User login
Achievable Benchmarks of Care for Pediatric Readmissions
Hospital readmission rates are a common metric for defining, evaluating, and benchmarking quality of care. The Centers for Medicare and Medicaid Services (CMS) publicly report hospital readmission rates for common adult conditions and reduces payments to hospitals with excessive readmissions.1 Recently, the focus on pediatric readmission rates has increased and the National Quality Forum (NQF) has endorsed at least two pediatric readmission-specific quality indicators which could be used by public and private payers in pay-for-performance programs aimed at institutions caring for children.2 While preventability of readmissions and their value as a marker of quality remains debated, their acceptance by the NQF and CMS has led public and private payers to propose readmission-related penalties for hospitals caring for children. 3-5
All-cause 30-day same-hospital readmission rates for pediatric conditions are half of the adult readmission rates, around 6% in most studies, compared to 12% in adults.6,7 The lower rates of pediatric readmissions makes it difficult to only use mean readmission rates to stratify hospitals into high- or low-performers and set target goals for improvement.8 While adult readmissions have been studied in depth, there are no consistent measures used to benchmark pediatric readmissions across hospital types.
Given the emphasis placed on readmissions, it is essential to understand patterns in pediatric readmission rates to determine optimal and achievable targets for improvement. Achievable Benchmarks of Care (ABCs) are one approach to understanding readmission rates and have an advantage over using mean or medians in performance improvement as they can stratify performance for conditions with low readmission rates and low volumes.9 When creating benchmarks, it is important that hospitals performance is evaluated among peer hospitals with similar patient populations, not just a cumulative average from all hospital types which may punish hospitals with a more complex patient case mix.10 The goal of this study was to calculate the readmission rates and the ABCs for common pediatric diagnoses by hospital type to identify priority conditions for quality improvement efforts using a previously published methodology.11-13
METHODS
Data Source
We conducted a retrospective analysis of patients less than 18 years of age in the Healthcare Utilization Project 2014 Nationwide Readmissions Database (NRD). The NRD includes public hospitals; academic medical centers; and specialty hospitals in obstetrics and gynecology, otolaryngology, orthopedics, and cancer; and pediatric, public, and academic medical hospitals. Excluded are long-term care facilities such as rehabilitation, long-term acute care, psychiatric, alcoholism, and chemical dependency hospitals. The readmissions data contains information from hospitals grouped by region, population census, and teaching status.14 Three hospital type classifications used in this study were metropolitan teaching hospitals, metropolitan nonteaching hospitals, and nonmetropolitan hospitals. These three hospital type classifications follow the reporting format in the NRD.
Study Population
Patients less than 18 years old were included if they were discharged from January 1, 2014 through November 30, 2014 and had a readmission to the index hospital within 30 days. We limited inclusion to discharges through November 30 so we could identify patients with a 30-day readmission as patient identifiers do not link across years in the NRD.
Exposure
We included 30-day, all-cause, same-hospital readmissions to the index acute care hospital, excluding labor and delivery, normal newborn care, chemotherapy, transfers, and mortalities. Intrahospital discharge and admissions within the same hospital system were not defined as a readmission, but rather as a “same-day event.”15 For example, institutions with inpatient mental health facilities, medical unit discharges and admission to the mental health unit were not identified as a readmission in this dataset.
Outcome
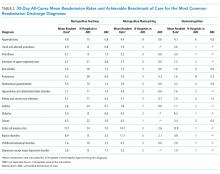
For each hospital type, we measured same-hospital, all-cause, 30-day readmission rates and achievable benchmark of care for the 17 most commonly readmitted pediatric discharge diagnoses. To identify the target readmission diagnoses and all-cause, 30-day readmissions based on their index hospitalizations, All-Patient Refined Diagnosis-Related Groups (APR-DRG), version 25 (3M Health Information Systems, Salt Lake City, Utah) were ordered by frequency for each hospital type. The 20 most common APR-DRGs were the same across all hospital types. The authors then evaluated these 20 APR-DRGs for clinical consistency of included diagnoses identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes within each APR-DRG. Three diagnosis-related groups were excluded from the analysis (major hematologic/immunologic disease except for sickle cell, other anemia and disorders of blood and blood forming organs, and other digestive system diagnoses) due to the heterogeneity of the diagnoses identified by the ICD-9-CM codes within each APR-DRG. We refer to each APR-DRG as a “diagnosis” throughout the article.
Analysis
The demographic characteristics of the patients seen at the three hospital types were summarized using frequencies and percentages. Reports were generated for patient age, gender, payer source, patient residence, median household income, patient complexity, and discharge disposition. Patient complexity was defined using complex chronic condition (CCC) and the number of chronic conditions (CCI).16,17 As previously defined in the literature, a complex chronic condition is “any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one organ system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.”16 Whereas, the Agency for Healthcare Research and Quality’s Chronic Condition Indicator (CCI) defines single, non-CCCs (eg, allergic rhinitis).17
For each diagnosis, we calculated the mean readmission rate for hospitals in each hospital type category. We then calculated an ABC for each diagnosis in each hospital type using a four-step process.13,18 First, to control for hospitals with small sample sizes, we adjusted all readmission rates using an adjusted performance fraction ([numerator+1]/[denominator +2]), where the numerator is the number of all-cause 30-day readmissions and the denominator is the number of discharges for the selected diagnosis. Then the hospitals were ordered from lowest (best performing) to highest (worst performing) using the adjusted readmission rate. Third, the number of discharges from the best performing hospital to the worst performing hospital was summed until at least 10% of the total discharges had been accounted for. Finally, we computed the ABC as the average of these best performing hospitals. We only report ABCs for which at least three hospitals were included as best performers in the calculation.13
To evaluate hospital performance on ABCs for each diagnosis, we identified the percent of hospitals in each setting that were outliers. We defined an outlier as any hospital whose 95% confidence interval for their readmission rate for a given diagnosis did not contain the ABC for their hospital type. All the statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, North Carolina).
This project was reviewed by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and determined to be nonhuman subjects research.
RESULTS
Hospital-Type Demographics
The 690,949 discharges from 1,664 hospitals were categorized into 525 metropolitan teaching (550,039 discharges, 79.6% of discharges), 552 metropolitan nonteaching (97,207 discharges, 14% of discharges), and 587 nonmetropolitan hospitals (43,703 discharges, 6.3% of discharges; Table 1). There were significant differences in the patient composition among the three hospital settings. Nonmetropolitan hospitals had a larger percentage of younger patients (aged 0-4 years, P < .001), prominence of first and second quartile median household income, and fewer medically complex patients (48.3% No CCC/No CCI versus 25.5% metropolitan teaching and 33.7% nonteaching, P < .001). Disposition home was over 96% in all three hospital types; however, the metropolitan teaching had a greater percentage of patients discharged to home health versus metropolitan nonteaching and nonmetropolitan hospitals (2.3% versus 0.5%; P < .001).
Readmission Rates
The 17 most common diagnoses based on the number of all-cause 30-day same-hospital readmissions, were categorized into two surgical, seven acute/infectious, four chronic, and four mental health diagnoses (Table 2). Readmission rates varied based on diagnosis and hospital type (Table 2). Overall, mean readmission rates were low, especially in acute respiratory tract related diseases. For chronic diseases, asthma readmissions were consistently low in all three hospital types, whereas sickle cell disease had the highest readmission rate in all three hospital types.
Achievable Benchmarks of Care by Hospital Type
The diagnoses for which ABC could be calculated across all three hospital types included appendectomy and four acute conditions (bronchiolitis, pneumonia, nonbacterial gastroenteritis, and kidney/urinary tract infections). For these conditions, metropolitan teaching hospitals had a more significant percentage of outlier hospitals compared to metropolitan nonteaching and nonmetropolitan hospitals. The percent of outlier hospitals varied by diagnosis and hospital type (Figure).
Metropolitan Teaching
The readmission ABC was calculated for all 17 diagnoses (Table 2). The ABC ranged from 0.4% in acute kidney and urinary tract infection to 7.0% in sickle cell anemia crisis. Bipolar disorder, major depressive disorders and other psychoses, and sickle cell disease (SCD) had the highest percent of outlier hospitals whose mean readmission rates confidence interval did not contain the ABC; tonsil and adenoid procedures and viral illness had the lowest.1
Metropolitan Nonteaching
The ABC was calculated for 13 of the 17 diagnoses because ABCs were not calculated when there were fewer than three best practicing hospitals. This was the case for tonsil and adenoid procedures, diabetes, seizures, and depression except for major depressive disorder (Table 2). Seven of the 13 diagnoses had an ABC of 0.0%: viral illness, infections of the upper respiratory tract, bronchiolitis, gastroenteritis, hypovolemia and electrolyte disorders, asthma, and childhood behavioral disorders. Like the findings at the metropolitan teaching hospitals, ABCs were lowest for surgical and acute conditions while bipolar disorder, major depressive disorders and other psychoses, and SCD had the highest percent of outlier hospitals with readmission rates beyond the 95% confidence interval of their hospital type’s ABC.
Nonmetropolitan
There was a sufficient number of best practicing hospitals to calculate the ABC for six of the 17 diagnoses (Table 2). For conditions where readmission ABCs could be calculated, they were low: 0.0% for appendectomy, bronchiolitis, gastroenteritis, and seizure; 0.3% for pneumonia; and 1.3% in kidney and urinary tract disorders. None of the conditions with the highest ABCs in other hospital settings (bipolar disease, sickle cell anemia crisis, and major depressive disorders and other psychoses) could be calculated in this setting. Seizure-related readmissions exhibited the most outlier hospitals yet were less than 5%.1
DISCUSSION
Among a nationally representative sample of different hospital types that deliver care to children, we report the mean readmission rates and ABCs for 30-day all-cause, same-hospital readmissions for the most commonly readmitted pediatric diagnoses based on hospital type. Previous studies have shown patient variables such as race, ethnicity, and insurance type influencing readmission rates.19,20 However, hospital type has also been associated with a higher risk of readmission due to the varying complexity of patients at different hospital types.21,22 Our analyses provide hospital-type specific national estimates of pediatric readmission ABCs for medical and surgical conditions, many less than 1%. While commonly encountered pediatric conditions like asthma and bronchiolitis had low mean readmission rates and ABCs across all hospital types, the mean rates and ABCs for SCD and mental health disorders were much higher with more hospitals performing far from the ABCs.
Diagnoses with a larger percentage of outlier hospitals may represent a national opportunity to improve care for children. Conditions such as SCD and mental illnesses have the highest percentage of hospitals whose readmission rates fall outside of the ABCs in both metropolitan teaching and metropolitan nonteaching hospitals. Hospital performance on SCD and mental health disorders may not reflect deficits in hospital quality or poor adherence to evidence-based best practices, but rather the complex interplay of factors on various levels from government policy and insurance plans, to patient and family resources, to access and availability of medical and mental health specific care. Most importantly, these diseases may represent a significant opportunity for quality improvementin hospitals across the United States.
Sickle cell disease is predominantly a disease among African-Americans, a demographic risk factor for decreased access to care and limited patient and family resources.23-26 In previous studies evaluating the disparity in readmission rates for Black children with asthma, socioeconomic variables explained 53% of the observed disparity and readmission rates were inversely related to the childhood opportunity index of the patient’s census tract and positively related with geographic social risk.27,28 Likewise, with SCD affecting a specific demographic and being a chronic disease, best practice policies need to account for the child’s medical needs and include the patient and family resources to ensure access to care and enhanced case management for chronic disease if we aim to improve performance among the outlier hospitals.
Similarly, barriers to care for children with mental illnesses in the United States need attention.29,30 While there is a paucity of data on the prevalence of mental health disorders in children, one national report estimates that one in 10 American adolescents have depression.29,31 The American Academy of Pediatrics has developed a policy statement on mental health competencies and a mental health tool-kit for primary care pediatricians; however, no such guidelines or policy statements exist for hospitalized patients with acute or chronic psychiatric conditions.32,33 Moreover, hospitals are increasingly facing “boarding” of children with acute psychiatric illness in inpatient units and emergency departments.34 The American Medical Association and the American College of Emergency Physicians have expressed concerns regarding the boarding of children with acute psychiatric illness because nonpsychiatric hospitals do not have adequate resources to evaluate, manage, and place these children who deserve appropriate facilities for further management. Coordinated case management and “bundled” discharge planning in other chronic illnesses have shown benefit in cost reduction and readmission.35-37 Evidence-based practices around pediatric readmissions in other diagnoses should be explored as possible interventions in these conditions.38
There are several limitations to this study. Our data is limited to one calendar year; therefore, admissions in January do not account for potential readmissions from December of the previous year, as patient identifiers do not link across years in the NRD. We also limited our evaluation to the conventional 30-day readmission window, but recent publications may indicate that readmission windows with different timelines could be a more accurate reflection of medically preventable readmissions versus a reflection of social determinants of health leading to readmissions.24 Newborn index admissions were not an allowable index admission; therefore, we may be underreporting readmissions in the neonatal age group. We also chose to include all-cause readmissions, a conventional method to evaluate readmission within an institution, but which may not reflect the quality of care delivered in the index admission. For example, an asthmatic discharged after an acute exacerbation readmitted for dehydration secondary to gastroenteritis may not reflect a lack of quality in asthma inpatient care. Readmissions were limited to the same hospital; therefore, this study cannot account for readmissions at other institutions, which may cause us to underestimate readmission rates. However, end-users of our findings most likely have access only to their own institution’s data. The inclusion of observation status admissions in the database varies from state to state; therefore, this percent of admissions in the database is unknown.
The use of the ABC methodology has some inherent limitations. One hospital with a significant volume diagnosis and low readmission rate within a hospital type may prohibit the reporting of an ABC if less than three hospitals composed the total of the ‘best performing’ hospitals. This was a significant limitation leading to the exclusion of many ABCs in nonmetropolitan institutions. The limitation of calculating and reporting an ABC then prohibits the calculation of outlier hospitals within a hospital type for a given diagnosis. However, when the ABCs are not available, we do provide the mean readmission rate for the diagnosis within the hospital type. While the hospital groupings by population and teaching status for ABCs provide meaningful comparisons for within each hospital setting, it should be noted that there may be vast differences among hospitals within each type (eg, tertiary children’s hospitals compared to teaching hospitals with a pediatric floor in the metropolitan teaching hospital category).39,40
As healthcare moves from a fee-for-service model to a population-health centered, value-based model, reduction in readmission rates will be more than a quality measure and will have potential financial implications.41 In the Medicare fee-for-service patients, the Hospital Readmission Reduction Program (HRRP) penalize hospitals with excess readmissions for acute myocardial infarction, heart failure, and pneumonia. The hospitals subject to penalties in the HRRP had greater reduction in readmission rates in the targeted, and even nontargeted conditions, compared with hospitals not subject to penalties.42 Similarly, we believe that our data on low readmission rates and ABCs for conditions such as asthma, bronchiolitis, and appendicitis could represent decades of quality improvement work for the most common pediatric conditions among hospitalized children. Sickle cell disease and mental health problems remain as outliers and merit further attention. To move to a true population-health model, hospitals will need to explore outlier conditions including evaluating patient-level readmission patterns across institutions. This moves readmission from a hospital quality measure to a patient-centric quality measure, and perhaps will provide value to the patient and the healthcare system alike.
CONCLUSIONS
The readmission ABCs for the most commonly readmitted pediatric diagnoses are low, regardless of the hospital setting. The highest pediatric readmission rates in SCD, bipolar disorders, and major depressive disorder were lower than the most common adult readmission diagnoses. However, mental health conditions and SCD remain as outliers for pediatric readmissions, burden hospital systems, and perhaps warrant national-level attention. The ABCs stratified by hospital type in this study facilitate comparisons and identify opportunities for population-level interventions to meaningfully improve patient care.
Disclosures
The authors have nothing to disclose.
1. Medicare. 30-day death and readmission measures data. https://www.medicare.gov/hospitalcompare/Data/30-day-measures.html. Accessed October 24, 2017.
2. National Quality Forum. Performance Measures; 2016 https://www.quality fourm.org/Measuring_Performance/Endorsed_Performance_Measures_Maintenance.aspx. Accessed October 24, 2017.
3. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
4. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182-e20154182. https://doi.org/10.1542/peds.2015-4182.
5. Halfon P, Eggli Y, Prêtre-Rohrbach I, et al. Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972-981. https://doi.org/10.1097/01.mlr.0000228002.43688.c2.
6. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619. https://doi.org/10.1016/j.jpeds.2014.10.052.
7. Berry JG, Gay JC, Joynt Maddox KJ, et al. Age trends in 30 day hospital readmissions: US national retrospective analysis. BMJ. 2018;360:k497. https://doi.org/10.1136/bmj.k497.
8. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527d.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
10. Gohil SK, Datta R, Cao C, et al. Impact of hospital population case-mix, including poverty, on hospital all-cause and infection-related 30-day readmission rates. Clin Infect Dis. 2015;61(8):1235-1243. https://doi.org/10.1093/cid/civ539.
11. Parikh K, Hall M, Mittal V, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052.
12. Reyes M, Paulus E, Hronek C, et al. Choosing wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029.
13. Kiefe CI, Weissman NW, Allison JJ, et al. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care. 1998;10(5):443-447. https://doi.org/10.1093/intqhc/10.5.443.
14. Agency for Healthcare Research and Quality. Nationwide Readmissions Database Availability of Data Elements. . https://www.hcup-us.ahrq.gov/partner/MOARef/HCUPdata_elements.pdf. Accessed 2018 Jun 6
15. Healthcare Cost and Utilization Project. HCUP NRD description of data elements. Agency Healthc Res Qual. https://www.hcup-us.ahrq.gov/db/vars/samedayevent/nrdnote.jsp. Accessed 2018 Jun 6; 2015.
16. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
17. Agency for Healthcare Research and Quality. HCUP chronic condition indicator. Healthc Cost Util Proj. https://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed 2016 Apr 26; 2009.
18. Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract. 1999;5(3):269-281. https://doi.org/10.1046/j.1365-2753.1999.00203.x.
19. Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675-681. https://doi.org/10.1001/jama.2011.123.
20. Kenyon CC, Melvin PR, Chiang VW, et al. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003.
21. Sobota A, Graham DA, Neufeld EJ, Heeney MM. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children’s hospitals: risk factors and hospital variation. Pediatr Blood Cancer. 2012;58(1):61-65. https://doi.org/10.1002/pbc.23221.
22. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
23. Ginde AA, Espinola JA, Camargo CA. Improved overall trends but persistent racial disparities in emergency department visits for acute asthma, 1993-2005. J Allergy Clin Immunol. 2008;122(2):313-318. https://doi.org/10.1016/j.jaci.2008.04.024.
24. Parikh K, Berry J, Hall M, et al. Racial and ethnic differences in pediatric readmissions for common chronic conditions. J Pediatr. 2017;186. https://doi.org/10.1016/j.jpeds.2017.03.046.
25. Chen BK, Hibbert J, Cheng X, Bennett K. Travel distance and sociodemographic correlates of potentially avoidable emergency department visits in California, 2006-2010: an observational study. Int J Equity Health. 2015;14(1):30. https://doi.org/10.1186/s12939-015-0158-y.
26. Ray KN, Chari AV, Engberg J, et al. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175(12):175(12):1983-1986. https://doi.org/10.1001/jamainternmed.2015.4468.
27. Beck AF, Huang B, Wheeler K, et al. The child opportunity index and disparities in pediatric asthma hospitalizations across one Ohio metropolitan area. J Pediatr. 2011-2013;190:200-206. https://doi.org/10.1016/j.jpeds.2017.08.007.
28. Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: area-based socioeconomic measures for assessing the risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012;102(12):2308-2314. https://doi.org/10.2105/AJPH.2012.300806.
29. Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37-44.e2. https://doi.org/10.1016/j.jaac.2014.10.010.
30. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. https://doi.org/10.1542/peds.2017-1571.
31. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989. https://doi.org/10.1016/j.jaac.2010.05.017.
32. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part II. Treatment and ongoing management. Pediatrics. 2018;141(3):e20174082. https://doi.org/10.1542/peds.2017-4082.
33. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. Practice preparation, identification, assessment, and initial management. Pediatrics. 2018;141(3):e20174081. https://doi.org/10.1542/peds.2017-4081.
34. Dolan MA, Fein JA, Committee on Pediatric Emergency Medicine. Pediatric and adolescent mental health emergencies in the emergency Medical Services system. Pediatrics. 2011;127(5):e1356-e1366. https://doi.org/10.1542/peds.2011-0522.
35. Collaborative Healthcare Strategies. Hospital Guide to Reducing Medicaid Readmissions. Rockville, MD: 2014. https://www.ahrq.gov/sites/default/files/publications/files/medreadmissions.pdf. Accessed 2017 Oct 11.
36. Hilbert K, Payne R, Wooton S. Children’s Hospitals’ Solutions for Patient Safety. Readmissions Bundle Tools. Cincinnati, OH; 2014.
37. Nuckols TK, Keeler E, Morton S, et al. Economic evaluation of quality improvement interventions designed to prevent hospital readmission: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(7):975-985. https://doi.org/10.1001/jamainternmed.2017.1136.
38. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962. https://doi.org/10.1001/jamapediatrics.2014.891.
39. Chen HF, Carlson E, Popoola T, Suzuki S. The impact of rurality on 30-day preventable readmission, illness severity, and risk of mortality for heart failure Medicare home health beneficiaries. J Rural Health. 2016;32(2):176-187. https://doi.org/10.1111/jrh.12142.
40. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-hospital readmission rates as a measure of pediatric quality of care. JAMA Pediatr. 2015;169(10):905-912. https://doi.org/10.1001/jamapediatrics.2015.1129.
41. Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30(4):636-645. https://doi.org/10.1377/hlthaff.2010.0526.
42. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533.
Hospital readmission rates are a common metric for defining, evaluating, and benchmarking quality of care. The Centers for Medicare and Medicaid Services (CMS) publicly report hospital readmission rates for common adult conditions and reduces payments to hospitals with excessive readmissions.1 Recently, the focus on pediatric readmission rates has increased and the National Quality Forum (NQF) has endorsed at least two pediatric readmission-specific quality indicators which could be used by public and private payers in pay-for-performance programs aimed at institutions caring for children.2 While preventability of readmissions and their value as a marker of quality remains debated, their acceptance by the NQF and CMS has led public and private payers to propose readmission-related penalties for hospitals caring for children. 3-5
All-cause 30-day same-hospital readmission rates for pediatric conditions are half of the adult readmission rates, around 6% in most studies, compared to 12% in adults.6,7 The lower rates of pediatric readmissions makes it difficult to only use mean readmission rates to stratify hospitals into high- or low-performers and set target goals for improvement.8 While adult readmissions have been studied in depth, there are no consistent measures used to benchmark pediatric readmissions across hospital types.
Given the emphasis placed on readmissions, it is essential to understand patterns in pediatric readmission rates to determine optimal and achievable targets for improvement. Achievable Benchmarks of Care (ABCs) are one approach to understanding readmission rates and have an advantage over using mean or medians in performance improvement as they can stratify performance for conditions with low readmission rates and low volumes.9 When creating benchmarks, it is important that hospitals performance is evaluated among peer hospitals with similar patient populations, not just a cumulative average from all hospital types which may punish hospitals with a more complex patient case mix.10 The goal of this study was to calculate the readmission rates and the ABCs for common pediatric diagnoses by hospital type to identify priority conditions for quality improvement efforts using a previously published methodology.11-13
METHODS
Data Source
We conducted a retrospective analysis of patients less than 18 years of age in the Healthcare Utilization Project 2014 Nationwide Readmissions Database (NRD). The NRD includes public hospitals; academic medical centers; and specialty hospitals in obstetrics and gynecology, otolaryngology, orthopedics, and cancer; and pediatric, public, and academic medical hospitals. Excluded are long-term care facilities such as rehabilitation, long-term acute care, psychiatric, alcoholism, and chemical dependency hospitals. The readmissions data contains information from hospitals grouped by region, population census, and teaching status.14 Three hospital type classifications used in this study were metropolitan teaching hospitals, metropolitan nonteaching hospitals, and nonmetropolitan hospitals. These three hospital type classifications follow the reporting format in the NRD.
Study Population
Patients less than 18 years old were included if they were discharged from January 1, 2014 through November 30, 2014 and had a readmission to the index hospital within 30 days. We limited inclusion to discharges through November 30 so we could identify patients with a 30-day readmission as patient identifiers do not link across years in the NRD.
Exposure
We included 30-day, all-cause, same-hospital readmissions to the index acute care hospital, excluding labor and delivery, normal newborn care, chemotherapy, transfers, and mortalities. Intrahospital discharge and admissions within the same hospital system were not defined as a readmission, but rather as a “same-day event.”15 For example, institutions with inpatient mental health facilities, medical unit discharges and admission to the mental health unit were not identified as a readmission in this dataset.
Outcome
For each hospital type, we measured same-hospital, all-cause, 30-day readmission rates and achievable benchmark of care for the 17 most commonly readmitted pediatric discharge diagnoses. To identify the target readmission diagnoses and all-cause, 30-day readmissions based on their index hospitalizations, All-Patient Refined Diagnosis-Related Groups (APR-DRG), version 25 (3M Health Information Systems, Salt Lake City, Utah) were ordered by frequency for each hospital type. The 20 most common APR-DRGs were the same across all hospital types. The authors then evaluated these 20 APR-DRGs for clinical consistency of included diagnoses identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes within each APR-DRG. Three diagnosis-related groups were excluded from the analysis (major hematologic/immunologic disease except for sickle cell, other anemia and disorders of blood and blood forming organs, and other digestive system diagnoses) due to the heterogeneity of the diagnoses identified by the ICD-9-CM codes within each APR-DRG. We refer to each APR-DRG as a “diagnosis” throughout the article.
Analysis
The demographic characteristics of the patients seen at the three hospital types were summarized using frequencies and percentages. Reports were generated for patient age, gender, payer source, patient residence, median household income, patient complexity, and discharge disposition. Patient complexity was defined using complex chronic condition (CCC) and the number of chronic conditions (CCI).16,17 As previously defined in the literature, a complex chronic condition is “any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one organ system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.”16 Whereas, the Agency for Healthcare Research and Quality’s Chronic Condition Indicator (CCI) defines single, non-CCCs (eg, allergic rhinitis).17
For each diagnosis, we calculated the mean readmission rate for hospitals in each hospital type category. We then calculated an ABC for each diagnosis in each hospital type using a four-step process.13,18 First, to control for hospitals with small sample sizes, we adjusted all readmission rates using an adjusted performance fraction ([numerator+1]/[denominator +2]), where the numerator is the number of all-cause 30-day readmissions and the denominator is the number of discharges for the selected diagnosis. Then the hospitals were ordered from lowest (best performing) to highest (worst performing) using the adjusted readmission rate. Third, the number of discharges from the best performing hospital to the worst performing hospital was summed until at least 10% of the total discharges had been accounted for. Finally, we computed the ABC as the average of these best performing hospitals. We only report ABCs for which at least three hospitals were included as best performers in the calculation.13
To evaluate hospital performance on ABCs for each diagnosis, we identified the percent of hospitals in each setting that were outliers. We defined an outlier as any hospital whose 95% confidence interval for their readmission rate for a given diagnosis did not contain the ABC for their hospital type. All the statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, North Carolina).
This project was reviewed by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and determined to be nonhuman subjects research.
RESULTS
Hospital-Type Demographics
The 690,949 discharges from 1,664 hospitals were categorized into 525 metropolitan teaching (550,039 discharges, 79.6% of discharges), 552 metropolitan nonteaching (97,207 discharges, 14% of discharges), and 587 nonmetropolitan hospitals (43,703 discharges, 6.3% of discharges; Table 1). There were significant differences in the patient composition among the three hospital settings. Nonmetropolitan hospitals had a larger percentage of younger patients (aged 0-4 years, P < .001), prominence of first and second quartile median household income, and fewer medically complex patients (48.3% No CCC/No CCI versus 25.5% metropolitan teaching and 33.7% nonteaching, P < .001). Disposition home was over 96% in all three hospital types; however, the metropolitan teaching had a greater percentage of patients discharged to home health versus metropolitan nonteaching and nonmetropolitan hospitals (2.3% versus 0.5%; P < .001).
Readmission Rates
The 17 most common diagnoses based on the number of all-cause 30-day same-hospital readmissions, were categorized into two surgical, seven acute/infectious, four chronic, and four mental health diagnoses (Table 2). Readmission rates varied based on diagnosis and hospital type (Table 2). Overall, mean readmission rates were low, especially in acute respiratory tract related diseases. For chronic diseases, asthma readmissions were consistently low in all three hospital types, whereas sickle cell disease had the highest readmission rate in all three hospital types.
Achievable Benchmarks of Care by Hospital Type
The diagnoses for which ABC could be calculated across all three hospital types included appendectomy and four acute conditions (bronchiolitis, pneumonia, nonbacterial gastroenteritis, and kidney/urinary tract infections). For these conditions, metropolitan teaching hospitals had a more significant percentage of outlier hospitals compared to metropolitan nonteaching and nonmetropolitan hospitals. The percent of outlier hospitals varied by diagnosis and hospital type (Figure).
Metropolitan Teaching
The readmission ABC was calculated for all 17 diagnoses (Table 2). The ABC ranged from 0.4% in acute kidney and urinary tract infection to 7.0% in sickle cell anemia crisis. Bipolar disorder, major depressive disorders and other psychoses, and sickle cell disease (SCD) had the highest percent of outlier hospitals whose mean readmission rates confidence interval did not contain the ABC; tonsil and adenoid procedures and viral illness had the lowest.1
Metropolitan Nonteaching
The ABC was calculated for 13 of the 17 diagnoses because ABCs were not calculated when there were fewer than three best practicing hospitals. This was the case for tonsil and adenoid procedures, diabetes, seizures, and depression except for major depressive disorder (Table 2). Seven of the 13 diagnoses had an ABC of 0.0%: viral illness, infections of the upper respiratory tract, bronchiolitis, gastroenteritis, hypovolemia and electrolyte disorders, asthma, and childhood behavioral disorders. Like the findings at the metropolitan teaching hospitals, ABCs were lowest for surgical and acute conditions while bipolar disorder, major depressive disorders and other psychoses, and SCD had the highest percent of outlier hospitals with readmission rates beyond the 95% confidence interval of their hospital type’s ABC.
Nonmetropolitan
There was a sufficient number of best practicing hospitals to calculate the ABC for six of the 17 diagnoses (Table 2). For conditions where readmission ABCs could be calculated, they were low: 0.0% for appendectomy, bronchiolitis, gastroenteritis, and seizure; 0.3% for pneumonia; and 1.3% in kidney and urinary tract disorders. None of the conditions with the highest ABCs in other hospital settings (bipolar disease, sickle cell anemia crisis, and major depressive disorders and other psychoses) could be calculated in this setting. Seizure-related readmissions exhibited the most outlier hospitals yet were less than 5%.1
DISCUSSION
Among a nationally representative sample of different hospital types that deliver care to children, we report the mean readmission rates and ABCs for 30-day all-cause, same-hospital readmissions for the most commonly readmitted pediatric diagnoses based on hospital type. Previous studies have shown patient variables such as race, ethnicity, and insurance type influencing readmission rates.19,20 However, hospital type has also been associated with a higher risk of readmission due to the varying complexity of patients at different hospital types.21,22 Our analyses provide hospital-type specific national estimates of pediatric readmission ABCs for medical and surgical conditions, many less than 1%. While commonly encountered pediatric conditions like asthma and bronchiolitis had low mean readmission rates and ABCs across all hospital types, the mean rates and ABCs for SCD and mental health disorders were much higher with more hospitals performing far from the ABCs.
Diagnoses with a larger percentage of outlier hospitals may represent a national opportunity to improve care for children. Conditions such as SCD and mental illnesses have the highest percentage of hospitals whose readmission rates fall outside of the ABCs in both metropolitan teaching and metropolitan nonteaching hospitals. Hospital performance on SCD and mental health disorders may not reflect deficits in hospital quality or poor adherence to evidence-based best practices, but rather the complex interplay of factors on various levels from government policy and insurance plans, to patient and family resources, to access and availability of medical and mental health specific care. Most importantly, these diseases may represent a significant opportunity for quality improvementin hospitals across the United States.
Sickle cell disease is predominantly a disease among African-Americans, a demographic risk factor for decreased access to care and limited patient and family resources.23-26 In previous studies evaluating the disparity in readmission rates for Black children with asthma, socioeconomic variables explained 53% of the observed disparity and readmission rates were inversely related to the childhood opportunity index of the patient’s census tract and positively related with geographic social risk.27,28 Likewise, with SCD affecting a specific demographic and being a chronic disease, best practice policies need to account for the child’s medical needs and include the patient and family resources to ensure access to care and enhanced case management for chronic disease if we aim to improve performance among the outlier hospitals.
Similarly, barriers to care for children with mental illnesses in the United States need attention.29,30 While there is a paucity of data on the prevalence of mental health disorders in children, one national report estimates that one in 10 American adolescents have depression.29,31 The American Academy of Pediatrics has developed a policy statement on mental health competencies and a mental health tool-kit for primary care pediatricians; however, no such guidelines or policy statements exist for hospitalized patients with acute or chronic psychiatric conditions.32,33 Moreover, hospitals are increasingly facing “boarding” of children with acute psychiatric illness in inpatient units and emergency departments.34 The American Medical Association and the American College of Emergency Physicians have expressed concerns regarding the boarding of children with acute psychiatric illness because nonpsychiatric hospitals do not have adequate resources to evaluate, manage, and place these children who deserve appropriate facilities for further management. Coordinated case management and “bundled” discharge planning in other chronic illnesses have shown benefit in cost reduction and readmission.35-37 Evidence-based practices around pediatric readmissions in other diagnoses should be explored as possible interventions in these conditions.38
There are several limitations to this study. Our data is limited to one calendar year; therefore, admissions in January do not account for potential readmissions from December of the previous year, as patient identifiers do not link across years in the NRD. We also limited our evaluation to the conventional 30-day readmission window, but recent publications may indicate that readmission windows with different timelines could be a more accurate reflection of medically preventable readmissions versus a reflection of social determinants of health leading to readmissions.24 Newborn index admissions were not an allowable index admission; therefore, we may be underreporting readmissions in the neonatal age group. We also chose to include all-cause readmissions, a conventional method to evaluate readmission within an institution, but which may not reflect the quality of care delivered in the index admission. For example, an asthmatic discharged after an acute exacerbation readmitted for dehydration secondary to gastroenteritis may not reflect a lack of quality in asthma inpatient care. Readmissions were limited to the same hospital; therefore, this study cannot account for readmissions at other institutions, which may cause us to underestimate readmission rates. However, end-users of our findings most likely have access only to their own institution’s data. The inclusion of observation status admissions in the database varies from state to state; therefore, this percent of admissions in the database is unknown.
The use of the ABC methodology has some inherent limitations. One hospital with a significant volume diagnosis and low readmission rate within a hospital type may prohibit the reporting of an ABC if less than three hospitals composed the total of the ‘best performing’ hospitals. This was a significant limitation leading to the exclusion of many ABCs in nonmetropolitan institutions. The limitation of calculating and reporting an ABC then prohibits the calculation of outlier hospitals within a hospital type for a given diagnosis. However, when the ABCs are not available, we do provide the mean readmission rate for the diagnosis within the hospital type. While the hospital groupings by population and teaching status for ABCs provide meaningful comparisons for within each hospital setting, it should be noted that there may be vast differences among hospitals within each type (eg, tertiary children’s hospitals compared to teaching hospitals with a pediatric floor in the metropolitan teaching hospital category).39,40
As healthcare moves from a fee-for-service model to a population-health centered, value-based model, reduction in readmission rates will be more than a quality measure and will have potential financial implications.41 In the Medicare fee-for-service patients, the Hospital Readmission Reduction Program (HRRP) penalize hospitals with excess readmissions for acute myocardial infarction, heart failure, and pneumonia. The hospitals subject to penalties in the HRRP had greater reduction in readmission rates in the targeted, and even nontargeted conditions, compared with hospitals not subject to penalties.42 Similarly, we believe that our data on low readmission rates and ABCs for conditions such as asthma, bronchiolitis, and appendicitis could represent decades of quality improvement work for the most common pediatric conditions among hospitalized children. Sickle cell disease and mental health problems remain as outliers and merit further attention. To move to a true population-health model, hospitals will need to explore outlier conditions including evaluating patient-level readmission patterns across institutions. This moves readmission from a hospital quality measure to a patient-centric quality measure, and perhaps will provide value to the patient and the healthcare system alike.
CONCLUSIONS
The readmission ABCs for the most commonly readmitted pediatric diagnoses are low, regardless of the hospital setting. The highest pediatric readmission rates in SCD, bipolar disorders, and major depressive disorder were lower than the most common adult readmission diagnoses. However, mental health conditions and SCD remain as outliers for pediatric readmissions, burden hospital systems, and perhaps warrant national-level attention. The ABCs stratified by hospital type in this study facilitate comparisons and identify opportunities for population-level interventions to meaningfully improve patient care.
Disclosures
The authors have nothing to disclose.
Hospital readmission rates are a common metric for defining, evaluating, and benchmarking quality of care. The Centers for Medicare and Medicaid Services (CMS) publicly report hospital readmission rates for common adult conditions and reduces payments to hospitals with excessive readmissions.1 Recently, the focus on pediatric readmission rates has increased and the National Quality Forum (NQF) has endorsed at least two pediatric readmission-specific quality indicators which could be used by public and private payers in pay-for-performance programs aimed at institutions caring for children.2 While preventability of readmissions and their value as a marker of quality remains debated, their acceptance by the NQF and CMS has led public and private payers to propose readmission-related penalties for hospitals caring for children. 3-5
All-cause 30-day same-hospital readmission rates for pediatric conditions are half of the adult readmission rates, around 6% in most studies, compared to 12% in adults.6,7 The lower rates of pediatric readmissions makes it difficult to only use mean readmission rates to stratify hospitals into high- or low-performers and set target goals for improvement.8 While adult readmissions have been studied in depth, there are no consistent measures used to benchmark pediatric readmissions across hospital types.
Given the emphasis placed on readmissions, it is essential to understand patterns in pediatric readmission rates to determine optimal and achievable targets for improvement. Achievable Benchmarks of Care (ABCs) are one approach to understanding readmission rates and have an advantage over using mean or medians in performance improvement as they can stratify performance for conditions with low readmission rates and low volumes.9 When creating benchmarks, it is important that hospitals performance is evaluated among peer hospitals with similar patient populations, not just a cumulative average from all hospital types which may punish hospitals with a more complex patient case mix.10 The goal of this study was to calculate the readmission rates and the ABCs for common pediatric diagnoses by hospital type to identify priority conditions for quality improvement efforts using a previously published methodology.11-13
METHODS
Data Source
We conducted a retrospective analysis of patients less than 18 years of age in the Healthcare Utilization Project 2014 Nationwide Readmissions Database (NRD). The NRD includes public hospitals; academic medical centers; and specialty hospitals in obstetrics and gynecology, otolaryngology, orthopedics, and cancer; and pediatric, public, and academic medical hospitals. Excluded are long-term care facilities such as rehabilitation, long-term acute care, psychiatric, alcoholism, and chemical dependency hospitals. The readmissions data contains information from hospitals grouped by region, population census, and teaching status.14 Three hospital type classifications used in this study were metropolitan teaching hospitals, metropolitan nonteaching hospitals, and nonmetropolitan hospitals. These three hospital type classifications follow the reporting format in the NRD.
Study Population
Patients less than 18 years old were included if they were discharged from January 1, 2014 through November 30, 2014 and had a readmission to the index hospital within 30 days. We limited inclusion to discharges through November 30 so we could identify patients with a 30-day readmission as patient identifiers do not link across years in the NRD.
Exposure
We included 30-day, all-cause, same-hospital readmissions to the index acute care hospital, excluding labor and delivery, normal newborn care, chemotherapy, transfers, and mortalities. Intrahospital discharge and admissions within the same hospital system were not defined as a readmission, but rather as a “same-day event.”15 For example, institutions with inpatient mental health facilities, medical unit discharges and admission to the mental health unit were not identified as a readmission in this dataset.
Outcome
For each hospital type, we measured same-hospital, all-cause, 30-day readmission rates and achievable benchmark of care for the 17 most commonly readmitted pediatric discharge diagnoses. To identify the target readmission diagnoses and all-cause, 30-day readmissions based on their index hospitalizations, All-Patient Refined Diagnosis-Related Groups (APR-DRG), version 25 (3M Health Information Systems, Salt Lake City, Utah) were ordered by frequency for each hospital type. The 20 most common APR-DRGs were the same across all hospital types. The authors then evaluated these 20 APR-DRGs for clinical consistency of included diagnoses identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes within each APR-DRG. Three diagnosis-related groups were excluded from the analysis (major hematologic/immunologic disease except for sickle cell, other anemia and disorders of blood and blood forming organs, and other digestive system diagnoses) due to the heterogeneity of the diagnoses identified by the ICD-9-CM codes within each APR-DRG. We refer to each APR-DRG as a “diagnosis” throughout the article.
Analysis
The demographic characteristics of the patients seen at the three hospital types were summarized using frequencies and percentages. Reports were generated for patient age, gender, payer source, patient residence, median household income, patient complexity, and discharge disposition. Patient complexity was defined using complex chronic condition (CCC) and the number of chronic conditions (CCI).16,17 As previously defined in the literature, a complex chronic condition is “any medical condition that can be reasonably expected to last at least 12 months (unless death intervenes) and to involve either several different organ systems or one organ system severely enough to require specialty pediatric care and probably some period of hospitalization in a tertiary care center.”16 Whereas, the Agency for Healthcare Research and Quality’s Chronic Condition Indicator (CCI) defines single, non-CCCs (eg, allergic rhinitis).17
For each diagnosis, we calculated the mean readmission rate for hospitals in each hospital type category. We then calculated an ABC for each diagnosis in each hospital type using a four-step process.13,18 First, to control for hospitals with small sample sizes, we adjusted all readmission rates using an adjusted performance fraction ([numerator+1]/[denominator +2]), where the numerator is the number of all-cause 30-day readmissions and the denominator is the number of discharges for the selected diagnosis. Then the hospitals were ordered from lowest (best performing) to highest (worst performing) using the adjusted readmission rate. Third, the number of discharges from the best performing hospital to the worst performing hospital was summed until at least 10% of the total discharges had been accounted for. Finally, we computed the ABC as the average of these best performing hospitals. We only report ABCs for which at least three hospitals were included as best performers in the calculation.13
To evaluate hospital performance on ABCs for each diagnosis, we identified the percent of hospitals in each setting that were outliers. We defined an outlier as any hospital whose 95% confidence interval for their readmission rate for a given diagnosis did not contain the ABC for their hospital type. All the statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, North Carolina).
This project was reviewed by the Cincinnati Children’s Hospital Medical Center Institutional Review Board and determined to be nonhuman subjects research.
RESULTS
Hospital-Type Demographics
The 690,949 discharges from 1,664 hospitals were categorized into 525 metropolitan teaching (550,039 discharges, 79.6% of discharges), 552 metropolitan nonteaching (97,207 discharges, 14% of discharges), and 587 nonmetropolitan hospitals (43,703 discharges, 6.3% of discharges; Table 1). There were significant differences in the patient composition among the three hospital settings. Nonmetropolitan hospitals had a larger percentage of younger patients (aged 0-4 years, P < .001), prominence of first and second quartile median household income, and fewer medically complex patients (48.3% No CCC/No CCI versus 25.5% metropolitan teaching and 33.7% nonteaching, P < .001). Disposition home was over 96% in all three hospital types; however, the metropolitan teaching had a greater percentage of patients discharged to home health versus metropolitan nonteaching and nonmetropolitan hospitals (2.3% versus 0.5%; P < .001).
Readmission Rates
The 17 most common diagnoses based on the number of all-cause 30-day same-hospital readmissions, were categorized into two surgical, seven acute/infectious, four chronic, and four mental health diagnoses (Table 2). Readmission rates varied based on diagnosis and hospital type (Table 2). Overall, mean readmission rates were low, especially in acute respiratory tract related diseases. For chronic diseases, asthma readmissions were consistently low in all three hospital types, whereas sickle cell disease had the highest readmission rate in all three hospital types.
Achievable Benchmarks of Care by Hospital Type
The diagnoses for which ABC could be calculated across all three hospital types included appendectomy and four acute conditions (bronchiolitis, pneumonia, nonbacterial gastroenteritis, and kidney/urinary tract infections). For these conditions, metropolitan teaching hospitals had a more significant percentage of outlier hospitals compared to metropolitan nonteaching and nonmetropolitan hospitals. The percent of outlier hospitals varied by diagnosis and hospital type (Figure).
Metropolitan Teaching
The readmission ABC was calculated for all 17 diagnoses (Table 2). The ABC ranged from 0.4% in acute kidney and urinary tract infection to 7.0% in sickle cell anemia crisis. Bipolar disorder, major depressive disorders and other psychoses, and sickle cell disease (SCD) had the highest percent of outlier hospitals whose mean readmission rates confidence interval did not contain the ABC; tonsil and adenoid procedures and viral illness had the lowest.1
Metropolitan Nonteaching
The ABC was calculated for 13 of the 17 diagnoses because ABCs were not calculated when there were fewer than three best practicing hospitals. This was the case for tonsil and adenoid procedures, diabetes, seizures, and depression except for major depressive disorder (Table 2). Seven of the 13 diagnoses had an ABC of 0.0%: viral illness, infections of the upper respiratory tract, bronchiolitis, gastroenteritis, hypovolemia and electrolyte disorders, asthma, and childhood behavioral disorders. Like the findings at the metropolitan teaching hospitals, ABCs were lowest for surgical and acute conditions while bipolar disorder, major depressive disorders and other psychoses, and SCD had the highest percent of outlier hospitals with readmission rates beyond the 95% confidence interval of their hospital type’s ABC.
Nonmetropolitan
There was a sufficient number of best practicing hospitals to calculate the ABC for six of the 17 diagnoses (Table 2). For conditions where readmission ABCs could be calculated, they were low: 0.0% for appendectomy, bronchiolitis, gastroenteritis, and seizure; 0.3% for pneumonia; and 1.3% in kidney and urinary tract disorders. None of the conditions with the highest ABCs in other hospital settings (bipolar disease, sickle cell anemia crisis, and major depressive disorders and other psychoses) could be calculated in this setting. Seizure-related readmissions exhibited the most outlier hospitals yet were less than 5%.1
DISCUSSION
Among a nationally representative sample of different hospital types that deliver care to children, we report the mean readmission rates and ABCs for 30-day all-cause, same-hospital readmissions for the most commonly readmitted pediatric diagnoses based on hospital type. Previous studies have shown patient variables such as race, ethnicity, and insurance type influencing readmission rates.19,20 However, hospital type has also been associated with a higher risk of readmission due to the varying complexity of patients at different hospital types.21,22 Our analyses provide hospital-type specific national estimates of pediatric readmission ABCs for medical and surgical conditions, many less than 1%. While commonly encountered pediatric conditions like asthma and bronchiolitis had low mean readmission rates and ABCs across all hospital types, the mean rates and ABCs for SCD and mental health disorders were much higher with more hospitals performing far from the ABCs.
Diagnoses with a larger percentage of outlier hospitals may represent a national opportunity to improve care for children. Conditions such as SCD and mental illnesses have the highest percentage of hospitals whose readmission rates fall outside of the ABCs in both metropolitan teaching and metropolitan nonteaching hospitals. Hospital performance on SCD and mental health disorders may not reflect deficits in hospital quality or poor adherence to evidence-based best practices, but rather the complex interplay of factors on various levels from government policy and insurance plans, to patient and family resources, to access and availability of medical and mental health specific care. Most importantly, these diseases may represent a significant opportunity for quality improvementin hospitals across the United States.
Sickle cell disease is predominantly a disease among African-Americans, a demographic risk factor for decreased access to care and limited patient and family resources.23-26 In previous studies evaluating the disparity in readmission rates for Black children with asthma, socioeconomic variables explained 53% of the observed disparity and readmission rates were inversely related to the childhood opportunity index of the patient’s census tract and positively related with geographic social risk.27,28 Likewise, with SCD affecting a specific demographic and being a chronic disease, best practice policies need to account for the child’s medical needs and include the patient and family resources to ensure access to care and enhanced case management for chronic disease if we aim to improve performance among the outlier hospitals.
Similarly, barriers to care for children with mental illnesses in the United States need attention.29,30 While there is a paucity of data on the prevalence of mental health disorders in children, one national report estimates that one in 10 American adolescents have depression.29,31 The American Academy of Pediatrics has developed a policy statement on mental health competencies and a mental health tool-kit for primary care pediatricians; however, no such guidelines or policy statements exist for hospitalized patients with acute or chronic psychiatric conditions.32,33 Moreover, hospitals are increasingly facing “boarding” of children with acute psychiatric illness in inpatient units and emergency departments.34 The American Medical Association and the American College of Emergency Physicians have expressed concerns regarding the boarding of children with acute psychiatric illness because nonpsychiatric hospitals do not have adequate resources to evaluate, manage, and place these children who deserve appropriate facilities for further management. Coordinated case management and “bundled” discharge planning in other chronic illnesses have shown benefit in cost reduction and readmission.35-37 Evidence-based practices around pediatric readmissions in other diagnoses should be explored as possible interventions in these conditions.38
There are several limitations to this study. Our data is limited to one calendar year; therefore, admissions in January do not account for potential readmissions from December of the previous year, as patient identifiers do not link across years in the NRD. We also limited our evaluation to the conventional 30-day readmission window, but recent publications may indicate that readmission windows with different timelines could be a more accurate reflection of medically preventable readmissions versus a reflection of social determinants of health leading to readmissions.24 Newborn index admissions were not an allowable index admission; therefore, we may be underreporting readmissions in the neonatal age group. We also chose to include all-cause readmissions, a conventional method to evaluate readmission within an institution, but which may not reflect the quality of care delivered in the index admission. For example, an asthmatic discharged after an acute exacerbation readmitted for dehydration secondary to gastroenteritis may not reflect a lack of quality in asthma inpatient care. Readmissions were limited to the same hospital; therefore, this study cannot account for readmissions at other institutions, which may cause us to underestimate readmission rates. However, end-users of our findings most likely have access only to their own institution’s data. The inclusion of observation status admissions in the database varies from state to state; therefore, this percent of admissions in the database is unknown.
The use of the ABC methodology has some inherent limitations. One hospital with a significant volume diagnosis and low readmission rate within a hospital type may prohibit the reporting of an ABC if less than three hospitals composed the total of the ‘best performing’ hospitals. This was a significant limitation leading to the exclusion of many ABCs in nonmetropolitan institutions. The limitation of calculating and reporting an ABC then prohibits the calculation of outlier hospitals within a hospital type for a given diagnosis. However, when the ABCs are not available, we do provide the mean readmission rate for the diagnosis within the hospital type. While the hospital groupings by population and teaching status for ABCs provide meaningful comparisons for within each hospital setting, it should be noted that there may be vast differences among hospitals within each type (eg, tertiary children’s hospitals compared to teaching hospitals with a pediatric floor in the metropolitan teaching hospital category).39,40
As healthcare moves from a fee-for-service model to a population-health centered, value-based model, reduction in readmission rates will be more than a quality measure and will have potential financial implications.41 In the Medicare fee-for-service patients, the Hospital Readmission Reduction Program (HRRP) penalize hospitals with excess readmissions for acute myocardial infarction, heart failure, and pneumonia. The hospitals subject to penalties in the HRRP had greater reduction in readmission rates in the targeted, and even nontargeted conditions, compared with hospitals not subject to penalties.42 Similarly, we believe that our data on low readmission rates and ABCs for conditions such as asthma, bronchiolitis, and appendicitis could represent decades of quality improvement work for the most common pediatric conditions among hospitalized children. Sickle cell disease and mental health problems remain as outliers and merit further attention. To move to a true population-health model, hospitals will need to explore outlier conditions including evaluating patient-level readmission patterns across institutions. This moves readmission from a hospital quality measure to a patient-centric quality measure, and perhaps will provide value to the patient and the healthcare system alike.
CONCLUSIONS
The readmission ABCs for the most commonly readmitted pediatric diagnoses are low, regardless of the hospital setting. The highest pediatric readmission rates in SCD, bipolar disorders, and major depressive disorder were lower than the most common adult readmission diagnoses. However, mental health conditions and SCD remain as outliers for pediatric readmissions, burden hospital systems, and perhaps warrant national-level attention. The ABCs stratified by hospital type in this study facilitate comparisons and identify opportunities for population-level interventions to meaningfully improve patient care.
Disclosures
The authors have nothing to disclose.
1. Medicare. 30-day death and readmission measures data. https://www.medicare.gov/hospitalcompare/Data/30-day-measures.html. Accessed October 24, 2017.
2. National Quality Forum. Performance Measures; 2016 https://www.quality fourm.org/Measuring_Performance/Endorsed_Performance_Measures_Maintenance.aspx. Accessed October 24, 2017.
3. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
4. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182-e20154182. https://doi.org/10.1542/peds.2015-4182.
5. Halfon P, Eggli Y, Prêtre-Rohrbach I, et al. Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972-981. https://doi.org/10.1097/01.mlr.0000228002.43688.c2.
6. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619. https://doi.org/10.1016/j.jpeds.2014.10.052.
7. Berry JG, Gay JC, Joynt Maddox KJ, et al. Age trends in 30 day hospital readmissions: US national retrospective analysis. BMJ. 2018;360:k497. https://doi.org/10.1136/bmj.k497.
8. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527d.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
10. Gohil SK, Datta R, Cao C, et al. Impact of hospital population case-mix, including poverty, on hospital all-cause and infection-related 30-day readmission rates. Clin Infect Dis. 2015;61(8):1235-1243. https://doi.org/10.1093/cid/civ539.
11. Parikh K, Hall M, Mittal V, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052.
12. Reyes M, Paulus E, Hronek C, et al. Choosing wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029.
13. Kiefe CI, Weissman NW, Allison JJ, et al. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care. 1998;10(5):443-447. https://doi.org/10.1093/intqhc/10.5.443.
14. Agency for Healthcare Research and Quality. Nationwide Readmissions Database Availability of Data Elements. . https://www.hcup-us.ahrq.gov/partner/MOARef/HCUPdata_elements.pdf. Accessed 2018 Jun 6
15. Healthcare Cost and Utilization Project. HCUP NRD description of data elements. Agency Healthc Res Qual. https://www.hcup-us.ahrq.gov/db/vars/samedayevent/nrdnote.jsp. Accessed 2018 Jun 6; 2015.
16. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
17. Agency for Healthcare Research and Quality. HCUP chronic condition indicator. Healthc Cost Util Proj. https://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed 2016 Apr 26; 2009.
18. Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract. 1999;5(3):269-281. https://doi.org/10.1046/j.1365-2753.1999.00203.x.
19. Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675-681. https://doi.org/10.1001/jama.2011.123.
20. Kenyon CC, Melvin PR, Chiang VW, et al. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003.
21. Sobota A, Graham DA, Neufeld EJ, Heeney MM. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children’s hospitals: risk factors and hospital variation. Pediatr Blood Cancer. 2012;58(1):61-65. https://doi.org/10.1002/pbc.23221.
22. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
23. Ginde AA, Espinola JA, Camargo CA. Improved overall trends but persistent racial disparities in emergency department visits for acute asthma, 1993-2005. J Allergy Clin Immunol. 2008;122(2):313-318. https://doi.org/10.1016/j.jaci.2008.04.024.
24. Parikh K, Berry J, Hall M, et al. Racial and ethnic differences in pediatric readmissions for common chronic conditions. J Pediatr. 2017;186. https://doi.org/10.1016/j.jpeds.2017.03.046.
25. Chen BK, Hibbert J, Cheng X, Bennett K. Travel distance and sociodemographic correlates of potentially avoidable emergency department visits in California, 2006-2010: an observational study. Int J Equity Health. 2015;14(1):30. https://doi.org/10.1186/s12939-015-0158-y.
26. Ray KN, Chari AV, Engberg J, et al. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175(12):175(12):1983-1986. https://doi.org/10.1001/jamainternmed.2015.4468.
27. Beck AF, Huang B, Wheeler K, et al. The child opportunity index and disparities in pediatric asthma hospitalizations across one Ohio metropolitan area. J Pediatr. 2011-2013;190:200-206. https://doi.org/10.1016/j.jpeds.2017.08.007.
28. Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: area-based socioeconomic measures for assessing the risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012;102(12):2308-2314. https://doi.org/10.2105/AJPH.2012.300806.
29. Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37-44.e2. https://doi.org/10.1016/j.jaac.2014.10.010.
30. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. https://doi.org/10.1542/peds.2017-1571.
31. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989. https://doi.org/10.1016/j.jaac.2010.05.017.
32. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part II. Treatment and ongoing management. Pediatrics. 2018;141(3):e20174082. https://doi.org/10.1542/peds.2017-4082.
33. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. Practice preparation, identification, assessment, and initial management. Pediatrics. 2018;141(3):e20174081. https://doi.org/10.1542/peds.2017-4081.
34. Dolan MA, Fein JA, Committee on Pediatric Emergency Medicine. Pediatric and adolescent mental health emergencies in the emergency Medical Services system. Pediatrics. 2011;127(5):e1356-e1366. https://doi.org/10.1542/peds.2011-0522.
35. Collaborative Healthcare Strategies. Hospital Guide to Reducing Medicaid Readmissions. Rockville, MD: 2014. https://www.ahrq.gov/sites/default/files/publications/files/medreadmissions.pdf. Accessed 2017 Oct 11.
36. Hilbert K, Payne R, Wooton S. Children’s Hospitals’ Solutions for Patient Safety. Readmissions Bundle Tools. Cincinnati, OH; 2014.
37. Nuckols TK, Keeler E, Morton S, et al. Economic evaluation of quality improvement interventions designed to prevent hospital readmission: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(7):975-985. https://doi.org/10.1001/jamainternmed.2017.1136.
38. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962. https://doi.org/10.1001/jamapediatrics.2014.891.
39. Chen HF, Carlson E, Popoola T, Suzuki S. The impact of rurality on 30-day preventable readmission, illness severity, and risk of mortality for heart failure Medicare home health beneficiaries. J Rural Health. 2016;32(2):176-187. https://doi.org/10.1111/jrh.12142.
40. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-hospital readmission rates as a measure of pediatric quality of care. JAMA Pediatr. 2015;169(10):905-912. https://doi.org/10.1001/jamapediatrics.2015.1129.
41. Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30(4):636-645. https://doi.org/10.1377/hlthaff.2010.0526.
42. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533.
1. Medicare. 30-day death and readmission measures data. https://www.medicare.gov/hospitalcompare/Data/30-day-measures.html. Accessed October 24, 2017.
2. National Quality Forum. Performance Measures; 2016 https://www.quality fourm.org/Measuring_Performance/Endorsed_Performance_Measures_Maintenance.aspx. Accessed October 24, 2017.
3. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (re)admissions network. Pediatrics. 2015;135(1):164-175. https://doi.org/10.1542/peds.2014-1887.
4. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2):e20154182-e20154182. https://doi.org/10.1542/peds.2015-4182.
5. Halfon P, Eggli Y, Prêtre-Rohrbach I, et al. Validation of the potentially avoidable hospital readmission rate as a routine indicator of the quality of hospital care. Med Care. 2006;44(11):972-981. https://doi.org/10.1097/01.mlr.0000228002.43688.c2.
6. Gay JC, Agrawal R, Auger KA, et al. Rates and impact of potentially preventable readmissions at children’s hospitals. J Pediatr. 2015;166(3):613-619. https://doi.org/10.1016/j.jpeds.2014.10.052.
7. Berry JG, Gay JC, Joynt Maddox KJ, et al. Age trends in 30 day hospital readmissions: US national retrospective analysis. BMJ. 2018;360:k497. https://doi.org/10.1136/bmj.k497.
8. Bardach NS, Vittinghoff E, Asteria-Penaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. https://doi.org/10.1542/peds.2012-3527d.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. https://doi.org/10.1001/jama.2012.188351.
10. Gohil SK, Datta R, Cao C, et al. Impact of hospital population case-mix, including poverty, on hospital all-cause and infection-related 30-day readmission rates. Clin Infect Dis. 2015;61(8):1235-1243. https://doi.org/10.1093/cid/civ539.
11. Parikh K, Hall M, Mittal V, et al. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics. 2014;134(3):555-562. https://doi.org/10.1542/peds.2014-1052.
12. Reyes M, Paulus E, Hronek C, et al. Choosing wisely campaign: report card and achievable benchmarks of care for children’s hospitals. Hosp Pediatr. 2017;7(11):633-641. https://doi.org/10.1542/hpeds.2017-0029.
13. Kiefe CI, Weissman NW, Allison JJ, et al. Identifying achievable benchmarks of care: concepts and methodology. Int J Qual Health Care. 1998;10(5):443-447. https://doi.org/10.1093/intqhc/10.5.443.
14. Agency for Healthcare Research and Quality. Nationwide Readmissions Database Availability of Data Elements. . https://www.hcup-us.ahrq.gov/partner/MOARef/HCUPdata_elements.pdf. Accessed 2018 Jun 6
15. Healthcare Cost and Utilization Project. HCUP NRD description of data elements. Agency Healthc Res Qual. https://www.hcup-us.ahrq.gov/db/vars/samedayevent/nrdnote.jsp. Accessed 2018 Jun 6; 2015.
16. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. https://doi.org/10.1186/1471-2431-14-199.
17. Agency for Healthcare Research and Quality. HCUP chronic condition indicator. Healthc Cost Util Proj. https://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Accessed 2016 Apr 26; 2009.
18. Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract. 1999;5(3):269-281. https://doi.org/10.1046/j.1365-2753.1999.00203.x.
19. Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675-681. https://doi.org/10.1001/jama.2011.123.
20. Kenyon CC, Melvin PR, Chiang VW, et al. Rehospitalization for childhood asthma: timing, variation, and opportunities for intervention. J Pediatr. 2014;164(2):300-305. https://doi.org/10.1016/j.jpeds.2013.10.003.
21. Sobota A, Graham DA, Neufeld EJ, Heeney MM. Thirty-day readmission rates following hospitalization for pediatric sickle cell crisis at freestanding children’s hospitals: risk factors and hospital variation. Pediatr Blood Cancer. 2012;58(1):61-65. https://doi.org/10.1002/pbc.23221.
22. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. https://doi.org/10.1001/jama.2011.122.
23. Ginde AA, Espinola JA, Camargo CA. Improved overall trends but persistent racial disparities in emergency department visits for acute asthma, 1993-2005. J Allergy Clin Immunol. 2008;122(2):313-318. https://doi.org/10.1016/j.jaci.2008.04.024.
24. Parikh K, Berry J, Hall M, et al. Racial and ethnic differences in pediatric readmissions for common chronic conditions. J Pediatr. 2017;186. https://doi.org/10.1016/j.jpeds.2017.03.046.
25. Chen BK, Hibbert J, Cheng X, Bennett K. Travel distance and sociodemographic correlates of potentially avoidable emergency department visits in California, 2006-2010: an observational study. Int J Equity Health. 2015;14(1):30. https://doi.org/10.1186/s12939-015-0158-y.
26. Ray KN, Chari AV, Engberg J, et al. Disparities in time spent seeking medical care in the United States. JAMA Intern Med. 2015;175(12):175(12):1983-1986. https://doi.org/10.1001/jamainternmed.2015.4468.
27. Beck AF, Huang B, Wheeler K, et al. The child opportunity index and disparities in pediatric asthma hospitalizations across one Ohio metropolitan area. J Pediatr. 2011-2013;190:200-206. https://doi.org/10.1016/j.jpeds.2017.08.007.
28. Beck AF, Simmons JM, Huang B, Kahn RS. Geomedicine: area-based socioeconomic measures for assessing the risk of hospital reutilization among children admitted for asthma. Am J Public Health. 2012;102(12):2308-2314. https://doi.org/10.2105/AJPH.2012.300806.
29. Avenevoli S, Swendsen J, He JP, Burstein M, Merikangas KR. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J Am Acad Child Adolesc Psychiatry. 2015;54(1):37-44.e2. https://doi.org/10.1016/j.jaac.2014.10.010.
30. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. https://doi.org/10.1542/peds.2017-1571.
31. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989. https://doi.org/10.1016/j.jaac.2010.05.017.
32. Cheung AH, Zuckerbrot RA, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part II. Treatment and ongoing management. Pediatrics. 2018;141(3):e20174082. https://doi.org/10.1542/peds.2017-4082.
33. Zuckerbrot RA, Cheung A, Jensen PS, et al. Guidelines for adolescent depression in primary care (GLAD-PC): Part I. Practice preparation, identification, assessment, and initial management. Pediatrics. 2018;141(3):e20174081. https://doi.org/10.1542/peds.2017-4081.
34. Dolan MA, Fein JA, Committee on Pediatric Emergency Medicine. Pediatric and adolescent mental health emergencies in the emergency Medical Services system. Pediatrics. 2011;127(5):e1356-e1366. https://doi.org/10.1542/peds.2011-0522.
35. Collaborative Healthcare Strategies. Hospital Guide to Reducing Medicaid Readmissions. Rockville, MD: 2014. https://www.ahrq.gov/sites/default/files/publications/files/medreadmissions.pdf. Accessed 2017 Oct 11.
36. Hilbert K, Payne R, Wooton S. Children’s Hospitals’ Solutions for Patient Safety. Readmissions Bundle Tools. Cincinnati, OH; 2014.
37. Nuckols TK, Keeler E, Morton S, et al. Economic evaluation of quality improvement interventions designed to prevent hospital readmission: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(7):975-985. https://doi.org/10.1001/jamainternmed.2017.1136.
38. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962. https://doi.org/10.1001/jamapediatrics.2014.891.
39. Chen HF, Carlson E, Popoola T, Suzuki S. The impact of rurality on 30-day preventable readmission, illness severity, and risk of mortality for heart failure Medicare home health beneficiaries. J Rural Health. 2016;32(2):176-187. https://doi.org/10.1111/jrh.12142.
40. Khan A, Nakamura MM, Zaslavsky AM, et al. Same-hospital readmission rates as a measure of pediatric quality of care. JAMA Pediatr. 2015;169(10):905-912. https://doi.org/10.1001/jamapediatrics.2015.1129.
41. Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff. 2011;30(4):636-645. https://doi.org/10.1377/hlthaff.2010.0526.
42. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533.
© 2019 Society of Hospital Medicine
An Acute Care for Elders Quality Improvement Program for Complex, High-Cost Patients Yields Savings for the System
In 2016, 15.2% of older Americans were hospitalized compared with 7% of the overall population and their length of stay (LOS) was 0.7 days greater.1 Geriatric hospitalizations frequently result in complications, functional decline, nursing home transfers, and increased cost.2-4 This pattern of decline has been termed “hospitalitis” or dysfunctional syndrome.5,6 Hospitals need data-driven approaches to improve outcomes for elders. The Acute Care for Elders (ACE) program, which has been in existence for roughly 25 years, is one such model. ACE features include an environment prepared for older adults, patient-centered care to prevent functional and cognitive decline, frequent medical review to prevent iatrogenic injury or new geriatric syndromes, and early discharge and rehabilitation planning to maximize the likelihood of return to the community.7 Although published data vary somewhat, ACE programs have robust evidence documenting improved safety, quality, and value.8-15 A recent meta-analysis found that ACE programs decrease LOS, costs, new nursing home discharges, falls, delirium, and functional decline.16 However, of the 13 ACE trials reported to date, only five were published in the last decade. Recent rising pressure to decrease hospitalizations and reduce LOS has shifted some care to other settings and it is unclear whether the same results would persist in today’s rapid-paced hospitals.
ACE programs require enhanced resources and restructured care processes but there is a notable lack of data to guide patient selection. Admission criteria vary among the published reports, and information on whether comorbidity burden impacts the magnitude of benefit is scarce. One ACE investigator commented, “We were not able to identify a subgroup of patients who were most likely to benefit.”17 Not all hospitalized older adults can receive ACE care, and some units have closed due to financial and logistic pressures; thus, criteria to target this scarce resource are urgently needed. Our hospital implemented an ACE program in 2014 and we have measured and internally benchmarked important quality improvement metrics. Using this data, we conducted an exploratory analysis to generate hypotheses on the differential impact across the spectrum of cost, LOS, 30-day readmissions, and variations across quartiles of comorbidity severity.
METHODS
Setting and Patients
In September 2014, our 716-bed teaching hospital in Springfield, Massachusetts launched an ACE program to improve care for older adults on a single medical unit. The program succeeded in engaging the senior leadership, and geriatrics was identified as a priority in Baystate’s 5-year strategic plan. ACE patients ≥70 years were admitted from the emergency department with inpatient status. Patients transferred from other units or with advanced dementia or nearing death were excluded. Core components of the ACE program were derived from published summaries (see supplementary material).7,16
Interprofessional ”ACE Rounds”
Interprofessional ACE Rounds occurred every weekday. As one ACE analyst has noted, “the interdisciplinary team…ensures that the multifactorial nature of functional decline is met with a multicomponent plan to prevent it.”18 Rounds participants shifted over time but always included a geriatrics physician assistant (PA) or geriatrician (team leader), a pharmacist, staff nurses, and a chaplain. The nurse educator, dietician, research assistant, and patient advocate/volunteers attended intermittently. Before rounds, the PA reviewed the admission notes for new ACE patients. Initially, rounds were lengthy and included nurse coaching. Later, nurses’ presentations were structured by the SPICES tool (Sleep, Problems with eating/feeding, Incontinence, Confusion, Evidence of falls, Skin Breakdown)19 and tracking and reporting templates. Coaching and education, along with conversations that did not require the full team, were removed from rounds. Thus, the time required for rounds declined from about 75 minutes to 35 minutes, which allowed more patients to be discussed efficiently. This change was critical as the number of ACE patients rose following the shift to the larger unit. The pharmacist reviewed medications focusing on potentially inappropriate drugs. Following rounds, the nurses and pharmacist conveyed recommendations to the hospitalists.
Patient-Centered Activities to Prevent Functional and Cognitive Decline
Project leaders coached staff about the importance of mobility, sleep, and delirium prevention and identification. The nurses screened patients using the Confusion Assessment Method (CAM) and reported delirium promptly. Specific care sets for ACE patients were implemented (see supplementary material).
The project was enhanced by several palliative care components, ie tracking pain, noting psychiatric symptoms, and considering prognosis by posing the “Surprise Question” during rounds.20 (“Would you be surprised if this patient died in the next year?”). As far as staffing and logistics allowed, the goals of care conversation were held by a geriatrics PA with patients/families who “screened in.”
Prepared Environment
The ACE program’s unit was remodeled to facilitate physical and cognitive functioning and promote sleep at night (quiet hours: 10 PM-6 AM).
In accordance with quality improvement processes, iterative shifts were implemented over time in terms of checklist, presentation format, timing, and team participation. In December 2016, the program relocated to a unit with 34 ACE beds and 5 end-of-life beds; this move markedly increased the number of eligible ACE patients.
Study Design, Data Source, and Patients
Since we were implementing and measuring our ACE program with a quality improvement lens, we chose a descriptive cross-sectional study design to generate hypotheses regarding our program’s impact compared to usual care. Using a hospital-wide billing database (McKesson Performance Analytics, v19, Alpharetta, Georgia) we sampled inpatients aged >70 years with a medical Diagnosis Related Group (DRGs) admitted through the emergency department and discharged from a medical unit from September 22, 2014 to August 31, 2017. These criteria mirrored those in the ACE unit. Older adults requiring specialized care (eg, those with myocardial infarct) were excluded, as were those with billing codes for mechanical ventilation, admission to critical care units, or discharge to hospice. Because one of our outcomes was readmission, we excluded patients who died during hospitalization. Patient characteristics collected included demographics and insurance category. To evaluate comorbidity burden, we collected ICD-9/ICD-10 diagnostic codes and generated a combined comorbidity score as described by Gagne, et al.21 This score was devised to predict mortality and 30-day readmissions and had better predictive ability in elders than the Elixhauser or Charlson scores. Scores ranged from −2 to 26, although values >20 are rare.
Exposure
Subjects were categorized as either discharged from the ACE or discharged from usual care. ACE discharges were tracked daily on a spreadsheet that was linked into our sample of eligible subjects.
Outcomes
Total cost of hospitalization (direct plus indirect costs), LOS, and all-cause 30-day readmissions were queried from the same billing database.
Statistical Analysis
As this study was a quality improvement project, analyses were descriptive and exploratory; no statistical hypothesis testing was conducted. We initially evaluated subject characteristics and comorbidities across study groups to determine group balance and comparability using means and standard deviations for continuous data and frequencies and percentages for categorical data. To analyze total cost and LOS, we utilized quantile regression with clustered standard errors to account for clustering by patient. We calculated the median difference between hospitalization cost and LOS for usual care versus ACE patients (with ACE as the referent group). To explore variations across the distributions of outcomes, we determined differences in cost and LOS and their 95% confidence intervals at the 25th, 50th, 75th, and 90th percentiles. Thirty-day readmission risk was estimated using a generalized estimating equation model with a logit link and binomial family. Readmission risk is presented along with 95% confidence intervals. For all models, we initially evaluated change over time (by quarter). After establishing the absence of time trends, we collapsed results into a comparison of usual care versus ACE care. Model estimates are presented both unadjusted and adjusted for age and comorbidity score. Following our initial analyses of cost, LOS, and 30-day readmission risk; we explored differences across quartiles of combined comorbidity scores. We used the same unadjusted models described above but incorporated an interaction term to generate estimates stratified by quartile of comorbidity score. We performed two additional analyses to evaluate the robustness of our findings. First, because hemiplegia prevalence was higher in the usual-care group than in the ACE group and can result in higher cost of care, we repeated the analysis after excluding those patients with hemiplegia. Second, because we were unable to control for functional capacity in the entire sample, we evaluated group differences in mobility for a subsample obtained prior to October 2015 using ICD-9 diagnostic codes, which can be considered surrogate markers for mobility.22 The results of our first analysis did not substantively change our main findings; in our second analysis, groups were balanced by mobility factors which suggested that confounding by functional capacity would be limited in our full sample. The results of these analyses are reported in the supplemental material.
Analysis was completed using Stata v15.1 (StataCorp, LP College Station, Texas). The Baystate Medical Center Institutional Review Board determined that the initiative was quality improvement and “not research.”
RESULTS
A total of 13,209 patients met the initial inclusion criteria; 1,621 were excluded, resulting in a sample of 11,588 patients. Over the 3-year study period, 1,429 (12.3%) were discharged from ACE and 10,159 (87.7%) were discharged from usual care. The groups were similar in age, sex, race and insurance status. Compared with the usual-care group, ACE patients had a higher median comorbidity score (3 vs 2 for usual care) and higher rates for anemia, dementia, fluid and electrolyte disorders, hypertension, and chronic obstructive pulmonary disease (COPD). However, ACE patients had lower rates of hemiplegia (0.9% vs 3%), arrhythmias, and pulmonary circulation disorders than those with usual care (Table 1).
The median cost per ACE patient was slightly lower at $6,258 (interquartile range [IQR] = $4,683-$8,547) versus $6,858 (IQR = $4,855-$10,478) in usual care. Across the cost distribution, the ACE program had lower costs than usual care; however, these differences became more pronounced at the higher end of the distribution. For example, compared with the ACE group, the usual-care group’s unadjusted cost difference was $171 higher at the 25th percentile, $600 higher at the median, $1,932 higher at the 75th percentile, and $3,687 higher at the 90th percentile. The ACE median LOS was 3.7 days (IQR = 2.7-5.0) compared with 3.8 days (IQR = 2.7-6.0) for non-ACE patients. Similar to cost, LOS differences rose at higher percentiles of the distribution, with shorter stays for the ACE patients within each grouping. Compared with the ACE group, the unadjusted LOS difference for usual-care patients ranged from 0 days at the 25th percentile to 0.2 day longer at the median, 1.0 day longer at the 75th percentile, and 1.9 days longer at the 90th percentile. For both cost and LOS models, estimates remained stable after adjusting for age and combined comorbidity score (Table 2).
We explored the impact of increasing comorbidity burden on these outcomes using the following quartiles of the combined comorbidity score: −2 to 0 (387 ACE vs 3,322 usual-care patients), 1 to 2 (264 ACE vs 1,856 usual-care patients), 3 to 5 (476 ACE vs 2,859 usual-care patients), and 6 to 15 (301 ACE vs 2,122 usual-care patients). It was not surprising that cost and LOS paralleled each other, with the greatest cost and LOS benefits in the highest quartile of the combined comorbidity score (Figure 1). For example, at the 90th percentile, the cost difference approached $6,000 higher for the usual-care group in the top quartile of combined comorbidity score compared with nearly $3,000 higher for the lowest quartile. Similarly, at the 90th percentile, LOS for usual-care patients was 2.9 days longer at the top quartile compared with 1.7 days longer at the lowest quartile.
The all-cause 30-day readmission risk was similar for both groups, with an absolute risk difference of −0.7% (95% CI = −2.6% to 1.3%). Adjustment for age and comorbidity score did not substantially change this result. Following stratification by quartile of combined comorbidity scores, we observed similar readmission risks at each quartile (Figure 2).
DISCUSSION
This quality improvement initiative evaluated which ACE admissions yielded the greatest value and found the largest reductions in LOS and cost in patients with the greatest comorbidity scores (frequently referred to as “high need, high cost”).23,24 Based on prior literature, we had anticipated that moderate risk patients would show the maximum benefit.15,25 In contrast to our findings, a University of Alabama (UAB) ACE program subgroup analysis using the CMS Case Mix Index (CMI) found a cost reduction for patients with low or intermediate CMI scores but not for those with high scores.15 The Hospital Elder Life Program (HELP) has yielded maximal impact for patients at moderate risk for delirium.26 Our results are supported by a University of Texas, Houston, study revealing lower LOS and cost for ACE patients, despite high CMI scores and endemic frailty, although it did not report outcomes across a range of comorbidities or costs.27 Our results may be determined by the specific characteristics of the Baystate ACE initiative. Our emphasis on considering prognosis and encouraging advance care planning could have contributed to the improved metrics for more complicated patients. It is possible that patients with high comorbidity burden were more likely to screen in with the surprise question, leading to more frequent goals of care discussions by the hospitalists or geriatrics team, which, in turn, may have resulted in less aggressive care and consequently lower costs. The emphasis on prognosis and palliative care was not a feature of the UAB or Texas studies. Additional components, such as the delirium screening and the presence of volunteer advocates, could also have impacted the results. Our tiered approach during rounds with rapid reviews for most patients and longer discussions for those at highest risk may have further contributed to the findings. Finally, although we did not track the recommendation acceptance rate for the entire study period, in the first 9 months of the project, 9,325 recommendations were made with an acceptance rate of >85%. We previously reported a similar acceptance rate for medication recommendations.28 Another factor contributing to our results may be the ways in which we categorized patients and calculated costs. We used the Gagne combined comorbidity score, which includes only prior conditions;21 the UAB study used CMI, which includes severity of presenting illness and complications, as well as baseline comorbidities. We also compared total cost, while UAB reported variable direct cost.
This study has a number of limitations. First, it was conducted at a single site and may not apply to other hospitals. Second, as a quality improvement program, its design, processes, and personnel evolved over time, and, as in any multicomponent initiative, the effect of individual factors on the outcomes is unknown. Third, this is an observational study with the aim of generating hypotheses for more rigorous studies in the future and residual confounding factors may exist despite efforts to adjust for variables present in an administrative database. Thus, we were unable to completely adjust for potentially important social factors, presence of delirium, or baseline functional status.
To our knowledge, this study is the first report on the differential impact of comorbidity scores and cost distribution on ACE total cost and LOS reductions. Despite its limitations, it contributes to the existing literature by suggesting that the Gagne comorbidity score can help identify which admissions will yield the greatest value. The Gagne score could be calculated at admission using the ePrognosis risk calculator or incorporated and automated in the EMR.29 Many health systems are reluctant to designate beds for specific subpopulations since doing so decreases flexibility and complicates the admission process. A dynamic tension exists between increasing income streams now and generating future savings by supporting initiatives with upfront costs. Other successful acute care geriatrics programs, such as NICHE,30 HELP,31 MACE,32 and consultation teams, exist.33 Studies reporting the outcomes of combining ACE units with these other approaches in a “portfolio approach” will inform the design of the most efficient and impactful programs.34 Scrupulous attention to symptom control and advance care planning are key features of our program, and, given the high prevalence of advanced serious illness in hospitalized older adults, this consideration may be critical for success.
As ACE units can only care for a small fraction of hospitalized older adults, determining which patients will maximally benefit from the structured, team-based care on ACE units is crucial. We found that the greatest impact on LOS and costs occurred in the subgroup with the highest comorbidity scores and overall cost. ACE care for the most vulnerable patients appeared to yield the greatest value for the system; thus, these older adults may need to be prioritized for admission. This improvement may enhance quality and value outcomes, maximize a scarce resource, and secure results needed to sustain the “clinician-led and data-driven” ACE model in the face of changing clinical and financial landscapes.35
Acknowledgments
All those with significant contributions to this work are included as authors.
The authors express their deep appreciation to all their Baystate collaborators, particularly to Rebecca Starr, MD, the first geriatrics medical director of the program, Ms. Virginia Chipps, RN, the program’s first nurse manager, and Tasmiah Chowdhury, PharmD, the program’s first pharmacist. We are also deeply grateful to those persons who provided programmatic advice and input on model ACE programs elsewhere, including Kyle Allen, MD, Michael Malone MD, Robert Palmer MD, and, especially, Kellie Flood, MD.
Disclosures
None of the authors have any existing or potential personal or financial conflicts relevant to this paper to report.
Funding
This work was supported in part by a Geriatric Workforce Enhancement Program award (grant # U1QHP28702) from the Health Resources and Services Administration and by internal support from Baystate Health
1. National Hospital Survey: number and rate of hospital discharge 2010 table. 2010; https://www.cdc.gov/nchs/fastats/hospital.htm. Accessed February, 10th 2019.
2. Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, Newhouse JP, Weiler PC, Hiatt HH. Incidence of adverse events and negligence in hospitalized patients. results of the Harvard medical practice study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
3. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
4. Levinson D. Adverse vents in hospitals: National incidence among Medicare beneficiaries; US Department of Health and Human Services, Office of the Inspector General 2010. Accessed February, 10th, 2019.
5. Palmer RM, Counsell S, Landefeld CS. Clinical intervention trials: the ACE unit. Clin Geriatr Med. 1998;14(4):831-849. PubMed
6. Landefeld CS. Foreword. In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York Humana Press; 2014:v-xii.
7. Fox MT, Persaud M, Maimets I, O’Brien K, Brooks D, Tregunno D, Schraa E. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. https://doi.org/10.1111/jgs.12028.
8. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344. https://doi.org/10.1056/NEJM199505183322006.
9. Covinsky KE, King JT, Jr., Quinn LM, Siddique R, Palmer R, Kresevic DM, Fortinsky RH, Kowal J, Landefeld CS. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc. 1997;45(6):729-734. PubMed
10. Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, Quinn LM, Allen KR, Covinsky KE, Landefeld CS. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572-1581. PubMed
11. Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, Blom JO, Angquist KA. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc. 2000;48(11):1381-1388. PubMed
12. Saltvedt I, Mo ES, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792-798. PubMed
13. Jayadevappa R, Chhatre S, Weiner M, Raziano DB. Health resource utilization and medical care cost of Acute Care Elderly unit patients. Value Health. 2006;9(3):186-192. https://doi.org/10.1111/j.1524-4733.2006.00099.x.
14. Barnes DE, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, Chren MM, Landefeld CS. Acute Care for Elders units produced shorter hospital stays at lower cost while maintaining patients’ functional status. Health Aff (Millwood). 2012;31(6):1227-1236. https://doi.org/10.1377/hlthaff.2012.0142.
15. Flood KL, Maclennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an Acute Care for Elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013;173(11):981-987. https://doi.org/10.1001/jamainternmed.2013.524.
16. Fox MT, Sidani S, Persaud M, Tregunno D, Maimets I, Brooks D, O’Brien K. Acute Care for Elders components of acute geriatric unit care: systematic descriptive review. J Am Geriatr Soc. 2013;61(6):939-946. https://doi.org/10.1111/jgs.12282.
17. Palmer MR, Kresevic DM. The Acute Care for Elders unit In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:92.
18. Pierluissi E, Francis D, Covinsky KE. Patient and hospital factors that lead to adverse outcomes in hospitalized elders In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:42.
19. Fulmer T. How to try this: Fulmer SPICES. Am J Nurs. 2007;107(10):40-48; quiz 48-49. https://doi.org/10.1097/01.NAJ.0000292197.76076.e1.
20. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484-E493. https://doi.org/10.1503/cmaj.160775.
21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2010;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004.
22. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716-722. https://doi.org/10.1097/MLR.0000000000000729.
23. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375(10):909-911. https://doi.org/10.1056/NEJMp1804276.
24. Blumenthal D. Caring for high-need, high-cost patients: what makes for a successful care management program? . https://www.commonwealthfund.org/publications/journal-article/2016/jul/caring-high-need-high-cost-patients-urgent-priority. Accessed March, 20th 2019.
25. Ahmed NN, Pearce SE. Acute Care for the Elderly: a literature review. Popul Health Manag. 2010;13(4):219-225. https://doi.org/10.1089/pop.2009.0058.
26. Inouye SK, Bogardus ST, Jr., Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM, Jr. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
27. Ahmed N, Taylor K, McDaniel Y, Dyer CB. The role of an Acute Care for the Elderly unit in achieving hospital quality indicators while caring for frail hospitalized elders. Popul Health Manag. 2012;15(4):236-240. https://doi.org/10.1089/pop.2011.0055.
28. Chowdhury TP, Starr R, Brennan M, Knee A, Ehresman M, Velayutham L, Malanowski AJ, Courtney HA, Stefan MS. A quality improvement initiative to improve medication management in an Acute Care for Elders program through integration of a clinical pharmacist. J Pharm Pract. 2018:897190018786618. https://doi.org/10.1177/0897190018786618.
29. Lee S, Smith A, Widera E. ePrognosis -Gagne index. https://eprognosis.ucsf.edu/gagne.php. Accessed March 20th, 2019.
30. Turner JT, Lee V, Fletcher K, Hudson K, Barton D. Measuring quality of care with an inpatient elderly population. The geriatric resource nurse model. J Gerontol Nurs. 2001;27(3):8-18. PubMed
31. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. https://doi.org/10.1016/j.jagp.2018.06.007.
32. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. https://doi.org/10.1001/jamainternmed.2013.478.
33. Sennour Y, Counsell SR, Jones J, Weiner M. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139-2145. https://doi.org/10.1111/j.1532-5415.2009.02496.x.
34. Capezuti E, Boltz M. An overview of hospital-based models of care. In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders. New York: Humana Press 2014:49-68.
35. Malone ML, Yoo JW, Goodwin SJ. An introduction to the Acute Care for Elders In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:1-9.
In 2016, 15.2% of older Americans were hospitalized compared with 7% of the overall population and their length of stay (LOS) was 0.7 days greater.1 Geriatric hospitalizations frequently result in complications, functional decline, nursing home transfers, and increased cost.2-4 This pattern of decline has been termed “hospitalitis” or dysfunctional syndrome.5,6 Hospitals need data-driven approaches to improve outcomes for elders. The Acute Care for Elders (ACE) program, which has been in existence for roughly 25 years, is one such model. ACE features include an environment prepared for older adults, patient-centered care to prevent functional and cognitive decline, frequent medical review to prevent iatrogenic injury or new geriatric syndromes, and early discharge and rehabilitation planning to maximize the likelihood of return to the community.7 Although published data vary somewhat, ACE programs have robust evidence documenting improved safety, quality, and value.8-15 A recent meta-analysis found that ACE programs decrease LOS, costs, new nursing home discharges, falls, delirium, and functional decline.16 However, of the 13 ACE trials reported to date, only five were published in the last decade. Recent rising pressure to decrease hospitalizations and reduce LOS has shifted some care to other settings and it is unclear whether the same results would persist in today’s rapid-paced hospitals.
ACE programs require enhanced resources and restructured care processes but there is a notable lack of data to guide patient selection. Admission criteria vary among the published reports, and information on whether comorbidity burden impacts the magnitude of benefit is scarce. One ACE investigator commented, “We were not able to identify a subgroup of patients who were most likely to benefit.”17 Not all hospitalized older adults can receive ACE care, and some units have closed due to financial and logistic pressures; thus, criteria to target this scarce resource are urgently needed. Our hospital implemented an ACE program in 2014 and we have measured and internally benchmarked important quality improvement metrics. Using this data, we conducted an exploratory analysis to generate hypotheses on the differential impact across the spectrum of cost, LOS, 30-day readmissions, and variations across quartiles of comorbidity severity.
METHODS
Setting and Patients
In September 2014, our 716-bed teaching hospital in Springfield, Massachusetts launched an ACE program to improve care for older adults on a single medical unit. The program succeeded in engaging the senior leadership, and geriatrics was identified as a priority in Baystate’s 5-year strategic plan. ACE patients ≥70 years were admitted from the emergency department with inpatient status. Patients transferred from other units or with advanced dementia or nearing death were excluded. Core components of the ACE program were derived from published summaries (see supplementary material).7,16
Interprofessional ”ACE Rounds”
Interprofessional ACE Rounds occurred every weekday. As one ACE analyst has noted, “the interdisciplinary team…ensures that the multifactorial nature of functional decline is met with a multicomponent plan to prevent it.”18 Rounds participants shifted over time but always included a geriatrics physician assistant (PA) or geriatrician (team leader), a pharmacist, staff nurses, and a chaplain. The nurse educator, dietician, research assistant, and patient advocate/volunteers attended intermittently. Before rounds, the PA reviewed the admission notes for new ACE patients. Initially, rounds were lengthy and included nurse coaching. Later, nurses’ presentations were structured by the SPICES tool (Sleep, Problems with eating/feeding, Incontinence, Confusion, Evidence of falls, Skin Breakdown)19 and tracking and reporting templates. Coaching and education, along with conversations that did not require the full team, were removed from rounds. Thus, the time required for rounds declined from about 75 minutes to 35 minutes, which allowed more patients to be discussed efficiently. This change was critical as the number of ACE patients rose following the shift to the larger unit. The pharmacist reviewed medications focusing on potentially inappropriate drugs. Following rounds, the nurses and pharmacist conveyed recommendations to the hospitalists.
Patient-Centered Activities to Prevent Functional and Cognitive Decline
Project leaders coached staff about the importance of mobility, sleep, and delirium prevention and identification. The nurses screened patients using the Confusion Assessment Method (CAM) and reported delirium promptly. Specific care sets for ACE patients were implemented (see supplementary material).
The project was enhanced by several palliative care components, ie tracking pain, noting psychiatric symptoms, and considering prognosis by posing the “Surprise Question” during rounds.20 (“Would you be surprised if this patient died in the next year?”). As far as staffing and logistics allowed, the goals of care conversation were held by a geriatrics PA with patients/families who “screened in.”
Prepared Environment
The ACE program’s unit was remodeled to facilitate physical and cognitive functioning and promote sleep at night (quiet hours: 10 PM-6 AM).
In accordance with quality improvement processes, iterative shifts were implemented over time in terms of checklist, presentation format, timing, and team participation. In December 2016, the program relocated to a unit with 34 ACE beds and 5 end-of-life beds; this move markedly increased the number of eligible ACE patients.
Study Design, Data Source, and Patients
Since we were implementing and measuring our ACE program with a quality improvement lens, we chose a descriptive cross-sectional study design to generate hypotheses regarding our program’s impact compared to usual care. Using a hospital-wide billing database (McKesson Performance Analytics, v19, Alpharetta, Georgia) we sampled inpatients aged >70 years with a medical Diagnosis Related Group (DRGs) admitted through the emergency department and discharged from a medical unit from September 22, 2014 to August 31, 2017. These criteria mirrored those in the ACE unit. Older adults requiring specialized care (eg, those with myocardial infarct) were excluded, as were those with billing codes for mechanical ventilation, admission to critical care units, or discharge to hospice. Because one of our outcomes was readmission, we excluded patients who died during hospitalization. Patient characteristics collected included demographics and insurance category. To evaluate comorbidity burden, we collected ICD-9/ICD-10 diagnostic codes and generated a combined comorbidity score as described by Gagne, et al.21 This score was devised to predict mortality and 30-day readmissions and had better predictive ability in elders than the Elixhauser or Charlson scores. Scores ranged from −2 to 26, although values >20 are rare.
Exposure
Subjects were categorized as either discharged from the ACE or discharged from usual care. ACE discharges were tracked daily on a spreadsheet that was linked into our sample of eligible subjects.
Outcomes
Total cost of hospitalization (direct plus indirect costs), LOS, and all-cause 30-day readmissions were queried from the same billing database.
Statistical Analysis
As this study was a quality improvement project, analyses were descriptive and exploratory; no statistical hypothesis testing was conducted. We initially evaluated subject characteristics and comorbidities across study groups to determine group balance and comparability using means and standard deviations for continuous data and frequencies and percentages for categorical data. To analyze total cost and LOS, we utilized quantile regression with clustered standard errors to account for clustering by patient. We calculated the median difference between hospitalization cost and LOS for usual care versus ACE patients (with ACE as the referent group). To explore variations across the distributions of outcomes, we determined differences in cost and LOS and their 95% confidence intervals at the 25th, 50th, 75th, and 90th percentiles. Thirty-day readmission risk was estimated using a generalized estimating equation model with a logit link and binomial family. Readmission risk is presented along with 95% confidence intervals. For all models, we initially evaluated change over time (by quarter). After establishing the absence of time trends, we collapsed results into a comparison of usual care versus ACE care. Model estimates are presented both unadjusted and adjusted for age and comorbidity score. Following our initial analyses of cost, LOS, and 30-day readmission risk; we explored differences across quartiles of combined comorbidity scores. We used the same unadjusted models described above but incorporated an interaction term to generate estimates stratified by quartile of comorbidity score. We performed two additional analyses to evaluate the robustness of our findings. First, because hemiplegia prevalence was higher in the usual-care group than in the ACE group and can result in higher cost of care, we repeated the analysis after excluding those patients with hemiplegia. Second, because we were unable to control for functional capacity in the entire sample, we evaluated group differences in mobility for a subsample obtained prior to October 2015 using ICD-9 diagnostic codes, which can be considered surrogate markers for mobility.22 The results of our first analysis did not substantively change our main findings; in our second analysis, groups were balanced by mobility factors which suggested that confounding by functional capacity would be limited in our full sample. The results of these analyses are reported in the supplemental material.
Analysis was completed using Stata v15.1 (StataCorp, LP College Station, Texas). The Baystate Medical Center Institutional Review Board determined that the initiative was quality improvement and “not research.”
RESULTS
A total of 13,209 patients met the initial inclusion criteria; 1,621 were excluded, resulting in a sample of 11,588 patients. Over the 3-year study period, 1,429 (12.3%) were discharged from ACE and 10,159 (87.7%) were discharged from usual care. The groups were similar in age, sex, race and insurance status. Compared with the usual-care group, ACE patients had a higher median comorbidity score (3 vs 2 for usual care) and higher rates for anemia, dementia, fluid and electrolyte disorders, hypertension, and chronic obstructive pulmonary disease (COPD). However, ACE patients had lower rates of hemiplegia (0.9% vs 3%), arrhythmias, and pulmonary circulation disorders than those with usual care (Table 1).
The median cost per ACE patient was slightly lower at $6,258 (interquartile range [IQR] = $4,683-$8,547) versus $6,858 (IQR = $4,855-$10,478) in usual care. Across the cost distribution, the ACE program had lower costs than usual care; however, these differences became more pronounced at the higher end of the distribution. For example, compared with the ACE group, the usual-care group’s unadjusted cost difference was $171 higher at the 25th percentile, $600 higher at the median, $1,932 higher at the 75th percentile, and $3,687 higher at the 90th percentile. The ACE median LOS was 3.7 days (IQR = 2.7-5.0) compared with 3.8 days (IQR = 2.7-6.0) for non-ACE patients. Similar to cost, LOS differences rose at higher percentiles of the distribution, with shorter stays for the ACE patients within each grouping. Compared with the ACE group, the unadjusted LOS difference for usual-care patients ranged from 0 days at the 25th percentile to 0.2 day longer at the median, 1.0 day longer at the 75th percentile, and 1.9 days longer at the 90th percentile. For both cost and LOS models, estimates remained stable after adjusting for age and combined comorbidity score (Table 2).
We explored the impact of increasing comorbidity burden on these outcomes using the following quartiles of the combined comorbidity score: −2 to 0 (387 ACE vs 3,322 usual-care patients), 1 to 2 (264 ACE vs 1,856 usual-care patients), 3 to 5 (476 ACE vs 2,859 usual-care patients), and 6 to 15 (301 ACE vs 2,122 usual-care patients). It was not surprising that cost and LOS paralleled each other, with the greatest cost and LOS benefits in the highest quartile of the combined comorbidity score (Figure 1). For example, at the 90th percentile, the cost difference approached $6,000 higher for the usual-care group in the top quartile of combined comorbidity score compared with nearly $3,000 higher for the lowest quartile. Similarly, at the 90th percentile, LOS for usual-care patients was 2.9 days longer at the top quartile compared with 1.7 days longer at the lowest quartile.
The all-cause 30-day readmission risk was similar for both groups, with an absolute risk difference of −0.7% (95% CI = −2.6% to 1.3%). Adjustment for age and comorbidity score did not substantially change this result. Following stratification by quartile of combined comorbidity scores, we observed similar readmission risks at each quartile (Figure 2).
DISCUSSION
This quality improvement initiative evaluated which ACE admissions yielded the greatest value and found the largest reductions in LOS and cost in patients with the greatest comorbidity scores (frequently referred to as “high need, high cost”).23,24 Based on prior literature, we had anticipated that moderate risk patients would show the maximum benefit.15,25 In contrast to our findings, a University of Alabama (UAB) ACE program subgroup analysis using the CMS Case Mix Index (CMI) found a cost reduction for patients with low or intermediate CMI scores but not for those with high scores.15 The Hospital Elder Life Program (HELP) has yielded maximal impact for patients at moderate risk for delirium.26 Our results are supported by a University of Texas, Houston, study revealing lower LOS and cost for ACE patients, despite high CMI scores and endemic frailty, although it did not report outcomes across a range of comorbidities or costs.27 Our results may be determined by the specific characteristics of the Baystate ACE initiative. Our emphasis on considering prognosis and encouraging advance care planning could have contributed to the improved metrics for more complicated patients. It is possible that patients with high comorbidity burden were more likely to screen in with the surprise question, leading to more frequent goals of care discussions by the hospitalists or geriatrics team, which, in turn, may have resulted in less aggressive care and consequently lower costs. The emphasis on prognosis and palliative care was not a feature of the UAB or Texas studies. Additional components, such as the delirium screening and the presence of volunteer advocates, could also have impacted the results. Our tiered approach during rounds with rapid reviews for most patients and longer discussions for those at highest risk may have further contributed to the findings. Finally, although we did not track the recommendation acceptance rate for the entire study period, in the first 9 months of the project, 9,325 recommendations were made with an acceptance rate of >85%. We previously reported a similar acceptance rate for medication recommendations.28 Another factor contributing to our results may be the ways in which we categorized patients and calculated costs. We used the Gagne combined comorbidity score, which includes only prior conditions;21 the UAB study used CMI, which includes severity of presenting illness and complications, as well as baseline comorbidities. We also compared total cost, while UAB reported variable direct cost.
This study has a number of limitations. First, it was conducted at a single site and may not apply to other hospitals. Second, as a quality improvement program, its design, processes, and personnel evolved over time, and, as in any multicomponent initiative, the effect of individual factors on the outcomes is unknown. Third, this is an observational study with the aim of generating hypotheses for more rigorous studies in the future and residual confounding factors may exist despite efforts to adjust for variables present in an administrative database. Thus, we were unable to completely adjust for potentially important social factors, presence of delirium, or baseline functional status.
To our knowledge, this study is the first report on the differential impact of comorbidity scores and cost distribution on ACE total cost and LOS reductions. Despite its limitations, it contributes to the existing literature by suggesting that the Gagne comorbidity score can help identify which admissions will yield the greatest value. The Gagne score could be calculated at admission using the ePrognosis risk calculator or incorporated and automated in the EMR.29 Many health systems are reluctant to designate beds for specific subpopulations since doing so decreases flexibility and complicates the admission process. A dynamic tension exists between increasing income streams now and generating future savings by supporting initiatives with upfront costs. Other successful acute care geriatrics programs, such as NICHE,30 HELP,31 MACE,32 and consultation teams, exist.33 Studies reporting the outcomes of combining ACE units with these other approaches in a “portfolio approach” will inform the design of the most efficient and impactful programs.34 Scrupulous attention to symptom control and advance care planning are key features of our program, and, given the high prevalence of advanced serious illness in hospitalized older adults, this consideration may be critical for success.
As ACE units can only care for a small fraction of hospitalized older adults, determining which patients will maximally benefit from the structured, team-based care on ACE units is crucial. We found that the greatest impact on LOS and costs occurred in the subgroup with the highest comorbidity scores and overall cost. ACE care for the most vulnerable patients appeared to yield the greatest value for the system; thus, these older adults may need to be prioritized for admission. This improvement may enhance quality and value outcomes, maximize a scarce resource, and secure results needed to sustain the “clinician-led and data-driven” ACE model in the face of changing clinical and financial landscapes.35
Acknowledgments
All those with significant contributions to this work are included as authors.
The authors express their deep appreciation to all their Baystate collaborators, particularly to Rebecca Starr, MD, the first geriatrics medical director of the program, Ms. Virginia Chipps, RN, the program’s first nurse manager, and Tasmiah Chowdhury, PharmD, the program’s first pharmacist. We are also deeply grateful to those persons who provided programmatic advice and input on model ACE programs elsewhere, including Kyle Allen, MD, Michael Malone MD, Robert Palmer MD, and, especially, Kellie Flood, MD.
Disclosures
None of the authors have any existing or potential personal or financial conflicts relevant to this paper to report.
Funding
This work was supported in part by a Geriatric Workforce Enhancement Program award (grant # U1QHP28702) from the Health Resources and Services Administration and by internal support from Baystate Health
In 2016, 15.2% of older Americans were hospitalized compared with 7% of the overall population and their length of stay (LOS) was 0.7 days greater.1 Geriatric hospitalizations frequently result in complications, functional decline, nursing home transfers, and increased cost.2-4 This pattern of decline has been termed “hospitalitis” or dysfunctional syndrome.5,6 Hospitals need data-driven approaches to improve outcomes for elders. The Acute Care for Elders (ACE) program, which has been in existence for roughly 25 years, is one such model. ACE features include an environment prepared for older adults, patient-centered care to prevent functional and cognitive decline, frequent medical review to prevent iatrogenic injury or new geriatric syndromes, and early discharge and rehabilitation planning to maximize the likelihood of return to the community.7 Although published data vary somewhat, ACE programs have robust evidence documenting improved safety, quality, and value.8-15 A recent meta-analysis found that ACE programs decrease LOS, costs, new nursing home discharges, falls, delirium, and functional decline.16 However, of the 13 ACE trials reported to date, only five were published in the last decade. Recent rising pressure to decrease hospitalizations and reduce LOS has shifted some care to other settings and it is unclear whether the same results would persist in today’s rapid-paced hospitals.
ACE programs require enhanced resources and restructured care processes but there is a notable lack of data to guide patient selection. Admission criteria vary among the published reports, and information on whether comorbidity burden impacts the magnitude of benefit is scarce. One ACE investigator commented, “We were not able to identify a subgroup of patients who were most likely to benefit.”17 Not all hospitalized older adults can receive ACE care, and some units have closed due to financial and logistic pressures; thus, criteria to target this scarce resource are urgently needed. Our hospital implemented an ACE program in 2014 and we have measured and internally benchmarked important quality improvement metrics. Using this data, we conducted an exploratory analysis to generate hypotheses on the differential impact across the spectrum of cost, LOS, 30-day readmissions, and variations across quartiles of comorbidity severity.
METHODS
Setting and Patients
In September 2014, our 716-bed teaching hospital in Springfield, Massachusetts launched an ACE program to improve care for older adults on a single medical unit. The program succeeded in engaging the senior leadership, and geriatrics was identified as a priority in Baystate’s 5-year strategic plan. ACE patients ≥70 years were admitted from the emergency department with inpatient status. Patients transferred from other units or with advanced dementia or nearing death were excluded. Core components of the ACE program were derived from published summaries (see supplementary material).7,16
Interprofessional ”ACE Rounds”
Interprofessional ACE Rounds occurred every weekday. As one ACE analyst has noted, “the interdisciplinary team…ensures that the multifactorial nature of functional decline is met with a multicomponent plan to prevent it.”18 Rounds participants shifted over time but always included a geriatrics physician assistant (PA) or geriatrician (team leader), a pharmacist, staff nurses, and a chaplain. The nurse educator, dietician, research assistant, and patient advocate/volunteers attended intermittently. Before rounds, the PA reviewed the admission notes for new ACE patients. Initially, rounds were lengthy and included nurse coaching. Later, nurses’ presentations were structured by the SPICES tool (Sleep, Problems with eating/feeding, Incontinence, Confusion, Evidence of falls, Skin Breakdown)19 and tracking and reporting templates. Coaching and education, along with conversations that did not require the full team, were removed from rounds. Thus, the time required for rounds declined from about 75 minutes to 35 minutes, which allowed more patients to be discussed efficiently. This change was critical as the number of ACE patients rose following the shift to the larger unit. The pharmacist reviewed medications focusing on potentially inappropriate drugs. Following rounds, the nurses and pharmacist conveyed recommendations to the hospitalists.
Patient-Centered Activities to Prevent Functional and Cognitive Decline
Project leaders coached staff about the importance of mobility, sleep, and delirium prevention and identification. The nurses screened patients using the Confusion Assessment Method (CAM) and reported delirium promptly. Specific care sets for ACE patients were implemented (see supplementary material).
The project was enhanced by several palliative care components, ie tracking pain, noting psychiatric symptoms, and considering prognosis by posing the “Surprise Question” during rounds.20 (“Would you be surprised if this patient died in the next year?”). As far as staffing and logistics allowed, the goals of care conversation were held by a geriatrics PA with patients/families who “screened in.”
Prepared Environment
The ACE program’s unit was remodeled to facilitate physical and cognitive functioning and promote sleep at night (quiet hours: 10 PM-6 AM).
In accordance with quality improvement processes, iterative shifts were implemented over time in terms of checklist, presentation format, timing, and team participation. In December 2016, the program relocated to a unit with 34 ACE beds and 5 end-of-life beds; this move markedly increased the number of eligible ACE patients.
Study Design, Data Source, and Patients
Since we were implementing and measuring our ACE program with a quality improvement lens, we chose a descriptive cross-sectional study design to generate hypotheses regarding our program’s impact compared to usual care. Using a hospital-wide billing database (McKesson Performance Analytics, v19, Alpharetta, Georgia) we sampled inpatients aged >70 years with a medical Diagnosis Related Group (DRGs) admitted through the emergency department and discharged from a medical unit from September 22, 2014 to August 31, 2017. These criteria mirrored those in the ACE unit. Older adults requiring specialized care (eg, those with myocardial infarct) were excluded, as were those with billing codes for mechanical ventilation, admission to critical care units, or discharge to hospice. Because one of our outcomes was readmission, we excluded patients who died during hospitalization. Patient characteristics collected included demographics and insurance category. To evaluate comorbidity burden, we collected ICD-9/ICD-10 diagnostic codes and generated a combined comorbidity score as described by Gagne, et al.21 This score was devised to predict mortality and 30-day readmissions and had better predictive ability in elders than the Elixhauser or Charlson scores. Scores ranged from −2 to 26, although values >20 are rare.
Exposure
Subjects were categorized as either discharged from the ACE or discharged from usual care. ACE discharges were tracked daily on a spreadsheet that was linked into our sample of eligible subjects.
Outcomes
Total cost of hospitalization (direct plus indirect costs), LOS, and all-cause 30-day readmissions were queried from the same billing database.
Statistical Analysis
As this study was a quality improvement project, analyses were descriptive and exploratory; no statistical hypothesis testing was conducted. We initially evaluated subject characteristics and comorbidities across study groups to determine group balance and comparability using means and standard deviations for continuous data and frequencies and percentages for categorical data. To analyze total cost and LOS, we utilized quantile regression with clustered standard errors to account for clustering by patient. We calculated the median difference between hospitalization cost and LOS for usual care versus ACE patients (with ACE as the referent group). To explore variations across the distributions of outcomes, we determined differences in cost and LOS and their 95% confidence intervals at the 25th, 50th, 75th, and 90th percentiles. Thirty-day readmission risk was estimated using a generalized estimating equation model with a logit link and binomial family. Readmission risk is presented along with 95% confidence intervals. For all models, we initially evaluated change over time (by quarter). After establishing the absence of time trends, we collapsed results into a comparison of usual care versus ACE care. Model estimates are presented both unadjusted and adjusted for age and comorbidity score. Following our initial analyses of cost, LOS, and 30-day readmission risk; we explored differences across quartiles of combined comorbidity scores. We used the same unadjusted models described above but incorporated an interaction term to generate estimates stratified by quartile of comorbidity score. We performed two additional analyses to evaluate the robustness of our findings. First, because hemiplegia prevalence was higher in the usual-care group than in the ACE group and can result in higher cost of care, we repeated the analysis after excluding those patients with hemiplegia. Second, because we were unable to control for functional capacity in the entire sample, we evaluated group differences in mobility for a subsample obtained prior to October 2015 using ICD-9 diagnostic codes, which can be considered surrogate markers for mobility.22 The results of our first analysis did not substantively change our main findings; in our second analysis, groups were balanced by mobility factors which suggested that confounding by functional capacity would be limited in our full sample. The results of these analyses are reported in the supplemental material.
Analysis was completed using Stata v15.1 (StataCorp, LP College Station, Texas). The Baystate Medical Center Institutional Review Board determined that the initiative was quality improvement and “not research.”
RESULTS
A total of 13,209 patients met the initial inclusion criteria; 1,621 were excluded, resulting in a sample of 11,588 patients. Over the 3-year study period, 1,429 (12.3%) were discharged from ACE and 10,159 (87.7%) were discharged from usual care. The groups were similar in age, sex, race and insurance status. Compared with the usual-care group, ACE patients had a higher median comorbidity score (3 vs 2 for usual care) and higher rates for anemia, dementia, fluid and electrolyte disorders, hypertension, and chronic obstructive pulmonary disease (COPD). However, ACE patients had lower rates of hemiplegia (0.9% vs 3%), arrhythmias, and pulmonary circulation disorders than those with usual care (Table 1).
The median cost per ACE patient was slightly lower at $6,258 (interquartile range [IQR] = $4,683-$8,547) versus $6,858 (IQR = $4,855-$10,478) in usual care. Across the cost distribution, the ACE program had lower costs than usual care; however, these differences became more pronounced at the higher end of the distribution. For example, compared with the ACE group, the usual-care group’s unadjusted cost difference was $171 higher at the 25th percentile, $600 higher at the median, $1,932 higher at the 75th percentile, and $3,687 higher at the 90th percentile. The ACE median LOS was 3.7 days (IQR = 2.7-5.0) compared with 3.8 days (IQR = 2.7-6.0) for non-ACE patients. Similar to cost, LOS differences rose at higher percentiles of the distribution, with shorter stays for the ACE patients within each grouping. Compared with the ACE group, the unadjusted LOS difference for usual-care patients ranged from 0 days at the 25th percentile to 0.2 day longer at the median, 1.0 day longer at the 75th percentile, and 1.9 days longer at the 90th percentile. For both cost and LOS models, estimates remained stable after adjusting for age and combined comorbidity score (Table 2).
We explored the impact of increasing comorbidity burden on these outcomes using the following quartiles of the combined comorbidity score: −2 to 0 (387 ACE vs 3,322 usual-care patients), 1 to 2 (264 ACE vs 1,856 usual-care patients), 3 to 5 (476 ACE vs 2,859 usual-care patients), and 6 to 15 (301 ACE vs 2,122 usual-care patients). It was not surprising that cost and LOS paralleled each other, with the greatest cost and LOS benefits in the highest quartile of the combined comorbidity score (Figure 1). For example, at the 90th percentile, the cost difference approached $6,000 higher for the usual-care group in the top quartile of combined comorbidity score compared with nearly $3,000 higher for the lowest quartile. Similarly, at the 90th percentile, LOS for usual-care patients was 2.9 days longer at the top quartile compared with 1.7 days longer at the lowest quartile.
The all-cause 30-day readmission risk was similar for both groups, with an absolute risk difference of −0.7% (95% CI = −2.6% to 1.3%). Adjustment for age and comorbidity score did not substantially change this result. Following stratification by quartile of combined comorbidity scores, we observed similar readmission risks at each quartile (Figure 2).
DISCUSSION
This quality improvement initiative evaluated which ACE admissions yielded the greatest value and found the largest reductions in LOS and cost in patients with the greatest comorbidity scores (frequently referred to as “high need, high cost”).23,24 Based on prior literature, we had anticipated that moderate risk patients would show the maximum benefit.15,25 In contrast to our findings, a University of Alabama (UAB) ACE program subgroup analysis using the CMS Case Mix Index (CMI) found a cost reduction for patients with low or intermediate CMI scores but not for those with high scores.15 The Hospital Elder Life Program (HELP) has yielded maximal impact for patients at moderate risk for delirium.26 Our results are supported by a University of Texas, Houston, study revealing lower LOS and cost for ACE patients, despite high CMI scores and endemic frailty, although it did not report outcomes across a range of comorbidities or costs.27 Our results may be determined by the specific characteristics of the Baystate ACE initiative. Our emphasis on considering prognosis and encouraging advance care planning could have contributed to the improved metrics for more complicated patients. It is possible that patients with high comorbidity burden were more likely to screen in with the surprise question, leading to more frequent goals of care discussions by the hospitalists or geriatrics team, which, in turn, may have resulted in less aggressive care and consequently lower costs. The emphasis on prognosis and palliative care was not a feature of the UAB or Texas studies. Additional components, such as the delirium screening and the presence of volunteer advocates, could also have impacted the results. Our tiered approach during rounds with rapid reviews for most patients and longer discussions for those at highest risk may have further contributed to the findings. Finally, although we did not track the recommendation acceptance rate for the entire study period, in the first 9 months of the project, 9,325 recommendations were made with an acceptance rate of >85%. We previously reported a similar acceptance rate for medication recommendations.28 Another factor contributing to our results may be the ways in which we categorized patients and calculated costs. We used the Gagne combined comorbidity score, which includes only prior conditions;21 the UAB study used CMI, which includes severity of presenting illness and complications, as well as baseline comorbidities. We also compared total cost, while UAB reported variable direct cost.
This study has a number of limitations. First, it was conducted at a single site and may not apply to other hospitals. Second, as a quality improvement program, its design, processes, and personnel evolved over time, and, as in any multicomponent initiative, the effect of individual factors on the outcomes is unknown. Third, this is an observational study with the aim of generating hypotheses for more rigorous studies in the future and residual confounding factors may exist despite efforts to adjust for variables present in an administrative database. Thus, we were unable to completely adjust for potentially important social factors, presence of delirium, or baseline functional status.
To our knowledge, this study is the first report on the differential impact of comorbidity scores and cost distribution on ACE total cost and LOS reductions. Despite its limitations, it contributes to the existing literature by suggesting that the Gagne comorbidity score can help identify which admissions will yield the greatest value. The Gagne score could be calculated at admission using the ePrognosis risk calculator or incorporated and automated in the EMR.29 Many health systems are reluctant to designate beds for specific subpopulations since doing so decreases flexibility and complicates the admission process. A dynamic tension exists between increasing income streams now and generating future savings by supporting initiatives with upfront costs. Other successful acute care geriatrics programs, such as NICHE,30 HELP,31 MACE,32 and consultation teams, exist.33 Studies reporting the outcomes of combining ACE units with these other approaches in a “portfolio approach” will inform the design of the most efficient and impactful programs.34 Scrupulous attention to symptom control and advance care planning are key features of our program, and, given the high prevalence of advanced serious illness in hospitalized older adults, this consideration may be critical for success.
As ACE units can only care for a small fraction of hospitalized older adults, determining which patients will maximally benefit from the structured, team-based care on ACE units is crucial. We found that the greatest impact on LOS and costs occurred in the subgroup with the highest comorbidity scores and overall cost. ACE care for the most vulnerable patients appeared to yield the greatest value for the system; thus, these older adults may need to be prioritized for admission. This improvement may enhance quality and value outcomes, maximize a scarce resource, and secure results needed to sustain the “clinician-led and data-driven” ACE model in the face of changing clinical and financial landscapes.35
Acknowledgments
All those with significant contributions to this work are included as authors.
The authors express their deep appreciation to all their Baystate collaborators, particularly to Rebecca Starr, MD, the first geriatrics medical director of the program, Ms. Virginia Chipps, RN, the program’s first nurse manager, and Tasmiah Chowdhury, PharmD, the program’s first pharmacist. We are also deeply grateful to those persons who provided programmatic advice and input on model ACE programs elsewhere, including Kyle Allen, MD, Michael Malone MD, Robert Palmer MD, and, especially, Kellie Flood, MD.
Disclosures
None of the authors have any existing or potential personal or financial conflicts relevant to this paper to report.
Funding
This work was supported in part by a Geriatric Workforce Enhancement Program award (grant # U1QHP28702) from the Health Resources and Services Administration and by internal support from Baystate Health
1. National Hospital Survey: number and rate of hospital discharge 2010 table. 2010; https://www.cdc.gov/nchs/fastats/hospital.htm. Accessed February, 10th 2019.
2. Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, Newhouse JP, Weiler PC, Hiatt HH. Incidence of adverse events and negligence in hospitalized patients. results of the Harvard medical practice study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
3. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
4. Levinson D. Adverse vents in hospitals: National incidence among Medicare beneficiaries; US Department of Health and Human Services, Office of the Inspector General 2010. Accessed February, 10th, 2019.
5. Palmer RM, Counsell S, Landefeld CS. Clinical intervention trials: the ACE unit. Clin Geriatr Med. 1998;14(4):831-849. PubMed
6. Landefeld CS. Foreword. In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York Humana Press; 2014:v-xii.
7. Fox MT, Persaud M, Maimets I, O’Brien K, Brooks D, Tregunno D, Schraa E. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. https://doi.org/10.1111/jgs.12028.
8. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344. https://doi.org/10.1056/NEJM199505183322006.
9. Covinsky KE, King JT, Jr., Quinn LM, Siddique R, Palmer R, Kresevic DM, Fortinsky RH, Kowal J, Landefeld CS. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc. 1997;45(6):729-734. PubMed
10. Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, Quinn LM, Allen KR, Covinsky KE, Landefeld CS. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572-1581. PubMed
11. Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, Blom JO, Angquist KA. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc. 2000;48(11):1381-1388. PubMed
12. Saltvedt I, Mo ES, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792-798. PubMed
13. Jayadevappa R, Chhatre S, Weiner M, Raziano DB. Health resource utilization and medical care cost of Acute Care Elderly unit patients. Value Health. 2006;9(3):186-192. https://doi.org/10.1111/j.1524-4733.2006.00099.x.
14. Barnes DE, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, Chren MM, Landefeld CS. Acute Care for Elders units produced shorter hospital stays at lower cost while maintaining patients’ functional status. Health Aff (Millwood). 2012;31(6):1227-1236. https://doi.org/10.1377/hlthaff.2012.0142.
15. Flood KL, Maclennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an Acute Care for Elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013;173(11):981-987. https://doi.org/10.1001/jamainternmed.2013.524.
16. Fox MT, Sidani S, Persaud M, Tregunno D, Maimets I, Brooks D, O’Brien K. Acute Care for Elders components of acute geriatric unit care: systematic descriptive review. J Am Geriatr Soc. 2013;61(6):939-946. https://doi.org/10.1111/jgs.12282.
17. Palmer MR, Kresevic DM. The Acute Care for Elders unit In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:92.
18. Pierluissi E, Francis D, Covinsky KE. Patient and hospital factors that lead to adverse outcomes in hospitalized elders In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:42.
19. Fulmer T. How to try this: Fulmer SPICES. Am J Nurs. 2007;107(10):40-48; quiz 48-49. https://doi.org/10.1097/01.NAJ.0000292197.76076.e1.
20. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484-E493. https://doi.org/10.1503/cmaj.160775.
21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2010;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004.
22. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716-722. https://doi.org/10.1097/MLR.0000000000000729.
23. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375(10):909-911. https://doi.org/10.1056/NEJMp1804276.
24. Blumenthal D. Caring for high-need, high-cost patients: what makes for a successful care management program? . https://www.commonwealthfund.org/publications/journal-article/2016/jul/caring-high-need-high-cost-patients-urgent-priority. Accessed March, 20th 2019.
25. Ahmed NN, Pearce SE. Acute Care for the Elderly: a literature review. Popul Health Manag. 2010;13(4):219-225. https://doi.org/10.1089/pop.2009.0058.
26. Inouye SK, Bogardus ST, Jr., Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM, Jr. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
27. Ahmed N, Taylor K, McDaniel Y, Dyer CB. The role of an Acute Care for the Elderly unit in achieving hospital quality indicators while caring for frail hospitalized elders. Popul Health Manag. 2012;15(4):236-240. https://doi.org/10.1089/pop.2011.0055.
28. Chowdhury TP, Starr R, Brennan M, Knee A, Ehresman M, Velayutham L, Malanowski AJ, Courtney HA, Stefan MS. A quality improvement initiative to improve medication management in an Acute Care for Elders program through integration of a clinical pharmacist. J Pharm Pract. 2018:897190018786618. https://doi.org/10.1177/0897190018786618.
29. Lee S, Smith A, Widera E. ePrognosis -Gagne index. https://eprognosis.ucsf.edu/gagne.php. Accessed March 20th, 2019.
30. Turner JT, Lee V, Fletcher K, Hudson K, Barton D. Measuring quality of care with an inpatient elderly population. The geriatric resource nurse model. J Gerontol Nurs. 2001;27(3):8-18. PubMed
31. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. https://doi.org/10.1016/j.jagp.2018.06.007.
32. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. https://doi.org/10.1001/jamainternmed.2013.478.
33. Sennour Y, Counsell SR, Jones J, Weiner M. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139-2145. https://doi.org/10.1111/j.1532-5415.2009.02496.x.
34. Capezuti E, Boltz M. An overview of hospital-based models of care. In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders. New York: Humana Press 2014:49-68.
35. Malone ML, Yoo JW, Goodwin SJ. An introduction to the Acute Care for Elders In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:1-9.
1. National Hospital Survey: number and rate of hospital discharge 2010 table. 2010; https://www.cdc.gov/nchs/fastats/hospital.htm. Accessed February, 10th 2019.
2. Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, Newhouse JP, Weiler PC, Hiatt HH. Incidence of adverse events and negligence in hospitalized patients. results of the Harvard medical practice study I. N Engl J Med. 1991;324(6):370-376. https://doi.org/10.1056/NEJM199102073240604.
3. Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118(3):219-223. PubMed
4. Levinson D. Adverse vents in hospitals: National incidence among Medicare beneficiaries; US Department of Health and Human Services, Office of the Inspector General 2010. Accessed February, 10th, 2019.
5. Palmer RM, Counsell S, Landefeld CS. Clinical intervention trials: the ACE unit. Clin Geriatr Med. 1998;14(4):831-849. PubMed
6. Landefeld CS. Foreword. In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York Humana Press; 2014:v-xii.
7. Fox MT, Persaud M, Maimets I, O’Brien K, Brooks D, Tregunno D, Schraa E. Effectiveness of acute geriatric unit care using acute care for elders components: a systematic review and meta-analysis. J Am Geriatr Soc. 2012;60(12):2237-2245. https://doi.org/10.1111/jgs.12028.
8. Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332(20):1338-1344. https://doi.org/10.1056/NEJM199505183322006.
9. Covinsky KE, King JT, Jr., Quinn LM, Siddique R, Palmer R, Kresevic DM, Fortinsky RH, Kowal J, Landefeld CS. Do acute care for elders units increase hospital costs? A cost analysis using the hospital perspective. J Am Geriatr Soc. 1997;45(6):729-734. PubMed
10. Counsell SR, Holder CM, Liebenauer LL, Palmer RM, Fortinsky RH, Kresevic DM, Quinn LM, Allen KR, Covinsky KE, Landefeld CS. Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: a randomized controlled trial of Acute Care for Elders (ACE) in a community hospital. J Am Geriatr Soc. 2000;48(12):1572-1581. PubMed
11. Asplund K, Gustafson Y, Jacobsson C, Bucht G, Wahlin A, Peterson J, Blom JO, Angquist KA. Geriatric-based versus general wards for older acute medical patients: a randomized comparison of outcomes and use of resources. J Am Geriatr Soc. 2000;48(11):1381-1388. PubMed
12. Saltvedt I, Mo ES, Fayers P, Kaasa S, Sletvold O. Reduced mortality in treating acutely sick, frail older patients in a geriatric evaluation and management unit. A prospective randomized trial. J Am Geriatr Soc. 2002;50(5):792-798. PubMed
13. Jayadevappa R, Chhatre S, Weiner M, Raziano DB. Health resource utilization and medical care cost of Acute Care Elderly unit patients. Value Health. 2006;9(3):186-192. https://doi.org/10.1111/j.1524-4733.2006.00099.x.
14. Barnes DE, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J, Chren MM, Landefeld CS. Acute Care for Elders units produced shorter hospital stays at lower cost while maintaining patients’ functional status. Health Aff (Millwood). 2012;31(6):1227-1236. https://doi.org/10.1377/hlthaff.2012.0142.
15. Flood KL, Maclennan PA, McGrew D, Green D, Dodd C, Brown CJ. Effects of an Acute Care for Elders unit on costs and 30-day readmissions. JAMA Intern Med. 2013;173(11):981-987. https://doi.org/10.1001/jamainternmed.2013.524.
16. Fox MT, Sidani S, Persaud M, Tregunno D, Maimets I, Brooks D, O’Brien K. Acute Care for Elders components of acute geriatric unit care: systematic descriptive review. J Am Geriatr Soc. 2013;61(6):939-946. https://doi.org/10.1111/jgs.12282.
17. Palmer MR, Kresevic DM. The Acute Care for Elders unit In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:92.
18. Pierluissi E, Francis D, Covinsky KE. Patient and hospital factors that lead to adverse outcomes in hospitalized elders In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:42.
19. Fulmer T. How to try this: Fulmer SPICES. Am J Nurs. 2007;107(10):40-48; quiz 48-49. https://doi.org/10.1097/01.NAJ.0000292197.76076.e1.
20. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484-E493. https://doi.org/10.1503/cmaj.160775.
21. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2010;64(7):749-759. doi: 10.1016/j.jclinepi.2010.10.004.
22. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care. 2017;55(7):716-722. https://doi.org/10.1097/MLR.0000000000000729.
23. Blumenthal D, Chernof B, Fulmer T, Lumpkin J, Selberg J. Caring for high-need, high-cost patients - an urgent priority. N Engl J Med. 2016;375(10):909-911. https://doi.org/10.1056/NEJMp1804276.
24. Blumenthal D. Caring for high-need, high-cost patients: what makes for a successful care management program? . https://www.commonwealthfund.org/publications/journal-article/2016/jul/caring-high-need-high-cost-patients-urgent-priority. Accessed March, 20th 2019.
25. Ahmed NN, Pearce SE. Acute Care for the Elderly: a literature review. Popul Health Manag. 2010;13(4):219-225. https://doi.org/10.1089/pop.2009.0058.
26. Inouye SK, Bogardus ST, Jr., Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM, Jr. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
27. Ahmed N, Taylor K, McDaniel Y, Dyer CB. The role of an Acute Care for the Elderly unit in achieving hospital quality indicators while caring for frail hospitalized elders. Popul Health Manag. 2012;15(4):236-240. https://doi.org/10.1089/pop.2011.0055.
28. Chowdhury TP, Starr R, Brennan M, Knee A, Ehresman M, Velayutham L, Malanowski AJ, Courtney HA, Stefan MS. A quality improvement initiative to improve medication management in an Acute Care for Elders program through integration of a clinical pharmacist. J Pharm Pract. 2018:897190018786618. https://doi.org/10.1177/0897190018786618.
29. Lee S, Smith A, Widera E. ePrognosis -Gagne index. https://eprognosis.ucsf.edu/gagne.php. Accessed March 20th, 2019.
30. Turner JT, Lee V, Fletcher K, Hudson K, Barton D. Measuring quality of care with an inpatient elderly population. The geriatric resource nurse model. J Gerontol Nurs. 2001;27(3):8-18. PubMed
31. Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015-1033. https://doi.org/10.1016/j.jagp.2018.06.007.
32. Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA Intern Med. 2013;173(11):990-996. https://doi.org/10.1001/jamainternmed.2013.478.
33. Sennour Y, Counsell SR, Jones J, Weiner M. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57(11):2139-2145. https://doi.org/10.1111/j.1532-5415.2009.02496.x.
34. Capezuti E, Boltz M. An overview of hospital-based models of care. In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders. New York: Humana Press 2014:49-68.
35. Malone ML, Yoo JW, Goodwin SJ. An introduction to the Acute Care for Elders In: Malone ML, Palmer MR, Capezuti E, eds. Acute Care for Elders New York: Humana Press 2014:1-9.
© 2019 Society of Hospital Medicine
Retrospective Cohort Study of the Prevalence of Off-label Gabapentinoid Prescriptions in Hospitalized Medical Patients
In the1990s, gabapentin was licensed in the United States as an anticonvulsant and it became widely successful in the mid-2000s when marketed for the treatment of pain. Since then, prescriptions for gabapentinoids have accelerated dramatically.1,2 Between 2012 and 2016, the total spending on pregabalin in the United States increased from $1.9 to $4.4 billion, with pregabalin ranking eighth overall for specific drug spending.3
Despite a finite number of indications, there has been a steady rise in off-label use, with an increased risk of adverse drug events (ADEs).4,5 Several meta-analyses suggest either low-quality or no evidence of benefit for gabapentinoid use in settings including neuropathic pain in cancer, sciatica, and chronic low back pain.6-8 Lack of efficacy is compounded by adverse effects such as altered mental status, fluid retention, sedation, and increased risk of traumatic falls in older adults.6,9,10 Finally, dependency is a concern; opioids are coprescribed in up to 50% of patients,11 increasing the odds of opioid-related death by up to 60%.12
To better characterize gabapentinoid use in hospitalized patients, we analyzed a retrospective cohort of patients admitted to our tertiary care medical teaching unit, examining preadmission and in-hospital prescribing trends, off-label use, and deprescribing.
METHODS
Patient data were collected from a retrospective cohort, including all consecutive admissions to our 52-bed medical clinical teaching unit in Montréal, Canada, since December 2013.13 We reviewed admissions between December 17, 2013 and June 30, 2017 and identified three populations of gabapentinoid users from medication reconciliation documents: preadmission users continued at discharge, preadmission users deprescribed in hospital, and new in-hospital users continued at discharge. Deprescribing was defined as having the drug stopped at discharge or a prescribed taper that included stopping. The term “gabapentinoid users” refers to preadmission gabapentinoid use.
Gabapentinoid users were compared with nonusers with regard to demographic characteristics; select comorbidities; coprescription of opioids, benzodiazepines, and Z-drugs; length of stay (LOS); and inpatient mortality. Only the first eligible admission per patient was considered. Patients who had multiple admissions over the period of interest were classified as “users” in the patient-level analyses if they were taking a gabapentinoid at home or at discharge on at least one admission.
Doses and indications were collected from medication reconciliation performed by a clinical pharmacist, which included an interview with the patient or a proxy and a review of the indications for all drugs. These data were merged with any additional potential indications found in the admission notes (listing all chronic conditions from a detailed medical history) and review of the electronic medical record. The US Food and Drug Administration (FDA) approved the indications and the recommended doses were taken from product monographs and compared with doses prescribed to patients. When documented, the reason for new prescriptions and justification for deprescribing at discharge were manually abstracted from discharge summaries and medication reconciliation documents.
Continuous variables were expressed as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 test. Proportions of gabapentinoid use and deprescribing, including 95% confidence intervals around each proportion, were plotted and linear regression was performed versus fiscal quarter to evaluate for temporal trends. A two-sided α value of 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas). The McGill University Health Centre Research Ethics Board approved this study.
RESULTS
A total of 4,103 unique patients were admitted from December 2013 to July 2017, of whom 550 (13.4%) were receiving a gabapentinoid before admission. Two preadmission users were coprescribed gabapentin and pregabalin for a total of 552 prescriptions. The prevalence of preadmission gabapentinoid use remained steady during the period of interest (Appendix 1; P = .29 for temporal trend). There were no significant differences between gabapentinoid users and nonusers with regard to age or sex, but users had a higher prevalence of chronic disease (
The indications for gabapentinoid use are presented in Table 2. Only a minority (17% or 94/552) had an approved indication. Among these 94 patients, 38 (40%) received FDA-recommended doses, 47 (50%) received doses below those demonstrated to be effective, and 9 (10%) received higher-than-recommended doses. New prescriptions at discharge were observed in 1.5% of patients, with the majority given for off-label indications (Appendix 2).
DISCUSSION
In this large cohort study of hospitalized medical patients, preadmission gabapentinoid use was present in one in every eight admitted patients. Most patients had off-label indications, including the small number of patients who had the drug started in hospital. Even for approved indications, the doses were often lower than what trials have suggested to be effective. Finally, although we have demonstrated that deprescribing occurred, it was uncommon and either precipitated by an adverse event or the justification was poorly documented.
To our knowledge, our study is one of the first to examine what happens to gabapentinoids in hospitalized patients and we present important new data with respect to dosing and prescribing patterns. The low rates of discontinuation, intent to taper, or dose decreases in our cohort represent a potential area of improvement in deprescribing.
Deprescribing should be considered for patients with serious adverse events, for whom less serious adverse effects preclude achieving clinically effective doses, and for those who do not perceive benefit. Given the magnitude of the problems presented by polypharmacy, we propose that stopping priority be given to off-label use (especially when clinically ineffective) and for patients coprescribed opioids or sedatives. Up to a third of users in our cohort were coprescribed opioids or benzodiazepines, which is particularly concerning given the association with increased opioid-related mortality.12,15 Although we did not observe a difference in inpatient mortality, such a study is underpowered for this outcome especially when considering the competing risks of death in hospital. Importantly, when deprescribing, the drug should be tapered over several weeks to limit symptoms of withdrawal and to prevent seizure.11
Presumed off-label use and subtherapeutic doses were common in our cohort, with only 17% of users having a clearly documented FDA-approved indication, in agreement with a previous study that reported only 5% on-label use.4 High doses of gabapentinoids required for efficacy in clinical trials may be difficult to achieve because of dose-limiting side effects, which may explain the relatively low median doses recorded in our real-world cohort. Another possibility is that frail, older patients with renal dysfunction experience effectiveness at lower median doses than those quoted from study populations. In our study, patients on lower doses of gabapentinoids had a higher prevalence of stage IV or V chronic kidney disease (CKD). Stage IV/V CKD was identified in 16/47 (34.0%) patients on lower doses of gabapentinoids, compared to 4/38 (10.5%) on doses within the FDA-recommended range.
Our study has limitations; findings from a single Canadian tertiary care hospital may not be generalizable to other hospitals or countries, particularly given the differences between the Canadian and US health systems. Indications were extracted from the patient chart and even with the best possible medication history and thorough review, sometimes they had to be inferred. Caution should also be exercised when interpreting the omission of an indication as equating to a lack of justifiable medication use; however, the rate of off-label use in our cohort is in agreement with prior research.4 Moreover, with a retrospective design, the effectiveness of the drug on an individual basis could not be assessed, which would have allowed a more precise estimate of the proportion of patients for whom deprescribing might have been appropriate. The strengths of this study include a large sample of real-world, heterogeneous, general medical patients spanning several years and our use of trained pharmacists and physicians to determine the drug indication as opposed to reliance on administrative data.
CONCLUSION
Gabapentinoid use was frequent in our cohort of hospitalized medical patients, with a high prevalence of off-label use, subtherapeutic doses, and coadministration with opioids and benzodiazepines. Deprescribing at discharge was uncommon and often triggered by an adverse event. The identification of gabapentinoids during hospitalization is an opportunity to reevaluate the indication for the drug, assess for effectiveness, and consider deprescribing to help reduce polypharmacy and ideally ADEs.
Acknowledgment
For the purposes of authorship, Dr. McDonald and Dr. Lee contributed equally.
Disclosures
Dr. Emily McDonald and Dr. Todd Lee have a patent pending for MedSafer, a deprescribing software, and both receive research salary support from the Fonds de Recherche Santé du Québec. Dr. Gingras, Dr. Lieu, and Dr. Papillon-Ferland have nothing to disclose.
1. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. https://doi.org/10.1001/jamainternmed.2017.7856.
2. Kwok H, Khuu W, Fernandes K, et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019-1026. https://doi.org/10.1093/pm/pnw351.
3. Medicines use and spending in the U.S. — a review of 2016 and outlook to 2021: IMS Institute for Healthcare Informatics; 2017. https://structurecms-staging-psyclone.netdna-ssl.com/client_assets/dwonk/media/attachments/590c/6aa0/6970/2d2d/4182/0000/590c6aa069702d2d41820000.pdf?1493985952. Accessed March 21, 2019.
4. Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. J Manag Care Pharm. 2002;8(4):266-271. doi: 10.18553/jmcp.2002.8.4.266.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55-63. https://doi.org/10.1001/jamainternmed.2015.6058.
6. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. https://doi.org/10.1371/journal.pmed.1002369.
7. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793. https://doi.org/10.1503/cmaj.171333.
8. Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med. 2018;32(1):276-286. https://doi.org/10.1177/0269216317711826.
9. Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68(6):903-912. https://doi.org/10.1007/s00228-012-1213-x.
10. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359-376. https://doi.org/10.2165/11599460-000000000-00000.
11. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. https://doi.org/10.1007/s40265-017-0700-x.
12. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396.
13. McDonald EG, Saleh RR, Lee TC. Ezetimibe use remains common among medical inpatients. Am J Med. 2015;128(2):193-195. https://doi.org/10.1016/j.amjmed.2014.10.016.
14. U.S. Food and Drug Administration. LYRICA - Highlights of Prescribing Information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf. Accessed April 30, 2019.
15. U.S. Food and Drug Administration. NEURONTIN - Highlights of Prescribing Information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed April 30, 2019.
16. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732-734. https://doi.org/10.7326/M18-1136.
In the1990s, gabapentin was licensed in the United States as an anticonvulsant and it became widely successful in the mid-2000s when marketed for the treatment of pain. Since then, prescriptions for gabapentinoids have accelerated dramatically.1,2 Between 2012 and 2016, the total spending on pregabalin in the United States increased from $1.9 to $4.4 billion, with pregabalin ranking eighth overall for specific drug spending.3
Despite a finite number of indications, there has been a steady rise in off-label use, with an increased risk of adverse drug events (ADEs).4,5 Several meta-analyses suggest either low-quality or no evidence of benefit for gabapentinoid use in settings including neuropathic pain in cancer, sciatica, and chronic low back pain.6-8 Lack of efficacy is compounded by adverse effects such as altered mental status, fluid retention, sedation, and increased risk of traumatic falls in older adults.6,9,10 Finally, dependency is a concern; opioids are coprescribed in up to 50% of patients,11 increasing the odds of opioid-related death by up to 60%.12
To better characterize gabapentinoid use in hospitalized patients, we analyzed a retrospective cohort of patients admitted to our tertiary care medical teaching unit, examining preadmission and in-hospital prescribing trends, off-label use, and deprescribing.
METHODS
Patient data were collected from a retrospective cohort, including all consecutive admissions to our 52-bed medical clinical teaching unit in Montréal, Canada, since December 2013.13 We reviewed admissions between December 17, 2013 and June 30, 2017 and identified three populations of gabapentinoid users from medication reconciliation documents: preadmission users continued at discharge, preadmission users deprescribed in hospital, and new in-hospital users continued at discharge. Deprescribing was defined as having the drug stopped at discharge or a prescribed taper that included stopping. The term “gabapentinoid users” refers to preadmission gabapentinoid use.
Gabapentinoid users were compared with nonusers with regard to demographic characteristics; select comorbidities; coprescription of opioids, benzodiazepines, and Z-drugs; length of stay (LOS); and inpatient mortality. Only the first eligible admission per patient was considered. Patients who had multiple admissions over the period of interest were classified as “users” in the patient-level analyses if they were taking a gabapentinoid at home or at discharge on at least one admission.
Doses and indications were collected from medication reconciliation performed by a clinical pharmacist, which included an interview with the patient or a proxy and a review of the indications for all drugs. These data were merged with any additional potential indications found in the admission notes (listing all chronic conditions from a detailed medical history) and review of the electronic medical record. The US Food and Drug Administration (FDA) approved the indications and the recommended doses were taken from product monographs and compared with doses prescribed to patients. When documented, the reason for new prescriptions and justification for deprescribing at discharge were manually abstracted from discharge summaries and medication reconciliation documents.
Continuous variables were expressed as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 test. Proportions of gabapentinoid use and deprescribing, including 95% confidence intervals around each proportion, were plotted and linear regression was performed versus fiscal quarter to evaluate for temporal trends. A two-sided α value of 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas). The McGill University Health Centre Research Ethics Board approved this study.
RESULTS
A total of 4,103 unique patients were admitted from December 2013 to July 2017, of whom 550 (13.4%) were receiving a gabapentinoid before admission. Two preadmission users were coprescribed gabapentin and pregabalin for a total of 552 prescriptions. The prevalence of preadmission gabapentinoid use remained steady during the period of interest (Appendix 1; P = .29 for temporal trend). There were no significant differences between gabapentinoid users and nonusers with regard to age or sex, but users had a higher prevalence of chronic disease (
The indications for gabapentinoid use are presented in Table 2. Only a minority (17% or 94/552) had an approved indication. Among these 94 patients, 38 (40%) received FDA-recommended doses, 47 (50%) received doses below those demonstrated to be effective, and 9 (10%) received higher-than-recommended doses. New prescriptions at discharge were observed in 1.5% of patients, with the majority given for off-label indications (Appendix 2).
DISCUSSION
In this large cohort study of hospitalized medical patients, preadmission gabapentinoid use was present in one in every eight admitted patients. Most patients had off-label indications, including the small number of patients who had the drug started in hospital. Even for approved indications, the doses were often lower than what trials have suggested to be effective. Finally, although we have demonstrated that deprescribing occurred, it was uncommon and either precipitated by an adverse event or the justification was poorly documented.
To our knowledge, our study is one of the first to examine what happens to gabapentinoids in hospitalized patients and we present important new data with respect to dosing and prescribing patterns. The low rates of discontinuation, intent to taper, or dose decreases in our cohort represent a potential area of improvement in deprescribing.
Deprescribing should be considered for patients with serious adverse events, for whom less serious adverse effects preclude achieving clinically effective doses, and for those who do not perceive benefit. Given the magnitude of the problems presented by polypharmacy, we propose that stopping priority be given to off-label use (especially when clinically ineffective) and for patients coprescribed opioids or sedatives. Up to a third of users in our cohort were coprescribed opioids or benzodiazepines, which is particularly concerning given the association with increased opioid-related mortality.12,15 Although we did not observe a difference in inpatient mortality, such a study is underpowered for this outcome especially when considering the competing risks of death in hospital. Importantly, when deprescribing, the drug should be tapered over several weeks to limit symptoms of withdrawal and to prevent seizure.11
Presumed off-label use and subtherapeutic doses were common in our cohort, with only 17% of users having a clearly documented FDA-approved indication, in agreement with a previous study that reported only 5% on-label use.4 High doses of gabapentinoids required for efficacy in clinical trials may be difficult to achieve because of dose-limiting side effects, which may explain the relatively low median doses recorded in our real-world cohort. Another possibility is that frail, older patients with renal dysfunction experience effectiveness at lower median doses than those quoted from study populations. In our study, patients on lower doses of gabapentinoids had a higher prevalence of stage IV or V chronic kidney disease (CKD). Stage IV/V CKD was identified in 16/47 (34.0%) patients on lower doses of gabapentinoids, compared to 4/38 (10.5%) on doses within the FDA-recommended range.
Our study has limitations; findings from a single Canadian tertiary care hospital may not be generalizable to other hospitals or countries, particularly given the differences between the Canadian and US health systems. Indications were extracted from the patient chart and even with the best possible medication history and thorough review, sometimes they had to be inferred. Caution should also be exercised when interpreting the omission of an indication as equating to a lack of justifiable medication use; however, the rate of off-label use in our cohort is in agreement with prior research.4 Moreover, with a retrospective design, the effectiveness of the drug on an individual basis could not be assessed, which would have allowed a more precise estimate of the proportion of patients for whom deprescribing might have been appropriate. The strengths of this study include a large sample of real-world, heterogeneous, general medical patients spanning several years and our use of trained pharmacists and physicians to determine the drug indication as opposed to reliance on administrative data.
CONCLUSION
Gabapentinoid use was frequent in our cohort of hospitalized medical patients, with a high prevalence of off-label use, subtherapeutic doses, and coadministration with opioids and benzodiazepines. Deprescribing at discharge was uncommon and often triggered by an adverse event. The identification of gabapentinoids during hospitalization is an opportunity to reevaluate the indication for the drug, assess for effectiveness, and consider deprescribing to help reduce polypharmacy and ideally ADEs.
Acknowledgment
For the purposes of authorship, Dr. McDonald and Dr. Lee contributed equally.
Disclosures
Dr. Emily McDonald and Dr. Todd Lee have a patent pending for MedSafer, a deprescribing software, and both receive research salary support from the Fonds de Recherche Santé du Québec. Dr. Gingras, Dr. Lieu, and Dr. Papillon-Ferland have nothing to disclose.
In the1990s, gabapentin was licensed in the United States as an anticonvulsant and it became widely successful in the mid-2000s when marketed for the treatment of pain. Since then, prescriptions for gabapentinoids have accelerated dramatically.1,2 Between 2012 and 2016, the total spending on pregabalin in the United States increased from $1.9 to $4.4 billion, with pregabalin ranking eighth overall for specific drug spending.3
Despite a finite number of indications, there has been a steady rise in off-label use, with an increased risk of adverse drug events (ADEs).4,5 Several meta-analyses suggest either low-quality or no evidence of benefit for gabapentinoid use in settings including neuropathic pain in cancer, sciatica, and chronic low back pain.6-8 Lack of efficacy is compounded by adverse effects such as altered mental status, fluid retention, sedation, and increased risk of traumatic falls in older adults.6,9,10 Finally, dependency is a concern; opioids are coprescribed in up to 50% of patients,11 increasing the odds of opioid-related death by up to 60%.12
To better characterize gabapentinoid use in hospitalized patients, we analyzed a retrospective cohort of patients admitted to our tertiary care medical teaching unit, examining preadmission and in-hospital prescribing trends, off-label use, and deprescribing.
METHODS
Patient data were collected from a retrospective cohort, including all consecutive admissions to our 52-bed medical clinical teaching unit in Montréal, Canada, since December 2013.13 We reviewed admissions between December 17, 2013 and June 30, 2017 and identified three populations of gabapentinoid users from medication reconciliation documents: preadmission users continued at discharge, preadmission users deprescribed in hospital, and new in-hospital users continued at discharge. Deprescribing was defined as having the drug stopped at discharge or a prescribed taper that included stopping. The term “gabapentinoid users” refers to preadmission gabapentinoid use.
Gabapentinoid users were compared with nonusers with regard to demographic characteristics; select comorbidities; coprescription of opioids, benzodiazepines, and Z-drugs; length of stay (LOS); and inpatient mortality. Only the first eligible admission per patient was considered. Patients who had multiple admissions over the period of interest were classified as “users” in the patient-level analyses if they were taking a gabapentinoid at home or at discharge on at least one admission.
Doses and indications were collected from medication reconciliation performed by a clinical pharmacist, which included an interview with the patient or a proxy and a review of the indications for all drugs. These data were merged with any additional potential indications found in the admission notes (listing all chronic conditions from a detailed medical history) and review of the electronic medical record. The US Food and Drug Administration (FDA) approved the indications and the recommended doses were taken from product monographs and compared with doses prescribed to patients. When documented, the reason for new prescriptions and justification for deprescribing at discharge were manually abstracted from discharge summaries and medication reconciliation documents.
Continuous variables were expressed as median and interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 test. Proportions of gabapentinoid use and deprescribing, including 95% confidence intervals around each proportion, were plotted and linear regression was performed versus fiscal quarter to evaluate for temporal trends. A two-sided α value of 0.05 was considered to be statistically significant. Statistical analyses were performed using Stata version 15 (StataCorp LLC, College Station, Texas). The McGill University Health Centre Research Ethics Board approved this study.
RESULTS
A total of 4,103 unique patients were admitted from December 2013 to July 2017, of whom 550 (13.4%) were receiving a gabapentinoid before admission. Two preadmission users were coprescribed gabapentin and pregabalin for a total of 552 prescriptions. The prevalence of preadmission gabapentinoid use remained steady during the period of interest (Appendix 1; P = .29 for temporal trend). There were no significant differences between gabapentinoid users and nonusers with regard to age or sex, but users had a higher prevalence of chronic disease (
The indications for gabapentinoid use are presented in Table 2. Only a minority (17% or 94/552) had an approved indication. Among these 94 patients, 38 (40%) received FDA-recommended doses, 47 (50%) received doses below those demonstrated to be effective, and 9 (10%) received higher-than-recommended doses. New prescriptions at discharge were observed in 1.5% of patients, with the majority given for off-label indications (Appendix 2).
DISCUSSION
In this large cohort study of hospitalized medical patients, preadmission gabapentinoid use was present in one in every eight admitted patients. Most patients had off-label indications, including the small number of patients who had the drug started in hospital. Even for approved indications, the doses were often lower than what trials have suggested to be effective. Finally, although we have demonstrated that deprescribing occurred, it was uncommon and either precipitated by an adverse event or the justification was poorly documented.
To our knowledge, our study is one of the first to examine what happens to gabapentinoids in hospitalized patients and we present important new data with respect to dosing and prescribing patterns. The low rates of discontinuation, intent to taper, or dose decreases in our cohort represent a potential area of improvement in deprescribing.
Deprescribing should be considered for patients with serious adverse events, for whom less serious adverse effects preclude achieving clinically effective doses, and for those who do not perceive benefit. Given the magnitude of the problems presented by polypharmacy, we propose that stopping priority be given to off-label use (especially when clinically ineffective) and for patients coprescribed opioids or sedatives. Up to a third of users in our cohort were coprescribed opioids or benzodiazepines, which is particularly concerning given the association with increased opioid-related mortality.12,15 Although we did not observe a difference in inpatient mortality, such a study is underpowered for this outcome especially when considering the competing risks of death in hospital. Importantly, when deprescribing, the drug should be tapered over several weeks to limit symptoms of withdrawal and to prevent seizure.11
Presumed off-label use and subtherapeutic doses were common in our cohort, with only 17% of users having a clearly documented FDA-approved indication, in agreement with a previous study that reported only 5% on-label use.4 High doses of gabapentinoids required for efficacy in clinical trials may be difficult to achieve because of dose-limiting side effects, which may explain the relatively low median doses recorded in our real-world cohort. Another possibility is that frail, older patients with renal dysfunction experience effectiveness at lower median doses than those quoted from study populations. In our study, patients on lower doses of gabapentinoids had a higher prevalence of stage IV or V chronic kidney disease (CKD). Stage IV/V CKD was identified in 16/47 (34.0%) patients on lower doses of gabapentinoids, compared to 4/38 (10.5%) on doses within the FDA-recommended range.
Our study has limitations; findings from a single Canadian tertiary care hospital may not be generalizable to other hospitals or countries, particularly given the differences between the Canadian and US health systems. Indications were extracted from the patient chart and even with the best possible medication history and thorough review, sometimes they had to be inferred. Caution should also be exercised when interpreting the omission of an indication as equating to a lack of justifiable medication use; however, the rate of off-label use in our cohort is in agreement with prior research.4 Moreover, with a retrospective design, the effectiveness of the drug on an individual basis could not be assessed, which would have allowed a more precise estimate of the proportion of patients for whom deprescribing might have been appropriate. The strengths of this study include a large sample of real-world, heterogeneous, general medical patients spanning several years and our use of trained pharmacists and physicians to determine the drug indication as opposed to reliance on administrative data.
CONCLUSION
Gabapentinoid use was frequent in our cohort of hospitalized medical patients, with a high prevalence of off-label use, subtherapeutic doses, and coadministration with opioids and benzodiazepines. Deprescribing at discharge was uncommon and often triggered by an adverse event. The identification of gabapentinoids during hospitalization is an opportunity to reevaluate the indication for the drug, assess for effectiveness, and consider deprescribing to help reduce polypharmacy and ideally ADEs.
Acknowledgment
For the purposes of authorship, Dr. McDonald and Dr. Lee contributed equally.
Disclosures
Dr. Emily McDonald and Dr. Todd Lee have a patent pending for MedSafer, a deprescribing software, and both receive research salary support from the Fonds de Recherche Santé du Québec. Dr. Gingras, Dr. Lieu, and Dr. Papillon-Ferland have nothing to disclose.
1. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. https://doi.org/10.1001/jamainternmed.2017.7856.
2. Kwok H, Khuu W, Fernandes K, et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019-1026. https://doi.org/10.1093/pm/pnw351.
3. Medicines use and spending in the U.S. — a review of 2016 and outlook to 2021: IMS Institute for Healthcare Informatics; 2017. https://structurecms-staging-psyclone.netdna-ssl.com/client_assets/dwonk/media/attachments/590c/6aa0/6970/2d2d/4182/0000/590c6aa069702d2d41820000.pdf?1493985952. Accessed March 21, 2019.
4. Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. J Manag Care Pharm. 2002;8(4):266-271. doi: 10.18553/jmcp.2002.8.4.266.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55-63. https://doi.org/10.1001/jamainternmed.2015.6058.
6. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. https://doi.org/10.1371/journal.pmed.1002369.
7. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793. https://doi.org/10.1503/cmaj.171333.
8. Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med. 2018;32(1):276-286. https://doi.org/10.1177/0269216317711826.
9. Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68(6):903-912. https://doi.org/10.1007/s00228-012-1213-x.
10. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359-376. https://doi.org/10.2165/11599460-000000000-00000.
11. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. https://doi.org/10.1007/s40265-017-0700-x.
12. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396.
13. McDonald EG, Saleh RR, Lee TC. Ezetimibe use remains common among medical inpatients. Am J Med. 2015;128(2):193-195. https://doi.org/10.1016/j.amjmed.2014.10.016.
14. U.S. Food and Drug Administration. LYRICA - Highlights of Prescribing Information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf. Accessed April 30, 2019.
15. U.S. Food and Drug Administration. NEURONTIN - Highlights of Prescribing Information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed April 30, 2019.
16. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732-734. https://doi.org/10.7326/M18-1136.
1. Johansen ME. Gabapentinoid use in the United States 2002 through 2015. JAMA Intern Med. 2018;178(2):292-294. https://doi.org/10.1001/jamainternmed.2017.7856.
2. Kwok H, Khuu W, Fernandes K, et al. Impact of unrestricted access to pregabalin on the use of opioids and other CNS-active medications: a cross-sectional time series analysis. Pain Med. 2017;18(6):1019-1026. https://doi.org/10.1093/pm/pnw351.
3. Medicines use and spending in the U.S. — a review of 2016 and outlook to 2021: IMS Institute for Healthcare Informatics; 2017. https://structurecms-staging-psyclone.netdna-ssl.com/client_assets/dwonk/media/attachments/590c/6aa0/6970/2d2d/4182/0000/590c6aa069702d2d41820000.pdf?1493985952. Accessed March 21, 2019.
4. Hamer AM, Haxby DG, McFarland BH, Ketchum K. Gabapentin use in a managed medicaid population. J Manag Care Pharm. 2002;8(4):266-271. doi: 10.18553/jmcp.2002.8.4.266.
5. Eguale T, Buckeridge DL, Verma A, et al. Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;176(1):55-63. https://doi.org/10.1001/jamainternmed.2015.6058.
6. Shanthanna H, Gilron I, Rajarathinam M, et al. Benefits and safety of gabapentinoids in chronic low back pain: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2017;14(8):e1002369. https://doi.org/10.1371/journal.pmed.1002369.
7. Enke O, New HA, New CH, et al. Anticonvulsants in the treatment of low back pain and lumbar radicular pain: a systematic review and meta-analysis. CMAJ. 2018;190(26):E786-E793. https://doi.org/10.1503/cmaj.171333.
8. Kane CM, Mulvey MR, Wright S, Craigs C, Wright JM, Bennett MI. Opioids combined with antidepressants or antiepileptic drugs for cancer pain: systematic review and meta-analysis. Palliat Med. 2018;32(1):276-286. https://doi.org/10.1177/0269216317711826.
9. Zaccara G, Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur J Clin Pharmacol. 2012;68(6):903-912. https://doi.org/10.1007/s00228-012-1213-x.
10. Huang AR, Mallet L, Rochefort CM, Eguale T, Buckeridge DL, Tamblyn R. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359-376. https://doi.org/10.2165/11599460-000000000-00000.
11. Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. https://doi.org/10.1007/s40265-017-0700-x.
12. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017;14(10):e1002396. https://doi.org/10.1371/journal.pmed.1002396.
13. McDonald EG, Saleh RR, Lee TC. Ezetimibe use remains common among medical inpatients. Am J Med. 2015;128(2):193-195. https://doi.org/10.1016/j.amjmed.2014.10.016.
14. U.S. Food and Drug Administration. LYRICA - Highlights of Prescribing Information 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021446s028lbl.pdf. Accessed April 30, 2019.
15. U.S. Food and Drug Administration. NEURONTIN - Highlights of Prescribing Information 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020235s064_020882s047_021129s046lbl.pdf. Accessed April 30, 2019.
16. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann Intern Med. 2018;169(10):732-734. https://doi.org/10.7326/M18-1136.
© 2019 Society of Hospital Medicine
Association of Herpes Simplex Virus Testing with Hospital Length of Stay for Infants ≤60 Days of Age Undergoing Evaluation for Meningitis
Neonatal herpes simplex virus (HSV) is associated with significant morbidity and mortality,1 particularly when the diagnosis or treatment is delayed.2 Therefore, many infants aged ≤60 days being evaluated for meningitis undergo cerebrospinal fluid (CSF) HSV polymerase chain reaction (PCR) testing even though the risk of HSV infection is low [estimated at 0.4% of those undergoing evaluation for central nervous system (CNS) infection].3 A single-center study demonstrated that CSF HSV PCR testing increases the hospital length of stay (LOS) for infants aged ≤56 days,4 although these single-center findings may not be generalizable. To this end, we measured the association between CSF HSV PCR testing and LOS in a multicenter cohort of hospitalized young infants.
METHODS
Study Design
We conducted a planned secondary analysis of a retrospective cohort of infants aged ≤60 days who presented to the emergency department (ED) between January 1, 2005 and December 31, 2013, enrolled in the Pediatric Emergency Medicine Collaborative Research Committee (PEM CRC) HSV study.3 Our study was limited to the 20 hospitals that contributed hospital LOS data. The study protocol was approved by each site’s institutional review board with permission for data sharing.
Study Population
Eligible infants were identified at each site using a site-specific electronic search strategy. Infants were eligible for inclusion if a CSF culture was obtained in the ED or within 24 hours of ED arrival. We excluded infants who were discharged from the ED and those with missing hospital LOS data.
Data Collection
Site investigators extracted the following data elements either electronically or from medical records: patient demographics; ED arrival date and time; hospital discharge date and time; urinalysis results; peripheral and CSF cell counts; blood, urine, and CSF bacterial culture results; as well as the results of HSV PCR and viral cultures. Infants with growth of a pathogen in blood or CSF, or a catheterized urine culture with ≥50,000 colony-forming units (CFUs)/mL of a single pathogenic bacteria, or 10,000-50,000 CFUs/mL of a single pathogenic bacteria with an abnormal urinalysis (ie, positive nitrite or leukocyte esterase on urine dipstick or >5 white blood cells [WBCs] per high power field on urine microscopy) were classified as having a serious bacterial infection (SBI).5,6 Infants with a positive HSV PCR or viral culture from any site were classified as having HSV infection.3 Hospitalized infants who did not have an HSV PCR test performed were assumed not to have HSV disease if not diagnosed during the hospital stay or repeat ED encounter.3
Outcome Measures
The primary outcome was hospital LOS, defined at all hospitals as the time from ED arrival to provider signature of the hospital discharge order, calculated in minutes and then converted into days.
Statistical Analysis
We described LOS using medians with interquartile ranges (IQR) and compared between infants with and without a CSF HSV PCR test performed using the Mann–Whitney U test. To evaluate the association between performance of CSF HSV PCR testing and hospital LOS, we used negative binomial regression given the count variable outcome (LOS) with an overdispersed distribution. For this analysis, we clustered by hospital after adjusting for the following factors determined a priori: age, gender, study year, and presence of serious bacterial or HSV infection. Using the relative marginal modeled estimates of LOS (tested vs not tested), we determined the percentage increase in LOS. We then repeated the analyses after stratifying by the location of testing (ie, in-house vs send-out), age (≤28 days vs 29-60 days), and presence or absence of CSF pleocytosis (defined as a CSF WBC of ≥16 cells/mm3for infants aged ≤28 days and ≥10 cells/mm3for infants aged 29-60 days),7 because infants aged 29-60 days and those without CSF pleocytosis are reported to be at very low risk for CNS HSV infection.3,8 We utilized Stata Data Analysis and Statistical Software, version 15.0 (StataCorp, Inc.; College Station, Texas) for statistical analyses.
RESULTS
Of 24,103 infants with CSF cultures obtained at the 20 participating sites, we excluded 2,673 (11.1%) discharged from the ED or with missing disposition and 934 (3.9%) with missing LOS, leaving a study cohort of 20,496 infants (Figure). Overall, 1,780 infants (8.7%) had an SBI and 99 (0.5%) had an HSV infection, of which 46 (46.5%) had a CNS HSV infection.
Among the 20,496 study infants, 7,399 (36.1%) had a CSF HSV PCR test performed; 5,935 infants (80.2% of those tested) had in-house and 1,464 (19.8%) had send-out testing. Among infants with available CSF cell counts, a CSF HSV PCR test was more commonly performed in infants with CSF pleocytosis than in those without (1,865/4,439 [42.0%] with CSF pleocytosis vs 3,705/12,002 [30.9%] without CSF pleocytosis; odds ratio [OR] 1.6, 95% CI 1.5-1.7). Of the 7,399 infants who had a CSF HSV PCR test performed, 46 (0.6%) had a positive test. Of the tested infants, 5,570 (75.3%) had an available CSF WBC count; a positive CSF HSV PCR test was more common in infants with CSF pleocytosis than in those without (25 positive tests/1,865 infants with CSF pleocytosis [1.3%] vs 9/3,705 [0.2%] without CSF pleocytosis; OR 5.6, 95% CI 2.6-12.0). Among the 5,308 infants aged 29-60 days without CSF pleocytosis, 1,110 (20.9%) had a CSF HSV PCR test performed and only one infant (0.09% of those tested) had a positive test.
Without adjustment, infants with a CSF HSV PCR test had a longer median LOS than infants who were not tested (2.5 vs 2.3 days; P < .001). After adjustment, infants with a CSF HSV PCR test performed had a 23% longer duration of hospitalization. The association between testing and LOS was similar for older vs younger infants, infants with and without CSF pleocytosis, and in-house vs send-out testing (Table).
DISCUSSION
In a large, multicenter cohort of more than 20,000 hospitalized infants aged ≤60 days undergoing evaluation for meningitis, we examined the association of CSF HSV PCR testing with hospital LOS. Approximately one-third of study infants had a CSF HSV PCR test obtained. After adjustment for patient- and hospital-level factors, the treating clinician’s decision to obtain a CSF HSV PCR test was associated with a 23% longer hospital LOS (nearly one-half day).
Our findings are consistent with those of previous studies. First, our observed association of the decision to obtain a CSF HSV PCR test and LOS was similar in magnitude to that of a previous single-center investigation.4 Second, we also found that older infants and those without CSF pleocytosis were at very low risk of HSV infection.3,8 For the otherwise low-risk infants, the longer LOS may be due to delays in obtaining CSF HSV PCR test results, which should be explored in future research. Our study has greater generalizability than previous single-center studies by substantially increasing the population size as well as the variety of clinical settings. Ensuring clinicians’ access to rapid HSV PCR testing platforms will further mitigate the impact of HSV testing on LOS.
When deciding to perform a CSF HSV PCR test for infants aged ≤60 days, clinicians must balance the low incidence of neonatal HSV3 with the risk of delayed diagnosis and treatment of HSV infection, which include neurologic sequelae or even death.1,2 As infants with CNS HSV infection commonly present nonspecifically and only a minority of infected infants have skin vesicles,1 controversy exists as to which infants should be evaluated for HSV infection, resulting in considerable variability in HSV testing.3 Some clinicians advocate for more conservative testing strategies that include the performance of CSF HSV PCR testing in all febrile infants aged ≤21 days.9 Others suggest limiting testing to infants who meet high-risk criteria (eg, seizures, ill-appearance, or CSF pleocytosis).10,11 Further investigation will need to elucidate the clinical and laboratory predictors of HSV infection to identify those infants who would benefit most from HSV testing as well as the outcomes of infants not tested.
Our study has several limitations. First, we could not determine the reason why clinicians elected to obtain a CSF HSV PCR test, and we do not know the test turnaround time or the time when results became available to the clinical team. Second, we did not abstract clinical data such as ill-appearance or seizures. Although we adjusted for the presence of serious bacterial or HSV infection as proxy measures for illness severity, it is possible that other clinical factors were associated with HSV testing and LOS. Third, although we adjusted for patient- and hospital-level factors in our regression model, the potential for residual confounding persists. Fourth, we did not explore acyclovir administration as a factor associated with LOS as some sites did not provide data on acyclovir. Fifth, we did not evaluate the impact of HSV testing of other sample types (eg, blood or skin) on LOS. Sixth, our study was conducted primarily at children’s hospitals, and our findings may not be generalizable to general hospitals with hospitalized neonates.
CONCLUSIONS
For infants aged ≤60 days undergoing evaluation for meningitis, CSF HSV PCR testing was associated with a slightly longer hospital LOS. Improved methods to identify and target testing to higher risk infants may mitigate the impact on LOS for low-risk infants.
Acknowledgments
The authors acknowledge the following collaborators in the Pediatric Emergency Medicine Clinical Research Network (PEM CRC) Herpes Simplex Virus (HSV) Study Group who collected data for this study and/or the parent study: Joseph L Arms, MD (Minneapolis, Minnesota), Stuart A Bradin, DO (Ann Arbor, Michigan), Sarah J Curtis, MD, MSc (Edmonton, Alberta, Canada), Paul T Ishimine, MD (San Diego, California), Dina Kulik, MD (Toronto, Ontario, Canada), Prashant Mahajan, MD, MPH, MBA (Ann Arbor, Michigan), Aaron S Miller, MD, MSPH (St. Louis, Missouri), Pamela J Okada, MD (Dallas, Texas), Christopher M Pruitt, MD (Birmingham, Alabama), Suzanne M Schmidt, MD (Chicago, Illinois), David Schnadower, Amy D Thompson, MD (Wilmington, Delaware), Joanna E Thomson, MD, MPH (Cincinnati, Ohio), MD, MPH (St. Louis, Missouri), and Neil G. Uspal, MD (Seattle, Washington).
Disclosures
Dr. Aronson reports grants from the Agency for Healthcare Research and Quality during the conduct of the study. Dr. Shah reports grants from Patient-Centered Outcomes Research Institute, grants from the National Institute of Allergy and Infectious Diseases, and grants from the National Heart Lung Blood Institute, outside the submitted work. Dr. Shah is the Editor-in-Chief of the Journal of Hospital Medicine. All other authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Funding
This project was supported by the Section of Emergency Medicine of the American Academy of Pediatrics (AAP) and Baylor College of Medicine and by the grant number K08HS026006 (Aronson) from the Agency for Healthcare Research and Quality. The content is solely the responsibi
1. Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223-229. PubMed
2. Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153-1160. https://doi.org/10.1136/eb-2012-100674.
3. Cruz AT, Freedman SB, Kulik DM, et al. Herpes simplex virus infection in infants undergoing meningitis evaluation. Pediatrics. 2018;141(2):e20171688. https://doi.org/10.1542/peds.2017-1688.
4. Shah SS, Volk J, Mohamad Z, Hodinka RL, Zorc JJ. Herpes simplex virus testing and hospital length of stay in neonates and young infants. J Pediatr. 2010;156(5):738-743. https://doi.org/10.1016/j.jpeds.2009.11.079.
5. Mahajan P, Kuppermann N, Mejias A, et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846-857. https://doi.org/10.1001/jama.2016.9207.
6. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479.
7. Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018;141(3):e20173405. https://doi.org/10.1542/peds.2017-3405.
8. Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153(2):164-169. https://doi.org/10.1016/j.jpeds.2008.02.031.
9. Long SS. In defense of empiric acyclovir therapy in certain neonates. J Pediatr. 2008;153(2):157-158. https://doi.org/10.1016/j.jpeds.2008.04.071.
10. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166.
11. Kimberlin DW. When should you initiate acyclovir therapy in a neonate? J Pediatr. 2008;153(2):155-156. https://doi.org/10.1016/j.jpeds.2008.04.027.
Neonatal herpes simplex virus (HSV) is associated with significant morbidity and mortality,1 particularly when the diagnosis or treatment is delayed.2 Therefore, many infants aged ≤60 days being evaluated for meningitis undergo cerebrospinal fluid (CSF) HSV polymerase chain reaction (PCR) testing even though the risk of HSV infection is low [estimated at 0.4% of those undergoing evaluation for central nervous system (CNS) infection].3 A single-center study demonstrated that CSF HSV PCR testing increases the hospital length of stay (LOS) for infants aged ≤56 days,4 although these single-center findings may not be generalizable. To this end, we measured the association between CSF HSV PCR testing and LOS in a multicenter cohort of hospitalized young infants.
METHODS
Study Design
We conducted a planned secondary analysis of a retrospective cohort of infants aged ≤60 days who presented to the emergency department (ED) between January 1, 2005 and December 31, 2013, enrolled in the Pediatric Emergency Medicine Collaborative Research Committee (PEM CRC) HSV study.3 Our study was limited to the 20 hospitals that contributed hospital LOS data. The study protocol was approved by each site’s institutional review board with permission for data sharing.
Study Population
Eligible infants were identified at each site using a site-specific electronic search strategy. Infants were eligible for inclusion if a CSF culture was obtained in the ED or within 24 hours of ED arrival. We excluded infants who were discharged from the ED and those with missing hospital LOS data.
Data Collection
Site investigators extracted the following data elements either electronically or from medical records: patient demographics; ED arrival date and time; hospital discharge date and time; urinalysis results; peripheral and CSF cell counts; blood, urine, and CSF bacterial culture results; as well as the results of HSV PCR and viral cultures. Infants with growth of a pathogen in blood or CSF, or a catheterized urine culture with ≥50,000 colony-forming units (CFUs)/mL of a single pathogenic bacteria, or 10,000-50,000 CFUs/mL of a single pathogenic bacteria with an abnormal urinalysis (ie, positive nitrite or leukocyte esterase on urine dipstick or >5 white blood cells [WBCs] per high power field on urine microscopy) were classified as having a serious bacterial infection (SBI).5,6 Infants with a positive HSV PCR or viral culture from any site were classified as having HSV infection.3 Hospitalized infants who did not have an HSV PCR test performed were assumed not to have HSV disease if not diagnosed during the hospital stay or repeat ED encounter.3
Outcome Measures
The primary outcome was hospital LOS, defined at all hospitals as the time from ED arrival to provider signature of the hospital discharge order, calculated in minutes and then converted into days.
Statistical Analysis
We described LOS using medians with interquartile ranges (IQR) and compared between infants with and without a CSF HSV PCR test performed using the Mann–Whitney U test. To evaluate the association between performance of CSF HSV PCR testing and hospital LOS, we used negative binomial regression given the count variable outcome (LOS) with an overdispersed distribution. For this analysis, we clustered by hospital after adjusting for the following factors determined a priori: age, gender, study year, and presence of serious bacterial or HSV infection. Using the relative marginal modeled estimates of LOS (tested vs not tested), we determined the percentage increase in LOS. We then repeated the analyses after stratifying by the location of testing (ie, in-house vs send-out), age (≤28 days vs 29-60 days), and presence or absence of CSF pleocytosis (defined as a CSF WBC of ≥16 cells/mm3for infants aged ≤28 days and ≥10 cells/mm3for infants aged 29-60 days),7 because infants aged 29-60 days and those without CSF pleocytosis are reported to be at very low risk for CNS HSV infection.3,8 We utilized Stata Data Analysis and Statistical Software, version 15.0 (StataCorp, Inc.; College Station, Texas) for statistical analyses.
RESULTS
Of 24,103 infants with CSF cultures obtained at the 20 participating sites, we excluded 2,673 (11.1%) discharged from the ED or with missing disposition and 934 (3.9%) with missing LOS, leaving a study cohort of 20,496 infants (Figure). Overall, 1,780 infants (8.7%) had an SBI and 99 (0.5%) had an HSV infection, of which 46 (46.5%) had a CNS HSV infection.
Among the 20,496 study infants, 7,399 (36.1%) had a CSF HSV PCR test performed; 5,935 infants (80.2% of those tested) had in-house and 1,464 (19.8%) had send-out testing. Among infants with available CSF cell counts, a CSF HSV PCR test was more commonly performed in infants with CSF pleocytosis than in those without (1,865/4,439 [42.0%] with CSF pleocytosis vs 3,705/12,002 [30.9%] without CSF pleocytosis; odds ratio [OR] 1.6, 95% CI 1.5-1.7). Of the 7,399 infants who had a CSF HSV PCR test performed, 46 (0.6%) had a positive test. Of the tested infants, 5,570 (75.3%) had an available CSF WBC count; a positive CSF HSV PCR test was more common in infants with CSF pleocytosis than in those without (25 positive tests/1,865 infants with CSF pleocytosis [1.3%] vs 9/3,705 [0.2%] without CSF pleocytosis; OR 5.6, 95% CI 2.6-12.0). Among the 5,308 infants aged 29-60 days without CSF pleocytosis, 1,110 (20.9%) had a CSF HSV PCR test performed and only one infant (0.09% of those tested) had a positive test.
Without adjustment, infants with a CSF HSV PCR test had a longer median LOS than infants who were not tested (2.5 vs 2.3 days; P < .001). After adjustment, infants with a CSF HSV PCR test performed had a 23% longer duration of hospitalization. The association between testing and LOS was similar for older vs younger infants, infants with and without CSF pleocytosis, and in-house vs send-out testing (Table).
DISCUSSION
In a large, multicenter cohort of more than 20,000 hospitalized infants aged ≤60 days undergoing evaluation for meningitis, we examined the association of CSF HSV PCR testing with hospital LOS. Approximately one-third of study infants had a CSF HSV PCR test obtained. After adjustment for patient- and hospital-level factors, the treating clinician’s decision to obtain a CSF HSV PCR test was associated with a 23% longer hospital LOS (nearly one-half day).
Our findings are consistent with those of previous studies. First, our observed association of the decision to obtain a CSF HSV PCR test and LOS was similar in magnitude to that of a previous single-center investigation.4 Second, we also found that older infants and those without CSF pleocytosis were at very low risk of HSV infection.3,8 For the otherwise low-risk infants, the longer LOS may be due to delays in obtaining CSF HSV PCR test results, which should be explored in future research. Our study has greater generalizability than previous single-center studies by substantially increasing the population size as well as the variety of clinical settings. Ensuring clinicians’ access to rapid HSV PCR testing platforms will further mitigate the impact of HSV testing on LOS.
When deciding to perform a CSF HSV PCR test for infants aged ≤60 days, clinicians must balance the low incidence of neonatal HSV3 with the risk of delayed diagnosis and treatment of HSV infection, which include neurologic sequelae or even death.1,2 As infants with CNS HSV infection commonly present nonspecifically and only a minority of infected infants have skin vesicles,1 controversy exists as to which infants should be evaluated for HSV infection, resulting in considerable variability in HSV testing.3 Some clinicians advocate for more conservative testing strategies that include the performance of CSF HSV PCR testing in all febrile infants aged ≤21 days.9 Others suggest limiting testing to infants who meet high-risk criteria (eg, seizures, ill-appearance, or CSF pleocytosis).10,11 Further investigation will need to elucidate the clinical and laboratory predictors of HSV infection to identify those infants who would benefit most from HSV testing as well as the outcomes of infants not tested.
Our study has several limitations. First, we could not determine the reason why clinicians elected to obtain a CSF HSV PCR test, and we do not know the test turnaround time or the time when results became available to the clinical team. Second, we did not abstract clinical data such as ill-appearance or seizures. Although we adjusted for the presence of serious bacterial or HSV infection as proxy measures for illness severity, it is possible that other clinical factors were associated with HSV testing and LOS. Third, although we adjusted for patient- and hospital-level factors in our regression model, the potential for residual confounding persists. Fourth, we did not explore acyclovir administration as a factor associated with LOS as some sites did not provide data on acyclovir. Fifth, we did not evaluate the impact of HSV testing of other sample types (eg, blood or skin) on LOS. Sixth, our study was conducted primarily at children’s hospitals, and our findings may not be generalizable to general hospitals with hospitalized neonates.
CONCLUSIONS
For infants aged ≤60 days undergoing evaluation for meningitis, CSF HSV PCR testing was associated with a slightly longer hospital LOS. Improved methods to identify and target testing to higher risk infants may mitigate the impact on LOS for low-risk infants.
Acknowledgments
The authors acknowledge the following collaborators in the Pediatric Emergency Medicine Clinical Research Network (PEM CRC) Herpes Simplex Virus (HSV) Study Group who collected data for this study and/or the parent study: Joseph L Arms, MD (Minneapolis, Minnesota), Stuart A Bradin, DO (Ann Arbor, Michigan), Sarah J Curtis, MD, MSc (Edmonton, Alberta, Canada), Paul T Ishimine, MD (San Diego, California), Dina Kulik, MD (Toronto, Ontario, Canada), Prashant Mahajan, MD, MPH, MBA (Ann Arbor, Michigan), Aaron S Miller, MD, MSPH (St. Louis, Missouri), Pamela J Okada, MD (Dallas, Texas), Christopher M Pruitt, MD (Birmingham, Alabama), Suzanne M Schmidt, MD (Chicago, Illinois), David Schnadower, Amy D Thompson, MD (Wilmington, Delaware), Joanna E Thomson, MD, MPH (Cincinnati, Ohio), MD, MPH (St. Louis, Missouri), and Neil G. Uspal, MD (Seattle, Washington).
Disclosures
Dr. Aronson reports grants from the Agency for Healthcare Research and Quality during the conduct of the study. Dr. Shah reports grants from Patient-Centered Outcomes Research Institute, grants from the National Institute of Allergy and Infectious Diseases, and grants from the National Heart Lung Blood Institute, outside the submitted work. Dr. Shah is the Editor-in-Chief of the Journal of Hospital Medicine. All other authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Funding
This project was supported by the Section of Emergency Medicine of the American Academy of Pediatrics (AAP) and Baylor College of Medicine and by the grant number K08HS026006 (Aronson) from the Agency for Healthcare Research and Quality. The content is solely the responsibi
Neonatal herpes simplex virus (HSV) is associated with significant morbidity and mortality,1 particularly when the diagnosis or treatment is delayed.2 Therefore, many infants aged ≤60 days being evaluated for meningitis undergo cerebrospinal fluid (CSF) HSV polymerase chain reaction (PCR) testing even though the risk of HSV infection is low [estimated at 0.4% of those undergoing evaluation for central nervous system (CNS) infection].3 A single-center study demonstrated that CSF HSV PCR testing increases the hospital length of stay (LOS) for infants aged ≤56 days,4 although these single-center findings may not be generalizable. To this end, we measured the association between CSF HSV PCR testing and LOS in a multicenter cohort of hospitalized young infants.
METHODS
Study Design
We conducted a planned secondary analysis of a retrospective cohort of infants aged ≤60 days who presented to the emergency department (ED) between January 1, 2005 and December 31, 2013, enrolled in the Pediatric Emergency Medicine Collaborative Research Committee (PEM CRC) HSV study.3 Our study was limited to the 20 hospitals that contributed hospital LOS data. The study protocol was approved by each site’s institutional review board with permission for data sharing.
Study Population
Eligible infants were identified at each site using a site-specific electronic search strategy. Infants were eligible for inclusion if a CSF culture was obtained in the ED or within 24 hours of ED arrival. We excluded infants who were discharged from the ED and those with missing hospital LOS data.
Data Collection
Site investigators extracted the following data elements either electronically or from medical records: patient demographics; ED arrival date and time; hospital discharge date and time; urinalysis results; peripheral and CSF cell counts; blood, urine, and CSF bacterial culture results; as well as the results of HSV PCR and viral cultures. Infants with growth of a pathogen in blood or CSF, or a catheterized urine culture with ≥50,000 colony-forming units (CFUs)/mL of a single pathogenic bacteria, or 10,000-50,000 CFUs/mL of a single pathogenic bacteria with an abnormal urinalysis (ie, positive nitrite or leukocyte esterase on urine dipstick or >5 white blood cells [WBCs] per high power field on urine microscopy) were classified as having a serious bacterial infection (SBI).5,6 Infants with a positive HSV PCR or viral culture from any site were classified as having HSV infection.3 Hospitalized infants who did not have an HSV PCR test performed were assumed not to have HSV disease if not diagnosed during the hospital stay or repeat ED encounter.3
Outcome Measures
The primary outcome was hospital LOS, defined at all hospitals as the time from ED arrival to provider signature of the hospital discharge order, calculated in minutes and then converted into days.
Statistical Analysis
We described LOS using medians with interquartile ranges (IQR) and compared between infants with and without a CSF HSV PCR test performed using the Mann–Whitney U test. To evaluate the association between performance of CSF HSV PCR testing and hospital LOS, we used negative binomial regression given the count variable outcome (LOS) with an overdispersed distribution. For this analysis, we clustered by hospital after adjusting for the following factors determined a priori: age, gender, study year, and presence of serious bacterial or HSV infection. Using the relative marginal modeled estimates of LOS (tested vs not tested), we determined the percentage increase in LOS. We then repeated the analyses after stratifying by the location of testing (ie, in-house vs send-out), age (≤28 days vs 29-60 days), and presence or absence of CSF pleocytosis (defined as a CSF WBC of ≥16 cells/mm3for infants aged ≤28 days and ≥10 cells/mm3for infants aged 29-60 days),7 because infants aged 29-60 days and those without CSF pleocytosis are reported to be at very low risk for CNS HSV infection.3,8 We utilized Stata Data Analysis and Statistical Software, version 15.0 (StataCorp, Inc.; College Station, Texas) for statistical analyses.
RESULTS
Of 24,103 infants with CSF cultures obtained at the 20 participating sites, we excluded 2,673 (11.1%) discharged from the ED or with missing disposition and 934 (3.9%) with missing LOS, leaving a study cohort of 20,496 infants (Figure). Overall, 1,780 infants (8.7%) had an SBI and 99 (0.5%) had an HSV infection, of which 46 (46.5%) had a CNS HSV infection.
Among the 20,496 study infants, 7,399 (36.1%) had a CSF HSV PCR test performed; 5,935 infants (80.2% of those tested) had in-house and 1,464 (19.8%) had send-out testing. Among infants with available CSF cell counts, a CSF HSV PCR test was more commonly performed in infants with CSF pleocytosis than in those without (1,865/4,439 [42.0%] with CSF pleocytosis vs 3,705/12,002 [30.9%] without CSF pleocytosis; odds ratio [OR] 1.6, 95% CI 1.5-1.7). Of the 7,399 infants who had a CSF HSV PCR test performed, 46 (0.6%) had a positive test. Of the tested infants, 5,570 (75.3%) had an available CSF WBC count; a positive CSF HSV PCR test was more common in infants with CSF pleocytosis than in those without (25 positive tests/1,865 infants with CSF pleocytosis [1.3%] vs 9/3,705 [0.2%] without CSF pleocytosis; OR 5.6, 95% CI 2.6-12.0). Among the 5,308 infants aged 29-60 days without CSF pleocytosis, 1,110 (20.9%) had a CSF HSV PCR test performed and only one infant (0.09% of those tested) had a positive test.
Without adjustment, infants with a CSF HSV PCR test had a longer median LOS than infants who were not tested (2.5 vs 2.3 days; P < .001). After adjustment, infants with a CSF HSV PCR test performed had a 23% longer duration of hospitalization. The association between testing and LOS was similar for older vs younger infants, infants with and without CSF pleocytosis, and in-house vs send-out testing (Table).
DISCUSSION
In a large, multicenter cohort of more than 20,000 hospitalized infants aged ≤60 days undergoing evaluation for meningitis, we examined the association of CSF HSV PCR testing with hospital LOS. Approximately one-third of study infants had a CSF HSV PCR test obtained. After adjustment for patient- and hospital-level factors, the treating clinician’s decision to obtain a CSF HSV PCR test was associated with a 23% longer hospital LOS (nearly one-half day).
Our findings are consistent with those of previous studies. First, our observed association of the decision to obtain a CSF HSV PCR test and LOS was similar in magnitude to that of a previous single-center investigation.4 Second, we also found that older infants and those without CSF pleocytosis were at very low risk of HSV infection.3,8 For the otherwise low-risk infants, the longer LOS may be due to delays in obtaining CSF HSV PCR test results, which should be explored in future research. Our study has greater generalizability than previous single-center studies by substantially increasing the population size as well as the variety of clinical settings. Ensuring clinicians’ access to rapid HSV PCR testing platforms will further mitigate the impact of HSV testing on LOS.
When deciding to perform a CSF HSV PCR test for infants aged ≤60 days, clinicians must balance the low incidence of neonatal HSV3 with the risk of delayed diagnosis and treatment of HSV infection, which include neurologic sequelae or even death.1,2 As infants with CNS HSV infection commonly present nonspecifically and only a minority of infected infants have skin vesicles,1 controversy exists as to which infants should be evaluated for HSV infection, resulting in considerable variability in HSV testing.3 Some clinicians advocate for more conservative testing strategies that include the performance of CSF HSV PCR testing in all febrile infants aged ≤21 days.9 Others suggest limiting testing to infants who meet high-risk criteria (eg, seizures, ill-appearance, or CSF pleocytosis).10,11 Further investigation will need to elucidate the clinical and laboratory predictors of HSV infection to identify those infants who would benefit most from HSV testing as well as the outcomes of infants not tested.
Our study has several limitations. First, we could not determine the reason why clinicians elected to obtain a CSF HSV PCR test, and we do not know the test turnaround time or the time when results became available to the clinical team. Second, we did not abstract clinical data such as ill-appearance or seizures. Although we adjusted for the presence of serious bacterial or HSV infection as proxy measures for illness severity, it is possible that other clinical factors were associated with HSV testing and LOS. Third, although we adjusted for patient- and hospital-level factors in our regression model, the potential for residual confounding persists. Fourth, we did not explore acyclovir administration as a factor associated with LOS as some sites did not provide data on acyclovir. Fifth, we did not evaluate the impact of HSV testing of other sample types (eg, blood or skin) on LOS. Sixth, our study was conducted primarily at children’s hospitals, and our findings may not be generalizable to general hospitals with hospitalized neonates.
CONCLUSIONS
For infants aged ≤60 days undergoing evaluation for meningitis, CSF HSV PCR testing was associated with a slightly longer hospital LOS. Improved methods to identify and target testing to higher risk infants may mitigate the impact on LOS for low-risk infants.
Acknowledgments
The authors acknowledge the following collaborators in the Pediatric Emergency Medicine Clinical Research Network (PEM CRC) Herpes Simplex Virus (HSV) Study Group who collected data for this study and/or the parent study: Joseph L Arms, MD (Minneapolis, Minnesota), Stuart A Bradin, DO (Ann Arbor, Michigan), Sarah J Curtis, MD, MSc (Edmonton, Alberta, Canada), Paul T Ishimine, MD (San Diego, California), Dina Kulik, MD (Toronto, Ontario, Canada), Prashant Mahajan, MD, MPH, MBA (Ann Arbor, Michigan), Aaron S Miller, MD, MSPH (St. Louis, Missouri), Pamela J Okada, MD (Dallas, Texas), Christopher M Pruitt, MD (Birmingham, Alabama), Suzanne M Schmidt, MD (Chicago, Illinois), David Schnadower, Amy D Thompson, MD (Wilmington, Delaware), Joanna E Thomson, MD, MPH (Cincinnati, Ohio), MD, MPH (St. Louis, Missouri), and Neil G. Uspal, MD (Seattle, Washington).
Disclosures
Dr. Aronson reports grants from the Agency for Healthcare Research and Quality during the conduct of the study. Dr. Shah reports grants from Patient-Centered Outcomes Research Institute, grants from the National Institute of Allergy and Infectious Diseases, and grants from the National Heart Lung Blood Institute, outside the submitted work. Dr. Shah is the Editor-in-Chief of the Journal of Hospital Medicine. All other authors have no conflicts of interest or financial relationships relevant to this article to disclose.
Funding
This project was supported by the Section of Emergency Medicine of the American Academy of Pediatrics (AAP) and Baylor College of Medicine and by the grant number K08HS026006 (Aronson) from the Agency for Healthcare Research and Quality. The content is solely the responsibi
1. Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223-229. PubMed
2. Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153-1160. https://doi.org/10.1136/eb-2012-100674.
3. Cruz AT, Freedman SB, Kulik DM, et al. Herpes simplex virus infection in infants undergoing meningitis evaluation. Pediatrics. 2018;141(2):e20171688. https://doi.org/10.1542/peds.2017-1688.
4. Shah SS, Volk J, Mohamad Z, Hodinka RL, Zorc JJ. Herpes simplex virus testing and hospital length of stay in neonates and young infants. J Pediatr. 2010;156(5):738-743. https://doi.org/10.1016/j.jpeds.2009.11.079.
5. Mahajan P, Kuppermann N, Mejias A, et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846-857. https://doi.org/10.1001/jama.2016.9207.
6. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479.
7. Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018;141(3):e20173405. https://doi.org/10.1542/peds.2017-3405.
8. Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153(2):164-169. https://doi.org/10.1016/j.jpeds.2008.02.031.
9. Long SS. In defense of empiric acyclovir therapy in certain neonates. J Pediatr. 2008;153(2):157-158. https://doi.org/10.1016/j.jpeds.2008.04.071.
10. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166.
11. Kimberlin DW. When should you initiate acyclovir therapy in a neonate? J Pediatr. 2008;153(2):155-156. https://doi.org/10.1016/j.jpeds.2008.04.027.
1. Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223-229. PubMed
2. Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics. 2011;128(6):1153-1160. https://doi.org/10.1136/eb-2012-100674.
3. Cruz AT, Freedman SB, Kulik DM, et al. Herpes simplex virus infection in infants undergoing meningitis evaluation. Pediatrics. 2018;141(2):e20171688. https://doi.org/10.1542/peds.2017-1688.
4. Shah SS, Volk J, Mohamad Z, Hodinka RL, Zorc JJ. Herpes simplex virus testing and hospital length of stay in neonates and young infants. J Pediatr. 2010;156(5):738-743. https://doi.org/10.1016/j.jpeds.2009.11.079.
5. Mahajan P, Kuppermann N, Mejias A, et al. Association of RNA biosignatures with bacterial infections in febrile infants aged 60 days or younger. JAMA. 2016;316(8):846-857. https://doi.org/10.1001/jama.2016.9207.
6. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics. 2010;126(6):1074-1083. https://doi.org/10.1542/peds.2010-0479.
7. Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018;141(3):e20173405. https://doi.org/10.1542/peds.2017-3405.
8. Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr. 2008;153(2):164-169. https://doi.org/10.1016/j.jpeds.2008.02.031.
9. Long SS. In defense of empiric acyclovir therapy in certain neonates. J Pediatr. 2008;153(2):157-158. https://doi.org/10.1016/j.jpeds.2008.04.071.
10. Brower L, Schondelmeyer A, Wilson P, Shah SS. Testing and empiric treatment for neonatal herpes simplex virus: challenges and opportunities for improving the value of care. Hosp Pediatr. 2016;6(2):108-111. https://doi.org/10.1542/hpeds.2015-0166.
11. Kimberlin DW. When should you initiate acyclovir therapy in a neonate? J Pediatr. 2008;153(2):155-156. https://doi.org/10.1016/j.jpeds.2008.04.027.
© 2019 Society of Hospital Medicine
Senators question whether health IT regulation can keep up with innovation
It has been more than 10 years since Congress passed the HITECH Act to jump start the adoption of electronic health records. And yet interoperability, the key to making their adoption meaningful, remains an elusive target.
Members of the Senate Health, Education, Labor, and Pensions Committee questioned whether attempts to regulate interoperability – and health IT in general – are doing more harm than good in terms of creating a truly interoperable environment.
A key concern raised during a May 7 hearing examining the implementation of the health IT provisions of the 21st Century Cures Act (H.R. 34), signed into law in December 2016, was that the slow speed of government regulatory action simply cannot keep pace with innovation.
“In 2016, my question to a panel like this was, ‘How the hell are you going to do this?’ I think what you’ve heard is different variations of that going around the room,” Sen. Richard Burr (R-N.C) said. “The difference is we’re in 2019 and this is a discussion that we started in 2013 about how do we get systems to talk to other systems.”
“Here is my problem,” he continued, noting that between 2013 and 2019, “technology has exploded. It runs at an unbelievable pace. And here we are trying to set standards and set architecture of software ... that 12 months from now is going to be obsolete because that’s how fast technology is changing.”
Sen. Burr added that the problem is that government can’t regulate fast enough. “Our problem is we can’t get rules through when the technology that we are applying it to is in existence. By the time we get a rule through, technology’s changed, and it may or may not apply.”
Donald Rucker, MD, national coordinator for health information technology at the Health & Human Services department, testified that the current app marketplace is “stable” and “the entire point of what we are doing jointly is to allow these apps in the market economy to incorporate patient’s medical data into that.”
Centers for Medicare & Medicaid Services Chief Medical Officer Kate Goodrich, MD, agreed.
“I would absolutely agree with Don that the timing is really right for this because of where the state of play is with the standards, and I think it’s a good time to take advantage of that and move the field forward,” she testified at the hearing.
The Office of the National Coordinator for Health IT recently issued a proposed rule related to interoperability. The CMS has issued a concurrent proposed rule, also related to interoperability.
Sen. Burr, however, was not moved by these assurances.
“I am probably no less convinced that we are going to get to the finish line today than I was in 2016, though the tools that we have are much better,” he said, telling an anecdote about how providers and insurers took the initiative to improve the outcomes of diabetes patients without any prompting from legislative or regulatory action.
“It seems to me that that’s the most appropriate first place to go, is to create an incentive for the providers,” he said. “It is to their financial benefit to do that.”
Sen. Mitt Romney (R-Utah) also expressed doubt as to whether the goal of interoperability was achievable.
“I wonder exactly whether it is conceivable for us to achieve standards that will allow systems to talk to each other from one hospital system, for instance, to another provider, or whether that’s frankly a bridge too far at this stage,” he said.
Dr. Rucker acknowledged that there is “plenty of room for skepticism,” but he said that the goals of interoperability in health care are achieved broadly across most sectors in the economy today, and it’s about customizing what already works in the marketplace to the needs of the health care environment.
Dr. Goodrich agreed, noting that, in her time as a practicing physician, she has seen advances in the amount of data that can be transfered electronically.
“There is reason for optimism,” she said. “So, yes, plenty of room for skepticism, but also more optimism than probably any of us would have had a couple of years ago.”
It has been more than 10 years since Congress passed the HITECH Act to jump start the adoption of electronic health records. And yet interoperability, the key to making their adoption meaningful, remains an elusive target.
Members of the Senate Health, Education, Labor, and Pensions Committee questioned whether attempts to regulate interoperability – and health IT in general – are doing more harm than good in terms of creating a truly interoperable environment.
A key concern raised during a May 7 hearing examining the implementation of the health IT provisions of the 21st Century Cures Act (H.R. 34), signed into law in December 2016, was that the slow speed of government regulatory action simply cannot keep pace with innovation.
“In 2016, my question to a panel like this was, ‘How the hell are you going to do this?’ I think what you’ve heard is different variations of that going around the room,” Sen. Richard Burr (R-N.C) said. “The difference is we’re in 2019 and this is a discussion that we started in 2013 about how do we get systems to talk to other systems.”
“Here is my problem,” he continued, noting that between 2013 and 2019, “technology has exploded. It runs at an unbelievable pace. And here we are trying to set standards and set architecture of software ... that 12 months from now is going to be obsolete because that’s how fast technology is changing.”
Sen. Burr added that the problem is that government can’t regulate fast enough. “Our problem is we can’t get rules through when the technology that we are applying it to is in existence. By the time we get a rule through, technology’s changed, and it may or may not apply.”
Donald Rucker, MD, national coordinator for health information technology at the Health & Human Services department, testified that the current app marketplace is “stable” and “the entire point of what we are doing jointly is to allow these apps in the market economy to incorporate patient’s medical data into that.”
Centers for Medicare & Medicaid Services Chief Medical Officer Kate Goodrich, MD, agreed.
“I would absolutely agree with Don that the timing is really right for this because of where the state of play is with the standards, and I think it’s a good time to take advantage of that and move the field forward,” she testified at the hearing.
The Office of the National Coordinator for Health IT recently issued a proposed rule related to interoperability. The CMS has issued a concurrent proposed rule, also related to interoperability.
Sen. Burr, however, was not moved by these assurances.
“I am probably no less convinced that we are going to get to the finish line today than I was in 2016, though the tools that we have are much better,” he said, telling an anecdote about how providers and insurers took the initiative to improve the outcomes of diabetes patients without any prompting from legislative or regulatory action.
“It seems to me that that’s the most appropriate first place to go, is to create an incentive for the providers,” he said. “It is to their financial benefit to do that.”
Sen. Mitt Romney (R-Utah) also expressed doubt as to whether the goal of interoperability was achievable.
“I wonder exactly whether it is conceivable for us to achieve standards that will allow systems to talk to each other from one hospital system, for instance, to another provider, or whether that’s frankly a bridge too far at this stage,” he said.
Dr. Rucker acknowledged that there is “plenty of room for skepticism,” but he said that the goals of interoperability in health care are achieved broadly across most sectors in the economy today, and it’s about customizing what already works in the marketplace to the needs of the health care environment.
Dr. Goodrich agreed, noting that, in her time as a practicing physician, she has seen advances in the amount of data that can be transfered electronically.
“There is reason for optimism,” she said. “So, yes, plenty of room for skepticism, but also more optimism than probably any of us would have had a couple of years ago.”
It has been more than 10 years since Congress passed the HITECH Act to jump start the adoption of electronic health records. And yet interoperability, the key to making their adoption meaningful, remains an elusive target.
Members of the Senate Health, Education, Labor, and Pensions Committee questioned whether attempts to regulate interoperability – and health IT in general – are doing more harm than good in terms of creating a truly interoperable environment.
A key concern raised during a May 7 hearing examining the implementation of the health IT provisions of the 21st Century Cures Act (H.R. 34), signed into law in December 2016, was that the slow speed of government regulatory action simply cannot keep pace with innovation.
“In 2016, my question to a panel like this was, ‘How the hell are you going to do this?’ I think what you’ve heard is different variations of that going around the room,” Sen. Richard Burr (R-N.C) said. “The difference is we’re in 2019 and this is a discussion that we started in 2013 about how do we get systems to talk to other systems.”
“Here is my problem,” he continued, noting that between 2013 and 2019, “technology has exploded. It runs at an unbelievable pace. And here we are trying to set standards and set architecture of software ... that 12 months from now is going to be obsolete because that’s how fast technology is changing.”
Sen. Burr added that the problem is that government can’t regulate fast enough. “Our problem is we can’t get rules through when the technology that we are applying it to is in existence. By the time we get a rule through, technology’s changed, and it may or may not apply.”
Donald Rucker, MD, national coordinator for health information technology at the Health & Human Services department, testified that the current app marketplace is “stable” and “the entire point of what we are doing jointly is to allow these apps in the market economy to incorporate patient’s medical data into that.”
Centers for Medicare & Medicaid Services Chief Medical Officer Kate Goodrich, MD, agreed.
“I would absolutely agree with Don that the timing is really right for this because of where the state of play is with the standards, and I think it’s a good time to take advantage of that and move the field forward,” she testified at the hearing.
The Office of the National Coordinator for Health IT recently issued a proposed rule related to interoperability. The CMS has issued a concurrent proposed rule, also related to interoperability.
Sen. Burr, however, was not moved by these assurances.
“I am probably no less convinced that we are going to get to the finish line today than I was in 2016, though the tools that we have are much better,” he said, telling an anecdote about how providers and insurers took the initiative to improve the outcomes of diabetes patients without any prompting from legislative or regulatory action.
“It seems to me that that’s the most appropriate first place to go, is to create an incentive for the providers,” he said. “It is to their financial benefit to do that.”
Sen. Mitt Romney (R-Utah) also expressed doubt as to whether the goal of interoperability was achievable.
“I wonder exactly whether it is conceivable for us to achieve standards that will allow systems to talk to each other from one hospital system, for instance, to another provider, or whether that’s frankly a bridge too far at this stage,” he said.
Dr. Rucker acknowledged that there is “plenty of room for skepticism,” but he said that the goals of interoperability in health care are achieved broadly across most sectors in the economy today, and it’s about customizing what already works in the marketplace to the needs of the health care environment.
Dr. Goodrich agreed, noting that, in her time as a practicing physician, she has seen advances in the amount of data that can be transfered electronically.
“There is reason for optimism,” she said. “So, yes, plenty of room for skepticism, but also more optimism than probably any of us would have had a couple of years ago.”
REPORTING FROM A SENATE HELP COMMITTEE HEARING
Changes in brain networks may predict MS worsening
PHILADELPHIA – (MS), according to a study described at the annual meeting of the American Academy of Neurology.
Neurologists do not have reliable biomarkers to predict disease evolution in the medium or long term for patients with MS. The ability to predict disease evolution accurately could aid in the choice of treatment.
Maria Assunta Rocca, MD, head of the Neuroimaging of CNS White Matter Unit, Department of Neurology, Institute of Experimental Neurology, Scientific Institute Ospedale, San Raffaele, Milan, Italy, and colleagues sought to evaluate structural and functional network MRI measures as predictors of clinical deterioration over 6.5 years. They obtained conventional, 3D, T1-weighted, diffusion-weighted MRI, and resting-state functional MRI images at baseline from 233 patients with MS and 77 healthy controls. Patients underwent a neurologic examination at baseline and after a median follow-up period of 6.5 years. At follow-up, the researchers classified patients as clinically stable or worsened, according to their change in Expanded Disability Status Scale (EDSS) score. They also evaluated conversion to secondary progressive MS among patients with relapsing remitting MS at baseline.
To identify the main large-scale resting state functional connectivity networks, Dr. Rocca and colleagues applied spatial independent component analysis to resting state functional MRI data. They applied the same technique to gray matter probability maps and fractional anisotropy maps to identify the corresponding structural gray matter and white matter networks.
At follow-up, 105 patients with MS (45%) had significant EDSS worsening. Of 157 patients with relapsing remitting MS, 26 (16%) converted to secondary progressive MS. The multivariable model, after adjustment for follow-up duration, identified baseline EDSS score (odds ratio, 1.59), normalized gray matter volume (OR, 0.99), and abnormally high baseline resting state functional connectivity of the left precentral gyrus in the sensorimotor network (OR, 1.67) as predictors of EDSS worsening. These variables remained significant after the researchers adjusted for treatment effect. Independent variables associated with conversion to secondary progressive MS included baseline EDSS score (OR, 2.8) and atrophy of gray matter networks associated with sensory (OR, 0.5) and motor (OR, 0.4) functions.
Dr. Rocca received personal compensation from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva, Merck Serono, and Roche. Coauthors reported research support from Biogen, Merck Serono, Novartis, Teva, and Roche..
SOURCE: Filippi M et al. AAN 2019, Abstract S49.004.
PHILADELPHIA – (MS), according to a study described at the annual meeting of the American Academy of Neurology.
Neurologists do not have reliable biomarkers to predict disease evolution in the medium or long term for patients with MS. The ability to predict disease evolution accurately could aid in the choice of treatment.
Maria Assunta Rocca, MD, head of the Neuroimaging of CNS White Matter Unit, Department of Neurology, Institute of Experimental Neurology, Scientific Institute Ospedale, San Raffaele, Milan, Italy, and colleagues sought to evaluate structural and functional network MRI measures as predictors of clinical deterioration over 6.5 years. They obtained conventional, 3D, T1-weighted, diffusion-weighted MRI, and resting-state functional MRI images at baseline from 233 patients with MS and 77 healthy controls. Patients underwent a neurologic examination at baseline and after a median follow-up period of 6.5 years. At follow-up, the researchers classified patients as clinically stable or worsened, according to their change in Expanded Disability Status Scale (EDSS) score. They also evaluated conversion to secondary progressive MS among patients with relapsing remitting MS at baseline.
To identify the main large-scale resting state functional connectivity networks, Dr. Rocca and colleagues applied spatial independent component analysis to resting state functional MRI data. They applied the same technique to gray matter probability maps and fractional anisotropy maps to identify the corresponding structural gray matter and white matter networks.
At follow-up, 105 patients with MS (45%) had significant EDSS worsening. Of 157 patients with relapsing remitting MS, 26 (16%) converted to secondary progressive MS. The multivariable model, after adjustment for follow-up duration, identified baseline EDSS score (odds ratio, 1.59), normalized gray matter volume (OR, 0.99), and abnormally high baseline resting state functional connectivity of the left precentral gyrus in the sensorimotor network (OR, 1.67) as predictors of EDSS worsening. These variables remained significant after the researchers adjusted for treatment effect. Independent variables associated with conversion to secondary progressive MS included baseline EDSS score (OR, 2.8) and atrophy of gray matter networks associated with sensory (OR, 0.5) and motor (OR, 0.4) functions.
Dr. Rocca received personal compensation from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva, Merck Serono, and Roche. Coauthors reported research support from Biogen, Merck Serono, Novartis, Teva, and Roche..
SOURCE: Filippi M et al. AAN 2019, Abstract S49.004.
PHILADELPHIA – (MS), according to a study described at the annual meeting of the American Academy of Neurology.
Neurologists do not have reliable biomarkers to predict disease evolution in the medium or long term for patients with MS. The ability to predict disease evolution accurately could aid in the choice of treatment.
Maria Assunta Rocca, MD, head of the Neuroimaging of CNS White Matter Unit, Department of Neurology, Institute of Experimental Neurology, Scientific Institute Ospedale, San Raffaele, Milan, Italy, and colleagues sought to evaluate structural and functional network MRI measures as predictors of clinical deterioration over 6.5 years. They obtained conventional, 3D, T1-weighted, diffusion-weighted MRI, and resting-state functional MRI images at baseline from 233 patients with MS and 77 healthy controls. Patients underwent a neurologic examination at baseline and after a median follow-up period of 6.5 years. At follow-up, the researchers classified patients as clinically stable or worsened, according to their change in Expanded Disability Status Scale (EDSS) score. They also evaluated conversion to secondary progressive MS among patients with relapsing remitting MS at baseline.
To identify the main large-scale resting state functional connectivity networks, Dr. Rocca and colleagues applied spatial independent component analysis to resting state functional MRI data. They applied the same technique to gray matter probability maps and fractional anisotropy maps to identify the corresponding structural gray matter and white matter networks.
At follow-up, 105 patients with MS (45%) had significant EDSS worsening. Of 157 patients with relapsing remitting MS, 26 (16%) converted to secondary progressive MS. The multivariable model, after adjustment for follow-up duration, identified baseline EDSS score (odds ratio, 1.59), normalized gray matter volume (OR, 0.99), and abnormally high baseline resting state functional connectivity of the left precentral gyrus in the sensorimotor network (OR, 1.67) as predictors of EDSS worsening. These variables remained significant after the researchers adjusted for treatment effect. Independent variables associated with conversion to secondary progressive MS included baseline EDSS score (OR, 2.8) and atrophy of gray matter networks associated with sensory (OR, 0.5) and motor (OR, 0.4) functions.
Dr. Rocca received personal compensation from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva, Merck Serono, and Roche. Coauthors reported research support from Biogen, Merck Serono, Novartis, Teva, and Roche..
SOURCE: Filippi M et al. AAN 2019, Abstract S49.004.
REPORTING FROM AAN 2019
Key clinical point: Structural and functional network MRI measures predict long-term worsening in multiple sclerosis.
Major finding: The odds ratio of worsening for patients with abnormally high baseline resting state functional connectivity is 1.67.
Study details: A prospective imaging study of 233 patients with multiple sclerosis and 77 healthy controls.
Disclosures: Dr. Rocca received personal compensation from Biogen Idec, Novartis, Genzyme, Sanofi-Aventis, Teva, Merck Serono, and Roche. Coauthors reported research support from Biogen, Merck Serono, Novartis, Teva, and Roche.
Source: Filippi M et al. AAN 2019, Abstract S49.004.
WISE sheds light on angina in INOCA
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
NEW ORLEANS – A higher baseline average coronary peak flow velocity is an independent predictor of angina in women with symptomatic ischemia and nonobstructive coronary artery disease (INOCA), according to a new report from the WISE-CVD study.
WISE-CVD (the Women’s Ischemia Syndrome Evaluation: Coronary Vascular Dysfunction) project is a National Institutes of Health–sponsored series of studies. WISE investigators have previously shown that higher baseline average peak flow velocity (BAPV) is correlated with volumetric flow and is an independent predictor of major adverse cardiovascular events. However, until now the relationship between BAPV and anginal symptoms hadn’t been investigated, Nissi S. Suppogu, MD, observed at the annual meeting of the American College of Cardiology.
She reported on 260 women with angiographically evaluated symptomatic INOCA who participated in WISE-CVD. They were divided into two groups based upon their BAPV: 123 had a BAPV of 22 cm/sec or more, and 137 had a BAPV of less than 22 cm/sec.
Women in the high BAPV group had more frequent angina as shown by their average score of 50 on that domain of the Seattle Angina Questionnaire, compared with 60 in the low-BAPV group. The high-BAPV group also had significantly worse angina-related quality of life as reflected in their lower score on that dimension of a related instrument, the Seattle Angina Questionnaire–7.
Further support for the notion that high-BAPV women with INOCA have more severe angina than those with low BAPV comes from the finding that they were significantly more likely to use nitrates (37.6% of them did so, compared with 22.6% of low-BAPV women) and ranolazine, or Ranexa (7.9% versus 1.7%). In addition, the high-BAPV patients had numerically greater usage of other antianginal agents – beta-blockers, calcium channel blockers, and ACE inhibitors or angiotensin receptor blockers – although these differences didn’t reach statistical significance, reported Dr. Suppogu of Cedars-Sinai Medical Center in Los Angeles.
She reported having no financial conflicts regarding her presentation.
REPORTING FROM ACC 19
Cystic Scalp Lesion
The Diagnosis: Merkel Cell Carcinoma
An excisional biopsy revealed that the dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma (Figure 1). The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli. There was a tendency for smudgy artifacts at the periphery of the infiltrate, and the cells had relatively scant cytoplasm with slight streaming. Occasional apoptotic forms were present. Immunohistochemistry showed strong dotlike staining with cytokeratin 20 and moderate positivity with synaptophysin and chromogranin A (Figure 2). Unusually, there also was weak staining in a few tumor cells with thyroid transcription factor 1, a marker usually indicative of small cell carcinoma of the lungs that typically is negative in Merkel cell carcinoma (MCC). A second thyroid transcription factor 1 monoclonal antibody used in a double immunostain for lung adenocarcinomas was completely negative. This second antibody is more specific but less sensitive than the stand-alone version. The skin biopsy results confirmed the diagnosis of MCC. Given the patient's frailty and comorbidities, wide local excision was not performed and the patient was referred to radiation oncology. He died several months later from metastatic MCC.
dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma. The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli (H&E, original magnification ×200).
Merkel cell carcinoma (original magnification ×200).
Merkel cell carcinoma is an uncommon skin malignancy that can be easily mistaken for other conditions if the clinician is not familiar with its typical presentation. It most commonly is found on the head and neck in elderly individuals, most often aged 60 to 80 years,1 with a notable history of sun exposure and/or immunosuppression. It is an aggressive skin cancer that originally was thought to be due to pathogenic changes of Merkel cells,2 which are specialized touch receptors located at the dermoepidermal junction of the skin; however, newer evidence has suggested that MCC arises from malignant changes to skin stem cells.3 It shares more characteristics with extracutaneous neuroendocrine tumors and is more aptly labeled by pathologists as a primary neuroendocrine carcinoma of the skin.4
The frequency of MCC is highest in Australia, likely due to intense sun exposure, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100,000 individuals from 2006 to 2010.5 The lowest incidence rates were reported in Finland (0.11 and 0.12 per 100,000 males and females, respectively)6 and Denmark (2.2 cases per million person-years).7 The clinical features of MCC are summarized by the mnemonic AEIOU: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin.8 In a 2008 study of 195 patients, 89% of primary MCC lesions met 3 or more criteria, 32% met 4 or more criteria, and 7% met all 5 criteria.8
The classic presentation of MCC is a pink-red to violaceous nodule on the head or neck in an elderly patient, but there is a need to maintain suspicion of malignancy when examining a presumed infected cystic lesion, especially when a round of antibiotics has not ameliorated the symptoms. According to Heath et al,8 of 106 patients treated for MCC, 56% of first clinical impressions were benign. A PubMed and Scopus search was performed with the MeSH headings Merkel cell carcinoma +/- presentation to uncover similar unusual presentations between 1970 and the present day. Merkel cell carcinoma has been misdiagnosed as seemingly benign lesions including lipoma,9 allergic contact dermatitis,10 and atheroma.11 The differential diagnosis of MCC also includes cysts, amelanotic melanoma, basal cell carcinoma, dermatofibrosarcoma protuberans, squamous cell carcinoma, fungal kerion, leiomyosarcoma, neurothekeoma, abscesses, and cutaneous lymphoma.
Merkel cell polyomavirus has been implicated in the malignant transformation of MCC. It is a small, human, nonenveloped, double-stranded DNA virus1 and is found in approximately 70% to 80% of MCC cases.12 Merkel cell polyomavirus is a respiratory tract pathogen that is acquired by immunocompetent infants; it integrates itself into the host's genome and then enters a long latency period to later reactivate in immunocompromised adults.13
Wide local excision down to fascia is the mainstay of treatment of MCC, with recommended margins of 1 to 2 cm.14 Mohs micrographic surgery also can be considered.15 Similar to other neuroendocrine tumors, MCC is considered a radiosensitive tumor; radiation likely improves local control and is recommended in early-stage disease.16,17 It also has been described as the sole treatment modality in patients who are not candidates for surgery. The role of chemotherapy is more controversial, as responses do not appear to be long-lasting but should be considered in patients with advanced disease.14,18 There have been major advances in immunotherapy with the recent approvals of avelumab, an anti-PD-L1 inhibitor,19 and pembrolizumab,20 an anti-PD-1 inhibitor, for metastatic MCC. Clinical trials for MCC using kinase inhibitors and somatostatin analogues currently are ongoing.21
Several studies have demonstrated high rates of occult nodal disease in clinically node-negative patients, which has led to widespread use of sentinel lymph node biopsies.22,23 A sentinel lymph node biopsy is recommended at the time of surgery to aid with treatment decisions and prognosis.24
Merkel cell carcinoma is highly aggressive, and more than one-third of patients die from their disease, making it twice as lethal as melanoma. Overall survival rates remain low (5-year overall survival, 0%-18%) for advanced disease.5 Unfortunately, progression to metastasis is common and most often occurs within 2 years of diagnosis.17,25 Follow-up after treatment of MCC is crucial, with the 2019 National Comprehensive Cancer Network (NCCN) guidelines suggesting a physical examination with complete skin and complete lymph node examination every 3 to 6 months for 3 years and every 6 to 12 months thereafter.15
This case is an important reminder to include MCC in the differential diagnosis of presumed infected cysts, particularly on sun-exposed sites in elderly patients, as our patient was treated with antibiotics twice without improvement. An infected cyst with a lack of response to antibiotics should alert clinicians to the potential of malignancy.
- Sourvinos G, Mammas IN, Spandidos GA. 2015 Merkel cell polyoma virus infections in childhood. Arch Virol. 2015;160:887-892.
- Sibley RK, Rosai J, Foucar E, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. a histologic and ultrastructural study of two cases. Am J Surg Pathol. 1980;4:211-221.
- Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1-7.
- Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864-872.
- Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma--a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737-742.
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793-801.
- Heath M, Jaimes N, Lamos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis of 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;59:375-381.
- Sarma DP, Heagley DE, Chalupa J, et al. An unusual clinical presentation of Merkel cell carcinoma: a case report. Case Rep Med. 2010;2010:905414.
- Craven E, Alexandroff A, Liu JK, et al. Merkel cell carcinoma mistaken for allergic contact dermatitis. BMJ. 2015;351:h4635.
- Kinoshita A, Hoashi T, Okazaki S, et al. Atypical case of Merkel cell carcinoma difficult to diagnose clinically. J Dermatol. 2017;44:E158-E159.
- Donepudi S, DeConti LC, Samlowski WE. Recent advances in the understanding of the genetics, etiology, and treatment of Merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
- Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyoma virus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83:2220-2224.
- Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Merkel Cell Carcinoma. Version 2.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2019.
- Jabbour J. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943-1952.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204-208.
- Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755-767.
- Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15-19.
- FDA approves pembrolizumab for Merkel cell carcinoma. US Food & Drug Administration website. http://www.fda.gov/Drugs/Information OnDrugs/ApprovedDrugs/ucm628867.htm. Published December 19, 2018. Accessed April 23, 2019.
- Schadendorff D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy, and unmet medical needs. Eur J Cancer. 2017;71:53-69.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036-1041.
- Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624-1630.
- Gupta SG, Wang LC, Penas LC, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685-690.
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16:2985-2993.
The Diagnosis: Merkel Cell Carcinoma
An excisional biopsy revealed that the dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma (Figure 1). The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli. There was a tendency for smudgy artifacts at the periphery of the infiltrate, and the cells had relatively scant cytoplasm with slight streaming. Occasional apoptotic forms were present. Immunohistochemistry showed strong dotlike staining with cytokeratin 20 and moderate positivity with synaptophysin and chromogranin A (Figure 2). Unusually, there also was weak staining in a few tumor cells with thyroid transcription factor 1, a marker usually indicative of small cell carcinoma of the lungs that typically is negative in Merkel cell carcinoma (MCC). A second thyroid transcription factor 1 monoclonal antibody used in a double immunostain for lung adenocarcinomas was completely negative. This second antibody is more specific but less sensitive than the stand-alone version. The skin biopsy results confirmed the diagnosis of MCC. Given the patient's frailty and comorbidities, wide local excision was not performed and the patient was referred to radiation oncology. He died several months later from metastatic MCC.
dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma. The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli (H&E, original magnification ×200).
Merkel cell carcinoma (original magnification ×200).
Merkel cell carcinoma is an uncommon skin malignancy that can be easily mistaken for other conditions if the clinician is not familiar with its typical presentation. It most commonly is found on the head and neck in elderly individuals, most often aged 60 to 80 years,1 with a notable history of sun exposure and/or immunosuppression. It is an aggressive skin cancer that originally was thought to be due to pathogenic changes of Merkel cells,2 which are specialized touch receptors located at the dermoepidermal junction of the skin; however, newer evidence has suggested that MCC arises from malignant changes to skin stem cells.3 It shares more characteristics with extracutaneous neuroendocrine tumors and is more aptly labeled by pathologists as a primary neuroendocrine carcinoma of the skin.4
The frequency of MCC is highest in Australia, likely due to intense sun exposure, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100,000 individuals from 2006 to 2010.5 The lowest incidence rates were reported in Finland (0.11 and 0.12 per 100,000 males and females, respectively)6 and Denmark (2.2 cases per million person-years).7 The clinical features of MCC are summarized by the mnemonic AEIOU: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin.8 In a 2008 study of 195 patients, 89% of primary MCC lesions met 3 or more criteria, 32% met 4 or more criteria, and 7% met all 5 criteria.8
The classic presentation of MCC is a pink-red to violaceous nodule on the head or neck in an elderly patient, but there is a need to maintain suspicion of malignancy when examining a presumed infected cystic lesion, especially when a round of antibiotics has not ameliorated the symptoms. According to Heath et al,8 of 106 patients treated for MCC, 56% of first clinical impressions were benign. A PubMed and Scopus search was performed with the MeSH headings Merkel cell carcinoma +/- presentation to uncover similar unusual presentations between 1970 and the present day. Merkel cell carcinoma has been misdiagnosed as seemingly benign lesions including lipoma,9 allergic contact dermatitis,10 and atheroma.11 The differential diagnosis of MCC also includes cysts, amelanotic melanoma, basal cell carcinoma, dermatofibrosarcoma protuberans, squamous cell carcinoma, fungal kerion, leiomyosarcoma, neurothekeoma, abscesses, and cutaneous lymphoma.
Merkel cell polyomavirus has been implicated in the malignant transformation of MCC. It is a small, human, nonenveloped, double-stranded DNA virus1 and is found in approximately 70% to 80% of MCC cases.12 Merkel cell polyomavirus is a respiratory tract pathogen that is acquired by immunocompetent infants; it integrates itself into the host's genome and then enters a long latency period to later reactivate in immunocompromised adults.13
Wide local excision down to fascia is the mainstay of treatment of MCC, with recommended margins of 1 to 2 cm.14 Mohs micrographic surgery also can be considered.15 Similar to other neuroendocrine tumors, MCC is considered a radiosensitive tumor; radiation likely improves local control and is recommended in early-stage disease.16,17 It also has been described as the sole treatment modality in patients who are not candidates for surgery. The role of chemotherapy is more controversial, as responses do not appear to be long-lasting but should be considered in patients with advanced disease.14,18 There have been major advances in immunotherapy with the recent approvals of avelumab, an anti-PD-L1 inhibitor,19 and pembrolizumab,20 an anti-PD-1 inhibitor, for metastatic MCC. Clinical trials for MCC using kinase inhibitors and somatostatin analogues currently are ongoing.21
Several studies have demonstrated high rates of occult nodal disease in clinically node-negative patients, which has led to widespread use of sentinel lymph node biopsies.22,23 A sentinel lymph node biopsy is recommended at the time of surgery to aid with treatment decisions and prognosis.24
Merkel cell carcinoma is highly aggressive, and more than one-third of patients die from their disease, making it twice as lethal as melanoma. Overall survival rates remain low (5-year overall survival, 0%-18%) for advanced disease.5 Unfortunately, progression to metastasis is common and most often occurs within 2 years of diagnosis.17,25 Follow-up after treatment of MCC is crucial, with the 2019 National Comprehensive Cancer Network (NCCN) guidelines suggesting a physical examination with complete skin and complete lymph node examination every 3 to 6 months for 3 years and every 6 to 12 months thereafter.15
This case is an important reminder to include MCC in the differential diagnosis of presumed infected cysts, particularly on sun-exposed sites in elderly patients, as our patient was treated with antibiotics twice without improvement. An infected cyst with a lack of response to antibiotics should alert clinicians to the potential of malignancy.
The Diagnosis: Merkel Cell Carcinoma
An excisional biopsy revealed that the dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma (Figure 1). The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli. There was a tendency for smudgy artifacts at the periphery of the infiltrate, and the cells had relatively scant cytoplasm with slight streaming. Occasional apoptotic forms were present. Immunohistochemistry showed strong dotlike staining with cytokeratin 20 and moderate positivity with synaptophysin and chromogranin A (Figure 2). Unusually, there also was weak staining in a few tumor cells with thyroid transcription factor 1, a marker usually indicative of small cell carcinoma of the lungs that typically is negative in Merkel cell carcinoma (MCC). A second thyroid transcription factor 1 monoclonal antibody used in a double immunostain for lung adenocarcinomas was completely negative. This second antibody is more specific but less sensitive than the stand-alone version. The skin biopsy results confirmed the diagnosis of MCC. Given the patient's frailty and comorbidities, wide local excision was not performed and the patient was referred to radiation oncology. He died several months later from metastatic MCC.
dermis was mostly replaced by a malignant neoplastic infiltrate morphologically resembling small cell carcinoma. The cells had uniform hyperchromatic nuclei with fairly even chromatin and generally inconspicuous nucleoli (H&E, original magnification ×200).
Merkel cell carcinoma (original magnification ×200).
Merkel cell carcinoma is an uncommon skin malignancy that can be easily mistaken for other conditions if the clinician is not familiar with its typical presentation. It most commonly is found on the head and neck in elderly individuals, most often aged 60 to 80 years,1 with a notable history of sun exposure and/or immunosuppression. It is an aggressive skin cancer that originally was thought to be due to pathogenic changes of Merkel cells,2 which are specialized touch receptors located at the dermoepidermal junction of the skin; however, newer evidence has suggested that MCC arises from malignant changes to skin stem cells.3 It shares more characteristics with extracutaneous neuroendocrine tumors and is more aptly labeled by pathologists as a primary neuroendocrine carcinoma of the skin.4
The frequency of MCC is highest in Australia, likely due to intense sun exposure, where the age-adjusted incidence rate reported in Queensland was 1.6 per 100,000 individuals from 2006 to 2010.5 The lowest incidence rates were reported in Finland (0.11 and 0.12 per 100,000 males and females, respectively)6 and Denmark (2.2 cases per million person-years).7 The clinical features of MCC are summarized by the mnemonic AEIOU: asymptomatic/lack of tenderness, expanding rapidly, immune suppression, older than 50 years, UV-exposed site on a person with fair skin.8 In a 2008 study of 195 patients, 89% of primary MCC lesions met 3 or more criteria, 32% met 4 or more criteria, and 7% met all 5 criteria.8
The classic presentation of MCC is a pink-red to violaceous nodule on the head or neck in an elderly patient, but there is a need to maintain suspicion of malignancy when examining a presumed infected cystic lesion, especially when a round of antibiotics has not ameliorated the symptoms. According to Heath et al,8 of 106 patients treated for MCC, 56% of first clinical impressions were benign. A PubMed and Scopus search was performed with the MeSH headings Merkel cell carcinoma +/- presentation to uncover similar unusual presentations between 1970 and the present day. Merkel cell carcinoma has been misdiagnosed as seemingly benign lesions including lipoma,9 allergic contact dermatitis,10 and atheroma.11 The differential diagnosis of MCC also includes cysts, amelanotic melanoma, basal cell carcinoma, dermatofibrosarcoma protuberans, squamous cell carcinoma, fungal kerion, leiomyosarcoma, neurothekeoma, abscesses, and cutaneous lymphoma.
Merkel cell polyomavirus has been implicated in the malignant transformation of MCC. It is a small, human, nonenveloped, double-stranded DNA virus1 and is found in approximately 70% to 80% of MCC cases.12 Merkel cell polyomavirus is a respiratory tract pathogen that is acquired by immunocompetent infants; it integrates itself into the host's genome and then enters a long latency period to later reactivate in immunocompromised adults.13
Wide local excision down to fascia is the mainstay of treatment of MCC, with recommended margins of 1 to 2 cm.14 Mohs micrographic surgery also can be considered.15 Similar to other neuroendocrine tumors, MCC is considered a radiosensitive tumor; radiation likely improves local control and is recommended in early-stage disease.16,17 It also has been described as the sole treatment modality in patients who are not candidates for surgery. The role of chemotherapy is more controversial, as responses do not appear to be long-lasting but should be considered in patients with advanced disease.14,18 There have been major advances in immunotherapy with the recent approvals of avelumab, an anti-PD-L1 inhibitor,19 and pembrolizumab,20 an anti-PD-1 inhibitor, for metastatic MCC. Clinical trials for MCC using kinase inhibitors and somatostatin analogues currently are ongoing.21
Several studies have demonstrated high rates of occult nodal disease in clinically node-negative patients, which has led to widespread use of sentinel lymph node biopsies.22,23 A sentinel lymph node biopsy is recommended at the time of surgery to aid with treatment decisions and prognosis.24
Merkel cell carcinoma is highly aggressive, and more than one-third of patients die from their disease, making it twice as lethal as melanoma. Overall survival rates remain low (5-year overall survival, 0%-18%) for advanced disease.5 Unfortunately, progression to metastasis is common and most often occurs within 2 years of diagnosis.17,25 Follow-up after treatment of MCC is crucial, with the 2019 National Comprehensive Cancer Network (NCCN) guidelines suggesting a physical examination with complete skin and complete lymph node examination every 3 to 6 months for 3 years and every 6 to 12 months thereafter.15
This case is an important reminder to include MCC in the differential diagnosis of presumed infected cysts, particularly on sun-exposed sites in elderly patients, as our patient was treated with antibiotics twice without improvement. An infected cyst with a lack of response to antibiotics should alert clinicians to the potential of malignancy.
- Sourvinos G, Mammas IN, Spandidos GA. 2015 Merkel cell polyoma virus infections in childhood. Arch Virol. 2015;160:887-892.
- Sibley RK, Rosai J, Foucar E, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. a histologic and ultrastructural study of two cases. Am J Surg Pathol. 1980;4:211-221.
- Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1-7.
- Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864-872.
- Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma--a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737-742.
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793-801.
- Heath M, Jaimes N, Lamos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis of 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;59:375-381.
- Sarma DP, Heagley DE, Chalupa J, et al. An unusual clinical presentation of Merkel cell carcinoma: a case report. Case Rep Med. 2010;2010:905414.
- Craven E, Alexandroff A, Liu JK, et al. Merkel cell carcinoma mistaken for allergic contact dermatitis. BMJ. 2015;351:h4635.
- Kinoshita A, Hoashi T, Okazaki S, et al. Atypical case of Merkel cell carcinoma difficult to diagnose clinically. J Dermatol. 2017;44:E158-E159.
- Donepudi S, DeConti LC, Samlowski WE. Recent advances in the understanding of the genetics, etiology, and treatment of Merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
- Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyoma virus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83:2220-2224.
- Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Merkel Cell Carcinoma. Version 2.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2019.
- Jabbour J. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943-1952.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204-208.
- Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755-767.
- Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15-19.
- FDA approves pembrolizumab for Merkel cell carcinoma. US Food & Drug Administration website. http://www.fda.gov/Drugs/Information OnDrugs/ApprovedDrugs/ucm628867.htm. Published December 19, 2018. Accessed April 23, 2019.
- Schadendorff D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy, and unmet medical needs. Eur J Cancer. 2017;71:53-69.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036-1041.
- Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624-1630.
- Gupta SG, Wang LC, Penas LC, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685-690.
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16:2985-2993.
- Sourvinos G, Mammas IN, Spandidos GA. 2015 Merkel cell polyoma virus infections in childhood. Arch Virol. 2015;160:887-892.
- Sibley RK, Rosai J, Foucar E, et al. Neuroendocrine (Merkel cell) carcinoma of the skin. a histologic and ultrastructural study of two cases. Am J Surg Pathol. 1980;4:211-221.
- Tilling T, Moll I. Which are the cells of origin in Merkel cell carcinoma? J Skin Cancer. 2012;2012:1-7.
- Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166.
- Youlden DR, Soyer HP, Youl PH, et al. Incidence and survival for Merkel cell carcinoma in Queensland, Australia, 1993-2010. JAMA Dermatol. 2014;150:864-872.
- Kukko H, Böhling T, Koljonen V, et al. Merkel cell carcinoma--a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer. 2012;48:737-742.
- Kaae J, Hansen AV, Biggar RJ, et al. Merkel cell carcinoma: incidence, mortality, and risk of other cancers. J Natl Cancer Inst. 2010;102:793-801.
- Heath M, Jaimes N, Lamos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis of 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;59:375-381.
- Sarma DP, Heagley DE, Chalupa J, et al. An unusual clinical presentation of Merkel cell carcinoma: a case report. Case Rep Med. 2010;2010:905414.
- Craven E, Alexandroff A, Liu JK, et al. Merkel cell carcinoma mistaken for allergic contact dermatitis. BMJ. 2015;351:h4635.
- Kinoshita A, Hoashi T, Okazaki S, et al. Atypical case of Merkel cell carcinoma difficult to diagnose clinically. J Dermatol. 2017;44:E158-E159.
- Donepudi S, DeConti LC, Samlowski WE. Recent advances in the understanding of the genetics, etiology, and treatment of Merkel cell carcinoma. Semin Oncol. 2012;39:163-172.
- Abedi Kiasari B, Vallely PJ, Klapper PE. Merkel cell polyoma virus DNA in immunocompetent and immunocompromised patients with respiratory disease. J Med Virol. 2011;83:2220-2224.
- Tai P. A practical update of surgical management of Merkel cell carcinoma of the skin. ISRN Surg. 2013;2013:850797.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Merkel Cell Carcinoma. Version 2.2019. Fort Washington, PA: National Comprehensive Cancer Network; 2019.
- Jabbour J. Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004. Ann Surg Oncol. 2007;14:1943-1952.
- Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204-208.
- Akhtar S, Oza KK, Wright J. Merkel cell carcinoma: report of 10 cases and review of the literature. J Am Acad Dermatol 2000;43:755-767.
- Palla AR, Doll D. Immunotherapy in Merkel cell carcinoma: role of avelumab. Immunotargets Ther. 2018;7:15-19.
- FDA approves pembrolizumab for Merkel cell carcinoma. US Food & Drug Administration website. http://www.fda.gov/Drugs/Information OnDrugs/ApprovedDrugs/ucm628867.htm. Published December 19, 2018. Accessed April 23, 2019.
- Schadendorff D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy, and unmet medical needs. Eur J Cancer. 2017;71:53-69.
- Schwartz JL, Griffith KA, Lowe L, et al. Features predicting sentinel lymph node positivity in Merkel cell carcinoma. J Clin Oncol. 2011;29:1036-1041.
- Kachare SD, Wong JH, Vohra NA, et al. Sentinel lymph node biopsy is associated with improved survival in Merkel cell carcinoma. Ann Surg Oncol. 2014;21:1624-1630.
- Gupta SG, Wang LC, Penas LC, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685-690.
- Bajetta E, Celio L, Platania M, et al. Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone. Ann Surg Oncol. 2009;16:2985-2993.
A frail 85-year-old man presented to the emergency department for treatment of a 4.0.2 ×2.5-cm, erythematous, tender nodule on the scalp. The area was increasingly painful with persistent throbbing, which led to sleep disruption. The nodule did not express any material and was not aspirated or surgically treated. The lesion had been present for 1 to 2 years and was small and stable in size until it grew rapidly in the 6 weeks prior to presentation. The patient initially presented to his general practitioner during this period of rapid growth and was diagnosed with an infected sebaceous cyst that was treated with a course of oral cephalexin without improvement. Bacterial or fungal cultures were not performed. No other similar lesions were present, but there was 1 palpable lymph node in the right posterior cervical chain. At the time of presentation to the emergency department, the patient felt well and denied weight loss, night sweats, or fevers. He was given a dose of intravenous cefazolin by the emergency physician and then was referred to surgery for management of an infected sebaceous cyst.
Ibudilast’s efficacy differs in primary and secondary progressive MS
PHILADELPHIA – researchers reported at the annual meeting of the American Academy of Neurology.
The difference may be related to faster atrophy rates among patients with primary progressive MS who received placebo, compared with those with secondary progressive MS who received placebo.
The finding was surprising, said Andrew Goodman, MD, professor of neurology, chief of the neuroimmunology unit, and director of the multiple sclerosis center at the University of Rochester (N.Y.). “Going into the trial, it was my bias and expectation that both primary and secondary progressive MS would behave more similarly than different.”
The trial, SPRINT-MS, included more than 250 patients with progressive MS at 28 sites. Patients were aged 18-65 years and were followed for 96 weeks. Patients had primary progressive MS (n = 134) or secondary progressive MS (n = 121) and were randomized 1:1 to ibudilast or placebo.
Ibudilast is an orally administered small molecule that has been used in Japan for approximately 30 years for asthma and other indications, Dr. Goodman said. Preclinical models suggested that the drug may have neuroprotective effects.
The trial’s primary result – a 48% slowing in the rate of whole brain atrophy as measured by brain parenchymal fraction with ibudilast – was reported last year (N Engl J Med. 2018 Aug 30;379[9]:846-55).
The present study examined whether the treatment effect of ibudilast was similar by progressive disease type using a linear mixed model analytic approach.
The group with primary progressive MS included a smaller percentage of women. Patients with secondary progressive MS had longer disease duration and more brain atrophy at baseline.
“The overall benefit which we previously reported appears to be driven by subjects with primary progressive rather than secondary progressive MS,” Dr. Goodman said. Accounting for baseline covariates did not affect this result.
Among patients who received placebo, brain atrophy in those with secondary progressive MS was 57% slower than in those with primary progressive MS. The rate of atrophy for untreated patients with primary progressive MS “was roughly twice as fast as that in the secondary progressive MS group, which we think may explain in part the differential in efficacy,” Dr. Goodman said. “These findings may impact future trial design for progressive MS.”
The SPRINT-MS trial was funded by the National Institute of Neurological Disorders and Stroke. The National Multiple Sclerosis Society and MediciNova also supported the study. Dr. Goodman reported receiving research support from pharmaceutical companies, as well as personal compensation from companies for consulting, serving on a scientific advisory board, and speaking.
SOURCE: Goodman A et al. AAN 2019, Abstract S12.007.
PHILADELPHIA – researchers reported at the annual meeting of the American Academy of Neurology.
The difference may be related to faster atrophy rates among patients with primary progressive MS who received placebo, compared with those with secondary progressive MS who received placebo.
The finding was surprising, said Andrew Goodman, MD, professor of neurology, chief of the neuroimmunology unit, and director of the multiple sclerosis center at the University of Rochester (N.Y.). “Going into the trial, it was my bias and expectation that both primary and secondary progressive MS would behave more similarly than different.”
The trial, SPRINT-MS, included more than 250 patients with progressive MS at 28 sites. Patients were aged 18-65 years and were followed for 96 weeks. Patients had primary progressive MS (n = 134) or secondary progressive MS (n = 121) and were randomized 1:1 to ibudilast or placebo.
Ibudilast is an orally administered small molecule that has been used in Japan for approximately 30 years for asthma and other indications, Dr. Goodman said. Preclinical models suggested that the drug may have neuroprotective effects.
The trial’s primary result – a 48% slowing in the rate of whole brain atrophy as measured by brain parenchymal fraction with ibudilast – was reported last year (N Engl J Med. 2018 Aug 30;379[9]:846-55).
The present study examined whether the treatment effect of ibudilast was similar by progressive disease type using a linear mixed model analytic approach.
The group with primary progressive MS included a smaller percentage of women. Patients with secondary progressive MS had longer disease duration and more brain atrophy at baseline.
“The overall benefit which we previously reported appears to be driven by subjects with primary progressive rather than secondary progressive MS,” Dr. Goodman said. Accounting for baseline covariates did not affect this result.
Among patients who received placebo, brain atrophy in those with secondary progressive MS was 57% slower than in those with primary progressive MS. The rate of atrophy for untreated patients with primary progressive MS “was roughly twice as fast as that in the secondary progressive MS group, which we think may explain in part the differential in efficacy,” Dr. Goodman said. “These findings may impact future trial design for progressive MS.”
The SPRINT-MS trial was funded by the National Institute of Neurological Disorders and Stroke. The National Multiple Sclerosis Society and MediciNova also supported the study. Dr. Goodman reported receiving research support from pharmaceutical companies, as well as personal compensation from companies for consulting, serving on a scientific advisory board, and speaking.
SOURCE: Goodman A et al. AAN 2019, Abstract S12.007.
PHILADELPHIA – researchers reported at the annual meeting of the American Academy of Neurology.
The difference may be related to faster atrophy rates among patients with primary progressive MS who received placebo, compared with those with secondary progressive MS who received placebo.
The finding was surprising, said Andrew Goodman, MD, professor of neurology, chief of the neuroimmunology unit, and director of the multiple sclerosis center at the University of Rochester (N.Y.). “Going into the trial, it was my bias and expectation that both primary and secondary progressive MS would behave more similarly than different.”
The trial, SPRINT-MS, included more than 250 patients with progressive MS at 28 sites. Patients were aged 18-65 years and were followed for 96 weeks. Patients had primary progressive MS (n = 134) or secondary progressive MS (n = 121) and were randomized 1:1 to ibudilast or placebo.
Ibudilast is an orally administered small molecule that has been used in Japan for approximately 30 years for asthma and other indications, Dr. Goodman said. Preclinical models suggested that the drug may have neuroprotective effects.
The trial’s primary result – a 48% slowing in the rate of whole brain atrophy as measured by brain parenchymal fraction with ibudilast – was reported last year (N Engl J Med. 2018 Aug 30;379[9]:846-55).
The present study examined whether the treatment effect of ibudilast was similar by progressive disease type using a linear mixed model analytic approach.
The group with primary progressive MS included a smaller percentage of women. Patients with secondary progressive MS had longer disease duration and more brain atrophy at baseline.
“The overall benefit which we previously reported appears to be driven by subjects with primary progressive rather than secondary progressive MS,” Dr. Goodman said. Accounting for baseline covariates did not affect this result.
Among patients who received placebo, brain atrophy in those with secondary progressive MS was 57% slower than in those with primary progressive MS. The rate of atrophy for untreated patients with primary progressive MS “was roughly twice as fast as that in the secondary progressive MS group, which we think may explain in part the differential in efficacy,” Dr. Goodman said. “These findings may impact future trial design for progressive MS.”
The SPRINT-MS trial was funded by the National Institute of Neurological Disorders and Stroke. The National Multiple Sclerosis Society and MediciNova also supported the study. Dr. Goodman reported receiving research support from pharmaceutical companies, as well as personal compensation from companies for consulting, serving on a scientific advisory board, and speaking.
SOURCE: Goodman A et al. AAN 2019, Abstract S12.007.
REPORTING FROM AAN 2019
CBO predicts more Medicare spending with drug rebate proposal
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.
Medicare spending on pharmaceuticals is projected to increase if the Centers for Medicare & Medicaid Services finalizes changes to drug rebates in the Medicare program.
The Congressional Budget Office is estimating that Medicare spending would increase by $170 billion from 2020-2029 if the rebate rule goes into effect, according to a report released May 2.
The proposed rule, issued Jan. 31, would make it illegal for drug manufacturers to pay rebates to health plans and pharmacy benefit managers in return for better formulary placement. Instead of rebates, manufacturers could offer discounts directly to beneficiaries by lowering list prices or making a payment to the pharmacy for the full amount of the negotiated discount – a chargeback. Under the proposal, a beneficiary’s cost sharing would be based on the lower list price or the price after the chargeback.
The CBO’s projected spending increases are based on the assumption that manufacturers will withhold 15% of current-law rebates, as well as increases in federal subsidies for premiums, changes in annual thresholds to beneficiary cost sharing, and the cost of implementing the chargeback system.
The agency expects premiums to rise, as many plans currently use the rebates they receive from drug companies to lower premiums across the board.
However, some beneficiaries “would pay lower prices on their prescription drugs, and for some beneficiaries, those reductions would be greater than their premium increases,” the CBO stated in its report. For beneficiaries who use few drugs or who use drugs that have no significant rebates, “the premium increase would outweigh the price reduction.”
Another reason federal spending would increase under this proposal is an expected increase in utilization that would come with the lowering of prices.
“In CBO’s estimate, the additional Part D utilization stemming from implementing the proposed rule would increase federal spending for beneficiaries who are not enrolled in the low-income subsidy program over the 2020-2029 period by a total of about 2% or $10 billion,” the report noted.
But the increase in utilization would have a net positive effect on Medicare spending for this population, as more beneficiaries followed their drug regimens resulting in lower spending for physician and hospital services under Medicare Part A and Part B by an estimated $20 billion over the same period, according to the CBO.
“On net, those effects are projected to reduce Medicare spending by $10 billion over the 2020-2029 period,” according to the report.