User login
The Challenges of Normal Pressure Hydrocephalus: A Case-Based Review
CE/CME No: CR-1512
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize the common presenting symptoms of idiopathic normal pressure hydrocephalus (iNPH).
• Describe findings on brain imaging (MRI or CT) that are highly suggestive of a diagnosis of iNPH.
• Describe supplementary tests commonly used to help confirm a suspected diagnosis of iNPH.
• Discuss the prognosis and expected outcomes from ventriculoperitoneal shunt placement for iNPH.
FACULTY
Freddi Segal-Gidan is Director of the Rancho Los Amigos/University of Southern California (USC) Alzheimer’s Disease Center and Assistant Clinical Professor in the departments of Neurology and Family Medicine at Keck School of Medicine, USC, and in Gerontology at L. Davis School of Gerontology at USC, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2015.
Article begins on next page >>
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of dementia. Unfortunately, the symptoms of iNPH—cognitive impairment, gait change, and urinary incontinence—develop slowly and are often mistaken for those of other conditions or for normal aging. This article explains when to suspect iNPH and the steps you need to take when iNPH is in the differential.
Ten years ago, a 74-year-old semi-retired cardiologist self-referred to neurology for evaluation of forgetfulness that had increased in the previous two years. He remained functionally independent in all daily activities. Mental status screening with the Mini-Mental State Exam was within normal limits. He underwent comprehensive neuropsychologic testing, which revealed an estimated verbal IQ of 130, a word list recall in the low average range, and normal results for all other tests; the report also noted mild depression. He was seen one year later for follow-up and reported continued memory difficulties. A brain MRI showed ventricular dilatation with cerebral and cerebellar atrophy “consistent with age.” He was placed on an off-label trial of donepezil and vitamin E.
Two years later, he began to experience slowing of his gait and was noted to have “mild Parkinsonism” on neurologic examination. He was started on carbidopa/levodopa, with no improvement. Another MRI showed no progression from two years prior, but the “possibility of normal pressure hydrocephalus” (NPH) was noted in the radiology report. He underwent a lumbar drain procedure, after which he had slow improvement in gait over the next two months.
Four to 12 months following the lumbar drain procedure, he experienced worsening gait, balance problems, and urinary urgency, and he reported increasing memory difficulty. Neurologic examination was noteworthy for soft voice with hoarse quality, slightly increased tone in the upper extremities (right greater than left), and wide-based and unsteady gait with dragging of feet. Another brain MRI was done, with the report noting “ventriculomegaly out of proportion to volume loss … NPH cannot be excluded.” After review of the results for a second opinion, an MRI with cerebrospinal fluid (CSF) flow study was performed; based on the results, the patient was determined to be a good candidate for ventriculoperitoneal shunt placement. He underwent shunt placement without incident and had sustained improvement in gait and cognition over the next six years.
Idiopathic normal pressure hydrocephalus (iNPH) is an uncommon but important differential to consider in any older individual with cognitive decline. NPH was first discussed in the medical literature in 1965, when Adams and Hakim described the characteristic features of iNPH: the triad of walking impairment, “dementia,” and urinary incontinence in the presence of enlarged ventricles but normal intracranial pressure.1
With the continued aging of the population, an increasing number of individuals can be expected to experience cognitive decline, gait and balance difficulties, and urinary incontinence. Clinicians caring for patients who present with one or more of these symptoms must keep iNPH in mind for the differential diagnosis. iNPH is a treatable condition, and appropriate intervention can significantly improve affected patients’ lives, as well as reduce health care expenditures.2,3
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The underlying pathophysiology of iNPH is not completely understood. The symptoms are believed to arise from the slow, gradual, and insidious accumulation of CSF within the brain ventricles. The current understanding is that the CSF acts as a lymphatic drainage system for the brain, entering the brain parenchyma via paravascular spaces that surround penetrating arteries and clearing interstitial fluid along paravenous drainage pathways.4
CSF reabsorption into the blood is a dual process, with drainage via the arachnoid villi and granulations within the dural sinuses and slow drainage via lymphatic vessels in the perineural, otic, and ophthalmic regions. There is a pressure gradient of fluid in the subarachnoid space and ventricles, with the CSF pressure normally higher than the pressure of the venous system, allowing outflow of CSF.
In iNPH, outflow of CSF is at least partially disrupted, and there is decreased CSF reabsorption, resulting in a higher, normal baseline CSF volume over time. The underlying cause of reduced CSF reabsorption in iNPH remains uncertain, but it has been proposed that arachnoid granulations fail to maintain adequate removal of CSF, possibly due to fibrosis or scarring.5 In response to increased CSF volume, the ventricles distend and compress the brain parenchyma. Exactly how the pressure exerted by the ventricles leads to changes in gait, cognition, and urinary incontinence is not well understood.
Continue for epidemiology >>
EPIDEMIOLOGY
The prevalence of iNPH has been estimated at 21.9 cases per 100,000 persons.6 It occurs primarily in individuals older than age 606 and occurs more frequently with increasing age, as shown in a recent report in which the prevalence of probable iNPH was 0.2% in those ages 70 to 79 and 5.9% in those ages 80 and older.7 Based on these numbers, the authors estimated that approximately 700,000 Americans older than 70 may have iNPH. It is a rare cause of dementia among the population with dementia onset after age 65 (“senile onset”). No gender or racial/ethnic differences have been reported.
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of iNPH is based on clinical findings. Making the diagnosis can be challenging, as the symptoms overlap with common age-related changes and age-associated medical conditions, and there is no single diagnostic test. A high index of clinical suspicion or an incidental finding on neuroimaging done in the diagnostic work-up for cognitive impairment/dementia (or some other reason) are the usual triggers for further investigation.
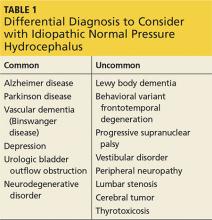
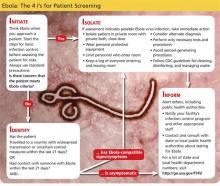
Clinicians should include iNPH in the differential, along with alternative diagnoses, when the history includes one or more of the three symptoms of iNPH: cognitive decline, gait disturbance, and/or urinary incontinence (see Table 1). While superficially appearing to be an easily recognizable condition, iNPH is actually a very complex disease that goes unrecognized and undiagnosed in many individuals.8 Evidence-based guidelines developed in 2005 attempted to devise a classification system based upon age, gait speed, nature of symptoms, neuroimaging changes, and CSF opening pressures.9
The symptoms of iNPH typically develop insidiously and progress slowly. The earliest symptom is most often gait disturbance. The gait disturbance associated with iNPH is described as “magnetic” or gait apraxia and includes trouble with initiation, reduced stride length, and a slow, cautious quality.10 Cognitive impairment typically has a frontosubcortical pattern, with psychomotor slowing, decreased attention or concentration, and problems with verbal fluency and executive function.11 Deficits in visuospatial and construction skills may also be observed.
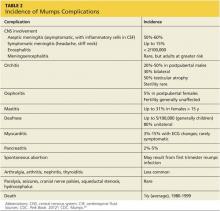
Memory decline, which predominates in Alzheimer disease, may be less pronounced in iNPH. Urinary incontinence is usually a combination of urgency and frequency, mostly due to detrusor overactivity.12 A majority of patients (62%) treated for iNPH have all three symptoms of the triad, but in some cases only one or two symptoms are present.13 Gait disturbance is the most common feature, present in 98% of cases, followed by urinary incontinence (79%) and cognitive impairment (78%).13
Physical examination should include a complete neurologic exam. Mental status testing will typically show slowing, with decreased comprehension and increased time required to complete tasks. Decreased short-term memory recall may be improved with cues. Speech may be slow but is without aphasia or dysarthria. The gait pattern often includes a wide stance; slow, small steps with decreased floor clearance; and retained arm swing. Motor examination of the lower extremities may demonstrate some increased tone and slightly brisk reflexes.
Continue for neuroimaging >>
NEUROIMAGING
Brain neuroimaging with CT or MRI is essential to the initial investigation and diagnostic evaluation of suspected iNPH. Neuroimaging is not diagnostic in itself, but the findings are important both to support a suspected diagnosis of iNPH and to exclude other conditions that could cause similar findings or contribute to the symptoms (eg, stroke or tumor).
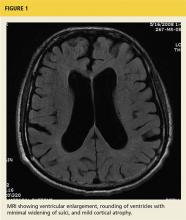
The key finding is enlargement of the lateral ventricles (ventriculomegaly) disproportionate to the degree of cortical atrophy (see Figure 1). Ventricular dilatation is characterized by rounding of the contour of the ventricles with a widened third ventricle. Normal volume of brain parenchyma is evidenced by the absence of sulci widening, which would be seen in the presence of cortical atrophy and the absence of obscured sulci. White matter changes, seen as periventricular white matter hyperintensity on MRI, has also been noted frequently on imaging consistent with iNPH.14 On MRI, a marked CSF flow void in the aqueduct of Sylvius and fourth ventricle, called a flow void, is usually seen.15
The Evans ratio, calculated by dividing the maximum width of the ventricular frontal horns on imaging by the widest skull diameter, is one criteria for diagnosis of iNPH on neuroimaging. An Evans ratio greater than 0.3 (signifying ventriculomegaly), within the appropriate clinical context, is considered indicative of iNPH.14,16
Continue for confirmatory studies >>
CONFIRMATORY STUDIES
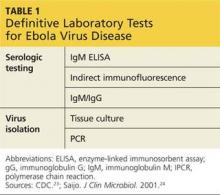
Beyond neuroimaging, a variety of specialty studies are used to increase diagnostic certainty and as predictors of outcome from surgical intervention. These include large-volume lumbar tap (“tap test”), external lumbar drainage, nuclear or CT cisternogram, and CSF flow imaging. Each of these tests has some risk, and no single test has been conclusively demonstrated by itself to be superior to one or a combination of the others. No CSF biomarkers have as yet been identified for the diagnosis of iNPH.17
The simplest supplemental test is the CSF tap test, which involves the removal of 40 to 50 mL of CSF via lumbar puncture. The patient is then assessed for improvement of symptoms by comparing gait and cognition prior to the test with that from 30 to 60 minutes after. Patients with significant symptomatic improvement (lasting at least a few weeks and up to months) have been found to be good candidates for shunt surgery.18 Patients who have high opening pressure (> 20 cm H2O) require further investigation for secondary causes of NPH (eg, meningitis).18 Routine CSF analysis should be done (cell count, protein, glucose) to rule out chronic meningitis, which can mimic NPH.
The external lumbar drainage (ELD) test involves placement of an indwelling external lumbar catheter (lumbar drain) for external drainage of approximately 300 mL/d of CSF over one to five days. It is useful in patients who do not have a significant response to the tap test and for whom a high index of suspicion for iNPH remains. A positive response to ELD has been found to predict a potentially positive shunt response.19 The ELD test has a high positive predictive value (80% to 100%).18
Nuclear or CT cisternography has been used to evaluate CSF reabsorption. In the presence of iNPH, cisternography demonstrates ventricular reflux with slow cortical uptake.20,21 A positive cisternogram combined with a radioisotope CT exam that shows normal cerebral blood flow is better than cisternography alone in predicting positive outcome from shunt surgery.22
CSF flow studies utilize T2-weighted images on MRI to estimate CSF flow through the ventricles. In the assessment of iNPH, evaluation of CSF flow by MRI is used in the preoperative evaluation and also in post–shunt-placement follow-up. Slow-moving CSF has an increased signal, while regions of fast-moving CSF, such as in a narrow cerebral aqueduct, have no signal. In the presence of iNPH, the cerebral aqueduct shows an increased pulsatile flow void, and there is a hypointense or absent signal in the proximal fourth ventricle on proton density–weighted images. The presence of an increased CSF flow void has been found to be highly predictive of a positive outcome from ventriculoperitoneal (VP) shunt placement.23
Another approach involves the direct measurement of the velocity of CSF stroke volume, which is the mean volume of CSF that passes through the aqueduct during systole and diastole. Studies have found that a CSF stroke volume of 42 µL or greater is an indicator for a good probability of improvement after VP shunt placement.24
Continue for management >>
MANAGEMENT
The definitive treatment of iNPH is CSF diversion with VP shunt placement. However, as with any surgical procedure, the benefits and risks must seriously be weighed. Since most cases of iNPH involve older adults, many with co-existing, chronic medical conditions, it is important that clinicians undertake a full assessment of the patient’s medical conditions and ability to withstand surgery.
Shunts are inserted into the frontal or occipital horn of the lateral ventricle of the nondominant hemisphere, with tubing connected by a one-way valve directed to the peritoneal cavity. Fixed medium-low pressure valves have largely been replaced by programmable valves that allow adjustment of flow rates. The incidence of shunt complications in recent years has been reduced to about 20%.25
Death or severe postsurgical morbidity occurs in approximately 7% of patients who undergo shunt surgery.26 Subdural hematoma is a common complication whose incidence has been greatly reduced with the use of dual-switch and programmable valves.27 Additional complications include intracranial infection, seizures, intracerebral hemorrhage, mechanical shunt failures, and abdominal injury (ascites, perforation), as well as signs and symptoms of shunt infections (headache, malaise, nausea, fever).
Continue for the prognosis >>
PROGNOSIS
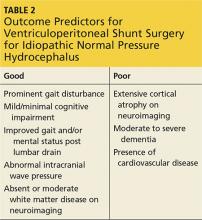
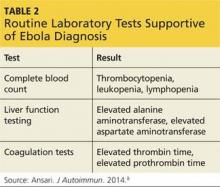
The symptoms of iNPH are slowly progressive. Early recognition and intervention have been shown to improve outcomes.28 Long-term improvement following shunt surgery has been reported in up to 75% of patients when there is proper patient selection.13 A large body of literature has focused on proper patient selection and outcome predictors for shunting (see Table 2).
Gait and imbalance have repeatedly been reported to improve the most from shunting, particularly when gait disturbance precedes cognitive decline.29,30 Cognitive impairment, particularly once it reaches the degree of dementia, is least responsive to shunt placement, with only about 50% of patients experiencing improvement in cognition postsurgery.31
The SINPHONI study (Study of Idiopathic Normal Pressure Hydrocephalus on Neurological Improvement) conducted in Japan found that mild impairment in any of the triad symptoms (gait, cognition, urinary incontinence) prior to shunt surgery predicted disappearance of symptoms following surgery; in addition, younger age was a predictor of disappearance of gait disturbance.32 Complete disappearance of symptoms is often not achievable, but significant improvement in symptoms may be a more attainable outcome goal. Long-term follow-up has found that symptom improvement is sustained in up to 25% to 47% of patients over three to five years.33,34
Continue for the conclusion >>
CONCLUSION
As the case illustrates, the diagnosis of iNPH is not always apparent or easy to make. In this instance, there was a five-year delay between onset of symptoms and diagnosis. Multiple providers were consulted, and several misdiagnoses (depression, Parkinson disease, Alzheimer disease) were pursued while the symptoms of iNPH continued to develop—a common occurrence in many iNPH cases.
Because of the insidious onset, symptoms of iNPH often go unnoticed or are ignored, minimized, or overlooked by both patients and providers. It is not uncommon for clinicians to misdiagnose gait instability as a sign of Parkinson disease and cognitive impairment as early dementia (especially Alzheimer disease), or to attribute urinary frequency and urgency to benign prostatic hypertrophy in men. A high index of suspicion among providers and early diagnosis are important, as it is now well established that early intervention with VP shunt can have a dramatic impact on symptoms in the majority of patients with iNPH.
1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273(3):117-126.
2. Klinge P, Hellström P, Tans J, Wikkelse C; European iNPH Multicenter Study Group. One year outcome in the European multicenter study on iNPH. Acta Neurol Scand. 2012;126:145-153.
3. Williams MA, Sharkey P, Van Doren D, et al. Influence of shunt surgery on health care expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007:107:21-28.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Bradley WG Jr. Diagnostic tools in hydrocephalus. Neurosurg Clin N Am. 2001;12(4):661-684.
6. Brean A, Edie P. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
7. Jaraj D, Rabiel K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449-1454.
8. Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is a patient. Clin Med. 2011;11(2):162-165.
9. Marmarou A, Bergsneider M, Relkin N, et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3 Suppl):S1-S3.
10. Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987;44(3):263-267.
11. Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neuropsychiatry. 1999;67(6):723-731.
12. Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507-510.
13. McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
14. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1186.
15. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16.
16. Gallia Gl, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375-381.
17. Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normalpressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392.
18. Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic test for the preoperative assessment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28.
19. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery. 1988;22(2):388-391.
20. Gado MH, Coleman RE, Lee KS, et al. Correlation between computerized transaxial tomography and radionuclide cisternography in dementia. Neurology. 1976;26(6 pt 1):555-560.
21. Patten D, Benson D. Cisternograpy. In: Schneider PB, Treves S. Nuclear Medicine in Clinical Practice. Amsterdam, Holland: Elsevier/North Holland Biomedical Press; 1978.
22. Chang CC, Kuwana N, Ito S, Ikegami T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol Med Chir. 1999;39(12):841-846.
23. Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
24. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
25. Kiefer M, Eymann R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl. 2010;106:107-112.
26. Vanneste J, Augustijn P, Dirven C, et al. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42(1):54-59.
27. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neuro Med Chir (Tokyo). 2003;43(4):197-200.
28. Andren K, Wikkelso C, Tisell M, Hellstrom P. Natural course of idiopathic normal pressure hydrocephalus. Neurol Neurosurg Psychiatry. 2014;85(7):806-810.
29. Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1987;43(9):940-942.
30. Cage T, Auguste K, Wrensch M, et al. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18(5):649-654.
31. Duinkerke A, Williams MA, Rigamonti D, Hilla AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17(3):179-184.
32. Kazui H, Mori E, Ohkawa S, et al. Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci. 2013;328(1-2):64-69.
33. Malm J, Kristensen B, Stegmayr B, et al. Three-year survival and functional outcome of patients with idiopathic normal pressure hydrocephalus syndrome. Neurology. 2000;55(4):576-578.
34. Klinge P, Marmarou A, Bergsneider M, et al. Outcome of shunting in idiopathic normal pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(3 Suppl):S40-S52.
CE/CME No: CR-1512
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize the common presenting symptoms of idiopathic normal pressure hydrocephalus (iNPH).
• Describe findings on brain imaging (MRI or CT) that are highly suggestive of a diagnosis of iNPH.
• Describe supplementary tests commonly used to help confirm a suspected diagnosis of iNPH.
• Discuss the prognosis and expected outcomes from ventriculoperitoneal shunt placement for iNPH.
FACULTY
Freddi Segal-Gidan is Director of the Rancho Los Amigos/University of Southern California (USC) Alzheimer’s Disease Center and Assistant Clinical Professor in the departments of Neurology and Family Medicine at Keck School of Medicine, USC, and in Gerontology at L. Davis School of Gerontology at USC, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2015.
Article begins on next page >>
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of dementia. Unfortunately, the symptoms of iNPH—cognitive impairment, gait change, and urinary incontinence—develop slowly and are often mistaken for those of other conditions or for normal aging. This article explains when to suspect iNPH and the steps you need to take when iNPH is in the differential.
Ten years ago, a 74-year-old semi-retired cardiologist self-referred to neurology for evaluation of forgetfulness that had increased in the previous two years. He remained functionally independent in all daily activities. Mental status screening with the Mini-Mental State Exam was within normal limits. He underwent comprehensive neuropsychologic testing, which revealed an estimated verbal IQ of 130, a word list recall in the low average range, and normal results for all other tests; the report also noted mild depression. He was seen one year later for follow-up and reported continued memory difficulties. A brain MRI showed ventricular dilatation with cerebral and cerebellar atrophy “consistent with age.” He was placed on an off-label trial of donepezil and vitamin E.
Two years later, he began to experience slowing of his gait and was noted to have “mild Parkinsonism” on neurologic examination. He was started on carbidopa/levodopa, with no improvement. Another MRI showed no progression from two years prior, but the “possibility of normal pressure hydrocephalus” (NPH) was noted in the radiology report. He underwent a lumbar drain procedure, after which he had slow improvement in gait over the next two months.
Four to 12 months following the lumbar drain procedure, he experienced worsening gait, balance problems, and urinary urgency, and he reported increasing memory difficulty. Neurologic examination was noteworthy for soft voice with hoarse quality, slightly increased tone in the upper extremities (right greater than left), and wide-based and unsteady gait with dragging of feet. Another brain MRI was done, with the report noting “ventriculomegaly out of proportion to volume loss … NPH cannot be excluded.” After review of the results for a second opinion, an MRI with cerebrospinal fluid (CSF) flow study was performed; based on the results, the patient was determined to be a good candidate for ventriculoperitoneal shunt placement. He underwent shunt placement without incident and had sustained improvement in gait and cognition over the next six years.
Idiopathic normal pressure hydrocephalus (iNPH) is an uncommon but important differential to consider in any older individual with cognitive decline. NPH was first discussed in the medical literature in 1965, when Adams and Hakim described the characteristic features of iNPH: the triad of walking impairment, “dementia,” and urinary incontinence in the presence of enlarged ventricles but normal intracranial pressure.1
With the continued aging of the population, an increasing number of individuals can be expected to experience cognitive decline, gait and balance difficulties, and urinary incontinence. Clinicians caring for patients who present with one or more of these symptoms must keep iNPH in mind for the differential diagnosis. iNPH is a treatable condition, and appropriate intervention can significantly improve affected patients’ lives, as well as reduce health care expenditures.2,3
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The underlying pathophysiology of iNPH is not completely understood. The symptoms are believed to arise from the slow, gradual, and insidious accumulation of CSF within the brain ventricles. The current understanding is that the CSF acts as a lymphatic drainage system for the brain, entering the brain parenchyma via paravascular spaces that surround penetrating arteries and clearing interstitial fluid along paravenous drainage pathways.4
CSF reabsorption into the blood is a dual process, with drainage via the arachnoid villi and granulations within the dural sinuses and slow drainage via lymphatic vessels in the perineural, otic, and ophthalmic regions. There is a pressure gradient of fluid in the subarachnoid space and ventricles, with the CSF pressure normally higher than the pressure of the venous system, allowing outflow of CSF.
In iNPH, outflow of CSF is at least partially disrupted, and there is decreased CSF reabsorption, resulting in a higher, normal baseline CSF volume over time. The underlying cause of reduced CSF reabsorption in iNPH remains uncertain, but it has been proposed that arachnoid granulations fail to maintain adequate removal of CSF, possibly due to fibrosis or scarring.5 In response to increased CSF volume, the ventricles distend and compress the brain parenchyma. Exactly how the pressure exerted by the ventricles leads to changes in gait, cognition, and urinary incontinence is not well understood.
Continue for epidemiology >>
EPIDEMIOLOGY
The prevalence of iNPH has been estimated at 21.9 cases per 100,000 persons.6 It occurs primarily in individuals older than age 606 and occurs more frequently with increasing age, as shown in a recent report in which the prevalence of probable iNPH was 0.2% in those ages 70 to 79 and 5.9% in those ages 80 and older.7 Based on these numbers, the authors estimated that approximately 700,000 Americans older than 70 may have iNPH. It is a rare cause of dementia among the population with dementia onset after age 65 (“senile onset”). No gender or racial/ethnic differences have been reported.
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of iNPH is based on clinical findings. Making the diagnosis can be challenging, as the symptoms overlap with common age-related changes and age-associated medical conditions, and there is no single diagnostic test. A high index of clinical suspicion or an incidental finding on neuroimaging done in the diagnostic work-up for cognitive impairment/dementia (or some other reason) are the usual triggers for further investigation.
Clinicians should include iNPH in the differential, along with alternative diagnoses, when the history includes one or more of the three symptoms of iNPH: cognitive decline, gait disturbance, and/or urinary incontinence (see Table 1). While superficially appearing to be an easily recognizable condition, iNPH is actually a very complex disease that goes unrecognized and undiagnosed in many individuals.8 Evidence-based guidelines developed in 2005 attempted to devise a classification system based upon age, gait speed, nature of symptoms, neuroimaging changes, and CSF opening pressures.9
The symptoms of iNPH typically develop insidiously and progress slowly. The earliest symptom is most often gait disturbance. The gait disturbance associated with iNPH is described as “magnetic” or gait apraxia and includes trouble with initiation, reduced stride length, and a slow, cautious quality.10 Cognitive impairment typically has a frontosubcortical pattern, with psychomotor slowing, decreased attention or concentration, and problems with verbal fluency and executive function.11 Deficits in visuospatial and construction skills may also be observed.
Memory decline, which predominates in Alzheimer disease, may be less pronounced in iNPH. Urinary incontinence is usually a combination of urgency and frequency, mostly due to detrusor overactivity.12 A majority of patients (62%) treated for iNPH have all three symptoms of the triad, but in some cases only one or two symptoms are present.13 Gait disturbance is the most common feature, present in 98% of cases, followed by urinary incontinence (79%) and cognitive impairment (78%).13
Physical examination should include a complete neurologic exam. Mental status testing will typically show slowing, with decreased comprehension and increased time required to complete tasks. Decreased short-term memory recall may be improved with cues. Speech may be slow but is without aphasia or dysarthria. The gait pattern often includes a wide stance; slow, small steps with decreased floor clearance; and retained arm swing. Motor examination of the lower extremities may demonstrate some increased tone and slightly brisk reflexes.
Continue for neuroimaging >>
NEUROIMAGING
Brain neuroimaging with CT or MRI is essential to the initial investigation and diagnostic evaluation of suspected iNPH. Neuroimaging is not diagnostic in itself, but the findings are important both to support a suspected diagnosis of iNPH and to exclude other conditions that could cause similar findings or contribute to the symptoms (eg, stroke or tumor).
The key finding is enlargement of the lateral ventricles (ventriculomegaly) disproportionate to the degree of cortical atrophy (see Figure 1). Ventricular dilatation is characterized by rounding of the contour of the ventricles with a widened third ventricle. Normal volume of brain parenchyma is evidenced by the absence of sulci widening, which would be seen in the presence of cortical atrophy and the absence of obscured sulci. White matter changes, seen as periventricular white matter hyperintensity on MRI, has also been noted frequently on imaging consistent with iNPH.14 On MRI, a marked CSF flow void in the aqueduct of Sylvius and fourth ventricle, called a flow void, is usually seen.15
The Evans ratio, calculated by dividing the maximum width of the ventricular frontal horns on imaging by the widest skull diameter, is one criteria for diagnosis of iNPH on neuroimaging. An Evans ratio greater than 0.3 (signifying ventriculomegaly), within the appropriate clinical context, is considered indicative of iNPH.14,16
Continue for confirmatory studies >>
CONFIRMATORY STUDIES
Beyond neuroimaging, a variety of specialty studies are used to increase diagnostic certainty and as predictors of outcome from surgical intervention. These include large-volume lumbar tap (“tap test”), external lumbar drainage, nuclear or CT cisternogram, and CSF flow imaging. Each of these tests has some risk, and no single test has been conclusively demonstrated by itself to be superior to one or a combination of the others. No CSF biomarkers have as yet been identified for the diagnosis of iNPH.17
The simplest supplemental test is the CSF tap test, which involves the removal of 40 to 50 mL of CSF via lumbar puncture. The patient is then assessed for improvement of symptoms by comparing gait and cognition prior to the test with that from 30 to 60 minutes after. Patients with significant symptomatic improvement (lasting at least a few weeks and up to months) have been found to be good candidates for shunt surgery.18 Patients who have high opening pressure (> 20 cm H2O) require further investigation for secondary causes of NPH (eg, meningitis).18 Routine CSF analysis should be done (cell count, protein, glucose) to rule out chronic meningitis, which can mimic NPH.
The external lumbar drainage (ELD) test involves placement of an indwelling external lumbar catheter (lumbar drain) for external drainage of approximately 300 mL/d of CSF over one to five days. It is useful in patients who do not have a significant response to the tap test and for whom a high index of suspicion for iNPH remains. A positive response to ELD has been found to predict a potentially positive shunt response.19 The ELD test has a high positive predictive value (80% to 100%).18
Nuclear or CT cisternography has been used to evaluate CSF reabsorption. In the presence of iNPH, cisternography demonstrates ventricular reflux with slow cortical uptake.20,21 A positive cisternogram combined with a radioisotope CT exam that shows normal cerebral blood flow is better than cisternography alone in predicting positive outcome from shunt surgery.22
CSF flow studies utilize T2-weighted images on MRI to estimate CSF flow through the ventricles. In the assessment of iNPH, evaluation of CSF flow by MRI is used in the preoperative evaluation and also in post–shunt-placement follow-up. Slow-moving CSF has an increased signal, while regions of fast-moving CSF, such as in a narrow cerebral aqueduct, have no signal. In the presence of iNPH, the cerebral aqueduct shows an increased pulsatile flow void, and there is a hypointense or absent signal in the proximal fourth ventricle on proton density–weighted images. The presence of an increased CSF flow void has been found to be highly predictive of a positive outcome from ventriculoperitoneal (VP) shunt placement.23
Another approach involves the direct measurement of the velocity of CSF stroke volume, which is the mean volume of CSF that passes through the aqueduct during systole and diastole. Studies have found that a CSF stroke volume of 42 µL or greater is an indicator for a good probability of improvement after VP shunt placement.24
Continue for management >>
MANAGEMENT
The definitive treatment of iNPH is CSF diversion with VP shunt placement. However, as with any surgical procedure, the benefits and risks must seriously be weighed. Since most cases of iNPH involve older adults, many with co-existing, chronic medical conditions, it is important that clinicians undertake a full assessment of the patient’s medical conditions and ability to withstand surgery.
Shunts are inserted into the frontal or occipital horn of the lateral ventricle of the nondominant hemisphere, with tubing connected by a one-way valve directed to the peritoneal cavity. Fixed medium-low pressure valves have largely been replaced by programmable valves that allow adjustment of flow rates. The incidence of shunt complications in recent years has been reduced to about 20%.25
Death or severe postsurgical morbidity occurs in approximately 7% of patients who undergo shunt surgery.26 Subdural hematoma is a common complication whose incidence has been greatly reduced with the use of dual-switch and programmable valves.27 Additional complications include intracranial infection, seizures, intracerebral hemorrhage, mechanical shunt failures, and abdominal injury (ascites, perforation), as well as signs and symptoms of shunt infections (headache, malaise, nausea, fever).
Continue for the prognosis >>
PROGNOSIS
The symptoms of iNPH are slowly progressive. Early recognition and intervention have been shown to improve outcomes.28 Long-term improvement following shunt surgery has been reported in up to 75% of patients when there is proper patient selection.13 A large body of literature has focused on proper patient selection and outcome predictors for shunting (see Table 2).
Gait and imbalance have repeatedly been reported to improve the most from shunting, particularly when gait disturbance precedes cognitive decline.29,30 Cognitive impairment, particularly once it reaches the degree of dementia, is least responsive to shunt placement, with only about 50% of patients experiencing improvement in cognition postsurgery.31
The SINPHONI study (Study of Idiopathic Normal Pressure Hydrocephalus on Neurological Improvement) conducted in Japan found that mild impairment in any of the triad symptoms (gait, cognition, urinary incontinence) prior to shunt surgery predicted disappearance of symptoms following surgery; in addition, younger age was a predictor of disappearance of gait disturbance.32 Complete disappearance of symptoms is often not achievable, but significant improvement in symptoms may be a more attainable outcome goal. Long-term follow-up has found that symptom improvement is sustained in up to 25% to 47% of patients over three to five years.33,34
Continue for the conclusion >>
CONCLUSION
As the case illustrates, the diagnosis of iNPH is not always apparent or easy to make. In this instance, there was a five-year delay between onset of symptoms and diagnosis. Multiple providers were consulted, and several misdiagnoses (depression, Parkinson disease, Alzheimer disease) were pursued while the symptoms of iNPH continued to develop—a common occurrence in many iNPH cases.
Because of the insidious onset, symptoms of iNPH often go unnoticed or are ignored, minimized, or overlooked by both patients and providers. It is not uncommon for clinicians to misdiagnose gait instability as a sign of Parkinson disease and cognitive impairment as early dementia (especially Alzheimer disease), or to attribute urinary frequency and urgency to benign prostatic hypertrophy in men. A high index of suspicion among providers and early diagnosis are important, as it is now well established that early intervention with VP shunt can have a dramatic impact on symptoms in the majority of patients with iNPH.
CE/CME No: CR-1512
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Recognize the common presenting symptoms of idiopathic normal pressure hydrocephalus (iNPH).
• Describe findings on brain imaging (MRI or CT) that are highly suggestive of a diagnosis of iNPH.
• Describe supplementary tests commonly used to help confirm a suspected diagnosis of iNPH.
• Discuss the prognosis and expected outcomes from ventriculoperitoneal shunt placement for iNPH.
FACULTY
Freddi Segal-Gidan is Director of the Rancho Los Amigos/University of Southern California (USC) Alzheimer’s Disease Center and Assistant Clinical Professor in the departments of Neurology and Family Medicine at Keck School of Medicine, USC, and in Gerontology at L. Davis School of Gerontology at USC, Los Angeles.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2015.
Article begins on next page >>
Idiopathic normal pressure hydrocephalus (iNPH) is one of the few reversible causes of dementia. Unfortunately, the symptoms of iNPH—cognitive impairment, gait change, and urinary incontinence—develop slowly and are often mistaken for those of other conditions or for normal aging. This article explains when to suspect iNPH and the steps you need to take when iNPH is in the differential.
Ten years ago, a 74-year-old semi-retired cardiologist self-referred to neurology for evaluation of forgetfulness that had increased in the previous two years. He remained functionally independent in all daily activities. Mental status screening with the Mini-Mental State Exam was within normal limits. He underwent comprehensive neuropsychologic testing, which revealed an estimated verbal IQ of 130, a word list recall in the low average range, and normal results for all other tests; the report also noted mild depression. He was seen one year later for follow-up and reported continued memory difficulties. A brain MRI showed ventricular dilatation with cerebral and cerebellar atrophy “consistent with age.” He was placed on an off-label trial of donepezil and vitamin E.
Two years later, he began to experience slowing of his gait and was noted to have “mild Parkinsonism” on neurologic examination. He was started on carbidopa/levodopa, with no improvement. Another MRI showed no progression from two years prior, but the “possibility of normal pressure hydrocephalus” (NPH) was noted in the radiology report. He underwent a lumbar drain procedure, after which he had slow improvement in gait over the next two months.
Four to 12 months following the lumbar drain procedure, he experienced worsening gait, balance problems, and urinary urgency, and he reported increasing memory difficulty. Neurologic examination was noteworthy for soft voice with hoarse quality, slightly increased tone in the upper extremities (right greater than left), and wide-based and unsteady gait with dragging of feet. Another brain MRI was done, with the report noting “ventriculomegaly out of proportion to volume loss … NPH cannot be excluded.” After review of the results for a second opinion, an MRI with cerebrospinal fluid (CSF) flow study was performed; based on the results, the patient was determined to be a good candidate for ventriculoperitoneal shunt placement. He underwent shunt placement without incident and had sustained improvement in gait and cognition over the next six years.
Idiopathic normal pressure hydrocephalus (iNPH) is an uncommon but important differential to consider in any older individual with cognitive decline. NPH was first discussed in the medical literature in 1965, when Adams and Hakim described the characteristic features of iNPH: the triad of walking impairment, “dementia,” and urinary incontinence in the presence of enlarged ventricles but normal intracranial pressure.1
With the continued aging of the population, an increasing number of individuals can be expected to experience cognitive decline, gait and balance difficulties, and urinary incontinence. Clinicians caring for patients who present with one or more of these symptoms must keep iNPH in mind for the differential diagnosis. iNPH is a treatable condition, and appropriate intervention can significantly improve affected patients’ lives, as well as reduce health care expenditures.2,3
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The underlying pathophysiology of iNPH is not completely understood. The symptoms are believed to arise from the slow, gradual, and insidious accumulation of CSF within the brain ventricles. The current understanding is that the CSF acts as a lymphatic drainage system for the brain, entering the brain parenchyma via paravascular spaces that surround penetrating arteries and clearing interstitial fluid along paravenous drainage pathways.4
CSF reabsorption into the blood is a dual process, with drainage via the arachnoid villi and granulations within the dural sinuses and slow drainage via lymphatic vessels in the perineural, otic, and ophthalmic regions. There is a pressure gradient of fluid in the subarachnoid space and ventricles, with the CSF pressure normally higher than the pressure of the venous system, allowing outflow of CSF.
In iNPH, outflow of CSF is at least partially disrupted, and there is decreased CSF reabsorption, resulting in a higher, normal baseline CSF volume over time. The underlying cause of reduced CSF reabsorption in iNPH remains uncertain, but it has been proposed that arachnoid granulations fail to maintain adequate removal of CSF, possibly due to fibrosis or scarring.5 In response to increased CSF volume, the ventricles distend and compress the brain parenchyma. Exactly how the pressure exerted by the ventricles leads to changes in gait, cognition, and urinary incontinence is not well understood.
Continue for epidemiology >>
EPIDEMIOLOGY
The prevalence of iNPH has been estimated at 21.9 cases per 100,000 persons.6 It occurs primarily in individuals older than age 606 and occurs more frequently with increasing age, as shown in a recent report in which the prevalence of probable iNPH was 0.2% in those ages 70 to 79 and 5.9% in those ages 80 and older.7 Based on these numbers, the authors estimated that approximately 700,000 Americans older than 70 may have iNPH. It is a rare cause of dementia among the population with dementia onset after age 65 (“senile onset”). No gender or racial/ethnic differences have been reported.
Continue for the diagnosis >>
DIAGNOSIS
The diagnosis of iNPH is based on clinical findings. Making the diagnosis can be challenging, as the symptoms overlap with common age-related changes and age-associated medical conditions, and there is no single diagnostic test. A high index of clinical suspicion or an incidental finding on neuroimaging done in the diagnostic work-up for cognitive impairment/dementia (or some other reason) are the usual triggers for further investigation.
Clinicians should include iNPH in the differential, along with alternative diagnoses, when the history includes one or more of the three symptoms of iNPH: cognitive decline, gait disturbance, and/or urinary incontinence (see Table 1). While superficially appearing to be an easily recognizable condition, iNPH is actually a very complex disease that goes unrecognized and undiagnosed in many individuals.8 Evidence-based guidelines developed in 2005 attempted to devise a classification system based upon age, gait speed, nature of symptoms, neuroimaging changes, and CSF opening pressures.9
The symptoms of iNPH typically develop insidiously and progress slowly. The earliest symptom is most often gait disturbance. The gait disturbance associated with iNPH is described as “magnetic” or gait apraxia and includes trouble with initiation, reduced stride length, and a slow, cautious quality.10 Cognitive impairment typically has a frontosubcortical pattern, with psychomotor slowing, decreased attention or concentration, and problems with verbal fluency and executive function.11 Deficits in visuospatial and construction skills may also be observed.
Memory decline, which predominates in Alzheimer disease, may be less pronounced in iNPH. Urinary incontinence is usually a combination of urgency and frequency, mostly due to detrusor overactivity.12 A majority of patients (62%) treated for iNPH have all three symptoms of the triad, but in some cases only one or two symptoms are present.13 Gait disturbance is the most common feature, present in 98% of cases, followed by urinary incontinence (79%) and cognitive impairment (78%).13
Physical examination should include a complete neurologic exam. Mental status testing will typically show slowing, with decreased comprehension and increased time required to complete tasks. Decreased short-term memory recall may be improved with cues. Speech may be slow but is without aphasia or dysarthria. The gait pattern often includes a wide stance; slow, small steps with decreased floor clearance; and retained arm swing. Motor examination of the lower extremities may demonstrate some increased tone and slightly brisk reflexes.
Continue for neuroimaging >>
NEUROIMAGING
Brain neuroimaging with CT or MRI is essential to the initial investigation and diagnostic evaluation of suspected iNPH. Neuroimaging is not diagnostic in itself, but the findings are important both to support a suspected diagnosis of iNPH and to exclude other conditions that could cause similar findings or contribute to the symptoms (eg, stroke or tumor).
The key finding is enlargement of the lateral ventricles (ventriculomegaly) disproportionate to the degree of cortical atrophy (see Figure 1). Ventricular dilatation is characterized by rounding of the contour of the ventricles with a widened third ventricle. Normal volume of brain parenchyma is evidenced by the absence of sulci widening, which would be seen in the presence of cortical atrophy and the absence of obscured sulci. White matter changes, seen as periventricular white matter hyperintensity on MRI, has also been noted frequently on imaging consistent with iNPH.14 On MRI, a marked CSF flow void in the aqueduct of Sylvius and fourth ventricle, called a flow void, is usually seen.15
The Evans ratio, calculated by dividing the maximum width of the ventricular frontal horns on imaging by the widest skull diameter, is one criteria for diagnosis of iNPH on neuroimaging. An Evans ratio greater than 0.3 (signifying ventriculomegaly), within the appropriate clinical context, is considered indicative of iNPH.14,16
Continue for confirmatory studies >>
CONFIRMATORY STUDIES
Beyond neuroimaging, a variety of specialty studies are used to increase diagnostic certainty and as predictors of outcome from surgical intervention. These include large-volume lumbar tap (“tap test”), external lumbar drainage, nuclear or CT cisternogram, and CSF flow imaging. Each of these tests has some risk, and no single test has been conclusively demonstrated by itself to be superior to one or a combination of the others. No CSF biomarkers have as yet been identified for the diagnosis of iNPH.17
The simplest supplemental test is the CSF tap test, which involves the removal of 40 to 50 mL of CSF via lumbar puncture. The patient is then assessed for improvement of symptoms by comparing gait and cognition prior to the test with that from 30 to 60 minutes after. Patients with significant symptomatic improvement (lasting at least a few weeks and up to months) have been found to be good candidates for shunt surgery.18 Patients who have high opening pressure (> 20 cm H2O) require further investigation for secondary causes of NPH (eg, meningitis).18 Routine CSF analysis should be done (cell count, protein, glucose) to rule out chronic meningitis, which can mimic NPH.
The external lumbar drainage (ELD) test involves placement of an indwelling external lumbar catheter (lumbar drain) for external drainage of approximately 300 mL/d of CSF over one to five days. It is useful in patients who do not have a significant response to the tap test and for whom a high index of suspicion for iNPH remains. A positive response to ELD has been found to predict a potentially positive shunt response.19 The ELD test has a high positive predictive value (80% to 100%).18
Nuclear or CT cisternography has been used to evaluate CSF reabsorption. In the presence of iNPH, cisternography demonstrates ventricular reflux with slow cortical uptake.20,21 A positive cisternogram combined with a radioisotope CT exam that shows normal cerebral blood flow is better than cisternography alone in predicting positive outcome from shunt surgery.22
CSF flow studies utilize T2-weighted images on MRI to estimate CSF flow through the ventricles. In the assessment of iNPH, evaluation of CSF flow by MRI is used in the preoperative evaluation and also in post–shunt-placement follow-up. Slow-moving CSF has an increased signal, while regions of fast-moving CSF, such as in a narrow cerebral aqueduct, have no signal. In the presence of iNPH, the cerebral aqueduct shows an increased pulsatile flow void, and there is a hypointense or absent signal in the proximal fourth ventricle on proton density–weighted images. The presence of an increased CSF flow void has been found to be highly predictive of a positive outcome from ventriculoperitoneal (VP) shunt placement.23
Another approach involves the direct measurement of the velocity of CSF stroke volume, which is the mean volume of CSF that passes through the aqueduct during systole and diastole. Studies have found that a CSF stroke volume of 42 µL or greater is an indicator for a good probability of improvement after VP shunt placement.24
Continue for management >>
MANAGEMENT
The definitive treatment of iNPH is CSF diversion with VP shunt placement. However, as with any surgical procedure, the benefits and risks must seriously be weighed. Since most cases of iNPH involve older adults, many with co-existing, chronic medical conditions, it is important that clinicians undertake a full assessment of the patient’s medical conditions and ability to withstand surgery.
Shunts are inserted into the frontal or occipital horn of the lateral ventricle of the nondominant hemisphere, with tubing connected by a one-way valve directed to the peritoneal cavity. Fixed medium-low pressure valves have largely been replaced by programmable valves that allow adjustment of flow rates. The incidence of shunt complications in recent years has been reduced to about 20%.25
Death or severe postsurgical morbidity occurs in approximately 7% of patients who undergo shunt surgery.26 Subdural hematoma is a common complication whose incidence has been greatly reduced with the use of dual-switch and programmable valves.27 Additional complications include intracranial infection, seizures, intracerebral hemorrhage, mechanical shunt failures, and abdominal injury (ascites, perforation), as well as signs and symptoms of shunt infections (headache, malaise, nausea, fever).
Continue for the prognosis >>
PROGNOSIS
The symptoms of iNPH are slowly progressive. Early recognition and intervention have been shown to improve outcomes.28 Long-term improvement following shunt surgery has been reported in up to 75% of patients when there is proper patient selection.13 A large body of literature has focused on proper patient selection and outcome predictors for shunting (see Table 2).
Gait and imbalance have repeatedly been reported to improve the most from shunting, particularly when gait disturbance precedes cognitive decline.29,30 Cognitive impairment, particularly once it reaches the degree of dementia, is least responsive to shunt placement, with only about 50% of patients experiencing improvement in cognition postsurgery.31
The SINPHONI study (Study of Idiopathic Normal Pressure Hydrocephalus on Neurological Improvement) conducted in Japan found that mild impairment in any of the triad symptoms (gait, cognition, urinary incontinence) prior to shunt surgery predicted disappearance of symptoms following surgery; in addition, younger age was a predictor of disappearance of gait disturbance.32 Complete disappearance of symptoms is often not achievable, but significant improvement in symptoms may be a more attainable outcome goal. Long-term follow-up has found that symptom improvement is sustained in up to 25% to 47% of patients over three to five years.33,34
Continue for the conclusion >>
CONCLUSION
As the case illustrates, the diagnosis of iNPH is not always apparent or easy to make. In this instance, there was a five-year delay between onset of symptoms and diagnosis. Multiple providers were consulted, and several misdiagnoses (depression, Parkinson disease, Alzheimer disease) were pursued while the symptoms of iNPH continued to develop—a common occurrence in many iNPH cases.
Because of the insidious onset, symptoms of iNPH often go unnoticed or are ignored, minimized, or overlooked by both patients and providers. It is not uncommon for clinicians to misdiagnose gait instability as a sign of Parkinson disease and cognitive impairment as early dementia (especially Alzheimer disease), or to attribute urinary frequency and urgency to benign prostatic hypertrophy in men. A high index of suspicion among providers and early diagnosis are important, as it is now well established that early intervention with VP shunt can have a dramatic impact on symptoms in the majority of patients with iNPH.
1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273(3):117-126.
2. Klinge P, Hellström P, Tans J, Wikkelse C; European iNPH Multicenter Study Group. One year outcome in the European multicenter study on iNPH. Acta Neurol Scand. 2012;126:145-153.
3. Williams MA, Sharkey P, Van Doren D, et al. Influence of shunt surgery on health care expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007:107:21-28.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Bradley WG Jr. Diagnostic tools in hydrocephalus. Neurosurg Clin N Am. 2001;12(4):661-684.
6. Brean A, Edie P. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
7. Jaraj D, Rabiel K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449-1454.
8. Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is a patient. Clin Med. 2011;11(2):162-165.
9. Marmarou A, Bergsneider M, Relkin N, et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3 Suppl):S1-S3.
10. Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987;44(3):263-267.
11. Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neuropsychiatry. 1999;67(6):723-731.
12. Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507-510.
13. McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
14. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1186.
15. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16.
16. Gallia Gl, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375-381.
17. Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normalpressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392.
18. Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic test for the preoperative assessment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28.
19. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery. 1988;22(2):388-391.
20. Gado MH, Coleman RE, Lee KS, et al. Correlation between computerized transaxial tomography and radionuclide cisternography in dementia. Neurology. 1976;26(6 pt 1):555-560.
21. Patten D, Benson D. Cisternograpy. In: Schneider PB, Treves S. Nuclear Medicine in Clinical Practice. Amsterdam, Holland: Elsevier/North Holland Biomedical Press; 1978.
22. Chang CC, Kuwana N, Ito S, Ikegami T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol Med Chir. 1999;39(12):841-846.
23. Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
24. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
25. Kiefer M, Eymann R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl. 2010;106:107-112.
26. Vanneste J, Augustijn P, Dirven C, et al. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42(1):54-59.
27. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neuro Med Chir (Tokyo). 2003;43(4):197-200.
28. Andren K, Wikkelso C, Tisell M, Hellstrom P. Natural course of idiopathic normal pressure hydrocephalus. Neurol Neurosurg Psychiatry. 2014;85(7):806-810.
29. Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1987;43(9):940-942.
30. Cage T, Auguste K, Wrensch M, et al. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18(5):649-654.
31. Duinkerke A, Williams MA, Rigamonti D, Hilla AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17(3):179-184.
32. Kazui H, Mori E, Ohkawa S, et al. Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci. 2013;328(1-2):64-69.
33. Malm J, Kristensen B, Stegmayr B, et al. Three-year survival and functional outcome of patients with idiopathic normal pressure hydrocephalus syndrome. Neurology. 2000;55(4):576-578.
34. Klinge P, Marmarou A, Bergsneider M, et al. Outcome of shunting in idiopathic normal pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(3 Suppl):S40-S52.
1. Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure: a treatable syndrome. N Engl J Med. 1965;273(3):117-126.
2. Klinge P, Hellström P, Tans J, Wikkelse C; European iNPH Multicenter Study Group. One year outcome in the European multicenter study on iNPH. Acta Neurol Scand. 2012;126:145-153.
3. Williams MA, Sharkey P, Van Doren D, et al. Influence of shunt surgery on health care expenditures of elderly fee-for-service Medicare beneficiaries with hydrocephalus. J Neurosurg. 2007:107:21-28.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Bradley WG Jr. Diagnostic tools in hydrocephalus. Neurosurg Clin N Am. 2001;12(4):661-684.
6. Brean A, Edie P. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(1):48-53.
7. Jaraj D, Rabiel K, Marlow T, et al. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. 2014;82(16):1449-1454.
8. Conn HO. Normal pressure hydrocephalus (NPH): more about NPH by a physician who is a patient. Clin Med. 2011;11(2):162-165.
9. Marmarou A, Bergsneider M, Relkin N, et al. Development of guidelines for idiopathic normal-pressure hydrocephalus: introduction. Neurosurgery. 2005;57(3 Suppl):S1-S3.
10. Sudarsky L, Simon S. Gait disorder in late-life hydrocephalus. Arch Neurol. 1987;44(3):263-267.
11. Iddon JL, Pickard JD, Cross JJ, et al. Specific patterns of cognitive impairment in patients with idiopathic normal pressure hydrocephalus and Alzheimer’s disease: a pilot study. J Neurol Neuropsychiatry. 1999;67(6):723-731.
12. Sakakibara R, Kanda T, Sekido T, et al. Mechanism of bladder dysfunction in idiopathic normal pressure hydrocephalus. Neurourol Urodyn. 2008;27(6):507-510.
13. McGirt MJ, Woodworth G, Coon AL, et al. Diagnosis, treatment and analysis of long-term outcomes in idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):699-705.
14. Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery. 2001;49(5):1166-1186.
15. Relkin N, Marmarou A, Klinge P, et al. Diagnosing idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S4-S16.
16. Gallia Gl, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2(7):375-381.
17. Jeppsson A, Zetterberg H, Blennow K, Wikkelso C. Idiopathic normalpressure hydrocephalus: pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385-1392.
18. Marmarou A, Bergsneider M, Klinge P, et al. The value of supplemental prognostic test for the preoperative assessment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S17-S28.
19. Haan J, Thomeer RT. Predictive value of temporary external lumbar drainage in normal pressure hydrocephalus. Neurosurgery. 1988;22(2):388-391.
20. Gado MH, Coleman RE, Lee KS, et al. Correlation between computerized transaxial tomography and radionuclide cisternography in dementia. Neurology. 1976;26(6 pt 1):555-560.
21. Patten D, Benson D. Cisternograpy. In: Schneider PB, Treves S. Nuclear Medicine in Clinical Practice. Amsterdam, Holland: Elsevier/North Holland Biomedical Press; 1978.
22. Chang CC, Kuwana N, Ito S, Ikegami T. Prediction of effectiveness of shunting in patients with normal pressure hydrocephalus by cerebral blood flow measurement and computed tomography cisternography. Neurol Med Chir. 1999;39(12):841-846.
23. Bradley WG Jr, Whittemore AR, Kortman KE, et al. Marked cerebrospinal fluid void: indicator of successful shunt in patients with suspected normal-pressure hydrocephalus. Radiology. 1991;178(2):459-466.
24. Bradley WG, Scalzo D, Queralt J, et al. Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology. 1996;198(2):523-529.
25. Kiefer M, Eymann R. Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl. 2010;106:107-112.
26. Vanneste J, Augustijn P, Dirven C, et al. Shunting normal-pressure hydrocephalus: do the benefits outweigh the risks? A multicenter study and literature review. Neurology. 1992;42(1):54-59.
27. Kamiryo T, Hamada J, Fuwa I, Ushio Y. Acute subdural hematoma after lumboperitoneal shunt placement in patients with normal pressure hydrocephalus. Neuro Med Chir (Tokyo). 2003;43(4):197-200.
28. Andren K, Wikkelso C, Tisell M, Hellstrom P. Natural course of idiopathic normal pressure hydrocephalus. Neurol Neurosurg Psychiatry. 2014;85(7):806-810.
29. Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol. 1987;43(9):940-942.
30. Cage T, Auguste K, Wrensch M, et al. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18(5):649-654.
31. Duinkerke A, Williams MA, Rigamonti D, Hilla AE. Cognitive recovery in idiopathic normal pressure hydrocephalus after shunt. Cogn Behav Neurol. 2004;17(3):179-184.
32. Kazui H, Mori E, Ohkawa S, et al. Predictors of the disappearance of triad symptoms in patients with idiopathic normal pressure hydrocephalus after shunt surgery. J Neurol Sci. 2013;328(1-2):64-69.
33. Malm J, Kristensen B, Stegmayr B, et al. Three-year survival and functional outcome of patients with idiopathic normal pressure hydrocephalus syndrome. Neurology. 2000;55(4):576-578.
34. Klinge P, Marmarou A, Bergsneider M, et al. Outcome of shunting in idiopathic normal pressure hydrocephalus and the value of outcome assessment in shunted patients. Neurosurgery. 2005;57(3 Suppl):S40-S52.
November 2015: Click for Credit
Here are 8 articles in the November issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Low-risk Prostate Cancer: Immediate Contemplation, Not Immediate Intervention
To take the posttest, go to http://bit.ly/1Vz6Cok
VITALS
Key clinical point: Men with favorable-risk prostate cancer have a low risk for progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program, overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
2. Diabetes in Seniors Increases Dementia Risk
To take the posttest, go to http://bit.ly/1Q1bITm
VITALS
Key clinical point: Even short-term hyperglycemia in late life can trigger or accelerate cognitive decline, and incident diabetes is a risk factor for dementia after adjustment for differences in cardiovascular disease and other common risk factors.
Major finding: Individuals diagnosed with diabetes later in life have a 16% higher risk for dementia than do those without diabetes.
Data source: A population-based matched cohort study in 225,045 seniors newly diagnosed with diabetes and 668,070 nondiabetic controls.
Disclosures: The Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the University of Toronto, and the Ontario Ministry of Health and Long-Term Care supported the study. One author reported an unrestricted grant from Amgen, but there were no other conflicts of interest declared.
3. Extremes of Sleep Linked With Early Signs of CVD
To take the posttest, go to http://bit.ly/1FSvLmw
VITALS
Key clinical point: Individuals with very long or short sleep, or poor sleep quality, showed signs of early cardiovascular disease.
Major finding: Extremely short and extremely long sleep duration were associated with significantly increased levels of coronary artery calcification (CAC) and increased brachial-ankle pulse wave velocity (baPWV).
Data source: Cross-sectional study of more than 47,000 healthy adult men and women who reported sleep duration and quality and underwent either measurement of CAC.
Disclosures: The funding source was not reported. The authors reported no disclosures.
4. Sunscreens With DNA Repair Enzymes Might Lessen AK Progression
To take the posttest, go to http://bit.ly/1LdZWFf
VITALS
Key clinical point: Sunscreen containing DNA repair enzymes might prevent malignant progression of actinic keratosis better than sunscreen alone.
Major finding: Field cancerization and cyclobutane pyrimidine dimer levels improved significantly more with sunscreen plus enzymes than with sunscreen only (P < .0001 for each).
Data source: Six-month randomized trial of 28 patients with actinic keratosis.
Disclosures: Biodue S.p.A. provided the methyl aminolevulinate used in the study. Dr. Enzo Emanuele, the study’s senior author, is a major shareholder of Living Research S.A.S., a privately held biomedical research organization that provided funding for the work. The other researchers reported no conflicts of interest.
5. Breastfeeding Protects Against Postpartum MS Relapse
To take the posttest, go to http://bit.ly/1OSYU49
VITALS
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first six postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least two months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for one year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer HealthCare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
6. S aureus Seen in 1% of Pediatric CAP Cases
To take the posttest, go to http://bit.ly/1FPJnQ3
VITALS
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended firstline narrow-spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, seven had S aureus infections, six classified as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
7. Higher Arrhythmia Risk for Psoriasis Patients
To take the posttest, go to http://bit.ly/1VBdbS6
VITALS
Key clinical point: Patients with psoriasis are at increased risk for arrhythmia compared to those without psoriasis.
Major finding: After researchers adjusted for history and medication use, patients with psoriasis were at increased risk for overall arrhythmia (adjusted hazard ratio, 1.34; 95% confidence interval, 1.29-1.39).
Data source: A retrospective cohort study using data from almost 41,000 psoriasis patients identified from the Taiwan National Health Insurance Research Database, and almost 163,000 age- and sex-matched cohorts from the same database.
Disclosures: The study was institutionally funded. Dr. Chiu, Ms. Chang, and three other authors had no disclosures; one author disclosed having conducted clinical trials or received honoraria from several companies, including Pfizer and Novartis, and having received speaking fees from AbbVie.
8. Hepatitis C Drove Steep Rises in Cirrhosis, HCC, and Related Deaths
To take the posttest, go to http://bit.ly/1jyNrdp
VITALS
Key clinical point: Cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality rose substantially among Veterans Affairs (VA) patients over the past 12 years, mainly driven by hepatitis C virus infection.
Major finding: The prevalence of cirrhosis nearly doubled between 2001 and 2013, while cirrhosis-related deaths rose by about 50% and the incidence of HCC almost tripled.
Data source: A retrospective cohort study of 129,998 VA patients with cirrhosis and 21,326 VA patients with HCC between 2001 and 2013.
Disclosures: The Department of VA and the Veterans Health Administration funded the study. The investigators declared no competing interests.
Here are 8 articles in the November issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Low-risk Prostate Cancer: Immediate Contemplation, Not Immediate Intervention
To take the posttest, go to http://bit.ly/1Vz6Cok
VITALS
Key clinical point: Men with favorable-risk prostate cancer have a low risk for progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program, overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
2. Diabetes in Seniors Increases Dementia Risk
To take the posttest, go to http://bit.ly/1Q1bITm
VITALS
Key clinical point: Even short-term hyperglycemia in late life can trigger or accelerate cognitive decline, and incident diabetes is a risk factor for dementia after adjustment for differences in cardiovascular disease and other common risk factors.
Major finding: Individuals diagnosed with diabetes later in life have a 16% higher risk for dementia than do those without diabetes.
Data source: A population-based matched cohort study in 225,045 seniors newly diagnosed with diabetes and 668,070 nondiabetic controls.
Disclosures: The Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the University of Toronto, and the Ontario Ministry of Health and Long-Term Care supported the study. One author reported an unrestricted grant from Amgen, but there were no other conflicts of interest declared.
3. Extremes of Sleep Linked With Early Signs of CVD
To take the posttest, go to http://bit.ly/1FSvLmw
VITALS
Key clinical point: Individuals with very long or short sleep, or poor sleep quality, showed signs of early cardiovascular disease.
Major finding: Extremely short and extremely long sleep duration were associated with significantly increased levels of coronary artery calcification (CAC) and increased brachial-ankle pulse wave velocity (baPWV).
Data source: Cross-sectional study of more than 47,000 healthy adult men and women who reported sleep duration and quality and underwent either measurement of CAC.
Disclosures: The funding source was not reported. The authors reported no disclosures.
4. Sunscreens With DNA Repair Enzymes Might Lessen AK Progression
To take the posttest, go to http://bit.ly/1LdZWFf
VITALS
Key clinical point: Sunscreen containing DNA repair enzymes might prevent malignant progression of actinic keratosis better than sunscreen alone.
Major finding: Field cancerization and cyclobutane pyrimidine dimer levels improved significantly more with sunscreen plus enzymes than with sunscreen only (P < .0001 for each).
Data source: Six-month randomized trial of 28 patients with actinic keratosis.
Disclosures: Biodue S.p.A. provided the methyl aminolevulinate used in the study. Dr. Enzo Emanuele, the study’s senior author, is a major shareholder of Living Research S.A.S., a privately held biomedical research organization that provided funding for the work. The other researchers reported no conflicts of interest.
5. Breastfeeding Protects Against Postpartum MS Relapse
To take the posttest, go to http://bit.ly/1OSYU49
VITALS
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first six postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least two months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for one year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer HealthCare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
6. S aureus Seen in 1% of Pediatric CAP Cases
To take the posttest, go to http://bit.ly/1FPJnQ3
VITALS
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended firstline narrow-spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, seven had S aureus infections, six classified as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
7. Higher Arrhythmia Risk for Psoriasis Patients
To take the posttest, go to http://bit.ly/1VBdbS6
VITALS
Key clinical point: Patients with psoriasis are at increased risk for arrhythmia compared to those without psoriasis.
Major finding: After researchers adjusted for history and medication use, patients with psoriasis were at increased risk for overall arrhythmia (adjusted hazard ratio, 1.34; 95% confidence interval, 1.29-1.39).
Data source: A retrospective cohort study using data from almost 41,000 psoriasis patients identified from the Taiwan National Health Insurance Research Database, and almost 163,000 age- and sex-matched cohorts from the same database.
Disclosures: The study was institutionally funded. Dr. Chiu, Ms. Chang, and three other authors had no disclosures; one author disclosed having conducted clinical trials or received honoraria from several companies, including Pfizer and Novartis, and having received speaking fees from AbbVie.
8. Hepatitis C Drove Steep Rises in Cirrhosis, HCC, and Related Deaths
To take the posttest, go to http://bit.ly/1jyNrdp
VITALS
Key clinical point: Cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality rose substantially among Veterans Affairs (VA) patients over the past 12 years, mainly driven by hepatitis C virus infection.
Major finding: The prevalence of cirrhosis nearly doubled between 2001 and 2013, while cirrhosis-related deaths rose by about 50% and the incidence of HCC almost tripled.
Data source: A retrospective cohort study of 129,998 VA patients with cirrhosis and 21,326 VA patients with HCC between 2001 and 2013.
Disclosures: The Department of VA and the Veterans Health Administration funded the study. The investigators declared no competing interests.
Here are 8 articles in the November issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Low-risk Prostate Cancer: Immediate Contemplation, Not Immediate Intervention
To take the posttest, go to http://bit.ly/1Vz6Cok
VITALS
Key clinical point: Men with favorable-risk prostate cancer have a low risk for progression to a lethal phenotype and should consider active surveillance.
Major finding: Of 1,298 men with favorable-risk prostate cancer who were enrolled in an active surveillance program, overall, cancer-specific, and metastasis-free survival rates were 69%, 99.9%, and 99.4%, respectively, at 15 years.
Data source: A follow-up of a cohort of men with favorable-risk prostate cancer receiving active surveillance at a single institution that used a clearly defined protocol for enrollment, monitoring, and intervention.
Disclosures: There were no outside funding sources reported. Some coauthors reported consulting or advisory roles with Metamark Genetics, MDxHealth, Dianon Systems, DAKO, Trock, SonaCare Medical, Myriad Genetics, Rochon Genova, Rothwell Figg, and Roche.
2. Diabetes in Seniors Increases Dementia Risk
To take the posttest, go to http://bit.ly/1Q1bITm
VITALS
Key clinical point: Even short-term hyperglycemia in late life can trigger or accelerate cognitive decline, and incident diabetes is a risk factor for dementia after adjustment for differences in cardiovascular disease and other common risk factors.
Major finding: Individuals diagnosed with diabetes later in life have a 16% higher risk for dementia than do those without diabetes.
Data source: A population-based matched cohort study in 225,045 seniors newly diagnosed with diabetes and 668,070 nondiabetic controls.
Disclosures: The Canadian Institutes of Health Research, the Heart and Stroke Foundation of Ontario, the Canadian Institutes of Health Research, the University of Toronto, and the Ontario Ministry of Health and Long-Term Care supported the study. One author reported an unrestricted grant from Amgen, but there were no other conflicts of interest declared.
3. Extremes of Sleep Linked With Early Signs of CVD
To take the posttest, go to http://bit.ly/1FSvLmw
VITALS
Key clinical point: Individuals with very long or short sleep, or poor sleep quality, showed signs of early cardiovascular disease.
Major finding: Extremely short and extremely long sleep duration were associated with significantly increased levels of coronary artery calcification (CAC) and increased brachial-ankle pulse wave velocity (baPWV).
Data source: Cross-sectional study of more than 47,000 healthy adult men and women who reported sleep duration and quality and underwent either measurement of CAC.
Disclosures: The funding source was not reported. The authors reported no disclosures.
4. Sunscreens With DNA Repair Enzymes Might Lessen AK Progression
To take the posttest, go to http://bit.ly/1LdZWFf
VITALS
Key clinical point: Sunscreen containing DNA repair enzymes might prevent malignant progression of actinic keratosis better than sunscreen alone.
Major finding: Field cancerization and cyclobutane pyrimidine dimer levels improved significantly more with sunscreen plus enzymes than with sunscreen only (P < .0001 for each).
Data source: Six-month randomized trial of 28 patients with actinic keratosis.
Disclosures: Biodue S.p.A. provided the methyl aminolevulinate used in the study. Dr. Enzo Emanuele, the study’s senior author, is a major shareholder of Living Research S.A.S., a privately held biomedical research organization that provided funding for the work. The other researchers reported no conflicts of interest.
5. Breastfeeding Protects Against Postpartum MS Relapse
To take the posttest, go to http://bit.ly/1OSYU49
VITALS
Key clinical point: Don’t discourage new mothers with multiple sclerosis from breastfeeding.
Major finding: Among 81 women who did not breastfeed or who supplemented breastfeeding early on, 31 (38.3%) had an MS relapse within the first six postpartum months, compared with 29 women (24.2%) among the 120 who intended to breastfeed their children exclusively for at least two months (adjusted HR, 1.70).
Data source: A prospective study of 201 pregnant women with relapsing-remitting MS who were followed for one year post partum.
Disclosures: The work was funded by the German Research Foundation. The German MS and pregnancy registry was partly supported by Bayer HealthCare, Biogen Idec, Merck Serono, Novartis Pharma, and Genzyme Pharmaceuticals. Five of the researchers reported receiving speaker honoraria or other financial support from pharmaceutical companies.
6. S aureus Seen in 1% of Pediatric CAP Cases
To take the posttest, go to http://bit.ly/1FPJnQ3
VITALS
Key clinical point: About 1% of children presenting to a hospital with community-acquired pneumonia had Staphylococcus aureus infections, which do not respond to recommended firstline narrow-spectrum antibiotics for CAP.
Major finding: In a cohort of 554 children admitted with CAP, seven had S aureus infections, six classified as complicated. All received vancomycin within 24 hours of admission; anemia incidence was significantly higher in S aureus patients than for the rest of the cohort.
Data source: Retrospective cohort study of more than 3,400 children.
Disclosures: The study received no outside funding, and Dr. Hofto disclosed no conflicts of interest.
7. Higher Arrhythmia Risk for Psoriasis Patients
To take the posttest, go to http://bit.ly/1VBdbS6
VITALS
Key clinical point: Patients with psoriasis are at increased risk for arrhythmia compared to those without psoriasis.
Major finding: After researchers adjusted for history and medication use, patients with psoriasis were at increased risk for overall arrhythmia (adjusted hazard ratio, 1.34; 95% confidence interval, 1.29-1.39).
Data source: A retrospective cohort study using data from almost 41,000 psoriasis patients identified from the Taiwan National Health Insurance Research Database, and almost 163,000 age- and sex-matched cohorts from the same database.
Disclosures: The study was institutionally funded. Dr. Chiu, Ms. Chang, and three other authors had no disclosures; one author disclosed having conducted clinical trials or received honoraria from several companies, including Pfizer and Novartis, and having received speaking fees from AbbVie.
8. Hepatitis C Drove Steep Rises in Cirrhosis, HCC, and Related Deaths
To take the posttest, go to http://bit.ly/1jyNrdp
VITALS
Key clinical point: Cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality rose substantially among Veterans Affairs (VA) patients over the past 12 years, mainly driven by hepatitis C virus infection.
Major finding: The prevalence of cirrhosis nearly doubled between 2001 and 2013, while cirrhosis-related deaths rose by about 50% and the incidence of HCC almost tripled.
Data source: A retrospective cohort study of 129,998 VA patients with cirrhosis and 21,326 VA patients with HCC between 2001 and 2013.
Disclosures: The Department of VA and the Veterans Health Administration funded the study. The investigators declared no competing interests.
Prediabetes and Metabolic Syndrome: Current Trend
CE/CME No: CR-1510
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the pathophysiology of and risk factors for diabetes.
• Identify the current diagnostic criteria for prediabetes.
• Discuss health risks associated with metabolic syndrome.
• Describe the management of prediabetes and metabolic syndrome.
FACULTY
Annie Abraham is an Assistant Clinical Professor at Texas Woman's University, Dallas and Doctor of Nursing Practice candidate at Texas Christian University, Fort Worth, Texas. Susan Chaney is a Professor, Allison Huffman is an Assistant Clinical Professor, and Kathryn Kremer is an Associate Clinical Professor, at Texas Woman’s University, Dallas.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of October 2015.
Article begins on next page >>
Prediabetes and metabolic syndrome are growing health concerns in the United States and around the world. Lack of awareness of current recommendations may lead to delays in treatment and subsequent increases in diabetes and cardiovascular disease. Evidence-based recommendations for the management of prediabetes and metabolic syndrome focus on lifestyle changes. The most effective strategies for the prevention of type 2 diabetes and management of metabolic syndrome are reviewed here.
The global prevalence of type 2 diabetes mellitus (T2DM) in adults has reached epidemic proportions. Approximately 285 million adults (ages 20-79), or 6.4% of the adult population, around the world are affected, and these numbers are expected to rise to 439 million (7.7%) by 2030.1 In the United States, however, the prevalence is even higher, at 8.3% of the adult population.2
T2DM is a chronic disease that can cause significant morbidity and mortality. Diabetes is associated with macrovascular (eg, heart disease and stroke) as well as microvascular (eg, retinopathy, neuropathy, and microalbuminuria) complications.3 The economic cost of diagnosed diabetes in the US was approximately $245 billion in 2012.4 Early identification and management of prediabetes by nurse practitioners and physician assistants is essential to minimize disease prevalence and progression and to reduce the tremendous economic burden associated with T2DM.
PATHOPHYSIOLOGY AND RISK FACTORS FOR DIABETES
Diabetes is a complex disorder characterized by hyperglycemia resulting from insufficient insulin secretion and/or decreased tissue response to insulin.5 The progression of normal glucose tolerance to diabetes is a continuous process involving many organ systems. The disease develops primarily as a result of impaired insulin action in muscle, impaired insulin secretion from the pancreatic β-cells, and increased hepatic glucose production.6
In addition, defects in other organs, including increased free fatty acid production in the adipose tissue, decreased incretin effect in the digestive tract, increased glucagon secretion from the pancreatic α-cells, increased glucose reabsorption from the kidneys, and neurotransmitter dysfunction, lead to progression of diabetes.6 This phenomenon is called the ominous octet.6 Gradual increases in glucose levels have been detected as early as 13 years prior to diagnosis of diabetes, with an abrupt increase occurring in the final two to six years before diagnosis.7
Modifiable risk factors such as obesity, poor dietary intake, and a sedentary lifestyle have been associated with increased risk for T2DM.2 Certain antipsychotic medications may also play a part in disease progression.3
According to the American Diabetes Association (ADA), screening for diabetes should begin at age 45 and be repeated every three years.8 For adults younger than 45, screening should be done in those with a BMI of 25 or higher and at least one of the following additional risk factors:
• Family history (first-degree relative with diabetes)
• Ethnicity (high-risk populations include African American, Latino, Native American, Asian American, Pacific Islander)
• History of polycystic ovary syndrome
• History of gestational diabetes (GDM) or delivering a baby weighing more than 9 lb
• Physical inactivity
• Hypertension and/or hyperlipidemia
• History of cardiovascular disease (CVD)
• Insulin resistance.8
IDENTIFICATION/DIAGNOSIS OF PREDIABETES
Diabetes is preceded by an asymptomatic phase known as prediabetes. In prediabetes, blood glucose levels are higher than normal but do not meet the criteria for diabetes.8 According to the American Association of Clinical Endocrinologists (AACE) and the ADA, prediabetes is defined as impaired fasting glucose (fasting plasma glucose level of 100-125 mg/dL) and/or impaired glucose tolerance (two-hour plasma glucose value of 140-199 mg/dL after a 75-g oral glucose tolerance test).8,9 The ADA also considers a hemoglobin A1C value of 5.7% to 6.4% to be indicative of prediabetes.8
In the US, it is estimated that more than 79 million adults have prediabetes.2 Approximately 70% of individuals with prediabetes will progress to T2DM.7 Prediabetes not only increases the risk for T2DM but is also associated with an increased risk for microvascular and macrovascular complications and end-organ damage.3 Impaired fasting glucose and impaired glucose tolerance are linked with obesity, lipid abnormalities, and hypertension.8 Early identification and treatment of prediabetes is therefore important to prevent or delay the onset of diabetes as well as its complications.3
Continue for health risks and the metabolic syndrome >>
HEALTH RISKS AND THE METABOLIC SYNDROME
It is estimated that up to 25% of the general nonobese, nondiabetic population has insulin resistance patterns similar to those seen in T2DM.10 These persons are at much higher risk for T2DM than are insulin-sensitive persons and also often have elevated plasma triglycerides, low levels of high-density lipoproteins (HDLs), and higher blood pressure. This clustering of metabolic risk factors is termed metabolic syndrome. The five criteria for metabolic syndrome are
• Large waistline or abdominal obesity
• High triglyceride level
• Low HDL cholesterol level High blood pressure
• High fasting blood sugar.11
Over time, the list of factors associated with the metabolic syndrome has been expanded to include small, dense, low-density lipoproteins (LDLs);12 hyperuricemia;13 prothrombotic state with increased levels of plasminogen activator inhibitor type 1;14 and proinflammatory states.14 These metabolic abnormalities significantly increase the risk for atherosclerotic disease.10
A number of health risks are associated with metabolic syndrome, including low-grade inflammation leading to bone loss in men, hypertension, hypertriglyceridemia, low LDL, abdominal obesity, xanthomas, heart disease, diabetes, fatty liver, cancers (including breast cancer), obstructive sleep apnea, and recurrent preeclampsia.11 Importantly, the risk for heart disease, diabetes, and stroke increases; patients with metabolic syndrome are two times more likely to develop heart disease and five times more likely to develop diabetes than those without it.15
A combination of factors contributes to the propensity for certain individuals to develop metabolic abnormalities. Nonmodifiable risk factors for metabolic syndrome include age, gender, ethnicity, and family history or genetic predisposition. Mexican Americans have the highest overall prevalence of metabolic syndrome at 31.9%.11 In general, the incidence is slightly higher in females; Hispanic and African American women are 1.5 times more likely to be affected than non-Hispanic Caucasian women.11
Some research suggests that criteria parameters should be adjusted for the nonmodifiable risk factors. For example, BMI, often used as a marker for obesity and a tool for predicting cardiometabolic risk, is much lower among Asian Americans compared with other ethnic groups, yet Asian Americans have a significantly higher prevalence of metabolic syndrome in all BMI categories compared with non-Hispanic Caucasians.16 This finding suggests that lower BMIs should be used for defining overweight/obesity in Asian Americans.16
Hypertension
Metabolic syndrome has been associated with an increased risk for hypertension as well as an increase in adverse cardiovascular events.10 The relationship between obesity and hypertension is also well established. With obesity, factors such as an increase in intravascular volume, elevated cardiac output, activation of the renin-angiotensin system, and elevated sympathetic outflow all can contribute to the development of hypertension. Weight control/reduction measures can result in lowered blood pressure.10
Obesity/overweight
Weight gain and abdominal adiposity have been associated with increased prevalence and incidence of metabolic syndrome. While as much as a quarter of the general population meets the criteria for metabolic syndrome, the distribution in relation to weight reflects a prevalence of 4.6% in normal-weight individuals (BMI < 25), 22.4% in those who are overweight (BMI 25-29.9), and nearly 60% in those who are obese (BMI > 30).17
A longitudinal study of mean risk factors for metabolic syndrome demonstrated that young adults whose BMI increased over a 15-year period had steadily worsening levels of all metabolic components, regardless of their baseline BMI.18 Conversely, those with a stable or decreased BMI had no or only minimal worsening of risk factor levels, also independent from baseline.18 Thus, young adults who can maintain a stable BMI into middle age (when the incidence of metabolic syndrome peaks) may prevent the progression of other cardiovascular risk factors and the development of metabolic syndrome and T2DM, even if they are already overweight.
Screening for risk for macrovascular events
The original Framingham Heart Study (FHS) gave rise to a screening tool for identifying persons at risk for atherosclerotic cardiovascular disease (ASCVD) based on risk factors. These risk factors include hypertension, smoking, hyperlipidemia, and postmenopausal status. Nonmodifiable factors include male gender, increasing age, family history, and African American ethnicity. Contributing factors include obesity, T2DM, and stress.19 Although the risk assessment tool based on the FHS data was widely used for many years, cardiovascular events that were unexpected for given risk stratifications highlighted inconsistencies in the tool’s risk identification process.
To more accurately identify patients at risk for ASCVD and suggest preventive strategies, the American College of Cardiology (ACC) and American Heart Association (AHA) convened an expert panel in 2013 to develop new guidelines for screening and treatment of high cholesterol.20 These guidelines introduced a new screening tool for estimating risk for a cardiovascular event based on gender, age, race, total cholesterol, HDL cholesterol, systolic blood pressure, need for treatment for hypertension, presence of diabetes, and smoking status. The lifetime risk estimate tool is only appropriate for individuals ages 20 to 59 but can serve as an educational guideline for demonstrating how lifestyle factors can positively or negatively affect risk for a cardiovascular event. Based on the risk stratification generated by the ASCVD screening tool, clinicians can recommend and implement treatment strategies to lower the risk for a future event (see Figure).21
Continue for management of prediabetes and metabolic syndrome >>
MANAGEMENT OF PREDIABETES AND METABOLIC SYNDROME
Lifestyle interventions
Early intervention in prediabetes will delay and even prevent diabetes. Only 11.1% of American adults with prediabetes are aware of their diagnosis.22 Therefore, the initial step in the management of prediabetes is increasing patient awareness.23 Lifestyle changes improve insulin sensitivity and preserve β-cell function and therefore must be the cornerstone of any diabetes prevention program.6,24
According to AACE, weight loss is essential for the management of prediabetes.9 Intensive lifestyle interventions such as dietary changes, exercise, and weight loss can reduce the rate of conversion to T2DM by approximately 58% after three years.8 In addition, lifestyle modification is recommended as a firstline measure before pharmacologic therapies are started and should be continued throughout the process of any hypertension treatment plan.25
Activity level. Physical inactivity is a major risk factor for metabolic syndrome. As such, physical activity can become a therapeutic strategy to reduce body weight and increase fitness in adults with metabolic syndrome.26 Obesity incidence has increased globally, largely due to a combination of poor dietary habits and a sedentary lifestyle.
The clinical benefits of physical activity and exercise programs include weight loss, increased insulin sensitivity, improved glycemic control, and a reduction in all-cause mortality risk.26 When an exercise intervention is maintained, there is also an improvement in the lipid profile and a decrease in mean arterial blood pressure.26 Even when not combined with dietary restrictions, endurance-type exercise reduces body weight, waist circumference, and visceral adipose tissue mass in obese individuals, although the reductions are less than those seen with diet–exercise interventions.26
There is a strong relationship between visceral obesity and risk for CVD, but individuals with visceral obesity who are physically active have a 24% lower mortality risk than their sedentary counterparts.27 Exercise interventions for metabolic syndrome also improve insulin sensitivity and decrease blood A1C, which in turn leads to a reduced risk for microvascular and macrovascular disease and premature death.26
The goals for individuals with prediabetes should include a 7% weight loss and at least 30 min/d of walking at least five times per week, or participation in other moderate-intensity exercise for a minimum of 150 min/wk.8,28 Many guidelines recommend exercising three to five times per week, but few studies demonstrate the level of activity necessary to generate adventitious effects. Hansen and colleagues conducted a literature review focusing on the effects of exercise interventions on metabolic syndrome and related conditions and found that prolonged low-intensity exercise sessions are at least as effective as high-intensity exercise performed for a shorter duration in persons with known metabolic syndrome.26
Dietary considerations. Dietary education should include instruction regarding portion control and use of the glycemic index.29 Maintenance of a food and exercise diary, with regular review by an educator or primary care provider, may encourage compliance in at-risk individuals. Many different diet types have been studied, and the therapeutic efficacy of certain diets has been demonstrated for multiple components of metabolic syndrome.
The Dietary Approaches to Stop Hypertension (DASH) diet has been shown to be the most effective diet for lowering blood pressure. The DASH recommendations include consuming a diet that emphasizes high intake of vegetables, fruits, whole grains, low-fat dairy products, poultry, nontropical vegetable oils, nuts, fish, and legumes.30 The DASH diet limits the intake of sweets, sugar-sweetened beverages, and red meats and also limits sodium intake to a desirable level of 1,500 mg/d.
Numerous studies have demonstrated the effects of “heart healthy” diets in reducing metabolic risk factors, with recommendations for low-fat, low-carbohydrate, and low-sugar meals, but relatively few studies have evaluated specific dietary alterations. Dhingra and colleagues examined the link between the obesity epidemic and the rising consumption of soft drinks and found a more than 50% higher incidence of metabolic syndrome among persons who drank at least one regular or diet soft drink per day as compared with those who drank less than one soft drink per week.31 High-fructose corn syrup, the primary added sweetener in soft drinks, contains approximately 55% fructose and can lead to weight gain, increased insulin resistance, a decrease in HDL cholesterol, and an increase in triglycerides.31 In this study, however, both regular and diet soft drinks led to similar metabolic derangements, suggesting that additional factors may be involved.31
Geographic studies of metabolic syndrome have given rise to research in regional diets and their beneficial effects. The low incidence of coronary heart disease in Mediterranean countries drove the research for the PREDIMED trial, which compared the effects of two Mediterranean-style diets with a typical low-fat diet. The term “low fat” is often misleading, because it suggests that all fats are bad for the body. While the general principles of Mediterranean-type diets include eating more fruits, vegetables, whole grains, legumes, and nuts, their main component is the use of olive oil in place of butter. Olive oil is a rich source of monounsaturated fatty acids, which have proven beneficial effects on cardiovascular risk factors, obesity, and diabetes.32
In the PREDIMED trial, the participating high-risk individuals on all three diet interventions experienced a decrease in body weight and adiposity measurements, with no observed differences in outcomes for subgroups defined by age, sex, ethnicity, baseline weight, or activity level.32 Compared with the low-fat diet group, participants in the Mediterranean diet groups had decreased systolic and diastolic blood pressures, improved lipid profiles, decreased insulin resistance, and reduced concentrations of inflammatory molecules.32 Since low-fat diets tend to lower both LDL and HDL cholesterol, a fat-rich Mediterranean diet may be more appropriate for high-risk individuals because it decreases LDL cholesterol, triglycerides, and total cholesterol while increasing HDL cholesterol.32 These findings challenge the efficacy of low-fat diets centered on carbohydrate intake by demonstrating greater benefits through carbohydrate replacement with dietary fats.
Pharmacologic interventions
Diabetes. Many studies have shown that pharmacologic intervention can delay the onset of T2DM in those at high risk. Several classes of medications have been studied to evaluate their effectiveness in diabetes prevention. In the Diabetes Prevention Program study, metformin reduced the incidence of T2DM by 31% when compared with placebo; lifestyle intervention reduced the incidence by 58%.24 Metformin reduces the risk for diabetes by inhibiting glucose production in the liver while improving peripheral muscle tissue sensitivity to insulin.6
Other oral agents, such as thiazolidinediones, α-glucosidase inhibitors, and the lipase inhibitor orlistat, have been shown to decrease the incidence of T2DM. However, because studies have demonstrated that medications such as metformin are not as effective as diet and exercise in delaying the onset of diabetes, their use must be limited to high-risk individuals, such as those with a history of GDM, those who are extremely obese, and/or those with uncontrolled hyperglycemia.8
Dyslipidemia. Persons with prediabetes are prone to progression to T2DM and experience cardiovascular events.33 Dyslipidemias—or abnormal blood cholesterol levels—commonly occur in persons with prediabetes and are strongly associated with macrovascular events such as MI or CVD.
The 2013 ACC/AHA guidelines for lowering cholesterol to reduce ASCVD risk in adults are unlike previous guidelines in that they do not provide hard and fast rules about reducing the LDL cholesterol (LDL-C) level to a specific number.20 Instead, the guidelines focus on using statins to reduce the risk for primary and secondary cardiovascular events in those most likely to benefit.20 The panel described four groups who would most likely benefit from statin therapy:
• Persons who have clinical ASCVD
• Persons with LDL-C levels ≥ 190 mg/dL
• Persons ages 40 to 75 with diabetes and LDL-C levels of 70-189 mg/dL
• And (most pertinent to those with prediabetes) persons ages 40 to 75 who do not have clinical ASCVD or diabetes but have LDL-C levels of 70-189 mg/dL and an estimated 10-year ASCVD risk of 7.5% or higher.20
Based on risk stratification, treatment strategies such as statins are recommended to lower an individual’s risk for a future event. High-intensity statin therapy should be initiated in persons with a lifetime risk of 7.5% or higher.20
Hypertension. Metabolic syndrome has a strong relationship with the development of hypertension.10 Pharmacologic intervention for hypertension may be appropriate if lifestyle changes alone do not provide adequate control of blood pressure. Evidence-based guidelines for hypertension management were released in 2014 by the panel members appointed to the Eighth Joint National Committee (JNC 8).25 The JNC 8 guidelines include new, specific recommendations aimed at managing high blood pressure in adults.
For individuals ages 60 and older, pharmacologic therapy should be initiated at a blood pressure of 150/90 mm Hg or higher. For adults younger than 60 or those with comorbidities such as diabetes and chronic kidney disease, the guidelines recommend initiating pharmacologic therapy at a blood pressure of 140/90 mm Hg or higher.
Firstline drug recommendations vary among individuals. For nonblack adults, even those with diabetes, the recommended initial medications include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or angiotensin receptor blocker. For the general black population, including those with diabetes, initial treatment with either a thiazide-type diuretic or calcium channel blocker is preferred.25
Continue for other interventions >>
Other interventions
Group-based educational interventions. Innovative methods are needed to successfully implement diabetes prevention measures in the general population. The ADA recommends diabetes self-management education to help those with prediabetes make behavioral changes.8
Education provided in a group setting using a patient-centered approach has been shown to be effective in improving health care outcomes and increasing self-management.8,29,34 Group-based diabetes education programs have also been found to be cost-effective over the long term.8,29 The ultimate goal of a group-based educational program is to improve the lifestyle practices of the participants through healthier eating and higher activity levels, thus preventing or delaying the onset of T2DM.
Culture-specific interventions. Health care providers should consider cultural variations when formulating treatment plans to improve disease outcomes.35 Patients often find it difficult to adopt a new lifestyle that they see as irrelevant to their cultural practices. For example, in one study, low-income Latina women did not consider cervical cancer screenings necessary due to absence of symptoms such as pain.35
Cultural influences are present in patients’ values and beliefs about disease processes, symptoms, prevention, treatment, self-management, and patient-provider relationships.35 Primary care providers must consider these values and beliefs, as well as their own, to avoid bias.
A culturally oriented approach to the management of prediabetes ensures that care is tailored to individual patients’ needs.36 Measures aimed at fostering culturally competent care include programs that provide culture-specific training for health care providers, use of language-appropriate patient education materials, recruitment of bicultural health care providers, and use of interpreter services.35 Culturally relevant care improves the success rate of sustained lifestyle changes in individuals at risk for chronic conditions such as diabetes.36
Surgical intervention. Bariatric surgery, reserved for high-risk individuals (BMI ≥ 35), has been shown to significantly decrease the incidence of T2DM in morbidly obese individuals.8 These benefits are probably related to the immediate metabolic changes that occur following the surgery, as well as the long-term sustained weight loss. For patients who undergo bariatric surgery, it is important to maintain regular follow-up to promote and maintain behavioral changes and identify barriers to a healthy lifestyle.
CONCLUSION
Clinicians must be able to effectively diagnose and treat prediabetes and metabolic syndrome. Due to its chronic nature and the resources needed to manage the disease and its complications, diabetes places an enormous burden on society. Efforts to prevent or delay the onset of diabetes are therefore essential to curb the costs and burden associated with this chronic disease.
While lifestyle changes are considered the mainstay of prediabetes and metabolic syndrome management, pharmacologic treatment may be considered in high-risk individuals. Patient education, though important, will not improve glycemic control or modify learned behaviors on its own. Health care providers should use a patient-centered approach to guide implementation of evidence-based guidelines in individuals with prediabetes and/or metabolic syndrome.8
Furthermore, innovative methods are needed to successfully implement diabetes and CVD prevention measures in the general population. Health care policies must be put in place to proactively encourage disease prevention rather than just focusing on acute care.37 It is important for NPs and PAs to devise viable strategies for the management of prediabetes and metabolic syndrome.
Environmental change must be made at the population level, incorporating lifestyle changes outside the health care system. There must be collaboration among various governmental and social organizations to ensure a society that promotes a healthy lifestyle.
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4-14.
2. American Association of Diabetes Educators. AADE position statement: primary prevention of type 2 diabetes. Diabetes Educ. 2012;38(1):147-150.
3. Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia—when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology (ACE) and the American Association of Clinical Endocrinologists (AACE). Endocr Pract. 2008;14(7):933-946.
4. American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):S81-S90.
6. DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-112.
7. Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279-2290.
8. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(1):S1-S94.
9. Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract. 2013;19(suppl 2):1-48. www.aace.com/files/algorithm-07-11-2013.pdf. Accessed September 18, 2015.
10. Masharani U. Diabetes mellitus & hypoglycemia. In: Papadakis MA, McPhee SJ, eds. Current Medical Diagnosis & Treatment 2014. 53rd ed. New York, NY: McGraw-Hill Education; 2014:1154-1159.
11. Cash JC, Hall M. Endocrine guidelines. In: Cash JC, Glass CA. Family Practice Guidelines. 3rd ed. New York, NY: Springer Publishing Company; 2014:649-651.
12. Reaven GM, Chen Y-DI, Jeppesen J, et al. Insulin resistance and hyperinsulinemia in individuals with small, dense, low density lipoprotein particles. J Clin Invest. 1993;92:141-146.
13. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008-3011.
14. Grundy SM, Brewer HB, Cleemen JI, et al; for the Conference Participants. Definition of metabolic syndrome. Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433-438.
15. National Heart, Lung, and Blood Institute. What is metabolic syndrome? www.nhlbi.nih.gov/health/health-topics/topics/ms. Accessed September 18, 2015.
16. Palaniappan LP, Wong EC, Shin JJ, et al. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond). 2011;35(3):393-400.
17. Park Y, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163(4):427-436.
18. Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115:1004-1011.
19. Boudi FB, Ahsan CH. Risk factors for coronary artery disease. MedScape. http://emedicine.medscape.com/article/164163-overview#a4. Accessed September 18, 2015.
20. Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
21. American College of Cardiology/American Heart Association. ASCVD risk estimator. http://tools.cardiosource.org/ASCVD-Risk-Estimator/. Accessed July 19, 2015.
22. CDC. Awareness of prediabetes—United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209-212.
23. Geiss LS, James C, Gregg EW, et al. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am J Prev Med. 2010;38:403-409.
24. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6): 393-403.
25. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
26. Hansen D, Dendale P, van Loon LJC, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med. 2010;40(11):921-940.
27. Tjonna AE, Nilsen TIL, Slordahl SA, et al. The association of metabolic clustering and physical activity with cardiovascular mortality: the HUNT study in Norway. J Epidemiol Community Health. 2010;64(8):690-695.
28. Kawahara T, Takahashi K, Inazu T, et al. Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2-day in-hospital diabetes educational program: the Joetsu Diabetes Prevention Trial. Diabetes Care. 2008;31(10):1949-1954.
29. Imai S, Kozai H, Naruse Y, et al. Randomized controlled trial of two forms of self-management group education in Japanese people with impaired glucose tolerance. J Clin Biochem Nutr. 2008;43(2):82-87.
30. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
31. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480-488.
32. Estruch R, Martínez-González MA, Corella D, et al; PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1-11.
33. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635-643.
34. Davis AM, Sawyer DR, Vinci LM. The potential of group visits in diabetes care. Clin Diabetes. 2008;26(2):58-62.
35. Shaw SJ, Huebner C, Armin J, et al. The role of culture in health literacy and chronic disease screening and management. J Immigr Minor Health. 2009;11(6):460-467.
36. Orzech KM, Vivian J, Huebner Torres C, et al. Diet and exercise adherence and practices among medically underserved patients with chronic disease: variation across four ethnic groups. Health Educ Behav. 2013;40(1): 56-66.
37. Narayan KM, Echouffo-Tcheugui J, Mohan V, Ali MK. Analysis & commentary: Global prevention and control of type 2 diabetes will require paradigm shifts in policies within and among countries. Health Aff. 2012;31(1):84-92.
CE/CME No: CR-1510
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the pathophysiology of and risk factors for diabetes.
• Identify the current diagnostic criteria for prediabetes.
• Discuss health risks associated with metabolic syndrome.
• Describe the management of prediabetes and metabolic syndrome.
FACULTY
Annie Abraham is an Assistant Clinical Professor at Texas Woman's University, Dallas and Doctor of Nursing Practice candidate at Texas Christian University, Fort Worth, Texas. Susan Chaney is a Professor, Allison Huffman is an Assistant Clinical Professor, and Kathryn Kremer is an Associate Clinical Professor, at Texas Woman’s University, Dallas.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of October 2015.
Article begins on next page >>
Prediabetes and metabolic syndrome are growing health concerns in the United States and around the world. Lack of awareness of current recommendations may lead to delays in treatment and subsequent increases in diabetes and cardiovascular disease. Evidence-based recommendations for the management of prediabetes and metabolic syndrome focus on lifestyle changes. The most effective strategies for the prevention of type 2 diabetes and management of metabolic syndrome are reviewed here.
The global prevalence of type 2 diabetes mellitus (T2DM) in adults has reached epidemic proportions. Approximately 285 million adults (ages 20-79), or 6.4% of the adult population, around the world are affected, and these numbers are expected to rise to 439 million (7.7%) by 2030.1 In the United States, however, the prevalence is even higher, at 8.3% of the adult population.2
T2DM is a chronic disease that can cause significant morbidity and mortality. Diabetes is associated with macrovascular (eg, heart disease and stroke) as well as microvascular (eg, retinopathy, neuropathy, and microalbuminuria) complications.3 The economic cost of diagnosed diabetes in the US was approximately $245 billion in 2012.4 Early identification and management of prediabetes by nurse practitioners and physician assistants is essential to minimize disease prevalence and progression and to reduce the tremendous economic burden associated with T2DM.
PATHOPHYSIOLOGY AND RISK FACTORS FOR DIABETES
Diabetes is a complex disorder characterized by hyperglycemia resulting from insufficient insulin secretion and/or decreased tissue response to insulin.5 The progression of normal glucose tolerance to diabetes is a continuous process involving many organ systems. The disease develops primarily as a result of impaired insulin action in muscle, impaired insulin secretion from the pancreatic β-cells, and increased hepatic glucose production.6
In addition, defects in other organs, including increased free fatty acid production in the adipose tissue, decreased incretin effect in the digestive tract, increased glucagon secretion from the pancreatic α-cells, increased glucose reabsorption from the kidneys, and neurotransmitter dysfunction, lead to progression of diabetes.6 This phenomenon is called the ominous octet.6 Gradual increases in glucose levels have been detected as early as 13 years prior to diagnosis of diabetes, with an abrupt increase occurring in the final two to six years before diagnosis.7
Modifiable risk factors such as obesity, poor dietary intake, and a sedentary lifestyle have been associated with increased risk for T2DM.2 Certain antipsychotic medications may also play a part in disease progression.3
According to the American Diabetes Association (ADA), screening for diabetes should begin at age 45 and be repeated every three years.8 For adults younger than 45, screening should be done in those with a BMI of 25 or higher and at least one of the following additional risk factors:
• Family history (first-degree relative with diabetes)
• Ethnicity (high-risk populations include African American, Latino, Native American, Asian American, Pacific Islander)
• History of polycystic ovary syndrome
• History of gestational diabetes (GDM) or delivering a baby weighing more than 9 lb
• Physical inactivity
• Hypertension and/or hyperlipidemia
• History of cardiovascular disease (CVD)
• Insulin resistance.8
IDENTIFICATION/DIAGNOSIS OF PREDIABETES
Diabetes is preceded by an asymptomatic phase known as prediabetes. In prediabetes, blood glucose levels are higher than normal but do not meet the criteria for diabetes.8 According to the American Association of Clinical Endocrinologists (AACE) and the ADA, prediabetes is defined as impaired fasting glucose (fasting plasma glucose level of 100-125 mg/dL) and/or impaired glucose tolerance (two-hour plasma glucose value of 140-199 mg/dL after a 75-g oral glucose tolerance test).8,9 The ADA also considers a hemoglobin A1C value of 5.7% to 6.4% to be indicative of prediabetes.8
In the US, it is estimated that more than 79 million adults have prediabetes.2 Approximately 70% of individuals with prediabetes will progress to T2DM.7 Prediabetes not only increases the risk for T2DM but is also associated with an increased risk for microvascular and macrovascular complications and end-organ damage.3 Impaired fasting glucose and impaired glucose tolerance are linked with obesity, lipid abnormalities, and hypertension.8 Early identification and treatment of prediabetes is therefore important to prevent or delay the onset of diabetes as well as its complications.3
Continue for health risks and the metabolic syndrome >>
HEALTH RISKS AND THE METABOLIC SYNDROME
It is estimated that up to 25% of the general nonobese, nondiabetic population has insulin resistance patterns similar to those seen in T2DM.10 These persons are at much higher risk for T2DM than are insulin-sensitive persons and also often have elevated plasma triglycerides, low levels of high-density lipoproteins (HDLs), and higher blood pressure. This clustering of metabolic risk factors is termed metabolic syndrome. The five criteria for metabolic syndrome are
• Large waistline or abdominal obesity
• High triglyceride level
• Low HDL cholesterol level High blood pressure
• High fasting blood sugar.11
Over time, the list of factors associated with the metabolic syndrome has been expanded to include small, dense, low-density lipoproteins (LDLs);12 hyperuricemia;13 prothrombotic state with increased levels of plasminogen activator inhibitor type 1;14 and proinflammatory states.14 These metabolic abnormalities significantly increase the risk for atherosclerotic disease.10
A number of health risks are associated with metabolic syndrome, including low-grade inflammation leading to bone loss in men, hypertension, hypertriglyceridemia, low LDL, abdominal obesity, xanthomas, heart disease, diabetes, fatty liver, cancers (including breast cancer), obstructive sleep apnea, and recurrent preeclampsia.11 Importantly, the risk for heart disease, diabetes, and stroke increases; patients with metabolic syndrome are two times more likely to develop heart disease and five times more likely to develop diabetes than those without it.15
A combination of factors contributes to the propensity for certain individuals to develop metabolic abnormalities. Nonmodifiable risk factors for metabolic syndrome include age, gender, ethnicity, and family history or genetic predisposition. Mexican Americans have the highest overall prevalence of metabolic syndrome at 31.9%.11 In general, the incidence is slightly higher in females; Hispanic and African American women are 1.5 times more likely to be affected than non-Hispanic Caucasian women.11
Some research suggests that criteria parameters should be adjusted for the nonmodifiable risk factors. For example, BMI, often used as a marker for obesity and a tool for predicting cardiometabolic risk, is much lower among Asian Americans compared with other ethnic groups, yet Asian Americans have a significantly higher prevalence of metabolic syndrome in all BMI categories compared with non-Hispanic Caucasians.16 This finding suggests that lower BMIs should be used for defining overweight/obesity in Asian Americans.16
Hypertension
Metabolic syndrome has been associated with an increased risk for hypertension as well as an increase in adverse cardiovascular events.10 The relationship between obesity and hypertension is also well established. With obesity, factors such as an increase in intravascular volume, elevated cardiac output, activation of the renin-angiotensin system, and elevated sympathetic outflow all can contribute to the development of hypertension. Weight control/reduction measures can result in lowered blood pressure.10
Obesity/overweight
Weight gain and abdominal adiposity have been associated with increased prevalence and incidence of metabolic syndrome. While as much as a quarter of the general population meets the criteria for metabolic syndrome, the distribution in relation to weight reflects a prevalence of 4.6% in normal-weight individuals (BMI < 25), 22.4% in those who are overweight (BMI 25-29.9), and nearly 60% in those who are obese (BMI > 30).17
A longitudinal study of mean risk factors for metabolic syndrome demonstrated that young adults whose BMI increased over a 15-year period had steadily worsening levels of all metabolic components, regardless of their baseline BMI.18 Conversely, those with a stable or decreased BMI had no or only minimal worsening of risk factor levels, also independent from baseline.18 Thus, young adults who can maintain a stable BMI into middle age (when the incidence of metabolic syndrome peaks) may prevent the progression of other cardiovascular risk factors and the development of metabolic syndrome and T2DM, even if they are already overweight.
Screening for risk for macrovascular events
The original Framingham Heart Study (FHS) gave rise to a screening tool for identifying persons at risk for atherosclerotic cardiovascular disease (ASCVD) based on risk factors. These risk factors include hypertension, smoking, hyperlipidemia, and postmenopausal status. Nonmodifiable factors include male gender, increasing age, family history, and African American ethnicity. Contributing factors include obesity, T2DM, and stress.19 Although the risk assessment tool based on the FHS data was widely used for many years, cardiovascular events that were unexpected for given risk stratifications highlighted inconsistencies in the tool’s risk identification process.
To more accurately identify patients at risk for ASCVD and suggest preventive strategies, the American College of Cardiology (ACC) and American Heart Association (AHA) convened an expert panel in 2013 to develop new guidelines for screening and treatment of high cholesterol.20 These guidelines introduced a new screening tool for estimating risk for a cardiovascular event based on gender, age, race, total cholesterol, HDL cholesterol, systolic blood pressure, need for treatment for hypertension, presence of diabetes, and smoking status. The lifetime risk estimate tool is only appropriate for individuals ages 20 to 59 but can serve as an educational guideline for demonstrating how lifestyle factors can positively or negatively affect risk for a cardiovascular event. Based on the risk stratification generated by the ASCVD screening tool, clinicians can recommend and implement treatment strategies to lower the risk for a future event (see Figure).21
Continue for management of prediabetes and metabolic syndrome >>
MANAGEMENT OF PREDIABETES AND METABOLIC SYNDROME
Lifestyle interventions
Early intervention in prediabetes will delay and even prevent diabetes. Only 11.1% of American adults with prediabetes are aware of their diagnosis.22 Therefore, the initial step in the management of prediabetes is increasing patient awareness.23 Lifestyle changes improve insulin sensitivity and preserve β-cell function and therefore must be the cornerstone of any diabetes prevention program.6,24
According to AACE, weight loss is essential for the management of prediabetes.9 Intensive lifestyle interventions such as dietary changes, exercise, and weight loss can reduce the rate of conversion to T2DM by approximately 58% after three years.8 In addition, lifestyle modification is recommended as a firstline measure before pharmacologic therapies are started and should be continued throughout the process of any hypertension treatment plan.25
Activity level. Physical inactivity is a major risk factor for metabolic syndrome. As such, physical activity can become a therapeutic strategy to reduce body weight and increase fitness in adults with metabolic syndrome.26 Obesity incidence has increased globally, largely due to a combination of poor dietary habits and a sedentary lifestyle.
The clinical benefits of physical activity and exercise programs include weight loss, increased insulin sensitivity, improved glycemic control, and a reduction in all-cause mortality risk.26 When an exercise intervention is maintained, there is also an improvement in the lipid profile and a decrease in mean arterial blood pressure.26 Even when not combined with dietary restrictions, endurance-type exercise reduces body weight, waist circumference, and visceral adipose tissue mass in obese individuals, although the reductions are less than those seen with diet–exercise interventions.26
There is a strong relationship between visceral obesity and risk for CVD, but individuals with visceral obesity who are physically active have a 24% lower mortality risk than their sedentary counterparts.27 Exercise interventions for metabolic syndrome also improve insulin sensitivity and decrease blood A1C, which in turn leads to a reduced risk for microvascular and macrovascular disease and premature death.26
The goals for individuals with prediabetes should include a 7% weight loss and at least 30 min/d of walking at least five times per week, or participation in other moderate-intensity exercise for a minimum of 150 min/wk.8,28 Many guidelines recommend exercising three to five times per week, but few studies demonstrate the level of activity necessary to generate adventitious effects. Hansen and colleagues conducted a literature review focusing on the effects of exercise interventions on metabolic syndrome and related conditions and found that prolonged low-intensity exercise sessions are at least as effective as high-intensity exercise performed for a shorter duration in persons with known metabolic syndrome.26
Dietary considerations. Dietary education should include instruction regarding portion control and use of the glycemic index.29 Maintenance of a food and exercise diary, with regular review by an educator or primary care provider, may encourage compliance in at-risk individuals. Many different diet types have been studied, and the therapeutic efficacy of certain diets has been demonstrated for multiple components of metabolic syndrome.
The Dietary Approaches to Stop Hypertension (DASH) diet has been shown to be the most effective diet for lowering blood pressure. The DASH recommendations include consuming a diet that emphasizes high intake of vegetables, fruits, whole grains, low-fat dairy products, poultry, nontropical vegetable oils, nuts, fish, and legumes.30 The DASH diet limits the intake of sweets, sugar-sweetened beverages, and red meats and also limits sodium intake to a desirable level of 1,500 mg/d.
Numerous studies have demonstrated the effects of “heart healthy” diets in reducing metabolic risk factors, with recommendations for low-fat, low-carbohydrate, and low-sugar meals, but relatively few studies have evaluated specific dietary alterations. Dhingra and colleagues examined the link between the obesity epidemic and the rising consumption of soft drinks and found a more than 50% higher incidence of metabolic syndrome among persons who drank at least one regular or diet soft drink per day as compared with those who drank less than one soft drink per week.31 High-fructose corn syrup, the primary added sweetener in soft drinks, contains approximately 55% fructose and can lead to weight gain, increased insulin resistance, a decrease in HDL cholesterol, and an increase in triglycerides.31 In this study, however, both regular and diet soft drinks led to similar metabolic derangements, suggesting that additional factors may be involved.31
Geographic studies of metabolic syndrome have given rise to research in regional diets and their beneficial effects. The low incidence of coronary heart disease in Mediterranean countries drove the research for the PREDIMED trial, which compared the effects of two Mediterranean-style diets with a typical low-fat diet. The term “low fat” is often misleading, because it suggests that all fats are bad for the body. While the general principles of Mediterranean-type diets include eating more fruits, vegetables, whole grains, legumes, and nuts, their main component is the use of olive oil in place of butter. Olive oil is a rich source of monounsaturated fatty acids, which have proven beneficial effects on cardiovascular risk factors, obesity, and diabetes.32
In the PREDIMED trial, the participating high-risk individuals on all three diet interventions experienced a decrease in body weight and adiposity measurements, with no observed differences in outcomes for subgroups defined by age, sex, ethnicity, baseline weight, or activity level.32 Compared with the low-fat diet group, participants in the Mediterranean diet groups had decreased systolic and diastolic blood pressures, improved lipid profiles, decreased insulin resistance, and reduced concentrations of inflammatory molecules.32 Since low-fat diets tend to lower both LDL and HDL cholesterol, a fat-rich Mediterranean diet may be more appropriate for high-risk individuals because it decreases LDL cholesterol, triglycerides, and total cholesterol while increasing HDL cholesterol.32 These findings challenge the efficacy of low-fat diets centered on carbohydrate intake by demonstrating greater benefits through carbohydrate replacement with dietary fats.
Pharmacologic interventions
Diabetes. Many studies have shown that pharmacologic intervention can delay the onset of T2DM in those at high risk. Several classes of medications have been studied to evaluate their effectiveness in diabetes prevention. In the Diabetes Prevention Program study, metformin reduced the incidence of T2DM by 31% when compared with placebo; lifestyle intervention reduced the incidence by 58%.24 Metformin reduces the risk for diabetes by inhibiting glucose production in the liver while improving peripheral muscle tissue sensitivity to insulin.6
Other oral agents, such as thiazolidinediones, α-glucosidase inhibitors, and the lipase inhibitor orlistat, have been shown to decrease the incidence of T2DM. However, because studies have demonstrated that medications such as metformin are not as effective as diet and exercise in delaying the onset of diabetes, their use must be limited to high-risk individuals, such as those with a history of GDM, those who are extremely obese, and/or those with uncontrolled hyperglycemia.8
Dyslipidemia. Persons with prediabetes are prone to progression to T2DM and experience cardiovascular events.33 Dyslipidemias—or abnormal blood cholesterol levels—commonly occur in persons with prediabetes and are strongly associated with macrovascular events such as MI or CVD.
The 2013 ACC/AHA guidelines for lowering cholesterol to reduce ASCVD risk in adults are unlike previous guidelines in that they do not provide hard and fast rules about reducing the LDL cholesterol (LDL-C) level to a specific number.20 Instead, the guidelines focus on using statins to reduce the risk for primary and secondary cardiovascular events in those most likely to benefit.20 The panel described four groups who would most likely benefit from statin therapy:
• Persons who have clinical ASCVD
• Persons with LDL-C levels ≥ 190 mg/dL
• Persons ages 40 to 75 with diabetes and LDL-C levels of 70-189 mg/dL
• And (most pertinent to those with prediabetes) persons ages 40 to 75 who do not have clinical ASCVD or diabetes but have LDL-C levels of 70-189 mg/dL and an estimated 10-year ASCVD risk of 7.5% or higher.20
Based on risk stratification, treatment strategies such as statins are recommended to lower an individual’s risk for a future event. High-intensity statin therapy should be initiated in persons with a lifetime risk of 7.5% or higher.20
Hypertension. Metabolic syndrome has a strong relationship with the development of hypertension.10 Pharmacologic intervention for hypertension may be appropriate if lifestyle changes alone do not provide adequate control of blood pressure. Evidence-based guidelines for hypertension management were released in 2014 by the panel members appointed to the Eighth Joint National Committee (JNC 8).25 The JNC 8 guidelines include new, specific recommendations aimed at managing high blood pressure in adults.
For individuals ages 60 and older, pharmacologic therapy should be initiated at a blood pressure of 150/90 mm Hg or higher. For adults younger than 60 or those with comorbidities such as diabetes and chronic kidney disease, the guidelines recommend initiating pharmacologic therapy at a blood pressure of 140/90 mm Hg or higher.
Firstline drug recommendations vary among individuals. For nonblack adults, even those with diabetes, the recommended initial medications include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or angiotensin receptor blocker. For the general black population, including those with diabetes, initial treatment with either a thiazide-type diuretic or calcium channel blocker is preferred.25
Continue for other interventions >>
Other interventions
Group-based educational interventions. Innovative methods are needed to successfully implement diabetes prevention measures in the general population. The ADA recommends diabetes self-management education to help those with prediabetes make behavioral changes.8
Education provided in a group setting using a patient-centered approach has been shown to be effective in improving health care outcomes and increasing self-management.8,29,34 Group-based diabetes education programs have also been found to be cost-effective over the long term.8,29 The ultimate goal of a group-based educational program is to improve the lifestyle practices of the participants through healthier eating and higher activity levels, thus preventing or delaying the onset of T2DM.
Culture-specific interventions. Health care providers should consider cultural variations when formulating treatment plans to improve disease outcomes.35 Patients often find it difficult to adopt a new lifestyle that they see as irrelevant to their cultural practices. For example, in one study, low-income Latina women did not consider cervical cancer screenings necessary due to absence of symptoms such as pain.35
Cultural influences are present in patients’ values and beliefs about disease processes, symptoms, prevention, treatment, self-management, and patient-provider relationships.35 Primary care providers must consider these values and beliefs, as well as their own, to avoid bias.
A culturally oriented approach to the management of prediabetes ensures that care is tailored to individual patients’ needs.36 Measures aimed at fostering culturally competent care include programs that provide culture-specific training for health care providers, use of language-appropriate patient education materials, recruitment of bicultural health care providers, and use of interpreter services.35 Culturally relevant care improves the success rate of sustained lifestyle changes in individuals at risk for chronic conditions such as diabetes.36
Surgical intervention. Bariatric surgery, reserved for high-risk individuals (BMI ≥ 35), has been shown to significantly decrease the incidence of T2DM in morbidly obese individuals.8 These benefits are probably related to the immediate metabolic changes that occur following the surgery, as well as the long-term sustained weight loss. For patients who undergo bariatric surgery, it is important to maintain regular follow-up to promote and maintain behavioral changes and identify barriers to a healthy lifestyle.
CONCLUSION
Clinicians must be able to effectively diagnose and treat prediabetes and metabolic syndrome. Due to its chronic nature and the resources needed to manage the disease and its complications, diabetes places an enormous burden on society. Efforts to prevent or delay the onset of diabetes are therefore essential to curb the costs and burden associated with this chronic disease.
While lifestyle changes are considered the mainstay of prediabetes and metabolic syndrome management, pharmacologic treatment may be considered in high-risk individuals. Patient education, though important, will not improve glycemic control or modify learned behaviors on its own. Health care providers should use a patient-centered approach to guide implementation of evidence-based guidelines in individuals with prediabetes and/or metabolic syndrome.8
Furthermore, innovative methods are needed to successfully implement diabetes and CVD prevention measures in the general population. Health care policies must be put in place to proactively encourage disease prevention rather than just focusing on acute care.37 It is important for NPs and PAs to devise viable strategies for the management of prediabetes and metabolic syndrome.
Environmental change must be made at the population level, incorporating lifestyle changes outside the health care system. There must be collaboration among various governmental and social organizations to ensure a society that promotes a healthy lifestyle.
CE/CME No: CR-1510
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the pathophysiology of and risk factors for diabetes.
• Identify the current diagnostic criteria for prediabetes.
• Discuss health risks associated with metabolic syndrome.
• Describe the management of prediabetes and metabolic syndrome.
FACULTY
Annie Abraham is an Assistant Clinical Professor at Texas Woman's University, Dallas and Doctor of Nursing Practice candidate at Texas Christian University, Fort Worth, Texas. Susan Chaney is a Professor, Allison Huffman is an Assistant Clinical Professor, and Kathryn Kremer is an Associate Clinical Professor, at Texas Woman’s University, Dallas.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of October 2015.
Article begins on next page >>
Prediabetes and metabolic syndrome are growing health concerns in the United States and around the world. Lack of awareness of current recommendations may lead to delays in treatment and subsequent increases in diabetes and cardiovascular disease. Evidence-based recommendations for the management of prediabetes and metabolic syndrome focus on lifestyle changes. The most effective strategies for the prevention of type 2 diabetes and management of metabolic syndrome are reviewed here.
The global prevalence of type 2 diabetes mellitus (T2DM) in adults has reached epidemic proportions. Approximately 285 million adults (ages 20-79), or 6.4% of the adult population, around the world are affected, and these numbers are expected to rise to 439 million (7.7%) by 2030.1 In the United States, however, the prevalence is even higher, at 8.3% of the adult population.2
T2DM is a chronic disease that can cause significant morbidity and mortality. Diabetes is associated with macrovascular (eg, heart disease and stroke) as well as microvascular (eg, retinopathy, neuropathy, and microalbuminuria) complications.3 The economic cost of diagnosed diabetes in the US was approximately $245 billion in 2012.4 Early identification and management of prediabetes by nurse practitioners and physician assistants is essential to minimize disease prevalence and progression and to reduce the tremendous economic burden associated with T2DM.
PATHOPHYSIOLOGY AND RISK FACTORS FOR DIABETES
Diabetes is a complex disorder characterized by hyperglycemia resulting from insufficient insulin secretion and/or decreased tissue response to insulin.5 The progression of normal glucose tolerance to diabetes is a continuous process involving many organ systems. The disease develops primarily as a result of impaired insulin action in muscle, impaired insulin secretion from the pancreatic β-cells, and increased hepatic glucose production.6
In addition, defects in other organs, including increased free fatty acid production in the adipose tissue, decreased incretin effect in the digestive tract, increased glucagon secretion from the pancreatic α-cells, increased glucose reabsorption from the kidneys, and neurotransmitter dysfunction, lead to progression of diabetes.6 This phenomenon is called the ominous octet.6 Gradual increases in glucose levels have been detected as early as 13 years prior to diagnosis of diabetes, with an abrupt increase occurring in the final two to six years before diagnosis.7
Modifiable risk factors such as obesity, poor dietary intake, and a sedentary lifestyle have been associated with increased risk for T2DM.2 Certain antipsychotic medications may also play a part in disease progression.3
According to the American Diabetes Association (ADA), screening for diabetes should begin at age 45 and be repeated every three years.8 For adults younger than 45, screening should be done in those with a BMI of 25 or higher and at least one of the following additional risk factors:
• Family history (first-degree relative with diabetes)
• Ethnicity (high-risk populations include African American, Latino, Native American, Asian American, Pacific Islander)
• History of polycystic ovary syndrome
• History of gestational diabetes (GDM) or delivering a baby weighing more than 9 lb
• Physical inactivity
• Hypertension and/or hyperlipidemia
• History of cardiovascular disease (CVD)
• Insulin resistance.8
IDENTIFICATION/DIAGNOSIS OF PREDIABETES
Diabetes is preceded by an asymptomatic phase known as prediabetes. In prediabetes, blood glucose levels are higher than normal but do not meet the criteria for diabetes.8 According to the American Association of Clinical Endocrinologists (AACE) and the ADA, prediabetes is defined as impaired fasting glucose (fasting plasma glucose level of 100-125 mg/dL) and/or impaired glucose tolerance (two-hour plasma glucose value of 140-199 mg/dL after a 75-g oral glucose tolerance test).8,9 The ADA also considers a hemoglobin A1C value of 5.7% to 6.4% to be indicative of prediabetes.8
In the US, it is estimated that more than 79 million adults have prediabetes.2 Approximately 70% of individuals with prediabetes will progress to T2DM.7 Prediabetes not only increases the risk for T2DM but is also associated with an increased risk for microvascular and macrovascular complications and end-organ damage.3 Impaired fasting glucose and impaired glucose tolerance are linked with obesity, lipid abnormalities, and hypertension.8 Early identification and treatment of prediabetes is therefore important to prevent or delay the onset of diabetes as well as its complications.3
Continue for health risks and the metabolic syndrome >>
HEALTH RISKS AND THE METABOLIC SYNDROME
It is estimated that up to 25% of the general nonobese, nondiabetic population has insulin resistance patterns similar to those seen in T2DM.10 These persons are at much higher risk for T2DM than are insulin-sensitive persons and also often have elevated plasma triglycerides, low levels of high-density lipoproteins (HDLs), and higher blood pressure. This clustering of metabolic risk factors is termed metabolic syndrome. The five criteria for metabolic syndrome are
• Large waistline or abdominal obesity
• High triglyceride level
• Low HDL cholesterol level High blood pressure
• High fasting blood sugar.11
Over time, the list of factors associated with the metabolic syndrome has been expanded to include small, dense, low-density lipoproteins (LDLs);12 hyperuricemia;13 prothrombotic state with increased levels of plasminogen activator inhibitor type 1;14 and proinflammatory states.14 These metabolic abnormalities significantly increase the risk for atherosclerotic disease.10
A number of health risks are associated with metabolic syndrome, including low-grade inflammation leading to bone loss in men, hypertension, hypertriglyceridemia, low LDL, abdominal obesity, xanthomas, heart disease, diabetes, fatty liver, cancers (including breast cancer), obstructive sleep apnea, and recurrent preeclampsia.11 Importantly, the risk for heart disease, diabetes, and stroke increases; patients with metabolic syndrome are two times more likely to develop heart disease and five times more likely to develop diabetes than those without it.15
A combination of factors contributes to the propensity for certain individuals to develop metabolic abnormalities. Nonmodifiable risk factors for metabolic syndrome include age, gender, ethnicity, and family history or genetic predisposition. Mexican Americans have the highest overall prevalence of metabolic syndrome at 31.9%.11 In general, the incidence is slightly higher in females; Hispanic and African American women are 1.5 times more likely to be affected than non-Hispanic Caucasian women.11
Some research suggests that criteria parameters should be adjusted for the nonmodifiable risk factors. For example, BMI, often used as a marker for obesity and a tool for predicting cardiometabolic risk, is much lower among Asian Americans compared with other ethnic groups, yet Asian Americans have a significantly higher prevalence of metabolic syndrome in all BMI categories compared with non-Hispanic Caucasians.16 This finding suggests that lower BMIs should be used for defining overweight/obesity in Asian Americans.16
Hypertension
Metabolic syndrome has been associated with an increased risk for hypertension as well as an increase in adverse cardiovascular events.10 The relationship between obesity and hypertension is also well established. With obesity, factors such as an increase in intravascular volume, elevated cardiac output, activation of the renin-angiotensin system, and elevated sympathetic outflow all can contribute to the development of hypertension. Weight control/reduction measures can result in lowered blood pressure.10
Obesity/overweight
Weight gain and abdominal adiposity have been associated with increased prevalence and incidence of metabolic syndrome. While as much as a quarter of the general population meets the criteria for metabolic syndrome, the distribution in relation to weight reflects a prevalence of 4.6% in normal-weight individuals (BMI < 25), 22.4% in those who are overweight (BMI 25-29.9), and nearly 60% in those who are obese (BMI > 30).17
A longitudinal study of mean risk factors for metabolic syndrome demonstrated that young adults whose BMI increased over a 15-year period had steadily worsening levels of all metabolic components, regardless of their baseline BMI.18 Conversely, those with a stable or decreased BMI had no or only minimal worsening of risk factor levels, also independent from baseline.18 Thus, young adults who can maintain a stable BMI into middle age (when the incidence of metabolic syndrome peaks) may prevent the progression of other cardiovascular risk factors and the development of metabolic syndrome and T2DM, even if they are already overweight.
Screening for risk for macrovascular events
The original Framingham Heart Study (FHS) gave rise to a screening tool for identifying persons at risk for atherosclerotic cardiovascular disease (ASCVD) based on risk factors. These risk factors include hypertension, smoking, hyperlipidemia, and postmenopausal status. Nonmodifiable factors include male gender, increasing age, family history, and African American ethnicity. Contributing factors include obesity, T2DM, and stress.19 Although the risk assessment tool based on the FHS data was widely used for many years, cardiovascular events that were unexpected for given risk stratifications highlighted inconsistencies in the tool’s risk identification process.
To more accurately identify patients at risk for ASCVD and suggest preventive strategies, the American College of Cardiology (ACC) and American Heart Association (AHA) convened an expert panel in 2013 to develop new guidelines for screening and treatment of high cholesterol.20 These guidelines introduced a new screening tool for estimating risk for a cardiovascular event based on gender, age, race, total cholesterol, HDL cholesterol, systolic blood pressure, need for treatment for hypertension, presence of diabetes, and smoking status. The lifetime risk estimate tool is only appropriate for individuals ages 20 to 59 but can serve as an educational guideline for demonstrating how lifestyle factors can positively or negatively affect risk for a cardiovascular event. Based on the risk stratification generated by the ASCVD screening tool, clinicians can recommend and implement treatment strategies to lower the risk for a future event (see Figure).21
Continue for management of prediabetes and metabolic syndrome >>
MANAGEMENT OF PREDIABETES AND METABOLIC SYNDROME
Lifestyle interventions
Early intervention in prediabetes will delay and even prevent diabetes. Only 11.1% of American adults with prediabetes are aware of their diagnosis.22 Therefore, the initial step in the management of prediabetes is increasing patient awareness.23 Lifestyle changes improve insulin sensitivity and preserve β-cell function and therefore must be the cornerstone of any diabetes prevention program.6,24
According to AACE, weight loss is essential for the management of prediabetes.9 Intensive lifestyle interventions such as dietary changes, exercise, and weight loss can reduce the rate of conversion to T2DM by approximately 58% after three years.8 In addition, lifestyle modification is recommended as a firstline measure before pharmacologic therapies are started and should be continued throughout the process of any hypertension treatment plan.25
Activity level. Physical inactivity is a major risk factor for metabolic syndrome. As such, physical activity can become a therapeutic strategy to reduce body weight and increase fitness in adults with metabolic syndrome.26 Obesity incidence has increased globally, largely due to a combination of poor dietary habits and a sedentary lifestyle.
The clinical benefits of physical activity and exercise programs include weight loss, increased insulin sensitivity, improved glycemic control, and a reduction in all-cause mortality risk.26 When an exercise intervention is maintained, there is also an improvement in the lipid profile and a decrease in mean arterial blood pressure.26 Even when not combined with dietary restrictions, endurance-type exercise reduces body weight, waist circumference, and visceral adipose tissue mass in obese individuals, although the reductions are less than those seen with diet–exercise interventions.26
There is a strong relationship between visceral obesity and risk for CVD, but individuals with visceral obesity who are physically active have a 24% lower mortality risk than their sedentary counterparts.27 Exercise interventions for metabolic syndrome also improve insulin sensitivity and decrease blood A1C, which in turn leads to a reduced risk for microvascular and macrovascular disease and premature death.26
The goals for individuals with prediabetes should include a 7% weight loss and at least 30 min/d of walking at least five times per week, or participation in other moderate-intensity exercise for a minimum of 150 min/wk.8,28 Many guidelines recommend exercising three to five times per week, but few studies demonstrate the level of activity necessary to generate adventitious effects. Hansen and colleagues conducted a literature review focusing on the effects of exercise interventions on metabolic syndrome and related conditions and found that prolonged low-intensity exercise sessions are at least as effective as high-intensity exercise performed for a shorter duration in persons with known metabolic syndrome.26
Dietary considerations. Dietary education should include instruction regarding portion control and use of the glycemic index.29 Maintenance of a food and exercise diary, with regular review by an educator or primary care provider, may encourage compliance in at-risk individuals. Many different diet types have been studied, and the therapeutic efficacy of certain diets has been demonstrated for multiple components of metabolic syndrome.
The Dietary Approaches to Stop Hypertension (DASH) diet has been shown to be the most effective diet for lowering blood pressure. The DASH recommendations include consuming a diet that emphasizes high intake of vegetables, fruits, whole grains, low-fat dairy products, poultry, nontropical vegetable oils, nuts, fish, and legumes.30 The DASH diet limits the intake of sweets, sugar-sweetened beverages, and red meats and also limits sodium intake to a desirable level of 1,500 mg/d.
Numerous studies have demonstrated the effects of “heart healthy” diets in reducing metabolic risk factors, with recommendations for low-fat, low-carbohydrate, and low-sugar meals, but relatively few studies have evaluated specific dietary alterations. Dhingra and colleagues examined the link between the obesity epidemic and the rising consumption of soft drinks and found a more than 50% higher incidence of metabolic syndrome among persons who drank at least one regular or diet soft drink per day as compared with those who drank less than one soft drink per week.31 High-fructose corn syrup, the primary added sweetener in soft drinks, contains approximately 55% fructose and can lead to weight gain, increased insulin resistance, a decrease in HDL cholesterol, and an increase in triglycerides.31 In this study, however, both regular and diet soft drinks led to similar metabolic derangements, suggesting that additional factors may be involved.31
Geographic studies of metabolic syndrome have given rise to research in regional diets and their beneficial effects. The low incidence of coronary heart disease in Mediterranean countries drove the research for the PREDIMED trial, which compared the effects of two Mediterranean-style diets with a typical low-fat diet. The term “low fat” is often misleading, because it suggests that all fats are bad for the body. While the general principles of Mediterranean-type diets include eating more fruits, vegetables, whole grains, legumes, and nuts, their main component is the use of olive oil in place of butter. Olive oil is a rich source of monounsaturated fatty acids, which have proven beneficial effects on cardiovascular risk factors, obesity, and diabetes.32
In the PREDIMED trial, the participating high-risk individuals on all three diet interventions experienced a decrease in body weight and adiposity measurements, with no observed differences in outcomes for subgroups defined by age, sex, ethnicity, baseline weight, or activity level.32 Compared with the low-fat diet group, participants in the Mediterranean diet groups had decreased systolic and diastolic blood pressures, improved lipid profiles, decreased insulin resistance, and reduced concentrations of inflammatory molecules.32 Since low-fat diets tend to lower both LDL and HDL cholesterol, a fat-rich Mediterranean diet may be more appropriate for high-risk individuals because it decreases LDL cholesterol, triglycerides, and total cholesterol while increasing HDL cholesterol.32 These findings challenge the efficacy of low-fat diets centered on carbohydrate intake by demonstrating greater benefits through carbohydrate replacement with dietary fats.
Pharmacologic interventions
Diabetes. Many studies have shown that pharmacologic intervention can delay the onset of T2DM in those at high risk. Several classes of medications have been studied to evaluate their effectiveness in diabetes prevention. In the Diabetes Prevention Program study, metformin reduced the incidence of T2DM by 31% when compared with placebo; lifestyle intervention reduced the incidence by 58%.24 Metformin reduces the risk for diabetes by inhibiting glucose production in the liver while improving peripheral muscle tissue sensitivity to insulin.6
Other oral agents, such as thiazolidinediones, α-glucosidase inhibitors, and the lipase inhibitor orlistat, have been shown to decrease the incidence of T2DM. However, because studies have demonstrated that medications such as metformin are not as effective as diet and exercise in delaying the onset of diabetes, their use must be limited to high-risk individuals, such as those with a history of GDM, those who are extremely obese, and/or those with uncontrolled hyperglycemia.8
Dyslipidemia. Persons with prediabetes are prone to progression to T2DM and experience cardiovascular events.33 Dyslipidemias—or abnormal blood cholesterol levels—commonly occur in persons with prediabetes and are strongly associated with macrovascular events such as MI or CVD.
The 2013 ACC/AHA guidelines for lowering cholesterol to reduce ASCVD risk in adults are unlike previous guidelines in that they do not provide hard and fast rules about reducing the LDL cholesterol (LDL-C) level to a specific number.20 Instead, the guidelines focus on using statins to reduce the risk for primary and secondary cardiovascular events in those most likely to benefit.20 The panel described four groups who would most likely benefit from statin therapy:
• Persons who have clinical ASCVD
• Persons with LDL-C levels ≥ 190 mg/dL
• Persons ages 40 to 75 with diabetes and LDL-C levels of 70-189 mg/dL
• And (most pertinent to those with prediabetes) persons ages 40 to 75 who do not have clinical ASCVD or diabetes but have LDL-C levels of 70-189 mg/dL and an estimated 10-year ASCVD risk of 7.5% or higher.20
Based on risk stratification, treatment strategies such as statins are recommended to lower an individual’s risk for a future event. High-intensity statin therapy should be initiated in persons with a lifetime risk of 7.5% or higher.20
Hypertension. Metabolic syndrome has a strong relationship with the development of hypertension.10 Pharmacologic intervention for hypertension may be appropriate if lifestyle changes alone do not provide adequate control of blood pressure. Evidence-based guidelines for hypertension management were released in 2014 by the panel members appointed to the Eighth Joint National Committee (JNC 8).25 The JNC 8 guidelines include new, specific recommendations aimed at managing high blood pressure in adults.
For individuals ages 60 and older, pharmacologic therapy should be initiated at a blood pressure of 150/90 mm Hg or higher. For adults younger than 60 or those with comorbidities such as diabetes and chronic kidney disease, the guidelines recommend initiating pharmacologic therapy at a blood pressure of 140/90 mm Hg or higher.
Firstline drug recommendations vary among individuals. For nonblack adults, even those with diabetes, the recommended initial medications include a thiazide-type diuretic, calcium channel blocker, ACE inhibitor, or angiotensin receptor blocker. For the general black population, including those with diabetes, initial treatment with either a thiazide-type diuretic or calcium channel blocker is preferred.25
Continue for other interventions >>
Other interventions
Group-based educational interventions. Innovative methods are needed to successfully implement diabetes prevention measures in the general population. The ADA recommends diabetes self-management education to help those with prediabetes make behavioral changes.8
Education provided in a group setting using a patient-centered approach has been shown to be effective in improving health care outcomes and increasing self-management.8,29,34 Group-based diabetes education programs have also been found to be cost-effective over the long term.8,29 The ultimate goal of a group-based educational program is to improve the lifestyle practices of the participants through healthier eating and higher activity levels, thus preventing or delaying the onset of T2DM.
Culture-specific interventions. Health care providers should consider cultural variations when formulating treatment plans to improve disease outcomes.35 Patients often find it difficult to adopt a new lifestyle that they see as irrelevant to their cultural practices. For example, in one study, low-income Latina women did not consider cervical cancer screenings necessary due to absence of symptoms such as pain.35
Cultural influences are present in patients’ values and beliefs about disease processes, symptoms, prevention, treatment, self-management, and patient-provider relationships.35 Primary care providers must consider these values and beliefs, as well as their own, to avoid bias.
A culturally oriented approach to the management of prediabetes ensures that care is tailored to individual patients’ needs.36 Measures aimed at fostering culturally competent care include programs that provide culture-specific training for health care providers, use of language-appropriate patient education materials, recruitment of bicultural health care providers, and use of interpreter services.35 Culturally relevant care improves the success rate of sustained lifestyle changes in individuals at risk for chronic conditions such as diabetes.36
Surgical intervention. Bariatric surgery, reserved for high-risk individuals (BMI ≥ 35), has been shown to significantly decrease the incidence of T2DM in morbidly obese individuals.8 These benefits are probably related to the immediate metabolic changes that occur following the surgery, as well as the long-term sustained weight loss. For patients who undergo bariatric surgery, it is important to maintain regular follow-up to promote and maintain behavioral changes and identify barriers to a healthy lifestyle.
CONCLUSION
Clinicians must be able to effectively diagnose and treat prediabetes and metabolic syndrome. Due to its chronic nature and the resources needed to manage the disease and its complications, diabetes places an enormous burden on society. Efforts to prevent or delay the onset of diabetes are therefore essential to curb the costs and burden associated with this chronic disease.
While lifestyle changes are considered the mainstay of prediabetes and metabolic syndrome management, pharmacologic treatment may be considered in high-risk individuals. Patient education, though important, will not improve glycemic control or modify learned behaviors on its own. Health care providers should use a patient-centered approach to guide implementation of evidence-based guidelines in individuals with prediabetes and/or metabolic syndrome.8
Furthermore, innovative methods are needed to successfully implement diabetes and CVD prevention measures in the general population. Health care policies must be put in place to proactively encourage disease prevention rather than just focusing on acute care.37 It is important for NPs and PAs to devise viable strategies for the management of prediabetes and metabolic syndrome.
Environmental change must be made at the population level, incorporating lifestyle changes outside the health care system. There must be collaboration among various governmental and social organizations to ensure a society that promotes a healthy lifestyle.
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4-14.
2. American Association of Diabetes Educators. AADE position statement: primary prevention of type 2 diabetes. Diabetes Educ. 2012;38(1):147-150.
3. Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia—when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology (ACE) and the American Association of Clinical Endocrinologists (AACE). Endocr Pract. 2008;14(7):933-946.
4. American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):S81-S90.
6. DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-112.
7. Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279-2290.
8. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(1):S1-S94.
9. Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract. 2013;19(suppl 2):1-48. www.aace.com/files/algorithm-07-11-2013.pdf. Accessed September 18, 2015.
10. Masharani U. Diabetes mellitus & hypoglycemia. In: Papadakis MA, McPhee SJ, eds. Current Medical Diagnosis & Treatment 2014. 53rd ed. New York, NY: McGraw-Hill Education; 2014:1154-1159.
11. Cash JC, Hall M. Endocrine guidelines. In: Cash JC, Glass CA. Family Practice Guidelines. 3rd ed. New York, NY: Springer Publishing Company; 2014:649-651.
12. Reaven GM, Chen Y-DI, Jeppesen J, et al. Insulin resistance and hyperinsulinemia in individuals with small, dense, low density lipoprotein particles. J Clin Invest. 1993;92:141-146.
13. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008-3011.
14. Grundy SM, Brewer HB, Cleemen JI, et al; for the Conference Participants. Definition of metabolic syndrome. Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433-438.
15. National Heart, Lung, and Blood Institute. What is metabolic syndrome? www.nhlbi.nih.gov/health/health-topics/topics/ms. Accessed September 18, 2015.
16. Palaniappan LP, Wong EC, Shin JJ, et al. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond). 2011;35(3):393-400.
17. Park Y, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163(4):427-436.
18. Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115:1004-1011.
19. Boudi FB, Ahsan CH. Risk factors for coronary artery disease. MedScape. http://emedicine.medscape.com/article/164163-overview#a4. Accessed September 18, 2015.
20. Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
21. American College of Cardiology/American Heart Association. ASCVD risk estimator. http://tools.cardiosource.org/ASCVD-Risk-Estimator/. Accessed July 19, 2015.
22. CDC. Awareness of prediabetes—United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209-212.
23. Geiss LS, James C, Gregg EW, et al. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am J Prev Med. 2010;38:403-409.
24. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6): 393-403.
25. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
26. Hansen D, Dendale P, van Loon LJC, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med. 2010;40(11):921-940.
27. Tjonna AE, Nilsen TIL, Slordahl SA, et al. The association of metabolic clustering and physical activity with cardiovascular mortality: the HUNT study in Norway. J Epidemiol Community Health. 2010;64(8):690-695.
28. Kawahara T, Takahashi K, Inazu T, et al. Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2-day in-hospital diabetes educational program: the Joetsu Diabetes Prevention Trial. Diabetes Care. 2008;31(10):1949-1954.
29. Imai S, Kozai H, Naruse Y, et al. Randomized controlled trial of two forms of self-management group education in Japanese people with impaired glucose tolerance. J Clin Biochem Nutr. 2008;43(2):82-87.
30. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
31. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480-488.
32. Estruch R, Martínez-González MA, Corella D, et al; PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1-11.
33. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635-643.
34. Davis AM, Sawyer DR, Vinci LM. The potential of group visits in diabetes care. Clin Diabetes. 2008;26(2):58-62.
35. Shaw SJ, Huebner C, Armin J, et al. The role of culture in health literacy and chronic disease screening and management. J Immigr Minor Health. 2009;11(6):460-467.
36. Orzech KM, Vivian J, Huebner Torres C, et al. Diet and exercise adherence and practices among medically underserved patients with chronic disease: variation across four ethnic groups. Health Educ Behav. 2013;40(1): 56-66.
37. Narayan KM, Echouffo-Tcheugui J, Mohan V, Ali MK. Analysis & commentary: Global prevention and control of type 2 diabetes will require paradigm shifts in policies within and among countries. Health Aff. 2012;31(1):84-92.
1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4-14.
2. American Association of Diabetes Educators. AADE position statement: primary prevention of type 2 diabetes. Diabetes Educ. 2012;38(1):147-150.
3. Garber AJ, Handelsman Y, Einhorn D, et al. Diagnosis and management of prediabetes in the continuum of hyperglycemia—when do the risks of diabetes begin? A consensus statement from the American College of Endocrinology (ACE) and the American Association of Clinical Endocrinologists (AACE). Endocr Pract. 2008;14(7):933-946.
4. American Diabetes Association. Economic costs of diabetes in the US in 2012. Diabetes Care. 2013;36(4):1033-1046.
5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(1):S81-S90.
6. DeFronzo RA, Triplitt CL, Abdul-Ghani M, Cersosimo E. Novel agents for the treatment of type 2 diabetes. Diabetes Spectr. 2014;27(2):100-112.
7. Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279-2290.
8. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(1):S1-S94.
9. Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Endocrinologists’ comprehensive diabetes management algorithm 2013 consensus statement. Endocr Pract. 2013;19(suppl 2):1-48. www.aace.com/files/algorithm-07-11-2013.pdf. Accessed September 18, 2015.
10. Masharani U. Diabetes mellitus & hypoglycemia. In: Papadakis MA, McPhee SJ, eds. Current Medical Diagnosis & Treatment 2014. 53rd ed. New York, NY: McGraw-Hill Education; 2014:1154-1159.
11. Cash JC, Hall M. Endocrine guidelines. In: Cash JC, Glass CA. Family Practice Guidelines. 3rd ed. New York, NY: Springer Publishing Company; 2014:649-651.
12. Reaven GM, Chen Y-DI, Jeppesen J, et al. Insulin resistance and hyperinsulinemia in individuals with small, dense, low density lipoprotein particles. J Clin Invest. 1993;92:141-146.
13. Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266(21):3008-3011.
14. Grundy SM, Brewer HB, Cleemen JI, et al; for the Conference Participants. Definition of metabolic syndrome. Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433-438.
15. National Heart, Lung, and Blood Institute. What is metabolic syndrome? www.nhlbi.nih.gov/health/health-topics/topics/ms. Accessed September 18, 2015.
16. Palaniappan LP, Wong EC, Shin JJ, et al. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond). 2011;35(3):393-400.
17. Park Y, Zhu S, Palaniappan L, et al. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163(4):427-436.
18. Lloyd-Jones DM, Liu K, Colangelo LA, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation. 2007;115:1004-1011.
19. Boudi FB, Ahsan CH. Risk factors for coronary artery disease. MedScape. http://emedicine.medscape.com/article/164163-overview#a4. Accessed September 18, 2015.
20. Stone NJ, Robinson J, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
21. American College of Cardiology/American Heart Association. ASCVD risk estimator. http://tools.cardiosource.org/ASCVD-Risk-Estimator/. Accessed July 19, 2015.
22. CDC. Awareness of prediabetes—United States, 2005-2010. MMWR Morb Mortal Wkly Rep. 2013;62(11):209-212.
23. Geiss LS, James C, Gregg EW, et al. Diabetes risk reduction behaviors among U.S. adults with prediabetes. Am J Prev Med. 2010;38:403-409.
24. Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6): 393-403.
25. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
26. Hansen D, Dendale P, van Loon LJC, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med. 2010;40(11):921-940.
27. Tjonna AE, Nilsen TIL, Slordahl SA, et al. The association of metabolic clustering and physical activity with cardiovascular mortality: the HUNT study in Norway. J Epidemiol Community Health. 2010;64(8):690-695.
28. Kawahara T, Takahashi K, Inazu T, et al. Reduced progression to type 2 diabetes from impaired glucose tolerance after a 2-day in-hospital diabetes educational program: the Joetsu Diabetes Prevention Trial. Diabetes Care. 2008;31(10):1949-1954.
29. Imai S, Kozai H, Naruse Y, et al. Randomized controlled trial of two forms of self-management group education in Japanese people with impaired glucose tolerance. J Clin Biochem Nutr. 2008;43(2):82-87.
30. Eckel RH, Jakicic JM, Ard JD, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S76-S99.
31. Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116(5):480-488.
32. Estruch R, Martínez-González MA, Corella D, et al; PREDIMED Study Investigators. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1-11.
33. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59(7):635-643.
34. Davis AM, Sawyer DR, Vinci LM. The potential of group visits in diabetes care. Clin Diabetes. 2008;26(2):58-62.
35. Shaw SJ, Huebner C, Armin J, et al. The role of culture in health literacy and chronic disease screening and management. J Immigr Minor Health. 2009;11(6):460-467.
36. Orzech KM, Vivian J, Huebner Torres C, et al. Diet and exercise adherence and practices among medically underserved patients with chronic disease: variation across four ethnic groups. Health Educ Behav. 2013;40(1): 56-66.
37. Narayan KM, Echouffo-Tcheugui J, Mohan V, Ali MK. Analysis & commentary: Global prevention and control of type 2 diabetes will require paradigm shifts in policies within and among countries. Health Aff. 2012;31(1):84-92.
Ebola: Lessons from the Latest Pandemic
CE/CME No: CR-1509
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients at high risk for exposure to and contraction of Ebola virus disease based on patient history, physical exam, and laboratory findings.
• Respond appropriately to high-risk patients by utilizing personal protective equipment, employing isolation strategies, and immediately reporting cases to hospital infection control and local health departments.
• Reassure domestic patients about the low risk of contracting Ebola virus in the United States.
FACULTY
Catherine B. Silver, Erin L. Leon, and Sarah A. Zaino are recent graduates of the Pace University Physician Assistant Program in New York City, where Ellen D. Mandel is Clinical Professor.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of September 2015.
Article begins on next page >>
The 2014 re-emergence of Ebola virus disease (EVD) quickly became the largest and deadliest outbreak of the disease ever recorded. Originating in Guinea, it spread to neighboring countries and others around the globe. As potentially the firstline health care contacts during a pandemic, all primary care providers need to be aware of the signs and symptoms of EVD so that they can quickly identify, isolate, and treat affected patients. This article describes the history, pathophysiology, diagnosis, and treatment of the disease.
In 1918, influenza virus—in the most deadly pandemic in the past century—killed an estimated 20 to 50 million people worldwide.1 A more recent example of a devastating pandemic that is still sweeping the globe with high morbidity and mortality is HIV/AIDS. According to the World Health Organization (WHO), in 2013, 35 million people were living with HIV/AIDS worldwide and 1.5 million people died from HIV/AIDS–related illnesses.2 Similarly, emerging respiratory infectious diseases such as avian influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and H7N9 influenza have all been named as possible threats due to their high fatality rates.3
In 1999, the WHO created a preparedness plan for pandemic influenza (updated in 2005) to provide information on reducing the risk for infection and informing government and health care organizations of proper outbreak response.3 The virulence and high mortality associated with Ebola virus disease (EVD) necessitate a similarly detailed preparedness plan, including international collaboration and commitment to providing research, training, support, and personnel to combat the current outbreak and prevent future outbreaks (see “‘Present’: Ebola's Impact on PAs in Liberia” for an interview with the President of the Liberia National Physician Assistants Association).
All primary care providers (PCPs) need to be aware of the signs and symptoms of EVD so that they can properly identify suspected cases, take necessary precautions to avoid transmission, and quickly transfer patients to facilities equipped to provide isolation and appropriate supportive treatment. PCPs may be the first providers to come into contact with patients infected with a pathogen during a pandemic, especially if the initial symptoms are mild enough not to warrant a visit to the emergency department. In 2003, the first case of SARS was diagnosed and treated by a Canadian family physician, and the initial case of H1N1 in Japan during the 2009 pandemic was first seen by a family physician as well.3
If PCPs are not sufficiently prepared to deal with a patient exhibiting signs or symptoms of EVD, it is likely that they, their staff, and other patients will be at greater risk for contraction and transmission. It has been found that PCPs are less likely to be prepared for dealing with pandemics, especially since high-level personal protective equipment (PPE)—eg, N95 masks, gowns, eye protection—are stocked at a lower rate in outpatient clinics than in hospitals.3 PCPs should prepare by familiarizing themselves with the signs and symptoms of EVD and by stocking high-level PPE.
In March 2014, the WHO received reports of a developing epidemic of EVD in Guinea in West Africa. The outbreak started in two districts of that country during December 2013; from there, it spread to Liberia and Sierra Leone, with scattered cases in Nigeria, Mali, Senegal, Spain, the United Kingdom, and the United States, making it the largest EVD epidemic ever recorded. The outbreak’s morbidity and mortality surpass that of all previous EVD epidemics in the past 38 years combined.4 As of August 19, 2015, there have been 15,188 laboratory-confirmed cases (the total number of cases is estimated at 28,000) and 11,286 deaths in the current epidemic.5
The first recorded outbreak of EVD occurred in 1976 in a village called Yambuku, located near the Ebola River in Zaire (now known as the Democratic Republic of Congo). At that time, a team of scientists from the CDC was sent to Zaire to identify the agent responsible for a deadly hemorrhagic fever that was ravaging the local hospital.6 This and subsequent outbreaks in central Africa were contained due to rapid coordinated efforts to stop the spread of the disease through a number of strategies.
Among these strategies, quick diagnosis, isolation of contacts, and quarantine of the greater area played a significant role in stemming the outbreak.4 Additional steps that helped curb disease spread included rapid burials with disinfectants and home visits by health workers, with patient education provided to help to assuage any fear villagers may have had of foreign health workers.6 Finally, health workers and surveillance teams were provided with PPE and were encouraged to continue their work despite the outbreak, with the promise that they would receive treatment equal to that given to foreign aid workers if they too fell ill.6 Each of these measures utilized in tandem allowed for control of the initial outbreak.
Despite being similar to previous outbreaks in terms of transmission rate, incubation period, fatality rate, and estimated basic reproduction number (R0, the estimated number of people infected by a single patient), the number of persons affected by the current epidemic eclipses any previous outbreak. Thus, political, economic, and social issues, rather than biologic characteristics, have made this epidemic the largest in history.4 The lack of medical infrastructure in the most severely affected nations has hindered efforts to provide care to those infected, and the number of patients requiring medical treatment vastly exceeds the number of hospital beds available.4
The WHO estimates that cutting transmission rates by 50% through the rapid and rigorous employment of sophisticated infection-control practices will halt the growth of the epidemic and eventually eradicate the virus from the human population.4 There is, however, the danger that if control measures are not implemented soon, EVD will become endemic in West Africa.4 In the US, early recognition, a well-informed public, and advanced medical infrastructure will allow for quick identification and containment of the virus. Public awareness, especially among health workers, is essential to stopping the epidemic’s spread.
Continue for pathophysiology and transmission >>
PATHOPHYSIOLOGY AND TRANSMISSION
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae.8 Five viruses of the Ebolavirus genus have been described: EBOV, Tai Forest virus, Reston virus, Sudan virus, and Bundibugyo virus. Except for Reston virus, each of these viruses causes hemorrhagic fever with high mortality.
EVD is a zoonotic disease, meaning that outbreaks typically begin by passage of the virus from an animal vector to a human host. In this case, it is thought that the viral reservoir consists of several species of fruit- and insect-eating bats native to West Africa.8,9 The vector that transmits the virus from bats to humans is not well understood, but reports name nonhuman primates (NHP) and pigs as possible culprits.8 Though EBOV is not typically transmitted through food, the practice of consuming “bushmeat”—hunted wild animals such as bats, monkeys, and rodents—has been linked to transmission of the virus. According to the CDC, the mechanism for this mode of transmission is likely through the butchering and processing of infected animals.10 It is important to note that only wild animals hunted in endemic regions of Africa carry the risk for transmission. To date, there have been no reports of EBOV transmission via contact with any animal, wild or domestic, in this country.
Once the virus has infected a human host, transmission of the disease continues from person to person via contact with infected bodily fluids. The three main modalities of virus transmission in underdeveloped countries include nosocomial transmission (improper sterilization techniques), funeral preparation, and community transmission.11 The most infectious substances are blood, feces, and vomit, but the virus has also been found in saliva, tears, breast milk, sweat, urine, and semen.12
Though controversial, evidence now suggests that EBOV can survive in semen for more than three months, even in patients who have fully recovered from the disease.13 To prevent sexually transmitted EBOV exposure, the WHO recommends that convalescent EVD patients use barrier methods such as condoms and female condoms to prevent the exchange of bodily fluids during sexual activity.13
Like other pathogens requiring droplet precautions, EBOV can only enter an uninfected individual through nonintact skin or mucous membranes, or parenterally. Transmission may also take place via fomites, or contaminated surfaces and objects which have not been properly sanitized.12 Studies suggest that the virus cannot survive on fomites for extended periods at room temperature; however, when refrigerated to 40°F, EBOV survived for more than three weeks.14 The incubation period for EBOV ranges from two to 21 days, with an average of 11 days.8
Current research indicates that the virus is not transmissible until symptoms appear, and therefore, infected patients are not contagious during the incubation period.15 The amount of EBOV in body fluid is referred to as viral load and has been determined to be a contributing factor in the transmission of the virus. As the viral load rises, symptoms worsen and the patient becomes more contagious.16 Patients with EVD are most contagious in the later stages of the disease (when viral load is highest) and shortly after death.16
With the recent infection of health care workers in Spain and Texas, there has also developed public concern regarding the possibility of contracting EBOV infection from pets. Currently, the CDC has no documented cases of domesticated animals contracting EVD or spreading the virus.17 Nonetheless, any pets in the home of EVD patients will be evaluated and managed by local health officials (via quarantine, surveillance, and possible euthanasia).17 In Spain, a nurses’ aide infected with the disease lost the fight to keep her dog, and health officials euthanized the 12-year-old mixed breed while his owner was in quarantine.18 By contrast, the King Charles Spaniel of Texas nurse Nina Pham was quarantined for three weeks and later reunited with his family.19 The divergent treatment of pets in the two cases illustrates how public concern about EVD ultimately influences decision-making.
Detailed study of the pathophysiology of EVD is difficult due to the virulence of EBOV and its high mortality, which are reflected by its classification as a biosafety level 4 (BSL-4) organism. Handling and study of organisms with BSL-4 designation require sophisticated laboratory equipment and advanced safety technology only available in developed countries. Further, ethical concerns dictate that the virus be studied in animal models rather than in humans. As such, mouse, guinea pig, and NHP models provide most of the available data.
EBOV evades immune system detection and destruction because of its extensively glycosylated lipid bilayer envelope.8 Once inside a suitable host, the virus reproduces by hijacking immune cells: monocytes, macrophages, and dendritic cells. Simultaneously, infection incites large-scale inflammation via cytokines, lymphocyte apoptosis resulting in lymphopenia, inhibition of innate and acquired humoral and cellular immune responses, and disruption of the clotting cascade.8
In later stages of infection, EBOV targets hepatocytes and endothelial cells.8 Liver dysfunction leads to interruption of clotting factor production, thus causing coagulopathy. Endothelial dysfunction is responsible for “leakage” of blood from vessels into skin, mucous membranes, and the gastrointestinal tract.8
Continue for the diagnosis >>
DIAGNOSIS
Patient history
To be diagnosed with EVD, patients must have a history of travel to an EBOV-affected region in the previous 21 days.20 Of particular importance in the US is gathering an accurate travel history from potential EBOV patients. According to the CDC, countries affected by the outbreak include Guinea, Liberia, and Sierra Leone; countries with travel-related cases include Nigeria, Spain, the US, the UK, Mali, and Senegal.21 Practitioners abroad should inquire about patients’ encounters with body fluid of infected individuals, contact with contaminated objects, and interaction with infected animals.
Physical exam
Initial symptoms are nonspecific, with a classic viral prodrome of fever, chills, muscle aches, and general malaise.8 Stage two is characterized by abdominal pain, nausea, vomiting, and diarrhea.8 In the final hemorrhagic stage of the disease, clotting dysfunction leads to subcutaneous and internal bleeding (epistaxis, petechiae, ecchymoses, hematochezia, and melena) and conjunctival hemorrhage.8,22 In this terminal stage of EVD, extreme blood loss causes organ failure, disseminated intravascular coagulation, shock, and death.8
Laboratory testing
Several methods of laboratory diagnosis exist, but all testing must be performed several days after the onset of symptoms; thus, patients with suspected EVD should remain isolated pending test results. At the outset of symptoms, the following laboratory diagnostic tests may be used to determine whether a patient is infected with EBOV:
• Antigen-capture enzyme-linked immunosorbent assay (ELISA)
• Immunoglobulin (IgM) ELISA
• Polymerase chain reaction (PCR)
• Virus isolation.23
IgM and IgG antibodies may be isolated from patients who have recovered from the disease.23 Finally, postmortem testing may be done via immunohistochemistry testing, PCR, or virus isolation.23 The CDC standard is IgG ELISA, which has 93% sensitivity and 98% specificity for EBOV antibody detection (see Table 1).24
Though not definitive, routine laboratory tests may support an EVD diagnosis. The complete blood count of a person with EVD reveals evidence of thrombocytopenia, leukopenia, and lymphopenia.8 Viral attack on hepatocytes results in elevated alanine aminotransferase and aspartate aminotransferase levels, while coagulopathy is reflected by elevated thrombin and prothrombin times (see Table 2).8 A drawback to any type of testing is that it requires advanced technology and safety precautions that are not widely available in the underdeveloped countries where the outbreak is currently taking place.8
Reporting
The CDC recommends immediate isolation of suspected EVD patients and the employment of standard, contact, and droplet precautions, including the use of gowns, gloves, masks, and face protection. Once the patient has been isolated, health care providers should notify their hospital’s Infection Control Program and immediately contact their local health department.20
Treatment
At present, the standard treatment for EVD is supportive care. The CDC recommends the use of IV fluid hydration and the maintenance of electrolytes, oxygen status, and blood pressure, as well as the treatment of any concurrent infection.25 These supportive measures, though noncurative, appear to significantly reduce mortality.
Another proposed treatment for EVD is transfusion of whole blood or plasma from recovered patients in the convalescent phase of infection. Through this technique, patients with early EVD benefit from the effective immune response of recovered individuals via passive immunization. Per WHO recommendations, only patients who have tested negative for EVD twice and have been out of the hospital for 28 days are eligible as potential donors.26 As with all blood product transfusions, the blood of the donor and the recipient must be typed and screened for compatibility.
No vaccines for the prevention of EVD have been approved by the FDA, but several vaccines are undergoing extensive research. Among them are prevaccines and postvaccines. Prevaccines, also known as preventive vaccines, are designed to be administered prior to pathogen exposure. Postvaccines, also referred to as therapeutic vaccines, are used after a person has sustained pathogen exposure, with the goal of stimulating the patient’s immune system to fight the infection.8
EVD vaccines are categorized into two classes: replicating and nonreplicating. Currently available replicating vaccines include recombinant vesicular stomatitis virus, recombinant human parainfluenza virus type 3, rabies virus, and cytomegalovirus.27 Nonreplicating vaccines include inactivated vaccines, replicons, DNA vaccines, recombinant adenoviruses, subunit vaccines, and replication-deficient ebola viruses.27
One prevaccine in particular, the recombinant adenovirus, has produced positive results in providing vaccine protection in NHPs. This vaccine is capable of protecting against multiple strains of ebola viruses, but because the vaccine is based on adenovirus serotype 5, for which a large proportion of the human population has immunity, its overall efficacy is significantly reduced.8,27 Significant progress has been made with the therapeutic vaccine ZMapp in the treatment of EVD in NHPs. ZMapp is a combination of three monoclonal antibodies that, when administered to an infected NHP, cling to the virus and prevent it from further invading healthy cells.8 Because this vaccine has not yet undergone human trials and is still in early experimental stages, special permission from the FDA is required to obtain it.8
Finally, researchers are optimistic that AVI-7357, an antiviral in late stages of clinical trials, will be an effective therapeutic agent for EVD. Its mechanism of action is thought to be inhibition of the VP24 protein; this viral protein is thought to play a role in the switch from viral replication to transcription, and blocking it is believed to effectively obstruct replication of the virus.28 Although much research is underway in the treatment of EVD, none of the proposed treatments has met the standards of FDA approval.
Continue for the prognosis >>
PROGNOSIS
Of the five identified ebola virus species, each differs in its virulence, morbidity, mortality, and prognosis. The mildest species is the nonfatal Reston ebolavirus, which is found in Asia and apparently causes asymptomatic infection in humans. Bundibugyo ebolavirus has a mortality rate of less than 40%, while Sudan ebolavirus has a mortality rate of about 50%.29 The mortality rate of Tai Forest ebolavirus is unknown because there has been only one recorded case of human infection. The current outbreak is caused by a strain of Zaire ebolavirus, which has the highest mortality rate at 70% to 90% (see Table 3).29
Despite the differing mortality rates among the ebolaviruses, fatality rate also depends on factors beyond the biologic characteristics of the species of ebolavirus responsible for the infection. According to WHO data collected during the first nine months of the current epidemic, the fatality rate among hospitalized patients in Liberia, Guinea, and Sierra Leone is 64.3%, lower than the average fatality rate of 70.8% in these countries.4 This data, however, represents only patients treated in the affected countries in Africa.
Given the lack of medical and governmental infrastructure in the nations where the research took place, it can be assumed that better, faster diagnosis and supportive treatment could increase survival in countries with robust health care systems, such as those in the US and Europe. In addition, demographic factors such as age affect mortality, with older age (> 45) carrying a worse prognosis.4 Other risk factors for increased mortality include general symptoms such as diarrhea, conjunctivitis, dyspnea, dysphagia, confusion, and unconsciousness or coma, as well as hemorrhagic symptoms.4
Due to a lack of health care infrastructure in affected West African nations, patients with EVD are receiving insufficient supportive treatment. In order to increase survival, it is essential to treat hypovolemia and electrolyte imbalance with therapies such as IV fluids and electrolyte repletion.30 All health care providers must be encouraged to use every tool at their disposal for providing supportive care for patients with EVD.
CONCLUSION
The US has a robust health care system capable of providing the training and resources necessary for containing outbreaks of diseases like EVD. Recognition of this can help to maintain public calm in the event of a full-scale epidemic of EVD in the US (however unlikely this may be). EVD is highly transmissible in its symptomatic stages, and recent cases in Texas and New York illustrate the need for PCPs and hospitals to be on alert for patients with possible exposure. Similarly, patient care teams must work together, exercise effective communication, and utilize pre-established plans for identification, isolation, and treatment in epidemics. Patients exhibiting fever and other signs and symptoms of EBOV must be asked about any recent travel to Liberia, Sierra Leone, and Guinea, and if they have had any contact with sick persons prior to their symptoms. Health care workers play an important role in epidemic control. As such, they should be familiar with risks, precautions, and protocols set forth by the WHO, CDC, and local health authorities.
1. CDC. Reconstruction of the 1918 influenza pandemic virus. www.cdc.gov/flu/about/qa/1918flupandemic.htm. Accessed August 24, 2015.
2. World Health Organization. Global Health Observatory (GHO) data: HIV/AIDS. www.who.int/gho/hiv/en/. Accessed August 24, 2015.
3. Tomizuka T, Kanatani Y, Kawahara K. Insufficient preparedness of primary care practices for pandemic influenza and the effect of a preparedness plan in Japan: a prefecture-wide cross-sectional study. BMC Fam Pract. 2013;14:174.
4. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481-1495.
5. CDC. 2014 Ebola outbreak in West Africa. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed June 11, 2015.
6. Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663-1666.
7. Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014;25(3):128-129.
8. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1-9.
9. Saez AM, Weiss S, Nowak K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7(1):17-23.
10. CDC. Facts about Ebola and bushmeat. www.cdc.gov/vhf/ebola/pdf/bushmeat-and-ebola.pdf. Accessed August 24, 2015.
11. MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6(6):e1546.
12. World Health Organization. What we know about transmission of the Ebola virus among humans. www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed August 24, 2015.
13. World Health Organization. Sexual transmission of the Ebola Virus: evidence and knowledge gaps. www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed August 24, 2015.
14. Piercy TJ, Smither SJ, Steward JA, et al. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109(5):1531-1539.
15. Ki M. What do we really fear? The epidemiological characteristics of Ebola and our preparedness. Epidemiol Health. 2014;36:e2014014.
16. Yamin D, Gertler S, Ndeffo-Mbah ML, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162(1):11-17.
17. CDC. Questions and answers about Ebola and pets. www.cdc.gov/vhf/ebola/transmission/qas-pets.html. Accessed August 24, 2015.
18. Wilson J. ‘Save Excalibur’ fails: Madrid euthanizes Ebola patient’s dog. CNN. www.cnn.com/2014/10/08/health/save-excalibur-ebola-dog/ Accessed June 12, 2015.
19. Serjeant J. New York doctor with Ebola improves, nurse reunited with dog. Reuters. www.reuters.com/article/2014/11/01/us-health-ebola-usa-idUSKBN0II1SP20141101. Accessed August 24, 2015.
20. CDC. Ebola virus disease (Ebola) algorithm for evaluation of the returned traveler. www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf. Accessed August 24, 2015.
21. CDC. 2014 Ebola outbreak in West Africa: outbreak distribution map. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Accessed August 24, 2015.
22. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(pt 8):1619-1624.
23. CDC. Ebola virus disease: diagnosis. www.cdc.gov/vhf/ebola/diagnosis/. Accessed August 24, 2015.
24. Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol. 2001;39(1):1-7.
25. CDC. Ebola (Ebola Virus Disease). www.cdc.gov/vhf/ebola/treatment/. Accessed August 24, 2015.
26. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Version 1.0. September 2014. Accessed August 24, 2015.
27. Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859-872.
28. Iversen PL, Warren TK, Wells JB, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806-2830.
29. Feldmann H. Ebola—a growing threat? N Engl J Med. 2014;371(15):1375-1378.
30. Lamontagne F, Clement C, Fletcher T, et al. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014; 371(17):1565-1566.
CE/CME No: CR-1509
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients at high risk for exposure to and contraction of Ebola virus disease based on patient history, physical exam, and laboratory findings.
• Respond appropriately to high-risk patients by utilizing personal protective equipment, employing isolation strategies, and immediately reporting cases to hospital infection control and local health departments.
• Reassure domestic patients about the low risk of contracting Ebola virus in the United States.
FACULTY
Catherine B. Silver, Erin L. Leon, and Sarah A. Zaino are recent graduates of the Pace University Physician Assistant Program in New York City, where Ellen D. Mandel is Clinical Professor.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of September 2015.
Article begins on next page >>
The 2014 re-emergence of Ebola virus disease (EVD) quickly became the largest and deadliest outbreak of the disease ever recorded. Originating in Guinea, it spread to neighboring countries and others around the globe. As potentially the firstline health care contacts during a pandemic, all primary care providers need to be aware of the signs and symptoms of EVD so that they can quickly identify, isolate, and treat affected patients. This article describes the history, pathophysiology, diagnosis, and treatment of the disease.
In 1918, influenza virus—in the most deadly pandemic in the past century—killed an estimated 20 to 50 million people worldwide.1 A more recent example of a devastating pandemic that is still sweeping the globe with high morbidity and mortality is HIV/AIDS. According to the World Health Organization (WHO), in 2013, 35 million people were living with HIV/AIDS worldwide and 1.5 million people died from HIV/AIDS–related illnesses.2 Similarly, emerging respiratory infectious diseases such as avian influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and H7N9 influenza have all been named as possible threats due to their high fatality rates.3
In 1999, the WHO created a preparedness plan for pandemic influenza (updated in 2005) to provide information on reducing the risk for infection and informing government and health care organizations of proper outbreak response.3 The virulence and high mortality associated with Ebola virus disease (EVD) necessitate a similarly detailed preparedness plan, including international collaboration and commitment to providing research, training, support, and personnel to combat the current outbreak and prevent future outbreaks (see “‘Present’: Ebola's Impact on PAs in Liberia” for an interview with the President of the Liberia National Physician Assistants Association).
All primary care providers (PCPs) need to be aware of the signs and symptoms of EVD so that they can properly identify suspected cases, take necessary precautions to avoid transmission, and quickly transfer patients to facilities equipped to provide isolation and appropriate supportive treatment. PCPs may be the first providers to come into contact with patients infected with a pathogen during a pandemic, especially if the initial symptoms are mild enough not to warrant a visit to the emergency department. In 2003, the first case of SARS was diagnosed and treated by a Canadian family physician, and the initial case of H1N1 in Japan during the 2009 pandemic was first seen by a family physician as well.3
If PCPs are not sufficiently prepared to deal with a patient exhibiting signs or symptoms of EVD, it is likely that they, their staff, and other patients will be at greater risk for contraction and transmission. It has been found that PCPs are less likely to be prepared for dealing with pandemics, especially since high-level personal protective equipment (PPE)—eg, N95 masks, gowns, eye protection—are stocked at a lower rate in outpatient clinics than in hospitals.3 PCPs should prepare by familiarizing themselves with the signs and symptoms of EVD and by stocking high-level PPE.
In March 2014, the WHO received reports of a developing epidemic of EVD in Guinea in West Africa. The outbreak started in two districts of that country during December 2013; from there, it spread to Liberia and Sierra Leone, with scattered cases in Nigeria, Mali, Senegal, Spain, the United Kingdom, and the United States, making it the largest EVD epidemic ever recorded. The outbreak’s morbidity and mortality surpass that of all previous EVD epidemics in the past 38 years combined.4 As of August 19, 2015, there have been 15,188 laboratory-confirmed cases (the total number of cases is estimated at 28,000) and 11,286 deaths in the current epidemic.5
The first recorded outbreak of EVD occurred in 1976 in a village called Yambuku, located near the Ebola River in Zaire (now known as the Democratic Republic of Congo). At that time, a team of scientists from the CDC was sent to Zaire to identify the agent responsible for a deadly hemorrhagic fever that was ravaging the local hospital.6 This and subsequent outbreaks in central Africa were contained due to rapid coordinated efforts to stop the spread of the disease through a number of strategies.
Among these strategies, quick diagnosis, isolation of contacts, and quarantine of the greater area played a significant role in stemming the outbreak.4 Additional steps that helped curb disease spread included rapid burials with disinfectants and home visits by health workers, with patient education provided to help to assuage any fear villagers may have had of foreign health workers.6 Finally, health workers and surveillance teams were provided with PPE and were encouraged to continue their work despite the outbreak, with the promise that they would receive treatment equal to that given to foreign aid workers if they too fell ill.6 Each of these measures utilized in tandem allowed for control of the initial outbreak.
Despite being similar to previous outbreaks in terms of transmission rate, incubation period, fatality rate, and estimated basic reproduction number (R0, the estimated number of people infected by a single patient), the number of persons affected by the current epidemic eclipses any previous outbreak. Thus, political, economic, and social issues, rather than biologic characteristics, have made this epidemic the largest in history.4 The lack of medical infrastructure in the most severely affected nations has hindered efforts to provide care to those infected, and the number of patients requiring medical treatment vastly exceeds the number of hospital beds available.4
The WHO estimates that cutting transmission rates by 50% through the rapid and rigorous employment of sophisticated infection-control practices will halt the growth of the epidemic and eventually eradicate the virus from the human population.4 There is, however, the danger that if control measures are not implemented soon, EVD will become endemic in West Africa.4 In the US, early recognition, a well-informed public, and advanced medical infrastructure will allow for quick identification and containment of the virus. Public awareness, especially among health workers, is essential to stopping the epidemic’s spread.
Continue for pathophysiology and transmission >>
PATHOPHYSIOLOGY AND TRANSMISSION
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae.8 Five viruses of the Ebolavirus genus have been described: EBOV, Tai Forest virus, Reston virus, Sudan virus, and Bundibugyo virus. Except for Reston virus, each of these viruses causes hemorrhagic fever with high mortality.
EVD is a zoonotic disease, meaning that outbreaks typically begin by passage of the virus from an animal vector to a human host. In this case, it is thought that the viral reservoir consists of several species of fruit- and insect-eating bats native to West Africa.8,9 The vector that transmits the virus from bats to humans is not well understood, but reports name nonhuman primates (NHP) and pigs as possible culprits.8 Though EBOV is not typically transmitted through food, the practice of consuming “bushmeat”—hunted wild animals such as bats, monkeys, and rodents—has been linked to transmission of the virus. According to the CDC, the mechanism for this mode of transmission is likely through the butchering and processing of infected animals.10 It is important to note that only wild animals hunted in endemic regions of Africa carry the risk for transmission. To date, there have been no reports of EBOV transmission via contact with any animal, wild or domestic, in this country.
Once the virus has infected a human host, transmission of the disease continues from person to person via contact with infected bodily fluids. The three main modalities of virus transmission in underdeveloped countries include nosocomial transmission (improper sterilization techniques), funeral preparation, and community transmission.11 The most infectious substances are blood, feces, and vomit, but the virus has also been found in saliva, tears, breast milk, sweat, urine, and semen.12
Though controversial, evidence now suggests that EBOV can survive in semen for more than three months, even in patients who have fully recovered from the disease.13 To prevent sexually transmitted EBOV exposure, the WHO recommends that convalescent EVD patients use barrier methods such as condoms and female condoms to prevent the exchange of bodily fluids during sexual activity.13
Like other pathogens requiring droplet precautions, EBOV can only enter an uninfected individual through nonintact skin or mucous membranes, or parenterally. Transmission may also take place via fomites, or contaminated surfaces and objects which have not been properly sanitized.12 Studies suggest that the virus cannot survive on fomites for extended periods at room temperature; however, when refrigerated to 40°F, EBOV survived for more than three weeks.14 The incubation period for EBOV ranges from two to 21 days, with an average of 11 days.8
Current research indicates that the virus is not transmissible until symptoms appear, and therefore, infected patients are not contagious during the incubation period.15 The amount of EBOV in body fluid is referred to as viral load and has been determined to be a contributing factor in the transmission of the virus. As the viral load rises, symptoms worsen and the patient becomes more contagious.16 Patients with EVD are most contagious in the later stages of the disease (when viral load is highest) and shortly after death.16
With the recent infection of health care workers in Spain and Texas, there has also developed public concern regarding the possibility of contracting EBOV infection from pets. Currently, the CDC has no documented cases of domesticated animals contracting EVD or spreading the virus.17 Nonetheless, any pets in the home of EVD patients will be evaluated and managed by local health officials (via quarantine, surveillance, and possible euthanasia).17 In Spain, a nurses’ aide infected with the disease lost the fight to keep her dog, and health officials euthanized the 12-year-old mixed breed while his owner was in quarantine.18 By contrast, the King Charles Spaniel of Texas nurse Nina Pham was quarantined for three weeks and later reunited with his family.19 The divergent treatment of pets in the two cases illustrates how public concern about EVD ultimately influences decision-making.
Detailed study of the pathophysiology of EVD is difficult due to the virulence of EBOV and its high mortality, which are reflected by its classification as a biosafety level 4 (BSL-4) organism. Handling and study of organisms with BSL-4 designation require sophisticated laboratory equipment and advanced safety technology only available in developed countries. Further, ethical concerns dictate that the virus be studied in animal models rather than in humans. As such, mouse, guinea pig, and NHP models provide most of the available data.
EBOV evades immune system detection and destruction because of its extensively glycosylated lipid bilayer envelope.8 Once inside a suitable host, the virus reproduces by hijacking immune cells: monocytes, macrophages, and dendritic cells. Simultaneously, infection incites large-scale inflammation via cytokines, lymphocyte apoptosis resulting in lymphopenia, inhibition of innate and acquired humoral and cellular immune responses, and disruption of the clotting cascade.8
In later stages of infection, EBOV targets hepatocytes and endothelial cells.8 Liver dysfunction leads to interruption of clotting factor production, thus causing coagulopathy. Endothelial dysfunction is responsible for “leakage” of blood from vessels into skin, mucous membranes, and the gastrointestinal tract.8
Continue for the diagnosis >>
DIAGNOSIS
Patient history
To be diagnosed with EVD, patients must have a history of travel to an EBOV-affected region in the previous 21 days.20 Of particular importance in the US is gathering an accurate travel history from potential EBOV patients. According to the CDC, countries affected by the outbreak include Guinea, Liberia, and Sierra Leone; countries with travel-related cases include Nigeria, Spain, the US, the UK, Mali, and Senegal.21 Practitioners abroad should inquire about patients’ encounters with body fluid of infected individuals, contact with contaminated objects, and interaction with infected animals.
Physical exam
Initial symptoms are nonspecific, with a classic viral prodrome of fever, chills, muscle aches, and general malaise.8 Stage two is characterized by abdominal pain, nausea, vomiting, and diarrhea.8 In the final hemorrhagic stage of the disease, clotting dysfunction leads to subcutaneous and internal bleeding (epistaxis, petechiae, ecchymoses, hematochezia, and melena) and conjunctival hemorrhage.8,22 In this terminal stage of EVD, extreme blood loss causes organ failure, disseminated intravascular coagulation, shock, and death.8
Laboratory testing
Several methods of laboratory diagnosis exist, but all testing must be performed several days after the onset of symptoms; thus, patients with suspected EVD should remain isolated pending test results. At the outset of symptoms, the following laboratory diagnostic tests may be used to determine whether a patient is infected with EBOV:
• Antigen-capture enzyme-linked immunosorbent assay (ELISA)
• Immunoglobulin (IgM) ELISA
• Polymerase chain reaction (PCR)
• Virus isolation.23
IgM and IgG antibodies may be isolated from patients who have recovered from the disease.23 Finally, postmortem testing may be done via immunohistochemistry testing, PCR, or virus isolation.23 The CDC standard is IgG ELISA, which has 93% sensitivity and 98% specificity for EBOV antibody detection (see Table 1).24
Though not definitive, routine laboratory tests may support an EVD diagnosis. The complete blood count of a person with EVD reveals evidence of thrombocytopenia, leukopenia, and lymphopenia.8 Viral attack on hepatocytes results in elevated alanine aminotransferase and aspartate aminotransferase levels, while coagulopathy is reflected by elevated thrombin and prothrombin times (see Table 2).8 A drawback to any type of testing is that it requires advanced technology and safety precautions that are not widely available in the underdeveloped countries where the outbreak is currently taking place.8
Reporting
The CDC recommends immediate isolation of suspected EVD patients and the employment of standard, contact, and droplet precautions, including the use of gowns, gloves, masks, and face protection. Once the patient has been isolated, health care providers should notify their hospital’s Infection Control Program and immediately contact their local health department.20
Treatment
At present, the standard treatment for EVD is supportive care. The CDC recommends the use of IV fluid hydration and the maintenance of electrolytes, oxygen status, and blood pressure, as well as the treatment of any concurrent infection.25 These supportive measures, though noncurative, appear to significantly reduce mortality.
Another proposed treatment for EVD is transfusion of whole blood or plasma from recovered patients in the convalescent phase of infection. Through this technique, patients with early EVD benefit from the effective immune response of recovered individuals via passive immunization. Per WHO recommendations, only patients who have tested negative for EVD twice and have been out of the hospital for 28 days are eligible as potential donors.26 As with all blood product transfusions, the blood of the donor and the recipient must be typed and screened for compatibility.
No vaccines for the prevention of EVD have been approved by the FDA, but several vaccines are undergoing extensive research. Among them are prevaccines and postvaccines. Prevaccines, also known as preventive vaccines, are designed to be administered prior to pathogen exposure. Postvaccines, also referred to as therapeutic vaccines, are used after a person has sustained pathogen exposure, with the goal of stimulating the patient’s immune system to fight the infection.8
EVD vaccines are categorized into two classes: replicating and nonreplicating. Currently available replicating vaccines include recombinant vesicular stomatitis virus, recombinant human parainfluenza virus type 3, rabies virus, and cytomegalovirus.27 Nonreplicating vaccines include inactivated vaccines, replicons, DNA vaccines, recombinant adenoviruses, subunit vaccines, and replication-deficient ebola viruses.27
One prevaccine in particular, the recombinant adenovirus, has produced positive results in providing vaccine protection in NHPs. This vaccine is capable of protecting against multiple strains of ebola viruses, but because the vaccine is based on adenovirus serotype 5, for which a large proportion of the human population has immunity, its overall efficacy is significantly reduced.8,27 Significant progress has been made with the therapeutic vaccine ZMapp in the treatment of EVD in NHPs. ZMapp is a combination of three monoclonal antibodies that, when administered to an infected NHP, cling to the virus and prevent it from further invading healthy cells.8 Because this vaccine has not yet undergone human trials and is still in early experimental stages, special permission from the FDA is required to obtain it.8
Finally, researchers are optimistic that AVI-7357, an antiviral in late stages of clinical trials, will be an effective therapeutic agent for EVD. Its mechanism of action is thought to be inhibition of the VP24 protein; this viral protein is thought to play a role in the switch from viral replication to transcription, and blocking it is believed to effectively obstruct replication of the virus.28 Although much research is underway in the treatment of EVD, none of the proposed treatments has met the standards of FDA approval.
Continue for the prognosis >>
PROGNOSIS
Of the five identified ebola virus species, each differs in its virulence, morbidity, mortality, and prognosis. The mildest species is the nonfatal Reston ebolavirus, which is found in Asia and apparently causes asymptomatic infection in humans. Bundibugyo ebolavirus has a mortality rate of less than 40%, while Sudan ebolavirus has a mortality rate of about 50%.29 The mortality rate of Tai Forest ebolavirus is unknown because there has been only one recorded case of human infection. The current outbreak is caused by a strain of Zaire ebolavirus, which has the highest mortality rate at 70% to 90% (see Table 3).29
Despite the differing mortality rates among the ebolaviruses, fatality rate also depends on factors beyond the biologic characteristics of the species of ebolavirus responsible for the infection. According to WHO data collected during the first nine months of the current epidemic, the fatality rate among hospitalized patients in Liberia, Guinea, and Sierra Leone is 64.3%, lower than the average fatality rate of 70.8% in these countries.4 This data, however, represents only patients treated in the affected countries in Africa.
Given the lack of medical and governmental infrastructure in the nations where the research took place, it can be assumed that better, faster diagnosis and supportive treatment could increase survival in countries with robust health care systems, such as those in the US and Europe. In addition, demographic factors such as age affect mortality, with older age (> 45) carrying a worse prognosis.4 Other risk factors for increased mortality include general symptoms such as diarrhea, conjunctivitis, dyspnea, dysphagia, confusion, and unconsciousness or coma, as well as hemorrhagic symptoms.4
Due to a lack of health care infrastructure in affected West African nations, patients with EVD are receiving insufficient supportive treatment. In order to increase survival, it is essential to treat hypovolemia and electrolyte imbalance with therapies such as IV fluids and electrolyte repletion.30 All health care providers must be encouraged to use every tool at their disposal for providing supportive care for patients with EVD.
CONCLUSION
The US has a robust health care system capable of providing the training and resources necessary for containing outbreaks of diseases like EVD. Recognition of this can help to maintain public calm in the event of a full-scale epidemic of EVD in the US (however unlikely this may be). EVD is highly transmissible in its symptomatic stages, and recent cases in Texas and New York illustrate the need for PCPs and hospitals to be on alert for patients with possible exposure. Similarly, patient care teams must work together, exercise effective communication, and utilize pre-established plans for identification, isolation, and treatment in epidemics. Patients exhibiting fever and other signs and symptoms of EBOV must be asked about any recent travel to Liberia, Sierra Leone, and Guinea, and if they have had any contact with sick persons prior to their symptoms. Health care workers play an important role in epidemic control. As such, they should be familiar with risks, precautions, and protocols set forth by the WHO, CDC, and local health authorities.
CE/CME No: CR-1509
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify patients at high risk for exposure to and contraction of Ebola virus disease based on patient history, physical exam, and laboratory findings.
• Respond appropriately to high-risk patients by utilizing personal protective equipment, employing isolation strategies, and immediately reporting cases to hospital infection control and local health departments.
• Reassure domestic patients about the low risk of contracting Ebola virus in the United States.
FACULTY
Catherine B. Silver, Erin L. Leon, and Sarah A. Zaino are recent graduates of the Pace University Physician Assistant Program in New York City, where Ellen D. Mandel is Clinical Professor.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of September 2015.
Article begins on next page >>
The 2014 re-emergence of Ebola virus disease (EVD) quickly became the largest and deadliest outbreak of the disease ever recorded. Originating in Guinea, it spread to neighboring countries and others around the globe. As potentially the firstline health care contacts during a pandemic, all primary care providers need to be aware of the signs and symptoms of EVD so that they can quickly identify, isolate, and treat affected patients. This article describes the history, pathophysiology, diagnosis, and treatment of the disease.
In 1918, influenza virus—in the most deadly pandemic in the past century—killed an estimated 20 to 50 million people worldwide.1 A more recent example of a devastating pandemic that is still sweeping the globe with high morbidity and mortality is HIV/AIDS. According to the World Health Organization (WHO), in 2013, 35 million people were living with HIV/AIDS worldwide and 1.5 million people died from HIV/AIDS–related illnesses.2 Similarly, emerging respiratory infectious diseases such as avian influenza, Middle East respiratory syndrome (MERS), severe acute respiratory syndrome (SARS), and H7N9 influenza have all been named as possible threats due to their high fatality rates.3
In 1999, the WHO created a preparedness plan for pandemic influenza (updated in 2005) to provide information on reducing the risk for infection and informing government and health care organizations of proper outbreak response.3 The virulence and high mortality associated with Ebola virus disease (EVD) necessitate a similarly detailed preparedness plan, including international collaboration and commitment to providing research, training, support, and personnel to combat the current outbreak and prevent future outbreaks (see “‘Present’: Ebola's Impact on PAs in Liberia” for an interview with the President of the Liberia National Physician Assistants Association).
All primary care providers (PCPs) need to be aware of the signs and symptoms of EVD so that they can properly identify suspected cases, take necessary precautions to avoid transmission, and quickly transfer patients to facilities equipped to provide isolation and appropriate supportive treatment. PCPs may be the first providers to come into contact with patients infected with a pathogen during a pandemic, especially if the initial symptoms are mild enough not to warrant a visit to the emergency department. In 2003, the first case of SARS was diagnosed and treated by a Canadian family physician, and the initial case of H1N1 in Japan during the 2009 pandemic was first seen by a family physician as well.3
If PCPs are not sufficiently prepared to deal with a patient exhibiting signs or symptoms of EVD, it is likely that they, their staff, and other patients will be at greater risk for contraction and transmission. It has been found that PCPs are less likely to be prepared for dealing with pandemics, especially since high-level personal protective equipment (PPE)—eg, N95 masks, gowns, eye protection—are stocked at a lower rate in outpatient clinics than in hospitals.3 PCPs should prepare by familiarizing themselves with the signs and symptoms of EVD and by stocking high-level PPE.
In March 2014, the WHO received reports of a developing epidemic of EVD in Guinea in West Africa. The outbreak started in two districts of that country during December 2013; from there, it spread to Liberia and Sierra Leone, with scattered cases in Nigeria, Mali, Senegal, Spain, the United Kingdom, and the United States, making it the largest EVD epidemic ever recorded. The outbreak’s morbidity and mortality surpass that of all previous EVD epidemics in the past 38 years combined.4 As of August 19, 2015, there have been 15,188 laboratory-confirmed cases (the total number of cases is estimated at 28,000) and 11,286 deaths in the current epidemic.5
The first recorded outbreak of EVD occurred in 1976 in a village called Yambuku, located near the Ebola River in Zaire (now known as the Democratic Republic of Congo). At that time, a team of scientists from the CDC was sent to Zaire to identify the agent responsible for a deadly hemorrhagic fever that was ravaging the local hospital.6 This and subsequent outbreaks in central Africa were contained due to rapid coordinated efforts to stop the spread of the disease through a number of strategies.
Among these strategies, quick diagnosis, isolation of contacts, and quarantine of the greater area played a significant role in stemming the outbreak.4 Additional steps that helped curb disease spread included rapid burials with disinfectants and home visits by health workers, with patient education provided to help to assuage any fear villagers may have had of foreign health workers.6 Finally, health workers and surveillance teams were provided with PPE and were encouraged to continue their work despite the outbreak, with the promise that they would receive treatment equal to that given to foreign aid workers if they too fell ill.6 Each of these measures utilized in tandem allowed for control of the initial outbreak.
Despite being similar to previous outbreaks in terms of transmission rate, incubation period, fatality rate, and estimated basic reproduction number (R0, the estimated number of people infected by a single patient), the number of persons affected by the current epidemic eclipses any previous outbreak. Thus, political, economic, and social issues, rather than biologic characteristics, have made this epidemic the largest in history.4 The lack of medical infrastructure in the most severely affected nations has hindered efforts to provide care to those infected, and the number of patients requiring medical treatment vastly exceeds the number of hospital beds available.4
The WHO estimates that cutting transmission rates by 50% through the rapid and rigorous employment of sophisticated infection-control practices will halt the growth of the epidemic and eventually eradicate the virus from the human population.4 There is, however, the danger that if control measures are not implemented soon, EVD will become endemic in West Africa.4 In the US, early recognition, a well-informed public, and advanced medical infrastructure will allow for quick identification and containment of the virus. Public awareness, especially among health workers, is essential to stopping the epidemic’s spread.
Continue for pathophysiology and transmission >>
PATHOPHYSIOLOGY AND TRANSMISSION
Ebola virus (EBOV) is an enveloped RNA virus of the family Filoviridae.8 Five viruses of the Ebolavirus genus have been described: EBOV, Tai Forest virus, Reston virus, Sudan virus, and Bundibugyo virus. Except for Reston virus, each of these viruses causes hemorrhagic fever with high mortality.
EVD is a zoonotic disease, meaning that outbreaks typically begin by passage of the virus from an animal vector to a human host. In this case, it is thought that the viral reservoir consists of several species of fruit- and insect-eating bats native to West Africa.8,9 The vector that transmits the virus from bats to humans is not well understood, but reports name nonhuman primates (NHP) and pigs as possible culprits.8 Though EBOV is not typically transmitted through food, the practice of consuming “bushmeat”—hunted wild animals such as bats, monkeys, and rodents—has been linked to transmission of the virus. According to the CDC, the mechanism for this mode of transmission is likely through the butchering and processing of infected animals.10 It is important to note that only wild animals hunted in endemic regions of Africa carry the risk for transmission. To date, there have been no reports of EBOV transmission via contact with any animal, wild or domestic, in this country.
Once the virus has infected a human host, transmission of the disease continues from person to person via contact with infected bodily fluids. The three main modalities of virus transmission in underdeveloped countries include nosocomial transmission (improper sterilization techniques), funeral preparation, and community transmission.11 The most infectious substances are blood, feces, and vomit, but the virus has also been found in saliva, tears, breast milk, sweat, urine, and semen.12
Though controversial, evidence now suggests that EBOV can survive in semen for more than three months, even in patients who have fully recovered from the disease.13 To prevent sexually transmitted EBOV exposure, the WHO recommends that convalescent EVD patients use barrier methods such as condoms and female condoms to prevent the exchange of bodily fluids during sexual activity.13
Like other pathogens requiring droplet precautions, EBOV can only enter an uninfected individual through nonintact skin or mucous membranes, or parenterally. Transmission may also take place via fomites, or contaminated surfaces and objects which have not been properly sanitized.12 Studies suggest that the virus cannot survive on fomites for extended periods at room temperature; however, when refrigerated to 40°F, EBOV survived for more than three weeks.14 The incubation period for EBOV ranges from two to 21 days, with an average of 11 days.8
Current research indicates that the virus is not transmissible until symptoms appear, and therefore, infected patients are not contagious during the incubation period.15 The amount of EBOV in body fluid is referred to as viral load and has been determined to be a contributing factor in the transmission of the virus. As the viral load rises, symptoms worsen and the patient becomes more contagious.16 Patients with EVD are most contagious in the later stages of the disease (when viral load is highest) and shortly after death.16
With the recent infection of health care workers in Spain and Texas, there has also developed public concern regarding the possibility of contracting EBOV infection from pets. Currently, the CDC has no documented cases of domesticated animals contracting EVD or spreading the virus.17 Nonetheless, any pets in the home of EVD patients will be evaluated and managed by local health officials (via quarantine, surveillance, and possible euthanasia).17 In Spain, a nurses’ aide infected with the disease lost the fight to keep her dog, and health officials euthanized the 12-year-old mixed breed while his owner was in quarantine.18 By contrast, the King Charles Spaniel of Texas nurse Nina Pham was quarantined for three weeks and later reunited with his family.19 The divergent treatment of pets in the two cases illustrates how public concern about EVD ultimately influences decision-making.
Detailed study of the pathophysiology of EVD is difficult due to the virulence of EBOV and its high mortality, which are reflected by its classification as a biosafety level 4 (BSL-4) organism. Handling and study of organisms with BSL-4 designation require sophisticated laboratory equipment and advanced safety technology only available in developed countries. Further, ethical concerns dictate that the virus be studied in animal models rather than in humans. As such, mouse, guinea pig, and NHP models provide most of the available data.
EBOV evades immune system detection and destruction because of its extensively glycosylated lipid bilayer envelope.8 Once inside a suitable host, the virus reproduces by hijacking immune cells: monocytes, macrophages, and dendritic cells. Simultaneously, infection incites large-scale inflammation via cytokines, lymphocyte apoptosis resulting in lymphopenia, inhibition of innate and acquired humoral and cellular immune responses, and disruption of the clotting cascade.8
In later stages of infection, EBOV targets hepatocytes and endothelial cells.8 Liver dysfunction leads to interruption of clotting factor production, thus causing coagulopathy. Endothelial dysfunction is responsible for “leakage” of blood from vessels into skin, mucous membranes, and the gastrointestinal tract.8
Continue for the diagnosis >>
DIAGNOSIS
Patient history
To be diagnosed with EVD, patients must have a history of travel to an EBOV-affected region in the previous 21 days.20 Of particular importance in the US is gathering an accurate travel history from potential EBOV patients. According to the CDC, countries affected by the outbreak include Guinea, Liberia, and Sierra Leone; countries with travel-related cases include Nigeria, Spain, the US, the UK, Mali, and Senegal.21 Practitioners abroad should inquire about patients’ encounters with body fluid of infected individuals, contact with contaminated objects, and interaction with infected animals.
Physical exam
Initial symptoms are nonspecific, with a classic viral prodrome of fever, chills, muscle aches, and general malaise.8 Stage two is characterized by abdominal pain, nausea, vomiting, and diarrhea.8 In the final hemorrhagic stage of the disease, clotting dysfunction leads to subcutaneous and internal bleeding (epistaxis, petechiae, ecchymoses, hematochezia, and melena) and conjunctival hemorrhage.8,22 In this terminal stage of EVD, extreme blood loss causes organ failure, disseminated intravascular coagulation, shock, and death.8
Laboratory testing
Several methods of laboratory diagnosis exist, but all testing must be performed several days after the onset of symptoms; thus, patients with suspected EVD should remain isolated pending test results. At the outset of symptoms, the following laboratory diagnostic tests may be used to determine whether a patient is infected with EBOV:
• Antigen-capture enzyme-linked immunosorbent assay (ELISA)
• Immunoglobulin (IgM) ELISA
• Polymerase chain reaction (PCR)
• Virus isolation.23
IgM and IgG antibodies may be isolated from patients who have recovered from the disease.23 Finally, postmortem testing may be done via immunohistochemistry testing, PCR, or virus isolation.23 The CDC standard is IgG ELISA, which has 93% sensitivity and 98% specificity for EBOV antibody detection (see Table 1).24
Though not definitive, routine laboratory tests may support an EVD diagnosis. The complete blood count of a person with EVD reveals evidence of thrombocytopenia, leukopenia, and lymphopenia.8 Viral attack on hepatocytes results in elevated alanine aminotransferase and aspartate aminotransferase levels, while coagulopathy is reflected by elevated thrombin and prothrombin times (see Table 2).8 A drawback to any type of testing is that it requires advanced technology and safety precautions that are not widely available in the underdeveloped countries where the outbreak is currently taking place.8
Reporting
The CDC recommends immediate isolation of suspected EVD patients and the employment of standard, contact, and droplet precautions, including the use of gowns, gloves, masks, and face protection. Once the patient has been isolated, health care providers should notify their hospital’s Infection Control Program and immediately contact their local health department.20
Treatment
At present, the standard treatment for EVD is supportive care. The CDC recommends the use of IV fluid hydration and the maintenance of electrolytes, oxygen status, and blood pressure, as well as the treatment of any concurrent infection.25 These supportive measures, though noncurative, appear to significantly reduce mortality.
Another proposed treatment for EVD is transfusion of whole blood or plasma from recovered patients in the convalescent phase of infection. Through this technique, patients with early EVD benefit from the effective immune response of recovered individuals via passive immunization. Per WHO recommendations, only patients who have tested negative for EVD twice and have been out of the hospital for 28 days are eligible as potential donors.26 As with all blood product transfusions, the blood of the donor and the recipient must be typed and screened for compatibility.
No vaccines for the prevention of EVD have been approved by the FDA, but several vaccines are undergoing extensive research. Among them are prevaccines and postvaccines. Prevaccines, also known as preventive vaccines, are designed to be administered prior to pathogen exposure. Postvaccines, also referred to as therapeutic vaccines, are used after a person has sustained pathogen exposure, with the goal of stimulating the patient’s immune system to fight the infection.8
EVD vaccines are categorized into two classes: replicating and nonreplicating. Currently available replicating vaccines include recombinant vesicular stomatitis virus, recombinant human parainfluenza virus type 3, rabies virus, and cytomegalovirus.27 Nonreplicating vaccines include inactivated vaccines, replicons, DNA vaccines, recombinant adenoviruses, subunit vaccines, and replication-deficient ebola viruses.27
One prevaccine in particular, the recombinant adenovirus, has produced positive results in providing vaccine protection in NHPs. This vaccine is capable of protecting against multiple strains of ebola viruses, but because the vaccine is based on adenovirus serotype 5, for which a large proportion of the human population has immunity, its overall efficacy is significantly reduced.8,27 Significant progress has been made with the therapeutic vaccine ZMapp in the treatment of EVD in NHPs. ZMapp is a combination of three monoclonal antibodies that, when administered to an infected NHP, cling to the virus and prevent it from further invading healthy cells.8 Because this vaccine has not yet undergone human trials and is still in early experimental stages, special permission from the FDA is required to obtain it.8
Finally, researchers are optimistic that AVI-7357, an antiviral in late stages of clinical trials, will be an effective therapeutic agent for EVD. Its mechanism of action is thought to be inhibition of the VP24 protein; this viral protein is thought to play a role in the switch from viral replication to transcription, and blocking it is believed to effectively obstruct replication of the virus.28 Although much research is underway in the treatment of EVD, none of the proposed treatments has met the standards of FDA approval.
Continue for the prognosis >>
PROGNOSIS
Of the five identified ebola virus species, each differs in its virulence, morbidity, mortality, and prognosis. The mildest species is the nonfatal Reston ebolavirus, which is found in Asia and apparently causes asymptomatic infection in humans. Bundibugyo ebolavirus has a mortality rate of less than 40%, while Sudan ebolavirus has a mortality rate of about 50%.29 The mortality rate of Tai Forest ebolavirus is unknown because there has been only one recorded case of human infection. The current outbreak is caused by a strain of Zaire ebolavirus, which has the highest mortality rate at 70% to 90% (see Table 3).29
Despite the differing mortality rates among the ebolaviruses, fatality rate also depends on factors beyond the biologic characteristics of the species of ebolavirus responsible for the infection. According to WHO data collected during the first nine months of the current epidemic, the fatality rate among hospitalized patients in Liberia, Guinea, and Sierra Leone is 64.3%, lower than the average fatality rate of 70.8% in these countries.4 This data, however, represents only patients treated in the affected countries in Africa.
Given the lack of medical and governmental infrastructure in the nations where the research took place, it can be assumed that better, faster diagnosis and supportive treatment could increase survival in countries with robust health care systems, such as those in the US and Europe. In addition, demographic factors such as age affect mortality, with older age (> 45) carrying a worse prognosis.4 Other risk factors for increased mortality include general symptoms such as diarrhea, conjunctivitis, dyspnea, dysphagia, confusion, and unconsciousness or coma, as well as hemorrhagic symptoms.4
Due to a lack of health care infrastructure in affected West African nations, patients with EVD are receiving insufficient supportive treatment. In order to increase survival, it is essential to treat hypovolemia and electrolyte imbalance with therapies such as IV fluids and electrolyte repletion.30 All health care providers must be encouraged to use every tool at their disposal for providing supportive care for patients with EVD.
CONCLUSION
The US has a robust health care system capable of providing the training and resources necessary for containing outbreaks of diseases like EVD. Recognition of this can help to maintain public calm in the event of a full-scale epidemic of EVD in the US (however unlikely this may be). EVD is highly transmissible in its symptomatic stages, and recent cases in Texas and New York illustrate the need for PCPs and hospitals to be on alert for patients with possible exposure. Similarly, patient care teams must work together, exercise effective communication, and utilize pre-established plans for identification, isolation, and treatment in epidemics. Patients exhibiting fever and other signs and symptoms of EBOV must be asked about any recent travel to Liberia, Sierra Leone, and Guinea, and if they have had any contact with sick persons prior to their symptoms. Health care workers play an important role in epidemic control. As such, they should be familiar with risks, precautions, and protocols set forth by the WHO, CDC, and local health authorities.
1. CDC. Reconstruction of the 1918 influenza pandemic virus. www.cdc.gov/flu/about/qa/1918flupandemic.htm. Accessed August 24, 2015.
2. World Health Organization. Global Health Observatory (GHO) data: HIV/AIDS. www.who.int/gho/hiv/en/. Accessed August 24, 2015.
3. Tomizuka T, Kanatani Y, Kawahara K. Insufficient preparedness of primary care practices for pandemic influenza and the effect of a preparedness plan in Japan: a prefecture-wide cross-sectional study. BMC Fam Pract. 2013;14:174.
4. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481-1495.
5. CDC. 2014 Ebola outbreak in West Africa. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed June 11, 2015.
6. Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663-1666.
7. Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014;25(3):128-129.
8. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1-9.
9. Saez AM, Weiss S, Nowak K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7(1):17-23.
10. CDC. Facts about Ebola and bushmeat. www.cdc.gov/vhf/ebola/pdf/bushmeat-and-ebola.pdf. Accessed August 24, 2015.
11. MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6(6):e1546.
12. World Health Organization. What we know about transmission of the Ebola virus among humans. www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed August 24, 2015.
13. World Health Organization. Sexual transmission of the Ebola Virus: evidence and knowledge gaps. www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed August 24, 2015.
14. Piercy TJ, Smither SJ, Steward JA, et al. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109(5):1531-1539.
15. Ki M. What do we really fear? The epidemiological characteristics of Ebola and our preparedness. Epidemiol Health. 2014;36:e2014014.
16. Yamin D, Gertler S, Ndeffo-Mbah ML, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162(1):11-17.
17. CDC. Questions and answers about Ebola and pets. www.cdc.gov/vhf/ebola/transmission/qas-pets.html. Accessed August 24, 2015.
18. Wilson J. ‘Save Excalibur’ fails: Madrid euthanizes Ebola patient’s dog. CNN. www.cnn.com/2014/10/08/health/save-excalibur-ebola-dog/ Accessed June 12, 2015.
19. Serjeant J. New York doctor with Ebola improves, nurse reunited with dog. Reuters. www.reuters.com/article/2014/11/01/us-health-ebola-usa-idUSKBN0II1SP20141101. Accessed August 24, 2015.
20. CDC. Ebola virus disease (Ebola) algorithm for evaluation of the returned traveler. www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf. Accessed August 24, 2015.
21. CDC. 2014 Ebola outbreak in West Africa: outbreak distribution map. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Accessed August 24, 2015.
22. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(pt 8):1619-1624.
23. CDC. Ebola virus disease: diagnosis. www.cdc.gov/vhf/ebola/diagnosis/. Accessed August 24, 2015.
24. Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol. 2001;39(1):1-7.
25. CDC. Ebola (Ebola Virus Disease). www.cdc.gov/vhf/ebola/treatment/. Accessed August 24, 2015.
26. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Version 1.0. September 2014. Accessed August 24, 2015.
27. Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859-872.
28. Iversen PL, Warren TK, Wells JB, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806-2830.
29. Feldmann H. Ebola—a growing threat? N Engl J Med. 2014;371(15):1375-1378.
30. Lamontagne F, Clement C, Fletcher T, et al. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014; 371(17):1565-1566.
1. CDC. Reconstruction of the 1918 influenza pandemic virus. www.cdc.gov/flu/about/qa/1918flupandemic.htm. Accessed August 24, 2015.
2. World Health Organization. Global Health Observatory (GHO) data: HIV/AIDS. www.who.int/gho/hiv/en/. Accessed August 24, 2015.
3. Tomizuka T, Kanatani Y, Kawahara K. Insufficient preparedness of primary care practices for pandemic influenza and the effect of a preparedness plan in Japan: a prefecture-wide cross-sectional study. BMC Fam Pract. 2013;14:174.
4. WHO Ebola Response Team. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med. 2014;371(16):1481-1495.
5. CDC. 2014 Ebola outbreak in West Africa. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/index.html. Accessed June 11, 2015.
6. Breman JG, Johnson KM. Ebola then and now. N Engl J Med. 2014;371(18):1663-1666.
7. Laupland KB, Valiquette L. Ebola virus disease. Can J Infect Dis Med Microbiol. 2014;25(3):128-129.
8. Ansari AA. Clinical features and pathobiology of Ebolavirus infection. J Autoimmun. 2014;55:1-9.
9. Saez AM, Weiss S, Nowak K, et al. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7(1):17-23.
10. CDC. Facts about Ebola and bushmeat. www.cdc.gov/vhf/ebola/pdf/bushmeat-and-ebola.pdf. Accessed August 24, 2015.
11. MacNeil A, Rollin PE. Ebola and Marburg hemorrhagic fevers: neglected tropical diseases? PLoS Negl Trop Dis. 2012;6(6):e1546.
12. World Health Organization. What we know about transmission of the Ebola virus among humans. www.who.int/mediacentre/news/ebola/06-october-2014/en/. Accessed August 24, 2015.
13. World Health Organization. Sexual transmission of the Ebola Virus: evidence and knowledge gaps. www.who.int/reproductivehealth/topics/rtis/ebola-virus-semen/en/. Accessed August 24, 2015.
14. Piercy TJ, Smither SJ, Steward JA, et al. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J Appl Microbiol. 2010;109(5):1531-1539.
15. Ki M. What do we really fear? The epidemiological characteristics of Ebola and our preparedness. Epidemiol Health. 2014;36:e2014014.
16. Yamin D, Gertler S, Ndeffo-Mbah ML, et al. Effect of Ebola progression on transmission and control in Liberia. Ann Intern Med. 2015;162(1):11-17.
17. CDC. Questions and answers about Ebola and pets. www.cdc.gov/vhf/ebola/transmission/qas-pets.html. Accessed August 24, 2015.
18. Wilson J. ‘Save Excalibur’ fails: Madrid euthanizes Ebola patient’s dog. CNN. www.cnn.com/2014/10/08/health/save-excalibur-ebola-dog/ Accessed June 12, 2015.
19. Serjeant J. New York doctor with Ebola improves, nurse reunited with dog. Reuters. www.reuters.com/article/2014/11/01/us-health-ebola-usa-idUSKBN0II1SP20141101. Accessed August 24, 2015.
20. CDC. Ebola virus disease (Ebola) algorithm for evaluation of the returned traveler. www.cdc.gov/vhf/ebola/pdf/ebola-algorithm.pdf. Accessed August 24, 2015.
21. CDC. 2014 Ebola outbreak in West Africa: outbreak distribution map. www.cdc.gov/vhf/ebola/outbreaks/2014-west-africa/distribution-map.html. Accessed August 24, 2015.
22. Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95(pt 8):1619-1624.
23. CDC. Ebola virus disease: diagnosis. www.cdc.gov/vhf/ebola/diagnosis/. Accessed August 24, 2015.
24. Saijo M, Niikura M, Morikawa S, et al. Enzyme-linked immunosorbent assays for detection of antibodies to Ebola and Marburg viruses using recombinant nucleoproteins. J Clin Microbiol. 2001;39(1):1-7.
25. CDC. Ebola (Ebola Virus Disease). www.cdc.gov/vhf/ebola/treatment/. Accessed August 24, 2015.
26. World Health Organization. Use of convalescent whole blood or plasma collected from patients recovered from Ebola virus disease for transfusion, as an empirical treatment during outbreaks. http://apps.who.int/iris/bitstream/10665/135591/1/WHO_HIS_SDS_2014.8_eng.pdf?ua=1. Version 1.0. September 2014. Accessed August 24, 2015.
27. Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859-872.
28. Iversen PL, Warren TK, Wells JB, et al. Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections. Viruses. 2012;4(11):2806-2830.
29. Feldmann H. Ebola—a growing threat? N Engl J Med. 2014;371(15):1375-1378.
30. Lamontagne F, Clement C, Fletcher T, et al. Doing today’s work superbly well—treating Ebola with current tools. N Engl J Med. 2014; 371(17):1565-1566.
Obstructive Sleep Apnea: Evaluation & Management
CE/CME No: CR-1508
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the diagnostic criteria and clinical presentation of obstructive sleep apnea (OSA).
• Discuss the common screening questionnaires used in clinical practice to identify patients at risk for OSA.
• Describe the common comorbidities associated with OSA.
• Identify the features of pharyngeal structures used in the modified Mallampati classification.
• Know how to provide support and education for the patient in regard to treatment options, weight loss, residual daytime sleepiness, and smoking and alcohol cessation.
FACULTY
Bonnie Dadig is the Chair and Program Director of the Georgia Regents University (GRU) Physician Assistant Department and a PA in the GRU Department of Family Medicine outpatient clinic, Augusta. Morgan Edwards is a recently graduated PA student from GRU. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of August 2015.
Article begins on next page >>
Nearly 22 million Americans are affected by obstructive sleep apnea (OSA), making it the most common sleep disorder. Patients with undiagnosed and untreated OSA are at increased risk for cardiovascular and cerebrovascular health consequences and excessive daytime sleepiness. Here’s how you can address the symptoms and complications that make OSA a major public health concern, and as a result, decrease economic burdens and increase quality of life.
Obstructive sleep apnea (OSA) is an increasingly common diagnosis, with nearly 22 million Americans currently affected.1 In 2010, the diagnosis of OSA was recorded in 5.8 million office visits made by patients ages 20 or older—a 427% increase since 1999.2 While nearly 50% of patients in primary care settings complain of sleep disturbances, up to 80% of OSA cases remain undiagnosed.1,3 The high prevalence of sleep disorders in both the general population and in primary care settings, along with the underdiagnosis of OSA, illustrates the essential role of primary care providers in recognizing the signs and symptoms of OSA and evaluating patients for this condition.1-3
BACKGROUND AND PATHOPHYSIOLOGY
OSA is the most common sleep disorder. Characterized by recurrent episodes of upper airway obstruction during sleep, it results in significantly reduced airflow (hypopnea) or complete cessation of breathing (apnea) for intervals lasting ≥ 10 seconds.4,5 The total number of apneas or hypopneas per hour of sleep—the apnea-hypopnea index (AHI)—is one domain that can be used to classify the severity of OSA (see Table 1).5
Because the human upper airway lacks rigid or bony support, under normal circumstances, the pharyngeal dilator muscles act to oppose the negative pressure within the upper airway during inspiration when awake. And although the striated nature of the pharyngeal dilator muscles leads to a reduction in airway muscle tone during sleep, enough opposition is maintained to prevent airway collapse. In OSA patients, however, the reduced pharyngeal muscle tone fails to offset the negative airway pressure generated during inspiration while asleep, causing recurrent airway obstructions.6 In addition, anatomically narrowed upper airways can further impede airway patency during sleep.4 Causes include macroglossia and/or tonsillar hypertrophy, obesity with fat tissue deposits in the neck that compress the airway, and bony craniofacial abnormalities such as retrognathia.7
Disruptions in breathing from apneas and hypopneas result in hypercapnia and hypoxia, which trigger arousal centers in the central nervous system to increase sympathetic activity. As a result, the patient briefly awakens from sleep, which increases respiratory and pharyngeal dilator muscle tone and ultimately restores airway patency, and ventilation resumes.4
The sympathetic response associated with apneic and hypopneic episodes causes concomitant cardiac stimulation. This leads to transient vasoconstriction and elevations in heart rate and blood pressure. Vascular changes occur over time and result in persistent hypertension and other cardiovascular impairments.4
Undiagnosed and untreated OSA predisposes individuals to a multitude of health consequences and ultimately results in substantial public health and economic burdens. A recent study estimated that the economic cost of moderate-to-severe OSA in the United States is between $65 billion and $165 billion annually.8 Patients with OSA are at increased risk for complications such as systemic and pulmonary hypertension, congestive heart failure, cardiac arrhythmias, coronary artery disease, myocardial infarction, cerebrovascular accident, diabetes, and metabolic syndrome.9 Excessive daytime sleepiness from OSA has also been shown to contribute to increased rates of motor vehicle accidents, poor job performance, impaired cognitive function, and decreased quality-of-life measures.9
Continue for clinical evaluation >>
CLINICAL EVALUATION
Patient History
Clinicians become aware of patients with OSA when they present with associated symptoms or complications. These symptoms are encountered in several settings—as a part of the routine health maintenance examination, as the patient’s presenting complaint, or as a part of the evaluation for another complaint or medical condition.
Regardless of how the symptoms are discovered, all patients with symptoms of and/or positive risk factors for OSA should be assessed via a comprehensive sleep oriented history and physical examination. Symptoms suggestive of OSA include witnessed or reported apneas, snoring, gasping/choking at night, excessive daytime sleepiness not explained by other factors, severity of sleepiness (as determined by the Epworth Sleepiness Scale), nonrefreshing sleep, sleep fragmentation/maintenance insomnia, nocturia, morning headaches, decreased concentration, memory loss, decreased libido, and irritability.10 A recently published study found that nocturnal gasping or choking is the most reliable indicator for identifying patients with OSA.11
The clinical presentation of OSA in certain populations may differ from that of typical adult OSA, requiring a higher degree of clinical suspicion. For instance, pediatric OSA should be suspected in any child who presents with enuresis, poor school performance, failure to thrive, attention-deficit/hyperactivity disorder, or learning disabilities.9 Alternatively, females with OSA have a propensity to emphasize complaints of tiredness, fatigue, or lack of energy rather than report excessive sleepiness per se. These complaints frequently misdirect clinicians toward psychiatric and endocrine diagnoses, without considering the possibility of a sleep disorder.12
Risk Factors
In addition to symptomatology, there are several risk factors associated with OSA that should be considered. The most important are male gender, increasing age (40 to 70), obesity, large neck circumference, and craniofacial and upper airway abnormalities.13 Among the intermediate risk factors are family history of OSA, pregnancy, and Hispanic, Asian, and African-American ethnicity.
Other risk factors associated with OSA include cigarette smoking, alcohol use before sleep, and use of sedative medications.13 Comorbid medical conditions have also been shown to exacerbate and to contribute to OSA; these include GERD, diabetes, congestive heart failure, treatment-refractory hypertension, hypothyroidism, acromegaly, atrial fibrillation, stroke, Down syndrome (due to macroglossia), and pulmonary hypertension.4,10
Screening Questionnaires
Several questionnaires have been validated to screen for OSA. They can be used clinically as part of the sleep-oriented history to quantify a patient’s subjective complaint of sleepiness; however, they are typically more helpful for ruling out sleep apnea than for diagnosing it.11 Two of the most commonly utilized are the Epworth Sleepiness Scale (ESS; http://epworthsleepinessscale.com/epworth-sleepiness-scale.pdf) and the Berlin Questionnaire (www.sleepapnea.org/assets/files/pdf/Berlin%20Questionnaire.pdf).
The ESS was developed to identify patients with excessive daytime sleepiness. It asks patients to rate—on a scale of 0 (would never doze) to 4 (high chance of dozing)—how likely they are to doze off or fall asleep during eight everyday scenarios (ie, sitting and reading; watching TV; sitting inactive in a public place; being a passenger in a vehicle for one or more hours; lying down in the afternoon; sitting and talking to someone; sitting quietly after lunch; or while stopped for a few minutes while driving in traffic).14 The total score can range from 0 to 24. Patients scoring higher than 10 should be referred to a sleep specialist. The ESS can be used to detect symptoms suggestive of OSA; however, it is not specific to OSA and was designed to screen for all sleep disorders.14
In contrast, the Berlin Questionnaire consists of 10 questions that quantify and qualify a patient’s snoring and sleepiness, and it was developed specifically to evaluate for OSA. The questionnaire measures three categories of symptoms: snoring, fatigue, and blood pressure and BMI. A patient is deemed at high risk for OSA when scoring positive in two or more symptom categories, and at low risk when scoring positive in one category.15 However, when OSA is defined as an AHI of ≥ 5 events/hr, the Berlin Questionnaire is 80% sensitive and 46% specific for OSA.11 Therefore, screening questionnaires are useful as supplementary tools but are of limited utility as the sole method for evaluating and diagnosing OSA.
Physical Examination
Physical examination should include assessment for risk factors and comorbidities associated with OSA; this includes measurement of blood pressure, blood glucose, and neck circumference; calculation of BMI and waist-to-hip ratio; and assessment for craniofacial abnormalities. Additionally, cardiovascular, respiratory, and neurologic systems should be evaluated for potential alterations due to OSA.10
Physical exam findings suggestive of OSA are BMI ≥ 30, neck circumference > 17 in for males and > 16 in for females, and craniofacial and upper airway abnormalities. Such anatomic variations include retrognathia, macroglossia (eg, Down syndrome patients), tonsillar hypertrophy (eg, pediatric patients), lateral peritonsillar narrowing, high-arched or narrow hard palate, enlarged uvula, nasal polyps, nasal deviation, turbinate hypertrophy, and oropharynx anatomy consistent with modified Mallampati class III or IV.10 Although OSA is more prevalent in obese patients (those with a BMI ≥ 30), it should be noted that 14.6% of OSA patients are normal weight (BMI < 25) and 34.5%, overweight (BMI 25 to 30).16
The modified Mallampati classification is a straightforward scoring method that was designed to estimate the difficulty of oral intubation based on the extent of mouth opening relative to the size of the tongue.17 According to the protocol set forth by Mallampati and colleagues, assessment is performed with the patient sitting upright with the head in a neutral position, the mouth open, and the tongue maximally protruded without the patient speaking or saying “ahh.”18 The examiner then inspects the pharyngeal structures and classifies the patient’s airway according to the structures visualized. Class I is present when the soft palate, uvula, and pillars are visible; class II when the soft palate and uvula are visible; class III when the soft palate and base of the uvula are visible; class IV when the soft palate is not visible at all (see Figure 1).17,18
An alternative method for observing the pharyngeal structures is via nasopharyngoscopy.19 This procedure can be done in the office setting and utilizes a flexible scope inserted into the nasal cavity and advanced into the pharynx. By providing direct visualization of the upper airway structures, nasopharyngoscopy allows the operator to determine the extent and the location of airway collapse. Imaging by nasopharyngoscopy does not involve any radiation exposure and can be done while the patient is sedated or awake.19
LABORATORY WORKUP AND DIAGNOSIS
Patients deemed high-risk by the sleep-oriented history and physical examination should be subsequently referred to a sleep specialist for objective testing to establish the diagnosis and determine the severity of OSA. Despite the utilization of screening questionnaires, currently there is no consensus on risk stratification to determine when to refer patients with suspected OSA. In other words, no clear thresholds exist on whether a patient is at “low risk” or at “high risk” for OSA based on history and physical examination findings. Therefore, referral is made at the clinician’s discretion, based on the number and severity of OSA symptoms and risk factors.
There are two standard objective sleep study tests: in-laboratory polysomnography (PSG) and home-based sleep studies. The in-laboratory PSG is the gold standard for the diagnosis of OSA.4 The following physiologic signals are measured and interpreted during in-laboratory PSG: electroencephalogram, electro-oculogram, chin electromyogram, airflow, oxygen saturation, respiratory effort, and electrocardiogram.4
Because PSG is resource intensive, home-based sleep studies are an acceptable alternative; however, they should only be considered in patients with a high probability of moderate-to-severe OSA without comorbid sleep disorders or medical conditions. During home-based sleep studies, airflow, respiratory effort, and blood oxygenation are documented.10
The diagnosis of OSA is validated if the AHI on PSG or on home-based sleep studies is > 15 events/h or > 5 events/h with any of the following coexisting signs or symptoms: excessive daytime sleepiness; unintentional sleep episodes during wakefulness; unrefreshing sleep; fatigue; insomnia; waking up holding breath, gasping, or choking; or loud snoring, breathing interruptions, or both, described by the bed partner.10 In addition to diagnosing OSA, PSG and home-based sleep studies are useful in ruling out a variety of conditions that similarly cause excessive daytime sleepiness due to disrupted sleep.
The differential diagnosis of OSA includes, but is not limited to, periodic limb movement disorder, restless legs syndrome, narcolepsy, central sleep apnea, circadian rhythm sleep-wake disorders, respiratory diseases (ie, COPD, asthma), primary snoring, depression, and substance abuse.20
Continue for treatment options >>
TREATMENT OPTIONS
Because OSA is a chronic condition with no definitive cure, lifelong management and follow-up are needed to evaluate treatment adherence/response and the development or resolution of comorbidities. Treatment options for OSA consist primarily of medical (positive airway pressure, use of oral appliances, and pharmacotherapy), behavioral (weight loss, positional therapy, and substance avoidance), and surgical approaches. The patient should actively participate in the treatment and management of this chronic condition.10
Positive Airway Pressure
Regardless of OSA severity, the treatment of choice is positive airway pressure (PAP) during sleep, with continuous PAP (CPAP) being the recommended mode of delivery. PAP is delivered via a face or nasal mask attached to a machine that maintains a constant upper airway pressure, which in turn prevents pharyngeal collapse during sleep. As a result, the upper airway remains patent, and apneas and hypopneas are inhibited.4
PAP can be delivered in three different modes: CPAP, bilevel (BPAP), or autotitrating (APAP). CPAP provides PAP at a fixed rate throughout the respiratory cycle. In contrast, BPAP delivers a preset PAP that is greater during inspiration than during expiration. BPAP is used to alleviate the discomfort of exhaling against the fixed airway pressure of traditional CPAP by delivering lower pressure during expiration than during inspiration. APAP delivers fluctuating positive airway pressures according to changes in the patient’s breathing patterns and is used in patients with complex breathing patterns. CPAP is the recommended mode of airway pressure delivery; however, BPAP or APAP can be considered in patients who cannot tolerate CPAP.10,21
The amount of pressure needed to maintain airway patency is determined during PSG, and the CPAP system is titrated up to this optimal level. Patient adherence is a commonly encountered problem with CPAP therapy due to adverse effects, such as skin irritation, misfit of mask, dry mouth, nasal congestion, and rhinorrhea. Humidification, nasal saline sprays, and mask resizing can help to alleviate such symptoms.4 Follow-up in the early stages should be frequent enough to troubleshoot and remediate problems with the PAP mask, the machine, and adherence. Long-term follow-up for maintenance, adherence, and signs and symptoms of worsening OSA should be scheduled yearly.10
Oral Appliances
Oral appliances (OAs) are an alternative treatment option for OSA patients who are nonadherent with CPAP, fail CPAP or behavioral therapies, experience adverse effects from CPAP, or prefer OAs. Two types of OAs exist: mandibular repositioning appliances and tongue retaining devices. The former anteriorly displaces the mandible relative to the maxilla; the latter anteriorly displaces the tongue only. Regardless of the type selected, OAs are designed to increase the diameter of the pharynx and decrease pharyngeal collapsibility.10
Although less efficacious than traditional CPAP therapy, OAs may be considered in patients with mild-to-moderate OSA.10 Patients with severe OSA should not be offered OAs as a treatment option. Current evidence suggests that OAs can substantially reduce the severity of OSA as well as daytime sleepiness. Additionally, OAs are superior to CPAP in patient preference, which could improve long-term patient adherence to treatment.22
Prior to initiating therapy with an OA, patients should have a dental examination to assess candidacy for, and proper fit of, an OA. Regular follow-ups are required initially to ensure proper fit and comfort of the device. Patients should be instructed to make an office visit if they experience adverse effects such as temporomandibular joint discomfort, tooth tenderness, excessive salivation, and gum irritation.22 Once final adjustments have been made to the fit of the OA, a PSG should be performed to ensure maximal therapeutic efficacy. Follow-up with a dental specialist is advised every six months for the first year and yearly thereafter.10
Pharmacology
According to the practice parameters of the American Academy of Sleep Medicine (AASM), there are currently no pharmacologic agents that prevent or overcome upper airway obstruction well enough to be considered a primary treatment option for OSA.23 The most widely studied pharmacologic agents—selective serotonergic reuptake inhibitors, protriptyline, methylxanthine derivatives (aminophylline and theophylline), estrogen therapy (with or without progesterone), supplemental oxygen, and short-acting nasal decongestants—are not recommended for the primary treatment of OSA.23 Pharmacologic therapy is therefore most beneficial when used as adjuvant therapy (which targets a residual symptom) rather than primary therapy (which targets the underlying cause of the disorder).
There are currently two FDA-approved medications for the treatment of residual excessive daytime sleepiness in patients who are receiving adequate primary treatment (eg, PAP, OAs) and who do not have any other identifiable cause for the sleepiness: modafinil and armodafinil.23,24 The exact mechanism of action of these agents has not been fully characterized, but they are known to act on the central nervous system to enhance alertness (likely by enhancing dopamine signaling).24
Prior to prescribing these medications, other causes of sleepiness must be ruled out; these include suboptimal adherence with PAP, poorly fitting PAP mask, poor sleep hygiene, insufficient sleep, other sleep disorders, and depression.10 Patients should be informed that these drugs should be used in conjunction with pre-existing CPAP therapy. Common adverse effects of modafinil include headache, dizziness, gastrointestinal upset, and insomnia. This medication should be used cautiously in patients with a history of cardiovascular and/or psychologic problems because it can cause serious adverse effects, including chest pain, palpitations, hypertension, hallucinations, aggression, anxiety, depression, and severe skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.4,24
Weight Loss
Because obesity is one of the most prevalent risk factors for OSA, weight loss should be recommended for all overweight and obese OSA patients.13,23 Weight loss can be achieved by several different methods, including exercise programs, dietary programs, a combination of the two, and bariatric surgery. A recent meta-analysis found that exercise alone is less successful in reducing AHI compared to dietary modifications; however, a combination of exercise and dietary changes is superior to both individual interventions in reducing AHI.25 This meta-analysis also found that weight-loss interventions improve, but fail to normalize, AHI.25 Thus, weight-loss interventions should be an adjuvant therapy in the treatment of OSA.23
Another recent meta-analysis found that lifestyle interventions, specifically exercise training, might improve OSA severity and symptoms even in the absence of significant weight loss. According to this study, exercise training produced a 32% reduction in AHI in OSA patients and a 42% reduction in AHI when compared to OSA patients who did not partake in exercise training.26 Additionally, OSA patients experienced significant improvements in cardiorespiratory fitness, daytime sleepiness, and sleep efficiency. Because there was no significant reduction in BMI with exercise training, it is likely that there are additional benefits of exercise training that are independent of the effects on weight loss.26
Positional Therapy
Approximately 50% to 60% of OSA patients have positional OSA, which is classified as an increase of 50% or more in the AHI when sleeping in the supine position compared with nonsupine sleep positions.27 It is thought that positional therapy, consisting of any technique that maintains the patient in a nonsupine sleep position, decreases the tendency for the tongue to prolapse and for the airway to collapse.27 Several positional devices exist, including pillows, tennis balls, backpacks, and vibratory chest alarms. Additionally, sleeping in the lateral recumbent position can function as a form of positional therapy.4,21
According to the AASM, less obese, younger patients and those with less severe OSA are more likely to achieve a normalized AHI by sleeping in a nonsupine position; thus, these patients are more likely to benefit from positional therapy than their OSA counterparts.23,27 A recent study found that CPAP therapy is superior to positional therapy in reducing apneic episodes (ie, AHI) in patients with positional OSA.27 Thus, positional therapy is an effective secondary treatment that can be used as an adjuvant therapy to CPAP in patients with mild positional OSA.23,27
Substance Avoidance
All patients with OSA should be advised to avoid alcohol, as it has been shown to increase the duration and frequency of apneic episodes and lower arterial oxyhemoglobin saturation during sleep.28 Not only does alcohol exacerbate preexisting OSA, but it has also been shown to induce frank OSA in patients who snore but do not have OSA at baseline.28 Sedative medications (eg, benzodiazepines) have similar effects on the central nervous system and should also be avoided if possible. Both of these substances have inhibitory actions on the central nervous system, thereby relaxing the pharyngeal muscles and promoting upper airway collapse during sleep.
Because cigarette smoking is an independent risk factor for snoring and thus a presumed risk factor for OSA, smoking cessation is a necessary component of the treatment of OSA.13,29 Cigarette smoking is thought to increase airway inflammation, exacerbate preexisting lung conditions such as COPD and asthma, and induce sleep fragmentation due to the effects of nicotine withdrawal during sleep.29 Alcohol, sedative medications, and cigarettes have all been shown to exacerbate OSA; thus, patient education about the harmful effects of these substances and the benefits of avoiding them is a crucial aspect of OSA therapy.
Surgical Therapies
When both CPAP and OA are inadequate in the management of OSA and/or when obstructive or functional anatomic abnormalities exist, upper airway surgery can be considered for patients. There is currently no consensus about the role of surgery in OSA patients, nor are there clear guidelines or screening questionnaires that accurately predict which patients will benefit the most from upper airway surgery. The appropriate surgical procedure depends on the site of anatomic abnormality and could be a nasal, oral, oropharyngeal, nasopharyngeal, hypopharyngeal, laryngeal, or global airway procedure.10 Some of the most common surgical procedures for the management of OSA are described below.
Uvulopalatopharyngoplasty (UPPP). This surgical procedure involves resecting the entire uvula and the obstructive portion of the soft palate while resizing and reorienting the tonsillar pillars. As a sole procedure, with or without tonsillectomy, UPPP does not reliably normalize the AHI when treating moderate-to-severe OSA.30
Radiofrequency ablation (RFA). RFA is a less invasive variation of UPPP that involves using a temperature-controlled probe to deliver energy to the upper airway tissue (typically the tongue base and/or the soft palate) in an effort to induce palatal stiffening. This procedure can be considered in patients with mild-to-moderate OSA who cannot tolerate or are unwilling to adhere to PAP or OA therapies.30
Maxillo-mandibular advancement (MMA). This operation involves indirect advancement of the anterior pharyngeal tissues (ie, the soft palate, tongue base, and suprahyoid musculature) via their attachment to the maxilla, mandible, and hyoid bone. The simultaneous advancement of the maxilla and mandible are accomplished by sagittal split osteotomies that are stabilized with plates, screws, or bone grafts.31 This surgery is designed to enlarge the retrolingual airway and provide some advancement of the retropalate without directly manipulating the pharyngeal tissues. MMA is indicated for patients with severe OSA who cannot tolerate or are unwilling to adhere to PAP therapy or in whom OAs have been found ineffective.30
Tracheostomy. This procedure consists of creating an airway through the anterior neck into the upper trachea. This opening bypasses the entire upper airway obstruction and thus is 100% effective in curing OSA. However, tracheostomy is typically last-line due to the resulting undesirable alterations in the patient’s physical appearance and to the risks associated with the procedure.5 According to the AASM, this operation should only be considered when other options do not exist, have failed, or are refused, or when this operation is deemed necessary by clinical urgency.30
Hypoglossal nerve stimulation. This therapy uses a surgically implanted device, approved by the FDA in 2014, to detect the patient’s breathing pattern and stimulate the hypoglossal nerve, causing tongue protrusion during inspiration. Tongue movement is controlled, and thus, the airway remains patent during inspiration.32 It is indicated for use in patients with moderate-to-severe OSA who have refused CPAP or for whom CPAP treatment has been unsuccessful.
According to a recently published study, a 68% decrease in median AHI at 12 months postimplantation was reported among those receiving hypoglossal nerve stimulation.33 Additionally, OSA patients subjectively reported decreased sleepiness and an increased quality of life compared to baseline.
The most common adverse effects were transient tongue weakness postoperatively, discomfort related to stimulation, and tongue soreness.33 Its use is contraindicated in patients who are pregnant or plan to become pregnant and in those who will require MRI, who have other implantable devices that may interact with the stimulation system, who have any condition that may affect neurologic control of the upper airway, and who have any anatomic abnormalities that may prevent effective performance of the upper airway stimulation (eg, the presence of complete concentric collapse at the retropalatal airway during endoscopy).32,33 Although its use is promising for the treatment of OSA, hypoglossal nerve stimulation is still a novel option that is not yet readily employed.33
OUTCOMES ASSESSMENT
Clinical judgment should be used to determine the appropriate treatment(s) based on OSA severity and patient preference. Regardless of the treatment option chosen, all OSA patients should have an outcomes assessment performed after the initiation of therapy. Indicators to monitor include resolution of sleepiness, OSA-specific quality-of-life measures, adherence to therapy, avoidance of factors exacerbating OSA (eg, alcohol, tobacco, sedative medications), amount of sleep being obtained, sleep hygiene practices, weight loss, and patient and spousal satisfaction.10 Patients with all levels of OSA severity should receive ongoing management to ensure long-term resolution of symptoms and adherence to treatment. Improvements in primary care treatment, follow-up, and outcomes evaluation are becoming increasingly important to address the symptoms and complications that make OSA a major public health concern.
1. American Sleep Apnea Association. A very short course on sleep apnea. www.sleepapnea.org/i-am-a-health-care-professional.html. Accessed July 21, 2015.
2. Ford ES, Wheaton AG, Cunningham TJ, et al. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care Survey 1999-2010. Sleep. 2014;37(8):1283-1293.
3. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. NIH Consens Sci Statements. 2005;22(2):1-30.
4. Carlucci M, Smith M, Corbridge SJ. Poor sleep, hazardous breathing: an overview of obstructive sleep apnea. Nurse Pract. 2013;38(3):20-28.
5. Institute for Clinical Systems Improvement. Health Care Guideline: Diagnosis and Treatment of Obstructive Sleep Apnea. 6th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2008.
6. Douglas NJ. Sleep apnea. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:2186-2188.
7. US Department of Health and Human Services, National Heart, Lung, and Blood Institute. What causes sleep apnea? www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/causes.html. Accessed uly 21, 2015.
8. Harvard Medical School, Division of Sleep Medicine. The price of fatigue: the surprising economic costs of unmanaged sleep apnea. December 2010. https://sleep.med.harvard.edu/file_download/100. Accessed July 21, 2015.
9. Pagel JF. Obstructive sleep apnea (OSA) in primary care: evidence-based practice. J Am Board Fam Med. 2007;20(4):392-398.
10. Epstein LJ, Kristo D, Strollo PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
11. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea: the rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
12. Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10(10):1075-1084.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013-2016.
14. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
15. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485-491.
16. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441-446.
17. Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487-490.
18. Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434.
19. Gregorio MG, Jacomelli M, Figueiredo AC, et al. Evaluation of airway obstruction by nasopharyngoscopy: comparison of the Müller maneuver versus induced sleep. Braz J Otorhinolaryngol. 2007;73(5):618-622.
20. Kline LR. Clinical presentation and diagnosis of obstructive sleep apnea in adults. Up-to-Date. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-obstructive-sleep-apnea-in-adults. Accessed July 21, 2015.
21. Kryger MH, Malhotra A. Management of obstructive sleep apnea in adults. Up-to-Date. www.uptodate.com/contents/management-of-obstructive-sleep-apnea-in-adults. Accessed July 21, 2015.
22. Lim J, Lasserson TJ, Fleetham J, Wright JJ. Oral appliances for obstructive sleep apneoa. Cochrane Database Syst Rev. 2006;1:CD004435.
23. Morgenthaler TI, Kapen S, Lee-Chiong T, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8): 1031-1035.
24. Pepin JL. Evaluation and management of residual sleepiness in obstructive sleep apnea. Up-to-Date. www.uptodate.com/contents/evaluation-and-management-of-residual-sleepiness-in-obstructive-sleep-apnea. Accessed July 21, 2015.
25. Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36(10):1553-1562.
26. Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175-184.
27. Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014; 18(1):19-24.
28. Issa FG, Sullivan CE. Alcohol, snoring, and sleep apnea. J Neurol Neurosurg Psychiatry. 1982;45(4):353-359.
29. Lin Y, Li QY, Zhang XJ. Interaction between smoking and obstructive sleep apnea: not just participants. Chin Med J. 2012;125(17):3150-3156.
30. Aurora RN, Casey KR, Kristo D, et al; American Academy of Sleep Medicine. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408-1413.
31. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396-1407.
32. US Food and Drug Administration. Recently-approved devices. Inspire upper airway stimulation - P130008. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm398321.htm. Accessed July 21, 2015.
33. Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
CE/CME No: CR-1508
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the diagnostic criteria and clinical presentation of obstructive sleep apnea (OSA).
• Discuss the common screening questionnaires used in clinical practice to identify patients at risk for OSA.
• Describe the common comorbidities associated with OSA.
• Identify the features of pharyngeal structures used in the modified Mallampati classification.
• Know how to provide support and education for the patient in regard to treatment options, weight loss, residual daytime sleepiness, and smoking and alcohol cessation.
FACULTY
Bonnie Dadig is the Chair and Program Director of the Georgia Regents University (GRU) Physician Assistant Department and a PA in the GRU Department of Family Medicine outpatient clinic, Augusta. Morgan Edwards is a recently graduated PA student from GRU. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of August 2015.
Article begins on next page >>
Nearly 22 million Americans are affected by obstructive sleep apnea (OSA), making it the most common sleep disorder. Patients with undiagnosed and untreated OSA are at increased risk for cardiovascular and cerebrovascular health consequences and excessive daytime sleepiness. Here’s how you can address the symptoms and complications that make OSA a major public health concern, and as a result, decrease economic burdens and increase quality of life.
Obstructive sleep apnea (OSA) is an increasingly common diagnosis, with nearly 22 million Americans currently affected.1 In 2010, the diagnosis of OSA was recorded in 5.8 million office visits made by patients ages 20 or older—a 427% increase since 1999.2 While nearly 50% of patients in primary care settings complain of sleep disturbances, up to 80% of OSA cases remain undiagnosed.1,3 The high prevalence of sleep disorders in both the general population and in primary care settings, along with the underdiagnosis of OSA, illustrates the essential role of primary care providers in recognizing the signs and symptoms of OSA and evaluating patients for this condition.1-3
BACKGROUND AND PATHOPHYSIOLOGY
OSA is the most common sleep disorder. Characterized by recurrent episodes of upper airway obstruction during sleep, it results in significantly reduced airflow (hypopnea) or complete cessation of breathing (apnea) for intervals lasting ≥ 10 seconds.4,5 The total number of apneas or hypopneas per hour of sleep—the apnea-hypopnea index (AHI)—is one domain that can be used to classify the severity of OSA (see Table 1).5
Because the human upper airway lacks rigid or bony support, under normal circumstances, the pharyngeal dilator muscles act to oppose the negative pressure within the upper airway during inspiration when awake. And although the striated nature of the pharyngeal dilator muscles leads to a reduction in airway muscle tone during sleep, enough opposition is maintained to prevent airway collapse. In OSA patients, however, the reduced pharyngeal muscle tone fails to offset the negative airway pressure generated during inspiration while asleep, causing recurrent airway obstructions.6 In addition, anatomically narrowed upper airways can further impede airway patency during sleep.4 Causes include macroglossia and/or tonsillar hypertrophy, obesity with fat tissue deposits in the neck that compress the airway, and bony craniofacial abnormalities such as retrognathia.7
Disruptions in breathing from apneas and hypopneas result in hypercapnia and hypoxia, which trigger arousal centers in the central nervous system to increase sympathetic activity. As a result, the patient briefly awakens from sleep, which increases respiratory and pharyngeal dilator muscle tone and ultimately restores airway patency, and ventilation resumes.4
The sympathetic response associated with apneic and hypopneic episodes causes concomitant cardiac stimulation. This leads to transient vasoconstriction and elevations in heart rate and blood pressure. Vascular changes occur over time and result in persistent hypertension and other cardiovascular impairments.4
Undiagnosed and untreated OSA predisposes individuals to a multitude of health consequences and ultimately results in substantial public health and economic burdens. A recent study estimated that the economic cost of moderate-to-severe OSA in the United States is between $65 billion and $165 billion annually.8 Patients with OSA are at increased risk for complications such as systemic and pulmonary hypertension, congestive heart failure, cardiac arrhythmias, coronary artery disease, myocardial infarction, cerebrovascular accident, diabetes, and metabolic syndrome.9 Excessive daytime sleepiness from OSA has also been shown to contribute to increased rates of motor vehicle accidents, poor job performance, impaired cognitive function, and decreased quality-of-life measures.9
Continue for clinical evaluation >>
CLINICAL EVALUATION
Patient History
Clinicians become aware of patients with OSA when they present with associated symptoms or complications. These symptoms are encountered in several settings—as a part of the routine health maintenance examination, as the patient’s presenting complaint, or as a part of the evaluation for another complaint or medical condition.
Regardless of how the symptoms are discovered, all patients with symptoms of and/or positive risk factors for OSA should be assessed via a comprehensive sleep oriented history and physical examination. Symptoms suggestive of OSA include witnessed or reported apneas, snoring, gasping/choking at night, excessive daytime sleepiness not explained by other factors, severity of sleepiness (as determined by the Epworth Sleepiness Scale), nonrefreshing sleep, sleep fragmentation/maintenance insomnia, nocturia, morning headaches, decreased concentration, memory loss, decreased libido, and irritability.10 A recently published study found that nocturnal gasping or choking is the most reliable indicator for identifying patients with OSA.11
The clinical presentation of OSA in certain populations may differ from that of typical adult OSA, requiring a higher degree of clinical suspicion. For instance, pediatric OSA should be suspected in any child who presents with enuresis, poor school performance, failure to thrive, attention-deficit/hyperactivity disorder, or learning disabilities.9 Alternatively, females with OSA have a propensity to emphasize complaints of tiredness, fatigue, or lack of energy rather than report excessive sleepiness per se. These complaints frequently misdirect clinicians toward psychiatric and endocrine diagnoses, without considering the possibility of a sleep disorder.12
Risk Factors
In addition to symptomatology, there are several risk factors associated with OSA that should be considered. The most important are male gender, increasing age (40 to 70), obesity, large neck circumference, and craniofacial and upper airway abnormalities.13 Among the intermediate risk factors are family history of OSA, pregnancy, and Hispanic, Asian, and African-American ethnicity.
Other risk factors associated with OSA include cigarette smoking, alcohol use before sleep, and use of sedative medications.13 Comorbid medical conditions have also been shown to exacerbate and to contribute to OSA; these include GERD, diabetes, congestive heart failure, treatment-refractory hypertension, hypothyroidism, acromegaly, atrial fibrillation, stroke, Down syndrome (due to macroglossia), and pulmonary hypertension.4,10
Screening Questionnaires
Several questionnaires have been validated to screen for OSA. They can be used clinically as part of the sleep-oriented history to quantify a patient’s subjective complaint of sleepiness; however, they are typically more helpful for ruling out sleep apnea than for diagnosing it.11 Two of the most commonly utilized are the Epworth Sleepiness Scale (ESS; http://epworthsleepinessscale.com/epworth-sleepiness-scale.pdf) and the Berlin Questionnaire (www.sleepapnea.org/assets/files/pdf/Berlin%20Questionnaire.pdf).
The ESS was developed to identify patients with excessive daytime sleepiness. It asks patients to rate—on a scale of 0 (would never doze) to 4 (high chance of dozing)—how likely they are to doze off or fall asleep during eight everyday scenarios (ie, sitting and reading; watching TV; sitting inactive in a public place; being a passenger in a vehicle for one or more hours; lying down in the afternoon; sitting and talking to someone; sitting quietly after lunch; or while stopped for a few minutes while driving in traffic).14 The total score can range from 0 to 24. Patients scoring higher than 10 should be referred to a sleep specialist. The ESS can be used to detect symptoms suggestive of OSA; however, it is not specific to OSA and was designed to screen for all sleep disorders.14
In contrast, the Berlin Questionnaire consists of 10 questions that quantify and qualify a patient’s snoring and sleepiness, and it was developed specifically to evaluate for OSA. The questionnaire measures three categories of symptoms: snoring, fatigue, and blood pressure and BMI. A patient is deemed at high risk for OSA when scoring positive in two or more symptom categories, and at low risk when scoring positive in one category.15 However, when OSA is defined as an AHI of ≥ 5 events/hr, the Berlin Questionnaire is 80% sensitive and 46% specific for OSA.11 Therefore, screening questionnaires are useful as supplementary tools but are of limited utility as the sole method for evaluating and diagnosing OSA.
Physical Examination
Physical examination should include assessment for risk factors and comorbidities associated with OSA; this includes measurement of blood pressure, blood glucose, and neck circumference; calculation of BMI and waist-to-hip ratio; and assessment for craniofacial abnormalities. Additionally, cardiovascular, respiratory, and neurologic systems should be evaluated for potential alterations due to OSA.10
Physical exam findings suggestive of OSA are BMI ≥ 30, neck circumference > 17 in for males and > 16 in for females, and craniofacial and upper airway abnormalities. Such anatomic variations include retrognathia, macroglossia (eg, Down syndrome patients), tonsillar hypertrophy (eg, pediatric patients), lateral peritonsillar narrowing, high-arched or narrow hard palate, enlarged uvula, nasal polyps, nasal deviation, turbinate hypertrophy, and oropharynx anatomy consistent with modified Mallampati class III or IV.10 Although OSA is more prevalent in obese patients (those with a BMI ≥ 30), it should be noted that 14.6% of OSA patients are normal weight (BMI < 25) and 34.5%, overweight (BMI 25 to 30).16
The modified Mallampati classification is a straightforward scoring method that was designed to estimate the difficulty of oral intubation based on the extent of mouth opening relative to the size of the tongue.17 According to the protocol set forth by Mallampati and colleagues, assessment is performed with the patient sitting upright with the head in a neutral position, the mouth open, and the tongue maximally protruded without the patient speaking or saying “ahh.”18 The examiner then inspects the pharyngeal structures and classifies the patient’s airway according to the structures visualized. Class I is present when the soft palate, uvula, and pillars are visible; class II when the soft palate and uvula are visible; class III when the soft palate and base of the uvula are visible; class IV when the soft palate is not visible at all (see Figure 1).17,18
An alternative method for observing the pharyngeal structures is via nasopharyngoscopy.19 This procedure can be done in the office setting and utilizes a flexible scope inserted into the nasal cavity and advanced into the pharynx. By providing direct visualization of the upper airway structures, nasopharyngoscopy allows the operator to determine the extent and the location of airway collapse. Imaging by nasopharyngoscopy does not involve any radiation exposure and can be done while the patient is sedated or awake.19
LABORATORY WORKUP AND DIAGNOSIS
Patients deemed high-risk by the sleep-oriented history and physical examination should be subsequently referred to a sleep specialist for objective testing to establish the diagnosis and determine the severity of OSA. Despite the utilization of screening questionnaires, currently there is no consensus on risk stratification to determine when to refer patients with suspected OSA. In other words, no clear thresholds exist on whether a patient is at “low risk” or at “high risk” for OSA based on history and physical examination findings. Therefore, referral is made at the clinician’s discretion, based on the number and severity of OSA symptoms and risk factors.
There are two standard objective sleep study tests: in-laboratory polysomnography (PSG) and home-based sleep studies. The in-laboratory PSG is the gold standard for the diagnosis of OSA.4 The following physiologic signals are measured and interpreted during in-laboratory PSG: electroencephalogram, electro-oculogram, chin electromyogram, airflow, oxygen saturation, respiratory effort, and electrocardiogram.4
Because PSG is resource intensive, home-based sleep studies are an acceptable alternative; however, they should only be considered in patients with a high probability of moderate-to-severe OSA without comorbid sleep disorders or medical conditions. During home-based sleep studies, airflow, respiratory effort, and blood oxygenation are documented.10
The diagnosis of OSA is validated if the AHI on PSG or on home-based sleep studies is > 15 events/h or > 5 events/h with any of the following coexisting signs or symptoms: excessive daytime sleepiness; unintentional sleep episodes during wakefulness; unrefreshing sleep; fatigue; insomnia; waking up holding breath, gasping, or choking; or loud snoring, breathing interruptions, or both, described by the bed partner.10 In addition to diagnosing OSA, PSG and home-based sleep studies are useful in ruling out a variety of conditions that similarly cause excessive daytime sleepiness due to disrupted sleep.
The differential diagnosis of OSA includes, but is not limited to, periodic limb movement disorder, restless legs syndrome, narcolepsy, central sleep apnea, circadian rhythm sleep-wake disorders, respiratory diseases (ie, COPD, asthma), primary snoring, depression, and substance abuse.20
Continue for treatment options >>
TREATMENT OPTIONS
Because OSA is a chronic condition with no definitive cure, lifelong management and follow-up are needed to evaluate treatment adherence/response and the development or resolution of comorbidities. Treatment options for OSA consist primarily of medical (positive airway pressure, use of oral appliances, and pharmacotherapy), behavioral (weight loss, positional therapy, and substance avoidance), and surgical approaches. The patient should actively participate in the treatment and management of this chronic condition.10
Positive Airway Pressure
Regardless of OSA severity, the treatment of choice is positive airway pressure (PAP) during sleep, with continuous PAP (CPAP) being the recommended mode of delivery. PAP is delivered via a face or nasal mask attached to a machine that maintains a constant upper airway pressure, which in turn prevents pharyngeal collapse during sleep. As a result, the upper airway remains patent, and apneas and hypopneas are inhibited.4
PAP can be delivered in three different modes: CPAP, bilevel (BPAP), or autotitrating (APAP). CPAP provides PAP at a fixed rate throughout the respiratory cycle. In contrast, BPAP delivers a preset PAP that is greater during inspiration than during expiration. BPAP is used to alleviate the discomfort of exhaling against the fixed airway pressure of traditional CPAP by delivering lower pressure during expiration than during inspiration. APAP delivers fluctuating positive airway pressures according to changes in the patient’s breathing patterns and is used in patients with complex breathing patterns. CPAP is the recommended mode of airway pressure delivery; however, BPAP or APAP can be considered in patients who cannot tolerate CPAP.10,21
The amount of pressure needed to maintain airway patency is determined during PSG, and the CPAP system is titrated up to this optimal level. Patient adherence is a commonly encountered problem with CPAP therapy due to adverse effects, such as skin irritation, misfit of mask, dry mouth, nasal congestion, and rhinorrhea. Humidification, nasal saline sprays, and mask resizing can help to alleviate such symptoms.4 Follow-up in the early stages should be frequent enough to troubleshoot and remediate problems with the PAP mask, the machine, and adherence. Long-term follow-up for maintenance, adherence, and signs and symptoms of worsening OSA should be scheduled yearly.10
Oral Appliances
Oral appliances (OAs) are an alternative treatment option for OSA patients who are nonadherent with CPAP, fail CPAP or behavioral therapies, experience adverse effects from CPAP, or prefer OAs. Two types of OAs exist: mandibular repositioning appliances and tongue retaining devices. The former anteriorly displaces the mandible relative to the maxilla; the latter anteriorly displaces the tongue only. Regardless of the type selected, OAs are designed to increase the diameter of the pharynx and decrease pharyngeal collapsibility.10
Although less efficacious than traditional CPAP therapy, OAs may be considered in patients with mild-to-moderate OSA.10 Patients with severe OSA should not be offered OAs as a treatment option. Current evidence suggests that OAs can substantially reduce the severity of OSA as well as daytime sleepiness. Additionally, OAs are superior to CPAP in patient preference, which could improve long-term patient adherence to treatment.22
Prior to initiating therapy with an OA, patients should have a dental examination to assess candidacy for, and proper fit of, an OA. Regular follow-ups are required initially to ensure proper fit and comfort of the device. Patients should be instructed to make an office visit if they experience adverse effects such as temporomandibular joint discomfort, tooth tenderness, excessive salivation, and gum irritation.22 Once final adjustments have been made to the fit of the OA, a PSG should be performed to ensure maximal therapeutic efficacy. Follow-up with a dental specialist is advised every six months for the first year and yearly thereafter.10
Pharmacology
According to the practice parameters of the American Academy of Sleep Medicine (AASM), there are currently no pharmacologic agents that prevent or overcome upper airway obstruction well enough to be considered a primary treatment option for OSA.23 The most widely studied pharmacologic agents—selective serotonergic reuptake inhibitors, protriptyline, methylxanthine derivatives (aminophylline and theophylline), estrogen therapy (with or without progesterone), supplemental oxygen, and short-acting nasal decongestants—are not recommended for the primary treatment of OSA.23 Pharmacologic therapy is therefore most beneficial when used as adjuvant therapy (which targets a residual symptom) rather than primary therapy (which targets the underlying cause of the disorder).
There are currently two FDA-approved medications for the treatment of residual excessive daytime sleepiness in patients who are receiving adequate primary treatment (eg, PAP, OAs) and who do not have any other identifiable cause for the sleepiness: modafinil and armodafinil.23,24 The exact mechanism of action of these agents has not been fully characterized, but they are known to act on the central nervous system to enhance alertness (likely by enhancing dopamine signaling).24
Prior to prescribing these medications, other causes of sleepiness must be ruled out; these include suboptimal adherence with PAP, poorly fitting PAP mask, poor sleep hygiene, insufficient sleep, other sleep disorders, and depression.10 Patients should be informed that these drugs should be used in conjunction with pre-existing CPAP therapy. Common adverse effects of modafinil include headache, dizziness, gastrointestinal upset, and insomnia. This medication should be used cautiously in patients with a history of cardiovascular and/or psychologic problems because it can cause serious adverse effects, including chest pain, palpitations, hypertension, hallucinations, aggression, anxiety, depression, and severe skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.4,24
Weight Loss
Because obesity is one of the most prevalent risk factors for OSA, weight loss should be recommended for all overweight and obese OSA patients.13,23 Weight loss can be achieved by several different methods, including exercise programs, dietary programs, a combination of the two, and bariatric surgery. A recent meta-analysis found that exercise alone is less successful in reducing AHI compared to dietary modifications; however, a combination of exercise and dietary changes is superior to both individual interventions in reducing AHI.25 This meta-analysis also found that weight-loss interventions improve, but fail to normalize, AHI.25 Thus, weight-loss interventions should be an adjuvant therapy in the treatment of OSA.23
Another recent meta-analysis found that lifestyle interventions, specifically exercise training, might improve OSA severity and symptoms even in the absence of significant weight loss. According to this study, exercise training produced a 32% reduction in AHI in OSA patients and a 42% reduction in AHI when compared to OSA patients who did not partake in exercise training.26 Additionally, OSA patients experienced significant improvements in cardiorespiratory fitness, daytime sleepiness, and sleep efficiency. Because there was no significant reduction in BMI with exercise training, it is likely that there are additional benefits of exercise training that are independent of the effects on weight loss.26
Positional Therapy
Approximately 50% to 60% of OSA patients have positional OSA, which is classified as an increase of 50% or more in the AHI when sleeping in the supine position compared with nonsupine sleep positions.27 It is thought that positional therapy, consisting of any technique that maintains the patient in a nonsupine sleep position, decreases the tendency for the tongue to prolapse and for the airway to collapse.27 Several positional devices exist, including pillows, tennis balls, backpacks, and vibratory chest alarms. Additionally, sleeping in the lateral recumbent position can function as a form of positional therapy.4,21
According to the AASM, less obese, younger patients and those with less severe OSA are more likely to achieve a normalized AHI by sleeping in a nonsupine position; thus, these patients are more likely to benefit from positional therapy than their OSA counterparts.23,27 A recent study found that CPAP therapy is superior to positional therapy in reducing apneic episodes (ie, AHI) in patients with positional OSA.27 Thus, positional therapy is an effective secondary treatment that can be used as an adjuvant therapy to CPAP in patients with mild positional OSA.23,27
Substance Avoidance
All patients with OSA should be advised to avoid alcohol, as it has been shown to increase the duration and frequency of apneic episodes and lower arterial oxyhemoglobin saturation during sleep.28 Not only does alcohol exacerbate preexisting OSA, but it has also been shown to induce frank OSA in patients who snore but do not have OSA at baseline.28 Sedative medications (eg, benzodiazepines) have similar effects on the central nervous system and should also be avoided if possible. Both of these substances have inhibitory actions on the central nervous system, thereby relaxing the pharyngeal muscles and promoting upper airway collapse during sleep.
Because cigarette smoking is an independent risk factor for snoring and thus a presumed risk factor for OSA, smoking cessation is a necessary component of the treatment of OSA.13,29 Cigarette smoking is thought to increase airway inflammation, exacerbate preexisting lung conditions such as COPD and asthma, and induce sleep fragmentation due to the effects of nicotine withdrawal during sleep.29 Alcohol, sedative medications, and cigarettes have all been shown to exacerbate OSA; thus, patient education about the harmful effects of these substances and the benefits of avoiding them is a crucial aspect of OSA therapy.
Surgical Therapies
When both CPAP and OA are inadequate in the management of OSA and/or when obstructive or functional anatomic abnormalities exist, upper airway surgery can be considered for patients. There is currently no consensus about the role of surgery in OSA patients, nor are there clear guidelines or screening questionnaires that accurately predict which patients will benefit the most from upper airway surgery. The appropriate surgical procedure depends on the site of anatomic abnormality and could be a nasal, oral, oropharyngeal, nasopharyngeal, hypopharyngeal, laryngeal, or global airway procedure.10 Some of the most common surgical procedures for the management of OSA are described below.
Uvulopalatopharyngoplasty (UPPP). This surgical procedure involves resecting the entire uvula and the obstructive portion of the soft palate while resizing and reorienting the tonsillar pillars. As a sole procedure, with or without tonsillectomy, UPPP does not reliably normalize the AHI when treating moderate-to-severe OSA.30
Radiofrequency ablation (RFA). RFA is a less invasive variation of UPPP that involves using a temperature-controlled probe to deliver energy to the upper airway tissue (typically the tongue base and/or the soft palate) in an effort to induce palatal stiffening. This procedure can be considered in patients with mild-to-moderate OSA who cannot tolerate or are unwilling to adhere to PAP or OA therapies.30
Maxillo-mandibular advancement (MMA). This operation involves indirect advancement of the anterior pharyngeal tissues (ie, the soft palate, tongue base, and suprahyoid musculature) via their attachment to the maxilla, mandible, and hyoid bone. The simultaneous advancement of the maxilla and mandible are accomplished by sagittal split osteotomies that are stabilized with plates, screws, or bone grafts.31 This surgery is designed to enlarge the retrolingual airway and provide some advancement of the retropalate without directly manipulating the pharyngeal tissues. MMA is indicated for patients with severe OSA who cannot tolerate or are unwilling to adhere to PAP therapy or in whom OAs have been found ineffective.30
Tracheostomy. This procedure consists of creating an airway through the anterior neck into the upper trachea. This opening bypasses the entire upper airway obstruction and thus is 100% effective in curing OSA. However, tracheostomy is typically last-line due to the resulting undesirable alterations in the patient’s physical appearance and to the risks associated with the procedure.5 According to the AASM, this operation should only be considered when other options do not exist, have failed, or are refused, or when this operation is deemed necessary by clinical urgency.30
Hypoglossal nerve stimulation. This therapy uses a surgically implanted device, approved by the FDA in 2014, to detect the patient’s breathing pattern and stimulate the hypoglossal nerve, causing tongue protrusion during inspiration. Tongue movement is controlled, and thus, the airway remains patent during inspiration.32 It is indicated for use in patients with moderate-to-severe OSA who have refused CPAP or for whom CPAP treatment has been unsuccessful.
According to a recently published study, a 68% decrease in median AHI at 12 months postimplantation was reported among those receiving hypoglossal nerve stimulation.33 Additionally, OSA patients subjectively reported decreased sleepiness and an increased quality of life compared to baseline.
The most common adverse effects were transient tongue weakness postoperatively, discomfort related to stimulation, and tongue soreness.33 Its use is contraindicated in patients who are pregnant or plan to become pregnant and in those who will require MRI, who have other implantable devices that may interact with the stimulation system, who have any condition that may affect neurologic control of the upper airway, and who have any anatomic abnormalities that may prevent effective performance of the upper airway stimulation (eg, the presence of complete concentric collapse at the retropalatal airway during endoscopy).32,33 Although its use is promising for the treatment of OSA, hypoglossal nerve stimulation is still a novel option that is not yet readily employed.33
OUTCOMES ASSESSMENT
Clinical judgment should be used to determine the appropriate treatment(s) based on OSA severity and patient preference. Regardless of the treatment option chosen, all OSA patients should have an outcomes assessment performed after the initiation of therapy. Indicators to monitor include resolution of sleepiness, OSA-specific quality-of-life measures, adherence to therapy, avoidance of factors exacerbating OSA (eg, alcohol, tobacco, sedative medications), amount of sleep being obtained, sleep hygiene practices, weight loss, and patient and spousal satisfaction.10 Patients with all levels of OSA severity should receive ongoing management to ensure long-term resolution of symptoms and adherence to treatment. Improvements in primary care treatment, follow-up, and outcomes evaluation are becoming increasingly important to address the symptoms and complications that make OSA a major public health concern.
CE/CME No: CR-1508
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the diagnostic criteria and clinical presentation of obstructive sleep apnea (OSA).
• Discuss the common screening questionnaires used in clinical practice to identify patients at risk for OSA.
• Describe the common comorbidities associated with OSA.
• Identify the features of pharyngeal structures used in the modified Mallampati classification.
• Know how to provide support and education for the patient in regard to treatment options, weight loss, residual daytime sleepiness, and smoking and alcohol cessation.
FACULTY
Bonnie Dadig is the Chair and Program Director of the Georgia Regents University (GRU) Physician Assistant Department and a PA in the GRU Department of Family Medicine outpatient clinic, Augusta. Morgan Edwards is a recently graduated PA student from GRU. The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of August 2015.
Article begins on next page >>
Nearly 22 million Americans are affected by obstructive sleep apnea (OSA), making it the most common sleep disorder. Patients with undiagnosed and untreated OSA are at increased risk for cardiovascular and cerebrovascular health consequences and excessive daytime sleepiness. Here’s how you can address the symptoms and complications that make OSA a major public health concern, and as a result, decrease economic burdens and increase quality of life.
Obstructive sleep apnea (OSA) is an increasingly common diagnosis, with nearly 22 million Americans currently affected.1 In 2010, the diagnosis of OSA was recorded in 5.8 million office visits made by patients ages 20 or older—a 427% increase since 1999.2 While nearly 50% of patients in primary care settings complain of sleep disturbances, up to 80% of OSA cases remain undiagnosed.1,3 The high prevalence of sleep disorders in both the general population and in primary care settings, along with the underdiagnosis of OSA, illustrates the essential role of primary care providers in recognizing the signs and symptoms of OSA and evaluating patients for this condition.1-3
BACKGROUND AND PATHOPHYSIOLOGY
OSA is the most common sleep disorder. Characterized by recurrent episodes of upper airway obstruction during sleep, it results in significantly reduced airflow (hypopnea) or complete cessation of breathing (apnea) for intervals lasting ≥ 10 seconds.4,5 The total number of apneas or hypopneas per hour of sleep—the apnea-hypopnea index (AHI)—is one domain that can be used to classify the severity of OSA (see Table 1).5
Because the human upper airway lacks rigid or bony support, under normal circumstances, the pharyngeal dilator muscles act to oppose the negative pressure within the upper airway during inspiration when awake. And although the striated nature of the pharyngeal dilator muscles leads to a reduction in airway muscle tone during sleep, enough opposition is maintained to prevent airway collapse. In OSA patients, however, the reduced pharyngeal muscle tone fails to offset the negative airway pressure generated during inspiration while asleep, causing recurrent airway obstructions.6 In addition, anatomically narrowed upper airways can further impede airway patency during sleep.4 Causes include macroglossia and/or tonsillar hypertrophy, obesity with fat tissue deposits in the neck that compress the airway, and bony craniofacial abnormalities such as retrognathia.7
Disruptions in breathing from apneas and hypopneas result in hypercapnia and hypoxia, which trigger arousal centers in the central nervous system to increase sympathetic activity. As a result, the patient briefly awakens from sleep, which increases respiratory and pharyngeal dilator muscle tone and ultimately restores airway patency, and ventilation resumes.4
The sympathetic response associated with apneic and hypopneic episodes causes concomitant cardiac stimulation. This leads to transient vasoconstriction and elevations in heart rate and blood pressure. Vascular changes occur over time and result in persistent hypertension and other cardiovascular impairments.4
Undiagnosed and untreated OSA predisposes individuals to a multitude of health consequences and ultimately results in substantial public health and economic burdens. A recent study estimated that the economic cost of moderate-to-severe OSA in the United States is between $65 billion and $165 billion annually.8 Patients with OSA are at increased risk for complications such as systemic and pulmonary hypertension, congestive heart failure, cardiac arrhythmias, coronary artery disease, myocardial infarction, cerebrovascular accident, diabetes, and metabolic syndrome.9 Excessive daytime sleepiness from OSA has also been shown to contribute to increased rates of motor vehicle accidents, poor job performance, impaired cognitive function, and decreased quality-of-life measures.9
Continue for clinical evaluation >>
CLINICAL EVALUATION
Patient History
Clinicians become aware of patients with OSA when they present with associated symptoms or complications. These symptoms are encountered in several settings—as a part of the routine health maintenance examination, as the patient’s presenting complaint, or as a part of the evaluation for another complaint or medical condition.
Regardless of how the symptoms are discovered, all patients with symptoms of and/or positive risk factors for OSA should be assessed via a comprehensive sleep oriented history and physical examination. Symptoms suggestive of OSA include witnessed or reported apneas, snoring, gasping/choking at night, excessive daytime sleepiness not explained by other factors, severity of sleepiness (as determined by the Epworth Sleepiness Scale), nonrefreshing sleep, sleep fragmentation/maintenance insomnia, nocturia, morning headaches, decreased concentration, memory loss, decreased libido, and irritability.10 A recently published study found that nocturnal gasping or choking is the most reliable indicator for identifying patients with OSA.11
The clinical presentation of OSA in certain populations may differ from that of typical adult OSA, requiring a higher degree of clinical suspicion. For instance, pediatric OSA should be suspected in any child who presents with enuresis, poor school performance, failure to thrive, attention-deficit/hyperactivity disorder, or learning disabilities.9 Alternatively, females with OSA have a propensity to emphasize complaints of tiredness, fatigue, or lack of energy rather than report excessive sleepiness per se. These complaints frequently misdirect clinicians toward psychiatric and endocrine diagnoses, without considering the possibility of a sleep disorder.12
Risk Factors
In addition to symptomatology, there are several risk factors associated with OSA that should be considered. The most important are male gender, increasing age (40 to 70), obesity, large neck circumference, and craniofacial and upper airway abnormalities.13 Among the intermediate risk factors are family history of OSA, pregnancy, and Hispanic, Asian, and African-American ethnicity.
Other risk factors associated with OSA include cigarette smoking, alcohol use before sleep, and use of sedative medications.13 Comorbid medical conditions have also been shown to exacerbate and to contribute to OSA; these include GERD, diabetes, congestive heart failure, treatment-refractory hypertension, hypothyroidism, acromegaly, atrial fibrillation, stroke, Down syndrome (due to macroglossia), and pulmonary hypertension.4,10
Screening Questionnaires
Several questionnaires have been validated to screen for OSA. They can be used clinically as part of the sleep-oriented history to quantify a patient’s subjective complaint of sleepiness; however, they are typically more helpful for ruling out sleep apnea than for diagnosing it.11 Two of the most commonly utilized are the Epworth Sleepiness Scale (ESS; http://epworthsleepinessscale.com/epworth-sleepiness-scale.pdf) and the Berlin Questionnaire (www.sleepapnea.org/assets/files/pdf/Berlin%20Questionnaire.pdf).
The ESS was developed to identify patients with excessive daytime sleepiness. It asks patients to rate—on a scale of 0 (would never doze) to 4 (high chance of dozing)—how likely they are to doze off or fall asleep during eight everyday scenarios (ie, sitting and reading; watching TV; sitting inactive in a public place; being a passenger in a vehicle for one or more hours; lying down in the afternoon; sitting and talking to someone; sitting quietly after lunch; or while stopped for a few minutes while driving in traffic).14 The total score can range from 0 to 24. Patients scoring higher than 10 should be referred to a sleep specialist. The ESS can be used to detect symptoms suggestive of OSA; however, it is not specific to OSA and was designed to screen for all sleep disorders.14
In contrast, the Berlin Questionnaire consists of 10 questions that quantify and qualify a patient’s snoring and sleepiness, and it was developed specifically to evaluate for OSA. The questionnaire measures three categories of symptoms: snoring, fatigue, and blood pressure and BMI. A patient is deemed at high risk for OSA when scoring positive in two or more symptom categories, and at low risk when scoring positive in one category.15 However, when OSA is defined as an AHI of ≥ 5 events/hr, the Berlin Questionnaire is 80% sensitive and 46% specific for OSA.11 Therefore, screening questionnaires are useful as supplementary tools but are of limited utility as the sole method for evaluating and diagnosing OSA.
Physical Examination
Physical examination should include assessment for risk factors and comorbidities associated with OSA; this includes measurement of blood pressure, blood glucose, and neck circumference; calculation of BMI and waist-to-hip ratio; and assessment for craniofacial abnormalities. Additionally, cardiovascular, respiratory, and neurologic systems should be evaluated for potential alterations due to OSA.10
Physical exam findings suggestive of OSA are BMI ≥ 30, neck circumference > 17 in for males and > 16 in for females, and craniofacial and upper airway abnormalities. Such anatomic variations include retrognathia, macroglossia (eg, Down syndrome patients), tonsillar hypertrophy (eg, pediatric patients), lateral peritonsillar narrowing, high-arched or narrow hard palate, enlarged uvula, nasal polyps, nasal deviation, turbinate hypertrophy, and oropharynx anatomy consistent with modified Mallampati class III or IV.10 Although OSA is more prevalent in obese patients (those with a BMI ≥ 30), it should be noted that 14.6% of OSA patients are normal weight (BMI < 25) and 34.5%, overweight (BMI 25 to 30).16
The modified Mallampati classification is a straightforward scoring method that was designed to estimate the difficulty of oral intubation based on the extent of mouth opening relative to the size of the tongue.17 According to the protocol set forth by Mallampati and colleagues, assessment is performed with the patient sitting upright with the head in a neutral position, the mouth open, and the tongue maximally protruded without the patient speaking or saying “ahh.”18 The examiner then inspects the pharyngeal structures and classifies the patient’s airway according to the structures visualized. Class I is present when the soft palate, uvula, and pillars are visible; class II when the soft palate and uvula are visible; class III when the soft palate and base of the uvula are visible; class IV when the soft palate is not visible at all (see Figure 1).17,18
An alternative method for observing the pharyngeal structures is via nasopharyngoscopy.19 This procedure can be done in the office setting and utilizes a flexible scope inserted into the nasal cavity and advanced into the pharynx. By providing direct visualization of the upper airway structures, nasopharyngoscopy allows the operator to determine the extent and the location of airway collapse. Imaging by nasopharyngoscopy does not involve any radiation exposure and can be done while the patient is sedated or awake.19
LABORATORY WORKUP AND DIAGNOSIS
Patients deemed high-risk by the sleep-oriented history and physical examination should be subsequently referred to a sleep specialist for objective testing to establish the diagnosis and determine the severity of OSA. Despite the utilization of screening questionnaires, currently there is no consensus on risk stratification to determine when to refer patients with suspected OSA. In other words, no clear thresholds exist on whether a patient is at “low risk” or at “high risk” for OSA based on history and physical examination findings. Therefore, referral is made at the clinician’s discretion, based on the number and severity of OSA symptoms and risk factors.
There are two standard objective sleep study tests: in-laboratory polysomnography (PSG) and home-based sleep studies. The in-laboratory PSG is the gold standard for the diagnosis of OSA.4 The following physiologic signals are measured and interpreted during in-laboratory PSG: electroencephalogram, electro-oculogram, chin electromyogram, airflow, oxygen saturation, respiratory effort, and electrocardiogram.4
Because PSG is resource intensive, home-based sleep studies are an acceptable alternative; however, they should only be considered in patients with a high probability of moderate-to-severe OSA without comorbid sleep disorders or medical conditions. During home-based sleep studies, airflow, respiratory effort, and blood oxygenation are documented.10
The diagnosis of OSA is validated if the AHI on PSG or on home-based sleep studies is > 15 events/h or > 5 events/h with any of the following coexisting signs or symptoms: excessive daytime sleepiness; unintentional sleep episodes during wakefulness; unrefreshing sleep; fatigue; insomnia; waking up holding breath, gasping, or choking; or loud snoring, breathing interruptions, or both, described by the bed partner.10 In addition to diagnosing OSA, PSG and home-based sleep studies are useful in ruling out a variety of conditions that similarly cause excessive daytime sleepiness due to disrupted sleep.
The differential diagnosis of OSA includes, but is not limited to, periodic limb movement disorder, restless legs syndrome, narcolepsy, central sleep apnea, circadian rhythm sleep-wake disorders, respiratory diseases (ie, COPD, asthma), primary snoring, depression, and substance abuse.20
Continue for treatment options >>
TREATMENT OPTIONS
Because OSA is a chronic condition with no definitive cure, lifelong management and follow-up are needed to evaluate treatment adherence/response and the development or resolution of comorbidities. Treatment options for OSA consist primarily of medical (positive airway pressure, use of oral appliances, and pharmacotherapy), behavioral (weight loss, positional therapy, and substance avoidance), and surgical approaches. The patient should actively participate in the treatment and management of this chronic condition.10
Positive Airway Pressure
Regardless of OSA severity, the treatment of choice is positive airway pressure (PAP) during sleep, with continuous PAP (CPAP) being the recommended mode of delivery. PAP is delivered via a face or nasal mask attached to a machine that maintains a constant upper airway pressure, which in turn prevents pharyngeal collapse during sleep. As a result, the upper airway remains patent, and apneas and hypopneas are inhibited.4
PAP can be delivered in three different modes: CPAP, bilevel (BPAP), or autotitrating (APAP). CPAP provides PAP at a fixed rate throughout the respiratory cycle. In contrast, BPAP delivers a preset PAP that is greater during inspiration than during expiration. BPAP is used to alleviate the discomfort of exhaling against the fixed airway pressure of traditional CPAP by delivering lower pressure during expiration than during inspiration. APAP delivers fluctuating positive airway pressures according to changes in the patient’s breathing patterns and is used in patients with complex breathing patterns. CPAP is the recommended mode of airway pressure delivery; however, BPAP or APAP can be considered in patients who cannot tolerate CPAP.10,21
The amount of pressure needed to maintain airway patency is determined during PSG, and the CPAP system is titrated up to this optimal level. Patient adherence is a commonly encountered problem with CPAP therapy due to adverse effects, such as skin irritation, misfit of mask, dry mouth, nasal congestion, and rhinorrhea. Humidification, nasal saline sprays, and mask resizing can help to alleviate such symptoms.4 Follow-up in the early stages should be frequent enough to troubleshoot and remediate problems with the PAP mask, the machine, and adherence. Long-term follow-up for maintenance, adherence, and signs and symptoms of worsening OSA should be scheduled yearly.10
Oral Appliances
Oral appliances (OAs) are an alternative treatment option for OSA patients who are nonadherent with CPAP, fail CPAP or behavioral therapies, experience adverse effects from CPAP, or prefer OAs. Two types of OAs exist: mandibular repositioning appliances and tongue retaining devices. The former anteriorly displaces the mandible relative to the maxilla; the latter anteriorly displaces the tongue only. Regardless of the type selected, OAs are designed to increase the diameter of the pharynx and decrease pharyngeal collapsibility.10
Although less efficacious than traditional CPAP therapy, OAs may be considered in patients with mild-to-moderate OSA.10 Patients with severe OSA should not be offered OAs as a treatment option. Current evidence suggests that OAs can substantially reduce the severity of OSA as well as daytime sleepiness. Additionally, OAs are superior to CPAP in patient preference, which could improve long-term patient adherence to treatment.22
Prior to initiating therapy with an OA, patients should have a dental examination to assess candidacy for, and proper fit of, an OA. Regular follow-ups are required initially to ensure proper fit and comfort of the device. Patients should be instructed to make an office visit if they experience adverse effects such as temporomandibular joint discomfort, tooth tenderness, excessive salivation, and gum irritation.22 Once final adjustments have been made to the fit of the OA, a PSG should be performed to ensure maximal therapeutic efficacy. Follow-up with a dental specialist is advised every six months for the first year and yearly thereafter.10
Pharmacology
According to the practice parameters of the American Academy of Sleep Medicine (AASM), there are currently no pharmacologic agents that prevent or overcome upper airway obstruction well enough to be considered a primary treatment option for OSA.23 The most widely studied pharmacologic agents—selective serotonergic reuptake inhibitors, protriptyline, methylxanthine derivatives (aminophylline and theophylline), estrogen therapy (with or without progesterone), supplemental oxygen, and short-acting nasal decongestants—are not recommended for the primary treatment of OSA.23 Pharmacologic therapy is therefore most beneficial when used as adjuvant therapy (which targets a residual symptom) rather than primary therapy (which targets the underlying cause of the disorder).
There are currently two FDA-approved medications for the treatment of residual excessive daytime sleepiness in patients who are receiving adequate primary treatment (eg, PAP, OAs) and who do not have any other identifiable cause for the sleepiness: modafinil and armodafinil.23,24 The exact mechanism of action of these agents has not been fully characterized, but they are known to act on the central nervous system to enhance alertness (likely by enhancing dopamine signaling).24
Prior to prescribing these medications, other causes of sleepiness must be ruled out; these include suboptimal adherence with PAP, poorly fitting PAP mask, poor sleep hygiene, insufficient sleep, other sleep disorders, and depression.10 Patients should be informed that these drugs should be used in conjunction with pre-existing CPAP therapy. Common adverse effects of modafinil include headache, dizziness, gastrointestinal upset, and insomnia. This medication should be used cautiously in patients with a history of cardiovascular and/or psychologic problems because it can cause serious adverse effects, including chest pain, palpitations, hypertension, hallucinations, aggression, anxiety, depression, and severe skin reactions, such as Stevens-Johnson syndrome and toxic epidermal necrolysis.4,24
Weight Loss
Because obesity is one of the most prevalent risk factors for OSA, weight loss should be recommended for all overweight and obese OSA patients.13,23 Weight loss can be achieved by several different methods, including exercise programs, dietary programs, a combination of the two, and bariatric surgery. A recent meta-analysis found that exercise alone is less successful in reducing AHI compared to dietary modifications; however, a combination of exercise and dietary changes is superior to both individual interventions in reducing AHI.25 This meta-analysis also found that weight-loss interventions improve, but fail to normalize, AHI.25 Thus, weight-loss interventions should be an adjuvant therapy in the treatment of OSA.23
Another recent meta-analysis found that lifestyle interventions, specifically exercise training, might improve OSA severity and symptoms even in the absence of significant weight loss. According to this study, exercise training produced a 32% reduction in AHI in OSA patients and a 42% reduction in AHI when compared to OSA patients who did not partake in exercise training.26 Additionally, OSA patients experienced significant improvements in cardiorespiratory fitness, daytime sleepiness, and sleep efficiency. Because there was no significant reduction in BMI with exercise training, it is likely that there are additional benefits of exercise training that are independent of the effects on weight loss.26
Positional Therapy
Approximately 50% to 60% of OSA patients have positional OSA, which is classified as an increase of 50% or more in the AHI when sleeping in the supine position compared with nonsupine sleep positions.27 It is thought that positional therapy, consisting of any technique that maintains the patient in a nonsupine sleep position, decreases the tendency for the tongue to prolapse and for the airway to collapse.27 Several positional devices exist, including pillows, tennis balls, backpacks, and vibratory chest alarms. Additionally, sleeping in the lateral recumbent position can function as a form of positional therapy.4,21
According to the AASM, less obese, younger patients and those with less severe OSA are more likely to achieve a normalized AHI by sleeping in a nonsupine position; thus, these patients are more likely to benefit from positional therapy than their OSA counterparts.23,27 A recent study found that CPAP therapy is superior to positional therapy in reducing apneic episodes (ie, AHI) in patients with positional OSA.27 Thus, positional therapy is an effective secondary treatment that can be used as an adjuvant therapy to CPAP in patients with mild positional OSA.23,27
Substance Avoidance
All patients with OSA should be advised to avoid alcohol, as it has been shown to increase the duration and frequency of apneic episodes and lower arterial oxyhemoglobin saturation during sleep.28 Not only does alcohol exacerbate preexisting OSA, but it has also been shown to induce frank OSA in patients who snore but do not have OSA at baseline.28 Sedative medications (eg, benzodiazepines) have similar effects on the central nervous system and should also be avoided if possible. Both of these substances have inhibitory actions on the central nervous system, thereby relaxing the pharyngeal muscles and promoting upper airway collapse during sleep.
Because cigarette smoking is an independent risk factor for snoring and thus a presumed risk factor for OSA, smoking cessation is a necessary component of the treatment of OSA.13,29 Cigarette smoking is thought to increase airway inflammation, exacerbate preexisting lung conditions such as COPD and asthma, and induce sleep fragmentation due to the effects of nicotine withdrawal during sleep.29 Alcohol, sedative medications, and cigarettes have all been shown to exacerbate OSA; thus, patient education about the harmful effects of these substances and the benefits of avoiding them is a crucial aspect of OSA therapy.
Surgical Therapies
When both CPAP and OA are inadequate in the management of OSA and/or when obstructive or functional anatomic abnormalities exist, upper airway surgery can be considered for patients. There is currently no consensus about the role of surgery in OSA patients, nor are there clear guidelines or screening questionnaires that accurately predict which patients will benefit the most from upper airway surgery. The appropriate surgical procedure depends on the site of anatomic abnormality and could be a nasal, oral, oropharyngeal, nasopharyngeal, hypopharyngeal, laryngeal, or global airway procedure.10 Some of the most common surgical procedures for the management of OSA are described below.
Uvulopalatopharyngoplasty (UPPP). This surgical procedure involves resecting the entire uvula and the obstructive portion of the soft palate while resizing and reorienting the tonsillar pillars. As a sole procedure, with or without tonsillectomy, UPPP does not reliably normalize the AHI when treating moderate-to-severe OSA.30
Radiofrequency ablation (RFA). RFA is a less invasive variation of UPPP that involves using a temperature-controlled probe to deliver energy to the upper airway tissue (typically the tongue base and/or the soft palate) in an effort to induce palatal stiffening. This procedure can be considered in patients with mild-to-moderate OSA who cannot tolerate or are unwilling to adhere to PAP or OA therapies.30
Maxillo-mandibular advancement (MMA). This operation involves indirect advancement of the anterior pharyngeal tissues (ie, the soft palate, tongue base, and suprahyoid musculature) via their attachment to the maxilla, mandible, and hyoid bone. The simultaneous advancement of the maxilla and mandible are accomplished by sagittal split osteotomies that are stabilized with plates, screws, or bone grafts.31 This surgery is designed to enlarge the retrolingual airway and provide some advancement of the retropalate without directly manipulating the pharyngeal tissues. MMA is indicated for patients with severe OSA who cannot tolerate or are unwilling to adhere to PAP therapy or in whom OAs have been found ineffective.30
Tracheostomy. This procedure consists of creating an airway through the anterior neck into the upper trachea. This opening bypasses the entire upper airway obstruction and thus is 100% effective in curing OSA. However, tracheostomy is typically last-line due to the resulting undesirable alterations in the patient’s physical appearance and to the risks associated with the procedure.5 According to the AASM, this operation should only be considered when other options do not exist, have failed, or are refused, or when this operation is deemed necessary by clinical urgency.30
Hypoglossal nerve stimulation. This therapy uses a surgically implanted device, approved by the FDA in 2014, to detect the patient’s breathing pattern and stimulate the hypoglossal nerve, causing tongue protrusion during inspiration. Tongue movement is controlled, and thus, the airway remains patent during inspiration.32 It is indicated for use in patients with moderate-to-severe OSA who have refused CPAP or for whom CPAP treatment has been unsuccessful.
According to a recently published study, a 68% decrease in median AHI at 12 months postimplantation was reported among those receiving hypoglossal nerve stimulation.33 Additionally, OSA patients subjectively reported decreased sleepiness and an increased quality of life compared to baseline.
The most common adverse effects were transient tongue weakness postoperatively, discomfort related to stimulation, and tongue soreness.33 Its use is contraindicated in patients who are pregnant or plan to become pregnant and in those who will require MRI, who have other implantable devices that may interact with the stimulation system, who have any condition that may affect neurologic control of the upper airway, and who have any anatomic abnormalities that may prevent effective performance of the upper airway stimulation (eg, the presence of complete concentric collapse at the retropalatal airway during endoscopy).32,33 Although its use is promising for the treatment of OSA, hypoglossal nerve stimulation is still a novel option that is not yet readily employed.33
OUTCOMES ASSESSMENT
Clinical judgment should be used to determine the appropriate treatment(s) based on OSA severity and patient preference. Regardless of the treatment option chosen, all OSA patients should have an outcomes assessment performed after the initiation of therapy. Indicators to monitor include resolution of sleepiness, OSA-specific quality-of-life measures, adherence to therapy, avoidance of factors exacerbating OSA (eg, alcohol, tobacco, sedative medications), amount of sleep being obtained, sleep hygiene practices, weight loss, and patient and spousal satisfaction.10 Patients with all levels of OSA severity should receive ongoing management to ensure long-term resolution of symptoms and adherence to treatment. Improvements in primary care treatment, follow-up, and outcomes evaluation are becoming increasingly important to address the symptoms and complications that make OSA a major public health concern.
1. American Sleep Apnea Association. A very short course on sleep apnea. www.sleepapnea.org/i-am-a-health-care-professional.html. Accessed July 21, 2015.
2. Ford ES, Wheaton AG, Cunningham TJ, et al. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care Survey 1999-2010. Sleep. 2014;37(8):1283-1293.
3. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. NIH Consens Sci Statements. 2005;22(2):1-30.
4. Carlucci M, Smith M, Corbridge SJ. Poor sleep, hazardous breathing: an overview of obstructive sleep apnea. Nurse Pract. 2013;38(3):20-28.
5. Institute for Clinical Systems Improvement. Health Care Guideline: Diagnosis and Treatment of Obstructive Sleep Apnea. 6th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2008.
6. Douglas NJ. Sleep apnea. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:2186-2188.
7. US Department of Health and Human Services, National Heart, Lung, and Blood Institute. What causes sleep apnea? www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/causes.html. Accessed uly 21, 2015.
8. Harvard Medical School, Division of Sleep Medicine. The price of fatigue: the surprising economic costs of unmanaged sleep apnea. December 2010. https://sleep.med.harvard.edu/file_download/100. Accessed July 21, 2015.
9. Pagel JF. Obstructive sleep apnea (OSA) in primary care: evidence-based practice. J Am Board Fam Med. 2007;20(4):392-398.
10. Epstein LJ, Kristo D, Strollo PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
11. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea: the rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
12. Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10(10):1075-1084.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013-2016.
14. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
15. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485-491.
16. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441-446.
17. Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487-490.
18. Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434.
19. Gregorio MG, Jacomelli M, Figueiredo AC, et al. Evaluation of airway obstruction by nasopharyngoscopy: comparison of the Müller maneuver versus induced sleep. Braz J Otorhinolaryngol. 2007;73(5):618-622.
20. Kline LR. Clinical presentation and diagnosis of obstructive sleep apnea in adults. Up-to-Date. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-obstructive-sleep-apnea-in-adults. Accessed July 21, 2015.
21. Kryger MH, Malhotra A. Management of obstructive sleep apnea in adults. Up-to-Date. www.uptodate.com/contents/management-of-obstructive-sleep-apnea-in-adults. Accessed July 21, 2015.
22. Lim J, Lasserson TJ, Fleetham J, Wright JJ. Oral appliances for obstructive sleep apneoa. Cochrane Database Syst Rev. 2006;1:CD004435.
23. Morgenthaler TI, Kapen S, Lee-Chiong T, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8): 1031-1035.
24. Pepin JL. Evaluation and management of residual sleepiness in obstructive sleep apnea. Up-to-Date. www.uptodate.com/contents/evaluation-and-management-of-residual-sleepiness-in-obstructive-sleep-apnea. Accessed July 21, 2015.
25. Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36(10):1553-1562.
26. Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175-184.
27. Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014; 18(1):19-24.
28. Issa FG, Sullivan CE. Alcohol, snoring, and sleep apnea. J Neurol Neurosurg Psychiatry. 1982;45(4):353-359.
29. Lin Y, Li QY, Zhang XJ. Interaction between smoking and obstructive sleep apnea: not just participants. Chin Med J. 2012;125(17):3150-3156.
30. Aurora RN, Casey KR, Kristo D, et al; American Academy of Sleep Medicine. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408-1413.
31. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396-1407.
32. US Food and Drug Administration. Recently-approved devices. Inspire upper airway stimulation - P130008. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm398321.htm. Accessed July 21, 2015.
33. Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
1. American Sleep Apnea Association. A very short course on sleep apnea. www.sleepapnea.org/i-am-a-health-care-professional.html. Accessed July 21, 2015.
2. Ford ES, Wheaton AG, Cunningham TJ, et al. Trends in outpatient visits for insomnia, sleep apnea, and prescriptions for sleep medications among US adults: findings from the National Ambulatory Medical Care Survey 1999-2010. Sleep. 2014;37(8):1283-1293.
3. NIH State-of-the-Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. NIH Consens Sci Statements. 2005;22(2):1-30.
4. Carlucci M, Smith M, Corbridge SJ. Poor sleep, hazardous breathing: an overview of obstructive sleep apnea. Nurse Pract. 2013;38(3):20-28.
5. Institute for Clinical Systems Improvement. Health Care Guideline: Diagnosis and Treatment of Obstructive Sleep Apnea. 6th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2008.
6. Douglas NJ. Sleep apnea. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012:2186-2188.
7. US Department of Health and Human Services, National Heart, Lung, and Blood Institute. What causes sleep apnea? www.nhlbi.nih.gov/health/health-topics/topics/sleepapnea/causes.html. Accessed uly 21, 2015.
8. Harvard Medical School, Division of Sleep Medicine. The price of fatigue: the surprising economic costs of unmanaged sleep apnea. December 2010. https://sleep.med.harvard.edu/file_download/100. Accessed July 21, 2015.
9. Pagel JF. Obstructive sleep apnea (OSA) in primary care: evidence-based practice. J Am Board Fam Med. 2007;20(4):392-398.
10. Epstein LJ, Kristo D, Strollo PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management, and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
11. Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea: the rational clinical examination systematic review. JAMA. 2013;310(7):731-741.
12. Ye L, Pien GW, Weaver TE. Gender differences in the clinical manifestation of obstructive sleep apnea. Sleep Med. 2009;10(10):1075-1084.
13. Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013-2016.
14. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545.
15. Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485-491.
16. Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441-446.
17. Samsoon GL, Young JR. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487-490.
18. Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434.
19. Gregorio MG, Jacomelli M, Figueiredo AC, et al. Evaluation of airway obstruction by nasopharyngoscopy: comparison of the Müller maneuver versus induced sleep. Braz J Otorhinolaryngol. 2007;73(5):618-622.
20. Kline LR. Clinical presentation and diagnosis of obstructive sleep apnea in adults. Up-to-Date. www.uptodate.com/contents/clinical-presentation-and-diagnosis-of-obstructive-sleep-apnea-in-adults. Accessed July 21, 2015.
21. Kryger MH, Malhotra A. Management of obstructive sleep apnea in adults. Up-to-Date. www.uptodate.com/contents/management-of-obstructive-sleep-apnea-in-adults. Accessed July 21, 2015.
22. Lim J, Lasserson TJ, Fleetham J, Wright JJ. Oral appliances for obstructive sleep apneoa. Cochrane Database Syst Rev. 2006;1:CD004435.
23. Morgenthaler TI, Kapen S, Lee-Chiong T, et al; Standards of Practice Committee; American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8): 1031-1035.
24. Pepin JL. Evaluation and management of residual sleepiness in obstructive sleep apnea. Up-to-Date. www.uptodate.com/contents/evaluation-and-management-of-residual-sleepiness-in-obstructive-sleep-apnea. Accessed July 21, 2015.
25. Araghi MH, Chen YF, Jagielski A, et al. Effectiveness of lifestyle interventions on obstructive sleep apnea (OSA): systematic review and meta-analysis. Sleep. 2013;36(10):1553-1562.
26. Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175-184.
27. Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014; 18(1):19-24.
28. Issa FG, Sullivan CE. Alcohol, snoring, and sleep apnea. J Neurol Neurosurg Psychiatry. 1982;45(4):353-359.
29. Lin Y, Li QY, Zhang XJ. Interaction between smoking and obstructive sleep apnea: not just participants. Chin Med J. 2012;125(17):3150-3156.
30. Aurora RN, Casey KR, Kristo D, et al; American Academy of Sleep Medicine. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408-1413.
31. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33(10):1396-1407.
32. US Food and Drug Administration. Recently-approved devices. Inspire upper airway stimulation - P130008. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm398321.htm. Accessed July 21, 2015.
33. Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
July 2015: Click for Credit
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
Here are 7 articles in the July issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. BSR: Multiple Benefits Seen With Intensive Psoriatic Arthritis Therapy
Multiple joint and skin benefits can be achieved by intensively treating patients with psoriatic arthritis (PsA) until they achieve a set of minimal disease activity (MDA) criteria (see Table), an expert said at the British Society for Rheumatology annual conference.
To take the posttest, go to: http://bit.ly/1KaikxW
2. Subclinical Hyperthyroidism Linked to Higher Fracture Risk
Individuals with subclinical hyperthyroidism are at increased risk for hip and other fractures, according to the authors of a meta-analysis. The researchers examined data from 70,298 individuals—4,092 with subclinical hypothyroidism and 2,219 with subclinical hyperthyroidism—enrolled in 13 prospective cohort studies.
To take the posttest, go to: http://bit.ly/1H13j0t
3. Newer Oral Contraceptives Pose Higher VTE Risk
The risk for venous thromboembolism (VTE) is generally greater for women using oral contraceptives with newer types of progestogen hormones than for those taking older, second-generation birth control pills, study results showed.
To take the posttest, go to: http://bit.ly/1AKQert
4. Statins, Fibrates Lower Stroke Risk in Elderly
Both statin and fibrate therapies taken to improve lipid profiles decreased risk for stroke by 30% in a community-dwelling population of elderly people, according to a prospective European study published online in the British Medical Journal.
To take the posttest, go to: http://bit.ly/1FuyYCb
5. Cystic Fibrosis–related Diabetes Requires Different Approach
Cystic fibrosis–related diabetes (CFRD) is a unique disease that requires a different mindset on the part of the treating clinician.
To take the posttest, go to: http://bit.ly/1BKGZCm
6. CVD Risk Persists for 40 Years in Hodgkin Survivors
People who survive Hodgkin lymphoma in adolescence or young adulthood remain at very high risk for cardiovascular disease (CVD) for at least 40 years—the longest period for which they have been followed, according to the results of a retrospective cohort study of more than 2,500 patients.
To take the posttest, go to: http://bit.ly/1M5ymYG
7. Asymptomatic Carotid Stenosis and Central Sleep Apnea Linked
More than two-thirds of patients with asymptomatic carotid stenosis are likely to have sleep apnea, according to an observational study. The polysomnography results of 96 patients with asymptomatic extracranial carotid stenosis revealed that 69% had sleep apnea: 42% had obstructive sleep apnea (OSA) and 27%, central sleep apnea (CSA).
To take the posttest, go to: http://bit.ly/1SWGPmb
June 2015: Click for Credit
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
Here are 6 articles in the June issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. ACCP and CTS Issue Joint Guideline on COPD Exacerbations
To take the posttest, go to: http://bit.ly/1EIadmv
VITALS
Key clinical point: The American College of Chest Physicians and the Canadian Thoracic Society have issued a guideline for prevention of acute exacerbations of COPD.
Major finding: COPD exacerbations are acute, trajectory changing, and often deadly manifestations of a chronic disease.
Data source: A comprehensive literature review on prevention of acute COPD exacerbations and a compilation of 33 recommendations and suggestions for clinicians in clinical practice.
Disclosures: The American College of Chest Physicians, the Canadian Thoracic Society, and the American Thoracic Society supported the project. Dr Criner reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
2. GI Symptoms Common in Parkinson Disease and Related Disorders
To take the posttest, go to: http://bit.ly/1AZSaXA
VITALS
Key clinical point: GI symptoms were linked with specific motor and nonmotor features of Parkinson disease and related disorders.
Major finding: Constipation was the most common symptom among all three parkinsonian disorders.
Data source: Multicenter, cross-sectional survey of 473 patients with Parkinson disease, atypical parkinsonism, or vascular parkinsonism.
Disclosures: The Collaborative Clinical Research Fund of Boramae Medical Center partially funded the work. The authors declared no relevant conflicts of interest.
3. Aerosolized Measles Vaccine Inferior to Subcutaneous
To take the posttest, go to: http://bit.ly/1RKJizC
VITALS
Key clinical point: An aerosolized measles vaccine was immunogenic but inferior to the subcutaneous vaccine at inducing seropositivity among babies residing in rural India.
Major finding: The primary endpoint—seropositivity for antibodies against measles at 91 days after vaccination—was 85.4% for aerosolized vaccine and 94.6% for subcutaneous.
Data source: An open-label, randomized noninferiority trial comparing aerosolized vs subcutaneous measles vaccination in 2,004 infants ages 9 to 11.9 months in villages in India.
Disclosures: This study was funded by the Bill and Melinda Gates Foundation. The Serum Institute of India provided vaccines free of charge, and Aerogen provided the delivery devices free of charge. Dr Low reported several grants plus monies paid to her institution from the World Health Organization for projects about vaccines and sexually transmitted infections; her associates reported ties to the Serum Institute of India, Aerogen, and Dance Biopharm. One associate has a patent pending on an aerosol device licensed to Novartis and another has a patent pending related to vaccine nebulizers.
4. Unrecognized Diabetes Common in Acute MI
To take the posttest, go to: http://bit.ly/1IB9sC8
VITALS
Key clinical point: Many patients presenting with acute MI had unrecognized diabetes, which, in most cases, remained undiagnosed, untreated, and unrecorded.
Major finding: Of 2,854 (10%) patients enrolled in an MI registry, 287 had A1C levels of 6.5% or higher on routine laboratory testing during hospitalization for acute MI, but treating physicians recognized only 101 of these cases of diabetes (35%).
Data source: A retrospective cohort study involving 2,854 adults presenting with acute MI to 24 US medical centers in a 3.5-year period.
Disclosures: This study was sponsored by the National Heart, Lung, and Blood Institute and supported by a research grant from Genentech. Dr Arnold reported receiving honoraria from Novartis; her associates reported ties to numerous industry sources.
5. Methotrexate and Biologics Linked to Higher Zoster Risk in Psoriasis
To take the posttest, go to: http://bit.ly/1AZScyF
VITALS
Key clinical point: The combination of methotrexate and biologics for the treatment of psoriasis may increase risk for herpes zoster.
Major finding: Combination therapy with both biologic medications and methotrexate was associated with a significant 66% increase in the incidence of herpes zoster over more than 11 years of follow-up.
Data source: Analysis of medical records for 95,941 patients with psoriasis.
Disclosures: One author reported consultancies and research grants from a range of pharmaceutical companies. There were no other disclosures.
6. ACP: Avoid ECG, MPI Cardiac Screening in Low-risk Patients
To take the posttest, go to: http://bit.ly/1e3NLha
May 2015: Click for Credit
Here are 5 articles in the May issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Clindamycin, TMP-SMX Are Equally Effective for Skin Infections
To take the posttest, go to: http://bit.ly/1cVxR85
VITALS
Key clinical point: Clindamycin and TMP-SMX had similar efficacy and adverse-effect profiles for treating uncomplicated skin infections, including both abscesses and cellulitis.
Major finding: At 7-10 days after therapy completion, the rates of cure in the evaluable population were 89.5% with clindamycin and 88.2% with TMP-SMX.
Data source: A prospective, multicenter, randomized, double-blind clinical trial involving 524 adults and children followed for 1 month after treatment.
Disclosures: This trial was supported by the National Institutes of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (NCT00730028). Dr Miller reported receiving consulting fees from Cubist, Durata, and Pfizer; his associates reported ties to Cubist, Pfizer, EMMES, Theravance, AstraZeneca, Trius, Merck, and Cerexa.
2. Hormone Therapy 10 Years Postmenopause Increases Risks
To take the posttest, go to: http://bit.ly/1OBYcUe
VITALS
Key clinical point: Hormone therapy in postmenopausal women increases stroke risk.
Major finding: Stroke increased by 24%, venous thromboembolism by 92%, and pulmonary embolism by 81% in postmenopausal women receiving hormone therapy.
Data source: A review and meta-analysis of 19 randomized controlled trials involving 40,140 postmenopausal women who received orally administered hormone therapy, placebo, or no treatment for prevention of cardiovascular disease.
Disclosures: One study was funded by Wyeth-Ayerst. Two studies received partial funding from Novo-Nordisk Pharmaceutical, and one study was funded by the National Institutes of Health with support from Wyeth-Ayerst, Hoffman-La Roche, Pharmacia, and Upjohn. Eight other studies used medication provided by various pharmaceutical companies.
3. "Perfect storm" of Depression, Stress Raises Risk for MI, Death
To take the posttest, go to: http://bit.ly/1yM2HtF
VITALS
Key clinical point: Concurrent depression and stress in coronary heart disease patients may increase early risk for MI and death.
Major finding: CHD patients with high depressive symptoms and high stress at baseline had an increased risk for MI and death early during follow-up (adjusted HR, 1.48).
Data source: A prospective cohort study of 4,487 adults.
Disclosures: The National Institute of Neurological Disorders and Stroke and the National Heart, Lung, and Blood Institute supported the study. Dr Alcántara reported having no disclosures; two other authors received salary support from Amgen for research, and one served as a consultant for DiaDexus.
4. Type 2 Diabetes Lower in Familial Hypercholesterolemia
To take the posttest, go to: http://bit.ly/1bplTDc
VITALS
Key clinical point: The prevalence of type 2 diabetes appears to be significantly lower in patients with familial hypercholesterolemia than in their unaffected relatives.
Major finding: The prevalence of type 2 diabetes was 1.75% in 25,137 patients with familial hypercholesterolemia, compared with 2.93% in 38,183 of their unaffected relatives.
Data source: An observational cross-sectional analysis of data for 63,320 people in the Dutch national registry of familial hypercholesterolemia.
Disclosures: The study sponsor was not specified; the familial hypercholesterolemia registry is subsidized by the Dutch government. Dr Besseling reported having no financial disclosures; his associates reported ties to Aegerion, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Eli Lilly, Genzyme, JSiS, MSD, Novartis, Pfizer, Regeneron, Roche, and Sanofi.
5. Mongersen Induces 55%-65% Remission Rates in Crohn’s
To take the posttest, go to: http://bit.ly/1DctonL
VITALS
Key clinical point: Mongersen, an oral SMAD7 antisense oligonucleotide, induced remission rates as high as 55%-65% in a small 2-week phase II clinical trial.
Major finding: Rates of remission were 65% in the 43 participants who received 160 mg of mongersen, 55% in the 40 who received 40 mg, 12% in the 41 who received 10 mg, and 10% in the 42 who received placebo.
Data source: A randomized, placebo-controlled, double-blind phase II clinical trial involving 166 adults at 17 medical centers in Italy and Germany.
Disclosures: This study was sponsored by Giuliani, acting under contract to Nogra Pharma. Dr Monteleone reported ties to Giuliani, Novo Nordisk, Teva, Sirtris, Lycera, Sofar, and Zambon, and holds a patent related to the use of SMAD7 antisense oligonucleotides in Crohn’s disease. His associates reported financial ties to numerous industry sources.
Here are 5 articles in the May issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Clindamycin, TMP-SMX Are Equally Effective for Skin Infections
To take the posttest, go to: http://bit.ly/1cVxR85
VITALS
Key clinical point: Clindamycin and TMP-SMX had similar efficacy and adverse-effect profiles for treating uncomplicated skin infections, including both abscesses and cellulitis.
Major finding: At 7-10 days after therapy completion, the rates of cure in the evaluable population were 89.5% with clindamycin and 88.2% with TMP-SMX.
Data source: A prospective, multicenter, randomized, double-blind clinical trial involving 524 adults and children followed for 1 month after treatment.
Disclosures: This trial was supported by the National Institutes of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (NCT00730028). Dr Miller reported receiving consulting fees from Cubist, Durata, and Pfizer; his associates reported ties to Cubist, Pfizer, EMMES, Theravance, AstraZeneca, Trius, Merck, and Cerexa.
2. Hormone Therapy 10 Years Postmenopause Increases Risks
To take the posttest, go to: http://bit.ly/1OBYcUe
VITALS
Key clinical point: Hormone therapy in postmenopausal women increases stroke risk.
Major finding: Stroke increased by 24%, venous thromboembolism by 92%, and pulmonary embolism by 81% in postmenopausal women receiving hormone therapy.
Data source: A review and meta-analysis of 19 randomized controlled trials involving 40,140 postmenopausal women who received orally administered hormone therapy, placebo, or no treatment for prevention of cardiovascular disease.
Disclosures: One study was funded by Wyeth-Ayerst. Two studies received partial funding from Novo-Nordisk Pharmaceutical, and one study was funded by the National Institutes of Health with support from Wyeth-Ayerst, Hoffman-La Roche, Pharmacia, and Upjohn. Eight other studies used medication provided by various pharmaceutical companies.
3. "Perfect storm" of Depression, Stress Raises Risk for MI, Death
To take the posttest, go to: http://bit.ly/1yM2HtF
VITALS
Key clinical point: Concurrent depression and stress in coronary heart disease patients may increase early risk for MI and death.
Major finding: CHD patients with high depressive symptoms and high stress at baseline had an increased risk for MI and death early during follow-up (adjusted HR, 1.48).
Data source: A prospective cohort study of 4,487 adults.
Disclosures: The National Institute of Neurological Disorders and Stroke and the National Heart, Lung, and Blood Institute supported the study. Dr Alcántara reported having no disclosures; two other authors received salary support from Amgen for research, and one served as a consultant for DiaDexus.
4. Type 2 Diabetes Lower in Familial Hypercholesterolemia
To take the posttest, go to: http://bit.ly/1bplTDc
VITALS
Key clinical point: The prevalence of type 2 diabetes appears to be significantly lower in patients with familial hypercholesterolemia than in their unaffected relatives.
Major finding: The prevalence of type 2 diabetes was 1.75% in 25,137 patients with familial hypercholesterolemia, compared with 2.93% in 38,183 of their unaffected relatives.
Data source: An observational cross-sectional analysis of data for 63,320 people in the Dutch national registry of familial hypercholesterolemia.
Disclosures: The study sponsor was not specified; the familial hypercholesterolemia registry is subsidized by the Dutch government. Dr Besseling reported having no financial disclosures; his associates reported ties to Aegerion, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Eli Lilly, Genzyme, JSiS, MSD, Novartis, Pfizer, Regeneron, Roche, and Sanofi.
5. Mongersen Induces 55%-65% Remission Rates in Crohn’s
To take the posttest, go to: http://bit.ly/1DctonL
VITALS
Key clinical point: Mongersen, an oral SMAD7 antisense oligonucleotide, induced remission rates as high as 55%-65% in a small 2-week phase II clinical trial.
Major finding: Rates of remission were 65% in the 43 participants who received 160 mg of mongersen, 55% in the 40 who received 40 mg, 12% in the 41 who received 10 mg, and 10% in the 42 who received placebo.
Data source: A randomized, placebo-controlled, double-blind phase II clinical trial involving 166 adults at 17 medical centers in Italy and Germany.
Disclosures: This study was sponsored by Giuliani, acting under contract to Nogra Pharma. Dr Monteleone reported ties to Giuliani, Novo Nordisk, Teva, Sirtris, Lycera, Sofar, and Zambon, and holds a patent related to the use of SMAD7 antisense oligonucleotides in Crohn’s disease. His associates reported financial ties to numerous industry sources.
Here are 5 articles in the May issue of Clinician Reviews (accreditation valid until January 1, 2016):
1. Clindamycin, TMP-SMX Are Equally Effective for Skin Infections
To take the posttest, go to: http://bit.ly/1cVxR85
VITALS
Key clinical point: Clindamycin and TMP-SMX had similar efficacy and adverse-effect profiles for treating uncomplicated skin infections, including both abscesses and cellulitis.
Major finding: At 7-10 days after therapy completion, the rates of cure in the evaluable population were 89.5% with clindamycin and 88.2% with TMP-SMX.
Data source: A prospective, multicenter, randomized, double-blind clinical trial involving 524 adults and children followed for 1 month after treatment.
Disclosures: This trial was supported by the National Institutes of Allergy and Infectious Diseases and the National Center for Advancing Translational Sciences (NCT00730028). Dr Miller reported receiving consulting fees from Cubist, Durata, and Pfizer; his associates reported ties to Cubist, Pfizer, EMMES, Theravance, AstraZeneca, Trius, Merck, and Cerexa.
2. Hormone Therapy 10 Years Postmenopause Increases Risks
To take the posttest, go to: http://bit.ly/1OBYcUe
VITALS
Key clinical point: Hormone therapy in postmenopausal women increases stroke risk.
Major finding: Stroke increased by 24%, venous thromboembolism by 92%, and pulmonary embolism by 81% in postmenopausal women receiving hormone therapy.
Data source: A review and meta-analysis of 19 randomized controlled trials involving 40,140 postmenopausal women who received orally administered hormone therapy, placebo, or no treatment for prevention of cardiovascular disease.
Disclosures: One study was funded by Wyeth-Ayerst. Two studies received partial funding from Novo-Nordisk Pharmaceutical, and one study was funded by the National Institutes of Health with support from Wyeth-Ayerst, Hoffman-La Roche, Pharmacia, and Upjohn. Eight other studies used medication provided by various pharmaceutical companies.
3. "Perfect storm" of Depression, Stress Raises Risk for MI, Death
To take the posttest, go to: http://bit.ly/1yM2HtF
VITALS
Key clinical point: Concurrent depression and stress in coronary heart disease patients may increase early risk for MI and death.
Major finding: CHD patients with high depressive symptoms and high stress at baseline had an increased risk for MI and death early during follow-up (adjusted HR, 1.48).
Data source: A prospective cohort study of 4,487 adults.
Disclosures: The National Institute of Neurological Disorders and Stroke and the National Heart, Lung, and Blood Institute supported the study. Dr Alcántara reported having no disclosures; two other authors received salary support from Amgen for research, and one served as a consultant for DiaDexus.
4. Type 2 Diabetes Lower in Familial Hypercholesterolemia
To take the posttest, go to: http://bit.ly/1bplTDc
VITALS
Key clinical point: The prevalence of type 2 diabetes appears to be significantly lower in patients with familial hypercholesterolemia than in their unaffected relatives.
Major finding: The prevalence of type 2 diabetes was 1.75% in 25,137 patients with familial hypercholesterolemia, compared with 2.93% in 38,183 of their unaffected relatives.
Data source: An observational cross-sectional analysis of data for 63,320 people in the Dutch national registry of familial hypercholesterolemia.
Disclosures: The study sponsor was not specified; the familial hypercholesterolemia registry is subsidized by the Dutch government. Dr Besseling reported having no financial disclosures; his associates reported ties to Aegerion, Amgen, AstraZeneca, Boehringer Ingelheim, Cerenis, Eli Lilly, Genzyme, JSiS, MSD, Novartis, Pfizer, Regeneron, Roche, and Sanofi.
5. Mongersen Induces 55%-65% Remission Rates in Crohn’s
To take the posttest, go to: http://bit.ly/1DctonL
VITALS
Key clinical point: Mongersen, an oral SMAD7 antisense oligonucleotide, induced remission rates as high as 55%-65% in a small 2-week phase II clinical trial.
Major finding: Rates of remission were 65% in the 43 participants who received 160 mg of mongersen, 55% in the 40 who received 40 mg, 12% in the 41 who received 10 mg, and 10% in the 42 who received placebo.
Data source: A randomized, placebo-controlled, double-blind phase II clinical trial involving 166 adults at 17 medical centers in Italy and Germany.
Disclosures: This study was sponsored by Giuliani, acting under contract to Nogra Pharma. Dr Monteleone reported ties to Giuliani, Novo Nordisk, Teva, Sirtris, Lycera, Sofar, and Zambon, and holds a patent related to the use of SMAD7 antisense oligonucleotides in Crohn’s disease. His associates reported financial ties to numerous industry sources.
Mumps–It’s Back!
CE/CME No: CR-1504
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the likely causes of mumps outbreaks.
• Explain the various possible manifestations of mumps illness.
• Identify the testing methods available to confirm a mumps diagnosis.
• Describe the potential complications of mumps and their incidence.
• Know what to do in the event of a mumps outbreak.
FACULTY
Jo Hanna Friend D’Epiro is a primary care PA in Student Health Services and a doctoral student, Workforce Development and Education, at The Ohio State University in Columbus. The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of April 2015.
Article begins on next page >>
Although overshadowed in the headlines by a sharp increase in measles cases, mumps too is making a comeback, with outbreaks throughout 2014 and early 2015. Many of today’s clinicians have never seen a case of mumps, let alone experienced an outbreak. Here’s what to look for and what to do if mumps makes its appearance in your practice.
In 2014, 1,151 cases of mumps were reported in the United States.1 By contrast, the typical annual rate has been in the low hundreds since 1989, when the CDC recommended a two-dose measles-mumps-rubella (MMR) vaccination regimen.1,2
Yet mumps has resurged in the past decade, with large outbreaks in 2006 (6,584 cases) and 2009-2010 (4,603 cases).3 Mumps outbreaks tend to occur among vaccinated young adults, such as college students, sports players, and campers, who live in close quarters.4
The 2014 outbreak centered around the Ohio State University campus in Columbus.5 That outbreak was declared over in October, with a total of 484 cases—more than in the entire US in 2013.1 In late 2014, at least 20 players and two officials in the National Hockey League became infected with mumps.6 More recently, Idaho announced that a 21-case outbreak that began at the University of Idaho’s Moscow campus had spread to Washington, with two additional cases reported there.7 What is responsible for these outbreaks, and what can the primary care clinician do to prevent or mitigate them?
EPIDEMIOLOGY
The mumps virus is part of the Rubulavirus genus of the Paramyxoviridae family. It affects the central nervous system (CNS) and glands—most commonly, the parotids. Uniquely human, mumps virus is found in saliva, cerebrospinal fluid, blood, breast milk, infected tissues, and urine.8,9 It is transmitted through contact with respiratory secretions and/or saliva, direct contact, or through fomites (eg, bedding, doorknobs).10
Before development of an effective vaccine, mumps was a universal childhood disease in the US; by age 14, most children had been infected.11 In the absence of widespread vaccination, mumps epidemics will occur every three to five years—as they still do in parts of the world without effective vaccination programs.12
As a result of widespread vaccination in the US, mumps incidence declined from 152,209 cases in 1967 to 2,982 cases in 1985 (see “Mumps and the MMR Vaccine”). Cases were reduced even further when administration of a second MMR dose was introduced in 1990.13 By 2000, the Healthy People 2010 goal was to eliminate mumps altogether.14
Continue for patient presentation >>
PATIENT PRESENTATION
Parotitis is the classic (but not universal) physical exam finding in mumps. Parotid gland inflammation causes generalized swelling anterior to the ear and inferior to the mastoid process, with jaw angle obliteration (see Figure 1).9 If only one parotid gland is involved, the patient’s face appears asymmetric. Other significant exam findings may include fever and erythematous swelling of the Stensen (parotid) duct.10
Nonspecific symptoms—including respiratory symptoms, myalgia, anorexia, malaise, headache, and low-grade fever—may occur in more than 50% of cases.8 CNS involvement may cause nuchal rigidity (stiff neck). In postpubertal males, testicular swelling and/or induration, pain, tenderness, and enlarged inguinal lymph nodes may be present.
Mumps can be challenging to diagnose based on clinical presentation alone; for example, parotitis occurs in only 30% to 40% of cases.8 Other viruses, such as parainfluenza virus 1 and 3, coxsackievirus, adenovirus, influenza A, cytomegalovirus, and HIV, can also cause swelling of the parotid glands, but mumps is the only virus known to cause parotitis on an epidemic scale.4 Furthermore, up to 20% of cases may be asymptomatic.8,11 Because mumps is highly contagious, a history of exposure to an affected individual is a compelling factor in making the diagnosis.
The incubation period for mumps is 12 to 25 days, with parotitis usually developing 16 to 18 days after exposure.4 This relatively lengthy incubation period increases the likelihood of viral spread. The virus is contagious from three days prior to symptom onset to day 4 of active disease.8 To prevent disease transmission, it is recommended that individuals remain isolated from others until five days after the onset of salivary gland swelling.4
Next page: Laboratory confirmation >>
LABORATORY CONFIRMATION
The CDC recommends determination of any one of the following to help confirm the diagnosis of acute mumps infection.
• Presence of serum mumps IgM antibodies
• Significant rise in IgG antibody titer between the acute and convalescent-phase serum specimens
• IgG seroconversion
• Positive mumps virus culture
• Detection of virus by real-time reverse transcriptase polymerase chain reaction (RT-PCR)4
Antibody testing
At the initial visit, a serum specimen should be obtained to test for mumps IgM antibodies.4 The CDC recommends enzyme immunoassay (EIA) testing for IgM antibodies to confirm acute mumps infection.4
IgM antibodies are detectable five days after onset of symptoms and, after reaching a maximum level, remain elevated for several weeks. If the initial IgM test is negative, the test can be repeated in five to seven days.4
Either EIA or immunofluorescence antibody assay (IFA) testing for mumps IgG antibodies should be performed on both acute- and convalescent-phase serum samples. Laboratory confirmation requires a fourfold rise in the antibody titer using a quantitative assay4 or seroconversion from negative to positive.
Virus detection
If possible, mumps virus samples should be obtained no more than three to eight days after symptom onset because delay may result in a low viral yield.4 The best viral samples are obtained via parotid duct swabs (see Figure 2). Before swabbing the buccal cavity, the parotid gland should be massaged for 30 seconds to ensure that the specimen contains gland secretions.4
Mumps virus can be detected by RT-PCR or culture. The RT-PCR is currently the most sensitive test for mumps, but most RT-PCR testing is done by public health laboratories and the CDC, and results may not be available until after the illness has resolved.4
Laboratory tests, however, are not always helpful in confirming a clinical diagnosis of mumps. Vaccinated persons may not mount a secondary immune response to mumps and consequently may not have a significant IgM response. It is also possible that a high level of IgG antibodies will cause a false-positive IgM test results.4
Other laboratory tests that may support a diagnosis of mumps include a complete blood cell count, which may reveal a leukopenia with relative lymphocytosis or neutrophil leukocytosis,16 and a measurement of serum amylase level which, if elevated, may confirm the inflammatory process.10 See Table 1 for a summary of tests that confirm or support a mumps diagnosis.
Mumps is a reportable illness, and the local health department should be contacted for assistance with determining where and how to ship specimens.17 However, in the absence of laboratory confirmation, only clinical cases with parotitis, other salivary gland involvement, or mumps-related complications are notifiable.11
Continue for mumps manifestations >>
MUMPS MANIFESTATIONS
The continuum of mumps illness ranges from asymptomatic infection to parotitis (the most well-known manifestation) to rare but severe complications.8 Table 2 lists potential complications of mumps in order of frequency. Complications vary by age and sex but tend to occur more often in adults.4,11
Immunization modifies the clinical presentation of mumps11 and likely decreases complications. Although one analysis of the 2006 outbreak identified no difference in complication rates between vaccinated and unvaccinated patients, the authors attributed this to misclassification of patients’ vaccination status; they did find lower reported rates of mumps complications compared with complication rates before widespread vaccination.13 A study of the 2009-2010 outbreak found that complication rates were lower among vaccinated patients.18
RISK FACTORS FOR OUTBREAKS
Of the three components of the MMR vaccine, the least effective is the mumps portion. One dose confers 78% immunity and two doses, 88%, which the CDC characterizes as incomplete protection.1 Compare this to the measles vaccine, which is 97% effective with two doses,19 and the rubella vaccine, which is 97% effective after a single dose in conferring immunity.11
In 2000, as a result of high rates of vaccination, the US determined that endemic measles had been eliminated. A similar conclusion was reached about rubella in 2004, and both determinations were reaffirmed in 2011.20 In contrast, mumps has never been eliminated.1
Waning immunity
While antibodies to mumps as a result of vaccination persist into adulthood, they decline over time. A 2009 CDC study found that, 12 years after a second MMR dose, mumps antibody levels in adolescents and young adults had declined to levels similar to those measured before the second dose.21 Other analyses of major outbreaks suggested the need for further studies to determine optimal timing for the second MMR dose (eg, at a later age) or if a third dose would provide longer-lasting immunity.13,22 Waning immunity among young adults, coupled with high-density living environments that intensify exposure to the virus, increase risk for the disease.23
Further complicating the situation is the lack of evidence about the required level of antibodies needed to confer protection against mumps infection.17 To date, the antibody titer threshold of mumps-specific IgG at which an individual is protected from the disease is unknown.11,17
Imported risks
Mumps remains endemic in many parts of the world, with vaccination employed in only 61% of countries belonging to the World Health Organization.4 Several recent outbreaks were traced to index cases originating outside the US.13 It is likely that importation of the virus from abroad will continue.
Next page: Outbreak management >>
OUTBREAK MANAGEMENT
A mumps outbreak is defined as three or more cases linked by time and place.4 The keys to managing an outbreak are to define the population(s) at risk and their transmission setting(s) and to rapidly identify and vaccinate vulnerable individuals without evidence of immunity.4
Presumptive evidence of mumps immunity includes11
• Documentation of vaccination with two doses of live mumps virus–containing vaccine
• Laboratory evidence of immunity
• Laboratory confirmation of disease
• Birth year before 1957.
Documentation of two doses of MMR constitutes evidence of adequate vaccination for school-age children and adolescents and for young adults attending postsecondary institutions. During an outbreak, susceptible (ie, unvaccinated) students should be excluded from attendance until they have been vaccinated; those with one dose may attend but should receive the second dose.4 Those declining vaccination for medical, religious, or other reasons should be excluded until at least 26 days after the onset of parotitis in the last person with mumps at the institution.24
If the outbreak threatens the wider community (eg, preschool-age children and adults), a second MMR dose should be considered for children ages 1 to 4 or for adults who have received one MMR dose. Similarly, MMR vaccination should be considered for adults born before 1957 who have no other evidence of immunity and are at risk for exposure to the virus.11
In the workplace, health care workers’ (HCWs’) immunity status should be known, documented, and accessible in advance of an outbreak.11 If an HCW without evidence of immunity is exposed to mumps, he or she should be excluded from patient care from the time of first unprotected exposure through the 25th day after the last exposure.25 Although individuals born before 1957 are generally considered immune, if a nosocomial mumps outbreak occurs, the two-dose MMR regimen should be administered to these HCW as well.4
In 1991, the US military began to immunize recruits routinely with MMR, regardless of their immunization status.26 During the 2006 mumps outbreak, the incidence of mumps among military personnel was minimal compared to that among their civilian counterparts—perhaps due to administration of a third MMR dose to an unknown number of recruits.22
CDC researchers studied the impact of a third MMR dose for mumps outbreak control in 2012 and concluded that, while a third dose may help control outbreaks among populations with preexisting high two-dose vaccine coverage, further study is needed.27
Although insufficient data exist on which to base a recommendation for or against a third MMR dose for mumps outbreak control, the CDC has issued guidance for public health departments for targeted administration during outbreaks. Considerations include
• Intense exposure settings
• High two-dose vaccination coverage (ie, > 90%)
• High attack rates (> 5 cases per 1,000 population)
• Evidence of ongoing transmission for at least two weeks in the target population.4
TREATMENT
There is no specific treatment for mumps. Care is supportive and in the outpatient setting includes rest, cold or heat to the affected areas, and OTC pain relievers. Ice can be used to help relieve the pain of orchitis. Acidic foods may stimulate the parotid glands, causing pain and difficulty swallowing, and should be avoided.
Isolation of infectious patients is vital to preventing the spread of mumps.4 In the clinician’s office, a separate waiting area should be used for a potential mumps patient, or the patient should be located at least three feet from other patients and asked to wear a surgical mask. HCW working with potential mumps patients should follow droplet precautions (eg, wear personal protective equipment) in addition to standard precautions and should be hypervigilant about hand washing.24
CONCLUSION
Mumps is a usually benign, self-limited infectious disease that can potentially result in serious complications. It is also prone to periodic outbreaks. Control of mumps can best be accomplished by remembering these five “Ps”:
• Prevention—through widespread two-dose MMR vaccination
• Parotitis—recognize it as the primary symptom of mumps and make the diagnosis in a timely manner
• Persistence—in making the diagnosis clinically and in weighing laboratory results within the context of clinical disease
• Personal protective equipment—use it consistently in the health care setting or as needed in the home
• Protection—isolate patients with mumps to avoid spreading the disease
1. CDC. Mumps cases and outbreaks. www.cdc.gov/mumps/outbreaks.html. Accessed March 19, 2015.
2. CDC. Measles prevention: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Morb Mortal Wkly Rep. 1989;38(suppl 9):1-18.
3. CDC. Summary of notifiable diseases—United States. 1993-2012. MMWR Morb Mortal Wkly Rep. www.cdc.gov/mmwr/mmwr_nd/index.html. Accessed March 19, 2015.
4. CDC. Mumps, chap 9. In: Manual for the Surveillance of Vaccine-Preventable Diseases. August 2012. www.cdc.gov/vaccines/pubs/surv-manual/chpt09-mumps.html. Accessed March 19, 2015.
5. Pyle E. Central Ohio mumps outbreak finally loses steam. The Columbus Dispatch. October 11, 2014. www.dispatch.com/content/stories/local/2014/10/10/Health-officials-declare-mumps-outbreak-over.html. Accessed March 19, 2015.
6. Hascup J. Three more mumps cases: Penguins’ Steve Downie, Thomas Greiss, Devils’ Patrik Elias, Michael Ryder. USA TODAY. December 27, 2014. www.usatoday.com/story/sports/nhl/2014/12/26/steve-downie-thomas-greiss-mumps-pittsburgh-penguins/20911031/. Accessed March 19, 2015.
7. Idaho public health officials warn that mumps outbreak involving University students has spread to Boise and State of Washington [news release]. Boise, ID: Idaho Department of Health and Welfare Public Information Office; February 6, 2015. www.healthandwelfare.idaho.gov/AboutUs/Newsroom/tabid/130/ctl/ArticleView/mid/3061/articleId/1819/Idaho-Public-Health-Officials-Warn-that-Mumps-Outbreak-Involving-University-Students-Has-Spread-to-Boise-and-State-of-Washington.aspx. Accessed March 19, 2015.
8. CDC. Epidemiology and prevention of vaccine-preventable diseases. Mumps, chap 12. In: The Pink Book: Course Textbook. 12th ed, 2012. www.cdc.gov/vaccines/pubs/pinkbook/mumps.html. Accessed March 19, 2015.
9. Albrecht MA. Epidemiology, clinical manifestations, diagnosis, and management of mumps. www.uptodate.com/contents/epidemiology-clin ical-manifestations-diagnosis-and-management-of-mumps. Accessed March 19, 2015.
10. Defendi GL. Mumps. http://reference.medscape.com/article/966678-overview. Accessed March 19, 2015.
11. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR04):1-34.
12. Rubin S, Carbone KM. Mumps. In: Longo DL, Fauci AS, Kasper DL et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: The McGraw-Hill Companies, Inc.; 2012:chap 194.
13. Dayan G, Quinlisk M, Parker A, et al. Recent resurgence of mumps in the United States. New Engl J Med. 2008;358:1580-1589.
14. US Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2010: Objectives for Improving Health. www.healthypeople.gov/2010/Document/HTML/Volume1 /14Immunization.htm#_Toc494510239. Accessed March 19, 2015.
15. CDC. Measles, mumps, and rubella: vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1998;47(RR-8):1-57.
16. Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ. 2005;(330):1132-1135.
17. CDC. Laboratory testing for mumps infection. www.cdc.gov/mumps/lab/qa-lab-test-infect.html. Accessed March 19, 2015.
18. Barskey AE, Schulte C, Rosen JB, et al. Mumps outbreak in Orthodox Jewish communities in the United States. New Engl J Med. 2012;367(18): 1704-1713.
19. CDC. Frequently asked questions about measles in the U.S. www.cdc.gov/measles/about/faqs.html. Accessed March 19, 2015.
20. Papania MJ, Wallace GS, Rota PA, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatr. 2014;168(2):148-155.
21. LeBaron CW, Forghani B, Beck C, et al. Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis. 2009; 199(4):552-560.
22. Anis E, Grotto I, Moerman L, et al. Mumps outbreak in Israel’s highly vaccinated society: are two doses enough? Epidemiol Infect. 2012; 140:439-446.
23. Sane J, Gouma S, Koopmans M, et al. Epidemic of mumps among vaccinated persons, The Netherlands, 2009-2012. Emerg Infect Dis. 2014;20(4):643-648.
24. CDC. Exposure and response. www.cdc.gov/mumps/clinical/qa-exposure-response.html. Accessed March 19, 2015.
25. CDC. Mumps prevention and control in healthcare settings. www.cdc.gov/mumps/prev-control-settings/background.html. Accessed March 19, 2015.
26. Barskey AE, Glasser JW, LeBaron CW. Mumps resurgence in the United States: a historical perspective on unexpected elements. Vaccine. 2009;27:6186-6195.
27. Ogbuanu IU, Kutty PK, Hudson JM, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics. 2012;130:e1567-e1574.
28. CDC. Physical findings and complications. www.cdc.gov/mumps/clinical/qa-physical-complic.html. Accessed March 19, 2015.
29. Conniff R. A forgotten pioneer of vaccines. The New York Times. May 6, 2013. www.nytimes.com/2013/05/07/health/maurice-hilleman-mmr-vaccines-forgotten-hero.html?_r=0. Accessed March 19, 2015.
30. CDC. MMR vaccine for mumps. www.cdc.gov/vaccines/vpd-vac/mumps/vac-faqs-tech.htm. Accessed March 19, 2015.
31. The College of Physicians of Philadelphia. The history of vaccines. www.historyofvaccines.org/content/timelines/all. Accessed March 19, 2015.
32. CDC. Travelers’ health. Mumps. wwwnc.cdc.gov/travel/diseases/mumps. Accessed March 19, 2015.
33. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children [retracted in Lancet. 2010;375:445]. Lancet. 1998;351:637-641.
34. Murch SH, Anthony A, Casson DH, et al. Retraction of an interpretation. Lancet. 2004;363(9411):750.
35. Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:1-16.
36. The Editors of The Lancet. Notice of retraction of "Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children" [retraction of: Wakefield AJ, Murch SH, Anthony A, et al. In: Lancet. 1998;351:637-641]. Lancet. 2010;375:445.
37. Whalen J. UK bans doctor who linked autism to vaccine. The Wall Street Journal. May 24, 2010. www.wsj.com/articles/SB10001424052748704113504575263994195318772. Accessed March 19, 2015.
38. CDC. Vaccine safety: measles, mumps and rubella (MMR) vaccine. www.cdc.gov/vaccinesafety/vaccines/mmr/mmr.html. Accessed March 19, 2015.
39. Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347:1477-1482.
40. DeStefano F, Bhasin TK, Thompson WW, et al. Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan Atlanta. Pediatrics. 2004;113(2):259-266.
41. Richler J, Luyster R. Risi S, et al. Is there a ‘regressive phenotype’ of autism spectrum disorder associated with the measles-mumps-rubella vaccine? A CPEA study. J Autism Dev Disord. 2006;36(3):299-316.
42. Nyhan B, Reifler J, Richey S, Freed G. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014:133:1-8.
43. Haberman C. A discredited vaccine study’s continuing impact on public health. The New York Times. February 1, 2015. www.nytimes.com/2015/02/02/us/a-discredited-vaccine-studys-continuing-impact- on-public- health.html?_r=0. Accessed March 19, 2015.
CE/CME No: CR-1504
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the likely causes of mumps outbreaks.
• Explain the various possible manifestations of mumps illness.
• Identify the testing methods available to confirm a mumps diagnosis.
• Describe the potential complications of mumps and their incidence.
• Know what to do in the event of a mumps outbreak.
FACULTY
Jo Hanna Friend D’Epiro is a primary care PA in Student Health Services and a doctoral student, Workforce Development and Education, at The Ohio State University in Columbus. The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of April 2015.
Article begins on next page >>
Although overshadowed in the headlines by a sharp increase in measles cases, mumps too is making a comeback, with outbreaks throughout 2014 and early 2015. Many of today’s clinicians have never seen a case of mumps, let alone experienced an outbreak. Here’s what to look for and what to do if mumps makes its appearance in your practice.
In 2014, 1,151 cases of mumps were reported in the United States.1 By contrast, the typical annual rate has been in the low hundreds since 1989, when the CDC recommended a two-dose measles-mumps-rubella (MMR) vaccination regimen.1,2
Yet mumps has resurged in the past decade, with large outbreaks in 2006 (6,584 cases) and 2009-2010 (4,603 cases).3 Mumps outbreaks tend to occur among vaccinated young adults, such as college students, sports players, and campers, who live in close quarters.4
The 2014 outbreak centered around the Ohio State University campus in Columbus.5 That outbreak was declared over in October, with a total of 484 cases—more than in the entire US in 2013.1 In late 2014, at least 20 players and two officials in the National Hockey League became infected with mumps.6 More recently, Idaho announced that a 21-case outbreak that began at the University of Idaho’s Moscow campus had spread to Washington, with two additional cases reported there.7 What is responsible for these outbreaks, and what can the primary care clinician do to prevent or mitigate them?
EPIDEMIOLOGY
The mumps virus is part of the Rubulavirus genus of the Paramyxoviridae family. It affects the central nervous system (CNS) and glands—most commonly, the parotids. Uniquely human, mumps virus is found in saliva, cerebrospinal fluid, blood, breast milk, infected tissues, and urine.8,9 It is transmitted through contact with respiratory secretions and/or saliva, direct contact, or through fomites (eg, bedding, doorknobs).10
Before development of an effective vaccine, mumps was a universal childhood disease in the US; by age 14, most children had been infected.11 In the absence of widespread vaccination, mumps epidemics will occur every three to five years—as they still do in parts of the world without effective vaccination programs.12
As a result of widespread vaccination in the US, mumps incidence declined from 152,209 cases in 1967 to 2,982 cases in 1985 (see “Mumps and the MMR Vaccine”). Cases were reduced even further when administration of a second MMR dose was introduced in 1990.13 By 2000, the Healthy People 2010 goal was to eliminate mumps altogether.14
Continue for patient presentation >>
PATIENT PRESENTATION
Parotitis is the classic (but not universal) physical exam finding in mumps. Parotid gland inflammation causes generalized swelling anterior to the ear and inferior to the mastoid process, with jaw angle obliteration (see Figure 1).9 If only one parotid gland is involved, the patient’s face appears asymmetric. Other significant exam findings may include fever and erythematous swelling of the Stensen (parotid) duct.10
Nonspecific symptoms—including respiratory symptoms, myalgia, anorexia, malaise, headache, and low-grade fever—may occur in more than 50% of cases.8 CNS involvement may cause nuchal rigidity (stiff neck). In postpubertal males, testicular swelling and/or induration, pain, tenderness, and enlarged inguinal lymph nodes may be present.
Mumps can be challenging to diagnose based on clinical presentation alone; for example, parotitis occurs in only 30% to 40% of cases.8 Other viruses, such as parainfluenza virus 1 and 3, coxsackievirus, adenovirus, influenza A, cytomegalovirus, and HIV, can also cause swelling of the parotid glands, but mumps is the only virus known to cause parotitis on an epidemic scale.4 Furthermore, up to 20% of cases may be asymptomatic.8,11 Because mumps is highly contagious, a history of exposure to an affected individual is a compelling factor in making the diagnosis.
The incubation period for mumps is 12 to 25 days, with parotitis usually developing 16 to 18 days after exposure.4 This relatively lengthy incubation period increases the likelihood of viral spread. The virus is contagious from three days prior to symptom onset to day 4 of active disease.8 To prevent disease transmission, it is recommended that individuals remain isolated from others until five days after the onset of salivary gland swelling.4
Next page: Laboratory confirmation >>
LABORATORY CONFIRMATION
The CDC recommends determination of any one of the following to help confirm the diagnosis of acute mumps infection.
• Presence of serum mumps IgM antibodies
• Significant rise in IgG antibody titer between the acute and convalescent-phase serum specimens
• IgG seroconversion
• Positive mumps virus culture
• Detection of virus by real-time reverse transcriptase polymerase chain reaction (RT-PCR)4
Antibody testing
At the initial visit, a serum specimen should be obtained to test for mumps IgM antibodies.4 The CDC recommends enzyme immunoassay (EIA) testing for IgM antibodies to confirm acute mumps infection.4
IgM antibodies are detectable five days after onset of symptoms and, after reaching a maximum level, remain elevated for several weeks. If the initial IgM test is negative, the test can be repeated in five to seven days.4
Either EIA or immunofluorescence antibody assay (IFA) testing for mumps IgG antibodies should be performed on both acute- and convalescent-phase serum samples. Laboratory confirmation requires a fourfold rise in the antibody titer using a quantitative assay4 or seroconversion from negative to positive.
Virus detection
If possible, mumps virus samples should be obtained no more than three to eight days after symptom onset because delay may result in a low viral yield.4 The best viral samples are obtained via parotid duct swabs (see Figure 2). Before swabbing the buccal cavity, the parotid gland should be massaged for 30 seconds to ensure that the specimen contains gland secretions.4
Mumps virus can be detected by RT-PCR or culture. The RT-PCR is currently the most sensitive test for mumps, but most RT-PCR testing is done by public health laboratories and the CDC, and results may not be available until after the illness has resolved.4
Laboratory tests, however, are not always helpful in confirming a clinical diagnosis of mumps. Vaccinated persons may not mount a secondary immune response to mumps and consequently may not have a significant IgM response. It is also possible that a high level of IgG antibodies will cause a false-positive IgM test results.4
Other laboratory tests that may support a diagnosis of mumps include a complete blood cell count, which may reveal a leukopenia with relative lymphocytosis or neutrophil leukocytosis,16 and a measurement of serum amylase level which, if elevated, may confirm the inflammatory process.10 See Table 1 for a summary of tests that confirm or support a mumps diagnosis.
Mumps is a reportable illness, and the local health department should be contacted for assistance with determining where and how to ship specimens.17 However, in the absence of laboratory confirmation, only clinical cases with parotitis, other salivary gland involvement, or mumps-related complications are notifiable.11
Continue for mumps manifestations >>
MUMPS MANIFESTATIONS
The continuum of mumps illness ranges from asymptomatic infection to parotitis (the most well-known manifestation) to rare but severe complications.8 Table 2 lists potential complications of mumps in order of frequency. Complications vary by age and sex but tend to occur more often in adults.4,11
Immunization modifies the clinical presentation of mumps11 and likely decreases complications. Although one analysis of the 2006 outbreak identified no difference in complication rates between vaccinated and unvaccinated patients, the authors attributed this to misclassification of patients’ vaccination status; they did find lower reported rates of mumps complications compared with complication rates before widespread vaccination.13 A study of the 2009-2010 outbreak found that complication rates were lower among vaccinated patients.18
RISK FACTORS FOR OUTBREAKS
Of the three components of the MMR vaccine, the least effective is the mumps portion. One dose confers 78% immunity and two doses, 88%, which the CDC characterizes as incomplete protection.1 Compare this to the measles vaccine, which is 97% effective with two doses,19 and the rubella vaccine, which is 97% effective after a single dose in conferring immunity.11
In 2000, as a result of high rates of vaccination, the US determined that endemic measles had been eliminated. A similar conclusion was reached about rubella in 2004, and both determinations were reaffirmed in 2011.20 In contrast, mumps has never been eliminated.1
Waning immunity
While antibodies to mumps as a result of vaccination persist into adulthood, they decline over time. A 2009 CDC study found that, 12 years after a second MMR dose, mumps antibody levels in adolescents and young adults had declined to levels similar to those measured before the second dose.21 Other analyses of major outbreaks suggested the need for further studies to determine optimal timing for the second MMR dose (eg, at a later age) or if a third dose would provide longer-lasting immunity.13,22 Waning immunity among young adults, coupled with high-density living environments that intensify exposure to the virus, increase risk for the disease.23
Further complicating the situation is the lack of evidence about the required level of antibodies needed to confer protection against mumps infection.17 To date, the antibody titer threshold of mumps-specific IgG at which an individual is protected from the disease is unknown.11,17
Imported risks
Mumps remains endemic in many parts of the world, with vaccination employed in only 61% of countries belonging to the World Health Organization.4 Several recent outbreaks were traced to index cases originating outside the US.13 It is likely that importation of the virus from abroad will continue.
Next page: Outbreak management >>
OUTBREAK MANAGEMENT
A mumps outbreak is defined as three or more cases linked by time and place.4 The keys to managing an outbreak are to define the population(s) at risk and their transmission setting(s) and to rapidly identify and vaccinate vulnerable individuals without evidence of immunity.4
Presumptive evidence of mumps immunity includes11
• Documentation of vaccination with two doses of live mumps virus–containing vaccine
• Laboratory evidence of immunity
• Laboratory confirmation of disease
• Birth year before 1957.
Documentation of two doses of MMR constitutes evidence of adequate vaccination for school-age children and adolescents and for young adults attending postsecondary institutions. During an outbreak, susceptible (ie, unvaccinated) students should be excluded from attendance until they have been vaccinated; those with one dose may attend but should receive the second dose.4 Those declining vaccination for medical, religious, or other reasons should be excluded until at least 26 days after the onset of parotitis in the last person with mumps at the institution.24
If the outbreak threatens the wider community (eg, preschool-age children and adults), a second MMR dose should be considered for children ages 1 to 4 or for adults who have received one MMR dose. Similarly, MMR vaccination should be considered for adults born before 1957 who have no other evidence of immunity and are at risk for exposure to the virus.11
In the workplace, health care workers’ (HCWs’) immunity status should be known, documented, and accessible in advance of an outbreak.11 If an HCW without evidence of immunity is exposed to mumps, he or she should be excluded from patient care from the time of first unprotected exposure through the 25th day after the last exposure.25 Although individuals born before 1957 are generally considered immune, if a nosocomial mumps outbreak occurs, the two-dose MMR regimen should be administered to these HCW as well.4
In 1991, the US military began to immunize recruits routinely with MMR, regardless of their immunization status.26 During the 2006 mumps outbreak, the incidence of mumps among military personnel was minimal compared to that among their civilian counterparts—perhaps due to administration of a third MMR dose to an unknown number of recruits.22
CDC researchers studied the impact of a third MMR dose for mumps outbreak control in 2012 and concluded that, while a third dose may help control outbreaks among populations with preexisting high two-dose vaccine coverage, further study is needed.27
Although insufficient data exist on which to base a recommendation for or against a third MMR dose for mumps outbreak control, the CDC has issued guidance for public health departments for targeted administration during outbreaks. Considerations include
• Intense exposure settings
• High two-dose vaccination coverage (ie, > 90%)
• High attack rates (> 5 cases per 1,000 population)
• Evidence of ongoing transmission for at least two weeks in the target population.4
TREATMENT
There is no specific treatment for mumps. Care is supportive and in the outpatient setting includes rest, cold or heat to the affected areas, and OTC pain relievers. Ice can be used to help relieve the pain of orchitis. Acidic foods may stimulate the parotid glands, causing pain and difficulty swallowing, and should be avoided.
Isolation of infectious patients is vital to preventing the spread of mumps.4 In the clinician’s office, a separate waiting area should be used for a potential mumps patient, or the patient should be located at least three feet from other patients and asked to wear a surgical mask. HCW working with potential mumps patients should follow droplet precautions (eg, wear personal protective equipment) in addition to standard precautions and should be hypervigilant about hand washing.24
CONCLUSION
Mumps is a usually benign, self-limited infectious disease that can potentially result in serious complications. It is also prone to periodic outbreaks. Control of mumps can best be accomplished by remembering these five “Ps”:
• Prevention—through widespread two-dose MMR vaccination
• Parotitis—recognize it as the primary symptom of mumps and make the diagnosis in a timely manner
• Persistence—in making the diagnosis clinically and in weighing laboratory results within the context of clinical disease
• Personal protective equipment—use it consistently in the health care setting or as needed in the home
• Protection—isolate patients with mumps to avoid spreading the disease
CE/CME No: CR-1504
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the likely causes of mumps outbreaks.
• Explain the various possible manifestations of mumps illness.
• Identify the testing methods available to confirm a mumps diagnosis.
• Describe the potential complications of mumps and their incidence.
• Know what to do in the event of a mumps outbreak.
FACULTY
Jo Hanna Friend D’Epiro is a primary care PA in Student Health Services and a doctoral student, Workforce Development and Education, at The Ohio State University in Columbus. The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of April 2015.
Article begins on next page >>
Although overshadowed in the headlines by a sharp increase in measles cases, mumps too is making a comeback, with outbreaks throughout 2014 and early 2015. Many of today’s clinicians have never seen a case of mumps, let alone experienced an outbreak. Here’s what to look for and what to do if mumps makes its appearance in your practice.
In 2014, 1,151 cases of mumps were reported in the United States.1 By contrast, the typical annual rate has been in the low hundreds since 1989, when the CDC recommended a two-dose measles-mumps-rubella (MMR) vaccination regimen.1,2
Yet mumps has resurged in the past decade, with large outbreaks in 2006 (6,584 cases) and 2009-2010 (4,603 cases).3 Mumps outbreaks tend to occur among vaccinated young adults, such as college students, sports players, and campers, who live in close quarters.4
The 2014 outbreak centered around the Ohio State University campus in Columbus.5 That outbreak was declared over in October, with a total of 484 cases—more than in the entire US in 2013.1 In late 2014, at least 20 players and two officials in the National Hockey League became infected with mumps.6 More recently, Idaho announced that a 21-case outbreak that began at the University of Idaho’s Moscow campus had spread to Washington, with two additional cases reported there.7 What is responsible for these outbreaks, and what can the primary care clinician do to prevent or mitigate them?
EPIDEMIOLOGY
The mumps virus is part of the Rubulavirus genus of the Paramyxoviridae family. It affects the central nervous system (CNS) and glands—most commonly, the parotids. Uniquely human, mumps virus is found in saliva, cerebrospinal fluid, blood, breast milk, infected tissues, and urine.8,9 It is transmitted through contact with respiratory secretions and/or saliva, direct contact, or through fomites (eg, bedding, doorknobs).10
Before development of an effective vaccine, mumps was a universal childhood disease in the US; by age 14, most children had been infected.11 In the absence of widespread vaccination, mumps epidemics will occur every three to five years—as they still do in parts of the world without effective vaccination programs.12
As a result of widespread vaccination in the US, mumps incidence declined from 152,209 cases in 1967 to 2,982 cases in 1985 (see “Mumps and the MMR Vaccine”). Cases were reduced even further when administration of a second MMR dose was introduced in 1990.13 By 2000, the Healthy People 2010 goal was to eliminate mumps altogether.14
Continue for patient presentation >>
PATIENT PRESENTATION
Parotitis is the classic (but not universal) physical exam finding in mumps. Parotid gland inflammation causes generalized swelling anterior to the ear and inferior to the mastoid process, with jaw angle obliteration (see Figure 1).9 If only one parotid gland is involved, the patient’s face appears asymmetric. Other significant exam findings may include fever and erythematous swelling of the Stensen (parotid) duct.10
Nonspecific symptoms—including respiratory symptoms, myalgia, anorexia, malaise, headache, and low-grade fever—may occur in more than 50% of cases.8 CNS involvement may cause nuchal rigidity (stiff neck). In postpubertal males, testicular swelling and/or induration, pain, tenderness, and enlarged inguinal lymph nodes may be present.
Mumps can be challenging to diagnose based on clinical presentation alone; for example, parotitis occurs in only 30% to 40% of cases.8 Other viruses, such as parainfluenza virus 1 and 3, coxsackievirus, adenovirus, influenza A, cytomegalovirus, and HIV, can also cause swelling of the parotid glands, but mumps is the only virus known to cause parotitis on an epidemic scale.4 Furthermore, up to 20% of cases may be asymptomatic.8,11 Because mumps is highly contagious, a history of exposure to an affected individual is a compelling factor in making the diagnosis.
The incubation period for mumps is 12 to 25 days, with parotitis usually developing 16 to 18 days after exposure.4 This relatively lengthy incubation period increases the likelihood of viral spread. The virus is contagious from three days prior to symptom onset to day 4 of active disease.8 To prevent disease transmission, it is recommended that individuals remain isolated from others until five days after the onset of salivary gland swelling.4
Next page: Laboratory confirmation >>
LABORATORY CONFIRMATION
The CDC recommends determination of any one of the following to help confirm the diagnosis of acute mumps infection.
• Presence of serum mumps IgM antibodies
• Significant rise in IgG antibody titer between the acute and convalescent-phase serum specimens
• IgG seroconversion
• Positive mumps virus culture
• Detection of virus by real-time reverse transcriptase polymerase chain reaction (RT-PCR)4
Antibody testing
At the initial visit, a serum specimen should be obtained to test for mumps IgM antibodies.4 The CDC recommends enzyme immunoassay (EIA) testing for IgM antibodies to confirm acute mumps infection.4
IgM antibodies are detectable five days after onset of symptoms and, after reaching a maximum level, remain elevated for several weeks. If the initial IgM test is negative, the test can be repeated in five to seven days.4
Either EIA or immunofluorescence antibody assay (IFA) testing for mumps IgG antibodies should be performed on both acute- and convalescent-phase serum samples. Laboratory confirmation requires a fourfold rise in the antibody titer using a quantitative assay4 or seroconversion from negative to positive.
Virus detection
If possible, mumps virus samples should be obtained no more than three to eight days after symptom onset because delay may result in a low viral yield.4 The best viral samples are obtained via parotid duct swabs (see Figure 2). Before swabbing the buccal cavity, the parotid gland should be massaged for 30 seconds to ensure that the specimen contains gland secretions.4
Mumps virus can be detected by RT-PCR or culture. The RT-PCR is currently the most sensitive test for mumps, but most RT-PCR testing is done by public health laboratories and the CDC, and results may not be available until after the illness has resolved.4
Laboratory tests, however, are not always helpful in confirming a clinical diagnosis of mumps. Vaccinated persons may not mount a secondary immune response to mumps and consequently may not have a significant IgM response. It is also possible that a high level of IgG antibodies will cause a false-positive IgM test results.4
Other laboratory tests that may support a diagnosis of mumps include a complete blood cell count, which may reveal a leukopenia with relative lymphocytosis or neutrophil leukocytosis,16 and a measurement of serum amylase level which, if elevated, may confirm the inflammatory process.10 See Table 1 for a summary of tests that confirm or support a mumps diagnosis.
Mumps is a reportable illness, and the local health department should be contacted for assistance with determining where and how to ship specimens.17 However, in the absence of laboratory confirmation, only clinical cases with parotitis, other salivary gland involvement, or mumps-related complications are notifiable.11
Continue for mumps manifestations >>
MUMPS MANIFESTATIONS
The continuum of mumps illness ranges from asymptomatic infection to parotitis (the most well-known manifestation) to rare but severe complications.8 Table 2 lists potential complications of mumps in order of frequency. Complications vary by age and sex but tend to occur more often in adults.4,11
Immunization modifies the clinical presentation of mumps11 and likely decreases complications. Although one analysis of the 2006 outbreak identified no difference in complication rates between vaccinated and unvaccinated patients, the authors attributed this to misclassification of patients’ vaccination status; they did find lower reported rates of mumps complications compared with complication rates before widespread vaccination.13 A study of the 2009-2010 outbreak found that complication rates were lower among vaccinated patients.18
RISK FACTORS FOR OUTBREAKS
Of the three components of the MMR vaccine, the least effective is the mumps portion. One dose confers 78% immunity and two doses, 88%, which the CDC characterizes as incomplete protection.1 Compare this to the measles vaccine, which is 97% effective with two doses,19 and the rubella vaccine, which is 97% effective after a single dose in conferring immunity.11
In 2000, as a result of high rates of vaccination, the US determined that endemic measles had been eliminated. A similar conclusion was reached about rubella in 2004, and both determinations were reaffirmed in 2011.20 In contrast, mumps has never been eliminated.1
Waning immunity
While antibodies to mumps as a result of vaccination persist into adulthood, they decline over time. A 2009 CDC study found that, 12 years after a second MMR dose, mumps antibody levels in adolescents and young adults had declined to levels similar to those measured before the second dose.21 Other analyses of major outbreaks suggested the need for further studies to determine optimal timing for the second MMR dose (eg, at a later age) or if a third dose would provide longer-lasting immunity.13,22 Waning immunity among young adults, coupled with high-density living environments that intensify exposure to the virus, increase risk for the disease.23
Further complicating the situation is the lack of evidence about the required level of antibodies needed to confer protection against mumps infection.17 To date, the antibody titer threshold of mumps-specific IgG at which an individual is protected from the disease is unknown.11,17
Imported risks
Mumps remains endemic in many parts of the world, with vaccination employed in only 61% of countries belonging to the World Health Organization.4 Several recent outbreaks were traced to index cases originating outside the US.13 It is likely that importation of the virus from abroad will continue.
Next page: Outbreak management >>
OUTBREAK MANAGEMENT
A mumps outbreak is defined as three or more cases linked by time and place.4 The keys to managing an outbreak are to define the population(s) at risk and their transmission setting(s) and to rapidly identify and vaccinate vulnerable individuals without evidence of immunity.4
Presumptive evidence of mumps immunity includes11
• Documentation of vaccination with two doses of live mumps virus–containing vaccine
• Laboratory evidence of immunity
• Laboratory confirmation of disease
• Birth year before 1957.
Documentation of two doses of MMR constitutes evidence of adequate vaccination for school-age children and adolescents and for young adults attending postsecondary institutions. During an outbreak, susceptible (ie, unvaccinated) students should be excluded from attendance until they have been vaccinated; those with one dose may attend but should receive the second dose.4 Those declining vaccination for medical, religious, or other reasons should be excluded until at least 26 days after the onset of parotitis in the last person with mumps at the institution.24
If the outbreak threatens the wider community (eg, preschool-age children and adults), a second MMR dose should be considered for children ages 1 to 4 or for adults who have received one MMR dose. Similarly, MMR vaccination should be considered for adults born before 1957 who have no other evidence of immunity and are at risk for exposure to the virus.11
In the workplace, health care workers’ (HCWs’) immunity status should be known, documented, and accessible in advance of an outbreak.11 If an HCW without evidence of immunity is exposed to mumps, he or she should be excluded from patient care from the time of first unprotected exposure through the 25th day after the last exposure.25 Although individuals born before 1957 are generally considered immune, if a nosocomial mumps outbreak occurs, the two-dose MMR regimen should be administered to these HCW as well.4
In 1991, the US military began to immunize recruits routinely with MMR, regardless of their immunization status.26 During the 2006 mumps outbreak, the incidence of mumps among military personnel was minimal compared to that among their civilian counterparts—perhaps due to administration of a third MMR dose to an unknown number of recruits.22
CDC researchers studied the impact of a third MMR dose for mumps outbreak control in 2012 and concluded that, while a third dose may help control outbreaks among populations with preexisting high two-dose vaccine coverage, further study is needed.27
Although insufficient data exist on which to base a recommendation for or against a third MMR dose for mumps outbreak control, the CDC has issued guidance for public health departments for targeted administration during outbreaks. Considerations include
• Intense exposure settings
• High two-dose vaccination coverage (ie, > 90%)
• High attack rates (> 5 cases per 1,000 population)
• Evidence of ongoing transmission for at least two weeks in the target population.4
TREATMENT
There is no specific treatment for mumps. Care is supportive and in the outpatient setting includes rest, cold or heat to the affected areas, and OTC pain relievers. Ice can be used to help relieve the pain of orchitis. Acidic foods may stimulate the parotid glands, causing pain and difficulty swallowing, and should be avoided.
Isolation of infectious patients is vital to preventing the spread of mumps.4 In the clinician’s office, a separate waiting area should be used for a potential mumps patient, or the patient should be located at least three feet from other patients and asked to wear a surgical mask. HCW working with potential mumps patients should follow droplet precautions (eg, wear personal protective equipment) in addition to standard precautions and should be hypervigilant about hand washing.24
CONCLUSION
Mumps is a usually benign, self-limited infectious disease that can potentially result in serious complications. It is also prone to periodic outbreaks. Control of mumps can best be accomplished by remembering these five “Ps”:
• Prevention—through widespread two-dose MMR vaccination
• Parotitis—recognize it as the primary symptom of mumps and make the diagnosis in a timely manner
• Persistence—in making the diagnosis clinically and in weighing laboratory results within the context of clinical disease
• Personal protective equipment—use it consistently in the health care setting or as needed in the home
• Protection—isolate patients with mumps to avoid spreading the disease
1. CDC. Mumps cases and outbreaks. www.cdc.gov/mumps/outbreaks.html. Accessed March 19, 2015.
2. CDC. Measles prevention: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Morb Mortal Wkly Rep. 1989;38(suppl 9):1-18.
3. CDC. Summary of notifiable diseases—United States. 1993-2012. MMWR Morb Mortal Wkly Rep. www.cdc.gov/mmwr/mmwr_nd/index.html. Accessed March 19, 2015.
4. CDC. Mumps, chap 9. In: Manual for the Surveillance of Vaccine-Preventable Diseases. August 2012. www.cdc.gov/vaccines/pubs/surv-manual/chpt09-mumps.html. Accessed March 19, 2015.
5. Pyle E. Central Ohio mumps outbreak finally loses steam. The Columbus Dispatch. October 11, 2014. www.dispatch.com/content/stories/local/2014/10/10/Health-officials-declare-mumps-outbreak-over.html. Accessed March 19, 2015.
6. Hascup J. Three more mumps cases: Penguins’ Steve Downie, Thomas Greiss, Devils’ Patrik Elias, Michael Ryder. USA TODAY. December 27, 2014. www.usatoday.com/story/sports/nhl/2014/12/26/steve-downie-thomas-greiss-mumps-pittsburgh-penguins/20911031/. Accessed March 19, 2015.
7. Idaho public health officials warn that mumps outbreak involving University students has spread to Boise and State of Washington [news release]. Boise, ID: Idaho Department of Health and Welfare Public Information Office; February 6, 2015. www.healthandwelfare.idaho.gov/AboutUs/Newsroom/tabid/130/ctl/ArticleView/mid/3061/articleId/1819/Idaho-Public-Health-Officials-Warn-that-Mumps-Outbreak-Involving-University-Students-Has-Spread-to-Boise-and-State-of-Washington.aspx. Accessed March 19, 2015.
8. CDC. Epidemiology and prevention of vaccine-preventable diseases. Mumps, chap 12. In: The Pink Book: Course Textbook. 12th ed, 2012. www.cdc.gov/vaccines/pubs/pinkbook/mumps.html. Accessed March 19, 2015.
9. Albrecht MA. Epidemiology, clinical manifestations, diagnosis, and management of mumps. www.uptodate.com/contents/epidemiology-clin ical-manifestations-diagnosis-and-management-of-mumps. Accessed March 19, 2015.
10. Defendi GL. Mumps. http://reference.medscape.com/article/966678-overview. Accessed March 19, 2015.
11. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR04):1-34.
12. Rubin S, Carbone KM. Mumps. In: Longo DL, Fauci AS, Kasper DL et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: The McGraw-Hill Companies, Inc.; 2012:chap 194.
13. Dayan G, Quinlisk M, Parker A, et al. Recent resurgence of mumps in the United States. New Engl J Med. 2008;358:1580-1589.
14. US Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2010: Objectives for Improving Health. www.healthypeople.gov/2010/Document/HTML/Volume1 /14Immunization.htm#_Toc494510239. Accessed March 19, 2015.
15. CDC. Measles, mumps, and rubella: vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1998;47(RR-8):1-57.
16. Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ. 2005;(330):1132-1135.
17. CDC. Laboratory testing for mumps infection. www.cdc.gov/mumps/lab/qa-lab-test-infect.html. Accessed March 19, 2015.
18. Barskey AE, Schulte C, Rosen JB, et al. Mumps outbreak in Orthodox Jewish communities in the United States. New Engl J Med. 2012;367(18): 1704-1713.
19. CDC. Frequently asked questions about measles in the U.S. www.cdc.gov/measles/about/faqs.html. Accessed March 19, 2015.
20. Papania MJ, Wallace GS, Rota PA, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatr. 2014;168(2):148-155.
21. LeBaron CW, Forghani B, Beck C, et al. Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis. 2009; 199(4):552-560.
22. Anis E, Grotto I, Moerman L, et al. Mumps outbreak in Israel’s highly vaccinated society: are two doses enough? Epidemiol Infect. 2012; 140:439-446.
23. Sane J, Gouma S, Koopmans M, et al. Epidemic of mumps among vaccinated persons, The Netherlands, 2009-2012. Emerg Infect Dis. 2014;20(4):643-648.
24. CDC. Exposure and response. www.cdc.gov/mumps/clinical/qa-exposure-response.html. Accessed March 19, 2015.
25. CDC. Mumps prevention and control in healthcare settings. www.cdc.gov/mumps/prev-control-settings/background.html. Accessed March 19, 2015.
26. Barskey AE, Glasser JW, LeBaron CW. Mumps resurgence in the United States: a historical perspective on unexpected elements. Vaccine. 2009;27:6186-6195.
27. Ogbuanu IU, Kutty PK, Hudson JM, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics. 2012;130:e1567-e1574.
28. CDC. Physical findings and complications. www.cdc.gov/mumps/clinical/qa-physical-complic.html. Accessed March 19, 2015.
29. Conniff R. A forgotten pioneer of vaccines. The New York Times. May 6, 2013. www.nytimes.com/2013/05/07/health/maurice-hilleman-mmr-vaccines-forgotten-hero.html?_r=0. Accessed March 19, 2015.
30. CDC. MMR vaccine for mumps. www.cdc.gov/vaccines/vpd-vac/mumps/vac-faqs-tech.htm. Accessed March 19, 2015.
31. The College of Physicians of Philadelphia. The history of vaccines. www.historyofvaccines.org/content/timelines/all. Accessed March 19, 2015.
32. CDC. Travelers’ health. Mumps. wwwnc.cdc.gov/travel/diseases/mumps. Accessed March 19, 2015.
33. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children [retracted in Lancet. 2010;375:445]. Lancet. 1998;351:637-641.
34. Murch SH, Anthony A, Casson DH, et al. Retraction of an interpretation. Lancet. 2004;363(9411):750.
35. Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:1-16.
36. The Editors of The Lancet. Notice of retraction of "Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children" [retraction of: Wakefield AJ, Murch SH, Anthony A, et al. In: Lancet. 1998;351:637-641]. Lancet. 2010;375:445.
37. Whalen J. UK bans doctor who linked autism to vaccine. The Wall Street Journal. May 24, 2010. www.wsj.com/articles/SB10001424052748704113504575263994195318772. Accessed March 19, 2015.
38. CDC. Vaccine safety: measles, mumps and rubella (MMR) vaccine. www.cdc.gov/vaccinesafety/vaccines/mmr/mmr.html. Accessed March 19, 2015.
39. Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347:1477-1482.
40. DeStefano F, Bhasin TK, Thompson WW, et al. Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan Atlanta. Pediatrics. 2004;113(2):259-266.
41. Richler J, Luyster R. Risi S, et al. Is there a ‘regressive phenotype’ of autism spectrum disorder associated with the measles-mumps-rubella vaccine? A CPEA study. J Autism Dev Disord. 2006;36(3):299-316.
42. Nyhan B, Reifler J, Richey S, Freed G. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014:133:1-8.
43. Haberman C. A discredited vaccine study’s continuing impact on public health. The New York Times. February 1, 2015. www.nytimes.com/2015/02/02/us/a-discredited-vaccine-studys-continuing-impact- on-public- health.html?_r=0. Accessed March 19, 2015.
1. CDC. Mumps cases and outbreaks. www.cdc.gov/mumps/outbreaks.html. Accessed March 19, 2015.
2. CDC. Measles prevention: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Morb Mortal Wkly Rep. 1989;38(suppl 9):1-18.
3. CDC. Summary of notifiable diseases—United States. 1993-2012. MMWR Morb Mortal Wkly Rep. www.cdc.gov/mmwr/mmwr_nd/index.html. Accessed March 19, 2015.
4. CDC. Mumps, chap 9. In: Manual for the Surveillance of Vaccine-Preventable Diseases. August 2012. www.cdc.gov/vaccines/pubs/surv-manual/chpt09-mumps.html. Accessed March 19, 2015.
5. Pyle E. Central Ohio mumps outbreak finally loses steam. The Columbus Dispatch. October 11, 2014. www.dispatch.com/content/stories/local/2014/10/10/Health-officials-declare-mumps-outbreak-over.html. Accessed March 19, 2015.
6. Hascup J. Three more mumps cases: Penguins’ Steve Downie, Thomas Greiss, Devils’ Patrik Elias, Michael Ryder. USA TODAY. December 27, 2014. www.usatoday.com/story/sports/nhl/2014/12/26/steve-downie-thomas-greiss-mumps-pittsburgh-penguins/20911031/. Accessed March 19, 2015.
7. Idaho public health officials warn that mumps outbreak involving University students has spread to Boise and State of Washington [news release]. Boise, ID: Idaho Department of Health and Welfare Public Information Office; February 6, 2015. www.healthandwelfare.idaho.gov/AboutUs/Newsroom/tabid/130/ctl/ArticleView/mid/3061/articleId/1819/Idaho-Public-Health-Officials-Warn-that-Mumps-Outbreak-Involving-University-Students-Has-Spread-to-Boise-and-State-of-Washington.aspx. Accessed March 19, 2015.
8. CDC. Epidemiology and prevention of vaccine-preventable diseases. Mumps, chap 12. In: The Pink Book: Course Textbook. 12th ed, 2012. www.cdc.gov/vaccines/pubs/pinkbook/mumps.html. Accessed March 19, 2015.
9. Albrecht MA. Epidemiology, clinical manifestations, diagnosis, and management of mumps. www.uptodate.com/contents/epidemiology-clin ical-manifestations-diagnosis-and-management-of-mumps. Accessed March 19, 2015.
10. Defendi GL. Mumps. http://reference.medscape.com/article/966678-overview. Accessed March 19, 2015.
11. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(RR04):1-34.
12. Rubin S, Carbone KM. Mumps. In: Longo DL, Fauci AS, Kasper DL et al, eds. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: The McGraw-Hill Companies, Inc.; 2012:chap 194.
13. Dayan G, Quinlisk M, Parker A, et al. Recent resurgence of mumps in the United States. New Engl J Med. 2008;358:1580-1589.
14. US Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2010: Objectives for Improving Health. www.healthypeople.gov/2010/Document/HTML/Volume1 /14Immunization.htm#_Toc494510239. Accessed March 19, 2015.
15. CDC. Measles, mumps, and rubella: vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1998;47(RR-8):1-57.
16. Gupta RK, Best J, MacMahon E. Mumps and the UK epidemic 2005. BMJ. 2005;(330):1132-1135.
17. CDC. Laboratory testing for mumps infection. www.cdc.gov/mumps/lab/qa-lab-test-infect.html. Accessed March 19, 2015.
18. Barskey AE, Schulte C, Rosen JB, et al. Mumps outbreak in Orthodox Jewish communities in the United States. New Engl J Med. 2012;367(18): 1704-1713.
19. CDC. Frequently asked questions about measles in the U.S. www.cdc.gov/measles/about/faqs.html. Accessed March 19, 2015.
20. Papania MJ, Wallace GS, Rota PA, et al. Elimination of endemic measles, rubella, and congenital rubella syndrome from the Western hemisphere: the US experience. JAMA Pediatr. 2014;168(2):148-155.
21. LeBaron CW, Forghani B, Beck C, et al. Persistence of mumps antibodies after 2 doses of measles-mumps-rubella vaccine. J Infect Dis. 2009; 199(4):552-560.
22. Anis E, Grotto I, Moerman L, et al. Mumps outbreak in Israel’s highly vaccinated society: are two doses enough? Epidemiol Infect. 2012; 140:439-446.
23. Sane J, Gouma S, Koopmans M, et al. Epidemic of mumps among vaccinated persons, The Netherlands, 2009-2012. Emerg Infect Dis. 2014;20(4):643-648.
24. CDC. Exposure and response. www.cdc.gov/mumps/clinical/qa-exposure-response.html. Accessed March 19, 2015.
25. CDC. Mumps prevention and control in healthcare settings. www.cdc.gov/mumps/prev-control-settings/background.html. Accessed March 19, 2015.
26. Barskey AE, Glasser JW, LeBaron CW. Mumps resurgence in the United States: a historical perspective on unexpected elements. Vaccine. 2009;27:6186-6195.
27. Ogbuanu IU, Kutty PK, Hudson JM, et al. Impact of a third dose of measles-mumps-rubella vaccine on a mumps outbreak. Pediatrics. 2012;130:e1567-e1574.
28. CDC. Physical findings and complications. www.cdc.gov/mumps/clinical/qa-physical-complic.html. Accessed March 19, 2015.
29. Conniff R. A forgotten pioneer of vaccines. The New York Times. May 6, 2013. www.nytimes.com/2013/05/07/health/maurice-hilleman-mmr-vaccines-forgotten-hero.html?_r=0. Accessed March 19, 2015.
30. CDC. MMR vaccine for mumps. www.cdc.gov/vaccines/vpd-vac/mumps/vac-faqs-tech.htm. Accessed March 19, 2015.
31. The College of Physicians of Philadelphia. The history of vaccines. www.historyofvaccines.org/content/timelines/all. Accessed March 19, 2015.
32. CDC. Travelers’ health. Mumps. wwwnc.cdc.gov/travel/diseases/mumps. Accessed March 19, 2015.
33. Wakefield AJ, Murch SH, Anthony A, et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children [retracted in Lancet. 2010;375:445]. Lancet. 1998;351:637-641.
34. Murch SH, Anthony A, Casson DH, et al. Retraction of an interpretation. Lancet. 2004;363(9411):750.
35. Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:1-16.
36. The Editors of The Lancet. Notice of retraction of "Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children" [retraction of: Wakefield AJ, Murch SH, Anthony A, et al. In: Lancet. 1998;351:637-641]. Lancet. 2010;375:445.
37. Whalen J. UK bans doctor who linked autism to vaccine. The Wall Street Journal. May 24, 2010. www.wsj.com/articles/SB10001424052748704113504575263994195318772. Accessed March 19, 2015.
38. CDC. Vaccine safety: measles, mumps and rubella (MMR) vaccine. www.cdc.gov/vaccinesafety/vaccines/mmr/mmr.html. Accessed March 19, 2015.
39. Madsen KM, Hviid A, Vestergaard M, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002;347:1477-1482.
40. DeStefano F, Bhasin TK, Thompson WW, et al. Age at first measles-mumps-rubella vaccination in children with autism and school-matched control subjects: a population-based study in metropolitan Atlanta. Pediatrics. 2004;113(2):259-266.
41. Richler J, Luyster R. Risi S, et al. Is there a ‘regressive phenotype’ of autism spectrum disorder associated with the measles-mumps-rubella vaccine? A CPEA study. J Autism Dev Disord. 2006;36(3):299-316.
42. Nyhan B, Reifler J, Richey S, Freed G. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014:133:1-8.
43. Haberman C. A discredited vaccine study’s continuing impact on public health. The New York Times. February 1, 2015. www.nytimes.com/2015/02/02/us/a-discredited-vaccine-studys-continuing-impact- on-public- health.html?_r=0. Accessed March 19, 2015.
Pediatric T2DM: A Growing Threat to US Health
CE/CME No: CR-1503
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between pediatric type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and definitively establish the diagnosis.
• Understand the link between pediatric obesity and T2DM and help the patient and family incorporate healthy eating and physical activity habits into their lifestyle.
• Describe the scope of treatment options for pediatric T2DM and the importance of monitoring glycemic control to ensure that treatment goals are met.
• Explain the long-term health risks associated with pediatric T2DM and how to screen for complications in order to initiate early treatment.
• Establish a health care team with the primary care clinician, T2DM specialists, and the patient’s family to create an individualized plan of care for your pediatric patient with T2DM.
FACULTY
Ashlyn Smith is an endocrinology PA at Endocrinology Associates in Scottsdale, Arizona. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2015.
Article begins on next page >>
The increasing prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents is a serious health threat that urgently requires effective lifestyle intervention in at-risk patients, vigilant disease management for those diagnosed, and further research to support treatment decisions in the pediatric population.
Over the past several decades, National Health and Nutrition Examination Survey (NHANES) data have documented a sharp rise in the prevalence of obesity in children and adolescents ages 2 to 19: from 5.5% during 1976-1980 to 16.9% in 2009-2010.1 Cardiovascular damage once seen only in adults is occurring in obese children, along with other obesity-related comorbidities such as dyslipidemia and insulin resistance.2 Similarly, type 2 diabetes mellitus (T2DM), once a disease of middle-aged or older adults, is being diagnosed with growing frequency in the pediatric population.3
While much is known about adult T2DM, less has been established about pediatric T2DM because of its relatively recent emergence. Areas requiring further study in the pediatric population include the determination of optimal target A1C levels; the most effective treatments for both T2DM and coexisting conditions; and the long-term impact on morbidity and mortality when T2DM is diagnosed so early in life. Increasing evidence suggests that T2DM in young people is an “aggressive” form of diabetes,4 with significant comorbidities that may already be present at diagnosis.5
In an effort to document long-term outcomes for patients diagnosed with “young-onset type 2 diabetes mellitus” (defined as T2DM diagnosed between ages 15 and 30), researchers reviewed records from the Royal Prince Alfred Hospital’s Diabetes Clinical Database, established in 1986, that were matched against the Australian National Death Index through June 2011. They identified 470 cases of type 1 diabetes mellitus (T1DM) and 354 cases of T2DM, with a median observation period of more than 20 years for patients in both groups, and compared morbidity and mortality outcomes.6 The authors found that unfavorable cardiovascular risk factors were more prevalent in the T2DM group and developed earlier in the disease process—in some cases, as early as two years after diagnosis—than in patients with T1DM. Diabetic complications (eg, albuminuria and neuropathy) were more prevalent in the T2DM group, but the rates of retinopathy were about the same in both groups.6
In terms of mortality, 11% of the patients with T2DM and 6.8% of those with T1DM had died, and the deaths in the T2DM group occurred after a significantly shorter duration of disease (26.9 v 36.5 y). Cardiovascular causes of death predominated in both groups but were more common in patients with T2DM (50.0%) than with T1DM (30.3%). The authors concluded that T2DM is “the more lethal phenotype” of diabetes in young people and requires intensive intervention directed at both glycemic control and cardiovascular risk management.6
EPIDEMIOLOGY
In 2012, an estimated 208,000 Americans younger than 20 were diagnosed with diabetes7; approximately 10.5% of them (21,000) were diagnosed with T2DM.8 While these numbers are a small fraction of the 29.1 million Americans living with diabetes,7 researchers note that both the incidence and prevalence of T2DM in young people are increasing.
For the years 2002-2003, the SEARCH for Diabetes in Youth Study Group estimated the annual incidence of new cases of diabetes in persons younger than 20 to be 15,000 for T1DM and 3,700 for T2DM.3 By 2008-2009, those estimates had grown to 18,436 per year for T1DM and 5,089 per year for T2DM.7
It has been projected that, if incidence rates remain constant, the number of young people diagnosed with T2DM in the United States will increase by 49% by 2050. If incidence grows by 2.3% annually, however, the increase could be fourfold by 2050.9
Prevalence has also increased. The number of persons age 19 or younger living with T1DM increased by 21.1% between 2001 and 2009; prevalence of T2DM in this age-group grew by 30.5% during the same period.10 T2DM prevalence also varies by race and ethnicity, ranging from 0.17/1,000 in non-Hispanic white youth to 1.20/1,000 among young people of Native American heritage (see Table, above).10
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The progression toward T2DM is influenced by both genetic and lifestyle risk factors that lead to insulin resistance and eventual pancreatic β-cell dysfunction. While aerobic exercise increases insulin sensitivity, a diet high in carbohydrates and fat increases the demand for insulin. So a sedentary lifestyle, combined with a high-carbohydrate, high-fat diet, gradually results in a state of insulin resistance. Compensatory hyperinsulinism will maintain normoglycemia for an indeterminate amount of time; but eventually, pancreatic “burnout” leads to pancreatic β-cell dysfunction and decreased insulin secretion. Relative insulin deficiency then causes decreased cellular glucose uptake, hyperglycemia, and ultimately, T2DM.11
Additional pathophysiologic deficiencies and malfunctions that contribute to hyperglycemia include increased glucagon secretion, decreased incretin effect, and increased renal glucose reabsorption.12
CLINICAL PRESENTATION
Clinical presentation of T2DM varies greatly among pediatric patients. Classic symptoms may include polydipsia and polyuria related to hyperglycemia. In addition, the patient or his/her family may note frequent infections or visual disturbances (eg, blurred or diminished vision). However, particularly in the pediatric population, the patient is often asymptomatic.13
On examination, the clinician may observe that the child or adolescent is overweight or obese. He or she may have mild to severe acanthosis nigricans on the posterior neck, axillae, abdomen, or thighs and over the antecubital fossa. However, acanthosis nigricans may not manifest in patients with fairer skin. In severe cases, patients with T2DM may present with hyperglycemic hyperosmolar nonketotic coma or diabetic ketoacidosis.14
Next page: Diagnosis >>
DIAGNOSIS
Identification of children at risk for T2DM is the first step in delaying or preventing pediatric T2DM and its complications. The American Diabetes Association’s Standards of Medical Care in Diabetes—2015 indicate that asymptomatic children and adolescents who are overweight (BMI above the 85th percentile for age and sex, weight for height above the 85th percentile, or weight greater than 120% of ideal for height) and who meet at least two of the following criteria should be tested for T2DM15:
• Family history of T2DM in first- or second-degree relative (74% to 100% have a first- or second-degree relative with diabetes14)
• Non-European ancestry (Native American, African-American, Latino, Asian American, Pacific Islander)
• Signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome [PCOS], or small-for-gestational-age birth weight)
• Maternal history of diabetes or gestational diabetes during pregnancy.
Screening should begin when the child is 10 or when puberty is attained, with retesting every three years thereafter.
Testing
The diagnosis of diabetes may be confirmed by any one of the following test results4,15
• A1C level ≥ 6.5%
• Fasting blood glucose (FBG) ≥ 126 mg/dL
• Two-hour oral glucose tolerance test (OGTT) level ≥ 200 mg/dL
• Random serum glucose ≥ 200 mg/dL with symptoms of hyperglycemia.
Type 1 or type 2?
In some newly diagnosed pediatric patients with diabetes, distinguishing T1DM from T2DM may be difficult; 25% of cases of T2DM are misclassified as T1DM.4,14 For example, an obese child who presents with ketosis appears to have features of both T1DM and T2DM.4 The distinction needs to be made as soon as possible because management of the diseases is quite different.
Measurement of C-peptide level (low in T1DM, normal or high in T2DM) is suggestive but not 100% reliable for this purpose. Similarly, ketosis is an unreliable indicator; adolescents with T2DM present with ketoacidosis in 5% to 25% of cases.4,14
Another means to differentiate T1DM and T2DM is determining the levels of diabetes autoantibodies (DAA) to insulin. Pediatric patients with suspected DM can be screened for the islet cell autoantibodies GAD (glutamic acid decarboxylase)-65 and IA (insulin antibody)-2. In addition, a new DAA assay that tests for the zinc transporter 8 autoantibody [ZnT8Ab] was recently approved by the FDA for marketing.16 Detection of these antibodies is generally—but not always—an indicator of T1DM, an autoimmune disease of the pancreas.5
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group found that, of 1,206 obese (BMI in the 85th percentile or higher) participants with T2DM, 9.8% tested positive for either or both of the GAD-65 and IA-2 antibodies. It was unclear if the DAA-positive patients had both T1DM and T2DM or T1DM with insulin resistance due to obesity. They concluded that testing for islet cell autoantibodies was needed to reliably identify patients with autoimmune diabetes.17
In addition to establishing the correct diagnosis, the clinician should order tests to rule out a concomitant thyroid disorder and to assess the patient for diabetic complications. Testing should include a thyroid panel, lipid panel, urine microalbumin level, and renal and hepatic function tests.18
Continue for treatment of T2DM >>
TREATMENT OF T2DM
Because relevant clinical data are limited, blood glucose (BG) goals should be individualized for each child with T2DM. As a rule of thumb, guidelines for BG goals in pediatric T1DM can serve as a reference for establishing BG goals in T2DM. Once adulthood is reached, BG goals reflect adult ranges.
FBG and A1C goals in pediatric T1DM are
• Younger than 4 years: 80 to 200 mg/dL and < 8.5%
• Between 5 and 11: 70 to 180 mg/dL and < 8.0%
• 12 and older: 70 to 150 mg/dL and < 7.5%.18
Primary care clinicians who manage pediatric patients with T2DM should consult with diabetes specialists at diagnosis and at least annually thereafter. Consultation is encouraged when treatment goals are not met.4
Lifestyle changes
The cornerstone of pediatric T2DM treatment is effective lifestyle intervention aimed at achievement of the patient’s BG goals and reduction of risks for microvascular and macrovascular complications.
Dietary modification methods can vary, depending on the caregivers’ and patient’s previous knowledge of nutrition. For families with little nutritional knowledge, the clinician may recommend the “plate method,” with half a plate designated for vegetables, one quarter for lean meats and protein, and one quarter for carbohydrates. As an alternative, the clinician may advise simple portion control, including smaller plate sizes and only one serving per meal. In addition, all sugared beverages should be avoided and daily milk intake should be limited.19
Behaviors surrounding meal preparation and snacking may also contribute to excessive caloric intake. Each day’s meals should be planned, and eating while at the computer, doing homework, or watching TV should be avoided.4
As time and the family’s comfort level permit, intensification of nutritional therapy can improve glycemic control through specific instruction regarding calorie counting and selection of low-fat and low-carbohydrate foods. Calorie intake of 900 to 1,200 kcal/d for children ages 6 to 12 and a minimum of 1,200 kcal/d for adolescents ages 13 to 18 are recommended for weight loss and improved body composition (see "Resources for Weight Management and Nutrition").4
Physical activity in the pediatric population can be derived from multiple sources, including physical education classes, after-school programs, sports and dance programs, and walks with the family or the dog. When discussing exercise recommendations, it is essential to consider any limitations imposed by the family’s finances and family members’ schedules.
Cardiovascular activity is recommended for 30 to 60 min or more every day or most days.4,14 To decrease excessive pursuit of sedentary activities, parents are urged to limit children’s nonacademic “screen time” to a maximum of two hours per day and to discourage the placement of video screens and television sets in children’s bedrooms.4
While lifestyle intervention remains the first-line treatment choice for pediatric T2DM, expert consensus is that less than 10% of pediatric patients reach glycemic control goals with lifestyle modifications alone.4
Next page: Treatment with metformin >>
First-line medication: Metformin
If treatment goals are not met with lifestyle changes, treatment with metformin should be initiated.4,14 Metformin is currently the only FDA-approved oral agent for the treatment of T2DM in children, although it is not recommended for use in those younger than 10.20 Metformin improves glycemic control by increasing insulin action, decreasing gluconeogenesis, and decreasing glucagon secretion.11 Metformin may also regulate ovulation in patients with PCOS, increasing the patient’s fertility. Adolescent female patients being treated with metformin should avoid pregnancy because its use is not recommended during pregnancy.14,20
Because metformin carries a black box warning for a rare but life-threatening complication—lactic acidosis—the clinician should confirm normal renal function before treatment initiation. Renal function should be monitored regularly (at least annually during metformin treatment and more often if impaired renal function is anticipated) and metformin discontinued if impaired renal function is present. Risk is also reduced by use of the lowest effective dose.20
To minimize common gastrointestinal adverse effects, metformin should be taken with meals. Treatment should be initiated at a dose of 500 mg/d,4 with slow titration upward by 500 mg/wk, as needed and tolerated, until an effective maintenance dose is reached. Maintenance doses may range from 500 mg/d to 2,000 mg/d (taken in divided doses).20
Metformin use can result in moderate decreases in BG and A1C levels. In addition, because metformin does not stimulate insulin secretion, the risk for hypoglycemia is minimal.20 The clinician may consider monitoring vitamin B12 levels, particularly in a population already at risk for peripheral neuropathy, because metformin increases risk for vitamin B12 deficiency.20
As T2DM progresses, metformin alone may be insufficient for maintenance of glycemic control.
Insulin therapy
Treatment with insulin therapy, rather than metformin, should be initiated in pediatric diabetes patients if the diagnosis of T2DM is not confirmed or for those patients who present with ketosis or ketoacidosis.4
Insulin therapy should also be initiated for patients with confirmed T2DM if random tests for plasma BG levels are 250 mg/dL or higher or A1C levels are more than 9.0%.4 Reflecting the lack of consensus on pediatric T2DM treatment, the ADA recommends insulin therapy when the A1C level is greater than 8.5%.13 Insulin may be used in conjunction with metformin, which may decrease the insulin dosage that would otherwise be needed due to metformin’s ability to increase insulin sensitivity.4
Insulin regimens
Selection of the appropriate insulin regimen depends on clinician judgment, patient and family comfort level, and lifestyle considerations. Initial barriers for the patient and family may include resistance to injecting medication, difficulty understanding instructions for insulin therapy, and fear of weight gain or hypoglycemia. Close monitoring for significant day-to-day fluctuations in the pediatric patient’s activity levels or diet is essential in order to adjust insulin dosage as needed and prevent hyperglycemia or hypoglycemia.
The types of insulin therapy include
Basal. Basal insulin at bedtime with insulin detemir or glargine is a straightforward method of insulin delivery, with lower risk for hypoglycemia. The ADA recommends initial dosing at 0.3 to 0.4 U/kg/d for the pediatric population, titrating slowly until FBG is at goal without hypoglycemia.13
Basal-bolus. If postprandial BG or A1C levels remain elevated, basal insulin provides the groundwork for intensification to a physiologic basal-bolus regimen with addition of insulin lispro, aspart, or glulisine. Bolus insulin therapy can be in the form of a fixed mealtime dose or as an insulin-to-carbohydrate ratio, as caretaker comfort and patient lifestyle permit.11
In patients who are not able to adhere to a basal-bolus regimen due to multiple daily injections, consider conservative treatment with twice-daily insulin NPH as basal therapy, with or without short-acting insulin at breakfast and dinner.11
An alternative for adults that is occasionally used in youth (although not yet FDA-approved for this use) is twice-daily premixed insulin aspart 70/30 or lispro 75/25. While this regimen can improve compliance, it does not permit mealtime adjustments in insulin dosing and requires a snack between meals to prevent hypoglycemia.11
Families will sometimes choose insulin pump therapy for a pediatric patient with T2DM.11 For these patients, use of the insulin pump requires a motivated family committed to BG monitoring at least four times a day and in possession of a good working knowledge of basic diabetes management.21
Continue for additional treatment options >>
Additional treatment options
While metformin and insulin are the only FDA-approved medications for the treatment of T2DM in the pediatric population, additional medications may be used if needed because of deteriorating glycemic control.4 If treated with noninsulin agents (including metformin), adolescent female patients should be counseled to avoid pregnancy because long-term data are lacking about the safety to the fetus of noninsulin agents.22
Metformin-rosiglitazone. In the TODAY trial,23 699 participants (ages 10 to 17) with T2DM were randomly assigned one of three treatment options: metformin alone, metformin plus rosiglitazone, and metformin with lifestyle intervention. The study found that monotherapy with metformin was often insufficient to achieve glycemic control, with a rate of treatment failure of 51.8%. Rates of failure for the other groups were 38.6% and 46.6%, respectively.
While the combination of metformin and rosiglitazone was found to be most effective, at the time these results were published, access to rosiglitazone was restricted by the FDA because of concerns about the drug’s cardiovascular safety. The FDA has since determined that rosiglitazone’s cardiovascular risks are comparable to those of other diabetes drugs and directed in November 2013 that the restrictions be removed.24 In this context, the TODAY study researchers concluded that it was unclear if the results were specific to rosiglitazone, to the thiazolidinedione drug class as a whole, or to some attribute of combination therapy; they suggested, however, that combination therapy may be superior for pediatric T2DM treatment.23
Incretin-based therapies. Another potential therapeutic option is the use of incretin-based therapies, such as the glucagon-like peptide (GLP-1) agonists exenatide or liraglutide. These have been shown to be effective at lowering BG levels in the adult population and have the added benefit of causing weight loss.
Although not approved for pediatric use, GLP-1 agonists would provide a means of therapy intensification without weight gain, which can worsen insulin resistance and propel the disease process forward. Further research is needed, and there is an ongoing multicenter safety and efficacy trial of exenatide use in adolescents with T2DM.13
Self-monitoring of blood glucoseInitiation and frequency of self-monitoring of BG (SMBG) in pediatric T2DM patients depends on the treatment regimen and achievement of BG goals.
The American Academy of Pediatrics recommends initiation of SMBG when the patient is 1) taking insulin or medications with risk for hypoglycemia; 2) initiating or changing a treatment regimen; 3) not meeting treatment goals; or 4) experiencing an intercurrent illness.4 In addition, a patient with hyperglycemic or hypoglycemic symptoms should perform SMBG at least for the duration of the symptoms.
For all patients newly diagnosed with T2DM, SMBG is recommended before meals (including morning fasting) and at bedtime until target BG levels are achieved.4 For patients whose disease is well controlled with metformin, SMBG can be performed on an infrequent or intermittent basis.
In most cases, the clinician should advise the patient to perform morning and bedtime SMBG to assess fasting and postprandial glucose levels. Postprandial SMBG will allow both caregivers and clinicians to assess for glycemic excursions, especially in cases in which A1C is above goal, despite an FBG level that is at goal.4 Premeal SMBG may also be temporarily utilized during acute illness and to monitor for glycemic excursions.
Patients receiving multiple daily injections or insulin pump therapy require BG testing prior to every meal and often postprandial or bedtime SMBG as well.
Therapy intensification
At any point during treatment, if glycemic goals are not being met, the clinician should analyze the patient’s current medication(s), lifestyle interventions, and treatment adherence for opportunities to optimize treatment effectiveness. Therapy intensification may require more frequent office visits and SMBG, augmenting or adding medication, and referral to a nutritionist or diabetes educator. The need for treatment individualization and enhancement is likely to arise frequently, and it is often beneficial to set this expectation early in the T2DM management process with the pediatric patient and his/her family.4
SCREENING FOR AND MANAGEMENT OF COMPLICATIONS
Due in part to the prolonged duration of illness, patients diagnosed with T2DM as children or adolescents are at higher risk than adults for microvascular and macrovascular complications. Therefore, regular screening for retinopathy, nephropathy, hypertension, and dyslipidemia is essential, as follows:
• A dilated eye exam for diabetic retinopathy is recommended at diagnosis, annually, and more frequently as needed, depending on findings.13
• Annual screening for microalbuminuria will assess for early renal impairment and allow treatment to prevent diabetic nephropathy. As in adults, pediatric cases of microalbuminuria are treated with ACE inhibitors or angiotensin receptor blockers (ARBs). ACE inhibitors are dosed at 0.05-0.15 mg/kg/d in pediatric patients.11 Adolescent female patients should avoid pregnancy if taking an ACE inhibitor or ARB because of risks to the fetus from these Category D medications.13
• In children and adolescents, the diagnosis of hypertension is made using age-, height-, and gender-adjusted percentiles. The 2011 National Heart, Lung and Blood Institute’s (NHLBI’s) integrated guidelines for cardiovascular health and risk reduction in children and adolescents include updated blood pressure tables and diagnostic algorithms for this purpose.25 Pediatric hypertension is treated with ACE inhibitors and ARBs, and dosing recommendations are also available in the NHLBI guidelines.25
• Acceptable lipid levels for children and adolescents are as follows: LDL, < 110 mg/dL; HDL, > 45 mg/dL; and triglycerides, < 75 mg/dL (those ages 0 to 9) and < 90 mg/dL (those ages 10 to 19.)25 For dyslipidemia, diagnostic algorithms and treatment recommendations may be found in the NHLBI guidelines.25
Next page: Transitioning to adult care >>
TRANSITIONING TO ADULT CARE
As adolescents transition into adulthood, changes in finances, insurance, living situation, occupation, and education produce a potential gap in care for those with T2DM. In the year prior to the transition to adult care, the clinician should set clear expectations for the patient’s responsibilities when he or she assumes self-care.26
In addition, the clinician should provide the transitioning patient and family with an up-to-date summary of the patient’s health status, medications, and dates and results of the most recent examinations and screenings. If the patient has been under the care of a pediatric clinician, referral to a trusted adult health care provider may be helpful.
Transition planning checklists, resources, and information forms that can be provided to the new health care team are available from the National Diabetes Education Program (a partner of the NIH and CDC) at http://ndep.nih.gov/transitions.
CONCLUSION
Primary care clinicians will likely see a growing number of pediatric patients with T2DM. Consultation with or referral to pediatric medical subspecialists, ongoing comanagement with experts in the evolving pediatric T2DM field, and strong partnerships with parents and caregivers, will ensure that optimal care of the pediatric patient’s lifetime health needs is initiated and maintained.
1. Fryar CD, Carroll MD, Ogden CL. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2009-2010. CDC. National Center for Health Statistics, Health E-Stat, September 2012.
2. Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62(15):1309-1319.
3. Dabelea D, Bell RA, D’Agostino Jr RB, et al; the SEARCH for Diabetes in Youth Writing Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716-2724.
4. Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
5. American Diabetes Association. Children and adolescents. Sec. 11. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S70-S76.
6. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diabetes Care. 2013;36:3863-3869.
7. CDC. National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. www.cdc.gov/diabetes/pubs/statsre port14/national-diabetes-report-web.pdf. Accessed February 17, 2015.
8. Pettitt DJ, Talton J, Dabelea D, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in US youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402-408.
9. Imperatore G, Boyle JP, Thompson TJ, et al; for the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged < 20 years through 2050. Diabetes Care. 2012;35:2515-2520.
10. Dabelea D, Mayer-Davis EJ, Saydah S, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786.
11. Lifshitz F (ed). Pediatric Endocrinology. Vol 1. 5th ed. New York; Informa Healthcare: 2006.
12. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773-795.
13. Flint A, Arslanian B. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(suppl 2):S177-S183.
14. American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23(3):381-389.
15. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S8-S16.
16. FDA. FDA allows marketing of first ZnT8Ab autoantibody test to help diagnose type 1 diabetes. Press release. August 20, 2014.
17. Klingensmith GJ, Pyle L, Arslanian S, et al; the TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype-results from the TODAY study. Diabetes Care. 2010;33(9):1-6.
18. Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28(1):186-212.
19. Academy of Nutrition and Dietetics. Pediatric weight management guideline (2007). www.andeal.org/topic.cfm?cat=2721. Accessed February 17, 2015.
20. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
21. American Association of Clinical Endocrinologists/American College of Endocrinology. Consensus statement by the AACE/ACE insulin pump management task force. Endocr Prac. 2014;20(5):463-489.
22. American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S77-S79.
23. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256.
24. FDA. Drug safety communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. www.fda.gov/downloads/Drugs/DrugSafety/UCM381108.pdf. Accessed February 17, 2015.
25. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(suppl 5):S213-S256.
26. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care. 2011;34(11):2477-2485.
CE/CME No: CR-1503
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between pediatric type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and definitively establish the diagnosis.
• Understand the link between pediatric obesity and T2DM and help the patient and family incorporate healthy eating and physical activity habits into their lifestyle.
• Describe the scope of treatment options for pediatric T2DM and the importance of monitoring glycemic control to ensure that treatment goals are met.
• Explain the long-term health risks associated with pediatric T2DM and how to screen for complications in order to initiate early treatment.
• Establish a health care team with the primary care clinician, T2DM specialists, and the patient’s family to create an individualized plan of care for your pediatric patient with T2DM.
FACULTY
Ashlyn Smith is an endocrinology PA at Endocrinology Associates in Scottsdale, Arizona. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2015.
Article begins on next page >>
The increasing prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents is a serious health threat that urgently requires effective lifestyle intervention in at-risk patients, vigilant disease management for those diagnosed, and further research to support treatment decisions in the pediatric population.
Over the past several decades, National Health and Nutrition Examination Survey (NHANES) data have documented a sharp rise in the prevalence of obesity in children and adolescents ages 2 to 19: from 5.5% during 1976-1980 to 16.9% in 2009-2010.1 Cardiovascular damage once seen only in adults is occurring in obese children, along with other obesity-related comorbidities such as dyslipidemia and insulin resistance.2 Similarly, type 2 diabetes mellitus (T2DM), once a disease of middle-aged or older adults, is being diagnosed with growing frequency in the pediatric population.3
While much is known about adult T2DM, less has been established about pediatric T2DM because of its relatively recent emergence. Areas requiring further study in the pediatric population include the determination of optimal target A1C levels; the most effective treatments for both T2DM and coexisting conditions; and the long-term impact on morbidity and mortality when T2DM is diagnosed so early in life. Increasing evidence suggests that T2DM in young people is an “aggressive” form of diabetes,4 with significant comorbidities that may already be present at diagnosis.5
In an effort to document long-term outcomes for patients diagnosed with “young-onset type 2 diabetes mellitus” (defined as T2DM diagnosed between ages 15 and 30), researchers reviewed records from the Royal Prince Alfred Hospital’s Diabetes Clinical Database, established in 1986, that were matched against the Australian National Death Index through June 2011. They identified 470 cases of type 1 diabetes mellitus (T1DM) and 354 cases of T2DM, with a median observation period of more than 20 years for patients in both groups, and compared morbidity and mortality outcomes.6 The authors found that unfavorable cardiovascular risk factors were more prevalent in the T2DM group and developed earlier in the disease process—in some cases, as early as two years after diagnosis—than in patients with T1DM. Diabetic complications (eg, albuminuria and neuropathy) were more prevalent in the T2DM group, but the rates of retinopathy were about the same in both groups.6
In terms of mortality, 11% of the patients with T2DM and 6.8% of those with T1DM had died, and the deaths in the T2DM group occurred after a significantly shorter duration of disease (26.9 v 36.5 y). Cardiovascular causes of death predominated in both groups but were more common in patients with T2DM (50.0%) than with T1DM (30.3%). The authors concluded that T2DM is “the more lethal phenotype” of diabetes in young people and requires intensive intervention directed at both glycemic control and cardiovascular risk management.6
EPIDEMIOLOGY
In 2012, an estimated 208,000 Americans younger than 20 were diagnosed with diabetes7; approximately 10.5% of them (21,000) were diagnosed with T2DM.8 While these numbers are a small fraction of the 29.1 million Americans living with diabetes,7 researchers note that both the incidence and prevalence of T2DM in young people are increasing.
For the years 2002-2003, the SEARCH for Diabetes in Youth Study Group estimated the annual incidence of new cases of diabetes in persons younger than 20 to be 15,000 for T1DM and 3,700 for T2DM.3 By 2008-2009, those estimates had grown to 18,436 per year for T1DM and 5,089 per year for T2DM.7
It has been projected that, if incidence rates remain constant, the number of young people diagnosed with T2DM in the United States will increase by 49% by 2050. If incidence grows by 2.3% annually, however, the increase could be fourfold by 2050.9
Prevalence has also increased. The number of persons age 19 or younger living with T1DM increased by 21.1% between 2001 and 2009; prevalence of T2DM in this age-group grew by 30.5% during the same period.10 T2DM prevalence also varies by race and ethnicity, ranging from 0.17/1,000 in non-Hispanic white youth to 1.20/1,000 among young people of Native American heritage (see Table, above).10
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The progression toward T2DM is influenced by both genetic and lifestyle risk factors that lead to insulin resistance and eventual pancreatic β-cell dysfunction. While aerobic exercise increases insulin sensitivity, a diet high in carbohydrates and fat increases the demand for insulin. So a sedentary lifestyle, combined with a high-carbohydrate, high-fat diet, gradually results in a state of insulin resistance. Compensatory hyperinsulinism will maintain normoglycemia for an indeterminate amount of time; but eventually, pancreatic “burnout” leads to pancreatic β-cell dysfunction and decreased insulin secretion. Relative insulin deficiency then causes decreased cellular glucose uptake, hyperglycemia, and ultimately, T2DM.11
Additional pathophysiologic deficiencies and malfunctions that contribute to hyperglycemia include increased glucagon secretion, decreased incretin effect, and increased renal glucose reabsorption.12
CLINICAL PRESENTATION
Clinical presentation of T2DM varies greatly among pediatric patients. Classic symptoms may include polydipsia and polyuria related to hyperglycemia. In addition, the patient or his/her family may note frequent infections or visual disturbances (eg, blurred or diminished vision). However, particularly in the pediatric population, the patient is often asymptomatic.13
On examination, the clinician may observe that the child or adolescent is overweight or obese. He or she may have mild to severe acanthosis nigricans on the posterior neck, axillae, abdomen, or thighs and over the antecubital fossa. However, acanthosis nigricans may not manifest in patients with fairer skin. In severe cases, patients with T2DM may present with hyperglycemic hyperosmolar nonketotic coma or diabetic ketoacidosis.14
Next page: Diagnosis >>
DIAGNOSIS
Identification of children at risk for T2DM is the first step in delaying or preventing pediatric T2DM and its complications. The American Diabetes Association’s Standards of Medical Care in Diabetes—2015 indicate that asymptomatic children and adolescents who are overweight (BMI above the 85th percentile for age and sex, weight for height above the 85th percentile, or weight greater than 120% of ideal for height) and who meet at least two of the following criteria should be tested for T2DM15:
• Family history of T2DM in first- or second-degree relative (74% to 100% have a first- or second-degree relative with diabetes14)
• Non-European ancestry (Native American, African-American, Latino, Asian American, Pacific Islander)
• Signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome [PCOS], or small-for-gestational-age birth weight)
• Maternal history of diabetes or gestational diabetes during pregnancy.
Screening should begin when the child is 10 or when puberty is attained, with retesting every three years thereafter.
Testing
The diagnosis of diabetes may be confirmed by any one of the following test results4,15
• A1C level ≥ 6.5%
• Fasting blood glucose (FBG) ≥ 126 mg/dL
• Two-hour oral glucose tolerance test (OGTT) level ≥ 200 mg/dL
• Random serum glucose ≥ 200 mg/dL with symptoms of hyperglycemia.
Type 1 or type 2?
In some newly diagnosed pediatric patients with diabetes, distinguishing T1DM from T2DM may be difficult; 25% of cases of T2DM are misclassified as T1DM.4,14 For example, an obese child who presents with ketosis appears to have features of both T1DM and T2DM.4 The distinction needs to be made as soon as possible because management of the diseases is quite different.
Measurement of C-peptide level (low in T1DM, normal or high in T2DM) is suggestive but not 100% reliable for this purpose. Similarly, ketosis is an unreliable indicator; adolescents with T2DM present with ketoacidosis in 5% to 25% of cases.4,14
Another means to differentiate T1DM and T2DM is determining the levels of diabetes autoantibodies (DAA) to insulin. Pediatric patients with suspected DM can be screened for the islet cell autoantibodies GAD (glutamic acid decarboxylase)-65 and IA (insulin antibody)-2. In addition, a new DAA assay that tests for the zinc transporter 8 autoantibody [ZnT8Ab] was recently approved by the FDA for marketing.16 Detection of these antibodies is generally—but not always—an indicator of T1DM, an autoimmune disease of the pancreas.5
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group found that, of 1,206 obese (BMI in the 85th percentile or higher) participants with T2DM, 9.8% tested positive for either or both of the GAD-65 and IA-2 antibodies. It was unclear if the DAA-positive patients had both T1DM and T2DM or T1DM with insulin resistance due to obesity. They concluded that testing for islet cell autoantibodies was needed to reliably identify patients with autoimmune diabetes.17
In addition to establishing the correct diagnosis, the clinician should order tests to rule out a concomitant thyroid disorder and to assess the patient for diabetic complications. Testing should include a thyroid panel, lipid panel, urine microalbumin level, and renal and hepatic function tests.18
Continue for treatment of T2DM >>
TREATMENT OF T2DM
Because relevant clinical data are limited, blood glucose (BG) goals should be individualized for each child with T2DM. As a rule of thumb, guidelines for BG goals in pediatric T1DM can serve as a reference for establishing BG goals in T2DM. Once adulthood is reached, BG goals reflect adult ranges.
FBG and A1C goals in pediatric T1DM are
• Younger than 4 years: 80 to 200 mg/dL and < 8.5%
• Between 5 and 11: 70 to 180 mg/dL and < 8.0%
• 12 and older: 70 to 150 mg/dL and < 7.5%.18
Primary care clinicians who manage pediatric patients with T2DM should consult with diabetes specialists at diagnosis and at least annually thereafter. Consultation is encouraged when treatment goals are not met.4
Lifestyle changes
The cornerstone of pediatric T2DM treatment is effective lifestyle intervention aimed at achievement of the patient’s BG goals and reduction of risks for microvascular and macrovascular complications.
Dietary modification methods can vary, depending on the caregivers’ and patient’s previous knowledge of nutrition. For families with little nutritional knowledge, the clinician may recommend the “plate method,” with half a plate designated for vegetables, one quarter for lean meats and protein, and one quarter for carbohydrates. As an alternative, the clinician may advise simple portion control, including smaller plate sizes and only one serving per meal. In addition, all sugared beverages should be avoided and daily milk intake should be limited.19
Behaviors surrounding meal preparation and snacking may also contribute to excessive caloric intake. Each day’s meals should be planned, and eating while at the computer, doing homework, or watching TV should be avoided.4
As time and the family’s comfort level permit, intensification of nutritional therapy can improve glycemic control through specific instruction regarding calorie counting and selection of low-fat and low-carbohydrate foods. Calorie intake of 900 to 1,200 kcal/d for children ages 6 to 12 and a minimum of 1,200 kcal/d for adolescents ages 13 to 18 are recommended for weight loss and improved body composition (see "Resources for Weight Management and Nutrition").4
Physical activity in the pediatric population can be derived from multiple sources, including physical education classes, after-school programs, sports and dance programs, and walks with the family or the dog. When discussing exercise recommendations, it is essential to consider any limitations imposed by the family’s finances and family members’ schedules.
Cardiovascular activity is recommended for 30 to 60 min or more every day or most days.4,14 To decrease excessive pursuit of sedentary activities, parents are urged to limit children’s nonacademic “screen time” to a maximum of two hours per day and to discourage the placement of video screens and television sets in children’s bedrooms.4
While lifestyle intervention remains the first-line treatment choice for pediatric T2DM, expert consensus is that less than 10% of pediatric patients reach glycemic control goals with lifestyle modifications alone.4
Next page: Treatment with metformin >>
First-line medication: Metformin
If treatment goals are not met with lifestyle changes, treatment with metformin should be initiated.4,14 Metformin is currently the only FDA-approved oral agent for the treatment of T2DM in children, although it is not recommended for use in those younger than 10.20 Metformin improves glycemic control by increasing insulin action, decreasing gluconeogenesis, and decreasing glucagon secretion.11 Metformin may also regulate ovulation in patients with PCOS, increasing the patient’s fertility. Adolescent female patients being treated with metformin should avoid pregnancy because its use is not recommended during pregnancy.14,20
Because metformin carries a black box warning for a rare but life-threatening complication—lactic acidosis—the clinician should confirm normal renal function before treatment initiation. Renal function should be monitored regularly (at least annually during metformin treatment and more often if impaired renal function is anticipated) and metformin discontinued if impaired renal function is present. Risk is also reduced by use of the lowest effective dose.20
To minimize common gastrointestinal adverse effects, metformin should be taken with meals. Treatment should be initiated at a dose of 500 mg/d,4 with slow titration upward by 500 mg/wk, as needed and tolerated, until an effective maintenance dose is reached. Maintenance doses may range from 500 mg/d to 2,000 mg/d (taken in divided doses).20
Metformin use can result in moderate decreases in BG and A1C levels. In addition, because metformin does not stimulate insulin secretion, the risk for hypoglycemia is minimal.20 The clinician may consider monitoring vitamin B12 levels, particularly in a population already at risk for peripheral neuropathy, because metformin increases risk for vitamin B12 deficiency.20
As T2DM progresses, metformin alone may be insufficient for maintenance of glycemic control.
Insulin therapy
Treatment with insulin therapy, rather than metformin, should be initiated in pediatric diabetes patients if the diagnosis of T2DM is not confirmed or for those patients who present with ketosis or ketoacidosis.4
Insulin therapy should also be initiated for patients with confirmed T2DM if random tests for plasma BG levels are 250 mg/dL or higher or A1C levels are more than 9.0%.4 Reflecting the lack of consensus on pediatric T2DM treatment, the ADA recommends insulin therapy when the A1C level is greater than 8.5%.13 Insulin may be used in conjunction with metformin, which may decrease the insulin dosage that would otherwise be needed due to metformin’s ability to increase insulin sensitivity.4
Insulin regimens
Selection of the appropriate insulin regimen depends on clinician judgment, patient and family comfort level, and lifestyle considerations. Initial barriers for the patient and family may include resistance to injecting medication, difficulty understanding instructions for insulin therapy, and fear of weight gain or hypoglycemia. Close monitoring for significant day-to-day fluctuations in the pediatric patient’s activity levels or diet is essential in order to adjust insulin dosage as needed and prevent hyperglycemia or hypoglycemia.
The types of insulin therapy include
Basal. Basal insulin at bedtime with insulin detemir or glargine is a straightforward method of insulin delivery, with lower risk for hypoglycemia. The ADA recommends initial dosing at 0.3 to 0.4 U/kg/d for the pediatric population, titrating slowly until FBG is at goal without hypoglycemia.13
Basal-bolus. If postprandial BG or A1C levels remain elevated, basal insulin provides the groundwork for intensification to a physiologic basal-bolus regimen with addition of insulin lispro, aspart, or glulisine. Bolus insulin therapy can be in the form of a fixed mealtime dose or as an insulin-to-carbohydrate ratio, as caretaker comfort and patient lifestyle permit.11
In patients who are not able to adhere to a basal-bolus regimen due to multiple daily injections, consider conservative treatment with twice-daily insulin NPH as basal therapy, with or without short-acting insulin at breakfast and dinner.11
An alternative for adults that is occasionally used in youth (although not yet FDA-approved for this use) is twice-daily premixed insulin aspart 70/30 or lispro 75/25. While this regimen can improve compliance, it does not permit mealtime adjustments in insulin dosing and requires a snack between meals to prevent hypoglycemia.11
Families will sometimes choose insulin pump therapy for a pediatric patient with T2DM.11 For these patients, use of the insulin pump requires a motivated family committed to BG monitoring at least four times a day and in possession of a good working knowledge of basic diabetes management.21
Continue for additional treatment options >>
Additional treatment options
While metformin and insulin are the only FDA-approved medications for the treatment of T2DM in the pediatric population, additional medications may be used if needed because of deteriorating glycemic control.4 If treated with noninsulin agents (including metformin), adolescent female patients should be counseled to avoid pregnancy because long-term data are lacking about the safety to the fetus of noninsulin agents.22
Metformin-rosiglitazone. In the TODAY trial,23 699 participants (ages 10 to 17) with T2DM were randomly assigned one of three treatment options: metformin alone, metformin plus rosiglitazone, and metformin with lifestyle intervention. The study found that monotherapy with metformin was often insufficient to achieve glycemic control, with a rate of treatment failure of 51.8%. Rates of failure for the other groups were 38.6% and 46.6%, respectively.
While the combination of metformin and rosiglitazone was found to be most effective, at the time these results were published, access to rosiglitazone was restricted by the FDA because of concerns about the drug’s cardiovascular safety. The FDA has since determined that rosiglitazone’s cardiovascular risks are comparable to those of other diabetes drugs and directed in November 2013 that the restrictions be removed.24 In this context, the TODAY study researchers concluded that it was unclear if the results were specific to rosiglitazone, to the thiazolidinedione drug class as a whole, or to some attribute of combination therapy; they suggested, however, that combination therapy may be superior for pediatric T2DM treatment.23
Incretin-based therapies. Another potential therapeutic option is the use of incretin-based therapies, such as the glucagon-like peptide (GLP-1) agonists exenatide or liraglutide. These have been shown to be effective at lowering BG levels in the adult population and have the added benefit of causing weight loss.
Although not approved for pediatric use, GLP-1 agonists would provide a means of therapy intensification without weight gain, which can worsen insulin resistance and propel the disease process forward. Further research is needed, and there is an ongoing multicenter safety and efficacy trial of exenatide use in adolescents with T2DM.13
Self-monitoring of blood glucoseInitiation and frequency of self-monitoring of BG (SMBG) in pediatric T2DM patients depends on the treatment regimen and achievement of BG goals.
The American Academy of Pediatrics recommends initiation of SMBG when the patient is 1) taking insulin or medications with risk for hypoglycemia; 2) initiating or changing a treatment regimen; 3) not meeting treatment goals; or 4) experiencing an intercurrent illness.4 In addition, a patient with hyperglycemic or hypoglycemic symptoms should perform SMBG at least for the duration of the symptoms.
For all patients newly diagnosed with T2DM, SMBG is recommended before meals (including morning fasting) and at bedtime until target BG levels are achieved.4 For patients whose disease is well controlled with metformin, SMBG can be performed on an infrequent or intermittent basis.
In most cases, the clinician should advise the patient to perform morning and bedtime SMBG to assess fasting and postprandial glucose levels. Postprandial SMBG will allow both caregivers and clinicians to assess for glycemic excursions, especially in cases in which A1C is above goal, despite an FBG level that is at goal.4 Premeal SMBG may also be temporarily utilized during acute illness and to monitor for glycemic excursions.
Patients receiving multiple daily injections or insulin pump therapy require BG testing prior to every meal and often postprandial or bedtime SMBG as well.
Therapy intensification
At any point during treatment, if glycemic goals are not being met, the clinician should analyze the patient’s current medication(s), lifestyle interventions, and treatment adherence for opportunities to optimize treatment effectiveness. Therapy intensification may require more frequent office visits and SMBG, augmenting or adding medication, and referral to a nutritionist or diabetes educator. The need for treatment individualization and enhancement is likely to arise frequently, and it is often beneficial to set this expectation early in the T2DM management process with the pediatric patient and his/her family.4
SCREENING FOR AND MANAGEMENT OF COMPLICATIONS
Due in part to the prolonged duration of illness, patients diagnosed with T2DM as children or adolescents are at higher risk than adults for microvascular and macrovascular complications. Therefore, regular screening for retinopathy, nephropathy, hypertension, and dyslipidemia is essential, as follows:
• A dilated eye exam for diabetic retinopathy is recommended at diagnosis, annually, and more frequently as needed, depending on findings.13
• Annual screening for microalbuminuria will assess for early renal impairment and allow treatment to prevent diabetic nephropathy. As in adults, pediatric cases of microalbuminuria are treated with ACE inhibitors or angiotensin receptor blockers (ARBs). ACE inhibitors are dosed at 0.05-0.15 mg/kg/d in pediatric patients.11 Adolescent female patients should avoid pregnancy if taking an ACE inhibitor or ARB because of risks to the fetus from these Category D medications.13
• In children and adolescents, the diagnosis of hypertension is made using age-, height-, and gender-adjusted percentiles. The 2011 National Heart, Lung and Blood Institute’s (NHLBI’s) integrated guidelines for cardiovascular health and risk reduction in children and adolescents include updated blood pressure tables and diagnostic algorithms for this purpose.25 Pediatric hypertension is treated with ACE inhibitors and ARBs, and dosing recommendations are also available in the NHLBI guidelines.25
• Acceptable lipid levels for children and adolescents are as follows: LDL, < 110 mg/dL; HDL, > 45 mg/dL; and triglycerides, < 75 mg/dL (those ages 0 to 9) and < 90 mg/dL (those ages 10 to 19.)25 For dyslipidemia, diagnostic algorithms and treatment recommendations may be found in the NHLBI guidelines.25
Next page: Transitioning to adult care >>
TRANSITIONING TO ADULT CARE
As adolescents transition into adulthood, changes in finances, insurance, living situation, occupation, and education produce a potential gap in care for those with T2DM. In the year prior to the transition to adult care, the clinician should set clear expectations for the patient’s responsibilities when he or she assumes self-care.26
In addition, the clinician should provide the transitioning patient and family with an up-to-date summary of the patient’s health status, medications, and dates and results of the most recent examinations and screenings. If the patient has been under the care of a pediatric clinician, referral to a trusted adult health care provider may be helpful.
Transition planning checklists, resources, and information forms that can be provided to the new health care team are available from the National Diabetes Education Program (a partner of the NIH and CDC) at http://ndep.nih.gov/transitions.
CONCLUSION
Primary care clinicians will likely see a growing number of pediatric patients with T2DM. Consultation with or referral to pediatric medical subspecialists, ongoing comanagement with experts in the evolving pediatric T2DM field, and strong partnerships with parents and caregivers, will ensure that optimal care of the pediatric patient’s lifetime health needs is initiated and maintained.
CE/CME No: CR-1503
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Differentiate between pediatric type 1 diabetes mellitus (T1DM) and type 2 DM (T2DM) and definitively establish the diagnosis.
• Understand the link between pediatric obesity and T2DM and help the patient and family incorporate healthy eating and physical activity habits into their lifestyle.
• Describe the scope of treatment options for pediatric T2DM and the importance of monitoring glycemic control to ensure that treatment goals are met.
• Explain the long-term health risks associated with pediatric T2DM and how to screen for complications in order to initiate early treatment.
• Establish a health care team with the primary care clinician, T2DM specialists, and the patient’s family to create an individualized plan of care for your pediatric patient with T2DM.
FACULTY
Ashlyn Smith is an endocrinology PA at Endocrinology Associates in Scottsdale, Arizona. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2015.
Article begins on next page >>
The increasing prevalence of type 2 diabetes mellitus (T2DM) in children and adolescents is a serious health threat that urgently requires effective lifestyle intervention in at-risk patients, vigilant disease management for those diagnosed, and further research to support treatment decisions in the pediatric population.
Over the past several decades, National Health and Nutrition Examination Survey (NHANES) data have documented a sharp rise in the prevalence of obesity in children and adolescents ages 2 to 19: from 5.5% during 1976-1980 to 16.9% in 2009-2010.1 Cardiovascular damage once seen only in adults is occurring in obese children, along with other obesity-related comorbidities such as dyslipidemia and insulin resistance.2 Similarly, type 2 diabetes mellitus (T2DM), once a disease of middle-aged or older adults, is being diagnosed with growing frequency in the pediatric population.3
While much is known about adult T2DM, less has been established about pediatric T2DM because of its relatively recent emergence. Areas requiring further study in the pediatric population include the determination of optimal target A1C levels; the most effective treatments for both T2DM and coexisting conditions; and the long-term impact on morbidity and mortality when T2DM is diagnosed so early in life. Increasing evidence suggests that T2DM in young people is an “aggressive” form of diabetes,4 with significant comorbidities that may already be present at diagnosis.5
In an effort to document long-term outcomes for patients diagnosed with “young-onset type 2 diabetes mellitus” (defined as T2DM diagnosed between ages 15 and 30), researchers reviewed records from the Royal Prince Alfred Hospital’s Diabetes Clinical Database, established in 1986, that were matched against the Australian National Death Index through June 2011. They identified 470 cases of type 1 diabetes mellitus (T1DM) and 354 cases of T2DM, with a median observation period of more than 20 years for patients in both groups, and compared morbidity and mortality outcomes.6 The authors found that unfavorable cardiovascular risk factors were more prevalent in the T2DM group and developed earlier in the disease process—in some cases, as early as two years after diagnosis—than in patients with T1DM. Diabetic complications (eg, albuminuria and neuropathy) were more prevalent in the T2DM group, but the rates of retinopathy were about the same in both groups.6
In terms of mortality, 11% of the patients with T2DM and 6.8% of those with T1DM had died, and the deaths in the T2DM group occurred after a significantly shorter duration of disease (26.9 v 36.5 y). Cardiovascular causes of death predominated in both groups but were more common in patients with T2DM (50.0%) than with T1DM (30.3%). The authors concluded that T2DM is “the more lethal phenotype” of diabetes in young people and requires intensive intervention directed at both glycemic control and cardiovascular risk management.6
EPIDEMIOLOGY
In 2012, an estimated 208,000 Americans younger than 20 were diagnosed with diabetes7; approximately 10.5% of them (21,000) were diagnosed with T2DM.8 While these numbers are a small fraction of the 29.1 million Americans living with diabetes,7 researchers note that both the incidence and prevalence of T2DM in young people are increasing.
For the years 2002-2003, the SEARCH for Diabetes in Youth Study Group estimated the annual incidence of new cases of diabetes in persons younger than 20 to be 15,000 for T1DM and 3,700 for T2DM.3 By 2008-2009, those estimates had grown to 18,436 per year for T1DM and 5,089 per year for T2DM.7
It has been projected that, if incidence rates remain constant, the number of young people diagnosed with T2DM in the United States will increase by 49% by 2050. If incidence grows by 2.3% annually, however, the increase could be fourfold by 2050.9
Prevalence has also increased. The number of persons age 19 or younger living with T1DM increased by 21.1% between 2001 and 2009; prevalence of T2DM in this age-group grew by 30.5% during the same period.10 T2DM prevalence also varies by race and ethnicity, ranging from 0.17/1,000 in non-Hispanic white youth to 1.20/1,000 among young people of Native American heritage (see Table, above).10
Continue for pathophysiology >>
PATHOPHYSIOLOGY
The progression toward T2DM is influenced by both genetic and lifestyle risk factors that lead to insulin resistance and eventual pancreatic β-cell dysfunction. While aerobic exercise increases insulin sensitivity, a diet high in carbohydrates and fat increases the demand for insulin. So a sedentary lifestyle, combined with a high-carbohydrate, high-fat diet, gradually results in a state of insulin resistance. Compensatory hyperinsulinism will maintain normoglycemia for an indeterminate amount of time; but eventually, pancreatic “burnout” leads to pancreatic β-cell dysfunction and decreased insulin secretion. Relative insulin deficiency then causes decreased cellular glucose uptake, hyperglycemia, and ultimately, T2DM.11
Additional pathophysiologic deficiencies and malfunctions that contribute to hyperglycemia include increased glucagon secretion, decreased incretin effect, and increased renal glucose reabsorption.12
CLINICAL PRESENTATION
Clinical presentation of T2DM varies greatly among pediatric patients. Classic symptoms may include polydipsia and polyuria related to hyperglycemia. In addition, the patient or his/her family may note frequent infections or visual disturbances (eg, blurred or diminished vision). However, particularly in the pediatric population, the patient is often asymptomatic.13
On examination, the clinician may observe that the child or adolescent is overweight or obese. He or she may have mild to severe acanthosis nigricans on the posterior neck, axillae, abdomen, or thighs and over the antecubital fossa. However, acanthosis nigricans may not manifest in patients with fairer skin. In severe cases, patients with T2DM may present with hyperglycemic hyperosmolar nonketotic coma or diabetic ketoacidosis.14
Next page: Diagnosis >>
DIAGNOSIS
Identification of children at risk for T2DM is the first step in delaying or preventing pediatric T2DM and its complications. The American Diabetes Association’s Standards of Medical Care in Diabetes—2015 indicate that asymptomatic children and adolescents who are overweight (BMI above the 85th percentile for age and sex, weight for height above the 85th percentile, or weight greater than 120% of ideal for height) and who meet at least two of the following criteria should be tested for T2DM15:
• Family history of T2DM in first- or second-degree relative (74% to 100% have a first- or second-degree relative with diabetes14)
• Non-European ancestry (Native American, African-American, Latino, Asian American, Pacific Islander)
• Signs of insulin resistance or associated conditions (acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome [PCOS], or small-for-gestational-age birth weight)
• Maternal history of diabetes or gestational diabetes during pregnancy.
Screening should begin when the child is 10 or when puberty is attained, with retesting every three years thereafter.
Testing
The diagnosis of diabetes may be confirmed by any one of the following test results4,15
• A1C level ≥ 6.5%
• Fasting blood glucose (FBG) ≥ 126 mg/dL
• Two-hour oral glucose tolerance test (OGTT) level ≥ 200 mg/dL
• Random serum glucose ≥ 200 mg/dL with symptoms of hyperglycemia.
Type 1 or type 2?
In some newly diagnosed pediatric patients with diabetes, distinguishing T1DM from T2DM may be difficult; 25% of cases of T2DM are misclassified as T1DM.4,14 For example, an obese child who presents with ketosis appears to have features of both T1DM and T2DM.4 The distinction needs to be made as soon as possible because management of the diseases is quite different.
Measurement of C-peptide level (low in T1DM, normal or high in T2DM) is suggestive but not 100% reliable for this purpose. Similarly, ketosis is an unreliable indicator; adolescents with T2DM present with ketoacidosis in 5% to 25% of cases.4,14
Another means to differentiate T1DM and T2DM is determining the levels of diabetes autoantibodies (DAA) to insulin. Pediatric patients with suspected DM can be screened for the islet cell autoantibodies GAD (glutamic acid decarboxylase)-65 and IA (insulin antibody)-2. In addition, a new DAA assay that tests for the zinc transporter 8 autoantibody [ZnT8Ab] was recently approved by the FDA for marketing.16 Detection of these antibodies is generally—but not always—an indicator of T1DM, an autoimmune disease of the pancreas.5
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study Group found that, of 1,206 obese (BMI in the 85th percentile or higher) participants with T2DM, 9.8% tested positive for either or both of the GAD-65 and IA-2 antibodies. It was unclear if the DAA-positive patients had both T1DM and T2DM or T1DM with insulin resistance due to obesity. They concluded that testing for islet cell autoantibodies was needed to reliably identify patients with autoimmune diabetes.17
In addition to establishing the correct diagnosis, the clinician should order tests to rule out a concomitant thyroid disorder and to assess the patient for diabetic complications. Testing should include a thyroid panel, lipid panel, urine microalbumin level, and renal and hepatic function tests.18
Continue for treatment of T2DM >>
TREATMENT OF T2DM
Because relevant clinical data are limited, blood glucose (BG) goals should be individualized for each child with T2DM. As a rule of thumb, guidelines for BG goals in pediatric T1DM can serve as a reference for establishing BG goals in T2DM. Once adulthood is reached, BG goals reflect adult ranges.
FBG and A1C goals in pediatric T1DM are
• Younger than 4 years: 80 to 200 mg/dL and < 8.5%
• Between 5 and 11: 70 to 180 mg/dL and < 8.0%
• 12 and older: 70 to 150 mg/dL and < 7.5%.18
Primary care clinicians who manage pediatric patients with T2DM should consult with diabetes specialists at diagnosis and at least annually thereafter. Consultation is encouraged when treatment goals are not met.4
Lifestyle changes
The cornerstone of pediatric T2DM treatment is effective lifestyle intervention aimed at achievement of the patient’s BG goals and reduction of risks for microvascular and macrovascular complications.
Dietary modification methods can vary, depending on the caregivers’ and patient’s previous knowledge of nutrition. For families with little nutritional knowledge, the clinician may recommend the “plate method,” with half a plate designated for vegetables, one quarter for lean meats and protein, and one quarter for carbohydrates. As an alternative, the clinician may advise simple portion control, including smaller plate sizes and only one serving per meal. In addition, all sugared beverages should be avoided and daily milk intake should be limited.19
Behaviors surrounding meal preparation and snacking may also contribute to excessive caloric intake. Each day’s meals should be planned, and eating while at the computer, doing homework, or watching TV should be avoided.4
As time and the family’s comfort level permit, intensification of nutritional therapy can improve glycemic control through specific instruction regarding calorie counting and selection of low-fat and low-carbohydrate foods. Calorie intake of 900 to 1,200 kcal/d for children ages 6 to 12 and a minimum of 1,200 kcal/d for adolescents ages 13 to 18 are recommended for weight loss and improved body composition (see "Resources for Weight Management and Nutrition").4
Physical activity in the pediatric population can be derived from multiple sources, including physical education classes, after-school programs, sports and dance programs, and walks with the family or the dog. When discussing exercise recommendations, it is essential to consider any limitations imposed by the family’s finances and family members’ schedules.
Cardiovascular activity is recommended for 30 to 60 min or more every day or most days.4,14 To decrease excessive pursuit of sedentary activities, parents are urged to limit children’s nonacademic “screen time” to a maximum of two hours per day and to discourage the placement of video screens and television sets in children’s bedrooms.4
While lifestyle intervention remains the first-line treatment choice for pediatric T2DM, expert consensus is that less than 10% of pediatric patients reach glycemic control goals with lifestyle modifications alone.4
Next page: Treatment with metformin >>
First-line medication: Metformin
If treatment goals are not met with lifestyle changes, treatment with metformin should be initiated.4,14 Metformin is currently the only FDA-approved oral agent for the treatment of T2DM in children, although it is not recommended for use in those younger than 10.20 Metformin improves glycemic control by increasing insulin action, decreasing gluconeogenesis, and decreasing glucagon secretion.11 Metformin may also regulate ovulation in patients with PCOS, increasing the patient’s fertility. Adolescent female patients being treated with metformin should avoid pregnancy because its use is not recommended during pregnancy.14,20
Because metformin carries a black box warning for a rare but life-threatening complication—lactic acidosis—the clinician should confirm normal renal function before treatment initiation. Renal function should be monitored regularly (at least annually during metformin treatment and more often if impaired renal function is anticipated) and metformin discontinued if impaired renal function is present. Risk is also reduced by use of the lowest effective dose.20
To minimize common gastrointestinal adverse effects, metformin should be taken with meals. Treatment should be initiated at a dose of 500 mg/d,4 with slow titration upward by 500 mg/wk, as needed and tolerated, until an effective maintenance dose is reached. Maintenance doses may range from 500 mg/d to 2,000 mg/d (taken in divided doses).20
Metformin use can result in moderate decreases in BG and A1C levels. In addition, because metformin does not stimulate insulin secretion, the risk for hypoglycemia is minimal.20 The clinician may consider monitoring vitamin B12 levels, particularly in a population already at risk for peripheral neuropathy, because metformin increases risk for vitamin B12 deficiency.20
As T2DM progresses, metformin alone may be insufficient for maintenance of glycemic control.
Insulin therapy
Treatment with insulin therapy, rather than metformin, should be initiated in pediatric diabetes patients if the diagnosis of T2DM is not confirmed or for those patients who present with ketosis or ketoacidosis.4
Insulin therapy should also be initiated for patients with confirmed T2DM if random tests for plasma BG levels are 250 mg/dL or higher or A1C levels are more than 9.0%.4 Reflecting the lack of consensus on pediatric T2DM treatment, the ADA recommends insulin therapy when the A1C level is greater than 8.5%.13 Insulin may be used in conjunction with metformin, which may decrease the insulin dosage that would otherwise be needed due to metformin’s ability to increase insulin sensitivity.4
Insulin regimens
Selection of the appropriate insulin regimen depends on clinician judgment, patient and family comfort level, and lifestyle considerations. Initial barriers for the patient and family may include resistance to injecting medication, difficulty understanding instructions for insulin therapy, and fear of weight gain or hypoglycemia. Close monitoring for significant day-to-day fluctuations in the pediatric patient’s activity levels or diet is essential in order to adjust insulin dosage as needed and prevent hyperglycemia or hypoglycemia.
The types of insulin therapy include
Basal. Basal insulin at bedtime with insulin detemir or glargine is a straightforward method of insulin delivery, with lower risk for hypoglycemia. The ADA recommends initial dosing at 0.3 to 0.4 U/kg/d for the pediatric population, titrating slowly until FBG is at goal without hypoglycemia.13
Basal-bolus. If postprandial BG or A1C levels remain elevated, basal insulin provides the groundwork for intensification to a physiologic basal-bolus regimen with addition of insulin lispro, aspart, or glulisine. Bolus insulin therapy can be in the form of a fixed mealtime dose or as an insulin-to-carbohydrate ratio, as caretaker comfort and patient lifestyle permit.11
In patients who are not able to adhere to a basal-bolus regimen due to multiple daily injections, consider conservative treatment with twice-daily insulin NPH as basal therapy, with or without short-acting insulin at breakfast and dinner.11
An alternative for adults that is occasionally used in youth (although not yet FDA-approved for this use) is twice-daily premixed insulin aspart 70/30 or lispro 75/25. While this regimen can improve compliance, it does not permit mealtime adjustments in insulin dosing and requires a snack between meals to prevent hypoglycemia.11
Families will sometimes choose insulin pump therapy for a pediatric patient with T2DM.11 For these patients, use of the insulin pump requires a motivated family committed to BG monitoring at least four times a day and in possession of a good working knowledge of basic diabetes management.21
Continue for additional treatment options >>
Additional treatment options
While metformin and insulin are the only FDA-approved medications for the treatment of T2DM in the pediatric population, additional medications may be used if needed because of deteriorating glycemic control.4 If treated with noninsulin agents (including metformin), adolescent female patients should be counseled to avoid pregnancy because long-term data are lacking about the safety to the fetus of noninsulin agents.22
Metformin-rosiglitazone. In the TODAY trial,23 699 participants (ages 10 to 17) with T2DM were randomly assigned one of three treatment options: metformin alone, metformin plus rosiglitazone, and metformin with lifestyle intervention. The study found that monotherapy with metformin was often insufficient to achieve glycemic control, with a rate of treatment failure of 51.8%. Rates of failure for the other groups were 38.6% and 46.6%, respectively.
While the combination of metformin and rosiglitazone was found to be most effective, at the time these results were published, access to rosiglitazone was restricted by the FDA because of concerns about the drug’s cardiovascular safety. The FDA has since determined that rosiglitazone’s cardiovascular risks are comparable to those of other diabetes drugs and directed in November 2013 that the restrictions be removed.24 In this context, the TODAY study researchers concluded that it was unclear if the results were specific to rosiglitazone, to the thiazolidinedione drug class as a whole, or to some attribute of combination therapy; they suggested, however, that combination therapy may be superior for pediatric T2DM treatment.23
Incretin-based therapies. Another potential therapeutic option is the use of incretin-based therapies, such as the glucagon-like peptide (GLP-1) agonists exenatide or liraglutide. These have been shown to be effective at lowering BG levels in the adult population and have the added benefit of causing weight loss.
Although not approved for pediatric use, GLP-1 agonists would provide a means of therapy intensification without weight gain, which can worsen insulin resistance and propel the disease process forward. Further research is needed, and there is an ongoing multicenter safety and efficacy trial of exenatide use in adolescents with T2DM.13
Self-monitoring of blood glucoseInitiation and frequency of self-monitoring of BG (SMBG) in pediatric T2DM patients depends on the treatment regimen and achievement of BG goals.
The American Academy of Pediatrics recommends initiation of SMBG when the patient is 1) taking insulin or medications with risk for hypoglycemia; 2) initiating or changing a treatment regimen; 3) not meeting treatment goals; or 4) experiencing an intercurrent illness.4 In addition, a patient with hyperglycemic or hypoglycemic symptoms should perform SMBG at least for the duration of the symptoms.
For all patients newly diagnosed with T2DM, SMBG is recommended before meals (including morning fasting) and at bedtime until target BG levels are achieved.4 For patients whose disease is well controlled with metformin, SMBG can be performed on an infrequent or intermittent basis.
In most cases, the clinician should advise the patient to perform morning and bedtime SMBG to assess fasting and postprandial glucose levels. Postprandial SMBG will allow both caregivers and clinicians to assess for glycemic excursions, especially in cases in which A1C is above goal, despite an FBG level that is at goal.4 Premeal SMBG may also be temporarily utilized during acute illness and to monitor for glycemic excursions.
Patients receiving multiple daily injections or insulin pump therapy require BG testing prior to every meal and often postprandial or bedtime SMBG as well.
Therapy intensification
At any point during treatment, if glycemic goals are not being met, the clinician should analyze the patient’s current medication(s), lifestyle interventions, and treatment adherence for opportunities to optimize treatment effectiveness. Therapy intensification may require more frequent office visits and SMBG, augmenting or adding medication, and referral to a nutritionist or diabetes educator. The need for treatment individualization and enhancement is likely to arise frequently, and it is often beneficial to set this expectation early in the T2DM management process with the pediatric patient and his/her family.4
SCREENING FOR AND MANAGEMENT OF COMPLICATIONS
Due in part to the prolonged duration of illness, patients diagnosed with T2DM as children or adolescents are at higher risk than adults for microvascular and macrovascular complications. Therefore, regular screening for retinopathy, nephropathy, hypertension, and dyslipidemia is essential, as follows:
• A dilated eye exam for diabetic retinopathy is recommended at diagnosis, annually, and more frequently as needed, depending on findings.13
• Annual screening for microalbuminuria will assess for early renal impairment and allow treatment to prevent diabetic nephropathy. As in adults, pediatric cases of microalbuminuria are treated with ACE inhibitors or angiotensin receptor blockers (ARBs). ACE inhibitors are dosed at 0.05-0.15 mg/kg/d in pediatric patients.11 Adolescent female patients should avoid pregnancy if taking an ACE inhibitor or ARB because of risks to the fetus from these Category D medications.13
• In children and adolescents, the diagnosis of hypertension is made using age-, height-, and gender-adjusted percentiles. The 2011 National Heart, Lung and Blood Institute’s (NHLBI’s) integrated guidelines for cardiovascular health and risk reduction in children and adolescents include updated blood pressure tables and diagnostic algorithms for this purpose.25 Pediatric hypertension is treated with ACE inhibitors and ARBs, and dosing recommendations are also available in the NHLBI guidelines.25
• Acceptable lipid levels for children and adolescents are as follows: LDL, < 110 mg/dL; HDL, > 45 mg/dL; and triglycerides, < 75 mg/dL (those ages 0 to 9) and < 90 mg/dL (those ages 10 to 19.)25 For dyslipidemia, diagnostic algorithms and treatment recommendations may be found in the NHLBI guidelines.25
Next page: Transitioning to adult care >>
TRANSITIONING TO ADULT CARE
As adolescents transition into adulthood, changes in finances, insurance, living situation, occupation, and education produce a potential gap in care for those with T2DM. In the year prior to the transition to adult care, the clinician should set clear expectations for the patient’s responsibilities when he or she assumes self-care.26
In addition, the clinician should provide the transitioning patient and family with an up-to-date summary of the patient’s health status, medications, and dates and results of the most recent examinations and screenings. If the patient has been under the care of a pediatric clinician, referral to a trusted adult health care provider may be helpful.
Transition planning checklists, resources, and information forms that can be provided to the new health care team are available from the National Diabetes Education Program (a partner of the NIH and CDC) at http://ndep.nih.gov/transitions.
CONCLUSION
Primary care clinicians will likely see a growing number of pediatric patients with T2DM. Consultation with or referral to pediatric medical subspecialists, ongoing comanagement with experts in the evolving pediatric T2DM field, and strong partnerships with parents and caregivers, will ensure that optimal care of the pediatric patient’s lifetime health needs is initiated and maintained.
1. Fryar CD, Carroll MD, Ogden CL. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2009-2010. CDC. National Center for Health Statistics, Health E-Stat, September 2012.
2. Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62(15):1309-1319.
3. Dabelea D, Bell RA, D’Agostino Jr RB, et al; the SEARCH for Diabetes in Youth Writing Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716-2724.
4. Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
5. American Diabetes Association. Children and adolescents. Sec. 11. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S70-S76.
6. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diabetes Care. 2013;36:3863-3869.
7. CDC. National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. www.cdc.gov/diabetes/pubs/statsre port14/national-diabetes-report-web.pdf. Accessed February 17, 2015.
8. Pettitt DJ, Talton J, Dabelea D, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in US youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402-408.
9. Imperatore G, Boyle JP, Thompson TJ, et al; for the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged < 20 years through 2050. Diabetes Care. 2012;35:2515-2520.
10. Dabelea D, Mayer-Davis EJ, Saydah S, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786.
11. Lifshitz F (ed). Pediatric Endocrinology. Vol 1. 5th ed. New York; Informa Healthcare: 2006.
12. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773-795.
13. Flint A, Arslanian B. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(suppl 2):S177-S183.
14. American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23(3):381-389.
15. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S8-S16.
16. FDA. FDA allows marketing of first ZnT8Ab autoantibody test to help diagnose type 1 diabetes. Press release. August 20, 2014.
17. Klingensmith GJ, Pyle L, Arslanian S, et al; the TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype-results from the TODAY study. Diabetes Care. 2010;33(9):1-6.
18. Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28(1):186-212.
19. Academy of Nutrition and Dietetics. Pediatric weight management guideline (2007). www.andeal.org/topic.cfm?cat=2721. Accessed February 17, 2015.
20. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
21. American Association of Clinical Endocrinologists/American College of Endocrinology. Consensus statement by the AACE/ACE insulin pump management task force. Endocr Prac. 2014;20(5):463-489.
22. American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S77-S79.
23. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256.
24. FDA. Drug safety communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. www.fda.gov/downloads/Drugs/DrugSafety/UCM381108.pdf. Accessed February 17, 2015.
25. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(suppl 5):S213-S256.
26. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care. 2011;34(11):2477-2485.
1. Fryar CD, Carroll MD, Ogden CL. Prevalence of obesity among children and adolescents: United States, trends 1963-1965 through 2009-2010. CDC. National Center for Health Statistics, Health E-Stat, September 2012.
2. Cote AT, Harris KC, Panagiotopoulos C, et al. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62(15):1309-1319.
3. Dabelea D, Bell RA, D’Agostino Jr RB, et al; the SEARCH for Diabetes in Youth Writing Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297(24):2716-2724.
4. Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 diabetes mellitus (T2DM) in children and adolescents. Pediatrics. 2013;131(2):364-382.
5. American Diabetes Association. Children and adolescents. Sec. 11. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S70-S76.
6. Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes. Diabetes Care. 2013;36:3863-3869.
7. CDC. National Diabetes Statistics Report, 2014: Estimates of Diabetes and its Burden in the United States. www.cdc.gov/diabetes/pubs/statsre port14/national-diabetes-report-web.pdf. Accessed February 17, 2015.
8. Pettitt DJ, Talton J, Dabelea D, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of diabetes in US youth in 2009: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402-408.
9. Imperatore G, Boyle JP, Thompson TJ, et al; for the SEARCH for Diabetes in Youth Study Group. Projections of type 1 and type 2 diabetes burden in the U.S. population aged < 20 years through 2050. Diabetes Care. 2012;35:2515-2520.
10. Dabelea D, Mayer-Davis EJ, Saydah S, et al; for the SEARCH for Diabetes in Youth Study Group. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778-1786.
11. Lifshitz F (ed). Pediatric Endocrinology. Vol 1. 5th ed. New York; Informa Healthcare: 2006.
12. DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes. Diabetes. 2009;58:773-795.
13. Flint A, Arslanian B. Treatment of type 2 diabetes in youth. Diabetes Care. 2011;34(suppl 2):S177-S183.
14. American Diabetes Association. Type 2 diabetes in children and adolescents: consensus statement. Diabetes Care. 2000;23(3):381-389.
15. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S8-S16.
16. FDA. FDA allows marketing of first ZnT8Ab autoantibody test to help diagnose type 1 diabetes. Press release. August 20, 2014.
17. Klingensmith GJ, Pyle L, Arslanian S, et al; the TODAY Study Group. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype-results from the TODAY study. Diabetes Care. 2010;33(9):1-6.
18. Silverstein J, Klingensmith G, Copeland K, et al. American Diabetes Association. Care of children and adolescents with type 1 diabetes. Diabetes Care. 2005;28(1):186-212.
19. Academy of Nutrition and Dietetics. Pediatric weight management guideline (2007). www.andeal.org/topic.cfm?cat=2721. Accessed February 17, 2015.
20. Glucophage [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
21. American Association of Clinical Endocrinologists/American College of Endocrinology. Consensus statement by the AACE/ACE insulin pump management task force. Endocr Prac. 2014;20(5):463-489.
22. American Diabetes Association. Management of diabetes in pregnancy. Sec. 12. In: Standards of Medical Care in Diabetes—2015. Diabetes Care. 2015;38(suppl 1):S77-S79.
23. TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247-2256.
24. FDA. Drug safety communication. FDA requires removal of some prescribing and dispensing restrictions for rosiglitazone-containing diabetes medicines. www.fda.gov/downloads/Drugs/DrugSafety/UCM381108.pdf. Accessed February 17, 2015.
25. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics. 2011;128(suppl 5):S213-S256.
26. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems. Diabetes Care. 2011;34(11):2477-2485.