User login
Liquid Fasting Mitigates Negative Pre-Surgery Impact of Semaglutide
These findings suggest that patients taking GLP-1 receptor agonists (GLP-1RAs) may benefit from a 24-hour liquid fast before anesthetic procedures without the need for a medication hold, reported lead author Haarika Korlipara, MD, of NewYork–Presbyterian/Weill Cornell Medical Center, New York, and colleagues.
“[T]he effects of delayed gastric emptying in patients on long-acting GLP-1RAs are clinically important in the management of anesthetized patients, who may develop periprocedural complications in the setting of retained solid gastric contents,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
The researchers retrospectively analyzed clinical data from 1,212 patients undergoing upper endoscopy at a tertiary care center. Among them, 602 were on semaglutide for more than four weeks, while 610 were controls not taking the medication.
The primary outcome was the presence of retained solid gastric contents. Secondary outcomes included the need for intubation, early procedure termination, and recommendations for repeat endoscopy.
Semaglutide use was an independent predictor of retained solid gastric contents (odds ratio [OR], 4.74; 95% CI, 2.40-9.35; P less than .0001). Multivariable propensity-matched analysis showed a 6% absolute increase in retained gastric contents in the semaglutide group compared to controls (P less than .0001).
This increase appeared clinically relevant, as semaglutide use was associated with a higher rate of early procedure termination (OR, 3.09; P = 0.02) and recommendations for repeat endoscopies (OR, 3.61; P = 0.02), “indicating the degree of retained solid gastric contents was enough to limit the intended gastric mucosal examination,” the investigators wrote.
However, patients who underwent same-day colonoscopy, which included a 24-hour clear liquid fast leading up to the procedure, were less likely to have retained gastric contents (OR, 0.41; 95% CI, 0.23-0.73; P = 0.003), suggesting that extended fasting protocols may mitigate the risk of procedural complications.
“Patients with a history of gastroparesis are often advised to stop ingesting solid foods and maintain a clear liquid diet for a longer period than standard ASA guidance before anesthetized procedures,” Dr. Korlipara and colleagues wrote. “In our opinion, this recommendation should be considered in patients on long-term GLP-1RA therapy, in response to the findings reported in this study and others about the protective effects of a 24-hour liquid fast.”
Point-of-care gastric ultrasound may also be considered to evaluate patients at higher risk of retained stomach contents, they added, especially in patients with additional risk factors for delayed gastric emptying.
“Previously published data have linked prolonged gastric emptying delays in patients chronically using these medications,” they wrote. “Considering the effect on blood sugar and associated procedural risk, especially in patients taking this medication for diabetes management, more studies are warranted to determine the effect of medication on periprocedural complications and recommend repeat evaluation.”
After this study was released, new clinical guidance on the use of GLP-1RAs before surgery was co-published by AGA and four other societies. The guidance notes that, in most cases, patients can continue to take GLP-1RAs, but individual risk factors for complications should be assessed prior to surgery. The guidance cautions that patients at high risk for significant GI side effects should follow a liquid diet for 24 hours before a procedure and the anesthesia plan be adjusted accordingly. In rare cases, the procedure should be delayed.
Dr. Korlipara disclosed no conflicts of interest.
These findings suggest that patients taking GLP-1 receptor agonists (GLP-1RAs) may benefit from a 24-hour liquid fast before anesthetic procedures without the need for a medication hold, reported lead author Haarika Korlipara, MD, of NewYork–Presbyterian/Weill Cornell Medical Center, New York, and colleagues.
“[T]he effects of delayed gastric emptying in patients on long-acting GLP-1RAs are clinically important in the management of anesthetized patients, who may develop periprocedural complications in the setting of retained solid gastric contents,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
The researchers retrospectively analyzed clinical data from 1,212 patients undergoing upper endoscopy at a tertiary care center. Among them, 602 were on semaglutide for more than four weeks, while 610 were controls not taking the medication.
The primary outcome was the presence of retained solid gastric contents. Secondary outcomes included the need for intubation, early procedure termination, and recommendations for repeat endoscopy.
Semaglutide use was an independent predictor of retained solid gastric contents (odds ratio [OR], 4.74; 95% CI, 2.40-9.35; P less than .0001). Multivariable propensity-matched analysis showed a 6% absolute increase in retained gastric contents in the semaglutide group compared to controls (P less than .0001).
This increase appeared clinically relevant, as semaglutide use was associated with a higher rate of early procedure termination (OR, 3.09; P = 0.02) and recommendations for repeat endoscopies (OR, 3.61; P = 0.02), “indicating the degree of retained solid gastric contents was enough to limit the intended gastric mucosal examination,” the investigators wrote.
However, patients who underwent same-day colonoscopy, which included a 24-hour clear liquid fast leading up to the procedure, were less likely to have retained gastric contents (OR, 0.41; 95% CI, 0.23-0.73; P = 0.003), suggesting that extended fasting protocols may mitigate the risk of procedural complications.
“Patients with a history of gastroparesis are often advised to stop ingesting solid foods and maintain a clear liquid diet for a longer period than standard ASA guidance before anesthetized procedures,” Dr. Korlipara and colleagues wrote. “In our opinion, this recommendation should be considered in patients on long-term GLP-1RA therapy, in response to the findings reported in this study and others about the protective effects of a 24-hour liquid fast.”
Point-of-care gastric ultrasound may also be considered to evaluate patients at higher risk of retained stomach contents, they added, especially in patients with additional risk factors for delayed gastric emptying.
“Previously published data have linked prolonged gastric emptying delays in patients chronically using these medications,” they wrote. “Considering the effect on blood sugar and associated procedural risk, especially in patients taking this medication for diabetes management, more studies are warranted to determine the effect of medication on periprocedural complications and recommend repeat evaluation.”
After this study was released, new clinical guidance on the use of GLP-1RAs before surgery was co-published by AGA and four other societies. The guidance notes that, in most cases, patients can continue to take GLP-1RAs, but individual risk factors for complications should be assessed prior to surgery. The guidance cautions that patients at high risk for significant GI side effects should follow a liquid diet for 24 hours before a procedure and the anesthesia plan be adjusted accordingly. In rare cases, the procedure should be delayed.
Dr. Korlipara disclosed no conflicts of interest.
These findings suggest that patients taking GLP-1 receptor agonists (GLP-1RAs) may benefit from a 24-hour liquid fast before anesthetic procedures without the need for a medication hold, reported lead author Haarika Korlipara, MD, of NewYork–Presbyterian/Weill Cornell Medical Center, New York, and colleagues.
“[T]he effects of delayed gastric emptying in patients on long-acting GLP-1RAs are clinically important in the management of anesthetized patients, who may develop periprocedural complications in the setting of retained solid gastric contents,” the investigators wrote in Techniques and Innovations in Gastrointestinal Endoscopy.
The researchers retrospectively analyzed clinical data from 1,212 patients undergoing upper endoscopy at a tertiary care center. Among them, 602 were on semaglutide for more than four weeks, while 610 were controls not taking the medication.
The primary outcome was the presence of retained solid gastric contents. Secondary outcomes included the need for intubation, early procedure termination, and recommendations for repeat endoscopy.
Semaglutide use was an independent predictor of retained solid gastric contents (odds ratio [OR], 4.74; 95% CI, 2.40-9.35; P less than .0001). Multivariable propensity-matched analysis showed a 6% absolute increase in retained gastric contents in the semaglutide group compared to controls (P less than .0001).
This increase appeared clinically relevant, as semaglutide use was associated with a higher rate of early procedure termination (OR, 3.09; P = 0.02) and recommendations for repeat endoscopies (OR, 3.61; P = 0.02), “indicating the degree of retained solid gastric contents was enough to limit the intended gastric mucosal examination,” the investigators wrote.
However, patients who underwent same-day colonoscopy, which included a 24-hour clear liquid fast leading up to the procedure, were less likely to have retained gastric contents (OR, 0.41; 95% CI, 0.23-0.73; P = 0.003), suggesting that extended fasting protocols may mitigate the risk of procedural complications.
“Patients with a history of gastroparesis are often advised to stop ingesting solid foods and maintain a clear liquid diet for a longer period than standard ASA guidance before anesthetized procedures,” Dr. Korlipara and colleagues wrote. “In our opinion, this recommendation should be considered in patients on long-term GLP-1RA therapy, in response to the findings reported in this study and others about the protective effects of a 24-hour liquid fast.”
Point-of-care gastric ultrasound may also be considered to evaluate patients at higher risk of retained stomach contents, they added, especially in patients with additional risk factors for delayed gastric emptying.
“Previously published data have linked prolonged gastric emptying delays in patients chronically using these medications,” they wrote. “Considering the effect on blood sugar and associated procedural risk, especially in patients taking this medication for diabetes management, more studies are warranted to determine the effect of medication on periprocedural complications and recommend repeat evaluation.”
After this study was released, new clinical guidance on the use of GLP-1RAs before surgery was co-published by AGA and four other societies. The guidance notes that, in most cases, patients can continue to take GLP-1RAs, but individual risk factors for complications should be assessed prior to surgery. The guidance cautions that patients at high risk for significant GI side effects should follow a liquid diet for 24 hours before a procedure and the anesthesia plan be adjusted accordingly. In rare cases, the procedure should be delayed.
Dr. Korlipara disclosed no conflicts of interest.
FROM TECHNIQUES AND INNOVATIONS IN GASTROINTESTINAL ENDOSCOPY
Plastic Pollution’s Next Victim: The Human Urinary Tract
Although a 2019 World Health Organization (WHO) report concluded that microplastics in drinking water posed no risk to human health, accumulating evidence is beginning to challenge these findings.
Since plastics became widely used in the mid-20th century, they have evolved from a novel substance to an essential component in countless applications, with global production reaching 368 million tons in 2019 and expected to double by 2039. The production and degradation of plastics involve physical, chemical, and biological processes, leading to the formation of tiny fragments known as microplastics (MPs) and nanoplastics (NPs), which accumulate in the environment. Beyond the well-documented environmental harms of MPs and NPs, growing evidence of their presence within the human body raises concerns about their potential to trigger various harmful biological processes. Their detection in the urinary tract and their potential links to kidney and bladder diseases, as shown in animal studies, are particularly alarming.
Impacts Becoming Apparent
As the impact of plastic pollution becomes increasingly apparent, the need for standardized international definitions of MPs and NPs is pressing. Government publications reveal notable discrepancies between organizations in defining these fragmented plastics. The lack of consensus among regulatory bodies highlights the challenges in mitigating the environmental and health impacts of MPs and NPs. The International Organization for Standardization offers the most precise classification, defining MPs as solid, insoluble plastic particles ranging from 1 µm to 1 mm and NPs as particles smaller than 1 µm.
The intrusion of MPs and NPs into the human body, whether through inhalation, ingestion, or skin exposure (via wounds, hair follicles, or sweat glands), has been linked to harmful biological effects, including inflammation, alterations in cellular metabolism, physical cellular damage, and reduced cell viability.
Urinary Tract Plastics
The detection of MPs and NPs in the human urinary tract, combined with limited understanding of their effects, is a growing concern. An exploratory study published earlier this year aimed to systematically summarize the existing literature regarding the presence of MPs and NPs in the urinary tract and their potential consequences, guided by these research questions:
- What are the characteristics of the plastics detected in the human urinary tract?
- How are MPs and NPs defined in the current literature?
- What methodologies are used to explore the presence and effects of MPs and NPs?
- What are the pathophysiologic consequences of the presence of MPs and NPs in the human urinary tract?
For this study, the “urinary tract” included the kidneys, bladder, ureter, urethra, and urine. By focusing on the urinary tract, the study aimed to consolidate current understanding of MPs and NPs, raise awareness of this emerging issue, and lay the groundwork for further research that could contribute to public health policies and clinical practice guidelines.
The researchers conducted a scoping literature review following the recommendations of the JBI [formerly known as the Joanna Briggs Institute). They systematically searched five databases — PubMed, Scopus, CINAHL, Web of Science, and Embase — as well as gray literature sources.
Concerning Study Results
Eighteen articles were identified. The authors represent seven countries: Pakistan (n = 1), the Netherlands (n = 1), the US (n = 1), Taiwan (n = 1), Germany (n = 3), China (n = 5), and Italy (n = 6). Among these studies, six investigated and characterized the presence of MPs and NPs in the human urinary tract. MPs and NPs were detected in urine samples (n = 5), kidney cancer samples (n = 2), and bladder cancer samples (n = 1).
Additionally, 12 studies examined the effects of MPs and NPs on human urinary tract cell lines. Their findings suggest that MPs and NPs have cytotoxic effects, increase inflammation, reduce cell viability, and alter mitogen-activated protein kinase signaling pathways.
Raman spectroscopy was the primary method used to detect and characterize MPs and NPs in human samples (five out of six studies; 83%). Alternatively, pyrolysis-gas chromatography-mass spectrometry combined with direct laser infrared spectroscopy was used in one study.
Further Research Needed
This exploratory study underscores the urgent need for further research and policy development to address the challenges posed by microplastic contamination. It highlights the rapidly emerging threat of human urinary tract contamination by microplastics, questioning the WHO’s claim that microplastics pose no public health risk. The documented cytotoxic effects of microplastics, and their ability to induce inflammation, reduce cell viability, and disrupt signaling pathways, raise significant public health concerns related to bladder cancer, chronic kidney disease, chronic urinary infections, and incontinence.
Bernard-Alex Gauzere, retired physician formerly with the national health system in France (intensive care unit, tropical medicine), has disclosed no relevant financial relationships.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Although a 2019 World Health Organization (WHO) report concluded that microplastics in drinking water posed no risk to human health, accumulating evidence is beginning to challenge these findings.
Since plastics became widely used in the mid-20th century, they have evolved from a novel substance to an essential component in countless applications, with global production reaching 368 million tons in 2019 and expected to double by 2039. The production and degradation of plastics involve physical, chemical, and biological processes, leading to the formation of tiny fragments known as microplastics (MPs) and nanoplastics (NPs), which accumulate in the environment. Beyond the well-documented environmental harms of MPs and NPs, growing evidence of their presence within the human body raises concerns about their potential to trigger various harmful biological processes. Their detection in the urinary tract and their potential links to kidney and bladder diseases, as shown in animal studies, are particularly alarming.
Impacts Becoming Apparent
As the impact of plastic pollution becomes increasingly apparent, the need for standardized international definitions of MPs and NPs is pressing. Government publications reveal notable discrepancies between organizations in defining these fragmented plastics. The lack of consensus among regulatory bodies highlights the challenges in mitigating the environmental and health impacts of MPs and NPs. The International Organization for Standardization offers the most precise classification, defining MPs as solid, insoluble plastic particles ranging from 1 µm to 1 mm and NPs as particles smaller than 1 µm.
The intrusion of MPs and NPs into the human body, whether through inhalation, ingestion, or skin exposure (via wounds, hair follicles, or sweat glands), has been linked to harmful biological effects, including inflammation, alterations in cellular metabolism, physical cellular damage, and reduced cell viability.
Urinary Tract Plastics
The detection of MPs and NPs in the human urinary tract, combined with limited understanding of their effects, is a growing concern. An exploratory study published earlier this year aimed to systematically summarize the existing literature regarding the presence of MPs and NPs in the urinary tract and their potential consequences, guided by these research questions:
- What are the characteristics of the plastics detected in the human urinary tract?
- How are MPs and NPs defined in the current literature?
- What methodologies are used to explore the presence and effects of MPs and NPs?
- What are the pathophysiologic consequences of the presence of MPs and NPs in the human urinary tract?
For this study, the “urinary tract” included the kidneys, bladder, ureter, urethra, and urine. By focusing on the urinary tract, the study aimed to consolidate current understanding of MPs and NPs, raise awareness of this emerging issue, and lay the groundwork for further research that could contribute to public health policies and clinical practice guidelines.
The researchers conducted a scoping literature review following the recommendations of the JBI [formerly known as the Joanna Briggs Institute). They systematically searched five databases — PubMed, Scopus, CINAHL, Web of Science, and Embase — as well as gray literature sources.
Concerning Study Results
Eighteen articles were identified. The authors represent seven countries: Pakistan (n = 1), the Netherlands (n = 1), the US (n = 1), Taiwan (n = 1), Germany (n = 3), China (n = 5), and Italy (n = 6). Among these studies, six investigated and characterized the presence of MPs and NPs in the human urinary tract. MPs and NPs were detected in urine samples (n = 5), kidney cancer samples (n = 2), and bladder cancer samples (n = 1).
Additionally, 12 studies examined the effects of MPs and NPs on human urinary tract cell lines. Their findings suggest that MPs and NPs have cytotoxic effects, increase inflammation, reduce cell viability, and alter mitogen-activated protein kinase signaling pathways.
Raman spectroscopy was the primary method used to detect and characterize MPs and NPs in human samples (five out of six studies; 83%). Alternatively, pyrolysis-gas chromatography-mass spectrometry combined with direct laser infrared spectroscopy was used in one study.
Further Research Needed
This exploratory study underscores the urgent need for further research and policy development to address the challenges posed by microplastic contamination. It highlights the rapidly emerging threat of human urinary tract contamination by microplastics, questioning the WHO’s claim that microplastics pose no public health risk. The documented cytotoxic effects of microplastics, and their ability to induce inflammation, reduce cell viability, and disrupt signaling pathways, raise significant public health concerns related to bladder cancer, chronic kidney disease, chronic urinary infections, and incontinence.
Bernard-Alex Gauzere, retired physician formerly with the national health system in France (intensive care unit, tropical medicine), has disclosed no relevant financial relationships.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Although a 2019 World Health Organization (WHO) report concluded that microplastics in drinking water posed no risk to human health, accumulating evidence is beginning to challenge these findings.
Since plastics became widely used in the mid-20th century, they have evolved from a novel substance to an essential component in countless applications, with global production reaching 368 million tons in 2019 and expected to double by 2039. The production and degradation of plastics involve physical, chemical, and biological processes, leading to the formation of tiny fragments known as microplastics (MPs) and nanoplastics (NPs), which accumulate in the environment. Beyond the well-documented environmental harms of MPs and NPs, growing evidence of their presence within the human body raises concerns about their potential to trigger various harmful biological processes. Their detection in the urinary tract and their potential links to kidney and bladder diseases, as shown in animal studies, are particularly alarming.
Impacts Becoming Apparent
As the impact of plastic pollution becomes increasingly apparent, the need for standardized international definitions of MPs and NPs is pressing. Government publications reveal notable discrepancies between organizations in defining these fragmented plastics. The lack of consensus among regulatory bodies highlights the challenges in mitigating the environmental and health impacts of MPs and NPs. The International Organization for Standardization offers the most precise classification, defining MPs as solid, insoluble plastic particles ranging from 1 µm to 1 mm and NPs as particles smaller than 1 µm.
The intrusion of MPs and NPs into the human body, whether through inhalation, ingestion, or skin exposure (via wounds, hair follicles, or sweat glands), has been linked to harmful biological effects, including inflammation, alterations in cellular metabolism, physical cellular damage, and reduced cell viability.
Urinary Tract Plastics
The detection of MPs and NPs in the human urinary tract, combined with limited understanding of their effects, is a growing concern. An exploratory study published earlier this year aimed to systematically summarize the existing literature regarding the presence of MPs and NPs in the urinary tract and their potential consequences, guided by these research questions:
- What are the characteristics of the plastics detected in the human urinary tract?
- How are MPs and NPs defined in the current literature?
- What methodologies are used to explore the presence and effects of MPs and NPs?
- What are the pathophysiologic consequences of the presence of MPs and NPs in the human urinary tract?
For this study, the “urinary tract” included the kidneys, bladder, ureter, urethra, and urine. By focusing on the urinary tract, the study aimed to consolidate current understanding of MPs and NPs, raise awareness of this emerging issue, and lay the groundwork for further research that could contribute to public health policies and clinical practice guidelines.
The researchers conducted a scoping literature review following the recommendations of the JBI [formerly known as the Joanna Briggs Institute). They systematically searched five databases — PubMed, Scopus, CINAHL, Web of Science, and Embase — as well as gray literature sources.
Concerning Study Results
Eighteen articles were identified. The authors represent seven countries: Pakistan (n = 1), the Netherlands (n = 1), the US (n = 1), Taiwan (n = 1), Germany (n = 3), China (n = 5), and Italy (n = 6). Among these studies, six investigated and characterized the presence of MPs and NPs in the human urinary tract. MPs and NPs were detected in urine samples (n = 5), kidney cancer samples (n = 2), and bladder cancer samples (n = 1).
Additionally, 12 studies examined the effects of MPs and NPs on human urinary tract cell lines. Their findings suggest that MPs and NPs have cytotoxic effects, increase inflammation, reduce cell viability, and alter mitogen-activated protein kinase signaling pathways.
Raman spectroscopy was the primary method used to detect and characterize MPs and NPs in human samples (five out of six studies; 83%). Alternatively, pyrolysis-gas chromatography-mass spectrometry combined with direct laser infrared spectroscopy was used in one study.
Further Research Needed
This exploratory study underscores the urgent need for further research and policy development to address the challenges posed by microplastic contamination. It highlights the rapidly emerging threat of human urinary tract contamination by microplastics, questioning the WHO’s claim that microplastics pose no public health risk. The documented cytotoxic effects of microplastics, and their ability to induce inflammation, reduce cell viability, and disrupt signaling pathways, raise significant public health concerns related to bladder cancer, chronic kidney disease, chronic urinary infections, and incontinence.
Bernard-Alex Gauzere, retired physician formerly with the national health system in France (intensive care unit, tropical medicine), has disclosed no relevant financial relationships.
This story was translated from JIM using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Postpartum Depression Common After Cesarean Delivery
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
About one in six women experience symptoms of postpartum depression (PPD) 2 months after cesarean delivery, with certain obstetric factors such as emergency cesarean delivery before labor, cesarean delivery after labor induction, lack of social support in the operating room, and severe postoperative pain influencing the risk.
METHODOLOGY:
- Researchers conducted a prospective ancillary cohort study of the Tranexamic Acid for Preventing Postpartum Hemorrhage after Cesarean Delivery (TRAAP2) trial to examine the prevalence of PPD 2 months after cesarean delivery and associated risk factors.
- A total of 2793 women (median age, 33.5 years) were included who had a cesarean delivery at 34 or more weeks of gestation; they completed the Edinburgh Postnatal Depression Scale (EPDS), a self-administered questionnaire, at 2 months after delivery.
- Information about the cesarean delivery, postpartum blood loss, immediate postpartum period, psychiatric history, and memories of delivery and postoperative pain were prospectively collected.
- Medical records were used to obtain details about characteristics of patients; 5.0% had a psychiatric history (2.4% composed of depression).
- The main endpoint was a positive screening for symptoms consistent with this depression — defined as a PPD diagnosis — 2 months after caesarian delivery, with an EPDS score of 13 or higher.
TAKEAWAY:
- The prevalence of a provisional PPD diagnosis at 2 months after cesarean delivery was 16.4% (95% CI, 14.9-18.0) with an EPDS score of 13 or higher and was 23.1% (95% CI, 21.4-24.9%) with a cutoff value of 11 or higher.
- Women who had an emergency cesarean delivery before labor had a higher risk for PPD than those who had a normal cesarean delivery before labor started (adjusted odds ratio [aOR], 1.70; 95% CI, 1.15-2.50); women who had started labor after induction but then had a cesarean delivery also had a higher risk for PPD than those who had a cesarean delivery before going into labor (aOR, 1.36; 95% CI, 1.03-1.84).
- Severe pain during the postpartum stay (aOR, 1.73; 95% CI, 1.32-2.26) and bad memories of delivery (aOR, 1.67; 95% CI, 1.14-2.45) were also risk factors for PPD.
- However, women who had social support in the operating room showed a 27% lower risk for PPD (P = .02).
IN PRACTICE:
“Identifying subgroups of women at risk for PPD based on aspects of their obstetric experience could help to screen for women who might benefit from early screening and interventions,” the authors wrote.
SOURCE:
This study was led by Alizée Froeliger, MD, MPH, of the Department of Obstetrics and Gynecology at Bordeaux University Hospital in France, and was published online in American Journal of Obstetrics & Gynecology.
LIMITATIONS:
The study population was derived from a randomized controlled trial, which may have underestimated the prevalence of PPD. The use of a self-administered questionnaire for PPD screening may not have provided a definitive diagnosis. Moreover, this study did not assess the prevalence of depressive symptoms during pregnancy.
DISCLOSURES:
The TRAAP2 trial was supported by a grant from the French Ministry of Health under its Clinical Research Hospital Program. One author reported carrying out consultancy work and lecturing for Ferring Laboratories, GlaxoSmithKline, and other pharmaceutical companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Telehealth Adoption in Primary Care: Reducing Low-Value Services
TOPLINE:
Increased telehealth use in primary care practices is associated with reduced rates of low-value cervical cancer screening and thyroid testing. No significant association is found between telehealth use and most other low-value care services.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using Medicare fee-for-service claims data from January 1, 2019, to December 31, 2022.
- A total of 577,928 Medicare beneficiaries attributed to 2552 primary care practices in Michigan were included in the study.
- Telehealth use was stratified into low, medium, and high tertiles based on the number of telehealth services per 1000 beneficiaries in 2022.
- Low-value care was assessed using eight claims-based measures relevant to primary care, grouped into office-based, laboratory-based, imaging-based, and mixed-modality services.
- Poisson regression models were used to estimate the association between practice-level telehealth use and rates of low-value care services, controlling for practice-level characteristics.
TAKEAWAY:
- High practice-level telehealth use was associated with lower rates of low-value cervical cancer screening (–2.9 services per 1000 beneficiaries; 95% CI, –5.3 to –0.4).
- High practice-level telehealth use was associated with lower rates of low-value thyroid testing (–40 tests per 1000 beneficiaries; 95% CI, –70 to –9).
- No significant association was found between practice-level telehealth use and rates of other low-value care services.
- The findings suggested that telehealth can be used to deliver primary care services without introducing wasteful or unnecessary care and can even reduce low-value care.
IN PRACTICE:
“While the rapid growth of telehealth has enhanced access to care for individuals, it has also raised concern for unintended consequences in the form of wasteful or unnecessary care, ie, low-value care. Our study suggests that increased practice-level telehealth use was not associated with the delivery of low-value care services in primary care and may even help reduce office-based low-value care,” the authors of the study wrote.
SOURCE:
This study was led by Terrence Liu, MD, MS, University of Michigan, Ann Arbor. It was published online in JAMA Network Open.
LIMITATIONS:
This study was performed among Medicare fee-for-service beneficiaries with a Michigan residence and may not be generalizable to the broader Medicare beneficiary population. Administrative claims data do not include clinical information, which limited the ability to measure overall quality of care. The study defined telehealth use at the practice level and did not assess individual outcomes. Additional research is needed at a national level to determine the impact of telehealth on low-value care services in primary care.
DISCLOSURES:
This study was supported by grants from the Agency for Healthcare Research and Quality. Liu received funding from the University of Michigan National Clinician Scholars Program and Veterans Affairs Center for Clinical Management Research. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Increased telehealth use in primary care practices is associated with reduced rates of low-value cervical cancer screening and thyroid testing. No significant association is found between telehealth use and most other low-value care services.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using Medicare fee-for-service claims data from January 1, 2019, to December 31, 2022.
- A total of 577,928 Medicare beneficiaries attributed to 2552 primary care practices in Michigan were included in the study.
- Telehealth use was stratified into low, medium, and high tertiles based on the number of telehealth services per 1000 beneficiaries in 2022.
- Low-value care was assessed using eight claims-based measures relevant to primary care, grouped into office-based, laboratory-based, imaging-based, and mixed-modality services.
- Poisson regression models were used to estimate the association between practice-level telehealth use and rates of low-value care services, controlling for practice-level characteristics.
TAKEAWAY:
- High practice-level telehealth use was associated with lower rates of low-value cervical cancer screening (–2.9 services per 1000 beneficiaries; 95% CI, –5.3 to –0.4).
- High practice-level telehealth use was associated with lower rates of low-value thyroid testing (–40 tests per 1000 beneficiaries; 95% CI, –70 to –9).
- No significant association was found between practice-level telehealth use and rates of other low-value care services.
- The findings suggested that telehealth can be used to deliver primary care services without introducing wasteful or unnecessary care and can even reduce low-value care.
IN PRACTICE:
“While the rapid growth of telehealth has enhanced access to care for individuals, it has also raised concern for unintended consequences in the form of wasteful or unnecessary care, ie, low-value care. Our study suggests that increased practice-level telehealth use was not associated with the delivery of low-value care services in primary care and may even help reduce office-based low-value care,” the authors of the study wrote.
SOURCE:
This study was led by Terrence Liu, MD, MS, University of Michigan, Ann Arbor. It was published online in JAMA Network Open.
LIMITATIONS:
This study was performed among Medicare fee-for-service beneficiaries with a Michigan residence and may not be generalizable to the broader Medicare beneficiary population. Administrative claims data do not include clinical information, which limited the ability to measure overall quality of care. The study defined telehealth use at the practice level and did not assess individual outcomes. Additional research is needed at a national level to determine the impact of telehealth on low-value care services in primary care.
DISCLOSURES:
This study was supported by grants from the Agency for Healthcare Research and Quality. Liu received funding from the University of Michigan National Clinician Scholars Program and Veterans Affairs Center for Clinical Management Research. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Increased telehealth use in primary care practices is associated with reduced rates of low-value cervical cancer screening and thyroid testing. No significant association is found between telehealth use and most other low-value care services.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using Medicare fee-for-service claims data from January 1, 2019, to December 31, 2022.
- A total of 577,928 Medicare beneficiaries attributed to 2552 primary care practices in Michigan were included in the study.
- Telehealth use was stratified into low, medium, and high tertiles based on the number of telehealth services per 1000 beneficiaries in 2022.
- Low-value care was assessed using eight claims-based measures relevant to primary care, grouped into office-based, laboratory-based, imaging-based, and mixed-modality services.
- Poisson regression models were used to estimate the association between practice-level telehealth use and rates of low-value care services, controlling for practice-level characteristics.
TAKEAWAY:
- High practice-level telehealth use was associated with lower rates of low-value cervical cancer screening (–2.9 services per 1000 beneficiaries; 95% CI, –5.3 to –0.4).
- High practice-level telehealth use was associated with lower rates of low-value thyroid testing (–40 tests per 1000 beneficiaries; 95% CI, –70 to –9).
- No significant association was found between practice-level telehealth use and rates of other low-value care services.
- The findings suggested that telehealth can be used to deliver primary care services without introducing wasteful or unnecessary care and can even reduce low-value care.
IN PRACTICE:
“While the rapid growth of telehealth has enhanced access to care for individuals, it has also raised concern for unintended consequences in the form of wasteful or unnecessary care, ie, low-value care. Our study suggests that increased practice-level telehealth use was not associated with the delivery of low-value care services in primary care and may even help reduce office-based low-value care,” the authors of the study wrote.
SOURCE:
This study was led by Terrence Liu, MD, MS, University of Michigan, Ann Arbor. It was published online in JAMA Network Open.
LIMITATIONS:
This study was performed among Medicare fee-for-service beneficiaries with a Michigan residence and may not be generalizable to the broader Medicare beneficiary population. Administrative claims data do not include clinical information, which limited the ability to measure overall quality of care. The study defined telehealth use at the practice level and did not assess individual outcomes. Additional research is needed at a national level to determine the impact of telehealth on low-value care services in primary care.
DISCLOSURES:
This study was supported by grants from the Agency for Healthcare Research and Quality. Liu received funding from the University of Michigan National Clinician Scholars Program and Veterans Affairs Center for Clinical Management Research. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
GLP-1 RAs Safe in the Perioperative Period: New Guidance
The new guidance, contrasting with earlier recommendations, says these incrementally used agents can be taken up until the day of surgery, but patients are advised to follow a liquid diet for 24 hours before the procedure. The decision to proceed with endoscopy and other procedures should be based on shared decision-making with the patient and interdisciplinary care teams in conjunction with minimization of the aspiration risk from delayed gastric emptying, the guidance stresses.
The five endorsing organizations are the American Society for Metabolic and Bariatric Surgery, American Society of Anesthesiologists (ASA), American Gastroenterological Association, International Society of Perioperative Care of Patients with Obesity, and Society of American Gastrointestinal and Endoscopic Surgeons. The societies emphasize that the statement is intended as guidance only and is not an evidence-based formal guideline.
GLP-1 RAs are known to delay gastric emptying, raising concerns about regurgitation, aspiration, and airway compromise during anesthesia. Rare serious adverse events have also been observed, prompting the ASA in 2023 to recommend holding these agents for 1 week for the injectable form and 1 day for the oral form before all procedures requiring anesthesia.
That abundance of caution, however, had negative impacts of its own. “This guidance has led to cancellations and postponements of many endoscopic and surgical procedures or required patients to undergo general anesthesia who may otherwise have had their procedures performed under moderate sedation,” said guidance coauthor Allison R. Schulman, MD, MPH, an associate professor of medicine and surgery and chief of endoscopy at the University of Michigan in Ann Arbor. “Nearly all institutions have been forced to revise preprocedural protocols, despite a lack of high-level evidence to suggest that these adjustments are necessary.”
“Studies have yielded mixed results as to whether patients on GLP-1s are at increased risk of these events, and the limited data available are inconsistent,” Schulman said. “As a result, there are inconsistencies in the recommendations from various societies leading to growing uncertainty with proceduralists on how to provide safe, effective, and timely procedural care to patients taking GLP-1 RAs.”
The new joint-society guidance may alleviate some of the uncertainty. Among the recommendations:
- Continuing GLP-1 RAs in the perioperative period should be based on shared decision-making with the patient and all care teams balancing the metabolic need for the GLP-1 RA with individual patient risk.
- Certain variables may increase the risk for delayed gastric emptying and aspiration with the periprocedural use of GLP-1 RAs: escalation phase — This phase vs the maintenance phase is associated with a higher risk for delayed gastric emptying; higher dose — the higher the dose, the greater the risk for gastrointestinal (GI) side effects; weekly dosing — GI side effects are more common with weekly vs daily formulations; presence of GI symptoms — nausea, vomiting, abdominal pain, dyspepsia, and constipation may suggest delayed gastric emptying; and medical problems beyond GLP-1 RA indications with GI effects — assess for such conditions as bowel dysmotility, gastroparesis, and Parkinson’s disease.
- Risk factors should be assessed in advance to allow sufficient time to adjust preoperative care, including diet modification and medication bridging if GLP-1 RA cessation is deemed advisable.
- If retained gastric contents are a concern on the day of a procedure, point-of-care gastric ultrasound could be used to assess aspiration risk, resources permitting.
- The aspiration risk from delayed gastric emptying should be minimized by preoperative diet modification and/or altering the anesthesia plan to consider rapid sequence induction of general anesthesia for tracheal intubation. A 24-hour preoperative liquid diet, as before colonoscopy and bariatric surgery, can be utilized when delayed gastric emptying is a concern.
- When concern about retained gastric contents exists on procedure day, providers should engage patients in a shared decision-making model and consider the benefits and risks of rapid-sequence induction of general anesthesia for tracheal intubation to minimize aspiration risk vs procedure cancellation.
“Safe continuation of surgery and gastrointestinal endoscopy, and prevention of procedure cancellation, for patients on GLP-1 RAs can be prioritized following the recommendations above, as would occur for other patient populations with gastroparesis,” the guidance panel wrote.
Commenting on the statement but not involved in it, David B. Purow, MD, managing director of the Digestive Health Center at Northwell Health/Huntington Hospital in Huntington, New York, said the recommendations will encourage clinicians to be more discerning about actual risk in individual cases rather than follow the previous blanket recommendation to stop these agents before procedures requiring sedation.
While GLP-1 RAs were prescribed for the relatively small number of patients with diabetes, he said, the risk was not apparent but became clearer with the widespread use of these agents for weight loss — often unregulated and undisclosed to care providers.
“The pendulum shifted too far the other way, and now it’s shifted back,” he said in an interview. “The new guidance is great because now we can be more thoughtful about managing individual patients.” He cited, for instance, the recommendations on the greater risk in patients in the dose escalation phase or on higher doses, and the risk-reducing measure of a liquid diet for 24 hours before surgery.
His center is already using point-of-care ultrasound and recently had a case in which a patient who forgot and took his GLP-1 RA before a scheduled procedure was found on ultrasound to have a full stomach. “In some cases, these drugs can cause an almost gastroparesis level of delayed emptying,” Purow said.
Purow thinks this early guidance will probably progress to firm guidelines within a year. Schulman is more cautious. “Our understanding of this complex topic is increasing rapidly, and ongoing clinical research will ultimately lead to evidence-based guidelines in this changing landscape,” she said.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Schulman is a consultant for Apollo Endosurgery, Boston Scientific, Olympus, Microtech, and Fractyl. Purow had no competing interests to declare.
A version of this article first appeared on Medscape.com.
The new guidance, contrasting with earlier recommendations, says these incrementally used agents can be taken up until the day of surgery, but patients are advised to follow a liquid diet for 24 hours before the procedure. The decision to proceed with endoscopy and other procedures should be based on shared decision-making with the patient and interdisciplinary care teams in conjunction with minimization of the aspiration risk from delayed gastric emptying, the guidance stresses.
The five endorsing organizations are the American Society for Metabolic and Bariatric Surgery, American Society of Anesthesiologists (ASA), American Gastroenterological Association, International Society of Perioperative Care of Patients with Obesity, and Society of American Gastrointestinal and Endoscopic Surgeons. The societies emphasize that the statement is intended as guidance only and is not an evidence-based formal guideline.
GLP-1 RAs are known to delay gastric emptying, raising concerns about regurgitation, aspiration, and airway compromise during anesthesia. Rare serious adverse events have also been observed, prompting the ASA in 2023 to recommend holding these agents for 1 week for the injectable form and 1 day for the oral form before all procedures requiring anesthesia.
That abundance of caution, however, had negative impacts of its own. “This guidance has led to cancellations and postponements of many endoscopic and surgical procedures or required patients to undergo general anesthesia who may otherwise have had their procedures performed under moderate sedation,” said guidance coauthor Allison R. Schulman, MD, MPH, an associate professor of medicine and surgery and chief of endoscopy at the University of Michigan in Ann Arbor. “Nearly all institutions have been forced to revise preprocedural protocols, despite a lack of high-level evidence to suggest that these adjustments are necessary.”
“Studies have yielded mixed results as to whether patients on GLP-1s are at increased risk of these events, and the limited data available are inconsistent,” Schulman said. “As a result, there are inconsistencies in the recommendations from various societies leading to growing uncertainty with proceduralists on how to provide safe, effective, and timely procedural care to patients taking GLP-1 RAs.”
The new joint-society guidance may alleviate some of the uncertainty. Among the recommendations:
- Continuing GLP-1 RAs in the perioperative period should be based on shared decision-making with the patient and all care teams balancing the metabolic need for the GLP-1 RA with individual patient risk.
- Certain variables may increase the risk for delayed gastric emptying and aspiration with the periprocedural use of GLP-1 RAs: escalation phase — This phase vs the maintenance phase is associated with a higher risk for delayed gastric emptying; higher dose — the higher the dose, the greater the risk for gastrointestinal (GI) side effects; weekly dosing — GI side effects are more common with weekly vs daily formulations; presence of GI symptoms — nausea, vomiting, abdominal pain, dyspepsia, and constipation may suggest delayed gastric emptying; and medical problems beyond GLP-1 RA indications with GI effects — assess for such conditions as bowel dysmotility, gastroparesis, and Parkinson’s disease.
- Risk factors should be assessed in advance to allow sufficient time to adjust preoperative care, including diet modification and medication bridging if GLP-1 RA cessation is deemed advisable.
- If retained gastric contents are a concern on the day of a procedure, point-of-care gastric ultrasound could be used to assess aspiration risk, resources permitting.
- The aspiration risk from delayed gastric emptying should be minimized by preoperative diet modification and/or altering the anesthesia plan to consider rapid sequence induction of general anesthesia for tracheal intubation. A 24-hour preoperative liquid diet, as before colonoscopy and bariatric surgery, can be utilized when delayed gastric emptying is a concern.
- When concern about retained gastric contents exists on procedure day, providers should engage patients in a shared decision-making model and consider the benefits and risks of rapid-sequence induction of general anesthesia for tracheal intubation to minimize aspiration risk vs procedure cancellation.
“Safe continuation of surgery and gastrointestinal endoscopy, and prevention of procedure cancellation, for patients on GLP-1 RAs can be prioritized following the recommendations above, as would occur for other patient populations with gastroparesis,” the guidance panel wrote.
Commenting on the statement but not involved in it, David B. Purow, MD, managing director of the Digestive Health Center at Northwell Health/Huntington Hospital in Huntington, New York, said the recommendations will encourage clinicians to be more discerning about actual risk in individual cases rather than follow the previous blanket recommendation to stop these agents before procedures requiring sedation.
While GLP-1 RAs were prescribed for the relatively small number of patients with diabetes, he said, the risk was not apparent but became clearer with the widespread use of these agents for weight loss — often unregulated and undisclosed to care providers.
“The pendulum shifted too far the other way, and now it’s shifted back,” he said in an interview. “The new guidance is great because now we can be more thoughtful about managing individual patients.” He cited, for instance, the recommendations on the greater risk in patients in the dose escalation phase or on higher doses, and the risk-reducing measure of a liquid diet for 24 hours before surgery.
His center is already using point-of-care ultrasound and recently had a case in which a patient who forgot and took his GLP-1 RA before a scheduled procedure was found on ultrasound to have a full stomach. “In some cases, these drugs can cause an almost gastroparesis level of delayed emptying,” Purow said.
Purow thinks this early guidance will probably progress to firm guidelines within a year. Schulman is more cautious. “Our understanding of this complex topic is increasing rapidly, and ongoing clinical research will ultimately lead to evidence-based guidelines in this changing landscape,” she said.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Schulman is a consultant for Apollo Endosurgery, Boston Scientific, Olympus, Microtech, and Fractyl. Purow had no competing interests to declare.
A version of this article first appeared on Medscape.com.
The new guidance, contrasting with earlier recommendations, says these incrementally used agents can be taken up until the day of surgery, but patients are advised to follow a liquid diet for 24 hours before the procedure. The decision to proceed with endoscopy and other procedures should be based on shared decision-making with the patient and interdisciplinary care teams in conjunction with minimization of the aspiration risk from delayed gastric emptying, the guidance stresses.
The five endorsing organizations are the American Society for Metabolic and Bariatric Surgery, American Society of Anesthesiologists (ASA), American Gastroenterological Association, International Society of Perioperative Care of Patients with Obesity, and Society of American Gastrointestinal and Endoscopic Surgeons. The societies emphasize that the statement is intended as guidance only and is not an evidence-based formal guideline.
GLP-1 RAs are known to delay gastric emptying, raising concerns about regurgitation, aspiration, and airway compromise during anesthesia. Rare serious adverse events have also been observed, prompting the ASA in 2023 to recommend holding these agents for 1 week for the injectable form and 1 day for the oral form before all procedures requiring anesthesia.
That abundance of caution, however, had negative impacts of its own. “This guidance has led to cancellations and postponements of many endoscopic and surgical procedures or required patients to undergo general anesthesia who may otherwise have had their procedures performed under moderate sedation,” said guidance coauthor Allison R. Schulman, MD, MPH, an associate professor of medicine and surgery and chief of endoscopy at the University of Michigan in Ann Arbor. “Nearly all institutions have been forced to revise preprocedural protocols, despite a lack of high-level evidence to suggest that these adjustments are necessary.”
“Studies have yielded mixed results as to whether patients on GLP-1s are at increased risk of these events, and the limited data available are inconsistent,” Schulman said. “As a result, there are inconsistencies in the recommendations from various societies leading to growing uncertainty with proceduralists on how to provide safe, effective, and timely procedural care to patients taking GLP-1 RAs.”
The new joint-society guidance may alleviate some of the uncertainty. Among the recommendations:
- Continuing GLP-1 RAs in the perioperative period should be based on shared decision-making with the patient and all care teams balancing the metabolic need for the GLP-1 RA with individual patient risk.
- Certain variables may increase the risk for delayed gastric emptying and aspiration with the periprocedural use of GLP-1 RAs: escalation phase — This phase vs the maintenance phase is associated with a higher risk for delayed gastric emptying; higher dose — the higher the dose, the greater the risk for gastrointestinal (GI) side effects; weekly dosing — GI side effects are more common with weekly vs daily formulations; presence of GI symptoms — nausea, vomiting, abdominal pain, dyspepsia, and constipation may suggest delayed gastric emptying; and medical problems beyond GLP-1 RA indications with GI effects — assess for such conditions as bowel dysmotility, gastroparesis, and Parkinson’s disease.
- Risk factors should be assessed in advance to allow sufficient time to adjust preoperative care, including diet modification and medication bridging if GLP-1 RA cessation is deemed advisable.
- If retained gastric contents are a concern on the day of a procedure, point-of-care gastric ultrasound could be used to assess aspiration risk, resources permitting.
- The aspiration risk from delayed gastric emptying should be minimized by preoperative diet modification and/or altering the anesthesia plan to consider rapid sequence induction of general anesthesia for tracheal intubation. A 24-hour preoperative liquid diet, as before colonoscopy and bariatric surgery, can be utilized when delayed gastric emptying is a concern.
- When concern about retained gastric contents exists on procedure day, providers should engage patients in a shared decision-making model and consider the benefits and risks of rapid-sequence induction of general anesthesia for tracheal intubation to minimize aspiration risk vs procedure cancellation.
“Safe continuation of surgery and gastrointestinal endoscopy, and prevention of procedure cancellation, for patients on GLP-1 RAs can be prioritized following the recommendations above, as would occur for other patient populations with gastroparesis,” the guidance panel wrote.
Commenting on the statement but not involved in it, David B. Purow, MD, managing director of the Digestive Health Center at Northwell Health/Huntington Hospital in Huntington, New York, said the recommendations will encourage clinicians to be more discerning about actual risk in individual cases rather than follow the previous blanket recommendation to stop these agents before procedures requiring sedation.
While GLP-1 RAs were prescribed for the relatively small number of patients with diabetes, he said, the risk was not apparent but became clearer with the widespread use of these agents for weight loss — often unregulated and undisclosed to care providers.
“The pendulum shifted too far the other way, and now it’s shifted back,” he said in an interview. “The new guidance is great because now we can be more thoughtful about managing individual patients.” He cited, for instance, the recommendations on the greater risk in patients in the dose escalation phase or on higher doses, and the risk-reducing measure of a liquid diet for 24 hours before surgery.
His center is already using point-of-care ultrasound and recently had a case in which a patient who forgot and took his GLP-1 RA before a scheduled procedure was found on ultrasound to have a full stomach. “In some cases, these drugs can cause an almost gastroparesis level of delayed emptying,” Purow said.
Purow thinks this early guidance will probably progress to firm guidelines within a year. Schulman is more cautious. “Our understanding of this complex topic is increasing rapidly, and ongoing clinical research will ultimately lead to evidence-based guidelines in this changing landscape,” she said.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Schulman is a consultant for Apollo Endosurgery, Boston Scientific, Olympus, Microtech, and Fractyl. Purow had no competing interests to declare.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Shorter H pylori Treatment With Vonoprazan Shows Better Results
PHILADELPHIA — with omeprazole, amoxicillin, and clarithromycin, according to the results of a randomized, multicenter study.
In addition, the triple therapy regimen with vonoprazan was generally better tolerated than the 14-day omeprazole-based regimen.
The new treatment combination was created to tackle the two main reasons that patients with H pylori experience treatment failure: Inadequate acid suppressant activity and antibiotic resistance, said principal investigator Kachonsak Yongwatana, MD, from Phramongkutklao Hospital in Bangkok, Thailand.
“Vonoprazan” is the more potent option for acid suppression, and “levofloxacin” addresses antibiotic resistance, he explained.
Yongwatana presented the findings (Abstract 41) at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting. The ACG recently released a clinical guideline on the treatment of H pylori infection.
Robust Eradication Rates
Yongwatana and colleagues enrolled adult patients with H pylori infections at four hospitals in Thailand between December 2022 and September 2023. The presence of H pylori was confirmed by upper gastrointestinal endoscopy with positive rapid urease test or positive test on tissue biopsy.
Patients were then randomized into two treatment groups: The 10-day VAL group (vonoprazan 20 mg twice daily, amoxicillin 1000 mg twice daily, and levofloxacin 500 mg once daily for 10 days) and the 14-day OAC group (omeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily for 14 days). Eradication was assessed by urea breath test 4 weeks after completion of treatment.
There were 280 patients in total, with 140 in each group. There were no significant differences in baseline characteristics between the groups. The most common endoscopic findings among all participants included erosive gastritis (38%), nonerosive gastritis (27%), and gastric ulcer (17%).
In comparing the treatments, the researchers found that 10-day VAL led to significantly greater H pylori eradication rate than the 14-day OAC group in both intention-to-treat analysis (91.4 % vs 80.7%, P = .009) and per-protocol analysis (93.4% vs 83.7%, P = .012).
Vonoprazan-based therapy was also well tolerated by participants. Patients in the 10-day VAL group had significantly lower rates of experiencing a bitter taste (2.1% vs 42.9%, P < .001) and bloating (5% vs 12.1%, P = .033) than those in the 14-day OAC group.
Isolating the BMI Effect
The researchers conducted a subgroup analysis on potential factors influencing response, which revealed that having a body mass index (BMI) < 23.5 was significantly associated with a higher chance at successful H pylori eradication (relative risk [RR], 2.27; P = .049).
They then analyzed whether this BMI threshold was predictive in the separate treatment regimens. Although having a BMI < 23.5 was significantly associated with a higher eradication rate in the 14-day OAC group (RR, 3.34; P = .026), no such effect was noted in the 10-day VAL group (RR, 1.10; P = .888).
The influence of BMI could be caused by the bioavailability of the treatments used in the regimen, Younwatana said in an interview. He and his colleagues recommended against using the 14-day OAC regimen in those with BMI ≥ 23.5.
“In patients with a high BMI, we should be concerned that normal proton pump inhibitors may not work,” he said. “You have to step up to the higher-potency options.”
Seeking Confirmation in Other Populations
Session comoderator Felice Schnoll-Sussman, MD, MSc, professor of clinical medicine and the director of the Jay Monahan Center for Gastrointestinal Health, director of the DIGEST program, and the associate chair of medicine for Outreach and Network at New York–Presbyterian Brooklyn Methodist Hospital in New York City, said in an interview that the promising results merit confirmation in other populations.
“When you see a study that is coming out of one country, when there could be issues related to antibiotic sensitivity in H pylori, it really is important to decide whether or not this is applicable to other patient populations,” said Schnoll-Sussman, who was not involved in the study.
She noted that this is also true of the findings from the subgroup as it is unclear whether average rates of BMI are notably lower in Thailand from other countries.
“As we know, BMI affects so many things with disease states. So, it’s a possibility in a country where the BMI is actually lower, there may be something else about these individuals in terms of their wellness status that could be underlying the effect.”
The study had no specific funding, although Takeda supplied treatments used in the analysis. Yongwatana reported no relevant financial relationships. Schnoll-Sussman reported serving as an advisory committee/board member for Braintree, Ethicon, Implantica, and Phathom.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — with omeprazole, amoxicillin, and clarithromycin, according to the results of a randomized, multicenter study.
In addition, the triple therapy regimen with vonoprazan was generally better tolerated than the 14-day omeprazole-based regimen.
The new treatment combination was created to tackle the two main reasons that patients with H pylori experience treatment failure: Inadequate acid suppressant activity and antibiotic resistance, said principal investigator Kachonsak Yongwatana, MD, from Phramongkutklao Hospital in Bangkok, Thailand.
“Vonoprazan” is the more potent option for acid suppression, and “levofloxacin” addresses antibiotic resistance, he explained.
Yongwatana presented the findings (Abstract 41) at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting. The ACG recently released a clinical guideline on the treatment of H pylori infection.
Robust Eradication Rates
Yongwatana and colleagues enrolled adult patients with H pylori infections at four hospitals in Thailand between December 2022 and September 2023. The presence of H pylori was confirmed by upper gastrointestinal endoscopy with positive rapid urease test or positive test on tissue biopsy.
Patients were then randomized into two treatment groups: The 10-day VAL group (vonoprazan 20 mg twice daily, amoxicillin 1000 mg twice daily, and levofloxacin 500 mg once daily for 10 days) and the 14-day OAC group (omeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily for 14 days). Eradication was assessed by urea breath test 4 weeks after completion of treatment.
There were 280 patients in total, with 140 in each group. There were no significant differences in baseline characteristics between the groups. The most common endoscopic findings among all participants included erosive gastritis (38%), nonerosive gastritis (27%), and gastric ulcer (17%).
In comparing the treatments, the researchers found that 10-day VAL led to significantly greater H pylori eradication rate than the 14-day OAC group in both intention-to-treat analysis (91.4 % vs 80.7%, P = .009) and per-protocol analysis (93.4% vs 83.7%, P = .012).
Vonoprazan-based therapy was also well tolerated by participants. Patients in the 10-day VAL group had significantly lower rates of experiencing a bitter taste (2.1% vs 42.9%, P < .001) and bloating (5% vs 12.1%, P = .033) than those in the 14-day OAC group.
Isolating the BMI Effect
The researchers conducted a subgroup analysis on potential factors influencing response, which revealed that having a body mass index (BMI) < 23.5 was significantly associated with a higher chance at successful H pylori eradication (relative risk [RR], 2.27; P = .049).
They then analyzed whether this BMI threshold was predictive in the separate treatment regimens. Although having a BMI < 23.5 was significantly associated with a higher eradication rate in the 14-day OAC group (RR, 3.34; P = .026), no such effect was noted in the 10-day VAL group (RR, 1.10; P = .888).
The influence of BMI could be caused by the bioavailability of the treatments used in the regimen, Younwatana said in an interview. He and his colleagues recommended against using the 14-day OAC regimen in those with BMI ≥ 23.5.
“In patients with a high BMI, we should be concerned that normal proton pump inhibitors may not work,” he said. “You have to step up to the higher-potency options.”
Seeking Confirmation in Other Populations
Session comoderator Felice Schnoll-Sussman, MD, MSc, professor of clinical medicine and the director of the Jay Monahan Center for Gastrointestinal Health, director of the DIGEST program, and the associate chair of medicine for Outreach and Network at New York–Presbyterian Brooklyn Methodist Hospital in New York City, said in an interview that the promising results merit confirmation in other populations.
“When you see a study that is coming out of one country, when there could be issues related to antibiotic sensitivity in H pylori, it really is important to decide whether or not this is applicable to other patient populations,” said Schnoll-Sussman, who was not involved in the study.
She noted that this is also true of the findings from the subgroup as it is unclear whether average rates of BMI are notably lower in Thailand from other countries.
“As we know, BMI affects so many things with disease states. So, it’s a possibility in a country where the BMI is actually lower, there may be something else about these individuals in terms of their wellness status that could be underlying the effect.”
The study had no specific funding, although Takeda supplied treatments used in the analysis. Yongwatana reported no relevant financial relationships. Schnoll-Sussman reported serving as an advisory committee/board member for Braintree, Ethicon, Implantica, and Phathom.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — with omeprazole, amoxicillin, and clarithromycin, according to the results of a randomized, multicenter study.
In addition, the triple therapy regimen with vonoprazan was generally better tolerated than the 14-day omeprazole-based regimen.
The new treatment combination was created to tackle the two main reasons that patients with H pylori experience treatment failure: Inadequate acid suppressant activity and antibiotic resistance, said principal investigator Kachonsak Yongwatana, MD, from Phramongkutklao Hospital in Bangkok, Thailand.
“Vonoprazan” is the more potent option for acid suppression, and “levofloxacin” addresses antibiotic resistance, he explained.
Yongwatana presented the findings (Abstract 41) at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting. The ACG recently released a clinical guideline on the treatment of H pylori infection.
Robust Eradication Rates
Yongwatana and colleagues enrolled adult patients with H pylori infections at four hospitals in Thailand between December 2022 and September 2023. The presence of H pylori was confirmed by upper gastrointestinal endoscopy with positive rapid urease test or positive test on tissue biopsy.
Patients were then randomized into two treatment groups: The 10-day VAL group (vonoprazan 20 mg twice daily, amoxicillin 1000 mg twice daily, and levofloxacin 500 mg once daily for 10 days) and the 14-day OAC group (omeprazole 20 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily for 14 days). Eradication was assessed by urea breath test 4 weeks after completion of treatment.
There were 280 patients in total, with 140 in each group. There were no significant differences in baseline characteristics between the groups. The most common endoscopic findings among all participants included erosive gastritis (38%), nonerosive gastritis (27%), and gastric ulcer (17%).
In comparing the treatments, the researchers found that 10-day VAL led to significantly greater H pylori eradication rate than the 14-day OAC group in both intention-to-treat analysis (91.4 % vs 80.7%, P = .009) and per-protocol analysis (93.4% vs 83.7%, P = .012).
Vonoprazan-based therapy was also well tolerated by participants. Patients in the 10-day VAL group had significantly lower rates of experiencing a bitter taste (2.1% vs 42.9%, P < .001) and bloating (5% vs 12.1%, P = .033) than those in the 14-day OAC group.
Isolating the BMI Effect
The researchers conducted a subgroup analysis on potential factors influencing response, which revealed that having a body mass index (BMI) < 23.5 was significantly associated with a higher chance at successful H pylori eradication (relative risk [RR], 2.27; P = .049).
They then analyzed whether this BMI threshold was predictive in the separate treatment regimens. Although having a BMI < 23.5 was significantly associated with a higher eradication rate in the 14-day OAC group (RR, 3.34; P = .026), no such effect was noted in the 10-day VAL group (RR, 1.10; P = .888).
The influence of BMI could be caused by the bioavailability of the treatments used in the regimen, Younwatana said in an interview. He and his colleagues recommended against using the 14-day OAC regimen in those with BMI ≥ 23.5.
“In patients with a high BMI, we should be concerned that normal proton pump inhibitors may not work,” he said. “You have to step up to the higher-potency options.”
Seeking Confirmation in Other Populations
Session comoderator Felice Schnoll-Sussman, MD, MSc, professor of clinical medicine and the director of the Jay Monahan Center for Gastrointestinal Health, director of the DIGEST program, and the associate chair of medicine for Outreach and Network at New York–Presbyterian Brooklyn Methodist Hospital in New York City, said in an interview that the promising results merit confirmation in other populations.
“When you see a study that is coming out of one country, when there could be issues related to antibiotic sensitivity in H pylori, it really is important to decide whether or not this is applicable to other patient populations,” said Schnoll-Sussman, who was not involved in the study.
She noted that this is also true of the findings from the subgroup as it is unclear whether average rates of BMI are notably lower in Thailand from other countries.
“As we know, BMI affects so many things with disease states. So, it’s a possibility in a country where the BMI is actually lower, there may be something else about these individuals in terms of their wellness status that could be underlying the effect.”
The study had no specific funding, although Takeda supplied treatments used in the analysis. Yongwatana reported no relevant financial relationships. Schnoll-Sussman reported serving as an advisory committee/board member for Braintree, Ethicon, Implantica, and Phathom.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Disparities in Skin Cancer Outcomes in the Latine/Hispanic Population
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13
More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18
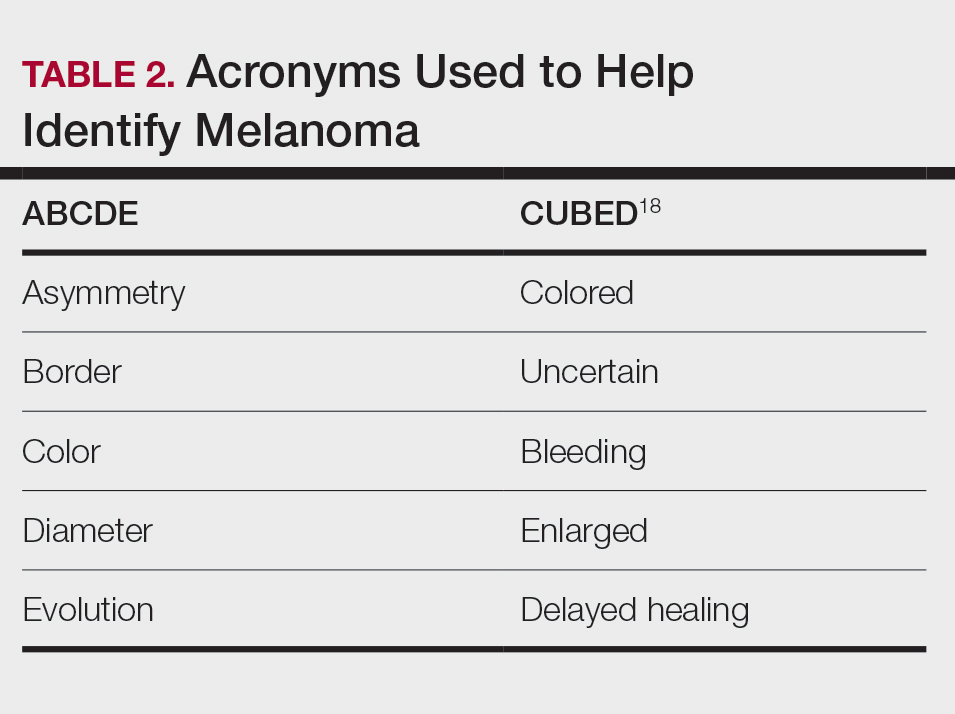
Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws.Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13

More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18

Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws.Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
The Latine/Hispanic population in the United States comprises one of the largest and youngest skin of color communities.1,2 In 2020, this group accounted for 19% of all Americans—a percentage expected to increase to more than 25% by 2060.3
It must be emphasized that the Latine/Hispanic community in the United States is incredibly diverse.4 Approximately one-third of individuals in this group are foreign-born, and this community is made up of people from all racialized groups, religions, languages, and cultural identities.2 The heterogeneity of the Latine/Hispanic population translates into a wide representation of skin tones, reflecting a rich range of ancestries, ethnicities, and cultures. The percentage of individuals from each origin group may differ according to where they live in the United States; for instance, individuals who identify as Mexican comprise more than 80% of the Latine/Hispanic population in both Texas and California but only 17% in Florida, where more than half of Latine/Hispanic people identify as Cuban or Puerto Rican.4,5 As a result, when it comes to skin cancer epidemiology, variations in incidence and mortality may exist within each of these subgroups who identify as part of the Latine/Hispanic community, as reported for other cancers.6,7 Further research is needed to investigate these potential differences.Unfortunately, considerable health disparities persist among this rapidly growing population, including increased morbidity and mortality from melanoma and keratinocyte carcinomas (KCs) despite overall low lifetime incidence.8,9 In this review, the epidemiology, clinical manifestation, and ethnic disparities for skin cancer among the US Latine/Hispanic population are summarized; other factors impacting overall health and health care, including sociocultural factors, also are briefly discussed.
Terminology
Before a meaningful dialogue can be had about skin cancer in the Latine/Hispanic population, it is important to contextualize the terms used to identify this patient population, including Latino/Latine and Hispanic. In the early 1970s, the United States adopted the term Hispanic as a way of conglomerating Spanish-speaking individuals from Spain, the Caribbean, and Central and South America. The goal was to implement a common identifier that enabled the US government to study the economic and social development of these groups.10 Nevertheless, considerable differences (eg, variations in skin pigmentation, sun sensitivity) exist among Hispanic communities, with some having stronger European, African, or Amerindian influences due to colonization of their distinct countries.11
In contrast, Latino is a geographic term and refers to people with roots in Latin America and the Caribbean (Table 1).12,13 For example, a person from Brazil may be considered Latino but not Hispanic as Brazilians speak Portuguese; alternatively, Spaniards (who are considered Hispanic) are not Latino because Spain is not a Latin American country. A person from Mexico would be considered both Latino and Hispanic.13

More recently, the term Latine has been introduced as an alternative to the gender binary inherent in the Spanish language.12 For the purposes of this article, the terms Latine and Hispanic will be used interchangeably (unless otherwise specified) depending on how they are cited in the existing literature. Furthermore, the term non-Hispanic White (NHW) will be used to refer to individuals who have been socially ascribed or who self-identify as White in terms of race or ethnicity.
Melanoma
Melanoma, the deadliest form of skin cancer, is more likely to metastasize compared to other forms of skin cancer, including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). For Latine/Hispanic individuals living in the United States, the lifetime risk for melanoma is 1 in 200 compared to 1 in 33 for NHW individuals.14 While the lifetime risk for melanoma is low for the Latine/Hispanic population, Hispanic individuals are diagnosed with melanoma at an earlier age (mean, 56 years), and the rate of new cases is marginally higher for women (4.9 per 100,000) compared to men (4.8 per 100,000).15,16
Typical sites of melanoma manifestation in Latine/Hispanic individuals include the torso (most common site in Hispanic men), lower extremities (most common site in Hispanic women), and acral sites (palms, soles, and nails).9,16,17 Anatomic location also can vary according to age for both men and women. For men, the incidence of melanoma on the trunk appears to decrease with age, while the incidence on the head and neck may increase. For women, the incidence of melanoma on the lower extremities and hip increases with age. Cutaneous melanoma may manifest as a lesion with asymmetry, irregular borders, variation in pigmentation, large diameter (>6 mm), and evolution over time. In patients with skin of color, melanoma easily can be missed, as it also typically mimics more benign skin conditions and may develop from an existing black- or dark brown–pigmented macule.18 The most common histologic subtype reported among Latine/Hispanic individuals in the United States is superficial spreading melanoma (20%–23%) followed by nodular melanoma and acral lentiginous melanoma.16,19 Until additional risk factors associated with melanoma susceptibility in Hispanic/Latine people are better elucidated, it may be appropriate to use an alternative acronym, such as CUBED (Table 2), in addition to the standard ABCDE system to help recognize potential melanoma on acral sites.18

Although the lifetime risk for melanoma among Hispanic individuals in the United States is lower than that for NHW individuals, Hispanic patients who are diagnosed with melanoma are more likely to present with increased tumor thickness and later-stage diagnosis compared to NHW individuals.8,16,20 In a recent study by Qian et al,8 advanced stage melanoma—defined as regional or distant stage disease—was present in 12.6% of NHW individuals. In contrast, the percentage of Hispanics with advanced disease was higher at 21%.8 Even after controlling for insurance and poverty status, Hispanic individuals were at greater risk than NHW individuals for late-stage diagnosis.16,20
Morbidity and mortality also have been shown to be higher in Hispanic patients with cutaneous melanoma.9,17 Reasons for this are multifactorial, with studies specific to melanoma citing challenges associated with early detection in individuals with deeply pigmented skin, a lack of awareness and knowledge about skin cancer among Latine/Hispanic patients, and treatment disparities.21-23 Moreover, very few studies have reported comprehensive data on patients from Africa and Latin America. Studies examining the role of genetic ancestry, epigenetic variants, and skin pigmentation and the risk for melanoma among the Latine/Hispanic population therefore are much needed.24
Keratinocyte Carcinomas
Keratinocyte carcinomas, also known as nonmelanoma skin cancers, include BCC and SCC. In comparison to the high-quality data available for melanoma from cancer registries, there are less reliable incidence data for KCs, especially among individuals with skin of color.25 As a result, KC epidemiology in the United States is drawn largely from case series (especially for individuals with skin of color) or claims data from small data sets often from geographically restricted regions within the United States.25,26
Basal Cell Carcinoma—Basal cell carcinoma is the most common malignant skin cancer in Latine/Hispanic individuals. Among those with lighter skin tones, the lifetime risk for BCC is about 30%.27,28 Men typically are affected at a higher rate than women, and the median age for diagnosis is 68 years.29 The development of BCC primarily is linked to lifetime accumulated UV radiation exposure. Even though BCC has a low mortality rate, it can lead to substantial morbidity due to factors such as tumor location, size, and rate of invasion, resulting in cosmetic and functional issues. Given its low metastatic potential, treatment of BCC typically is aimed at local control.30 Options for treatment include Mohs micrographic surgery (MMS), curettage and electrodessication, cryosurgery, photodynamic therapy, radiation therapy, and topical therapies. Systemic therapies are reserved for patients with locally advanced or metastatic disease.30
Latine/Hispanic patients characteristically present with BCCs on sun-exposed areas of the skin such as the head and neck region. In most patients, BCC manifests as a translucent pearly nodule with superficial telangiectasias and/or a nonhealing ulcer with a central depression and rolled nontender borders. However, in patients with skin of color, 66% of BCCs manifest with pigmentation; in fact, pigmented BCC (a subtype of BCC) has been shown to have a higher prevalence among Hispanic individuals, with an incidence twice as frequent as in NHW individuals.31 In addition, there are reports of increased tendency among Latine/Hispanic individuals to develop multiple BCCs.32,33
The relationship between UV exposure and KCs could explain the relatively higher incidence in populations with skin of color living in warmer climates, including Hispanic individuals.34 Even so, the development of BCCs appears to correlate directly with the degree of pigmentation in the skin, as it is most common in individuals with lighter skin tones within the Hispanic population.25,34,35 Other risk factors associated with BCC development include albinism, arsenic ingestion, chronic infections, immunosuppression, history of radiation treatment, and history of scars or ulcers due to physical/thermal trauma.35-37
Squamous Cell Carcinoma—Squamous cell carcinoma is the second most common skin cancer among Latine/Hispanic patients. In contrast with NHW patients, evidence supporting the role of UV exposure as a primary risk factor for SCC in patients with skin of color remains limited.25,38 Reports linking UV exposure and KCs in Hispanic and Black individuals predominantly include case series or population-based studies that do not consider levels of UV exposure.25
More recently, genetic ancestry analyses of a large multiethnic cohort found an increased risk for cutaneous SCC among Latine/Hispanic individuals with European ancestry compared to those with Native American or African ancestry; however, these genetic ancestry associations were attenuated (although not eliminated) after considering skin pigmentation (using loci associated with skin pigmentation), history of sun exposure (using actinic keratoses as a covariate for chronic sun exposure), and sun-protected vs sun-exposed anatomic sites, supporting the role of other environmental or sociocultural factors in the development of SCC.39 Similar to BCCs, immunosuppression, chronic scarring, skin irritation, and inflammatory disease also are documented risk factors.9,32
Among NHW individuals with lighter skin tones, SCC characteristically manifests on sun-exposed areas of the skin such as the head and neck region. Typically, a lesion may appear as a scaly erythematous papule or plaque that may be verrucous in nature or a nonhealing bleeding ulcer. In patients with more deeply pigmented skin, SCC tends to develop in the perianal region and on the penis and lower legs; pigmented lesions also may be present (as commonly reported in BCCs).9,32,36
Unfortunately, the lower incidence of KCs and lack of surveillance in populations with skin of color result in a low index of clinical suspicion, leading to delayed diagnoses and increased morbidity.40 Keratinocyte carcinomas are more costly to treat and require more health care resources for Latine/Hispanic and Black patients compared to their NHW counterparts; for example, KCs are associated with more ambulatory visits, more prescription medications, and greater cost on a per-person, per-year basis in Latine/Hispanic and Black patients compared with NHW patients.41 Moreover, a recent multicenter retrospective study found Hispanic patients had 17% larger MMS defects following treatment for KCs compared to NHW patients after adjustment for age, sex, and insurance type.42
Hispanic patients tend to present initially with SCCs in areas associated with advanced disease, such as the anogenital region, penis, and the lower extremities. Latine and Black men have the highest incidence of penile SCC, which is rare with high morbidity and mortality.32,43,44 The higher incidence of penile SCC among Hispanic individuals living in southern states could correspond to circumcision or HPV infection rates,44 ultimately impacting incidence.45
Dermatofibrosarcoma Protuberans
Dermatofibrosarcoma protuberans (DFSP) is a rare locally aggressive cutaneous sarcoma. According to population studies, overall incidence of DFSP is around 4.1 to 4.2 per million in the United States. Population-based studies on DFSP are limited, but available data suggest that Black patients as well as women have the highest incidence.46
Dermatofibrosarcoma protuberans is characterized by its capacity to invade surrounding tissues in a tentaclelike pattern.47 This characteristic often leads to inadequate initial resection of the lesion as well as a high recurrence rate despite its low metastatic potential.48 In early stages, DFSP typically manifests as an asymptomatic plaque with a slow growth rate. The color of the lesion ranges from reddish brown to flesh colored. The pigmented form of DFSP, known as Bednar tumor, is the most common among Black patients.47 As the tumor grows, it tends to become firm and nodular. The most common location for
Although current guidelines designate MMS as the first-line treatment for DFSP, the procedure may be inaccessible for certain populations.49 Patients with skin of color are more likely to undergo wide local excision (WLE) than MMS; however, WLE is less effective, with a recurrence rate of 30% compared with 3% in those treated with MMS.50 A retrospective cohort study of more than 2000 patients revealed that Hispanic and Black patients were less likely to undergo MMS. In addition, the authors noted that WLE recipients more commonly were deceased at the end of the study.51
Despite undergoing treatment for a primary DFSP, Hispanic patients also appear to be at increased risk for a second surgery.52 Additional studies are needed to elucidate the reasons behind higher recurrence rates in Latine/Hispanic patients compared to NHW individuals.
Factors Influencing Skin Cancer Outcomes
In recent years, racial and ethnic disparities in health care use, medical treatment, and quality of care among minoritized populations (including Latine/Hispanic groups) have been documented in the medical literature.53,54 These systemic inequities, which are rooted in structural racism,55 have contributed to poorer health outcomes, worse health status, and lower-quality care for minoritized patients living in the United States, including those impacted by dermatologic conditions.8,43,55-57 Becoming familiar with the sociocultural factors influencing skin cancer outcomes in the Latine/Hispanic community (including the lack of or inadequate health insurance, medical mistrust, language, and other cultural elements) and the paucity of research in this domain could help eliminate existing health inequities in this population.
Health Insurance Coverage—Although the uninsured rates in the Latine population have decreased since the passage of the Affordable Care Act (from 30% in 2013 to a low of 19% in 2017),58 inadequate health insurance coverage remains one of the largest barriers to health care access and a contributor to health disparities among the Latine community. Nearly 1 in 5 Latine individuals in the United States are uninsured compared to 8% of NHW individuals.58 Even though Latine individuals are more likely than non-Latine individuals to be part of the workforce, Latine employees are less likely to receive employer-sponsored coverage (27% vs 53% for NHW individuals).59
Not surprisingly, noncitizens are far less likely to be insured; this includes lawfully present immigrants (ie, permanent residents or green card holders, refugees, asylees, and others who are authorized to live in the United States temporarily or permanently) and undocumented immigrants (including individuals who entered the country without authorization and individuals who entered the country lawfully and stayed after their visa or status expired). The higher uninsured rate among noncitizens reflects not only limited access to employer-sponsored coverage but includes immigrant eligibility restrictions for federal programs such as Medicaid, the Children’s Health Insurance Program, and the Affordable Care Act Marketplace coverage.60
With approximately 9 million Americans living in mixed-status families (and nearly 10% of babies born each year with at least one undocumented parent), restrictive federal or state health care policies may extend beyond their stated target and impact both Latine citizens and noncitizens.61-65 For instance, Vargas et al64 found that both Latine citizens and noncitizens who lived in states with a high number of immigration-related laws had decreased odds of reporting optimal health as compared to Latine respondents in states with fewer immigration-related laws.Other barriers to enrollment include fears and confusion about program qualification, even if eligible.58
Medical Mistrust and Unfamiliarity—Mistrust of medical professionals has been shown to reduce patient adherence to treatment as prescribed by their medical provider and can negatively influence health outcomes.53 For racial/ethnic minoritized groups (including Latine/Hispanic patients), medical mistrust may be rooted in patients’ experience of discrimination in the health care setting. In a recent cross-sectional study, results from a survey of California adults (including 704 non-Hispanic Black, 711 Hispanic, and 913 NHW adults) found links between levels of medical mistrust and perceived discrimination based on race/ethnicity and language as well as perceived discrimination due to income level and type or lack of insurance.53 Interestingly, discrimination attributed to income level and insurance status remained after controlling for race/ethnicity and language. As expected, patients reliant on public insurance programs such as Medicare have been reported to have greater medical mistrust and suspicion compared with private insurance holders.65 Together, these findings support the notion that individuals who have low socioeconomic status and lack insurance coverage—disproportionately historically marginalized populations—are more likely to perceive discrimination in health care settings, have greater medical mistrust, and experience poorer health outcomes.53
It also is important for health care providers to consider that the US health care system is unfamiliar to many Latine/Hispanic individuals. Costs of medical services tend to be substantially higher in the United States, which can contribute to mistrust in the system.66 In addition, unethical medical experimentations have negatively affected both Latine and especially non-Hispanic Black populations, with long-lasting perceptions of deception and exploitation.67 These beliefs have undermined the trust that these populations have in clinicians and the health care system.54,67
Language and Other Cultural Elements—The inability to effectively communicate with health care providers could contribute to disparities in access to and use of health care services among Latine/Hispanic individuals. In a Medical Expenditure Panel Survey analysis, half of Hispanic patients with limited comfort speaking English did not have a usual source of care, and almost 90% of those with a usual source of care had a provider who spoke Spanish or used interpreters—indicating that few Hispanic individuals with limited comfort speaking English selected a usual source of care without language assistance.68,69 In other examples, language barriers contributed to disparities in cancer screening, and individuals with limited English proficiency were more likely to have difficulty understanding their physician due to language barriers.68,70
Improving cultural misconceptions regarding skin conditions, especially skin cancer, is another important consideration in the Latine/Hispanic community. Many Latine/Hispanic individuals wrongly believe they cannot develop skin cancer due to their darker skin tones and lack of family history.26 Moreover, multiple studies assessing melanoma knowledge and perception among participants with skin of color (including one with an equal number of Latine/Hispanic, Black/African American, and Asian individuals for a total of 120 participants) revealed that many were unaware of the risk for melanoma on acral sites.71 Participants expressed a need for more culturally relevant content from both clinicians and public materials (eg, images of acral melanoma in a person with skin of color).71-73
Paucity of Research—There is limited research regarding skin cancer risks and methods of prevention for patients with skin of color, including the Latine/Hispanic population. Efforts to engage and include patients from these communities, as well as clinicians or investigators from similar backgrounds, in clinical studies are desperately needed. It also is important that clinical studies collect data beyond population descriptors to account for both clinical and genetic variations observed in the Latine/Hispanic population.
Latine/Hispanic individuals are quite diverse with many variable factors that may influence skin cancer outcomes. Often, cancer surveillance data are available in aggregate only, which could mask this heterogeneity.74 Rigorous studies that collect more granular data, including objective measures of skin pigmentation beyond self-reported Fitzpatrick skin type, culture/beliefs, lifestyle/behavior, geographic location, socioeconomic status, genetics, or epigenetics could help fully elucidate skin cancer risks and mitigate health disparities among individuals who identify as part of this population.
Final Thoughts
The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by dermatologic health disparities. We hope this review helps to increase recognition of the clinical manifestations of skin cancer in Latine/Hispanic patients. Other factors that may impact skin cancer outcomes in this population include (but are not limited to) lack of or inadequate health insurance, medical mistrust, linguistic barriers and/or individual/cultural perspectives, along with limited research. Recognizing and addressing these (albeit complex) barriers that contribute to the inequitable access to health care in this population remains a critical step toward improving skin cancer outcomes.
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
- Noe-Bustamnate L, Lopez MH, Krogstad JM. US Hispanic population surpassed 60 million in 2019, but growth has slowed. July 7, 2020. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2020/07/07/u-s-hispanic-population-surpassed-60-million-in-2019-but-growth-has-slowed/
- Frank C, Lopez MH. Hispanic Americans’ trust in and engagement with science. Pew Research Center. June 14, 2022. Accessed September 3, 2024. https://www.pewresearch.org/wp-content/uploads/sites/20/2022/06/PS_2022.06.14_hispanic-americans-science_REPORT.pdf
- US Census Bureau. Projections of the size and composition of the US population: 2014 to 2060. US Government Printing Office; 2015. Accessed September 5, 2024. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Zong J. A mosaic, not a monolith: a profile of the U.S. Latino population, 2000-2020. October 26, 2022. Accessed September 3, 2024. https://latino.ucla.edu/research/latino-population-2000-2020/
- Latinos in California, Texas, New York, Florida and New Jersey. Pew Research Center. March 19, 2004. Accessed September 3, 2024. https://www.pewresearch.org/hispanic/2004/03/19/latinos-in-california-texas-new-york-florida-and-new-jersey/
- Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation US Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162-2169.
- Pinheiro PS, Callahan KE, Kobetz EN. Disaggregated Hispanic groups and cancer: importance, methodology, and current knowledge. In: Ramirez AG, Trapido EJ, eds. Advancing the Science of Cancer in Latinos. Springer; 2020:17-34.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526.
- Cruzval-O’Reilly E, Lugo-Somolinos A. Melanoma in Hispanics: we may have it all wrong. Cutis. 2020;106:28-30.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:474-480.
- Lopez MH, Krogstad JM, Passel JS. Who is Hispanic? September 5, 2023. Accessed September 3, 2024. https://www.pewresearch.org/short-reads/2023/09/05/who-is-hispanic/
- Carrasquillo OY, Lambert J, Merritt BG. Comment on “Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study.”J Am Acad Dermatol. 2022;87:E129-E130.
- American Cancer Society. Key statistics for melanoma skin cancer. Updated January 17, 2024. Accessed September 3, 2024. https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/key-statistics.html
- National Cancer Institute. Melanoma of the skin: recent trends in SEER age-adjusted incidence rates, 2000-2021. Updated June 27, 2024. Accessed September 3, 2024. https://seer.cancer.gov/statistics-network/explorer/application.htmlsite=53&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=6&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_display=2
- Garnett E, Townsend J, Steele B, et al. Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA. Cancer Causes Control. 2016;27:647-659.
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801.
- Bristow IR, de Berker DA, Acland KM, et al. Clinical guidelines for the recognition of melanoma of the foot and nail unit. J Foot Ankle Res. 2010;3:25.
- Fernandez JM, Mata EM, Behbahani S, et al. Survival of Hispanic patients with cutaneous melanoma: a retrospective cohort analysis of 6016 cases from the National Cancer Database. J Am Acad Dermatol. 2023;88:1135-1138.
- Hu S, Sherman R, Arheart K, et al. Predictors of neighborhood risk for late-stage melanoma: addressing disparities through spatial analysis and area-based measures. J Investigative Dermatol. 2014;134:937-945.
- Buster KJ, You Z, Fouad M, et al. Skin cancer risk perceptions: a comparison across ethnicity, age, education, gender, and income. J Am Acad Dermatol. 2012;66:771-779.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncology. 2008;9:222-231.
- Weiss J, Kirsner RS, Hu S. Trends in primary skin cancer prevention among US Hispanics: a systematic review. J Drugs Dermatol. 2012;11:580-586.
- Carvalho LAD, Aguiar FC, Smalley KSM, et al. Acral melanoma: new insights into the immune and genomic landscape. Neoplasia. 2023;46:100947.
- Kolitz E, Lopes F, Arffa M, et al. UV Exposure and the risk of keratinocyte carcinoma in skin of color: a systematic review. JAMA Dermatol. 2022;158:542-546.
- Lukowiak TM, Aizman L, Perz A, et al. Association of age, sex, race, and geographic region with variation of the ratio of basal cell to cutaneous squamous cell carcinomas in the United States. JAMA Dermatol. 2020;156:1192-1198.
- Basset-Seguin N, Herms F. Update in the management of basal cell carcinoma. Acta Derm Venereol. 2020;100:adv00140.
- McDaniel B, Badri T, Steele RB. Basal cell carcinoma. StatPearls [Internet]. Updated March 13, 2024. Accessed September 3, 2024. https://www.ncbi.nlm.nih.gov/books/NBK482439/
- Dessinioti C, Antoniou C, Katsambas A, et al. Basal cell carcinoma: what’s new under the sun. Photochem Photobiol. 2010;86:481-491.
- Kim DP, Kus KJB, Ruiz E. Basal cell carcinoma review. Hematol Oncol Clin North Am. 2019;33:13-24.
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34(5 pt 1):751-752.
- Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910.
- Byrd-Miles K, Toombs EL, Peck GL. Skin cancer in individuals of African, Asian, Latin-American, and American-Indian descent: differences in incidence, clinical presentation, and survival compared to Caucasians. J Drugs Dermatol. 2007;6:10-16.
- Rivas M, Rojas E, Calaf GM, et al. Association between non-melanoma and melanoma skin cancer rates, vitamin D and latitude. Oncol Lett. 2017;13:3787-3792.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206.
- Davis DS, Robinson C, Callender VD. Skin cancer in women of color: epidemiology, pathogenesis and clinical manifestations. Int J Womens Dermatol. 2021;7:127-134.
- Maafs E, De la Barreda F, Delgado R, et al. Basal cell carcinoma of trunk and extremities. Int J Dermatol. 1997;36:622-628.
- Munjal A, Ferguson N. Skin cancer in skin of color. Dermatol Clin. 2023;41:481-489.
- Jorgenson E, Choquet H, Yin J, et al. Genetic ancestry, skin pigmentation, and the risk of cutaneous squamous cell carcinoma in Hispanic/Latino and non-Hispanic white populations. Commun Biol. 2020;3:765.
- Soliman YS, Mieczkowska K, Zhu TR, et al. Characterizing basal cell carcinoma in Hispanic individuals undergoing Mohs micrographic surgery: a 7-year retrospective review at an academic institution in the Bronx. Brit J Dermatol. 2022;187:597-599.
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study. J Am Acad Dermatol. 2022;86:373-378.
- Blumenthal LY, Arzeno J, Syder N, et al. Disparities in nonmelanoma skin cancer in Hispanic/Latino patients based on Mohs micrographic surgery defect size: a multicenter retrospective study. J Am Acad Dermatol. 2022;86:353-358.
- Slopnick EA, Kim SP, Kiechle JE, et al. Racial disparities differ for African Americans and Hispanics in the diagnosis and treatment of penile cancer. Urology. 2016;96:22-28.
- Goodman MT, Hernandez BY, Shvetsov YB. Demographic and pathologic differences in the incidence of invasive penile cancer in the United States, 1995-2003. Cancer Epidemiol Biomarkers Prev. 2007;16:1833-1839.
- Thompson EL, Rosen BL, Maness SB. Social determinants of health and human papillomavirus vaccination among young adults, National Health Interview Survey 2016. J Community Health. 2019;44:149-158.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Mosallaei D, Lee EB, Lobl M, et al. Rare cutaneous malignancies in skin of color. Dermatol Surg. 2022;48:606-612.
- Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152:1365-1371.
- Orenstein LAV, Nelson MM, Wolner Z, et al. Differences in outpatient dermatology encounter work relative value units and net payments by patient race, sex, and age. JAMA Dermatol. 2021;157:406-412.
- Lowe GC, Onajin O, Baum CL, et al. A comparison of Mohs micrographic surgery and wide local excision for treatment of dermatofibrosarcoma protuberans with long-term follow-up: the Mayo Clinic experience. Dermatol Surg. 2017;43:98-106.
- Moore KJ, Chang MS, Weiss J, et al. Racial and ethnic differences in the surgical treatment of dermatofibrosarcoma protuberans: a retrospective cohort analysis. J Am Acad Dermatol. 2022;87:245-247.
- Trofymenko O, Bordeaux JS, Zeitouni NC. Survival in patients with primary dermatofibrosarcoma protuberans: National Cancer Database analysis. J Am Acad Dermatol. 2018;78:1125-1134.
- Bazargan M, Cobb S, Assari S. Discrimination and medical mistrust in a racially and ethnically diverse sample of California adults. Ann Fam Med. 2021;19:4-15.
- Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC; 2003.
- Bailey ZD, Krieger N, Agenor M, et al. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453-1463.
- Tackett KJ, Jenkins F, Morrell DS, et al. Structural racism and its influence on the severity of atopic dermatitis in African American children. Pediatric Dermatol. 2020;37:142-146.
- Greif C, Srivastava D, Nijhawan RI. A retrospective cohort study of dermatofibrosarcoma protuberans at a large metropolitan academic center. JAAD Int. 2022;6:104-106.
- Office of the Assistant Secretary for Planning and Evaluation. Health insurance coverage and access to care among Latinos: recent rrends and key challenges (HP-2021-22). October 8, 2021. Accessed September 3, 2024. https://aspe.hhs.gov/reports/health-insurance-coverage-access-care-among-latinos
- Keisler-Starkey K, Bunch LN. Health insurance coverage in the United States: 2020 (Current Population Reports No. P60-274). US Census Bureau; 2021. https://www.census.gov/content/dam/Census/library/publications/2021/demo/p60-274.pdf
- Kaiser Family Foundation. Key facts on health coverage of immigrants. Updated June 26, 2024. Accessed September 3, 2024. https://www.kff.org/racial-equity-and-health-policy/fact-sheet/key-facts-on-health-coverage-of-immigrants/
- Pew Research Center. Unauthorized immigrants: length of residency, patterns of parenthood. Published December 1, 2011. Accessed October 28, 2024. https://www.pewresearch.org/race-and-ethnicity/2011/12/01/unauthorized-immigrants-length-of-residency-patterns-of-parenthood/
- Schneider J, Schmitt M. Understanding the relationship between racial discrimination and mental health among African American adults: a review. SAGE Open. 2015;5:1-10.
- Philbin MM, Flake M, Hatzenbuehler ML, et al. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc Sci Med. 2018;199:29-38.
- Vargas ED, Sanchez GR, Juarez M. The impact of punitive immigrant laws on the health of Latina/o Populations. Polit Policy. 2017;45:312-337.
- Sutton AL, He J, Edmonds MC, et al. Medical mistrust in Black breast cancer patients: acknowledging the roles of the trustor and the trustee. J Cancer Educ. 2019;34:600-607.
- Jacobs J. An overview of Latin American healthcare systems. Pacific Prime Latin America. July 31, 2023. Accessed September 3, 2024. https://www.pacificprime.lat/blog/an-overview-of-latin-american-healthcare-systems/
- CDC. Unfair and unjust practices and conditions harm Hispanic and Latino people and drive health disparities. May 15, 2024. Accessed September 3, 2024. https://www.cdc.gov/tobacco-health-equity/collection/hispanic-latino-unfair-and-unjust.html
- Hall IJ, Rim SH, Dasari S. Preventive care use among Hispanic adults with limited comfort speaking English: an analysis of the Medical Expenditure Panel Survey data. Prev Med. 2022;159:107042.
- Brach C, Chevarley FM. Demographics and health care access and utilization of limited-English-proficient and English-proficient Hispanics. Agency for Healthcare Research and Quality. February 2008. http://meps.ahrq.gov/mepsweb/data_files/publications//rf28/rf28.pdf
- Berdahl TA, Kirby JB. Patient-provider communication disparities by limited English proficiency (LEP): trends from the US Medical Expenditure Panel Survey, 2006-2015. J General Intern Med. 2019;34:1434-1440.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Robinson JK, Nodal M, Chavez L, et al. Enhancing the relevance of skin self-examination for Latinos. JAMA Dermatol. 2017;153:717-718.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Madrigal JM, Correa-Mendez M, Arias JD, et al. Hispanic, Latino/a, Latinx, Latine: disentangling the identities of Hispanic/Latino Americans. National Cancer Institute Division of Cancer Epidemiology & Genetics. October 20, 2022. Accessed September 3, 2024. https://dceg.cancer.gov/about/diversity-inclusion/inclusivity-minute/2022/disentangling-identities-hispanic-latino-americans
Practice Points
- The Latine/Hispanic community—the largest ethnic minoritized group in the United States—is disproportionately affected by disparities in skin cancer outcomes.
- Factors influencing skin cancer outcomes in Latine/Hispanic patients in the United States are complex and multidimensional, including lack of familiarity among dermatologists with skin cancer manifestation in this population compared to non-Hispanic White individuals as well as limited data elucidating risk factors for skin cancer in patients with skin of color and sociocultural factors.
Pinto Bean Pressure Wraps: A Novel Approach to Treating Digital Warts
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.
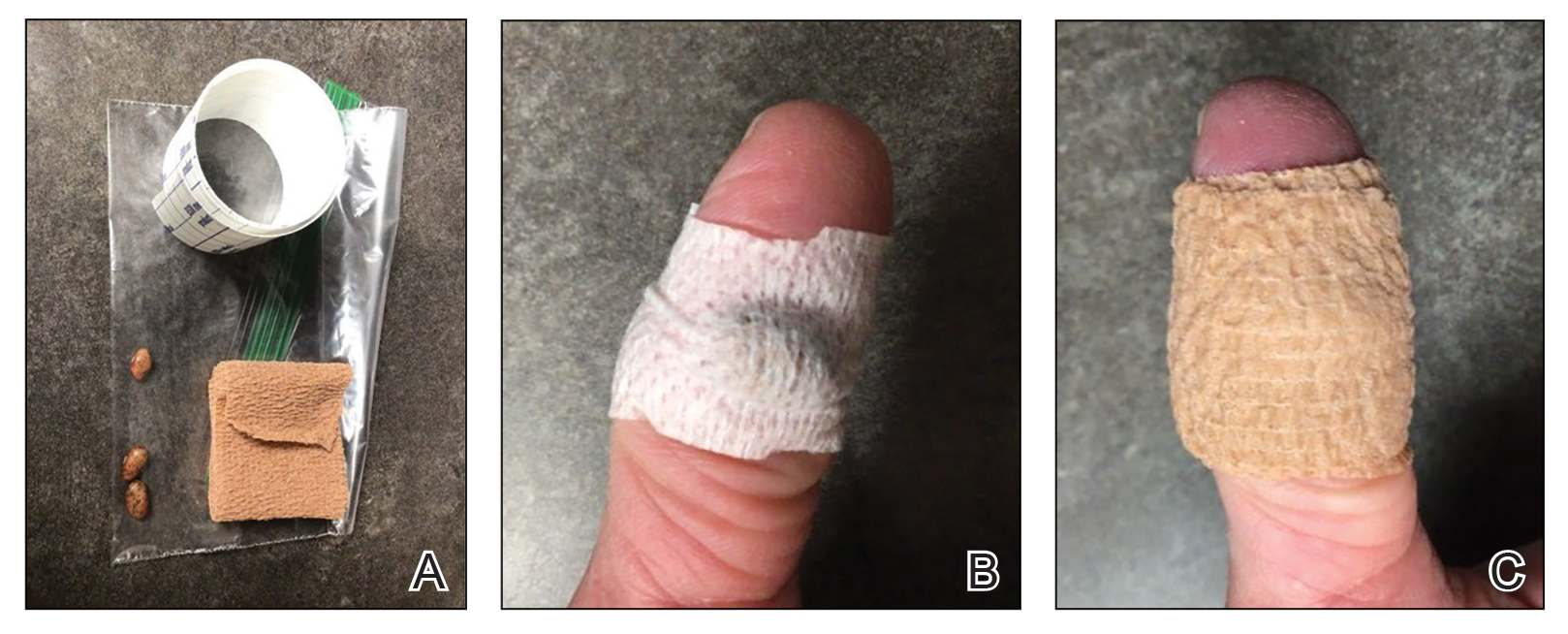
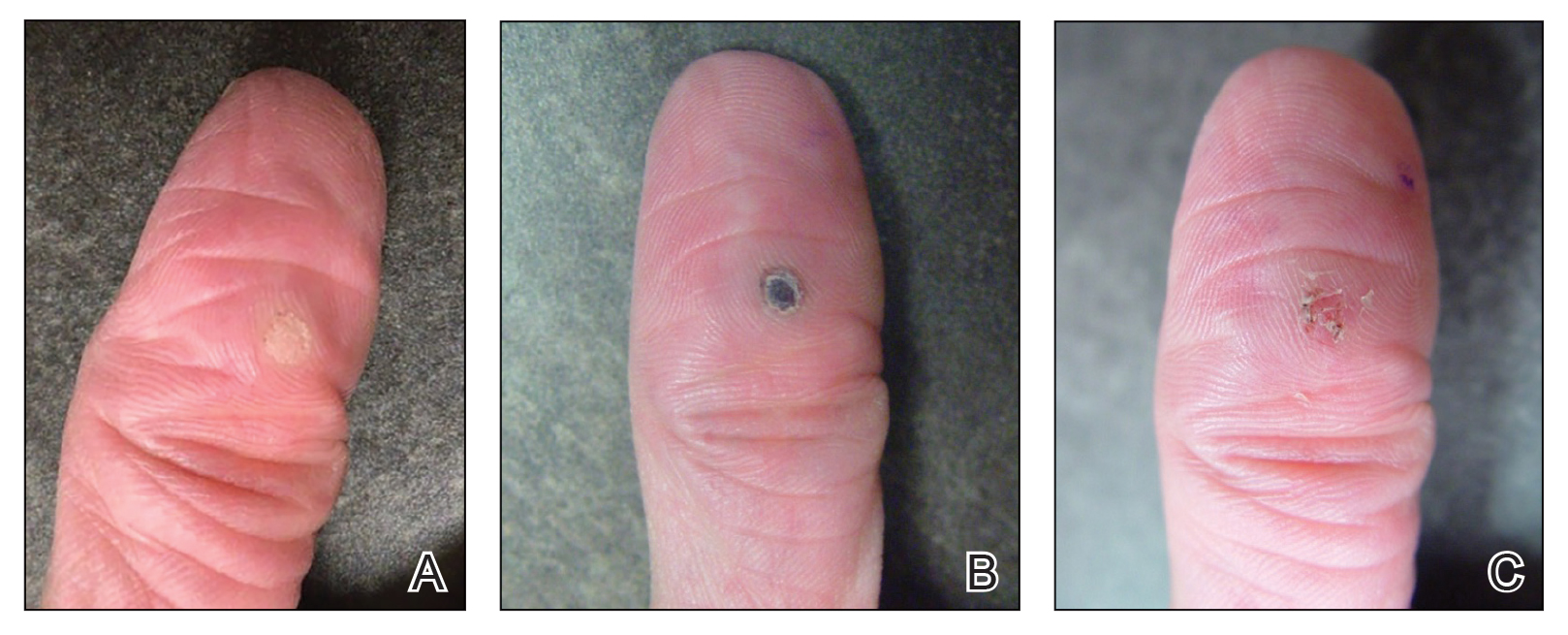
What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.


What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
Practice Gap
Verruca vulgaris is a common dermatologic challenge due to its high prevalence and tendency to recur following routinely employed destructive modalities (eg, cryotherapy, electrosurgery), which can incur a considerable amount of pain and some risk for scarring.1,2 Other treatment methods for warts such as topical salicylic acid preparations, topical immunotherapy, or intralesional allergen injections often require multiple treatment sessions.3,4 Furthermore, the financial burden of traditional wart treatment can be substantial.4 Better techniques are needed to improve the clinician’s approach to treating warts. We describe a home-based technique to treat common digital warts using pinto bean pressure wraps to induce ischemic changes in wart tissue with similar response rates to commonly used modalities.
Technique
Our technique utilizes a small, hard, convex object that is applied directly over the digital wart. A simple self-adhesive wrap is used to cover the object and maintain constant pressure on the wart overnight. We typically use a dried pinto bean (a variety of the common bean Phaseolus vulgaris) acquired from a local grocery store due to its ideal size, hard surface, and convex shape (Figure 1). The bean is taped in place directly overlying the wart and covered with a self-adhesive wrap overnight. The wrap is removed in the morning, and often no further treatment is needed. The ischemic wart tissue is allowed to slough spontaneously over 1 to 2 weeks. No wound care or dressing is necessary (Figure 2). Larger warts may require application of the pressure wraps for 2 to 3 additional nights. While most warts resolve with this technique, we have observed a recurrence rate similar to that for cryotherapy. Patients are advised that any recurrent warts can be re-treated monthly, if needed, until resolution.


What to Use and How to Prepare—Any small, hard, convex object can be used for the pressure wrap; we also have used appropriately sized and shaped plastic shirt buttons with similar results. Home kits can be assembled in advance and provided to patients at their initial visit along with appropriate instructions (Figure 1A).
Effects on the Skin and Distal Digit—Application of pressure wraps does not harm normal skin; however, care should be taken when the self-adherent wrap is applied so as not to induce ischemia of the distal digit. The wrap should be applied using gentle pressure with patients experiencing minimal discomfort from the overnight application.
Indications—This pressure wrap technique can be employed on most digital warts, including periungual warts, which can be difficult to treat by other means. However, in our experience this technique is not effective for nondigital warts, likely due to the inability to maintain adequate pressure with the overlying dressing. Patients at risk for compromised digital perfusion, such as those with Raynaud phenomenon or systemic sclerosis, should not be treated with pressure wraps due to possible digital ischemia.
Precautions—Patients should be advised that the pinto bean should only be used if dry and should not be ingested. The bean can be a choking hazard for small children, therefore appropriate precautions should be used. Allergic contact dermatitis to the materials used in this technique is possible, but we have never observed this. The pinto bean can be reused for future application as long as it remains dry and provides a hard convex surface.
Practice Implications
The probable mechanism of the ischemic changes to the wart tissue likely is the occlusion of tortuous blood vessels in the dermal papillae, which are intrinsic to wart tissue and absent in normal skin.1 This pressure-induced ischemic injury allows for selective destruction of the wart tissue with sparing of the normal skin. Our technique is fairly novel, although at least one report in the literature has described the use of a mechanical device to induce ischemic changes in skin tags.5
The use of pinto bean pressure wraps to induce ischemic change in digital warts provides a low-risk and nearly pain-free alternative to more expensive and invasive treatment methods. Moreover, this technique allows for a low-cost home-based therapy that can be repeated easily for other digital sites or if recurrence is noted.
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
- Cardoso J, Calonje E. Cutaneous manifestations of human papillomaviruses: a review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:145-154.
- Lipke M. An armamentarium of wart treatments. Clin Med Res. 2006;4:273-293. doi:10.3121/cmr.4.4.273
- Muse M, Stiff K, Glines K, et al. A review of intralesional wart therapy. Dermatol Online J. 2020;26:2. doi:10.5070/D3263048027
- Berna R, Margolis D, Barbieri J. Annual health care utilization and costs for treatment of cutaneous and anogenital warts among a commercially insured population in the US, 2017-2019. JAMA Dermatol. 2022;158:695-697. doi:10.1001/jamadermatol.2022.0964
- Fredriksson C, Ilias M, Anderson C. New mechanical device for effective removal of skin tags in routine health care. Dermatol Online J. 2009;15:9. doi:10.5070/D37tj2800k
No Link Between PPI Use and Risk for Cardiovascular Events
TOPLINE:
There is no significant association between the use of proton pump inhibitors (PPIs) and risk for cardiovascular events, a meta-analysis shows. However, patients with gastroesophageal reflux disease (GERD) do experience a slight increase in cardiovascular events with PPI use.
METHODOLOGY:
- PPIs are commonly used gastric acid suppressants; however, they have pleiotropic effects, some of which have been hypothesized to augment cardiovascular disorders.
- Researchers conducted a meta-analysis of randomized clinical trials with at least 100 patients and treatment durations > 30 days, which compared groups receiving PPIs to those on placebo or other active treatments.
- The primary outcome was a composite of nonfatal myocardial infarctions, nonfatal strokes, fatal cardiovascular adverse events, coronary revascularizations, and hospitalizations for unstable angina.
TAKEAWAY:
- Researchers included data from 52 placebo-controlled trials, with 14,988 patients and 8323 patients randomized to receive a PPI or placebo, respectively; the mean treatment duration was 0.45 person-years for those treated with PPIs and 0.32 person-years for those treated with placebo.
- Among placebo-controlled trials, 24 were conducted in patients with GERD.
- Researchers also included 61 active-controlled trials that compared PPIs with histamine-2 receptor antagonists (51 trials) or other active treatments.
- The incidence rate ratio for the primary outcome was 0.72 when comparing PPI to placebo, indicating no significant association between PPI and cardiovascular events.
- Among patients with GERD, cardiovascular events occurred only in those treated with PPIs, leading to approximately one excess cardiovascular event per 100 person-years of PPI treatment relative to placebo.
- Researchers found no association between PPI treatment and the risk for cardiovascular events in trials comparing PPIs with other active treatments.
IN PRACTICE:
“We found no association of cardiovascular events with PPI treatment,” the authors wrote. “Cardiovascular events appeared more frequent with PPI treatment in GERD trials, but results from this subgroup should be interpreted with the limitations of the analysis in mind.”
SOURCE:
The study, led by Andrew D. Mosholder, MD, MPH, Division of Epidemiology, US Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring, Maryland, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study lacked individual patient data, which precluded a time-to-event analysis or an analysis accounting for patient characteristics such as age or sex. The mean duration of PPI treatment in these trials was a few months, limiting the assessment of cardiovascular risk with extended use. The risk estimates were influenced the most by data on omeprazole and esomeprazole.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
There is no significant association between the use of proton pump inhibitors (PPIs) and risk for cardiovascular events, a meta-analysis shows. However, patients with gastroesophageal reflux disease (GERD) do experience a slight increase in cardiovascular events with PPI use.
METHODOLOGY:
- PPIs are commonly used gastric acid suppressants; however, they have pleiotropic effects, some of which have been hypothesized to augment cardiovascular disorders.
- Researchers conducted a meta-analysis of randomized clinical trials with at least 100 patients and treatment durations > 30 days, which compared groups receiving PPIs to those on placebo or other active treatments.
- The primary outcome was a composite of nonfatal myocardial infarctions, nonfatal strokes, fatal cardiovascular adverse events, coronary revascularizations, and hospitalizations for unstable angina.
TAKEAWAY:
- Researchers included data from 52 placebo-controlled trials, with 14,988 patients and 8323 patients randomized to receive a PPI or placebo, respectively; the mean treatment duration was 0.45 person-years for those treated with PPIs and 0.32 person-years for those treated with placebo.
- Among placebo-controlled trials, 24 were conducted in patients with GERD.
- Researchers also included 61 active-controlled trials that compared PPIs with histamine-2 receptor antagonists (51 trials) or other active treatments.
- The incidence rate ratio for the primary outcome was 0.72 when comparing PPI to placebo, indicating no significant association between PPI and cardiovascular events.
- Among patients with GERD, cardiovascular events occurred only in those treated with PPIs, leading to approximately one excess cardiovascular event per 100 person-years of PPI treatment relative to placebo.
- Researchers found no association between PPI treatment and the risk for cardiovascular events in trials comparing PPIs with other active treatments.
IN PRACTICE:
“We found no association of cardiovascular events with PPI treatment,” the authors wrote. “Cardiovascular events appeared more frequent with PPI treatment in GERD trials, but results from this subgroup should be interpreted with the limitations of the analysis in mind.”
SOURCE:
The study, led by Andrew D. Mosholder, MD, MPH, Division of Epidemiology, US Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring, Maryland, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study lacked individual patient data, which precluded a time-to-event analysis or an analysis accounting for patient characteristics such as age or sex. The mean duration of PPI treatment in these trials was a few months, limiting the assessment of cardiovascular risk with extended use. The risk estimates were influenced the most by data on omeprazole and esomeprazole.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
There is no significant association between the use of proton pump inhibitors (PPIs) and risk for cardiovascular events, a meta-analysis shows. However, patients with gastroesophageal reflux disease (GERD) do experience a slight increase in cardiovascular events with PPI use.
METHODOLOGY:
- PPIs are commonly used gastric acid suppressants; however, they have pleiotropic effects, some of which have been hypothesized to augment cardiovascular disorders.
- Researchers conducted a meta-analysis of randomized clinical trials with at least 100 patients and treatment durations > 30 days, which compared groups receiving PPIs to those on placebo or other active treatments.
- The primary outcome was a composite of nonfatal myocardial infarctions, nonfatal strokes, fatal cardiovascular adverse events, coronary revascularizations, and hospitalizations for unstable angina.
TAKEAWAY:
- Researchers included data from 52 placebo-controlled trials, with 14,988 patients and 8323 patients randomized to receive a PPI or placebo, respectively; the mean treatment duration was 0.45 person-years for those treated with PPIs and 0.32 person-years for those treated with placebo.
- Among placebo-controlled trials, 24 were conducted in patients with GERD.
- Researchers also included 61 active-controlled trials that compared PPIs with histamine-2 receptor antagonists (51 trials) or other active treatments.
- The incidence rate ratio for the primary outcome was 0.72 when comparing PPI to placebo, indicating no significant association between PPI and cardiovascular events.
- Among patients with GERD, cardiovascular events occurred only in those treated with PPIs, leading to approximately one excess cardiovascular event per 100 person-years of PPI treatment relative to placebo.
- Researchers found no association between PPI treatment and the risk for cardiovascular events in trials comparing PPIs with other active treatments.
IN PRACTICE:
“We found no association of cardiovascular events with PPI treatment,” the authors wrote. “Cardiovascular events appeared more frequent with PPI treatment in GERD trials, but results from this subgroup should be interpreted with the limitations of the analysis in mind.”
SOURCE:
The study, led by Andrew D. Mosholder, MD, MPH, Division of Epidemiology, US Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring, Maryland, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study lacked individual patient data, which precluded a time-to-event analysis or an analysis accounting for patient characteristics such as age or sex. The mean duration of PPI treatment in these trials was a few months, limiting the assessment of cardiovascular risk with extended use. The risk estimates were influenced the most by data on omeprazole and esomeprazole.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Alopecia Induced by Poly-L-Lactic Acid Injection
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.
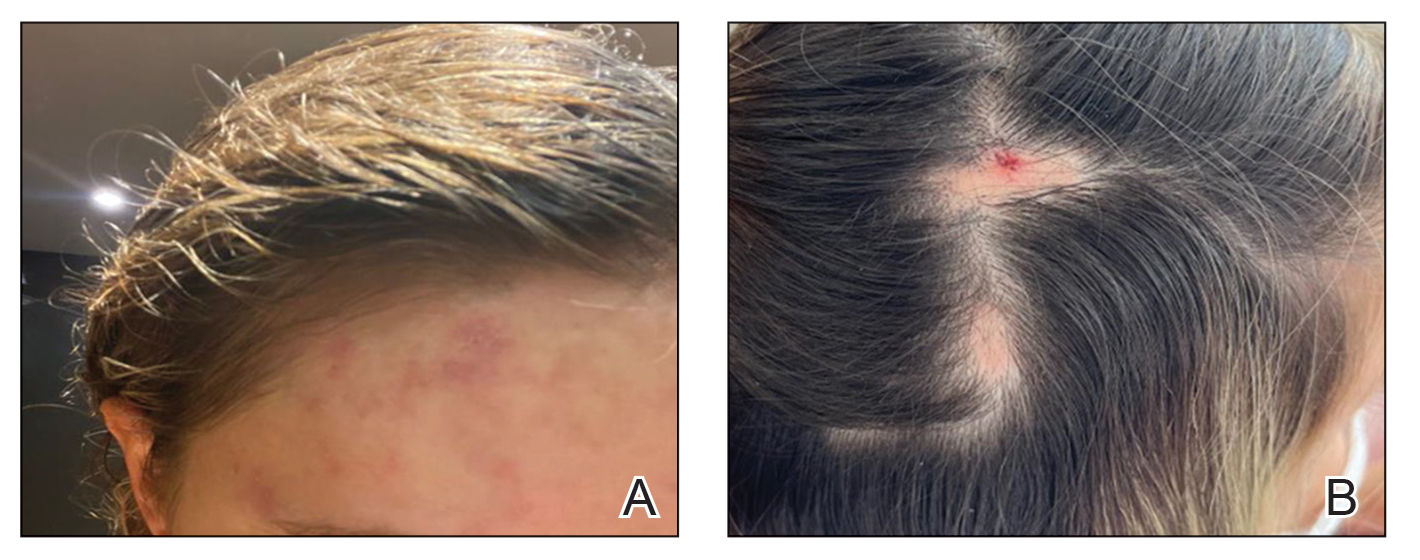
The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.
Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.

The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.


Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
Cosmetic procedures carry inherent risks of adverse events. Transient and permanent alopecia are rare complications of these procedures. Although they have not been fully elucidated, several pathologic mechanisms for hair loss following cosmetic procedures have been proposed, including extravascular compression (a phenomenon that has been well documented in bedridden patients) as well as intravascular occlusion leading to inflammation and necrosis, which has been associated with hyaluronic acid (HA) fillers.¹ Cases of alopecia also have been reported following mesotherapy and calcium hydroxyapatite, deoxycholic acid, and botulinum toxin injections.² We report a case of alopecia resulting from poly-L-lactic acid (PLLA) injection in a 35-year-old woman with the intent to raise awareness of this rare adverse event.
Case Report
A healthy 35-year-old woman received aesthetic PLLA injections on the face and frontal hairline performed by an outside dermatologist using the vector technique. During the procedure, the patient experienced intense itchiness at the right temporal artery vascular territory and reported a substantial headache the next day. She also presented with erythema and edema of the frontal and right parietal scalp with a well-delimited livedoid vascular area along the temporal artery territory on the right side of the head 1 day after the procedure (Figure 1). These signs were reported to the outside dermatologist who performed the procedure, but they were not assumed to be adverse events at that time.

The condition persisted for 4 days followed by the development of an irregular 3×2-cm patch of alopecia on the right parietal scalp. A 3-day course of self-administered oral prednisolone 0.2 mg/kg/d was prescribed.
Twenty-seven days after the procedure, the patient presented to our trichology clinic for evaluation of a single patch of nonscarring alopecia on the right parietal scalp. Trichoscopy showed multiple yellow and black dots, broken hairs, pigment deposits, and an erythematous background mainly composed of linear telangiectatic vessels (Figure 2). Histopathologic analysis revealed a lymphocytic inflammatory infiltrate surrounding the follicular units that was compatible with an alopecia areata–like pattern as well as PLLA deposits in the subcutaneous tissue forming foreign body granulomas (Figure 3). The diagnosis of PLLA-induced alopecia was made based on the detection of PLLA at the biopsy site within the patchy alopecia.


Intralesional triamcinolone acetonide 5 mg/mL was administered at 1-cm intervals in the subdermal space (0.1 mL/puncture site). After 14 days, the patient developed an additional patch of alopecia in the same vascular territory as the right temporal artery, positioned just beneath the initial patch, with similar trichoscopy findings. The patches were treated with intralesional triamcinolone acetonide for 3 additional sessions, administered every 4 weeks. Long-term monitoring of the patient revealed regrowth with comparable hair count to the unaffected contralateral scalp, indicative of a nonscarring alopecia.
Comment
Poly-L-lactic acid is a biostimulator synthesized from the α-hydroxy acid family in 1954 that has been safely used in suture materials, resorbable plates, and orthopedic screws.4 Alopecia has been reported as a systemic allergic reaction to biodegradable screws following an orthopedic procedure.5 Prior reports of embolization and retinal ischemia with PLLA have raised concerns regarding its occlusive potential.6-9
Approved by the US Food and Drug Administration in 2004 for soft tissue restoration in HIV-related lipoatrophy, PLLA was expanded to cosmetic applications in 2009. As previously reported with HA fillers, we hypothesize that extravascular compression resulting from the placement of the filler material (due to the volume injected in the scalp area) contributes to the development of alopecia plus PLLA embolism–induced ischemic alopecia in the affected areas.10 In our case, the diagnosis of PLLA-induced alopecia was confirmed based on the finding of the filler material in the subcutaneous tissue on histopathology, probably due to embolization. Moreover, trichoscopic findings were all similar to those described after HA embolization.11 The features found in our patient due to the PLLA local reaction were similar to those seen in other conditions such as alopecia areata, pressure alopecia, and chemotherapy-induced alopecia; therefore, histopathology confirmation is mandatory in cases of hair loss associated with PLLA.
The emergence of a secondary patch of alopecia prompts consideration of an intrinsic late inflammatory propensity of PLLA. Immune cells recognize PLLA as a foreign body, and subclinical inflammatory foreign body reactions can cause PLLA-induced collagen synthesis.12 This phenomenon underscores the need for further investigation into the immunologic implications of PLLA in alopecia pathogenesis.
The angiogenic properties of the anagen phase require an adequate blood supply for effective hair growth; therefore, the lack of blood and nutrient supply to the hair bulb triggers miniaturization, a possible explanation for the hair thinning found in the alopecic patch.13
Conclusion
Alopecia as an adverse effect of cosmetic procedures can be distressing for patients, even when reversible. A detailed understanding of scalp anatomy is critical for satisfactory outcomes with aesthetic procedures. Physicians must pay attention to the amount and area of material injected in order to avoid possible mechanisms of ischemia—embolization and/or extravascular compression—especially in highly vascularized areas.
We present a rare report of alopecia as an adverse event of PLLA injection. Dermatologists must be aware of this rare condition, and trichoscopy combined with histopathologic analysis are encouraged for early recognition and proper management.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
- Issa NT, Kaiser M, Martinez-Velasco A, et al. Alopecia after cosmetic injection procedures: a review. Dermatol Surg. 2022;48:855-861.
- Alopecia with foreign body granulomas induced by Radiesse injection: a case report. J Cosmet Laser Ther. 2018;20:462-464.
- Munia C, Parada M, de Alvarenga Morais MH. Changes in facial morphology using poly-L-lactic acid application according to vector technique: a case series. J Clin Aesthet Dermatol. 2022;15:38-42.
- Attenello NH, Maas CS. Injectable fillers: review of material and properties. Facial Plast Surg. 2015;31:29-34.
- Mastrokalos DS, Paessler HH. Allergic reaction to biodegradable interference poly-L-lactic acid screws after anterior cruciate ligament reconstruction with bone-patellar tendon-bone graft. Arthroscopy. 2008;24:732-733.
- Wu CW, Wu HJ. Retinal artery occlusion following cosmetic injection of poly-L-lactic acid. Taiwan J Ophthalmol. 2021;11:317-320.
- Yuan JT, Chang TW, Yu SS, et al. Mental artery occlusion from poly-L-lactic acid injection at the lateral chin. Dermatol Surg. 2017;43:1402-1405.
- Ragam A, Agemy SA, Dave SB, et al. Ipsilateral ophthalmic and cerebral infarctions after cosmetic polylactic acid injection into the forehead. J Neuroophthalmol. 2017;37:77-80.
- Witmanowski H, Błochowiak K. Another face of dermal fillers. Postepy Dermatol Alergol. 2020;37:651-659.
- Yang Q, Qiu L, Yi C, et al. Reversible alopecia with localized scalp necrosis after accidental embolization of the parietal artery with hyaluronic acid. Aesthetic Plast Surg. 2017;41:695-699.
- Asz-Sigall D, Iñigo-Gomez K, Ortega-Springall MF, et al. Alopecia secondary to hyaluronic acid embolization: trichoscopic findings. Skin Appendage Disord. 2019;5:396-400.
- Oh S, Lee JH, Kim HM, et al. Poly-L-lactic acid fillers improved dermal collagen synthesis by modulating M2 macrophage polarization in aged animal skin. Cells. 2023;12:1320. doi:10.3390/cells12091320
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and mechanistic approach to the hair growth cycle and hair loss. J Clin Med. 2023;12:893.2. Liu RF, Kuo TT, Chao YY, et al.
Practice Points
- Alopecia is a potential adverse event of poly-L-lactic acid (PLLA) injection, and prior reports of embolization and retinal ischemia with PLLA use raise the concern of its occlusive potential.
- The combination of extravascular compression due to the presence of the filler material in the subcutaneous tissue as well as intravascular PLLA embolism may contribute to tissue ischemia–induced alopecia in the affected areas.
- Poly-L-lactic acid also may cause a local inflammatory reaction that is alopecia areata–like, which would explain its similar trichoscopy findings.