User login
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
2021 Update on minimally invasive gynecologic surgery

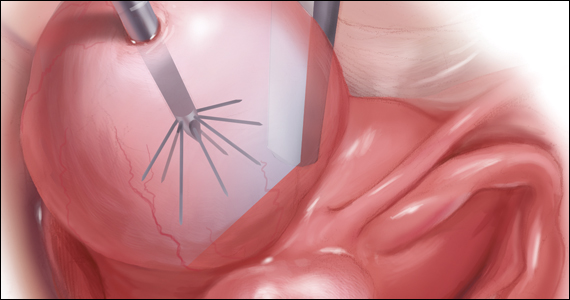
Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5
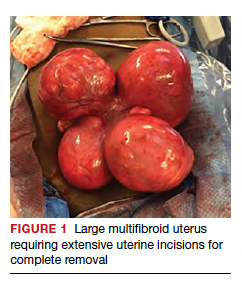
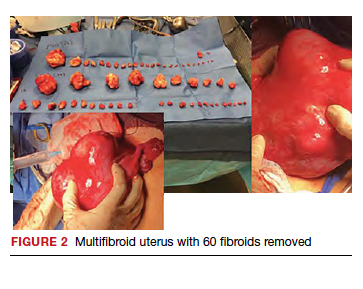
For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.
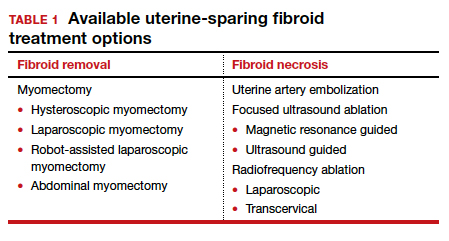
Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
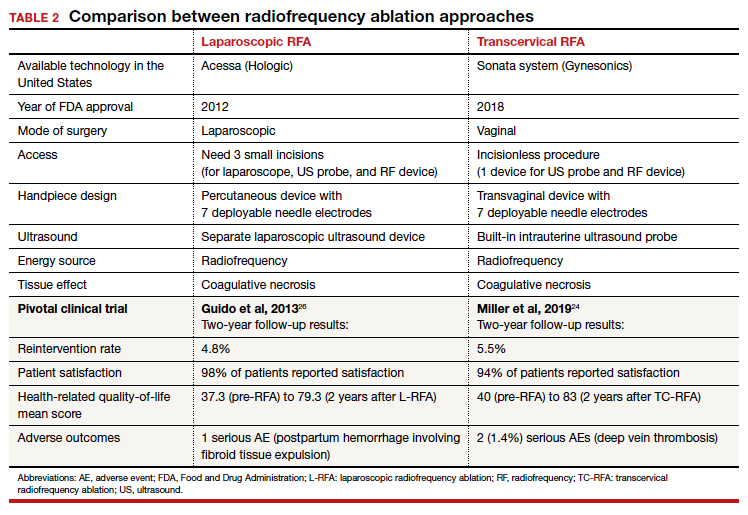
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.
Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.

Uterine fibroids are a common condition that affects up to 80% of reproductive-age women.1 Many women with fibroids are asymptomatic, but some experience symptoms that profoundly disrupt their lives, such as abnormal uterine bleeding, pelvic pain, and bulk symptoms including bladder and bowel dysfunction.2 Although hysterectomy remains the definitive treatment for symptomatic fibroids, many women seek more conservative management. Hormonal treatment, such as contraceptive pills, levonorgestrel intrauterine devices, and gonadotropin-releasing hormone analogs, can improve heavy menstrual bleeding and anemia.3 Additionally, uterine artery embolization is a nonsurgical uterine-sparing option. However, these treatments are not ideal options for women who want to conceive.4 For reproductive-age women who desire future fertility, myomectomy has been the standard of care. Unfortunately, by the time patients become symptomatic from their fibroids and seek care, they may have numerous and/or sizable fibroids that result in high blood loss, surgical scarring, and the probable need for cesarean delivery (FIGURES 1 and 2).5


For patients who desire future conception, treatment of uterine fibroids poses a challenge in which optimizing symptomatic improvement must be balanced with protecting fertility and improving reproductive outcomes. In recent years, high-intensity focused ultrasound (FUS) and radiofrequency ablation (RFA) have been presented as less invasive, uterine-sparing alternatives for fibroid treatment that could potentially provide that balance.
In this article, we briefly review the available uterine-sparing fibroid treatments and their outcomes and then focus specifically on RFA as a possible option to address the fibroid treatment gap for reproductive-age women who desire future fertility.
Overview of uterine-sparing treatments
Two approaches can be pursued for conservative fibroid treatment: fibroid removal and fibroid necrosis (TABLE 1). We focus this review on outcomes for the most widely available of these treatments.

Myomectomy
For reproductive-age women who wish to conceive, surgical removal of fibroids has been the standard of care for symptomatic patients. Myomectomy can be performed via laparotomy, laparoscopy, robot-assisted surgery, and hysteroscopy. The mode of surgery depends on the fibroid characteristics (size, number, and location) and the surgeon’s skill set. Although some variation in the data exists, overall surgical outcomes, including blood loss, postoperative pain, and length of stay, are generally more favorable for minimally invasive approaches compared with laparotomy, with no significant differences in fibroid recurrence or reproductive outcomes (live birth rate, miscarriage rate, and cesarean delivery rate).6 This comes at the expense of longer operating time compared with laparotomy.7
While improvement in abnormal uterine bleeding and pelvic pain is reliable and usually significant after myomectomy,8 reproductive implications also warrant consideration. Myomectomy is associated with subsequent uterine adhesion formation, with some studies finding rates up to 83% to 94% depending on the surgical approach and the number of fibroids removed.9 These adhesions can impair fertility success.10 Myomectomy also is associated with high rates of cesarean delivery,5 invasive placentation (including placenta accreta spectrum),11 and uterine rupture.12 While the latter 2 complications are rare, they potentially can be catastrophic and should be kept in mind.
Continue to: Uterine artery embolization...
Uterine artery embolization
As a nonsurgical alternative to myomectomy, uterine artery embolization (UAE) has gained popularity as a conservative fibroid treatment since it was introduced in 1995. It is less invasive than myomectomy, a benefit for patients who decline surgery or are not ideal candidates for surgery.13 Evidence suggests that UAE produces overall comparable symptomatic improvement compared with myomectomy. One study showed no significant differences between UAE and myomectomy in terms of decreased uterine volume and menstrual bleeding at 6-month follow-up.14 In terms of long-term outcomes, a large multicenter study showed no significant difference in reintervention rates at 7 years posttreatment between UAE and myomectomy (8.9% vs 11.2%, respectively), and a significantly higher rate of improved menstrual bleeding with UAE (79.4% vs 49.5%), with no significant difference in bulk symptoms.15 The evidence is not entirely consistent, as other studies have shown increased rates of reintervention with UAE,8,16 but overall UAE can be considered a reasonable alternative to myomectomy in terms of symptomatic improvement.
Pregnancy outcomes data, however, are mixed, and UAE often is not recommended for patients with future fertility plans. In a large review article that compared minimally invasive fibroid treatments, UAE was associated with a lower live birth rate compared with myomectomy and ablation techniques (60.6% for UAE, 75.6% for myomectomy, and 70.5% for ablation), and it also had the highest rate of miscarriage (27.4% for UAE vs 19.0% for myomectomy and 11.9% for ablation) and abnormal placentation.12 While UAE remains an effective option for conservative treatment of symptomatic fibroids, it appears to have a worse impact on reproductive outcomes compared with myomectomy or ablative treatments.
Magnetic resonance–guided focused ultrasound
Emerging as a noninvasive ablation treatment for fibroids, magnetic resonance–guided focused ultrasound (MRgFUS) uses targeted high-intensity ultrasound pulses to cause thermal and mechanical fibroid tissue disruption.17 Data on this treatment are less robust given that it is newer than myomectomy or UAE. One study showed a decrease in fibroid volume by 12% at 1 month and 15% at 6 months, with 37.1% of patients reporting marked improvement in symptoms and an additional 31.4% reporting partial improvement; these are modest numbers compared with other treatment approaches.18 Another study showed more favorable outcomes, with 74% of patients reporting clinically significant improvement in bleeding and pain, and a 12.7% reintervention rate, comparable to rates reported for UAE and myomectomy.19
Because MRgFUS is newer than UAE or myomectomy, data are limited in terms of pregnancy outcomes, particularly because initial trials excluded women with future fertility plans due to lack of knowledge regarding pregnancy safety. A follow-up case series from one of the initial studies showed a decreased miscarriage rate compared with UAE, a term delivery rate of 93%, and a similar rate of abnormal placentation.20 A more recent systematic review concluded that reproductive outcomes were noninferior to myomectomy; however, the outcomes data for MRgFUS were heterogenous and many studies did not report pregnancy rates.21
Overall, MRgFUS appears to be an effective alternative approach for symptomatic fibroids, but the long-term data are not yet conclusive and information on pregnancy safety and outcomes largely is lacking. Recent reviews have not made definitive statements on whether MRgFUS should be offered to patients desiring future fertility.
Continue to: RFA is a promising option...
RFA is a promising option
RFA is another noninvasive fibroid ablation technique that has become more widely adopted in recent years. Here, we describe the basics of RFA and its impact on fibroid symptoms and reproductive outcomes.
The RFA technique
RFA uses hyperthermic energy from a handpiece and real-time ultrasound for targeted coagulative necrosis via a laparoscopic (L-RFA) or transcervical (TC-RFA) approach.22 A comparison between the 2 devices available on the market in the United States is shown in TABLE 2. Ultrasound guidance allows placement of radiofrequency needles directly into the fibroid to target local treatment to the fibroid tissue only. Once the fibroid undergoes coagulative necrosis, the process of fibroid resorption and volume reduction occurs over weeks to months, depending on the fibroid size.

Impact on fibroid symptoms
Both laparoscopic and transcervical RFA approaches have shown significant decreases in pelvic pain and heavy menstrual bleeding associated with fibroids and a low reintervention rate that emphasizes the durability of their impact.
A feasibility and safety study of a TC-RFA device prior to the primary clinical trials found only a 4.3% reintervention rate in the first 18 months postprocedure.23 The pivotal clinical trial of a TC-RFA device that followed also reported a low 5.5% reintervention rate in the first 24 months postprocedure, with significant improvement in health-related quality-of-life and high patient satisfaction24 (results shown in TABLE 2, along with trial results for an L-RFA device). A subsequent study of TC-RFA reported that symptomatic improvement persisted at 3-year follow-up, with a 9.2% reintervention rate comparable to existing fibroid treatments such as myomectomy and UAE.25 The original L-RFA trial also has shown similar positive results at 2-year follow-up, with a low reintervention rate of 4.8% after treatment, and similar patient satisfaction and quality-of-life improvements as TC-RFA.26 While long-term data are limited by only recent approval by the Food and Drug Administration (FDA) of a TC-RFA device in 2018, one study followed clinical trial patients for a mean duration of 64 months. This study found no surgical reinterventions in the first 3.5 years posttreatment and a persistent reduction in fibroid symptoms from baseline 64.9 points to 27.6 points, as assessed by a validated symptom severity scale (out of 100 points).27 Similar improvements in health-related quality-of life-were also found to persist for years posttreatment.4
In a large systematic review that compared L-RFA, MRgFUS, UAE, and myomectomy, L-RFA had similar improvement rates in quality-of-life and symptom severity scores compared with myomectomy, with no significant difference in reintervention rates.28 This review also noted minimal heterogeneity among RFA meta-analyses data in contrast to significant heterogeneity among UAE and myomectomy data.
Reproductive outcomes
Similar to MRgFUS, the initial studies of RFA devices largely excluded women with future fertility plans, as data on safety were lacking. However, many RFA devices are now on the market across the globe, and subsequent pregnancies have been tracked and reported.
A large case series that included clinical trials and commercial settings reported a miscarriage rate (13.3%) similar to that of the general obstetric population and no cases of uterine rupture, invasive placentation, preterm delivery, or placental abruption.29 Other case series have reported live birth rates similar those with myomectomy, and safe and favorable pregnancy outcomes with RFA have been supported by larger systematic reviews of all ablation techniques.12
Continue to: Uterine impact...
Uterine impact
One study of TC-RFA patients showed a greater than 65% reduction in fibroid volume (with a 90% reduction in fibroid volume for fibroids larger than 6 cm prior to RFA), and 54% of patients reported complete resolution of symptoms, with another 36% reporting decreased symptoms.30 Similar decreases in fibroid volume, ranging from 65% to 84%, have been reported in numerous follow-up studies, with significant decreases in bleeding and pain in 78% to 88% of patients.23,31-33 Additionally, a large secondary analysis of a TC-RFA clinical trial showed that patients did not have any significant decrease in uterine wall thickness or integrity on follow-up with magnetic resonance imaging compared with baseline measurements, and they did not have any new myometrial scars (assessed as nonperfused linear areas).22
As with other ablation techniques, most data on RFA pregnancy outcomes come from case series, and further research and evaluation are needed. Existing studies, however, have demonstrated promising aspects of RFA that argue its usefulness in women with fertility plans.
A prospective trial that evaluated intrauterine adhesion formation with use of a TC-RFA device found no new adhesions on 6-week follow-up hysteroscopy compared with baseline pre-RFA hysteroscopy.34 Because intrauterine adhesion formation and uterine rupture are both significant concerns with other uterine-sparing fibroid treatment approaches such as myomectomy, these findings suggest that RFA may be a better alternative for women who are planning future pregnancies, as they may have increased fertility success and decreased catastrophic complications.
The consensus is growing that RFA is a safe and effective option for women who desire minimally invasive fibroid treatment and want to preserve fertility.
Unique benefits of RFA
In this article, we highlight RFA as an emerging treatment option for fibroid management, particularly for women who desire a uterine-sparing approach to preserve their reproductive options. Although myomectomy has been the standard of care for many years, with UAE as the alternative nonsurgical treatment, neither approach provides the best balance between symptomatic improvement and reproductive outcomes, and neither is without pregnancy risks. In addition, many women with symptomatic fibroids do not desire future conception but decline fibroid removal for religious or personal reasons. RFA offers these women an alternative minimally invasive option for uterine-sparing fibroid treatment.
RFA presents a unique “incision-free” fibroid treatment that is truly minimally invasive. This technique minimizes the risks associated with myomectomy, such as intra-abdominal adhesions, intrauterine adhesions (Asherman syndrome), need for cesarean delivery, and pregnancy complications such as uterine rupture or invasive placentation. Furthermore, the evolution of an RFA transcervical approach has enabled treatment with no abdominal or uterine incisions, thus offering all the above reproductive benefits as well as the operative benefits of a faster recovery, less pain, and less risk of intraperitoneal surgical complications.
While many women desire uterine-sparing fibroid treatment even without future fertility plans, the larger question is whether we should treat fibroids more strategically for women who desire future fertility. Myomectomy and UAE are effective and reliable in terms of fibroid symptomatic improvement, but RFA promises more beneficial reproductive outcomes. The ability to avoid uterine myometrial incisions and still attain significant symptomatic improvement should be prioritized in these patients.
Currently, RFA is not approved by the FDA as a fertility-enabling treatment, and these patients have been largely excluded from RFA studies. However, the reproductive-age patient who desires future conception may benefit most from RFA. Furthermore, RFA technology also could address the gap in uterine-sparing treatment for reproductive-age women with adenomyosis. Although a complete review of adenomyosis treatment is beyond the scope of this article, recent studies show that RFA produces similar improvement in both uterine volume and symptom severity in women with adenomyosis.35-37 ●
The RFA data suggest that both laparoscopic and transcervical RFA offer a safe and effective alternative treatment option for patients with symptomatic fibroids who seek uterine-sparing treatment, and transcervical RFA offers the least invasive treatment option. Women with fibroids who wish to conceive currently face a challenging treatment gap in clinical medicine, and future research is needed to address this concern in these patients. RFA is promising and appears to be a better fertility-enabling conservative fibroid treatment than the current options of myomectomy or UAE.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
- Baird DD, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100-107.
- Stewart EA. Clinical practice. Uterine fibroids. N Engl J Med. 2015;372:1646-1655.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387-400.
- Gupta JK, Sinha A, Lumsden MA, et al. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2014;CD005073.
- Paul GP, Naik SA, Madhu KN, et al. Complications of laparoscopic myomectomy: a single surgeon’s series of 1001 cases. Aust N Z J Obstet Gynaecol. 2010;50:385-390.
- Flyckt R, Coyne K, Falcone T. Minimally invasive myomectomy. Clin Obstet Gynecol. 2017;60:252-272.
- Bean EM, Cutner A, Holland T, et al. Laparoscopic myomectomy: a single-center retrospective review of 514 patients. J Minim Invasive Gynecol. 2017;24:485-493.
- Broder MS, Goodwin S, Chen G, et al. Comparison of longterm outcomes of myomectomy and uterine artery embolization. Obstet Gynecol. 2002;100(5 pt 1):864-868.
- Torng PL. Adhesion prevention in laparoscopic myomectomy. Gynecol Minim Invasive Ther. 2014;3:7-11.
- Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9:190-197.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Khaw SC, Anderson RA, Lui MW. Systematic review of pregnancy outcomes after fertility-preserving treatment of uterine fibroids. Reprod Biomed Online. 2020;40:429-444.
- Spies JB, Ascher SA, Roth AR, et al. Uterine artery embolization for leiomyomata. Obstet Gynecol. 2001;98:29-34.
- Goodwin SC, Bradley LD, Lipman JC, et al. Uterine artery embolization versus myomectomy: a multicenter comparative study. Fertil Steril. 2006;85:14-21
- Jia JB, Nguyen ET, Ravilla A, et al. Comparison of uterine artery embolization and myomectomy: a long-term analysis of 863 patients. Am J Interv Radiol. 2020;5:1.
- Huang JY, Kafy S, Dugas A, et al. Failure of uterine fibroid embolization. Fertil Steril. 2006;85:30-35.
- Hesley GK, Gorny KR, Woodrum DA. MR-guided focused ultrasound for the treatment of uterine fibroids. Cardiovasc Intervent Radiol. 2013;36:5-13.
- Rabinovici J, Inbar Y, Revel A, et al. Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol. 2007;30:771-777.
- Mindjuk I, Trumm CG, Herzog P, et al. MRI predictors of clinical success in MR-guided focused ultrasound (MRgFUS) treatments of uterine fibroids: results from a single centre. Eur Radiol. 2015;25:1317-1328.
- Rabinovici J, David M, Fukunishi H, et al; MRgFUS Study Group. Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril. 2010;93:199-209.
- Anneveldt KJ, Oever HJV, Nijholt IM, et al. Systematic review of reproductive outcomes after high intensity focused ultrasound treatment of uterine fibroids. Eur J Radiol. 2021;141:109801.
- Bongers M, Gupta J, Garza-Leal JG, et al. The INTEGRITY trial: preservation of uterine-wall integrity 12 months after transcervical fibroid ablation with the Sonata system. J Gynecol Surg. 2019;35:299-303.
- Kim CH, Kim SR, Lee HA, et al. Transvaginal ultrasound-guided radiofrequency myolysis for uterine myomas. Hum Reprod. 2011;26:559–563.
- Miller CE, Osman KM. Transcervical radiofrequency ablation of symptomatic uterine fibroids: 2-year results of the Sonata pivotal trial. J Gynecol Surg. 2019;35:345-349.
- Lukes A, Green MA. Three-year results of the Sonata pivotal trial of transcervical fibroid ablation for symptomatic uterine myomata. J Gynecol Surg. 2020;36:228-233.
- Guido RS, Macer JA, Abbott K, et al. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Qual Life Outcomes. 2013;11:139.
- Garza-Leal JG. Long-term clinical outcomes of transcervical radiofrequency ablation of uterine fibroids: the VITALITY study. J Gynecol Surg. 2019;35:19-23.
- Cope AG, Young RJ, Stewart EA. Non-extirpative treatments for uterine myomas: measuring success. J Minim Invasive Gynecol. 2021;28:442-452.e4.
- Berman JM, Shashoua A, Olson C, et al. Case series of reproductive outcomes after laparoscopic radiofrequency ablation of symptomatic myomas. J Minim Invasive Gynecol. 2020;27:639-645.
- Jones S, O’Donovan P, Toub D. Radiofrequency ablation for treatment of symptomatic uterine fibroids. Obstet Gynecol Int. 2012;2012:194839.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192:768-773.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21:2081-2085.
- Szydłowska I, Starczewski A. Laparoscopic coagulation of uterine myomas with the use of a unipolar electrode. Surg Laparosc Endosc Percutan Tech. 2007;17:99-103.
- Bongers M, Quinn SD, Mueller MD et al. Evaluation of uterine patency following transcervical uterine fibroid ablation with the Sonata system (the OPEN clinical trial). Eur J Obstet Gynecol Reprod Biol. 2019;242:122-125.
- Hai N, Hou Q, Ding X, et al. Ultrasound-guided transcervical radiofrequency ablation for symptomatic uterine adenomyosis. Br J Radiol. 2017;90:201601132.
- Polin M, Krenitsky N, Hur HC. Transcervical radiofrequency ablation for symptomatic adenomyosis: a case report. J Minim Invasive Gyn. 2021;28:S152-S153.
- Scarperi S, Pontrelli G, Campana C, et al. Laparoscopic radiofrequency thermal ablation for uterine adenomyosis. JSLS. 2015;19:e2015.00071.
Relugolix combination therapy: A novel hormonal treatment for AUB associated with uterine fibroids
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.
Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.
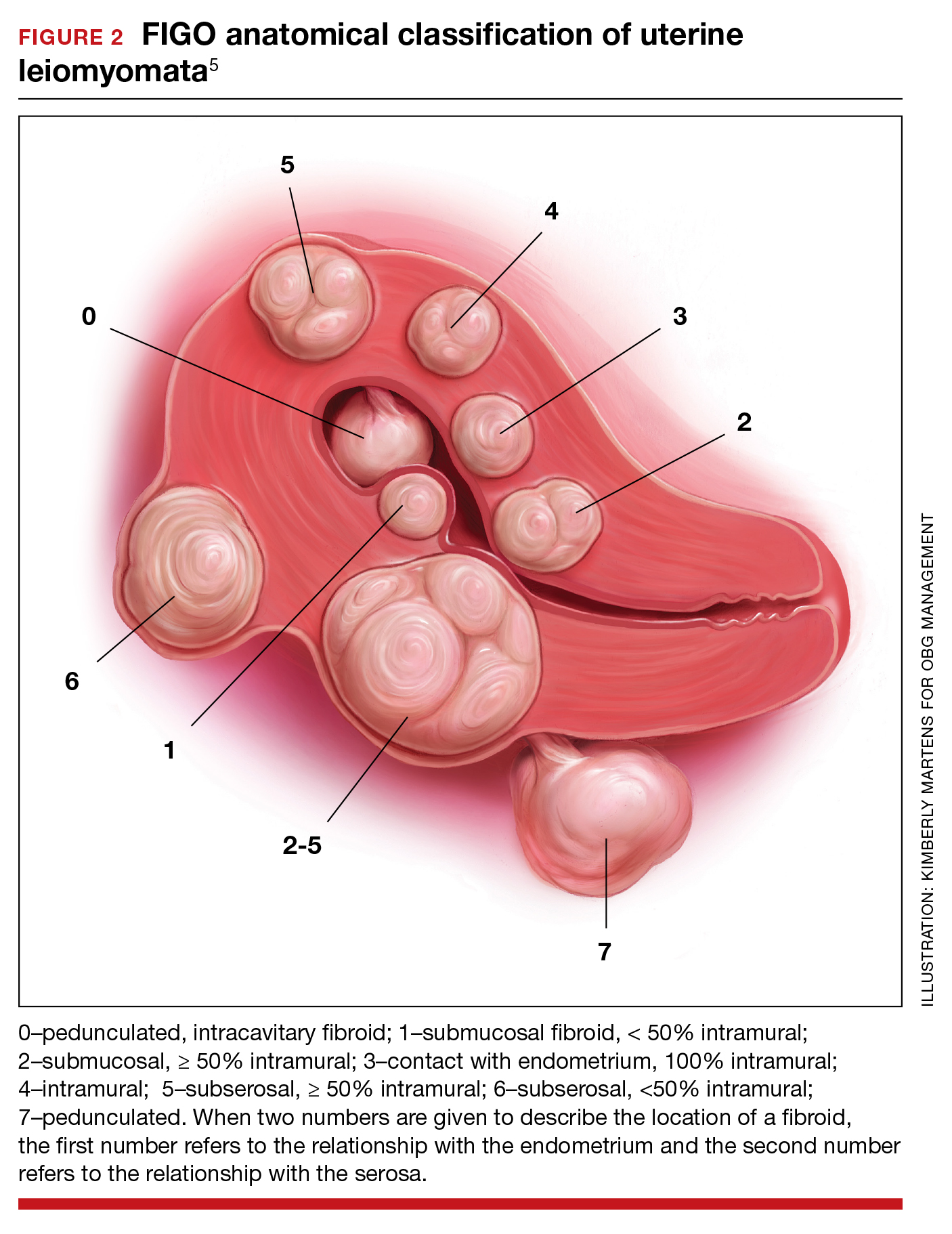
The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
When gonadotropin-releasing hormone (GnRH) agonist and antagonist peptide medications were first approved for use in the 1980s and 1990s, the available agents could only be administered by injection or nasal spray. The innovative development of orally active, nonpeptide GnRH antagonists, including relugolix and elagolix (FIGURE 1), is a major breakthrough in women’s health. Orally active GnRH antagonists provide gynecologists with a unique way to regulate hypothalamic-pituitary-ovarian-uterus function. GnRH antagonists bind to the pituitary GnRH receptor, reducing pituitary secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). In turn, reduction in LH and FSH suppresses ovarian follicle development, reducing ovarian secretion of estradiol and progesterone. The uterine endometrium becomes less active in response to low levels of estradiol and progesterone, resulting in oligomenorrhea or amenorrhea. The hypoestrogenic adverse effects of GnRH antagonist treatment, including bone loss and vasomotor symptoms can be minimized by adding back a low dose of estrogen and progestin, such as oral estradiol 1 mg and norethindrone acetate 0.5 mg.

Recently, the US Food and Drug Administration (FDA) approved oral relugolix combination therapy (Myfembree, Myovant Sciences and Pfizer Inc; relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) once daily for the treatment of abnormal uterine bleeding (AUB) associated with uterine leiomyomata (fibroids) in premenopausal women for up to 24 months.1 This editorial will focus on key clinical issues when using relugolix combination therapy.
Relugolix combination treatment is superior to placebo for AUB from fibroids
In 2 clinical trials, 770 women with symptomatic uterine fibroids were randomly assigned to 1 of 3 groups2:
- placebo for 24 weeks
- relugolix combination therapy (consisting of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg) daily for 24 weeks
- relugolix monotherapy (40 mg daily for 12 weeks) followed by relugolix combination therapy for 12 additional weeks (delayed combination therapy group).
The women’s mean age was approximately 42 years, and they had a mean menstrual blood loss at baseline of approximately 230 mL and mean uterine volume by ultrasound measurement of 408 cm3.2 Prior to entry into the study all the women had an endometrial biopsy and a transvaginal ultrasound study of the pelvis. Women with a baseline bone mineral density Z-score of less than -2.0 at the spine, total hip, or femoral neck were excluded from the study because of low bone mass.2
At 24 weeks of treatment, approximately 72% of the women in the relugolix combination therapy groups had less than 80 mL of menstrual blood volume loss and ≥50% reduction in menstrual blood loss from baseline compared with 17% of women in the placebo group.2 At 8 weeks of treatment mean percent changes in menstrual blood loss from baseline were approximately 80% and 20% for the women receiving relugolix combination and placebo, respectively. Those differences persisted from 8 weeks through 24 weeks of treatment.1 In the last 35 days of treatment, amenorrhea was reported by approximately 51% and 4.5% of women receiving relugolix combination or placebo treatment, respectively.2 Compared with the placebo group, the relugolix combination groups reported significant improvement in bleeding and pelvic discomfort and had a higher hemoglobin concentration. Compared with placebo, relugolix combination treatment resulted in a greater percentage decrease in uterine volume (-12.9% vs +2.2%, respectively; P< .001).2
Continue to: Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy...
Relugolix combination treatment is associated with fewer side effects than relugolix monotherapy
Compared with relugolix combination therapy, women treated with relugolix monotherapy for 12 weeks followed by 12 weeks of relugolix combination therapy lost more bone density as measured by dual-energy X-ray absorptiometry and reported more vasomotor symptoms. This is an expected finding because GnRH antagonist monotherapy is known to significantly reduce ovarian estradiol and progesterone levels, causing bone loss and vasomotor symptoms. Relugolix combination treatment minimizes bone density loss and vasomotor symptoms because the combination of estradiol and norethindrone helps to preserve bone density and reduce hot flashes. Based on these and other findings, the FDA approved relugolix combination therapy for up to 24 months of treatment.1
Contraindications
Contraindications to relugolix combination therapy include: 1) pregnancy, 2) undiagnosed abnormal uterine bleeding, 3) current or personal history of breast cancer or other hormone-sensitive cancer, 4) known osteoporosis, 5) liver disease, 6) high risk of thrombosis, and 7) hypersensitivity to components of the medication.1
Adverse reactions
Serious adverse reactions were reported by 3.1% and 2.3% of women treated with the relugolix combination and placebo, respectively. Women taking relugolix combination reported the following adverse effects: 10.6% hot flashes, 6.3% AUB, 3.5% alopecia, and 3.1% decreased libido. Women taking placebo reported the following adverse effects: 6.6% hot flashes, 1.2% AUB, 0.8% alopecia, and 0.4% decreased libido. Among women taking relugolix combination, the following events occurred, each reported once by different women: myoma expulsion with menorrhagia, myoma prolapse without menorrhagia, cholecystitis, and pelvic pain.1
Bone loss
In women taking relugolix combination or placebo for 6 months, lumbar spine bone density change from baseline, as measured by DEXA, were -0.23% and +0.18%, respectively.1 After 12 months of relugolix combination treatment, lumbar spine bone density had decreased by -0.8% from baseline. These changes in lumbar bone density are minimal, and in my opinion of no clinical importance.
Reported mental health effects
Compared with placebo, more women taking relugolix combination reported depression, depressed mood, or mood swings (2.4% vs 0.8%), irritability (2.4% vs 0%), and anxiety (1.2% vs 0.8%).1
Continue to: Options for the treatment of AUB caused by fibroids...
Options for the treatment of AUB caused by fibroids
There are many options for the treatment of AUB caused by fibroids, including surgical, hormonal, and nonhormonal therapies. Women with bothersome fibroids strongly prefer to be involved in the decision-making process and select the treatment plan that is best for their situation.3 The patient’s preferences can be explored by discussing the main benefits and common complications and side effects of each treatment option.
Surgical options for the treatment of AUB caused by fibroids include, but are not limited to, hysterectomy (laparoscopic, vaginal, or laparotomy), myomectomy (hysteroscopic, laparoscopic, or laparotomy), uterine artery embolization, focused ultrasound surgery, radiofrequency ablation, cryomyolysis, endometrial ablation, and occlusion of the uterine arteries.4 The FIGO classification system provides a consensus nomenclature for describing fibroid location (see FIGURE 2).5 The selection of a treatment option is greatly influenced by the location of the fibroids in the uterus.6 Most experts recommend hysteroscopic myomectomy to treat Type 0 and Type 1 fibroids causing AUB.6 For Type 2 fibroids, hysteroscopic myomectomy, if technically feasible, is associated with a high rate of resolution of AUB with minimal complications. Hormonal treatment of Type 0 and Type 1 fibroids may result in red degeneration of the fibroid with significant menorrhagia.7,8 In my practice, I generally advise patients that hysteroscopic myomectomy is the first-line treatment option for Types 0, 1, and 2 fibroids causing AUB.

The FDA has approved the hormonal options of relugolix combination therapy (Myfembree)2 and elagolix combination therapy (Oriahnn)9,10 for the treatment of AUB associated with fibroids. Of note, elagolix combination therapy contains the same daily dose of estradiol (1 mg) and norethindrone acetate (0.5 mg) as relugolix combination therapy. Relugolix and elagolix combination therapy for fibroids are good options for women who have FIGO Type 2 to 5 fibroids and who prefer a nonsurgical option. If GnRH antagonist combination therapy results in a meaningful reduction in AUB, treatment can be continued for up to 2 years. If the patient reports an insufficient decrease in AUB, an alternative surgical, hormonal, or nonhormonal option can be offered.
Other hormonal treatments that may reduce AUB due to fibroids include combination estrogen-progestin contraceptives,11 the levonorgestrel-releasing intrauterine device (LNG-IUD),12 progestins, and leuprolide.13 Leuprolide plus iron therapy is approved by the FDA for improving red blood cell concentration prior to surgery in women with fibroids, AUB, and anemia.14 The Mirena LNG-IUD is FDA approved for the treatment of heavy menstrual bleeding among women who choose to use an IUD for contraception.15 However, a recent systematic review and meta-analysis concluded that because of very low-quality evidence it was difficult to assess the efficacy of the LNG-IUD and progestins for the treatment of fibroids.16 Tranexamic acid is a nonhormonal option, FDA approved for the treatment of cyclic heavy management of AUB caused by fibroids, and may be an option for women who are near menopause.
New hormonal treatment adds options for women
Fibroids are the most common pelvic tumor of women. Women with fibroids often present for clinical care due to AUB, pelvic pain, and/or lower abdominal discomfort. For women with symptomatic fibroids it may be difficult to effectively complete employment-related tasks and home responsibilities. In one study, women with symptomatic fibroids reported that their symptoms negatively impacted approximately 20 hours per month of employment-related work and 12 hours per month of home responsibilities, reducing productivity in both settings.19 Relugolix combination therapy adds another important option for the hormonal treatment of the problems caused by these prevalent and bothersome tumors, improving health and the quality of contributions at work and home. ●
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
- Orgovyx [package insert]. Brisbane, CA: Myovant Sciences, Inc; December 2020.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Treatment of uterine fibroid symptoms with relugolix combination therapy. N Engl J Med. 2021;384:630-642. doi: 10.1056/NEJMoa2008283.
- Solberg LI, Asche SE, Anderson LH, et al. Evaluating preference-sensitive care for uterine fibroids: it’s not so simple. J Women’s Health. 2009;18:1071-1079. doi: 10.1089/jwh.2008.0948.
- Stewart EA. Uterine Fibroids. N Engl J Med. 2015;372:1646-1655. doi: 10.1056/NEJMcp1411029.
- Munro MG, Critchley HO, Broder MS, et al. FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int J Gynaecol Obstet. 2011;113:3-13. doi: 10.1016/j.ijgo.2010.11.011.
- Donnez J, Dolmans MM. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665-686. doi: 10.1093/humupd/dmw023.
- Furui T, Imai A, Takagi A, et al. Differential efficacy of gonadotropin-releasing hormone (GnRH) agonist treatment on pedunculated and degenerated myomas: a retrospective study of 630 women. J Obstet Gynaecol. 2000;20:504-506. doi: 10.1080/014436100434703.
- Takeuchi M, Matsuzaki K, Bando Y, et al. Evaluation of red degeneration of uterine leiomyoma with susceptibility-weighted MR imaging. Magn Reson Med Sci. 2019;18:158-162. doi: 10.2463/mrms.mp.2018-0074.
- Schlaff WD, Ackerman RT, Al-Hendy A, et al. Elagolix for heavy menstrual bleeding in women with uterine fibroids. N Engl J Med. 2020;382:328-340. doi: 10.1056/NEJMoa1904351.
- Simon JA, Al-Hendy A, Archer DE, et al. Elagolix treatment for up to 12 months in women with heavy menstrual bleeding and uterine leiomyomas. Obstet Gynecol. 2020;135:1313-1326. doi: 10.1097/AOG.0000000000003869.
- Yao X, Stewart EA, Laughlin-Tommaso SK, et al. Medical therapies for heavy menstrual bleeding in women with uterine fibroids: a retrospective analysis of a large commercially insured population in the USA. BJOG. 2017;124:322-330. doi: 10.1111/1471-0528.14383.
- Zapata LB, Whiteman MK, Tepper NK, et al. Intrauterine device use among women with uterine fibroids: a systematic review. Contraception. 2010;82:41-55. doi: 10.1016/j.contraception.2010.02.011.
- Donnez J, Tomaszewski J, Vazquez F, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421-432. doi: 10.1056/NEJMoa1103180.
- Lupron Depot [package insert]. Osaka, Japan: Takeda; Revised March 2012.
- Mirena [package insert]. Whippany, NJ: Bayer Healthcare Pharmaceuticals, Inc; Revised August 2020.
- Sangkormkamhang US, Lumbiganon P, Pattanittum P. Progestogens or progestogen-releasing intrauterine systems for uterine fibroids (other than preoperative medical therapy). Cochrane Database Syst Rev. 2020;CD008994. doi: 10.1002/14651858.CD008994.pub3.
- Lysteda [package insert]. Parsippany, NJ: Ferring Pharmaceuticals Inc; Revised October 2013.
- Eder S, Baker J, Gersten J, et al. Efficacy and safety of oral tranexamic acid in women with heavy menstrual bleeding and fibroids. Women’s Health. 2013;9:397-403. doi: 10.2217/whe.13.28.
Solimon AM, Anand SB, Coyne KS, et al. Examining the relationship between symptomatic burden and self-reported productivity losses among patients with uterine fibroids in the United States. J Occup Environ Med. 2017;59:974-981. doi: 10.1097/JOM.0000000000001105.
Primary ovarian insufficiency requires long-term management of sequelae
Primary ovarian insufficiency is not your mother’s early menopause, according to Laurie McKenzie, MD, a reproductive endocrinologist and associate professor of ob.gyn. at the University of Texas MD Anderson Cancer Center with a joint appointment at Baylor College of Medicine, both in Houston.
Known previously as primary ovarian failure, the syndrome of primary ovarian insufficiency (POI) no longer refers to a failure in part because of the term’s negative connotations but mostly because it’s not precisely accurate, Dr. McKenzie told attendees at the 2021 annual meeting of the American College of Obstetricians and Gynecologists on May 1.
“Many of these women, especially early on in diagnosis, may be experiencing some intermittent ovarian function, so it may not be a complete failure of the ovaries,” Dr. McKenzie said.
Although the condition is not common, affecting about 1% of the female population, “it’s the kind of thing that when a gynecologist has someone who has this walk into their office, you really need to know how to address it because these women are understandably very distressed.” Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, said in an interview after attending the talk.
Women who develop POI lose ovarian activity before age 40, characterized by menstrual disturbance with raised gonadotropins and low estradiol. Symptoms include the hot flushes and night sweats characteristic of estrogen deficiency as well as vaginal symptoms, including dyspareunia and dryness. Other symptoms can include sleep disturbance, mood changes, poor concentration, stiffness, dry eyes, altered urinary frequency, low libido, and lack of energy.
Dr. McKenzie urged doctors to ask women about their symptoms if they present with amenorrhea because young women with primary amenorrhea rarely experience symptoms at presentation, “implying that these symptoms are due to estrogen withdrawal rather than estrogen deficiency,” she said. Diagnosis involves confirmation of 4-6 months of amenorrhea or oligomenorrhea and two measurements of elevated follicle-stimulating hormone (FSH). Following this work-up, clinicians should seek the cause of the condition.
Etiology of POI and associated conditions
A wide range of conditions or genetic factors can cause POI or be more likely in patients with POI, Dr. McKenzie said. Many women diagnosed with POI have chromosomal abnormalities, and there is no cutoff for genetic testing, she said. Most of these genetic causes (94%) are X chromosome abnormalities, including Turners-associated dysmorphic features, gonadal dysgenesis, and FMR1 anomalies. Autosomal gene mutations could also play a role in POI.
Although women with the full FMR1 mutation (Fragile X syndrome) do not have an increased risk of POI, those with the premutation (55-200 repeats) have a 13%-26% increased risk of developing POI, albeit no increased risk of intellectual disability. About 0.8%-7.5% of women with sporadic POI and up to 13% of women with a family history of POI have this genetic anomaly.
Autoimmune conditions may also develop or be related to POI, including hypothyroidism and adrenal insufficiency, Dr. McKenzie said. About 20% of adults with POI will develop hypothyroidism, so testing every 1-2 years is reasonable, though no formal screening guidelines exist. In women whose cause of POI is unknown or in whom you suspect an immune disorder, clinicians may consider screening for 21OH-Ab or adrenocortical antibodies. Patients with a positive 21OH-Ab or adrenocortical antibodies test should be referred to an endocrinologist to test adrenal function and rule out Addison disease.
Though diabetes mellitus has been linked to POI, not enough evidence exists to recommend screening women with POI for diabetes. There’s similarly no indication for infection screening, but infections can cause POI. Mumps oophoritis, for example, accounts for 3%-7% of POI cases. Cancer therapy, including radiotherapy and chemotherapy, and surgical treatment for cancer can result in POI.
“Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause but are not readily diagnosable causes of POI,” Dr. McKenzie said. “Although not proven to cause POI, cigarette smoking is toxic to the ovaries and has been linked to an earlier age at menopause.” Then there are many women whose cause of POI is unknown.
To take all these possibilities into account, Dr. McKenzie described the complete diagnostic work-up recommended by ACOG:
- Menstrual irregularity for at least 3-4 months
- Test FSH and estradiol
- Test hCG, TSH, and prolactin
- If diagnosis is confirmed, test karyotype, FMR1 premutation, adrenal antibodies, and a pelvic sonogram.
However, she added during the Q&A after her talk, she is not sure why a sonogram is recommended or what additional information it might provide.
Long-term consequences of POI
Dr McKenzie noted that one study found a 2-year reduction in life expectancy among women who developed menopause before age 40. The reduced life expectancy linked to untreated POI is primarily caused by cardiovascular disease, she said. Women who undergo menopause aged between 35 and 40 years have a 50% greater risk of death related to ischemic heart disease than those ages 49-51, after adjusting for other comorbidities and confounders.
“Women with primary ovarian insufficiency should be advised on how to reduce cardiovascular risk factors by not smoking, taking regular exercise, and maintaining a healthy weight,” Dr McKenzie said.
No interventions have been shown to increase ovarian activity
Though fertility is substantially reduced in women with POI, it may not be completely gone. Several studies have found pregnancy rates ranging from 1.5% to 4.8%, and one study found that 25% of women with idiopathic POI had some evidence of ovarian function. Clinicians should therefore recommend women with POI use contraception if they do not want to conceive. Egg donation is an option for preserving fertility in women with POI but only before POI is solidly established.
“No interventions have been reliably shown to increase ovarian activity and natural conception rates,” Dr. McKenzie said.
For women who survive childhood or adolescent cancer and become pregnant, no evidence has shown an increased risk of congenital anomalies, but risk of low birth weight is elevated in babies whose mothers received anthracyclines. Treatment with anthracyclines and mediastinal radiotherapy have also been linked with cardiomyopathy and heart failure, so an echocardiogram prior to pregnancy is indicated in women with exposure to these or high-dose cyclophosphamide.
Abdominopelvic radiotherapy, however, has been linked to poor uterine function with a greater risk of late miscarriage, prematurity, low birth weight, stillbirth, neonatal hemorrhage, and postpartum hemorrhage.
“Pregnancies in women with Turner syndrome are very high risk and may have a maternal mortality as high as 3.5%,” Dr. McKenzie said, so these pregnancies require involvement of a cardiologist.
Other sequelae of POI can include increased bone resorption, net loss of bone (2%-3% annually soon after menopause) and reduced bone mineral density. Women should be getting 1,000 mg/day of calcium and 800 IU/day of vitamin D, but bone screening remains controversial in the field. Finally, providers should not ignore psychosocial effects of POI, including grief, diminished self esteem, and sadness, even more so, potentially, among adolescents.
Treatment of POI
Managing POI involves a two-pronged strategy of providing enough estrogen (estradiol, ethinyl estradiol, or conjugated equine estrogens) to mimic normal physiology and enough progestogen (synthetic or progesterone) to protect the endometrium from the mitogenic effect of estrogen.
The two primary options are hormone therapy and combination oral contraceptives. Hormone therapy might allow ovulation and pregnancy in some women, but combination oral contraceptive may feel less stigmatized in those who are still young, albeit with a potential risk for venous thromboembolism.
Continuous treatment tends to be easier and can involve breakthrough bleeding in younger patients; in postmenopausal women, breast cancer risk is higher but endometrial cancer risk is lower. Cyclic treatment mimics the endometrium’s normal function, resulting in bleeding that may help some women feel more “normal” and aids in knowing about a pregnancy. Those wanting to avoid bleeds and use contraception can use the levonorgestrel IUD off label.
Dr. Streicher said in an interview, “Not only is it critically important to recognize [long-term consequences] in this small group of women, but the lessons learned from young women who go though menopause can absolutely be extrapolated to women who go through menopause at an appropriate time.”
Dr. McKenzie had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceutical.
Primary ovarian insufficiency is not your mother’s early menopause, according to Laurie McKenzie, MD, a reproductive endocrinologist and associate professor of ob.gyn. at the University of Texas MD Anderson Cancer Center with a joint appointment at Baylor College of Medicine, both in Houston.
Known previously as primary ovarian failure, the syndrome of primary ovarian insufficiency (POI) no longer refers to a failure in part because of the term’s negative connotations but mostly because it’s not precisely accurate, Dr. McKenzie told attendees at the 2021 annual meeting of the American College of Obstetricians and Gynecologists on May 1.
“Many of these women, especially early on in diagnosis, may be experiencing some intermittent ovarian function, so it may not be a complete failure of the ovaries,” Dr. McKenzie said.
Although the condition is not common, affecting about 1% of the female population, “it’s the kind of thing that when a gynecologist has someone who has this walk into their office, you really need to know how to address it because these women are understandably very distressed.” Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, said in an interview after attending the talk.
Women who develop POI lose ovarian activity before age 40, characterized by menstrual disturbance with raised gonadotropins and low estradiol. Symptoms include the hot flushes and night sweats characteristic of estrogen deficiency as well as vaginal symptoms, including dyspareunia and dryness. Other symptoms can include sleep disturbance, mood changes, poor concentration, stiffness, dry eyes, altered urinary frequency, low libido, and lack of energy.
Dr. McKenzie urged doctors to ask women about their symptoms if they present with amenorrhea because young women with primary amenorrhea rarely experience symptoms at presentation, “implying that these symptoms are due to estrogen withdrawal rather than estrogen deficiency,” she said. Diagnosis involves confirmation of 4-6 months of amenorrhea or oligomenorrhea and two measurements of elevated follicle-stimulating hormone (FSH). Following this work-up, clinicians should seek the cause of the condition.
Etiology of POI and associated conditions
A wide range of conditions or genetic factors can cause POI or be more likely in patients with POI, Dr. McKenzie said. Many women diagnosed with POI have chromosomal abnormalities, and there is no cutoff for genetic testing, she said. Most of these genetic causes (94%) are X chromosome abnormalities, including Turners-associated dysmorphic features, gonadal dysgenesis, and FMR1 anomalies. Autosomal gene mutations could also play a role in POI.
Although women with the full FMR1 mutation (Fragile X syndrome) do not have an increased risk of POI, those with the premutation (55-200 repeats) have a 13%-26% increased risk of developing POI, albeit no increased risk of intellectual disability. About 0.8%-7.5% of women with sporadic POI and up to 13% of women with a family history of POI have this genetic anomaly.
Autoimmune conditions may also develop or be related to POI, including hypothyroidism and adrenal insufficiency, Dr. McKenzie said. About 20% of adults with POI will develop hypothyroidism, so testing every 1-2 years is reasonable, though no formal screening guidelines exist. In women whose cause of POI is unknown or in whom you suspect an immune disorder, clinicians may consider screening for 21OH-Ab or adrenocortical antibodies. Patients with a positive 21OH-Ab or adrenocortical antibodies test should be referred to an endocrinologist to test adrenal function and rule out Addison disease.
Though diabetes mellitus has been linked to POI, not enough evidence exists to recommend screening women with POI for diabetes. There’s similarly no indication for infection screening, but infections can cause POI. Mumps oophoritis, for example, accounts for 3%-7% of POI cases. Cancer therapy, including radiotherapy and chemotherapy, and surgical treatment for cancer can result in POI.
“Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause but are not readily diagnosable causes of POI,” Dr. McKenzie said. “Although not proven to cause POI, cigarette smoking is toxic to the ovaries and has been linked to an earlier age at menopause.” Then there are many women whose cause of POI is unknown.
To take all these possibilities into account, Dr. McKenzie described the complete diagnostic work-up recommended by ACOG:
- Menstrual irregularity for at least 3-4 months
- Test FSH and estradiol
- Test hCG, TSH, and prolactin
- If diagnosis is confirmed, test karyotype, FMR1 premutation, adrenal antibodies, and a pelvic sonogram.
However, she added during the Q&A after her talk, she is not sure why a sonogram is recommended or what additional information it might provide.
Long-term consequences of POI
Dr McKenzie noted that one study found a 2-year reduction in life expectancy among women who developed menopause before age 40. The reduced life expectancy linked to untreated POI is primarily caused by cardiovascular disease, she said. Women who undergo menopause aged between 35 and 40 years have a 50% greater risk of death related to ischemic heart disease than those ages 49-51, after adjusting for other comorbidities and confounders.
“Women with primary ovarian insufficiency should be advised on how to reduce cardiovascular risk factors by not smoking, taking regular exercise, and maintaining a healthy weight,” Dr McKenzie said.
No interventions have been shown to increase ovarian activity
Though fertility is substantially reduced in women with POI, it may not be completely gone. Several studies have found pregnancy rates ranging from 1.5% to 4.8%, and one study found that 25% of women with idiopathic POI had some evidence of ovarian function. Clinicians should therefore recommend women with POI use contraception if they do not want to conceive. Egg donation is an option for preserving fertility in women with POI but only before POI is solidly established.
“No interventions have been reliably shown to increase ovarian activity and natural conception rates,” Dr. McKenzie said.
For women who survive childhood or adolescent cancer and become pregnant, no evidence has shown an increased risk of congenital anomalies, but risk of low birth weight is elevated in babies whose mothers received anthracyclines. Treatment with anthracyclines and mediastinal radiotherapy have also been linked with cardiomyopathy and heart failure, so an echocardiogram prior to pregnancy is indicated in women with exposure to these or high-dose cyclophosphamide.
Abdominopelvic radiotherapy, however, has been linked to poor uterine function with a greater risk of late miscarriage, prematurity, low birth weight, stillbirth, neonatal hemorrhage, and postpartum hemorrhage.
“Pregnancies in women with Turner syndrome are very high risk and may have a maternal mortality as high as 3.5%,” Dr. McKenzie said, so these pregnancies require involvement of a cardiologist.
Other sequelae of POI can include increased bone resorption, net loss of bone (2%-3% annually soon after menopause) and reduced bone mineral density. Women should be getting 1,000 mg/day of calcium and 800 IU/day of vitamin D, but bone screening remains controversial in the field. Finally, providers should not ignore psychosocial effects of POI, including grief, diminished self esteem, and sadness, even more so, potentially, among adolescents.
Treatment of POI
Managing POI involves a two-pronged strategy of providing enough estrogen (estradiol, ethinyl estradiol, or conjugated equine estrogens) to mimic normal physiology and enough progestogen (synthetic or progesterone) to protect the endometrium from the mitogenic effect of estrogen.
The two primary options are hormone therapy and combination oral contraceptives. Hormone therapy might allow ovulation and pregnancy in some women, but combination oral contraceptive may feel less stigmatized in those who are still young, albeit with a potential risk for venous thromboembolism.
Continuous treatment tends to be easier and can involve breakthrough bleeding in younger patients; in postmenopausal women, breast cancer risk is higher but endometrial cancer risk is lower. Cyclic treatment mimics the endometrium’s normal function, resulting in bleeding that may help some women feel more “normal” and aids in knowing about a pregnancy. Those wanting to avoid bleeds and use contraception can use the levonorgestrel IUD off label.
Dr. Streicher said in an interview, “Not only is it critically important to recognize [long-term consequences] in this small group of women, but the lessons learned from young women who go though menopause can absolutely be extrapolated to women who go through menopause at an appropriate time.”
Dr. McKenzie had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceutical.
Primary ovarian insufficiency is not your mother’s early menopause, according to Laurie McKenzie, MD, a reproductive endocrinologist and associate professor of ob.gyn. at the University of Texas MD Anderson Cancer Center with a joint appointment at Baylor College of Medicine, both in Houston.
Known previously as primary ovarian failure, the syndrome of primary ovarian insufficiency (POI) no longer refers to a failure in part because of the term’s negative connotations but mostly because it’s not precisely accurate, Dr. McKenzie told attendees at the 2021 annual meeting of the American College of Obstetricians and Gynecologists on May 1.
“Many of these women, especially early on in diagnosis, may be experiencing some intermittent ovarian function, so it may not be a complete failure of the ovaries,” Dr. McKenzie said.
Although the condition is not common, affecting about 1% of the female population, “it’s the kind of thing that when a gynecologist has someone who has this walk into their office, you really need to know how to address it because these women are understandably very distressed.” Lauren Streicher, MD, a clinical professor of obstetrics and gynecology at Northwestern University, Chicago, said in an interview after attending the talk.
Women who develop POI lose ovarian activity before age 40, characterized by menstrual disturbance with raised gonadotropins and low estradiol. Symptoms include the hot flushes and night sweats characteristic of estrogen deficiency as well as vaginal symptoms, including dyspareunia and dryness. Other symptoms can include sleep disturbance, mood changes, poor concentration, stiffness, dry eyes, altered urinary frequency, low libido, and lack of energy.
Dr. McKenzie urged doctors to ask women about their symptoms if they present with amenorrhea because young women with primary amenorrhea rarely experience symptoms at presentation, “implying that these symptoms are due to estrogen withdrawal rather than estrogen deficiency,” she said. Diagnosis involves confirmation of 4-6 months of amenorrhea or oligomenorrhea and two measurements of elevated follicle-stimulating hormone (FSH). Following this work-up, clinicians should seek the cause of the condition.
Etiology of POI and associated conditions
A wide range of conditions or genetic factors can cause POI or be more likely in patients with POI, Dr. McKenzie said. Many women diagnosed with POI have chromosomal abnormalities, and there is no cutoff for genetic testing, she said. Most of these genetic causes (94%) are X chromosome abnormalities, including Turners-associated dysmorphic features, gonadal dysgenesis, and FMR1 anomalies. Autosomal gene mutations could also play a role in POI.
Although women with the full FMR1 mutation (Fragile X syndrome) do not have an increased risk of POI, those with the premutation (55-200 repeats) have a 13%-26% increased risk of developing POI, albeit no increased risk of intellectual disability. About 0.8%-7.5% of women with sporadic POI and up to 13% of women with a family history of POI have this genetic anomaly.
Autoimmune conditions may also develop or be related to POI, including hypothyroidism and adrenal insufficiency, Dr. McKenzie said. About 20% of adults with POI will develop hypothyroidism, so testing every 1-2 years is reasonable, though no formal screening guidelines exist. In women whose cause of POI is unknown or in whom you suspect an immune disorder, clinicians may consider screening for 21OH-Ab or adrenocortical antibodies. Patients with a positive 21OH-Ab or adrenocortical antibodies test should be referred to an endocrinologist to test adrenal function and rule out Addison disease.
Though diabetes mellitus has been linked to POI, not enough evidence exists to recommend screening women with POI for diabetes. There’s similarly no indication for infection screening, but infections can cause POI. Mumps oophoritis, for example, accounts for 3%-7% of POI cases. Cancer therapy, including radiotherapy and chemotherapy, and surgical treatment for cancer can result in POI.
“Smoking, alcohol, nutrition, and exposure to endocrine disruptors are implicated as influencing the age of menopause but are not readily diagnosable causes of POI,” Dr. McKenzie said. “Although not proven to cause POI, cigarette smoking is toxic to the ovaries and has been linked to an earlier age at menopause.” Then there are many women whose cause of POI is unknown.
To take all these possibilities into account, Dr. McKenzie described the complete diagnostic work-up recommended by ACOG:
- Menstrual irregularity for at least 3-4 months
- Test FSH and estradiol
- Test hCG, TSH, and prolactin
- If diagnosis is confirmed, test karyotype, FMR1 premutation, adrenal antibodies, and a pelvic sonogram.
However, she added during the Q&A after her talk, she is not sure why a sonogram is recommended or what additional information it might provide.
Long-term consequences of POI
Dr McKenzie noted that one study found a 2-year reduction in life expectancy among women who developed menopause before age 40. The reduced life expectancy linked to untreated POI is primarily caused by cardiovascular disease, she said. Women who undergo menopause aged between 35 and 40 years have a 50% greater risk of death related to ischemic heart disease than those ages 49-51, after adjusting for other comorbidities and confounders.
“Women with primary ovarian insufficiency should be advised on how to reduce cardiovascular risk factors by not smoking, taking regular exercise, and maintaining a healthy weight,” Dr McKenzie said.
No interventions have been shown to increase ovarian activity
Though fertility is substantially reduced in women with POI, it may not be completely gone. Several studies have found pregnancy rates ranging from 1.5% to 4.8%, and one study found that 25% of women with idiopathic POI had some evidence of ovarian function. Clinicians should therefore recommend women with POI use contraception if they do not want to conceive. Egg donation is an option for preserving fertility in women with POI but only before POI is solidly established.
“No interventions have been reliably shown to increase ovarian activity and natural conception rates,” Dr. McKenzie said.
For women who survive childhood or adolescent cancer and become pregnant, no evidence has shown an increased risk of congenital anomalies, but risk of low birth weight is elevated in babies whose mothers received anthracyclines. Treatment with anthracyclines and mediastinal radiotherapy have also been linked with cardiomyopathy and heart failure, so an echocardiogram prior to pregnancy is indicated in women with exposure to these or high-dose cyclophosphamide.
Abdominopelvic radiotherapy, however, has been linked to poor uterine function with a greater risk of late miscarriage, prematurity, low birth weight, stillbirth, neonatal hemorrhage, and postpartum hemorrhage.
“Pregnancies in women with Turner syndrome are very high risk and may have a maternal mortality as high as 3.5%,” Dr. McKenzie said, so these pregnancies require involvement of a cardiologist.
Other sequelae of POI can include increased bone resorption, net loss of bone (2%-3% annually soon after menopause) and reduced bone mineral density. Women should be getting 1,000 mg/day of calcium and 800 IU/day of vitamin D, but bone screening remains controversial in the field. Finally, providers should not ignore psychosocial effects of POI, including grief, diminished self esteem, and sadness, even more so, potentially, among adolescents.
Treatment of POI
Managing POI involves a two-pronged strategy of providing enough estrogen (estradiol, ethinyl estradiol, or conjugated equine estrogens) to mimic normal physiology and enough progestogen (synthetic or progesterone) to protect the endometrium from the mitogenic effect of estrogen.
The two primary options are hormone therapy and combination oral contraceptives. Hormone therapy might allow ovulation and pregnancy in some women, but combination oral contraceptive may feel less stigmatized in those who are still young, albeit with a potential risk for venous thromboembolism.
Continuous treatment tends to be easier and can involve breakthrough bleeding in younger patients; in postmenopausal women, breast cancer risk is higher but endometrial cancer risk is lower. Cyclic treatment mimics the endometrium’s normal function, resulting in bleeding that may help some women feel more “normal” and aids in knowing about a pregnancy. Those wanting to avoid bleeds and use contraception can use the levonorgestrel IUD off label.
Dr. Streicher said in an interview, “Not only is it critically important to recognize [long-term consequences] in this small group of women, but the lessons learned from young women who go though menopause can absolutely be extrapolated to women who go through menopause at an appropriate time.”
Dr. McKenzie had no disclosures. Dr. Streicher has consulted for Astellas Pharma and Church & Dwight, and she owns investments in InControl Medical and Sermonix Pharmaceutical.
FROM ACOG 2021
Optimize your treatment of endometriosis by using an FDA-approved hormonal medication
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
Women with endometriosis often present for medical care for one or more of the following health issues: pelvic pain, infertility, and/or an adnexal cyst (endometrioma). For women with moderate or severe pelvic pain and laparoscopically diagnosed endometriosis, hormone therapy is often necessary to achieve maximal long-term reduction in pain and optimize health. I focus on opportunities to optimize hormonal treatment of endometriosis in this editorial.
When plan A is not working, move expeditiously to plan B
Cyclic or continuous combination estrogen-progestin contraceptives are commonly prescribed to treat pelvic pain caused by endometriosis. Although endometriosis pain may initially improve with estrogen-progestin contraceptives, many women on this medication will eventually report that they have worsening pelvic pain that adversely impacts their daily activities. Surprisingly, clinicians often continue to prescribe estrogen-progestin contraceptives even after the patient reports that the treatment is not effective, and their pain continues to be bothersome.
Patients benefit when they have access to the full range of hormone treatments that have been approved by the FDA for the treatment of moderate to severe pelvic pain caused by endometriosis (TABLE). In the situation where an estrogen-progestin contraceptive is no longer effective at reducing the pelvic pain, I will often offer the patient the option of norethindrone acetate (NEA) or elagolix treatment. My experience is that stopping the estrogen-progestin contraceptive and starting NEA or elagolix will result in a significant decrease in pain symptoms and improvement in the patient’s quality of life.

Other FDA-approved options to treat pelvic pain caused by endometriosis include depot medroxyprogesterone acetate injectable suspension, depot leuprolide acetate, goserelin implant, and danazol. I do not routinely prescribe depot medroxyprogesterone acetate because some patients report new onset or worsening symptoms of depression on the medication. I prescribe depot-leuprolide acetate less often than in the past, because many patients report moderate to severe hypoestrogenic symptoms on this medication. In women taking depot-leuprolide acetate, moderate to severe vasomotor symptoms can be improved by prescribing NEA pills, but the alternative of norethindrone monotherapy is less expensive. I seldom use goserelin or danazol in my practice. The needle required to place the goserelin implant has a diameter of approximately 1.7 mm (16 gauge) or 2.1 mm (14 gauge), for the 3.6 mg and 10 mg doses, respectively. The large diameter of the needle can cause pain and bruising at the implant site. As a comparison, the progestin subdermal implant needle is approximately 2.1 mm in diameter. Danazol is associated with weight gain, and most women prefer to avoid this side effect.
Continue to: Norethindrone acetate...
Norethindrone acetate
NEA 5 mg daily is approved by the FDA to treat endometriosis.1 NEA was approved at a time when large controlled clinical trials were not routinely required for a medicine to be approved. The data to support NEA treatment of pelvic pain caused by endometriosis is based on cohort studies. In a study of 194 women, median age 21 years with moderate to severe pelvic pain and surgically proven endometriosis, the effect of NEA on pelvic pain was explored.2 The initial dose of NEA was 5 mg daily. If the patient did not achieve a reduction in pelvic pain and amenorrhea on the NEA dose of 5 mg daily, the dose was increased by 2.5 mg every 2 weeks, up to a maximum of 15 mg, until amenorrhea and/or a decrease in pelvic pain was achieved. Ninety-five percent of the women in this cohort had previously been treated with an estrogen-progestin contraceptive or a GnRH antagonist and had discontinued those medications because of inadequate control of pelvic pain or because of side effects of the medication.
In this large cohort, 65% of women reported significant improvement in pelvic pain, with a median pain score of 5 before treatment and 0 following NEA treatment. About 55% of the women reported no side effects. The most commonly reported side effects were weight gain (16%; mean weight gain, 3.1 kg), acne (10%), mood lability (9%), hot flashes (8%), depression (6%), scalp hair loss (4%), headache (4%), nausea (3%), and deepening of the voice (1%). (In this study women could report more than one side effect.)
In another cohort study of 52 women with pelvic pain and surgically confirmed endometriosis, NEA treatment resulted in pain relief in 94% of the women.3 Breakthrough bleeding was a common side effect, reported by 58% of participants. The investigators concluded that NEA treatment was a “cost-effective alternative with relatively mild side effects in the treatment of symptomatic endometriosis.” A conclusion which I endorse.
NEA has been reported to effectively treat ovarian endometriomas and rectovaginal endometriosis.4,5 In a cohort of 18 women who had previously had the surgical resection of an ovarian endometriosis cyst and had postoperative recurrence of pelvic pain and ovarian endometriosis, treatment was initiated with an escalating NEA regimen.4 Treatment was initiated with NEA 5 mg daily, with the dosage increased every 2 weeks by 2.5 mg until amenorrhea was established. Most women achieved amenorrhea with NEA 5 mg daily, and 89% had reduced pelvic pain. The investigators reported complete regression of the endometriosis cyst(s) in 74% of the women. In my experience, NEA does not result in complete regression of endometriosis cysts, but it does cause a reduction in cyst diameter and total volume.
In a retrospective cohort study, 61 women with pelvic pain and rectovaginal endometriosis had 5 years of treatment with NEA 2.5 mg or 5.0 mg daily.5 NEA treatment resulted in a decrease in dysmenorrhea, deep dyspareunia, and dyschezia. The most common side effects attributed to NEA treatment were weight gain (30%), vaginal bleeding (23%), decreased libido (11%), headache (9%), bloating or swelling (8%), depression (7%), and acne (5%). In women who had sequential imaging studies, NEA treatment resulted in a decrease in rectovaginal lesion volume, stable disease volume, or an increase in lesion volume in 56%, 32%, and 12% of the women, respectively. The investigators concluded that for women with rectovaginal endometriosis, NEA treatment is a low-cost option for long-term treatment.
In my practice, I do not prescribe NEA at doses greater than 5 mg daily. There are case reports that NEA at a dose of ≥10 mg daily is associated with the development of a hepatic adenoma,6 elevated liver transaminase concentration,7 and jaundice.8 If NEA 5 mg daily is not effective in controlling pelvic pain caused by endometriosis, I stop the NEA and start a GnRH analogue, most often elagolix.
NEA 5 mg is not FDA approved as a contraceptive. However, norethindrone 0.35 mg daily, also known as the “mini-pill”, is approved as a progestin-only contraceptive.9 NEA is rapidly and completely deacetylated to norethindrone, and the disposition of oral NEA is indistinguishable from that of norethindrone.1 Since norethindrone 0.35 mg daily is approved as a contraceptive, it is highly likely that NEA 5 mg has contraceptive properties if taken daily.
Continue to: Elagolix...
Elagolix
Elagolix is FDA approved for the treatment of pelvic pain caused by endometriosis. I reviewed the key studies resulting in FDA approval in the November 2018 issue of
In the Elaris Endometriosis-I study, 872 women with endometriosis and pelvic pain were randomly assigned to treatment with 1 of 2 doses of elagolix (high-dose [200 mg twice daily] and low-dose [150 mg once daily]) or placebo.11 After 3 months of therapy, a clinically meaningful reduction in dysmenorrhea pain was reported by 76%, 46%, and 20% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.001 for comparisons of elagolix to placebo). After 3 months of therapy, a clinically meaningful reduction in nonmenstrual pain or decreased or stable use of rescue analgesics was reported by 55%, 50%, and 37% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively (P<.01 low-dose elagolix vs placebo and P<.001 high-dose elagolix vs placebo).
Hot flashes that were severe enough to be reported as an adverse event by the study participants were reported by 42%, 24%, and 7% of the women in the high-dose elagolix, low-dose elagolix, and placebo groups. Bone density was measured at baseline and after 6 months of treatment. Lumbar bone density changes were -2.61%, -0.32%, and +0.47% and hip femoral neck bone density changes were -1.89%, -0.39%, and +0.02% in the high-dose elagolix, low-dose elagolix, and placebo groups, respectively.
Another large clinical trial of elagolix for the treatment of pelvic pain caused by endometriosis, Elaris EM-II, involving 817 women, produced results very similar to those reported in Elaris EM-I. The elagolix continuation studies, Elaris EM-III and -IV, demonstrated efficacy and safety of elagolix through 12 months of treatment.12
In my 2018 review,10 I noted that elagolix dose adjustment can be utilized to attempt to achieve maximal pain relief with minimal vasomotor symptoms. Elagolix at 200 mg twice daily produces a mean estradiol concentration of 12 pg/mL, whereas elagolix at 150 mg daily resulted in a mean estradiol concentration of 41 pg/mL.13 The estrogen threshold hypothesis posits that in women with endometriosis a stable estradiol concentration of 20 to 30 pg/mL is often associated with decreased pain and fewer vasomotor events.14 To achieve the target estradiol range of 20 to 30 pg/mL, I often initiate elagolix treatment with 200 mg twice daily. This enables a rapid onset of amenorrhea and a reduction in pelvic pain. Once amenorrhea has been achieved and a decrease in pelvic pain has occurred, I adjust the dose downward to 200 mg twice daily on even calendar days of each month and 200 mg once daily on odd calendar days each month. Some women will have continued pain relief and amenorrhea when the dose is further decreased to 200 mg once daily. If bothersome bleeding recurs and/or pain symptoms increase in severity, the dose can be increased to 200 mg twice daily or an alternating regimen of 200 mg twice daily and 200 mg once daily, every 2 days. An alternative to dose adjustment is to combine elagolix with NEA, which can reduce the severity of hot flashes and reduce bone loss caused by hypoestrogenism.15,16
Health insurers and pharmacy benefits managers may require a prior authorization before approving and dispensing elagolix. The prior authorization process can be burdensome for clinicians, consuming limited healthcare resources, contributing to burnout and frustrating patients.17 Elagolix is less expensive than depot-leuprolide acetate and nafarelin nasal spray and somewhat more expensive than a goserelin implant.18,19
Elagolix is not approved as a contraceptive. In the Elaris EM-I and -II trials women were advised to use 2 forms of contraception, although pregnancies did occur. There were 6 pregnancies among 475 women taking elagolix 150 mg daily and 2 pregnancies among 477 women taking elagolix 200 mg twice daily.20 Women taking elagolix should be advised to use a contraceptive, but not an estrogen-progestin contraceptive.
Continue to: Do not use opioids to treat chronic pelvic pain caused by endometriosis...
Do not use opioids to treat chronic pelvic pain caused by endometriosis
One of the greatest public health tragedies of our era is the opioid misuse epidemic. Hundreds of thousands of deaths have been caused by opioid misuse. The Centers for Disease Control and Prevention reported that for the 12-month period ending in May 2020, there were 81,000 opioid-related deaths, the greatest number ever reported in a 12-month period.21 Many authorities believe that in the United States opioid medications have been over-prescribed, contributing to the opioid misuse epidemic. There is little evidence that chronic pelvic pain is optimally managed by chronic treatment with an opioid.22,23 Prescribing opioids to vulnerable individuals to treat chronic pelvic pain may result in opioid dependency and adversely affect the patient’s health. It is best to pledge not to prescribe an opioid medication for a woman with chronic pelvic pain caused by endometriosis. In situations when pelvic pain is difficult to control with hormonal therapy and nonopioid pain medications, referral to a specialty pain practice may be warranted.
Post–conservative surgery hormone treatment reduces pelvic pain recurrence
In a meta-analysis of 14 studies that reported on endometriosis recurrence rates following conservative surgery, recurrence (defined as recurrent pelvic pain or an imaging study showing recurrent endometriosis) was significantly reduced with the use of hormone treatment compared with expectant management or placebo treatment.24 The postoperative relative risk of endometriosis recurrence was reduced by 83% with progestin treatment, 64% with estrogen-progestin contraceptive treatment, and 38% with GnRH analogue treatment. Overall, the number of patients that needed to be treated to prevent one endometriosis recurrence was 10, assuming a recurrence rate of 25% in the placebo treatment or expectant management groups.
For women with pelvic pain caused by endometriosis who develop a recurrence of pelvic pain while on postoperative hormone treatment, it is important for the prescribing clinician to be flexible and consider changing the hormone regimen. For example, if a postoperative patient is treated with a continuous estrogen-progestin contraceptive and develops recurrent pain, I will stop the contraceptive and initiate treatment with either NEA or elagolix.
Capitalize on opportunities to improve the medical care of women with endometriosis
Early diagnosis of endometriosis can be facilitated by recognizing that the condition is a common cause of moderate to severe dysmenorrhea. In 5 studies involving 1,187 women, the mean length of time from onset of pelvic pain symptoms to diagnosis of endometriosis was 8.6 years.25 If a woman with pelvic pain caused by endometriosis has not had sufficient pain relief with one brand of continuous estrogen-progestin contraceptive, it is best not to prescribe an alternative brand but rather to switch to a progestin-only treatment or a GnRH antagonist. If plan A is not working, move expeditiously to plan B. ●
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
- Aygestin [package insert]. Barr Laboratories: Pomona, NY; 2007.
- Kaser DJ, Missmer SA, Berry KF, et al. Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms. J Pediatr Adolesc Gynecol. 2012;25:105-108.
- Muneyyirci-Delale O, Karacan M. Effect of norethindrone acetate in the treatment of symptomatic endometriosis. Int J Fertil Womens Med. 1998;43:24-27.
- Muneyyirci-Delale O, Anopa J, Charles C, et al. Medical management of recurrent endometrioma with long-term norethindrone acetate. Int J Women Health. 2012;4:149-154.
- Morotti M, Venturini PL, Biscaldi E, et al. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur J Obstet Gynecol Repro Biol. 2017;213:4-10.
- Brady PC, Missmer SA, Laufer MR. Hepatic adenomas in adolescents and young women with endometriosis treated with norethindrone acetate. J Pediatr Adolesc Gynecol. 2017;30:422-424.
- Choudhary NS, Bodh V, Chaudhari S, et al. Norethisterone related drug induced liver injury: a series of 3 cases. J Clin Exp Hepatol. 2017;7:266- 268.
- Perez-Mera RA, Shields CE. Jaundice associated with norethindrone acetate therapy. N Engl J Med. 1962;267:1137-1138.
- Camila [package insert]. Mayne Pharma Inc: Greenville, NC; 2018.
- Barbieri RL. Elagolix: a new treatment for pelvic pain caused by endometriosis. OBG Manag. 2018;30:10,12-14, 20.
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Surrey E, Taylor HS, Giudice L, et al. Long-term outcomes of elagolix in women with endometriosis: results from two extension studies. Obstet Gynecol. 2018;132:147-160.
- Orilissa [package insert]. AbbVie Inc; North Chicago, IL; 2018.
- Barbieri RL. Hormonal treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
- Hornstein MD, Surrey ES, Weisberg GW, et al. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
- Gallagher JS, Missmer SA, Hornstein MD, et al. Long-term effects of gonadotropin-releasing hormone agonists and add-back in adolescent endometriosis. J Pediatr Adolesc Gynecol. 2018;31:376- 381.
- Miller A, Shor R, Waites T, et al. Prior authorization reform for better patient care. J Am Coll Cardiol. 2018;71:1937-1939.
- Depot-leuprolide acetate. Good Rx website. https://www.goodrx.com/. Accessed January 22, 2021.
- Goserelin. Good Rx website. https://www .goodrx.com/. Accessed January 22, 2021
- Taylor HS, Giudice LC, Lessey BA, et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N Engl J Med. 2017;377:28-40.
- Centers for Disease Control and Prevention. Overdose deaths accelerating during COVID19. https://www.cdc.gov/media/releases/2020 /p1218-overdose-deaths-covid-19.html. Reviewed December 18, 2020. Accessed March 24, 2021.
- Till SR, As-Sanie S. 3 cases of chronic pelvic pain with nonsurgical, nonopioid therapies. OBG Manag. 2018;30:41-48.
- Steele A. Opioid use and depression in chronic pelvic pain. Obstet Gynecol Clin North Am. 2014;41:491-501.
- Zakhari A, Delpero E, McKeown S, et al. Endometriosis recurrence following post-operative hormonal suppression: a systematic review and meta-analysis. Hum Reprod Update. 2021;27:96- 107.
- Barbieri RL. Why are there delays in the diagnosis of endometriosis? OBG Manag. 2017;29:8, 10-11, 16.
Cancer screening stopped by pandemic: Repercussions to come?
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Last year, cancer screening programs around the world ground to a halt as SARS-CoV-2 infection rates surged globally. The effect of this slowdown is now becoming clear.
Thousands of cancer diagnoses are “missing,” and oncologists worry that this will lead to more advanced cancers and higher mortality for years to come.
“I feel like this is an earthquake that’s rocked our health care system. My guess is that you’ll probably still see repercussions of this over the next couple of years at least,” said Sharon Chang, MD, an attending surgical oncologist in the Permanente Medical Group, Fremont, Calif.
She was senior author of a study that analyzed the effects of the slowdown in mammography screening as a result of California’s “shelter-in-place” order on March 17, 2020. In the 2 months that followed, there were 64% fewer breast cancer diagnoses at 21 Kaiser Permanente medical centers, compared with the same period in 2019 (250 vs. 703).
In effect, approximately 450 breast cancer patients had “disappeared,” said coauthor Annie Tang, MD, a research fellow at the University of California, San Francisco, East Bay surgery program.
“What surprised me most from our data was the sheer number of breast cancer patients that were missing,” Dr. Tang said in an interview.
A similar picture has emerged elsewhere.
In Boston, an estimated 1,438 cancerous and precancerous lesions “went missing” during the first 3 months of pandemic shutdown, according to a study from the Massachusetts General Brigham health care system.
In this study, the investigators assessed screening rates for five cancers – breast cancer (mammography), prostate cancer (prostate-specific antigen testing), colorectal cancer (colonoscopy), cervical cancer (Papanicolaou tests), and lung cancer (low-dose CT).
Screening rates during the first peak of the pandemic (March 2 to June 2, 2020) were compared with those during the preceding and following 3 months and during the same 3 months in 2019.
The results showed a pronounced drop in screening rates during the peak pandemic period, compared with the three control periods. Decreases occurred for all screening tests and ranged from –60% to –82%.
There were also significant decreases in cancer diagnoses resulting from the decreases in screening tests, ranging from –19% to –78%.
“Quantifying the actual problem made us realize how much work needs to be done to get us back to prepandemic numbers,” said senior author Quoc-Dien Trinh, MD, FACS, codirector of the Dana Farber/Brigham and Women’s prostate cancer program.
In the Canadian province of Alberta, a similar decrease in cancer diagnoses occurred during the early days of the pandemic.
By the end of 2020, Alberta was “missing” approximately 2,000 cases of invasive cancers and 1,000 cases of noninvasive cancers, Doug Stewart, MD, senior medical director at the Cancer Strategic Clinical Network (SCN) of Alberta Health Services, told this news organization.
Dr. Stewart is able to track cancer diagnoses in Alberta almost in real time through a mandatory cancer registry. Within a month of shutdown, there was a 30% decrease in diagnoses of invasive cancers and a 50% decrease “in the kind of preinvasive cancers that, for the most part, are picked up by screening programs,” said Dr. Stewart.
After the health care system opened up again in the summer, Stewart said, noninvasive cancer diagnoses continued to be 20% lower than expected. There was a 10% shortfall in invasive cancer diagnoses.
The number of diagnoses had returned to normal by December 2020. However, Dr. Stewart is worried that this fact conceals a terrible truth.
The worry is over the backlog. Although the number of diagnoses is now similar to what it was before the pandemic, “people are presenting later, and maybe the cancer is more advanced,” he speculated.
His team at Alberta Health Services is assessing whether the cancers that are being diagnosed now are more advanced. Initial results are anticipated by late April 2021.
In the United Kingdom, there was a similar halt in cancer screening as a result of the country’s lockdown. Researchers now predict an uptick in cancer diagnoses.
Ajay Aggarwal, MD, PhD, consultant clinical oncologist and associate professor at the London School of Hygiene and Tropical Medicine, and colleagues have estimated that at least 3,500 deaths from breast, colorectal, esophageal, and lung cancer will occur during the next 5 years in England that could have been avoided had it not been for the lockdown measures necessitated by the pandemic.
Speaking to this news organization, Dr. Aggarwal warned that these numbers, which are from a modeling study published in August 2020, are “extremely conservative,” because the investigators considered diagnostic delays over only a 3-month period, the analysis involved only four cancers, and it did not reflect deferral of cancer treatment.
“It felt like it was the tip of the iceberg,” Dr. Aggarwal said. He warns that more recent data suggest that “diagnostic delays are probably worse than we predicted.”
He suspects that there is more at play than screening cancellations.
In another study conducted in the United Kingdom, data show “a falling edge of referrals” from primary care to cancer centers early in the pandemic. In that study, investigators analyzed real-time weekly hospital data from eight large British hospitals and found that urgent cancer referrals fell 70% at their lowest point.
“It really surprised me that the urgent referrals dropped so drastically,” said lead author Alvina Lai, PhD, a lecturer in health data analytics at University College London.
She attributed this in part to patients’ adherence to lockdown rules. “Patients are trying to follow government guidelines to stay home and not go to [general practitioners] unless necessary,” Dr. Lai explained in an interview.
Canada, like the United Kingdom, has a publicly funded health care system. Dr. Stewart came to a similar conclusion. “Some patients who have been diagnosed with cancer ... have told me it took them an extra couple of months to even contact the family doc, because they ... didn’t want to bother the family doctor with something that wasn’t COVID, this kind of guilt. They want to do something good for society. You know, most people are just really nice people, and they don’t want to bother the health care system if they don’t have COVID,” Dr. Stewart said.
Shelley Fuld Nasso, CEO of the National Coalition for Cancer Survivorship, a nonprofit organization based in Silver Spring, Md., agreed that screening shutdowns are not the only danger. “While we agree that screening is really important, we also want to make sure patients are following up with their physicians about symptoms that they have,” she said.
“Some of the speculation or concern about increased mortality for cancer is related to screening, but some of it is related to delayed diagnosis because of not following up on symptoms. ... What concerns me is not everyone has that ability or willingness to advocate for themselves,” she said.
Speaking at a press briefing held by the American Society for Radiation Oncology on March 30, Dr. Nasso related a case involving a patient who experienced severe arm pain. In a teleconsultation with her primary care physician, her condition was diagnosed as arthritis. She was subsequently diagnosed in the ED as having multiple myeloma.
Patients who “feel fine” may postpone their checkups to avoid going to the hospital and risking exposure to COVID-19.
“Some patients are still hesitant about returning for their mammograms or coming in if they feel a breast lump,” Dr. Tang said. “That fear of COVID-19 is still out there, and we don’t know how long patients are going to delay.”
In London, Dr. Aggarwal saw a similar response to the pandemic. “People were overestimating quite significantly what their risk of death was from acquiring COVID-19, and I think that balance was never [redressed] explicitly,” he said.
Public health initiatives to rebalance the messaging are now underway.
Public Health England and National Health Service England launched their Help Us Help You campaign in October 2020. The public information campaign urges people to speak to their doctors if they were “worried about a symptom that could be cancer.”
In Canada, the provincial government in Alberta has launched a public awareness campaign that conveys the message, “cancer has not gone away.”
“Cancer is still the No. 1 cause of potential life-years lost, despite COVID,” Dr. Stewart said. “We need to do what we can to make sure there’s no slippage in survival rates.”
Dr. Tang, Dr. Chang, Dr. Lai, Dr. Stewart, and Dr. Aggarwal have disclosed no relevant financial relationship. Dr. Trinh has received personal fees from Astellas, Bayer, and Janssen and grants from Intuitive Surgical.
A version of this article first appeared on Medscape.com.
Retroperitoneal anatomy and parametrial dissection in robotic uterine artery-sparing radical trachelectomy
Replace routine preoperative testing with individualized risk assessment and indicated testing
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8
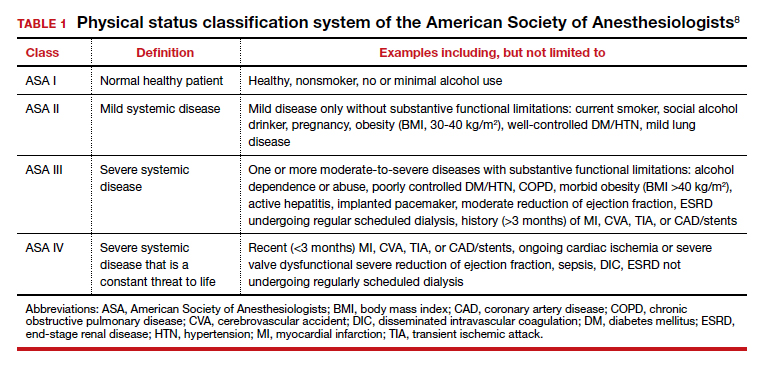
In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11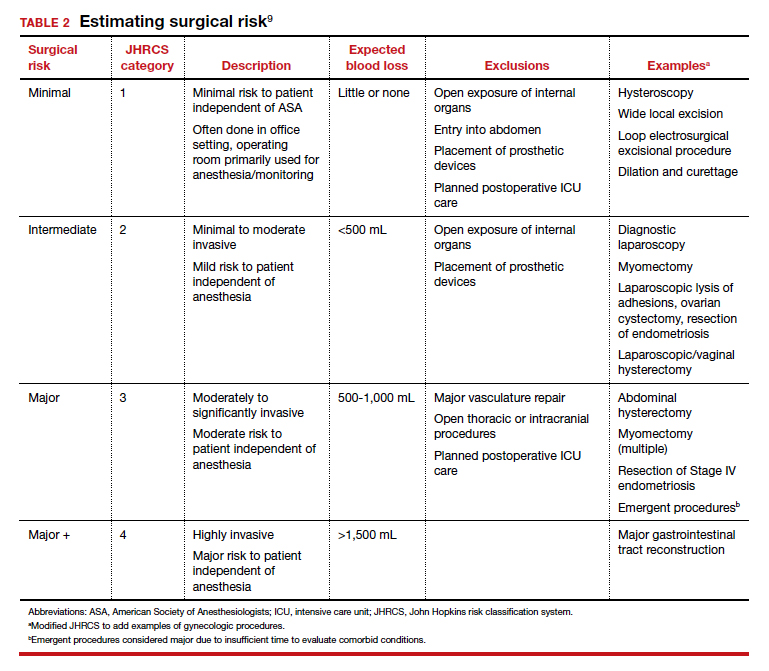
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14
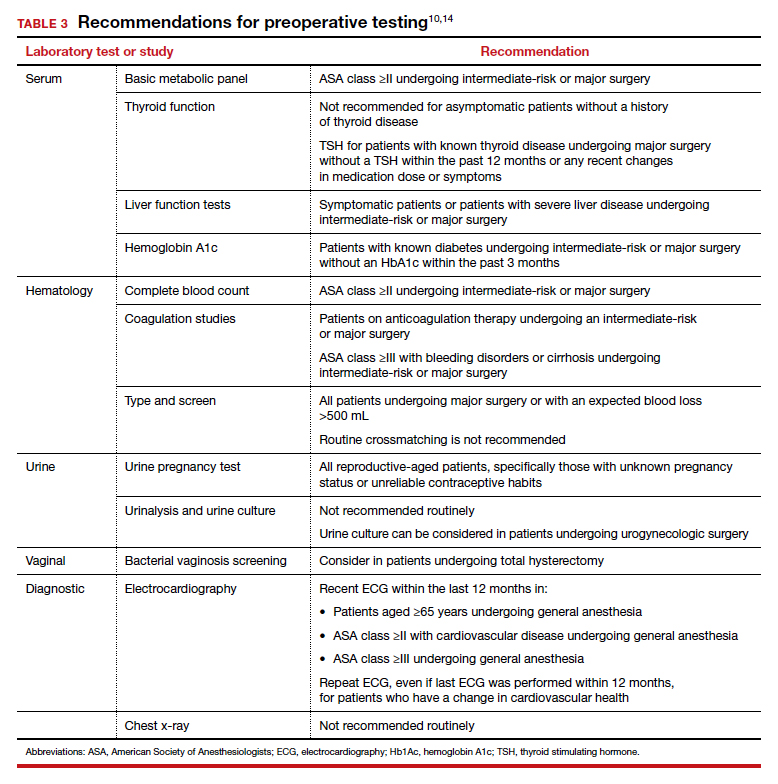
The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8

In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14

The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
CASE Patient questions need for preoperative tests
A healthy 42-year-old woman (G2P2) with abnormal uterine bleeding and a 2-cm endometrial polyp is scheduled for hysteroscopic polypectomy. After your preoperative clinic visit, the patient receives her paperwork containing information about preoperative lab work and diagnostic studies. You are asked to come into the room because she has further questions. When you arrive, the patient holds the papers out and asks, “Is all this blood work and a chest x-ray necessary? I thought I was healthy and this was a fairly simple surgery. Is there more I should be worried about?”
How would you respond?
The goal of preoperative testing is to determine which patients may be at an increased risk for experiencing an adverse perioperative event, taking into account both the inherent risks of the surgical procedure and the health of the individual patient. In the literature, the general consensus is that physicians rely too heavily on unnecessary laboratory and diagnostic testing during their preoperative assessment.1 More than 50% of patients who underwent preoperative evaluation had at least 1 unindicated test.2 These tests may result in a high frequency of abnormal findings, with less than 3% of abnormalities having clinical value or leading to a change in management.3
With health care costs accounting for almost 20% of the gross domestic product in the United States (totaling about $3.5 billion in 2017), performing unindicated preoperative testing contributes to the economic burden on health care systems, with an estimated cost of $3 to $18 million annually.4,5 In addition, unindicated tests can increase patient anxiety and necessitate follow-up testing, possibly exposing physicians to increased liability if abnormal results are not adequately investigated.6
It is time to rethink our use of routine preoperative testing.
Which tests to consider—or not: Evidence-based guidance
Professional societies, including the American Board of Internal Medicine’s Choosing Wisely campaign, promote a move away from routine testing to avoid unnecessary visits and studies. In addition, the American Society of Anesthesiologists (ASA) has published recommendations to guide preoperative testing.7 To stratify patients’ surgical risk according to their pre-existing health conditions, the ASA created a physical status classification system (TABLE 1).8

In addition to individual patient characteristics, some guidelines similarly stratify surgical procedures into minor, intermediate, and major risk. The modified Johns Hopkins surgical criteria allocates surgical risk based on expected blood loss, insensible loss, and the inherent risk of a procedure separate from anesthesia (TABLE 2).9 Despite these guidelines, physicians responsible for preoperative evaluations continue to order laboratory and diagnostic tests that are not indicated, often over concerns of case delays or cancellations.10,11
The following evidence-based recommendations provide guidance to gynecologists performing surgery for benign indications to determine which preoperative studies should be performed.
Serum chemistries
Basic metabolic panel (BMP). In both contemporary studies and earlier prospective studies, a preoperative BMP has a low likelihood of changing the surgical procedure or the patient’s management, especially in patients who are classified as ASA I and are undergoing minor- and intermediate-risk procedures.12,13 Therefore, we recommend a BMP for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.14
Thyroid function. A basic tenet of preoperative evaluation is that asymptomatic patients should not be diagnosed according to lab values prior to surgical intervention. Therefore, we do not recommend routine preoperative thyroid function testing in patients without a history of thyroid disease.10 For patients with known thyroid disease, a thyroid stimulating hormone (TSH) level should be evaluated prior to major surgery, or with any changes in medication dose or symptoms, within the past year.15
Liver function tests (LFTs). Routine screening of asymptomatic individuals without risk factors for liver disease is not recommended because there is a significantly lower incidence of abnormal lab values for LFTs than for other lab tests.16 We recommend LFTs only in symptomatic patients or patients diagnosed with severe liver disease undergoing intermediate-risk or major procedures.14
Hemoglobin A1c (HbA1c). Poorly controlled diabetes is a risk factor for poor wound healing, hospital readmission, prolonged hospitalization, and adverse events following surgery.17 We recommend that HbA1c levels be drawn only for patients with known diabetes undergoing intermediate-risk or major surgery who do not have an available lab value within the past 3 months.14
Continue to: Hematologic studies...
Hematologic studies
Complete blood count (CBC). Many patients undergoing gynecologic procedures may have unreported or undiagnosed anemia secondary to abnormal uterine bleeding, which also may encompass heavy menstrual bleeding. With an abnormal CBC likely to affect preoperative management, assessment of preoperative hemoglobin levels is critical so that hemoglobin levels can be appropriately corrected before surgery. We therefore recommend obtaining a CBC for patients in class ASA II or higher who are undergoing intermediate-risk or major surgery.10,14
Coagulation studies. Preoperative coagulation studies are unlikely to uncover previously undiagnosed inherited coagulopathies, which are generally uncommon in the general population, and they do not predict operative bleeding when ordered unnecessarily.18,19 Therefore, we recommend preoperative coagulation studies only in patients 1) currently on anticoagulation therapy undergoing intermediate-risk or major surgery or 2) in class ASA III or higher with bleeding disorders or cirrhosis undergoing intermediate-risk or major surgery.14
Type and screen (T&S). Complicated algorithms have been proposed to determine when a preoperative T&S is necessary, but these may be impractical for busy gynecologists.20 We recommend a T&S within 72 hours, or on the day, of surgery for all patients undergoing major surgery, including hysterectomy, or with an anticipated blood loss of more than 500 mL; routine crossmatching of blood is not recommended.10,14
Urologic studies
Urine pregnancy test. Although the probability of a positive pregnancy test is likely very low, its occurrence frequently leads to the cancellation of surgery. We therefore recommend a preoperative urine pregnancy test, particularly in reproductive-aged patients with unknown pregnancy status or unreliable contraceptive habits.14 Preoperative urine pregnancy testing, even in patients who report sexual inactivity, ideally should be individualized and based on risk of fetal harm during or subsequent to surgery. Surgeries involving the uterus, or those involving possible teratogens like radiation, also should be considered when making recommendations for testing.
Urinalysis and urine culture. In asymptomatic patients undergoing general gynecologic procedures, a routine preoperative urinalysis and urine culture are of little value.18 However, among patients undergoing a urogynecologic surgical procedure, the risk of a postoperative urinary tract infection is higher than among patients undergoing a nonurogynecologic procedure.21,22 Therefore, we typically do not recommend routine preoperative urinalysis or urine culture, but a preoperative urine culture may be beneficial in patients undergoing urogynecologic surgery.14
Continue to: Diagnostic studies...
Diagnostic studies
Electrocardiography (ECG). The absolute difference in cardiovascular death is less than 1% among patients with and without ECG abnormalities undergoing a noncardiac procedure with minimal to moderate risk; therefore, routine ECG for low-risk patients should not be performed.23 Instead, ECG should be performed in patients with known coronary artery disease or structural heart disease and in patients aged 65 years and older, since age older than 65 years is an independent predictor of significant ECG abnormalities.24,25 We therefore recommend that the following individuals have an ECG within the last 12 months: patients aged 65 years and older, patients in class ASA II or higher with cardiovascular disease, and patients in class ASA III or higher undergoing general anesthesia. If there is a change in cardiovascular health since the most recent ECG—even if it was performed within 12 months—a repeat ECG is warranted.10,14
Chest x-ray. Despite a high rate of abnormalities seen on routine and indicated chest x-rays, there is no significant difference in perioperative pulmonary complications among patients with a normal or abnormal chest x-ray.16 Rather than changing surgical management, these abnormal results are more likely to lead to the cancellation or postponement of a surgical procedure.7 We therefore recommend against routine preoperative chest x-ray.14

The bottom line
Preoperative testing serves as an additional component of surgical planning. The fact is, however, that abnormal test results are common and frequently do not correlate with surgical outcomes.26 Instead, they can lead to unnecessary surgical procedure cancellations or postponements, undue anxiety in patients, increased liability among physicians, and rising health care costs.5-7
Rather than overly relying on routine laboratory or diagnostic studies, the history and physical examination should continue to be the cornerstone for surgeons responsible for assessing surgical risk. With individualized risk assessment, specific, indicated testing rather than routine nonspecific testing can be obtained.10,14 In short, low-risk patients undergoing noncardiac surgery are unlikely to benefit from preoperative ECG, chest x-ray, or routine laboratory testing without clinical indication. ●
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
- Kachalia A, Berg A, Fagerlin A, et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162:100-108.
- Onuoha OC, Hatch M, Miano TA, et al. The incidence of un-indicated preoperative testing in a tertiary academic ambulatory center: a retrospective cohort study. Perioper Med. 2015; 4:14.
- Kaplan EB, Sheiner LB, Boeckmann AJ, et al. The usefulness of preoperative laboratory screening. JAMA. 1985;253:3576-3581.
- Centers for Disease Control and Prevention National Center for Health Statistics. Table 42: Gross domestic product, national health expenditures, per capita amounts, percent distribution, and average annual percent change: United States, selected years 1960-2017. https://www.cdc.gov/nchs/ data/hus/2018/042.pdf. Accessed July 2020.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256:518-528.
- O’Neill F, Carter E, Pink N, et al. Routine preoperative tests for elective surgery: summary of updated NICE guidance. BMJ. 2016;354: i3292.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116:522-538.
- American Society of Anesthesiologists. ASA physical status classification system. https://www.asahq.org/standardsand-guidelines/asa-physical-status-classification-system. Accessed July 2020.
- Pasternak LR, Johns A. Ambulatory gynaecological surgery: risk and assessment. Best Pract Res Clin Obstet Gynaecol. 2005;19:663-679.
- Shields J, Lupo A, Walsh T, et al. Preoperative evaluation for gynecologic surgery: a guide to judicious, evidence-based testing. Curr Opin Obstet Gynecol. 2018;30:252-259.
- Sigmund AE, Stevens ER, Blitz JD, et al. Use of preoperative testing and physicians’ response to professional society guidance. JAMA Intern Med. 2015;175:1352-1359.
- St Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116:694-700.
- De Sousa Soares D, Brandao RR, Mourao MR, et al. Relevance of routine testing in low-risk patients undergoing minor and medium surgical procedures. Braz J Anesthesiol. 2013;63:197-201.
- Shields J, Kho KA. Preoperative evaluation for minimally invasive gynecologic surgery: what is the best evidence and recommendations for clinical practice. J Minim Invasive Gynecol. 2019;26:312-320.
- Palace MR. Perioperative management of thyroid dysfunction. Health Serv Insights. 2017;10:1178632916689677.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7-40.
- Jehan F, Khan M, Sakran JV, et al. Perioperative glycemic control and postoperative complications in patients undergoing emergency general surgery: what is the role of plasma hemoglobin A1c? J Trauma Acute Care Surg. 2018;84:112-117.
- Feely MA, Collins CS, Daniels PR, et al. Preoperative testing before noncardiac surgery: guidelines and recommendations. Am Fam Physician. 2013;87:414-418.
- Rusk MH. Avoiding unnecessary preoperative testing. Med Clin North Am. 2016;100:1003-1008.
- Dexter F, Ledolter J, Davis E, et al. Systematic criteria for type and screen based on procedure’s probability of erythrocyte transfusion. Anesthesiology. 2012;116:768-778.
- Gehrich AP, Lustik MB, Mehr AA, et al. Risk of postoperative urinary tract infections following midurethral sling operations in women undergoing hysterectomy. Int Urogynecol J. 2016;27:483-490.
- American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 195 summary: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:1177- 1179.
- Noordzij PG, Boersma E, Bax JJ, et al. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol, 2006;97: 1103-1106.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/ AHA guideline on perioperative cardiovascular examination and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2215-2245.
- Correll DJ, Hepner DL, Chang C, et al. Preoperative electrocardiograms: patient factors predictive of abnormalities. Anesthesiology. 2009;110:1217-1122.
- Fritsch G, Flamm M, Hepner DL, et al. Abnormal preoperative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56:339-350.
For obese postmenopausal women, what options may decrease endometrial cancer risk?
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
Prophylactic antibiotics for myomectomy?
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
In the 1990s, researchers found that patients undergoing any type of surgical procedure were more than twice as likely to die if they developed postsurgical infection.1 Work to reduce surgical site infection (SSI) has and does continue, with perioperative antibiotics representing a good part of that effort. The American College of Obstetricians and Gynecologists currently recommends such antibiotic therapy for women undergoing laparotomy and laparoscopic hysterectomy.2 ACOG does not, however, recommend prophylactic antibiotics for myomectomy procedures.3 Rates of infection for hysterectomy have been reported to be 3.9% for abdominal and 1.4% for minimally invasive approaches.4
To determine the current use of antibiotics during myomectomy and associated rates of SSI at their institutions, Dipti Banerjee, MD, and colleagues conducted a retrospective analysis of women undergoing laparoscopic or abdominal myomectomy between February 2013 and December 2017 at the University of California, Los Angeles and Hoag Memorial Hospital in Orange County, California. They presented their study results at AAGL’s 49th Global Congress on MIGS, held virtually November 6-14, 2020.3
Rate of SSI after myomectomy
A total of 620 women underwent laparoscopic myomectomy and 563 underwent open myomectomy during the study period. Antibiotics were used in 76.9% of cases. SSI developed within 6 weeks of surgery in 34 women (2.9%) overall. The women undergoing abdominal myomectomy without antibiotics were more likely to experience SSI than the women who received antibiotics (odds ratio [OR], 4.89; confidence interval [CI], 1.80–13.27; P = .0006). For laparoscopic myomectomy, antibiotic use did not affect the odds of developing SSI (OR, 1.08; CI, 0.35–3.35).
Antibiotics were more likely to be used in certain cases
Antibiotics were more likely to be administered for patients who:
- were obese (body mass index ≥30 kg/m2) (P = .009)
- underwent previous abdominal surgery (P = .001)
- underwent laparotomy (P <.0001)
- had endometrial cavity entry (P <.0001)
- had >1 fibroid (P = .0004) or an aggregate fibroid weight >500 g (P <.0001).
More data on antibiotics for myomectomy
In a retrospective study conducted at 2 academic hospitals in Boston, Massachusetts, 1,211 women underwent myomectomy from 2009 to 2016. (Exclusions were use of vaginal or hysteroscopic myomectomy, chromopertubation, or conversion to hysterectomy.) More than 92% of the women received perioperative antibiotics at the time of surgery. Although demographics were similar between women receiving and not receiving antibiotics, women who received antibiotics were more likely to have longer operative times (median 140 vs 85 min), a greater myoma burden (7 vs 2 myomas removed and weight 255 vs 53 g), and lose blood during the procedure (137 vs 50 mL). These women also were 4 times less likely to have surgical site infection (adjusted OR, 3.77; 95% CI, 1.30–10.97; P = .015).5,6
Banerjee and colleagues say that their California study demonstrates “that the majority of surgeons elect to use antibiotics prophylactically” during myomectomy, despite current ACOG guidelines, and that their findings of benefit for abdominal myomectomy but not for laparoscopic myomectomy should inform future guidance on antibiotics for myomectomy surgery.3
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.
- Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725-730.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol. 2018;131:e172-e189.
- Banerjee D, Dejbakhsh S, Patel HH, et al. Perioperative antibiotic prophylaxis in myomectomy surgery. Paper presented at 49th Annual Meeting of the AAGL; November 2020.
- Uppal S, Harris J, Al-Niaimi A. Prophylactic antibiotic choice and risk of surgical site infection after hysterectomy. Obstet Gynecol. 2016;127:321-329.
- Kim AJ, Clark NV, Jansen LJ, et al. Perioperative antibiotic use and associated infectious outcomes at the time of myomectomy. Obstet Gynecol. 2019;133:626-635.
- Rebar RW. Should perioperative antibiotics at myomectomy be universal? NEJM J Watch. March 11, 2019.