User login
Inhibitor may overcome ibrutinib resistance in MCL
Investigators have identified a mechanism of ibrutinib resistance in mantle cell lymphoma (MCL) and showed that a small molecule can overcome that resistance in vitro and in vivo.
The team found that ibrutinib-resistant MCL cells rely on oxidative phosphorylation (OXPHOS) and glutaminolysis to survive.
Targeting the OXPHOS pathway with a small molecule, IACS-010759, inhibited the proliferation of ibrutinib-resistant cells in vitro.
IACS-010759 also decreased tumor volume and improved survival in mouse models of ibrutinib-resistant MCL and double-hit B-cell lymphoma.
Now, IACS-10759 is being tested in phase 1 trials of lymphoma and solid tumors (NCT03291938) as well as acute myeloid leukemia (NCT02882321).
Liang Zhang, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted the preclinical research and described their findings in Science Translational Medicine.
The investigators sequenced samples from MCL patients with ibrutinib-sensitive and -resistant disease and found that “glutamine-fueled OXPHOS appears to be a prominent energy metabolism pathway in ibrutinib-resistant MCL cells.”
This finding prompted the team to test IACS-010759, an inhibitor of ETC complex I, in ibrutinib-resistant MCL. They theorized that the inhibitor would be effective because, during OXPHOS, electrons are transferred from electron donors to acceptors through the ETC in redox reactions that release energy to form ATP, and OXPHOS generates ATP to meet requirements for cell growth.
In experiments, IACS-010759 inhibited the proliferation of two ibrutinib-resistant MCL cell lines, Z-138 and Maver-1, in a dose-dependent manner.
The investigators also tested IACS-010759 in two mouse models of ibrutinib-resistant MCL. In both models, mice treated with IACS-010759 had a significant reduction in tumor volume, compared with controls. In one model, IACS-010759 extended survival by a median of 11 days.
Finally, the team tested IACS-010759 in a model of ibrutinib-resistant, double-hit (MYC and BCL-2) B-cell lymphoma with central nervous system involvement. Again, IACS-010759 significantly inhibited tumor growth. Compared to ibrutinib and vehicle control, IACS-010759 provided a median survival benefit of more than 20 days.
There were no toxicities associated with IACS-010759 treatment, according to the investigators.
This research was supported by the MD Anderson B Cell Lymphoma Moon Shot Project, Gary Rogers Foundation, Kinder Foundation, Cullen Foundation, Cancer Prevention Research Institute of Texas, and the National Institutes of Health. Most investigators reported having no competing interests, but two reported a patent (WO/2015/130790).
SOURCE: Zhang L et al. Sci Transl Med. 2019 May 8. doi: 10.1126/scitranslmed.aau1167.
Investigators have identified a mechanism of ibrutinib resistance in mantle cell lymphoma (MCL) and showed that a small molecule can overcome that resistance in vitro and in vivo.
The team found that ibrutinib-resistant MCL cells rely on oxidative phosphorylation (OXPHOS) and glutaminolysis to survive.
Targeting the OXPHOS pathway with a small molecule, IACS-010759, inhibited the proliferation of ibrutinib-resistant cells in vitro.
IACS-010759 also decreased tumor volume and improved survival in mouse models of ibrutinib-resistant MCL and double-hit B-cell lymphoma.
Now, IACS-10759 is being tested in phase 1 trials of lymphoma and solid tumors (NCT03291938) as well as acute myeloid leukemia (NCT02882321).
Liang Zhang, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted the preclinical research and described their findings in Science Translational Medicine.
The investigators sequenced samples from MCL patients with ibrutinib-sensitive and -resistant disease and found that “glutamine-fueled OXPHOS appears to be a prominent energy metabolism pathway in ibrutinib-resistant MCL cells.”
This finding prompted the team to test IACS-010759, an inhibitor of ETC complex I, in ibrutinib-resistant MCL. They theorized that the inhibitor would be effective because, during OXPHOS, electrons are transferred from electron donors to acceptors through the ETC in redox reactions that release energy to form ATP, and OXPHOS generates ATP to meet requirements for cell growth.
In experiments, IACS-010759 inhibited the proliferation of two ibrutinib-resistant MCL cell lines, Z-138 and Maver-1, in a dose-dependent manner.
The investigators also tested IACS-010759 in two mouse models of ibrutinib-resistant MCL. In both models, mice treated with IACS-010759 had a significant reduction in tumor volume, compared with controls. In one model, IACS-010759 extended survival by a median of 11 days.
Finally, the team tested IACS-010759 in a model of ibrutinib-resistant, double-hit (MYC and BCL-2) B-cell lymphoma with central nervous system involvement. Again, IACS-010759 significantly inhibited tumor growth. Compared to ibrutinib and vehicle control, IACS-010759 provided a median survival benefit of more than 20 days.
There were no toxicities associated with IACS-010759 treatment, according to the investigators.
This research was supported by the MD Anderson B Cell Lymphoma Moon Shot Project, Gary Rogers Foundation, Kinder Foundation, Cullen Foundation, Cancer Prevention Research Institute of Texas, and the National Institutes of Health. Most investigators reported having no competing interests, but two reported a patent (WO/2015/130790).
SOURCE: Zhang L et al. Sci Transl Med. 2019 May 8. doi: 10.1126/scitranslmed.aau1167.
Investigators have identified a mechanism of ibrutinib resistance in mantle cell lymphoma (MCL) and showed that a small molecule can overcome that resistance in vitro and in vivo.
The team found that ibrutinib-resistant MCL cells rely on oxidative phosphorylation (OXPHOS) and glutaminolysis to survive.
Targeting the OXPHOS pathway with a small molecule, IACS-010759, inhibited the proliferation of ibrutinib-resistant cells in vitro.
IACS-010759 also decreased tumor volume and improved survival in mouse models of ibrutinib-resistant MCL and double-hit B-cell lymphoma.
Now, IACS-10759 is being tested in phase 1 trials of lymphoma and solid tumors (NCT03291938) as well as acute myeloid leukemia (NCT02882321).
Liang Zhang, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston, and his colleagues conducted the preclinical research and described their findings in Science Translational Medicine.
The investigators sequenced samples from MCL patients with ibrutinib-sensitive and -resistant disease and found that “glutamine-fueled OXPHOS appears to be a prominent energy metabolism pathway in ibrutinib-resistant MCL cells.”
This finding prompted the team to test IACS-010759, an inhibitor of ETC complex I, in ibrutinib-resistant MCL. They theorized that the inhibitor would be effective because, during OXPHOS, electrons are transferred from electron donors to acceptors through the ETC in redox reactions that release energy to form ATP, and OXPHOS generates ATP to meet requirements for cell growth.
In experiments, IACS-010759 inhibited the proliferation of two ibrutinib-resistant MCL cell lines, Z-138 and Maver-1, in a dose-dependent manner.
The investigators also tested IACS-010759 in two mouse models of ibrutinib-resistant MCL. In both models, mice treated with IACS-010759 had a significant reduction in tumor volume, compared with controls. In one model, IACS-010759 extended survival by a median of 11 days.
Finally, the team tested IACS-010759 in a model of ibrutinib-resistant, double-hit (MYC and BCL-2) B-cell lymphoma with central nervous system involvement. Again, IACS-010759 significantly inhibited tumor growth. Compared to ibrutinib and vehicle control, IACS-010759 provided a median survival benefit of more than 20 days.
There were no toxicities associated with IACS-010759 treatment, according to the investigators.
This research was supported by the MD Anderson B Cell Lymphoma Moon Shot Project, Gary Rogers Foundation, Kinder Foundation, Cullen Foundation, Cancer Prevention Research Institute of Texas, and the National Institutes of Health. Most investigators reported having no competing interests, but two reported a patent (WO/2015/130790).
SOURCE: Zhang L et al. Sci Transl Med. 2019 May 8. doi: 10.1126/scitranslmed.aau1167.
FROM SCIENCE TRANSLATIONAL MEDICINE
Perplexing text messages may be the sole sign of stroke
PHILADELPHIA – described at the annual meeting of the American Academy of Neurology. The phenomenon, dubbed “dystextia” and characterized by confusing interpersonal electronic communications, is not new but is increasingly relevant to clinical practice now that smartphones are essentially ubiquitous, said the case report authors.
In fact, time to intervention may be positively impacted if access to a patient’s texts and emails can be obtained, said lead author Taylor R. Anderson, a medical student at Wayne State University, Detroit.
The findings sparked interest in further research into the underlying causes and implications of dystextia: “It will be interesting to see if there are specific regions of the brain that are responsible for texting, and how they relate to other forms of communication such as handwriting and typing,” the authors said in their poster presentation.
Mr. Anderson and colleagues described two patients evaluated at Ascension St. John Hospital and Medical Center in Detroit who had stroke presenting as difficulty in typing text messages.
One case was a 43-year-old woman who experienced headache consistent with her usual migraine and spelling errors in her texts and posts on Facebook. She had visuospatial anomalies and left facial droop on evaluation. A brain MRI revealed acute embolic infarcts in the parietal and right frontal lobes, according to Mr. Anderson and coauthors.
The second case was a 66-year-old woman who had difficulty writing texts and typed notes. A head CT done in an urgent care facility showed a left frontal subacute infarct, which, according to the authors, was likely related to risk factors including hypertension, diabetes, and dyslipidemia.
These cases show that dystextia can arise after lesions in either hemisphere, the authors said. However, they emphasized that both cases involved the dominant cerebral hemisphere, while by contrast, there have not been previous reports of dystextia due to nondominant hemispheric infarct.
It stands to reason that stroke would affect the ability to text, a multipurpose task that involves use of motor, language, and vision skills, the authors said. Strokes could affect not only skills needed to type, read, and express thoughts, but also visuospatial memory mapping to letters on the device’s keyboard, they noted.
The left frontal and superior parietal regions have been implicated in handwriting in neuroimaging experiments, the authors said, while the operculum and left second frontal convolution are involved in typing.
The authors had nothing to disclose.
SOURCE: Anderson T et al. AAN 2019. Abstract P5.3-062.
PHILADELPHIA – described at the annual meeting of the American Academy of Neurology. The phenomenon, dubbed “dystextia” and characterized by confusing interpersonal electronic communications, is not new but is increasingly relevant to clinical practice now that smartphones are essentially ubiquitous, said the case report authors.
In fact, time to intervention may be positively impacted if access to a patient’s texts and emails can be obtained, said lead author Taylor R. Anderson, a medical student at Wayne State University, Detroit.
The findings sparked interest in further research into the underlying causes and implications of dystextia: “It will be interesting to see if there are specific regions of the brain that are responsible for texting, and how they relate to other forms of communication such as handwriting and typing,” the authors said in their poster presentation.
Mr. Anderson and colleagues described two patients evaluated at Ascension St. John Hospital and Medical Center in Detroit who had stroke presenting as difficulty in typing text messages.
One case was a 43-year-old woman who experienced headache consistent with her usual migraine and spelling errors in her texts and posts on Facebook. She had visuospatial anomalies and left facial droop on evaluation. A brain MRI revealed acute embolic infarcts in the parietal and right frontal lobes, according to Mr. Anderson and coauthors.
The second case was a 66-year-old woman who had difficulty writing texts and typed notes. A head CT done in an urgent care facility showed a left frontal subacute infarct, which, according to the authors, was likely related to risk factors including hypertension, diabetes, and dyslipidemia.
These cases show that dystextia can arise after lesions in either hemisphere, the authors said. However, they emphasized that both cases involved the dominant cerebral hemisphere, while by contrast, there have not been previous reports of dystextia due to nondominant hemispheric infarct.
It stands to reason that stroke would affect the ability to text, a multipurpose task that involves use of motor, language, and vision skills, the authors said. Strokes could affect not only skills needed to type, read, and express thoughts, but also visuospatial memory mapping to letters on the device’s keyboard, they noted.
The left frontal and superior parietal regions have been implicated in handwriting in neuroimaging experiments, the authors said, while the operculum and left second frontal convolution are involved in typing.
The authors had nothing to disclose.
SOURCE: Anderson T et al. AAN 2019. Abstract P5.3-062.
PHILADELPHIA – described at the annual meeting of the American Academy of Neurology. The phenomenon, dubbed “dystextia” and characterized by confusing interpersonal electronic communications, is not new but is increasingly relevant to clinical practice now that smartphones are essentially ubiquitous, said the case report authors.
In fact, time to intervention may be positively impacted if access to a patient’s texts and emails can be obtained, said lead author Taylor R. Anderson, a medical student at Wayne State University, Detroit.
The findings sparked interest in further research into the underlying causes and implications of dystextia: “It will be interesting to see if there are specific regions of the brain that are responsible for texting, and how they relate to other forms of communication such as handwriting and typing,” the authors said in their poster presentation.
Mr. Anderson and colleagues described two patients evaluated at Ascension St. John Hospital and Medical Center in Detroit who had stroke presenting as difficulty in typing text messages.
One case was a 43-year-old woman who experienced headache consistent with her usual migraine and spelling errors in her texts and posts on Facebook. She had visuospatial anomalies and left facial droop on evaluation. A brain MRI revealed acute embolic infarcts in the parietal and right frontal lobes, according to Mr. Anderson and coauthors.
The second case was a 66-year-old woman who had difficulty writing texts and typed notes. A head CT done in an urgent care facility showed a left frontal subacute infarct, which, according to the authors, was likely related to risk factors including hypertension, diabetes, and dyslipidemia.
These cases show that dystextia can arise after lesions in either hemisphere, the authors said. However, they emphasized that both cases involved the dominant cerebral hemisphere, while by contrast, there have not been previous reports of dystextia due to nondominant hemispheric infarct.
It stands to reason that stroke would affect the ability to text, a multipurpose task that involves use of motor, language, and vision skills, the authors said. Strokes could affect not only skills needed to type, read, and express thoughts, but also visuospatial memory mapping to letters on the device’s keyboard, they noted.
The left frontal and superior parietal regions have been implicated in handwriting in neuroimaging experiments, the authors said, while the operculum and left second frontal convolution are involved in typing.
The authors had nothing to disclose.
SOURCE: Anderson T et al. AAN 2019. Abstract P5.3-062.
REPORTING FROM AAN 2019
BTK inhibitor reduces MS enhancing lesions
. However, there was no difference between the 25-mg once daily, 75-mg once daily, 75-mg twice daily, and placebo-treated groups in Expanded Disability Status Scale scores, according to a double-blind, randomized, phase 2 trial published in the New England Journal of Medicine (2019 May 10. doi: 10.1056/NEJMoa1901981).
We first reported on the results of this trial when they were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Find our coverage at the link below.
. However, there was no difference between the 25-mg once daily, 75-mg once daily, 75-mg twice daily, and placebo-treated groups in Expanded Disability Status Scale scores, according to a double-blind, randomized, phase 2 trial published in the New England Journal of Medicine (2019 May 10. doi: 10.1056/NEJMoa1901981).
We first reported on the results of this trial when they were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Find our coverage at the link below.
. However, there was no difference between the 25-mg once daily, 75-mg once daily, 75-mg twice daily, and placebo-treated groups in Expanded Disability Status Scale scores, according to a double-blind, randomized, phase 2 trial published in the New England Journal of Medicine (2019 May 10. doi: 10.1056/NEJMoa1901981).
We first reported on the results of this trial when they were presented at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Survey: High costs lead to skipped or postponed health care
Half of all Americans with employer-sponsored health benefits say that they or someone in their family has skipped or postponed care because of the cost, according to a survey by the Kaiser Family Foundation and the Los Angeles Times.
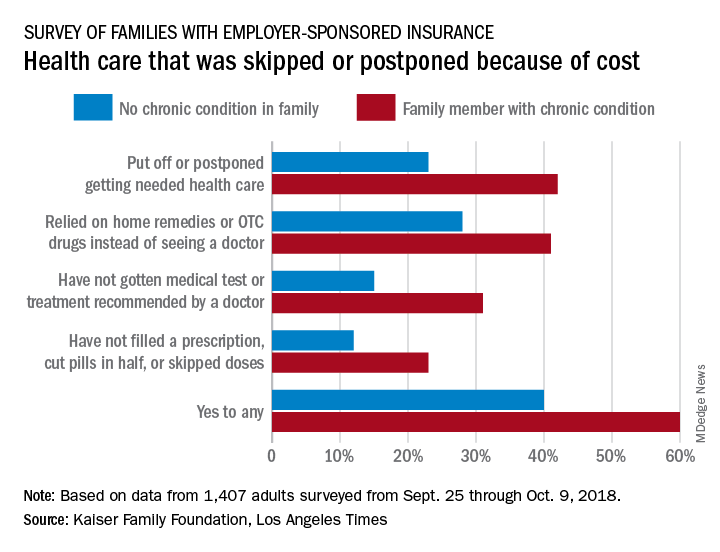
That number changes, however, when chronic conditions are considered. In the survey of Americans covered by employer-sponsored health insurance, 60% of those with a family member who had a chronic condition said that cost had altered the care of someone in the family over the previous 12 months, compared with 40% of those who had no chronic condition in their family, the KFF and L.A. Times noted in their report.
More specifically, families with an individual who had a chronic condition were more likely to put off or postpone needed care (42% vs. 23%) and to rely on home remedies or OTC drugs instead of visiting a physician (41% vs. 28%) than were families without chronic conditions, the report’s authors said.
When asked about the affordability of their health care, 49% of those in families with a chronic health condition said they had a problem paying for their coverage in the past year, compared with 29% of respondents in families with no chronic condition.
“Drilling down into the consequences of these affordability problems reveals more about the financial burden of health care on families with chronic conditions,” compared with those without chronic conditions: cut back spending on food, clothes, household items (35% vs. 16%); used up all or most of their savings (26% vs. 11%); and borrowed money from friends or family (14% vs. 6%), according to the researchers.
Although respondents felt “that the cost of health care for people like them is too high, more say the current U.S. health insurance system works well for people with employer coverage than say it works well for people on Medicare or Medicaid or those who purchase their own insurance. Asked who is to blame for high costs, majorities point the finger at pharmaceutical and insurance companies, while fewer see hospitals, doctors, or employers as deserving of blame,” the KFF and L.A. Times investigators wrote.
The survey involved a sample of 1,407 adults aged 18-64 years and was conducted from Sept. 25 through Oct. 9, 2018. The margin of the sampling error is ±3 percentage points.
Half of all Americans with employer-sponsored health benefits say that they or someone in their family has skipped or postponed care because of the cost, according to a survey by the Kaiser Family Foundation and the Los Angeles Times.

That number changes, however, when chronic conditions are considered. In the survey of Americans covered by employer-sponsored health insurance, 60% of those with a family member who had a chronic condition said that cost had altered the care of someone in the family over the previous 12 months, compared with 40% of those who had no chronic condition in their family, the KFF and L.A. Times noted in their report.
More specifically, families with an individual who had a chronic condition were more likely to put off or postpone needed care (42% vs. 23%) and to rely on home remedies or OTC drugs instead of visiting a physician (41% vs. 28%) than were families without chronic conditions, the report’s authors said.
When asked about the affordability of their health care, 49% of those in families with a chronic health condition said they had a problem paying for their coverage in the past year, compared with 29% of respondents in families with no chronic condition.
“Drilling down into the consequences of these affordability problems reveals more about the financial burden of health care on families with chronic conditions,” compared with those without chronic conditions: cut back spending on food, clothes, household items (35% vs. 16%); used up all or most of their savings (26% vs. 11%); and borrowed money from friends or family (14% vs. 6%), according to the researchers.
Although respondents felt “that the cost of health care for people like them is too high, more say the current U.S. health insurance system works well for people with employer coverage than say it works well for people on Medicare or Medicaid or those who purchase their own insurance. Asked who is to blame for high costs, majorities point the finger at pharmaceutical and insurance companies, while fewer see hospitals, doctors, or employers as deserving of blame,” the KFF and L.A. Times investigators wrote.
The survey involved a sample of 1,407 adults aged 18-64 years and was conducted from Sept. 25 through Oct. 9, 2018. The margin of the sampling error is ±3 percentage points.
Half of all Americans with employer-sponsored health benefits say that they or someone in their family has skipped or postponed care because of the cost, according to a survey by the Kaiser Family Foundation and the Los Angeles Times.

That number changes, however, when chronic conditions are considered. In the survey of Americans covered by employer-sponsored health insurance, 60% of those with a family member who had a chronic condition said that cost had altered the care of someone in the family over the previous 12 months, compared with 40% of those who had no chronic condition in their family, the KFF and L.A. Times noted in their report.
More specifically, families with an individual who had a chronic condition were more likely to put off or postpone needed care (42% vs. 23%) and to rely on home remedies or OTC drugs instead of visiting a physician (41% vs. 28%) than were families without chronic conditions, the report’s authors said.
When asked about the affordability of their health care, 49% of those in families with a chronic health condition said they had a problem paying for their coverage in the past year, compared with 29% of respondents in families with no chronic condition.
“Drilling down into the consequences of these affordability problems reveals more about the financial burden of health care on families with chronic conditions,” compared with those without chronic conditions: cut back spending on food, clothes, household items (35% vs. 16%); used up all or most of their savings (26% vs. 11%); and borrowed money from friends or family (14% vs. 6%), according to the researchers.
Although respondents felt “that the cost of health care for people like them is too high, more say the current U.S. health insurance system works well for people with employer coverage than say it works well for people on Medicare or Medicaid or those who purchase their own insurance. Asked who is to blame for high costs, majorities point the finger at pharmaceutical and insurance companies, while fewer see hospitals, doctors, or employers as deserving of blame,” the KFF and L.A. Times investigators wrote.
The survey involved a sample of 1,407 adults aged 18-64 years and was conducted from Sept. 25 through Oct. 9, 2018. The margin of the sampling error is ±3 percentage points.
Renal denervation boosts effectiveness of AFib catheter ablation
SAN FRANCISCO – Adding renal denervation when performing catheter ablation of paroxysmal atrial fibrillation in hypertensive patients substantially reduced their arrhythmia recurrence rate during the subsequent year in a multicenter, randomized trial with 302 patients.
The findings established renal denervation (RDN) as a “reasonable” tool to increase the success of atrial fibrillation (AFib) catheter ablation, Jonathan S. Steinberg, MD, said at the annual scientific sessions of the Heart Rhythm Society.
“The RDN procedure seems remarkably safe and seems to be reliably accomplished when an electrophysiologist does it,” said Dr. Steinberg, director of the Arrhythmia Center of the Summit Medical Group in Montclair, N.J. Given the evidence he reported that performing RDN simultaneously with AFib catheter ablation by pulmonary vein isolation significantly improved freedom from arrhythmia recurrence, this approach “is ready for clinical use at institutions that could mount this kind of program,” he declared.
The rate of freedom from arrhythmia recurrence while off antiarrhythmic drugs during the year following treatment was 57% among 138 patients treated with pulmonary vein isolation only, and 72% in 145 who underwent both pulmonary vein isolation and renal denervation. That’s “a pretty big difference in outcome” with no increased risk and with about 20 added minutes of procedure time, Dr. Steinberg said in a video interview. He acknowledged that, currently, no catheter is approved for U.S. marketing that is specifically designed for renal denervation, but the operators in the study he reported all used conventional radiofrequency ablation catheters with an irrigated tip, a design with U.S. availability.
The ERADICATE-AF (Renal Artery Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) study randomized 302 patients with paroxysmal AFib and hypertension uncontrolled by medication at three centers in Russia, one in Germany, and one in Poland. Enrolled patients averaged about 60 years of age, about 60% were men, and their average blood pressure was roughly 150/90 mm Hg while on treatment with a median of two antihypertensive drugs, including 100% on either an ACE inhibitor or angiotensin receptor blocker. The study operators performed RDN by placing an average of six lesions in a spiral pattern in each of the patient’s two renal arteries.
The investigators screened for arrhythmia recurrence with 7-day Holter monitoring at 3, 6, 9, and 12 months, with full 12-month follow-up available for 283 patients. After 12 months, blood pressures had declined by an average of 16/11 mm Hg among the patients who underwent RDN, with essentially no change in the patients who had pulmonary vein isolation only. Dr. Steinberg attributed the high success of the renal denervation procedures to the familiarity of the participating electrophysiologist operators with catheter-tip ablations.
“We have gone from treating patients with resistant hypertension to now treating patients with less severe hypertension,” Dr. Steinberg noted, and the next study he is planning will take this approach into patients with paroxysmal AFib but without hypertension, using RDN “solely as an anti-arrhythmic intervention,” he explained.
ERADICATE-AF did not receive commercial funding. Dr. Steinberg has been a consultant to Allergan, AtriCure, Biosense Webster, Corfigo, Medtronic, and Omron. He owns stock in AliveCor and receives salary from National Cardiac and G Medical.
ERADICATE-AF was a well-performed, informative, and provocative study that produced exciting results. I was very impressed that, despite the added complexity of performing an extra procedure, there appeared to be virtually no added risk to patients, with essentially identical complication rates in the two arms of the study. The 15.6% absolute difference in the rate of arrhythmia recurrences means that about six patients need to have renal denervation added to their catheter ablation to prevent one arrhythmia recurrence during 12 months, a pretty remarkable number-needed-to-treat.
Despite the successful outcome, adding renal denervation is not a panacea. These patients still had a 28% rate of recurrent atrial fibrillation during follow-up, and on average they also remained above their goal blood pressure despite the pressure reduction that renal denervation produced. The 43% arrhythmia recurrence rate among the patients who underwent only pulmonary vein isolation was consistent with prior reports on the efficacy of this treatment.
The findings raise the question of whether this approach would also work in AFib patients who are not hypertensive, and we must be cautious about the longer-term safety and durability of this treatment.
Cara N. Pellegrini, MD , is director of cardiac electrophysiology at the San Francisco VA Medical Center. She had no disclosures. She made these comments as designated discussant for ERADICATE-AF.
ERADICATE-AF was a well-performed, informative, and provocative study that produced exciting results. I was very impressed that, despite the added complexity of performing an extra procedure, there appeared to be virtually no added risk to patients, with essentially identical complication rates in the two arms of the study. The 15.6% absolute difference in the rate of arrhythmia recurrences means that about six patients need to have renal denervation added to their catheter ablation to prevent one arrhythmia recurrence during 12 months, a pretty remarkable number-needed-to-treat.
Despite the successful outcome, adding renal denervation is not a panacea. These patients still had a 28% rate of recurrent atrial fibrillation during follow-up, and on average they also remained above their goal blood pressure despite the pressure reduction that renal denervation produced. The 43% arrhythmia recurrence rate among the patients who underwent only pulmonary vein isolation was consistent with prior reports on the efficacy of this treatment.
The findings raise the question of whether this approach would also work in AFib patients who are not hypertensive, and we must be cautious about the longer-term safety and durability of this treatment.
Cara N. Pellegrini, MD , is director of cardiac electrophysiology at the San Francisco VA Medical Center. She had no disclosures. She made these comments as designated discussant for ERADICATE-AF.
ERADICATE-AF was a well-performed, informative, and provocative study that produced exciting results. I was very impressed that, despite the added complexity of performing an extra procedure, there appeared to be virtually no added risk to patients, with essentially identical complication rates in the two arms of the study. The 15.6% absolute difference in the rate of arrhythmia recurrences means that about six patients need to have renal denervation added to their catheter ablation to prevent one arrhythmia recurrence during 12 months, a pretty remarkable number-needed-to-treat.
Despite the successful outcome, adding renal denervation is not a panacea. These patients still had a 28% rate of recurrent atrial fibrillation during follow-up, and on average they also remained above their goal blood pressure despite the pressure reduction that renal denervation produced. The 43% arrhythmia recurrence rate among the patients who underwent only pulmonary vein isolation was consistent with prior reports on the efficacy of this treatment.
The findings raise the question of whether this approach would also work in AFib patients who are not hypertensive, and we must be cautious about the longer-term safety and durability of this treatment.
Cara N. Pellegrini, MD , is director of cardiac electrophysiology at the San Francisco VA Medical Center. She had no disclosures. She made these comments as designated discussant for ERADICATE-AF.
SAN FRANCISCO – Adding renal denervation when performing catheter ablation of paroxysmal atrial fibrillation in hypertensive patients substantially reduced their arrhythmia recurrence rate during the subsequent year in a multicenter, randomized trial with 302 patients.
The findings established renal denervation (RDN) as a “reasonable” tool to increase the success of atrial fibrillation (AFib) catheter ablation, Jonathan S. Steinberg, MD, said at the annual scientific sessions of the Heart Rhythm Society.
“The RDN procedure seems remarkably safe and seems to be reliably accomplished when an electrophysiologist does it,” said Dr. Steinberg, director of the Arrhythmia Center of the Summit Medical Group in Montclair, N.J. Given the evidence he reported that performing RDN simultaneously with AFib catheter ablation by pulmonary vein isolation significantly improved freedom from arrhythmia recurrence, this approach “is ready for clinical use at institutions that could mount this kind of program,” he declared.
The rate of freedom from arrhythmia recurrence while off antiarrhythmic drugs during the year following treatment was 57% among 138 patients treated with pulmonary vein isolation only, and 72% in 145 who underwent both pulmonary vein isolation and renal denervation. That’s “a pretty big difference in outcome” with no increased risk and with about 20 added minutes of procedure time, Dr. Steinberg said in a video interview. He acknowledged that, currently, no catheter is approved for U.S. marketing that is specifically designed for renal denervation, but the operators in the study he reported all used conventional radiofrequency ablation catheters with an irrigated tip, a design with U.S. availability.
The ERADICATE-AF (Renal Artery Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) study randomized 302 patients with paroxysmal AFib and hypertension uncontrolled by medication at three centers in Russia, one in Germany, and one in Poland. Enrolled patients averaged about 60 years of age, about 60% were men, and their average blood pressure was roughly 150/90 mm Hg while on treatment with a median of two antihypertensive drugs, including 100% on either an ACE inhibitor or angiotensin receptor blocker. The study operators performed RDN by placing an average of six lesions in a spiral pattern in each of the patient’s two renal arteries.
The investigators screened for arrhythmia recurrence with 7-day Holter monitoring at 3, 6, 9, and 12 months, with full 12-month follow-up available for 283 patients. After 12 months, blood pressures had declined by an average of 16/11 mm Hg among the patients who underwent RDN, with essentially no change in the patients who had pulmonary vein isolation only. Dr. Steinberg attributed the high success of the renal denervation procedures to the familiarity of the participating electrophysiologist operators with catheter-tip ablations.
“We have gone from treating patients with resistant hypertension to now treating patients with less severe hypertension,” Dr. Steinberg noted, and the next study he is planning will take this approach into patients with paroxysmal AFib but without hypertension, using RDN “solely as an anti-arrhythmic intervention,” he explained.
ERADICATE-AF did not receive commercial funding. Dr. Steinberg has been a consultant to Allergan, AtriCure, Biosense Webster, Corfigo, Medtronic, and Omron. He owns stock in AliveCor and receives salary from National Cardiac and G Medical.
SAN FRANCISCO – Adding renal denervation when performing catheter ablation of paroxysmal atrial fibrillation in hypertensive patients substantially reduced their arrhythmia recurrence rate during the subsequent year in a multicenter, randomized trial with 302 patients.
The findings established renal denervation (RDN) as a “reasonable” tool to increase the success of atrial fibrillation (AFib) catheter ablation, Jonathan S. Steinberg, MD, said at the annual scientific sessions of the Heart Rhythm Society.
“The RDN procedure seems remarkably safe and seems to be reliably accomplished when an electrophysiologist does it,” said Dr. Steinberg, director of the Arrhythmia Center of the Summit Medical Group in Montclair, N.J. Given the evidence he reported that performing RDN simultaneously with AFib catheter ablation by pulmonary vein isolation significantly improved freedom from arrhythmia recurrence, this approach “is ready for clinical use at institutions that could mount this kind of program,” he declared.
The rate of freedom from arrhythmia recurrence while off antiarrhythmic drugs during the year following treatment was 57% among 138 patients treated with pulmonary vein isolation only, and 72% in 145 who underwent both pulmonary vein isolation and renal denervation. That’s “a pretty big difference in outcome” with no increased risk and with about 20 added minutes of procedure time, Dr. Steinberg said in a video interview. He acknowledged that, currently, no catheter is approved for U.S. marketing that is specifically designed for renal denervation, but the operators in the study he reported all used conventional radiofrequency ablation catheters with an irrigated tip, a design with U.S. availability.
The ERADICATE-AF (Renal Artery Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) study randomized 302 patients with paroxysmal AFib and hypertension uncontrolled by medication at three centers in Russia, one in Germany, and one in Poland. Enrolled patients averaged about 60 years of age, about 60% were men, and their average blood pressure was roughly 150/90 mm Hg while on treatment with a median of two antihypertensive drugs, including 100% on either an ACE inhibitor or angiotensin receptor blocker. The study operators performed RDN by placing an average of six lesions in a spiral pattern in each of the patient’s two renal arteries.
The investigators screened for arrhythmia recurrence with 7-day Holter monitoring at 3, 6, 9, and 12 months, with full 12-month follow-up available for 283 patients. After 12 months, blood pressures had declined by an average of 16/11 mm Hg among the patients who underwent RDN, with essentially no change in the patients who had pulmonary vein isolation only. Dr. Steinberg attributed the high success of the renal denervation procedures to the familiarity of the participating electrophysiologist operators with catheter-tip ablations.
“We have gone from treating patients with resistant hypertension to now treating patients with less severe hypertension,” Dr. Steinberg noted, and the next study he is planning will take this approach into patients with paroxysmal AFib but without hypertension, using RDN “solely as an anti-arrhythmic intervention,” he explained.
ERADICATE-AF did not receive commercial funding. Dr. Steinberg has been a consultant to Allergan, AtriCure, Biosense Webster, Corfigo, Medtronic, and Omron. He owns stock in AliveCor and receives salary from National Cardiac and G Medical.
REPORTING FROM HEART RHYTHM 2019
Review hints at improved semen quality after bariatric surgery
LOS ANGELES – On the male fertility front, obesity seems to hurt semen quality. So does weight-loss surgery reverse the trend? A new review of existing research suggests that there may be an effect, but the findings aren’t conclusive.
“We found something,” said Sikarin Upala, MD, a second-year endocrinology fellow at the University of Chicago, who pointed out that three of the four reports he and his colleagues reviewed suggested improvement in semen motility. “But we still need to study more about whether bariatric surgery will affect infertility,” he continued.
Dr. Upala, who led the systematic review and meta-analysis of research into bariatric surgery and semen quality, spoke in an interview after his presentation at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
As researchers explained in a 2018 report, “conflicting results have been observed in studies evaluating the correlation between [body mass index] and sperm parameters, such as sperm concentration and total sperm count.” However, they noted that it is “generally accepted” that men with obesity seem to be at higher risk of having a low sperm count or having azoospermia, which is the total lack of sperm in semen.
It’s also not clear whether weight loss directly improves male fertility. “We do know that androgen levels improve after weight-loss surgery, and that might be one factor among several that may contribute to improved male fertility,” Edward Lin, DO, MBA, FACS, professor of surgery and chief of gastrointestinal and general surgery at Emory University, Atlanta, said in an interview.
In their review, Dr. Upala and his colleagues analyzed four studies published between 2012 and 2018 that evaluated the effect of bariatric surgery on semen quality. All of the studies examined semen volume and sperm morphology and motility, and three examined sperm concentration.
A meta-analysis found that motility and volume improved after surgery; however, some of the studies (two for volume, one for motility) failed to show a statistically significant change.
There was no statistically significant difference in sperm morphology or concentration overall, although one study showed a statistically significant improvement in both categories.
Overall, “there might be a little bit of positive effect, but we couldn’t reach a good conclusion because there were too few studies,” Dr. Upala said.
Dr. Lin, director of the Emory Bariatrics Center, agreed that the review findings are limited. He said that although the findings hint at a positive effect on semen quality, “the jury is still out” when it comes to a link between bariatric surgery and male infertility.
“Multiple factors contribute to semen quality,” he added, pointing to vitamin deficiencies, micronutrient levels in the body, enzyme signaling pathways, and sperm chromatin integrity. “In fact, surgically or diet-induced weight loss may be associated with permissive malnutrition, which further exacerbates these deficiencies. Deficiencies in these areas can sometimes take months, if not years, to correct by taking vitamin D or copper or zinc, for example.”
Dr. Lin referred to a small study in which reporters observed semen abnormalities and subfertility after weight-loss surgery despite improvements in androgenic and quality of life levels.
Dr. Upala reported having no relevant disclosures.
LOS ANGELES – On the male fertility front, obesity seems to hurt semen quality. So does weight-loss surgery reverse the trend? A new review of existing research suggests that there may be an effect, but the findings aren’t conclusive.
“We found something,” said Sikarin Upala, MD, a second-year endocrinology fellow at the University of Chicago, who pointed out that three of the four reports he and his colleagues reviewed suggested improvement in semen motility. “But we still need to study more about whether bariatric surgery will affect infertility,” he continued.
Dr. Upala, who led the systematic review and meta-analysis of research into bariatric surgery and semen quality, spoke in an interview after his presentation at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
As researchers explained in a 2018 report, “conflicting results have been observed in studies evaluating the correlation between [body mass index] and sperm parameters, such as sperm concentration and total sperm count.” However, they noted that it is “generally accepted” that men with obesity seem to be at higher risk of having a low sperm count or having azoospermia, which is the total lack of sperm in semen.
It’s also not clear whether weight loss directly improves male fertility. “We do know that androgen levels improve after weight-loss surgery, and that might be one factor among several that may contribute to improved male fertility,” Edward Lin, DO, MBA, FACS, professor of surgery and chief of gastrointestinal and general surgery at Emory University, Atlanta, said in an interview.
In their review, Dr. Upala and his colleagues analyzed four studies published between 2012 and 2018 that evaluated the effect of bariatric surgery on semen quality. All of the studies examined semen volume and sperm morphology and motility, and three examined sperm concentration.
A meta-analysis found that motility and volume improved after surgery; however, some of the studies (two for volume, one for motility) failed to show a statistically significant change.
There was no statistically significant difference in sperm morphology or concentration overall, although one study showed a statistically significant improvement in both categories.
Overall, “there might be a little bit of positive effect, but we couldn’t reach a good conclusion because there were too few studies,” Dr. Upala said.
Dr. Lin, director of the Emory Bariatrics Center, agreed that the review findings are limited. He said that although the findings hint at a positive effect on semen quality, “the jury is still out” when it comes to a link between bariatric surgery and male infertility.
“Multiple factors contribute to semen quality,” he added, pointing to vitamin deficiencies, micronutrient levels in the body, enzyme signaling pathways, and sperm chromatin integrity. “In fact, surgically or diet-induced weight loss may be associated with permissive malnutrition, which further exacerbates these deficiencies. Deficiencies in these areas can sometimes take months, if not years, to correct by taking vitamin D or copper or zinc, for example.”
Dr. Lin referred to a small study in which reporters observed semen abnormalities and subfertility after weight-loss surgery despite improvements in androgenic and quality of life levels.
Dr. Upala reported having no relevant disclosures.
LOS ANGELES – On the male fertility front, obesity seems to hurt semen quality. So does weight-loss surgery reverse the trend? A new review of existing research suggests that there may be an effect, but the findings aren’t conclusive.
“We found something,” said Sikarin Upala, MD, a second-year endocrinology fellow at the University of Chicago, who pointed out that three of the four reports he and his colleagues reviewed suggested improvement in semen motility. “But we still need to study more about whether bariatric surgery will affect infertility,” he continued.
Dr. Upala, who led the systematic review and meta-analysis of research into bariatric surgery and semen quality, spoke in an interview after his presentation at the annual scientific and clinical congress of the American Association of Clinical Endocrinologists.
As researchers explained in a 2018 report, “conflicting results have been observed in studies evaluating the correlation between [body mass index] and sperm parameters, such as sperm concentration and total sperm count.” However, they noted that it is “generally accepted” that men with obesity seem to be at higher risk of having a low sperm count or having azoospermia, which is the total lack of sperm in semen.
It’s also not clear whether weight loss directly improves male fertility. “We do know that androgen levels improve after weight-loss surgery, and that might be one factor among several that may contribute to improved male fertility,” Edward Lin, DO, MBA, FACS, professor of surgery and chief of gastrointestinal and general surgery at Emory University, Atlanta, said in an interview.
In their review, Dr. Upala and his colleagues analyzed four studies published between 2012 and 2018 that evaluated the effect of bariatric surgery on semen quality. All of the studies examined semen volume and sperm morphology and motility, and three examined sperm concentration.
A meta-analysis found that motility and volume improved after surgery; however, some of the studies (two for volume, one for motility) failed to show a statistically significant change.
There was no statistically significant difference in sperm morphology or concentration overall, although one study showed a statistically significant improvement in both categories.
Overall, “there might be a little bit of positive effect, but we couldn’t reach a good conclusion because there were too few studies,” Dr. Upala said.
Dr. Lin, director of the Emory Bariatrics Center, agreed that the review findings are limited. He said that although the findings hint at a positive effect on semen quality, “the jury is still out” when it comes to a link between bariatric surgery and male infertility.
“Multiple factors contribute to semen quality,” he added, pointing to vitamin deficiencies, micronutrient levels in the body, enzyme signaling pathways, and sperm chromatin integrity. “In fact, surgically or diet-induced weight loss may be associated with permissive malnutrition, which further exacerbates these deficiencies. Deficiencies in these areas can sometimes take months, if not years, to correct by taking vitamin D or copper or zinc, for example.”
Dr. Lin referred to a small study in which reporters observed semen abnormalities and subfertility after weight-loss surgery despite improvements in androgenic and quality of life levels.
Dr. Upala reported having no relevant disclosures.
REPORTING FROM AACE 2019
Intradermal etanercept improves discoid lupus
BIRMINGHAM, ENGLAND – Intradermal delivery of a tumor necrosis factor inhibitor (TNFi) could offer patients with discoid lupus erythematosus (DLE) a much-needed additional treatment option, according to results of a phase 2, “proof-of-concept” study.
Overall, 14 (56%) of the 25 patients in the study achieved a 20% or greater reduction in disease activity from baseline to week 12 via intradermal injection of etanercept (Enbrel), which was assessed via the modified limited Score of Activity and Damage in DLE (ML-SADDLE). About half (48%) and one-fifth (20%) also achieved greater reductions of 50% and 70%, respectively.
“Discoid lupus is a chronic form of cutaneous lupus. Usually it occurs in visible areas like the face and scalp, causing scarring, so it’s really disabling and affects patients’ quality of life,” observed the lead study investigator Md Yuzaiful Md Yusof, MBChB, PhD, NIHR Academic Clinical Lecturer at the University of Leeds, England.
“It’s also one of the most resistant manifestations of lupus,” he said during a poster presentation at the annual conference of the British Society for Rheumatology. “Usually, when people have discoid lupus, the dermatologist gives antimalarial treatment, but only 50% of people respond to these drugs. So, what happens to the rest of them?” Basically, it is trial and error, Dr. Md Yusof said; some patients may be given disease-modifying antirheumatic drugs and in some patients this may work well, but in others there may be toxicity that contraindicates treatment.
B-cell therapy with rituximab (Rituxan) has not been successful, he said. In a previous study of 35 patients with refractory discoid lupus, none of the patients responded to rituximab and half of them actually flared after taking the drug.
There is a pathologic case for using anti-TNF therapy in DLE, but the use of TNFis is not without concern. Such treatment can increase antinuclear antibody production and make lupus worse. “In order to overcome this, as the lesion is quite small, we don’t need to use a systemic approach,” Dr. Md Yusof explained in an interview. “If you give directly, it should just be confined to the lesion and not absorbed, that’s the whole idea of thinking outside the box.” He noted that if it worked, such treatment would be for inducing remission and not for maintenance.
The study, “Targeted therapy using intradermal injection of etanercept for remission induction in discoid lupus erythematosus” (TARGET-DLE) was designed to test the validity of using intradermal rather than subcutaneous TNFi therapy in patients with discoid lupus.
Dr. Md Yusof noted that only 25 patients needed to be recruited into the single-arm, prospective trial as a “Simon’s two-stage minimized design” was used (Control Clin Trials. 1989;10[1]:1-10). This involved treating the first few patients to see if a response occurred and if it did, carrying on with treating the others, but if no response occurred in at least two patients, the trial would stop completely.
Adult patients were eligible for inclusion if they had one or more active DLE lesions and had not responded to antimalarial treatment. Stable doses of DMARDs and up to 10 mg of oral prednisolone daily was permitted if already being taken prior to entering the study.
Etanercept was injected intradermally around the most symptomatic lesion once a week for up to 12 weeks. The dosage was determined based on the radius of the selected discoid lesion. Over an 18-month period, all 25 patients were recruited, including 18 women. The median age of patients was 47 years, and six had systemic lupus erythematosus. The median number of prior DMARDs was 5 but ranged from 1 to 16, indicating a very resistant patient population.
The primary endpoint was at least 6 of the 25 patients having at least a 20% reduction in ML-SADDLE at week 12; 14 (56%) patients achieved this.
“We didn’t use CLASI [Cutaneous Lupus Area and Severity Index Activity Score] because that only includes erythema and atrophy,” Dr. Md Yusof explained. “In discoid lupus, induration is quite important as well, so that’s why we used ML-SADDLE. We called it ‘modified limited’ because the original SADDLE score is based on the whole organ score, but we only calculated the one lesion that we wanted to treat.”
In addition to meeting the primary endpoint, several secondary endpoints were met, including significant improvements in scores on visual analog scales as determined by pre- and posttreatment scoring by physicians (53.1 mm vs. 23.2 mm; P less than .001) and patients (56.9 mm vs. 29.7 mm; P = .001). Mean Dermatology Life Quality Index (DLQI) score significantly improved between pre- and post treatment, as did blood perfusion under the skin based on laser Doppler imaging and infrared thermography. However, no difference was seen with optical coherence tomography.
“There were only four grade 3/4 toxicities, and importantly, none of the SLE patients got worse, and none with DLE only converted into SLE,” Dr. Md Yusof reported. Of the four grade 3/4 adverse events, two were chest infections, one was heart failure, and one was a worsening of chilblains.
“It was a full-powered phase 2 trial, and because it was positive, now we can go to phase 3 trial,” he added.
Before conducting a phase 3 trial, however, Dr. Md Yusof wants to refine how the TNFi is delivered. Perhaps an intradermal patch with microneedles could be used. This would be left on the skin for a short amount of time to allow drug delivery and then removed. It could help ensure that all patients comply with treatment and perhaps even self-administer, he noted.
“The median compliance rate was 80%, which is not too bad, but I think when we come to run a phase 3 trial, I’m looking to improve the drug delivery,” he said. Changing the delivery method will need to be validated before a phase 3 trial can be started.
The study was not commercially funded. Dr. Md Yusof had no disclosures. Pfizer provided the study drug free of charge.
SOURCE: Md Yusof MY et al. Rheumatology. 2019;58(suppl 3): Abstract 244. doi: 10.1093/rheumatology/kez107.060.
BIRMINGHAM, ENGLAND – Intradermal delivery of a tumor necrosis factor inhibitor (TNFi) could offer patients with discoid lupus erythematosus (DLE) a much-needed additional treatment option, according to results of a phase 2, “proof-of-concept” study.
Overall, 14 (56%) of the 25 patients in the study achieved a 20% or greater reduction in disease activity from baseline to week 12 via intradermal injection of etanercept (Enbrel), which was assessed via the modified limited Score of Activity and Damage in DLE (ML-SADDLE). About half (48%) and one-fifth (20%) also achieved greater reductions of 50% and 70%, respectively.
“Discoid lupus is a chronic form of cutaneous lupus. Usually it occurs in visible areas like the face and scalp, causing scarring, so it’s really disabling and affects patients’ quality of life,” observed the lead study investigator Md Yuzaiful Md Yusof, MBChB, PhD, NIHR Academic Clinical Lecturer at the University of Leeds, England.
“It’s also one of the most resistant manifestations of lupus,” he said during a poster presentation at the annual conference of the British Society for Rheumatology. “Usually, when people have discoid lupus, the dermatologist gives antimalarial treatment, but only 50% of people respond to these drugs. So, what happens to the rest of them?” Basically, it is trial and error, Dr. Md Yusof said; some patients may be given disease-modifying antirheumatic drugs and in some patients this may work well, but in others there may be toxicity that contraindicates treatment.
B-cell therapy with rituximab (Rituxan) has not been successful, he said. In a previous study of 35 patients with refractory discoid lupus, none of the patients responded to rituximab and half of them actually flared after taking the drug.
There is a pathologic case for using anti-TNF therapy in DLE, but the use of TNFis is not without concern. Such treatment can increase antinuclear antibody production and make lupus worse. “In order to overcome this, as the lesion is quite small, we don’t need to use a systemic approach,” Dr. Md Yusof explained in an interview. “If you give directly, it should just be confined to the lesion and not absorbed, that’s the whole idea of thinking outside the box.” He noted that if it worked, such treatment would be for inducing remission and not for maintenance.
The study, “Targeted therapy using intradermal injection of etanercept for remission induction in discoid lupus erythematosus” (TARGET-DLE) was designed to test the validity of using intradermal rather than subcutaneous TNFi therapy in patients with discoid lupus.
Dr. Md Yusof noted that only 25 patients needed to be recruited into the single-arm, prospective trial as a “Simon’s two-stage minimized design” was used (Control Clin Trials. 1989;10[1]:1-10). This involved treating the first few patients to see if a response occurred and if it did, carrying on with treating the others, but if no response occurred in at least two patients, the trial would stop completely.
Adult patients were eligible for inclusion if they had one or more active DLE lesions and had not responded to antimalarial treatment. Stable doses of DMARDs and up to 10 mg of oral prednisolone daily was permitted if already being taken prior to entering the study.
Etanercept was injected intradermally around the most symptomatic lesion once a week for up to 12 weeks. The dosage was determined based on the radius of the selected discoid lesion. Over an 18-month period, all 25 patients were recruited, including 18 women. The median age of patients was 47 years, and six had systemic lupus erythematosus. The median number of prior DMARDs was 5 but ranged from 1 to 16, indicating a very resistant patient population.
The primary endpoint was at least 6 of the 25 patients having at least a 20% reduction in ML-SADDLE at week 12; 14 (56%) patients achieved this.
“We didn’t use CLASI [Cutaneous Lupus Area and Severity Index Activity Score] because that only includes erythema and atrophy,” Dr. Md Yusof explained. “In discoid lupus, induration is quite important as well, so that’s why we used ML-SADDLE. We called it ‘modified limited’ because the original SADDLE score is based on the whole organ score, but we only calculated the one lesion that we wanted to treat.”
In addition to meeting the primary endpoint, several secondary endpoints were met, including significant improvements in scores on visual analog scales as determined by pre- and posttreatment scoring by physicians (53.1 mm vs. 23.2 mm; P less than .001) and patients (56.9 mm vs. 29.7 mm; P = .001). Mean Dermatology Life Quality Index (DLQI) score significantly improved between pre- and post treatment, as did blood perfusion under the skin based on laser Doppler imaging and infrared thermography. However, no difference was seen with optical coherence tomography.
“There were only four grade 3/4 toxicities, and importantly, none of the SLE patients got worse, and none with DLE only converted into SLE,” Dr. Md Yusof reported. Of the four grade 3/4 adverse events, two were chest infections, one was heart failure, and one was a worsening of chilblains.
“It was a full-powered phase 2 trial, and because it was positive, now we can go to phase 3 trial,” he added.
Before conducting a phase 3 trial, however, Dr. Md Yusof wants to refine how the TNFi is delivered. Perhaps an intradermal patch with microneedles could be used. This would be left on the skin for a short amount of time to allow drug delivery and then removed. It could help ensure that all patients comply with treatment and perhaps even self-administer, he noted.
“The median compliance rate was 80%, which is not too bad, but I think when we come to run a phase 3 trial, I’m looking to improve the drug delivery,” he said. Changing the delivery method will need to be validated before a phase 3 trial can be started.
The study was not commercially funded. Dr. Md Yusof had no disclosures. Pfizer provided the study drug free of charge.
SOURCE: Md Yusof MY et al. Rheumatology. 2019;58(suppl 3): Abstract 244. doi: 10.1093/rheumatology/kez107.060.
BIRMINGHAM, ENGLAND – Intradermal delivery of a tumor necrosis factor inhibitor (TNFi) could offer patients with discoid lupus erythematosus (DLE) a much-needed additional treatment option, according to results of a phase 2, “proof-of-concept” study.
Overall, 14 (56%) of the 25 patients in the study achieved a 20% or greater reduction in disease activity from baseline to week 12 via intradermal injection of etanercept (Enbrel), which was assessed via the modified limited Score of Activity and Damage in DLE (ML-SADDLE). About half (48%) and one-fifth (20%) also achieved greater reductions of 50% and 70%, respectively.
“Discoid lupus is a chronic form of cutaneous lupus. Usually it occurs in visible areas like the face and scalp, causing scarring, so it’s really disabling and affects patients’ quality of life,” observed the lead study investigator Md Yuzaiful Md Yusof, MBChB, PhD, NIHR Academic Clinical Lecturer at the University of Leeds, England.
“It’s also one of the most resistant manifestations of lupus,” he said during a poster presentation at the annual conference of the British Society for Rheumatology. “Usually, when people have discoid lupus, the dermatologist gives antimalarial treatment, but only 50% of people respond to these drugs. So, what happens to the rest of them?” Basically, it is trial and error, Dr. Md Yusof said; some patients may be given disease-modifying antirheumatic drugs and in some patients this may work well, but in others there may be toxicity that contraindicates treatment.
B-cell therapy with rituximab (Rituxan) has not been successful, he said. In a previous study of 35 patients with refractory discoid lupus, none of the patients responded to rituximab and half of them actually flared after taking the drug.
There is a pathologic case for using anti-TNF therapy in DLE, but the use of TNFis is not without concern. Such treatment can increase antinuclear antibody production and make lupus worse. “In order to overcome this, as the lesion is quite small, we don’t need to use a systemic approach,” Dr. Md Yusof explained in an interview. “If you give directly, it should just be confined to the lesion and not absorbed, that’s the whole idea of thinking outside the box.” He noted that if it worked, such treatment would be for inducing remission and not for maintenance.
The study, “Targeted therapy using intradermal injection of etanercept for remission induction in discoid lupus erythematosus” (TARGET-DLE) was designed to test the validity of using intradermal rather than subcutaneous TNFi therapy in patients with discoid lupus.
Dr. Md Yusof noted that only 25 patients needed to be recruited into the single-arm, prospective trial as a “Simon’s two-stage minimized design” was used (Control Clin Trials. 1989;10[1]:1-10). This involved treating the first few patients to see if a response occurred and if it did, carrying on with treating the others, but if no response occurred in at least two patients, the trial would stop completely.
Adult patients were eligible for inclusion if they had one or more active DLE lesions and had not responded to antimalarial treatment. Stable doses of DMARDs and up to 10 mg of oral prednisolone daily was permitted if already being taken prior to entering the study.
Etanercept was injected intradermally around the most symptomatic lesion once a week for up to 12 weeks. The dosage was determined based on the radius of the selected discoid lesion. Over an 18-month period, all 25 patients were recruited, including 18 women. The median age of patients was 47 years, and six had systemic lupus erythematosus. The median number of prior DMARDs was 5 but ranged from 1 to 16, indicating a very resistant patient population.
The primary endpoint was at least 6 of the 25 patients having at least a 20% reduction in ML-SADDLE at week 12; 14 (56%) patients achieved this.
“We didn’t use CLASI [Cutaneous Lupus Area and Severity Index Activity Score] because that only includes erythema and atrophy,” Dr. Md Yusof explained. “In discoid lupus, induration is quite important as well, so that’s why we used ML-SADDLE. We called it ‘modified limited’ because the original SADDLE score is based on the whole organ score, but we only calculated the one lesion that we wanted to treat.”
In addition to meeting the primary endpoint, several secondary endpoints were met, including significant improvements in scores on visual analog scales as determined by pre- and posttreatment scoring by physicians (53.1 mm vs. 23.2 mm; P less than .001) and patients (56.9 mm vs. 29.7 mm; P = .001). Mean Dermatology Life Quality Index (DLQI) score significantly improved between pre- and post treatment, as did blood perfusion under the skin based on laser Doppler imaging and infrared thermography. However, no difference was seen with optical coherence tomography.
“There were only four grade 3/4 toxicities, and importantly, none of the SLE patients got worse, and none with DLE only converted into SLE,” Dr. Md Yusof reported. Of the four grade 3/4 adverse events, two were chest infections, one was heart failure, and one was a worsening of chilblains.
“It was a full-powered phase 2 trial, and because it was positive, now we can go to phase 3 trial,” he added.
Before conducting a phase 3 trial, however, Dr. Md Yusof wants to refine how the TNFi is delivered. Perhaps an intradermal patch with microneedles could be used. This would be left on the skin for a short amount of time to allow drug delivery and then removed. It could help ensure that all patients comply with treatment and perhaps even self-administer, he noted.
“The median compliance rate was 80%, which is not too bad, but I think when we come to run a phase 3 trial, I’m looking to improve the drug delivery,” he said. Changing the delivery method will need to be validated before a phase 3 trial can be started.
The study was not commercially funded. Dr. Md Yusof had no disclosures. Pfizer provided the study drug free of charge.
SOURCE: Md Yusof MY et al. Rheumatology. 2019;58(suppl 3): Abstract 244. doi: 10.1093/rheumatology/kez107.060.
REPORTING FROM BSR 2019
Experts agree on optimal use of MRI in axSpA
BIRMINGHAM, ENGLAND – An evidence-based approach coupled with expert consensus has been used to determine the best way to use MRI for the diagnosis of axial spondyloarthritis (axSpA).
Working under the auspices of the British Society for Spondyloarthritis (BRITSpA), a task force of nine rheumatologists and nine musculoskeletal radiologists with an interest in axSpA developed a set of seven recommendations that provide guidance on how to best to acquire and then interpret MRI images of the spine and sacroiliac joints.
The recommendations, which were published online (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez173), cover how to perform MRI when axSpA is suspected, such as by imaging both the sacroiliac joints and the spine, and provide guidance on the sequences and order of MRI planes to be used, and what features may increase the diagnostic confidence of axSpA.
The recommendations are as follows:
• When requesting an MRI for suspected axSpA, imaging of both the sacroiliac joints and the spine is recommended.
• T1-weighted and fat-suppressed, fluid-sensitive sequences are recommended for suspected axSpA.
• The minimum protocol when requesting an MRI for suspected axSpA should include sagittal images of the spine with extended lateral coverage and images of the sacroiliac joints that are in an oblique coronal plane to the joint.
• In the sacroiliac joints, the presence of bone marrow edema, fatty infiltration, or erosion is suggestive of the diagnosis of axSpA. The presence of more than one of these features increases the diagnostic confidence of axSpA.
• In the spine, the presence of multiple corner inflammatory lesions and/or multiple corner fatty lesions increases the diagnostic confidence of axSpA.
• In the sacroiliac joints and/or spine, the presence of characteristic new bone formation increases the diagnostic confidence of axSpA.
• The full range and combination of active and structural lesions of the sacroiliac joints and spine should be taken into account when deciding if the MRI scan is suggestive of axSpA or not.
The recommendations “are intended to standardize practice around the use of MRI,” said Alexis Jones, MBBS, MS (Rheumatology), a senior clinical research fellow at University College London Hospitals NHS Foundation Trust. She presented the recommendations on behalf of the expert task force at the annual conference of the British Society for Rheumatology.
“MRI has become an essential tool in axial spondyloarthritis. It facilitates earlier diagnoses and therefore has allowed for earlier initiation of treatment. It can be used to monitor the burden of inflammation and may predict response to therapy,” Dr. Jones said. Despite this, “there is significant inconsistency in the use of MRI” in clinical practice.
For instance, a survey performed in the United Kingdom (J Rheumatol. 2017;44[6]:780-5) highlighted the need for better collaboration between rheumatology and radiology departments to identify axSpA MRI lesions and develop appropriate protocols.
That survey showed that a quarter of radiologists were not aware of the term axSpA, and just 31% and 25%, respectively, were aware of Assessment of Spondyloarthritis international Society (ASAS) criteria for a positive MRI of the sacroiliac joints and spine. Furthermore, 18% of radiologists did not recognize bone marrow edema as a diagnostic feature of axSpA.
The heterogeneity in the performance of MRI in clinical practice could lead to a delay in diagnosis and potentially misdiagnosis, the task force’s lead author and consultant rheumatologist, Pedro Machado, MD, said in an interview.
“I think everyone has been focusing on demonstrating the value of MRI in the condition but then they forgot to look at the standardization aspect,” said Dr. Machado, who works at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
With that in mind, the BRITSpA-endorsed task force was set up and met to determine the scope of the recommendations. They looked at the evidence for the use of MRI in the diagnosis of axSpA and used two overarching principles to draft the recommendations: 1) the diagnosis of axSpA is based on clinical, laboratory, and imaging features; 2) Some patients with axSpA have isolated inflammation of the sacroiliac joints or spine.
“All of the recommendations were met with a high level of agreement, indicating a strong consensus” among rheumatologists and radiologists, Dr. Jones noted.
“These recommendations can be immediately applied to clinical practice,” said Dr. Machado, who noted that they should standardize practice and decrease heterogeneity around the use of MRI. “This will help ensure a more informed and consistent approach to the diagnosis of axSpA.”
One of the potential impacts of the recommendations, if followed, is that they may actually help to reduce health care costs, Dr. Machado suggested, because an optimized protocol would be used, making MRI more cost effective by not including sequences that do not add value in the condition.
The next task is to share the recommendations more widely and make sure they are applied in clinical practice.
A systematic literature review on which the recommendations were based was published simultaneously with the conference presentation (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez172).
The work was supported by BRITSpA. The authors had no relevant disclosures.
SOURCE: Bray TJP et al. Rheumatology 2019;58(suppl 3): Abstract 033. doi: 10.1093/rheumatology/kez105.032.
BIRMINGHAM, ENGLAND – An evidence-based approach coupled with expert consensus has been used to determine the best way to use MRI for the diagnosis of axial spondyloarthritis (axSpA).
Working under the auspices of the British Society for Spondyloarthritis (BRITSpA), a task force of nine rheumatologists and nine musculoskeletal radiologists with an interest in axSpA developed a set of seven recommendations that provide guidance on how to best to acquire and then interpret MRI images of the spine and sacroiliac joints.
The recommendations, which were published online (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez173), cover how to perform MRI when axSpA is suspected, such as by imaging both the sacroiliac joints and the spine, and provide guidance on the sequences and order of MRI planes to be used, and what features may increase the diagnostic confidence of axSpA.
The recommendations are as follows:
• When requesting an MRI for suspected axSpA, imaging of both the sacroiliac joints and the spine is recommended.
• T1-weighted and fat-suppressed, fluid-sensitive sequences are recommended for suspected axSpA.
• The minimum protocol when requesting an MRI for suspected axSpA should include sagittal images of the spine with extended lateral coverage and images of the sacroiliac joints that are in an oblique coronal plane to the joint.
• In the sacroiliac joints, the presence of bone marrow edema, fatty infiltration, or erosion is suggestive of the diagnosis of axSpA. The presence of more than one of these features increases the diagnostic confidence of axSpA.
• In the spine, the presence of multiple corner inflammatory lesions and/or multiple corner fatty lesions increases the diagnostic confidence of axSpA.
• In the sacroiliac joints and/or spine, the presence of characteristic new bone formation increases the diagnostic confidence of axSpA.
• The full range and combination of active and structural lesions of the sacroiliac joints and spine should be taken into account when deciding if the MRI scan is suggestive of axSpA or not.
The recommendations “are intended to standardize practice around the use of MRI,” said Alexis Jones, MBBS, MS (Rheumatology), a senior clinical research fellow at University College London Hospitals NHS Foundation Trust. She presented the recommendations on behalf of the expert task force at the annual conference of the British Society for Rheumatology.
“MRI has become an essential tool in axial spondyloarthritis. It facilitates earlier diagnoses and therefore has allowed for earlier initiation of treatment. It can be used to monitor the burden of inflammation and may predict response to therapy,” Dr. Jones said. Despite this, “there is significant inconsistency in the use of MRI” in clinical practice.
For instance, a survey performed in the United Kingdom (J Rheumatol. 2017;44[6]:780-5) highlighted the need for better collaboration between rheumatology and radiology departments to identify axSpA MRI lesions and develop appropriate protocols.
That survey showed that a quarter of radiologists were not aware of the term axSpA, and just 31% and 25%, respectively, were aware of Assessment of Spondyloarthritis international Society (ASAS) criteria for a positive MRI of the sacroiliac joints and spine. Furthermore, 18% of radiologists did not recognize bone marrow edema as a diagnostic feature of axSpA.
The heterogeneity in the performance of MRI in clinical practice could lead to a delay in diagnosis and potentially misdiagnosis, the task force’s lead author and consultant rheumatologist, Pedro Machado, MD, said in an interview.
“I think everyone has been focusing on demonstrating the value of MRI in the condition but then they forgot to look at the standardization aspect,” said Dr. Machado, who works at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
With that in mind, the BRITSpA-endorsed task force was set up and met to determine the scope of the recommendations. They looked at the evidence for the use of MRI in the diagnosis of axSpA and used two overarching principles to draft the recommendations: 1) the diagnosis of axSpA is based on clinical, laboratory, and imaging features; 2) Some patients with axSpA have isolated inflammation of the sacroiliac joints or spine.
“All of the recommendations were met with a high level of agreement, indicating a strong consensus” among rheumatologists and radiologists, Dr. Jones noted.
“These recommendations can be immediately applied to clinical practice,” said Dr. Machado, who noted that they should standardize practice and decrease heterogeneity around the use of MRI. “This will help ensure a more informed and consistent approach to the diagnosis of axSpA.”
One of the potential impacts of the recommendations, if followed, is that they may actually help to reduce health care costs, Dr. Machado suggested, because an optimized protocol would be used, making MRI more cost effective by not including sequences that do not add value in the condition.
The next task is to share the recommendations more widely and make sure they are applied in clinical practice.
A systematic literature review on which the recommendations were based was published simultaneously with the conference presentation (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez172).
The work was supported by BRITSpA. The authors had no relevant disclosures.
SOURCE: Bray TJP et al. Rheumatology 2019;58(suppl 3): Abstract 033. doi: 10.1093/rheumatology/kez105.032.
BIRMINGHAM, ENGLAND – An evidence-based approach coupled with expert consensus has been used to determine the best way to use MRI for the diagnosis of axial spondyloarthritis (axSpA).
Working under the auspices of the British Society for Spondyloarthritis (BRITSpA), a task force of nine rheumatologists and nine musculoskeletal radiologists with an interest in axSpA developed a set of seven recommendations that provide guidance on how to best to acquire and then interpret MRI images of the spine and sacroiliac joints.
The recommendations, which were published online (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez173), cover how to perform MRI when axSpA is suspected, such as by imaging both the sacroiliac joints and the spine, and provide guidance on the sequences and order of MRI planes to be used, and what features may increase the diagnostic confidence of axSpA.
The recommendations are as follows:
• When requesting an MRI for suspected axSpA, imaging of both the sacroiliac joints and the spine is recommended.
• T1-weighted and fat-suppressed, fluid-sensitive sequences are recommended for suspected axSpA.
• The minimum protocol when requesting an MRI for suspected axSpA should include sagittal images of the spine with extended lateral coverage and images of the sacroiliac joints that are in an oblique coronal plane to the joint.
• In the sacroiliac joints, the presence of bone marrow edema, fatty infiltration, or erosion is suggestive of the diagnosis of axSpA. The presence of more than one of these features increases the diagnostic confidence of axSpA.
• In the spine, the presence of multiple corner inflammatory lesions and/or multiple corner fatty lesions increases the diagnostic confidence of axSpA.
• In the sacroiliac joints and/or spine, the presence of characteristic new bone formation increases the diagnostic confidence of axSpA.
• The full range and combination of active and structural lesions of the sacroiliac joints and spine should be taken into account when deciding if the MRI scan is suggestive of axSpA or not.
The recommendations “are intended to standardize practice around the use of MRI,” said Alexis Jones, MBBS, MS (Rheumatology), a senior clinical research fellow at University College London Hospitals NHS Foundation Trust. She presented the recommendations on behalf of the expert task force at the annual conference of the British Society for Rheumatology.
“MRI has become an essential tool in axial spondyloarthritis. It facilitates earlier diagnoses and therefore has allowed for earlier initiation of treatment. It can be used to monitor the burden of inflammation and may predict response to therapy,” Dr. Jones said. Despite this, “there is significant inconsistency in the use of MRI” in clinical practice.
For instance, a survey performed in the United Kingdom (J Rheumatol. 2017;44[6]:780-5) highlighted the need for better collaboration between rheumatology and radiology departments to identify axSpA MRI lesions and develop appropriate protocols.
That survey showed that a quarter of radiologists were not aware of the term axSpA, and just 31% and 25%, respectively, were aware of Assessment of Spondyloarthritis international Society (ASAS) criteria for a positive MRI of the sacroiliac joints and spine. Furthermore, 18% of radiologists did not recognize bone marrow edema as a diagnostic feature of axSpA.
The heterogeneity in the performance of MRI in clinical practice could lead to a delay in diagnosis and potentially misdiagnosis, the task force’s lead author and consultant rheumatologist, Pedro Machado, MD, said in an interview.
“I think everyone has been focusing on demonstrating the value of MRI in the condition but then they forgot to look at the standardization aspect,” said Dr. Machado, who works at University College Hospital and the National Hospital for Neurology and Neurosurgery in London.
With that in mind, the BRITSpA-endorsed task force was set up and met to determine the scope of the recommendations. They looked at the evidence for the use of MRI in the diagnosis of axSpA and used two overarching principles to draft the recommendations: 1) the diagnosis of axSpA is based on clinical, laboratory, and imaging features; 2) Some patients with axSpA have isolated inflammation of the sacroiliac joints or spine.
“All of the recommendations were met with a high level of agreement, indicating a strong consensus” among rheumatologists and radiologists, Dr. Jones noted.
“These recommendations can be immediately applied to clinical practice,” said Dr. Machado, who noted that they should standardize practice and decrease heterogeneity around the use of MRI. “This will help ensure a more informed and consistent approach to the diagnosis of axSpA.”
One of the potential impacts of the recommendations, if followed, is that they may actually help to reduce health care costs, Dr. Machado suggested, because an optimized protocol would be used, making MRI more cost effective by not including sequences that do not add value in the condition.
The next task is to share the recommendations more widely and make sure they are applied in clinical practice.
A systematic literature review on which the recommendations were based was published simultaneously with the conference presentation (Rheumatology. 2019 May 2. doi: 10.1093/rheumatology/kez172).
The work was supported by BRITSpA. The authors had no relevant disclosures.
SOURCE: Bray TJP et al. Rheumatology 2019;58(suppl 3): Abstract 033. doi: 10.1093/rheumatology/kez105.032.
REPORTING FROM BSR 2019
Interview with Stephen Krieger, MD, on the topographical model of multiple sclerosis
We interviewed Dr. Stephen Krieger to discuss his research in the implementation of the topographical model of Multiple Sclerosis.
What is the concept behind the topographical model of multiple sclerosis (MS)?
DR. KRIEGER: MS is an incredibly heterogeneous, and in many ways, unpredictable disease. Some people with MS will have a relapsing course, others will take a progressive course of disease, and many will have a disease course that spans both a relapsing phase and a progressive phase.
We have traditionally divided MS into phenotypes like relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), or primary-progressive MS (PPMS), and these phenotypes have been foundational in our field and used to define clinical trial cohorts and outcomes. They also have been used for the approval of our medicines. In practice, however, it sometimes can be difficult to know precisely what kind of MS, what phenotypes of MS, that an individual patient has.
The topographical model, which was proposed four years ago, tries to unify our concepts of MS in a way that spans across those phenotypes and animates the disease course in a more dynamic way to bridge from one phenotype to another.
As an individual patient, for example, develops clinically isolated syndrome (CIS) or first attack, and then RRMS, and then later SPMS, that gets depicted in a dynamic visualization through the topographical model. The model also makes use of the idea that where an MS lesion is in the central nervous system (CNS) defines the clinical symptoms that it causes.
This is something that we have long known, and the art of localization in neurology has existed for at least a couple hundred years. But we have not used that in the way we have depicted MS clinical course in recent decades. The topographical model tries to bring this idea of mapping an individual patient’s disease topography back into the clinical picture.
In the topographical model, the lesions are shown as different topographical peaks, via the hills and valleys of areas of MS damage across different regions of the CNS [image]. They are compensated for by reserve, by the ability that the nervous system has to compensate and to keep a disease process from crossing the clinical threshold and causing symptoms. What the topographical model displays is that patients with MS lose reserve as time passes.
We know that there is brain atrophy, brain stem atrophy, spinal cord atrophy, and retinal nerve fiber layer thinning in this disease. The topographical model takes the concept that MS causes a loss of tissue across the CNS and applies it to where the lesions are in the CNS. The coming together of those two things brings about the clinical picture unmasking the deficit from those lesions over time. The short version is a depiction of disease course in MS. The way it looks has been likened to a leaking swimming pool, where there is a shallow end and a deep end, and as reserve drains over time more and more of that subclinical disease becomes unmasked.
In the Laitman article (2018), you applied the model to real patients. What were the main findings from that study?
DR. KRIEGER: Until now, the topographical model has been conceptual with a visual depiction, and I think it has been important as an educational tool and an aid to help shape our thinking about MS in a unified way.
The Laitman et al research, is the first time we have applied the concepts of the model to individual patients to confirm whether we could map individual patients’ MS histories in the topographical model and see if we could depict their clinical course this way. We found that we could.
One of the most important points that the topographical model makes is the idea that as progression occurs and reserve is lost, there is an unmasking of underlying disease. Meaning, all of the signs and symptoms that a patient has had during their relapse when they were accumulating lesions should be re-revealed or recapitulated when reserve is lost and progression occurs.
To confirm this, we mapped ten patients in the topographical model. We characterized their signs and symptoms of relapses during the relapsing phase and we found that the vast majority of these symptoms had redeclared themselves at the time that these patients developed SPMS. Furthermore, those symptoms were continuing to worsen in their pattern; that is in the pattern of their disease topography as the years have continued to pass since they developed SPMS.
This was the first empirical study in real patients to show that the principles of the topographical model held true. This recapitulation hypothesis of symptoms in progressive disease was borne out, and that can help to lay the groundwork for future empirical studies to see how this model can be used as a predictive tool.
How does this new theory of MS disease progression better inform treatment decisions than the disease course theories that currently exist?
DR. KRIEGER: We have had the clinical phenotypes for 20 years and it has been very helpful to us in the development of treatments that we have shown are effective for RRMS and in more recent years for PPMS. What we don’t really have is a way of personalizing and predicting the individual person’s disease trajectory.
Although we have prognostic factors that we know are important, such as age and MRI disease burden, there is still great uncertainty of the clinical course in the individual patient. If the topographical model can be further empirically validated using real world data, that could help us to predict what is going to happen to an individual patient. That can help us to make better treatment decisions for them because it could inform our treatment decisions in a more personalized way.
Is there any other recent research that supports these concepts?
DR. KRIEGER: We talk a lot about the need for biomarkers in MS to help us predict disease course and the topographical model makes the case that lesion location is a crucial biomarker. That is, the patient that has lesions in the spinal cord and the brain stem is more likely to have progressive signs and symptoms referable to those lesions.
A separate piece of work recently done by Keegan and colleagues that was published in Multiple Sclerosis Journal, looked at their own cohort of patients that had at least one critically located lesion, typically in the high cervical spinal cord or the lower brain stem, as being the crucial driver of the development of motor dysfunction and progressive disability.
In an editorial I wrote with my colleague, Fred Lublin, called “Location, location, location,” we point out that this is in some ways the best data in support of the concept of the topographical model that I have seen. It outlines a framework or a methodology where the importance of lesion location in defining the clinical picture and the risk of progression for an individual patient can be studied.
We interviewed Dr. Stephen Krieger to discuss his research in the implementation of the topographical model of Multiple Sclerosis.
What is the concept behind the topographical model of multiple sclerosis (MS)?
DR. KRIEGER: MS is an incredibly heterogeneous, and in many ways, unpredictable disease. Some people with MS will have a relapsing course, others will take a progressive course of disease, and many will have a disease course that spans both a relapsing phase and a progressive phase.
We have traditionally divided MS into phenotypes like relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), or primary-progressive MS (PPMS), and these phenotypes have been foundational in our field and used to define clinical trial cohorts and outcomes. They also have been used for the approval of our medicines. In practice, however, it sometimes can be difficult to know precisely what kind of MS, what phenotypes of MS, that an individual patient has.
The topographical model, which was proposed four years ago, tries to unify our concepts of MS in a way that spans across those phenotypes and animates the disease course in a more dynamic way to bridge from one phenotype to another.
As an individual patient, for example, develops clinically isolated syndrome (CIS) or first attack, and then RRMS, and then later SPMS, that gets depicted in a dynamic visualization through the topographical model. The model also makes use of the idea that where an MS lesion is in the central nervous system (CNS) defines the clinical symptoms that it causes.
This is something that we have long known, and the art of localization in neurology has existed for at least a couple hundred years. But we have not used that in the way we have depicted MS clinical course in recent decades. The topographical model tries to bring this idea of mapping an individual patient’s disease topography back into the clinical picture.
In the topographical model, the lesions are shown as different topographical peaks, via the hills and valleys of areas of MS damage across different regions of the CNS [image]. They are compensated for by reserve, by the ability that the nervous system has to compensate and to keep a disease process from crossing the clinical threshold and causing symptoms. What the topographical model displays is that patients with MS lose reserve as time passes.
We know that there is brain atrophy, brain stem atrophy, spinal cord atrophy, and retinal nerve fiber layer thinning in this disease. The topographical model takes the concept that MS causes a loss of tissue across the CNS and applies it to where the lesions are in the CNS. The coming together of those two things brings about the clinical picture unmasking the deficit from those lesions over time. The short version is a depiction of disease course in MS. The way it looks has been likened to a leaking swimming pool, where there is a shallow end and a deep end, and as reserve drains over time more and more of that subclinical disease becomes unmasked.
In the Laitman article (2018), you applied the model to real patients. What were the main findings from that study?
DR. KRIEGER: Until now, the topographical model has been conceptual with a visual depiction, and I think it has been important as an educational tool and an aid to help shape our thinking about MS in a unified way.
The Laitman et al research, is the first time we have applied the concepts of the model to individual patients to confirm whether we could map individual patients’ MS histories in the topographical model and see if we could depict their clinical course this way. We found that we could.
One of the most important points that the topographical model makes is the idea that as progression occurs and reserve is lost, there is an unmasking of underlying disease. Meaning, all of the signs and symptoms that a patient has had during their relapse when they were accumulating lesions should be re-revealed or recapitulated when reserve is lost and progression occurs.
To confirm this, we mapped ten patients in the topographical model. We characterized their signs and symptoms of relapses during the relapsing phase and we found that the vast majority of these symptoms had redeclared themselves at the time that these patients developed SPMS. Furthermore, those symptoms were continuing to worsen in their pattern; that is in the pattern of their disease topography as the years have continued to pass since they developed SPMS.
This was the first empirical study in real patients to show that the principles of the topographical model held true. This recapitulation hypothesis of symptoms in progressive disease was borne out, and that can help to lay the groundwork for future empirical studies to see how this model can be used as a predictive tool.
How does this new theory of MS disease progression better inform treatment decisions than the disease course theories that currently exist?
DR. KRIEGER: We have had the clinical phenotypes for 20 years and it has been very helpful to us in the development of treatments that we have shown are effective for RRMS and in more recent years for PPMS. What we don’t really have is a way of personalizing and predicting the individual person’s disease trajectory.
Although we have prognostic factors that we know are important, such as age and MRI disease burden, there is still great uncertainty of the clinical course in the individual patient. If the topographical model can be further empirically validated using real world data, that could help us to predict what is going to happen to an individual patient. That can help us to make better treatment decisions for them because it could inform our treatment decisions in a more personalized way.
Is there any other recent research that supports these concepts?
DR. KRIEGER: We talk a lot about the need for biomarkers in MS to help us predict disease course and the topographical model makes the case that lesion location is a crucial biomarker. That is, the patient that has lesions in the spinal cord and the brain stem is more likely to have progressive signs and symptoms referable to those lesions.
A separate piece of work recently done by Keegan and colleagues that was published in Multiple Sclerosis Journal, looked at their own cohort of patients that had at least one critically located lesion, typically in the high cervical spinal cord or the lower brain stem, as being the crucial driver of the development of motor dysfunction and progressive disability.
In an editorial I wrote with my colleague, Fred Lublin, called “Location, location, location,” we point out that this is in some ways the best data in support of the concept of the topographical model that I have seen. It outlines a framework or a methodology where the importance of lesion location in defining the clinical picture and the risk of progression for an individual patient can be studied.
We interviewed Dr. Stephen Krieger to discuss his research in the implementation of the topographical model of Multiple Sclerosis.
What is the concept behind the topographical model of multiple sclerosis (MS)?
DR. KRIEGER: MS is an incredibly heterogeneous, and in many ways, unpredictable disease. Some people with MS will have a relapsing course, others will take a progressive course of disease, and many will have a disease course that spans both a relapsing phase and a progressive phase.
We have traditionally divided MS into phenotypes like relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), or primary-progressive MS (PPMS), and these phenotypes have been foundational in our field and used to define clinical trial cohorts and outcomes. They also have been used for the approval of our medicines. In practice, however, it sometimes can be difficult to know precisely what kind of MS, what phenotypes of MS, that an individual patient has.
The topographical model, which was proposed four years ago, tries to unify our concepts of MS in a way that spans across those phenotypes and animates the disease course in a more dynamic way to bridge from one phenotype to another.
As an individual patient, for example, develops clinically isolated syndrome (CIS) or first attack, and then RRMS, and then later SPMS, that gets depicted in a dynamic visualization through the topographical model. The model also makes use of the idea that where an MS lesion is in the central nervous system (CNS) defines the clinical symptoms that it causes.
This is something that we have long known, and the art of localization in neurology has existed for at least a couple hundred years. But we have not used that in the way we have depicted MS clinical course in recent decades. The topographical model tries to bring this idea of mapping an individual patient’s disease topography back into the clinical picture.
In the topographical model, the lesions are shown as different topographical peaks, via the hills and valleys of areas of MS damage across different regions of the CNS [image]. They are compensated for by reserve, by the ability that the nervous system has to compensate and to keep a disease process from crossing the clinical threshold and causing symptoms. What the topographical model displays is that patients with MS lose reserve as time passes.
We know that there is brain atrophy, brain stem atrophy, spinal cord atrophy, and retinal nerve fiber layer thinning in this disease. The topographical model takes the concept that MS causes a loss of tissue across the CNS and applies it to where the lesions are in the CNS. The coming together of those two things brings about the clinical picture unmasking the deficit from those lesions over time. The short version is a depiction of disease course in MS. The way it looks has been likened to a leaking swimming pool, where there is a shallow end and a deep end, and as reserve drains over time more and more of that subclinical disease becomes unmasked.
In the Laitman article (2018), you applied the model to real patients. What were the main findings from that study?
DR. KRIEGER: Until now, the topographical model has been conceptual with a visual depiction, and I think it has been important as an educational tool and an aid to help shape our thinking about MS in a unified way.
The Laitman et al research, is the first time we have applied the concepts of the model to individual patients to confirm whether we could map individual patients’ MS histories in the topographical model and see if we could depict their clinical course this way. We found that we could.
One of the most important points that the topographical model makes is the idea that as progression occurs and reserve is lost, there is an unmasking of underlying disease. Meaning, all of the signs and symptoms that a patient has had during their relapse when they were accumulating lesions should be re-revealed or recapitulated when reserve is lost and progression occurs.
To confirm this, we mapped ten patients in the topographical model. We characterized their signs and symptoms of relapses during the relapsing phase and we found that the vast majority of these symptoms had redeclared themselves at the time that these patients developed SPMS. Furthermore, those symptoms were continuing to worsen in their pattern; that is in the pattern of their disease topography as the years have continued to pass since they developed SPMS.
This was the first empirical study in real patients to show that the principles of the topographical model held true. This recapitulation hypothesis of symptoms in progressive disease was borne out, and that can help to lay the groundwork for future empirical studies to see how this model can be used as a predictive tool.
How does this new theory of MS disease progression better inform treatment decisions than the disease course theories that currently exist?
DR. KRIEGER: We have had the clinical phenotypes for 20 years and it has been very helpful to us in the development of treatments that we have shown are effective for RRMS and in more recent years for PPMS. What we don’t really have is a way of personalizing and predicting the individual person’s disease trajectory.
Although we have prognostic factors that we know are important, such as age and MRI disease burden, there is still great uncertainty of the clinical course in the individual patient. If the topographical model can be further empirically validated using real world data, that could help us to predict what is going to happen to an individual patient. That can help us to make better treatment decisions for them because it could inform our treatment decisions in a more personalized way.
Is there any other recent research that supports these concepts?
DR. KRIEGER: We talk a lot about the need for biomarkers in MS to help us predict disease course and the topographical model makes the case that lesion location is a crucial biomarker. That is, the patient that has lesions in the spinal cord and the brain stem is more likely to have progressive signs and symptoms referable to those lesions.
A separate piece of work recently done by Keegan and colleagues that was published in Multiple Sclerosis Journal, looked at their own cohort of patients that had at least one critically located lesion, typically in the high cervical spinal cord or the lower brain stem, as being the crucial driver of the development of motor dysfunction and progressive disability.
In an editorial I wrote with my colleague, Fred Lublin, called “Location, location, location,” we point out that this is in some ways the best data in support of the concept of the topographical model that I have seen. It outlines a framework or a methodology where the importance of lesion location in defining the clinical picture and the risk of progression for an individual patient can be studied.
Most postapproval trials explore novel indications
The majority of postapproval clinical trials conducted by pharmaceutical companies explored novel or expanded uses as opposed to monitoring original indications, according to results from a cross-sectional study.
“The U.S. Food and Drug Administration can use postmarketing requirements to mandate pharmaceutical companies to conduct clinical trials after the approval of novel therapeutics,” wrote Joshua J. Skydel of Tufts University, Boston, and colleagues in JAMA Network Open.
“From 2009 through 2012, the FDA approved 110 novel therapeutics for 120 indications,” the researchers reported.
Of those approved, 37 novel agents were identified that lacked requirements for postmarket monitoring. Data was obtained from the Drugs@FDA database and consisted of industry-sponsored postapproval clinical studies conducted in the United States.
Postapproval study information included 600 trials registered on ClinicalTrials.gov that were primarily aimed at producing additional efficacy and safety data in approved or unapproved uses. The majority of these trials were for novel therapeutics being evaluated in oncology- and hematology-related indications.
After analysis, the researchers found that most of these trials examined agents for novel or expanded indications (60.5%) or widened populations of the initially indicated condition (20.3%).
In addition, several of these studies were nonrandomized (59.8%), had small sample sizes (median, 44 participants), and did not include comparators (63.5%).
“Among 600 trials, only 12.0% exclusively evaluated the originally approved indication,” the authors wrote.
The researchers acknowledged that a key limitation of the study was the inclusion of only postapproval clinical studies affiliated with pharmaceutical companies.
“The FDA may need to impose additional postmarketing requirements to generate further evidence for the original indications,” they concluded.
The study was funded by the Tufts University School of Medicine and the Laura and John Arnold Foundation. The authors reported financial affiliations with Johnson & Johnson, Medtronic, the Blue Cross Blue Shield Association, the National Institutes of Health, the Laura and John Arnold Foundation, and the Food and Drug Administration.
SOURCE: Skydel JJ et al. JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410.
One question that remains from the study by Skydel et al. is why the Food and Drug Administration did not mandate postapproval clinical studies for more than one-third of the novel agents approved from 2009 to 2012.
Among 600 postapproval trials affiliated with pharmaceutical companies, the vast majority were for novel therapeutics being studied in oncology- and hematology-related indications. Many of these premarket trials are one-arm, nonrandomized studies approved with evidence from surrogate outcomes. Recent studies have revealed that many novel agents do not show an overall survival benefit 5 years post approval.
From a clinical perspective, interpreting safety and efficacy data from these trials is challenging because of various limitations, including small sample sizes, no control groups, and lack of randomization. High-quality evidence helping clinicians determine which patients will benefit from these novel therapeutics is needed.
In addition, the purpose of these sponsored studies has come into question. While they can be used to generate further clinical data, many other reasons are possible. The expectation could be that once these studies are complete, clinicians may prescribe the agent being examined, thereby increasing off-label prescribing. Recent studies have revealed that off-label prescribing has the potential to increase the risk of drug-related adverse events.
“Postapproval studies need to advance clinical care by answering unresolved questions,” Dr. Lexchin wrote. “Clinicians and patients deserve better.”
Joel Lexchin, MD, is associated with the School of Health Policy and Management at York University in Toronto. He reported financial affiliations with the Gordon and Betty Moore Foundation, the Government of Canada, the Ontario Supporting Patient Oriented Research Support Unit, the St Michael’s Hospital Foundation, the Canadian Institutes of Health Research, and the American Diabetes Association. These comments are adapted from his editorial. (JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410 ).
One question that remains from the study by Skydel et al. is why the Food and Drug Administration did not mandate postapproval clinical studies for more than one-third of the novel agents approved from 2009 to 2012.
Among 600 postapproval trials affiliated with pharmaceutical companies, the vast majority were for novel therapeutics being studied in oncology- and hematology-related indications. Many of these premarket trials are one-arm, nonrandomized studies approved with evidence from surrogate outcomes. Recent studies have revealed that many novel agents do not show an overall survival benefit 5 years post approval.
From a clinical perspective, interpreting safety and efficacy data from these trials is challenging because of various limitations, including small sample sizes, no control groups, and lack of randomization. High-quality evidence helping clinicians determine which patients will benefit from these novel therapeutics is needed.
In addition, the purpose of these sponsored studies has come into question. While they can be used to generate further clinical data, many other reasons are possible. The expectation could be that once these studies are complete, clinicians may prescribe the agent being examined, thereby increasing off-label prescribing. Recent studies have revealed that off-label prescribing has the potential to increase the risk of drug-related adverse events.
“Postapproval studies need to advance clinical care by answering unresolved questions,” Dr. Lexchin wrote. “Clinicians and patients deserve better.”
Joel Lexchin, MD, is associated with the School of Health Policy and Management at York University in Toronto. He reported financial affiliations with the Gordon and Betty Moore Foundation, the Government of Canada, the Ontario Supporting Patient Oriented Research Support Unit, the St Michael’s Hospital Foundation, the Canadian Institutes of Health Research, and the American Diabetes Association. These comments are adapted from his editorial. (JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410 ).
One question that remains from the study by Skydel et al. is why the Food and Drug Administration did not mandate postapproval clinical studies for more than one-third of the novel agents approved from 2009 to 2012.
Among 600 postapproval trials affiliated with pharmaceutical companies, the vast majority were for novel therapeutics being studied in oncology- and hematology-related indications. Many of these premarket trials are one-arm, nonrandomized studies approved with evidence from surrogate outcomes. Recent studies have revealed that many novel agents do not show an overall survival benefit 5 years post approval.
From a clinical perspective, interpreting safety and efficacy data from these trials is challenging because of various limitations, including small sample sizes, no control groups, and lack of randomization. High-quality evidence helping clinicians determine which patients will benefit from these novel therapeutics is needed.
In addition, the purpose of these sponsored studies has come into question. While they can be used to generate further clinical data, many other reasons are possible. The expectation could be that once these studies are complete, clinicians may prescribe the agent being examined, thereby increasing off-label prescribing. Recent studies have revealed that off-label prescribing has the potential to increase the risk of drug-related adverse events.
“Postapproval studies need to advance clinical care by answering unresolved questions,” Dr. Lexchin wrote. “Clinicians and patients deserve better.”
Joel Lexchin, MD, is associated with the School of Health Policy and Management at York University in Toronto. He reported financial affiliations with the Gordon and Betty Moore Foundation, the Government of Canada, the Ontario Supporting Patient Oriented Research Support Unit, the St Michael’s Hospital Foundation, the Canadian Institutes of Health Research, and the American Diabetes Association. These comments are adapted from his editorial. (JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410 ).
The majority of postapproval clinical trials conducted by pharmaceutical companies explored novel or expanded uses as opposed to monitoring original indications, according to results from a cross-sectional study.
“The U.S. Food and Drug Administration can use postmarketing requirements to mandate pharmaceutical companies to conduct clinical trials after the approval of novel therapeutics,” wrote Joshua J. Skydel of Tufts University, Boston, and colleagues in JAMA Network Open.
“From 2009 through 2012, the FDA approved 110 novel therapeutics for 120 indications,” the researchers reported.
Of those approved, 37 novel agents were identified that lacked requirements for postmarket monitoring. Data was obtained from the Drugs@FDA database and consisted of industry-sponsored postapproval clinical studies conducted in the United States.
Postapproval study information included 600 trials registered on ClinicalTrials.gov that were primarily aimed at producing additional efficacy and safety data in approved or unapproved uses. The majority of these trials were for novel therapeutics being evaluated in oncology- and hematology-related indications.
After analysis, the researchers found that most of these trials examined agents for novel or expanded indications (60.5%) or widened populations of the initially indicated condition (20.3%).
In addition, several of these studies were nonrandomized (59.8%), had small sample sizes (median, 44 participants), and did not include comparators (63.5%).
“Among 600 trials, only 12.0% exclusively evaluated the originally approved indication,” the authors wrote.
The researchers acknowledged that a key limitation of the study was the inclusion of only postapproval clinical studies affiliated with pharmaceutical companies.
“The FDA may need to impose additional postmarketing requirements to generate further evidence for the original indications,” they concluded.
The study was funded by the Tufts University School of Medicine and the Laura and John Arnold Foundation. The authors reported financial affiliations with Johnson & Johnson, Medtronic, the Blue Cross Blue Shield Association, the National Institutes of Health, the Laura and John Arnold Foundation, and the Food and Drug Administration.
SOURCE: Skydel JJ et al. JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410.
The majority of postapproval clinical trials conducted by pharmaceutical companies explored novel or expanded uses as opposed to monitoring original indications, according to results from a cross-sectional study.
“The U.S. Food and Drug Administration can use postmarketing requirements to mandate pharmaceutical companies to conduct clinical trials after the approval of novel therapeutics,” wrote Joshua J. Skydel of Tufts University, Boston, and colleagues in JAMA Network Open.
“From 2009 through 2012, the FDA approved 110 novel therapeutics for 120 indications,” the researchers reported.
Of those approved, 37 novel agents were identified that lacked requirements for postmarket monitoring. Data was obtained from the Drugs@FDA database and consisted of industry-sponsored postapproval clinical studies conducted in the United States.
Postapproval study information included 600 trials registered on ClinicalTrials.gov that were primarily aimed at producing additional efficacy and safety data in approved or unapproved uses. The majority of these trials were for novel therapeutics being evaluated in oncology- and hematology-related indications.
After analysis, the researchers found that most of these trials examined agents for novel or expanded indications (60.5%) or widened populations of the initially indicated condition (20.3%).
In addition, several of these studies were nonrandomized (59.8%), had small sample sizes (median, 44 participants), and did not include comparators (63.5%).
“Among 600 trials, only 12.0% exclusively evaluated the originally approved indication,” the authors wrote.
The researchers acknowledged that a key limitation of the study was the inclusion of only postapproval clinical studies affiliated with pharmaceutical companies.
“The FDA may need to impose additional postmarketing requirements to generate further evidence for the original indications,” they concluded.
The study was funded by the Tufts University School of Medicine and the Laura and John Arnold Foundation. The authors reported financial affiliations with Johnson & Johnson, Medtronic, the Blue Cross Blue Shield Association, the National Institutes of Health, the Laura and John Arnold Foundation, and the Food and Drug Administration.
SOURCE: Skydel JJ et al. JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410.
FROM JAMA NETWORK OPEN
Key clinical point: The majority of postapproval clinical trials conducted by pharmaceutical companies explored novel or expanded uses as opposed to monitoring original indications.
Major finding: Among 600 postapproval studies, only 12.0% solely examined the original therapeutic indication.
Study details: A cross-sectional analysis of 37 novel agents without postmarketing requirements.
Disclosures: The study was funded by the Tufts University School of Medicine and the Laura and John Arnold Foundation. The authors reported financial affiliations with Johnson & Johnson, Medtronic, the Blue Cross Blue Shield Association, the National Institutes of Health, the Laura and John Arnold Foundation, and the Food and Drug Administration.
Source: Skydel JJ et al. JAMA Netw Open. 2019 May 9. doi: 10.1001/jamanetworkopen.2019.3410.











