User login
Oral Anticoagulants and Nonvalvular A-fib: A Balancing Act
CE/CME No: CR-1502
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the presenting signs and symptoms of atrial fibrillation (A-fib).
• Define the differential and tests for diagnosing A-fib.
• Identify the risk factors associated with A-fib.
• Discuss the anticoagulant treatment options for nonvalvular A-fib.
• List aspects of A-fib management about which patients benefit from clinician instruction.
FACULTY
Deedra Harrington, Janis R. Guilbeau, and Christy McDonald Lenahan are Assistant Professors in the College of Nursing and Allied Health Professions at the University of Louisiana at Lafayette. Dr. Harrington is also a cardiology intensivist nurse practitioner at the Heart Hospital of Lafayette. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2015.
Article begins on next page >>
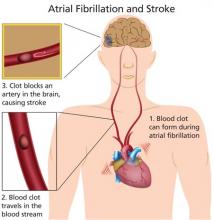
Patients with nonvalvular atrial fibrillation (A-fib) have a fivefold greater risk for ischemic stroke than those without. Newer oral anticoagulants reduce this risk—but also increase risk for serious bleeding, including intracranial hemorrhage. Here are the evidence-based guidelines to help you make the choice that’s best for your patient.
Atrial fibrillation (A-fib) is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation that results in ineffective atrial contraction; this causes inadequate ventricular rate control and variable ventricular filling.1 A common cardiac arrhythmia that is estimated to affect between 2.7 and 6.1 million Americans,2 A-fib is projected to affect as many as 12.1 million people by the year 2030.3 Incidence increases with age; while less than 1% of patients with A-fib are younger than 60, more than a third are 80 or older.1
Morbidity and mortality associated with A-fib are significant. The risk for an embolic event is particularly profound—five times that of persons without A-fib; again, this risk increases with age. In patients ages 50 to 59, 1.5% of strokes are attributed to A-fib; this percentage increases to 23.5% for those ages 80 to 89.2
Treatment of A-fib is aimed at rate control and rhythm conversion, generally through the use of drugs or ablation procedures, and stroke risk reduction, using oral anticoagulants to prevent thrombus formation. This review will focus on the use of newer oral anticoagulants for reduction of stroke risk associated with nonvalvular A-fib.
PATIENT PRESENTATION
Patients with new-onset A-fib may present with a variety of symptoms, including palpitations, chest pain, pressure or discomfort, shortness of breath, lightheadedness, fatigue, or exercise intolerance.4 Patients with chest pain, palpitations, and shortness of breath in particular should be assessed immediately for myocardial infarction before evaluating for A-fib. Poor perfusion may cause a decreased level of patient consciousness; therefore, hypotension or even Alzheimer disease should be ruled out.
Continue for diagnostic evaluation >>
DIAGNOSTIC EVALUATION
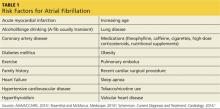
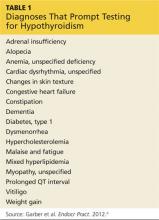
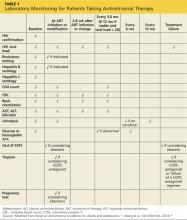
A complete patient history and thorough review of systems will enable the clinician to identify the risk factors for A-fib and establish a diagnosis (see Table 1).1,4,5 Evaluation should also include a detailed physical examination. Upon initial cardiovascular assessment, the patient’s apical pulse may be rapid, irregular, or disorganized during auscultation. If underlying A-fib is related to a valvular abnormality, an audible murmur may be auscultated.5
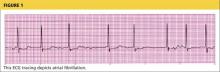
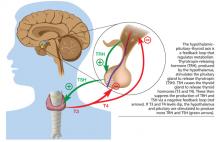
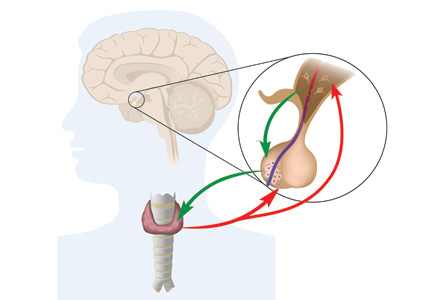
Workup for A-fib includes the standard 12-lead ECG, chest radiograph, thyroid function test, and echocardiogram. The 12-lead ECG is definitive for making the diagnosis of A-fib (see Figure 1). A-fib is characterized by irregular R-R intervals when atrioventricular conduction is present, absence of distinct repeating P waves, and irregular atrial activity.1
If the patient describes episodes consistent with A-fib that is not detectable at the office visit, 24- or 48-hour ambulatory Holter monitoring may be revealing. Event monitors can be used to determine the frequency with which the patient experiences A-fib over an extended period of time (up to 30 days).6
As part of the differential diagnosis of A-fib, clinicians need to consider other possible atrial conduction abnormalities, including atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia, multifocal atrial tachycardia, and Wolff-Parkinson-White syndrome.5
To rule out other etiologies, consider performing the following examinations and tests4
• A chest x-ray can rule out undiagnosed lung disease (eg, chronic obstructive pulmonary disease).
• To exclude hyperthyroidism as a cause of the patient’s symptoms, thyroid function testing and a physical examination for exophthalmos, carotid bruits, and thyromegaly are needed.
• Echocardiography is useful to exclude valvular abnormalities and/or heart failure.
• A complete blood cell count will rule out any infectious process or anemic state.
• Renal function studies and a comprehensive metabolic panel will detect signs of renal failure or electrolyte imbalance.
• Cardiac enzyme measurement can help rule out the occurrence of a myocardial event.
• A brain natriuretic peptide test can identify if heart failure is a contributing factor.
Continue for A-fib classification >>
A-FIB CLASSIFICATION
For purposes of choosing appropriate therapy, it is necessary to determine whether the cause of A-fib is valvular or nonvalvular. Valvular A-fib is described as A-fib that occurs in the presence of valvular heart disease or defect, such as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.1 In the absence of these types of conditions, A-fib is considered nonvalvular. The vast majority of patients have nonvalvular A-fib; in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) study, researchers found that, among 17,974 adults with A-fib who were members of a large California health maintenance organization, only 4.9% had valvular heart disease.8
A-fib is commonly classified into four subcategories, based on its duration: paroxysmal, persistent, longstanding persistent, and permanent.
Paroxysmal. The occurrence of at least two episodes that have terminated in less than seven days without treatment.
Persistent. An episode lasting more than seven days or less than seven days after electric or pharmacologic conversion.
Longstanding persistent. Continuous A-fib for more than one year.
Permanent. A category for patients in whom rhythm control is no longer being pursued.
This simplified classification is often used to choose between ablative or medication therapies. To ensure accuracy, however, underlying causes, risk factors, and mechanisms should be determined.9
Stroke risk calculation
Once nonvalvular A-fib is confirmed, the next step is to control the ventricular rate and attempt to convert the A-fib rhythm. To accomplish this, the patient’s risk for stroke must be estimated and the need for oral anticoagulation determined.
The CHADS2 risk stratification system for calculating an individual’s risk for ischemic stroke in A-fib was developed in 2001. The risk criteria used in the calculation are Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, transient ischemic attack, or thromboembolism.10
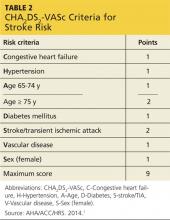
Recent additions to the criteria account for advanced age, gender, and known vascular disease.1,5 Known as the CHA2DS2-VASc, this scoring system is outlined in Table 2. If the patient’s score is 0, risk for stroke is low and anticoagulation therapy is not recommended. If the score is 1, the risk is intermediate, and the patient may be treated with aspirin therapy or anticoagulation. With a CHA2DS2-VASc score of 2 or greater, anticoagulation treatment is recommended to reduce the risk for stroke.1
While the expanded CHA2DS2-VASc criteria more clearly define the basis for an anticoagulation recommendation—particularly in older patients, women, and those with a vascular history—the superiority of one over the other is undetermined.11 However, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with A-fib recommend use of the CHA2DS2-VASc.1
Continue for anticoagulation therapy >>
ANTICOAGULATION THERAPY
The choice of anticoagulation treatment requires weighing the risks and benefits of oral anticoagulation therapy. Stroke and bleeding risks, cost, tolerability, potential for drug interactions, likelihood of patient adherence to the anticoagulation regimen, and patient preferences should be considered.1
The three oral anticoagulants recently approved by the FDA for the reduction of stroke and systemic embolism risks in nonvalvular A-fib are dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, both factor Xa inhibitors.12-14
The clinical trials upon which the FDA’s approval of these anticoagulants was based included only patients with nonvalvular A-fib. For patients with valvular disease, warfarin, a vitamin-K–dependent inhibitor, is currently recommended.1,15 It is also recommended for patients with both end-stage renal disease (ESRD) and either nonvalvular or valvular A-fib.1
A-fib and chronic kidney disease
It is estimated that one-third of patients with A-fib are also diagnosed with chronic kidney disease (CKD).16 Because patients with CKD have a greater risk for bleeding, anticoagulant therapy for these patients requires reduced dosing and close monitoring for bleeding.
The 2014 ACC/AHA/HRS practice guidelines include guidance for selection of oral anticoagulants for patients with nonvalvular A-fib and CKD (see Table 3).1,12-14,17 Dosing of dabigatran and rivaroxaban require evaluation of creatinine clearance before treatment is initiated.
When warfarin is indicated, dose adjustments for renal impairment are based on the prothrombin time/international normalized ratio (INR) value.1 Current guidelines recommend maintaining a therapeutic INR between 2.0 and 3.0 for nonvalvular A-fib in patients with CKD.1 Patients with difficulty maintaining therapeutic INR levels may benefit from alternate therapy with Xa inhibitors or a direct thrombin inhibitor except in the presence of ESRD.1
Continue for patient adherence >>
PATIENT ADHERENCE
Recent studies have indicated that adherence to anticoagulation therapy among A-fib patients drops by as much as 50% after one year of therapy.18 Causes are multifactorial and include complexity of treatment regimen, missed doses, patient unawareness of stroke risk, and fear of bleeding.19 Educating both patients and caregivers has been associated with significant improvements in medication compliance in these patients.19
Complex regimens
Treatment requirements, such as the serial laboratory testing and dosage adjustments associated with warfarin therapy, can be a major contributing factor to anticoagulation nonadherence.18,20 In this regard, the newer once-daily medications that require limited follow-up may be good alternatives to warfarin.21
In patients for whom warfarin therapy is indicated, educational interventions may include
• Written information for patients and caregivers about medication regimens and dosage scheduling
• Reinforcement of treatment goals and outcomes
• Use of dosing aids such as dated and timed pill dispensers
• Incorporating caregiver support to help patients adhere to the medication regimen.
These interventions have been shown to improve adherence with complex treatment regimens.22
Missed doses
Missing anticoagulant doses is not an uncommon occurrence, and patients should be advised of appropriate catch-up strategies when this occurs.
For dabigatran, the missed dose should be taken as soon as the patient remembers, but only if the next scheduled dose is more than six hours away.12 For rivaroxaban, missed doses should be taken as soon as the patient remembers, and the next dose should resume as scheduled.13 For apixaban, a missed dose should be taken as soon as possible but not in combination with any other doses.14
For patients taking warfarin, a missed dose should be taken as soon as possible on the same day.23 If more than 24 hours have elapsed, the patient should contact his or her health care provider before taking any medication.23
Stroke risk
Adherence to anticoagulation therapy significantly reduces the risk for stroke among A-fib patients. Estimates suggest that anticoagulants can reduce stroke risk by as much as 68% in patients with A-fib.24
Even with optimal anticoagulation therapy, however, stroke remains a major complication.25 Through group sessions or patient education pamphlets, patients and caregivers should be informed about the high risk for stroke associated with A-fib and should know its early symptoms.26 These include sudden onset of one or more of the following: confusion or difficulty understanding speech; numbness or weakness of the face or extremities, limited to one side of the body; severe headache; dizziness, loss of balance, or difficulty ambulating; and/or visual disturbances in one or both eyes.26
Next page: Bleeding risk >>
Bleeding risk
Patients should be advised of the major risk for bleeding associated with all anticoagulant therapies.27 Screening for bleeding includes assessment of Hypertension, Abnormal renal and/or liver function, previous Stroke, Bleeding history, Labile INR, being Elderly, and currently prescribed Drugs and/or excessive use of alcohol (known as HAS-BLED) (see Table 4).1,28 Use of a bleeding risk assessment tool such as HAS-BLED may help identify the patient’s risk but cannot be the basis for treatment decisions.1,29
Despite efforts to decrease bleeding risks, patients should understand that hemorrhagic complications can still occur. Patients taking anticoagulants should be familiar with early signs and symptoms of bleeding (eg, sudden, severe headache; melena; hematemesis; nosebleeds) and should notify their health care provider immediately if any of these symptoms occur.12-14,23
If bleeding occurs, it is recommended that anticoagulant treatment be stopped. In addition, depending on the severity of the bleeding, the clinician may elect to administer activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X to reverse the effects of newer oral anticoagulants.1 Vitamin K, the antidote for warfarin, is not effective on direct thrombin inhibitors or factor Xa inhibitors. Currently, there is no established means of reversing the anticoagulant effects of the newer oral anticoagulants.12-14
FOLLOW-UP
Since optimal utilization of cardiovascular medication occurs in only 50% of the patient population, appropriate follow-up must be implemented to improve overall outcomes of pharmacologic therapy.30 Follow-up protocols depend on multiple factors, including type of anticoagulation therapy, patient response to therapy, and patient comorbidities.31 Monitoring warfarin use is time-consuming and resource-intensive; laboratory monitoring requirements for the newer oral anticoagulants have not been established.32
Patients taking warfarin should be vigilant in follow-up with serial laboratory measurements and dosage adjustments.23 Once therapy is initiated, INR is monitored every two to four days until two therapeutic INR levels are obtained.31,33 Monitoring can then be changed to once weekly until two more therapeutic levels are obtained.31 The INR monitoring interval can then be increased to every two to four weeks, with two weeks being a more conservative strategy.33 The practitioner may want to consider advancing to four-week monitoring intervals once four therapeutic INR levels have been obtained.31 It may be necessary to return to two-to-four day monitoring of INR if a nontherapeutic INR is obtained, the patient becomes ill, a medication is changed, or the patient makes a significant dietary change.31
When to refer
Primary care practitioners can manage anticoagulation therapy safely and efficiently, but cardiology referral may be warranted in certain situations. For example, patients with complex cardiac disease may benefit from cardiology referral.7 Considerations for referral to a cardiologist for further evaluation may include
• Abnormal exercise stress test results
• Abnormal echocardiogram results
• 12-lead ECG that reveals rapid, irregular wide pre-excited QRS complexes5
Patients who are drug intolerant or who remain symptomatic on pharmacologic rate control should also be referred to cardiology.7 In addition, patients who may require a pacemaker or defibrillator or who may be candidates for ablation should also be referred to an electrophysiology specialist.7
Continue for conclusion >>
CONCLUSION
Nonvalvular A-fib is a common arrhythmia that contributes significantly to morbidity among older adults. Use of the most current clinical practice guidelines coupled with patient education will improve overall patient outcomes.
* Editor's note: At press time, the FDA had announced approval of another oral anticoagulant, edoxaban, for the reduction of stroke and systemic embolism risks in nonvalvular A-fib.
1. January CT, Wann S, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update. Circulation. 2014;129(3):e28-e292.
3. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013:112:1142-1147.
4. Rosenthal L, McManus DD. Atrial fibrillation workup. http://emedicine.medscape.com/article/151066-workup. Accessed January 19, 2015.
5. Scheinman MM. Atrial fibrillation. In: Crawford MH, ed. Current Diagnosis and Treatment: Cardiology. Fourth Edition. New York: McGraw-Hill Education; 2014: 141-149.
6. Berry E, Padgett H. Management of patients with atrial fibrillation: diagnosis and treatment. Nurs Stand. 2012;26(22):47-56.
7. Gutierrez C, Blanchard DG. Atrial fibrillation: diagnosis and treatment. Am Fam Physician. 2011;83(1):61-68.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
9. Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23(2):71-84.
10. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57-64.
11. Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:603.e1-603.e6.
12. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2010.
13. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
14. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
15. Verma A, Cairns JA, Mitchell LB, et al, for the CCC Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114-1130.
16. Hart RG, Eielboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013;29(7 suppl):S71-S78.
17. Engelbertz C, Reinecke H. Atrial fibrillation and oral anticoagulation in chronic kidney disease. J Atr Fibrillation. 2012;4(6):89-100.
18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban vs. warfarin among patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461-2469.
19. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600.
20. Albert NM. Use of novel oral anticoagulants for patients with atrial fibrillation: systematic review and clinical implications. Heart Lung. 2014;43:48-59.
21. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Preference and Adherence. 2010;4:52-60.
22. National Heart Foundation of Australia. Improving adherence in cardiovascular care. A toolkit for health professionals. www.heartfoundation.org.au/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf. Accessed January 19, 2015.
23. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1954.
24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254]. Arch Intern Med. 1994;154(13):1449-1457.
25. Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3),442-448.
26. Morimoto A, Miyamatsu, M, Okamura T, et al. Effects of intensive and moderate public education on knowledge of early stroke symptoms among a Japanese population: the Acquisition of Stroke Knowledge study. Stroke. 2013;44(10):2829-2834.
27. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70(10 suppl 1):S3-S11.
28. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
29. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683.
30. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thrombosis J. 2013;11(8):1-5.
31. Ivers N, Dorian P. Applying the atrial fibrillation guidelines update to manage your patients with atrial fibrillation. Can J Cardiol. 2014;30:1241-1244.
32. Wigle P, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician. 2013;87(8):556-566.
33. Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. 1999;59(3):635-646.
CE/CME No: CR-1502
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the presenting signs and symptoms of atrial fibrillation (A-fib).
• Define the differential and tests for diagnosing A-fib.
• Identify the risk factors associated with A-fib.
• Discuss the anticoagulant treatment options for nonvalvular A-fib.
• List aspects of A-fib management about which patients benefit from clinician instruction.
FACULTY
Deedra Harrington, Janis R. Guilbeau, and Christy McDonald Lenahan are Assistant Professors in the College of Nursing and Allied Health Professions at the University of Louisiana at Lafayette. Dr. Harrington is also a cardiology intensivist nurse practitioner at the Heart Hospital of Lafayette. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2015.
Article begins on next page >>
Patients with nonvalvular atrial fibrillation (A-fib) have a fivefold greater risk for ischemic stroke than those without. Newer oral anticoagulants reduce this risk—but also increase risk for serious bleeding, including intracranial hemorrhage. Here are the evidence-based guidelines to help you make the choice that’s best for your patient.
Atrial fibrillation (A-fib) is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation that results in ineffective atrial contraction; this causes inadequate ventricular rate control and variable ventricular filling.1 A common cardiac arrhythmia that is estimated to affect between 2.7 and 6.1 million Americans,2 A-fib is projected to affect as many as 12.1 million people by the year 2030.3 Incidence increases with age; while less than 1% of patients with A-fib are younger than 60, more than a third are 80 or older.1
Morbidity and mortality associated with A-fib are significant. The risk for an embolic event is particularly profound—five times that of persons without A-fib; again, this risk increases with age. In patients ages 50 to 59, 1.5% of strokes are attributed to A-fib; this percentage increases to 23.5% for those ages 80 to 89.2
Treatment of A-fib is aimed at rate control and rhythm conversion, generally through the use of drugs or ablation procedures, and stroke risk reduction, using oral anticoagulants to prevent thrombus formation. This review will focus on the use of newer oral anticoagulants for reduction of stroke risk associated with nonvalvular A-fib.
PATIENT PRESENTATION
Patients with new-onset A-fib may present with a variety of symptoms, including palpitations, chest pain, pressure or discomfort, shortness of breath, lightheadedness, fatigue, or exercise intolerance.4 Patients with chest pain, palpitations, and shortness of breath in particular should be assessed immediately for myocardial infarction before evaluating for A-fib. Poor perfusion may cause a decreased level of patient consciousness; therefore, hypotension or even Alzheimer disease should be ruled out.
Continue for diagnostic evaluation >>
DIAGNOSTIC EVALUATION
A complete patient history and thorough review of systems will enable the clinician to identify the risk factors for A-fib and establish a diagnosis (see Table 1).1,4,5 Evaluation should also include a detailed physical examination. Upon initial cardiovascular assessment, the patient’s apical pulse may be rapid, irregular, or disorganized during auscultation. If underlying A-fib is related to a valvular abnormality, an audible murmur may be auscultated.5
Workup for A-fib includes the standard 12-lead ECG, chest radiograph, thyroid function test, and echocardiogram. The 12-lead ECG is definitive for making the diagnosis of A-fib (see Figure 1). A-fib is characterized by irregular R-R intervals when atrioventricular conduction is present, absence of distinct repeating P waves, and irregular atrial activity.1
If the patient describes episodes consistent with A-fib that is not detectable at the office visit, 24- or 48-hour ambulatory Holter monitoring may be revealing. Event monitors can be used to determine the frequency with which the patient experiences A-fib over an extended period of time (up to 30 days).6
As part of the differential diagnosis of A-fib, clinicians need to consider other possible atrial conduction abnormalities, including atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia, multifocal atrial tachycardia, and Wolff-Parkinson-White syndrome.5
To rule out other etiologies, consider performing the following examinations and tests4
• A chest x-ray can rule out undiagnosed lung disease (eg, chronic obstructive pulmonary disease).
• To exclude hyperthyroidism as a cause of the patient’s symptoms, thyroid function testing and a physical examination for exophthalmos, carotid bruits, and thyromegaly are needed.
• Echocardiography is useful to exclude valvular abnormalities and/or heart failure.
• A complete blood cell count will rule out any infectious process or anemic state.
• Renal function studies and a comprehensive metabolic panel will detect signs of renal failure or electrolyte imbalance.
• Cardiac enzyme measurement can help rule out the occurrence of a myocardial event.
• A brain natriuretic peptide test can identify if heart failure is a contributing factor.
Continue for A-fib classification >>
A-FIB CLASSIFICATION
For purposes of choosing appropriate therapy, it is necessary to determine whether the cause of A-fib is valvular or nonvalvular. Valvular A-fib is described as A-fib that occurs in the presence of valvular heart disease or defect, such as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.1 In the absence of these types of conditions, A-fib is considered nonvalvular. The vast majority of patients have nonvalvular A-fib; in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) study, researchers found that, among 17,974 adults with A-fib who were members of a large California health maintenance organization, only 4.9% had valvular heart disease.8
A-fib is commonly classified into four subcategories, based on its duration: paroxysmal, persistent, longstanding persistent, and permanent.
Paroxysmal. The occurrence of at least two episodes that have terminated in less than seven days without treatment.
Persistent. An episode lasting more than seven days or less than seven days after electric or pharmacologic conversion.
Longstanding persistent. Continuous A-fib for more than one year.
Permanent. A category for patients in whom rhythm control is no longer being pursued.
This simplified classification is often used to choose between ablative or medication therapies. To ensure accuracy, however, underlying causes, risk factors, and mechanisms should be determined.9
Stroke risk calculation
Once nonvalvular A-fib is confirmed, the next step is to control the ventricular rate and attempt to convert the A-fib rhythm. To accomplish this, the patient’s risk for stroke must be estimated and the need for oral anticoagulation determined.
The CHADS2 risk stratification system for calculating an individual’s risk for ischemic stroke in A-fib was developed in 2001. The risk criteria used in the calculation are Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, transient ischemic attack, or thromboembolism.10
Recent additions to the criteria account for advanced age, gender, and known vascular disease.1,5 Known as the CHA2DS2-VASc, this scoring system is outlined in Table 2. If the patient’s score is 0, risk for stroke is low and anticoagulation therapy is not recommended. If the score is 1, the risk is intermediate, and the patient may be treated with aspirin therapy or anticoagulation. With a CHA2DS2-VASc score of 2 or greater, anticoagulation treatment is recommended to reduce the risk for stroke.1
While the expanded CHA2DS2-VASc criteria more clearly define the basis for an anticoagulation recommendation—particularly in older patients, women, and those with a vascular history—the superiority of one over the other is undetermined.11 However, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with A-fib recommend use of the CHA2DS2-VASc.1
Continue for anticoagulation therapy >>
ANTICOAGULATION THERAPY
The choice of anticoagulation treatment requires weighing the risks and benefits of oral anticoagulation therapy. Stroke and bleeding risks, cost, tolerability, potential for drug interactions, likelihood of patient adherence to the anticoagulation regimen, and patient preferences should be considered.1
The three oral anticoagulants recently approved by the FDA for the reduction of stroke and systemic embolism risks in nonvalvular A-fib are dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, both factor Xa inhibitors.12-14
The clinical trials upon which the FDA’s approval of these anticoagulants was based included only patients with nonvalvular A-fib. For patients with valvular disease, warfarin, a vitamin-K–dependent inhibitor, is currently recommended.1,15 It is also recommended for patients with both end-stage renal disease (ESRD) and either nonvalvular or valvular A-fib.1
A-fib and chronic kidney disease
It is estimated that one-third of patients with A-fib are also diagnosed with chronic kidney disease (CKD).16 Because patients with CKD have a greater risk for bleeding, anticoagulant therapy for these patients requires reduced dosing and close monitoring for bleeding.
The 2014 ACC/AHA/HRS practice guidelines include guidance for selection of oral anticoagulants for patients with nonvalvular A-fib and CKD (see Table 3).1,12-14,17 Dosing of dabigatran and rivaroxaban require evaluation of creatinine clearance before treatment is initiated.
When warfarin is indicated, dose adjustments for renal impairment are based on the prothrombin time/international normalized ratio (INR) value.1 Current guidelines recommend maintaining a therapeutic INR between 2.0 and 3.0 for nonvalvular A-fib in patients with CKD.1 Patients with difficulty maintaining therapeutic INR levels may benefit from alternate therapy with Xa inhibitors or a direct thrombin inhibitor except in the presence of ESRD.1
Continue for patient adherence >>
PATIENT ADHERENCE
Recent studies have indicated that adherence to anticoagulation therapy among A-fib patients drops by as much as 50% after one year of therapy.18 Causes are multifactorial and include complexity of treatment regimen, missed doses, patient unawareness of stroke risk, and fear of bleeding.19 Educating both patients and caregivers has been associated with significant improvements in medication compliance in these patients.19
Complex regimens
Treatment requirements, such as the serial laboratory testing and dosage adjustments associated with warfarin therapy, can be a major contributing factor to anticoagulation nonadherence.18,20 In this regard, the newer once-daily medications that require limited follow-up may be good alternatives to warfarin.21
In patients for whom warfarin therapy is indicated, educational interventions may include
• Written information for patients and caregivers about medication regimens and dosage scheduling
• Reinforcement of treatment goals and outcomes
• Use of dosing aids such as dated and timed pill dispensers
• Incorporating caregiver support to help patients adhere to the medication regimen.
These interventions have been shown to improve adherence with complex treatment regimens.22
Missed doses
Missing anticoagulant doses is not an uncommon occurrence, and patients should be advised of appropriate catch-up strategies when this occurs.
For dabigatran, the missed dose should be taken as soon as the patient remembers, but only if the next scheduled dose is more than six hours away.12 For rivaroxaban, missed doses should be taken as soon as the patient remembers, and the next dose should resume as scheduled.13 For apixaban, a missed dose should be taken as soon as possible but not in combination with any other doses.14
For patients taking warfarin, a missed dose should be taken as soon as possible on the same day.23 If more than 24 hours have elapsed, the patient should contact his or her health care provider before taking any medication.23
Stroke risk
Adherence to anticoagulation therapy significantly reduces the risk for stroke among A-fib patients. Estimates suggest that anticoagulants can reduce stroke risk by as much as 68% in patients with A-fib.24
Even with optimal anticoagulation therapy, however, stroke remains a major complication.25 Through group sessions or patient education pamphlets, patients and caregivers should be informed about the high risk for stroke associated with A-fib and should know its early symptoms.26 These include sudden onset of one or more of the following: confusion or difficulty understanding speech; numbness or weakness of the face or extremities, limited to one side of the body; severe headache; dizziness, loss of balance, or difficulty ambulating; and/or visual disturbances in one or both eyes.26
Next page: Bleeding risk >>
Bleeding risk
Patients should be advised of the major risk for bleeding associated with all anticoagulant therapies.27 Screening for bleeding includes assessment of Hypertension, Abnormal renal and/or liver function, previous Stroke, Bleeding history, Labile INR, being Elderly, and currently prescribed Drugs and/or excessive use of alcohol (known as HAS-BLED) (see Table 4).1,28 Use of a bleeding risk assessment tool such as HAS-BLED may help identify the patient’s risk but cannot be the basis for treatment decisions.1,29
Despite efforts to decrease bleeding risks, patients should understand that hemorrhagic complications can still occur. Patients taking anticoagulants should be familiar with early signs and symptoms of bleeding (eg, sudden, severe headache; melena; hematemesis; nosebleeds) and should notify their health care provider immediately if any of these symptoms occur.12-14,23
If bleeding occurs, it is recommended that anticoagulant treatment be stopped. In addition, depending on the severity of the bleeding, the clinician may elect to administer activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X to reverse the effects of newer oral anticoagulants.1 Vitamin K, the antidote for warfarin, is not effective on direct thrombin inhibitors or factor Xa inhibitors. Currently, there is no established means of reversing the anticoagulant effects of the newer oral anticoagulants.12-14
FOLLOW-UP
Since optimal utilization of cardiovascular medication occurs in only 50% of the patient population, appropriate follow-up must be implemented to improve overall outcomes of pharmacologic therapy.30 Follow-up protocols depend on multiple factors, including type of anticoagulation therapy, patient response to therapy, and patient comorbidities.31 Monitoring warfarin use is time-consuming and resource-intensive; laboratory monitoring requirements for the newer oral anticoagulants have not been established.32
Patients taking warfarin should be vigilant in follow-up with serial laboratory measurements and dosage adjustments.23 Once therapy is initiated, INR is monitored every two to four days until two therapeutic INR levels are obtained.31,33 Monitoring can then be changed to once weekly until two more therapeutic levels are obtained.31 The INR monitoring interval can then be increased to every two to four weeks, with two weeks being a more conservative strategy.33 The practitioner may want to consider advancing to four-week monitoring intervals once four therapeutic INR levels have been obtained.31 It may be necessary to return to two-to-four day monitoring of INR if a nontherapeutic INR is obtained, the patient becomes ill, a medication is changed, or the patient makes a significant dietary change.31
When to refer
Primary care practitioners can manage anticoagulation therapy safely and efficiently, but cardiology referral may be warranted in certain situations. For example, patients with complex cardiac disease may benefit from cardiology referral.7 Considerations for referral to a cardiologist for further evaluation may include
• Abnormal exercise stress test results
• Abnormal echocardiogram results
• 12-lead ECG that reveals rapid, irregular wide pre-excited QRS complexes5
Patients who are drug intolerant or who remain symptomatic on pharmacologic rate control should also be referred to cardiology.7 In addition, patients who may require a pacemaker or defibrillator or who may be candidates for ablation should also be referred to an electrophysiology specialist.7
Continue for conclusion >>
CONCLUSION
Nonvalvular A-fib is a common arrhythmia that contributes significantly to morbidity among older adults. Use of the most current clinical practice guidelines coupled with patient education will improve overall patient outcomes.
* Editor's note: At press time, the FDA had announced approval of another oral anticoagulant, edoxaban, for the reduction of stroke and systemic embolism risks in nonvalvular A-fib.
CE/CME No: CR-1502
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the presenting signs and symptoms of atrial fibrillation (A-fib).
• Define the differential and tests for diagnosing A-fib.
• Identify the risk factors associated with A-fib.
• Discuss the anticoagulant treatment options for nonvalvular A-fib.
• List aspects of A-fib management about which patients benefit from clinician instruction.
FACULTY
Deedra Harrington, Janis R. Guilbeau, and Christy McDonald Lenahan are Assistant Professors in the College of Nursing and Allied Health Professions at the University of Louisiana at Lafayette. Dr. Harrington is also a cardiology intensivist nurse practitioner at the Heart Hospital of Lafayette. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2015.
Article begins on next page >>
Patients with nonvalvular atrial fibrillation (A-fib) have a fivefold greater risk for ischemic stroke than those without. Newer oral anticoagulants reduce this risk—but also increase risk for serious bleeding, including intracranial hemorrhage. Here are the evidence-based guidelines to help you make the choice that’s best for your patient.
Atrial fibrillation (A-fib) is a supraventricular tachyarrhythmia characterized by uncoordinated atrial activation that results in ineffective atrial contraction; this causes inadequate ventricular rate control and variable ventricular filling.1 A common cardiac arrhythmia that is estimated to affect between 2.7 and 6.1 million Americans,2 A-fib is projected to affect as many as 12.1 million people by the year 2030.3 Incidence increases with age; while less than 1% of patients with A-fib are younger than 60, more than a third are 80 or older.1
Morbidity and mortality associated with A-fib are significant. The risk for an embolic event is particularly profound—five times that of persons without A-fib; again, this risk increases with age. In patients ages 50 to 59, 1.5% of strokes are attributed to A-fib; this percentage increases to 23.5% for those ages 80 to 89.2
Treatment of A-fib is aimed at rate control and rhythm conversion, generally through the use of drugs or ablation procedures, and stroke risk reduction, using oral anticoagulants to prevent thrombus formation. This review will focus on the use of newer oral anticoagulants for reduction of stroke risk associated with nonvalvular A-fib.
PATIENT PRESENTATION
Patients with new-onset A-fib may present with a variety of symptoms, including palpitations, chest pain, pressure or discomfort, shortness of breath, lightheadedness, fatigue, or exercise intolerance.4 Patients with chest pain, palpitations, and shortness of breath in particular should be assessed immediately for myocardial infarction before evaluating for A-fib. Poor perfusion may cause a decreased level of patient consciousness; therefore, hypotension or even Alzheimer disease should be ruled out.
Continue for diagnostic evaluation >>
DIAGNOSTIC EVALUATION
A complete patient history and thorough review of systems will enable the clinician to identify the risk factors for A-fib and establish a diagnosis (see Table 1).1,4,5 Evaluation should also include a detailed physical examination. Upon initial cardiovascular assessment, the patient’s apical pulse may be rapid, irregular, or disorganized during auscultation. If underlying A-fib is related to a valvular abnormality, an audible murmur may be auscultated.5
Workup for A-fib includes the standard 12-lead ECG, chest radiograph, thyroid function test, and echocardiogram. The 12-lead ECG is definitive for making the diagnosis of A-fib (see Figure 1). A-fib is characterized by irregular R-R intervals when atrioventricular conduction is present, absence of distinct repeating P waves, and irregular atrial activity.1
If the patient describes episodes consistent with A-fib that is not detectable at the office visit, 24- or 48-hour ambulatory Holter monitoring may be revealing. Event monitors can be used to determine the frequency with which the patient experiences A-fib over an extended period of time (up to 30 days).6
As part of the differential diagnosis of A-fib, clinicians need to consider other possible atrial conduction abnormalities, including atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia, multifocal atrial tachycardia, and Wolff-Parkinson-White syndrome.5
To rule out other etiologies, consider performing the following examinations and tests4
• A chest x-ray can rule out undiagnosed lung disease (eg, chronic obstructive pulmonary disease).
• To exclude hyperthyroidism as a cause of the patient’s symptoms, thyroid function testing and a physical examination for exophthalmos, carotid bruits, and thyromegaly are needed.
• Echocardiography is useful to exclude valvular abnormalities and/or heart failure.
• A complete blood cell count will rule out any infectious process or anemic state.
• Renal function studies and a comprehensive metabolic panel will detect signs of renal failure or electrolyte imbalance.
• Cardiac enzyme measurement can help rule out the occurrence of a myocardial event.
• A brain natriuretic peptide test can identify if heart failure is a contributing factor.
Continue for A-fib classification >>
A-FIB CLASSIFICATION
For purposes of choosing appropriate therapy, it is necessary to determine whether the cause of A-fib is valvular or nonvalvular. Valvular A-fib is described as A-fib that occurs in the presence of valvular heart disease or defect, such as rheumatic mitral stenosis, a mechanical or bioprosthetic heart valve, or mitral valve repair.1 In the absence of these types of conditions, A-fib is considered nonvalvular. The vast majority of patients have nonvalvular A-fib; in the ATRIA (AnTicoagulation and Risk Factors In Atrial Fibrillation) study, researchers found that, among 17,974 adults with A-fib who were members of a large California health maintenance organization, only 4.9% had valvular heart disease.8
A-fib is commonly classified into four subcategories, based on its duration: paroxysmal, persistent, longstanding persistent, and permanent.
Paroxysmal. The occurrence of at least two episodes that have terminated in less than seven days without treatment.
Persistent. An episode lasting more than seven days or less than seven days after electric or pharmacologic conversion.
Longstanding persistent. Continuous A-fib for more than one year.
Permanent. A category for patients in whom rhythm control is no longer being pursued.
This simplified classification is often used to choose between ablative or medication therapies. To ensure accuracy, however, underlying causes, risk factors, and mechanisms should be determined.9
Stroke risk calculation
Once nonvalvular A-fib is confirmed, the next step is to control the ventricular rate and attempt to convert the A-fib rhythm. To accomplish this, the patient’s risk for stroke must be estimated and the need for oral anticoagulation determined.
The CHADS2 risk stratification system for calculating an individual’s risk for ischemic stroke in A-fib was developed in 2001. The risk criteria used in the calculation are Congestive heart failure, Hypertension, Age ≥ 75 years, Diabetes mellitus, prior Stroke, transient ischemic attack, or thromboembolism.10
Recent additions to the criteria account for advanced age, gender, and known vascular disease.1,5 Known as the CHA2DS2-VASc, this scoring system is outlined in Table 2. If the patient’s score is 0, risk for stroke is low and anticoagulation therapy is not recommended. If the score is 1, the risk is intermediate, and the patient may be treated with aspirin therapy or anticoagulation. With a CHA2DS2-VASc score of 2 or greater, anticoagulation treatment is recommended to reduce the risk for stroke.1
While the expanded CHA2DS2-VASc criteria more clearly define the basis for an anticoagulation recommendation—particularly in older patients, women, and those with a vascular history—the superiority of one over the other is undetermined.11 However, the 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guidelines for the management of patients with A-fib recommend use of the CHA2DS2-VASc.1
Continue for anticoagulation therapy >>
ANTICOAGULATION THERAPY
The choice of anticoagulation treatment requires weighing the risks and benefits of oral anticoagulation therapy. Stroke and bleeding risks, cost, tolerability, potential for drug interactions, likelihood of patient adherence to the anticoagulation regimen, and patient preferences should be considered.1
The three oral anticoagulants recently approved by the FDA for the reduction of stroke and systemic embolism risks in nonvalvular A-fib are dabigatran, a direct thrombin inhibitor, and rivaroxaban and apixaban, both factor Xa inhibitors.12-14
The clinical trials upon which the FDA’s approval of these anticoagulants was based included only patients with nonvalvular A-fib. For patients with valvular disease, warfarin, a vitamin-K–dependent inhibitor, is currently recommended.1,15 It is also recommended for patients with both end-stage renal disease (ESRD) and either nonvalvular or valvular A-fib.1
A-fib and chronic kidney disease
It is estimated that one-third of patients with A-fib are also diagnosed with chronic kidney disease (CKD).16 Because patients with CKD have a greater risk for bleeding, anticoagulant therapy for these patients requires reduced dosing and close monitoring for bleeding.
The 2014 ACC/AHA/HRS practice guidelines include guidance for selection of oral anticoagulants for patients with nonvalvular A-fib and CKD (see Table 3).1,12-14,17 Dosing of dabigatran and rivaroxaban require evaluation of creatinine clearance before treatment is initiated.
When warfarin is indicated, dose adjustments for renal impairment are based on the prothrombin time/international normalized ratio (INR) value.1 Current guidelines recommend maintaining a therapeutic INR between 2.0 and 3.0 for nonvalvular A-fib in patients with CKD.1 Patients with difficulty maintaining therapeutic INR levels may benefit from alternate therapy with Xa inhibitors or a direct thrombin inhibitor except in the presence of ESRD.1
Continue for patient adherence >>
PATIENT ADHERENCE
Recent studies have indicated that adherence to anticoagulation therapy among A-fib patients drops by as much as 50% after one year of therapy.18 Causes are multifactorial and include complexity of treatment regimen, missed doses, patient unawareness of stroke risk, and fear of bleeding.19 Educating both patients and caregivers has been associated with significant improvements in medication compliance in these patients.19
Complex regimens
Treatment requirements, such as the serial laboratory testing and dosage adjustments associated with warfarin therapy, can be a major contributing factor to anticoagulation nonadherence.18,20 In this regard, the newer once-daily medications that require limited follow-up may be good alternatives to warfarin.21
In patients for whom warfarin therapy is indicated, educational interventions may include
• Written information for patients and caregivers about medication regimens and dosage scheduling
• Reinforcement of treatment goals and outcomes
• Use of dosing aids such as dated and timed pill dispensers
• Incorporating caregiver support to help patients adhere to the medication regimen.
These interventions have been shown to improve adherence with complex treatment regimens.22
Missed doses
Missing anticoagulant doses is not an uncommon occurrence, and patients should be advised of appropriate catch-up strategies when this occurs.
For dabigatran, the missed dose should be taken as soon as the patient remembers, but only if the next scheduled dose is more than six hours away.12 For rivaroxaban, missed doses should be taken as soon as the patient remembers, and the next dose should resume as scheduled.13 For apixaban, a missed dose should be taken as soon as possible but not in combination with any other doses.14
For patients taking warfarin, a missed dose should be taken as soon as possible on the same day.23 If more than 24 hours have elapsed, the patient should contact his or her health care provider before taking any medication.23
Stroke risk
Adherence to anticoagulation therapy significantly reduces the risk for stroke among A-fib patients. Estimates suggest that anticoagulants can reduce stroke risk by as much as 68% in patients with A-fib.24
Even with optimal anticoagulation therapy, however, stroke remains a major complication.25 Through group sessions or patient education pamphlets, patients and caregivers should be informed about the high risk for stroke associated with A-fib and should know its early symptoms.26 These include sudden onset of one or more of the following: confusion or difficulty understanding speech; numbness or weakness of the face or extremities, limited to one side of the body; severe headache; dizziness, loss of balance, or difficulty ambulating; and/or visual disturbances in one or both eyes.26
Next page: Bleeding risk >>
Bleeding risk
Patients should be advised of the major risk for bleeding associated with all anticoagulant therapies.27 Screening for bleeding includes assessment of Hypertension, Abnormal renal and/or liver function, previous Stroke, Bleeding history, Labile INR, being Elderly, and currently prescribed Drugs and/or excessive use of alcohol (known as HAS-BLED) (see Table 4).1,28 Use of a bleeding risk assessment tool such as HAS-BLED may help identify the patient’s risk but cannot be the basis for treatment decisions.1,29
Despite efforts to decrease bleeding risks, patients should understand that hemorrhagic complications can still occur. Patients taking anticoagulants should be familiar with early signs and symptoms of bleeding (eg, sudden, severe headache; melena; hematemesis; nosebleeds) and should notify their health care provider immediately if any of these symptoms occur.12-14,23
If bleeding occurs, it is recommended that anticoagulant treatment be stopped. In addition, depending on the severity of the bleeding, the clinician may elect to administer activated prothrombin complex concentrates, recombinant factor VIIa, or concentrates of factors II, IX, or X to reverse the effects of newer oral anticoagulants.1 Vitamin K, the antidote for warfarin, is not effective on direct thrombin inhibitors or factor Xa inhibitors. Currently, there is no established means of reversing the anticoagulant effects of the newer oral anticoagulants.12-14
FOLLOW-UP
Since optimal utilization of cardiovascular medication occurs in only 50% of the patient population, appropriate follow-up must be implemented to improve overall outcomes of pharmacologic therapy.30 Follow-up protocols depend on multiple factors, including type of anticoagulation therapy, patient response to therapy, and patient comorbidities.31 Monitoring warfarin use is time-consuming and resource-intensive; laboratory monitoring requirements for the newer oral anticoagulants have not been established.32
Patients taking warfarin should be vigilant in follow-up with serial laboratory measurements and dosage adjustments.23 Once therapy is initiated, INR is monitored every two to four days until two therapeutic INR levels are obtained.31,33 Monitoring can then be changed to once weekly until two more therapeutic levels are obtained.31 The INR monitoring interval can then be increased to every two to four weeks, with two weeks being a more conservative strategy.33 The practitioner may want to consider advancing to four-week monitoring intervals once four therapeutic INR levels have been obtained.31 It may be necessary to return to two-to-four day monitoring of INR if a nontherapeutic INR is obtained, the patient becomes ill, a medication is changed, or the patient makes a significant dietary change.31
When to refer
Primary care practitioners can manage anticoagulation therapy safely and efficiently, but cardiology referral may be warranted in certain situations. For example, patients with complex cardiac disease may benefit from cardiology referral.7 Considerations for referral to a cardiologist for further evaluation may include
• Abnormal exercise stress test results
• Abnormal echocardiogram results
• 12-lead ECG that reveals rapid, irregular wide pre-excited QRS complexes5
Patients who are drug intolerant or who remain symptomatic on pharmacologic rate control should also be referred to cardiology.7 In addition, patients who may require a pacemaker or defibrillator or who may be candidates for ablation should also be referred to an electrophysiology specialist.7
Continue for conclusion >>
CONCLUSION
Nonvalvular A-fib is a common arrhythmia that contributes significantly to morbidity among older adults. Use of the most current clinical practice guidelines coupled with patient education will improve overall patient outcomes.
* Editor's note: At press time, the FDA had announced approval of another oral anticoagulant, edoxaban, for the reduction of stroke and systemic embolism risks in nonvalvular A-fib.
1. January CT, Wann S, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update. Circulation. 2014;129(3):e28-e292.
3. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013:112:1142-1147.
4. Rosenthal L, McManus DD. Atrial fibrillation workup. http://emedicine.medscape.com/article/151066-workup. Accessed January 19, 2015.
5. Scheinman MM. Atrial fibrillation. In: Crawford MH, ed. Current Diagnosis and Treatment: Cardiology. Fourth Edition. New York: McGraw-Hill Education; 2014: 141-149.
6. Berry E, Padgett H. Management of patients with atrial fibrillation: diagnosis and treatment. Nurs Stand. 2012;26(22):47-56.
7. Gutierrez C, Blanchard DG. Atrial fibrillation: diagnosis and treatment. Am Fam Physician. 2011;83(1):61-68.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
9. Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23(2):71-84.
10. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57-64.
11. Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:603.e1-603.e6.
12. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2010.
13. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
14. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
15. Verma A, Cairns JA, Mitchell LB, et al, for the CCC Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114-1130.
16. Hart RG, Eielboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013;29(7 suppl):S71-S78.
17. Engelbertz C, Reinecke H. Atrial fibrillation and oral anticoagulation in chronic kidney disease. J Atr Fibrillation. 2012;4(6):89-100.
18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban vs. warfarin among patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461-2469.
19. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600.
20. Albert NM. Use of novel oral anticoagulants for patients with atrial fibrillation: systematic review and clinical implications. Heart Lung. 2014;43:48-59.
21. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Preference and Adherence. 2010;4:52-60.
22. National Heart Foundation of Australia. Improving adherence in cardiovascular care. A toolkit for health professionals. www.heartfoundation.org.au/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf. Accessed January 19, 2015.
23. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1954.
24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254]. Arch Intern Med. 1994;154(13):1449-1457.
25. Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3),442-448.
26. Morimoto A, Miyamatsu, M, Okamura T, et al. Effects of intensive and moderate public education on knowledge of early stroke symptoms among a Japanese population: the Acquisition of Stroke Knowledge study. Stroke. 2013;44(10):2829-2834.
27. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70(10 suppl 1):S3-S11.
28. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
29. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683.
30. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thrombosis J. 2013;11(8):1-5.
31. Ivers N, Dorian P. Applying the atrial fibrillation guidelines update to manage your patients with atrial fibrillation. Can J Cardiol. 2014;30:1241-1244.
32. Wigle P, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician. 2013;87(8):556-566.
33. Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. 1999;59(3):635-646.
1. January CT, Wann S, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246-2280.
2. Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2014 update. Circulation. 2014;129(3):e28-e292.
3. Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the US adult population. Am J Cardiol. 2013:112:1142-1147.
4. Rosenthal L, McManus DD. Atrial fibrillation workup. http://emedicine.medscape.com/article/151066-workup. Accessed January 19, 2015.
5. Scheinman MM. Atrial fibrillation. In: Crawford MH, ed. Current Diagnosis and Treatment: Cardiology. Fourth Edition. New York: McGraw-Hill Education; 2014: 141-149.
6. Berry E, Padgett H. Management of patients with atrial fibrillation: diagnosis and treatment. Nurs Stand. 2012;26(22):47-56.
7. Gutierrez C, Blanchard DG. Atrial fibrillation: diagnosis and treatment. Am Fam Physician. 2011;83(1):61-68.
8. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
9. Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovasc Pathol. 2014;23(2):71-84.
10. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension Age > 75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J. 2008;156(1):57-64.
11. Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med. 2012; 125:603.e1-603.e6.
12. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2010.
13. Xarelto [package insert]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2011.
14. Eliquis [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2012.
15. Verma A, Cairns JA, Mitchell LB, et al, for the CCC Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114-1130.
16. Hart RG, Eielboom JW, Brimble KS, et al. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can J Cardiol. 2013;29(7 suppl):S71-S78.
17. Engelbertz C, Reinecke H. Atrial fibrillation and oral anticoagulation in chronic kidney disease. J Atr Fibrillation. 2012;4(6):89-100.
18. Nelson WW, Song X, Coleman CI, et al. Medication persistence and discontinuation of rivaroxaban vs. warfarin among patients with nonvalvular atrial fibrillation. Curr Med Res Opin. 2014;30(12):2461-2469.
19. Clarkesmith DE, Pattison HM, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev. 2013;6:CD008600.
20. Albert NM. Use of novel oral anticoagulants for patients with atrial fibrillation: systematic review and clinical implications. Heart Lung. 2014;43:48-59.
21. Kneeland PP, Fang MC. Current issues in patient adherence and persistence: focus on anticoagulants for the treatment and prevention of thromboembolism. Patient Preference and Adherence. 2010;4:52-60.
22. National Heart Foundation of Australia. Improving adherence in cardiovascular care. A toolkit for health professionals. www.heartfoundation.org.au/SiteCollectionDocuments/Improving-adherence-in-cardiovascular-care-toolkit.pdf. Accessed January 19, 2015.
23. Coumadin [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 1954.
24. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials [published correction appears in Arch Intern Med. 1994;154(19):2254]. Arch Intern Med. 1994;154(13):1449-1457.
25. Kirchhof P, Breithardt G, Camm AJ, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3),442-448.
26. Morimoto A, Miyamatsu, M, Okamura T, et al. Effects of intensive and moderate public education on knowledge of early stroke symptoms among a Japanese population: the Acquisition of Stroke Knowledge study. Stroke. 2013;44(10):2829-2834.
27. Nutescu EA. Oral anticoagulant therapies: balancing the risks. Am J Health Syst Pharm. 2013;70(10 suppl 1):S3-S11.
28. Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126(7):860-865.
29. Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683.
30. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thrombosis J. 2013;11(8):1-5.
31. Ivers N, Dorian P. Applying the atrial fibrillation guidelines update to manage your patients with atrial fibrillation. Can J Cardiol. 2014;30:1241-1244.
32. Wigle P, Bloomfield HE, Tubb M, Doherty M. Updated guidelines on outpatient anticoagulation. Am Fam Physician. 2013;87(8):556-566.
33. Horton JD, Bushwick BM. Warfarin therapy: evolving strategies in anticoagulation. Am Fam Physician. 1999;59(3):635-646.
ADHD: Putting the Pieces Together
CE/CME No: CR-1501
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the attention-deficit/hyperactivity disorder (ADHD) diagnostic criteria of inattention, hyperactivity, and/or impulsivity as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR), and the DSM-5 update.
• Discuss the American Academy of Pediatrics clinical practice guideline for evaluation, diagnosis, treatment, and monitoring of children and adolescents with ADHD.
• Describe the classes of medications available to treat ADHD, the indications for each, and the forms available (eg, short- versus long-acting, tablets, capsules, patches, liquids).
• Identify environmental factors at home and in school that can affect a child’s ADHD and know how to address them.
• Understand and provide support for the parent’s role in managing a child’s ADHD.
FACULTY
Amy Chandler is a family nurse practitioner at Peninsula Surgical Group in Salisbury, Maryland. Mary Parsons is an Assistant Professor at the University of Maryland School of Nursing in Baltimore. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.25 hours of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of January 2015.
Article begins on next page >>
Hyperactivity, impulsivity, disruptive behavior, lack of focus—the symptoms of attention-deficit/hyperactivity disorder can negatively impact children and their families. Primary care providers have the opportunity to bring a semblance of order to chaos, and improve quality of life for all involved, by identifying affected patients and providing effective treatment options. Here is everything you need to know.
Attention-deficit/hyperactivity disorder (ADHD) is a diagnosis on the rise. In 2011, approximately 11% of school-aged children had ever been diagnosed with ADHD—an increase of 2 million children since 2003.1 Another analysis found that, while more children ages 8 to 15 years were diagnosed with ADHD than with any other mental health disorder, only about half had received treatment for it.2 Since the majority of children—65% to 85%—are diagnosed in the primary care setting,3 there is a clear need for primary care clinicians to be up-to-date on the diagnosis and treatment of this disorder.
To enhance both evidence-based practice and quality outcomes for the child and family dealing with this chronic and challenging disorder, an overview of the latest information about ADHD is presented here. In addition to diagnostic criteria, clinical presentation, screening methods, and treatment options, a discussion of the parental role in ADHD management and a medication reference guide are also provided (the latter to facilitate appropriate selection of initial pharmacologic therapy).
INTRODUCTION
ADHD is characterized by persistent patterns of hyperactivity-impulsivity and/or inattention4 and is diagnosed about twice as often in boys as in girls.2 The hyperactive-impulsive form usually manifests before age 7, while the inattentive form may not be apparent until age 8 or 9.5 Although symptoms may wane with maturity, they persist into adulthood for about 50% of patients.6
ADHD can impair academic performance, disrupt familial and interpersonal relationships, and lead to social isolation and low self-esteem.4 Clinicians should be mindful of the potential negative long-term effects of ADHD, which include increased risk for substance abuse and other antisocial activities and fewer vocational opportunities.7
The economic impact of ADHD is also significant. An often-cited 2007 cost-of-illness analysis estimated a minimum annual societal cost of $42.5 billion for pediatric ADHD. A more likely estimate is twice that and highlights the public health importance of ADHD to health care practitioners, families, and society.8
ETIOLOGY
Currently, a single cause for ADHD has not been established, although research supports a genetic basis, with secondary factors (eg, environmental influences) also involved.9 Ongoing studies have identified numerous genes that contribute to ADHD.10 The disorder has also been seen in children with brain damage, including perinatal brain damage, fetal alcohol syndrome, and Down syndrome.11
MRI studies have shown brain patterns that link ADHD with decreased functioning in the cingulo-frontal-parietal (CFP) cognitive-attention network. Specifically, impairment in the dorsal anterior midcingulate cortex, termed the daMCC, is responsible for inappropriate or excessive motor behavior, while alterations in the dorsolateral prefrontal cortex, or DLPFC, have adverse effects on the ability to think ahead, plan, and reason.12 With this knowledge, clinicians can choose medications that target specific areas of the brain, if appropriate.
Environmental influences
Two factors hypothesized to influence ADHD symptoms are nutrition and the home environment. Many studies have examined the effects of refined sugar, additives, and preservatives on ADHD symptoms. Results indicate that, while monitoring a child’s intake of these ingredients may be beneficial from a nutritional standpoint, it is unlikely that they significantly affect ADHD symptoms.10,13,14
Given ADHD’s genetic component, parents may have ADHD themselves—making it more difficult for them to provide consistency and structure in the home environment.15,16 Chaotic living situations can exacerbate ADHD symptoms,11 and research indicates that mothers with ADHD are more likely to engage in negative parenting, with higher demands and little praise, than mothers without the disorder.15 They may exhibit less patience and feel the need to control the child’s environment, even during playtime.17 Preschool hyperactivity has been identified in children whose parents exhibit coercive, overstimulating, negative, or inconsistent parenting.18
Next page: Making the Diagnosis >>
MAKING THE DIAGNOSIS
Presenting behaviors
Symptoms of ADHD typically manifest in academic settings. The greater frequency of ADHD diagnosis and treatment in boys may derive from differences in how the genders externalize behaviors.19 While boys often exhibit more impulsive and aggressive behavior, girls internalize symptoms and are more likely to be inattentive. As a result, girls are less likely to be disruptive in the classroom and may not be referred for evaluation. Boys, on the other hand, may be overdiagnosed due to their disruptive behaviors.19 Daley indicates that inattention (eg, daydreaming) and hyperactivity (eg, fidgeting) symptoms become more apparent in structured settings, such as a classroom.15
Any child who requires a mental health assessment should also be screened for ADHD; if the results of the screening suggest ADHD, a full evaluation is warranted.20
Diagnostic criteria
According to the American Psychiatric Association’s criteria—outlined in the Diagnostic and Statistical Manual of Mental Disorders–IV (Text Revision) (DSM-IV-TR) and the subsequent DSM-5—the diagnosis of ADHD requires that the patient have at least six symptoms of inattention, hyperactivity, and/or impulsivity.4,21,22 For patients ages 17 or older, the DSM-5 requires five symptoms of inattention or hyperactivity-impulsivity.21
Children are usually unable to complete one task prior to starting another and are easily distracted by noises otherwise ignored by others (eg, lawnmowing, background conversation).4 They may also exhibit excessive fidgeting and interrupt formal conversations or lectures.4 Inappropriate behavior relative to the child’s developmental level must have been present for at least six months prior to age 7 (according to DSM-IV-TR)4 or age 12 (DSM-5).21
In addition to exhibiting the above symptoms for a minimum of six months, impairment must be observed in at least two settings (home, school, or work), with obvious clinical impairment in social, academic, or occupational functioning.4 ADHD symptoms can vary depending on the setting; the child may have more difficulty paying attention and following directions in a classroom filled with children than in an environment with less stimuli (eg, one-on-one home situations).11
Children who exhibit ADHD symptoms in only one setting may actually have problems secondary to cognition, emotional maturity, or feelings of well-being in a particular setting.11 Therefore, the clinician must rely on multiple sources of information (eg, parents, teachers, other caregivers) in order to make the diagnosis of ADHD.23 A teacher should have had regular contact with the child for at least four to six months in order to provide an accurate evaluation of symptoms and their persistence.5
Assessment scales
Validated parent and teacher instruments, along with the DSM-IV-TR criteria, can be used to obtain data to support a diagnosis of ADHD.5,24
The Conners Comprehensive Behavior Rating Scales and the ADHD Rating Scale IV (DSM-IV) can be used by teachers and parents to document behaviors of preschool-aged children.5
The Vanderbilt Assessment Scales, which have been validated in both primary care and referral settings, may be used for children older than 4.5
More information about rating scales is available at the National Resource Center on ADHD website (www.help4adhd.org/en/treatment/scales).
In addition to parent- or teacher-reported data, further evidence of functional impairment may be gleaned through a review of report cards, standardized testing, and school records (eg, attendance or disciplinary actions).24
Differential diagnosis
The differential diagnosis should include other mental health conditions, sleep disorders, and any possible underlying medical or developmental problems.24 Hyperthyroidism and lead toxicity, as examples, could elicit symptoms consistent with ADHD.11 When the diagnosis is in doubt, coexisting conditions are present, or treatment options are in question, the primary care clinician should refer the patient to a pediatric or mental health specialist for further evaluation.23
BEFORE TREATMENT
After the diagnosis is made but before treatment commences, a complete review of systems, physical and psychologic evaluation, cardiac and sleep history, thorough family history pertaining to mental health and medical conditions, and an age-appropriate interview are required.24 The exam results assist in identifying other concerns that would indicate the need for appropriate diagnostic testing and/or referral (see Table 1).
Although stimulant treatment for ADHD has not been shown to increase cardiovascular events in otherwise healthy young people,25 experts differ in their opinions about the need for screening ECG prior to treatment initiation. The American Heart Association recommends a screening ECG before stimulant therapy is initiated,26 while the American Academy of Pediatrics (AAP) does not, as long as the history and exam results yield no cardiac findings.27 Since the Prescribing Information for most ADHD medications cautions against use in patients with known serious cardiac abnormalities, if exam findings suggest the possibility of cardiovascular disease, further evaluation with ECG and referral to a pediatric cardiologist are indicated.26,28
In the interview, the clinician should explore the patient’s perception of his or her behavior in response to family and social relationships and academic performance (eg, parent-teacher communication, report cards, detentions, suspensions). The psychosocial history may provide an explanation for acute onset of symptoms or coexisting disorders. Family history provides valuable insight regarding the health and cognitive abilities of family members, as well as the possibility of genetic influences on the child.24
Parents should be advised that ADHD is a chronic condition that requires a long-term treatment approach for symptoms that may last into adulthood. Identification of goals and treatment success depend on collaborative efforts with parents, teachers, clinicians, caregivers, and mental health clinicians.24
Continue for parents and ADHD >>
PARENTS AND ADHD
Stress
Caregivers for a child with ADHD are burdened with the ongoing challenges of the child’s inability to follow rules and his or her continual struggles with academics and peer relationships.29 These hardships stress parents’ patience, often resulting in parental impulsive reactivity (eg, physical punishment of the child).
In addition, social isolation for both children and parents is common because of the judgmental scrutiny of other parents whose children are not affected by ADHD.30 Parents’ career and social activities may be interrupted, adding to the sense of guilt, blame, burnout, and depression often associated with parenting a child with ADHD.29
While some parenting stress is expected, those who are unable to cope with everyday events are more likely to experience conflict and anger, further increasing the child’s anxiety and emotional state.31 This dysfunctional environment prohibits the adoption of positive parenting techniques and ultimately contributes to more psychologic distress and harm to the parent-child relationship.31
Support
Once a child is diagnosed with ADHD, parents require adequate education to bolster their understanding of how to manage their child’s symptoms.29 Establishing effective support systems—whether a spouse, family member, friend, or another parent whose child has ADHD—is imperative to enable parents to cope with the stress and to promote positive parenting, which has been shown to decrease symptomatic behavior.32
Furthermore, understanding the individual child’s traits will help the parents channel his or her energy into personal areas of interest, such as sports or creative outlets (art, dance, theater).32
Training
Parenting styles may play a role in the management of a child’s ADHD.9,11 Encouraging parents to learn how to change their responses to their child’s behavior through evidence-based behavioral training can be one of the most effective interventions for both parents and children.33 Parents who participate in this training gain greater behavioral understanding and treatment satisfaction, and their children experience significant improvement in conduct and other symptoms.23
TREATMENT OPTIONS
Choice of treatment depends on patient age, the severity of functional impairment, and the individual needs of the child. Treatment may include behavioral therapy, medication administration, or a combination of the two.23
Behavioral therapy
Behavioral therapists can provide parents and teachers with evidence-based training to understand and manage ADHD conduct.34 Parents and teachers are taught to recognize the effects of environmental factors on behavior and then to modify environments and daily schedules and set appropriate limits. They learn how to reinforce positive behavior, identify triggers, and decrease inappropriate behavior, using calm disciplinary approaches that lead to positive parent-child and teacher-student relationships.23,34
Collaborative efforts between parents and teachers are crucial to the child’s treatment plan, and individualized educational plans should be implemented to enhance academic performance, social skills, and self-esteem. For example, teachers can send home daily report cards to assist parents with monitoring core symptoms and treatment efficacy.35
Behavioral therapy classes for both parent and child, or for parents only, usually meet weekly for eight to 20 weeks. Parents learn how to build positive relationships, set limits, and respond consistently with rewards or punishments (eg, consequences, time-outs). Less effective outcomes were noted when such interventions were tried by parents without adequate training.14,24,33
Medications have been found to be more effective to treat ADHD symptoms than behavioral therapy alone, and parents and teachers report higher satisfaction with treatment plans that combine behavior modification with pharmacologic therapy.23
Next: Medication recommendations >>
MEDICATION RECOMMENDATIONS
AAP medication recommendations are age-specific and should be used only if the patient meets DSM-IV-TR criteria.24 An overview of both stimulant and nonstimulant treatment options for ADHD is provided in the Figure.
When an ADHD treatment regimen does not result in improved symptoms and functioning, the clinician should revisit the treatment plan and differential diagnosis. After reevaluation, the clinician may choose to add or change medication type or dose, adjust behavioral therapy, or consider the possibility of language or learning disabilities, mental health disorders, other psychosocial stressors, or poor adherence to the treatment plan.24
Parents may refuse stimulant therapy for their child, fearing future drug use (or abuse) in adolescence.24 Clinicians should respect parents’ decisions to defer stimulant therapy, while at the same time clarifying concerns or misunderstandings in order to provide optimal care for the child.24 Studies have demonstrated that stimulant treatment for ADHD has a protective effect, with outcomes suggesting significantly decreased risk for drug or alcohol dependence.36
Clinician-provided anticipatory education about dose titration, expected treatment results, and potential adverse effects will help prepare parents for the ADHD medication management process.23 The length of time needed for dose titration varies by class of ADHD medication (see Table 2). For stimulants, effects on core symptoms may be seen within the first week. Nonstimulants take longer: up to six weeks for atomoxetine and up to four weeks for the a2-adrenergic agonists.24
Stimulants
Evidence strongly supports stimulants as first-line treatment for ADHD in children ages 6 and older.23 Stimulants are safe and efficacious in reducing the core symptoms of inattention, hyperactivity, and impulsivity.23 The initial choice of stimulant depends on the individual child’s needs. It should be noted that, although decreased growth rates have been observed in children taking stimulants, this effect ends by the third year of treatment.38 If maximum doses are reached and desired effects are not achieved with the first stimulant, choosing an alternate drug in the stimulant class may be an effective option.24 Clinicians may also consider other drug classes based on the comprehensive exam, adverse effects profile, contraindications, and individual responses.
Nonstimulants
Nonstimulant medications include atomoxetine and the a2-adrenergic agonists extended-release clonidine and extended-release guanfacine.24
Atomoxetine. Atomoxetine is a selective norepinephrine-reuptake inhibitor that may be prescribed if substance abuse or diversion is suspected or if stimulant therapy is contraindicated, undesirable, or unsuccessful.24,39 Using weight-based dosing for titration,37 the first week of therapy should begin with a half-dose to minimize sedation or gastrointestinal symptoms.24 While atomoxetine can be quite effective, caution should be used when prescribing this agent, as it carries a black box warning for suicidal ideation.24
α2-Adrenergic agonists. Both extended-release clonidine and extended-release guanfacine are α2-adrenergic agonists that may be beneficial when stimulants and atomoxetine have failed or when there are coexisting conditions.37 Either clonidine or guanfacine can be given in combination with a stimulant, if stimulant or atomoxetine treatment alone is unsuccessful or if adverse effects are unacceptable.24,39 Sedative effects and withdrawal irritability are more common with clonidine than with guanfacine, and when discontinued, these medications should be tapered to avoid blood pressure changes.24,40
Age-specific recommendations
Medications should be selected based on age ranges. In preschool-age children (ages 4 to 5), methylphenidate is recommended when target behaviors and impaired functioning persist for nine months in both home and day care/school settings, despite behavioral therapy.23,24 As examples, medication may be warranted if the child poses a significant risk of injury to other children or caregivers and/or faces expulsion from preschool or day care because of his or her behaviors.34
Risks and benefits should be considered carefully before medical therapy is initiated in this group. Further, the lowest possible starting dose of methylphenidate is preferred for preschoolers due to their slower metabolism.24 While dextroamphetamine is FDA approved for children younger than 6, efficacy and safety have not been proven in this age group and the AAP does not recommend it.23
For children ages 6 to 18, the AAP recommends combined treatment with medication and evidence-based behavioral training for the patient, parent, and teacher. In the adolescent population, evidence supports use of stimulants as first-line therapy, but with close monitoring for medication misuse.23 Misuse may become apparent through increased prescription refills; if identified, ADHD medication should be discontinued and substance abuse treatment initiated before ADHD therapy recommences.24
Continue for dosing and special considerations >>
Dosing and special considerations
Initiation of ADHD medication is usually begun over the weekend to allow parents time to observe for adverse effects, to better manage the drug titration process, and to minimize academic disruptions.41 Although more expensive, the extended-release formulas are often selected to eliminate the need for multiple doses and to decrease perceived stigma associated with medication administration at school.24 Longer-acting formulations have also been shown to increase treatment adherence. Adding shorter-acting doses in late afternoon, however, may be helpful if the child experiences difficulty concentrating (eg, on homework, sports, driving).24
Although drug holidays are not usually recommended, children can be evaluated for this individually. For example, a child whose ADHD symptoms primarily involve inattention may only require medication on school days.37
Follow-up
During the titration process, which can take up to three months, weekly clinician contacts—some by phone—to monitor effectiveness, adherence, and adverse effects are recommended (see Table 3). Blood pressure and heart rate should be monitored carefully and titration continued as needed until optimal response to treatment has been achieved.24 Monitoring should continue every three months for the first year of treatment and biannually thereafter.24 After several years, it is reasonable to consider a closely monitored drug-free trial period to determine if medication is still necessary.24 Finally, clinicians should remain cognizant of the potential cardiovascular effects of longer-term stimulant use and adhere to clinical guidelines.42
If coexisting conditions such as severe mood or anxiety disorders are present and improvement is not noted in core ADHD symptoms after three months of treatment, the patient should be referred to a mental health specialist.23
CONCLUSION
Primary care providers must be knowledgeable about ADHD and offer appropriate evidence-based interventions, treatments, and supportive measures to patients and families dealing with this disorder. Clinicians should know what resources are available for parents of children with ADHD. Psychoeducation for parents, families, and teachers is imperative to ensure positive home and school environments. Each treatment plan requires an individual approach, and optimal use of all available resources is desirable. Implementing evidence-based recommendations will increase the overall quality of life for children and families dealing with ADHD and optimize behavioral outcomes.
The authors would like to thank Laurie Rockelli, PhD, RN, PMHCNS-BC, and William Campbell, EdD, RN, for their revisions and support in the completion of this article.
1. Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.
2. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
3. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85(9):890-896.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
5. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: clinical features and evaluation. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-clinical-features-and-evaluation?source=see_link. Accessed December 15, 2014.
6. Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122(5): 97-109.
7. Danckaerts M, Sonuga-Barke E, Coghill D, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83-105.
8. Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambul Pediatr. 2007;7(1 suppl):121-131.
9. Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD). Child Psychiatry Hum Dev. 2010;41(2):168-192.
10. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: epidemiology and pathogenesis. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-epidemiology-and-pathogenesis?source=see_link. Accessed December 15, 2014.
11. Marcdante KJ, Kliegman RM, Jenson HB, Behrman RE. Attention-deficit/hyperactivity disorder. In: Merrit J, Cicalese B, eds. Nelson Essentials of Pediatrics. 6th ed. Philadelphia, PA: Saunders Elsevier; 2011:50-52.
12. Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160-1167.
13. CDC. Facts about ADHD. www.cdc.gov/NCBDDD/adhd/facts.html. Accessed December 15, 2014.
14. Rajwan E, Chacko A, Moeller M. Nonpharmacological interventions for preschool ADHD: state of the evidence and implications for practice. Prof Psychol Res Pr. 2012;43(5):520-526.
15. Daley D. Attention deficit hyperactivity disorder: a review of the essential facts. Child Care Health Dev. 2006;32(2):193-204.
16. van der Oord S, Bögels S, Peijnenburg D. The effectiveness of mindfulness training for children with ADHD and mindful parenting for their parents. J Child Fam Stud. 2012;21(1):139-147.
17. Zisser AR, Eyberg SM. Maternal ADHD: parent-child interactions and relations with child disruptive behavior. Child Fam Behavior Ther. 2012;34(1):33-52.
18. Daley D, Jones K, Hutchings J, Thompson M. Attention deficit hyperactivity disorder in pre-school children: current findings, recommended interventions and future directions. Child Care Health Dev. 2009;35(6):754-766.
19. Bruchmüller K, Margraf J, Schneider S. Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J Consult Clin Psychol. 2012;80(1):128-138.
20. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
22. American Psychiatric Association. Highlights of Changes from DSM-IV-TR to DSM-5. www.dsm5.org/Documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf. Accessed December 15, 2014.
23. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
24. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Supplemental information. Implementing the key action statements: an algorithm and explanation for process of care for the evaluation, diagnosis, treatment, and monitoring of ADHD in children and adolescents. http://pediatrics.aappublications.org/content/suppl/2011/10/11/peds.2011-2654.DC1/zpe611117822p.pdf. Accessed December 15, 2014.
25. Olfson M, Huang C, Gerhard T, et al. Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(2):147-156.
26. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
27. Perrin JM, Friedman RA, Knilans TK, et al. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
28. Berger S. Cardiac evaluation of patients receiving pharmacotherapy for attention deficit hyperactivity disorder. www.uptodate.com/contents/cardiac-evaluation-of-patients-receiving-pharmacotherapy-for-attention-deficit-hyperactivity-disorder?source=see_link. Accessed December 15, 2014.
29. Ho SC, Chien WT, Wang L. Parents’ perceptions of care-giving to a child with attention deficit hyperactivity disorder: an exploratory study. Contemp Nurse. 2011;40(1):41-56.
30. Moen ØL, Hall-Lord ML, Hedelin B. Contending and adapting every day: Norwegian parents’ lived experience of having a child with ADHD. J Fam Nursing. 2011;17(4):441-462.
31. Theule J, Wiener J, Rogers M, Marton I. Predicting parenting stress in families of children with ADHD: parent and contextual factors. J Child Fam Stud. 2011;20(5):640-647.
32. Brown RP, Gerbarg PL. Non-Drug Treatments for ADHD. New York, NY: Norton & Company; 2012:5-26.
33. Jones K, Daley D, Hutchings J, et al. Efficacy of the Incredible Years Basic parent training programme as an early intervention for children with conduct problems and ADHD. Child Care Health Dev. 2007;33(6):749-756.
34. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: overview of treatment and prognosis. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-overview-of-treatment-and-prognosis?source=see_link. Accessed December 15, 2014.
35. Fabiano GA, Vujnovic RK, Pelham WE, et al. Enhancing the effectiveness of special education programming for children with attention deficit hyperactivity disorder using a daily report card. School Psychol Rev. 2010;39(2):219-239.
36. Wilens TE, Faraone SV, Biederman J, Gunawardene, S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003; 111(1):179-185.
37. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: treatment with medications. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-treatment-with-medications?source=see_link. Accessed November 10, 2014.
38. Swanson JM, Elliott GR, Greenhill LL, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1014–1026.
39. Krull KR. Pharmacology of drugs used to treat attention deficit hyperactivity disorder in children and adolescents. www.uptodate.com/contents/pharmacology-of-drugs-used-to-treat-attention-deficit-hyperactivity-disorder-in-children-and-adolescents?source=see_link. Accessed December 15, 2014.
40. Scahill L. Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs. 2009;23(suppl 1):43-49.
41. Krull KR. Patient information: treatment of attention deficit hyperactivity disorder in children (beyond the basics). www.uptodate.com/contents/treatment-of-attention-deficit-hyperactivity-disorder-in-children-beyond-the-basics?source=related_link. Accessed December 15, 2014.
42. Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50(10):978-990.
CE/CME No: CR-1501
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the attention-deficit/hyperactivity disorder (ADHD) diagnostic criteria of inattention, hyperactivity, and/or impulsivity as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR), and the DSM-5 update.
• Discuss the American Academy of Pediatrics clinical practice guideline for evaluation, diagnosis, treatment, and monitoring of children and adolescents with ADHD.
• Describe the classes of medications available to treat ADHD, the indications for each, and the forms available (eg, short- versus long-acting, tablets, capsules, patches, liquids).
• Identify environmental factors at home and in school that can affect a child’s ADHD and know how to address them.
• Understand and provide support for the parent’s role in managing a child’s ADHD.
FACULTY
Amy Chandler is a family nurse practitioner at Peninsula Surgical Group in Salisbury, Maryland. Mary Parsons is an Assistant Professor at the University of Maryland School of Nursing in Baltimore. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.25 hours of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of January 2015.
Article begins on next page >>
Hyperactivity, impulsivity, disruptive behavior, lack of focus—the symptoms of attention-deficit/hyperactivity disorder can negatively impact children and their families. Primary care providers have the opportunity to bring a semblance of order to chaos, and improve quality of life for all involved, by identifying affected patients and providing effective treatment options. Here is everything you need to know.
Attention-deficit/hyperactivity disorder (ADHD) is a diagnosis on the rise. In 2011, approximately 11% of school-aged children had ever been diagnosed with ADHD—an increase of 2 million children since 2003.1 Another analysis found that, while more children ages 8 to 15 years were diagnosed with ADHD than with any other mental health disorder, only about half had received treatment for it.2 Since the majority of children—65% to 85%—are diagnosed in the primary care setting,3 there is a clear need for primary care clinicians to be up-to-date on the diagnosis and treatment of this disorder.
To enhance both evidence-based practice and quality outcomes for the child and family dealing with this chronic and challenging disorder, an overview of the latest information about ADHD is presented here. In addition to diagnostic criteria, clinical presentation, screening methods, and treatment options, a discussion of the parental role in ADHD management and a medication reference guide are also provided (the latter to facilitate appropriate selection of initial pharmacologic therapy).
INTRODUCTION
ADHD is characterized by persistent patterns of hyperactivity-impulsivity and/or inattention4 and is diagnosed about twice as often in boys as in girls.2 The hyperactive-impulsive form usually manifests before age 7, while the inattentive form may not be apparent until age 8 or 9.5 Although symptoms may wane with maturity, they persist into adulthood for about 50% of patients.6
ADHD can impair academic performance, disrupt familial and interpersonal relationships, and lead to social isolation and low self-esteem.4 Clinicians should be mindful of the potential negative long-term effects of ADHD, which include increased risk for substance abuse and other antisocial activities and fewer vocational opportunities.7
The economic impact of ADHD is also significant. An often-cited 2007 cost-of-illness analysis estimated a minimum annual societal cost of $42.5 billion for pediatric ADHD. A more likely estimate is twice that and highlights the public health importance of ADHD to health care practitioners, families, and society.8
ETIOLOGY
Currently, a single cause for ADHD has not been established, although research supports a genetic basis, with secondary factors (eg, environmental influences) also involved.9 Ongoing studies have identified numerous genes that contribute to ADHD.10 The disorder has also been seen in children with brain damage, including perinatal brain damage, fetal alcohol syndrome, and Down syndrome.11
MRI studies have shown brain patterns that link ADHD with decreased functioning in the cingulo-frontal-parietal (CFP) cognitive-attention network. Specifically, impairment in the dorsal anterior midcingulate cortex, termed the daMCC, is responsible for inappropriate or excessive motor behavior, while alterations in the dorsolateral prefrontal cortex, or DLPFC, have adverse effects on the ability to think ahead, plan, and reason.12 With this knowledge, clinicians can choose medications that target specific areas of the brain, if appropriate.
Environmental influences
Two factors hypothesized to influence ADHD symptoms are nutrition and the home environment. Many studies have examined the effects of refined sugar, additives, and preservatives on ADHD symptoms. Results indicate that, while monitoring a child’s intake of these ingredients may be beneficial from a nutritional standpoint, it is unlikely that they significantly affect ADHD symptoms.10,13,14
Given ADHD’s genetic component, parents may have ADHD themselves—making it more difficult for them to provide consistency and structure in the home environment.15,16 Chaotic living situations can exacerbate ADHD symptoms,11 and research indicates that mothers with ADHD are more likely to engage in negative parenting, with higher demands and little praise, than mothers without the disorder.15 They may exhibit less patience and feel the need to control the child’s environment, even during playtime.17 Preschool hyperactivity has been identified in children whose parents exhibit coercive, overstimulating, negative, or inconsistent parenting.18
Next page: Making the Diagnosis >>
MAKING THE DIAGNOSIS
Presenting behaviors
Symptoms of ADHD typically manifest in academic settings. The greater frequency of ADHD diagnosis and treatment in boys may derive from differences in how the genders externalize behaviors.19 While boys often exhibit more impulsive and aggressive behavior, girls internalize symptoms and are more likely to be inattentive. As a result, girls are less likely to be disruptive in the classroom and may not be referred for evaluation. Boys, on the other hand, may be overdiagnosed due to their disruptive behaviors.19 Daley indicates that inattention (eg, daydreaming) and hyperactivity (eg, fidgeting) symptoms become more apparent in structured settings, such as a classroom.15
Any child who requires a mental health assessment should also be screened for ADHD; if the results of the screening suggest ADHD, a full evaluation is warranted.20
Diagnostic criteria
According to the American Psychiatric Association’s criteria—outlined in the Diagnostic and Statistical Manual of Mental Disorders–IV (Text Revision) (DSM-IV-TR) and the subsequent DSM-5—the diagnosis of ADHD requires that the patient have at least six symptoms of inattention, hyperactivity, and/or impulsivity.4,21,22 For patients ages 17 or older, the DSM-5 requires five symptoms of inattention or hyperactivity-impulsivity.21
Children are usually unable to complete one task prior to starting another and are easily distracted by noises otherwise ignored by others (eg, lawnmowing, background conversation).4 They may also exhibit excessive fidgeting and interrupt formal conversations or lectures.4 Inappropriate behavior relative to the child’s developmental level must have been present for at least six months prior to age 7 (according to DSM-IV-TR)4 or age 12 (DSM-5).21
In addition to exhibiting the above symptoms for a minimum of six months, impairment must be observed in at least two settings (home, school, or work), with obvious clinical impairment in social, academic, or occupational functioning.4 ADHD symptoms can vary depending on the setting; the child may have more difficulty paying attention and following directions in a classroom filled with children than in an environment with less stimuli (eg, one-on-one home situations).11
Children who exhibit ADHD symptoms in only one setting may actually have problems secondary to cognition, emotional maturity, or feelings of well-being in a particular setting.11 Therefore, the clinician must rely on multiple sources of information (eg, parents, teachers, other caregivers) in order to make the diagnosis of ADHD.23 A teacher should have had regular contact with the child for at least four to six months in order to provide an accurate evaluation of symptoms and their persistence.5
Assessment scales
Validated parent and teacher instruments, along with the DSM-IV-TR criteria, can be used to obtain data to support a diagnosis of ADHD.5,24
The Conners Comprehensive Behavior Rating Scales and the ADHD Rating Scale IV (DSM-IV) can be used by teachers and parents to document behaviors of preschool-aged children.5
The Vanderbilt Assessment Scales, which have been validated in both primary care and referral settings, may be used for children older than 4.5
More information about rating scales is available at the National Resource Center on ADHD website (www.help4adhd.org/en/treatment/scales).
In addition to parent- or teacher-reported data, further evidence of functional impairment may be gleaned through a review of report cards, standardized testing, and school records (eg, attendance or disciplinary actions).24
Differential diagnosis
The differential diagnosis should include other mental health conditions, sleep disorders, and any possible underlying medical or developmental problems.24 Hyperthyroidism and lead toxicity, as examples, could elicit symptoms consistent with ADHD.11 When the diagnosis is in doubt, coexisting conditions are present, or treatment options are in question, the primary care clinician should refer the patient to a pediatric or mental health specialist for further evaluation.23
BEFORE TREATMENT
After the diagnosis is made but before treatment commences, a complete review of systems, physical and psychologic evaluation, cardiac and sleep history, thorough family history pertaining to mental health and medical conditions, and an age-appropriate interview are required.24 The exam results assist in identifying other concerns that would indicate the need for appropriate diagnostic testing and/or referral (see Table 1).
Although stimulant treatment for ADHD has not been shown to increase cardiovascular events in otherwise healthy young people,25 experts differ in their opinions about the need for screening ECG prior to treatment initiation. The American Heart Association recommends a screening ECG before stimulant therapy is initiated,26 while the American Academy of Pediatrics (AAP) does not, as long as the history and exam results yield no cardiac findings.27 Since the Prescribing Information for most ADHD medications cautions against use in patients with known serious cardiac abnormalities, if exam findings suggest the possibility of cardiovascular disease, further evaluation with ECG and referral to a pediatric cardiologist are indicated.26,28
In the interview, the clinician should explore the patient’s perception of his or her behavior in response to family and social relationships and academic performance (eg, parent-teacher communication, report cards, detentions, suspensions). The psychosocial history may provide an explanation for acute onset of symptoms or coexisting disorders. Family history provides valuable insight regarding the health and cognitive abilities of family members, as well as the possibility of genetic influences on the child.24
Parents should be advised that ADHD is a chronic condition that requires a long-term treatment approach for symptoms that may last into adulthood. Identification of goals and treatment success depend on collaborative efforts with parents, teachers, clinicians, caregivers, and mental health clinicians.24
Continue for parents and ADHD >>
PARENTS AND ADHD
Stress
Caregivers for a child with ADHD are burdened with the ongoing challenges of the child’s inability to follow rules and his or her continual struggles with academics and peer relationships.29 These hardships stress parents’ patience, often resulting in parental impulsive reactivity (eg, physical punishment of the child).
In addition, social isolation for both children and parents is common because of the judgmental scrutiny of other parents whose children are not affected by ADHD.30 Parents’ career and social activities may be interrupted, adding to the sense of guilt, blame, burnout, and depression often associated with parenting a child with ADHD.29
While some parenting stress is expected, those who are unable to cope with everyday events are more likely to experience conflict and anger, further increasing the child’s anxiety and emotional state.31 This dysfunctional environment prohibits the adoption of positive parenting techniques and ultimately contributes to more psychologic distress and harm to the parent-child relationship.31
Support
Once a child is diagnosed with ADHD, parents require adequate education to bolster their understanding of how to manage their child’s symptoms.29 Establishing effective support systems—whether a spouse, family member, friend, or another parent whose child has ADHD—is imperative to enable parents to cope with the stress and to promote positive parenting, which has been shown to decrease symptomatic behavior.32
Furthermore, understanding the individual child’s traits will help the parents channel his or her energy into personal areas of interest, such as sports or creative outlets (art, dance, theater).32
Training
Parenting styles may play a role in the management of a child’s ADHD.9,11 Encouraging parents to learn how to change their responses to their child’s behavior through evidence-based behavioral training can be one of the most effective interventions for both parents and children.33 Parents who participate in this training gain greater behavioral understanding and treatment satisfaction, and their children experience significant improvement in conduct and other symptoms.23
TREATMENT OPTIONS
Choice of treatment depends on patient age, the severity of functional impairment, and the individual needs of the child. Treatment may include behavioral therapy, medication administration, or a combination of the two.23
Behavioral therapy
Behavioral therapists can provide parents and teachers with evidence-based training to understand and manage ADHD conduct.34 Parents and teachers are taught to recognize the effects of environmental factors on behavior and then to modify environments and daily schedules and set appropriate limits. They learn how to reinforce positive behavior, identify triggers, and decrease inappropriate behavior, using calm disciplinary approaches that lead to positive parent-child and teacher-student relationships.23,34
Collaborative efforts between parents and teachers are crucial to the child’s treatment plan, and individualized educational plans should be implemented to enhance academic performance, social skills, and self-esteem. For example, teachers can send home daily report cards to assist parents with monitoring core symptoms and treatment efficacy.35
Behavioral therapy classes for both parent and child, or for parents only, usually meet weekly for eight to 20 weeks. Parents learn how to build positive relationships, set limits, and respond consistently with rewards or punishments (eg, consequences, time-outs). Less effective outcomes were noted when such interventions were tried by parents without adequate training.14,24,33
Medications have been found to be more effective to treat ADHD symptoms than behavioral therapy alone, and parents and teachers report higher satisfaction with treatment plans that combine behavior modification with pharmacologic therapy.23
Next: Medication recommendations >>
MEDICATION RECOMMENDATIONS
AAP medication recommendations are age-specific and should be used only if the patient meets DSM-IV-TR criteria.24 An overview of both stimulant and nonstimulant treatment options for ADHD is provided in the Figure.
When an ADHD treatment regimen does not result in improved symptoms and functioning, the clinician should revisit the treatment plan and differential diagnosis. After reevaluation, the clinician may choose to add or change medication type or dose, adjust behavioral therapy, or consider the possibility of language or learning disabilities, mental health disorders, other psychosocial stressors, or poor adherence to the treatment plan.24
Parents may refuse stimulant therapy for their child, fearing future drug use (or abuse) in adolescence.24 Clinicians should respect parents’ decisions to defer stimulant therapy, while at the same time clarifying concerns or misunderstandings in order to provide optimal care for the child.24 Studies have demonstrated that stimulant treatment for ADHD has a protective effect, with outcomes suggesting significantly decreased risk for drug or alcohol dependence.36
Clinician-provided anticipatory education about dose titration, expected treatment results, and potential adverse effects will help prepare parents for the ADHD medication management process.23 The length of time needed for dose titration varies by class of ADHD medication (see Table 2). For stimulants, effects on core symptoms may be seen within the first week. Nonstimulants take longer: up to six weeks for atomoxetine and up to four weeks for the a2-adrenergic agonists.24
Stimulants
Evidence strongly supports stimulants as first-line treatment for ADHD in children ages 6 and older.23 Stimulants are safe and efficacious in reducing the core symptoms of inattention, hyperactivity, and impulsivity.23 The initial choice of stimulant depends on the individual child’s needs. It should be noted that, although decreased growth rates have been observed in children taking stimulants, this effect ends by the third year of treatment.38 If maximum doses are reached and desired effects are not achieved with the first stimulant, choosing an alternate drug in the stimulant class may be an effective option.24 Clinicians may also consider other drug classes based on the comprehensive exam, adverse effects profile, contraindications, and individual responses.
Nonstimulants
Nonstimulant medications include atomoxetine and the a2-adrenergic agonists extended-release clonidine and extended-release guanfacine.24
Atomoxetine. Atomoxetine is a selective norepinephrine-reuptake inhibitor that may be prescribed if substance abuse or diversion is suspected or if stimulant therapy is contraindicated, undesirable, or unsuccessful.24,39 Using weight-based dosing for titration,37 the first week of therapy should begin with a half-dose to minimize sedation or gastrointestinal symptoms.24 While atomoxetine can be quite effective, caution should be used when prescribing this agent, as it carries a black box warning for suicidal ideation.24
α2-Adrenergic agonists. Both extended-release clonidine and extended-release guanfacine are α2-adrenergic agonists that may be beneficial when stimulants and atomoxetine have failed or when there are coexisting conditions.37 Either clonidine or guanfacine can be given in combination with a stimulant, if stimulant or atomoxetine treatment alone is unsuccessful or if adverse effects are unacceptable.24,39 Sedative effects and withdrawal irritability are more common with clonidine than with guanfacine, and when discontinued, these medications should be tapered to avoid blood pressure changes.24,40
Age-specific recommendations
Medications should be selected based on age ranges. In preschool-age children (ages 4 to 5), methylphenidate is recommended when target behaviors and impaired functioning persist for nine months in both home and day care/school settings, despite behavioral therapy.23,24 As examples, medication may be warranted if the child poses a significant risk of injury to other children or caregivers and/or faces expulsion from preschool or day care because of his or her behaviors.34
Risks and benefits should be considered carefully before medical therapy is initiated in this group. Further, the lowest possible starting dose of methylphenidate is preferred for preschoolers due to their slower metabolism.24 While dextroamphetamine is FDA approved for children younger than 6, efficacy and safety have not been proven in this age group and the AAP does not recommend it.23
For children ages 6 to 18, the AAP recommends combined treatment with medication and evidence-based behavioral training for the patient, parent, and teacher. In the adolescent population, evidence supports use of stimulants as first-line therapy, but with close monitoring for medication misuse.23 Misuse may become apparent through increased prescription refills; if identified, ADHD medication should be discontinued and substance abuse treatment initiated before ADHD therapy recommences.24
Continue for dosing and special considerations >>
Dosing and special considerations
Initiation of ADHD medication is usually begun over the weekend to allow parents time to observe for adverse effects, to better manage the drug titration process, and to minimize academic disruptions.41 Although more expensive, the extended-release formulas are often selected to eliminate the need for multiple doses and to decrease perceived stigma associated with medication administration at school.24 Longer-acting formulations have also been shown to increase treatment adherence. Adding shorter-acting doses in late afternoon, however, may be helpful if the child experiences difficulty concentrating (eg, on homework, sports, driving).24
Although drug holidays are not usually recommended, children can be evaluated for this individually. For example, a child whose ADHD symptoms primarily involve inattention may only require medication on school days.37
Follow-up
During the titration process, which can take up to three months, weekly clinician contacts—some by phone—to monitor effectiveness, adherence, and adverse effects are recommended (see Table 3). Blood pressure and heart rate should be monitored carefully and titration continued as needed until optimal response to treatment has been achieved.24 Monitoring should continue every three months for the first year of treatment and biannually thereafter.24 After several years, it is reasonable to consider a closely monitored drug-free trial period to determine if medication is still necessary.24 Finally, clinicians should remain cognizant of the potential cardiovascular effects of longer-term stimulant use and adhere to clinical guidelines.42
If coexisting conditions such as severe mood or anxiety disorders are present and improvement is not noted in core ADHD symptoms after three months of treatment, the patient should be referred to a mental health specialist.23
CONCLUSION
Primary care providers must be knowledgeable about ADHD and offer appropriate evidence-based interventions, treatments, and supportive measures to patients and families dealing with this disorder. Clinicians should know what resources are available for parents of children with ADHD. Psychoeducation for parents, families, and teachers is imperative to ensure positive home and school environments. Each treatment plan requires an individual approach, and optimal use of all available resources is desirable. Implementing evidence-based recommendations will increase the overall quality of life for children and families dealing with ADHD and optimize behavioral outcomes.
The authors would like to thank Laurie Rockelli, PhD, RN, PMHCNS-BC, and William Campbell, EdD, RN, for their revisions and support in the completion of this article.
CE/CME No: CR-1501
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain the attention-deficit/hyperactivity disorder (ADHD) diagnostic criteria of inattention, hyperactivity, and/or impulsivity as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR), and the DSM-5 update.
• Discuss the American Academy of Pediatrics clinical practice guideline for evaluation, diagnosis, treatment, and monitoring of children and adolescents with ADHD.
• Describe the classes of medications available to treat ADHD, the indications for each, and the forms available (eg, short- versus long-acting, tablets, capsules, patches, liquids).
• Identify environmental factors at home and in school that can affect a child’s ADHD and know how to address them.
• Understand and provide support for the parent’s role in managing a child’s ADHD.
FACULTY
Amy Chandler is a family nurse practitioner at Peninsula Surgical Group in Salisbury, Maryland. Mary Parsons is an Assistant Professor at the University of Maryland School of Nursing in Baltimore. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.25 hours of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of January 2015.
Article begins on next page >>
Hyperactivity, impulsivity, disruptive behavior, lack of focus—the symptoms of attention-deficit/hyperactivity disorder can negatively impact children and their families. Primary care providers have the opportunity to bring a semblance of order to chaos, and improve quality of life for all involved, by identifying affected patients and providing effective treatment options. Here is everything you need to know.
Attention-deficit/hyperactivity disorder (ADHD) is a diagnosis on the rise. In 2011, approximately 11% of school-aged children had ever been diagnosed with ADHD—an increase of 2 million children since 2003.1 Another analysis found that, while more children ages 8 to 15 years were diagnosed with ADHD than with any other mental health disorder, only about half had received treatment for it.2 Since the majority of children—65% to 85%—are diagnosed in the primary care setting,3 there is a clear need for primary care clinicians to be up-to-date on the diagnosis and treatment of this disorder.
To enhance both evidence-based practice and quality outcomes for the child and family dealing with this chronic and challenging disorder, an overview of the latest information about ADHD is presented here. In addition to diagnostic criteria, clinical presentation, screening methods, and treatment options, a discussion of the parental role in ADHD management and a medication reference guide are also provided (the latter to facilitate appropriate selection of initial pharmacologic therapy).
INTRODUCTION
ADHD is characterized by persistent patterns of hyperactivity-impulsivity and/or inattention4 and is diagnosed about twice as often in boys as in girls.2 The hyperactive-impulsive form usually manifests before age 7, while the inattentive form may not be apparent until age 8 or 9.5 Although symptoms may wane with maturity, they persist into adulthood for about 50% of patients.6
ADHD can impair academic performance, disrupt familial and interpersonal relationships, and lead to social isolation and low self-esteem.4 Clinicians should be mindful of the potential negative long-term effects of ADHD, which include increased risk for substance abuse and other antisocial activities and fewer vocational opportunities.7
The economic impact of ADHD is also significant. An often-cited 2007 cost-of-illness analysis estimated a minimum annual societal cost of $42.5 billion for pediatric ADHD. A more likely estimate is twice that and highlights the public health importance of ADHD to health care practitioners, families, and society.8
ETIOLOGY
Currently, a single cause for ADHD has not been established, although research supports a genetic basis, with secondary factors (eg, environmental influences) also involved.9 Ongoing studies have identified numerous genes that contribute to ADHD.10 The disorder has also been seen in children with brain damage, including perinatal brain damage, fetal alcohol syndrome, and Down syndrome.11
MRI studies have shown brain patterns that link ADHD with decreased functioning in the cingulo-frontal-parietal (CFP) cognitive-attention network. Specifically, impairment in the dorsal anterior midcingulate cortex, termed the daMCC, is responsible for inappropriate or excessive motor behavior, while alterations in the dorsolateral prefrontal cortex, or DLPFC, have adverse effects on the ability to think ahead, plan, and reason.12 With this knowledge, clinicians can choose medications that target specific areas of the brain, if appropriate.
Environmental influences
Two factors hypothesized to influence ADHD symptoms are nutrition and the home environment. Many studies have examined the effects of refined sugar, additives, and preservatives on ADHD symptoms. Results indicate that, while monitoring a child’s intake of these ingredients may be beneficial from a nutritional standpoint, it is unlikely that they significantly affect ADHD symptoms.10,13,14
Given ADHD’s genetic component, parents may have ADHD themselves—making it more difficult for them to provide consistency and structure in the home environment.15,16 Chaotic living situations can exacerbate ADHD symptoms,11 and research indicates that mothers with ADHD are more likely to engage in negative parenting, with higher demands and little praise, than mothers without the disorder.15 They may exhibit less patience and feel the need to control the child’s environment, even during playtime.17 Preschool hyperactivity has been identified in children whose parents exhibit coercive, overstimulating, negative, or inconsistent parenting.18
Next page: Making the Diagnosis >>
MAKING THE DIAGNOSIS
Presenting behaviors
Symptoms of ADHD typically manifest in academic settings. The greater frequency of ADHD diagnosis and treatment in boys may derive from differences in how the genders externalize behaviors.19 While boys often exhibit more impulsive and aggressive behavior, girls internalize symptoms and are more likely to be inattentive. As a result, girls are less likely to be disruptive in the classroom and may not be referred for evaluation. Boys, on the other hand, may be overdiagnosed due to their disruptive behaviors.19 Daley indicates that inattention (eg, daydreaming) and hyperactivity (eg, fidgeting) symptoms become more apparent in structured settings, such as a classroom.15
Any child who requires a mental health assessment should also be screened for ADHD; if the results of the screening suggest ADHD, a full evaluation is warranted.20
Diagnostic criteria
According to the American Psychiatric Association’s criteria—outlined in the Diagnostic and Statistical Manual of Mental Disorders–IV (Text Revision) (DSM-IV-TR) and the subsequent DSM-5—the diagnosis of ADHD requires that the patient have at least six symptoms of inattention, hyperactivity, and/or impulsivity.4,21,22 For patients ages 17 or older, the DSM-5 requires five symptoms of inattention or hyperactivity-impulsivity.21
Children are usually unable to complete one task prior to starting another and are easily distracted by noises otherwise ignored by others (eg, lawnmowing, background conversation).4 They may also exhibit excessive fidgeting and interrupt formal conversations or lectures.4 Inappropriate behavior relative to the child’s developmental level must have been present for at least six months prior to age 7 (according to DSM-IV-TR)4 or age 12 (DSM-5).21
In addition to exhibiting the above symptoms for a minimum of six months, impairment must be observed in at least two settings (home, school, or work), with obvious clinical impairment in social, academic, or occupational functioning.4 ADHD symptoms can vary depending on the setting; the child may have more difficulty paying attention and following directions in a classroom filled with children than in an environment with less stimuli (eg, one-on-one home situations).11
Children who exhibit ADHD symptoms in only one setting may actually have problems secondary to cognition, emotional maturity, or feelings of well-being in a particular setting.11 Therefore, the clinician must rely on multiple sources of information (eg, parents, teachers, other caregivers) in order to make the diagnosis of ADHD.23 A teacher should have had regular contact with the child for at least four to six months in order to provide an accurate evaluation of symptoms and their persistence.5
Assessment scales
Validated parent and teacher instruments, along with the DSM-IV-TR criteria, can be used to obtain data to support a diagnosis of ADHD.5,24
The Conners Comprehensive Behavior Rating Scales and the ADHD Rating Scale IV (DSM-IV) can be used by teachers and parents to document behaviors of preschool-aged children.5
The Vanderbilt Assessment Scales, which have been validated in both primary care and referral settings, may be used for children older than 4.5
More information about rating scales is available at the National Resource Center on ADHD website (www.help4adhd.org/en/treatment/scales).
In addition to parent- or teacher-reported data, further evidence of functional impairment may be gleaned through a review of report cards, standardized testing, and school records (eg, attendance or disciplinary actions).24
Differential diagnosis
The differential diagnosis should include other mental health conditions, sleep disorders, and any possible underlying medical or developmental problems.24 Hyperthyroidism and lead toxicity, as examples, could elicit symptoms consistent with ADHD.11 When the diagnosis is in doubt, coexisting conditions are present, or treatment options are in question, the primary care clinician should refer the patient to a pediatric or mental health specialist for further evaluation.23
BEFORE TREATMENT
After the diagnosis is made but before treatment commences, a complete review of systems, physical and psychologic evaluation, cardiac and sleep history, thorough family history pertaining to mental health and medical conditions, and an age-appropriate interview are required.24 The exam results assist in identifying other concerns that would indicate the need for appropriate diagnostic testing and/or referral (see Table 1).
Although stimulant treatment for ADHD has not been shown to increase cardiovascular events in otherwise healthy young people,25 experts differ in their opinions about the need for screening ECG prior to treatment initiation. The American Heart Association recommends a screening ECG before stimulant therapy is initiated,26 while the American Academy of Pediatrics (AAP) does not, as long as the history and exam results yield no cardiac findings.27 Since the Prescribing Information for most ADHD medications cautions against use in patients with known serious cardiac abnormalities, if exam findings suggest the possibility of cardiovascular disease, further evaluation with ECG and referral to a pediatric cardiologist are indicated.26,28
In the interview, the clinician should explore the patient’s perception of his or her behavior in response to family and social relationships and academic performance (eg, parent-teacher communication, report cards, detentions, suspensions). The psychosocial history may provide an explanation for acute onset of symptoms or coexisting disorders. Family history provides valuable insight regarding the health and cognitive abilities of family members, as well as the possibility of genetic influences on the child.24
Parents should be advised that ADHD is a chronic condition that requires a long-term treatment approach for symptoms that may last into adulthood. Identification of goals and treatment success depend on collaborative efforts with parents, teachers, clinicians, caregivers, and mental health clinicians.24
Continue for parents and ADHD >>
PARENTS AND ADHD
Stress
Caregivers for a child with ADHD are burdened with the ongoing challenges of the child’s inability to follow rules and his or her continual struggles with academics and peer relationships.29 These hardships stress parents’ patience, often resulting in parental impulsive reactivity (eg, physical punishment of the child).
In addition, social isolation for both children and parents is common because of the judgmental scrutiny of other parents whose children are not affected by ADHD.30 Parents’ career and social activities may be interrupted, adding to the sense of guilt, blame, burnout, and depression often associated with parenting a child with ADHD.29
While some parenting stress is expected, those who are unable to cope with everyday events are more likely to experience conflict and anger, further increasing the child’s anxiety and emotional state.31 This dysfunctional environment prohibits the adoption of positive parenting techniques and ultimately contributes to more psychologic distress and harm to the parent-child relationship.31
Support
Once a child is diagnosed with ADHD, parents require adequate education to bolster their understanding of how to manage their child’s symptoms.29 Establishing effective support systems—whether a spouse, family member, friend, or another parent whose child has ADHD—is imperative to enable parents to cope with the stress and to promote positive parenting, which has been shown to decrease symptomatic behavior.32
Furthermore, understanding the individual child’s traits will help the parents channel his or her energy into personal areas of interest, such as sports or creative outlets (art, dance, theater).32
Training
Parenting styles may play a role in the management of a child’s ADHD.9,11 Encouraging parents to learn how to change their responses to their child’s behavior through evidence-based behavioral training can be one of the most effective interventions for both parents and children.33 Parents who participate in this training gain greater behavioral understanding and treatment satisfaction, and their children experience significant improvement in conduct and other symptoms.23
TREATMENT OPTIONS
Choice of treatment depends on patient age, the severity of functional impairment, and the individual needs of the child. Treatment may include behavioral therapy, medication administration, or a combination of the two.23
Behavioral therapy
Behavioral therapists can provide parents and teachers with evidence-based training to understand and manage ADHD conduct.34 Parents and teachers are taught to recognize the effects of environmental factors on behavior and then to modify environments and daily schedules and set appropriate limits. They learn how to reinforce positive behavior, identify triggers, and decrease inappropriate behavior, using calm disciplinary approaches that lead to positive parent-child and teacher-student relationships.23,34
Collaborative efforts between parents and teachers are crucial to the child’s treatment plan, and individualized educational plans should be implemented to enhance academic performance, social skills, and self-esteem. For example, teachers can send home daily report cards to assist parents with monitoring core symptoms and treatment efficacy.35
Behavioral therapy classes for both parent and child, or for parents only, usually meet weekly for eight to 20 weeks. Parents learn how to build positive relationships, set limits, and respond consistently with rewards or punishments (eg, consequences, time-outs). Less effective outcomes were noted when such interventions were tried by parents without adequate training.14,24,33
Medications have been found to be more effective to treat ADHD symptoms than behavioral therapy alone, and parents and teachers report higher satisfaction with treatment plans that combine behavior modification with pharmacologic therapy.23
Next: Medication recommendations >>
MEDICATION RECOMMENDATIONS
AAP medication recommendations are age-specific and should be used only if the patient meets DSM-IV-TR criteria.24 An overview of both stimulant and nonstimulant treatment options for ADHD is provided in the Figure.
When an ADHD treatment regimen does not result in improved symptoms and functioning, the clinician should revisit the treatment plan and differential diagnosis. After reevaluation, the clinician may choose to add or change medication type or dose, adjust behavioral therapy, or consider the possibility of language or learning disabilities, mental health disorders, other psychosocial stressors, or poor adherence to the treatment plan.24
Parents may refuse stimulant therapy for their child, fearing future drug use (or abuse) in adolescence.24 Clinicians should respect parents’ decisions to defer stimulant therapy, while at the same time clarifying concerns or misunderstandings in order to provide optimal care for the child.24 Studies have demonstrated that stimulant treatment for ADHD has a protective effect, with outcomes suggesting significantly decreased risk for drug or alcohol dependence.36
Clinician-provided anticipatory education about dose titration, expected treatment results, and potential adverse effects will help prepare parents for the ADHD medication management process.23 The length of time needed for dose titration varies by class of ADHD medication (see Table 2). For stimulants, effects on core symptoms may be seen within the first week. Nonstimulants take longer: up to six weeks for atomoxetine and up to four weeks for the a2-adrenergic agonists.24
Stimulants
Evidence strongly supports stimulants as first-line treatment for ADHD in children ages 6 and older.23 Stimulants are safe and efficacious in reducing the core symptoms of inattention, hyperactivity, and impulsivity.23 The initial choice of stimulant depends on the individual child’s needs. It should be noted that, although decreased growth rates have been observed in children taking stimulants, this effect ends by the third year of treatment.38 If maximum doses are reached and desired effects are not achieved with the first stimulant, choosing an alternate drug in the stimulant class may be an effective option.24 Clinicians may also consider other drug classes based on the comprehensive exam, adverse effects profile, contraindications, and individual responses.
Nonstimulants
Nonstimulant medications include atomoxetine and the a2-adrenergic agonists extended-release clonidine and extended-release guanfacine.24
Atomoxetine. Atomoxetine is a selective norepinephrine-reuptake inhibitor that may be prescribed if substance abuse or diversion is suspected or if stimulant therapy is contraindicated, undesirable, or unsuccessful.24,39 Using weight-based dosing for titration,37 the first week of therapy should begin with a half-dose to minimize sedation or gastrointestinal symptoms.24 While atomoxetine can be quite effective, caution should be used when prescribing this agent, as it carries a black box warning for suicidal ideation.24
α2-Adrenergic agonists. Both extended-release clonidine and extended-release guanfacine are α2-adrenergic agonists that may be beneficial when stimulants and atomoxetine have failed or when there are coexisting conditions.37 Either clonidine or guanfacine can be given in combination with a stimulant, if stimulant or atomoxetine treatment alone is unsuccessful or if adverse effects are unacceptable.24,39 Sedative effects and withdrawal irritability are more common with clonidine than with guanfacine, and when discontinued, these medications should be tapered to avoid blood pressure changes.24,40
Age-specific recommendations
Medications should be selected based on age ranges. In preschool-age children (ages 4 to 5), methylphenidate is recommended when target behaviors and impaired functioning persist for nine months in both home and day care/school settings, despite behavioral therapy.23,24 As examples, medication may be warranted if the child poses a significant risk of injury to other children or caregivers and/or faces expulsion from preschool or day care because of his or her behaviors.34
Risks and benefits should be considered carefully before medical therapy is initiated in this group. Further, the lowest possible starting dose of methylphenidate is preferred for preschoolers due to their slower metabolism.24 While dextroamphetamine is FDA approved for children younger than 6, efficacy and safety have not been proven in this age group and the AAP does not recommend it.23
For children ages 6 to 18, the AAP recommends combined treatment with medication and evidence-based behavioral training for the patient, parent, and teacher. In the adolescent population, evidence supports use of stimulants as first-line therapy, but with close monitoring for medication misuse.23 Misuse may become apparent through increased prescription refills; if identified, ADHD medication should be discontinued and substance abuse treatment initiated before ADHD therapy recommences.24
Continue for dosing and special considerations >>
Dosing and special considerations
Initiation of ADHD medication is usually begun over the weekend to allow parents time to observe for adverse effects, to better manage the drug titration process, and to minimize academic disruptions.41 Although more expensive, the extended-release formulas are often selected to eliminate the need for multiple doses and to decrease perceived stigma associated with medication administration at school.24 Longer-acting formulations have also been shown to increase treatment adherence. Adding shorter-acting doses in late afternoon, however, may be helpful if the child experiences difficulty concentrating (eg, on homework, sports, driving).24
Although drug holidays are not usually recommended, children can be evaluated for this individually. For example, a child whose ADHD symptoms primarily involve inattention may only require medication on school days.37
Follow-up
During the titration process, which can take up to three months, weekly clinician contacts—some by phone—to monitor effectiveness, adherence, and adverse effects are recommended (see Table 3). Blood pressure and heart rate should be monitored carefully and titration continued as needed until optimal response to treatment has been achieved.24 Monitoring should continue every three months for the first year of treatment and biannually thereafter.24 After several years, it is reasonable to consider a closely monitored drug-free trial period to determine if medication is still necessary.24 Finally, clinicians should remain cognizant of the potential cardiovascular effects of longer-term stimulant use and adhere to clinical guidelines.42
If coexisting conditions such as severe mood or anxiety disorders are present and improvement is not noted in core ADHD symptoms after three months of treatment, the patient should be referred to a mental health specialist.23
CONCLUSION
Primary care providers must be knowledgeable about ADHD and offer appropriate evidence-based interventions, treatments, and supportive measures to patients and families dealing with this disorder. Clinicians should know what resources are available for parents of children with ADHD. Psychoeducation for parents, families, and teachers is imperative to ensure positive home and school environments. Each treatment plan requires an individual approach, and optimal use of all available resources is desirable. Implementing evidence-based recommendations will increase the overall quality of life for children and families dealing with ADHD and optimize behavioral outcomes.
The authors would like to thank Laurie Rockelli, PhD, RN, PMHCNS-BC, and William Campbell, EdD, RN, for their revisions and support in the completion of this article.
1. Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.
2. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
3. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85(9):890-896.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
5. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: clinical features and evaluation. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-clinical-features-and-evaluation?source=see_link. Accessed December 15, 2014.
6. Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122(5): 97-109.
7. Danckaerts M, Sonuga-Barke E, Coghill D, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83-105.
8. Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambul Pediatr. 2007;7(1 suppl):121-131.
9. Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD). Child Psychiatry Hum Dev. 2010;41(2):168-192.
10. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: epidemiology and pathogenesis. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-epidemiology-and-pathogenesis?source=see_link. Accessed December 15, 2014.
11. Marcdante KJ, Kliegman RM, Jenson HB, Behrman RE. Attention-deficit/hyperactivity disorder. In: Merrit J, Cicalese B, eds. Nelson Essentials of Pediatrics. 6th ed. Philadelphia, PA: Saunders Elsevier; 2011:50-52.
12. Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160-1167.
13. CDC. Facts about ADHD. www.cdc.gov/NCBDDD/adhd/facts.html. Accessed December 15, 2014.
14. Rajwan E, Chacko A, Moeller M. Nonpharmacological interventions for preschool ADHD: state of the evidence and implications for practice. Prof Psychol Res Pr. 2012;43(5):520-526.
15. Daley D. Attention deficit hyperactivity disorder: a review of the essential facts. Child Care Health Dev. 2006;32(2):193-204.
16. van der Oord S, Bögels S, Peijnenburg D. The effectiveness of mindfulness training for children with ADHD and mindful parenting for their parents. J Child Fam Stud. 2012;21(1):139-147.
17. Zisser AR, Eyberg SM. Maternal ADHD: parent-child interactions and relations with child disruptive behavior. Child Fam Behavior Ther. 2012;34(1):33-52.
18. Daley D, Jones K, Hutchings J, Thompson M. Attention deficit hyperactivity disorder in pre-school children: current findings, recommended interventions and future directions. Child Care Health Dev. 2009;35(6):754-766.
19. Bruchmüller K, Margraf J, Schneider S. Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J Consult Clin Psychol. 2012;80(1):128-138.
20. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
22. American Psychiatric Association. Highlights of Changes from DSM-IV-TR to DSM-5. www.dsm5.org/Documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf. Accessed December 15, 2014.
23. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
24. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Supplemental information. Implementing the key action statements: an algorithm and explanation for process of care for the evaluation, diagnosis, treatment, and monitoring of ADHD in children and adolescents. http://pediatrics.aappublications.org/content/suppl/2011/10/11/peds.2011-2654.DC1/zpe611117822p.pdf. Accessed December 15, 2014.
25. Olfson M, Huang C, Gerhard T, et al. Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(2):147-156.
26. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
27. Perrin JM, Friedman RA, Knilans TK, et al. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
28. Berger S. Cardiac evaluation of patients receiving pharmacotherapy for attention deficit hyperactivity disorder. www.uptodate.com/contents/cardiac-evaluation-of-patients-receiving-pharmacotherapy-for-attention-deficit-hyperactivity-disorder?source=see_link. Accessed December 15, 2014.
29. Ho SC, Chien WT, Wang L. Parents’ perceptions of care-giving to a child with attention deficit hyperactivity disorder: an exploratory study. Contemp Nurse. 2011;40(1):41-56.
30. Moen ØL, Hall-Lord ML, Hedelin B. Contending and adapting every day: Norwegian parents’ lived experience of having a child with ADHD. J Fam Nursing. 2011;17(4):441-462.
31. Theule J, Wiener J, Rogers M, Marton I. Predicting parenting stress in families of children with ADHD: parent and contextual factors. J Child Fam Stud. 2011;20(5):640-647.
32. Brown RP, Gerbarg PL. Non-Drug Treatments for ADHD. New York, NY: Norton & Company; 2012:5-26.
33. Jones K, Daley D, Hutchings J, et al. Efficacy of the Incredible Years Basic parent training programme as an early intervention for children with conduct problems and ADHD. Child Care Health Dev. 2007;33(6):749-756.
34. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: overview of treatment and prognosis. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-overview-of-treatment-and-prognosis?source=see_link. Accessed December 15, 2014.
35. Fabiano GA, Vujnovic RK, Pelham WE, et al. Enhancing the effectiveness of special education programming for children with attention deficit hyperactivity disorder using a daily report card. School Psychol Rev. 2010;39(2):219-239.
36. Wilens TE, Faraone SV, Biederman J, Gunawardene, S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003; 111(1):179-185.
37. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: treatment with medications. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-treatment-with-medications?source=see_link. Accessed November 10, 2014.
38. Swanson JM, Elliott GR, Greenhill LL, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1014–1026.
39. Krull KR. Pharmacology of drugs used to treat attention deficit hyperactivity disorder in children and adolescents. www.uptodate.com/contents/pharmacology-of-drugs-used-to-treat-attention-deficit-hyperactivity-disorder-in-children-and-adolescents?source=see_link. Accessed December 15, 2014.
40. Scahill L. Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs. 2009;23(suppl 1):43-49.
41. Krull KR. Patient information: treatment of attention deficit hyperactivity disorder in children (beyond the basics). www.uptodate.com/contents/treatment-of-attention-deficit-hyperactivity-disorder-in-children-beyond-the-basics?source=related_link. Accessed December 15, 2014.
42. Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50(10):978-990.
1. Visser SN, Danielson ML, Bitsko RH, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.
2. Merikangas KR, He JP, Brody D, et al. Prevalence and treatment of mental disorders among US children in the 2001-2004 NHANES. Pediatrics. 2010;125(1):75-81.
3. Post RE, Kurlansik SL. Diagnosis and management of attention-deficit/hyperactivity disorder in adults. Am Fam Physician. 2012;85(9):890-896.
4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
5. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: clinical features and evaluation. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-clinical-features-and-evaluation?source=see_link. Accessed December 15, 2014.
6. Wilens TE, Spencer TJ. Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med. 2010;122(5): 97-109.
7. Danckaerts M, Sonuga-Barke E, Coghill D, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83-105.
8. Pelham WE, Foster EM, Robb JA. The economic impact of attention-deficit/hyperactivity disorder in children and adolescents. Ambul Pediatr. 2007;7(1 suppl):121-131.
9. Deault LC. A systematic review of parenting in relation to the development of comorbidities and functional impairments in children with attention-deficit/hyperactivity disorder (ADHD). Child Psychiatry Hum Dev. 2010;41(2):168-192.
10. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: epidemiology and pathogenesis. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-epidemiology-and-pathogenesis?source=see_link. Accessed December 15, 2014.
11. Marcdante KJ, Kliegman RM, Jenson HB, Behrman RE. Attention-deficit/hyperactivity disorder. In: Merrit J, Cicalese B, eds. Nelson Essentials of Pediatrics. 6th ed. Philadelphia, PA: Saunders Elsevier; 2011:50-52.
12. Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):1160-1167.
13. CDC. Facts about ADHD. www.cdc.gov/NCBDDD/adhd/facts.html. Accessed December 15, 2014.
14. Rajwan E, Chacko A, Moeller M. Nonpharmacological interventions for preschool ADHD: state of the evidence and implications for practice. Prof Psychol Res Pr. 2012;43(5):520-526.
15. Daley D. Attention deficit hyperactivity disorder: a review of the essential facts. Child Care Health Dev. 2006;32(2):193-204.
16. van der Oord S, Bögels S, Peijnenburg D. The effectiveness of mindfulness training for children with ADHD and mindful parenting for their parents. J Child Fam Stud. 2012;21(1):139-147.
17. Zisser AR, Eyberg SM. Maternal ADHD: parent-child interactions and relations with child disruptive behavior. Child Fam Behavior Ther. 2012;34(1):33-52.
18. Daley D, Jones K, Hutchings J, Thompson M. Attention deficit hyperactivity disorder in pre-school children: current findings, recommended interventions and future directions. Child Care Health Dev. 2009;35(6):754-766.
19. Bruchmüller K, Margraf J, Schneider S. Is ADHD diagnosed in accord with diagnostic criteria? Overdiagnosis and influence of client gender on diagnosis. J Consult Clin Psychol. 2012;80(1):128-138.
20. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
22. American Psychiatric Association. Highlights of Changes from DSM-IV-TR to DSM-5. www.dsm5.org/Documents/changes%20from%20dsm-iv-tr%20to%20dsm-5.pdf. Accessed December 15, 2014.
23. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007-1022.
24. American Academy of Pediatrics. Subcommittee on Attention-Deficit/Hyperactivity Disorder. Supplemental information. Implementing the key action statements: an algorithm and explanation for process of care for the evaluation, diagnosis, treatment, and monitoring of ADHD in children and adolescents. http://pediatrics.aappublications.org/content/suppl/2011/10/11/peds.2011-2654.DC1/zpe611117822p.pdf. Accessed December 15, 2014.
25. Olfson M, Huang C, Gerhard T, et al. Stimulants and cardiovascular events in youth with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(2):147-156.
26. Vetter VL, Elia J, Erickson C, et al. Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117(18):2407-2423.
27. Perrin JM, Friedman RA, Knilans TK, et al. Cardiovascular monitoring and stimulant drugs for attention-deficit/hyperactivity disorder. Pediatrics. 2008;122:451-453.
28. Berger S. Cardiac evaluation of patients receiving pharmacotherapy for attention deficit hyperactivity disorder. www.uptodate.com/contents/cardiac-evaluation-of-patients-receiving-pharmacotherapy-for-attention-deficit-hyperactivity-disorder?source=see_link. Accessed December 15, 2014.
29. Ho SC, Chien WT, Wang L. Parents’ perceptions of care-giving to a child with attention deficit hyperactivity disorder: an exploratory study. Contemp Nurse. 2011;40(1):41-56.
30. Moen ØL, Hall-Lord ML, Hedelin B. Contending and adapting every day: Norwegian parents’ lived experience of having a child with ADHD. J Fam Nursing. 2011;17(4):441-462.
31. Theule J, Wiener J, Rogers M, Marton I. Predicting parenting stress in families of children with ADHD: parent and contextual factors. J Child Fam Stud. 2011;20(5):640-647.
32. Brown RP, Gerbarg PL. Non-Drug Treatments for ADHD. New York, NY: Norton & Company; 2012:5-26.
33. Jones K, Daley D, Hutchings J, et al. Efficacy of the Incredible Years Basic parent training programme as an early intervention for children with conduct problems and ADHD. Child Care Health Dev. 2007;33(6):749-756.
34. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: overview of treatment and prognosis. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-overview-of-treatment-and-prognosis?source=see_link. Accessed December 15, 2014.
35. Fabiano GA, Vujnovic RK, Pelham WE, et al. Enhancing the effectiveness of special education programming for children with attention deficit hyperactivity disorder using a daily report card. School Psychol Rev. 2010;39(2):219-239.
36. Wilens TE, Faraone SV, Biederman J, Gunawardene, S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003; 111(1):179-185.
37. Krull KR. Attention deficit hyperactivity disorder in children and adolescents: treatment with medications. www.uptodate.com/contents/attention-deficit-hyperactivity-disorder-in-children-and-adolescents-treatment-with-medications?source=see_link. Accessed November 10, 2014.
38. Swanson JM, Elliott GR, Greenhill LL, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1014–1026.
39. Krull KR. Pharmacology of drugs used to treat attention deficit hyperactivity disorder in children and adolescents. www.uptodate.com/contents/pharmacology-of-drugs-used-to-treat-attention-deficit-hyperactivity-disorder-in-children-and-adolescents?source=see_link. Accessed December 15, 2014.
40. Scahill L. Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs. 2009;23(suppl 1):43-49.
41. Krull KR. Patient information: treatment of attention deficit hyperactivity disorder in children (beyond the basics). www.uptodate.com/contents/treatment-of-attention-deficit-hyperactivity-disorder-in-children-beyond-the-basics?source=related_link. Accessed December 15, 2014.
42. Hammerness PG, Perrin JM, Shelley-Abrahamson R, Wilens TE. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50(10):978-990.
Vocal Cord Dysfunction: Unmasking the Asthma Pretender
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
CE/CME No: CR-1412
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Discuss the evolution in thinking about the pathogenesis of and treatment for vocal cord dysfunction (VCD).
• Describe the three primary functions of the healthy vocal cords.
• List the conditions or factors that may trigger VCD.
• Explain how to differentiate VCD from asthma.
• Develop a treatment plan for VCD that addresses both patient-specific VCD triggers and management of symptomatic episodes.
FACULTY
Linda S. MacConnell is an Assistant Professor in the Department of Physician Assistant Studies and Randy D. Danielsen is a Professor and Dean at the Arizona School of Health Sciences, AT Still University, Mesa. Ms. MacConnell is also a clinical PA affiliated with Enticare, an otolaryngology practice in Chandler, Arizona. Susan Symington is a clinical PA with the Arizona Asthma & Allergy Institute, with which Dr. Danielsen is also affiliated.
Linda MacConnell and Randy Danielsen have no significant financial relationships to disclose. Susan Symington is a member of the speaker’s bureau for Teva Respiratory and Thermo Fisher Scientific, Inc.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of December 2014.
Article begins on next page >>
The symptoms of vocal cord dysfunction (VCD) can be mistaken for those
of asthma or other respiratory illnesses. As a result, VCD is often misdiagnosed,
leading to unnecessary, ineffective, costly, or even dangerous treatment. Here are
the facts that will enable you to avoid making an erroneous diagnosis, choosing
potentially harmful treatment, and delaying effective treatment.
A 33-year-old oncology nurse, JD, had moved from Seattle to Phoenix about six months earlier for a job opportunity. Shortly after starting her new job, she had developed intermittent dyspnea on exertion, with a cough lasting several minutes at a time, along with a sensation of heaviness over the larynx and a choking sensation. These symptoms were precipitated by gastroesophageal reflux disease (GERD), postnasal drainage, stress, and significant environmental change (ie, Seattle to Phoenix). She noticed that, since moving to Phoenix, she frequently cleared her throat but denied any hoarseness, dysphagia, chest tightness, chest pain, or wheezing. She noted nasal congestion and clear nasal discharge on exposure to inhaled irritants (eg, woodstove smoke) and strong fragrances (eg, perfume or cologne).
On physical examination, the patient was alert, oriented, and in no acute distress. She was coughing intermittently but was able to speak in complete sentences. No stridor or dyspnea was noted, either on exertion (jogging in place) or at rest.
HEENT examination was normal, with no scalp lesions or tenderness; face, symmetric; light reflex, symmetric; conjunctivae, clear; sclera white, without lesions or redness; pupils, equal, reactive to light and accommodation; tympanic membranes and canals, clear with intact landmarks; no nasal deformities; nasal mucosa, mildly erythematous with mild engorgement of the turbinates; no nasal polyps seen; nasal septum midline without perforation; no sinus tenderness on percussion; pharynx, clear without exudate; uvula rises on phonation; and oral mucosa and gingivae, pink without lesions. Neck was supple without masses or thyromegaly, and trachea was midline. Lungs were clear to auscultation with normal respiratory movement and no accessory muscle use, with normal anteroposterior diameter. Heart examination revealed regular rate and rhythm, without murmur, clicks, or gallops.
Examination of the skin was normal, without rashes, hives, swelling, petechiae, or significant ecchymosis. There was no palpable cervical, supraclavicular, or axillary adenopathy.
Results of laboratory studies included a normal complete blood count with differential and a normal IgE level of 46.3 IU/mL. Spirometry testing revealed normal values without obstruction; however, there was a flattening of the inspiratory flow loop, with no reversibility after bronchodilator, which was highly suggestive of vocal cord dysfunction (VCD). Perennial nonallergic rhinitis (formerly called vasomotor rhinitis) was confirmed because the patient experienced fewer symptoms to perfume after nasal corticosteroid use. The patient’s GERD was generally well controlled with esomeprazole but was likely a contributing factor to her vocal cord symptoms.
On laryngoscopy, abnormal vocal cord movement toward the midline during both inspiration and expiration was visualized, confirming the diagnosis of VCD.
BACKGROUND
VCD is a partial upper airway obstruction caused by paradoxical adduction (medial movement) of the vocal cords.1 Although it is primarily associated with inspiration, it sometimes manifests during expiration as well.
The true incidence of VCD is uncertain; different studies have found incidence rates varying from 2% to 27%, with higher rates in patients with asthma.1,2 However, highlighting the risk for misdiagnosis, some 10% of patients evaluated for asthma unresponsive to aggressive treatment were found, in fact, to have VCD alone.2
Similarly, although VCD is generally more common in women than in men, the reported female-to-male ratio has varied from 2:1 to 4:1.1,2,4 Some reports suggest that VCD is seen more frequently in younger women, with average ages at diagnosis of 14.5 in adolescents and 33 in adults.2,3 Others identify a broader age range, with most patients older than 50.4
Historically, VCD has been known by a variety of names and has been observed clinically since 1842. In that year, Dunglison referred to it as hysteric croup, describing a disorder of the laryngeal muscles brought on by “hysteria.”5 Later, Mackenzie was able to visualize adduction of the vocal cords during inspiration in patients with stridor by using a laryngoscope.6 Osler demonstrated his understanding of the condition in 1902, stating, “Spasm of muscles may occur with violent inspiratory effort and great distress, and may even lead to cyanosis. Extraordinary cries may be produced either inspiratory or expiratory.”7
More recently, in 1974, Patterson et al reported finding laryngoscopic evidence of VCD, which they termed Munchausen’s stridor.8 They used this descriptor to report on the case of a young woman with 15 hospital admissions for this condition. At the time, the etiology of the condition was believed to be largely psychologic, and its evaluation was consigned to psychiatrists and other mental health practitioners.
As laryngoscopy became more widely available in the 1970s and 1980s, diagnosis of VCD increased, although the condition remains underrecognized.9 Ibrahim et al suggest that primary care clinicians may not be as aware of VCD as they should be and may not consider laryngoscopy for possible VCD in patients whose asthma is poorly controlled.2
Disagreement persists with regard to the preferred name for the condition. Because numerous disorders involve abnormal vocal cord function, Christopher proposed moving away from the broad term VCD and toward a more descriptive term: paradoxical vocal fold motion (PVFM) disorder.10 Interestingly, use of the two terms seems to be divided along specialty lines: VCD is preferred by allergy, pulmonology, and mental health specialists, while PVFM is favored by otolaryngology specialists and speech-language pathologists.11
Further complicating awareness and recognition of VCD is its longstanding reputation as a psychologic disorder. In fact, the paradigm has shifted away from defining VCD as a purely psychopathologic entity to the identification of numerous functional etiologies for the disorder. This, however, has resulted in many new terms to describe the condition, including nonorganic upper airway obstruction, pseudoasthma, irritable larynx syndrome, factitious asthma, spasmodic croup, functional upper airway obstruction, episodic laryngeal dyskinesia, functional laryngeal obstruction, functional laryngeal stridor, and episodic paroxysmal laryngospasm.1
Regardless of its name, an understanding of VCD is essential for both primary care and specialty clinicians because of its frequent misdiagnosis as asthma, allergies, or severe upper airway obstruction. When it is misdiagnosed as asthma, aggressive asthma treatments—to which VCD does not respond—may be prescribed, including high-dose inhaled and systemic corticosteroids and bronchodilators. Patients may experience multiple emergency department (ED) visits and hospitalizations and, in some cases, may be subjected to tracheostomies and intubation.
Continue for vocal cord physiology and functions >>
VOCAL CORD PHYSIOLOGY AND FUNCTIONS
The vocal cords are located within the larynx. Abduction, or opening, of the cords is controlled by the posterior cricoarytenoid muscle; adduction, or closing, occurs via contraction of the lateral cricoarytenoid muscle. These muscles are innervated by the recurrent laryngeal nerve to control the width of the space—the rima glottidis—between the cords. During inspiration, the glottis opens; during expiration, it narrows but remains open.12
The vocal cords are involved in three main functions: protection of the airway, respiration, and phonation (vocal production). These functions are at least partially controlled involuntarily by brain stem reflexes; however, only airway protection—the most important of these functions—is reflexive and involuntary.12 Respiration may be controlled voluntarily, and phonation is primarily voluntary. Closure of the vocal cords is under the control of the laryngeal nerve branches of the vagal nerve.12,13
The vocal cords normally abduct during inspiration to allow air to pass through them into the trachea and the lungs. Sniffing, puffing, snuffling, and panting also cause the vocal cords to abduct. The vocal cords adduct with phonation (talking, singing), coughing, clearing the throat, performing the Valsalva maneuver, and swallowing. During expiration, 10% to 40% adduction is considered normal.14
VOCAL CORD DYSFUNCTION
Pathogenesis and etiology
VCD is a nonspecific term, and a number of factors may be involved in its development.15 Although the precise cause of VCD is unknown, it is believed to result from laryngeal hyperresponsiveness. This exaggerated responsiveness may be prompted by irritant and nonirritant triggers of the sensory receptors in the larynx, trachea, and large airways that mediate cough and glottis closure reflexes.16
VCD may be among a group of airway disorders triggered by occupational exposures, including irritants and psychologic stressors. For example, occupationally triggered VCD was diagnosed in rescue, recovery, and cleanup workers at the World Trade Center disaster site.4
A history of childhood sexual abuse has also been associated by some researchers with the development of VCD. For example, Freedman et al reported that, of 47 patients with VCD, 14 identified such a history and five were suspected of having been sexually abused as children.17
Paradoxical movement of the vocal cords causes them to close when they should open. (Click here for a video on normal and abnormal vocal cord movement.) VCD generally occurs during inspiration, causing obstruction of the incoming air through the larynx. Symptoms of VCD frequently include dyspnea, coughing, wheezing, hoarseness, and tightness or pain in the throat.
Examination of the flow-volume loops recorded when a patient experiences “wheezing” during spirometry testing reveals a flattened inspiratory loop, indicating a decrease of airflow into the lungs (see Figure 1).13,16 “Wheezing” is actually a misnomer in this situation because the term typically refers to sounds that occur during expiration.
Triggers
Physiologic, psychologic, and neurologic factors may all contribute to VCD.1,15 Conditions that can trigger VCD include
• Asthma
• Postnasal drip
• Recent upper respiratory illness (URI)
• Talking, singing
• Exercise
• Cough
• Voice strain
• Stress, anxiety, tension, elevated emotions
• Common irritants (eg, strong smells)
• Airborne irritants
• Rhinosinusitis
• GERD
• Use of certain medications
Identification of a particular patient’s triggers is key to successful management of VCD.
PATIENT PRESENTATION
Although there is no “typical” patient with VCD, the condition occurs more frequently in women, with the most common age at onset between 20 and 40 years. However, VCD has been seen in very young children and in adults as old as 83, and its diagnosis in the pediatric population is increasing.18
The patient may present with complaints of atypical chest pain, throat tightness, stridor, choking, difficult vocalization, cough and sometimes dysphagia, GERD, or rhinosinusitis (see Table 1). These signs and symptoms may occur without provocation, or patients may relate a history of triggers such as anxiety, irritant exposure, or exercise. In fact, about 14% of VCD is associated with exercise, particularly in young female athletes who experience shortness of breath and even stridor with exercise.19
A characteristic finding on physical examination is inspiratory stridor, along with respiratory distress.20 The stridor is best auscultated not over the anterior chest wall but over the tracheal area of the anterior neck.
Continue for differential diagnosis >>
DIFFERENTIAL DIAGNOSIS
Distinguishing VCD from other disorders can be challenging. Differential diagnosis should include
• Non–vocal cord adduction disorders, such as thyroid goiter, upper airway hemorrhage, caustic ingestion, neoplastic disorders, rheumatoid cricoarytenoid arthritis, pharyngeal abscess, angioedema, pulmonary embolus21
• Anatomic defects (eg, laryngomalacia, subglottic stenosis)
• Tracheal masses (eg, enlarged thyroid gland)
• Vocal cord polyps
• Laryngospasm
• Vocal cord paresis
• Neurologic causes (eg, brain stem compression, severe cortical injury, nuclear or lower motor neuron injury, movement disorders)
• Nonorganic causes (eg, factitious symptoms or malingering; conversion disorder)22
• Reactive airway disease.
Some disorders are easier to distinguish from VCD than others. For example, although laryngospasm may produce similar symptoms, episodes are brief, lasting seconds to minutes; VCD episodes may last hours to days.
Asthma
Even the most astute clinician will be unable to obtain adequate information from the patient history to differentiate VCD from asthma. There is a significant overlap of symptoms—shortness of breath, cough, wheezing—and frequently, the diseases coexist. History is often negative for chest pain, but it is common for patients with VCD, when asked to describe their symptoms, to report chest tightness. The clinician therefore needs to ask the patient to point to where the tightness is felt—in the chest or in the neck over the laryngeal area—to distinguish the source.
Asthma symptoms usually increase over a few hours, days, or weeks but respond to medications that open the airway and reduce inflammation (inhaled β-agonists and corticosteroids). VCD symptoms usually occur or decrease suddenly and do not respond well to traditional asthma treatments.
Other differences between asthma and VCD symptoms include voice changes and time of day when symptoms occur. The person with VCD will experience voice changes, such as hoarseness, as well as prolonged coughing episodes. Patients with asthma may awaken at night because of breathlessness, while most patients with VCD experience symptoms only during the day.
The diagnosis is generally confirmed if VCD is seen on direct laryngoscopic visualization during a symptomatic episode. In terms of adduction, the anterior cords will appear normal, but the posterior portion of the cords will display the classic “glottis chink” (see Figure 2).9
If the diagnosis is in question, videostroboscopy, a technique that provides a magnified slow-motion view of vocal cord vibration, can help identify or exclude pathologic conditions of the vocal cords.23
Convincing the patient of the validity of the diagnosis may be problematic if the patient has been previously diagnosed with and treated for another condition. The diagnosis should be explained and the patient counseled what appropriate care for VCD entails (see discussion under “Patient education and self-care”).
TREATMENT
Acute episode
During an acute VCD episode, offering the patient calm reassurance can be effective in resolving the episode. Simple breathing guidance may also be beneficial; instructing the patient to breathe rapidly and shallowly (ie, pant) can result in immediate resolution of symptoms.24 The patient can be advised to utilize other techniques, such as diaphragmatic breathing, breathing through the nose, breathing through a straw, pursed-lip breathing, and exhaling with a hissing sound.25
Long-term management
Although various strategies are employed in the management of VCD, well-designed studies on which to base treatment decisions have not been performed. Of course, control and management of possible underlying triggers or disorders should be implemented. Because etiology is rarely known, treatment for VCD is generally empiric.
Evidence does exist, however, to suggest that voice therapy, the treatment of choice for muscle tension dysphonia, is also effective for VCD. Speech therapy with specific voice and breathing exercises can enable the patient to manage the condition, thereby reducing ED visits, hospitalizations, and treatment costs.26
Patient education and self-care
Patient education is a critical component of VCD management. The clinician should explain the functions of the larynx to the patient, including the normal functioning of the vocal cords during respiration, speaking, swallowing, coughing, throat clearing, and breath holding. It may also enhance patients’ understanding of VCD to view their diagnostic laryngoscopy or videostroboscopy films.21
The patient should be advised to rest the voice, hydrate, utilize sialagogues (lozenges, gum) to stimulate salivation, reduce exposure to triggers when possible, and decrease stress. She should be encouraged to track VCD triggers by documenting what she is doing, where, and when, at the time of a VCD episode.
Two exercises—“paused breathing” and “belly breathing”—can be used by patients to learn how to relax the vocal cords (see “Patient Handout”). Patients should practice these exercises three times a day so that they can be easily recalled and performed during VCD episodes.
Continue for outcomes >>
OUTCOMES
Little is known about long-term outcomes for patients with VCD. The current literature consists of poorly described and conflicting case reports and results of small trials. Although documentation is lacking, the authors agree that, by educating the patient about the diagnosis, teaching effective VCD management strategies, and referring patients for voice therapy, clinicians can help patients achieve signicant improvement. Further investigation is needed to enhance our knowledge of the causes of VCD and to research additional diagnostic modalities and treatments.2
CASE PATIENT
After diagnosing VCD, the clinician explained the normal functioning of the vocal cords and how certain factors may cause them to close during inspiration. The patient then understood why bronchodilator therapy had failed to relieve her symptoms. She was counseled to continue her inhaled nasal steroid and proton pump inhibitor for her perennial nonallergic rhinitis and GERD, respectively, because these conditions may trigger her VCD, and to take steps to manage her stress. She learned breathing techniques to alleviate acute episodes of VCD and was informed of the option of voice therapy with a speech therapist if needed.
At six-week follow-up, the patient reported that she was complying with her medication regimen, had made an effort to relax more, and had experienced no acute attacks of VCD since her last visit.
CONCLUSION
Patients with symptoms suggestive of VCD require a thorough evaluation, including laryngoscopic examination, to ensure accurate diagnosis and avoid a too-common misdiagnosis. Primary care clinicians should know about VCD and, if not trained in the performance of flexible laryngoscopy, should refer the symptomatic patient to a specialist for appropriate work-up.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
1. Hoyte FCL. Vocal cord dysfunction. Immunol Allergy Clin N Am. 2013;33:1-22.
2. Ibrahim WH, Gheriani HA, Almohamed AA, Raza T. Paradoxical vocal cord motion disorder: past, present and future. Postgrad Med J. 2007;83:164-172.
3. Powell DM, Karanfilov BI, Beechler KB, et al. Paradoxical vocal cord dysfunction in juveniles. Arch Otolaryngol Head Neck Surg. 2000;126(1):29-34.
4. Husein OF, Husein TN, Gardner R, et al. Formal psychological testing in patients with paradoxical vocal fold dysfunction. Laryngoscope. 2008; 118(4):740-747.
5. Dunglison RD. The Practice of Medicine. Philadelphia, PA: Lea and Blanchard; 1842:257-258.
6. MacKenzie M. Use of Laryngoscopy in Diseases of the Throat. Philadelphia, PA: Lindsey and Blackeston; 1869:246-250.
7. Osler W. Hysteria. In: The Principles and Practice of Medicine. 4th ed. New York, NY: Appleton; 1902:1111-1112.
8. Patterson R, Schatz M, Horton M. Munchausen’s stridor: non-organic laryngeal obstruction. Clin Allergy. 1974;4:307-310.
9. Christopher KL, Wood RP 2nd, Eckert RC, et al. Vocal cord dysfunction presenting as asthma. N Engl J Med. 1983;308(26):1566-1570.
10. Christopher KL. Understanding vocal cord dysfunction: a step in the right direction with a long road ahead. Chest. 2006;129(4):842-843.
11. Christopher KL, Morris MJ. Vocal cord dysfunction, paradoxic vocal fold motion, or laryngomalacia? Our understanding requires an interdisciplinary approach. Otolaryngol Clin N Am. 2010;43:43-66.
12. Sasaki CT, Weaver EM. Physiology of the larynx. Am J Med. 1997;103:9S-18S.
13. Balkissoon R. Occupational upper airway disease. Clin Chest Med. 2002;23:717-725.
14. Murakami Y, Kirschner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol Rhinol Laryngol. 1972;81(1):59-71.
15. Forrest LA, Husein T, Husein O. Paradoxical vocal cord motion disorder: classification and treatment. Laryngoscope. 2012;122:844-853.
16. Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg. 2002;127(6):501-511.
17. Freedman MR, Rosenberg SJ, Schmaling KB. Childhood sexual abuse in patients with paradoxical vocal cord dysfunction. J Nerv Ment Dis. 1991;179(5):295-298.
18. Buddiga P. Vocal cord dysfunction. Medscape. http://emedicine.medscape.com/article/137782-overview. Accessed November 12, 2014.
19. Chiang T, Marcinow AM, deSilva BW, et al. Exercise-induced paradoxical vocal fold motion disorder: diagnosis and management. Laryngoscope. 2013;123:727-731.
20. Morris MJ, Deal LE, Bean DR, et al. Vocal cord dysfunction in patients with exertional dyspnea. Chest. 1999;116(6):1676-1682.
21. Hicks M, Brugman SM, Katial R. Vocal cord dysfunction/paradoxical vocal fold motion. Prim Care. 2008;35(1):81-103.
22. Maschka DA, Bauman NM, McCray PB, et al. A classification scheme for paradoxical vocal fold motion. Laryngoscope. 1997;107(11):1429-1435.
23. Uloza V, Vegiene A, Pribuisiene R, Saferis V. Quantitative evaluation of video laryngostroboscopy: reliability of the basic parameters. J Voice. 2013;27(3):361-368.
24. Pitchenik AF. Functional laryngeal obstruction relieved by panting. Chest. 1991;100(5):1465-1467.
25. Deckert J, Deckert L. Vocal cord dysfunction. Am Fam Physician. 2010;81(2):156-160.
26. Carding PN, Horsley IA, Docherty GJ. A study of the effectiveness of voice therapy in the treatment of 45 patients with nonorganic dysphonia. J Voice. 1999;13(1):72-104.
Chronic Hepatitis C Infection: Bane of Baby Boomers
CE/CME No: CR-1411
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the risk factors for HCV infection.
• Identify who should be screened for HCV infection.
• Discuss the symptoms, clinical course, diagnosis, and complications of chronic HCV infection.
• Differentiate between the treatment of acute and chronic HCV infection.
• Describe the challenges of treating HCV infection in patients who are coinfected with HIV.
FACULTY
Daniel Sturm and Samuel L. Gurevitz are Assistant Professors in the Physician Assistant Program, College of Pharmacy and Health Sciences, at Butler University in Indianapolis. Cassidy Davidson, Abigail Fritchley, and Audrey Wagaman are students in the PA Program at Butler University. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Several million Americans, primarily those in their fifties and sixties, contracted hepatitis C virus (HCV) many years ago and are unaware of their infection and their risk for HCV-related liver disease. Screening at-risk patients is important because newer treatment regimens are curative and can reduce associated morbidity and mortality.
Chronic infection with hepatitis C virus (HCV) is a major cause of liver disease in the United States. National Health and Nutrition Examination Survey data indicates that 2.7 million people have chronic HCV infection; the CDC estimates 3.2 million.1-3 Yet these individuals may be asymptomatic for years, despite slow progression of sequelae (eg, chronic liver disease, cirrhosis, hepatocellular carcinoma [HCC]) that may silently unfold. Left untreated, chronic HCV infection is associated with a 15% to 30% risk for cirrhosis within 20 years, which subsequently confers an annual risk for HCC of 2% to 4%.4 Additionally, chronic HCV infection is now the leading indication for liver transplantation.3
Of note, 70% of those with chronic HCV infection were born between 1945 and 1965.2 This is believed to be attributable to viral transmission via contaminated blood, blood products, and organ transplants prior to the implementation of universal precautions for blood supply screening in 1992; and to past injection drug use, even if it occurred only once.3
In a recent analysis, it was determined that the clinical and economic burdens of chronic HCV infection increased in the past decade, and this trend is likely to continue during the next decade.5 For example, HCV was responsible for 15,106 US deaths in 2007, surpassing deaths caused by HIV for the first time.6 Since then, the number of HCV-related deaths has continued to increase, to 17,721 in 2011.7 Economic costs solely attributable to HCV infection are difficult to calculate, but estimates range from several hundred million dollars to $30 billion annually.5 The World Health Organization (WHO) estimates that more than 185 million people worldwide are infected with HCV and that HCV is responsible for 350,000 deaths annually.4
The main goal of treatment is to achieve a sustained virologic response (SVR), defined as undetectable HCV RNA in serum 12 to 24 weeks after completion of treatment and thereby prevent or reduce the complications of HCV infection.3 Proactive screening, diagnosis, monitoring, and treatment of HCV infection can significantly reduce long-term morbidity and mortality.
INDICATIONS FOR HCV SCREENING
Recommendations for HCV screening have been developed by numerous organizations, including the WHO, CDC, US Preventive Services Task Force (USPSTF), American Association for the Study of Liver Diseases (AASLD), and the Infectious Diseases Society of America (IDSA) (see Table 1).3,4,8,9-11 Screening should be offered to all individuals meeting one or more of the criteria. A 2012 CDC update calls for one-time testing for all persons born between 1945 and 1965 because of the disproportionately high prevalence of HCV infection in this cohort, which is five times greater than in the general population.11
The initial screening tool for HCV infection is an HCV antibody test. A positive or reactive anti-HCV antibody result can signify either current or resolved infection, so a positive result should be followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present.4,8-10 See Figure on previous page for an HCV screening algorithm, which includes the points at which referrals to specialists are indicated.
A rapid HCV antibody test was approved by the FDA in 2011 and is favored because of its widespread availability, ease of use, rapid results, and low cost. Point-of-care testing is comparable in sensitivity and specificity to laboratory-based HCV assays and can utilize blood obtained via fingerstick or venipuncture.8 This form of testing facilitates HCV screening in a variety of settings, such as health fairs and emergency departments, as well as in high-risk settings such as methadone clinics.
Populations at increased risk for HCV infection are also at increased risk for hepatitis B virus (HBV), HIV, and tuberculosis infections; therefore, screening for these may also be warranted.4
PROGRESSION OF UNTREATED INFECTION
Just as with chronic HCV infection, patients newly infected with HCV are typically asymptomatic; the illness manifests with clinical symptoms in only 20% to 30% of cases.3 If symptoms do appear, they include fever, fatigue, dark urine, clay-colored stool, abdominal pain, loss of appetite, nausea, vomiting, joint pain, and jaundice. These acute phase symptoms may last two to 12 weeks.12
Spontaneous clearance of the virus occurs in only 15% to 25% of cases (see further discussion under “Acute HCV.”).2,3 Even if a patient clears an HCV infection, if he or she falls within one of the at-risk categories (see Table 1), then periodic screening should continue because prior HCV infection does not protect against future infection.3
The progression of chronic HCV is indolent and often subclinical, with fatigue being the most common complaint. Other nonspecific symptoms may include nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. One study noted that symptoms do not correlate with disease severity.13 In a 10-year prospective study, chronic HCV infection increased mortality when the infection was acquired at an early age (younger than 50) and/or when cirrhosis developed.14 Patients with cirrhosis progress to liver decompensation at a rate of 3% to 6% annually.15
In addition to liver disease, HCV infection is associated with an increased risk for non-Hodgkin lymphoma.3 HCV may also induce insulin resistance, which increases the risk for hepatic fibrosis. Other studies document HCV-induced cognitive impairment, but little scientific data is yet available as to its pathogenesis.16
DIAGNOSIS
Diagnostic testing for suspected HCV infection begins with an antibody test.8 A positive antibody test indicates one of three possibilities: active infection, resolved infection, or a false-positive result. Two drawbacks of HCV antibody testing are that immunocompromised patients may falsely test negative and that antibodies cannot be detected until eight to 12 weeks after the infection is acquired.11 A positive antibody test should be confirmed with an HCV polymerase chain reaction (PCR) RNA test.8,10
HCV RNA testing can detect the virus earlier than antibody testing—as early as two weeks after infection. Although a positive HCV RNA test confirms current HCV infection, its higher cost precludes its use as an initial diagnostic test for lower-risk patients.
In patients who test negative but for whom there is a high index of suspicion for HCV infection (eg, jaundiced patient with an elevated alanine aminotransferase level [ALT]), or for a health care worker with a recent bloodborne HCV exposure, testing for HCV antibodies, HCV RNA, and ALT levels should be ordered at regular intervals for a period of six months.10
Acute versus chronic infection
Distinguishing acute and chronic HCV is difficult. A determination that HCV is newly acquired requires a documented negative baseline antibody test, followed by laboratory evidence of seroconversion. This is typically only seen in cases where there has been a recent, known exposure to the virus.
Both HCV antibody and RNA testing are recommended when screening high-risk patients, including those who are immunocompromised, on hemodialysis, or have had a recent exposure to HCV-positive blood.10 Rapid PCR HCV RNA tests can assess both viral load and genotype (see discussion of HCV genotypes under “Chronic HCV”).17 This information helps guide and measure patient response to treatment.
Liver disease severity
Liver fibrosis and cirrhosis are serious complications of HCV; hepatomegaly or splenomegaly may or may not be present on physical examination and patients may require liver biopsies to evaluate disease stage.18 An assessment of the severity of liver damage can determine the urgency of treatment and predict treatment efficacy.
Biopsy results permit the grading of inflammation and nodularity and the staging of septal fibrosis, which can reliably predict future progression of the patient’s disease.19 However, liver biopsy is invasive, painful, and may contain sampling errors; complications may include bleeding, infection, and occasionally accidental injury to a nearby organ. An initial noninvasive assessment may be performed using vibration-controlled transient liver elastography, an ultrasound-based technology that measures liver stiffness, which correlates well with the degree of fibrosis or cirrhosis. Elastography, along with measurement of direct serum biomarkers that are produced by activated hepatic stellate cells involved in fibrosis, affords an accurate, noninvasive means of assessing liver damage.10
Continue for treatment options >>
TREATMENT
Acute HCV
To prevent progression of disease from acute to chronic infection, patients diagnosed with acute disease should be treated if
• They are likely to adhere to the treatment plan
• They have no contraindications to pegylated interferon a (PEG-IFN a) treatment.
Contraindications to PEG-IFN a treatment include uncontrolled depression, psychosis, or epilepsy; pregnancy; couples' unwillingness to use effective contraception during treatment; severe concurrent medical disease; and decompensated liver disease.20
For acute HCV infection, treatment with PEG-IFN a-2a 180 µg/wk or PEG-IFN a-2b 1.5 µg/kg/wk for 24 weeks is recommended.20 Treatment results in an SVR greater than 80%.21 Combination therapy with ribavirin (RBV) does not increase SVR in the treatment of acute HCV infection.22
The appropriate time to begin treatment has not been firmly established because, as previously noted, 15% to 25% of those infected will spontaneously clear the virus. The European Association for the Study of the Liver (EASL) suggests that patients who remain HCV positive at 12 weeks from the time of suspected infection should be treated.23
Chronic HCV
Treatment of chronic HCV is based on multiple considerations, many of them patient-specific (see Table 2). One key element in treatment choice is the HCV genotype with which the patient is infected.
The most prevalent in the US are genotypes 1 through 6; further, genotype 1 has two subtypes: 1a and 1b. In the US, genotype 1 is most common, infecting about 70% of patients; genotype 2, 16%; genotype 3, 12%; genotype 4, 1%; genotype 5, < 1%; and genotype 6, 1%.24 Infection with one genotype does not protect an individual from future infection with the same or a different HCV genotype.3
Other factors influencing treatment include viral load, ALT levels, coinfection (eg, HIV, HBV), comorbidities, and treatment contraindications.21 Treatment is recommended for those who have detectable HCV RNA levels, elevated ALT levels, progressive liver disease on biopsy, and the absence of any serious comorbid conditions or contraindications. ALT levels, however, can fluctuate and do not always correlate with disease severity.
For years, the standard treatment for chronic HCV infection has been PEG-IFN a/RBV for 48 weeks. This combination produced an SVR of 50% to 80%, depending on genotype.3,25 Recently, however, treatment protocols have changed considerably with the introduction of two new and very effective direct-acting antiviral agents (DAAs): simeprevir, an HCV NS3/4A protease inhibitor, and sofosbuvir, an HCV NS5B polymerase inhibitor.
Simeprevir was approved by the FDA in October 2013 for use in combination with PEG-IFN/RBV for treatment of chronic HCV genotype 1 infection in adults with compensated liver disease.27 Because the efficacy of simeprevir is reduced in patients with HCV genotype 1a with an NS3 Q80K polymorphism, screening for NS3 Q80K is recommended; alternative therapy should be considered when this mutation is present.27
Sofosbuvir received FDA approval in December 2013 for use in combination with other antiviral drugs for treatment of chronic HCV infection, with established efficacy for treatment of genotypes 1, 2, 3, and 4 and for HCV/HIV coinfection.28 For genotype 1, simeprevir and sofosbuvir achieve SVRs of 80% and 90%, respectively.27,28
Table 3 lists the current medications for treatment of chronic HCV, including their adverse effects, contraindications, and drug interactions.
Updated treatment guidelines
AASLD, IDSA, EASL, WHO, and the Department of Veterans Affairs National Hepatitis C Resource Center Program recently issued updated evidence-based recommendations for the treatment of chronic HCV.4,10,23,26 Highlights of the changes to the guidelines include
• With the introduction of simeprevir and sofosbuvir, the guidelines no longer recommend combination PEG-IFN a/RBV for 48 weeks as the standard treatment for chronic HCV infection11,23,26
• Treatment regimens utilizing the protease inhibitors telaprevir and boceprevir are no longer recommended (with the exception of the WHO guidelines, which include them in a “conditional” recommendation).4
Regimens that include telaprevir and boceprevir are associated with higher rates of serious adverse effects, such as skin reactions and anemia, and involve longer treatment duration, higher pill burden, several drug interactions, frequent dosing, intensive monitoring, and the need to be taken with food (for telaprevir, high-fat food).10,23,26
Finally, the EASL recommends an additional agent, daclatasvir, an NS5A replication complex inhibitor, as an option for treating HCV genotype 1, 3, and 4.23 At this time, it is approved for use in Europe but has not been approved by the FDA.
Regardless of the treatment regimen, all patients receiving HCV antiviral therapy should be tested regularly to assess effectiveness of treatment and to monitor for the occurrence of adverse effects. Recommended periodic laboratory testing should include HCV RNA, complete blood count with differential, liver function, TSH level, renal function, comprehensive metabolic panel, bilirubin level, and pregnancy.29
SPECIAL POPULATIONS
Patients with HIV/HCV coinfection, a history of injection drug use, and renal impairment require management tailored to their individual circumstances.4,23,29
HIV/HCV-coinfected patients
Approximately 25% to 33% of patients infected with HIV are coinfected with HCV. HCV infection progresses more rapidly in HIV-infected patients, and coinfected patients are at greater risk for cirrhosis, liver cancer, and liver failure.30
For patients with HIV/HCV coinfection, the decision to start treatment is more complex because of the high pill burden, overlapping toxicities, and interactions among the drugs used to treat HIV and HCV infections.4 HIV antiviral therapy should be started before HCV treatment to improve immune function, thereby decreasing the risks for both further infections and HIV transmission. This also allows the patient to adjust gradually to each regimen.
One exception to this is when an HIV treatment–naïve patient has a CD4 count > 500 cells/mL.30 In this situation, HCV treatment is sometimes completed prior to the start of HIV treatment.
Recommended treatment for HIV/HCV coinfection, by HCV genotype, is outlined below. For all regimens, the weight-based RBV dosage is calculated as follows:
• Weight < 75 kg, 1,000 mg/d
• Weight ≥ 75 kg, 1,200 mg/d10,30
• Genotypes 1 and 4 (IFN eligible): Sofosbuvir 400 mg/d and weight-based RBV plus weekly PEG-IFN a for 12 weeks
• Genotypes 1 and 4 (IFN ineligible): Sofosbuvir 400 mg/d and weight-based RBV for 24 weeks
• Genotypes 2 and 3 (regardless of IFN eligibility): Sofosbuvir 400 mg/d and weight-based RBV for 12 weeks for genotype 2 and 24 weeks for genotype 3
• Genotypes 5 and 6 (IFN eligible): Sofosbuvir 400 mg/d plus weight-based RBV plus weekly PEG-IFN a for 12 weeks.
Injection drug users
HCV infection among young injection drug users (IDUs) is an emerging epidemic that must be addressed by recognizing at-risk populations, screening for early disease, and providing treatment and education. Globally, it is estimated that approximately 67% of IDUs—approximately 10 million people—are infected with HCV.31 Treatment of HCV in IDUs requires integration of many services and health care professionals, including addiction specialists. Dependence on opiates, alcohol, or other substances is common in this patient population. Patients should be counseled on the importance of abstaining from alcohol. IDUs are at risk for hepatitis A (HAV) and HBV infections and should be vaccinated against these diseases.4
Treatment decisions should be based on an individualized evaluation of the patient’s social, lifestyle, and clinical factors.4,23,29 Consideration must also be given to potential drug interactions.4,23,29 In IDUs, treatment with PEG-IFN a/RBV should be considered because DAA studies have excluded active users.20 Evaluation of the safety and efficacy of new regimens containing PEG-IFN a, as well as PEG-IFN-a–free regimens, in IDUs is needed.20
Renal impairment
Both PEG-IFN a and RBV require dose adjustments in patients with a creatinine clearance less than 30 mL/min.4,10,23,26 Further, simeprevir and sofosbuvir have not been studied in HCV patients with creatinine clearance less than 30 mL/min.10,26
On the next page: Barriers to therapy >>
BARRIERS TO THERAPY
Patient-related
Barriers to treatment include lack of acceptance of treatment due to the absence of symptoms; lengthy duration of treatment; adverse effects of HCV drugs; and treatment costs.10 Potential strategies to overcome such obstacles include patient education; simplified dosing; better-tolerated treatments; and collaboration with pharmaceutical companies that offer patient assistance programs.
Drugs for HCV treatment can cause unpleasant adverse effects. Clinicians should encourage adherence for the entire duration of treatment and provide practical advice for coping with adverse effects such as fatigue, headache and other flulike symptoms, injection site reactions, cough, bad taste in mouth, oral ulcers, dry mouth, anorexia, nausea or vomiting, skin reactions, hair thinning or hair loss, and insomnia.10,32
Strategies that may help alleviate these undesirable effects include regular low-impact exercise; drinking plenty of fluids; eating a well-balanced diet; maintaining good sleep hygiene; taking acetaminophen or ibuprofen for myalgias or headaches; and rotating PEG-IFN a injection sites.
Substance abuse and psychiatric disorders are common in patients with HCV infection. These patients should be referred to mental health or substance abuse services.10
Clinician-related
Obstacles to successfully treating patients with chronic HCV infection include patient-related barriers; lack of expertise in HCV management; practitioner bias against or resistance to treating patients who use illicit drugs or abuse alcohol; and concerns about the costs of treatment.
Potential strategies to overcome clinician barriers include collaboration with specialists (eg, hepatologists), utilizing telemedicine if necessary; availability of accessible, clear HCV treatment guidelines; and use of computer-based clinical decision support tools (eg, pop-up reminders and standing orders).10
PATIENT COUNSELING
Patients undergoing treatment for chronic HCV infection should be counseled on the following topics33
• Risk for transmission to sex partners
• Not sharing personal items that might have blood on them, such as toothbrushes or razors, and covering any bleeding wounds to keep from spreading infectious blood or secretions
• Need for vaccinations against HAV and HBV if not immune
• Not donating blood, organs, tissue, or semen
• Stop using illicit drugs
• If continuing to inject drugs, avoid reusing or sharing syringes, needles, water, or drug preparation equipment
• Clean the injection site with a new swab prior to injection
• Safely dispose of syringes after one use
• Consider the benefits of joining a support group
• Avoid alcohol because it can accelerate cirrhosis and end-stage liver disease
• Not to start any new medicines, including OTC and herbal medicines, without checking with their health care professional.
FUTURE TREATMENTS
Treatments for HCV infection are evolving rapidly, and IFN-free options with excellent SVRs are emerging. Below are brief summaries of some of the current research that is focused on the study of IFN-free options. Other new regimens are awaiting approval by the FDA. These include
• The combination of sofosbuvir plus ledipasvir (an NS5A inhibitor) with and without RBV, for HCV genotype 1 infection for 8 or 12 weeks. The SVR in both groups was 93% to 95%. RBV had no effect on SVR.33
• An all-oral combination therapy of daclatasvir (an HCV NS5A replication complex inhibitor) plus sofosbuvir, with or without RBV, for HCV genotypes 1, 2, and 3 for 24 weeks. The SVR varied from 98% with genotype 1, 92% with genotype 2, and 89% for genotype 3. Patients who received RBV had an SVR of 94%; those who did not achieved an SVR of 98%.34
• The combination of ABT-450 (a protease inhibitor boosted with ritonavir), ombitasvir (NS5A inhibitor), and dasabuvir (a nonnucleoside inhibitor) with RBV in patients with HCV genotype 1 and no cirrhosis. At 12 weeks, an SVR of 96% was achieved.35
Despite years of research, a vaccine to prevent HCV infection has not yet been developed, although research continues. The major challenge is the number of genotypes and subtypes of HCV. A vaccine to prevent HCV infection will need to induce immunity to all genotypes and subtypes.36
CONCLUSION
Patients with chronic HCV infection are frequently unaware of this fact, even though the majority of them acquired the liver disease decades ago. Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease. Identification of infected patients enables treatment initiation and, in most cases, cure of the infection. All patients at risk for infection should be counseled about risk reduction and screened periodically.
Thanks to newer, more effective treatment options, patients with HCV have an excellent chance today of clearing the virus and ultimately being cured. This could lead to a dramatic reduction in future HCV-associated morbidity and mortality. Since most of those infected today have never been treated, screening of at-risk patients is essential.
* Editor's note: At press time, the FDA had announced approval of a combination pill (ledipasvir/sofosbuvir) for the treatment of patients with chronic HCV.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. CDC. Hepatitis C FAQs for Health Professionals. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed October 19, 2014.
4. The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed October 19, 2014.
5. Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39(5):518-531.
6. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
7. CDC. Surveillance for Viral Hepatitis—United States, 2012. www.cdc.gov/
hepatitis/Statistics/2012Surveillance/index.htm. Accessed October 19, 2014.
8. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. Moyer VA, on behalf of the US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed October 19, 2014.
11. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR. 2012;61(RR-04):1-18.
12. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006;174(5):649-659.
13. Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med. 1993;86(2):119-125.
14. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687-1695.
15. Marinho RT, Vitor S, Velosa J. Benefits of curing hepatitis C infection.
J Gastrointestin Liver Dis. 2014;23(1):85-90.
16. Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369-3374.
17. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int. 2011;31(suppl 1): 3-12.
18. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
19. Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23(6):1334-1340.
20. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed October 19, 2014.
21. Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207.
22. Wiegand J, Buggisch P, Boecher W, et al; German HEP-NET Acute HCV Study Group. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-256.
23. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392-420.
24. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. September 2014. Published online ahead of print.
25. Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
26. Department of Veterans Affairs. National Hepatitis C Resource Center Program and the Office of Public Health. Chronic hepatitis C virus infection: treatment considerations. www.hepatitis.va.gov/pdf/2014hcv.pdf. Accessed October 19, 2014.
27. Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1-3.
28. Sofosbuvir (Sovaldi) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1434):5-6.
29. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
30. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aids info.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed October 19, 2014.
31. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systemic reviews. Lancet. 2011;378(9791):571-583.
32. US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration. A treatment improvement protocol: addressing viral hepatitis in people with substance abuse disorders. Tip 53. 2011.
33. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8-12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879-1888.
34. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211-221.
35. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):
1594-1603.
36. Drummer HE. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol. 2014;5:329.
CE/CME No: CR-1411
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the risk factors for HCV infection.
• Identify who should be screened for HCV infection.
• Discuss the symptoms, clinical course, diagnosis, and complications of chronic HCV infection.
• Differentiate between the treatment of acute and chronic HCV infection.
• Describe the challenges of treating HCV infection in patients who are coinfected with HIV.
FACULTY
Daniel Sturm and Samuel L. Gurevitz are Assistant Professors in the Physician Assistant Program, College of Pharmacy and Health Sciences, at Butler University in Indianapolis. Cassidy Davidson, Abigail Fritchley, and Audrey Wagaman are students in the PA Program at Butler University. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Several million Americans, primarily those in their fifties and sixties, contracted hepatitis C virus (HCV) many years ago and are unaware of their infection and their risk for HCV-related liver disease. Screening at-risk patients is important because newer treatment regimens are curative and can reduce associated morbidity and mortality.
Chronic infection with hepatitis C virus (HCV) is a major cause of liver disease in the United States. National Health and Nutrition Examination Survey data indicates that 2.7 million people have chronic HCV infection; the CDC estimates 3.2 million.1-3 Yet these individuals may be asymptomatic for years, despite slow progression of sequelae (eg, chronic liver disease, cirrhosis, hepatocellular carcinoma [HCC]) that may silently unfold. Left untreated, chronic HCV infection is associated with a 15% to 30% risk for cirrhosis within 20 years, which subsequently confers an annual risk for HCC of 2% to 4%.4 Additionally, chronic HCV infection is now the leading indication for liver transplantation.3
Of note, 70% of those with chronic HCV infection were born between 1945 and 1965.2 This is believed to be attributable to viral transmission via contaminated blood, blood products, and organ transplants prior to the implementation of universal precautions for blood supply screening in 1992; and to past injection drug use, even if it occurred only once.3
In a recent analysis, it was determined that the clinical and economic burdens of chronic HCV infection increased in the past decade, and this trend is likely to continue during the next decade.5 For example, HCV was responsible for 15,106 US deaths in 2007, surpassing deaths caused by HIV for the first time.6 Since then, the number of HCV-related deaths has continued to increase, to 17,721 in 2011.7 Economic costs solely attributable to HCV infection are difficult to calculate, but estimates range from several hundred million dollars to $30 billion annually.5 The World Health Organization (WHO) estimates that more than 185 million people worldwide are infected with HCV and that HCV is responsible for 350,000 deaths annually.4
The main goal of treatment is to achieve a sustained virologic response (SVR), defined as undetectable HCV RNA in serum 12 to 24 weeks after completion of treatment and thereby prevent or reduce the complications of HCV infection.3 Proactive screening, diagnosis, monitoring, and treatment of HCV infection can significantly reduce long-term morbidity and mortality.
INDICATIONS FOR HCV SCREENING
Recommendations for HCV screening have been developed by numerous organizations, including the WHO, CDC, US Preventive Services Task Force (USPSTF), American Association for the Study of Liver Diseases (AASLD), and the Infectious Diseases Society of America (IDSA) (see Table 1).3,4,8,9-11 Screening should be offered to all individuals meeting one or more of the criteria. A 2012 CDC update calls for one-time testing for all persons born between 1945 and 1965 because of the disproportionately high prevalence of HCV infection in this cohort, which is five times greater than in the general population.11
The initial screening tool for HCV infection is an HCV antibody test. A positive or reactive anti-HCV antibody result can signify either current or resolved infection, so a positive result should be followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present.4,8-10 See Figure on previous page for an HCV screening algorithm, which includes the points at which referrals to specialists are indicated.
A rapid HCV antibody test was approved by the FDA in 2011 and is favored because of its widespread availability, ease of use, rapid results, and low cost. Point-of-care testing is comparable in sensitivity and specificity to laboratory-based HCV assays and can utilize blood obtained via fingerstick or venipuncture.8 This form of testing facilitates HCV screening in a variety of settings, such as health fairs and emergency departments, as well as in high-risk settings such as methadone clinics.
Populations at increased risk for HCV infection are also at increased risk for hepatitis B virus (HBV), HIV, and tuberculosis infections; therefore, screening for these may also be warranted.4
PROGRESSION OF UNTREATED INFECTION
Just as with chronic HCV infection, patients newly infected with HCV are typically asymptomatic; the illness manifests with clinical symptoms in only 20% to 30% of cases.3 If symptoms do appear, they include fever, fatigue, dark urine, clay-colored stool, abdominal pain, loss of appetite, nausea, vomiting, joint pain, and jaundice. These acute phase symptoms may last two to 12 weeks.12
Spontaneous clearance of the virus occurs in only 15% to 25% of cases (see further discussion under “Acute HCV.”).2,3 Even if a patient clears an HCV infection, if he or she falls within one of the at-risk categories (see Table 1), then periodic screening should continue because prior HCV infection does not protect against future infection.3
The progression of chronic HCV is indolent and often subclinical, with fatigue being the most common complaint. Other nonspecific symptoms may include nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. One study noted that symptoms do not correlate with disease severity.13 In a 10-year prospective study, chronic HCV infection increased mortality when the infection was acquired at an early age (younger than 50) and/or when cirrhosis developed.14 Patients with cirrhosis progress to liver decompensation at a rate of 3% to 6% annually.15
In addition to liver disease, HCV infection is associated with an increased risk for non-Hodgkin lymphoma.3 HCV may also induce insulin resistance, which increases the risk for hepatic fibrosis. Other studies document HCV-induced cognitive impairment, but little scientific data is yet available as to its pathogenesis.16
DIAGNOSIS
Diagnostic testing for suspected HCV infection begins with an antibody test.8 A positive antibody test indicates one of three possibilities: active infection, resolved infection, or a false-positive result. Two drawbacks of HCV antibody testing are that immunocompromised patients may falsely test negative and that antibodies cannot be detected until eight to 12 weeks after the infection is acquired.11 A positive antibody test should be confirmed with an HCV polymerase chain reaction (PCR) RNA test.8,10
HCV RNA testing can detect the virus earlier than antibody testing—as early as two weeks after infection. Although a positive HCV RNA test confirms current HCV infection, its higher cost precludes its use as an initial diagnostic test for lower-risk patients.
In patients who test negative but for whom there is a high index of suspicion for HCV infection (eg, jaundiced patient with an elevated alanine aminotransferase level [ALT]), or for a health care worker with a recent bloodborne HCV exposure, testing for HCV antibodies, HCV RNA, and ALT levels should be ordered at regular intervals for a period of six months.10
Acute versus chronic infection
Distinguishing acute and chronic HCV is difficult. A determination that HCV is newly acquired requires a documented negative baseline antibody test, followed by laboratory evidence of seroconversion. This is typically only seen in cases where there has been a recent, known exposure to the virus.
Both HCV antibody and RNA testing are recommended when screening high-risk patients, including those who are immunocompromised, on hemodialysis, or have had a recent exposure to HCV-positive blood.10 Rapid PCR HCV RNA tests can assess both viral load and genotype (see discussion of HCV genotypes under “Chronic HCV”).17 This information helps guide and measure patient response to treatment.
Liver disease severity
Liver fibrosis and cirrhosis are serious complications of HCV; hepatomegaly or splenomegaly may or may not be present on physical examination and patients may require liver biopsies to evaluate disease stage.18 An assessment of the severity of liver damage can determine the urgency of treatment and predict treatment efficacy.
Biopsy results permit the grading of inflammation and nodularity and the staging of septal fibrosis, which can reliably predict future progression of the patient’s disease.19 However, liver biopsy is invasive, painful, and may contain sampling errors; complications may include bleeding, infection, and occasionally accidental injury to a nearby organ. An initial noninvasive assessment may be performed using vibration-controlled transient liver elastography, an ultrasound-based technology that measures liver stiffness, which correlates well with the degree of fibrosis or cirrhosis. Elastography, along with measurement of direct serum biomarkers that are produced by activated hepatic stellate cells involved in fibrosis, affords an accurate, noninvasive means of assessing liver damage.10
Continue for treatment options >>
TREATMENT
Acute HCV
To prevent progression of disease from acute to chronic infection, patients diagnosed with acute disease should be treated if
• They are likely to adhere to the treatment plan
• They have no contraindications to pegylated interferon a (PEG-IFN a) treatment.
Contraindications to PEG-IFN a treatment include uncontrolled depression, psychosis, or epilepsy; pregnancy; couples' unwillingness to use effective contraception during treatment; severe concurrent medical disease; and decompensated liver disease.20
For acute HCV infection, treatment with PEG-IFN a-2a 180 µg/wk or PEG-IFN a-2b 1.5 µg/kg/wk for 24 weeks is recommended.20 Treatment results in an SVR greater than 80%.21 Combination therapy with ribavirin (RBV) does not increase SVR in the treatment of acute HCV infection.22
The appropriate time to begin treatment has not been firmly established because, as previously noted, 15% to 25% of those infected will spontaneously clear the virus. The European Association for the Study of the Liver (EASL) suggests that patients who remain HCV positive at 12 weeks from the time of suspected infection should be treated.23
Chronic HCV
Treatment of chronic HCV is based on multiple considerations, many of them patient-specific (see Table 2). One key element in treatment choice is the HCV genotype with which the patient is infected.
The most prevalent in the US are genotypes 1 through 6; further, genotype 1 has two subtypes: 1a and 1b. In the US, genotype 1 is most common, infecting about 70% of patients; genotype 2, 16%; genotype 3, 12%; genotype 4, 1%; genotype 5, < 1%; and genotype 6, 1%.24 Infection with one genotype does not protect an individual from future infection with the same or a different HCV genotype.3
Other factors influencing treatment include viral load, ALT levels, coinfection (eg, HIV, HBV), comorbidities, and treatment contraindications.21 Treatment is recommended for those who have detectable HCV RNA levels, elevated ALT levels, progressive liver disease on biopsy, and the absence of any serious comorbid conditions or contraindications. ALT levels, however, can fluctuate and do not always correlate with disease severity.
For years, the standard treatment for chronic HCV infection has been PEG-IFN a/RBV for 48 weeks. This combination produced an SVR of 50% to 80%, depending on genotype.3,25 Recently, however, treatment protocols have changed considerably with the introduction of two new and very effective direct-acting antiviral agents (DAAs): simeprevir, an HCV NS3/4A protease inhibitor, and sofosbuvir, an HCV NS5B polymerase inhibitor.
Simeprevir was approved by the FDA in October 2013 for use in combination with PEG-IFN/RBV for treatment of chronic HCV genotype 1 infection in adults with compensated liver disease.27 Because the efficacy of simeprevir is reduced in patients with HCV genotype 1a with an NS3 Q80K polymorphism, screening for NS3 Q80K is recommended; alternative therapy should be considered when this mutation is present.27
Sofosbuvir received FDA approval in December 2013 for use in combination with other antiviral drugs for treatment of chronic HCV infection, with established efficacy for treatment of genotypes 1, 2, 3, and 4 and for HCV/HIV coinfection.28 For genotype 1, simeprevir and sofosbuvir achieve SVRs of 80% and 90%, respectively.27,28
Table 3 lists the current medications for treatment of chronic HCV, including their adverse effects, contraindications, and drug interactions.
Updated treatment guidelines
AASLD, IDSA, EASL, WHO, and the Department of Veterans Affairs National Hepatitis C Resource Center Program recently issued updated evidence-based recommendations for the treatment of chronic HCV.4,10,23,26 Highlights of the changes to the guidelines include
• With the introduction of simeprevir and sofosbuvir, the guidelines no longer recommend combination PEG-IFN a/RBV for 48 weeks as the standard treatment for chronic HCV infection11,23,26
• Treatment regimens utilizing the protease inhibitors telaprevir and boceprevir are no longer recommended (with the exception of the WHO guidelines, which include them in a “conditional” recommendation).4
Regimens that include telaprevir and boceprevir are associated with higher rates of serious adverse effects, such as skin reactions and anemia, and involve longer treatment duration, higher pill burden, several drug interactions, frequent dosing, intensive monitoring, and the need to be taken with food (for telaprevir, high-fat food).10,23,26
Finally, the EASL recommends an additional agent, daclatasvir, an NS5A replication complex inhibitor, as an option for treating HCV genotype 1, 3, and 4.23 At this time, it is approved for use in Europe but has not been approved by the FDA.
Regardless of the treatment regimen, all patients receiving HCV antiviral therapy should be tested regularly to assess effectiveness of treatment and to monitor for the occurrence of adverse effects. Recommended periodic laboratory testing should include HCV RNA, complete blood count with differential, liver function, TSH level, renal function, comprehensive metabolic panel, bilirubin level, and pregnancy.29
SPECIAL POPULATIONS
Patients with HIV/HCV coinfection, a history of injection drug use, and renal impairment require management tailored to their individual circumstances.4,23,29
HIV/HCV-coinfected patients
Approximately 25% to 33% of patients infected with HIV are coinfected with HCV. HCV infection progresses more rapidly in HIV-infected patients, and coinfected patients are at greater risk for cirrhosis, liver cancer, and liver failure.30
For patients with HIV/HCV coinfection, the decision to start treatment is more complex because of the high pill burden, overlapping toxicities, and interactions among the drugs used to treat HIV and HCV infections.4 HIV antiviral therapy should be started before HCV treatment to improve immune function, thereby decreasing the risks for both further infections and HIV transmission. This also allows the patient to adjust gradually to each regimen.
One exception to this is when an HIV treatment–naïve patient has a CD4 count > 500 cells/mL.30 In this situation, HCV treatment is sometimes completed prior to the start of HIV treatment.
Recommended treatment for HIV/HCV coinfection, by HCV genotype, is outlined below. For all regimens, the weight-based RBV dosage is calculated as follows:
• Weight < 75 kg, 1,000 mg/d
• Weight ≥ 75 kg, 1,200 mg/d10,30
• Genotypes 1 and 4 (IFN eligible): Sofosbuvir 400 mg/d and weight-based RBV plus weekly PEG-IFN a for 12 weeks
• Genotypes 1 and 4 (IFN ineligible): Sofosbuvir 400 mg/d and weight-based RBV for 24 weeks
• Genotypes 2 and 3 (regardless of IFN eligibility): Sofosbuvir 400 mg/d and weight-based RBV for 12 weeks for genotype 2 and 24 weeks for genotype 3
• Genotypes 5 and 6 (IFN eligible): Sofosbuvir 400 mg/d plus weight-based RBV plus weekly PEG-IFN a for 12 weeks.
Injection drug users
HCV infection among young injection drug users (IDUs) is an emerging epidemic that must be addressed by recognizing at-risk populations, screening for early disease, and providing treatment and education. Globally, it is estimated that approximately 67% of IDUs—approximately 10 million people—are infected with HCV.31 Treatment of HCV in IDUs requires integration of many services and health care professionals, including addiction specialists. Dependence on opiates, alcohol, or other substances is common in this patient population. Patients should be counseled on the importance of abstaining from alcohol. IDUs are at risk for hepatitis A (HAV) and HBV infections and should be vaccinated against these diseases.4
Treatment decisions should be based on an individualized evaluation of the patient’s social, lifestyle, and clinical factors.4,23,29 Consideration must also be given to potential drug interactions.4,23,29 In IDUs, treatment with PEG-IFN a/RBV should be considered because DAA studies have excluded active users.20 Evaluation of the safety and efficacy of new regimens containing PEG-IFN a, as well as PEG-IFN-a–free regimens, in IDUs is needed.20
Renal impairment
Both PEG-IFN a and RBV require dose adjustments in patients with a creatinine clearance less than 30 mL/min.4,10,23,26 Further, simeprevir and sofosbuvir have not been studied in HCV patients with creatinine clearance less than 30 mL/min.10,26
On the next page: Barriers to therapy >>
BARRIERS TO THERAPY
Patient-related
Barriers to treatment include lack of acceptance of treatment due to the absence of symptoms; lengthy duration of treatment; adverse effects of HCV drugs; and treatment costs.10 Potential strategies to overcome such obstacles include patient education; simplified dosing; better-tolerated treatments; and collaboration with pharmaceutical companies that offer patient assistance programs.
Drugs for HCV treatment can cause unpleasant adverse effects. Clinicians should encourage adherence for the entire duration of treatment and provide practical advice for coping with adverse effects such as fatigue, headache and other flulike symptoms, injection site reactions, cough, bad taste in mouth, oral ulcers, dry mouth, anorexia, nausea or vomiting, skin reactions, hair thinning or hair loss, and insomnia.10,32
Strategies that may help alleviate these undesirable effects include regular low-impact exercise; drinking plenty of fluids; eating a well-balanced diet; maintaining good sleep hygiene; taking acetaminophen or ibuprofen for myalgias or headaches; and rotating PEG-IFN a injection sites.
Substance abuse and psychiatric disorders are common in patients with HCV infection. These patients should be referred to mental health or substance abuse services.10
Clinician-related
Obstacles to successfully treating patients with chronic HCV infection include patient-related barriers; lack of expertise in HCV management; practitioner bias against or resistance to treating patients who use illicit drugs or abuse alcohol; and concerns about the costs of treatment.
Potential strategies to overcome clinician barriers include collaboration with specialists (eg, hepatologists), utilizing telemedicine if necessary; availability of accessible, clear HCV treatment guidelines; and use of computer-based clinical decision support tools (eg, pop-up reminders and standing orders).10
PATIENT COUNSELING
Patients undergoing treatment for chronic HCV infection should be counseled on the following topics33
• Risk for transmission to sex partners
• Not sharing personal items that might have blood on them, such as toothbrushes or razors, and covering any bleeding wounds to keep from spreading infectious blood or secretions
• Need for vaccinations against HAV and HBV if not immune
• Not donating blood, organs, tissue, or semen
• Stop using illicit drugs
• If continuing to inject drugs, avoid reusing or sharing syringes, needles, water, or drug preparation equipment
• Clean the injection site with a new swab prior to injection
• Safely dispose of syringes after one use
• Consider the benefits of joining a support group
• Avoid alcohol because it can accelerate cirrhosis and end-stage liver disease
• Not to start any new medicines, including OTC and herbal medicines, without checking with their health care professional.
FUTURE TREATMENTS
Treatments for HCV infection are evolving rapidly, and IFN-free options with excellent SVRs are emerging. Below are brief summaries of some of the current research that is focused on the study of IFN-free options. Other new regimens are awaiting approval by the FDA. These include
• The combination of sofosbuvir plus ledipasvir (an NS5A inhibitor) with and without RBV, for HCV genotype 1 infection for 8 or 12 weeks. The SVR in both groups was 93% to 95%. RBV had no effect on SVR.33
• An all-oral combination therapy of daclatasvir (an HCV NS5A replication complex inhibitor) plus sofosbuvir, with or without RBV, for HCV genotypes 1, 2, and 3 for 24 weeks. The SVR varied from 98% with genotype 1, 92% with genotype 2, and 89% for genotype 3. Patients who received RBV had an SVR of 94%; those who did not achieved an SVR of 98%.34
• The combination of ABT-450 (a protease inhibitor boosted with ritonavir), ombitasvir (NS5A inhibitor), and dasabuvir (a nonnucleoside inhibitor) with RBV in patients with HCV genotype 1 and no cirrhosis. At 12 weeks, an SVR of 96% was achieved.35
Despite years of research, a vaccine to prevent HCV infection has not yet been developed, although research continues. The major challenge is the number of genotypes and subtypes of HCV. A vaccine to prevent HCV infection will need to induce immunity to all genotypes and subtypes.36
CONCLUSION
Patients with chronic HCV infection are frequently unaware of this fact, even though the majority of them acquired the liver disease decades ago. Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease. Identification of infected patients enables treatment initiation and, in most cases, cure of the infection. All patients at risk for infection should be counseled about risk reduction and screened periodically.
Thanks to newer, more effective treatment options, patients with HCV have an excellent chance today of clearing the virus and ultimately being cured. This could lead to a dramatic reduction in future HCV-associated morbidity and mortality. Since most of those infected today have never been treated, screening of at-risk patients is essential.
* Editor's note: At press time, the FDA had announced approval of a combination pill (ledipasvir/sofosbuvir) for the treatment of patients with chronic HCV.
CE/CME No: CR-1411
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the risk factors for HCV infection.
• Identify who should be screened for HCV infection.
• Discuss the symptoms, clinical course, diagnosis, and complications of chronic HCV infection.
• Differentiate between the treatment of acute and chronic HCV infection.
• Describe the challenges of treating HCV infection in patients who are coinfected with HIV.
FACULTY
Daniel Sturm and Samuel L. Gurevitz are Assistant Professors in the Physician Assistant Program, College of Pharmacy and Health Sciences, at Butler University in Indianapolis. Cassidy Davidson, Abigail Fritchley, and Audrey Wagaman are students in the PA Program at Butler University. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Several million Americans, primarily those in their fifties and sixties, contracted hepatitis C virus (HCV) many years ago and are unaware of their infection and their risk for HCV-related liver disease. Screening at-risk patients is important because newer treatment regimens are curative and can reduce associated morbidity and mortality.
Chronic infection with hepatitis C virus (HCV) is a major cause of liver disease in the United States. National Health and Nutrition Examination Survey data indicates that 2.7 million people have chronic HCV infection; the CDC estimates 3.2 million.1-3 Yet these individuals may be asymptomatic for years, despite slow progression of sequelae (eg, chronic liver disease, cirrhosis, hepatocellular carcinoma [HCC]) that may silently unfold. Left untreated, chronic HCV infection is associated with a 15% to 30% risk for cirrhosis within 20 years, which subsequently confers an annual risk for HCC of 2% to 4%.4 Additionally, chronic HCV infection is now the leading indication for liver transplantation.3
Of note, 70% of those with chronic HCV infection were born between 1945 and 1965.2 This is believed to be attributable to viral transmission via contaminated blood, blood products, and organ transplants prior to the implementation of universal precautions for blood supply screening in 1992; and to past injection drug use, even if it occurred only once.3
In a recent analysis, it was determined that the clinical and economic burdens of chronic HCV infection increased in the past decade, and this trend is likely to continue during the next decade.5 For example, HCV was responsible for 15,106 US deaths in 2007, surpassing deaths caused by HIV for the first time.6 Since then, the number of HCV-related deaths has continued to increase, to 17,721 in 2011.7 Economic costs solely attributable to HCV infection are difficult to calculate, but estimates range from several hundred million dollars to $30 billion annually.5 The World Health Organization (WHO) estimates that more than 185 million people worldwide are infected with HCV and that HCV is responsible for 350,000 deaths annually.4
The main goal of treatment is to achieve a sustained virologic response (SVR), defined as undetectable HCV RNA in serum 12 to 24 weeks after completion of treatment and thereby prevent or reduce the complications of HCV infection.3 Proactive screening, diagnosis, monitoring, and treatment of HCV infection can significantly reduce long-term morbidity and mortality.
INDICATIONS FOR HCV SCREENING
Recommendations for HCV screening have been developed by numerous organizations, including the WHO, CDC, US Preventive Services Task Force (USPSTF), American Association for the Study of Liver Diseases (AASLD), and the Infectious Diseases Society of America (IDSA) (see Table 1).3,4,8,9-11 Screening should be offered to all individuals meeting one or more of the criteria. A 2012 CDC update calls for one-time testing for all persons born between 1945 and 1965 because of the disproportionately high prevalence of HCV infection in this cohort, which is five times greater than in the general population.11
The initial screening tool for HCV infection is an HCV antibody test. A positive or reactive anti-HCV antibody result can signify either current or resolved infection, so a positive result should be followed with an HCV ribonucleic acid (RNA) test to determine if active infection is present.4,8-10 See Figure on previous page for an HCV screening algorithm, which includes the points at which referrals to specialists are indicated.
A rapid HCV antibody test was approved by the FDA in 2011 and is favored because of its widespread availability, ease of use, rapid results, and low cost. Point-of-care testing is comparable in sensitivity and specificity to laboratory-based HCV assays and can utilize blood obtained via fingerstick or venipuncture.8 This form of testing facilitates HCV screening in a variety of settings, such as health fairs and emergency departments, as well as in high-risk settings such as methadone clinics.
Populations at increased risk for HCV infection are also at increased risk for hepatitis B virus (HBV), HIV, and tuberculosis infections; therefore, screening for these may also be warranted.4
PROGRESSION OF UNTREATED INFECTION
Just as with chronic HCV infection, patients newly infected with HCV are typically asymptomatic; the illness manifests with clinical symptoms in only 20% to 30% of cases.3 If symptoms do appear, they include fever, fatigue, dark urine, clay-colored stool, abdominal pain, loss of appetite, nausea, vomiting, joint pain, and jaundice. These acute phase symptoms may last two to 12 weeks.12
Spontaneous clearance of the virus occurs in only 15% to 25% of cases (see further discussion under “Acute HCV.”).2,3 Even if a patient clears an HCV infection, if he or she falls within one of the at-risk categories (see Table 1), then periodic screening should continue because prior HCV infection does not protect against future infection.3
The progression of chronic HCV is indolent and often subclinical, with fatigue being the most common complaint. Other nonspecific symptoms may include nausea, anorexia, myalgia, arthralgia, weakness, and weight loss. One study noted that symptoms do not correlate with disease severity.13 In a 10-year prospective study, chronic HCV infection increased mortality when the infection was acquired at an early age (younger than 50) and/or when cirrhosis developed.14 Patients with cirrhosis progress to liver decompensation at a rate of 3% to 6% annually.15
In addition to liver disease, HCV infection is associated with an increased risk for non-Hodgkin lymphoma.3 HCV may also induce insulin resistance, which increases the risk for hepatic fibrosis. Other studies document HCV-induced cognitive impairment, but little scientific data is yet available as to its pathogenesis.16
DIAGNOSIS
Diagnostic testing for suspected HCV infection begins with an antibody test.8 A positive antibody test indicates one of three possibilities: active infection, resolved infection, or a false-positive result. Two drawbacks of HCV antibody testing are that immunocompromised patients may falsely test negative and that antibodies cannot be detected until eight to 12 weeks after the infection is acquired.11 A positive antibody test should be confirmed with an HCV polymerase chain reaction (PCR) RNA test.8,10
HCV RNA testing can detect the virus earlier than antibody testing—as early as two weeks after infection. Although a positive HCV RNA test confirms current HCV infection, its higher cost precludes its use as an initial diagnostic test for lower-risk patients.
In patients who test negative but for whom there is a high index of suspicion for HCV infection (eg, jaundiced patient with an elevated alanine aminotransferase level [ALT]), or for a health care worker with a recent bloodborne HCV exposure, testing for HCV antibodies, HCV RNA, and ALT levels should be ordered at regular intervals for a period of six months.10
Acute versus chronic infection
Distinguishing acute and chronic HCV is difficult. A determination that HCV is newly acquired requires a documented negative baseline antibody test, followed by laboratory evidence of seroconversion. This is typically only seen in cases where there has been a recent, known exposure to the virus.
Both HCV antibody and RNA testing are recommended when screening high-risk patients, including those who are immunocompromised, on hemodialysis, or have had a recent exposure to HCV-positive blood.10 Rapid PCR HCV RNA tests can assess both viral load and genotype (see discussion of HCV genotypes under “Chronic HCV”).17 This information helps guide and measure patient response to treatment.
Liver disease severity
Liver fibrosis and cirrhosis are serious complications of HCV; hepatomegaly or splenomegaly may or may not be present on physical examination and patients may require liver biopsies to evaluate disease stage.18 An assessment of the severity of liver damage can determine the urgency of treatment and predict treatment efficacy.
Biopsy results permit the grading of inflammation and nodularity and the staging of septal fibrosis, which can reliably predict future progression of the patient’s disease.19 However, liver biopsy is invasive, painful, and may contain sampling errors; complications may include bleeding, infection, and occasionally accidental injury to a nearby organ. An initial noninvasive assessment may be performed using vibration-controlled transient liver elastography, an ultrasound-based technology that measures liver stiffness, which correlates well with the degree of fibrosis or cirrhosis. Elastography, along with measurement of direct serum biomarkers that are produced by activated hepatic stellate cells involved in fibrosis, affords an accurate, noninvasive means of assessing liver damage.10
Continue for treatment options >>
TREATMENT
Acute HCV
To prevent progression of disease from acute to chronic infection, patients diagnosed with acute disease should be treated if
• They are likely to adhere to the treatment plan
• They have no contraindications to pegylated interferon a (PEG-IFN a) treatment.
Contraindications to PEG-IFN a treatment include uncontrolled depression, psychosis, or epilepsy; pregnancy; couples' unwillingness to use effective contraception during treatment; severe concurrent medical disease; and decompensated liver disease.20
For acute HCV infection, treatment with PEG-IFN a-2a 180 µg/wk or PEG-IFN a-2b 1.5 µg/kg/wk for 24 weeks is recommended.20 Treatment results in an SVR greater than 80%.21 Combination therapy with ribavirin (RBV) does not increase SVR in the treatment of acute HCV infection.22
The appropriate time to begin treatment has not been firmly established because, as previously noted, 15% to 25% of those infected will spontaneously clear the virus. The European Association for the Study of the Liver (EASL) suggests that patients who remain HCV positive at 12 weeks from the time of suspected infection should be treated.23
Chronic HCV
Treatment of chronic HCV is based on multiple considerations, many of them patient-specific (see Table 2). One key element in treatment choice is the HCV genotype with which the patient is infected.
The most prevalent in the US are genotypes 1 through 6; further, genotype 1 has two subtypes: 1a and 1b. In the US, genotype 1 is most common, infecting about 70% of patients; genotype 2, 16%; genotype 3, 12%; genotype 4, 1%; genotype 5, < 1%; and genotype 6, 1%.24 Infection with one genotype does not protect an individual from future infection with the same or a different HCV genotype.3
Other factors influencing treatment include viral load, ALT levels, coinfection (eg, HIV, HBV), comorbidities, and treatment contraindications.21 Treatment is recommended for those who have detectable HCV RNA levels, elevated ALT levels, progressive liver disease on biopsy, and the absence of any serious comorbid conditions or contraindications. ALT levels, however, can fluctuate and do not always correlate with disease severity.
For years, the standard treatment for chronic HCV infection has been PEG-IFN a/RBV for 48 weeks. This combination produced an SVR of 50% to 80%, depending on genotype.3,25 Recently, however, treatment protocols have changed considerably with the introduction of two new and very effective direct-acting antiviral agents (DAAs): simeprevir, an HCV NS3/4A protease inhibitor, and sofosbuvir, an HCV NS5B polymerase inhibitor.
Simeprevir was approved by the FDA in October 2013 for use in combination with PEG-IFN/RBV for treatment of chronic HCV genotype 1 infection in adults with compensated liver disease.27 Because the efficacy of simeprevir is reduced in patients with HCV genotype 1a with an NS3 Q80K polymorphism, screening for NS3 Q80K is recommended; alternative therapy should be considered when this mutation is present.27
Sofosbuvir received FDA approval in December 2013 for use in combination with other antiviral drugs for treatment of chronic HCV infection, with established efficacy for treatment of genotypes 1, 2, 3, and 4 and for HCV/HIV coinfection.28 For genotype 1, simeprevir and sofosbuvir achieve SVRs of 80% and 90%, respectively.27,28
Table 3 lists the current medications for treatment of chronic HCV, including their adverse effects, contraindications, and drug interactions.
Updated treatment guidelines
AASLD, IDSA, EASL, WHO, and the Department of Veterans Affairs National Hepatitis C Resource Center Program recently issued updated evidence-based recommendations for the treatment of chronic HCV.4,10,23,26 Highlights of the changes to the guidelines include
• With the introduction of simeprevir and sofosbuvir, the guidelines no longer recommend combination PEG-IFN a/RBV for 48 weeks as the standard treatment for chronic HCV infection11,23,26
• Treatment regimens utilizing the protease inhibitors telaprevir and boceprevir are no longer recommended (with the exception of the WHO guidelines, which include them in a “conditional” recommendation).4
Regimens that include telaprevir and boceprevir are associated with higher rates of serious adverse effects, such as skin reactions and anemia, and involve longer treatment duration, higher pill burden, several drug interactions, frequent dosing, intensive monitoring, and the need to be taken with food (for telaprevir, high-fat food).10,23,26
Finally, the EASL recommends an additional agent, daclatasvir, an NS5A replication complex inhibitor, as an option for treating HCV genotype 1, 3, and 4.23 At this time, it is approved for use in Europe but has not been approved by the FDA.
Regardless of the treatment regimen, all patients receiving HCV antiviral therapy should be tested regularly to assess effectiveness of treatment and to monitor for the occurrence of adverse effects. Recommended periodic laboratory testing should include HCV RNA, complete blood count with differential, liver function, TSH level, renal function, comprehensive metabolic panel, bilirubin level, and pregnancy.29
SPECIAL POPULATIONS
Patients with HIV/HCV coinfection, a history of injection drug use, and renal impairment require management tailored to their individual circumstances.4,23,29
HIV/HCV-coinfected patients
Approximately 25% to 33% of patients infected with HIV are coinfected with HCV. HCV infection progresses more rapidly in HIV-infected patients, and coinfected patients are at greater risk for cirrhosis, liver cancer, and liver failure.30
For patients with HIV/HCV coinfection, the decision to start treatment is more complex because of the high pill burden, overlapping toxicities, and interactions among the drugs used to treat HIV and HCV infections.4 HIV antiviral therapy should be started before HCV treatment to improve immune function, thereby decreasing the risks for both further infections and HIV transmission. This also allows the patient to adjust gradually to each regimen.
One exception to this is when an HIV treatment–naïve patient has a CD4 count > 500 cells/mL.30 In this situation, HCV treatment is sometimes completed prior to the start of HIV treatment.
Recommended treatment for HIV/HCV coinfection, by HCV genotype, is outlined below. For all regimens, the weight-based RBV dosage is calculated as follows:
• Weight < 75 kg, 1,000 mg/d
• Weight ≥ 75 kg, 1,200 mg/d10,30
• Genotypes 1 and 4 (IFN eligible): Sofosbuvir 400 mg/d and weight-based RBV plus weekly PEG-IFN a for 12 weeks
• Genotypes 1 and 4 (IFN ineligible): Sofosbuvir 400 mg/d and weight-based RBV for 24 weeks
• Genotypes 2 and 3 (regardless of IFN eligibility): Sofosbuvir 400 mg/d and weight-based RBV for 12 weeks for genotype 2 and 24 weeks for genotype 3
• Genotypes 5 and 6 (IFN eligible): Sofosbuvir 400 mg/d plus weight-based RBV plus weekly PEG-IFN a for 12 weeks.
Injection drug users
HCV infection among young injection drug users (IDUs) is an emerging epidemic that must be addressed by recognizing at-risk populations, screening for early disease, and providing treatment and education. Globally, it is estimated that approximately 67% of IDUs—approximately 10 million people—are infected with HCV.31 Treatment of HCV in IDUs requires integration of many services and health care professionals, including addiction specialists. Dependence on opiates, alcohol, or other substances is common in this patient population. Patients should be counseled on the importance of abstaining from alcohol. IDUs are at risk for hepatitis A (HAV) and HBV infections and should be vaccinated against these diseases.4
Treatment decisions should be based on an individualized evaluation of the patient’s social, lifestyle, and clinical factors.4,23,29 Consideration must also be given to potential drug interactions.4,23,29 In IDUs, treatment with PEG-IFN a/RBV should be considered because DAA studies have excluded active users.20 Evaluation of the safety and efficacy of new regimens containing PEG-IFN a, as well as PEG-IFN-a–free regimens, in IDUs is needed.20
Renal impairment
Both PEG-IFN a and RBV require dose adjustments in patients with a creatinine clearance less than 30 mL/min.4,10,23,26 Further, simeprevir and sofosbuvir have not been studied in HCV patients with creatinine clearance less than 30 mL/min.10,26
On the next page: Barriers to therapy >>
BARRIERS TO THERAPY
Patient-related
Barriers to treatment include lack of acceptance of treatment due to the absence of symptoms; lengthy duration of treatment; adverse effects of HCV drugs; and treatment costs.10 Potential strategies to overcome such obstacles include patient education; simplified dosing; better-tolerated treatments; and collaboration with pharmaceutical companies that offer patient assistance programs.
Drugs for HCV treatment can cause unpleasant adverse effects. Clinicians should encourage adherence for the entire duration of treatment and provide practical advice for coping with adverse effects such as fatigue, headache and other flulike symptoms, injection site reactions, cough, bad taste in mouth, oral ulcers, dry mouth, anorexia, nausea or vomiting, skin reactions, hair thinning or hair loss, and insomnia.10,32
Strategies that may help alleviate these undesirable effects include regular low-impact exercise; drinking plenty of fluids; eating a well-balanced diet; maintaining good sleep hygiene; taking acetaminophen or ibuprofen for myalgias or headaches; and rotating PEG-IFN a injection sites.
Substance abuse and psychiatric disorders are common in patients with HCV infection. These patients should be referred to mental health or substance abuse services.10
Clinician-related
Obstacles to successfully treating patients with chronic HCV infection include patient-related barriers; lack of expertise in HCV management; practitioner bias against or resistance to treating patients who use illicit drugs or abuse alcohol; and concerns about the costs of treatment.
Potential strategies to overcome clinician barriers include collaboration with specialists (eg, hepatologists), utilizing telemedicine if necessary; availability of accessible, clear HCV treatment guidelines; and use of computer-based clinical decision support tools (eg, pop-up reminders and standing orders).10
PATIENT COUNSELING
Patients undergoing treatment for chronic HCV infection should be counseled on the following topics33
• Risk for transmission to sex partners
• Not sharing personal items that might have blood on them, such as toothbrushes or razors, and covering any bleeding wounds to keep from spreading infectious blood or secretions
• Need for vaccinations against HAV and HBV if not immune
• Not donating blood, organs, tissue, or semen
• Stop using illicit drugs
• If continuing to inject drugs, avoid reusing or sharing syringes, needles, water, or drug preparation equipment
• Clean the injection site with a new swab prior to injection
• Safely dispose of syringes after one use
• Consider the benefits of joining a support group
• Avoid alcohol because it can accelerate cirrhosis and end-stage liver disease
• Not to start any new medicines, including OTC and herbal medicines, without checking with their health care professional.
FUTURE TREATMENTS
Treatments for HCV infection are evolving rapidly, and IFN-free options with excellent SVRs are emerging. Below are brief summaries of some of the current research that is focused on the study of IFN-free options. Other new regimens are awaiting approval by the FDA. These include
• The combination of sofosbuvir plus ledipasvir (an NS5A inhibitor) with and without RBV, for HCV genotype 1 infection for 8 or 12 weeks. The SVR in both groups was 93% to 95%. RBV had no effect on SVR.33
• An all-oral combination therapy of daclatasvir (an HCV NS5A replication complex inhibitor) plus sofosbuvir, with or without RBV, for HCV genotypes 1, 2, and 3 for 24 weeks. The SVR varied from 98% with genotype 1, 92% with genotype 2, and 89% for genotype 3. Patients who received RBV had an SVR of 94%; those who did not achieved an SVR of 98%.34
• The combination of ABT-450 (a protease inhibitor boosted with ritonavir), ombitasvir (NS5A inhibitor), and dasabuvir (a nonnucleoside inhibitor) with RBV in patients with HCV genotype 1 and no cirrhosis. At 12 weeks, an SVR of 96% was achieved.35
Despite years of research, a vaccine to prevent HCV infection has not yet been developed, although research continues. The major challenge is the number of genotypes and subtypes of HCV. A vaccine to prevent HCV infection will need to induce immunity to all genotypes and subtypes.36
CONCLUSION
Patients with chronic HCV infection are frequently unaware of this fact, even though the majority of them acquired the liver disease decades ago. Because of the potentially serious consequences of untreated chronic HCV, it is critical that primary care clinicians identify and screen patients who are at risk for having or acquiring the disease. Identification of infected patients enables treatment initiation and, in most cases, cure of the infection. All patients at risk for infection should be counseled about risk reduction and screened periodically.
Thanks to newer, more effective treatment options, patients with HCV have an excellent chance today of clearing the virus and ultimately being cured. This could lead to a dramatic reduction in future HCV-associated morbidity and mortality. Since most of those infected today have never been treated, screening of at-risk patients is essential.
* Editor's note: At press time, the FDA had announced approval of a combination pill (ledipasvir/sofosbuvir) for the treatment of patients with chronic HCV.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. CDC. Hepatitis C FAQs for Health Professionals. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed October 19, 2014.
4. The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed October 19, 2014.
5. Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39(5):518-531.
6. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
7. CDC. Surveillance for Viral Hepatitis—United States, 2012. www.cdc.gov/
hepatitis/Statistics/2012Surveillance/index.htm. Accessed October 19, 2014.
8. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. Moyer VA, on behalf of the US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed October 19, 2014.
11. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR. 2012;61(RR-04):1-18.
12. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006;174(5):649-659.
13. Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med. 1993;86(2):119-125.
14. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687-1695.
15. Marinho RT, Vitor S, Velosa J. Benefits of curing hepatitis C infection.
J Gastrointestin Liver Dis. 2014;23(1):85-90.
16. Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369-3374.
17. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int. 2011;31(suppl 1): 3-12.
18. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
19. Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23(6):1334-1340.
20. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed October 19, 2014.
21. Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207.
22. Wiegand J, Buggisch P, Boecher W, et al; German HEP-NET Acute HCV Study Group. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-256.
23. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392-420.
24. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. September 2014. Published online ahead of print.
25. Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
26. Department of Veterans Affairs. National Hepatitis C Resource Center Program and the Office of Public Health. Chronic hepatitis C virus infection: treatment considerations. www.hepatitis.va.gov/pdf/2014hcv.pdf. Accessed October 19, 2014.
27. Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1-3.
28. Sofosbuvir (Sovaldi) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1434):5-6.
29. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
30. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aids info.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed October 19, 2014.
31. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systemic reviews. Lancet. 2011;378(9791):571-583.
32. US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration. A treatment improvement protocol: addressing viral hepatitis in people with substance abuse disorders. Tip 53. 2011.
33. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8-12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879-1888.
34. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211-221.
35. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):
1594-1603.
36. Drummer HE. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol. 2014;5:329.
1. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. CDC. Hepatitis C FAQs for Health Professionals. www.cdc.gov/hepatitis/hcv/hcvfaq.htm. Accessed October 19, 2014.
4. The World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection. www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. Accessed October 19, 2014.
5. Younossi ZM, Kanwal F, Saab S, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther. 2014;39(5):518-531.
6. Ly KN, Xing J, Klevens M, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271-278.
7. CDC. Surveillance for Viral Hepatitis—United States, 2012. www.cdc.gov/
hepatitis/Statistics/2012Surveillance/index.htm. Accessed October 19, 2014.
8. CDC. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR. 2013;62(18):362-365.
9. Moyer VA, on behalf of the US Preventive Services Task Force. Screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
10. American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/sites/default/files/full_report.pdf. Accessed October 19, 2014.
11. Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR. 2012;61(RR-04):1-18.
12. Wong T, Lee SS. Hepatitis C: a review for primary care physicians. CMAJ. 2006;174(5):649-659.
13. Merican I, Sherlock S, McIntyre N, Dusheiko GM. Clinical, biochemical and histological features in 102 patients with chronic hepatitis C virus infection. Q J Med. 1993;86(2):119-125.
14. Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28(6):1687-1695.
15. Marinho RT, Vitor S, Velosa J. Benefits of curing hepatitis C infection.
J Gastrointestin Liver Dis. 2014;23(1):85-90.
16. Senzolo M, Schiff S, D’Aloiso CM, et al. Neuropsychological alterations in hepatitis C infection: the role of inflammation. World J Gastroenterol. 2011;17(29):3369-3374.
17. de Leuw P, Sarrazin C, Zeuzem S. How to use virological tools for the optimal management of chronic hepatitis C. Liver Int. 2011;31(suppl 1): 3-12.
18. Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
19. Yano M, Kumada H, Kage M, et al. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23(6):1334-1340.
20. European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C 2014. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed October 19, 2014.
21. Corey KE, Mendez-Navarro J, Gorospe EC, et al. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207.
22. Wiegand J, Buggisch P, Boecher W, et al; German HEP-NET Acute HCV Study Group. Early monotherapy with pegylated interferon alpha-2b for acute hepatitis C infection: the HEP-NET acute-HCV-II study. Hepatology. 2006;43(2):250-256.
23. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60(2):392-420.
24. Belousova V, Abd-Rabou AA, Mousa SA. Recent advances and future directions in the management of hepatitis C infections. Pharmacol Ther. September 2014. Published online ahead of print.
25. Fried MW, Shiffman ML, Rajender Reddy K, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
26. Department of Veterans Affairs. National Hepatitis C Resource Center Program and the Office of Public Health. Chronic hepatitis C virus infection: treatment considerations. www.hepatitis.va.gov/pdf/2014hcv.pdf. Accessed October 19, 2014.
27. Simeprevir (Olysio) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1433):1-3.
28. Sofosbuvir (Sovaldi) for chronic hepatitis C. Med Lett Drugs Ther. 2014;56(1434):5-6.
29. CDC. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
30. US Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aids info.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed October 19, 2014.
31. Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systemic reviews. Lancet. 2011;378(9791):571-583.
32. US Department of Health and Human Services: Substance Abuse and Mental Health Services Administration. A treatment improvement protocol: addressing viral hepatitis in people with substance abuse disorders. Tip 53. 2011.
33. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8-12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370: 1879-1888.
34. Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al; AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(15):211-221.
35. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):
1594-1603.
36. Drummer HE. Challenges to the development of vaccines to hepatitis C virus that elicit neutralizing antibodies. Front Microbiol. 2014;5:329.
Spondylolysis and Spondylolisthesis Primary Care Clinicians' Role
CE/CME No: CR-1410
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify risk factors for the development of spondylolysis and spondylolisthesis
• Discuss the differential diagnoses associated with low back pain
• Describe physical examination findings consistent with spondylolysis and spondylolisthesis
• List the imaging modalities that may be used to confirm these diagnoses and explain the indications for each
• Identify when a pediatric patient with low back pain should be referred to an orthopedic or neurologic specialist for further evaluation and treatment
FACULTY
Shannon P. More is a pediatric nurse practitioner in New York City. Rita Marie John is a Professor at the Columbia University School of Nursing in New York City. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
The most common causes of low back pain in adolescents, spondylolysis and spondylolisthesis can be tricky to diagnose. The primary care clinician plays a key role in optimal management so that full recovery, in most cases, is achieved.
Spondylolysis, the most common cause of low back pain in adolescents, occurs in approximately 8% to 14% of adolescent athletes.1 It is estimated that 15% to 25% of patients with spondylolysis will develop spondylolisthesis.2 This article discusses the definitions, pathophysiology, risk factors, differential diagnosis, and clinical presentation of spondylolysis and spondylolisthesis, including a review of diagnostic imaging, treatment, and the primary care clinician’s role in the optimal management of affected patients.
Spondylolysis is a congenital or acquired unilateral or bilateral defect in the portion of a vertebra—usually L5—called the pars interarticularis, the bony area between the superior and inferior articulating facets (see Figure 1a). Recurrent trauma, such as repeated flexion, hyperextension, or twisting, can weaken the pars interarticularis. During these movements, the inferior articular process of the cranial (upper) vertebra can come into contact with the pars interarticularis of the caudal (lower) vertebra. When this happens repeatedly, a stress reaction may occur in the pars interarticularis, which can result in a stress or complete nonunion fracture.
The paraspinal muscles around the lumbar spine may adjust to accommodate the weakened vertebra, resulting in postural alterations. This can predispose patients to further spinal injury because normal muscular control of that portion of the spine is impaired.3
When the break in the pars interarticularis causes slippage, or forward movement, of the upper vertebra (again, usually L5) over the vertebra (ie, S1) below it, spondylolisthesis occurs (see Figure 1b, above).2,4 This happens more frequently with bilateral spondylolysis and is often associated with an adolescent growth spurt.5
Spondylolisthesis can manifest anywhere in the spine but is most common in the lumbosacral region, with most (71% to 95%) cases occurring at L5 and the remainder at L4.6 Some patients develop these conditions spontaneously through strenuous activity or injury; others are predisposed but remain asymptomatic until the condition is exacerbated through athletic activities.
Wiltse first described spondylolisthesis in 1962 and 10 years later published a classification system based on etiology and anatomy; his research is still used today as the basis for diagnosis.7,8 The five types of spondylolisthesis are
• Type 1, dysplastic (congenital)
• Type 2, isthmic (defect in pars interarticularis)
• Type 3, degenerative (arthritic changes in older patients)
• Type 4, traumatic (acute injury)
• Type 5, pathologic (bone disease).8
Types 1 and 2 occur in pediatric patients, with 85% of such cases classified as type 2.4
The classification is further refined according to degree of severity, defined as the percentage of slippage of the upper over the lower vertebra. Grade 1 (first degree) entails a slippage of less than 25%; grade 2 (second degree), 26% to 50%; grade 3 (third degree), 51% to 75%; and grade 4 (fourth degree), greater than 75%.5
Risk Factors
In general, women are at greater risk for stress fractures than men are.9 Yet while pars interarticularis defects are twice as common in men as in women, women are more likely to progress to spondylolisthesis.10 Similarly, in patients with bilateral spondylolysis, women are significantly more likely than men (90.9% vs 66.2%) to develop spondylolisthesis.11 In general, those who repetitively hyperextend the lower back (eg, football players, rowers, dancers, gymnasts, soccer players, swimmers) are at increased risk for both spondylolysis and spondylolisthesis.
While a patient’s activities and environmental stressors play a role in the development of spondylolysis and spondylolisthesis, a genetic predisposition is believed to be a factor as well: A five-fold increase in the incidence of defects of the pars interarticularis has been noted in near relatives of patients with spondylolisthesis.7 In addition, patients with conditions such as spina bifida occulta, severe scoliosis, and osteogenesis imperfecta are at increased risk for these conditions.6,12
Differential DiagnosIs
Both spondylolysis and spondylolisthesis manifest as low back pain, for which the differential diagnosis is extensive. Pathologic causes of low back pain are much less common than causes related to structural weakness or trauma; however, carefully differentiating these is important so that pathology is identified promptly and treated appropriately.13
The differential diagnosis for low back pain in pediatric patients, by type of pain, includes
• Pain at night or with fever or other generalized symptoms: tumor or infection
• Acute pain: herniated disk, slipped apophysis, spondylolysis, vertebral fracture, or muscle strain
• Chronic pain: Scheuermann kyphosis, inflammatory spondyloarthropathies, or psychological problems
• Pain with spinal forward flexion: herniated disk or slipped apophysis
• Pain with spinal extension: spondylolysis, spondylolisthesis, or lesion or injury in the pedicle or lamina (posterior arch)
• Pain with recent-onset scoliosis: tumor, infection, herniated disk, syrinx, or idiopathic scoliosis
• Other pain: pyelonephritis or sickle cell crisis.13
Clinical Presentation
A systematic approach to a patient with a chief concern of low back pain is recommended. An initial assessment of the patient’s vital signs and growth parameters should be compared to those from previous visits to determine if there have been any changes in the usual pattern.
History
Use the standard HEEADSSS (Home, Education, Eating, Activities, Drugs, Sexuality, Suicide, Safety) adolescent psychosocial assessment as part of the patient history.14 In particular, focus on details about the physical activities and sports in which the patient participates and obtain data on the amount of time spent on each. When taking the family history, pay particular attention to any predisposition to musculoskeletal disorders. In the review of systems, note any history of traumatic injury.
Next, ask the patient to point with one finger to the location at which the pain is felt; in spondylolysis and spondylolisthesis, pain localizes to the waist. Obtain a detailed history of the pain, including onset, duration, frequency, location, and all alleviating or exacerbating factors.15
Physical examination
A brief summary of key features to assess during the physical examination is presented in Table 1. When completing the physical assessment, follow the typical head-to-toe approach, with special attention to evaluation of the presenting back pain.
Visual inspection. A visual inspection of the spine for scoliosis and kyphosis is key; also be sure to examine posture, assess symmetry, and observe for any midline defects.13 Hemangiomas, a line of hairy patches, or other abnormal markings along the body’s vertical axis may suggest an intraspinal anomaly.
Palpation. The entire spine should be palpated to confirm the location of the pain as identified by the patient. In particular, note if tenderness is felt over bony structures of the spine or in the paraspinal musculature.
Range of motion. After the locus of the pain is confirmed, instruct the patient to flex and extend the spine to assess for worsening pain. If noted, this finding is pathognomonic for spondylolysis and spondylolisthesis.13
Also useful for making the diagnosis is the one-leg hyperextension test, in which the patient is asked to raise one leg off the ground and lean backward. Pain elicited during this movement is indicative of back injury, including spondylolysis or spondylolisthesis. A positive result with the one-leg hyperextension test alone, however, is not a clinical marker for spondylolysis. Not only may the test elicit pain stemming from other pathologies, but the results are dependent on the patient’s subjective reporting of pain.16
Muscular signs and symptoms. Hamstring tightness is present in 80% of symptomatic patients.5 Consequently, the patient may have a somewhat waddling gait due to the inability to flex the hips and extend the knee simultaneously. For this reason, physical examination for spondylolysis and spondylolisthesis includes gait assessment. Other clinical signs of spondylolisthesis include a weak and drooping abdominal wall, paravertebral muscle hypertrophy, increased lumbar lordosis, hamstring muscle spasm, and pain during lateral trunk flexion/extension and double leg raising.17
Motor and sensory function. It is important to assess motor and sensory function to differentiate neurologic from orthopedic conditions. Deep tendon reflexes and lower extremity motor strength and sensory capabilities also need careful assessment.13 Sensation in the region of the cauda equinus requires further evaluation due to the possibility of cauda equinus compression. Hyperreflexia indicates an upper motor lesion, whereas hyporeflexia indicates a lower motor lesion—neither of which would be expected in spondylolisthesis or spondylolysis.
A patient with any positive neurologic signs should be referred to a neurologic specialist.
Diagnostic TESTING
Unfortunately, rigorous comparative research is lacking on which to base clinical practice guidelines for diagnosis (as well as treatment) of spondylolysis and spondylolisthesis.18 Nevertheless, current standards call for a detailed physical examination as an effective diagnostic tool and recommend radiologic evaluation for a definitive diagnosis.17,18 Lateral radiographs are also useful for identifying the degree of vertebral slippage when spondylolisthesis is diagnosed.17 The specialist may choose to utilize such diagnostic modalities as CT, single-photon-emission CT (SPECT), or MRI.
Radiologic evaluation
For complete radiologic evaluation, four x-ray views of the spine are necessary: anterior-posterior (AP), lateral, and bilateral oblique views. A fracture seen in the pars interarticularis is called the Scotty dog sign because it looks like a collar around the neck of a Scottish terrier (see Figures 2 and 3).2
Since treatment for nonspecific back pain and spondylolysis is essentially the same, it could be argued that radiographic imaging to confirm the clinical diagnosis exposes the patient to unnecessary radiation (a particular concern in the pediatric population). But a definitive diagnosis also rules out other pathologies that would require more aggressive treatment.
If x-rays reveal abnormalities, refer the patient to an orthopedic specialist for further evaluation. Referral to orthopedics should also be prompted if spondylolysis or spondylolisthesis is suspected but x-rays are insufficient to make the diagnosis.
CT and SPECT
Standaert and Herring suggest that CT combined with SPECT is the standard for diagnosis of a pars interarticularis lesion1; in many reported cases, CT test results may be negative even when SPECT results are abnormal, suggesting that both studies are needed. In other cases, CT can help identify the origin of an abnormality seen on SPECT.
The disadvantage to using both modalities is that the patient is exposed to additional ionizing radiation. If only one method is to be used, SPECT may be preferred; it exposes the patient to less ionizing radiation and seldom requires sedation.19
Magnetic resonance imaging
In terms of radiation exposure, MRI is preferred to CT and SPECT because it does not utilize ionizing radiation; unfortunately, it is also less effective at detection of spondylolysis and spondylolisthesis. A review of the literature indicates that MRI is not as sensitive as SPECT in identifying stress on the pars interarticularis.16,19
In general, MRI is superior for visualizing soft tissue pathology (eg, disk disease, nerve root compression, inflammation), while CT is superior for visualization of bone. In the context of back pain, MRI may be informative when etiologies other than spondylolysis and spondylolisthesis are suspected; Feldman et al recommend MRI for patients with constant back pain, radicular pain, nighttime pain, and/or abnormal neurologic examination results.16
The advantages and disadvantages of x-rays, SPECT, CT, and MRI for the diagnosis of spondylolysis and spondylolisthesis are summarized in Table 2.1,15,16,18,19
Laboratory studies
There are no laboratory tests to confirm the diagnosis of spondylolysis or spondylolisthesis. To eliminate other diagnoses, however, it is appropriate to order certain laboratory tests. In addition to radiographs, Bernstein and Cozen recommend a complete blood count, an erythrocyte sedimentation rate, and a C-reactive protein test as part of the diagnostic work-up if the history and physical are suspicious for underlying pathology, such as infection.13
Management and Treatment Options
Treatment for spondylolysis or spondylolisthesis ranges from basic strengthening programs to surgical intervention and is based on the severity of the patient’s condition. The goal is to alleviate symptoms and facilitate a return to normal activities. Treatment should be individualized based on the patient’s age, athletic level and demands, and severity of symptoms.10
Nonsurgical treatment
Conservative treatment options include rest, physical therapy, core strengthening, and antilordotic bracing for several months (eg, eight to 12 weeks).5 Most grade 1 and 2 cases can be successfully treated nonsurgically.
Studies of nonsurgical treatment of children and young adults with spondylolysis and/or mild spondylolisthesis (up to 25% slippage) were evaluated in a meta-analysis.20 The authors found that approximately 84% of such patients were pain free or nearly pain free with unrestricted activities within one year of treatment. While nonsurgical treatment does not usually resolve the pars interarticularis defect, it alleviates symptoms and enables the patient’s return to unrestricted activities. Further, the authors found no significant difference in outcomes between patients who were treated with or without bracing.20
In contrast, another study demonstrated that patients who wore braces achieved higher functional outcomes than those who did not.21 However, the patients who were braced were restricted from physical activity longer than were those who were not braced, so it could not be definitively determined if bracing or additional rest was the reason for the improved outcomes.
A significant limitation of bracing is that it is a restrictive and sometimes uncomfortable treatment, especially for an active child or adolescent. If clinicians can treat these patients effectively without bracing, greater compliance with treatment may result.
This study also found a strong correlation between early intervention and an increased incidence of bony healing. The researchers recommend the early use of sensitive diagnostic imaging so that treatment can begin early, increasing the possibility that the fracture will heal.20
Skeletally immature patients should be followed clinically at six-month to one-year intervals, including use of lateral x-rays for spinal evaluation, to ensure that progressive spondylolisthesis does not develop. Once skeletal development is complete, follow-up is no longer necessary because progressive spondylolisthesis is unlikely at or near skeletal maturity.10
Surgical treatment
When conservative treatment fails to alleviate the pediatric patient’s pain, if daily functioning is impaired, or if the spondylolisthesis is of a more severe grade or progresses, surgical correction to repair the pars interarticularis defect or laminectomy and spinal fusion may be necessary.5 Adolescent patients treated surgically are likely to have good long-term results but may still experience symptoms, including back pain, into adulthood.22 The procedure used in a particular case depends on the degree of severity and the patient’s specific presentation.
PRIMARY CARE IS KEY
As stated earlier, referral to an orthopedic specialist is indicated when spondylolysis or spondylolisthesis is suspected or confirmed or to a neurologic specialist when any neurologic signs are positive or deficits are noted. In these instances, the primary care clinician is key to early diagnosis and prompt treatment.
Primary health care practitioners are in a position to contribute to the management of spondylolysis or spondylolisthesis in a way that will help facilitate a full recovery. For example, the patient and parent or guardian should be counseled about the need for the child to refrain from athletic activity while awaiting an orthopedic or neurologic evaluation. Restriction of activity will prevent exacerbation of the injury and increased pain. Suggest OTC NSAIDs, taken with food to prevent gastrointestinal adverse effects, for pain relief.13
It can also be helpful to assess the patient’s psychosocial status to determine whether he or she might face barriers to recovery. Often a major obstacle in treating these conditions is pressure from parents, coaches, or the patients themselves to continue athletic activities despite pain and injury.
Clinicians can offer support by reinforcing the message that limiting physical activity is essential to recovery; it should not be compromised because of patient, parental, coach, or peer pressure. At the same time, it is important to recognize that a leave of absence from a competitive sport, no matter how short, can affect the patient’s mental health. If the patient feels angry or depressed about the diagnosis, mental health counseling options can be discussed.
Educating patients and parents about the numerous treatments for acute low back pain that have been proven to offer little benefit can be informative and reassuring. For example, adding spinal manipulation and chiropractic techniques to established medical treatments does not improve outcomes. Neither does the use of oral corticosteroids, acupuncture, massage, traction, or exercise programs. Bed rest should be avoided.23
Conclusion
In the primary care setting, low back pain is a common complaint with an extensive differential diagnosis. If a thorough history and physical examination prompt suspicion for spondylolysis or spondylolisthesis, x-rays will usually confirm the diagnosis. If the patient is referred to an orthopedic specialist, the primary care clinician can supplement and reinforce the treatment plan through patient and parent education about the diagnosis and its treatment. Compliance with conservative nonsurgical treatment may enable the patient to make a speedier return to his or her usual physical activities.
The authors would like to thank Jennifer Tareco, MD, and Robert More, MD, for their revisions and support in the completion of this article.
1. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
2. Spiegel DA, Dormans JP. Spondylolysis and spondylolisthesis. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE, eds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2011:1561-1571.
3. Smith J. Moving beyond the neutral spine: stabilizing the dancer with lumbar extension dysfunction. J Dance Med Sci. 2009;13(3):73-82.
4. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
5. Wicker A. Spondylolysis and spondylolisthesis in sports. Int Sports Med J. 2008;9(2):74-78.
6. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007; 6(1):62-66.
7. Wiltse LL. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539-560.
8. Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
9. Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767-778.
10. Tallarico RA, Madom IA, Palumbo MA. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16(1):32-38.
11. Takao S, Sakai T, Sairyo K, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 2010;57 (1-2):133-137.
12. Hatz D, Esposito PW, Schroeder B, et al. The incidence of spondylolysis and spondylolisthesis in children with osteogenesis imperfecta. J Pediatr Orthop. 2011;31(6):655-660.
13. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76(11):1669-1676.
14. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: The psychosocial interview for adolescents updated for a new century fueled by media. Contemp Pediatr. 2014;1:16-28.
15. Feldman DS, Straight JJ, Badra MI, et al. Evaluation of an algorithmic approach to pediatric back pain. J Pediatr Orthop. 2006;26(3):353-357.
16. Masci L, Pike J, Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
17. Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil. 2009;22(1):27-32.
18. Bhatia NN, Chow G, Timon SJ, Watts HG. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J Pediatr Orthop. 2008;28(2):230-233.
19. Zukotynski K, Curtis C, Grant FD, et al. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5(13):1-6.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop. 2009;29(2):146-156.
21. Alvarez-Diaz P, Alentorn-Geli E, Steinbacher G, et al. Conservative treatment of lumbar spondylolysis in young soccer players. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2111-2114.
22. Helenius I, Remes V, Lamberg T, et al. Long-term health-related quality of life after surgery for adolescent idiopathic scoliosis and spondylolisthesis. J Bone Joint Surg Am. 2008;90(6):1231-1239.
23. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
CE/CME No: CR-1410
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify risk factors for the development of spondylolysis and spondylolisthesis
• Discuss the differential diagnoses associated with low back pain
• Describe physical examination findings consistent with spondylolysis and spondylolisthesis
• List the imaging modalities that may be used to confirm these diagnoses and explain the indications for each
• Identify when a pediatric patient with low back pain should be referred to an orthopedic or neurologic specialist for further evaluation and treatment
FACULTY
Shannon P. More is a pediatric nurse practitioner in New York City. Rita Marie John is a Professor at the Columbia University School of Nursing in New York City. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
The most common causes of low back pain in adolescents, spondylolysis and spondylolisthesis can be tricky to diagnose. The primary care clinician plays a key role in optimal management so that full recovery, in most cases, is achieved.
Spondylolysis, the most common cause of low back pain in adolescents, occurs in approximately 8% to 14% of adolescent athletes.1 It is estimated that 15% to 25% of patients with spondylolysis will develop spondylolisthesis.2 This article discusses the definitions, pathophysiology, risk factors, differential diagnosis, and clinical presentation of spondylolysis and spondylolisthesis, including a review of diagnostic imaging, treatment, and the primary care clinician’s role in the optimal management of affected patients.
Spondylolysis is a congenital or acquired unilateral or bilateral defect in the portion of a vertebra—usually L5—called the pars interarticularis, the bony area between the superior and inferior articulating facets (see Figure 1a). Recurrent trauma, such as repeated flexion, hyperextension, or twisting, can weaken the pars interarticularis. During these movements, the inferior articular process of the cranial (upper) vertebra can come into contact with the pars interarticularis of the caudal (lower) vertebra. When this happens repeatedly, a stress reaction may occur in the pars interarticularis, which can result in a stress or complete nonunion fracture.
The paraspinal muscles around the lumbar spine may adjust to accommodate the weakened vertebra, resulting in postural alterations. This can predispose patients to further spinal injury because normal muscular control of that portion of the spine is impaired.3
When the break in the pars interarticularis causes slippage, or forward movement, of the upper vertebra (again, usually L5) over the vertebra (ie, S1) below it, spondylolisthesis occurs (see Figure 1b, above).2,4 This happens more frequently with bilateral spondylolysis and is often associated with an adolescent growth spurt.5
Spondylolisthesis can manifest anywhere in the spine but is most common in the lumbosacral region, with most (71% to 95%) cases occurring at L5 and the remainder at L4.6 Some patients develop these conditions spontaneously through strenuous activity or injury; others are predisposed but remain asymptomatic until the condition is exacerbated through athletic activities.
Wiltse first described spondylolisthesis in 1962 and 10 years later published a classification system based on etiology and anatomy; his research is still used today as the basis for diagnosis.7,8 The five types of spondylolisthesis are
• Type 1, dysplastic (congenital)
• Type 2, isthmic (defect in pars interarticularis)
• Type 3, degenerative (arthritic changes in older patients)
• Type 4, traumatic (acute injury)
• Type 5, pathologic (bone disease).8
Types 1 and 2 occur in pediatric patients, with 85% of such cases classified as type 2.4
The classification is further refined according to degree of severity, defined as the percentage of slippage of the upper over the lower vertebra. Grade 1 (first degree) entails a slippage of less than 25%; grade 2 (second degree), 26% to 50%; grade 3 (third degree), 51% to 75%; and grade 4 (fourth degree), greater than 75%.5
Risk Factors
In general, women are at greater risk for stress fractures than men are.9 Yet while pars interarticularis defects are twice as common in men as in women, women are more likely to progress to spondylolisthesis.10 Similarly, in patients with bilateral spondylolysis, women are significantly more likely than men (90.9% vs 66.2%) to develop spondylolisthesis.11 In general, those who repetitively hyperextend the lower back (eg, football players, rowers, dancers, gymnasts, soccer players, swimmers) are at increased risk for both spondylolysis and spondylolisthesis.
While a patient’s activities and environmental stressors play a role in the development of spondylolysis and spondylolisthesis, a genetic predisposition is believed to be a factor as well: A five-fold increase in the incidence of defects of the pars interarticularis has been noted in near relatives of patients with spondylolisthesis.7 In addition, patients with conditions such as spina bifida occulta, severe scoliosis, and osteogenesis imperfecta are at increased risk for these conditions.6,12
Differential DiagnosIs
Both spondylolysis and spondylolisthesis manifest as low back pain, for which the differential diagnosis is extensive. Pathologic causes of low back pain are much less common than causes related to structural weakness or trauma; however, carefully differentiating these is important so that pathology is identified promptly and treated appropriately.13
The differential diagnosis for low back pain in pediatric patients, by type of pain, includes
• Pain at night or with fever or other generalized symptoms: tumor or infection
• Acute pain: herniated disk, slipped apophysis, spondylolysis, vertebral fracture, or muscle strain
• Chronic pain: Scheuermann kyphosis, inflammatory spondyloarthropathies, or psychological problems
• Pain with spinal forward flexion: herniated disk or slipped apophysis
• Pain with spinal extension: spondylolysis, spondylolisthesis, or lesion or injury in the pedicle or lamina (posterior arch)
• Pain with recent-onset scoliosis: tumor, infection, herniated disk, syrinx, or idiopathic scoliosis
• Other pain: pyelonephritis or sickle cell crisis.13
Clinical Presentation
A systematic approach to a patient with a chief concern of low back pain is recommended. An initial assessment of the patient’s vital signs and growth parameters should be compared to those from previous visits to determine if there have been any changes in the usual pattern.
History
Use the standard HEEADSSS (Home, Education, Eating, Activities, Drugs, Sexuality, Suicide, Safety) adolescent psychosocial assessment as part of the patient history.14 In particular, focus on details about the physical activities and sports in which the patient participates and obtain data on the amount of time spent on each. When taking the family history, pay particular attention to any predisposition to musculoskeletal disorders. In the review of systems, note any history of traumatic injury.
Next, ask the patient to point with one finger to the location at which the pain is felt; in spondylolysis and spondylolisthesis, pain localizes to the waist. Obtain a detailed history of the pain, including onset, duration, frequency, location, and all alleviating or exacerbating factors.15
Physical examination
A brief summary of key features to assess during the physical examination is presented in Table 1. When completing the physical assessment, follow the typical head-to-toe approach, with special attention to evaluation of the presenting back pain.
Visual inspection. A visual inspection of the spine for scoliosis and kyphosis is key; also be sure to examine posture, assess symmetry, and observe for any midline defects.13 Hemangiomas, a line of hairy patches, or other abnormal markings along the body’s vertical axis may suggest an intraspinal anomaly.
Palpation. The entire spine should be palpated to confirm the location of the pain as identified by the patient. In particular, note if tenderness is felt over bony structures of the spine or in the paraspinal musculature.
Range of motion. After the locus of the pain is confirmed, instruct the patient to flex and extend the spine to assess for worsening pain. If noted, this finding is pathognomonic for spondylolysis and spondylolisthesis.13
Also useful for making the diagnosis is the one-leg hyperextension test, in which the patient is asked to raise one leg off the ground and lean backward. Pain elicited during this movement is indicative of back injury, including spondylolysis or spondylolisthesis. A positive result with the one-leg hyperextension test alone, however, is not a clinical marker for spondylolysis. Not only may the test elicit pain stemming from other pathologies, but the results are dependent on the patient’s subjective reporting of pain.16
Muscular signs and symptoms. Hamstring tightness is present in 80% of symptomatic patients.5 Consequently, the patient may have a somewhat waddling gait due to the inability to flex the hips and extend the knee simultaneously. For this reason, physical examination for spondylolysis and spondylolisthesis includes gait assessment. Other clinical signs of spondylolisthesis include a weak and drooping abdominal wall, paravertebral muscle hypertrophy, increased lumbar lordosis, hamstring muscle spasm, and pain during lateral trunk flexion/extension and double leg raising.17
Motor and sensory function. It is important to assess motor and sensory function to differentiate neurologic from orthopedic conditions. Deep tendon reflexes and lower extremity motor strength and sensory capabilities also need careful assessment.13 Sensation in the region of the cauda equinus requires further evaluation due to the possibility of cauda equinus compression. Hyperreflexia indicates an upper motor lesion, whereas hyporeflexia indicates a lower motor lesion—neither of which would be expected in spondylolisthesis or spondylolysis.
A patient with any positive neurologic signs should be referred to a neurologic specialist.
Diagnostic TESTING
Unfortunately, rigorous comparative research is lacking on which to base clinical practice guidelines for diagnosis (as well as treatment) of spondylolysis and spondylolisthesis.18 Nevertheless, current standards call for a detailed physical examination as an effective diagnostic tool and recommend radiologic evaluation for a definitive diagnosis.17,18 Lateral radiographs are also useful for identifying the degree of vertebral slippage when spondylolisthesis is diagnosed.17 The specialist may choose to utilize such diagnostic modalities as CT, single-photon-emission CT (SPECT), or MRI.
Radiologic evaluation
For complete radiologic evaluation, four x-ray views of the spine are necessary: anterior-posterior (AP), lateral, and bilateral oblique views. A fracture seen in the pars interarticularis is called the Scotty dog sign because it looks like a collar around the neck of a Scottish terrier (see Figures 2 and 3).2
Since treatment for nonspecific back pain and spondylolysis is essentially the same, it could be argued that radiographic imaging to confirm the clinical diagnosis exposes the patient to unnecessary radiation (a particular concern in the pediatric population). But a definitive diagnosis also rules out other pathologies that would require more aggressive treatment.
If x-rays reveal abnormalities, refer the patient to an orthopedic specialist for further evaluation. Referral to orthopedics should also be prompted if spondylolysis or spondylolisthesis is suspected but x-rays are insufficient to make the diagnosis.
CT and SPECT
Standaert and Herring suggest that CT combined with SPECT is the standard for diagnosis of a pars interarticularis lesion1; in many reported cases, CT test results may be negative even when SPECT results are abnormal, suggesting that both studies are needed. In other cases, CT can help identify the origin of an abnormality seen on SPECT.
The disadvantage to using both modalities is that the patient is exposed to additional ionizing radiation. If only one method is to be used, SPECT may be preferred; it exposes the patient to less ionizing radiation and seldom requires sedation.19
Magnetic resonance imaging
In terms of radiation exposure, MRI is preferred to CT and SPECT because it does not utilize ionizing radiation; unfortunately, it is also less effective at detection of spondylolysis and spondylolisthesis. A review of the literature indicates that MRI is not as sensitive as SPECT in identifying stress on the pars interarticularis.16,19
In general, MRI is superior for visualizing soft tissue pathology (eg, disk disease, nerve root compression, inflammation), while CT is superior for visualization of bone. In the context of back pain, MRI may be informative when etiologies other than spondylolysis and spondylolisthesis are suspected; Feldman et al recommend MRI for patients with constant back pain, radicular pain, nighttime pain, and/or abnormal neurologic examination results.16
The advantages and disadvantages of x-rays, SPECT, CT, and MRI for the diagnosis of spondylolysis and spondylolisthesis are summarized in Table 2.1,15,16,18,19
Laboratory studies
There are no laboratory tests to confirm the diagnosis of spondylolysis or spondylolisthesis. To eliminate other diagnoses, however, it is appropriate to order certain laboratory tests. In addition to radiographs, Bernstein and Cozen recommend a complete blood count, an erythrocyte sedimentation rate, and a C-reactive protein test as part of the diagnostic work-up if the history and physical are suspicious for underlying pathology, such as infection.13
Management and Treatment Options
Treatment for spondylolysis or spondylolisthesis ranges from basic strengthening programs to surgical intervention and is based on the severity of the patient’s condition. The goal is to alleviate symptoms and facilitate a return to normal activities. Treatment should be individualized based on the patient’s age, athletic level and demands, and severity of symptoms.10
Nonsurgical treatment
Conservative treatment options include rest, physical therapy, core strengthening, and antilordotic bracing for several months (eg, eight to 12 weeks).5 Most grade 1 and 2 cases can be successfully treated nonsurgically.
Studies of nonsurgical treatment of children and young adults with spondylolysis and/or mild spondylolisthesis (up to 25% slippage) were evaluated in a meta-analysis.20 The authors found that approximately 84% of such patients were pain free or nearly pain free with unrestricted activities within one year of treatment. While nonsurgical treatment does not usually resolve the pars interarticularis defect, it alleviates symptoms and enables the patient’s return to unrestricted activities. Further, the authors found no significant difference in outcomes between patients who were treated with or without bracing.20
In contrast, another study demonstrated that patients who wore braces achieved higher functional outcomes than those who did not.21 However, the patients who were braced were restricted from physical activity longer than were those who were not braced, so it could not be definitively determined if bracing or additional rest was the reason for the improved outcomes.
A significant limitation of bracing is that it is a restrictive and sometimes uncomfortable treatment, especially for an active child or adolescent. If clinicians can treat these patients effectively without bracing, greater compliance with treatment may result.
This study also found a strong correlation between early intervention and an increased incidence of bony healing. The researchers recommend the early use of sensitive diagnostic imaging so that treatment can begin early, increasing the possibility that the fracture will heal.20
Skeletally immature patients should be followed clinically at six-month to one-year intervals, including use of lateral x-rays for spinal evaluation, to ensure that progressive spondylolisthesis does not develop. Once skeletal development is complete, follow-up is no longer necessary because progressive spondylolisthesis is unlikely at or near skeletal maturity.10
Surgical treatment
When conservative treatment fails to alleviate the pediatric patient’s pain, if daily functioning is impaired, or if the spondylolisthesis is of a more severe grade or progresses, surgical correction to repair the pars interarticularis defect or laminectomy and spinal fusion may be necessary.5 Adolescent patients treated surgically are likely to have good long-term results but may still experience symptoms, including back pain, into adulthood.22 The procedure used in a particular case depends on the degree of severity and the patient’s specific presentation.
PRIMARY CARE IS KEY
As stated earlier, referral to an orthopedic specialist is indicated when spondylolysis or spondylolisthesis is suspected or confirmed or to a neurologic specialist when any neurologic signs are positive or deficits are noted. In these instances, the primary care clinician is key to early diagnosis and prompt treatment.
Primary health care practitioners are in a position to contribute to the management of spondylolysis or spondylolisthesis in a way that will help facilitate a full recovery. For example, the patient and parent or guardian should be counseled about the need for the child to refrain from athletic activity while awaiting an orthopedic or neurologic evaluation. Restriction of activity will prevent exacerbation of the injury and increased pain. Suggest OTC NSAIDs, taken with food to prevent gastrointestinal adverse effects, for pain relief.13
It can also be helpful to assess the patient’s psychosocial status to determine whether he or she might face barriers to recovery. Often a major obstacle in treating these conditions is pressure from parents, coaches, or the patients themselves to continue athletic activities despite pain and injury.
Clinicians can offer support by reinforcing the message that limiting physical activity is essential to recovery; it should not be compromised because of patient, parental, coach, or peer pressure. At the same time, it is important to recognize that a leave of absence from a competitive sport, no matter how short, can affect the patient’s mental health. If the patient feels angry or depressed about the diagnosis, mental health counseling options can be discussed.
Educating patients and parents about the numerous treatments for acute low back pain that have been proven to offer little benefit can be informative and reassuring. For example, adding spinal manipulation and chiropractic techniques to established medical treatments does not improve outcomes. Neither does the use of oral corticosteroids, acupuncture, massage, traction, or exercise programs. Bed rest should be avoided.23
Conclusion
In the primary care setting, low back pain is a common complaint with an extensive differential diagnosis. If a thorough history and physical examination prompt suspicion for spondylolysis or spondylolisthesis, x-rays will usually confirm the diagnosis. If the patient is referred to an orthopedic specialist, the primary care clinician can supplement and reinforce the treatment plan through patient and parent education about the diagnosis and its treatment. Compliance with conservative nonsurgical treatment may enable the patient to make a speedier return to his or her usual physical activities.
The authors would like to thank Jennifer Tareco, MD, and Robert More, MD, for their revisions and support in the completion of this article.
CE/CME No: CR-1410
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify risk factors for the development of spondylolysis and spondylolisthesis
• Discuss the differential diagnoses associated with low back pain
• Describe physical examination findings consistent with spondylolysis and spondylolisthesis
• List the imaging modalities that may be used to confirm these diagnoses and explain the indications for each
• Identify when a pediatric patient with low back pain should be referred to an orthopedic or neurologic specialist for further evaluation and treatment
FACULTY
Shannon P. More is a pediatric nurse practitioner in New York City. Rita Marie John is a Professor at the Columbia University School of Nursing in New York City. The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
The most common causes of low back pain in adolescents, spondylolysis and spondylolisthesis can be tricky to diagnose. The primary care clinician plays a key role in optimal management so that full recovery, in most cases, is achieved.
Spondylolysis, the most common cause of low back pain in adolescents, occurs in approximately 8% to 14% of adolescent athletes.1 It is estimated that 15% to 25% of patients with spondylolysis will develop spondylolisthesis.2 This article discusses the definitions, pathophysiology, risk factors, differential diagnosis, and clinical presentation of spondylolysis and spondylolisthesis, including a review of diagnostic imaging, treatment, and the primary care clinician’s role in the optimal management of affected patients.
Spondylolysis is a congenital or acquired unilateral or bilateral defect in the portion of a vertebra—usually L5—called the pars interarticularis, the bony area between the superior and inferior articulating facets (see Figure 1a). Recurrent trauma, such as repeated flexion, hyperextension, or twisting, can weaken the pars interarticularis. During these movements, the inferior articular process of the cranial (upper) vertebra can come into contact with the pars interarticularis of the caudal (lower) vertebra. When this happens repeatedly, a stress reaction may occur in the pars interarticularis, which can result in a stress or complete nonunion fracture.
The paraspinal muscles around the lumbar spine may adjust to accommodate the weakened vertebra, resulting in postural alterations. This can predispose patients to further spinal injury because normal muscular control of that portion of the spine is impaired.3
When the break in the pars interarticularis causes slippage, or forward movement, of the upper vertebra (again, usually L5) over the vertebra (ie, S1) below it, spondylolisthesis occurs (see Figure 1b, above).2,4 This happens more frequently with bilateral spondylolysis and is often associated with an adolescent growth spurt.5
Spondylolisthesis can manifest anywhere in the spine but is most common in the lumbosacral region, with most (71% to 95%) cases occurring at L5 and the remainder at L4.6 Some patients develop these conditions spontaneously through strenuous activity or injury; others are predisposed but remain asymptomatic until the condition is exacerbated through athletic activities.
Wiltse first described spondylolisthesis in 1962 and 10 years later published a classification system based on etiology and anatomy; his research is still used today as the basis for diagnosis.7,8 The five types of spondylolisthesis are
• Type 1, dysplastic (congenital)
• Type 2, isthmic (defect in pars interarticularis)
• Type 3, degenerative (arthritic changes in older patients)
• Type 4, traumatic (acute injury)
• Type 5, pathologic (bone disease).8
Types 1 and 2 occur in pediatric patients, with 85% of such cases classified as type 2.4
The classification is further refined according to degree of severity, defined as the percentage of slippage of the upper over the lower vertebra. Grade 1 (first degree) entails a slippage of less than 25%; grade 2 (second degree), 26% to 50%; grade 3 (third degree), 51% to 75%; and grade 4 (fourth degree), greater than 75%.5
Risk Factors
In general, women are at greater risk for stress fractures than men are.9 Yet while pars interarticularis defects are twice as common in men as in women, women are more likely to progress to spondylolisthesis.10 Similarly, in patients with bilateral spondylolysis, women are significantly more likely than men (90.9% vs 66.2%) to develop spondylolisthesis.11 In general, those who repetitively hyperextend the lower back (eg, football players, rowers, dancers, gymnasts, soccer players, swimmers) are at increased risk for both spondylolysis and spondylolisthesis.
While a patient’s activities and environmental stressors play a role in the development of spondylolysis and spondylolisthesis, a genetic predisposition is believed to be a factor as well: A five-fold increase in the incidence of defects of the pars interarticularis has been noted in near relatives of patients with spondylolisthesis.7 In addition, patients with conditions such as spina bifida occulta, severe scoliosis, and osteogenesis imperfecta are at increased risk for these conditions.6,12
Differential DiagnosIs
Both spondylolysis and spondylolisthesis manifest as low back pain, for which the differential diagnosis is extensive. Pathologic causes of low back pain are much less common than causes related to structural weakness or trauma; however, carefully differentiating these is important so that pathology is identified promptly and treated appropriately.13
The differential diagnosis for low back pain in pediatric patients, by type of pain, includes
• Pain at night or with fever or other generalized symptoms: tumor or infection
• Acute pain: herniated disk, slipped apophysis, spondylolysis, vertebral fracture, or muscle strain
• Chronic pain: Scheuermann kyphosis, inflammatory spondyloarthropathies, or psychological problems
• Pain with spinal forward flexion: herniated disk or slipped apophysis
• Pain with spinal extension: spondylolysis, spondylolisthesis, or lesion or injury in the pedicle or lamina (posterior arch)
• Pain with recent-onset scoliosis: tumor, infection, herniated disk, syrinx, or idiopathic scoliosis
• Other pain: pyelonephritis or sickle cell crisis.13
Clinical Presentation
A systematic approach to a patient with a chief concern of low back pain is recommended. An initial assessment of the patient’s vital signs and growth parameters should be compared to those from previous visits to determine if there have been any changes in the usual pattern.
History
Use the standard HEEADSSS (Home, Education, Eating, Activities, Drugs, Sexuality, Suicide, Safety) adolescent psychosocial assessment as part of the patient history.14 In particular, focus on details about the physical activities and sports in which the patient participates and obtain data on the amount of time spent on each. When taking the family history, pay particular attention to any predisposition to musculoskeletal disorders. In the review of systems, note any history of traumatic injury.
Next, ask the patient to point with one finger to the location at which the pain is felt; in spondylolysis and spondylolisthesis, pain localizes to the waist. Obtain a detailed history of the pain, including onset, duration, frequency, location, and all alleviating or exacerbating factors.15
Physical examination
A brief summary of key features to assess during the physical examination is presented in Table 1. When completing the physical assessment, follow the typical head-to-toe approach, with special attention to evaluation of the presenting back pain.
Visual inspection. A visual inspection of the spine for scoliosis and kyphosis is key; also be sure to examine posture, assess symmetry, and observe for any midline defects.13 Hemangiomas, a line of hairy patches, or other abnormal markings along the body’s vertical axis may suggest an intraspinal anomaly.
Palpation. The entire spine should be palpated to confirm the location of the pain as identified by the patient. In particular, note if tenderness is felt over bony structures of the spine or in the paraspinal musculature.
Range of motion. After the locus of the pain is confirmed, instruct the patient to flex and extend the spine to assess for worsening pain. If noted, this finding is pathognomonic for spondylolysis and spondylolisthesis.13
Also useful for making the diagnosis is the one-leg hyperextension test, in which the patient is asked to raise one leg off the ground and lean backward. Pain elicited during this movement is indicative of back injury, including spondylolysis or spondylolisthesis. A positive result with the one-leg hyperextension test alone, however, is not a clinical marker for spondylolysis. Not only may the test elicit pain stemming from other pathologies, but the results are dependent on the patient’s subjective reporting of pain.16
Muscular signs and symptoms. Hamstring tightness is present in 80% of symptomatic patients.5 Consequently, the patient may have a somewhat waddling gait due to the inability to flex the hips and extend the knee simultaneously. For this reason, physical examination for spondylolysis and spondylolisthesis includes gait assessment. Other clinical signs of spondylolisthesis include a weak and drooping abdominal wall, paravertebral muscle hypertrophy, increased lumbar lordosis, hamstring muscle spasm, and pain during lateral trunk flexion/extension and double leg raising.17
Motor and sensory function. It is important to assess motor and sensory function to differentiate neurologic from orthopedic conditions. Deep tendon reflexes and lower extremity motor strength and sensory capabilities also need careful assessment.13 Sensation in the region of the cauda equinus requires further evaluation due to the possibility of cauda equinus compression. Hyperreflexia indicates an upper motor lesion, whereas hyporeflexia indicates a lower motor lesion—neither of which would be expected in spondylolisthesis or spondylolysis.
A patient with any positive neurologic signs should be referred to a neurologic specialist.
Diagnostic TESTING
Unfortunately, rigorous comparative research is lacking on which to base clinical practice guidelines for diagnosis (as well as treatment) of spondylolysis and spondylolisthesis.18 Nevertheless, current standards call for a detailed physical examination as an effective diagnostic tool and recommend radiologic evaluation for a definitive diagnosis.17,18 Lateral radiographs are also useful for identifying the degree of vertebral slippage when spondylolisthesis is diagnosed.17 The specialist may choose to utilize such diagnostic modalities as CT, single-photon-emission CT (SPECT), or MRI.
Radiologic evaluation
For complete radiologic evaluation, four x-ray views of the spine are necessary: anterior-posterior (AP), lateral, and bilateral oblique views. A fracture seen in the pars interarticularis is called the Scotty dog sign because it looks like a collar around the neck of a Scottish terrier (see Figures 2 and 3).2
Since treatment for nonspecific back pain and spondylolysis is essentially the same, it could be argued that radiographic imaging to confirm the clinical diagnosis exposes the patient to unnecessary radiation (a particular concern in the pediatric population). But a definitive diagnosis also rules out other pathologies that would require more aggressive treatment.
If x-rays reveal abnormalities, refer the patient to an orthopedic specialist for further evaluation. Referral to orthopedics should also be prompted if spondylolysis or spondylolisthesis is suspected but x-rays are insufficient to make the diagnosis.
CT and SPECT
Standaert and Herring suggest that CT combined with SPECT is the standard for diagnosis of a pars interarticularis lesion1; in many reported cases, CT test results may be negative even when SPECT results are abnormal, suggesting that both studies are needed. In other cases, CT can help identify the origin of an abnormality seen on SPECT.
The disadvantage to using both modalities is that the patient is exposed to additional ionizing radiation. If only one method is to be used, SPECT may be preferred; it exposes the patient to less ionizing radiation and seldom requires sedation.19
Magnetic resonance imaging
In terms of radiation exposure, MRI is preferred to CT and SPECT because it does not utilize ionizing radiation; unfortunately, it is also less effective at detection of spondylolysis and spondylolisthesis. A review of the literature indicates that MRI is not as sensitive as SPECT in identifying stress on the pars interarticularis.16,19
In general, MRI is superior for visualizing soft tissue pathology (eg, disk disease, nerve root compression, inflammation), while CT is superior for visualization of bone. In the context of back pain, MRI may be informative when etiologies other than spondylolysis and spondylolisthesis are suspected; Feldman et al recommend MRI for patients with constant back pain, radicular pain, nighttime pain, and/or abnormal neurologic examination results.16
The advantages and disadvantages of x-rays, SPECT, CT, and MRI for the diagnosis of spondylolysis and spondylolisthesis are summarized in Table 2.1,15,16,18,19
Laboratory studies
There are no laboratory tests to confirm the diagnosis of spondylolysis or spondylolisthesis. To eliminate other diagnoses, however, it is appropriate to order certain laboratory tests. In addition to radiographs, Bernstein and Cozen recommend a complete blood count, an erythrocyte sedimentation rate, and a C-reactive protein test as part of the diagnostic work-up if the history and physical are suspicious for underlying pathology, such as infection.13
Management and Treatment Options
Treatment for spondylolysis or spondylolisthesis ranges from basic strengthening programs to surgical intervention and is based on the severity of the patient’s condition. The goal is to alleviate symptoms and facilitate a return to normal activities. Treatment should be individualized based on the patient’s age, athletic level and demands, and severity of symptoms.10
Nonsurgical treatment
Conservative treatment options include rest, physical therapy, core strengthening, and antilordotic bracing for several months (eg, eight to 12 weeks).5 Most grade 1 and 2 cases can be successfully treated nonsurgically.
Studies of nonsurgical treatment of children and young adults with spondylolysis and/or mild spondylolisthesis (up to 25% slippage) were evaluated in a meta-analysis.20 The authors found that approximately 84% of such patients were pain free or nearly pain free with unrestricted activities within one year of treatment. While nonsurgical treatment does not usually resolve the pars interarticularis defect, it alleviates symptoms and enables the patient’s return to unrestricted activities. Further, the authors found no significant difference in outcomes between patients who were treated with or without bracing.20
In contrast, another study demonstrated that patients who wore braces achieved higher functional outcomes than those who did not.21 However, the patients who were braced were restricted from physical activity longer than were those who were not braced, so it could not be definitively determined if bracing or additional rest was the reason for the improved outcomes.
A significant limitation of bracing is that it is a restrictive and sometimes uncomfortable treatment, especially for an active child or adolescent. If clinicians can treat these patients effectively without bracing, greater compliance with treatment may result.
This study also found a strong correlation between early intervention and an increased incidence of bony healing. The researchers recommend the early use of sensitive diagnostic imaging so that treatment can begin early, increasing the possibility that the fracture will heal.20
Skeletally immature patients should be followed clinically at six-month to one-year intervals, including use of lateral x-rays for spinal evaluation, to ensure that progressive spondylolisthesis does not develop. Once skeletal development is complete, follow-up is no longer necessary because progressive spondylolisthesis is unlikely at or near skeletal maturity.10
Surgical treatment
When conservative treatment fails to alleviate the pediatric patient’s pain, if daily functioning is impaired, or if the spondylolisthesis is of a more severe grade or progresses, surgical correction to repair the pars interarticularis defect or laminectomy and spinal fusion may be necessary.5 Adolescent patients treated surgically are likely to have good long-term results but may still experience symptoms, including back pain, into adulthood.22 The procedure used in a particular case depends on the degree of severity and the patient’s specific presentation.
PRIMARY CARE IS KEY
As stated earlier, referral to an orthopedic specialist is indicated when spondylolysis or spondylolisthesis is suspected or confirmed or to a neurologic specialist when any neurologic signs are positive or deficits are noted. In these instances, the primary care clinician is key to early diagnosis and prompt treatment.
Primary health care practitioners are in a position to contribute to the management of spondylolysis or spondylolisthesis in a way that will help facilitate a full recovery. For example, the patient and parent or guardian should be counseled about the need for the child to refrain from athletic activity while awaiting an orthopedic or neurologic evaluation. Restriction of activity will prevent exacerbation of the injury and increased pain. Suggest OTC NSAIDs, taken with food to prevent gastrointestinal adverse effects, for pain relief.13
It can also be helpful to assess the patient’s psychosocial status to determine whether he or she might face barriers to recovery. Often a major obstacle in treating these conditions is pressure from parents, coaches, or the patients themselves to continue athletic activities despite pain and injury.
Clinicians can offer support by reinforcing the message that limiting physical activity is essential to recovery; it should not be compromised because of patient, parental, coach, or peer pressure. At the same time, it is important to recognize that a leave of absence from a competitive sport, no matter how short, can affect the patient’s mental health. If the patient feels angry or depressed about the diagnosis, mental health counseling options can be discussed.
Educating patients and parents about the numerous treatments for acute low back pain that have been proven to offer little benefit can be informative and reassuring. For example, adding spinal manipulation and chiropractic techniques to established medical treatments does not improve outcomes. Neither does the use of oral corticosteroids, acupuncture, massage, traction, or exercise programs. Bed rest should be avoided.23
Conclusion
In the primary care setting, low back pain is a common complaint with an extensive differential diagnosis. If a thorough history and physical examination prompt suspicion for spondylolysis or spondylolisthesis, x-rays will usually confirm the diagnosis. If the patient is referred to an orthopedic specialist, the primary care clinician can supplement and reinforce the treatment plan through patient and parent education about the diagnosis and its treatment. Compliance with conservative nonsurgical treatment may enable the patient to make a speedier return to his or her usual physical activities.
The authors would like to thank Jennifer Tareco, MD, and Robert More, MD, for their revisions and support in the completion of this article.
1. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
2. Spiegel DA, Dormans JP. Spondylolysis and spondylolisthesis. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE, eds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2011:1561-1571.
3. Smith J. Moving beyond the neutral spine: stabilizing the dancer with lumbar extension dysfunction. J Dance Med Sci. 2009;13(3):73-82.
4. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
5. Wicker A. Spondylolysis and spondylolisthesis in sports. Int Sports Med J. 2008;9(2):74-78.
6. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007; 6(1):62-66.
7. Wiltse LL. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539-560.
8. Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
9. Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767-778.
10. Tallarico RA, Madom IA, Palumbo MA. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16(1):32-38.
11. Takao S, Sakai T, Sairyo K, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 2010;57 (1-2):133-137.
12. Hatz D, Esposito PW, Schroeder B, et al. The incidence of spondylolysis and spondylolisthesis in children with osteogenesis imperfecta. J Pediatr Orthop. 2011;31(6):655-660.
13. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76(11):1669-1676.
14. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: The psychosocial interview for adolescents updated for a new century fueled by media. Contemp Pediatr. 2014;1:16-28.
15. Feldman DS, Straight JJ, Badra MI, et al. Evaluation of an algorithmic approach to pediatric back pain. J Pediatr Orthop. 2006;26(3):353-357.
16. Masci L, Pike J, Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
17. Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil. 2009;22(1):27-32.
18. Bhatia NN, Chow G, Timon SJ, Watts HG. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J Pediatr Orthop. 2008;28(2):230-233.
19. Zukotynski K, Curtis C, Grant FD, et al. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5(13):1-6.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop. 2009;29(2):146-156.
21. Alvarez-Diaz P, Alentorn-Geli E, Steinbacher G, et al. Conservative treatment of lumbar spondylolysis in young soccer players. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2111-2114.
22. Helenius I, Remes V, Lamberg T, et al. Long-term health-related quality of life after surgery for adolescent idiopathic scoliosis and spondylolisthesis. J Bone Joint Surg Am. 2008;90(6):1231-1239.
23. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
1. Standaert CJ, Herring SA. Expert opinion and controversies in sports and musculoskeletal medicine: the diagnosis and treatment of spondylolysis in adolescent athletes. Arch Phys Med Rehabil. 2007;88(4):537-540.
2. Spiegel DA, Dormans JP. Spondylolysis and spondylolisthesis. In: Kliegman RM, Stanton BF, Schor NF, St. Geme III JW, Behrman RE, eds. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; 2011:1561-1571.
3. Smith J. Moving beyond the neutral spine: stabilizing the dancer with lumbar extension dysfunction. J Dance Med Sci. 2009;13(3):73-82.
4. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
5. Wicker A. Spondylolysis and spondylolisthesis in sports. Int Sports Med J. 2008;9(2):74-78.
6. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007; 6(1):62-66.
7. Wiltse LL. The etiology of spondylolisthesis. J Bone Joint Surg Am. 1962;44-A:539-560.
8. Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
9. Ivkovic A, Franic M, Bojanic I, Pecina M. Overuse injuries in female athletes. Croat Med J. 2007;48:767-778.
10. Tallarico RA, Madom IA, Palumbo MA. Spondylolysis and spondylolisthesis in the athlete. Sports Med Arthrosc. 2008;16(1):32-38.
11. Takao S, Sakai T, Sairyo K, et al. Radiographic comparison between male and female patients with lumbar spondylolysis. J Med Invest. 2010;57 (1-2):133-137.
12. Hatz D, Esposito PW, Schroeder B, et al. The incidence of spondylolysis and spondylolisthesis in children with osteogenesis imperfecta. J Pediatr Orthop. 2011;31(6):655-660.
13. Bernstein RM, Cozen H. Evaluation of back pain in children and adolescents. Am Fam Physician. 2007;76(11):1669-1676.
14. Klein DA, Goldenring JM, Adelman WP. HEEADSSS 3.0: The psychosocial interview for adolescents updated for a new century fueled by media. Contemp Pediatr. 2014;1:16-28.
15. Feldman DS, Straight JJ, Badra MI, et al. Evaluation of an algorithmic approach to pediatric back pain. J Pediatr Orthop. 2006;26(3):353-357.
16. Masci L, Pike J, Malara F, et al. Use of the one-legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-946.
17. Kalpakcioglu B, Altinbilek T, Senel K. Determination of spondylolisthesis in low back pain by clinical evaluation. J Back Musculoskelet Rehabil. 2009;22(1):27-32.
18. Bhatia NN, Chow G, Timon SJ, Watts HG. Diagnostic modalities for the evaluation of pediatric back pain: a prospective study. J Pediatr Orthop. 2008;28(2):230-233.
19. Zukotynski K, Curtis C, Grant FD, et al. The value of SPECT in the detection of stress injury to the pars interarticularis in patients with low back pain. J Orthop Surg Res. 2010;5(13):1-6.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults. J Pediatr Orthop. 2009;29(2):146-156.
21. Alvarez-Diaz P, Alentorn-Geli E, Steinbacher G, et al. Conservative treatment of lumbar spondylolysis in young soccer players. Knee Surg Sports Traumatol Arthrosc. 2011;19(12):2111-2114.
22. Helenius I, Remes V, Lamberg T, et al. Long-term health-related quality of life after surgery for adolescent idiopathic scoliosis and spondylolisthesis. J Bone Joint Surg Am. 2008;90(6):1231-1239.
23. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
Clinical Management of Sports-Related Pediatric Concussions
CE/CME No: CR-1409
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define the term concussion.
• Identify the major signs and symptoms of a concussion.
• Discuss the initial management of a pediatric patient with a suspected sports-related concussion.
• Describe the current recommendations for return to play after a pediatric concussion.
• Explain the main challenges clinicians face in the management of pediatric concussions
FACULTY
Sydney Meckler is pursuing her Doctor of Nursing Practice degree at Columbia University, New York City. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
This article describes the challenges of diagnosis and treatment of pediatric concussive injuries, with particular attention to the effects of concussion on the developing brain. It highlights the need for individualized, age-dependent care; standardized return-to-play and return-to-school protocols; and better-informed clinicians, parents, teachers, and coaches.
Pediatric concussions—those occurring in children younger than 18—account for almost half a million emergency department visits each year.1 Of these, more than 25% occur during sports-related activities.2 Yet it is believed that pediatric concussive injuries are significantly underreported because parents do not always seek medical attention for their concussed children and because little has been published on the management of sports-related concussions in pre–high-school-age athletes.2
The 4th International Consensus Conference on Concussion in Sport, the American Academy of Neurology, and the American Medical Society for Sports Medicine all recently updated their guidelines for concussion evaluation and management.3-5 However, the lack of data on concussions in younger pediatric athletes presents challenges for the optimal management of these patients.6 In fact, in a February 2014 report, the Institute of Medicine said that more research is needed to develop evidence-based sports-related concussion management guidelines for children and adolescents.7
In this article, the factors that distinguish concussions in children from those in adults will be described, as will the management techniques that primary care clinicians can use to effectively address them.
WHAT IS A CONCUSSION?
Although the term concussion has been used interchangeably with head trauma and brain injury, concussion is most often described as mild traumatic brain injury (mTBI). Variations in terminology reflect both the lack of agreement on a standard definition for concussion, especially as it applies to the pediatric population, and the complexities of concussion diagnosis and management.8 This inherent ambiguity can lead to suboptimal recognition and awareness of concussion among clinicians, patients, families, school personnel, and sports staff, especially of its serious aftereffects in the pediatric patient.
A blow to the head can propel the brain to strike one side of the skull and then bounce off the opposite side. This can damage or tear the axons—the signal transmitters of the neurons—from their cell bodies, disrupting brain cell communications and causing neurocognitive deficits.
While a concussion is a direct injury to the brain, it is also an evolving process comprised of several pathophysiologic components that are a consequence of biomechanical forces. The brain does not directly contact the skull but is surrounded by cerebrospinal fluid and fluid-filled vessels with a greater density than the brain itself. This intracranial space provides room for the brain to move upon impact by shifting and compressing from one side of the skull to the other.9 The concussive injury occurs as a result of either a direct strike to the head or from an indirect force traumatic enough to be transmitted to the brain. The strike or force produces a complex disturbance to the brain that, in most cases, does not result in loss of consciousness.3
THE GREATER VULNERABILITY OF THE PEDIATRIC BRAIN
Pathophysiology
For a concussion to occur, the biomechanical threshold at which nerve cells' adaptability is impeded must be crossed.10 This sets in motion a cascade of events, developing over minutes, hours, and even days, in which homeostasis is disrupted due to an imbalance of ionic, neuronal, metabolic, and pathophysiologic processes.5
While many studies have sought to explain the pathophysiology of pediatric sports-related concussions, much is still unknown. One hypothesis suggests that, after the injury, neuronal depolarization occurs, releasing excitatory neurotransmitters and altering metabolism by the immediate release of potassium. Restoration of homeostasis demands response by the sodium-potassium pump, requiring energy in the form of glucose. Coupled with axonal injury and alterations in cerebral blood flow, an imbalance between energy supply and demand occurs.5
Maugans et al found that a single pediatric concussion does not cause a structural or metabolic injury but instead a physiologic interruption—different from what occurs in adult concussions.10 They observed a statistically significant reduction in cerebral blood flow of more than 10% in a small group of postconcussion children, ages 11 to 15, compared with a control group. This alteration persisted for two weeks in two-thirds of the children and for up to a month in the remaining third.10 The delayed return of normal cerebral blood flow is believed to be a major contributing factor to pediatric concussion-related symptoms. However, little is known about the long-term effects of concussion on the developing brain.9
Crucial developmental years
Because the pediatric brain is still developing, children between the ages of 5 and 12 are at greater risk for sustaining a disruption in brain function from a concussion. In addition, this group is more susceptible in general to sports injuries compared to adults, because of their stage of growth and development.11
With a pediatric concussion, the individual child’s stage of brain development and the possible effects on his or her evolving neurocognitive functioning must be taken into account. Critical skills, such as language, emotion, memory, problem solving, and motor dexterity, emerge during childhood, and a disturbance during this time could have substantial consequences.12 Motor skills and primary senses usually mature by age 5, with language maturity occurring by age 10.9 However, many other vital skills and processes, such as emotion, judgment, and abstract reasoning, are not fully developed until adulthood. As a result, damage to cortical areas of the brain, such as the parietal lobe and frontal areas—involved in fine motor skills and complex thinking—can hinder or delay the development of these necessary processes.9
Many researchers suggest that, while a concussion to the developing brain may not significantly affect previously learned functions, it may notably delay development of new cognitive functions. As a result, recovery from a concussion during these years may be poorer.2,9,12
On the next page: Clinical presentation and post injury evaluation >>
CLINICAL PRESENTATION
Initial assessment of pediatric athletes with suspected concussive injuries can be particularly challenging because of the variability and evolving nature of associated signs and symptoms, resulting in a wide range of clinical presentations.3 These presenting signs and symptoms can, however, provide vital clues to the location of the injury within the brain because the consequences of concussion arise from injury to the brain’s cortical and subcortical systems (see Table 1).9
The cortical systems are located within the frontal, temporal, parietal, and occipital lobes, each of which houses control centers for specific learned skills and functions. The subcortical systems include the hypothalamus, trigeminal system, basal ganglia, and cerebellum. While a concussion most often causes diffuse harm rather than localized trauma, familiarity with the functions associated with specific brain locations aids in initial identification of the injury and allows for individualized concussion management.13
After a concussion occurs, it is essential to evaluate all domains of the child’s functioning through a comprehensive physical assessment, including a detailed concussion history and review of preinjury risk factors.3 In addition, there may be unobservable symptoms about which a child is unable to communicate due to his or her stage of emotional and cognitive development and restricted capacity to serve as a primary reporter.14
Signs and symptoms
The four categories of concussion signs and symptoms include physical, cognitive, emotional, and sleep (see Table 2).15 A concussed patient may experience any variation or combination of these signs and symptoms.
It is important to recognize that, although loss of consciousness is a serious sign of a concussion, it occurs in only about 10% of concussive injuries.5,15 In fact, current concussion guidelines no longer advocate the use of grading scales, which assessed the severity of a concussion based primarily on the presence or absence of a brief loss of consciousness. Evidence now shows that loss of consciousness does not predict clinical course or cognitive long-term impairment after a concussion.16
Duration of symptoms
The duration of concussion symptoms can vary, but in most cases symptoms last seven to 10 days, with 10% to 15% of patients reporting symptoms for more than 10 days.3,5 While recovery duration has not been studied sufficiently in children younger than 15, younger children require longer recovery periods. The resolution of symptoms, however, does not necessarily correlate with absolute cognitive recovery.5,17
Modifying and complicating factors
In addition to the patient’s presenting signs and symptoms, factors that may modify or complicate recovery from a concussion should be identified (see Table 3).3 These factors include preexisting conditions or disorders and the use of certain prescription drugs. Each factor should be assessed individually, taking into account the patient’s age.2
Some presenting symptoms may overlap with symptoms of common disorders in this group. It is important to note if symptoms of such conditions as attention-deficit/hyperactivity disorder (ADHD), sleep disorders, learning disabilities, and mood disorders were present prior to the concussion.5
Modifying factors provide essential information that can help predict the individual patient’s anticipated recovery process and must be taken into account throughout the process. They are particularly important when assessing the differences between a child’s preconcussion and postconcussion functioning.3,4
POST INJURY EVALUATION OF COGNITIVE FUNCTIONING
Concussion is diagnosed clinically, based on the mechanism of the sustained injury, and is supported primarily by reported and observed signs and symptoms. The ability to systematically evaluate the patient for manifestations of concussion is essential to pediatric concussion management.3,18
To aid in appropriately evaluating symptoms and assessing functional damage, many standardized assessment tools and neuropsychological tests have been developed. These tools have been modified for younger athletes due to differences in children’s neurocognitive development and in their ability to be symptom self-reporters.
Concussion assessment tools
The tools most commonly used to assess the effects of concussion in younger athletes are listed in Table 4. It was not until 2012, with the release of the Child Sport Concussion Assessment Tool Version 3 (Child-SCAT3), that an appropriately adapted and valid concussion assessment tool became available for children younger than 10.3
Assessment tools are useful but are not diagnostic. They do not take the place of a clinician’s thorough neurocognitive evaluation of a potentially concussed child.
Neuropsychologic testing
In order to properly manage pediatric concussions, postconcussion status must be assessed relative to preconcussion status in order to ensure optimal resolution of symptoms and cognitive recovery.19
The American Medical Society for Sports Medicine supports baseline testing for high-risk athletes, defined as those with a prior history of concussions or “confounding” conditions (learning disability, mood and attention disorders, migraine headaches).5 The International Consensus Statement on Concussion in Sport states that there is insufficient evidence to recommend routine baseline testing, but its early use after pediatric concussion may provide helpful information for determining when the child may return to school. The American Academy of Neurology suggests that clinicians “… might utilize baseline scores on concussion assessment tools, especially in younger athletes, those with prior concussions, or those with preexisting learning disabilities/ADHD, as doing so fosters better interpretation of postinjury scores.”4
Assessment tools can provide essential insight into the cognitive functional state of a concussed child athlete, even after symptom resolution.3,5,20 They are not stand-alone measures of recovery but can be useful components of the pediatric concussion evaluation and management process and an aid to clinical decision making.3,13
Paper-and-pencil or computerized neuropsychologic tests provide objective measures of brain-behavior relationships and are more sensitive in detecting subtle cognitive impairments than a clinical examination.5 Although neuropsychologic testing has not been validated as a diagnostic tool, it may be useful for both baseline (preinjury) testing of athletes and for monitoring recovery from a concussive event.5,20 (For more information on motor control assessment, see Dirks RP, McLeod TCV. Sport-related mild traumatic brain injury. Clinician Reviews. 2008;18[9]:22. http://bit.ly/1mjizIa.)
On the next page: Postconcussion management >>
POSTCONCUSSION MANAGEMENT
All concussion management guidelines concur that immediate removal from play of a pediatric athlete with a suspected concussion is the most important initial action. Regardless of how short the duration or mild the symptoms may be, same-day return to play should never occur.3-5 This is particularly true because acute concussion is an evolving injury and manifestation of symptoms, including cognitive deficits, is often delayed.3
The foundation of postconcussion recovery is rest, both physical and cognitive. Acute symptoms must diminish before a gradual resumption of activities.3,5
Cognitive rest
Cognitive rest is the cornerstone of concussion management.5 The latest guidelines stress the importance, particularly in the pediatric age-group, of decreasing any activities of daily living that may aggravate symptoms.3 This includes such common childhood pastimes as playing video games, watching television, and using a computer.15 More important, cognitive rest means academic rest, which is essential to postconcussion recovery and preparing the child for a “return to school” or “return to learn.”21, 22
Return to play
In pediatric concussion management, a conservative approach to the determination of when a concussed child may return to play (RTP) is key. RTP decisions are guided by the resolution of the child’s symptoms and are based on clinical judgment.3-5
Current guidelines outline a gradual, stepwise approach to RTP after full recovery, which must be individualized and age-appropriate.3,5 These include
• Light aerobic exercise: Increase heart rate to 70% maximum predicted by walking, swimming, or using stationary bike. No resistance training.
• Sport-specific exercise: Add movement with skating or running drills. No head impact activities.
• Noncontact training drills: Add exercise, coordination, and cognitive load with progression to more complex training drills (eg, passing drills in football and ice hockey). May introduce progressive resistance training.
• Full contact practice: To restore confidence and allow coaching staff to assess functional skills, permit participation in normal training activities after obtaining medical clearance.
At any step, if symptoms develop with activity, the process is stopped and, after a 24-hour period of rest, is restarted at the previous symptom-free step.3,5
The goal of this approach is to ensure that the pediatric brain recovers fully and can resume normal developmental acquisition of cognitive skills and functions. Further, as previously noted, children and adolescents require more time to recover from the effects of a concussion than adults do.2,3 If a child returns too quickly to activity postconcussion, clinical evidence suggests that worsening of cognitive deficits is likely.9 Therefore, cautious postconcussion management that allows sufficient recovery time before clearance is given for a return to sports participation is highly recommended.2,4,5 No child should RTP unless cleared by a health care provider trained in the evaluation and management of pediatric concussive injuries.4,5,15
Return to school
Inadequate knowledge of concussion on the part of parents, teachers, and school officials can be a barrier to appropriate return-to-school decisions after pediatric concussive injuries.22 Since pre–high-school-age children spend the majority of their time in school, it is essential that a child’s school attendance and workload demands be decreased during recovery from a concussion.
Evidence indicates that an increase in cognitive or physical activity before complete recovery—ie, before normal brain cellular function is restored—may prolong cognitive dysfunction.5 Arbogast et al found that, in many pediatric concussion patients, unresolved symptoms impeded learning and school-based functioning: 10% to 18% of the children studied experienced fatigue, difficulty concentrating, feeling foggy, and/or vision problems.21 Within the first two weeks postinjury, 80% reported an increase in symptom severity while at school.
Academic demands should be increased gradually, with adjustments made for the individual student as needed, in order to avoid the exacerbation of such school-setting symptoms as headache, dizziness, light and/or noise sensitivity, and difficulty concentrating or remembering.22
While RTP guidelines are widely understood and implemented by clinicians, one survey found that return-to-school guidance is provided less often.21 Clinicians should be mindful of the importance of both physical rest and cognitive rest during recovery from pediatric concussion and should provide parents with clear guidance for both.21
On the next page: Role of the primary care clinician >>
THE ROLE OF THE PRIMARY CARE CLINICIAN
Primary care clinicians are central to pediatric concussion management because they facilitate and oversee a child’s recovery. They may administer neuropsychologic testing, monitor symptom resolution, and supervise the concussed child’s gradual return to physical and academic activities through the individualized phases of RTP and return-to-school protocols.4,20,23
The NP or PA functions as the main point of contact within the heath care team, organizing the plan of care and coordinating with parents, teachers, and coaches throughout the recovery process. Close follow-up care, including detailed documentation, should take place at well-child examinations to help ensure that the child’s development continues to progress as expected.
Referrals
Although referrals to a specialist are not typically necessary, persistent symptoms—those lasting longer than 10 days and not usually specific to concussion—warrant immediate and appropriate referral.3,22 Approximately 30% of mTBI patients experience long-term, often significant aftereffects, underscoring that mTBI is not a single entity and that each patient is unique.24
Risk factors for protracted symptoms
Factors that may be associated with prolonged or persistent duration of symptoms include early posttraumatic headache, previous concussion, early amnesia, alteration in mental status, disorientation, fatigue/fogginess, developmental disorders (eg, learning disabilities, ADHD), and psychiatric disorders (eg, anxiety, depression).4,13 Studies have examined this association, and there is conflicting evidence as to whether prolonged symptoms are attributed to, or are a combination of, preconcussion modifying factors, concussive episode severity, and/or coexistent pathologies.3,4
Concussion complications
Postconcussion syndrome can occur in pediatric athletes after a single concussion.3,23 The syndrome is not clearly understood but is characterized by the persistence of multiple cognitive, physical, and/or emotional symptoms of a concussion for weeks or even months, making it difficult to both diagnose and manage.5,15
Second impact syndrome, only documented in pediatric athletes, occurs when a child sustains a concussion before fully recovering from a prior concussion. This can lead to cerebral vascular congestion, cerebral swelling, and in some cases, death.15 Immediate removal of a concussed child from play until completely asymptomatic is obviously essential to the prevention of this rare catastrophe.
Any child with persistent symptoms should be referred to a multidisciplinary team of providers experienced in concussion management. This team may include, but is not limited to, neurologists, developmental pediatricians, concussion specialists, and psychologic, cognitive, and/or physical therapists.3,5
CONCUSSION EDUCATION
Family
Family-centered education is another essential component of pediatric concussion management. Parents and guardians are extremely influential and important to a child’s overall recovery, especially a younger child’s. While older pre–high-school-age children are much more capable of self-reporting symptoms, the younger child may not yet be developmentally able to do so, making the parent or guardian’s assistance and input essential.14
Unfortunately, studies have shown that a significant number of parents lack a good understanding of what constitutes a concussion as well as of the appropriate steps to ensure their child’s safe return to sport and school.22,25 Many parents view concussion as meaning a less severe head injury than if mild TBI or minor TBI is used.26 It is imperative that clinicians ensure that parents understand the seriousness of their child’s concussive injury and how to judge when their child may safely return to school.
GAAME is an acronym for a simple concussion action plan for parents of young sports participants. The name is based on the first word of each step in the plan:
Get out, Assess, Ask, Medical attention, and Emergency situation.
The GAAME handout (see "Parent Guide") briefly explains the steps to take if a child experiences a blow to the head during a sports activity. It can be given to parents of all pre–high-schoolers, whether their children participate in organized sports or not.
GAAME utilizes a five-step approach that immediately pulls the child out of the game and highlights the important information a clinician will need when the child receives medical attention.3 Parents, guardians, and other involved parties serve as a child’s best advocate by being informed and alert to the early signs and symptoms of a concussion and by helping the child to identify his or her own symptoms.
School personnel
Teachers and other school officials must recognize both the necessity of cognitive rest during concussion recovery and their role in enabling a child’s gradual return to the cognitive demands of school. A child may require such adjustments as a shorter school day or week, a reduced workload, and extra time for tests to avoid exacerbation of concussion symptoms.5,22 In light of state concussion laws (see below), schools commonly ensure that personnel are well-informed about concussion and its aftereffects in the classroom.
Sports officials
Heightened awareness of the need for more effective concussion education and management in youth sports has led to enactment of laws on youth sports-related concussions in all 50 states and the District of Columbia.27 Most of these laws include
1. Inform and educate coaches, athletes, and their parents and guardians about concussion through training and/or a concussion information sheet.
2. An athlete who is believed to have a concussion is to be removed from play right away.
3. An athlete can only return to play or practice after at least 24 hours and with permission from a health care professional.
Clinicians should also be familiar with the laws in their states as they may affect clinical decisions (eg, timing of RTP protocol).
On the next page: Conclusion >>
CONCLUSION
Optimal management of pediatric concussions is a multifaceted challenge that is best addressed by age-appropriate, individualized patient care. Educational initiatives based on the latest concussion guidelines will increase awareness of appropriate concussion management by all involved in overseeing the recovery of the pre-high-school-age child.
A greater understanding of concussion, coupled with management based on current, standardized RTP and return-to-school protocols, will enhance coordination among the multidisciplinary team overseeing the child’s recovery and facilitate the return to full preconcussion functioning. More research about the effects of concussion on the developing brain is needed in order to create evidence-based guidelines specific to this pediatric population.
1. Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002-2006. www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. Accessed August 14, 2014.
2. Meehan WP, Taylor AM, Proctor M. The pediatric athlete: younger athletes with sports-related concussion. Clin Sports Med. 2011;30(1):1-11.
3. McCrory P, Meeuwisse WH, Aubry M, et al; Concussion in Sport Group. Consensus statement on concussion in sport: the 4th International Consensus Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
4. Giza CC, Kutcher JS, Ashwal S, et al; Guideline Development Subcommittee, American Academy of Neurology. Summary of evidence-based guideline update: evaluation and management of concussion in sports. Neurology. 2013;80(24):2250-2257.
5. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15-26.
6. Chrisman SP, Schiff MA, Rivara FP. Physician concussion knowledge and effect of mailing the CDC “heads up” toolkit. Clin Pediatr. 2011;50(11):1031-1039.
7. Graham R, Rivara FP, Ford MA, Spicer CM, eds. Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. Washington, DC: The National Academies Press; 2014.
8. Khurana VG, Kaye AH. An overview of concussion in sport. J Clin Neurosci. 2012;19(1):1-11.
9. Toledo E, Lebel A, Becerra L, et al. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci Biobehav Rev. 2012;36(6):1510-1531.
10. Maugans TA, Farley C, Altaye M, et al. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28-37.
11. Franklin CC, Weiss JM. Stopping sports injuries in kids: an overview of the last year in publications. Curr Opin Pediatr. 2012;24(1):64-67.
12. Cernak I, Chang T, Ahmed FA, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2012;32(5-6):442-453.
13. Gioia GA, Collins M, Isquith PK. Improving identification and diagnosis of mild traumatic brain injury with evidence: psychometric support for the acute concussion evaluation. J Head Trauma Rehabil. 2008;23(4):230-242.
14. Echemendia RJ, Iverson G, McCrea M, et al. Advances in neuropsychological assessment of sport-related concussion. Br J Sports Med. 2013;47(5):294-298.
15. Halstead M, Walter D. Clinical report: sports-related concussion in children and adolescents. Pediatrics. 2010;126(3):597-615.
16. Gomez JE, Hergenroeder AC. New guidelines for management of concussion in sport: special concern for youth. J Adolesc Health. 2013;53(3):311-313.
17. Grubenhoff JA, Kirkwood M, Gao D, et al. Evaluation of standardized assessment of concussion in a pediatric emergency department. Pediatrics. 2010;126(4):688-694.
18. Fung M, Willer B, Moreland D, Leddy J. A proposal for an evidence-based emergency department discharge form for mild traumatic brain injury. Brain Inj. 2006;20(9):889-994.
19. Apps JN, Walter KD. Pediatric and Adolescent Concussion: Diagnosis, Management, and Outcomes. New York: Springer Science+Business Media, LLC; 2012. http://midnurse.umsha.ac.ir/uploads/Pediatric&Adolescent_Concus sion.pdf. Accessed August 14, 2014.
20. Kirkwood MW, Yeates KO, Wilson PE. Pediatric sports-related concussion: a review of the clinical management of an oft-neglected population in children and adolescents. Pediatrics. 2006;117(4):1359-1371.
21. Arbogast KB, McGinley AD, Master CL, et al. Cognitive rest and school-based recommendations following pediatric concussion: the need for primary support tools. Clin Pediatr. 2013;452(5):397-402.
22. Halstead ME, McAvoy K, Devore CD, et al. Returning to learning following a concussion. Pediatrics. 2013;132(5):948-957.
23. Marsh AM, Fraser D, Marsh JP. Management of concussion in the pediatric patient. J Pediatr Health Care. 2013;27(6):499-504.
24. Rosenbaum SB, Lipton ML. Embracing chaos: the scope and importance of clinical and pathological heterogeneity in MTBI. Brain Imaging Behav. 2012;6:255-282.
25. Bakhos LL, Lockhart GR, Myers R, Linakis JG. Emergency department visits for concussion in young child athletes. Pediatrics. 2010;126(3):e550-e556.
26. Gordon KE, Dooley JM, Fitzpatrick EA, et al. Concussion or mild traumatic brain injury: parents appreciate the nuances of nosology. Pediatr Neurol. 2010;43(4):253-257.
27. CDC. Get a heads up on concussion in sports policies. www.cdc.gov/concussion/policies.html. Accessed August 14, 2014.
28. Gioia G, Collins M. Acute concussion evaluation (ACE). 2006. www.cdc.gov/concussion/headsup/pdf/ace-a.pdf. Accessed August 14, 2014.
29. ImPACT Applications Inc. ImPACT (Immediate Post-Concussion Assessment and Cognitive Testing). www.impacttest.com/about/?Overview-1. Accessed August 14, 2014.
CE/CME No: CR-1409
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define the term concussion.
• Identify the major signs and symptoms of a concussion.
• Discuss the initial management of a pediatric patient with a suspected sports-related concussion.
• Describe the current recommendations for return to play after a pediatric concussion.
• Explain the main challenges clinicians face in the management of pediatric concussions
FACULTY
Sydney Meckler is pursuing her Doctor of Nursing Practice degree at Columbia University, New York City. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
This article describes the challenges of diagnosis and treatment of pediatric concussive injuries, with particular attention to the effects of concussion on the developing brain. It highlights the need for individualized, age-dependent care; standardized return-to-play and return-to-school protocols; and better-informed clinicians, parents, teachers, and coaches.
Pediatric concussions—those occurring in children younger than 18—account for almost half a million emergency department visits each year.1 Of these, more than 25% occur during sports-related activities.2 Yet it is believed that pediatric concussive injuries are significantly underreported because parents do not always seek medical attention for their concussed children and because little has been published on the management of sports-related concussions in pre–high-school-age athletes.2
The 4th International Consensus Conference on Concussion in Sport, the American Academy of Neurology, and the American Medical Society for Sports Medicine all recently updated their guidelines for concussion evaluation and management.3-5 However, the lack of data on concussions in younger pediatric athletes presents challenges for the optimal management of these patients.6 In fact, in a February 2014 report, the Institute of Medicine said that more research is needed to develop evidence-based sports-related concussion management guidelines for children and adolescents.7
In this article, the factors that distinguish concussions in children from those in adults will be described, as will the management techniques that primary care clinicians can use to effectively address them.
WHAT IS A CONCUSSION?
Although the term concussion has been used interchangeably with head trauma and brain injury, concussion is most often described as mild traumatic brain injury (mTBI). Variations in terminology reflect both the lack of agreement on a standard definition for concussion, especially as it applies to the pediatric population, and the complexities of concussion diagnosis and management.8 This inherent ambiguity can lead to suboptimal recognition and awareness of concussion among clinicians, patients, families, school personnel, and sports staff, especially of its serious aftereffects in the pediatric patient.
A blow to the head can propel the brain to strike one side of the skull and then bounce off the opposite side. This can damage or tear the axons—the signal transmitters of the neurons—from their cell bodies, disrupting brain cell communications and causing neurocognitive deficits.
While a concussion is a direct injury to the brain, it is also an evolving process comprised of several pathophysiologic components that are a consequence of biomechanical forces. The brain does not directly contact the skull but is surrounded by cerebrospinal fluid and fluid-filled vessels with a greater density than the brain itself. This intracranial space provides room for the brain to move upon impact by shifting and compressing from one side of the skull to the other.9 The concussive injury occurs as a result of either a direct strike to the head or from an indirect force traumatic enough to be transmitted to the brain. The strike or force produces a complex disturbance to the brain that, in most cases, does not result in loss of consciousness.3
THE GREATER VULNERABILITY OF THE PEDIATRIC BRAIN
Pathophysiology
For a concussion to occur, the biomechanical threshold at which nerve cells' adaptability is impeded must be crossed.10 This sets in motion a cascade of events, developing over minutes, hours, and even days, in which homeostasis is disrupted due to an imbalance of ionic, neuronal, metabolic, and pathophysiologic processes.5
While many studies have sought to explain the pathophysiology of pediatric sports-related concussions, much is still unknown. One hypothesis suggests that, after the injury, neuronal depolarization occurs, releasing excitatory neurotransmitters and altering metabolism by the immediate release of potassium. Restoration of homeostasis demands response by the sodium-potassium pump, requiring energy in the form of glucose. Coupled with axonal injury and alterations in cerebral blood flow, an imbalance between energy supply and demand occurs.5
Maugans et al found that a single pediatric concussion does not cause a structural or metabolic injury but instead a physiologic interruption—different from what occurs in adult concussions.10 They observed a statistically significant reduction in cerebral blood flow of more than 10% in a small group of postconcussion children, ages 11 to 15, compared with a control group. This alteration persisted for two weeks in two-thirds of the children and for up to a month in the remaining third.10 The delayed return of normal cerebral blood flow is believed to be a major contributing factor to pediatric concussion-related symptoms. However, little is known about the long-term effects of concussion on the developing brain.9
Crucial developmental years
Because the pediatric brain is still developing, children between the ages of 5 and 12 are at greater risk for sustaining a disruption in brain function from a concussion. In addition, this group is more susceptible in general to sports injuries compared to adults, because of their stage of growth and development.11
With a pediatric concussion, the individual child’s stage of brain development and the possible effects on his or her evolving neurocognitive functioning must be taken into account. Critical skills, such as language, emotion, memory, problem solving, and motor dexterity, emerge during childhood, and a disturbance during this time could have substantial consequences.12 Motor skills and primary senses usually mature by age 5, with language maturity occurring by age 10.9 However, many other vital skills and processes, such as emotion, judgment, and abstract reasoning, are not fully developed until adulthood. As a result, damage to cortical areas of the brain, such as the parietal lobe and frontal areas—involved in fine motor skills and complex thinking—can hinder or delay the development of these necessary processes.9
Many researchers suggest that, while a concussion to the developing brain may not significantly affect previously learned functions, it may notably delay development of new cognitive functions. As a result, recovery from a concussion during these years may be poorer.2,9,12
On the next page: Clinical presentation and post injury evaluation >>
CLINICAL PRESENTATION
Initial assessment of pediatric athletes with suspected concussive injuries can be particularly challenging because of the variability and evolving nature of associated signs and symptoms, resulting in a wide range of clinical presentations.3 These presenting signs and symptoms can, however, provide vital clues to the location of the injury within the brain because the consequences of concussion arise from injury to the brain’s cortical and subcortical systems (see Table 1).9
The cortical systems are located within the frontal, temporal, parietal, and occipital lobes, each of which houses control centers for specific learned skills and functions. The subcortical systems include the hypothalamus, trigeminal system, basal ganglia, and cerebellum. While a concussion most often causes diffuse harm rather than localized trauma, familiarity with the functions associated with specific brain locations aids in initial identification of the injury and allows for individualized concussion management.13
After a concussion occurs, it is essential to evaluate all domains of the child’s functioning through a comprehensive physical assessment, including a detailed concussion history and review of preinjury risk factors.3 In addition, there may be unobservable symptoms about which a child is unable to communicate due to his or her stage of emotional and cognitive development and restricted capacity to serve as a primary reporter.14
Signs and symptoms
The four categories of concussion signs and symptoms include physical, cognitive, emotional, and sleep (see Table 2).15 A concussed patient may experience any variation or combination of these signs and symptoms.
It is important to recognize that, although loss of consciousness is a serious sign of a concussion, it occurs in only about 10% of concussive injuries.5,15 In fact, current concussion guidelines no longer advocate the use of grading scales, which assessed the severity of a concussion based primarily on the presence or absence of a brief loss of consciousness. Evidence now shows that loss of consciousness does not predict clinical course or cognitive long-term impairment after a concussion.16
Duration of symptoms
The duration of concussion symptoms can vary, but in most cases symptoms last seven to 10 days, with 10% to 15% of patients reporting symptoms for more than 10 days.3,5 While recovery duration has not been studied sufficiently in children younger than 15, younger children require longer recovery periods. The resolution of symptoms, however, does not necessarily correlate with absolute cognitive recovery.5,17
Modifying and complicating factors
In addition to the patient’s presenting signs and symptoms, factors that may modify or complicate recovery from a concussion should be identified (see Table 3).3 These factors include preexisting conditions or disorders and the use of certain prescription drugs. Each factor should be assessed individually, taking into account the patient’s age.2
Some presenting symptoms may overlap with symptoms of common disorders in this group. It is important to note if symptoms of such conditions as attention-deficit/hyperactivity disorder (ADHD), sleep disorders, learning disabilities, and mood disorders were present prior to the concussion.5
Modifying factors provide essential information that can help predict the individual patient’s anticipated recovery process and must be taken into account throughout the process. They are particularly important when assessing the differences between a child’s preconcussion and postconcussion functioning.3,4
POST INJURY EVALUATION OF COGNITIVE FUNCTIONING
Concussion is diagnosed clinically, based on the mechanism of the sustained injury, and is supported primarily by reported and observed signs and symptoms. The ability to systematically evaluate the patient for manifestations of concussion is essential to pediatric concussion management.3,18
To aid in appropriately evaluating symptoms and assessing functional damage, many standardized assessment tools and neuropsychological tests have been developed. These tools have been modified for younger athletes due to differences in children’s neurocognitive development and in their ability to be symptom self-reporters.
Concussion assessment tools
The tools most commonly used to assess the effects of concussion in younger athletes are listed in Table 4. It was not until 2012, with the release of the Child Sport Concussion Assessment Tool Version 3 (Child-SCAT3), that an appropriately adapted and valid concussion assessment tool became available for children younger than 10.3
Assessment tools are useful but are not diagnostic. They do not take the place of a clinician’s thorough neurocognitive evaluation of a potentially concussed child.
Neuropsychologic testing
In order to properly manage pediatric concussions, postconcussion status must be assessed relative to preconcussion status in order to ensure optimal resolution of symptoms and cognitive recovery.19
The American Medical Society for Sports Medicine supports baseline testing for high-risk athletes, defined as those with a prior history of concussions or “confounding” conditions (learning disability, mood and attention disorders, migraine headaches).5 The International Consensus Statement on Concussion in Sport states that there is insufficient evidence to recommend routine baseline testing, but its early use after pediatric concussion may provide helpful information for determining when the child may return to school. The American Academy of Neurology suggests that clinicians “… might utilize baseline scores on concussion assessment tools, especially in younger athletes, those with prior concussions, or those with preexisting learning disabilities/ADHD, as doing so fosters better interpretation of postinjury scores.”4
Assessment tools can provide essential insight into the cognitive functional state of a concussed child athlete, even after symptom resolution.3,5,20 They are not stand-alone measures of recovery but can be useful components of the pediatric concussion evaluation and management process and an aid to clinical decision making.3,13
Paper-and-pencil or computerized neuropsychologic tests provide objective measures of brain-behavior relationships and are more sensitive in detecting subtle cognitive impairments than a clinical examination.5 Although neuropsychologic testing has not been validated as a diagnostic tool, it may be useful for both baseline (preinjury) testing of athletes and for monitoring recovery from a concussive event.5,20 (For more information on motor control assessment, see Dirks RP, McLeod TCV. Sport-related mild traumatic brain injury. Clinician Reviews. 2008;18[9]:22. http://bit.ly/1mjizIa.)
On the next page: Postconcussion management >>
POSTCONCUSSION MANAGEMENT
All concussion management guidelines concur that immediate removal from play of a pediatric athlete with a suspected concussion is the most important initial action. Regardless of how short the duration or mild the symptoms may be, same-day return to play should never occur.3-5 This is particularly true because acute concussion is an evolving injury and manifestation of symptoms, including cognitive deficits, is often delayed.3
The foundation of postconcussion recovery is rest, both physical and cognitive. Acute symptoms must diminish before a gradual resumption of activities.3,5
Cognitive rest
Cognitive rest is the cornerstone of concussion management.5 The latest guidelines stress the importance, particularly in the pediatric age-group, of decreasing any activities of daily living that may aggravate symptoms.3 This includes such common childhood pastimes as playing video games, watching television, and using a computer.15 More important, cognitive rest means academic rest, which is essential to postconcussion recovery and preparing the child for a “return to school” or “return to learn.”21, 22
Return to play
In pediatric concussion management, a conservative approach to the determination of when a concussed child may return to play (RTP) is key. RTP decisions are guided by the resolution of the child’s symptoms and are based on clinical judgment.3-5
Current guidelines outline a gradual, stepwise approach to RTP after full recovery, which must be individualized and age-appropriate.3,5 These include
• Light aerobic exercise: Increase heart rate to 70% maximum predicted by walking, swimming, or using stationary bike. No resistance training.
• Sport-specific exercise: Add movement with skating or running drills. No head impact activities.
• Noncontact training drills: Add exercise, coordination, and cognitive load with progression to more complex training drills (eg, passing drills in football and ice hockey). May introduce progressive resistance training.
• Full contact practice: To restore confidence and allow coaching staff to assess functional skills, permit participation in normal training activities after obtaining medical clearance.
At any step, if symptoms develop with activity, the process is stopped and, after a 24-hour period of rest, is restarted at the previous symptom-free step.3,5
The goal of this approach is to ensure that the pediatric brain recovers fully and can resume normal developmental acquisition of cognitive skills and functions. Further, as previously noted, children and adolescents require more time to recover from the effects of a concussion than adults do.2,3 If a child returns too quickly to activity postconcussion, clinical evidence suggests that worsening of cognitive deficits is likely.9 Therefore, cautious postconcussion management that allows sufficient recovery time before clearance is given for a return to sports participation is highly recommended.2,4,5 No child should RTP unless cleared by a health care provider trained in the evaluation and management of pediatric concussive injuries.4,5,15
Return to school
Inadequate knowledge of concussion on the part of parents, teachers, and school officials can be a barrier to appropriate return-to-school decisions after pediatric concussive injuries.22 Since pre–high-school-age children spend the majority of their time in school, it is essential that a child’s school attendance and workload demands be decreased during recovery from a concussion.
Evidence indicates that an increase in cognitive or physical activity before complete recovery—ie, before normal brain cellular function is restored—may prolong cognitive dysfunction.5 Arbogast et al found that, in many pediatric concussion patients, unresolved symptoms impeded learning and school-based functioning: 10% to 18% of the children studied experienced fatigue, difficulty concentrating, feeling foggy, and/or vision problems.21 Within the first two weeks postinjury, 80% reported an increase in symptom severity while at school.
Academic demands should be increased gradually, with adjustments made for the individual student as needed, in order to avoid the exacerbation of such school-setting symptoms as headache, dizziness, light and/or noise sensitivity, and difficulty concentrating or remembering.22
While RTP guidelines are widely understood and implemented by clinicians, one survey found that return-to-school guidance is provided less often.21 Clinicians should be mindful of the importance of both physical rest and cognitive rest during recovery from pediatric concussion and should provide parents with clear guidance for both.21
On the next page: Role of the primary care clinician >>
THE ROLE OF THE PRIMARY CARE CLINICIAN
Primary care clinicians are central to pediatric concussion management because they facilitate and oversee a child’s recovery. They may administer neuropsychologic testing, monitor symptom resolution, and supervise the concussed child’s gradual return to physical and academic activities through the individualized phases of RTP and return-to-school protocols.4,20,23
The NP or PA functions as the main point of contact within the heath care team, organizing the plan of care and coordinating with parents, teachers, and coaches throughout the recovery process. Close follow-up care, including detailed documentation, should take place at well-child examinations to help ensure that the child’s development continues to progress as expected.
Referrals
Although referrals to a specialist are not typically necessary, persistent symptoms—those lasting longer than 10 days and not usually specific to concussion—warrant immediate and appropriate referral.3,22 Approximately 30% of mTBI patients experience long-term, often significant aftereffects, underscoring that mTBI is not a single entity and that each patient is unique.24
Risk factors for protracted symptoms
Factors that may be associated with prolonged or persistent duration of symptoms include early posttraumatic headache, previous concussion, early amnesia, alteration in mental status, disorientation, fatigue/fogginess, developmental disorders (eg, learning disabilities, ADHD), and psychiatric disorders (eg, anxiety, depression).4,13 Studies have examined this association, and there is conflicting evidence as to whether prolonged symptoms are attributed to, or are a combination of, preconcussion modifying factors, concussive episode severity, and/or coexistent pathologies.3,4
Concussion complications
Postconcussion syndrome can occur in pediatric athletes after a single concussion.3,23 The syndrome is not clearly understood but is characterized by the persistence of multiple cognitive, physical, and/or emotional symptoms of a concussion for weeks or even months, making it difficult to both diagnose and manage.5,15
Second impact syndrome, only documented in pediatric athletes, occurs when a child sustains a concussion before fully recovering from a prior concussion. This can lead to cerebral vascular congestion, cerebral swelling, and in some cases, death.15 Immediate removal of a concussed child from play until completely asymptomatic is obviously essential to the prevention of this rare catastrophe.
Any child with persistent symptoms should be referred to a multidisciplinary team of providers experienced in concussion management. This team may include, but is not limited to, neurologists, developmental pediatricians, concussion specialists, and psychologic, cognitive, and/or physical therapists.3,5
CONCUSSION EDUCATION
Family
Family-centered education is another essential component of pediatric concussion management. Parents and guardians are extremely influential and important to a child’s overall recovery, especially a younger child’s. While older pre–high-school-age children are much more capable of self-reporting symptoms, the younger child may not yet be developmentally able to do so, making the parent or guardian’s assistance and input essential.14
Unfortunately, studies have shown that a significant number of parents lack a good understanding of what constitutes a concussion as well as of the appropriate steps to ensure their child’s safe return to sport and school.22,25 Many parents view concussion as meaning a less severe head injury than if mild TBI or minor TBI is used.26 It is imperative that clinicians ensure that parents understand the seriousness of their child’s concussive injury and how to judge when their child may safely return to school.
GAAME is an acronym for a simple concussion action plan for parents of young sports participants. The name is based on the first word of each step in the plan:
Get out, Assess, Ask, Medical attention, and Emergency situation.
The GAAME handout (see "Parent Guide") briefly explains the steps to take if a child experiences a blow to the head during a sports activity. It can be given to parents of all pre–high-schoolers, whether their children participate in organized sports or not.
GAAME utilizes a five-step approach that immediately pulls the child out of the game and highlights the important information a clinician will need when the child receives medical attention.3 Parents, guardians, and other involved parties serve as a child’s best advocate by being informed and alert to the early signs and symptoms of a concussion and by helping the child to identify his or her own symptoms.
School personnel
Teachers and other school officials must recognize both the necessity of cognitive rest during concussion recovery and their role in enabling a child’s gradual return to the cognitive demands of school. A child may require such adjustments as a shorter school day or week, a reduced workload, and extra time for tests to avoid exacerbation of concussion symptoms.5,22 In light of state concussion laws (see below), schools commonly ensure that personnel are well-informed about concussion and its aftereffects in the classroom.
Sports officials
Heightened awareness of the need for more effective concussion education and management in youth sports has led to enactment of laws on youth sports-related concussions in all 50 states and the District of Columbia.27 Most of these laws include
1. Inform and educate coaches, athletes, and their parents and guardians about concussion through training and/or a concussion information sheet.
2. An athlete who is believed to have a concussion is to be removed from play right away.
3. An athlete can only return to play or practice after at least 24 hours and with permission from a health care professional.
Clinicians should also be familiar with the laws in their states as they may affect clinical decisions (eg, timing of RTP protocol).
On the next page: Conclusion >>
CONCLUSION
Optimal management of pediatric concussions is a multifaceted challenge that is best addressed by age-appropriate, individualized patient care. Educational initiatives based on the latest concussion guidelines will increase awareness of appropriate concussion management by all involved in overseeing the recovery of the pre-high-school-age child.
A greater understanding of concussion, coupled with management based on current, standardized RTP and return-to-school protocols, will enhance coordination among the multidisciplinary team overseeing the child’s recovery and facilitate the return to full preconcussion functioning. More research about the effects of concussion on the developing brain is needed in order to create evidence-based guidelines specific to this pediatric population.
CE/CME No: CR-1409
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define the term concussion.
• Identify the major signs and symptoms of a concussion.
• Discuss the initial management of a pediatric patient with a suspected sports-related concussion.
• Describe the current recommendations for return to play after a pediatric concussion.
• Explain the main challenges clinicians face in the management of pediatric concussions
FACULTY
Sydney Meckler is pursuing her Doctor of Nursing Practice degree at Columbia University, New York City. The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
This article describes the challenges of diagnosis and treatment of pediatric concussive injuries, with particular attention to the effects of concussion on the developing brain. It highlights the need for individualized, age-dependent care; standardized return-to-play and return-to-school protocols; and better-informed clinicians, parents, teachers, and coaches.
Pediatric concussions—those occurring in children younger than 18—account for almost half a million emergency department visits each year.1 Of these, more than 25% occur during sports-related activities.2 Yet it is believed that pediatric concussive injuries are significantly underreported because parents do not always seek medical attention for their concussed children and because little has been published on the management of sports-related concussions in pre–high-school-age athletes.2
The 4th International Consensus Conference on Concussion in Sport, the American Academy of Neurology, and the American Medical Society for Sports Medicine all recently updated their guidelines for concussion evaluation and management.3-5 However, the lack of data on concussions in younger pediatric athletes presents challenges for the optimal management of these patients.6 In fact, in a February 2014 report, the Institute of Medicine said that more research is needed to develop evidence-based sports-related concussion management guidelines for children and adolescents.7
In this article, the factors that distinguish concussions in children from those in adults will be described, as will the management techniques that primary care clinicians can use to effectively address them.
WHAT IS A CONCUSSION?
Although the term concussion has been used interchangeably with head trauma and brain injury, concussion is most often described as mild traumatic brain injury (mTBI). Variations in terminology reflect both the lack of agreement on a standard definition for concussion, especially as it applies to the pediatric population, and the complexities of concussion diagnosis and management.8 This inherent ambiguity can lead to suboptimal recognition and awareness of concussion among clinicians, patients, families, school personnel, and sports staff, especially of its serious aftereffects in the pediatric patient.
A blow to the head can propel the brain to strike one side of the skull and then bounce off the opposite side. This can damage or tear the axons—the signal transmitters of the neurons—from their cell bodies, disrupting brain cell communications and causing neurocognitive deficits.
While a concussion is a direct injury to the brain, it is also an evolving process comprised of several pathophysiologic components that are a consequence of biomechanical forces. The brain does not directly contact the skull but is surrounded by cerebrospinal fluid and fluid-filled vessels with a greater density than the brain itself. This intracranial space provides room for the brain to move upon impact by shifting and compressing from one side of the skull to the other.9 The concussive injury occurs as a result of either a direct strike to the head or from an indirect force traumatic enough to be transmitted to the brain. The strike or force produces a complex disturbance to the brain that, in most cases, does not result in loss of consciousness.3
THE GREATER VULNERABILITY OF THE PEDIATRIC BRAIN
Pathophysiology
For a concussion to occur, the biomechanical threshold at which nerve cells' adaptability is impeded must be crossed.10 This sets in motion a cascade of events, developing over minutes, hours, and even days, in which homeostasis is disrupted due to an imbalance of ionic, neuronal, metabolic, and pathophysiologic processes.5
While many studies have sought to explain the pathophysiology of pediatric sports-related concussions, much is still unknown. One hypothesis suggests that, after the injury, neuronal depolarization occurs, releasing excitatory neurotransmitters and altering metabolism by the immediate release of potassium. Restoration of homeostasis demands response by the sodium-potassium pump, requiring energy in the form of glucose. Coupled with axonal injury and alterations in cerebral blood flow, an imbalance between energy supply and demand occurs.5
Maugans et al found that a single pediatric concussion does not cause a structural or metabolic injury but instead a physiologic interruption—different from what occurs in adult concussions.10 They observed a statistically significant reduction in cerebral blood flow of more than 10% in a small group of postconcussion children, ages 11 to 15, compared with a control group. This alteration persisted for two weeks in two-thirds of the children and for up to a month in the remaining third.10 The delayed return of normal cerebral blood flow is believed to be a major contributing factor to pediatric concussion-related symptoms. However, little is known about the long-term effects of concussion on the developing brain.9
Crucial developmental years
Because the pediatric brain is still developing, children between the ages of 5 and 12 are at greater risk for sustaining a disruption in brain function from a concussion. In addition, this group is more susceptible in general to sports injuries compared to adults, because of their stage of growth and development.11
With a pediatric concussion, the individual child’s stage of brain development and the possible effects on his or her evolving neurocognitive functioning must be taken into account. Critical skills, such as language, emotion, memory, problem solving, and motor dexterity, emerge during childhood, and a disturbance during this time could have substantial consequences.12 Motor skills and primary senses usually mature by age 5, with language maturity occurring by age 10.9 However, many other vital skills and processes, such as emotion, judgment, and abstract reasoning, are not fully developed until adulthood. As a result, damage to cortical areas of the brain, such as the parietal lobe and frontal areas—involved in fine motor skills and complex thinking—can hinder or delay the development of these necessary processes.9
Many researchers suggest that, while a concussion to the developing brain may not significantly affect previously learned functions, it may notably delay development of new cognitive functions. As a result, recovery from a concussion during these years may be poorer.2,9,12
On the next page: Clinical presentation and post injury evaluation >>
CLINICAL PRESENTATION
Initial assessment of pediatric athletes with suspected concussive injuries can be particularly challenging because of the variability and evolving nature of associated signs and symptoms, resulting in a wide range of clinical presentations.3 These presenting signs and symptoms can, however, provide vital clues to the location of the injury within the brain because the consequences of concussion arise from injury to the brain’s cortical and subcortical systems (see Table 1).9
The cortical systems are located within the frontal, temporal, parietal, and occipital lobes, each of which houses control centers for specific learned skills and functions. The subcortical systems include the hypothalamus, trigeminal system, basal ganglia, and cerebellum. While a concussion most often causes diffuse harm rather than localized trauma, familiarity with the functions associated with specific brain locations aids in initial identification of the injury and allows for individualized concussion management.13
After a concussion occurs, it is essential to evaluate all domains of the child’s functioning through a comprehensive physical assessment, including a detailed concussion history and review of preinjury risk factors.3 In addition, there may be unobservable symptoms about which a child is unable to communicate due to his or her stage of emotional and cognitive development and restricted capacity to serve as a primary reporter.14
Signs and symptoms
The four categories of concussion signs and symptoms include physical, cognitive, emotional, and sleep (see Table 2).15 A concussed patient may experience any variation or combination of these signs and symptoms.
It is important to recognize that, although loss of consciousness is a serious sign of a concussion, it occurs in only about 10% of concussive injuries.5,15 In fact, current concussion guidelines no longer advocate the use of grading scales, which assessed the severity of a concussion based primarily on the presence or absence of a brief loss of consciousness. Evidence now shows that loss of consciousness does not predict clinical course or cognitive long-term impairment after a concussion.16
Duration of symptoms
The duration of concussion symptoms can vary, but in most cases symptoms last seven to 10 days, with 10% to 15% of patients reporting symptoms for more than 10 days.3,5 While recovery duration has not been studied sufficiently in children younger than 15, younger children require longer recovery periods. The resolution of symptoms, however, does not necessarily correlate with absolute cognitive recovery.5,17
Modifying and complicating factors
In addition to the patient’s presenting signs and symptoms, factors that may modify or complicate recovery from a concussion should be identified (see Table 3).3 These factors include preexisting conditions or disorders and the use of certain prescription drugs. Each factor should be assessed individually, taking into account the patient’s age.2
Some presenting symptoms may overlap with symptoms of common disorders in this group. It is important to note if symptoms of such conditions as attention-deficit/hyperactivity disorder (ADHD), sleep disorders, learning disabilities, and mood disorders were present prior to the concussion.5
Modifying factors provide essential information that can help predict the individual patient’s anticipated recovery process and must be taken into account throughout the process. They are particularly important when assessing the differences between a child’s preconcussion and postconcussion functioning.3,4
POST INJURY EVALUATION OF COGNITIVE FUNCTIONING
Concussion is diagnosed clinically, based on the mechanism of the sustained injury, and is supported primarily by reported and observed signs and symptoms. The ability to systematically evaluate the patient for manifestations of concussion is essential to pediatric concussion management.3,18
To aid in appropriately evaluating symptoms and assessing functional damage, many standardized assessment tools and neuropsychological tests have been developed. These tools have been modified for younger athletes due to differences in children’s neurocognitive development and in their ability to be symptom self-reporters.
Concussion assessment tools
The tools most commonly used to assess the effects of concussion in younger athletes are listed in Table 4. It was not until 2012, with the release of the Child Sport Concussion Assessment Tool Version 3 (Child-SCAT3), that an appropriately adapted and valid concussion assessment tool became available for children younger than 10.3
Assessment tools are useful but are not diagnostic. They do not take the place of a clinician’s thorough neurocognitive evaluation of a potentially concussed child.
Neuropsychologic testing
In order to properly manage pediatric concussions, postconcussion status must be assessed relative to preconcussion status in order to ensure optimal resolution of symptoms and cognitive recovery.19
The American Medical Society for Sports Medicine supports baseline testing for high-risk athletes, defined as those with a prior history of concussions or “confounding” conditions (learning disability, mood and attention disorders, migraine headaches).5 The International Consensus Statement on Concussion in Sport states that there is insufficient evidence to recommend routine baseline testing, but its early use after pediatric concussion may provide helpful information for determining when the child may return to school. The American Academy of Neurology suggests that clinicians “… might utilize baseline scores on concussion assessment tools, especially in younger athletes, those with prior concussions, or those with preexisting learning disabilities/ADHD, as doing so fosters better interpretation of postinjury scores.”4
Assessment tools can provide essential insight into the cognitive functional state of a concussed child athlete, even after symptom resolution.3,5,20 They are not stand-alone measures of recovery but can be useful components of the pediatric concussion evaluation and management process and an aid to clinical decision making.3,13
Paper-and-pencil or computerized neuropsychologic tests provide objective measures of brain-behavior relationships and are more sensitive in detecting subtle cognitive impairments than a clinical examination.5 Although neuropsychologic testing has not been validated as a diagnostic tool, it may be useful for both baseline (preinjury) testing of athletes and for monitoring recovery from a concussive event.5,20 (For more information on motor control assessment, see Dirks RP, McLeod TCV. Sport-related mild traumatic brain injury. Clinician Reviews. 2008;18[9]:22. http://bit.ly/1mjizIa.)
On the next page: Postconcussion management >>
POSTCONCUSSION MANAGEMENT
All concussion management guidelines concur that immediate removal from play of a pediatric athlete with a suspected concussion is the most important initial action. Regardless of how short the duration or mild the symptoms may be, same-day return to play should never occur.3-5 This is particularly true because acute concussion is an evolving injury and manifestation of symptoms, including cognitive deficits, is often delayed.3
The foundation of postconcussion recovery is rest, both physical and cognitive. Acute symptoms must diminish before a gradual resumption of activities.3,5
Cognitive rest
Cognitive rest is the cornerstone of concussion management.5 The latest guidelines stress the importance, particularly in the pediatric age-group, of decreasing any activities of daily living that may aggravate symptoms.3 This includes such common childhood pastimes as playing video games, watching television, and using a computer.15 More important, cognitive rest means academic rest, which is essential to postconcussion recovery and preparing the child for a “return to school” or “return to learn.”21, 22
Return to play
In pediatric concussion management, a conservative approach to the determination of when a concussed child may return to play (RTP) is key. RTP decisions are guided by the resolution of the child’s symptoms and are based on clinical judgment.3-5
Current guidelines outline a gradual, stepwise approach to RTP after full recovery, which must be individualized and age-appropriate.3,5 These include
• Light aerobic exercise: Increase heart rate to 70% maximum predicted by walking, swimming, or using stationary bike. No resistance training.
• Sport-specific exercise: Add movement with skating or running drills. No head impact activities.
• Noncontact training drills: Add exercise, coordination, and cognitive load with progression to more complex training drills (eg, passing drills in football and ice hockey). May introduce progressive resistance training.
• Full contact practice: To restore confidence and allow coaching staff to assess functional skills, permit participation in normal training activities after obtaining medical clearance.
At any step, if symptoms develop with activity, the process is stopped and, after a 24-hour period of rest, is restarted at the previous symptom-free step.3,5
The goal of this approach is to ensure that the pediatric brain recovers fully and can resume normal developmental acquisition of cognitive skills and functions. Further, as previously noted, children and adolescents require more time to recover from the effects of a concussion than adults do.2,3 If a child returns too quickly to activity postconcussion, clinical evidence suggests that worsening of cognitive deficits is likely.9 Therefore, cautious postconcussion management that allows sufficient recovery time before clearance is given for a return to sports participation is highly recommended.2,4,5 No child should RTP unless cleared by a health care provider trained in the evaluation and management of pediatric concussive injuries.4,5,15
Return to school
Inadequate knowledge of concussion on the part of parents, teachers, and school officials can be a barrier to appropriate return-to-school decisions after pediatric concussive injuries.22 Since pre–high-school-age children spend the majority of their time in school, it is essential that a child’s school attendance and workload demands be decreased during recovery from a concussion.
Evidence indicates that an increase in cognitive or physical activity before complete recovery—ie, before normal brain cellular function is restored—may prolong cognitive dysfunction.5 Arbogast et al found that, in many pediatric concussion patients, unresolved symptoms impeded learning and school-based functioning: 10% to 18% of the children studied experienced fatigue, difficulty concentrating, feeling foggy, and/or vision problems.21 Within the first two weeks postinjury, 80% reported an increase in symptom severity while at school.
Academic demands should be increased gradually, with adjustments made for the individual student as needed, in order to avoid the exacerbation of such school-setting symptoms as headache, dizziness, light and/or noise sensitivity, and difficulty concentrating or remembering.22
While RTP guidelines are widely understood and implemented by clinicians, one survey found that return-to-school guidance is provided less often.21 Clinicians should be mindful of the importance of both physical rest and cognitive rest during recovery from pediatric concussion and should provide parents with clear guidance for both.21
On the next page: Role of the primary care clinician >>
THE ROLE OF THE PRIMARY CARE CLINICIAN
Primary care clinicians are central to pediatric concussion management because they facilitate and oversee a child’s recovery. They may administer neuropsychologic testing, monitor symptom resolution, and supervise the concussed child’s gradual return to physical and academic activities through the individualized phases of RTP and return-to-school protocols.4,20,23
The NP or PA functions as the main point of contact within the heath care team, organizing the plan of care and coordinating with parents, teachers, and coaches throughout the recovery process. Close follow-up care, including detailed documentation, should take place at well-child examinations to help ensure that the child’s development continues to progress as expected.
Referrals
Although referrals to a specialist are not typically necessary, persistent symptoms—those lasting longer than 10 days and not usually specific to concussion—warrant immediate and appropriate referral.3,22 Approximately 30% of mTBI patients experience long-term, often significant aftereffects, underscoring that mTBI is not a single entity and that each patient is unique.24
Risk factors for protracted symptoms
Factors that may be associated with prolonged or persistent duration of symptoms include early posttraumatic headache, previous concussion, early amnesia, alteration in mental status, disorientation, fatigue/fogginess, developmental disorders (eg, learning disabilities, ADHD), and psychiatric disorders (eg, anxiety, depression).4,13 Studies have examined this association, and there is conflicting evidence as to whether prolonged symptoms are attributed to, or are a combination of, preconcussion modifying factors, concussive episode severity, and/or coexistent pathologies.3,4
Concussion complications
Postconcussion syndrome can occur in pediatric athletes after a single concussion.3,23 The syndrome is not clearly understood but is characterized by the persistence of multiple cognitive, physical, and/or emotional symptoms of a concussion for weeks or even months, making it difficult to both diagnose and manage.5,15
Second impact syndrome, only documented in pediatric athletes, occurs when a child sustains a concussion before fully recovering from a prior concussion. This can lead to cerebral vascular congestion, cerebral swelling, and in some cases, death.15 Immediate removal of a concussed child from play until completely asymptomatic is obviously essential to the prevention of this rare catastrophe.
Any child with persistent symptoms should be referred to a multidisciplinary team of providers experienced in concussion management. This team may include, but is not limited to, neurologists, developmental pediatricians, concussion specialists, and psychologic, cognitive, and/or physical therapists.3,5
CONCUSSION EDUCATION
Family
Family-centered education is another essential component of pediatric concussion management. Parents and guardians are extremely influential and important to a child’s overall recovery, especially a younger child’s. While older pre–high-school-age children are much more capable of self-reporting symptoms, the younger child may not yet be developmentally able to do so, making the parent or guardian’s assistance and input essential.14
Unfortunately, studies have shown that a significant number of parents lack a good understanding of what constitutes a concussion as well as of the appropriate steps to ensure their child’s safe return to sport and school.22,25 Many parents view concussion as meaning a less severe head injury than if mild TBI or minor TBI is used.26 It is imperative that clinicians ensure that parents understand the seriousness of their child’s concussive injury and how to judge when their child may safely return to school.
GAAME is an acronym for a simple concussion action plan for parents of young sports participants. The name is based on the first word of each step in the plan:
Get out, Assess, Ask, Medical attention, and Emergency situation.
The GAAME handout (see "Parent Guide") briefly explains the steps to take if a child experiences a blow to the head during a sports activity. It can be given to parents of all pre–high-schoolers, whether their children participate in organized sports or not.
GAAME utilizes a five-step approach that immediately pulls the child out of the game and highlights the important information a clinician will need when the child receives medical attention.3 Parents, guardians, and other involved parties serve as a child’s best advocate by being informed and alert to the early signs and symptoms of a concussion and by helping the child to identify his or her own symptoms.
School personnel
Teachers and other school officials must recognize both the necessity of cognitive rest during concussion recovery and their role in enabling a child’s gradual return to the cognitive demands of school. A child may require such adjustments as a shorter school day or week, a reduced workload, and extra time for tests to avoid exacerbation of concussion symptoms.5,22 In light of state concussion laws (see below), schools commonly ensure that personnel are well-informed about concussion and its aftereffects in the classroom.
Sports officials
Heightened awareness of the need for more effective concussion education and management in youth sports has led to enactment of laws on youth sports-related concussions in all 50 states and the District of Columbia.27 Most of these laws include
1. Inform and educate coaches, athletes, and their parents and guardians about concussion through training and/or a concussion information sheet.
2. An athlete who is believed to have a concussion is to be removed from play right away.
3. An athlete can only return to play or practice after at least 24 hours and with permission from a health care professional.
Clinicians should also be familiar with the laws in their states as they may affect clinical decisions (eg, timing of RTP protocol).
On the next page: Conclusion >>
CONCLUSION
Optimal management of pediatric concussions is a multifaceted challenge that is best addressed by age-appropriate, individualized patient care. Educational initiatives based on the latest concussion guidelines will increase awareness of appropriate concussion management by all involved in overseeing the recovery of the pre-high-school-age child.
A greater understanding of concussion, coupled with management based on current, standardized RTP and return-to-school protocols, will enhance coordination among the multidisciplinary team overseeing the child’s recovery and facilitate the return to full preconcussion functioning. More research about the effects of concussion on the developing brain is needed in order to create evidence-based guidelines specific to this pediatric population.
1. Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002-2006. www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. Accessed August 14, 2014.
2. Meehan WP, Taylor AM, Proctor M. The pediatric athlete: younger athletes with sports-related concussion. Clin Sports Med. 2011;30(1):1-11.
3. McCrory P, Meeuwisse WH, Aubry M, et al; Concussion in Sport Group. Consensus statement on concussion in sport: the 4th International Consensus Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
4. Giza CC, Kutcher JS, Ashwal S, et al; Guideline Development Subcommittee, American Academy of Neurology. Summary of evidence-based guideline update: evaluation and management of concussion in sports. Neurology. 2013;80(24):2250-2257.
5. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15-26.
6. Chrisman SP, Schiff MA, Rivara FP. Physician concussion knowledge and effect of mailing the CDC “heads up” toolkit. Clin Pediatr. 2011;50(11):1031-1039.
7. Graham R, Rivara FP, Ford MA, Spicer CM, eds. Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. Washington, DC: The National Academies Press; 2014.
8. Khurana VG, Kaye AH. An overview of concussion in sport. J Clin Neurosci. 2012;19(1):1-11.
9. Toledo E, Lebel A, Becerra L, et al. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci Biobehav Rev. 2012;36(6):1510-1531.
10. Maugans TA, Farley C, Altaye M, et al. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28-37.
11. Franklin CC, Weiss JM. Stopping sports injuries in kids: an overview of the last year in publications. Curr Opin Pediatr. 2012;24(1):64-67.
12. Cernak I, Chang T, Ahmed FA, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2012;32(5-6):442-453.
13. Gioia GA, Collins M, Isquith PK. Improving identification and diagnosis of mild traumatic brain injury with evidence: psychometric support for the acute concussion evaluation. J Head Trauma Rehabil. 2008;23(4):230-242.
14. Echemendia RJ, Iverson G, McCrea M, et al. Advances in neuropsychological assessment of sport-related concussion. Br J Sports Med. 2013;47(5):294-298.
15. Halstead M, Walter D. Clinical report: sports-related concussion in children and adolescents. Pediatrics. 2010;126(3):597-615.
16. Gomez JE, Hergenroeder AC. New guidelines for management of concussion in sport: special concern for youth. J Adolesc Health. 2013;53(3):311-313.
17. Grubenhoff JA, Kirkwood M, Gao D, et al. Evaluation of standardized assessment of concussion in a pediatric emergency department. Pediatrics. 2010;126(4):688-694.
18. Fung M, Willer B, Moreland D, Leddy J. A proposal for an evidence-based emergency department discharge form for mild traumatic brain injury. Brain Inj. 2006;20(9):889-994.
19. Apps JN, Walter KD. Pediatric and Adolescent Concussion: Diagnosis, Management, and Outcomes. New York: Springer Science+Business Media, LLC; 2012. http://midnurse.umsha.ac.ir/uploads/Pediatric&Adolescent_Concus sion.pdf. Accessed August 14, 2014.
20. Kirkwood MW, Yeates KO, Wilson PE. Pediatric sports-related concussion: a review of the clinical management of an oft-neglected population in children and adolescents. Pediatrics. 2006;117(4):1359-1371.
21. Arbogast KB, McGinley AD, Master CL, et al. Cognitive rest and school-based recommendations following pediatric concussion: the need for primary support tools. Clin Pediatr. 2013;452(5):397-402.
22. Halstead ME, McAvoy K, Devore CD, et al. Returning to learning following a concussion. Pediatrics. 2013;132(5):948-957.
23. Marsh AM, Fraser D, Marsh JP. Management of concussion in the pediatric patient. J Pediatr Health Care. 2013;27(6):499-504.
24. Rosenbaum SB, Lipton ML. Embracing chaos: the scope and importance of clinical and pathological heterogeneity in MTBI. Brain Imaging Behav. 2012;6:255-282.
25. Bakhos LL, Lockhart GR, Myers R, Linakis JG. Emergency department visits for concussion in young child athletes. Pediatrics. 2010;126(3):e550-e556.
26. Gordon KE, Dooley JM, Fitzpatrick EA, et al. Concussion or mild traumatic brain injury: parents appreciate the nuances of nosology. Pediatr Neurol. 2010;43(4):253-257.
27. CDC. Get a heads up on concussion in sports policies. www.cdc.gov/concussion/policies.html. Accessed August 14, 2014.
28. Gioia G, Collins M. Acute concussion evaluation (ACE). 2006. www.cdc.gov/concussion/headsup/pdf/ace-a.pdf. Accessed August 14, 2014.
29. ImPACT Applications Inc. ImPACT (Immediate Post-Concussion Assessment and Cognitive Testing). www.impacttest.com/about/?Overview-1. Accessed August 14, 2014.
1. Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002-2006. www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf. Accessed August 14, 2014.
2. Meehan WP, Taylor AM, Proctor M. The pediatric athlete: younger athletes with sports-related concussion. Clin Sports Med. 2011;30(1):1-11.
3. McCrory P, Meeuwisse WH, Aubry M, et al; Concussion in Sport Group. Consensus statement on concussion in sport: the 4th International Consensus Conference on Concussion in Sport held in Zurich, November 2012. Br J Sports Med. 2013;47(5):250-258.
4. Giza CC, Kutcher JS, Ashwal S, et al; Guideline Development Subcommittee, American Academy of Neurology. Summary of evidence-based guideline update: evaluation and management of concussion in sports. Neurology. 2013;80(24):2250-2257.
5. Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15-26.
6. Chrisman SP, Schiff MA, Rivara FP. Physician concussion knowledge and effect of mailing the CDC “heads up” toolkit. Clin Pediatr. 2011;50(11):1031-1039.
7. Graham R, Rivara FP, Ford MA, Spicer CM, eds. Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. Washington, DC: The National Academies Press; 2014.
8. Khurana VG, Kaye AH. An overview of concussion in sport. J Clin Neurosci. 2012;19(1):1-11.
9. Toledo E, Lebel A, Becerra L, et al. The young brain and concussion: imaging as a biomarker for diagnosis and prognosis. Neurosci Biobehav Rev. 2012;36(6):1510-1531.
10. Maugans TA, Farley C, Altaye M, et al. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012;129(1):28-37.
11. Franklin CC, Weiss JM. Stopping sports injuries in kids: an overview of the last year in publications. Curr Opin Pediatr. 2012;24(1):64-67.
12. Cernak I, Chang T, Ahmed FA, et al. Pathophysiological response to experimental diffuse brain trauma differs as a function of developmental age. Dev Neurosci. 2012;32(5-6):442-453.
13. Gioia GA, Collins M, Isquith PK. Improving identification and diagnosis of mild traumatic brain injury with evidence: psychometric support for the acute concussion evaluation. J Head Trauma Rehabil. 2008;23(4):230-242.
14. Echemendia RJ, Iverson G, McCrea M, et al. Advances in neuropsychological assessment of sport-related concussion. Br J Sports Med. 2013;47(5):294-298.
15. Halstead M, Walter D. Clinical report: sports-related concussion in children and adolescents. Pediatrics. 2010;126(3):597-615.
16. Gomez JE, Hergenroeder AC. New guidelines for management of concussion in sport: special concern for youth. J Adolesc Health. 2013;53(3):311-313.
17. Grubenhoff JA, Kirkwood M, Gao D, et al. Evaluation of standardized assessment of concussion in a pediatric emergency department. Pediatrics. 2010;126(4):688-694.
18. Fung M, Willer B, Moreland D, Leddy J. A proposal for an evidence-based emergency department discharge form for mild traumatic brain injury. Brain Inj. 2006;20(9):889-994.
19. Apps JN, Walter KD. Pediatric and Adolescent Concussion: Diagnosis, Management, and Outcomes. New York: Springer Science+Business Media, LLC; 2012. http://midnurse.umsha.ac.ir/uploads/Pediatric&Adolescent_Concus sion.pdf. Accessed August 14, 2014.
20. Kirkwood MW, Yeates KO, Wilson PE. Pediatric sports-related concussion: a review of the clinical management of an oft-neglected population in children and adolescents. Pediatrics. 2006;117(4):1359-1371.
21. Arbogast KB, McGinley AD, Master CL, et al. Cognitive rest and school-based recommendations following pediatric concussion: the need for primary support tools. Clin Pediatr. 2013;452(5):397-402.
22. Halstead ME, McAvoy K, Devore CD, et al. Returning to learning following a concussion. Pediatrics. 2013;132(5):948-957.
23. Marsh AM, Fraser D, Marsh JP. Management of concussion in the pediatric patient. J Pediatr Health Care. 2013;27(6):499-504.
24. Rosenbaum SB, Lipton ML. Embracing chaos: the scope and importance of clinical and pathological heterogeneity in MTBI. Brain Imaging Behav. 2012;6:255-282.
25. Bakhos LL, Lockhart GR, Myers R, Linakis JG. Emergency department visits for concussion in young child athletes. Pediatrics. 2010;126(3):e550-e556.
26. Gordon KE, Dooley JM, Fitzpatrick EA, et al. Concussion or mild traumatic brain injury: parents appreciate the nuances of nosology. Pediatr Neurol. 2010;43(4):253-257.
27. CDC. Get a heads up on concussion in sports policies. www.cdc.gov/concussion/policies.html. Accessed August 14, 2014.
28. Gioia G, Collins M. Acute concussion evaluation (ACE). 2006. www.cdc.gov/concussion/headsup/pdf/ace-a.pdf. Accessed August 14, 2014.
29. ImPACT Applications Inc. ImPACT (Immediate Post-Concussion Assessment and Cognitive Testing). www.impacttest.com/about/?Overview-1. Accessed August 14, 2014.
Preconception Health Care
CE/CME No: CR-1408
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define preconception and interconception health care and explain how these concepts relate to primary care.
• Describe three common clinical presentations in reproductive-age women that have implications for preconception and interconception health care.
• Explain the considerations, in terms of potential pregnancies, when prescribing pharmacologic treatment for reproductive-age women.
• Discuss examples of cultural factors and beliefs that may affect preconception health counseling provided to women of reproductive age.
FACULTY
Kathleen A. Ahonen is an Assistant Professor and Colleen Quinlan is an Associate Professor at the University of Toledo College of Nursing.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Because half of all pregnancies in the United States are unplanned, primary care constitutes preconception health care for women ages 15 to 44. Here are recommendations to incorporate into routine visits to improve outcomes of possible pregnancies.
Traditional interventions to improve pregnancy outcomes in the United States have focused on early, consistent prenatal care and on reducing the teenage pregnancy rate. Data indicate that early access to prenatal care has increased and that the teenage pregnancy rate has fallen.1,2
But while US rates of preterm births, low–birth-weight infants, and infant mortality have all declined since the mid-2000s,3 they remain higher than those in many other developed countries.4-6 Furthermore, there are significant differences in these rates by race and ethnicity.3
Many experts believe that waiting until a woman is pregnant to address the health needs and behaviors that adversely affect pregnancy outcomes is, at best, an incomplete solution to these problems. A more comprehensive view of women’s health care broadens this focus to stress the importance of preconception health care throughout the reproductive years. It is key to the achievement of optimal health, not only for women but also for their potential future children.7-9
The CDC defines preconception health simply as the health of women and men during their reproductive years. Preconception health care is defined as medical care during those years (for women, ages 15 to 44) that focuses on improving aspects of the patient’s health or health behaviors that can improve outcomes in the event of a pregnancy.10 The term interconception health care is also applied specifically to health care provided during the time between pregnancies.
This article highlights obesity, depression and other mood disorders, and nutritional deficiencies and reviews potential risk factors, particularly occurring very early in a pregnancy. Recommendations are offered for more effective health counseling at routine visits, with the goal of improving outcomes of potential pregnancies.
PRIMARY CARE IS PRECONCEPTION CARE
Researchers have reported that only about 50% of reproductive-age women receive counseling about health behaviors and pregnancy planning8 and that such counseling is even rarer for adolescent females.11,12 Because women of childbearing age commonly see both Ob-Gyn and family practice clinicians for health care, primary care providers are well positioned to deliver preconception health care by integrating health counseling, discussion of health behaviors, and primary and secondary prevention into patient care for reproductive-age women (see "Summary of CDC Recommendations to Improve Preconception Health and Health Care").6
On the next page: Obesity >>
OBESITY
Researchers in both the US and internationally have noted the connection between obesity and pregnancy complications such as gestational diabetes that increase the risk for poor pregnancy outcomes.13-15 Indeed, pregnancy outcomes can be altered for the better with weight loss in the preconception or interconception period.16
Weight loss is complex; it can be difficult to accomplish, and long-term maintenance also presents a challenge. Short-term weight loss is achievable, however, and can produce beneficial metabolic effects, such as a decrease in insulin resistance.17
Before weight loss attempts can be initiated, the overweight or obese patient’s weight must be acknowledged as a health problem. In one 2009 study, Callaway et al found that in their sample of 412 overweight and obese women, only 17% were advised by their health care providers to lose weight.18
Regular physical activity can facilitate the achievement and maintenance of a healthy weight. However, physical inactivity is common in nonpregnant women, especially those with higher BMIs. The CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS; www.cdc.gov/prams), widely regarded as an important source of data about women’s health behaviors, reveals that nearly 40% of US women do not meet national recommendations for routine physical activity in the three months prior to pregnancy.19
Recent obesity guidelines recommend initial weight loss goals of 5% to 10% of baseline weight in six months before pregnancy.17 Some women may not achieve these goals because they are unaware of the potentially short time period between discontinuation of contraception and conception. Others, especially those older than 30, intentionally plan for short interconception intervals as part of their family planning strategy, which does not allow enough time for safe weight loss.20
Primary care clinicians should counsel these patients on the importance of a healthy weight and regular physical activity to the maintenance of optimal health. Particular emphasis should be placed on achieving weight loss slowly and safely and then maintaining it, at least for the short term. This strategy may be effective in helping a female patient achieve a lower BMI prior to pregnancy.
Bariatric surgery
Because the number of women undergoing bariatric surgery for morbid obesity is rapidly increasing,21 it is important to educate them about the specific effects of such procedures on reproductive health. A period of rapid weight loss—such as occurs after bariatric surgery—is not a time to consider pregnancy, despite the improvement in eventual pregnancy outcomes associated with a healthier BMI.
Many of these women may have been anovulatory when morbidly obese and unaware that fertility increases in the postoperative year, after a reduced BMI is achieved. If ovulation and menses have not yet normalized, a woman may become pregnant and not know it. This could result in the inadvertent exposure of the developing fetus to such teratogenic risks as alcohol, tobacco, and certain prescription drugs. While a pregnancy may be welcome, better outcomes are likely if pregnancy is avoided for at least 12 months after bariatric surgery.22 Effective contraception should be used until ovulation cycles stabilize.
Some surgical weight loss procedures, such as the Roux-en-Y gastric bypass, may alter the absorption of medication, including oral contraceptives (OCs), making the use of OCs after such procedures less than ideal. The more reliably absorbed injectable medroxyprogesterone is an option, but women who have undergone bariatric surgery often wish to avoid the associated risk for weight gain. Nonhormonal long-acting contraceptive methods not associated with weight gain, such as the copper intrauterine device, may be preferable for use in the first year postprocedure.21
On the next page: Depression and other mood disorders >>
DEPRESSION AND OTHER MOOD DISORDERS
Mood disorders include depression, bipolar disorder, and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs, such as paroxetine or sertraline) or serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine or duloxetine) are often prescribed for primary care management of depression and other mood disorders.
When SSRIs or SNRIs are not fully effective, clinicians may refer patients to mental health specialists for consultation and possible ongoing management. Women of reproductive age who receive specialty care for mood disorders are encouraged to continue their regular visits to primary care clinicians.
Medication: Risk for birth defects
Anticonvulsants, such as valproate, carbamazepine, and lamotrigine, are commonly used to treat bipolar disorder.23 When taken during the first trimester of pregnancy, these drugs pose well-documented risks to the rapidly developing fetus. Most evidence relates to the risk for neural tube defects, such as spina bifida, but other evidence suggests a risk for general cognitive impairment after prenatal valproate exposure. While the latter is based primarily on studies of women taking anti-epileptic drugs for seizure control—not psychiatric diagnoses—first-trimester risks appear to be independent of maternal seizures.23 Although folic acid supplementation decreases the incidence of neural tube defects (see discussion under “Nutritional Deficiencies"), it is unknown if such supplementation is effective in mitigating the additional risks to the fetus from exposure to anticonvulsants.
Female patients of childbearing age must be advised of the potential effects of these commonly prescribed mood-stabilizing drugs, not only as they relate to the diagnosis being treated but also regarding their possible effects on an early, undiagnosed pregnancy. Unfortunately, evidence indicates that insufficient attention is given to counseling reproductive-age women about the risks and benefits of these drugs as they relate to potential conception, at least in the context of specialty care.23 Therefore, the primary care clinician and the specialist should utilize a team approach, emphasizing careful reproductive planning to avoid pregnancy while under treatment with these drugs to ensure the best possible outcomes.
In the context of potential pregnancy, the need to manage depression and other mood disorders effectively is particularly important: Prepregnancy depressive mood has been significantly associated with preterm birth, and at least 14.5% of women experience a new episode of depression during pregnancy.24 Thus, effective treatment of mood disorders should be a priority, both as part of preconception care and during pregnancy.
Similarly, treatment strategies for postpartum depression—widely estimated to affect 10% to 20% of new mothers—must consider the potential risks of pharmacologic therapy to a fetus should the patient conceive during treatment.
On the next page: Nutritional deficiencies >>
NUTRITIONAL DEFICIENCIES
While there is widespread public awareness, at least on a basic level, of the importance of good nutrition during pregnancy, what that constitutes is not necessarily clearly understood. Even less well recognized is the importance of a woman’s nutritional status at the time of conception, at preimplantation, and during the early weeks of placental development, before pregnancy is known or confirmed. During this crucial time—three to seven weeks after the last menstrual period—an inadequate diet may result in low–birth-weight infants with lifelong health problems.25 These may include respiratory problems associated with barotrauma from ventilation at birth; neural tube defects; and orofacial clefts.25
Because of inadequate intake of fresh fruits and vegetables, many reproductive-age women in the US are deficient in vitamins A, C, B6, and E, as well as calcium, iron, zinc, magnesium, and folic acid. Although vitamin and mineral supplements are readily available, little clinical research—with the exception of folic acid—has been done on the efficacy of such supplementation.26
Until more is known, intake of these dietary components is best achieved as part of a well-balanced diet; however, this recommendation may need to be modified for African-American women. In a recent retrospective study of almost 2,500 white and African-American women who took a multivitamin supplement consistently during the month before conception, supplementation was associated with increased infant birth weight in the infants born to African-American women but not in those born to white women.26
Folic acid
In the specific case of folic acid, the crucial importance of preconception intake by reproductive-age women is hard to overstate. A well-established body of research supports supplementation to reduce the incidence of neural tube defects that may occur very early in development, before many women are aware of a pregnancy.25-27 However, it is estimated that only a minority of reproductive-age women take a regular folic acid supplement. This may be particularly true of women who are actively avoiding pregnancy and using regular contraception. Patients need to be educated that, as effective as current contraceptive methods are known to be, each method has a typical user failure rate, meaning that actual effectiveness is lower than theoretical effectiveness.
Considering that half of US pregnancies are unintended, with some occurring as a result of contraceptive method or user failures, the FDA Advisory Committee unanimously endorsed the concept of using OCs as a vehicle for folate supplementation.27 There are currently two FDA-approved OCs fortified with the equivalent of 0.4 mg of folic acid. Both contain drospirenone and therefore present a somewhat elevated risk for blood clots, especially in the first year of use.28,29 While this risk is small compared to the incidence of blood clots during pregnancy, a careful history should be taken to avoid prescribing these products to patients already at increased risk for blood clots (eg, obese women, smokers [even light smokers], those with a history of a blood clot after surgery or a motor vehicle accident). For women without risk factors, folic acid–supplemented OCs may be very beneficial should they become pregnant unintentionally or quickly after stopping contraception.
On the next page: Cultural considerations and conclusion >>
CULTURAL CONSIDERATIONS
Not all racial, ethnic, and socioeconomic groups consider health and pregnancy in the same cultural context, and nonmedical factors may affect health behaviors and sources of health counseling. Studies of women of different cultural backgrounds are illustrative.
In one study, increasing women’s evidence-based knowledge of preconception and interconception health behaviors, using group education and peer support, was shown to produce attitudinal and behavior changes in a sample of reproductive-age rural white women, especially with regard to nutrition and physical activity in the preconception period.30
Another study of primarily low-income Latina women with low levels of acculturation revealed that they had good understanding of the importance of attention to health once pregnancy is confirmed. However, they expressed much less belief in the ability of a woman to control her own preconception general health.31
A third study involving a sample of African-American women found that the women saw no clear role for preconception or interconception care through health care visits with primary care clinicians; rather, they looked to their social and cultural communities and families for such support.32
These diverse results suggest that both community-wide education and one-to-one health counseling are needed to effect improvements in health behaviors and knowledge. Subtle differences in cultural context must be recognized by health care providers who interact with a broad range of reproductive-age women.
CONCLUSION
Guidelines from the American Academy of Pediatrics, the American Congress of Obstetricians and Gynecologists, and the CDC all endorse the integration of preconception care into primary care encounters.33-36 The concept of preconception health care offers clinicians the opportunity to greatly influence the health of reproductive-age women in the primary care setting, with the potential to achieve small but clinically significant changes in health behaviors. Complications of pregnancy and poor pregnancy outcomes may be reduced when the overall health status of women of reproductive age is addressed, with awareness and mitigation of factors known to produce negative pregnancy outcomes.
There is a need, however, for ongoing research to develop effective, evidence-based strategies for use by primary care clinicians in the effort to improve women’s preconception health and, ultimately, pregnancy outcomes.
1. National Center for Health Statistics. Table 7: Prenatal care for live births, by detailed race and Hispanic origin of mother: United States, selected years 1970–2004. In: Health, United States, 2006. Report No: 2006-1232. www.ncbi.nlm.nih.gov/books/NBK21003. Accessed July 17, 2014.
2. Office of Adolescent Health, US Department of Health and Human Services. Trends in teen pregnancy and childbearing. www.hhs.gov/ash/oah/adolescent-health-topics/reproductive-health/teen-pregnancy/trends.html#_ftn6. Accessed July 17, 2014.
3. Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Child Health USA 2013. http://mchb.hrsa.gov/chusa13. Accessed July 17, 2014.
4. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf. Accessed July 17, 2014.
5. Organization for Economic Cooperation and Development (OECD). Low birth weight infants, 2009 and change 1970-2009 (or nearest year). In: Health at a Glance 2011: OECD indicators. www.oecd.org/els/health-systems/49105858.pdf. Accessed July 17, 2014.
6. CDC. Infant mortality rates and international rankings. www.cdc.gov/nchs/data/hus/2013/016.pdf. Accessed July 17, 2014.
7. Livingood WC, Brady C, Pierce K, et al. Impact of pre-conception health care: evaluation of a social determinants focused intervention. Matern Child Health J. 2010;14:382-391.
8. Hillemeier MM, Weisman CS, Chase GA, et al. Women’s preconceptional health and use of health services: implications for preconception care. Health Serv Res. 2008;43(1):54-75.
9. Chuang CH, Weisman CS, Hillemeier MM, et al. Pregnancy intention and health behaviors: results from the Central Pennsylvania Women’s Health Study Cohort. Matern Child Health J. 2010;14:501-510.
10. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/overview.html. Accessed July 17, 2014.
11. Heavey E. Don’t miss preconception care opportunities for adolescents. Am J Matern Child Nurs. 2010;35(4):213-219.
12. Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health. 2010;46:324-330.
13. Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care—United States. MMWR Recomm Rep. 2006;55:1-23.
14. Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: is it time for meaningful research to inform preventive and management strategies? Brit J Obstet Gynecol. 2006;113:1134-1140.
15. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56-59.
16. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502-1505.
17. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee.full.pdf+html. Accessed July 17, 2014.
18. Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191(8):425-427.
19. Donahue SMA, Zimmerman FJ, Starr JR, Holt VL. Correlates of pre-pregnancy physical inactivity: results from the pregnancy risk assessment monitoring system. Matern Child Health J. 2010;14:235-244.
20. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64-71.
21. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2013;121(1):213-217.
22. Ciangura C, Corigliano N, Basdevant A, et al. Etonogestrel concentrations in morbidly obese women following Roux-en-Y gastric bypass surgery: three case reports. Contraception. 2011;84:649-651.
23. Wieck A, Rao S, Sein K, Haddad PM. A survey of antiepileptic prescribing to women of childbearing potential in psychiatry. Arch Womens Ment Health. 2007;10:83-85.
24. Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803-811.
25. Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol. 2008;S345-S353.
26. Burris HH, Mitchell AA, Werler MM. Periconceptional multivitamin use and infant birth weight disparities. Ann Epidemiol. 2010;20(3):233-240.
27. Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011:e1-e8.
28. Beyaz [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
29. Safyral [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
30. Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Womens Health Issues. 2008;18(6 suppl):S87-S96.
31. Coonrod DV, Bruce NC, Malcolm TD, et al. Knowledge and attitudes regarding preconception care in a predominantly low-income Mexican American population. Am J Obstet Gynecol. 2009;686:e1-e7.
32. Canady RB, Tiedje LB, Lauber C. Preconception care and pregnancy planning: voices of African American women. Matern Child Nurs. 2008;33(2):90-97.
33. Bellanca HK, Hunter MS. ONE KEY QUESTION: preventive reproductive health is part of high quality primary care. Contraception. 2013;88(1):3-6.
34. American Academy of Pediatrics. Preconception health care. www.healthychildren.org/English/ages-stages/prenatal/Pages/Reduce-the-Risk-of-Birth-Defects.aspx. Accessed July 17, 2014.
35. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. The importance of preconception care in the continuum of women’s health care. www.acog.org/Resources_And_Publi cations/Committee_Opinions/Committee_on_Gynecologic_Practice/The_Importance_of_Preconception_Care_in_the_Continuum_of_Wom ens_Health_Care. Accessed July 17, 2014.
36. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/hcp/. Accessed July 17, 2014.
CE/CME No: CR-1408
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define preconception and interconception health care and explain how these concepts relate to primary care.
• Describe three common clinical presentations in reproductive-age women that have implications for preconception and interconception health care.
• Explain the considerations, in terms of potential pregnancies, when prescribing pharmacologic treatment for reproductive-age women.
• Discuss examples of cultural factors and beliefs that may affect preconception health counseling provided to women of reproductive age.
FACULTY
Kathleen A. Ahonen is an Assistant Professor and Colleen Quinlan is an Associate Professor at the University of Toledo College of Nursing.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Because half of all pregnancies in the United States are unplanned, primary care constitutes preconception health care for women ages 15 to 44. Here are recommendations to incorporate into routine visits to improve outcomes of possible pregnancies.
Traditional interventions to improve pregnancy outcomes in the United States have focused on early, consistent prenatal care and on reducing the teenage pregnancy rate. Data indicate that early access to prenatal care has increased and that the teenage pregnancy rate has fallen.1,2
But while US rates of preterm births, low–birth-weight infants, and infant mortality have all declined since the mid-2000s,3 they remain higher than those in many other developed countries.4-6 Furthermore, there are significant differences in these rates by race and ethnicity.3
Many experts believe that waiting until a woman is pregnant to address the health needs and behaviors that adversely affect pregnancy outcomes is, at best, an incomplete solution to these problems. A more comprehensive view of women’s health care broadens this focus to stress the importance of preconception health care throughout the reproductive years. It is key to the achievement of optimal health, not only for women but also for their potential future children.7-9
The CDC defines preconception health simply as the health of women and men during their reproductive years. Preconception health care is defined as medical care during those years (for women, ages 15 to 44) that focuses on improving aspects of the patient’s health or health behaviors that can improve outcomes in the event of a pregnancy.10 The term interconception health care is also applied specifically to health care provided during the time between pregnancies.
This article highlights obesity, depression and other mood disorders, and nutritional deficiencies and reviews potential risk factors, particularly occurring very early in a pregnancy. Recommendations are offered for more effective health counseling at routine visits, with the goal of improving outcomes of potential pregnancies.
PRIMARY CARE IS PRECONCEPTION CARE
Researchers have reported that only about 50% of reproductive-age women receive counseling about health behaviors and pregnancy planning8 and that such counseling is even rarer for adolescent females.11,12 Because women of childbearing age commonly see both Ob-Gyn and family practice clinicians for health care, primary care providers are well positioned to deliver preconception health care by integrating health counseling, discussion of health behaviors, and primary and secondary prevention into patient care for reproductive-age women (see "Summary of CDC Recommendations to Improve Preconception Health and Health Care").6
On the next page: Obesity >>
OBESITY
Researchers in both the US and internationally have noted the connection between obesity and pregnancy complications such as gestational diabetes that increase the risk for poor pregnancy outcomes.13-15 Indeed, pregnancy outcomes can be altered for the better with weight loss in the preconception or interconception period.16
Weight loss is complex; it can be difficult to accomplish, and long-term maintenance also presents a challenge. Short-term weight loss is achievable, however, and can produce beneficial metabolic effects, such as a decrease in insulin resistance.17
Before weight loss attempts can be initiated, the overweight or obese patient’s weight must be acknowledged as a health problem. In one 2009 study, Callaway et al found that in their sample of 412 overweight and obese women, only 17% were advised by their health care providers to lose weight.18
Regular physical activity can facilitate the achievement and maintenance of a healthy weight. However, physical inactivity is common in nonpregnant women, especially those with higher BMIs. The CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS; www.cdc.gov/prams), widely regarded as an important source of data about women’s health behaviors, reveals that nearly 40% of US women do not meet national recommendations for routine physical activity in the three months prior to pregnancy.19
Recent obesity guidelines recommend initial weight loss goals of 5% to 10% of baseline weight in six months before pregnancy.17 Some women may not achieve these goals because they are unaware of the potentially short time period between discontinuation of contraception and conception. Others, especially those older than 30, intentionally plan for short interconception intervals as part of their family planning strategy, which does not allow enough time for safe weight loss.20
Primary care clinicians should counsel these patients on the importance of a healthy weight and regular physical activity to the maintenance of optimal health. Particular emphasis should be placed on achieving weight loss slowly and safely and then maintaining it, at least for the short term. This strategy may be effective in helping a female patient achieve a lower BMI prior to pregnancy.
Bariatric surgery
Because the number of women undergoing bariatric surgery for morbid obesity is rapidly increasing,21 it is important to educate them about the specific effects of such procedures on reproductive health. A period of rapid weight loss—such as occurs after bariatric surgery—is not a time to consider pregnancy, despite the improvement in eventual pregnancy outcomes associated with a healthier BMI.
Many of these women may have been anovulatory when morbidly obese and unaware that fertility increases in the postoperative year, after a reduced BMI is achieved. If ovulation and menses have not yet normalized, a woman may become pregnant and not know it. This could result in the inadvertent exposure of the developing fetus to such teratogenic risks as alcohol, tobacco, and certain prescription drugs. While a pregnancy may be welcome, better outcomes are likely if pregnancy is avoided for at least 12 months after bariatric surgery.22 Effective contraception should be used until ovulation cycles stabilize.
Some surgical weight loss procedures, such as the Roux-en-Y gastric bypass, may alter the absorption of medication, including oral contraceptives (OCs), making the use of OCs after such procedures less than ideal. The more reliably absorbed injectable medroxyprogesterone is an option, but women who have undergone bariatric surgery often wish to avoid the associated risk for weight gain. Nonhormonal long-acting contraceptive methods not associated with weight gain, such as the copper intrauterine device, may be preferable for use in the first year postprocedure.21
On the next page: Depression and other mood disorders >>
DEPRESSION AND OTHER MOOD DISORDERS
Mood disorders include depression, bipolar disorder, and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs, such as paroxetine or sertraline) or serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine or duloxetine) are often prescribed for primary care management of depression and other mood disorders.
When SSRIs or SNRIs are not fully effective, clinicians may refer patients to mental health specialists for consultation and possible ongoing management. Women of reproductive age who receive specialty care for mood disorders are encouraged to continue their regular visits to primary care clinicians.
Medication: Risk for birth defects
Anticonvulsants, such as valproate, carbamazepine, and lamotrigine, are commonly used to treat bipolar disorder.23 When taken during the first trimester of pregnancy, these drugs pose well-documented risks to the rapidly developing fetus. Most evidence relates to the risk for neural tube defects, such as spina bifida, but other evidence suggests a risk for general cognitive impairment after prenatal valproate exposure. While the latter is based primarily on studies of women taking anti-epileptic drugs for seizure control—not psychiatric diagnoses—first-trimester risks appear to be independent of maternal seizures.23 Although folic acid supplementation decreases the incidence of neural tube defects (see discussion under “Nutritional Deficiencies"), it is unknown if such supplementation is effective in mitigating the additional risks to the fetus from exposure to anticonvulsants.
Female patients of childbearing age must be advised of the potential effects of these commonly prescribed mood-stabilizing drugs, not only as they relate to the diagnosis being treated but also regarding their possible effects on an early, undiagnosed pregnancy. Unfortunately, evidence indicates that insufficient attention is given to counseling reproductive-age women about the risks and benefits of these drugs as they relate to potential conception, at least in the context of specialty care.23 Therefore, the primary care clinician and the specialist should utilize a team approach, emphasizing careful reproductive planning to avoid pregnancy while under treatment with these drugs to ensure the best possible outcomes.
In the context of potential pregnancy, the need to manage depression and other mood disorders effectively is particularly important: Prepregnancy depressive mood has been significantly associated with preterm birth, and at least 14.5% of women experience a new episode of depression during pregnancy.24 Thus, effective treatment of mood disorders should be a priority, both as part of preconception care and during pregnancy.
Similarly, treatment strategies for postpartum depression—widely estimated to affect 10% to 20% of new mothers—must consider the potential risks of pharmacologic therapy to a fetus should the patient conceive during treatment.
On the next page: Nutritional deficiencies >>
NUTRITIONAL DEFICIENCIES
While there is widespread public awareness, at least on a basic level, of the importance of good nutrition during pregnancy, what that constitutes is not necessarily clearly understood. Even less well recognized is the importance of a woman’s nutritional status at the time of conception, at preimplantation, and during the early weeks of placental development, before pregnancy is known or confirmed. During this crucial time—three to seven weeks after the last menstrual period—an inadequate diet may result in low–birth-weight infants with lifelong health problems.25 These may include respiratory problems associated with barotrauma from ventilation at birth; neural tube defects; and orofacial clefts.25
Because of inadequate intake of fresh fruits and vegetables, many reproductive-age women in the US are deficient in vitamins A, C, B6, and E, as well as calcium, iron, zinc, magnesium, and folic acid. Although vitamin and mineral supplements are readily available, little clinical research—with the exception of folic acid—has been done on the efficacy of such supplementation.26
Until more is known, intake of these dietary components is best achieved as part of a well-balanced diet; however, this recommendation may need to be modified for African-American women. In a recent retrospective study of almost 2,500 white and African-American women who took a multivitamin supplement consistently during the month before conception, supplementation was associated with increased infant birth weight in the infants born to African-American women but not in those born to white women.26
Folic acid
In the specific case of folic acid, the crucial importance of preconception intake by reproductive-age women is hard to overstate. A well-established body of research supports supplementation to reduce the incidence of neural tube defects that may occur very early in development, before many women are aware of a pregnancy.25-27 However, it is estimated that only a minority of reproductive-age women take a regular folic acid supplement. This may be particularly true of women who are actively avoiding pregnancy and using regular contraception. Patients need to be educated that, as effective as current contraceptive methods are known to be, each method has a typical user failure rate, meaning that actual effectiveness is lower than theoretical effectiveness.
Considering that half of US pregnancies are unintended, with some occurring as a result of contraceptive method or user failures, the FDA Advisory Committee unanimously endorsed the concept of using OCs as a vehicle for folate supplementation.27 There are currently two FDA-approved OCs fortified with the equivalent of 0.4 mg of folic acid. Both contain drospirenone and therefore present a somewhat elevated risk for blood clots, especially in the first year of use.28,29 While this risk is small compared to the incidence of blood clots during pregnancy, a careful history should be taken to avoid prescribing these products to patients already at increased risk for blood clots (eg, obese women, smokers [even light smokers], those with a history of a blood clot after surgery or a motor vehicle accident). For women without risk factors, folic acid–supplemented OCs may be very beneficial should they become pregnant unintentionally or quickly after stopping contraception.
On the next page: Cultural considerations and conclusion >>
CULTURAL CONSIDERATIONS
Not all racial, ethnic, and socioeconomic groups consider health and pregnancy in the same cultural context, and nonmedical factors may affect health behaviors and sources of health counseling. Studies of women of different cultural backgrounds are illustrative.
In one study, increasing women’s evidence-based knowledge of preconception and interconception health behaviors, using group education and peer support, was shown to produce attitudinal and behavior changes in a sample of reproductive-age rural white women, especially with regard to nutrition and physical activity in the preconception period.30
Another study of primarily low-income Latina women with low levels of acculturation revealed that they had good understanding of the importance of attention to health once pregnancy is confirmed. However, they expressed much less belief in the ability of a woman to control her own preconception general health.31
A third study involving a sample of African-American women found that the women saw no clear role for preconception or interconception care through health care visits with primary care clinicians; rather, they looked to their social and cultural communities and families for such support.32
These diverse results suggest that both community-wide education and one-to-one health counseling are needed to effect improvements in health behaviors and knowledge. Subtle differences in cultural context must be recognized by health care providers who interact with a broad range of reproductive-age women.
CONCLUSION
Guidelines from the American Academy of Pediatrics, the American Congress of Obstetricians and Gynecologists, and the CDC all endorse the integration of preconception care into primary care encounters.33-36 The concept of preconception health care offers clinicians the opportunity to greatly influence the health of reproductive-age women in the primary care setting, with the potential to achieve small but clinically significant changes in health behaviors. Complications of pregnancy and poor pregnancy outcomes may be reduced when the overall health status of women of reproductive age is addressed, with awareness and mitigation of factors known to produce negative pregnancy outcomes.
There is a need, however, for ongoing research to develop effective, evidence-based strategies for use by primary care clinicians in the effort to improve women’s preconception health and, ultimately, pregnancy outcomes.
CE/CME No: CR-1408
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Define preconception and interconception health care and explain how these concepts relate to primary care.
• Describe three common clinical presentations in reproductive-age women that have implications for preconception and interconception health care.
• Explain the considerations, in terms of potential pregnancies, when prescribing pharmacologic treatment for reproductive-age women.
• Discuss examples of cultural factors and beliefs that may affect preconception health counseling provided to women of reproductive age.
FACULTY
Kathleen A. Ahonen is an Assistant Professor and Colleen Quinlan is an Associate Professor at the University of Toledo College of Nursing.
The authors have no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Because half of all pregnancies in the United States are unplanned, primary care constitutes preconception health care for women ages 15 to 44. Here are recommendations to incorporate into routine visits to improve outcomes of possible pregnancies.
Traditional interventions to improve pregnancy outcomes in the United States have focused on early, consistent prenatal care and on reducing the teenage pregnancy rate. Data indicate that early access to prenatal care has increased and that the teenage pregnancy rate has fallen.1,2
But while US rates of preterm births, low–birth-weight infants, and infant mortality have all declined since the mid-2000s,3 they remain higher than those in many other developed countries.4-6 Furthermore, there are significant differences in these rates by race and ethnicity.3
Many experts believe that waiting until a woman is pregnant to address the health needs and behaviors that adversely affect pregnancy outcomes is, at best, an incomplete solution to these problems. A more comprehensive view of women’s health care broadens this focus to stress the importance of preconception health care throughout the reproductive years. It is key to the achievement of optimal health, not only for women but also for their potential future children.7-9
The CDC defines preconception health simply as the health of women and men during their reproductive years. Preconception health care is defined as medical care during those years (for women, ages 15 to 44) that focuses on improving aspects of the patient’s health or health behaviors that can improve outcomes in the event of a pregnancy.10 The term interconception health care is also applied specifically to health care provided during the time between pregnancies.
This article highlights obesity, depression and other mood disorders, and nutritional deficiencies and reviews potential risk factors, particularly occurring very early in a pregnancy. Recommendations are offered for more effective health counseling at routine visits, with the goal of improving outcomes of potential pregnancies.
PRIMARY CARE IS PRECONCEPTION CARE
Researchers have reported that only about 50% of reproductive-age women receive counseling about health behaviors and pregnancy planning8 and that such counseling is even rarer for adolescent females.11,12 Because women of childbearing age commonly see both Ob-Gyn and family practice clinicians for health care, primary care providers are well positioned to deliver preconception health care by integrating health counseling, discussion of health behaviors, and primary and secondary prevention into patient care for reproductive-age women (see "Summary of CDC Recommendations to Improve Preconception Health and Health Care").6
On the next page: Obesity >>
OBESITY
Researchers in both the US and internationally have noted the connection between obesity and pregnancy complications such as gestational diabetes that increase the risk for poor pregnancy outcomes.13-15 Indeed, pregnancy outcomes can be altered for the better with weight loss in the preconception or interconception period.16
Weight loss is complex; it can be difficult to accomplish, and long-term maintenance also presents a challenge. Short-term weight loss is achievable, however, and can produce beneficial metabolic effects, such as a decrease in insulin resistance.17
Before weight loss attempts can be initiated, the overweight or obese patient’s weight must be acknowledged as a health problem. In one 2009 study, Callaway et al found that in their sample of 412 overweight and obese women, only 17% were advised by their health care providers to lose weight.18
Regular physical activity can facilitate the achievement and maintenance of a healthy weight. However, physical inactivity is common in nonpregnant women, especially those with higher BMIs. The CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS; www.cdc.gov/prams), widely regarded as an important source of data about women’s health behaviors, reveals that nearly 40% of US women do not meet national recommendations for routine physical activity in the three months prior to pregnancy.19
Recent obesity guidelines recommend initial weight loss goals of 5% to 10% of baseline weight in six months before pregnancy.17 Some women may not achieve these goals because they are unaware of the potentially short time period between discontinuation of contraception and conception. Others, especially those older than 30, intentionally plan for short interconception intervals as part of their family planning strategy, which does not allow enough time for safe weight loss.20
Primary care clinicians should counsel these patients on the importance of a healthy weight and regular physical activity to the maintenance of optimal health. Particular emphasis should be placed on achieving weight loss slowly and safely and then maintaining it, at least for the short term. This strategy may be effective in helping a female patient achieve a lower BMI prior to pregnancy.
Bariatric surgery
Because the number of women undergoing bariatric surgery for morbid obesity is rapidly increasing,21 it is important to educate them about the specific effects of such procedures on reproductive health. A period of rapid weight loss—such as occurs after bariatric surgery—is not a time to consider pregnancy, despite the improvement in eventual pregnancy outcomes associated with a healthier BMI.
Many of these women may have been anovulatory when morbidly obese and unaware that fertility increases in the postoperative year, after a reduced BMI is achieved. If ovulation and menses have not yet normalized, a woman may become pregnant and not know it. This could result in the inadvertent exposure of the developing fetus to such teratogenic risks as alcohol, tobacco, and certain prescription drugs. While a pregnancy may be welcome, better outcomes are likely if pregnancy is avoided for at least 12 months after bariatric surgery.22 Effective contraception should be used until ovulation cycles stabilize.
Some surgical weight loss procedures, such as the Roux-en-Y gastric bypass, may alter the absorption of medication, including oral contraceptives (OCs), making the use of OCs after such procedures less than ideal. The more reliably absorbed injectable medroxyprogesterone is an option, but women who have undergone bariatric surgery often wish to avoid the associated risk for weight gain. Nonhormonal long-acting contraceptive methods not associated with weight gain, such as the copper intrauterine device, may be preferable for use in the first year postprocedure.21
On the next page: Depression and other mood disorders >>
DEPRESSION AND OTHER MOOD DISORDERS
Mood disorders include depression, bipolar disorder, and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs, such as paroxetine or sertraline) or serotonin and norepinephrine reuptake inhibitors (SNRIs, such as venlafaxine or duloxetine) are often prescribed for primary care management of depression and other mood disorders.
When SSRIs or SNRIs are not fully effective, clinicians may refer patients to mental health specialists for consultation and possible ongoing management. Women of reproductive age who receive specialty care for mood disorders are encouraged to continue their regular visits to primary care clinicians.
Medication: Risk for birth defects
Anticonvulsants, such as valproate, carbamazepine, and lamotrigine, are commonly used to treat bipolar disorder.23 When taken during the first trimester of pregnancy, these drugs pose well-documented risks to the rapidly developing fetus. Most evidence relates to the risk for neural tube defects, such as spina bifida, but other evidence suggests a risk for general cognitive impairment after prenatal valproate exposure. While the latter is based primarily on studies of women taking anti-epileptic drugs for seizure control—not psychiatric diagnoses—first-trimester risks appear to be independent of maternal seizures.23 Although folic acid supplementation decreases the incidence of neural tube defects (see discussion under “Nutritional Deficiencies"), it is unknown if such supplementation is effective in mitigating the additional risks to the fetus from exposure to anticonvulsants.
Female patients of childbearing age must be advised of the potential effects of these commonly prescribed mood-stabilizing drugs, not only as they relate to the diagnosis being treated but also regarding their possible effects on an early, undiagnosed pregnancy. Unfortunately, evidence indicates that insufficient attention is given to counseling reproductive-age women about the risks and benefits of these drugs as they relate to potential conception, at least in the context of specialty care.23 Therefore, the primary care clinician and the specialist should utilize a team approach, emphasizing careful reproductive planning to avoid pregnancy while under treatment with these drugs to ensure the best possible outcomes.
In the context of potential pregnancy, the need to manage depression and other mood disorders effectively is particularly important: Prepregnancy depressive mood has been significantly associated with preterm birth, and at least 14.5% of women experience a new episode of depression during pregnancy.24 Thus, effective treatment of mood disorders should be a priority, both as part of preconception care and during pregnancy.
Similarly, treatment strategies for postpartum depression—widely estimated to affect 10% to 20% of new mothers—must consider the potential risks of pharmacologic therapy to a fetus should the patient conceive during treatment.
On the next page: Nutritional deficiencies >>
NUTRITIONAL DEFICIENCIES
While there is widespread public awareness, at least on a basic level, of the importance of good nutrition during pregnancy, what that constitutes is not necessarily clearly understood. Even less well recognized is the importance of a woman’s nutritional status at the time of conception, at preimplantation, and during the early weeks of placental development, before pregnancy is known or confirmed. During this crucial time—three to seven weeks after the last menstrual period—an inadequate diet may result in low–birth-weight infants with lifelong health problems.25 These may include respiratory problems associated with barotrauma from ventilation at birth; neural tube defects; and orofacial clefts.25
Because of inadequate intake of fresh fruits and vegetables, many reproductive-age women in the US are deficient in vitamins A, C, B6, and E, as well as calcium, iron, zinc, magnesium, and folic acid. Although vitamin and mineral supplements are readily available, little clinical research—with the exception of folic acid—has been done on the efficacy of such supplementation.26
Until more is known, intake of these dietary components is best achieved as part of a well-balanced diet; however, this recommendation may need to be modified for African-American women. In a recent retrospective study of almost 2,500 white and African-American women who took a multivitamin supplement consistently during the month before conception, supplementation was associated with increased infant birth weight in the infants born to African-American women but not in those born to white women.26
Folic acid
In the specific case of folic acid, the crucial importance of preconception intake by reproductive-age women is hard to overstate. A well-established body of research supports supplementation to reduce the incidence of neural tube defects that may occur very early in development, before many women are aware of a pregnancy.25-27 However, it is estimated that only a minority of reproductive-age women take a regular folic acid supplement. This may be particularly true of women who are actively avoiding pregnancy and using regular contraception. Patients need to be educated that, as effective as current contraceptive methods are known to be, each method has a typical user failure rate, meaning that actual effectiveness is lower than theoretical effectiveness.
Considering that half of US pregnancies are unintended, with some occurring as a result of contraceptive method or user failures, the FDA Advisory Committee unanimously endorsed the concept of using OCs as a vehicle for folate supplementation.27 There are currently two FDA-approved OCs fortified with the equivalent of 0.4 mg of folic acid. Both contain drospirenone and therefore present a somewhat elevated risk for blood clots, especially in the first year of use.28,29 While this risk is small compared to the incidence of blood clots during pregnancy, a careful history should be taken to avoid prescribing these products to patients already at increased risk for blood clots (eg, obese women, smokers [even light smokers], those with a history of a blood clot after surgery or a motor vehicle accident). For women without risk factors, folic acid–supplemented OCs may be very beneficial should they become pregnant unintentionally or quickly after stopping contraception.
On the next page: Cultural considerations and conclusion >>
CULTURAL CONSIDERATIONS
Not all racial, ethnic, and socioeconomic groups consider health and pregnancy in the same cultural context, and nonmedical factors may affect health behaviors and sources of health counseling. Studies of women of different cultural backgrounds are illustrative.
In one study, increasing women’s evidence-based knowledge of preconception and interconception health behaviors, using group education and peer support, was shown to produce attitudinal and behavior changes in a sample of reproductive-age rural white women, especially with regard to nutrition and physical activity in the preconception period.30
Another study of primarily low-income Latina women with low levels of acculturation revealed that they had good understanding of the importance of attention to health once pregnancy is confirmed. However, they expressed much less belief in the ability of a woman to control her own preconception general health.31
A third study involving a sample of African-American women found that the women saw no clear role for preconception or interconception care through health care visits with primary care clinicians; rather, they looked to their social and cultural communities and families for such support.32
These diverse results suggest that both community-wide education and one-to-one health counseling are needed to effect improvements in health behaviors and knowledge. Subtle differences in cultural context must be recognized by health care providers who interact with a broad range of reproductive-age women.
CONCLUSION
Guidelines from the American Academy of Pediatrics, the American Congress of Obstetricians and Gynecologists, and the CDC all endorse the integration of preconception care into primary care encounters.33-36 The concept of preconception health care offers clinicians the opportunity to greatly influence the health of reproductive-age women in the primary care setting, with the potential to achieve small but clinically significant changes in health behaviors. Complications of pregnancy and poor pregnancy outcomes may be reduced when the overall health status of women of reproductive age is addressed, with awareness and mitigation of factors known to produce negative pregnancy outcomes.
There is a need, however, for ongoing research to develop effective, evidence-based strategies for use by primary care clinicians in the effort to improve women’s preconception health and, ultimately, pregnancy outcomes.
1. National Center for Health Statistics. Table 7: Prenatal care for live births, by detailed race and Hispanic origin of mother: United States, selected years 1970–2004. In: Health, United States, 2006. Report No: 2006-1232. www.ncbi.nlm.nih.gov/books/NBK21003. Accessed July 17, 2014.
2. Office of Adolescent Health, US Department of Health and Human Services. Trends in teen pregnancy and childbearing. www.hhs.gov/ash/oah/adolescent-health-topics/reproductive-health/teen-pregnancy/trends.html#_ftn6. Accessed July 17, 2014.
3. Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Child Health USA 2013. http://mchb.hrsa.gov/chusa13. Accessed July 17, 2014.
4. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf. Accessed July 17, 2014.
5. Organization for Economic Cooperation and Development (OECD). Low birth weight infants, 2009 and change 1970-2009 (or nearest year). In: Health at a Glance 2011: OECD indicators. www.oecd.org/els/health-systems/49105858.pdf. Accessed July 17, 2014.
6. CDC. Infant mortality rates and international rankings. www.cdc.gov/nchs/data/hus/2013/016.pdf. Accessed July 17, 2014.
7. Livingood WC, Brady C, Pierce K, et al. Impact of pre-conception health care: evaluation of a social determinants focused intervention. Matern Child Health J. 2010;14:382-391.
8. Hillemeier MM, Weisman CS, Chase GA, et al. Women’s preconceptional health and use of health services: implications for preconception care. Health Serv Res. 2008;43(1):54-75.
9. Chuang CH, Weisman CS, Hillemeier MM, et al. Pregnancy intention and health behaviors: results from the Central Pennsylvania Women’s Health Study Cohort. Matern Child Health J. 2010;14:501-510.
10. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/overview.html. Accessed July 17, 2014.
11. Heavey E. Don’t miss preconception care opportunities for adolescents. Am J Matern Child Nurs. 2010;35(4):213-219.
12. Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health. 2010;46:324-330.
13. Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care—United States. MMWR Recomm Rep. 2006;55:1-23.
14. Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: is it time for meaningful research to inform preventive and management strategies? Brit J Obstet Gynecol. 2006;113:1134-1140.
15. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56-59.
16. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502-1505.
17. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee.full.pdf+html. Accessed July 17, 2014.
18. Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191(8):425-427.
19. Donahue SMA, Zimmerman FJ, Starr JR, Holt VL. Correlates of pre-pregnancy physical inactivity: results from the pregnancy risk assessment monitoring system. Matern Child Health J. 2010;14:235-244.
20. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64-71.
21. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2013;121(1):213-217.
22. Ciangura C, Corigliano N, Basdevant A, et al. Etonogestrel concentrations in morbidly obese women following Roux-en-Y gastric bypass surgery: three case reports. Contraception. 2011;84:649-651.
23. Wieck A, Rao S, Sein K, Haddad PM. A survey of antiepileptic prescribing to women of childbearing potential in psychiatry. Arch Womens Ment Health. 2007;10:83-85.
24. Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803-811.
25. Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol. 2008;S345-S353.
26. Burris HH, Mitchell AA, Werler MM. Periconceptional multivitamin use and infant birth weight disparities. Ann Epidemiol. 2010;20(3):233-240.
27. Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011:e1-e8.
28. Beyaz [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
29. Safyral [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
30. Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Womens Health Issues. 2008;18(6 suppl):S87-S96.
31. Coonrod DV, Bruce NC, Malcolm TD, et al. Knowledge and attitudes regarding preconception care in a predominantly low-income Mexican American population. Am J Obstet Gynecol. 2009;686:e1-e7.
32. Canady RB, Tiedje LB, Lauber C. Preconception care and pregnancy planning: voices of African American women. Matern Child Nurs. 2008;33(2):90-97.
33. Bellanca HK, Hunter MS. ONE KEY QUESTION: preventive reproductive health is part of high quality primary care. Contraception. 2013;88(1):3-6.
34. American Academy of Pediatrics. Preconception health care. www.healthychildren.org/English/ages-stages/prenatal/Pages/Reduce-the-Risk-of-Birth-Defects.aspx. Accessed July 17, 2014.
35. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. The importance of preconception care in the continuum of women’s health care. www.acog.org/Resources_And_Publi cations/Committee_Opinions/Committee_on_Gynecologic_Practice/The_Importance_of_Preconception_Care_in_the_Continuum_of_Wom ens_Health_Care. Accessed July 17, 2014.
36. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/hcp/. Accessed July 17, 2014.
1. National Center for Health Statistics. Table 7: Prenatal care for live births, by detailed race and Hispanic origin of mother: United States, selected years 1970–2004. In: Health, United States, 2006. Report No: 2006-1232. www.ncbi.nlm.nih.gov/books/NBK21003. Accessed July 17, 2014.
2. Office of Adolescent Health, US Department of Health and Human Services. Trends in teen pregnancy and childbearing. www.hhs.gov/ash/oah/adolescent-health-topics/reproductive-health/teen-pregnancy/trends.html#_ftn6. Accessed July 17, 2014.
3. Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Child Health USA 2013. http://mchb.hrsa.gov/chusa13. Accessed July 17, 2014.
4. World Health Organization. Born Too Soon: The Global Action Report on Preterm Birth. www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf. Accessed July 17, 2014.
5. Organization for Economic Cooperation and Development (OECD). Low birth weight infants, 2009 and change 1970-2009 (or nearest year). In: Health at a Glance 2011: OECD indicators. www.oecd.org/els/health-systems/49105858.pdf. Accessed July 17, 2014.
6. CDC. Infant mortality rates and international rankings. www.cdc.gov/nchs/data/hus/2013/016.pdf. Accessed July 17, 2014.
7. Livingood WC, Brady C, Pierce K, et al. Impact of pre-conception health care: evaluation of a social determinants focused intervention. Matern Child Health J. 2010;14:382-391.
8. Hillemeier MM, Weisman CS, Chase GA, et al. Women’s preconceptional health and use of health services: implications for preconception care. Health Serv Res. 2008;43(1):54-75.
9. Chuang CH, Weisman CS, Hillemeier MM, et al. Pregnancy intention and health behaviors: results from the Central Pennsylvania Women’s Health Study Cohort. Matern Child Health J. 2010;14:501-510.
10. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/overview.html. Accessed July 17, 2014.
11. Heavey E. Don’t miss preconception care opportunities for adolescents. Am J Matern Child Nurs. 2010;35(4):213-219.
12. Hoover KW, Tao G, Berman S, Kent CK. Utilization of health services in physician offices and outpatient clinics by adolescents and young women in the United States: implications for improving access to reproductive health services. J Adolesc Health. 2010;46:324-330.
13. Johnson K, Posner SF, Biermann J. Recommendations to improve preconception health and health care—United States. MMWR Recomm Rep. 2006;55:1-23.
14. Krishnamoorthy U, Schram CM, Hill SR. Maternal obesity in pregnancy: is it time for meaningful research to inform preventive and management strategies? Brit J Obstet Gynecol. 2006;113:1134-1140.
15. Callaway LK, Prins JB, Chang AM, McIntyre HD. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med J Aust. 2006;184:56-59.
16. Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502-1505.
17. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the Obesity Society. Circulation. http://circ.ahajournals.org/content/early/2013/11/11/01.cir.0000437739.71477.ee.full.pdf+html. Accessed July 17, 2014.
18. Callaway LK, O’Callaghan MJ, McIntyre HD. Barriers to addressing overweight and obesity before conception. Med J Aust. 2009;191(8):425-427.
19. Donahue SMA, Zimmerman FJ, Starr JR, Holt VL. Correlates of pre-pregnancy physical inactivity: results from the pregnancy risk assessment monitoring system. Matern Child Health J. 2010;14:235-244.
20. Gemmill A, Lindberg LD. Short interpregnancy intervals in the United States. Obstet Gynecol. 2013;122(1):64-71.
21. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obesity in pregnancy. Obstet Gynecol. 2013;121(1):213-217.
22. Ciangura C, Corigliano N, Basdevant A, et al. Etonogestrel concentrations in morbidly obese women following Roux-en-Y gastric bypass surgery: three case reports. Contraception. 2011;84:649-651.
23. Wieck A, Rao S, Sein K, Haddad PM. A survey of antiepileptic prescribing to women of childbearing potential in psychiatry. Arch Womens Ment Health. 2007;10:83-85.
24. Gavin AR, Chae DH, Mustillo S, Kiefe CI. Prepregnancy depressive mood and preterm birth in black and white women: findings from the CARDIA study. J Womens Health. 2009;18(6):803-811.
25. Gardiner PM, Nelson L, Shellhaas CS, et al. The clinical content of preconception care: nutrition and dietary supplements. Am J Obstet Gynecol. 2008;S345-S353.
26. Burris HH, Mitchell AA, Werler MM. Periconceptional multivitamin use and infant birth weight disparities. Ann Epidemiol. 2010;20(3):233-240.
27. Taylor TN, Farkouh RA, Graham JB, et al. Potential reduction in neural tube defects associated with use of Metafolin-fortified oral contraceptives in the United States. Am J Obstet Gynecol. 2011:e1-e8.
28. Beyaz [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
29. Safyral [package insert]. Wayne, NJ: Bayer HealthCare, 2010.
30. Hillemeier MM, Downs DS, Feinberg ME, et al. Improving women’s preconceptional health: findings from a randomized trial of the Strong Healthy Women intervention in the Central Pennsylvania Women’s Health Study. Womens Health Issues. 2008;18(6 suppl):S87-S96.
31. Coonrod DV, Bruce NC, Malcolm TD, et al. Knowledge and attitudes regarding preconception care in a predominantly low-income Mexican American population. Am J Obstet Gynecol. 2009;686:e1-e7.
32. Canady RB, Tiedje LB, Lauber C. Preconception care and pregnancy planning: voices of African American women. Matern Child Nurs. 2008;33(2):90-97.
33. Bellanca HK, Hunter MS. ONE KEY QUESTION: preventive reproductive health is part of high quality primary care. Contraception. 2013;88(1):3-6.
34. American Academy of Pediatrics. Preconception health care. www.healthychildren.org/English/ages-stages/prenatal/Pages/Reduce-the-Risk-of-Birth-Defects.aspx. Accessed July 17, 2014.
35. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. The importance of preconception care in the continuum of women’s health care. www.acog.org/Resources_And_Publi cations/Committee_Opinions/Committee_on_Gynecologic_Practice/The_Importance_of_Preconception_Care_in_the_Continuum_of_Wom ens_Health_Care. Accessed July 17, 2014.
36. CDC. Preconception health and health care. www.cdc.gov/preconcep tion/hcp/. Accessed July 17, 2014.
Hypothyroidism: Clinical Challenges in Diagnosis and Treatment
CE/CME No: CR-1407
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify the signs and symptoms of and risk factors for hypothyroidism.
• Name the laboratory tests used to diagnose or rule out hypothyroidism.
• Describe current evidence-based and alternative treatments for hypothyroidism.
• Discuss appropriate long-term follow-up and monitoring of hypothyroidism.
• Instruct patients regarding optimal self-management of hypothyroidism.
FACULTY
Kara-anne Gregory Curl is an Adjunct Clinical Instructor of Medicine, Division of Endocrinology and Metabolism, with Medical Faculty Associates at George Washington University in Washington, DC.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Although hypothyroidism is common, its typically vague symptoms of fatigue, lack of energy, and weight gain are shared with many other conditions. Awareness of risk factors for hypothyroidism will aid in the differential diagnosis, and the patient’s symptoms can help guide the clinician to the appropriate diagnostic workup. Thyroid function test results are necessary to confirm or rule out the diagnosis.
Approximately 4.6% of the US population ages 12 and older has been diagnosed with hypothyroidism, making it the most frequently diagnosed thyroid disorder.1 Hypothyroidism is defined by an underproduction of thyroid hormones and can be either primary or secondary. Primary hypothyroidism is caused by the failure of the thyroid gland to produce adequate quantities of the hormones triiodothyronine (T3) and levorotatory thyroxine (T4). Secondary (central) hypothyroidism is a result of inadequate production of thyrotropin (TSH) by the pituitary gland; less often, it is caused by inadequate production of thyrotropin-releasing hormone by the hypothalamus. The great majority of patients with hypothyroidism have the primary form of the disease. In the US, the most common cause of
hypothyroidism is chronic autoimmune thyroiditis (Hashimoto thyroiditis); worldwide, it is iodine deficiency.
Because hypothyroid symptoms are vague, the disease can be difficult to diagnose. Patients most often present with complaints of fatigue and lack of energy, raising suspicion for hypothyroidism; but true symptomatic, overt hypothyroidism is rather rare, occurring in only 0.3% of the population.2
Subclinical hypothyroidism, in which TSH levels are elevated but T3 and T4 levels are normal and the patient experiences few, if any, symptoms, is much more common. This makes the use of diagnostic laboratory tests essential; hypothyroidism cannot be diagnosed based on clinical presentation alone. Signs and symptoms may include fatigue, lack of energy, cold intolerance, weight gain, thinning hair/hair loss, dry skin, constipation, menstrual cycle abnormalities, irritability, and depression. Bradycardia and hypotension are also possible. Physical examination may reveal depressed affect, eyelid edema, loss of lateral third of the eyebrow, thickened tongue, dry skin, and hyporeflexivity; however, most patients will have a benign and unrevealing physical examination.3
Hypothyroidism is more common in white people and in women and increases in incidence after age 60. Risk factors for hypothyroidism include
• Family history
• Autoimmune disorders (eg, type 1 diabetes, Addison disease)
• History of Graves disease treated with radioactive iodine or thyroidectomy
• Past external beam radiotherapy for head and neck malignancies
• Postpartum thyroiditis
• Turner and Down syndromes
• Multiple sclerosis
• Amiodarone or lithium use
• Iodine deficiency or previous residence in an iodine-deficient area.4
In addition, testing for hypothyroidism may be warranted in patients with any of the diagnoses in Table 1.
Because of its vague symptomatology, hypothyroidism can mimic many disease processes. However, the most pertinent include depression, anemia, and dementia/Alzheimer disease. Laboratory testing will almost always identify hypothyroidism.
On the next page: Laboratory workup >>
LABORATORY WORKUP
Thyrotropin
The diagnosis of hypothyroidism is based on the results of the TSH test, which is the primary screening test for thyroid dysfunction.4 TSH secretion is extremely sensitive to minor increases and decreases in T3 or T4, making it the most reliable laboratory test for the assessment of thyroid function. An elevated serum TSH level in the presence of hypothyroid symptoms is diagnostic of primary hypothyroidism. Other causes of elevated TSH, such as thyrotropin-secreting pituitary tumors, are rare, and their symptomatology is different.3
Free Levorotatory Thyroxine
Though elevated TSH levels occur before T4 abnormalities are detected, T4 measurement can sometimes be useful in the diagnosis of hypothyroidism, especially in cases of possible central hypothyroidism. As a diagnostic test, measurement of serum free T4 (FT4) is preferable to total T4 because T4 binds to specific proteins in serum, making obtaining an accurate total T4 level subject to factors that alter binding. By contrast, FT4, the metabolically active form of the hormone, is not affected by binding factors. In primary hypothyroidism, FT4 is low or normal.4
Measurement of the FT4 level will also confirm the diagnosis of central hypothyroidism, if the FT4 is low when TSH is normal or low. As FT4 decreases, the TSH should elevate to compensate; in the presence of a low FT4, even a normal TSH is indicative of hypothyroidism. In a patient with overt hypothyroid symptoms with a normal TSH, an FT4 should be ordered for further workup.4
Triiodothyronine
Measurement of the serum T3 level, whether total or free, is of little clinical utility because it often remains normal, even as TSH and T4 levels change.4
Typical diagnostic test results in primary and secondary hypothyroidism are summarized in Table 2.
Other Factors That May Affect Thyroid Function Test Results
The overall health status of the patient must be considered when evaluating the results of thyroid function tests because the results can be affected by other factors.
• Serum TSH may be low, often in combination with low FT4, in hospitalized patients with acute illness.
• TSH may increase to levels above normal during recovery from nonthyroid-related illness.
• Serum TSH typically falls (infrequently to below 0.1 mlU/L) during the first trimester of pregnancy due to the stimulatory effects of human chorionic gonadotropin on the thyroid.5 Levels typically return to normal in the second trimester.
• TSH and FT4 can be altered in the postpartum period secondary to postpartum thyroiditis. Levels will often resolve on their own without treatment.
• Patients with anorexia nervosa may have low TSH levels as well as low levels of FT4 secondary to pituitary and hypothalamic dysfunction.
• Mild TSH elevations may also be a normal manifestation of aging; TSH values above 3.0 mlU/L occur with increasing frequency with age.4
Thyroid Peroxidase Antibodies
Testing the patient for thyroid peroxidase (TPO) antibodies, although not required to make the hypothyroidism diagnosis, may provide additional useful information. A positive TPO antibody result is significantly associated with hypothyroidism; in particular, TPO antibodies are more likely to be present in patients with autoimmune thyroiditis, helping to confirm the diagnosis.2,4 However, positive antibody test results do not change clinical management decisions. Results will remain positive during treatment, and the continued presence of antibodies warrants no alteration in treatment or medication dose.
An elevated TPO antibody level does impart a risk for future transition to overt hypothyroidism, so this test is recommended for patients with subclinical hypothyroidism. In addition, for patients with other autoimmune diseases, such as type 1 diabetes or Addison disease, or with chromosomal disorders, such as Down or Turner syndromes, TPO antibodies suggest a propensity toward hypothyroidism. Current research also indicates that both pregnancy rates and pregnancy outcomes improve when TPO antibody–positive patients whose TSH levels are above 2.5 mlU/L are treated.5
On the next page: Diagnosis and treatment >>
DIAGNOSIS
The diagnosis of hypothyroidism is made on the basis of laboratory test results, but symptomatology can help guide the clinician to the appropriate laboratory workup. Symptoms alone, when laboratory values are within normal limits or even at the high end of the normal range, do not support a hypothyroidism diagnosis. In such a case, a differential diagnosis should be pursued.
Since overt symptoms of hypothyroidism are rare, clinicians may wonder if they should screen all their patients for hypothyroidism. Current recommendations, as set forth in the joint American Thyroid Association (ATA) and American Association of Clinical Endocrinologists (AACE) clinical practice guidelines, support “aggressive case finding” rather than universal screening because as yet, there is no consensus on screening guidelines.4 The ATA recommends screening all adults at age 35 and then every five years thereafter.6 In contrast, the AACE recommends routine TSH measurement in “older” patients, particularly women.7
There is, however, compelling evidence for testing patients with any of the following4
• An autoimmune disease
• Pernicious anemia
• A first-degree relative with autoimmune thyroid disease
• An abnormal thyroid examination
• Past radiation to the thyroid gland, including radioactive iodine therapy for hyperthyroidism
• Past external beam radiotherapy for head and neck malignancies
• History of thyroid surgery
• History of thyroid dysfunction
• One of the diagnoses listed in Table 1.
It is also suggested that patients with psychiatric disorders and patients taking amiodarone or lithium be screened.4
TREATMENT
The standard evidence-based treatment for hypothyroidism is hormone replacement with levothyroxine.4 This is a Grade A recommendation in the ATA/AACE joint guidelines. Levothyroxine is bioequivalent to T4 in the body and has a half-life of approximately six to seven days. It is stable and easily adjusted by monitoring TSH levels; adverse reactions or complications are minimal. The starting dose of levothyroxine is 1.6 µg/kg/d for both primary and secondary (central) hypothyroidism.
Dose increases are made in 12.5 or 25 µg increments. In primary hypothyroidism, TSH levels should be monitored to determine the need for dose adjustments. FT4 need not be checked unless there is a discrepancy in the TSH levels.4 In secondary hypothyroidism, TSH levels will always remain normal or low, and FT4 should be used to monitor therapy. Levels should be measured four to eight weeks after initiation of treatment or after subsequent dose adjustments.
Overt Hypothyroidism (Primary or Secondary)
All patients with symptomatic, overt hypothyroidism and an elevated TSH level should be treated. Treatment is lifelong, and the goal is to reduce patient symptomatology, improve well-being, and prevent complications. The treatment target is a TSH level in the normal range, approximately 0.45 to 4.5 mIU/L on most laboratory assays. However, NHANES III data revealed that the mean serum TSH level in the normal population is 1.5 mlU/L.2 Based on this fact and on their experience, many clinicians would argue that a more appropriate goal is a TSH target in the midnormal range, such as 0.5 to 2.5 mlU/L. There is little evidence to support a low- or subnormal TSH target in the treatment of hypothyroidism.4
Subclinical Hypothyroidism
For patients with the more common subclinical hypothyroidism (an elevated TSH level without symptoms), the benefits of treatment are less clear. It is suggested that if the TSH level is > 10 mIU/L, even patients without symptoms should be treated because risk for overt hypothyroidism is high.8 A TSH level > 10 mIU/L has also been shown to increase the patient’s coronary artery disease (CAD) risk.8 For individuals with TSH levels in the 4.5 to 10 mIU/L range who feel well and have no hypothyroid symptoms, the evidence is less clear. Treatment may be beneficial, but this has not been determined definitively.4 A watch-and-wait approach may be taken with these patients; if symptoms develop, treatment should be considered.
Patients with subclinical hypothyroidism generally do not require full replacement doses (ie, a starting dosage of 1.6 µg/kg/d). A dosage of 25 to 75 µg/d is typically sufficient to achieve goal TSH levels. For patients older than 60 with no CAD and for all patients with CAD, lower starting dosages of 50 µg/d and 12.5 to 25 µg/d, respectively, are recommended.4
Normal TSH, Positive TPO in Pregnancy
Current clinical evidence does not support treatment of patients who have normal TSH levels (2.5 to 4.5 mIU/L) but who are positive for TPO antibodies.4 The exception is pregnant women or those considering pregnancy. Research suggests that the rates of spontaneous miscarriage and preterm labor are higher in TPO-positive women in this TSH range, so treatment may reduce these risks.4
On the next page: Additional treatment considerations >>
ADDITIONAL TREATMENT CONSIDERATIONS
Overtreatment
The main complication of hypothyroidism treatment occurs when the patient receives more thyroid hormone than is required, which has been reported in 20% of cases.9 The primary risks of overtreatment are osteoporosis and atrial fibrillation. Care should be taken especially in vulnerable populations such as the elderly, who are particularly susceptible to atrial fibrillation, and postmenopausal women, who are prone to accelerated bone loss. Targeting a TSH level in the midnormal range (0.5-2.5 mIU/L) will help the clinician avoid overtreatment complications; if symptoms persist, other causes should be sought.
For persistent symptomatology in the presence of normal thyroid test results, clinicians should consider other potential causes.
Combination T4/T3 Treatment
Liothyronine is bioequivalent to T3 in the body. It is not recommended as a primary agent except in cases of thyroid suppression requiring quick reversal; its half-life is only approximately 18 hours. This short half-life makes it more difficult to monitor because T3 levels can vary substantially throughout the day.3
Recent media attention has focused on combination levothyroxine-liothyronine treatment for hypothyroidism, and patients may inquire about this treatment option. The healthy thyroid gland produces these hormones in a ratio of approximately 13:1; most of the active T3 in the body results from T4 to T3 conversion in the peripheral tissues.3
But is combination therapy an evidence-based treatment option for hypothyroidism? The ATA/AACE joint guidelines indicate that there is inadequate evidence to support the use of levothyroxine and liothyronine combinations to treat hypothyroidism. This recommendation was downgraded from Grade A to Grade B in the current guidelines.4 This is because a few studies suggest that some patients report feeling better on T4/T3 combinations, and it is possible that some patient subgroups may benefit from combination treatment.4 There are no data that clearly identify these subgroups, and it is unknown precisely why some patients report improvement; further research is required.4 Combination therapy is not recommended for pregnant women or those planning pregnancy because of the potential for harm to the fetus.4
Patients sometimes request a more “natural” treatment for hypothyroidism, and animal-derived desiccated thyroid is the one most often prescribed.4 The two commonly used forms of desiccated thyroid are porcine in origin. Each is a levothyroxine-liothyronine combination in a ratio of approximately 4:1. While a recent randomized, double-blind, crossover study compared desiccated thyroid extract (DTE) to levothyroxine treatment and found that 48.6% of study subjects preferred DTE therapy, the authors concluded that “DTE therapy may be relevant for some hypothyroid patients” without defining the characteristics of those patients.10
In addition, because desiccated thyroid is derived from a tissue product, there can be variability in dosing that may make it challenging to reach treatment goals consistently. Inquiring vegan and vegetarian patients would also need to be advised of the animal origin of desiccated thyroid.
Treatment When Tests are Normal
Clinicians commonly encounter patients who request treatment for hypothyroid symptoms in the absence of laboratory evidence of hypothyroidism. Although hypothyroid symptoms are common, vague, and nonspecific and pinpointing their precise etiology may be difficult, there is no benefit in treating patients for hypothyroidism when thyroid test results are normal. In fact, treatment may be harmful, as there is substantial risk for subclinical or overt hyperthyroidism.4
Generic vs Brand-Name Levothyroxine
Both generic and brand-name preparations of levothyroxine are available. Generic formulations are made by a variety of manufacturers, and formulations can vary in production; a brand name assures one manufacturer and consistency in production. It is difficult to accurately assess the bioequivalence and, therefore, the interchangeability, of the various manufacturers’ generic formulations.4 Differences in bioavailability of the drug may affect the dose the patient receives. Minor fluctuations may occur in thyroid function test results, which may or may not be clinically acceptable in an individual patient. Therefore, the current consensus encourages the use of a consistent levothyroxine preparation for individual patients to minimize variability from refill to refill.4 For patients for whom medication cost is a key factor, generic formulations can be considerably less expensive than their brand-name counterparts.
FOLLOW-UP TESTING
For primary hypothyroidism, the frequency of follow-up is dictated by symptoms and laboratory test values. Patients should be advised that symptoms will improve with treatment but that this improvement may not be noticeable for three to six months, even after TSH levels have reached the normal range.4
Thyroid function testing is typically repeated at four to eight weeks to assess initial dose titration; once an adequate replacement dose has been reached, testing may be repeated at six months and then annually thereafter unless symptoms arise.4 In pregnant women, TSH and FT4 levels should be measured every four weeks during the first half of pregnancy and at least once between 26 and 32 weeks’ gestation (levothyroxine dose requirements typically increase by 20% to 50% during pregnancy).4
Patients with secondary hypothyroidism should be referred for an endocrinology consultation for evaluation of their general pituitary or hypothalamic function.
Changes in Dose Requirements
Dose requirements may change over time in any given patient. Underlying thyroid function may wane, and any absorptive issues secondary to other diseases, such as celiac disease, may alter dose requirements. In pregnancy, dose requirements increase but generally revert back to baseline postpartum.4 Any addition or discontinuation of medications that affect plasma binding or metabolism will alter thyroid dosing.4 Increasing age or weight loss may require decreases in dosing. After any dose adjustment, thyroid function test results should be reevaluated in four to eight weeks, with the same follow-up schedule for repeat testing as after initiation of hypothyroidism treatment.
On the next page: Patient education and conclusion >>
PATIENT EDUCATION
Patient education should focus on treatment and follow-up. Patients need to be told that treatment is lifelong, and it may be helpful to describe levothyroxine as a replacement for their thyroid hormone, rather than as a treatment of their thyroid disease. They should be reminded that they will need to undergo repeat thyroid function testing periodically to assess their dose. While their dose may need to be adjusted over time, typically TSH levels will stabilize and laboratory testing will only be necessary every 12 months.4
Clinicians should also review with patients how and when to take levothyroxine. One tablet should be taken daily, with water, on an empty stomach, at least 30 to 60 minutes before eating or drinking or four hours after eating or drinking. Multivitamins or other supplements, especially those containing calcium and iron, should be taken at least four hours after taking levothyroxine. These are necessary requirements because both food and minerals can decrease absorption of the medication.4
Patients should also be informed that if they miss a dose of medication, two tablets may be taken the next day, but consistency in daily dosing is the goal. In patients with significant compliance problems, weekly dosing of levothyroxine results in similar safety, treatment outcomes, and TSH values as daily dosing.11
Finally, symptoms of both hypothyroidism and hyperthyroidism should be described and reviewed. Patients should be advised to notify their health care providers should any of these symptoms occur.
CONCLUSION
Hypothyroidism is a common illness encountered in the primary care setting. Clinicians must be familiar with the signs and symptoms, as well as the risk factors for the disease, because many patients have minimal symptomatology. Appropriate laboratory testing will clarify the diagnosis.
Patients with symptomatic hypothyroidism confirmed on laboratory testing should be treated. The benefit of treatment in other subgroups is less clear and should be guided by current evidence-based guidelines. The mainstay of medical management for hypothyroidism is levothyroxine; other treatment options require further research. Well-informed patients are key to effective management of hypothyroidism.
1. Golden SH, Robinson KA, Saldanha I, et al. Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
2. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4) and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
3. Gardner D, Shoback D. Thyroid gland. In: Greenspan’s Basic and Clinical Endocrinology. 9th ed. New York, NY: McGraw-Hill Medical; 2011:
163-226.
4. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028. www.aace.com/files/final-file-hypo-guidelines.pdf. Accessed June 18, 2014.
5. Negro R, Schwarz A, Gismondi R, et al. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab. 2011;96(6):E920-E924.
6. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction [published correction appears in Arch Intern Med. 2001;161(2):284]. Arch Intern Med. 2000;160(11):1573-1575.
7. Baskin HJ, Cobin RH, Duick DS, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism [published correction appears in Endocr Pract. 2008;14(6):802-803]. Endocr Pract. 2002;8(6):457-469.
8. Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365-1374.
9. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526-534.
10. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):
1982-1990.
11. Grebe SK, Cooke RR, Ford HC, et al. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82(3):870-875.
CE/CME No: CR-1407
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify the signs and symptoms of and risk factors for hypothyroidism.
• Name the laboratory tests used to diagnose or rule out hypothyroidism.
• Describe current evidence-based and alternative treatments for hypothyroidism.
• Discuss appropriate long-term follow-up and monitoring of hypothyroidism.
• Instruct patients regarding optimal self-management of hypothyroidism.
FACULTY
Kara-anne Gregory Curl is an Adjunct Clinical Instructor of Medicine, Division of Endocrinology and Metabolism, with Medical Faculty Associates at George Washington University in Washington, DC.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Although hypothyroidism is common, its typically vague symptoms of fatigue, lack of energy, and weight gain are shared with many other conditions. Awareness of risk factors for hypothyroidism will aid in the differential diagnosis, and the patient’s symptoms can help guide the clinician to the appropriate diagnostic workup. Thyroid function test results are necessary to confirm or rule out the diagnosis.
Approximately 4.6% of the US population ages 12 and older has been diagnosed with hypothyroidism, making it the most frequently diagnosed thyroid disorder.1 Hypothyroidism is defined by an underproduction of thyroid hormones and can be either primary or secondary. Primary hypothyroidism is caused by the failure of the thyroid gland to produce adequate quantities of the hormones triiodothyronine (T3) and levorotatory thyroxine (T4). Secondary (central) hypothyroidism is a result of inadequate production of thyrotropin (TSH) by the pituitary gland; less often, it is caused by inadequate production of thyrotropin-releasing hormone by the hypothalamus. The great majority of patients with hypothyroidism have the primary form of the disease. In the US, the most common cause of
hypothyroidism is chronic autoimmune thyroiditis (Hashimoto thyroiditis); worldwide, it is iodine deficiency.
Because hypothyroid symptoms are vague, the disease can be difficult to diagnose. Patients most often present with complaints of fatigue and lack of energy, raising suspicion for hypothyroidism; but true symptomatic, overt hypothyroidism is rather rare, occurring in only 0.3% of the population.2
Subclinical hypothyroidism, in which TSH levels are elevated but T3 and T4 levels are normal and the patient experiences few, if any, symptoms, is much more common. This makes the use of diagnostic laboratory tests essential; hypothyroidism cannot be diagnosed based on clinical presentation alone. Signs and symptoms may include fatigue, lack of energy, cold intolerance, weight gain, thinning hair/hair loss, dry skin, constipation, menstrual cycle abnormalities, irritability, and depression. Bradycardia and hypotension are also possible. Physical examination may reveal depressed affect, eyelid edema, loss of lateral third of the eyebrow, thickened tongue, dry skin, and hyporeflexivity; however, most patients will have a benign and unrevealing physical examination.3
Hypothyroidism is more common in white people and in women and increases in incidence after age 60. Risk factors for hypothyroidism include
• Family history
• Autoimmune disorders (eg, type 1 diabetes, Addison disease)
• History of Graves disease treated with radioactive iodine or thyroidectomy
• Past external beam radiotherapy for head and neck malignancies
• Postpartum thyroiditis
• Turner and Down syndromes
• Multiple sclerosis
• Amiodarone or lithium use
• Iodine deficiency or previous residence in an iodine-deficient area.4
In addition, testing for hypothyroidism may be warranted in patients with any of the diagnoses in Table 1.
Because of its vague symptomatology, hypothyroidism can mimic many disease processes. However, the most pertinent include depression, anemia, and dementia/Alzheimer disease. Laboratory testing will almost always identify hypothyroidism.
On the next page: Laboratory workup >>
LABORATORY WORKUP
Thyrotropin
The diagnosis of hypothyroidism is based on the results of the TSH test, which is the primary screening test for thyroid dysfunction.4 TSH secretion is extremely sensitive to minor increases and decreases in T3 or T4, making it the most reliable laboratory test for the assessment of thyroid function. An elevated serum TSH level in the presence of hypothyroid symptoms is diagnostic of primary hypothyroidism. Other causes of elevated TSH, such as thyrotropin-secreting pituitary tumors, are rare, and their symptomatology is different.3
Free Levorotatory Thyroxine
Though elevated TSH levels occur before T4 abnormalities are detected, T4 measurement can sometimes be useful in the diagnosis of hypothyroidism, especially in cases of possible central hypothyroidism. As a diagnostic test, measurement of serum free T4 (FT4) is preferable to total T4 because T4 binds to specific proteins in serum, making obtaining an accurate total T4 level subject to factors that alter binding. By contrast, FT4, the metabolically active form of the hormone, is not affected by binding factors. In primary hypothyroidism, FT4 is low or normal.4
Measurement of the FT4 level will also confirm the diagnosis of central hypothyroidism, if the FT4 is low when TSH is normal or low. As FT4 decreases, the TSH should elevate to compensate; in the presence of a low FT4, even a normal TSH is indicative of hypothyroidism. In a patient with overt hypothyroid symptoms with a normal TSH, an FT4 should be ordered for further workup.4
Triiodothyronine
Measurement of the serum T3 level, whether total or free, is of little clinical utility because it often remains normal, even as TSH and T4 levels change.4
Typical diagnostic test results in primary and secondary hypothyroidism are summarized in Table 2.
Other Factors That May Affect Thyroid Function Test Results
The overall health status of the patient must be considered when evaluating the results of thyroid function tests because the results can be affected by other factors.
• Serum TSH may be low, often in combination with low FT4, in hospitalized patients with acute illness.
• TSH may increase to levels above normal during recovery from nonthyroid-related illness.
• Serum TSH typically falls (infrequently to below 0.1 mlU/L) during the first trimester of pregnancy due to the stimulatory effects of human chorionic gonadotropin on the thyroid.5 Levels typically return to normal in the second trimester.
• TSH and FT4 can be altered in the postpartum period secondary to postpartum thyroiditis. Levels will often resolve on their own without treatment.
• Patients with anorexia nervosa may have low TSH levels as well as low levels of FT4 secondary to pituitary and hypothalamic dysfunction.
• Mild TSH elevations may also be a normal manifestation of aging; TSH values above 3.0 mlU/L occur with increasing frequency with age.4
Thyroid Peroxidase Antibodies
Testing the patient for thyroid peroxidase (TPO) antibodies, although not required to make the hypothyroidism diagnosis, may provide additional useful information. A positive TPO antibody result is significantly associated with hypothyroidism; in particular, TPO antibodies are more likely to be present in patients with autoimmune thyroiditis, helping to confirm the diagnosis.2,4 However, positive antibody test results do not change clinical management decisions. Results will remain positive during treatment, and the continued presence of antibodies warrants no alteration in treatment or medication dose.
An elevated TPO antibody level does impart a risk for future transition to overt hypothyroidism, so this test is recommended for patients with subclinical hypothyroidism. In addition, for patients with other autoimmune diseases, such as type 1 diabetes or Addison disease, or with chromosomal disorders, such as Down or Turner syndromes, TPO antibodies suggest a propensity toward hypothyroidism. Current research also indicates that both pregnancy rates and pregnancy outcomes improve when TPO antibody–positive patients whose TSH levels are above 2.5 mlU/L are treated.5
On the next page: Diagnosis and treatment >>
DIAGNOSIS
The diagnosis of hypothyroidism is made on the basis of laboratory test results, but symptomatology can help guide the clinician to the appropriate laboratory workup. Symptoms alone, when laboratory values are within normal limits or even at the high end of the normal range, do not support a hypothyroidism diagnosis. In such a case, a differential diagnosis should be pursued.
Since overt symptoms of hypothyroidism are rare, clinicians may wonder if they should screen all their patients for hypothyroidism. Current recommendations, as set forth in the joint American Thyroid Association (ATA) and American Association of Clinical Endocrinologists (AACE) clinical practice guidelines, support “aggressive case finding” rather than universal screening because as yet, there is no consensus on screening guidelines.4 The ATA recommends screening all adults at age 35 and then every five years thereafter.6 In contrast, the AACE recommends routine TSH measurement in “older” patients, particularly women.7
There is, however, compelling evidence for testing patients with any of the following4
• An autoimmune disease
• Pernicious anemia
• A first-degree relative with autoimmune thyroid disease
• An abnormal thyroid examination
• Past radiation to the thyroid gland, including radioactive iodine therapy for hyperthyroidism
• Past external beam radiotherapy for head and neck malignancies
• History of thyroid surgery
• History of thyroid dysfunction
• One of the diagnoses listed in Table 1.
It is also suggested that patients with psychiatric disorders and patients taking amiodarone or lithium be screened.4
TREATMENT
The standard evidence-based treatment for hypothyroidism is hormone replacement with levothyroxine.4 This is a Grade A recommendation in the ATA/AACE joint guidelines. Levothyroxine is bioequivalent to T4 in the body and has a half-life of approximately six to seven days. It is stable and easily adjusted by monitoring TSH levels; adverse reactions or complications are minimal. The starting dose of levothyroxine is 1.6 µg/kg/d for both primary and secondary (central) hypothyroidism.
Dose increases are made in 12.5 or 25 µg increments. In primary hypothyroidism, TSH levels should be monitored to determine the need for dose adjustments. FT4 need not be checked unless there is a discrepancy in the TSH levels.4 In secondary hypothyroidism, TSH levels will always remain normal or low, and FT4 should be used to monitor therapy. Levels should be measured four to eight weeks after initiation of treatment or after subsequent dose adjustments.
Overt Hypothyroidism (Primary or Secondary)
All patients with symptomatic, overt hypothyroidism and an elevated TSH level should be treated. Treatment is lifelong, and the goal is to reduce patient symptomatology, improve well-being, and prevent complications. The treatment target is a TSH level in the normal range, approximately 0.45 to 4.5 mIU/L on most laboratory assays. However, NHANES III data revealed that the mean serum TSH level in the normal population is 1.5 mlU/L.2 Based on this fact and on their experience, many clinicians would argue that a more appropriate goal is a TSH target in the midnormal range, such as 0.5 to 2.5 mlU/L. There is little evidence to support a low- or subnormal TSH target in the treatment of hypothyroidism.4
Subclinical Hypothyroidism
For patients with the more common subclinical hypothyroidism (an elevated TSH level without symptoms), the benefits of treatment are less clear. It is suggested that if the TSH level is > 10 mIU/L, even patients without symptoms should be treated because risk for overt hypothyroidism is high.8 A TSH level > 10 mIU/L has also been shown to increase the patient’s coronary artery disease (CAD) risk.8 For individuals with TSH levels in the 4.5 to 10 mIU/L range who feel well and have no hypothyroid symptoms, the evidence is less clear. Treatment may be beneficial, but this has not been determined definitively.4 A watch-and-wait approach may be taken with these patients; if symptoms develop, treatment should be considered.
Patients with subclinical hypothyroidism generally do not require full replacement doses (ie, a starting dosage of 1.6 µg/kg/d). A dosage of 25 to 75 µg/d is typically sufficient to achieve goal TSH levels. For patients older than 60 with no CAD and for all patients with CAD, lower starting dosages of 50 µg/d and 12.5 to 25 µg/d, respectively, are recommended.4
Normal TSH, Positive TPO in Pregnancy
Current clinical evidence does not support treatment of patients who have normal TSH levels (2.5 to 4.5 mIU/L) but who are positive for TPO antibodies.4 The exception is pregnant women or those considering pregnancy. Research suggests that the rates of spontaneous miscarriage and preterm labor are higher in TPO-positive women in this TSH range, so treatment may reduce these risks.4
On the next page: Additional treatment considerations >>
ADDITIONAL TREATMENT CONSIDERATIONS
Overtreatment
The main complication of hypothyroidism treatment occurs when the patient receives more thyroid hormone than is required, which has been reported in 20% of cases.9 The primary risks of overtreatment are osteoporosis and atrial fibrillation. Care should be taken especially in vulnerable populations such as the elderly, who are particularly susceptible to atrial fibrillation, and postmenopausal women, who are prone to accelerated bone loss. Targeting a TSH level in the midnormal range (0.5-2.5 mIU/L) will help the clinician avoid overtreatment complications; if symptoms persist, other causes should be sought.
For persistent symptomatology in the presence of normal thyroid test results, clinicians should consider other potential causes.
Combination T4/T3 Treatment
Liothyronine is bioequivalent to T3 in the body. It is not recommended as a primary agent except in cases of thyroid suppression requiring quick reversal; its half-life is only approximately 18 hours. This short half-life makes it more difficult to monitor because T3 levels can vary substantially throughout the day.3
Recent media attention has focused on combination levothyroxine-liothyronine treatment for hypothyroidism, and patients may inquire about this treatment option. The healthy thyroid gland produces these hormones in a ratio of approximately 13:1; most of the active T3 in the body results from T4 to T3 conversion in the peripheral tissues.3
But is combination therapy an evidence-based treatment option for hypothyroidism? The ATA/AACE joint guidelines indicate that there is inadequate evidence to support the use of levothyroxine and liothyronine combinations to treat hypothyroidism. This recommendation was downgraded from Grade A to Grade B in the current guidelines.4 This is because a few studies suggest that some patients report feeling better on T4/T3 combinations, and it is possible that some patient subgroups may benefit from combination treatment.4 There are no data that clearly identify these subgroups, and it is unknown precisely why some patients report improvement; further research is required.4 Combination therapy is not recommended for pregnant women or those planning pregnancy because of the potential for harm to the fetus.4
Patients sometimes request a more “natural” treatment for hypothyroidism, and animal-derived desiccated thyroid is the one most often prescribed.4 The two commonly used forms of desiccated thyroid are porcine in origin. Each is a levothyroxine-liothyronine combination in a ratio of approximately 4:1. While a recent randomized, double-blind, crossover study compared desiccated thyroid extract (DTE) to levothyroxine treatment and found that 48.6% of study subjects preferred DTE therapy, the authors concluded that “DTE therapy may be relevant for some hypothyroid patients” without defining the characteristics of those patients.10
In addition, because desiccated thyroid is derived from a tissue product, there can be variability in dosing that may make it challenging to reach treatment goals consistently. Inquiring vegan and vegetarian patients would also need to be advised of the animal origin of desiccated thyroid.
Treatment When Tests are Normal
Clinicians commonly encounter patients who request treatment for hypothyroid symptoms in the absence of laboratory evidence of hypothyroidism. Although hypothyroid symptoms are common, vague, and nonspecific and pinpointing their precise etiology may be difficult, there is no benefit in treating patients for hypothyroidism when thyroid test results are normal. In fact, treatment may be harmful, as there is substantial risk for subclinical or overt hyperthyroidism.4
Generic vs Brand-Name Levothyroxine
Both generic and brand-name preparations of levothyroxine are available. Generic formulations are made by a variety of manufacturers, and formulations can vary in production; a brand name assures one manufacturer and consistency in production. It is difficult to accurately assess the bioequivalence and, therefore, the interchangeability, of the various manufacturers’ generic formulations.4 Differences in bioavailability of the drug may affect the dose the patient receives. Minor fluctuations may occur in thyroid function test results, which may or may not be clinically acceptable in an individual patient. Therefore, the current consensus encourages the use of a consistent levothyroxine preparation for individual patients to minimize variability from refill to refill.4 For patients for whom medication cost is a key factor, generic formulations can be considerably less expensive than their brand-name counterparts.
FOLLOW-UP TESTING
For primary hypothyroidism, the frequency of follow-up is dictated by symptoms and laboratory test values. Patients should be advised that symptoms will improve with treatment but that this improvement may not be noticeable for three to six months, even after TSH levels have reached the normal range.4
Thyroid function testing is typically repeated at four to eight weeks to assess initial dose titration; once an adequate replacement dose has been reached, testing may be repeated at six months and then annually thereafter unless symptoms arise.4 In pregnant women, TSH and FT4 levels should be measured every four weeks during the first half of pregnancy and at least once between 26 and 32 weeks’ gestation (levothyroxine dose requirements typically increase by 20% to 50% during pregnancy).4
Patients with secondary hypothyroidism should be referred for an endocrinology consultation for evaluation of their general pituitary or hypothalamic function.
Changes in Dose Requirements
Dose requirements may change over time in any given patient. Underlying thyroid function may wane, and any absorptive issues secondary to other diseases, such as celiac disease, may alter dose requirements. In pregnancy, dose requirements increase but generally revert back to baseline postpartum.4 Any addition or discontinuation of medications that affect plasma binding or metabolism will alter thyroid dosing.4 Increasing age or weight loss may require decreases in dosing. After any dose adjustment, thyroid function test results should be reevaluated in four to eight weeks, with the same follow-up schedule for repeat testing as after initiation of hypothyroidism treatment.
On the next page: Patient education and conclusion >>
PATIENT EDUCATION
Patient education should focus on treatment and follow-up. Patients need to be told that treatment is lifelong, and it may be helpful to describe levothyroxine as a replacement for their thyroid hormone, rather than as a treatment of their thyroid disease. They should be reminded that they will need to undergo repeat thyroid function testing periodically to assess their dose. While their dose may need to be adjusted over time, typically TSH levels will stabilize and laboratory testing will only be necessary every 12 months.4
Clinicians should also review with patients how and when to take levothyroxine. One tablet should be taken daily, with water, on an empty stomach, at least 30 to 60 minutes before eating or drinking or four hours after eating or drinking. Multivitamins or other supplements, especially those containing calcium and iron, should be taken at least four hours after taking levothyroxine. These are necessary requirements because both food and minerals can decrease absorption of the medication.4
Patients should also be informed that if they miss a dose of medication, two tablets may be taken the next day, but consistency in daily dosing is the goal. In patients with significant compliance problems, weekly dosing of levothyroxine results in similar safety, treatment outcomes, and TSH values as daily dosing.11
Finally, symptoms of both hypothyroidism and hyperthyroidism should be described and reviewed. Patients should be advised to notify their health care providers should any of these symptoms occur.
CONCLUSION
Hypothyroidism is a common illness encountered in the primary care setting. Clinicians must be familiar with the signs and symptoms, as well as the risk factors for the disease, because many patients have minimal symptomatology. Appropriate laboratory testing will clarify the diagnosis.
Patients with symptomatic hypothyroidism confirmed on laboratory testing should be treated. The benefit of treatment in other subgroups is less clear and should be guided by current evidence-based guidelines. The mainstay of medical management for hypothyroidism is levothyroxine; other treatment options require further research. Well-informed patients are key to effective management of hypothyroidism.
CE/CME No: CR-1407
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify the signs and symptoms of and risk factors for hypothyroidism.
• Name the laboratory tests used to diagnose or rule out hypothyroidism.
• Describe current evidence-based and alternative treatments for hypothyroidism.
• Discuss appropriate long-term follow-up and monitoring of hypothyroidism.
• Instruct patients regarding optimal self-management of hypothyroidism.
FACULTY
Kara-anne Gregory Curl is an Adjunct Clinical Instructor of Medicine, Division of Endocrinology and Metabolism, with Medical Faculty Associates at George Washington University in Washington, DC.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Although hypothyroidism is common, its typically vague symptoms of fatigue, lack of energy, and weight gain are shared with many other conditions. Awareness of risk factors for hypothyroidism will aid in the differential diagnosis, and the patient’s symptoms can help guide the clinician to the appropriate diagnostic workup. Thyroid function test results are necessary to confirm or rule out the diagnosis.
Approximately 4.6% of the US population ages 12 and older has been diagnosed with hypothyroidism, making it the most frequently diagnosed thyroid disorder.1 Hypothyroidism is defined by an underproduction of thyroid hormones and can be either primary or secondary. Primary hypothyroidism is caused by the failure of the thyroid gland to produce adequate quantities of the hormones triiodothyronine (T3) and levorotatory thyroxine (T4). Secondary (central) hypothyroidism is a result of inadequate production of thyrotropin (TSH) by the pituitary gland; less often, it is caused by inadequate production of thyrotropin-releasing hormone by the hypothalamus. The great majority of patients with hypothyroidism have the primary form of the disease. In the US, the most common cause of
hypothyroidism is chronic autoimmune thyroiditis (Hashimoto thyroiditis); worldwide, it is iodine deficiency.
Because hypothyroid symptoms are vague, the disease can be difficult to diagnose. Patients most often present with complaints of fatigue and lack of energy, raising suspicion for hypothyroidism; but true symptomatic, overt hypothyroidism is rather rare, occurring in only 0.3% of the population.2
Subclinical hypothyroidism, in which TSH levels are elevated but T3 and T4 levels are normal and the patient experiences few, if any, symptoms, is much more common. This makes the use of diagnostic laboratory tests essential; hypothyroidism cannot be diagnosed based on clinical presentation alone. Signs and symptoms may include fatigue, lack of energy, cold intolerance, weight gain, thinning hair/hair loss, dry skin, constipation, menstrual cycle abnormalities, irritability, and depression. Bradycardia and hypotension are also possible. Physical examination may reveal depressed affect, eyelid edema, loss of lateral third of the eyebrow, thickened tongue, dry skin, and hyporeflexivity; however, most patients will have a benign and unrevealing physical examination.3
Hypothyroidism is more common in white people and in women and increases in incidence after age 60. Risk factors for hypothyroidism include
• Family history
• Autoimmune disorders (eg, type 1 diabetes, Addison disease)
• History of Graves disease treated with radioactive iodine or thyroidectomy
• Past external beam radiotherapy for head and neck malignancies
• Postpartum thyroiditis
• Turner and Down syndromes
• Multiple sclerosis
• Amiodarone or lithium use
• Iodine deficiency or previous residence in an iodine-deficient area.4
In addition, testing for hypothyroidism may be warranted in patients with any of the diagnoses in Table 1.
Because of its vague symptomatology, hypothyroidism can mimic many disease processes. However, the most pertinent include depression, anemia, and dementia/Alzheimer disease. Laboratory testing will almost always identify hypothyroidism.
On the next page: Laboratory workup >>
LABORATORY WORKUP
Thyrotropin
The diagnosis of hypothyroidism is based on the results of the TSH test, which is the primary screening test for thyroid dysfunction.4 TSH secretion is extremely sensitive to minor increases and decreases in T3 or T4, making it the most reliable laboratory test for the assessment of thyroid function. An elevated serum TSH level in the presence of hypothyroid symptoms is diagnostic of primary hypothyroidism. Other causes of elevated TSH, such as thyrotropin-secreting pituitary tumors, are rare, and their symptomatology is different.3
Free Levorotatory Thyroxine
Though elevated TSH levels occur before T4 abnormalities are detected, T4 measurement can sometimes be useful in the diagnosis of hypothyroidism, especially in cases of possible central hypothyroidism. As a diagnostic test, measurement of serum free T4 (FT4) is preferable to total T4 because T4 binds to specific proteins in serum, making obtaining an accurate total T4 level subject to factors that alter binding. By contrast, FT4, the metabolically active form of the hormone, is not affected by binding factors. In primary hypothyroidism, FT4 is low or normal.4
Measurement of the FT4 level will also confirm the diagnosis of central hypothyroidism, if the FT4 is low when TSH is normal or low. As FT4 decreases, the TSH should elevate to compensate; in the presence of a low FT4, even a normal TSH is indicative of hypothyroidism. In a patient with overt hypothyroid symptoms with a normal TSH, an FT4 should be ordered for further workup.4
Triiodothyronine
Measurement of the serum T3 level, whether total or free, is of little clinical utility because it often remains normal, even as TSH and T4 levels change.4
Typical diagnostic test results in primary and secondary hypothyroidism are summarized in Table 2.
Other Factors That May Affect Thyroid Function Test Results
The overall health status of the patient must be considered when evaluating the results of thyroid function tests because the results can be affected by other factors.
• Serum TSH may be low, often in combination with low FT4, in hospitalized patients with acute illness.
• TSH may increase to levels above normal during recovery from nonthyroid-related illness.
• Serum TSH typically falls (infrequently to below 0.1 mlU/L) during the first trimester of pregnancy due to the stimulatory effects of human chorionic gonadotropin on the thyroid.5 Levels typically return to normal in the second trimester.
• TSH and FT4 can be altered in the postpartum period secondary to postpartum thyroiditis. Levels will often resolve on their own without treatment.
• Patients with anorexia nervosa may have low TSH levels as well as low levels of FT4 secondary to pituitary and hypothalamic dysfunction.
• Mild TSH elevations may also be a normal manifestation of aging; TSH values above 3.0 mlU/L occur with increasing frequency with age.4
Thyroid Peroxidase Antibodies
Testing the patient for thyroid peroxidase (TPO) antibodies, although not required to make the hypothyroidism diagnosis, may provide additional useful information. A positive TPO antibody result is significantly associated with hypothyroidism; in particular, TPO antibodies are more likely to be present in patients with autoimmune thyroiditis, helping to confirm the diagnosis.2,4 However, positive antibody test results do not change clinical management decisions. Results will remain positive during treatment, and the continued presence of antibodies warrants no alteration in treatment or medication dose.
An elevated TPO antibody level does impart a risk for future transition to overt hypothyroidism, so this test is recommended for patients with subclinical hypothyroidism. In addition, for patients with other autoimmune diseases, such as type 1 diabetes or Addison disease, or with chromosomal disorders, such as Down or Turner syndromes, TPO antibodies suggest a propensity toward hypothyroidism. Current research also indicates that both pregnancy rates and pregnancy outcomes improve when TPO antibody–positive patients whose TSH levels are above 2.5 mlU/L are treated.5
On the next page: Diagnosis and treatment >>
DIAGNOSIS
The diagnosis of hypothyroidism is made on the basis of laboratory test results, but symptomatology can help guide the clinician to the appropriate laboratory workup. Symptoms alone, when laboratory values are within normal limits or even at the high end of the normal range, do not support a hypothyroidism diagnosis. In such a case, a differential diagnosis should be pursued.
Since overt symptoms of hypothyroidism are rare, clinicians may wonder if they should screen all their patients for hypothyroidism. Current recommendations, as set forth in the joint American Thyroid Association (ATA) and American Association of Clinical Endocrinologists (AACE) clinical practice guidelines, support “aggressive case finding” rather than universal screening because as yet, there is no consensus on screening guidelines.4 The ATA recommends screening all adults at age 35 and then every five years thereafter.6 In contrast, the AACE recommends routine TSH measurement in “older” patients, particularly women.7
There is, however, compelling evidence for testing patients with any of the following4
• An autoimmune disease
• Pernicious anemia
• A first-degree relative with autoimmune thyroid disease
• An abnormal thyroid examination
• Past radiation to the thyroid gland, including radioactive iodine therapy for hyperthyroidism
• Past external beam radiotherapy for head and neck malignancies
• History of thyroid surgery
• History of thyroid dysfunction
• One of the diagnoses listed in Table 1.
It is also suggested that patients with psychiatric disorders and patients taking amiodarone or lithium be screened.4
TREATMENT
The standard evidence-based treatment for hypothyroidism is hormone replacement with levothyroxine.4 This is a Grade A recommendation in the ATA/AACE joint guidelines. Levothyroxine is bioequivalent to T4 in the body and has a half-life of approximately six to seven days. It is stable and easily adjusted by monitoring TSH levels; adverse reactions or complications are minimal. The starting dose of levothyroxine is 1.6 µg/kg/d for both primary and secondary (central) hypothyroidism.
Dose increases are made in 12.5 or 25 µg increments. In primary hypothyroidism, TSH levels should be monitored to determine the need for dose adjustments. FT4 need not be checked unless there is a discrepancy in the TSH levels.4 In secondary hypothyroidism, TSH levels will always remain normal or low, and FT4 should be used to monitor therapy. Levels should be measured four to eight weeks after initiation of treatment or after subsequent dose adjustments.
Overt Hypothyroidism (Primary or Secondary)
All patients with symptomatic, overt hypothyroidism and an elevated TSH level should be treated. Treatment is lifelong, and the goal is to reduce patient symptomatology, improve well-being, and prevent complications. The treatment target is a TSH level in the normal range, approximately 0.45 to 4.5 mIU/L on most laboratory assays. However, NHANES III data revealed that the mean serum TSH level in the normal population is 1.5 mlU/L.2 Based on this fact and on their experience, many clinicians would argue that a more appropriate goal is a TSH target in the midnormal range, such as 0.5 to 2.5 mlU/L. There is little evidence to support a low- or subnormal TSH target in the treatment of hypothyroidism.4
Subclinical Hypothyroidism
For patients with the more common subclinical hypothyroidism (an elevated TSH level without symptoms), the benefits of treatment are less clear. It is suggested that if the TSH level is > 10 mIU/L, even patients without symptoms should be treated because risk for overt hypothyroidism is high.8 A TSH level > 10 mIU/L has also been shown to increase the patient’s coronary artery disease (CAD) risk.8 For individuals with TSH levels in the 4.5 to 10 mIU/L range who feel well and have no hypothyroid symptoms, the evidence is less clear. Treatment may be beneficial, but this has not been determined definitively.4 A watch-and-wait approach may be taken with these patients; if symptoms develop, treatment should be considered.
Patients with subclinical hypothyroidism generally do not require full replacement doses (ie, a starting dosage of 1.6 µg/kg/d). A dosage of 25 to 75 µg/d is typically sufficient to achieve goal TSH levels. For patients older than 60 with no CAD and for all patients with CAD, lower starting dosages of 50 µg/d and 12.5 to 25 µg/d, respectively, are recommended.4
Normal TSH, Positive TPO in Pregnancy
Current clinical evidence does not support treatment of patients who have normal TSH levels (2.5 to 4.5 mIU/L) but who are positive for TPO antibodies.4 The exception is pregnant women or those considering pregnancy. Research suggests that the rates of spontaneous miscarriage and preterm labor are higher in TPO-positive women in this TSH range, so treatment may reduce these risks.4
On the next page: Additional treatment considerations >>
ADDITIONAL TREATMENT CONSIDERATIONS
Overtreatment
The main complication of hypothyroidism treatment occurs when the patient receives more thyroid hormone than is required, which has been reported in 20% of cases.9 The primary risks of overtreatment are osteoporosis and atrial fibrillation. Care should be taken especially in vulnerable populations such as the elderly, who are particularly susceptible to atrial fibrillation, and postmenopausal women, who are prone to accelerated bone loss. Targeting a TSH level in the midnormal range (0.5-2.5 mIU/L) will help the clinician avoid overtreatment complications; if symptoms persist, other causes should be sought.
For persistent symptomatology in the presence of normal thyroid test results, clinicians should consider other potential causes.
Combination T4/T3 Treatment
Liothyronine is bioequivalent to T3 in the body. It is not recommended as a primary agent except in cases of thyroid suppression requiring quick reversal; its half-life is only approximately 18 hours. This short half-life makes it more difficult to monitor because T3 levels can vary substantially throughout the day.3
Recent media attention has focused on combination levothyroxine-liothyronine treatment for hypothyroidism, and patients may inquire about this treatment option. The healthy thyroid gland produces these hormones in a ratio of approximately 13:1; most of the active T3 in the body results from T4 to T3 conversion in the peripheral tissues.3
But is combination therapy an evidence-based treatment option for hypothyroidism? The ATA/AACE joint guidelines indicate that there is inadequate evidence to support the use of levothyroxine and liothyronine combinations to treat hypothyroidism. This recommendation was downgraded from Grade A to Grade B in the current guidelines.4 This is because a few studies suggest that some patients report feeling better on T4/T3 combinations, and it is possible that some patient subgroups may benefit from combination treatment.4 There are no data that clearly identify these subgroups, and it is unknown precisely why some patients report improvement; further research is required.4 Combination therapy is not recommended for pregnant women or those planning pregnancy because of the potential for harm to the fetus.4
Patients sometimes request a more “natural” treatment for hypothyroidism, and animal-derived desiccated thyroid is the one most often prescribed.4 The two commonly used forms of desiccated thyroid are porcine in origin. Each is a levothyroxine-liothyronine combination in a ratio of approximately 4:1. While a recent randomized, double-blind, crossover study compared desiccated thyroid extract (DTE) to levothyroxine treatment and found that 48.6% of study subjects preferred DTE therapy, the authors concluded that “DTE therapy may be relevant for some hypothyroid patients” without defining the characteristics of those patients.10
In addition, because desiccated thyroid is derived from a tissue product, there can be variability in dosing that may make it challenging to reach treatment goals consistently. Inquiring vegan and vegetarian patients would also need to be advised of the animal origin of desiccated thyroid.
Treatment When Tests are Normal
Clinicians commonly encounter patients who request treatment for hypothyroid symptoms in the absence of laboratory evidence of hypothyroidism. Although hypothyroid symptoms are common, vague, and nonspecific and pinpointing their precise etiology may be difficult, there is no benefit in treating patients for hypothyroidism when thyroid test results are normal. In fact, treatment may be harmful, as there is substantial risk for subclinical or overt hyperthyroidism.4
Generic vs Brand-Name Levothyroxine
Both generic and brand-name preparations of levothyroxine are available. Generic formulations are made by a variety of manufacturers, and formulations can vary in production; a brand name assures one manufacturer and consistency in production. It is difficult to accurately assess the bioequivalence and, therefore, the interchangeability, of the various manufacturers’ generic formulations.4 Differences in bioavailability of the drug may affect the dose the patient receives. Minor fluctuations may occur in thyroid function test results, which may or may not be clinically acceptable in an individual patient. Therefore, the current consensus encourages the use of a consistent levothyroxine preparation for individual patients to minimize variability from refill to refill.4 For patients for whom medication cost is a key factor, generic formulations can be considerably less expensive than their brand-name counterparts.
FOLLOW-UP TESTING
For primary hypothyroidism, the frequency of follow-up is dictated by symptoms and laboratory test values. Patients should be advised that symptoms will improve with treatment but that this improvement may not be noticeable for three to six months, even after TSH levels have reached the normal range.4
Thyroid function testing is typically repeated at four to eight weeks to assess initial dose titration; once an adequate replacement dose has been reached, testing may be repeated at six months and then annually thereafter unless symptoms arise.4 In pregnant women, TSH and FT4 levels should be measured every four weeks during the first half of pregnancy and at least once between 26 and 32 weeks’ gestation (levothyroxine dose requirements typically increase by 20% to 50% during pregnancy).4
Patients with secondary hypothyroidism should be referred for an endocrinology consultation for evaluation of their general pituitary or hypothalamic function.
Changes in Dose Requirements
Dose requirements may change over time in any given patient. Underlying thyroid function may wane, and any absorptive issues secondary to other diseases, such as celiac disease, may alter dose requirements. In pregnancy, dose requirements increase but generally revert back to baseline postpartum.4 Any addition or discontinuation of medications that affect plasma binding or metabolism will alter thyroid dosing.4 Increasing age or weight loss may require decreases in dosing. After any dose adjustment, thyroid function test results should be reevaluated in four to eight weeks, with the same follow-up schedule for repeat testing as after initiation of hypothyroidism treatment.
On the next page: Patient education and conclusion >>
PATIENT EDUCATION
Patient education should focus on treatment and follow-up. Patients need to be told that treatment is lifelong, and it may be helpful to describe levothyroxine as a replacement for their thyroid hormone, rather than as a treatment of their thyroid disease. They should be reminded that they will need to undergo repeat thyroid function testing periodically to assess their dose. While their dose may need to be adjusted over time, typically TSH levels will stabilize and laboratory testing will only be necessary every 12 months.4
Clinicians should also review with patients how and when to take levothyroxine. One tablet should be taken daily, with water, on an empty stomach, at least 30 to 60 minutes before eating or drinking or four hours after eating or drinking. Multivitamins or other supplements, especially those containing calcium and iron, should be taken at least four hours after taking levothyroxine. These are necessary requirements because both food and minerals can decrease absorption of the medication.4
Patients should also be informed that if they miss a dose of medication, two tablets may be taken the next day, but consistency in daily dosing is the goal. In patients with significant compliance problems, weekly dosing of levothyroxine results in similar safety, treatment outcomes, and TSH values as daily dosing.11
Finally, symptoms of both hypothyroidism and hyperthyroidism should be described and reviewed. Patients should be advised to notify their health care providers should any of these symptoms occur.
CONCLUSION
Hypothyroidism is a common illness encountered in the primary care setting. Clinicians must be familiar with the signs and symptoms, as well as the risk factors for the disease, because many patients have minimal symptomatology. Appropriate laboratory testing will clarify the diagnosis.
Patients with symptomatic hypothyroidism confirmed on laboratory testing should be treated. The benefit of treatment in other subgroups is less clear and should be guided by current evidence-based guidelines. The mainstay of medical management for hypothyroidism is levothyroxine; other treatment options require further research. Well-informed patients are key to effective management of hypothyroidism.
1. Golden SH, Robinson KA, Saldanha I, et al. Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
2. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4) and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
3. Gardner D, Shoback D. Thyroid gland. In: Greenspan’s Basic and Clinical Endocrinology. 9th ed. New York, NY: McGraw-Hill Medical; 2011:
163-226.
4. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028. www.aace.com/files/final-file-hypo-guidelines.pdf. Accessed June 18, 2014.
5. Negro R, Schwarz A, Gismondi R, et al. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab. 2011;96(6):E920-E924.
6. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction [published correction appears in Arch Intern Med. 2001;161(2):284]. Arch Intern Med. 2000;160(11):1573-1575.
7. Baskin HJ, Cobin RH, Duick DS, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism [published correction appears in Endocr Pract. 2008;14(6):802-803]. Endocr Pract. 2002;8(6):457-469.
8. Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365-1374.
9. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526-534.
10. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):
1982-1990.
11. Grebe SK, Cooke RR, Ford HC, et al. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82(3):870-875.
1. Golden SH, Robinson KA, Saldanha I, et al. Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853-1878.
2. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4) and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499.
3. Gardner D, Shoback D. Thyroid gland. In: Greenspan’s Basic and Clinical Endocrinology. 9th ed. New York, NY: McGraw-Hill Medical; 2011:
163-226.
4. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028. www.aace.com/files/final-file-hypo-guidelines.pdf. Accessed June 18, 2014.
5. Negro R, Schwarz A, Gismondi R, et al. Thyroid antibody positivity in the first trimester of pregnancy is associated with negative pregnancy outcomes. J Clin Endocrinol Metab. 2011;96(6):E920-E924.
6. Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction [published correction appears in Arch Intern Med. 2001;161(2):284]. Arch Intern Med. 2000;160(11):1573-1575.
7. Baskin HJ, Cobin RH, Duick DS, et al; American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism [published correction appears in Endocr Pract. 2008;14(6):802-803]. Endocr Pract. 2002;8(6):457-469.
8. Rodondi N, den Elzen WP, Bauer DC, et al; Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365-1374.
9. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526-534.
10. Hoang TD, Olsen CH, Mai VQ, et al. Desiccated thyroid extract compared with levothyroxine in the treatment of hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2013;98(5):
1982-1990.
11. Grebe SK, Cooke RR, Ford HC, et al. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab. 1997;82(3):870-875.
Dizziness and Vertigo: Recognizing Vestibular Migraine in the Primary Care Setting
CE/CME No: CR-1406
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the clinical manifestations of vestibular migraine (VM).
• List classifications of medications known to induce vestibular symptoms.
• Describe the office-based tests used to evaluate vestibular function.
• List the diagnostic criteria for VM.
• Discuss the differential diagnosis of VM, including peripheral and central causes of vertigo.
• Discuss pharmacologic and nonpharmacologic treatment options for VM.
FACULTY
Jennifer Hart is an Instructor of Nursing at Wor-Wic Community College in Salisbury, Maryland. Mary Parsons is Director of the Graduate and Second Degree Nursing Programs at Salisbury University in Maryland.
The authors have no financial information to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Vestibular migraine (VM) is the most common cause of recurrent dizziness and vertigo but is often unrecognized by health care providers. VM causes significant impairment in level of function and quality of life, and the diagnosis should be considered when symptoms cannot be explained by other etiologies. Information and guidance are provided to raise clinicians’ awareness of VM in order to increase accurate diagnosis, guide management decisions, and improve patient health outcomes.
Headache and dizziness are common reasons for primary care visits. In the general population, the prevalence of migraine is 13% to 16%, while dizziness and vertigo affect approximately 20% to 30%.1 Despite the prevalence of these conditions, many providers are unaware of vestibular migraine (VM) and may overlook it when considering differential diagnoses for these symptoms.
This is not surprising since, until recently, the International Headache Society’s (IHS) International Classification of Headache Disorders (ICHD)—considered the “gold standard” for defining and diagnosing headaches across all medical specialties—included no diagnostic criteria for VM.2 The second edition of the ICHD, ICHD-2, identified vertigo as a symptom of migraine only in the context of basilar migraine.1 Since fewer than 10% of patients with both vertigo and migraine met the criteria for basilar migraine, most VM patients could not be correctly classified under ICHD-2.1
In 2012, the Committee for Classification of Vestibular Disorders of the Bárány Society and the Migraine Classification Subcommittee of the IHS jointly published diagnostic criteria for VM.3 These criteria are included in the beta version of ICHD-3, published on the IHS website on July 3, 2013, for immediate use and field testing before ICHD-3 is finalized.4
DIAGNOSTIC CRITERIA FOR VM
The criteria for diagnosis of VM are as follows4:
A. At least five episodes fulfilling criteria C and D
B. A current or past history of migraine without aura or migraine with aura
C. Vestibular symptoms of moderate or severe intensity, lasting between 5 min and 72 h
D. At least 50% of episodes are associated with at least one of the following migrainous features:
- Headache with at least two of the following four characteristics:
a. Unilateral location
b. Pulsating quality
c. Moderate or severe intensity
d. Aggravation by routine physical activity - Photophobia and phonophobia
- Visual aura
E. Not better accounted for by another ICHD-3 diagnosis or by another vestibular disorder
Vestibular symptoms include3
• Spontaneous vertigo, which can be internal (false sensation of self-motion) or external (false sensation that surroundings are spinning)
• Positional vertigo (after change in head position)
• Visually induced vertigo (triggered by a complex or large moving visual stimulus)
• Head motion–induced vertigo
• Head motion–induced dizziness with nausea (dizziness is a sensation of disturbed spatial orientation)
Moderate vestibular symptoms interfere with, but do not prevent, daily activities; severe vestibular symptoms impede them.
On the next page: Diagnosis Overview >>
DIAGNOSIS: OVERVIEW
VM is a diagnosis of exclusion that is considered appropriate when no other peripheral or central vestibular disorder is present to account for the patient’s dizziness.5,6 Asking the patient the right questions will provide clues to the correct diagnosis.7 Most patients with VM present with normal physical, vestibular, and neurologic examinations, especially if examined when symptom-free.5 In contrast, examinations for competing diagnoses often reveal abnormal findings.
Vestibular vs nonvestibular vertigo. Typically, patients have difficulty describing their sensations with words more specific than “dizzy.”8 This lack of clarity is a challenge when the clinician is attempting to differentiate between true vertigo (ie, vertigo caused by vestibular dysfunction) and other types of nonvestibular dizziness. Specific categories of dizziness include vertigo, imbalance, presyncope, and lightheadedness; careful consideration must be given to the causes associated with each (see Table 1).9-11
Clinicians should focus on timing, duration of symptoms, triggers, and any other associated symptoms to determine the diagnosis.8,10 When evaluating a patient, a broad definition of vertigo, including spinning and/or rotational sensations as well as illusions of movement, is recommended.8
Peripheral vs central disorders. True vertigo must be further evaluated to determine whether its etiology is peripheral or central. In VM, a combination of both peripheral and central deficits can be seen.10 Clinicians must consider certain defining features to differentiate between these.
Peripheral Vertigo
If the cause of the patient's vertigo is peripheral, onset is often abrupt and the patient may experience mild-to-moderate imbalance that does not affect his or her ability to walk unassisted. Combined horizontal and rotational nystagmus may occur, which lessens or disappears with focused gaze. The nystagmus does not change direction when the patient gazes to either side and may fade after a few days.
The patient also may report experiencing tinnitus or hearing loss, accompanied by severe nausea or vomiting. Neurologic symptoms are rare in peripherally caused vertigo unless a concurrent diagnosis of migraine is confirmed.
Other symptoms may include weakness, dysarthria, changes in vision or hearing, paresthesias, changes in sensory or motor function, altered level of consciousness, and headache.10
Central Vertigo
Centrally caused vertigo often persists for hours to weeks, and neurologic symptoms are common. Patients have difficulty walking or standing still; problems with balance are severe. The nystagmus is purely horizontal, vertical, or rotational and may last for weeks to months. It is not inhibited with a focused gaze and may change direction with gaze. It is important to note that vertical nystagmus is 80% sensitive for vestibular nuclear or cerebellar central lesions.10
Tinnitus, if it occurs at all, is episodic, and nausea and/or vomiting vary from patient to patient. Patients presenting with central nervous system (CNS) disorders rarely complain of vertigo as their only symptom. Additional descriptions of dizziness may be used to describe the various cranial nerve abnormalities that suggest CNS etiology.9
DIFFERENTIAL DIAGNOSIS
Numerous conditions should be included in the differential when evaluating dizziness and vertigo symptoms. (See also Collie M. Vertigo: diagnosis and management. Clinician Reviews. 2013;23[12]:46-53.)
Peripheral Vertigo
Possible peripheral causes of vertigo include5,6,12,13
• Benign paroxysmal positional vertigo (BPPV)
• Vestibular neuritis
• Labyrinthitis
• Ménière disease
• Superior canal dehiscence
• Perilymphatic fistula
• Otitis media
• Aminoglycoside toxicity
• Trauma
BPPV is characterized by recurrent episodes of intense vertigo that last for seconds to one minute and are provoked by specific head movements. The Dix-Hallpike maneuver provokes vertigo and nystagmus and confirms the diagnosis.14 Vestibular neuritis and labyrinthitis present in similar ways. They are characterized by the onset of intense vertigo that can persist for several days, with nausea, vomiting, and imbalance also present. The main differentiating feature between them is associated hearing loss; vestibular neuritis is not associated with hearing loss, but labyrinthitis is. Both disorders are thought to be caused by inflammation of the vestibular nerve as the result of a viral infection. Patients may report recent upper respiratory infections or influenza like illnesses.10,12
Central Vertigo
Possible central causes of vertigo include5,6,13,15
• Acoustic neuroma (vestibular schwannoma)
• Cerebellar infarction
• Brainstem stroke
• Multiple sclerosis
• Episodic ataxia
• Psychogenic dizziness
Acoustic neuroma (vestibular schwannoma) is a benign growth on the vestibular nerve. Symptoms generally include progressive hearing loss and unilateral tinnitus accompanied by dizziness and imbalance.10 Acute attacks of vertigo are rare, but patients often complain of aural fullness, headache, and/or facial numbness.1,10
Cerebellar infarction usually presents with sudden onset of symptoms at maximal intensity. Risk factors for cerebellar infarction include a history of hypertension, coronary artery disease, diabetes, previous transient ischemic attack, smoking, alcohol consumption, atrial fibrillation, and hyperlipidemia.10
While only 5% of vertigo complaints are the result of CNS disorders, vertigo is an early symptom of brainstem stroke.15 Because of the significant morbidity and mortality associated with stroke, it must be considered in the initial diagnostic workup of vertigo.
Multiple sclerosis (MS) is an autoimmune disease in which the myelin axons in the CNS are destroyed. Dizziness and vertigo are common complaints associated with MS, but these patients will often present with other symptoms suggestive of CNS involvement. Common complaints include, but are not limited to, muscle weakness, fatigue, paresthesias, spasms, ataxia, pain, diplopia, dysarthria, heat intolerance, and urinary frequency.16
Episodic ataxia type 2 (EA2) is an inherited autosomal dominant disorder characterized by attacks of ataxia, vertigo, and nausea that can last from minutes to days. EA2 is often difficult to differentiate from VM due to symptom overlap. Fifty percent of patients with EA2 have a history of migraine headaches; often, there is a family history of similar symptoms.5
On the next page: Ménière Disease Versus VM >>
MÉNIÈRE DISEASE VERSUS VM
When headaches and dizziness coincide, VM is the most probable diagnosis.5 BPPV, Ménière disease, cerebellar disorders, motion sickness, and psychiatric syndromes (major depression and panic disorder) occur more often in patients with migraine than in those without.1 Ménière disease and VM often coexist, and up to 50% of patients with Ménière disease also meet criteria for migraine.5 (For more information, see Pearson T. Ménière disease: a lifelong merry-go-round. Clinician Reviews. 2013;23[10]:38-43.) Because many of their symptoms overlap, differentiating between Ménière disease and VM is critical; key differences in symptom presentation are as follows.17,18
Vertigo. The vertigo of Ménière disease is short-lived, lasting up to 24 hours.17 In contrast, episodes of vertigo with VM can last more than 24 hours; patients may experience a continuous rocking sensation for several weeks or even months.
Hearing loss. Sensorineural hearing loss in patients with Ménière disease is progressive and most often unilateral, but can be bilateral. In patients with VM, sensorineural hearing loss is rare; if it occurs, it is usually episodic and not progressive.17
Tinnitus. Tinnitus is a symptom of both Ménière disease and VM and may be unilateral or bilateral in both. In Ménière disease, patients report tinnitus of significant intensity, low in pitch, and “roaring,” whereas in VM, the tinnitus is usually high-pitched and unobtrusive.
Headache. Unless a concurrent diagnosis of migraine exists, patients with Ménière disease do not present with headache or photophobia. Many patients with VM, though not all, confirm a positive history of headache.
Phonophobia. Phonophobia is a frequent symptom in patients with VM.17
On the next page: Clinical Manifestations >>
CLINICAL MANIFESTATIONS OF VM
The clinical presentation of VM varies for each patient, as do the frequency and duration of episodes. Manifestations of VM may include5,19,20
• Vertigo associated with visual triggers
• Nausea and/or dizziness
• Spontaneous positional vertigo
• Head motion intolerance
• Motion sickness
• Lightheadedness
• Headache
• Chronic disequilibrium
• Inability to concentrate
• Mild hearing loss or tinnitus
• Cervicalgia
• Anxiety
• Panic
• Photophobia
• Phonophobia
• Sensory aura
Visual vertigo, described as vertigo worsened by visual stimulation such as moving scenes, scrolling patterns, and movement of large crowds or traffic, is highly suggestive of VM.19 Aura-type symptoms are of significant diagnostic importance because they may be the only apparent connection between vertigo and migraine.19
The duration of vertigo and dizziness may range from seconds to weeks. These episodes may have no temporal relationship with headaches. Vertigo and dizziness can continue for more than 24 hours for about half of VM patients; in some, symptoms persist for several weeks or more.12 In addition, vertigo and headache may never occur together, which further increases the diagnostic challenge for this disorder.1,5
Although many hypotheses exist, the pathophysiology of VM remains unclear.
Patient History
It is vital to obtain a detailed medical and social history in order to determine possible etiologies for symptoms. Medications in particular must be reviewed with care because many are known to induce vestibular-type symptoms.
Cardiac agents of potential concern for causing dizziness include β-blockers, diuretics, ACE inhibitors, β-blockers, and nitrates. CNS agents associated with dizziness include antipsychotics, dopaminergic drugs, opioids/analgesics, hypnotics, anticonvulsants, tricyclic antidepressants, and muscle relaxants. Anticholinergics, phosphodiesterase type 5 inhibitors, antibacterials, aminoglycosides, fluoroquinolones, and antineoplastics may also lead to dizziness; several of these medications are also known to be ototoxic. Dizziness can also be related to orthostatic hypotension, which is a common adverse effect of many of the listed medications, especially when used in combination.7,9
Diabetes, hypertension, vascular diseases, and neurologic disorders should be considered as causes for vision and proprioception problems.7 Patients may present with a personal history of headaches for years before vestibular symptoms develop.6 Motion sickness is suggestive of a possible migraine diagnosis.19
Positive family history is common for migraineurs.21 Patients ages 30 to 39 are affected most frequently, with an estimated prevalence of 7% in men and 24.4% in women.22
Physical Examination
The focused physical should include cranial nerve assessments, an otoscopic examination, hearing evaluation with a tuning fork, and audiometry, if a hearing deficit is detected. Minor oculomotor abnormalities, such as “weak” nystagmus with vertical, horizontal, torsional, or positional components, may be noted in approximately 70% of patients with VM.5 Various in-office tests (see Table 2) should also be conducted to narrow the possible causes for vertigo and dizziness and rule out more serious disorders.7,10 ,11,23
Diagnostic Workup
Laboratory tests are generally not recommended because they identify the cause of vertigo in only approximately 1% of patients.23 Similarly, routine use of MRI or CT is not recommended, but these modalities may be indicated for patients with focal neurologic deficits or risk factors for cerebrovascular disease, or if acute treatments for peripheral vertigo are unsuccessful.10
On the next page: Treatment >>
TREATMENT OF VM
Nonpharmacologic
Nonpharmacologic measures to prevent VM attacks include the avoidance of “triggers” through dietary restrictions, stress reduction, and healthy lifestyle modifications. Diaries can be used to help identify common triggers to avoid (see Table 3); patients should understand that it may take up to three months before noticeable improvement in symptoms is seen. Once symptoms have resolved, cautious re-introduction of suspect foods may be attempted.24
Vestibular rehabilitation therapy promotes CNS compensation for inner ear deficits and reduces symptoms of disequilibrium and dizziness.25 It is helpful for complications of VM, such as anxiety, visual dependence, or loss of confidence with balance.10
Pharmacologic
Prophylactic drug therapy is the mainstay of medical management for VM when nonpharmacologic measures are inadequate; episodes are frequent and severe; or symptoms are of long duration.5 The drugs’ adverse effect profiles, as well as patient comorbidities, should guide the choice of therapy.26
Abortive migraine therapies have not been shown to be effective for symptoms of dizziness17 and may cause rebound symptoms. Vestibular suppressants (promethazine, dimenhydrinate, and meclizine) can be used for acute episodes of VM.5
According to evidence-based guidelines for the preventive treatment of migraine in adults,26 the pharmacologic options include multiple drug classes stratified by level, depending on the quality of evidence supporting their effectiveness in migraine prevention.
Level A comprises medications with established efficacy. These include divalproex sodium, sodium valproate, topiramate, frovatriptan (for menstrual migraine), metoprolol, propanolol, and timolol.
Level B medications that are “probably effective” include naratriptan, zolmitriptan, amitriptyline, venlafaxine, atenolol, and nadolol.
Medications that are “possibly effective” are in the Level C category and include carbamazepine, nebivolol, pindolol, lisinopril, candesartan, clonidine, guanfacine, and cyproheptadine.
The Level U category drugs are labeled “inadequate or conflicting data to support or refute medication use” and include fluvoxamine, fluoxetine, protriptyline, gabapentin, bisoprolol, cyclandelate, acenocoumarol, warfarin, picotamide, acetazolamide, nicardipine, nifedipine, nimodipine, and verapamil.
Medications that are established as possibly or probably ineffective fall under the category of Other and include clomipramine, lamotrigine, acebutolol, clonazepam, nabumetone, oxcarbazepine, and telmisartan.
When initiating therapy, low doses are recommended because migraineurs’ sensory hypersensitivity is thought to extend to medications.5 Sequence and dosing information, along with contraindications, should be considered when choosing medical therapies (see Table 4).5,12,19,21,26 Patient response to treatment is evaluated after one to three months, and medications should be discontinued if symptoms persist, maximum dose is reached, or significant adverse effects occur.5 A reasonable goal of therapy is to reduce episode frequency by more than 50%.19 Should both lifestyle modifications and medications fail, referral to a specialist for reevaluation of the diagnosis is warranted.8
On the next page: Conclusion >>
CONCLUSION
While VM is considered the most common cause of recurrent vertigo and dizziness, few primary care providers are familiar with the diagnosis. Differentiating among the various causes for these symptoms and determining if the cause is a CNS or peripheral system disorder are essential to narrowing the differential diagnoses.
The inclusion of VM in the ICHD-3 system will increase both clinician awareness and accurate diagnosis of the disorder. Office-based assessments can be performed to evaluate these common complaints, and numerous medical therapies are available to successfully treat patients with VM. Through greater awareness of VM and use of evidence-based diagnostic and treatment guidelines, clinicians can significantly improve quality of life and health outcomes for patients with the disorder.
1. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol. 2009;256(3):333-338.
2. The International Headache Society. The International Headache Classification. 2nd ed. www.ihs-classification.org/en/01_einleitung/02_einleitung/. Accessed May 16, 2014.
3. Lempert T, Oleson J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22:167-172.
4. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. 3rd ed (beta version). Cephalalgia. 2013;33(9):629-808.
5. Cherchi M, Hain T. Migraine-associated vertigo. Otolaryngol Clin Am. 2011;44:367-375.
6. Honaker J, Samy R. Migraine-associated vestibulopathy. Curr Opin Otolaryngol Head Neck Surg. 2008;16:412-415.
7. Shaia WT. Dizziness evaluation. http://emedicine.medscape.com/article/1831429-overview. Accessed May 16, 2014.
8. Watson S. Vertigo and migraine: "how can it be a migraine if I don’t have a headache?" Med Today. 2011;12(12):36-43.
9. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82(4):361-368.
10. Furman M, Rizzolo D. Evaluating the patient with vertigo: a complex complaint made simple. JAAPA. 2011;24(10):52-58.
11. Kuo CH, Pang T, Chang R. Vertigo – part 1 - assessment in general practice. Aust Fam Physician. 2008;37(5):341-347.
12. Hain, T. Migraine associated vertigo: vestibular migraine. www.dizziness-and-balance.com/disorders/central/migraine/mav.html. Accessed May 16, 2014.
13. Cha YH, Kane MJ, Baloh RW. Familial clustering of migraine, episodic vertigo, and Ménière’s disease. Otol Neurotol. 2008;29(1):93-96.
14. Clark MM. How to sort out a complaint of dizziness. Patient Care. 2003;37: 44-52.
15. Hain TC. Brainstem strokes associated with vertigo or hearing symptoms. www.dizziness-and- balance.com/disorders/central/strokes/brainstem%20strokes.htm. Accessed May 16, 2014.
16. Luzzio C, Dangond F. Multiple sclerosis. http://emedicine.medscape.com/article/1146199-overview. Accessed May 16, 2014.
17. Benson AG. Migraine-associated vertigo. http://emedicine.medscape.com/article/884136-overview#aw2aab6b5. Accessed May 16, 2014.
18. Teggi R, Fabiano B, Recanata P, et al. Case reports on two patients with episodic vertigo, fluctuating hearing loss, and migraine responding to prophylactic drugs for migraine. Ménière disease or migraine-associated vertigo? Acta Otorhinolaryngol Ital. 2010;30(4):217-220.
19. Neuhauser H, Lempert T. Vestibular migraine. Neurol Clin. 2009;27:379-391.
20. Kramer J, Buskirk J. Vestibular migraine a/k/a migraine-associated vertigo. www.vestibular.org/migraine-associated-vertigo-mav. Accessed April 1, 2014.
21. Chawla J. Migraine headache. http://emedicine.medscape.com/article/1142556-overview. Accessed May 16, 2014.
22. Cutrer MF, Bajwa ZH, Sabahat A. Pathophysiology, clinical manifestations, and diagnosis of migraine in adults. www.uptodate.com/contents/patho physiology-clinical-manifestations-and-diagnosis-of-migraine-in-adults. Accessed May 16, 2014.
23. Labuguen R. Initial evaluation of vertigo [published correction appears in Am Fam Physician. 2006;73(10):1704]. Am Fam Physician. 2006;73(2):244-251.
24. Texiedo M. Common migraine food triggers. http://deedee.dbi.udel.edu/MichaelTeixidoMD/patientInfo/migrainesFoodTrig.html. Accessed May 16, 2014.
25. Bisdorff AR. Management of vestibular migraine. Ther Adv Neurol Disord. 2011;4(3):183-191.
26. Silberstein S, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults [published correction appears in Neurology. 2013;80:871]. Neurology. 2012;78(17):1337-1345.
CE/CME No: CR-1406
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the clinical manifestations of vestibular migraine (VM).
• List classifications of medications known to induce vestibular symptoms.
• Describe the office-based tests used to evaluate vestibular function.
• List the diagnostic criteria for VM.
• Discuss the differential diagnosis of VM, including peripheral and central causes of vertigo.
• Discuss pharmacologic and nonpharmacologic treatment options for VM.
FACULTY
Jennifer Hart is an Instructor of Nursing at Wor-Wic Community College in Salisbury, Maryland. Mary Parsons is Director of the Graduate and Second Degree Nursing Programs at Salisbury University in Maryland.
The authors have no financial information to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Vestibular migraine (VM) is the most common cause of recurrent dizziness and vertigo but is often unrecognized by health care providers. VM causes significant impairment in level of function and quality of life, and the diagnosis should be considered when symptoms cannot be explained by other etiologies. Information and guidance are provided to raise clinicians’ awareness of VM in order to increase accurate diagnosis, guide management decisions, and improve patient health outcomes.
Headache and dizziness are common reasons for primary care visits. In the general population, the prevalence of migraine is 13% to 16%, while dizziness and vertigo affect approximately 20% to 30%.1 Despite the prevalence of these conditions, many providers are unaware of vestibular migraine (VM) and may overlook it when considering differential diagnoses for these symptoms.
This is not surprising since, until recently, the International Headache Society’s (IHS) International Classification of Headache Disorders (ICHD)—considered the “gold standard” for defining and diagnosing headaches across all medical specialties—included no diagnostic criteria for VM.2 The second edition of the ICHD, ICHD-2, identified vertigo as a symptom of migraine only in the context of basilar migraine.1 Since fewer than 10% of patients with both vertigo and migraine met the criteria for basilar migraine, most VM patients could not be correctly classified under ICHD-2.1
In 2012, the Committee for Classification of Vestibular Disorders of the Bárány Society and the Migraine Classification Subcommittee of the IHS jointly published diagnostic criteria for VM.3 These criteria are included in the beta version of ICHD-3, published on the IHS website on July 3, 2013, for immediate use and field testing before ICHD-3 is finalized.4
DIAGNOSTIC CRITERIA FOR VM
The criteria for diagnosis of VM are as follows4:
A. At least five episodes fulfilling criteria C and D
B. A current or past history of migraine without aura or migraine with aura
C. Vestibular symptoms of moderate or severe intensity, lasting between 5 min and 72 h
D. At least 50% of episodes are associated with at least one of the following migrainous features:
- Headache with at least two of the following four characteristics:
a. Unilateral location
b. Pulsating quality
c. Moderate or severe intensity
d. Aggravation by routine physical activity - Photophobia and phonophobia
- Visual aura
E. Not better accounted for by another ICHD-3 diagnosis or by another vestibular disorder
Vestibular symptoms include3
• Spontaneous vertigo, which can be internal (false sensation of self-motion) or external (false sensation that surroundings are spinning)
• Positional vertigo (after change in head position)
• Visually induced vertigo (triggered by a complex or large moving visual stimulus)
• Head motion–induced vertigo
• Head motion–induced dizziness with nausea (dizziness is a sensation of disturbed spatial orientation)
Moderate vestibular symptoms interfere with, but do not prevent, daily activities; severe vestibular symptoms impede them.
On the next page: Diagnosis Overview >>
DIAGNOSIS: OVERVIEW
VM is a diagnosis of exclusion that is considered appropriate when no other peripheral or central vestibular disorder is present to account for the patient’s dizziness.5,6 Asking the patient the right questions will provide clues to the correct diagnosis.7 Most patients with VM present with normal physical, vestibular, and neurologic examinations, especially if examined when symptom-free.5 In contrast, examinations for competing diagnoses often reveal abnormal findings.
Vestibular vs nonvestibular vertigo. Typically, patients have difficulty describing their sensations with words more specific than “dizzy.”8 This lack of clarity is a challenge when the clinician is attempting to differentiate between true vertigo (ie, vertigo caused by vestibular dysfunction) and other types of nonvestibular dizziness. Specific categories of dizziness include vertigo, imbalance, presyncope, and lightheadedness; careful consideration must be given to the causes associated with each (see Table 1).9-11
Clinicians should focus on timing, duration of symptoms, triggers, and any other associated symptoms to determine the diagnosis.8,10 When evaluating a patient, a broad definition of vertigo, including spinning and/or rotational sensations as well as illusions of movement, is recommended.8
Peripheral vs central disorders. True vertigo must be further evaluated to determine whether its etiology is peripheral or central. In VM, a combination of both peripheral and central deficits can be seen.10 Clinicians must consider certain defining features to differentiate between these.
Peripheral Vertigo
If the cause of the patient's vertigo is peripheral, onset is often abrupt and the patient may experience mild-to-moderate imbalance that does not affect his or her ability to walk unassisted. Combined horizontal and rotational nystagmus may occur, which lessens or disappears with focused gaze. The nystagmus does not change direction when the patient gazes to either side and may fade after a few days.
The patient also may report experiencing tinnitus or hearing loss, accompanied by severe nausea or vomiting. Neurologic symptoms are rare in peripherally caused vertigo unless a concurrent diagnosis of migraine is confirmed.
Other symptoms may include weakness, dysarthria, changes in vision or hearing, paresthesias, changes in sensory or motor function, altered level of consciousness, and headache.10
Central Vertigo
Centrally caused vertigo often persists for hours to weeks, and neurologic symptoms are common. Patients have difficulty walking or standing still; problems with balance are severe. The nystagmus is purely horizontal, vertical, or rotational and may last for weeks to months. It is not inhibited with a focused gaze and may change direction with gaze. It is important to note that vertical nystagmus is 80% sensitive for vestibular nuclear or cerebellar central lesions.10
Tinnitus, if it occurs at all, is episodic, and nausea and/or vomiting vary from patient to patient. Patients presenting with central nervous system (CNS) disorders rarely complain of vertigo as their only symptom. Additional descriptions of dizziness may be used to describe the various cranial nerve abnormalities that suggest CNS etiology.9
DIFFERENTIAL DIAGNOSIS
Numerous conditions should be included in the differential when evaluating dizziness and vertigo symptoms. (See also Collie M. Vertigo: diagnosis and management. Clinician Reviews. 2013;23[12]:46-53.)
Peripheral Vertigo
Possible peripheral causes of vertigo include5,6,12,13
• Benign paroxysmal positional vertigo (BPPV)
• Vestibular neuritis
• Labyrinthitis
• Ménière disease
• Superior canal dehiscence
• Perilymphatic fistula
• Otitis media
• Aminoglycoside toxicity
• Trauma
BPPV is characterized by recurrent episodes of intense vertigo that last for seconds to one minute and are provoked by specific head movements. The Dix-Hallpike maneuver provokes vertigo and nystagmus and confirms the diagnosis.14 Vestibular neuritis and labyrinthitis present in similar ways. They are characterized by the onset of intense vertigo that can persist for several days, with nausea, vomiting, and imbalance also present. The main differentiating feature between them is associated hearing loss; vestibular neuritis is not associated with hearing loss, but labyrinthitis is. Both disorders are thought to be caused by inflammation of the vestibular nerve as the result of a viral infection. Patients may report recent upper respiratory infections or influenza like illnesses.10,12
Central Vertigo
Possible central causes of vertigo include5,6,13,15
• Acoustic neuroma (vestibular schwannoma)
• Cerebellar infarction
• Brainstem stroke
• Multiple sclerosis
• Episodic ataxia
• Psychogenic dizziness
Acoustic neuroma (vestibular schwannoma) is a benign growth on the vestibular nerve. Symptoms generally include progressive hearing loss and unilateral tinnitus accompanied by dizziness and imbalance.10 Acute attacks of vertigo are rare, but patients often complain of aural fullness, headache, and/or facial numbness.1,10
Cerebellar infarction usually presents with sudden onset of symptoms at maximal intensity. Risk factors for cerebellar infarction include a history of hypertension, coronary artery disease, diabetes, previous transient ischemic attack, smoking, alcohol consumption, atrial fibrillation, and hyperlipidemia.10
While only 5% of vertigo complaints are the result of CNS disorders, vertigo is an early symptom of brainstem stroke.15 Because of the significant morbidity and mortality associated with stroke, it must be considered in the initial diagnostic workup of vertigo.
Multiple sclerosis (MS) is an autoimmune disease in which the myelin axons in the CNS are destroyed. Dizziness and vertigo are common complaints associated with MS, but these patients will often present with other symptoms suggestive of CNS involvement. Common complaints include, but are not limited to, muscle weakness, fatigue, paresthesias, spasms, ataxia, pain, diplopia, dysarthria, heat intolerance, and urinary frequency.16
Episodic ataxia type 2 (EA2) is an inherited autosomal dominant disorder characterized by attacks of ataxia, vertigo, and nausea that can last from minutes to days. EA2 is often difficult to differentiate from VM due to symptom overlap. Fifty percent of patients with EA2 have a history of migraine headaches; often, there is a family history of similar symptoms.5
On the next page: Ménière Disease Versus VM >>
MÉNIÈRE DISEASE VERSUS VM
When headaches and dizziness coincide, VM is the most probable diagnosis.5 BPPV, Ménière disease, cerebellar disorders, motion sickness, and psychiatric syndromes (major depression and panic disorder) occur more often in patients with migraine than in those without.1 Ménière disease and VM often coexist, and up to 50% of patients with Ménière disease also meet criteria for migraine.5 (For more information, see Pearson T. Ménière disease: a lifelong merry-go-round. Clinician Reviews. 2013;23[10]:38-43.) Because many of their symptoms overlap, differentiating between Ménière disease and VM is critical; key differences in symptom presentation are as follows.17,18
Vertigo. The vertigo of Ménière disease is short-lived, lasting up to 24 hours.17 In contrast, episodes of vertigo with VM can last more than 24 hours; patients may experience a continuous rocking sensation for several weeks or even months.
Hearing loss. Sensorineural hearing loss in patients with Ménière disease is progressive and most often unilateral, but can be bilateral. In patients with VM, sensorineural hearing loss is rare; if it occurs, it is usually episodic and not progressive.17
Tinnitus. Tinnitus is a symptom of both Ménière disease and VM and may be unilateral or bilateral in both. In Ménière disease, patients report tinnitus of significant intensity, low in pitch, and “roaring,” whereas in VM, the tinnitus is usually high-pitched and unobtrusive.
Headache. Unless a concurrent diagnosis of migraine exists, patients with Ménière disease do not present with headache or photophobia. Many patients with VM, though not all, confirm a positive history of headache.
Phonophobia. Phonophobia is a frequent symptom in patients with VM.17
On the next page: Clinical Manifestations >>
CLINICAL MANIFESTATIONS OF VM
The clinical presentation of VM varies for each patient, as do the frequency and duration of episodes. Manifestations of VM may include5,19,20
• Vertigo associated with visual triggers
• Nausea and/or dizziness
• Spontaneous positional vertigo
• Head motion intolerance
• Motion sickness
• Lightheadedness
• Headache
• Chronic disequilibrium
• Inability to concentrate
• Mild hearing loss or tinnitus
• Cervicalgia
• Anxiety
• Panic
• Photophobia
• Phonophobia
• Sensory aura
Visual vertigo, described as vertigo worsened by visual stimulation such as moving scenes, scrolling patterns, and movement of large crowds or traffic, is highly suggestive of VM.19 Aura-type symptoms are of significant diagnostic importance because they may be the only apparent connection between vertigo and migraine.19
The duration of vertigo and dizziness may range from seconds to weeks. These episodes may have no temporal relationship with headaches. Vertigo and dizziness can continue for more than 24 hours for about half of VM patients; in some, symptoms persist for several weeks or more.12 In addition, vertigo and headache may never occur together, which further increases the diagnostic challenge for this disorder.1,5
Although many hypotheses exist, the pathophysiology of VM remains unclear.
Patient History
It is vital to obtain a detailed medical and social history in order to determine possible etiologies for symptoms. Medications in particular must be reviewed with care because many are known to induce vestibular-type symptoms.
Cardiac agents of potential concern for causing dizziness include β-blockers, diuretics, ACE inhibitors, β-blockers, and nitrates. CNS agents associated with dizziness include antipsychotics, dopaminergic drugs, opioids/analgesics, hypnotics, anticonvulsants, tricyclic antidepressants, and muscle relaxants. Anticholinergics, phosphodiesterase type 5 inhibitors, antibacterials, aminoglycosides, fluoroquinolones, and antineoplastics may also lead to dizziness; several of these medications are also known to be ototoxic. Dizziness can also be related to orthostatic hypotension, which is a common adverse effect of many of the listed medications, especially when used in combination.7,9
Diabetes, hypertension, vascular diseases, and neurologic disorders should be considered as causes for vision and proprioception problems.7 Patients may present with a personal history of headaches for years before vestibular symptoms develop.6 Motion sickness is suggestive of a possible migraine diagnosis.19
Positive family history is common for migraineurs.21 Patients ages 30 to 39 are affected most frequently, with an estimated prevalence of 7% in men and 24.4% in women.22
Physical Examination
The focused physical should include cranial nerve assessments, an otoscopic examination, hearing evaluation with a tuning fork, and audiometry, if a hearing deficit is detected. Minor oculomotor abnormalities, such as “weak” nystagmus with vertical, horizontal, torsional, or positional components, may be noted in approximately 70% of patients with VM.5 Various in-office tests (see Table 2) should also be conducted to narrow the possible causes for vertigo and dizziness and rule out more serious disorders.7,10 ,11,23
Diagnostic Workup
Laboratory tests are generally not recommended because they identify the cause of vertigo in only approximately 1% of patients.23 Similarly, routine use of MRI or CT is not recommended, but these modalities may be indicated for patients with focal neurologic deficits or risk factors for cerebrovascular disease, or if acute treatments for peripheral vertigo are unsuccessful.10
On the next page: Treatment >>
TREATMENT OF VM
Nonpharmacologic
Nonpharmacologic measures to prevent VM attacks include the avoidance of “triggers” through dietary restrictions, stress reduction, and healthy lifestyle modifications. Diaries can be used to help identify common triggers to avoid (see Table 3); patients should understand that it may take up to three months before noticeable improvement in symptoms is seen. Once symptoms have resolved, cautious re-introduction of suspect foods may be attempted.24
Vestibular rehabilitation therapy promotes CNS compensation for inner ear deficits and reduces symptoms of disequilibrium and dizziness.25 It is helpful for complications of VM, such as anxiety, visual dependence, or loss of confidence with balance.10
Pharmacologic
Prophylactic drug therapy is the mainstay of medical management for VM when nonpharmacologic measures are inadequate; episodes are frequent and severe; or symptoms are of long duration.5 The drugs’ adverse effect profiles, as well as patient comorbidities, should guide the choice of therapy.26
Abortive migraine therapies have not been shown to be effective for symptoms of dizziness17 and may cause rebound symptoms. Vestibular suppressants (promethazine, dimenhydrinate, and meclizine) can be used for acute episodes of VM.5
According to evidence-based guidelines for the preventive treatment of migraine in adults,26 the pharmacologic options include multiple drug classes stratified by level, depending on the quality of evidence supporting their effectiveness in migraine prevention.
Level A comprises medications with established efficacy. These include divalproex sodium, sodium valproate, topiramate, frovatriptan (for menstrual migraine), metoprolol, propanolol, and timolol.
Level B medications that are “probably effective” include naratriptan, zolmitriptan, amitriptyline, venlafaxine, atenolol, and nadolol.
Medications that are “possibly effective” are in the Level C category and include carbamazepine, nebivolol, pindolol, lisinopril, candesartan, clonidine, guanfacine, and cyproheptadine.
The Level U category drugs are labeled “inadequate or conflicting data to support or refute medication use” and include fluvoxamine, fluoxetine, protriptyline, gabapentin, bisoprolol, cyclandelate, acenocoumarol, warfarin, picotamide, acetazolamide, nicardipine, nifedipine, nimodipine, and verapamil.
Medications that are established as possibly or probably ineffective fall under the category of Other and include clomipramine, lamotrigine, acebutolol, clonazepam, nabumetone, oxcarbazepine, and telmisartan.
When initiating therapy, low doses are recommended because migraineurs’ sensory hypersensitivity is thought to extend to medications.5 Sequence and dosing information, along with contraindications, should be considered when choosing medical therapies (see Table 4).5,12,19,21,26 Patient response to treatment is evaluated after one to three months, and medications should be discontinued if symptoms persist, maximum dose is reached, or significant adverse effects occur.5 A reasonable goal of therapy is to reduce episode frequency by more than 50%.19 Should both lifestyle modifications and medications fail, referral to a specialist for reevaluation of the diagnosis is warranted.8
On the next page: Conclusion >>
CONCLUSION
While VM is considered the most common cause of recurrent vertigo and dizziness, few primary care providers are familiar with the diagnosis. Differentiating among the various causes for these symptoms and determining if the cause is a CNS or peripheral system disorder are essential to narrowing the differential diagnoses.
The inclusion of VM in the ICHD-3 system will increase both clinician awareness and accurate diagnosis of the disorder. Office-based assessments can be performed to evaluate these common complaints, and numerous medical therapies are available to successfully treat patients with VM. Through greater awareness of VM and use of evidence-based diagnostic and treatment guidelines, clinicians can significantly improve quality of life and health outcomes for patients with the disorder.
CE/CME No: CR-1406
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the clinical manifestations of vestibular migraine (VM).
• List classifications of medications known to induce vestibular symptoms.
• Describe the office-based tests used to evaluate vestibular function.
• List the diagnostic criteria for VM.
• Discuss the differential diagnosis of VM, including peripheral and central causes of vertigo.
• Discuss pharmacologic and nonpharmacologic treatment options for VM.
FACULTY
Jennifer Hart is an Instructor of Nursing at Wor-Wic Community College in Salisbury, Maryland. Mary Parsons is Director of the Graduate and Second Degree Nursing Programs at Salisbury University in Maryland.
The authors have no financial information to disclose.
ACCREDITATION STATEMENT
Article begins on next page >>
Vestibular migraine (VM) is the most common cause of recurrent dizziness and vertigo but is often unrecognized by health care providers. VM causes significant impairment in level of function and quality of life, and the diagnosis should be considered when symptoms cannot be explained by other etiologies. Information and guidance are provided to raise clinicians’ awareness of VM in order to increase accurate diagnosis, guide management decisions, and improve patient health outcomes.
Headache and dizziness are common reasons for primary care visits. In the general population, the prevalence of migraine is 13% to 16%, while dizziness and vertigo affect approximately 20% to 30%.1 Despite the prevalence of these conditions, many providers are unaware of vestibular migraine (VM) and may overlook it when considering differential diagnoses for these symptoms.
This is not surprising since, until recently, the International Headache Society’s (IHS) International Classification of Headache Disorders (ICHD)—considered the “gold standard” for defining and diagnosing headaches across all medical specialties—included no diagnostic criteria for VM.2 The second edition of the ICHD, ICHD-2, identified vertigo as a symptom of migraine only in the context of basilar migraine.1 Since fewer than 10% of patients with both vertigo and migraine met the criteria for basilar migraine, most VM patients could not be correctly classified under ICHD-2.1
In 2012, the Committee for Classification of Vestibular Disorders of the Bárány Society and the Migraine Classification Subcommittee of the IHS jointly published diagnostic criteria for VM.3 These criteria are included in the beta version of ICHD-3, published on the IHS website on July 3, 2013, for immediate use and field testing before ICHD-3 is finalized.4
DIAGNOSTIC CRITERIA FOR VM
The criteria for diagnosis of VM are as follows4:
A. At least five episodes fulfilling criteria C and D
B. A current or past history of migraine without aura or migraine with aura
C. Vestibular symptoms of moderate or severe intensity, lasting between 5 min and 72 h
D. At least 50% of episodes are associated with at least one of the following migrainous features:
- Headache with at least two of the following four characteristics:
a. Unilateral location
b. Pulsating quality
c. Moderate or severe intensity
d. Aggravation by routine physical activity - Photophobia and phonophobia
- Visual aura
E. Not better accounted for by another ICHD-3 diagnosis or by another vestibular disorder
Vestibular symptoms include3
• Spontaneous vertigo, which can be internal (false sensation of self-motion) or external (false sensation that surroundings are spinning)
• Positional vertigo (after change in head position)
• Visually induced vertigo (triggered by a complex or large moving visual stimulus)
• Head motion–induced vertigo
• Head motion–induced dizziness with nausea (dizziness is a sensation of disturbed spatial orientation)
Moderate vestibular symptoms interfere with, but do not prevent, daily activities; severe vestibular symptoms impede them.
On the next page: Diagnosis Overview >>
DIAGNOSIS: OVERVIEW
VM is a diagnosis of exclusion that is considered appropriate when no other peripheral or central vestibular disorder is present to account for the patient’s dizziness.5,6 Asking the patient the right questions will provide clues to the correct diagnosis.7 Most patients with VM present with normal physical, vestibular, and neurologic examinations, especially if examined when symptom-free.5 In contrast, examinations for competing diagnoses often reveal abnormal findings.
Vestibular vs nonvestibular vertigo. Typically, patients have difficulty describing their sensations with words more specific than “dizzy.”8 This lack of clarity is a challenge when the clinician is attempting to differentiate between true vertigo (ie, vertigo caused by vestibular dysfunction) and other types of nonvestibular dizziness. Specific categories of dizziness include vertigo, imbalance, presyncope, and lightheadedness; careful consideration must be given to the causes associated with each (see Table 1).9-11
Clinicians should focus on timing, duration of symptoms, triggers, and any other associated symptoms to determine the diagnosis.8,10 When evaluating a patient, a broad definition of vertigo, including spinning and/or rotational sensations as well as illusions of movement, is recommended.8
Peripheral vs central disorders. True vertigo must be further evaluated to determine whether its etiology is peripheral or central. In VM, a combination of both peripheral and central deficits can be seen.10 Clinicians must consider certain defining features to differentiate between these.
Peripheral Vertigo
If the cause of the patient's vertigo is peripheral, onset is often abrupt and the patient may experience mild-to-moderate imbalance that does not affect his or her ability to walk unassisted. Combined horizontal and rotational nystagmus may occur, which lessens or disappears with focused gaze. The nystagmus does not change direction when the patient gazes to either side and may fade after a few days.
The patient also may report experiencing tinnitus or hearing loss, accompanied by severe nausea or vomiting. Neurologic symptoms are rare in peripherally caused vertigo unless a concurrent diagnosis of migraine is confirmed.
Other symptoms may include weakness, dysarthria, changes in vision or hearing, paresthesias, changes in sensory or motor function, altered level of consciousness, and headache.10
Central Vertigo
Centrally caused vertigo often persists for hours to weeks, and neurologic symptoms are common. Patients have difficulty walking or standing still; problems with balance are severe. The nystagmus is purely horizontal, vertical, or rotational and may last for weeks to months. It is not inhibited with a focused gaze and may change direction with gaze. It is important to note that vertical nystagmus is 80% sensitive for vestibular nuclear or cerebellar central lesions.10
Tinnitus, if it occurs at all, is episodic, and nausea and/or vomiting vary from patient to patient. Patients presenting with central nervous system (CNS) disorders rarely complain of vertigo as their only symptom. Additional descriptions of dizziness may be used to describe the various cranial nerve abnormalities that suggest CNS etiology.9
DIFFERENTIAL DIAGNOSIS
Numerous conditions should be included in the differential when evaluating dizziness and vertigo symptoms. (See also Collie M. Vertigo: diagnosis and management. Clinician Reviews. 2013;23[12]:46-53.)
Peripheral Vertigo
Possible peripheral causes of vertigo include5,6,12,13
• Benign paroxysmal positional vertigo (BPPV)
• Vestibular neuritis
• Labyrinthitis
• Ménière disease
• Superior canal dehiscence
• Perilymphatic fistula
• Otitis media
• Aminoglycoside toxicity
• Trauma
BPPV is characterized by recurrent episodes of intense vertigo that last for seconds to one minute and are provoked by specific head movements. The Dix-Hallpike maneuver provokes vertigo and nystagmus and confirms the diagnosis.14 Vestibular neuritis and labyrinthitis present in similar ways. They are characterized by the onset of intense vertigo that can persist for several days, with nausea, vomiting, and imbalance also present. The main differentiating feature between them is associated hearing loss; vestibular neuritis is not associated with hearing loss, but labyrinthitis is. Both disorders are thought to be caused by inflammation of the vestibular nerve as the result of a viral infection. Patients may report recent upper respiratory infections or influenza like illnesses.10,12
Central Vertigo
Possible central causes of vertigo include5,6,13,15
• Acoustic neuroma (vestibular schwannoma)
• Cerebellar infarction
• Brainstem stroke
• Multiple sclerosis
• Episodic ataxia
• Psychogenic dizziness
Acoustic neuroma (vestibular schwannoma) is a benign growth on the vestibular nerve. Symptoms generally include progressive hearing loss and unilateral tinnitus accompanied by dizziness and imbalance.10 Acute attacks of vertigo are rare, but patients often complain of aural fullness, headache, and/or facial numbness.1,10
Cerebellar infarction usually presents with sudden onset of symptoms at maximal intensity. Risk factors for cerebellar infarction include a history of hypertension, coronary artery disease, diabetes, previous transient ischemic attack, smoking, alcohol consumption, atrial fibrillation, and hyperlipidemia.10
While only 5% of vertigo complaints are the result of CNS disorders, vertigo is an early symptom of brainstem stroke.15 Because of the significant morbidity and mortality associated with stroke, it must be considered in the initial diagnostic workup of vertigo.
Multiple sclerosis (MS) is an autoimmune disease in which the myelin axons in the CNS are destroyed. Dizziness and vertigo are common complaints associated with MS, but these patients will often present with other symptoms suggestive of CNS involvement. Common complaints include, but are not limited to, muscle weakness, fatigue, paresthesias, spasms, ataxia, pain, diplopia, dysarthria, heat intolerance, and urinary frequency.16
Episodic ataxia type 2 (EA2) is an inherited autosomal dominant disorder characterized by attacks of ataxia, vertigo, and nausea that can last from minutes to days. EA2 is often difficult to differentiate from VM due to symptom overlap. Fifty percent of patients with EA2 have a history of migraine headaches; often, there is a family history of similar symptoms.5
On the next page: Ménière Disease Versus VM >>
MÉNIÈRE DISEASE VERSUS VM
When headaches and dizziness coincide, VM is the most probable diagnosis.5 BPPV, Ménière disease, cerebellar disorders, motion sickness, and psychiatric syndromes (major depression and panic disorder) occur more often in patients with migraine than in those without.1 Ménière disease and VM often coexist, and up to 50% of patients with Ménière disease also meet criteria for migraine.5 (For more information, see Pearson T. Ménière disease: a lifelong merry-go-round. Clinician Reviews. 2013;23[10]:38-43.) Because many of their symptoms overlap, differentiating between Ménière disease and VM is critical; key differences in symptom presentation are as follows.17,18
Vertigo. The vertigo of Ménière disease is short-lived, lasting up to 24 hours.17 In contrast, episodes of vertigo with VM can last more than 24 hours; patients may experience a continuous rocking sensation for several weeks or even months.
Hearing loss. Sensorineural hearing loss in patients with Ménière disease is progressive and most often unilateral, but can be bilateral. In patients with VM, sensorineural hearing loss is rare; if it occurs, it is usually episodic and not progressive.17
Tinnitus. Tinnitus is a symptom of both Ménière disease and VM and may be unilateral or bilateral in both. In Ménière disease, patients report tinnitus of significant intensity, low in pitch, and “roaring,” whereas in VM, the tinnitus is usually high-pitched and unobtrusive.
Headache. Unless a concurrent diagnosis of migraine exists, patients with Ménière disease do not present with headache or photophobia. Many patients with VM, though not all, confirm a positive history of headache.
Phonophobia. Phonophobia is a frequent symptom in patients with VM.17
On the next page: Clinical Manifestations >>
CLINICAL MANIFESTATIONS OF VM
The clinical presentation of VM varies for each patient, as do the frequency and duration of episodes. Manifestations of VM may include5,19,20
• Vertigo associated with visual triggers
• Nausea and/or dizziness
• Spontaneous positional vertigo
• Head motion intolerance
• Motion sickness
• Lightheadedness
• Headache
• Chronic disequilibrium
• Inability to concentrate
• Mild hearing loss or tinnitus
• Cervicalgia
• Anxiety
• Panic
• Photophobia
• Phonophobia
• Sensory aura
Visual vertigo, described as vertigo worsened by visual stimulation such as moving scenes, scrolling patterns, and movement of large crowds or traffic, is highly suggestive of VM.19 Aura-type symptoms are of significant diagnostic importance because they may be the only apparent connection between vertigo and migraine.19
The duration of vertigo and dizziness may range from seconds to weeks. These episodes may have no temporal relationship with headaches. Vertigo and dizziness can continue for more than 24 hours for about half of VM patients; in some, symptoms persist for several weeks or more.12 In addition, vertigo and headache may never occur together, which further increases the diagnostic challenge for this disorder.1,5
Although many hypotheses exist, the pathophysiology of VM remains unclear.
Patient History
It is vital to obtain a detailed medical and social history in order to determine possible etiologies for symptoms. Medications in particular must be reviewed with care because many are known to induce vestibular-type symptoms.
Cardiac agents of potential concern for causing dizziness include β-blockers, diuretics, ACE inhibitors, β-blockers, and nitrates. CNS agents associated with dizziness include antipsychotics, dopaminergic drugs, opioids/analgesics, hypnotics, anticonvulsants, tricyclic antidepressants, and muscle relaxants. Anticholinergics, phosphodiesterase type 5 inhibitors, antibacterials, aminoglycosides, fluoroquinolones, and antineoplastics may also lead to dizziness; several of these medications are also known to be ototoxic. Dizziness can also be related to orthostatic hypotension, which is a common adverse effect of many of the listed medications, especially when used in combination.7,9
Diabetes, hypertension, vascular diseases, and neurologic disorders should be considered as causes for vision and proprioception problems.7 Patients may present with a personal history of headaches for years before vestibular symptoms develop.6 Motion sickness is suggestive of a possible migraine diagnosis.19
Positive family history is common for migraineurs.21 Patients ages 30 to 39 are affected most frequently, with an estimated prevalence of 7% in men and 24.4% in women.22
Physical Examination
The focused physical should include cranial nerve assessments, an otoscopic examination, hearing evaluation with a tuning fork, and audiometry, if a hearing deficit is detected. Minor oculomotor abnormalities, such as “weak” nystagmus with vertical, horizontal, torsional, or positional components, may be noted in approximately 70% of patients with VM.5 Various in-office tests (see Table 2) should also be conducted to narrow the possible causes for vertigo and dizziness and rule out more serious disorders.7,10 ,11,23
Diagnostic Workup
Laboratory tests are generally not recommended because they identify the cause of vertigo in only approximately 1% of patients.23 Similarly, routine use of MRI or CT is not recommended, but these modalities may be indicated for patients with focal neurologic deficits or risk factors for cerebrovascular disease, or if acute treatments for peripheral vertigo are unsuccessful.10
On the next page: Treatment >>
TREATMENT OF VM
Nonpharmacologic
Nonpharmacologic measures to prevent VM attacks include the avoidance of “triggers” through dietary restrictions, stress reduction, and healthy lifestyle modifications. Diaries can be used to help identify common triggers to avoid (see Table 3); patients should understand that it may take up to three months before noticeable improvement in symptoms is seen. Once symptoms have resolved, cautious re-introduction of suspect foods may be attempted.24
Vestibular rehabilitation therapy promotes CNS compensation for inner ear deficits and reduces symptoms of disequilibrium and dizziness.25 It is helpful for complications of VM, such as anxiety, visual dependence, or loss of confidence with balance.10
Pharmacologic
Prophylactic drug therapy is the mainstay of medical management for VM when nonpharmacologic measures are inadequate; episodes are frequent and severe; or symptoms are of long duration.5 The drugs’ adverse effect profiles, as well as patient comorbidities, should guide the choice of therapy.26
Abortive migraine therapies have not been shown to be effective for symptoms of dizziness17 and may cause rebound symptoms. Vestibular suppressants (promethazine, dimenhydrinate, and meclizine) can be used for acute episodes of VM.5
According to evidence-based guidelines for the preventive treatment of migraine in adults,26 the pharmacologic options include multiple drug classes stratified by level, depending on the quality of evidence supporting their effectiveness in migraine prevention.
Level A comprises medications with established efficacy. These include divalproex sodium, sodium valproate, topiramate, frovatriptan (for menstrual migraine), metoprolol, propanolol, and timolol.
Level B medications that are “probably effective” include naratriptan, zolmitriptan, amitriptyline, venlafaxine, atenolol, and nadolol.
Medications that are “possibly effective” are in the Level C category and include carbamazepine, nebivolol, pindolol, lisinopril, candesartan, clonidine, guanfacine, and cyproheptadine.
The Level U category drugs are labeled “inadequate or conflicting data to support or refute medication use” and include fluvoxamine, fluoxetine, protriptyline, gabapentin, bisoprolol, cyclandelate, acenocoumarol, warfarin, picotamide, acetazolamide, nicardipine, nifedipine, nimodipine, and verapamil.
Medications that are established as possibly or probably ineffective fall under the category of Other and include clomipramine, lamotrigine, acebutolol, clonazepam, nabumetone, oxcarbazepine, and telmisartan.
When initiating therapy, low doses are recommended because migraineurs’ sensory hypersensitivity is thought to extend to medications.5 Sequence and dosing information, along with contraindications, should be considered when choosing medical therapies (see Table 4).5,12,19,21,26 Patient response to treatment is evaluated after one to three months, and medications should be discontinued if symptoms persist, maximum dose is reached, or significant adverse effects occur.5 A reasonable goal of therapy is to reduce episode frequency by more than 50%.19 Should both lifestyle modifications and medications fail, referral to a specialist for reevaluation of the diagnosis is warranted.8
On the next page: Conclusion >>
CONCLUSION
While VM is considered the most common cause of recurrent vertigo and dizziness, few primary care providers are familiar with the diagnosis. Differentiating among the various causes for these symptoms and determining if the cause is a CNS or peripheral system disorder are essential to narrowing the differential diagnoses.
The inclusion of VM in the ICHD-3 system will increase both clinician awareness and accurate diagnosis of the disorder. Office-based assessments can be performed to evaluate these common complaints, and numerous medical therapies are available to successfully treat patients with VM. Through greater awareness of VM and use of evidence-based diagnostic and treatment guidelines, clinicians can significantly improve quality of life and health outcomes for patients with the disorder.
1. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol. 2009;256(3):333-338.
2. The International Headache Society. The International Headache Classification. 2nd ed. www.ihs-classification.org/en/01_einleitung/02_einleitung/. Accessed May 16, 2014.
3. Lempert T, Oleson J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22:167-172.
4. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. 3rd ed (beta version). Cephalalgia. 2013;33(9):629-808.
5. Cherchi M, Hain T. Migraine-associated vertigo. Otolaryngol Clin Am. 2011;44:367-375.
6. Honaker J, Samy R. Migraine-associated vestibulopathy. Curr Opin Otolaryngol Head Neck Surg. 2008;16:412-415.
7. Shaia WT. Dizziness evaluation. http://emedicine.medscape.com/article/1831429-overview. Accessed May 16, 2014.
8. Watson S. Vertigo and migraine: "how can it be a migraine if I don’t have a headache?" Med Today. 2011;12(12):36-43.
9. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82(4):361-368.
10. Furman M, Rizzolo D. Evaluating the patient with vertigo: a complex complaint made simple. JAAPA. 2011;24(10):52-58.
11. Kuo CH, Pang T, Chang R. Vertigo – part 1 - assessment in general practice. Aust Fam Physician. 2008;37(5):341-347.
12. Hain, T. Migraine associated vertigo: vestibular migraine. www.dizziness-and-balance.com/disorders/central/migraine/mav.html. Accessed May 16, 2014.
13. Cha YH, Kane MJ, Baloh RW. Familial clustering of migraine, episodic vertigo, and Ménière’s disease. Otol Neurotol. 2008;29(1):93-96.
14. Clark MM. How to sort out a complaint of dizziness. Patient Care. 2003;37: 44-52.
15. Hain TC. Brainstem strokes associated with vertigo or hearing symptoms. www.dizziness-and- balance.com/disorders/central/strokes/brainstem%20strokes.htm. Accessed May 16, 2014.
16. Luzzio C, Dangond F. Multiple sclerosis. http://emedicine.medscape.com/article/1146199-overview. Accessed May 16, 2014.
17. Benson AG. Migraine-associated vertigo. http://emedicine.medscape.com/article/884136-overview#aw2aab6b5. Accessed May 16, 2014.
18. Teggi R, Fabiano B, Recanata P, et al. Case reports on two patients with episodic vertigo, fluctuating hearing loss, and migraine responding to prophylactic drugs for migraine. Ménière disease or migraine-associated vertigo? Acta Otorhinolaryngol Ital. 2010;30(4):217-220.
19. Neuhauser H, Lempert T. Vestibular migraine. Neurol Clin. 2009;27:379-391.
20. Kramer J, Buskirk J. Vestibular migraine a/k/a migraine-associated vertigo. www.vestibular.org/migraine-associated-vertigo-mav. Accessed April 1, 2014.
21. Chawla J. Migraine headache. http://emedicine.medscape.com/article/1142556-overview. Accessed May 16, 2014.
22. Cutrer MF, Bajwa ZH, Sabahat A. Pathophysiology, clinical manifestations, and diagnosis of migraine in adults. www.uptodate.com/contents/patho physiology-clinical-manifestations-and-diagnosis-of-migraine-in-adults. Accessed May 16, 2014.
23. Labuguen R. Initial evaluation of vertigo [published correction appears in Am Fam Physician. 2006;73(10):1704]. Am Fam Physician. 2006;73(2):244-251.
24. Texiedo M. Common migraine food triggers. http://deedee.dbi.udel.edu/MichaelTeixidoMD/patientInfo/migrainesFoodTrig.html. Accessed May 16, 2014.
25. Bisdorff AR. Management of vestibular migraine. Ther Adv Neurol Disord. 2011;4(3):183-191.
26. Silberstein S, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults [published correction appears in Neurology. 2013;80:871]. Neurology. 2012;78(17):1337-1345.
1. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol. 2009;256(3):333-338.
2. The International Headache Society. The International Headache Classification. 2nd ed. www.ihs-classification.org/en/01_einleitung/02_einleitung/. Accessed May 16, 2014.
3. Lempert T, Oleson J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22:167-172.
4. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders. 3rd ed (beta version). Cephalalgia. 2013;33(9):629-808.
5. Cherchi M, Hain T. Migraine-associated vertigo. Otolaryngol Clin Am. 2011;44:367-375.
6. Honaker J, Samy R. Migraine-associated vestibulopathy. Curr Opin Otolaryngol Head Neck Surg. 2008;16:412-415.
7. Shaia WT. Dizziness evaluation. http://emedicine.medscape.com/article/1831429-overview. Accessed May 16, 2014.
8. Watson S. Vertigo and migraine: "how can it be a migraine if I don’t have a headache?" Med Today. 2011;12(12):36-43.
9. Post RE, Dickerson LM. Dizziness: a diagnostic approach. Am Fam Physician. 2010;82(4):361-368.
10. Furman M, Rizzolo D. Evaluating the patient with vertigo: a complex complaint made simple. JAAPA. 2011;24(10):52-58.
11. Kuo CH, Pang T, Chang R. Vertigo – part 1 - assessment in general practice. Aust Fam Physician. 2008;37(5):341-347.
12. Hain, T. Migraine associated vertigo: vestibular migraine. www.dizziness-and-balance.com/disorders/central/migraine/mav.html. Accessed May 16, 2014.
13. Cha YH, Kane MJ, Baloh RW. Familial clustering of migraine, episodic vertigo, and Ménière’s disease. Otol Neurotol. 2008;29(1):93-96.
14. Clark MM. How to sort out a complaint of dizziness. Patient Care. 2003;37: 44-52.
15. Hain TC. Brainstem strokes associated with vertigo or hearing symptoms. www.dizziness-and- balance.com/disorders/central/strokes/brainstem%20strokes.htm. Accessed May 16, 2014.
16. Luzzio C, Dangond F. Multiple sclerosis. http://emedicine.medscape.com/article/1146199-overview. Accessed May 16, 2014.
17. Benson AG. Migraine-associated vertigo. http://emedicine.medscape.com/article/884136-overview#aw2aab6b5. Accessed May 16, 2014.
18. Teggi R, Fabiano B, Recanata P, et al. Case reports on two patients with episodic vertigo, fluctuating hearing loss, and migraine responding to prophylactic drugs for migraine. Ménière disease or migraine-associated vertigo? Acta Otorhinolaryngol Ital. 2010;30(4):217-220.
19. Neuhauser H, Lempert T. Vestibular migraine. Neurol Clin. 2009;27:379-391.
20. Kramer J, Buskirk J. Vestibular migraine a/k/a migraine-associated vertigo. www.vestibular.org/migraine-associated-vertigo-mav. Accessed April 1, 2014.
21. Chawla J. Migraine headache. http://emedicine.medscape.com/article/1142556-overview. Accessed May 16, 2014.
22. Cutrer MF, Bajwa ZH, Sabahat A. Pathophysiology, clinical manifestations, and diagnosis of migraine in adults. www.uptodate.com/contents/patho physiology-clinical-manifestations-and-diagnosis-of-migraine-in-adults. Accessed May 16, 2014.
23. Labuguen R. Initial evaluation of vertigo [published correction appears in Am Fam Physician. 2006;73(10):1704]. Am Fam Physician. 2006;73(2):244-251.
24. Texiedo M. Common migraine food triggers. http://deedee.dbi.udel.edu/MichaelTeixidoMD/patientInfo/migrainesFoodTrig.html. Accessed May 16, 2014.
25. Bisdorff AR. Management of vestibular migraine. Ther Adv Neurol Disord. 2011;4(3):183-191.
26. Silberstein S, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults [published correction appears in Neurology. 2013;80:871]. Neurology. 2012;78(17):1337-1345.
HIV Infection: What Primary Care Providers Need to Know
CE/CME No: CR-1405
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the current recommendations for HIV screening.
• Describe the current recommendations for pre- and postexposure HIV prophylaxis.
• Recognize the constellation of symptoms and signs that may represent a patient presenting with acute (primary) HIV infection.
• Discuss the initial evaluation and management of a patient with HIV.
• Compare and contrast the preferred regimens for previously untreated patients with HIV in terms of the key features of the regimens’ components and the rationale for using one versus another.
FACULTY
Susan LeLacheur is an Associate Professor of PA Studies at The George Washington University School of Medicine and Health Sciences in Washington, DC.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.5 hours of American Academy of Physician Assistants (AAPA) Category I CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2014.
Article begins on next page >>
Over the decades, HIV infection has transitioned from an almost universally deadly infection to a chronic, manageable disease. Increased survival, along with improved access to health care and screening, has allowed far more patients to live relatively normal lives. But primary care providers need to stay up to date on all aspects of the disease in order to provide the best possible care to those affected and aid efforts to stem the spread of disease.
Patients with HIV infection whose disease is well controlled can now live a normal lifespan. Because of this increase in lifespan, along with increased health care coverage through the Affordable Care Act, primary care clinicians will be increasingly responsible for the care of patients with HIV. In order to offer optimal treatment, however, clinicians must remain current on HIV screening, initial treatment, and ongoing management. New therapies and simplified regimens have improved HIV care, but diligence in monitoring for complications of HIV and its treatment is needed in order to optimize care.
Consider, for example, a 19-year-old man who comes into your primary care office complaining of a sore throat, malaise, and a generalized rash. On exam, you note cervical adenopathy and a widely scattered rash, mainly on his trunk. His throat is mildly erythematous without exudates. You do a rapid test for strep and for mononucleosis, both of which are negative. A viral syndrome seems the most likely diagnosis, but if you neglect to get a sexual and drug history, you might not think of HIV as the virus in question. And even if routine HIV antibody screening is done as recommended, HIV antibodies have likely not yet developed. This is a classic presentation of acute retroviral syndrome, easily confused with a myriad of less consequential infections. Most cases of HIV are not detected at this stage, although it is estimated that between 50% and 90% of patients with acute HIV infection seek medical care.1-4 The patient with acute HIV and potentially many others can be spared significant harm if HIV is part of your differential diagnosis.
Primary care clinicians play a key role in all aspects of HIV care, starting with HIV prevention through screening, helping HIV-negative patients reduce the risk for infection, and helping to assure that HIV-positive patients are less likely to spread the disease because their own infection is fully suppressed. Primary care providers will also become increasingly involved in the care of patients who are living with HIV. Just as with diabetes or hypertension, patients already HIV infected can be largely managed by knowledgeable primary care clinicians. The required knowledge includes an understanding of the latest recommendations for screening, both pre-exposure and postexposure prophylaxis, and the initial work-up and ongoing management of patients diagnosed with HIV. Expert consultation is often helpful and will be critical in many cases, but all providers should have a working knowledge of HIV care.
On the next page: Screening and prevention >>
SCREENING AND PREVENTION
When discussing many disease processes, prevention is relegated to the end of the discussion—but with HIV, prevention must be a primary concern for patients and providers. HIV prevention includes two critical strategies: finding previously undiagnosed cases of the infection and preventing transmission through treatment of those with the disease, safer sex practices, or prophylactic antiretroviral therapy for those at highest risk. (Chemoprophylaxis for those who have had a potential exposure is referred to as postexposure prophylaxis, or PEP. Chemoprophylaxis for those at high risk for exposure is termed pre-exposure prophylaxis, or PrEP.)
Guidelines published by the CDC in 2006 recommended that clinicians include HIV screening as a routine part of medical care for all patients and that barriers to routine HIV testing be removed. Since then, the estimated proportion of people infected with HIV who are unaware of their HIV-positive status has dropped from about 25% to 16%.5-7 In applying these recommendations to a primary care setting, the practitioner simply adds an HIV antibody test to the standard panel given to new patients. In addition, established patients who have not previously been tested should be advised to have a baseline HIV test regardless of risk.
Clinicians should offer HIV testing on an opt-out basis and inform patients that the test is recommended; patients can refuse the test should they choose to do so. Patients at higher risk for HIV should undergo retesting annually—or more frequently if indicated. Routine testing need not be accompanied by prevention counseling or special consent. While counseling on safer sex or other risk reduction strategies is always appropriate, it should not be seen as an impediment to routine screening.
Newer fourth-generation immunofluorescence assays (IA) detect both HIV-1 and HIV-2 earlier after infection because they are able to detect the HIV viral capsid protein p24 as well as HIV antibodies. If the initial assay is positive, subsequent confirmatory testing with a discrimination assay will distinguish between the two types of HIV. Because the newer IA tests detect antibody much earlier, using the discrimination assay as the confirmatory test rather than a Western blot has become commonplace, although either test may be used. Home testing for HIV is also available. Patients who obtain a positive result are instructed to have the result confirmed by their health care provider.
Neither rapid tests nor home tests will pick up new infections during initial infection (known as the window period prior to the development of detectable antibodies) but they can be very helpful in screening programs since the patient need not return for a second visit to obtain results. Whatever test is initially used, it is important to confirm its results with a second test; for instance, the first test might be a fourth-generation IA (either a rapid test or traditional), confirmed by a discrimination assay or Western blot. If the confirmatory test is negative or indeterminate, an HIV RNA (viral load) test should be considered.8 HIV RNA should also be used whenever a new infection is suspected.
PRE- AND POSTEXPOSURE PROPHYLAXIS
PEP to prevent HIV seroconversion following a possible or confirmed HIV exposure is now well accepted, whether the exposure is occupational or not.9,10 Clinicians should start PEP as soon as possible after exposure, preferably within two hours, but no later than 72 hours after exposure. Do not delay treatment even if the source's or exposed patient’s HIV status or other factors are unknown. Treatment can always be discontinued or modified when further information becomes available.
The most recent update issued by the US Public Health Service includes a change to prior guidelines. They now recommend that all potentially exposed individuals receive a three-drug combination of tenofovir 300 mg orally once daily, emtricitabine 200 mg orally once daily (coformulated as Truvada), and raltegravir 400 mg orally twice daily. Patients should be instructed to continue antiretroviral medications for four weeks. Raltegravir, an integrase inhibitor, has replaced previously recommended drugs used in a three-drug regimen because of its lower incidence of adverse effects as compared to older antiretrovirals. Alternative regimens may be used for a variety of reasons, but the need for an alternative should generally spur expert consultation.
The use of three drugs in all cases is based both on a better understanding of the pathophysiology of HIV and on the availability of medications that are better tolerated. Expert consultation is always helpful in cases of PEP, but particularly in those complicated by pregnancy or breastfeeding, known or suspected drug resistance in the source patient, unknown HIV status of the source patient, delayed report of exposure (> 72 hours), or significant medical illness of the exposed patient. In addition, any toxicity experienced should spur expert consultation. If local expert resources are unavailable, immediate assistance can be obtained through the National Clinicians' Postexposure Prophylaxis Hotline (PEPline) at (888) 448-4911.
Whenever possible, the source patient should undergo testing if his or her HIV status is unknown. If the exposed patient chooses to receive PEP, it is helpful to evaluate the source patient’s history of medication resistance, which should be done by a clinician with HIV expertise. In all cases, the likelihood of HIV conversion after an exposure is low, ranging from approximately 0.3% for a percutaneous exposure to 0.09% for a mucous membrane exposure.11,12
Clinicians should test the exposed patient for HIV and hepatitis B and C, and women should have a pregnancy test performed. For patients being treated, renal and hepatic function should be evaluated and hematology tests done to monitor for adverse reactions to therapy at baseline, two weeks, and completion of treatment. The exposed patient must be advised to take precautions to avoid any potential for transmission of HIV: use barrier contraception and avoid blood or tissue donation or breastfeeding until after the final follow-up visit. Initial follow-up should occur within 72 hours to reevaluate the need for therapy and reinforce the need for adherence if therapy is continued. Clinicians should also assess the patient regarding his or her emotional as well as physical well-being and access to resources to aid in coping with the event. If a fourth-generation test is used, follow-up HIV testing should be done at six weeks and four months after exposure. Otherwise, follow-up HIV testing should occur at six weeks, 12 weeks, and six months.
PrEP using tenofovir 300 mg orally once daily and emtricitabine 200 mg orally once daily is recommended in interim CDC guidelines for those at particularly high risk for HIV exposure, such as homosexual or heterosexual partners of HIV-positive individuals or injection drug users. For PrEP to be effective, medication adherence is critical. Because PrEP involves daily therapy, patients should be carefully selected and expert consultation is advised.13,14 All patients on PrEP require regular follow-up and HIV testing. On follow-up, side effects and continued risk should be evaluated. PrEP should be considered part of a broader prevention strategy that includes condom use, prevention and treatment of other sexually transmitted infections, and frequent reassessment of risk. There are often significant difficulties in obtaining insurance coverage for PrEP, but this may change as further research is done and health care coverage broadens.
On the next page: Recognizing and managing acute HIV >>
RECOGNIZING AND MANAGING ACUTE HIV
As noted above, the symptoms of acute (or primary) retroviral syndrome can suggest numerous other infectious processes, including mononucleosis, influenza, or the common cold. The most common symptoms are fever, fatigue, and malaise, but arthralgias, headache, anorexia, nausea, diarrhea, and pharyngitis are also common. The severity of symptoms can range widely, sometimes causing very mild illness and occasionally causing symptoms sufficiently severe to require hospitalization. In a patient presenting with symptoms that may result from acute retroviral syndrome, an HIV RNA test should be performed as it is the earliest indicator of HIV infection. It is critical that a patient suspected of having acute HIV be counseled regarding prevention of transmission since his or her infectivity during the initial infection is extremely high.
The latest US Department of Health and Human Services (HHS) HIV guidelines, published in February 2013, recommend that all patients newly diagnosed with HIV be offered antiretroviral treatment.15 It is likely that earlier initiation of therapy will reduce the viral set point (the level of virus reached after an immune response to initial infection) and potentially reduce overall damage to the immune system.16 As with all HIV-positive patients, those with new infection must be prepared to commit to ongoing antiretroviral therapy, with the goal of achieving an undetectable viral load.
RECOGNIZING AND MANAGING CHRONIC HIV
Most people with HIV infection will be asymptomatic up until approximately 10 years after the initial infection (hence the importance of screening), but some clinical symptoms and signs should bring HIV to mind as a possible cause. Persistent or recurrent fungal infections, especially oral thrush but also vaginal candidiasis, herpes zoster in an otherwise healthy patient, seborrheic dermatitis, unexplained weight loss, or persistent lymphadenopathy should trigger a suspicion of HIV during the differential diagnosis. Patients may also present very late in their HIV infection with far more serious opportunistic complications, including Pneumocystis jiroveci (formally carinii) pneumonia, infectious esophagitis, cryptococcal meningitis, tuberculosis, Kaposi sarcoma, or any of a myriad of other infectious or neoplastic complications of HIV-related immunosuppression. Most of these very serious illnesses will occur only in patients with a severely depressed immune system (CD4 < 200 cells/mm3), but some patients with very low CD4 cell counts may have no or only very mild symptoms.
Because improved screening has been shown to reduce the proportion of people presenting late in the course of HIV infection,17 patients presenting with severe manifestations of HIV-related immune dysfunction should become increasingly rare. Nonetheless, clinicians should include HIV in the differential for any patient presenting with a significant infection or cancer. A full discussion of HIV-related opportunistic infections and cancers is beyond the scope of this article, but further information can be found at www.aidsinfo.nih.gov.
On the next page: Initiating care >>
INITIATING CARE
Rapport is, of course, critical with all patients, but especially so in patients with HIV. The patient’s psychosocial as well as medical needs must be met in order to optimize adherence both to medication and to ongoing clinical care. HIV, even more than other life-changing chronic diseases, carries a stigma that patients can experience in a variety of ways. There are two messages that clinicians will want to convey to patients: HIV is a chronic, manageable condition, and support is available, either at the clinician’s office or through resources provided by the clinician upon diagnosis.
Underlying mental health problems, whether temporary or more chronic, can have a significant impact on the patient’s ability to adhere to the HIV treatment regimen. Addressing psychosocial needs may also help with patient adherence to clinical care. One of the most salient problems in HIV care today is retention in care. Only 25% of 1.1 million people living with HIV in the US are retained in care and achieve viral suppression (see Figure).18-20 Initial linkage to care and subsequent retention are areas in which all health care providers can play a role. Emergency and urgent care providers should have a protocol for immediate and direct referral of patients who test positive for HIV. Primary care providers can optimally treat their patients by closely monitoring response to therapy and reinforcing adherence to medication and to follow-up visits, both of which will substantially improve long-term survival.21,22
HIV treatment may begin as soon as the diagnosis is made and resistance test results become available, but the patient’s readiness to begin therapy is key. The patient needs to understand the chronic nature of the disease and the need for lifelong therapy so that he or she can commit to fully engaging in the management of the disease. While earlier HIV treatment improves the patient’s prognosis, the urgency of treatment increases gradually as the function of the immune system declines, particularly as the patient’s CD4 count drops below 350 cells/mm3.
The first step in managing a patient with HIV is to perform a complete history and physical examination. It must include a good family history; any concomitant medical conditions; current medications including herbal, alternative, and home therapies; and a complete social and behavioral history. Physical examination should be comprehensive, as manifestations of HIV may be subtle and can occur in any organ system. Physical manifestations of HIV are dependent on the patient’s immune status, but particular attention should be given to the skin and mucous membranes of the mouth and genitals as well as the neurologic examination. Neurologic complications of HIV may include sensory changes and cognitive dysfunction. Persistent nontender lymphadenopathy, which develops with initial infection, is frequently present. Of course, any clinical complaints should spur closer evaluation of the relevant system or systems.
Baseline laboratory testing should include confirmation of HIV if results were obtained anonymously or documentation is not available (Table 1). A resistance test (HIV genotype or GenoSure) should be performed at entry. Resistance mutations can be transmitted from person to person, and the baseline genotype offers the best opportunity to evaluate the patient for transmitted mutations. Resistance to a medication will determine antiretroviral therapy options for the patient. Hepatitis B and C status should be checked in all patients prior to starting therapy because results will impact therapeutic decisions. Patients co-infected with HIV and chronic hepatitis B should be evaluated by a specialist, since medication regimens for HIV and hepatitis B have overlapping activity against these viruses; it is generally advisable to fully treat both infections. Similarly, patients co-infected with hepatitis C and HIV should have a specialist consult. New advances in hepatitis C therapy are revolutionizing our approach to the disease, but HCV and HIV medications may have drug interactions.15,23
The most critical tests for evaluating the patient’s HIV status and response to therapy are the HIV RNA (viral load) and CD4 lymphocyte subset (also known as T cells, T4 cells, or CD4 cells). The CD4 count gives an estimate of the patient’s immune function, while the viral load is the best measure of the effect of medication. In general, the viral load should reach an undetectable level within the first few months of therapy. Other tests that should be done include chemistries and a urinalysis to evaluate renal function, liver enzymes, fasting lipids, and glucose (or A1C).
Other screening tests recommended at baseline include those for exposure to tuberculosis, toxoplasmosis, syphilis, gonorrhea, and chlamydia. Additionally, all women with HIV should have a baseline cervical cancer screening. Cytomegalovirus infection may be assumed in men having sex with men or injection drug users, but a screening immunoglobulin G antibody test should be done in patients at lower risk. Patients without a history of varicella-zoster virus infection or vaccination should be screened for that disease and may be considered for vaccination if they are older than 8 years with a CD4 count higher than 200 or ages 1 to 8 with a CD4 percentage higher than 15%.15,24 Additionally, a test for the HLA-B*5701 allele is recommended prior to starting a regimen containing abacavir. Performing this test at baseline will allow the patient and clinician a broader set of treatment options if the test is negative.
Ongoing monitoring of a patient with HIV depends on many factors. Early in treatment, it is important to keep a regular schedule of visits to monitor continued response to therapy. Additionally, clinicians should monitor for complications of HIV and its treatment, including renal and hepatic effects. Some medications used in HIV therapy can alter laboratory parameters with or without an effect on function. For example, atazanavir will often increase levels of bilirubin. The change is generally mild and not associated with clinical effects. Similarly, elvitegravir and dolutegravir both cause an initial increase in serum creatinine that has not, to date, been associated with clinical renal disease.
The most significant complications faced by patients with HIV as they age are cardiac, metabolic, renal, and bone disease that tend to occur earlier and with greater severity than in the general population. HIV medications are associated with osteoporosis, lipid abnormalities, abnormal glucose metabolism, and renal toxicity. Older patients and those with personal or familial risk factors should be monitored for these complications according to standard prevention guidelines. The new primary care guidelines for the care of patients with HIV published by the Infectious Diseases Society of America acknowledge the far more manageable nature of HIV and extended lifespan of those with the disease. The guidelines recommend extending follow-up intervals to every six to 12 months for those who have no other health complication and whose viral load is undetectable
(< 20 copies/mL) for two to three years.15,24
All patients should receive standard vaccines, including influenza (annually), Tdap (tetanus, diphtheria, pertussis), and human papillomavirus (ages 9 to 26 for females and 9 to 21 for males). In addition, patients with HIV should receive a pneumococcal vaccine and, if antibody negative, a hepatitis A and/or B vaccine.
On the next page: Initiating antiretroviral therapy >>
INITIATING ANTIRETROVIRAL THERAPY
It is always helpful to consult an HIV expert prior to initiating antiretroviral therapy, but for patients with no baseline resistance, medical complications, or potential for drug interactions, initial therapy is fairly straightforward. The overall goals of therapy are to maximize the patient’s quality and quantity of life, improve immune function, suppress viral replication, and prevent HIV transmission.15 HIV transmission is significantly reduced when the infected patient is effectively treated and the viral load is suppressed to undetectable. Immune reconstitution as measured by the CD4 count is variable, but most patients will achieve some improvement once viral replication is controlled. Starting antiretroviral therapy is appropriate at any CD4 cell count level, but data regarding the need for therapy as the immune system is depleted are increasingly strong. The strongest data are for patients with CD4 counts below 350 cells/mm3, but large observational studies show benefit to patients starting earlier. There is also a public health benefit of reducing HIV transmission through treatment of patients already infected.25
Therapy cannot, at this time, eradicate the disease, but optimal viral suppression to an undetectable viral load is the most certain indication that therapy is working and should be monitored closely, generally every three or four months. The baseline viral load will respond quickly, and it is helpful to evaluate it shortly after initiating therapy. Scheduling a visit with the patient two weeks after initiating therapy offers the opportunity to perform a viral load test and to discuss any problems the patient has with therapy, including adherence, adverse effects, and any changes in concomitant medications. Alternately, the patient can be scheduled for an HIV RNA test at two weeks, with a clinical visit set for a time when results will be available (generally another week or two). Sharing viral load results is an excellent tool for supporting the patient’s self-efficacy. The dramatic reduction in virus helps both to assure the patient that he or she can successfully control HIV and to reinforce adherence to both medication and clinical follow-up.
Medication regimens for HIV have become increasingly easy to manage for both the patient and clinician. Most clinicians will generally use those listed as preferred regimens in the HHS guidelines (Table 2). Each recommended regimen contains three antiretroviral medications. Some also contain one additional medicine to pharmacologically boost the activity of one of the other medications by inhibiting metabolism. All recommended regimens include a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs), with either a nonnucleoside reverse transcriptase inhibitor (NNRTI), an integrase strand transfer inhibitor, or a protease inhibitor (PI). All PIs and one of the recommended integrase inhibitor regimens will also include a boosting agent, either ritonavir or cobicistat.15,26
Four nucleoside backbone agents are among those listed as preferred: tenofovir, abacavir, emtricitabine, and lamivudine. Emtricitabine and lamivudine are nearly identical in action and should never be used together. Either may be combined with tenofovir or abacavir, but the availability of fixed-dose coformulations dictates that either tenofovir/emtricitabine (Truvada, also contained in Atripla and Complera, discussed below) or abacavir/lamivudine (Epzicom) is used in practice. Tenofovir is associated with renal toxicity and with osteoporosis. Abacavir is associated with a rare hypersensitivity reaction in patients with a positive HLA-B*5701 mutation. The only abacavir/lamivudine–containing regimen currently included as preferred in the HHS guidelines is with the integrase inhibitor dolutegravir, based on studies done with that combination. With the exception of abacavir, all these medications require an adjustment for patients with renal impairment.15
The one NNRTI-based regimen listed as preferred is a one-pill, once-daily combination of efavirenz, tenofovir, and emtricitabine, coformulated under the trade name Atripla. The efavirenz component of this combination has some teratogenic potential and should generally not be used in women who are likely to become pregnant. Efavirenz has the potential to cause sleep disturbance and other central nervous system (CNS) effects; these often improve after a week or two of therapy. One additional consideration with an efavirenz-containing regimen is the long half-life of the drug relative to other components of the regimen. If a patient is inconsistently adherent, there will be periods when only efavirenz will remain in his or her system, leading to a high potential for the development of resistance. For this reason, it may not be the optimal initial choice for a patient who is known to have difficulty with medication adherence.15
There is another NNRTI-based regimen, a single-pill, once-daily coformulation that includes rilpivirine, tenofovir, and emtricitabine (Complera). It is currently included on the alternative, not the preferred, list of options, primarily because a high rate of virologic failure (failure to achieve an undetectable HIV RNA level) was seen in patients who started the regimen when their HIV RNA levels were greater than 100,000 copies/mL at baseline. In patients with lower HIV RNA levels at baseline, this regimen provides a reasonable alternative for women considering pregnancy or patients with pre-existing psychiatric disorders or sleep disturbances, who are at particular risk for the CNS adverse effects of efavirenz. Like efavirenz, rilpivirine has also been associated with depressive disorders. It must be taken with a full meal (400 cal), so the patient’s eating pattern and access to food should be considered.
Two PI-based regimens are included as preferred: atazanavir and darunavir. Both must be boosted with a low dose (100 mg/d in a treatment-naïve patient) of ritonavir and combined with tenofovir/emtricitabine. While either regimen requires three separate pills daily, all three may be taken together along with food to improve absorption. As a class, boosted PIs provide a strong barrier to resistance and have a similar half-life to the NRTI backbone, making them a good choice for a patient with a history of medication nonadherence. The most common adverse reactions, particularly to the ritonavir portion of the regimen, are gastrointestinal disturbances, such as nausea and diarrhea. PIs are also associated with lipid abnormalities. This is less of a problem with atazanavir than with most other medications in the class. Atazanavir is associated with a benign increase in bilirubin that is generally asymptomatic. A coformulation of darunavir with tenofovir/emtricitabine and boosted with cobicistat rather than ritonavir is likely to be approved and available in the near future, providing the first one-pill, once-daily option for a PI-based regimen.
The newest class of HIV antiretroviral medications are the integrase strand transfer inhibitors: raltegravir, elvitegravir, and dolutegravir. Elvitegravir is available in a fixed-dose, one-pill, once-daily coformulation with tenofovir/emtricitabine and boosted with cobicistat. Raltegravir is currently dosed twice daily in combination with once daily tenofovir/etricitabine, but research on a once-daily formulation is in progress. Raltegravir is metabolized differently from most other medications used for HIV and may be better for use in patients on statins, opioids, oral contraceptives, and many other drugs. Dolutegravir is taken once daily with either tenofovir/emtricitabine or abacavir/lamivudine.15,26
A one-pill, once-daily coformulation of dolutegravir with abacavir/lamivudine will likely be available soon. Though most patients tolerate these drugs well, common adverse effects include nausea, diz-ziness, headache, and insomnia. Dolutegravir and elvitegravir will both cause an initial increase in the serum creatinine level, not associated with renal tubular dysfunction, that will stabilize after a few weeks.26
All preferred regimens are effective and should bring the viral load under control fairly rapidly, although full viral suppression may take weeks or months. Even after viral suppression is achieved, “blips” or temporary increases in the viral load can occur during therapy. However, a persistent viral load exceeding 200 copies/mL may indicate problems with the therapy. Clinicians should assess and reinforce adherence to the regimen, but if the viral load remains detectable, an expert should be
consulted.27
All medications used to control HIV have the potential for drug interactions, both with each other and with other commonly used medications, including OTC and herbal medicines. For this reason, it is important to carefully review all medicines for interactions; a full list may be found at http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adoles cent-arv-guidelines/32/drug-interactions or www.hiv-druginteractions.org/.
On the next page: When to refer and conclusion >>
WHEN TO REFER
Expert assistance with initiation and management of HIV care is always helpful, but primary care clinicians can provide the majority of care. Obtain expert consultation when
• Initial resistance testing shows resistance to NRTI backbone medication(s) listed as preferred initial options
• There is a significant comorbidity, especially one that involves medications that may interact with an HIV regimen
• Hepatitis B or C is present
• Pregnancy is present or planned
• HIV is not controlled by the initial regimen or a regimen fails.
CONCLUSION
HIV is now viewed as a chronic, manageable disease. As improved screening continues to identify an increasing proportion of HIV-positive patients, improving therapies keep these patients alive and well. As access to care continues to improve, primary care clinicians will provide more of the care of HIV patients. Some patients will require consultation with an HIV specialist, but primary care clinicians must build a strong foundation of knowledge regarding the treatment of HIV and remain current as progress in the field continues.
1. Daar ES, Little S, Pitt J, et al. Diagnosis of primary HIV-1 infection: Los Angeles county primary HIV infection recruitment network. Ann Intern Med. 2001;134:25-29.
2. Hightow-Weidman LB, Golin CE, Green K, et al. Identifying people with acute HIV infection: demographic features, risk factors, and use of health care among individuals with AHI in North Carolina. AIDS Behav. 2009;13:1075-1083.
3. Richey LE, Halperin J. Acute human immunodeficiency virus infection. Am J Med Sci. 2013;345(2):136-142.
4. Weintraub AC, Giner J, Menezes P, et al. Infrequent diagnosis of primary human immunodeficiency virus infection: missed opportunities in acute care settings. Arch Intern Med. 2003;163:2097-2100.
5. Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs.
J Acquir Immune Defic Syndr. 2005;39:446-453.
6. CDC. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. www.cdc.gov/hiv/topics/surveillance/resourc es/reports/#supplemental. Accessed April 15, 2014.
7. CDC. National HIV prevention progress report, 2013. www.cdc.gov/hiv/pdf/policies_NationalProgressReport.pdf. Published December 2013. Accessed April 15, 2014.
8. CDC. Draft recommendations: diagnostic laboratory testing for HIV infection in the United States. www.cdc.gov/hiv/pdf/policies_Draft_HIV_Testing_Alg_Rec_508.2.pdf. Accessed April 15, 2014.
9. Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
10. Pinkerton SD, Martin JN, Roland ME, et al. Cost-effectiveness of HIV postexposure prophylaxis following sexual or injection drug exposure in 96 metropolitan areas in the United States. AIDS. 2004;18:2065-2073.
11. CDC. Update to interim guidance for preexposure prophylaxis (PrEP) for the prevention of HIV infection: PrEP for injecting drug users. MMWR. 2013;62:463-465.
12. Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med. 1997;102(suppl 5B):9-15.
13. CDC. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR. 2012;61:586-589.
14. CDC. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR. 2011;60:65-68.
15. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed April 15, 2014.
16. Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009; 360:1815-1826.
17. Castel AD, Magnus M, Peterson J, et al. Implementing a novel citywide rapid HIV testing campaign in Washington, DC: findings and lessons learned. Public Health Rep. 2012;127:422-431.
18. CDC. HIV in the United States: stages of care. www.cdc.gov/hiv/pdf/research_mmp_StagesofCare.pdf. Accessed April 15, 2014.
19. Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793-800.
20. Mugavero MJ, Westfall AO, Zinski A, et al. Retention in Care (RIC) Study Group. Measuring retention in HIV care: the elusive gold standard.
J Acquir Immune Defic Syndr. 2012;61:574-580.
21. Fleishman JA, Yehia BR, Moore RD, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249-259.
22. Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62:356-362.
23. American Association for the Study of Liver Diseases. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed April 15, 2014.
24. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1-e34.
25. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
26. Panel on Antiretroviral Guidelines for Adults and Adolescents. Recommendation on integrase inhibitor use in antiretroviral treatment-naive HIV-infected individuals from the HHS panel on antiretroviral guidelines for adults and adolescents. http://aidsinfo.nih.gov/contentfiles/upload/AdultARV_INSTIRecommendations.pdf. Updated 2013. Accessed April 15, 2014.
27. Chesney MA. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S149-155.
CE/CME No: CR-1405
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the current recommendations for HIV screening.
• Describe the current recommendations for pre- and postexposure HIV prophylaxis.
• Recognize the constellation of symptoms and signs that may represent a patient presenting with acute (primary) HIV infection.
• Discuss the initial evaluation and management of a patient with HIV.
• Compare and contrast the preferred regimens for previously untreated patients with HIV in terms of the key features of the regimens’ components and the rationale for using one versus another.
FACULTY
Susan LeLacheur is an Associate Professor of PA Studies at The George Washington University School of Medicine and Health Sciences in Washington, DC.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.5 hours of American Academy of Physician Assistants (AAPA) Category I CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2014.
Article begins on next page >>
Over the decades, HIV infection has transitioned from an almost universally deadly infection to a chronic, manageable disease. Increased survival, along with improved access to health care and screening, has allowed far more patients to live relatively normal lives. But primary care providers need to stay up to date on all aspects of the disease in order to provide the best possible care to those affected and aid efforts to stem the spread of disease.
Patients with HIV infection whose disease is well controlled can now live a normal lifespan. Because of this increase in lifespan, along with increased health care coverage through the Affordable Care Act, primary care clinicians will be increasingly responsible for the care of patients with HIV. In order to offer optimal treatment, however, clinicians must remain current on HIV screening, initial treatment, and ongoing management. New therapies and simplified regimens have improved HIV care, but diligence in monitoring for complications of HIV and its treatment is needed in order to optimize care.
Consider, for example, a 19-year-old man who comes into your primary care office complaining of a sore throat, malaise, and a generalized rash. On exam, you note cervical adenopathy and a widely scattered rash, mainly on his trunk. His throat is mildly erythematous without exudates. You do a rapid test for strep and for mononucleosis, both of which are negative. A viral syndrome seems the most likely diagnosis, but if you neglect to get a sexual and drug history, you might not think of HIV as the virus in question. And even if routine HIV antibody screening is done as recommended, HIV antibodies have likely not yet developed. This is a classic presentation of acute retroviral syndrome, easily confused with a myriad of less consequential infections. Most cases of HIV are not detected at this stage, although it is estimated that between 50% and 90% of patients with acute HIV infection seek medical care.1-4 The patient with acute HIV and potentially many others can be spared significant harm if HIV is part of your differential diagnosis.
Primary care clinicians play a key role in all aspects of HIV care, starting with HIV prevention through screening, helping HIV-negative patients reduce the risk for infection, and helping to assure that HIV-positive patients are less likely to spread the disease because their own infection is fully suppressed. Primary care providers will also become increasingly involved in the care of patients who are living with HIV. Just as with diabetes or hypertension, patients already HIV infected can be largely managed by knowledgeable primary care clinicians. The required knowledge includes an understanding of the latest recommendations for screening, both pre-exposure and postexposure prophylaxis, and the initial work-up and ongoing management of patients diagnosed with HIV. Expert consultation is often helpful and will be critical in many cases, but all providers should have a working knowledge of HIV care.
On the next page: Screening and prevention >>
SCREENING AND PREVENTION
When discussing many disease processes, prevention is relegated to the end of the discussion—but with HIV, prevention must be a primary concern for patients and providers. HIV prevention includes two critical strategies: finding previously undiagnosed cases of the infection and preventing transmission through treatment of those with the disease, safer sex practices, or prophylactic antiretroviral therapy for those at highest risk. (Chemoprophylaxis for those who have had a potential exposure is referred to as postexposure prophylaxis, or PEP. Chemoprophylaxis for those at high risk for exposure is termed pre-exposure prophylaxis, or PrEP.)
Guidelines published by the CDC in 2006 recommended that clinicians include HIV screening as a routine part of medical care for all patients and that barriers to routine HIV testing be removed. Since then, the estimated proportion of people infected with HIV who are unaware of their HIV-positive status has dropped from about 25% to 16%.5-7 In applying these recommendations to a primary care setting, the practitioner simply adds an HIV antibody test to the standard panel given to new patients. In addition, established patients who have not previously been tested should be advised to have a baseline HIV test regardless of risk.
Clinicians should offer HIV testing on an opt-out basis and inform patients that the test is recommended; patients can refuse the test should they choose to do so. Patients at higher risk for HIV should undergo retesting annually—or more frequently if indicated. Routine testing need not be accompanied by prevention counseling or special consent. While counseling on safer sex or other risk reduction strategies is always appropriate, it should not be seen as an impediment to routine screening.
Newer fourth-generation immunofluorescence assays (IA) detect both HIV-1 and HIV-2 earlier after infection because they are able to detect the HIV viral capsid protein p24 as well as HIV antibodies. If the initial assay is positive, subsequent confirmatory testing with a discrimination assay will distinguish between the two types of HIV. Because the newer IA tests detect antibody much earlier, using the discrimination assay as the confirmatory test rather than a Western blot has become commonplace, although either test may be used. Home testing for HIV is also available. Patients who obtain a positive result are instructed to have the result confirmed by their health care provider.
Neither rapid tests nor home tests will pick up new infections during initial infection (known as the window period prior to the development of detectable antibodies) but they can be very helpful in screening programs since the patient need not return for a second visit to obtain results. Whatever test is initially used, it is important to confirm its results with a second test; for instance, the first test might be a fourth-generation IA (either a rapid test or traditional), confirmed by a discrimination assay or Western blot. If the confirmatory test is negative or indeterminate, an HIV RNA (viral load) test should be considered.8 HIV RNA should also be used whenever a new infection is suspected.
PRE- AND POSTEXPOSURE PROPHYLAXIS
PEP to prevent HIV seroconversion following a possible or confirmed HIV exposure is now well accepted, whether the exposure is occupational or not.9,10 Clinicians should start PEP as soon as possible after exposure, preferably within two hours, but no later than 72 hours after exposure. Do not delay treatment even if the source's or exposed patient’s HIV status or other factors are unknown. Treatment can always be discontinued or modified when further information becomes available.
The most recent update issued by the US Public Health Service includes a change to prior guidelines. They now recommend that all potentially exposed individuals receive a three-drug combination of tenofovir 300 mg orally once daily, emtricitabine 200 mg orally once daily (coformulated as Truvada), and raltegravir 400 mg orally twice daily. Patients should be instructed to continue antiretroviral medications for four weeks. Raltegravir, an integrase inhibitor, has replaced previously recommended drugs used in a three-drug regimen because of its lower incidence of adverse effects as compared to older antiretrovirals. Alternative regimens may be used for a variety of reasons, but the need for an alternative should generally spur expert consultation.
The use of three drugs in all cases is based both on a better understanding of the pathophysiology of HIV and on the availability of medications that are better tolerated. Expert consultation is always helpful in cases of PEP, but particularly in those complicated by pregnancy or breastfeeding, known or suspected drug resistance in the source patient, unknown HIV status of the source patient, delayed report of exposure (> 72 hours), or significant medical illness of the exposed patient. In addition, any toxicity experienced should spur expert consultation. If local expert resources are unavailable, immediate assistance can be obtained through the National Clinicians' Postexposure Prophylaxis Hotline (PEPline) at (888) 448-4911.
Whenever possible, the source patient should undergo testing if his or her HIV status is unknown. If the exposed patient chooses to receive PEP, it is helpful to evaluate the source patient’s history of medication resistance, which should be done by a clinician with HIV expertise. In all cases, the likelihood of HIV conversion after an exposure is low, ranging from approximately 0.3% for a percutaneous exposure to 0.09% for a mucous membrane exposure.11,12
Clinicians should test the exposed patient for HIV and hepatitis B and C, and women should have a pregnancy test performed. For patients being treated, renal and hepatic function should be evaluated and hematology tests done to monitor for adverse reactions to therapy at baseline, two weeks, and completion of treatment. The exposed patient must be advised to take precautions to avoid any potential for transmission of HIV: use barrier contraception and avoid blood or tissue donation or breastfeeding until after the final follow-up visit. Initial follow-up should occur within 72 hours to reevaluate the need for therapy and reinforce the need for adherence if therapy is continued. Clinicians should also assess the patient regarding his or her emotional as well as physical well-being and access to resources to aid in coping with the event. If a fourth-generation test is used, follow-up HIV testing should be done at six weeks and four months after exposure. Otherwise, follow-up HIV testing should occur at six weeks, 12 weeks, and six months.
PrEP using tenofovir 300 mg orally once daily and emtricitabine 200 mg orally once daily is recommended in interim CDC guidelines for those at particularly high risk for HIV exposure, such as homosexual or heterosexual partners of HIV-positive individuals or injection drug users. For PrEP to be effective, medication adherence is critical. Because PrEP involves daily therapy, patients should be carefully selected and expert consultation is advised.13,14 All patients on PrEP require regular follow-up and HIV testing. On follow-up, side effects and continued risk should be evaluated. PrEP should be considered part of a broader prevention strategy that includes condom use, prevention and treatment of other sexually transmitted infections, and frequent reassessment of risk. There are often significant difficulties in obtaining insurance coverage for PrEP, but this may change as further research is done and health care coverage broadens.
On the next page: Recognizing and managing acute HIV >>
RECOGNIZING AND MANAGING ACUTE HIV
As noted above, the symptoms of acute (or primary) retroviral syndrome can suggest numerous other infectious processes, including mononucleosis, influenza, or the common cold. The most common symptoms are fever, fatigue, and malaise, but arthralgias, headache, anorexia, nausea, diarrhea, and pharyngitis are also common. The severity of symptoms can range widely, sometimes causing very mild illness and occasionally causing symptoms sufficiently severe to require hospitalization. In a patient presenting with symptoms that may result from acute retroviral syndrome, an HIV RNA test should be performed as it is the earliest indicator of HIV infection. It is critical that a patient suspected of having acute HIV be counseled regarding prevention of transmission since his or her infectivity during the initial infection is extremely high.
The latest US Department of Health and Human Services (HHS) HIV guidelines, published in February 2013, recommend that all patients newly diagnosed with HIV be offered antiretroviral treatment.15 It is likely that earlier initiation of therapy will reduce the viral set point (the level of virus reached after an immune response to initial infection) and potentially reduce overall damage to the immune system.16 As with all HIV-positive patients, those with new infection must be prepared to commit to ongoing antiretroviral therapy, with the goal of achieving an undetectable viral load.
RECOGNIZING AND MANAGING CHRONIC HIV
Most people with HIV infection will be asymptomatic up until approximately 10 years after the initial infection (hence the importance of screening), but some clinical symptoms and signs should bring HIV to mind as a possible cause. Persistent or recurrent fungal infections, especially oral thrush but also vaginal candidiasis, herpes zoster in an otherwise healthy patient, seborrheic dermatitis, unexplained weight loss, or persistent lymphadenopathy should trigger a suspicion of HIV during the differential diagnosis. Patients may also present very late in their HIV infection with far more serious opportunistic complications, including Pneumocystis jiroveci (formally carinii) pneumonia, infectious esophagitis, cryptococcal meningitis, tuberculosis, Kaposi sarcoma, or any of a myriad of other infectious or neoplastic complications of HIV-related immunosuppression. Most of these very serious illnesses will occur only in patients with a severely depressed immune system (CD4 < 200 cells/mm3), but some patients with very low CD4 cell counts may have no or only very mild symptoms.
Because improved screening has been shown to reduce the proportion of people presenting late in the course of HIV infection,17 patients presenting with severe manifestations of HIV-related immune dysfunction should become increasingly rare. Nonetheless, clinicians should include HIV in the differential for any patient presenting with a significant infection or cancer. A full discussion of HIV-related opportunistic infections and cancers is beyond the scope of this article, but further information can be found at www.aidsinfo.nih.gov.
On the next page: Initiating care >>
INITIATING CARE
Rapport is, of course, critical with all patients, but especially so in patients with HIV. The patient’s psychosocial as well as medical needs must be met in order to optimize adherence both to medication and to ongoing clinical care. HIV, even more than other life-changing chronic diseases, carries a stigma that patients can experience in a variety of ways. There are two messages that clinicians will want to convey to patients: HIV is a chronic, manageable condition, and support is available, either at the clinician’s office or through resources provided by the clinician upon diagnosis.
Underlying mental health problems, whether temporary or more chronic, can have a significant impact on the patient’s ability to adhere to the HIV treatment regimen. Addressing psychosocial needs may also help with patient adherence to clinical care. One of the most salient problems in HIV care today is retention in care. Only 25% of 1.1 million people living with HIV in the US are retained in care and achieve viral suppression (see Figure).18-20 Initial linkage to care and subsequent retention are areas in which all health care providers can play a role. Emergency and urgent care providers should have a protocol for immediate and direct referral of patients who test positive for HIV. Primary care providers can optimally treat their patients by closely monitoring response to therapy and reinforcing adherence to medication and to follow-up visits, both of which will substantially improve long-term survival.21,22
HIV treatment may begin as soon as the diagnosis is made and resistance test results become available, but the patient’s readiness to begin therapy is key. The patient needs to understand the chronic nature of the disease and the need for lifelong therapy so that he or she can commit to fully engaging in the management of the disease. While earlier HIV treatment improves the patient’s prognosis, the urgency of treatment increases gradually as the function of the immune system declines, particularly as the patient’s CD4 count drops below 350 cells/mm3.
The first step in managing a patient with HIV is to perform a complete history and physical examination. It must include a good family history; any concomitant medical conditions; current medications including herbal, alternative, and home therapies; and a complete social and behavioral history. Physical examination should be comprehensive, as manifestations of HIV may be subtle and can occur in any organ system. Physical manifestations of HIV are dependent on the patient’s immune status, but particular attention should be given to the skin and mucous membranes of the mouth and genitals as well as the neurologic examination. Neurologic complications of HIV may include sensory changes and cognitive dysfunction. Persistent nontender lymphadenopathy, which develops with initial infection, is frequently present. Of course, any clinical complaints should spur closer evaluation of the relevant system or systems.
Baseline laboratory testing should include confirmation of HIV if results were obtained anonymously or documentation is not available (Table 1). A resistance test (HIV genotype or GenoSure) should be performed at entry. Resistance mutations can be transmitted from person to person, and the baseline genotype offers the best opportunity to evaluate the patient for transmitted mutations. Resistance to a medication will determine antiretroviral therapy options for the patient. Hepatitis B and C status should be checked in all patients prior to starting therapy because results will impact therapeutic decisions. Patients co-infected with HIV and chronic hepatitis B should be evaluated by a specialist, since medication regimens for HIV and hepatitis B have overlapping activity against these viruses; it is generally advisable to fully treat both infections. Similarly, patients co-infected with hepatitis C and HIV should have a specialist consult. New advances in hepatitis C therapy are revolutionizing our approach to the disease, but HCV and HIV medications may have drug interactions.15,23
The most critical tests for evaluating the patient’s HIV status and response to therapy are the HIV RNA (viral load) and CD4 lymphocyte subset (also known as T cells, T4 cells, or CD4 cells). The CD4 count gives an estimate of the patient’s immune function, while the viral load is the best measure of the effect of medication. In general, the viral load should reach an undetectable level within the first few months of therapy. Other tests that should be done include chemistries and a urinalysis to evaluate renal function, liver enzymes, fasting lipids, and glucose (or A1C).
Other screening tests recommended at baseline include those for exposure to tuberculosis, toxoplasmosis, syphilis, gonorrhea, and chlamydia. Additionally, all women with HIV should have a baseline cervical cancer screening. Cytomegalovirus infection may be assumed in men having sex with men or injection drug users, but a screening immunoglobulin G antibody test should be done in patients at lower risk. Patients without a history of varicella-zoster virus infection or vaccination should be screened for that disease and may be considered for vaccination if they are older than 8 years with a CD4 count higher than 200 or ages 1 to 8 with a CD4 percentage higher than 15%.15,24 Additionally, a test for the HLA-B*5701 allele is recommended prior to starting a regimen containing abacavir. Performing this test at baseline will allow the patient and clinician a broader set of treatment options if the test is negative.
Ongoing monitoring of a patient with HIV depends on many factors. Early in treatment, it is important to keep a regular schedule of visits to monitor continued response to therapy. Additionally, clinicians should monitor for complications of HIV and its treatment, including renal and hepatic effects. Some medications used in HIV therapy can alter laboratory parameters with or without an effect on function. For example, atazanavir will often increase levels of bilirubin. The change is generally mild and not associated with clinical effects. Similarly, elvitegravir and dolutegravir both cause an initial increase in serum creatinine that has not, to date, been associated with clinical renal disease.
The most significant complications faced by patients with HIV as they age are cardiac, metabolic, renal, and bone disease that tend to occur earlier and with greater severity than in the general population. HIV medications are associated with osteoporosis, lipid abnormalities, abnormal glucose metabolism, and renal toxicity. Older patients and those with personal or familial risk factors should be monitored for these complications according to standard prevention guidelines. The new primary care guidelines for the care of patients with HIV published by the Infectious Diseases Society of America acknowledge the far more manageable nature of HIV and extended lifespan of those with the disease. The guidelines recommend extending follow-up intervals to every six to 12 months for those who have no other health complication and whose viral load is undetectable
(< 20 copies/mL) for two to three years.15,24
All patients should receive standard vaccines, including influenza (annually), Tdap (tetanus, diphtheria, pertussis), and human papillomavirus (ages 9 to 26 for females and 9 to 21 for males). In addition, patients with HIV should receive a pneumococcal vaccine and, if antibody negative, a hepatitis A and/or B vaccine.
On the next page: Initiating antiretroviral therapy >>
INITIATING ANTIRETROVIRAL THERAPY
It is always helpful to consult an HIV expert prior to initiating antiretroviral therapy, but for patients with no baseline resistance, medical complications, or potential for drug interactions, initial therapy is fairly straightforward. The overall goals of therapy are to maximize the patient’s quality and quantity of life, improve immune function, suppress viral replication, and prevent HIV transmission.15 HIV transmission is significantly reduced when the infected patient is effectively treated and the viral load is suppressed to undetectable. Immune reconstitution as measured by the CD4 count is variable, but most patients will achieve some improvement once viral replication is controlled. Starting antiretroviral therapy is appropriate at any CD4 cell count level, but data regarding the need for therapy as the immune system is depleted are increasingly strong. The strongest data are for patients with CD4 counts below 350 cells/mm3, but large observational studies show benefit to patients starting earlier. There is also a public health benefit of reducing HIV transmission through treatment of patients already infected.25
Therapy cannot, at this time, eradicate the disease, but optimal viral suppression to an undetectable viral load is the most certain indication that therapy is working and should be monitored closely, generally every three or four months. The baseline viral load will respond quickly, and it is helpful to evaluate it shortly after initiating therapy. Scheduling a visit with the patient two weeks after initiating therapy offers the opportunity to perform a viral load test and to discuss any problems the patient has with therapy, including adherence, adverse effects, and any changes in concomitant medications. Alternately, the patient can be scheduled for an HIV RNA test at two weeks, with a clinical visit set for a time when results will be available (generally another week or two). Sharing viral load results is an excellent tool for supporting the patient’s self-efficacy. The dramatic reduction in virus helps both to assure the patient that he or she can successfully control HIV and to reinforce adherence to both medication and clinical follow-up.
Medication regimens for HIV have become increasingly easy to manage for both the patient and clinician. Most clinicians will generally use those listed as preferred regimens in the HHS guidelines (Table 2). Each recommended regimen contains three antiretroviral medications. Some also contain one additional medicine to pharmacologically boost the activity of one of the other medications by inhibiting metabolism. All recommended regimens include a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs), with either a nonnucleoside reverse transcriptase inhibitor (NNRTI), an integrase strand transfer inhibitor, or a protease inhibitor (PI). All PIs and one of the recommended integrase inhibitor regimens will also include a boosting agent, either ritonavir or cobicistat.15,26
Four nucleoside backbone agents are among those listed as preferred: tenofovir, abacavir, emtricitabine, and lamivudine. Emtricitabine and lamivudine are nearly identical in action and should never be used together. Either may be combined with tenofovir or abacavir, but the availability of fixed-dose coformulations dictates that either tenofovir/emtricitabine (Truvada, also contained in Atripla and Complera, discussed below) or abacavir/lamivudine (Epzicom) is used in practice. Tenofovir is associated with renal toxicity and with osteoporosis. Abacavir is associated with a rare hypersensitivity reaction in patients with a positive HLA-B*5701 mutation. The only abacavir/lamivudine–containing regimen currently included as preferred in the HHS guidelines is with the integrase inhibitor dolutegravir, based on studies done with that combination. With the exception of abacavir, all these medications require an adjustment for patients with renal impairment.15
The one NNRTI-based regimen listed as preferred is a one-pill, once-daily combination of efavirenz, tenofovir, and emtricitabine, coformulated under the trade name Atripla. The efavirenz component of this combination has some teratogenic potential and should generally not be used in women who are likely to become pregnant. Efavirenz has the potential to cause sleep disturbance and other central nervous system (CNS) effects; these often improve after a week or two of therapy. One additional consideration with an efavirenz-containing regimen is the long half-life of the drug relative to other components of the regimen. If a patient is inconsistently adherent, there will be periods when only efavirenz will remain in his or her system, leading to a high potential for the development of resistance. For this reason, it may not be the optimal initial choice for a patient who is known to have difficulty with medication adherence.15
There is another NNRTI-based regimen, a single-pill, once-daily coformulation that includes rilpivirine, tenofovir, and emtricitabine (Complera). It is currently included on the alternative, not the preferred, list of options, primarily because a high rate of virologic failure (failure to achieve an undetectable HIV RNA level) was seen in patients who started the regimen when their HIV RNA levels were greater than 100,000 copies/mL at baseline. In patients with lower HIV RNA levels at baseline, this regimen provides a reasonable alternative for women considering pregnancy or patients with pre-existing psychiatric disorders or sleep disturbances, who are at particular risk for the CNS adverse effects of efavirenz. Like efavirenz, rilpivirine has also been associated with depressive disorders. It must be taken with a full meal (400 cal), so the patient’s eating pattern and access to food should be considered.
Two PI-based regimens are included as preferred: atazanavir and darunavir. Both must be boosted with a low dose (100 mg/d in a treatment-naïve patient) of ritonavir and combined with tenofovir/emtricitabine. While either regimen requires three separate pills daily, all three may be taken together along with food to improve absorption. As a class, boosted PIs provide a strong barrier to resistance and have a similar half-life to the NRTI backbone, making them a good choice for a patient with a history of medication nonadherence. The most common adverse reactions, particularly to the ritonavir portion of the regimen, are gastrointestinal disturbances, such as nausea and diarrhea. PIs are also associated with lipid abnormalities. This is less of a problem with atazanavir than with most other medications in the class. Atazanavir is associated with a benign increase in bilirubin that is generally asymptomatic. A coformulation of darunavir with tenofovir/emtricitabine and boosted with cobicistat rather than ritonavir is likely to be approved and available in the near future, providing the first one-pill, once-daily option for a PI-based regimen.
The newest class of HIV antiretroviral medications are the integrase strand transfer inhibitors: raltegravir, elvitegravir, and dolutegravir. Elvitegravir is available in a fixed-dose, one-pill, once-daily coformulation with tenofovir/emtricitabine and boosted with cobicistat. Raltegravir is currently dosed twice daily in combination with once daily tenofovir/etricitabine, but research on a once-daily formulation is in progress. Raltegravir is metabolized differently from most other medications used for HIV and may be better for use in patients on statins, opioids, oral contraceptives, and many other drugs. Dolutegravir is taken once daily with either tenofovir/emtricitabine or abacavir/lamivudine.15,26
A one-pill, once-daily coformulation of dolutegravir with abacavir/lamivudine will likely be available soon. Though most patients tolerate these drugs well, common adverse effects include nausea, diz-ziness, headache, and insomnia. Dolutegravir and elvitegravir will both cause an initial increase in the serum creatinine level, not associated with renal tubular dysfunction, that will stabilize after a few weeks.26
All preferred regimens are effective and should bring the viral load under control fairly rapidly, although full viral suppression may take weeks or months. Even after viral suppression is achieved, “blips” or temporary increases in the viral load can occur during therapy. However, a persistent viral load exceeding 200 copies/mL may indicate problems with the therapy. Clinicians should assess and reinforce adherence to the regimen, but if the viral load remains detectable, an expert should be
consulted.27
All medications used to control HIV have the potential for drug interactions, both with each other and with other commonly used medications, including OTC and herbal medicines. For this reason, it is important to carefully review all medicines for interactions; a full list may be found at http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adoles cent-arv-guidelines/32/drug-interactions or www.hiv-druginteractions.org/.
On the next page: When to refer and conclusion >>
WHEN TO REFER
Expert assistance with initiation and management of HIV care is always helpful, but primary care clinicians can provide the majority of care. Obtain expert consultation when
• Initial resistance testing shows resistance to NRTI backbone medication(s) listed as preferred initial options
• There is a significant comorbidity, especially one that involves medications that may interact with an HIV regimen
• Hepatitis B or C is present
• Pregnancy is present or planned
• HIV is not controlled by the initial regimen or a regimen fails.
CONCLUSION
HIV is now viewed as a chronic, manageable disease. As improved screening continues to identify an increasing proportion of HIV-positive patients, improving therapies keep these patients alive and well. As access to care continues to improve, primary care clinicians will provide more of the care of HIV patients. Some patients will require consultation with an HIV specialist, but primary care clinicians must build a strong foundation of knowledge regarding the treatment of HIV and remain current as progress in the field continues.
CE/CME No: CR-1405
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the current recommendations for HIV screening.
• Describe the current recommendations for pre- and postexposure HIV prophylaxis.
• Recognize the constellation of symptoms and signs that may represent a patient presenting with acute (primary) HIV infection.
• Discuss the initial evaluation and management of a patient with HIV.
• Compare and contrast the preferred regimens for previously untreated patients with HIV in terms of the key features of the regimens’ components and the rationale for using one versus another.
FACULTY
Susan LeLacheur is an Associate Professor of PA Studies at The George Washington University School of Medicine and Health Sciences in Washington, DC.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.5 hours of American Academy of Physician Assistants (AAPA) Category I CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2014.
Article begins on next page >>
Over the decades, HIV infection has transitioned from an almost universally deadly infection to a chronic, manageable disease. Increased survival, along with improved access to health care and screening, has allowed far more patients to live relatively normal lives. But primary care providers need to stay up to date on all aspects of the disease in order to provide the best possible care to those affected and aid efforts to stem the spread of disease.
Patients with HIV infection whose disease is well controlled can now live a normal lifespan. Because of this increase in lifespan, along with increased health care coverage through the Affordable Care Act, primary care clinicians will be increasingly responsible for the care of patients with HIV. In order to offer optimal treatment, however, clinicians must remain current on HIV screening, initial treatment, and ongoing management. New therapies and simplified regimens have improved HIV care, but diligence in monitoring for complications of HIV and its treatment is needed in order to optimize care.
Consider, for example, a 19-year-old man who comes into your primary care office complaining of a sore throat, malaise, and a generalized rash. On exam, you note cervical adenopathy and a widely scattered rash, mainly on his trunk. His throat is mildly erythematous without exudates. You do a rapid test for strep and for mononucleosis, both of which are negative. A viral syndrome seems the most likely diagnosis, but if you neglect to get a sexual and drug history, you might not think of HIV as the virus in question. And even if routine HIV antibody screening is done as recommended, HIV antibodies have likely not yet developed. This is a classic presentation of acute retroviral syndrome, easily confused with a myriad of less consequential infections. Most cases of HIV are not detected at this stage, although it is estimated that between 50% and 90% of patients with acute HIV infection seek medical care.1-4 The patient with acute HIV and potentially many others can be spared significant harm if HIV is part of your differential diagnosis.
Primary care clinicians play a key role in all aspects of HIV care, starting with HIV prevention through screening, helping HIV-negative patients reduce the risk for infection, and helping to assure that HIV-positive patients are less likely to spread the disease because their own infection is fully suppressed. Primary care providers will also become increasingly involved in the care of patients who are living with HIV. Just as with diabetes or hypertension, patients already HIV infected can be largely managed by knowledgeable primary care clinicians. The required knowledge includes an understanding of the latest recommendations for screening, both pre-exposure and postexposure prophylaxis, and the initial work-up and ongoing management of patients diagnosed with HIV. Expert consultation is often helpful and will be critical in many cases, but all providers should have a working knowledge of HIV care.
On the next page: Screening and prevention >>
SCREENING AND PREVENTION
When discussing many disease processes, prevention is relegated to the end of the discussion—but with HIV, prevention must be a primary concern for patients and providers. HIV prevention includes two critical strategies: finding previously undiagnosed cases of the infection and preventing transmission through treatment of those with the disease, safer sex practices, or prophylactic antiretroviral therapy for those at highest risk. (Chemoprophylaxis for those who have had a potential exposure is referred to as postexposure prophylaxis, or PEP. Chemoprophylaxis for those at high risk for exposure is termed pre-exposure prophylaxis, or PrEP.)
Guidelines published by the CDC in 2006 recommended that clinicians include HIV screening as a routine part of medical care for all patients and that barriers to routine HIV testing be removed. Since then, the estimated proportion of people infected with HIV who are unaware of their HIV-positive status has dropped from about 25% to 16%.5-7 In applying these recommendations to a primary care setting, the practitioner simply adds an HIV antibody test to the standard panel given to new patients. In addition, established patients who have not previously been tested should be advised to have a baseline HIV test regardless of risk.
Clinicians should offer HIV testing on an opt-out basis and inform patients that the test is recommended; patients can refuse the test should they choose to do so. Patients at higher risk for HIV should undergo retesting annually—or more frequently if indicated. Routine testing need not be accompanied by prevention counseling or special consent. While counseling on safer sex or other risk reduction strategies is always appropriate, it should not be seen as an impediment to routine screening.
Newer fourth-generation immunofluorescence assays (IA) detect both HIV-1 and HIV-2 earlier after infection because they are able to detect the HIV viral capsid protein p24 as well as HIV antibodies. If the initial assay is positive, subsequent confirmatory testing with a discrimination assay will distinguish between the two types of HIV. Because the newer IA tests detect antibody much earlier, using the discrimination assay as the confirmatory test rather than a Western blot has become commonplace, although either test may be used. Home testing for HIV is also available. Patients who obtain a positive result are instructed to have the result confirmed by their health care provider.
Neither rapid tests nor home tests will pick up new infections during initial infection (known as the window period prior to the development of detectable antibodies) but they can be very helpful in screening programs since the patient need not return for a second visit to obtain results. Whatever test is initially used, it is important to confirm its results with a second test; for instance, the first test might be a fourth-generation IA (either a rapid test or traditional), confirmed by a discrimination assay or Western blot. If the confirmatory test is negative or indeterminate, an HIV RNA (viral load) test should be considered.8 HIV RNA should also be used whenever a new infection is suspected.
PRE- AND POSTEXPOSURE PROPHYLAXIS
PEP to prevent HIV seroconversion following a possible or confirmed HIV exposure is now well accepted, whether the exposure is occupational or not.9,10 Clinicians should start PEP as soon as possible after exposure, preferably within two hours, but no later than 72 hours after exposure. Do not delay treatment even if the source's or exposed patient’s HIV status or other factors are unknown. Treatment can always be discontinued or modified when further information becomes available.
The most recent update issued by the US Public Health Service includes a change to prior guidelines. They now recommend that all potentially exposed individuals receive a three-drug combination of tenofovir 300 mg orally once daily, emtricitabine 200 mg orally once daily (coformulated as Truvada), and raltegravir 400 mg orally twice daily. Patients should be instructed to continue antiretroviral medications for four weeks. Raltegravir, an integrase inhibitor, has replaced previously recommended drugs used in a three-drug regimen because of its lower incidence of adverse effects as compared to older antiretrovirals. Alternative regimens may be used for a variety of reasons, but the need for an alternative should generally spur expert consultation.
The use of three drugs in all cases is based both on a better understanding of the pathophysiology of HIV and on the availability of medications that are better tolerated. Expert consultation is always helpful in cases of PEP, but particularly in those complicated by pregnancy or breastfeeding, known or suspected drug resistance in the source patient, unknown HIV status of the source patient, delayed report of exposure (> 72 hours), or significant medical illness of the exposed patient. In addition, any toxicity experienced should spur expert consultation. If local expert resources are unavailable, immediate assistance can be obtained through the National Clinicians' Postexposure Prophylaxis Hotline (PEPline) at (888) 448-4911.
Whenever possible, the source patient should undergo testing if his or her HIV status is unknown. If the exposed patient chooses to receive PEP, it is helpful to evaluate the source patient’s history of medication resistance, which should be done by a clinician with HIV expertise. In all cases, the likelihood of HIV conversion after an exposure is low, ranging from approximately 0.3% for a percutaneous exposure to 0.09% for a mucous membrane exposure.11,12
Clinicians should test the exposed patient for HIV and hepatitis B and C, and women should have a pregnancy test performed. For patients being treated, renal and hepatic function should be evaluated and hematology tests done to monitor for adverse reactions to therapy at baseline, two weeks, and completion of treatment. The exposed patient must be advised to take precautions to avoid any potential for transmission of HIV: use barrier contraception and avoid blood or tissue donation or breastfeeding until after the final follow-up visit. Initial follow-up should occur within 72 hours to reevaluate the need for therapy and reinforce the need for adherence if therapy is continued. Clinicians should also assess the patient regarding his or her emotional as well as physical well-being and access to resources to aid in coping with the event. If a fourth-generation test is used, follow-up HIV testing should be done at six weeks and four months after exposure. Otherwise, follow-up HIV testing should occur at six weeks, 12 weeks, and six months.
PrEP using tenofovir 300 mg orally once daily and emtricitabine 200 mg orally once daily is recommended in interim CDC guidelines for those at particularly high risk for HIV exposure, such as homosexual or heterosexual partners of HIV-positive individuals or injection drug users. For PrEP to be effective, medication adherence is critical. Because PrEP involves daily therapy, patients should be carefully selected and expert consultation is advised.13,14 All patients on PrEP require regular follow-up and HIV testing. On follow-up, side effects and continued risk should be evaluated. PrEP should be considered part of a broader prevention strategy that includes condom use, prevention and treatment of other sexually transmitted infections, and frequent reassessment of risk. There are often significant difficulties in obtaining insurance coverage for PrEP, but this may change as further research is done and health care coverage broadens.
On the next page: Recognizing and managing acute HIV >>
RECOGNIZING AND MANAGING ACUTE HIV
As noted above, the symptoms of acute (or primary) retroviral syndrome can suggest numerous other infectious processes, including mononucleosis, influenza, or the common cold. The most common symptoms are fever, fatigue, and malaise, but arthralgias, headache, anorexia, nausea, diarrhea, and pharyngitis are also common. The severity of symptoms can range widely, sometimes causing very mild illness and occasionally causing symptoms sufficiently severe to require hospitalization. In a patient presenting with symptoms that may result from acute retroviral syndrome, an HIV RNA test should be performed as it is the earliest indicator of HIV infection. It is critical that a patient suspected of having acute HIV be counseled regarding prevention of transmission since his or her infectivity during the initial infection is extremely high.
The latest US Department of Health and Human Services (HHS) HIV guidelines, published in February 2013, recommend that all patients newly diagnosed with HIV be offered antiretroviral treatment.15 It is likely that earlier initiation of therapy will reduce the viral set point (the level of virus reached after an immune response to initial infection) and potentially reduce overall damage to the immune system.16 As with all HIV-positive patients, those with new infection must be prepared to commit to ongoing antiretroviral therapy, with the goal of achieving an undetectable viral load.
RECOGNIZING AND MANAGING CHRONIC HIV
Most people with HIV infection will be asymptomatic up until approximately 10 years after the initial infection (hence the importance of screening), but some clinical symptoms and signs should bring HIV to mind as a possible cause. Persistent or recurrent fungal infections, especially oral thrush but also vaginal candidiasis, herpes zoster in an otherwise healthy patient, seborrheic dermatitis, unexplained weight loss, or persistent lymphadenopathy should trigger a suspicion of HIV during the differential diagnosis. Patients may also present very late in their HIV infection with far more serious opportunistic complications, including Pneumocystis jiroveci (formally carinii) pneumonia, infectious esophagitis, cryptococcal meningitis, tuberculosis, Kaposi sarcoma, or any of a myriad of other infectious or neoplastic complications of HIV-related immunosuppression. Most of these very serious illnesses will occur only in patients with a severely depressed immune system (CD4 < 200 cells/mm3), but some patients with very low CD4 cell counts may have no or only very mild symptoms.
Because improved screening has been shown to reduce the proportion of people presenting late in the course of HIV infection,17 patients presenting with severe manifestations of HIV-related immune dysfunction should become increasingly rare. Nonetheless, clinicians should include HIV in the differential for any patient presenting with a significant infection or cancer. A full discussion of HIV-related opportunistic infections and cancers is beyond the scope of this article, but further information can be found at www.aidsinfo.nih.gov.
On the next page: Initiating care >>
INITIATING CARE
Rapport is, of course, critical with all patients, but especially so in patients with HIV. The patient’s psychosocial as well as medical needs must be met in order to optimize adherence both to medication and to ongoing clinical care. HIV, even more than other life-changing chronic diseases, carries a stigma that patients can experience in a variety of ways. There are two messages that clinicians will want to convey to patients: HIV is a chronic, manageable condition, and support is available, either at the clinician’s office or through resources provided by the clinician upon diagnosis.
Underlying mental health problems, whether temporary or more chronic, can have a significant impact on the patient’s ability to adhere to the HIV treatment regimen. Addressing psychosocial needs may also help with patient adherence to clinical care. One of the most salient problems in HIV care today is retention in care. Only 25% of 1.1 million people living with HIV in the US are retained in care and achieve viral suppression (see Figure).18-20 Initial linkage to care and subsequent retention are areas in which all health care providers can play a role. Emergency and urgent care providers should have a protocol for immediate and direct referral of patients who test positive for HIV. Primary care providers can optimally treat their patients by closely monitoring response to therapy and reinforcing adherence to medication and to follow-up visits, both of which will substantially improve long-term survival.21,22
HIV treatment may begin as soon as the diagnosis is made and resistance test results become available, but the patient’s readiness to begin therapy is key. The patient needs to understand the chronic nature of the disease and the need for lifelong therapy so that he or she can commit to fully engaging in the management of the disease. While earlier HIV treatment improves the patient’s prognosis, the urgency of treatment increases gradually as the function of the immune system declines, particularly as the patient’s CD4 count drops below 350 cells/mm3.
The first step in managing a patient with HIV is to perform a complete history and physical examination. It must include a good family history; any concomitant medical conditions; current medications including herbal, alternative, and home therapies; and a complete social and behavioral history. Physical examination should be comprehensive, as manifestations of HIV may be subtle and can occur in any organ system. Physical manifestations of HIV are dependent on the patient’s immune status, but particular attention should be given to the skin and mucous membranes of the mouth and genitals as well as the neurologic examination. Neurologic complications of HIV may include sensory changes and cognitive dysfunction. Persistent nontender lymphadenopathy, which develops with initial infection, is frequently present. Of course, any clinical complaints should spur closer evaluation of the relevant system or systems.
Baseline laboratory testing should include confirmation of HIV if results were obtained anonymously or documentation is not available (Table 1). A resistance test (HIV genotype or GenoSure) should be performed at entry. Resistance mutations can be transmitted from person to person, and the baseline genotype offers the best opportunity to evaluate the patient for transmitted mutations. Resistance to a medication will determine antiretroviral therapy options for the patient. Hepatitis B and C status should be checked in all patients prior to starting therapy because results will impact therapeutic decisions. Patients co-infected with HIV and chronic hepatitis B should be evaluated by a specialist, since medication regimens for HIV and hepatitis B have overlapping activity against these viruses; it is generally advisable to fully treat both infections. Similarly, patients co-infected with hepatitis C and HIV should have a specialist consult. New advances in hepatitis C therapy are revolutionizing our approach to the disease, but HCV and HIV medications may have drug interactions.15,23
The most critical tests for evaluating the patient’s HIV status and response to therapy are the HIV RNA (viral load) and CD4 lymphocyte subset (also known as T cells, T4 cells, or CD4 cells). The CD4 count gives an estimate of the patient’s immune function, while the viral load is the best measure of the effect of medication. In general, the viral load should reach an undetectable level within the first few months of therapy. Other tests that should be done include chemistries and a urinalysis to evaluate renal function, liver enzymes, fasting lipids, and glucose (or A1C).
Other screening tests recommended at baseline include those for exposure to tuberculosis, toxoplasmosis, syphilis, gonorrhea, and chlamydia. Additionally, all women with HIV should have a baseline cervical cancer screening. Cytomegalovirus infection may be assumed in men having sex with men or injection drug users, but a screening immunoglobulin G antibody test should be done in patients at lower risk. Patients without a history of varicella-zoster virus infection or vaccination should be screened for that disease and may be considered for vaccination if they are older than 8 years with a CD4 count higher than 200 or ages 1 to 8 with a CD4 percentage higher than 15%.15,24 Additionally, a test for the HLA-B*5701 allele is recommended prior to starting a regimen containing abacavir. Performing this test at baseline will allow the patient and clinician a broader set of treatment options if the test is negative.
Ongoing monitoring of a patient with HIV depends on many factors. Early in treatment, it is important to keep a regular schedule of visits to monitor continued response to therapy. Additionally, clinicians should monitor for complications of HIV and its treatment, including renal and hepatic effects. Some medications used in HIV therapy can alter laboratory parameters with or without an effect on function. For example, atazanavir will often increase levels of bilirubin. The change is generally mild and not associated with clinical effects. Similarly, elvitegravir and dolutegravir both cause an initial increase in serum creatinine that has not, to date, been associated with clinical renal disease.
The most significant complications faced by patients with HIV as they age are cardiac, metabolic, renal, and bone disease that tend to occur earlier and with greater severity than in the general population. HIV medications are associated with osteoporosis, lipid abnormalities, abnormal glucose metabolism, and renal toxicity. Older patients and those with personal or familial risk factors should be monitored for these complications according to standard prevention guidelines. The new primary care guidelines for the care of patients with HIV published by the Infectious Diseases Society of America acknowledge the far more manageable nature of HIV and extended lifespan of those with the disease. The guidelines recommend extending follow-up intervals to every six to 12 months for those who have no other health complication and whose viral load is undetectable
(< 20 copies/mL) for two to three years.15,24
All patients should receive standard vaccines, including influenza (annually), Tdap (tetanus, diphtheria, pertussis), and human papillomavirus (ages 9 to 26 for females and 9 to 21 for males). In addition, patients with HIV should receive a pneumococcal vaccine and, if antibody negative, a hepatitis A and/or B vaccine.
On the next page: Initiating antiretroviral therapy >>
INITIATING ANTIRETROVIRAL THERAPY
It is always helpful to consult an HIV expert prior to initiating antiretroviral therapy, but for patients with no baseline resistance, medical complications, or potential for drug interactions, initial therapy is fairly straightforward. The overall goals of therapy are to maximize the patient’s quality and quantity of life, improve immune function, suppress viral replication, and prevent HIV transmission.15 HIV transmission is significantly reduced when the infected patient is effectively treated and the viral load is suppressed to undetectable. Immune reconstitution as measured by the CD4 count is variable, but most patients will achieve some improvement once viral replication is controlled. Starting antiretroviral therapy is appropriate at any CD4 cell count level, but data regarding the need for therapy as the immune system is depleted are increasingly strong. The strongest data are for patients with CD4 counts below 350 cells/mm3, but large observational studies show benefit to patients starting earlier. There is also a public health benefit of reducing HIV transmission through treatment of patients already infected.25
Therapy cannot, at this time, eradicate the disease, but optimal viral suppression to an undetectable viral load is the most certain indication that therapy is working and should be monitored closely, generally every three or four months. The baseline viral load will respond quickly, and it is helpful to evaluate it shortly after initiating therapy. Scheduling a visit with the patient two weeks after initiating therapy offers the opportunity to perform a viral load test and to discuss any problems the patient has with therapy, including adherence, adverse effects, and any changes in concomitant medications. Alternately, the patient can be scheduled for an HIV RNA test at two weeks, with a clinical visit set for a time when results will be available (generally another week or two). Sharing viral load results is an excellent tool for supporting the patient’s self-efficacy. The dramatic reduction in virus helps both to assure the patient that he or she can successfully control HIV and to reinforce adherence to both medication and clinical follow-up.
Medication regimens for HIV have become increasingly easy to manage for both the patient and clinician. Most clinicians will generally use those listed as preferred regimens in the HHS guidelines (Table 2). Each recommended regimen contains three antiretroviral medications. Some also contain one additional medicine to pharmacologically boost the activity of one of the other medications by inhibiting metabolism. All recommended regimens include a backbone of two nucleoside reverse transcriptase inhibitors (NRTIs), with either a nonnucleoside reverse transcriptase inhibitor (NNRTI), an integrase strand transfer inhibitor, or a protease inhibitor (PI). All PIs and one of the recommended integrase inhibitor regimens will also include a boosting agent, either ritonavir or cobicistat.15,26
Four nucleoside backbone agents are among those listed as preferred: tenofovir, abacavir, emtricitabine, and lamivudine. Emtricitabine and lamivudine are nearly identical in action and should never be used together. Either may be combined with tenofovir or abacavir, but the availability of fixed-dose coformulations dictates that either tenofovir/emtricitabine (Truvada, also contained in Atripla and Complera, discussed below) or abacavir/lamivudine (Epzicom) is used in practice. Tenofovir is associated with renal toxicity and with osteoporosis. Abacavir is associated with a rare hypersensitivity reaction in patients with a positive HLA-B*5701 mutation. The only abacavir/lamivudine–containing regimen currently included as preferred in the HHS guidelines is with the integrase inhibitor dolutegravir, based on studies done with that combination. With the exception of abacavir, all these medications require an adjustment for patients with renal impairment.15
The one NNRTI-based regimen listed as preferred is a one-pill, once-daily combination of efavirenz, tenofovir, and emtricitabine, coformulated under the trade name Atripla. The efavirenz component of this combination has some teratogenic potential and should generally not be used in women who are likely to become pregnant. Efavirenz has the potential to cause sleep disturbance and other central nervous system (CNS) effects; these often improve after a week or two of therapy. One additional consideration with an efavirenz-containing regimen is the long half-life of the drug relative to other components of the regimen. If a patient is inconsistently adherent, there will be periods when only efavirenz will remain in his or her system, leading to a high potential for the development of resistance. For this reason, it may not be the optimal initial choice for a patient who is known to have difficulty with medication adherence.15
There is another NNRTI-based regimen, a single-pill, once-daily coformulation that includes rilpivirine, tenofovir, and emtricitabine (Complera). It is currently included on the alternative, not the preferred, list of options, primarily because a high rate of virologic failure (failure to achieve an undetectable HIV RNA level) was seen in patients who started the regimen when their HIV RNA levels were greater than 100,000 copies/mL at baseline. In patients with lower HIV RNA levels at baseline, this regimen provides a reasonable alternative for women considering pregnancy or patients with pre-existing psychiatric disorders or sleep disturbances, who are at particular risk for the CNS adverse effects of efavirenz. Like efavirenz, rilpivirine has also been associated with depressive disorders. It must be taken with a full meal (400 cal), so the patient’s eating pattern and access to food should be considered.
Two PI-based regimens are included as preferred: atazanavir and darunavir. Both must be boosted with a low dose (100 mg/d in a treatment-naïve patient) of ritonavir and combined with tenofovir/emtricitabine. While either regimen requires three separate pills daily, all three may be taken together along with food to improve absorption. As a class, boosted PIs provide a strong barrier to resistance and have a similar half-life to the NRTI backbone, making them a good choice for a patient with a history of medication nonadherence. The most common adverse reactions, particularly to the ritonavir portion of the regimen, are gastrointestinal disturbances, such as nausea and diarrhea. PIs are also associated with lipid abnormalities. This is less of a problem with atazanavir than with most other medications in the class. Atazanavir is associated with a benign increase in bilirubin that is generally asymptomatic. A coformulation of darunavir with tenofovir/emtricitabine and boosted with cobicistat rather than ritonavir is likely to be approved and available in the near future, providing the first one-pill, once-daily option for a PI-based regimen.
The newest class of HIV antiretroviral medications are the integrase strand transfer inhibitors: raltegravir, elvitegravir, and dolutegravir. Elvitegravir is available in a fixed-dose, one-pill, once-daily coformulation with tenofovir/emtricitabine and boosted with cobicistat. Raltegravir is currently dosed twice daily in combination with once daily tenofovir/etricitabine, but research on a once-daily formulation is in progress. Raltegravir is metabolized differently from most other medications used for HIV and may be better for use in patients on statins, opioids, oral contraceptives, and many other drugs. Dolutegravir is taken once daily with either tenofovir/emtricitabine or abacavir/lamivudine.15,26
A one-pill, once-daily coformulation of dolutegravir with abacavir/lamivudine will likely be available soon. Though most patients tolerate these drugs well, common adverse effects include nausea, diz-ziness, headache, and insomnia. Dolutegravir and elvitegravir will both cause an initial increase in the serum creatinine level, not associated with renal tubular dysfunction, that will stabilize after a few weeks.26
All preferred regimens are effective and should bring the viral load under control fairly rapidly, although full viral suppression may take weeks or months. Even after viral suppression is achieved, “blips” or temporary increases in the viral load can occur during therapy. However, a persistent viral load exceeding 200 copies/mL may indicate problems with the therapy. Clinicians should assess and reinforce adherence to the regimen, but if the viral load remains detectable, an expert should be
consulted.27
All medications used to control HIV have the potential for drug interactions, both with each other and with other commonly used medications, including OTC and herbal medicines. For this reason, it is important to carefully review all medicines for interactions; a full list may be found at http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adoles cent-arv-guidelines/32/drug-interactions or www.hiv-druginteractions.org/.
On the next page: When to refer and conclusion >>
WHEN TO REFER
Expert assistance with initiation and management of HIV care is always helpful, but primary care clinicians can provide the majority of care. Obtain expert consultation when
• Initial resistance testing shows resistance to NRTI backbone medication(s) listed as preferred initial options
• There is a significant comorbidity, especially one that involves medications that may interact with an HIV regimen
• Hepatitis B or C is present
• Pregnancy is present or planned
• HIV is not controlled by the initial regimen or a regimen fails.
CONCLUSION
HIV is now viewed as a chronic, manageable disease. As improved screening continues to identify an increasing proportion of HIV-positive patients, improving therapies keep these patients alive and well. As access to care continues to improve, primary care clinicians will provide more of the care of HIV patients. Some patients will require consultation with an HIV specialist, but primary care clinicians must build a strong foundation of knowledge regarding the treatment of HIV and remain current as progress in the field continues.
1. Daar ES, Little S, Pitt J, et al. Diagnosis of primary HIV-1 infection: Los Angeles county primary HIV infection recruitment network. Ann Intern Med. 2001;134:25-29.
2. Hightow-Weidman LB, Golin CE, Green K, et al. Identifying people with acute HIV infection: demographic features, risk factors, and use of health care among individuals with AHI in North Carolina. AIDS Behav. 2009;13:1075-1083.
3. Richey LE, Halperin J. Acute human immunodeficiency virus infection. Am J Med Sci. 2013;345(2):136-142.
4. Weintraub AC, Giner J, Menezes P, et al. Infrequent diagnosis of primary human immunodeficiency virus infection: missed opportunities in acute care settings. Arch Intern Med. 2003;163:2097-2100.
5. Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs.
J Acquir Immune Defic Syndr. 2005;39:446-453.
6. CDC. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. www.cdc.gov/hiv/topics/surveillance/resourc es/reports/#supplemental. Accessed April 15, 2014.
7. CDC. National HIV prevention progress report, 2013. www.cdc.gov/hiv/pdf/policies_NationalProgressReport.pdf. Published December 2013. Accessed April 15, 2014.
8. CDC. Draft recommendations: diagnostic laboratory testing for HIV infection in the United States. www.cdc.gov/hiv/pdf/policies_Draft_HIV_Testing_Alg_Rec_508.2.pdf. Accessed April 15, 2014.
9. Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
10. Pinkerton SD, Martin JN, Roland ME, et al. Cost-effectiveness of HIV postexposure prophylaxis following sexual or injection drug exposure in 96 metropolitan areas in the United States. AIDS. 2004;18:2065-2073.
11. CDC. Update to interim guidance for preexposure prophylaxis (PrEP) for the prevention of HIV infection: PrEP for injecting drug users. MMWR. 2013;62:463-465.
12. Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med. 1997;102(suppl 5B):9-15.
13. CDC. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR. 2012;61:586-589.
14. CDC. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR. 2011;60:65-68.
15. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed April 15, 2014.
16. Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009; 360:1815-1826.
17. Castel AD, Magnus M, Peterson J, et al. Implementing a novel citywide rapid HIV testing campaign in Washington, DC: findings and lessons learned. Public Health Rep. 2012;127:422-431.
18. CDC. HIV in the United States: stages of care. www.cdc.gov/hiv/pdf/research_mmp_StagesofCare.pdf. Accessed April 15, 2014.
19. Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793-800.
20. Mugavero MJ, Westfall AO, Zinski A, et al. Retention in Care (RIC) Study Group. Measuring retention in HIV care: the elusive gold standard.
J Acquir Immune Defic Syndr. 2012;61:574-580.
21. Fleishman JA, Yehia BR, Moore RD, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249-259.
22. Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62:356-362.
23. American Association for the Study of Liver Diseases. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed April 15, 2014.
24. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1-e34.
25. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
26. Panel on Antiretroviral Guidelines for Adults and Adolescents. Recommendation on integrase inhibitor use in antiretroviral treatment-naive HIV-infected individuals from the HHS panel on antiretroviral guidelines for adults and adolescents. http://aidsinfo.nih.gov/contentfiles/upload/AdultARV_INSTIRecommendations.pdf. Updated 2013. Accessed April 15, 2014.
27. Chesney MA. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S149-155.
1. Daar ES, Little S, Pitt J, et al. Diagnosis of primary HIV-1 infection: Los Angeles county primary HIV infection recruitment network. Ann Intern Med. 2001;134:25-29.
2. Hightow-Weidman LB, Golin CE, Green K, et al. Identifying people with acute HIV infection: demographic features, risk factors, and use of health care among individuals with AHI in North Carolina. AIDS Behav. 2009;13:1075-1083.
3. Richey LE, Halperin J. Acute human immunodeficiency virus infection. Am J Med Sci. 2013;345(2):136-142.
4. Weintraub AC, Giner J, Menezes P, et al. Infrequent diagnosis of primary human immunodeficiency virus infection: missed opportunities in acute care settings. Arch Intern Med. 2003;163:2097-2100.
5. Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs.
J Acquir Immune Defic Syndr. 2005;39:446-453.
6. CDC. Estimated HIV incidence among adults and adolescents in the United States, 2007–2010. www.cdc.gov/hiv/topics/surveillance/resourc es/reports/#supplemental. Accessed April 15, 2014.
7. CDC. National HIV prevention progress report, 2013. www.cdc.gov/hiv/pdf/policies_NationalProgressReport.pdf. Published December 2013. Accessed April 15, 2014.
8. CDC. Draft recommendations: diagnostic laboratory testing for HIV infection in the United States. www.cdc.gov/hiv/pdf/policies_Draft_HIV_Testing_Alg_Rec_508.2.pdf. Accessed April 15, 2014.
9. Kuhar DT, Henderson DK, Struble KA, et al. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
10. Pinkerton SD, Martin JN, Roland ME, et al. Cost-effectiveness of HIV postexposure prophylaxis following sexual or injection drug exposure in 96 metropolitan areas in the United States. AIDS. 2004;18:2065-2073.
11. CDC. Update to interim guidance for preexposure prophylaxis (PrEP) for the prevention of HIV infection: PrEP for injecting drug users. MMWR. 2013;62:463-465.
12. Bell DM. Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview. Am J Med. 1997;102(suppl 5B):9-15.
13. CDC. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR. 2012;61:586-589.
14. CDC. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR. 2011;60:65-68.
15. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed April 15, 2014.
16. Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009; 360:1815-1826.
17. Castel AD, Magnus M, Peterson J, et al. Implementing a novel citywide rapid HIV testing campaign in Washington, DC: findings and lessons learned. Public Health Rep. 2012;127:422-431.
18. CDC. HIV in the United States: stages of care. www.cdc.gov/hiv/pdf/research_mmp_StagesofCare.pdf. Accessed April 15, 2014.
19. Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793-800.
20. Mugavero MJ, Westfall AO, Zinski A, et al. Retention in Care (RIC) Study Group. Measuring retention in HIV care: the elusive gold standard.
J Acquir Immune Defic Syndr. 2012;61:574-580.
21. Fleishman JA, Yehia BR, Moore RD, et al. Establishment, retention, and loss to follow-up in outpatient HIV care. J Acquir Immune Defic Syndr. 2012;60:249-259.
22. Rebeiro P, Althoff KN, Buchacz K, et al. Retention among North American HIV-infected persons in clinical care, 2000-2008. J Acquir Immune Defic Syndr. 2013;62:356-362.
23. American Association for the Study of Liver Diseases. Recommendations for testing, managing, and treating hepatitis C. www.hcvguidelines.org/. Accessed April 15, 2014.
24. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1-e34.
25. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493-505.
26. Panel on Antiretroviral Guidelines for Adults and Adolescents. Recommendation on integrase inhibitor use in antiretroviral treatment-naive HIV-infected individuals from the HHS panel on antiretroviral guidelines for adults and adolescents. http://aidsinfo.nih.gov/contentfiles/upload/AdultARV_INSTIRecommendations.pdf. Updated 2013. Accessed April 15, 2014.
27. Chesney MA. The elusive gold standard: future perspectives for HIV adherence assessment and intervention. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S149-155.