serving as a consultant to Amgen, Mithra, Sojournix, and TherapeuticsMD. In addition, his institution is receiving support from Bayer and the National Institutes of Health for a clinical trial.
PHILADELPHIA – Postmenopausal women are at risk of numerous medical conditions after the onset of menopause, but many ob.gyns feel uncomfortable treating those patients, according to a keynote speaker at the annual meeting of the American Society for Reproductive Medicine.
The population of women entering menopause continues to rise, and they are at risk for developing chronic diseases about a decade after the onset of menopause, said Rogerio A. Lobo, MD, professor of obstetrics and gynecology at Columbia University, New York.
“For me, from a primary care perspective, there’s a major opportunity for all of us providers at the onset of menopause to identify risks and initiate preventive strategies,” he said.
These newly menopausal women are at risk for diseases across multiple specialty areas, which include obesity, metabolic syndrome, diabetes, cardiovascular disease, osteoporosis, chronic arthritis, dementia, cognitive decline, depression, and cancer. “My focus along these lines is really longevity, reduction in mortality as well as quality of life,” said Dr. Lobo.
Understanding of the benefits of estrogen therapy for postmenopausal women began with work in two studies in the 1990s. One paper by Meir J. Stampfer, MD, and associates on 15 studies examining the effects of hormone therapy on coronary heart disease (CHD) found that the relative risk of estrogen therapy on the disease was 0.50 (95% confidence interval, 0.43-0.56) after adjusting for only prospective and angiographic studies (Prev Med. 1991;20[1]:47-63).
A second paper by Deborah Grady, MD, MPH, and associates found that hormone therapy with estrogen plus progestin decreased the risk of CHD and hip fracture in women but increased the risk of endometrial and breast cancer, and carried a recommendation for using estrogen plus progestin for women who have received a hysterectomy or who are at high risk for CHD (Ann Intern Med. 1992 Dec 15;117[12]:1016-37).
In the early 2000s, data from the Women’s Health Initiative (WHI) began to show a different story: Therapy with estrogen plus progestin was shown to carry risks of early harm in postmenopausal women, and one study by JoAnn E. Manson, MD, DrPH, and associates had a hazard ratio of 1.24 (nominal 95% confidence interval, 1.00-1.54) for CHD in postmenopausal women aged 50-79 years receiving the combined therapy (N Engl J Med. 2003;349:523-34).
The confidence intervals were later adjusted so the association was not significant, but the results led to conclusions that hormone therapy was harmful to women and increased risk of breast cancer, declining cognition, and dementia, as well as cardiovascular diseases such as coronary disease, stroke and thrombosis.
“That was the dogma for many people to this day, but it was clearly a rush to judgment,” said Dr. Lobo. “More harm than good was done for the field.”
The contradictory findings from the WHI and other studies may be explained by the timing of hormone therapy, Dr. Lobo explained. In the ELITE trial, 643 postmenopausal women, stratified into early-postmenopausal (less than 6 years) and late-postmenopausal (equal to or greater than 10 years) groups, received 1 mg of daily oral 17-beta-estradiol with 45 mg of progesterone vaginal gel or placebo. Researchers found that it was beneficial for preventing the progression of subclinical atherosclerosis when therapy was initiated in early but not in late menopause (N Eng J Med. 2016; 374:1221-31).
Estrogen also has benefits for the brain, and might help improve rates of cognitive decline and Alzheimer’s disease in postmenopausal women, Dr. Lobo said. Of the 1,768 women in the Cache County Study who described their use of hormone therapy after menopause, 176 women developed Alzheimer’s; however, use of hormone therapy within 5 years of menopause was associated with a 30% reduced risk of Alzheimer’s (95% confidence interval, 0.49-0.99) and had better benefits for long-term use up to 10 years. But this effect was not present in women who started hormone therapy 5 years or more after onset of menopause (Neurology. 2012 Oct 30. doi: 10.1212/WNL.0b013e318271f823).
Clinicians also should look at the risk of hormone therapy in terms of absolute real risk rather than relative risk. “In WHI, even though many of these events were not statistically significant, even if they assumed they were, the absolute numbers were 7-8 events per 10,000 women per year,” he said. “Those, according to WHI, are rare events if they’re even true.”
“For breast cancer, which is a big concern a lot of women have, endogenous risk factors are much higher than what hormones do,” he added.
Yet clinicians continue to act on data from the WHI, Dr. Lobo noted. In fact, many ob.gyns. report that they are uncomfortable treating women with symptoms associated with menopause.
“Three out of four women who seek help for symptoms don’t receive it. The practice of menopause has largely disappeared from for many, many practices.”
The American Society for Reproductive Medicine used to have a “menopause day,” but the society no longer offers a track for menopause, Dr. Lobo said. One solution aimed at addressing the absence of training might be a menopause curriculum for ob.gyn. residents to help them initiate prevention strategies for postmenopausal women and have the confidence to manage this patient population. Dr. Lobo cited one study from Johns Hopkins where ob.gyn. residents underwent a 2-year menopause medicine curriculum and scored significantly higher on posttest scores after completing the program (78.7% vs. 57.3%; P less than .05). After the curriculum, 85.7% also reported they were more comfortable treating patients with menopause (Menopause. 2016 Mar. 23[3]:275-9).
Work also needs to be done on the front of understanding which hormone therapies are most effective for postmenopausal women. While there is currently no one hormone therapy to specifically recommend, in the future, pharmacogenetics and genetic or molecular risk analyses will play a role in knowing which products to prescribe. “It can be done, to be able to have a clear path for longevity and improved quality of life,” Dr. Lobo said.
Dr. Lobo reported serving as a consultant to Amgen, Mithra, Sojournix, and TherapeuticsMD. In addition, his institution is receiving support from Bayer and the National Institutes of Health for a clinical trial.
PHILADELPHIA – Postmenopausal women are at risk of numerous medical conditions after the onset of menopause, but many ob.gyns feel uncomfortable treating those patients, according to a keynote speaker at the annual meeting of the American Society for Reproductive Medicine.
The population of women entering menopause continues to rise, and they are at risk for developing chronic diseases about a decade after the onset of menopause, said Rogerio A. Lobo, MD, professor of obstetrics and gynecology at Columbia University, New York.
“For me, from a primary care perspective, there’s a major opportunity for all of us providers at the onset of menopause to identify risks and initiate preventive strategies,” he said.
These newly menopausal women are at risk for diseases across multiple specialty areas, which include obesity, metabolic syndrome, diabetes, cardiovascular disease, osteoporosis, chronic arthritis, dementia, cognitive decline, depression, and cancer. “My focus along these lines is really longevity, reduction in mortality as well as quality of life,” said Dr. Lobo.
Understanding of the benefits of estrogen therapy for postmenopausal women began with work in two studies in the 1990s. One paper by Meir J. Stampfer, MD, and associates on 15 studies examining the effects of hormone therapy on coronary heart disease (CHD) found that the relative risk of estrogen therapy on the disease was 0.50 (95% confidence interval, 0.43-0.56) after adjusting for only prospective and angiographic studies (Prev Med. 1991;20[1]:47-63).
A second paper by Deborah Grady, MD, MPH, and associates found that hormone therapy with estrogen plus progestin decreased the risk of CHD and hip fracture in women but increased the risk of endometrial and breast cancer, and carried a recommendation for using estrogen plus progestin for women who have received a hysterectomy or who are at high risk for CHD (Ann Intern Med. 1992 Dec 15;117[12]:1016-37).
In the early 2000s, data from the Women’s Health Initiative (WHI) began to show a different story: Therapy with estrogen plus progestin was shown to carry risks of early harm in postmenopausal women, and one study by JoAnn E. Manson, MD, DrPH, and associates had a hazard ratio of 1.24 (nominal 95% confidence interval, 1.00-1.54) for CHD in postmenopausal women aged 50-79 years receiving the combined therapy (N Engl J Med. 2003;349:523-34).
The confidence intervals were later adjusted so the association was not significant, but the results led to conclusions that hormone therapy was harmful to women and increased risk of breast cancer, declining cognition, and dementia, as well as cardiovascular diseases such as coronary disease, stroke and thrombosis.
“That was the dogma for many people to this day, but it was clearly a rush to judgment,” said Dr. Lobo. “More harm than good was done for the field.”
The contradictory findings from the WHI and other studies may be explained by the timing of hormone therapy, Dr. Lobo explained. In the ELITE trial, 643 postmenopausal women, stratified into early-postmenopausal (less than 6 years) and late-postmenopausal (equal to or greater than 10 years) groups, received 1 mg of daily oral 17-beta-estradiol with 45 mg of progesterone vaginal gel or placebo. Researchers found that it was beneficial for preventing the progression of subclinical atherosclerosis when therapy was initiated in early but not in late menopause (N Eng J Med. 2016; 374:1221-31).
Estrogen also has benefits for the brain, and might help improve rates of cognitive decline and Alzheimer’s disease in postmenopausal women, Dr. Lobo said. Of the 1,768 women in the Cache County Study who described their use of hormone therapy after menopause, 176 women developed Alzheimer’s; however, use of hormone therapy within 5 years of menopause was associated with a 30% reduced risk of Alzheimer’s (95% confidence interval, 0.49-0.99) and had better benefits for long-term use up to 10 years. But this effect was not present in women who started hormone therapy 5 years or more after onset of menopause (Neurology. 2012 Oct 30. doi: 10.1212/WNL.0b013e318271f823).
Clinicians also should look at the risk of hormone therapy in terms of absolute real risk rather than relative risk. “In WHI, even though many of these events were not statistically significant, even if they assumed they were, the absolute numbers were 7-8 events per 10,000 women per year,” he said. “Those, according to WHI, are rare events if they’re even true.”
“For breast cancer, which is a big concern a lot of women have, endogenous risk factors are much higher than what hormones do,” he added.
Yet clinicians continue to act on data from the WHI, Dr. Lobo noted. In fact, many ob.gyns. report that they are uncomfortable treating women with symptoms associated with menopause.
“Three out of four women who seek help for symptoms don’t receive it. The practice of menopause has largely disappeared from for many, many practices.”
The American Society for Reproductive Medicine used to have a “menopause day,” but the society no longer offers a track for menopause, Dr. Lobo said. One solution aimed at addressing the absence of training might be a menopause curriculum for ob.gyn. residents to help them initiate prevention strategies for postmenopausal women and have the confidence to manage this patient population. Dr. Lobo cited one study from Johns Hopkins where ob.gyn. residents underwent a 2-year menopause medicine curriculum and scored significantly higher on posttest scores after completing the program (78.7% vs. 57.3%; P less than .05). After the curriculum, 85.7% also reported they were more comfortable treating patients with menopause (Menopause. 2016 Mar. 23[3]:275-9).
Work also needs to be done on the front of understanding which hormone therapies are most effective for postmenopausal women. While there is currently no one hormone therapy to specifically recommend, in the future, pharmacogenetics and genetic or molecular risk analyses will play a role in knowing which products to prescribe. “It can be done, to be able to have a clear path for longevity and improved quality of life,” Dr. Lobo said.
Dr. Lobo reported serving as a consultant to Amgen, Mithra, Sojournix, and TherapeuticsMD. In addition, his institution is receiving support from Bayer and the National Institutes of Health for a clinical trial.
EXPERT ANALYSIS FROM ASRM 2019








