User login
Using Co-Design Methods to Create a Patient-Oriented Discharge Summary
From the OpenLab, University Health Network, Toronto, Canada (Dr. Hahn-Goldberg, Dr. Okrainec, Dr. Abrams, Mr. Huynh); School of Health Policy and Management, York University, Toronto (Dr. Hahn-Goldberg); Toronto Central Local Health Integration Network (Ms. Damba); Centre for Addiction and Mental Health, Toronto (Ms. Solomon); and the Department of Medicine, University Health Network, Toronto (Dr. Okrainec, Dr. Abrams).
Abstract
- Objective: To describe the co-design process we under-took to create a patient-oriented discharge summary (PODS) with patients, caregivers, and providers.
- Method: Descriptive report.
- Results: We designed and produced a prototype PODS, based on best practices in information design, graphic design, and patient education. Through a co-design process, patients, health care providers, designers and system planners worked together to establish what content needed to be included, as well as how it would be organized and presented. From an initial prototype, we then refined the PODS through an iterative participatory design process involving patients, including those from hard-to-reach groups such as patients with language barriers and/or low health literacy and patients with a primary psychiatric diagnosis.
- Conclusion: Co-design events and targeted focus groups are very useful for engaging patients and caregivers in the design and development of solutions aimed at improving their experience of care. It is important to include all users, especially those who are harder to reach, such as patients with language barriers and mental health conditions. Engaging health care providers is essential to ensure feasibility of those solutions.
Traditional discharge summaries are written primarily for the patient’s primary care provider and are not designed as tools to support communication between the clinician and patient regarding instructions for patients to follow at home post discharge. A more patient-centered version of the discharge summary is needed to complement the traditional format.
To enhance our patients’ care experience in the post-discharge period, we set out to co-design a patient-oriented discharge summary (PODS) with patients, caregivers, and providers. The main objective of this project was to develop a prototype PODS that not only addressed critical information that patients felt was the most important to know following discharge, but also provided this information in the most comprehensible format at hospitals within the Toronto area. The project focused on reformatting patient-care instructions for patients discharged from inpatient medical wards as these instructions presented the best opportunity for improvement.
This project drew on work done in countries such as India, South Africa, and Pakistan, where challenges with general and health literacy have led to the introduction of simplified discharge forms and medication instructions that place a greater emphasis on visual communication to improve information comprehension. For example, the use of pictograms in patient materials has been shown to increase patient understanding of and compliance with care instructions in these countries [1–3].
We have provided an overview of the PODS project elsewhere [4]. In this article, we describe the co-design process we undertook with patients, health care providers, and designers in creating a PODS.
Design Methods
We used several methods to design and develop the PODS and to engage multiple stakeholders in the design process. Among these methods were innovative techniques for understanding the patient experience of discharge, such as cultural probes [5], where patients were given journals and cameras to document their time at home after discharge, as described by Hahn-Goldberg et al [4]. Other key methods for determining the design of the PODS included:
Patient education consultation. A patient education representative with training and expertise in designing materials for patients with low health literacy was added to the team advisory committee to act as a consultant at all stages of the study. This helped to ensure that we would be following best practices in design for our target population.
Review of literature. We reviewed the literature pertaining to design for patients with low health literacy and language barriers, including the resources available through the patient education department at the University Health Network.
Review of Toronto Central Local Health Integration Network (TCLHIN) hospitals’ tools: current discharge summaries, components they included and how they were formatted.
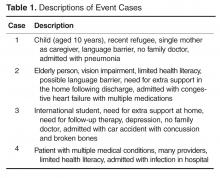
Co-design event. We held an interprofessional design event where teams of patients, health care providers, and designers worked together to create draft PODS for 4 hypothetical patient cases.
Focus groups and surveys. We used feedback from focus groups and surveys to revise and improve the first PODS prototype.
Insights from the Literature
Studies have shown that multiple interventions tend to increase adherence, that self-management should be encouraged, and that modes of communication other than verbal must be used [6]. Visual cues, such as pictures or symbols, are useful to help with recall of medications and instructions for people with language barriers or limited health literacy [7]. Simplified written instructions and larger fonts have been found to be effective in patients with language barriers or limited health literacy, as have use of illustrated medication schedules [8]. Other guidelines include using short words and sentences, writing directly to the reader, listing important points in list format, and using left justification so there is even spacing between words.
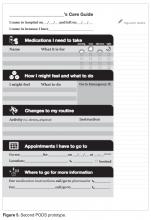
The literature is consistent with our findings from speaking with patient education representatives and patients. Patients and caregivers noted that the PODS should be written in plain language, use large fonts, include illustrations of care and medication schedules, and include headings that are meaningful to the patient. Patients also expressed the desire for charts and lists that they could use while completing their follow-up care plan.
“My mom made me a chart of when to drink water and how much (patient).”
“We were given a sheet to record all feedings (caregiver).”
“We can provide a list of patient meds in a grid format with days of the week and times of day. We use our judgement to give this to patients. It is not standard practice (pharmacist).”
“A discharge form in ‘plain English’ should be standardized (patient).”
The Co-design Event
Solutions resulting from the design event ranged from more traditional discharge summaries that were enhanced with multiple languages and images to make things clear for patients, to solutions including interactive patient portals. There were solutions that came with stickers to color-code your medications, areas for patients to write notes, and checklists for them to keep track of all their follow-up plans.
Refining the Design Using Focus Groups and Surveys
The ideas and concepts generated during the design event were analyzed by the interdisciplinary advisory team at OpenLab. In addition, several team sessions were held with health care
This is a great piece. You guys are doing an awesome job. This would have saved me so much anxiety and fear of doing something wrong when I was discharged. I didn’t want to bother my doctors and went on a hope and prayer. Even my home care people weren’t always sure of what to do. Again this would be a great step forward in easing patients’ fears, especially senior citizens. GREAT WORK. THANKS FOR CARING.
Discussion
Innovative methods such as co-design events and targeted focus groups are very useful for
Future Plans
The PODS template has now been adapted and implemented in several hospitals in Toronto, Canada, using a supported early adopter process [10]. Future plans are to test the impact of the PODS on patient experience and health outcomes using a randomized controlled trial. Also, for now, we have focused on a paper version of PODS, but with the increasing prevalence of electronic health records and consumer-oriented health care apps, future consideration for a digital and mobile version of PODS is warranted.
Conclusion
Patients need to be prepared for discharge so that they can engage in supported self-management once they return home.
Corresponding author: Shoshana Hahn-Goldberg, PhD, 294 Mullen Dr., Thornhill, ON L4J 2P2.
Funding/support: The PODS project has been funded by the Toronto Central Local Health Integration Network.
Financial disclosures: None.
1. Rajesh R, Vidyasagar S, Varma M, Sharma S. Design And evaluation of pictograms for communicating information about adverse drug reactions to antiretroviral therapy in Indian human immunodeficiency virus positive patients. J Pharm Biomed Sci 2012;16:1–11.
2. Dowse R, Ehlers MS. The evaluation of pharmaceutical pictograms in a low-literate South African population. Patient Educ Couns 2001;45:87–99.
3. Clayton M, Syed F, Rashid A, Fayyaz U. Improving illiterate patients’ understanding and adherence to discharge medications. BMJ Qual Improv Rep 2012;1:u496.w167.
4. Hahn-Goldberg S, Okrainec K, Huynh T, et al. Co-creating patient-oriented instructions with patients, caregivers, and providers. J Hosp Med 2015;10:804–7.
5. Gaver B, Dunne T, Pacenti E. Design: cultural probes. Interactions 1999;6:21–9.
6. Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54.
7. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medications in an anticoagulation clinic: A comparison of verbal vs. visual assessment. J Health Commun 2006;11:651–4.
8. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage 2009;25:11–32.
9. IDEO. Design thinking for educators toolkit. 2nd ed. New York: IDEObooks; 2013.
10. Hahn-Goldberg S, Okrainec K, Damba C, et al. Implementing patient oriented discharge summaries (PODS): A multi-site pilot across early adopter hospitals. Healthc Q 2016;19:42–8.
From the OpenLab, University Health Network, Toronto, Canada (Dr. Hahn-Goldberg, Dr. Okrainec, Dr. Abrams, Mr. Huynh); School of Health Policy and Management, York University, Toronto (Dr. Hahn-Goldberg); Toronto Central Local Health Integration Network (Ms. Damba); Centre for Addiction and Mental Health, Toronto (Ms. Solomon); and the Department of Medicine, University Health Network, Toronto (Dr. Okrainec, Dr. Abrams).
Abstract
- Objective: To describe the co-design process we under-took to create a patient-oriented discharge summary (PODS) with patients, caregivers, and providers.
- Method: Descriptive report.
- Results: We designed and produced a prototype PODS, based on best practices in information design, graphic design, and patient education. Through a co-design process, patients, health care providers, designers and system planners worked together to establish what content needed to be included, as well as how it would be organized and presented. From an initial prototype, we then refined the PODS through an iterative participatory design process involving patients, including those from hard-to-reach groups such as patients with language barriers and/or low health literacy and patients with a primary psychiatric diagnosis.
- Conclusion: Co-design events and targeted focus groups are very useful for engaging patients and caregivers in the design and development of solutions aimed at improving their experience of care. It is important to include all users, especially those who are harder to reach, such as patients with language barriers and mental health conditions. Engaging health care providers is essential to ensure feasibility of those solutions.
Traditional discharge summaries are written primarily for the patient’s primary care provider and are not designed as tools to support communication between the clinician and patient regarding instructions for patients to follow at home post discharge. A more patient-centered version of the discharge summary is needed to complement the traditional format.
To enhance our patients’ care experience in the post-discharge period, we set out to co-design a patient-oriented discharge summary (PODS) with patients, caregivers, and providers. The main objective of this project was to develop a prototype PODS that not only addressed critical information that patients felt was the most important to know following discharge, but also provided this information in the most comprehensible format at hospitals within the Toronto area. The project focused on reformatting patient-care instructions for patients discharged from inpatient medical wards as these instructions presented the best opportunity for improvement.
This project drew on work done in countries such as India, South Africa, and Pakistan, where challenges with general and health literacy have led to the introduction of simplified discharge forms and medication instructions that place a greater emphasis on visual communication to improve information comprehension. For example, the use of pictograms in patient materials has been shown to increase patient understanding of and compliance with care instructions in these countries [1–3].
We have provided an overview of the PODS project elsewhere [4]. In this article, we describe the co-design process we undertook with patients, health care providers, and designers in creating a PODS.
Design Methods
We used several methods to design and develop the PODS and to engage multiple stakeholders in the design process. Among these methods were innovative techniques for understanding the patient experience of discharge, such as cultural probes [5], where patients were given journals and cameras to document their time at home after discharge, as described by Hahn-Goldberg et al [4]. Other key methods for determining the design of the PODS included:
Patient education consultation. A patient education representative with training and expertise in designing materials for patients with low health literacy was added to the team advisory committee to act as a consultant at all stages of the study. This helped to ensure that we would be following best practices in design for our target population.
Review of literature. We reviewed the literature pertaining to design for patients with low health literacy and language barriers, including the resources available through the patient education department at the University Health Network.
Review of Toronto Central Local Health Integration Network (TCLHIN) hospitals’ tools: current discharge summaries, components they included and how they were formatted.
Co-design event. We held an interprofessional design event where teams of patients, health care providers, and designers worked together to create draft PODS for 4 hypothetical patient cases.
Focus groups and surveys. We used feedback from focus groups and surveys to revise and improve the first PODS prototype.
Insights from the Literature
Studies have shown that multiple interventions tend to increase adherence, that self-management should be encouraged, and that modes of communication other than verbal must be used [6]. Visual cues, such as pictures or symbols, are useful to help with recall of medications and instructions for people with language barriers or limited health literacy [7]. Simplified written instructions and larger fonts have been found to be effective in patients with language barriers or limited health literacy, as have use of illustrated medication schedules [8]. Other guidelines include using short words and sentences, writing directly to the reader, listing important points in list format, and using left justification so there is even spacing between words.
The literature is consistent with our findings from speaking with patient education representatives and patients. Patients and caregivers noted that the PODS should be written in plain language, use large fonts, include illustrations of care and medication schedules, and include headings that are meaningful to the patient. Patients also expressed the desire for charts and lists that they could use while completing their follow-up care plan.
“My mom made me a chart of when to drink water and how much (patient).”
“We were given a sheet to record all feedings (caregiver).”
“We can provide a list of patient meds in a grid format with days of the week and times of day. We use our judgement to give this to patients. It is not standard practice (pharmacist).”
“A discharge form in ‘plain English’ should be standardized (patient).”
The Co-design Event
Solutions resulting from the design event ranged from more traditional discharge summaries that were enhanced with multiple languages and images to make things clear for patients, to solutions including interactive patient portals. There were solutions that came with stickers to color-code your medications, areas for patients to write notes, and checklists for them to keep track of all their follow-up plans.
Refining the Design Using Focus Groups and Surveys
The ideas and concepts generated during the design event were analyzed by the interdisciplinary advisory team at OpenLab. In addition, several team sessions were held with health care
This is a great piece. You guys are doing an awesome job. This would have saved me so much anxiety and fear of doing something wrong when I was discharged. I didn’t want to bother my doctors and went on a hope and prayer. Even my home care people weren’t always sure of what to do. Again this would be a great step forward in easing patients’ fears, especially senior citizens. GREAT WORK. THANKS FOR CARING.
Discussion
Innovative methods such as co-design events and targeted focus groups are very useful for
Future Plans
The PODS template has now been adapted and implemented in several hospitals in Toronto, Canada, using a supported early adopter process [10]. Future plans are to test the impact of the PODS on patient experience and health outcomes using a randomized controlled trial. Also, for now, we have focused on a paper version of PODS, but with the increasing prevalence of electronic health records and consumer-oriented health care apps, future consideration for a digital and mobile version of PODS is warranted.
Conclusion
Patients need to be prepared for discharge so that they can engage in supported self-management once they return home.
Corresponding author: Shoshana Hahn-Goldberg, PhD, 294 Mullen Dr., Thornhill, ON L4J 2P2.
Funding/support: The PODS project has been funded by the Toronto Central Local Health Integration Network.
Financial disclosures: None.
From the OpenLab, University Health Network, Toronto, Canada (Dr. Hahn-Goldberg, Dr. Okrainec, Dr. Abrams, Mr. Huynh); School of Health Policy and Management, York University, Toronto (Dr. Hahn-Goldberg); Toronto Central Local Health Integration Network (Ms. Damba); Centre for Addiction and Mental Health, Toronto (Ms. Solomon); and the Department of Medicine, University Health Network, Toronto (Dr. Okrainec, Dr. Abrams).
Abstract
- Objective: To describe the co-design process we under-took to create a patient-oriented discharge summary (PODS) with patients, caregivers, and providers.
- Method: Descriptive report.
- Results: We designed and produced a prototype PODS, based on best practices in information design, graphic design, and patient education. Through a co-design process, patients, health care providers, designers and system planners worked together to establish what content needed to be included, as well as how it would be organized and presented. From an initial prototype, we then refined the PODS through an iterative participatory design process involving patients, including those from hard-to-reach groups such as patients with language barriers and/or low health literacy and patients with a primary psychiatric diagnosis.
- Conclusion: Co-design events and targeted focus groups are very useful for engaging patients and caregivers in the design and development of solutions aimed at improving their experience of care. It is important to include all users, especially those who are harder to reach, such as patients with language barriers and mental health conditions. Engaging health care providers is essential to ensure feasibility of those solutions.
Traditional discharge summaries are written primarily for the patient’s primary care provider and are not designed as tools to support communication between the clinician and patient regarding instructions for patients to follow at home post discharge. A more patient-centered version of the discharge summary is needed to complement the traditional format.
To enhance our patients’ care experience in the post-discharge period, we set out to co-design a patient-oriented discharge summary (PODS) with patients, caregivers, and providers. The main objective of this project was to develop a prototype PODS that not only addressed critical information that patients felt was the most important to know following discharge, but also provided this information in the most comprehensible format at hospitals within the Toronto area. The project focused on reformatting patient-care instructions for patients discharged from inpatient medical wards as these instructions presented the best opportunity for improvement.
This project drew on work done in countries such as India, South Africa, and Pakistan, where challenges with general and health literacy have led to the introduction of simplified discharge forms and medication instructions that place a greater emphasis on visual communication to improve information comprehension. For example, the use of pictograms in patient materials has been shown to increase patient understanding of and compliance with care instructions in these countries [1–3].
We have provided an overview of the PODS project elsewhere [4]. In this article, we describe the co-design process we undertook with patients, health care providers, and designers in creating a PODS.
Design Methods
We used several methods to design and develop the PODS and to engage multiple stakeholders in the design process. Among these methods were innovative techniques for understanding the patient experience of discharge, such as cultural probes [5], where patients were given journals and cameras to document their time at home after discharge, as described by Hahn-Goldberg et al [4]. Other key methods for determining the design of the PODS included:
Patient education consultation. A patient education representative with training and expertise in designing materials for patients with low health literacy was added to the team advisory committee to act as a consultant at all stages of the study. This helped to ensure that we would be following best practices in design for our target population.
Review of literature. We reviewed the literature pertaining to design for patients with low health literacy and language barriers, including the resources available through the patient education department at the University Health Network.
Review of Toronto Central Local Health Integration Network (TCLHIN) hospitals’ tools: current discharge summaries, components they included and how they were formatted.
Co-design event. We held an interprofessional design event where teams of patients, health care providers, and designers worked together to create draft PODS for 4 hypothetical patient cases.
Focus groups and surveys. We used feedback from focus groups and surveys to revise and improve the first PODS prototype.
Insights from the Literature
Studies have shown that multiple interventions tend to increase adherence, that self-management should be encouraged, and that modes of communication other than verbal must be used [6]. Visual cues, such as pictures or symbols, are useful to help with recall of medications and instructions for people with language barriers or limited health literacy [7]. Simplified written instructions and larger fonts have been found to be effective in patients with language barriers or limited health literacy, as have use of illustrated medication schedules [8]. Other guidelines include using short words and sentences, writing directly to the reader, listing important points in list format, and using left justification so there is even spacing between words.
The literature is consistent with our findings from speaking with patient education representatives and patients. Patients and caregivers noted that the PODS should be written in plain language, use large fonts, include illustrations of care and medication schedules, and include headings that are meaningful to the patient. Patients also expressed the desire for charts and lists that they could use while completing their follow-up care plan.
“My mom made me a chart of when to drink water and how much (patient).”
“We were given a sheet to record all feedings (caregiver).”
“We can provide a list of patient meds in a grid format with days of the week and times of day. We use our judgement to give this to patients. It is not standard practice (pharmacist).”
“A discharge form in ‘plain English’ should be standardized (patient).”
The Co-design Event
Solutions resulting from the design event ranged from more traditional discharge summaries that were enhanced with multiple languages and images to make things clear for patients, to solutions including interactive patient portals. There were solutions that came with stickers to color-code your medications, areas for patients to write notes, and checklists for them to keep track of all their follow-up plans.
Refining the Design Using Focus Groups and Surveys
The ideas and concepts generated during the design event were analyzed by the interdisciplinary advisory team at OpenLab. In addition, several team sessions were held with health care
This is a great piece. You guys are doing an awesome job. This would have saved me so much anxiety and fear of doing something wrong when I was discharged. I didn’t want to bother my doctors and went on a hope and prayer. Even my home care people weren’t always sure of what to do. Again this would be a great step forward in easing patients’ fears, especially senior citizens. GREAT WORK. THANKS FOR CARING.
Discussion
Innovative methods such as co-design events and targeted focus groups are very useful for
Future Plans
The PODS template has now been adapted and implemented in several hospitals in Toronto, Canada, using a supported early adopter process [10]. Future plans are to test the impact of the PODS on patient experience and health outcomes using a randomized controlled trial. Also, for now, we have focused on a paper version of PODS, but with the increasing prevalence of electronic health records and consumer-oriented health care apps, future consideration for a digital and mobile version of PODS is warranted.
Conclusion
Patients need to be prepared for discharge so that they can engage in supported self-management once they return home.
Corresponding author: Shoshana Hahn-Goldberg, PhD, 294 Mullen Dr., Thornhill, ON L4J 2P2.
Funding/support: The PODS project has been funded by the Toronto Central Local Health Integration Network.
Financial disclosures: None.
1. Rajesh R, Vidyasagar S, Varma M, Sharma S. Design And evaluation of pictograms for communicating information about adverse drug reactions to antiretroviral therapy in Indian human immunodeficiency virus positive patients. J Pharm Biomed Sci 2012;16:1–11.
2. Dowse R, Ehlers MS. The evaluation of pharmaceutical pictograms in a low-literate South African population. Patient Educ Couns 2001;45:87–99.
3. Clayton M, Syed F, Rashid A, Fayyaz U. Improving illiterate patients’ understanding and adherence to discharge medications. BMJ Qual Improv Rep 2012;1:u496.w167.
4. Hahn-Goldberg S, Okrainec K, Huynh T, et al. Co-creating patient-oriented instructions with patients, caregivers, and providers. J Hosp Med 2015;10:804–7.
5. Gaver B, Dunne T, Pacenti E. Design: cultural probes. Interactions 1999;6:21–9.
6. Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54.
7. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medications in an anticoagulation clinic: A comparison of verbal vs. visual assessment. J Health Commun 2006;11:651–4.
8. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage 2009;25:11–32.
9. IDEO. Design thinking for educators toolkit. 2nd ed. New York: IDEObooks; 2013.
10. Hahn-Goldberg S, Okrainec K, Damba C, et al. Implementing patient oriented discharge summaries (PODS): A multi-site pilot across early adopter hospitals. Healthc Q 2016;19:42–8.
1. Rajesh R, Vidyasagar S, Varma M, Sharma S. Design And evaluation of pictograms for communicating information about adverse drug reactions to antiretroviral therapy in Indian human immunodeficiency virus positive patients. J Pharm Biomed Sci 2012;16:1–11.
2. Dowse R, Ehlers MS. The evaluation of pharmaceutical pictograms in a low-literate South African population. Patient Educ Couns 2001;45:87–99.
3. Clayton M, Syed F, Rashid A, Fayyaz U. Improving illiterate patients’ understanding and adherence to discharge medications. BMJ Qual Improv Rep 2012;1:u496.w167.
4. Hahn-Goldberg S, Okrainec K, Huynh T, et al. Co-creating patient-oriented instructions with patients, caregivers, and providers. J Hosp Med 2015;10:804–7.
5. Gaver B, Dunne T, Pacenti E. Design: cultural probes. Interactions 1999;6:21–9.
6. Sheridan SL, Halpern DJ, Viera AJ, et al. Interventions for individuals with low health literacy: a systematic review. J Health Commun 2011;16 Suppl 3:30–54.
7. Schillinger D, Machtinger EL, Wang F, et al. Language, literacy, and communication regarding medications in an anticoagulation clinic: A comparison of verbal vs. visual assessment. J Health Commun 2006;11:651–4.
8. Chugh A, Williams MV, Grigsby J, Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage 2009;25:11–32.
9. IDEO. Design thinking for educators toolkit. 2nd ed. New York: IDEObooks; 2013.
10. Hahn-Goldberg S, Okrainec K, Damba C, et al. Implementing patient oriented discharge summaries (PODS): A multi-site pilot across early adopter hospitals. Healthc Q 2016;19:42–8.
Using the Common Sense Model in Daily Clinical Practice for Improving Medication Adherence
From Genoa-QoL Healthcare and the University of Michigan College of Pharmacy, Ann Arbor, MI.
Abstract
- Objective: To review the Common Sense Model, a framework that can be used for understanding patients’ behavior, including taking or not taking medications as prescribed.
- Methods: Descriptive report.
- Results: Medication adherence, a critical component of achieving good patient outcomes and reducing medical costs, is dependent upon patient illness beliefs. The Common Sense Model holds that these beliefs can be categorized as illness identity, cause, consequence, control, and timeline. Effective communication is necessary to understand the beliefs that patients hold and help them understand their condition. Good communication also can allay fears and other emotions that can be disruptive to achieving good outcomes.
- Conclusion: Clinicians should seek to understand their patients’ illness beliefs and collaborate with them to achieve desired health outcomes.
Clinical practice is based on scientific evidence, by which medical problems are diagnosed and treatment recommendations are made. However, the role of the patient may not be completely recognized as an integral part of the process of patient care. The impact of failing to adequately recognize the patient perspective is evident in medication nonadherence. Health psychology research can provide clinicians insight into patients’ perceptions and behavior. This paper reviews the Common Sense Model (CSM), a behavioral model that provides a framework that can be used in understanding patients’ behavior. In this paper I will discuss the model and how it can be a possible strategy for improving adherence.
Making the Case for CSM in Daily Practice
It can be difficult to realize that persons seeking medical attention would not take medications as prescribed by a physician. In fact, studies reveal that on average, 16.4% of prescribed medications will not be picked up from the pharmacy [1]. Of those patients who do pick up their medication, approximately 1 out of 4 will not take them as prescribed [2]. Such medication nonadherence leads to poor health outcomes and increased health care costs [3,4]. There are many reasons for medication nonadherence [5], and there is no single solution to improving medication adherence [6]. A Cochrane review of randomized controlled trials evaluating various interventions intended to enhance patient adherence to prescribed medications for medical conditions found them to have limited effectiveness. Interventions assessed included health and medication information, reminder calls, follow-up assessment of medication therapy, social support, and simplification of the treatment regimen [6]. In an exploratory study of patients with chronic health conditions, Kucukarslan et al found patients’ beliefs about their illness and their medication are integral to their health care decisions [7]. Their findings were consistent with the CSM, which is based on Leventhal’s theory of self-regulation.
Self-regulation theory states that rational people will make decisions to reduce their health threat. Patients’ perceptions of their selves and environments drives their behavior. So in the presence of a health threat, a person will seek to eliminate or reduce that threat. However, coping behavior is complex. A person may decide to follow the advice of his clinician, follow some other advice (from family, friends, advertising, etc.), or do nothing. The premise of self-regulation is that people will choose a common sense approach to their health threat [8]. Therefore, clinicians must understand their patients’ viewpoint of themselves and their health condition so they may help guide them toward healthy outcomes.
The Common Sense Model
The CSM is a framework for understanding patient behavior when faced with a health threat. It holds that patients form common sense representations of their illness using information from 5 domains [8]: (1) the identity of the illness (the label the patient gives to the condition and symptoms); (2) the cause of the illness; (3) the consequences of the illness (beliefs about how the illness will impact the patient’s well-being); (4) whether the illness can be controlled or cured; and (5) timeline (beliefs about how long the condition will last). A patient may either act to address the health threat or choose to ignore it. Patient emotions are proposed to have a role on patient behavior along with the 5 dimensions of illness perception.
Illness Identity
Illness identity is the label patients place on the health threat; it is most likely not the same as the signs and symptoms clinicians use. Therefore, the first misconnect between physician and patient may be in describing the illness. Chen et al studied illness identity as perceived by patients with hypertension [9,10]. Illness identity was defined as (1) hypertension-related symptoms, (2) symptoms experienced before and after their diagnosis; and (3) symptoms used to predict high blood pressure. Although hypertension is asymptomatic, patients do perceive symptoms such as headache associated with their hypertension. The researchers found those patients who identified more symptoms were more likely to believe that their symptoms caused the hypertension and were correspondingly less likely to use their medication. For them, when the headache subsides, so does the hypertension.
Physicians should find out how patients assess their health condition and provide them tools for evaluating their response to medication. In the case of hypertension, the physician could have the patient check their blood pressure with and without the headache to demonstrate that hypertension occurs even when the patient is not “symptomatic.” The point is to converse with the patient to learn how they view their condition. Clinicians should resist the “urge” to correct patients. Taking time to help patients better understand their condition is important. A misstep:
Patient: I can tell when my blood pressure is high. I get a pounding headache.
Doctor: High blood pressure is an asymptomatic condition. Your headaches are not caused by your high blood pressure.
Patients may choose to ignore the clinician if they feel strongly about how they define their illness. It is better to listen to the patient and offer steps to learn about their health condition. Here is a better response from the physician:
Doctor: You are telling me that you can tell when your blood pressure is high. So when your head aches your pressure is high, right?
Patient: Yes.
Doctor: Let me tell you more about high blood pressure. High blood pressure is also present without headaches...
Illness Causes
There are multiple causative factors patients may associate with their disease. Causes attributed to disease may be based on patient experiences, input from family and friends, and cultural factors. Causes may include emotional state, stress or worry, overwork, genetic predisposition, or environmental factors (eg, pollution). Jessop and Rutter found patients who perceive their condition as due to uncontrollable factors, such as chance, germs, or pollution, were less likely to take their medication [11]. Similar findings were published by Chen et al [9]. They found psychological factors, environmental risk factors (eg, smoking, diet), and even bad luck or chance associated with less likelihood of taking medications as prescribed. Clinicians should explore patients’ perceptions of causes of a condition. Patients strive to eliminate the perceived cause, thus eliminating the need to take medication. In some cultures, bad luck or chance drives patients’ decisions to not take medication, or they believe in fate and do not accept treatment. Whether they feel they can control their condition by eliminating the cause or have a fatalistic view that the cause of their condition is not within their control, the clinician must work with the patient to reduce the impact of misperceptions or significance of perceived causes.
Illness Consequence
Consequence associated with the health condition is an important factor in patient behavior [12]. Patients must understand the specific threats to their health if a condition is left untreated or uncontrolled. Patients’ view of illness consequence may be formed by their own perceived vulnerability or susceptibility and the perceived seriousness of the condition. For example, patients with hypertension should be informed about the impact of high blood pressure on their bodies and the consequence of disability from stroke, dependency on dialysis from kidney failure, or death. They may not consider themselves susceptible to illness since they “feel healthy” and may decide to delay treatment. Patients with conditions such as asthma or heart failure may believe they are cured when their symptoms abate and therefore believe they have no more need for medication. Such patients need education to understand that they are asymptomatic because they are well controlled with medication.
Illness Control
Patients may feel they can control their health condition by changing their behavior, changing their environment, and/or by taking prescribed medication. As discussed earlier, cause and control both work together to form patient beliefs and actions. Patients will take their medications as prescribed if they believe in the effectiveness of medication to control their condition [11,13–15]. Interestingly, Ross found those who felt they had more control over their illness were more likely not to take their medication as prescribed [12]. These persons are more likely to not want to become “dependent” on medication. Their feeling was that they can make changes in their lives and thereby improve their health condition.
Physicians should invite patients’ thoughts as to what should be done to improve their health condition, and collaborate with the patient on an action plan for change if change is expected to improve/control the health condition. Follow-up to assess the patient’s health status longitudinally is necessary.
In this exchange, the patient feels he can control his hypertension on his own:
Doctor: I recommend that you start taking medication to control your blood pressure. Uncontrolled high blood pressure can lead to many health problems.
Patient: I am not ready to start taking medication.
Doctor: What are your reasons?
Patient: I am under a lot of stress at work. Once I get control of this stress, my blood pressure will go down.
Doctor: Getting control of your stress at work is important. Let me tell you more about high blood pressure.
Patient: Okay.
Doctor: There is no one cause of your high blood pressure. Eliminating your work stress will most likely not reduce your blood pressure....
Timeline
Health conditions can be acute, chronic, or cyclical (ie, seasonal); however, patients may have different perceptions of the duration of their health condition. In Kucukarslan et al, some patients did not believe their hypertension was a lifelong condition because they felt they would be able to cure it [7]. For example, as illustrated above, patients may believe that stress causes their hypertension, and if the stress could be controlled, then their blood pressure would normalize. Conversely, Ross et al found that patients who viewed their hypertension as a long-term condition were more likely to believe their medications were necessary and thus more likely to take their medication as prescribed [12]. A lifelong or chronic health condition is a difficult concept for patients to accept, especially ones who may view themselves as too young to have the condition.
Emotions
After being informed about their health condition, patients may feel emotions that are not apparent to the practitioner. These may include worry, depression, anger, anxiety, or fear. Emotions may impact their decision to take medication [12,14]. Listening for patients’ responses to health information provided by the clinician and letting patients know they have been heard will help allay strong negative emotions [16]. Good communication builds trust between the clinician and patient.
Conclusion
Patients receive medical advice from clinicians that may be inconsistent with their beliefs and understanding of their health condition. Studies of medication nonadherence find many factors contribute to it and no one tool to improve medication adherence exists. However, the consequence of medication nonadherence are great and include include worsening condition, increased comorbid disease, and increased health care costs. Understanding patients’ beliefs about their health condition is an important step toward reducing medication nonadherence. The CSM provides a framework for clinicians to guide patients toward effective decision-making. Listening to the patient explain how they view their condition—how they define it, the causes, consequences, how to control it, and how long it will last or if it will progress—are important to the process of working with the patient manage their condition effectively. Clinicians’ reaction to these perceptions are important, and dismissing them may alienate patients. Effective communication is necessary to understand patients’ perspectives and to help them manage their health condition.
Corresponding author: Suzan N. Kucukarslan, PhD, RPh, skucukar@gmail.com.
Financial disclosures: None.
1. Gadkari AS, McHorney CA. Medication non-fulfillment rates and reasons: a narrative systematic review. Curr Med Res Opin 2010;26:683–785.
2. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200–9.
3. Ho PM, Rumsfeld JS, Masoudi FA, et al. The effect of medication non-adherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166;1836–41.
4. Benjamin RM. Medication adherence: Helping patients take their medicines as directed. Pub Health Rep 2012;2–3.
5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97.
6. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008;(2):CD000011.
7. Kucukarslan SN, Lewis NJW, Shimp LA, et al. Exploring patient experiences with prescription medicines to identify unmet patient needs: implications for research and practice. Res Social Adm Pharm 2012;8:321–332.
8. Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychol Health 1998;13:717–33.
9. Chen S-L, Tsai J-C, Chou K-R. Illness perceptions and adherence to therapeutic regimens among patients with hypertension: A structural model approach. Int J Nurs Stud 2011;48:235–45.
10. Chen S-L, Tsai J-C, Lee W-L. The impact of illness perception on adherence to therapeutic regimens of patients with hypertension in Taiwan. J Clin Nurs 2009;18:2234–44.
11. Jessop DC, Rutter DR. Adherence to asthma medication: the role of illness representations. Psychol Health 2003;18:595–612.
12. Ross S, Walker A, MacLeod M. Patient compliance in hypertension:role of illness perceptions and treatment beliefs. J Hum Hypertension 2004;18:607–13.
13 Searle A, Norman P. Thompson R. Vedhara K. A prospective examination of illness belies and coping in patients with type 2 diabetes. Br J Health Psychol 2007;12:621–38.
14. Zugelj U, Zuparnicic M, Komidar L, et al. Self-reported adherence behavior in adolescent hypertensive patients: the role of illness representation and personality. J Pediatr Psychol 2010;35:1049–60.
15. Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perception and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health 2002;17:17–32.
16. Northouse LL, Northouse PG. Health communication: strategies for health professionals. Stamford: Prentice Hall; 1998.
From Genoa-QoL Healthcare and the University of Michigan College of Pharmacy, Ann Arbor, MI.
Abstract
- Objective: To review the Common Sense Model, a framework that can be used for understanding patients’ behavior, including taking or not taking medications as prescribed.
- Methods: Descriptive report.
- Results: Medication adherence, a critical component of achieving good patient outcomes and reducing medical costs, is dependent upon patient illness beliefs. The Common Sense Model holds that these beliefs can be categorized as illness identity, cause, consequence, control, and timeline. Effective communication is necessary to understand the beliefs that patients hold and help them understand their condition. Good communication also can allay fears and other emotions that can be disruptive to achieving good outcomes.
- Conclusion: Clinicians should seek to understand their patients’ illness beliefs and collaborate with them to achieve desired health outcomes.
Clinical practice is based on scientific evidence, by which medical problems are diagnosed and treatment recommendations are made. However, the role of the patient may not be completely recognized as an integral part of the process of patient care. The impact of failing to adequately recognize the patient perspective is evident in medication nonadherence. Health psychology research can provide clinicians insight into patients’ perceptions and behavior. This paper reviews the Common Sense Model (CSM), a behavioral model that provides a framework that can be used in understanding patients’ behavior. In this paper I will discuss the model and how it can be a possible strategy for improving adherence.
Making the Case for CSM in Daily Practice
It can be difficult to realize that persons seeking medical attention would not take medications as prescribed by a physician. In fact, studies reveal that on average, 16.4% of prescribed medications will not be picked up from the pharmacy [1]. Of those patients who do pick up their medication, approximately 1 out of 4 will not take them as prescribed [2]. Such medication nonadherence leads to poor health outcomes and increased health care costs [3,4]. There are many reasons for medication nonadherence [5], and there is no single solution to improving medication adherence [6]. A Cochrane review of randomized controlled trials evaluating various interventions intended to enhance patient adherence to prescribed medications for medical conditions found them to have limited effectiveness. Interventions assessed included health and medication information, reminder calls, follow-up assessment of medication therapy, social support, and simplification of the treatment regimen [6]. In an exploratory study of patients with chronic health conditions, Kucukarslan et al found patients’ beliefs about their illness and their medication are integral to their health care decisions [7]. Their findings were consistent with the CSM, which is based on Leventhal’s theory of self-regulation.
Self-regulation theory states that rational people will make decisions to reduce their health threat. Patients’ perceptions of their selves and environments drives their behavior. So in the presence of a health threat, a person will seek to eliminate or reduce that threat. However, coping behavior is complex. A person may decide to follow the advice of his clinician, follow some other advice (from family, friends, advertising, etc.), or do nothing. The premise of self-regulation is that people will choose a common sense approach to their health threat [8]. Therefore, clinicians must understand their patients’ viewpoint of themselves and their health condition so they may help guide them toward healthy outcomes.
The Common Sense Model
The CSM is a framework for understanding patient behavior when faced with a health threat. It holds that patients form common sense representations of their illness using information from 5 domains [8]: (1) the identity of the illness (the label the patient gives to the condition and symptoms); (2) the cause of the illness; (3) the consequences of the illness (beliefs about how the illness will impact the patient’s well-being); (4) whether the illness can be controlled or cured; and (5) timeline (beliefs about how long the condition will last). A patient may either act to address the health threat or choose to ignore it. Patient emotions are proposed to have a role on patient behavior along with the 5 dimensions of illness perception.
Illness Identity
Illness identity is the label patients place on the health threat; it is most likely not the same as the signs and symptoms clinicians use. Therefore, the first misconnect between physician and patient may be in describing the illness. Chen et al studied illness identity as perceived by patients with hypertension [9,10]. Illness identity was defined as (1) hypertension-related symptoms, (2) symptoms experienced before and after their diagnosis; and (3) symptoms used to predict high blood pressure. Although hypertension is asymptomatic, patients do perceive symptoms such as headache associated with their hypertension. The researchers found those patients who identified more symptoms were more likely to believe that their symptoms caused the hypertension and were correspondingly less likely to use their medication. For them, when the headache subsides, so does the hypertension.
Physicians should find out how patients assess their health condition and provide them tools for evaluating their response to medication. In the case of hypertension, the physician could have the patient check their blood pressure with and without the headache to demonstrate that hypertension occurs even when the patient is not “symptomatic.” The point is to converse with the patient to learn how they view their condition. Clinicians should resist the “urge” to correct patients. Taking time to help patients better understand their condition is important. A misstep:
Patient: I can tell when my blood pressure is high. I get a pounding headache.
Doctor: High blood pressure is an asymptomatic condition. Your headaches are not caused by your high blood pressure.
Patients may choose to ignore the clinician if they feel strongly about how they define their illness. It is better to listen to the patient and offer steps to learn about their health condition. Here is a better response from the physician:
Doctor: You are telling me that you can tell when your blood pressure is high. So when your head aches your pressure is high, right?
Patient: Yes.
Doctor: Let me tell you more about high blood pressure. High blood pressure is also present without headaches...
Illness Causes
There are multiple causative factors patients may associate with their disease. Causes attributed to disease may be based on patient experiences, input from family and friends, and cultural factors. Causes may include emotional state, stress or worry, overwork, genetic predisposition, or environmental factors (eg, pollution). Jessop and Rutter found patients who perceive their condition as due to uncontrollable factors, such as chance, germs, or pollution, were less likely to take their medication [11]. Similar findings were published by Chen et al [9]. They found psychological factors, environmental risk factors (eg, smoking, diet), and even bad luck or chance associated with less likelihood of taking medications as prescribed. Clinicians should explore patients’ perceptions of causes of a condition. Patients strive to eliminate the perceived cause, thus eliminating the need to take medication. In some cultures, bad luck or chance drives patients’ decisions to not take medication, or they believe in fate and do not accept treatment. Whether they feel they can control their condition by eliminating the cause or have a fatalistic view that the cause of their condition is not within their control, the clinician must work with the patient to reduce the impact of misperceptions or significance of perceived causes.
Illness Consequence
Consequence associated with the health condition is an important factor in patient behavior [12]. Patients must understand the specific threats to their health if a condition is left untreated or uncontrolled. Patients’ view of illness consequence may be formed by their own perceived vulnerability or susceptibility and the perceived seriousness of the condition. For example, patients with hypertension should be informed about the impact of high blood pressure on their bodies and the consequence of disability from stroke, dependency on dialysis from kidney failure, or death. They may not consider themselves susceptible to illness since they “feel healthy” and may decide to delay treatment. Patients with conditions such as asthma or heart failure may believe they are cured when their symptoms abate and therefore believe they have no more need for medication. Such patients need education to understand that they are asymptomatic because they are well controlled with medication.
Illness Control
Patients may feel they can control their health condition by changing their behavior, changing their environment, and/or by taking prescribed medication. As discussed earlier, cause and control both work together to form patient beliefs and actions. Patients will take their medications as prescribed if they believe in the effectiveness of medication to control their condition [11,13–15]. Interestingly, Ross found those who felt they had more control over their illness were more likely not to take their medication as prescribed [12]. These persons are more likely to not want to become “dependent” on medication. Their feeling was that they can make changes in their lives and thereby improve their health condition.
Physicians should invite patients’ thoughts as to what should be done to improve their health condition, and collaborate with the patient on an action plan for change if change is expected to improve/control the health condition. Follow-up to assess the patient’s health status longitudinally is necessary.
In this exchange, the patient feels he can control his hypertension on his own:
Doctor: I recommend that you start taking medication to control your blood pressure. Uncontrolled high blood pressure can lead to many health problems.
Patient: I am not ready to start taking medication.
Doctor: What are your reasons?
Patient: I am under a lot of stress at work. Once I get control of this stress, my blood pressure will go down.
Doctor: Getting control of your stress at work is important. Let me tell you more about high blood pressure.
Patient: Okay.
Doctor: There is no one cause of your high blood pressure. Eliminating your work stress will most likely not reduce your blood pressure....
Timeline
Health conditions can be acute, chronic, or cyclical (ie, seasonal); however, patients may have different perceptions of the duration of their health condition. In Kucukarslan et al, some patients did not believe their hypertension was a lifelong condition because they felt they would be able to cure it [7]. For example, as illustrated above, patients may believe that stress causes their hypertension, and if the stress could be controlled, then their blood pressure would normalize. Conversely, Ross et al found that patients who viewed their hypertension as a long-term condition were more likely to believe their medications were necessary and thus more likely to take their medication as prescribed [12]. A lifelong or chronic health condition is a difficult concept for patients to accept, especially ones who may view themselves as too young to have the condition.
Emotions
After being informed about their health condition, patients may feel emotions that are not apparent to the practitioner. These may include worry, depression, anger, anxiety, or fear. Emotions may impact their decision to take medication [12,14]. Listening for patients’ responses to health information provided by the clinician and letting patients know they have been heard will help allay strong negative emotions [16]. Good communication builds trust between the clinician and patient.
Conclusion
Patients receive medical advice from clinicians that may be inconsistent with their beliefs and understanding of their health condition. Studies of medication nonadherence find many factors contribute to it and no one tool to improve medication adherence exists. However, the consequence of medication nonadherence are great and include include worsening condition, increased comorbid disease, and increased health care costs. Understanding patients’ beliefs about their health condition is an important step toward reducing medication nonadherence. The CSM provides a framework for clinicians to guide patients toward effective decision-making. Listening to the patient explain how they view their condition—how they define it, the causes, consequences, how to control it, and how long it will last or if it will progress—are important to the process of working with the patient manage their condition effectively. Clinicians’ reaction to these perceptions are important, and dismissing them may alienate patients. Effective communication is necessary to understand patients’ perspectives and to help them manage their health condition.
Corresponding author: Suzan N. Kucukarslan, PhD, RPh, skucukar@gmail.com.
Financial disclosures: None.
From Genoa-QoL Healthcare and the University of Michigan College of Pharmacy, Ann Arbor, MI.
Abstract
- Objective: To review the Common Sense Model, a framework that can be used for understanding patients’ behavior, including taking or not taking medications as prescribed.
- Methods: Descriptive report.
- Results: Medication adherence, a critical component of achieving good patient outcomes and reducing medical costs, is dependent upon patient illness beliefs. The Common Sense Model holds that these beliefs can be categorized as illness identity, cause, consequence, control, and timeline. Effective communication is necessary to understand the beliefs that patients hold and help them understand their condition. Good communication also can allay fears and other emotions that can be disruptive to achieving good outcomes.
- Conclusion: Clinicians should seek to understand their patients’ illness beliefs and collaborate with them to achieve desired health outcomes.
Clinical practice is based on scientific evidence, by which medical problems are diagnosed and treatment recommendations are made. However, the role of the patient may not be completely recognized as an integral part of the process of patient care. The impact of failing to adequately recognize the patient perspective is evident in medication nonadherence. Health psychology research can provide clinicians insight into patients’ perceptions and behavior. This paper reviews the Common Sense Model (CSM), a behavioral model that provides a framework that can be used in understanding patients’ behavior. In this paper I will discuss the model and how it can be a possible strategy for improving adherence.
Making the Case for CSM in Daily Practice
It can be difficult to realize that persons seeking medical attention would not take medications as prescribed by a physician. In fact, studies reveal that on average, 16.4% of prescribed medications will not be picked up from the pharmacy [1]. Of those patients who do pick up their medication, approximately 1 out of 4 will not take them as prescribed [2]. Such medication nonadherence leads to poor health outcomes and increased health care costs [3,4]. There are many reasons for medication nonadherence [5], and there is no single solution to improving medication adherence [6]. A Cochrane review of randomized controlled trials evaluating various interventions intended to enhance patient adherence to prescribed medications for medical conditions found them to have limited effectiveness. Interventions assessed included health and medication information, reminder calls, follow-up assessment of medication therapy, social support, and simplification of the treatment regimen [6]. In an exploratory study of patients with chronic health conditions, Kucukarslan et al found patients’ beliefs about their illness and their medication are integral to their health care decisions [7]. Their findings were consistent with the CSM, which is based on Leventhal’s theory of self-regulation.
Self-regulation theory states that rational people will make decisions to reduce their health threat. Patients’ perceptions of their selves and environments drives their behavior. So in the presence of a health threat, a person will seek to eliminate or reduce that threat. However, coping behavior is complex. A person may decide to follow the advice of his clinician, follow some other advice (from family, friends, advertising, etc.), or do nothing. The premise of self-regulation is that people will choose a common sense approach to their health threat [8]. Therefore, clinicians must understand their patients’ viewpoint of themselves and their health condition so they may help guide them toward healthy outcomes.
The Common Sense Model
The CSM is a framework for understanding patient behavior when faced with a health threat. It holds that patients form common sense representations of their illness using information from 5 domains [8]: (1) the identity of the illness (the label the patient gives to the condition and symptoms); (2) the cause of the illness; (3) the consequences of the illness (beliefs about how the illness will impact the patient’s well-being); (4) whether the illness can be controlled or cured; and (5) timeline (beliefs about how long the condition will last). A patient may either act to address the health threat or choose to ignore it. Patient emotions are proposed to have a role on patient behavior along with the 5 dimensions of illness perception.
Illness Identity
Illness identity is the label patients place on the health threat; it is most likely not the same as the signs and symptoms clinicians use. Therefore, the first misconnect between physician and patient may be in describing the illness. Chen et al studied illness identity as perceived by patients with hypertension [9,10]. Illness identity was defined as (1) hypertension-related symptoms, (2) symptoms experienced before and after their diagnosis; and (3) symptoms used to predict high blood pressure. Although hypertension is asymptomatic, patients do perceive symptoms such as headache associated with their hypertension. The researchers found those patients who identified more symptoms were more likely to believe that their symptoms caused the hypertension and were correspondingly less likely to use their medication. For them, when the headache subsides, so does the hypertension.
Physicians should find out how patients assess their health condition and provide them tools for evaluating their response to medication. In the case of hypertension, the physician could have the patient check their blood pressure with and without the headache to demonstrate that hypertension occurs even when the patient is not “symptomatic.” The point is to converse with the patient to learn how they view their condition. Clinicians should resist the “urge” to correct patients. Taking time to help patients better understand their condition is important. A misstep:
Patient: I can tell when my blood pressure is high. I get a pounding headache.
Doctor: High blood pressure is an asymptomatic condition. Your headaches are not caused by your high blood pressure.
Patients may choose to ignore the clinician if they feel strongly about how they define their illness. It is better to listen to the patient and offer steps to learn about their health condition. Here is a better response from the physician:
Doctor: You are telling me that you can tell when your blood pressure is high. So when your head aches your pressure is high, right?
Patient: Yes.
Doctor: Let me tell you more about high blood pressure. High blood pressure is also present without headaches...
Illness Causes
There are multiple causative factors patients may associate with their disease. Causes attributed to disease may be based on patient experiences, input from family and friends, and cultural factors. Causes may include emotional state, stress or worry, overwork, genetic predisposition, or environmental factors (eg, pollution). Jessop and Rutter found patients who perceive their condition as due to uncontrollable factors, such as chance, germs, or pollution, were less likely to take their medication [11]. Similar findings were published by Chen et al [9]. They found psychological factors, environmental risk factors (eg, smoking, diet), and even bad luck or chance associated with less likelihood of taking medications as prescribed. Clinicians should explore patients’ perceptions of causes of a condition. Patients strive to eliminate the perceived cause, thus eliminating the need to take medication. In some cultures, bad luck or chance drives patients’ decisions to not take medication, or they believe in fate and do not accept treatment. Whether they feel they can control their condition by eliminating the cause or have a fatalistic view that the cause of their condition is not within their control, the clinician must work with the patient to reduce the impact of misperceptions or significance of perceived causes.
Illness Consequence
Consequence associated with the health condition is an important factor in patient behavior [12]. Patients must understand the specific threats to their health if a condition is left untreated or uncontrolled. Patients’ view of illness consequence may be formed by their own perceived vulnerability or susceptibility and the perceived seriousness of the condition. For example, patients with hypertension should be informed about the impact of high blood pressure on their bodies and the consequence of disability from stroke, dependency on dialysis from kidney failure, or death. They may not consider themselves susceptible to illness since they “feel healthy” and may decide to delay treatment. Patients with conditions such as asthma or heart failure may believe they are cured when their symptoms abate and therefore believe they have no more need for medication. Such patients need education to understand that they are asymptomatic because they are well controlled with medication.
Illness Control
Patients may feel they can control their health condition by changing their behavior, changing their environment, and/or by taking prescribed medication. As discussed earlier, cause and control both work together to form patient beliefs and actions. Patients will take their medications as prescribed if they believe in the effectiveness of medication to control their condition [11,13–15]. Interestingly, Ross found those who felt they had more control over their illness were more likely not to take their medication as prescribed [12]. These persons are more likely to not want to become “dependent” on medication. Their feeling was that they can make changes in their lives and thereby improve their health condition.
Physicians should invite patients’ thoughts as to what should be done to improve their health condition, and collaborate with the patient on an action plan for change if change is expected to improve/control the health condition. Follow-up to assess the patient’s health status longitudinally is necessary.
In this exchange, the patient feels he can control his hypertension on his own:
Doctor: I recommend that you start taking medication to control your blood pressure. Uncontrolled high blood pressure can lead to many health problems.
Patient: I am not ready to start taking medication.
Doctor: What are your reasons?
Patient: I am under a lot of stress at work. Once I get control of this stress, my blood pressure will go down.
Doctor: Getting control of your stress at work is important. Let me tell you more about high blood pressure.
Patient: Okay.
Doctor: There is no one cause of your high blood pressure. Eliminating your work stress will most likely not reduce your blood pressure....
Timeline
Health conditions can be acute, chronic, or cyclical (ie, seasonal); however, patients may have different perceptions of the duration of their health condition. In Kucukarslan et al, some patients did not believe their hypertension was a lifelong condition because they felt they would be able to cure it [7]. For example, as illustrated above, patients may believe that stress causes their hypertension, and if the stress could be controlled, then their blood pressure would normalize. Conversely, Ross et al found that patients who viewed their hypertension as a long-term condition were more likely to believe their medications were necessary and thus more likely to take their medication as prescribed [12]. A lifelong or chronic health condition is a difficult concept for patients to accept, especially ones who may view themselves as too young to have the condition.
Emotions
After being informed about their health condition, patients may feel emotions that are not apparent to the practitioner. These may include worry, depression, anger, anxiety, or fear. Emotions may impact their decision to take medication [12,14]. Listening for patients’ responses to health information provided by the clinician and letting patients know they have been heard will help allay strong negative emotions [16]. Good communication builds trust between the clinician and patient.
Conclusion
Patients receive medical advice from clinicians that may be inconsistent with their beliefs and understanding of their health condition. Studies of medication nonadherence find many factors contribute to it and no one tool to improve medication adherence exists. However, the consequence of medication nonadherence are great and include include worsening condition, increased comorbid disease, and increased health care costs. Understanding patients’ beliefs about their health condition is an important step toward reducing medication nonadherence. The CSM provides a framework for clinicians to guide patients toward effective decision-making. Listening to the patient explain how they view their condition—how they define it, the causes, consequences, how to control it, and how long it will last or if it will progress—are important to the process of working with the patient manage their condition effectively. Clinicians’ reaction to these perceptions are important, and dismissing them may alienate patients. Effective communication is necessary to understand patients’ perspectives and to help them manage their health condition.
Corresponding author: Suzan N. Kucukarslan, PhD, RPh, skucukar@gmail.com.
Financial disclosures: None.
1. Gadkari AS, McHorney CA. Medication non-fulfillment rates and reasons: a narrative systematic review. Curr Med Res Opin 2010;26:683–785.
2. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200–9.
3. Ho PM, Rumsfeld JS, Masoudi FA, et al. The effect of medication non-adherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166;1836–41.
4. Benjamin RM. Medication adherence: Helping patients take their medicines as directed. Pub Health Rep 2012;2–3.
5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97.
6. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008;(2):CD000011.
7. Kucukarslan SN, Lewis NJW, Shimp LA, et al. Exploring patient experiences with prescription medicines to identify unmet patient needs: implications for research and practice. Res Social Adm Pharm 2012;8:321–332.
8. Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychol Health 1998;13:717–33.
9. Chen S-L, Tsai J-C, Chou K-R. Illness perceptions and adherence to therapeutic regimens among patients with hypertension: A structural model approach. Int J Nurs Stud 2011;48:235–45.
10. Chen S-L, Tsai J-C, Lee W-L. The impact of illness perception on adherence to therapeutic regimens of patients with hypertension in Taiwan. J Clin Nurs 2009;18:2234–44.
11. Jessop DC, Rutter DR. Adherence to asthma medication: the role of illness representations. Psychol Health 2003;18:595–612.
12. Ross S, Walker A, MacLeod M. Patient compliance in hypertension:role of illness perceptions and treatment beliefs. J Hum Hypertension 2004;18:607–13.
13 Searle A, Norman P. Thompson R. Vedhara K. A prospective examination of illness belies and coping in patients with type 2 diabetes. Br J Health Psychol 2007;12:621–38.
14. Zugelj U, Zuparnicic M, Komidar L, et al. Self-reported adherence behavior in adolescent hypertensive patients: the role of illness representation and personality. J Pediatr Psychol 2010;35:1049–60.
15. Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perception and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health 2002;17:17–32.
16. Northouse LL, Northouse PG. Health communication: strategies for health professionals. Stamford: Prentice Hall; 1998.
1. Gadkari AS, McHorney CA. Medication non-fulfillment rates and reasons: a narrative systematic review. Curr Med Res Opin 2010;26:683–785.
2. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200–9.
3. Ho PM, Rumsfeld JS, Masoudi FA, et al. The effect of medication non-adherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166;1836–41.
4. Benjamin RM. Medication adherence: Helping patients take their medicines as directed. Pub Health Rep 2012;2–3.
5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97.
6. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008;(2):CD000011.
7. Kucukarslan SN, Lewis NJW, Shimp LA, et al. Exploring patient experiences with prescription medicines to identify unmet patient needs: implications for research and practice. Res Social Adm Pharm 2012;8:321–332.
8. Leventhal H, Leventhal EA, Contrada RJ. Self-regulation, health, and behavior: a perceptual-cognitive approach. Psychol Health 1998;13:717–33.
9. Chen S-L, Tsai J-C, Chou K-R. Illness perceptions and adherence to therapeutic regimens among patients with hypertension: A structural model approach. Int J Nurs Stud 2011;48:235–45.
10. Chen S-L, Tsai J-C, Lee W-L. The impact of illness perception on adherence to therapeutic regimens of patients with hypertension in Taiwan. J Clin Nurs 2009;18:2234–44.
11. Jessop DC, Rutter DR. Adherence to asthma medication: the role of illness representations. Psychol Health 2003;18:595–612.
12. Ross S, Walker A, MacLeod M. Patient compliance in hypertension:role of illness perceptions and treatment beliefs. J Hum Hypertension 2004;18:607–13.
13 Searle A, Norman P. Thompson R. Vedhara K. A prospective examination of illness belies and coping in patients with type 2 diabetes. Br J Health Psychol 2007;12:621–38.
14. Zugelj U, Zuparnicic M, Komidar L, et al. Self-reported adherence behavior in adolescent hypertensive patients: the role of illness representation and personality. J Pediatr Psychol 2010;35:1049–60.
15. Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perception and treatment beliefs in explaining non-adherence to preventer medication. Psychol Health 2002;17:17–32.
16. Northouse LL, Northouse PG. Health communication: strategies for health professionals. Stamford: Prentice Hall; 1998.
Patients, Persistence, and Partnership: Creating and Sustaining Patient and Family Advisory Councils in a Hospital Setting
From the Center for Patients and Families (Dr. Fagan, Ms. Wong, Ms. Morrison, Ms. Carnie), and the Division of Women’s Health and Gender Biology (Dr. Lewis-O’Connor), Brigham and Women’s Hospital, Boston, MA.
Abstract
- Objective: To describe and illustrate the phases of creating, recruiting, launching, and sustaining a successful Patient and Family Advisory Council (PFAC) in a hospital setting.
- Method: Descriptive report.
- Results: There are 4 stages in creating and establishing a PFAC: council preparation, patient/family advisor recruitment, council launch, and sustaining an established council. Each stage poses challenges that need to be addressed in order to progress to the next stage. The ability for hospital leadership to authentically partner with patient and family advisors is key to maintaining and sustaining PFACs.
- Conclusion: The success of a PFAC is based on leadership support, advisors’ commitment to their PFAC, and the ability to sustain the council. PFACs can promote patient- and family-centered care and shift the model of care from a prescribed model to one that embraces partnerships with patients while advancing care delivery. As patient- and family-centered care advances, it is important that best practices and resources for building and sustaining PFACs are developed and made available to ensure all hospitals have access to this valuable resource.
Throughout the country, families, patients, and health care professionals are working together in new ways, including within patient and family advisory councils (PFACs). First established in the 1990s, PFACs became widespread after patient-centeredness was identified by the Institute of Medicine as one of the 6 aims of quality health care [1]. PFACs were created to institutionalize a partnership between hospital leadership, clinicians, patients, and families to improve care delivery. Through this partnership, PFACs facilitate the sharing of patient perspectives and input on hospital policies and programs; serve as a resource to providers; and promote relationships between staff, patients, and family members [2]. PFACs also play an important role in promoting patient- and family-centered care, ensuring that patient needs and values are at the center of the care delivery system.
The BWH Center for Patients and Families includes the executive director, a project manager, and a senior patient advisor, who together oversee the PFACs. The project manager provides logistical support and is available as concerns arise, working closely with the senior patient advisor to ensure patient/family advisors are acclimated to their role and all PFACs run smoothly. The senior patient advisor is a volunteer advisor and patient advocate with long term experience creating and sustaining PFACs and mentoring patient/family advisors. Her role as a mentor includes attending PFAC meetings to model skills and behaviors for other advisors and working with them to ensure they are comfortable in their PFAC. Her advocacy work includes listening to and helping to articulate lived patient/family experiences as compelling narratives, which can be shared with hospital leadership and used as exemplars to spur change. Together, the team recruits and trains patient/family advisors, support all phases of PFAC development, and represent the patient/family voice within the hospital.
In this article, we describe and illustrate the 4 stages of creating and sustaining a successful PFAC to provide guidance and lessons learned to organizations seeking to develop this valuable resource. These stages are: (1) council preparation, (2) patient/family advisor recruitment, (3) council launch, and (4) sustaining an established council.
Council Preparation
Preparation of the PFAC occurs once hospital or service line leadership has identified a need for and is committed to having a PFAC. Leadership contacts the executive director of the Center for Patients and Families to discuss the strategy and vision of the council. The executive director describes the attributes sought in an advisor and the core principles of patient- and family-centered care. This discussion includes recruitment methods, meeting logistics, and who will serve as council chair. The council chair should be in a leadership role and willing to champion the PFAC for their service line. The executive director discusses what is being planned with relevant clinicians and staff at a staff meeting in order to foster buy-in and involve them in the council recruitment process.
The BWH Center for Patients and Families has adapted Institute for Patient- and Family-Centered Care recommendations for PFAC logistics and structure [3]. Councils generally meet monthly for 90 minutes excluding August and December. As volunteers, our advisors receive no monetary compensation but receive complimentary parking and are often provided meals. Advisors are asked to commit to a 3-year term with the understanding that personal issues can arise and their commitment may change; after their term, they are welcome to continue. We recommend to leadership that PFACs be comprised of no more than 1 staff member to every 4 advisors. This ratio seeks to address any potential power imbalance and promotes a feeling of ownership of their council . The project manager works with leadership in creating council guidelines, including the council’s goals, expectations, and each member’s role.
Patient/Family Advisor Recruitment
We ask service line providers and staff to nominate patients and family members they believe would be suitable advisors. The attributes we look for in an advisor include their ability to: (1) share personal experiences in ways that professional and support staff can learn from, (2) see the big picture of a challenge or scenario and give advice using the lens of the patient or family member, (3) be interested in more than one agenda item, (4) speak to multiple operational topics, (5) listen to other points of view and be empathetic, (6) connect with other advisors and staff, and (7) have a good sense of humor. Candidates with both positive and negative experiences are sought so that we can learn and improve from their experience [2].
To find advisors with these attributes, we ask providers to review their schedule and think about who they look forward to seeing or connect with on a personal level. This method has proven successful at producing candidates that have the attributes we seek. It is vital that the patients we recruit are able to see past their own personal experiences to understand broader objectives and how they fit into the bigger picture, enabling them to participate in a variety of projects and committees. There are no educational or specific skill requirements to become an advisor; the only requirement is that candidates must have experience as a patient or caregiver (family member) of a patient at BWH.
Recommended patients receive a letter notifying them that they have been nominated to be an advisor on a PFAC by their treating clinician. The project manager contacts potential advisors to see if they are interested and provides a brief description of PFACs and the role of patient/family advisors. The project manager emphasizes the importance of patient/family input to the hospital, describing the opportunities patient/family advisors have to contribute their expertise as a patient or caregiver to decisions and projects that will positively affect future patient care. Examples of past successful PFAC projects are shared to give a sense of the importance of the advisor role within the hospital and the appreciation hospital leadership has for PFAC contributions. The project manager reiterates that their clinician nominated them to the council to encourage the candidate to feel that their voice deserves to be heard.
Interested candidates are interviewed by phone by the team. Each candidate is asked the same questions: (1) How long have you been a patient in the clinic or unit?; (2) Describe your experiences in this clinic/service; and (3) Describe what works well and what could be improved in your care. During the interview, we listen to their personal narrative and their perspective on their care, which allows us to assess whether they have the attributes of a successful patient/family advisor. Candidate’s narratives illustrate how they would share their concerns, contribute to solutions, and if they have the ability to see beyond their own personal agenda. We also listen carefully for themes of tolerance, operational insight, empathy, and problem-solving capabilities. Interviews take about 15–20 minutes, depending on how many follow-up questions we have for the candidate and if they have questions for us.
After the phone interview, the team determines whether the candidate would be an appropriate patient/family advisor. If there are any concerns and more information is needed, the project manager reaches out to the staff and contacts the candidate to invite them for an in-person interview. Of the interviewed candidates, about 75% to 80% are invited to join. Candidates who are not chosen are generally unable to clearly articulate issues they see within the hospital/clinic, may have personal relationships with the staff (ie, friends with the physician), or cannot see pass their own issues and are inflexible in their thinking. Those not chosen receive a note thanking them for their time and interest. The candidates chosen to be advisors are on boarded through BWH volunteer services and must attend a 3-hour BWH volunteer orientation, be HIPAA compliant, and be cleared by occupational health before receiving their advisor ID badge and beginning service.
Council Launch
Once the advisors have been recruited and oriented, the council enters the launching stage, which lasts from the council’s first meeting until the 1-year anniversary. The first meeting agenda is designed to introduce staff and advisors to each other. Advisors each share their health care narratives and the staff shares their motivations for participating in the council. The council chair reviews the purpose and goals of the council.
During the first year, the council gains experience working together as a team. Council projects are initially chosen by the council chair and should be reasonably simple to accomplish and meaningful to advisors so that advisors recognize that their feedback is being heard and acted on. Example projects include creating clearer directional sign-age, assessing recliners for patient rooms, and providing feedback on patient handouts to ensure patient friendliness.
As the council advances, projects can be initiated by the advisors. This process is facilitated when an advisor is added as council co-chair, which usually occurs at the end of the first year. Projects often arise from similar concerns shared by advisors during the recruitment interview process.
Sustaining an Established Council
A council is considered established when it enters its second year and has named a patient advisor as a co-chair. Established councils have undertaken projects such as improving the layout of the whiteboards in patient rooms and providing feedback to staff on how to manage challenging patients. In addition, established councils may be tapped when service lines without a PFAC seek to gather advisor feedback for a project. For example, one of our established councils has provided feedback on two patient safety research projects.
Councils are sustained by continually engaging advisors in projects that are of value to them, both in their department and hospital-wide. Advisors should be given the opportunity to prioritize and set new council goals. One of the overarching goals for all our PFACs is to improve communication between patients and staff. Councils at this stage often participate in grand rounds or attend staff meetings to share their narratives, enabling providers to understand their perspective. The council can also be engaged in grant-funded research initiatives. Having PFACs involved in various projects allows advisors to bring their narratives to a wider audience and be a part of change from numerous avenues within the hospital.
Patient and Family Advisory Councils in Practice
BWH has 16 PFACs in various stages of growth. To illustrate the variety in council structure and function, we describe 3 PFACs below. Each has unique composition and goals based on the needs of service line leadership.
Shapiro Cardiovascular PFAC
The Shapiro Cardiovascular Center, a LEED silver-certified building and with private patient rooms that welcome family members to stay with their loved ones [4], opened in 2008. The chief nursing officer felt the care provided in this new space should promote and embody PFCC. With the assistance of the Center for Patients and Families, the associate chief nursing officer was charged with creating the Shapiro Cardiovascular PFAC. Launched in May 2011, this PFAC provides input to improve the patient experience for inpatient and ambulatory care housed in the Shapiro Center.
The Shapiro PFAC originally consisted of medical/surgical cardiac and heart transplant patients; renal transplant recipients and donors later joined. Initially, this council worked on patient/visitor guidelines for the inpatient units. As the council became more experienced, advisors interviewed nursing director candidates for cardiac surgery ICU and organized two PFCC nursing grand rounds. These grand rounds featured a panel of Shapiro advisors sharing their perspective of their hospital care and reflections on their healing process. This council has also provided feedback on hospital-wide projects, such as the refinement of a nursing fall prevention tool and the development of patient-informed measures of a successful surgery. As advisors became more experienced, they were recruited by the executive director to be part of other committees and research projects.
The Shapiro PFAC is one of the oldest councils at BWH, consisting of 12 advisors and 3 staff members, with most of the inaugural advisors remaining. Because the council chair has changed twice since 2011, this council does not have a formal advisor co-chair but the council remains a cohesive team as they work in partnership with the newest chair. To sustain this PFAC, leadership has consistently engaged the council in operational projects. For example, the associate chief nursing officer has suggested advisors be part of unit-based councils composed of staff nurses and educators who work to improve patient care within their unit. Advisors have also been invited to participate in staff and nursing director meetings to share their narratives and allow staff to reflect on the care they provide patients.
LGBTQ PFAC
In the fall of 2014, BWH held an educational Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) patient experience forum prompted by a complaint from the wife of a maternity patient that the care they received was not patient/family-centered. During this panel discussion, 4 LGBTQ patients described their care at BWH, including what went well and what their providers could have done better. There was an acknowledgement by a majority of providers in the audience that they did not receive training on inclusive care for LGBTQ patients and their families. The providers identified the need to be educated on LGBTQ issues and care concerns, and their desire to work towards creating a warm and inclusive environment that better serves LGBTQ patients. This organic request for education was met with enthusiasm from the panel participants and led to a commitment to form an LGBTQ PFAC at BWH.
The LGBTQ PFAC is co-chaired by the executive director of the Center for Patients and Families and a LGBTQ patient advisor who receives his care at BWH. It was important for this council to be co-led by an advisor from the beginning to acknowledge and validate LGBTQ experiences of care which had previously been marginalized. Because LGBTQ patients interact with all service lines at BWH, it made sense that a central operational leader with significant experience listening and responding to the patient voice co-leads the group. This council is composed of LGBTQ patients, their caregivers/partners/spouses, BWH LGBTQ staff that also receive healthcare from BWH, and LGBTQ academic stakeholders who provide historical contextualization to inform change.
The LGBTQ PFAC began the preparation phase in April 2015 and launched as a hospital-wide council in October 2015. This launch was widely publicized so that all BWH employees would know this council was created to elevate LGBTQ patients and caregivers into the mainstream hospital consciousness. The goals for this year are to partner with the existing LGBTQ employee group to create a standardized LGBTQ provider directory, educate staff on the healthcare needs of the community, and promote educational awareness, compassionate understanding, and improved care for transgender patients. As this council matures into the established stage, new projects will be taken on in line with the needs seen by members.
Women’s Health Council
The Women’s Health Council is a unique PFAC established in 2012. The council serves a population of trauma survivors cared for by the Coordinated Approach to Recovery and Empowerment (C.A.R.E.) clinic at BWH, also founded in 2012. Patients who receive care in this clinic have experienced violence and trauma, including domestic and sexual violence, child maltreatment, and human trafficking. Due to previous experience leading a PFAC, the C.A.R.E. Director understood the importance of patient input and engaged patients as advisors while forming the clinic.
The C.A.R.E. clinic serves both men and women but the majority of survivors served are female; thus, the patient advisors on its PFAC are all female. To recruit advisors, clinicians, and social workers at the clinic refer potential candidates to the C.A.R.E. Director, who then interviews them. The criteria for advisors for this council include being a female survivor of violence and trauma, being physically and mentally able to serve, and able to participate in a way that does not re-traumatize them. There are currently 14 advisors on the council with a goal to grow to 30 advisors. Experience has shown that members become busy with family, school and careers and may need to step away for short periods of time; thus, the council seeks to continually recruit to ensure robust membership.
Instead of the usual monthly scheduled meetings, this council holds “meetings on demand.” Advisors are polled via email to find a time in the near future that works for the group. The PFAC generally uses a web conferencing platform for their meetings and has an in-person meeting once or twice a year. Also unlike other councils, this council does not require their advisors to share their personal narratives; it is up to each advisor to decide what to share.
This council has accomplished numerous goals since its inception, including its first task of giving the C.A.R.E. clinic its name. The council has provided feedback on the development of the C.A.R.E. brochure and website and serves as key informants in all aspects of policy and procedures for the C.A.R.E. clinic. Additionally, they have provided input on how to create a safe environment for patients and screen patients to identify a victim of violence or human trafficking [5]. This council has been sustained by the strong community fostered by the director and projects led by the advisors, as each advisor has a vested interest in ensuring the clinic provides a safe environment for patients seeking care. This year, the council is hoping to host experts from the Boston Health Commission to share best practices in providing services to victims of abuse and violence.
Lessons Learned
The BWH Center for Patients and Families has encountered challenges when creating and sustaining PFACs, such as recruiting advisors from diverse ethnic, cultural, and economic backgrounds. Currently, our advisor population is primarily comprised of Caucasian patient/family members from middle and upper economic backgrounds, though it has increasingly diversified as the program has grown. We believe the lack of representation from other backgrounds is due to scheduling difficulties, the lack of payment for advisors, visibility of the PFAC program, and, potentially, cultural norms that promote deference to medical expertise. We have worked to increase PFAC diversity by asking providers to specifically seek out and nominate patients that will broaden our reach as a council.
Retaining and recruiting advisors after the PFACs have launched can also present a challenge. Some advisors have had to resign due to job demands, relocation, health issues, or the need to take care of family. To resolve this issue, we have asked PFAC chairs to continuously actively recruit advisors. By doing so, the councils gain new perspectives and ensure there are adequate number of advisors should a vacancy occur.
Sustaining PFACs once they are established requires time, effort, and commitment of leadership, advisors, and dedicated staff resources. The council needs to be continuously engaged in meaningful projects and feel that their participation is impactful and creates change. It is important that clinical leadership stays actively involved and attends all PFAC meetings. If there is a change in leadership as we experienced on our Shapiro PFAC, it is critical that the interim chair participates and supports the goal of the council. Regardless, leadership must show sustained enthusiasm for PFAC engagement and achievement.
Employing technology can also help sustain councils. Although we prefer in-person meetings, the option to attend meetings through online or phone conferencing should be made available to support advisors who are unable to attend in person. At this time, only one of our councils uses web conferencing, while several of our councils offer an option to call in via a conference line. The conference line has been beneficial in helping us retain and engage advisors who travel a significant distance to attend meetings.
We recognize that BWH has many resources available due to its status as a large, academic medical center in an urban center. Nonetheless, PFACs can play a vital role in hospitals no matter the setting, location, or size as long as there is buy in from hospital leadership. Although BWH has 16 PFACs, it is not necessary to have this many councils. Having one PFAC may be sufficient for smaller hospitals; the ideal number of councils depends on the size and complexity of the institution. Hospitals without a dedicated department like the Center for Patients and Families can create PFACs by partnering with volunteer services, patient engagement, or quality and safety departments. Existing departments with the capability to train advisors and provide meeting resources to support patient/family recruitment and engagement should be harnessed whenever possible. It is, however, important to have a dedicated staff member to serve as a point person for the advisors should they have any questions or concerns. Technology, such as web conferencing described above, can facilitate attendance by patient/family advisors who have limited time or resources and will be valuable for hospitals in a rural setting. The stages we have described are critical to the success of creating and sustaining a PFAC regardless of where they are developed and can be adapted to fit the unique needs and environments of any healthcare setting.
Conclusion
BWH’s Center for Patients and Families has created 16 PFACs since 2008, which are in various stages of development. Our PFACs are successful for many reasons, including a rigorous recruitment and interview process, leadership support, advisors’ commitment to their PFAC, and making modifications made based on lessons learned, as illustrated by the 3 PFACs discussed. We are able to sustain our councils by continually engagingadvisors, having leadership partner with advisors, setting feasible goals, and recruiting new advisors for a fresh perspective. PFACs promote patient- and family-centered care and can shift the model of care from a prescribed model to one that embraces collaboration with patients while advancing care delivery. As patient- and family-centered care advances, it is important that best practices for building and sustaining PFACs are developed and made available to ensure all hospitals have access to this valuable resource.
Corresponding author: Celene Wong, MHA, Center for Patients and Families, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115, cwong3@partners.org.
Financial disclosures: None.
1. Institute of Medicine (US). Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press; 2001. Accessed 6 Apr 2016 at www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf.
2. Institute for Patient- and Family-Centered Care. Institute for Patient- and Family-Centered Care creating patient and family advisory councils. Oct 2010. Accessed 10 Jan 2016 at www.ipfcc.org/advance/Advisory_Councils.pdf.
3. Institute for Patient- and Family-Centered Care. Institute for Patient- and Family-Centered Care core concepts. Dec 2010. Accessed 10 Jan 2016 at www.ipfcc.org/faq.html.
4. NBBJ. Caring to connect. 2016. Accessed 22 Feb 2016 at www.nbbj.com/work/brigham-and-womens-hospital-shapiro/.
5. Lewis-O’Connor A, Chadwick M. Engaging the voice of patients affected by gender-based violence: informing practice and policy. J Forensic Nurs 2015;11:240–9.
From the Center for Patients and Families (Dr. Fagan, Ms. Wong, Ms. Morrison, Ms. Carnie), and the Division of Women’s Health and Gender Biology (Dr. Lewis-O’Connor), Brigham and Women’s Hospital, Boston, MA.
Abstract
- Objective: To describe and illustrate the phases of creating, recruiting, launching, and sustaining a successful Patient and Family Advisory Council (PFAC) in a hospital setting.
- Method: Descriptive report.
- Results: There are 4 stages in creating and establishing a PFAC: council preparation, patient/family advisor recruitment, council launch, and sustaining an established council. Each stage poses challenges that need to be addressed in order to progress to the next stage. The ability for hospital leadership to authentically partner with patient and family advisors is key to maintaining and sustaining PFACs.
- Conclusion: The success of a PFAC is based on leadership support, advisors’ commitment to their PFAC, and the ability to sustain the council. PFACs can promote patient- and family-centered care and shift the model of care from a prescribed model to one that embraces partnerships with patients while advancing care delivery. As patient- and family-centered care advances, it is important that best practices and resources for building and sustaining PFACs are developed and made available to ensure all hospitals have access to this valuable resource.
Throughout the country, families, patients, and health care professionals are working together in new ways, including within patient and family advisory councils (PFACs). First established in the 1990s, PFACs became widespread after patient-centeredness was identified by the Institute of Medicine as one of the 6 aims of quality health care [1]. PFACs were created to institutionalize a partnership between hospital leadership, clinicians, patients, and families to improve care delivery. Through this partnership, PFACs facilitate the sharing of patient perspectives and input on hospital policies and programs; serve as a resource to providers; and promote relationships between staff, patients, and family members [2]. PFACs also play an important role in promoting patient- and family-centered care, ensuring that patient needs and values are at the center of the care delivery system.
The BWH Center for Patients and Families includes the executive director, a project manager, and a senior patient advisor, who together oversee the PFACs. The project manager provides logistical support and is available as concerns arise, working closely with the senior patient advisor to ensure patient/family advisors are acclimated to their role and all PFACs run smoothly. The senior patient advisor is a volunteer advisor and patient advocate with long term experience creating and sustaining PFACs and mentoring patient/family advisors. Her role as a mentor includes attending PFAC meetings to model skills and behaviors for other advisors and working with them to ensure they are comfortable in their PFAC. Her advocacy work includes listening to and helping to articulate lived patient/family experiences as compelling narratives, which can be shared with hospital leadership and used as exemplars to spur change. Together, the team recruits and trains patient/family advisors, support all phases of PFAC development, and represent the patient/family voice within the hospital.
In this article, we describe and illustrate the 4 stages of creating and sustaining a successful PFAC to provide guidance and lessons learned to organizations seeking to develop this valuable resource. These stages are: (1) council preparation, (2) patient/family advisor recruitment, (3) council launch, and (4) sustaining an established council.
Council Preparation
Preparation of the PFAC occurs once hospital or service line leadership has identified a need for and is committed to having a PFAC. Leadership contacts the executive director of the Center for Patients and Families to discuss the strategy and vision of the council. The executive director describes the attributes sought in an advisor and the core principles of patient- and family-centered care. This discussion includes recruitment methods, meeting logistics, and who will serve as council chair. The council chair should be in a leadership role and willing to champion the PFAC for their service line. The executive director discusses what is being planned with relevant clinicians and staff at a staff meeting in order to foster buy-in and involve them in the council recruitment process.
The BWH Center for Patients and Families has adapted Institute for Patient- and Family-Centered Care recommendations for PFAC logistics and structure [3]. Councils generally meet monthly for 90 minutes excluding August and December. As volunteers, our advisors receive no monetary compensation but receive complimentary parking and are often provided meals. Advisors are asked to commit to a 3-year term with the understanding that personal issues can arise and their commitment may change; after their term, they are welcome to continue. We recommend to leadership that PFACs be comprised of no more than 1 staff member to every 4 advisors. This ratio seeks to address any potential power imbalance and promotes a feeling of ownership of their council . The project manager works with leadership in creating council guidelines, including the council’s goals, expectations, and each member’s role.
Patient/Family Advisor Recruitment
We ask service line providers and staff to nominate patients and family members they believe would be suitable advisors. The attributes we look for in an advisor include their ability to: (1) share personal experiences in ways that professional and support staff can learn from, (2) see the big picture of a challenge or scenario and give advice using the lens of the patient or family member, (3) be interested in more than one agenda item, (4) speak to multiple operational topics, (5) listen to other points of view and be empathetic, (6) connect with other advisors and staff, and (7) have a good sense of humor. Candidates with both positive and negative experiences are sought so that we can learn and improve from their experience [2].
To find advisors with these attributes, we ask providers to review their schedule and think about who they look forward to seeing or connect with on a personal level. This method has proven successful at producing candidates that have the attributes we seek. It is vital that the patients we recruit are able to see past their own personal experiences to understand broader objectives and how they fit into the bigger picture, enabling them to participate in a variety of projects and committees. There are no educational or specific skill requirements to become an advisor; the only requirement is that candidates must have experience as a patient or caregiver (family member) of a patient at BWH.
Recommended patients receive a letter notifying them that they have been nominated to be an advisor on a PFAC by their treating clinician. The project manager contacts potential advisors to see if they are interested and provides a brief description of PFACs and the role of patient/family advisors. The project manager emphasizes the importance of patient/family input to the hospital, describing the opportunities patient/family advisors have to contribute their expertise as a patient or caregiver to decisions and projects that will positively affect future patient care. Examples of past successful PFAC projects are shared to give a sense of the importance of the advisor role within the hospital and the appreciation hospital leadership has for PFAC contributions. The project manager reiterates that their clinician nominated them to the council to encourage the candidate to feel that their voice deserves to be heard.
Interested candidates are interviewed by phone by the team. Each candidate is asked the same questions: (1) How long have you been a patient in the clinic or unit?; (2) Describe your experiences in this clinic/service; and (3) Describe what works well and what could be improved in your care. During the interview, we listen to their personal narrative and their perspective on their care, which allows us to assess whether they have the attributes of a successful patient/family advisor. Candidate’s narratives illustrate how they would share their concerns, contribute to solutions, and if they have the ability to see beyond their own personal agenda. We also listen carefully for themes of tolerance, operational insight, empathy, and problem-solving capabilities. Interviews take about 15–20 minutes, depending on how many follow-up questions we have for the candidate and if they have questions for us.
After the phone interview, the team determines whether the candidate would be an appropriate patient/family advisor. If there are any concerns and more information is needed, the project manager reaches out to the staff and contacts the candidate to invite them for an in-person interview. Of the interviewed candidates, about 75% to 80% are invited to join. Candidates who are not chosen are generally unable to clearly articulate issues they see within the hospital/clinic, may have personal relationships with the staff (ie, friends with the physician), or cannot see pass their own issues and are inflexible in their thinking. Those not chosen receive a note thanking them for their time and interest. The candidates chosen to be advisors are on boarded through BWH volunteer services and must attend a 3-hour BWH volunteer orientation, be HIPAA compliant, and be cleared by occupational health before receiving their advisor ID badge and beginning service.
Council Launch
Once the advisors have been recruited and oriented, the council enters the launching stage, which lasts from the council’s first meeting until the 1-year anniversary. The first meeting agenda is designed to introduce staff and advisors to each other. Advisors each share their health care narratives and the staff shares their motivations for participating in the council. The council chair reviews the purpose and goals of the council.
During the first year, the council gains experience working together as a team. Council projects are initially chosen by the council chair and should be reasonably simple to accomplish and meaningful to advisors so that advisors recognize that their feedback is being heard and acted on. Example projects include creating clearer directional sign-age, assessing recliners for patient rooms, and providing feedback on patient handouts to ensure patient friendliness.
As the council advances, projects can be initiated by the advisors. This process is facilitated when an advisor is added as council co-chair, which usually occurs at the end of the first year. Projects often arise from similar concerns shared by advisors during the recruitment interview process.
Sustaining an Established Council
A council is considered established when it enters its second year and has named a patient advisor as a co-chair. Established councils have undertaken projects such as improving the layout of the whiteboards in patient rooms and providing feedback to staff on how to manage challenging patients. In addition, established councils may be tapped when service lines without a PFAC seek to gather advisor feedback for a project. For example, one of our established councils has provided feedback on two patient safety research projects.
Councils are sustained by continually engaging advisors in projects that are of value to them, both in their department and hospital-wide. Advisors should be given the opportunity to prioritize and set new council goals. One of the overarching goals for all our PFACs is to improve communication between patients and staff. Councils at this stage often participate in grand rounds or attend staff meetings to share their narratives, enabling providers to understand their perspective. The council can also be engaged in grant-funded research initiatives. Having PFACs involved in various projects allows advisors to bring their narratives to a wider audience and be a part of change from numerous avenues within the hospital.
Patient and Family Advisory Councils in Practice
BWH has 16 PFACs in various stages of growth. To illustrate the variety in council structure and function, we describe 3 PFACs below. Each has unique composition and goals based on the needs of service line leadership.
Shapiro Cardiovascular PFAC
The Shapiro Cardiovascular Center, a LEED silver-certified building and with private patient rooms that welcome family members to stay with their loved ones [4], opened in 2008. The chief nursing officer felt the care provided in this new space should promote and embody PFCC. With the assistance of the Center for Patients and Families, the associate chief nursing officer was charged with creating the Shapiro Cardiovascular PFAC. Launched in May 2011, this PFAC provides input to improve the patient experience for inpatient and ambulatory care housed in the Shapiro Center.
The Shapiro PFAC originally consisted of medical/surgical cardiac and heart transplant patients; renal transplant recipients and donors later joined. Initially, this council worked on patient/visitor guidelines for the inpatient units. As the council became more experienced, advisors interviewed nursing director candidates for cardiac surgery ICU and organized two PFCC nursing grand rounds. These grand rounds featured a panel of Shapiro advisors sharing their perspective of their hospital care and reflections on their healing process. This council has also provided feedback on hospital-wide projects, such as the refinement of a nursing fall prevention tool and the development of patient-informed measures of a successful surgery. As advisors became more experienced, they were recruited by the executive director to be part of other committees and research projects.
The Shapiro PFAC is one of the oldest councils at BWH, consisting of 12 advisors and 3 staff members, with most of the inaugural advisors remaining. Because the council chair has changed twice since 2011, this council does not have a formal advisor co-chair but the council remains a cohesive team as they work in partnership with the newest chair. To sustain this PFAC, leadership has consistently engaged the council in operational projects. For example, the associate chief nursing officer has suggested advisors be part of unit-based councils composed of staff nurses and educators who work to improve patient care within their unit. Advisors have also been invited to participate in staff and nursing director meetings to share their narratives and allow staff to reflect on the care they provide patients.
LGBTQ PFAC
In the fall of 2014, BWH held an educational Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) patient experience forum prompted by a complaint from the wife of a maternity patient that the care they received was not patient/family-centered. During this panel discussion, 4 LGBTQ patients described their care at BWH, including what went well and what their providers could have done better. There was an acknowledgement by a majority of providers in the audience that they did not receive training on inclusive care for LGBTQ patients and their families. The providers identified the need to be educated on LGBTQ issues and care concerns, and their desire to work towards creating a warm and inclusive environment that better serves LGBTQ patients. This organic request for education was met with enthusiasm from the panel participants and led to a commitment to form an LGBTQ PFAC at BWH.
The LGBTQ PFAC is co-chaired by the executive director of the Center for Patients and Families and a LGBTQ patient advisor who receives his care at BWH. It was important for this council to be co-led by an advisor from the beginning to acknowledge and validate LGBTQ experiences of care which had previously been marginalized. Because LGBTQ patients interact with all service lines at BWH, it made sense that a central operational leader with significant experience listening and responding to the patient voice co-leads the group. This council is composed of LGBTQ patients, their caregivers/partners/spouses, BWH LGBTQ staff that also receive healthcare from BWH, and LGBTQ academic stakeholders who provide historical contextualization to inform change.
The LGBTQ PFAC began the preparation phase in April 2015 and launched as a hospital-wide council in October 2015. This launch was widely publicized so that all BWH employees would know this council was created to elevate LGBTQ patients and caregivers into the mainstream hospital consciousness. The goals for this year are to partner with the existing LGBTQ employee group to create a standardized LGBTQ provider directory, educate staff on the healthcare needs of the community, and promote educational awareness, compassionate understanding, and improved care for transgender patients. As this council matures into the established stage, new projects will be taken on in line with the needs seen by members.
Women’s Health Council
The Women’s Health Council is a unique PFAC established in 2012. The council serves a population of trauma survivors cared for by the Coordinated Approach to Recovery and Empowerment (C.A.R.E.) clinic at BWH, also founded in 2012. Patients who receive care in this clinic have experienced violence and trauma, including domestic and sexual violence, child maltreatment, and human trafficking. Due to previous experience leading a PFAC, the C.A.R.E. Director understood the importance of patient input and engaged patients as advisors while forming the clinic.
The C.A.R.E. clinic serves both men and women but the majority of survivors served are female; thus, the patient advisors on its PFAC are all female. To recruit advisors, clinicians, and social workers at the clinic refer potential candidates to the C.A.R.E. Director, who then interviews them. The criteria for advisors for this council include being a female survivor of violence and trauma, being physically and mentally able to serve, and able to participate in a way that does not re-traumatize them. There are currently 14 advisors on the council with a goal to grow to 30 advisors. Experience has shown that members become busy with family, school and careers and may need to step away for short periods of time; thus, the council seeks to continually recruit to ensure robust membership.
Instead of the usual monthly scheduled meetings, this council holds “meetings on demand.” Advisors are polled via email to find a time in the near future that works for the group. The PFAC generally uses a web conferencing platform for their meetings and has an in-person meeting once or twice a year. Also unlike other councils, this council does not require their advisors to share their personal narratives; it is up to each advisor to decide what to share.
This council has accomplished numerous goals since its inception, including its first task of giving the C.A.R.E. clinic its name. The council has provided feedback on the development of the C.A.R.E. brochure and website and serves as key informants in all aspects of policy and procedures for the C.A.R.E. clinic. Additionally, they have provided input on how to create a safe environment for patients and screen patients to identify a victim of violence or human trafficking [5]. This council has been sustained by the strong community fostered by the director and projects led by the advisors, as each advisor has a vested interest in ensuring the clinic provides a safe environment for patients seeking care. This year, the council is hoping to host experts from the Boston Health Commission to share best practices in providing services to victims of abuse and violence.
Lessons Learned
The BWH Center for Patients and Families has encountered challenges when creating and sustaining PFACs, such as recruiting advisors from diverse ethnic, cultural, and economic backgrounds. Currently, our advisor population is primarily comprised of Caucasian patient/family members from middle and upper economic backgrounds, though it has increasingly diversified as the program has grown. We believe the lack of representation from other backgrounds is due to scheduling difficulties, the lack of payment for advisors, visibility of the PFAC program, and, potentially, cultural norms that promote deference to medical expertise. We have worked to increase PFAC diversity by asking providers to specifically seek out and nominate patients that will broaden our reach as a council.
Retaining and recruiting advisors after the PFACs have launched can also present a challenge. Some advisors have had to resign due to job demands, relocation, health issues, or the need to take care of family. To resolve this issue, we have asked PFAC chairs to continuously actively recruit advisors. By doing so, the councils gain new perspectives and ensure there are adequate number of advisors should a vacancy occur.
Sustaining PFACs once they are established requires time, effort, and commitment of leadership, advisors, and dedicated staff resources. The council needs to be continuously engaged in meaningful projects and feel that their participation is impactful and creates change. It is important that clinical leadership stays actively involved and attends all PFAC meetings. If there is a change in leadership as we experienced on our Shapiro PFAC, it is critical that the interim chair participates and supports the goal of the council. Regardless, leadership must show sustained enthusiasm for PFAC engagement and achievement.
Employing technology can also help sustain councils. Although we prefer in-person meetings, the option to attend meetings through online or phone conferencing should be made available to support advisors who are unable to attend in person. At this time, only one of our councils uses web conferencing, while several of our councils offer an option to call in via a conference line. The conference line has been beneficial in helping us retain and engage advisors who travel a significant distance to attend meetings.
We recognize that BWH has many resources available due to its status as a large, academic medical center in an urban center. Nonetheless, PFACs can play a vital role in hospitals no matter the setting, location, or size as long as there is buy in from hospital leadership. Although BWH has 16 PFACs, it is not necessary to have this many councils. Having one PFAC may be sufficient for smaller hospitals; the ideal number of councils depends on the size and complexity of the institution. Hospitals without a dedicated department like the Center for Patients and Families can create PFACs by partnering with volunteer services, patient engagement, or quality and safety departments. Existing departments with the capability to train advisors and provide meeting resources to support patient/family recruitment and engagement should be harnessed whenever possible. It is, however, important to have a dedicated staff member to serve as a point person for the advisors should they have any questions or concerns. Technology, such as web conferencing described above, can facilitate attendance by patient/family advisors who have limited time or resources and will be valuable for hospitals in a rural setting. The stages we have described are critical to the success of creating and sustaining a PFAC regardless of where they are developed and can be adapted to fit the unique needs and environments of any healthcare setting.
Conclusion
BWH’s Center for Patients and Families has created 16 PFACs since 2008, which are in various stages of development. Our PFACs are successful for many reasons, including a rigorous recruitment and interview process, leadership support, advisors’ commitment to their PFAC, and making modifications made based on lessons learned, as illustrated by the 3 PFACs discussed. We are able to sustain our councils by continually engagingadvisors, having leadership partner with advisors, setting feasible goals, and recruiting new advisors for a fresh perspective. PFACs promote patient- and family-centered care and can shift the model of care from a prescribed model to one that embraces collaboration with patients while advancing care delivery. As patient- and family-centered care advances, it is important that best practices for building and sustaining PFACs are developed and made available to ensure all hospitals have access to this valuable resource.
Corresponding author: Celene Wong, MHA, Center for Patients and Families, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115, cwong3@partners.org.
Financial disclosures: None.
From the Center for Patients and Families (Dr. Fagan, Ms. Wong, Ms. Morrison, Ms. Carnie), and the Division of Women’s Health and Gender Biology (Dr. Lewis-O’Connor), Brigham and Women’s Hospital, Boston, MA.
Abstract
- Objective: To describe and illustrate the phases of creating, recruiting, launching, and sustaining a successful Patient and Family Advisory Council (PFAC) in a hospital setting.
- Method: Descriptive report.
- Results: There are 4 stages in creating and establishing a PFAC: council preparation, patient/family advisor recruitment, council launch, and sustaining an established council. Each stage poses challenges that need to be addressed in order to progress to the next stage. The ability for hospital leadership to authentically partner with patient and family advisors is key to maintaining and sustaining PFACs.
- Conclusion: The success of a PFAC is based on leadership support, advisors’ commitment to their PFAC, and the ability to sustain the council. PFACs can promote patient- and family-centered care and shift the model of care from a prescribed model to one that embraces partnerships with patients while advancing care delivery. As patient- and family-centered care advances, it is important that best practices and resources for building and sustaining PFACs are developed and made available to ensure all hospitals have access to this valuable resource.
Throughout the country, families, patients, and health care professionals are working together in new ways, including within patient and family advisory councils (PFACs). First established in the 1990s, PFACs became widespread after patient-centeredness was identified by the Institute of Medicine as one of the 6 aims of quality health care [1]. PFACs were created to institutionalize a partnership between hospital leadership, clinicians, patients, and families to improve care delivery. Through this partnership, PFACs facilitate the sharing of patient perspectives and input on hospital policies and programs; serve as a resource to providers; and promote relationships between staff, patients, and family members [2]. PFACs also play an important role in promoting patient- and family-centered care, ensuring that patient needs and values are at the center of the care delivery system.
The BWH Center for Patients and Families includes the executive director, a project manager, and a senior patient advisor, who together oversee the PFACs. The project manager provides logistical support and is available as concerns arise, working closely with the senior patient advisor to ensure patient/family advisors are acclimated to their role and all PFACs run smoothly. The senior patient advisor is a volunteer advisor and patient advocate with long term experience creating and sustaining PFACs and mentoring patient/family advisors. Her role as a mentor includes attending PFAC meetings to model skills and behaviors for other advisors and working with them to ensure they are comfortable in their PFAC. Her advocacy work includes listening to and helping to articulate lived patient/family experiences as compelling narratives, which can be shared with hospital leadership and used as exemplars to spur change. Together, the team recruits and trains patient/family advisors, support all phases of PFAC development, and represent the patient/family voice within the hospital.
In this article, we describe and illustrate the 4 stages of creating and sustaining a successful PFAC to provide guidance and lessons learned to organizations seeking to develop this valuable resource. These stages are: (1) council preparation, (2) patient/family advisor recruitment, (3) council launch, and (4) sustaining an established council.
Council Preparation
Preparation of the PFAC occurs once hospital or service line leadership has identified a need for and is committed to having a PFAC. Leadership contacts the executive director of the Center for Patients and Families to discuss the strategy and vision of the council. The executive director describes the attributes sought in an advisor and the core principles of patient- and family-centered care. This discussion includes recruitment methods, meeting logistics, and who will serve as council chair. The council chair should be in a leadership role and willing to champion the PFAC for their service line. The executive director discusses what is being planned with relevant clinicians and staff at a staff meeting in order to foster buy-in and involve them in the council recruitment process.
The BWH Center for Patients and Families has adapted Institute for Patient- and Family-Centered Care recommendations for PFAC logistics and structure [3]. Councils generally meet monthly for 90 minutes excluding August and December. As volunteers, our advisors receive no monetary compensation but receive complimentary parking and are often provided meals. Advisors are asked to commit to a 3-year term with the understanding that personal issues can arise and their commitment may change; after their term, they are welcome to continue. We recommend to leadership that PFACs be comprised of no more than 1 staff member to every 4 advisors. This ratio seeks to address any potential power imbalance and promotes a feeling of ownership of their council . The project manager works with leadership in creating council guidelines, including the council’s goals, expectations, and each member’s role.
Patient/Family Advisor Recruitment
We ask service line providers and staff to nominate patients and family members they believe would be suitable advisors. The attributes we look for in an advisor include their ability to: (1) share personal experiences in ways that professional and support staff can learn from, (2) see the big picture of a challenge or scenario and give advice using the lens of the patient or family member, (3) be interested in more than one agenda item, (4) speak to multiple operational topics, (5) listen to other points of view and be empathetic, (6) connect with other advisors and staff, and (7) have a good sense of humor. Candidates with both positive and negative experiences are sought so that we can learn and improve from their experience [2].
To find advisors with these attributes, we ask providers to review their schedule and think about who they look forward to seeing or connect with on a personal level. This method has proven successful at producing candidates that have the attributes we seek. It is vital that the patients we recruit are able to see past their own personal experiences to understand broader objectives and how they fit into the bigger picture, enabling them to participate in a variety of projects and committees. There are no educational or specific skill requirements to become an advisor; the only requirement is that candidates must have experience as a patient or caregiver (family member) of a patient at BWH.
Recommended patients receive a letter notifying them that they have been nominated to be an advisor on a PFAC by their treating clinician. The project manager contacts potential advisors to see if they are interested and provides a brief description of PFACs and the role of patient/family advisors. The project manager emphasizes the importance of patient/family input to the hospital, describing the opportunities patient/family advisors have to contribute their expertise as a patient or caregiver to decisions and projects that will positively affect future patient care. Examples of past successful PFAC projects are shared to give a sense of the importance of the advisor role within the hospital and the appreciation hospital leadership has for PFAC contributions. The project manager reiterates that their clinician nominated them to the council to encourage the candidate to feel that their voice deserves to be heard.
Interested candidates are interviewed by phone by the team. Each candidate is asked the same questions: (1) How long have you been a patient in the clinic or unit?; (2) Describe your experiences in this clinic/service; and (3) Describe what works well and what could be improved in your care. During the interview, we listen to their personal narrative and their perspective on their care, which allows us to assess whether they have the attributes of a successful patient/family advisor. Candidate’s narratives illustrate how they would share their concerns, contribute to solutions, and if they have the ability to see beyond their own personal agenda. We also listen carefully for themes of tolerance, operational insight, empathy, and problem-solving capabilities. Interviews take about 15–20 minutes, depending on how many follow-up questions we have for the candidate and if they have questions for us.
After the phone interview, the team determines whether the candidate would be an appropriate patient/family advisor. If there are any concerns and more information is needed, the project manager reaches out to the staff and contacts the candidate to invite them for an in-person interview. Of the interviewed candidates, about 75% to 80% are invited to join. Candidates who are not chosen are generally unable to clearly articulate issues they see within the hospital/clinic, may have personal relationships with the staff (ie, friends with the physician), or cannot see pass their own issues and are inflexible in their thinking. Those not chosen receive a note thanking them for their time and interest. The candidates chosen to be advisors are on boarded through BWH volunteer services and must attend a 3-hour BWH volunteer orientation, be HIPAA compliant, and be cleared by occupational health before receiving their advisor ID badge and beginning service.
Council Launch
Once the advisors have been recruited and oriented, the council enters the launching stage, which lasts from the council’s first meeting until the 1-year anniversary. The first meeting agenda is designed to introduce staff and advisors to each other. Advisors each share their health care narratives and the staff shares their motivations for participating in the council. The council chair reviews the purpose and goals of the council.
During the first year, the council gains experience working together as a team. Council projects are initially chosen by the council chair and should be reasonably simple to accomplish and meaningful to advisors so that advisors recognize that their feedback is being heard and acted on. Example projects include creating clearer directional sign-age, assessing recliners for patient rooms, and providing feedback on patient handouts to ensure patient friendliness.
As the council advances, projects can be initiated by the advisors. This process is facilitated when an advisor is added as council co-chair, which usually occurs at the end of the first year. Projects often arise from similar concerns shared by advisors during the recruitment interview process.
Sustaining an Established Council
A council is considered established when it enters its second year and has named a patient advisor as a co-chair. Established councils have undertaken projects such as improving the layout of the whiteboards in patient rooms and providing feedback to staff on how to manage challenging patients. In addition, established councils may be tapped when service lines without a PFAC seek to gather advisor feedback for a project. For example, one of our established councils has provided feedback on two patient safety research projects.
Councils are sustained by continually engaging advisors in projects that are of value to them, both in their department and hospital-wide. Advisors should be given the opportunity to prioritize and set new council goals. One of the overarching goals for all our PFACs is to improve communication between patients and staff. Councils at this stage often participate in grand rounds or attend staff meetings to share their narratives, enabling providers to understand their perspective. The council can also be engaged in grant-funded research initiatives. Having PFACs involved in various projects allows advisors to bring their narratives to a wider audience and be a part of change from numerous avenues within the hospital.
Patient and Family Advisory Councils in Practice
BWH has 16 PFACs in various stages of growth. To illustrate the variety in council structure and function, we describe 3 PFACs below. Each has unique composition and goals based on the needs of service line leadership.
Shapiro Cardiovascular PFAC
The Shapiro Cardiovascular Center, a LEED silver-certified building and with private patient rooms that welcome family members to stay with their loved ones [4], opened in 2008. The chief nursing officer felt the care provided in this new space should promote and embody PFCC. With the assistance of the Center for Patients and Families, the associate chief nursing officer was charged with creating the Shapiro Cardiovascular PFAC. Launched in May 2011, this PFAC provides input to improve the patient experience for inpatient and ambulatory care housed in the Shapiro Center.
The Shapiro PFAC originally consisted of medical/surgical cardiac and heart transplant patients; renal transplant recipients and donors later joined. Initially, this council worked on patient/visitor guidelines for the inpatient units. As the council became more experienced, advisors interviewed nursing director candidates for cardiac surgery ICU and organized two PFCC nursing grand rounds. These grand rounds featured a panel of Shapiro advisors sharing their perspective of their hospital care and reflections on their healing process. This council has also provided feedback on hospital-wide projects, such as the refinement of a nursing fall prevention tool and the development of patient-informed measures of a successful surgery. As advisors became more experienced, they were recruited by the executive director to be part of other committees and research projects.
The Shapiro PFAC is one of the oldest councils at BWH, consisting of 12 advisors and 3 staff members, with most of the inaugural advisors remaining. Because the council chair has changed twice since 2011, this council does not have a formal advisor co-chair but the council remains a cohesive team as they work in partnership with the newest chair. To sustain this PFAC, leadership has consistently engaged the council in operational projects. For example, the associate chief nursing officer has suggested advisors be part of unit-based councils composed of staff nurses and educators who work to improve patient care within their unit. Advisors have also been invited to participate in staff and nursing director meetings to share their narratives and allow staff to reflect on the care they provide patients.
LGBTQ PFAC
In the fall of 2014, BWH held an educational Lesbian, Gay, Bisexual, Transgender, and Queer (LGBTQ) patient experience forum prompted by a complaint from the wife of a maternity patient that the care they received was not patient/family-centered. During this panel discussion, 4 LGBTQ patients described their care at BWH, including what went well and what their providers could have done better. There was an acknowledgement by a majority of providers in the audience that they did not receive training on inclusive care for LGBTQ patients and their families. The providers identified the need to be educated on LGBTQ issues and care concerns, and their desire to work towards creating a warm and inclusive environment that better serves LGBTQ patients. This organic request for education was met with enthusiasm from the panel participants and led to a commitment to form an LGBTQ PFAC at BWH.
The LGBTQ PFAC is co-chaired by the executive director of the Center for Patients and Families and a LGBTQ patient advisor who receives his care at BWH. It was important for this council to be co-led by an advisor from the beginning to acknowledge and validate LGBTQ experiences of care which had previously been marginalized. Because LGBTQ patients interact with all service lines at BWH, it made sense that a central operational leader with significant experience listening and responding to the patient voice co-leads the group. This council is composed of LGBTQ patients, their caregivers/partners/spouses, BWH LGBTQ staff that also receive healthcare from BWH, and LGBTQ academic stakeholders who provide historical contextualization to inform change.
The LGBTQ PFAC began the preparation phase in April 2015 and launched as a hospital-wide council in October 2015. This launch was widely publicized so that all BWH employees would know this council was created to elevate LGBTQ patients and caregivers into the mainstream hospital consciousness. The goals for this year are to partner with the existing LGBTQ employee group to create a standardized LGBTQ provider directory, educate staff on the healthcare needs of the community, and promote educational awareness, compassionate understanding, and improved care for transgender patients. As this council matures into the established stage, new projects will be taken on in line with the needs seen by members.
Women’s Health Council
The Women’s Health Council is a unique PFAC established in 2012. The council serves a population of trauma survivors cared for by the Coordinated Approach to Recovery and Empowerment (C.A.R.E.) clinic at BWH, also founded in 2012. Patients who receive care in this clinic have experienced violence and trauma, including domestic and sexual violence, child maltreatment, and human trafficking. Due to previous experience leading a PFAC, the C.A.R.E. Director understood the importance of patient input and engaged patients as advisors while forming the clinic.
The C.A.R.E. clinic serves both men and women but the majority of survivors served are female; thus, the patient advisors on its PFAC are all female. To recruit advisors, clinicians, and social workers at the clinic refer potential candidates to the C.A.R.E. Director, who then interviews them. The criteria for advisors for this council include being a female survivor of violence and trauma, being physically and mentally able to serve, and able to participate in a way that does not re-traumatize them. There are currently 14 advisors on the council with a goal to grow to 30 advisors. Experience has shown that members become busy with family, school and careers and may need to step away for short periods of time; thus, the council seeks to continually recruit to ensure robust membership.
Instead of the usual monthly scheduled meetings, this council holds “meetings on demand.” Advisors are polled via email to find a time in the near future that works for the group. The PFAC generally uses a web conferencing platform for their meetings and has an in-person meeting once or twice a year. Also unlike other councils, this council does not require their advisors to share their personal narratives; it is up to each advisor to decide what to share.
This council has accomplished numerous goals since its inception, including its first task of giving the C.A.R.E. clinic its name. The council has provided feedback on the development of the C.A.R.E. brochure and website and serves as key informants in all aspects of policy and procedures for the C.A.R.E. clinic. Additionally, they have provided input on how to create a safe environment for patients and screen patients to identify a victim of violence or human trafficking [5]. This council has been sustained by the strong community fostered by the director and projects led by the advisors, as each advisor has a vested interest in ensuring the clinic provides a safe environment for patients seeking care. This year, the council is hoping to host experts from the Boston Health Commission to share best practices in providing services to victims of abuse and violence.
Lessons Learned
The BWH Center for Patients and Families has encountered challenges when creating and sustaining PFACs, such as recruiting advisors from diverse ethnic, cultural, and economic backgrounds. Currently, our advisor population is primarily comprised of Caucasian patient/family members from middle and upper economic backgrounds, though it has increasingly diversified as the program has grown. We believe the lack of representation from other backgrounds is due to scheduling difficulties, the lack of payment for advisors, visibility of the PFAC program, and, potentially, cultural norms that promote deference to medical expertise. We have worked to increase PFAC diversity by asking providers to specifically seek out and nominate patients that will broaden our reach as a council.
Retaining and recruiting advisors after the PFACs have launched can also present a challenge. Some advisors have had to resign due to job demands, relocation, health issues, or the need to take care of family. To resolve this issue, we have asked PFAC chairs to continuously actively recruit advisors. By doing so, the councils gain new perspectives and ensure there are adequate number of advisors should a vacancy occur.
Sustaining PFACs once they are established requires time, effort, and commitment of leadership, advisors, and dedicated staff resources. The council needs to be continuously engaged in meaningful projects and feel that their participation is impactful and creates change. It is important that clinical leadership stays actively involved and attends all PFAC meetings. If there is a change in leadership as we experienced on our Shapiro PFAC, it is critical that the interim chair participates and supports the goal of the council. Regardless, leadership must show sustained enthusiasm for PFAC engagement and achievement.
Employing technology can also help sustain councils. Although we prefer in-person meetings, the option to attend meetings through online or phone conferencing should be made available to support advisors who are unable to attend in person. At this time, only one of our councils uses web conferencing, while several of our councils offer an option to call in via a conference line. The conference line has been beneficial in helping us retain and engage advisors who travel a significant distance to attend meetings.
We recognize that BWH has many resources available due to its status as a large, academic medical center in an urban center. Nonetheless, PFACs can play a vital role in hospitals no matter the setting, location, or size as long as there is buy in from hospital leadership. Although BWH has 16 PFACs, it is not necessary to have this many councils. Having one PFAC may be sufficient for smaller hospitals; the ideal number of councils depends on the size and complexity of the institution. Hospitals without a dedicated department like the Center for Patients and Families can create PFACs by partnering with volunteer services, patient engagement, or quality and safety departments. Existing departments with the capability to train advisors and provide meeting resources to support patient/family recruitment and engagement should be harnessed whenever possible. It is, however, important to have a dedicated staff member to serve as a point person for the advisors should they have any questions or concerns. Technology, such as web conferencing described above, can facilitate attendance by patient/family advisors who have limited time or resources and will be valuable for hospitals in a rural setting. The stages we have described are critical to the success of creating and sustaining a PFAC regardless of where they are developed and can be adapted to fit the unique needs and environments of any healthcare setting.
Conclusion
BWH’s Center for Patients and Families has created 16 PFACs since 2008, which are in various stages of development. Our PFACs are successful for many reasons, including a rigorous recruitment and interview process, leadership support, advisors’ commitment to their PFAC, and making modifications made based on lessons learned, as illustrated by the 3 PFACs discussed. We are able to sustain our councils by continually engagingadvisors, having leadership partner with advisors, setting feasible goals, and recruiting new advisors for a fresh perspective. PFACs promote patient- and family-centered care and can shift the model of care from a prescribed model to one that embraces collaboration with patients while advancing care delivery. As patient- and family-centered care advances, it is important that best practices for building and sustaining PFACs are developed and made available to ensure all hospitals have access to this valuable resource.
Corresponding author: Celene Wong, MHA, Center for Patients and Families, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115, cwong3@partners.org.
Financial disclosures: None.
1. Institute of Medicine (US). Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press; 2001. Accessed 6 Apr 2016 at www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf.
2. Institute for Patient- and Family-Centered Care. Institute for Patient- and Family-Centered Care creating patient and family advisory councils. Oct 2010. Accessed 10 Jan 2016 at www.ipfcc.org/advance/Advisory_Councils.pdf.
3. Institute for Patient- and Family-Centered Care. Institute for Patient- and Family-Centered Care core concepts. Dec 2010. Accessed 10 Jan 2016 at www.ipfcc.org/faq.html.
4. NBBJ. Caring to connect. 2016. Accessed 22 Feb 2016 at www.nbbj.com/work/brigham-and-womens-hospital-shapiro/.
5. Lewis-O’Connor A, Chadwick M. Engaging the voice of patients affected by gender-based violence: informing practice and policy. J Forensic Nurs 2015;11:240–9.
1. Institute of Medicine (US). Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academy Press; 2001. Accessed 6 Apr 2016 at www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf.
2. Institute for Patient- and Family-Centered Care. Institute for Patient- and Family-Centered Care creating patient and family advisory councils. Oct 2010. Accessed 10 Jan 2016 at www.ipfcc.org/advance/Advisory_Councils.pdf.
3. Institute for Patient- and Family-Centered Care. Institute for Patient- and Family-Centered Care core concepts. Dec 2010. Accessed 10 Jan 2016 at www.ipfcc.org/faq.html.
4. NBBJ. Caring to connect. 2016. Accessed 22 Feb 2016 at www.nbbj.com/work/brigham-and-womens-hospital-shapiro/.
5. Lewis-O’Connor A, Chadwick M. Engaging the voice of patients affected by gender-based violence: informing practice and policy. J Forensic Nurs 2015;11:240–9.
Pharmacists’ Involvement in Medication Management Along the Continuum of Care: Challenges, Lessons Learned, and Implications for Health Systems
From The Johns Hopkins Hospital, Baltimore, MD.
The project described was supported by grant # 1C1CMS331053-01-00 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Findings may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
Abstract
- Background: Medication management is becoming more complex, with new medications entering the market, drug prices increasing, and patients transferring into and out of the hospital. Transitions of care services are being implemented to prevent readmissions and increase patient satisfaction. Pharmacists play a key role by expanding clinical services provided to patients around medication management.
- Objective: To describe a pharmacy transitions of care model at a large academic teaching hospital and lessons learned during implementation.
- Methods: A pharmacy bundle of services was initially developed in a medical patient population and included medication reconciliation, patient education targeting high-risk medications, post-discharge follow-up phone calls, and bedside discharge prescription delivery. This bundle was expanded to other patient populations through the use of residency-trained pharmacists, pharmacy residents, pharmacy students, and certified pharmacy technicians.
- Results: Challenges were faced when implementing our transitions of care services, including expanding care coordination team coverage with existing resources, training pharmacy staff in new roles, determining the needs of patients cared for by teams we had not previously been integrated into, and creating our discharge prescription delivery program. During this process, we learned to rethink the role of pharmacists on our team, value the support within our institution to create change in order to improve patient care, and continuously evaluate this process.
- Conclusion: We are at an opportune time to expand the scope of the inpatient pharmacist to provide advanced medication-related services to patients. Residency training is creating individuals who will thrive in these new models.
Medication management around the acute care inpatient stay is a challenging but crucial task to ensure patient safety and desired clinical outcomes. The first step in successful medication management is to understand the patient’s medication regimen in the home environment. Patients may take medications differently than prescribed; skip medication doses intentionally to make a supply last longer; use over-the-counter medications, herbal supplements, or someone else’s medication based on the recommendation of family or friends; or discontinue medications based on side effects or media influence. Over the course of the inpatient stay, medication management involves adjusting doses based on changes in organ function, detecting side effects and potential drug interactions, and monitoring clinical outcomes to ensure appropriate drug therapy is being prescribed. As the patient approaches discharge, ensuring the patient understands the indications for his/her medications, has self-monitoring techniques to recognize efficacy or adverse effects, and has access to discharge medications is important. Lastly, long-term medication management includes patient access to pharmaceutical expertise over time. Pharmacists’ involvement in medication therapy management services and patient-centered medical homes is key to ensuring safe and effective medication use over time.
In 2009, the Johns Hopkins Health System Readmission Prevention Task Force developed strategies to reduce preventable readmissions and improve transitions of care. In 2011, a new multidisciplinary approach to patient care was implemented at the Johns Hopkins Hospital (JHH) to optimize care coordination and improve acute care management. Using this approach, care coordination teams composed of physicians, nurses, pharmacists, nurse case managers, social workers, physical/occupational therapists, nutritionists, and home care coordinators meet on a daily basis to discuss the inpatient and discharge care needs of patients in order to improve care transitions and reduce preventable readmissions. In 2012, JHH was awarded a 3-year innovation grant from the Centers for Medicaid & Medicare Services (CMS) that would assist with expansion of these care coordination teams to every unit of the hospital. Prior to implementation of the care coordination model at JHH in 2011, there were 3 pharmacists who consistently rounded on 3 inpatient medicine teams (one pharmacist also had operational responsibilities). Pharmacists were deemed by the task force to be key providers of medication management and, thus, essential members of the care coordination team. Due to an inability to hire a new pharmacist for every care coordination team, the department of pharmacy needed to determine how to provide consistent pharmacist coverage utilizing current resources. This report describes the challenges faced and lessons learned by our adult inpatient pharmacy division when implementing a pharmacy bundle of services to improve care transitions for an adult patient population.
Setting
JHH is an 1192-bed academic teaching hospital located in Baltimore, Maryland. At JHH, the department of pharmacy has 4 inpatient divisions that service the medication needs of different patient populations: medicine, critical care/surgery, oncology, and pediatrics. The adultinpatient pharmacy division covers medicine units in addition to obstetrics, neurology, and surgery units. It is responsible for 486 inpatient beds on 22 units and was the first division to provide the pharmacy bundle of services described below. Currently, 11 rounding and 5 operational pharmacists provide care coordination and order verification support, respectively, for the division during day shift.
Program Overview
Rounding pharmacists on care coordination teams address acute care medication issues and provide a bundle of services that includes targeted patient education, medication reconciliation, post-discharge follow-up phone calls, and discharge prescription planning. The full details of these services have been described [1]. Briefly, patients newly initiated on medications deemed ”high-risk” (eg, anticoagulation, insulin, metered dose inhalers, dual antiplatelets) receive education by a member of the pharmacy team (ie, pharmacist, pharmacy resident, or pharmacy student) prior to discharge. Those patients who receive education are offered a post-discharge follow-up phone call to assess for any questions or issues. Patients who accept this service are contacted 48 to 72 hours post-discharge. Specific patient populations (eg, patients with congestive heart failure, diabetes) are also targeted for completion of medication reconciliation. If patients are being discharged with prescriptions, they are offered our “Meds for Home” service. Patients who accept this service have their prescriptions filled at one of our outpatient pharmacies and delivered to the unit prior to discharge. Highly trained certified pharmacy technicians, called “Meds for Home” coordinators (MHCs; previously known as transitions pharmacist extenders), facilitate this process.
Challenges Faced
Care Coordination Team Coverage
One challenge to implementation of the pharmacy bundle of services was providing consistent team coverage with adequately trained pharmacists. It was not feasible to hire a pharmacist to cover each of the care coordination teams. To address gaps in coverage, we initially utilized postgraduate year 1 and 2 (PGY1 and 2) pharmacy residents on an internal medicine rotation to cover care coordination teams without a rounding pharmacist. However, this method proved unreliable as a pharmacy resident was not scheduled for an internal medicine rotation each month. In the beginning, our division had 3 rounding and 9 operational pharmacists during day shift. To provide sufficient clinical coverage while still adequately addressing order verification needs, a major restructuring of our pharmacy model was necessary. We increased the bed-to-pharmacist ratio for order verification, which allowed the number of operational pharmacists to decrease from 9 to 5. Those 4 remaining pharmacists were now available to serve as rounding pharmacists. Along with hiring 2 additional rounding pharmacists with funding from the CMS innovation grant, we were able to increase the number of care coordination teams consistently covered from 3 to 9.
Although we expanded pharmacist coverage of care coordination teams, time constraints prevented all patients who met criteria for patient education or medication reconciliation to have these services completed in a timely manner or at all by a pharmacist. Our rounding pharmacists’ responsibilities also included participation in high-level activities such as order set reviews for a new provider order entry system, ambulatory clinic time, stewardship activities, and quality improvement projects. In order to increase our scope, we utilized pharmacy technicians, students, and residents to assist with completing these tasks. All pharmacy students and residents on rotation within our division participated in a daily huddle Monday through Friday. Rounding pharmacists whose unit had patient education needs that could not be met by that pharmacist submitted requests by a set time. Those patient education tasks were then divided amongst the pharmacy learners at the huddle for completion. Prior to being allowed to independently counsel patients, pharmacy learners’ patient education skills were evaluated by preceptors. To facilitate timely completion of medication histories, technicians were hired. These medication history technicians are available Monday through Friday to complete medication histories for patients admitted to specific medicine units, ideally within 24 hours of admission. Rounding pharmacists are notified of completion of medication histories via our electronic medical record and reconcile that list with the patient’s inpatient medication list. Any clinically relevant discrepancies are communicated to providers. Pharmacy learners may also collect medication histories.
Training Rounding Pharmacists
Another challenge we faced was providing adequate training for operational pharmacists transitioning to a rounding position. Residency training is crucial in providing the level of skill necessary to identify complex drug therapy problems, adjust treatment regimens, and create plans where limited data exist to drive drug therapy recommendations [2,3]. Rotations during the final year of pharmacy school provide exposure to interacting with patients and providers. Completion of PGY1 residency training allows a pharmacist to practice as a generalist with a broad range of experiences provided during the year to identify medication-related problems. PGY2 residency training allows the pharmacist to spend a concentrated year in the chosen area of expertise and gain a deeper knowledge of medication use in a specific patient population or area of practice [2]. After 2 years of clinical residency training, pharmacists have the skills to interact with patients and multidisciplinary teams to optimize medication regimens, provide medication education, and measure the value they bring to the health care of patients.
Some of the operational pharmacists who were transitioning to the rounding pharmacist role had no training beyond pharmacy school or had only completed a PGY1 pharmacy residency. Initially, training for this new role lasted only a few days and consisted of orientation to the unit and observation of care coordination rounds. We learned that this brief amount of training was insufficient, even for those with PGY1 pharmacy residency training. In order to ensure that these rounding pharmacists could successfully provide the bundle of services and meet the high clinical demands of the inpatient service, we developed a comprehensive training program. Those interested in transitioning from an operational to a rounding pharmacist role must now complete a 6-week training program. The first 2 weeks consists of improving patient education and medication history skills. The remaining 4 weeks are spent honing clinical rounding skills. Rounding pharmacists-in-training also receive a formal review of their performance utilizing an evaluation form developed by the American Society of Health-System Pharmacists (ASHP) for pharmacy residents.
Establishing a Pharmacy Bundle and the Role of a Rounding Pharmacist on New Units
Some of the units implementing care coordination teams, such as neurology, did not previously have a pharmacist rounding on those units. Furthermore, these units had a high patient census (eg, 60 patients), which made it difficult for one pharmacist to clinically evaluate every patient. Multiple specialty teams also admitted patients to a single unit, which made it challenging for the pharmacist to develop strong working relationships with providers. As such, rounding pharmacists deployed to those units had difficulty establishing their role on the team, especially for those pharmacists without or with only 1 year of postgraduate training. To address this issue, a PGY2-trained pharmacist rounded on the unit to assess which areas/teams had the greatest need for a pharmacist. Completing this needs assessment on these units allowed for the rounding pharmacist to more effectively use his/her time. It also allowed for a smoother transition from operational to rounding pharmacist by removing the burden of establishing a brand new role and identifying necessary tasks to be completed throughout the day.
We also discovered on these new care coordination units that our patient criteria for education and medication reconciliation were not universal. We developed and initiated our pharmacy bundle of services in a medical patient population. As we expanded these services to other patient care areas, the targeted list of medications/conditions changed. For example, surgical patients had a greater need for education around opioid therapy and complex bowel regimens while neurology patients needed education regarding antiepileptic regimens. Similarly, patients requiring medication reconciliation also changed. Nurses were performing medication reconciliation for patients with elective surgeries and had a system that worked for that population. Therefore, we did not need to focus efforts for this population around medication reconciliation and could shift our focus more towards medication education.
Optimizing the Delivery of Discharge Prescriptions
The Meds for Home workflow has been updated multiple times since implementation. These changes resulted from early and frequent meetings with nurses, case managers, providers, and the pharmacy team. The Meds for Home service uses an outpatient pharmacy located within the hospital that has high prescription volumes at baseline to fill discharge prescriptions. Due to the volume of out-patient prescriptions and unexpected discharges, delays in prescription delivery occurred. To improve efficiency, a separate workflow and space were designated for filling Meds for Home prescriptions. Initially, MHCs were visiting floors to pick-up and deliver prescriptions at set times (ie, 10 am, 2 pm, and 5 pm). Instead of using set pick-up and delivery times, the Meds for Home service now uses a rolling 2-hour turnaround time during service hours. Additionally, providers, case managers, and units were educated to provide discharge prescriptions, especially those requiring prior authorization, as early as possible to expedite service. By identifying these issues early in the process, we were able to develop a different strategy that worked for the units, providers, and pharmacy.
Lessons Learned
The time of transition from one level of care to another is a vulnerable time for patients, as it is a time when medication-related problems often arise. In an elderly patient population, one study demonstrated that contributing factors for medication discrepancies following hospital discharge included unintended nonadherence and inadequate discharge instructions, and patients experiencing a medication discrepancy were at a significantly higher risk of readmission [4]. Hospital readmissions have also been linked to a lack of adequate follow-up in the outpatient setting [5]. Pharmacists should become more involved in preventing medication-related problems during the times of transition by performing activities such as medication reconciliation, patient education, and assessment of patient outcomes post-discharge [6,7]. Studies have demonstrated that pharmacists are able to reduce medication-related adverse events during and after hospitalization by completing these activities [8–10]. Residency-trained pharmacists are well-equipped to provide these services and are needed to create new processes and models to meet the ever changing demands of health care payers and accrediting bodies. ASHP recommends pharmacists entering into careers in health systems be at least PGY1-trained while the American College of Clinical Pharmacy (ACCP) envisions all pharmacists involved in direct patient care complete residency training [11,12]. Health systems will continue to be challenged with transforming pharmacy models to allow for this influx of highly trained individuals in a time of budget constraints. Below, we describe the lessons we learned while implementing our pharmacy bundle of services and think are essential for other institutions to consider when initiating their own services.
Rethink the Role of the Pharmacist
As health systems acquire smaller hospitals, the role of the pharmacist may need to be redefined and reinvented. The responsibilities of a pharmacist in a large academic hospital may be different than those of a pharmacist with the same skill set in a community hospital. However, despite the difference in practice setting, the same core pharmacy services around medication use can still be deployed. Participation in transitions of care activities is a relatively new concept for many pharmacists as residency training programs traditionally focused on caring for patients within a defined setting such as the intensive care unit or ambulatory care. The pharmacy profession should define the role of the clinical pharmacist in order to make the incorporation of transitions of care responsibilities into job expectations easier for all. The ACCP outlines this need and sets forth recommendations for clinical pharmacists’ responsibilities within the health care team to include assessing patients and medication regimens, developing and implementing medication-related therapy plans, and evaluating clinical outcomes [13]. Pharmacy leadership organizations, including ASHP and ACCP, offer resources providing the vision of pharmacy practice and expectations for which institutions should be reaching. Pharmacy departments should use these resources to complete gap analyses of current processes and those envisioned for the future to help guide efforts for change at their own institutions.
Obtain Support Within Your Institution
Gaining support from hospital leadership for advancing pharmacists’ involvement in patient care is instrumental. Without leadership support at both the institutional and department of pharmacy levels, pharmacists with advanced training may be hindered from practicing at the top of their license. Furthermore, support by leadership of pharmacy residency programs and experiential student learning sites at the institution is also important. Pharmacy residents and students became indispensable in our model and allowed us to expand our reach to more patients. We used residents to cover additional teams that were previously uncovered by a rounding pharmacist and, along with students, provide medication reconciliation, patient education, and follow-up phone calls to more patients. Requiring participation in the pharmacy bundle of services for rotations also allowed us to train these individuals about the value of transitions of care and see the challenges patients face in gaining access to medications. In a survey of academic medical center executives, pharmacy directors, and pharmacists at 8 institutions, residents were noted to add value to the institution through decreasing drug-related errors and drug costs, expansion of clinical services, and enhancing opportunities for research [14].
Support from other disciplines is also essential. Collaborating with other disciplines should occur prior to, during, and beyond implementation. We collaborated with providers, nurses, case managers, social workers and many other disciplines during all phases of the process. Being inclusive during the planning process allowed everyone to understand each other’s role and to provide input on how we could work together to best utilize everyone’s resources. This multidisciplinary approach to developing pharmacy services also allowed an opportunity to collaborate on research and evaluate our processes with other disciplines.
Tracking interventions will demonstrate the value of pharmacists, technicians, and other pharmacy team members participating in these advanced roles. This information will be useful when justifying the practice model to hospital leadership and for recruiting new pharmacists, residents, and technicians to the institution. Additionally, defining both outcome (eg, 30-day readmission rates, HCAHPS scores) and process (eg, number of patient education sessions performed, number of medication discrepancies reconciled) measures upfront is important in order for those involved to understand how their work will be assessed. These data will be useful in determining whether the intervention is making an impact early on and allow for restructuring of the process if not.
Create Depth in Your Team While Engaging Current Resources
We spent a significant amount of time planning the implementation of our pharmacy bundle of services, collaborating with other disciplines, and training our pharmacy team members. We hired highly trained and competent people into new positions and ensured every-one clearly understood their responsibilities. This was a critical step in order to ensure we were providing optimal care to our patients and integrating leaders into our team. We also utilized our current workforce to fill new clinical rounding pharmacist or technician roles. For those pharmacists who had not completed a residency, we required the pharmacists to complete a compact training program similar to that required of our residents [1]. This training ensured that important services were being performed adequately by each rounding pharmacist. Similarly, technicians transitioning from a primarily medication dispensing role to a MHC or medication history role received extensive training to assist with developing their new skill set.
Creating relationships with an outpatient pharmacy is essential to ensure patients are discharged from the hospital on medications they can afford long-term. We are fortunate to have 5 outpatient pharmacies on the JHH campus that are under the Johns Hopkins Health System umbrella, which made collaboration between the inpatient and outpatient teams seamless. However, many hospitals may not be directly affiliated with an outpatient pharmacy with which to collaborate or may contract with a retail chain pharmacy. In the latter case, inpatient and outpatient pharmacies must work together to define roles around transitions of care and how to best serve the patient in a collaborative manner. If no onsite outpatient pharmacy exists, dedicated resources should be acquired to serve as a liaison between the inpatient team and the outpatient pharmacy. These resources may work through issues such as formulary preferences, prior authorization requests, and connecting the patient to the medication either through bedside delivery or filling at the patient’s community pharmacy. Community pharmacies recognize the cost benefit they could gain through 340B pricing and specialty drug dispensing when working in collaboration with healthcare systems. However, health systems must be aware that collaborating with outpatient pharmacy partnerships will create further challenges as providers ensure patient preference for use of a particular pharmacy is honored and cost-sharing is incorporated into models.
Continuously Reevaluate Your Services
As implementation of our pharmacy bundle of services began, meeting early and often was essential to identify issues and adjust our workflow to resolve those issues quickly. When the inpatient component of the pharmacy bundle of services was first implemented, rounding pharmacists and pharmacy leadership initially met on a weekly basis to provide feedback on the practice model and develop resolutions for any issues. However, it is important to also include other disciplines in the evaluation process. For the Meds for Home program, pharmacy leadership not only met with MHCs but also with providers, nurses, case managers, and social workers for feedback on how to improve the service. Although the workflow of our pharmacy bundle of services are more established, evaluations still occur albeit less frequently.
Conclusion
Pharmacists’ involvement in transitions of care should become part of the daily responsibility. Health systems should understand how efforts to expand pharmacists’ interventions align with overall hospital goals. Many hospitals may already have programs in place to help with transitions of care. Pharmacists can help expand current efforts through increased visibility to physicians and patients as well as collaboration with outpatient pharmacies to ensure medications are effective and affordable for patients long-term.
Note: The project described was supported by grant no. 1C1CMS331053-01-00 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Findings may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
Corresponding author: Vi Gilmore, PharmD, BCPS, Clinical Pharmacy Specialist, Internal Medicine, The Johns Hopkins Hospital, 600 N. Wolfe Street, Carnegie 180, Baltimore, MD 21287, vdo1@jhmi.edu.
Financial disclosures: None.
1. Gilmore V, Efird L, Fu D, et al. Implementation of transitions-of-care services through acute care and outpatient pharmacy collaboration. Am J Health Syst Pharm 2015;72:737–44.
2. American College of Clinical Pharmacy, Burke JM, Miller WA, et al. Clinical pharmacist competencies. Pharmacotherapy 2008;28:806–15.
3. American Society of Health-System Pharmacists. ASHP accreditation standard for postgraduate year one (PGY1) pharmacy residency programs. Available at www.ashp.org/DocLibrary/Accreditation/Newly-approved-PGY1-Standard-September-2014.pdf.
4. Coleman EA, Smith JD, Raha D, Min S. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005:165:1842–7.
5. Jackson C, Shahsahebi M, Wedlake T, DuBard C. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med 2015;13:115–22.
6. American College of Clinical Pharmacy, Hume AL, Kirwin J, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy 2012;32:e326–37.
7. Cobaugh DJ, Amin A, Bookwalter T, et al. ASHP-SHM joint statement on hospitalist-pharmacist collaboration. Am J Health Syst Pharm 2008;65:260–3.
8. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166:565–71.
9. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization. Ann Intern Med 2009;150:178–87.
10. Murphy EM, Oxencis CJ, Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm 2009;66:2126–31.
11. Myers CE. ASHP Health-System Pharmacy 2015 Initiative. Am J Health-Syst Pharm 2004;61:657.
12. Murphy JE, Nappi JM, Bosso JA, et al. ACCP position statement. American College of Clinical Pharmacy’s vision of the future: postgraduate pharmacy residency training as a prerequisite for direct patient care. Pharmacotherapy 2006;26:
722–33.
13. Harris IM, Phillips B, Boyce E, et al. Clinical pharmacy should adopt a consistent process of direct patient care. Pharmacotherapy 2014;34:e133–48.
14. Fuller PD, Smith KM, Hinman RK, et al. Value of pharmacy residency training: a survey of the academic medical center perspective. Am J Health Syst Pharm 2012;69:158–65.
From The Johns Hopkins Hospital, Baltimore, MD.
The project described was supported by grant # 1C1CMS331053-01-00 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Findings may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
Abstract
- Background: Medication management is becoming more complex, with new medications entering the market, drug prices increasing, and patients transferring into and out of the hospital. Transitions of care services are being implemented to prevent readmissions and increase patient satisfaction. Pharmacists play a key role by expanding clinical services provided to patients around medication management.
- Objective: To describe a pharmacy transitions of care model at a large academic teaching hospital and lessons learned during implementation.
- Methods: A pharmacy bundle of services was initially developed in a medical patient population and included medication reconciliation, patient education targeting high-risk medications, post-discharge follow-up phone calls, and bedside discharge prescription delivery. This bundle was expanded to other patient populations through the use of residency-trained pharmacists, pharmacy residents, pharmacy students, and certified pharmacy technicians.
- Results: Challenges were faced when implementing our transitions of care services, including expanding care coordination team coverage with existing resources, training pharmacy staff in new roles, determining the needs of patients cared for by teams we had not previously been integrated into, and creating our discharge prescription delivery program. During this process, we learned to rethink the role of pharmacists on our team, value the support within our institution to create change in order to improve patient care, and continuously evaluate this process.
- Conclusion: We are at an opportune time to expand the scope of the inpatient pharmacist to provide advanced medication-related services to patients. Residency training is creating individuals who will thrive in these new models.
Medication management around the acute care inpatient stay is a challenging but crucial task to ensure patient safety and desired clinical outcomes. The first step in successful medication management is to understand the patient’s medication regimen in the home environment. Patients may take medications differently than prescribed; skip medication doses intentionally to make a supply last longer; use over-the-counter medications, herbal supplements, or someone else’s medication based on the recommendation of family or friends; or discontinue medications based on side effects or media influence. Over the course of the inpatient stay, medication management involves adjusting doses based on changes in organ function, detecting side effects and potential drug interactions, and monitoring clinical outcomes to ensure appropriate drug therapy is being prescribed. As the patient approaches discharge, ensuring the patient understands the indications for his/her medications, has self-monitoring techniques to recognize efficacy or adverse effects, and has access to discharge medications is important. Lastly, long-term medication management includes patient access to pharmaceutical expertise over time. Pharmacists’ involvement in medication therapy management services and patient-centered medical homes is key to ensuring safe and effective medication use over time.
In 2009, the Johns Hopkins Health System Readmission Prevention Task Force developed strategies to reduce preventable readmissions and improve transitions of care. In 2011, a new multidisciplinary approach to patient care was implemented at the Johns Hopkins Hospital (JHH) to optimize care coordination and improve acute care management. Using this approach, care coordination teams composed of physicians, nurses, pharmacists, nurse case managers, social workers, physical/occupational therapists, nutritionists, and home care coordinators meet on a daily basis to discuss the inpatient and discharge care needs of patients in order to improve care transitions and reduce preventable readmissions. In 2012, JHH was awarded a 3-year innovation grant from the Centers for Medicaid & Medicare Services (CMS) that would assist with expansion of these care coordination teams to every unit of the hospital. Prior to implementation of the care coordination model at JHH in 2011, there were 3 pharmacists who consistently rounded on 3 inpatient medicine teams (one pharmacist also had operational responsibilities). Pharmacists were deemed by the task force to be key providers of medication management and, thus, essential members of the care coordination team. Due to an inability to hire a new pharmacist for every care coordination team, the department of pharmacy needed to determine how to provide consistent pharmacist coverage utilizing current resources. This report describes the challenges faced and lessons learned by our adult inpatient pharmacy division when implementing a pharmacy bundle of services to improve care transitions for an adult patient population.
Setting
JHH is an 1192-bed academic teaching hospital located in Baltimore, Maryland. At JHH, the department of pharmacy has 4 inpatient divisions that service the medication needs of different patient populations: medicine, critical care/surgery, oncology, and pediatrics. The adultinpatient pharmacy division covers medicine units in addition to obstetrics, neurology, and surgery units. It is responsible for 486 inpatient beds on 22 units and was the first division to provide the pharmacy bundle of services described below. Currently, 11 rounding and 5 operational pharmacists provide care coordination and order verification support, respectively, for the division during day shift.
Program Overview
Rounding pharmacists on care coordination teams address acute care medication issues and provide a bundle of services that includes targeted patient education, medication reconciliation, post-discharge follow-up phone calls, and discharge prescription planning. The full details of these services have been described [1]. Briefly, patients newly initiated on medications deemed ”high-risk” (eg, anticoagulation, insulin, metered dose inhalers, dual antiplatelets) receive education by a member of the pharmacy team (ie, pharmacist, pharmacy resident, or pharmacy student) prior to discharge. Those patients who receive education are offered a post-discharge follow-up phone call to assess for any questions or issues. Patients who accept this service are contacted 48 to 72 hours post-discharge. Specific patient populations (eg, patients with congestive heart failure, diabetes) are also targeted for completion of medication reconciliation. If patients are being discharged with prescriptions, they are offered our “Meds for Home” service. Patients who accept this service have their prescriptions filled at one of our outpatient pharmacies and delivered to the unit prior to discharge. Highly trained certified pharmacy technicians, called “Meds for Home” coordinators (MHCs; previously known as transitions pharmacist extenders), facilitate this process.
Challenges Faced
Care Coordination Team Coverage
One challenge to implementation of the pharmacy bundle of services was providing consistent team coverage with adequately trained pharmacists. It was not feasible to hire a pharmacist to cover each of the care coordination teams. To address gaps in coverage, we initially utilized postgraduate year 1 and 2 (PGY1 and 2) pharmacy residents on an internal medicine rotation to cover care coordination teams without a rounding pharmacist. However, this method proved unreliable as a pharmacy resident was not scheduled for an internal medicine rotation each month. In the beginning, our division had 3 rounding and 9 operational pharmacists during day shift. To provide sufficient clinical coverage while still adequately addressing order verification needs, a major restructuring of our pharmacy model was necessary. We increased the bed-to-pharmacist ratio for order verification, which allowed the number of operational pharmacists to decrease from 9 to 5. Those 4 remaining pharmacists were now available to serve as rounding pharmacists. Along with hiring 2 additional rounding pharmacists with funding from the CMS innovation grant, we were able to increase the number of care coordination teams consistently covered from 3 to 9.
Although we expanded pharmacist coverage of care coordination teams, time constraints prevented all patients who met criteria for patient education or medication reconciliation to have these services completed in a timely manner or at all by a pharmacist. Our rounding pharmacists’ responsibilities also included participation in high-level activities such as order set reviews for a new provider order entry system, ambulatory clinic time, stewardship activities, and quality improvement projects. In order to increase our scope, we utilized pharmacy technicians, students, and residents to assist with completing these tasks. All pharmacy students and residents on rotation within our division participated in a daily huddle Monday through Friday. Rounding pharmacists whose unit had patient education needs that could not be met by that pharmacist submitted requests by a set time. Those patient education tasks were then divided amongst the pharmacy learners at the huddle for completion. Prior to being allowed to independently counsel patients, pharmacy learners’ patient education skills were evaluated by preceptors. To facilitate timely completion of medication histories, technicians were hired. These medication history technicians are available Monday through Friday to complete medication histories for patients admitted to specific medicine units, ideally within 24 hours of admission. Rounding pharmacists are notified of completion of medication histories via our electronic medical record and reconcile that list with the patient’s inpatient medication list. Any clinically relevant discrepancies are communicated to providers. Pharmacy learners may also collect medication histories.
Training Rounding Pharmacists
Another challenge we faced was providing adequate training for operational pharmacists transitioning to a rounding position. Residency training is crucial in providing the level of skill necessary to identify complex drug therapy problems, adjust treatment regimens, and create plans where limited data exist to drive drug therapy recommendations [2,3]. Rotations during the final year of pharmacy school provide exposure to interacting with patients and providers. Completion of PGY1 residency training allows a pharmacist to practice as a generalist with a broad range of experiences provided during the year to identify medication-related problems. PGY2 residency training allows the pharmacist to spend a concentrated year in the chosen area of expertise and gain a deeper knowledge of medication use in a specific patient population or area of practice [2]. After 2 years of clinical residency training, pharmacists have the skills to interact with patients and multidisciplinary teams to optimize medication regimens, provide medication education, and measure the value they bring to the health care of patients.
Some of the operational pharmacists who were transitioning to the rounding pharmacist role had no training beyond pharmacy school or had only completed a PGY1 pharmacy residency. Initially, training for this new role lasted only a few days and consisted of orientation to the unit and observation of care coordination rounds. We learned that this brief amount of training was insufficient, even for those with PGY1 pharmacy residency training. In order to ensure that these rounding pharmacists could successfully provide the bundle of services and meet the high clinical demands of the inpatient service, we developed a comprehensive training program. Those interested in transitioning from an operational to a rounding pharmacist role must now complete a 6-week training program. The first 2 weeks consists of improving patient education and medication history skills. The remaining 4 weeks are spent honing clinical rounding skills. Rounding pharmacists-in-training also receive a formal review of their performance utilizing an evaluation form developed by the American Society of Health-System Pharmacists (ASHP) for pharmacy residents.
Establishing a Pharmacy Bundle and the Role of a Rounding Pharmacist on New Units
Some of the units implementing care coordination teams, such as neurology, did not previously have a pharmacist rounding on those units. Furthermore, these units had a high patient census (eg, 60 patients), which made it difficult for one pharmacist to clinically evaluate every patient. Multiple specialty teams also admitted patients to a single unit, which made it challenging for the pharmacist to develop strong working relationships with providers. As such, rounding pharmacists deployed to those units had difficulty establishing their role on the team, especially for those pharmacists without or with only 1 year of postgraduate training. To address this issue, a PGY2-trained pharmacist rounded on the unit to assess which areas/teams had the greatest need for a pharmacist. Completing this needs assessment on these units allowed for the rounding pharmacist to more effectively use his/her time. It also allowed for a smoother transition from operational to rounding pharmacist by removing the burden of establishing a brand new role and identifying necessary tasks to be completed throughout the day.
We also discovered on these new care coordination units that our patient criteria for education and medication reconciliation were not universal. We developed and initiated our pharmacy bundle of services in a medical patient population. As we expanded these services to other patient care areas, the targeted list of medications/conditions changed. For example, surgical patients had a greater need for education around opioid therapy and complex bowel regimens while neurology patients needed education regarding antiepileptic regimens. Similarly, patients requiring medication reconciliation also changed. Nurses were performing medication reconciliation for patients with elective surgeries and had a system that worked for that population. Therefore, we did not need to focus efforts for this population around medication reconciliation and could shift our focus more towards medication education.
Optimizing the Delivery of Discharge Prescriptions
The Meds for Home workflow has been updated multiple times since implementation. These changes resulted from early and frequent meetings with nurses, case managers, providers, and the pharmacy team. The Meds for Home service uses an outpatient pharmacy located within the hospital that has high prescription volumes at baseline to fill discharge prescriptions. Due to the volume of out-patient prescriptions and unexpected discharges, delays in prescription delivery occurred. To improve efficiency, a separate workflow and space were designated for filling Meds for Home prescriptions. Initially, MHCs were visiting floors to pick-up and deliver prescriptions at set times (ie, 10 am, 2 pm, and 5 pm). Instead of using set pick-up and delivery times, the Meds for Home service now uses a rolling 2-hour turnaround time during service hours. Additionally, providers, case managers, and units were educated to provide discharge prescriptions, especially those requiring prior authorization, as early as possible to expedite service. By identifying these issues early in the process, we were able to develop a different strategy that worked for the units, providers, and pharmacy.
Lessons Learned
The time of transition from one level of care to another is a vulnerable time for patients, as it is a time when medication-related problems often arise. In an elderly patient population, one study demonstrated that contributing factors for medication discrepancies following hospital discharge included unintended nonadherence and inadequate discharge instructions, and patients experiencing a medication discrepancy were at a significantly higher risk of readmission [4]. Hospital readmissions have also been linked to a lack of adequate follow-up in the outpatient setting [5]. Pharmacists should become more involved in preventing medication-related problems during the times of transition by performing activities such as medication reconciliation, patient education, and assessment of patient outcomes post-discharge [6,7]. Studies have demonstrated that pharmacists are able to reduce medication-related adverse events during and after hospitalization by completing these activities [8–10]. Residency-trained pharmacists are well-equipped to provide these services and are needed to create new processes and models to meet the ever changing demands of health care payers and accrediting bodies. ASHP recommends pharmacists entering into careers in health systems be at least PGY1-trained while the American College of Clinical Pharmacy (ACCP) envisions all pharmacists involved in direct patient care complete residency training [11,12]. Health systems will continue to be challenged with transforming pharmacy models to allow for this influx of highly trained individuals in a time of budget constraints. Below, we describe the lessons we learned while implementing our pharmacy bundle of services and think are essential for other institutions to consider when initiating their own services.
Rethink the Role of the Pharmacist
As health systems acquire smaller hospitals, the role of the pharmacist may need to be redefined and reinvented. The responsibilities of a pharmacist in a large academic hospital may be different than those of a pharmacist with the same skill set in a community hospital. However, despite the difference in practice setting, the same core pharmacy services around medication use can still be deployed. Participation in transitions of care activities is a relatively new concept for many pharmacists as residency training programs traditionally focused on caring for patients within a defined setting such as the intensive care unit or ambulatory care. The pharmacy profession should define the role of the clinical pharmacist in order to make the incorporation of transitions of care responsibilities into job expectations easier for all. The ACCP outlines this need and sets forth recommendations for clinical pharmacists’ responsibilities within the health care team to include assessing patients and medication regimens, developing and implementing medication-related therapy plans, and evaluating clinical outcomes [13]. Pharmacy leadership organizations, including ASHP and ACCP, offer resources providing the vision of pharmacy practice and expectations for which institutions should be reaching. Pharmacy departments should use these resources to complete gap analyses of current processes and those envisioned for the future to help guide efforts for change at their own institutions.
Obtain Support Within Your Institution
Gaining support from hospital leadership for advancing pharmacists’ involvement in patient care is instrumental. Without leadership support at both the institutional and department of pharmacy levels, pharmacists with advanced training may be hindered from practicing at the top of their license. Furthermore, support by leadership of pharmacy residency programs and experiential student learning sites at the institution is also important. Pharmacy residents and students became indispensable in our model and allowed us to expand our reach to more patients. We used residents to cover additional teams that were previously uncovered by a rounding pharmacist and, along with students, provide medication reconciliation, patient education, and follow-up phone calls to more patients. Requiring participation in the pharmacy bundle of services for rotations also allowed us to train these individuals about the value of transitions of care and see the challenges patients face in gaining access to medications. In a survey of academic medical center executives, pharmacy directors, and pharmacists at 8 institutions, residents were noted to add value to the institution through decreasing drug-related errors and drug costs, expansion of clinical services, and enhancing opportunities for research [14].
Support from other disciplines is also essential. Collaborating with other disciplines should occur prior to, during, and beyond implementation. We collaborated with providers, nurses, case managers, social workers and many other disciplines during all phases of the process. Being inclusive during the planning process allowed everyone to understand each other’s role and to provide input on how we could work together to best utilize everyone’s resources. This multidisciplinary approach to developing pharmacy services also allowed an opportunity to collaborate on research and evaluate our processes with other disciplines.
Tracking interventions will demonstrate the value of pharmacists, technicians, and other pharmacy team members participating in these advanced roles. This information will be useful when justifying the practice model to hospital leadership and for recruiting new pharmacists, residents, and technicians to the institution. Additionally, defining both outcome (eg, 30-day readmission rates, HCAHPS scores) and process (eg, number of patient education sessions performed, number of medication discrepancies reconciled) measures upfront is important in order for those involved to understand how their work will be assessed. These data will be useful in determining whether the intervention is making an impact early on and allow for restructuring of the process if not.
Create Depth in Your Team While Engaging Current Resources
We spent a significant amount of time planning the implementation of our pharmacy bundle of services, collaborating with other disciplines, and training our pharmacy team members. We hired highly trained and competent people into new positions and ensured every-one clearly understood their responsibilities. This was a critical step in order to ensure we were providing optimal care to our patients and integrating leaders into our team. We also utilized our current workforce to fill new clinical rounding pharmacist or technician roles. For those pharmacists who had not completed a residency, we required the pharmacists to complete a compact training program similar to that required of our residents [1]. This training ensured that important services were being performed adequately by each rounding pharmacist. Similarly, technicians transitioning from a primarily medication dispensing role to a MHC or medication history role received extensive training to assist with developing their new skill set.
Creating relationships with an outpatient pharmacy is essential to ensure patients are discharged from the hospital on medications they can afford long-term. We are fortunate to have 5 outpatient pharmacies on the JHH campus that are under the Johns Hopkins Health System umbrella, which made collaboration between the inpatient and outpatient teams seamless. However, many hospitals may not be directly affiliated with an outpatient pharmacy with which to collaborate or may contract with a retail chain pharmacy. In the latter case, inpatient and outpatient pharmacies must work together to define roles around transitions of care and how to best serve the patient in a collaborative manner. If no onsite outpatient pharmacy exists, dedicated resources should be acquired to serve as a liaison between the inpatient team and the outpatient pharmacy. These resources may work through issues such as formulary preferences, prior authorization requests, and connecting the patient to the medication either through bedside delivery or filling at the patient’s community pharmacy. Community pharmacies recognize the cost benefit they could gain through 340B pricing and specialty drug dispensing when working in collaboration with healthcare systems. However, health systems must be aware that collaborating with outpatient pharmacy partnerships will create further challenges as providers ensure patient preference for use of a particular pharmacy is honored and cost-sharing is incorporated into models.
Continuously Reevaluate Your Services
As implementation of our pharmacy bundle of services began, meeting early and often was essential to identify issues and adjust our workflow to resolve those issues quickly. When the inpatient component of the pharmacy bundle of services was first implemented, rounding pharmacists and pharmacy leadership initially met on a weekly basis to provide feedback on the practice model and develop resolutions for any issues. However, it is important to also include other disciplines in the evaluation process. For the Meds for Home program, pharmacy leadership not only met with MHCs but also with providers, nurses, case managers, and social workers for feedback on how to improve the service. Although the workflow of our pharmacy bundle of services are more established, evaluations still occur albeit less frequently.
Conclusion
Pharmacists’ involvement in transitions of care should become part of the daily responsibility. Health systems should understand how efforts to expand pharmacists’ interventions align with overall hospital goals. Many hospitals may already have programs in place to help with transitions of care. Pharmacists can help expand current efforts through increased visibility to physicians and patients as well as collaboration with outpatient pharmacies to ensure medications are effective and affordable for patients long-term.
Note: The project described was supported by grant no. 1C1CMS331053-01-00 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Findings may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
Corresponding author: Vi Gilmore, PharmD, BCPS, Clinical Pharmacy Specialist, Internal Medicine, The Johns Hopkins Hospital, 600 N. Wolfe Street, Carnegie 180, Baltimore, MD 21287, vdo1@jhmi.edu.
Financial disclosures: None.
From The Johns Hopkins Hospital, Baltimore, MD.
The project described was supported by grant # 1C1CMS331053-01-00 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Findings may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
Abstract
- Background: Medication management is becoming more complex, with new medications entering the market, drug prices increasing, and patients transferring into and out of the hospital. Transitions of care services are being implemented to prevent readmissions and increase patient satisfaction. Pharmacists play a key role by expanding clinical services provided to patients around medication management.
- Objective: To describe a pharmacy transitions of care model at a large academic teaching hospital and lessons learned during implementation.
- Methods: A pharmacy bundle of services was initially developed in a medical patient population and included medication reconciliation, patient education targeting high-risk medications, post-discharge follow-up phone calls, and bedside discharge prescription delivery. This bundle was expanded to other patient populations through the use of residency-trained pharmacists, pharmacy residents, pharmacy students, and certified pharmacy technicians.
- Results: Challenges were faced when implementing our transitions of care services, including expanding care coordination team coverage with existing resources, training pharmacy staff in new roles, determining the needs of patients cared for by teams we had not previously been integrated into, and creating our discharge prescription delivery program. During this process, we learned to rethink the role of pharmacists on our team, value the support within our institution to create change in order to improve patient care, and continuously evaluate this process.
- Conclusion: We are at an opportune time to expand the scope of the inpatient pharmacist to provide advanced medication-related services to patients. Residency training is creating individuals who will thrive in these new models.
Medication management around the acute care inpatient stay is a challenging but crucial task to ensure patient safety and desired clinical outcomes. The first step in successful medication management is to understand the patient’s medication regimen in the home environment. Patients may take medications differently than prescribed; skip medication doses intentionally to make a supply last longer; use over-the-counter medications, herbal supplements, or someone else’s medication based on the recommendation of family or friends; or discontinue medications based on side effects or media influence. Over the course of the inpatient stay, medication management involves adjusting doses based on changes in organ function, detecting side effects and potential drug interactions, and monitoring clinical outcomes to ensure appropriate drug therapy is being prescribed. As the patient approaches discharge, ensuring the patient understands the indications for his/her medications, has self-monitoring techniques to recognize efficacy or adverse effects, and has access to discharge medications is important. Lastly, long-term medication management includes patient access to pharmaceutical expertise over time. Pharmacists’ involvement in medication therapy management services and patient-centered medical homes is key to ensuring safe and effective medication use over time.
In 2009, the Johns Hopkins Health System Readmission Prevention Task Force developed strategies to reduce preventable readmissions and improve transitions of care. In 2011, a new multidisciplinary approach to patient care was implemented at the Johns Hopkins Hospital (JHH) to optimize care coordination and improve acute care management. Using this approach, care coordination teams composed of physicians, nurses, pharmacists, nurse case managers, social workers, physical/occupational therapists, nutritionists, and home care coordinators meet on a daily basis to discuss the inpatient and discharge care needs of patients in order to improve care transitions and reduce preventable readmissions. In 2012, JHH was awarded a 3-year innovation grant from the Centers for Medicaid & Medicare Services (CMS) that would assist with expansion of these care coordination teams to every unit of the hospital. Prior to implementation of the care coordination model at JHH in 2011, there were 3 pharmacists who consistently rounded on 3 inpatient medicine teams (one pharmacist also had operational responsibilities). Pharmacists were deemed by the task force to be key providers of medication management and, thus, essential members of the care coordination team. Due to an inability to hire a new pharmacist for every care coordination team, the department of pharmacy needed to determine how to provide consistent pharmacist coverage utilizing current resources. This report describes the challenges faced and lessons learned by our adult inpatient pharmacy division when implementing a pharmacy bundle of services to improve care transitions for an adult patient population.
Setting
JHH is an 1192-bed academic teaching hospital located in Baltimore, Maryland. At JHH, the department of pharmacy has 4 inpatient divisions that service the medication needs of different patient populations: medicine, critical care/surgery, oncology, and pediatrics. The adultinpatient pharmacy division covers medicine units in addition to obstetrics, neurology, and surgery units. It is responsible for 486 inpatient beds on 22 units and was the first division to provide the pharmacy bundle of services described below. Currently, 11 rounding and 5 operational pharmacists provide care coordination and order verification support, respectively, for the division during day shift.
Program Overview
Rounding pharmacists on care coordination teams address acute care medication issues and provide a bundle of services that includes targeted patient education, medication reconciliation, post-discharge follow-up phone calls, and discharge prescription planning. The full details of these services have been described [1]. Briefly, patients newly initiated on medications deemed ”high-risk” (eg, anticoagulation, insulin, metered dose inhalers, dual antiplatelets) receive education by a member of the pharmacy team (ie, pharmacist, pharmacy resident, or pharmacy student) prior to discharge. Those patients who receive education are offered a post-discharge follow-up phone call to assess for any questions or issues. Patients who accept this service are contacted 48 to 72 hours post-discharge. Specific patient populations (eg, patients with congestive heart failure, diabetes) are also targeted for completion of medication reconciliation. If patients are being discharged with prescriptions, they are offered our “Meds for Home” service. Patients who accept this service have their prescriptions filled at one of our outpatient pharmacies and delivered to the unit prior to discharge. Highly trained certified pharmacy technicians, called “Meds for Home” coordinators (MHCs; previously known as transitions pharmacist extenders), facilitate this process.
Challenges Faced
Care Coordination Team Coverage
One challenge to implementation of the pharmacy bundle of services was providing consistent team coverage with adequately trained pharmacists. It was not feasible to hire a pharmacist to cover each of the care coordination teams. To address gaps in coverage, we initially utilized postgraduate year 1 and 2 (PGY1 and 2) pharmacy residents on an internal medicine rotation to cover care coordination teams without a rounding pharmacist. However, this method proved unreliable as a pharmacy resident was not scheduled for an internal medicine rotation each month. In the beginning, our division had 3 rounding and 9 operational pharmacists during day shift. To provide sufficient clinical coverage while still adequately addressing order verification needs, a major restructuring of our pharmacy model was necessary. We increased the bed-to-pharmacist ratio for order verification, which allowed the number of operational pharmacists to decrease from 9 to 5. Those 4 remaining pharmacists were now available to serve as rounding pharmacists. Along with hiring 2 additional rounding pharmacists with funding from the CMS innovation grant, we were able to increase the number of care coordination teams consistently covered from 3 to 9.
Although we expanded pharmacist coverage of care coordination teams, time constraints prevented all patients who met criteria for patient education or medication reconciliation to have these services completed in a timely manner or at all by a pharmacist. Our rounding pharmacists’ responsibilities also included participation in high-level activities such as order set reviews for a new provider order entry system, ambulatory clinic time, stewardship activities, and quality improvement projects. In order to increase our scope, we utilized pharmacy technicians, students, and residents to assist with completing these tasks. All pharmacy students and residents on rotation within our division participated in a daily huddle Monday through Friday. Rounding pharmacists whose unit had patient education needs that could not be met by that pharmacist submitted requests by a set time. Those patient education tasks were then divided amongst the pharmacy learners at the huddle for completion. Prior to being allowed to independently counsel patients, pharmacy learners’ patient education skills were evaluated by preceptors. To facilitate timely completion of medication histories, technicians were hired. These medication history technicians are available Monday through Friday to complete medication histories for patients admitted to specific medicine units, ideally within 24 hours of admission. Rounding pharmacists are notified of completion of medication histories via our electronic medical record and reconcile that list with the patient’s inpatient medication list. Any clinically relevant discrepancies are communicated to providers. Pharmacy learners may also collect medication histories.
Training Rounding Pharmacists
Another challenge we faced was providing adequate training for operational pharmacists transitioning to a rounding position. Residency training is crucial in providing the level of skill necessary to identify complex drug therapy problems, adjust treatment regimens, and create plans where limited data exist to drive drug therapy recommendations [2,3]. Rotations during the final year of pharmacy school provide exposure to interacting with patients and providers. Completion of PGY1 residency training allows a pharmacist to practice as a generalist with a broad range of experiences provided during the year to identify medication-related problems. PGY2 residency training allows the pharmacist to spend a concentrated year in the chosen area of expertise and gain a deeper knowledge of medication use in a specific patient population or area of practice [2]. After 2 years of clinical residency training, pharmacists have the skills to interact with patients and multidisciplinary teams to optimize medication regimens, provide medication education, and measure the value they bring to the health care of patients.
Some of the operational pharmacists who were transitioning to the rounding pharmacist role had no training beyond pharmacy school or had only completed a PGY1 pharmacy residency. Initially, training for this new role lasted only a few days and consisted of orientation to the unit and observation of care coordination rounds. We learned that this brief amount of training was insufficient, even for those with PGY1 pharmacy residency training. In order to ensure that these rounding pharmacists could successfully provide the bundle of services and meet the high clinical demands of the inpatient service, we developed a comprehensive training program. Those interested in transitioning from an operational to a rounding pharmacist role must now complete a 6-week training program. The first 2 weeks consists of improving patient education and medication history skills. The remaining 4 weeks are spent honing clinical rounding skills. Rounding pharmacists-in-training also receive a formal review of their performance utilizing an evaluation form developed by the American Society of Health-System Pharmacists (ASHP) for pharmacy residents.
Establishing a Pharmacy Bundle and the Role of a Rounding Pharmacist on New Units
Some of the units implementing care coordination teams, such as neurology, did not previously have a pharmacist rounding on those units. Furthermore, these units had a high patient census (eg, 60 patients), which made it difficult for one pharmacist to clinically evaluate every patient. Multiple specialty teams also admitted patients to a single unit, which made it challenging for the pharmacist to develop strong working relationships with providers. As such, rounding pharmacists deployed to those units had difficulty establishing their role on the team, especially for those pharmacists without or with only 1 year of postgraduate training. To address this issue, a PGY2-trained pharmacist rounded on the unit to assess which areas/teams had the greatest need for a pharmacist. Completing this needs assessment on these units allowed for the rounding pharmacist to more effectively use his/her time. It also allowed for a smoother transition from operational to rounding pharmacist by removing the burden of establishing a brand new role and identifying necessary tasks to be completed throughout the day.
We also discovered on these new care coordination units that our patient criteria for education and medication reconciliation were not universal. We developed and initiated our pharmacy bundle of services in a medical patient population. As we expanded these services to other patient care areas, the targeted list of medications/conditions changed. For example, surgical patients had a greater need for education around opioid therapy and complex bowel regimens while neurology patients needed education regarding antiepileptic regimens. Similarly, patients requiring medication reconciliation also changed. Nurses were performing medication reconciliation for patients with elective surgeries and had a system that worked for that population. Therefore, we did not need to focus efforts for this population around medication reconciliation and could shift our focus more towards medication education.
Optimizing the Delivery of Discharge Prescriptions
The Meds for Home workflow has been updated multiple times since implementation. These changes resulted from early and frequent meetings with nurses, case managers, providers, and the pharmacy team. The Meds for Home service uses an outpatient pharmacy located within the hospital that has high prescription volumes at baseline to fill discharge prescriptions. Due to the volume of out-patient prescriptions and unexpected discharges, delays in prescription delivery occurred. To improve efficiency, a separate workflow and space were designated for filling Meds for Home prescriptions. Initially, MHCs were visiting floors to pick-up and deliver prescriptions at set times (ie, 10 am, 2 pm, and 5 pm). Instead of using set pick-up and delivery times, the Meds for Home service now uses a rolling 2-hour turnaround time during service hours. Additionally, providers, case managers, and units were educated to provide discharge prescriptions, especially those requiring prior authorization, as early as possible to expedite service. By identifying these issues early in the process, we were able to develop a different strategy that worked for the units, providers, and pharmacy.
Lessons Learned
The time of transition from one level of care to another is a vulnerable time for patients, as it is a time when medication-related problems often arise. In an elderly patient population, one study demonstrated that contributing factors for medication discrepancies following hospital discharge included unintended nonadherence and inadequate discharge instructions, and patients experiencing a medication discrepancy were at a significantly higher risk of readmission [4]. Hospital readmissions have also been linked to a lack of adequate follow-up in the outpatient setting [5]. Pharmacists should become more involved in preventing medication-related problems during the times of transition by performing activities such as medication reconciliation, patient education, and assessment of patient outcomes post-discharge [6,7]. Studies have demonstrated that pharmacists are able to reduce medication-related adverse events during and after hospitalization by completing these activities [8–10]. Residency-trained pharmacists are well-equipped to provide these services and are needed to create new processes and models to meet the ever changing demands of health care payers and accrediting bodies. ASHP recommends pharmacists entering into careers in health systems be at least PGY1-trained while the American College of Clinical Pharmacy (ACCP) envisions all pharmacists involved in direct patient care complete residency training [11,12]. Health systems will continue to be challenged with transforming pharmacy models to allow for this influx of highly trained individuals in a time of budget constraints. Below, we describe the lessons we learned while implementing our pharmacy bundle of services and think are essential for other institutions to consider when initiating their own services.
Rethink the Role of the Pharmacist
As health systems acquire smaller hospitals, the role of the pharmacist may need to be redefined and reinvented. The responsibilities of a pharmacist in a large academic hospital may be different than those of a pharmacist with the same skill set in a community hospital. However, despite the difference in practice setting, the same core pharmacy services around medication use can still be deployed. Participation in transitions of care activities is a relatively new concept for many pharmacists as residency training programs traditionally focused on caring for patients within a defined setting such as the intensive care unit or ambulatory care. The pharmacy profession should define the role of the clinical pharmacist in order to make the incorporation of transitions of care responsibilities into job expectations easier for all. The ACCP outlines this need and sets forth recommendations for clinical pharmacists’ responsibilities within the health care team to include assessing patients and medication regimens, developing and implementing medication-related therapy plans, and evaluating clinical outcomes [13]. Pharmacy leadership organizations, including ASHP and ACCP, offer resources providing the vision of pharmacy practice and expectations for which institutions should be reaching. Pharmacy departments should use these resources to complete gap analyses of current processes and those envisioned for the future to help guide efforts for change at their own institutions.
Obtain Support Within Your Institution
Gaining support from hospital leadership for advancing pharmacists’ involvement in patient care is instrumental. Without leadership support at both the institutional and department of pharmacy levels, pharmacists with advanced training may be hindered from practicing at the top of their license. Furthermore, support by leadership of pharmacy residency programs and experiential student learning sites at the institution is also important. Pharmacy residents and students became indispensable in our model and allowed us to expand our reach to more patients. We used residents to cover additional teams that were previously uncovered by a rounding pharmacist and, along with students, provide medication reconciliation, patient education, and follow-up phone calls to more patients. Requiring participation in the pharmacy bundle of services for rotations also allowed us to train these individuals about the value of transitions of care and see the challenges patients face in gaining access to medications. In a survey of academic medical center executives, pharmacy directors, and pharmacists at 8 institutions, residents were noted to add value to the institution through decreasing drug-related errors and drug costs, expansion of clinical services, and enhancing opportunities for research [14].
Support from other disciplines is also essential. Collaborating with other disciplines should occur prior to, during, and beyond implementation. We collaborated with providers, nurses, case managers, social workers and many other disciplines during all phases of the process. Being inclusive during the planning process allowed everyone to understand each other’s role and to provide input on how we could work together to best utilize everyone’s resources. This multidisciplinary approach to developing pharmacy services also allowed an opportunity to collaborate on research and evaluate our processes with other disciplines.
Tracking interventions will demonstrate the value of pharmacists, technicians, and other pharmacy team members participating in these advanced roles. This information will be useful when justifying the practice model to hospital leadership and for recruiting new pharmacists, residents, and technicians to the institution. Additionally, defining both outcome (eg, 30-day readmission rates, HCAHPS scores) and process (eg, number of patient education sessions performed, number of medication discrepancies reconciled) measures upfront is important in order for those involved to understand how their work will be assessed. These data will be useful in determining whether the intervention is making an impact early on and allow for restructuring of the process if not.
Create Depth in Your Team While Engaging Current Resources
We spent a significant amount of time planning the implementation of our pharmacy bundle of services, collaborating with other disciplines, and training our pharmacy team members. We hired highly trained and competent people into new positions and ensured every-one clearly understood their responsibilities. This was a critical step in order to ensure we were providing optimal care to our patients and integrating leaders into our team. We also utilized our current workforce to fill new clinical rounding pharmacist or technician roles. For those pharmacists who had not completed a residency, we required the pharmacists to complete a compact training program similar to that required of our residents [1]. This training ensured that important services were being performed adequately by each rounding pharmacist. Similarly, technicians transitioning from a primarily medication dispensing role to a MHC or medication history role received extensive training to assist with developing their new skill set.
Creating relationships with an outpatient pharmacy is essential to ensure patients are discharged from the hospital on medications they can afford long-term. We are fortunate to have 5 outpatient pharmacies on the JHH campus that are under the Johns Hopkins Health System umbrella, which made collaboration between the inpatient and outpatient teams seamless. However, many hospitals may not be directly affiliated with an outpatient pharmacy with which to collaborate or may contract with a retail chain pharmacy. In the latter case, inpatient and outpatient pharmacies must work together to define roles around transitions of care and how to best serve the patient in a collaborative manner. If no onsite outpatient pharmacy exists, dedicated resources should be acquired to serve as a liaison between the inpatient team and the outpatient pharmacy. These resources may work through issues such as formulary preferences, prior authorization requests, and connecting the patient to the medication either through bedside delivery or filling at the patient’s community pharmacy. Community pharmacies recognize the cost benefit they could gain through 340B pricing and specialty drug dispensing when working in collaboration with healthcare systems. However, health systems must be aware that collaborating with outpatient pharmacy partnerships will create further challenges as providers ensure patient preference for use of a particular pharmacy is honored and cost-sharing is incorporated into models.
Continuously Reevaluate Your Services
As implementation of our pharmacy bundle of services began, meeting early and often was essential to identify issues and adjust our workflow to resolve those issues quickly. When the inpatient component of the pharmacy bundle of services was first implemented, rounding pharmacists and pharmacy leadership initially met on a weekly basis to provide feedback on the practice model and develop resolutions for any issues. However, it is important to also include other disciplines in the evaluation process. For the Meds for Home program, pharmacy leadership not only met with MHCs but also with providers, nurses, case managers, and social workers for feedback on how to improve the service. Although the workflow of our pharmacy bundle of services are more established, evaluations still occur albeit less frequently.
Conclusion
Pharmacists’ involvement in transitions of care should become part of the daily responsibility. Health systems should understand how efforts to expand pharmacists’ interventions align with overall hospital goals. Many hospitals may already have programs in place to help with transitions of care. Pharmacists can help expand current efforts through increased visibility to physicians and patients as well as collaboration with outpatient pharmacies to ensure medications are effective and affordable for patients long-term.
Note: The project described was supported by grant no. 1C1CMS331053-01-00 from the U.S. Department of Health and Human Services, Centers for Medicare & Medicaid Services. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Department of Health and Human Services or any of its agencies. The research presented was conducted by the awardee. Findings may or may not be consistent with or confirmed by the findings of the independent evaluation contractor.
Corresponding author: Vi Gilmore, PharmD, BCPS, Clinical Pharmacy Specialist, Internal Medicine, The Johns Hopkins Hospital, 600 N. Wolfe Street, Carnegie 180, Baltimore, MD 21287, vdo1@jhmi.edu.
Financial disclosures: None.
1. Gilmore V, Efird L, Fu D, et al. Implementation of transitions-of-care services through acute care and outpatient pharmacy collaboration. Am J Health Syst Pharm 2015;72:737–44.
2. American College of Clinical Pharmacy, Burke JM, Miller WA, et al. Clinical pharmacist competencies. Pharmacotherapy 2008;28:806–15.
3. American Society of Health-System Pharmacists. ASHP accreditation standard for postgraduate year one (PGY1) pharmacy residency programs. Available at www.ashp.org/DocLibrary/Accreditation/Newly-approved-PGY1-Standard-September-2014.pdf.
4. Coleman EA, Smith JD, Raha D, Min S. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005:165:1842–7.
5. Jackson C, Shahsahebi M, Wedlake T, DuBard C. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med 2015;13:115–22.
6. American College of Clinical Pharmacy, Hume AL, Kirwin J, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy 2012;32:e326–37.
7. Cobaugh DJ, Amin A, Bookwalter T, et al. ASHP-SHM joint statement on hospitalist-pharmacist collaboration. Am J Health Syst Pharm 2008;65:260–3.
8. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166:565–71.
9. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization. Ann Intern Med 2009;150:178–87.
10. Murphy EM, Oxencis CJ, Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm 2009;66:2126–31.
11. Myers CE. ASHP Health-System Pharmacy 2015 Initiative. Am J Health-Syst Pharm 2004;61:657.
12. Murphy JE, Nappi JM, Bosso JA, et al. ACCP position statement. American College of Clinical Pharmacy’s vision of the future: postgraduate pharmacy residency training as a prerequisite for direct patient care. Pharmacotherapy 2006;26:
722–33.
13. Harris IM, Phillips B, Boyce E, et al. Clinical pharmacy should adopt a consistent process of direct patient care. Pharmacotherapy 2014;34:e133–48.
14. Fuller PD, Smith KM, Hinman RK, et al. Value of pharmacy residency training: a survey of the academic medical center perspective. Am J Health Syst Pharm 2012;69:158–65.
1. Gilmore V, Efird L, Fu D, et al. Implementation of transitions-of-care services through acute care and outpatient pharmacy collaboration. Am J Health Syst Pharm 2015;72:737–44.
2. American College of Clinical Pharmacy, Burke JM, Miller WA, et al. Clinical pharmacist competencies. Pharmacotherapy 2008;28:806–15.
3. American Society of Health-System Pharmacists. ASHP accreditation standard for postgraduate year one (PGY1) pharmacy residency programs. Available at www.ashp.org/DocLibrary/Accreditation/Newly-approved-PGY1-Standard-September-2014.pdf.
4. Coleman EA, Smith JD, Raha D, Min S. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med 2005:165:1842–7.
5. Jackson C, Shahsahebi M, Wedlake T, DuBard C. Timeliness of outpatient follow-up: an evidence-based approach for planning after hospital discharge. Ann Fam Med 2015;13:115–22.
6. American College of Clinical Pharmacy, Hume AL, Kirwin J, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy 2012;32:e326–37.
7. Cobaugh DJ, Amin A, Bookwalter T, et al. ASHP-SHM joint statement on hospitalist-pharmacist collaboration. Am J Health Syst Pharm 2008;65:260–3.
8. Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006;166:565–71.
9. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization. Ann Intern Med 2009;150:178–87.
10. Murphy EM, Oxencis CJ, Klauck JA, et al. Medication reconciliation at an academic medical center: implementation of a comprehensive program from admission to discharge. Am J Health Syst Pharm 2009;66:2126–31.
11. Myers CE. ASHP Health-System Pharmacy 2015 Initiative. Am J Health-Syst Pharm 2004;61:657.
12. Murphy JE, Nappi JM, Bosso JA, et al. ACCP position statement. American College of Clinical Pharmacy’s vision of the future: postgraduate pharmacy residency training as a prerequisite for direct patient care. Pharmacotherapy 2006;26:
722–33.
13. Harris IM, Phillips B, Boyce E, et al. Clinical pharmacy should adopt a consistent process of direct patient care. Pharmacotherapy 2014;34:e133–48.
14. Fuller PD, Smith KM, Hinman RK, et al. Value of pharmacy residency training: a survey of the academic medical center perspective. Am J Health Syst Pharm 2012;69:158–65.
How to Manage Family-Centered Rounds
From the Department of Pediatrics, George Washington University and Children’s National Medical Center, Washington, DC (Dr. Kern), the Department of Pediatrics, Children’s Hospital Los Angeles and University of Southern California Keck School of Medicine, Los Angeles, CA (Dr. Gay), and the Department of Pediatrics, University of Texas Southwestern Medical Center and Children’s Health System, Dallas, TX (Dr. Mittal).
Abstract
- Objective: To present a model for operationalizing successful family-centered rounds (FCRs).
- Methods: Literature review and experience with leading FCR workshops at national meetings.
- Results: FCRs are multidisciplinary rounds that involve patients and families in decision-making. The model has gained substantial momentum nationally and is widely practiced in US pediatric hospitals. Many quality improvement–related FCR benefits have been identified, including improved parental satisfaction, communication, team-based practice, incorporation of practice guidelines, prevention of medication errors, and improved trainee and staff education and satisfaction. Physical and time constraints, variability in attending FCR style and teaching style, lack of FCR structure and process, specific and sensitive patient conditions, and language barriers are key challenges to implementing FCRs. Operationalizing a successful FCR program requires key stakeholders developing and defining a FCR process and structure, including developing a strong faculty development program.
- Conclusion: FCR benefits for a health care system are many. Key stakeholders involvement, developing FCR "ground rules," troubleshooting FCR barriers, and developing a strong faculty development program are key to managing successful FCRs.
The practice of medicine is a team sport and no team is complete without the patient and family being an integral part of it. Over the past 15 years, health care and the practice of medicine has slowly moved away from physician-centered care to patient- and family-centered care (FCC). This change has been a gradual shift in our culture and FCC has become a widely adopted philosophy within the US health care system [1]. FCC has been recognized and embraced by numerous medical and professional societies, including the Institute of Medicine (IOM), the American Academy of Pediatrics (AAP), and family advocacy organizations such as Family Voices and the Institute for Patient- and Family-Centered Care [1,2]. At its most basic, “family-centered care” occurs when patients/families and medical providers partner together to formulate medical plans that are built upon the sharing of open and unbiased information and that account for the diversity and individual strengths and needs of each patient and family unit [3]. FCC in the inpatient setting for hospitalized patients is most exemplified by the practice of family-centered (bedside) rounds, or FCRs [1].
Interestingly, FCC as a philosophy of care developed during a time when bedside rounds, and by extension clinical teaching, moved away from the bedside. Rounds are an integral part of how work is done in the inpatient setting. They come in many different flavors, from “pre-rounds” to “card-flip rounds” to “attending rounds,” “table/conference room rounds,” “hallway rounds,” “bedside rounds,” and the aforementioned family-centered rounds. In the first half of the 20th century,the majority of teaching rounds took place at the patient’s bedside, in the model advocated by Sir William Osler [4]. Indeed, as Dr. Osler wrote in 1903, “there should be no teaching without a patient for a text, and the best teaching is that taught by the patient himself” [5]. By the late 1970s through the mid-1990s, however, the proportion of clinical teaching occurring at the bedside had decreased to as low as 16% [6–8]. Many reasons behind the change have been speculated, including faculty comfort with lecture-based teaching and desire to control the content of teaching discussions, as well as technological advancement necessitating access to computers during case review.
In contrast, the patient-and family-centered movement began in the mid-20th century as a response to the separation trauma experienced by hospitalized children and their families [9]. Hospitals responded by liberalizing their visiting policies and encouraging direct care-giving by parents. FCC was further bolstered by consumer-led movements in the 1960s and 1970s, and by federal legislation in the 1980s targeting children with special health care needs. FCC gained national recognition in 2001 when the Institute of Medicine emphasized that involving patients and families in health care decisions increased the quality of their care [2]. Subsequently, the AAP endorsed FCC as a guiding approach to pediatric care in their 2003 report “Family-centered care and the pediatrician’s role” [1]. As part of this report, the AAP recommended that bedside presentations with active engagement of families become the standard of care. FCRs developed at several children’s hospitals in the US in the following years, with the first conceptual model of FCR published by Muething et al in 2007 [10].
Definition of Family-Centered Rounds
While no consensus definition of FCR exists, the most frequently cited description comes from Sisterhen et al who describe FCR as “interdisciplinary work rounds at the bedside in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself” [11]. Three key features should be noted in this definition. First, FCR requires the active participation of family members, not merely their presence. In this way, patient and family voices are heard and their preferences solicited with respect to clinical decision-making. Second, FCR take place at the bedside, in alignment with the 2003 AAP policy statement that standard practice should be to conduct attending rounds with full case presentations in patient rooms in the presence of family. Third, FCR are typically interdisciplinary, involving patients and their families, physicians and trainees, nurses, and other ancillary staff (such as interpreters, case managers, and pharmacists) [1,10,11,12].
Since the IOM report, FCRs have gained substantial national momentum. A PRIS (Pediatric Research in Inpatient Setting) network study in 2010 published the first survey of pediatric hospitalist rounding practices in the US and Canada [12]. The study reported that 44% of pediatric hospitalists conducted FCRs, and about a quarter conducted rounds as hallway rounds or sit down rounds. Academic hospitalists were significantly more likely to conduct FCRs compared with non-academic (48% vs. 31%; P < 0.05) hospitalists. In accordance with Muething et al’s experience with FCRs in the Cincinnati model, the survey respondents did not associate FCR with prolonged rounding duration [10,12]. FCRs were also associated with greater bedside nurse participation [12]. Given the momentum behind FCC and the oft-cited benefits of FCR, it can only be presumed that the number of pediatric hospitals conducting FCR has significantly increased since the PRIS study was published in 2010.
FCRs Can Improve Quality of Care for Hospitalized Children
FCRs bring together multiple stakeholders involved in the patient’s care in the same place at the same time everyday. This allows for shared-decision making, identification of medical teams by families, and allows for direct and open communication between parents and medical teams [1,10–12]. The key stakeholders on a FCR team include the patient and family members and the medical team. The medical team includes attending physician, fellow, resident, and students, bedside nurse, care coordinator/case manager and other ancillary services. Although not enough data is available on who should attend rounds, case mangers and bedside nurse along with medical team and patients and families were found to be crucial in the general inpatient setting [12].
Integrating FCRs into the daily workflow in the inpatient setting provides several benefits for patients and families and the medical team, including trainees. Improvements in family-centered care principles, parental satisfaction, interdisciplinary team communication, efficiency, patient safety, and resident and medical student education have been reported consistently [9–23].
FCR Benefits for Patients and Families
Muething et al described increased patient-family satis-faction with higher levels of family participation in rounds and earlier discharge times [10]. On FCRs, families report having the opportunity to communicate directly with the entire care team, clarify misinformation and better understand care plans including discharge goals, leading to higher levels family satisfaction [10,14,24]. Both English and limited-English-proficient families report positive experiences with FCRs [21–23]. Families express appreciation with learning opportunities on FCRs, as well as the opportunity to serve as teachers to the medical team [14,16,21]. Families reported comfort with trainees being on rounds and appreciated seeing the medical personnel working as team [21]. They also report trust, comfort, and accountability towards the system and providers as they saw them working together as teams. They felt respected and involved as the medical teams involved them during rounds. Parents also report comfort with diversity of providers and feel that having multidisciplinary and diverse teams help with cultural competencies. Parents appreciated trainees being led by attending physician and felt that attending FCRs made them understand the medical process and the steps involved in caring for their child. They also reported that attending FCRs helps trainees learn about answering the kind of questions that parents usually ask. Contrary to the popular belief, parental participation has not increased the duration of FCRs and parental presence during rounds decreases time spent discussing each patient [14,25].
FCRs and Staff Satisfaction
Staff satisfaction with FCRs has been consistently high [13,14,18–23]. Nursing and medical staffs report valuing FCRs as they foster a sense of teamwork, improve understanding of the patient’s care plan and enhance communication between the care team and families [14]. FCRs significantly increase bedside nurse participation during rounds [12]. Presence of nursing and ancillary staff on FCRs improves efficiency by providing valuable information and helping address discharge goal [10]. Anecdotal data suggests that FCRs reduces number of pages trainees receive from nurses.
FCRs and Outcomes
FCRs have been perceived to improve in patient safety including errors in history taking and miscommunication, and incorrect information; and promote medication reconciliation, safety and adherence [17,20,21]. FCRs have shown to improve patient satisfaction, communication, and coordination of care and trainee education [10,14,21].
Educational Benefits of FCRs
families (Table 1) [26].
FCR Benefits for Hospitals and Health Care Systems
As health care prepares to fully adopt reforms and shift from volume-based to value-based payment systems, creating value in every patient encounter is vital. Conducting daily FCRs provide an dynamic venue for hospitals where daily rounds can incorporate evidence-based practice guidelines, prevent medication errors, ensure safety, reduce unnecessary tests and treatments, and improve transparency and accountability in care. This model can help hospital financially by meeting key quality and safety metrics and also help provide cost effective care through use and reinforcement of clinical pathways during rounds.
FCR Barriers
While many hospitals have adopted FCRs, many barriers to FCR implementation exist [10–14,18–23] (Table 1). Understanding these barriers and overcoming them are crucial for successful implementation. Conducting FCRs involve many aspects of care that happen during rounds. These include discussions about history, physical examinations, labs, and other tests; clinical decision-making and communication between parents and providers; team communication; teaching of trainees; discharge planning; and coordination of care [20]. Given all these aspects of care involved during rounds, being able to conduct multidisciplinary rounds in a timely and efficient way can be a challenge in a busy and dynamic inpatient setting.
Key identified FCR barriers have included physical constraints such as small patient rooms, large team size, patients being on multiple floors or units, infection control precautions leading to increased time involved with teams gowning and gloving; lack of training on FCRs for trainees and faculty; language and cultural barriers; family/patient concerns of privacy/disclosure of sensitive information; trainee’s fears of not appearing knowledgeable in front of families; and variability in attending physicians’ teaching style and approach to FCR [10–15,21].
Operationalizing Successful FCRs
Forming FCR Steering Committee: Developing Ground Rules
While there are many barriers to conducting efficient FCRs there are some that are unique to each institution. Therefore, for those institutions planning to initiate FCRs, the first step might be to form a FCR steering committee of key stakeholders who could review the current state, do a needs assessment for initiating FCRs, develop a structured and standardized FCR process and revise the FCR process periodically to meet the needs of the dynamic inpatient setting [10,12,14].
Defining and Identifying the FCR Process: Who, Where, and When of FCRs
The steering committee should clearly define FCRs and identify what FCRs would involve. For example, should FCRs involve complete case presentations and discussion in front of the parent or focused relevent H&P in a language that the parent understands? The steering committee should identify key elements/aspects of FCRs that would happen on daily rounds. For example: how should each patient receive information about FCRs? Should FCRs be offered to all patients? Do patients have options to opt-in or opt-out of FCRs on a daily basis or a one-time basis? Who should attend FCRs? For example, other than medical team, the bedside nurse and case manager should attend FCRs on a general pediatric service. Should the team round based on nursing assignments or resident assignments or in the order of room numbers? What should a typical rounding encounter involve? For example, each encounter should begin with the intern knocking on the door, asking parental permission for FCR team to enter the room, who should present, who should lead the rounds (the senior resident or the attending), who should stand where in the room? What should each encounter involve—for example, case presentation and discussion, parental involvement in decision-making, clarification of any parental questions, plan for that day, criteria for discharge and discharge needs assessment, teaching of resident and students, use of lay language etc. How should each rounding encounter end? Should the intern ask if parents have additional questions? It is important that the steering committee clearly identify these minute rounding details. Additionally, the committee should identify the rounding wards/area, the timing and duration of FCRs, how information about FCRs will be shared with patients and families, how trainees and attendees will be educated about FCRs and when are FCRs appropriate and when not. Defining the process early through stakeholder identification can reduce variability and create some standardization yet allow for individual style variations within the constraints of standardization. This will help reduced attending variability, which was cited as the most common FCR barrier by trainees.
As Seltz et al described, Latino families reported positive experiences with FCRs when a Spanish-speaking provider was involved. However, they report less satisfaction with telephone interpreters and did not feel empowered at times on FCRs due to language differences [23]. Addressing the language needs based on demographics and cultural needs will promote greater acceptance of FCRs [23].
Identifying and Defining Trainee Role
Participating in the FCR can create anxiety for medical students and residents. Therefore, educating them about the FCR process and structure beforehand and clearly defining roles can help them conceptualize their roles and expectation and ease their anxiety with FCRs. This will require the steering committee to collaboratively discuss how each encounter would look during FCR from a trainee’s perspective. Who will present the case? The third- year medical student versus the fourth-year medical student or the intern or based on case allocations? How should the case be presented? Should it be short and pointed presentation versus complete history and physical examination on each patient? How long should an encounter last on a new patient and on a follow-up patient? Who will examine the patient? The student who is presenting the case, the attending, the intern who overlooks the student, or the senior resident? Who will answer the follow-up questions from a parent initially? Should the senior resident lead the team under the attending guidance? How will the senior resident be prepared for morning rounds? Using lay language when talking to parents should be encouraged and taught to trainees routinely during FCRs.
Identifying and Defining Clinical Teaching Styles
Faculty Development Program and Importance of “Safe Environment”
Developing an educational program to train faculty, trainee and staff about FCRs can help streamline FCRs. Conducting FCRs is a cultural change and focusing on early adopters is crucial. Muething et al’s model showed better acceptance of FCRs by interns than by senior residents. Being patient during change management is key to successful implementation. Anecdotal discussions during PAS workshops suggests that on an average programs have required 3 years to get significant buy-in and streamlining of FCRs [10,12].
Suboptimal attending behavior such as attending variability in the FCRs process and teaching strategies have been reported as FCR barriers [14,21]. Residents report attending physician as an important factor determining success of FCRs. As attending physicians typically are the leaders of the FCRs team, training faculty about conducting effective and efficient FCRs is crucial to successful FCRs. [12,21]. Key aspects of faculty development should include: (1) education about the FCR standard process for the institution, (2) importance of time management during rounds, including tips and strategies to be efficient, (3) teaching styles during FCRs, including demonstrating role modeling, and (4) direct observation of trainees and individual and team feedback to streamline FCRs. Role-plays or simulated FCRs might be a venue to explore for faculty development on FCRs [14,21].
Creating a “safe environment” during FCRs where each person feels comfortable and secure is vital to team work [7,12,21]. Often trainees are apprehensive or afraid due to medical hierarchy and this might prevent developing a teaching and learning environment. Trainees fear not appearing knowledgeable in front of families and student rotate too often to adapt to different attending styles [21]. Therefore, reassuring trainees that the goal of FCRs is to conduct daily inpatient rounding to ensure key aspects of FCRs are met without disrespecting and insulting any person on rounds and clarifying and reassuring trainees that their fear of not appearing knowledgeable is real and it will be respected, might help create a safe environment where FCR teams are not only conducting the daily ritual of inpatient rounding, and teaching but also ensuring that trainees are enjoying being the clinician and physicians that they want to be. Therefore, attending role modeling is crucial and it is no surprise that in multiple studies variability in attending rounding and teaching style was identified consistently as a FCR barrier.
Preparing for Daily FCRS: Team Work, Efficiency, and Time Management
Conducting daily timely and efficient rounds require daily preparation by teams. Prior to FCRs, teams should know about all of the patients on whom FCRs will be conducted including those who refused FCRs, if any. This can be done via a pre-round or card-flip rounding method where the teams discuss key diagnoses, indication for admission, and identify any outliers to conducting FCRs such as sensitive patient condition, patients refused FCRs, etc. Some institutions have incorporated these at “morning check out” or at morning “huddles.” These help faculty avoid any last minute surprises during rounds and helps with time management during FCRs [12]. Faculty can then plan on some anticipated “teaching moments” before rounds to keep the rounds flowing, for example, a physical exam finding, a clarifying history that can clinch a diagnoses, a clinical pearl, a complex medical case where the parent might share their story and knowledge, an interesting interpretation of a lab, an x-ray or MRI finding. Faculties are multitasking during FCRs by diagnosing and managing patient and learners and leading effective efficient and timely rounds where parental questions are answered, orders are written, to-do work is identified, discharge planning and care coordination is done and trainees stay focused and attend noon conference on time. This requires thoughtful planning before starting FCRs. Time management and managing priorities is key to positive team experiences of FCRs. Both starting and ending FCRs on time should be emphasized and reinforced continually.
Nurse Preparation for FCRs
Nurses are the frontline providers and educating them about FCRs process can help them better explain FCRs to patients and families. Nurses often know the minute details such as timing of an MRI, if the patient has vomited in the morning, or when the parents are coming, etc. This important information sharing during FCRs can help team prepare for the day and provide patients and families’ expectations for the day. Nursing participation can also enhance their knowledge about the thought process behind decisions and care plans and avoid additional time paging house staff to obtain clarification [12–15,21].
Trainee Preparation for FCRs
While pediatric residents do report that FCRs leads to fewer requests for clarifications from families and nurses after FCRs, many still harbor concerns about the time required for FCRs and the overall efficiency of rounds [14]. Educating trainees about the FCR process and explaining why FCRs are beneficial can help alleviate trainee anxiety around FCRs. Involving trainees in the FCR communication and creating a safe and nurturing environment during FCRs can further reduce trainee anxiety [21]. Parents who have attended FCRs with trainees report understanding that trainees are in training and that they have felt comfortable to see attending physician lead the trainees.
FCRs and Technology
Use of technology during FCRs can be helpful to write orders in real time, follow-up and share lab values and or imaging study with parents or teach students. The increasing use of technology on FCRs, such as computers and handheld devices, can help with rounding and teaching; however, it also has the potential to be a distractor and requires that the medical team remain vigilant that the patient and family are the focus of FCRs [26].
Efficiency Pearls
Certain strategies can be utilized to keep FCRs efficient:
- Orient the FCR team about FCR process
- Identify rounding sequence for the day so team can move efficiently between rooms. Identifying potential discharges for the following morning and discharging those patients before rounds can reduce rounding census and provide additional rounding time. Teams can identify approximate time spent in each room based on census, as rounding time is constant.
- Starting and ending FCRs at the allocated time is key to success of FCRs. Sometimes this might require the attending and senior resident splitting the last 1–2 patients to finish rounds on time.
- Prepare students and interns for effective and efficient yet complete presentations during rounds that reflect their knowledge and thought process rather than presenting the entire H&P.
- Keep teaching during rounds focused. As a resident reported, “attendings should keep it short and not go off on a half hour lecture during FCRs. On FCRs I want to hear bam…bam…bam! tidbits, little hints, clinical pearls. Things that you would not know and only see and know when you were there in the room [21].”
- Encourage and teach senior residents’ role as a leader and teacher [21].
- With a situation requiring more time talking to families, request to go back later in the afternoon so as to stay on track on FCR time.
- Faculty can review lab results and history and physical findings on new admissions before rounds to avoid surprises during FCRs and to save time. This can be done during pre-round/card flip/or morning huddle.
Limitations
This article is based on the authors’ review of literature, experience in conducting FCRs, and experience from leading and attending FCR-related workshops at annual pediatric academic societies’ meetings and annual pediatric hospital medicine meetings between 2010 and 2015. There are several limitations to this work. Firstly, the majority of FCR literature is based on perceptions and are not measured outcomes. In addition, how FCRs will apply on services with complex patients needs more study. Different institutions have different physical constraints as well as sociodemographic and cultural factors that might affect FCRs. Daily census among hospitals varies and rounding duration may vary for them.
Conclusion
Family-centered rounds are widely accepted among pediatric hospitalists in the US. Reported benefits of FCRs include improved parent satisfaction, communication, better team communication, improved patient safety and better education for trainees. Many barriers to efficient FCRs exist, and for programs planning to incorporate FCRs in their daily rounds it is crucial to understand FCR benefits and barriers and assess their current state, including physical environment, when planning FCRs. Having a period to plan for FCR implementation through key stakeholder involvement helps define FCR process and lay down a conceptual model suited to individual organization. Educating the team members including families about FCRs and developing a strong faculty development program can further strengthen FCR implementation. Special focus should be given to time management, teaching styles during FCRs, and creating a safe and nurturing environment for FCRs to succeed.
Corresponding author: Vineeta Mittal, MD, MBA, 1935 Medical District Dr., Dallas, TX 75235, vineeta.mittal@childrens.com.
1. American Academy of Pediatrics Committee on Hospital Care. Family-centered care and the pediatrician’s role. Pediatrics 2003;112:691–7.
2. Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001.
3. Kuo DZ, Joutrow AJ, Arango P, et al. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J 2012;16:297–305.
4. Reichsman F, Browning FE, Hinshaw JR. Observations of undergraduate clinical teaching in action. J Med Educ 1964;39:147–63.
5. Osler W. On the need of a radical reform in our methods of teaching senior students. Med News 1903;82:49–53.
6. Collins GF, Cassie JM, Dagget CJ. The role of the attending physician in clinical training. J Med Educ 1978;53:429–31.
7. Lacombe MA. On bedside teaching. Ann Intern Med 1997;126:217–20.
8. Linfors EW, Neelon FA. Sounding board. The case of bedside rounds. N Engl J Med 1980;303:1230–3.
9. Jolley J, Shields J. The evolution of family-centered care. J Pediatr Nursing 2009;42:164–70.
10. Muething SE, Kotagal UR, Schoettker PJ, et al. Family-centered rounds: a new approach to patient care and teaching. Pediatrics 2007;119:829–32.
11. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teach Learn Med 2007;19:319–22.
12. Mittal V, Sigrest T, Ottolini M, et al. Family-centered rounds on pediatric wards: a PRIS network survey of Canadian and US hospitalists. Pediatrics 2010;126:37–43.
13. Rosen P, Stenger E, Bochkoris M, et al. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics 2009;123:e603–8.
14. Rappaport DI, Ketterer TA, Nilforoshan V, Sharif I. Family-centered rounds: views of families, nurses, trainees, and attending physicians. Clin Pediatr (Phila) 2012;51:260–6.
15. Young HN, Schumacher JB, Moreno MA, et al. Medical student self-efficacy with family-centered care during bedside rounds. Acad Med 2012;87:767–75.
16. Beck J, Meyer R, Kind T, Bhansali B. The importance of situational awareness: a qualitative study of family members’ and nurses’ perspectives on teaching during family-centered rounds. Acad Med 2015 Jul 21. Epub ahead of print.
17. Benjamin J, Cox E, Trapskin P, et al. Family-initiated dialogue about medicaitons during family-centered rounds. Pediatrics 2015;135:94–100.
18. Cox E, Schumacher J, Young H, et al. Medical student outcomes after family-centered bedside rounds. Acad Pediatri 2011;11:403–8.
19. Latta LC, Dick R, Parry C, Tamura GS. Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med 2008;83:292–7.
20. Mittal V. Family-centered rounds. Pediatr Clin North Am 2014;61:663–70.
21. Mittal V, Krieger E, Lee B, et al. Pediatric residents’ perspectives on family-centered rounds - a qualitative study at 2 children’s hospitals. J Grad Med Educ 2013;5:81–7.
22. Lion KC, Mangione-Smith R, Martyn M, et al. Comprehension on family-centered rounds for limited English proficient families. Acad Pediatr 2013;13:236–42.
23. Seltz LB, Zimmer L, Ochoa-Nunez L, et al. Latino families’ experiences with family-centered rounds at an academic children’s hospital. Acad Pediatr 2011;11:432–8.
24. Kuo DZ, Sisterhen LL, Sigrest TE, et al. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics 2012;130:299–305.
25. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr 2013;3:31–8.
26. Kern J, Bhansali P. Handheld electronic device use by pediatric hospitalists on family centered rounds. J Med Syst 2016;40:9.
From the Department of Pediatrics, George Washington University and Children’s National Medical Center, Washington, DC (Dr. Kern), the Department of Pediatrics, Children’s Hospital Los Angeles and University of Southern California Keck School of Medicine, Los Angeles, CA (Dr. Gay), and the Department of Pediatrics, University of Texas Southwestern Medical Center and Children’s Health System, Dallas, TX (Dr. Mittal).
Abstract
- Objective: To present a model for operationalizing successful family-centered rounds (FCRs).
- Methods: Literature review and experience with leading FCR workshops at national meetings.
- Results: FCRs are multidisciplinary rounds that involve patients and families in decision-making. The model has gained substantial momentum nationally and is widely practiced in US pediatric hospitals. Many quality improvement–related FCR benefits have been identified, including improved parental satisfaction, communication, team-based practice, incorporation of practice guidelines, prevention of medication errors, and improved trainee and staff education and satisfaction. Physical and time constraints, variability in attending FCR style and teaching style, lack of FCR structure and process, specific and sensitive patient conditions, and language barriers are key challenges to implementing FCRs. Operationalizing a successful FCR program requires key stakeholders developing and defining a FCR process and structure, including developing a strong faculty development program.
- Conclusion: FCR benefits for a health care system are many. Key stakeholders involvement, developing FCR "ground rules," troubleshooting FCR barriers, and developing a strong faculty development program are key to managing successful FCRs.
The practice of medicine is a team sport and no team is complete without the patient and family being an integral part of it. Over the past 15 years, health care and the practice of medicine has slowly moved away from physician-centered care to patient- and family-centered care (FCC). This change has been a gradual shift in our culture and FCC has become a widely adopted philosophy within the US health care system [1]. FCC has been recognized and embraced by numerous medical and professional societies, including the Institute of Medicine (IOM), the American Academy of Pediatrics (AAP), and family advocacy organizations such as Family Voices and the Institute for Patient- and Family-Centered Care [1,2]. At its most basic, “family-centered care” occurs when patients/families and medical providers partner together to formulate medical plans that are built upon the sharing of open and unbiased information and that account for the diversity and individual strengths and needs of each patient and family unit [3]. FCC in the inpatient setting for hospitalized patients is most exemplified by the practice of family-centered (bedside) rounds, or FCRs [1].
Interestingly, FCC as a philosophy of care developed during a time when bedside rounds, and by extension clinical teaching, moved away from the bedside. Rounds are an integral part of how work is done in the inpatient setting. They come in many different flavors, from “pre-rounds” to “card-flip rounds” to “attending rounds,” “table/conference room rounds,” “hallway rounds,” “bedside rounds,” and the aforementioned family-centered rounds. In the first half of the 20th century,the majority of teaching rounds took place at the patient’s bedside, in the model advocated by Sir William Osler [4]. Indeed, as Dr. Osler wrote in 1903, “there should be no teaching without a patient for a text, and the best teaching is that taught by the patient himself” [5]. By the late 1970s through the mid-1990s, however, the proportion of clinical teaching occurring at the bedside had decreased to as low as 16% [6–8]. Many reasons behind the change have been speculated, including faculty comfort with lecture-based teaching and desire to control the content of teaching discussions, as well as technological advancement necessitating access to computers during case review.
In contrast, the patient-and family-centered movement began in the mid-20th century as a response to the separation trauma experienced by hospitalized children and their families [9]. Hospitals responded by liberalizing their visiting policies and encouraging direct care-giving by parents. FCC was further bolstered by consumer-led movements in the 1960s and 1970s, and by federal legislation in the 1980s targeting children with special health care needs. FCC gained national recognition in 2001 when the Institute of Medicine emphasized that involving patients and families in health care decisions increased the quality of their care [2]. Subsequently, the AAP endorsed FCC as a guiding approach to pediatric care in their 2003 report “Family-centered care and the pediatrician’s role” [1]. As part of this report, the AAP recommended that bedside presentations with active engagement of families become the standard of care. FCRs developed at several children’s hospitals in the US in the following years, with the first conceptual model of FCR published by Muething et al in 2007 [10].
Definition of Family-Centered Rounds
While no consensus definition of FCR exists, the most frequently cited description comes from Sisterhen et al who describe FCR as “interdisciplinary work rounds at the bedside in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself” [11]. Three key features should be noted in this definition. First, FCR requires the active participation of family members, not merely their presence. In this way, patient and family voices are heard and their preferences solicited with respect to clinical decision-making. Second, FCR take place at the bedside, in alignment with the 2003 AAP policy statement that standard practice should be to conduct attending rounds with full case presentations in patient rooms in the presence of family. Third, FCR are typically interdisciplinary, involving patients and their families, physicians and trainees, nurses, and other ancillary staff (such as interpreters, case managers, and pharmacists) [1,10,11,12].
Since the IOM report, FCRs have gained substantial national momentum. A PRIS (Pediatric Research in Inpatient Setting) network study in 2010 published the first survey of pediatric hospitalist rounding practices in the US and Canada [12]. The study reported that 44% of pediatric hospitalists conducted FCRs, and about a quarter conducted rounds as hallway rounds or sit down rounds. Academic hospitalists were significantly more likely to conduct FCRs compared with non-academic (48% vs. 31%; P < 0.05) hospitalists. In accordance with Muething et al’s experience with FCRs in the Cincinnati model, the survey respondents did not associate FCR with prolonged rounding duration [10,12]. FCRs were also associated with greater bedside nurse participation [12]. Given the momentum behind FCC and the oft-cited benefits of FCR, it can only be presumed that the number of pediatric hospitals conducting FCR has significantly increased since the PRIS study was published in 2010.
FCRs Can Improve Quality of Care for Hospitalized Children
FCRs bring together multiple stakeholders involved in the patient’s care in the same place at the same time everyday. This allows for shared-decision making, identification of medical teams by families, and allows for direct and open communication between parents and medical teams [1,10–12]. The key stakeholders on a FCR team include the patient and family members and the medical team. The medical team includes attending physician, fellow, resident, and students, bedside nurse, care coordinator/case manager and other ancillary services. Although not enough data is available on who should attend rounds, case mangers and bedside nurse along with medical team and patients and families were found to be crucial in the general inpatient setting [12].
Integrating FCRs into the daily workflow in the inpatient setting provides several benefits for patients and families and the medical team, including trainees. Improvements in family-centered care principles, parental satisfaction, interdisciplinary team communication, efficiency, patient safety, and resident and medical student education have been reported consistently [9–23].
FCR Benefits for Patients and Families
Muething et al described increased patient-family satis-faction with higher levels of family participation in rounds and earlier discharge times [10]. On FCRs, families report having the opportunity to communicate directly with the entire care team, clarify misinformation and better understand care plans including discharge goals, leading to higher levels family satisfaction [10,14,24]. Both English and limited-English-proficient families report positive experiences with FCRs [21–23]. Families express appreciation with learning opportunities on FCRs, as well as the opportunity to serve as teachers to the medical team [14,16,21]. Families reported comfort with trainees being on rounds and appreciated seeing the medical personnel working as team [21]. They also report trust, comfort, and accountability towards the system and providers as they saw them working together as teams. They felt respected and involved as the medical teams involved them during rounds. Parents also report comfort with diversity of providers and feel that having multidisciplinary and diverse teams help with cultural competencies. Parents appreciated trainees being led by attending physician and felt that attending FCRs made them understand the medical process and the steps involved in caring for their child. They also reported that attending FCRs helps trainees learn about answering the kind of questions that parents usually ask. Contrary to the popular belief, parental participation has not increased the duration of FCRs and parental presence during rounds decreases time spent discussing each patient [14,25].
FCRs and Staff Satisfaction
Staff satisfaction with FCRs has been consistently high [13,14,18–23]. Nursing and medical staffs report valuing FCRs as they foster a sense of teamwork, improve understanding of the patient’s care plan and enhance communication between the care team and families [14]. FCRs significantly increase bedside nurse participation during rounds [12]. Presence of nursing and ancillary staff on FCRs improves efficiency by providing valuable information and helping address discharge goal [10]. Anecdotal data suggests that FCRs reduces number of pages trainees receive from nurses.
FCRs and Outcomes
FCRs have been perceived to improve in patient safety including errors in history taking and miscommunication, and incorrect information; and promote medication reconciliation, safety and adherence [17,20,21]. FCRs have shown to improve patient satisfaction, communication, and coordination of care and trainee education [10,14,21].
Educational Benefits of FCRs
families (Table 1) [26].
FCR Benefits for Hospitals and Health Care Systems
As health care prepares to fully adopt reforms and shift from volume-based to value-based payment systems, creating value in every patient encounter is vital. Conducting daily FCRs provide an dynamic venue for hospitals where daily rounds can incorporate evidence-based practice guidelines, prevent medication errors, ensure safety, reduce unnecessary tests and treatments, and improve transparency and accountability in care. This model can help hospital financially by meeting key quality and safety metrics and also help provide cost effective care through use and reinforcement of clinical pathways during rounds.
FCR Barriers
While many hospitals have adopted FCRs, many barriers to FCR implementation exist [10–14,18–23] (Table 1). Understanding these barriers and overcoming them are crucial for successful implementation. Conducting FCRs involve many aspects of care that happen during rounds. These include discussions about history, physical examinations, labs, and other tests; clinical decision-making and communication between parents and providers; team communication; teaching of trainees; discharge planning; and coordination of care [20]. Given all these aspects of care involved during rounds, being able to conduct multidisciplinary rounds in a timely and efficient way can be a challenge in a busy and dynamic inpatient setting.
Key identified FCR barriers have included physical constraints such as small patient rooms, large team size, patients being on multiple floors or units, infection control precautions leading to increased time involved with teams gowning and gloving; lack of training on FCRs for trainees and faculty; language and cultural barriers; family/patient concerns of privacy/disclosure of sensitive information; trainee’s fears of not appearing knowledgeable in front of families; and variability in attending physicians’ teaching style and approach to FCR [10–15,21].
Operationalizing Successful FCRs
Forming FCR Steering Committee: Developing Ground Rules
While there are many barriers to conducting efficient FCRs there are some that are unique to each institution. Therefore, for those institutions planning to initiate FCRs, the first step might be to form a FCR steering committee of key stakeholders who could review the current state, do a needs assessment for initiating FCRs, develop a structured and standardized FCR process and revise the FCR process periodically to meet the needs of the dynamic inpatient setting [10,12,14].
Defining and Identifying the FCR Process: Who, Where, and When of FCRs
The steering committee should clearly define FCRs and identify what FCRs would involve. For example, should FCRs involve complete case presentations and discussion in front of the parent or focused relevent H&P in a language that the parent understands? The steering committee should identify key elements/aspects of FCRs that would happen on daily rounds. For example: how should each patient receive information about FCRs? Should FCRs be offered to all patients? Do patients have options to opt-in or opt-out of FCRs on a daily basis or a one-time basis? Who should attend FCRs? For example, other than medical team, the bedside nurse and case manager should attend FCRs on a general pediatric service. Should the team round based on nursing assignments or resident assignments or in the order of room numbers? What should a typical rounding encounter involve? For example, each encounter should begin with the intern knocking on the door, asking parental permission for FCR team to enter the room, who should present, who should lead the rounds (the senior resident or the attending), who should stand where in the room? What should each encounter involve—for example, case presentation and discussion, parental involvement in decision-making, clarification of any parental questions, plan for that day, criteria for discharge and discharge needs assessment, teaching of resident and students, use of lay language etc. How should each rounding encounter end? Should the intern ask if parents have additional questions? It is important that the steering committee clearly identify these minute rounding details. Additionally, the committee should identify the rounding wards/area, the timing and duration of FCRs, how information about FCRs will be shared with patients and families, how trainees and attendees will be educated about FCRs and when are FCRs appropriate and when not. Defining the process early through stakeholder identification can reduce variability and create some standardization yet allow for individual style variations within the constraints of standardization. This will help reduced attending variability, which was cited as the most common FCR barrier by trainees.
As Seltz et al described, Latino families reported positive experiences with FCRs when a Spanish-speaking provider was involved. However, they report less satisfaction with telephone interpreters and did not feel empowered at times on FCRs due to language differences [23]. Addressing the language needs based on demographics and cultural needs will promote greater acceptance of FCRs [23].
Identifying and Defining Trainee Role
Participating in the FCR can create anxiety for medical students and residents. Therefore, educating them about the FCR process and structure beforehand and clearly defining roles can help them conceptualize their roles and expectation and ease their anxiety with FCRs. This will require the steering committee to collaboratively discuss how each encounter would look during FCR from a trainee’s perspective. Who will present the case? The third- year medical student versus the fourth-year medical student or the intern or based on case allocations? How should the case be presented? Should it be short and pointed presentation versus complete history and physical examination on each patient? How long should an encounter last on a new patient and on a follow-up patient? Who will examine the patient? The student who is presenting the case, the attending, the intern who overlooks the student, or the senior resident? Who will answer the follow-up questions from a parent initially? Should the senior resident lead the team under the attending guidance? How will the senior resident be prepared for morning rounds? Using lay language when talking to parents should be encouraged and taught to trainees routinely during FCRs.
Identifying and Defining Clinical Teaching Styles
Faculty Development Program and Importance of “Safe Environment”
Developing an educational program to train faculty, trainee and staff about FCRs can help streamline FCRs. Conducting FCRs is a cultural change and focusing on early adopters is crucial. Muething et al’s model showed better acceptance of FCRs by interns than by senior residents. Being patient during change management is key to successful implementation. Anecdotal discussions during PAS workshops suggests that on an average programs have required 3 years to get significant buy-in and streamlining of FCRs [10,12].
Suboptimal attending behavior such as attending variability in the FCRs process and teaching strategies have been reported as FCR barriers [14,21]. Residents report attending physician as an important factor determining success of FCRs. As attending physicians typically are the leaders of the FCRs team, training faculty about conducting effective and efficient FCRs is crucial to successful FCRs. [12,21]. Key aspects of faculty development should include: (1) education about the FCR standard process for the institution, (2) importance of time management during rounds, including tips and strategies to be efficient, (3) teaching styles during FCRs, including demonstrating role modeling, and (4) direct observation of trainees and individual and team feedback to streamline FCRs. Role-plays or simulated FCRs might be a venue to explore for faculty development on FCRs [14,21].
Creating a “safe environment” during FCRs where each person feels comfortable and secure is vital to team work [7,12,21]. Often trainees are apprehensive or afraid due to medical hierarchy and this might prevent developing a teaching and learning environment. Trainees fear not appearing knowledgeable in front of families and student rotate too often to adapt to different attending styles [21]. Therefore, reassuring trainees that the goal of FCRs is to conduct daily inpatient rounding to ensure key aspects of FCRs are met without disrespecting and insulting any person on rounds and clarifying and reassuring trainees that their fear of not appearing knowledgeable is real and it will be respected, might help create a safe environment where FCR teams are not only conducting the daily ritual of inpatient rounding, and teaching but also ensuring that trainees are enjoying being the clinician and physicians that they want to be. Therefore, attending role modeling is crucial and it is no surprise that in multiple studies variability in attending rounding and teaching style was identified consistently as a FCR barrier.
Preparing for Daily FCRS: Team Work, Efficiency, and Time Management
Conducting daily timely and efficient rounds require daily preparation by teams. Prior to FCRs, teams should know about all of the patients on whom FCRs will be conducted including those who refused FCRs, if any. This can be done via a pre-round or card-flip rounding method where the teams discuss key diagnoses, indication for admission, and identify any outliers to conducting FCRs such as sensitive patient condition, patients refused FCRs, etc. Some institutions have incorporated these at “morning check out” or at morning “huddles.” These help faculty avoid any last minute surprises during rounds and helps with time management during FCRs [12]. Faculty can then plan on some anticipated “teaching moments” before rounds to keep the rounds flowing, for example, a physical exam finding, a clarifying history that can clinch a diagnoses, a clinical pearl, a complex medical case where the parent might share their story and knowledge, an interesting interpretation of a lab, an x-ray or MRI finding. Faculties are multitasking during FCRs by diagnosing and managing patient and learners and leading effective efficient and timely rounds where parental questions are answered, orders are written, to-do work is identified, discharge planning and care coordination is done and trainees stay focused and attend noon conference on time. This requires thoughtful planning before starting FCRs. Time management and managing priorities is key to positive team experiences of FCRs. Both starting and ending FCRs on time should be emphasized and reinforced continually.
Nurse Preparation for FCRs
Nurses are the frontline providers and educating them about FCRs process can help them better explain FCRs to patients and families. Nurses often know the minute details such as timing of an MRI, if the patient has vomited in the morning, or when the parents are coming, etc. This important information sharing during FCRs can help team prepare for the day and provide patients and families’ expectations for the day. Nursing participation can also enhance their knowledge about the thought process behind decisions and care plans and avoid additional time paging house staff to obtain clarification [12–15,21].
Trainee Preparation for FCRs
While pediatric residents do report that FCRs leads to fewer requests for clarifications from families and nurses after FCRs, many still harbor concerns about the time required for FCRs and the overall efficiency of rounds [14]. Educating trainees about the FCR process and explaining why FCRs are beneficial can help alleviate trainee anxiety around FCRs. Involving trainees in the FCR communication and creating a safe and nurturing environment during FCRs can further reduce trainee anxiety [21]. Parents who have attended FCRs with trainees report understanding that trainees are in training and that they have felt comfortable to see attending physician lead the trainees.
FCRs and Technology
Use of technology during FCRs can be helpful to write orders in real time, follow-up and share lab values and or imaging study with parents or teach students. The increasing use of technology on FCRs, such as computers and handheld devices, can help with rounding and teaching; however, it also has the potential to be a distractor and requires that the medical team remain vigilant that the patient and family are the focus of FCRs [26].
Efficiency Pearls
Certain strategies can be utilized to keep FCRs efficient:
- Orient the FCR team about FCR process
- Identify rounding sequence for the day so team can move efficiently between rooms. Identifying potential discharges for the following morning and discharging those patients before rounds can reduce rounding census and provide additional rounding time. Teams can identify approximate time spent in each room based on census, as rounding time is constant.
- Starting and ending FCRs at the allocated time is key to success of FCRs. Sometimes this might require the attending and senior resident splitting the last 1–2 patients to finish rounds on time.
- Prepare students and interns for effective and efficient yet complete presentations during rounds that reflect their knowledge and thought process rather than presenting the entire H&P.
- Keep teaching during rounds focused. As a resident reported, “attendings should keep it short and not go off on a half hour lecture during FCRs. On FCRs I want to hear bam…bam…bam! tidbits, little hints, clinical pearls. Things that you would not know and only see and know when you were there in the room [21].”
- Encourage and teach senior residents’ role as a leader and teacher [21].
- With a situation requiring more time talking to families, request to go back later in the afternoon so as to stay on track on FCR time.
- Faculty can review lab results and history and physical findings on new admissions before rounds to avoid surprises during FCRs and to save time. This can be done during pre-round/card flip/or morning huddle.
Limitations
This article is based on the authors’ review of literature, experience in conducting FCRs, and experience from leading and attending FCR-related workshops at annual pediatric academic societies’ meetings and annual pediatric hospital medicine meetings between 2010 and 2015. There are several limitations to this work. Firstly, the majority of FCR literature is based on perceptions and are not measured outcomes. In addition, how FCRs will apply on services with complex patients needs more study. Different institutions have different physical constraints as well as sociodemographic and cultural factors that might affect FCRs. Daily census among hospitals varies and rounding duration may vary for them.
Conclusion
Family-centered rounds are widely accepted among pediatric hospitalists in the US. Reported benefits of FCRs include improved parent satisfaction, communication, better team communication, improved patient safety and better education for trainees. Many barriers to efficient FCRs exist, and for programs planning to incorporate FCRs in their daily rounds it is crucial to understand FCR benefits and barriers and assess their current state, including physical environment, when planning FCRs. Having a period to plan for FCR implementation through key stakeholder involvement helps define FCR process and lay down a conceptual model suited to individual organization. Educating the team members including families about FCRs and developing a strong faculty development program can further strengthen FCR implementation. Special focus should be given to time management, teaching styles during FCRs, and creating a safe and nurturing environment for FCRs to succeed.
Corresponding author: Vineeta Mittal, MD, MBA, 1935 Medical District Dr., Dallas, TX 75235, vineeta.mittal@childrens.com.
From the Department of Pediatrics, George Washington University and Children’s National Medical Center, Washington, DC (Dr. Kern), the Department of Pediatrics, Children’s Hospital Los Angeles and University of Southern California Keck School of Medicine, Los Angeles, CA (Dr. Gay), and the Department of Pediatrics, University of Texas Southwestern Medical Center and Children’s Health System, Dallas, TX (Dr. Mittal).
Abstract
- Objective: To present a model for operationalizing successful family-centered rounds (FCRs).
- Methods: Literature review and experience with leading FCR workshops at national meetings.
- Results: FCRs are multidisciplinary rounds that involve patients and families in decision-making. The model has gained substantial momentum nationally and is widely practiced in US pediatric hospitals. Many quality improvement–related FCR benefits have been identified, including improved parental satisfaction, communication, team-based practice, incorporation of practice guidelines, prevention of medication errors, and improved trainee and staff education and satisfaction. Physical and time constraints, variability in attending FCR style and teaching style, lack of FCR structure and process, specific and sensitive patient conditions, and language barriers are key challenges to implementing FCRs. Operationalizing a successful FCR program requires key stakeholders developing and defining a FCR process and structure, including developing a strong faculty development program.
- Conclusion: FCR benefits for a health care system are many. Key stakeholders involvement, developing FCR "ground rules," troubleshooting FCR barriers, and developing a strong faculty development program are key to managing successful FCRs.
The practice of medicine is a team sport and no team is complete without the patient and family being an integral part of it. Over the past 15 years, health care and the practice of medicine has slowly moved away from physician-centered care to patient- and family-centered care (FCC). This change has been a gradual shift in our culture and FCC has become a widely adopted philosophy within the US health care system [1]. FCC has been recognized and embraced by numerous medical and professional societies, including the Institute of Medicine (IOM), the American Academy of Pediatrics (AAP), and family advocacy organizations such as Family Voices and the Institute for Patient- and Family-Centered Care [1,2]. At its most basic, “family-centered care” occurs when patients/families and medical providers partner together to formulate medical plans that are built upon the sharing of open and unbiased information and that account for the diversity and individual strengths and needs of each patient and family unit [3]. FCC in the inpatient setting for hospitalized patients is most exemplified by the practice of family-centered (bedside) rounds, or FCRs [1].
Interestingly, FCC as a philosophy of care developed during a time when bedside rounds, and by extension clinical teaching, moved away from the bedside. Rounds are an integral part of how work is done in the inpatient setting. They come in many different flavors, from “pre-rounds” to “card-flip rounds” to “attending rounds,” “table/conference room rounds,” “hallway rounds,” “bedside rounds,” and the aforementioned family-centered rounds. In the first half of the 20th century,the majority of teaching rounds took place at the patient’s bedside, in the model advocated by Sir William Osler [4]. Indeed, as Dr. Osler wrote in 1903, “there should be no teaching without a patient for a text, and the best teaching is that taught by the patient himself” [5]. By the late 1970s through the mid-1990s, however, the proportion of clinical teaching occurring at the bedside had decreased to as low as 16% [6–8]. Many reasons behind the change have been speculated, including faculty comfort with lecture-based teaching and desire to control the content of teaching discussions, as well as technological advancement necessitating access to computers during case review.
In contrast, the patient-and family-centered movement began in the mid-20th century as a response to the separation trauma experienced by hospitalized children and their families [9]. Hospitals responded by liberalizing their visiting policies and encouraging direct care-giving by parents. FCC was further bolstered by consumer-led movements in the 1960s and 1970s, and by federal legislation in the 1980s targeting children with special health care needs. FCC gained national recognition in 2001 when the Institute of Medicine emphasized that involving patients and families in health care decisions increased the quality of their care [2]. Subsequently, the AAP endorsed FCC as a guiding approach to pediatric care in their 2003 report “Family-centered care and the pediatrician’s role” [1]. As part of this report, the AAP recommended that bedside presentations with active engagement of families become the standard of care. FCRs developed at several children’s hospitals in the US in the following years, with the first conceptual model of FCR published by Muething et al in 2007 [10].
Definition of Family-Centered Rounds
While no consensus definition of FCR exists, the most frequently cited description comes from Sisterhen et al who describe FCR as “interdisciplinary work rounds at the bedside in which the patient and family share in the control of the management plan as well as in the evaluation of the process itself” [11]. Three key features should be noted in this definition. First, FCR requires the active participation of family members, not merely their presence. In this way, patient and family voices are heard and their preferences solicited with respect to clinical decision-making. Second, FCR take place at the bedside, in alignment with the 2003 AAP policy statement that standard practice should be to conduct attending rounds with full case presentations in patient rooms in the presence of family. Third, FCR are typically interdisciplinary, involving patients and their families, physicians and trainees, nurses, and other ancillary staff (such as interpreters, case managers, and pharmacists) [1,10,11,12].
Since the IOM report, FCRs have gained substantial national momentum. A PRIS (Pediatric Research in Inpatient Setting) network study in 2010 published the first survey of pediatric hospitalist rounding practices in the US and Canada [12]. The study reported that 44% of pediatric hospitalists conducted FCRs, and about a quarter conducted rounds as hallway rounds or sit down rounds. Academic hospitalists were significantly more likely to conduct FCRs compared with non-academic (48% vs. 31%; P < 0.05) hospitalists. In accordance with Muething et al’s experience with FCRs in the Cincinnati model, the survey respondents did not associate FCR with prolonged rounding duration [10,12]. FCRs were also associated with greater bedside nurse participation [12]. Given the momentum behind FCC and the oft-cited benefits of FCR, it can only be presumed that the number of pediatric hospitals conducting FCR has significantly increased since the PRIS study was published in 2010.
FCRs Can Improve Quality of Care for Hospitalized Children
FCRs bring together multiple stakeholders involved in the patient’s care in the same place at the same time everyday. This allows for shared-decision making, identification of medical teams by families, and allows for direct and open communication between parents and medical teams [1,10–12]. The key stakeholders on a FCR team include the patient and family members and the medical team. The medical team includes attending physician, fellow, resident, and students, bedside nurse, care coordinator/case manager and other ancillary services. Although not enough data is available on who should attend rounds, case mangers and bedside nurse along with medical team and patients and families were found to be crucial in the general inpatient setting [12].
Integrating FCRs into the daily workflow in the inpatient setting provides several benefits for patients and families and the medical team, including trainees. Improvements in family-centered care principles, parental satisfaction, interdisciplinary team communication, efficiency, patient safety, and resident and medical student education have been reported consistently [9–23].
FCR Benefits for Patients and Families
Muething et al described increased patient-family satis-faction with higher levels of family participation in rounds and earlier discharge times [10]. On FCRs, families report having the opportunity to communicate directly with the entire care team, clarify misinformation and better understand care plans including discharge goals, leading to higher levels family satisfaction [10,14,24]. Both English and limited-English-proficient families report positive experiences with FCRs [21–23]. Families express appreciation with learning opportunities on FCRs, as well as the opportunity to serve as teachers to the medical team [14,16,21]. Families reported comfort with trainees being on rounds and appreciated seeing the medical personnel working as team [21]. They also report trust, comfort, and accountability towards the system and providers as they saw them working together as teams. They felt respected and involved as the medical teams involved them during rounds. Parents also report comfort with diversity of providers and feel that having multidisciplinary and diverse teams help with cultural competencies. Parents appreciated trainees being led by attending physician and felt that attending FCRs made them understand the medical process and the steps involved in caring for their child. They also reported that attending FCRs helps trainees learn about answering the kind of questions that parents usually ask. Contrary to the popular belief, parental participation has not increased the duration of FCRs and parental presence during rounds decreases time spent discussing each patient [14,25].
FCRs and Staff Satisfaction
Staff satisfaction with FCRs has been consistently high [13,14,18–23]. Nursing and medical staffs report valuing FCRs as they foster a sense of teamwork, improve understanding of the patient’s care plan and enhance communication between the care team and families [14]. FCRs significantly increase bedside nurse participation during rounds [12]. Presence of nursing and ancillary staff on FCRs improves efficiency by providing valuable information and helping address discharge goal [10]. Anecdotal data suggests that FCRs reduces number of pages trainees receive from nurses.
FCRs and Outcomes
FCRs have been perceived to improve in patient safety including errors in history taking and miscommunication, and incorrect information; and promote medication reconciliation, safety and adherence [17,20,21]. FCRs have shown to improve patient satisfaction, communication, and coordination of care and trainee education [10,14,21].
Educational Benefits of FCRs
families (Table 1) [26].
FCR Benefits for Hospitals and Health Care Systems
As health care prepares to fully adopt reforms and shift from volume-based to value-based payment systems, creating value in every patient encounter is vital. Conducting daily FCRs provide an dynamic venue for hospitals where daily rounds can incorporate evidence-based practice guidelines, prevent medication errors, ensure safety, reduce unnecessary tests and treatments, and improve transparency and accountability in care. This model can help hospital financially by meeting key quality and safety metrics and also help provide cost effective care through use and reinforcement of clinical pathways during rounds.
FCR Barriers
While many hospitals have adopted FCRs, many barriers to FCR implementation exist [10–14,18–23] (Table 1). Understanding these barriers and overcoming them are crucial for successful implementation. Conducting FCRs involve many aspects of care that happen during rounds. These include discussions about history, physical examinations, labs, and other tests; clinical decision-making and communication between parents and providers; team communication; teaching of trainees; discharge planning; and coordination of care [20]. Given all these aspects of care involved during rounds, being able to conduct multidisciplinary rounds in a timely and efficient way can be a challenge in a busy and dynamic inpatient setting.
Key identified FCR barriers have included physical constraints such as small patient rooms, large team size, patients being on multiple floors or units, infection control precautions leading to increased time involved with teams gowning and gloving; lack of training on FCRs for trainees and faculty; language and cultural barriers; family/patient concerns of privacy/disclosure of sensitive information; trainee’s fears of not appearing knowledgeable in front of families; and variability in attending physicians’ teaching style and approach to FCR [10–15,21].
Operationalizing Successful FCRs
Forming FCR Steering Committee: Developing Ground Rules
While there are many barriers to conducting efficient FCRs there are some that are unique to each institution. Therefore, for those institutions planning to initiate FCRs, the first step might be to form a FCR steering committee of key stakeholders who could review the current state, do a needs assessment for initiating FCRs, develop a structured and standardized FCR process and revise the FCR process periodically to meet the needs of the dynamic inpatient setting [10,12,14].
Defining and Identifying the FCR Process: Who, Where, and When of FCRs
The steering committee should clearly define FCRs and identify what FCRs would involve. For example, should FCRs involve complete case presentations and discussion in front of the parent or focused relevent H&P in a language that the parent understands? The steering committee should identify key elements/aspects of FCRs that would happen on daily rounds. For example: how should each patient receive information about FCRs? Should FCRs be offered to all patients? Do patients have options to opt-in or opt-out of FCRs on a daily basis or a one-time basis? Who should attend FCRs? For example, other than medical team, the bedside nurse and case manager should attend FCRs on a general pediatric service. Should the team round based on nursing assignments or resident assignments or in the order of room numbers? What should a typical rounding encounter involve? For example, each encounter should begin with the intern knocking on the door, asking parental permission for FCR team to enter the room, who should present, who should lead the rounds (the senior resident or the attending), who should stand where in the room? What should each encounter involve—for example, case presentation and discussion, parental involvement in decision-making, clarification of any parental questions, plan for that day, criteria for discharge and discharge needs assessment, teaching of resident and students, use of lay language etc. How should each rounding encounter end? Should the intern ask if parents have additional questions? It is important that the steering committee clearly identify these minute rounding details. Additionally, the committee should identify the rounding wards/area, the timing and duration of FCRs, how information about FCRs will be shared with patients and families, how trainees and attendees will be educated about FCRs and when are FCRs appropriate and when not. Defining the process early through stakeholder identification can reduce variability and create some standardization yet allow for individual style variations within the constraints of standardization. This will help reduced attending variability, which was cited as the most common FCR barrier by trainees.
As Seltz et al described, Latino families reported positive experiences with FCRs when a Spanish-speaking provider was involved. However, they report less satisfaction with telephone interpreters and did not feel empowered at times on FCRs due to language differences [23]. Addressing the language needs based on demographics and cultural needs will promote greater acceptance of FCRs [23].
Identifying and Defining Trainee Role
Participating in the FCR can create anxiety for medical students and residents. Therefore, educating them about the FCR process and structure beforehand and clearly defining roles can help them conceptualize their roles and expectation and ease their anxiety with FCRs. This will require the steering committee to collaboratively discuss how each encounter would look during FCR from a trainee’s perspective. Who will present the case? The third- year medical student versus the fourth-year medical student or the intern or based on case allocations? How should the case be presented? Should it be short and pointed presentation versus complete history and physical examination on each patient? How long should an encounter last on a new patient and on a follow-up patient? Who will examine the patient? The student who is presenting the case, the attending, the intern who overlooks the student, or the senior resident? Who will answer the follow-up questions from a parent initially? Should the senior resident lead the team under the attending guidance? How will the senior resident be prepared for morning rounds? Using lay language when talking to parents should be encouraged and taught to trainees routinely during FCRs.
Identifying and Defining Clinical Teaching Styles
Faculty Development Program and Importance of “Safe Environment”
Developing an educational program to train faculty, trainee and staff about FCRs can help streamline FCRs. Conducting FCRs is a cultural change and focusing on early adopters is crucial. Muething et al’s model showed better acceptance of FCRs by interns than by senior residents. Being patient during change management is key to successful implementation. Anecdotal discussions during PAS workshops suggests that on an average programs have required 3 years to get significant buy-in and streamlining of FCRs [10,12].
Suboptimal attending behavior such as attending variability in the FCRs process and teaching strategies have been reported as FCR barriers [14,21]. Residents report attending physician as an important factor determining success of FCRs. As attending physicians typically are the leaders of the FCRs team, training faculty about conducting effective and efficient FCRs is crucial to successful FCRs. [12,21]. Key aspects of faculty development should include: (1) education about the FCR standard process for the institution, (2) importance of time management during rounds, including tips and strategies to be efficient, (3) teaching styles during FCRs, including demonstrating role modeling, and (4) direct observation of trainees and individual and team feedback to streamline FCRs. Role-plays or simulated FCRs might be a venue to explore for faculty development on FCRs [14,21].
Creating a “safe environment” during FCRs where each person feels comfortable and secure is vital to team work [7,12,21]. Often trainees are apprehensive or afraid due to medical hierarchy and this might prevent developing a teaching and learning environment. Trainees fear not appearing knowledgeable in front of families and student rotate too often to adapt to different attending styles [21]. Therefore, reassuring trainees that the goal of FCRs is to conduct daily inpatient rounding to ensure key aspects of FCRs are met without disrespecting and insulting any person on rounds and clarifying and reassuring trainees that their fear of not appearing knowledgeable is real and it will be respected, might help create a safe environment where FCR teams are not only conducting the daily ritual of inpatient rounding, and teaching but also ensuring that trainees are enjoying being the clinician and physicians that they want to be. Therefore, attending role modeling is crucial and it is no surprise that in multiple studies variability in attending rounding and teaching style was identified consistently as a FCR barrier.
Preparing for Daily FCRS: Team Work, Efficiency, and Time Management
Conducting daily timely and efficient rounds require daily preparation by teams. Prior to FCRs, teams should know about all of the patients on whom FCRs will be conducted including those who refused FCRs, if any. This can be done via a pre-round or card-flip rounding method where the teams discuss key diagnoses, indication for admission, and identify any outliers to conducting FCRs such as sensitive patient condition, patients refused FCRs, etc. Some institutions have incorporated these at “morning check out” or at morning “huddles.” These help faculty avoid any last minute surprises during rounds and helps with time management during FCRs [12]. Faculty can then plan on some anticipated “teaching moments” before rounds to keep the rounds flowing, for example, a physical exam finding, a clarifying history that can clinch a diagnoses, a clinical pearl, a complex medical case where the parent might share their story and knowledge, an interesting interpretation of a lab, an x-ray or MRI finding. Faculties are multitasking during FCRs by diagnosing and managing patient and learners and leading effective efficient and timely rounds where parental questions are answered, orders are written, to-do work is identified, discharge planning and care coordination is done and trainees stay focused and attend noon conference on time. This requires thoughtful planning before starting FCRs. Time management and managing priorities is key to positive team experiences of FCRs. Both starting and ending FCRs on time should be emphasized and reinforced continually.
Nurse Preparation for FCRs
Nurses are the frontline providers and educating them about FCRs process can help them better explain FCRs to patients and families. Nurses often know the minute details such as timing of an MRI, if the patient has vomited in the morning, or when the parents are coming, etc. This important information sharing during FCRs can help team prepare for the day and provide patients and families’ expectations for the day. Nursing participation can also enhance their knowledge about the thought process behind decisions and care plans and avoid additional time paging house staff to obtain clarification [12–15,21].
Trainee Preparation for FCRs
While pediatric residents do report that FCRs leads to fewer requests for clarifications from families and nurses after FCRs, many still harbor concerns about the time required for FCRs and the overall efficiency of rounds [14]. Educating trainees about the FCR process and explaining why FCRs are beneficial can help alleviate trainee anxiety around FCRs. Involving trainees in the FCR communication and creating a safe and nurturing environment during FCRs can further reduce trainee anxiety [21]. Parents who have attended FCRs with trainees report understanding that trainees are in training and that they have felt comfortable to see attending physician lead the trainees.
FCRs and Technology
Use of technology during FCRs can be helpful to write orders in real time, follow-up and share lab values and or imaging study with parents or teach students. The increasing use of technology on FCRs, such as computers and handheld devices, can help with rounding and teaching; however, it also has the potential to be a distractor and requires that the medical team remain vigilant that the patient and family are the focus of FCRs [26].
Efficiency Pearls
Certain strategies can be utilized to keep FCRs efficient:
- Orient the FCR team about FCR process
- Identify rounding sequence for the day so team can move efficiently between rooms. Identifying potential discharges for the following morning and discharging those patients before rounds can reduce rounding census and provide additional rounding time. Teams can identify approximate time spent in each room based on census, as rounding time is constant.
- Starting and ending FCRs at the allocated time is key to success of FCRs. Sometimes this might require the attending and senior resident splitting the last 1–2 patients to finish rounds on time.
- Prepare students and interns for effective and efficient yet complete presentations during rounds that reflect their knowledge and thought process rather than presenting the entire H&P.
- Keep teaching during rounds focused. As a resident reported, “attendings should keep it short and not go off on a half hour lecture during FCRs. On FCRs I want to hear bam…bam…bam! tidbits, little hints, clinical pearls. Things that you would not know and only see and know when you were there in the room [21].”
- Encourage and teach senior residents’ role as a leader and teacher [21].
- With a situation requiring more time talking to families, request to go back later in the afternoon so as to stay on track on FCR time.
- Faculty can review lab results and history and physical findings on new admissions before rounds to avoid surprises during FCRs and to save time. This can be done during pre-round/card flip/or morning huddle.
Limitations
This article is based on the authors’ review of literature, experience in conducting FCRs, and experience from leading and attending FCR-related workshops at annual pediatric academic societies’ meetings and annual pediatric hospital medicine meetings between 2010 and 2015. There are several limitations to this work. Firstly, the majority of FCR literature is based on perceptions and are not measured outcomes. In addition, how FCRs will apply on services with complex patients needs more study. Different institutions have different physical constraints as well as sociodemographic and cultural factors that might affect FCRs. Daily census among hospitals varies and rounding duration may vary for them.
Conclusion
Family-centered rounds are widely accepted among pediatric hospitalists in the US. Reported benefits of FCRs include improved parent satisfaction, communication, better team communication, improved patient safety and better education for trainees. Many barriers to efficient FCRs exist, and for programs planning to incorporate FCRs in their daily rounds it is crucial to understand FCR benefits and barriers and assess their current state, including physical environment, when planning FCRs. Having a period to plan for FCR implementation through key stakeholder involvement helps define FCR process and lay down a conceptual model suited to individual organization. Educating the team members including families about FCRs and developing a strong faculty development program can further strengthen FCR implementation. Special focus should be given to time management, teaching styles during FCRs, and creating a safe and nurturing environment for FCRs to succeed.
Corresponding author: Vineeta Mittal, MD, MBA, 1935 Medical District Dr., Dallas, TX 75235, vineeta.mittal@childrens.com.
1. American Academy of Pediatrics Committee on Hospital Care. Family-centered care and the pediatrician’s role. Pediatrics 2003;112:691–7.
2. Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001.
3. Kuo DZ, Joutrow AJ, Arango P, et al. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J 2012;16:297–305.
4. Reichsman F, Browning FE, Hinshaw JR. Observations of undergraduate clinical teaching in action. J Med Educ 1964;39:147–63.
5. Osler W. On the need of a radical reform in our methods of teaching senior students. Med News 1903;82:49–53.
6. Collins GF, Cassie JM, Dagget CJ. The role of the attending physician in clinical training. J Med Educ 1978;53:429–31.
7. Lacombe MA. On bedside teaching. Ann Intern Med 1997;126:217–20.
8. Linfors EW, Neelon FA. Sounding board. The case of bedside rounds. N Engl J Med 1980;303:1230–3.
9. Jolley J, Shields J. The evolution of family-centered care. J Pediatr Nursing 2009;42:164–70.
10. Muething SE, Kotagal UR, Schoettker PJ, et al. Family-centered rounds: a new approach to patient care and teaching. Pediatrics 2007;119:829–32.
11. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teach Learn Med 2007;19:319–22.
12. Mittal V, Sigrest T, Ottolini M, et al. Family-centered rounds on pediatric wards: a PRIS network survey of Canadian and US hospitalists. Pediatrics 2010;126:37–43.
13. Rosen P, Stenger E, Bochkoris M, et al. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics 2009;123:e603–8.
14. Rappaport DI, Ketterer TA, Nilforoshan V, Sharif I. Family-centered rounds: views of families, nurses, trainees, and attending physicians. Clin Pediatr (Phila) 2012;51:260–6.
15. Young HN, Schumacher JB, Moreno MA, et al. Medical student self-efficacy with family-centered care during bedside rounds. Acad Med 2012;87:767–75.
16. Beck J, Meyer R, Kind T, Bhansali B. The importance of situational awareness: a qualitative study of family members’ and nurses’ perspectives on teaching during family-centered rounds. Acad Med 2015 Jul 21. Epub ahead of print.
17. Benjamin J, Cox E, Trapskin P, et al. Family-initiated dialogue about medicaitons during family-centered rounds. Pediatrics 2015;135:94–100.
18. Cox E, Schumacher J, Young H, et al. Medical student outcomes after family-centered bedside rounds. Acad Pediatri 2011;11:403–8.
19. Latta LC, Dick R, Parry C, Tamura GS. Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med 2008;83:292–7.
20. Mittal V. Family-centered rounds. Pediatr Clin North Am 2014;61:663–70.
21. Mittal V, Krieger E, Lee B, et al. Pediatric residents’ perspectives on family-centered rounds - a qualitative study at 2 children’s hospitals. J Grad Med Educ 2013;5:81–7.
22. Lion KC, Mangione-Smith R, Martyn M, et al. Comprehension on family-centered rounds for limited English proficient families. Acad Pediatr 2013;13:236–42.
23. Seltz LB, Zimmer L, Ochoa-Nunez L, et al. Latino families’ experiences with family-centered rounds at an academic children’s hospital. Acad Pediatr 2011;11:432–8.
24. Kuo DZ, Sisterhen LL, Sigrest TE, et al. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics 2012;130:299–305.
25. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr 2013;3:31–8.
26. Kern J, Bhansali P. Handheld electronic device use by pediatric hospitalists on family centered rounds. J Med Syst 2016;40:9.
1. American Academy of Pediatrics Committee on Hospital Care. Family-centered care and the pediatrician’s role. Pediatrics 2003;112:691–7.
2. Institute of Medicine, Committee on Quality Health Care in America. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: The National Academies Press; 2001.
3. Kuo DZ, Joutrow AJ, Arango P, et al. Family-centered care: current applications and future directions in pediatric health care. Matern Child Health J 2012;16:297–305.
4. Reichsman F, Browning FE, Hinshaw JR. Observations of undergraduate clinical teaching in action. J Med Educ 1964;39:147–63.
5. Osler W. On the need of a radical reform in our methods of teaching senior students. Med News 1903;82:49–53.
6. Collins GF, Cassie JM, Dagget CJ. The role of the attending physician in clinical training. J Med Educ 1978;53:429–31.
7. Lacombe MA. On bedside teaching. Ann Intern Med 1997;126:217–20.
8. Linfors EW, Neelon FA. Sounding board. The case of bedside rounds. N Engl J Med 1980;303:1230–3.
9. Jolley J, Shields J. The evolution of family-centered care. J Pediatr Nursing 2009;42:164–70.
10. Muething SE, Kotagal UR, Schoettker PJ, et al. Family-centered rounds: a new approach to patient care and teaching. Pediatrics 2007;119:829–32.
11. Sisterhen LL, Blaszak RT, Woods MB, Smith CE. Defining family-centered rounds. Teach Learn Med 2007;19:319–22.
12. Mittal V, Sigrest T, Ottolini M, et al. Family-centered rounds on pediatric wards: a PRIS network survey of Canadian and US hospitalists. Pediatrics 2010;126:37–43.
13. Rosen P, Stenger E, Bochkoris M, et al. Family-centered multidisciplinary rounds enhance the team approach in pediatrics. Pediatrics 2009;123:e603–8.
14. Rappaport DI, Ketterer TA, Nilforoshan V, Sharif I. Family-centered rounds: views of families, nurses, trainees, and attending physicians. Clin Pediatr (Phila) 2012;51:260–6.
15. Young HN, Schumacher JB, Moreno MA, et al. Medical student self-efficacy with family-centered care during bedside rounds. Acad Med 2012;87:767–75.
16. Beck J, Meyer R, Kind T, Bhansali B. The importance of situational awareness: a qualitative study of family members’ and nurses’ perspectives on teaching during family-centered rounds. Acad Med 2015 Jul 21. Epub ahead of print.
17. Benjamin J, Cox E, Trapskin P, et al. Family-initiated dialogue about medicaitons during family-centered rounds. Pediatrics 2015;135:94–100.
18. Cox E, Schumacher J, Young H, et al. Medical student outcomes after family-centered bedside rounds. Acad Pediatri 2011;11:403–8.
19. Latta LC, Dick R, Parry C, Tamura GS. Parental responses to involvement in rounds on a pediatric inpatient unit at a teaching hospital: a qualitative study. Acad Med 2008;83:292–7.
20. Mittal V. Family-centered rounds. Pediatr Clin North Am 2014;61:663–70.
21. Mittal V, Krieger E, Lee B, et al. Pediatric residents’ perspectives on family-centered rounds - a qualitative study at 2 children’s hospitals. J Grad Med Educ 2013;5:81–7.
22. Lion KC, Mangione-Smith R, Martyn M, et al. Comprehension on family-centered rounds for limited English proficient families. Acad Pediatr 2013;13:236–42.
23. Seltz LB, Zimmer L, Ochoa-Nunez L, et al. Latino families’ experiences with family-centered rounds at an academic children’s hospital. Acad Pediatr 2011;11:432–8.
24. Kuo DZ, Sisterhen LL, Sigrest TE, et al. Family experiences and pediatric health services use associated with family-centered rounds. Pediatrics 2012;130:299–305.
25. Bhansali P, Birch S, Campbell JK, et al. A time-motion study of inpatient rounds using a family-centered rounds model. Hosp Pediatr 2013;3:31–8.
26. Kern J, Bhansali P. Handheld electronic device use by pediatric hospitalists on family centered rounds. J Med Syst 2016;40:9.
Reducing Surgical Site Infections in a Children’s Hospital: The Fuzzy Elements of Change
From the Hospital for Sick Children, Toronto, ON.
Abstract
- Objective: To describe the iterative and adaptive process used in implementing strategies to reduce surgical site infections (SSI) in a pediatric academic health science center.
- Methods: A multidisciplinary group was tasked with implementing strategies to reduce SSI with a focus on evaluating the use of a guideline for the use of prophylactic antibiotics and determining the rate of SSI.
- Results: The task force initially addressed surgical preparation solution, hair removal, oxygenation, and normothermia. The task force subsequently revised a guideline for the use of prophylactic antibiotics and implemented the guideline iteratively with multiple strategies including audit and feedback, communication and dissemination, and computerised order entry. The appropriate use of the guideline was associated with a 30% reduction in the rate of SSI.
- Conclusion: Using iterative and adaptive strategies over many years, the SSI rate was reduced by 30%.
Improving quality of care is a prime concern for clinicians, patients, families, and health systems [1]. Quality improvement methods are used widely in medicine for studying and addressing problems with care and have successfully addressed gaps in quality. The challenges include defining quality, obtaining complete and accurate data about quality, developing meaningful and cost-effective interventions to improve quality, and to successfully change clinician’s behaviour with commensurate improvement in quality of care.
Quality improvement in health care involves effecting and assessing change in a setting of complexity and uncertainty. Whereas the randomized trial may be used to measure the effectiveness of a particular treatment, quality improvement implementation involves an iterative and adaptive process in response to local events as the implementation proceeds [2]. These context-specific iterative changes to the implementation process are the fuzzy elements of change. This article describes a quality improvement initative to to reduce surgical site infections at an academic health science center with a focus on the fuzziness inherent in the process and our iterative responses to local events.
Setting
The Hospital for Sick Children (Sickkids) is a childrens’ academic health science center in Toronto, Ontario, Canada. The largest children’s hospital in Canada, with 8000 health care professionals, scientists, trainees, administrative and support staff, it has approximately 300 beds, 15,000 inpatient admissions, 12,000 surgical procedures, 70,000 emergency visits, and 300,000 outpatient visits annually. The hospital is a Level 1 trauma unit and performs the full spectrum of pediatric surgical care including transplant and cardiac procedures. The hospital and physician staffs are affiliated with the University of Toronto. The hospital has 16 theatre operating rooms, with 11 perioperative divisions and departments.
The departmental and divisional structure of the hospital, which emulates the university organizational structure, does not represent the size and level of clinical activity of the groups. For example, the department of otolaryngology, head and neck surgery has 5 surgeons whereas the division of orthopedics (as one of 6 divisions in the department of surgery) has 9 orthopedic surgeons. Furthermore, a divisional and departmental structure arguably does not match the institutional operational aims related to patient care delivery. Thus, in 2007 the 3 departments of surgery, the departments of critical care, anaesthesia and pain medicine, and dentistry were clustered together as “perioperative services,” reporting to a chief of perioperative services who in turn reported directly to the CEO. The chief of perioperative services, responsible for all operational issues, was concurrently the surgeon-in-chief.
Physicians at Sickkids are not paid fee-for-service. Each division/department receives compensation according to their specific speciality on a full-time equivalent (FTE) basis. While clinical and academic productivity is measured, physicians do not receive activity-based compensation. The perioperative service chiefs have primary responsibility for the clinical operations and academic activity. A perioperative care unit (POCU) executive has primarily responsibility for policy and financial oversight of the operating rooms.
As this was primarily a quality improvement initiative, we obtained institutional approval through that process.
Defining the Target for Quality improvement
To determine shared objectives for quality improvement, the surgeon-in-chief organized a daylong retreat in 2005 of all physicians (of the 11 divisions and departments that was later called perioperative services), nurses, and other disciplines involved in delivering surgical care. All scheduled clinics and OR activity were cancelled. The start and end of the retreat day matched the nursing day shift with a voluntary social event at the end. In the morning after meeting together, the 3 disciplines of nursing, surgery and anaesthesia met to discuss speciality-specific issues. In the afternoon, the 3 disciplines reconvened in small multidisciplinary groups of 8 to 10 individuals to discuss the objectives for improvement using the Institute of Medicine framework [1]. Outcomes of the small group discussions were presented to, and discussed by, the entire group, and those initiatives that achieved general endorsement were approved. A report summarising all recommendations arising from the day was widely circulated for comment. Recommendations were grouped, where appropriate, and assigned to task forces. Task forces were multidisciplinary groups co-led by 2 disciplines, with specific objectives arising from the retreat recommendations with measurable goals and a timeline of 12 to 18 months for completion of the recommendations.
The retreat of the perioperative services group recognized that many aspects of high quality care were hampered by variable diagnoses, comorbidities, and multiple and complex interventions with a critical lack of easily measured and cogent outcomes. The 4 areas that were relevant to all disciplines, most amenable to evaluation, and where significant quality gains were perceived to be necessary and possible were safety, perioperative pain, access to surgery, and surgical site infection (SSI). This paper reports on the SSI QI program.
Initial Task Force Work
An SSI task force initially addressed surgical preparation solution, hair clipping, oxygenation and normothermia. All razors were physically removed from the ORs and replaced by electric clippers. Multi-use proviodine preparation solution was replaced by single-use 70% isopropyl alcohol with 2% chlorhexidene (except for open wounds and neonates). Pilot studies of patients arriving in the POCU revealed that hypoxia was not an issue and normothermia was seldom an issue. Thereafter the prime focus shifted to the use of prophylactic antibiotics to reduce SSI.
Compliance with Antibiotic Prophylaxis Guideline
Guideline Update Process
A guideline for the use of prophylactic antibiotics to prevent SSI had been in place at Sickkids for many years. However, a chart review revealed only 40% of patients were receiving the correct drug, dose, duration, and time of administration relative to the incision, and few patients were receiving appropriate intraoperative top-ups [3]. In addition, the existing guideline was incomplete for all specialities and procedures, did not consider the issue of beta-lactam antibiotic allergy, and had no specific dosing for neonates. Therefore, the guideline needed to be updated and be more comprehensive before any attempts to increase compliance with the guideline was initiated. The infection control specialist and pharmacist reviewed evidence-based guidelines from the literature on adults to create a guideline comprehensive for speciality and procedure with specific dosing for neonates and alternative antibiotics for patients allergic to penicillin [3]. Updating the guidelines took almost a year.
The next step was to seek endorsement of all the surgical subspecialities. The guidelines were circulated to all specialities for comments. While a few specialists provided minor comments, as discussed further below, this step did not result in substantive feedback and again took almost a year.
The final guidelines were discussed at multiple meetings of the members of perioperative services and approved by the hospital drug and therapeutics committee. A date was set to introduce the new guideline and announced at departmental meetings, in emails, and on banners in the OR.
The revised guidelines replaced the old guidelines on the e-formulary. Hard copies were attached to the anaesthetic machine in each OR and the need for antibiotics was made part of the “time-out” before commencement of the procedure.
Early Monitoring of Guideline Use
To monitor the use of the guidelines, the use of an antibiotic and the timing related to the surgical incision became part of charting by nurses. Nurses charted many aspects of the surgical procedure through a surgical information management system (SIS, Alpharetta, GA). While documentation of the specific drug and dose was considered important information, the additional charting burden for nurses was considered to be too great. Thus the compromise was to chart if a drug was given and the time of administration to allow determination if the drug was given within an hour of the surgical incision.
Early results from monitoring of antibiotic administration revealed that drugs often were given well in advance of the 1-hour target. To address this issue, first, antibiotics given “on call to OR” was eliminated (because the duration from the call to go to the OR and until the surgical incision was never less than 1 hour) and thereafter all antibiotics were given in the OR. Second, due to prolonged anesthetic times prior to surgical start for complex cases, anesthetists changed their practise to give antibiotics as one of the final steps prior to start of surgery.
The next step was to monitor the use and timing of antibiotics by surgical division/department automatically using data from SIS. Concurrent with the efforts to improve the use of prophylactic antibiotic, a score card had been created to monitor quality and efficiency activities within perioperative services. The use and timing of prophylactic antibiotics became part of that monthly report. While the appropriate use of antibiotics improved over 6 months, a repeat audit revealed that compliance with the guideline for patients to receive, or not receive, antibiotics was only moderately improved [5]. Furthermore, whereas the guideline stated that antibiotics were needed only intra-operatively for the majority of procedures, antibiotics were extended postoperatively for periods ranging from 24 to 72 hours.
Addressing Compliance Issues
First, semi-annual mandatory lectures were presented to residents and fellows delineating the importance of the guidelines, with a specific focus on correct duration of antibiotics. Furthermore a “stop warning” was added to the computerized physician order entry system (orders are completed almost exclusively by house staff). In addition, we introduced an individual audit and feedback mechanism (see below).
Automated Audit and Feedback Process and Results
Each surgeon and anesthetist received an automated email the morning after the procedure detailing whether antibiotics had been indicated and whether they had been given or held appropriately. To accomplish this required that all surgical procedures (entered on SIS by the nurses) were matched to the guidelines. With the assistance of each division and department, each SIS procedural code was matched to the guideline as to whether antibiotics were indicated or not. In the case of multiple procedures, if any of the procedures warranted antibiotics then antibiotics were indicated for that patient. The automatic email sent to the staff acknowledged potential errors due to incorrect matching of the surgical procedure to guideline, incorrect charting by nurses, and incorrect indication of the guideline to receive (or not receive) antibiotics.
The response to this email had several impacts. First, the response identified many errors related to matching of SIS procedure to guidelines. Second, the email served as impetus to improve nurse charting. Third, through the automated emails we determined that some patients were on antibiotics for a pre-existing infection. Thus a separate notation in the SIS charting by the nursing staff was added to indicate a pre-existing infection (to prevent an automated email). Fourth, while circulation of the guidelines to all divisions and departments had provided little feedback to the final draft of guideline, responses to the emails resulted in refinement of ambiguities in guideline related to procedure description, and in some cases changes to the guideline based on the use of antibiotics. Fifth, the emails improved compliance with the guideline [3].
While audit and feedback resulted in a substantial rise in the appropriate use and timing of antibiotics, the nurses were often harassed about their charting, placing them in the uncomfortable position of seen to be enforcing the guideline. Also, some surgeons vehemently disliked the emails, pointing to occasional inaccuracies of the emails. Finally, the audit and feedback provided feedback after the surgical event, and while increasing attention on the guideline, did nothing for the individual patient. An alternative proposed strategy was that at the time of SIS charting of the procedure that SIS could serve as a decision tool and indicate whether antibiotics were indicated, and indicate the correct antibiotic. However SIS is proprietary software and we were unable to make the necessary programming changes.
Measuring SSI Rate
Concurrently with focusing on the process measures of the appropriate use of antibiotics, we also developed a mechanism to measure SSI [4]. Prior to this quality improvement initiative, the existing mechanism to measure institutional SSI was based on daily visits to surgical wards by infection control practitioners (ICPs) supplemented by identification of patients by positive wound cultures in microbiology. Due to the expense of active monitoring across all surgical disciplines, this program had been restricted to neurosurgery, cardiac surgery, and spine surgery (areas of high risk for SSI identified in the past). Because the hospital did not have the resources to expand ICP monitoring to all surgical areas, an alternative strategy of using health record coders was explored as a means to provide comprehensive rates of SSI for all disciplines.
The first step in using health records as a means to identify SSI was to perform a review of all SSIs identified by health records in the 3 priority areas monitored by the ICPs. All health records identified “SSI” were reviewed by a surgeon to determine which were and were not SSI, according to the Centers for Disease Control criteria [5]. The review identified that the International Classification of Disease (ICD−10) coding for SSI included, in addition to SSI, multiple types of infections such as sepsis and central line infections. The review also identified that the health record coders had no specific criteria and therefore were variable in how they coded “SSI.” The review identified that the ICPs missed some true infections that were identified by health record coders.
To address the ambiguity of ICD coding, extension codes to the ICD codes were added to code specifically for SSI. To address the lack of criteria for SSI, the health record coders were trained by ICPs to use Centers for Disease Control criteria for SSI [5]. While both of these steps improved the identification of SSI by health record coders, a subsequent chart audit identified false positive and false negative recording of SSI by both ICPs and health record coders. The task force accepted that no method was completely accurate and that health record coding for SSI was financially feasible and provided SSI rates for all surgical disciplines. The task force concluded that health record coding would serve the purpose of monitoring trends in SSIs.
Impact of Guideline Compliance
The final step in the quality improvement initiative of reducing SSI was to evaluate trends in use of prophylactic antibiotics and the relationship with SSI. Through the multiple iterative strategies described above, the administration of an antibiotic within an hour of the incision increased to over 80% of patients. To evaluate the impact of guideline compliance, approximately 9000 procedures were reviewed over a 21-month period [4]. In the approximately 4500 patients who had a guideline-based indication to receive antibiotics, the 80% who received correct administration of an antibiotic within 1 hour of the incision had a reduction in the rate of SSI by one third compared with the 20% who didn’t receive antibiotics. Of the approximately 4500 patients who did not have an indication for antibiotics, 80% did not receive antibiotics (20% did receive despite no indication) and had a (statistically insignificant) lower rate of SSI compared to the 20% who received antibiotics inappropriately. In summary, only 50% of children having surgery had an indication for antibiotics, and not receiving antibiotics saved money, reduced antibiotic exposure, and did not increase the rate of SSI. In the 50% of patients who received antibiotics according to the guidelines the rate of SSI was reduced by 30% [6].
Discussion
Duration of Project
The total duration of the Sickkids effort to measure and reduce the rate of SSI and thereby improve the quality of surgical care took almost 8 years. The duration, which ideally should have been about one quarter of that time, was due to multiple issues. First, there were many simultaneous competing demands to improve quality in other IOM domains such as safety and efficiency. Second, no one on the task force had protected time and thus meetings could be no more than monthly because people could not complete tasks in a shorter time frame. Third, many of the steps relied on wider physician involvement such as reviewing the revised guidelines. The physicians were slow to respond and only after all 9 surgical disciplines had signed off on the guidelines could implementation proceed. Finally, many of the important issues came up only after implementation of a specific step. For example, the recognition of the need for an individual audit and feedback mechanism created the need of mapping the procedures to guidelines to SIS procedures, a process that took more than a year to complete. Also the responses to the emails created the need for revisions to the guideline with subsequent delays for re-approval with hospital and IT support for eformulary changes.
Success Factors and Impediments
The factors that in retrospect seem critical to effecting positive change started with a general endorsement of the perioperative services group for improving quality and specifically SSI. The retreat and an open forum involving multiple disciplines was critical in creating a mandate for change. Second, the task force not only had multiple and key discipline representation for each aspect of the change management strategy, but the task force members were passionate about the importance of reducing SSI. Third, the multiple strategies used for change needed to be adaptive and iterative to new findings as they arose. While the task force attempted to anticipate barriers to change, only once the quality initiative started did the task force truly understand the barriers and respond in turn. Finally, the need for relentless energy by the leaders and task force was critical to seeing the project to completion.
While the appropriate use of antibiotics increased with a reduction in SSI, several aspects of this initiative were not successful. First, despite the surgeon-in-chief’s semi-annual lectures, this initiative did not successfully engage the majority of the house staff manifested by their continued habit of prescribing postoperative antibiotics for hours to days despite the guideline advice. Second, because nurses were tasked with asking about and recording the use of antibiotics, an unintended consequence was that they took the brunt of disgruntled physicians. Despite all our attempts, many nurses felt this initiative brought negative responses of physicians toward their charting duties. Third, while audit and feedback was an important strategy to improve guideline compliance, many physicians saw the daily emails in response to noncompliance with the guidelines as intrusive and irritating. Also we could not program SIS to make it a decision support in real time rather than documenting an event after the fact and, thereby, not enhancing care for that individual patient. Finally, we adopted a strategy of health record coding for SSI due to the prohibitive expenses of a comprehensive active monitoring strategies by ICPs.
Exportability
The strategies used in this quality improvement project to reduce SSI may be exportable to other hospitals with similar results. However, the emphasis on which element of change management strategy is most important would likely vary by context [2,6]. The elements most essential for success were a mechanism to develop group buy-in, a dedicated multidisciplinary task force with leader(s) with relentless commitment to achieving meaningful change, and a mechanism to evaluate both the process measures and the final outcome. The elements of change would vary by site and including consideration of the mechanism for physician compensation, commitment of physicians to institutional initiatives to enhance quality, and institutional resources to support quality initiatives.
None of the observed changes in this quality improvement initiative can be confidently attributed to any of the specific interventions. The interventions were completed in stages, but most importantly were constantly changed, emphasized and de-emphasized according to the responses. This is the fuzzy nature of change whereby leaders take reasonable steps to effect change but have to constantly adapt to barriers to change. While a specific change strategy generalizable to all contexts would be ideal, in the end at an institutional level, positive change is the ultimate aim rather than determining which interventions are effective. This response to events as they arise as illustrated in our quality improvement journey, is the fuzzy side of change management.
Conclusion
In conclusion, through a long period with a multitude of strategies, use of a guideline for prophylactic antibiotics increased and was associated with a reduction in SSI. Future directions need to consider cost-effective strategies to actively monitor SSI and testing of other strategies to reduce SSI. Institutions embarking on change need to consider that initiatives will likely need to adapt to specific contextual responses.
Corresponding author: James G. Wright, MD, PMH, FRCS, Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford Botnar Research Centre, Windmill Road, Oxford, OX3 7LD, UK, james.wright@ndorms.ox.ac.uk.
Funding/support: RB Salter Chair in Paediatric Surgical Research.
1. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
2. Grol R, Wensing M, Eccles M, Davis D, editors. Improving patient care: the implementation of change in health care. 2nd ed. Wiley Blackwell; 2013.
3. So JP, Aleem IS, Tsang DS, et al. Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg 2015;262:403–8.
4. Khoshbin A, So JP, Aleem IS, et al. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg 2015;262:397–402.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132.
6. Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof 2011;31:174–80.
From the Hospital for Sick Children, Toronto, ON.
Abstract
- Objective: To describe the iterative and adaptive process used in implementing strategies to reduce surgical site infections (SSI) in a pediatric academic health science center.
- Methods: A multidisciplinary group was tasked with implementing strategies to reduce SSI with a focus on evaluating the use of a guideline for the use of prophylactic antibiotics and determining the rate of SSI.
- Results: The task force initially addressed surgical preparation solution, hair removal, oxygenation, and normothermia. The task force subsequently revised a guideline for the use of prophylactic antibiotics and implemented the guideline iteratively with multiple strategies including audit and feedback, communication and dissemination, and computerised order entry. The appropriate use of the guideline was associated with a 30% reduction in the rate of SSI.
- Conclusion: Using iterative and adaptive strategies over many years, the SSI rate was reduced by 30%.
Improving quality of care is a prime concern for clinicians, patients, families, and health systems [1]. Quality improvement methods are used widely in medicine for studying and addressing problems with care and have successfully addressed gaps in quality. The challenges include defining quality, obtaining complete and accurate data about quality, developing meaningful and cost-effective interventions to improve quality, and to successfully change clinician’s behaviour with commensurate improvement in quality of care.
Quality improvement in health care involves effecting and assessing change in a setting of complexity and uncertainty. Whereas the randomized trial may be used to measure the effectiveness of a particular treatment, quality improvement implementation involves an iterative and adaptive process in response to local events as the implementation proceeds [2]. These context-specific iterative changes to the implementation process are the fuzzy elements of change. This article describes a quality improvement initative to to reduce surgical site infections at an academic health science center with a focus on the fuzziness inherent in the process and our iterative responses to local events.
Setting
The Hospital for Sick Children (Sickkids) is a childrens’ academic health science center in Toronto, Ontario, Canada. The largest children’s hospital in Canada, with 8000 health care professionals, scientists, trainees, administrative and support staff, it has approximately 300 beds, 15,000 inpatient admissions, 12,000 surgical procedures, 70,000 emergency visits, and 300,000 outpatient visits annually. The hospital is a Level 1 trauma unit and performs the full spectrum of pediatric surgical care including transplant and cardiac procedures. The hospital and physician staffs are affiliated with the University of Toronto. The hospital has 16 theatre operating rooms, with 11 perioperative divisions and departments.
The departmental and divisional structure of the hospital, which emulates the university organizational structure, does not represent the size and level of clinical activity of the groups. For example, the department of otolaryngology, head and neck surgery has 5 surgeons whereas the division of orthopedics (as one of 6 divisions in the department of surgery) has 9 orthopedic surgeons. Furthermore, a divisional and departmental structure arguably does not match the institutional operational aims related to patient care delivery. Thus, in 2007 the 3 departments of surgery, the departments of critical care, anaesthesia and pain medicine, and dentistry were clustered together as “perioperative services,” reporting to a chief of perioperative services who in turn reported directly to the CEO. The chief of perioperative services, responsible for all operational issues, was concurrently the surgeon-in-chief.
Physicians at Sickkids are not paid fee-for-service. Each division/department receives compensation according to their specific speciality on a full-time equivalent (FTE) basis. While clinical and academic productivity is measured, physicians do not receive activity-based compensation. The perioperative service chiefs have primary responsibility for the clinical operations and academic activity. A perioperative care unit (POCU) executive has primarily responsibility for policy and financial oversight of the operating rooms.
As this was primarily a quality improvement initiative, we obtained institutional approval through that process.
Defining the Target for Quality improvement
To determine shared objectives for quality improvement, the surgeon-in-chief organized a daylong retreat in 2005 of all physicians (of the 11 divisions and departments that was later called perioperative services), nurses, and other disciplines involved in delivering surgical care. All scheduled clinics and OR activity were cancelled. The start and end of the retreat day matched the nursing day shift with a voluntary social event at the end. In the morning after meeting together, the 3 disciplines of nursing, surgery and anaesthesia met to discuss speciality-specific issues. In the afternoon, the 3 disciplines reconvened in small multidisciplinary groups of 8 to 10 individuals to discuss the objectives for improvement using the Institute of Medicine framework [1]. Outcomes of the small group discussions were presented to, and discussed by, the entire group, and those initiatives that achieved general endorsement were approved. A report summarising all recommendations arising from the day was widely circulated for comment. Recommendations were grouped, where appropriate, and assigned to task forces. Task forces were multidisciplinary groups co-led by 2 disciplines, with specific objectives arising from the retreat recommendations with measurable goals and a timeline of 12 to 18 months for completion of the recommendations.
The retreat of the perioperative services group recognized that many aspects of high quality care were hampered by variable diagnoses, comorbidities, and multiple and complex interventions with a critical lack of easily measured and cogent outcomes. The 4 areas that were relevant to all disciplines, most amenable to evaluation, and where significant quality gains were perceived to be necessary and possible were safety, perioperative pain, access to surgery, and surgical site infection (SSI). This paper reports on the SSI QI program.
Initial Task Force Work
An SSI task force initially addressed surgical preparation solution, hair clipping, oxygenation and normothermia. All razors were physically removed from the ORs and replaced by electric clippers. Multi-use proviodine preparation solution was replaced by single-use 70% isopropyl alcohol with 2% chlorhexidene (except for open wounds and neonates). Pilot studies of patients arriving in the POCU revealed that hypoxia was not an issue and normothermia was seldom an issue. Thereafter the prime focus shifted to the use of prophylactic antibiotics to reduce SSI.
Compliance with Antibiotic Prophylaxis Guideline
Guideline Update Process
A guideline for the use of prophylactic antibiotics to prevent SSI had been in place at Sickkids for many years. However, a chart review revealed only 40% of patients were receiving the correct drug, dose, duration, and time of administration relative to the incision, and few patients were receiving appropriate intraoperative top-ups [3]. In addition, the existing guideline was incomplete for all specialities and procedures, did not consider the issue of beta-lactam antibiotic allergy, and had no specific dosing for neonates. Therefore, the guideline needed to be updated and be more comprehensive before any attempts to increase compliance with the guideline was initiated. The infection control specialist and pharmacist reviewed evidence-based guidelines from the literature on adults to create a guideline comprehensive for speciality and procedure with specific dosing for neonates and alternative antibiotics for patients allergic to penicillin [3]. Updating the guidelines took almost a year.
The next step was to seek endorsement of all the surgical subspecialities. The guidelines were circulated to all specialities for comments. While a few specialists provided minor comments, as discussed further below, this step did not result in substantive feedback and again took almost a year.
The final guidelines were discussed at multiple meetings of the members of perioperative services and approved by the hospital drug and therapeutics committee. A date was set to introduce the new guideline and announced at departmental meetings, in emails, and on banners in the OR.
The revised guidelines replaced the old guidelines on the e-formulary. Hard copies were attached to the anaesthetic machine in each OR and the need for antibiotics was made part of the “time-out” before commencement of the procedure.
Early Monitoring of Guideline Use
To monitor the use of the guidelines, the use of an antibiotic and the timing related to the surgical incision became part of charting by nurses. Nurses charted many aspects of the surgical procedure through a surgical information management system (SIS, Alpharetta, GA). While documentation of the specific drug and dose was considered important information, the additional charting burden for nurses was considered to be too great. Thus the compromise was to chart if a drug was given and the time of administration to allow determination if the drug was given within an hour of the surgical incision.
Early results from monitoring of antibiotic administration revealed that drugs often were given well in advance of the 1-hour target. To address this issue, first, antibiotics given “on call to OR” was eliminated (because the duration from the call to go to the OR and until the surgical incision was never less than 1 hour) and thereafter all antibiotics were given in the OR. Second, due to prolonged anesthetic times prior to surgical start for complex cases, anesthetists changed their practise to give antibiotics as one of the final steps prior to start of surgery.
The next step was to monitor the use and timing of antibiotics by surgical division/department automatically using data from SIS. Concurrent with the efforts to improve the use of prophylactic antibiotic, a score card had been created to monitor quality and efficiency activities within perioperative services. The use and timing of prophylactic antibiotics became part of that monthly report. While the appropriate use of antibiotics improved over 6 months, a repeat audit revealed that compliance with the guideline for patients to receive, or not receive, antibiotics was only moderately improved [5]. Furthermore, whereas the guideline stated that antibiotics were needed only intra-operatively for the majority of procedures, antibiotics were extended postoperatively for periods ranging from 24 to 72 hours.
Addressing Compliance Issues
First, semi-annual mandatory lectures were presented to residents and fellows delineating the importance of the guidelines, with a specific focus on correct duration of antibiotics. Furthermore a “stop warning” was added to the computerized physician order entry system (orders are completed almost exclusively by house staff). In addition, we introduced an individual audit and feedback mechanism (see below).
Automated Audit and Feedback Process and Results
Each surgeon and anesthetist received an automated email the morning after the procedure detailing whether antibiotics had been indicated and whether they had been given or held appropriately. To accomplish this required that all surgical procedures (entered on SIS by the nurses) were matched to the guidelines. With the assistance of each division and department, each SIS procedural code was matched to the guideline as to whether antibiotics were indicated or not. In the case of multiple procedures, if any of the procedures warranted antibiotics then antibiotics were indicated for that patient. The automatic email sent to the staff acknowledged potential errors due to incorrect matching of the surgical procedure to guideline, incorrect charting by nurses, and incorrect indication of the guideline to receive (or not receive) antibiotics.
The response to this email had several impacts. First, the response identified many errors related to matching of SIS procedure to guidelines. Second, the email served as impetus to improve nurse charting. Third, through the automated emails we determined that some patients were on antibiotics for a pre-existing infection. Thus a separate notation in the SIS charting by the nursing staff was added to indicate a pre-existing infection (to prevent an automated email). Fourth, while circulation of the guidelines to all divisions and departments had provided little feedback to the final draft of guideline, responses to the emails resulted in refinement of ambiguities in guideline related to procedure description, and in some cases changes to the guideline based on the use of antibiotics. Fifth, the emails improved compliance with the guideline [3].
While audit and feedback resulted in a substantial rise in the appropriate use and timing of antibiotics, the nurses were often harassed about their charting, placing them in the uncomfortable position of seen to be enforcing the guideline. Also, some surgeons vehemently disliked the emails, pointing to occasional inaccuracies of the emails. Finally, the audit and feedback provided feedback after the surgical event, and while increasing attention on the guideline, did nothing for the individual patient. An alternative proposed strategy was that at the time of SIS charting of the procedure that SIS could serve as a decision tool and indicate whether antibiotics were indicated, and indicate the correct antibiotic. However SIS is proprietary software and we were unable to make the necessary programming changes.
Measuring SSI Rate
Concurrently with focusing on the process measures of the appropriate use of antibiotics, we also developed a mechanism to measure SSI [4]. Prior to this quality improvement initiative, the existing mechanism to measure institutional SSI was based on daily visits to surgical wards by infection control practitioners (ICPs) supplemented by identification of patients by positive wound cultures in microbiology. Due to the expense of active monitoring across all surgical disciplines, this program had been restricted to neurosurgery, cardiac surgery, and spine surgery (areas of high risk for SSI identified in the past). Because the hospital did not have the resources to expand ICP monitoring to all surgical areas, an alternative strategy of using health record coders was explored as a means to provide comprehensive rates of SSI for all disciplines.
The first step in using health records as a means to identify SSI was to perform a review of all SSIs identified by health records in the 3 priority areas monitored by the ICPs. All health records identified “SSI” were reviewed by a surgeon to determine which were and were not SSI, according to the Centers for Disease Control criteria [5]. The review identified that the International Classification of Disease (ICD−10) coding for SSI included, in addition to SSI, multiple types of infections such as sepsis and central line infections. The review also identified that the health record coders had no specific criteria and therefore were variable in how they coded “SSI.” The review identified that the ICPs missed some true infections that were identified by health record coders.
To address the ambiguity of ICD coding, extension codes to the ICD codes were added to code specifically for SSI. To address the lack of criteria for SSI, the health record coders were trained by ICPs to use Centers for Disease Control criteria for SSI [5]. While both of these steps improved the identification of SSI by health record coders, a subsequent chart audit identified false positive and false negative recording of SSI by both ICPs and health record coders. The task force accepted that no method was completely accurate and that health record coding for SSI was financially feasible and provided SSI rates for all surgical disciplines. The task force concluded that health record coding would serve the purpose of monitoring trends in SSIs.
Impact of Guideline Compliance
The final step in the quality improvement initiative of reducing SSI was to evaluate trends in use of prophylactic antibiotics and the relationship with SSI. Through the multiple iterative strategies described above, the administration of an antibiotic within an hour of the incision increased to over 80% of patients. To evaluate the impact of guideline compliance, approximately 9000 procedures were reviewed over a 21-month period [4]. In the approximately 4500 patients who had a guideline-based indication to receive antibiotics, the 80% who received correct administration of an antibiotic within 1 hour of the incision had a reduction in the rate of SSI by one third compared with the 20% who didn’t receive antibiotics. Of the approximately 4500 patients who did not have an indication for antibiotics, 80% did not receive antibiotics (20% did receive despite no indication) and had a (statistically insignificant) lower rate of SSI compared to the 20% who received antibiotics inappropriately. In summary, only 50% of children having surgery had an indication for antibiotics, and not receiving antibiotics saved money, reduced antibiotic exposure, and did not increase the rate of SSI. In the 50% of patients who received antibiotics according to the guidelines the rate of SSI was reduced by 30% [6].
Discussion
Duration of Project
The total duration of the Sickkids effort to measure and reduce the rate of SSI and thereby improve the quality of surgical care took almost 8 years. The duration, which ideally should have been about one quarter of that time, was due to multiple issues. First, there were many simultaneous competing demands to improve quality in other IOM domains such as safety and efficiency. Second, no one on the task force had protected time and thus meetings could be no more than monthly because people could not complete tasks in a shorter time frame. Third, many of the steps relied on wider physician involvement such as reviewing the revised guidelines. The physicians were slow to respond and only after all 9 surgical disciplines had signed off on the guidelines could implementation proceed. Finally, many of the important issues came up only after implementation of a specific step. For example, the recognition of the need for an individual audit and feedback mechanism created the need of mapping the procedures to guidelines to SIS procedures, a process that took more than a year to complete. Also the responses to the emails created the need for revisions to the guideline with subsequent delays for re-approval with hospital and IT support for eformulary changes.
Success Factors and Impediments
The factors that in retrospect seem critical to effecting positive change started with a general endorsement of the perioperative services group for improving quality and specifically SSI. The retreat and an open forum involving multiple disciplines was critical in creating a mandate for change. Second, the task force not only had multiple and key discipline representation for each aspect of the change management strategy, but the task force members were passionate about the importance of reducing SSI. Third, the multiple strategies used for change needed to be adaptive and iterative to new findings as they arose. While the task force attempted to anticipate barriers to change, only once the quality initiative started did the task force truly understand the barriers and respond in turn. Finally, the need for relentless energy by the leaders and task force was critical to seeing the project to completion.
While the appropriate use of antibiotics increased with a reduction in SSI, several aspects of this initiative were not successful. First, despite the surgeon-in-chief’s semi-annual lectures, this initiative did not successfully engage the majority of the house staff manifested by their continued habit of prescribing postoperative antibiotics for hours to days despite the guideline advice. Second, because nurses were tasked with asking about and recording the use of antibiotics, an unintended consequence was that they took the brunt of disgruntled physicians. Despite all our attempts, many nurses felt this initiative brought negative responses of physicians toward their charting duties. Third, while audit and feedback was an important strategy to improve guideline compliance, many physicians saw the daily emails in response to noncompliance with the guidelines as intrusive and irritating. Also we could not program SIS to make it a decision support in real time rather than documenting an event after the fact and, thereby, not enhancing care for that individual patient. Finally, we adopted a strategy of health record coding for SSI due to the prohibitive expenses of a comprehensive active monitoring strategies by ICPs.
Exportability
The strategies used in this quality improvement project to reduce SSI may be exportable to other hospitals with similar results. However, the emphasis on which element of change management strategy is most important would likely vary by context [2,6]. The elements most essential for success were a mechanism to develop group buy-in, a dedicated multidisciplinary task force with leader(s) with relentless commitment to achieving meaningful change, and a mechanism to evaluate both the process measures and the final outcome. The elements of change would vary by site and including consideration of the mechanism for physician compensation, commitment of physicians to institutional initiatives to enhance quality, and institutional resources to support quality initiatives.
None of the observed changes in this quality improvement initiative can be confidently attributed to any of the specific interventions. The interventions were completed in stages, but most importantly were constantly changed, emphasized and de-emphasized according to the responses. This is the fuzzy nature of change whereby leaders take reasonable steps to effect change but have to constantly adapt to barriers to change. While a specific change strategy generalizable to all contexts would be ideal, in the end at an institutional level, positive change is the ultimate aim rather than determining which interventions are effective. This response to events as they arise as illustrated in our quality improvement journey, is the fuzzy side of change management.
Conclusion
In conclusion, through a long period with a multitude of strategies, use of a guideline for prophylactic antibiotics increased and was associated with a reduction in SSI. Future directions need to consider cost-effective strategies to actively monitor SSI and testing of other strategies to reduce SSI. Institutions embarking on change need to consider that initiatives will likely need to adapt to specific contextual responses.
Corresponding author: James G. Wright, MD, PMH, FRCS, Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford Botnar Research Centre, Windmill Road, Oxford, OX3 7LD, UK, james.wright@ndorms.ox.ac.uk.
Funding/support: RB Salter Chair in Paediatric Surgical Research.
From the Hospital for Sick Children, Toronto, ON.
Abstract
- Objective: To describe the iterative and adaptive process used in implementing strategies to reduce surgical site infections (SSI) in a pediatric academic health science center.
- Methods: A multidisciplinary group was tasked with implementing strategies to reduce SSI with a focus on evaluating the use of a guideline for the use of prophylactic antibiotics and determining the rate of SSI.
- Results: The task force initially addressed surgical preparation solution, hair removal, oxygenation, and normothermia. The task force subsequently revised a guideline for the use of prophylactic antibiotics and implemented the guideline iteratively with multiple strategies including audit and feedback, communication and dissemination, and computerised order entry. The appropriate use of the guideline was associated with a 30% reduction in the rate of SSI.
- Conclusion: Using iterative and adaptive strategies over many years, the SSI rate was reduced by 30%.
Improving quality of care is a prime concern for clinicians, patients, families, and health systems [1]. Quality improvement methods are used widely in medicine for studying and addressing problems with care and have successfully addressed gaps in quality. The challenges include defining quality, obtaining complete and accurate data about quality, developing meaningful and cost-effective interventions to improve quality, and to successfully change clinician’s behaviour with commensurate improvement in quality of care.
Quality improvement in health care involves effecting and assessing change in a setting of complexity and uncertainty. Whereas the randomized trial may be used to measure the effectiveness of a particular treatment, quality improvement implementation involves an iterative and adaptive process in response to local events as the implementation proceeds [2]. These context-specific iterative changes to the implementation process are the fuzzy elements of change. This article describes a quality improvement initative to to reduce surgical site infections at an academic health science center with a focus on the fuzziness inherent in the process and our iterative responses to local events.
Setting
The Hospital for Sick Children (Sickkids) is a childrens’ academic health science center in Toronto, Ontario, Canada. The largest children’s hospital in Canada, with 8000 health care professionals, scientists, trainees, administrative and support staff, it has approximately 300 beds, 15,000 inpatient admissions, 12,000 surgical procedures, 70,000 emergency visits, and 300,000 outpatient visits annually. The hospital is a Level 1 trauma unit and performs the full spectrum of pediatric surgical care including transplant and cardiac procedures. The hospital and physician staffs are affiliated with the University of Toronto. The hospital has 16 theatre operating rooms, with 11 perioperative divisions and departments.
The departmental and divisional structure of the hospital, which emulates the university organizational structure, does not represent the size and level of clinical activity of the groups. For example, the department of otolaryngology, head and neck surgery has 5 surgeons whereas the division of orthopedics (as one of 6 divisions in the department of surgery) has 9 orthopedic surgeons. Furthermore, a divisional and departmental structure arguably does not match the institutional operational aims related to patient care delivery. Thus, in 2007 the 3 departments of surgery, the departments of critical care, anaesthesia and pain medicine, and dentistry were clustered together as “perioperative services,” reporting to a chief of perioperative services who in turn reported directly to the CEO. The chief of perioperative services, responsible for all operational issues, was concurrently the surgeon-in-chief.
Physicians at Sickkids are not paid fee-for-service. Each division/department receives compensation according to their specific speciality on a full-time equivalent (FTE) basis. While clinical and academic productivity is measured, physicians do not receive activity-based compensation. The perioperative service chiefs have primary responsibility for the clinical operations and academic activity. A perioperative care unit (POCU) executive has primarily responsibility for policy and financial oversight of the operating rooms.
As this was primarily a quality improvement initiative, we obtained institutional approval through that process.
Defining the Target for Quality improvement
To determine shared objectives for quality improvement, the surgeon-in-chief organized a daylong retreat in 2005 of all physicians (of the 11 divisions and departments that was later called perioperative services), nurses, and other disciplines involved in delivering surgical care. All scheduled clinics and OR activity were cancelled. The start and end of the retreat day matched the nursing day shift with a voluntary social event at the end. In the morning after meeting together, the 3 disciplines of nursing, surgery and anaesthesia met to discuss speciality-specific issues. In the afternoon, the 3 disciplines reconvened in small multidisciplinary groups of 8 to 10 individuals to discuss the objectives for improvement using the Institute of Medicine framework [1]. Outcomes of the small group discussions were presented to, and discussed by, the entire group, and those initiatives that achieved general endorsement were approved. A report summarising all recommendations arising from the day was widely circulated for comment. Recommendations were grouped, where appropriate, and assigned to task forces. Task forces were multidisciplinary groups co-led by 2 disciplines, with specific objectives arising from the retreat recommendations with measurable goals and a timeline of 12 to 18 months for completion of the recommendations.
The retreat of the perioperative services group recognized that many aspects of high quality care were hampered by variable diagnoses, comorbidities, and multiple and complex interventions with a critical lack of easily measured and cogent outcomes. The 4 areas that were relevant to all disciplines, most amenable to evaluation, and where significant quality gains were perceived to be necessary and possible were safety, perioperative pain, access to surgery, and surgical site infection (SSI). This paper reports on the SSI QI program.
Initial Task Force Work
An SSI task force initially addressed surgical preparation solution, hair clipping, oxygenation and normothermia. All razors were physically removed from the ORs and replaced by electric clippers. Multi-use proviodine preparation solution was replaced by single-use 70% isopropyl alcohol with 2% chlorhexidene (except for open wounds and neonates). Pilot studies of patients arriving in the POCU revealed that hypoxia was not an issue and normothermia was seldom an issue. Thereafter the prime focus shifted to the use of prophylactic antibiotics to reduce SSI.
Compliance with Antibiotic Prophylaxis Guideline
Guideline Update Process
A guideline for the use of prophylactic antibiotics to prevent SSI had been in place at Sickkids for many years. However, a chart review revealed only 40% of patients were receiving the correct drug, dose, duration, and time of administration relative to the incision, and few patients were receiving appropriate intraoperative top-ups [3]. In addition, the existing guideline was incomplete for all specialities and procedures, did not consider the issue of beta-lactam antibiotic allergy, and had no specific dosing for neonates. Therefore, the guideline needed to be updated and be more comprehensive before any attempts to increase compliance with the guideline was initiated. The infection control specialist and pharmacist reviewed evidence-based guidelines from the literature on adults to create a guideline comprehensive for speciality and procedure with specific dosing for neonates and alternative antibiotics for patients allergic to penicillin [3]. Updating the guidelines took almost a year.
The next step was to seek endorsement of all the surgical subspecialities. The guidelines were circulated to all specialities for comments. While a few specialists provided minor comments, as discussed further below, this step did not result in substantive feedback and again took almost a year.
The final guidelines were discussed at multiple meetings of the members of perioperative services and approved by the hospital drug and therapeutics committee. A date was set to introduce the new guideline and announced at departmental meetings, in emails, and on banners in the OR.
The revised guidelines replaced the old guidelines on the e-formulary. Hard copies were attached to the anaesthetic machine in each OR and the need for antibiotics was made part of the “time-out” before commencement of the procedure.
Early Monitoring of Guideline Use
To monitor the use of the guidelines, the use of an antibiotic and the timing related to the surgical incision became part of charting by nurses. Nurses charted many aspects of the surgical procedure through a surgical information management system (SIS, Alpharetta, GA). While documentation of the specific drug and dose was considered important information, the additional charting burden for nurses was considered to be too great. Thus the compromise was to chart if a drug was given and the time of administration to allow determination if the drug was given within an hour of the surgical incision.
Early results from monitoring of antibiotic administration revealed that drugs often were given well in advance of the 1-hour target. To address this issue, first, antibiotics given “on call to OR” was eliminated (because the duration from the call to go to the OR and until the surgical incision was never less than 1 hour) and thereafter all antibiotics were given in the OR. Second, due to prolonged anesthetic times prior to surgical start for complex cases, anesthetists changed their practise to give antibiotics as one of the final steps prior to start of surgery.
The next step was to monitor the use and timing of antibiotics by surgical division/department automatically using data from SIS. Concurrent with the efforts to improve the use of prophylactic antibiotic, a score card had been created to monitor quality and efficiency activities within perioperative services. The use and timing of prophylactic antibiotics became part of that monthly report. While the appropriate use of antibiotics improved over 6 months, a repeat audit revealed that compliance with the guideline for patients to receive, or not receive, antibiotics was only moderately improved [5]. Furthermore, whereas the guideline stated that antibiotics were needed only intra-operatively for the majority of procedures, antibiotics were extended postoperatively for periods ranging from 24 to 72 hours.
Addressing Compliance Issues
First, semi-annual mandatory lectures were presented to residents and fellows delineating the importance of the guidelines, with a specific focus on correct duration of antibiotics. Furthermore a “stop warning” was added to the computerized physician order entry system (orders are completed almost exclusively by house staff). In addition, we introduced an individual audit and feedback mechanism (see below).
Automated Audit and Feedback Process and Results
Each surgeon and anesthetist received an automated email the morning after the procedure detailing whether antibiotics had been indicated and whether they had been given or held appropriately. To accomplish this required that all surgical procedures (entered on SIS by the nurses) were matched to the guidelines. With the assistance of each division and department, each SIS procedural code was matched to the guideline as to whether antibiotics were indicated or not. In the case of multiple procedures, if any of the procedures warranted antibiotics then antibiotics were indicated for that patient. The automatic email sent to the staff acknowledged potential errors due to incorrect matching of the surgical procedure to guideline, incorrect charting by nurses, and incorrect indication of the guideline to receive (or not receive) antibiotics.
The response to this email had several impacts. First, the response identified many errors related to matching of SIS procedure to guidelines. Second, the email served as impetus to improve nurse charting. Third, through the automated emails we determined that some patients were on antibiotics for a pre-existing infection. Thus a separate notation in the SIS charting by the nursing staff was added to indicate a pre-existing infection (to prevent an automated email). Fourth, while circulation of the guidelines to all divisions and departments had provided little feedback to the final draft of guideline, responses to the emails resulted in refinement of ambiguities in guideline related to procedure description, and in some cases changes to the guideline based on the use of antibiotics. Fifth, the emails improved compliance with the guideline [3].
While audit and feedback resulted in a substantial rise in the appropriate use and timing of antibiotics, the nurses were often harassed about their charting, placing them in the uncomfortable position of seen to be enforcing the guideline. Also, some surgeons vehemently disliked the emails, pointing to occasional inaccuracies of the emails. Finally, the audit and feedback provided feedback after the surgical event, and while increasing attention on the guideline, did nothing for the individual patient. An alternative proposed strategy was that at the time of SIS charting of the procedure that SIS could serve as a decision tool and indicate whether antibiotics were indicated, and indicate the correct antibiotic. However SIS is proprietary software and we were unable to make the necessary programming changes.
Measuring SSI Rate
Concurrently with focusing on the process measures of the appropriate use of antibiotics, we also developed a mechanism to measure SSI [4]. Prior to this quality improvement initiative, the existing mechanism to measure institutional SSI was based on daily visits to surgical wards by infection control practitioners (ICPs) supplemented by identification of patients by positive wound cultures in microbiology. Due to the expense of active monitoring across all surgical disciplines, this program had been restricted to neurosurgery, cardiac surgery, and spine surgery (areas of high risk for SSI identified in the past). Because the hospital did not have the resources to expand ICP monitoring to all surgical areas, an alternative strategy of using health record coders was explored as a means to provide comprehensive rates of SSI for all disciplines.
The first step in using health records as a means to identify SSI was to perform a review of all SSIs identified by health records in the 3 priority areas monitored by the ICPs. All health records identified “SSI” were reviewed by a surgeon to determine which were and were not SSI, according to the Centers for Disease Control criteria [5]. The review identified that the International Classification of Disease (ICD−10) coding for SSI included, in addition to SSI, multiple types of infections such as sepsis and central line infections. The review also identified that the health record coders had no specific criteria and therefore were variable in how they coded “SSI.” The review identified that the ICPs missed some true infections that were identified by health record coders.
To address the ambiguity of ICD coding, extension codes to the ICD codes were added to code specifically for SSI. To address the lack of criteria for SSI, the health record coders were trained by ICPs to use Centers for Disease Control criteria for SSI [5]. While both of these steps improved the identification of SSI by health record coders, a subsequent chart audit identified false positive and false negative recording of SSI by both ICPs and health record coders. The task force accepted that no method was completely accurate and that health record coding for SSI was financially feasible and provided SSI rates for all surgical disciplines. The task force concluded that health record coding would serve the purpose of monitoring trends in SSIs.
Impact of Guideline Compliance
The final step in the quality improvement initiative of reducing SSI was to evaluate trends in use of prophylactic antibiotics and the relationship with SSI. Through the multiple iterative strategies described above, the administration of an antibiotic within an hour of the incision increased to over 80% of patients. To evaluate the impact of guideline compliance, approximately 9000 procedures were reviewed over a 21-month period [4]. In the approximately 4500 patients who had a guideline-based indication to receive antibiotics, the 80% who received correct administration of an antibiotic within 1 hour of the incision had a reduction in the rate of SSI by one third compared with the 20% who didn’t receive antibiotics. Of the approximately 4500 patients who did not have an indication for antibiotics, 80% did not receive antibiotics (20% did receive despite no indication) and had a (statistically insignificant) lower rate of SSI compared to the 20% who received antibiotics inappropriately. In summary, only 50% of children having surgery had an indication for antibiotics, and not receiving antibiotics saved money, reduced antibiotic exposure, and did not increase the rate of SSI. In the 50% of patients who received antibiotics according to the guidelines the rate of SSI was reduced by 30% [6].
Discussion
Duration of Project
The total duration of the Sickkids effort to measure and reduce the rate of SSI and thereby improve the quality of surgical care took almost 8 years. The duration, which ideally should have been about one quarter of that time, was due to multiple issues. First, there were many simultaneous competing demands to improve quality in other IOM domains such as safety and efficiency. Second, no one on the task force had protected time and thus meetings could be no more than monthly because people could not complete tasks in a shorter time frame. Third, many of the steps relied on wider physician involvement such as reviewing the revised guidelines. The physicians were slow to respond and only after all 9 surgical disciplines had signed off on the guidelines could implementation proceed. Finally, many of the important issues came up only after implementation of a specific step. For example, the recognition of the need for an individual audit and feedback mechanism created the need of mapping the procedures to guidelines to SIS procedures, a process that took more than a year to complete. Also the responses to the emails created the need for revisions to the guideline with subsequent delays for re-approval with hospital and IT support for eformulary changes.
Success Factors and Impediments
The factors that in retrospect seem critical to effecting positive change started with a general endorsement of the perioperative services group for improving quality and specifically SSI. The retreat and an open forum involving multiple disciplines was critical in creating a mandate for change. Second, the task force not only had multiple and key discipline representation for each aspect of the change management strategy, but the task force members were passionate about the importance of reducing SSI. Third, the multiple strategies used for change needed to be adaptive and iterative to new findings as they arose. While the task force attempted to anticipate barriers to change, only once the quality initiative started did the task force truly understand the barriers and respond in turn. Finally, the need for relentless energy by the leaders and task force was critical to seeing the project to completion.
While the appropriate use of antibiotics increased with a reduction in SSI, several aspects of this initiative were not successful. First, despite the surgeon-in-chief’s semi-annual lectures, this initiative did not successfully engage the majority of the house staff manifested by their continued habit of prescribing postoperative antibiotics for hours to days despite the guideline advice. Second, because nurses were tasked with asking about and recording the use of antibiotics, an unintended consequence was that they took the brunt of disgruntled physicians. Despite all our attempts, many nurses felt this initiative brought negative responses of physicians toward their charting duties. Third, while audit and feedback was an important strategy to improve guideline compliance, many physicians saw the daily emails in response to noncompliance with the guidelines as intrusive and irritating. Also we could not program SIS to make it a decision support in real time rather than documenting an event after the fact and, thereby, not enhancing care for that individual patient. Finally, we adopted a strategy of health record coding for SSI due to the prohibitive expenses of a comprehensive active monitoring strategies by ICPs.
Exportability
The strategies used in this quality improvement project to reduce SSI may be exportable to other hospitals with similar results. However, the emphasis on which element of change management strategy is most important would likely vary by context [2,6]. The elements most essential for success were a mechanism to develop group buy-in, a dedicated multidisciplinary task force with leader(s) with relentless commitment to achieving meaningful change, and a mechanism to evaluate both the process measures and the final outcome. The elements of change would vary by site and including consideration of the mechanism for physician compensation, commitment of physicians to institutional initiatives to enhance quality, and institutional resources to support quality initiatives.
None of the observed changes in this quality improvement initiative can be confidently attributed to any of the specific interventions. The interventions were completed in stages, but most importantly were constantly changed, emphasized and de-emphasized according to the responses. This is the fuzzy nature of change whereby leaders take reasonable steps to effect change but have to constantly adapt to barriers to change. While a specific change strategy generalizable to all contexts would be ideal, in the end at an institutional level, positive change is the ultimate aim rather than determining which interventions are effective. This response to events as they arise as illustrated in our quality improvement journey, is the fuzzy side of change management.
Conclusion
In conclusion, through a long period with a multitude of strategies, use of a guideline for prophylactic antibiotics increased and was associated with a reduction in SSI. Future directions need to consider cost-effective strategies to actively monitor SSI and testing of other strategies to reduce SSI. Institutions embarking on change need to consider that initiatives will likely need to adapt to specific contextual responses.
Corresponding author: James G. Wright, MD, PMH, FRCS, Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford Botnar Research Centre, Windmill Road, Oxford, OX3 7LD, UK, james.wright@ndorms.ox.ac.uk.
Funding/support: RB Salter Chair in Paediatric Surgical Research.
1. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
2. Grol R, Wensing M, Eccles M, Davis D, editors. Improving patient care: the implementation of change in health care. 2nd ed. Wiley Blackwell; 2013.
3. So JP, Aleem IS, Tsang DS, et al. Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg 2015;262:403–8.
4. Khoshbin A, So JP, Aleem IS, et al. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg 2015;262:397–402.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132.
6. Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof 2011;31:174–80.
1. Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
2. Grol R, Wensing M, Eccles M, Davis D, editors. Improving patient care: the implementation of change in health care. 2nd ed. Wiley Blackwell; 2013.
3. So JP, Aleem IS, Tsang DS, et al. Increasing compliance with an antibiotic prophylaxis guideline to prevent pediatric surgical site infection: before and after study. Ann Surg 2015;262:403–8.
4. Khoshbin A, So JP, Aleem IS, et al. Antibiotic prophylaxis to prevent surgical site infections in children: a prospective cohort study. Ann Surg 2015;262:397–402.
5. Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132.
6. Curran JA, Grimshaw JM, Hayden JA, Campbell B. Knowledge translation research: the science of moving research into policy and practice. J Contin Educ Health Prof 2011;31:174–80.
Attitudes Surrounding Continuous Telemetry Utilization by Providers at an Academic Tertiary Medical Center
From the Johns Hopkins Bayview Medical Center, Baltimore, MD (Drs. Johnson, Knight, Maygers, and Zakaria), and Duke University Hospital, Durham, NC (Dr. Mock).
Abstract
- Objective: To determine patterns of telemetry use at a tertiary academic institution and identify factors contributing to noncompliance with guidelines regarding telemetry use.
- Methods: Web-based survey of 180 providers, including internal medicine residents and cardiovascular disease fellows, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners, and physician assistants.
- Results: Of the 180 providers surveyed, 67 (37%) replied. Most providers (76%) were unaware of guidelines regarding appropriate telemetry use and 85% selected inappropriate diagnoses as warranting telemetry. Only 21% routinely discontinued the telemetry order within 48 hours.
- Conclusions: Many providers at a tertiary academic institution utilize continuous telemetry inappropriately and are unaware of telemetry guidelines. These findings should guide interventions to improve telemetry utilization.
For many decades, telemetry has been widely used in the management and monitoring of patients with possible acute coronary syndromes (ACS), arrhythmias, cardiac events, and strokes [1]. In addition, telemetry has often been used in other clinical scenarios with less rigorous data supporting its use [2–4]. As a result, in 2004 the American Heart Association (AHA) issued guidelines providing recommendations for best practices in hospital ECG monitoring. Indications for telemetry were classified into 3 diagnosis-driven groups: class I (indicated in all patients), class II (indicated in most patients, may be of benefit) and class III (not indicated, no therapeutic benefit) [2]. However, these recommendations have not been widely followed and telemetry is inappropriately used for many inpatients [5,6].
There are several reasons why clinicians fail to adhere to guidelines, including knowledge deficits, attitudes regarding the current guidelines, and institution-specific factors influencing practitioner behaviors [7]. In response to reports of widespread telemetry overuse, the Choosing Wisely Campaign of the American Board of Internal Medicine Foundation has championed judicious telemetry use, advocating evidence-based, protocol-driven telemetry management for patients not in intensive care units who do not meet guideline-based criteria for continuous telemetry [8].
In order to understand patterns of telemetry use at our academic institution and identify factors associated with this practice, we systematically analyzed telemetry use perceptions through provider surveys. We hypothesized that providers have misperceptions about appropriate use of telemetry and that this knowledge gap results in overuse of telemetry at our institution.
Methods
Setting
Johns Hopkins Bayview Medical Center is a 400-bed academic medical center serving southeastern Baltimore. Providers included internal medicine residents and cardiovascular disease fellows who rotate to the medical center and Johns Hopkins Hospital, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners (NPs), and physician assistants (PAs).
Current Telemetry Practice
Remote telemetric monitoring is available in all adult, non-intensive care units of the hospital except for the psychiatry unit. However, the number of monitors are limited and it is not possible to monitor every patient if the wards are at capacity. Obstetrics uses its own unique cardiac monitoring system and thus was not included in the survey. Each monitor (IntelliVue, Philips Healthcare, Amsterdam, Netherlands) is attached to the patient using 5 lead wires, with electrocardiographic data transmitted to a monitoring station based in the progressive care unit, a cardio-pulmonary step-down unit. Monitors can be ordered in one of 3 manners, as mandated by hospital policy:
- Continuous telemetry – Telemetry monitoring is uninterrupted until discontinued by a provider.
- Telemetry protocol – Within 12 hours of telemetry placement, a monitor technician generates a report, which is reviewed by the nurse caring for the patient. The nurse performs an electrocardiogram (ECG) if the patient meets pre-specified criteria for telemetry discontinuation, which includes the absence of arrhythmias, troponin elevations, chest pain, or hemodynamic instability. The repeat ECG is then read and signed by the provider. After these criteria are met, telemetry can be discontinued.
- Stroke telemetry protocol – Telemetry is applied for 48 hours, mainly for detection of paroxysmal atrial fibrillation. Monitoring can be temporarily discontinued if the patient requires magnetic resonance imaging, which interferes with the telemetric monitors.
When entering any of the 3 possible telemetry orders in our computerized provider order entry system (Meditech, Westwood, MA), the ordering provider is required to indicate baseline rhythm, pacemaker presence, and desired heart rate warning parameters. Once the order is electronically signed, a monitor technician notes the order in a logbook and assigns the patient a telemeter, which is applied by the patient’s nurse.
If a monitored patient develops any predefined abnormal rhythm, audible alerts notify monitor technicians and an alert is sent to a portable telephone carried by the patient’s assigned nurse. Either the monitoring technician or the nurse then has the discretion to silence the alarm, note it in the chart, and/or contact the patient’s provider. If alerts are recorded, then a sample telemetry monitoring strip is saved into the patient’s paper medical chart.
Survey Instrument
After approval from the Johns Hopkins institutional review board, we queried providers who worked on the medicine and cardiology wards to assess the context and culture in which telemetry monitoring is used (see Appendix). The study was exempt from requiring informed consent. All staff had the option to decline study participation. We administered the survey using an online survey software program (SurveyMonkey, Palo Alto, CA), sending survey links via email to all internal medicine residents, cardiovascular disease fellows, internal medicine and cardiology teaching attending physicians, hospitalists, NPs, and PAs. Respondents completed the survey anonymously. To increase response rates, providers were sent a monthly reminder email. The survey was open from March 2014 to May 2014 for a total of 3 months.
Analysis
The survey data were compiled and analyzed using Microsoft Excel (Mac version 14.4; Microsoft, Redmond, WA). Variables are displayed as numbers and percentages, as appropriate.
Results
All providers reported having ordered telemetry, but almost all were either unaware of (76%) or only somewhat familiar with (21%) the AHA guidelines for appropriate telemetry use. Notably, the vast majority of fellows and residents reported that they were not at all familiar with the guidelines (100% and 96%, respectively). When asked why providers do not adhere to telemetry guidelines, lack of awareness of and lack of familiarity with the guidelines were the top 2 choices among respondents (Figure 1).
Additionally, most providers acknowledged experiencing adverse effects of telemetry: 86% (57/66) had experienced delayed patient transfers from the emergency department to inpatient floors due to telemetry unavailability and 97% (65/67) had experienced some delay in obtaining tests or studies for their telemetry-monitored patients. Despite acknowledging the potential consequences of telemetry use, only 21% (14/66) of providers routinely (ie, > 75% of the time) discontinued telemetry within 48 hours. Fifteen percent (10/65) routinely allowed telemetry to continue until the time of patient discharge. When discontinued, it was mainly due to the provider’s decision (57%); however, respondents noted that nurses prompted telemetry discontinuation 28% of the time.
Discussion
Consistent with previous studies [3–5,9–15], the majority of providers at our institution do not think continuous telemetry is appropriately utilized. Most survey respondents acknowledged a lack of awareness surrounding current guideline recommendations, which could explain why providers often do not follow them. Despite conceding their knowledge deficits, providers assumed their practice patterns for ordering telemetry were “appropriate”(ie, guideline-supported). This assertion may be incorrect as the majority of providers in our survey chose at least 1 non–guideline-supported indication for telemetry. Other studies have suggested additional reasons for inappropriate telemetry utilization. Providers may disagree with guideline recommendations, may assign lesser importance to guidelines when caring for an individual patient, or may fall victim to inertia (ie, not ordering telemetry appropriately simply because changing one’s practice pattern is difficult) [7].
In addition, the majority of our providers perceived telemetry overuse, which has been well-recognized nationwide [4]. While we did not assess this directly, other studies suggest that providers may overuse telemetry to provide a sense of reassurance when caring for a sick patient, since continuous telemetry is perceived to provide a higher level of care [6,15–17]. Unfortunately, no study has shown a benefit for continuous telemetry when placed for non-guideline-based diagnoses—whether for cardiac or non-cardiac diagnoses [3,9–11,13,14]. Likewise, the guidelines suggest that telemetry use should be time-limited, since the majority of benefit is accrued in the first 48 hours. Beyond that time, no study has shown a clear benefit to continuous telemetry [2]. Therefore, telemetry overuse may lead to unnecessarily increased costs without added benefits [3,9–11,13–15,18].
Our conclusions are tempered by the nature of our survey data. We recognize that our survey has not been previously validated. In addition, our response rates were low. This low sample size may lead to under-representation of diverse ideas. Also, our survey results may not be generalizable, since our study was conducted at a single academic hospital. Our institution’s telemetry ordering culture may differ from others, therefore making our results less applicable to other centers.
Despite these limitations, our results aid in understanding attitudes that surround the use of continuous telemetry, which can shape formal educational interventions to encourage appropriate guideline-based telemetry use. Since our providers agree on the need for more education about the guidelines, components such as online modules or in-person lecture educational sessions, newsletters, email communications, and incorporation of AHA guidelines into the institution’s automated computer order entry system could be utilized [17]. Didactic interventions could be designed especially for trainees given their overall lack of familiarity with the guidelines. Another potential intervention could include supplying providers with publically shared personalized measures of their own practices, since providers benefit from reinforcement and individualized feedback on appropriate utilization practices [19]. Previous studies have suggested that a multidisciplinary approach to patient care leads to positive outcomes [20,21], and in our experience, nursing input is absolutely critical in outlining potential problems and in developing solutions. Our findings suggest that nurses could play an active role in alerting providers when patients have telemetry in use and identifying patients who may no longer need it.
In summary, we have shown that many providers at a tertiary academic institution utilized continuous telemetry inappropriately, and were unaware of guidelines surrounding telemetry use. Future interventions aimed at educating providers, encouraging dialogue between staff, and enabling guideline-supported utilization may increase appropriate telemetry use leading to lower cost and improved quality of patient care.
Acknowledgment: The authors wish to thank Dr. Colleen Christmas, Dr. Panagis Galiatsatos, Mrs. Barbara Brigade, Ms. Joetta Love, Ms. Terri Rigsby, and Mrs. Lisa Shirk for their invaluable technical and administrative support.
Corresponding author: Amber Johnson, MD, MBA, 200 Lothrop St., S-553 Scaife Hall, Pittsburgh, PA 15213, amberjohn@gmail.com.
Financial disclosures: None.
1. Day H. Preliminary studies of an acute coronary care area. J Lancet 1963;83:53–5.
2. Drew B, Califf R, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110:2721–46.
3. Estrada C, Battilana G, Alexander M, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med 2000;15:51–5.
4. Henriques-Forsythe M, Ivonye C, Jamched U, et al. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med 2009;76:368–72.
5. Chen E, Hollander, J. When do patients need admission to a telemetry bed? J Emerg Med 2007;33:53–60.
6. Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012;172:1349–50.
7. Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines?: A framework for improvement. JAMA 1999;282:1458–65.
8. Adult hospital medicine. Five things physicians and patients should question. 15 Aug 2013. Available at www.choosingwisely.org/doctor-patient-lists/society-of-hospital-medicine-adult-hospital-medicine/
9. Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: A prospective cohort study of risk stratification and outcomes. Am J Med 2001;110:7–11.
10. Estrada C, Rosman H, Prasad N, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995;76:960–5.
11. Hollander J, Sites F, Pollack C, Shofer F. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71–6.
12. Ivonye C, Ohuabunwo C, Henriques-Forsythe M, et al. Evaluation of telemetry utilization, policy, and outcomes in an inner-city academic medical center. J Natl Med Assoc 2010;102:598–604.
13. Schull M, Redelmeier D. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000;7:647–52.
14. Sivaram C, Summers J, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998;21:503–5.
15. Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002;122:517–23.
16. Chen S, Zakaria S. Behind the monitor-The trouble with telemetry: a teachable moment. JAMA Intern Med 2015;175:894.
17. Dressler R, Dryer M, Coletti C, et al. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014;174:1852–4.
18. Benjamin E, Klugman R, Luckmann R, et al. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care 2013;19:e225–32.
19. Solomon D, Hashimoto H, Daltroy L, Liang M. Techniques to improve physicians use of diagnostic tests: A new conceptual framework. JAMA 1998;280:2020–7.
20. Richeson J, Johnson J. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung 1992;21:18–24.
21. Curley C, McEachern J, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care 1998;36:AS4–12.
From the Johns Hopkins Bayview Medical Center, Baltimore, MD (Drs. Johnson, Knight, Maygers, and Zakaria), and Duke University Hospital, Durham, NC (Dr. Mock).
Abstract
- Objective: To determine patterns of telemetry use at a tertiary academic institution and identify factors contributing to noncompliance with guidelines regarding telemetry use.
- Methods: Web-based survey of 180 providers, including internal medicine residents and cardiovascular disease fellows, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners, and physician assistants.
- Results: Of the 180 providers surveyed, 67 (37%) replied. Most providers (76%) were unaware of guidelines regarding appropriate telemetry use and 85% selected inappropriate diagnoses as warranting telemetry. Only 21% routinely discontinued the telemetry order within 48 hours.
- Conclusions: Many providers at a tertiary academic institution utilize continuous telemetry inappropriately and are unaware of telemetry guidelines. These findings should guide interventions to improve telemetry utilization.
For many decades, telemetry has been widely used in the management and monitoring of patients with possible acute coronary syndromes (ACS), arrhythmias, cardiac events, and strokes [1]. In addition, telemetry has often been used in other clinical scenarios with less rigorous data supporting its use [2–4]. As a result, in 2004 the American Heart Association (AHA) issued guidelines providing recommendations for best practices in hospital ECG monitoring. Indications for telemetry were classified into 3 diagnosis-driven groups: class I (indicated in all patients), class II (indicated in most patients, may be of benefit) and class III (not indicated, no therapeutic benefit) [2]. However, these recommendations have not been widely followed and telemetry is inappropriately used for many inpatients [5,6].
There are several reasons why clinicians fail to adhere to guidelines, including knowledge deficits, attitudes regarding the current guidelines, and institution-specific factors influencing practitioner behaviors [7]. In response to reports of widespread telemetry overuse, the Choosing Wisely Campaign of the American Board of Internal Medicine Foundation has championed judicious telemetry use, advocating evidence-based, protocol-driven telemetry management for patients not in intensive care units who do not meet guideline-based criteria for continuous telemetry [8].
In order to understand patterns of telemetry use at our academic institution and identify factors associated with this practice, we systematically analyzed telemetry use perceptions through provider surveys. We hypothesized that providers have misperceptions about appropriate use of telemetry and that this knowledge gap results in overuse of telemetry at our institution.
Methods
Setting
Johns Hopkins Bayview Medical Center is a 400-bed academic medical center serving southeastern Baltimore. Providers included internal medicine residents and cardiovascular disease fellows who rotate to the medical center and Johns Hopkins Hospital, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners (NPs), and physician assistants (PAs).
Current Telemetry Practice
Remote telemetric monitoring is available in all adult, non-intensive care units of the hospital except for the psychiatry unit. However, the number of monitors are limited and it is not possible to monitor every patient if the wards are at capacity. Obstetrics uses its own unique cardiac monitoring system and thus was not included in the survey. Each monitor (IntelliVue, Philips Healthcare, Amsterdam, Netherlands) is attached to the patient using 5 lead wires, with electrocardiographic data transmitted to a monitoring station based in the progressive care unit, a cardio-pulmonary step-down unit. Monitors can be ordered in one of 3 manners, as mandated by hospital policy:
- Continuous telemetry – Telemetry monitoring is uninterrupted until discontinued by a provider.
- Telemetry protocol – Within 12 hours of telemetry placement, a monitor technician generates a report, which is reviewed by the nurse caring for the patient. The nurse performs an electrocardiogram (ECG) if the patient meets pre-specified criteria for telemetry discontinuation, which includes the absence of arrhythmias, troponin elevations, chest pain, or hemodynamic instability. The repeat ECG is then read and signed by the provider. After these criteria are met, telemetry can be discontinued.
- Stroke telemetry protocol – Telemetry is applied for 48 hours, mainly for detection of paroxysmal atrial fibrillation. Monitoring can be temporarily discontinued if the patient requires magnetic resonance imaging, which interferes with the telemetric monitors.
When entering any of the 3 possible telemetry orders in our computerized provider order entry system (Meditech, Westwood, MA), the ordering provider is required to indicate baseline rhythm, pacemaker presence, and desired heart rate warning parameters. Once the order is electronically signed, a monitor technician notes the order in a logbook and assigns the patient a telemeter, which is applied by the patient’s nurse.
If a monitored patient develops any predefined abnormal rhythm, audible alerts notify monitor technicians and an alert is sent to a portable telephone carried by the patient’s assigned nurse. Either the monitoring technician or the nurse then has the discretion to silence the alarm, note it in the chart, and/or contact the patient’s provider. If alerts are recorded, then a sample telemetry monitoring strip is saved into the patient’s paper medical chart.
Survey Instrument
After approval from the Johns Hopkins institutional review board, we queried providers who worked on the medicine and cardiology wards to assess the context and culture in which telemetry monitoring is used (see Appendix). The study was exempt from requiring informed consent. All staff had the option to decline study participation. We administered the survey using an online survey software program (SurveyMonkey, Palo Alto, CA), sending survey links via email to all internal medicine residents, cardiovascular disease fellows, internal medicine and cardiology teaching attending physicians, hospitalists, NPs, and PAs. Respondents completed the survey anonymously. To increase response rates, providers were sent a monthly reminder email. The survey was open from March 2014 to May 2014 for a total of 3 months.
Analysis
The survey data were compiled and analyzed using Microsoft Excel (Mac version 14.4; Microsoft, Redmond, WA). Variables are displayed as numbers and percentages, as appropriate.
Results
All providers reported having ordered telemetry, but almost all were either unaware of (76%) or only somewhat familiar with (21%) the AHA guidelines for appropriate telemetry use. Notably, the vast majority of fellows and residents reported that they were not at all familiar with the guidelines (100% and 96%, respectively). When asked why providers do not adhere to telemetry guidelines, lack of awareness of and lack of familiarity with the guidelines were the top 2 choices among respondents (Figure 1).
Additionally, most providers acknowledged experiencing adverse effects of telemetry: 86% (57/66) had experienced delayed patient transfers from the emergency department to inpatient floors due to telemetry unavailability and 97% (65/67) had experienced some delay in obtaining tests or studies for their telemetry-monitored patients. Despite acknowledging the potential consequences of telemetry use, only 21% (14/66) of providers routinely (ie, > 75% of the time) discontinued telemetry within 48 hours. Fifteen percent (10/65) routinely allowed telemetry to continue until the time of patient discharge. When discontinued, it was mainly due to the provider’s decision (57%); however, respondents noted that nurses prompted telemetry discontinuation 28% of the time.
Discussion
Consistent with previous studies [3–5,9–15], the majority of providers at our institution do not think continuous telemetry is appropriately utilized. Most survey respondents acknowledged a lack of awareness surrounding current guideline recommendations, which could explain why providers often do not follow them. Despite conceding their knowledge deficits, providers assumed their practice patterns for ordering telemetry were “appropriate”(ie, guideline-supported). This assertion may be incorrect as the majority of providers in our survey chose at least 1 non–guideline-supported indication for telemetry. Other studies have suggested additional reasons for inappropriate telemetry utilization. Providers may disagree with guideline recommendations, may assign lesser importance to guidelines when caring for an individual patient, or may fall victim to inertia (ie, not ordering telemetry appropriately simply because changing one’s practice pattern is difficult) [7].
In addition, the majority of our providers perceived telemetry overuse, which has been well-recognized nationwide [4]. While we did not assess this directly, other studies suggest that providers may overuse telemetry to provide a sense of reassurance when caring for a sick patient, since continuous telemetry is perceived to provide a higher level of care [6,15–17]. Unfortunately, no study has shown a benefit for continuous telemetry when placed for non-guideline-based diagnoses—whether for cardiac or non-cardiac diagnoses [3,9–11,13,14]. Likewise, the guidelines suggest that telemetry use should be time-limited, since the majority of benefit is accrued in the first 48 hours. Beyond that time, no study has shown a clear benefit to continuous telemetry [2]. Therefore, telemetry overuse may lead to unnecessarily increased costs without added benefits [3,9–11,13–15,18].
Our conclusions are tempered by the nature of our survey data. We recognize that our survey has not been previously validated. In addition, our response rates were low. This low sample size may lead to under-representation of diverse ideas. Also, our survey results may not be generalizable, since our study was conducted at a single academic hospital. Our institution’s telemetry ordering culture may differ from others, therefore making our results less applicable to other centers.
Despite these limitations, our results aid in understanding attitudes that surround the use of continuous telemetry, which can shape formal educational interventions to encourage appropriate guideline-based telemetry use. Since our providers agree on the need for more education about the guidelines, components such as online modules or in-person lecture educational sessions, newsletters, email communications, and incorporation of AHA guidelines into the institution’s automated computer order entry system could be utilized [17]. Didactic interventions could be designed especially for trainees given their overall lack of familiarity with the guidelines. Another potential intervention could include supplying providers with publically shared personalized measures of their own practices, since providers benefit from reinforcement and individualized feedback on appropriate utilization practices [19]. Previous studies have suggested that a multidisciplinary approach to patient care leads to positive outcomes [20,21], and in our experience, nursing input is absolutely critical in outlining potential problems and in developing solutions. Our findings suggest that nurses could play an active role in alerting providers when patients have telemetry in use and identifying patients who may no longer need it.
In summary, we have shown that many providers at a tertiary academic institution utilized continuous telemetry inappropriately, and were unaware of guidelines surrounding telemetry use. Future interventions aimed at educating providers, encouraging dialogue between staff, and enabling guideline-supported utilization may increase appropriate telemetry use leading to lower cost and improved quality of patient care.
Acknowledgment: The authors wish to thank Dr. Colleen Christmas, Dr. Panagis Galiatsatos, Mrs. Barbara Brigade, Ms. Joetta Love, Ms. Terri Rigsby, and Mrs. Lisa Shirk for their invaluable technical and administrative support.
Corresponding author: Amber Johnson, MD, MBA, 200 Lothrop St., S-553 Scaife Hall, Pittsburgh, PA 15213, amberjohn@gmail.com.
Financial disclosures: None.
From the Johns Hopkins Bayview Medical Center, Baltimore, MD (Drs. Johnson, Knight, Maygers, and Zakaria), and Duke University Hospital, Durham, NC (Dr. Mock).
Abstract
- Objective: To determine patterns of telemetry use at a tertiary academic institution and identify factors contributing to noncompliance with guidelines regarding telemetry use.
- Methods: Web-based survey of 180 providers, including internal medicine residents and cardiovascular disease fellows, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners, and physician assistants.
- Results: Of the 180 providers surveyed, 67 (37%) replied. Most providers (76%) were unaware of guidelines regarding appropriate telemetry use and 85% selected inappropriate diagnoses as warranting telemetry. Only 21% routinely discontinued the telemetry order within 48 hours.
- Conclusions: Many providers at a tertiary academic institution utilize continuous telemetry inappropriately and are unaware of telemetry guidelines. These findings should guide interventions to improve telemetry utilization.
For many decades, telemetry has been widely used in the management and monitoring of patients with possible acute coronary syndromes (ACS), arrhythmias, cardiac events, and strokes [1]. In addition, telemetry has often been used in other clinical scenarios with less rigorous data supporting its use [2–4]. As a result, in 2004 the American Heart Association (AHA) issued guidelines providing recommendations for best practices in hospital ECG monitoring. Indications for telemetry were classified into 3 diagnosis-driven groups: class I (indicated in all patients), class II (indicated in most patients, may be of benefit) and class III (not indicated, no therapeutic benefit) [2]. However, these recommendations have not been widely followed and telemetry is inappropriately used for many inpatients [5,6].
There are several reasons why clinicians fail to adhere to guidelines, including knowledge deficits, attitudes regarding the current guidelines, and institution-specific factors influencing practitioner behaviors [7]. In response to reports of widespread telemetry overuse, the Choosing Wisely Campaign of the American Board of Internal Medicine Foundation has championed judicious telemetry use, advocating evidence-based, protocol-driven telemetry management for patients not in intensive care units who do not meet guideline-based criteria for continuous telemetry [8].
In order to understand patterns of telemetry use at our academic institution and identify factors associated with this practice, we systematically analyzed telemetry use perceptions through provider surveys. We hypothesized that providers have misperceptions about appropriate use of telemetry and that this knowledge gap results in overuse of telemetry at our institution.
Methods
Setting
Johns Hopkins Bayview Medical Center is a 400-bed academic medical center serving southeastern Baltimore. Providers included internal medicine residents and cardiovascular disease fellows who rotate to the medical center and Johns Hopkins Hospital, hospitalists, non-hospitalist teaching attending physicians, nurse practitioners (NPs), and physician assistants (PAs).
Current Telemetry Practice
Remote telemetric monitoring is available in all adult, non-intensive care units of the hospital except for the psychiatry unit. However, the number of monitors are limited and it is not possible to monitor every patient if the wards are at capacity. Obstetrics uses its own unique cardiac monitoring system and thus was not included in the survey. Each monitor (IntelliVue, Philips Healthcare, Amsterdam, Netherlands) is attached to the patient using 5 lead wires, with electrocardiographic data transmitted to a monitoring station based in the progressive care unit, a cardio-pulmonary step-down unit. Monitors can be ordered in one of 3 manners, as mandated by hospital policy:
- Continuous telemetry – Telemetry monitoring is uninterrupted until discontinued by a provider.
- Telemetry protocol – Within 12 hours of telemetry placement, a monitor technician generates a report, which is reviewed by the nurse caring for the patient. The nurse performs an electrocardiogram (ECG) if the patient meets pre-specified criteria for telemetry discontinuation, which includes the absence of arrhythmias, troponin elevations, chest pain, or hemodynamic instability. The repeat ECG is then read and signed by the provider. After these criteria are met, telemetry can be discontinued.
- Stroke telemetry protocol – Telemetry is applied for 48 hours, mainly for detection of paroxysmal atrial fibrillation. Monitoring can be temporarily discontinued if the patient requires magnetic resonance imaging, which interferes with the telemetric monitors.
When entering any of the 3 possible telemetry orders in our computerized provider order entry system (Meditech, Westwood, MA), the ordering provider is required to indicate baseline rhythm, pacemaker presence, and desired heart rate warning parameters. Once the order is electronically signed, a monitor technician notes the order in a logbook and assigns the patient a telemeter, which is applied by the patient’s nurse.
If a monitored patient develops any predefined abnormal rhythm, audible alerts notify monitor technicians and an alert is sent to a portable telephone carried by the patient’s assigned nurse. Either the monitoring technician or the nurse then has the discretion to silence the alarm, note it in the chart, and/or contact the patient’s provider. If alerts are recorded, then a sample telemetry monitoring strip is saved into the patient’s paper medical chart.
Survey Instrument
After approval from the Johns Hopkins institutional review board, we queried providers who worked on the medicine and cardiology wards to assess the context and culture in which telemetry monitoring is used (see Appendix). The study was exempt from requiring informed consent. All staff had the option to decline study participation. We administered the survey using an online survey software program (SurveyMonkey, Palo Alto, CA), sending survey links via email to all internal medicine residents, cardiovascular disease fellows, internal medicine and cardiology teaching attending physicians, hospitalists, NPs, and PAs. Respondents completed the survey anonymously. To increase response rates, providers were sent a monthly reminder email. The survey was open from March 2014 to May 2014 for a total of 3 months.
Analysis
The survey data were compiled and analyzed using Microsoft Excel (Mac version 14.4; Microsoft, Redmond, WA). Variables are displayed as numbers and percentages, as appropriate.
Results
All providers reported having ordered telemetry, but almost all were either unaware of (76%) or only somewhat familiar with (21%) the AHA guidelines for appropriate telemetry use. Notably, the vast majority of fellows and residents reported that they were not at all familiar with the guidelines (100% and 96%, respectively). When asked why providers do not adhere to telemetry guidelines, lack of awareness of and lack of familiarity with the guidelines were the top 2 choices among respondents (Figure 1).
Additionally, most providers acknowledged experiencing adverse effects of telemetry: 86% (57/66) had experienced delayed patient transfers from the emergency department to inpatient floors due to telemetry unavailability and 97% (65/67) had experienced some delay in obtaining tests or studies for their telemetry-monitored patients. Despite acknowledging the potential consequences of telemetry use, only 21% (14/66) of providers routinely (ie, > 75% of the time) discontinued telemetry within 48 hours. Fifteen percent (10/65) routinely allowed telemetry to continue until the time of patient discharge. When discontinued, it was mainly due to the provider’s decision (57%); however, respondents noted that nurses prompted telemetry discontinuation 28% of the time.
Discussion
Consistent with previous studies [3–5,9–15], the majority of providers at our institution do not think continuous telemetry is appropriately utilized. Most survey respondents acknowledged a lack of awareness surrounding current guideline recommendations, which could explain why providers often do not follow them. Despite conceding their knowledge deficits, providers assumed their practice patterns for ordering telemetry were “appropriate”(ie, guideline-supported). This assertion may be incorrect as the majority of providers in our survey chose at least 1 non–guideline-supported indication for telemetry. Other studies have suggested additional reasons for inappropriate telemetry utilization. Providers may disagree with guideline recommendations, may assign lesser importance to guidelines when caring for an individual patient, or may fall victim to inertia (ie, not ordering telemetry appropriately simply because changing one’s practice pattern is difficult) [7].
In addition, the majority of our providers perceived telemetry overuse, which has been well-recognized nationwide [4]. While we did not assess this directly, other studies suggest that providers may overuse telemetry to provide a sense of reassurance when caring for a sick patient, since continuous telemetry is perceived to provide a higher level of care [6,15–17]. Unfortunately, no study has shown a benefit for continuous telemetry when placed for non-guideline-based diagnoses—whether for cardiac or non-cardiac diagnoses [3,9–11,13,14]. Likewise, the guidelines suggest that telemetry use should be time-limited, since the majority of benefit is accrued in the first 48 hours. Beyond that time, no study has shown a clear benefit to continuous telemetry [2]. Therefore, telemetry overuse may lead to unnecessarily increased costs without added benefits [3,9–11,13–15,18].
Our conclusions are tempered by the nature of our survey data. We recognize that our survey has not been previously validated. In addition, our response rates were low. This low sample size may lead to under-representation of diverse ideas. Also, our survey results may not be generalizable, since our study was conducted at a single academic hospital. Our institution’s telemetry ordering culture may differ from others, therefore making our results less applicable to other centers.
Despite these limitations, our results aid in understanding attitudes that surround the use of continuous telemetry, which can shape formal educational interventions to encourage appropriate guideline-based telemetry use. Since our providers agree on the need for more education about the guidelines, components such as online modules or in-person lecture educational sessions, newsletters, email communications, and incorporation of AHA guidelines into the institution’s automated computer order entry system could be utilized [17]. Didactic interventions could be designed especially for trainees given their overall lack of familiarity with the guidelines. Another potential intervention could include supplying providers with publically shared personalized measures of their own practices, since providers benefit from reinforcement and individualized feedback on appropriate utilization practices [19]. Previous studies have suggested that a multidisciplinary approach to patient care leads to positive outcomes [20,21], and in our experience, nursing input is absolutely critical in outlining potential problems and in developing solutions. Our findings suggest that nurses could play an active role in alerting providers when patients have telemetry in use and identifying patients who may no longer need it.
In summary, we have shown that many providers at a tertiary academic institution utilized continuous telemetry inappropriately, and were unaware of guidelines surrounding telemetry use. Future interventions aimed at educating providers, encouraging dialogue between staff, and enabling guideline-supported utilization may increase appropriate telemetry use leading to lower cost and improved quality of patient care.
Acknowledgment: The authors wish to thank Dr. Colleen Christmas, Dr. Panagis Galiatsatos, Mrs. Barbara Brigade, Ms. Joetta Love, Ms. Terri Rigsby, and Mrs. Lisa Shirk for their invaluable technical and administrative support.
Corresponding author: Amber Johnson, MD, MBA, 200 Lothrop St., S-553 Scaife Hall, Pittsburgh, PA 15213, amberjohn@gmail.com.
Financial disclosures: None.
1. Day H. Preliminary studies of an acute coronary care area. J Lancet 1963;83:53–5.
2. Drew B, Califf R, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110:2721–46.
3. Estrada C, Battilana G, Alexander M, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med 2000;15:51–5.
4. Henriques-Forsythe M, Ivonye C, Jamched U, et al. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med 2009;76:368–72.
5. Chen E, Hollander, J. When do patients need admission to a telemetry bed? J Emerg Med 2007;33:53–60.
6. Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012;172:1349–50.
7. Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines?: A framework for improvement. JAMA 1999;282:1458–65.
8. Adult hospital medicine. Five things physicians and patients should question. 15 Aug 2013. Available at www.choosingwisely.org/doctor-patient-lists/society-of-hospital-medicine-adult-hospital-medicine/
9. Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: A prospective cohort study of risk stratification and outcomes. Am J Med 2001;110:7–11.
10. Estrada C, Rosman H, Prasad N, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995;76:960–5.
11. Hollander J, Sites F, Pollack C, Shofer F. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71–6.
12. Ivonye C, Ohuabunwo C, Henriques-Forsythe M, et al. Evaluation of telemetry utilization, policy, and outcomes in an inner-city academic medical center. J Natl Med Assoc 2010;102:598–604.
13. Schull M, Redelmeier D. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000;7:647–52.
14. Sivaram C, Summers J, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998;21:503–5.
15. Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002;122:517–23.
16. Chen S, Zakaria S. Behind the monitor-The trouble with telemetry: a teachable moment. JAMA Intern Med 2015;175:894.
17. Dressler R, Dryer M, Coletti C, et al. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014;174:1852–4.
18. Benjamin E, Klugman R, Luckmann R, et al. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care 2013;19:e225–32.
19. Solomon D, Hashimoto H, Daltroy L, Liang M. Techniques to improve physicians use of diagnostic tests: A new conceptual framework. JAMA 1998;280:2020–7.
20. Richeson J, Johnson J. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung 1992;21:18–24.
21. Curley C, McEachern J, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care 1998;36:AS4–12.
1. Day H. Preliminary studies of an acute coronary care area. J Lancet 1963;83:53–5.
2. Drew B, Califf R, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004;110:2721–46.
3. Estrada C, Battilana G, Alexander M, et al. Evaluation of guidelines for the use of telemetry in the non-intensive-care setting. J Gen Intern Med 2000;15:51–5.
4. Henriques-Forsythe M, Ivonye C, Jamched U, et al. Is telemetry overused? Is it as helpful as thought? Cleve Clin J Med 2009;76:368–72.
5. Chen E, Hollander, J. When do patients need admission to a telemetry bed? J Emerg Med 2007;33:53–60.
6. Najafi N, Auerbach A. Use and outcomes of telemetry monitoring on a medicine service. Arch Intern Med 2012;172:1349–50.
7. Cabana M, Rand C, Powe N, et al. Why don't physicians follow clinical practice guidelines?: A framework for improvement. JAMA 1999;282:1458–65.
8. Adult hospital medicine. Five things physicians and patients should question. 15 Aug 2013. Available at www.choosingwisely.org/doctor-patient-lists/society-of-hospital-medicine-adult-hospital-medicine/
9. Durairaj L, Reilly B, Das K, et al. Emergency department admissions to inpatient cardiac telemetry beds: A prospective cohort study of risk stratification and outcomes. Am J Med 2001;110:7–11.
10. Estrada C, Rosman H, Prasad N, et al. Role of telemetry monitoring in the non-intensive care unit. Am J Cardiol 1995;76:960–5.
11. Hollander J, Sites F, Pollack C, Shofer F. Lack of utility of telemetry monitoring for identification of cardiac death and life-threatening ventricular dysrhythmias in low-risk patients with chest pain. Ann Emerg Med 2004;43:71–6.
12. Ivonye C, Ohuabunwo C, Henriques-Forsythe M, et al. Evaluation of telemetry utilization, policy, and outcomes in an inner-city academic medical center. J Natl Med Assoc 2010;102:598–604.
13. Schull M, Redelmeier D. Continuous electrocardiographic monitoring and cardiac arrest outcomes in 8,932 telemetry ward patients. Acad Emerg Med 2000;7:647–52.
14. Sivaram C, Summers J, Ahmed N. Telemetry outside critical care units: patterns of utilization and influence on management decisions. Clin Cardiol 1998;21:503–5.
15. Snider A, Papaleo M, Beldner S, et al. Is telemetry monitoring necessary in low-risk suspected acute chest pain syndromes? Chest 2002;122:517–23.
16. Chen S, Zakaria S. Behind the monitor-The trouble with telemetry: a teachable moment. JAMA Intern Med 2015;175:894.
17. Dressler R, Dryer M, Coletti C, et al. Altering overuse of cardiac telemetry in non-intensive care unit settings by hardwiring the use of American Heart Association guidelines. JAMA Intern Med 2014;174:1852–4.
18. Benjamin E, Klugman R, Luckmann R, et al. Impact of cardiac telemetry on patient safety and cost. Am J Manag Care 2013;19:e225–32.
19. Solomon D, Hashimoto H, Daltroy L, Liang M. Techniques to improve physicians use of diagnostic tests: A new conceptual framework. JAMA 1998;280:2020–7.
20. Richeson J, Johnson J. The association between interdisciplinary collaboration and patient outcomes in a medical intensive care unit. Heart Lung 1992;21:18–24.
21. Curley C, McEachern J, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care 1998;36:AS4–12.
Applying a Quality Improvement Framework to Operating Room Efficiency in an Academic-Practice Partnership
From the Case Western Reserve University School of Medicine, Cleveland, OH.
Abstract
- Objective: To improve operating room (OR) scheduling efficiency at a large academic institution through the use of an academic-practice partnership and quality improvement (QI) methods.
- Methods: The OR administrative team at a large academic hospital partnered with students in a graduate level QI course to apply QI tools to the problem of OR efficiency.
- Results: The team found wide variation in the way that surgeries were scheduled and other factors that contributed to inefficient OR utilization. A plan-do-study-act (PDSA) cycle was applied to the problem of discrepancy in surgeons’ interpretation of case length, resulting in poor case length accuracy. Our intervention, adding time on the schedule for cases, did not show consistent improvement in case length accuracy.
- Conclusion: Although our intervention did not lead to sustained improvements in OR scheduling efficiency, our project demonstrates how QI tools can be taught and applied in an academic course to address a management problem. Further research is needed to study the impact of student teams on health care improvement.
Operating rooms are one of the most costly departments of a hospital. At University Hospitals Case Medical Center (UHCMC), as at many hospitals, operating room utilization is a key area of focus for both operating room (OR) and hospital administrators. Efficient use of the OR is an important aspect of a hospital’s finances and patient-centeredness.
UHCMC uses block scheduling, a common OR scheduling design. Each surgical department is allotted a certain number of blocks (hours of reserved OR time) that they are responsible for filling with surgical cases and that the hospital is responsible for staffing. Block utilization rate is a metric commonly used to measure OR efficiency. It divides the time that the OR is in use by the total block time allocated to the department (while accounting for room turnaround time). An industry benchmark is 75% block utilization [1], which was adopted as an internal target at UHCMC. Achieving this metric is necessary because the hospital (rather than each individual surgical department) is responsible for ensuring that the appropriate amount of non-surgeon staff (eg, anesthesiologists, nurses, scrub techs, and facilities staff) is available. Poor utilization rates indicate that the staff and equipment are inefficiently used, which can impact the hospital’s financial well-being [2]. Block utilization is the result of a complex system, making it challenging to improve. Many people are involved in scheduling, and a large degree of inherent uncertainty exists in the system.
At UHCMC, block utilization rates by department ranged from 52% to 80%, with an overall utilization of 64% from February to July 2014. Given this wide variation, higher level management staff in the OR initiated a project in which OR administrators partnered with students in a graduate level QI course in an effort to improve overall block utilization. They believed that improving block utilization rate would improve the effectiveness, patient-centeredness, and efficiency of care, health care delivery goals described by the Institute of Medicine [3].
Methods
Setting
The OR at UHCMC contains 4 operating suites that serve over 25,000 patients per year and train over 900 residents each year. Nearly 250 surgeons in 23 departments use the OR. The OR schedule at our institution is coordinated by block scheduling, as described above. If a surgical department cannot fill the block, they must release the time to central scheduling for re-allocation of the time to another department.
Application of QI Process
This QI project was an academic-practice collaboration between UHCMC and a graduate level course at Case Western Reserve University called The Continual Improvement of Healthcare: an Interdisciplinary Course [4]. Faculty course instructors solicit applications of QI projects from departments at UHCMC. The project team consisted of 4 students (from medicine, social work, public health, and bioethics), 2 administrative staff from UHCMC, and a QI coach who is on the faculty at Case Western. Guidance was provided by 2 faculty facilitators. The students attended 15 weekly class sessions, 4 meetings with the project team, numerous data gathering sessions with other hospital staff, and held a handful of outside-class student team meetings. An early class session was devoted to team skills and the Seven-Step meeting process [5]. Each classroom session consisted of structured group activities to practice the tools of the QI process.
Tool 1: Global Aim
The team first established a global aim: to improve the OR block utilization rate at UHCMC. This aim was based on the initial project proposal from UHCMC. The global aim explains the reason that the project team was established, and frames all future work [7].
Tool 2: Industry Assessment
Based on the global aim, the student team performed an industry assessment in order to understand strategies for improving block utilization rate in use at other institutions. Peer-reviewed journal articles and case reports were reviewed and the student team was able to contact a team at another institution working on similar issues.
Overall, 2 broad categories of interventions to improve block utilization were identified. Some institutions addressed the way time in the OR was scheduled. They made improvements to how block time was allotted, timing of cases, and dealing with add-on cases [8]. Others focused on using time in the OR more efficiently by addressing room turnover, delays including waiting for surgeons, and waiting for hospital beds [9]. Because the specific case mix of each hospital is so distinct, hospitals that successfully made changes all used a variety of interventions [10–12]. After the industry assessment, the student team realized that there would be a large number of possible approaches to the problem of block utilization, and a better understanding of the actual process of scheduling at UHCMC was necessary to find an area of focus.
Tool 3: Process Map
As the project team began to address the global aim of improving OR block utilization at UHCMC, they needed to have a thorough understanding of how OR time was allotted and used. To do this, the student team created a process map by interviewing process stakeholders, including the OR managers and department schedulers in orthopedics, general surgery, and urology, as suggested by the OR managers. The perspective of these staff were critical to understanding the process of operating room scheduling.
Through the creation of the process map, the project team found that there was wide variation in the process and structure for scheduling surgeries. Some departments used one central scheduler while others used individual secretaries for each surgeon. Some surgeons maintained control over changing their schedule, while others did not. Further, the project team learned that the metric of block utilization rate was of varying importance to people working on the ground.
Tool 4: Fishbone Diagram
After understanding the process, the project team considered all of the factors that
Tool 5: Specific Aim
Though the global aim was to improve block utilization, the project team needed to chose a specific aim that met S.M.A.R.T criteria: Specific, Measureable, Achievable, Results-focused, and Time-bound [7]. After considering multiple potential areas of initial focus, the OR staff suggested focusing on the issue of case length accuracy. In qualitative interviews, the student team had found that the surgery request forms ask for “case length,” and the schedulers were not sure how the surgeons defined it. When the OR is booked for an operation, the amount of time blocked out is the time from when the patient is brought into the operating room to the time that the patient leaves the room, or WIWO (Wheels In Wheels Out). This WIWO time includes anesthesia induction and preparations for surgery such as positioning. Some surgeons think about case length as only the time that the patient is operated on, or CTC (Cut to Close). Thus, the surgeon may be requesting less time than is really necessary for the case if he or she is only thinking about CTC time. The student team created a survey and found that 2 urology surgeons considered case length to be WIWO, and 4 considered case length to mean CTC.
Tools 6 and 7: PDSA Cycle and Control Charts
The Plan-Do-Study-Act cycle is an iterative plan of action for designing and testing a specific change [7]. This part of the QI cycle involved implementing and testing a change to address our specific aim. As the first cycle of change, the team requested that the scheduler add 15 minutes to the surgeons’ requested case time over 1 week. Of the urologists scheduled that week, one had used CTC and the other had not completed the student team’s survey. In order to study the change, the project team used control charts for the 2 surgeons whose case times were adapted. Prior to the intervention, the surgeons averaged at least 20 minutes over their scheduled time, with wide variation. Surgeons were infrequently completing cases at or below their requested case time. Most of the inaccuracy came from going long. The team used control charts to understand the impact of the change. The control charts showed that after the change in scheduling time, the 2 surgeons still went over their allotted case time, but to a lesser degree.
After gaining new information, the next step in the PDSA cycle is to determine the next test of change. The student team recommended sharing these data with the surgeons to consider next steps in improving block utilization, though time constraints of the semester limited continued involvement of the student team in the next PDSA cycle.
Discussion
Through the application of QI tools, new insight was gained about OR efficiency and potential improvements. The student team talked to numerous staff involved in scheduling and each discussion increased understanding of the issues that lead to OR inefficiency. The process map and fishbone diagram provided a visual expression of how small issues could impact the overall OR system. Application of QI tools also led the team to the discovery that surgeons may be interpreting case length in disparate ways, contributing to problems with scheduling.
Though the intervention did not have significant impact over 1 week, more time for subsequent PDSA cycles may have resulted in clinical improvements. Despite the limitations, the student team uncovered an important aspect of the block scheduling process, providing valuable information and insight for the department around this scheduling issue. The student team’s work was shared between multiple surgical departments, and the QI work in the department is ongoing.
Implications for Health Care Institutions
Nontraditional Projects Can Work
The issue of OR utilization is perhaps not a “traditional” QI project given the macro nature of the problem. Once it was broken down into discrete processes, problems such as OR turnover, scheduling redundancies, and others look much more like traditional QI projects. It may be beneficial to institutions to broaden the scope of QI to problems that may, at first glance, seem out of the realm of process mapping, fishbone diagramming, and SMART aims. QI tools can turn management problems into projects that can be tackled by small teams, creating an culture of change in an organization [13].
Benefits of Student Teams
There are clear benefits to the institution working with students. Our hospital-based team members found it beneficial to have independent observers review the process and recommend improvements. Students were able to challenge the status quo and point out inefficiencies that have remained due to institutional complacency and lack of resources. The hospital employees were impressed and surprised that the students found the misunderstanding about case length, and noted that it suggests that there may be other places where there are miscommunications between various people involved in OR scheduling. The students’ energy and time was supported by the QI expertise of the course instructors, and the practical knowledge of the hospital-based team members. Similar benefits have been noted by others utilizing collaborative QI educational models [14,15].
Benefits for Students
For the students on the team, the opportunity to apply QI concepts to the real world was a unique learning opportunity. First, the project was truly interdisciplinary. The students were from varied fields and they worked with schedulers, surgeons, and office managers providing the students with insight into the meaning and perspectives of interprofessional collaboration. The students appreciated the complexity and tensions of the OR staff who were working to balance the schedules of nurses, anesthesiologists, and other OR support staff. Additionally, interdisciplinary collaboration in health care is of increasing importance in everyday practice [16,17]. A strong understanding of collaboration across professions will be a cornerstone of the students’ credentials as they move into the workforce.
There is also value in adding real work experience to academics. The students were able to appreciate not only the concepts of QI but the actual challenges of implementing QI methodology in an institution where people had varying levels of buy-in. Quality improvement is about more than sitting at a whiteboard coming up with charts—it is about enacting actual change and understanding specific real-world situations. The hospital collaboration allowed the students to gain experience that is impossible to replicate in the classroom.
Limitations and Barriers
As noted in other academic-practice collaborations, the limitation of completing the project in one semester presents a barrier to collaboration; the working world does not operate on an academic timeline [14]. Students were limited to only testing one cycle of change. This part of the semester was disappointing as the students would have liked to implement multiple PDSA cycles. The OR managers faced barriers as well; they invested time in educating students who would soon move on, and would have to repeat the process with a new group of students. The department has continued on with this work, but losing the students who they oriented was not ideal.
The course instructors were flexible in allowing the project team to spend the majority of time breaking down the problem of OR block utilization into testable changes, which was the bulk of our work. However, the skill that the team was able to dedicate the least amount time to, testing and implementing change, is useful for the students to learn and beneficial for the organization. Moving forward, allowing teams to build on the previous semester’s work, and even implementing a student handoff, might be tried.
Future Directions
Although our intervention did not lead to sustained improvements in OR scheduling efficiency, our project demonstrates how QI tools can be taught and applied in an academic course to address a management problem. Research to specifically understand institutional benefits of academic-practice collaborations would be helpful in recruiting partners and furthering best practices for participants in these partnerships. Research is also needed to understand the impact of QI collaborative models such as the one described in this paper on improving interprofessional teamwork and communication skills, as called for by health care professional educators [16].
Corresponding author: Danielle O’Rourke-Suchoff, BA, Case Western Reserve University School of Medicine, Office of Student Affairs, 10900 Euclid Ave., Cleveland, OH 44106, dko@case.edu.
Financial disclosures: none.
1. The right strategies can help increase OR utilization. OR Manager 2013;29:21–2.
2. Jackson RL. The business of surgery. Managing the OR as a profit center requires more than just IT. It requires a profit-making mindset, too. Health Manage Technol 2002;23:20–2.
3. Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington (DC): National Academy Press; 2001.
4. Hand R, Dolansky MA, Hanahan E, Tinsley N. Quality comes alive: an interdisciplinary student team’s quality improvement experience in learning by doing—health care education case study. Qual Approaches Higher Educ 2014;5:26–32.
5. Scholtes PR, Joiner BL, Streibel BJ. The team handbook. Oriel; 2003.
6. Institute for Healthcare Improvement. Open School. 2015. Accessed 13 Apr 2015 at www.ihi.org/education/ihiopenschool/Pages/default.aspx.
7. Ogrinc GS, Headrick LA, Moore SM, et al. Fundamentals of health care improvement: A guide to improving your patients’ care. 2nd ed. Oakbrook Terrace, IL: Joint Commission Resources and the Institute for Healthcare Improvement; 2012.
8. Managing patient flow: Smoothing OR schedule can ease capacity crunches, researchers say. OR Manager 2003;19:1,9–10.
9. Harders M, Malangoni MA, Weight S, Sidhu T. Improving operating room efficiency through process redesign. Surgery 2006;140:509–16.
10. Paynter J, Horne W, Sizemore R. Realizing revenue opportunities in the operating room. 2015. Accessed 13 Apr 2015 at www.ihi.org/resources/Pages/ImprovementStories/RealizingRevenueOpportunitiesintheOperatingRoom.aspx.
11. Cima RR, Brown MJ, Hebl JR, et al. Use of Lean and Six Sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J Am Coll Surg 2011;213:83–92.
12. Day R, Garfinkel R, Thompson S. Integrated block sharing: a win–win strategy for hospitals and surgeons. Manufact Serv Op Manage 2012;14:567–83.
13. Pardini-Kiely K, Greenlee E, Hopkins J, et al. Improving and Sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Improv Pt Safety 2010;36:387–98.
14. Hall LW, Headrick LA, Cox KR, et al. Linking health professional learners and health care workers on action-based improvement teams. Qual Manag Health Care 2009;18:194–201.
15. Ogrinc GS, Nierenberg DW, Batalden PB. Building experiential learning about quality improvement into a medical school curriculum: The Dartmouth Experience. Health Aff 2011;30:716–22.
16. Interprofessional Education Collaborative Expert Panel. Core competencies for interprofessional collaborative practice. Washington, DC: Interprofessional Education Collaborative; 2011.
17. World Health Organization. Framework for action on inerprofessional education and collaborative practice. Geneva: World Health Organization; 2010.
From the Case Western Reserve University School of Medicine, Cleveland, OH.
Abstract
- Objective: To improve operating room (OR) scheduling efficiency at a large academic institution through the use of an academic-practice partnership and quality improvement (QI) methods.
- Methods: The OR administrative team at a large academic hospital partnered with students in a graduate level QI course to apply QI tools to the problem of OR efficiency.
- Results: The team found wide variation in the way that surgeries were scheduled and other factors that contributed to inefficient OR utilization. A plan-do-study-act (PDSA) cycle was applied to the problem of discrepancy in surgeons’ interpretation of case length, resulting in poor case length accuracy. Our intervention, adding time on the schedule for cases, did not show consistent improvement in case length accuracy.
- Conclusion: Although our intervention did not lead to sustained improvements in OR scheduling efficiency, our project demonstrates how QI tools can be taught and applied in an academic course to address a management problem. Further research is needed to study the impact of student teams on health care improvement.
Operating rooms are one of the most costly departments of a hospital. At University Hospitals Case Medical Center (UHCMC), as at many hospitals, operating room utilization is a key area of focus for both operating room (OR) and hospital administrators. Efficient use of the OR is an important aspect of a hospital’s finances and patient-centeredness.
UHCMC uses block scheduling, a common OR scheduling design. Each surgical department is allotted a certain number of blocks (hours of reserved OR time) that they are responsible for filling with surgical cases and that the hospital is responsible for staffing. Block utilization rate is a metric commonly used to measure OR efficiency. It divides the time that the OR is in use by the total block time allocated to the department (while accounting for room turnaround time). An industry benchmark is 75% block utilization [1], which was adopted as an internal target at UHCMC. Achieving this metric is necessary because the hospital (rather than each individual surgical department) is responsible for ensuring that the appropriate amount of non-surgeon staff (eg, anesthesiologists, nurses, scrub techs, and facilities staff) is available. Poor utilization rates indicate that the staff and equipment are inefficiently used, which can impact the hospital’s financial well-being [2]. Block utilization is the result of a complex system, making it challenging to improve. Many people are involved in scheduling, and a large degree of inherent uncertainty exists in the system.
At UHCMC, block utilization rates by department ranged from 52% to 80%, with an overall utilization of 64% from February to July 2014. Given this wide variation, higher level management staff in the OR initiated a project in which OR administrators partnered with students in a graduate level QI course in an effort to improve overall block utilization. They believed that improving block utilization rate would improve the effectiveness, patient-centeredness, and efficiency of care, health care delivery goals described by the Institute of Medicine [3].
Methods
Setting
The OR at UHCMC contains 4 operating suites that serve over 25,000 patients per year and train over 900 residents each year. Nearly 250 surgeons in 23 departments use the OR. The OR schedule at our institution is coordinated by block scheduling, as described above. If a surgical department cannot fill the block, they must release the time to central scheduling for re-allocation of the time to another department.
Application of QI Process
This QI project was an academic-practice collaboration between UHCMC and a graduate level course at Case Western Reserve University called The Continual Improvement of Healthcare: an Interdisciplinary Course [4]. Faculty course instructors solicit applications of QI projects from departments at UHCMC. The project team consisted of 4 students (from medicine, social work, public health, and bioethics), 2 administrative staff from UHCMC, and a QI coach who is on the faculty at Case Western. Guidance was provided by 2 faculty facilitators. The students attended 15 weekly class sessions, 4 meetings with the project team, numerous data gathering sessions with other hospital staff, and held a handful of outside-class student team meetings. An early class session was devoted to team skills and the Seven-Step meeting process [5]. Each classroom session consisted of structured group activities to practice the tools of the QI process.
Tool 1: Global Aim
The team first established a global aim: to improve the OR block utilization rate at UHCMC. This aim was based on the initial project proposal from UHCMC. The global aim explains the reason that the project team was established, and frames all future work [7].
Tool 2: Industry Assessment
Based on the global aim, the student team performed an industry assessment in order to understand strategies for improving block utilization rate in use at other institutions. Peer-reviewed journal articles and case reports were reviewed and the student team was able to contact a team at another institution working on similar issues.
Overall, 2 broad categories of interventions to improve block utilization were identified. Some institutions addressed the way time in the OR was scheduled. They made improvements to how block time was allotted, timing of cases, and dealing with add-on cases [8]. Others focused on using time in the OR more efficiently by addressing room turnover, delays including waiting for surgeons, and waiting for hospital beds [9]. Because the specific case mix of each hospital is so distinct, hospitals that successfully made changes all used a variety of interventions [10–12]. After the industry assessment, the student team realized that there would be a large number of possible approaches to the problem of block utilization, and a better understanding of the actual process of scheduling at UHCMC was necessary to find an area of focus.
Tool 3: Process Map
As the project team began to address the global aim of improving OR block utilization at UHCMC, they needed to have a thorough understanding of how OR time was allotted and used. To do this, the student team created a process map by interviewing process stakeholders, including the OR managers and department schedulers in orthopedics, general surgery, and urology, as suggested by the OR managers. The perspective of these staff were critical to understanding the process of operating room scheduling.
Through the creation of the process map, the project team found that there was wide variation in the process and structure for scheduling surgeries. Some departments used one central scheduler while others used individual secretaries for each surgeon. Some surgeons maintained control over changing their schedule, while others did not. Further, the project team learned that the metric of block utilization rate was of varying importance to people working on the ground.
Tool 4: Fishbone Diagram
After understanding the process, the project team considered all of the factors that
Tool 5: Specific Aim
Though the global aim was to improve block utilization, the project team needed to chose a specific aim that met S.M.A.R.T criteria: Specific, Measureable, Achievable, Results-focused, and Time-bound [7]. After considering multiple potential areas of initial focus, the OR staff suggested focusing on the issue of case length accuracy. In qualitative interviews, the student team had found that the surgery request forms ask for “case length,” and the schedulers were not sure how the surgeons defined it. When the OR is booked for an operation, the amount of time blocked out is the time from when the patient is brought into the operating room to the time that the patient leaves the room, or WIWO (Wheels In Wheels Out). This WIWO time includes anesthesia induction and preparations for surgery such as positioning. Some surgeons think about case length as only the time that the patient is operated on, or CTC (Cut to Close). Thus, the surgeon may be requesting less time than is really necessary for the case if he or she is only thinking about CTC time. The student team created a survey and found that 2 urology surgeons considered case length to be WIWO, and 4 considered case length to mean CTC.
Tools 6 and 7: PDSA Cycle and Control Charts
The Plan-Do-Study-Act cycle is an iterative plan of action for designing and testing a specific change [7]. This part of the QI cycle involved implementing and testing a change to address our specific aim. As the first cycle of change, the team requested that the scheduler add 15 minutes to the surgeons’ requested case time over 1 week. Of the urologists scheduled that week, one had used CTC and the other had not completed the student team’s survey. In order to study the change, the project team used control charts for the 2 surgeons whose case times were adapted. Prior to the intervention, the surgeons averaged at least 20 minutes over their scheduled time, with wide variation. Surgeons were infrequently completing cases at or below their requested case time. Most of the inaccuracy came from going long. The team used control charts to understand the impact of the change. The control charts showed that after the change in scheduling time, the 2 surgeons still went over their allotted case time, but to a lesser degree.
After gaining new information, the next step in the PDSA cycle is to determine the next test of change. The student team recommended sharing these data with the surgeons to consider next steps in improving block utilization, though time constraints of the semester limited continued involvement of the student team in the next PDSA cycle.
Discussion
Through the application of QI tools, new insight was gained about OR efficiency and potential improvements. The student team talked to numerous staff involved in scheduling and each discussion increased understanding of the issues that lead to OR inefficiency. The process map and fishbone diagram provided a visual expression of how small issues could impact the overall OR system. Application of QI tools also led the team to the discovery that surgeons may be interpreting case length in disparate ways, contributing to problems with scheduling.
Though the intervention did not have significant impact over 1 week, more time for subsequent PDSA cycles may have resulted in clinical improvements. Despite the limitations, the student team uncovered an important aspect of the block scheduling process, providing valuable information and insight for the department around this scheduling issue. The student team’s work was shared between multiple surgical departments, and the QI work in the department is ongoing.
Implications for Health Care Institutions
Nontraditional Projects Can Work
The issue of OR utilization is perhaps not a “traditional” QI project given the macro nature of the problem. Once it was broken down into discrete processes, problems such as OR turnover, scheduling redundancies, and others look much more like traditional QI projects. It may be beneficial to institutions to broaden the scope of QI to problems that may, at first glance, seem out of the realm of process mapping, fishbone diagramming, and SMART aims. QI tools can turn management problems into projects that can be tackled by small teams, creating an culture of change in an organization [13].
Benefits of Student Teams
There are clear benefits to the institution working with students. Our hospital-based team members found it beneficial to have independent observers review the process and recommend improvements. Students were able to challenge the status quo and point out inefficiencies that have remained due to institutional complacency and lack of resources. The hospital employees were impressed and surprised that the students found the misunderstanding about case length, and noted that it suggests that there may be other places where there are miscommunications between various people involved in OR scheduling. The students’ energy and time was supported by the QI expertise of the course instructors, and the practical knowledge of the hospital-based team members. Similar benefits have been noted by others utilizing collaborative QI educational models [14,15].
Benefits for Students
For the students on the team, the opportunity to apply QI concepts to the real world was a unique learning opportunity. First, the project was truly interdisciplinary. The students were from varied fields and they worked with schedulers, surgeons, and office managers providing the students with insight into the meaning and perspectives of interprofessional collaboration. The students appreciated the complexity and tensions of the OR staff who were working to balance the schedules of nurses, anesthesiologists, and other OR support staff. Additionally, interdisciplinary collaboration in health care is of increasing importance in everyday practice [16,17]. A strong understanding of collaboration across professions will be a cornerstone of the students’ credentials as they move into the workforce.
There is also value in adding real work experience to academics. The students were able to appreciate not only the concepts of QI but the actual challenges of implementing QI methodology in an institution where people had varying levels of buy-in. Quality improvement is about more than sitting at a whiteboard coming up with charts—it is about enacting actual change and understanding specific real-world situations. The hospital collaboration allowed the students to gain experience that is impossible to replicate in the classroom.
Limitations and Barriers
As noted in other academic-practice collaborations, the limitation of completing the project in one semester presents a barrier to collaboration; the working world does not operate on an academic timeline [14]. Students were limited to only testing one cycle of change. This part of the semester was disappointing as the students would have liked to implement multiple PDSA cycles. The OR managers faced barriers as well; they invested time in educating students who would soon move on, and would have to repeat the process with a new group of students. The department has continued on with this work, but losing the students who they oriented was not ideal.
The course instructors were flexible in allowing the project team to spend the majority of time breaking down the problem of OR block utilization into testable changes, which was the bulk of our work. However, the skill that the team was able to dedicate the least amount time to, testing and implementing change, is useful for the students to learn and beneficial for the organization. Moving forward, allowing teams to build on the previous semester’s work, and even implementing a student handoff, might be tried.
Future Directions
Although our intervention did not lead to sustained improvements in OR scheduling efficiency, our project demonstrates how QI tools can be taught and applied in an academic course to address a management problem. Research to specifically understand institutional benefits of academic-practice collaborations would be helpful in recruiting partners and furthering best practices for participants in these partnerships. Research is also needed to understand the impact of QI collaborative models such as the one described in this paper on improving interprofessional teamwork and communication skills, as called for by health care professional educators [16].
Corresponding author: Danielle O’Rourke-Suchoff, BA, Case Western Reserve University School of Medicine, Office of Student Affairs, 10900 Euclid Ave., Cleveland, OH 44106, dko@case.edu.
Financial disclosures: none.
From the Case Western Reserve University School of Medicine, Cleveland, OH.
Abstract
- Objective: To improve operating room (OR) scheduling efficiency at a large academic institution through the use of an academic-practice partnership and quality improvement (QI) methods.
- Methods: The OR administrative team at a large academic hospital partnered with students in a graduate level QI course to apply QI tools to the problem of OR efficiency.
- Results: The team found wide variation in the way that surgeries were scheduled and other factors that contributed to inefficient OR utilization. A plan-do-study-act (PDSA) cycle was applied to the problem of discrepancy in surgeons’ interpretation of case length, resulting in poor case length accuracy. Our intervention, adding time on the schedule for cases, did not show consistent improvement in case length accuracy.
- Conclusion: Although our intervention did not lead to sustained improvements in OR scheduling efficiency, our project demonstrates how QI tools can be taught and applied in an academic course to address a management problem. Further research is needed to study the impact of student teams on health care improvement.
Operating rooms are one of the most costly departments of a hospital. At University Hospitals Case Medical Center (UHCMC), as at many hospitals, operating room utilization is a key area of focus for both operating room (OR) and hospital administrators. Efficient use of the OR is an important aspect of a hospital’s finances and patient-centeredness.
UHCMC uses block scheduling, a common OR scheduling design. Each surgical department is allotted a certain number of blocks (hours of reserved OR time) that they are responsible for filling with surgical cases and that the hospital is responsible for staffing. Block utilization rate is a metric commonly used to measure OR efficiency. It divides the time that the OR is in use by the total block time allocated to the department (while accounting for room turnaround time). An industry benchmark is 75% block utilization [1], which was adopted as an internal target at UHCMC. Achieving this metric is necessary because the hospital (rather than each individual surgical department) is responsible for ensuring that the appropriate amount of non-surgeon staff (eg, anesthesiologists, nurses, scrub techs, and facilities staff) is available. Poor utilization rates indicate that the staff and equipment are inefficiently used, which can impact the hospital’s financial well-being [2]. Block utilization is the result of a complex system, making it challenging to improve. Many people are involved in scheduling, and a large degree of inherent uncertainty exists in the system.
At UHCMC, block utilization rates by department ranged from 52% to 80%, with an overall utilization of 64% from February to July 2014. Given this wide variation, higher level management staff in the OR initiated a project in which OR administrators partnered with students in a graduate level QI course in an effort to improve overall block utilization. They believed that improving block utilization rate would improve the effectiveness, patient-centeredness, and efficiency of care, health care delivery goals described by the Institute of Medicine [3].
Methods
Setting
The OR at UHCMC contains 4 operating suites that serve over 25,000 patients per year and train over 900 residents each year. Nearly 250 surgeons in 23 departments use the OR. The OR schedule at our institution is coordinated by block scheduling, as described above. If a surgical department cannot fill the block, they must release the time to central scheduling for re-allocation of the time to another department.
Application of QI Process
This QI project was an academic-practice collaboration between UHCMC and a graduate level course at Case Western Reserve University called The Continual Improvement of Healthcare: an Interdisciplinary Course [4]. Faculty course instructors solicit applications of QI projects from departments at UHCMC. The project team consisted of 4 students (from medicine, social work, public health, and bioethics), 2 administrative staff from UHCMC, and a QI coach who is on the faculty at Case Western. Guidance was provided by 2 faculty facilitators. The students attended 15 weekly class sessions, 4 meetings with the project team, numerous data gathering sessions with other hospital staff, and held a handful of outside-class student team meetings. An early class session was devoted to team skills and the Seven-Step meeting process [5]. Each classroom session consisted of structured group activities to practice the tools of the QI process.
Tool 1: Global Aim
The team first established a global aim: to improve the OR block utilization rate at UHCMC. This aim was based on the initial project proposal from UHCMC. The global aim explains the reason that the project team was established, and frames all future work [7].
Tool 2: Industry Assessment
Based on the global aim, the student team performed an industry assessment in order to understand strategies for improving block utilization rate in use at other institutions. Peer-reviewed journal articles and case reports were reviewed and the student team was able to contact a team at another institution working on similar issues.
Overall, 2 broad categories of interventions to improve block utilization were identified. Some institutions addressed the way time in the OR was scheduled. They made improvements to how block time was allotted, timing of cases, and dealing with add-on cases [8]. Others focused on using time in the OR more efficiently by addressing room turnover, delays including waiting for surgeons, and waiting for hospital beds [9]. Because the specific case mix of each hospital is so distinct, hospitals that successfully made changes all used a variety of interventions [10–12]. After the industry assessment, the student team realized that there would be a large number of possible approaches to the problem of block utilization, and a better understanding of the actual process of scheduling at UHCMC was necessary to find an area of focus.
Tool 3: Process Map
As the project team began to address the global aim of improving OR block utilization at UHCMC, they needed to have a thorough understanding of how OR time was allotted and used. To do this, the student team created a process map by interviewing process stakeholders, including the OR managers and department schedulers in orthopedics, general surgery, and urology, as suggested by the OR managers. The perspective of these staff were critical to understanding the process of operating room scheduling.
Through the creation of the process map, the project team found that there was wide variation in the process and structure for scheduling surgeries. Some departments used one central scheduler while others used individual secretaries for each surgeon. Some surgeons maintained control over changing their schedule, while others did not. Further, the project team learned that the metric of block utilization rate was of varying importance to people working on the ground.
Tool 4: Fishbone Diagram
After understanding the process, the project team considered all of the factors that
Tool 5: Specific Aim
Though the global aim was to improve block utilization, the project team needed to chose a specific aim that met S.M.A.R.T criteria: Specific, Measureable, Achievable, Results-focused, and Time-bound [7]. After considering multiple potential areas of initial focus, the OR staff suggested focusing on the issue of case length accuracy. In qualitative interviews, the student team had found that the surgery request forms ask for “case length,” and the schedulers were not sure how the surgeons defined it. When the OR is booked for an operation, the amount of time blocked out is the time from when the patient is brought into the operating room to the time that the patient leaves the room, or WIWO (Wheels In Wheels Out). This WIWO time includes anesthesia induction and preparations for surgery such as positioning. Some surgeons think about case length as only the time that the patient is operated on, or CTC (Cut to Close). Thus, the surgeon may be requesting less time than is really necessary for the case if he or she is only thinking about CTC time. The student team created a survey and found that 2 urology surgeons considered case length to be WIWO, and 4 considered case length to mean CTC.
Tools 6 and 7: PDSA Cycle and Control Charts
The Plan-Do-Study-Act cycle is an iterative plan of action for designing and testing a specific change [7]. This part of the QI cycle involved implementing and testing a change to address our specific aim. As the first cycle of change, the team requested that the scheduler add 15 minutes to the surgeons’ requested case time over 1 week. Of the urologists scheduled that week, one had used CTC and the other had not completed the student team’s survey. In order to study the change, the project team used control charts for the 2 surgeons whose case times were adapted. Prior to the intervention, the surgeons averaged at least 20 minutes over their scheduled time, with wide variation. Surgeons were infrequently completing cases at or below their requested case time. Most of the inaccuracy came from going long. The team used control charts to understand the impact of the change. The control charts showed that after the change in scheduling time, the 2 surgeons still went over their allotted case time, but to a lesser degree.
After gaining new information, the next step in the PDSA cycle is to determine the next test of change. The student team recommended sharing these data with the surgeons to consider next steps in improving block utilization, though time constraints of the semester limited continued involvement of the student team in the next PDSA cycle.
Discussion
Through the application of QI tools, new insight was gained about OR efficiency and potential improvements. The student team talked to numerous staff involved in scheduling and each discussion increased understanding of the issues that lead to OR inefficiency. The process map and fishbone diagram provided a visual expression of how small issues could impact the overall OR system. Application of QI tools also led the team to the discovery that surgeons may be interpreting case length in disparate ways, contributing to problems with scheduling.
Though the intervention did not have significant impact over 1 week, more time for subsequent PDSA cycles may have resulted in clinical improvements. Despite the limitations, the student team uncovered an important aspect of the block scheduling process, providing valuable information and insight for the department around this scheduling issue. The student team’s work was shared between multiple surgical departments, and the QI work in the department is ongoing.
Implications for Health Care Institutions
Nontraditional Projects Can Work
The issue of OR utilization is perhaps not a “traditional” QI project given the macro nature of the problem. Once it was broken down into discrete processes, problems such as OR turnover, scheduling redundancies, and others look much more like traditional QI projects. It may be beneficial to institutions to broaden the scope of QI to problems that may, at first glance, seem out of the realm of process mapping, fishbone diagramming, and SMART aims. QI tools can turn management problems into projects that can be tackled by small teams, creating an culture of change in an organization [13].
Benefits of Student Teams
There are clear benefits to the institution working with students. Our hospital-based team members found it beneficial to have independent observers review the process and recommend improvements. Students were able to challenge the status quo and point out inefficiencies that have remained due to institutional complacency and lack of resources. The hospital employees were impressed and surprised that the students found the misunderstanding about case length, and noted that it suggests that there may be other places where there are miscommunications between various people involved in OR scheduling. The students’ energy and time was supported by the QI expertise of the course instructors, and the practical knowledge of the hospital-based team members. Similar benefits have been noted by others utilizing collaborative QI educational models [14,15].
Benefits for Students
For the students on the team, the opportunity to apply QI concepts to the real world was a unique learning opportunity. First, the project was truly interdisciplinary. The students were from varied fields and they worked with schedulers, surgeons, and office managers providing the students with insight into the meaning and perspectives of interprofessional collaboration. The students appreciated the complexity and tensions of the OR staff who were working to balance the schedules of nurses, anesthesiologists, and other OR support staff. Additionally, interdisciplinary collaboration in health care is of increasing importance in everyday practice [16,17]. A strong understanding of collaboration across professions will be a cornerstone of the students’ credentials as they move into the workforce.
There is also value in adding real work experience to academics. The students were able to appreciate not only the concepts of QI but the actual challenges of implementing QI methodology in an institution where people had varying levels of buy-in. Quality improvement is about more than sitting at a whiteboard coming up with charts—it is about enacting actual change and understanding specific real-world situations. The hospital collaboration allowed the students to gain experience that is impossible to replicate in the classroom.
Limitations and Barriers
As noted in other academic-practice collaborations, the limitation of completing the project in one semester presents a barrier to collaboration; the working world does not operate on an academic timeline [14]. Students were limited to only testing one cycle of change. This part of the semester was disappointing as the students would have liked to implement multiple PDSA cycles. The OR managers faced barriers as well; they invested time in educating students who would soon move on, and would have to repeat the process with a new group of students. The department has continued on with this work, but losing the students who they oriented was not ideal.
The course instructors were flexible in allowing the project team to spend the majority of time breaking down the problem of OR block utilization into testable changes, which was the bulk of our work. However, the skill that the team was able to dedicate the least amount time to, testing and implementing change, is useful for the students to learn and beneficial for the organization. Moving forward, allowing teams to build on the previous semester’s work, and even implementing a student handoff, might be tried.
Future Directions
Although our intervention did not lead to sustained improvements in OR scheduling efficiency, our project demonstrates how QI tools can be taught and applied in an academic course to address a management problem. Research to specifically understand institutional benefits of academic-practice collaborations would be helpful in recruiting partners and furthering best practices for participants in these partnerships. Research is also needed to understand the impact of QI collaborative models such as the one described in this paper on improving interprofessional teamwork and communication skills, as called for by health care professional educators [16].
Corresponding author: Danielle O’Rourke-Suchoff, BA, Case Western Reserve University School of Medicine, Office of Student Affairs, 10900 Euclid Ave., Cleveland, OH 44106, dko@case.edu.
Financial disclosures: none.
1. The right strategies can help increase OR utilization. OR Manager 2013;29:21–2.
2. Jackson RL. The business of surgery. Managing the OR as a profit center requires more than just IT. It requires a profit-making mindset, too. Health Manage Technol 2002;23:20–2.
3. Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington (DC): National Academy Press; 2001.
4. Hand R, Dolansky MA, Hanahan E, Tinsley N. Quality comes alive: an interdisciplinary student team’s quality improvement experience in learning by doing—health care education case study. Qual Approaches Higher Educ 2014;5:26–32.
5. Scholtes PR, Joiner BL, Streibel BJ. The team handbook. Oriel; 2003.
6. Institute for Healthcare Improvement. Open School. 2015. Accessed 13 Apr 2015 at www.ihi.org/education/ihiopenschool/Pages/default.aspx.
7. Ogrinc GS, Headrick LA, Moore SM, et al. Fundamentals of health care improvement: A guide to improving your patients’ care. 2nd ed. Oakbrook Terrace, IL: Joint Commission Resources and the Institute for Healthcare Improvement; 2012.
8. Managing patient flow: Smoothing OR schedule can ease capacity crunches, researchers say. OR Manager 2003;19:1,9–10.
9. Harders M, Malangoni MA, Weight S, Sidhu T. Improving operating room efficiency through process redesign. Surgery 2006;140:509–16.
10. Paynter J, Horne W, Sizemore R. Realizing revenue opportunities in the operating room. 2015. Accessed 13 Apr 2015 at www.ihi.org/resources/Pages/ImprovementStories/RealizingRevenueOpportunitiesintheOperatingRoom.aspx.
11. Cima RR, Brown MJ, Hebl JR, et al. Use of Lean and Six Sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J Am Coll Surg 2011;213:83–92.
12. Day R, Garfinkel R, Thompson S. Integrated block sharing: a win–win strategy for hospitals and surgeons. Manufact Serv Op Manage 2012;14:567–83.
13. Pardini-Kiely K, Greenlee E, Hopkins J, et al. Improving and Sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Improv Pt Safety 2010;36:387–98.
14. Hall LW, Headrick LA, Cox KR, et al. Linking health professional learners and health care workers on action-based improvement teams. Qual Manag Health Care 2009;18:194–201.
15. Ogrinc GS, Nierenberg DW, Batalden PB. Building experiential learning about quality improvement into a medical school curriculum: The Dartmouth Experience. Health Aff 2011;30:716–22.
16. Interprofessional Education Collaborative Expert Panel. Core competencies for interprofessional collaborative practice. Washington, DC: Interprofessional Education Collaborative; 2011.
17. World Health Organization. Framework for action on inerprofessional education and collaborative practice. Geneva: World Health Organization; 2010.
1. The right strategies can help increase OR utilization. OR Manager 2013;29:21–2.
2. Jackson RL. The business of surgery. Managing the OR as a profit center requires more than just IT. It requires a profit-making mindset, too. Health Manage Technol 2002;23:20–2.
3. Institute of Medicine. Crossing the quality chasm: A new health system for the 21st century. Washington (DC): National Academy Press; 2001.
4. Hand R, Dolansky MA, Hanahan E, Tinsley N. Quality comes alive: an interdisciplinary student team’s quality improvement experience in learning by doing—health care education case study. Qual Approaches Higher Educ 2014;5:26–32.
5. Scholtes PR, Joiner BL, Streibel BJ. The team handbook. Oriel; 2003.
6. Institute for Healthcare Improvement. Open School. 2015. Accessed 13 Apr 2015 at www.ihi.org/education/ihiopenschool/Pages/default.aspx.
7. Ogrinc GS, Headrick LA, Moore SM, et al. Fundamentals of health care improvement: A guide to improving your patients’ care. 2nd ed. Oakbrook Terrace, IL: Joint Commission Resources and the Institute for Healthcare Improvement; 2012.
8. Managing patient flow: Smoothing OR schedule can ease capacity crunches, researchers say. OR Manager 2003;19:1,9–10.
9. Harders M, Malangoni MA, Weight S, Sidhu T. Improving operating room efficiency through process redesign. Surgery 2006;140:509–16.
10. Paynter J, Horne W, Sizemore R. Realizing revenue opportunities in the operating room. 2015. Accessed 13 Apr 2015 at www.ihi.org/resources/Pages/ImprovementStories/RealizingRevenueOpportunitiesintheOperatingRoom.aspx.
11. Cima RR, Brown MJ, Hebl JR, et al. Use of Lean and Six Sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J Am Coll Surg 2011;213:83–92.
12. Day R, Garfinkel R, Thompson S. Integrated block sharing: a win–win strategy for hospitals and surgeons. Manufact Serv Op Manage 2012;14:567–83.
13. Pardini-Kiely K, Greenlee E, Hopkins J, et al. Improving and Sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Improv Pt Safety 2010;36:387–98.
14. Hall LW, Headrick LA, Cox KR, et al. Linking health professional learners and health care workers on action-based improvement teams. Qual Manag Health Care 2009;18:194–201.
15. Ogrinc GS, Nierenberg DW, Batalden PB. Building experiential learning about quality improvement into a medical school curriculum: The Dartmouth Experience. Health Aff 2011;30:716–22.
16. Interprofessional Education Collaborative Expert Panel. Core competencies for interprofessional collaborative practice. Washington, DC: Interprofessional Education Collaborative; 2011.
17. World Health Organization. Framework for action on inerprofessional education and collaborative practice. Geneva: World Health Organization; 2010.
Interdisciplinary Geriatric Difficult Case Conference: Innovative Education Across the Continuum
From Wheaton Franciscan Healthcare (Ms. Fedel), Aspirus (Ms. Hackbarth), and Aurora Health Care (Mr. Malsch and Ms. Pagel).
Abstract
- Background: There is a nationwide shortage of geriatric prepared providers. Caring for complex older adults is challenging.
- Objective: To develop an efficient and affordable way to educate members of the interdisciplinary team involved in the care of geriatric patients.
- Methods: A team from 3 area health systems developed a plan to present monthly case studies via teleconference. Cases are presented by a direct caregiver using the Wisconsin Star Method to facilitate analysis of the case. A geriatric expert and another member of the team presents teaching points, and questions are elicited and discussed.
- Results: The team has completed 18 consecutive monthly teleconferences. Participant satisfaction has been favorable. Participation on the call has increased approximately 300% since the initiation of the program.
- Conclusion: The case teleconference provides an accessible and affordable educational forum that provides learners an opportunity to improve their knowledge in care of older adults.
The number of older adults in the United States will nearly double between 2005 and 2030 [1] as the baby boom generation begins turning 65 and as life expectancy for older Americans increases. The Institute of Medicine’s (IOM) landmark report Retooling for an Aging America: Building the Health Care Workforce states that “unless action is taken immediately, the health care workforce will lack the capacity (in both size and ability) to meet the needs of older patients in the future [1].” One of their recommendations is to explore ways to widen the duties and responsibilities of workers at various levels of training. More health care providers need to be trained in the basics of geriatric care and should be capable of caring for older patients.
Team-based care is becoming more prevalent. Care delivered by interdisciplinary teams have been shown to improve patient outcomes [2]. A team led by one of the authors (PF) developed an intervention to increase the geriatric and teamwork competencies of interdisciplinary teams who serve patients throughout Wisconsin. The Interdisciplinary Geriatric Difficult Case Conference Call (IGDCC) is sponsored monthly by 3 Wisconsin health systems. The purpose is to provide opportunities to discuss clinical cases, to learn from one another and from experts, and to elevate the level of geriatric care in the states of Wisconsin, Michigan, and beyond. Each month a difficult case is presented by a clinician involved in that patient’s care. Time is allotted for participants to ask questions, and teaching points are shared by a clinical expert to highlight concepts and provide additional context. The IGDCC is meant to be a joint learning exercise to explore a specific difficult patient situation and learn skills and knowledge to improve care and transitions for older adults. The conference call is not a critique of the care, but rather an opportunity to jointly learn from the challenging situations all experience.
Background
The IGDCC was created by four members of 3 health systems in Wisconsin: Wheaton Franciscan Healthcare, Aspirus, and Aurora Health Care. The health systems serve and partially overlap on a broad geographic and demographic area of Wisconsin. The 4 members collaborated on numerous projects in the past, including Nurses Improving Case for Health System Elders (NICHE) implementation [3]. A common concern among the team is the management of challenging geriatric clinical patients and having a prepared workforce to meet those challenges.
Problem/Issue
As mentioned above, the older adult population is increasing, and these statistics are reflected in our service area [4]. Exacerbating these demographic changes is a shortage of health care workers in all disciplines, inadequate geriatric training, and the increased prevalence of multiple chronic conditions. Older adults also have higher rates of 30-day readmissions as well as higher rates of functional decline and medical errors during hospital stays [5,6]. Effective interprofessional teamwork is essential for the delivery of high-quality patient care in an increasingly complex health environment [7]. The IOM’s Future of Nursing report recommends that nurses, who represent the largest segment of the US health workforce, should achieve higher levels of training and be full partners in redesigning health care [8]. Unfortunately, effective care is hampered by poor coordination, limited communication, boundary infringement, and lack of understanding of roles [9]. Meta-analyses have demonstrated that there is a positive relationship between team training interventions and outcomes [10,11].
Objectives
The objective of the IGDCC is to elevate the level of geriatric care in the region by providing an accessible and affordable forum for the education of health care workers involved in the care of our most vulnerable population. To meet this challenge, the 4 founding members of IGDCC utilized the Aurora Health Care Geriatric Fellow’s Most Difficult Case (GFMCC) conference format as a model [12,13]. All disciplines are encouraged to participate, with announcements sent out via the leadership at the participating hospital systems. Participants have the option to call into the conference and teleconference via their own personal telephone and computer; in addition, each participating hospital system frequently hosts an open forum teleconference room where participants also may join a group.
Conference Components
The team uses the Wisconsin Star Method framework for presentation and discussion of the case. The Star Method, developed by Timothy Howell, enables clinical data about a person to be mapped out onto a single field with 5 domains: medications, medical, behavioral, personal, and social [14], creating a visual representation of the complicated and interacting physical, emotional, and social issues of older adults (Figure). By becoming comfortable using this method, the learner can use a similar approach in their clinical practice to address the needs of the patient in a holistic manner.
The case call concludes with expert teaching points from both a geriatric expert and a member of the interdisciplinary team. The interdisciplinary team member is chosen based on the key issues raised by the case. For example, cases that are made complex due to polypharmacy and adverse drug reactions might have a pharmacist presenting pertinent take-home message for the learner. In addition, geriatric teaching experts (ie, a geriatrician or advanced practice geriatric nursing specialist) provide the learner with insights that they can apply to their future practice. Often times the teaching points consist of an analysis of the various geriatric syndromes and how they can be managed in the complex older adult.
Implementation
Implementation of the IGDCC is coordinated by an oversight team with representation from each of the 3 sponsoring health systems. The oversight team currently includes 4 members: 3 geriatric clinical nurse specialists and a geriatric service line administrator. The team is responsible for:
- Planning the conference call schedule
- Making arrangements for case presenters and experts to contribute teaching points
- Registering participants and sharing written materials with participants
- Publicizing and encouraging attendance
- Soliciting feedback for continual improvement
- Exploring and implementing new ways to maximize learning.
Team members share duties and rotate case presentations. The Aurora and Wheaton Franciscan systems provide the geriatric specialists who provide the expert teaching points. The Aspirus system provides the conference line and webinar application and supports publicity and evaluations. All 3 systems are supported by a geriatric clinical nurse specialist who identifies and helps prepare presenters, case presentations, and call participants. Over time, the conference call format has evolved into a webinar format, allowing participants to either phone into the call for audio only or participate via both audio and visual. The visual allows participants to watch on their computer screens while the case is presented using the Star Method. During the call, a member of the oversight team adds clinical details by typing into a Word template of a blank star, adding information for each of the 5 domains in real-time as the case is discussed. Another member of the team facilitates the call, introducing presenters and experts, describing the Star Method, and offering “housekeeping” announcements. The facilitator also watches the timing to make certain the agenda is followed and the call begins and ends on time. During the call, another member of the team updates the attendance spreadsheet and makes a recording of each session.
Some participating facilities reserve a meeting room and project the webinar onto a screen for shared viewing. One of the participating sites has done this quite successfully with a growing group of participants coming together to watch the case during their lunch hour. This allows an opportunity for group discussion—when the conference call is on “mute” so as not to disrupt learners at other locations.
Measurement/Analysis
Attendance has steadily increased. In CY2015 from January to September, the mean attendance per month was 29.1 (mode, 17). The maximum per month was 62 (September 2015). The program enjoyed a boost in attendance beginning in July 2015 when Nurses Improving Care of Healthsystem Elders (NICHE) [3] began promoting the call-in opportunity to its NICHE Coordinators at member health systems. In June 2015, the technology was improved to allow for recorded sessions, and the recordings are growing in popularity from 2 listeners per month in July 2014 to 23 listeners per month in September 2015.
Lessons Learned
In comparing the IGDCC with similar conference call educational offerings, the team found that the program was unique in 2 areas. First, in addition to having a rich discussion in the care of frail older adults with experts in the field, the team also sought to help our staff learn how to present a difficult case to their peers. Three of our 4 committee members are geriatric clinical nurse specialists (a fourth is a clinical nurse specialist from Aspirus who assists periodically) who have been able to mentor, guide, and encourage interdisciplinary team members to present a challenging case. Many presenters had never presented a difficult case in this format. Presenters found the process fun and rewarding and have offered to present cases again in the future.
A second unique feature was utilizing the Wisconsin Star Method rather than focusing on a typical medical model framework for discussing a challenging case. The Star Method allows participants to increase their proficiency in providing comprehensive care while being more confident and mindful in addressing the complicated interacting physical, emotional and social issues of older adults [13].
A monthly post-call debriefing with committee members to review the strengths and weakness of the call was key to growing the program. The committee was able to critically review the process of the call, review participant surveys and discuss next steps. Adding a webinar approach, automatic email notification of calls, participant electronic survey, recording the call, and the addition of offering contact hours were some of the action items that were a result of monthly debriefing calls.
The team also found the 3-system collaboration to be beneficial. Aspirus has a large rural population, and Wheaton and Aurora have a diverse population, and each adds to the participant’s experience. Each IGDCC was rotated between the systems, which did not put the burden on any one health system. An annual call assignment listing was maintained for noting which system was responsible for the case each month and whether the geriatric expert was assigned/confirmed. Identifying the committee’s individual and collective group expertise was helpful in the overall project planning. The committee also developed a standard presenter guide and template and an expert teaching guide so the monthly IGDCC were consistent.
Challenges
The committee did not have a budget. Participation on the committee was in-kind funding from each system. Aspirus used its electronic system in place at the time to support the project. Interactive conference call education platform can be challenging with multiple participants on an open line who may not mute their phone. Often times, when a group of participants are calling in from one phone line it is difficult to know how many people are attending the IGDCC. It can be challenging at times to facilitate the call during the discussion component as participants occasionally talk over each other.
Current Status/Future Directions
The team has completed 18 consecutive monthly IGDCCs. Our participation rate has tripled. Participant satisfaction remains favorable. The team is now offering 1 contact hour to participants, and our invitations to participate have been extended to national health care groups. Challenging cases will be presented from community sources outside the hospital. Focusing attention on elevating the level of geriatric care in our region using a community educational approach will give us new opportunities for collaborating on best practice in multiple settings across the care continuum.
Acknowledgment: The planning team acknowledges Evalyn Michira, MSN, RN, PHN, AGCNS-BC, for her assistance in call presentations.
Corresponding author: Margie Hackbarth, MBA, margie.hackbarth@aspirus.org.
Financial disclosures: none.
1. Institute of Medicine. Retooling for an aging America: Building the health care workforce. Washington, DC: National Academies Press; 2008.
2. Mitchell P, Wynia M, Golden R, et al. Core principles and values of effective team-based health care. Discussion paper. Washington, DC; Institute of Medicine; 2012.
3. Nurses Improving Care for Healthsystem Elders. Accessed 1 Dec 2015 at www.nicheprogram.org/.
4. Wisconsin Department of Health Services. Southeastern region population report: 1 Jul 2013. Accessed 16 Feb 2015 at www.dhs.wisconsin.gov/sites/default/files/legacy/population/13data/southeastern.pdf.
5. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA 2003;289:1371–3.
6. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;(29):1–20, 24.
7. Nembhard IM, Edmondson AC. Making it safe: The effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav 2006; 27:941–66.
8. Institute of Medicine. The future of nursing: leading change, advancing health. National Academies Press; 2011.
9. Reeves S, Zwarenstein M, Goldman et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2013;3:CD002213.
10. Salas E, Diaz Granados D, Klein C, et al. Does team training improve team performance? A meta-analysis. Hum Factors 2008;50:903–33.
11. Strasser DD, Burridge AB, Falconer JA, et al. Toward spanning the quality chasm: an examination of team functioning measures. Arch Phys Med Rehabil 2014;95:2220–3.
12. Roche VM, Torregosa H, Howell T, Malone ML. Establishing a treatment plan for an elder with a complex and incomplete medical history and multiple medical providers, diagnoses, and medications. Ann Long-Term Care 2012;20(9).
13. Roche VM, Arnouville J, Danto-Nocton ES, et al. Optimal management of an older patient with multiple comorbidities and a complex psychosocial history. Ann Long-Term Care 2011;19(9).
14. Wisconsin Geriatric Psychiatry Initiative. The Wisconsin Star Method. Accessed 19 Jan 2015 at wgpi.wisc.edu/wisconsin-star-method/.
From Wheaton Franciscan Healthcare (Ms. Fedel), Aspirus (Ms. Hackbarth), and Aurora Health Care (Mr. Malsch and Ms. Pagel).
Abstract
- Background: There is a nationwide shortage of geriatric prepared providers. Caring for complex older adults is challenging.
- Objective: To develop an efficient and affordable way to educate members of the interdisciplinary team involved in the care of geriatric patients.
- Methods: A team from 3 area health systems developed a plan to present monthly case studies via teleconference. Cases are presented by a direct caregiver using the Wisconsin Star Method to facilitate analysis of the case. A geriatric expert and another member of the team presents teaching points, and questions are elicited and discussed.
- Results: The team has completed 18 consecutive monthly teleconferences. Participant satisfaction has been favorable. Participation on the call has increased approximately 300% since the initiation of the program.
- Conclusion: The case teleconference provides an accessible and affordable educational forum that provides learners an opportunity to improve their knowledge in care of older adults.
The number of older adults in the United States will nearly double between 2005 and 2030 [1] as the baby boom generation begins turning 65 and as life expectancy for older Americans increases. The Institute of Medicine’s (IOM) landmark report Retooling for an Aging America: Building the Health Care Workforce states that “unless action is taken immediately, the health care workforce will lack the capacity (in both size and ability) to meet the needs of older patients in the future [1].” One of their recommendations is to explore ways to widen the duties and responsibilities of workers at various levels of training. More health care providers need to be trained in the basics of geriatric care and should be capable of caring for older patients.
Team-based care is becoming more prevalent. Care delivered by interdisciplinary teams have been shown to improve patient outcomes [2]. A team led by one of the authors (PF) developed an intervention to increase the geriatric and teamwork competencies of interdisciplinary teams who serve patients throughout Wisconsin. The Interdisciplinary Geriatric Difficult Case Conference Call (IGDCC) is sponsored monthly by 3 Wisconsin health systems. The purpose is to provide opportunities to discuss clinical cases, to learn from one another and from experts, and to elevate the level of geriatric care in the states of Wisconsin, Michigan, and beyond. Each month a difficult case is presented by a clinician involved in that patient’s care. Time is allotted for participants to ask questions, and teaching points are shared by a clinical expert to highlight concepts and provide additional context. The IGDCC is meant to be a joint learning exercise to explore a specific difficult patient situation and learn skills and knowledge to improve care and transitions for older adults. The conference call is not a critique of the care, but rather an opportunity to jointly learn from the challenging situations all experience.
Background
The IGDCC was created by four members of 3 health systems in Wisconsin: Wheaton Franciscan Healthcare, Aspirus, and Aurora Health Care. The health systems serve and partially overlap on a broad geographic and demographic area of Wisconsin. The 4 members collaborated on numerous projects in the past, including Nurses Improving Case for Health System Elders (NICHE) implementation [3]. A common concern among the team is the management of challenging geriatric clinical patients and having a prepared workforce to meet those challenges.
Problem/Issue
As mentioned above, the older adult population is increasing, and these statistics are reflected in our service area [4]. Exacerbating these demographic changes is a shortage of health care workers in all disciplines, inadequate geriatric training, and the increased prevalence of multiple chronic conditions. Older adults also have higher rates of 30-day readmissions as well as higher rates of functional decline and medical errors during hospital stays [5,6]. Effective interprofessional teamwork is essential for the delivery of high-quality patient care in an increasingly complex health environment [7]. The IOM’s Future of Nursing report recommends that nurses, who represent the largest segment of the US health workforce, should achieve higher levels of training and be full partners in redesigning health care [8]. Unfortunately, effective care is hampered by poor coordination, limited communication, boundary infringement, and lack of understanding of roles [9]. Meta-analyses have demonstrated that there is a positive relationship between team training interventions and outcomes [10,11].
Objectives
The objective of the IGDCC is to elevate the level of geriatric care in the region by providing an accessible and affordable forum for the education of health care workers involved in the care of our most vulnerable population. To meet this challenge, the 4 founding members of IGDCC utilized the Aurora Health Care Geriatric Fellow’s Most Difficult Case (GFMCC) conference format as a model [12,13]. All disciplines are encouraged to participate, with announcements sent out via the leadership at the participating hospital systems. Participants have the option to call into the conference and teleconference via their own personal telephone and computer; in addition, each participating hospital system frequently hosts an open forum teleconference room where participants also may join a group.
Conference Components
The team uses the Wisconsin Star Method framework for presentation and discussion of the case. The Star Method, developed by Timothy Howell, enables clinical data about a person to be mapped out onto a single field with 5 domains: medications, medical, behavioral, personal, and social [14], creating a visual representation of the complicated and interacting physical, emotional, and social issues of older adults (Figure). By becoming comfortable using this method, the learner can use a similar approach in their clinical practice to address the needs of the patient in a holistic manner.
The case call concludes with expert teaching points from both a geriatric expert and a member of the interdisciplinary team. The interdisciplinary team member is chosen based on the key issues raised by the case. For example, cases that are made complex due to polypharmacy and adverse drug reactions might have a pharmacist presenting pertinent take-home message for the learner. In addition, geriatric teaching experts (ie, a geriatrician or advanced practice geriatric nursing specialist) provide the learner with insights that they can apply to their future practice. Often times the teaching points consist of an analysis of the various geriatric syndromes and how they can be managed in the complex older adult.
Implementation
Implementation of the IGDCC is coordinated by an oversight team with representation from each of the 3 sponsoring health systems. The oversight team currently includes 4 members: 3 geriatric clinical nurse specialists and a geriatric service line administrator. The team is responsible for:
- Planning the conference call schedule
- Making arrangements for case presenters and experts to contribute teaching points
- Registering participants and sharing written materials with participants
- Publicizing and encouraging attendance
- Soliciting feedback for continual improvement
- Exploring and implementing new ways to maximize learning.
Team members share duties and rotate case presentations. The Aurora and Wheaton Franciscan systems provide the geriatric specialists who provide the expert teaching points. The Aspirus system provides the conference line and webinar application and supports publicity and evaluations. All 3 systems are supported by a geriatric clinical nurse specialist who identifies and helps prepare presenters, case presentations, and call participants. Over time, the conference call format has evolved into a webinar format, allowing participants to either phone into the call for audio only or participate via both audio and visual. The visual allows participants to watch on their computer screens while the case is presented using the Star Method. During the call, a member of the oversight team adds clinical details by typing into a Word template of a blank star, adding information for each of the 5 domains in real-time as the case is discussed. Another member of the team facilitates the call, introducing presenters and experts, describing the Star Method, and offering “housekeeping” announcements. The facilitator also watches the timing to make certain the agenda is followed and the call begins and ends on time. During the call, another member of the team updates the attendance spreadsheet and makes a recording of each session.
Some participating facilities reserve a meeting room and project the webinar onto a screen for shared viewing. One of the participating sites has done this quite successfully with a growing group of participants coming together to watch the case during their lunch hour. This allows an opportunity for group discussion—when the conference call is on “mute” so as not to disrupt learners at other locations.
Measurement/Analysis
Attendance has steadily increased. In CY2015 from January to September, the mean attendance per month was 29.1 (mode, 17). The maximum per month was 62 (September 2015). The program enjoyed a boost in attendance beginning in July 2015 when Nurses Improving Care of Healthsystem Elders (NICHE) [3] began promoting the call-in opportunity to its NICHE Coordinators at member health systems. In June 2015, the technology was improved to allow for recorded sessions, and the recordings are growing in popularity from 2 listeners per month in July 2014 to 23 listeners per month in September 2015.
Lessons Learned
In comparing the IGDCC with similar conference call educational offerings, the team found that the program was unique in 2 areas. First, in addition to having a rich discussion in the care of frail older adults with experts in the field, the team also sought to help our staff learn how to present a difficult case to their peers. Three of our 4 committee members are geriatric clinical nurse specialists (a fourth is a clinical nurse specialist from Aspirus who assists periodically) who have been able to mentor, guide, and encourage interdisciplinary team members to present a challenging case. Many presenters had never presented a difficult case in this format. Presenters found the process fun and rewarding and have offered to present cases again in the future.
A second unique feature was utilizing the Wisconsin Star Method rather than focusing on a typical medical model framework for discussing a challenging case. The Star Method allows participants to increase their proficiency in providing comprehensive care while being more confident and mindful in addressing the complicated interacting physical, emotional and social issues of older adults [13].
A monthly post-call debriefing with committee members to review the strengths and weakness of the call was key to growing the program. The committee was able to critically review the process of the call, review participant surveys and discuss next steps. Adding a webinar approach, automatic email notification of calls, participant electronic survey, recording the call, and the addition of offering contact hours were some of the action items that were a result of monthly debriefing calls.
The team also found the 3-system collaboration to be beneficial. Aspirus has a large rural population, and Wheaton and Aurora have a diverse population, and each adds to the participant’s experience. Each IGDCC was rotated between the systems, which did not put the burden on any one health system. An annual call assignment listing was maintained for noting which system was responsible for the case each month and whether the geriatric expert was assigned/confirmed. Identifying the committee’s individual and collective group expertise was helpful in the overall project planning. The committee also developed a standard presenter guide and template and an expert teaching guide so the monthly IGDCC were consistent.
Challenges
The committee did not have a budget. Participation on the committee was in-kind funding from each system. Aspirus used its electronic system in place at the time to support the project. Interactive conference call education platform can be challenging with multiple participants on an open line who may not mute their phone. Often times, when a group of participants are calling in from one phone line it is difficult to know how many people are attending the IGDCC. It can be challenging at times to facilitate the call during the discussion component as participants occasionally talk over each other.
Current Status/Future Directions
The team has completed 18 consecutive monthly IGDCCs. Our participation rate has tripled. Participant satisfaction remains favorable. The team is now offering 1 contact hour to participants, and our invitations to participate have been extended to national health care groups. Challenging cases will be presented from community sources outside the hospital. Focusing attention on elevating the level of geriatric care in our region using a community educational approach will give us new opportunities for collaborating on best practice in multiple settings across the care continuum.
Acknowledgment: The planning team acknowledges Evalyn Michira, MSN, RN, PHN, AGCNS-BC, for her assistance in call presentations.
Corresponding author: Margie Hackbarth, MBA, margie.hackbarth@aspirus.org.
Financial disclosures: none.
From Wheaton Franciscan Healthcare (Ms. Fedel), Aspirus (Ms. Hackbarth), and Aurora Health Care (Mr. Malsch and Ms. Pagel).
Abstract
- Background: There is a nationwide shortage of geriatric prepared providers. Caring for complex older adults is challenging.
- Objective: To develop an efficient and affordable way to educate members of the interdisciplinary team involved in the care of geriatric patients.
- Methods: A team from 3 area health systems developed a plan to present monthly case studies via teleconference. Cases are presented by a direct caregiver using the Wisconsin Star Method to facilitate analysis of the case. A geriatric expert and another member of the team presents teaching points, and questions are elicited and discussed.
- Results: The team has completed 18 consecutive monthly teleconferences. Participant satisfaction has been favorable. Participation on the call has increased approximately 300% since the initiation of the program.
- Conclusion: The case teleconference provides an accessible and affordable educational forum that provides learners an opportunity to improve their knowledge in care of older adults.
The number of older adults in the United States will nearly double between 2005 and 2030 [1] as the baby boom generation begins turning 65 and as life expectancy for older Americans increases. The Institute of Medicine’s (IOM) landmark report Retooling for an Aging America: Building the Health Care Workforce states that “unless action is taken immediately, the health care workforce will lack the capacity (in both size and ability) to meet the needs of older patients in the future [1].” One of their recommendations is to explore ways to widen the duties and responsibilities of workers at various levels of training. More health care providers need to be trained in the basics of geriatric care and should be capable of caring for older patients.
Team-based care is becoming more prevalent. Care delivered by interdisciplinary teams have been shown to improve patient outcomes [2]. A team led by one of the authors (PF) developed an intervention to increase the geriatric and teamwork competencies of interdisciplinary teams who serve patients throughout Wisconsin. The Interdisciplinary Geriatric Difficult Case Conference Call (IGDCC) is sponsored monthly by 3 Wisconsin health systems. The purpose is to provide opportunities to discuss clinical cases, to learn from one another and from experts, and to elevate the level of geriatric care in the states of Wisconsin, Michigan, and beyond. Each month a difficult case is presented by a clinician involved in that patient’s care. Time is allotted for participants to ask questions, and teaching points are shared by a clinical expert to highlight concepts and provide additional context. The IGDCC is meant to be a joint learning exercise to explore a specific difficult patient situation and learn skills and knowledge to improve care and transitions for older adults. The conference call is not a critique of the care, but rather an opportunity to jointly learn from the challenging situations all experience.
Background
The IGDCC was created by four members of 3 health systems in Wisconsin: Wheaton Franciscan Healthcare, Aspirus, and Aurora Health Care. The health systems serve and partially overlap on a broad geographic and demographic area of Wisconsin. The 4 members collaborated on numerous projects in the past, including Nurses Improving Case for Health System Elders (NICHE) implementation [3]. A common concern among the team is the management of challenging geriatric clinical patients and having a prepared workforce to meet those challenges.
Problem/Issue
As mentioned above, the older adult population is increasing, and these statistics are reflected in our service area [4]. Exacerbating these demographic changes is a shortage of health care workers in all disciplines, inadequate geriatric training, and the increased prevalence of multiple chronic conditions. Older adults also have higher rates of 30-day readmissions as well as higher rates of functional decline and medical errors during hospital stays [5,6]. Effective interprofessional teamwork is essential for the delivery of high-quality patient care in an increasingly complex health environment [7]. The IOM’s Future of Nursing report recommends that nurses, who represent the largest segment of the US health workforce, should achieve higher levels of training and be full partners in redesigning health care [8]. Unfortunately, effective care is hampered by poor coordination, limited communication, boundary infringement, and lack of understanding of roles [9]. Meta-analyses have demonstrated that there is a positive relationship between team training interventions and outcomes [10,11].
Objectives
The objective of the IGDCC is to elevate the level of geriatric care in the region by providing an accessible and affordable forum for the education of health care workers involved in the care of our most vulnerable population. To meet this challenge, the 4 founding members of IGDCC utilized the Aurora Health Care Geriatric Fellow’s Most Difficult Case (GFMCC) conference format as a model [12,13]. All disciplines are encouraged to participate, with announcements sent out via the leadership at the participating hospital systems. Participants have the option to call into the conference and teleconference via their own personal telephone and computer; in addition, each participating hospital system frequently hosts an open forum teleconference room where participants also may join a group.
Conference Components
The team uses the Wisconsin Star Method framework for presentation and discussion of the case. The Star Method, developed by Timothy Howell, enables clinical data about a person to be mapped out onto a single field with 5 domains: medications, medical, behavioral, personal, and social [14], creating a visual representation of the complicated and interacting physical, emotional, and social issues of older adults (Figure). By becoming comfortable using this method, the learner can use a similar approach in their clinical practice to address the needs of the patient in a holistic manner.
The case call concludes with expert teaching points from both a geriatric expert and a member of the interdisciplinary team. The interdisciplinary team member is chosen based on the key issues raised by the case. For example, cases that are made complex due to polypharmacy and adverse drug reactions might have a pharmacist presenting pertinent take-home message for the learner. In addition, geriatric teaching experts (ie, a geriatrician or advanced practice geriatric nursing specialist) provide the learner with insights that they can apply to their future practice. Often times the teaching points consist of an analysis of the various geriatric syndromes and how they can be managed in the complex older adult.
Implementation
Implementation of the IGDCC is coordinated by an oversight team with representation from each of the 3 sponsoring health systems. The oversight team currently includes 4 members: 3 geriatric clinical nurse specialists and a geriatric service line administrator. The team is responsible for:
- Planning the conference call schedule
- Making arrangements for case presenters and experts to contribute teaching points
- Registering participants and sharing written materials with participants
- Publicizing and encouraging attendance
- Soliciting feedback for continual improvement
- Exploring and implementing new ways to maximize learning.
Team members share duties and rotate case presentations. The Aurora and Wheaton Franciscan systems provide the geriatric specialists who provide the expert teaching points. The Aspirus system provides the conference line and webinar application and supports publicity and evaluations. All 3 systems are supported by a geriatric clinical nurse specialist who identifies and helps prepare presenters, case presentations, and call participants. Over time, the conference call format has evolved into a webinar format, allowing participants to either phone into the call for audio only or participate via both audio and visual. The visual allows participants to watch on their computer screens while the case is presented using the Star Method. During the call, a member of the oversight team adds clinical details by typing into a Word template of a blank star, adding information for each of the 5 domains in real-time as the case is discussed. Another member of the team facilitates the call, introducing presenters and experts, describing the Star Method, and offering “housekeeping” announcements. The facilitator also watches the timing to make certain the agenda is followed and the call begins and ends on time. During the call, another member of the team updates the attendance spreadsheet and makes a recording of each session.
Some participating facilities reserve a meeting room and project the webinar onto a screen for shared viewing. One of the participating sites has done this quite successfully with a growing group of participants coming together to watch the case during their lunch hour. This allows an opportunity for group discussion—when the conference call is on “mute” so as not to disrupt learners at other locations.
Measurement/Analysis
Attendance has steadily increased. In CY2015 from January to September, the mean attendance per month was 29.1 (mode, 17). The maximum per month was 62 (September 2015). The program enjoyed a boost in attendance beginning in July 2015 when Nurses Improving Care of Healthsystem Elders (NICHE) [3] began promoting the call-in opportunity to its NICHE Coordinators at member health systems. In June 2015, the technology was improved to allow for recorded sessions, and the recordings are growing in popularity from 2 listeners per month in July 2014 to 23 listeners per month in September 2015.
Lessons Learned
In comparing the IGDCC with similar conference call educational offerings, the team found that the program was unique in 2 areas. First, in addition to having a rich discussion in the care of frail older adults with experts in the field, the team also sought to help our staff learn how to present a difficult case to their peers. Three of our 4 committee members are geriatric clinical nurse specialists (a fourth is a clinical nurse specialist from Aspirus who assists periodically) who have been able to mentor, guide, and encourage interdisciplinary team members to present a challenging case. Many presenters had never presented a difficult case in this format. Presenters found the process fun and rewarding and have offered to present cases again in the future.
A second unique feature was utilizing the Wisconsin Star Method rather than focusing on a typical medical model framework for discussing a challenging case. The Star Method allows participants to increase their proficiency in providing comprehensive care while being more confident and mindful in addressing the complicated interacting physical, emotional and social issues of older adults [13].
A monthly post-call debriefing with committee members to review the strengths and weakness of the call was key to growing the program. The committee was able to critically review the process of the call, review participant surveys and discuss next steps. Adding a webinar approach, automatic email notification of calls, participant electronic survey, recording the call, and the addition of offering contact hours were some of the action items that were a result of monthly debriefing calls.
The team also found the 3-system collaboration to be beneficial. Aspirus has a large rural population, and Wheaton and Aurora have a diverse population, and each adds to the participant’s experience. Each IGDCC was rotated between the systems, which did not put the burden on any one health system. An annual call assignment listing was maintained for noting which system was responsible for the case each month and whether the geriatric expert was assigned/confirmed. Identifying the committee’s individual and collective group expertise was helpful in the overall project planning. The committee also developed a standard presenter guide and template and an expert teaching guide so the monthly IGDCC were consistent.
Challenges
The committee did not have a budget. Participation on the committee was in-kind funding from each system. Aspirus used its electronic system in place at the time to support the project. Interactive conference call education platform can be challenging with multiple participants on an open line who may not mute their phone. Often times, when a group of participants are calling in from one phone line it is difficult to know how many people are attending the IGDCC. It can be challenging at times to facilitate the call during the discussion component as participants occasionally talk over each other.
Current Status/Future Directions
The team has completed 18 consecutive monthly IGDCCs. Our participation rate has tripled. Participant satisfaction remains favorable. The team is now offering 1 contact hour to participants, and our invitations to participate have been extended to national health care groups. Challenging cases will be presented from community sources outside the hospital. Focusing attention on elevating the level of geriatric care in our region using a community educational approach will give us new opportunities for collaborating on best practice in multiple settings across the care continuum.
Acknowledgment: The planning team acknowledges Evalyn Michira, MSN, RN, PHN, AGCNS-BC, for her assistance in call presentations.
Corresponding author: Margie Hackbarth, MBA, margie.hackbarth@aspirus.org.
Financial disclosures: none.
1. Institute of Medicine. Retooling for an aging America: Building the health care workforce. Washington, DC: National Academies Press; 2008.
2. Mitchell P, Wynia M, Golden R, et al. Core principles and values of effective team-based health care. Discussion paper. Washington, DC; Institute of Medicine; 2012.
3. Nurses Improving Care for Healthsystem Elders. Accessed 1 Dec 2015 at www.nicheprogram.org/.
4. Wisconsin Department of Health Services. Southeastern region population report: 1 Jul 2013. Accessed 16 Feb 2015 at www.dhs.wisconsin.gov/sites/default/files/legacy/population/13data/southeastern.pdf.
5. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA 2003;289:1371–3.
6. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;(29):1–20, 24.
7. Nembhard IM, Edmondson AC. Making it safe: The effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav 2006; 27:941–66.
8. Institute of Medicine. The future of nursing: leading change, advancing health. National Academies Press; 2011.
9. Reeves S, Zwarenstein M, Goldman et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2013;3:CD002213.
10. Salas E, Diaz Granados D, Klein C, et al. Does team training improve team performance? A meta-analysis. Hum Factors 2008;50:903–33.
11. Strasser DD, Burridge AB, Falconer JA, et al. Toward spanning the quality chasm: an examination of team functioning measures. Arch Phys Med Rehabil 2014;95:2220–3.
12. Roche VM, Torregosa H, Howell T, Malone ML. Establishing a treatment plan for an elder with a complex and incomplete medical history and multiple medical providers, diagnoses, and medications. Ann Long-Term Care 2012;20(9).
13. Roche VM, Arnouville J, Danto-Nocton ES, et al. Optimal management of an older patient with multiple comorbidities and a complex psychosocial history. Ann Long-Term Care 2011;19(9).
14. Wisconsin Geriatric Psychiatry Initiative. The Wisconsin Star Method. Accessed 19 Jan 2015 at wgpi.wisc.edu/wisconsin-star-method/.
1. Institute of Medicine. Retooling for an aging America: Building the health care workforce. Washington, DC: National Academies Press; 2008.
2. Mitchell P, Wynia M, Golden R, et al. Core principles and values of effective team-based health care. Discussion paper. Washington, DC; Institute of Medicine; 2012.
3. Nurses Improving Care for Healthsystem Elders. Accessed 1 Dec 2015 at www.nicheprogram.org/.
4. Wisconsin Department of Health Services. Southeastern region population report: 1 Jul 2013. Accessed 16 Feb 2015 at www.dhs.wisconsin.gov/sites/default/files/legacy/population/13data/southeastern.pdf.
5. From the Centers for Disease Control and Prevention. Public health and aging: trends in aging--United States and worldwide. JAMA 2003;289:1371–3.
6. Hall MJ, DeFrances CJ, Williams SN, et al. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010;(29):1–20, 24.
7. Nembhard IM, Edmondson AC. Making it safe: The effects of leader inclusiveness and professional status on psychological safety and improvement efforts in health care teams. J Organiz Behav 2006; 27:941–66.
8. Institute of Medicine. The future of nursing: leading change, advancing health. National Academies Press; 2011.
9. Reeves S, Zwarenstein M, Goldman et al. Interprofessional education: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2013;3:CD002213.
10. Salas E, Diaz Granados D, Klein C, et al. Does team training improve team performance? A meta-analysis. Hum Factors 2008;50:903–33.
11. Strasser DD, Burridge AB, Falconer JA, et al. Toward spanning the quality chasm: an examination of team functioning measures. Arch Phys Med Rehabil 2014;95:2220–3.
12. Roche VM, Torregosa H, Howell T, Malone ML. Establishing a treatment plan for an elder with a complex and incomplete medical history and multiple medical providers, diagnoses, and medications. Ann Long-Term Care 2012;20(9).
13. Roche VM, Arnouville J, Danto-Nocton ES, et al. Optimal management of an older patient with multiple comorbidities and a complex psychosocial history. Ann Long-Term Care 2011;19(9).
14. Wisconsin Geriatric Psychiatry Initiative. The Wisconsin Star Method. Accessed 19 Jan 2015 at wgpi.wisc.edu/wisconsin-star-method/.
Clinician Telephone Training to Reduce Family Tobacco Use: Analysis of Transcribed Recordings
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.
After the data were coded into themes, the coded data were analyzed by the first author using qualitative description. Qualitative description is a method of analyzing coded qualitative data by looking at the words and meanings expressed by respondents [27]. Through this method of analysis, we were able to understand what concerns the clinicians and staff voiced about aspects of the CEASE intervention.
Ethics
The study was approved institutional review boards at Massachusetts General Hospital, the AAP, and the health care practices that required local IRB approval. The quotes used in this paper have been anonymized and cleaned to remove any identifying information, such as location and names.
Peer-to-Peer Training Calls
The peer-to-peer training calls were conducted after training and study materials arrived. The project leader (a pediatrician in the practice who was interested in spearheading the CEASE intervention) was asked to watch the training video. Using an evidence-based, previously developed call script [28], the principal investigator trained the project leader in key aspects of addressing family tobacco use and exposure, such as using an electronic tablet screener survey to identify family members who smoke, exploring techniques for prescribing or recommending NRT, and identifying ways to connect family members to free tobacco cessation counseling and support services. On occasion, other staff from the pediatric office (eg, a nurse or office manager) joined the call.
The principal investigator presented information, clarified points in the video, explained the materials, and asked questions and elicited relevant experiences from the project leader. In addition to teaching the project leader about the tobacco control strategies used in CEASE, the peer-to-peer calls prepared the project leader to train the rest of their own practice clinicians and staff in the CEASE intervention.
Whole Office Training Calls
Each practice’s local project leader led the whole office training calls, but CEASE study staff were on the call to introduce themselves to office staff, answer any questions that staff may have raised that the project leader could not answer, give information about data collection, and to generally support the implementation of the CEASE intervention and research program. During this call, the project leader watched the video with the group and tailored the training for his or her practice, focusing on issues of relevance for patients and staff.
Training Calls as Research Data
As many practices struggle with research burden [29], finding innovative and unobtrusive methods of collecting data is especially useful for research teams and participating practices. During both calls, clinicians and staff were asked open-ended questions to learn about their concerns regarding intervention implementation, share their own experiences with tobacco and tobacco control, and explore practice-specific methods to address family smoking. CEASE staff used this opportunity to help practices tailor the intervention to the local setting, such as by offering quitline enrollment sheets in another language. Clinician and staff answers to open-ended questions provided qualitative data for this manuscript.
Results and Discussion
The research team used training call data to explore clinician and staff concerns and desires related to family-centered tobacco control. The most common themes were: (1) prescribing, dosing, and insurance coverage of NRT, (2) motivation for and methods to help families become tobacco-free, and (3) the impact of the CEASE intervention on the day-to-day operations of the practice.
Nicotine Replacement Therapy
Prescribing or recommending NRT is one of the best ways to help families become tobacco-free and is a crucial component of the CEASE intervention [30–32]. Through the telephone trainings, clinicians and staff were trained to prescribe NRT using pre-printed prescription sheets, presented information about the effectiveness of NRT for smoking cessation, and referred to an information sheet on NRT to answer other questions as needed.
During the calls, it became clear that the pediatric clinicians were interested in prescribing NRT to help smokers quit, but lacked the skills and knowledge to do so:
I’m writing all this down [about NRT], because I don’t know any of this. (IN peer-to-peer)
Is 4 mg the strongest the gum comes in? (NC whole office)
This lack of knowledge may be a barrier to prescribing NRT in the pediatric setting. A national survey revealed that while smoking parents would accept prescriptions for NRT from their child’s doctor, very few received a prescription [33]. The calls provided an opportunity to have clinicians’ questions about NRT be answered by a pediatric tobacco control expert.
Clinicians were interested in helping parents stop smoking with medication, but were worried about access to medication; one of the most common questions voiced was not about how or why to prescribe NRT but how to help low-income parents get NRT for free or low-cost.
Some people—they don’t have insurance, so, how much it costs, they need to know that. (TN peer-to-peer)
I just know I’ve got a bunch ... Obamacare doesn’t work down here, so—I’ve still got families who don’t have any insurance, and you’re like, “Oh, I was hoping you could get something,” and they’re like, “Well, we can’t.” I have a fair number of kids who—are on some type of insurance, but the parents don’t have any coverage for NRT. (VA peer-to-peer)
While NRT is covered under the Affordable Care Act, many states have not expanded their Medicaid coverage [34]; this leaves many low-income families without access to health insurance or to free or low-cost NRT. While NRT remains one of the best and most common smoking cessation tools [35] there was no way to reassure practices that parents would be able to obtain the prescribed NRT without guaranteed coverage. In a previous study, the cost of NRT was seen by smokers as a barrier to using NRT to quit smoking [32]. Clinicians’ concerns about the cost of NRT reveal an understanding of the needs and issues relevant to their patient population.
Motivation for and Methods to Help Families Become Tobacco-Free
Clinicians and office staff were motivated to help families become tobacco-free and were interested in various ways to do so. The motivation and interest were personal, clinical, and organizational, relating to the ways in which care in the pediatric office could be altered to address tobacco in a more systematic way.
Motivation
The interest in smoking cessation stems from the desire to protect children from the harmful effects tobacco smoke and to prevent children themselves from taking up smoking:
We’d always talked about the smoking, and the parents finally quit. Probably not like I helped them—I just had been harping on them—but by that point the boy was smoking. When he was little he was like, “Oh, that’s nasty. I can’t believe my parents smoke.” Then by the time he was 14-15 and the parents actually did manage to quit, he was smoking, and I was like, “Ugh, really?” (VA peer-to-peer)
I totally understand the dire need for this project, in both the tobacco in the households, as well as the teenagers smoking. I heard one stat[istic], that one of our high schools had 80% of children using tobacco products… And that’s on my watch… I understand and I share the same passion that you do, for personal reasons, as well as reasons to help the whole community. (NC peer-to-peer)
Pediatricians saw themselves as responsible for protecting children’s health through reducing their tobacco smoke exposure, for working to prevent teen smoking, and for the overall health of their communities. Helping prevent childhood exposure to tobacco smoke and teen smoking initiation are crucial tasks for pediatricians; the 2015 AAP tobacco policy statement strongly recommends that pediatric offices include tobacco use prevention messages when talking to children and teens to help prevent smoking initiation, as well as helping families establish smoking bans for homes and cars [36]. By participating in the CEASE telephone trainings, clinicians and office staff were learning skills and tools to help them act on their motivation to protect families from the harms of tobacco.
Strategies
Pediatricians and office staff were interested in learning specific strategies and tools to help parents stop smoking. Practices wanted to know how and when to set a quit date with families, how to use services to help families become smoke-free, and how to tailor assistance to specific populations.
Yeah, we’re wondering about other languages, because we do have a large Hispanic patient population and a sizable group of folks that come from Saudi Arabia, and I know that some of them do smoke. (TN peer-to-peer)
Set[ting] a quit date for the patient —so how long we want to set the date? 6 months, 3 months, 1 year, 2 years, what? (TN peer-to-peer)
If you have a mom who lives with grandma and grandpa, the mom may not smoke but grandma and grandpa smoke, but they still live in that home… But anyone who comes in, we’re going to help. Does that sound right? (VA peer-to-peer)
By participating in the study, the clinicians and office staff were actively seeking to improve their knowledge of tobacco-related issues; past research has shown that pediatric residents saw lack of training in tobacco control as a key reason for inconsistent tobacco control outreach and intervention [37]. The training calls were an opportunity to gain information more specifically related to the pediatric practice’s population and office setting, building upon the other CEASE training materials. The training calls were also a chance for the CEASE research team to adapt strategies and tools to the practices, for example by providing materials that met the practices’ needs.
Impact of Intervention on Day-To-Day Operations
The training calls revealed that integrating CEASE into office workflows was a major concern. Integrating preventive services into routine office practice is a frequent concern of primary care providers [38–41]. These concerns about office flow reflect worries about financing [42] and benchmarking [43–45].
I think they’re going to have some of the same questions [that I initially had] in terms of how this might work with workflow. But as we’ve talked through all of this, I think we can make it work, and make it just sort of incorporated as part of our everyday questions that we ask. And it shouldn’t really slow things down. And I think that’ll be the main thing the providers would be focusing on is, how’s this going to impact me and all the other things I have to do in the course of a visit? This [phone call] answers a lot of questions I had in terms of that. (IN peer-to-peer)
As wait time was a performance measure for many of the practices, the clinicians and staff were hesitant to add any activities to check-in that might increase wait time.
I know, so especially, we’re trying to do a care team right now... don’t want them to spend too much time in the waiting room. (OH whole office)
During the calls, clinicians and office staff were asked to reflect on their practices and discuss ways that their practice would implement the CEASE intervention. This moment of reflection is a benefit of research participation, as it allows practices to improve the care they provide [46]. The calls allowed for on-the-spot tailoring of the intervention to meet the specific needs of the practice, an opportunity for the research staff and practice to work together to make the intervention fit their particular office situation and flow. Data collected from the training calls were also reviewed during the CEASE implementation process to support practices with specific concerns.
Strengths and Limitations
As these data were collected during training calls and subject to social desirability bias, the concerns raised may not be an exhaustive list of all concerns that clinicians and office staff had. However, the concerns that were raised by clinicians became a natural and essential part of the training process. As the practices’ initial concerns were identified early in the study, it was possible to address these concerns throughout the early implementation phases of CEASE. Transcribing calls and analyzing training call data as quickly as possible during the training phases of an intervention could prove beneficial for strengthening the implementation.
Dedicating the extra time and effort to record the training calls as a source of data formalized and strengthened the implementation process. By recording training calls, the study team was able to document the practices’ concerns and share them among the research team, including those who were not on training calls. This effort was a significant source of quality improvement data for the research team and helped ensure that we were responsive to the articulated needs of clinicians and practices.
Conclusion
The training call data revealed both the concerns as well as the interests of child health care clinicians in regard to addressing family tobacco use. While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco free, they expressed concerns that could threaten full implementation of family tobacco control strategies. These concerns and interests related to the coverage and affordability of NRT, integrating tobacco control strategies into the practice flow, and learning strategies to address family-wide tobacco use, such as helping grandparents quit smoking or addressing tobacco use with those who were not native English speakers. The concerns and interests of clinicians and office staff revealed that they were genuinely interested in learning ways to tailor strategies to address tobacco use for their practices and patient populations. By recording the training calls, the study team was better able to help them tailor the intervention to their practice, both during the calls and during subsequent implementation by providing new materials and additional information on subjects of concern to the practice. Carefully documenting training calls with health care practices are an ideal opportunity to collect information on issues that may impact full implementation of future interventions.
Corresponding author: Jonathan P. Winickoff, jwinickoff@mgh.harvard.edu
1. U.S. Department of Health and Human Services. The health consequences of involuntary tobacco smoke: a report of the Surgeon General. 2006.
2. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85.
3. Polanska K, Hanke W, Ronchetti R, et al. Environmental tobacco smoke exposure and children’s health. Acta Paediatr Suppl 2006;95:86–92.
4. American Academy of Pediatrics, Committee on Substance Abuse. Tobacco’s toll: implications for the pediatrician. Pediatrics 2001;107:794–8.
5. U.S. Department of Health and Human Services. Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2007.
6. Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics 2011;127:85–92.
7. Taylor SM, Ross NA, Cummings KM, et al. Community intervention trial for smoking cessation (COMMIT): changes in community attitudes toward cigarette smoking. Health Educ Res 1998;13:109-22.
8. Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics 2010;125:518–25.
9. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
10. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
11. Ossip DJ, Chang Y, Nabi-Burza E, et al. Strict smoke-free home policies among smoking parents in pediatric settings. Acad Pediatr 2013;13:517–23.
12. Winickoff JP, Park ER, Hipple BJ, et al. Clinical effort against secondhand smoke exposure: development of framework and intervention. Pediatrics 2008;122:e363–e75.
13. Nabi-Burza E, Winickoff JP, Finch S, Regan S. Triple tobacco screen: opportunity to help families become smokefree. Am J Prev Med 2013;45:728–31.
14. Winickoff JP. Pediatrician-led program increases provision of smoking cessation support, boosts quit rates among parents. Innovations in Medicine 2011. Accessed 24 Nov 2015 at https://innovations.ahrq.gov/profiles/pediatrician-led-program-increases-provision-smoking-cessation-support-boosts-quit-rates.
15. Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000.
16. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;134:933-41.
17. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024.
18. Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475–80.
19. Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36:764–8.
20.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med 1998;339:673–9.
21. Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: Results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun 2012;17 Suppl 1:44-53.
22. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med 2003;157:295–302.
23. Orleans CT, Schoenbach VJ, Wagner EH. Self-help quit smoking interventions: effects of self-help materials, social support materials, social support instructions and telephone counseling. J Consult Clin Psychol 1991;59:439–48.
24. An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166:536–42.
25. Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114:847–55.
26. Creswell, JW. Qualitative inquiry and research design: choosing among five approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007.
27. Sandelowski M. Focus on research methods: whatever happened to qualitative description. Res Nurs Health 2000;23:334–40.
28. Winickoff JP, Hipple B, Drehmer J, et al. The clinical effort against secondhand smoke exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
29. Clark T, Sinclair R. The costs and benefits of acting as a research site. Evid Policy A J Res Debate Pract 2008;4:105–19.
30. Zhu S, Melcer T, Sun J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305–11.
31. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res 2006;8:661–9.
32. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119–22.
33. Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics 2005;115:1013–7.
34. Kaiser Family Foundation. Status of state action on the medicaid expansion decision. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.
35. U.S. Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014.
36. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:998–1007.
37. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr 2007;150:547–52.
38. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med 1996;5:108–15.
39. Swartz SH, Hays JT. Office-based intervention for tobacco dependence. Med Clin North Am 2004;88:1623–41.
40. Bordley WC, Margolis PA, Stuart J, et al. Improving preventive service delivery through office systems. Pediatrics 2001;108:E41.
41. Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 25:w555–w71.
42. Rigotti NA, Quinn VP, Stevens VJ, et al. Tobacco-control policies in 11 leading managed care organizations: progress and challenges. Eff Clin Pract 2002;5:130–6.
43. Curry SJ. Organizational interventions to encourage guideline implementation. Chest 2000;118(2 Suppl):40S–6S.
44. Berg M, Meijerink Y, Gras M, et al. Feasibility first: developing public performance indicators on patient safety and clinical effectiveness for Dutch hospitals. Health Policy 2005;75:59–73.
45. Gandhi TK, Puopolo a L, Dasse P, et al. Obstacles to collaborative quality improvement: the case of ambulatory general medical care. Int J Qual Health Care 2000;12:115–23.
46. Mol A. Proving or improving: on health care research as a form of self-reflection. Qual Health Res 2006;16:405–14.
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.
After the data were coded into themes, the coded data were analyzed by the first author using qualitative description. Qualitative description is a method of analyzing coded qualitative data by looking at the words and meanings expressed by respondents [27]. Through this method of analysis, we were able to understand what concerns the clinicians and staff voiced about aspects of the CEASE intervention.
Ethics
The study was approved institutional review boards at Massachusetts General Hospital, the AAP, and the health care practices that required local IRB approval. The quotes used in this paper have been anonymized and cleaned to remove any identifying information, such as location and names.
Peer-to-Peer Training Calls
The peer-to-peer training calls were conducted after training and study materials arrived. The project leader (a pediatrician in the practice who was interested in spearheading the CEASE intervention) was asked to watch the training video. Using an evidence-based, previously developed call script [28], the principal investigator trained the project leader in key aspects of addressing family tobacco use and exposure, such as using an electronic tablet screener survey to identify family members who smoke, exploring techniques for prescribing or recommending NRT, and identifying ways to connect family members to free tobacco cessation counseling and support services. On occasion, other staff from the pediatric office (eg, a nurse or office manager) joined the call.
The principal investigator presented information, clarified points in the video, explained the materials, and asked questions and elicited relevant experiences from the project leader. In addition to teaching the project leader about the tobacco control strategies used in CEASE, the peer-to-peer calls prepared the project leader to train the rest of their own practice clinicians and staff in the CEASE intervention.
Whole Office Training Calls
Each practice’s local project leader led the whole office training calls, but CEASE study staff were on the call to introduce themselves to office staff, answer any questions that staff may have raised that the project leader could not answer, give information about data collection, and to generally support the implementation of the CEASE intervention and research program. During this call, the project leader watched the video with the group and tailored the training for his or her practice, focusing on issues of relevance for patients and staff.
Training Calls as Research Data
As many practices struggle with research burden [29], finding innovative and unobtrusive methods of collecting data is especially useful for research teams and participating practices. During both calls, clinicians and staff were asked open-ended questions to learn about their concerns regarding intervention implementation, share their own experiences with tobacco and tobacco control, and explore practice-specific methods to address family smoking. CEASE staff used this opportunity to help practices tailor the intervention to the local setting, such as by offering quitline enrollment sheets in another language. Clinician and staff answers to open-ended questions provided qualitative data for this manuscript.
Results and Discussion
The research team used training call data to explore clinician and staff concerns and desires related to family-centered tobacco control. The most common themes were: (1) prescribing, dosing, and insurance coverage of NRT, (2) motivation for and methods to help families become tobacco-free, and (3) the impact of the CEASE intervention on the day-to-day operations of the practice.
Nicotine Replacement Therapy
Prescribing or recommending NRT is one of the best ways to help families become tobacco-free and is a crucial component of the CEASE intervention [30–32]. Through the telephone trainings, clinicians and staff were trained to prescribe NRT using pre-printed prescription sheets, presented information about the effectiveness of NRT for smoking cessation, and referred to an information sheet on NRT to answer other questions as needed.
During the calls, it became clear that the pediatric clinicians were interested in prescribing NRT to help smokers quit, but lacked the skills and knowledge to do so:
I’m writing all this down [about NRT], because I don’t know any of this. (IN peer-to-peer)
Is 4 mg the strongest the gum comes in? (NC whole office)
This lack of knowledge may be a barrier to prescribing NRT in the pediatric setting. A national survey revealed that while smoking parents would accept prescriptions for NRT from their child’s doctor, very few received a prescription [33]. The calls provided an opportunity to have clinicians’ questions about NRT be answered by a pediatric tobacco control expert.
Clinicians were interested in helping parents stop smoking with medication, but were worried about access to medication; one of the most common questions voiced was not about how or why to prescribe NRT but how to help low-income parents get NRT for free or low-cost.
Some people—they don’t have insurance, so, how much it costs, they need to know that. (TN peer-to-peer)
I just know I’ve got a bunch ... Obamacare doesn’t work down here, so—I’ve still got families who don’t have any insurance, and you’re like, “Oh, I was hoping you could get something,” and they’re like, “Well, we can’t.” I have a fair number of kids who—are on some type of insurance, but the parents don’t have any coverage for NRT. (VA peer-to-peer)
While NRT is covered under the Affordable Care Act, many states have not expanded their Medicaid coverage [34]; this leaves many low-income families without access to health insurance or to free or low-cost NRT. While NRT remains one of the best and most common smoking cessation tools [35] there was no way to reassure practices that parents would be able to obtain the prescribed NRT without guaranteed coverage. In a previous study, the cost of NRT was seen by smokers as a barrier to using NRT to quit smoking [32]. Clinicians’ concerns about the cost of NRT reveal an understanding of the needs and issues relevant to their patient population.
Motivation for and Methods to Help Families Become Tobacco-Free
Clinicians and office staff were motivated to help families become tobacco-free and were interested in various ways to do so. The motivation and interest were personal, clinical, and organizational, relating to the ways in which care in the pediatric office could be altered to address tobacco in a more systematic way.
Motivation
The interest in smoking cessation stems from the desire to protect children from the harmful effects tobacco smoke and to prevent children themselves from taking up smoking:
We’d always talked about the smoking, and the parents finally quit. Probably not like I helped them—I just had been harping on them—but by that point the boy was smoking. When he was little he was like, “Oh, that’s nasty. I can’t believe my parents smoke.” Then by the time he was 14-15 and the parents actually did manage to quit, he was smoking, and I was like, “Ugh, really?” (VA peer-to-peer)
I totally understand the dire need for this project, in both the tobacco in the households, as well as the teenagers smoking. I heard one stat[istic], that one of our high schools had 80% of children using tobacco products… And that’s on my watch… I understand and I share the same passion that you do, for personal reasons, as well as reasons to help the whole community. (NC peer-to-peer)
Pediatricians saw themselves as responsible for protecting children’s health through reducing their tobacco smoke exposure, for working to prevent teen smoking, and for the overall health of their communities. Helping prevent childhood exposure to tobacco smoke and teen smoking initiation are crucial tasks for pediatricians; the 2015 AAP tobacco policy statement strongly recommends that pediatric offices include tobacco use prevention messages when talking to children and teens to help prevent smoking initiation, as well as helping families establish smoking bans for homes and cars [36]. By participating in the CEASE telephone trainings, clinicians and office staff were learning skills and tools to help them act on their motivation to protect families from the harms of tobacco.
Strategies
Pediatricians and office staff were interested in learning specific strategies and tools to help parents stop smoking. Practices wanted to know how and when to set a quit date with families, how to use services to help families become smoke-free, and how to tailor assistance to specific populations.
Yeah, we’re wondering about other languages, because we do have a large Hispanic patient population and a sizable group of folks that come from Saudi Arabia, and I know that some of them do smoke. (TN peer-to-peer)
Set[ting] a quit date for the patient —so how long we want to set the date? 6 months, 3 months, 1 year, 2 years, what? (TN peer-to-peer)
If you have a mom who lives with grandma and grandpa, the mom may not smoke but grandma and grandpa smoke, but they still live in that home… But anyone who comes in, we’re going to help. Does that sound right? (VA peer-to-peer)
By participating in the study, the clinicians and office staff were actively seeking to improve their knowledge of tobacco-related issues; past research has shown that pediatric residents saw lack of training in tobacco control as a key reason for inconsistent tobacco control outreach and intervention [37]. The training calls were an opportunity to gain information more specifically related to the pediatric practice’s population and office setting, building upon the other CEASE training materials. The training calls were also a chance for the CEASE research team to adapt strategies and tools to the practices, for example by providing materials that met the practices’ needs.
Impact of Intervention on Day-To-Day Operations
The training calls revealed that integrating CEASE into office workflows was a major concern. Integrating preventive services into routine office practice is a frequent concern of primary care providers [38–41]. These concerns about office flow reflect worries about financing [42] and benchmarking [43–45].
I think they’re going to have some of the same questions [that I initially had] in terms of how this might work with workflow. But as we’ve talked through all of this, I think we can make it work, and make it just sort of incorporated as part of our everyday questions that we ask. And it shouldn’t really slow things down. And I think that’ll be the main thing the providers would be focusing on is, how’s this going to impact me and all the other things I have to do in the course of a visit? This [phone call] answers a lot of questions I had in terms of that. (IN peer-to-peer)
As wait time was a performance measure for many of the practices, the clinicians and staff were hesitant to add any activities to check-in that might increase wait time.
I know, so especially, we’re trying to do a care team right now... don’t want them to spend too much time in the waiting room. (OH whole office)
During the calls, clinicians and office staff were asked to reflect on their practices and discuss ways that their practice would implement the CEASE intervention. This moment of reflection is a benefit of research participation, as it allows practices to improve the care they provide [46]. The calls allowed for on-the-spot tailoring of the intervention to meet the specific needs of the practice, an opportunity for the research staff and practice to work together to make the intervention fit their particular office situation and flow. Data collected from the training calls were also reviewed during the CEASE implementation process to support practices with specific concerns.
Strengths and Limitations
As these data were collected during training calls and subject to social desirability bias, the concerns raised may not be an exhaustive list of all concerns that clinicians and office staff had. However, the concerns that were raised by clinicians became a natural and essential part of the training process. As the practices’ initial concerns were identified early in the study, it was possible to address these concerns throughout the early implementation phases of CEASE. Transcribing calls and analyzing training call data as quickly as possible during the training phases of an intervention could prove beneficial for strengthening the implementation.
Dedicating the extra time and effort to record the training calls as a source of data formalized and strengthened the implementation process. By recording training calls, the study team was able to document the practices’ concerns and share them among the research team, including those who were not on training calls. This effort was a significant source of quality improvement data for the research team and helped ensure that we were responsive to the articulated needs of clinicians and practices.
Conclusion
The training call data revealed both the concerns as well as the interests of child health care clinicians in regard to addressing family tobacco use. While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco free, they expressed concerns that could threaten full implementation of family tobacco control strategies. These concerns and interests related to the coverage and affordability of NRT, integrating tobacco control strategies into the practice flow, and learning strategies to address family-wide tobacco use, such as helping grandparents quit smoking or addressing tobacco use with those who were not native English speakers. The concerns and interests of clinicians and office staff revealed that they were genuinely interested in learning ways to tailor strategies to address tobacco use for their practices and patient populations. By recording the training calls, the study team was better able to help them tailor the intervention to their practice, both during the calls and during subsequent implementation by providing new materials and additional information on subjects of concern to the practice. Carefully documenting training calls with health care practices are an ideal opportunity to collect information on issues that may impact full implementation of future interventions.
Corresponding author: Jonathan P. Winickoff, jwinickoff@mgh.harvard.edu
From the Massachusetts General Hospital for Children, Boston, MA (Walters, Drehmer, Nabi-Burza, Winickoff), the University of Rochester School of Medicine, Rochester, NY (Ossip), and the American Academy of Pediatrics Julius B. Richmond Center of Excellence, Elk Grove Village, IL (Whitmore, Gorzkowski). †Deceased 31 December 2015.
Abstract
- Background: Family tobacco use and exposure are significant threats to the health of children and their families. However, few pediatric clinicians address family tobacco use and exposure in a routine and effective manner. The Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice.
- Objective: To review the main considerations and questions that clinicians and office staff expressed during telephone training to participate in CEASE.
- Methods: This study was conducted in pediatric practices in 5 US states. Practices were recruited by the American Academy of Pediatrics (10 intervention, 10 control). Ten training calls were recorded and transcribed. The data was then coded inductively based on themes found in the transcripts.
- Results: The data revealed that clinicians and staff were concerned about prescribing, dosing, and insurance coverage of nicotine replacement therapy; motivation for and methods to help families become tobacco-free; and the impact of the intervention on practice operations.
- Conclusion: While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco-free, they expressed concerns that could threaten implementation of family tobacco control strategies.
The devastating health consequences of smoking and exposure to tobacco smoke have been well demonstrated. As declared in the 2006 Surgeon General’s Report, there is no safe level of exposure to tobacco [1]. Children are especially at risk for exposure to toxins and toxicants in tobacco smoke [1,2]. Exposure to tobacco smoke is associated with higher levels of asthma, increased risk of sudden infant death syndrome, increased rates of upper respiratory infections, and behavioral issues [3–5]. Recent research shows that over 70% of children in the United States have some level of exposure to tobacco smoke [6]; parents and other family members are commonly the cause of this exposure, especially in young children. Children and parents benefit when parents stop smoking; parent life expectancy increases by an average of 7 years [7], the risk of tobacco-related poor pregnancy outcomes is reduced, and future children are spared from exposure to tobacco smoke [8].
There is a growing movement to address tobacco use and exposure in the pediatric office setting; the 2015 American Academy of Pediatrics tobacco policy statement Clinical Practice Policy to Protect Children From Tobacco, Nicotine, and Tobacco Smoke recommends that pediatricians ask about children’s exposure to tobacco and address parental tobacco use by implementing office-wide systems to deliver advice, counseling, referral to cessation resources, and smoking cessation medication to smokers [9].
Despite significant risks of tobacco smoke exposure to children, we found in a previous paper that only 3.5% of parents in control practices received any tobacco control assistance [10]. Through a systematic and ongoing line of research, the Clinical Effort Against Secondhand Smoke Exposure (CEASE) intervention was developed to tackle this gap between clinical need and clinical practice. The CEASE intervention has been successfully shown to train and equip pediatric officesfrom a distance to address family tobacco use within existing office systems [10–14]. An enhanced CEASE intervention is undergoing testing in pediatric practices in 5 US states.
One of the more innovative aspects of CEASE has been the use of training calls. In studies of CEASE, the peer-to-peer call was conducted by the principal investigator with the project leader at the practice using a train-the-trainer model. After the project leader was trained through the peer-to-peer call, the project leader then led the whole office training call, with the support of CEASE staff by phone. The training calls worked in conjunction with the other aspects of the training, as shown in the Table. The training calls for the practices provided a valuable research opportunity. We examined the concerns and issues that clinicians and office staff had about implementing an office-wide tobacco control program through a qualitative analysis of the call transcripts. This paper outlines the main considerations and questions that clinicians and office staff expressed during the training calls. Understanding the points of view of clinicians and staff will help researchers and clinical educators strengthen the design of tobacco control interventions.
Methods
Study Aims
The data for this paper were collected as part of a larger mixed-methods controlled trial. The overarching aims of the trial were to study implementation and sustainability of tobacco-control services delivered at the clinic level, to facilitate behavior change among parents and evaluate cost-per-quit among parents who smoke, and to study systems changes and the processes that affect them at the practice level. The study was conducted in 5 intervention and 5 control pediatric primary care practices in 5 states; this paper reports on data collected in intervention practices and focuses on understanding the systems changes and processes that are instituted when implementing a tobacco control program at the clinician and practice level.
Practice Recruitment and Eligibility
Practices were recruited through the American Academy of Pediatrics using direct emails, newsletter/listserv articles, phone calls to members, and in-person recruitment at national meetings. Eligible practices were located in a non–hospital-based setting, had an average patient flow of at least 50 patients per day, used an electronic medical record (EMR) system, and were matched in each state based on practice size and smoking rate. Interested practices also had to be willing to host a research assistant to collect exit interview data from parents. Practices were excluded if they took part in previous CEASE studies or were actively enrolling participants into other tobacco control research studies. Based on these criteria, 18 eligible practices from Indiana, North Carolina, Ohio, Tennessee, Michigan, and Virginia agreed to participate in the study. Of the 6 states, one state was chosen as a replacement state. Five practices from the remaining states were assigned to the intervention group, 5 to the control group, and 5 were assigned to the replacement group in case an intervention or control practice in their state withdrew from the study. Each intervention practice participated in a peer-to-peer training call and a whole office training call. Data analyzed in this paper was collected from all 10 intervention practice training calls.
Training Calls Data Collection
The peer-to-peer and whole office training calls were recorded and transcribed. Permission to record the calls was requested by the trainer (the principal investigator of the study) and given verbally by each person being trained. The training call recordings were then transcribed verbatim by a commercial service; the transcriptions were spot-checked for accuracy.
The transcripts were first read closely by the first author (BHW), then coded inductively into relevant themes that emerged from the calls. The inductive coding was guided by the questions and concerns that the clinicians raised during the training, as well as the ways in which the trainer addressed these concerns and tailored the training to the needs and interests of the pediatric clinicians [26]. The coding was reviewed and confirmed by the other study team members.
After the data were coded into themes, the coded data were analyzed by the first author using qualitative description. Qualitative description is a method of analyzing coded qualitative data by looking at the words and meanings expressed by respondents [27]. Through this method of analysis, we were able to understand what concerns the clinicians and staff voiced about aspects of the CEASE intervention.
Ethics
The study was approved institutional review boards at Massachusetts General Hospital, the AAP, and the health care practices that required local IRB approval. The quotes used in this paper have been anonymized and cleaned to remove any identifying information, such as location and names.
Peer-to-Peer Training Calls
The peer-to-peer training calls were conducted after training and study materials arrived. The project leader (a pediatrician in the practice who was interested in spearheading the CEASE intervention) was asked to watch the training video. Using an evidence-based, previously developed call script [28], the principal investigator trained the project leader in key aspects of addressing family tobacco use and exposure, such as using an electronic tablet screener survey to identify family members who smoke, exploring techniques for prescribing or recommending NRT, and identifying ways to connect family members to free tobacco cessation counseling and support services. On occasion, other staff from the pediatric office (eg, a nurse or office manager) joined the call.
The principal investigator presented information, clarified points in the video, explained the materials, and asked questions and elicited relevant experiences from the project leader. In addition to teaching the project leader about the tobacco control strategies used in CEASE, the peer-to-peer calls prepared the project leader to train the rest of their own practice clinicians and staff in the CEASE intervention.
Whole Office Training Calls
Each practice’s local project leader led the whole office training calls, but CEASE study staff were on the call to introduce themselves to office staff, answer any questions that staff may have raised that the project leader could not answer, give information about data collection, and to generally support the implementation of the CEASE intervention and research program. During this call, the project leader watched the video with the group and tailored the training for his or her practice, focusing on issues of relevance for patients and staff.
Training Calls as Research Data
As many practices struggle with research burden [29], finding innovative and unobtrusive methods of collecting data is especially useful for research teams and participating practices. During both calls, clinicians and staff were asked open-ended questions to learn about their concerns regarding intervention implementation, share their own experiences with tobacco and tobacco control, and explore practice-specific methods to address family smoking. CEASE staff used this opportunity to help practices tailor the intervention to the local setting, such as by offering quitline enrollment sheets in another language. Clinician and staff answers to open-ended questions provided qualitative data for this manuscript.
Results and Discussion
The research team used training call data to explore clinician and staff concerns and desires related to family-centered tobacco control. The most common themes were: (1) prescribing, dosing, and insurance coverage of NRT, (2) motivation for and methods to help families become tobacco-free, and (3) the impact of the CEASE intervention on the day-to-day operations of the practice.
Nicotine Replacement Therapy
Prescribing or recommending NRT is one of the best ways to help families become tobacco-free and is a crucial component of the CEASE intervention [30–32]. Through the telephone trainings, clinicians and staff were trained to prescribe NRT using pre-printed prescription sheets, presented information about the effectiveness of NRT for smoking cessation, and referred to an information sheet on NRT to answer other questions as needed.
During the calls, it became clear that the pediatric clinicians were interested in prescribing NRT to help smokers quit, but lacked the skills and knowledge to do so:
I’m writing all this down [about NRT], because I don’t know any of this. (IN peer-to-peer)
Is 4 mg the strongest the gum comes in? (NC whole office)
This lack of knowledge may be a barrier to prescribing NRT in the pediatric setting. A national survey revealed that while smoking parents would accept prescriptions for NRT from their child’s doctor, very few received a prescription [33]. The calls provided an opportunity to have clinicians’ questions about NRT be answered by a pediatric tobacco control expert.
Clinicians were interested in helping parents stop smoking with medication, but were worried about access to medication; one of the most common questions voiced was not about how or why to prescribe NRT but how to help low-income parents get NRT for free or low-cost.
Some people—they don’t have insurance, so, how much it costs, they need to know that. (TN peer-to-peer)
I just know I’ve got a bunch ... Obamacare doesn’t work down here, so—I’ve still got families who don’t have any insurance, and you’re like, “Oh, I was hoping you could get something,” and they’re like, “Well, we can’t.” I have a fair number of kids who—are on some type of insurance, but the parents don’t have any coverage for NRT. (VA peer-to-peer)
While NRT is covered under the Affordable Care Act, many states have not expanded their Medicaid coverage [34]; this leaves many low-income families without access to health insurance or to free or low-cost NRT. While NRT remains one of the best and most common smoking cessation tools [35] there was no way to reassure practices that parents would be able to obtain the prescribed NRT without guaranteed coverage. In a previous study, the cost of NRT was seen by smokers as a barrier to using NRT to quit smoking [32]. Clinicians’ concerns about the cost of NRT reveal an understanding of the needs and issues relevant to their patient population.
Motivation for and Methods to Help Families Become Tobacco-Free
Clinicians and office staff were motivated to help families become tobacco-free and were interested in various ways to do so. The motivation and interest were personal, clinical, and organizational, relating to the ways in which care in the pediatric office could be altered to address tobacco in a more systematic way.
Motivation
The interest in smoking cessation stems from the desire to protect children from the harmful effects tobacco smoke and to prevent children themselves from taking up smoking:
We’d always talked about the smoking, and the parents finally quit. Probably not like I helped them—I just had been harping on them—but by that point the boy was smoking. When he was little he was like, “Oh, that’s nasty. I can’t believe my parents smoke.” Then by the time he was 14-15 and the parents actually did manage to quit, he was smoking, and I was like, “Ugh, really?” (VA peer-to-peer)
I totally understand the dire need for this project, in both the tobacco in the households, as well as the teenagers smoking. I heard one stat[istic], that one of our high schools had 80% of children using tobacco products… And that’s on my watch… I understand and I share the same passion that you do, for personal reasons, as well as reasons to help the whole community. (NC peer-to-peer)
Pediatricians saw themselves as responsible for protecting children’s health through reducing their tobacco smoke exposure, for working to prevent teen smoking, and for the overall health of their communities. Helping prevent childhood exposure to tobacco smoke and teen smoking initiation are crucial tasks for pediatricians; the 2015 AAP tobacco policy statement strongly recommends that pediatric offices include tobacco use prevention messages when talking to children and teens to help prevent smoking initiation, as well as helping families establish smoking bans for homes and cars [36]. By participating in the CEASE telephone trainings, clinicians and office staff were learning skills and tools to help them act on their motivation to protect families from the harms of tobacco.
Strategies
Pediatricians and office staff were interested in learning specific strategies and tools to help parents stop smoking. Practices wanted to know how and when to set a quit date with families, how to use services to help families become smoke-free, and how to tailor assistance to specific populations.
Yeah, we’re wondering about other languages, because we do have a large Hispanic patient population and a sizable group of folks that come from Saudi Arabia, and I know that some of them do smoke. (TN peer-to-peer)
Set[ting] a quit date for the patient —so how long we want to set the date? 6 months, 3 months, 1 year, 2 years, what? (TN peer-to-peer)
If you have a mom who lives with grandma and grandpa, the mom may not smoke but grandma and grandpa smoke, but they still live in that home… But anyone who comes in, we’re going to help. Does that sound right? (VA peer-to-peer)
By participating in the study, the clinicians and office staff were actively seeking to improve their knowledge of tobacco-related issues; past research has shown that pediatric residents saw lack of training in tobacco control as a key reason for inconsistent tobacco control outreach and intervention [37]. The training calls were an opportunity to gain information more specifically related to the pediatric practice’s population and office setting, building upon the other CEASE training materials. The training calls were also a chance for the CEASE research team to adapt strategies and tools to the practices, for example by providing materials that met the practices’ needs.
Impact of Intervention on Day-To-Day Operations
The training calls revealed that integrating CEASE into office workflows was a major concern. Integrating preventive services into routine office practice is a frequent concern of primary care providers [38–41]. These concerns about office flow reflect worries about financing [42] and benchmarking [43–45].
I think they’re going to have some of the same questions [that I initially had] in terms of how this might work with workflow. But as we’ve talked through all of this, I think we can make it work, and make it just sort of incorporated as part of our everyday questions that we ask. And it shouldn’t really slow things down. And I think that’ll be the main thing the providers would be focusing on is, how’s this going to impact me and all the other things I have to do in the course of a visit? This [phone call] answers a lot of questions I had in terms of that. (IN peer-to-peer)
As wait time was a performance measure for many of the practices, the clinicians and staff were hesitant to add any activities to check-in that might increase wait time.
I know, so especially, we’re trying to do a care team right now... don’t want them to spend too much time in the waiting room. (OH whole office)
During the calls, clinicians and office staff were asked to reflect on their practices and discuss ways that their practice would implement the CEASE intervention. This moment of reflection is a benefit of research participation, as it allows practices to improve the care they provide [46]. The calls allowed for on-the-spot tailoring of the intervention to meet the specific needs of the practice, an opportunity for the research staff and practice to work together to make the intervention fit their particular office situation and flow. Data collected from the training calls were also reviewed during the CEASE implementation process to support practices with specific concerns.
Strengths and Limitations
As these data were collected during training calls and subject to social desirability bias, the concerns raised may not be an exhaustive list of all concerns that clinicians and office staff had. However, the concerns that were raised by clinicians became a natural and essential part of the training process. As the practices’ initial concerns were identified early in the study, it was possible to address these concerns throughout the early implementation phases of CEASE. Transcribing calls and analyzing training call data as quickly as possible during the training phases of an intervention could prove beneficial for strengthening the implementation.
Dedicating the extra time and effort to record the training calls as a source of data formalized and strengthened the implementation process. By recording training calls, the study team was able to document the practices’ concerns and share them among the research team, including those who were not on training calls. This effort was a significant source of quality improvement data for the research team and helped ensure that we were responsive to the articulated needs of clinicians and practices.
Conclusion
The training call data revealed both the concerns as well as the interests of child health care clinicians in regard to addressing family tobacco use. While the majority of clinicians and office staff were interested and enthusiastic about helping families become tobacco free, they expressed concerns that could threaten full implementation of family tobacco control strategies. These concerns and interests related to the coverage and affordability of NRT, integrating tobacco control strategies into the practice flow, and learning strategies to address family-wide tobacco use, such as helping grandparents quit smoking or addressing tobacco use with those who were not native English speakers. The concerns and interests of clinicians and office staff revealed that they were genuinely interested in learning ways to tailor strategies to address tobacco use for their practices and patient populations. By recording the training calls, the study team was better able to help them tailor the intervention to their practice, both during the calls and during subsequent implementation by providing new materials and additional information on subjects of concern to the practice. Carefully documenting training calls with health care practices are an ideal opportunity to collect information on issues that may impact full implementation of future interventions.
Corresponding author: Jonathan P. Winickoff, jwinickoff@mgh.harvard.edu
1. U.S. Department of Health and Human Services. The health consequences of involuntary tobacco smoke: a report of the Surgeon General. 2006.
2. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85.
3. Polanska K, Hanke W, Ronchetti R, et al. Environmental tobacco smoke exposure and children’s health. Acta Paediatr Suppl 2006;95:86–92.
4. American Academy of Pediatrics, Committee on Substance Abuse. Tobacco’s toll: implications for the pediatrician. Pediatrics 2001;107:794–8.
5. U.S. Department of Health and Human Services. Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2007.
6. Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics 2011;127:85–92.
7. Taylor SM, Ross NA, Cummings KM, et al. Community intervention trial for smoking cessation (COMMIT): changes in community attitudes toward cigarette smoking. Health Educ Res 1998;13:109-22.
8. Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics 2010;125:518–25.
9. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
10. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
11. Ossip DJ, Chang Y, Nabi-Burza E, et al. Strict smoke-free home policies among smoking parents in pediatric settings. Acad Pediatr 2013;13:517–23.
12. Winickoff JP, Park ER, Hipple BJ, et al. Clinical effort against secondhand smoke exposure: development of framework and intervention. Pediatrics 2008;122:e363–e75.
13. Nabi-Burza E, Winickoff JP, Finch S, Regan S. Triple tobacco screen: opportunity to help families become smokefree. Am J Prev Med 2013;45:728–31.
14. Winickoff JP. Pediatrician-led program increases provision of smoking cessation support, boosts quit rates among parents. Innovations in Medicine 2011. Accessed 24 Nov 2015 at https://innovations.ahrq.gov/profiles/pediatrician-led-program-increases-provision-smoking-cessation-support-boosts-quit-rates.
15. Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000.
16. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;134:933-41.
17. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024.
18. Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475–80.
19. Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36:764–8.
20.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med 1998;339:673–9.
21. Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: Results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun 2012;17 Suppl 1:44-53.
22. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med 2003;157:295–302.
23. Orleans CT, Schoenbach VJ, Wagner EH. Self-help quit smoking interventions: effects of self-help materials, social support materials, social support instructions and telephone counseling. J Consult Clin Psychol 1991;59:439–48.
24. An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166:536–42.
25. Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114:847–55.
26. Creswell, JW. Qualitative inquiry and research design: choosing among five approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007.
27. Sandelowski M. Focus on research methods: whatever happened to qualitative description. Res Nurs Health 2000;23:334–40.
28. Winickoff JP, Hipple B, Drehmer J, et al. The clinical effort against secondhand smoke exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
29. Clark T, Sinclair R. The costs and benefits of acting as a research site. Evid Policy A J Res Debate Pract 2008;4:105–19.
30. Zhu S, Melcer T, Sun J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305–11.
31. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res 2006;8:661–9.
32. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119–22.
33. Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics 2005;115:1013–7.
34. Kaiser Family Foundation. Status of state action on the medicaid expansion decision. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.
35. U.S. Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014.
36. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:998–1007.
37. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr 2007;150:547–52.
38. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med 1996;5:108–15.
39. Swartz SH, Hays JT. Office-based intervention for tobacco dependence. Med Clin North Am 2004;88:1623–41.
40. Bordley WC, Margolis PA, Stuart J, et al. Improving preventive service delivery through office systems. Pediatrics 2001;108:E41.
41. Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 25:w555–w71.
42. Rigotti NA, Quinn VP, Stevens VJ, et al. Tobacco-control policies in 11 leading managed care organizations: progress and challenges. Eff Clin Pract 2002;5:130–6.
43. Curry SJ. Organizational interventions to encourage guideline implementation. Chest 2000;118(2 Suppl):40S–6S.
44. Berg M, Meijerink Y, Gras M, et al. Feasibility first: developing public performance indicators on patient safety and clinical effectiveness for Dutch hospitals. Health Policy 2005;75:59–73.
45. Gandhi TK, Puopolo a L, Dasse P, et al. Obstacles to collaborative quality improvement: the case of ambulatory general medical care. Int J Qual Health Care 2000;12:115–23.
46. Mol A. Proving or improving: on health care research as a form of self-reflection. Qual Health Res 2006;16:405–14.
1. U.S. Department of Health and Human Services. The health consequences of involuntary tobacco smoke: a report of the Surgeon General. 2006.
2. Rauh VA, Whyatt RM, Garfinkel R, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol 2004;26:373–85.
3. Polanska K, Hanke W, Ronchetti R, et al. Environmental tobacco smoke exposure and children’s health. Acta Paediatr Suppl 2006;95:86–92.
4. American Academy of Pediatrics, Committee on Substance Abuse. Tobacco’s toll: implications for the pediatrician. Pediatrics 2001;107:794–8.
5. U.S. Department of Health and Human Services. Children and secondhand smoke exposure. Excerpts from the health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2007.
6. Wilson KM, Klein JD, Blumkin AK, et al. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics 2011;127:85–92.
7. Taylor SM, Ross NA, Cummings KM, et al. Community intervention trial for smoking cessation (COMMIT): changes in community attitudes toward cigarette smoking. Health Educ Res 1998;13:109-22.
8. Winickoff JP, Healey EA, Regan S, et al. Using the postpartum hospital stay to address mothers’ and fathers’ smoking: the NEWS study. Pediatrics 2010;125:518–25.
9. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:1008–17.
10. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control intervention in pediatric practice. Pediatrics 2013;132:109–17.
11. Ossip DJ, Chang Y, Nabi-Burza E, et al. Strict smoke-free home policies among smoking parents in pediatric settings. Acad Pediatr 2013;13:517–23.
12. Winickoff JP, Park ER, Hipple BJ, et al. Clinical effort against secondhand smoke exposure: development of framework and intervention. Pediatrics 2008;122:e363–e75.
13. Nabi-Burza E, Winickoff JP, Finch S, Regan S. Triple tobacco screen: opportunity to help families become smokefree. Am J Prev Med 2013;45:728–31.
14. Winickoff JP. Pediatrician-led program increases provision of smoking cessation support, boosts quit rates among parents. Innovations in Medicine 2011. Accessed 24 Nov 2015 at https://innovations.ahrq.gov/profiles/pediatrician-led-program-increases-provision-smoking-cessation-support-boosts-quit-rates.
15. Fiore MC, Bailey WC, Cohen SJ. Treating tobacco use and dependence. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2000.
16. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics 2014;134:933-41.
17. Moore D, Aveyard P, Connock M, et al. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ 2009;338:b1024.
18. Aveyard P, Wang D, Connock M, et al. Assessing the outcomes of prolonged cessation-induction and aid-to-cessation trials: floating prolonged abstinence. Nicotine Tob Res 2009;11:475–80.
19. Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav 2011;36:764–8.
20.Curry SJ, Grothaus LC, McAfee T, Pabiniak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med 1998;339:673–9.
21. Abroms LC, Ahuja M, Kodl Y, et al. Text2Quit: Results from a pilot test of a personalized, interactive mobile health smoking cessation program. J Health Commun 2012;17 Suppl 1:44-53.
22. Curry SJ, Ludman EJ, Graham E, et al. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med 2003;157:295–302.
23. Orleans CT, Schoenbach VJ, Wagner EH. Self-help quit smoking interventions: effects of self-help materials, social support materials, social support instructions and telephone counseling. J Consult Clin Psychol 1991;59:439–48.
24. An LC, Zhu SH, Nelson DB, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Arch Intern Med 2006;166:536–42.
25. Warner DO, Klesges RC, Dale LC, et al. Clinician-delivered intervention to facilitate tobacco quitline use by surgical patients. Anesthesiology 2011;114:847–55.
26. Creswell, JW. Qualitative inquiry and research design: choosing among five approaches. 2nd ed. Thousand Oaks, CA: Sage; 2007.
27. Sandelowski M. Focus on research methods: whatever happened to qualitative description. Res Nurs Health 2000;23:334–40.
28. Winickoff JP, Hipple B, Drehmer J, et al. The clinical effort against secondhand smoke exposure (CEASE) intervention: A decade of lessons learned. J Clin Outcomes Manag 2012;19:414–9.
29. Clark T, Sinclair R. The costs and benefits of acting as a research site. Evid Policy A J Res Debate Pract 2008;4:105–19.
30. Zhu S, Melcer T, Sun J. Smoking cessation with and without assistance: a population-based analysis. Am J Prev Med 2000;18:305–11.
31. Gilpin EA, Messer K, Pierce JP. Population effectiveness of pharmaceutical aids for smoking cessation: what is associated with increased success? Nicotine Tob Res 2006;8:661–9.
32. Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: results from the National Health Interview Survey, 2000. Am J Prev Med 2005;28:119–22.
33. Winickoff JP, Tanski SE, McMillen RC, et al. Child health care clinicians’ use of medications to help parents quit smoking: a national parent survey. Pediatrics 2005;115:1013–7.
34. Kaiser Family Foundation. Status of state action on the medicaid expansion decision. Available at http://kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/.
35. U.S. Department of Health and Human Services. The health consequences of smoking- 50 years of progress: a report of the Surgeon General. 2014.
36. American Academy of Pediatrics, Section on Tobacco Control. Policy statement: Public policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics 2015;136:998–1007.
37. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr 2007;150:547–52.
38. Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med 1996;5:108–15.
39. Swartz SH, Hays JT. Office-based intervention for tobacco dependence. Med Clin North Am 2004;88:1623–41.
40. Bordley WC, Margolis PA, Stuart J, et al. Improving preventive service delivery through office systems. Pediatrics 2001;108:E41.
41. Schoen C, Osborn R, Huynh PT, et al. On the front lines of care: primary care doctors’ office systems, experiences, and views in seven countries. Health Aff (Millwood) 25:w555–w71.
42. Rigotti NA, Quinn VP, Stevens VJ, et al. Tobacco-control policies in 11 leading managed care organizations: progress and challenges. Eff Clin Pract 2002;5:130–6.
43. Curry SJ. Organizational interventions to encourage guideline implementation. Chest 2000;118(2 Suppl):40S–6S.
44. Berg M, Meijerink Y, Gras M, et al. Feasibility first: developing public performance indicators on patient safety and clinical effectiveness for Dutch hospitals. Health Policy 2005;75:59–73.
45. Gandhi TK, Puopolo a L, Dasse P, et al. Obstacles to collaborative quality improvement: the case of ambulatory general medical care. Int J Qual Health Care 2000;12:115–23.
46. Mol A. Proving or improving: on health care research as a form of self-reflection. Qual Health Res 2006;16:405–14.