User login
Clinical Progress Note: Rhythm Control for Patients With Atrial Fibrillation
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
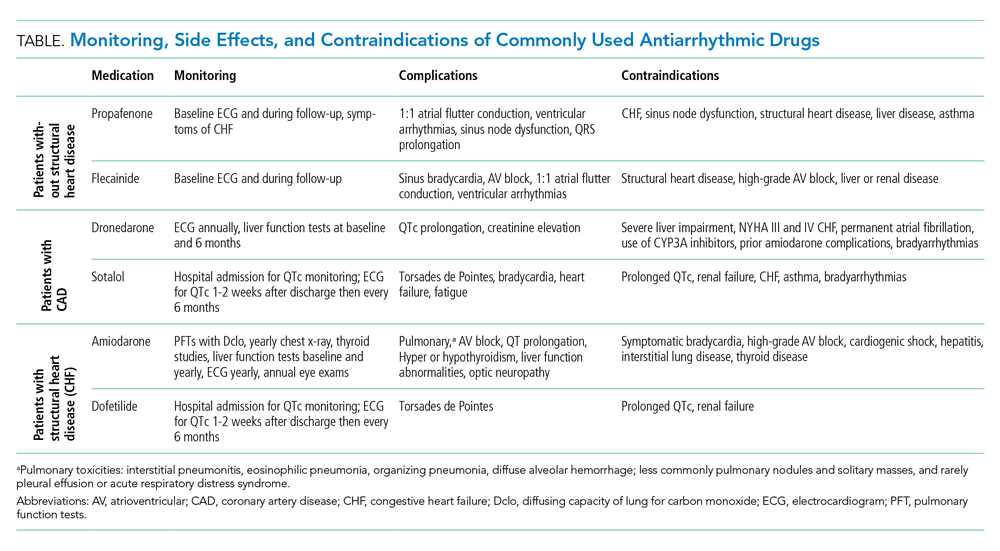
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3
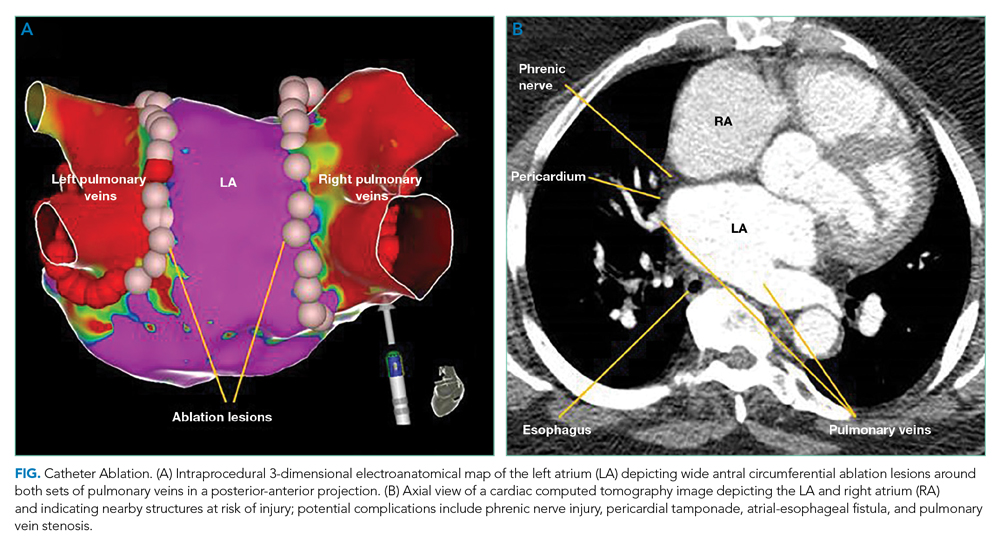
Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7
Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
It has been 19 years since the publication of the landmark AFFIRM trial.1 At the time of publication, a “rhythm control” strategy was the preferred therapy, with a rate control approach an accepted alternative. AFFIRM showed no mortality benefit of rhythm control over rate control, and its result dramatically shifted the paradigm of atrial fibrillation (AF) management. However, the high crossover rate between treatment arms may have biased the study toward the null hypothesis. Post hoc analyses of AFFIRM and other observational studies indicate that sinus rhythm was associated with a lower risk of death.2 Since AFFIRM, technical advances and procedural experience have improved the safety and efficacy of catheter ablation (CA), and recently published randomized trials have shown improved outcomes with rhythm control. This Progress Note summarizes the recent evidence, updating hospitalists on the management of AF, including inpatient cardioversion, patient selection for CA, use of antiarrhythmic drugs (AADs), and lifestyle modifications associated with maintenance of sinus rhythm.
Search Strategy
A PubMed search for recent publications using combined the MeSH terms “atrial fibrillation” with “catheter ablation,” “antiarrhythmic drugs,” and “lifestyle modifications.” Our review filtered for randomized trials, guidelines, and selected reviews.
Should I pursue inpatient cardioversion for my patient?
Urgent cardioversion is recommended for those with hemodynamic instability, AF associated ischemia, or acute heart failure.3 Whether to perform elective cardioversion depends on AF duration, symptoms, and the initial evaluation for structural heart disease or reversible causes of AF. Evaluation for new-onset AF includes eliciting a history of AF-associated comorbidities (hypertension, alcohol use, obstructive sleep apnea) and an echocardiogram and thyroid, renal, and liver function tests.3 Stable patients with AF precipitated by high-catecholamine states (eg, postoperative AF, sepsis, hyperthyroidism, pulmonary embolism, substance use) require management of the underlying condition before considering rhythm control. Inpatient electrical or pharmacologic cardioversion may be considered for patients with stable, new-onset AF sufficiently symptomatic to require hospitalization. Pre-procedure anticoagulation and a transesophageal echocardiogram to rule out left atrial thrombus before cardioversion is preferred for a first episode of AF suspected of lasting longer than 48 hours but requires anesthesia and considerable resources. In resource-constrained settings, patients asymptomatic once rate controlled may be safely discharged with a referral for outpatient cardioversion.
For patients with structural heart disease (left atrial dilation), previously failed cardioversion, or recurrent AF, initiating AADs (eg, ibutilide, amiodarone) before electrical cardioversion can improve the success rate of cardioversion.3 Ibutilide infusion requires cardiology consultation and postinfusion hemodynamic and QTc monitoring. Defer immediate cardioversion among stable patients unable to continue a minimum of 4 weeks of anticoagulation or with comorbidities for which risks of cardioversion outweigh benefits.
Is a rhythm control strategy best for my patient?
Successful maintenance of sinus rhythm is associated with reduced symptom burden and improved quality of life and is recommended for patients with persistent symptoms, failure of rate control, younger age, first episode of AF, or patient preference for rhythm control.3 Since AF progression results in irreversible cardiac remodeling, earlier rhythm control may prevent further atrial remodeling and atrial myopathy.
The EAST-AFNET 4 trial evaluated a rhythm-control strategy in patients with AF duration <12 months and who met two of the following: age > 65 years, female sex, heart failure, hypertension, diabetes, coronary artery disease, and chronic kidney disease.4 Maintenance of sinus rhythm was associated with a lower composite outcome of adverse cardiovascular outcomes and death from cardiovascular causes over 5 years compared to rate control (3.9/100 person-years vs 5.0/100 person-years, P = .005). Interestingly, roughly 20% of patients underwent CA and the remainder received AADs. The large proportion of patients treated with AADs raises the question of why the results differed from AFFIRM. There are four primary differences between these trials to consider. First, EAST-AFNET 4 used an early rhythm-control strategy (<12 months). Second, nearly all patients in EAST-AFNET 4 continued guideline-recommend anticoagulation compared to 70% receiving rhythm control in AFFIRM. Third, in AFFIRM, 62.8% of patients received amiodarone, which has significant long-term adverse effects compared to 11.8% by the end of EAST-AFNET 4. Finally, increased use of CA in EAST-AFNET 4 may have contributed to the success of rhythm control. In patients with cardiovascular disease or cardiovascular risk factors, a rhythm-control strategy will be best if implemented early (<12 months), before the development of long-standing persistent AF, and if clinicians adhere to anticoagulation recommendations.
Should my patient receive antiarrhythmics, catheter ablation, or both?
Antiarrhythmic Drugs
Antiarrhythmic drug use prior to CA remains the cornerstone of a rhythm-control strategy for patients meeting EAST-AFNET 4 trial criteria or patient preference for medical management. Hospitalists’ knowledge of key differences between AADs used in EAST-AFNET 4 and AFFIRM as well as American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline recommendations help avoid harmful AAD prescribing. Notably, 21.9% of patients in AFFIRM received AADs no longer recommended to maintain sinus rhythm in the AHA/ACC/HRS guidelines (quinidine, disopyramide, procainamide, moricizine).3 For patients without structural heart disease, flecainide, propafenone, sotalol, or dronedarone are preferred. Dronedarone and sotalol remain an option for those with coronary artery disease. For patients with heart failure with reduced ejection fraction (HFrEF), amiodarone and dofetilide are preferred (Table).3

Catheter Ablation
The AHA/ACC/HRS guidelines offer a Ia recommendation for CA in patients with recurrent, symptomatic AF who failed AAD therapy. Initial CA is a IIa recommendation and is increasingly common for patients with paroxysmal AF who prefer this strategy to long-term AAD use.3 Recent trials evaluated CA as a primary treatment modality in patients with heart failure and as initial management before AADs.
Initial Catheter Ablation
The CABANA trial compared CA with AADs as an initial approach for maintaining sinus rhythm.5 In the intention-to-treat analysis, there was no difference in all death or disabling stroke between AAD therapy and CA at 5-year follow-up. The results are limited by a 27.5% crossover rate from drug therapy to CA. The per-protocol analysis based on the treatment received favored CA for the primary composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest at 12 months. The STOP-AF and EARLY-AF trials found that initial CA was more successful in maintaining freedom from atrial arrhythmias (74.6% vs 45.0%, P < .001)6 and fewer symptomatic atrial arrhythmias among patients with paroxysmal AF compared to AADs, without significant CA-associated adverse events.6,7

Catheter Ablation Plus Antiarrhythmics
Ongoing AADs following CA may suppress AF triggers, especially in patients with persistent AF or high-risk for recurrence post ablation (left atrial dilation). The AMIO-CAT trial found that 4 weeks of amiodarone after ablation reduced early AF recurrence at 3 months (34% vs 53%, P = .006), arrhythmia-related hospitalizations, and need for cardioversion in patients with paroxysmal and persistent AF.8 However, amiodarone did not reduce recurrent atrial tachyarrhythmias at 6 months. The POWDER-AF trial evaluated AAD use for 1 year after CA in patients with drug-refractory paroxysmal AF.9 Continuation of class IC (eg, flecainide) and III (eg, amiodarone) AADs resulted in a near 20% absolute risk reduction in recurrent atrial arrhythmias and reduced the need for repeat CA. These trials suggest that discharging patients on adjunctive AADs decreases early recurrence of AF and arrhythmia-related hospitalizations; however, studies evaluating additional clinical outcomes are needed.
Heart Failure
The AATAC trial found CA was superior to amiodarone therapy at maintaining freedom from AF and reducing unplanned hospitalizations and mortality among patients with persistent AF and HFrEF.10 The larger CASTLE-AF trial randomized patients with an ejection fraction below 35% and NYHA class II or greater symptoms with symptomatic paroxysmal AF or persistent AF in whom AAD therapy failed to CA or medical therapy.11 The CA group experienced lower cardiovascular mortality (11.2% vs 22.3%, P = .009) and fewer heart failure hospitalizations (20.7% vs 35.9%, P = .004). The subsequent AMICA trial did not find a benefit of CA in patients with HFrEF and persistent or long-standing persistent AF; however, this trial was limited to 12 months, whereas the benefit of CA in CASTLE-AF was observed after 12 months.12 Also, AMICA enrolled patients with higher NYHA class. Therefore, hospitalists should refer AF patients with left ventricular systolic dysfunction and NYHA II or III symptoms for CA. Comparing AMICA and CASTLE-AF suggests earlier referral for CA, prior to the development of worsening heart failure symptoms, may improve outcomes.
Data for patients with heart failure with preserved EF (HFpEF) is limited. One small trial showed reduced heart failure hospitalizations in HFpEF patients treated with CA compared to AADs or beta-blockers.13 It is reasonable to refer HFpEF patients with persisting symptoms or reduced quality of life for CA.
What long-term risk-modification should I recommend?
The AHA Scientific Statement on Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation delineates risk factors that increase the incidence of AF, including alcohol consumption, obstructive sleep apnea, hypertension, and obesity.14 Among regular alcohol consumers with paroxysmal or persistent AF managed with a rhythm-control strategy, cessation of alcohol has been shown to significantly lower the incidence of recurrent AF (53.0% vs 73.0%, P = .005), and lead to a longer time until recurrence of AF compared to patients regularly consuming alcohol.15 Among patients with obstructive sleep apnea, a systematic review of nonrandomized studies showed continuous positive airway pressure is associated with maintenance of sinus rhythm.14 Control of these risk factors is associated with up to approximately 40% of patients maintaining sinus rhythm without intervention, and hospitalists should encourage lifestyle modification to maximize the probability of maintaining sinus rhythm.
Summary
Hospitalists frequently determine the best initial management strategy for patients admitted with new-onset AF, and recent literature may shift more patients towards management with rhythm control. Based on the trials reviewed in this Progress Note, hospitalists should recommend a rhythm-control strategy for patients with symptomatic, paroxysmal, or persistent AF of <12 months’ duration and refer patients with HFrEF for CA. Adherence to guideline recommendations is essential when prescribing AADs to avoid adverse drug events. It is vital to ensure patients managed with a rhythm-control strategy receive anticoagulation for 4 weeks post cardioversion or 2 months post CA with long-term anticoagulation based on CHA2DS2-VASc score. Finally, admissions for AF should serve as a catalyst to communicate to patients the importance of addressing obstructive sleep apnea, obesity, and alcohol use disorders. Applying these evidence-based practices will enable hospitalists to make clinical decisions that improve symptom burden and survival for patients with AF.
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
1. Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825-1833. https://doi.org/10.1056/NEJMoa021328
2. Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509-1513. https://doi.org/10.1161/01.Cir.0000121736.16643.11
3. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Circulation. 2014;130(23):e199-e267. https://doi.org/10.1161/CIR.0000000000000041
4. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305-1316. https://doi.org/10.1056/NEJMoa2019422
5. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321(13):1261-1274. https://doi.org/doi:10.1001/jama.2019.0693
6. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384(4):316-324. https://doi.org/10.1056/NEJMoa2029554
7. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384(4):305-315. https://doi.org/10.1056/NEJMoa2029980
8. Darkner S, Chen X, Hansen J, et al. Recurrence of arrhythmia following short-term oral AMIOdarone after CATheter ablation for atrial fibrillation: a double-blind, randomized, placebo-controlled study (AMIO-CAT trial). Eur Heart J. 2014;35(47):3356-3364. https://doi.org/10.1093/eurheartj/ehu354
9. Duytschaever M, Demolder A, Phlips T, et al. PulmOnary vein isolation with vs. without continued antiarrhythmic drug treatment in subjects with recurrent atrial fibrillation (POWDER AF): results from a multicentre randomized trial. Eur Heart J. 2018;39(16):1429-1437. https://doi.org/10.1093/eurheartj/ehx666
10. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133(17):1637-1344. https://doi.org/10.1161/circulationaha.115.019406
11. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417-427. https://doi.org/10.1056/NEJMoa1707855
12. Kuck KH, Merkely B, Zahn R, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA Trial. Circ Arrhythm Electrophysiol. 2019;12(12):e007731. d https://doi.org/10.1161/circep.119.007731
13. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. 2020;31(3):682-688. https://doi.org/10.1111/jce.14369
14. Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American Heart Association. Circulation. 2020;141(16):e750-e772. https://doi.org/10.1161/CIR.0000000000000748
15. Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. 2020;382(1):20-28. https://doi.org/10.1056/NEJMoa1817591
© 2021 Society of Hospital Medicine
Clinical Progress Note: Consolidated Guidelines on Management of Coagulopathy and Antithrombotic Agents for Common Bedside Procedures
The practice of internal medicine includes bedside procedures such as paracentesis, thoracentesis, and lumbar puncture (LP). The American Board of Internal Medicine requires graduates of internal medicine residency programs to be competent in the cognitive components of procedural training (eg, indications, contraindications, complications) and considers it essential that trainees have opportunities to perform procedures relevant to their intended career direction.1 Whether or not the performance of procedures is part of a given hospitalist’s practice, it is necessary that hospitalists understand each procedure’s risks and mitigation strategies to prevent a range of periprocedural complications, including clinically significant bleeding. Numerous recommendations and guidelines exist describing bleeding risk for common procedures. In this Progress Note, we summarize and consolidate this literature, covering a range of scenarios common to the hospital setting, including thrombocytopenia, elevated international normalized ratio (INR), and the use of medications such as antiplatelet and anticoagulant agents (Table 1 and Table 2). We performed electronic searches in PubMed, focusing on literature published since 2016. Key search terms included paracentesis, thoracentesis, lumbar puncture, anticoagulant, antiplatelet, coagulopathy, INR, thrombocytopenia, and guideline. In addition, we used the following MeSH terms: spinal puncture AND blood coagulation disorders, spinal puncture AND platelet aggregation inhibitors, spinal puncture AND anticoagulants, paracentesis AND blood coagulation disorders, paracentesis AND platelet aggregation inhibitors, paracentesis AND anticoagulants, thoracentesis AND blood coagulation disorders, thoracentesis AND platelet aggregation inhibitors, and thoracentesis AND anticoagulants.

GENERAL CONCEPTS
Weighing Risks and Benefits

Hepatic and Renal Dysfunction
In the setting of chronic liver disease, thrombocytopenia and elevated INR are generally not reliable indicators of bleeding risk.13 The included recommendations for INR and platelet count thresholds in the setting of chronic liver disease are derived from the referenced guidelines and supplemental personal communication with the guideline authors. Many antiplatelet and anticoagulant medications are partially cleared or metabolized by the liver, suggesting that hepatic dysfunction may impact drug clearance, but this has not been well studied. Impaired renal function should also be considered when determining appropriate hold times for antithrombotic drugs that are partially renally cleared. The periprocedural hold and restart times outlined in Table 2 are specific to patients without clinically significant hepatic or renal dysfunction. For patients with these conditions, further information on hold time adjustment can be found in the individual references.

Bridging Therapy
Resuming Therapy
Other Considerations
Some guidelines referenced in this article are based on data collected on procedures performed by interventional radiologists, which may or may not accurately reflect the bleeding risks of bedside procedures performed by hospitalists. In the case of LP, we included some regional anesthesia and pain procedure guidelines based on the assumption that certain procedures are analogous to LP and associated with similar bleeding risks.
PARACENTESIS
Paracentesis is a common procedure that can be performed safely at the bedside. The overall rate of serious complications is low (1%-2%), with severe hemorrhage accounting for the majority of those complications (0.97%).15 Bleeding usually occurs from puncture of an abdominal wall vein, a mesenteric varix, or an inferior epigastric artery. Certain techniques may help to mitigate serious bleeding, including the use of ultrasound to avoid overlying vessels. Paracentesis is frequently performed in patients with cirrhosis, a population at increased risk for coagulopathy, although INR and platelet counts may not reflect aggregate bleeding risk in patients with cirrhosis. The American Association for the Study of Liver Diseases released new guidelines in 2021, stating that elevated prothrombin time or thrombocytopenia is not a contraindication to paracentesis.6 The most liberal guidelines for patients without chronic liver disease suggest correcting to an INR of 2.0 to 3.0, with multiple societies suggesting that a platelet count as low as 20,000/µL is safe.2,3 As shown in Table 2, most guidelines recommend continuation of antiplatelet agents such as aspirin and thienopyridines (eg, clopidogrel, prasugrel), whereas recommendations vary regarding continuation of anticoagulant agents.
THORACENTESIS
Akin to paracentesis, thoracentesis is generally considered to be a safe bedside procedure, with an incidence of thoracentesis-associated bleeding of less than 1%.15 Certain techniques may help to mitigate serious bleeding, including the insertion of the needle over the superior aspect of the rib in an effort to avoid the intercostal neurovascular bundle, which runs along the inferior aspect of each rib. Various clinical societies have proposed INR and platelet thresholds at which the risk of bleeding from thoracentesis is thought to be acceptable. The most liberal guidelines include a target INR of
LUMBAR PUNCTURE
Compared to thoracentesis and paracentesis, LP is generally considered to be a higher-risk procedure owing to the rare possibility of spinal hematoma with associated neurologic compromise. In one retrospective review of more than 49,000 patients without coagulopathy who underwent LP, the risk for developing a spinal hematoma by 30 days post procedure was 0.20%.16 Certain techniques may help to mitigate serious bleeding, including the use of image guidance in patients with large body habitus or those with difficult anatomy. Compared with paracentesis and thoracentesis, guideline recommendations for safe INR and platelet thresholds in patients undergoing LP are based on a more limited body of evidence. Guidelines also suggest a target INR of anywhere from ≤1.5 to the most liberal suggestion of 2.0 to 3.0.2-4 The SIR guidelines categorize LP as a low–bleeding risk procedure, with a platelet threshold of 20,000/µL but note that most other societies and guidelines regard LP as a high–bleeding risk procedure with more conservative platelet thresholds.2 The Association of British Neurologists (ABN), however, allows platelets to be 40,000/µL or greater than 20,000/µL with an additional risk-benefit discussion.7 In contrast to paracentesis and thoracentesis, recommendations regarding hold times of antithrombotic medications prior to LP are more variable and sometimes more conservative. For example, some guidelines indicate that the thienopyridines can be continued, whereas others recommend holding them for up to 1 week prior to LP.2,4,7
GAPS IN KNOWLEDGE
A theme throughout the recent literature and recommendations from clinical societies is that it is uncommon for there to be one unifying recommendation for every situation, especially regarding LP. Recent guidelines remain largely based on studies that are decades old. With bedside ultrasound becoming more accessible and established in daily practice, the risk of bleeding has been decreasing, potentially making periprocedural coagulopathies and antithrombotic agents less of a concern. For example, in a retrospective study of 69,859 paracenteses, ultrasound guidance reduced the risk of bleeding complications by 68%, an odds ratio of 0.32 (95% CI, 0.25-0.41).17 More research is needed to assess procedural bleeding risks in the context of current practice standards. This article focuses on a subset of bedside procedures most commonly performed by hospitalists. Similar references for other common bedside procedures, such as arthrocentesis, central venous catheter, and arterial line placement, would be helpful. Finally, this article does not capture such nuances as needle gauge, operator experience, availability of (and comfort with) ultrasound, and variations in patient anatomy, all of which are factors that can contribute to the complexities and risks of these bedside procedures.
CONCLUSION
Although not every internal medicine physician performs bedside procedures in their practice, it is vital that all understand the cognitive aspects of common bedside procedures. This necessitates the understanding of periprocedural risks and possible complications and applying that to individual patients. Correcting coagulopathy and stopping or reversing antithrombotic agents are mitigation strategies that are associated with risk. It is therefore important to understand when coagulopathy should be corrected and when antithrombotic agents should be held and for how long. With multiple existing and sometimes conflicting guidelines regarding periprocedural management of coagulopathy and antithrombotic agents, we hope that providing consolidated tables with this information will increase efficiency, aid in risk-benefit discussions between patients and care teams, and enhance patient safety.
1. Nichani S, Fitterman N, Lukela M, Crocker J. The core competencies in hospital medicine 2017 Revision. Section 2: procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54. https://doi.org/10.12788/jhm.2728
2. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part ii: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
3. Hadi M, Walker C, Desborough M, et al. CIRSE standards of practice on peri-operative anticoagulation management during interventional radiology procedures. Cardiovasc Intervent Radiol. 2021;44(4):523-536. https://doi.org/10.1007/s00270-020-02763-4
4. Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25(2):144-156. https://doi.org/10.5152/dir.2019.18291
5. Demirci NY, Koksal D, Bilaceroglu S, et al. Management of bleeding risk before pleural procedures: a consensus statement of Turkish Respiratory Society—Pleura study group. Consensus Report. Eurasian J Pulmonol. 2020;22(2):73-78. https://doi.org/10.4103/ejop.ejop_28_20
6. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
7. Dodd KC, Emsley HCA, Desborough MJR, Chhetri SK. Periprocedural antithrombotic management for lumbar puncture: Association of British Neurologists clinical guideline. Pract Neurol. 2018;18(6):436-446. https://doi.org/10.1136/practneurol-2017-001820
8. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. https://doi.org/10.1097/aap.0000000000000763
9. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225-262. https://doi.org/:10.1097/aap.0000000000000700
10. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847-1948. https://doi.org/10.1016/j.cjca.2020.09.001
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. https://doi.org/10.1016/j.jacc.2016.11.024
12. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/chest.11-2298
13. Crowe B, Tahhan SG, Lacy C, Grzankowski J, Lessing JN. Things we do for no reason™: Routine correction of elevated INR and thrombocytopenia prior to paracentesis in patients with cirrhosis. J Hosp Med. 2021;16(2):102-104. https://doi.org/10.12788/jhm.3458
14. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441-449. https://doi.org/10.1002/clc.23336
15. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023
16. Bodilsen J, Mariager T, Vestergaard HH, et al. Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA. 2020;324(14):1419-1428. https://doi.org/10.1001/jama.2020.14895
17. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
The practice of internal medicine includes bedside procedures such as paracentesis, thoracentesis, and lumbar puncture (LP). The American Board of Internal Medicine requires graduates of internal medicine residency programs to be competent in the cognitive components of procedural training (eg, indications, contraindications, complications) and considers it essential that trainees have opportunities to perform procedures relevant to their intended career direction.1 Whether or not the performance of procedures is part of a given hospitalist’s practice, it is necessary that hospitalists understand each procedure’s risks and mitigation strategies to prevent a range of periprocedural complications, including clinically significant bleeding. Numerous recommendations and guidelines exist describing bleeding risk for common procedures. In this Progress Note, we summarize and consolidate this literature, covering a range of scenarios common to the hospital setting, including thrombocytopenia, elevated international normalized ratio (INR), and the use of medications such as antiplatelet and anticoagulant agents (Table 1 and Table 2). We performed electronic searches in PubMed, focusing on literature published since 2016. Key search terms included paracentesis, thoracentesis, lumbar puncture, anticoagulant, antiplatelet, coagulopathy, INR, thrombocytopenia, and guideline. In addition, we used the following MeSH terms: spinal puncture AND blood coagulation disorders, spinal puncture AND platelet aggregation inhibitors, spinal puncture AND anticoagulants, paracentesis AND blood coagulation disorders, paracentesis AND platelet aggregation inhibitors, paracentesis AND anticoagulants, thoracentesis AND blood coagulation disorders, thoracentesis AND platelet aggregation inhibitors, and thoracentesis AND anticoagulants.

GENERAL CONCEPTS
Weighing Risks and Benefits

Hepatic and Renal Dysfunction
In the setting of chronic liver disease, thrombocytopenia and elevated INR are generally not reliable indicators of bleeding risk.13 The included recommendations for INR and platelet count thresholds in the setting of chronic liver disease are derived from the referenced guidelines and supplemental personal communication with the guideline authors. Many antiplatelet and anticoagulant medications are partially cleared or metabolized by the liver, suggesting that hepatic dysfunction may impact drug clearance, but this has not been well studied. Impaired renal function should also be considered when determining appropriate hold times for antithrombotic drugs that are partially renally cleared. The periprocedural hold and restart times outlined in Table 2 are specific to patients without clinically significant hepatic or renal dysfunction. For patients with these conditions, further information on hold time adjustment can be found in the individual references.

Bridging Therapy
Resuming Therapy
Other Considerations
Some guidelines referenced in this article are based on data collected on procedures performed by interventional radiologists, which may or may not accurately reflect the bleeding risks of bedside procedures performed by hospitalists. In the case of LP, we included some regional anesthesia and pain procedure guidelines based on the assumption that certain procedures are analogous to LP and associated with similar bleeding risks.
PARACENTESIS
Paracentesis is a common procedure that can be performed safely at the bedside. The overall rate of serious complications is low (1%-2%), with severe hemorrhage accounting for the majority of those complications (0.97%).15 Bleeding usually occurs from puncture of an abdominal wall vein, a mesenteric varix, or an inferior epigastric artery. Certain techniques may help to mitigate serious bleeding, including the use of ultrasound to avoid overlying vessels. Paracentesis is frequently performed in patients with cirrhosis, a population at increased risk for coagulopathy, although INR and platelet counts may not reflect aggregate bleeding risk in patients with cirrhosis. The American Association for the Study of Liver Diseases released new guidelines in 2021, stating that elevated prothrombin time or thrombocytopenia is not a contraindication to paracentesis.6 The most liberal guidelines for patients without chronic liver disease suggest correcting to an INR of 2.0 to 3.0, with multiple societies suggesting that a platelet count as low as 20,000/µL is safe.2,3 As shown in Table 2, most guidelines recommend continuation of antiplatelet agents such as aspirin and thienopyridines (eg, clopidogrel, prasugrel), whereas recommendations vary regarding continuation of anticoagulant agents.
THORACENTESIS
Akin to paracentesis, thoracentesis is generally considered to be a safe bedside procedure, with an incidence of thoracentesis-associated bleeding of less than 1%.15 Certain techniques may help to mitigate serious bleeding, including the insertion of the needle over the superior aspect of the rib in an effort to avoid the intercostal neurovascular bundle, which runs along the inferior aspect of each rib. Various clinical societies have proposed INR and platelet thresholds at which the risk of bleeding from thoracentesis is thought to be acceptable. The most liberal guidelines include a target INR of
LUMBAR PUNCTURE
Compared to thoracentesis and paracentesis, LP is generally considered to be a higher-risk procedure owing to the rare possibility of spinal hematoma with associated neurologic compromise. In one retrospective review of more than 49,000 patients without coagulopathy who underwent LP, the risk for developing a spinal hematoma by 30 days post procedure was 0.20%.16 Certain techniques may help to mitigate serious bleeding, including the use of image guidance in patients with large body habitus or those with difficult anatomy. Compared with paracentesis and thoracentesis, guideline recommendations for safe INR and platelet thresholds in patients undergoing LP are based on a more limited body of evidence. Guidelines also suggest a target INR of anywhere from ≤1.5 to the most liberal suggestion of 2.0 to 3.0.2-4 The SIR guidelines categorize LP as a low–bleeding risk procedure, with a platelet threshold of 20,000/µL but note that most other societies and guidelines regard LP as a high–bleeding risk procedure with more conservative platelet thresholds.2 The Association of British Neurologists (ABN), however, allows platelets to be 40,000/µL or greater than 20,000/µL with an additional risk-benefit discussion.7 In contrast to paracentesis and thoracentesis, recommendations regarding hold times of antithrombotic medications prior to LP are more variable and sometimes more conservative. For example, some guidelines indicate that the thienopyridines can be continued, whereas others recommend holding them for up to 1 week prior to LP.2,4,7
GAPS IN KNOWLEDGE
A theme throughout the recent literature and recommendations from clinical societies is that it is uncommon for there to be one unifying recommendation for every situation, especially regarding LP. Recent guidelines remain largely based on studies that are decades old. With bedside ultrasound becoming more accessible and established in daily practice, the risk of bleeding has been decreasing, potentially making periprocedural coagulopathies and antithrombotic agents less of a concern. For example, in a retrospective study of 69,859 paracenteses, ultrasound guidance reduced the risk of bleeding complications by 68%, an odds ratio of 0.32 (95% CI, 0.25-0.41).17 More research is needed to assess procedural bleeding risks in the context of current practice standards. This article focuses on a subset of bedside procedures most commonly performed by hospitalists. Similar references for other common bedside procedures, such as arthrocentesis, central venous catheter, and arterial line placement, would be helpful. Finally, this article does not capture such nuances as needle gauge, operator experience, availability of (and comfort with) ultrasound, and variations in patient anatomy, all of which are factors that can contribute to the complexities and risks of these bedside procedures.
CONCLUSION
Although not every internal medicine physician performs bedside procedures in their practice, it is vital that all understand the cognitive aspects of common bedside procedures. This necessitates the understanding of periprocedural risks and possible complications and applying that to individual patients. Correcting coagulopathy and stopping or reversing antithrombotic agents are mitigation strategies that are associated with risk. It is therefore important to understand when coagulopathy should be corrected and when antithrombotic agents should be held and for how long. With multiple existing and sometimes conflicting guidelines regarding periprocedural management of coagulopathy and antithrombotic agents, we hope that providing consolidated tables with this information will increase efficiency, aid in risk-benefit discussions between patients and care teams, and enhance patient safety.
The practice of internal medicine includes bedside procedures such as paracentesis, thoracentesis, and lumbar puncture (LP). The American Board of Internal Medicine requires graduates of internal medicine residency programs to be competent in the cognitive components of procedural training (eg, indications, contraindications, complications) and considers it essential that trainees have opportunities to perform procedures relevant to their intended career direction.1 Whether or not the performance of procedures is part of a given hospitalist’s practice, it is necessary that hospitalists understand each procedure’s risks and mitigation strategies to prevent a range of periprocedural complications, including clinically significant bleeding. Numerous recommendations and guidelines exist describing bleeding risk for common procedures. In this Progress Note, we summarize and consolidate this literature, covering a range of scenarios common to the hospital setting, including thrombocytopenia, elevated international normalized ratio (INR), and the use of medications such as antiplatelet and anticoagulant agents (Table 1 and Table 2). We performed electronic searches in PubMed, focusing on literature published since 2016. Key search terms included paracentesis, thoracentesis, lumbar puncture, anticoagulant, antiplatelet, coagulopathy, INR, thrombocytopenia, and guideline. In addition, we used the following MeSH terms: spinal puncture AND blood coagulation disorders, spinal puncture AND platelet aggregation inhibitors, spinal puncture AND anticoagulants, paracentesis AND blood coagulation disorders, paracentesis AND platelet aggregation inhibitors, paracentesis AND anticoagulants, thoracentesis AND blood coagulation disorders, thoracentesis AND platelet aggregation inhibitors, and thoracentesis AND anticoagulants.

GENERAL CONCEPTS
Weighing Risks and Benefits

Hepatic and Renal Dysfunction
In the setting of chronic liver disease, thrombocytopenia and elevated INR are generally not reliable indicators of bleeding risk.13 The included recommendations for INR and platelet count thresholds in the setting of chronic liver disease are derived from the referenced guidelines and supplemental personal communication with the guideline authors. Many antiplatelet and anticoagulant medications are partially cleared or metabolized by the liver, suggesting that hepatic dysfunction may impact drug clearance, but this has not been well studied. Impaired renal function should also be considered when determining appropriate hold times for antithrombotic drugs that are partially renally cleared. The periprocedural hold and restart times outlined in Table 2 are specific to patients without clinically significant hepatic or renal dysfunction. For patients with these conditions, further information on hold time adjustment can be found in the individual references.

Bridging Therapy
Resuming Therapy
Other Considerations
Some guidelines referenced in this article are based on data collected on procedures performed by interventional radiologists, which may or may not accurately reflect the bleeding risks of bedside procedures performed by hospitalists. In the case of LP, we included some regional anesthesia and pain procedure guidelines based on the assumption that certain procedures are analogous to LP and associated with similar bleeding risks.
PARACENTESIS
Paracentesis is a common procedure that can be performed safely at the bedside. The overall rate of serious complications is low (1%-2%), with severe hemorrhage accounting for the majority of those complications (0.97%).15 Bleeding usually occurs from puncture of an abdominal wall vein, a mesenteric varix, or an inferior epigastric artery. Certain techniques may help to mitigate serious bleeding, including the use of ultrasound to avoid overlying vessels. Paracentesis is frequently performed in patients with cirrhosis, a population at increased risk for coagulopathy, although INR and platelet counts may not reflect aggregate bleeding risk in patients with cirrhosis. The American Association for the Study of Liver Diseases released new guidelines in 2021, stating that elevated prothrombin time or thrombocytopenia is not a contraindication to paracentesis.6 The most liberal guidelines for patients without chronic liver disease suggest correcting to an INR of 2.0 to 3.0, with multiple societies suggesting that a platelet count as low as 20,000/µL is safe.2,3 As shown in Table 2, most guidelines recommend continuation of antiplatelet agents such as aspirin and thienopyridines (eg, clopidogrel, prasugrel), whereas recommendations vary regarding continuation of anticoagulant agents.
THORACENTESIS
Akin to paracentesis, thoracentesis is generally considered to be a safe bedside procedure, with an incidence of thoracentesis-associated bleeding of less than 1%.15 Certain techniques may help to mitigate serious bleeding, including the insertion of the needle over the superior aspect of the rib in an effort to avoid the intercostal neurovascular bundle, which runs along the inferior aspect of each rib. Various clinical societies have proposed INR and platelet thresholds at which the risk of bleeding from thoracentesis is thought to be acceptable. The most liberal guidelines include a target INR of
LUMBAR PUNCTURE
Compared to thoracentesis and paracentesis, LP is generally considered to be a higher-risk procedure owing to the rare possibility of spinal hematoma with associated neurologic compromise. In one retrospective review of more than 49,000 patients without coagulopathy who underwent LP, the risk for developing a spinal hematoma by 30 days post procedure was 0.20%.16 Certain techniques may help to mitigate serious bleeding, including the use of image guidance in patients with large body habitus or those with difficult anatomy. Compared with paracentesis and thoracentesis, guideline recommendations for safe INR and platelet thresholds in patients undergoing LP are based on a more limited body of evidence. Guidelines also suggest a target INR of anywhere from ≤1.5 to the most liberal suggestion of 2.0 to 3.0.2-4 The SIR guidelines categorize LP as a low–bleeding risk procedure, with a platelet threshold of 20,000/µL but note that most other societies and guidelines regard LP as a high–bleeding risk procedure with more conservative platelet thresholds.2 The Association of British Neurologists (ABN), however, allows platelets to be 40,000/µL or greater than 20,000/µL with an additional risk-benefit discussion.7 In contrast to paracentesis and thoracentesis, recommendations regarding hold times of antithrombotic medications prior to LP are more variable and sometimes more conservative. For example, some guidelines indicate that the thienopyridines can be continued, whereas others recommend holding them for up to 1 week prior to LP.2,4,7
GAPS IN KNOWLEDGE
A theme throughout the recent literature and recommendations from clinical societies is that it is uncommon for there to be one unifying recommendation for every situation, especially regarding LP. Recent guidelines remain largely based on studies that are decades old. With bedside ultrasound becoming more accessible and established in daily practice, the risk of bleeding has been decreasing, potentially making periprocedural coagulopathies and antithrombotic agents less of a concern. For example, in a retrospective study of 69,859 paracenteses, ultrasound guidance reduced the risk of bleeding complications by 68%, an odds ratio of 0.32 (95% CI, 0.25-0.41).17 More research is needed to assess procedural bleeding risks in the context of current practice standards. This article focuses on a subset of bedside procedures most commonly performed by hospitalists. Similar references for other common bedside procedures, such as arthrocentesis, central venous catheter, and arterial line placement, would be helpful. Finally, this article does not capture such nuances as needle gauge, operator experience, availability of (and comfort with) ultrasound, and variations in patient anatomy, all of which are factors that can contribute to the complexities and risks of these bedside procedures.
CONCLUSION
Although not every internal medicine physician performs bedside procedures in their practice, it is vital that all understand the cognitive aspects of common bedside procedures. This necessitates the understanding of periprocedural risks and possible complications and applying that to individual patients. Correcting coagulopathy and stopping or reversing antithrombotic agents are mitigation strategies that are associated with risk. It is therefore important to understand when coagulopathy should be corrected and when antithrombotic agents should be held and for how long. With multiple existing and sometimes conflicting guidelines regarding periprocedural management of coagulopathy and antithrombotic agents, we hope that providing consolidated tables with this information will increase efficiency, aid in risk-benefit discussions between patients and care teams, and enhance patient safety.
1. Nichani S, Fitterman N, Lukela M, Crocker J. The core competencies in hospital medicine 2017 Revision. Section 2: procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54. https://doi.org/10.12788/jhm.2728
2. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part ii: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
3. Hadi M, Walker C, Desborough M, et al. CIRSE standards of practice on peri-operative anticoagulation management during interventional radiology procedures. Cardiovasc Intervent Radiol. 2021;44(4):523-536. https://doi.org/10.1007/s00270-020-02763-4
4. Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25(2):144-156. https://doi.org/10.5152/dir.2019.18291
5. Demirci NY, Koksal D, Bilaceroglu S, et al. Management of bleeding risk before pleural procedures: a consensus statement of Turkish Respiratory Society—Pleura study group. Consensus Report. Eurasian J Pulmonol. 2020;22(2):73-78. https://doi.org/10.4103/ejop.ejop_28_20
6. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
7. Dodd KC, Emsley HCA, Desborough MJR, Chhetri SK. Periprocedural antithrombotic management for lumbar puncture: Association of British Neurologists clinical guideline. Pract Neurol. 2018;18(6):436-446. https://doi.org/10.1136/practneurol-2017-001820
8. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. https://doi.org/10.1097/aap.0000000000000763
9. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225-262. https://doi.org/:10.1097/aap.0000000000000700
10. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847-1948. https://doi.org/10.1016/j.cjca.2020.09.001
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. https://doi.org/10.1016/j.jacc.2016.11.024
12. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/chest.11-2298
13. Crowe B, Tahhan SG, Lacy C, Grzankowski J, Lessing JN. Things we do for no reason™: Routine correction of elevated INR and thrombocytopenia prior to paracentesis in patients with cirrhosis. J Hosp Med. 2021;16(2):102-104. https://doi.org/10.12788/jhm.3458
14. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441-449. https://doi.org/10.1002/clc.23336
15. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023
16. Bodilsen J, Mariager T, Vestergaard HH, et al. Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA. 2020;324(14):1419-1428. https://doi.org/10.1001/jama.2020.14895
17. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
1. Nichani S, Fitterman N, Lukela M, Crocker J. The core competencies in hospital medicine 2017 Revision. Section 2: procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54. https://doi.org/10.12788/jhm.2728
2. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part ii: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
3. Hadi M, Walker C, Desborough M, et al. CIRSE standards of practice on peri-operative anticoagulation management during interventional radiology procedures. Cardiovasc Intervent Radiol. 2021;44(4):523-536. https://doi.org/10.1007/s00270-020-02763-4
4. Özütemiz C, Rykken JB. Lumbar puncture under fluoroscopy guidance: a technical review for radiologists. Diagn Interv Radiol. 2019;25(2):144-156. https://doi.org/10.5152/dir.2019.18291
5. Demirci NY, Koksal D, Bilaceroglu S, et al. Management of bleeding risk before pleural procedures: a consensus statement of Turkish Respiratory Society—Pleura study group. Consensus Report. Eurasian J Pulmonol. 2020;22(2):73-78. https://doi.org/10.4103/ejop.ejop_28_20
6. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
7. Dodd KC, Emsley HCA, Desborough MJR, Chhetri SK. Periprocedural antithrombotic management for lumbar puncture: Association of British Neurologists clinical guideline. Pract Neurol. 2018;18(6):436-446. https://doi.org/10.1136/practneurol-2017-001820
8. Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Fourth Edition). Reg Anesth Pain Med. 2018;43(3):263-309. https://doi.org/10.1097/aap.0000000000000763
9. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (Second Edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43(3):225-262. https://doi.org/:10.1097/aap.0000000000000700
10. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol. 2020;36(12):1847-1948. https://doi.org/10.1016/j.cjca.2020.09.001
11. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. https://doi.org/10.1016/j.jacc.2016.11.024
12. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S-e350S. https://doi.org/10.1378/chest.11-2298
13. Crowe B, Tahhan SG, Lacy C, Grzankowski J, Lessing JN. Things we do for no reason™: Routine correction of elevated INR and thrombocytopenia prior to paracentesis in patients with cirrhosis. J Hosp Med. 2021;16(2):102-104. https://doi.org/10.12788/jhm.3458
14. Kuo HC, Liu FL, Chen JT, Cherng YG, Tam KW, Tai YH. Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic review and meta-analysis. Clin Cardiol. 2020;43(5):441-449. https://doi.org/10.1002/clc.23336
15. Wolfe KS, Kress JP. Risk of procedural hemorrhage. Chest. 2016;150(1):237-246. https://doi.org/10.1016/j.chest.2016.01.023
16. Bodilsen J, Mariager T, Vestergaard HH, et al. Association of lumbar puncture with spinal hematoma in patients with and without coagulopathy. JAMA. 2020;324(14):1419-1428. https://doi.org/10.1001/jama.2020.14895
17. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. https://doi.org/10.1378/chest.12-0447
© 2021 Society of Hospital Medicine
Methodologic Progress Note: A Clinician’s Guide to Logistic Regression
The ability to read and correctly interpret research is an essential skill, but most hospitalists—and physicians in general—do not receive formal training in biostatistics during their medical education.1-3 In addition to straightforward statistical tests that compare a single exposure and outcome, researchers commonly use statistical models to identify and quantify complex relationships among many exposures (eg, demographics, clinical characteristics, interventions, or other variables) and an outcome. Understanding statistical models can be challenging. Still, it is important to recognize the advantages and limitations of statistical models, how to interpret their results, and the potential implications of findings on current clinical practice.
In the article “Rates and Characteristics of Medical Malpractice Claims Against Hospitalists” published in the July 2021 issue of the Journal of Hospital Medicine, Schaffer et al4 used the Comparative Benchmarking System database, which is maintained by a malpractice insurer, to characterize malpractice claims against hospitalists. The authors used multiple logistic regression models to understand the relationship among clinical factors and indemnity payments. In this Progress Note, we describe situations in which logistic regression is the proper statistical method to analyze a data set, explain results from logistic regression analyses, and equip readers with skills to critically appraise conclusions drawn from these models.
Choosing an Appropriate Statistical Model
Statistical models often are used to describe the relationship among one or more exposure variables (ie, independent variables) and an outcome (ie, dependent variable). These models allow researchers to evaluate the effects of multiple exposure variables simultaneously, which in turn allows them to “isolate” the effect of each variable; in other words, models facilitate an understanding of the relationship between each exposure variable and the outcome, adjusted for (ie, independent of) the other exposure variables in the model.
Several statistical models can be used to quantify relationships within the data, but each type of model has certain assumptions that must be satisfied. Two important assumptions include characteristics of the outcome (eg, the type and distribution) and the nature of the relationships among the outcome and independent variables (eg, linear vs nonlinear). Simple linear regression, one of the most basic statistical models used in research,5 assumes that (a) the outcome is continuous (ie, any numeric value is possible) and normally distributed (ie, its histogram is a bell-shaped curve) and (b) the relationship between the independent variable and the outcome is linear (ie, follows a straight line). If an investigator wanted to understand how weight is related to height, a simple linear regression could be used to develop a mathematical equation that tells us how the outcome (weight) generally increases as the independent variable (height) increases.
Often, the outcome in a study is not a continuous variable but a simple success/failure variable (ie, dichotomous variable that can be one of two possible values). Schaffer et al4 examined the binary outcome of whether a malpractice claim case would end in an indemnity payment or no payment. Linear regression models are not equipped to handle dichotomous outcomes. Instead, we need to use a different statistical model: logistic regression. In logistic regression, the probability (p) of a defined outcome event is estimated by creating a regression model.
The Logistic Model
A probability (p) is a measure of how likely an event (eg, a malpractice claim ends in an indemnity payment or not) is to occur. It is always between 0 (ie, the event will definitely not occur) and 1 (ie, the event will definitely occur). A p of 0.5 means there is a 50/50 chance that the event will occur (ie, equivalent to a coin flip). Because p is a probability, we need to make sure it is always between 0 and 1. If we were to try to model p with a linear regression, the model would assume that p could extend beyond 0 and 1. What can we do?
Applying a transformation is a commonly used tool in statistics to make data work better within statistical models.6 In this case, we will transform the variable p. In logistic regression, we model the probability of experiencing the outcome through a transformation called a logit. The logit represents the natural logarithm (ln) of the ratio of the probability of experiencing the outcome (p) vs the probability of not experiencing the outcome (1 – p), with the ratio being the odds of the event occurring.
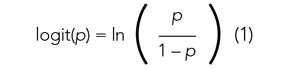
This transformation works well for dichotomous outcomes because the logit transformation approximates a straight line as long as p is not too large or too small (between 0.05 and 0.95).
If we are performing a logistic regression with only one independent variable (x) and want to understand the relationship between this variable (x) and the probability of an outcome event (p), then our model is the equation of a line. The equation for the base model of logistic regression with one independent variable (x) is
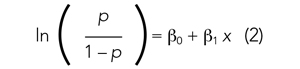
where β0 is the y-intercept and β1 is the slope of the line. Equation (2) is identical to the algebraic equation y = mx + b for a line, just rearranged slightly. In this algebraic equation, m is the slope (the same as β1) and b is the y-intercept (the same as β0). We will see that β0 and β1 are estimated (ie, assigned numeric values) from the data collected to help us understand how x and
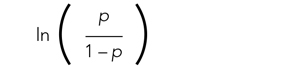
are related and are the basis for estimating odds ratios.
We can build more complex models using multivariable logistic regression by adding more independent variables to the right side of equation (2). Essentially, this is what S
There are two notable techniques used frequently with multivariable logistic regression models. The first involves choosing which independent variables to include in the model. One way to select variables for multivariable models is defining them a priori, that is deciding which variables are clinically or conceptually associated with the outcome before looking at the data. With this approach, we can test specific hypotheses about the relationships between the independent variables and the outcome. Another common approach is to look at the data and identify the variables that vary significantly between the two outcome groups. Schaffer et al4 used an a priori approach to define variables in their multivariable model (ie, “variables for inclusion into the multivariable model were determined a priori”).
A second technique is the evaluation of collinearity, which helps us understand whether the i
Understanding the Results of the Logistic Model
Fitting the model is the process by which statistical software (eg, SAS, Stata, R, SPSS) estimates the relationships among independent variables in the model and the outcome within a specific dataset. In equation (2), this essentially means that the software will evaluate the data and provide us with the best estimates for β0 (the y-intercept) and β1 (the slope) that describe the relationship between the variable x and
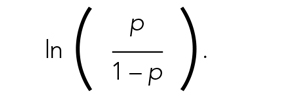
Modeling can be iterative, and part of the process may include removing variables from the model that are not significantly associated with the outcome to create a simpler solution, a process known as model reduction. The results from models describe the independent association between a specific characteristic and the outcome, meaning that the relationship has been adjusted for all the other characteristics in the model.
The relationships among the independent variables and outcome are most often represented as an odds ratio (OR), which quantifies the strength of the association between two variables and is directly calculated from the β values in the model. As the name suggests, an OR is a ratio of odds. But what are odds? Simply, the odds of an outcome (such as mortality) is the probability of experiencing the event divided by the probability of not experiencing that event; in other words, it is the ratio:

The concept of odds is often unfamiliar, so it can be helpful to consider the definition in the context of games of chance. For example, in horse race betting, the outcome of interest is that a horse will lose a race. Imagine that the probability of a horse losing a race is 0.8 and the probability of winning is 0.2. The odds of losing are
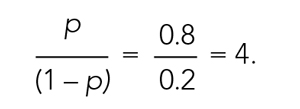
These odds usually are listed as 4-to-1, meaning that out of 5 races (ie, 4 + 1) the horse is expected to lose 4 times and win once. When odds are listed this way, we can easily calculate the associated probability by recognizing that the total number of expected races is the sum of two numbers (probability of losing: 4 races out of 5, or 0.80 vs probability of winning: 1 race out of 5, or 0.20).
In medical research, the OR typically represents the odds for one group of patients (A) compared with the odds for another group of patients (B) experiencing an outcome. If the odds of the outcome are the same for group A and group B, then OR = 1.0, meaning that the probability of the outcome is the same between the two groups. If the patients in group A have greater odds of experiencing the outcome compared with group B patients (and a greater probability of the outcome), then the OR will be >1. If the opposite is true, then the OR will be <1.
Schaffer et al4 estimated that the OR of an indemnity payment in malpractice cases involving errors in clinical judgment as a contributing factor was 5.01 (95% CI, 3.37-7.45). This means that malpractice cases involving errors in clinical judgement had a 5.01 times greater odds of indemnity payment compared with those without these errors after adjusting for all other variables in the model (eg, age, severity). Note that the 95% CI does not include 1.0. This indicates that the OR is statistically >1, and we can conclude that there is a significant relationship between errors in clinical judgment and payment that is unlikely to be attributed to chance alone.
In logistic regression for categorical independent variables, all categories are compared with a reference group within that variable, with the reference group serving as the denominator of the OR. The authors4 did not incorporate continuous independent variables in their multivariable logistic regression model. However, if the authors examined length of hospitalization as a contributing factor in indemnity payments, for example, the OR would represent a 1-unit increase in this variable (eg, 1-day increase in length of stay).
Conclusion
Logistic regression describes the relationships in data and is an important statistical model across many types of research. This Progress Note emphasizes the importance of weighing the advantages and limitations of logistic regression, provides a common approach to data transformation, and guides the correct interpretation of logistic regression model results.
1. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010. https://doi.org/10.1001/jama.298.9.1010
2. MacDougall M, Cameron HS, Maxwell SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. 2019;20(1):1. https://doi.org/10.1186/s12909-019-1842-1
3. Montori VM. Progress in evidence-based medicine. JAMA. 2008;300(15):1814-1816. https://doi.org/10.1001/jama.300.15.1814
4. Schaffer AC, Yu-Moe CW, Babayan A, Wachter RM, Einbinder JS. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021;16(7):390-396. https://doi.org/10.12788/jhm.3557
5. Lane DM, Scott D, Hebl M, Guerra R, Osherson D, Zimmer H. Introducton to Statistics. Accessed April 13, 2021. https://onlinestatbook.com/Online_Statistics_Education.pdf
6. Marill KA. Advanced statistics: linear regression, part II: multiple linear regression. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11(1):94-102. https://doi.org/10.1197/j.aem.2003.09.006
The ability to read and correctly interpret research is an essential skill, but most hospitalists—and physicians in general—do not receive formal training in biostatistics during their medical education.1-3 In addition to straightforward statistical tests that compare a single exposure and outcome, researchers commonly use statistical models to identify and quantify complex relationships among many exposures (eg, demographics, clinical characteristics, interventions, or other variables) and an outcome. Understanding statistical models can be challenging. Still, it is important to recognize the advantages and limitations of statistical models, how to interpret their results, and the potential implications of findings on current clinical practice.
In the article “Rates and Characteristics of Medical Malpractice Claims Against Hospitalists” published in the July 2021 issue of the Journal of Hospital Medicine, Schaffer et al4 used the Comparative Benchmarking System database, which is maintained by a malpractice insurer, to characterize malpractice claims against hospitalists. The authors used multiple logistic regression models to understand the relationship among clinical factors and indemnity payments. In this Progress Note, we describe situations in which logistic regression is the proper statistical method to analyze a data set, explain results from logistic regression analyses, and equip readers with skills to critically appraise conclusions drawn from these models.
Choosing an Appropriate Statistical Model
Statistical models often are used to describe the relationship among one or more exposure variables (ie, independent variables) and an outcome (ie, dependent variable). These models allow researchers to evaluate the effects of multiple exposure variables simultaneously, which in turn allows them to “isolate” the effect of each variable; in other words, models facilitate an understanding of the relationship between each exposure variable and the outcome, adjusted for (ie, independent of) the other exposure variables in the model.
Several statistical models can be used to quantify relationships within the data, but each type of model has certain assumptions that must be satisfied. Two important assumptions include characteristics of the outcome (eg, the type and distribution) and the nature of the relationships among the outcome and independent variables (eg, linear vs nonlinear). Simple linear regression, one of the most basic statistical models used in research,5 assumes that (a) the outcome is continuous (ie, any numeric value is possible) and normally distributed (ie, its histogram is a bell-shaped curve) and (b) the relationship between the independent variable and the outcome is linear (ie, follows a straight line). If an investigator wanted to understand how weight is related to height, a simple linear regression could be used to develop a mathematical equation that tells us how the outcome (weight) generally increases as the independent variable (height) increases.
Often, the outcome in a study is not a continuous variable but a simple success/failure variable (ie, dichotomous variable that can be one of two possible values). Schaffer et al4 examined the binary outcome of whether a malpractice claim case would end in an indemnity payment or no payment. Linear regression models are not equipped to handle dichotomous outcomes. Instead, we need to use a different statistical model: logistic regression. In logistic regression, the probability (p) of a defined outcome event is estimated by creating a regression model.
The Logistic Model
A probability (p) is a measure of how likely an event (eg, a malpractice claim ends in an indemnity payment or not) is to occur. It is always between 0 (ie, the event will definitely not occur) and 1 (ie, the event will definitely occur). A p of 0.5 means there is a 50/50 chance that the event will occur (ie, equivalent to a coin flip). Because p is a probability, we need to make sure it is always between 0 and 1. If we were to try to model p with a linear regression, the model would assume that p could extend beyond 0 and 1. What can we do?
Applying a transformation is a commonly used tool in statistics to make data work better within statistical models.6 In this case, we will transform the variable p. In logistic regression, we model the probability of experiencing the outcome through a transformation called a logit. The logit represents the natural logarithm (ln) of the ratio of the probability of experiencing the outcome (p) vs the probability of not experiencing the outcome (1 – p), with the ratio being the odds of the event occurring.
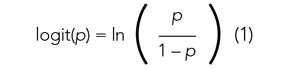
This transformation works well for dichotomous outcomes because the logit transformation approximates a straight line as long as p is not too large or too small (between 0.05 and 0.95).
If we are performing a logistic regression with only one independent variable (x) and want to understand the relationship between this variable (x) and the probability of an outcome event (p), then our model is the equation of a line. The equation for the base model of logistic regression with one independent variable (x) is
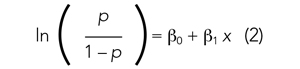
where β0 is the y-intercept and β1 is the slope of the line. Equation (2) is identical to the algebraic equation y = mx + b for a line, just rearranged slightly. In this algebraic equation, m is the slope (the same as β1) and b is the y-intercept (the same as β0). We will see that β0 and β1 are estimated (ie, assigned numeric values) from the data collected to help us understand how x and
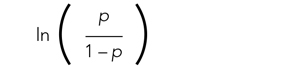
are related and are the basis for estimating odds ratios.
We can build more complex models using multivariable logistic regression by adding more independent variables to the right side of equation (2). Essentially, this is what S
There are two notable techniques used frequently with multivariable logistic regression models. The first involves choosing which independent variables to include in the model. One way to select variables for multivariable models is defining them a priori, that is deciding which variables are clinically or conceptually associated with the outcome before looking at the data. With this approach, we can test specific hypotheses about the relationships between the independent variables and the outcome. Another common approach is to look at the data and identify the variables that vary significantly between the two outcome groups. Schaffer et al4 used an a priori approach to define variables in their multivariable model (ie, “variables for inclusion into the multivariable model were determined a priori”).
A second technique is the evaluation of collinearity, which helps us understand whether the i
Understanding the Results of the Logistic Model
Fitting the model is the process by which statistical software (eg, SAS, Stata, R, SPSS) estimates the relationships among independent variables in the model and the outcome within a specific dataset. In equation (2), this essentially means that the software will evaluate the data and provide us with the best estimates for β0 (the y-intercept) and β1 (the slope) that describe the relationship between the variable x and
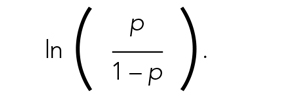
Modeling can be iterative, and part of the process may include removing variables from the model that are not significantly associated with the outcome to create a simpler solution, a process known as model reduction. The results from models describe the independent association between a specific characteristic and the outcome, meaning that the relationship has been adjusted for all the other characteristics in the model.
The relationships among the independent variables and outcome are most often represented as an odds ratio (OR), which quantifies the strength of the association between two variables and is directly calculated from the β values in the model. As the name suggests, an OR is a ratio of odds. But what are odds? Simply, the odds of an outcome (such as mortality) is the probability of experiencing the event divided by the probability of not experiencing that event; in other words, it is the ratio:

The concept of odds is often unfamiliar, so it can be helpful to consider the definition in the context of games of chance. For example, in horse race betting, the outcome of interest is that a horse will lose a race. Imagine that the probability of a horse losing a race is 0.8 and the probability of winning is 0.2. The odds of losing are
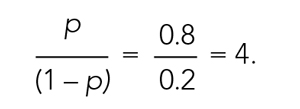
These odds usually are listed as 4-to-1, meaning that out of 5 races (ie, 4 + 1) the horse is expected to lose 4 times and win once. When odds are listed this way, we can easily calculate the associated probability by recognizing that the total number of expected races is the sum of two numbers (probability of losing: 4 races out of 5, or 0.80 vs probability of winning: 1 race out of 5, or 0.20).
In medical research, the OR typically represents the odds for one group of patients (A) compared with the odds for another group of patients (B) experiencing an outcome. If the odds of the outcome are the same for group A and group B, then OR = 1.0, meaning that the probability of the outcome is the same between the two groups. If the patients in group A have greater odds of experiencing the outcome compared with group B patients (and a greater probability of the outcome), then the OR will be >1. If the opposite is true, then the OR will be <1.
Schaffer et al4 estimated that the OR of an indemnity payment in malpractice cases involving errors in clinical judgment as a contributing factor was 5.01 (95% CI, 3.37-7.45). This means that malpractice cases involving errors in clinical judgement had a 5.01 times greater odds of indemnity payment compared with those without these errors after adjusting for all other variables in the model (eg, age, severity). Note that the 95% CI does not include 1.0. This indicates that the OR is statistically >1, and we can conclude that there is a significant relationship between errors in clinical judgment and payment that is unlikely to be attributed to chance alone.
In logistic regression for categorical independent variables, all categories are compared with a reference group within that variable, with the reference group serving as the denominator of the OR. The authors4 did not incorporate continuous independent variables in their multivariable logistic regression model. However, if the authors examined length of hospitalization as a contributing factor in indemnity payments, for example, the OR would represent a 1-unit increase in this variable (eg, 1-day increase in length of stay).
Conclusion
Logistic regression describes the relationships in data and is an important statistical model across many types of research. This Progress Note emphasizes the importance of weighing the advantages and limitations of logistic regression, provides a common approach to data transformation, and guides the correct interpretation of logistic regression model results.
The ability to read and correctly interpret research is an essential skill, but most hospitalists—and physicians in general—do not receive formal training in biostatistics during their medical education.1-3 In addition to straightforward statistical tests that compare a single exposure and outcome, researchers commonly use statistical models to identify and quantify complex relationships among many exposures (eg, demographics, clinical characteristics, interventions, or other variables) and an outcome. Understanding statistical models can be challenging. Still, it is important to recognize the advantages and limitations of statistical models, how to interpret their results, and the potential implications of findings on current clinical practice.
In the article “Rates and Characteristics of Medical Malpractice Claims Against Hospitalists” published in the July 2021 issue of the Journal of Hospital Medicine, Schaffer et al4 used the Comparative Benchmarking System database, which is maintained by a malpractice insurer, to characterize malpractice claims against hospitalists. The authors used multiple logistic regression models to understand the relationship among clinical factors and indemnity payments. In this Progress Note, we describe situations in which logistic regression is the proper statistical method to analyze a data set, explain results from logistic regression analyses, and equip readers with skills to critically appraise conclusions drawn from these models.
Choosing an Appropriate Statistical Model
Statistical models often are used to describe the relationship among one or more exposure variables (ie, independent variables) and an outcome (ie, dependent variable). These models allow researchers to evaluate the effects of multiple exposure variables simultaneously, which in turn allows them to “isolate” the effect of each variable; in other words, models facilitate an understanding of the relationship between each exposure variable and the outcome, adjusted for (ie, independent of) the other exposure variables in the model.
Several statistical models can be used to quantify relationships within the data, but each type of model has certain assumptions that must be satisfied. Two important assumptions include characteristics of the outcome (eg, the type and distribution) and the nature of the relationships among the outcome and independent variables (eg, linear vs nonlinear). Simple linear regression, one of the most basic statistical models used in research,5 assumes that (a) the outcome is continuous (ie, any numeric value is possible) and normally distributed (ie, its histogram is a bell-shaped curve) and (b) the relationship between the independent variable and the outcome is linear (ie, follows a straight line). If an investigator wanted to understand how weight is related to height, a simple linear regression could be used to develop a mathematical equation that tells us how the outcome (weight) generally increases as the independent variable (height) increases.
Often, the outcome in a study is not a continuous variable but a simple success/failure variable (ie, dichotomous variable that can be one of two possible values). Schaffer et al4 examined the binary outcome of whether a malpractice claim case would end in an indemnity payment or no payment. Linear regression models are not equipped to handle dichotomous outcomes. Instead, we need to use a different statistical model: logistic regression. In logistic regression, the probability (p) of a defined outcome event is estimated by creating a regression model.
The Logistic Model
A probability (p) is a measure of how likely an event (eg, a malpractice claim ends in an indemnity payment or not) is to occur. It is always between 0 (ie, the event will definitely not occur) and 1 (ie, the event will definitely occur). A p of 0.5 means there is a 50/50 chance that the event will occur (ie, equivalent to a coin flip). Because p is a probability, we need to make sure it is always between 0 and 1. If we were to try to model p with a linear regression, the model would assume that p could extend beyond 0 and 1. What can we do?
Applying a transformation is a commonly used tool in statistics to make data work better within statistical models.6 In this case, we will transform the variable p. In logistic regression, we model the probability of experiencing the outcome through a transformation called a logit. The logit represents the natural logarithm (ln) of the ratio of the probability of experiencing the outcome (p) vs the probability of not experiencing the outcome (1 – p), with the ratio being the odds of the event occurring.
This transformation works well for dichotomous outcomes because the logit transformation approximates a straight line as long as p is not too large or too small (between 0.05 and 0.95).
If we are performing a logistic regression with only one independent variable (x) and want to understand the relationship between this variable (x) and the probability of an outcome event (p), then our model is the equation of a line. The equation for the base model of logistic regression with one independent variable (x) is
where β0 is the y-intercept and β1 is the slope of the line. Equation (2) is identical to the algebraic equation y = mx + b for a line, just rearranged slightly. In this algebraic equation, m is the slope (the same as β1) and b is the y-intercept (the same as β0). We will see that β0 and β1 are estimated (ie, assigned numeric values) from the data collected to help us understand how x and
are related and are the basis for estimating odds ratios.
We can build more complex models using multivariable logistic regression by adding more independent variables to the right side of equation (2). Essentially, this is what S
There are two notable techniques used frequently with multivariable logistic regression models. The first involves choosing which independent variables to include in the model. One way to select variables for multivariable models is defining them a priori, that is deciding which variables are clinically or conceptually associated with the outcome before looking at the data. With this approach, we can test specific hypotheses about the relationships between the independent variables and the outcome. Another common approach is to look at the data and identify the variables that vary significantly between the two outcome groups. Schaffer et al4 used an a priori approach to define variables in their multivariable model (ie, “variables for inclusion into the multivariable model were determined a priori”).
A second technique is the evaluation of collinearity, which helps us understand whether the i
Understanding the Results of the Logistic Model
Fitting the model is the process by which statistical software (eg, SAS, Stata, R, SPSS) estimates the relationships among independent variables in the model and the outcome within a specific dataset. In equation (2), this essentially means that the software will evaluate the data and provide us with the best estimates for β0 (the y-intercept) and β1 (the slope) that describe the relationship between the variable x and
Modeling can be iterative, and part of the process may include removing variables from the model that are not significantly associated with the outcome to create a simpler solution, a process known as model reduction. The results from models describe the independent association between a specific characteristic and the outcome, meaning that the relationship has been adjusted for all the other characteristics in the model.
The relationships among the independent variables and outcome are most often represented as an odds ratio (OR), which quantifies the strength of the association between two variables and is directly calculated from the β values in the model. As the name suggests, an OR is a ratio of odds. But what are odds? Simply, the odds of an outcome (such as mortality) is the probability of experiencing the event divided by the probability of not experiencing that event; in other words, it is the ratio:

The concept of odds is often unfamiliar, so it can be helpful to consider the definition in the context of games of chance. For example, in horse race betting, the outcome of interest is that a horse will lose a race. Imagine that the probability of a horse losing a race is 0.8 and the probability of winning is 0.2. The odds of losing are
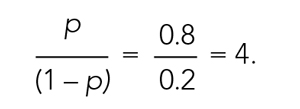
These odds usually are listed as 4-to-1, meaning that out of 5 races (ie, 4 + 1) the horse is expected to lose 4 times and win once. When odds are listed this way, we can easily calculate the associated probability by recognizing that the total number of expected races is the sum of two numbers (probability of losing: 4 races out of 5, or 0.80 vs probability of winning: 1 race out of 5, or 0.20).
In medical research, the OR typically represents the odds for one group of patients (A) compared with the odds for another group of patients (B) experiencing an outcome. If the odds of the outcome are the same for group A and group B, then OR = 1.0, meaning that the probability of the outcome is the same between the two groups. If the patients in group A have greater odds of experiencing the outcome compared with group B patients (and a greater probability of the outcome), then the OR will be >1. If the opposite is true, then the OR will be <1.
Schaffer et al4 estimated that the OR of an indemnity payment in malpractice cases involving errors in clinical judgment as a contributing factor was 5.01 (95% CI, 3.37-7.45). This means that malpractice cases involving errors in clinical judgement had a 5.01 times greater odds of indemnity payment compared with those without these errors after adjusting for all other variables in the model (eg, age, severity). Note that the 95% CI does not include 1.0. This indicates that the OR is statistically >1, and we can conclude that there is a significant relationship between errors in clinical judgment and payment that is unlikely to be attributed to chance alone.
In logistic regression for categorical independent variables, all categories are compared with a reference group within that variable, with the reference group serving as the denominator of the OR. The authors4 did not incorporate continuous independent variables in their multivariable logistic regression model. However, if the authors examined length of hospitalization as a contributing factor in indemnity payments, for example, the OR would represent a 1-unit increase in this variable (eg, 1-day increase in length of stay).
Conclusion
Logistic regression describes the relationships in data and is an important statistical model across many types of research. This Progress Note emphasizes the importance of weighing the advantages and limitations of logistic regression, provides a common approach to data transformation, and guides the correct interpretation of logistic regression model results.
1. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010. https://doi.org/10.1001/jama.298.9.1010
2. MacDougall M, Cameron HS, Maxwell SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. 2019;20(1):1. https://doi.org/10.1186/s12909-019-1842-1
3. Montori VM. Progress in evidence-based medicine. JAMA. 2008;300(15):1814-1816. https://doi.org/10.1001/jama.300.15.1814
4. Schaffer AC, Yu-Moe CW, Babayan A, Wachter RM, Einbinder JS. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021;16(7):390-396. https://doi.org/10.12788/jhm.3557
5. Lane DM, Scott D, Hebl M, Guerra R, Osherson D, Zimmer H. Introducton to Statistics. Accessed April 13, 2021. https://onlinestatbook.com/Online_Statistics_Education.pdf
6. Marill KA. Advanced statistics: linear regression, part II: multiple linear regression. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11(1):94-102. https://doi.org/10.1197/j.aem.2003.09.006
1. Windish DM, Huot SJ, Green ML. Medicine residents’ understanding of the biostatistics and results in the medical literature. JAMA. 2007;298(9):1010. https://doi.org/10.1001/jama.298.9.1010
2. MacDougall M, Cameron HS, Maxwell SRJ. Medical graduate views on statistical learning needs for clinical practice: a comprehensive survey. BMC Med Educ. 2019;20(1):1. https://doi.org/10.1186/s12909-019-1842-1
3. Montori VM. Progress in evidence-based medicine. JAMA. 2008;300(15):1814-1816. https://doi.org/10.1001/jama.300.15.1814
4. Schaffer AC, Yu-Moe CW, Babayan A, Wachter RM, Einbinder JS. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021;16(7):390-396. https://doi.org/10.12788/jhm.3557
5. Lane DM, Scott D, Hebl M, Guerra R, Osherson D, Zimmer H. Introducton to Statistics. Accessed April 13, 2021. https://onlinestatbook.com/Online_Statistics_Education.pdf
6. Marill KA. Advanced statistics: linear regression, part II: multiple linear regression. Acad Emerg Med Off J Soc Acad Emerg Med. 2004;11(1):94-102. https://doi.org/10.1197/j.aem.2003.09.006
© 2021 Society of Hospital Medicine
Clinical Progress Note: Intravenous Human Albumin in Patients With Cirrhosis
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).
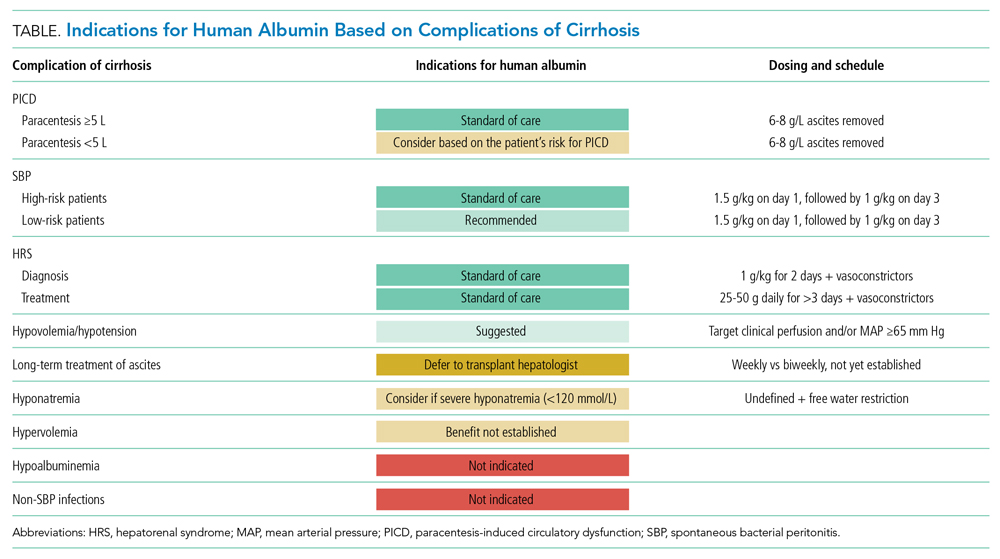
Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
The burden of chronic liver disease (CLD) in the United States is growing, and it is currently the fourth leading cause of death in adults aged 45 to 64 years.1 From 2012 to 2016, there were 538,720 hospitalizations in the United States for patients with cirrhosis, with almost a quarter having at least one cirrhosis-related complication. Inpatient hospitalizations for cirrhosis contribute to healthcare resource utilization, with a mean cost per CLD-related hospitalization of $16,271, and the presence of cirrhosis results in higher mortality and cost burden.1
In hospitalized patients with decompensated cirrhosis with ascites, intravenous human albumin (HA) infusion has been utilized for decades for a variety of indications. Current guidance by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommends the use of albumin for the prevention of paracentesis-induced circulatory dysfunction (PICD) for the prevention of kidney injury in spontaneous bacterial peritonitis (SBP) and for the diagnosis and treatment of hepatorenal syndrome (HRS).2,3 There have been several major trials in recent years studying the use of HA for other indications in patients with cirrhosis, and the Society of Critical Care Medicine (SCCM) updated their guidelines in 2020 to recommend HA administration in resuscitation of critically ill patients with liver failure with hypoalbuminemia.4This Clinical Progress Note addresses the use of albumin in hospitalized patients with cirrhosis, focusing on current indications and discussing potential uses published after the 2018 EASL guidelines. We conducted a literature search via the PubMed database. The authors began by using the Medical Subject Heading (MeSH) terms albumins/administration AND dosage; organization AND administration; adverse effects; and therapeutic use combined with liver cirrhosis as a MeSH major topic, which yielded 107 English-language articles published in the previous 10 years, and MeSH major topics of albumins and liver cirrhosis, which yielded 461 English-language articles, with 178 published in the previous 10 years. The search results were reviewed for applicability to albumin strategies for patients with cirrhosis.
CURRENT EVIDENCE-BASED INDICATIONS FOR USE OF ALBUMIN IN PATIENTS WITH CIRRHOSIS
There are three widely accepted and evidence-based indications for HA infusion in patients with cirrhosis, considered standard of care (Table).

Prevention of PICD
Therapeutic large-volume paracentesis (LVP) leads to a rise in plasma renin activity (PRA) centrally through several mechanisms and is not impacted by the rate of ascites removal.5 LVP relieves abdominal pressure, increasing venous return to the heart and cardiac output, and the corresponding drop in systemic vascular resistance with splanchnic vasodilation decreases effective circulating volume and activates the renin-angiotensin system. This PRA activation and circulatory dysfunction are associated with reaccumulating ascites, renal impairment, hypervolemic hyponatremia, and increased mortality.6 A large meta-analysis of 17 trials with 1225 patients found that HA infusion improves outcomes and reduces mortality for patients undergoing LVP (odds ratio [OR], 0.64; 95% CI, 0.41-0.98), reduces the risk of PICD more than other volume expanders tested, and lowers the incidence of hyponatremia.6 More recently, in 2017, Kütting et al7 analyzed 21 trials with 1277 patients and did not observe a significant mortality benefit for HA after LVP (OR, 0.78; 95% CI, 0.55-1.11). However, negative outcomes such as rise in PRA (OR, 0.53; 95% CI, 0.29-0.97) and hyponatremia (OR, 0.62; 95% CI, 0.42-0.94) were prevented. Guidelines recommend HA after LVP ≥5 L to prevent PICD, with a replacement volume of 6 to 8 g of albumin per liter of ascitic fluid removed.2,3 Some patients may be at higher risk for PICD with less ascites removed, and the AASLD supports the use of HA to prevent PICD after smaller-volume paracentesis in patients who are already hypotensive (systolic blood pressure <90 mm Hg) or hyponatremic (<130 mmol/L), or have acute kidney injury.3
Spontaneous Bacterial Peritonitis
Spontaneous bacterial peritonitis is diagnosed by paracentesis, defined as ascitic neutrophil count ≥250 cells/µL with or without bacterascites (positive bacteriological culture). Bacterascites may be a precursor to the development of SBP, with the fluid neutrophil count of ≥250 determining the need for SBP treatment.2 SBP can lead to circulatory dysfunction, hepatic encephalopathy, and HRS. Treating SBP with HA in addition to antibiotics reduces the risk of kidney injury compared with antibiotics alone (OR for kidney injury with antibiotics alone, 4.6; 95% CI, 1.3-16.1) and also reduces the risk of death (OR for mortality with antibiotics alone, 4.5; 95% CI, 1.0-20.9).8 The AASLD recommends albumin in addition to antibiotics in SBP to prevent HRS and acute kidney injury, and high-risk patients who already have kidney dysfunction (creatinine >1 mg/dL) or jaundice (total bilirubin >5 mg/dL) are more likely to benefit from albumin. The treatment schedule is 25% HA at 1.5 g/kg on day 1 and 1 g/kg on day 3.3 The EASL recommends administering HA to all patients with cirrhosis with SBP regardless of renal or liver indices. They acknowledge, however, that the incidence of SBP-associated acute kidney injury will be low in patients without severe hepatic disease or baseline renal impairment.2
Hepatorenal Syndrome
Albumin combined with vasoconstrictors is effective in treating HRS with a response rate of 20% to 80% (average, 50%).3 Vasoactive medications can include combination midodrine and octreotide or norepinephrine (or terlipressin outside of the United States). In patients with suspected HRS, the recommended dosing of 25% HA is 1 g/kg (to a maximum of 100 g of albumin) on day 1 and then 40 to 50 g daily for at least 3 days after the diagnosis is confirmed.3 The optimal duration of therapy beyond 3 days of combined therapy with midodrine, albumin, and octreotide is not established. Terlipressin treatment is recommended for a maximum of 14 days in cases of partial response or nonresponse in renal recovery.2
INDICATIONS FOR ALBUMIN WITHOUT CLEAR EVIDENCE OF EFFICACY
Hypoalbuminemia
Albumin administration to raise serum albumin levels in hospitalized patients has been a common practice. However, new evidence suggests that treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis does not protect patients from risk and causes harm. The Albumin To prevenT Infection in chronic liveR (ATTIRE) trial, published in 2021, randomly assigned 777 patients across 35 centers in the United Kingdom to receive daily 20% HA to target a serum albumin level of 3.0 g/dL vs standard care, including HA for established indications.2,3 The primary end point was a composite of infection, kidney dysfunction, and death within 3 to 15 days of initiating treatment. There were no differences in the primary end point; secondary end points of death at 28 days, 3 months, or 6 months; or duration of hospitalization. The treatment group received 10 times more albumin than the control group and reported more adverse events, including pulmonary edema.9
Long-Term Treatment in Patients With Ascites
The human Albumin for the treatmeNt of aScites in patients With hEpatic ciRrhosis (ANSWER) trial, published in 2018, found improved 18-month survival in patients with cirrhosis and ascites treated with diuretics who received long-term albumin. This was an open-label trial of 431 patients at 33 sites in Italy, and the treatment arm received weekly infusions of 40 g of 20% HA. They observed a 38% reduction in mortality hazard ratio and half the number of hospital days annually.10 Based on these data and those from a 2006 Italian study with similar design and results, the Italian Association for the Study of the Liver (AISF) strongly recommends long-term albumin treatment in patients with cirrhosis with ascites.11 The lead author on the ANSWER trial also authored the AISF statement, although this recommendation has not been adopted by the EASL or the AASLD.
Conversely, the Midodrine and Albumin for CirrHoTic patients (MACHT) trial, also published in 2018, randomly assigned 173 patients with ascites awaiting liver transplant to receive 40 g of HA every 15 days and midodrine in addition to standard care vs placebo. MACHT found no difference in mortality or complications at 1 year.12
Long-term albumin therapy as a preventive measure may be a disease modifier, taking advantage of the pleiotropic effects of albumin, though the differing conclusions from ANSWER and MACHT necessitate additional trials. The ongoing PRECIOSA study in Spain is assessing dosage and schedule for this therapy.13
Augmenting Diuresis
Loop diuretics are highly protein-bound, and, with hypoalbuminemia, there is less effective drug delivered to the site of action. One clinical approach is to augment diuretics with concomitant HA infusion. This approach is not supported by strong evidence or guidelines.
Hyponatremia
In a retrospective cohort study of 2435 hospitalized patients with cirrhosis, 1126 of whom had hyponatremia, those patients with sodium <130 mmol/L who received HA were more likely to have resolution of hyponatremia to >135 mmol/L. This was associated with improved 30-day survival.14 From this observational data, the AASLD supports the use of albumin combined with extreme fluid restriction (<1000 mL/d) for patients with severe hyponatremia (<120 mmol/L).3
Non-SBP Infections
A 2019 meta-analysis found no evidence of a benefit of HA for bacterial infections other than SBP. However, only three trials encompassing 407 patients met the inclusion criteria.15
NEW GUIDELINE-SUGGESTED USE FOR ALBUMIN IN PATIENTS WITH CIRRHOSIS
SCCM Guideline Update: Hypoalbuminemia and Hypotension
The 2020 SCCM Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU “suggest using albumin for resuscitation of patients [with liver failure] over other fluids, especially when serum albumin is low (<3 g/dL).” Acute-on-chronic liver failure is decompensation of cirrhosis combined with organ dysfunction (eg, coagulopathy, encephalopathy, kidney injury), a scenario that is frequently encountered by hospitalists outside of intensive care settings. In hypotensive patients with cirrhosis, the SCCM recommends administering albumin to a target mean arterial pressure of 65 mm Hg or otherwise adequate perfusion. This new recommendation is conditional, based on expert consensus, and derives from low-quality evidence, with acknowledgement that “costs may be prohibitive.”4
While the ATTIRE study demonstrated no benefit in treating hypoalbuminemia with infusion of HA in hospitalized patients with decompensated cirrhosis, the 2020 SCCM guidelines, released prior to the publication of the ATTIRE study, focused on more acutely ill patients. In the ATTIRE study, only 2% to 3% of the study population was in an intensive care unit.4,9 The use of albumin infusion in the critically ill, hypoalbuminemic, hypotensive patient is not well studied, and the SCCM acknowledges the lack of supportive evidence for this practice in their guideline statement.
CONCLUSION
The three cardinal clinical indications for human albumin in patients with cirrhosis—prevention of PICD after LVP, in SBP, and for HRS—remain supported by the literature and guidelines, with the most recent guidance adding more nuance in patient selection based on individual risk (Table). With the publication of several large-scale studies in the past few years and a 2021 update to the AASLD guidance statement, clinicians have more evidence to guide their use of HA in patients with cirrhosis. In particular, the practice of treating isolated hypoalbuminemia with HA is no longer supported by the best evidence and is potentially harmful. A professional society recommendation to preferentially use albumin as a resuscitation fluid in hypoalbuminemia was made without the benefit of the results of the 2021 ATTIRE trial. On the horizon, additional results from ongoing and upcoming studies exploring concepts of effective albumin concentration and the pleiotropic properties of HA will impact the use of this therapy in hospitalized patients with cirrhosis.
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
1. Hirode G, Saab S, Wong RJ. Trends in the burden of chronic liver disease among hospitalized US adults. JAMA Netw Open. 2020;3(4):e201997. https://doi.org/10.1001/jamanetworkopen.2020.1997
2. European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460. https://doi.org/10.1016/j.jhep.2018.03.024
3. Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014-1048. https://doi.org/10.1002/hep.31884
4. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the management of adult acute and acute-on-chronic liver failure in the ICU: cardiovascular, endocrine, hematologic, pulmonary, and renal considerations. Crit Care Med. 2020;48(3):e173-e191. https://doi.org/10.1097/CCM.0000000000004192
5. Elsabaawy MM, Abdelhamid SR, Alsebaey A, et al. The impact of paracentesis flow rate in patients with liver cirrhosis on the development of paracentesis induced circulatory dysfunction. Clin Mol Hepatol. 2015;21(4):365-371. https://doi.org/10.3350/cmh.2015.21.4.365
6. Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55(4):1172-1181. https://doi.org/10.1002/hep.24786
7. Kütting F, Schubert J, Franklin J, et al. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J Gastroenterol Hepatol. 2017;32(2):327-338. https://doi.org/10.1111/jgh.13421
8. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341(6):403-409. https://doi.org/10.1056/NEJM199908053410603
9. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. https://doi.org/10.1056/NEJMoa2022166
10. Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet. 2018;391(10138):2417-2429. https://doi.org/10.1016/S0140-6736(18)30840-7
11. Caraceni P, Angeli P, Prati D, et al. AISF-SIMTI position paper on the appropriate use of albumin in patients with liver cirrhosis: a 2020 update. Blood Transfus. 2021;19(1):9-13. https://doi.org/10.2450/2020.0414-20
12. Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol. 2018;69(6):1250-1259. https://doi.org/10.1016/j.jhep.2018.08.006
13. Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology. 2019;157(1):149-162. https://doi.org/10.1053/j.gastro.2019.03.021
14. Bajaj JS, Tandon P, O’Leary JG, et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113(9):1339. https://doi.org/10.1038/s41395-018-0119-3
15. Leão GS, Neto GJ, Jotz RdF, de Mattos AA, de Mattos ÂZ. Albumin for cirrhotic patients with extraperitoneal infections: a meta-analysis. J Gastroenterol Hepatol. 2019;34(12):2071-2076. https://doi.org/10.1111/jgh.14791
© 2021 Society of Hospital Medicine
Clinical Progress Note: E-cigarette, or Vaping, Product Use-Associated Lung Injury
E-cigarettes are handheld devices that are used to aerosolize a liquid that commonly contains nicotine, flavorings, and polyethylene glycol and/or vegetable glycerin. These products vary widely in design and style (Figure 1); from the disposable “cigalikes” to vape pens, mods, tanks, and pod systems such as JUUL, there has been a dramatic increase in the recognition, use, sale, and variety of products.1 In addition to the known risks of e-cigarette use, with youth nicotine addiction and progression to cigarette smoking, there is evidence of a wide range of health concerns, including pulmonary and cardiovascular effects, immune dysfunction, and carcinogenesis.1 The emergence of patients with severe lung injury in the summer of 2019 highlighted the harmful health effects specific to these tobacco products.2 Ultimately named EVALI (e-cigarette, or vaping, product use-associated lung injury), there have been 2,807 hospitalized patients with 68 deaths reported to the Centers for Disease Control and Prevention (CDC).2,3 This clinical progress note reviews the epidemiology and clinical course of EVALI and strategies to distinguish the disease from other illnesses. This is particularly timely with the emergence of and surges in COVID-19 cases.4
SEARCH STRATEGY
As the first reports of patients with e-cigarette–associated lung injury were made in the summer of 2019, and the CDC defined EVALI in the fall of 2019, a PubMed search was performed for studies published from June 2019 to June 2020, using the search terms “EVALI” or “e-cigarette–associated lung injury.” In addition, the authors reviewed the CDC and US Food and Drug Administration (FDA) website and presentations on EVALI available in the public domain. Articles discussing COVID-19 and EVALI that the authors became aware of were also included. This update is intended for hospitalists as well as researchers and public health advocates.
DEFINING EVALI
Standard diagnostic criteria do not yet exist, and EVALI remains a diagnosis of exclusion. For epidemiologic (and not diagnostic) purposes, however, the CDC developed the following definitions.3 A confirmed EVALI case must include all of the following criteria:
- Vaping or dabbing within 90 days prior to symptoms. Vaping refers to using e-cigarettes, while dabbing denotes inhaling concentrated tetrahydrocannabinol (THC) products, also known as wax, shatter, or oil
- Pulmonary infiltrates on chest X-ray (CXR) or ground-glass opacities on computed tomography (CT) scan
- Absence of pulmonary infection (including negative respiratory viral panel and influenza testing)
- Negative respiratory infectious disease testing, as clinically indicated
- No evidence in the medical record to suggest an alternative diagnosis
The criteria for a probable EVALI case are similar, except that an infection may be identified but thought not to be the sole cause of lung injury, or the minimum criteria to rule out infection may not be met.
EPIDEMIOLOGY AND DEMOGRAPHICS
Although cases have been reported in all 50 states, the District of Columbia, and two US territories, geographic heterogeneity has been observed.3 Hospital admissions for EVALI reported to the CDC peaked in mid-September 2019 and declined through February 2020.3,8 Although the CDC is no longer reporting weekly numbers, cases continue to be reported in the literature, and current numbers are unclear.4,9,10 The decrease in cases since the peak is thought to be due to increased public awareness of the dangers associated with vaping (particularly with THC-containing products), law enforcement actions, and removal of vitamin E acetate from products.3,8
Risk factors associated with EVALI include younger age, male sex, and use of THC products.5,6 The median age of hospitalized patients diagnosed with EVALI is 24 years, with patients ranging from 13 to 85 years old.3 Overall, 66% of all EVALI patients were male, 82% reported use of a THC-containing product, and 57% reported use of a nicotine-containing product. Approximately 14% of patients reported exclusive nicotine use.3
Nearly half (44%) of hospitalized EVALI patients reported to the CDC required intensive care.7 Of the 68 fatal cases reported to the CDC, the patients were older, with a median age of 51 years (range, 15-75 years), and had increased rates of preexisting conditions, including obesity, asthma, cardiac disease, chronic obstructive pulmonary disease, and mental health disorders.7
HISTORICAL FEATURES
Patients with EVALI may initially present with a variety of respiratory, gastrointestinal, and constitutional symptoms (including fever, muscle aches, and fatigue).11 For this reason, clinicians should universally ask about vaping or dabbing as part of an exposure history, taking care to ensure confidentiality, especially in the adolescent or youth population.12 If the patient reports use, details, including the types of devices, how they were obtained and used, the ingredients in the e-cigarette solution (e-liquid), and the presence of additives or flavorings, should all be noted.3,5,9,12 This history may not be volunteered by the patient, which could result in a delay in diagnosing EVALI.9,12 Although the CDC uses vaping within 90 days in the criteria for diagnosis,3 the likelihood of EVALI decreases with increased time from last use; longer than 1 month is unlikely to be related.11
PHYSICAL EXAM AND LABORATORY STUDIES
Physical assessment of a patient with EVALI may be notable for fever, tachypnea, hypoxemia, or tachycardia; rales may be present, but the exam is often otherwise unrevealing.5,11,12Lab studies may show a mild leukocytosis with neutrophilic predominance and elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein. Procalcitonin may be normal or mildly increased, and, rarely, impaired renal function, hyponatremia, and mild transaminitis may also be present.5,7 As EVALI remains a diagnosis of exclusion, an infectious workup must be completed, which should include evaluation of respiratory viruses and influenza, as well as SARS-CoV-2 testing.11,12
IMAGING AND ADVANCED DIAGNOSTICS
CXR may show bilateral consolidative opacities.11 If the CXR is normal but EVALI is suspected, a CT scan can be considered for diagnostic purposes. Ground-glass opacities are often present on CT imaging (Figure 2), occasionally with subpleural sparing, although this finding is also nonspecific. Less frequently, pneumomediastinum, pleural effusion, or pneumothorax may occur.6,11
Finally, bronchoscopy may be used to exclude other diagnoses if less invasive measures are not conclusive; pulmonary lipid-laden macrophages are associated with EVALI but are nonspecific.5 Cytology and/or biopsy can be used to eliminate other diagnoses but cannot confirm a diagnosis of EVALI.5
DIFFERENTIAL DIAGNOSIS
Hospitalists care for many patients with respiratory symptoms, particularly in the midst of the COVID-19 pandemic and influenza season. Common infectious etiologies that may present similarly include COVID-19, community-acquired pneumonia, influenza, and other viral respiratory illnesses. Hospitalists may rely on microbiologic testing to rule out these causes. If there is a history of vaping and dabbing and this testing is negative, EVALI must be considered more strongly. Recent case studies report that patients with EVALI have been presumed to have COVID-19, despite negative SARS-CoV-2 testing, resulting in delayed diagnosis.4,9 Two small case series suggest that leukocytosis, subpleural sparing on CT scan, vitamin E acetate or macrophages in bronchoalveolar lavage (BAL) fluid, and quick improvement with steroids may suggest a diagnosis of EVALI, as opposed to COVID-19.4,10
Consultation with pulmonary, infectious disease, and toxicology specialists may be of benefit when the diagnosis remains unclear, and specific patient characteristics should guide additional evaluation. Less common diagnoses may need to be considered depending on specific patient factors. For example, patients in certain geographical areas may need testing for endemic fungi, adolescents with recurrent respiratory illnesses may benefit from evaluation for structural lung disease or immunodeficiencies, and patients with impaired immune function need evaluation for Pneumocystis jiroveci infection.5 Diagnostic and treatment algorithms have been developed by the CDC; Kalininskiy et al11 have also proposed a clinical algorithm.12,13
TREATMENT AND CLINICAL COURSE
Empiric treatment for typical infectious pathogens is often provided until evaluation is complete.11,12 Although no randomized clinical trials exist, the CDC and other treatment algorithms recommend supportive care and abstinence from vaping.11-13 Although there are limited data regarding dose and duration, case reports have noted clinical improvement with corticosteroids.6,11-13 Use of steroids can be considered in consultation with a pulmonologist based on the clinical picture, including severity of illness, coexisting infections, and comorbidities.6,11-13 Overall, the clinical course for hospitalized patients with EVALI is variable, but the majority improve with supportive therapy.11,12
Substance use and mental health screening should be performed during hospitalization, as appropriate social support and tobacco use treatment are essential components of care.13 The FDA and CDC recommend universal abstention from all THC-containing products, particularly from informal sources. These agencies also recommend that all nonsmoking adults, including youth and women who are pregnant, abstain from the use of any e-cigarette products.3 Resources for patients who are tobacco users include the nationally available quit line, 1-800-QUIT-NOW, and Smokefree.gov. Similarly, follow-up with a primary care provider within 48 hours of discharge, as well as a visit with a pulmonologist within 4 weeks, is recommended by the CDC per the discharge readiness checklist, with the goal of improving management through earlier follow-up.13 Hospitalists should report confirmed or presumed cases to their local or state health department. Correct medical coding should also be used with diagnosis to better track and care for patients with EVALI; as of April 1, 2020, the World Health Organization established a new International Classification of Diseases, 10th Revision (ICD-10) code, U07.0, for vaping-related injury.14
FUTURE RESEARCH
As EVALI has only recently been described, further research on prevention, etiology, pathophysiology, treatment, and outcomes is needed Although the precise pathophysiology of EVALI remains unknown, vitamin E acetate, a diluent used in some THC-containing e-cigarette solutions, was detected in the BAL of 48 of 51 patients with EVALI (94%) in one study.15 However, available evidence is not sufficient to rule out other toxins found in e-cigarette solution.3 Longitudinal studies should be done to follow patients with EVALI with an emphasis on sustained tobacco use treatment, as the long-term effects of e-cigarette use remain unknown. Furthermore, although corticosteroids are often used, there have been no clinical trials on their efficacy, dose, or duration. Finally, since the CDC is no longer reporting cases, continued epidemiologic studies are necessary.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL CARE
EVALI, first reported in August 2019, is associated with vaping and e-cigarette use and may present with respiratory, gastrointestinal, and constitutional symptoms similar to COVID-19. Healthcare teams should universally screen patients for tobacco, vaping, and e-cigarette use. The majority of patients with EVALI improve with supportive care and abstinence from vaping and e-cigarettes. Tobacco cessation treatment, which includes access to pharmacotherapy and counseling, is critical for patients with EVALI. Additional treatment may include steroids in consultation with subspecialists. The pathophysiology and long-term effects of EVALI remain unclear. Hospitalists should continue to report cases to their local or state health department and use the ICD-10 code for EVALI.
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6):e20182741. https://doi.org/10.1542/peds.2018-2741
2. Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784-786. https://doi.org/10.15585/mmwr.mm6836e1
3. Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed June 5, 2020.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
4. Callahan SJ, Harris D, Collingridge DS, et al. Diagnosing EVALI in the time of COVID-19. Chest. 2020;158(5):2034-2037. https://doi.org/10.1016/j.chest.2020.06.029
5. Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. https://doi.org/10.1016/j.chest.2020.02.018
6. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin —final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/NEJMoa1911614
7. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589-1598. https://doi.org/10.1056/NEJMoa1915314
8. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(3):90-94. https://doi.org/10.15585/mmwr.mm6903e2
9. Armatas C, Heinzerling A, Wilken JA. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response—California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801-802. https://doi.org/10.15585/mmwr.mm6925a5
10. Kazachkov M, Pirzada M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020;8(12):1169-1170. https://doi.org/10.1016/S2213-2600(20)30450-1
11. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/S2213-2600(19)30415-1
12. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081-1086. https://doi.org/10.15585/mmwr.mm6846e2
13. Evans ME, Twentyman E, Click ES, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
14. AAP Division of Health Care Finance. Start using new diagnosis code for vaping-related disorder on April 1. American Academy of Pediatrics website. Accessed June 17, 2020. https://www.aappublications.org/news/aapnewsmag/2020/03/03/coding030320.full.pdf
15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/NEJMoa1916433
E-cigarettes are handheld devices that are used to aerosolize a liquid that commonly contains nicotine, flavorings, and polyethylene glycol and/or vegetable glycerin. These products vary widely in design and style (Figure 1); from the disposable “cigalikes” to vape pens, mods, tanks, and pod systems such as JUUL, there has been a dramatic increase in the recognition, use, sale, and variety of products.1 In addition to the known risks of e-cigarette use, with youth nicotine addiction and progression to cigarette smoking, there is evidence of a wide range of health concerns, including pulmonary and cardiovascular effects, immune dysfunction, and carcinogenesis.1 The emergence of patients with severe lung injury in the summer of 2019 highlighted the harmful health effects specific to these tobacco products.2 Ultimately named EVALI (e-cigarette, or vaping, product use-associated lung injury), there have been 2,807 hospitalized patients with 68 deaths reported to the Centers for Disease Control and Prevention (CDC).2,3 This clinical progress note reviews the epidemiology and clinical course of EVALI and strategies to distinguish the disease from other illnesses. This is particularly timely with the emergence of and surges in COVID-19 cases.4
SEARCH STRATEGY
As the first reports of patients with e-cigarette–associated lung injury were made in the summer of 2019, and the CDC defined EVALI in the fall of 2019, a PubMed search was performed for studies published from June 2019 to June 2020, using the search terms “EVALI” or “e-cigarette–associated lung injury.” In addition, the authors reviewed the CDC and US Food and Drug Administration (FDA) website and presentations on EVALI available in the public domain. Articles discussing COVID-19 and EVALI that the authors became aware of were also included. This update is intended for hospitalists as well as researchers and public health advocates.
DEFINING EVALI
Standard diagnostic criteria do not yet exist, and EVALI remains a diagnosis of exclusion. For epidemiologic (and not diagnostic) purposes, however, the CDC developed the following definitions.3 A confirmed EVALI case must include all of the following criteria:
- Vaping or dabbing within 90 days prior to symptoms. Vaping refers to using e-cigarettes, while dabbing denotes inhaling concentrated tetrahydrocannabinol (THC) products, also known as wax, shatter, or oil
- Pulmonary infiltrates on chest X-ray (CXR) or ground-glass opacities on computed tomography (CT) scan
- Absence of pulmonary infection (including negative respiratory viral panel and influenza testing)
- Negative respiratory infectious disease testing, as clinically indicated
- No evidence in the medical record to suggest an alternative diagnosis
The criteria for a probable EVALI case are similar, except that an infection may be identified but thought not to be the sole cause of lung injury, or the minimum criteria to rule out infection may not be met.
EPIDEMIOLOGY AND DEMOGRAPHICS
Although cases have been reported in all 50 states, the District of Columbia, and two US territories, geographic heterogeneity has been observed.3 Hospital admissions for EVALI reported to the CDC peaked in mid-September 2019 and declined through February 2020.3,8 Although the CDC is no longer reporting weekly numbers, cases continue to be reported in the literature, and current numbers are unclear.4,9,10 The decrease in cases since the peak is thought to be due to increased public awareness of the dangers associated with vaping (particularly with THC-containing products), law enforcement actions, and removal of vitamin E acetate from products.3,8
Risk factors associated with EVALI include younger age, male sex, and use of THC products.5,6 The median age of hospitalized patients diagnosed with EVALI is 24 years, with patients ranging from 13 to 85 years old.3 Overall, 66% of all EVALI patients were male, 82% reported use of a THC-containing product, and 57% reported use of a nicotine-containing product. Approximately 14% of patients reported exclusive nicotine use.3
Nearly half (44%) of hospitalized EVALI patients reported to the CDC required intensive care.7 Of the 68 fatal cases reported to the CDC, the patients were older, with a median age of 51 years (range, 15-75 years), and had increased rates of preexisting conditions, including obesity, asthma, cardiac disease, chronic obstructive pulmonary disease, and mental health disorders.7
HISTORICAL FEATURES
Patients with EVALI may initially present with a variety of respiratory, gastrointestinal, and constitutional symptoms (including fever, muscle aches, and fatigue).11 For this reason, clinicians should universally ask about vaping or dabbing as part of an exposure history, taking care to ensure confidentiality, especially in the adolescent or youth population.12 If the patient reports use, details, including the types of devices, how they were obtained and used, the ingredients in the e-cigarette solution (e-liquid), and the presence of additives or flavorings, should all be noted.3,5,9,12 This history may not be volunteered by the patient, which could result in a delay in diagnosing EVALI.9,12 Although the CDC uses vaping within 90 days in the criteria for diagnosis,3 the likelihood of EVALI decreases with increased time from last use; longer than 1 month is unlikely to be related.11
PHYSICAL EXAM AND LABORATORY STUDIES
Physical assessment of a patient with EVALI may be notable for fever, tachypnea, hypoxemia, or tachycardia; rales may be present, but the exam is often otherwise unrevealing.5,11,12Lab studies may show a mild leukocytosis with neutrophilic predominance and elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein. Procalcitonin may be normal or mildly increased, and, rarely, impaired renal function, hyponatremia, and mild transaminitis may also be present.5,7 As EVALI remains a diagnosis of exclusion, an infectious workup must be completed, which should include evaluation of respiratory viruses and influenza, as well as SARS-CoV-2 testing.11,12
IMAGING AND ADVANCED DIAGNOSTICS
CXR may show bilateral consolidative opacities.11 If the CXR is normal but EVALI is suspected, a CT scan can be considered for diagnostic purposes. Ground-glass opacities are often present on CT imaging (Figure 2), occasionally with subpleural sparing, although this finding is also nonspecific. Less frequently, pneumomediastinum, pleural effusion, or pneumothorax may occur.6,11
Finally, bronchoscopy may be used to exclude other diagnoses if less invasive measures are not conclusive; pulmonary lipid-laden macrophages are associated with EVALI but are nonspecific.5 Cytology and/or biopsy can be used to eliminate other diagnoses but cannot confirm a diagnosis of EVALI.5
DIFFERENTIAL DIAGNOSIS
Hospitalists care for many patients with respiratory symptoms, particularly in the midst of the COVID-19 pandemic and influenza season. Common infectious etiologies that may present similarly include COVID-19, community-acquired pneumonia, influenza, and other viral respiratory illnesses. Hospitalists may rely on microbiologic testing to rule out these causes. If there is a history of vaping and dabbing and this testing is negative, EVALI must be considered more strongly. Recent case studies report that patients with EVALI have been presumed to have COVID-19, despite negative SARS-CoV-2 testing, resulting in delayed diagnosis.4,9 Two small case series suggest that leukocytosis, subpleural sparing on CT scan, vitamin E acetate or macrophages in bronchoalveolar lavage (BAL) fluid, and quick improvement with steroids may suggest a diagnosis of EVALI, as opposed to COVID-19.4,10
Consultation with pulmonary, infectious disease, and toxicology specialists may be of benefit when the diagnosis remains unclear, and specific patient characteristics should guide additional evaluation. Less common diagnoses may need to be considered depending on specific patient factors. For example, patients in certain geographical areas may need testing for endemic fungi, adolescents with recurrent respiratory illnesses may benefit from evaluation for structural lung disease or immunodeficiencies, and patients with impaired immune function need evaluation for Pneumocystis jiroveci infection.5 Diagnostic and treatment algorithms have been developed by the CDC; Kalininskiy et al11 have also proposed a clinical algorithm.12,13
TREATMENT AND CLINICAL COURSE
Empiric treatment for typical infectious pathogens is often provided until evaluation is complete.11,12 Although no randomized clinical trials exist, the CDC and other treatment algorithms recommend supportive care and abstinence from vaping.11-13 Although there are limited data regarding dose and duration, case reports have noted clinical improvement with corticosteroids.6,11-13 Use of steroids can be considered in consultation with a pulmonologist based on the clinical picture, including severity of illness, coexisting infections, and comorbidities.6,11-13 Overall, the clinical course for hospitalized patients with EVALI is variable, but the majority improve with supportive therapy.11,12
Substance use and mental health screening should be performed during hospitalization, as appropriate social support and tobacco use treatment are essential components of care.13 The FDA and CDC recommend universal abstention from all THC-containing products, particularly from informal sources. These agencies also recommend that all nonsmoking adults, including youth and women who are pregnant, abstain from the use of any e-cigarette products.3 Resources for patients who are tobacco users include the nationally available quit line, 1-800-QUIT-NOW, and Smokefree.gov. Similarly, follow-up with a primary care provider within 48 hours of discharge, as well as a visit with a pulmonologist within 4 weeks, is recommended by the CDC per the discharge readiness checklist, with the goal of improving management through earlier follow-up.13 Hospitalists should report confirmed or presumed cases to their local or state health department. Correct medical coding should also be used with diagnosis to better track and care for patients with EVALI; as of April 1, 2020, the World Health Organization established a new International Classification of Diseases, 10th Revision (ICD-10) code, U07.0, for vaping-related injury.14
FUTURE RESEARCH
As EVALI has only recently been described, further research on prevention, etiology, pathophysiology, treatment, and outcomes is needed Although the precise pathophysiology of EVALI remains unknown, vitamin E acetate, a diluent used in some THC-containing e-cigarette solutions, was detected in the BAL of 48 of 51 patients with EVALI (94%) in one study.15 However, available evidence is not sufficient to rule out other toxins found in e-cigarette solution.3 Longitudinal studies should be done to follow patients with EVALI with an emphasis on sustained tobacco use treatment, as the long-term effects of e-cigarette use remain unknown. Furthermore, although corticosteroids are often used, there have been no clinical trials on their efficacy, dose, or duration. Finally, since the CDC is no longer reporting cases, continued epidemiologic studies are necessary.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL CARE
EVALI, first reported in August 2019, is associated with vaping and e-cigarette use and may present with respiratory, gastrointestinal, and constitutional symptoms similar to COVID-19. Healthcare teams should universally screen patients for tobacco, vaping, and e-cigarette use. The majority of patients with EVALI improve with supportive care and abstinence from vaping and e-cigarettes. Tobacco cessation treatment, which includes access to pharmacotherapy and counseling, is critical for patients with EVALI. Additional treatment may include steroids in consultation with subspecialists. The pathophysiology and long-term effects of EVALI remain unclear. Hospitalists should continue to report cases to their local or state health department and use the ICD-10 code for EVALI.
E-cigarettes are handheld devices that are used to aerosolize a liquid that commonly contains nicotine, flavorings, and polyethylene glycol and/or vegetable glycerin. These products vary widely in design and style (Figure 1); from the disposable “cigalikes” to vape pens, mods, tanks, and pod systems such as JUUL, there has been a dramatic increase in the recognition, use, sale, and variety of products.1 In addition to the known risks of e-cigarette use, with youth nicotine addiction and progression to cigarette smoking, there is evidence of a wide range of health concerns, including pulmonary and cardiovascular effects, immune dysfunction, and carcinogenesis.1 The emergence of patients with severe lung injury in the summer of 2019 highlighted the harmful health effects specific to these tobacco products.2 Ultimately named EVALI (e-cigarette, or vaping, product use-associated lung injury), there have been 2,807 hospitalized patients with 68 deaths reported to the Centers for Disease Control and Prevention (CDC).2,3 This clinical progress note reviews the epidemiology and clinical course of EVALI and strategies to distinguish the disease from other illnesses. This is particularly timely with the emergence of and surges in COVID-19 cases.4
SEARCH STRATEGY
As the first reports of patients with e-cigarette–associated lung injury were made in the summer of 2019, and the CDC defined EVALI in the fall of 2019, a PubMed search was performed for studies published from June 2019 to June 2020, using the search terms “EVALI” or “e-cigarette–associated lung injury.” In addition, the authors reviewed the CDC and US Food and Drug Administration (FDA) website and presentations on EVALI available in the public domain. Articles discussing COVID-19 and EVALI that the authors became aware of were also included. This update is intended for hospitalists as well as researchers and public health advocates.
DEFINING EVALI
Standard diagnostic criteria do not yet exist, and EVALI remains a diagnosis of exclusion. For epidemiologic (and not diagnostic) purposes, however, the CDC developed the following definitions.3 A confirmed EVALI case must include all of the following criteria:
- Vaping or dabbing within 90 days prior to symptoms. Vaping refers to using e-cigarettes, while dabbing denotes inhaling concentrated tetrahydrocannabinol (THC) products, also known as wax, shatter, or oil
- Pulmonary infiltrates on chest X-ray (CXR) or ground-glass opacities on computed tomography (CT) scan
- Absence of pulmonary infection (including negative respiratory viral panel and influenza testing)
- Negative respiratory infectious disease testing, as clinically indicated
- No evidence in the medical record to suggest an alternative diagnosis
The criteria for a probable EVALI case are similar, except that an infection may be identified but thought not to be the sole cause of lung injury, or the minimum criteria to rule out infection may not be met.
EPIDEMIOLOGY AND DEMOGRAPHICS
Although cases have been reported in all 50 states, the District of Columbia, and two US territories, geographic heterogeneity has been observed.3 Hospital admissions for EVALI reported to the CDC peaked in mid-September 2019 and declined through February 2020.3,8 Although the CDC is no longer reporting weekly numbers, cases continue to be reported in the literature, and current numbers are unclear.4,9,10 The decrease in cases since the peak is thought to be due to increased public awareness of the dangers associated with vaping (particularly with THC-containing products), law enforcement actions, and removal of vitamin E acetate from products.3,8
Risk factors associated with EVALI include younger age, male sex, and use of THC products.5,6 The median age of hospitalized patients diagnosed with EVALI is 24 years, with patients ranging from 13 to 85 years old.3 Overall, 66% of all EVALI patients were male, 82% reported use of a THC-containing product, and 57% reported use of a nicotine-containing product. Approximately 14% of patients reported exclusive nicotine use.3
Nearly half (44%) of hospitalized EVALI patients reported to the CDC required intensive care.7 Of the 68 fatal cases reported to the CDC, the patients were older, with a median age of 51 years (range, 15-75 years), and had increased rates of preexisting conditions, including obesity, asthma, cardiac disease, chronic obstructive pulmonary disease, and mental health disorders.7
HISTORICAL FEATURES
Patients with EVALI may initially present with a variety of respiratory, gastrointestinal, and constitutional symptoms (including fever, muscle aches, and fatigue).11 For this reason, clinicians should universally ask about vaping or dabbing as part of an exposure history, taking care to ensure confidentiality, especially in the adolescent or youth population.12 If the patient reports use, details, including the types of devices, how they were obtained and used, the ingredients in the e-cigarette solution (e-liquid), and the presence of additives or flavorings, should all be noted.3,5,9,12 This history may not be volunteered by the patient, which could result in a delay in diagnosing EVALI.9,12 Although the CDC uses vaping within 90 days in the criteria for diagnosis,3 the likelihood of EVALI decreases with increased time from last use; longer than 1 month is unlikely to be related.11
PHYSICAL EXAM AND LABORATORY STUDIES
Physical assessment of a patient with EVALI may be notable for fever, tachypnea, hypoxemia, or tachycardia; rales may be present, but the exam is often otherwise unrevealing.5,11,12Lab studies may show a mild leukocytosis with neutrophilic predominance and elevated inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein. Procalcitonin may be normal or mildly increased, and, rarely, impaired renal function, hyponatremia, and mild transaminitis may also be present.5,7 As EVALI remains a diagnosis of exclusion, an infectious workup must be completed, which should include evaluation of respiratory viruses and influenza, as well as SARS-CoV-2 testing.11,12
IMAGING AND ADVANCED DIAGNOSTICS
CXR may show bilateral consolidative opacities.11 If the CXR is normal but EVALI is suspected, a CT scan can be considered for diagnostic purposes. Ground-glass opacities are often present on CT imaging (Figure 2), occasionally with subpleural sparing, although this finding is also nonspecific. Less frequently, pneumomediastinum, pleural effusion, or pneumothorax may occur.6,11
Finally, bronchoscopy may be used to exclude other diagnoses if less invasive measures are not conclusive; pulmonary lipid-laden macrophages are associated with EVALI but are nonspecific.5 Cytology and/or biopsy can be used to eliminate other diagnoses but cannot confirm a diagnosis of EVALI.5
DIFFERENTIAL DIAGNOSIS
Hospitalists care for many patients with respiratory symptoms, particularly in the midst of the COVID-19 pandemic and influenza season. Common infectious etiologies that may present similarly include COVID-19, community-acquired pneumonia, influenza, and other viral respiratory illnesses. Hospitalists may rely on microbiologic testing to rule out these causes. If there is a history of vaping and dabbing and this testing is negative, EVALI must be considered more strongly. Recent case studies report that patients with EVALI have been presumed to have COVID-19, despite negative SARS-CoV-2 testing, resulting in delayed diagnosis.4,9 Two small case series suggest that leukocytosis, subpleural sparing on CT scan, vitamin E acetate or macrophages in bronchoalveolar lavage (BAL) fluid, and quick improvement with steroids may suggest a diagnosis of EVALI, as opposed to COVID-19.4,10
Consultation with pulmonary, infectious disease, and toxicology specialists may be of benefit when the diagnosis remains unclear, and specific patient characteristics should guide additional evaluation. Less common diagnoses may need to be considered depending on specific patient factors. For example, patients in certain geographical areas may need testing for endemic fungi, adolescents with recurrent respiratory illnesses may benefit from evaluation for structural lung disease or immunodeficiencies, and patients with impaired immune function need evaluation for Pneumocystis jiroveci infection.5 Diagnostic and treatment algorithms have been developed by the CDC; Kalininskiy et al11 have also proposed a clinical algorithm.12,13
TREATMENT AND CLINICAL COURSE
Empiric treatment for typical infectious pathogens is often provided until evaluation is complete.11,12 Although no randomized clinical trials exist, the CDC and other treatment algorithms recommend supportive care and abstinence from vaping.11-13 Although there are limited data regarding dose and duration, case reports have noted clinical improvement with corticosteroids.6,11-13 Use of steroids can be considered in consultation with a pulmonologist based on the clinical picture, including severity of illness, coexisting infections, and comorbidities.6,11-13 Overall, the clinical course for hospitalized patients with EVALI is variable, but the majority improve with supportive therapy.11,12
Substance use and mental health screening should be performed during hospitalization, as appropriate social support and tobacco use treatment are essential components of care.13 The FDA and CDC recommend universal abstention from all THC-containing products, particularly from informal sources. These agencies also recommend that all nonsmoking adults, including youth and women who are pregnant, abstain from the use of any e-cigarette products.3 Resources for patients who are tobacco users include the nationally available quit line, 1-800-QUIT-NOW, and Smokefree.gov. Similarly, follow-up with a primary care provider within 48 hours of discharge, as well as a visit with a pulmonologist within 4 weeks, is recommended by the CDC per the discharge readiness checklist, with the goal of improving management through earlier follow-up.13 Hospitalists should report confirmed or presumed cases to their local or state health department. Correct medical coding should also be used with diagnosis to better track and care for patients with EVALI; as of April 1, 2020, the World Health Organization established a new International Classification of Diseases, 10th Revision (ICD-10) code, U07.0, for vaping-related injury.14
FUTURE RESEARCH
As EVALI has only recently been described, further research on prevention, etiology, pathophysiology, treatment, and outcomes is needed Although the precise pathophysiology of EVALI remains unknown, vitamin E acetate, a diluent used in some THC-containing e-cigarette solutions, was detected in the BAL of 48 of 51 patients with EVALI (94%) in one study.15 However, available evidence is not sufficient to rule out other toxins found in e-cigarette solution.3 Longitudinal studies should be done to follow patients with EVALI with an emphasis on sustained tobacco use treatment, as the long-term effects of e-cigarette use remain unknown. Furthermore, although corticosteroids are often used, there have been no clinical trials on their efficacy, dose, or duration. Finally, since the CDC is no longer reporting cases, continued epidemiologic studies are necessary.
CONCLUSIONS AND IMPLICATIONS FOR CLINICAL CARE
EVALI, first reported in August 2019, is associated with vaping and e-cigarette use and may present with respiratory, gastrointestinal, and constitutional symptoms similar to COVID-19. Healthcare teams should universally screen patients for tobacco, vaping, and e-cigarette use. The majority of patients with EVALI improve with supportive care and abstinence from vaping and e-cigarettes. Tobacco cessation treatment, which includes access to pharmacotherapy and counseling, is critical for patients with EVALI. Additional treatment may include steroids in consultation with subspecialists. The pathophysiology and long-term effects of EVALI remain unclear. Hospitalists should continue to report cases to their local or state health department and use the ICD-10 code for EVALI.
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6):e20182741. https://doi.org/10.1542/peds.2018-2741
2. Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784-786. https://doi.org/10.15585/mmwr.mm6836e1
3. Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed June 5, 2020.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
4. Callahan SJ, Harris D, Collingridge DS, et al. Diagnosing EVALI in the time of COVID-19. Chest. 2020;158(5):2034-2037. https://doi.org/10.1016/j.chest.2020.06.029
5. Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. https://doi.org/10.1016/j.chest.2020.02.018
6. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin —final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/NEJMoa1911614
7. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589-1598. https://doi.org/10.1056/NEJMoa1915314
8. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(3):90-94. https://doi.org/10.15585/mmwr.mm6903e2
9. Armatas C, Heinzerling A, Wilken JA. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response—California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801-802. https://doi.org/10.15585/mmwr.mm6925a5
10. Kazachkov M, Pirzada M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020;8(12):1169-1170. https://doi.org/10.1016/S2213-2600(20)30450-1
11. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/S2213-2600(19)30415-1
12. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081-1086. https://doi.org/10.15585/mmwr.mm6846e2
13. Evans ME, Twentyman E, Click ES, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
14. AAP Division of Health Care Finance. Start using new diagnosis code for vaping-related disorder on April 1. American Academy of Pediatrics website. Accessed June 17, 2020. https://www.aappublications.org/news/aapnewsmag/2020/03/03/coding030320.full.pdf
15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/NEJMoa1916433
1. Walley SC, Wilson KM, Winickoff JP, Groner J. A public health crisis: electronic cigarettes, vape, and JUUL. Pediatrics. 2019;143(6):e20182741. https://doi.org/10.1542/peds.2018-2741
2. Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep. 2019;68(36):784-786. https://doi.org/10.15585/mmwr.mm6836e1
3. Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed June 5, 2020.https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
4. Callahan SJ, Harris D, Collingridge DS, et al. Diagnosing EVALI in the time of COVID-19. Chest. 2020;158(5):2034-2037. https://doi.org/10.1016/j.chest.2020.06.029
5. Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: general approach and the role of bronchoscopy. Chest. 2020;158(2):820-827. https://doi.org/10.1016/j.chest.2020.02.018
6. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin —final report. N Engl J Med. 2020;382(10):903-916. https://doi.org/10.1056/NEJMoa1911614
7. Werner AK, Koumans EH, Chatham-Stephens K, et al. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589-1598. https://doi.org/10.1056/NEJMoa1915314
8. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury—United States, August 2019-January 2020. MMWR Morb Mortal Wkly Rep. 2020;69(3):90-94. https://doi.org/10.15585/mmwr.mm6903e2
9. Armatas C, Heinzerling A, Wilken JA. Notes from the field: e-cigarette, or vaping, product use-associated lung injury cases during the COVID-19 response—California, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(25):801-802. https://doi.org/10.15585/mmwr.mm6925a5
10. Kazachkov M, Pirzada M. Diagnosis of EVALI in the COVID-19 era. Lancet Respir Med. 2020;8(12):1169-1170. https://doi.org/10.1016/S2213-2600(20)30450-1
11. Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019;7(12):1017-1026. https://doi.org/10.1016/S2213-2600(19)30415-1
12. Jatlaoui TC, Wiltz JL, Kabbani S, et al. Update: interim guidance for health care providers for managing patients with suspected e-cigarette, or vaping, product use-associated lung injury—United States, November 2019. MMWR Morb Mortal Wkly Rep. 2019;68(46):1081-1086. https://doi.org/10.15585/mmwr.mm6846e2
13. Evans ME, Twentyman E, Click ES, et al. Update: interim guidance for health care professionals evaluating and caring for patients with suspected e-cigarette, or vaping, product use-associated lung injury and for reducing the risk for rehospitalization and death following hospital discharge—United States, December 2019. MMWR Morb Mortal Wkly Rep. 2020;68(5152):1189-1194. https://doi.org/10.15585/mmwr.mm685152e2
14. AAP Division of Health Care Finance. Start using new diagnosis code for vaping-related disorder on April 1. American Academy of Pediatrics website. Accessed June 17, 2020. https://www.aappublications.org/news/aapnewsmag/2020/03/03/coding030320.full.pdf
15. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697-705. https://doi.org/10.1056/NEJMoa1916433
© 2021 Society of Hospital Medicine
Methodological Progress Note: Interrupted Time Series
Hospital medicine research often asks the question whether an intervention, such as a policy or guideline, has improved quality of care and/or whether there were any unintended consequences. Alternatively, investigators may be interested in understanding the impact of an event, such as a natural disaster or a pandemic, on hospital care. The study design that provides the best estimate of the causal effect of the intervention is the randomized controlled trial (RCT). The goal of randomization, which can be implemented at the patient or cluster level (eg, hospitals), is attaining a balance of the known and unknown confounders between study groups.
However, an RCT may not be feasible for several reasons: complexity, insufficient setup time or funding, ethical barriers to randomization, unwillingness of funders or payers to withhold the intervention from patients (ie, the control group), or anticipated contamination of the intervention into the control group (eg, provider practice change interventions). In addition, it may be impossible to conduct an RCT because the investigator does not have control over the design of an intervention or because they are studying an event, such as a pandemic.
In the June 2020 issue of the Journal of Hospital Medicine, Coon et al1 use a type of quasi-experimental design (QED)—specifically, the interrupted time series (ITS)—to examine the impact of the adoption of ward-based high-flow nasal cannula protocols on intensive care unit (ICU) admission for bronchiolitis at children’s hospitals. In this methodologic progress note, we discuss QEDs for evaluating the impact of healthcare interventions or events and focus on ITS, one of the strongest QEDs.
WHAT IS A QUASI-EXPERIMENTAL DESIGN?
Quasi-experimental design refers to a broad range of nonrandomized or partially randomized pre- vs postintervention studies.2 In order to test a causal hypothesis without randomization, QEDs define a comparison group or a time period in which an intervention has not been implemented, as well as at least one group or time period in which an intervention has been implemented. In a QED, the control may lack similarity with the intervention group or time period because of differences in the patients, sites, or time period (sometimes referred to as having a “nonequivalent control group”). Several design and analytic approaches are available to enhance the extent to which the study is able to make conclusions about the causal impact of the intervention.2,3 Because randomization is not necessary, QEDs allow for inclusion of a broader population than that which is feasible by RCTs, which increases the applicability and generalizability of the results. Therefore, they are a powerful research design to test the effectiveness of interventions in real-world settings.
The choice of which QED depends on whether the investigators are conducting a prospective evaluation and have control over the study design (ie, the ordering of the intervention, selection of sites or individuals, and/or timing and frequency of the data collection) or whether the investigators do not have control over the intervention, which is also known as a “natural experiment.”4,5 Some studies may also incorporate two QEDs in tandem.6 The Table provides a brief summary of different QEDs, ordered by methodologic strength, and distinguishes those that can be used to study natural experiments. In the study by Coon et al,1 an ITS is used as opposed to a methodologically stronger QED, such as the stepped-wedge design, because the investigators did not have control over the rollout of heated high-flow nasal canula protocols across hospitals.
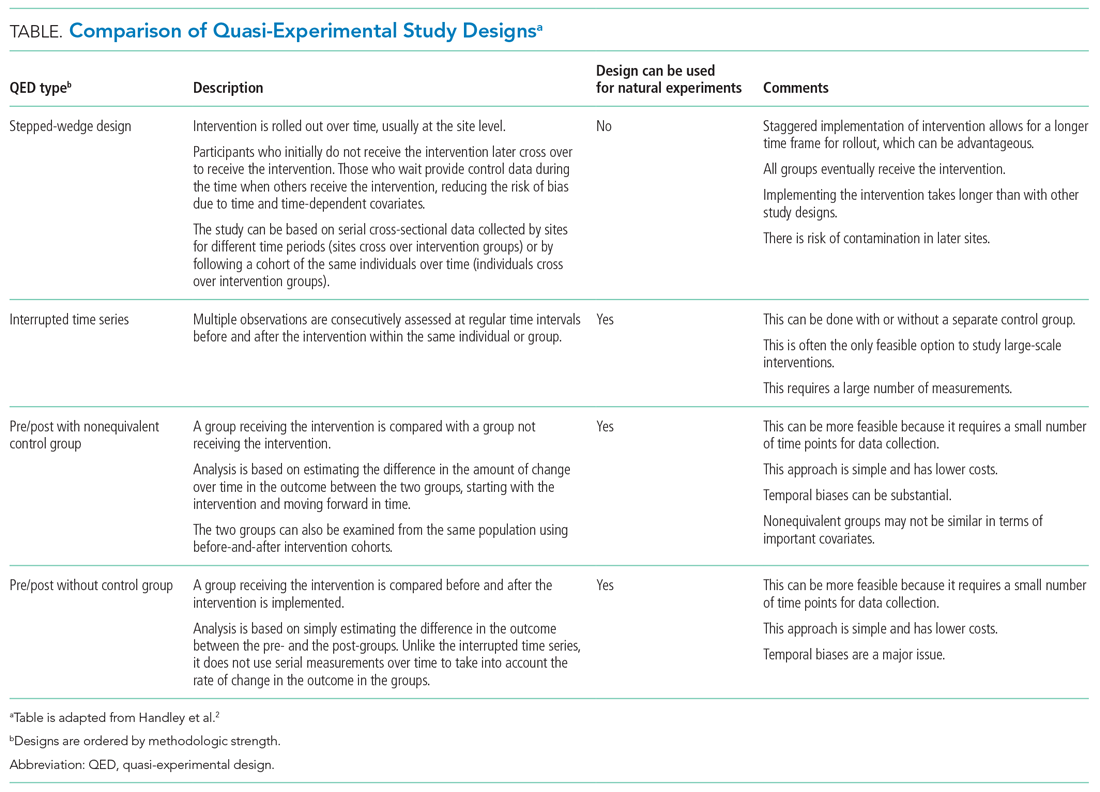
WHAT IS AN INTERRUPTED TIME SERIES?
Interrupted time series designs use repeated observations of an outcome over time. This method then divides, or “interrupts,” the series of data into two time periods: before the intervention or event and after. Using data from the preintervention period, an underlying trend in the outcome is estimated and assumed to continue forward into the postintervention period to estimate what would have occurred without the intervention. Any significant change in the outcome at the beginning of the postintervention period or change in the trend in the postintervention is then attributed to the intervention.
There are several important methodologic considerations when designing an ITS study, as detailed in other review papers.2,3,7,8 An ITS design can be retrospective or prospective. It can be of a single center or include multiple sites, as in Coon et al. It can be conducted with or without a control. The inclusion of a control, when appropriately chosen, improves the strength of the study design because it can account for seasonal trends and potential confounders that vary over time. The control can be a different group of hospitals or participants that are similar but did not receive the intervention, or it can be a different outcome in the same group of hospitals or participants that are not expected to be affected by the intervention. The ITS design may also be set up to estimate the individual effects of multicomponent interventions. If the different components are phased in sequentially over time, then it may be possible to interrupt the time series at these points and estimate the impact of each intervention component.
Other examples of ITS studies in hospital medicine include those that evaluated the impact of a readmission-reduction program,9 of state sepsis regulations on in-hospital mortality,10 of resident duty-hour reform on mortality among hospitalized patients,11 of a quality-improvement initiative on early discharge,12 and of national guidelines on pediatric pneumonia antibiotic selection.13 There are several types of ITS analysis, and in this article, we focus on segmented regression without a control group.7,8
WHAT IS A SEGMENTED REGRESSION ITS?
Segmented regression is the statistical model used to measure (a) the immediate change in the outcome (level) at the start of the intervention and (b) the change in the trend of the outcome (slope) in the postintervention period vs that in the preintervention period. Therefore, the intervention effect size is expressed in terms of the level change and the slope change. To function properly, the models require several repeated (eg, monthly) measurements of the outcome before and after the intervention. Some experts suggest a minimum of 4 to 12 observations, depending on a number of factors including the stability of the outcome and seasonal variations.7,8 If changes before and after more than one intervention are being examined, there should be the minimum number of observations separating them. Unlike typical regression models, time-series models can correct for autocorrelation if it is present in the data. Autocorrelation is the type of correlation that arises when data are collected over time, with those closest in time being more strongly correlated (there are also other types of autocorrelation, such as seasonal patterns). Using available statistical software, autocorrelation can be detected and, if present, it can be controlled for in the segmented regression models.
HOW ARE SEGMENTED REGRESSION RESULTS PRESENTED?
Coon et al present results of their ITS analysis in a panel of figures detailing each study outcome, ICU admission, ICU length of stay, total length of stay, and rates of mechanical ventilation. Each panel shows the rate of change in the outcome per season across hospitals, before and after adoption of heated high-flow nasal cannula protocols, and the level change at the time of adoption.
To further explain how segmented regression results are presented, in the Figure we detail the structure of a segmented regression figure evaluating the impact of an intervention without a control group. In addition to the regression figure, authors typically provide 95% CIs around the rates, level change, and the difference between the postintervention and preintervention periods, along with P values demonstrating whether the rates, level change, and the differences between period slopes differ significantly from zero.
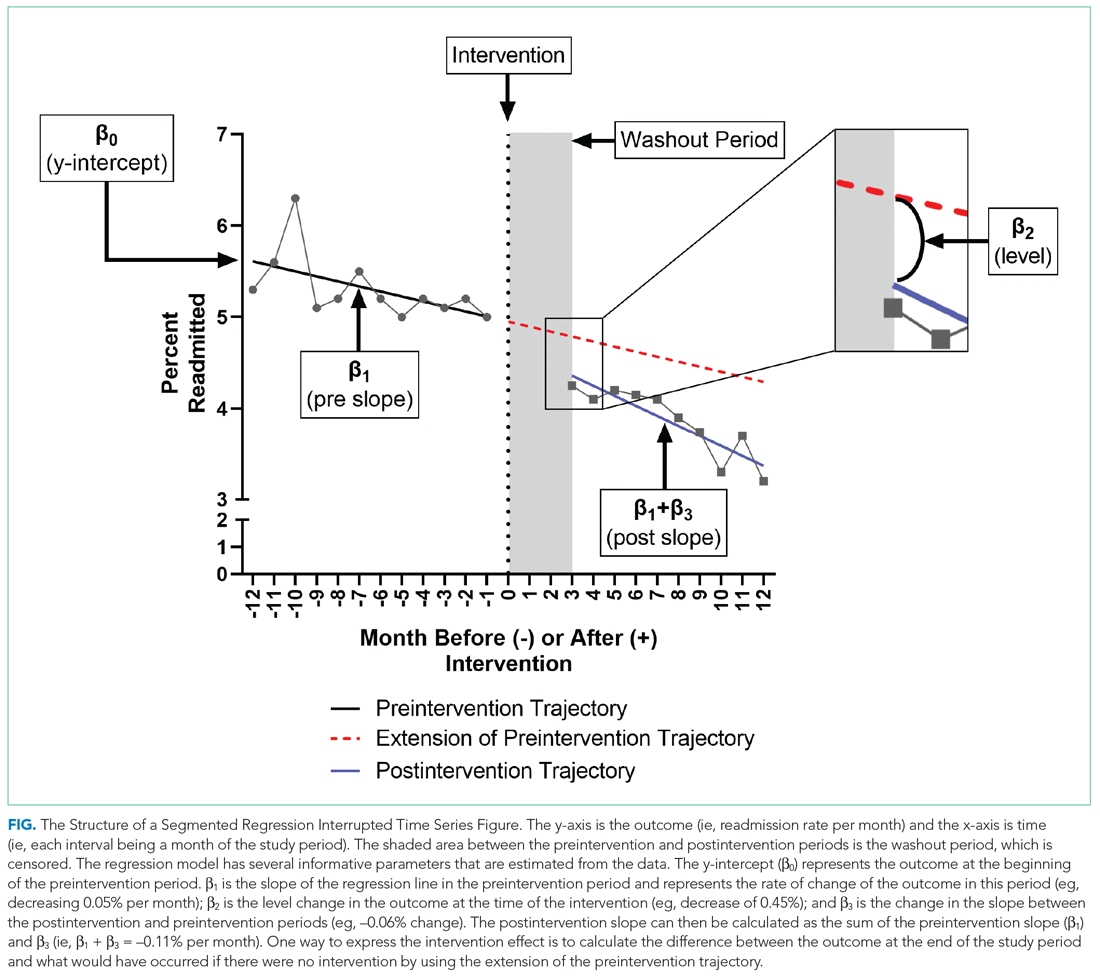
WHAT ARE THE UNDERLYING ASSUMPTIONS OF THE SEGMENTED REGRESSION ITS?
Segmented regression models assume a linear trend in the outcome. If the outcome follows a nonlinear pattern (eg, exponential spread of a disease during a pandemic), then using different distributions in the modeling or transformations of the data may be necessary. The validity of the comparison between the pre- and postintervention groups relies on the similarity between the populations. When there is imbalance, investigators can consider matching based on important characteristics or applying risk adjustment as necessary. Another important assumption is that the outcome of interest is unchanged in the absence of the intervention. Finally, the analysis assumes that the intervention is fully implemented at the time the postintervention period begins. Often, there is a washout period during which the old approach is stopped and the new approach (the intervention) is being implemented and can easily be taken into account.
WHAT ARE THE STRENGTHS OF THE SEGMENTED REGRESSION ITS?
There are several strengths of the ITS analysis and segmented regression.7,8 First, this approach accounts for a possible secular trend in the outcome measure that may have been present prior to the intervention. For example, investigators might conclude that a readmissions program was effective in reducing readmissions if they found that the mean readmission percentage in the period after the intervention was significantly lower than before using a simple pre/post study design. However, what if the readmission rate was already going down prior to the intervention? Using an ITS approach, they may have found that the rate of readmissions simply continued to decrease after the intervention at the same rate that it was decreasing prior to the intervention and, therefore, conclude that the intervention was not effective. Second, because the ITS approach evaluates changes in rates of an outcome at a population level, confounding by individual-level variables will not introduce serious bias unless the confounding occurred at the same time as the intervention. Third, ITS can be used to measure the unintended consequences of interventions or events, and investigators can construct separate time-series analyses for different outcomes. Fourth, ITS can be used to evaluate the impact of the intervention on subpopulations (eg, those grouped by age, sex, race) by conducting stratified analysis. Fifth, ITS provides simple and clear graphical results that can be easily understood by various audiences.
WHAT ARE THE IMPORTANT LIMITATIONS OF AN ITS?
By accounting for preintervention trends, ITS studies permit stronger causal inference than do cross-sectional or simple pre/post QEDs, but they may by prone to confounding by cointerventions or by changes in the population composition. Causal inference based on the ITS analysis is only valid to the extent to which the intervention was the only thing that changed at the point in time between the preintervention and postintervention periods. It is important for investigators to consider this in the design and discuss any coincident interventions. If there are multiple interventions over time, it is possible to account for these changes in the study design by creating multiple points of interruption provided there are sufficient measurements of the outcome between interventions. If the composition of the population changes at the same time as the intervention, this introduces bias. Changes in the ability to measure the outcome or changes to its definition also threaten the validity of the study’s inferences. Finally, it is also important to remember that when the outcome is a population-level measurement, inferences about individual-level outcomes are inappropriate due to ecological fallacies (ie, when inferences about individuals are deduced from inferences about the group to which those individuals belong). For example, Coon et al found that infants with bronchiolitis in the ward-based high-flow nasal cannula protocol group had greater ICU admission rates. It would be inappropriate to conclude that, based on this, an individual infant in a hospital on a ward-based protocol is more likely to be admitted to the ICU.
CONCLUSION
Studies evaluating interventions and events are important for informing healthcare practice, policy, and public health. While an RCT is the preferred method for such evaluations, investigators must often consider alternative study designs when an RCT is not feasible or when more real-world outcome evaluation is desired. Quasi-experimental designs are employed in studies that do not use randomization to study the impact of interventions in real-world settings, and an interrupted time series is a strong QED for the evaluation of interventions and natural experiments.
1. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
2. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5-25. https://doi.org/10.1146/annurev-publhealth-040617-014128
3. Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39-56. https://doi.org/10.1146/annurev-publhealth-031816-044327
4. Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health. 2012;66(12):1182-1186. https://doi.org/10.1136/jech-2011-200375
5. Coly A, Parry G. Evaluating Complex Health Interventions: A Guide to Rigorous Research Designs. AcademyHealth; 2017.
6. Orenstein EW, Rasooly IR, Mai MV, et al. Influence of simulation on electronic health record use patterns among pediatric residents. J Am Med Inform Assoc. 2018;25(11):1501-1506. https://doi.org/10.1093/jamia/ocy105
7. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
8. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. https://doi.org/10.1046/j.1365-2710.2002.00430.x
9. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533
10. Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. https://doi.org/10.1001/jama.2019.9021
11. Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975-983. https://doi.org/10.1001/jama.298.9.975
12. Destino L, Bennett D, Wood M, et al. Improving patient flow: analysis of an initiative to improve early discharge. J Hosp Med. 2019;14(1):22-27. https://doi.org/10.12788/jhm.3133
13. Williams DJ, Hall M, Gerber JS, et al; Pediatric Research in Inpatient Settings Network. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
Hospital medicine research often asks the question whether an intervention, such as a policy or guideline, has improved quality of care and/or whether there were any unintended consequences. Alternatively, investigators may be interested in understanding the impact of an event, such as a natural disaster or a pandemic, on hospital care. The study design that provides the best estimate of the causal effect of the intervention is the randomized controlled trial (RCT). The goal of randomization, which can be implemented at the patient or cluster level (eg, hospitals), is attaining a balance of the known and unknown confounders between study groups.
However, an RCT may not be feasible for several reasons: complexity, insufficient setup time or funding, ethical barriers to randomization, unwillingness of funders or payers to withhold the intervention from patients (ie, the control group), or anticipated contamination of the intervention into the control group (eg, provider practice change interventions). In addition, it may be impossible to conduct an RCT because the investigator does not have control over the design of an intervention or because they are studying an event, such as a pandemic.
In the June 2020 issue of the Journal of Hospital Medicine, Coon et al1 use a type of quasi-experimental design (QED)—specifically, the interrupted time series (ITS)—to examine the impact of the adoption of ward-based high-flow nasal cannula protocols on intensive care unit (ICU) admission for bronchiolitis at children’s hospitals. In this methodologic progress note, we discuss QEDs for evaluating the impact of healthcare interventions or events and focus on ITS, one of the strongest QEDs.
WHAT IS A QUASI-EXPERIMENTAL DESIGN?
Quasi-experimental design refers to a broad range of nonrandomized or partially randomized pre- vs postintervention studies.2 In order to test a causal hypothesis without randomization, QEDs define a comparison group or a time period in which an intervention has not been implemented, as well as at least one group or time period in which an intervention has been implemented. In a QED, the control may lack similarity with the intervention group or time period because of differences in the patients, sites, or time period (sometimes referred to as having a “nonequivalent control group”). Several design and analytic approaches are available to enhance the extent to which the study is able to make conclusions about the causal impact of the intervention.2,3 Because randomization is not necessary, QEDs allow for inclusion of a broader population than that which is feasible by RCTs, which increases the applicability and generalizability of the results. Therefore, they are a powerful research design to test the effectiveness of interventions in real-world settings.
The choice of which QED depends on whether the investigators are conducting a prospective evaluation and have control over the study design (ie, the ordering of the intervention, selection of sites or individuals, and/or timing and frequency of the data collection) or whether the investigators do not have control over the intervention, which is also known as a “natural experiment.”4,5 Some studies may also incorporate two QEDs in tandem.6 The Table provides a brief summary of different QEDs, ordered by methodologic strength, and distinguishes those that can be used to study natural experiments. In the study by Coon et al,1 an ITS is used as opposed to a methodologically stronger QED, such as the stepped-wedge design, because the investigators did not have control over the rollout of heated high-flow nasal canula protocols across hospitals.

WHAT IS AN INTERRUPTED TIME SERIES?
Interrupted time series designs use repeated observations of an outcome over time. This method then divides, or “interrupts,” the series of data into two time periods: before the intervention or event and after. Using data from the preintervention period, an underlying trend in the outcome is estimated and assumed to continue forward into the postintervention period to estimate what would have occurred without the intervention. Any significant change in the outcome at the beginning of the postintervention period or change in the trend in the postintervention is then attributed to the intervention.
There are several important methodologic considerations when designing an ITS study, as detailed in other review papers.2,3,7,8 An ITS design can be retrospective or prospective. It can be of a single center or include multiple sites, as in Coon et al. It can be conducted with or without a control. The inclusion of a control, when appropriately chosen, improves the strength of the study design because it can account for seasonal trends and potential confounders that vary over time. The control can be a different group of hospitals or participants that are similar but did not receive the intervention, or it can be a different outcome in the same group of hospitals or participants that are not expected to be affected by the intervention. The ITS design may also be set up to estimate the individual effects of multicomponent interventions. If the different components are phased in sequentially over time, then it may be possible to interrupt the time series at these points and estimate the impact of each intervention component.
Other examples of ITS studies in hospital medicine include those that evaluated the impact of a readmission-reduction program,9 of state sepsis regulations on in-hospital mortality,10 of resident duty-hour reform on mortality among hospitalized patients,11 of a quality-improvement initiative on early discharge,12 and of national guidelines on pediatric pneumonia antibiotic selection.13 There are several types of ITS analysis, and in this article, we focus on segmented regression without a control group.7,8
WHAT IS A SEGMENTED REGRESSION ITS?
Segmented regression is the statistical model used to measure (a) the immediate change in the outcome (level) at the start of the intervention and (b) the change in the trend of the outcome (slope) in the postintervention period vs that in the preintervention period. Therefore, the intervention effect size is expressed in terms of the level change and the slope change. To function properly, the models require several repeated (eg, monthly) measurements of the outcome before and after the intervention. Some experts suggest a minimum of 4 to 12 observations, depending on a number of factors including the stability of the outcome and seasonal variations.7,8 If changes before and after more than one intervention are being examined, there should be the minimum number of observations separating them. Unlike typical regression models, time-series models can correct for autocorrelation if it is present in the data. Autocorrelation is the type of correlation that arises when data are collected over time, with those closest in time being more strongly correlated (there are also other types of autocorrelation, such as seasonal patterns). Using available statistical software, autocorrelation can be detected and, if present, it can be controlled for in the segmented regression models.
HOW ARE SEGMENTED REGRESSION RESULTS PRESENTED?
Coon et al present results of their ITS analysis in a panel of figures detailing each study outcome, ICU admission, ICU length of stay, total length of stay, and rates of mechanical ventilation. Each panel shows the rate of change in the outcome per season across hospitals, before and after adoption of heated high-flow nasal cannula protocols, and the level change at the time of adoption.
To further explain how segmented regression results are presented, in the Figure we detail the structure of a segmented regression figure evaluating the impact of an intervention without a control group. In addition to the regression figure, authors typically provide 95% CIs around the rates, level change, and the difference between the postintervention and preintervention periods, along with P values demonstrating whether the rates, level change, and the differences between period slopes differ significantly from zero.

WHAT ARE THE UNDERLYING ASSUMPTIONS OF THE SEGMENTED REGRESSION ITS?
Segmented regression models assume a linear trend in the outcome. If the outcome follows a nonlinear pattern (eg, exponential spread of a disease during a pandemic), then using different distributions in the modeling or transformations of the data may be necessary. The validity of the comparison between the pre- and postintervention groups relies on the similarity between the populations. When there is imbalance, investigators can consider matching based on important characteristics or applying risk adjustment as necessary. Another important assumption is that the outcome of interest is unchanged in the absence of the intervention. Finally, the analysis assumes that the intervention is fully implemented at the time the postintervention period begins. Often, there is a washout period during which the old approach is stopped and the new approach (the intervention) is being implemented and can easily be taken into account.
WHAT ARE THE STRENGTHS OF THE SEGMENTED REGRESSION ITS?
There are several strengths of the ITS analysis and segmented regression.7,8 First, this approach accounts for a possible secular trend in the outcome measure that may have been present prior to the intervention. For example, investigators might conclude that a readmissions program was effective in reducing readmissions if they found that the mean readmission percentage in the period after the intervention was significantly lower than before using a simple pre/post study design. However, what if the readmission rate was already going down prior to the intervention? Using an ITS approach, they may have found that the rate of readmissions simply continued to decrease after the intervention at the same rate that it was decreasing prior to the intervention and, therefore, conclude that the intervention was not effective. Second, because the ITS approach evaluates changes in rates of an outcome at a population level, confounding by individual-level variables will not introduce serious bias unless the confounding occurred at the same time as the intervention. Third, ITS can be used to measure the unintended consequences of interventions or events, and investigators can construct separate time-series analyses for different outcomes. Fourth, ITS can be used to evaluate the impact of the intervention on subpopulations (eg, those grouped by age, sex, race) by conducting stratified analysis. Fifth, ITS provides simple and clear graphical results that can be easily understood by various audiences.
WHAT ARE THE IMPORTANT LIMITATIONS OF AN ITS?
By accounting for preintervention trends, ITS studies permit stronger causal inference than do cross-sectional or simple pre/post QEDs, but they may by prone to confounding by cointerventions or by changes in the population composition. Causal inference based on the ITS analysis is only valid to the extent to which the intervention was the only thing that changed at the point in time between the preintervention and postintervention periods. It is important for investigators to consider this in the design and discuss any coincident interventions. If there are multiple interventions over time, it is possible to account for these changes in the study design by creating multiple points of interruption provided there are sufficient measurements of the outcome between interventions. If the composition of the population changes at the same time as the intervention, this introduces bias. Changes in the ability to measure the outcome or changes to its definition also threaten the validity of the study’s inferences. Finally, it is also important to remember that when the outcome is a population-level measurement, inferences about individual-level outcomes are inappropriate due to ecological fallacies (ie, when inferences about individuals are deduced from inferences about the group to which those individuals belong). For example, Coon et al found that infants with bronchiolitis in the ward-based high-flow nasal cannula protocol group had greater ICU admission rates. It would be inappropriate to conclude that, based on this, an individual infant in a hospital on a ward-based protocol is more likely to be admitted to the ICU.
CONCLUSION
Studies evaluating interventions and events are important for informing healthcare practice, policy, and public health. While an RCT is the preferred method for such evaluations, investigators must often consider alternative study designs when an RCT is not feasible or when more real-world outcome evaluation is desired. Quasi-experimental designs are employed in studies that do not use randomization to study the impact of interventions in real-world settings, and an interrupted time series is a strong QED for the evaluation of interventions and natural experiments.
Hospital medicine research often asks the question whether an intervention, such as a policy or guideline, has improved quality of care and/or whether there were any unintended consequences. Alternatively, investigators may be interested in understanding the impact of an event, such as a natural disaster or a pandemic, on hospital care. The study design that provides the best estimate of the causal effect of the intervention is the randomized controlled trial (RCT). The goal of randomization, which can be implemented at the patient or cluster level (eg, hospitals), is attaining a balance of the known and unknown confounders between study groups.
However, an RCT may not be feasible for several reasons: complexity, insufficient setup time or funding, ethical barriers to randomization, unwillingness of funders or payers to withhold the intervention from patients (ie, the control group), or anticipated contamination of the intervention into the control group (eg, provider practice change interventions). In addition, it may be impossible to conduct an RCT because the investigator does not have control over the design of an intervention or because they are studying an event, such as a pandemic.
In the June 2020 issue of the Journal of Hospital Medicine, Coon et al1 use a type of quasi-experimental design (QED)—specifically, the interrupted time series (ITS)—to examine the impact of the adoption of ward-based high-flow nasal cannula protocols on intensive care unit (ICU) admission for bronchiolitis at children’s hospitals. In this methodologic progress note, we discuss QEDs for evaluating the impact of healthcare interventions or events and focus on ITS, one of the strongest QEDs.
WHAT IS A QUASI-EXPERIMENTAL DESIGN?
Quasi-experimental design refers to a broad range of nonrandomized or partially randomized pre- vs postintervention studies.2 In order to test a causal hypothesis without randomization, QEDs define a comparison group or a time period in which an intervention has not been implemented, as well as at least one group or time period in which an intervention has been implemented. In a QED, the control may lack similarity with the intervention group or time period because of differences in the patients, sites, or time period (sometimes referred to as having a “nonequivalent control group”). Several design and analytic approaches are available to enhance the extent to which the study is able to make conclusions about the causal impact of the intervention.2,3 Because randomization is not necessary, QEDs allow for inclusion of a broader population than that which is feasible by RCTs, which increases the applicability and generalizability of the results. Therefore, they are a powerful research design to test the effectiveness of interventions in real-world settings.
The choice of which QED depends on whether the investigators are conducting a prospective evaluation and have control over the study design (ie, the ordering of the intervention, selection of sites or individuals, and/or timing and frequency of the data collection) or whether the investigators do not have control over the intervention, which is also known as a “natural experiment.”4,5 Some studies may also incorporate two QEDs in tandem.6 The Table provides a brief summary of different QEDs, ordered by methodologic strength, and distinguishes those that can be used to study natural experiments. In the study by Coon et al,1 an ITS is used as opposed to a methodologically stronger QED, such as the stepped-wedge design, because the investigators did not have control over the rollout of heated high-flow nasal canula protocols across hospitals.

WHAT IS AN INTERRUPTED TIME SERIES?
Interrupted time series designs use repeated observations of an outcome over time. This method then divides, or “interrupts,” the series of data into two time periods: before the intervention or event and after. Using data from the preintervention period, an underlying trend in the outcome is estimated and assumed to continue forward into the postintervention period to estimate what would have occurred without the intervention. Any significant change in the outcome at the beginning of the postintervention period or change in the trend in the postintervention is then attributed to the intervention.
There are several important methodologic considerations when designing an ITS study, as detailed in other review papers.2,3,7,8 An ITS design can be retrospective or prospective. It can be of a single center or include multiple sites, as in Coon et al. It can be conducted with or without a control. The inclusion of a control, when appropriately chosen, improves the strength of the study design because it can account for seasonal trends and potential confounders that vary over time. The control can be a different group of hospitals or participants that are similar but did not receive the intervention, or it can be a different outcome in the same group of hospitals or participants that are not expected to be affected by the intervention. The ITS design may also be set up to estimate the individual effects of multicomponent interventions. If the different components are phased in sequentially over time, then it may be possible to interrupt the time series at these points and estimate the impact of each intervention component.
Other examples of ITS studies in hospital medicine include those that evaluated the impact of a readmission-reduction program,9 of state sepsis regulations on in-hospital mortality,10 of resident duty-hour reform on mortality among hospitalized patients,11 of a quality-improvement initiative on early discharge,12 and of national guidelines on pediatric pneumonia antibiotic selection.13 There are several types of ITS analysis, and in this article, we focus on segmented regression without a control group.7,8
WHAT IS A SEGMENTED REGRESSION ITS?
Segmented regression is the statistical model used to measure (a) the immediate change in the outcome (level) at the start of the intervention and (b) the change in the trend of the outcome (slope) in the postintervention period vs that in the preintervention period. Therefore, the intervention effect size is expressed in terms of the level change and the slope change. To function properly, the models require several repeated (eg, monthly) measurements of the outcome before and after the intervention. Some experts suggest a minimum of 4 to 12 observations, depending on a number of factors including the stability of the outcome and seasonal variations.7,8 If changes before and after more than one intervention are being examined, there should be the minimum number of observations separating them. Unlike typical regression models, time-series models can correct for autocorrelation if it is present in the data. Autocorrelation is the type of correlation that arises when data are collected over time, with those closest in time being more strongly correlated (there are also other types of autocorrelation, such as seasonal patterns). Using available statistical software, autocorrelation can be detected and, if present, it can be controlled for in the segmented regression models.
HOW ARE SEGMENTED REGRESSION RESULTS PRESENTED?
Coon et al present results of their ITS analysis in a panel of figures detailing each study outcome, ICU admission, ICU length of stay, total length of stay, and rates of mechanical ventilation. Each panel shows the rate of change in the outcome per season across hospitals, before and after adoption of heated high-flow nasal cannula protocols, and the level change at the time of adoption.
To further explain how segmented regression results are presented, in the Figure we detail the structure of a segmented regression figure evaluating the impact of an intervention without a control group. In addition to the regression figure, authors typically provide 95% CIs around the rates, level change, and the difference between the postintervention and preintervention periods, along with P values demonstrating whether the rates, level change, and the differences between period slopes differ significantly from zero.

WHAT ARE THE UNDERLYING ASSUMPTIONS OF THE SEGMENTED REGRESSION ITS?
Segmented regression models assume a linear trend in the outcome. If the outcome follows a nonlinear pattern (eg, exponential spread of a disease during a pandemic), then using different distributions in the modeling or transformations of the data may be necessary. The validity of the comparison between the pre- and postintervention groups relies on the similarity between the populations. When there is imbalance, investigators can consider matching based on important characteristics or applying risk adjustment as necessary. Another important assumption is that the outcome of interest is unchanged in the absence of the intervention. Finally, the analysis assumes that the intervention is fully implemented at the time the postintervention period begins. Often, there is a washout period during which the old approach is stopped and the new approach (the intervention) is being implemented and can easily be taken into account.
WHAT ARE THE STRENGTHS OF THE SEGMENTED REGRESSION ITS?
There are several strengths of the ITS analysis and segmented regression.7,8 First, this approach accounts for a possible secular trend in the outcome measure that may have been present prior to the intervention. For example, investigators might conclude that a readmissions program was effective in reducing readmissions if they found that the mean readmission percentage in the period after the intervention was significantly lower than before using a simple pre/post study design. However, what if the readmission rate was already going down prior to the intervention? Using an ITS approach, they may have found that the rate of readmissions simply continued to decrease after the intervention at the same rate that it was decreasing prior to the intervention and, therefore, conclude that the intervention was not effective. Second, because the ITS approach evaluates changes in rates of an outcome at a population level, confounding by individual-level variables will not introduce serious bias unless the confounding occurred at the same time as the intervention. Third, ITS can be used to measure the unintended consequences of interventions or events, and investigators can construct separate time-series analyses for different outcomes. Fourth, ITS can be used to evaluate the impact of the intervention on subpopulations (eg, those grouped by age, sex, race) by conducting stratified analysis. Fifth, ITS provides simple and clear graphical results that can be easily understood by various audiences.
WHAT ARE THE IMPORTANT LIMITATIONS OF AN ITS?
By accounting for preintervention trends, ITS studies permit stronger causal inference than do cross-sectional or simple pre/post QEDs, but they may by prone to confounding by cointerventions or by changes in the population composition. Causal inference based on the ITS analysis is only valid to the extent to which the intervention was the only thing that changed at the point in time between the preintervention and postintervention periods. It is important for investigators to consider this in the design and discuss any coincident interventions. If there are multiple interventions over time, it is possible to account for these changes in the study design by creating multiple points of interruption provided there are sufficient measurements of the outcome between interventions. If the composition of the population changes at the same time as the intervention, this introduces bias. Changes in the ability to measure the outcome or changes to its definition also threaten the validity of the study’s inferences. Finally, it is also important to remember that when the outcome is a population-level measurement, inferences about individual-level outcomes are inappropriate due to ecological fallacies (ie, when inferences about individuals are deduced from inferences about the group to which those individuals belong). For example, Coon et al found that infants with bronchiolitis in the ward-based high-flow nasal cannula protocol group had greater ICU admission rates. It would be inappropriate to conclude that, based on this, an individual infant in a hospital on a ward-based protocol is more likely to be admitted to the ICU.
CONCLUSION
Studies evaluating interventions and events are important for informing healthcare practice, policy, and public health. While an RCT is the preferred method for such evaluations, investigators must often consider alternative study designs when an RCT is not feasible or when more real-world outcome evaluation is desired. Quasi-experimental designs are employed in studies that do not use randomization to study the impact of interventions in real-world settings, and an interrupted time series is a strong QED for the evaluation of interventions and natural experiments.
1. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
2. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5-25. https://doi.org/10.1146/annurev-publhealth-040617-014128
3. Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39-56. https://doi.org/10.1146/annurev-publhealth-031816-044327
4. Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health. 2012;66(12):1182-1186. https://doi.org/10.1136/jech-2011-200375
5. Coly A, Parry G. Evaluating Complex Health Interventions: A Guide to Rigorous Research Designs. AcademyHealth; 2017.
6. Orenstein EW, Rasooly IR, Mai MV, et al. Influence of simulation on electronic health record use patterns among pediatric residents. J Am Med Inform Assoc. 2018;25(11):1501-1506. https://doi.org/10.1093/jamia/ocy105
7. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
8. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. https://doi.org/10.1046/j.1365-2710.2002.00430.x
9. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533
10. Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. https://doi.org/10.1001/jama.2019.9021
11. Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975-983. https://doi.org/10.1001/jama.298.9.975
12. Destino L, Bennett D, Wood M, et al. Improving patient flow: analysis of an initiative to improve early discharge. J Hosp Med. 2019;14(1):22-27. https://doi.org/10.12788/jhm.3133
13. Williams DJ, Hall M, Gerber JS, et al; Pediatric Research in Inpatient Settings Network. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
1. Coon ER, Stoddard G, Brady PW. Intensive care unit utilization after adoption of a ward-based high flow nasal cannula protocol. J Hosp Med. 2020;15(6):325-330. https://doi.org/10.12788/jhm.3417
2. Handley MA, Lyles CR, McCulloch C, Cattamanchi A. Selecting and improving quasi-experimental designs in effectiveness and implementation research. Annu Rev Public Health. 2018;39:5-25. https://doi.org/10.1146/annurev-publhealth-040617-014128
3. Craig P, Katikireddi SV, Leyland A, Popham F. Natural experiments: an overview of methods, approaches, and contributions to public health intervention research. Annu Rev Public Health. 2017;38:39-56. https://doi.org/10.1146/annurev-publhealth-031816-044327
4. Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: new Medical Research Council guidance. J Epidemiol Community Health. 2012;66(12):1182-1186. https://doi.org/10.1136/jech-2011-200375
5. Coly A, Parry G. Evaluating Complex Health Interventions: A Guide to Rigorous Research Designs. AcademyHealth; 2017.
6. Orenstein EW, Rasooly IR, Mai MV, et al. Influence of simulation on electronic health record use patterns among pediatric residents. J Am Med Inform Assoc. 2018;25(11):1501-1506. https://doi.org/10.1093/jamia/ocy105
7. Penfold RB, Zhang F. Use of interrupted time series analysis in evaluating health care quality improvements. Acad Pediatr. 2013;13(6 Suppl):S38-S44. https://doi.org/10.1016/j.acap.2013.08.002
8. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299-309. https://doi.org/10.1046/j.1365-2710.2002.00430.x
9. Desai NR, Ross JS, Kwon JY, et al. Association between hospital penalty status under the hospital readmission reduction program and readmission rates for target and nontarget conditions. JAMA. 2016;316(24):2647-2656. https://doi.org/10.1001/jama.2016.18533
10. Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. https://doi.org/10.1001/jama.2019.9021
11. Volpp KG, Rosen AK, Rosenbaum PR, et al. Mortality among hospitalized Medicare beneficiaries in the first 2 years following ACGME resident duty hour reform. JAMA. 2007;298(9):975-983. https://doi.org/10.1001/jama.298.9.975
12. Destino L, Bennett D, Wood M, et al. Improving patient flow: analysis of an initiative to improve early discharge. J Hosp Med. 2019;14(1):22-27. https://doi.org/10.12788/jhm.3133
13. Williams DJ, Hall M, Gerber JS, et al; Pediatric Research in Inpatient Settings Network. Impact of a national guideline on antibiotic selection for hospitalized pneumonia. Pediatrics. 2017;139(4):e20163231. https://doi.org/10.1542/peds.2016-3231
© 2021 Society of Hospital Medicine
Clinical Progress Note: Vascular Access Appropriateness Guidance for Pediatric Hospitalists
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table
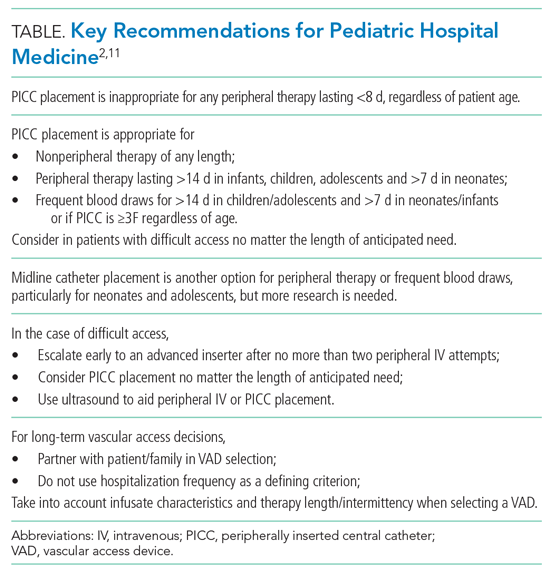
Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table

Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
Hospitalized pediatric patients often require vascular access for necessary therapies, such as antibiotics. However, vascular access devices (VADs) are also associated with harm, ranging from insertion complications to life-threatening bloodstream infections or thrombosis.1 Pediatric hospitalists often guide VAD placement. There is a paucity of evidence to guide VAD selection based on the relative benefits and risks.2 The Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics (miniMAGIC)2 offers the first set of standards. Like its adult predecessor guideline (MAGIC) published in 2015, it provides guidance on appropriate VAD selection based on current evidence and expertise from a multidisciplinary panel.2 The guideline informs device selection, device characteristics, and insertion technique for the pediatric population (term neonates to adolescents) and across a wide range of clinical indications.2 This review highlights key recommendations for pediatric hospitalists to help their decision-making.
METHODS USED IN PREPARING THE GUIDELINE
miniMAGIC was developed using the RAND/UCLA Appropriateness Method, a method proven to reduce inappropriate (ie, overused or underused) healthcare interventions.3 It combines rigorous evidence review with multidisciplinary expert opinion on real-world clinical scenarios to provide recommendations about an intervention’s appropriateness.3 This is particularly useful for clinical scenarios that lack high-quality evidence to guide decision-making. The RAND/UCLA method deems an intervention appropriate if the benefits outweigh the risks by a wide enough margin that proceeding is worthwhile, and it does not take cost into account.2 The method design consists of five phases: (1) defining the scope and key terms, (2) reviewing and synthesizing the literature, (3) selecting an expert panel, (4) developing case scenarios, and (5) conducting two rounds of appropriateness ratings by the expert panel for each clinical scenario.3 The guideline’s scope included term neonates (aged 0-30 days), infants (aged 31 days-1 year), children (aged 1-12 years), and adolescents (aged 12-18 years). Infants receiving care in the neonatal intensive care unit or special care nursery were excluded. Other specialized populations addressed based on setting or diagnosis were general hospitalized patients and patients with congenital cardiac disease, critical illness, oncologic and hematologic conditions, and long-term VAD-dependent conditions.3
A total of 133 studies or clinical practice guidelines (CPGs) met the eligibility criteria for the systematic review.4 Although the systematic review was conducted per the RAND/UCLA method using two independent reviewers who evaluated the methodologic quality, transparency, and relevancy of each article, there was no formal assessment of evidence quality. The recommendations were based primarily on observational studies and CPGs because there were few randomized controlled trials or systematic reviews on VAD selection for pediatric patients in the literature. Pediatric evidence was limited for certain scenarios or populations (eg, term neonates, midline catheters, difficult venous access, long-term VAD), so adult and/or neonatal evidence was included when applicable.
The panel included 14 pediatric clinical experts from cardiology, vascular access, critical care, hematology/oncology, emergency medicine, general surgery, hospital medicine, anesthesia, interventional radiology, pharmacology, and infectious diseases. The panel also included nonvoting panel members such as the panel facilitators, a methodologist, and a patient representative.
RESULTS OF THE CLINICAL REVIEW
We review four common clinical scenarios encountered by pediatric hospitalists and summarize key recommendations (Table

Peripherally Inserted Central Catheter
Patients may require peripherally inserted central catheters (PICCs) to facilitate a longer duration of intravenous (IV) therapy, such as delivery of antibiotics, or frequent blood draws. The need for prolonged vascular access is decreasing, as studies show many infections in children previously treated with prolonged IV antibiotics can be safely and equally effectively managed with early transition to oral therapy.5-8 These studies highlight the higher rate of complications and risks associated with PICCs, including thrombosis, infection, and mechanical issues, as well as the added healthcare utilization needed to evaluate and manage the complications. PICC-associated complication rates also increase with duration.4
However, there are some clinical scenarios that still warrant prolonged therapy and/or access; PICC recommendations are summarized in the Appendix Figure. The miniMAGIC panel deemed PICC lines appropriate for any nonperipheral therapy of any length. For peripherally compatible therapy, the panel rated PICC placement as inappropriate for therapy lasting less than 8 days, regardless of patient age. PICC placement in infants, children, and adolescents was rated appropriate for therapy with a duration exceeding 14 days, but the panel was uncertain about therapy expected to last between 8 and 14 days. Recognizing the additional challenges with maintaining peripheral IV catheter access in neonates, PICCs were deemed appropriate for neonates needing peripheral therapy lasting longer than 7 days.
The panel rated PICC placement appropriate for frequent blood draws (defined as more than one time per day) for more than 7 days in neonates or infants and more than 14 days in children and adolescents. But regardless of patient age, the PICC caliber must be at least 3F.
The miniMAGIC panel found that a single lumen is appropriate in most cases, highlighting that multilumen catheters increase the risk for infection, occlusion, and venous thrombosis.4 Multilumen catheters were rated as inappropriate in the case of reserving a lumen for blood products and blood sampling. When reserving a lumen for lipids and parenteral nutrition (PN), the panel was uncertain given the lack of evidence regarding the risks/benefits of the complications associated with the infusions themselves versus those of the device. Regardless, collaboration with a pharmacist and vascular access specialist is recommended to aid in choosing the most appropriate device characteristics.
Midline Catheters
Midline catheters are inserted in a peripheral vein, but the catheter tip terminates in the proximal extremity. Compared with peripheral IV catheters, midline catheters last longer and have lower rates of phlebitis. In addition, midline catheter placement does not require sedation or fluoroscopy and has lower rates of infection compared with PICC lines.9 Although there is good evidence in adults, and multiple panelists reported success in using midline catheters in various age groups, the evidence for their safe and efficacious use in pediatrics is limited, particularly for infants. Midline catheters were rated as appropriate for peripheral therapy lasting less than 8 days in neonates and less than 15 days in children and adolescents. Use in infants was deemed uncertain based on lack of published evidence. Midline catheters were also rated as appropriate for frequent blood draws of less than 8 days in neonates and less than 15 days in adolescents, but uncertain for children and infants.
Difficult Access and Insertion Procedure
The panel rated three or more attempts for peripheral IV catheter insertion by a single clinician as inappropriate and recommended early escalation to a more experienced inserter after 0 to 2 attempts by a single provider. The goal is to preserve insertion sites and reduce patient discomfort. If a patient loses access when only 1 day of therapy remains, the provider should transition to oral or intramuscular therapy when appropriate, particularly if there are no advanced insertion staff available or after two or more attempts at re-insertion are unsuccessful. There is high-quality evidence that supports vessel visualization (primarily ultrasound) with peripheral IV catheter and PICC placement.2 In the case of two or more unsuccessful attempts at peripheral IV catheter placement by an advanced inserter using technology assistance (ultrasound), PICC placement is considered appropriate by the panel to avoid delays in treatment and limit patient discomfort associated with repeat attempts.
Long-term Vascular Access
Children with medical complexity or chronic illness may require long-term (>2 months) or very-long-term (>1 year) vascular access. Common themes for VAD selection in this heterogeneous population include a focus on vessel preservation and complication prevention.2 The panel strongly recommended that clinicians partner with the patient and caregivers in the decision-making process. Shared decision-making is necessary to meet both the short-term and evolving needs of the of the patient and family. The panel also believed the frequency of hospitalization should not be used as a criterion for VAD selection since acute hospitalization is an unreliable proxy for disease severity in pediatric chronic disease conditions.2 Rather, the infusate characteristics and length/intermittency of therapy should be primary influencers of VAD selection. In general, the panel rated cuffed tunneled central VADs (CVADs) as appropriate for all age groups for long-term PN, long-term continuous infusions, and long-term intermittent therapies. For continuous non-PN infusions, appropriate ratings were given to PICCs for infants and children and total implanted venous devices (TIVDs) in children and adolescents. For intermittent (but at least daily) access, TIVDs and PICC lines were both rated as appropriate for children and adolescents but uncertain for neonates and infants. Peripheral devices were deemed inappropriate for all long-term complex therapies. For children and adolescents needing intermittent, regular peripheral treatments (eg, steroids or antibiotics), peripheral IVs and TIVDs were rated appropriate for short duration (<7 days) therapies. PICCs and midlines for this indication were uncertain because of the lack of evidence. For medium-duration intermittent therapies (8-14 days), PICCs, tunneled cuffed CVADs, and TIVDs were rated as appropriate. A recently released mobile application can help guide the clinician through many varied clinical scenarios and indications.10
LIMITATIONS AND GAPS
The guideline recommendations were more often reliant on clinical practice guidelines and expert panel opinion given the lack of high-quality pediatric evidence for most scenarios. The panel members were from the United States and Australia, so the recommendations may not be generalizable to care systems in other countries. Although the panel included experts from many specialties that care for patient populations needing VADs, not all subspecialty populations were considered, particularly those with long-term vascular access–dependent conditions who may be commonly hospitalized. Scenarios with disagreement or uncertainty highlight gaps in need of future study (eg, midline catheter use and device selection for blood draws).
CONCLUSIONS AND APPLICATION
miniMAGIC is the first appropriateness guideline to help standardize the safe use of VADs in children. Although some gaps remain, the authors intend it to be a living document that will need revisions as new evidence is published. A mobile health application facilitates use of the recommendations, providing quick, point-of-care decision support based on clinical features.10 Pediatric hospitalists should collaborate with their institutions to examine their current VAD use in hospitalized children and identify opportunities for practice change and standardization. Use of these recommendations may help hospitalists improve the care of hospitalized children by decreasing unnecessary PICC placement and better partner with patients and caregivers to limit discomfort surrounding VAD placement.
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
1. Ullman AJ, Marsh N, Mihala G, Cooke M, Rickard CM. Complications of central venous access devices: a systematic review. Pediatrics. 2015;136(5):e1331-e1344. https://doi.org/10.1542/peds.2015-1507
2. Ullman AJ, Bernstein SJ, Brown E, et al. The Michigan appropriateness guide for intravenous catheters in pediatrics: miniMAGIC. Pediatrics. 2020;145(Suppl 3):S269-S284. https://doi.org/10.1542/peds.2019-3474I
3. Ullman AJ, Chopra V, Brown E, et al. Developing appropriateness criteria for pediatric vascular access. Pediatrics. 2020;145(Suppl 3):S233-S242. https://doi.org/10.1542/peds.2019-3474G
4. Paterson RS, Chopra V, Brown E, et al. Selection and insertion of vascular access devices in pediatrics: a systematic review. Pediatrics. 2020;145(Suppl 3):S243-S268. https://doi.org/10.1542/peds.2019-3474H
5. Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomeyelitis in children. JAMA Pediatr. 2015;169(2):120-128. https://doi.org/10.1001/jamapediatrics.2014.2822
6. Shah SS, Srivastava R, Wu S, et al. Intravenous versus oral antibiotics for postdischarge treatment of complicated pneumonia. Pediatrics. 2016;138(6):e20161692. https://doi.org/10.1542/peds.2016-1692
7. Rangel SJ, Anderson BR, Srivastava R, et al. Intravenous versus oral antibiotics for the prevention of treatment failure in children with complicated appendicitis: has the abandonment of peripherally inserted catheters been justified? Ann Surg. 2017;266(2):361-368. https://doi.org/10.1097/SLA.0000000000001923
8. Desai S, Aronson PL, Shabanova V, et al. Parenteral antibiotic therapy duration in young infants with bacteremic urinary tract infections. Pediatrics. 2019;144(3):e20183844. https://doi.org/10.1542/peds.2018-3844
9. Anderson J, Greenwell A, Louderback J, Polivka BJ, Herron Behr J. Comparison of outcomes of extended dwell/midline peripheral intravenous catheters and peripherally inserted central catheters in children. J Assoc Vasc Access. 2016;21(3):158-164. https://doi.org/10.1016/j.java.2016.03.007
10. miniMAGIC: the Michigan Appropriateness Guide for Intravenous Catheters in Pediatrics app. Version 1.0.0. Alliance for Vascular Access Teaching and Research.
11. Shaughnessy EE, Morton K, Shah SS. Vascular access in hospitalized children. Pediatrics. 2020;145(Suppl 3):S298-S299. https://doi.org/10.1542/peds.2019-3474P
© 2021 Society of Hospital Medicine
Clinical Progress Note: Direct Oral Anticoagulants for Treatment of Venous Thromboembolism in Children
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.
The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.

The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
Venous thromboembolism (VTE) is a life-threatening event occurring with increasing frequency in hospitalized children and an incidence of more than 58 events per 10,000 hospitalizations.1 In pediatric patients, VTEs occur less often than in adults, have bimodal peaks in neonates and adolescents, and are typically provoked, with central venous access as the most common risk factor.1,
Treatment of pediatric VTE includes unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), and vitamin K antagonists (ie, warfarin). These agents have limitations, including parenteral administration, frequent lab monitoring, and drug/dietary interactions complicating use. Only recently have there been pediatric studies to assess these agents’ pharmacokinetics, pharmacodynamics, safety, and efficacy.2
Direct oral anticoagulants (DOACs) commonly used to treat VTE in adults have two mechanisms of action: direct thrombin (activated factor II) inhibition (ie, dabigatran) and activated factor X (Xa) inhibition (ie, rivaroxaban, apixaban, edoxaban, betrixaban). DOACs offer practical advantages over and efficacy similar to that of warfarin and heparin products, including oral administration, predictable pharmacology, no required lab monitoring, and fewer drug/dietary interactions. DOACs are already approved for VTE treatment in patients 18 years and older.3
This clinical practice update synthesizes 6 years (2014-2020) of literature regarding DOACs for treatment of VTE, focusing on their current role in patients 18 years and older and their emerging role in pediatric patients.
USE IN ADULTS
DOACs are approved by the US Food and Drug Administration (FDA) for multiple anticoagulation indications in adults, including treatment and prevention of acute VTE and prevention of stroke in nonvalvular atrial fibrillation (Table). DOACs are well tolerated by most adults; however, use in certain populations, including patients with liver disease with coagulopathy, advanced renal disease (creatinine clearance <30 mL/min), and class III obesity (body mass index [BMI] >40 kg/m2), requires caution.4,5 For adult patients with VTE without contraindications, DOACs are considered equivalent to warfarin; current CHEST guidelines even suggest preference of DOACs over warfarin.5 While it is prudent to exercise caution when extrapolating adult data to children, these data have informed ongoing pediatric DOAC clinical trials.

The efficacy and safety of each of the DOACs (aside from betrixaban, which is indicated only for prophylaxis) have compared with warfarin for treatment of VTE in adults.6 A meta-analysis of six clinical trials determined DOACs are noninferior to warfarin for VTE treatment.3 Only two of six trials included patients with provoked VTEs. The meta-analysis found no difference in rates of recurrent symptomatic VTE (primary outcome; relative risk [RR], 0.91; 95% CI, 0.79-1.06) or all-cause mortality (secondary outcome; RR, 0.98; 95% CI, 0.84-1.14). Additionally, DOACs were shown as possibly safer than warfarin due to fewer major bleeding events, particularly fatal bleeding (RR, 0.36; 95% CI, 0.15-0.84) and intracranial bleeding (RR, 0.34; 95% CI, 0.17-0.69). For clinically relevant nonmajor bleeding (eg, gastrointestinal bleeding requiring <2 U packed red blood cells), results were similar (RR, 0.73; 95% CI, 0.58-0.93).
DOACs appear to have effectiveness comparable with that of warfarin. A retrospective matched cohort study of 59,525 patients with acute VTE compared outcomes of patients on DOACs (95% on rivaroxaban) with those of patients on warfarin.6 There were no differences in all-cause mortality or major bleeding. Another retrospective cohort study of 62,431 patients with acute VTE compared rivaroxaban and apixaban with warfarin, as well as rivaroxaban and apixaban with each other.7 There were no differences in 3- and 6-month mortality between warfarin and DOAC users or between rivaroxaban and apixaban users.
Initial approval of DOACs brought concerns about reversibility in the setting of bleeding or urgent procedural need. Clinical practice guidelines, primarily based on observational studies and laboratory parameters in vitro or in healthy volunteers, recommend activated prothrombin complex concentrates as a first-line intervention.8 However, specific agents have now been FDA-approved for DOAC reversal.
Idarucizumab is an FDA-approved (2015) monoclonal antibody with high affinity for dabigatran. Approval was based on a multicenter prospective cohort study of 503 patients taking dabigatran who presented with major bleeding (301 patients) or requiring an urgent surgery (202 patients).9 Idarucizumab resulted in a median time to bleeding cessation of 2.5 hours for those 134 patients in whom time to bleeding cessation could be assessed. Patients with intracranial bleeding were excluded from the timed portion because follow up imaging was not mandated. For those requiring surgery, 93% had normal periprocedural hemostasis.
Andexanet alfa is an FDA-approved (2018) drug for reversal of apixaban and rivaroxaban that acts as a catalytically inactive decoy Xa molecule, binding Xa inhibitors with high affinity. A multicenter prospective cohort study of 352 patients on Xa inhibitors with major bleeding found administration of andexanet alfa resulted in excellent or good hemostasis in 82% of patients (204/249 patients) at 12 hours.10 There was no difference between rivaroxaban and apixaban patients. Both idarucizumab and andexanet alfa remain expensive and not universally available, but availability and use will likely increase with time.
EVIDENCE FOR USE IN CHILDREN
In pediatric patients, most VTEs are provoked, with the most common risk factor being presence of a central line. Frequency of this risk factor varies based on age (>60% of cases in older children and nearly 90% in neonates).1 The most recent American Society of Hematology guidelines recommend treating pediatric symptomatic VTE with anticoagulation and treating asymptomatic VTE instead of observation.2 These recommendations rely on evidence in adult patients due to the current paucity of evidence in pediatrics.
“Pediatric investigation plans” are the cornerstone for ongoing clinical trials of DOACs in pediatrics. While studies evaluating safety and efficacy of standard anticoagulants (UFH, LMWH, and warfarin) in pediatrics exist, clinical trials at the time of drug development did not include pediatric patients. This means none of the currently used anticoagulants were initially developed or approved for children.1 Under the Pediatric Research Equity Act of 2007, the FDA requires pharmaceutical companies to submit a New Drug Application to perform pediatric studies of drugs deemed likely for use in pediatric patients. Pediatric investigation plans allow for establishing safety, efficacy, dosing, and administration routes in pediatric populations. All four DOACs currently approved for treatment of VTE in adults have ongoing efficacy and safety clinical trials for children.
The first and only published clinical trial of DOAC efficacy and safety in pediatrics compared rivaroxaban to standard treatment of acute VTE (Appendix Table).11 The industry-sponsored, open-label EINSTEIN-Jr trial randomized patients aged 0 to 17 years 2:1 to weight-based rivaroxaban or standard treatment after receiving initial parenteral therapy for 5 to 9 days. While most patients were treated for at least 3 months, patients younger than 2 years with line-related thrombosis were treated for only 1 month. The study population mostly consisted of patients with initial, symptomatic, provoked VTE, with types ranging from cerebral venous sinus thrombosis to catheter-associated thrombosis. VTE risk factors, which varied by age, included presence of a central line, major infection, surgery, or trauma. While most VTEs in pediatric patients are expected to be central-line related, in the EINSTEIN-Jr trial only 25.2% of VTEs were central line–associated. The study evaluated symptomatic recurrent VTE (primary efficacy outcome) and clinically relevant bleeding (safety outcome). No significant difference was found between treatment groups in efficacy or safety outcomes, and there were no treatment-related deaths. While the trial was not powered to assess noninferiority due to low incidence of VTE in pediatrics, the absolute number of symptomatic recurrent VTEs was lower in the rivaroxaban group compared with the standard-care group (1% vs 3%). The investigators concluded that rivaroxaban is similarly efficacious and safe in children as compared with adults. FDA approval of rivaroxaban in pediatrics is expected given the trial’s favorable results. Clinicians may wish to consider whether the studied population is comparable with their own patients because the trial had a lower percentage of line-associated VTE than previously reported in the pediatric population.
Multiple clinical trials evaluating the efficacy and safety of other DOACs in pediatric patients are currently underway (Appendix Table).12-14 Apixaban and edoxaban have active multicenter, randomized, open-label clinical trials recruiting patients up to age 17 who have imaging-confirmed acute VTE. A similar trial for dabigatran has recently completed recruitment. Outcome measures include recurrent VTE, VTE-related mortality, and major or clinically relevant non-major bleeding. Like EINSTEIN-Jr, patients in the dabigatran and edoxaban trials were treated with parenteral therapy for at least 5 days prior to randomization.12,14 In the apixaban trial, participants can be randomized without initial parenteral treatment.13 Betrixaban, the newest DOAC approved in adults, does not currently have any open pediatric trials.
AREAS IN NEED OF FUTURE STUDY
Lack of approved reversal agents may initially limit DOAC use in children. An open-label study examining idarucizumab safety has completed enrollment, but it has not yet published results.15 To date, there are no pediatric clinical trials examining andexanet alpha. Future work will need to establish efficacy and safety of reversal agents in pediatrics.
DOACs have not been adequately studied in populations of patients with comorbidities, such as liver disease, renal disease, altered enteral absorption, and BMI higher than 40. Physiologic differences in children with cancer and in neonates merit further evaluation of DOAC safety and efficacy. While ongoing trials established weight-based dosing regimens for children, longitudinal studies will need to ensure adequate anticoagulation, especially in the populations listed here.
The safety outcomes in most DOAC studies include clinically relevant bleeding and VTE-related mortality. These outcomes are much less common in pediatric patients than they are in adults, and future studies may need to expand safety outcomes to those more frequently seen in children. Primary and secondary endpoint variability in pediatric DOAC clinical trials presents challenges interpreting and comparing study results.
SUMMARY
VTE is an increasingly common complication in hospitalized children contributing to significant morbidity.1 For decades, the only treatment options have been UFH, LMWH, or warfarin. DOACs offer many advantages compared with standard anticoagulation options. The only clinical trial evaluating efficacy and safety of DOACs published to date demonstrates that pediatric patients taking rivaroxaban have outcomes similar to those of patients receiving standard care. It is expected that DOACs will gain FDA approval for treatment of VTE in pediatric patients in the near future; therefore, hospitalists should understand indications for use of these medications.
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
1. Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399-404. https://doi.org/10.1182/asheducation-2018.1.399
2. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292-3316. https://doi.org/10.1182/bloodadvances.2018024786
3. Gómez-Outes A, Terleira-Fernández AI, Lecumberri R, Suárez-Gea ML, Vargas-Castrillón E. Direct oral anticoagulants in the treatment of acute venous thromboembolism: a systematic review and meta-analysis. Thromb Res. 2014;134(4):774-782. https://doi.org/10.1016/j.thromres.2014.06.020
4. Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14(6):1308-1313. https://doi.org/10.1111/jth.13323
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352. https://doi.org/10.1016/j.chest.2015.11.026
6. Jun M, Lix LM, Durand M, et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population based, observational study. BMJ. 2017;359:j4323. https://doi.org/10.1136/bmj.j4323
7. Roetker NS, Lutsey PL, Zakai NA, Alonso A, Adam TJ, MacLehose RF. All-cause mortality risk with direct oral anticoagulants and warfarin in the primary treatment of venous thromboembolism. Thromb Haemost. 2018;118(9):1637-1645. https://doi.org/10.1055/s-0038-1668521
8. Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg Med. 2018;11(1):55. https://doi.org/10.1186/s12245-018-0215-6
9. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(5):431-441. https://doi.org/10.1056/nejmoa1707278
10. Connolly SJ, Crowther M, Eikelboom JW, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326-1335. https://doi.org/10.1056/nejmoa1814051
11. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18-e27. https://doi.org/10.1016/s2352-3026(19)30219-4
12. Open label study comparing efficacy and safety of dabigatran etexilate to standard of care in paediatric patients with venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT01895777. Posted July 11, 2013. Updated July 7, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT01895777
13. Apixaban for the acute treatment of venous thromboembolism in children. ClinicalTrials.gov identifier: NCT02464969. Posted June 8, 2015. Updated September 10, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02464969
14. Hokusai study in pediatric patients with confirmed venous thromboembolism (VTE). ClinicalTrials.gov identifier: NCT02798471. Posted June 14, 2016. Update March 6, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02798471
15. Reversal dabigatran anticoagulant effect with idarucizumab. ClinicalTrials.gov Identifier: NCT02815670. Posted June 28, 2016. Updated April 14, 2020. Accessed September 23, 2020. https://clinicaltrials.gov/ct2/show/NCT02815670
© 2021 Society of Hospital Medicine

