User login
Maintaining and reclaiming hemostasis in laparoscopic surgery

Additional videos from SGS are available here, including these recent offerings:
- Safety and efficiency in the laparoscopic hysterectomy: Techniques to optimize the surgical approach
- Laparoscopic management of bladder fibroids: Surgical tips and tricks
- Laparoscopic techniques for Essure device removal

Additional videos from SGS are available here, including these recent offerings:
- Safety and efficiency in the laparoscopic hysterectomy: Techniques to optimize the surgical approach
- Laparoscopic management of bladder fibroids: Surgical tips and tricks
- Laparoscopic techniques for Essure device removal

Additional videos from SGS are available here, including these recent offerings:
- Safety and efficiency in the laparoscopic hysterectomy: Techniques to optimize the surgical approach
- Laparoscopic management of bladder fibroids: Surgical tips and tricks
- Laparoscopic techniques for Essure device removal
Two-drug combo should be first-line standard of care in advanced endometrial cancer
The combination proved to be noninferior to paclitaxel-doxorubicin-cisplatin (TAP) in terms of response, progression-free survival, and overall survival, and with lower toxicity.
Overall survival was a median of 37 months for TC and 41 months for TAP, and there were more adverse events of grade 3 or higher with TAP.
The data were initially presented at the 2012 annual meeting of the Society of Gynecologic Oncology. In the original presentation, lead author David Scott Miller, MD, said this combination should be the standard of care in this setting. Dr. Miller is professor of obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas.
“This subsequent long-term follow-up publication confirmed that,” he said in an interview. “TAP is now rarely used.”
The results have now been published in the Journal of Clinical Oncology.
The Gynecologic Oncology Group 177 trial established TAP about a decade earlier as the standard for systemic treatment of stage III-IV and recurrent endometrial cancer. However, the regimen was associated with substantially more toxicity than doxorubicin-cisplatin.
Phase 2 trials of TC suggested that the combination was active in endometrial cancer, the authors noted. They hypothesized that doxorubicin could be omitted from the regimen and that carboplatin could be substituted for cisplatin.
Equivalent survival, lower toxicity
In the current trial, Dr. Miller and colleagues sought to determine whether TC was therapeutically equivalent or noninferior to TAP with regard to survival outcomes. Secondary endpoints involved the toxicity profile of TC, compared with TAP. The two regimens were also compared with respect to patient-reported neurotoxicity and health-related quality of life (HRQoL).
From 2003 to 2009, 1,381 women with stage III, stage IV, and recurrent endometrial cancers were enrolled in the phase 3 GOG0209 trial. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony–stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles.
After treatment was completed, patients were followed quarterly for 2 years, semiannually for 3 years, and then annually until death. Most of the patients (61%) had measurable or recurrent disease at baseline.
In this updated analysis, with a median follow-up of 124 months, about two-thirds (>65%) of the patients had died; 28% remain alive without evidence of cancer. The adjusted ratio of death hazard of TC versus TAP was 1.002; for progression, the HR of TC to TAP was 1.032.
Median progression-free survival for TAP versus TC was 14 months versus 13 months, and for overall survival, 41 months versus 37 months.
As for adverse events, neutropenic fever was reported in 7% of patients who received TAP and in 6% of those who received TC. The rate of sensory neuropathy was greater among patients who received TAP (26% vs. 20%; P = .40), as was the rate of thrombocytopenia of grade ≥3 (23% vs. 12%), vomiting (7% vs. 4%), diarrhea (6% vs. 2%), and metabolic toxicities (14% vs. 8%). The rate of neutropenia was greater with TC (52% vs. 80%).
Data on HRQoL were collected from the first 538 patients enrolled before March 26, 2007. HRQoL was assessed at baseline and then at 6 weeks, 15 weeks, and 26 weeks. At 6 weeks, the TC group had higher scores on physical well-being and functional well-being (2.1-point difference; 0.3 to approximately 3.9 points; P = .009; effect size, 0.19). There were no statistically significant differences between groups at 15 and 26 weeks.
On the Functional Assessment of Cancer Therapy/GOG-Neurotoxicity four-item measure of sensory neuropathy (FACT/GOG-Ntx) subscale, scores were higher (indicating fewer neurotoxic symptoms) for patients in the TC group by 1.4 points (0.4 to approximately 2.5 points; P = .003; effect size, 0.64) at 26 weeks. There were no statistically significant differences between the groups at 6 and 15 weeks.
Dr. Miller noted that TC became the “backbone or control arm for most subsequent trials,” such as those evaluating immunotherapy and other agents in this setting.
The study was supported by National Cancer Institute grants to the GOG Administrative Office, the GOG Statistical Office, NRG Oncology (1 U10 CA180822), NRG Operations, and the National Cancer Institute Community Oncology Research Program. Dr. Miller has had a consulting or advisory role with Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, and Merck Sharp & Dohme; has been on the speakers’ bureaus for Clovis Oncology and Genentech; and has received institutional research funding from US Biotest, Advenchen Laboratories, Millennium, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, and Merck Sharp & Dohme.
A version of this article originally appeared on Medscape.com.
The combination proved to be noninferior to paclitaxel-doxorubicin-cisplatin (TAP) in terms of response, progression-free survival, and overall survival, and with lower toxicity.
Overall survival was a median of 37 months for TC and 41 months for TAP, and there were more adverse events of grade 3 or higher with TAP.
The data were initially presented at the 2012 annual meeting of the Society of Gynecologic Oncology. In the original presentation, lead author David Scott Miller, MD, said this combination should be the standard of care in this setting. Dr. Miller is professor of obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas.
“This subsequent long-term follow-up publication confirmed that,” he said in an interview. “TAP is now rarely used.”
The results have now been published in the Journal of Clinical Oncology.
The Gynecologic Oncology Group 177 trial established TAP about a decade earlier as the standard for systemic treatment of stage III-IV and recurrent endometrial cancer. However, the regimen was associated with substantially more toxicity than doxorubicin-cisplatin.
Phase 2 trials of TC suggested that the combination was active in endometrial cancer, the authors noted. They hypothesized that doxorubicin could be omitted from the regimen and that carboplatin could be substituted for cisplatin.
Equivalent survival, lower toxicity
In the current trial, Dr. Miller and colleagues sought to determine whether TC was therapeutically equivalent or noninferior to TAP with regard to survival outcomes. Secondary endpoints involved the toxicity profile of TC, compared with TAP. The two regimens were also compared with respect to patient-reported neurotoxicity and health-related quality of life (HRQoL).
From 2003 to 2009, 1,381 women with stage III, stage IV, and recurrent endometrial cancers were enrolled in the phase 3 GOG0209 trial. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony–stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles.
After treatment was completed, patients were followed quarterly for 2 years, semiannually for 3 years, and then annually until death. Most of the patients (61%) had measurable or recurrent disease at baseline.
In this updated analysis, with a median follow-up of 124 months, about two-thirds (>65%) of the patients had died; 28% remain alive without evidence of cancer. The adjusted ratio of death hazard of TC versus TAP was 1.002; for progression, the HR of TC to TAP was 1.032.
Median progression-free survival for TAP versus TC was 14 months versus 13 months, and for overall survival, 41 months versus 37 months.
As for adverse events, neutropenic fever was reported in 7% of patients who received TAP and in 6% of those who received TC. The rate of sensory neuropathy was greater among patients who received TAP (26% vs. 20%; P = .40), as was the rate of thrombocytopenia of grade ≥3 (23% vs. 12%), vomiting (7% vs. 4%), diarrhea (6% vs. 2%), and metabolic toxicities (14% vs. 8%). The rate of neutropenia was greater with TC (52% vs. 80%).
Data on HRQoL were collected from the first 538 patients enrolled before March 26, 2007. HRQoL was assessed at baseline and then at 6 weeks, 15 weeks, and 26 weeks. At 6 weeks, the TC group had higher scores on physical well-being and functional well-being (2.1-point difference; 0.3 to approximately 3.9 points; P = .009; effect size, 0.19). There were no statistically significant differences between groups at 15 and 26 weeks.
On the Functional Assessment of Cancer Therapy/GOG-Neurotoxicity four-item measure of sensory neuropathy (FACT/GOG-Ntx) subscale, scores were higher (indicating fewer neurotoxic symptoms) for patients in the TC group by 1.4 points (0.4 to approximately 2.5 points; P = .003; effect size, 0.64) at 26 weeks. There were no statistically significant differences between the groups at 6 and 15 weeks.
Dr. Miller noted that TC became the “backbone or control arm for most subsequent trials,” such as those evaluating immunotherapy and other agents in this setting.
The study was supported by National Cancer Institute grants to the GOG Administrative Office, the GOG Statistical Office, NRG Oncology (1 U10 CA180822), NRG Operations, and the National Cancer Institute Community Oncology Research Program. Dr. Miller has had a consulting or advisory role with Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, and Merck Sharp & Dohme; has been on the speakers’ bureaus for Clovis Oncology and Genentech; and has received institutional research funding from US Biotest, Advenchen Laboratories, Millennium, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, and Merck Sharp & Dohme.
A version of this article originally appeared on Medscape.com.
The combination proved to be noninferior to paclitaxel-doxorubicin-cisplatin (TAP) in terms of response, progression-free survival, and overall survival, and with lower toxicity.
Overall survival was a median of 37 months for TC and 41 months for TAP, and there were more adverse events of grade 3 or higher with TAP.
The data were initially presented at the 2012 annual meeting of the Society of Gynecologic Oncology. In the original presentation, lead author David Scott Miller, MD, said this combination should be the standard of care in this setting. Dr. Miller is professor of obstetrics and gynecology at the University of Texas Southwestern Medical Center, Dallas.
“This subsequent long-term follow-up publication confirmed that,” he said in an interview. “TAP is now rarely used.”
The results have now been published in the Journal of Clinical Oncology.
The Gynecologic Oncology Group 177 trial established TAP about a decade earlier as the standard for systemic treatment of stage III-IV and recurrent endometrial cancer. However, the regimen was associated with substantially more toxicity than doxorubicin-cisplatin.
Phase 2 trials of TC suggested that the combination was active in endometrial cancer, the authors noted. They hypothesized that doxorubicin could be omitted from the regimen and that carboplatin could be substituted for cisplatin.
Equivalent survival, lower toxicity
In the current trial, Dr. Miller and colleagues sought to determine whether TC was therapeutically equivalent or noninferior to TAP with regard to survival outcomes. Secondary endpoints involved the toxicity profile of TC, compared with TAP. The two regimens were also compared with respect to patient-reported neurotoxicity and health-related quality of life (HRQoL).
From 2003 to 2009, 1,381 women with stage III, stage IV, and recurrent endometrial cancers were enrolled in the phase 3 GOG0209 trial. Patients were treated with doxorubicin 45 mg/m2 and cisplatin 50 mg/m2 (day 1), followed by paclitaxel 160 mg/m2 (day 2) with granulocyte colony–stimulating factor or paclitaxel 175 mg/m2 and carboplatin area under the curve 6 (day 1) every 21 days for seven cycles.
After treatment was completed, patients were followed quarterly for 2 years, semiannually for 3 years, and then annually until death. Most of the patients (61%) had measurable or recurrent disease at baseline.
In this updated analysis, with a median follow-up of 124 months, about two-thirds (>65%) of the patients had died; 28% remain alive without evidence of cancer. The adjusted ratio of death hazard of TC versus TAP was 1.002; for progression, the HR of TC to TAP was 1.032.
Median progression-free survival for TAP versus TC was 14 months versus 13 months, and for overall survival, 41 months versus 37 months.
As for adverse events, neutropenic fever was reported in 7% of patients who received TAP and in 6% of those who received TC. The rate of sensory neuropathy was greater among patients who received TAP (26% vs. 20%; P = .40), as was the rate of thrombocytopenia of grade ≥3 (23% vs. 12%), vomiting (7% vs. 4%), diarrhea (6% vs. 2%), and metabolic toxicities (14% vs. 8%). The rate of neutropenia was greater with TC (52% vs. 80%).
Data on HRQoL were collected from the first 538 patients enrolled before March 26, 2007. HRQoL was assessed at baseline and then at 6 weeks, 15 weeks, and 26 weeks. At 6 weeks, the TC group had higher scores on physical well-being and functional well-being (2.1-point difference; 0.3 to approximately 3.9 points; P = .009; effect size, 0.19). There were no statistically significant differences between groups at 15 and 26 weeks.
On the Functional Assessment of Cancer Therapy/GOG-Neurotoxicity four-item measure of sensory neuropathy (FACT/GOG-Ntx) subscale, scores were higher (indicating fewer neurotoxic symptoms) for patients in the TC group by 1.4 points (0.4 to approximately 2.5 points; P = .003; effect size, 0.64) at 26 weeks. There were no statistically significant differences between the groups at 6 and 15 weeks.
Dr. Miller noted that TC became the “backbone or control arm for most subsequent trials,” such as those evaluating immunotherapy and other agents in this setting.
The study was supported by National Cancer Institute grants to the GOG Administrative Office, the GOG Statistical Office, NRG Oncology (1 U10 CA180822), NRG Operations, and the National Cancer Institute Community Oncology Research Program. Dr. Miller has had a consulting or advisory role with Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Guardant Health, Janssen, Alexion Pharmaceuticals, Clovis Oncology, and Merck Sharp & Dohme; has been on the speakers’ bureaus for Clovis Oncology and Genentech; and has received institutional research funding from US Biotest, Advenchen Laboratories, Millennium, Tesaro, Xenetic Biosciences, Advaxis, Janssen, Aeterna Zentaris, TRACON Pharma, Pfizer, Immunogen, Mateon Therapeutics, and Merck Sharp & Dohme.
A version of this article originally appeared on Medscape.com.
Menstrual irregularity appears to be predictor of early death
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
than women with regular or short cycles, reported Yi-Xin Wang, PhD, of Harvard TH Chan School of Public Health, Boston, and associates. This is particularly true in the presence of cardiovascular disease and a history of smoking.
In a peer-reviewed observational study of 79,505 premenopausal women enrolled in the Nurses’ Health Study II, the researchers sought to determine whether a life-long history of irregular or long menstrual cycles was associated with premature death. Patients averaged a mean age of 37.7 years and had no history of cardiovascular disease, cancer, or diabetes at enrollment.
Although irregular and long menstrual cycles are common and frequently linked with an increased risk of major chronic diseases – such as ovarian cancer, coronary heart disease, type 2 diabetes, and mental health problems – in women of reproductive age, actual evidence linking irregular or long menstrual cycles with mortality is scant, the researchers noted in the BMJ.
During the study, participants checked in at ages 14-17 years, 18-22 years, and 29-46 years to report the usual length and regularity of their menstrual cycles. Over 24 years of follow-up, a total of 1,975 premature deaths were noted, including 894 from cancer and 172 from cardiovascular disease.
Irregular cycles appear to bring risks
After considering other possible factors of influence, including age, weight, lifestyle, and family medical history, Dr. Wang and associates noted higher rates of mortality among those consistently reporting irregular cycles than women in the same age ranges with very regular cycles. Specifically, women aged 18-22 years and 29-46 years with cycles of 40 days or more were at greater risk of dying prematurely than were those in the same age ranges with cycles of 26-31 days.
Cardiovascular disease was a stronger predictor of death than cancer or other causes. Also included in the higher-risk group were those who currently smoked.
Among women reporting very regular cycles and women reporting always irregular cycles, mortality rates per 1,000 person-years were 1.05 and 1.23 at ages 14-17 years, 1.00 and 1.37 at ages 18-22 years, and 1.00 and 1.68 at ages 29-46 years, respectively.
The study also found that women reporting irregular cycles or no periods had a higher body mass indexes (28.2 vs. 25.0 kg/m2); were more likely to have conditions including hypertension (13.2% vs. 6.2%), high blood cholesterol levels (23.9% vs. 14.9%), hirsutism (8.4%
vs. 1.8%), or endometriosis (5.9% vs. 4.5%); and uterine fibroids (10.0% vs. 7.8%); and a higher prevalence of family history of diabetes (19.4% vs. 15.8%).
Dr. Wang and associates also observed – using multivariable Cox models – a greater risk of premature death across all categories and all age ranges in women with decreasing menstrual cycle regularity. In models that were fully adjusted, cycle lengths that were 40 days or more or too irregular to estimate from ages 18-22 and 29-46 expressed hazard ratios for premature death at the time of follow-up of 1.34 and 1.40, compared with women in the same age ranges reporting cycle lengths of 26-31 days.
Of note, Dr. Wang and colleagues unexpectedly discovered an increased risk of premature death in women who had used contraceptives between 14-17 years. They suggested that a greater number of women self-reporting contraceptive use in adolescence may have been using contraceptives to manage symptoms of polycystic ovary syndrome (PCOS) and other conditions such as endometriosis.
Relying on the potential inaccuracy inherent in patient recall of their menstrual cycle characteristics, and the likelihood for other unmeasured factors, may have affected study results. Study strengths included the significant number of participants who had a high follow-up rate over many years, and the availability of menstrual cycle data at three different points across the reproductive lifespan.
Because the mechanisms underlying these associations are likely related to the disrupted hormonal environment, the study results “emphasize the need for primary care providers to include menstrual cycle characteristics throughout the reproductive life span as additional vital signs in assessing women’s general health status,” Dr. Wang and colleagues cautioned.
Expert suggests a probable underlying link
“Irregular menstrual cycles in women have long been known to be associated with significant morbidities, including the leading causes of mortality worldwide such as cardiovascular disease and cancer,” Reshef Tal, MD, PhD, assistant professor of obstetrics, gynecology & reproductive sciences at Yale University, New Haven, Conn., said in an interview. “The findings of this large study that irregular menstrual cycles are associated with premature death, most strongly from cardiovascular causes, are therefore not surprising.”
Dr. Tal acknowledged that one probable underlying link is PCOS, which is recognized as the most common hormonal disorder affecting women of reproductive age. The irregular periods that characterize PCOS are tied to a number of metabolic risk factors, including obesity, insulin resistance, dyslipidemia, and hypertension, which increase the long-term risk of cardiovascular disease and cancer of the uterus.
“The study did not have information on patients’ pelvic ultrasound findings and male hormone levels, which would have helped to establish PCOS diagnosis. However, women in this study who had irregular cycles tended to have more hirsutism, high cholesterol, hypertension as well as higher BMI, suggesting that PCOS is at least partly responsible for the observed association with cardiovascular disease. Interestingly, the association between irregular cycles and early mortality was independent of BMI, indicating that mechanisms other than metabolic factors may also play a role,” observed Dr. Tal, who was asked to comment on the study.
“Irregular periods are a symptom and not a disease, so it is important to identify underlying metabolic risk factors. Furthermore, physicians are advised to counsel patients experiencing menstrual irregularity, [to advise them to] maintain a healthy lifestyle and be alert to health changes,” Dr. Tal suggested.
The study was funded by the National Institutes of Health. The investigators had no relevant financial disclosures. Dr. Tal said he had no relevant financial disclosures.
SOURCE: Chavarro J et al. BMJ. 2020. doi: 10.1136/bmj.m3464.
FROM THE BMJ
2020 Update on abnormal uterine bleeding
Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.

Abnormal uterine bleeding (AUB) continues to be a top reason that women present for gynecologic care. In general, our approach to the management of AUB is to diagnose causes before we prescribe therapy and to offer conservative therapies initially and progress to more invasive measures if indicated.
In this Update, we highlight several new studies that provide evidence for preferential use of certain medical and surgical therapies. In considering conservative therapy for the treatment of AUB, we take a closer look at the efficacy of cyclic progestogens. Another important issue, as more types of endometrial ablation (EA) are being developed and are coming into the market, is the need for additional guidance regarding decisions about EA versus progestin-releasing intrauterine devices (IUDs). Lastly, an unintended consequence of an increased cesarean delivery rate is the development of isthmocele, also known as cesarean scar defect or uterine niche. These defects, which can be bothersome and cause abnormal bleeding, are treated with various techniques. Within the last year, 2 systematic reviews that compare the efficacy of several different approaches and provide guidance have been published.
Is it time to retire cyclic progestogens for the treatment of heavy menstrual bleeding?
Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
In a recent Cochrane Database Systematic Review, Bofill Rodriguez and colleagues looked at the efficacy, safety, and tolerability of oral progestogen therapy for heavy menstrual bleeding.1 They considered progestogen (medroxyprogesterone acetate or norethisterone) in short-cycle use (7 to 10 days in the luteal phase) and long-cycle use (21 days per cycle) in a review of 15 randomized clinical trials (RCTs) that included a total of 1,071 women. As this topic had not been updated in 12 years, this review was essential in demonstrating changes that occurred over the past decade.
The primary outcomes of the analysis were menstrual blood loss and treatment satisfaction. Secondary outcomes included the number of days of bleeding, quality of life, adherence and acceptability of treatment, adverse events, and costs.
Classic progestogens fall short compared with newer approaches
Analysis of the data revealed that short-cycle progestogen was inferior to treatment with tranexamic acid, danazol, and the 65-µg progesterone-releasing IUD (Pg-IUD). Of note, the 65-µg Pg-IUD has been off the market since 2001, and danazol is rarely used in current practice. Furthermore, based on 2 trials, cyclic progestogens demonstrated no clear benefit over nonsteroidal anti-inflammatory drugs. Additionally, long-cycle progestogen therapy was found to be inferior to the 52-mg levonorgestrel-releasing IUD (LNG-IUD), tranexamic acid, and ormeloxifene.
It should be noted that the quality of evidence is still lacking for progestogen therapy, and this study's main limitation is bias, as the women and the researchers were aware of the treatments that were given. This review is helpful, however, for emphasizing the advantage of tranexamic acid and LNG-IUD use in clinical care.
The takeaway. Although it may not necessarily be time to retire the use of cyclic oral progestogens, the 52-mg LNG-IUD or tranexamic acid may be more successful for treating AUB in women who are appropriate candidates.
Cyclic progestogen therapy appears to be less effective for the treatment of AUB when compared with tranexamic acid and the LNG-IUD. It does not appear to be more helpful than nonsteroidal anti-inflammatory drugs. We frequently offer and prescribe tranexamic acid, 1,300 mg 3 times daily, as a medical alternative to hormonal therapy for up to 5 days monthly for women without thromboembolism risk. Lukes and colleagues published an RCT in 2010 that demonstrated a 40% reduction of bleeding in tranexamic acid–treated women compared with an 8.2% reduction in the placebo group.2
Continue to: Endometrial ablation...
Endometrial ablation: New evidence informs when it could (and could not) be the best option
Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
Two systematic reviews evaluated the efficacy of EA in women with abnormal uterine bleeding. One compared EA with the LNG-IUD and reported on safety and efficacy, while the other compared EA with hysterectomy and reported on quality of life.
Bergeron and colleagues reviewed 13 studies that included 884 women to compare the efficacy and safety of EA or resection with the LNG-IUD for the treatment of premenopausal women with AUB.3 They found no significant differences between EA and the LNG-IUD in terms of subsequent hysterectomy (risk ratio [RR] = 1.3; 95% confidence interval [CI], 0.60-2.11). It was not surprising that, when looking at age, EA was associated with a higher risk for hysterectomy in women younger than age 42 (RR = 5.26; 95% CI, 1.21-22.91). Conversely, subsequent hysterectomy was less likely with EA compared to LNG-IUD use in women older than 42 years. However, statistical significance was not reached in the older group (RR = 0.51; 95% CI, 0.21-1.24).
In the systematic review by Vitale and colleagues, 9 studies met inclusion criteria for a comparison of EA and hysterectomy, with the objective of ascertaining improvement in quality of life and several other measures.4
Although there was significant heterogeneity between assessment tools, both treatment groups experienced similar improvements in quality of life during the first year. However, hysterectomy was more advantageous in terms of improving uterine bleeding and satisfaction in the long term when compared with EA.4
As EA is considered, it is important to continue to counsel about the efficacy of the LNG-IUD, as well as its decreased associated morbidity. Additionally, EA is particularly less effective in younger women.
Continue to: Laparoscopy is best approach for isthomocele management, with caveats...
Laparoscopy is best approach for isthomocele management, with caveats
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
The isthmocele (cesarean scar defect, uterine niche), a known complication of cesarean delivery, represents a myometrial defect in the anterior uterine wall that often presents as abnormal uterine bleeding. It also can be a site for pregnancy-related complications, such as invasive placentation, placenta previa, and uterine rupture.
Two systematic reviews compared surgical strategies for treating isthmocele, including laparoscopy, hysteroscopy, combined laparoscopy and hysteroscopy, laparotomy, and vaginal repair.
Laparoscopy reduced isthmocele-associated AUB better than other techniques
A review by He and colleagues analyzed data from 10 pertinent studies (4 RCTs and 6 observational studies) that included 858 patients in total.5 Treatments compared were laparoscopy, hysteroscopy, combined laparoscopy with hysteroscopy, and vaginal repair for reduction of AUB and isthmocele and diverticulum depth.
The authors found no difference in intraoperative bleeding between the 4 surgical methods (laparotomy was not included in this review). Hysteroscopic surgery was associated with the shortest operative time, while laparoscopy was the longest surgery. In terms of reducing intermittent abnormal bleeding and scar depth, laparoscopic surgery performed better than the other 3 methods.
Approach considerations in isthmocele repair
Vitale and colleagues conducted a systematic review that included 33 publications (28 focused on a single surgical technique, 5 compared different techniques) to examine the effectiveness and risks of various surgical approaches for isthmocele in women with AUB, infertility, or for prevention of obstetric complications.6
Results of their analysis in general favored a laparoscopic approach for patients who desired future fertility, with an improvement rate of 92.7%. Hysteroscopic correction had an 85% improvement rate, and vaginal correction had an 82.5% improvement rate.
Although there were no high-level data to suggest a threshold for myometrial thickness in recommending a surgical approach, the authors provided a helpful algorithm for choosing a route based on a patient's fertility desires. For the asymptomatic patient, they suggest no treatment. In symptomatic patients, the laparoscopic approach is the gold standard but requires significant laparoscopic surgical skill, and a hysteroscopic approach may be considered as an alternative route if the residual myometrial defect is greater than 2.5 to 3.5 mm. For patients who are not considering future reproduction, hysteroscopy is the gold standard as long as the residual myometrial thickness is greater than 2.5 to 3.5 mm.
The takeaway. Of the several methods used for surgical isthmocele management, the laparoscopic approach reduced intermittent abnormal bleeding and scar depth better than other methods. It also was associated with the longest surgical duration. Hysteroscopic surgery was the quickest procedure to perform and is effective in removing the upper valve to promote the elimination of the hematocele and symptoms of abnormal bleeding; however, it does not change the anatomic aspects of the isthmocele in terms of myometrial thickness. Some authors suggested that deciding on the surgical route should be based on fertility desires and the residual thickness of the myometrium. ●
In terms of isthmocele repair, the laparoscopic approach is preferred in patients who desire fertility, as long as the surgeon possesses the skill set to perform this difficult surgery, and as long as the residual myometrium is thicker than 2.5 to 3.5 mm.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
- Bofill Rodriguez M, Lethaby A, Low C, et al. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2019;(8):CD001016.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled study. Obstet Gynecol. 2010;116:865-875.
- Bergeron C, Laberge PY, Boutin A, et al. Endometrial ablation or resection versus levonorgestrel intra-uterine system for the treatment of women with heavy menstrual bleeding and a normal uterine cavity: a systematic review with meta-analysis. Hum Reprod Update. 2020;26:302-311.
- Vitale SG, Ferrero S, Ciebiera M, et al. Hysteroscopic endometrial resection vs hysterectomy for abnormal uterine bleeding: impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr Opin Obstet Gynecol. 2020;32:159-165.
- He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
- Vitale SG, Ludwin A, Vilos GA, et al. From hysteroscopy to laparoendoscopic surgery: what is the best surgical approach for symptomatic isthmocele? A systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301:33-52.
How effective is elagolix treatment in women with fibroids and HMB?
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
Simon JA, Al-Hendy A, Archer DF, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326.
Expert Commentary
Uterine fibroids are common (occurring in up to 80% of reproductive-age women),1,2 and often associated with heavy menstrual bleeding (HMB). There are surgical and medical options, but typically medical options are used for short periods of time. Elagolix with hormonal add-back therapy was recently approved (May 29, 2020) by the US Food and Drug Administration (FDA) for treatment of HMB in women with uterine fibroids for up to 24 months.
Elagolix is an oral, nonpeptide gonadotropin-releasing hormone antagonist that results in a dose-dependent reduction of gonadotropins and ovarian sex hormones. There are now 2 approved products containing elagolix, with different indications:
- Orilissa. Elagolix was approved in 2018 by the FDA for moderate to severe pain associated with endometriosis. For that indication there are 2 dose options of elagolix (150 mg for up to 2 years and 200 mg for up to 6 months) and there is no hormonal add-back therapy.
- Oriahnn. Elagolix and hormonal add-back therapy was approved in 2020 for HMB associated with uterine fibroids for up to 24 months. The total daily dose of elagolix is 600 mg (elagolix 300 mg in the morning with estradiol 1 mg/norethindrone acetate 0.5 mg and then in the evening elagolix 300 mg and no hormonal add-back).
This new class of drug, GnRH antagonist, is an important one for women’s health, and emerging science will continue to expand its potential uses, such as in reproductive health, as well as long-term efficacy and safety. The difference in daily dose of elagolix for endometriosis (150 mg for 24 months) compared with HMB associated with fibroids (600 mg for 24 months) is why the hormonal add-back therapy is important and allows for protection of bone density.
This is an important manuscript because it highlights a medical option for women with HMB associated with fibroids, which can be used for a long period of time. Further, the improvement in bleeding is both impressive and maintained in the extension study. Approximately 90% of women show improvement in their menstrual bleeding associated with fibroids.
The question of what to do after 24 months of therapy with elagolix and hormonal add-back therapy is an important one, but providers should recognize that the limiting factor with this elagolix and hormonal add-back therapy is bone mineral density (BMD). We will only learn more and more moving forward if this is a clinically meaningful reason for stopping treatment at 24 months. The FDA takes a strict view of safety, and providers must weigh this with the benefit of therapy.
One other highlight between the 2 approved medications is that Orilissa does not have a black box warning, given that there is no hormonal add-back therapy. Oriahnn does have a warning, regarding thromboembolic disorders and vascular events:
- Estrogen and progestin combinations, including Oriahnn, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events.
- Oriahnn is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension.
Continue to: Details about the study...
Details about the study
The study by Simon et al is an extension study (UF-EXTEND), in that women could participate if they had completed 1 of the 2 pivotal studies on elagolix. The pivotal studies (Elaris UF1 and UF2) were both randomized, double-blinded, placebo-controlled studies with up to 6 months of therapy; for UF-EXTEND, however, participants were randomly assigned to either combined elagolix and hormone replacement therapy or elagolix alone for an additional 6 months of therapy. Although it was known that all participants would receive elagolix in UF-EXTEND, those who received hormonal add-back therapy were blinded. All women were then followed up for an additional 12 months after treatment ended.
The efficacy of elagolix was measured by the objective alkaline hematin method for menstrual blood loss with the a priori coprimary endpoints. The elagolix and hormonal add-back therapy group showed objective improvement in menstrual blood loss at 12 months in 87.9% of women in the extension study (89.4% in the elagolix alone group). This compares with 72.2% improvement at 6 months of treatment in the UF1 and UF2 studies for those taking elagolix and hormonal add-back therapy. These findings illustrate maintenance of the efficacy seen within the 6-month pivotal studies using elagolix over an extended amount of time.
The safety of elagolix also was demonstrated in UF-EXTEND. The 3 most common adverse events were similar to those found in Elaris UF1 and UF2 and included hot flushes, headache, and nausea. In the elagolix and hormonal add-back therapy group during the extension study, the percentage with hot flushes was 7%, headache 6%, and nausea 4%. These are small percentages, which is encouraging for providers and women with HMB associated with fibroids.
Effects on bone density
Bone density was evaluated at baseline in the UF1 and UF2 studies, through treatment, and then 12 months after the extended treatment was stopped. The hormonal add-back therapy of estradiol 1 mg/norethindrone acetate 0.5 mg significantly protected bone density. Some women did not have a decrease in bone density, but for those who did the average was less than 5% for the lumbar spine. The lumbar spine is considered the most reactive, so this illustrates the safety that combined therapy offers women with HMB and fibroids.
The lumbar spine is considered the most reactive, so this site is often used as the main focus with BMD studies. As Simon et al show, the lumbar spine mean BMD percent change from baseline for the elagolix with add-back therapy was -1.5% (95% confidence interval [CI], -1.9 to -1.0) in women who received up to 12 months of treatment at month 6 in the extension study. After stopping elagolix with add-back therapy, at 6 months the elagolix with add-back therapy had a Z-score of -0.6% (95% CI, -1.1 to -0.1). This shows a trend toward baseline, or a recovery within a short time from stopping medication.
Continue to: Study strengths and limitations...
Study strengths and limitations
Strengths of this study include its overall design; efficacy endpoints, which were all established a priori; the fact that measurement of menstrual blood loss was done with the objective alkaline hematin method; and the statistical analysis, which is thorough and well presented. This extension study allowed further evaluation of efficacy and safety for elagolix. Although the authors point out that there may be some selection bias in an extension study, the fact that so many women elected to continue into the extended study is a positive reflection of the treatment.
As providers learn of new therapies for management of HMB associated with fibroids, it is important to consider who will benefit the most. In my opinion, any woman with heavy periods associated with fibroids could be a candidate for elagolix with add-back therapy. This treatment is highly effective, well tolerated, and safe. My approach to management includes educating a woman on all potential therapies and this new option of elagolix and add-back therapy is an important one. The decision for an individual woman on how to manage heavy periods associated with fibroids should consider her contraceptive needs, medical issues, and the risk and benefit of individual therapies. ●
Elagolix and hormonal add-back therapy offer a long-term medical option for women with HMB associated with fibroids that is both effective and safe.
ANDREA S. LUKES, MD, MHSc
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA, Nicholson WK, Bradley L, et al. The burden of uterine fibroids for African-American women: results of a national survey. J Women’s Health. 2013;22:807-816.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
Evidence-based management of early pregnancy loss
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.
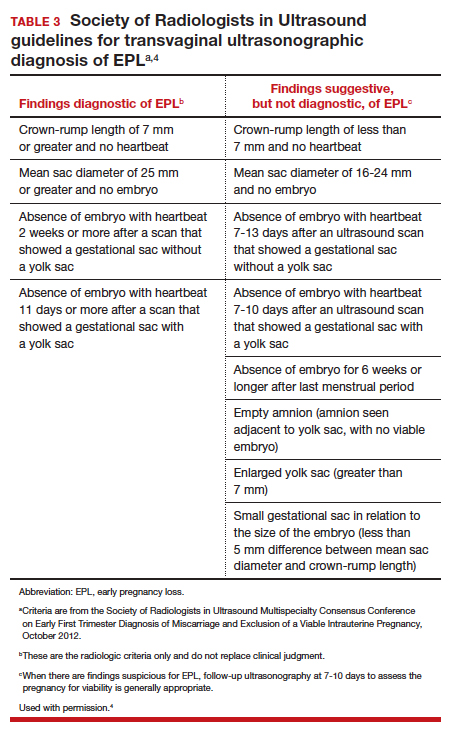
Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.
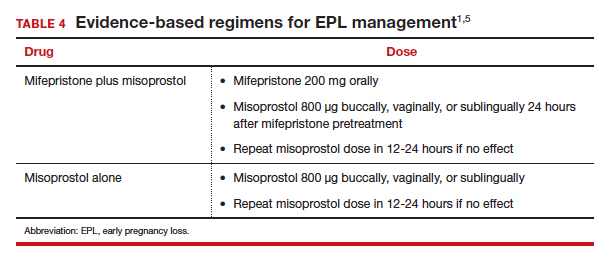
Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
The American College of Obstetricians and Gynecologists (ACOG) defines early pregnancy loss (EPL) as a nonviable, intrauterine pregnancy up to 12 6/7 weeks’ gestation.1 The term EPL has been used interchangeably with miscarriage, spontaneous abortion, and early pregnancy failure; the preferred terms among US women who experience pregnancy loss are EPL and miscarriage.2 EPL is the most common complication of early pregnancy and accounts for up to 15% to 20% of clinically recognized pregnancies.3
The most common cause of EPL is a chromosomal abnormality (TABLE 1). Other common etiologies include structural abnormalities, such as uterine fibroids or polyps. Risk factors for EPL include maternal age, prior pregnancy loss, and various maternal conditions and medication and substance use (TABLE 2).


Definitive diagnosis of EPL often requires more than 1 ultrasonography scan or other examination to determine whether a pregnancy is nonviable versus too early to confirm viability. The consensus guidelines from the Society of Radiologists in Ultrasound provide transvaginal ultrasonographic criteria to diagnose EPL (TABLE 3).4 Two of the diagnostic criteria require only 1 ultrasonography scan while the others require repeat ultrasonography.

Note that a definitive diagnosis may be more important to some patients than others due to differing pregnancy intent and/or desirableness. Patients may choose to take action in terms of medication or uterine aspiration based on suspicion of EPL, or they may wish to end the pregnancy regardless of EPL diagnosis.
Management options for EPL
EPL can be managed expectantly, with medication, or with uterine aspiration. These methods have different risks and benefits, and in most cases all should be made available to women who experience EPL.5-7
Expectant management
Expectant management involves waiting for the body to spontaneously expel the nonviable pregnancy. In the absence of any signs of infection, hemodynamic instability, or other medical instability, it is safe and reasonable to wait a month or more before intervening, according to patient choice. Expectant management is up to 80% effective.8
Medication management
Medication management entails using mifepristone and misoprostol, or misoprostol alone, to cause uterine contractions to expel the pregnancy. A landmark study demonstrated that medication management of EPL with the combination of mifepristone and misoprostol is significantly more effective than misoprostol alone.9 While the mean cost of mifepristone is approximately $90 per dose, its addition is cost-effective given the increased efficacy.10
The evidence-based combination regimen is to provide mifepristone 200 mg orally, followed 24 hours later by misoprostol 800 µg vaginally, for a success rate of 87.8% by 8 days, and 91.2% by 30 days posttreatment. Success rates can be increased further by adding a second dose of misoprostol to take as needed.5
We strongly recommend using the combination regimen if you have access to mifepristone. If you do not have access to mifepristone in your clinical setting, perhaps this indication for use can help facilitate getting it onto your formulary. (See “Ordering mifepristone” below.)
Without access to mifepristone, medication abortion still should be offered after discussing the decreased efficacy with patients. The first-trimester misoprostol-only regimen for EPL is to give misoprostol 800 µg buccally, vaginally, or sublingually, with a second dose if there is no effect (TABLE 4).1,5 For losses after 9 weeks, some data suggest adding additional doses of misoprostol 400 µg every 3 hours until expulsion.11
- There are 2 distributors of mifepristone in the United States. Danco (www.earlyoptionpill.com) distributes the branded Mifeprex and GenBioPro (www.genbiopro.com) distributes generic mifepristone.
- To order mifepristone, 1 health care provider from your clinic or facility must read and sign the distributor’s prescriber agreement and account setup form. These forms and instructions can be found on each distributor’s website. Future orders can be made by calling the distributor directly (Danco: 1-877-432-7596; GenBioPro: 1-855-643-3463).
- The shelf life of mifepristone is 18 months.
- Each patient who receives mifepristone needs to read and sign a patient agreement (available on distributor websites), as required by the US Food and Drug Administration–approved Risk Evaluation and Mitigation Strategy (REMS) program.

Continue to: Uterine aspiration...
Uterine aspiration
Uterine aspiration is the third management option for EPL and is virtually 100% successful. Although aspiration is used when expectant or medication management fails, it is also a first-line option based on patient choice or contraindications to the other 2 management options.
We recommend either manual vacuum aspiration (MVA) or electric vacuum aspiration (EVA); sharp curettage almost never should be used. Uterine aspiration can be performed safely in a clinic, emergency department, or operating room (OR) setting, depending on patient characteristics and desires.12-14 For various reasons, many patients prefer outpatient management. These reasons may include avoiding the costs and delays associated with OR management, wanting more control over who performs the procedure, or avoiding more significant/general anesthesia. MVA in the outpatient setting is the most cost-effective approach to uterine aspiration.15
Choosing a management approach
There are virtually no contraindications for uterine aspiration. Expectant and medication management are contraindicated (and uterine aspiration is recommended) in the setting of bleeding disorders, anticoagulation, suspected intrauterine infection, suspected molar pregnancy, significant cardiopulmonary disease, or any condition for which heavy, unsupervised bleeding might be dangerous.1 Uterine aspiration offers immediate resolution, with a procedure usually lasting 3 to 10 minutes. By contrast, expectant and medication management offer a less predictable time to resolution and, often, a more prolonged period of active pregnancy expulsion.
In the absence of a contraindication, patient choice should determine which management option is used. All 3 options are similarly safe and effective, and the differences that do exist are acceptable to patients as long as they are allowed to access their preferred EPL management method.5,6,16 Patient satisfaction is associated directly with the ability to choose the method of preference.
Managing pain
Pain management should be offered to all women diagnosed with EPL. Those who choose expectant or medication management likely will require only oral nonsteroidal anti-inflammatory drugs (NSAIDs). A minority may require the addition of a small number of narcotic pain pills.17
Women who choose uterine aspiration also should be offered pain management. All patients should be given a paracervical block; other medications can include NSAIDs, an oral benzodiazepine, intravenous (IV) sedation, or even general anesthesia/monitored airway care.17
Patients’ expectations about pain management should be addressed directly during initial counseling. This may help patients decide what type of management and treatment location they might prefer.
Checking blood type: Is it necessary?
The ACOG practice bulletin for EPL states, “administration of Rh D immune globulin should be considered in cases of early pregnancy loss, especially those that are later in the first trimester.”1 A growing body of evidence indicates that Rho(D) immune globulin likely is unnecessary in early pregnancy.
A recent prospective cohort study of 42 women who were at 5 to 12 weeks’ gestation found that the fetal red blood cell concentration was below the calculated threshold for Rh sensitization.18 In light of recent evidence, the National Abortion Federation now recommends foregoing Rh testing and provision of Rh immune globulin at less than 8 weeks’ gestation for uterine aspiration and at less than 10 weeks’ gestation for medication abortion.19
We feel there is sufficient evidence to forego Rh testing in EPL at similar gestational ages, although this is not yet reflected in US societal guidelines. (It is already standard practice in some countries.) Although the risk of Rh alloimmunization is low, the risk of significant consequences in the event of Rh alloimmunization is high. Currently, it also is reasonable to continue giving Rho(D) immune globulin to Rh-negative patients who experience EPL at any gestational age. A lower dose (50 µg) is sufficient for EPL; the standard 300-µg dose also is acceptable.20
We anticipate that society and ACOG guidelines will change in the next few years as the body of evidence increases, and practice should change to reflect new guidance.
Continue to: Prophylactic antibiotics...
Prophylactic antibiotics
The risk of infection with EPL is low overall regardless of the management approach.1 Prophylactic antibiotics are recommended for patients undergoing uterine aspiration but are not necessary in the setting of expectant or medication management. We recommend prophylaxis with 1 dose of oral doxycycline 200 mg or oral azithromycin 500 mg approximately 30 minutes to 1 hour prior to uterine aspiration.21 Alternatives include 1 dose of oral metronidazole 500 mg or, if the patient is unable to take oral medications, IV cefazolin 2 g.
A multisite international randomized controlled trial concluded that antibiotic prophylaxis before uterine aspiration for EPL did not significantly reduce the risk of infection.22 However, there was a significant reduction in pelvic infection with antibiotic administration for the subgroup of women who underwent MVA, which is our recommended approach (along with EVA, and opposed to sharp curettage) for outpatient EPL management.
Follow-up after EPL
In-person follow-up after treatment of EPL is not medically necessary. A repeat ultrasonography 1 to 2 weeks after expectant or medication management can be helpful to confirm completion of the process, and clinicians should focus on presence or absence of a gestational sac to determine if further management is needed.1
Follow-up by telemedicine or phone also is an option and may be preferred in the following situations:
- the patient lives far from the clinic
- travel to the clinic is difficult or expensive
- the patient has child-care issues
- there is a global pandemic necessitating physical distancing.
If the patient’s reported history and symptoms are consistent with a completed process, no further intervention is indicated.
If ongoing EPL is a concern, ask the patient to come in for an evaluation and ultrasonography. If visiting the clinic is still a challenge, following with urine or serum human chorionic gonadotropin (HCG) levels also is acceptable. Experts recommend waiting 4 weeks before expecting a negative urine HCG measurement, although up to 25% of women with a completed EPL will still have a positive test at 4 weeks.23,24
A postprocedure serum HCG is more helpful if a preprocedure HCG level already is known. Numerous studies have evaluated phone follow-up after medication abortion and it is reasonable to translate these practices to follow-up after EPL, recognizing that direct data looking at alternative EPL follow-up are much more limited.23,25-30
The benefit of HCG follow-up at a scheduled time (such as 1 week) is less clear for EPL than for medication abortion, as HCG trends are less predictable in the setting of EPL. However, if the pregnancy has passed, a significant drop in the HCG level would be expected. It is important to take into account the patient’s history and clinical symptoms and consider in-person evaluation with possible ultrasonography if there is concern that the pregnancy tissue has not passed.
Pay attention to mental health
It is critical to assess the patient’s mental and emotional health. This should be done both at the time of EPL diagnosis and management and again at follow-up. Both patients and their partners can struggle after experiencing EPL, and they may suffer from prolonged posttraumatic stress.31
Often, EPL occurs before people have shared the news about their pregnancy. This can amplify the sense of isolation and sadness many women report. Equally critical is recognizing that not all women who experience EPL grieve, and clinicians should normalize patient experiences and feelings. Provider language is important. We recommend use of these questions and phrases:
- I’m so sorry for your loss.
- How are you feeling?
- How have you been doing since I saw you last?
- Your friends/family/partner may be grieving differently or at a different pace than you—this is normal.
- Just because the EPL process is complete doesn’t necessarily mean your processing and/or grieving is over.
- Whatever you’re feeling is okay.
Continue to: Address desire for future pregnancy or contraception...
Address desire for future pregnancy or contraception
No additional workup is necessary after EPL unless a patient is experiencing recurrent pregnancy loss. We do recommend discussing plans for future conception. If a patient wants to conceive again as soon as possible, she can start trying when she feels emotionally ready (even before her next menstrual period). One study found that the ability to conceive and those pregnancy outcomes were the same when patients were randomly assigned to start trying immediately versus waiting 3 months after EPL.32
Alternatively, a patient may want to prevent pregnancy after EPL, and this information should be explicitly elicited and addressed with comprehensive contraception counseling as needed. All forms of contraception can be initiated immediately on successful management of EPL. All contraceptive methods, including an intrauterine device, can be initiated immediately following uterine aspiration.1,33,34
Patients should be reminded that if they delay contraception initiation by more than 7 days, they are potentially at risk for pregnancy.35 Most importantly, clinicians should not make assumptions about future pregnancy desires and should ask open-ended questions to provide appropriate patient counseling.
Finally, patients may feel additional anxiety in a subsequent pregnancy. It is helpful to acknowledge this and perhaps even offer earlier and more frequent visits in early pregnancy to help reduce anxiety. EPL is commonly experienced, and unfortunately it is sometimes poorly addressed by clinicians.
We hope this guidance will help you provide excellent, evidence-based, and sensitive care that will not only manage your patient’s EPL but also make the experience as positive as possible. ●
- Early pregnancy loss (EPL) is common, occurring in up to 15% to 20% of clinically recognized pregnancies.
- EPL can be managed expectantly, with medication, or by uterine aspiration.
- There are virtually no contraindications to uterine aspiration.
- Contraindications to expectant or medication management include any situation in which heavy, unsupervised bleeding might be dangerous.
- In the absence of contraindications, patient preference should dictate the management approach.
- Mifepristone-misoprostol is more effective than misoprostol alone.
- Manual uterine aspiration in the outpatient setting is the most cost-effective approach to uterine evacuation.
- Rh testing is not necessary at less than 8 weeks’ gestation if choosing uterine aspiration, or at less than 10 weeks’ gestation if choosing expectant or medication management.
- Antibiotic prophylaxis is indicated for uterine aspiration, but not for expectant or medication management.
- Ultrasonography follow-up should focus on presence or absence of gestational sac.
- There are viable telemedicine and phone follow-up options that do not require repeat ultrasonography or in-person evaluation.
- There is no need to delay future conception once EPL management is confirmed to be complete.
- It is okay to initiate any contraceptive method immediately on completed management of EPL.
- Feelings toward EPL can be complex and varied; it is helpful to normalize your patients’ experiences, ask open-ended questions, and provide support as needed.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 200: early pregnancy loss. Obstet Gynecol. 2018;132:e197-e207.
- Clement EG, Horvath S, McAllister A, et al. The language of first-trimester nonviable pregnancy: patient-reported preferences and clarity. Obstet Gynecol. 2019;133:149-154.
- Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60:1-21.
- Doubilet PM, Benson CB, Bourne T, et al; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Uterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369:1443-1451.
- Zhang J, Gilles JM, Barnhart K, et al. A comparison of medical management with misoprostol and surgical management for early pregnancy failure. N Engl J Med. 2005;353:761-769.
- Nanda K, Peloggia A, Grimes D, et al. Expectant care versus surgical treatment for miscarriage. Cochrane Database Syst Rev. 2006(2):CD003518.
- Neilson JP, Hickey M, Vazquez J. Medical treatment for early fetal death (less than 24 weeks). Cochrane Database Syst Rev. 2006(3):CD002253.
- Luise C, Jermy K, May C, et al. Outcome of expectant management of spontaneous first trimester miscarriage: observational study. BMJ. 2002;324:873-875.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170.
- Nagendra D, Koelper N, Loza-Avalos SE, et al. Cost-effectiveness of mifepristone pretreatment for the medical management of nonviable early pregnancy: secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e201594.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed. Geneva, Switzerland: World Health Organization; 2012.
- Wiebe E, Janssen P. Management of spontaneous abortion in family practices and hospitals. Fam Med. 1998;30:293-296.
- Harris LH, Dalton VK, Johnson TR. Surgical management of early pregnancy failure: history, politics, and safe, cost-effective care. Am J Obstet Gynecol. 2007;196:445.e1-e5.
- Dalton VK, Harris L, Weisman CS, et al. Patient preferences, satisfaction, and resource use in office evacuation of early pregnancy failure. Obstet Gynecol. 2006;108:103-110.
- Rausch M, Lorch S, Chung K, et al. A cost-effectiveness analysis of surgical versus medical management of early pregnancy loss. Fertil Steril. 2012;97:355-360.
- Trinder J, Brocklehurst P, Porter R, et al. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (Miscarriage Treatment [MIST] trial). BMJ. 2006;332:1235-1240.
- Calvache JA, Delgado-Noguera MF, Lesaffre E, et al. Anaesthesia for evacuation of incomplete miscarriage. Cochrane Database System Rev. 2012(4):CD008681.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- National Abortion Federation. 2020 clinical policy guidelines for abortion care. https://www.prochoice.org/education-and-advocacy/cpg. Accessed June 9, 2020.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet Gynecol. 2017;130:e59-e70.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 104: antibiotic prophylaxis for gynecologic procedures. Obstet Gynecol. 2009;113:1180-1189.
- Lissauer D, Wilson A, Hewitt CA, et al. A randomized trial of prophylactic antibiotics for miscarriage surgery. N Engl J Med. 2019;380:1012-1021.
- Perriera L, Reeves MF, Chen BA, et al. Feasibility of telephone follow-up after medical abortion. Contraception. 2010:81:143-149.
- Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
- Chen MJ, Rounds KM, Creinin MD, et al. Comparing office and telephone follow-up after medical abortion. Contraception. 2016;94:122-126.
- Clark W, Bracken H, Tanenhaus J, et al. Alternatives to a routine follow-up visit for early medical abortion. Obstet Gynecol. 2010;115(2 pt 1):264-272.
- Jackson AV, Dayananda I, Fortin JM, et al. Can women accurately assess the outcome of medical abortion based on symptoms alone? Contraception. 2012;85:192-197.
- Raymond EG, Tan YL, Grant M, et al. Self-assessment of medical abortion outcome using symptoms and home pregnancy testing. Contraception. 2018;97:324-328.
- Raymond EG, Shochet T, Bracken H. Low-sensitivity urine pregnancy testing to assess medical abortion outcome: a systematic review. Contraception. 2018;98:30-35.
- Raymond EG, Grossman D, Mark A, et al. Commentary: no-test medication abortion: a sample protocol for increasing access during a pandemic and beyond. Contraception. 2020;101:361-366.
- Farren J, Jalmbrant M, Ameye L, et al. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
- Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204-212. DOI: 0.1097/AOG.0000000000001159.
- American College of Obstetricians and Gynecologists. Committee opinion No. 642: increasing access to contraceptive implants and intrauterine devices to reduce unintended pregnancy. Obstet Gynecol. 2015;126:e44-e48.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. US medical eligibility criteria (US MEC) for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
BMD preserved with investigational drug for uterine fibroid bleeding
Combination therapy with relugolix, an investigational oral gonadotropin-releasing hormone antagonist, estradiol, and norethindrone acetate effectively preserved bone mineral density (BMD) in two replicate phase 3 studies enrolling women with heavy menstrual bleeding associated with uterine fibroids.
The BMD findings, released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, build upon previously reported positive primary endpoint data from the LIBERTY 1 and LIBERTY 2 studies. ACOG canceled the meeting and released abstracts for press coverage.
The developer of the drug, Myovant Sciences, plans to submit a new drug application to the Food and Drug Administration for approval of the single-tablet combination therapy for women with uterine fibroids, according to Albert Liao, the company’s director of corporate communications.
The two multinational LIBERTY studies randomized women who had a monthly menstrual blood loss volume of at least 80 mL in two consecutive cycles (or 160 mL in one cycle) in a 1:1:1 ratio to one of three groups: relugolix combination therapy for 24 weeks (once-daily relugolix 40 mg plus estradiol 1.0 mg plus norethindrone acetate 0.5 mg); relugolix alone (40 mg once daily) for 12 weeks followed by relugolix combination therapy for 12 weeks; or placebo for 24 weeks.
In October 2019 at the American Society for Reproductive Medicine Scientific Congress, investigators reported that 73% of women receiving combination therapy in the LIBERTY 1 trial achieved a menstrual blood loss of less than 80 mL and a 50% or greater reduction from baseline over the last 35 days of treatment, compared with 19% in the placebo group. The mean percent reduction in menstrual blood loss from baseline at week 24 was 84% for combination therapy and 23% for placebo.
Earlier in 2019, Myovant Sciences announced that, in the LIBERTY 2 study, 71% of women receiving combination therapy met the primary endpoints, compared with 15% in the placebo group. The reduction in menstrual blood loss in this study’s combination therapy arm was also 84%, according to a company press release from June 2019.
Each of the two clinical trials enrolled upwards of 380 women.
The new abstract released for press coverage by ACOG and published in Obstetrics & Gynecology reports that women receiving relugolix combination therapy in the LIBERTY 1 and LIBERTY 2 studies had a mean change in lumbar spine BMD of –0.36% and –0.13%, respectively, from baseline to 24 weeks. Percent change in lumbar spine BMD in the delayed combination therapy groups (12 initial weeks of relugolix monotherapy) was –1.82% and –2.12%. In the placebo groups, the change was 0.05% and 0.32%.
Michael R. McClung, MD, who is the lead author of the abstract and was scheduled to present the findings at the ACOG meeting, said in an interview that the slight decreases in lumbar spine BMD with combination therapy were noted largely at week 12 and are “clinically insignificant in my opinion.” BMD by dual-energy x-ray absorptiometry was assessed at weeks 12 and 24.
“There was no further increase [after week 12] and [in some patients] there was even a return to baseline,” said Dr. McClung, of the Oregon Osteoporosis Center in Portland.
The safety and efficacy of longer-term treatment with relugolix combination therapy has been investigated thus far through an open-label extension study that brought the treatment period to 52 weeks. The 1-year data has been positive and will be presented or published soon, said Mr. Liao. In addition, a “second, 52-week randomized withdrawal study has been designed to provide 2-year safety and efficacy data … and to evaluate the need for maintenance therapy.”
It’s important, Dr. McClung said, “for clinicians to be confident that BMD loss is prevented or minimized with longer-term relugolix combination therapy since treatment for uterine fibroids is not a short-term proposition. Given the stability of BMD values between weeks 12 and 24 in the LIBERTY studies, I’d anticipate that we will see stable values with longer-term treatment.”
Dr. McClung disclosed that he has served as a consultant/advisory board member and speaker for Amgen and a consultant/advisory board member for Myovant. Several of his coauthors disclosed employment and ownerships interests in Myovant.
SOURCE: McClung MR et al. Obstet Gynecol. 2020 May. doi: 10.1097/01.AOG.0000662944.34860.b4.
Combination therapy with relugolix, an investigational oral gonadotropin-releasing hormone antagonist, estradiol, and norethindrone acetate effectively preserved bone mineral density (BMD) in two replicate phase 3 studies enrolling women with heavy menstrual bleeding associated with uterine fibroids.
The BMD findings, released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, build upon previously reported positive primary endpoint data from the LIBERTY 1 and LIBERTY 2 studies. ACOG canceled the meeting and released abstracts for press coverage.
The developer of the drug, Myovant Sciences, plans to submit a new drug application to the Food and Drug Administration for approval of the single-tablet combination therapy for women with uterine fibroids, according to Albert Liao, the company’s director of corporate communications.
The two multinational LIBERTY studies randomized women who had a monthly menstrual blood loss volume of at least 80 mL in two consecutive cycles (or 160 mL in one cycle) in a 1:1:1 ratio to one of three groups: relugolix combination therapy for 24 weeks (once-daily relugolix 40 mg plus estradiol 1.0 mg plus norethindrone acetate 0.5 mg); relugolix alone (40 mg once daily) for 12 weeks followed by relugolix combination therapy for 12 weeks; or placebo for 24 weeks.
In October 2019 at the American Society for Reproductive Medicine Scientific Congress, investigators reported that 73% of women receiving combination therapy in the LIBERTY 1 trial achieved a menstrual blood loss of less than 80 mL and a 50% or greater reduction from baseline over the last 35 days of treatment, compared with 19% in the placebo group. The mean percent reduction in menstrual blood loss from baseline at week 24 was 84% for combination therapy and 23% for placebo.
Earlier in 2019, Myovant Sciences announced that, in the LIBERTY 2 study, 71% of women receiving combination therapy met the primary endpoints, compared with 15% in the placebo group. The reduction in menstrual blood loss in this study’s combination therapy arm was also 84%, according to a company press release from June 2019.
Each of the two clinical trials enrolled upwards of 380 women.
The new abstract released for press coverage by ACOG and published in Obstetrics & Gynecology reports that women receiving relugolix combination therapy in the LIBERTY 1 and LIBERTY 2 studies had a mean change in lumbar spine BMD of –0.36% and –0.13%, respectively, from baseline to 24 weeks. Percent change in lumbar spine BMD in the delayed combination therapy groups (12 initial weeks of relugolix monotherapy) was –1.82% and –2.12%. In the placebo groups, the change was 0.05% and 0.32%.
Michael R. McClung, MD, who is the lead author of the abstract and was scheduled to present the findings at the ACOG meeting, said in an interview that the slight decreases in lumbar spine BMD with combination therapy were noted largely at week 12 and are “clinically insignificant in my opinion.” BMD by dual-energy x-ray absorptiometry was assessed at weeks 12 and 24.
“There was no further increase [after week 12] and [in some patients] there was even a return to baseline,” said Dr. McClung, of the Oregon Osteoporosis Center in Portland.
The safety and efficacy of longer-term treatment with relugolix combination therapy has been investigated thus far through an open-label extension study that brought the treatment period to 52 weeks. The 1-year data has been positive and will be presented or published soon, said Mr. Liao. In addition, a “second, 52-week randomized withdrawal study has been designed to provide 2-year safety and efficacy data … and to evaluate the need for maintenance therapy.”
It’s important, Dr. McClung said, “for clinicians to be confident that BMD loss is prevented or minimized with longer-term relugolix combination therapy since treatment for uterine fibroids is not a short-term proposition. Given the stability of BMD values between weeks 12 and 24 in the LIBERTY studies, I’d anticipate that we will see stable values with longer-term treatment.”
Dr. McClung disclosed that he has served as a consultant/advisory board member and speaker for Amgen and a consultant/advisory board member for Myovant. Several of his coauthors disclosed employment and ownerships interests in Myovant.
SOURCE: McClung MR et al. Obstet Gynecol. 2020 May. doi: 10.1097/01.AOG.0000662944.34860.b4.
Combination therapy with relugolix, an investigational oral gonadotropin-releasing hormone antagonist, estradiol, and norethindrone acetate effectively preserved bone mineral density (BMD) in two replicate phase 3 studies enrolling women with heavy menstrual bleeding associated with uterine fibroids.
The BMD findings, released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists, build upon previously reported positive primary endpoint data from the LIBERTY 1 and LIBERTY 2 studies. ACOG canceled the meeting and released abstracts for press coverage.
The developer of the drug, Myovant Sciences, plans to submit a new drug application to the Food and Drug Administration for approval of the single-tablet combination therapy for women with uterine fibroids, according to Albert Liao, the company’s director of corporate communications.
The two multinational LIBERTY studies randomized women who had a monthly menstrual blood loss volume of at least 80 mL in two consecutive cycles (or 160 mL in one cycle) in a 1:1:1 ratio to one of three groups: relugolix combination therapy for 24 weeks (once-daily relugolix 40 mg plus estradiol 1.0 mg plus norethindrone acetate 0.5 mg); relugolix alone (40 mg once daily) for 12 weeks followed by relugolix combination therapy for 12 weeks; or placebo for 24 weeks.
In October 2019 at the American Society for Reproductive Medicine Scientific Congress, investigators reported that 73% of women receiving combination therapy in the LIBERTY 1 trial achieved a menstrual blood loss of less than 80 mL and a 50% or greater reduction from baseline over the last 35 days of treatment, compared with 19% in the placebo group. The mean percent reduction in menstrual blood loss from baseline at week 24 was 84% for combination therapy and 23% for placebo.
Earlier in 2019, Myovant Sciences announced that, in the LIBERTY 2 study, 71% of women receiving combination therapy met the primary endpoints, compared with 15% in the placebo group. The reduction in menstrual blood loss in this study’s combination therapy arm was also 84%, according to a company press release from June 2019.
Each of the two clinical trials enrolled upwards of 380 women.
The new abstract released for press coverage by ACOG and published in Obstetrics & Gynecology reports that women receiving relugolix combination therapy in the LIBERTY 1 and LIBERTY 2 studies had a mean change in lumbar spine BMD of –0.36% and –0.13%, respectively, from baseline to 24 weeks. Percent change in lumbar spine BMD in the delayed combination therapy groups (12 initial weeks of relugolix monotherapy) was –1.82% and –2.12%. In the placebo groups, the change was 0.05% and 0.32%.
Michael R. McClung, MD, who is the lead author of the abstract and was scheduled to present the findings at the ACOG meeting, said in an interview that the slight decreases in lumbar spine BMD with combination therapy were noted largely at week 12 and are “clinically insignificant in my opinion.” BMD by dual-energy x-ray absorptiometry was assessed at weeks 12 and 24.
“There was no further increase [after week 12] and [in some patients] there was even a return to baseline,” said Dr. McClung, of the Oregon Osteoporosis Center in Portland.
The safety and efficacy of longer-term treatment with relugolix combination therapy has been investigated thus far through an open-label extension study that brought the treatment period to 52 weeks. The 1-year data has been positive and will be presented or published soon, said Mr. Liao. In addition, a “second, 52-week randomized withdrawal study has been designed to provide 2-year safety and efficacy data … and to evaluate the need for maintenance therapy.”
It’s important, Dr. McClung said, “for clinicians to be confident that BMD loss is prevented or minimized with longer-term relugolix combination therapy since treatment for uterine fibroids is not a short-term proposition. Given the stability of BMD values between weeks 12 and 24 in the LIBERTY studies, I’d anticipate that we will see stable values with longer-term treatment.”
Dr. McClung disclosed that he has served as a consultant/advisory board member and speaker for Amgen and a consultant/advisory board member for Myovant. Several of his coauthors disclosed employment and ownerships interests in Myovant.
SOURCE: McClung MR et al. Obstet Gynecol. 2020 May. doi: 10.1097/01.AOG.0000662944.34860.b4.
FROM ACOG 2020
In the management of cesarean scar defects, is there a superior surgical method for treatment?
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
He Y, Zhong J, Zhou W, et al. Four surgical strategies for the treatment of cesarean scar defect: a systematic review and network meta-analysis. J Minim Invasive Gynecol. 2020;27:593-602.
EXPERT COMMENTARY
With the increase in cesarean deliveries performed over the decades, the sequelae of the surgery are now arising. Cesarean scar defects (CSDs) are a complication seen when the endometrium and muscular layers from a prior uterine scar are damaged. This damage in the uterine scar can lead to abnormal uterine bleeding and the implantation of an ectopic pregnancy, which can be life-threatening. Ultrasonography can be used to diagnose this defect, which can appear as a hypoechoic space filled with postmenstrual blood, representing a myometrial tear at the wound site.1 There are several risk factors for CSD, including multiple cesarean deliveries, cesarean delivery during advanced stages of labor, and uterine incisions near the cervix. Elevated body mass index as well as gestational diabetes also have been found to be associated with inadequate healing of the prior cesarean incision.2 Studies have shown that both single- and double-layer closure of the hysterotomy during a cesarean delivery have similar incidences of CSDs.3,4 There are multiple ways to correct a CSD; however, there is no gold standard that has been identified in the literature.
Details about the study
The study by He and colleagues is a meta-analysis aimed at comparing the treatment of CSDs via laparoscopy, hysteroscopy, combined hysteroscopy and laparoscopy, and vaginal repair. The primary outcome measures were reduction in abnormal uterine bleeding and scar defect depth. A total of 10 studies (n = 858) were reviewed: 4 randomized controlled trials (RCTs) and 6 observational studies. The studies analyzed varied in terms of which techniques were compared.
Patients who underwent uterine scar resection by combined laparoscopy and hysteroscopy had a shorter duration of abnormal uterine bleeding when compared with hysteroscopy alone (standardized mean difference [SMD] = 1.36; 95% confidence interval [CI], 0.37−2.36; P = .007) and vaginal repair (SMD = 1.58; 95% CI, 0.97−2.19; P<.0001). Combined laparoscopic and hysteroscopic technique also was found to reduce the diverticulum depth more than in vaginal repair (SMD = 1.57; 95% CI, 0.54−2.61; P = .003).
Continue to: Study strengths and weaknesses...
Study strengths and weaknesses
This is the first meta-analysis to compare the different surgical techniques to correct a CSD. The authors were able to compare many of the characteristics regarding the routes of repair, including hysteroscopy, laparoscopy, and vaginal. The authors were able to analyze the combined laparoscopic and hysteroscopic approach, which facilitates evaluation of the location and satisfaction of defect repair during the procedure.
Some weaknesses of this study include the limited amount of RCTs available for review. All studies were also from China, where the rate of CSDs is higher. Therefore, the results may not be generalizable to all populations. Given that the included studies were done at different sites, it is difficult to determine surgical expertise and surgical technique. Additionally, the studies analyzed varied by which techniques were compared; therefore, indirect analyses were conducted to compare certain techniques. There was limited follow-up for these patients (anywhere from 3 to 6 months), so long-term data and future pregnancy data are needed to determine the efficacy of these procedures.
CSDs are a rising concern due to the increasing cesarean delivery rate. It is critical to be able to identify as well as correct these defects. This is the first systematic review to compare 4 techniques of managing CSDs. Based on this article, there may be some additional benefit from combined hysteroscopic and laparoscopic repair of these defects in terms of decreasing bleeding and decreasing the scar defect depth. However, how these results translate into long-term outcomes for patients and their future pregnancies is still unknown, and further research must be done.
STEPHANIE DELGADO, MD, AND XIAOMING GUAN, MD, PHD
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
- Woźniak A, Pyra K, Tinto HR, et al. Ultrasonographic criteria of cesarean scar defect evaluation. J Ultrason. 2018;18: 162-165.
- Antila-Långsjö RM, Mäenpää JU, Huhtala HS, et al. Cesarean scar defect: a prospective study on risk factors. Am J Obstet Gynecol. 2018:219:458e1-e8.
- Di Spiezio Sardo A, Saccone G, McCurdy R, et al. Risk of cesarean scar defect following single- vs double-layer uterine closure: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. 2017;50:578-583.
- Roberge S, Demers S, Berghella V, et al. Impact of single- vs double-layer closure on adverse outcomes and uterine scar defect: a systematic review and meta-analysis. Am J Obstet Gynecol. 2014;211:453-460.
The role of hysteroscopy in diagnosing endometrial cancer
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).
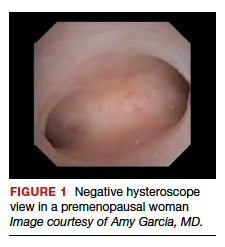
Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1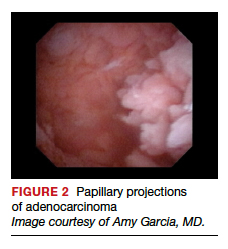
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4
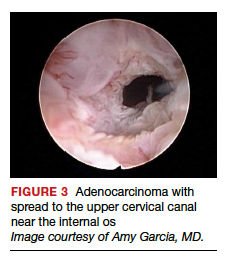
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5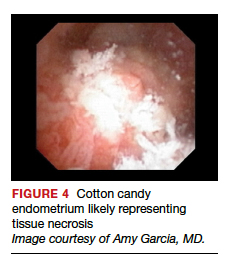
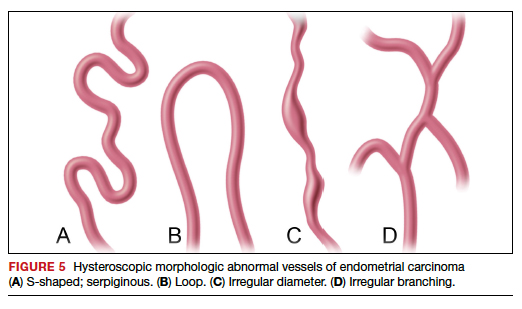
Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).
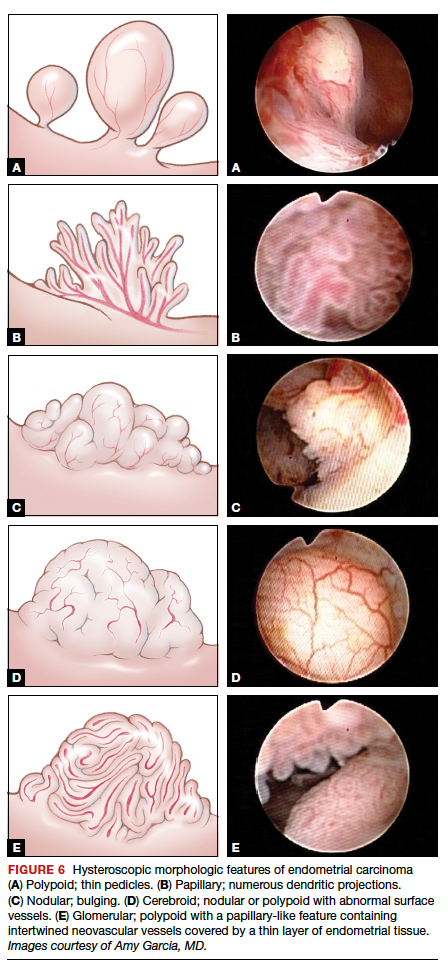
TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.
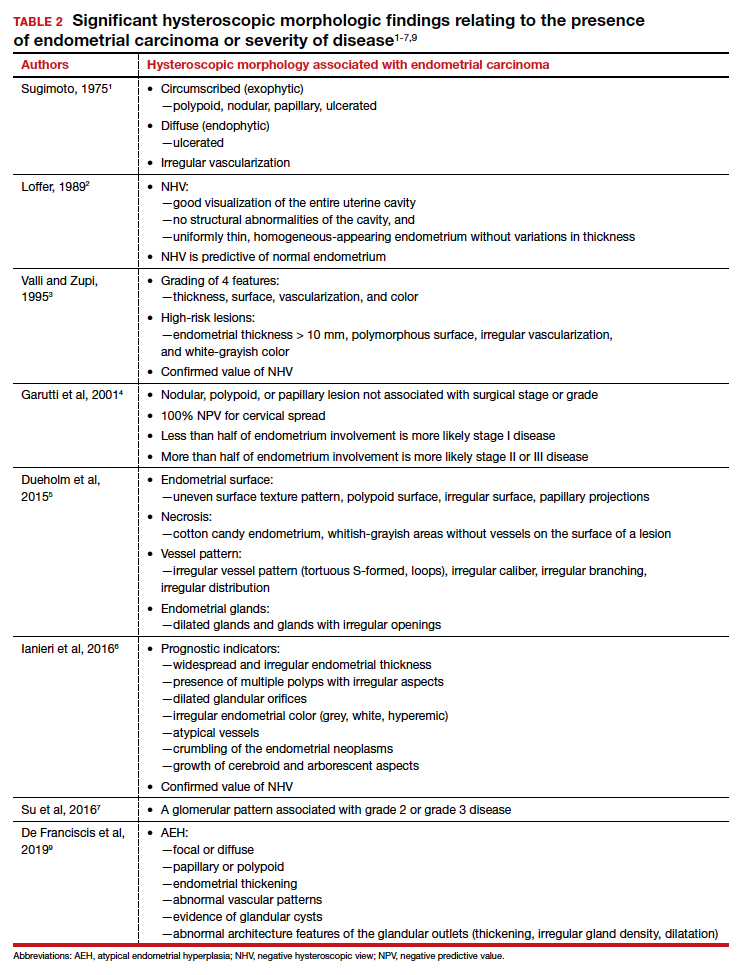
Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
For more than 45 years, gynecologists have used hysteroscopy to diagnose endometrial carcinoma and to associate morphologic descriptive terms with visual findings.1 Today, considerably more clinical evidence supports visual pattern recognition to assess the risk for and presence of endometrial carcinoma, improving observer-dependent biopsy of the most suspect lesions (VIDEO 1).
In this article, I discuss the clinical evolution of hysteroscopic pattern recognition of endometrial disease and review the visual findings that correlate with the likelihood of endometrial carcinoma. In addition, I have provided 9 short videos that show hysteroscopic views of various endometrial pathologies in the online version of this article at https://www.mdedge.com/obgyn.
Video 1. Endometrial carcinoma and visually directed biopsy

The negative hysteroscopic view defined
In 1989, Dr. Frank Loffer confirmed the diagnostic superiority of visually directed biopsy. He demonstrated the advantages of using hysteroscopy and directed biopsy in the evaluation of abnormal uterine bleeding (AUB) to obtain a more accurate diagnosis compared with dilation and curettage (D&C) alone (sensitivity, 98% vs 65%, respectively).2
Also derived from this work is the clinical application of the “negative hysteroscopic view” (NHV). Loffer used the following criteria to define the NHV: good visualization of the entire uterine cavity, no structural abnormalities of the cavity, and a uniformly thin, homogeneous-appearing endometrium without variations in thickness (TABLE 1). The last criterion can be expected to occur only in the early proliferative phase or in postmenopausal women.
Use of hysteroscopy therefore can predict accurately the absence of intrauterine and endometrial pathology when visual findings are negative and tissue sampling is not warranted (FIGURE 1, VIDEO 2).

Video 2. Negative hysteroscopic view

Efforts in hysteroscopic classification of endometrial carcinoma
Lesion morphologic characteristics. Sugimoto was among the first to describe the hysteroscopic identification of visual morphologic features that are most likely to be associated with endometrial carcinoma.1 Patients with AUB were evaluated with hysteroscopy as first-line management to describe lesion morphology and confirm biopsy with histopathology. Sugimoto classified endometrial carcinoma as circumscribed or exophytic with distinct forms, such as polypoid, nodular, papillary, and ulcerated (FIGURE 2). Diffuse or endophytic carcinoma is defined by an ulcerated type of lesion that indicates necrosis; this is most likely to represent an undifferentiated tumor. Sugimoto also described abnormal vascularity that often is associated with carcinoma.1
Endometrial features. Valli and Zupi created a nomenclature and classification for hysteroscopic endometrial lesions by prospectively grading 4 features: thickness, surface, vascularization, and color.3 Features were scored based on the degree of abnormality and could be considered to be of low or high risk for the presence of carcinoma. High-risk hysteroscopic features included endometrial thickness greater than 10 mm, polymorphous surface, irregular vascularization, and white-grayish color. The sensitivity for accurately diagnosing endometrial lesions was 86.9% for mild lesions and 96% for severe lesions.3 Also, these investigators confirmed the clinical value of the NHV and associated overall risk of precancer or cancer of the endometrium.
Continue to: Amount of endometrial involvement...
Amount of endometrial involvement. A few years later, Garuti and colleagues retrospectively related the hysteroscopic tumor features of known endometrial adenocarcinoma to stage, grade, and overall survival.4 In this system, they focused on classification of tumor morphology as nodular (bulging), polypoid (thin pedicles), or papillary (numerous dendritic projections), as well as whether the amount of abnormal tissue present was less than or more than half of the endometrium and if the lesion involved the cervix.
Several important findings associated with this system may improve visual diagnosis. First, hysteroscopic evaluation had a 100% negative predictive value for the cervical spread of disease (FIGURE 3, VIDEO 3). Second, the hysteroscopic morphologic tumor type did not relate to surgical stage or pathologic grade. Third, when less than half of the endometrium was involved, stage I disease was found (97%, 33 of 34). Last, when more than half of the endometrium was involved, advanced disease beyond stage I was found (9 of 26, 6 of whom had poorly differentiated disease).4

Video 3. Cervical spread of adenocarcinoma and visually directed biopsy

Structured pattern analysis. Recently, Dueholm and co-investigators published a prospective evaluation of women with postmenopausal bleeding and an endometrial thickness of 5 mm or greater.5 They used a structured system of visual pattern analysis during hysteroscopy that they termed the hysteroscopic cancer (HYCA) scoring system. The HYCA scoring system is based on surface outline (uneven, polypoid, and papillary projections), necrosis (cotton candy endometrium [FIGURE 4], whitish-grayish areas without vessels on the surface), and vessel pattern (tortuous S-shaped, loops, irregular caliber, irregular branching, and irregular distribution [FIGURE 5]). Structured pattern analysis predicted cancer with higher accuracy than subjective evaluation.5

Morphologic variables as indicators. In 2016, Ianieri and colleagues published a retrospective study on a risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma via hysteroscopy.6 They created a statistical risk model for development of the scoring system. A number of morphologic variables were prognostic indicators of atypical endometrial hyperplasia (AEH) and adenocarcinoma. These included widespread and irregular endometrial thickness, presence of multiple polyps with irregular aspects, dilated glandular orifices, irregular endometrial color (grey, white, or hyperemic), atypical vessels, crumbling of the endometrial neoplasms, and growth of cerebroid and arborescent aspects (VIDEO 4).
Video 4. Endometrial adenocarcinoma

The scoring system for endometrial adenocarcinoma correctly classified 42 of 44 cancers (sensitivity, 95.4%; specificity, 98.2%), and AEH had a sensitivity of 63.3% and a specificity of 90.4%.6 These investigators also showed a high negative predictive value of 99.5% for endometrial adenocarcinoma associated with a negative view at hysteroscopy. Similar to the Dueholm data, Ianieri and colleagues’ morphologic pattern analysis predicted cancer with high accuracy.
Glomerular pattern association. Su and colleagues also showed that pattern recognition could aid in the accurate hysteroscopic diagnosis of endometrial adenocarcinoma.7 They used the hysteroscopic presence of a glomerular pattern to predict the association with endometrial adenocarcinoma. A glomerular pattern was described as polypoid endometrium with a papillary-like feature, containing an abnormal neovascularization feature with “intertwined neovascular vessels covered by a thin layer of endometrial tissue” (FIGURE 6). The presence of a glomerular pattern indicated grade 2 or grade 3 disease in 25 of 26 women (96%; sensitivity, 84.6%, specificity, 81.8%)7 (see video 4).

TABLE 2 summarizes significant morphologic findings relating to the presences of endometrial carcinoma.

Continue to: Atypical endometrial hyperplasia: A difficult diagnosis...
Atypical endometrial hyperplasia: A difficult diagnosis
The most common type of endometrial cancer is endometrioid adenocarcinoma (type 1 endometrial carcinoma), and it accounts for approximately 75% to 80% of endometrial cancer diagnoses.8 Risk factors include prolonged unopposed estrogen exposure, obesity, diabetes, and age. Type 1 endometrial carcinoma follows a progressive continuum of histopathologic change: from endometrial hyperplasia without atypia to endometrial hyperplasia with atypia (AEH) to well-differentiated endometrial cancer. Therefore, it is possible for endometrial carcinoma to be present simultaneously with AEH. The reported prevalence of concurrent endometrial carcinoma among patients with AEH on biopsy is between 17% and 52%.8 Thus, the clinical consideration is for hysterectomy, especially in the postmenopausal patient with a diagnosis of AEH.
Hysteroscopic diagnosis of AEH, however, is more difficult than identification of endometrial carcinoma because a range of morphologic characteristics exist that resemble normal endometrium as well as more progressive disease (VIDEO 5). De Franciscis and colleagues based a hysteroscopic diagnosis of hyperplasia on one or more of the following findings: focal or diffuse, papillary or polypoid, endometrial thickening; abnormal vascular patterns; evidence of glandular cysts; and abnormal architecture features of the glandular outlets (thickening, irregular gland density, or dilatation)9 (VIDEO 6).
Video 5. Endometrial polyp and atypical hyperplasia

Additional studies, including that from Ianieri and colleagues, also have determined that AEH is difficult to discern visually from normal endometrium and other endometrial pathologies.6 In another investigation, Lasmar and coauthors reported a retrospective analysis of 4,054 hysteroscopic procedures with directed biopsies evaluating for concordance between the hysteroscopic view and histopathology.10 Agreement was 56.3% for AEH versus 94% for endometrial carcinoma. Among those with a histologic diagnosis of AEH, in 35.4% benign disease was suspected; in 2.1%, endometrial carcinoma was suspected; and in 6%, normal findings were presumed.10
Video 6. Nodular, polypoid atypical hyperplasia

Because of the similarities in morphologic features between AEH and endometrial carcinoma, tissue biopsy under direct visualization is warranted to assure sampling of the most significantly abnormal tissue and to confirm visual interpretation of findings.
Techniques for hysteroscopic-directed biopsy
Using a visual assessment of endometrial abnormalities allows the surgeon to examine the entire uterine cavity and to biopsy the most suspicious and concerning lesions. The directed biopsy technique can involve a simple grasping maneuver: With the jaws of a small grasper open, push slightly forward to accumulate tissue within the jaw, close the jaw, and remove the tissue carefully through the cervix (VIDEO 7). The size of the sample may be limited, and multiple samples may be needed, depending on the quantity of the tissue retrieved.
Video 7. Visually directed endometrial biopsy

Another technique involves first creating a plane of tissue to be removed with scissors and subsequently grasping and removing the tissue (see video 1 and video 3). This particular technique will yield more tissue with one pass of the hysteroscope into the cavity. Careful removal of tissue through the cervix is facilitated by withdrawing the sample in the grasper and the hysteroscope together at the same time, without pulling the sample through the operative channel of the hysteroscope. Also, by turning off the inflow port, the stream of saline does not wash the sample off the grasper at hysteroscope removal from the cervix.
Blind biopsy. If visual inspection reveals a diffuse process within the uterine cavity such that no normal endometrium is noted and the abnormality is of equal degree throughout the endometrial surface, a decision can be made to replace directed biopsy with a blind biopsy. In this scenario, the blind biopsy is certain to sample the representative disease process and not potentially miss significant lesions (see video 4 and video 6). Otherwise, the hysteroscope-directed biopsy would be preferable.
Continue to: Potential for intraperitoneal dissemination of endometrial cancer...
Potential for intraperitoneal dissemination of endometrial cancer
There is some concern about intraperitoneal dissemination of endometrial carcinoma at the time of hysteroscopy and effect on disease prognosis. Chang and colleagues conducted a large meta-analysis and found that hysteroscopy performed in the presence of type 1 endometrial carcinoma statistically significantly increased the likelihood of positive intraperitoneal cytology.11 In the included studies that reported survival rates (6 of 19), positive cytology did not alter the clinical outcome. The investigators recommended that hysteroscopy not be avoided for this reason, as it helps in the diagnosis of endometrial carcinoma, especially in the early stages of disease.11
In a recent retrospective analysis, Namazov and colleagues included only stage I endometrial carcinoma (to exclude the adverse effect of advanced stage on survival) and evaluated the assumed isolated effect of hysteroscopy on survival.12 They compared women in whom stage I endometrial carcinoma was diagnosed: 355 by hysteroscopy and 969 by a nonhysteroscopy method (D&C or office endometrial biopsy). Tumors were classified and grouped as low grade (endometrioid grade 1-2 and villoglandular) and high grade, consisting of endometrioid grade 3 and type 2 endometrial carcinoma (serous carcinoma, clear cell carcinoma, and carcinosarcoma) (VIDEOS 8 and 9). Positive intraperitoneal cytology at the time of surgery was 2.3% and 2.1% (P = .832), with an average interval from diagnosis to surgery of 34.6 days (range, 7–43 days).
Video 8. Carcinosarcoma

The authors proposed several explanations for the low rate of intraperitoneal cytology with hysteroscopy. One possibility is having lower mean intrauterine pressure below 100 mm Hg for saline uterine distension, although this was not standardized for all surgeons in the study but rather was a custom of the institution. In addition, the length of time between hysteroscopy and surgery may allow the immune-reactive peritoneum to respond to the cellular insult, thus decreasing the biologic burden at the time of surgery. The median follow-up was 52 months (range, 12–120 months), and there were no differences between the hysteroscopy and the nonhysteroscopy groups in the 5-year recurrence-free survival (90.2% vs 88.2%; P = .53), disease-specific survival (93.4% vs 91.7%; P = .5), and overall survival (86.2% vs 80.6%; P = .22). The authors concluded that hysteroscopy does not compromise the survival of patients with early-stage endometrial cancer.12
Video 9. Carcinosarcoma

Retrospective data from Chen and colleagues regarding type 2 endometrial carcinoma indicated a statistically significant increase in positive intraperitoneal cytology for carcinomas evaluated by hysteroscopy versus D&C (30% vs 12%; P = .008).13 Among the patients who died, there was no difference in disease-specific survival (53 months for hysteroscopy and 63.5 months for D&C; P = .34), and there was no difference in overall recurrence rates.13 Compared with type 1 endometrial carcinoma, type 2 endometrial carcinoma behaves more aggressively, with a higher incidence of extrauterine disease and an increased propensity for recurrence and poor outcome even in the early stages of the disease. This makes it difficult to determine the role of hysteroscopy in the prognosis of these carcinomas, especially in this study where most patients were diagnosed at a later stage.
Key takeaways
Hysteroscopy and directed biopsy are highly effective for visual and histopathologic diagnosis of atypical endometrial hyperplasia and endometrial carcinoma, and they are recommended in the evaluation of AUB, especially in the postmenopausal woman. When the hysteroscopic view is negative, there is a high correlation with the absence of uterine cavity and endometrial pathology. Hysteroscopic diagnostic accuracy is improved with structured use of visual grading scales, well-defined descriptors of endometrial pathology, and hysteroscopist experience.
Low operating intrauterine pressure may decrease the intraperitoneal spread of carcinoma cells during hysteroscopy, and current evidence suggests that there is no change in type 1 endometrial carcinoma prognosis and overall outcomes. Type 2 endometrial carcinoma is more aggressive and is associated with poor outcomes even in early stages, and the effect on disease progression by intraperitoneal spread of carcinoma cells at hysteroscopy is not yet known. Hysteroscopic evaluation of the uterine cavity and directed biopsy is easily and safely performed in the office and adds significantly to the evaluation and management of endometrial carcinoma.
Access them in the article online at mdedge.com/obgyn
Video 1. Endometrial carcinoma and visually directed biopsy
Nodular endometrioid adenocarcinoma grade 1 (type 1 endometrial carcinoma), benign endometrial polyps, and endometrial atrophy in a postmenopausal woman with bleeding. This video demonstrates visually directed biopsy to assure sampling of the most significant lesion.
Video 2. Negative hysteroscopic view
Digital flexible diagnostic hysteroscopy showing a negative hysteroscopic view in a premenopausal woman.
Video 3. Cervical spread of adenocarcinoma and visually directed biopsy
Diffuse endometrioid adenocarcinoma spread to the upper cervical canal near the internal cervical os. Hysteroscopic directed biopsy is performed.
Video 4. Endometrial adenocarcinoma
Fiberoptic flexible diagnostic hysteroscopy demonstrating diffuse endometrioid adenocarcinoma grade 3 with multiple morphologic features: polypoid, nodular, papillary, and glomerular with areas of necrosis.
Video 5. Endometrial polyp and atypical hyperplasia
Large benign endometrial polyp in an asymptomatic postmenopausal woman with enlarged endometrial stripe on pelvic ultrasound. The endometrium is atrophic except for a small whitish area on the anterior wall, which is atypical hyperplasia. This video highlights the need for visually directed biopsy to assure sampling of the most significant lesion.
Video 6. Nodular, polypoid atypical hyperplasia
Fiberoptic flexible diagnostic hysteroscopy showing diffuse nodular and polypoid atypical hyperplasia with abnormal glandular openings in a postmenopausal woman. Hysterectomy was performed secondary to the significant likelihood of concomitant endometrial carcinoma.
Video 7. Visually directed endometrial biopsy
Hysteroscopic-directed biopsy showing the technique of grasping and removing tissue of a benign adenomyosis cyst and proliferative endometrium.
Video 8. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a large intracavitary mass with soft, polypoid-like tissue in a symptomatic postmenopausal woman with bleeding.
Video 9. Carcinosarcoma
Carcinosarcoma (type 2 endometrial carcinoma) presents as a dense mass in a symptomatic postmenopausal woman with bleeding. This video shows the mass is nodular. These cancers typically grow into a spherical mass within the cavity
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
- Sugimoto O. Hysteroscopic diagnosis of endometrial carcinoma. A report of fifty-three cases examined at the Women’s Clinic of Kyoto University Hospital. Am J Obstet Gynecol. 1975;121:105-113.
- Loffer FD. Hysteroscopy with selective endometrial sampling compared with D&C for abnormal uterine bleeding: the value of a negative hysteroscopic view. Obstet Gynecol. 1989;73:16-20.
- Valli E, Zupi E. A new hysteroscopic classification of and nomenclature for endometrial lesions. J Am Assoc Gynecol Laparosc. 1995;2:279-283.
- Garuti G, De Giorgi O, Sambruni I, et al. Prognostic significance of hysteroscopic imaging in endometrioid endometrial adenocarcinoma. Gynecol Oncol. 2001;81: 408-413.
- Dueholm M, Hjorth IMD, Secher P, et al. Structured hysteroscopic evaluation of endometrium in women with postmenopausal bleeding. J Minim Invasive Gynecol. 2015;22:1215-1224.
- Ianieri MM, Staniscia T, Pontrelli G, et al. A new hysteroscopic risk scoring system for diagnosing endometrial hyperplasia and adenocarcinoma. J Minim Invasive Gynecol. 2016;23: 712-718.
- Su H, Pandey D, Liu L-Y, et al. Pattern recognition to prognosticate endometrial cancer: the science behind the art of office hysteroscopy—a retrospective study. Int J Gynecol Cancer. 2016;26:705-710.
- Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812-819.
- De Franciscis P, Riemma G, Schiattarella A, et al. Concordance between the hysteroscopic diagnosis of endometrial hyperplasia and histopathological examination. Diagnostics (Basel). 2019;9(4).
- Lasmar RB, Barrozo PRM, de Oliveira MAP, et al. Validation of hysteroscopic view in cases of endometrial hyperplasia and cancer in patients with abnormal uterine bleeding. J Minim Invasive Gynecol. 2006;13:409-412.
- Chang Y-N, Zhang Y, Wang Y-J, et al. Effect of hysteroscopy on the peritoneal dissemination of endometrial cancer cells: a meta-analysis. Fertil Steril. 2011;96:957-961.
- Namazov A, Gemer O, Helpman L, et al. The oncological safety of hysteroscopy in the diagnosis of early-stage endometrial cancer: an Israel Gynecologic Oncology Group study. Eur J Obstet Gynecol Reprod Biol. 2019;243:120-124.
- Chen J, Clark LH, Kong W-M, et al. Does hysteroscopy worsen prognosis in women with type II endometrial carcinoma? PLoS One. 2017;12(3):e0174226.
Medical management of abnormal uterine bleeding in reproductive-age women
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).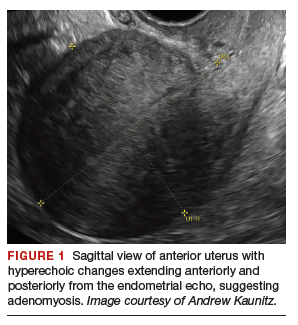
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).
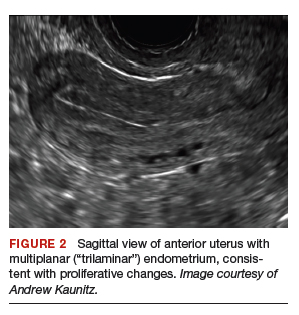

After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6
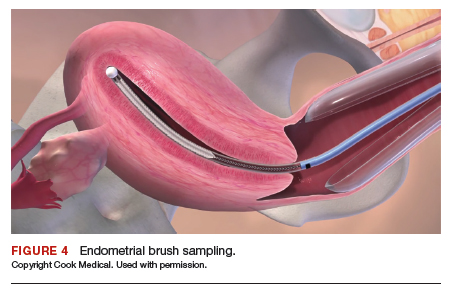
Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.
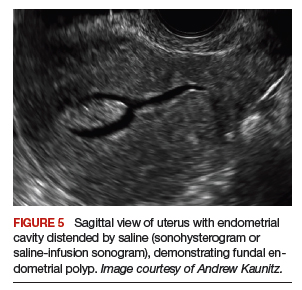
Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16
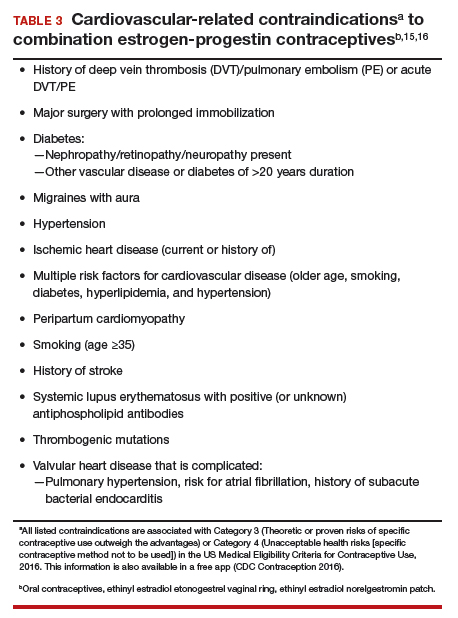
Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
Case 1 Multiparous woman presents with heavy regular menses
Over the past several years, a 34-year-old woman has noted increasing intensity and duration of menstrual flow, which now persists for 8 days and includes clots “the size of quarters” and soaks a pad within 1 hour. Sometimes she misses or leaves work on her heaviest days of flow. She reports that menstrual cramps prior to and during flow are increasingly bothersome and do not respond adequately to ibuprofen. She intermittently uses condoms for contraception. She does not wish to be pregnant currently; however, she recently entered into a new relationship and may wish to conceive in the future.
On bimanual examination, the uterus appears bulky. Her hemoglobin is 10.9 g/dL with low mean corpuscular volume and a serum ferritin level indicating iron depletion. Pelvic ultrasonography suggests uterine adenomyosis; no fibroids are imaged (FIGURE 1).
You advise the patient to take ferrous sulfate 325 mg every other day. After discussion with the patient regarding different treatment options, she chooses to proceed with placement of a 52-mg levonorgestrel (LNG) intrauterine device (IUD; Mirena or Liletta).
Case 2 Older adolescent presents with irregular bleeding
A 19-year-old patient reports approximately 6 bleeding episodes each year. She reports the duration of her bleeding as variable, and sometimes the bleeding is heavy with small clots passed. She has been previously diagnosed with polycystic ovary syndrome (PCOS). Combination estrogen-progestin oral contraceptives have been prescribed several times in the past, but she always has discontinued them due to nausea. The patient is in a same-sex relationship and does not anticipate being sexually active with a male. She reports having to shave her mustache and chin twice weekly for the past 1 to 2 years.
On physical examination, the patient is obese (body mass index [BMI], 32 kg/m2), facial acne and hirsutism are present, and hair extends from the mons toward the umbilicus. Bimanual examination reveals a normal size, mobile, nontender uterus without obvious adnexal pathology. Pelvic ultrasonography demonstrates a normal-appearing uterus with multiplanar endometrium (consistent with proliferative changes) (FIGURE 2). Ovarian imaging demonstrates ≥12 follicles per image (FIGURE 3).


After reviewing various treatment options, you prescribe oral medroxyprogesterone acetate 20 mg (two 10-mg tablets) daily in a continuous fashion. You counsel her that she should not be surprised or concerned if frequent or even continuous bleeding occurs initially, and that she should continue this medication despite the occurrence of such.
About one-third of all women experience abnormal uterine bleeding (AUB) sometime during their lifetime and AUB can impair quality of life.1 Surgical management, including hysterectomy and endometrial ablation, plays an important role in the management of AUB in patients who do not desire future pregnancies. However, many cases of AUB occur in women who may not have completed childbearing or in women who prefer to avoid surgery.2 AUB can be managed effectively medically in most cases.1 Accordingly, in this review, we focus on nonsurgical management of AUB.
Continue to: Because previously used terms, including...
Because previously used terms, including menorrhagia and meno-metrorrhagia, were inconsistently defined and confusing, the International Federation of Gynecology and Obstetrics introduced updated terminology in 2011 to better describe and characterize AUB in nonpregnant women. Heavy menstrual bleeding (HMB) refers to ovulatory (cyclic) bleeding that is more than 8 days’ duration, or sufficiently heavy to impair a woman’s quality of life. HMB is a pattern of AUB distinct from the irregular bleeding pattern typically caused by ovulatory dysfunction (AUB-O).1
Clinical evaluation
Obtain menstrual history. In addition to a medical, surgical, and gynecologic history, a thorough menstrual history should be obtained to further characterize the patient’s bleeding pattern. In contrast to the cyclical or ovulatory bleeding seen with HMB, bleeding associated with inconsistent ovulation (AUB-O) is unpredictable or irregular, and is commonly associated with PCOS. AUB-O is also encountered in recently menarchal girls (secondary to immaturity of the hypothalamic-pituitary-gonadal axis) and in those who are perimenopausal. In addition, medications that can induce hyperprolactinemia (such as certain antipsychotics) can cause AUB-O.
Evaluate for all sources of bleeding. Be sure to evaluate for extrauterine causes of bleeding, including the cervix, vagina, vulva, or the urinary or gastrointestinal tracts for bleeding. Intermenstrual bleeding occurring between normal regular menses may be caused by an endometrial polyp, submucosal fibroid, endometritis, or an IUD. The patient report of postcoital bleeding suggests that cervical disease (cervicitis, polyp, or malignancy) may be present. Uterine leiomyoma or adenomyosis represent common causes of HMB. However, HMB also may be caused by a copper IUD, coagulation disorders (including von Willebrand disease), or use of anticoagulant medications. Hormonal contraceptives also can cause irregular bleeding.
Perform a pelvic examination and measure vital signs. The presence of fever suggests the possible presence of pelvic inflammatory disease (PID), while orthostatic hypotension raises the possibility of hypovolemia. When vaginal speculum examination is performed, a cervical cause of abnormal bleeding may be noted. The presence of fresh or old blood or finding clots in the vaginal vault or at the cervical os are all consistent with AUB. A bimanual examination that reveals an enlarged or lobular uterus suggests leiomyoma or adenomyosis. Cervical or adnexal tenderness is often noted in women with PID, which itself may be associated with endometritis. The presence of hyperandrogenic signs on physical examination (eg, acne, hirsutism, or clitoromegaly) suggests PCOS. The finding of galactorrhea suggests that hyperprolactinemia may be present.
Laboratory assessment
Test for pregnancy, cervical disease, and sexually transmitted infection when appropriate. Pregnancy testing is appropriate for women with AUB aged 55 years or younger. If patients with AUB are not up to date with normal cervical cancer screening results, cervical cytology and/or human papillomavirus testing should be performed. Testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis should be performed in patients:
- younger than 25 years
- when the history indicates new or multiple sexual partners, or
- when vaginal discharge, cervicitis, cervical motion, or adnexal tenderness is present.
Continue to: Obtain a complete blood count and serum ferritin levels...
Obtain a complete blood count and serum ferritin levels. In women presenting with HMB, iron depletion and iron deficiency anemia are common. The finding of leukocytosis raises the possibility of PID or postpartum endometritis. In women with presumptive AUB-O, checking the levels of thyroid-stimulating hormone, free T4, and prolactin should be performed.
Screen for a hemostasis disorder. Women with excessive menstrual bleeding should be clinically screened for an underlying disorder of hemostasis (TABLE 1).3 When a hemostasis disorder is suspected, initial laboratory evaluation includes a partial thromboplastin time, prothrombin time, activated partial thromboplastin time, and fibrinogen. Women who have a positive clinical screen for a possible bleeding disorder or abnormal initial laboratory test results for disorders of hemostasis should undergo further laboratory evaluation, including von Willebrand factor antigen, ristocetin cofactor assay, and factor VIII. Consultation with a hematologist should be considered in these cases.

Perform endometrial biopsy when indicated
After excluding pregnancy, endometrial biopsy (through pipelle biospy or brush sampling; FIGURE 4) should be performed in women with AUB who are at increased risk for endometrial neoplasia. The prevalence of endometrial neoplasia is substantially higher among women ≥45 years of age4 and among patients with AUB who are also obese (BMI, ≥30 kg/m2).5 In addition, AUB patients with unopposed estrogen exposure (presumed anovulation/PCOS), as well as those with persistent AUB or failed medical management, should undergo endometrial biopsy.6

Utilize transvaginal ultrasonography
Transvaginal ultrasonography is often useful in the evaluation of patients with AUB, as it may identify uterine fibroids or adenomyosis, suggest intracavitary pathology (such as an endometrial polyp or submucosal fibroid), or raise the possibility of PCOS. In virginal patients or those in whom vaginal ultrasound is not appropriate, abdominal pelvic ultrasonography represents appropriate imaging. If unenhanced ultrasound suggests endometrial polyps or fibroids within the endometrial cavity, an office-based saline infusion sonogram (sonohysterogram) (FIGURE 5) or hysteroscopy should be performed. Targeted endometrial sampling and biopsy of intracavitary pathology can be performed at the time of hysteroscopy.

Treatment
When HMB impairs quality of life, is bothersome to the patient, or results in anemia, treatment is appropriate. Although bleeding episodes in women with AUB-O may be infrequent (as with Case 2), treatment prevents heavy or prolonged bleeding episodes as well as endometrial neoplasia that may otherwise occur in anovulatory women.
Many women with AUB can be managed medically. However, treatment choices will vary with respect to the patient’s desire for future fertility, medical comorbidities, personal preferences, and financial barriers. While many women may prefer outpatient medical management (TABLE 2),7-14 others might desire surgical therapy, including endometrial ablation or hysterectomy.

Oral contraceptives
Combination estrogen-progestin oral contraceptives represent appropriate initial therapy for many women in the reproductive-age group with AUB, whether women have HMB or AUB-O. However, contraceptive doses of estrogen are not appropriate for some women with risk factors for cardiovascular disease, including those who smoke cigarettes and are age ≥35 years or those who have hypertension (TABLE 3).15,16

Continue to: Menopausal dosages of HT...
Menopausal dosages of HT
If use of contraceptive doses of estrogen is not appropriate, continuous off-label use of menopausal combination formulations (physiologic dosage) of hormonal therapy (HT; ie, lower doses of estrogen than contraceptives) may be effective in reducing or eliminating AUB. Options for menopausal combination formulations include generic ethinyl estradiol 5 µg/norethindrone acetate 1 mg or estradiol 1 mg/norethindrone acetate 0.5 mg.7 High-dose oral progestin therapy (norethindrone acetate 5 mg tablet once daily or medroxyprogesterone acetate 10 mg tablets 1–3 times daily) also can be used when combination contraceptives are contraindicated and may be more effective than lower-dose combination formulations.
Package labeling, as well as some guidelines, indicate that oral progestins used to treat AUB should be taken cyclically.8 However, continuous daily use is easier for many patients and may be more effective in reducing bleeding. Accordingly, we counsel patients with AUB who are using progestins and who do not wish to conceive to take these medications continuously. High-dose oral progestin therapy may cause bloating, dysphoria, and increased appetite/weight gain. Women initiating hormonal management (including the progestin IUDs detailed below) for AUB should be counseled that irregular or even continuous light bleeding/spotting is common initially, but this bleeding pattern typically decreases with continued use.
IUDs
The LNG 52 mg IUD (Mirena or Liletta) effectively treats HMB, reducing bleeding in a manner comparable to that of endometrial ablation.9,10 The Mirena IUD is approved for treatment of HMB in women desiring intrauterine contraception. In contrast to oral medications, use of progestin IUDs does not involve daily administration and may represent an attractive option for women with HMB who would like to avoid surgery or preserve fertility. With ongoing use, continuous oral or intrauterine hormonal management may result in amenorrhea in some women with AUB.
When the LNG 52 mg IUD is used to treat HMB, the menstrual suppression impact may begin to attenuate after approximately 4 years of use; in this setting, replacing the IUD often restores effective menstrual suppression.11 The LNG 52 mg IUD effectively suppresses menses in women with coagulation disorders; if menstrual suppression with the progestin IUD is not adequate in this setting, it may be appropriate to add an oral combination estrogen-progestin contraceptive or high-dose oral progestin.11,12
NSAIDs and tranexamic acid
Off-label use of nonsteroidal anti-inflammatory drugs (naproxen 500–1,000 mg daily for 5 days beginning at the onset of menstrual flow or tranexamic acid two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can suppress HMB and is useful for women who prefer to avoid or have contraindications to hormonal treatments.13,14 Unfortunately, these agents are not as effective as hormonal management in treating AUB.
Iron supplementation is often needed
Iron depletion commonly results from HMB, often resulting in iron deficiency anemia. When iron depletion (readily identified by checking a serum ferritin level) or iron deficiency anemia is identified, iron supplementation should be recommended. Every-other-day administration of iron supplements maximizes iron absorption while minimizing the adverse effects of unabsorbed iron, such as nausea. Sixty mg of elemental iron (ferrous sulfate 325 mg) administered every other day represents an inexpensive and effective treatment for iron deficiency/anemia.17 In patients who cannot tolerate oral iron supplementation or for those in whom oral therapy is not appropriate or effective, newer intravenous iron formulations are safe and effective.18
Continue to: Case 1 Follow-up...
Case 1 Follow-up
The patient noted marked improvement in her menstrual cramps following LNG-containing IUD placement. Although she also reported that she no longer experienced heavy menstrual flow or cramps, she was bothered by frequent, unpredictable light bleeding/spotting. You prescribed norethindrone acetate (NETA) 5-mg tablet orally once daily, to be used in addition to her IUD. After using the IUD with concomitant NETA for 2 months’ duration, she noted that her bleeding/spotting almost completely resolved; however, she did report feeling irritable with use of the progestin tablets. She subsequently stopped the NETA tablets and, after 6 months of additional follow-up, reported only minimal spotting and no cramps.
At this later follow-up visit, you noted that her hemoglobin level increased to 12.6 g/dL, and the ferritin level no longer indicated iron depletion. After the IUD had been in place for 4 years, she reported that she was beginning to experience frequent light bleeding again. A follow-up vaginal sonogram noted a well-positioned IUD, there was no suggestion of intracavitary pathology, and adenomyosis continued to be imaged. She underwent IUD removal and placement of a new LNG 52 mg IUD. This resulted in marked reduction in her bleeding.
Case 2 Follow-up
Two weeks after beginning continuous oral progestin therapy, the patient called reporting frequent irregular bleeding. She was reassured that this was not unexpected and encouraged to continue oral progestin therapy. During a 3-month follow-up visit, the patient noted little, if any, bleeding over the previous 2 months and was pleased with this result. She continued to note acne and hirsutism and asked about the possibility of adding spironolactone to her oral progestin regimen.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int J Gynecol Obstet. 2018;143:393-408.
- Kaunitz AM. Abnormal uterine bleeding in reproductive-age women. JAMA. 2019;321:2126-2127.
- American College of Obstetricians and Gynecologists. ACOG committee opinion no. 557: management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891-896.
- National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Uterine Cancer. http://seer.cancer.gov/statfacts/html/corp.html. Accessed October 10, 2019.
- Wise MR, Gill P, Lensen S, et al. Body mass index trumps age in decision for endometrial biopsy: cohort study of symptomatic premenopausal women. Am J Obstet Gynecol. 2016;215:598.e1-598.e8.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins—Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197-206.
- The North American Menopause Society. Menopause Practice–A Clinician’s Guide. 5th ed. NAMS: Mayfield Heights, OH; 2014.
- National Institute for Health and Care Excellence. Heavy menstrual bleeding: assessment and management. https://www.nice.org.uk/guidance/ng88. Accessed October 10, 2019.
- Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625-632.
- Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104-1116.
- Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193-215.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8.
- Ylikorkala O, Pekonen F. Naproxen reduces idiopathic but not fibromyoma-induced menorrhagia. Obstet Gynecol. 1986;68:10-12.
- Lukes AS, Moore KA, Muse KN, et al. Tranexamic acid treatment for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:865-875.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1–103.
- ACOG Practice Bulletin no. 206: use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128-e150.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31-38.