User login
European marketing of Picato suspended while skin cancer risk reviewed
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
Treating AKs with PDT, other options
SEATTLE – While David Pariser, MD, said during a presentation at the annual Coastal Dermatology Symposium.
“My personal view is that, no matter how good other treatments are eventually going to be, we’re never going to give that up,” Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
During the presentation, he emphasized that it isn’t always clear which actinic keratosis (AK) should be treated and which can be left alone, since most AKs don’t progress to squamous cell carcinoma (SCC). “We know that most squamous cell carcinomas arise near AKs, and many of them have histologic evidence” of AK/SCC continuum at the periphery, he said. Sun protection reduces the incidence of AKs and the incidence of nonmelanoma skin cancer, “so it’s a logical conclusion that treating AKs reduces the development of SCCs, but there are no data to show that.”
Generally, treatment decisions are made based on the presence of symptoms, location, or appearance; if the area is irritated; or there is a progressive or unusual appearance, especially if hyperkeratotic. Physician or patient concerns about cancer can prompt treatment, as should a history of multiple skin cancers or the presence of immunosuppression, he said.
Treatment options include cryosurgery, surgery, topical agents, and photodynamic therapy (PDT); Dr. Pariser focused on the latter because it is a special interest of his.
Field cancerization is based on the idea that a broad area of cells may be at risk for developing into SCC, rather than just individual AKs. Treatment with methyl 5-aminolevulinate (MAL) can reveal the extent of a problem. In some patients, “you can see a lot of fluorescence in areas that look reasonably clinically normal. So this is a piece of evidence of this field cancerization, that maybe we shouldn’t be treating individual AKs, but larger areas,” Dr. Pariser said.
With PDT, there has been some debate about how long to leave the photosensitizer on the skin before applying the light. The longer it remains, the more it spreads to nerves, which can lead to pain during the procedure. A clinical trial comparing 1-, 2-, and 3-hour wait times showed no difference in efficacy. “So 1 hour is what I do for AKs, that’s it,” Dr. Pariser said.
There are two Food and Drug Administration–approved PDT systems, a blue-light system combined with aminolevulinic acid (ALA) and a newer red-light system combined with a nanoemulsion of ALA 7.8% and a proprietary 635-nm red LED light. The nanoemulsion has the theoretical advantage in that it can penetrate more deeply into the epidermis, though this isn’t really an issue when treating AKs, according to Dr. Pariser.
A study comparing nanoemulsion of ALA, compared with a MAL cream, found the nanoemulsion to be superior in achieving complete clearance of all lesions at 12 weeks (78.2% vs. 64.2%; P less than .05). Both treatments achieved best efficacy with LED lamps, and the proprietary red light may reduce pain by allowing use of lower light intensity (Br J Dermatol. 2012 Jan; 166[1]:137-46).
Another study, Dr. Pariser said, looked at whether occlusion during drug incubation improves outcomes of blue light ALA-PDT (J Drugs Dermatol. 2012;11[12]:1483-9). Patients underwent split occlusion on the upper extremities before undergoing blue-light treatment. The median clearance rate of AKs at 8 weeks was higher with occlusion, compared with the nonoccluded areas (75% vs. 47%; P = .006), and at 12 weeks, after a second treatment (89% vs. 70%; P = .00029). There was a higher efficacy with a 3-hour incubation period, compared with studies using a 2-hour incubation period.
Application of heat can also boost success rates by increasing the synthesis of the photoactive agent, Dr. Pariser said. One study found that a simple heating pad applied to the area treated with ALA-PDT and blue light led to an 88% reduction in lesions at 8 and 24 weeks, compared with a reduction of 71% at 8 weeks and 68% at 24 weeks without heat (P less than .0001). “So if you want to give PDT a little extra oomph, add occlusion and heat,” he commented.
He also pointed out the availability of a new 4% 5-fluorouracil cream that contains peanut oil, which has similar efficacy to 5% 5-fluorouracil cream but has been associated with less pruritus, stinging/burning, edema, crusting, scaling/dryness, erosion, and erythema (J Drugs Dermatol. 2016 Oct 1;15[10]: 1218-24).
Dr. Pariser is an investigator and consultant for DUSA/Sun Pharma, Photocure, LEO Pharma, and Biofrontera. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – While David Pariser, MD, said during a presentation at the annual Coastal Dermatology Symposium.
“My personal view is that, no matter how good other treatments are eventually going to be, we’re never going to give that up,” Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
During the presentation, he emphasized that it isn’t always clear which actinic keratosis (AK) should be treated and which can be left alone, since most AKs don’t progress to squamous cell carcinoma (SCC). “We know that most squamous cell carcinomas arise near AKs, and many of them have histologic evidence” of AK/SCC continuum at the periphery, he said. Sun protection reduces the incidence of AKs and the incidence of nonmelanoma skin cancer, “so it’s a logical conclusion that treating AKs reduces the development of SCCs, but there are no data to show that.”
Generally, treatment decisions are made based on the presence of symptoms, location, or appearance; if the area is irritated; or there is a progressive or unusual appearance, especially if hyperkeratotic. Physician or patient concerns about cancer can prompt treatment, as should a history of multiple skin cancers or the presence of immunosuppression, he said.
Treatment options include cryosurgery, surgery, topical agents, and photodynamic therapy (PDT); Dr. Pariser focused on the latter because it is a special interest of his.
Field cancerization is based on the idea that a broad area of cells may be at risk for developing into SCC, rather than just individual AKs. Treatment with methyl 5-aminolevulinate (MAL) can reveal the extent of a problem. In some patients, “you can see a lot of fluorescence in areas that look reasonably clinically normal. So this is a piece of evidence of this field cancerization, that maybe we shouldn’t be treating individual AKs, but larger areas,” Dr. Pariser said.
With PDT, there has been some debate about how long to leave the photosensitizer on the skin before applying the light. The longer it remains, the more it spreads to nerves, which can lead to pain during the procedure. A clinical trial comparing 1-, 2-, and 3-hour wait times showed no difference in efficacy. “So 1 hour is what I do for AKs, that’s it,” Dr. Pariser said.
There are two Food and Drug Administration–approved PDT systems, a blue-light system combined with aminolevulinic acid (ALA) and a newer red-light system combined with a nanoemulsion of ALA 7.8% and a proprietary 635-nm red LED light. The nanoemulsion has the theoretical advantage in that it can penetrate more deeply into the epidermis, though this isn’t really an issue when treating AKs, according to Dr. Pariser.
A study comparing nanoemulsion of ALA, compared with a MAL cream, found the nanoemulsion to be superior in achieving complete clearance of all lesions at 12 weeks (78.2% vs. 64.2%; P less than .05). Both treatments achieved best efficacy with LED lamps, and the proprietary red light may reduce pain by allowing use of lower light intensity (Br J Dermatol. 2012 Jan; 166[1]:137-46).
Another study, Dr. Pariser said, looked at whether occlusion during drug incubation improves outcomes of blue light ALA-PDT (J Drugs Dermatol. 2012;11[12]:1483-9). Patients underwent split occlusion on the upper extremities before undergoing blue-light treatment. The median clearance rate of AKs at 8 weeks was higher with occlusion, compared with the nonoccluded areas (75% vs. 47%; P = .006), and at 12 weeks, after a second treatment (89% vs. 70%; P = .00029). There was a higher efficacy with a 3-hour incubation period, compared with studies using a 2-hour incubation period.
Application of heat can also boost success rates by increasing the synthesis of the photoactive agent, Dr. Pariser said. One study found that a simple heating pad applied to the area treated with ALA-PDT and blue light led to an 88% reduction in lesions at 8 and 24 weeks, compared with a reduction of 71% at 8 weeks and 68% at 24 weeks without heat (P less than .0001). “So if you want to give PDT a little extra oomph, add occlusion and heat,” he commented.
He also pointed out the availability of a new 4% 5-fluorouracil cream that contains peanut oil, which has similar efficacy to 5% 5-fluorouracil cream but has been associated with less pruritus, stinging/burning, edema, crusting, scaling/dryness, erosion, and erythema (J Drugs Dermatol. 2016 Oct 1;15[10]: 1218-24).
Dr. Pariser is an investigator and consultant for DUSA/Sun Pharma, Photocure, LEO Pharma, and Biofrontera. This publication and Global Academy for Medical Education are owned by the same parent company.
SEATTLE – While David Pariser, MD, said during a presentation at the annual Coastal Dermatology Symposium.
“My personal view is that, no matter how good other treatments are eventually going to be, we’re never going to give that up,” Dr. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education.
During the presentation, he emphasized that it isn’t always clear which actinic keratosis (AK) should be treated and which can be left alone, since most AKs don’t progress to squamous cell carcinoma (SCC). “We know that most squamous cell carcinomas arise near AKs, and many of them have histologic evidence” of AK/SCC continuum at the periphery, he said. Sun protection reduces the incidence of AKs and the incidence of nonmelanoma skin cancer, “so it’s a logical conclusion that treating AKs reduces the development of SCCs, but there are no data to show that.”
Generally, treatment decisions are made based on the presence of symptoms, location, or appearance; if the area is irritated; or there is a progressive or unusual appearance, especially if hyperkeratotic. Physician or patient concerns about cancer can prompt treatment, as should a history of multiple skin cancers or the presence of immunosuppression, he said.
Treatment options include cryosurgery, surgery, topical agents, and photodynamic therapy (PDT); Dr. Pariser focused on the latter because it is a special interest of his.
Field cancerization is based on the idea that a broad area of cells may be at risk for developing into SCC, rather than just individual AKs. Treatment with methyl 5-aminolevulinate (MAL) can reveal the extent of a problem. In some patients, “you can see a lot of fluorescence in areas that look reasonably clinically normal. So this is a piece of evidence of this field cancerization, that maybe we shouldn’t be treating individual AKs, but larger areas,” Dr. Pariser said.
With PDT, there has been some debate about how long to leave the photosensitizer on the skin before applying the light. The longer it remains, the more it spreads to nerves, which can lead to pain during the procedure. A clinical trial comparing 1-, 2-, and 3-hour wait times showed no difference in efficacy. “So 1 hour is what I do for AKs, that’s it,” Dr. Pariser said.
There are two Food and Drug Administration–approved PDT systems, a blue-light system combined with aminolevulinic acid (ALA) and a newer red-light system combined with a nanoemulsion of ALA 7.8% and a proprietary 635-nm red LED light. The nanoemulsion has the theoretical advantage in that it can penetrate more deeply into the epidermis, though this isn’t really an issue when treating AKs, according to Dr. Pariser.
A study comparing nanoemulsion of ALA, compared with a MAL cream, found the nanoemulsion to be superior in achieving complete clearance of all lesions at 12 weeks (78.2% vs. 64.2%; P less than .05). Both treatments achieved best efficacy with LED lamps, and the proprietary red light may reduce pain by allowing use of lower light intensity (Br J Dermatol. 2012 Jan; 166[1]:137-46).
Another study, Dr. Pariser said, looked at whether occlusion during drug incubation improves outcomes of blue light ALA-PDT (J Drugs Dermatol. 2012;11[12]:1483-9). Patients underwent split occlusion on the upper extremities before undergoing blue-light treatment. The median clearance rate of AKs at 8 weeks was higher with occlusion, compared with the nonoccluded areas (75% vs. 47%; P = .006), and at 12 weeks, after a second treatment (89% vs. 70%; P = .00029). There was a higher efficacy with a 3-hour incubation period, compared with studies using a 2-hour incubation period.
Application of heat can also boost success rates by increasing the synthesis of the photoactive agent, Dr. Pariser said. One study found that a simple heating pad applied to the area treated with ALA-PDT and blue light led to an 88% reduction in lesions at 8 and 24 weeks, compared with a reduction of 71% at 8 weeks and 68% at 24 weeks without heat (P less than .0001). “So if you want to give PDT a little extra oomph, add occlusion and heat,” he commented.
He also pointed out the availability of a new 4% 5-fluorouracil cream that contains peanut oil, which has similar efficacy to 5% 5-fluorouracil cream but has been associated with less pruritus, stinging/burning, edema, crusting, scaling/dryness, erosion, and erythema (J Drugs Dermatol. 2016 Oct 1;15[10]: 1218-24).
Dr. Pariser is an investigator and consultant for DUSA/Sun Pharma, Photocure, LEO Pharma, and Biofrontera. This publication and Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM COASTAL DERM
Twitter Chat: Skin Cancer

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.
Fluorouracil beats other actinic keratosis treatments in head-to-head trial
A head-to-head comparison of four commonly used field-directed treatments for actinic keratosis (AK) found that 5% fluorouracil cream was the most effective at reducing the size of lesions.
In a study published in the March 7 issue of the New England Journal of Medicine, researchers reported the outcomes of a multicenter, single-blind trial in 602 patients with five or more AK lesions in one continuous area on the head measuring 25-100 cm2. Patients were randomized to treatment with either 5% fluorouracil cream, 5% imiquimod cream, methyl aminolevulinate photodynamic therapy (MAL-PDT), or 0.015% ingenol mebutate gel.
Overall, 74.7% of patients who received fluorouracil cream achieved treatment success – defined as at least a 75% reduction in lesion size at 12 months after the end of treatment – compared with 53.9% of patients treated with imiquimod, 37.7% of those treated with MAL-PDT, and 28.9% of those treated with ingenol mebutate. The differences between fluorouracil and the other treatments was significant.
Maud H.E. Jansen, MD, and Janneke P.H.M. Kessels, MD, of the department of dermatology at Maastricht (the Netherlands) University Medical Center and their coauthors pointed out that, while there was plenty of literature about different AK treatments, there were few head-to-head comparisons and many studies were underpowered or had different outcome measures.
Even when the analysis was restricted to patients with grade I or II lesions, fluorouracil remained the most effective treatment, with 75.3% of patients achieving treatment success, compared with 52.6% with imiquimod, 38.7% with MAL-PDT, and 30.2% with ingenol mebutate.
There were not enough patients with more severe grade III lesions to enable a separate analysis of their outcomes; 49 (7.9%) of patients in the study had at least one grade III lesion.
The authors noted that many previous studies had excluded patients with grade III lesions. “Exclusion of patients with grade III lesions was associated with slightly higher rates of success in the fluorouracil, MAL-PDT, and ingenol mebutate groups than the rates in the unrestricted analysis,” they wrote. The inclusion of patients with grade III AK lesions in this trial made it “more representative of patients seen in daily practice,” they added.
Treatment failure – less than 75% clearance of actinic keratosis at 3 months after the final treatment – was seen after one treatment cycle in 14.8% of patients treated with fluorouracil, 37.2% of patients on imiquimod, 34.6% of patients given photodynamic therapy, and 47.8% of patients on ingenol mebutate therapy.
All these patients were offered a second treatment cycle, but those treated with imiquimod, PDT, and ingenol mebutate were less likely to undergo a second treatment.
The authors suggested that the higher proportion of patients in the fluorouracil group who were willing to undergo a second round of therapy suggests they may have experienced less discomfort and inconvenience with the therapy to begin with, compared with those treated with the other regimens.
Full adherence to treatment was more common in the ingenol mebutate (98.7% of patients) and MAL-PDT (96.8%) groups, compared with the fluorouracil (88.7%) and imiquimod (88.2%) groups. However, patients in the fluorouracil group reported greater levels of patient satisfaction and improvements in health-related quality of life than did patients in the other treatment arms of the study.
No serious adverse events were reported with any of the treatments, and no patients stopped treatment because of adverse events. However, reports of moderate or severe crusts were highest among patients treated with imiquimod, and moderate to severe vesicles or bullae were highest among those treated with ingenol mebutate. Severe pain and severe burning sensation were significantly more common among those treated with MAL-PDT.
While the study had some limitations, the results “could affect treatment choices in both dermatology and primary care,” the authors wrote, pointing out how common AKs are in practice, accounting for 5 million dermatology visits in the United States every year. When considering treatment costs, “fluorouracil is also the most attractive option,” they added. “It is expected that a substantial cost reduction could be achieved with more uniformity in care and the choice for effective therapy.”
The study was supported by the Netherlands Organization for Health Research and Development. Five of the 11 authors declared conference costs, advisory board fees, or trial supplies from private industry, including from manufacturers of some of the products in the study. The remaining authors had no disclosures.
SOURCE: Jansen M et al. N Engl J Med. 2019;380:935-46.
A head-to-head comparison of four commonly used field-directed treatments for actinic keratosis (AK) found that 5% fluorouracil cream was the most effective at reducing the size of lesions.
In a study published in the March 7 issue of the New England Journal of Medicine, researchers reported the outcomes of a multicenter, single-blind trial in 602 patients with five or more AK lesions in one continuous area on the head measuring 25-100 cm2. Patients were randomized to treatment with either 5% fluorouracil cream, 5% imiquimod cream, methyl aminolevulinate photodynamic therapy (MAL-PDT), or 0.015% ingenol mebutate gel.
Overall, 74.7% of patients who received fluorouracil cream achieved treatment success – defined as at least a 75% reduction in lesion size at 12 months after the end of treatment – compared with 53.9% of patients treated with imiquimod, 37.7% of those treated with MAL-PDT, and 28.9% of those treated with ingenol mebutate. The differences between fluorouracil and the other treatments was significant.
Maud H.E. Jansen, MD, and Janneke P.H.M. Kessels, MD, of the department of dermatology at Maastricht (the Netherlands) University Medical Center and their coauthors pointed out that, while there was plenty of literature about different AK treatments, there were few head-to-head comparisons and many studies were underpowered or had different outcome measures.
Even when the analysis was restricted to patients with grade I or II lesions, fluorouracil remained the most effective treatment, with 75.3% of patients achieving treatment success, compared with 52.6% with imiquimod, 38.7% with MAL-PDT, and 30.2% with ingenol mebutate.
There were not enough patients with more severe grade III lesions to enable a separate analysis of their outcomes; 49 (7.9%) of patients in the study had at least one grade III lesion.
The authors noted that many previous studies had excluded patients with grade III lesions. “Exclusion of patients with grade III lesions was associated with slightly higher rates of success in the fluorouracil, MAL-PDT, and ingenol mebutate groups than the rates in the unrestricted analysis,” they wrote. The inclusion of patients with grade III AK lesions in this trial made it “more representative of patients seen in daily practice,” they added.
Treatment failure – less than 75% clearance of actinic keratosis at 3 months after the final treatment – was seen after one treatment cycle in 14.8% of patients treated with fluorouracil, 37.2% of patients on imiquimod, 34.6% of patients given photodynamic therapy, and 47.8% of patients on ingenol mebutate therapy.
All these patients were offered a second treatment cycle, but those treated with imiquimod, PDT, and ingenol mebutate were less likely to undergo a second treatment.
The authors suggested that the higher proportion of patients in the fluorouracil group who were willing to undergo a second round of therapy suggests they may have experienced less discomfort and inconvenience with the therapy to begin with, compared with those treated with the other regimens.
Full adherence to treatment was more common in the ingenol mebutate (98.7% of patients) and MAL-PDT (96.8%) groups, compared with the fluorouracil (88.7%) and imiquimod (88.2%) groups. However, patients in the fluorouracil group reported greater levels of patient satisfaction and improvements in health-related quality of life than did patients in the other treatment arms of the study.
No serious adverse events were reported with any of the treatments, and no patients stopped treatment because of adverse events. However, reports of moderate or severe crusts were highest among patients treated with imiquimod, and moderate to severe vesicles or bullae were highest among those treated with ingenol mebutate. Severe pain and severe burning sensation were significantly more common among those treated with MAL-PDT.
While the study had some limitations, the results “could affect treatment choices in both dermatology and primary care,” the authors wrote, pointing out how common AKs are in practice, accounting for 5 million dermatology visits in the United States every year. When considering treatment costs, “fluorouracil is also the most attractive option,” they added. “It is expected that a substantial cost reduction could be achieved with more uniformity in care and the choice for effective therapy.”
The study was supported by the Netherlands Organization for Health Research and Development. Five of the 11 authors declared conference costs, advisory board fees, or trial supplies from private industry, including from manufacturers of some of the products in the study. The remaining authors had no disclosures.
SOURCE: Jansen M et al. N Engl J Med. 2019;380:935-46.
A head-to-head comparison of four commonly used field-directed treatments for actinic keratosis (AK) found that 5% fluorouracil cream was the most effective at reducing the size of lesions.
In a study published in the March 7 issue of the New England Journal of Medicine, researchers reported the outcomes of a multicenter, single-blind trial in 602 patients with five or more AK lesions in one continuous area on the head measuring 25-100 cm2. Patients were randomized to treatment with either 5% fluorouracil cream, 5% imiquimod cream, methyl aminolevulinate photodynamic therapy (MAL-PDT), or 0.015% ingenol mebutate gel.
Overall, 74.7% of patients who received fluorouracil cream achieved treatment success – defined as at least a 75% reduction in lesion size at 12 months after the end of treatment – compared with 53.9% of patients treated with imiquimod, 37.7% of those treated with MAL-PDT, and 28.9% of those treated with ingenol mebutate. The differences between fluorouracil and the other treatments was significant.
Maud H.E. Jansen, MD, and Janneke P.H.M. Kessels, MD, of the department of dermatology at Maastricht (the Netherlands) University Medical Center and their coauthors pointed out that, while there was plenty of literature about different AK treatments, there were few head-to-head comparisons and many studies were underpowered or had different outcome measures.
Even when the analysis was restricted to patients with grade I or II lesions, fluorouracil remained the most effective treatment, with 75.3% of patients achieving treatment success, compared with 52.6% with imiquimod, 38.7% with MAL-PDT, and 30.2% with ingenol mebutate.
There were not enough patients with more severe grade III lesions to enable a separate analysis of their outcomes; 49 (7.9%) of patients in the study had at least one grade III lesion.
The authors noted that many previous studies had excluded patients with grade III lesions. “Exclusion of patients with grade III lesions was associated with slightly higher rates of success in the fluorouracil, MAL-PDT, and ingenol mebutate groups than the rates in the unrestricted analysis,” they wrote. The inclusion of patients with grade III AK lesions in this trial made it “more representative of patients seen in daily practice,” they added.
Treatment failure – less than 75% clearance of actinic keratosis at 3 months after the final treatment – was seen after one treatment cycle in 14.8% of patients treated with fluorouracil, 37.2% of patients on imiquimod, 34.6% of patients given photodynamic therapy, and 47.8% of patients on ingenol mebutate therapy.
All these patients were offered a second treatment cycle, but those treated with imiquimod, PDT, and ingenol mebutate were less likely to undergo a second treatment.
The authors suggested that the higher proportion of patients in the fluorouracil group who were willing to undergo a second round of therapy suggests they may have experienced less discomfort and inconvenience with the therapy to begin with, compared with those treated with the other regimens.
Full adherence to treatment was more common in the ingenol mebutate (98.7% of patients) and MAL-PDT (96.8%) groups, compared with the fluorouracil (88.7%) and imiquimod (88.2%) groups. However, patients in the fluorouracil group reported greater levels of patient satisfaction and improvements in health-related quality of life than did patients in the other treatment arms of the study.
No serious adverse events were reported with any of the treatments, and no patients stopped treatment because of adverse events. However, reports of moderate or severe crusts were highest among patients treated with imiquimod, and moderate to severe vesicles or bullae were highest among those treated with ingenol mebutate. Severe pain and severe burning sensation were significantly more common among those treated with MAL-PDT.
While the study had some limitations, the results “could affect treatment choices in both dermatology and primary care,” the authors wrote, pointing out how common AKs are in practice, accounting for 5 million dermatology visits in the United States every year. When considering treatment costs, “fluorouracil is also the most attractive option,” they added. “It is expected that a substantial cost reduction could be achieved with more uniformity in care and the choice for effective therapy.”
The study was supported by the Netherlands Organization for Health Research and Development. Five of the 11 authors declared conference costs, advisory board fees, or trial supplies from private industry, including from manufacturers of some of the products in the study. The remaining authors had no disclosures.
SOURCE: Jansen M et al. N Engl J Med. 2019;380:935-46.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Elephantiasis Nostras Verrucosa Secondary to Scleroderma
To the Editor:
Elephantiasis nostras verrucosa (ENV) is a skin disorder caused by marked underlying lymphedema that leads to hyperkeratosis, papillomatosis, and verrucous growths on the epidermis.1 The pathophysiology of ENV relates to noninfectious lymphatic obstruction and lymphatic fibrosis secondary to venous stasis, malignancy, radiation therapy, or trauma.2 We present an unusual case of lymphedema and subsequent ENV limited to the arms and hands in a patient with scleroderma, an autoimmune fibrosing disorder.
A 54-year-old woman with a 5-year history of scleroderma presented to our dermatology clinic for treatment of progressive skin changes including pruritus, tightness, finger ulcerations, and pus exuding from papules on the dorsal arms and hands. She had been experiencing several systemic symptoms including dysphagia and lung involvement, necessitating oxygen therapy and a continuous positive airway pressure device for pulmonary arterial hypertension. A computed tomography scan of the lungs demonstrated an increase in ground-glass infiltrates in the right lower lobe and an air-fluid level in the esophagus. At the time of presentation, she was being treated with bosentan and sildenafil for pulmonary arterial hypertension, in addition to prednisone, venlafaxine, lansoprazole, metoclopramide, levothyroxine, temazepam, aspirin, and oxycodone. In the 2 years prior to presentation, she had been treated with intravenous cyclophosphamide once monthly for 6 months, adalimumab for 1 year, and 1 session of photodynamic therapy to the arms, all without benefit.
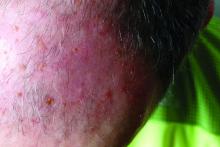
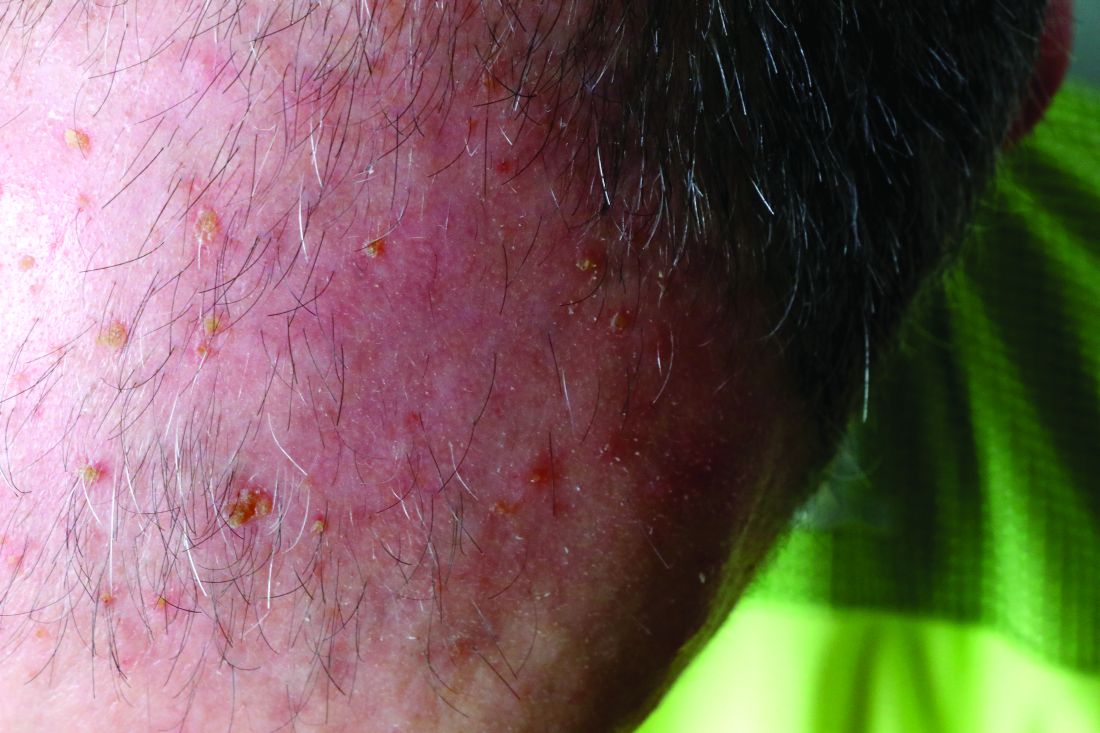
Physical examination showed cutaneous signs of scleroderma including marked sclerosis of the skin on the face, hands, V of the neck, proximal arms, and mid and proximal thighs. Excoriated papules with overlying crusting and pustulation were superimposed on the sclerotic skin of the arms (Figure 1).
A superinfection was diagnosed and treated with cephalexin 500 mg 4 times daily for 2 weeks; thereafter, mupirocin cream twice daily was used as needed. She was prescribed fexofenadine 180 mg twice daily and doxepin 20 mg at bedtime for pruritus.
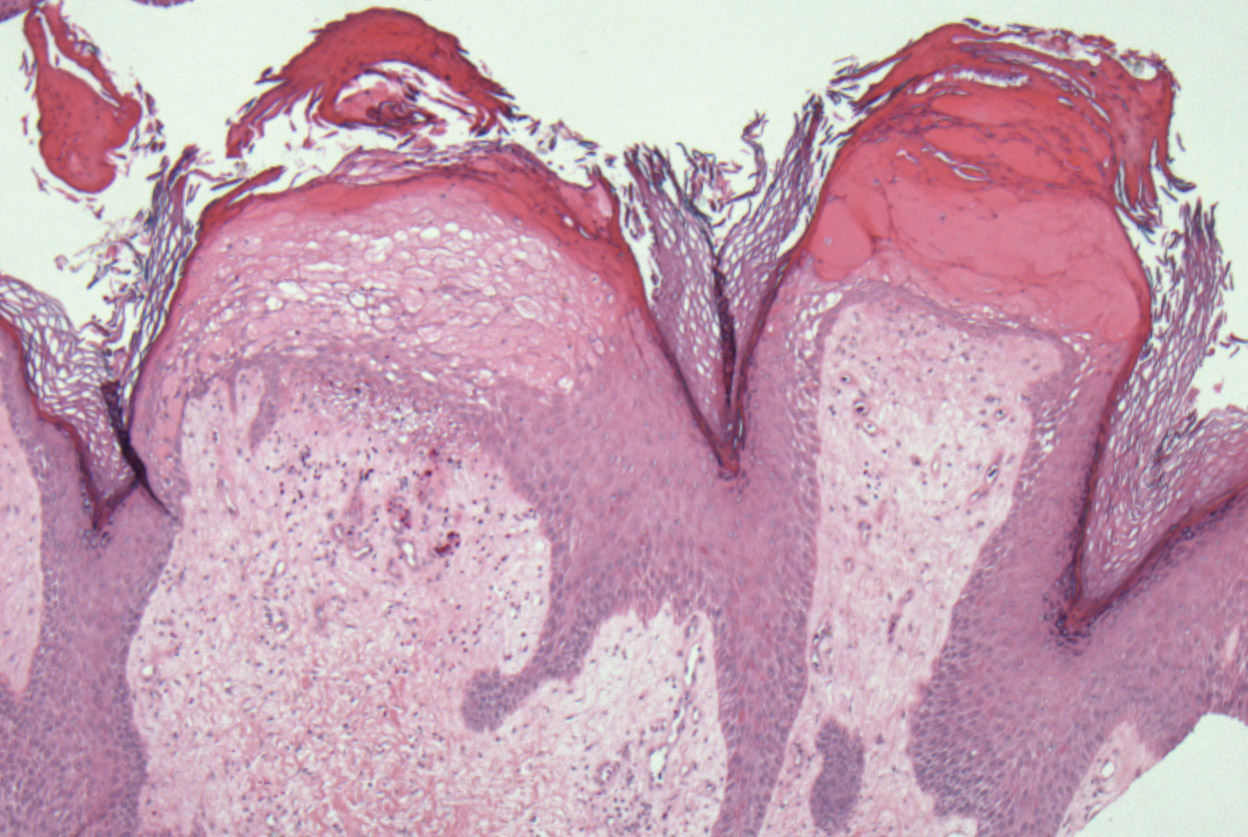
At 3-week follow-up, a trial of narrowband UVB therapy was recommended for control of pruritus. Two weeks later, a modified wet-wrap regimen using clobetasol ointment 0.5% twice daily covered with wet gauze followed by a self-adherent dressing was initiated only on the right arm for comparison purposes. This treatment was not successful. A biopsy taken from the left arm showed lymphedema with perivascular fibroplasia and epidermal hyperplasia consistent with ENV (Figure 2).
Two months after her initial presentation, we instituted treatment with tazarotene gel 0.1% twice daily to the arms as well as a water-based topical emulsion to the finger ulcerations and a healing ointment to the hands. A month later, the patient reported no benefit with tazarotene. She desired more flexibility in her arms and hands; therefore, after a discussion with her rheumatologist, biweekly psoralen plus UVA (PUVA) therapy was initiated. Five months after presentation, methotrexate (MTX) 15 mg once weekly with folic acid 1 mg once daily was added. The PUVA therapy and MTX were stopped 3 months later due to lack of treatment benefit.
The patient was referred to vascular medicine for possible compression therapy. It was determined that her vasculature was intact, but compression therapy was contraindicated due to underlying systemic sclerosis. She was subsequently prescribed mycophenolate mofetil 1000 mg twice daily by her rheumatologist. The options of serial excisions or laser resurfacing were presented, but she declined.
Elephantiasis nostras verrucosa is differentiated from elephantiasis tropica, which is caused by a filarial infection of the lymphatic system. The chronic obstructive lymphedema characteristic of ENV can present as a result of various primary or secondary etiologies including trauma, malignancy, venous stasis, inflammation, or infection.3 In systemic sclerosis, extravascular fibrosis theoretically can lead to lymphatic obstruction and subsequent lymphatic stasis. In turn, the pathophysiology of dermal and subcutaneous fibrosis likely reflects autoantibodies (eg, anticardiolipin antibodies) that can damage lymphatic and nonlymphatic vessels.4,5
As the underlying mechanism of ENV, fibrosis of lymphatic vessels in systemic sclerosis is not well documented. Characteristic features of systemic sclerosis include extensive fibrosis, fibroproliferative vasculopathy, and inflammation, which are all possible mechanisms for the internal lymphatic obstruction resulting in the skin changes observed in ENV.6 It seemed unusual that the fibrotic changes of lymphatic vessels in our patient were extensive enough to cause ENV of the upper extremities; lower extremity involvement is the more common presentation because of the greater likelihood of lymphedema manifesting in the legs and feet. Lower extremity ENV has been reported in association with scleroderma.7,8
Regarding therapeutic options, Boyd et al9 reported a good response in a patient with ENV of the abdomen who was treated with topical tazarotene. Additionally, PUVA and MTX have been reported to be beneficial for the progressive skin changes of systemic sclerosis.10 Mycophenolate mofetil has been used in patients who fail MTX therapy because of its antifibrotic properties without the side-effect profiles of other immunosuppressives, such as imatinib.10,11 In our patient, skin lesions persisted following these varied approaches, and compression therapy was not advised due to the underlying sclerosis.
Because options for medical treatment of severe ENV are limited, surgical debridement of the affected limb often remains the only viable option in advanced cases.12 A PubMed search of articles indexed for MEDLINE using the terms elephantiasis (MeSH terms) or elephantiasis (all fields) and scleroderma, systemic (MeSH terms) or scleroderma (all fields) and systemic (all fields) or systemic scleroderma (all fields) or scleroderma (all fields) or scleroderma, localized (MeSH terms) or scleroderma (all fields) and localized (all fields) or localized scleroderma (all fields) yielded only 1 other case report of lower extremity ENV in a patient with systemic sclerosis who ultimately required bilateral leg amputation.8 When possible, avoiding lymphostasis through compression and control of any underlying infections is important in the treatment and prevention of ENV.3
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Duckworth A, Husain J, DeHeer P. Elephantiasis nostras verrucosa or ‘mossy foot lesions’ in lymphedema praecox. J Am Podiatr Med Assoc. 2008;98:66-69.
- Assous N, Allanore Y, Batteaux F, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199-204.
- Derrett-Smith EC, Dooley A, Gilbane AJ, et al. Endothelial injury in a transforming growth-factor-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013;65:2928-2939.
- Pattanaik M, Brown M, Postlethwaite A. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105-125.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis and treatment guidelines. J Am Acad Dermatol. 2008;59:324-331.
- Chatterjee S, Karai L. Elephantiasis nostras verrucosa in a patient with systemic sclerosis. Clin Exp Dermatol. 2009;34:e696-e698.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63:e102-e104.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941.
To the Editor:
Elephantiasis nostras verrucosa (ENV) is a skin disorder caused by marked underlying lymphedema that leads to hyperkeratosis, papillomatosis, and verrucous growths on the epidermis.1 The pathophysiology of ENV relates to noninfectious lymphatic obstruction and lymphatic fibrosis secondary to venous stasis, malignancy, radiation therapy, or trauma.2 We present an unusual case of lymphedema and subsequent ENV limited to the arms and hands in a patient with scleroderma, an autoimmune fibrosing disorder.
A 54-year-old woman with a 5-year history of scleroderma presented to our dermatology clinic for treatment of progressive skin changes including pruritus, tightness, finger ulcerations, and pus exuding from papules on the dorsal arms and hands. She had been experiencing several systemic symptoms including dysphagia and lung involvement, necessitating oxygen therapy and a continuous positive airway pressure device for pulmonary arterial hypertension. A computed tomography scan of the lungs demonstrated an increase in ground-glass infiltrates in the right lower lobe and an air-fluid level in the esophagus. At the time of presentation, she was being treated with bosentan and sildenafil for pulmonary arterial hypertension, in addition to prednisone, venlafaxine, lansoprazole, metoclopramide, levothyroxine, temazepam, aspirin, and oxycodone. In the 2 years prior to presentation, she had been treated with intravenous cyclophosphamide once monthly for 6 months, adalimumab for 1 year, and 1 session of photodynamic therapy to the arms, all without benefit.
Physical examination showed cutaneous signs of scleroderma including marked sclerosis of the skin on the face, hands, V of the neck, proximal arms, and mid and proximal thighs. Excoriated papules with overlying crusting and pustulation were superimposed on the sclerotic skin of the arms (Figure 1).
A superinfection was diagnosed and treated with cephalexin 500 mg 4 times daily for 2 weeks; thereafter, mupirocin cream twice daily was used as needed. She was prescribed fexofenadine 180 mg twice daily and doxepin 20 mg at bedtime for pruritus.
At 3-week follow-up, a trial of narrowband UVB therapy was recommended for control of pruritus. Two weeks later, a modified wet-wrap regimen using clobetasol ointment 0.5% twice daily covered with wet gauze followed by a self-adherent dressing was initiated only on the right arm for comparison purposes. This treatment was not successful. A biopsy taken from the left arm showed lymphedema with perivascular fibroplasia and epidermal hyperplasia consistent with ENV (Figure 2).
Two months after her initial presentation, we instituted treatment with tazarotene gel 0.1% twice daily to the arms as well as a water-based topical emulsion to the finger ulcerations and a healing ointment to the hands. A month later, the patient reported no benefit with tazarotene. She desired more flexibility in her arms and hands; therefore, after a discussion with her rheumatologist, biweekly psoralen plus UVA (PUVA) therapy was initiated. Five months after presentation, methotrexate (MTX) 15 mg once weekly with folic acid 1 mg once daily was added. The PUVA therapy and MTX were stopped 3 months later due to lack of treatment benefit.
The patient was referred to vascular medicine for possible compression therapy. It was determined that her vasculature was intact, but compression therapy was contraindicated due to underlying systemic sclerosis. She was subsequently prescribed mycophenolate mofetil 1000 mg twice daily by her rheumatologist. The options of serial excisions or laser resurfacing were presented, but she declined.
Elephantiasis nostras verrucosa is differentiated from elephantiasis tropica, which is caused by a filarial infection of the lymphatic system. The chronic obstructive lymphedema characteristic of ENV can present as a result of various primary or secondary etiologies including trauma, malignancy, venous stasis, inflammation, or infection.3 In systemic sclerosis, extravascular fibrosis theoretically can lead to lymphatic obstruction and subsequent lymphatic stasis. In turn, the pathophysiology of dermal and subcutaneous fibrosis likely reflects autoantibodies (eg, anticardiolipin antibodies) that can damage lymphatic and nonlymphatic vessels.4,5
As the underlying mechanism of ENV, fibrosis of lymphatic vessels in systemic sclerosis is not well documented. Characteristic features of systemic sclerosis include extensive fibrosis, fibroproliferative vasculopathy, and inflammation, which are all possible mechanisms for the internal lymphatic obstruction resulting in the skin changes observed in ENV.6 It seemed unusual that the fibrotic changes of lymphatic vessels in our patient were extensive enough to cause ENV of the upper extremities; lower extremity involvement is the more common presentation because of the greater likelihood of lymphedema manifesting in the legs and feet. Lower extremity ENV has been reported in association with scleroderma.7,8
Regarding therapeutic options, Boyd et al9 reported a good response in a patient with ENV of the abdomen who was treated with topical tazarotene. Additionally, PUVA and MTX have been reported to be beneficial for the progressive skin changes of systemic sclerosis.10 Mycophenolate mofetil has been used in patients who fail MTX therapy because of its antifibrotic properties without the side-effect profiles of other immunosuppressives, such as imatinib.10,11 In our patient, skin lesions persisted following these varied approaches, and compression therapy was not advised due to the underlying sclerosis.
Because options for medical treatment of severe ENV are limited, surgical debridement of the affected limb often remains the only viable option in advanced cases.12 A PubMed search of articles indexed for MEDLINE using the terms elephantiasis (MeSH terms) or elephantiasis (all fields) and scleroderma, systemic (MeSH terms) or scleroderma (all fields) and systemic (all fields) or systemic scleroderma (all fields) or scleroderma (all fields) or scleroderma, localized (MeSH terms) or scleroderma (all fields) and localized (all fields) or localized scleroderma (all fields) yielded only 1 other case report of lower extremity ENV in a patient with systemic sclerosis who ultimately required bilateral leg amputation.8 When possible, avoiding lymphostasis through compression and control of any underlying infections is important in the treatment and prevention of ENV.3
To the Editor:
Elephantiasis nostras verrucosa (ENV) is a skin disorder caused by marked underlying lymphedema that leads to hyperkeratosis, papillomatosis, and verrucous growths on the epidermis.1 The pathophysiology of ENV relates to noninfectious lymphatic obstruction and lymphatic fibrosis secondary to venous stasis, malignancy, radiation therapy, or trauma.2 We present an unusual case of lymphedema and subsequent ENV limited to the arms and hands in a patient with scleroderma, an autoimmune fibrosing disorder.
A 54-year-old woman with a 5-year history of scleroderma presented to our dermatology clinic for treatment of progressive skin changes including pruritus, tightness, finger ulcerations, and pus exuding from papules on the dorsal arms and hands. She had been experiencing several systemic symptoms including dysphagia and lung involvement, necessitating oxygen therapy and a continuous positive airway pressure device for pulmonary arterial hypertension. A computed tomography scan of the lungs demonstrated an increase in ground-glass infiltrates in the right lower lobe and an air-fluid level in the esophagus. At the time of presentation, she was being treated with bosentan and sildenafil for pulmonary arterial hypertension, in addition to prednisone, venlafaxine, lansoprazole, metoclopramide, levothyroxine, temazepam, aspirin, and oxycodone. In the 2 years prior to presentation, she had been treated with intravenous cyclophosphamide once monthly for 6 months, adalimumab for 1 year, and 1 session of photodynamic therapy to the arms, all without benefit.
Physical examination showed cutaneous signs of scleroderma including marked sclerosis of the skin on the face, hands, V of the neck, proximal arms, and mid and proximal thighs. Excoriated papules with overlying crusting and pustulation were superimposed on the sclerotic skin of the arms (Figure 1).
A superinfection was diagnosed and treated with cephalexin 500 mg 4 times daily for 2 weeks; thereafter, mupirocin cream twice daily was used as needed. She was prescribed fexofenadine 180 mg twice daily and doxepin 20 mg at bedtime for pruritus.
At 3-week follow-up, a trial of narrowband UVB therapy was recommended for control of pruritus. Two weeks later, a modified wet-wrap regimen using clobetasol ointment 0.5% twice daily covered with wet gauze followed by a self-adherent dressing was initiated only on the right arm for comparison purposes. This treatment was not successful. A biopsy taken from the left arm showed lymphedema with perivascular fibroplasia and epidermal hyperplasia consistent with ENV (Figure 2).
Two months after her initial presentation, we instituted treatment with tazarotene gel 0.1% twice daily to the arms as well as a water-based topical emulsion to the finger ulcerations and a healing ointment to the hands. A month later, the patient reported no benefit with tazarotene. She desired more flexibility in her arms and hands; therefore, after a discussion with her rheumatologist, biweekly psoralen plus UVA (PUVA) therapy was initiated. Five months after presentation, methotrexate (MTX) 15 mg once weekly with folic acid 1 mg once daily was added. The PUVA therapy and MTX were stopped 3 months later due to lack of treatment benefit.
The patient was referred to vascular medicine for possible compression therapy. It was determined that her vasculature was intact, but compression therapy was contraindicated due to underlying systemic sclerosis. She was subsequently prescribed mycophenolate mofetil 1000 mg twice daily by her rheumatologist. The options of serial excisions or laser resurfacing were presented, but she declined.
Elephantiasis nostras verrucosa is differentiated from elephantiasis tropica, which is caused by a filarial infection of the lymphatic system. The chronic obstructive lymphedema characteristic of ENV can present as a result of various primary or secondary etiologies including trauma, malignancy, venous stasis, inflammation, or infection.3 In systemic sclerosis, extravascular fibrosis theoretically can lead to lymphatic obstruction and subsequent lymphatic stasis. In turn, the pathophysiology of dermal and subcutaneous fibrosis likely reflects autoantibodies (eg, anticardiolipin antibodies) that can damage lymphatic and nonlymphatic vessels.4,5
As the underlying mechanism of ENV, fibrosis of lymphatic vessels in systemic sclerosis is not well documented. Characteristic features of systemic sclerosis include extensive fibrosis, fibroproliferative vasculopathy, and inflammation, which are all possible mechanisms for the internal lymphatic obstruction resulting in the skin changes observed in ENV.6 It seemed unusual that the fibrotic changes of lymphatic vessels in our patient were extensive enough to cause ENV of the upper extremities; lower extremity involvement is the more common presentation because of the greater likelihood of lymphedema manifesting in the legs and feet. Lower extremity ENV has been reported in association with scleroderma.7,8
Regarding therapeutic options, Boyd et al9 reported a good response in a patient with ENV of the abdomen who was treated with topical tazarotene. Additionally, PUVA and MTX have been reported to be beneficial for the progressive skin changes of systemic sclerosis.10 Mycophenolate mofetil has been used in patients who fail MTX therapy because of its antifibrotic properties without the side-effect profiles of other immunosuppressives, such as imatinib.10,11 In our patient, skin lesions persisted following these varied approaches, and compression therapy was not advised due to the underlying sclerosis.
Because options for medical treatment of severe ENV are limited, surgical debridement of the affected limb often remains the only viable option in advanced cases.12 A PubMed search of articles indexed for MEDLINE using the terms elephantiasis (MeSH terms) or elephantiasis (all fields) and scleroderma, systemic (MeSH terms) or scleroderma (all fields) and systemic (all fields) or systemic scleroderma (all fields) or scleroderma (all fields) or scleroderma, localized (MeSH terms) or scleroderma (all fields) and localized (all fields) or localized scleroderma (all fields) yielded only 1 other case report of lower extremity ENV in a patient with systemic sclerosis who ultimately required bilateral leg amputation.8 When possible, avoiding lymphostasis through compression and control of any underlying infections is important in the treatment and prevention of ENV.3
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Duckworth A, Husain J, DeHeer P. Elephantiasis nostras verrucosa or ‘mossy foot lesions’ in lymphedema praecox. J Am Podiatr Med Assoc. 2008;98:66-69.
- Assous N, Allanore Y, Batteaux F, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199-204.
- Derrett-Smith EC, Dooley A, Gilbane AJ, et al. Endothelial injury in a transforming growth-factor-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013;65:2928-2939.
- Pattanaik M, Brown M, Postlethwaite A. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105-125.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis and treatment guidelines. J Am Acad Dermatol. 2008;59:324-331.
- Chatterjee S, Karai L. Elephantiasis nostras verrucosa in a patient with systemic sclerosis. Clin Exp Dermatol. 2009;34:e696-e698.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63:e102-e104.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941.
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Duckworth A, Husain J, DeHeer P. Elephantiasis nostras verrucosa or ‘mossy foot lesions’ in lymphedema praecox. J Am Podiatr Med Assoc. 2008;98:66-69.
- Assous N, Allanore Y, Batteaux F, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199-204.
- Derrett-Smith EC, Dooley A, Gilbane AJ, et al. Endothelial injury in a transforming growth-factor-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 2013;65:2928-2939.
- Pattanaik M, Brown M, Postlethwaite A. Vascular involvement in systemic sclerosis (scleroderma). J Inflamm Res. 2011;4:105-125.
- Kerchner K, Fleischer A, Yosipovitch G. Lower extremity lymphedema update: pathophysiology, diagnosis and treatment guidelines. J Am Acad Dermatol. 2008;59:324-331.
- Chatterjee S, Karai L. Elephantiasis nostras verrucosa in a patient with systemic sclerosis. Clin Exp Dermatol. 2009;34:e696-e698.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Fett, N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437.
- Moinzadeh P, Krieg T, Hunzelmann N. Imatinib treatment of generalized localized scleroderma (morphea). J Am Acad Dermatol. 2010;63:e102-e104.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941.
Practice Points
- Scleroderma rarely may lead to elephantiasis nostras verrucosa (ENV) of the upper extremities.
- Avoiding lymphostasis through compression and control of concomitant skin and soft tissue infections is important in the treatment and prevention of ENV.
Fine-tune staging for better SCC risk stratification
ORLANDO – When caring for individuals with sun-damaged skin, dermatologists need comfort with the full spectrum of photo-related skin disease. From assessment and treatment of actinic keratoses (AKs) and field cancerization, to long-term follow-up of cutaneous squamous cell carcinomas (SCCs), appropriate treatment and staging can improve patient quality of life and reduce health care costs, Vishal Patel, MD, said at the Orlando Dermatology Aesthetic and Clinical Conference.
said Dr. Patel, director of cutaneous oncology at George Washington University Cancer Center, Washington. On the other hand, he added, “field disease can be a marker for invasive squamous cell carcinoma risk, and it requires field treatment.” Treatment that reduces field disease is primary prevention because it decreases the formation of invasive SCC, he noted.
“But this level of disease – AKs and SCC in situ – doesn’t kill people,” he emphasized. “I want to leave you with an ability to stage this disease,” said Dr. Patel, noting that SCC mortality may eventually surpass melanoma mortality as deaths from the latter decline and numbers of older Americans with high ultraviolet light exposure and other risk factors climb.
While the majority of AKs regress within 5 years, he looks at the total burden of AKs as a marker for field cancerization “because having less than five in situ or actinic lesions puts you at less than a 1% risk of squamous cell carcinoma formation. Having more than 20 increases that risk 20-fold to 20%,” he said. “That’s the way we need to start thinking about this: Is this a disease – or a symptom?”
Rather than thinking of each AK or SCC in situ as a separate disease event, “the disease we need to be focusing on and treating is field cancerization,” he continued. Within this context, “we should not be thinking that … we need to be aggressive in our management,” which is what results in high costs.
“The reality is that this is a big quality of life issue for our patients. So what do we do?” Field treatment is appropriate for field disease, he said. Dr. Patel said that at GW only field treatment is used; destructive treatment for AKs and SCC in situ is not used. In the absence of patient and lesion characteristics that elevate risk,“surgery is really not the standard of care for in situ lesions for us,” he commented.
“We start by discerning the field disease from the invasive disease” with an initial round of field treatment and, if needed, adjunctive oral chemoprophylaxis. “We lather, rinse, and repeat” the field therapy, continuously if needed, Dr. Patel said.
“We like to do that because we can then identify those specific lesions we want to go after. No cryosurgery, no destructive therapy, because we run the risk of burying those tumors under the scar. They may recur and make it more difficult to accurately stage them in the future,” he noted.
“I like to be more sophisticated in thinking about our approach to the outcomes of these individual lesions,” he said. When it comes to excising lesions that have been biopsied and show invasive SCC, “disc excision may be a more cost-effective way to treat many low-risk SCCs,” he noted. In any case, “removal with clear surgical margins is key.”
Primary tumors with such low-risk attributes as diameter under a centimeter and thickness under 2 mm; well-defined borders; location on the trunk, neck, or extremities; well-differentiated histology; and lack of perineural invasion can all be considered for a disc technique, especially if the patient is immunocompetent without background chronic inflammation or a history of prior radiation therapy.
Staging SCCs, said Dr. Patel, is where things really get tricky. Older staging systems for SCC “led us to overtreat nonaggressive disease and undertreat aggressive disease. I think we have the responsibility to lead the charge to having a more sophisticated approach.” For example, patients whose tumors were staged T2 in the American Joint Commission on Cancer (AJCC) 7 classification system were most likely to have poor outcomes – in part because so few tumors were staged higher – which meant AJCC 7 didn’t provide adequate differentiation for useful risk prognostication.
A group of researchers at the Brigham and Women’s Hospital (BWH), Boston, “came up with a better system to better differentiate those T2 tumors into a high-risk and a low-risk subtype,” according to Dr. Patel.
With use of validated risk factors, the investigators applied a long list of risk factors to 2,000 tumors to see which risk factors, taken individually, were really contributing to poor outcomes. Eventually, four risk factors that made the most difference were identified: size greater than 2 cm, poor tumor differentiation, perineural invasion greater than 0.1 mm in diameter, and tumor invasion beyond subcutaneous fat. “I really want to highlight the size portion of those risk factors,” said Dr. Patel. “Something I’d like you to do in your clinical practice is to measure and document the size of the lesion. … That really, clearly helps” with risk prognostication.
These four factors were then used to break out a T2a stage for tumors with one risk factor and a T2b stage for tumors with two or three risk factors. Tumors with no risk factors are stage T1, and those with all four risk factors are stage T3. In situ SCC is T0.
Applying this new staging system to a 2,000-patient cohort with SCC yielded clear separation in outcomes including recurrence, nodal metastasis, disease-specific death, and overall survival between patients with the T2a and T2b tumors (P less than .001 for all; J Clin Oncol. 2014 Feb 1;32[4]:327-34).
While AJCC 8 is “significantly better” than AJCC 7 in its incorporation of meaningful risk factors into the SCC staging system, “it still underperforms in comparison” with the BWH staging system using the 2000 patient cohort, he said. Recent work has shown the BWH classification system to have superior specificity and positive predictive value in detecting nodal metastasis and disease-specific death in higher-grade tumors. But both BWH and AJCC 8 need further refinement.
“So what are the staging pearls to take home?” Dr. Patel asked. “First, utilize a staging system.” “Staging of SCC utilizing should be done routinely. Most data seems to suggest that the BWH system appears to outperform AJCC 8, and it is what we currently use routinely at GW,” he said.
Patients who are T1 by BWH criteria, with no risk factors, are at low or even no risk, he noted. He pointed out that of the nearly 1,400 patients who met T1 criteria, there were just eight local recurrences, one nodal metastasis, and no distant metastases or deaths. Knowing this should guide physicians on a treatment path that will reduce costs and provide patients with peace of mind, he said.
In the BWH schema, T2a patients fared almost as well, with a 2% risk of nodal metastasis and an overall 1% risk of disease-specific death. “T2a disease is low risk, in my mind. Most of these patients will go on to do well,” he said.
By contrast, “there may be a number of tumors that you are missing” that are candidates for close follow-up if the BWH criteria are not being used, said Dr. Patel. These are the T2b tumors. “For those patients, we want to aggressively follow them and think about a more aggressive management plan.”
The bottom line is that BWH T2b and T3 tumors are both high risk, and management needs to acknowledge this, he said. The current protocol in our cutaneous oncology program includes using routine radiologic nodal staging in patients with BWH stage 2b and above SCCs and considering sentinel lymph node biopsy for certain individuals.
For patients with BWH T2b and T3 tumors, dermatologists should give consideration to tertiary care or cancer center referrals so they have access to the full spectrum of diagnostic and therapeutic modalities and the opportunity to participate in clinical trials, Dr. Patel said.
Dr. Patel reported that he is a speaker for Regeneron/Sanofi and a cofounder of the Skin Cancer Outcomes (SCOUT) consortium.
This article was updated 2/9/2019
ORLANDO – When caring for individuals with sun-damaged skin, dermatologists need comfort with the full spectrum of photo-related skin disease. From assessment and treatment of actinic keratoses (AKs) and field cancerization, to long-term follow-up of cutaneous squamous cell carcinomas (SCCs), appropriate treatment and staging can improve patient quality of life and reduce health care costs, Vishal Patel, MD, said at the Orlando Dermatology Aesthetic and Clinical Conference.
said Dr. Patel, director of cutaneous oncology at George Washington University Cancer Center, Washington. On the other hand, he added, “field disease can be a marker for invasive squamous cell carcinoma risk, and it requires field treatment.” Treatment that reduces field disease is primary prevention because it decreases the formation of invasive SCC, he noted.
“But this level of disease – AKs and SCC in situ – doesn’t kill people,” he emphasized. “I want to leave you with an ability to stage this disease,” said Dr. Patel, noting that SCC mortality may eventually surpass melanoma mortality as deaths from the latter decline and numbers of older Americans with high ultraviolet light exposure and other risk factors climb.
While the majority of AKs regress within 5 years, he looks at the total burden of AKs as a marker for field cancerization “because having less than five in situ or actinic lesions puts you at less than a 1% risk of squamous cell carcinoma formation. Having more than 20 increases that risk 20-fold to 20%,” he said. “That’s the way we need to start thinking about this: Is this a disease – or a symptom?”
Rather than thinking of each AK or SCC in situ as a separate disease event, “the disease we need to be focusing on and treating is field cancerization,” he continued. Within this context, “we should not be thinking that … we need to be aggressive in our management,” which is what results in high costs.
“The reality is that this is a big quality of life issue for our patients. So what do we do?” Field treatment is appropriate for field disease, he said. Dr. Patel said that at GW only field treatment is used; destructive treatment for AKs and SCC in situ is not used. In the absence of patient and lesion characteristics that elevate risk,“surgery is really not the standard of care for in situ lesions for us,” he commented.
“We start by discerning the field disease from the invasive disease” with an initial round of field treatment and, if needed, adjunctive oral chemoprophylaxis. “We lather, rinse, and repeat” the field therapy, continuously if needed, Dr. Patel said.
“We like to do that because we can then identify those specific lesions we want to go after. No cryosurgery, no destructive therapy, because we run the risk of burying those tumors under the scar. They may recur and make it more difficult to accurately stage them in the future,” he noted.
“I like to be more sophisticated in thinking about our approach to the outcomes of these individual lesions,” he said. When it comes to excising lesions that have been biopsied and show invasive SCC, “disc excision may be a more cost-effective way to treat many low-risk SCCs,” he noted. In any case, “removal with clear surgical margins is key.”
Primary tumors with such low-risk attributes as diameter under a centimeter and thickness under 2 mm; well-defined borders; location on the trunk, neck, or extremities; well-differentiated histology; and lack of perineural invasion can all be considered for a disc technique, especially if the patient is immunocompetent without background chronic inflammation or a history of prior radiation therapy.
Staging SCCs, said Dr. Patel, is where things really get tricky. Older staging systems for SCC “led us to overtreat nonaggressive disease and undertreat aggressive disease. I think we have the responsibility to lead the charge to having a more sophisticated approach.” For example, patients whose tumors were staged T2 in the American Joint Commission on Cancer (AJCC) 7 classification system were most likely to have poor outcomes – in part because so few tumors were staged higher – which meant AJCC 7 didn’t provide adequate differentiation for useful risk prognostication.
A group of researchers at the Brigham and Women’s Hospital (BWH), Boston, “came up with a better system to better differentiate those T2 tumors into a high-risk and a low-risk subtype,” according to Dr. Patel.
With use of validated risk factors, the investigators applied a long list of risk factors to 2,000 tumors to see which risk factors, taken individually, were really contributing to poor outcomes. Eventually, four risk factors that made the most difference were identified: size greater than 2 cm, poor tumor differentiation, perineural invasion greater than 0.1 mm in diameter, and tumor invasion beyond subcutaneous fat. “I really want to highlight the size portion of those risk factors,” said Dr. Patel. “Something I’d like you to do in your clinical practice is to measure and document the size of the lesion. … That really, clearly helps” with risk prognostication.
These four factors were then used to break out a T2a stage for tumors with one risk factor and a T2b stage for tumors with two or three risk factors. Tumors with no risk factors are stage T1, and those with all four risk factors are stage T3. In situ SCC is T0.
Applying this new staging system to a 2,000-patient cohort with SCC yielded clear separation in outcomes including recurrence, nodal metastasis, disease-specific death, and overall survival between patients with the T2a and T2b tumors (P less than .001 for all; J Clin Oncol. 2014 Feb 1;32[4]:327-34).
While AJCC 8 is “significantly better” than AJCC 7 in its incorporation of meaningful risk factors into the SCC staging system, “it still underperforms in comparison” with the BWH staging system using the 2000 patient cohort, he said. Recent work has shown the BWH classification system to have superior specificity and positive predictive value in detecting nodal metastasis and disease-specific death in higher-grade tumors. But both BWH and AJCC 8 need further refinement.
“So what are the staging pearls to take home?” Dr. Patel asked. “First, utilize a staging system.” “Staging of SCC utilizing should be done routinely. Most data seems to suggest that the BWH system appears to outperform AJCC 8, and it is what we currently use routinely at GW,” he said.
Patients who are T1 by BWH criteria, with no risk factors, are at low or even no risk, he noted. He pointed out that of the nearly 1,400 patients who met T1 criteria, there were just eight local recurrences, one nodal metastasis, and no distant metastases or deaths. Knowing this should guide physicians on a treatment path that will reduce costs and provide patients with peace of mind, he said.
In the BWH schema, T2a patients fared almost as well, with a 2% risk of nodal metastasis and an overall 1% risk of disease-specific death. “T2a disease is low risk, in my mind. Most of these patients will go on to do well,” he said.
By contrast, “there may be a number of tumors that you are missing” that are candidates for close follow-up if the BWH criteria are not being used, said Dr. Patel. These are the T2b tumors. “For those patients, we want to aggressively follow them and think about a more aggressive management plan.”
The bottom line is that BWH T2b and T3 tumors are both high risk, and management needs to acknowledge this, he said. The current protocol in our cutaneous oncology program includes using routine radiologic nodal staging in patients with BWH stage 2b and above SCCs and considering sentinel lymph node biopsy for certain individuals.
For patients with BWH T2b and T3 tumors, dermatologists should give consideration to tertiary care or cancer center referrals so they have access to the full spectrum of diagnostic and therapeutic modalities and the opportunity to participate in clinical trials, Dr. Patel said.
Dr. Patel reported that he is a speaker for Regeneron/Sanofi and a cofounder of the Skin Cancer Outcomes (SCOUT) consortium.
This article was updated 2/9/2019
ORLANDO – When caring for individuals with sun-damaged skin, dermatologists need comfort with the full spectrum of photo-related skin disease. From assessment and treatment of actinic keratoses (AKs) and field cancerization, to long-term follow-up of cutaneous squamous cell carcinomas (SCCs), appropriate treatment and staging can improve patient quality of life and reduce health care costs, Vishal Patel, MD, said at the Orlando Dermatology Aesthetic and Clinical Conference.
said Dr. Patel, director of cutaneous oncology at George Washington University Cancer Center, Washington. On the other hand, he added, “field disease can be a marker for invasive squamous cell carcinoma risk, and it requires field treatment.” Treatment that reduces field disease is primary prevention because it decreases the formation of invasive SCC, he noted.
“But this level of disease – AKs and SCC in situ – doesn’t kill people,” he emphasized. “I want to leave you with an ability to stage this disease,” said Dr. Patel, noting that SCC mortality may eventually surpass melanoma mortality as deaths from the latter decline and numbers of older Americans with high ultraviolet light exposure and other risk factors climb.
While the majority of AKs regress within 5 years, he looks at the total burden of AKs as a marker for field cancerization “because having less than five in situ or actinic lesions puts you at less than a 1% risk of squamous cell carcinoma formation. Having more than 20 increases that risk 20-fold to 20%,” he said. “That’s the way we need to start thinking about this: Is this a disease – or a symptom?”
Rather than thinking of each AK or SCC in situ as a separate disease event, “the disease we need to be focusing on and treating is field cancerization,” he continued. Within this context, “we should not be thinking that … we need to be aggressive in our management,” which is what results in high costs.
“The reality is that this is a big quality of life issue for our patients. So what do we do?” Field treatment is appropriate for field disease, he said. Dr. Patel said that at GW only field treatment is used; destructive treatment for AKs and SCC in situ is not used. In the absence of patient and lesion characteristics that elevate risk,“surgery is really not the standard of care for in situ lesions for us,” he commented.
“We start by discerning the field disease from the invasive disease” with an initial round of field treatment and, if needed, adjunctive oral chemoprophylaxis. “We lather, rinse, and repeat” the field therapy, continuously if needed, Dr. Patel said.
“We like to do that because we can then identify those specific lesions we want to go after. No cryosurgery, no destructive therapy, because we run the risk of burying those tumors under the scar. They may recur and make it more difficult to accurately stage them in the future,” he noted.
“I like to be more sophisticated in thinking about our approach to the outcomes of these individual lesions,” he said. When it comes to excising lesions that have been biopsied and show invasive SCC, “disc excision may be a more cost-effective way to treat many low-risk SCCs,” he noted. In any case, “removal with clear surgical margins is key.”
Primary tumors with such low-risk attributes as diameter under a centimeter and thickness under 2 mm; well-defined borders; location on the trunk, neck, or extremities; well-differentiated histology; and lack of perineural invasion can all be considered for a disc technique, especially if the patient is immunocompetent without background chronic inflammation or a history of prior radiation therapy.
Staging SCCs, said Dr. Patel, is where things really get tricky. Older staging systems for SCC “led us to overtreat nonaggressive disease and undertreat aggressive disease. I think we have the responsibility to lead the charge to having a more sophisticated approach.” For example, patients whose tumors were staged T2 in the American Joint Commission on Cancer (AJCC) 7 classification system were most likely to have poor outcomes – in part because so few tumors were staged higher – which meant AJCC 7 didn’t provide adequate differentiation for useful risk prognostication.
A group of researchers at the Brigham and Women’s Hospital (BWH), Boston, “came up with a better system to better differentiate those T2 tumors into a high-risk and a low-risk subtype,” according to Dr. Patel.
With use of validated risk factors, the investigators applied a long list of risk factors to 2,000 tumors to see which risk factors, taken individually, were really contributing to poor outcomes. Eventually, four risk factors that made the most difference were identified: size greater than 2 cm, poor tumor differentiation, perineural invasion greater than 0.1 mm in diameter, and tumor invasion beyond subcutaneous fat. “I really want to highlight the size portion of those risk factors,” said Dr. Patel. “Something I’d like you to do in your clinical practice is to measure and document the size of the lesion. … That really, clearly helps” with risk prognostication.
These four factors were then used to break out a T2a stage for tumors with one risk factor and a T2b stage for tumors with two or three risk factors. Tumors with no risk factors are stage T1, and those with all four risk factors are stage T3. In situ SCC is T0.
Applying this new staging system to a 2,000-patient cohort with SCC yielded clear separation in outcomes including recurrence, nodal metastasis, disease-specific death, and overall survival between patients with the T2a and T2b tumors (P less than .001 for all; J Clin Oncol. 2014 Feb 1;32[4]:327-34).
While AJCC 8 is “significantly better” than AJCC 7 in its incorporation of meaningful risk factors into the SCC staging system, “it still underperforms in comparison” with the BWH staging system using the 2000 patient cohort, he said. Recent work has shown the BWH classification system to have superior specificity and positive predictive value in detecting nodal metastasis and disease-specific death in higher-grade tumors. But both BWH and AJCC 8 need further refinement.
“So what are the staging pearls to take home?” Dr. Patel asked. “First, utilize a staging system.” “Staging of SCC utilizing should be done routinely. Most data seems to suggest that the BWH system appears to outperform AJCC 8, and it is what we currently use routinely at GW,” he said.
Patients who are T1 by BWH criteria, with no risk factors, are at low or even no risk, he noted. He pointed out that of the nearly 1,400 patients who met T1 criteria, there were just eight local recurrences, one nodal metastasis, and no distant metastases or deaths. Knowing this should guide physicians on a treatment path that will reduce costs and provide patients with peace of mind, he said.
In the BWH schema, T2a patients fared almost as well, with a 2% risk of nodal metastasis and an overall 1% risk of disease-specific death. “T2a disease is low risk, in my mind. Most of these patients will go on to do well,” he said.
By contrast, “there may be a number of tumors that you are missing” that are candidates for close follow-up if the BWH criteria are not being used, said Dr. Patel. These are the T2b tumors. “For those patients, we want to aggressively follow them and think about a more aggressive management plan.”
The bottom line is that BWH T2b and T3 tumors are both high risk, and management needs to acknowledge this, he said. The current protocol in our cutaneous oncology program includes using routine radiologic nodal staging in patients with BWH stage 2b and above SCCs and considering sentinel lymph node biopsy for certain individuals.
For patients with BWH T2b and T3 tumors, dermatologists should give consideration to tertiary care or cancer center referrals so they have access to the full spectrum of diagnostic and therapeutic modalities and the opportunity to participate in clinical trials, Dr. Patel said.
Dr. Patel reported that he is a speaker for Regeneron/Sanofi and a cofounder of the Skin Cancer Outcomes (SCOUT) consortium.
This article was updated 2/9/2019
EXPERT ANALYSIS FROM ODAC 2019
Case report: Longstanding actinic keratosis responds to kanuka honey
GRAND CAYMAN, CAYMAN ISLANDS – Not all honeys are created equal, Theodore Rosen, MD, said at the meeting provided by Global Academy for Medical Education.
“It seems that kanuka is the new manuka,” said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston. These lesser-known New Zealand bush honeys may be something to watch because research and case reports continue to provide intriguing hints of how these honeys exert their immunomodulatory effects on skin, he commented, describing a recent case report describing the elimination of a large, long-standing actinic keratosis (AK) with application of kanuka honey.
Manuka (Leptospermum scoparium) is a large bush native to both Australia and New Zealand. Kanuka (Kunzea ericoides) is quite similar in size and appearance, but native only to New Zealand. Honey made from the flowers of these bushes possesses some unique properties that make it an attractive addition to wound healing regimens, according to a 2014 study (Int J Gen Med. 2014;7:149-58).
The study examined samples of manuka, kanuka, a manuka/kanuka blend, and clover honey. The investigators found that kanuka honey, and to a lesser extent manuka honey, exerted a potent anti-inflammatory effect in human embryonic kidney cells. The honeys interfered with toll-like receptor 1 and 2 signaling, which would reduce the production of proinflammatory cytokines.
Kanuka’s potency seems directly related to its unusually high level of arabinogalactan, according to Saras Mane, MD, primary author of the AK case report (Case Rep Dermatol Med. 2018 May 31;2018:4628971). Dr. Mane is with the Medical Research Institute of New Zealand in Wellington.
“The immunomodulatory properties of kanuka honey in particular are thought to be more potent than other New Zealand honeys due to the relatively high concentrations of arabinogalactan proteins present,” Dr. Mane and his coauthors wrote in the case report. “These proteins have been shown to stimulate release of TNF-alpha from monocytic cell lines in vitro.”
The report involved a 66-year-old man who was enrolled in a randomized trial of a commercialized medical-grade kanuka honey ointment (Honevo, 90% kanuka honey, 10% glycerin; Honeylab NZ) for rosacea.
The patient also had multiple AKs, including a raised, crusted, scaly lesion measuring 20 mm by 21 mm with marginal erythema on the back of one hand. The lesion had been present and dormant for a number of years, but it had recently begun to grow.
“This gentleman decided he’d just try the honey on his AK, too,” Dr. Rosen said. The man reported applying a small amount to the lesion and erythematous area once a day, leaving it on for about 30 to 60 minutes. After 5 days, he stopped because the lesion became tender. During the next two days, the patient reported “picking at” the lesion, which was softening. He repeated this cycle of treatment for 3 months with no other therapy to the lesion.
“The lesion gradually reduced in size with an initial rapid reduction in its dry, crusted nature,” the authors reported. “After 3 months, residual appearance of the lesion was a 20 mm by 17 mm area of pink skin with no elements of hypertrophy, crusting, or loss of skin integrity,” they noted. “At 6 months, there were no signs of recurrence. At 9 months, the appearance of the skin had fully returned to normal. A telephone follow-up was conducted at 2 years after treatment, and the patient reported that his skin in the area was still completely normal and that there were no signs of recurrence.”
Dr. Mane noted that they had only clinical evidence, and no histology of the lesion either before or after its change. “The AK was diagnosed and treated in primary care, where it is not usual for AKs to be biopsied, and the decision to write up the case was made after the course of treatment had finished,” they said.
“Immunomodulatory topical agents are already widely used in the treatment of AK as an immune component is evident in its etiology,” they wrote. “Immunocompromised patients have 250 times the risk of developing an AK than the general population.”
Dr. Rosen said that kanuka honey is also being investigated in psoriasis, eczema, acne, herpes simplex virus, and diaper dermatitis. It is also being studied for rosacea.
Dr. Mane declared no conflicts of interest. Some coauthors disclosed that they have previously received funding from HoneyLab NZ. Dr. Rosen has no commercial interest in HoneyLab.
The meeting was sponsored by Global Academy for Medical Education; Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – Not all honeys are created equal, Theodore Rosen, MD, said at the meeting provided by Global Academy for Medical Education.
“It seems that kanuka is the new manuka,” said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston. These lesser-known New Zealand bush honeys may be something to watch because research and case reports continue to provide intriguing hints of how these honeys exert their immunomodulatory effects on skin, he commented, describing a recent case report describing the elimination of a large, long-standing actinic keratosis (AK) with application of kanuka honey.
Manuka (Leptospermum scoparium) is a large bush native to both Australia and New Zealand. Kanuka (Kunzea ericoides) is quite similar in size and appearance, but native only to New Zealand. Honey made from the flowers of these bushes possesses some unique properties that make it an attractive addition to wound healing regimens, according to a 2014 study (Int J Gen Med. 2014;7:149-58).
The study examined samples of manuka, kanuka, a manuka/kanuka blend, and clover honey. The investigators found that kanuka honey, and to a lesser extent manuka honey, exerted a potent anti-inflammatory effect in human embryonic kidney cells. The honeys interfered with toll-like receptor 1 and 2 signaling, which would reduce the production of proinflammatory cytokines.
Kanuka’s potency seems directly related to its unusually high level of arabinogalactan, according to Saras Mane, MD, primary author of the AK case report (Case Rep Dermatol Med. 2018 May 31;2018:4628971). Dr. Mane is with the Medical Research Institute of New Zealand in Wellington.

“The immunomodulatory properties of kanuka honey in particular are thought to be more potent than other New Zealand honeys due to the relatively high concentrations of arabinogalactan proteins present,” Dr. Mane and his coauthors wrote in the case report. “These proteins have been shown to stimulate release of TNF-alpha from monocytic cell lines in vitro.”
The report involved a 66-year-old man who was enrolled in a randomized trial of a commercialized medical-grade kanuka honey ointment (Honevo, 90% kanuka honey, 10% glycerin; Honeylab NZ) for rosacea.
The patient also had multiple AKs, including a raised, crusted, scaly lesion measuring 20 mm by 21 mm with marginal erythema on the back of one hand. The lesion had been present and dormant for a number of years, but it had recently begun to grow.
“This gentleman decided he’d just try the honey on his AK, too,” Dr. Rosen said. The man reported applying a small amount to the lesion and erythematous area once a day, leaving it on for about 30 to 60 minutes. After 5 days, he stopped because the lesion became tender. During the next two days, the patient reported “picking at” the lesion, which was softening. He repeated this cycle of treatment for 3 months with no other therapy to the lesion.
“The lesion gradually reduced in size with an initial rapid reduction in its dry, crusted nature,” the authors reported. “After 3 months, residual appearance of the lesion was a 20 mm by 17 mm area of pink skin with no elements of hypertrophy, crusting, or loss of skin integrity,” they noted. “At 6 months, there were no signs of recurrence. At 9 months, the appearance of the skin had fully returned to normal. A telephone follow-up was conducted at 2 years after treatment, and the patient reported that his skin in the area was still completely normal and that there were no signs of recurrence.”
Dr. Mane noted that they had only clinical evidence, and no histology of the lesion either before or after its change. “The AK was diagnosed and treated in primary care, where it is not usual for AKs to be biopsied, and the decision to write up the case was made after the course of treatment had finished,” they said.
“Immunomodulatory topical agents are already widely used in the treatment of AK as an immune component is evident in its etiology,” they wrote. “Immunocompromised patients have 250 times the risk of developing an AK than the general population.”
Dr. Rosen said that kanuka honey is also being investigated in psoriasis, eczema, acne, herpes simplex virus, and diaper dermatitis. It is also being studied for rosacea.
Dr. Mane declared no conflicts of interest. Some coauthors disclosed that they have previously received funding from HoneyLab NZ. Dr. Rosen has no commercial interest in HoneyLab.
The meeting was sponsored by Global Academy for Medical Education; Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – Not all honeys are created equal, Theodore Rosen, MD, said at the meeting provided by Global Academy for Medical Education.
“It seems that kanuka is the new manuka,” said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston. These lesser-known New Zealand bush honeys may be something to watch because research and case reports continue to provide intriguing hints of how these honeys exert their immunomodulatory effects on skin, he commented, describing a recent case report describing the elimination of a large, long-standing actinic keratosis (AK) with application of kanuka honey.
Manuka (Leptospermum scoparium) is a large bush native to both Australia and New Zealand. Kanuka (Kunzea ericoides) is quite similar in size and appearance, but native only to New Zealand. Honey made from the flowers of these bushes possesses some unique properties that make it an attractive addition to wound healing regimens, according to a 2014 study (Int J Gen Med. 2014;7:149-58).
The study examined samples of manuka, kanuka, a manuka/kanuka blend, and clover honey. The investigators found that kanuka honey, and to a lesser extent manuka honey, exerted a potent anti-inflammatory effect in human embryonic kidney cells. The honeys interfered with toll-like receptor 1 and 2 signaling, which would reduce the production of proinflammatory cytokines.
Kanuka’s potency seems directly related to its unusually high level of arabinogalactan, according to Saras Mane, MD, primary author of the AK case report (Case Rep Dermatol Med. 2018 May 31;2018:4628971). Dr. Mane is with the Medical Research Institute of New Zealand in Wellington.

“The immunomodulatory properties of kanuka honey in particular are thought to be more potent than other New Zealand honeys due to the relatively high concentrations of arabinogalactan proteins present,” Dr. Mane and his coauthors wrote in the case report. “These proteins have been shown to stimulate release of TNF-alpha from monocytic cell lines in vitro.”
The report involved a 66-year-old man who was enrolled in a randomized trial of a commercialized medical-grade kanuka honey ointment (Honevo, 90% kanuka honey, 10% glycerin; Honeylab NZ) for rosacea.
The patient also had multiple AKs, including a raised, crusted, scaly lesion measuring 20 mm by 21 mm with marginal erythema on the back of one hand. The lesion had been present and dormant for a number of years, but it had recently begun to grow.
“This gentleman decided he’d just try the honey on his AK, too,” Dr. Rosen said. The man reported applying a small amount to the lesion and erythematous area once a day, leaving it on for about 30 to 60 minutes. After 5 days, he stopped because the lesion became tender. During the next two days, the patient reported “picking at” the lesion, which was softening. He repeated this cycle of treatment for 3 months with no other therapy to the lesion.
“The lesion gradually reduced in size with an initial rapid reduction in its dry, crusted nature,” the authors reported. “After 3 months, residual appearance of the lesion was a 20 mm by 17 mm area of pink skin with no elements of hypertrophy, crusting, or loss of skin integrity,” they noted. “At 6 months, there were no signs of recurrence. At 9 months, the appearance of the skin had fully returned to normal. A telephone follow-up was conducted at 2 years after treatment, and the patient reported that his skin in the area was still completely normal and that there were no signs of recurrence.”
Dr. Mane noted that they had only clinical evidence, and no histology of the lesion either before or after its change. “The AK was diagnosed and treated in primary care, where it is not usual for AKs to be biopsied, and the decision to write up the case was made after the course of treatment had finished,” they said.
“Immunomodulatory topical agents are already widely used in the treatment of AK as an immune component is evident in its etiology,” they wrote. “Immunocompromised patients have 250 times the risk of developing an AK than the general population.”
Dr. Rosen said that kanuka honey is also being investigated in psoriasis, eczema, acne, herpes simplex virus, and diaper dermatitis. It is also being studied for rosacea.
Dr. Mane declared no conflicts of interest. Some coauthors disclosed that they have previously received funding from HoneyLab NZ. Dr. Rosen has no commercial interest in HoneyLab.
The meeting was sponsored by Global Academy for Medical Education; Global Academy and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL CARIBBEAN DERMATOLOGY SYMPOSIUM
Will microneedling enhance the impact of photodynamic therapy?
ORLANDO –
Dr. Spencer, who practices in St. Petersburg, Fla., and is cochair of the conference, gave attendees a roundup of what’s new in adjuncts and delivery methods for photodynamic therapy (PDT). Among the updates is the promise of PDT delivered by means of an ultrashort incubation time of 10-20 minutes, followed by prolonged blue light exposure time of 1 hour. “The idea is that the enzymatic conversion is occurring during the light exposure,” Dr. Spencer said, adding that reports of this approach are mostly anecdotal.
A variation on the ultrashort incubation adds microneedling, he said. In one recent study, 33 patients who had facial actinic keratoses (AKs) were randomized to 10 or 20 minutes of incubation after application of aminolevulinic acid (ALA), followed by 1,000 seconds of exposure to blue light. However, in this split-face study, participants each had one side of their faces treated with microneedling and the other half with a sham treatment before ALA was applied.
Those who had the shorter incubation time had 43% of AKs cleared on the side that received microneedling, compared with 38% on the sham side. For those who received 20 minutes of ALA incubation, rates were higher, with 76% AK clearance on the treated side and 58% on the sham side. “Patients reported that the procedure was virtually painless on both sides,” said Dr. Spencer.
Though the addition of microneedling to PDT is a newer trend, there’s one that’s been a mainstay in Europe for some time: daylight PDT. He cited a review article published in 2016, which identified 17 studies on the use of daylight PDT (Dermatol Surg. 2016 Mar;42[3]:286-95).
Advantages of daylight PDT, he said, include less time in the office for patients and “supposedly less pain.” European protocols vary, but most use methyl aminolevulinate, which he said is a “little more lipophilic than ALA,” with incubation times ranging from 0 to 30 minutes. Exposure time is also variable, but will usually range from 1.5 to 2.5 hours. Most patients receive just one treatment, but some protocols will include up to three treatments.
Overall, studies show a range from 46% to almost 90% complete response rates when AKs are treated with daylight PDT. One study that looked at daylight PDT for small basal cell carcinomas showed that 94% of patients had clinical clearance of their lesions after two treatment sessions; however, the recurrence rate at 12 months post therapy was 21%, Dr. Spencer said.
He shared results of a recent head-to-head study of conventional and daylight PDT; conducted in Greece, the study enrolled patients with “high sun exposure” and used a split-face design.
Of the 46 patients who received MAL on both sides of their faces, response rates were similar at both 3 and 12 months, with slightly numerically higher clearance rates for conventional versus daylight PDT. The 3-month clearance rate for conventional PDT was 80.6%, compared with 78.0% for daylight PDT. At 12 months, the respective clearance rates were 73.7% and 71.8% (J Eur Acad Dermatol Venereol. 2018 Apr;32[4]:595-600). However, “significantly less pain was reported with daylight PDT,” Dr. Spencer said.
Daylight PDT hasn’t caught on the United States. Physicians have concern about the lack of control of UV dosing, and, he pointed out, “this, of course, is not billable.”
Dr. Spencer reported that he serves on the speakers bureau for Genentech.
ORLANDO –
Dr. Spencer, who practices in St. Petersburg, Fla., and is cochair of the conference, gave attendees a roundup of what’s new in adjuncts and delivery methods for photodynamic therapy (PDT). Among the updates is the promise of PDT delivered by means of an ultrashort incubation time of 10-20 minutes, followed by prolonged blue light exposure time of 1 hour. “The idea is that the enzymatic conversion is occurring during the light exposure,” Dr. Spencer said, adding that reports of this approach are mostly anecdotal.
A variation on the ultrashort incubation adds microneedling, he said. In one recent study, 33 patients who had facial actinic keratoses (AKs) were randomized to 10 or 20 minutes of incubation after application of aminolevulinic acid (ALA), followed by 1,000 seconds of exposure to blue light. However, in this split-face study, participants each had one side of their faces treated with microneedling and the other half with a sham treatment before ALA was applied.
Those who had the shorter incubation time had 43% of AKs cleared on the side that received microneedling, compared with 38% on the sham side. For those who received 20 minutes of ALA incubation, rates were higher, with 76% AK clearance on the treated side and 58% on the sham side. “Patients reported that the procedure was virtually painless on both sides,” said Dr. Spencer.
Though the addition of microneedling to PDT is a newer trend, there’s one that’s been a mainstay in Europe for some time: daylight PDT. He cited a review article published in 2016, which identified 17 studies on the use of daylight PDT (Dermatol Surg. 2016 Mar;42[3]:286-95).
Advantages of daylight PDT, he said, include less time in the office for patients and “supposedly less pain.” European protocols vary, but most use methyl aminolevulinate, which he said is a “little more lipophilic than ALA,” with incubation times ranging from 0 to 30 minutes. Exposure time is also variable, but will usually range from 1.5 to 2.5 hours. Most patients receive just one treatment, but some protocols will include up to three treatments.
Overall, studies show a range from 46% to almost 90% complete response rates when AKs are treated with daylight PDT. One study that looked at daylight PDT for small basal cell carcinomas showed that 94% of patients had clinical clearance of their lesions after two treatment sessions; however, the recurrence rate at 12 months post therapy was 21%, Dr. Spencer said.
He shared results of a recent head-to-head study of conventional and daylight PDT; conducted in Greece, the study enrolled patients with “high sun exposure” and used a split-face design.
Of the 46 patients who received MAL on both sides of their faces, response rates were similar at both 3 and 12 months, with slightly numerically higher clearance rates for conventional versus daylight PDT. The 3-month clearance rate for conventional PDT was 80.6%, compared with 78.0% for daylight PDT. At 12 months, the respective clearance rates were 73.7% and 71.8% (J Eur Acad Dermatol Venereol. 2018 Apr;32[4]:595-600). However, “significantly less pain was reported with daylight PDT,” Dr. Spencer said.
Daylight PDT hasn’t caught on the United States. Physicians have concern about the lack of control of UV dosing, and, he pointed out, “this, of course, is not billable.”
Dr. Spencer reported that he serves on the speakers bureau for Genentech.
ORLANDO –
Dr. Spencer, who practices in St. Petersburg, Fla., and is cochair of the conference, gave attendees a roundup of what’s new in adjuncts and delivery methods for photodynamic therapy (PDT). Among the updates is the promise of PDT delivered by means of an ultrashort incubation time of 10-20 minutes, followed by prolonged blue light exposure time of 1 hour. “The idea is that the enzymatic conversion is occurring during the light exposure,” Dr. Spencer said, adding that reports of this approach are mostly anecdotal.
A variation on the ultrashort incubation adds microneedling, he said. In one recent study, 33 patients who had facial actinic keratoses (AKs) were randomized to 10 or 20 minutes of incubation after application of aminolevulinic acid (ALA), followed by 1,000 seconds of exposure to blue light. However, in this split-face study, participants each had one side of their faces treated with microneedling and the other half with a sham treatment before ALA was applied.
Those who had the shorter incubation time had 43% of AKs cleared on the side that received microneedling, compared with 38% on the sham side. For those who received 20 minutes of ALA incubation, rates were higher, with 76% AK clearance on the treated side and 58% on the sham side. “Patients reported that the procedure was virtually painless on both sides,” said Dr. Spencer.
Though the addition of microneedling to PDT is a newer trend, there’s one that’s been a mainstay in Europe for some time: daylight PDT. He cited a review article published in 2016, which identified 17 studies on the use of daylight PDT (Dermatol Surg. 2016 Mar;42[3]:286-95).
Advantages of daylight PDT, he said, include less time in the office for patients and “supposedly less pain.” European protocols vary, but most use methyl aminolevulinate, which he said is a “little more lipophilic than ALA,” with incubation times ranging from 0 to 30 minutes. Exposure time is also variable, but will usually range from 1.5 to 2.5 hours. Most patients receive just one treatment, but some protocols will include up to three treatments.
Overall, studies show a range from 46% to almost 90% complete response rates when AKs are treated with daylight PDT. One study that looked at daylight PDT for small basal cell carcinomas showed that 94% of patients had clinical clearance of their lesions after two treatment sessions; however, the recurrence rate at 12 months post therapy was 21%, Dr. Spencer said.
He shared results of a recent head-to-head study of conventional and daylight PDT; conducted in Greece, the study enrolled patients with “high sun exposure” and used a split-face design.
Of the 46 patients who received MAL on both sides of their faces, response rates were similar at both 3 and 12 months, with slightly numerically higher clearance rates for conventional versus daylight PDT. The 3-month clearance rate for conventional PDT was 80.6%, compared with 78.0% for daylight PDT. At 12 months, the respective clearance rates were 73.7% and 71.8% (J Eur Acad Dermatol Venereol. 2018 Apr;32[4]:595-600). However, “significantly less pain was reported with daylight PDT,” Dr. Spencer said.
Daylight PDT hasn’t caught on the United States. Physicians have concern about the lack of control of UV dosing, and, he pointed out, “this, of course, is not billable.”
Dr. Spencer reported that he serves on the speakers bureau for Genentech.
EXPERT ANALYSIS FROM ODAC 2019
Products being developed for AKs therapy may appeal to patients
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
REPORTING FROM CARIBBEAN DERMATOLOGY SYMPOSIUM
Imiquimod-Induced Hypopigmentation Following Treatment of Periungual Verruca Vulgaris
Imiquimod is derived from the imidazoquinoline family and works by activating both innate and adaptive immune pathways. Imiquimod binds to toll-like receptor 7 located on monocytes, macrophages, and dendritic cells,1 which allows nuclear factor κβ light chain enhancer of activated B cells to induce production of proinflammatory cytokines, including IFN-α and tumor necrosis factor α, as well as IL-1, IL-6, IL-8, IL-10, and IL-12.2 These proinflammatory cytokines play a role in the innate immunity, triggering upregulation of the adaptive immune pathway and activating type 1 helper T cells, cytotoxic T cells, and natural killer cells. These cells have antiviral and antitumoral effects that lend to their significance in coordinating innate and adaptive immune mechanisms.3 More specifically, imiquimod enhances dendritic cell migration to regional lymph nodes and induces apoptosis via activation of proapoptotic B-cell lymphoma 2 proteins.1,2 Imiquimod has been approved by the US Food and Drug Administration (FDA) to treat external genitalia and perianal condyloma acuminata, actinic keratoses (AKs), and superficial basal cell carcinoma (BCC). It often is used off label for antiviral or antitumoral therapy in Bowen disease, squamous cell carcinoma, lentigo maligna, vulvar intraepithelial neoplasia, molluscum contagiosum, common warts, and leishmaniasis.1,2 Imiquimod is generally well tolerated; erythema and irritation at the application site are the most common side effects, with pigmentary change being less common.
Case Report
A 51-year-old man with a medical history of vitamin D deficiency, vitamin B12 deficiency, tinea pedis, and BCC presented with periungual verruca vulgaris on the right fifth digit and left thumb (Figure 1). The patient was prescribed imiquimod cream 5% to be applied 3 times weekly for 3 months. At 5-month follow-up the patient reported new-onset vitiligolike patches of depigmentation on the hands and feet that abruptly began 3 months after initiating treatment with imiquimod. On examination he had several depigmented patches with well-defined irregular borders on the bilateral dorsal hands and right foot as well as the right elbow (Figure 2). There was no personal or family history of vitiligo, thyroid disease, or autoimmune disease. Thyroid function studies and autoimmune panel were unremarkable. The patient also denied applying imiquimod to areas other than the periungual region of the right fifth digit and left thumb. He declined a biopsy of the lesions and was given a prescription for tacrolimus ointment 0.1% for twice-daily application. At 3-month follow-up the depigmented patches had spread. The patient is currently on 5-fluorouracil cream 5%. Despite loss of pigmentation, the periungual verruca vulgaris has persisted as well as depigmentation.
Comment
Imiquimod therapy is commonly used to treat conditions for which an antiviral or antitumor immune response is necessary for treatment and full resolution of skin conditions. It can yield positive results in conditions that are difficult to treat, such as periungual verruca vulgaris.4 The most common adverse effects of imiquimod include localized inflammation and application-site reactions. Pigment changes, though less common, also have been reported. From 1997 to 2003, 1257 cases of imiquimod adverse effects were reported to the FDA. There were 68 reported cases of pigmentary change, of which 51 documented vitiligo, hypopigmentation, or depigmentation. The others reported hyperpigmentation following imiquimod use.4 The imiquimod package insert lists application-site hypopigmentation as a possible adverse effect.5 Imiquimod-induced hypopigmentation and depigmentation have been reported in the peer-reviewed literature.4,6-14 Pigment loss has been reported in imiquimod treatment of condyloma acuminata, superficial BCC, nodular BCC, and extramammary Paget disease.6-8 Duration of therapy to onset of pigment loss ranged from 7 to 28 weeks.9 Imiquimod dosing varied among reported cases, ranging from 3 times weekly to daily application. Interestingly, hypopigmentation or depigmentation are not commonly associated with imiquimod use for the treatment of AKs, which Burnett and Kouba9 proposed may be due to the twice weekly imiquimod dosing regimen recommended by the FDA for the treatment of AK (below the minimum threshold for pigment loss). Our patient applied imiquimod cream 5% to periungual verruca vulgaris 3 times weekly for 3 months and may have developed vitiligolike depigmentation because he met this theoretical dosage threshold. Further research is necessary to confirm a dosage-related threshold for the development of depigmentation. Imiquimod-induced pigment loss has mainly been limited to the site of application.
Depigmentation was limited to the application site the majority of the time; however, depigmentation at adjacent sites has been reported.10 This finding was consistent with the proposed notion that cytokines induced by imiquimod have localized paracrine activity.11 Our patient was unique in that his depigmentation was present at the site of application, adjacent to the site of application, and at distant sites. He applied imiquimod only to the periungual area of the right fifth digit and left thumb but experienced depigmentation at several other sites. Although it is possible that our patient unintentionally spread imiquimod on the distant sites, it is less likely that the application would have been sufficient to cause depigmentation. Although systemic absorption of topical medications varies depending on multiple factors, the systemic absorption of imiquimod is minimal with mild systemic side effects reported, including headache, myalgia, and influenzalike symptoms.5 Thus, it is possible that our patient developed distant vitiligolike depigmentation as a systemic side effect of imiquimod therapy. Although our patient declined to have a biopsy performed, Gowda et al15 reported biopsy-proven vitiligo, demonstrating the absence of melanin and melanocytes following the use of imiquimod.
Several mechanisms have been proposed for imiquimod-induced depigmentation. For example, imiquimod may induce melanocyte apoptosis by increasing the levels of several proinflammatory and proapoptotic cytokines.16 Imiquimod-induced melanocyte apoptosis appears to involve elevated caspase-3, decreased B-cell lymphoma 2, altered mitogen-activated protein kinase expression, and ubiquitin-mediated proteolysis.13,17 Additionally, increased levels of IL-6 appear to increase melanocyte-binding molecules and increase melanocyte-leukocyte interactions. Another proposed theory targets toll-like receptor 7 on melanocytes that are acted on directly by imiquimod.11,17 In contrast, development of vitiligo following trauma (Koebner phenomenon) is not uncommon, and the immune effects induced by imiquimod may mimic those seen with trauma.14 Further research is needed to elucidate the mechanism by which imiquimod causes vitiligolike depigmentation.
Unfortunately, the depigmentation seen with imiquimod generally is permanent. Stefanaki et al10 showed repigmentation on cessation of imiquimod use. Our patient’s depigmentation remains unchanged despite treatment with tacrolimus ointment. Although it is possible for vitiligo to occur de novo without obvious inciting event or laboratory abnormality, the timeline and number of other cases in the literature make ours highly suspect for imiquimod-induced depigmentation.
Conclusion
Imiquimod is a commonly used immune-enhancing medication with an increasing list of off-label uses. Prior to prescribing imiquimod for a benign skin condition, clinicians should be cognizant of the potential for localized or possibly even distant depigmentation. We report a case of distant depigmentation following the use of imiquimod for periungual verruca vulgaris.
- Ganjian S, Ourian AJ, Shamtoub G, et al. Off-label indications for imiquimod. Dermatol Online J. 2009;15:4.
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21:291-300.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, eds. Janeway’s Immunobiology. 7th ed. New York, NY: Garland Science. 2008:39-108.
- Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715-716.
- Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2007.
- Kwon HH, Cho KH. Induction of vitiligo-like hypopigmentation after imiquimod treatment of extramammary Paget’s disease. Ann Dermatol. 2012;24:482-484.
- Mendonca CO, Yates VM. Permanent facial hypopigmentation following treatment with imiquimod. Clin Exp Dermatol. 2006;31:721-722.
- Zhang R, Zhu W. Genital vitiligo following use of imiquimod 5% cream. Indian J Dermatol. 2011;56:335-336.
- Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38:1872-1875.
- Stefanaki C, Nicolaidou E, Hadjivassiliou M. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755-756.
- Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10.
- Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2007;33:74-76.
- Grahovac M, Ehmann LM, Flaig M, et al. Giant basal cell carcinoma. Improvement and vitiligo-like hypopigmentation after intermittent treatment with 5% imiquimod. Acta Dermatovenerol Croat. 2012;20:275-278.
- Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342-343.
- Gowda S, Tillman DK, Fitzpatrick JE, et al. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878-881.
- Kim CH, Ahn JH, Kang SU, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301-306.
- Eapen BR. Vitiligo, psoriasis, and imiquimod: fitting all into the same pathway. Indian J Dermatol Venereol Leprol. 2008;74:169.
Imiquimod is derived from the imidazoquinoline family and works by activating both innate and adaptive immune pathways. Imiquimod binds to toll-like receptor 7 located on monocytes, macrophages, and dendritic cells,1 which allows nuclear factor κβ light chain enhancer of activated B cells to induce production of proinflammatory cytokines, including IFN-α and tumor necrosis factor α, as well as IL-1, IL-6, IL-8, IL-10, and IL-12.2 These proinflammatory cytokines play a role in the innate immunity, triggering upregulation of the adaptive immune pathway and activating type 1 helper T cells, cytotoxic T cells, and natural killer cells. These cells have antiviral and antitumoral effects that lend to their significance in coordinating innate and adaptive immune mechanisms.3 More specifically, imiquimod enhances dendritic cell migration to regional lymph nodes and induces apoptosis via activation of proapoptotic B-cell lymphoma 2 proteins.1,2 Imiquimod has been approved by the US Food and Drug Administration (FDA) to treat external genitalia and perianal condyloma acuminata, actinic keratoses (AKs), and superficial basal cell carcinoma (BCC). It often is used off label for antiviral or antitumoral therapy in Bowen disease, squamous cell carcinoma, lentigo maligna, vulvar intraepithelial neoplasia, molluscum contagiosum, common warts, and leishmaniasis.1,2 Imiquimod is generally well tolerated; erythema and irritation at the application site are the most common side effects, with pigmentary change being less common.
Case Report
A 51-year-old man with a medical history of vitamin D deficiency, vitamin B12 deficiency, tinea pedis, and BCC presented with periungual verruca vulgaris on the right fifth digit and left thumb (Figure 1). The patient was prescribed imiquimod cream 5% to be applied 3 times weekly for 3 months. At 5-month follow-up the patient reported new-onset vitiligolike patches of depigmentation on the hands and feet that abruptly began 3 months after initiating treatment with imiquimod. On examination he had several depigmented patches with well-defined irregular borders on the bilateral dorsal hands and right foot as well as the right elbow (Figure 2). There was no personal or family history of vitiligo, thyroid disease, or autoimmune disease. Thyroid function studies and autoimmune panel were unremarkable. The patient also denied applying imiquimod to areas other than the periungual region of the right fifth digit and left thumb. He declined a biopsy of the lesions and was given a prescription for tacrolimus ointment 0.1% for twice-daily application. At 3-month follow-up the depigmented patches had spread. The patient is currently on 5-fluorouracil cream 5%. Despite loss of pigmentation, the periungual verruca vulgaris has persisted as well as depigmentation.


Comment
Imiquimod therapy is commonly used to treat conditions for which an antiviral or antitumor immune response is necessary for treatment and full resolution of skin conditions. It can yield positive results in conditions that are difficult to treat, such as periungual verruca vulgaris.4 The most common adverse effects of imiquimod include localized inflammation and application-site reactions. Pigment changes, though less common, also have been reported. From 1997 to 2003, 1257 cases of imiquimod adverse effects were reported to the FDA. There were 68 reported cases of pigmentary change, of which 51 documented vitiligo, hypopigmentation, or depigmentation. The others reported hyperpigmentation following imiquimod use.4 The imiquimod package insert lists application-site hypopigmentation as a possible adverse effect.5 Imiquimod-induced hypopigmentation and depigmentation have been reported in the peer-reviewed literature.4,6-14 Pigment loss has been reported in imiquimod treatment of condyloma acuminata, superficial BCC, nodular BCC, and extramammary Paget disease.6-8 Duration of therapy to onset of pigment loss ranged from 7 to 28 weeks.9 Imiquimod dosing varied among reported cases, ranging from 3 times weekly to daily application. Interestingly, hypopigmentation or depigmentation are not commonly associated with imiquimod use for the treatment of AKs, which Burnett and Kouba9 proposed may be due to the twice weekly imiquimod dosing regimen recommended by the FDA for the treatment of AK (below the minimum threshold for pigment loss). Our patient applied imiquimod cream 5% to periungual verruca vulgaris 3 times weekly for 3 months and may have developed vitiligolike depigmentation because he met this theoretical dosage threshold. Further research is necessary to confirm a dosage-related threshold for the development of depigmentation. Imiquimod-induced pigment loss has mainly been limited to the site of application.
Depigmentation was limited to the application site the majority of the time; however, depigmentation at adjacent sites has been reported.10 This finding was consistent with the proposed notion that cytokines induced by imiquimod have localized paracrine activity.11 Our patient was unique in that his depigmentation was present at the site of application, adjacent to the site of application, and at distant sites. He applied imiquimod only to the periungual area of the right fifth digit and left thumb but experienced depigmentation at several other sites. Although it is possible that our patient unintentionally spread imiquimod on the distant sites, it is less likely that the application would have been sufficient to cause depigmentation. Although systemic absorption of topical medications varies depending on multiple factors, the systemic absorption of imiquimod is minimal with mild systemic side effects reported, including headache, myalgia, and influenzalike symptoms.5 Thus, it is possible that our patient developed distant vitiligolike depigmentation as a systemic side effect of imiquimod therapy. Although our patient declined to have a biopsy performed, Gowda et al15 reported biopsy-proven vitiligo, demonstrating the absence of melanin and melanocytes following the use of imiquimod.
Several mechanisms have been proposed for imiquimod-induced depigmentation. For example, imiquimod may induce melanocyte apoptosis by increasing the levels of several proinflammatory and proapoptotic cytokines.16 Imiquimod-induced melanocyte apoptosis appears to involve elevated caspase-3, decreased B-cell lymphoma 2, altered mitogen-activated protein kinase expression, and ubiquitin-mediated proteolysis.13,17 Additionally, increased levels of IL-6 appear to increase melanocyte-binding molecules and increase melanocyte-leukocyte interactions. Another proposed theory targets toll-like receptor 7 on melanocytes that are acted on directly by imiquimod.11,17 In contrast, development of vitiligo following trauma (Koebner phenomenon) is not uncommon, and the immune effects induced by imiquimod may mimic those seen with trauma.14 Further research is needed to elucidate the mechanism by which imiquimod causes vitiligolike depigmentation.
Unfortunately, the depigmentation seen with imiquimod generally is permanent. Stefanaki et al10 showed repigmentation on cessation of imiquimod use. Our patient’s depigmentation remains unchanged despite treatment with tacrolimus ointment. Although it is possible for vitiligo to occur de novo without obvious inciting event or laboratory abnormality, the timeline and number of other cases in the literature make ours highly suspect for imiquimod-induced depigmentation.
Conclusion
Imiquimod is a commonly used immune-enhancing medication with an increasing list of off-label uses. Prior to prescribing imiquimod for a benign skin condition, clinicians should be cognizant of the potential for localized or possibly even distant depigmentation. We report a case of distant depigmentation following the use of imiquimod for periungual verruca vulgaris.
Imiquimod is derived from the imidazoquinoline family and works by activating both innate and adaptive immune pathways. Imiquimod binds to toll-like receptor 7 located on monocytes, macrophages, and dendritic cells,1 which allows nuclear factor κβ light chain enhancer of activated B cells to induce production of proinflammatory cytokines, including IFN-α and tumor necrosis factor α, as well as IL-1, IL-6, IL-8, IL-10, and IL-12.2 These proinflammatory cytokines play a role in the innate immunity, triggering upregulation of the adaptive immune pathway and activating type 1 helper T cells, cytotoxic T cells, and natural killer cells. These cells have antiviral and antitumoral effects that lend to their significance in coordinating innate and adaptive immune mechanisms.3 More specifically, imiquimod enhances dendritic cell migration to regional lymph nodes and induces apoptosis via activation of proapoptotic B-cell lymphoma 2 proteins.1,2 Imiquimod has been approved by the US Food and Drug Administration (FDA) to treat external genitalia and perianal condyloma acuminata, actinic keratoses (AKs), and superficial basal cell carcinoma (BCC). It often is used off label for antiviral or antitumoral therapy in Bowen disease, squamous cell carcinoma, lentigo maligna, vulvar intraepithelial neoplasia, molluscum contagiosum, common warts, and leishmaniasis.1,2 Imiquimod is generally well tolerated; erythema and irritation at the application site are the most common side effects, with pigmentary change being less common.
Case Report
A 51-year-old man with a medical history of vitamin D deficiency, vitamin B12 deficiency, tinea pedis, and BCC presented with periungual verruca vulgaris on the right fifth digit and left thumb (Figure 1). The patient was prescribed imiquimod cream 5% to be applied 3 times weekly for 3 months. At 5-month follow-up the patient reported new-onset vitiligolike patches of depigmentation on the hands and feet that abruptly began 3 months after initiating treatment with imiquimod. On examination he had several depigmented patches with well-defined irregular borders on the bilateral dorsal hands and right foot as well as the right elbow (Figure 2). There was no personal or family history of vitiligo, thyroid disease, or autoimmune disease. Thyroid function studies and autoimmune panel were unremarkable. The patient also denied applying imiquimod to areas other than the periungual region of the right fifth digit and left thumb. He declined a biopsy of the lesions and was given a prescription for tacrolimus ointment 0.1% for twice-daily application. At 3-month follow-up the depigmented patches had spread. The patient is currently on 5-fluorouracil cream 5%. Despite loss of pigmentation, the periungual verruca vulgaris has persisted as well as depigmentation.


Comment
Imiquimod therapy is commonly used to treat conditions for which an antiviral or antitumor immune response is necessary for treatment and full resolution of skin conditions. It can yield positive results in conditions that are difficult to treat, such as periungual verruca vulgaris.4 The most common adverse effects of imiquimod include localized inflammation and application-site reactions. Pigment changes, though less common, also have been reported. From 1997 to 2003, 1257 cases of imiquimod adverse effects were reported to the FDA. There were 68 reported cases of pigmentary change, of which 51 documented vitiligo, hypopigmentation, or depigmentation. The others reported hyperpigmentation following imiquimod use.4 The imiquimod package insert lists application-site hypopigmentation as a possible adverse effect.5 Imiquimod-induced hypopigmentation and depigmentation have been reported in the peer-reviewed literature.4,6-14 Pigment loss has been reported in imiquimod treatment of condyloma acuminata, superficial BCC, nodular BCC, and extramammary Paget disease.6-8 Duration of therapy to onset of pigment loss ranged from 7 to 28 weeks.9 Imiquimod dosing varied among reported cases, ranging from 3 times weekly to daily application. Interestingly, hypopigmentation or depigmentation are not commonly associated with imiquimod use for the treatment of AKs, which Burnett and Kouba9 proposed may be due to the twice weekly imiquimod dosing regimen recommended by the FDA for the treatment of AK (below the minimum threshold for pigment loss). Our patient applied imiquimod cream 5% to periungual verruca vulgaris 3 times weekly for 3 months and may have developed vitiligolike depigmentation because he met this theoretical dosage threshold. Further research is necessary to confirm a dosage-related threshold for the development of depigmentation. Imiquimod-induced pigment loss has mainly been limited to the site of application.
Depigmentation was limited to the application site the majority of the time; however, depigmentation at adjacent sites has been reported.10 This finding was consistent with the proposed notion that cytokines induced by imiquimod have localized paracrine activity.11 Our patient was unique in that his depigmentation was present at the site of application, adjacent to the site of application, and at distant sites. He applied imiquimod only to the periungual area of the right fifth digit and left thumb but experienced depigmentation at several other sites. Although it is possible that our patient unintentionally spread imiquimod on the distant sites, it is less likely that the application would have been sufficient to cause depigmentation. Although systemic absorption of topical medications varies depending on multiple factors, the systemic absorption of imiquimod is minimal with mild systemic side effects reported, including headache, myalgia, and influenzalike symptoms.5 Thus, it is possible that our patient developed distant vitiligolike depigmentation as a systemic side effect of imiquimod therapy. Although our patient declined to have a biopsy performed, Gowda et al15 reported biopsy-proven vitiligo, demonstrating the absence of melanin and melanocytes following the use of imiquimod.
Several mechanisms have been proposed for imiquimod-induced depigmentation. For example, imiquimod may induce melanocyte apoptosis by increasing the levels of several proinflammatory and proapoptotic cytokines.16 Imiquimod-induced melanocyte apoptosis appears to involve elevated caspase-3, decreased B-cell lymphoma 2, altered mitogen-activated protein kinase expression, and ubiquitin-mediated proteolysis.13,17 Additionally, increased levels of IL-6 appear to increase melanocyte-binding molecules and increase melanocyte-leukocyte interactions. Another proposed theory targets toll-like receptor 7 on melanocytes that are acted on directly by imiquimod.11,17 In contrast, development of vitiligo following trauma (Koebner phenomenon) is not uncommon, and the immune effects induced by imiquimod may mimic those seen with trauma.14 Further research is needed to elucidate the mechanism by which imiquimod causes vitiligolike depigmentation.
Unfortunately, the depigmentation seen with imiquimod generally is permanent. Stefanaki et al10 showed repigmentation on cessation of imiquimod use. Our patient’s depigmentation remains unchanged despite treatment with tacrolimus ointment. Although it is possible for vitiligo to occur de novo without obvious inciting event or laboratory abnormality, the timeline and number of other cases in the literature make ours highly suspect for imiquimod-induced depigmentation.
Conclusion
Imiquimod is a commonly used immune-enhancing medication with an increasing list of off-label uses. Prior to prescribing imiquimod for a benign skin condition, clinicians should be cognizant of the potential for localized or possibly even distant depigmentation. We report a case of distant depigmentation following the use of imiquimod for periungual verruca vulgaris.
- Ganjian S, Ourian AJ, Shamtoub G, et al. Off-label indications for imiquimod. Dermatol Online J. 2009;15:4.
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21:291-300.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, eds. Janeway’s Immunobiology. 7th ed. New York, NY: Garland Science. 2008:39-108.
- Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715-716.
- Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2007.
- Kwon HH, Cho KH. Induction of vitiligo-like hypopigmentation after imiquimod treatment of extramammary Paget’s disease. Ann Dermatol. 2012;24:482-484.
- Mendonca CO, Yates VM. Permanent facial hypopigmentation following treatment with imiquimod. Clin Exp Dermatol. 2006;31:721-722.
- Zhang R, Zhu W. Genital vitiligo following use of imiquimod 5% cream. Indian J Dermatol. 2011;56:335-336.
- Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38:1872-1875.
- Stefanaki C, Nicolaidou E, Hadjivassiliou M. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755-756.
- Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10.
- Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2007;33:74-76.
- Grahovac M, Ehmann LM, Flaig M, et al. Giant basal cell carcinoma. Improvement and vitiligo-like hypopigmentation after intermittent treatment with 5% imiquimod. Acta Dermatovenerol Croat. 2012;20:275-278.
- Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342-343.
- Gowda S, Tillman DK, Fitzpatrick JE, et al. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878-881.
- Kim CH, Ahn JH, Kang SU, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301-306.
- Eapen BR. Vitiligo, psoriasis, and imiquimod: fitting all into the same pathway. Indian J Dermatol Venereol Leprol. 2008;74:169.
- Ganjian S, Ourian AJ, Shamtoub G, et al. Off-label indications for imiquimod. Dermatol Online J. 2009;15:4.
- Skinner RB Jr. Imiquimod. Dermatol Clin. 2003;21:291-300.
- Murphy K, Travers P, Walport M. Innate immunity. In: Murphy K, Travers P, Walport M, eds. Janeway’s Immunobiology. 7th ed. New York, NY: Garland Science. 2008:39-108.
- Brown T, Zirvi M, Cotsarelis G, et al. Vitiligo-like hypopigmentation associated with imiquimod treatment of genital warts. J Am Acad Dermatol. 2005;52:715-716.
- Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2007.
- Kwon HH, Cho KH. Induction of vitiligo-like hypopigmentation after imiquimod treatment of extramammary Paget’s disease. Ann Dermatol. 2012;24:482-484.
- Mendonca CO, Yates VM. Permanent facial hypopigmentation following treatment with imiquimod. Clin Exp Dermatol. 2006;31:721-722.
- Zhang R, Zhu W. Genital vitiligo following use of imiquimod 5% cream. Indian J Dermatol. 2011;56:335-336.
- Burnett CT, Kouba DJ. Imiquimod-induced depigmentation: report of two cases and review of the literature. Dermatol Surg. 2012;38:1872-1875.
- Stefanaki C, Nicolaidou E, Hadjivassiliou M. Imiquimod-induced vitiligo in a patient with genital warts. J Eur Acad Dermatol Venereol. 2006;20:755-756.
- Al-Dujaili Z, Hsu S. Imiquimod-induced vitiligo. Dermatol Online J. 2007;13:10.
- Mashiah J, Brenner S. Possible mechanisms in the induction of vitiligo-like hypopigmentation by topical imiquimod. Clin Exp Dermatol. 2007;33:74-76.
- Grahovac M, Ehmann LM, Flaig M, et al. Giant basal cell carcinoma. Improvement and vitiligo-like hypopigmentation after intermittent treatment with 5% imiquimod. Acta Dermatovenerol Croat. 2012;20:275-278.
- Serrão VV, Páris FR, Feio AB. Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol. 2008;18:342-343.
- Gowda S, Tillman DK, Fitzpatrick JE, et al. Imiquimod-induced vitiligo after treatment of nodular basal cell carcinoma. J Cutan Pathol. 2009;36:878-881.
- Kim CH, Ahn JH, Kang SU, et al. Imiquimod induces apoptosis of human melanocytes. Arch Dermatol Res. 2010;302:301-306.
- Eapen BR. Vitiligo, psoriasis, and imiquimod: fitting all into the same pathway. Indian J Dermatol Venereol Leprol. 2008;74:169.
Practice Points
- Imiquimod commonly is used off label to treat viral and neoplastic processes.
- Clinicians should be aware of the potential for dyspigmentation or depigmentation as a side effect from treatment.