User login
Whole Health Oncology—Just Do It: Making Whole Person Cancer Care Routine and Regular at the Dayton VA Medical Center (DVAMC)
Background
VA Whole Health (WH) is an approach that empowers and equips people to take charge of their health and well-being. In 2020, 18 WH Flagship sites demonstrated reduced opiate use and smaller increases in pharmacy costs as well as favorable veteran self-reported measures. VA mandated WH integration into mental health and primary care. Purose: To incorporate WH within Dayton VA cancer care, using the Personal Health Inventory (PHI) as an intake tool, a tumor-agnostic WH oncology clinic was established.
Methods
Led by an oncologist, a referral-based clinic opened in 2021. Pre-work included EHR items (stop codes/templates), staff training and leverage of mental health integration. VA’s generic PHI was utilized until an oncology-specific PHI was developed by leaders in the field.(3-5) Clinic data was tracked.
Results
170 visits offered (June 2021-May 2024). 32 referrals received (one without cancer; deaths: two pre-intake/five post-intake); 70 appointments occurred among 30 veterans (30 intake/40 follow-up) for 41% fill rate (up 5% from 1st six months). 96% PHI completion rate. Referral sources: fellows (43%), attendings (17%), PCP (3%), Survivorship Clinic (3%), self-referral (33%)--40% of these from cancer support group members. Cancer types (one dual-diagnosis; total >100%): 24% breast, 17% prostate, 17% NSCLC, 10% NHL, 10% pancreatic, 7% Head/Neck, 7% SCLC, 3% each colon/esophageal/kidney. Cancer Stages represented: I (10%), II (20%), III (23%) and IV (47%). Participant info: Age range (36-85); 69% male and 31% female with 86% on active cancer therapy (hormonal, immune-, chemo- or chemoradiation). Supplements were discussed at 26% of visits and referrals ordered at 27% (4-massage therapy, 1-acupuncture, 1-chiropractic, 2-health coaching, 1-cardiology, 1-lymphedema therapy, 1-social work, 1-survivorship clinic, 1-yoga, 1-diabetes education, 1-ENT, 1-nutrition, 1-pathology, 1-pulmonary, 1-prosthetics).
Conclusions
WH within cancer care is feasible for veterans on active treatment (all types/stages) and at a non-Flagship/unfunded site. Veterans gain introduction to WH through the PHI and Complementary-Integrative Health referrals (VA Directive 1137). Cancer support group attendance prompts WH clinic self-referrals. Next steps at DVAMC are to offer mind-body approaches such as virtual reality experiences in the infusion room and VA CALM sessions via asynchronous online delivery; funding would support WH evolution in oncology.
Background
VA Whole Health (WH) is an approach that empowers and equips people to take charge of their health and well-being. In 2020, 18 WH Flagship sites demonstrated reduced opiate use and smaller increases in pharmacy costs as well as favorable veteran self-reported measures. VA mandated WH integration into mental health and primary care. Purose: To incorporate WH within Dayton VA cancer care, using the Personal Health Inventory (PHI) as an intake tool, a tumor-agnostic WH oncology clinic was established.
Methods
Led by an oncologist, a referral-based clinic opened in 2021. Pre-work included EHR items (stop codes/templates), staff training and leverage of mental health integration. VA’s generic PHI was utilized until an oncology-specific PHI was developed by leaders in the field.(3-5) Clinic data was tracked.
Results
170 visits offered (June 2021-May 2024). 32 referrals received (one without cancer; deaths: two pre-intake/five post-intake); 70 appointments occurred among 30 veterans (30 intake/40 follow-up) for 41% fill rate (up 5% from 1st six months). 96% PHI completion rate. Referral sources: fellows (43%), attendings (17%), PCP (3%), Survivorship Clinic (3%), self-referral (33%)--40% of these from cancer support group members. Cancer types (one dual-diagnosis; total >100%): 24% breast, 17% prostate, 17% NSCLC, 10% NHL, 10% pancreatic, 7% Head/Neck, 7% SCLC, 3% each colon/esophageal/kidney. Cancer Stages represented: I (10%), II (20%), III (23%) and IV (47%). Participant info: Age range (36-85); 69% male and 31% female with 86% on active cancer therapy (hormonal, immune-, chemo- or chemoradiation). Supplements were discussed at 26% of visits and referrals ordered at 27% (4-massage therapy, 1-acupuncture, 1-chiropractic, 2-health coaching, 1-cardiology, 1-lymphedema therapy, 1-social work, 1-survivorship clinic, 1-yoga, 1-diabetes education, 1-ENT, 1-nutrition, 1-pathology, 1-pulmonary, 1-prosthetics).
Conclusions
WH within cancer care is feasible for veterans on active treatment (all types/stages) and at a non-Flagship/unfunded site. Veterans gain introduction to WH through the PHI and Complementary-Integrative Health referrals (VA Directive 1137). Cancer support group attendance prompts WH clinic self-referrals. Next steps at DVAMC are to offer mind-body approaches such as virtual reality experiences in the infusion room and VA CALM sessions via asynchronous online delivery; funding would support WH evolution in oncology.
Background
VA Whole Health (WH) is an approach that empowers and equips people to take charge of their health and well-being. In 2020, 18 WH Flagship sites demonstrated reduced opiate use and smaller increases in pharmacy costs as well as favorable veteran self-reported measures. VA mandated WH integration into mental health and primary care. Purose: To incorporate WH within Dayton VA cancer care, using the Personal Health Inventory (PHI) as an intake tool, a tumor-agnostic WH oncology clinic was established.
Methods
Led by an oncologist, a referral-based clinic opened in 2021. Pre-work included EHR items (stop codes/templates), staff training and leverage of mental health integration. VA’s generic PHI was utilized until an oncology-specific PHI was developed by leaders in the field.(3-5) Clinic data was tracked.
Results
170 visits offered (June 2021-May 2024). 32 referrals received (one without cancer; deaths: two pre-intake/five post-intake); 70 appointments occurred among 30 veterans (30 intake/40 follow-up) for 41% fill rate (up 5% from 1st six months). 96% PHI completion rate. Referral sources: fellows (43%), attendings (17%), PCP (3%), Survivorship Clinic (3%), self-referral (33%)--40% of these from cancer support group members. Cancer types (one dual-diagnosis; total >100%): 24% breast, 17% prostate, 17% NSCLC, 10% NHL, 10% pancreatic, 7% Head/Neck, 7% SCLC, 3% each colon/esophageal/kidney. Cancer Stages represented: I (10%), II (20%), III (23%) and IV (47%). Participant info: Age range (36-85); 69% male and 31% female with 86% on active cancer therapy (hormonal, immune-, chemo- or chemoradiation). Supplements were discussed at 26% of visits and referrals ordered at 27% (4-massage therapy, 1-acupuncture, 1-chiropractic, 2-health coaching, 1-cardiology, 1-lymphedema therapy, 1-social work, 1-survivorship clinic, 1-yoga, 1-diabetes education, 1-ENT, 1-nutrition, 1-pathology, 1-pulmonary, 1-prosthetics).
Conclusions
WH within cancer care is feasible for veterans on active treatment (all types/stages) and at a non-Flagship/unfunded site. Veterans gain introduction to WH through the PHI and Complementary-Integrative Health referrals (VA Directive 1137). Cancer support group attendance prompts WH clinic self-referrals. Next steps at DVAMC are to offer mind-body approaches such as virtual reality experiences in the infusion room and VA CALM sessions via asynchronous online delivery; funding would support WH evolution in oncology.
Nephrology–Palliative Care Collaboration to Promote Outpatient Hemodialysis Goals of Care Conversations
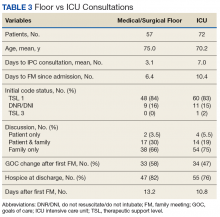
Estimates of chronic kidney disease (CKD) among veterans range between 34% and 47% higher than in the general population.1 As patients progress to end-stage kidney disease and begin chronic dialysis, they often experience further functional and cognitive decline and a high symptom burden, leading to poor quality of life.2 Clinicians should initiate goals of care conversations (GOCCs) to support high-risk patients on dialysis to ensure that the interventions they receive align with their goals and preferences, since many patients on dialysis prefer measures focused on pain relief and discomfort.3,4 While proactive GOCCs are supported among nephrology associations, few such conversations take place.5,6 In one study, more than half of patients on dialysis stated they had not discussed end-of-life preferences in the past 12 months.4 As a result, patients may not consider the larger implications of receiving dialysis indefinitely as a life-sustaining treatment (LST).
In May 2018, the US Department of Veterans Affairs (VA) National Center for Ethics in Health Care rolled out the Life-Sustaining Treatment Decisions Initiative to proactively engage patients with serious illnesses, such as those with end-stage kidney disease, in GOCCs to clarify their preferences for LSTs.7 After comprehensive training, a preliminary audit at the Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois, revealed that only 27% of patients on dialysis had LST preferences documented in a standardized LST note.
Nephrologists cite multiple barriers to proactively addressing goals of care with patients with advanced CKD, including clinician discomfort, perceived lack of time, infrastructure, and training.8,9 Similarly, the absence of a multidisciplinary advance care planning approach—specifically bringing together palliative care (PC) clinicians with nephrologists—has been highlighted but not as well studied.10,11
In this quality improvement (QI) project, we aimed to establish a workflow to enhance collaboration between nephrology and PC and to increase the percentage of VA patients on outpatient hemodialysis who engaged in GOCCs, as documented by completion of an LST progress note in the VA’s electronic health record (EHR). We developed a collaboration among PC, nephrology, and social work to improve the rates of documented GOCCs and LST patients on dialysis.
Implementation
EHJVAH is a 1A facility with > 80 patients who receive outpatient hemodialysis on campus. At the time of this collaboration in the fall of 2019, the collaborative dialysis team comprised 2 social workers and a nephrologist. The PC team included a coordinator, 2 nurse practitioners, and 3 physicians. A QI nurse was involved in the initial data gathering for this project.
The PC and nephrology medical directors developed a workflow process that reflected organizational and clinical steps in planning, initiating, and completing GOCCs with patients on outpatient dialysis (Table 1). The proposed process engaged an interdisciplinary PC and nephrology group and was revised to incorporate staff suggestions.
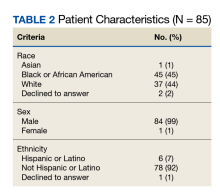
A prospective review of 85 EHJVAH hemodialysis unit patient records was conducted between September 1, 2019, and September 30, 2020 (Table 2). We reviewed LST completion rates for all patients receiving dialysis within this timeframe. During the intervention period, the PC team approached 40 patients without LST notes to engage in GOCCs.
Discussion
Over the 13-month collaboration, LST note completion rates increased from 27% to 81%, with 69 of 85 patients having a documented LST progress note in the EHR. PC approached nearly half of all patients on dialysis. Most patients agreed to be seen by the PC team, with 72% of those approached agreeing to a PC consultation. Previous research has suggested that having a trusted dialysis staff member included in GOCCs contributes to high acceptance rates.12
PC is a relatively uncommon partnership for nephrologists, and PC and hospice services are underutilized in patients on dialysis both nationally and within the VA.13-15 Our outcomes could be replicated, as PC is required at all VA sites.
Conclusions
The innovation of an interdisciplinary nephrology–PC collaboration was an important step in increasing high-quality GOCCs and eliciting patient preferences for LSTs among patients on dialysis. PC integration for patients on dialysis is associated with improved symptom management, fewer aggressive health care measures, and a higher likelihood of dying in one’s preferred setting.16 While this partnership focused on patients already receiving dialysis, successful PC interventions are felt most keenly upstream, before dialysis initiation.
Acknowledgments
The authors acknowledge the contributions of their colleague, Mary McCabe, DNP, Quality Systems Improvement, Edward Hines, Jr. Veterans Affairs Hospital. The authors also acknowledge the clinical dedication of the dialysis social workers, Sarah Adam, LCSW, and Sarah Kraner, LCSW, without which this collaboration would not have been possible.
1. Patel N, Golzy M, Nainani N, et al. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38(2):204-208. doi:10.3109/0886022X.2015.1117924
2. Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345-1352. doi:10.1093/ndt/gfg105
3. Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206-1214. doi:10.1001/jamainternmed.2013.6036
4. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. doi:10.2215/CJN.05960809
5. Williams AW, Dwyer AC, Eddy AA, et al; American Society of Nephrology Quality, and Patient Safety Task Force. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. doi:10.2215/CJN.04970512
6. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Renal Physicians Association; 2010.
7. US Department of Veterans Affairs, National Center for Ethics in Health Care. Goals of care conversations training for nurses, social workers, psychologists, and chaplains. Updated October 9, 2018. Accessed August 31, 2023. https://www.ethics.va.gov/goalsofcaretraining/team.asp
8. Goff SL, Unruh ML, Klingensmith J, et al. Advance care planning with patients on hemodialysis: an implementation study. BMC Palliat Care. 2019;18(1):64. Published 2019 Jul 26. doi:10.1186/s12904-019-0437-2
9. O’Hare AM, Szarka J, McFarland LV, et al. Provider perspectives on advance care planning for patients with kidney disease: whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855-866. doi:10.2215/CJN.11351015
10. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis. 2016;67(4):688-695. doi:10.1053/j.ajkd.2015.09.032
11. Holley JL, Davison SN. Advance care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015;10(3):344-346. doi:10.2215/CJN.00290115
12. Davison SN. Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. doi:10.2215/CJN.01050306
13. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248-1255. doi:10.2215/CJN.00970306
14. Williams ME, Sandeep J, Catic A. Aging and ESRD demographics: consequences for the practice of dialysis. Semin Dial. 2012;25(6):617-622. doi:10.1111/sdi.12029
15. US Dept of Veterans Affairs. FY 2020 annual report. Palliative and hospice care.
16. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614. doi:10.1093/ndt/gfq630
17. Fadem SZ, Fadem J. HD mortality predictor. Accessed August 31, 2023. http://touchcalc.com/calculators/sq
18. National Center for Ethics in Health Care. Setting health care goals: a guide for people with hearth problems. Updated June 2016. Accessed August 31, 2023. https://www.ethics.va.gov/docs/GoCC/lst_booklet_for_patients_setting_health_care_goals_final.pdf
Estimates of chronic kidney disease (CKD) among veterans range between 34% and 47% higher than in the general population.1 As patients progress to end-stage kidney disease and begin chronic dialysis, they often experience further functional and cognitive decline and a high symptom burden, leading to poor quality of life.2 Clinicians should initiate goals of care conversations (GOCCs) to support high-risk patients on dialysis to ensure that the interventions they receive align with their goals and preferences, since many patients on dialysis prefer measures focused on pain relief and discomfort.3,4 While proactive GOCCs are supported among nephrology associations, few such conversations take place.5,6 In one study, more than half of patients on dialysis stated they had not discussed end-of-life preferences in the past 12 months.4 As a result, patients may not consider the larger implications of receiving dialysis indefinitely as a life-sustaining treatment (LST).
In May 2018, the US Department of Veterans Affairs (VA) National Center for Ethics in Health Care rolled out the Life-Sustaining Treatment Decisions Initiative to proactively engage patients with serious illnesses, such as those with end-stage kidney disease, in GOCCs to clarify their preferences for LSTs.7 After comprehensive training, a preliminary audit at the Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois, revealed that only 27% of patients on dialysis had LST preferences documented in a standardized LST note.
Nephrologists cite multiple barriers to proactively addressing goals of care with patients with advanced CKD, including clinician discomfort, perceived lack of time, infrastructure, and training.8,9 Similarly, the absence of a multidisciplinary advance care planning approach—specifically bringing together palliative care (PC) clinicians with nephrologists—has been highlighted but not as well studied.10,11
In this quality improvement (QI) project, we aimed to establish a workflow to enhance collaboration between nephrology and PC and to increase the percentage of VA patients on outpatient hemodialysis who engaged in GOCCs, as documented by completion of an LST progress note in the VA’s electronic health record (EHR). We developed a collaboration among PC, nephrology, and social work to improve the rates of documented GOCCs and LST patients on dialysis.
Implementation
EHJVAH is a 1A facility with > 80 patients who receive outpatient hemodialysis on campus. At the time of this collaboration in the fall of 2019, the collaborative dialysis team comprised 2 social workers and a nephrologist. The PC team included a coordinator, 2 nurse practitioners, and 3 physicians. A QI nurse was involved in the initial data gathering for this project.
The PC and nephrology medical directors developed a workflow process that reflected organizational and clinical steps in planning, initiating, and completing GOCCs with patients on outpatient dialysis (Table 1). The proposed process engaged an interdisciplinary PC and nephrology group and was revised to incorporate staff suggestions.
A prospective review of 85 EHJVAH hemodialysis unit patient records was conducted between September 1, 2019, and September 30, 2020 (Table 2). We reviewed LST completion rates for all patients receiving dialysis within this timeframe. During the intervention period, the PC team approached 40 patients without LST notes to engage in GOCCs.
Discussion
Over the 13-month collaboration, LST note completion rates increased from 27% to 81%, with 69 of 85 patients having a documented LST progress note in the EHR. PC approached nearly half of all patients on dialysis. Most patients agreed to be seen by the PC team, with 72% of those approached agreeing to a PC consultation. Previous research has suggested that having a trusted dialysis staff member included in GOCCs contributes to high acceptance rates.12
PC is a relatively uncommon partnership for nephrologists, and PC and hospice services are underutilized in patients on dialysis both nationally and within the VA.13-15 Our outcomes could be replicated, as PC is required at all VA sites.
Conclusions
The innovation of an interdisciplinary nephrology–PC collaboration was an important step in increasing high-quality GOCCs and eliciting patient preferences for LSTs among patients on dialysis. PC integration for patients on dialysis is associated with improved symptom management, fewer aggressive health care measures, and a higher likelihood of dying in one’s preferred setting.16 While this partnership focused on patients already receiving dialysis, successful PC interventions are felt most keenly upstream, before dialysis initiation.
Acknowledgments
The authors acknowledge the contributions of their colleague, Mary McCabe, DNP, Quality Systems Improvement, Edward Hines, Jr. Veterans Affairs Hospital. The authors also acknowledge the clinical dedication of the dialysis social workers, Sarah Adam, LCSW, and Sarah Kraner, LCSW, without which this collaboration would not have been possible.
Estimates of chronic kidney disease (CKD) among veterans range between 34% and 47% higher than in the general population.1 As patients progress to end-stage kidney disease and begin chronic dialysis, they often experience further functional and cognitive decline and a high symptom burden, leading to poor quality of life.2 Clinicians should initiate goals of care conversations (GOCCs) to support high-risk patients on dialysis to ensure that the interventions they receive align with their goals and preferences, since many patients on dialysis prefer measures focused on pain relief and discomfort.3,4 While proactive GOCCs are supported among nephrology associations, few such conversations take place.5,6 In one study, more than half of patients on dialysis stated they had not discussed end-of-life preferences in the past 12 months.4 As a result, patients may not consider the larger implications of receiving dialysis indefinitely as a life-sustaining treatment (LST).
In May 2018, the US Department of Veterans Affairs (VA) National Center for Ethics in Health Care rolled out the Life-Sustaining Treatment Decisions Initiative to proactively engage patients with serious illnesses, such as those with end-stage kidney disease, in GOCCs to clarify their preferences for LSTs.7 After comprehensive training, a preliminary audit at the Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois, revealed that only 27% of patients on dialysis had LST preferences documented in a standardized LST note.
Nephrologists cite multiple barriers to proactively addressing goals of care with patients with advanced CKD, including clinician discomfort, perceived lack of time, infrastructure, and training.8,9 Similarly, the absence of a multidisciplinary advance care planning approach—specifically bringing together palliative care (PC) clinicians with nephrologists—has been highlighted but not as well studied.10,11
In this quality improvement (QI) project, we aimed to establish a workflow to enhance collaboration between nephrology and PC and to increase the percentage of VA patients on outpatient hemodialysis who engaged in GOCCs, as documented by completion of an LST progress note in the VA’s electronic health record (EHR). We developed a collaboration among PC, nephrology, and social work to improve the rates of documented GOCCs and LST patients on dialysis.
Implementation
EHJVAH is a 1A facility with > 80 patients who receive outpatient hemodialysis on campus. At the time of this collaboration in the fall of 2019, the collaborative dialysis team comprised 2 social workers and a nephrologist. The PC team included a coordinator, 2 nurse practitioners, and 3 physicians. A QI nurse was involved in the initial data gathering for this project.
The PC and nephrology medical directors developed a workflow process that reflected organizational and clinical steps in planning, initiating, and completing GOCCs with patients on outpatient dialysis (Table 1). The proposed process engaged an interdisciplinary PC and nephrology group and was revised to incorporate staff suggestions.
A prospective review of 85 EHJVAH hemodialysis unit patient records was conducted between September 1, 2019, and September 30, 2020 (Table 2). We reviewed LST completion rates for all patients receiving dialysis within this timeframe. During the intervention period, the PC team approached 40 patients without LST notes to engage in GOCCs.
Discussion
Over the 13-month collaboration, LST note completion rates increased from 27% to 81%, with 69 of 85 patients having a documented LST progress note in the EHR. PC approached nearly half of all patients on dialysis. Most patients agreed to be seen by the PC team, with 72% of those approached agreeing to a PC consultation. Previous research has suggested that having a trusted dialysis staff member included in GOCCs contributes to high acceptance rates.12
PC is a relatively uncommon partnership for nephrologists, and PC and hospice services are underutilized in patients on dialysis both nationally and within the VA.13-15 Our outcomes could be replicated, as PC is required at all VA sites.
Conclusions
The innovation of an interdisciplinary nephrology–PC collaboration was an important step in increasing high-quality GOCCs and eliciting patient preferences for LSTs among patients on dialysis. PC integration for patients on dialysis is associated with improved symptom management, fewer aggressive health care measures, and a higher likelihood of dying in one’s preferred setting.16 While this partnership focused on patients already receiving dialysis, successful PC interventions are felt most keenly upstream, before dialysis initiation.
Acknowledgments
The authors acknowledge the contributions of their colleague, Mary McCabe, DNP, Quality Systems Improvement, Edward Hines, Jr. Veterans Affairs Hospital. The authors also acknowledge the clinical dedication of the dialysis social workers, Sarah Adam, LCSW, and Sarah Kraner, LCSW, without which this collaboration would not have been possible.
1. Patel N, Golzy M, Nainani N, et al. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38(2):204-208. doi:10.3109/0886022X.2015.1117924
2. Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345-1352. doi:10.1093/ndt/gfg105
3. Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206-1214. doi:10.1001/jamainternmed.2013.6036
4. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. doi:10.2215/CJN.05960809
5. Williams AW, Dwyer AC, Eddy AA, et al; American Society of Nephrology Quality, and Patient Safety Task Force. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. doi:10.2215/CJN.04970512
6. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Renal Physicians Association; 2010.
7. US Department of Veterans Affairs, National Center for Ethics in Health Care. Goals of care conversations training for nurses, social workers, psychologists, and chaplains. Updated October 9, 2018. Accessed August 31, 2023. https://www.ethics.va.gov/goalsofcaretraining/team.asp
8. Goff SL, Unruh ML, Klingensmith J, et al. Advance care planning with patients on hemodialysis: an implementation study. BMC Palliat Care. 2019;18(1):64. Published 2019 Jul 26. doi:10.1186/s12904-019-0437-2
9. O’Hare AM, Szarka J, McFarland LV, et al. Provider perspectives on advance care planning for patients with kidney disease: whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855-866. doi:10.2215/CJN.11351015
10. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis. 2016;67(4):688-695. doi:10.1053/j.ajkd.2015.09.032
11. Holley JL, Davison SN. Advance care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015;10(3):344-346. doi:10.2215/CJN.00290115
12. Davison SN. Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. doi:10.2215/CJN.01050306
13. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248-1255. doi:10.2215/CJN.00970306
14. Williams ME, Sandeep J, Catic A. Aging and ESRD demographics: consequences for the practice of dialysis. Semin Dial. 2012;25(6):617-622. doi:10.1111/sdi.12029
15. US Dept of Veterans Affairs. FY 2020 annual report. Palliative and hospice care.
16. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614. doi:10.1093/ndt/gfq630
17. Fadem SZ, Fadem J. HD mortality predictor. Accessed August 31, 2023. http://touchcalc.com/calculators/sq
18. National Center for Ethics in Health Care. Setting health care goals: a guide for people with hearth problems. Updated June 2016. Accessed August 31, 2023. https://www.ethics.va.gov/docs/GoCC/lst_booklet_for_patients_setting_health_care_goals_final.pdf
1. Patel N, Golzy M, Nainani N, et al. Prevalence of various comorbidities among veterans with chronic kidney disease and its comparison with other datasets. Ren Fail. 2016;38(2):204-208. doi:10.3109/0886022X.2015.1117924
2. Weisbord SD, Carmody SS, Bruns FJ, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345-1352. doi:10.1093/ndt/gfg105
3. Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206-1214. doi:10.1001/jamainternmed.2013.6036
4. Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. doi:10.2215/CJN.05960809
5. Williams AW, Dwyer AC, Eddy AA, et al; American Society of Nephrology Quality, and Patient Safety Task Force. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephrol. 2012;7(10):1664-1672. doi:10.2215/CJN.04970512
6. Renal Physicians Association. Shared Decision-Making in the Appropriate Initiation of and Withdrawal from Dialysis. 2nd ed. Renal Physicians Association; 2010.
7. US Department of Veterans Affairs, National Center for Ethics in Health Care. Goals of care conversations training for nurses, social workers, psychologists, and chaplains. Updated October 9, 2018. Accessed August 31, 2023. https://www.ethics.va.gov/goalsofcaretraining/team.asp
8. Goff SL, Unruh ML, Klingensmith J, et al. Advance care planning with patients on hemodialysis: an implementation study. BMC Palliat Care. 2019;18(1):64. Published 2019 Jul 26. doi:10.1186/s12904-019-0437-2
9. O’Hare AM, Szarka J, McFarland LV, et al. Provider perspectives on advance care planning for patients with kidney disease: whose job is it anyway? Clin J Am Soc Nephrol. 2016;11(5):855-866. doi:10.2215/CJN.11351015
10. Koncicki HM, Schell JO. Communication skills and decision making for elderly patients with advanced kidney disease: a guide for nephrologists. Am J Kidney Dis. 2016;67(4):688-695. doi:10.1053/j.ajkd.2015.09.032
11. Holley JL, Davison SN. Advance care planning for patients with advanced CKD: a need to move forward. Clin J Am Soc Nephrol. 2015;10(3):344-346. doi:10.2215/CJN.00290115
12. Davison SN. Facilitating advance care planning for patients with end-stage renal disease: the patient perspective. Clin J Am Soc Nephrol. 2006;1(5):1023-1028. doi:10.2215/CJN.01050306
13. Murray AM, Arko C, Chen SC, Gilbertson DT, Moss AH. Use of hospice in the United States dialysis population. Clin J Am Soc Nephrol. 2006;1(6):1248-1255. doi:10.2215/CJN.00970306
14. Williams ME, Sandeep J, Catic A. Aging and ESRD demographics: consequences for the practice of dialysis. Semin Dial. 2012;25(6):617-622. doi:10.1111/sdi.12029
15. US Dept of Veterans Affairs. FY 2020 annual report. Palliative and hospice care.
16. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608-1614. doi:10.1093/ndt/gfq630
17. Fadem SZ, Fadem J. HD mortality predictor. Accessed August 31, 2023. http://touchcalc.com/calculators/sq
18. National Center for Ethics in Health Care. Setting health care goals: a guide for people with hearth problems. Updated June 2016. Accessed August 31, 2023. https://www.ethics.va.gov/docs/GoCC/lst_booklet_for_patients_setting_health_care_goals_final.pdf
Use of Mobile Messaging System for Self-Management of Chemotherapy Symptoms in Patients with Advanced Cancer (FULL)
Cancer and cancer-related treatment can cause a myriad of adverse effects.1,2 Early identification and management of these symptoms is paramount to the success of cancer treatment completion; however, clinic and telephonic strategies for addressing symptoms often result in delays in care.1 New strategies for patient engagement in the management of cancer and treatment-related symptoms are needed.
The use of online self-management tools can result in improvement in symptoms, reduce cancer symptom distress, improve quality-of-life, and improve medication adherence.3-9 A meta-analysis concluded that online interventions showed promise, but optimizing interventions would require additional research.10 Another meta-analysis found that online self-management was effective in managing several symptoms.11 An e-health method of collecting patient self-reported symptoms has been found to be acceptable to patients and feasible for use.12-14 We postulated that a mobile text messaging strategy may be an effective modality for augmenting symptom management for cancer patients in real time.
In the US Departmant of Veterans Affairs (VA), “Annie,” a self-care tool utilizing a text-messaging system has been implemented. Annie was developed modeling “Flo,” a messaging system in the United Kingdom that has been used for case management of chronic obstructive pulmonary disease, heart failure, stress incontinence, asthma, as a medication reminder tool, and to provide support for weight loss or post-operatively.15-17 Using Annie in the US, veterans have the ability to receive and track health information. Use of the Annie program has demonstrated improved continuous positive airway pressure monitor utilization in veterans with traumatic brain injury.18 Other uses within the Veterans Health Administration (VHA) include assisting patients with anger management, liver disease, anxiety, asthma, diabetes, HIV, hypertension, weight loss, and smoking cessation.
Methods
The Hematology/Oncology division of the Minneapolis VA Healthcare System (MVAHCS) is a tertiary care facility that administers about 260 new chemotherapy regimens annually. The MVAHCS interdisciplinary hematology/oncology group initiated a quality improvement project to determine the feasibility, acceptability, and experience of tailoring the Annie tool for self-management of cancer symptoms. The group consisted of 2 physicians, 3 advanced practice registered nurses, 1 physician assistant, 2 registered nurses, and 2 Annie program team members.
We first created a symptom management pilot protocol as a result of multidisciplinary team discussions. Examples of discussion points for consideration included, but were not limited to, timing of texts, amount of information to ask for and provide, what potential symptoms to consider, and which patient population to pilot first.
The initial protocol was agreed upon and is as follows: Patients were sent text messages twice daily Monday through Friday, and asked to rate 2 symptoms per day, using a severity scale of 0 to 4 (absent, mild, moderate, severe, or disabling): nausea/vomiting, mouth sores, fatigue (Figure 1), trouble breathing, appetite, constipation, diarrhea (Figure 2), numbness/tingling, pain. In addition, patients were asked whether they had had a fever or not. Based on their response to the symptom inquiries, the patient received an automated text response. The text may have provided positive affirmation that they were doing well, given them advice for home management, referred them to an educational hyperlink, asked them to call a direct number to the clinic, or instructed them to report directly to the emergency department (ED). Patients could input a particular symptom on any day, even if they were not specifically asked about that symptom on that day. Patients also were instructed to text, only if it was not an inconvenience to them, as we wanted the intervention to be helpful and not a burden.
Results
Through screening new patient consults or those referred for chemotherapy education, 15 male veterans enrolled in the symptom monitoring program over an 8 month period. There were additional patients who were not offered the program or chose not to participate; often due to not having texting capabilities on their phone or not liking the texting feature. The majority of those who participated in the program (n = 14) were enrolled at the start of Cycle 1; the other patient was enrolled at the start of Cycle 2. Patients were enrolled an average of 89 days (range 8-204). Average response rate was 84.2% (range 30-100%).
Although symptoms were not reviewed in real time, we reviewed responses to determine the utilization of the instructions given for the program. No veteran had 0 symptoms reported. There were numerous occurrences of a score of 1 or 2. Many of these patients had baseline symptoms due to their underlying cancer. A score of 3 or 4 on the system prompted the patient to call the clinic or go to the ED. Seven patients (some with multiple occurrences) were prompted to call; only 4 of these made the follow-up call to the clinic. All were offered a same day visit, but each declined. Only 1 patient reported a symptom on a day not prompted for that symptom. Symptoms that were reported are listed in order of frequency: fatigue, appetite loss, numbness, pain, mouth sore, and breathing difficulty. There were no visits to the ED.
Program Evaluation
An evaluation was conducted 30 to 60 days after program enrollment. We elicited feedback to determine who was reading and responding to the text message: the patient, a family member, or a caregiver; whether they found the prompts helpful and took action; how they felt about the number of texts; if they felt the program was helpful; and any other feedback that would improve the program. In general, the patients (8) answered the texts independently. In 4 cases, the spouse answered the texts, and 3 patients answered the texts together with their spouses. Most patients (11) found the amount of texting to be “just right.” However, 3 found it to be too many texts and 1 didn’t find the amount of texting to be enough.
Three veterans did not have enough symptoms to feel the program was of benefit to them, but they did feel it would have been helpful if they had been more symptomatic. One veteran recalled taking loperamide as needed, as a result of prompting. No veterans felt as though the texting feature was difficult to use; and overall, were very positive about the program. Several appreciated receiving messages that validated when they were doing well, and they felt empowered by self-management. One of the spouses was a registered nurse and found the information too basic to be of use.
Discussion
Initial evaluation of the program via survey found no technology challenges. Patients have been very positive about the program including ease of use, appreciation of messages that validated when they were doing well, empowerment of self-management, and some utilization of the texting advice for symptom management. Educational hyperlinks for constipation, fatigue, diarrhea, and nausea/vomiting were added after this evaluation, and patients felt that these additions provided a higher level of education.
Staff time for this intervention was minimal. A nurse navigator offered the texting program to the patient during chemotherapy education, along with some instructions, which generally took about 5 minutes. One of the Annie program staff enrolled the patient. From that point forward, this was a self-management tool, beyond checking to ensure that the patient was successful in starting the program and evaluating use for the purposes of this quality improvement project. This self-management tool did not replace any other mechanism that a patient would normally have in our department for seeking help for symptoms. The MVAHSC typical process for symptom management is to have patients call a 24/7 nurse line. If the triage nurse feels the symptoms are related to the patient’s cancer or cancer treatment, they are referred to the physician assistant who is assigned to take those calls and has the option to see the patient the same day. Patients could continue to call the nurse line or speak with providers at the next appointment at their discretion.
Conclusion
Although Annie has the option of using either text messaging or a mobile application, this project only utilized text messaging. The study by Basch and colleagues was the closest randomized trial we could identify to compare to our quality improvement intervention.5 The 2 main, distinct differences were that Basch and colleagues utilized online monitoring; and nurses were utilized to screen and intervene on responses, as appropriate.
The ability of our program to text patients without the use of an application or tablet, may enable more patients to participate due to ease of use. There would be no increased in expected workload for clinical staff, and may lead to decreased call burden. Since our program is automated, while still providing patients with the option to call and speak with a staff member as needed, this is a cost-effective, first-line option for symptom management for those experiencing cancer-related symptoms. We believe this text messaging tool can have system wide use and benefit throughout the VHA.
1. Bruera E, Dev R. Overview of managing common non-pain symptoms in palliative care. https://www.uptodate.com/contents/overview-of-managing-common-non-pain-symptoms-in-palliative-care. Updated June 12, 2019. Accessed July 18, 2019.
2. Pirschel C. The crucial role of symptom management in cancer care. https://voice.ons.org/news-and-views/the-crucial-role-of-symptom-management-in-cancer-care. Published December 14, 2017. Accessed July 18, 2019.
3. Adam R, Burton CD, Bond CM, de Bruin M, Murchie P. Can patient-reported measurements of pain be used to improve cancer pain management? A systematic review and meta-analysis. BMJ Support Palliat Care. 2017;7(4):373-382.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Berry DL, Blonquist TM, Patel RA, Halpenny B, McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: Impact on symptom distress. J Med Internet Res. 2015;3(7):e136.
6. Kolb NA, Smith AG, Singleton JR, et al. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26(5):1607-1615
7. Kamdar MM, Centi AJ, Fischer N, Jetwani K. A randomized controlled trial of a novel artificial-intelligence based smartphone application to optimize the management of cancer-related pain. Presented at: 2018 Palliative and Supportive Care in Oncology Symposium; November 16-17, 2018; San Diego, CA.
8. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537-546.
9. Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506.
10. Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):3370-351.
11. Kim AR, Park HA. Web-based self-management support intervention for cancer survivors: a systematic review and meta-analysis. Stud Health Technol Inform. 2015;216:142-147.
12. Girgis A, Durcinoska I, Levesque JV, et al; PROMPT-Care Program Group. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res. 2017;19(10):e330.
13. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed methods study. JMIR Cancer. 2018;4(2): e10932.
14. Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res. 2017;19(7):e219.
15. The Health Foundation. Overview of Florence simple telehealth text messaging system. https://www.health.org.uk/article/overview-of-the-florence-simple-telehealth-text-messaging-system. Accessed July 31, 2019.
16. Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B…Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: a feasibility study. 2017;9(9):193-199.
17. O’Connell P. Annie-the VA’s self-care game changer. http://www.simple.uk.net/home/blog/blogcontent/annie-thevasself-caregamechanger. Published April 21, 2016. Accessed August 2, 2019.
18. Kataria L, Sundahl, C, Skalina L, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study (P1.097). Neurology, 2018; 90(suppl 15):P1.097.
Cancer and cancer-related treatment can cause a myriad of adverse effects.1,2 Early identification and management of these symptoms is paramount to the success of cancer treatment completion; however, clinic and telephonic strategies for addressing symptoms often result in delays in care.1 New strategies for patient engagement in the management of cancer and treatment-related symptoms are needed.
The use of online self-management tools can result in improvement in symptoms, reduce cancer symptom distress, improve quality-of-life, and improve medication adherence.3-9 A meta-analysis concluded that online interventions showed promise, but optimizing interventions would require additional research.10 Another meta-analysis found that online self-management was effective in managing several symptoms.11 An e-health method of collecting patient self-reported symptoms has been found to be acceptable to patients and feasible for use.12-14 We postulated that a mobile text messaging strategy may be an effective modality for augmenting symptom management for cancer patients in real time.
In the US Departmant of Veterans Affairs (VA), “Annie,” a self-care tool utilizing a text-messaging system has been implemented. Annie was developed modeling “Flo,” a messaging system in the United Kingdom that has been used for case management of chronic obstructive pulmonary disease, heart failure, stress incontinence, asthma, as a medication reminder tool, and to provide support for weight loss or post-operatively.15-17 Using Annie in the US, veterans have the ability to receive and track health information. Use of the Annie program has demonstrated improved continuous positive airway pressure monitor utilization in veterans with traumatic brain injury.18 Other uses within the Veterans Health Administration (VHA) include assisting patients with anger management, liver disease, anxiety, asthma, diabetes, HIV, hypertension, weight loss, and smoking cessation.
Methods
The Hematology/Oncology division of the Minneapolis VA Healthcare System (MVAHCS) is a tertiary care facility that administers about 260 new chemotherapy regimens annually. The MVAHCS interdisciplinary hematology/oncology group initiated a quality improvement project to determine the feasibility, acceptability, and experience of tailoring the Annie tool for self-management of cancer symptoms. The group consisted of 2 physicians, 3 advanced practice registered nurses, 1 physician assistant, 2 registered nurses, and 2 Annie program team members.
We first created a symptom management pilot protocol as a result of multidisciplinary team discussions. Examples of discussion points for consideration included, but were not limited to, timing of texts, amount of information to ask for and provide, what potential symptoms to consider, and which patient population to pilot first.
The initial protocol was agreed upon and is as follows: Patients were sent text messages twice daily Monday through Friday, and asked to rate 2 symptoms per day, using a severity scale of 0 to 4 (absent, mild, moderate, severe, or disabling): nausea/vomiting, mouth sores, fatigue (Figure 1), trouble breathing, appetite, constipation, diarrhea (Figure 2), numbness/tingling, pain. In addition, patients were asked whether they had had a fever or not. Based on their response to the symptom inquiries, the patient received an automated text response. The text may have provided positive affirmation that they were doing well, given them advice for home management, referred them to an educational hyperlink, asked them to call a direct number to the clinic, or instructed them to report directly to the emergency department (ED). Patients could input a particular symptom on any day, even if they were not specifically asked about that symptom on that day. Patients also were instructed to text, only if it was not an inconvenience to them, as we wanted the intervention to be helpful and not a burden.
Results
Through screening new patient consults or those referred for chemotherapy education, 15 male veterans enrolled in the symptom monitoring program over an 8 month period. There were additional patients who were not offered the program or chose not to participate; often due to not having texting capabilities on their phone or not liking the texting feature. The majority of those who participated in the program (n = 14) were enrolled at the start of Cycle 1; the other patient was enrolled at the start of Cycle 2. Patients were enrolled an average of 89 days (range 8-204). Average response rate was 84.2% (range 30-100%).
Although symptoms were not reviewed in real time, we reviewed responses to determine the utilization of the instructions given for the program. No veteran had 0 symptoms reported. There were numerous occurrences of a score of 1 or 2. Many of these patients had baseline symptoms due to their underlying cancer. A score of 3 or 4 on the system prompted the patient to call the clinic or go to the ED. Seven patients (some with multiple occurrences) were prompted to call; only 4 of these made the follow-up call to the clinic. All were offered a same day visit, but each declined. Only 1 patient reported a symptom on a day not prompted for that symptom. Symptoms that were reported are listed in order of frequency: fatigue, appetite loss, numbness, pain, mouth sore, and breathing difficulty. There were no visits to the ED.
Program Evaluation
An evaluation was conducted 30 to 60 days after program enrollment. We elicited feedback to determine who was reading and responding to the text message: the patient, a family member, or a caregiver; whether they found the prompts helpful and took action; how they felt about the number of texts; if they felt the program was helpful; and any other feedback that would improve the program. In general, the patients (8) answered the texts independently. In 4 cases, the spouse answered the texts, and 3 patients answered the texts together with their spouses. Most patients (11) found the amount of texting to be “just right.” However, 3 found it to be too many texts and 1 didn’t find the amount of texting to be enough.
Three veterans did not have enough symptoms to feel the program was of benefit to them, but they did feel it would have been helpful if they had been more symptomatic. One veteran recalled taking loperamide as needed, as a result of prompting. No veterans felt as though the texting feature was difficult to use; and overall, were very positive about the program. Several appreciated receiving messages that validated when they were doing well, and they felt empowered by self-management. One of the spouses was a registered nurse and found the information too basic to be of use.
Discussion
Initial evaluation of the program via survey found no technology challenges. Patients have been very positive about the program including ease of use, appreciation of messages that validated when they were doing well, empowerment of self-management, and some utilization of the texting advice for symptom management. Educational hyperlinks for constipation, fatigue, diarrhea, and nausea/vomiting were added after this evaluation, and patients felt that these additions provided a higher level of education.
Staff time for this intervention was minimal. A nurse navigator offered the texting program to the patient during chemotherapy education, along with some instructions, which generally took about 5 minutes. One of the Annie program staff enrolled the patient. From that point forward, this was a self-management tool, beyond checking to ensure that the patient was successful in starting the program and evaluating use for the purposes of this quality improvement project. This self-management tool did not replace any other mechanism that a patient would normally have in our department for seeking help for symptoms. The MVAHSC typical process for symptom management is to have patients call a 24/7 nurse line. If the triage nurse feels the symptoms are related to the patient’s cancer or cancer treatment, they are referred to the physician assistant who is assigned to take those calls and has the option to see the patient the same day. Patients could continue to call the nurse line or speak with providers at the next appointment at their discretion.
Conclusion
Although Annie has the option of using either text messaging or a mobile application, this project only utilized text messaging. The study by Basch and colleagues was the closest randomized trial we could identify to compare to our quality improvement intervention.5 The 2 main, distinct differences were that Basch and colleagues utilized online monitoring; and nurses were utilized to screen and intervene on responses, as appropriate.
The ability of our program to text patients without the use of an application or tablet, may enable more patients to participate due to ease of use. There would be no increased in expected workload for clinical staff, and may lead to decreased call burden. Since our program is automated, while still providing patients with the option to call and speak with a staff member as needed, this is a cost-effective, first-line option for symptom management for those experiencing cancer-related symptoms. We believe this text messaging tool can have system wide use and benefit throughout the VHA.
Cancer and cancer-related treatment can cause a myriad of adverse effects.1,2 Early identification and management of these symptoms is paramount to the success of cancer treatment completion; however, clinic and telephonic strategies for addressing symptoms often result in delays in care.1 New strategies for patient engagement in the management of cancer and treatment-related symptoms are needed.
The use of online self-management tools can result in improvement in symptoms, reduce cancer symptom distress, improve quality-of-life, and improve medication adherence.3-9 A meta-analysis concluded that online interventions showed promise, but optimizing interventions would require additional research.10 Another meta-analysis found that online self-management was effective in managing several symptoms.11 An e-health method of collecting patient self-reported symptoms has been found to be acceptable to patients and feasible for use.12-14 We postulated that a mobile text messaging strategy may be an effective modality for augmenting symptom management for cancer patients in real time.
In the US Departmant of Veterans Affairs (VA), “Annie,” a self-care tool utilizing a text-messaging system has been implemented. Annie was developed modeling “Flo,” a messaging system in the United Kingdom that has been used for case management of chronic obstructive pulmonary disease, heart failure, stress incontinence, asthma, as a medication reminder tool, and to provide support for weight loss or post-operatively.15-17 Using Annie in the US, veterans have the ability to receive and track health information. Use of the Annie program has demonstrated improved continuous positive airway pressure monitor utilization in veterans with traumatic brain injury.18 Other uses within the Veterans Health Administration (VHA) include assisting patients with anger management, liver disease, anxiety, asthma, diabetes, HIV, hypertension, weight loss, and smoking cessation.
Methods
The Hematology/Oncology division of the Minneapolis VA Healthcare System (MVAHCS) is a tertiary care facility that administers about 260 new chemotherapy regimens annually. The MVAHCS interdisciplinary hematology/oncology group initiated a quality improvement project to determine the feasibility, acceptability, and experience of tailoring the Annie tool for self-management of cancer symptoms. The group consisted of 2 physicians, 3 advanced practice registered nurses, 1 physician assistant, 2 registered nurses, and 2 Annie program team members.
We first created a symptom management pilot protocol as a result of multidisciplinary team discussions. Examples of discussion points for consideration included, but were not limited to, timing of texts, amount of information to ask for and provide, what potential symptoms to consider, and which patient population to pilot first.
The initial protocol was agreed upon and is as follows: Patients were sent text messages twice daily Monday through Friday, and asked to rate 2 symptoms per day, using a severity scale of 0 to 4 (absent, mild, moderate, severe, or disabling): nausea/vomiting, mouth sores, fatigue (Figure 1), trouble breathing, appetite, constipation, diarrhea (Figure 2), numbness/tingling, pain. In addition, patients were asked whether they had had a fever or not. Based on their response to the symptom inquiries, the patient received an automated text response. The text may have provided positive affirmation that they were doing well, given them advice for home management, referred them to an educational hyperlink, asked them to call a direct number to the clinic, or instructed them to report directly to the emergency department (ED). Patients could input a particular symptom on any day, even if they were not specifically asked about that symptom on that day. Patients also were instructed to text, only if it was not an inconvenience to them, as we wanted the intervention to be helpful and not a burden.
Results
Through screening new patient consults or those referred for chemotherapy education, 15 male veterans enrolled in the symptom monitoring program over an 8 month period. There were additional patients who were not offered the program or chose not to participate; often due to not having texting capabilities on their phone or not liking the texting feature. The majority of those who participated in the program (n = 14) were enrolled at the start of Cycle 1; the other patient was enrolled at the start of Cycle 2. Patients were enrolled an average of 89 days (range 8-204). Average response rate was 84.2% (range 30-100%).
Although symptoms were not reviewed in real time, we reviewed responses to determine the utilization of the instructions given for the program. No veteran had 0 symptoms reported. There were numerous occurrences of a score of 1 or 2. Many of these patients had baseline symptoms due to their underlying cancer. A score of 3 or 4 on the system prompted the patient to call the clinic or go to the ED. Seven patients (some with multiple occurrences) were prompted to call; only 4 of these made the follow-up call to the clinic. All were offered a same day visit, but each declined. Only 1 patient reported a symptom on a day not prompted for that symptom. Symptoms that were reported are listed in order of frequency: fatigue, appetite loss, numbness, pain, mouth sore, and breathing difficulty. There were no visits to the ED.
Program Evaluation
An evaluation was conducted 30 to 60 days after program enrollment. We elicited feedback to determine who was reading and responding to the text message: the patient, a family member, or a caregiver; whether they found the prompts helpful and took action; how they felt about the number of texts; if they felt the program was helpful; and any other feedback that would improve the program. In general, the patients (8) answered the texts independently. In 4 cases, the spouse answered the texts, and 3 patients answered the texts together with their spouses. Most patients (11) found the amount of texting to be “just right.” However, 3 found it to be too many texts and 1 didn’t find the amount of texting to be enough.
Three veterans did not have enough symptoms to feel the program was of benefit to them, but they did feel it would have been helpful if they had been more symptomatic. One veteran recalled taking loperamide as needed, as a result of prompting. No veterans felt as though the texting feature was difficult to use; and overall, were very positive about the program. Several appreciated receiving messages that validated when they were doing well, and they felt empowered by self-management. One of the spouses was a registered nurse and found the information too basic to be of use.
Discussion
Initial evaluation of the program via survey found no technology challenges. Patients have been very positive about the program including ease of use, appreciation of messages that validated when they were doing well, empowerment of self-management, and some utilization of the texting advice for symptom management. Educational hyperlinks for constipation, fatigue, diarrhea, and nausea/vomiting were added after this evaluation, and patients felt that these additions provided a higher level of education.
Staff time for this intervention was minimal. A nurse navigator offered the texting program to the patient during chemotherapy education, along with some instructions, which generally took about 5 minutes. One of the Annie program staff enrolled the patient. From that point forward, this was a self-management tool, beyond checking to ensure that the patient was successful in starting the program and evaluating use for the purposes of this quality improvement project. This self-management tool did not replace any other mechanism that a patient would normally have in our department for seeking help for symptoms. The MVAHSC typical process for symptom management is to have patients call a 24/7 nurse line. If the triage nurse feels the symptoms are related to the patient’s cancer or cancer treatment, they are referred to the physician assistant who is assigned to take those calls and has the option to see the patient the same day. Patients could continue to call the nurse line or speak with providers at the next appointment at their discretion.
Conclusion
Although Annie has the option of using either text messaging or a mobile application, this project only utilized text messaging. The study by Basch and colleagues was the closest randomized trial we could identify to compare to our quality improvement intervention.5 The 2 main, distinct differences were that Basch and colleagues utilized online monitoring; and nurses were utilized to screen and intervene on responses, as appropriate.
The ability of our program to text patients without the use of an application or tablet, may enable more patients to participate due to ease of use. There would be no increased in expected workload for clinical staff, and may lead to decreased call burden. Since our program is automated, while still providing patients with the option to call and speak with a staff member as needed, this is a cost-effective, first-line option for symptom management for those experiencing cancer-related symptoms. We believe this text messaging tool can have system wide use and benefit throughout the VHA.
1. Bruera E, Dev R. Overview of managing common non-pain symptoms in palliative care. https://www.uptodate.com/contents/overview-of-managing-common-non-pain-symptoms-in-palliative-care. Updated June 12, 2019. Accessed July 18, 2019.
2. Pirschel C. The crucial role of symptom management in cancer care. https://voice.ons.org/news-and-views/the-crucial-role-of-symptom-management-in-cancer-care. Published December 14, 2017. Accessed July 18, 2019.
3. Adam R, Burton CD, Bond CM, de Bruin M, Murchie P. Can patient-reported measurements of pain be used to improve cancer pain management? A systematic review and meta-analysis. BMJ Support Palliat Care. 2017;7(4):373-382.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Berry DL, Blonquist TM, Patel RA, Halpenny B, McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: Impact on symptom distress. J Med Internet Res. 2015;3(7):e136.
6. Kolb NA, Smith AG, Singleton JR, et al. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26(5):1607-1615
7. Kamdar MM, Centi AJ, Fischer N, Jetwani K. A randomized controlled trial of a novel artificial-intelligence based smartphone application to optimize the management of cancer-related pain. Presented at: 2018 Palliative and Supportive Care in Oncology Symposium; November 16-17, 2018; San Diego, CA.
8. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537-546.
9. Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506.
10. Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):3370-351.
11. Kim AR, Park HA. Web-based self-management support intervention for cancer survivors: a systematic review and meta-analysis. Stud Health Technol Inform. 2015;216:142-147.
12. Girgis A, Durcinoska I, Levesque JV, et al; PROMPT-Care Program Group. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res. 2017;19(10):e330.
13. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed methods study. JMIR Cancer. 2018;4(2): e10932.
14. Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res. 2017;19(7):e219.
15. The Health Foundation. Overview of Florence simple telehealth text messaging system. https://www.health.org.uk/article/overview-of-the-florence-simple-telehealth-text-messaging-system. Accessed July 31, 2019.
16. Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B…Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: a feasibility study. 2017;9(9):193-199.
17. O’Connell P. Annie-the VA’s self-care game changer. http://www.simple.uk.net/home/blog/blogcontent/annie-thevasself-caregamechanger. Published April 21, 2016. Accessed August 2, 2019.
18. Kataria L, Sundahl, C, Skalina L, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study (P1.097). Neurology, 2018; 90(suppl 15):P1.097.
1. Bruera E, Dev R. Overview of managing common non-pain symptoms in palliative care. https://www.uptodate.com/contents/overview-of-managing-common-non-pain-symptoms-in-palliative-care. Updated June 12, 2019. Accessed July 18, 2019.
2. Pirschel C. The crucial role of symptom management in cancer care. https://voice.ons.org/news-and-views/the-crucial-role-of-symptom-management-in-cancer-care. Published December 14, 2017. Accessed July 18, 2019.
3. Adam R, Burton CD, Bond CM, de Bruin M, Murchie P. Can patient-reported measurements of pain be used to improve cancer pain management? A systematic review and meta-analysis. BMJ Support Palliat Care. 2017;7(4):373-382.
4. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557-565.
5. Berry DL, Blonquist TM, Patel RA, Halpenny B, McReynolds J. Exposure to a patient-centered, Web-based intervention for managing cancer symptom and quality of life issues: Impact on symptom distress. J Med Internet Res. 2015;3(7):e136.
6. Kolb NA, Smith AG, Singleton JR, et al. Chemotherapy-related neuropathic symptom management: a randomized trial of an automated symptom-monitoring system paired with nurse practitioner follow-up. Support Care Cancer. 2018;26(5):1607-1615
7. Kamdar MM, Centi AJ, Fischer N, Jetwani K. A randomized controlled trial of a novel artificial-intelligence based smartphone application to optimize the management of cancer-related pain. Presented at: 2018 Palliative and Supportive Care in Oncology Symposium; November 16-17, 2018; San Diego, CA.
8. Mooney KH, Beck SL, Wong B, et al. Automated home monitoring and management of patient-reported symptoms during chemotherapy: results of the symptom care at home RCT. Cancer Med. 2017;6(3):537-546.
9. Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health. 2016;22(6):497-506.
10. Fridriksdottir N, Gunnarsdottir S, Zoëga S, Ingadottir B, Hafsteinsdottir EJG. Effects of web-based interventions on cancer patients’ symptoms: review of randomized trials. Support Care Cancer. 2018;26(2):3370-351.
11. Kim AR, Park HA. Web-based self-management support intervention for cancer survivors: a systematic review and meta-analysis. Stud Health Technol Inform. 2015;216:142-147.
12. Girgis A, Durcinoska I, Levesque JV, et al; PROMPT-Care Program Group. eHealth system for collecting and utilizing patient reported outcome measures for personalized treatment and care (PROMPT-Care) among cancer patients: mixed methods approach to evaluate feasibility and acceptability. J Med Internet Res. 2017;19(10):e330.
13. Moradian S, Krzyzanowska MK, Maguire R, et al. Usability evaluation of a mobile phone-based system for remote monitoring and management of chemotherapy-related side effects in cancer patients: Mixed methods study. JMIR Cancer. 2018;4(2): e10932.
14. Voruganti T, Grunfeld E, Jamieson T, et al. My team of care study: a pilot randomized controlled trial of a web-based communication tool for collaborative care in patients with advanced cancer. J Med Internet Res. 2017;19(7):e219.
15. The Health Foundation. Overview of Florence simple telehealth text messaging system. https://www.health.org.uk/article/overview-of-the-florence-simple-telehealth-text-messaging-system. Accessed July 31, 2019.
16. Bragg DD, Edis H, Clark S, Parsons SL, Perumpalath B…Maxwell-Armstrong CA. Development of a telehealth monitoring service after colorectal surgery: a feasibility study. 2017;9(9):193-199.
17. O’Connell P. Annie-the VA’s self-care game changer. http://www.simple.uk.net/home/blog/blogcontent/annie-thevasself-caregamechanger. Published April 21, 2016. Accessed August 2, 2019.
18. Kataria L, Sundahl, C, Skalina L, et al. Text message reminders and intensive education improves positive airway pressure compliance and cognition in veterans with traumatic brain injury and obstructive sleep apnea: ANNIE pilot study (P1.097). Neurology, 2018; 90(suppl 15):P1.097.
A Novel Pharmaceutical Care Model for High-Risk Patients
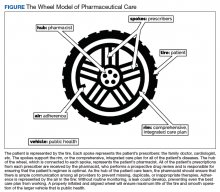
Nonadherence is a significant problem that has a negative impact on both patients and public health. Patients with multiple diseases often have complicated medication regimens, which can be difficult for them to manage. Unfortunately, nonadherence in these high-risk patients can have drastic consequences, including disease progression, hospitalization, and death, resulting in billions of dollars in unnecessary costs nationwide.1,2 The Wheel Model of Pharmaceutical Care (Figure) is a novel care model developed at the Gallup Indian Medical Center (GIMC) in New Mexico to address these problems by positioning pharmacy as a proactive service. The Wheel Model of Pharmaceutical Care was designed to improve adherence and patient outcomes and to encourage communication among the patient, pharmacists, prescribers, and other health care team members.
Pharmacists are central to managing patients’ medication therapies and coordinating communication among the health care providers (HCPs).1,3 Medication therapy management (MTM), a required component of Medicare Part D plans, helps ensure appropriate drug use and reduce the risk of adverse events.3 Since pharmacists receive prescriptions from all of the patient’s HCPs, patients may see pharmacists more often than they see any other HCP. GIMC is currently piloting a new clinic, the Medication Optimization, Synchronization, and Adherence Improvement Clinic (MOSAIC), that was created to implement the Wheel Model of Pharmaceutical Care. MOSAIC aims to provide proactive pharmacy services and continuous MTM to high-risk patients and will enable the effectiveness of this new pharmaceutical care model to be assessed.
Methods
Studies have identified certain populations who are at an increased risk for nonadherence: the elderly, patients with complex or extensive medication regimens, patients with multiple chronic medical conditions, substance misusers, certain ethnicities, patients of lower socioeconomic status, patients with limited literacy, and the homeless.2,4 Federal regulations require that Medicare Part D plans target beneficiaries who meet specific criteria for MTM programs. Under these rules, plans must target beneficiaries with ≥ 3 chronic diseases and ≥ 8 chronic medications, although plans also may include patients with fewer medications and diseases.3 Although the Wheel Model of Pharmaceutical Care is postulated to be an accurate model for the ideal care of all patients, initial implementation should be targeted toward populations who are likely to benefit the most from intervention. For these reasons, elderly Native American patients who have ≥ 2 chronic diseases and who take ≥ 5 chronic medications were targeted for initial enrollment in MOSAIC at GIMC.
Overview
In MOSAIC, pharmacists act as the hub of the pharmaceutical care wheel. Pharmacists work to ensure optimization of the patient’s comprehensive, integrated care plan—the rim of the wheel. As a part of this optimization process, MOSAIC pharmacists facilitate synchronization of the patient’s prescriptions to a monthly or quarterly target fill date. The patient’s current medication therapy is organized, and pharmacists track which medications are due to be filled instead of depending on the patient to request each prescription refill. This process effectively changes pharmacy from a requested service to a provided service.
Pharmacists also monitor the air in the tire to promote adherence. This is accomplished by providing efficient monthly or quarterly telephone or in-person consultations, which helps the patient better understand his or her comprehensive, integrated care plan. MOSAIC eliminates the possibility of nonadherence due to running out of refills. Specialized packaging, such as pill boxes or blister packs, can also improve adherence for certain patients.
MOSAIC ensures that pharmacists stay connected with the spokes, which represent a patient’s numerous prescribers, and close communication loops. Pharmacists can make prescribers aware of potential gaps or overlaps in treatment and assist them in the optimization and development of the patient’s comprehensive, integrated care plan. Pharmacists also make sure that the patient’s medication profile is current and accurate in the electronic health record (EHR). Any pertinent information discovered during MOSAIC encounters, such as abnormal laboratory results or changes in medications or disease, is documented in an EHR note. The patient’s prescribers are made aware of this information by tagging them as additional signers to the note in the EHR.
Keeping patients—the tires—healthy will ensure smooth operation of the vehicle and have a positive impact on public health. MOSAIC is expected to not only improve individual patient outcomes, but also decrease health care costs for patients and society due to nonadherence, suboptimal regimens, stockpiled home medications, and preventable hospital admissions.
Traditionally, pharmacy has been a requested service: A patient requests each of their prescriptions to be refilled, and the pharmacy fills the prescription. Ideally, pharmacy must become a provided service, with pharmacists keeping track of when a patient’s medications are due to be filled and actively looking for medication therapy optimization opportunities. This is accomplished by synchronizing the patient’s medications to the same monthly or quarterly fill date; screening for any potentially inappropriate medications, including high-risk medications in elderly patients, duplications, and omissions; verifying any medication changes with the patient each fill; and then providing all needed medications to the patient at a scheduled time.
To facilitate this process, custom software was developed for MOSAIC. In addition, a collaborative practice agreement (CPA) was drafted that allowed MOSAIC pharmacists to make certain medication therapy optimizations on behalf of the patient’s primary care provider. As part of this CPA, pharmacists also may order and act on certain laboratory tests, which helps to monitor disease progression, ensure safe medication use, and meet Government Performance and Results Act (GPRA) measures. As a novel model of pharmaceutical care, the effects of this approach are not yet known; however, research suggests that increased communication among HCPs and patient-centered approaches to care are beneficial to patient outcomes, adherence, and public health.1,5
Investigated Outcomes
As patients continue to enroll in MOSAIC, the effectiveness of the clinic will be evaluated. Specifically, quality of life, patient and HCP satisfaction with the program, adherence metrics, hospitalization rates, and all-cause mortality will be assessed for patients enrolled in MOSAIC as well as similar patients who are not enrolled in MOSAIC. Also, pharmacists will log all recommended medication therapy interventions so that the optimization component of MOSAIC may be quantified. GPRA measures and the financial implications of the interventions made by MOSAIC will also be evaluated.
Discussion
There are a number of factors, such as MTM services and interprofessional care teams, that research has shown to independently improve patient outcomes, adherence, or public health. By synthesizing these factors, a completely new approach—the Wheel Model of Pharmaceutical Care—was developed. This model presents a radical departure from traditional, requested-service practices and posits pharmacy as a provided service instead. Although the ideas of MTM and interprofessional care teams are not new, there has never been a practical way to truly integrate community pharmacists into the patient care team or to ensure adequate communication among all of the patient’s HCPs. The Wheel Model of Pharmaceutical Care includes public health as one of its core components and provides a framework for pharmacies to meaningfully impact health outcomes for patients.
The Wheel Model of Pharmaceutical Care was designed to minimize the likelihood of nonadherence. Despite this, patients might willfully choose to be nonadherent, forget to take their medications, or neglect to pick up their medications. Additionally, in health care systems where patients must pay for their medications, prescription drug costs might be a barrier to adherence.
When nonadherence is suspected, the Wheel Model of Pharmaceutical Care directs pharmacists in MOSAIC to take action. First, the underlying cause of the nonadherence must be determined. For example, if a patient is nonadherent because of an adverse drug reaction, a therapy change may be indicated. If a patient is nonadherent due to apathy toward their health or therapy, the patient may benefit from education about their condition and treatment options; thus, the patient can make shared, informed decisions and feel more actively involved with his or her health. If a patients is nonadherent due to forgetfulness, adherence packaging dispense methods should be considered as an alternative to traditional vials. Depending on the services offered by a given pharmacy, adherence packaging options may include blister packs, pill boxes, or strips prepared by robotic dispensing systems. The use of medication reminders, whether in the form of a smartphone application or a simple alarm clock, should be discussed with the patient. If the patient does not pick up their medications on time, a pharmacist can contact the patient to determine why the medications were not picked up and to assess any nonadherence. In this case, mail order pharmacy services, if available, should be offered to patients as a more convenient option.
The medication regimen optimization component of MOSAIC helps reduce the workload of primary care providers and allows pharmacists to act autonomously based on clinical judgment, within the scope of the CPA. This can prevent delays in care caused by no refills remaining on a prescription. The laboratory monitoring component allows pharmacists to track diseases and take action if necessary, which should have a favorable impact on GPRA measures. Medication optimizations can reduce wasted resources by identifying cost-saving formulary alternatives, potentially inappropriate medications, and suboptimal doses.
Since many Indian Health Service beneficiaries do not have private insurance and therefore do not generate third-party reimbursements for services and care provided by GIMC, keeping patients healthy and out of the hospital is a top priority. As more patients are enrolled in MOSAIC, the program is expected to have a favorable impact on pharmacy workload and workflow as well. Prescriptions are anticipated and filled in advance, which decreases the amount of patients calling and presenting to the pharmacy for same-day refill requests. Scheduling when MOSAIC patients’ medications are to be filled and dispensed creates a predictable workload that allows the pharmacy staff to be managed more efficiently.
Conclusion
Adherence is the responsibility of the patient, but the Wheel Model of Pharmaceutical Care aims to provide pharmacists with a framework to monitor and encourage adherence in their patients. By taking this patient-centered approach, MOSAIC is expected to improve outcomes and decrease hospitalizations for high-risk patients who simply need a little extra help with their medications.
1. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
2. Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. Case Manager. 2005;16(2):47-51.
3. Drug utilization management, quality assurance, and medication therapy management programs (MTMPs). Fed Regist. 2012;77(71):2207-22175. To be codified at 42 CFR § 423.153.
4. Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275-1282.
5. Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123-1133.
Nonadherence is a significant problem that has a negative impact on both patients and public health. Patients with multiple diseases often have complicated medication regimens, which can be difficult for them to manage. Unfortunately, nonadherence in these high-risk patients can have drastic consequences, including disease progression, hospitalization, and death, resulting in billions of dollars in unnecessary costs nationwide.1,2 The Wheel Model of Pharmaceutical Care (Figure) is a novel care model developed at the Gallup Indian Medical Center (GIMC) in New Mexico to address these problems by positioning pharmacy as a proactive service. The Wheel Model of Pharmaceutical Care was designed to improve adherence and patient outcomes and to encourage communication among the patient, pharmacists, prescribers, and other health care team members.
Pharmacists are central to managing patients’ medication therapies and coordinating communication among the health care providers (HCPs).1,3 Medication therapy management (MTM), a required component of Medicare Part D plans, helps ensure appropriate drug use and reduce the risk of adverse events.3 Since pharmacists receive prescriptions from all of the patient’s HCPs, patients may see pharmacists more often than they see any other HCP. GIMC is currently piloting a new clinic, the Medication Optimization, Synchronization, and Adherence Improvement Clinic (MOSAIC), that was created to implement the Wheel Model of Pharmaceutical Care. MOSAIC aims to provide proactive pharmacy services and continuous MTM to high-risk patients and will enable the effectiveness of this new pharmaceutical care model to be assessed.
Methods
Studies have identified certain populations who are at an increased risk for nonadherence: the elderly, patients with complex or extensive medication regimens, patients with multiple chronic medical conditions, substance misusers, certain ethnicities, patients of lower socioeconomic status, patients with limited literacy, and the homeless.2,4 Federal regulations require that Medicare Part D plans target beneficiaries who meet specific criteria for MTM programs. Under these rules, plans must target beneficiaries with ≥ 3 chronic diseases and ≥ 8 chronic medications, although plans also may include patients with fewer medications and diseases.3 Although the Wheel Model of Pharmaceutical Care is postulated to be an accurate model for the ideal care of all patients, initial implementation should be targeted toward populations who are likely to benefit the most from intervention. For these reasons, elderly Native American patients who have ≥ 2 chronic diseases and who take ≥ 5 chronic medications were targeted for initial enrollment in MOSAIC at GIMC.
Overview
In MOSAIC, pharmacists act as the hub of the pharmaceutical care wheel. Pharmacists work to ensure optimization of the patient’s comprehensive, integrated care plan—the rim of the wheel. As a part of this optimization process, MOSAIC pharmacists facilitate synchronization of the patient’s prescriptions to a monthly or quarterly target fill date. The patient’s current medication therapy is organized, and pharmacists track which medications are due to be filled instead of depending on the patient to request each prescription refill. This process effectively changes pharmacy from a requested service to a provided service.
Pharmacists also monitor the air in the tire to promote adherence. This is accomplished by providing efficient monthly or quarterly telephone or in-person consultations, which helps the patient better understand his or her comprehensive, integrated care plan. MOSAIC eliminates the possibility of nonadherence due to running out of refills. Specialized packaging, such as pill boxes or blister packs, can also improve adherence for certain patients.
MOSAIC ensures that pharmacists stay connected with the spokes, which represent a patient’s numerous prescribers, and close communication loops. Pharmacists can make prescribers aware of potential gaps or overlaps in treatment and assist them in the optimization and development of the patient’s comprehensive, integrated care plan. Pharmacists also make sure that the patient’s medication profile is current and accurate in the electronic health record (EHR). Any pertinent information discovered during MOSAIC encounters, such as abnormal laboratory results or changes in medications or disease, is documented in an EHR note. The patient’s prescribers are made aware of this information by tagging them as additional signers to the note in the EHR.
Keeping patients—the tires—healthy will ensure smooth operation of the vehicle and have a positive impact on public health. MOSAIC is expected to not only improve individual patient outcomes, but also decrease health care costs for patients and society due to nonadherence, suboptimal regimens, stockpiled home medications, and preventable hospital admissions.
Traditionally, pharmacy has been a requested service: A patient requests each of their prescriptions to be refilled, and the pharmacy fills the prescription. Ideally, pharmacy must become a provided service, with pharmacists keeping track of when a patient’s medications are due to be filled and actively looking for medication therapy optimization opportunities. This is accomplished by synchronizing the patient’s medications to the same monthly or quarterly fill date; screening for any potentially inappropriate medications, including high-risk medications in elderly patients, duplications, and omissions; verifying any medication changes with the patient each fill; and then providing all needed medications to the patient at a scheduled time.
To facilitate this process, custom software was developed for MOSAIC. In addition, a collaborative practice agreement (CPA) was drafted that allowed MOSAIC pharmacists to make certain medication therapy optimizations on behalf of the patient’s primary care provider. As part of this CPA, pharmacists also may order and act on certain laboratory tests, which helps to monitor disease progression, ensure safe medication use, and meet Government Performance and Results Act (GPRA) measures. As a novel model of pharmaceutical care, the effects of this approach are not yet known; however, research suggests that increased communication among HCPs and patient-centered approaches to care are beneficial to patient outcomes, adherence, and public health.1,5
Investigated Outcomes
As patients continue to enroll in MOSAIC, the effectiveness of the clinic will be evaluated. Specifically, quality of life, patient and HCP satisfaction with the program, adherence metrics, hospitalization rates, and all-cause mortality will be assessed for patients enrolled in MOSAIC as well as similar patients who are not enrolled in MOSAIC. Also, pharmacists will log all recommended medication therapy interventions so that the optimization component of MOSAIC may be quantified. GPRA measures and the financial implications of the interventions made by MOSAIC will also be evaluated.
Discussion
There are a number of factors, such as MTM services and interprofessional care teams, that research has shown to independently improve patient outcomes, adherence, or public health. By synthesizing these factors, a completely new approach—the Wheel Model of Pharmaceutical Care—was developed. This model presents a radical departure from traditional, requested-service practices and posits pharmacy as a provided service instead. Although the ideas of MTM and interprofessional care teams are not new, there has never been a practical way to truly integrate community pharmacists into the patient care team or to ensure adequate communication among all of the patient’s HCPs. The Wheel Model of Pharmaceutical Care includes public health as one of its core components and provides a framework for pharmacies to meaningfully impact health outcomes for patients.
The Wheel Model of Pharmaceutical Care was designed to minimize the likelihood of nonadherence. Despite this, patients might willfully choose to be nonadherent, forget to take their medications, or neglect to pick up their medications. Additionally, in health care systems where patients must pay for their medications, prescription drug costs might be a barrier to adherence.
When nonadherence is suspected, the Wheel Model of Pharmaceutical Care directs pharmacists in MOSAIC to take action. First, the underlying cause of the nonadherence must be determined. For example, if a patient is nonadherent because of an adverse drug reaction, a therapy change may be indicated. If a patient is nonadherent due to apathy toward their health or therapy, the patient may benefit from education about their condition and treatment options; thus, the patient can make shared, informed decisions and feel more actively involved with his or her health. If a patients is nonadherent due to forgetfulness, adherence packaging dispense methods should be considered as an alternative to traditional vials. Depending on the services offered by a given pharmacy, adherence packaging options may include blister packs, pill boxes, or strips prepared by robotic dispensing systems. The use of medication reminders, whether in the form of a smartphone application or a simple alarm clock, should be discussed with the patient. If the patient does not pick up their medications on time, a pharmacist can contact the patient to determine why the medications were not picked up and to assess any nonadherence. In this case, mail order pharmacy services, if available, should be offered to patients as a more convenient option.
The medication regimen optimization component of MOSAIC helps reduce the workload of primary care providers and allows pharmacists to act autonomously based on clinical judgment, within the scope of the CPA. This can prevent delays in care caused by no refills remaining on a prescription. The laboratory monitoring component allows pharmacists to track diseases and take action if necessary, which should have a favorable impact on GPRA measures. Medication optimizations can reduce wasted resources by identifying cost-saving formulary alternatives, potentially inappropriate medications, and suboptimal doses.
Since many Indian Health Service beneficiaries do not have private insurance and therefore do not generate third-party reimbursements for services and care provided by GIMC, keeping patients healthy and out of the hospital is a top priority. As more patients are enrolled in MOSAIC, the program is expected to have a favorable impact on pharmacy workload and workflow as well. Prescriptions are anticipated and filled in advance, which decreases the amount of patients calling and presenting to the pharmacy for same-day refill requests. Scheduling when MOSAIC patients’ medications are to be filled and dispensed creates a predictable workload that allows the pharmacy staff to be managed more efficiently.
Conclusion
Adherence is the responsibility of the patient, but the Wheel Model of Pharmaceutical Care aims to provide pharmacists with a framework to monitor and encourage adherence in their patients. By taking this patient-centered approach, MOSAIC is expected to improve outcomes and decrease hospitalizations for high-risk patients who simply need a little extra help with their medications.
Nonadherence is a significant problem that has a negative impact on both patients and public health. Patients with multiple diseases often have complicated medication regimens, which can be difficult for them to manage. Unfortunately, nonadherence in these high-risk patients can have drastic consequences, including disease progression, hospitalization, and death, resulting in billions of dollars in unnecessary costs nationwide.1,2 The Wheel Model of Pharmaceutical Care (Figure) is a novel care model developed at the Gallup Indian Medical Center (GIMC) in New Mexico to address these problems by positioning pharmacy as a proactive service. The Wheel Model of Pharmaceutical Care was designed to improve adherence and patient outcomes and to encourage communication among the patient, pharmacists, prescribers, and other health care team members.
Pharmacists are central to managing patients’ medication therapies and coordinating communication among the health care providers (HCPs).1,3 Medication therapy management (MTM), a required component of Medicare Part D plans, helps ensure appropriate drug use and reduce the risk of adverse events.3 Since pharmacists receive prescriptions from all of the patient’s HCPs, patients may see pharmacists more often than they see any other HCP. GIMC is currently piloting a new clinic, the Medication Optimization, Synchronization, and Adherence Improvement Clinic (MOSAIC), that was created to implement the Wheel Model of Pharmaceutical Care. MOSAIC aims to provide proactive pharmacy services and continuous MTM to high-risk patients and will enable the effectiveness of this new pharmaceutical care model to be assessed.
Methods
Studies have identified certain populations who are at an increased risk for nonadherence: the elderly, patients with complex or extensive medication regimens, patients with multiple chronic medical conditions, substance misusers, certain ethnicities, patients of lower socioeconomic status, patients with limited literacy, and the homeless.2,4 Federal regulations require that Medicare Part D plans target beneficiaries who meet specific criteria for MTM programs. Under these rules, plans must target beneficiaries with ≥ 3 chronic diseases and ≥ 8 chronic medications, although plans also may include patients with fewer medications and diseases.3 Although the Wheel Model of Pharmaceutical Care is postulated to be an accurate model for the ideal care of all patients, initial implementation should be targeted toward populations who are likely to benefit the most from intervention. For these reasons, elderly Native American patients who have ≥ 2 chronic diseases and who take ≥ 5 chronic medications were targeted for initial enrollment in MOSAIC at GIMC.
Overview
In MOSAIC, pharmacists act as the hub of the pharmaceutical care wheel. Pharmacists work to ensure optimization of the patient’s comprehensive, integrated care plan—the rim of the wheel. As a part of this optimization process, MOSAIC pharmacists facilitate synchronization of the patient’s prescriptions to a monthly or quarterly target fill date. The patient’s current medication therapy is organized, and pharmacists track which medications are due to be filled instead of depending on the patient to request each prescription refill. This process effectively changes pharmacy from a requested service to a provided service.
Pharmacists also monitor the air in the tire to promote adherence. This is accomplished by providing efficient monthly or quarterly telephone or in-person consultations, which helps the patient better understand his or her comprehensive, integrated care plan. MOSAIC eliminates the possibility of nonadherence due to running out of refills. Specialized packaging, such as pill boxes or blister packs, can also improve adherence for certain patients.
MOSAIC ensures that pharmacists stay connected with the spokes, which represent a patient’s numerous prescribers, and close communication loops. Pharmacists can make prescribers aware of potential gaps or overlaps in treatment and assist them in the optimization and development of the patient’s comprehensive, integrated care plan. Pharmacists also make sure that the patient’s medication profile is current and accurate in the electronic health record (EHR). Any pertinent information discovered during MOSAIC encounters, such as abnormal laboratory results or changes in medications or disease, is documented in an EHR note. The patient’s prescribers are made aware of this information by tagging them as additional signers to the note in the EHR.
Keeping patients—the tires—healthy will ensure smooth operation of the vehicle and have a positive impact on public health. MOSAIC is expected to not only improve individual patient outcomes, but also decrease health care costs for patients and society due to nonadherence, suboptimal regimens, stockpiled home medications, and preventable hospital admissions.
Traditionally, pharmacy has been a requested service: A patient requests each of their prescriptions to be refilled, and the pharmacy fills the prescription. Ideally, pharmacy must become a provided service, with pharmacists keeping track of when a patient’s medications are due to be filled and actively looking for medication therapy optimization opportunities. This is accomplished by synchronizing the patient’s medications to the same monthly or quarterly fill date; screening for any potentially inappropriate medications, including high-risk medications in elderly patients, duplications, and omissions; verifying any medication changes with the patient each fill; and then providing all needed medications to the patient at a scheduled time.
To facilitate this process, custom software was developed for MOSAIC. In addition, a collaborative practice agreement (CPA) was drafted that allowed MOSAIC pharmacists to make certain medication therapy optimizations on behalf of the patient’s primary care provider. As part of this CPA, pharmacists also may order and act on certain laboratory tests, which helps to monitor disease progression, ensure safe medication use, and meet Government Performance and Results Act (GPRA) measures. As a novel model of pharmaceutical care, the effects of this approach are not yet known; however, research suggests that increased communication among HCPs and patient-centered approaches to care are beneficial to patient outcomes, adherence, and public health.1,5
Investigated Outcomes
As patients continue to enroll in MOSAIC, the effectiveness of the clinic will be evaluated. Specifically, quality of life, patient and HCP satisfaction with the program, adherence metrics, hospitalization rates, and all-cause mortality will be assessed for patients enrolled in MOSAIC as well as similar patients who are not enrolled in MOSAIC. Also, pharmacists will log all recommended medication therapy interventions so that the optimization component of MOSAIC may be quantified. GPRA measures and the financial implications of the interventions made by MOSAIC will also be evaluated.
Discussion
There are a number of factors, such as MTM services and interprofessional care teams, that research has shown to independently improve patient outcomes, adherence, or public health. By synthesizing these factors, a completely new approach—the Wheel Model of Pharmaceutical Care—was developed. This model presents a radical departure from traditional, requested-service practices and posits pharmacy as a provided service instead. Although the ideas of MTM and interprofessional care teams are not new, there has never been a practical way to truly integrate community pharmacists into the patient care team or to ensure adequate communication among all of the patient’s HCPs. The Wheel Model of Pharmaceutical Care includes public health as one of its core components and provides a framework for pharmacies to meaningfully impact health outcomes for patients.
The Wheel Model of Pharmaceutical Care was designed to minimize the likelihood of nonadherence. Despite this, patients might willfully choose to be nonadherent, forget to take their medications, or neglect to pick up their medications. Additionally, in health care systems where patients must pay for their medications, prescription drug costs might be a barrier to adherence.
When nonadherence is suspected, the Wheel Model of Pharmaceutical Care directs pharmacists in MOSAIC to take action. First, the underlying cause of the nonadherence must be determined. For example, if a patient is nonadherent because of an adverse drug reaction, a therapy change may be indicated. If a patient is nonadherent due to apathy toward their health or therapy, the patient may benefit from education about their condition and treatment options; thus, the patient can make shared, informed decisions and feel more actively involved with his or her health. If a patients is nonadherent due to forgetfulness, adherence packaging dispense methods should be considered as an alternative to traditional vials. Depending on the services offered by a given pharmacy, adherence packaging options may include blister packs, pill boxes, or strips prepared by robotic dispensing systems. The use of medication reminders, whether in the form of a smartphone application or a simple alarm clock, should be discussed with the patient. If the patient does not pick up their medications on time, a pharmacist can contact the patient to determine why the medications were not picked up and to assess any nonadherence. In this case, mail order pharmacy services, if available, should be offered to patients as a more convenient option.
The medication regimen optimization component of MOSAIC helps reduce the workload of primary care providers and allows pharmacists to act autonomously based on clinical judgment, within the scope of the CPA. This can prevent delays in care caused by no refills remaining on a prescription. The laboratory monitoring component allows pharmacists to track diseases and take action if necessary, which should have a favorable impact on GPRA measures. Medication optimizations can reduce wasted resources by identifying cost-saving formulary alternatives, potentially inappropriate medications, and suboptimal doses.
Since many Indian Health Service beneficiaries do not have private insurance and therefore do not generate third-party reimbursements for services and care provided by GIMC, keeping patients healthy and out of the hospital is a top priority. As more patients are enrolled in MOSAIC, the program is expected to have a favorable impact on pharmacy workload and workflow as well. Prescriptions are anticipated and filled in advance, which decreases the amount of patients calling and presenting to the pharmacy for same-day refill requests. Scheduling when MOSAIC patients’ medications are to be filled and dispensed creates a predictable workload that allows the pharmacy staff to be managed more efficiently.
Conclusion
Adherence is the responsibility of the patient, but the Wheel Model of Pharmaceutical Care aims to provide pharmacists with a framework to monitor and encourage adherence in their patients. By taking this patient-centered approach, MOSAIC is expected to improve outcomes and decrease hospitalizations for high-risk patients who simply need a little extra help with their medications.
1. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
2. Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. Case Manager. 2005;16(2):47-51.
3. Drug utilization management, quality assurance, and medication therapy management programs (MTMPs). Fed Regist. 2012;77(71):2207-22175. To be codified at 42 CFR § 423.153.
4. Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275-1282.
5. Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123-1133.
1. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
2. Vlasnik JJ, Aliotta SL, DeLor B. Medication adherence: factors influencing compliance with prescribed medication plans. Case Manager. 2005;16(2):47-51.
3. Drug utilization management, quality assurance, and medication therapy management programs (MTMPs). Fed Regist. 2012;77(71):2207-22175. To be codified at 42 CFR § 423.153.
4. Thiruchselvam T, Naglie G, Moineddin R, et al. Risk factors for medication nonadherence in older adults with cognitive impairment who live alone. Int J Geriatr Psychiatry. 2012;27(12):1275-1282.
5. Liddy C, Blazkho V, Mill K. Challenges of self-management when living with multiple chronic conditions: systematic review of the qualitative literature. Can Fam Physician. 2014;60(12):1123-1133.
A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury (FULL)
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
An estimated 282,000 people in the US are living with spinal cord injury (SCI).1 Damage to the cervical spinal cord is the most prevalent. Among cervical spinal cord trauma, injury to levels C4, C5, and C6 have the highest occurrence.1 Damage to these levels has significant implications for functional status. Depending on pathology, patients’ functional status can range from requiring assistance for all activities of daily living (ADL) to potentially living independently.
Improving upper-limb function is vital to achieving independence. About half of people with tetraplegia judge hand and arm function to be the top factor that would improve quality of life (QOL).2 Persons with traumatic cervical SCI may lose the ability to use their hands from motor deficits, sensory dysfunction, proprioception problem, and/or loss of coordination. In addition, they may develop joint contracture, spasticity, pain, and other complications. Thus, their independence and ADL are affected significantly by multiple mechanisms of pathology.
Upper-extremity rehabilitation that emphasizes strengthening and maintaining functional range of motion (ROM) is fundamental to SCI rehabilitation. Rehabilitation to restore partial hand function has included ROM exercises, splinting, surgical procedures in the form of tendon transfers and various electrical stimulation devices, such as implantable neuroprostheses.2-7 These interventions improve the ability to grasp, hold, and release objects in selected individuals; however, they have not been universally accepted. Traditional modalities, such as active ROM (AROM) and passive ROM (PROM) and electrical stimulation remain highly used in upper-extremity rehabilitation. Devices have been developed to provide either PROM or electrical stimulation to improve hand function and to prevent muscle atrophy. Therapist- and caregiver-directed PROM exercises are time consuming and labor intensive. An innovative therapeutic approach that can provide all these modalities more efficiently is needed in SCI rehabilitation.
Until now, a single device that combines AROM and PROM simultaneously has not been available. A robotic system, the FES Hand Glove 200 (Robotix Hand Therapy Inc, Colorado Springs, CO), was developed to improve hand function (Figure).
Methods
This prospective safety study evaluated the occurrence of adverse effects (AEs) associated with the use of the FES Hand Glove 200. The study was performed in the Occupational Therapy Section of the Spinal Cord Injury Center at the James A. Haley Veterans’ Hospital (JAHVH) and approved by the JAHVH Research and Development Committee as well as the University of South Florida Investigational Review Board. For recruitment, the goals of the study as well as the inclusion and exclusion criteria were presented to the Spinal Cord Injury Center health care providers. Potential candidates of the study were referred to the study team from these providers.
Screening of the referred candidates was conducted by physicians during inpatient evaluations. All subjects signed a consent form. Participants included active-duty military or veterans with traumatic SCI at levels C4 to C8 and American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, and D. Participants were aged 18 to 60 years, at least 1-month post-SCI, medically stable, and had impairments in upper-extremities strength and ROM or function, including hand.
Subjects were excluded if any of the following were present: seizure within 3 months of study; active cancer; heterotopic ossification below the shoulder; new acute hand injuries of the study limb; unhealed fractures of the study limb; myocardial infarction within 12 months; severe cognitive impairment determined by a Modified Rancho Score below VI8; severe aphasia; pregnancy; skin irritations or open wounds in the study limb; fixed contractures of > 40° of the metacarpophalangeal (MP) or proximal interphalangeal (PIP) joints of the study hand; unwillingness to perform all of the therapies and assessments required for the study; active implant device (eg, pacemaker, implanted cardiac defibrillator, neurostimulator or drug infusion device); major psychological disorder; severe residual spasticity despite maximal medical therapy; muscle power grade of more than 3+ on wrist and finger extensors and flexors of the study limb; recent or current participation in research that could influence study response; pain that prevents participation in the study; or concurrent use of transcutaneous electrical stimulation on the study arm.
The following data were documented: level of SCI, AIS-score; complete medical history; physical examination (including skin integrity); and vital signs of bilateral upper extremities. A nurse practitioner (NP) certified in Functional Independent Measure (FIM) conducted chart reviews and/or in-person interviews of each subject to establish a FIM score before and after 6 weeks of research treatment. Two experienced occupational therapists (OTs) conducted detailed hand evaluations before the research treatment interventions. An OT provided subjects with education on the use, care, and precautions of the FES Hand Glove 200. The OT adjusted the device on the subject’s hand for proper fitting, including initial available PROM, and optimal muscle stimulation.
The OT then implemented the treatment protocol using the FES Hand Glove 200 in 1 hand per the subjects’ preference. The subjects received 30 minutes of PROM only on the FES Hand Glove 200, followed by an additional 30 minutes of PROM with FES for 1 hour of therapy per session. The study participants were treated 4 times per week for 6 weeks. Before and after each session, OTs evaluated and documented any loss of skin integrity and pain. Autonomic dysreflexia occurred when systolic BP increased > 20 to 30 mm Hg with symptoms such as headache, profuse sweating, or blurred vision was reported.9 The FES Hand Glove 200 was set up for PROM to the thumb and to digits 2 to 5 and for electrical muscle stimulation of the finger extensors and flexors. No other therapeutic exercise was performed during the study period on the other extremity. Primary and secondary outcomes were collected at the end of the 6-week intervention.
Primary outcomes included complications from the use of FES Hand Glove 200, including skin integrity and any joint deformity as drawn on a figure, changes of pain level by visual analog scale (VAS), and total number of autonomic dyreflexia episodes. Secondary measured outcomes included changes in PROM and AROM of wrist, metacarpal joint and interphalangeal joints of thumbs and digits 2 to 5 ≥ 10°; hand and pinch strength decline of > 1 lb; decline in manual muscle test, and FIM score, which is a validated measurement of disability and the level of assistance required for ADL.10
Statistical analyses were performed using SAS version 4 (Cary, NC) to assess the degree of change in the improvement score, which was defined as the postintervention score minus the preintervention score. However, because of the large standard error due to small sample sizes, the normality assumption was not satisfied for all the outcomes considered.
Results
Of the 20 participants screened, 14 men aged between 19 and 66 years with cervical SCI level of C4 to C6 AIS grades A to D were enrolled in the study. Three did not complete the 6-week trial due to SCI-related medical complications, which were unrelated to the use of the FES Hand Glove 200. They continued with regular OT treatment or self-directed home exercises after they were seen by the treating physician. (Table 1)
Skin integrity of all subjects was maintained throughout the study. One subject had a right-elbow wound before the intervention, which was unchanged at the end of the study. After 6 weeks of experimental intervention, there was no wrist or finger joint deformity noted and no increase in pain level except for 1 subject who reported increased pain that was unrelated to use of the device. No occurrence of autonomic dysreflexia was recorded during the use of FES Hand Glove 200 (Table 2).
For the secondary outcomes, there was no significant decrease in AROM or PROM ≥ 10° in forearm, wrist, or finger joints in any participants. There was no loss of strength > 1 lb as measured by gross grasp, pinch tip, 3-point, or lateral grip. There was no decline in motor strength per manual muscle testing. No worsening of FIM score was noted (Table 3).
Although this was not an efficacy study primarily, participants improved in several areas. Improvements included active and passive movements in the forearm, wrist, and hand. There also was significant improvement in strength of the extensor digitorum communis (EDC) muscle. Data are available on request to the authors.
Discussion
Passive ROM and AROM exercises and FES are common strategies to improve certain hand functions in people with cervical SCI. Many people, however, may experience limited duration or efficiency of rehabilitation secondary to lack of resources. Technologic advancement allowed the combination of PROM exercise and FES using the FES Hand Glove 200 device. The eventual goal of using this device is to enhance QOL by improving upper-extremity function. Because this device is not commercially available, its safety and tolerability are being tested prior to clinical use. Although 3 subjects withdrew from the study due to nondevice-related medical reasons, 11 subjects completed the study. Potential AEs included skin wounds, burns, tendon sprain or rupture, edema, and pain. At the end of the 6-week study period, there was no loss of skin integrity, no joint deformity, and no increase in hand or finger edema in all subjects. Increase in pain level at 6 weeks was noted in only 1 subject.
One concern was that overuse of such devices could potentially cause muscle fatigue, leading to decreased strength. Pinch grasp and manual muscle testing were evaluated, and no decrease in any of these parameters was noted at the end of study. Although this was not an efficacy study, there was some evidence of improved ROM of multiple wrist and finger joints as well as the EDC muscle strength.
Limitations
Limitations of the study included the duration of treatment of eight 30-minute sessions per week over a 6-week period. A longer treatment duration could result in repetition-related injuries and should be tested in future trials. Finally, the sample size of this study was relatively small. Future studies of different treatment frequency, longer duration of use and monitoring, and using a larger sample size are suggested. An efficacy study of this device using a randomized controlled design is indicated. As people with cervical SCI rank upper-extremity dysfunction as one of the top impairments that negatively impacts QOL, rehabilitation strategy to improve such functions should continue to be a research priority.2
Conclusion
This study supports the safety and tolerability of a 6-week course using FES Hand Glove 200 in traumatic SCI tetraplegic subjects. Additionally, data from this study suggest possible efficacy in enhancing ROM of various wrist and finger joints as well as certain muscle group. Further studies of efficacy with larger numbers of subjects are warranted.
Click here to read the digital edition.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
1. NSCISC National Spinal Cord Injury Statistic Center. 2016 annual report—public version. https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20-%20Complete%20Public%20Version.pdf. Published 2016. Accessed March 19, 2018.
2. Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37(1):32-36.
3. O’Driscoll SW, Giori NJ. Continuous passive motion (CPM): theory and principles of clinical application. J Rehabil Res Dev. 2000;37(2):179-188.
4. Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21(3):207-215.
5. de Kroon JR, Ijzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592-600.
6. Alon G, McBride K, Levitt AF. Feasibility of randomised clinical trial of early initiation and prolonged, home-base FES training to enhance upper limb functional recovery following stroke. https://www.researchgate.net /publication/237724608_Feasibility_of_randomised_clinical_trial_of_early _initiation_and_prolonged_home-based_FES_training_to_enhance_upper_limb _functional_recovery_following_stroke. Published 2004. Accessed March 21, 2018.
7. Alon G, McBride K. Persons with C5-C6 tetraplegia achieve selected functional gains using a neuroprosthesis. Arch Phys Med Rehabil. 2003;84(1):119-124.
8. Hagen C, Malkmus D, Durham P. Rancho Los Amigos Cognitive Scale. http://file .lacounty.gov/SDSInter/dhs/218118_RLOCFProfessionalReferenceCard-English .pdf. Published 1979. Accessed March 19, 2018.
9. Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81(4):506-516.
10. Grey N, Kennedy P. The Functional Independence Measure: a comparative study of clinician and self rating. Paraplegia. 1993;31(7):457-461.
Reducing COPD Readmission Rates: Using a COPD Care Service During Care Transitions
A chronic obstructive pulmonary disease care service improves timely access to follow-up care and patient education at the time of transition from hospital to home.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide and has an associated treatment cost of $9,800 per patient per year in the US.1-3 Within 5 years of hospital discharge for a COPD exacerbation, the rehospitalization risk is 44%, and the mortality rate is 55%.4 COPD affects more than 11 million Americans, and the disease prevalence among US veterans is 3-fold higher.5,6
Patients hospitalized for COPD have a 30-day readmission rate of 22.6%.7 Given the high patient burden, COPD was added to the Medicare Hospital Readmission Reductions Program in 2015, resulting in financial penalties for COPD readmissions within 30 days of hospital discharge.8 Ensuring timely access to follow-up care has been shown to significantly reduce risk for hospital readmissions.9 However, in a national review of Medicare claims, only 50% of patients readmitted to the hospital had a primary care provider (PCP) follow-up visit within 30 days of their hospital discharge.10 Despite the need to provide prompt patient follow-up during the transition from hospital to home, gaps within the health care system create barriers to providing timely postdischarge care.10-12 These gaps include breakdowns in practitioner and patient communication, lengthy time to follow-up, and incomplete medication reconciliation.13 To address this unmet need, clinics and hospitals require solutions that can be implemented quickly, using the resources of their current clinical models.
Pharmacists and registered nurses (RNs) within the US federal health care system are well positioned for involvement in the postdischarge care of high-risk patients with COPD. Ambulatory care practitioners within the US Department of Veterans Affairs (VA) health care system are integrated into patient aligned care teams (PACT). Each team consists of a PCP, pharmacist, RN, social worker, dietitian, licensed practical nurse, and medical scheduling support assistant.14 Each PACT team works together to provide patient education, chronic disease management, and medication optimization, and each team member contributes their unique training and expertise.
Interprofessional care is considered an integral method to improve health outcomes through effective teamwork and communication.15 Although interprofessional interventions are cited extensively in the literature highlighting medicine and nursing, a gap exists in the exploration of pharmacist contributions within interprofessional teams.16 The incorporation of clinical pharmacists in the literature is especially limited when considering transitions of care and the patient medical home.17 Given the critical and collaborative role pharmacists play within the PACT medical home, the COPD CARE (Chronic Obstructive Pulmonary Disease Coordinated Access to Reduce Exacerbations) service provides an opportunity to leverage pharmacists as prescribers with a scope of practice who coordinate transitions of care for patients with COPD.18 The service was designed to be collaborative within the PACT model and with the intent of reducing 30-day readmissions to the hospital or emergency department (ED) due to a COPD exacerbation.
This evaluation involved the identifying patients recently hospitalized for COPD; clinic follow-up, coordinated by a clinical pharmacist and nurse, within 30 days of hospital or ED discharge; the use of a COPD action plan; and timely triage of patients at high risk for COPD reexacerbation or with comorbid symptoms to PCPs. The COPD CARE service, leveraged the patient-centered medical home (PCMH) model for transitions of care after COPD exacerbations. The PCMH is a primary care model focused on the following functions: (1) comprehensive care; (2) patient-centered care; (3) coordinated care; (4) accessible service; and (5) quality and safety.19
Methods
The COPD CARE service was implemented on October 1, 2015, and evaluated through March 1, 2016 (Figure 1). All veterans receiving primary care through the pilot clinic site with a hospital admission or ED visit for COPD exacerbation were offered this intervention.
Patient Eligibility and Recruitment
Patients were excluded from the service if COPD or COPD-related diagnoses were not listed in their electronic health record (EHR) problem list. Patients who had previously received components of the intervention through consultation with specialty services were excluded. If a patient declined the service, they received the standard of care. This project was undertaken for programmatic evaluation and qualified for quality improvement (QI) exemption; as such an internal review board approval was not required.
Intervention
Participants enrolled in the COPD CARE service were scheduled for an interprofessional postdischarge follow-up visit with a pharmacist and nurse at the pilot outpatient clinic site, and this visit was termed the COPD CARE health visit. Participants ideally were seen within 30 days of discharge. The goal was to improve access to care while preventing a 30-day readmission. Within this 30-day window, the target follow-up period was 2 to 3 weeks postdischarge for the face-to-face visit. Patients who required postdischarge care for additional medical conditions received a clinic appointment with their PCP on the same day as their COPD CARE health visit. The COPD CARE health visit focused on 3 objectives: (1) COPD disease management and referrals; (2) COPD plan development; and (3) inhaler technique review and teaching.20,21
COPD Monitoring
During the 45-minute COPD CARE health visit, the pharmacist provided extensive disease management based on the GOLD guideline recommendation.22 In addition, the pharmacist administered the COPD Assessment Test (CAT) and reviewed patient COPD exacerbation history to guide prescribing.22 The patient and pharmacist also reviewed previous spirometry results if obtained within the past 2 years. COPD triggers and symptoms were assessed along with opportunities for therapeutic and lifestyle modifications.
Plan Development
Patients in the COPD CARE service also were given a COPD plan to improve health outcomes. (Figure 2). The plan included patient instructions to initiate steroid and antibiotic therapy if the patient experienced symptoms of increased cough, mucus production, and purulence, thereby reaching the high-yellow zone.
Patient Referrals
Patient referrals also were a critical component of the COPD CARE service. Pharmacists placed referrals for tobacco treatment services, pulmonary rehabilitation, a COPD group education class, and referral to specialty care if needed.
Inhaler Technique Review
Either the pharmacist or RN review the inhaler technique, and corrections and teachback methods used to ensure patient understanding.23 Patients were encouraged to bring home inhalers into clinic for technique assessment. Demonstration inhalers also were available and used by pharmacists and nurses for inhaler teaching as needed. The pharmacist indicated through chart documentation whether the patient’s inhaler technique was correct or whether modifications were made to improve medication delivery. Medication reconciliation also was performed for inhaled devices to insure patients were using medications as prescribed.
Outcomes
The primary outcome of this evaluation was an assessment of interventions made by the interprofessional care team during the COPD CARE health visit. Secondary outcomes included assessment of 30-day readmission rates as well as patient access to the primary care team using this interprofessional care model.
Data were collected after study completion through review of the EHR at baseline and at the end of the evaluation period. Baseline demographic information was collected through a retrospective chart review. Readmission rates were calculated as a composite of ED visits and rehospitalization within 30 days of discharge due to a COPD exacerbation.
Patients’ spirometry results were used in composite with clinical symptoms and risk of exacerbations to calculate GOLD staging.24
Results
A total of 19 patients admitted to the hospital or ED received follow-up through the COPD CARE service. Patients included in this analysis were primarily older adult white males.
Referrals were placed for 53% of patients in the COPD CARE service, with 21% of patients accepting referral to tobacco treatment clinic, and 32% of patients accepting referral to pulmonary rehabilitation. COPD plans were issued to all of patients in this service. Pharmacists modified therapy 58% of the time, with a review of medications prescribed by the clinical pharmacist (eApendixes 1 and 2, available at mdedge.com/fedprac).
Patients had a 0% composite readmission rate to the ED or hospital for a COPD exacerbation within 30-days of discharge. Access to care, defined as a visit with the primary care PACT team within 30 days of discharge, was achieved in 14 of the 19 patients (73.7%). Additionally, 12 of 19 patients (63.2%) in the COPD CARE service no longer needed to see their PCP following discharge, saving their provider a visit.
The pharmacist corrected patient inhaler technique in 52.6% of the patients participating in the service.
Discussion
The intent of this QI initiative was to assess a novel clinic intervention for a high-risk patient population during COPD care transitions. The strengths of this intervention involved a rapid cycle implementation using the existing medical home model and its multiprong approach to coordinating care. This approach involved coordinating self-direction COPD plans, timely hospital follow-up, and the innovative use of the interprofessional primary care team.
The COPD CARE service improved patient access to follow-up with no COPD readmissions in the intervention group. The COPD CARE service also validated the use of a coordinated medical home consisting of clinical pharmacists and nurses who provided the initial COPD disease monitoring and plan development. This intervention also resulted in patients receiving greater access to their PACT teams within 30 days of discharge and a higher rate of referrals to tobacco cessation clinics within the COPD CARE group. In addition, use of tools that enabled patients to self-manage their care, such as the COPD plan, was greater in the COPD CARE group.
The interventions made in-clinic likely contributed to service results (eAppendix 3).
In addition, the COPD CARE service provided necessary referrals to pulmonary rehabilitation, nutrition, and tobacco treatment clinics at a higher rate than those patients in the standard of care group. The high percentage of referrals placed to tobacco treatment clinic and pulmonary rehabilitation contributes to improvements in COPD disease control long-term.25
The COPD CARE service may best be described as a model for application of the interprofessional team in clinical practice, with the clinical pharmacist uniquely positioned for chronic disease management in the postacute care setting.26 Previously, literature has documented pharmacists as integral members of the team during patient care transitions. Pharmacist completion of medication reconciliation compared with usual care has shown a 28% relative risk (RR) reduction in ED visits and a 67% RR reduction in adverse drug event-related hospital revisits.27 Findings of the COPD CARE service are consistent with the literature and advance the role of pharmacists within the medical home model as prescribers for disease management.27
The interprofessional, team-based design of the COPD CARE service also is supported by recent recommendations from the COPD Foundation, as detailed in the 2nd National COPD Readmission Summit.28 Use of a proactive, team-based care model is emphasized as a central element to coordinating care transitions, with an expectation of 360 degree accountability by all team members for the patients care both during and after hospitalization. The clearly defined roles of each team member within the COPD CARE service, coupled with the expectation that each team member practices with autonomy and accountability, exemplifies the COPD Foundation vision for enhancing COPD care. In addition, the COPD CARE service uses many of the best practices detailed by the COPD Foundation, including the use of spirometry, referrals to pulmonary rehabilitation, and use of motivational interviewing for tobacco treatment clinic referral.
Limitations
This QI initiative has several limitations. By virtue of the study being designed as a practice improvement intervention with rapid implementation, the existing clinic referral structures were used to offer the service to eligible patients. This standard of care included routine telephone contact by a nurse case manager following hospital discharge. Although all patients in the COPD CARE service received the intervention, 5 patients were not seen within the 30-day window, resulting in an implementation rate of 73%. Of the 5 patients that were not seen, 4 were discharged from the ED. Timely follow-up in primary care clinic from the ED required the use of a time-intensive chart review for referral and subsequent delay in intervention delivery.A streamlined clinic referral process from the ED likely would further improve patient scheduling and result in a greater number of patients who would receive the intervention within 30 days of discharge. Despite this limitation, the COPD CARE service was able to see a large percentage of patients within the 30-day time frame postdischarge.
Future Directions
Although a major objective of this service was to reduce readmissions 30 days postdischarge, it is possible interventions made in clinic may have long-term beneficial effects.25,29 Future research should evaluate the impact of this interprofessional service on long-term disease outcomes, thereby determining whether the promising readmission results are sustained beyond 30 days postdischarge.30 In addition, incorporation of respiratory therapy and inpatient pharmacists during hospital discharge could provide a more effective and sustainable transition from hospital to home before the COPD CARE clinic visit.
Future implementations and evaluations of this COPD CARE service will in turn benefit from a key component of our intervention, which includes the collection of timely CAT scores, spirometry data, and adherence rates for COPD patients.31 Furthermore, the intervention was successfully delivered to a population recently hospitalized or seen in the ED, and therefore, at high risk for future COPD exacerbations. This initiative provides positive proof of a concept QI project using the existing PACT team model to reduce 30-day readmission rates in patients with COPD at high risk for exacerbation. Future efforts will focus on delivering this intervention to patients with mild, moderate, and severe COPD within a wide range of primary clinics.
Conslusion
The COPD CARE service involved the coordinated postdischarge care facilitated by an interprofessional team of clinical pharmacists, nurses and PCPs. The COPD CARE service leveraged an interprofessional team, centered on the PACT medical home, to make clinic interventions resulting in a 0% readmission rate and 63.2% increase in PCP access. The COPD CARE service further demonstrated the impact of coordinated efforts by interprofessional teams to optimize care for COPD management.
Acknowledgments
The authors thank Stephanie Gruber, PharmD; Lieneke Hafeman, RN; Molly Obermark, PharmD; Julia Peek, RT; Mark Regan, MD; Chris Roelke, RN; Steve Shoyer, PharmD; John Thielemann, RN; Sandy Tompkins, BS; and Wendi Wenger, RN, for their integral roles in the COPD CARE service.
1. World Health Organization. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en. Updated May 24, 2018. Accessed May 30, 2018.
2. Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged > 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31-45.
3. American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. http://www.lung.org/assets/documents/research/copd-trend-report.pdf. Published March 2013. Accessed May 30, 2018.
4. McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748-1755.
5. COPD Foundation. Patient groups back bill supporting US veterans with COPD. https://www.copdfoundation.org/About-Us/Press-Room/Press-Releases/Article/722/Patient-Groups-Back-Bill-Supporting-US-Veterans-with-COPD.aspx. Published November 9, 2010. Accessed May 30, 2018.
6. American Lung Association. Lung health and disease: how serious is COPD. http://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/learn-about-copd/how-serious-is-copd.html. Published 2016. Accessed May 30, 2018.
7. Shah T, Press V, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926.
8. Mcllvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;13(20):1796-1803.
9. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-122.
10. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
11. Hitch B, Parlier AB, Reed L, Galvin SL, Fagan EB, Wilson CG. Evaluation of a team-based, transition-of-care management service on 30-day readmission rates. N C Med J. 2016;77(2):87-92.
12. Stone J, Hoffman G. Medicare hospital readmissions: issues, policy options. In: Turner PM ed. Medicare: Background, Benefits and Issues. Nova Science Pub Inc; 2011:123-150.
13. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323.
14. Rosland A-M, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
15. World Health Organization. Nursing and midwifery. http://www.who.int/hrh/nursing_midwifery/en. Accessed September 18, 2018.
16. Supper I, Catala O, Lustman M, Chemla C, Bourgueil Y, Letrilliart L. Interprofessional collaboration in primary health care: a review of facilitators and barriers perceived by involved actors. J Public Health (Oxf). 2015;37(4):716-727.
17. Melody KT, McCartney E, Sen S, Duenas G. Optimizing care transitions: the role of the community pharmacist. Integr Pharm Res Pract. 2016;5:43-51.
18. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
19. US Department of Health and Human Services. Agency for Healthcare Research and Quality. Defining the PCMH. https://pcmh.ahrq.gov/page/defining-pcmh. Accessed May 29, 2018.
20. Kaplan A. The COPD action plan. Can Fam Physician. 2009;55(1):58-59.
21. Turnock AC, Walters EH, Walters JA, Wood-Baker R. Action plans for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(4):CD005074.
22. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2017 report). http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed May 29, 2018.
23. Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1:9.
24. Buist AS, Anzueto A, Calverley P, DeGuia TS, Fukuch Y. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2006.
25. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
26. ASHP Research and Education Foundation. Pharmacy forecast 2016-2020: strategic planning advice. http://www.ashpfoundation.org/PharmacyForecast2016. Published December 2015. Accessed May 29, 2018.
27. Mekonnen AB, McLachlan AJ, Brien JE. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003.
28. Willard KS, Sullivan JB, Thomashow BM, et al. The 2nd national COPD readmissions summit and beyond: from theory to implementation. Chronic Obstr Pulm Dis. 2016;3(4):778-790.
29. Scanlon PD, Connett JE, Waller LA, et al; Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The lung health study. Am J Respir Crit Care Med. 2000;161(2, pt 1):381-390.
30. Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926.
31. GlaxoSmithKline. COPD Assessment Test (CAT). Castest Online. http://www.catestonline.org/images/UserGuides/CATHCPUser%20guideEn.pdf. Updated October 2016.
A chronic obstructive pulmonary disease care service improves timely access to follow-up care and patient education at the time of transition from hospital to home.
A chronic obstructive pulmonary disease care service improves timely access to follow-up care and patient education at the time of transition from hospital to home.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide and has an associated treatment cost of $9,800 per patient per year in the US.1-3 Within 5 years of hospital discharge for a COPD exacerbation, the rehospitalization risk is 44%, and the mortality rate is 55%.4 COPD affects more than 11 million Americans, and the disease prevalence among US veterans is 3-fold higher.5,6
Patients hospitalized for COPD have a 30-day readmission rate of 22.6%.7 Given the high patient burden, COPD was added to the Medicare Hospital Readmission Reductions Program in 2015, resulting in financial penalties for COPD readmissions within 30 days of hospital discharge.8 Ensuring timely access to follow-up care has been shown to significantly reduce risk for hospital readmissions.9 However, in a national review of Medicare claims, only 50% of patients readmitted to the hospital had a primary care provider (PCP) follow-up visit within 30 days of their hospital discharge.10 Despite the need to provide prompt patient follow-up during the transition from hospital to home, gaps within the health care system create barriers to providing timely postdischarge care.10-12 These gaps include breakdowns in practitioner and patient communication, lengthy time to follow-up, and incomplete medication reconciliation.13 To address this unmet need, clinics and hospitals require solutions that can be implemented quickly, using the resources of their current clinical models.
Pharmacists and registered nurses (RNs) within the US federal health care system are well positioned for involvement in the postdischarge care of high-risk patients with COPD. Ambulatory care practitioners within the US Department of Veterans Affairs (VA) health care system are integrated into patient aligned care teams (PACT). Each team consists of a PCP, pharmacist, RN, social worker, dietitian, licensed practical nurse, and medical scheduling support assistant.14 Each PACT team works together to provide patient education, chronic disease management, and medication optimization, and each team member contributes their unique training and expertise.
Interprofessional care is considered an integral method to improve health outcomes through effective teamwork and communication.15 Although interprofessional interventions are cited extensively in the literature highlighting medicine and nursing, a gap exists in the exploration of pharmacist contributions within interprofessional teams.16 The incorporation of clinical pharmacists in the literature is especially limited when considering transitions of care and the patient medical home.17 Given the critical and collaborative role pharmacists play within the PACT medical home, the COPD CARE (Chronic Obstructive Pulmonary Disease Coordinated Access to Reduce Exacerbations) service provides an opportunity to leverage pharmacists as prescribers with a scope of practice who coordinate transitions of care for patients with COPD.18 The service was designed to be collaborative within the PACT model and with the intent of reducing 30-day readmissions to the hospital or emergency department (ED) due to a COPD exacerbation.
This evaluation involved the identifying patients recently hospitalized for COPD; clinic follow-up, coordinated by a clinical pharmacist and nurse, within 30 days of hospital or ED discharge; the use of a COPD action plan; and timely triage of patients at high risk for COPD reexacerbation or with comorbid symptoms to PCPs. The COPD CARE service, leveraged the patient-centered medical home (PCMH) model for transitions of care after COPD exacerbations. The PCMH is a primary care model focused on the following functions: (1) comprehensive care; (2) patient-centered care; (3) coordinated care; (4) accessible service; and (5) quality and safety.19
Methods
The COPD CARE service was implemented on October 1, 2015, and evaluated through March 1, 2016 (Figure 1). All veterans receiving primary care through the pilot clinic site with a hospital admission or ED visit for COPD exacerbation were offered this intervention.
Patient Eligibility and Recruitment
Patients were excluded from the service if COPD or COPD-related diagnoses were not listed in their electronic health record (EHR) problem list. Patients who had previously received components of the intervention through consultation with specialty services were excluded. If a patient declined the service, they received the standard of care. This project was undertaken for programmatic evaluation and qualified for quality improvement (QI) exemption; as such an internal review board approval was not required.
Intervention
Participants enrolled in the COPD CARE service were scheduled for an interprofessional postdischarge follow-up visit with a pharmacist and nurse at the pilot outpatient clinic site, and this visit was termed the COPD CARE health visit. Participants ideally were seen within 30 days of discharge. The goal was to improve access to care while preventing a 30-day readmission. Within this 30-day window, the target follow-up period was 2 to 3 weeks postdischarge for the face-to-face visit. Patients who required postdischarge care for additional medical conditions received a clinic appointment with their PCP on the same day as their COPD CARE health visit. The COPD CARE health visit focused on 3 objectives: (1) COPD disease management and referrals; (2) COPD plan development; and (3) inhaler technique review and teaching.20,21
COPD Monitoring
During the 45-minute COPD CARE health visit, the pharmacist provided extensive disease management based on the GOLD guideline recommendation.22 In addition, the pharmacist administered the COPD Assessment Test (CAT) and reviewed patient COPD exacerbation history to guide prescribing.22 The patient and pharmacist also reviewed previous spirometry results if obtained within the past 2 years. COPD triggers and symptoms were assessed along with opportunities for therapeutic and lifestyle modifications.
Plan Development
Patients in the COPD CARE service also were given a COPD plan to improve health outcomes. (Figure 2). The plan included patient instructions to initiate steroid and antibiotic therapy if the patient experienced symptoms of increased cough, mucus production, and purulence, thereby reaching the high-yellow zone.
Patient Referrals
Patient referrals also were a critical component of the COPD CARE service. Pharmacists placed referrals for tobacco treatment services, pulmonary rehabilitation, a COPD group education class, and referral to specialty care if needed.
Inhaler Technique Review
Either the pharmacist or RN review the inhaler technique, and corrections and teachback methods used to ensure patient understanding.23 Patients were encouraged to bring home inhalers into clinic for technique assessment. Demonstration inhalers also were available and used by pharmacists and nurses for inhaler teaching as needed. The pharmacist indicated through chart documentation whether the patient’s inhaler technique was correct or whether modifications were made to improve medication delivery. Medication reconciliation also was performed for inhaled devices to insure patients were using medications as prescribed.
Outcomes
The primary outcome of this evaluation was an assessment of interventions made by the interprofessional care team during the COPD CARE health visit. Secondary outcomes included assessment of 30-day readmission rates as well as patient access to the primary care team using this interprofessional care model.
Data were collected after study completion through review of the EHR at baseline and at the end of the evaluation period. Baseline demographic information was collected through a retrospective chart review. Readmission rates were calculated as a composite of ED visits and rehospitalization within 30 days of discharge due to a COPD exacerbation.
Patients’ spirometry results were used in composite with clinical symptoms and risk of exacerbations to calculate GOLD staging.24
Results
A total of 19 patients admitted to the hospital or ED received follow-up through the COPD CARE service. Patients included in this analysis were primarily older adult white males.
Referrals were placed for 53% of patients in the COPD CARE service, with 21% of patients accepting referral to tobacco treatment clinic, and 32% of patients accepting referral to pulmonary rehabilitation. COPD plans were issued to all of patients in this service. Pharmacists modified therapy 58% of the time, with a review of medications prescribed by the clinical pharmacist (eApendixes 1 and 2, available at mdedge.com/fedprac).
Patients had a 0% composite readmission rate to the ED or hospital for a COPD exacerbation within 30-days of discharge. Access to care, defined as a visit with the primary care PACT team within 30 days of discharge, was achieved in 14 of the 19 patients (73.7%). Additionally, 12 of 19 patients (63.2%) in the COPD CARE service no longer needed to see their PCP following discharge, saving their provider a visit.
The pharmacist corrected patient inhaler technique in 52.6% of the patients participating in the service.
Discussion
The intent of this QI initiative was to assess a novel clinic intervention for a high-risk patient population during COPD care transitions. The strengths of this intervention involved a rapid cycle implementation using the existing medical home model and its multiprong approach to coordinating care. This approach involved coordinating self-direction COPD plans, timely hospital follow-up, and the innovative use of the interprofessional primary care team.
The COPD CARE service improved patient access to follow-up with no COPD readmissions in the intervention group. The COPD CARE service also validated the use of a coordinated medical home consisting of clinical pharmacists and nurses who provided the initial COPD disease monitoring and plan development. This intervention also resulted in patients receiving greater access to their PACT teams within 30 days of discharge and a higher rate of referrals to tobacco cessation clinics within the COPD CARE group. In addition, use of tools that enabled patients to self-manage their care, such as the COPD plan, was greater in the COPD CARE group.
The interventions made in-clinic likely contributed to service results (eAppendix 3).
In addition, the COPD CARE service provided necessary referrals to pulmonary rehabilitation, nutrition, and tobacco treatment clinics at a higher rate than those patients in the standard of care group. The high percentage of referrals placed to tobacco treatment clinic and pulmonary rehabilitation contributes to improvements in COPD disease control long-term.25
The COPD CARE service may best be described as a model for application of the interprofessional team in clinical practice, with the clinical pharmacist uniquely positioned for chronic disease management in the postacute care setting.26 Previously, literature has documented pharmacists as integral members of the team during patient care transitions. Pharmacist completion of medication reconciliation compared with usual care has shown a 28% relative risk (RR) reduction in ED visits and a 67% RR reduction in adverse drug event-related hospital revisits.27 Findings of the COPD CARE service are consistent with the literature and advance the role of pharmacists within the medical home model as prescribers for disease management.27
The interprofessional, team-based design of the COPD CARE service also is supported by recent recommendations from the COPD Foundation, as detailed in the 2nd National COPD Readmission Summit.28 Use of a proactive, team-based care model is emphasized as a central element to coordinating care transitions, with an expectation of 360 degree accountability by all team members for the patients care both during and after hospitalization. The clearly defined roles of each team member within the COPD CARE service, coupled with the expectation that each team member practices with autonomy and accountability, exemplifies the COPD Foundation vision for enhancing COPD care. In addition, the COPD CARE service uses many of the best practices detailed by the COPD Foundation, including the use of spirometry, referrals to pulmonary rehabilitation, and use of motivational interviewing for tobacco treatment clinic referral.
Limitations
This QI initiative has several limitations. By virtue of the study being designed as a practice improvement intervention with rapid implementation, the existing clinic referral structures were used to offer the service to eligible patients. This standard of care included routine telephone contact by a nurse case manager following hospital discharge. Although all patients in the COPD CARE service received the intervention, 5 patients were not seen within the 30-day window, resulting in an implementation rate of 73%. Of the 5 patients that were not seen, 4 were discharged from the ED. Timely follow-up in primary care clinic from the ED required the use of a time-intensive chart review for referral and subsequent delay in intervention delivery.A streamlined clinic referral process from the ED likely would further improve patient scheduling and result in a greater number of patients who would receive the intervention within 30 days of discharge. Despite this limitation, the COPD CARE service was able to see a large percentage of patients within the 30-day time frame postdischarge.
Future Directions
Although a major objective of this service was to reduce readmissions 30 days postdischarge, it is possible interventions made in clinic may have long-term beneficial effects.25,29 Future research should evaluate the impact of this interprofessional service on long-term disease outcomes, thereby determining whether the promising readmission results are sustained beyond 30 days postdischarge.30 In addition, incorporation of respiratory therapy and inpatient pharmacists during hospital discharge could provide a more effective and sustainable transition from hospital to home before the COPD CARE clinic visit.
Future implementations and evaluations of this COPD CARE service will in turn benefit from a key component of our intervention, which includes the collection of timely CAT scores, spirometry data, and adherence rates for COPD patients.31 Furthermore, the intervention was successfully delivered to a population recently hospitalized or seen in the ED, and therefore, at high risk for future COPD exacerbations. This initiative provides positive proof of a concept QI project using the existing PACT team model to reduce 30-day readmission rates in patients with COPD at high risk for exacerbation. Future efforts will focus on delivering this intervention to patients with mild, moderate, and severe COPD within a wide range of primary clinics.
Conslusion
The COPD CARE service involved the coordinated postdischarge care facilitated by an interprofessional team of clinical pharmacists, nurses and PCPs. The COPD CARE service leveraged an interprofessional team, centered on the PACT medical home, to make clinic interventions resulting in a 0% readmission rate and 63.2% increase in PCP access. The COPD CARE service further demonstrated the impact of coordinated efforts by interprofessional teams to optimize care for COPD management.
Acknowledgments
The authors thank Stephanie Gruber, PharmD; Lieneke Hafeman, RN; Molly Obermark, PharmD; Julia Peek, RT; Mark Regan, MD; Chris Roelke, RN; Steve Shoyer, PharmD; John Thielemann, RN; Sandy Tompkins, BS; and Wendi Wenger, RN, for their integral roles in the COPD CARE service.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide and has an associated treatment cost of $9,800 per patient per year in the US.1-3 Within 5 years of hospital discharge for a COPD exacerbation, the rehospitalization risk is 44%, and the mortality rate is 55%.4 COPD affects more than 11 million Americans, and the disease prevalence among US veterans is 3-fold higher.5,6
Patients hospitalized for COPD have a 30-day readmission rate of 22.6%.7 Given the high patient burden, COPD was added to the Medicare Hospital Readmission Reductions Program in 2015, resulting in financial penalties for COPD readmissions within 30 days of hospital discharge.8 Ensuring timely access to follow-up care has been shown to significantly reduce risk for hospital readmissions.9 However, in a national review of Medicare claims, only 50% of patients readmitted to the hospital had a primary care provider (PCP) follow-up visit within 30 days of their hospital discharge.10 Despite the need to provide prompt patient follow-up during the transition from hospital to home, gaps within the health care system create barriers to providing timely postdischarge care.10-12 These gaps include breakdowns in practitioner and patient communication, lengthy time to follow-up, and incomplete medication reconciliation.13 To address this unmet need, clinics and hospitals require solutions that can be implemented quickly, using the resources of their current clinical models.
Pharmacists and registered nurses (RNs) within the US federal health care system are well positioned for involvement in the postdischarge care of high-risk patients with COPD. Ambulatory care practitioners within the US Department of Veterans Affairs (VA) health care system are integrated into patient aligned care teams (PACT). Each team consists of a PCP, pharmacist, RN, social worker, dietitian, licensed practical nurse, and medical scheduling support assistant.14 Each PACT team works together to provide patient education, chronic disease management, and medication optimization, and each team member contributes their unique training and expertise.
Interprofessional care is considered an integral method to improve health outcomes through effective teamwork and communication.15 Although interprofessional interventions are cited extensively in the literature highlighting medicine and nursing, a gap exists in the exploration of pharmacist contributions within interprofessional teams.16 The incorporation of clinical pharmacists in the literature is especially limited when considering transitions of care and the patient medical home.17 Given the critical and collaborative role pharmacists play within the PACT medical home, the COPD CARE (Chronic Obstructive Pulmonary Disease Coordinated Access to Reduce Exacerbations) service provides an opportunity to leverage pharmacists as prescribers with a scope of practice who coordinate transitions of care for patients with COPD.18 The service was designed to be collaborative within the PACT model and with the intent of reducing 30-day readmissions to the hospital or emergency department (ED) due to a COPD exacerbation.
This evaluation involved the identifying patients recently hospitalized for COPD; clinic follow-up, coordinated by a clinical pharmacist and nurse, within 30 days of hospital or ED discharge; the use of a COPD action plan; and timely triage of patients at high risk for COPD reexacerbation or with comorbid symptoms to PCPs. The COPD CARE service, leveraged the patient-centered medical home (PCMH) model for transitions of care after COPD exacerbations. The PCMH is a primary care model focused on the following functions: (1) comprehensive care; (2) patient-centered care; (3) coordinated care; (4) accessible service; and (5) quality and safety.19
Methods
The COPD CARE service was implemented on October 1, 2015, and evaluated through March 1, 2016 (Figure 1). All veterans receiving primary care through the pilot clinic site with a hospital admission or ED visit for COPD exacerbation were offered this intervention.
Patient Eligibility and Recruitment
Patients were excluded from the service if COPD or COPD-related diagnoses were not listed in their electronic health record (EHR) problem list. Patients who had previously received components of the intervention through consultation with specialty services were excluded. If a patient declined the service, they received the standard of care. This project was undertaken for programmatic evaluation and qualified for quality improvement (QI) exemption; as such an internal review board approval was not required.
Intervention
Participants enrolled in the COPD CARE service were scheduled for an interprofessional postdischarge follow-up visit with a pharmacist and nurse at the pilot outpatient clinic site, and this visit was termed the COPD CARE health visit. Participants ideally were seen within 30 days of discharge. The goal was to improve access to care while preventing a 30-day readmission. Within this 30-day window, the target follow-up period was 2 to 3 weeks postdischarge for the face-to-face visit. Patients who required postdischarge care for additional medical conditions received a clinic appointment with their PCP on the same day as their COPD CARE health visit. The COPD CARE health visit focused on 3 objectives: (1) COPD disease management and referrals; (2) COPD plan development; and (3) inhaler technique review and teaching.20,21
COPD Monitoring
During the 45-minute COPD CARE health visit, the pharmacist provided extensive disease management based on the GOLD guideline recommendation.22 In addition, the pharmacist administered the COPD Assessment Test (CAT) and reviewed patient COPD exacerbation history to guide prescribing.22 The patient and pharmacist also reviewed previous spirometry results if obtained within the past 2 years. COPD triggers and symptoms were assessed along with opportunities for therapeutic and lifestyle modifications.
Plan Development
Patients in the COPD CARE service also were given a COPD plan to improve health outcomes. (Figure 2). The plan included patient instructions to initiate steroid and antibiotic therapy if the patient experienced symptoms of increased cough, mucus production, and purulence, thereby reaching the high-yellow zone.
Patient Referrals
Patient referrals also were a critical component of the COPD CARE service. Pharmacists placed referrals for tobacco treatment services, pulmonary rehabilitation, a COPD group education class, and referral to specialty care if needed.
Inhaler Technique Review
Either the pharmacist or RN review the inhaler technique, and corrections and teachback methods used to ensure patient understanding.23 Patients were encouraged to bring home inhalers into clinic for technique assessment. Demonstration inhalers also were available and used by pharmacists and nurses for inhaler teaching as needed. The pharmacist indicated through chart documentation whether the patient’s inhaler technique was correct or whether modifications were made to improve medication delivery. Medication reconciliation also was performed for inhaled devices to insure patients were using medications as prescribed.
Outcomes
The primary outcome of this evaluation was an assessment of interventions made by the interprofessional care team during the COPD CARE health visit. Secondary outcomes included assessment of 30-day readmission rates as well as patient access to the primary care team using this interprofessional care model.
Data were collected after study completion through review of the EHR at baseline and at the end of the evaluation period. Baseline demographic information was collected through a retrospective chart review. Readmission rates were calculated as a composite of ED visits and rehospitalization within 30 days of discharge due to a COPD exacerbation.
Patients’ spirometry results were used in composite with clinical symptoms and risk of exacerbations to calculate GOLD staging.24
Results
A total of 19 patients admitted to the hospital or ED received follow-up through the COPD CARE service. Patients included in this analysis were primarily older adult white males.
Referrals were placed for 53% of patients in the COPD CARE service, with 21% of patients accepting referral to tobacco treatment clinic, and 32% of patients accepting referral to pulmonary rehabilitation. COPD plans were issued to all of patients in this service. Pharmacists modified therapy 58% of the time, with a review of medications prescribed by the clinical pharmacist (eApendixes 1 and 2, available at mdedge.com/fedprac).
Patients had a 0% composite readmission rate to the ED or hospital for a COPD exacerbation within 30-days of discharge. Access to care, defined as a visit with the primary care PACT team within 30 days of discharge, was achieved in 14 of the 19 patients (73.7%). Additionally, 12 of 19 patients (63.2%) in the COPD CARE service no longer needed to see their PCP following discharge, saving their provider a visit.
The pharmacist corrected patient inhaler technique in 52.6% of the patients participating in the service.
Discussion
The intent of this QI initiative was to assess a novel clinic intervention for a high-risk patient population during COPD care transitions. The strengths of this intervention involved a rapid cycle implementation using the existing medical home model and its multiprong approach to coordinating care. This approach involved coordinating self-direction COPD plans, timely hospital follow-up, and the innovative use of the interprofessional primary care team.
The COPD CARE service improved patient access to follow-up with no COPD readmissions in the intervention group. The COPD CARE service also validated the use of a coordinated medical home consisting of clinical pharmacists and nurses who provided the initial COPD disease monitoring and plan development. This intervention also resulted in patients receiving greater access to their PACT teams within 30 days of discharge and a higher rate of referrals to tobacco cessation clinics within the COPD CARE group. In addition, use of tools that enabled patients to self-manage their care, such as the COPD plan, was greater in the COPD CARE group.
The interventions made in-clinic likely contributed to service results (eAppendix 3).
In addition, the COPD CARE service provided necessary referrals to pulmonary rehabilitation, nutrition, and tobacco treatment clinics at a higher rate than those patients in the standard of care group. The high percentage of referrals placed to tobacco treatment clinic and pulmonary rehabilitation contributes to improvements in COPD disease control long-term.25
The COPD CARE service may best be described as a model for application of the interprofessional team in clinical practice, with the clinical pharmacist uniquely positioned for chronic disease management in the postacute care setting.26 Previously, literature has documented pharmacists as integral members of the team during patient care transitions. Pharmacist completion of medication reconciliation compared with usual care has shown a 28% relative risk (RR) reduction in ED visits and a 67% RR reduction in adverse drug event-related hospital revisits.27 Findings of the COPD CARE service are consistent with the literature and advance the role of pharmacists within the medical home model as prescribers for disease management.27
The interprofessional, team-based design of the COPD CARE service also is supported by recent recommendations from the COPD Foundation, as detailed in the 2nd National COPD Readmission Summit.28 Use of a proactive, team-based care model is emphasized as a central element to coordinating care transitions, with an expectation of 360 degree accountability by all team members for the patients care both during and after hospitalization. The clearly defined roles of each team member within the COPD CARE service, coupled with the expectation that each team member practices with autonomy and accountability, exemplifies the COPD Foundation vision for enhancing COPD care. In addition, the COPD CARE service uses many of the best practices detailed by the COPD Foundation, including the use of spirometry, referrals to pulmonary rehabilitation, and use of motivational interviewing for tobacco treatment clinic referral.
Limitations
This QI initiative has several limitations. By virtue of the study being designed as a practice improvement intervention with rapid implementation, the existing clinic referral structures were used to offer the service to eligible patients. This standard of care included routine telephone contact by a nurse case manager following hospital discharge. Although all patients in the COPD CARE service received the intervention, 5 patients were not seen within the 30-day window, resulting in an implementation rate of 73%. Of the 5 patients that were not seen, 4 were discharged from the ED. Timely follow-up in primary care clinic from the ED required the use of a time-intensive chart review for referral and subsequent delay in intervention delivery.A streamlined clinic referral process from the ED likely would further improve patient scheduling and result in a greater number of patients who would receive the intervention within 30 days of discharge. Despite this limitation, the COPD CARE service was able to see a large percentage of patients within the 30-day time frame postdischarge.
Future Directions
Although a major objective of this service was to reduce readmissions 30 days postdischarge, it is possible interventions made in clinic may have long-term beneficial effects.25,29 Future research should evaluate the impact of this interprofessional service on long-term disease outcomes, thereby determining whether the promising readmission results are sustained beyond 30 days postdischarge.30 In addition, incorporation of respiratory therapy and inpatient pharmacists during hospital discharge could provide a more effective and sustainable transition from hospital to home before the COPD CARE clinic visit.
Future implementations and evaluations of this COPD CARE service will in turn benefit from a key component of our intervention, which includes the collection of timely CAT scores, spirometry data, and adherence rates for COPD patients.31 Furthermore, the intervention was successfully delivered to a population recently hospitalized or seen in the ED, and therefore, at high risk for future COPD exacerbations. This initiative provides positive proof of a concept QI project using the existing PACT team model to reduce 30-day readmission rates in patients with COPD at high risk for exacerbation. Future efforts will focus on delivering this intervention to patients with mild, moderate, and severe COPD within a wide range of primary clinics.
Conslusion
The COPD CARE service involved the coordinated postdischarge care facilitated by an interprofessional team of clinical pharmacists, nurses and PCPs. The COPD CARE service leveraged an interprofessional team, centered on the PACT medical home, to make clinic interventions resulting in a 0% readmission rate and 63.2% increase in PCP access. The COPD CARE service further demonstrated the impact of coordinated efforts by interprofessional teams to optimize care for COPD management.
Acknowledgments
The authors thank Stephanie Gruber, PharmD; Lieneke Hafeman, RN; Molly Obermark, PharmD; Julia Peek, RT; Mark Regan, MD; Chris Roelke, RN; Steve Shoyer, PharmD; John Thielemann, RN; Sandy Tompkins, BS; and Wendi Wenger, RN, for their integral roles in the COPD CARE service.
1. World Health Organization. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en. Updated May 24, 2018. Accessed May 30, 2018.
2. Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged > 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31-45.
3. American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. http://www.lung.org/assets/documents/research/copd-trend-report.pdf. Published March 2013. Accessed May 30, 2018.
4. McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748-1755.
5. COPD Foundation. Patient groups back bill supporting US veterans with COPD. https://www.copdfoundation.org/About-Us/Press-Room/Press-Releases/Article/722/Patient-Groups-Back-Bill-Supporting-US-Veterans-with-COPD.aspx. Published November 9, 2010. Accessed May 30, 2018.
6. American Lung Association. Lung health and disease: how serious is COPD. http://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/learn-about-copd/how-serious-is-copd.html. Published 2016. Accessed May 30, 2018.
7. Shah T, Press V, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926.
8. Mcllvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;13(20):1796-1803.
9. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-122.
10. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
11. Hitch B, Parlier AB, Reed L, Galvin SL, Fagan EB, Wilson CG. Evaluation of a team-based, transition-of-care management service on 30-day readmission rates. N C Med J. 2016;77(2):87-92.
12. Stone J, Hoffman G. Medicare hospital readmissions: issues, policy options. In: Turner PM ed. Medicare: Background, Benefits and Issues. Nova Science Pub Inc; 2011:123-150.
13. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323.
14. Rosland A-M, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
15. World Health Organization. Nursing and midwifery. http://www.who.int/hrh/nursing_midwifery/en. Accessed September 18, 2018.
16. Supper I, Catala O, Lustman M, Chemla C, Bourgueil Y, Letrilliart L. Interprofessional collaboration in primary health care: a review of facilitators and barriers perceived by involved actors. J Public Health (Oxf). 2015;37(4):716-727.
17. Melody KT, McCartney E, Sen S, Duenas G. Optimizing care transitions: the role of the community pharmacist. Integr Pharm Res Pract. 2016;5:43-51.
18. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
19. US Department of Health and Human Services. Agency for Healthcare Research and Quality. Defining the PCMH. https://pcmh.ahrq.gov/page/defining-pcmh. Accessed May 29, 2018.
20. Kaplan A. The COPD action plan. Can Fam Physician. 2009;55(1):58-59.
21. Turnock AC, Walters EH, Walters JA, Wood-Baker R. Action plans for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(4):CD005074.
22. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2017 report). http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed May 29, 2018.
23. Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1:9.
24. Buist AS, Anzueto A, Calverley P, DeGuia TS, Fukuch Y. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2006.
25. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
26. ASHP Research and Education Foundation. Pharmacy forecast 2016-2020: strategic planning advice. http://www.ashpfoundation.org/PharmacyForecast2016. Published December 2015. Accessed May 29, 2018.
27. Mekonnen AB, McLachlan AJ, Brien JE. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003.
28. Willard KS, Sullivan JB, Thomashow BM, et al. The 2nd national COPD readmissions summit and beyond: from theory to implementation. Chronic Obstr Pulm Dis. 2016;3(4):778-790.
29. Scanlon PD, Connett JE, Waller LA, et al; Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The lung health study. Am J Respir Crit Care Med. 2000;161(2, pt 1):381-390.
30. Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926.
31. GlaxoSmithKline. COPD Assessment Test (CAT). Castest Online. http://www.catestonline.org/images/UserGuides/CATHCPUser%20guideEn.pdf. Updated October 2016.
1. World Health Organization. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en. Updated May 24, 2018. Accessed May 30, 2018.
2. Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged > 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31-45.
3. American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. http://www.lung.org/assets/documents/research/copd-trend-report.pdf. Published March 2013. Accessed May 30, 2018.
4. McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748-1755.
5. COPD Foundation. Patient groups back bill supporting US veterans with COPD. https://www.copdfoundation.org/About-Us/Press-Room/Press-Releases/Article/722/Patient-Groups-Back-Bill-Supporting-US-Veterans-with-COPD.aspx. Published November 9, 2010. Accessed May 30, 2018.
6. American Lung Association. Lung health and disease: how serious is COPD. http://www.lung.org/lung-health-and-diseases/lung-disease-lookup/copd/learn-about-copd/how-serious-is-copd.html. Published 2016. Accessed May 30, 2018.
7. Shah T, Press V, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926.
8. Mcllvennan CK, Eapen ZJ, Allen LA. Hospital readmissions reduction program. Circulation. 2015;13(20):1796-1803.
9. Jackson C, Shahsahebi M, Wedlake T, DuBard CA. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med. 2015;13(2):115-122.
10. Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
11. Hitch B, Parlier AB, Reed L, Galvin SL, Fagan EB, Wilson CG. Evaluation of a team-based, transition-of-care management service on 30-day readmission rates. N C Med J. 2016;77(2):87-92.
12. Stone J, Hoffman G. Medicare hospital readmissions: issues, policy options. In: Turner PM ed. Medicare: Background, Benefits and Issues. Nova Science Pub Inc; 2011:123-150.
13. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323.
14. Rosland A-M, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272.
15. World Health Organization. Nursing and midwifery. http://www.who.int/hrh/nursing_midwifery/en. Accessed September 18, 2018.
16. Supper I, Catala O, Lustman M, Chemla C, Bourgueil Y, Letrilliart L. Interprofessional collaboration in primary health care: a review of facilitators and barriers perceived by involved actors. J Public Health (Oxf). 2015;37(4):716-727.
17. Melody KT, McCartney E, Sen S, Duenas G. Optimizing care transitions: the role of the community pharmacist. Integr Pharm Res Pract. 2016;5:43-51.
18. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
19. US Department of Health and Human Services. Agency for Healthcare Research and Quality. Defining the PCMH. https://pcmh.ahrq.gov/page/defining-pcmh. Accessed May 29, 2018.
20. Kaplan A. The COPD action plan. Can Fam Physician. 2009;55(1):58-59.
21. Turnock AC, Walters EH, Walters JA, Wood-Baker R. Action plans for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(4):CD005074.
22. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2017 report). http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed May 29, 2018.
23. Bonini M, Usmani OS. The importance of inhaler devices in the treatment of COPD. COPD Res Pract. 2015;1:9.
24. Buist AS, Anzueto A, Calverley P, DeGuia TS, Fukuch Y. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2006.
25. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(2):CD003793.
26. ASHP Research and Education Foundation. Pharmacy forecast 2016-2020: strategic planning advice. http://www.ashpfoundation.org/PharmacyForecast2016. Published December 2015. Accessed May 29, 2018.
27. Mekonnen AB, McLachlan AJ, Brien JE. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003.
28. Willard KS, Sullivan JB, Thomashow BM, et al. The 2nd national COPD readmissions summit and beyond: from theory to implementation. Chronic Obstr Pulm Dis. 2016;3(4):778-790.
29. Scanlon PD, Connett JE, Waller LA, et al; Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The lung health study. Am J Respir Crit Care Med. 2000;161(2, pt 1):381-390.
30. Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916-926.
31. GlaxoSmithKline. COPD Assessment Test (CAT). Castest Online. http://www.catestonline.org/images/UserGuides/CATHCPUser%20guideEn.pdf. Updated October 2016.
Improving Team-Based Care Coordination Delivery and Documentation in the Health Record
Chronic diseases affect a substantial proportion of the US population, with 25% of adults diagnosed with 2 or more chronic health conditions.1 In 2010, 2 chronic diseases, heart disease and cancer, accounted for nearly 48% of deaths.2 Due to the significant public heath burden, strategies to improve chronic disease management have attracted a great deal of focus.3,4 Within increasingly complex health care delivery systems, policy makers are promoting care coordination (CC) as a tool to reduce fragmented care for patients with multiple comorbidities, improve patient experience and quality of care, and decrease costs and risks for error.3-8
Background
The Agency for Healthcare Research and Quality (AHRQ) defines care coordination as “deliberately organizing patient care activities and sharing information among all of the participants concerned with a patient’s care to achieve safer and more effective care.”5 Nationally, large scale investments have expanded health care models that provide team-based CC, such as patient-centered medical homes, known as patient-aligned care teams (PACTs) within the Department of Veterans Affairs (VA), accountable care organizations, and other complex care management programs.9-12 Additionally, incentives that reimburse for CC, such as Medicare’s chronic care management and transition care management billing codes, also are emerging.13,14
While there is significant interest and investment in promoting CC, little data about the specific activities and time required to provide necessary CC exist, which limits the ability of health care teams to optimize CC delivery.6 Understanding the components of CC has implications for human resource allocation, labor mapping, reimbursement, staff training, and optimizing collaborative networks for health care systems, which may improve the quality of CC and health outcomes for patients. To date, few tools exist that can be used to identify and track the CC services delivered by interdisciplinary teams within and outside of the health care setting.
This article describes the development and preliminary results of the implementation of a CC Template that was created in the VA Computerized Patient Record System (CPRS) to identify and track the components of CC services, delivered by a multidisciplinary team, as part of a quality improvement (QI) pilot project. Through use of the template, the team sought a formative understanding of the following questions: (1) Is it feasible to use the CC Template during routine workflow? (2) What specific types of CC services are provided by the team? (3) How much time does it take to perform these activities? (4) Who is the team collaborating with inside and outside of the health care setting and how are they communicating? (5) Given new reimbursement incentives, can the provision of CC be standardized and documented for broad applicability?
In complex systems, where coordination is needed among primary, specialty, hospital, emergency, and nonclinical care settings, a tool such as the CC Template offers a sustainable and replicable way to standardize documentation and knowledge about CC components. This foundational information can be used to optimize team structure, training, and resource allocation, to improve the quality of CC and to link elements of CC with clinical and operational outcomes.
Pact Intensive Management
Despite the implementation of PACT within VA, patients with complex medical conditions combined with socioeconomic stressors, mental health comorbidities, and low health literacy are at high risk for preventable hospitalizations and acute care utilization.17,18 Due to unmet needs that are beyond what PACTs are able to deliver, these high-risk patients may benefit from additional services to coordinate care within and outside the VA health care system, as suggest by the Extended Chronic Care Model.19-21
In 2014, the Office of Primary Care Services sponsored a QI initiative at 5 VA demonstration sites to develop PACT Intensive Management (PIM) interventions targeting patients at high risk for hospitalization and acute care utilization within VA. The PIM program design is based on work described previously, with patients identified for enrollment based on 90-day hospitalization risk ≥ 90th percentile, based on a VA risk modeling tool, and an acute care episode in the previous 6 months. 19 A common component of all PIM programs is the provision of intensive care management and CC by an interdisciplinary team working in conjunction with PACT. The CC Template was developed to assist in documenting and rigorously understanding the implementation of CC by the PIM team.
Local Setting
The Atlanta VA Medical Center (AVAMC) was chosen as one of the PIM demonstration sites. The Atlanta PIM team identified and enrolled a random sample of eligible, high-risk patients from 1 community-based outpatient clinic (CBOC) in an urban location with 7,524 unique patients. Between September 2014 and September 2016, 300 patients were identified, and 86 patients agreed to participate in the PIM program.
In the CC Template pilot, the Atlanta PIM team included 2 nurse practitioners (NP), 2 social workers (SW), and 1 telehealth registered nurse (RN). Upon enrollment, members of the PIM team conducted comprehensive home assessments and offered intensive care management for medical, social, and behavioral needs. The main pillars of care management offered to high-risk patients were based on previous work done both inside and outside VA and included home visits, telephone-based disease management, co-attending appointments with patients, transition care management, and interdisciplinary team meetings with a focus on care coordination between PACT and all services required by patients.11,19
The Atlanta PIM team performed a variety of tasks to coordinate care for enrolled patients that included simple, 1-step tasks, such as chart reviews, and multistep, complex tasks that required the expertise of multiple team members (Figure 1).
Additionally, inconsistency in delivery of CC between PIM team members was noted. For example, there was significant variability in CC services provided by different team members in the provision of transition care management (TCM) and coordinating care from hospitalization back to home. Some PIM staff coordinated care and communicated with the patient, hospital team, home-care service, and primary-care team, while other staff only reviewed the chart and placed orders in CPRS. Additionally, much of the CC work was documented in administrative notes that did not trigger workload credit. This made it difficult to show how to appropriately labor map PIM staff or how staff were spending their time caring for patients.
In order to standardize documentation of the interdisciplinary CC activities performed by the PIM team and account for staff time, the Atlanta PIM team decided to develop a CPRS CC Template. The objective of the CC Template was to facilitate documentation of CC activities in the EHR, describe the types of CC activities performed by PIM team members, and track the time to perform these activities for patients with various chronic diseases.
Template Design and Implementation
The original design of the template was informed by the Atlanta PIM team after several informal focus groups and process mapping of CC pathways in the fall of 2015. The participants were all members of the Atlanta PIM team, 2 primary care physicians working with PIM, an AVAMC documentation specialist and a clinical applications coordinator (CAC) assigned to work with PIM. The major themes that arose during the brainstorming discussion were that the template should: (1) be feasible to use during their daily clinic workflow; (2) improve documentation of CC; and (3) have value for spread to other VA sites. Discussion centered on creating a CC Template versatile enough to:
- Decrease the number of steps for documenting CC;
- Consist only of check boxes, with very little need for free text, with the option to enter narrative free text after template completion;
- Document time spent in aggregate for completing complex CC encounters;
- Document various types of CC work and modes of communication;
- Allow for use by all PIM staff;
- Identify all team members that participated in the CC encounter to reduce redundant documentation by multiple staff;
- Adapt to different clinic sites based on the varied disciplines participating in other locations;
- Use evidence-based checklists to help standardize delivery of CC for certain activities such as TCM; and
- Extract data without extensive chart reviews to inform current CC and future QI work.
Following the brainstorming sessions, the authors performed a literature review to identify and integrate CC best practices. The AHRQ Care Coordination Atlas served as the main resource in the design of the logic model that depicted the delivery and subsequent documentation of high-quality, evidence-based CC in the CC Template (Figure 2).6
After reaching consensus about the key components of the CC Template, the CAC created a pilot version (Figure 3). All of the elements within the CC Template allowed for data abstraction from the VA Corporate Data Warehouse (CDW) via discrete data elements known as health factors.
Over the course of implementation, the team became more enthusiastic about using the CC template to document previously unrecognized CC workload. Because the CC Template only was used to document CC workload and excluded encounters for clinical evaluation and management, specific notes were created and linked with the CC Template for optimal capture of encounters.
All components of the template were mandatory to eliminate the possibility of missing data. The Atlanta PIM site principal investigator developed a multicomponent training designed to increase support for the template by describing its value and to mitigate the potential for variability in how data are captured. Training included a face-to-face session with the team to review the template and work through sample CC cases. Additionally, a training manual with clear operational definitions and examples of how to complete each element of the CC Template was disseminated. The training was subsequently conducted with the San Francisco VA Medical Center PIM team, a spread site, via video conference. The spread site offered significant feedback on clarifying the training documents and adapting the CC template for their distinct care team structure. This feedback was incorporated into the final CC Template design to increase adaptability.
Implementation Evaluation
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)framework served as the basis for evaluation of CC Template implementation. The RE-AIM framework is well established and able to evaluate the implementation and potential successful spread of new programs.23,24 Using RE-AIM, the authors planned to analyze data to explore the reach effectiveness, adoption, implementation, and maintenance of the CC Template use while providing complex care management for high-risk patients.
All data for the evaluation was extracted from the CDW by a data analyst and stored on a secure server. A statistical process control (SPC) chart was used to analyze the implementation process to assess variation in template use.
Results
After implementation, 35 weeks of CC Template pilot data were analyzed from June 1, 2015 to January 5, 2016. The PIM team completed 393 CC Templates over this collection period. After week 23, the CC template was linked to specific CC notes automatically. From weeks 23 to 35 an average of 20.3 CC Templates were completed per week by the team. The RE-AIM was used to assess the implementation of the CC Template.
Reach was determined by the number of patients enrolled in PIM with CC Template documentation. Of patients enrolled in Atlanta PIM, 90.1% had ≥ 1 CC encounter documented by the CC Template; 74.4% of Atlanta PIM patients had ≥ 1 CC encounter documented; 15.5% of patients had > 10 CC encounters documented; and 1 patient had > 25 CC encounters documented by the CC template.
Effectiveness for describing CC activities was captured through data from CC Template. The CC Template documentation by the PIM team showed that 79.4% of CC encounters were < 20 minutes, and 9.9% of encounters were > 61 minutes. Telephone communication was involved in 50.4% of CC encounters, and 24% required multiple modes of communication such as face-to-face, instant messenger, chart-based communication. Care coordination during hospitalization and discharge accounted for 5.9% of template use. Of the CC encounters documenting hospital transitions, 94.4% documented communication with the inpatient team, 58.3% documented coordination with social support, and only 11.1% documented communication with primary care teams. Improving communication with PACT teams after hospital discharge was identified as a future QI project based on these data. The PIM team initiated 83.2% of CC encounters.
Adoption was determined by the use of the CC Template by the team. All 5 team members used the CC template to document at least 1 CC encounter.
Implementation allowed for improvement based on feedback from the PIM team. Mean completion of CC Templates rose from 10.9 per week to 20.3 per week after automatically linking the CC Template to specific CC notes. (Figure 4)
Maintenance was monitored over the course of the pilot. Consistent use of the CC Template over 35 full work weeks of data collection was seen, and mean utilization per week nearly doubled in the latter half of the pilot period.
Because several elements were added to the CC Template over the course of the pilot period, our ability to analyze the data for descriptive statistics about the types of CC services, related diagnoses, collaborators, and PIM staff involved in CC encounters was limited.
Discussion
Though all components of CC encounters could not be assessed during the pilot phase due to continuous improvement of the CC Template, the authors showed that it is feasible to use this tool to document and describe granular details about team-based CC. Pilot data from AVAMC show that the use of the CC Template standardized team CC documentation in a busy clinic setting provided data about the complexity of coordination activities and duration of CC activities. It also informed future CC QI projects, such as improving communication with primary care during the hospital discharge process.
Future evaluation of CC Template data can be used to (1) describe types of CC activities for high-risk PIM patients; (2) quantify the time required to complete CC activities to assist with staff labor mapping; (3) describe staff roles and referrals needed to complete specific CC activities inside and outside VA; (4) describe modes of communication between PIM and collaborators; (5) relate patient demographics and associated diagnoses with quantity of CC encounters; and (6) quantify frequency and time frame of CC after hospitalizations and ED care and subsequent impact on repeat hospitalizations and ED visits. Future research also can explore the link between CC activities and effort with clinical and patient-reported outcomes.
Social network analysis could be used with CC Template data to understand the network of referrals and collaborators involved in the care of a CC team’s patients. This type of analysis would assist teams to strengthen and formalize ties with collaborators as appropriate. For example, if data show that the team frequently collaborates with the cardiology clinic for a large subset of its patients, they may consider creating a CC agreement with formalized modes of communication that would streamline collaboration.
In order to improve the quality of the CC Template and to assess factors that may lead to sustainable use in clinical practice, qualitative assessment through survey, interview, or usability testing with staff would be beneficial to identify strategies to increase its adoption among clinical providers. This type of assessment will add knowledge about the CC Template implementation process, including contextual barriers or facilitators, feasibility of use during day-to-day operations, versatility of template use within construct of team-based care, and overall satisfaction with the template.
Limitations
Though the CC Template offers a large amount of data about the components of CC delivery, the information is based on self-report by staff. Training to ensure that all team members are documenting in the same manner is crucial to maintain the internal validity of the data. The template is limited to the fields currently developed, and future research could explore additional data elements that are critical to include based on feedback from VA staff.
Conclusion
To our knowledge, this VA medical center CC Template is the first tool described in the literature that standardizes and captures data about CC components in the EHR. This pilot data show that the template is feasible for use in a busy clinic setting and can streamline the process for capturing CC data that may otherwise not be documented.
During the pilot phase, the CC template allowed the PIM team to identify a small subset of patients within the PIM complex management who have a high level of CC needs. By identifying these patients, further work can be done to understand the specific needs of these higher utilizers and the types of CC activities required to assist them so that resources can be directed appropriately to that smaller subset. Telephone CC accounted for a large proportion of delivery, which has implications for ensuring that staff have access to mobile phones and EHR capability to document this additional workload. The PIM staff maintained use of the template throughout the pilot period and increased documentation when the CC Template was easily accessible and already linked to their CPRS notes, suggesting that in future implementation, ensuring that the template is linked to notes in use by the care team will be important for successful spread.
Additionally, CC Template data identified gaps in high-quality, evidence-based CC that can be addressed in real time, for example during the discharge process. Data from the CC Template showed that only 11.1% of CC encounters had documentation of communication between the PIM and primary care teams during transitions from hospital to home. Improving communication with PACT teams after hospital discharge was identified as a future PIM QI project based on these data. By improving documentation of CC in the EHR, the resulting information is foundational for future work that can improve the quality of team-based CC; plan staffing, team composition, and labor mapping; determine the cost of CC activities and improve reimbursement in certain settings; and assess outcomes of CC.
This tool has potential for application beyond the PIM team in the VA. The CC Template and training manual is scalable to any setting with team-based CC, including PACT, homeless programs, palliative care, Mental Health Intensive Case Management (MHICM) programs, nurse navigator programs, and other complex care delivery models involving care coordinators. Future study of its implementation and data may inform initiatives to develop ongoing team-based care coordination programs.
Acknowledgments
The authors thank the following colleagues for their input and support: Florence Longchamp, RN, Clinical Applications Coordinator at the Atlanta VA Medical Center without whom the CC Template would not have been created; the Atlanta and San Francisco VA PIM teams for their thoughtful comments and enthusiastic embrace of the CC Template; and the PIM National Evaluation Center for their support of this QI project. PACT Intensive Management demonstration sites are funded by the VA Office of Patient Care Services. During the implementation of the CC Template pilot and the preparation of this paper, the primary author was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Advanced Fellowships, VA Quality Scholars Program.
1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
2. Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated May 3, 2017. Accessed August 8, 2018.
3. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
4. US Department of Health and Human Services. Healthy people 2010: general data issues. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_general_data_issues.pdf. Published 2010. Accessed August 1, 2018.
5. McDonald KM, Sunderam V, Bravata DM, et al. Closing the quality gap: a critical analysis of quality improvement strategies, Vol 7: care coordination. Agency for Healthcare Research and Quality. https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. Published June 2007. Accessed August 1, 2018.
6. McDonald KM, Schultz E, Albin L, et al. Care coordination measures atlas. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed August 2, 2018.
7. Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating care across diseases, settings, and clinicians: a key role for the generalist in practice. Ann Intern Med. 2005;142(8):700-708.
8. Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15(5):329-336.
9. National Committee for Quality Assurance. The future of patient-centered medical homes: foundation for a better health care system. https://www.ncqa.org/Portals/0/Public%20Policy/2014%20PDFS/The_Future_of_PCMH.pdf. Accessed August 2, 2018.
10. US Department of Veterans Affairs, Veterans Health Administration. Patient Aligned Care Team (PACT) Handbook. VHA Handbook 1101.10:1–65. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2977. Updated May 26, 2017. Accessed August 2, 2018.
11. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
12. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19.
13. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Chronic care management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Published December 2016. Accessed August 2, 2018.
14. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Frequently asked questions about billing the Medicare physician fee schedule for transitional care management services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-TCMS.pdf. Published March 17, 2016. Accessed August 2, 2018.
15. Antonelli RC, Stille CJ, Antonelli DM. Care coordination for children and youth with special health care needs: a descriptive, multisite study of activities, personnel costs, and outcomes. Pediatrics. 2008;122(1):e209-e216.
16. Antonelli RC, Antonelli DM. Providing a medical home: the cost of care coordination services in a community-based, general pediatric practice. Pediatrics. 2004;113( suppl 5 ):1522-1528.
17. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
18. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an intensive management patient-aligned care team (ImPACT). J Gen Intern Med. 2014;29(suppl 4):S861-S869.
20. Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166-175.
21. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73-82.
22. US Department of Health & Human Services, Agency for Healthcare Research and Quality. Re-engineered discharge (RED) toolkit. http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html. Updated May 2017. Accessed August 3, 2018.
23. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327.
24. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-e46.
Chronic diseases affect a substantial proportion of the US population, with 25% of adults diagnosed with 2 or more chronic health conditions.1 In 2010, 2 chronic diseases, heart disease and cancer, accounted for nearly 48% of deaths.2 Due to the significant public heath burden, strategies to improve chronic disease management have attracted a great deal of focus.3,4 Within increasingly complex health care delivery systems, policy makers are promoting care coordination (CC) as a tool to reduce fragmented care for patients with multiple comorbidities, improve patient experience and quality of care, and decrease costs and risks for error.3-8
Background
The Agency for Healthcare Research and Quality (AHRQ) defines care coordination as “deliberately organizing patient care activities and sharing information among all of the participants concerned with a patient’s care to achieve safer and more effective care.”5 Nationally, large scale investments have expanded health care models that provide team-based CC, such as patient-centered medical homes, known as patient-aligned care teams (PACTs) within the Department of Veterans Affairs (VA), accountable care organizations, and other complex care management programs.9-12 Additionally, incentives that reimburse for CC, such as Medicare’s chronic care management and transition care management billing codes, also are emerging.13,14
While there is significant interest and investment in promoting CC, little data about the specific activities and time required to provide necessary CC exist, which limits the ability of health care teams to optimize CC delivery.6 Understanding the components of CC has implications for human resource allocation, labor mapping, reimbursement, staff training, and optimizing collaborative networks for health care systems, which may improve the quality of CC and health outcomes for patients. To date, few tools exist that can be used to identify and track the CC services delivered by interdisciplinary teams within and outside of the health care setting.
This article describes the development and preliminary results of the implementation of a CC Template that was created in the VA Computerized Patient Record System (CPRS) to identify and track the components of CC services, delivered by a multidisciplinary team, as part of a quality improvement (QI) pilot project. Through use of the template, the team sought a formative understanding of the following questions: (1) Is it feasible to use the CC Template during routine workflow? (2) What specific types of CC services are provided by the team? (3) How much time does it take to perform these activities? (4) Who is the team collaborating with inside and outside of the health care setting and how are they communicating? (5) Given new reimbursement incentives, can the provision of CC be standardized and documented for broad applicability?
In complex systems, where coordination is needed among primary, specialty, hospital, emergency, and nonclinical care settings, a tool such as the CC Template offers a sustainable and replicable way to standardize documentation and knowledge about CC components. This foundational information can be used to optimize team structure, training, and resource allocation, to improve the quality of CC and to link elements of CC with clinical and operational outcomes.
Pact Intensive Management
Despite the implementation of PACT within VA, patients with complex medical conditions combined with socioeconomic stressors, mental health comorbidities, and low health literacy are at high risk for preventable hospitalizations and acute care utilization.17,18 Due to unmet needs that are beyond what PACTs are able to deliver, these high-risk patients may benefit from additional services to coordinate care within and outside the VA health care system, as suggest by the Extended Chronic Care Model.19-21
In 2014, the Office of Primary Care Services sponsored a QI initiative at 5 VA demonstration sites to develop PACT Intensive Management (PIM) interventions targeting patients at high risk for hospitalization and acute care utilization within VA. The PIM program design is based on work described previously, with patients identified for enrollment based on 90-day hospitalization risk ≥ 90th percentile, based on a VA risk modeling tool, and an acute care episode in the previous 6 months. 19 A common component of all PIM programs is the provision of intensive care management and CC by an interdisciplinary team working in conjunction with PACT. The CC Template was developed to assist in documenting and rigorously understanding the implementation of CC by the PIM team.
Local Setting
The Atlanta VA Medical Center (AVAMC) was chosen as one of the PIM demonstration sites. The Atlanta PIM team identified and enrolled a random sample of eligible, high-risk patients from 1 community-based outpatient clinic (CBOC) in an urban location with 7,524 unique patients. Between September 2014 and September 2016, 300 patients were identified, and 86 patients agreed to participate in the PIM program.
In the CC Template pilot, the Atlanta PIM team included 2 nurse practitioners (NP), 2 social workers (SW), and 1 telehealth registered nurse (RN). Upon enrollment, members of the PIM team conducted comprehensive home assessments and offered intensive care management for medical, social, and behavioral needs. The main pillars of care management offered to high-risk patients were based on previous work done both inside and outside VA and included home visits, telephone-based disease management, co-attending appointments with patients, transition care management, and interdisciplinary team meetings with a focus on care coordination between PACT and all services required by patients.11,19
The Atlanta PIM team performed a variety of tasks to coordinate care for enrolled patients that included simple, 1-step tasks, such as chart reviews, and multistep, complex tasks that required the expertise of multiple team members (Figure 1).
Additionally, inconsistency in delivery of CC between PIM team members was noted. For example, there was significant variability in CC services provided by different team members in the provision of transition care management (TCM) and coordinating care from hospitalization back to home. Some PIM staff coordinated care and communicated with the patient, hospital team, home-care service, and primary-care team, while other staff only reviewed the chart and placed orders in CPRS. Additionally, much of the CC work was documented in administrative notes that did not trigger workload credit. This made it difficult to show how to appropriately labor map PIM staff or how staff were spending their time caring for patients.
In order to standardize documentation of the interdisciplinary CC activities performed by the PIM team and account for staff time, the Atlanta PIM team decided to develop a CPRS CC Template. The objective of the CC Template was to facilitate documentation of CC activities in the EHR, describe the types of CC activities performed by PIM team members, and track the time to perform these activities for patients with various chronic diseases.
Template Design and Implementation
The original design of the template was informed by the Atlanta PIM team after several informal focus groups and process mapping of CC pathways in the fall of 2015. The participants were all members of the Atlanta PIM team, 2 primary care physicians working with PIM, an AVAMC documentation specialist and a clinical applications coordinator (CAC) assigned to work with PIM. The major themes that arose during the brainstorming discussion were that the template should: (1) be feasible to use during their daily clinic workflow; (2) improve documentation of CC; and (3) have value for spread to other VA sites. Discussion centered on creating a CC Template versatile enough to:
- Decrease the number of steps for documenting CC;
- Consist only of check boxes, with very little need for free text, with the option to enter narrative free text after template completion;
- Document time spent in aggregate for completing complex CC encounters;
- Document various types of CC work and modes of communication;
- Allow for use by all PIM staff;
- Identify all team members that participated in the CC encounter to reduce redundant documentation by multiple staff;
- Adapt to different clinic sites based on the varied disciplines participating in other locations;
- Use evidence-based checklists to help standardize delivery of CC for certain activities such as TCM; and
- Extract data without extensive chart reviews to inform current CC and future QI work.
Following the brainstorming sessions, the authors performed a literature review to identify and integrate CC best practices. The AHRQ Care Coordination Atlas served as the main resource in the design of the logic model that depicted the delivery and subsequent documentation of high-quality, evidence-based CC in the CC Template (Figure 2).6
After reaching consensus about the key components of the CC Template, the CAC created a pilot version (Figure 3). All of the elements within the CC Template allowed for data abstraction from the VA Corporate Data Warehouse (CDW) via discrete data elements known as health factors.
Over the course of implementation, the team became more enthusiastic about using the CC template to document previously unrecognized CC workload. Because the CC Template only was used to document CC workload and excluded encounters for clinical evaluation and management, specific notes were created and linked with the CC Template for optimal capture of encounters.
All components of the template were mandatory to eliminate the possibility of missing data. The Atlanta PIM site principal investigator developed a multicomponent training designed to increase support for the template by describing its value and to mitigate the potential for variability in how data are captured. Training included a face-to-face session with the team to review the template and work through sample CC cases. Additionally, a training manual with clear operational definitions and examples of how to complete each element of the CC Template was disseminated. The training was subsequently conducted with the San Francisco VA Medical Center PIM team, a spread site, via video conference. The spread site offered significant feedback on clarifying the training documents and adapting the CC template for their distinct care team structure. This feedback was incorporated into the final CC Template design to increase adaptability.
Implementation Evaluation
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)framework served as the basis for evaluation of CC Template implementation. The RE-AIM framework is well established and able to evaluate the implementation and potential successful spread of new programs.23,24 Using RE-AIM, the authors planned to analyze data to explore the reach effectiveness, adoption, implementation, and maintenance of the CC Template use while providing complex care management for high-risk patients.
All data for the evaluation was extracted from the CDW by a data analyst and stored on a secure server. A statistical process control (SPC) chart was used to analyze the implementation process to assess variation in template use.
Results
After implementation, 35 weeks of CC Template pilot data were analyzed from June 1, 2015 to January 5, 2016. The PIM team completed 393 CC Templates over this collection period. After week 23, the CC template was linked to specific CC notes automatically. From weeks 23 to 35 an average of 20.3 CC Templates were completed per week by the team. The RE-AIM was used to assess the implementation of the CC Template.
Reach was determined by the number of patients enrolled in PIM with CC Template documentation. Of patients enrolled in Atlanta PIM, 90.1% had ≥ 1 CC encounter documented by the CC Template; 74.4% of Atlanta PIM patients had ≥ 1 CC encounter documented; 15.5% of patients had > 10 CC encounters documented; and 1 patient had > 25 CC encounters documented by the CC template.
Effectiveness for describing CC activities was captured through data from CC Template. The CC Template documentation by the PIM team showed that 79.4% of CC encounters were < 20 minutes, and 9.9% of encounters were > 61 minutes. Telephone communication was involved in 50.4% of CC encounters, and 24% required multiple modes of communication such as face-to-face, instant messenger, chart-based communication. Care coordination during hospitalization and discharge accounted for 5.9% of template use. Of the CC encounters documenting hospital transitions, 94.4% documented communication with the inpatient team, 58.3% documented coordination with social support, and only 11.1% documented communication with primary care teams. Improving communication with PACT teams after hospital discharge was identified as a future QI project based on these data. The PIM team initiated 83.2% of CC encounters.
Adoption was determined by the use of the CC Template by the team. All 5 team members used the CC template to document at least 1 CC encounter.
Implementation allowed for improvement based on feedback from the PIM team. Mean completion of CC Templates rose from 10.9 per week to 20.3 per week after automatically linking the CC Template to specific CC notes. (Figure 4)
Maintenance was monitored over the course of the pilot. Consistent use of the CC Template over 35 full work weeks of data collection was seen, and mean utilization per week nearly doubled in the latter half of the pilot period.
Because several elements were added to the CC Template over the course of the pilot period, our ability to analyze the data for descriptive statistics about the types of CC services, related diagnoses, collaborators, and PIM staff involved in CC encounters was limited.
Discussion
Though all components of CC encounters could not be assessed during the pilot phase due to continuous improvement of the CC Template, the authors showed that it is feasible to use this tool to document and describe granular details about team-based CC. Pilot data from AVAMC show that the use of the CC Template standardized team CC documentation in a busy clinic setting provided data about the complexity of coordination activities and duration of CC activities. It also informed future CC QI projects, such as improving communication with primary care during the hospital discharge process.
Future evaluation of CC Template data can be used to (1) describe types of CC activities for high-risk PIM patients; (2) quantify the time required to complete CC activities to assist with staff labor mapping; (3) describe staff roles and referrals needed to complete specific CC activities inside and outside VA; (4) describe modes of communication between PIM and collaborators; (5) relate patient demographics and associated diagnoses with quantity of CC encounters; and (6) quantify frequency and time frame of CC after hospitalizations and ED care and subsequent impact on repeat hospitalizations and ED visits. Future research also can explore the link between CC activities and effort with clinical and patient-reported outcomes.
Social network analysis could be used with CC Template data to understand the network of referrals and collaborators involved in the care of a CC team’s patients. This type of analysis would assist teams to strengthen and formalize ties with collaborators as appropriate. For example, if data show that the team frequently collaborates with the cardiology clinic for a large subset of its patients, they may consider creating a CC agreement with formalized modes of communication that would streamline collaboration.
In order to improve the quality of the CC Template and to assess factors that may lead to sustainable use in clinical practice, qualitative assessment through survey, interview, or usability testing with staff would be beneficial to identify strategies to increase its adoption among clinical providers. This type of assessment will add knowledge about the CC Template implementation process, including contextual barriers or facilitators, feasibility of use during day-to-day operations, versatility of template use within construct of team-based care, and overall satisfaction with the template.
Limitations
Though the CC Template offers a large amount of data about the components of CC delivery, the information is based on self-report by staff. Training to ensure that all team members are documenting in the same manner is crucial to maintain the internal validity of the data. The template is limited to the fields currently developed, and future research could explore additional data elements that are critical to include based on feedback from VA staff.
Conclusion
To our knowledge, this VA medical center CC Template is the first tool described in the literature that standardizes and captures data about CC components in the EHR. This pilot data show that the template is feasible for use in a busy clinic setting and can streamline the process for capturing CC data that may otherwise not be documented.
During the pilot phase, the CC template allowed the PIM team to identify a small subset of patients within the PIM complex management who have a high level of CC needs. By identifying these patients, further work can be done to understand the specific needs of these higher utilizers and the types of CC activities required to assist them so that resources can be directed appropriately to that smaller subset. Telephone CC accounted for a large proportion of delivery, which has implications for ensuring that staff have access to mobile phones and EHR capability to document this additional workload. The PIM staff maintained use of the template throughout the pilot period and increased documentation when the CC Template was easily accessible and already linked to their CPRS notes, suggesting that in future implementation, ensuring that the template is linked to notes in use by the care team will be important for successful spread.
Additionally, CC Template data identified gaps in high-quality, evidence-based CC that can be addressed in real time, for example during the discharge process. Data from the CC Template showed that only 11.1% of CC encounters had documentation of communication between the PIM and primary care teams during transitions from hospital to home. Improving communication with PACT teams after hospital discharge was identified as a future PIM QI project based on these data. By improving documentation of CC in the EHR, the resulting information is foundational for future work that can improve the quality of team-based CC; plan staffing, team composition, and labor mapping; determine the cost of CC activities and improve reimbursement in certain settings; and assess outcomes of CC.
This tool has potential for application beyond the PIM team in the VA. The CC Template and training manual is scalable to any setting with team-based CC, including PACT, homeless programs, palliative care, Mental Health Intensive Case Management (MHICM) programs, nurse navigator programs, and other complex care delivery models involving care coordinators. Future study of its implementation and data may inform initiatives to develop ongoing team-based care coordination programs.
Acknowledgments
The authors thank the following colleagues for their input and support: Florence Longchamp, RN, Clinical Applications Coordinator at the Atlanta VA Medical Center without whom the CC Template would not have been created; the Atlanta and San Francisco VA PIM teams for their thoughtful comments and enthusiastic embrace of the CC Template; and the PIM National Evaluation Center for their support of this QI project. PACT Intensive Management demonstration sites are funded by the VA Office of Patient Care Services. During the implementation of the CC Template pilot and the preparation of this paper, the primary author was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Advanced Fellowships, VA Quality Scholars Program.
Chronic diseases affect a substantial proportion of the US population, with 25% of adults diagnosed with 2 or more chronic health conditions.1 In 2010, 2 chronic diseases, heart disease and cancer, accounted for nearly 48% of deaths.2 Due to the significant public heath burden, strategies to improve chronic disease management have attracted a great deal of focus.3,4 Within increasingly complex health care delivery systems, policy makers are promoting care coordination (CC) as a tool to reduce fragmented care for patients with multiple comorbidities, improve patient experience and quality of care, and decrease costs and risks for error.3-8
Background
The Agency for Healthcare Research and Quality (AHRQ) defines care coordination as “deliberately organizing patient care activities and sharing information among all of the participants concerned with a patient’s care to achieve safer and more effective care.”5 Nationally, large scale investments have expanded health care models that provide team-based CC, such as patient-centered medical homes, known as patient-aligned care teams (PACTs) within the Department of Veterans Affairs (VA), accountable care organizations, and other complex care management programs.9-12 Additionally, incentives that reimburse for CC, such as Medicare’s chronic care management and transition care management billing codes, also are emerging.13,14
While there is significant interest and investment in promoting CC, little data about the specific activities and time required to provide necessary CC exist, which limits the ability of health care teams to optimize CC delivery.6 Understanding the components of CC has implications for human resource allocation, labor mapping, reimbursement, staff training, and optimizing collaborative networks for health care systems, which may improve the quality of CC and health outcomes for patients. To date, few tools exist that can be used to identify and track the CC services delivered by interdisciplinary teams within and outside of the health care setting.
This article describes the development and preliminary results of the implementation of a CC Template that was created in the VA Computerized Patient Record System (CPRS) to identify and track the components of CC services, delivered by a multidisciplinary team, as part of a quality improvement (QI) pilot project. Through use of the template, the team sought a formative understanding of the following questions: (1) Is it feasible to use the CC Template during routine workflow? (2) What specific types of CC services are provided by the team? (3) How much time does it take to perform these activities? (4) Who is the team collaborating with inside and outside of the health care setting and how are they communicating? (5) Given new reimbursement incentives, can the provision of CC be standardized and documented for broad applicability?
In complex systems, where coordination is needed among primary, specialty, hospital, emergency, and nonclinical care settings, a tool such as the CC Template offers a sustainable and replicable way to standardize documentation and knowledge about CC components. This foundational information can be used to optimize team structure, training, and resource allocation, to improve the quality of CC and to link elements of CC with clinical and operational outcomes.
Pact Intensive Management
Despite the implementation of PACT within VA, patients with complex medical conditions combined with socioeconomic stressors, mental health comorbidities, and low health literacy are at high risk for preventable hospitalizations and acute care utilization.17,18 Due to unmet needs that are beyond what PACTs are able to deliver, these high-risk patients may benefit from additional services to coordinate care within and outside the VA health care system, as suggest by the Extended Chronic Care Model.19-21
In 2014, the Office of Primary Care Services sponsored a QI initiative at 5 VA demonstration sites to develop PACT Intensive Management (PIM) interventions targeting patients at high risk for hospitalization and acute care utilization within VA. The PIM program design is based on work described previously, with patients identified for enrollment based on 90-day hospitalization risk ≥ 90th percentile, based on a VA risk modeling tool, and an acute care episode in the previous 6 months. 19 A common component of all PIM programs is the provision of intensive care management and CC by an interdisciplinary team working in conjunction with PACT. The CC Template was developed to assist in documenting and rigorously understanding the implementation of CC by the PIM team.
Local Setting
The Atlanta VA Medical Center (AVAMC) was chosen as one of the PIM demonstration sites. The Atlanta PIM team identified and enrolled a random sample of eligible, high-risk patients from 1 community-based outpatient clinic (CBOC) in an urban location with 7,524 unique patients. Between September 2014 and September 2016, 300 patients were identified, and 86 patients agreed to participate in the PIM program.
In the CC Template pilot, the Atlanta PIM team included 2 nurse practitioners (NP), 2 social workers (SW), and 1 telehealth registered nurse (RN). Upon enrollment, members of the PIM team conducted comprehensive home assessments and offered intensive care management for medical, social, and behavioral needs. The main pillars of care management offered to high-risk patients were based on previous work done both inside and outside VA and included home visits, telephone-based disease management, co-attending appointments with patients, transition care management, and interdisciplinary team meetings with a focus on care coordination between PACT and all services required by patients.11,19
The Atlanta PIM team performed a variety of tasks to coordinate care for enrolled patients that included simple, 1-step tasks, such as chart reviews, and multistep, complex tasks that required the expertise of multiple team members (Figure 1).
Additionally, inconsistency in delivery of CC between PIM team members was noted. For example, there was significant variability in CC services provided by different team members in the provision of transition care management (TCM) and coordinating care from hospitalization back to home. Some PIM staff coordinated care and communicated with the patient, hospital team, home-care service, and primary-care team, while other staff only reviewed the chart and placed orders in CPRS. Additionally, much of the CC work was documented in administrative notes that did not trigger workload credit. This made it difficult to show how to appropriately labor map PIM staff or how staff were spending their time caring for patients.
In order to standardize documentation of the interdisciplinary CC activities performed by the PIM team and account for staff time, the Atlanta PIM team decided to develop a CPRS CC Template. The objective of the CC Template was to facilitate documentation of CC activities in the EHR, describe the types of CC activities performed by PIM team members, and track the time to perform these activities for patients with various chronic diseases.
Template Design and Implementation
The original design of the template was informed by the Atlanta PIM team after several informal focus groups and process mapping of CC pathways in the fall of 2015. The participants were all members of the Atlanta PIM team, 2 primary care physicians working with PIM, an AVAMC documentation specialist and a clinical applications coordinator (CAC) assigned to work with PIM. The major themes that arose during the brainstorming discussion were that the template should: (1) be feasible to use during their daily clinic workflow; (2) improve documentation of CC; and (3) have value for spread to other VA sites. Discussion centered on creating a CC Template versatile enough to:
- Decrease the number of steps for documenting CC;
- Consist only of check boxes, with very little need for free text, with the option to enter narrative free text after template completion;
- Document time spent in aggregate for completing complex CC encounters;
- Document various types of CC work and modes of communication;
- Allow for use by all PIM staff;
- Identify all team members that participated in the CC encounter to reduce redundant documentation by multiple staff;
- Adapt to different clinic sites based on the varied disciplines participating in other locations;
- Use evidence-based checklists to help standardize delivery of CC for certain activities such as TCM; and
- Extract data without extensive chart reviews to inform current CC and future QI work.
Following the brainstorming sessions, the authors performed a literature review to identify and integrate CC best practices. The AHRQ Care Coordination Atlas served as the main resource in the design of the logic model that depicted the delivery and subsequent documentation of high-quality, evidence-based CC in the CC Template (Figure 2).6
After reaching consensus about the key components of the CC Template, the CAC created a pilot version (Figure 3). All of the elements within the CC Template allowed for data abstraction from the VA Corporate Data Warehouse (CDW) via discrete data elements known as health factors.
Over the course of implementation, the team became more enthusiastic about using the CC template to document previously unrecognized CC workload. Because the CC Template only was used to document CC workload and excluded encounters for clinical evaluation and management, specific notes were created and linked with the CC Template for optimal capture of encounters.
All components of the template were mandatory to eliminate the possibility of missing data. The Atlanta PIM site principal investigator developed a multicomponent training designed to increase support for the template by describing its value and to mitigate the potential for variability in how data are captured. Training included a face-to-face session with the team to review the template and work through sample CC cases. Additionally, a training manual with clear operational definitions and examples of how to complete each element of the CC Template was disseminated. The training was subsequently conducted with the San Francisco VA Medical Center PIM team, a spread site, via video conference. The spread site offered significant feedback on clarifying the training documents and adapting the CC template for their distinct care team structure. This feedback was incorporated into the final CC Template design to increase adaptability.
Implementation Evaluation
The RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)framework served as the basis for evaluation of CC Template implementation. The RE-AIM framework is well established and able to evaluate the implementation and potential successful spread of new programs.23,24 Using RE-AIM, the authors planned to analyze data to explore the reach effectiveness, adoption, implementation, and maintenance of the CC Template use while providing complex care management for high-risk patients.
All data for the evaluation was extracted from the CDW by a data analyst and stored on a secure server. A statistical process control (SPC) chart was used to analyze the implementation process to assess variation in template use.
Results
After implementation, 35 weeks of CC Template pilot data were analyzed from June 1, 2015 to January 5, 2016. The PIM team completed 393 CC Templates over this collection period. After week 23, the CC template was linked to specific CC notes automatically. From weeks 23 to 35 an average of 20.3 CC Templates were completed per week by the team. The RE-AIM was used to assess the implementation of the CC Template.
Reach was determined by the number of patients enrolled in PIM with CC Template documentation. Of patients enrolled in Atlanta PIM, 90.1% had ≥ 1 CC encounter documented by the CC Template; 74.4% of Atlanta PIM patients had ≥ 1 CC encounter documented; 15.5% of patients had > 10 CC encounters documented; and 1 patient had > 25 CC encounters documented by the CC template.
Effectiveness for describing CC activities was captured through data from CC Template. The CC Template documentation by the PIM team showed that 79.4% of CC encounters were < 20 minutes, and 9.9% of encounters were > 61 minutes. Telephone communication was involved in 50.4% of CC encounters, and 24% required multiple modes of communication such as face-to-face, instant messenger, chart-based communication. Care coordination during hospitalization and discharge accounted for 5.9% of template use. Of the CC encounters documenting hospital transitions, 94.4% documented communication with the inpatient team, 58.3% documented coordination with social support, and only 11.1% documented communication with primary care teams. Improving communication with PACT teams after hospital discharge was identified as a future QI project based on these data. The PIM team initiated 83.2% of CC encounters.
Adoption was determined by the use of the CC Template by the team. All 5 team members used the CC template to document at least 1 CC encounter.
Implementation allowed for improvement based on feedback from the PIM team. Mean completion of CC Templates rose from 10.9 per week to 20.3 per week after automatically linking the CC Template to specific CC notes. (Figure 4)
Maintenance was monitored over the course of the pilot. Consistent use of the CC Template over 35 full work weeks of data collection was seen, and mean utilization per week nearly doubled in the latter half of the pilot period.
Because several elements were added to the CC Template over the course of the pilot period, our ability to analyze the data for descriptive statistics about the types of CC services, related diagnoses, collaborators, and PIM staff involved in CC encounters was limited.
Discussion
Though all components of CC encounters could not be assessed during the pilot phase due to continuous improvement of the CC Template, the authors showed that it is feasible to use this tool to document and describe granular details about team-based CC. Pilot data from AVAMC show that the use of the CC Template standardized team CC documentation in a busy clinic setting provided data about the complexity of coordination activities and duration of CC activities. It also informed future CC QI projects, such as improving communication with primary care during the hospital discharge process.
Future evaluation of CC Template data can be used to (1) describe types of CC activities for high-risk PIM patients; (2) quantify the time required to complete CC activities to assist with staff labor mapping; (3) describe staff roles and referrals needed to complete specific CC activities inside and outside VA; (4) describe modes of communication between PIM and collaborators; (5) relate patient demographics and associated diagnoses with quantity of CC encounters; and (6) quantify frequency and time frame of CC after hospitalizations and ED care and subsequent impact on repeat hospitalizations and ED visits. Future research also can explore the link between CC activities and effort with clinical and patient-reported outcomes.
Social network analysis could be used with CC Template data to understand the network of referrals and collaborators involved in the care of a CC team’s patients. This type of analysis would assist teams to strengthen and formalize ties with collaborators as appropriate. For example, if data show that the team frequently collaborates with the cardiology clinic for a large subset of its patients, they may consider creating a CC agreement with formalized modes of communication that would streamline collaboration.
In order to improve the quality of the CC Template and to assess factors that may lead to sustainable use in clinical practice, qualitative assessment through survey, interview, or usability testing with staff would be beneficial to identify strategies to increase its adoption among clinical providers. This type of assessment will add knowledge about the CC Template implementation process, including contextual barriers or facilitators, feasibility of use during day-to-day operations, versatility of template use within construct of team-based care, and overall satisfaction with the template.
Limitations
Though the CC Template offers a large amount of data about the components of CC delivery, the information is based on self-report by staff. Training to ensure that all team members are documenting in the same manner is crucial to maintain the internal validity of the data. The template is limited to the fields currently developed, and future research could explore additional data elements that are critical to include based on feedback from VA staff.
Conclusion
To our knowledge, this VA medical center CC Template is the first tool described in the literature that standardizes and captures data about CC components in the EHR. This pilot data show that the template is feasible for use in a busy clinic setting and can streamline the process for capturing CC data that may otherwise not be documented.
During the pilot phase, the CC template allowed the PIM team to identify a small subset of patients within the PIM complex management who have a high level of CC needs. By identifying these patients, further work can be done to understand the specific needs of these higher utilizers and the types of CC activities required to assist them so that resources can be directed appropriately to that smaller subset. Telephone CC accounted for a large proportion of delivery, which has implications for ensuring that staff have access to mobile phones and EHR capability to document this additional workload. The PIM staff maintained use of the template throughout the pilot period and increased documentation when the CC Template was easily accessible and already linked to their CPRS notes, suggesting that in future implementation, ensuring that the template is linked to notes in use by the care team will be important for successful spread.
Additionally, CC Template data identified gaps in high-quality, evidence-based CC that can be addressed in real time, for example during the discharge process. Data from the CC Template showed that only 11.1% of CC encounters had documentation of communication between the PIM and primary care teams during transitions from hospital to home. Improving communication with PACT teams after hospital discharge was identified as a future PIM QI project based on these data. By improving documentation of CC in the EHR, the resulting information is foundational for future work that can improve the quality of team-based CC; plan staffing, team composition, and labor mapping; determine the cost of CC activities and improve reimbursement in certain settings; and assess outcomes of CC.
This tool has potential for application beyond the PIM team in the VA. The CC Template and training manual is scalable to any setting with team-based CC, including PACT, homeless programs, palliative care, Mental Health Intensive Case Management (MHICM) programs, nurse navigator programs, and other complex care delivery models involving care coordinators. Future study of its implementation and data may inform initiatives to develop ongoing team-based care coordination programs.
Acknowledgments
The authors thank the following colleagues for their input and support: Florence Longchamp, RN, Clinical Applications Coordinator at the Atlanta VA Medical Center without whom the CC Template would not have been created; the Atlanta and San Francisco VA PIM teams for their thoughtful comments and enthusiastic embrace of the CC Template; and the PIM National Evaluation Center for their support of this QI project. PACT Intensive Management demonstration sites are funded by the VA Office of Patient Care Services. During the implementation of the CC Template pilot and the preparation of this paper, the primary author was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations, Advanced Fellowships, VA Quality Scholars Program.
1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
2. Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated May 3, 2017. Accessed August 8, 2018.
3. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
4. US Department of Health and Human Services. Healthy people 2010: general data issues. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_general_data_issues.pdf. Published 2010. Accessed August 1, 2018.
5. McDonald KM, Sunderam V, Bravata DM, et al. Closing the quality gap: a critical analysis of quality improvement strategies, Vol 7: care coordination. Agency for Healthcare Research and Quality. https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. Published June 2007. Accessed August 1, 2018.
6. McDonald KM, Schultz E, Albin L, et al. Care coordination measures atlas. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed August 2, 2018.
7. Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating care across diseases, settings, and clinicians: a key role for the generalist in practice. Ann Intern Med. 2005;142(8):700-708.
8. Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15(5):329-336.
9. National Committee for Quality Assurance. The future of patient-centered medical homes: foundation for a better health care system. https://www.ncqa.org/Portals/0/Public%20Policy/2014%20PDFS/The_Future_of_PCMH.pdf. Accessed August 2, 2018.
10. US Department of Veterans Affairs, Veterans Health Administration. Patient Aligned Care Team (PACT) Handbook. VHA Handbook 1101.10:1–65. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2977. Updated May 26, 2017. Accessed August 2, 2018.
11. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
12. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19.
13. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Chronic care management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Published December 2016. Accessed August 2, 2018.
14. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Frequently asked questions about billing the Medicare physician fee schedule for transitional care management services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-TCMS.pdf. Published March 17, 2016. Accessed August 2, 2018.
15. Antonelli RC, Stille CJ, Antonelli DM. Care coordination for children and youth with special health care needs: a descriptive, multisite study of activities, personnel costs, and outcomes. Pediatrics. 2008;122(1):e209-e216.
16. Antonelli RC, Antonelli DM. Providing a medical home: the cost of care coordination services in a community-based, general pediatric practice. Pediatrics. 2004;113( suppl 5 ):1522-1528.
17. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
18. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an intensive management patient-aligned care team (ImPACT). J Gen Intern Med. 2014;29(suppl 4):S861-S869.
20. Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166-175.
21. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73-82.
22. US Department of Health & Human Services, Agency for Healthcare Research and Quality. Re-engineered discharge (RED) toolkit. http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html. Updated May 2017. Accessed August 3, 2018.
23. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327.
24. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-e46.
1. Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62.
2. Centers for Disease Control and Prevention. Deaths and mortality. https://www.cdc.gov/nchs/fastats/deaths.htm. Updated May 3, 2017. Accessed August 8, 2018.
3. Committee on Quality of Health Care in America, Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
4. US Department of Health and Human Services. Healthy people 2010: general data issues. https://www.cdc.gov/nchs/data/hpdata2010/hp2010_general_data_issues.pdf. Published 2010. Accessed August 1, 2018.
5. McDonald KM, Sunderam V, Bravata DM, et al. Closing the quality gap: a critical analysis of quality improvement strategies, Vol 7: care coordination. Agency for Healthcare Research and Quality. https://www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf. Published June 2007. Accessed August 1, 2018.
6. McDonald KM, Schultz E, Albin L, et al. Care coordination measures atlas. https://www.ahrq.gov/sites/default/files/publications/files/ccm_atlas.pdf. Updated June 2014. Accessed August 2, 2018.
7. Stille CJ, Jerant A, Bell D, Meltzer D, Elmore JG. Coordinating care across diseases, settings, and clinicians: a key role for the generalist in practice. Ann Intern Med. 2005;142(8):700-708.
8. Schillinger D, Bibbins-Domingo K, Vranizan K, Bacchetti P, Luce JM, Bindman AB. Effects of primary care coordination on public hospital patients. J Gen Intern Med. 2000;15(5):329-336.
9. National Committee for Quality Assurance. The future of patient-centered medical homes: foundation for a better health care system. https://www.ncqa.org/Portals/0/Public%20Policy/2014%20PDFS/The_Future_of_PCMH.pdf. Accessed August 2, 2018.
10. US Department of Veterans Affairs, Veterans Health Administration. Patient Aligned Care Team (PACT) Handbook. VHA Handbook 1101.10:1–65. http://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=2977. Updated May 26, 2017. Accessed August 2, 2018.
11. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
12. Hong CS, Siegel AL, Ferris TG. Caring for high-need, high-cost patients: what makes for a successful care management program? Issue Brief (Commonw Fund). 2014;19:1-19.
13. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Chronic care management services. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf. Published December 2016. Accessed August 2, 2018.
14. US Department of Health and Human Services, Centers for Medicare & Medicaid Services. Frequently asked questions about billing the Medicare physician fee schedule for transitional care management services. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/Downloads/FAQ-TCMS.pdf. Published March 17, 2016. Accessed August 2, 2018.
15. Antonelli RC, Stille CJ, Antonelli DM. Care coordination for children and youth with special health care needs: a descriptive, multisite study of activities, personnel costs, and outcomes. Pediatrics. 2008;122(1):e209-e216.
16. Antonelli RC, Antonelli DM. Providing a medical home: the cost of care coordination services in a community-based, general pediatric practice. Pediatrics. 2004;113( suppl 5 ):1522-1528.
17. Zulman DM, Pal Chee C, Wagner TH, et al. Multimorbidity and healthcare utilisation among high-cost patients in the US Veterans Affairs Health Care System. BMJ Open. 2015;5(4):e007771.
18. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
19. Zulman DM, Ezeji-Okoye SC, Shaw JG, et al. Partnered research in healthcare delivery redesign for high-need, high-cost patients: development and feasibility of an intensive management patient-aligned care team (ImPACT). J Gen Intern Med. 2014;29(suppl 4):S861-S869.
20. Zulman DM, Pal Chee C, Ezeji-Okoye SC, et al. Effect of an intensive outpatient program to augment primary care for high-need Veterans Affairs patients: a randomized clinical trial. JAMA Intern Med. 2017;177(2):166-175.
21. Barr VJ, Robinson S, Marin-Link B, et al. The expanded Chronic Care Model: an integration of concepts and strategies from population health promotion and the Chronic Care Model. Hosp Q. 2003;7(1):73-82.
22. US Department of Health & Human Services, Agency for Healthcare Research and Quality. Re-engineered discharge (RED) toolkit. http://www.ahrq.gov/professionals/systems/hospital/red/toolkit/index.html. Updated May 2017. Accessed August 3, 2018.
23. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322-1327.
24. Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38-e46.
Outcomes of Palliative Care Consults With Hospitalized Veterans
Families and patients receive emotional support and better care planning after palliative care consultations.
Inpatient palliative care (IPC) consultation services have been widely adopted in US hospitals. Outcomes research has demonstrated improved quality of life (QOL) for palliative inpatients for symptom control and satisfaction with care.1-5 Families benefit from emotional support, care planning, and transitions of care.4,6-8 Outcomes, including hospital length of stay, hospital costs, and discharge dispositionalso seem to improve.9-17 The Department of Veterans Affairs (VA) provides palliative care (PC) consultation teams at its hospitals nationwide; however, few studies exist to show how a PC service is used at a VA hospital. The following study of a PC consult team at an urban VA facility provides a unique picture of how a PC team is used.
Methods
The John Cochran Division of the VA St. Louis Health Care System (VASLHCS) in Missouri is a 509-bed adult acute care hospital with medical and surgical specialties and subspecialties available for veterans, including an intensive care unit (ICU). The PC team is one of the subspecialty teams following patients after consultation and consists of a PC physician, nurse practitioner, chaplain, social worker, and psychologist.
Data Collection
This study was exempt from internal review board approval. The attending physician kept track of each IPC encounter between September 2014 and April 2016. Data were retrieved from the Computerized Patient Record System by identifying charts that included family meeting notes during the specified time. All 130 patients included in this study were followed by the PC team. Patient charts were reviewed, and information was uploaded to spreadsheets, which became the database for this study. The data included age, patient location, diagnosis, number of days between admission and PC consultation, and number of days between admission and family meeting. Other data included code status changes and discharge dispositions. Only consultations that resulted in direct patient contact were included.
The VASLHCS requires therapeutic support level (TSL), or code status, documentation by the attending physician regarding the discussion with a competent patient or valid representative if the patient is incapacitated. Levels of support are TSL I ‘‘no limitation on care,’’ TSL II ‘‘partial code,’’ that is, usually no cardiopulmonary resuscitation or do not intubate with selected medical measures to continue, and TSL III ‘‘comfort measures only.’’ If a patient’s code level changed after IPC consultation, the change is recorded.
Data Analysis
The files were purged of all unique personal health history. Because there was no control group, multivariable analyses of association were not warranted. Analysis was confined to descriptive measures.
Results
A total of 130 patients with IPC consultations were included in this retrospective study conducted from September 2014 to April 2016 (Table 1).
The scope of IPC consultations usually include medical recommendations about symptom management, discharge planning, discussion about goals of care (GOC), code status and prognosis, managing expected in-hospital expirations (deaths), and determination of hospice eligibility. Of the IPC cohort, 74% were aged > 65 years; 26.1% were aged < 65 years (Table 2).
The mean days for an initial IPC consultation following admission was 3 on the medical/surgical floors and 7 days for ICU (P = .003; 95% CI, -6.37 to 1.36).
Discussion
Although small, the proportion of patients with serious illness or multiple chronic conditions account for a disproportionately large portion of health care spending.18 Despite the high cost, evidence demonstrates that these patients receive health care of inadequate quality characterized by fragmentation, overuse, medical errors, and poor QOL. Multiple studies show that IPC consultation provides improved patient outcomes and decreased hospital costs.9-17
From a purely outcomes-based interpretation, IPC consultation was associated with 83% of patients receiving a change in code status from full code/TSL 1. The study team drew 2 main conclusions from the data: (1) The IPC consultation is an effective way to broach GOC discussion and adjust code status; and (2) These data suggest room for earlier PC involvement. Remarkably, only 3 patients (2%) expired while inpatient with full code status.
The data also provide a unique comparison of timing of PC referrals. Pantilat and colleagues published characteristics of PC consultation services in California hospitals, and on average, patients were in the hospital 5.9 days (median 5.5; SD 3.3) prior to referral.19 This study’s average number of days for initial IPC consultation following admission was 3 days on the medical/surgical floors and 7 days in the ICU. Both time frames seem reasonable but again indicate some potential improvement for earlier IPC utilization.
Although the time frame of the intervention limited the number of patients in this study, early PC consultations in the acute care setting are a helpful intervention for veterans and families to better understand the complexity of their medical condition and prognosis and allow for a frank and open discussion about realistic goals. The importance of these discussions also were reflected in the high percentage of patients transitioning to hospice level of care (80%) and the low number of patients who remained full code (3 of 130). Other studies have shown conflicting results when interventions have been exclusively for cancer patients. In this study, 45% of patients were admitted with diagnoses other than cancer compared with 24% of patients with related diagnoses in a study by Gonsalves and colleagues.20
In this study, the majority (71.6%) of family meetings were held only with family (no patient involvement), resulting in missed opportunities for earlier patient and PC involvement especially for those patients with serious medical illnesses.
A systematic review published by Wendler and colleagues found that surrogate decision makers often find that role troubling and traumatizing even with advance directive documents.21 Earlier identification and PC consultations could initiate discussions between patients and their loved ones to decide “when enough is enough,” and about whether or not to prolong the dying process, when compatible with the patient’s wishes.
Early PC consultations also could highlight a potential highly vulnerable population of medically unbefriended patients (elder orphans). These patients may have no one in their lives to act as surrogate decision makers. This situation calls for further interventions regarding early identification of these patients and better processes to assist in their decision making. Many physicians believe it is not appropriate to begin advance directive planning on an outpatient basis. However, multiple studies have shown that patients want their doctors to discuss advance care planning with them before they become ill.22 Many other doctors have shown a positive response from patients when advance directive discussions are held during outpatient visits.23
The goals of this study were to evaluate the effectiveness of IPC consultation on goals of care and to address code status with patients and their families. Along with these conversations, the study team provided comprehensive PC evaluation. The PC team focused on providing excellent symptom management. The team of PC physicians, pain specialists, pain pharmacists, a chaplain, psychologists, and social workers addressed all the bio-psycho-social needs of patients/families and provided comprehensive recommendations. This multidimensional approach has gained significant acceptance.24
At VASLHCS, the program has grown to about 600 new consults per year, with a dedicated inpatient hospice unit, daily outpatient clinic, and myriad learning opportunities for trainees; the center has become a main site of rotation for hospice and palliative care fellows from training programs in St. Louis.
Utilization of PC consultation to help meeting the veterans’ needs at the bio-psycho-social level will also provide a benefit for the facility as it will decrease observed/expected standardized mortality ratio (SMR) data. This reduction of SMR data will be a result of successful patient transitions to hospice level of care at least 12 months prior to their passing or if their level of care is changed to inpatient hospice after they are admitted, the patients won’t be included as acute care mortality. However, with this initial small group of patients it was not possible to retrospectively calculate the impact on SMR or SAIL (Strategic Analytics for Improvement and Learning) indicators. The long-term expectation is to have a positive impact on those indicators represented by decreased inpatient mortality and improved SAIL.
Limitations
This study was a single-institution study, but every institution has its own internal culture. The team did not have a concurrent or historic control for comparison or use a questionnaire for patients and families rating their satisfaction.
Conclusion
This study provides multiple future directions of research as the authors now have baseline data about how the service is used. Future areas of interest would be to study the effectiveness of early palliative care interventions, such as a provider education series, implementation of consultation criteria, and prospective measurement of the impact of palliative care consultations on the SMR and SAIL indicators. This research could help identify which early interventions show the best efficacy, an area where research is notably lacking.25
1. El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9(3):87-94.
2. Higginson IJ, Finlay I, Goodwin DM, et al. Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage. 2002;23(2):96-106.
3. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190.
4. Benzar E, Hansen L, Kneitel AW, Fromme EK. Discharge planning for palliative care patients: a qualitative analysis. J Palliat Med. 2011;14(1):65-69.
5. Enguidanos S, Housen P, Penido M, Mejia B, Miller JA. Family members’ perceptions of inpatient palliative care consult services: a qualitative study. Palliat Med. 2014;28(1):42-48.
6. Granda-Cameron C, Viola SR, Lynch MP, Polomano RC. Measuring patient-oriented outcomes in palliative care: functionality and quality of life. Clin J Oncol Nurs. 2008;12(1):65-77.
7. Chand P, Gabriel T, Wallace CL, Nelson CM. Inpatient palliative care consultation: describing patient satisfaction. Perm J. 2013;17(1):53-55.
8. Tangeman JC, Rudra CB, Kerr CW, Grant PC. A hospice-hospital partnership: reducing hospitalization costs and 30-day readmissions among seriously ill adults. J Palliat Med. 2014;17(9):1005-1010.
9. Fromme EK, Bascom PB, Smith MD, et al. Survival, mortality, and location of death for patients seen by a hospital-based palliative care team. J Palliat Med. 2006;9(4):903-911.
10. Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973-979.
11. Ranganathan A, Dougherty M, Waite D, Casarett D. Can palliative home care reduce 30-day readmissions? Results of a propensity score matched cohort study. J Palliat Med. 2013;16(10):1290-1293.
12. Starks H, Wang S, Farber S, Owens DA, Curtis JR. Cost savings vary by length of stay for inpatients receiving palliative care consultation services. J Palliat Med. 2013;16(10):1215-1220.
13. Goldenheim A, Oates D, Parker V, Russell M, Winter M, Silliman RA. Rehospitalization of older adults discharged to home hospice care. J Palliat Med. 2014;17(7):841-844.
14. May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med. 2014;17(9):1054-1063.
15. Granda-Cameron C, Behta M, Hovinga M, Rundio A, Mintzer D. Risk factors associated with unplanned hospital readmissions in adults with cancer. Oncol Nurs Forum. 2015;42(3):e257-e268.
16. Brody AA, Ciemins E, Newman J, Harrington C. The effects of an inpatient palliative care team on discharge disposition. J Palliat Med. 2010;13(5):541-548.
17. Penrod JD, Deb P, Luhrs C, et al. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9(4):855-860.
18. Aldridge MD, Kelley AS. Appendix E, Epidemiology of serious illness and high utilization of health care. In: Institute of Medicine, Committee on Approaching Death: Addressing Key End of Life Issues. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
19. Pantilat SZ, Kerr KM, Billings JA, Bruno KA, O’Riordan DL. Characteristics of palliative care consultation services in California hospitals. J Palliat Med. 2012;15(5):555-560.
20. Gonsalves WI, Tashi T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veterans Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
21. Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336-346.
22. American Bar Association Commission on Law and Aging. Myths and facts about health care advance directives. https://www.americanbar.org/content/dam/aba/publications/bifocal/BIFOCALSept-Oct2015.authcheckdam.pdf. Accessed July 10, 2018.
23. Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou X-H, Wolinsky FD. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16(1):32-40.
24. Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life’s end in inpatient settings: the BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
25. Dalgaard K, Bergenholtz H, Nielsen M, Timm H. Early integration of palliative care in hospitals: a systematic review on methods, barriers, and outcome. Palliat Support Care. 2014;12(6):495-513.
Families and patients receive emotional support and better care planning after palliative care consultations.
Families and patients receive emotional support and better care planning after palliative care consultations.
Inpatient palliative care (IPC) consultation services have been widely adopted in US hospitals. Outcomes research has demonstrated improved quality of life (QOL) for palliative inpatients for symptom control and satisfaction with care.1-5 Families benefit from emotional support, care planning, and transitions of care.4,6-8 Outcomes, including hospital length of stay, hospital costs, and discharge dispositionalso seem to improve.9-17 The Department of Veterans Affairs (VA) provides palliative care (PC) consultation teams at its hospitals nationwide; however, few studies exist to show how a PC service is used at a VA hospital. The following study of a PC consult team at an urban VA facility provides a unique picture of how a PC team is used.
Methods
The John Cochran Division of the VA St. Louis Health Care System (VASLHCS) in Missouri is a 509-bed adult acute care hospital with medical and surgical specialties and subspecialties available for veterans, including an intensive care unit (ICU). The PC team is one of the subspecialty teams following patients after consultation and consists of a PC physician, nurse practitioner, chaplain, social worker, and psychologist.
Data Collection
This study was exempt from internal review board approval. The attending physician kept track of each IPC encounter between September 2014 and April 2016. Data were retrieved from the Computerized Patient Record System by identifying charts that included family meeting notes during the specified time. All 130 patients included in this study were followed by the PC team. Patient charts were reviewed, and information was uploaded to spreadsheets, which became the database for this study. The data included age, patient location, diagnosis, number of days between admission and PC consultation, and number of days between admission and family meeting. Other data included code status changes and discharge dispositions. Only consultations that resulted in direct patient contact were included.
The VASLHCS requires therapeutic support level (TSL), or code status, documentation by the attending physician regarding the discussion with a competent patient or valid representative if the patient is incapacitated. Levels of support are TSL I ‘‘no limitation on care,’’ TSL II ‘‘partial code,’’ that is, usually no cardiopulmonary resuscitation or do not intubate with selected medical measures to continue, and TSL III ‘‘comfort measures only.’’ If a patient’s code level changed after IPC consultation, the change is recorded.
Data Analysis
The files were purged of all unique personal health history. Because there was no control group, multivariable analyses of association were not warranted. Analysis was confined to descriptive measures.
Results
A total of 130 patients with IPC consultations were included in this retrospective study conducted from September 2014 to April 2016 (Table 1).
The scope of IPC consultations usually include medical recommendations about symptom management, discharge planning, discussion about goals of care (GOC), code status and prognosis, managing expected in-hospital expirations (deaths), and determination of hospice eligibility. Of the IPC cohort, 74% were aged > 65 years; 26.1% were aged < 65 years (Table 2).
The mean days for an initial IPC consultation following admission was 3 on the medical/surgical floors and 7 days for ICU (P = .003; 95% CI, -6.37 to 1.36).
Discussion
Although small, the proportion of patients with serious illness or multiple chronic conditions account for a disproportionately large portion of health care spending.18 Despite the high cost, evidence demonstrates that these patients receive health care of inadequate quality characterized by fragmentation, overuse, medical errors, and poor QOL. Multiple studies show that IPC consultation provides improved patient outcomes and decreased hospital costs.9-17
From a purely outcomes-based interpretation, IPC consultation was associated with 83% of patients receiving a change in code status from full code/TSL 1. The study team drew 2 main conclusions from the data: (1) The IPC consultation is an effective way to broach GOC discussion and adjust code status; and (2) These data suggest room for earlier PC involvement. Remarkably, only 3 patients (2%) expired while inpatient with full code status.
The data also provide a unique comparison of timing of PC referrals. Pantilat and colleagues published characteristics of PC consultation services in California hospitals, and on average, patients were in the hospital 5.9 days (median 5.5; SD 3.3) prior to referral.19 This study’s average number of days for initial IPC consultation following admission was 3 days on the medical/surgical floors and 7 days in the ICU. Both time frames seem reasonable but again indicate some potential improvement for earlier IPC utilization.
Although the time frame of the intervention limited the number of patients in this study, early PC consultations in the acute care setting are a helpful intervention for veterans and families to better understand the complexity of their medical condition and prognosis and allow for a frank and open discussion about realistic goals. The importance of these discussions also were reflected in the high percentage of patients transitioning to hospice level of care (80%) and the low number of patients who remained full code (3 of 130). Other studies have shown conflicting results when interventions have been exclusively for cancer patients. In this study, 45% of patients were admitted with diagnoses other than cancer compared with 24% of patients with related diagnoses in a study by Gonsalves and colleagues.20
In this study, the majority (71.6%) of family meetings were held only with family (no patient involvement), resulting in missed opportunities for earlier patient and PC involvement especially for those patients with serious medical illnesses.
A systematic review published by Wendler and colleagues found that surrogate decision makers often find that role troubling and traumatizing even with advance directive documents.21 Earlier identification and PC consultations could initiate discussions between patients and their loved ones to decide “when enough is enough,” and about whether or not to prolong the dying process, when compatible with the patient’s wishes.
Early PC consultations also could highlight a potential highly vulnerable population of medically unbefriended patients (elder orphans). These patients may have no one in their lives to act as surrogate decision makers. This situation calls for further interventions regarding early identification of these patients and better processes to assist in their decision making. Many physicians believe it is not appropriate to begin advance directive planning on an outpatient basis. However, multiple studies have shown that patients want their doctors to discuss advance care planning with them before they become ill.22 Many other doctors have shown a positive response from patients when advance directive discussions are held during outpatient visits.23
The goals of this study were to evaluate the effectiveness of IPC consultation on goals of care and to address code status with patients and their families. Along with these conversations, the study team provided comprehensive PC evaluation. The PC team focused on providing excellent symptom management. The team of PC physicians, pain specialists, pain pharmacists, a chaplain, psychologists, and social workers addressed all the bio-psycho-social needs of patients/families and provided comprehensive recommendations. This multidimensional approach has gained significant acceptance.24
At VASLHCS, the program has grown to about 600 new consults per year, with a dedicated inpatient hospice unit, daily outpatient clinic, and myriad learning opportunities for trainees; the center has become a main site of rotation for hospice and palliative care fellows from training programs in St. Louis.
Utilization of PC consultation to help meeting the veterans’ needs at the bio-psycho-social level will also provide a benefit for the facility as it will decrease observed/expected standardized mortality ratio (SMR) data. This reduction of SMR data will be a result of successful patient transitions to hospice level of care at least 12 months prior to their passing or if their level of care is changed to inpatient hospice after they are admitted, the patients won’t be included as acute care mortality. However, with this initial small group of patients it was not possible to retrospectively calculate the impact on SMR or SAIL (Strategic Analytics for Improvement and Learning) indicators. The long-term expectation is to have a positive impact on those indicators represented by decreased inpatient mortality and improved SAIL.
Limitations
This study was a single-institution study, but every institution has its own internal culture. The team did not have a concurrent or historic control for comparison or use a questionnaire for patients and families rating their satisfaction.
Conclusion
This study provides multiple future directions of research as the authors now have baseline data about how the service is used. Future areas of interest would be to study the effectiveness of early palliative care interventions, such as a provider education series, implementation of consultation criteria, and prospective measurement of the impact of palliative care consultations on the SMR and SAIL indicators. This research could help identify which early interventions show the best efficacy, an area where research is notably lacking.25
Inpatient palliative care (IPC) consultation services have been widely adopted in US hospitals. Outcomes research has demonstrated improved quality of life (QOL) for palliative inpatients for symptom control and satisfaction with care.1-5 Families benefit from emotional support, care planning, and transitions of care.4,6-8 Outcomes, including hospital length of stay, hospital costs, and discharge dispositionalso seem to improve.9-17 The Department of Veterans Affairs (VA) provides palliative care (PC) consultation teams at its hospitals nationwide; however, few studies exist to show how a PC service is used at a VA hospital. The following study of a PC consult team at an urban VA facility provides a unique picture of how a PC team is used.
Methods
The John Cochran Division of the VA St. Louis Health Care System (VASLHCS) in Missouri is a 509-bed adult acute care hospital with medical and surgical specialties and subspecialties available for veterans, including an intensive care unit (ICU). The PC team is one of the subspecialty teams following patients after consultation and consists of a PC physician, nurse practitioner, chaplain, social worker, and psychologist.
Data Collection
This study was exempt from internal review board approval. The attending physician kept track of each IPC encounter between September 2014 and April 2016. Data were retrieved from the Computerized Patient Record System by identifying charts that included family meeting notes during the specified time. All 130 patients included in this study were followed by the PC team. Patient charts were reviewed, and information was uploaded to spreadsheets, which became the database for this study. The data included age, patient location, diagnosis, number of days between admission and PC consultation, and number of days between admission and family meeting. Other data included code status changes and discharge dispositions. Only consultations that resulted in direct patient contact were included.
The VASLHCS requires therapeutic support level (TSL), or code status, documentation by the attending physician regarding the discussion with a competent patient or valid representative if the patient is incapacitated. Levels of support are TSL I ‘‘no limitation on care,’’ TSL II ‘‘partial code,’’ that is, usually no cardiopulmonary resuscitation or do not intubate with selected medical measures to continue, and TSL III ‘‘comfort measures only.’’ If a patient’s code level changed after IPC consultation, the change is recorded.
Data Analysis
The files were purged of all unique personal health history. Because there was no control group, multivariable analyses of association were not warranted. Analysis was confined to descriptive measures.
Results
A total of 130 patients with IPC consultations were included in this retrospective study conducted from September 2014 to April 2016 (Table 1).
The scope of IPC consultations usually include medical recommendations about symptom management, discharge planning, discussion about goals of care (GOC), code status and prognosis, managing expected in-hospital expirations (deaths), and determination of hospice eligibility. Of the IPC cohort, 74% were aged > 65 years; 26.1% were aged < 65 years (Table 2).
The mean days for an initial IPC consultation following admission was 3 on the medical/surgical floors and 7 days for ICU (P = .003; 95% CI, -6.37 to 1.36).
Discussion
Although small, the proportion of patients with serious illness or multiple chronic conditions account for a disproportionately large portion of health care spending.18 Despite the high cost, evidence demonstrates that these patients receive health care of inadequate quality characterized by fragmentation, overuse, medical errors, and poor QOL. Multiple studies show that IPC consultation provides improved patient outcomes and decreased hospital costs.9-17
From a purely outcomes-based interpretation, IPC consultation was associated with 83% of patients receiving a change in code status from full code/TSL 1. The study team drew 2 main conclusions from the data: (1) The IPC consultation is an effective way to broach GOC discussion and adjust code status; and (2) These data suggest room for earlier PC involvement. Remarkably, only 3 patients (2%) expired while inpatient with full code status.
The data also provide a unique comparison of timing of PC referrals. Pantilat and colleagues published characteristics of PC consultation services in California hospitals, and on average, patients were in the hospital 5.9 days (median 5.5; SD 3.3) prior to referral.19 This study’s average number of days for initial IPC consultation following admission was 3 days on the medical/surgical floors and 7 days in the ICU. Both time frames seem reasonable but again indicate some potential improvement for earlier IPC utilization.
Although the time frame of the intervention limited the number of patients in this study, early PC consultations in the acute care setting are a helpful intervention for veterans and families to better understand the complexity of their medical condition and prognosis and allow for a frank and open discussion about realistic goals. The importance of these discussions also were reflected in the high percentage of patients transitioning to hospice level of care (80%) and the low number of patients who remained full code (3 of 130). Other studies have shown conflicting results when interventions have been exclusively for cancer patients. In this study, 45% of patients were admitted with diagnoses other than cancer compared with 24% of patients with related diagnoses in a study by Gonsalves and colleagues.20
In this study, the majority (71.6%) of family meetings were held only with family (no patient involvement), resulting in missed opportunities for earlier patient and PC involvement especially for those patients with serious medical illnesses.
A systematic review published by Wendler and colleagues found that surrogate decision makers often find that role troubling and traumatizing even with advance directive documents.21 Earlier identification and PC consultations could initiate discussions between patients and their loved ones to decide “when enough is enough,” and about whether or not to prolong the dying process, when compatible with the patient’s wishes.
Early PC consultations also could highlight a potential highly vulnerable population of medically unbefriended patients (elder orphans). These patients may have no one in their lives to act as surrogate decision makers. This situation calls for further interventions regarding early identification of these patients and better processes to assist in their decision making. Many physicians believe it is not appropriate to begin advance directive planning on an outpatient basis. However, multiple studies have shown that patients want their doctors to discuss advance care planning with them before they become ill.22 Many other doctors have shown a positive response from patients when advance directive discussions are held during outpatient visits.23
The goals of this study were to evaluate the effectiveness of IPC consultation on goals of care and to address code status with patients and their families. Along with these conversations, the study team provided comprehensive PC evaluation. The PC team focused on providing excellent symptom management. The team of PC physicians, pain specialists, pain pharmacists, a chaplain, psychologists, and social workers addressed all the bio-psycho-social needs of patients/families and provided comprehensive recommendations. This multidimensional approach has gained significant acceptance.24
At VASLHCS, the program has grown to about 600 new consults per year, with a dedicated inpatient hospice unit, daily outpatient clinic, and myriad learning opportunities for trainees; the center has become a main site of rotation for hospice and palliative care fellows from training programs in St. Louis.
Utilization of PC consultation to help meeting the veterans’ needs at the bio-psycho-social level will also provide a benefit for the facility as it will decrease observed/expected standardized mortality ratio (SMR) data. This reduction of SMR data will be a result of successful patient transitions to hospice level of care at least 12 months prior to their passing or if their level of care is changed to inpatient hospice after they are admitted, the patients won’t be included as acute care mortality. However, with this initial small group of patients it was not possible to retrospectively calculate the impact on SMR or SAIL (Strategic Analytics for Improvement and Learning) indicators. The long-term expectation is to have a positive impact on those indicators represented by decreased inpatient mortality and improved SAIL.
Limitations
This study was a single-institution study, but every institution has its own internal culture. The team did not have a concurrent or historic control for comparison or use a questionnaire for patients and families rating their satisfaction.
Conclusion
This study provides multiple future directions of research as the authors now have baseline data about how the service is used. Future areas of interest would be to study the effectiveness of early palliative care interventions, such as a provider education series, implementation of consultation criteria, and prospective measurement of the impact of palliative care consultations on the SMR and SAIL indicators. This research could help identify which early interventions show the best efficacy, an area where research is notably lacking.25
1. El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9(3):87-94.
2. Higginson IJ, Finlay I, Goodwin DM, et al. Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage. 2002;23(2):96-106.
3. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190.
4. Benzar E, Hansen L, Kneitel AW, Fromme EK. Discharge planning for palliative care patients: a qualitative analysis. J Palliat Med. 2011;14(1):65-69.
5. Enguidanos S, Housen P, Penido M, Mejia B, Miller JA. Family members’ perceptions of inpatient palliative care consult services: a qualitative study. Palliat Med. 2014;28(1):42-48.
6. Granda-Cameron C, Viola SR, Lynch MP, Polomano RC. Measuring patient-oriented outcomes in palliative care: functionality and quality of life. Clin J Oncol Nurs. 2008;12(1):65-77.
7. Chand P, Gabriel T, Wallace CL, Nelson CM. Inpatient palliative care consultation: describing patient satisfaction. Perm J. 2013;17(1):53-55.
8. Tangeman JC, Rudra CB, Kerr CW, Grant PC. A hospice-hospital partnership: reducing hospitalization costs and 30-day readmissions among seriously ill adults. J Palliat Med. 2014;17(9):1005-1010.
9. Fromme EK, Bascom PB, Smith MD, et al. Survival, mortality, and location of death for patients seen by a hospital-based palliative care team. J Palliat Med. 2006;9(4):903-911.
10. Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973-979.
11. Ranganathan A, Dougherty M, Waite D, Casarett D. Can palliative home care reduce 30-day readmissions? Results of a propensity score matched cohort study. J Palliat Med. 2013;16(10):1290-1293.
12. Starks H, Wang S, Farber S, Owens DA, Curtis JR. Cost savings vary by length of stay for inpatients receiving palliative care consultation services. J Palliat Med. 2013;16(10):1215-1220.
13. Goldenheim A, Oates D, Parker V, Russell M, Winter M, Silliman RA. Rehospitalization of older adults discharged to home hospice care. J Palliat Med. 2014;17(7):841-844.
14. May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med. 2014;17(9):1054-1063.
15. Granda-Cameron C, Behta M, Hovinga M, Rundio A, Mintzer D. Risk factors associated with unplanned hospital readmissions in adults with cancer. Oncol Nurs Forum. 2015;42(3):e257-e268.
16. Brody AA, Ciemins E, Newman J, Harrington C. The effects of an inpatient palliative care team on discharge disposition. J Palliat Med. 2010;13(5):541-548.
17. Penrod JD, Deb P, Luhrs C, et al. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9(4):855-860.
18. Aldridge MD, Kelley AS. Appendix E, Epidemiology of serious illness and high utilization of health care. In: Institute of Medicine, Committee on Approaching Death: Addressing Key End of Life Issues. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
19. Pantilat SZ, Kerr KM, Billings JA, Bruno KA, O’Riordan DL. Characteristics of palliative care consultation services in California hospitals. J Palliat Med. 2012;15(5):555-560.
20. Gonsalves WI, Tashi T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veterans Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
21. Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336-346.
22. American Bar Association Commission on Law and Aging. Myths and facts about health care advance directives. https://www.americanbar.org/content/dam/aba/publications/bifocal/BIFOCALSept-Oct2015.authcheckdam.pdf. Accessed July 10, 2018.
23. Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou X-H, Wolinsky FD. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16(1):32-40.
24. Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life’s end in inpatient settings: the BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
25. Dalgaard K, Bergenholtz H, Nielsen M, Timm H. Early integration of palliative care in hospitals: a systematic review on methods, barriers, and outcome. Palliat Support Care. 2014;12(6):495-513.
1. El-Jawahri A, Greer JA, Temel JS. Does palliative care improve outcomes for patients with incurable illness? A review of the evidence. J Support Oncol. 2011;9(3):87-94.
2. Higginson IJ, Finlay I, Goodwin DM, et al. Do hospital-based palliative teams improve care for patients or families at the end of life? J Pain Symptom Manage. 2002;23(2):96-106.
3. Gade G, Venohr I, Conner D, et al. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180-190.
4. Benzar E, Hansen L, Kneitel AW, Fromme EK. Discharge planning for palliative care patients: a qualitative analysis. J Palliat Med. 2011;14(1):65-69.
5. Enguidanos S, Housen P, Penido M, Mejia B, Miller JA. Family members’ perceptions of inpatient palliative care consult services: a qualitative study. Palliat Med. 2014;28(1):42-48.
6. Granda-Cameron C, Viola SR, Lynch MP, Polomano RC. Measuring patient-oriented outcomes in palliative care: functionality and quality of life. Clin J Oncol Nurs. 2008;12(1):65-77.
7. Chand P, Gabriel T, Wallace CL, Nelson CM. Inpatient palliative care consultation: describing patient satisfaction. Perm J. 2013;17(1):53-55.
8. Tangeman JC, Rudra CB, Kerr CW, Grant PC. A hospice-hospital partnership: reducing hospitalization costs and 30-day readmissions among seriously ill adults. J Palliat Med. 2014;17(9):1005-1010.
9. Fromme EK, Bascom PB, Smith MD, et al. Survival, mortality, and location of death for patients seen by a hospital-based palliative care team. J Palliat Med. 2006;9(4):903-911.
10. Penrod JD, Deb P, Dellenbaugh C, et al. Hospital-based palliative care consultation: effects on hospital cost. J Palliat Med. 2010;13(8):973-979.
11. Ranganathan A, Dougherty M, Waite D, Casarett D. Can palliative home care reduce 30-day readmissions? Results of a propensity score matched cohort study. J Palliat Med. 2013;16(10):1290-1293.
12. Starks H, Wang S, Farber S, Owens DA, Curtis JR. Cost savings vary by length of stay for inpatients receiving palliative care consultation services. J Palliat Med. 2013;16(10):1215-1220.
13. Goldenheim A, Oates D, Parker V, Russell M, Winter M, Silliman RA. Rehospitalization of older adults discharged to home hospice care. J Palliat Med. 2014;17(7):841-844.
14. May P, Normand C, Morrison RS. Economic impact of hospital inpatient palliative care consultation: review of current evidence and directions for future research. J Palliat Med. 2014;17(9):1054-1063.
15. Granda-Cameron C, Behta M, Hovinga M, Rundio A, Mintzer D. Risk factors associated with unplanned hospital readmissions in adults with cancer. Oncol Nurs Forum. 2015;42(3):e257-e268.
16. Brody AA, Ciemins E, Newman J, Harrington C. The effects of an inpatient palliative care team on discharge disposition. J Palliat Med. 2010;13(5):541-548.
17. Penrod JD, Deb P, Luhrs C, et al. Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med. 2006;9(4):855-860.
18. Aldridge MD, Kelley AS. Appendix E, Epidemiology of serious illness and high utilization of health care. In: Institute of Medicine, Committee on Approaching Death: Addressing Key End of Life Issues. Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: National Academies Press; 2015.
19. Pantilat SZ, Kerr KM, Billings JA, Bruno KA, O’Riordan DL. Characteristics of palliative care consultation services in California hospitals. J Palliat Med. 2012;15(5):555-560.
20. Gonsalves WI, Tashi T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veterans Affairs cancer population. J Palliat Med. 2011;14(11):1231-1235.
21. Wendler D, Rid A. Systematic review: the effect on surrogates of making treatment decisions for others. Ann Intern Med. 2011;154(5):336-346.
22. American Bar Association Commission on Law and Aging. Myths and facts about health care advance directives. https://www.americanbar.org/content/dam/aba/publications/bifocal/BIFOCALSept-Oct2015.authcheckdam.pdf. Accessed July 10, 2018.
23. Tierney WM, Dexter PR, Gramelspacher GP, Perkins AJ, Zhou X-H, Wolinsky FD. The effect of discussions about advance directives on patients’ satisfaction with primary care. J Gen Intern Med. 2001;16(1):32-40.
24. Bailey FA, Williams BR, Woodby LL, et al. Intervention to improve care at life’s end in inpatient settings: the BEACON trial. J Gen Intern Med. 2014;29(6):836-843.
25. Dalgaard K, Bergenholtz H, Nielsen M, Timm H. Early integration of palliative care in hospitals: a systematic review on methods, barriers, and outcome. Palliat Support Care. 2014;12(6):495-513.
Cognitive Behavioral Therapy for Veterans With Tinnitus
Chronic tinnitus is defined as nonsensical, persistent sound in the head or ears with no external sound source that persists for more than 6 months.1 It is most commonly associated with sound trauma, aging, head injury, and damage to the ear structures.2 Tinnitus affects up to 30% of military veterans, a prevalence rate that is twice that of the nonveteran population.3 It also is the most common service-connected disability for veterans.4 In 2016, more than 1.6 million veterans had service-connected tinnitus.
Clinical management of tinnitus is the purview of audiologists, although their role in providing this service is not well defined.5 Following an audiologic evaluation for hearing loss, devices such as hearing aids, ear-level sound generators, or sounds played through speakers may be prescribed. However, effectiveness of these devices has been shown only when coupled with counseling.6 Counseling provided by audiologists often includes education about tinnitus etiology, maintaining hearing health, and use of sound to manage tinnitus. Length, content of care, and follow-up services vary among audiologists.
Given the importance of counseling and the added complexities of mental health and behavioral health comorbidities (eg, depression, anxiety, sleep disorders), various psychological therapies delivered by mental health specialists for tinnitus management have been explored.7-11 In fact, only psychological therapies have been documented to be efficacious for mitigating the negative effects of tinnitus on sleep, concentration, communication, and emotions.12 Among these approaches, cognitive behavioral therapy (CBT) has the strongest empirical support, particularly in terms of improving quality of life (QOL) and reducing depressive symptoms.11,12 Cognitive behavioral therapy for tinnitus is derived from social cognitive theory (SCT) and modeled after CBT for depression, anxiety, pain, and insomnia.11,13-15
Cognitive behavioral therapy helps patients with tinnitus reconceptualize the auditory problem as manageable and encourages acquisition, practice, and use of a range of specific tinnitus coping strategies to enhance perceptions of self-control and self-efficacy. Cognitive behavioral therapy involves a number of distinct therapeutic components, and there is no consensus about the efficacious components of CBT for tinnitus. For example, use of sound and purposeful exposure to tinnitus varies among providers.16 Additional questions about CBT pertain to its clinical implementation (eg, group vs individual sessions, frequency and length of sessions, in-person sessions vs delivery via telephone or Internet).
Programs offered at the Department of Veterans Affairs (VA) facilities take veteran-specific factors into account to promote optimal engagement and outcomes. Factors that differentiate veterans from civilians include (1) increased probability of low health literacy and low socioeconomic status among seniors17; (2) increased likelihood of acoustic and/or psychological trauma3,18; (3) overrepresentation of males; and (4) increased probability of mental health diagnoses.19,20 At the same time, veterans are highly diverse with respect to age, academic achievement, cultural background, medical and mental health comorbidities, and economic resources.17 In spite of this diversity, most veterans are unified by their sense of camaraderie, loyalty to country, and adherence to discipline and order.21 Peer support and other variables such as compassionate understanding of their military experiences may be especially important to consider when designing behavioral interventions for veterans.
The present phenomenologic study was motivated by the need to develop and test a veteran-specific CBT for tinnitus protocol (VET CBT-T). To examine veterans’ experiences with VET CBT-T, we designed a small pilot randomized controlled trial (RCT) of VET CBT-T comparing it to structured audiologist counseling (AC). A mixed quantitative and qualitative approach was employed, including assessing veterans’ acceptance of care, identifying aspects that can be modified to improve outcomes, and obtaining feasibility data to guide refinements to VET CBT-T to inform a larger RCT.
Methods
The study used a single-blind, randomized, parallel treatment (VET CBT-T vs AC) concurrent design complemented by collection of qualitative data. This study was approved by the institutional review board at the VA Connecticut Healthcare System (VACHS) and was registered at ClinicalTrials.gov (NCT00724152).
Measures
Four standardized measures were administered to potential participants. Callers were screened for eligibility by the research coordinator using Section A of the Tinnitus and Hearing Survey (THS), which had been developed to screen candidates for tinnitus studies by phone.22 The THS contains 3 sections to identify problems related to tinnitus (Section A), hearing (Section B), and sound tolerance (Section C). Section A contains 4 items, each with a possible score of 0 to 4, which identifies tinnitus problems. Callers who were veterans and who met the necessary cutoff of 4 out of 16 possible points on Section A were invited to meet in-person with the research coordinator to obtain written informed consent and conduct a thorough assessment of eligibility.23
Three additional assessment measures were then administered sequentially to determine eligibility. The first 2 of these measures were readministered to eligible candidates who agreed to participate in the study and were used to examine outcomes:
(1) Tinnitus Handicap Inventory (THI): The 25-item THI provides an index score (0-100), with higher scores reflecting poorer QOL and more perceived functional limitations due to tinnitus.24 Candidates with scores of ≤ 19 were excluded.
(2) Tinnitus Reaction Questionnaire (TRQ): The TRQ measures perceived impact of tinnitus on QOL with emphasis on emotional consequences of tinnitus.25 Higher scores indicate more severe impact. Scores of ≥ 17 points purportedly indicate significant tinnitus disturbance. Candidates scoring < 17 were excluded.
(3) Structured Clinical Interview for Diagnosis, Abbreviated-Interview/Nonpatient (SCIDa-I/NP): The SCIDa-I/NP is a measure used to assess symptoms of psychopathology.26 Candidates with a lifetime history of psychosis were excluded.
Interventions
Each of the interventions was offered using 6 group sessions, with 2 or more participants, on an approximate weekly schedule.
VET CBT-T. Two primary texts served as resources for developing the VET CBT-T protocol: (1) the Psychological Management of Chronic Tinnitus: A Cognitive-Behavioral Approach14; and (2) a manual for providing CBT for the treatment of chronic pain.27,28 Draft materials were developed by the clinical psychologist, including a clinician’s manual for providing VET CBT-T. Handouts were provided to each veteran. Components were compared to another unpublished CBT for tinnitus workbook.10
Key components targeted psychological difficulties including sleep disturbance, reduced functioning, low mood, nervousness, reduced pleasure from activities, and negative changes in relationships. The protocol emphasized basic information about tinnitus; relationships among tinnitus, acoustic trauma, and mental health symptoms; veterans’ sense of camaraderie and loyalty; and goal setting, making use of discipline and order veterans may desire.
To build rapport, the psychologist used reflective listening, encouraged interactive dialogue, and promoted participant understanding. Since tinnitus is thought to be exacerbated by stress, participants learned ways to manage stress and practiced relaxation exercises. Participants learned to create individualized, intersession goals that were realistic, specific, and measurable to promote self-efficacy. They also learned to list and increase pleasant activities to distract from tinnitus, improve QOL, and enable behavioral activation. Motivational interviewing addressed readiness, ambivalence, and resistance to achieving these specific goals. Participants also learned to identify and modify unhelpful thoughts about tinnitus (ie, cognitive restructuring).
Audiologist Counseling (AC). To control for patient contact in VET CBT-T, the study audiologist created a 6-session counseling protocol. The AC emphasized education about tinnitus and available tinnitus interventions. Participants were encouraged to ask questions, share their tinnitus experiences, and cope with tinnitus by avoiding silence.
Quantitative Analysis
To assess baseline between-group differences, pretreatment (t1;immediately prior to attending the first group session) administrations of the THI and TRQ were compared using t tests. Demographics between groups were compared using the chi-square test.
The THI and TRQ were readministered following completion of the last group session (t2) and about 8 weeks after the last group session (t3). Because of the small sample size, required assumptions for analyzing parametric tests of linear effects were not met. Thus, only descriptive results and mean differences in scores on the THI and TRQ between these 3 assessment periods are presented. SPSS PASW Statistics 18.0 (Hong Kong, China) was used for analyses.
Qualitative Analysis
VET CBT-T. After each VET CBT-T session, veterans’ acceptance of the protocol was assessed using 4 questions: (1) Was the information presented to you today easy to understand? Yes/No. If no, why not? (2) Were the examples (if there were any) useful? Yes/No. If no, why not? (3) Was the way the information was presented by the group leader helpful? Yes/No. If no, why not? and (4) Please tell us what you thought about today’s group. Participants were encouraged to be honest and provide detailed feedback. To facilitate unbiased responding, the group leader left the room while a research assistant collected the comments.
Participants’ experiences and acceptance of the interventions were explored using a stepwise content analysis approach.29,30First, the feedback data were prepared for analysis by entering verbatim responses into a spreadsheet. Next, these responses were reviewed by the clinical psychologist who identified themes. Examination of data involved qualitative analyses in which the phenomenon of interest was veterans’ acceptance of the interventions. This process presumed that most veterans would respond favorably to the intervention and, thus, acknowledged that the clinical psychologist designed and delivered the intervention.
Any contrary or negative comments were flagged. However, neutral and positive comments also were analyzed and tallied. Notations within each comment were used to calculate the number of occurrences of themes. Similar themes with few responses were collapsed into a single theme when appropriate. Final themes and tallies were shared with an auditor familiar with tinnitus, psychology, and qualitative methods who made comments and checked tallies within each theme. The themes were revised based on this audit and retallied. The clinical psychologist then summarized the themes in text, which was reviewed by the auditor for accuracy in capturing the important emergent themes.
Next, themes were used to examine typed verbatim transcripts from the second and fifth sessions of the intervention. Thematic content derived from the above feedback was extracted from the transcripts by the clinical psychologist. Then the study audiologist read the transcripts to confirm or reject these comments as relevant to the themes. Additional comments were nominated by the study audiologist and reviewed by the clinical psychologist who finalized feedback thought to best represent the themes.
Results
One hundred ninety-six persons inquired about the study (Figure 1).
After being deemed eligible by the otologist as having tinnitus associated with sound trauma, the remaining 25 eligible and interested candidates were randomized into the 2 treatment arms. To reduce attrition, formation of groups took into account participants’ preferred appointment days and times. When 3 or more participants were allocated to a treatment arm, group sessions were scheduled. Five (20%) participants were lost to attrition, including 1 randomized to VET CBT-T and 2 to AC who dropped out prior to receiving any intervention, and 2 randomized to VET CBT-T who dropped out after attending 1 session. Reasons stated for dropping out included concerns they would not learn new information, inability to tolerate sound in a group, unexpected moves out of the area, lack of time, loss of interest, and family emergencies.
Quantitative Analysis
Participants in the 2 treatment arms did not differ significantly on the pretreatment (t1) THI (t = 1.39, df = 18, P = .18) or TRQ (t = 0.99, df = 18, P = .33) scores. Differences in THI scores were computed from pre- to posttreatment (VET CBT-Tt1−t2 = 6.2; ACt1−t2 = 10.0) and from pretreatment to 8-week follow-up (VET CBT-Tt1−t3 = 4.4; ACt1−t3 = 9.1). Similarly, differences in mean TRQ index scores were computed from pre- to posttreatment (VET CBT-Tt1−t2 = 5.3; ACt1−t2 = 7.6) and from pretreatment to 8 weeks follow-up (VET CBT-Tt1−t3 = 1.5; ACt1−t3 = 5.6). These mean differences reveal consistent reductions in mean index scores (improved tinnitus-related QOL) between time points for each treatment arm, but do not indicate statistically or clinically significant reductions of distress.
Qualitative Analysis
The 89 comments that participants provided after the 18 VET CBT-T sessions (3 series of 6 sessions each) were mostly positive (67 responses, 75%) (eTable 2).
Components of VET CBT-T and Number of Sessions (n = 53)
Psychoeducation and Sound Enrichment. Participants generally appreciated the psychoeducation component of VET CBT-T, including basic information about the prevalence of tinnitus, mechanics of the ear and hearing, and how an enriched sound environment may assist with coping (5 comments).
Stress Reduction. Two of the 14 participants who commented on the stress reduction session (via relaxation exercises) noted that it was helpful in their lives overall but “had no effect on tinnitus.”
Goal Setting. Nine participants noted that they valued the goal-setting strategies and long-term relapse planning, one writing that he was “…glad to be encouraged to document and set specific, documented goals each week.”
Cognitive Restructuring and Acceptance. Participants reported that the cognitive restructuring session was important for “accepting” their tinnitus (9 comments). Overall cognitive restructuring appeared to be well received by participants as a concept when learning ways to cope with tinnitus.
Distraction. Of the 8 comments that regarded the increasing-pleasant-activities session, 1 participant reported that the pleasant activities worksheet had a female bias and that activities on one of the worksheets “were not helpful and overlapped too much.” A participant suggested that group leaders allow participants to brainstorm pleasant activities using an open-ended format instead of using categories of pleasant activities. Participants generally found the increasing-pleasant-activities component very beneficial for managing tinnitus.
Self-Hypnosis and Exposure Therapy. Two components not presented during VET CBT-T were identified by participants as potentially desirable. One participant suggested providing information on self-hypnosis and another stated that he focuses on his tinnitus to cope, much like exposure therapy for tinnitus.
Number of Sessions. One participant wrote after the last (sixth) session that he was “glad it’s over” possibly suggesting the intervention was too long. Another participant stated at the fifth session, “I basically want to get out of here—out of this, out of these meetings.” Conversely a participant stated, “I was kind of hoping there was one more after next week.” Another participant explained the intervention itself can be in conflict with its own stated goals of attending less to tinnitus.
Leader, Materials, and Presentations (n = 16).
Overall, the participants had positive experiences (15 comments) with their study therapist. They noted that the group leader was “flexible” and “did a great job of facilitating discussion.” One participant commented on the need for better visual presentations of the information and the need for the group leader to “use her own words” rather than reading the content.
Previous Use of CBT Skills (n = 5).
Five participants noted that information presented was “not new” and that they had acquired the coping skills spontaneously years earlier. Two expressed relief that some of the ways they had been dealing with their tinnitus prior to the intervention were actually recommended. One discussed that reviewing skills that he had already implemented was helpful.
Hope, Anger, and Mental Health (n = 8).
Three participants indicated the intervention gave them “hope” that they will learn ways to cope with tinnitus. One noted that the discussion regarding depression as a commonly co-occurring condition with tinnitus should include a better description of depressive symptoms. Two expressed relief to receive help for their frustration and anger resulting from tinnitus, and 1 participant discussed the added frustration of hearing loss with tinnitus. Excessive alcohol use to cope with tinnitus and the comorbidity of tinnitus and PTSD also were discussed.
Group Cohesiveness (n = 8)/Discord (n = 2).
Many group members commented that they enjoyed listening to information about tinnitus and sharing their experiences. There was friendly interaction and discussion among most of the participants. They commented that it was informative to see that others were coming forward for help and were interested to hear others’ experiences. The group format was mostly welcomed and appreciated. However, group discord occurred when topics other than tinnitus were shared, such as recovery from substance abuse. Two feedback comments indicated that this discussion was unwelcomed.
Discussion
This pilot study provides evidence that at least some of the veterans who were eligible and participated accrued benefit from either AC or VET CBT-T. There also is evidence that a structured and intensive counseling approach by an audiologist that does not include sound therapy is beneficial. Participants generally found that the interventions were acceptable for understanding basic information about tinnitus, and those in the VET CBT-T treatment arm reported improvements in problem solving and coping.
The planned qualitative analyses enabled in-depth examination of intervention feedback from participants and revealed important themes for modifying the VET CBT-T intervention, which occurred following completion of this pilot study. Further, these themes echo the success in creating a unique, veteran-centric, CBT intervention for tinnitus management.
Primarily, participants indicated that education about tinnitus prevalence, etiology, and sound enrichment assisted in coping with tinnitus. This theme also reflects the protocol’s emphasis on health literacy. The wide availability of misinformation on tinnitus, along with the potential for monetary scams, underlies the need for well-designed and research-informed tinnitus education programs. Helping veterans distinguish facts and myths is a potentially important element of tinnitus management. Experiences coping with trauma were salient as participants diverged in their opinions regarding the appropriateness of discussing these issues during a tinnitus management group.
Specific themes emerged regarding the relevance of posttraumatic stress disorder (PTSD) symptoms and substance use to cope with tinnitus. Comfort levels when hearing and discussing these symptoms varied among participants. Due to the high comorbidities of mental health disorders and tinnitus, this theme is important to consider when designing tinnitus management programs. Other qualitative themes served to validate peer support and that the veteran-centric protocol was acceptable, including positive regard for goal setting, indicating that adherence to discipline and order had been addressed.
Identified VET CBT-T Modifications
There were multiple indicators that the rationale for sessions needed to be clarified. Participants highlighted the need to hear clear expectations about what the coping skills would address and multiple reminders regarding the goals of relaxation exercises for tinnitus. Some expressed concern about their lack of concentration during the relaxation exercises. A concern that was discussed with participants only during the informed consent process was the possibility that the intervention could increase focus on tinnitus and, thus, temporarily increase tinnitus distress while receiving the intervention.
Need for Interdisciplinary Care
Perhaps the fact that both interventions were beneficial is not surprising since AC as delivered in this study was designed to be an active intervention that incorporated education and support, including some components similar to those included in VET CBT-T. It appears that AC designed to match the CBT intervention in terms of number of sessions among other enhancements suggests that this intervention may be efficacious. The AC intervention offered support and education about tinnitus; however, sound therapy was not provided despite some empirical support for its use.31 Future research should examine whether sound therapy adds benefit to an intensive and structured AC approach.
It has been proposed that a combination of CBT delivered by a mental health provider and AC should be implemented as an optimal tinnitus management protocol.31,32 Results of this and a number of other recent studies 16,32,33 encourage an integrated, interdisciplinary approach.
Findings of the present study were applied toward development of a hierarchical and interdisciplinary tinnitus management protocol, Progressive Tinnitus Management (PTM).22,34 Components of VET CBT-T were selected for the intervention provided with PTM, modified as per the feedback from participants in this study. This approach was combined with components of the AC intervention, further complemented by sound therapy. The new combined protocol was tested in a pilot telephone study of PTM for veterans and military members with positive results.32 Two VA-supported RCTs of PTM have since been completed.35,36 Results indicate that relative to wait list control, PTM is effective in mitigating negative effects of tinnitus.
Last, after this pilot study, the protocol was modified to address participants’ concerns that their primary care and other providers were unaware that interventions for tinnitus exist. Providers offering tinnitus care are now encouraged to share its availability with veterans and other clinicians. This study provides compelling evidence that veterans respond to messages of hope from providers that they do not needlessly have to suffer alone. Rather than telling veterans to “just live with it,” primary care providers should be able to offer ideas and services for learning how to live with tinnitus.
Future Directions
Future revisions of VET CBT-T could incorporate “acceptance” as a concept for coping with tinnitus, components such as self-hypnosis and exposure therapy, and strategies for coping with hearing loss and communication difficulties. Reexamination of the number and length of sessions also is encouraged, as well as integration of evidence-based interventions for comorbid conditions such as PTSD, substance use disorders, and insomnia. Perhaps increasing the use of peer support services would help reach veterans concerned by the stigma of receiving mental health care. Some veterans were dissatisfied with hearing about others’ mental health concerns. It may be beneficial to offer tinnitus interventions to cohorts of veterans identified with specific comorbid mental health or substance use disorders. However, peer support may be more important than protecting veterans from hearing about others’ mental health concerns as social cognitive theory suggests.
Limitations
This feasibility study’s strict inclusion criteria resulted in a small but well-defined sample that may not be representative of the larger population of veterans who could potentially benefit from these interventions. The extensive evaluation requirements and eligibility requirements likely enhanced the internal validity of the trial but may have compromised the external validity and generalizability of the study findings.
While a few women were assessed during the eligibility process, unfortunately, only men were deemed eligible. It is therefore unknown how women veterans would receive this protocol. Currently there is a bias in selecting male participants for tinnitus research. It is hoped that in the future a sample of women veterans could also provide feedback on this tinnitus management protocol developed by the VHA.
The fact that a large proportion of those who made initial contact about the study decided not to follow up is not inconsistent with studies of similar psychological interventions.37 Challenges related to engaging otherwise appropriate candidates in psychological interventions for a range of chronic health concerns have been well described and may apply to tinnitus management.
Conclusion
The qualitative feedback from participants was generally positive for both protocols. Emergent themes confirmed the need for a veteran-specific intervention while highlighting individual veterans’ needs. Transcripts from sessions provided additional descriptive information that identified changes to the protocols that would improve veterans’ receipt of care. Due to the abundance of veterans with tinnitus and the needs of veterans in terms of health care delivery and receipt, an interdisciplinary CBT-plus-AC protocol was created that is specific to and accepted by veterans with tinnitus.
Refinements to the VET CBT-T protocol were identified that led to development of PTM that was the subject of another small study, which then led to the authors’ 2 larger RCTs. Attempts were made to include greater attention to the mental health concerns of veterans both in terms of education and in offering sensitive delivery of the protocol. Session content was used to create an organized, visual presentation that follows an organized workbook.22,35 Refinements to the protocol are ongoing.
Acknowledgments
This material was based on work supported by US Department of Veterans Affairs, Rehabilitation Research and Development (RR&D): (1) R.D. Kerns’ Pilot Merit Grant #C6324P and (2) C.J. (Kendall) Schmidt’s Career Development Award-1 #D6848M. The authors would like to acknowledge the assistance from Rebecca Czlapinski, MA; Kathryn LaChappelle, MPH; and Emily Thielman, MS, for the conduct of the study, data collection and management, and preparation of this manuscript.
1. Henry JA. “Measurement” of tinnitus. Otol Neurotol. 2016;37(8):e276-e285.
2. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In Snow JB, ed. Tinnitus: Theory and Management. Hamilton, Canada: BC Becker; 2004:16-41.
3. Folmer RL, Theodoroff SM, Martin WH, Shi Y. Experimental, controversial, and futuristic treatments for chronic tinnitus. J Am Acad Audiol. 2014;25(1):106-125.
4. US Department of Veterans Affairs. Veterans Benefits Administration annual benefits report fiscal year 2016. https://www.benefits.va.gov/REPORTS/abr/ABR-All_Sec tions_FY16_06292017.pdf . Accessed June 19, 2018.
5. Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12(3):170-187.
6. Hoare DJ, Searchfield GD, El Refaie A, Henry JA. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62-75.
7. Andersson G, Porsaeus D, Wiklund M, Kaldo V, Larsen HC. Treatment of tinnitus in the elderly: a controlled trial of cognitive behavior therapy. Int J Audiol. 2005;44(11):671-675.
8. Henry JL, Wilson PH. Coping with tinnitus: two studies of psychological and audiological characteristics of patients with high and low tinnitus-related distress. Int Tinnitus J. 1995;1(2):85-92.
9. Henry JL, Wilson PH. The psychological management of tinnitus: comparison of a combined cognitive educational program, education alone and a waiting-list control. Int Tinnitus J. 1996;2:9-20.
10. Robinson SK, Viirre ES, Bailey KA, et al. A randomized controlled trial of cognitive-behavior therapy for tinnitus. Int Tinnitus J. 2008;14(2):119-126.
11. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010(9):CD005233.
12. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(Suppl)(2):S1-S40.
13. Beck JS. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. New York, NY: Guilford Press; 2011.
14. Henry JL, Wilson PH. The Psychological Management of Chronic Tinnitus : A Cognitive-Behavioral Approach. Boston, MA: Allyn and Bacon; 2001.
15. Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143-164.
16. Cima RF, Andersson G, Schmidt CJ, Henry JA. Cognitive-behavioral treatments for tinnitus: a review of the literature. J Am Acad Audiol. 2014;25(1):29-61.
17. Rodriguez V, Andrade AD, García-Retamero R, et al. Health literacy, numeracy, and graphical literacy among veterans in primary care and their effect on shared decision making and trust in physicians. J Health Commun. 2013;18(suppl 1):273-289.
18. Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002-2008. Am J Public Health. 2009;99(9):1651-1658.
19. Thomas JL, Wilk JE, Riviere LA, et al. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614-623.
20. Zivin K, McCarthy JF, McCammon RJ, et al. Health-related quality of life and utilities among patients with depression in the Department of Veterans Affairs. Psychiatr Serv. 2008;59(11):1331-1334.
21. Costa DL, Kahn ME. Health, wartime stress, and unit cohesion: evidence from Union Army veterans. Demography. 2010;47(1):45-66.
22. Henry JA, Zaugg TL, Myers PJ, Kendall (Schmidt) CJ. Progressive Tinnitus Management: Clinical Handbook for Audiologists. San Diego, CA: Plural Publishing; 2010.
23. Henry JA, Schechter MA, Loovis CL, et al. Clinical management of tinnitus using a “progressive intervention” approach. J Rehabil Res Dev. 2005;42(4 suppl 2):95-116.
24. Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148.
25. Wilson PH, Henry JL, Bowen M, Haralambous G. Tinnitus Reaction Questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res. 1991;34(1):197-201.
26. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Clinical Version, Administration Booklet. Washington, DC: American Psychiatric Press; 2012.
27. Kerns RD, Thorn BE, Dixon KE. Psychological treatments for persistent pain: an introduction. J Clin Psychol. 2006;62(11):1327-1331.
28. Otis JD. Managing Chronic Pain: A Cognitive-Behavioral Therapy Approach. New York, NY: Oxford; 2007.
29. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
30. Mertens DM. Research and Evaluation in Education and Psychology: Integrating Diversity With Quantitative, Qualitative, and Mixed Methods. 3rd ed. Los Angeles, CA: Sage; 2009 .
31. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
32. Henry JA, Zaugg TL, Myers PJ, et al. Pilot study to develop telehealth tinnitus management for persons with and without traumatic brain injury. J Rehabil Res Dev. 2012;49(7):1025-1042.
33. Weise C, Heinecke K, Rief W. Biofeedback-based behavioral treatment for chronic tinnitus: results of a randomized controlled trial. J Consult Clin Psychol. 2008;76(6):1046-1057.
34. Henry JA, Zaugg TL, Myers PJ, Kendall (Schmidt) CJ. How to Manage Your Tinnitus: A Step-by-Step Workbook, 3rd ed. San Diego, CA: Plural Publishing; 2010.
35. Henry JA, Thielman EJ, Zaugg TL, et al. Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with Progressive Tinnitus Management. J Speech Lang Hear Res. 2017;60(5):1378-1397.
36. Henry, JA, Thielman, E, Zaugg, et al. Telephone-based progressive tinnitus management for persons with and without traumatic brain injury: a randomized controlled trial. Ear Hear. 2018. [Epub ahead of print.]
37. Chang MW, Nitzke S, Brown R, et al. Recruitment challenges and enrollment observations from a community based intervention (Mothers In Motion) for low-income overweight and obese women. Contemp Clin Trials Commun. 2017;5:26-33.
Chronic tinnitus is defined as nonsensical, persistent sound in the head or ears with no external sound source that persists for more than 6 months.1 It is most commonly associated with sound trauma, aging, head injury, and damage to the ear structures.2 Tinnitus affects up to 30% of military veterans, a prevalence rate that is twice that of the nonveteran population.3 It also is the most common service-connected disability for veterans.4 In 2016, more than 1.6 million veterans had service-connected tinnitus.
Clinical management of tinnitus is the purview of audiologists, although their role in providing this service is not well defined.5 Following an audiologic evaluation for hearing loss, devices such as hearing aids, ear-level sound generators, or sounds played through speakers may be prescribed. However, effectiveness of these devices has been shown only when coupled with counseling.6 Counseling provided by audiologists often includes education about tinnitus etiology, maintaining hearing health, and use of sound to manage tinnitus. Length, content of care, and follow-up services vary among audiologists.
Given the importance of counseling and the added complexities of mental health and behavioral health comorbidities (eg, depression, anxiety, sleep disorders), various psychological therapies delivered by mental health specialists for tinnitus management have been explored.7-11 In fact, only psychological therapies have been documented to be efficacious for mitigating the negative effects of tinnitus on sleep, concentration, communication, and emotions.12 Among these approaches, cognitive behavioral therapy (CBT) has the strongest empirical support, particularly in terms of improving quality of life (QOL) and reducing depressive symptoms.11,12 Cognitive behavioral therapy for tinnitus is derived from social cognitive theory (SCT) and modeled after CBT for depression, anxiety, pain, and insomnia.11,13-15
Cognitive behavioral therapy helps patients with tinnitus reconceptualize the auditory problem as manageable and encourages acquisition, practice, and use of a range of specific tinnitus coping strategies to enhance perceptions of self-control and self-efficacy. Cognitive behavioral therapy involves a number of distinct therapeutic components, and there is no consensus about the efficacious components of CBT for tinnitus. For example, use of sound and purposeful exposure to tinnitus varies among providers.16 Additional questions about CBT pertain to its clinical implementation (eg, group vs individual sessions, frequency and length of sessions, in-person sessions vs delivery via telephone or Internet).
Programs offered at the Department of Veterans Affairs (VA) facilities take veteran-specific factors into account to promote optimal engagement and outcomes. Factors that differentiate veterans from civilians include (1) increased probability of low health literacy and low socioeconomic status among seniors17; (2) increased likelihood of acoustic and/or psychological trauma3,18; (3) overrepresentation of males; and (4) increased probability of mental health diagnoses.19,20 At the same time, veterans are highly diverse with respect to age, academic achievement, cultural background, medical and mental health comorbidities, and economic resources.17 In spite of this diversity, most veterans are unified by their sense of camaraderie, loyalty to country, and adherence to discipline and order.21 Peer support and other variables such as compassionate understanding of their military experiences may be especially important to consider when designing behavioral interventions for veterans.
The present phenomenologic study was motivated by the need to develop and test a veteran-specific CBT for tinnitus protocol (VET CBT-T). To examine veterans’ experiences with VET CBT-T, we designed a small pilot randomized controlled trial (RCT) of VET CBT-T comparing it to structured audiologist counseling (AC). A mixed quantitative and qualitative approach was employed, including assessing veterans’ acceptance of care, identifying aspects that can be modified to improve outcomes, and obtaining feasibility data to guide refinements to VET CBT-T to inform a larger RCT.
Methods
The study used a single-blind, randomized, parallel treatment (VET CBT-T vs AC) concurrent design complemented by collection of qualitative data. This study was approved by the institutional review board at the VA Connecticut Healthcare System (VACHS) and was registered at ClinicalTrials.gov (NCT00724152).
Measures
Four standardized measures were administered to potential participants. Callers were screened for eligibility by the research coordinator using Section A of the Tinnitus and Hearing Survey (THS), which had been developed to screen candidates for tinnitus studies by phone.22 The THS contains 3 sections to identify problems related to tinnitus (Section A), hearing (Section B), and sound tolerance (Section C). Section A contains 4 items, each with a possible score of 0 to 4, which identifies tinnitus problems. Callers who were veterans and who met the necessary cutoff of 4 out of 16 possible points on Section A were invited to meet in-person with the research coordinator to obtain written informed consent and conduct a thorough assessment of eligibility.23
Three additional assessment measures were then administered sequentially to determine eligibility. The first 2 of these measures were readministered to eligible candidates who agreed to participate in the study and were used to examine outcomes:
(1) Tinnitus Handicap Inventory (THI): The 25-item THI provides an index score (0-100), with higher scores reflecting poorer QOL and more perceived functional limitations due to tinnitus.24 Candidates with scores of ≤ 19 were excluded.
(2) Tinnitus Reaction Questionnaire (TRQ): The TRQ measures perceived impact of tinnitus on QOL with emphasis on emotional consequences of tinnitus.25 Higher scores indicate more severe impact. Scores of ≥ 17 points purportedly indicate significant tinnitus disturbance. Candidates scoring < 17 were excluded.
(3) Structured Clinical Interview for Diagnosis, Abbreviated-Interview/Nonpatient (SCIDa-I/NP): The SCIDa-I/NP is a measure used to assess symptoms of psychopathology.26 Candidates with a lifetime history of psychosis were excluded.
Interventions
Each of the interventions was offered using 6 group sessions, with 2 or more participants, on an approximate weekly schedule.
VET CBT-T. Two primary texts served as resources for developing the VET CBT-T protocol: (1) the Psychological Management of Chronic Tinnitus: A Cognitive-Behavioral Approach14; and (2) a manual for providing CBT for the treatment of chronic pain.27,28 Draft materials were developed by the clinical psychologist, including a clinician’s manual for providing VET CBT-T. Handouts were provided to each veteran. Components were compared to another unpublished CBT for tinnitus workbook.10
Key components targeted psychological difficulties including sleep disturbance, reduced functioning, low mood, nervousness, reduced pleasure from activities, and negative changes in relationships. The protocol emphasized basic information about tinnitus; relationships among tinnitus, acoustic trauma, and mental health symptoms; veterans’ sense of camaraderie and loyalty; and goal setting, making use of discipline and order veterans may desire.
To build rapport, the psychologist used reflective listening, encouraged interactive dialogue, and promoted participant understanding. Since tinnitus is thought to be exacerbated by stress, participants learned ways to manage stress and practiced relaxation exercises. Participants learned to create individualized, intersession goals that were realistic, specific, and measurable to promote self-efficacy. They also learned to list and increase pleasant activities to distract from tinnitus, improve QOL, and enable behavioral activation. Motivational interviewing addressed readiness, ambivalence, and resistance to achieving these specific goals. Participants also learned to identify and modify unhelpful thoughts about tinnitus (ie, cognitive restructuring).
Audiologist Counseling (AC). To control for patient contact in VET CBT-T, the study audiologist created a 6-session counseling protocol. The AC emphasized education about tinnitus and available tinnitus interventions. Participants were encouraged to ask questions, share their tinnitus experiences, and cope with tinnitus by avoiding silence.
Quantitative Analysis
To assess baseline between-group differences, pretreatment (t1;immediately prior to attending the first group session) administrations of the THI and TRQ were compared using t tests. Demographics between groups were compared using the chi-square test.
The THI and TRQ were readministered following completion of the last group session (t2) and about 8 weeks after the last group session (t3). Because of the small sample size, required assumptions for analyzing parametric tests of linear effects were not met. Thus, only descriptive results and mean differences in scores on the THI and TRQ between these 3 assessment periods are presented. SPSS PASW Statistics 18.0 (Hong Kong, China) was used for analyses.
Qualitative Analysis
VET CBT-T. After each VET CBT-T session, veterans’ acceptance of the protocol was assessed using 4 questions: (1) Was the information presented to you today easy to understand? Yes/No. If no, why not? (2) Were the examples (if there were any) useful? Yes/No. If no, why not? (3) Was the way the information was presented by the group leader helpful? Yes/No. If no, why not? and (4) Please tell us what you thought about today’s group. Participants were encouraged to be honest and provide detailed feedback. To facilitate unbiased responding, the group leader left the room while a research assistant collected the comments.
Participants’ experiences and acceptance of the interventions were explored using a stepwise content analysis approach.29,30First, the feedback data were prepared for analysis by entering verbatim responses into a spreadsheet. Next, these responses were reviewed by the clinical psychologist who identified themes. Examination of data involved qualitative analyses in which the phenomenon of interest was veterans’ acceptance of the interventions. This process presumed that most veterans would respond favorably to the intervention and, thus, acknowledged that the clinical psychologist designed and delivered the intervention.
Any contrary or negative comments were flagged. However, neutral and positive comments also were analyzed and tallied. Notations within each comment were used to calculate the number of occurrences of themes. Similar themes with few responses were collapsed into a single theme when appropriate. Final themes and tallies were shared with an auditor familiar with tinnitus, psychology, and qualitative methods who made comments and checked tallies within each theme. The themes were revised based on this audit and retallied. The clinical psychologist then summarized the themes in text, which was reviewed by the auditor for accuracy in capturing the important emergent themes.
Next, themes were used to examine typed verbatim transcripts from the second and fifth sessions of the intervention. Thematic content derived from the above feedback was extracted from the transcripts by the clinical psychologist. Then the study audiologist read the transcripts to confirm or reject these comments as relevant to the themes. Additional comments were nominated by the study audiologist and reviewed by the clinical psychologist who finalized feedback thought to best represent the themes.
Results
One hundred ninety-six persons inquired about the study (Figure 1).
After being deemed eligible by the otologist as having tinnitus associated with sound trauma, the remaining 25 eligible and interested candidates were randomized into the 2 treatment arms. To reduce attrition, formation of groups took into account participants’ preferred appointment days and times. When 3 or more participants were allocated to a treatment arm, group sessions were scheduled. Five (20%) participants were lost to attrition, including 1 randomized to VET CBT-T and 2 to AC who dropped out prior to receiving any intervention, and 2 randomized to VET CBT-T who dropped out after attending 1 session. Reasons stated for dropping out included concerns they would not learn new information, inability to tolerate sound in a group, unexpected moves out of the area, lack of time, loss of interest, and family emergencies.
Quantitative Analysis
Participants in the 2 treatment arms did not differ significantly on the pretreatment (t1) THI (t = 1.39, df = 18, P = .18) or TRQ (t = 0.99, df = 18, P = .33) scores. Differences in THI scores were computed from pre- to posttreatment (VET CBT-Tt1−t2 = 6.2; ACt1−t2 = 10.0) and from pretreatment to 8-week follow-up (VET CBT-Tt1−t3 = 4.4; ACt1−t3 = 9.1). Similarly, differences in mean TRQ index scores were computed from pre- to posttreatment (VET CBT-Tt1−t2 = 5.3; ACt1−t2 = 7.6) and from pretreatment to 8 weeks follow-up (VET CBT-Tt1−t3 = 1.5; ACt1−t3 = 5.6). These mean differences reveal consistent reductions in mean index scores (improved tinnitus-related QOL) between time points for each treatment arm, but do not indicate statistically or clinically significant reductions of distress.
Qualitative Analysis
The 89 comments that participants provided after the 18 VET CBT-T sessions (3 series of 6 sessions each) were mostly positive (67 responses, 75%) (eTable 2).
Components of VET CBT-T and Number of Sessions (n = 53)
Psychoeducation and Sound Enrichment. Participants generally appreciated the psychoeducation component of VET CBT-T, including basic information about the prevalence of tinnitus, mechanics of the ear and hearing, and how an enriched sound environment may assist with coping (5 comments).
Stress Reduction. Two of the 14 participants who commented on the stress reduction session (via relaxation exercises) noted that it was helpful in their lives overall but “had no effect on tinnitus.”
Goal Setting. Nine participants noted that they valued the goal-setting strategies and long-term relapse planning, one writing that he was “…glad to be encouraged to document and set specific, documented goals each week.”
Cognitive Restructuring and Acceptance. Participants reported that the cognitive restructuring session was important for “accepting” their tinnitus (9 comments). Overall cognitive restructuring appeared to be well received by participants as a concept when learning ways to cope with tinnitus.
Distraction. Of the 8 comments that regarded the increasing-pleasant-activities session, 1 participant reported that the pleasant activities worksheet had a female bias and that activities on one of the worksheets “were not helpful and overlapped too much.” A participant suggested that group leaders allow participants to brainstorm pleasant activities using an open-ended format instead of using categories of pleasant activities. Participants generally found the increasing-pleasant-activities component very beneficial for managing tinnitus.
Self-Hypnosis and Exposure Therapy. Two components not presented during VET CBT-T were identified by participants as potentially desirable. One participant suggested providing information on self-hypnosis and another stated that he focuses on his tinnitus to cope, much like exposure therapy for tinnitus.
Number of Sessions. One participant wrote after the last (sixth) session that he was “glad it’s over” possibly suggesting the intervention was too long. Another participant stated at the fifth session, “I basically want to get out of here—out of this, out of these meetings.” Conversely a participant stated, “I was kind of hoping there was one more after next week.” Another participant explained the intervention itself can be in conflict with its own stated goals of attending less to tinnitus.
Leader, Materials, and Presentations (n = 16).
Overall, the participants had positive experiences (15 comments) with their study therapist. They noted that the group leader was “flexible” and “did a great job of facilitating discussion.” One participant commented on the need for better visual presentations of the information and the need for the group leader to “use her own words” rather than reading the content.
Previous Use of CBT Skills (n = 5).
Five participants noted that information presented was “not new” and that they had acquired the coping skills spontaneously years earlier. Two expressed relief that some of the ways they had been dealing with their tinnitus prior to the intervention were actually recommended. One discussed that reviewing skills that he had already implemented was helpful.
Hope, Anger, and Mental Health (n = 8).
Three participants indicated the intervention gave them “hope” that they will learn ways to cope with tinnitus. One noted that the discussion regarding depression as a commonly co-occurring condition with tinnitus should include a better description of depressive symptoms. Two expressed relief to receive help for their frustration and anger resulting from tinnitus, and 1 participant discussed the added frustration of hearing loss with tinnitus. Excessive alcohol use to cope with tinnitus and the comorbidity of tinnitus and PTSD also were discussed.
Group Cohesiveness (n = 8)/Discord (n = 2).
Many group members commented that they enjoyed listening to information about tinnitus and sharing their experiences. There was friendly interaction and discussion among most of the participants. They commented that it was informative to see that others were coming forward for help and were interested to hear others’ experiences. The group format was mostly welcomed and appreciated. However, group discord occurred when topics other than tinnitus were shared, such as recovery from substance abuse. Two feedback comments indicated that this discussion was unwelcomed.
Discussion
This pilot study provides evidence that at least some of the veterans who were eligible and participated accrued benefit from either AC or VET CBT-T. There also is evidence that a structured and intensive counseling approach by an audiologist that does not include sound therapy is beneficial. Participants generally found that the interventions were acceptable for understanding basic information about tinnitus, and those in the VET CBT-T treatment arm reported improvements in problem solving and coping.
The planned qualitative analyses enabled in-depth examination of intervention feedback from participants and revealed important themes for modifying the VET CBT-T intervention, which occurred following completion of this pilot study. Further, these themes echo the success in creating a unique, veteran-centric, CBT intervention for tinnitus management.
Primarily, participants indicated that education about tinnitus prevalence, etiology, and sound enrichment assisted in coping with tinnitus. This theme also reflects the protocol’s emphasis on health literacy. The wide availability of misinformation on tinnitus, along with the potential for monetary scams, underlies the need for well-designed and research-informed tinnitus education programs. Helping veterans distinguish facts and myths is a potentially important element of tinnitus management. Experiences coping with trauma were salient as participants diverged in their opinions regarding the appropriateness of discussing these issues during a tinnitus management group.
Specific themes emerged regarding the relevance of posttraumatic stress disorder (PTSD) symptoms and substance use to cope with tinnitus. Comfort levels when hearing and discussing these symptoms varied among participants. Due to the high comorbidities of mental health disorders and tinnitus, this theme is important to consider when designing tinnitus management programs. Other qualitative themes served to validate peer support and that the veteran-centric protocol was acceptable, including positive regard for goal setting, indicating that adherence to discipline and order had been addressed.
Identified VET CBT-T Modifications
There were multiple indicators that the rationale for sessions needed to be clarified. Participants highlighted the need to hear clear expectations about what the coping skills would address and multiple reminders regarding the goals of relaxation exercises for tinnitus. Some expressed concern about their lack of concentration during the relaxation exercises. A concern that was discussed with participants only during the informed consent process was the possibility that the intervention could increase focus on tinnitus and, thus, temporarily increase tinnitus distress while receiving the intervention.
Need for Interdisciplinary Care
Perhaps the fact that both interventions were beneficial is not surprising since AC as delivered in this study was designed to be an active intervention that incorporated education and support, including some components similar to those included in VET CBT-T. It appears that AC designed to match the CBT intervention in terms of number of sessions among other enhancements suggests that this intervention may be efficacious. The AC intervention offered support and education about tinnitus; however, sound therapy was not provided despite some empirical support for its use.31 Future research should examine whether sound therapy adds benefit to an intensive and structured AC approach.
It has been proposed that a combination of CBT delivered by a mental health provider and AC should be implemented as an optimal tinnitus management protocol.31,32 Results of this and a number of other recent studies 16,32,33 encourage an integrated, interdisciplinary approach.
Findings of the present study were applied toward development of a hierarchical and interdisciplinary tinnitus management protocol, Progressive Tinnitus Management (PTM).22,34 Components of VET CBT-T were selected for the intervention provided with PTM, modified as per the feedback from participants in this study. This approach was combined with components of the AC intervention, further complemented by sound therapy. The new combined protocol was tested in a pilot telephone study of PTM for veterans and military members with positive results.32 Two VA-supported RCTs of PTM have since been completed.35,36 Results indicate that relative to wait list control, PTM is effective in mitigating negative effects of tinnitus.
Last, after this pilot study, the protocol was modified to address participants’ concerns that their primary care and other providers were unaware that interventions for tinnitus exist. Providers offering tinnitus care are now encouraged to share its availability with veterans and other clinicians. This study provides compelling evidence that veterans respond to messages of hope from providers that they do not needlessly have to suffer alone. Rather than telling veterans to “just live with it,” primary care providers should be able to offer ideas and services for learning how to live with tinnitus.
Future Directions
Future revisions of VET CBT-T could incorporate “acceptance” as a concept for coping with tinnitus, components such as self-hypnosis and exposure therapy, and strategies for coping with hearing loss and communication difficulties. Reexamination of the number and length of sessions also is encouraged, as well as integration of evidence-based interventions for comorbid conditions such as PTSD, substance use disorders, and insomnia. Perhaps increasing the use of peer support services would help reach veterans concerned by the stigma of receiving mental health care. Some veterans were dissatisfied with hearing about others’ mental health concerns. It may be beneficial to offer tinnitus interventions to cohorts of veterans identified with specific comorbid mental health or substance use disorders. However, peer support may be more important than protecting veterans from hearing about others’ mental health concerns as social cognitive theory suggests.
Limitations
This feasibility study’s strict inclusion criteria resulted in a small but well-defined sample that may not be representative of the larger population of veterans who could potentially benefit from these interventions. The extensive evaluation requirements and eligibility requirements likely enhanced the internal validity of the trial but may have compromised the external validity and generalizability of the study findings.
While a few women were assessed during the eligibility process, unfortunately, only men were deemed eligible. It is therefore unknown how women veterans would receive this protocol. Currently there is a bias in selecting male participants for tinnitus research. It is hoped that in the future a sample of women veterans could also provide feedback on this tinnitus management protocol developed by the VHA.
The fact that a large proportion of those who made initial contact about the study decided not to follow up is not inconsistent with studies of similar psychological interventions.37 Challenges related to engaging otherwise appropriate candidates in psychological interventions for a range of chronic health concerns have been well described and may apply to tinnitus management.
Conclusion
The qualitative feedback from participants was generally positive for both protocols. Emergent themes confirmed the need for a veteran-specific intervention while highlighting individual veterans’ needs. Transcripts from sessions provided additional descriptive information that identified changes to the protocols that would improve veterans’ receipt of care. Due to the abundance of veterans with tinnitus and the needs of veterans in terms of health care delivery and receipt, an interdisciplinary CBT-plus-AC protocol was created that is specific to and accepted by veterans with tinnitus.
Refinements to the VET CBT-T protocol were identified that led to development of PTM that was the subject of another small study, which then led to the authors’ 2 larger RCTs. Attempts were made to include greater attention to the mental health concerns of veterans both in terms of education and in offering sensitive delivery of the protocol. Session content was used to create an organized, visual presentation that follows an organized workbook.22,35 Refinements to the protocol are ongoing.
Acknowledgments
This material was based on work supported by US Department of Veterans Affairs, Rehabilitation Research and Development (RR&D): (1) R.D. Kerns’ Pilot Merit Grant #C6324P and (2) C.J. (Kendall) Schmidt’s Career Development Award-1 #D6848M. The authors would like to acknowledge the assistance from Rebecca Czlapinski, MA; Kathryn LaChappelle, MPH; and Emily Thielman, MS, for the conduct of the study, data collection and management, and preparation of this manuscript.
Chronic tinnitus is defined as nonsensical, persistent sound in the head or ears with no external sound source that persists for more than 6 months.1 It is most commonly associated with sound trauma, aging, head injury, and damage to the ear structures.2 Tinnitus affects up to 30% of military veterans, a prevalence rate that is twice that of the nonveteran population.3 It also is the most common service-connected disability for veterans.4 In 2016, more than 1.6 million veterans had service-connected tinnitus.
Clinical management of tinnitus is the purview of audiologists, although their role in providing this service is not well defined.5 Following an audiologic evaluation for hearing loss, devices such as hearing aids, ear-level sound generators, or sounds played through speakers may be prescribed. However, effectiveness of these devices has been shown only when coupled with counseling.6 Counseling provided by audiologists often includes education about tinnitus etiology, maintaining hearing health, and use of sound to manage tinnitus. Length, content of care, and follow-up services vary among audiologists.
Given the importance of counseling and the added complexities of mental health and behavioral health comorbidities (eg, depression, anxiety, sleep disorders), various psychological therapies delivered by mental health specialists for tinnitus management have been explored.7-11 In fact, only psychological therapies have been documented to be efficacious for mitigating the negative effects of tinnitus on sleep, concentration, communication, and emotions.12 Among these approaches, cognitive behavioral therapy (CBT) has the strongest empirical support, particularly in terms of improving quality of life (QOL) and reducing depressive symptoms.11,12 Cognitive behavioral therapy for tinnitus is derived from social cognitive theory (SCT) and modeled after CBT for depression, anxiety, pain, and insomnia.11,13-15
Cognitive behavioral therapy helps patients with tinnitus reconceptualize the auditory problem as manageable and encourages acquisition, practice, and use of a range of specific tinnitus coping strategies to enhance perceptions of self-control and self-efficacy. Cognitive behavioral therapy involves a number of distinct therapeutic components, and there is no consensus about the efficacious components of CBT for tinnitus. For example, use of sound and purposeful exposure to tinnitus varies among providers.16 Additional questions about CBT pertain to its clinical implementation (eg, group vs individual sessions, frequency and length of sessions, in-person sessions vs delivery via telephone or Internet).
Programs offered at the Department of Veterans Affairs (VA) facilities take veteran-specific factors into account to promote optimal engagement and outcomes. Factors that differentiate veterans from civilians include (1) increased probability of low health literacy and low socioeconomic status among seniors17; (2) increased likelihood of acoustic and/or psychological trauma3,18; (3) overrepresentation of males; and (4) increased probability of mental health diagnoses.19,20 At the same time, veterans are highly diverse with respect to age, academic achievement, cultural background, medical and mental health comorbidities, and economic resources.17 In spite of this diversity, most veterans are unified by their sense of camaraderie, loyalty to country, and adherence to discipline and order.21 Peer support and other variables such as compassionate understanding of their military experiences may be especially important to consider when designing behavioral interventions for veterans.
The present phenomenologic study was motivated by the need to develop and test a veteran-specific CBT for tinnitus protocol (VET CBT-T). To examine veterans’ experiences with VET CBT-T, we designed a small pilot randomized controlled trial (RCT) of VET CBT-T comparing it to structured audiologist counseling (AC). A mixed quantitative and qualitative approach was employed, including assessing veterans’ acceptance of care, identifying aspects that can be modified to improve outcomes, and obtaining feasibility data to guide refinements to VET CBT-T to inform a larger RCT.
Methods
The study used a single-blind, randomized, parallel treatment (VET CBT-T vs AC) concurrent design complemented by collection of qualitative data. This study was approved by the institutional review board at the VA Connecticut Healthcare System (VACHS) and was registered at ClinicalTrials.gov (NCT00724152).
Measures
Four standardized measures were administered to potential participants. Callers were screened for eligibility by the research coordinator using Section A of the Tinnitus and Hearing Survey (THS), which had been developed to screen candidates for tinnitus studies by phone.22 The THS contains 3 sections to identify problems related to tinnitus (Section A), hearing (Section B), and sound tolerance (Section C). Section A contains 4 items, each with a possible score of 0 to 4, which identifies tinnitus problems. Callers who were veterans and who met the necessary cutoff of 4 out of 16 possible points on Section A were invited to meet in-person with the research coordinator to obtain written informed consent and conduct a thorough assessment of eligibility.23
Three additional assessment measures were then administered sequentially to determine eligibility. The first 2 of these measures were readministered to eligible candidates who agreed to participate in the study and were used to examine outcomes:
(1) Tinnitus Handicap Inventory (THI): The 25-item THI provides an index score (0-100), with higher scores reflecting poorer QOL and more perceived functional limitations due to tinnitus.24 Candidates with scores of ≤ 19 were excluded.
(2) Tinnitus Reaction Questionnaire (TRQ): The TRQ measures perceived impact of tinnitus on QOL with emphasis on emotional consequences of tinnitus.25 Higher scores indicate more severe impact. Scores of ≥ 17 points purportedly indicate significant tinnitus disturbance. Candidates scoring < 17 were excluded.
(3) Structured Clinical Interview for Diagnosis, Abbreviated-Interview/Nonpatient (SCIDa-I/NP): The SCIDa-I/NP is a measure used to assess symptoms of psychopathology.26 Candidates with a lifetime history of psychosis were excluded.
Interventions
Each of the interventions was offered using 6 group sessions, with 2 or more participants, on an approximate weekly schedule.
VET CBT-T. Two primary texts served as resources for developing the VET CBT-T protocol: (1) the Psychological Management of Chronic Tinnitus: A Cognitive-Behavioral Approach14; and (2) a manual for providing CBT for the treatment of chronic pain.27,28 Draft materials were developed by the clinical psychologist, including a clinician’s manual for providing VET CBT-T. Handouts were provided to each veteran. Components were compared to another unpublished CBT for tinnitus workbook.10
Key components targeted psychological difficulties including sleep disturbance, reduced functioning, low mood, nervousness, reduced pleasure from activities, and negative changes in relationships. The protocol emphasized basic information about tinnitus; relationships among tinnitus, acoustic trauma, and mental health symptoms; veterans’ sense of camaraderie and loyalty; and goal setting, making use of discipline and order veterans may desire.
To build rapport, the psychologist used reflective listening, encouraged interactive dialogue, and promoted participant understanding. Since tinnitus is thought to be exacerbated by stress, participants learned ways to manage stress and practiced relaxation exercises. Participants learned to create individualized, intersession goals that were realistic, specific, and measurable to promote self-efficacy. They also learned to list and increase pleasant activities to distract from tinnitus, improve QOL, and enable behavioral activation. Motivational interviewing addressed readiness, ambivalence, and resistance to achieving these specific goals. Participants also learned to identify and modify unhelpful thoughts about tinnitus (ie, cognitive restructuring).
Audiologist Counseling (AC). To control for patient contact in VET CBT-T, the study audiologist created a 6-session counseling protocol. The AC emphasized education about tinnitus and available tinnitus interventions. Participants were encouraged to ask questions, share their tinnitus experiences, and cope with tinnitus by avoiding silence.
Quantitative Analysis
To assess baseline between-group differences, pretreatment (t1;immediately prior to attending the first group session) administrations of the THI and TRQ were compared using t tests. Demographics between groups were compared using the chi-square test.
The THI and TRQ were readministered following completion of the last group session (t2) and about 8 weeks after the last group session (t3). Because of the small sample size, required assumptions for analyzing parametric tests of linear effects were not met. Thus, only descriptive results and mean differences in scores on the THI and TRQ between these 3 assessment periods are presented. SPSS PASW Statistics 18.0 (Hong Kong, China) was used for analyses.
Qualitative Analysis
VET CBT-T. After each VET CBT-T session, veterans’ acceptance of the protocol was assessed using 4 questions: (1) Was the information presented to you today easy to understand? Yes/No. If no, why not? (2) Were the examples (if there were any) useful? Yes/No. If no, why not? (3) Was the way the information was presented by the group leader helpful? Yes/No. If no, why not? and (4) Please tell us what you thought about today’s group. Participants were encouraged to be honest and provide detailed feedback. To facilitate unbiased responding, the group leader left the room while a research assistant collected the comments.
Participants’ experiences and acceptance of the interventions were explored using a stepwise content analysis approach.29,30First, the feedback data were prepared for analysis by entering verbatim responses into a spreadsheet. Next, these responses were reviewed by the clinical psychologist who identified themes. Examination of data involved qualitative analyses in which the phenomenon of interest was veterans’ acceptance of the interventions. This process presumed that most veterans would respond favorably to the intervention and, thus, acknowledged that the clinical psychologist designed and delivered the intervention.
Any contrary or negative comments were flagged. However, neutral and positive comments also were analyzed and tallied. Notations within each comment were used to calculate the number of occurrences of themes. Similar themes with few responses were collapsed into a single theme when appropriate. Final themes and tallies were shared with an auditor familiar with tinnitus, psychology, and qualitative methods who made comments and checked tallies within each theme. The themes were revised based on this audit and retallied. The clinical psychologist then summarized the themes in text, which was reviewed by the auditor for accuracy in capturing the important emergent themes.
Next, themes were used to examine typed verbatim transcripts from the second and fifth sessions of the intervention. Thematic content derived from the above feedback was extracted from the transcripts by the clinical psychologist. Then the study audiologist read the transcripts to confirm or reject these comments as relevant to the themes. Additional comments were nominated by the study audiologist and reviewed by the clinical psychologist who finalized feedback thought to best represent the themes.
Results
One hundred ninety-six persons inquired about the study (Figure 1).
After being deemed eligible by the otologist as having tinnitus associated with sound trauma, the remaining 25 eligible and interested candidates were randomized into the 2 treatment arms. To reduce attrition, formation of groups took into account participants’ preferred appointment days and times. When 3 or more participants were allocated to a treatment arm, group sessions were scheduled. Five (20%) participants were lost to attrition, including 1 randomized to VET CBT-T and 2 to AC who dropped out prior to receiving any intervention, and 2 randomized to VET CBT-T who dropped out after attending 1 session. Reasons stated for dropping out included concerns they would not learn new information, inability to tolerate sound in a group, unexpected moves out of the area, lack of time, loss of interest, and family emergencies.
Quantitative Analysis
Participants in the 2 treatment arms did not differ significantly on the pretreatment (t1) THI (t = 1.39, df = 18, P = .18) or TRQ (t = 0.99, df = 18, P = .33) scores. Differences in THI scores were computed from pre- to posttreatment (VET CBT-Tt1−t2 = 6.2; ACt1−t2 = 10.0) and from pretreatment to 8-week follow-up (VET CBT-Tt1−t3 = 4.4; ACt1−t3 = 9.1). Similarly, differences in mean TRQ index scores were computed from pre- to posttreatment (VET CBT-Tt1−t2 = 5.3; ACt1−t2 = 7.6) and from pretreatment to 8 weeks follow-up (VET CBT-Tt1−t3 = 1.5; ACt1−t3 = 5.6). These mean differences reveal consistent reductions in mean index scores (improved tinnitus-related QOL) between time points for each treatment arm, but do not indicate statistically or clinically significant reductions of distress.
Qualitative Analysis
The 89 comments that participants provided after the 18 VET CBT-T sessions (3 series of 6 sessions each) were mostly positive (67 responses, 75%) (eTable 2).
Components of VET CBT-T and Number of Sessions (n = 53)
Psychoeducation and Sound Enrichment. Participants generally appreciated the psychoeducation component of VET CBT-T, including basic information about the prevalence of tinnitus, mechanics of the ear and hearing, and how an enriched sound environment may assist with coping (5 comments).
Stress Reduction. Two of the 14 participants who commented on the stress reduction session (via relaxation exercises) noted that it was helpful in their lives overall but “had no effect on tinnitus.”
Goal Setting. Nine participants noted that they valued the goal-setting strategies and long-term relapse planning, one writing that he was “…glad to be encouraged to document and set specific, documented goals each week.”
Cognitive Restructuring and Acceptance. Participants reported that the cognitive restructuring session was important for “accepting” their tinnitus (9 comments). Overall cognitive restructuring appeared to be well received by participants as a concept when learning ways to cope with tinnitus.
Distraction. Of the 8 comments that regarded the increasing-pleasant-activities session, 1 participant reported that the pleasant activities worksheet had a female bias and that activities on one of the worksheets “were not helpful and overlapped too much.” A participant suggested that group leaders allow participants to brainstorm pleasant activities using an open-ended format instead of using categories of pleasant activities. Participants generally found the increasing-pleasant-activities component very beneficial for managing tinnitus.
Self-Hypnosis and Exposure Therapy. Two components not presented during VET CBT-T were identified by participants as potentially desirable. One participant suggested providing information on self-hypnosis and another stated that he focuses on his tinnitus to cope, much like exposure therapy for tinnitus.
Number of Sessions. One participant wrote after the last (sixth) session that he was “glad it’s over” possibly suggesting the intervention was too long. Another participant stated at the fifth session, “I basically want to get out of here—out of this, out of these meetings.” Conversely a participant stated, “I was kind of hoping there was one more after next week.” Another participant explained the intervention itself can be in conflict with its own stated goals of attending less to tinnitus.
Leader, Materials, and Presentations (n = 16).
Overall, the participants had positive experiences (15 comments) with their study therapist. They noted that the group leader was “flexible” and “did a great job of facilitating discussion.” One participant commented on the need for better visual presentations of the information and the need for the group leader to “use her own words” rather than reading the content.
Previous Use of CBT Skills (n = 5).
Five participants noted that information presented was “not new” and that they had acquired the coping skills spontaneously years earlier. Two expressed relief that some of the ways they had been dealing with their tinnitus prior to the intervention were actually recommended. One discussed that reviewing skills that he had already implemented was helpful.
Hope, Anger, and Mental Health (n = 8).
Three participants indicated the intervention gave them “hope” that they will learn ways to cope with tinnitus. One noted that the discussion regarding depression as a commonly co-occurring condition with tinnitus should include a better description of depressive symptoms. Two expressed relief to receive help for their frustration and anger resulting from tinnitus, and 1 participant discussed the added frustration of hearing loss with tinnitus. Excessive alcohol use to cope with tinnitus and the comorbidity of tinnitus and PTSD also were discussed.
Group Cohesiveness (n = 8)/Discord (n = 2).
Many group members commented that they enjoyed listening to information about tinnitus and sharing their experiences. There was friendly interaction and discussion among most of the participants. They commented that it was informative to see that others were coming forward for help and were interested to hear others’ experiences. The group format was mostly welcomed and appreciated. However, group discord occurred when topics other than tinnitus were shared, such as recovery from substance abuse. Two feedback comments indicated that this discussion was unwelcomed.
Discussion
This pilot study provides evidence that at least some of the veterans who were eligible and participated accrued benefit from either AC or VET CBT-T. There also is evidence that a structured and intensive counseling approach by an audiologist that does not include sound therapy is beneficial. Participants generally found that the interventions were acceptable for understanding basic information about tinnitus, and those in the VET CBT-T treatment arm reported improvements in problem solving and coping.
The planned qualitative analyses enabled in-depth examination of intervention feedback from participants and revealed important themes for modifying the VET CBT-T intervention, which occurred following completion of this pilot study. Further, these themes echo the success in creating a unique, veteran-centric, CBT intervention for tinnitus management.
Primarily, participants indicated that education about tinnitus prevalence, etiology, and sound enrichment assisted in coping with tinnitus. This theme also reflects the protocol’s emphasis on health literacy. The wide availability of misinformation on tinnitus, along with the potential for monetary scams, underlies the need for well-designed and research-informed tinnitus education programs. Helping veterans distinguish facts and myths is a potentially important element of tinnitus management. Experiences coping with trauma were salient as participants diverged in their opinions regarding the appropriateness of discussing these issues during a tinnitus management group.
Specific themes emerged regarding the relevance of posttraumatic stress disorder (PTSD) symptoms and substance use to cope with tinnitus. Comfort levels when hearing and discussing these symptoms varied among participants. Due to the high comorbidities of mental health disorders and tinnitus, this theme is important to consider when designing tinnitus management programs. Other qualitative themes served to validate peer support and that the veteran-centric protocol was acceptable, including positive regard for goal setting, indicating that adherence to discipline and order had been addressed.
Identified VET CBT-T Modifications
There were multiple indicators that the rationale for sessions needed to be clarified. Participants highlighted the need to hear clear expectations about what the coping skills would address and multiple reminders regarding the goals of relaxation exercises for tinnitus. Some expressed concern about their lack of concentration during the relaxation exercises. A concern that was discussed with participants only during the informed consent process was the possibility that the intervention could increase focus on tinnitus and, thus, temporarily increase tinnitus distress while receiving the intervention.
Need for Interdisciplinary Care
Perhaps the fact that both interventions were beneficial is not surprising since AC as delivered in this study was designed to be an active intervention that incorporated education and support, including some components similar to those included in VET CBT-T. It appears that AC designed to match the CBT intervention in terms of number of sessions among other enhancements suggests that this intervention may be efficacious. The AC intervention offered support and education about tinnitus; however, sound therapy was not provided despite some empirical support for its use.31 Future research should examine whether sound therapy adds benefit to an intensive and structured AC approach.
It has been proposed that a combination of CBT delivered by a mental health provider and AC should be implemented as an optimal tinnitus management protocol.31,32 Results of this and a number of other recent studies 16,32,33 encourage an integrated, interdisciplinary approach.
Findings of the present study were applied toward development of a hierarchical and interdisciplinary tinnitus management protocol, Progressive Tinnitus Management (PTM).22,34 Components of VET CBT-T were selected for the intervention provided with PTM, modified as per the feedback from participants in this study. This approach was combined with components of the AC intervention, further complemented by sound therapy. The new combined protocol was tested in a pilot telephone study of PTM for veterans and military members with positive results.32 Two VA-supported RCTs of PTM have since been completed.35,36 Results indicate that relative to wait list control, PTM is effective in mitigating negative effects of tinnitus.
Last, after this pilot study, the protocol was modified to address participants’ concerns that their primary care and other providers were unaware that interventions for tinnitus exist. Providers offering tinnitus care are now encouraged to share its availability with veterans and other clinicians. This study provides compelling evidence that veterans respond to messages of hope from providers that they do not needlessly have to suffer alone. Rather than telling veterans to “just live with it,” primary care providers should be able to offer ideas and services for learning how to live with tinnitus.
Future Directions
Future revisions of VET CBT-T could incorporate “acceptance” as a concept for coping with tinnitus, components such as self-hypnosis and exposure therapy, and strategies for coping with hearing loss and communication difficulties. Reexamination of the number and length of sessions also is encouraged, as well as integration of evidence-based interventions for comorbid conditions such as PTSD, substance use disorders, and insomnia. Perhaps increasing the use of peer support services would help reach veterans concerned by the stigma of receiving mental health care. Some veterans were dissatisfied with hearing about others’ mental health concerns. It may be beneficial to offer tinnitus interventions to cohorts of veterans identified with specific comorbid mental health or substance use disorders. However, peer support may be more important than protecting veterans from hearing about others’ mental health concerns as social cognitive theory suggests.
Limitations
This feasibility study’s strict inclusion criteria resulted in a small but well-defined sample that may not be representative of the larger population of veterans who could potentially benefit from these interventions. The extensive evaluation requirements and eligibility requirements likely enhanced the internal validity of the trial but may have compromised the external validity and generalizability of the study findings.
While a few women were assessed during the eligibility process, unfortunately, only men were deemed eligible. It is therefore unknown how women veterans would receive this protocol. Currently there is a bias in selecting male participants for tinnitus research. It is hoped that in the future a sample of women veterans could also provide feedback on this tinnitus management protocol developed by the VHA.
The fact that a large proportion of those who made initial contact about the study decided not to follow up is not inconsistent with studies of similar psychological interventions.37 Challenges related to engaging otherwise appropriate candidates in psychological interventions for a range of chronic health concerns have been well described and may apply to tinnitus management.
Conclusion
The qualitative feedback from participants was generally positive for both protocols. Emergent themes confirmed the need for a veteran-specific intervention while highlighting individual veterans’ needs. Transcripts from sessions provided additional descriptive information that identified changes to the protocols that would improve veterans’ receipt of care. Due to the abundance of veterans with tinnitus and the needs of veterans in terms of health care delivery and receipt, an interdisciplinary CBT-plus-AC protocol was created that is specific to and accepted by veterans with tinnitus.
Refinements to the VET CBT-T protocol were identified that led to development of PTM that was the subject of another small study, which then led to the authors’ 2 larger RCTs. Attempts were made to include greater attention to the mental health concerns of veterans both in terms of education and in offering sensitive delivery of the protocol. Session content was used to create an organized, visual presentation that follows an organized workbook.22,35 Refinements to the protocol are ongoing.
Acknowledgments
This material was based on work supported by US Department of Veterans Affairs, Rehabilitation Research and Development (RR&D): (1) R.D. Kerns’ Pilot Merit Grant #C6324P and (2) C.J. (Kendall) Schmidt’s Career Development Award-1 #D6848M. The authors would like to acknowledge the assistance from Rebecca Czlapinski, MA; Kathryn LaChappelle, MPH; and Emily Thielman, MS, for the conduct of the study, data collection and management, and preparation of this manuscript.
1. Henry JA. “Measurement” of tinnitus. Otol Neurotol. 2016;37(8):e276-e285.
2. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In Snow JB, ed. Tinnitus: Theory and Management. Hamilton, Canada: BC Becker; 2004:16-41.
3. Folmer RL, Theodoroff SM, Martin WH, Shi Y. Experimental, controversial, and futuristic treatments for chronic tinnitus. J Am Acad Audiol. 2014;25(1):106-125.
4. US Department of Veterans Affairs. Veterans Benefits Administration annual benefits report fiscal year 2016. https://www.benefits.va.gov/REPORTS/abr/ABR-All_Sec tions_FY16_06292017.pdf . Accessed June 19, 2018.
5. Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12(3):170-187.
6. Hoare DJ, Searchfield GD, El Refaie A, Henry JA. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62-75.
7. Andersson G, Porsaeus D, Wiklund M, Kaldo V, Larsen HC. Treatment of tinnitus in the elderly: a controlled trial of cognitive behavior therapy. Int J Audiol. 2005;44(11):671-675.
8. Henry JL, Wilson PH. Coping with tinnitus: two studies of psychological and audiological characteristics of patients with high and low tinnitus-related distress. Int Tinnitus J. 1995;1(2):85-92.
9. Henry JL, Wilson PH. The psychological management of tinnitus: comparison of a combined cognitive educational program, education alone and a waiting-list control. Int Tinnitus J. 1996;2:9-20.
10. Robinson SK, Viirre ES, Bailey KA, et al. A randomized controlled trial of cognitive-behavior therapy for tinnitus. Int Tinnitus J. 2008;14(2):119-126.
11. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010(9):CD005233.
12. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(Suppl)(2):S1-S40.
13. Beck JS. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. New York, NY: Guilford Press; 2011.
14. Henry JL, Wilson PH. The Psychological Management of Chronic Tinnitus : A Cognitive-Behavioral Approach. Boston, MA: Allyn and Bacon; 2001.
15. Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143-164.
16. Cima RF, Andersson G, Schmidt CJ, Henry JA. Cognitive-behavioral treatments for tinnitus: a review of the literature. J Am Acad Audiol. 2014;25(1):29-61.
17. Rodriguez V, Andrade AD, García-Retamero R, et al. Health literacy, numeracy, and graphical literacy among veterans in primary care and their effect on shared decision making and trust in physicians. J Health Commun. 2013;18(suppl 1):273-289.
18. Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002-2008. Am J Public Health. 2009;99(9):1651-1658.
19. Thomas JL, Wilk JE, Riviere LA, et al. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614-623.
20. Zivin K, McCarthy JF, McCammon RJ, et al. Health-related quality of life and utilities among patients with depression in the Department of Veterans Affairs. Psychiatr Serv. 2008;59(11):1331-1334.
21. Costa DL, Kahn ME. Health, wartime stress, and unit cohesion: evidence from Union Army veterans. Demography. 2010;47(1):45-66.
22. Henry JA, Zaugg TL, Myers PJ, Kendall (Schmidt) CJ. Progressive Tinnitus Management: Clinical Handbook for Audiologists. San Diego, CA: Plural Publishing; 2010.
23. Henry JA, Schechter MA, Loovis CL, et al. Clinical management of tinnitus using a “progressive intervention” approach. J Rehabil Res Dev. 2005;42(4 suppl 2):95-116.
24. Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148.
25. Wilson PH, Henry JL, Bowen M, Haralambous G. Tinnitus Reaction Questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res. 1991;34(1):197-201.
26. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Clinical Version, Administration Booklet. Washington, DC: American Psychiatric Press; 2012.
27. Kerns RD, Thorn BE, Dixon KE. Psychological treatments for persistent pain: an introduction. J Clin Psychol. 2006;62(11):1327-1331.
28. Otis JD. Managing Chronic Pain: A Cognitive-Behavioral Therapy Approach. New York, NY: Oxford; 2007.
29. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
30. Mertens DM. Research and Evaluation in Education and Psychology: Integrating Diversity With Quantitative, Qualitative, and Mixed Methods. 3rd ed. Los Angeles, CA: Sage; 2009 .
31. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
32. Henry JA, Zaugg TL, Myers PJ, et al. Pilot study to develop telehealth tinnitus management for persons with and without traumatic brain injury. J Rehabil Res Dev. 2012;49(7):1025-1042.
33. Weise C, Heinecke K, Rief W. Biofeedback-based behavioral treatment for chronic tinnitus: results of a randomized controlled trial. J Consult Clin Psychol. 2008;76(6):1046-1057.
34. Henry JA, Zaugg TL, Myers PJ, Kendall (Schmidt) CJ. How to Manage Your Tinnitus: A Step-by-Step Workbook, 3rd ed. San Diego, CA: Plural Publishing; 2010.
35. Henry JA, Thielman EJ, Zaugg TL, et al. Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with Progressive Tinnitus Management. J Speech Lang Hear Res. 2017;60(5):1378-1397.
36. Henry, JA, Thielman, E, Zaugg, et al. Telephone-based progressive tinnitus management for persons with and without traumatic brain injury: a randomized controlled trial. Ear Hear. 2018. [Epub ahead of print.]
37. Chang MW, Nitzke S, Brown R, et al. Recruitment challenges and enrollment observations from a community based intervention (Mothers In Motion) for low-income overweight and obese women. Contemp Clin Trials Commun. 2017;5:26-33.
1. Henry JA. “Measurement” of tinnitus. Otol Neurotol. 2016;37(8):e276-e285.
2. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In Snow JB, ed. Tinnitus: Theory and Management. Hamilton, Canada: BC Becker; 2004:16-41.
3. Folmer RL, Theodoroff SM, Martin WH, Shi Y. Experimental, controversial, and futuristic treatments for chronic tinnitus. J Am Acad Audiol. 2014;25(1):106-125.
4. US Department of Veterans Affairs. Veterans Benefits Administration annual benefits report fiscal year 2016. https://www.benefits.va.gov/REPORTS/abr/ABR-All_Sec tions_FY16_06292017.pdf . Accessed June 19, 2018.
5. Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12(3):170-187.
6. Hoare DJ, Searchfield GD, El Refaie A, Henry JA. Sound therapy for tinnitus management: practicable options. J Am Acad Audiol. 2014;25(1):62-75.
7. Andersson G, Porsaeus D, Wiklund M, Kaldo V, Larsen HC. Treatment of tinnitus in the elderly: a controlled trial of cognitive behavior therapy. Int J Audiol. 2005;44(11):671-675.
8. Henry JL, Wilson PH. Coping with tinnitus: two studies of psychological and audiological characteristics of patients with high and low tinnitus-related distress. Int Tinnitus J. 1995;1(2):85-92.
9. Henry JL, Wilson PH. The psychological management of tinnitus: comparison of a combined cognitive educational program, education alone and a waiting-list control. Int Tinnitus J. 1996;2:9-20.
10. Robinson SK, Viirre ES, Bailey KA, et al. A randomized controlled trial of cognitive-behavior therapy for tinnitus. Int Tinnitus J. 2008;14(2):119-126.
11. Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010(9):CD005233.
12. Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: tinnitus. Otolaryngol Head Neck Surg. 2014;151(Suppl)(2):S1-S40.
13. Beck JS. Cognitive Behavior Therapy: Basics and Beyond. 2nd ed. New York, NY: Guilford Press; 2011.
14. Henry JL, Wilson PH. The Psychological Management of Chronic Tinnitus : A Cognitive-Behavioral Approach. Boston, MA: Allyn and Bacon; 2001.
15. Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143-164.
16. Cima RF, Andersson G, Schmidt CJ, Henry JA. Cognitive-behavioral treatments for tinnitus: a review of the literature. J Am Acad Audiol. 2014;25(1):29-61.
17. Rodriguez V, Andrade AD, García-Retamero R, et al. Health literacy, numeracy, and graphical literacy among veterans in primary care and their effect on shared decision making and trust in physicians. J Health Commun. 2013;18(suppl 1):273-289.
18. Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002-2008. Am J Public Health. 2009;99(9):1651-1658.
19. Thomas JL, Wilk JE, Riviere LA, et al. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67(6):614-623.
20. Zivin K, McCarthy JF, McCammon RJ, et al. Health-related quality of life and utilities among patients with depression in the Department of Veterans Affairs. Psychiatr Serv. 2008;59(11):1331-1334.
21. Costa DL, Kahn ME. Health, wartime stress, and unit cohesion: evidence from Union Army veterans. Demography. 2010;47(1):45-66.
22. Henry JA, Zaugg TL, Myers PJ, Kendall (Schmidt) CJ. Progressive Tinnitus Management: Clinical Handbook for Audiologists. San Diego, CA: Plural Publishing; 2010.
23. Henry JA, Schechter MA, Loovis CL, et al. Clinical management of tinnitus using a “progressive intervention” approach. J Rehabil Res Dev. 2005;42(4 suppl 2):95-116.
24. Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148.
25. Wilson PH, Henry JL, Bowen M, Haralambous G. Tinnitus Reaction Questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Hear Res. 1991;34(1):197-201.
26. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Clinical Version, Administration Booklet. Washington, DC: American Psychiatric Press; 2012.
27. Kerns RD, Thorn BE, Dixon KE. Psychological treatments for persistent pain: an introduction. J Clin Psychol. 2006;62(11):1327-1331.
28. Otis JD. Managing Chronic Pain: A Cognitive-Behavioral Therapy Approach. New York, NY: Oxford; 2007.
29. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health. 2000;23(4):334-340.
30. Mertens DM. Research and Evaluation in Education and Psychology: Integrating Diversity With Quantitative, Qualitative, and Mixed Methods. 3rd ed. Los Angeles, CA: Sage; 2009 .
31. Hobson J, Chisholm E, El Refaie A. Sound therapy (masking) in the management of tinnitus in adults. Cochrane Database Syst Rev. 2010;(12):CD006371.
32. Henry JA, Zaugg TL, Myers PJ, et al. Pilot study to develop telehealth tinnitus management for persons with and without traumatic brain injury. J Rehabil Res Dev. 2012;49(7):1025-1042.
33. Weise C, Heinecke K, Rief W. Biofeedback-based behavioral treatment for chronic tinnitus: results of a randomized controlled trial. J Consult Clin Psychol. 2008;76(6):1046-1057.
34. Henry JA, Zaugg TL, Myers PJ, Kendall (Schmidt) CJ. How to Manage Your Tinnitus: A Step-by-Step Workbook, 3rd ed. San Diego, CA: Plural Publishing; 2010.
35. Henry JA, Thielman EJ, Zaugg TL, et al. Randomized controlled trial in clinical settings to evaluate effectiveness of coping skills education used with Progressive Tinnitus Management. J Speech Lang Hear Res. 2017;60(5):1378-1397.
36. Henry, JA, Thielman, E, Zaugg, et al. Telephone-based progressive tinnitus management for persons with and without traumatic brain injury: a randomized controlled trial. Ear Hear. 2018. [Epub ahead of print.]
37. Chang MW, Nitzke S, Brown R, et al. Recruitment challenges and enrollment observations from a community based intervention (Mothers In Motion) for low-income overweight and obese women. Contemp Clin Trials Commun. 2017;5:26-33.
Development of a Pharmacist-Led Emergency Department Antimicrobial Surveillance Program
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
On September 18, 2014, President Barack Obama signed an executive order that made addressing antibiotic-resistant bacteria a national security policy.1 This legislation resulted in the creation of a large multidepartment task force to combat the global and domestic problem of antimicrobial resistance. The order required hospitals and other inpatient health care delivery facilities, including the Department of Veterans Affairs (VA), to implement robust antimicrobial stewardship programs that adhere to best practices, such as those identified by the Centers for Disease Control and Prevention (CDC). More specifically, the VA was mandated to take steps to encourage other areas of health care, such as ambulatory surgery centers and outpatient clinics, to adopt antimicrobial stewardship programs.1 This order also reinforced the importance for VA facilities to continue to develop, improve, and sustain efforts in antimicrobial stewardship.
Prior to the order, in 2012 the Richard L. Roudebush VA Medical Center (RRVAMC) in Indianapolis, Indiana, implemented an inpatient antimicrobial stewardship program that included thrice-weekly meetings to review inpatient records and make stewardship recommendations with an infectious diseases physician champion and clinical pharmacists. These efforts led to the improved use of antimicrobial agents on the inpatient side of the medical center. During the first 4 years of implementation, the program helped to decrease the defined daily doses of broad-spectrum antibiotics per 1,000 patient days nearly 36%, from 532 in 2012 to 343 in 2015, as well as decrease the days of therapy of fluoroquinolones per 1,000 patient days 28.75%, from 80 in 2012 to 57 in 2015. Additionally, the program showed a significant decrease in the standardized antimicrobial administration ratio, a benchmark measure developed by the CDC to reflect a facility’s actual antimicrobial use to the expected use of a similar facility based on bed size, number of intensive care unit beds, location type, and medical school affiliation.2
While the RRVAMC antimicrobial stewardship team has been able to intervene on most of the inpatients admitted to the medical center, the outpatient arena has had few antimicrobial stewardship interventions. Recognizing a need to establish and expand pharmacy services and for improvement of outpatient antimicrobial stewardship, RRVAMC leadership decided to establish a pharmacist-led outpatient antimicrobial surveillance program, starting specifically within the emergency department (ED).
Clinical pharmacists in the ED setting are uniquely positioned to improve patient care and encourage the judicious use of antimicrobials for empiric treatment of urinary tract infections (UTIs). The CDC’s Core Elements of Outpatient Antibiotic Stewardship recommends pharmacist availability in the ED setting, and previous literature has demonstrated pharmacist utility in ED postdischarge culture monitoring and surveillance.3-5
This article will highlight one such program review at the RRVAMC and demonstrate the need for pharmacist-led antimicrobial stewardship and monitoring in the ED. The purpose of this study was to test the hypothesis that pharmacist intervention would be necessary to prospectively check for “bug-drug mismatch” and assure proper follow-up of urine cultures at this institution. The project was deemed to be quality improvement and thereby granted exemption by the RRVAMC Institutional Review Board.
Methods
This project took place at the RRVAMC, a 229-bed tertiary academic medical center that serves > 60,000 patients annually. The RRVAMC ED has 20 beds and received about 29,000 visits in 2014. Patients were eligible for initial evaluation if they had a urine culture collected in the ED within the 91-day period from September 1, 2015 to November 30, 2015. Patients were included for data analysis if it was documented that they were treated for actual or clinically suspected, based on signs and symptoms, uncomplicated UTI, complicated UTI, or UTI with pyelonephritis. Patients did not need to have a positive urine culture for inclusion, as infections could still be present despite negative culture results.6 Patients with positive cultures who were not clinically symptomatic of a UTI and were not treated as such by the ED provider (ie, asymptomatic bacteriuria) were excluded from the study.
Data collection took place via daily chart review of patient records in both the Computerized Patient Record System and Decentralized Hospital Computer Program medical applications as urine cultures were performed. Data were gathered and assessed by a postgraduate year-2 internal medicine pharmacy resident on rotation in the ED who reviewed cultures daily and made interventions based on the results as needed. The pharmacy resident was physically present within the ED during the first 30 days of the project. The pharmacy resident was not within the direct practice area during the final 61 days of the project but was in a different area of the hospital and available for consultation.
Primary data collected included urine culture results and susceptibilities, empiric antimicrobial choices, and admission status. Other data collected included duration of treatment and secondary antibiotics chosen, each of which specifically evaluated those patients who were not admitted to the hospital and were thus treated as outpatients. Additional data generated from this study were used to identify empiric antibiotics utilized for the treatment of UTIs and assess for appropriate selection and duration of therapy within this institution.
Results
During the study period, 722 urine cultures were collected in the ED and were included for initial evaluation. Of these, 127 were treated by the ED provider pursuant to one of the indications specified and were included in the data analysis. Treatment with an antimicrobial agent provided adequate coverage for the identified pathogen in 112 patients, yielding a match rate of 88%. As all included cultures were collected in suspicion of an infection, those cultures yielding no growth were considered to have been adequately covered.
Nearly half (45%) of treatment plans included a fluoroquinolone. Of those treated on an outpatient basis, fluoroquinolones were even more frequently used, comprising 50 of 82 (61%) courses. Ciprofloxacin was the most frequently used treatment, used in 39 of the 82 outpatient regimens (48%). Cephalexin was the second most common and was used in 14 outpatient regimens (17%), followed by levofloxacin (15%) (Figure 1).
Mismatched cultures, or those where the prescribed antibiotic did not provide adequate coverage of the identified pathogens based on susceptibilities, occurred at a rate of 12%. Follow-up on these cultures was determined largely by the patient’s admission status. The majority of mismatched cultures were addressed by the inpatient team (10/15) upon admission.
Discussion
Empiric antibiotic selection for the treatment of UTIs continues to be the cornerstone of antibiotic management for the treatment of such a disease state.7 The noted drug-bug match rate of 88% in this study demonstrates effective initial empiric coverage and ensures a vast majority of veterans receive adequate coverage for identified pathogens. Additionally, this rate shows that the current system seems to be functioning appropriately and refutes the author’s preconceived ideas that the mismatch rate was higher at RRVAMC. However, these findings also demonstrate a predominant use of fluoroquinolones for empiric treatment in a majority of patients who could be better served with narrower spectrum agents. Only 2 of the outpatient regimens were for the treatment of pyelonephritis, the only indication in which a fluoroquinolone would be the standard of care per guideline recommendations.7
These findings were consistent with a similar study in which 83% of ED collected urine cultures ultimately grew bacteria susceptibleto empiric treatment.8 This number was similar to the current study despite the latter study consisting of predominantly female patients (93%) and excluding patients with a history of benign prostatic hypertrophy, catheter use, or history of genitourinary cancer, which are frequently found within the VA population. Thus, despite having a differing patient population at the current study’s facility with characteristics that would classify most to be treated as a complicated UTI, empiric coverage rates remained similar. The lower than anticipated intervention rate by the pharmacist on rotation in the ED can be directly attributed to this high empiric match rate, which could in turn be attributed to the extensive use of broad-spectrum antibiotics for treatment.
Empiric antimicrobial selection is based largely on local resistance patterns.7 Of particular importance is the resistance patterns of E coli, as it is the primary isolate responsible for UTIs worldwide. Thus, it is not unexpected that the most frequently isolated pathogen in the current study also was E coli. While clinical practice guidelines state that hospital-wide antibiograms often are skewed by cultures collected from inpatients or those with complicated infection, the current study found hospital-wide E coli resistance patterns, specifically those related to fluoroquinolone use, to be similar to those collected in the ED alone (78.5% hospital-wide susceptibility vs. 75% ED susceptibility). This was expected, as similar studies comparing E coli resistance patterns from ED-collected urine cultures to those institution-wide also have found similar rates of resistance.8,9 These findings are of particular importance as E coli resistance is noted to be increasing, varies with geographic area, and local resistance patterns are rarely known.7 Thus, these findings may aid ED providers in their empiric antimicrobial selections.
Ciprofloxacin was the most frequently used medication for the treatment of UTIs. While overall empiric selections were found to have favorable resistance patterns, it is difficult to interpret the appropriateness of ciprofloxacin’s use in the present study. First, there is a distinct lack of US-based clinical practice guidelines for the treatment of complicated UTIs. As the majority of this study population was male, it is difficult to directly extrapolate from the current Infectious Diseases Society of America treatment guidelines for uncomplicated cystitis and apply to the study population. Although recommended for the treatment of pyelonephritis, it is unclear whether ciprofloxacin should be utilized as a first-line empiric option for the treatment of UTIs in males.
Despite the lack of disease-specific recommendations for ciprofloxacin, recommendations exist regarding its use when local resistance patterns are known.7 It is currently recommended that these agents not be used when resistance rates of E coli exceed 20% for trimethoprim-sulfamethoxazole or 10% for fluoroquinolones. As this study demonstrated a nearly 25% resistance rate for E coli to fluoroquinolones in both the ED and institution-wide sample populations, it could potentially be ascertained that ciprofloxacin is an inappropriate choice for the empiric treatment of UTIs in this patient population. However, as noted, it is unknown whether this recommendation would still be applicable when applied to the treatment of complicated cystitis and greater male population, as overall rates of susceptible cultures to all organisms was similar to other published studies.8,9
While there is scant specific guidance related to the treatment of complicated UTIs, there is emerging guidance on the use of fluoroquinolones, both in general and specifically related to the treatment of UTIs. In July 2016, the FDA issued a drug safety communication regarding the use of and warnings for fluoroquinolones, which explicitly stated that “health care professionals should not prescribe systemic fluoroquinolones to patients who have other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated UTIs because the risks outweigh the benefits in these patients.”10
This guidance has the potential to impact fluoroquinolone prescribing significantly at RRVAMC. Given the large number of fluoroquinolones prescribed for UTIs, the downstream effects that this shift in prescribing would have is unknown. As most nonfluoroquinolones used for UTI typically are narrower in antimicrobial spectrum (eg, trimethoprim/sulfamethoxazole, nitrofurantoin, cephalexin, etc) the possibility exists that the match rate for empiric therapy may decrease. Thus, a larger need for closer follow-up to assure adequate coverage may arise, posing a more expanded role for an ED-based pharmacist than was demonstrated in the current study.
This new guidance also may place providers in an area of larger uncertainty with regards to treating both complicated and uncomplicated cystitis. Given the enhanced warnings on fluoroquinolone use, it is unknown whether prescribers would gravitate to utilizing similar options as their peers as alternatives to fluoroquinolones. Similarly, duration of therapy with nonfluoroquinolone agents is unclear as well; as the present study demonstrated a large range in treatment duration of outpatients (3-14 days). While the average observed duration of 8.3 days is intuitively fitting, as the majority of cases were in males, no published guideline exists that affirms the appropriateness of this finding. Such uncertainty and potential inconsistency between providers affords a large opportunity for developing a standardized treatment pathway for the treatment of UTIs to ensure both effective and guideline concordant treatment for patients, specifically with regards to antimicrobial selection and duration of treatment.
It is noteworthy to mention that all follow-ups on positive cultures inadequately covered by empiric therapy took place on the day organism identification and susceptibility data were released. This finding was somewhat surprising, as it was originally theorized that most ED-collected urine cultures were not monitored to completion by a pharmacist and that would be necessary in order to ensure proper follow-up of culture results. What is not clear is whether there is a robust process for the follow-up of urine cultures in the ED. Most of the bug-drug mismatches coincidentally were admitted to the inpatient teams where there were appropriate personnel to follow up and adjust the antibiotic selection. If there was a bug-drug mismatch, and the patient was not admitted, it is unclear whether there is a consistent process for follow-up.
Given the limited number of mismatched cultures that required change in therapy, it is unknown if this role would expand if more narrow-spectrum agents were utilized, theoretically leading to a higher mismatch rate and necessitating closer follow-up. Furthermore, given the common practice of mailed prescriptions at the VA, it is all the more imperative that the cultures be acted upon on the day they were identified, as the mailing and processing time of prescriptions may limit the clinical utility in switching from a more broad-spectrum agent, to one more targeted for an identified organism. While a patient traveling back to the medical center for expedited prescription pickup at the pharmacy would alleviate this problem, many patients at the facility travel great distances or may not have readily available travel means to return to the medical center.
Future Directions
While minimal follow-up was required after a patient had left the ED, this study demonstrated a fundamental need for further refinements in antimicrobial stewardship activities within the ED. Duration of therapy, empiric selection, and proper dosing are key areas where the ED-based pharmacy resident was able to intervene during the time physically stationed in the ED. The data collected from this study demonstrated this and was ultimately combined with other ED-based interventions and utilized as supporting evidence in the pharmacy service business plan, outlining the necessity of a full-time pharmacy presence in the ED. The business plan submission, along with other ongoing RRVAMC initiatives, ultimately led to the approval for clinical pharmacy specialists to expand practice into the ED. These positions will continue to advance pharmacy practice within the ED, while affording opportunities for pharmacists to practice at the top of their licensure, provide individualized provider education, and deliver real-time antimicrobial stewardship interventions. Furthermore, as the majority of the study period was monitored outside of the ED, the project may provide a model for other VA institutions without full-time ED pharmacists to implement as a means to improve antimicrobial stewardship and further build an evidence base for expanding their pharmacy services to the ED.
Given the large number of fluoroquinolones utilized in the ED, this study has raised the question of what prescribing patterns look like with regards to outpatient UTI treatment within the realm of primary care at RRVAMC. Despite the great strides made with regards to antimicrobial stewardship at this facility on the inpatient side, no formal antimicrobial stewardship program exists for review in the outpatient setting, where literature suggests the majority of antibiotics are prescribed.3,11 While more robust protocols are in place for follow-up of culture data in the primary care realm at this facility, the prescribing patterns are relatively unknown.
A recent study completed at a similar VA facility found that 60% of antibiotics prescribed for cystitis, pharyngitis, or sinusitis on an outpatient basis were guideline-discordant, and CDC guidance has further recommended specific focus should be undertaken with regards to outpatient stewardship practices in the treatment of genitourinary infections.3,12 These findings highlight the need for outpatient antimicrobial stewardship and presents a compelling reason to further investigate outpatient prescribing within primary care at RRVAMC.
Strengths and Limitations
Strengths of the current study include the ability to monitor urine cultures in real time and to provide timely interventions in the event of a rare bug-drug mismatch. The evaluation of cultures in this study shows that the majority of cases had a drug selected with adequate coverage. The study did assure ED providers that, even though guidelines may suggest otherwise, urine cultures drawn in the ED at RRVAMC followed similar resistance patterns seen for the facility as a whole. Moreover, it is valuable as it captures data that are directly applicable to the VA patient population, in which there is little published data with regards to UTI treatment and no formal VA guidance.
A primary limitation of this study is the lack of differentiation between cultures collected from patients with or without indwelling catheters. However, only including patients who presented with signs and/or symptoms of a UTI limits the number of cultures that could potentially be deemed as colonization, thus minimizing the potential for nonpathogenic organisms to confound the results. This study also did not differentiate the setting from which the patient presented (eg, community, extended care facility, etc) that could have potentially provided guidance on resistance patterns for community-acquired UTIs and whether this may have differed from hospital-acquired or facility-acquired UTIs. Another limitation was the relatively short time frame for data collection. A data collection period greater than 91 days would allow for a larger sample size, thus making the data more robust and potentially allowing for the identification of other trends not seen in the current study. A longer data collection period also would have afforded the opportunity to track more robust clinical outcomes throughout the study, identifying whether treatment failure may have been linked to the use of certain classes or spectrums of activity of antibiotics.
Conclusion
Despite the E coli resistance rate to ciprofloxacin (> 20%), the empiric treatments chosen were > 85% effective, needing minimal follow-up once a patient left the ED. Nonetheless, a change in prescribing patterns based on recent national recommendations may provide expanded opportunities in antimicrobial stewardship for ED-based pharmacists. Further research is needed in antimicrobial stewardship within this facility’s outpatient primary care realm, potentially uncovering other opportunities for pharmacist intervention to assure guideline concordant care for the treatment of UTIs as well as other infections treated in primary care patients.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.
1. Obama B. Executive order–combating antibiotic-resistant bacteria. https://www.whitehouse.gov/the -press-office/2014/09/18/executive-order-combating-antibiotic-resistant-bacteria. Published September 18, 2014. Accessed June 7, 2018.
2. Livorsi DJ, O’Leary E, Pierce T, et al. A novel metric to monitor the influence of antimicrobial stewardship activities. Infect Control Hosp Epidemiol. 2017;38(6):721-723.
3. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hick LA. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
4. Wymore ES, Casanova TJ, Broekenmeier RL, Martin JK Jr. Clinical pharmacist’s daily role in the emergency department of a community hospital. Am J Health-Syst Pharm. 2008;65(5):395-396, 398-399.
5. Frandzel S. ED pharmacists’ value on display at ASHP Midyear. http://www.pharmacypracticenews.com/ViewArticle.aspx?ses=ogst&d_id=53&a_id=22524. Published February 14, 2013. Accessed June 15, 2018.
6. Heytens S, DeSutter A, Coorevits L, et al. Women with symptoms of a urinary tract infection but a negative urine culture: PCR-based quantification of Escherichia coli suggests infection in most cases. Clin Microbiol Infect 2017;23(9)647-652.
7. Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120.
8. Lingenfelter E, Drapkin Z, Fritz K, Youngquist S, Madsen T, Fix M. ED pharmacist monitoring of provider antibiotic selection aids appropriate treatment for outpatient urinary tract infection. Am J Emerg Med. 2016;34(8):1600-1603.
9. Zatorski C, Jordan JA, Cosgrove SE, Zocchi M, May L. Comparison of antibiotic susceptibility of Escherichia coli in urinary isolates from an emergency department with other institutional susceptibility data. Am J Health-Syst Pharm. 2015;72(24):2176-2180.
10. US Food and Drug Administration. FDA drug safety communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Updated March 8, 2018. Accessed June 13, 2018.
11. Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
12. Meyer HE, Lund BC, Heintz BH, Alexander B, Egge JA, Livorsi DJ. Identifying opportunities to improve guideline-concordant antibiotic prescribing in veterans with acute respiratory infections or cystitis. Infect Control Hosp Epidemiol. 2017;38(6):724-728.