User login
Energy-Based Devices for Actinic Keratosis Field Therapy
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
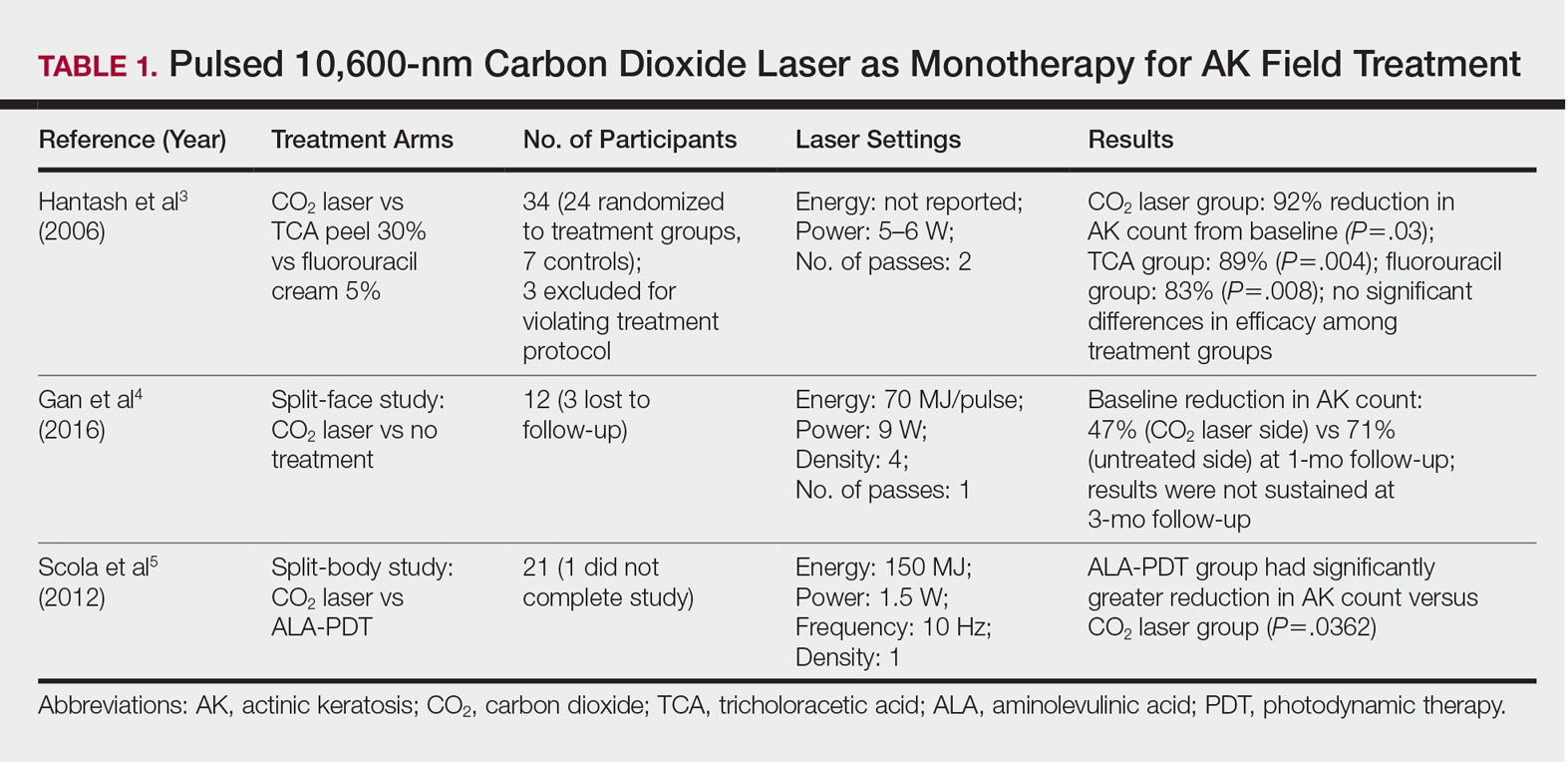
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
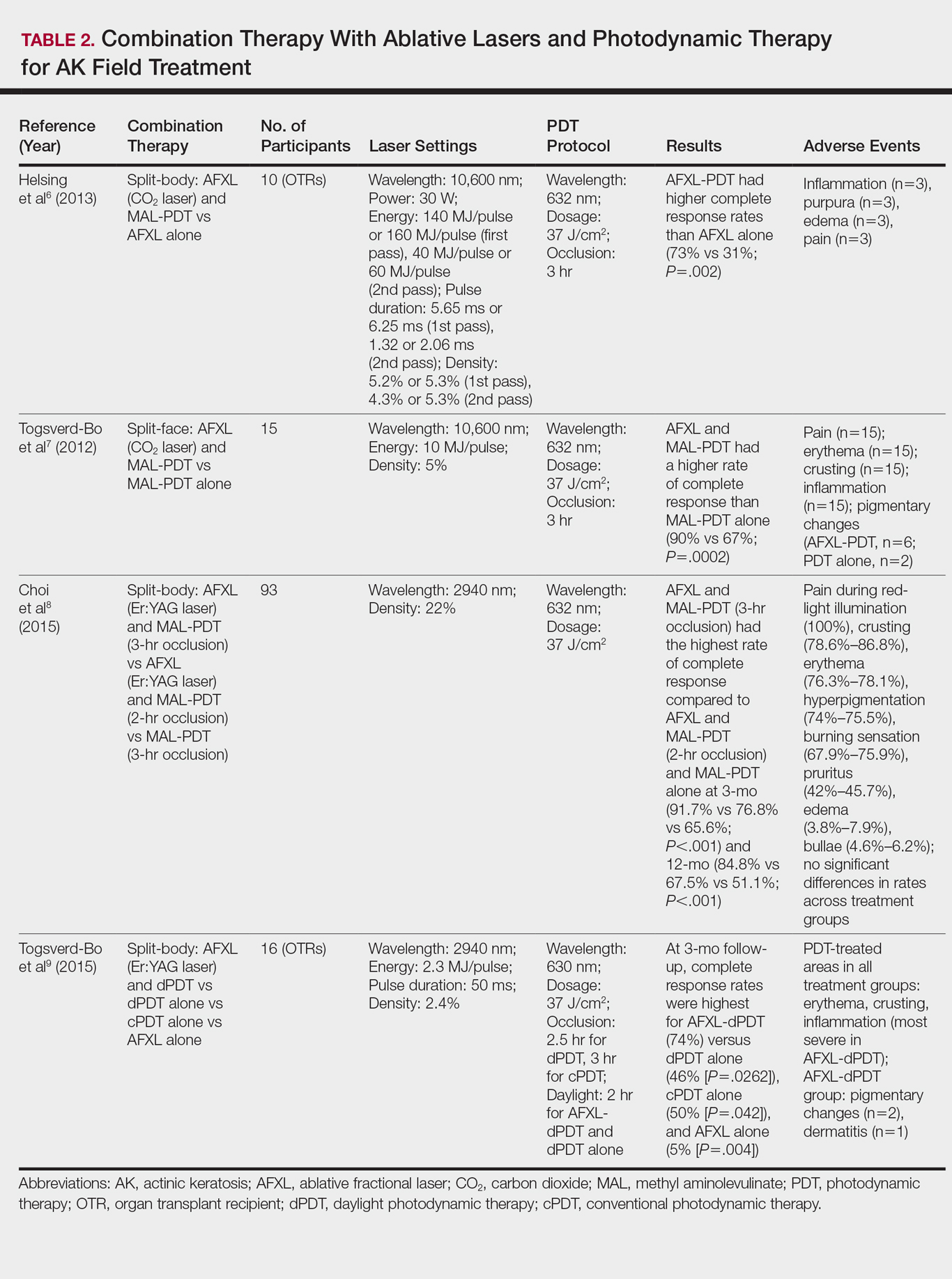
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
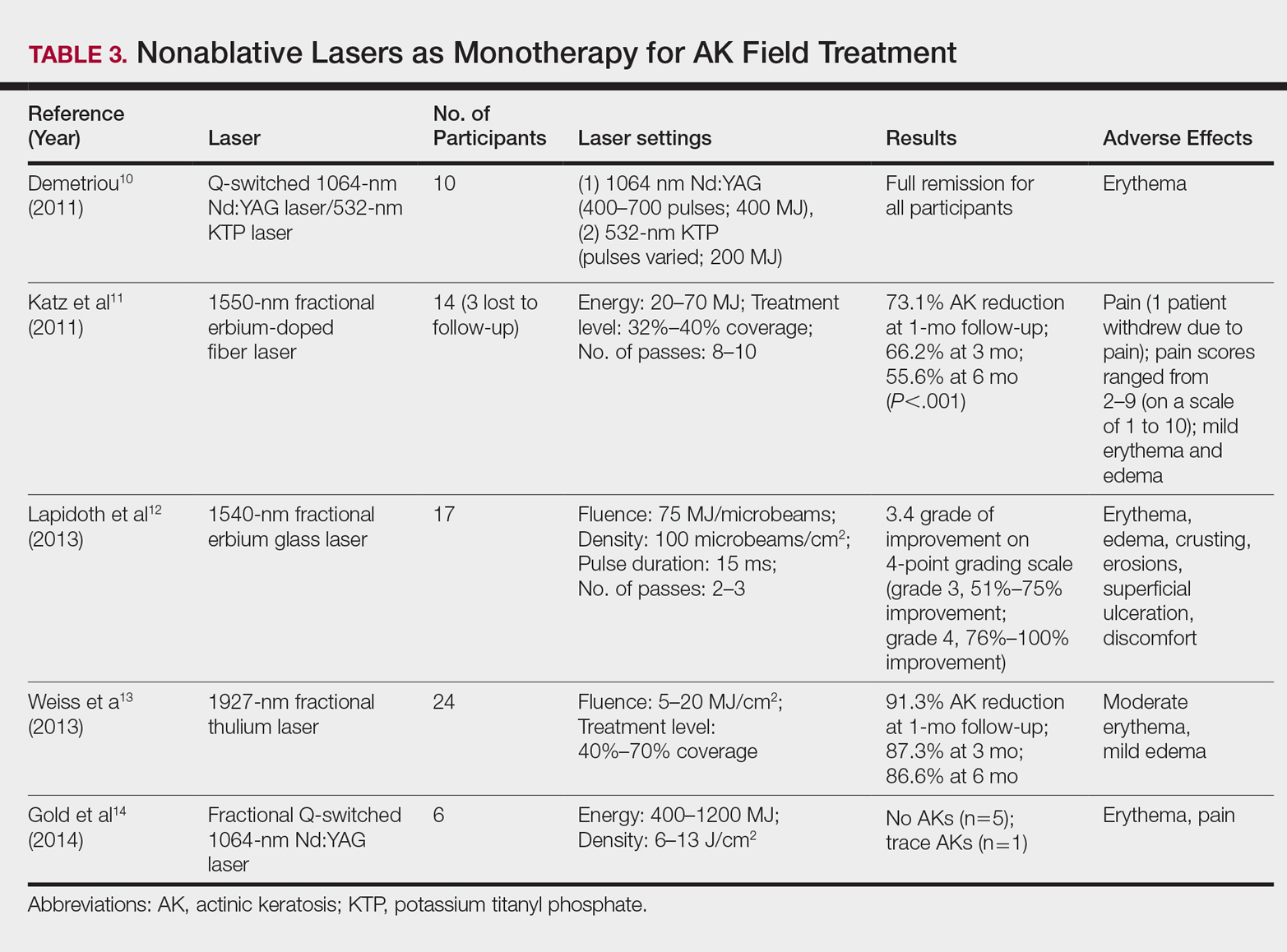
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
In cutaneous field cancerization, focal treatments such as cryotherapy are impractical, thus necessitating the use of field-directed therapies over the lesion and the surrounding skin field. Although evidence-based guidelines do not exist, field-directed therapy has been proposed in cases of 3 or more actinic keratoses (AKs) in a 25-cm2 area or larger.1 It can be further speculated that patients who are vulnerable to aggressive phenotypes of cutaneous malignancies, such as those with a genodermatosis or who are immunocompromised, necessitate a higher index of suspicion for field effect with even 1 or 2 AKs.
Current field-directed therapies include topical agents (imiquimod, fluorouracil, ingenol mebutate, and diclo-fenac), photodynamic therapy (PDT), and resurfacing procedures (lasers, chemical peels, dermabrasion). Although topical agents and PDT currently are gold standards in field treatment, the use of energy-based devices (ie, ablative and nonablative lasers) are attractive options as monotherapy or as part of a combination therapy. These devices are attractive options for field-directed therapy because they offer defined, customizable control of settings, allowing for optimal cosmesis and precision of therapy.
Principally, lasers function by damaging skin tissue to induce resurfacing, neocollagenesis, and vascular restructuring. Fractional versions of ablative and nonablative systems are available to target a fraction of the treatment area in evenly spaced microthermal zones and to minimize overall thermal damage.2
Given recent advances in laser systems and numerous investigations reported in the literature, a review of ablative and nonablative lasers that have been studied as treatment options for cutaneous field cancerization is provided, with a focus on treatment efficacy.
Ablative Lasers
Ablative lasers operate at higher wavelengths than nonablative lasers to destroy epidermal and dermal tissue. The 10,600-nm carbon dioxide (CO2) and 2940-nm Er:YAG lasers have been heavily investigated for field therapy for multiple AKs, both as monotherapies (Table 1) and in combination with PDT (Table 2).
Monotherapy
One randomized trial with 5-year follow-up compared the efficacy of full-face pulsed CO2 laser therapy, full-face trichloroacetic acid (TCA) peel 30%, and fluorouracil cream 5% (twice daily for 3 weeks) on AKs on the face and head.3 Thirty-one participants were randomized to the 3 treatment arms and a negative control arm. The mean AK counts at baseline for the CO2, TCA, and fluorouracil treatment groups were 78.0, 83.7, and 61.8, respectively. At 3-month follow-up, all treatment groups had significant reductions in the mean AK count from baseline (CO2 group, 92% [P=.03]; TCA group, 89% [P=.004]; fluorouracil group, 83% [P=.008]). No significant differences in efficacy among the treatment groups were noted. All 3 treatment groups had a demonstrably lower incidence of nonmelanoma skin cancer over 5-year follow-up compared to the control group (P<.001).3
In contrast to these promising results, the pulsed CO2 laser showed only short-term efficacy in a split-face study of 12 participants with at least 5 facial or scalp AKs on each of 2 symmetric facial sides who were randomized to 1 treatment side.4 At 1-month follow-up, the treatment side exhibited significantly fewer AKs compared to the control side (47% vs 71% at baseline; P=.01), but the improvement was not sustained at 3-month follow-up (49% vs 57%; P=.47).4
In another study, the CO2 laser was found to be inferior to 5-aminolevulinic acid PDT.5 Twenty-one participants who had at least 4 AKs in each symmetric half of a body region (head, hands, forearms) were randomized to PDT on 1 side and CO2 laser therapy on the other. Median baseline AK counts for the PDT and CO2 laser groups were 6 and 8, respectively. Both treatment groups exhibited significant median AK reduction from baseline 4 weeks posttreatment (PDT group, 82.1% [P<.05], CO2 laser group, 100% [P<.05]); however. at 3 months posttreatment the PDT group had significantly higher absolute (P=.0155) and relative (P=.0362) reductions in AK count compared to the CO2 laser group. One participant received a topical antibiotic for superficial infection on the PDT treatment side.5
Many questions remain regarding the practical application of laser ablation monotherapy for multiple AKs. More studies are needed to determine the practicality and long-term clinical efficacy of these devices.
PDT Combination Therapy
Laser ablation may be combined with PDT to increase efficacy and prolong remission rates. In fact, laser ablation may be thought of as a physical drug-delivery system to boost uptake of topical agents—in this case, aminolevulinic acid and methyl aminolevulinate (MAL)—given that it disrupts the skin barrier.
In a comparative study of ablative fractional laser (AFXL)–assisted PDT and AFXL alone in 10 organ transplant recipients on immunosuppression with at least 5 AKs on each dorsal hand, participants were randomized to AFXL-PDT on one treatment side and PDT on the other side.6 Participants received AFXL in an initial lesion-directed pass and then a second field-directed pass of a fractional CO2 laser. After AFXL exposure, methyl aminolevulinate was applied to the AFXL-PDT treatment side, with 3-hour occlusion. A total of 680 AKs were treated (335 in the AFXL-PDT group, 345 in the PDT group); results were stratified by the clinical grade of the lesion (1, slightly palpable; 2, moderately thick; 3, very thick or obvious). At 4-month follow-up, the AFXL-PDT group had a significantly higher median complete response rate of 73% compared to 31% in the AFXL group (P=.002). Interestingly, AFXL-PDT was also significantly more efficacious compared to AFXL for grades 1 (80% vs 37%; P=.02) and 2 (53% vs 7%, P=.009) AKs but not grade 3 AKs (4% vs 0%, P=.17).6
The combination of fractional CO2 laser and PDT also demonstrated superiority to PDT.7 In a split-face investigation, 15 participants with bilateral symmetric areas of 2 to 10 AKs on the face or scalp were randomized to receive fractional CO2 laser and MAL-PDT combination therapy on 1 treatment side and conventional MAL-PDT on the other side.7 The AFXL-PDT treatment side received laser ablation with immediate subsequent application of MAL to both treatment sides under 3-hour occlusion. At baseline, 103 AKs were treated by AFXL-PDT and 109 AKs were treated with conventional PDT. At 3-month follow-up, the AFXL-PDT treatment group exhibited a significantly higher rate of complete response (90%) compared to the conventional PDT group (67%)(P=.0002).7
Like the CO2 laser, the Er:YAG laser has demonstrated superior results when used in combination with PDT to treat field cancerization compared to either treatment alone. In a comparison study, 93 patients with 2 to 10 AK lesions on the face or scalp were randomized to treatment with AFXL (Er:YAG laser) and MAL-PDT with 3-hour occlusion, AFXL (Er:YAG laser) and MAL-PDT with 2-hour occlusion, and MAL-PDT with 3-hour occlusion.8 A total of 440 baseline AK lesions on the face or scalp were treated. At 3-month follow-up, the AFXL-PDT (3-hour occlusion) group had the highest rate of complete response (91.7%), compared to 76.8% (P=.001) in the AFXL-PDT (2-hour occlusion) and 65.6% (P=.001) in the PDT groups, regardless of the grade of AK lesion. The AFXL-PDT (2-hour occlusion) treatment was also superior to PDT alone (P=.038). These findings were sustained at 12-month follow-up (84.8% in the AFXL-PDT [3-hour occlusion] group [P<.001, compared to others]; 67.5% in the AFXL-PDT [2-hour occlusion] group [P<.001, compared to 3-hour PDT]; 51.1% in the PDT group). Importantly, the AK lesion recurrence rate was also lowest in the AFL-PDT (3-hour occlusion) group (7.5% vs 12.1% and 22.1% in the AFXL-PDT [2-hour occlusion] and PDT groups, respectively; P=.007).8
Combination therapy with AFXL and daylight PDT (dPDT) may improve the tolerability of PDT and the efficacy rate of field therapy in organ transplant recipients. One study demonstrated the superiority of this combination therapy in a population of 16 organ transplant recipients on immunosuppressants with at least 2 moderate to severely thick AKs in each of 4 comparable areas in the same anatomic region.9 The 4 areas were randomized to a single session of AFXL-dPDT, dPDT alone, conventional PDT, or AFXL alone. Ablation was performed with a fractional Er:YAG laser. The AFXL-dPDT and dPDT alone groups received MAL for 2.5 hours without occlusion, and the conventional PDT group received MAL for 3 hours with occlusion. Daylight exposure in dPDT groups was initiated 30 minutes after MAL application for 2 hours total. A baseline total of 542 AKs were treated. At 3-month follow-up, the complete response rate was highest for the AFXL-dPDT group (74%) compared to dPDT alone (46%; P=.0262), conventional PDT (50%; P=.042), and AFXL alone (5%; P=.004). Pain scores for AFXL–dPDT and dPDT alone were significantly lower than for conventional PDT and AFXL alone (P<.001).9
Nonablative Lasers
By heating the dermis to induce neogenesis without destruction, nonablative lasers offer superior healing times compared to their ablative counterparts. Multiple treatments with nonablative lasers may be necessary for maximal effect. Four nonablative laser devices have demonstrated efficacy in the treatment of multiple AKs10-14: (1) the Q-switched 1064-nm Nd:YAG laser, with or without a 532-nm potassium titanyl phosphate (KTP) laser; (2) the 1540-nm fractional erbium glass laser; (3) the 1550-nm fractional erbium-doped fiber laser; and (4) the 1927-nm fractional thulium laser (Table 3).
In a proof-of-concept study of the Q-switched Nd:YAG laser with the 532-nm KTP laser, 1 treatment session induced full remission of AKs in 10 patients at follow-up day 20, although the investigator did not grade improvement on a numerical scale.10 In a study of the fractional Q-switched 1064-nm Nd:YAG laser alone, 6 patients with trace or mild AKs received 4 treatment sessions at approximately 2-week intervals.14 All but 1 patient (who had trace AKs) had no AKs at 3-month follow-up.
The efficacy of the 1540-nm fractional erbium glass laser was examined in 17 participants with investigator-rated moderate-to-severe AK involvement of the scalp and face.12 Participants were given 2 or 3 treatment sessions at 3- to 4-week intervals and were graded by blinded dermatologists on a quartile scale of 0 (no improvement), 1 (1%–25% improvement), 2 (26%–50% improvement), 3 (51%–75% improvement), or 4 (76%–100% improvement). At 3 months posttreatment, the average grade of improvement was 3.4.12
The 1550-nm fractional erbium-doped fiber laser was tested in 14 men with multiple facial AKs (range, 9–44 AKs [mean, 22.1 AKs]).11 Participants received 5 treatment sessions at 2- to 4-week intervals, with majority energies used at 70 MJ and treatment level 11. The mean AK count was reduced significantly by 73.1%, 66.2%, and 55.6% at 1-, 3-, and 6-month follow-up, respectively (P<.001).11
The 1927-nm fractional thulium laser showed promising results in 24 participants with facial AKs.13 Participants received up to 4 treatment sessions at intervals from 2 to 6 weeks at the investigators’ discretion. At baseline, patients had an average of 14.04 facial AKs. At 1-, 3-, and 6-month follow-up, participants exhibited 91.3%, 87.3%, and 86.6% reduction in AK counts, respectively. The mean AK count at 3-month follow-up was 1.88.13
Due to limited sample sizes and/or lack of quantifiable results and controls in these studies, more studies are needed to fully elucidate the role of nonablative lasers in the treatment of AK.
Future Directions
Iontophoresis involves the noninvasive induction of an electrical current to facilitate ion movement through the skin and may be a novel method to boost the efficacy of current field therapies. In the first known study of its kisnd, iontophoresis-assisted AFXL-PDT was found to be noninferior to conventional AFXL-PDT15; however, additional studies demonstrating its superiority are needed before more widespread clinical use is considered.
Pretreatment with AFXL prior to topical field-directed therapies also has been proposed.16 In a case series of 13 patients, combination therapy with AFXL and ingenol mebutate was shown to be superior to ingenol mebutate alone (AK clearance rate, 89.2% vs 72.1%, respectively; P<.001).16 Randomized studies with longer follow-up time are needed.
Conclusion
Ablative and nonablative laser systems have yielded limited data about their potential as monotherapies for treatment of multiple AKs and are unlikely to replace topical agents and PDT as a first-line modality in field-directed treatment at this time. More studies with a larger number of participants and long-term follow-up are needed for further clarification of efficacy, safety, and clinical feasibility. Nevertheless, fractional ablative lasers in combination with PDT have shown robust efficacy and a favorable safety profile for treatment of multiple AKs.6-9 Further, this combination therapy exhibited a superior clearance rate and lower lesion recurrence in organ transplant recipients—a demographic that classically is difficult to treat.6-9
With continued rapid evolution of laser systems and more widespread use in dermatology, monotherapy and combination therapy may offer a dynamic new option in field cancerization that can decrease disease burden and treatment frequency.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
- Peris K, Calzavara-Pinton PG, Neri L, et al. Italian expert consensus for the management of actinic keratosis in immunocompetent patients. J Eur Acad Dermatol Venereol. 2016;30:1077-1084.
- Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J Am Acad Dermatol. 2008;58:719-737; quiz 738-740.
- Hantash BM, Stewart DB, Cooper ZA, et al. Facial resurfacing for nonmelanoma skin cancer prophylaxis. Arch Dermatol. 2006;142:976-982.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Scola N, Terras S, Georgas D, et al. A randomized, half-side comparative study of aminolaevulinate photodynamic therapy vs. CO(2) laser ablation in immunocompetent patients with multiple actinic keratoses. Br J Dermatol. 2012;167:1366-1373.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Choi SH, Kim KH, Song KH. Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. 2015;29:1598-1605.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Demetriou C. Reversing precancerous actinic damage by mixing wavelengths (1064 nm, 532 nm). J Cosmet Laser Ther. 2011;13:113-119.
- Katz TM, Goldberg LH, Marquez D, et al. Nonablative fractional photothermolysis for facial actinic keratoses: 6-month follow-up with histologic evaluation. J Am Acad Dermatol. 2011;65:349-356.
- Lapidoth M, Adatto M, Halachmi S. Treatment of actinic keratoses and photodamage with non-contact fractional 1540-nm laser quasi-ablation: an ex vivo and clinical evaluation. Lasers Med Sci. 2013;28:537-542.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Gold MH, Sensing W, Biron J. Fractional Q-switched 1,064-nm laser for the treatment of photoaged-photodamaged skin. J Cosmet Laser Ther. 2014;16:69-76.
- Choi SH, Kim TH, Song KH. Efficacy of iontophoresis-assisted ablative fractional laser photodynamic therapy with short incubation time for the treatment of actinic keratosis: 12-month follow-up results of a prospective, randomised, comparative trial. Photodiagnosis Photodyn Ther. 2017;18:105-110.
- Nisticò S, Sannino M, Del Duca E, et al. Ablative fractional laser improves treatment of actinic keratoses with ingenol mebutate. Eur J Inflamm. 2016;14:200-205.
Practice Points
- Ablative fractional laser therapy in combination with photodynamic therapy has demonstrated increased efficacy in treating field actinic keratoses (AKs) for up to 12 months of follow-up over either modality alone.
- Ablative and nonablative lasers as monotherapy in treating field AKs require further studies with larger sample sizes to determine efficacy and safety.
VIDEO: Painless PDT and other AK tricks
KAUAI, HAWAII – Imiquimod on the lip can be extremely painful, but it works wonders for actinic cheilitis, sometimes with only a few treatments.
Also, and how long patients stay under the lamp, advised Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
These are just a few of the tips Dr. Rosen shared in a presentation about AKs at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In a video interview after his talk, he went into detail about the use of imiquimod for actinic cheilitis – AKs of the lip – and painless PDT, as well as how to discuss AKs with patients – and a quick, clever way to help distinguish AKs from squamous cell carcinoma.
Dr. Rosen is an adviser to Aclaris, Cipher, Pfizer, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Imiquimod on the lip can be extremely painful, but it works wonders for actinic cheilitis, sometimes with only a few treatments.
Also, and how long patients stay under the lamp, advised Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
These are just a few of the tips Dr. Rosen shared in a presentation about AKs at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In a video interview after his talk, he went into detail about the use of imiquimod for actinic cheilitis – AKs of the lip – and painless PDT, as well as how to discuss AKs with patients – and a quick, clever way to help distinguish AKs from squamous cell carcinoma.
Dr. Rosen is an adviser to Aclaris, Cipher, Pfizer, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – Imiquimod on the lip can be extremely painful, but it works wonders for actinic cheilitis, sometimes with only a few treatments.
Also, and how long patients stay under the lamp, advised Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
These are just a few of the tips Dr. Rosen shared in a presentation about AKs at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
In a video interview after his talk, he went into detail about the use of imiquimod for actinic cheilitis – AKs of the lip – and painless PDT, as well as how to discuss AKs with patients – and a quick, clever way to help distinguish AKs from squamous cell carcinoma.
Dr. Rosen is an adviser to Aclaris, Cipher, Pfizer, and Valeant.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Best practices address latest trends in PDT, skin cancer treatment
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
MIAMI – Pearls for providers of photodynamic therapy (PDT) include tips on skin preparation, eye protection, and use of three new codes to maximize reimbursement. Also trending in medical dermatology are best practices for intralesional injections of 5-FU to treat the often challenging isomorphic squamous cell carcinomas (SCCs) or keratoacanthomas on the lower leg, as well as use of neoadjuvant hedgehog inhibitors to shrink large skin cancer lesions, according to Glenn David Goldman, MD.
“This talk is about what you can do medically as a dermatologic surgeon,” Dr. Goldman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Use new billing codes for photodynamic therapy
There are now three new PDT billing codes. “Make sure your coders are using these properly. They are active now, and if you don’t use them, you won’t get paid properly,” said Dr. Goldman, professor and medical director of dermatology at the University of Vermont, Burlington. Specifically, 96567 is for standard PDT applied by staff; 96573 is for PDT applied by a physician; and 96574 is for PDT and curettage performed by a physician.
“Be involved, don’t delegate,” Dr. Goldman added. “If you do, you will get paid half as much as you used to, which means you will lose money on every single patient you treat.”
What type of PDT physicians choose to use in their practice remains controversial. “Do you do short-contact PDT, do you do daylight PDT? We’ve gone back and forth in our practice,” Dr. Goldman said. “I’m not impressed with daylight PDT. I know this is at odds with some of the people here, but at least in Vermont, it doesn’t work very well.”
The way PDT was described in the original trials (a photosensitizer applied in the office followed by PDT) “works the best, with one caveat,” Dr. Goldman said. The caveat is that dermatologists should aim for a PDT clearance that approaches the efficacy of 5-fluorouracil (5-FU). “If you can get to that – which is difficult by the way – I think your patients will really appreciate this.”
An additional PDT pearl Dr. Goldman shared involves skin preparation: the use of acetone to defat the skin, even in patients with very thick lesions. Apply acetone with gauze to the site for 5 minutes and “all of that hyperkeratosis just wipes away,” curette off any residual hyperkeratosis – and consider a ring anesthetic block to control pain for the patient with severe disease, he advised.
Another tip is to forgo the goggles that come with most PDT kits. Instead, purchase smaller, disposable laser eye shields for PDT patients, Dr. Goldman said. “They work better. You can get closer to the eye … and they are more comfortable for the patient.”
Dr. Goldman’s practice is providing more PDT and much less 5-FU for patient convenience. “I believe if someone is willing to go through 3 weeks of 5-FU or 12-16 weeks of imiquimod, they get the best results. However, most people don’t want to do that if they can sit in front of a light for 15 minutes.”
Consider intralesional injections for SCCs and KAs on the legs
An ongoing challenge in medical dermatology is preventing rapid recurrence of SCCs and/or keratoacanthomas (KAs) near sites of previous excision on the legs. “We all see this quite a bit. Often you get lesions on the leg, you cut them out, and they come right back” close to the excision site, Dr. Goldman said.
He does not recommend methotrexate injections for these lesions. “Methotrexate does not work. It doesn’t hurt, but I’ve injected methotrexate into squamous cell carcinomas many times and they’ve never gone away.” In contrast, 5-FU “works incredibly well. They go away, I’ve had tremendous success. This has changed the way we treat these lesions.” 5-FU is inexpensive and can be obtained from oncology pharmacies. One caveat is 5-FU injections can be painful and patients require anesthesia prior to injection.
Using a 25-gauge or 27-gauge needle, Dr. Goldman injects 5-FU “exactly as I would a hypertrophic scar. I inject a squamous cell carcinoma carefully and ‘expand’ the tumor.” He typically injects a lesion every 2 weeks until it resolves completely, which typically takes two or three sessions.
“I want to emphasize that that’s really true about intralesional 5-FU for those KAs and scars on the legs,” said session moderator James Spencer, MD, a dermatologist in private practice in St. Petersburg, Florida. “Otherwise, you’re just chasing your tail trying to cut them out. You’ll do much better with the intralesional 5-FU; it’s easy to get, it’s affordable, it comes as 50 mg/mL … just keep it in the office.”
A recommended role for hedgehog inhibitors
Hedgehog inhibitors work best as neoadjuvant therapy to shrink large skin cancer tumors prior to excision, Dr. Goldman said. “Hedgehog inhibitors don’t cure anything … except for rare cases of small basal cell carcinomas.” For most lesions, however, the strategy is not curative.
“I don’t believe in hedgehog inhibitors for things that are readily resectable. We use them to shrink things down,” he added.
Dr. Goldman recommended treating patients with neoadjuvant hedgehog inhibitors to achieve the maximum tumor shrinking effect. Adverse effects tend to develop slowly over time, typically after a 6-week “grace period.” Nighttime leg muscle cramps, loss of taste, hair loss, and weight loss can occur. Also, electrolyte imbalances can occur, particularly in older patients with renal clearance issues. When the patient can no longer reasonably tolerate the adverse effects, which is usually the case, “then you do the surgery,” Dr. Goldman said.
“The benefits of neoadjuvant hedgehog inhibitors include predictable shrinkage of tumors,” and manufacturers have been helpful with financial issues, he noted.
Dr. Goldman had no relevant financial disclosures. Dr. Spencer has served on the speakers bureau for Genentech and Leo Pharma.
REPORTING FROM ODAC 2018
Topical 5-Fluorouracil Made Easy?
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
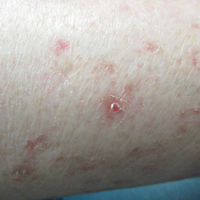
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
What is the recent research behind 5-fluorouracil cream 5% combined with calcipotriol ointment 0.005% for actinic keratoses?
Cunningham et al published a randomized double-blind study in which 131 patients with actinic keratoses (AKs) were assigned to either 5-fluorouracil (5-FU) cream 5% combined with calcipotriol (calcipotriene) ointment 0.005% twice daily to the face, scalp, and arms for 4 days, or 5-FU 5% combined with petrolatum applied in the same fashion. There was an 87.8% versus 26.3% mean reduction in the number of AKs and less severe pain, crusting, and ulceration in the study cohort compared to the 5-FU plus petrolatum group.
The same study also investigated immune parameters in these patients and found that the study group preferentially displayed activated thymic stromal lymphopoietin and a CD4 T cell-mediated reaction, among other effects. In prior studies, thymic stromal lymphopoietin has been shown to be upregulated in barrier-defective skin, displays antitumor activity, and is enhanced by topical calcipotriol application based on its original indication for psoriasis.
How do these study results impact patient care?
In a perfect world, every patient could tolerate and afford chemopreventative measures such as 5-FU cream, apply it diffusely to sun-exposed skin, and experience no severe irritant reactions and/or social pariah status. We all know that this product is effective, and we all overprepare patients to use it, knowing that they will call our offices panicked and fearful that they are allergic to or are becoming infected by this cream.
Although further study clearly is needed to determine the optimal application amount, duration of use, and vehicle mix, this new compound utilizing 2 topicals that are familiar to us--5-FU cream approved for AKs and early squamous cell skin cancers and calcipotriol ointment (though available only in cream in the United States currently) for psoriasis--is an encouraging step. Home therapy for AKs and possibly early nonmelanoma skin cancers that is more tolerable, of shorter duration, and in turn more effective than the current options would lessen the burden of treating these lesions surgically or rescheduling 5-FU patients often for irritation reaction education.
How do patients respond to this regimen?
In my own anecdotal experience, this regimen has been well received by patients and often is covered by most insurances when written as 2 separate prescriptions (both in cream vehicle). They still report some irritation, but I prefer to utilize it segmentally instead of treating all sun-exposed areas at once (ie, treat one side of the face/scalp twice daily for 4 days, then the other, or even divide it into smaller segments once the prior segment has healed). This combination, in addition to, for example, adding nicotinamide 500 mg twice daily to a patient's skin cancer chemopreventative sequence, is in my opinion a novel but safe, effective, and well-tolerated field therapy recommendation.
American Academy of Dermatology Actinic Keratosis Overview
American Academy of Family Physicians Actinic Keratoses Information
Suggested Readings
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116.
- Demehri S, Turkoz A, Manivasagam S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494-505.
- Rosamilia LL. Three Cheers for B3? Cutis. July 7, 2015. http://www.mdedge.com/cutis/article/101102/nonmelanoma-skin-cancer/three-cheers-b3. Accessed November 20, 2017.
- Sato-Deguchi E, Imafuku S, Chou B, et al. Topical vitamin D(3) analogues induce thymic stromal lymphopoietin and cathelicidin in psoriatic skin lesions. Br J Dermatol. 2012;167:77-84.
Debunking Actinic Keratosis Myths: Do All Actinic Keratoses Progress to Squamous Cell Carcinoma?
Myth: Hypertrophic actinic keratoses are more likely to progress to squamous cell carcinoma
Actinic keratosis (AK) indicates cumulative UV exposure and is the initial lesion in the majority of invasive cutaneous squamous cell carcinomas (SCCs). However, most AKs do not progress to invasive SCC and it currently is not possible to clinically or histopathologically determine which AK lesions will progress to SCC.
The rates of progression of individual AK lesions to SCC vary. In 2011, Feldman and Fleischer summarized 6 studies of 560 to 6691 patients with AK. The AK progression to SCC was found to range from 0.075% per year per lesion to 14% over 5 years.
Criscione et al found that the risk of progression of AK to primary SCC was 0.60% at 1 year and 2.57% at 4 years. In this study, 187 primary SCCs were diagnosed after enrollment, with 65% arising in previously clinically diagnosed and documented AKs. Therefore, although the authors noted low risks of progression of AK to SCC, they observed that the majority of SCCs arose from AKs.
The risk for progression of AK to invasive SCC with the potential for metastasis warrants treatment of AK with lesion- or field-directed therapy or a combined approach when indicated. Dermatologists also should monitor AK patients closely.
Expert Commentary
It’s a myth that hypertrophic lesions are more likely to turn into SCC. In fact, it’s the lesions with follicular extension that are more likely to progress to SCC. These may be hypertrophic or atrophic or simple AKs. The genetics of AKs that become hypertrophic may be different from those that become invasive. These lesions may be more likely to first grow outward before becoming invasive.
—Gary Goldenberg (New York, New York)
Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115:2523-2530.
Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
Pandey S, Mercer SE, Dallas K, et al. Evaluation of the prognostic significance of follicular extension in actinic keratoses. J Clin Aesthet Dermatol. 2012;5:25-28.
Myth: Hypertrophic actinic keratoses are more likely to progress to squamous cell carcinoma
Actinic keratosis (AK) indicates cumulative UV exposure and is the initial lesion in the majority of invasive cutaneous squamous cell carcinomas (SCCs). However, most AKs do not progress to invasive SCC and it currently is not possible to clinically or histopathologically determine which AK lesions will progress to SCC.
The rates of progression of individual AK lesions to SCC vary. In 2011, Feldman and Fleischer summarized 6 studies of 560 to 6691 patients with AK. The AK progression to SCC was found to range from 0.075% per year per lesion to 14% over 5 years.
Criscione et al found that the risk of progression of AK to primary SCC was 0.60% at 1 year and 2.57% at 4 years. In this study, 187 primary SCCs were diagnosed after enrollment, with 65% arising in previously clinically diagnosed and documented AKs. Therefore, although the authors noted low risks of progression of AK to SCC, they observed that the majority of SCCs arose from AKs.
The risk for progression of AK to invasive SCC with the potential for metastasis warrants treatment of AK with lesion- or field-directed therapy or a combined approach when indicated. Dermatologists also should monitor AK patients closely.
Expert Commentary
It’s a myth that hypertrophic lesions are more likely to turn into SCC. In fact, it’s the lesions with follicular extension that are more likely to progress to SCC. These may be hypertrophic or atrophic or simple AKs. The genetics of AKs that become hypertrophic may be different from those that become invasive. These lesions may be more likely to first grow outward before becoming invasive.
—Gary Goldenberg (New York, New York)
Myth: Hypertrophic actinic keratoses are more likely to progress to squamous cell carcinoma
Actinic keratosis (AK) indicates cumulative UV exposure and is the initial lesion in the majority of invasive cutaneous squamous cell carcinomas (SCCs). However, most AKs do not progress to invasive SCC and it currently is not possible to clinically or histopathologically determine which AK lesions will progress to SCC.
The rates of progression of individual AK lesions to SCC vary. In 2011, Feldman and Fleischer summarized 6 studies of 560 to 6691 patients with AK. The AK progression to SCC was found to range from 0.075% per year per lesion to 14% over 5 years.
Criscione et al found that the risk of progression of AK to primary SCC was 0.60% at 1 year and 2.57% at 4 years. In this study, 187 primary SCCs were diagnosed after enrollment, with 65% arising in previously clinically diagnosed and documented AKs. Therefore, although the authors noted low risks of progression of AK to SCC, they observed that the majority of SCCs arose from AKs.
The risk for progression of AK to invasive SCC with the potential for metastasis warrants treatment of AK with lesion- or field-directed therapy or a combined approach when indicated. Dermatologists also should monitor AK patients closely.
Expert Commentary
It’s a myth that hypertrophic lesions are more likely to turn into SCC. In fact, it’s the lesions with follicular extension that are more likely to progress to SCC. These may be hypertrophic or atrophic or simple AKs. The genetics of AKs that become hypertrophic may be different from those that become invasive. These lesions may be more likely to first grow outward before becoming invasive.
—Gary Goldenberg (New York, New York)
Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115:2523-2530.
Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
Pandey S, Mercer SE, Dallas K, et al. Evaluation of the prognostic significance of follicular extension in actinic keratoses. J Clin Aesthet Dermatol. 2012;5:25-28.
Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention trial. Cancer. 2009;115:2523-2530.
Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011;87:201-207.
Pandey S, Mercer SE, Dallas K, et al. Evaluation of the prognostic significance of follicular extension in actinic keratoses. J Clin Aesthet Dermatol. 2012;5:25-28.
Debunking Actinic Keratosis Myths: Are Patients With Darker Skin At Risk for Actinic Keratoses?
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Myth: Actinic keratoses are only seen in patients with lighter skin
Actinic keratoses (AKs) are precancerous lesions that may turn into squamous cell carcinoma if left untreated. UV rays cause AKs, either from outdoor sun exposure or tanning beds. According to the American Academy of Dermatology, AKs are more likely to develop in patients 40 years or older with fair skin; hair color that is naturally blonde or red; eye color that is naturally blue, green, or hazel; skin that freckles or burns when in the sun; a weakened immune system; and occupations involving substances that contain polycyclic aromatic hydrocarbons such as coal or tar.
A 2007 study compared the most common diagnoses among patients of different racial and ethnic groups in New York City. Alexis et al found that AK was in the top 10 diagnoses in white patients but not for black patients. They postulated that photoprotective factors in darkly pigmented skin such as larger and more numerous melanosomes that contain more melanin and are more dispersed throughout the epidermis result in a lower incidence of skin cancers in the skin of color (SOC) population.
RELATED ARTICLE: Common Dermatologic Disorders in Skin of Color: A Comparative Practice Survey
However, a recent skin cancer awareness study in Cutis reported that even though SOC populations have lower incidences of skin cancer such as melanoma, basal cell carcinoma, and squamous cell carcinoma, they exhibit higher death rates. Furthermore, black individuals are more likely to present with advanced-stage melanoma and acral lentiginous melanomas compared to white individuals. Kailas et al stated, “Overall, SOC patients have the poorest skin cancer prognosis, and the data suggest that the reason for this paradox is delayed diagnosis.” They evaluated several knowledge-based interventions for increasing skin cancer awareness, knowledge, and protective behaviors in SOC populations, including the use of visuals such as photographs to allow SOC patients to visualize different skin tones, educational interventions in another language, and pamphlets.
Dermatologists should be aware that education of SOC patients is important to eradicate the common misconception that these patients do not have to worry about AKs and other skin cancers. Remind these patients that they need to protect their skin from the sun, just as patients with fair skin do. Further research in the dermatology community should focus on educational interventions that will help increase knowledge regarding skin cancer in SOC populations.
Expert Commentary
Although more common in patients with lighter skin, actinic keratosis and skin cancer can be seen in patients of all skin types. Many patients are unaware of this risk and do not use sunscreen and other sun-protective measures. We, as a specialty, have to educate our patients and the public of the risk for actinic keratosis and skin cancer in all skin types.
—Gary Goldenberg, MD (New York, New York)
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
American Academy of Dermatology. Actinic keratosis. https://www.aad.org/public/diseases/scaly-skin/actinic-keratosis. Accessed October 17, 2017.
Kailas A, Botwin AL, Pritchett EN, et al. Assessing the effectiveness of knowledge-based interventions in increasing skin cancer awareness, knowledge, and protective behaviors in skin of color populations. Cutis. 2017;100:235-240.
Microneedle pretreatment shortened ALA incubation time
Pretreatment with microneedles provided a faster, less painful, way to treat facial actinic keratoses (AKs) with photodynamic therapy, with similar clearance rates as would be expected with traditional therapy, in a study of 33 patients.
This approach reduced the incubation time of aminolevulinic acid (ALA) to 20 minutes, with comparable results to one-hour ALA incubation times. “Interestingly, the secondary outcome of pain associated with blue light exposure during photodynamic therapy was nominal and not significantly different from the sham side,” reported Tatyana A. Petukhova, MD, and her associates in the department of dermatology at the University of California, Davis (JAMA Dermatol. 2017 May 17. doi: 10.1001/jamadermatol.2017.0849).
“Pain associated with PDT is the most severe adverse effect and may lead to interruption or discontinuation of treatment, resulting in refusal to repeat the process at a future date owing to unbearable discomfort,” they wrote. Patients also reported little to no swelling or pain after treatment and minimal erythema and peeling.
The randomized, split-face, single-blinded controlled trial enrolled 33 patients, with at least eight AKs on their faces, from a university dermatology outpatient clinic from 2015 to 2016. They were randomized to receive either 10 minutes or 20 minutes incubation time with ALA, and 32 completed the study. Those in the 20-minute group had a mean of 25 grade II facial AKs, and those in the 10-minute group had an average of 31 grade II facial AKs.
Before administration of ALA, each patient received pretreatment with a microneedle roller on one side of their face and a sham roller on the other side. On each half of their faces, the microneedle device (a single-use sterile array of microneedles measuring 200 mcm) or sham roller was rolled forward and backward eight times in four directions.
They were exposed to blue light for 1,000 seconds at an average wavelength of 478 nm, an overall fluence of 10 J/cm2, and advised to avoid sun exposure for 36 hours after treatment.
At follow-up one month later, among the patients with a 20-minute ALA incubation time, the mean AK clearance rate was 76% on the side with microneedle pretreatment, compared with 58% on the sham side (P less than .01). This included three patients with complete clearance. The efficacy of microneedle pretreatment with a 20-minute incubation time is similar to that of 1-hour incubation times with ALA. PDT typically uses 1-4 hours of incubation time.
Among the patients who received a 10-minute ALA incubation time, a mean of 43% of AKs on the microneedle side and 38% on the sham side cleared, a difference that was not statistically significant. Participants did not rate the pain as significantly different between each side of their face and both groups reported low levels of pain overall.
One limitation of the study was the short follow-up time. “Because actinic damage is cumulative, there is potential for thicker AKs to recur or for new lesions to develop during a longer follow-up period,” the authors noted.
The research was partly funded by an author’s University of California, Davis, Medical Student Research Fellowship. The authors reported having no disclosures.
The effectiveness of photodynamic therapy (PDT) as a field therapy option for actinic keratoses (AKs) has been well documented. However, treatment is limited by poor or variable transepidermal absorption owing to the hydrophilic nature of aminolevulinic acid (ALA).
To overcome the problem of transepidermal delivery, patients wait for hours at a time between ALA application and the light treatment. These prolonged incubation times limit usefulness. Petukhova et al. hypothesized that microneedles would enhance penetration and decrease incubation period without compromising safety and efficacy. The results suggest that an effective and safe PDT treatment can be achieved by using microneedles as means to expedite drug penetration. These results also suggest that further reduction of incubation time will not be achieved by better transepidermal penetration.
As envisioned in 1971, microneedles can have an important clinical role and can be an important tool in the dermatologist’s armamentarium. Microneedles are painless when compared with hypodermic needles, do not require specific training, minimize risk of needlestick injuries, can potentially reduce cost, and improve patient compliance and access. Therefore, microneedle-based devices can potentially be used at home by patients in a safe manner. Mostly in preclinical trials, as well as in some clinical trials, microneedles have been successfully used to deliver various drugs and vaccines.
Importantly, dermatologists are uniquely positioned to research this novel drug delivery method because they routinely treat cutaneous disease. This can be leveraged by dermatologists to use microneedles not only for dermal rejuvenation purposes but also to enhance drug delivery for dermatological indications. Therefore, the study by Petukhova et al. not only provides practical information for treatment of AKs with PDT but can also be viewed as a call for action to all dermatologists to lead innovation in the field and revolutionize dermatological drug delivery.
These comments are adapted from an accompanying editorial by Hadar Lev-Tov, MD, of the University of Florida, Miami. He reported having no disclosures.
The effectiveness of photodynamic therapy (PDT) as a field therapy option for actinic keratoses (AKs) has been well documented. However, treatment is limited by poor or variable transepidermal absorption owing to the hydrophilic nature of aminolevulinic acid (ALA).
To overcome the problem of transepidermal delivery, patients wait for hours at a time between ALA application and the light treatment. These prolonged incubation times limit usefulness. Petukhova et al. hypothesized that microneedles would enhance penetration and decrease incubation period without compromising safety and efficacy. The results suggest that an effective and safe PDT treatment can be achieved by using microneedles as means to expedite drug penetration. These results also suggest that further reduction of incubation time will not be achieved by better transepidermal penetration.
As envisioned in 1971, microneedles can have an important clinical role and can be an important tool in the dermatologist’s armamentarium. Microneedles are painless when compared with hypodermic needles, do not require specific training, minimize risk of needlestick injuries, can potentially reduce cost, and improve patient compliance and access. Therefore, microneedle-based devices can potentially be used at home by patients in a safe manner. Mostly in preclinical trials, as well as in some clinical trials, microneedles have been successfully used to deliver various drugs and vaccines.
Importantly, dermatologists are uniquely positioned to research this novel drug delivery method because they routinely treat cutaneous disease. This can be leveraged by dermatologists to use microneedles not only for dermal rejuvenation purposes but also to enhance drug delivery for dermatological indications. Therefore, the study by Petukhova et al. not only provides practical information for treatment of AKs with PDT but can also be viewed as a call for action to all dermatologists to lead innovation in the field and revolutionize dermatological drug delivery.
These comments are adapted from an accompanying editorial by Hadar Lev-Tov, MD, of the University of Florida, Miami. He reported having no disclosures.
The effectiveness of photodynamic therapy (PDT) as a field therapy option for actinic keratoses (AKs) has been well documented. However, treatment is limited by poor or variable transepidermal absorption owing to the hydrophilic nature of aminolevulinic acid (ALA).
To overcome the problem of transepidermal delivery, patients wait for hours at a time between ALA application and the light treatment. These prolonged incubation times limit usefulness. Petukhova et al. hypothesized that microneedles would enhance penetration and decrease incubation period without compromising safety and efficacy. The results suggest that an effective and safe PDT treatment can be achieved by using microneedles as means to expedite drug penetration. These results also suggest that further reduction of incubation time will not be achieved by better transepidermal penetration.
As envisioned in 1971, microneedles can have an important clinical role and can be an important tool in the dermatologist’s armamentarium. Microneedles are painless when compared with hypodermic needles, do not require specific training, minimize risk of needlestick injuries, can potentially reduce cost, and improve patient compliance and access. Therefore, microneedle-based devices can potentially be used at home by patients in a safe manner. Mostly in preclinical trials, as well as in some clinical trials, microneedles have been successfully used to deliver various drugs and vaccines.
Importantly, dermatologists are uniquely positioned to research this novel drug delivery method because they routinely treat cutaneous disease. This can be leveraged by dermatologists to use microneedles not only for dermal rejuvenation purposes but also to enhance drug delivery for dermatological indications. Therefore, the study by Petukhova et al. not only provides practical information for treatment of AKs with PDT but can also be viewed as a call for action to all dermatologists to lead innovation in the field and revolutionize dermatological drug delivery.
These comments are adapted from an accompanying editorial by Hadar Lev-Tov, MD, of the University of Florida, Miami. He reported having no disclosures.
Pretreatment with microneedles provided a faster, less painful, way to treat facial actinic keratoses (AKs) with photodynamic therapy, with similar clearance rates as would be expected with traditional therapy, in a study of 33 patients.
This approach reduced the incubation time of aminolevulinic acid (ALA) to 20 minutes, with comparable results to one-hour ALA incubation times. “Interestingly, the secondary outcome of pain associated with blue light exposure during photodynamic therapy was nominal and not significantly different from the sham side,” reported Tatyana A. Petukhova, MD, and her associates in the department of dermatology at the University of California, Davis (JAMA Dermatol. 2017 May 17. doi: 10.1001/jamadermatol.2017.0849).
“Pain associated with PDT is the most severe adverse effect and may lead to interruption or discontinuation of treatment, resulting in refusal to repeat the process at a future date owing to unbearable discomfort,” they wrote. Patients also reported little to no swelling or pain after treatment and minimal erythema and peeling.
The randomized, split-face, single-blinded controlled trial enrolled 33 patients, with at least eight AKs on their faces, from a university dermatology outpatient clinic from 2015 to 2016. They were randomized to receive either 10 minutes or 20 minutes incubation time with ALA, and 32 completed the study. Those in the 20-minute group had a mean of 25 grade II facial AKs, and those in the 10-minute group had an average of 31 grade II facial AKs.
Before administration of ALA, each patient received pretreatment with a microneedle roller on one side of their face and a sham roller on the other side. On each half of their faces, the microneedle device (a single-use sterile array of microneedles measuring 200 mcm) or sham roller was rolled forward and backward eight times in four directions.
They were exposed to blue light for 1,000 seconds at an average wavelength of 478 nm, an overall fluence of 10 J/cm2, and advised to avoid sun exposure for 36 hours after treatment.
At follow-up one month later, among the patients with a 20-minute ALA incubation time, the mean AK clearance rate was 76% on the side with microneedle pretreatment, compared with 58% on the sham side (P less than .01). This included three patients with complete clearance. The efficacy of microneedle pretreatment with a 20-minute incubation time is similar to that of 1-hour incubation times with ALA. PDT typically uses 1-4 hours of incubation time.
Among the patients who received a 10-minute ALA incubation time, a mean of 43% of AKs on the microneedle side and 38% on the sham side cleared, a difference that was not statistically significant. Participants did not rate the pain as significantly different between each side of their face and both groups reported low levels of pain overall.
One limitation of the study was the short follow-up time. “Because actinic damage is cumulative, there is potential for thicker AKs to recur or for new lesions to develop during a longer follow-up period,” the authors noted.
The research was partly funded by an author’s University of California, Davis, Medical Student Research Fellowship. The authors reported having no disclosures.
Pretreatment with microneedles provided a faster, less painful, way to treat facial actinic keratoses (AKs) with photodynamic therapy, with similar clearance rates as would be expected with traditional therapy, in a study of 33 patients.
This approach reduced the incubation time of aminolevulinic acid (ALA) to 20 minutes, with comparable results to one-hour ALA incubation times. “Interestingly, the secondary outcome of pain associated with blue light exposure during photodynamic therapy was nominal and not significantly different from the sham side,” reported Tatyana A. Petukhova, MD, and her associates in the department of dermatology at the University of California, Davis (JAMA Dermatol. 2017 May 17. doi: 10.1001/jamadermatol.2017.0849).
“Pain associated with PDT is the most severe adverse effect and may lead to interruption or discontinuation of treatment, resulting in refusal to repeat the process at a future date owing to unbearable discomfort,” they wrote. Patients also reported little to no swelling or pain after treatment and minimal erythema and peeling.
The randomized, split-face, single-blinded controlled trial enrolled 33 patients, with at least eight AKs on their faces, from a university dermatology outpatient clinic from 2015 to 2016. They were randomized to receive either 10 minutes or 20 minutes incubation time with ALA, and 32 completed the study. Those in the 20-minute group had a mean of 25 grade II facial AKs, and those in the 10-minute group had an average of 31 grade II facial AKs.
Before administration of ALA, each patient received pretreatment with a microneedle roller on one side of their face and a sham roller on the other side. On each half of their faces, the microneedle device (a single-use sterile array of microneedles measuring 200 mcm) or sham roller was rolled forward and backward eight times in four directions.
They were exposed to blue light for 1,000 seconds at an average wavelength of 478 nm, an overall fluence of 10 J/cm2, and advised to avoid sun exposure for 36 hours after treatment.
At follow-up one month later, among the patients with a 20-minute ALA incubation time, the mean AK clearance rate was 76% on the side with microneedle pretreatment, compared with 58% on the sham side (P less than .01). This included three patients with complete clearance. The efficacy of microneedle pretreatment with a 20-minute incubation time is similar to that of 1-hour incubation times with ALA. PDT typically uses 1-4 hours of incubation time.
Among the patients who received a 10-minute ALA incubation time, a mean of 43% of AKs on the microneedle side and 38% on the sham side cleared, a difference that was not statistically significant. Participants did not rate the pain as significantly different between each side of their face and both groups reported low levels of pain overall.
One limitation of the study was the short follow-up time. “Because actinic damage is cumulative, there is potential for thicker AKs to recur or for new lesions to develop during a longer follow-up period,” the authors noted.
The research was partly funded by an author’s University of California, Davis, Medical Student Research Fellowship. The authors reported having no disclosures.
Key clinical point:
Major finding: The mean facial AK clearance rate one month after microneedle pretreatment and 20 minutes incubation of ALA was 76%.
Data source: The findings are based on a randomized, controlled, single-blinded study of 33 outpatients with actinic keratoses.
Disclosures: The research was partly funded by an author’s University of California, Davis, Medical Student Research Fellowship. The authors reported having no disclosures.
5-Fluorouracil failed four separate measures of photoaging
PORTLAND – A standard course of topical 5-fluorouracil (5-FU) does not noticeably improve visual signs of facial photoaging, such as forehead lines and crow’s feet, according to the results of a blinded, controlled study of 281 elderly white men.
Four validated photonumeric measures revealed no statistically significant differences between the intervention and vehicle control arms at 6, 12, or 18 months’ follow-up, Kaveri Korgavkar, MD, said at the annual meeting of the Society for Investigative Dermatology. “This might be a true lack of impact, or current scales may not be sensitive enough to capture aspects of aging that are improved by 5-fluorouracil,” commented Dr. Korgavkar, who presented the findings on behalf of the VAKCCT (the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial) work group.
The treatment and control groups resembled each other demographically and clinically at baseline. Participants averaged 71.5 years of age (standard deviation, 0.57 years), 97% were male, 99% were white, and all had clinically meaningful histories of sun damage with at least two keratinocyte carcinomas in the previous 2 years, including at least one lesion on the face or ears. Previously, the VAKCCT investigators reported positive results for 5-FU as a chemopreventive – for example, it was associated with about a 60% reduction in actinic keratoses, compared with placebo, and the effects persisted for up to 3 years.
However, none of the four photonumeric scales of photoaging uncovered significant differences between the treatment and control groups at 6, 12, or 18 months’ follow-up, Dr. Korgavkar reported. That finding belies the results of two other previous studies, but they were small and uncontrolled, she added. One study of 19 patients reported statistically significant improvements over time in wrinkling, hyperpigmentation, lentigines, and sallowness based on the Griffith’s scale, while a second prospective study of 32 patients reported significant improvements in visual signs of photoaging on the forearms, with a corresponding rise in levels of procollagen 1 and a decrease in dermal elastosis at 1 month.
Existing scales might more effectively capture some aspects of photoaging – such as wrinkles or crow’s feet – than others, Dr. Korgavkar said in an interview. Therefore, she and her associates are working to construct more sensitive and comprehensive visual scales of photoaging, she said.
The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
PORTLAND – A standard course of topical 5-fluorouracil (5-FU) does not noticeably improve visual signs of facial photoaging, such as forehead lines and crow’s feet, according to the results of a blinded, controlled study of 281 elderly white men.
Four validated photonumeric measures revealed no statistically significant differences between the intervention and vehicle control arms at 6, 12, or 18 months’ follow-up, Kaveri Korgavkar, MD, said at the annual meeting of the Society for Investigative Dermatology. “This might be a true lack of impact, or current scales may not be sensitive enough to capture aspects of aging that are improved by 5-fluorouracil,” commented Dr. Korgavkar, who presented the findings on behalf of the VAKCCT (the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial) work group.
The treatment and control groups resembled each other demographically and clinically at baseline. Participants averaged 71.5 years of age (standard deviation, 0.57 years), 97% were male, 99% were white, and all had clinically meaningful histories of sun damage with at least two keratinocyte carcinomas in the previous 2 years, including at least one lesion on the face or ears. Previously, the VAKCCT investigators reported positive results for 5-FU as a chemopreventive – for example, it was associated with about a 60% reduction in actinic keratoses, compared with placebo, and the effects persisted for up to 3 years.
However, none of the four photonumeric scales of photoaging uncovered significant differences between the treatment and control groups at 6, 12, or 18 months’ follow-up, Dr. Korgavkar reported. That finding belies the results of two other previous studies, but they were small and uncontrolled, she added. One study of 19 patients reported statistically significant improvements over time in wrinkling, hyperpigmentation, lentigines, and sallowness based on the Griffith’s scale, while a second prospective study of 32 patients reported significant improvements in visual signs of photoaging on the forearms, with a corresponding rise in levels of procollagen 1 and a decrease in dermal elastosis at 1 month.
Existing scales might more effectively capture some aspects of photoaging – such as wrinkles or crow’s feet – than others, Dr. Korgavkar said in an interview. Therefore, she and her associates are working to construct more sensitive and comprehensive visual scales of photoaging, she said.
The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
PORTLAND – A standard course of topical 5-fluorouracil (5-FU) does not noticeably improve visual signs of facial photoaging, such as forehead lines and crow’s feet, according to the results of a blinded, controlled study of 281 elderly white men.
Four validated photonumeric measures revealed no statistically significant differences between the intervention and vehicle control arms at 6, 12, or 18 months’ follow-up, Kaveri Korgavkar, MD, said at the annual meeting of the Society for Investigative Dermatology. “This might be a true lack of impact, or current scales may not be sensitive enough to capture aspects of aging that are improved by 5-fluorouracil,” commented Dr. Korgavkar, who presented the findings on behalf of the VAKCCT (the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial) work group.
The treatment and control groups resembled each other demographically and clinically at baseline. Participants averaged 71.5 years of age (standard deviation, 0.57 years), 97% were male, 99% were white, and all had clinically meaningful histories of sun damage with at least two keratinocyte carcinomas in the previous 2 years, including at least one lesion on the face or ears. Previously, the VAKCCT investigators reported positive results for 5-FU as a chemopreventive – for example, it was associated with about a 60% reduction in actinic keratoses, compared with placebo, and the effects persisted for up to 3 years.
However, none of the four photonumeric scales of photoaging uncovered significant differences between the treatment and control groups at 6, 12, or 18 months’ follow-up, Dr. Korgavkar reported. That finding belies the results of two other previous studies, but they were small and uncontrolled, she added. One study of 19 patients reported statistically significant improvements over time in wrinkling, hyperpigmentation, lentigines, and sallowness based on the Griffith’s scale, while a second prospective study of 32 patients reported significant improvements in visual signs of photoaging on the forearms, with a corresponding rise in levels of procollagen 1 and a decrease in dermal elastosis at 1 month.
Existing scales might more effectively capture some aspects of photoaging – such as wrinkles or crow’s feet – than others, Dr. Korgavkar said in an interview. Therefore, she and her associates are working to construct more sensitive and comprehensive visual scales of photoaging, she said.
The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
AT SID 2017
Key clinical point: A standard topical course of 5-fluorouracil did not noticeably improve visual signs of photoaging, such as forehead lines and crow’s feet.
Major finding: Four validated photonumeric measures of photoaging revealed no statistically significant differences between the intervention and the vehicle control at 6, 12, or 18 months’ follow-up.
Data source: An analysis of data from 281 participants in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention trial (VAKCCT).
Disclosures: The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
New topical field therapies on the way for actinic keratoses
WAILEA, HAWAII – The search is on for new topical therapies for actinic keratoses (AKs) that patients will find more appealing than what’s now available, according to Neal Bhatia, MD.
Compliance with current topical field agents for actinic keratoses is not great. Many patients balk at the intense local skin reactions these agents elicit. There is a misplaced sense, especially among patients who don’t grasp the relationship between AKs and squamous cell carcinoma, that the treatment is worse than the disease, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
One that he said he is particularly excited about is a novel formulation of 4% 5-fluorouracil (5-FU) in an aqueous vehicle cream containing peanut oil. In a recently published, double-blind, multicenter clinical trial involving 841 patients, once-daily application of this product for 4 weeks provided better outcomes and superior tolerability, compared with the old-school regimen of 5% 5-FU cream twice daily.
One hundred percent of patients on 4% 5-FU in peanut oil achieved at least 75% clearance of AKs, as did 95% of those on twice daily 5% 5-FU. The incidence of application site skin irritation in the group on the novel product was only 30%, compared with 60% in the comparison group (J Drugs Dermatol. 2016 Oct 1;15[10]:1218-24).
The peanut oil provided a moisturizing effect and was safe even in patients with peanut allergy, Dr. Bhatia noted.
Another product in development as a topical field therapy for AKs is ingenol disoxate gel, a relative of ingenol mebutate (Picato). Unlike ingenol mebutate, ingenol disoxate remains stable without refrigeration. It’s also a more potent activator of protein kinase C. In mouse models, it shows significantly more cytotoxic potency than does ingenol mebutate.
Based upon the favorable results of a short-term, double-blind phase II study, Leo Pharma now has ingenol disoxate in larger clinical trials as field therapy for AKs on the full face, scalp, or chest, with treatment of larger surface areas than those for which ingenol mebutate is approved (J Dermatolog Treat. 2017 Apr 4:1-7).
Actikerall is also in the developmental pipeline. It consists of 0.5% 5-FU, in combination with 10% salicylic acid, in a film-forming base. Developed by Almirall, it is marketed in Canada by Cipher Pharmaceuticals.
SR-T100 gel utilizes as its active ingredient an antiproliferative extract of Solanum lycocarpum, the Brazilian wolf apple. Taiwan-based G & E Herbal Biotechnology is developing the product, which is in an ongoing phase II clinical trial.
Other novel agents for topical field therapy in phase II studies are KX2-391 ointment, a dual Src kinase/tubulin polymerization inhibitor that causes apoptosis of hyperproliferating cells, under development by Athenex, and Vidac Pharma’s VDA-1102 ointment, which selectively triggers apoptosis in neoplastic cells by modulating voltage-dependent anion channel 1/hexokinase enzyme 2, with minimal impact upon surrounding normal cells.
The principle underlying topical field therapy, compared with simply freezing AKs once they arise, is straightforward, Dr. Bhatia stressed. “It’s the difference between treating only what we can see and treating what’s also on the way.”
Dr. Bhatia reported having financial relationships with more than two dozen pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – The search is on for new topical therapies for actinic keratoses (AKs) that patients will find more appealing than what’s now available, according to Neal Bhatia, MD.
Compliance with current topical field agents for actinic keratoses is not great. Many patients balk at the intense local skin reactions these agents elicit. There is a misplaced sense, especially among patients who don’t grasp the relationship between AKs and squamous cell carcinoma, that the treatment is worse than the disease, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
One that he said he is particularly excited about is a novel formulation of 4% 5-fluorouracil (5-FU) in an aqueous vehicle cream containing peanut oil. In a recently published, double-blind, multicenter clinical trial involving 841 patients, once-daily application of this product for 4 weeks provided better outcomes and superior tolerability, compared with the old-school regimen of 5% 5-FU cream twice daily.
One hundred percent of patients on 4% 5-FU in peanut oil achieved at least 75% clearance of AKs, as did 95% of those on twice daily 5% 5-FU. The incidence of application site skin irritation in the group on the novel product was only 30%, compared with 60% in the comparison group (J Drugs Dermatol. 2016 Oct 1;15[10]:1218-24).
The peanut oil provided a moisturizing effect and was safe even in patients with peanut allergy, Dr. Bhatia noted.
Another product in development as a topical field therapy for AKs is ingenol disoxate gel, a relative of ingenol mebutate (Picato). Unlike ingenol mebutate, ingenol disoxate remains stable without refrigeration. It’s also a more potent activator of protein kinase C. In mouse models, it shows significantly more cytotoxic potency than does ingenol mebutate.
Based upon the favorable results of a short-term, double-blind phase II study, Leo Pharma now has ingenol disoxate in larger clinical trials as field therapy for AKs on the full face, scalp, or chest, with treatment of larger surface areas than those for which ingenol mebutate is approved (J Dermatolog Treat. 2017 Apr 4:1-7).
Actikerall is also in the developmental pipeline. It consists of 0.5% 5-FU, in combination with 10% salicylic acid, in a film-forming base. Developed by Almirall, it is marketed in Canada by Cipher Pharmaceuticals.
SR-T100 gel utilizes as its active ingredient an antiproliferative extract of Solanum lycocarpum, the Brazilian wolf apple. Taiwan-based G & E Herbal Biotechnology is developing the product, which is in an ongoing phase II clinical trial.
Other novel agents for topical field therapy in phase II studies are KX2-391 ointment, a dual Src kinase/tubulin polymerization inhibitor that causes apoptosis of hyperproliferating cells, under development by Athenex, and Vidac Pharma’s VDA-1102 ointment, which selectively triggers apoptosis in neoplastic cells by modulating voltage-dependent anion channel 1/hexokinase enzyme 2, with minimal impact upon surrounding normal cells.
The principle underlying topical field therapy, compared with simply freezing AKs once they arise, is straightforward, Dr. Bhatia stressed. “It’s the difference between treating only what we can see and treating what’s also on the way.”
Dr. Bhatia reported having financial relationships with more than two dozen pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – The search is on for new topical therapies for actinic keratoses (AKs) that patients will find more appealing than what’s now available, according to Neal Bhatia, MD.
Compliance with current topical field agents for actinic keratoses is not great. Many patients balk at the intense local skin reactions these agents elicit. There is a misplaced sense, especially among patients who don’t grasp the relationship between AKs and squamous cell carcinoma, that the treatment is worse than the disease, Dr. Bhatia said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
One that he said he is particularly excited about is a novel formulation of 4% 5-fluorouracil (5-FU) in an aqueous vehicle cream containing peanut oil. In a recently published, double-blind, multicenter clinical trial involving 841 patients, once-daily application of this product for 4 weeks provided better outcomes and superior tolerability, compared with the old-school regimen of 5% 5-FU cream twice daily.
One hundred percent of patients on 4% 5-FU in peanut oil achieved at least 75% clearance of AKs, as did 95% of those on twice daily 5% 5-FU. The incidence of application site skin irritation in the group on the novel product was only 30%, compared with 60% in the comparison group (J Drugs Dermatol. 2016 Oct 1;15[10]:1218-24).
The peanut oil provided a moisturizing effect and was safe even in patients with peanut allergy, Dr. Bhatia noted.
Another product in development as a topical field therapy for AKs is ingenol disoxate gel, a relative of ingenol mebutate (Picato). Unlike ingenol mebutate, ingenol disoxate remains stable without refrigeration. It’s also a more potent activator of protein kinase C. In mouse models, it shows significantly more cytotoxic potency than does ingenol mebutate.
Based upon the favorable results of a short-term, double-blind phase II study, Leo Pharma now has ingenol disoxate in larger clinical trials as field therapy for AKs on the full face, scalp, or chest, with treatment of larger surface areas than those for which ingenol mebutate is approved (J Dermatolog Treat. 2017 Apr 4:1-7).
Actikerall is also in the developmental pipeline. It consists of 0.5% 5-FU, in combination with 10% salicylic acid, in a film-forming base. Developed by Almirall, it is marketed in Canada by Cipher Pharmaceuticals.
SR-T100 gel utilizes as its active ingredient an antiproliferative extract of Solanum lycocarpum, the Brazilian wolf apple. Taiwan-based G & E Herbal Biotechnology is developing the product, which is in an ongoing phase II clinical trial.
Other novel agents for topical field therapy in phase II studies are KX2-391 ointment, a dual Src kinase/tubulin polymerization inhibitor that causes apoptosis of hyperproliferating cells, under development by Athenex, and Vidac Pharma’s VDA-1102 ointment, which selectively triggers apoptosis in neoplastic cells by modulating voltage-dependent anion channel 1/hexokinase enzyme 2, with minimal impact upon surrounding normal cells.
The principle underlying topical field therapy, compared with simply freezing AKs once they arise, is straightforward, Dr. Bhatia stressed. “It’s the difference between treating only what we can see and treating what’s also on the way.”
Dr. Bhatia reported having financial relationships with more than two dozen pharmaceutical companies. SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Disseminated Superficial Actinic Porokeratosis Treated With Ingenol Mebutate Gel 0.05%
Disseminated superficial actinic porokeratosis (DSAP) is a chronic condition characterized by numerous atrophic papules and patches with a distinctive peripheral keratotic ridge, typically found on sun-exposed areas.1,2 Treatment of DSAP is warranted not only for cosmetic and symptomatic benefits but also to prevent malignant transformation.3,4 Successful treatment of DSAP often is difficult and frequently requires the use of multiple modalities. Ingenol mebutate gel 0.05% is a topical medication primarily used for the treatment of actinic keratosis (AK) by inducing cell death.5 We report a case of DSAP treated effectively with ingenol mebutate gel 0.05%.
Case Report
A 37-year-old woman was referred to the dermatology department for counseling for pseudoxanthoma elasticum (PXE), which had been proven on biopsy by an outside dermatologist 2 years prior. Physical examination revealed yellow papules on the neck that were characteristic of PXE, but no lesions were noted on the arms or legs. The only other cutaneous finding was a soft nodule on the right hip consistent with a lipoma. The patient returned to our institution 6 years later with lesions on both lower legs. She reported that these lesions had been present for 3 years and were exacerbated by sun exposure. On physical examination, multiple scattered, erythematous, annular, scaling papules and plaques were noted on the bilateral legs. A biopsy showed the histopathologic findings of DSAP (Figure 1). The patient had no family history of DSAP or PXE.
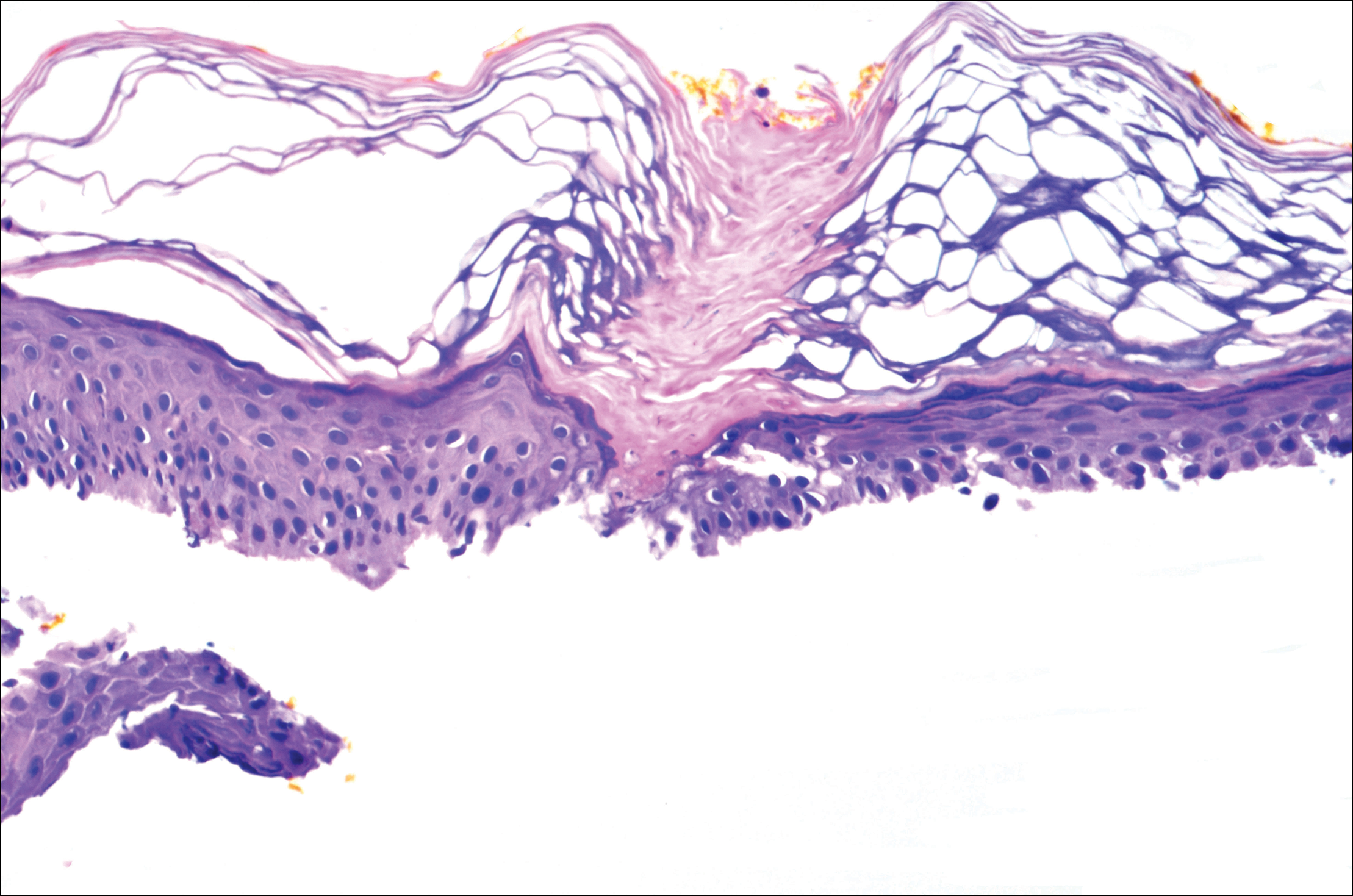
To determine the best treatment modality, we treated 4 test areas on both upper and lower legs: one with trichloroacetic acid (TCA), one with cryotherapy, one with imiquimod cream 5%, and one with tretinoin cream 0.1%. The patient returned 4 weeks later and showed modest response to TCA, cryotherapy, and tretinoin cream. Because cryotherapy was determined to be most effective, 20 more lesions were frozen at that visit. Over the next 2 years, the patient was treated with TCA, imiquimod cream 5%, and tretinoin cream 0.1%, but all ultimately proved ineffective for DSAP.
The patient returned 2 years after treatment failure (age 47 years) and was prescribed ingenol mebutate gel 0.05% for 2 days over an area of 25 cm2 on the right lower leg (Figure 2A). She returned for follow-up at days 3, 15, 30, and 60. At day 3, the patient developed an inflammatory response to the medication with moderate erythema and scaling of individual lesions. No vesiculation, pustulation, edema, or ulceration was exhibited (Figure 2B). At day 30, there was a marked reduction in scaling with some postinflammatory erythema (Figure 2C). At day 60, much of the erythema had faded and the scale remained notably reduced (Figure 2D).
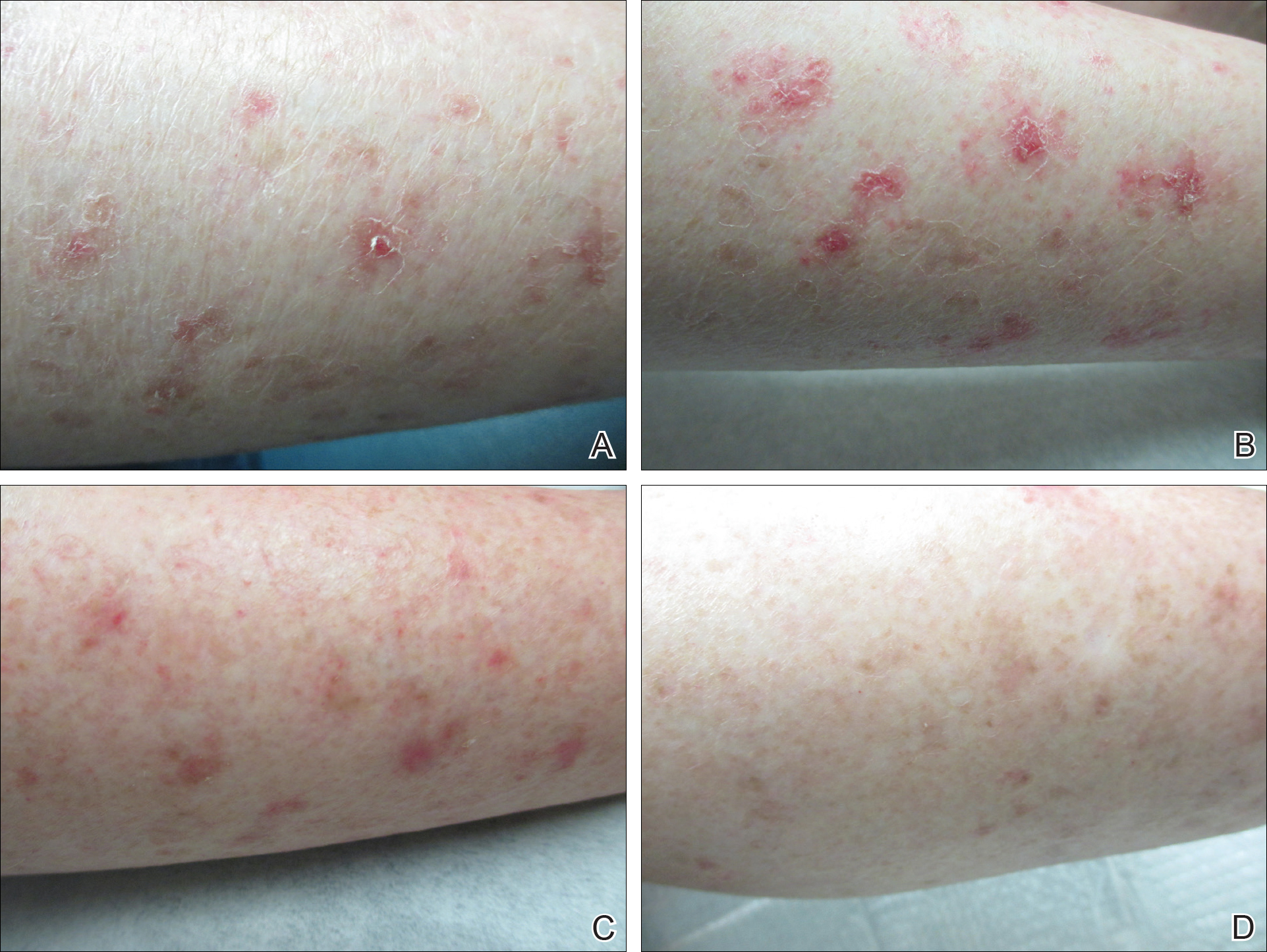
Comment
Disseminated superficial actinic porokeratosis is the most common subtype of porokeratosis, a keratinization disorder. There are 6 subtypes of porokeratosis identified in the literature: DSAP, disseminated superficial porokeratosis, classic porokeratosis of Mibelli, porokeratosis plantaris palmaris et disseminata, linear porokeratosis, and punctate porokeratosis.6 Disseminated superficial actinic porokeratosis has a female predominance (1.8:1 ratio)7 and generally appears in the third or fourth decades of life. Clonal proliferations of atypical keratinocytes have been implicated in the etiology of DSAP; however, the exact pathogenesis is unclear. Risk factors for DSAP include genetic susceptibility (eg, autosomal-dominant inheritance pattern), exposure to UV radiation, and drug-related immunosuppression or immunodeficiency.7 Other proposed etiologic risk factors include trauma and infection.8 Clinical diagnosis of DSAP is confirmed by the histological presence of a cornoid lamella (a thin column ofparakeratotic cells), a thinning epidermis, an absent or thinned granular cell layer, and a prominent dermal lymphocytic infiltrate.9,10
Disseminated superficial actinic porokeratosis clinically presents as small atrophic scaly papules and/or patches with raised peripheral ridges symmetrically dispersed on sun-exposed areas of the arms, legs, back, and shoulders. Although these lesions are extensive, they typically spare the mucous membranes, palms, and soles11; only a small percentage of cases report facial lesions,12 which often are asymptomatic but cosmetically bothersome. Additionally, approximately half of patients report symptoms of pruritus and/or stinging,13 thus treatment of DSAP is mainly indicated for symptomatic relief and cosmetic purposes. Malignant degeneration14,15 occurs in approximately 7.5% to 11% of porokeratosis cases,10,16 warranting treatment for preventative measures.
Management of DSAP is dependent on the extent of the disease and the level of concern for malignant transformation. Localized disease can be treated with cryotherapy, CO2 laser, and/or ablative techniques (eg, excision, curettage, dermabrasion) with variable degrees of success but high risk for scarring.1 More extensive disease requires treatment with topical retinoids, topical 5-fluorouracil, imiquimod cream 5%, diclofenac gel 3%, topical vitamin D3 analogues, and photodynamic therapy.1 Several other therapies have been reported in the literature with partial and/or complete success, including systemic retinoids (eg, acitretin), Q-switched ruby laser, Nd:YAG laser, fractional photothermolysis, Grenz rays, pulsed dye laser, fractional photothermolysis, topical corticosteroids, and fluor-hydroxy pulse peel.6 Although there is an extensive array of therapies for DSAP, treatment results are variable with mostly limited success. Successful treatment of DSAP is difficult and often requires the use of multiple modalities.
Ingenol mebutate is the active compound found in the sap of Euphorbia peplus used for the topical treatment of various skin conditions, including AKs.17 Ingenol mebutate gel 0.05% once daily for 2 days has been approved by the US Food and Drug Administration for the topical treatment of AKs. The mechanism of action of ingenol mebutate in AK therapy is not yet fully understood. In vivo and in vitro models have demonstrated both an induction of local lesion cell death and promotion of lesion-specific inflammatory response.18 When used in the treatment of AKs, ingenol mebutate gel 0.05% may cause a mild to moderate localized inflammatory response (eg, erythema, flaking/scaling, crusting, vesiculation/pustulation, erosion/ulceration, edema).
Our case is a rare report of successful treatment of DSAP with ingenol mebutate gel 0.05%. We found that treatment with ingenol mebutate gel 0.05% resulted in clinical improvement of DSAP lesions with minimal discomfort and good cosmetic response. This 2-day regimen is easy to use and patient friendly, improving medication compliance in such a cumbersome disease. We hope this case suggests that ingenol mebutate gel 0.05% could be a useful treatment alternative for DSAP, but future clinical studies should be conducted.
- Martin-Clavijo A, Kanelleas A, Vlachou C, et al. Porokeratoses. In: Lebwohl M, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease Comprehensive Therapeutic Strategies. 3rd ed. China: Elsevier Limited; 2010:584-586.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatology Online J. 2012;18:24.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- O’Regan GM, Irvine AD. Porokeratosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:442-446.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Brauer JA, Mandal R, Walters R, et al. Disseminated superficial porokeratosis. Dermatology Online J. 2010;16:20.
- Tallon B. Porokeratosis pathology. DermNet New Zealand website. http://www.dermnet.org.nz/pathology/porokeratosis-path.html. Updated December 2016. Accessed January 12, 2017.
- Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review [published online October 22, 2010]. J Dermatolog Treat. 2012;23:52-56.
- Spencer LV. Porokeratosis. UpToDate web site. https://eresources.library.mssm.edu:3285/contents/porokeratosis?source=search_result&search=porokeratosis&selectedTitle=1~22. Updated September 1, 2016. Accessed April 3, 2017.
- Sawyer R, Picou KA. Facial presentation of disseminated superficial actinic porokeratosis. Ear Nose Throat J. 1989;68:57-59.
- Schwarz T, Seiser A, Gschnait F. Disseminated superficial “actinic” porokeratosis. J Am Acad Dermatol. 1984;11(4, pt 2):724-730.
- Maubec E, Duvillard P, Margulis A, et al. Common skin cancers in porokeratosis. Br J Dermatol. 2005;152:1389-1391.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis [published online November 3, 2011]. Ann Dermatol. 2011;23:536-538.
- Kumari S, Mathur M. Disseminated superficial actinic porokeratosis. Nepal J Dermatol Venereol Leprol. 2010;9:22-24.
- Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149:666-670.
- Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
Disseminated superficial actinic porokeratosis (DSAP) is a chronic condition characterized by numerous atrophic papules and patches with a distinctive peripheral keratotic ridge, typically found on sun-exposed areas.1,2 Treatment of DSAP is warranted not only for cosmetic and symptomatic benefits but also to prevent malignant transformation.3,4 Successful treatment of DSAP often is difficult and frequently requires the use of multiple modalities. Ingenol mebutate gel 0.05% is a topical medication primarily used for the treatment of actinic keratosis (AK) by inducing cell death.5 We report a case of DSAP treated effectively with ingenol mebutate gel 0.05%.
Case Report
A 37-year-old woman was referred to the dermatology department for counseling for pseudoxanthoma elasticum (PXE), which had been proven on biopsy by an outside dermatologist 2 years prior. Physical examination revealed yellow papules on the neck that were characteristic of PXE, but no lesions were noted on the arms or legs. The only other cutaneous finding was a soft nodule on the right hip consistent with a lipoma. The patient returned to our institution 6 years later with lesions on both lower legs. She reported that these lesions had been present for 3 years and were exacerbated by sun exposure. On physical examination, multiple scattered, erythematous, annular, scaling papules and plaques were noted on the bilateral legs. A biopsy showed the histopathologic findings of DSAP (Figure 1). The patient had no family history of DSAP or PXE.

To determine the best treatment modality, we treated 4 test areas on both upper and lower legs: one with trichloroacetic acid (TCA), one with cryotherapy, one with imiquimod cream 5%, and one with tretinoin cream 0.1%. The patient returned 4 weeks later and showed modest response to TCA, cryotherapy, and tretinoin cream. Because cryotherapy was determined to be most effective, 20 more lesions were frozen at that visit. Over the next 2 years, the patient was treated with TCA, imiquimod cream 5%, and tretinoin cream 0.1%, but all ultimately proved ineffective for DSAP.
The patient returned 2 years after treatment failure (age 47 years) and was prescribed ingenol mebutate gel 0.05% for 2 days over an area of 25 cm2 on the right lower leg (Figure 2A). She returned for follow-up at days 3, 15, 30, and 60. At day 3, the patient developed an inflammatory response to the medication with moderate erythema and scaling of individual lesions. No vesiculation, pustulation, edema, or ulceration was exhibited (Figure 2B). At day 30, there was a marked reduction in scaling with some postinflammatory erythema (Figure 2C). At day 60, much of the erythema had faded and the scale remained notably reduced (Figure 2D).

Comment
Disseminated superficial actinic porokeratosis is the most common subtype of porokeratosis, a keratinization disorder. There are 6 subtypes of porokeratosis identified in the literature: DSAP, disseminated superficial porokeratosis, classic porokeratosis of Mibelli, porokeratosis plantaris palmaris et disseminata, linear porokeratosis, and punctate porokeratosis.6 Disseminated superficial actinic porokeratosis has a female predominance (1.8:1 ratio)7 and generally appears in the third or fourth decades of life. Clonal proliferations of atypical keratinocytes have been implicated in the etiology of DSAP; however, the exact pathogenesis is unclear. Risk factors for DSAP include genetic susceptibility (eg, autosomal-dominant inheritance pattern), exposure to UV radiation, and drug-related immunosuppression or immunodeficiency.7 Other proposed etiologic risk factors include trauma and infection.8 Clinical diagnosis of DSAP is confirmed by the histological presence of a cornoid lamella (a thin column ofparakeratotic cells), a thinning epidermis, an absent or thinned granular cell layer, and a prominent dermal lymphocytic infiltrate.9,10
Disseminated superficial actinic porokeratosis clinically presents as small atrophic scaly papules and/or patches with raised peripheral ridges symmetrically dispersed on sun-exposed areas of the arms, legs, back, and shoulders. Although these lesions are extensive, they typically spare the mucous membranes, palms, and soles11; only a small percentage of cases report facial lesions,12 which often are asymptomatic but cosmetically bothersome. Additionally, approximately half of patients report symptoms of pruritus and/or stinging,13 thus treatment of DSAP is mainly indicated for symptomatic relief and cosmetic purposes. Malignant degeneration14,15 occurs in approximately 7.5% to 11% of porokeratosis cases,10,16 warranting treatment for preventative measures.
Management of DSAP is dependent on the extent of the disease and the level of concern for malignant transformation. Localized disease can be treated with cryotherapy, CO2 laser, and/or ablative techniques (eg, excision, curettage, dermabrasion) with variable degrees of success but high risk for scarring.1 More extensive disease requires treatment with topical retinoids, topical 5-fluorouracil, imiquimod cream 5%, diclofenac gel 3%, topical vitamin D3 analogues, and photodynamic therapy.1 Several other therapies have been reported in the literature with partial and/or complete success, including systemic retinoids (eg, acitretin), Q-switched ruby laser, Nd:YAG laser, fractional photothermolysis, Grenz rays, pulsed dye laser, fractional photothermolysis, topical corticosteroids, and fluor-hydroxy pulse peel.6 Although there is an extensive array of therapies for DSAP, treatment results are variable with mostly limited success. Successful treatment of DSAP is difficult and often requires the use of multiple modalities.
Ingenol mebutate is the active compound found in the sap of Euphorbia peplus used for the topical treatment of various skin conditions, including AKs.17 Ingenol mebutate gel 0.05% once daily for 2 days has been approved by the US Food and Drug Administration for the topical treatment of AKs. The mechanism of action of ingenol mebutate in AK therapy is not yet fully understood. In vivo and in vitro models have demonstrated both an induction of local lesion cell death and promotion of lesion-specific inflammatory response.18 When used in the treatment of AKs, ingenol mebutate gel 0.05% may cause a mild to moderate localized inflammatory response (eg, erythema, flaking/scaling, crusting, vesiculation/pustulation, erosion/ulceration, edema).
Our case is a rare report of successful treatment of DSAP with ingenol mebutate gel 0.05%. We found that treatment with ingenol mebutate gel 0.05% resulted in clinical improvement of DSAP lesions with minimal discomfort and good cosmetic response. This 2-day regimen is easy to use and patient friendly, improving medication compliance in such a cumbersome disease. We hope this case suggests that ingenol mebutate gel 0.05% could be a useful treatment alternative for DSAP, but future clinical studies should be conducted.
Disseminated superficial actinic porokeratosis (DSAP) is a chronic condition characterized by numerous atrophic papules and patches with a distinctive peripheral keratotic ridge, typically found on sun-exposed areas.1,2 Treatment of DSAP is warranted not only for cosmetic and symptomatic benefits but also to prevent malignant transformation.3,4 Successful treatment of DSAP often is difficult and frequently requires the use of multiple modalities. Ingenol mebutate gel 0.05% is a topical medication primarily used for the treatment of actinic keratosis (AK) by inducing cell death.5 We report a case of DSAP treated effectively with ingenol mebutate gel 0.05%.
Case Report
A 37-year-old woman was referred to the dermatology department for counseling for pseudoxanthoma elasticum (PXE), which had been proven on biopsy by an outside dermatologist 2 years prior. Physical examination revealed yellow papules on the neck that were characteristic of PXE, but no lesions were noted on the arms or legs. The only other cutaneous finding was a soft nodule on the right hip consistent with a lipoma. The patient returned to our institution 6 years later with lesions on both lower legs. She reported that these lesions had been present for 3 years and were exacerbated by sun exposure. On physical examination, multiple scattered, erythematous, annular, scaling papules and plaques were noted on the bilateral legs. A biopsy showed the histopathologic findings of DSAP (Figure 1). The patient had no family history of DSAP or PXE.

To determine the best treatment modality, we treated 4 test areas on both upper and lower legs: one with trichloroacetic acid (TCA), one with cryotherapy, one with imiquimod cream 5%, and one with tretinoin cream 0.1%. The patient returned 4 weeks later and showed modest response to TCA, cryotherapy, and tretinoin cream. Because cryotherapy was determined to be most effective, 20 more lesions were frozen at that visit. Over the next 2 years, the patient was treated with TCA, imiquimod cream 5%, and tretinoin cream 0.1%, but all ultimately proved ineffective for DSAP.
The patient returned 2 years after treatment failure (age 47 years) and was prescribed ingenol mebutate gel 0.05% for 2 days over an area of 25 cm2 on the right lower leg (Figure 2A). She returned for follow-up at days 3, 15, 30, and 60. At day 3, the patient developed an inflammatory response to the medication with moderate erythema and scaling of individual lesions. No vesiculation, pustulation, edema, or ulceration was exhibited (Figure 2B). At day 30, there was a marked reduction in scaling with some postinflammatory erythema (Figure 2C). At day 60, much of the erythema had faded and the scale remained notably reduced (Figure 2D).

Comment
Disseminated superficial actinic porokeratosis is the most common subtype of porokeratosis, a keratinization disorder. There are 6 subtypes of porokeratosis identified in the literature: DSAP, disseminated superficial porokeratosis, classic porokeratosis of Mibelli, porokeratosis plantaris palmaris et disseminata, linear porokeratosis, and punctate porokeratosis.6 Disseminated superficial actinic porokeratosis has a female predominance (1.8:1 ratio)7 and generally appears in the third or fourth decades of life. Clonal proliferations of atypical keratinocytes have been implicated in the etiology of DSAP; however, the exact pathogenesis is unclear. Risk factors for DSAP include genetic susceptibility (eg, autosomal-dominant inheritance pattern), exposure to UV radiation, and drug-related immunosuppression or immunodeficiency.7 Other proposed etiologic risk factors include trauma and infection.8 Clinical diagnosis of DSAP is confirmed by the histological presence of a cornoid lamella (a thin column ofparakeratotic cells), a thinning epidermis, an absent or thinned granular cell layer, and a prominent dermal lymphocytic infiltrate.9,10
Disseminated superficial actinic porokeratosis clinically presents as small atrophic scaly papules and/or patches with raised peripheral ridges symmetrically dispersed on sun-exposed areas of the arms, legs, back, and shoulders. Although these lesions are extensive, they typically spare the mucous membranes, palms, and soles11; only a small percentage of cases report facial lesions,12 which often are asymptomatic but cosmetically bothersome. Additionally, approximately half of patients report symptoms of pruritus and/or stinging,13 thus treatment of DSAP is mainly indicated for symptomatic relief and cosmetic purposes. Malignant degeneration14,15 occurs in approximately 7.5% to 11% of porokeratosis cases,10,16 warranting treatment for preventative measures.
Management of DSAP is dependent on the extent of the disease and the level of concern for malignant transformation. Localized disease can be treated with cryotherapy, CO2 laser, and/or ablative techniques (eg, excision, curettage, dermabrasion) with variable degrees of success but high risk for scarring.1 More extensive disease requires treatment with topical retinoids, topical 5-fluorouracil, imiquimod cream 5%, diclofenac gel 3%, topical vitamin D3 analogues, and photodynamic therapy.1 Several other therapies have been reported in the literature with partial and/or complete success, including systemic retinoids (eg, acitretin), Q-switched ruby laser, Nd:YAG laser, fractional photothermolysis, Grenz rays, pulsed dye laser, fractional photothermolysis, topical corticosteroids, and fluor-hydroxy pulse peel.6 Although there is an extensive array of therapies for DSAP, treatment results are variable with mostly limited success. Successful treatment of DSAP is difficult and often requires the use of multiple modalities.
Ingenol mebutate is the active compound found in the sap of Euphorbia peplus used for the topical treatment of various skin conditions, including AKs.17 Ingenol mebutate gel 0.05% once daily for 2 days has been approved by the US Food and Drug Administration for the topical treatment of AKs. The mechanism of action of ingenol mebutate in AK therapy is not yet fully understood. In vivo and in vitro models have demonstrated both an induction of local lesion cell death and promotion of lesion-specific inflammatory response.18 When used in the treatment of AKs, ingenol mebutate gel 0.05% may cause a mild to moderate localized inflammatory response (eg, erythema, flaking/scaling, crusting, vesiculation/pustulation, erosion/ulceration, edema).
Our case is a rare report of successful treatment of DSAP with ingenol mebutate gel 0.05%. We found that treatment with ingenol mebutate gel 0.05% resulted in clinical improvement of DSAP lesions with minimal discomfort and good cosmetic response. This 2-day regimen is easy to use and patient friendly, improving medication compliance in such a cumbersome disease. We hope this case suggests that ingenol mebutate gel 0.05% could be a useful treatment alternative for DSAP, but future clinical studies should be conducted.
- Martin-Clavijo A, Kanelleas A, Vlachou C, et al. Porokeratoses. In: Lebwohl M, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease Comprehensive Therapeutic Strategies. 3rd ed. China: Elsevier Limited; 2010:584-586.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatology Online J. 2012;18:24.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- O’Regan GM, Irvine AD. Porokeratosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:442-446.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Brauer JA, Mandal R, Walters R, et al. Disseminated superficial porokeratosis. Dermatology Online J. 2010;16:20.
- Tallon B. Porokeratosis pathology. DermNet New Zealand website. http://www.dermnet.org.nz/pathology/porokeratosis-path.html. Updated December 2016. Accessed January 12, 2017.
- Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review [published online October 22, 2010]. J Dermatolog Treat. 2012;23:52-56.
- Spencer LV. Porokeratosis. UpToDate web site. https://eresources.library.mssm.edu:3285/contents/porokeratosis?source=search_result&search=porokeratosis&selectedTitle=1~22. Updated September 1, 2016. Accessed April 3, 2017.
- Sawyer R, Picou KA. Facial presentation of disseminated superficial actinic porokeratosis. Ear Nose Throat J. 1989;68:57-59.
- Schwarz T, Seiser A, Gschnait F. Disseminated superficial “actinic” porokeratosis. J Am Acad Dermatol. 1984;11(4, pt 2):724-730.
- Maubec E, Duvillard P, Margulis A, et al. Common skin cancers in porokeratosis. Br J Dermatol. 2005;152:1389-1391.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis [published online November 3, 2011]. Ann Dermatol. 2011;23:536-538.
- Kumari S, Mathur M. Disseminated superficial actinic porokeratosis. Nepal J Dermatol Venereol Leprol. 2010;9:22-24.
- Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149:666-670.
- Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
- Martin-Clavijo A, Kanelleas A, Vlachou C, et al. Porokeratoses. In: Lebwohl M, Heymann WR, Berth-Jones J, et al, eds. Treatment of Skin Disease Comprehensive Therapeutic Strategies. 3rd ed. China: Elsevier Limited; 2010:584-586.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatology Online J. 2012;18:24.
- Sasson M, Krain AD. Porokeratosis and cutaneous malignancy. a review. Dermatol Surg. 1996;22:339-342.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
- O’Regan GM, Irvine AD. Porokeratosis. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:442-446.
- Sertznig P, von Felbert V, Megahed M. Porokeratosis: present concepts. J Eur Acad Dermatol Venereol. 2012;26:404-412.
- Brauer JA, Mandal R, Walters R, et al. Disseminated superficial porokeratosis. Dermatology Online J. 2010;16:20.
- Tallon B. Porokeratosis pathology. DermNet New Zealand website. http://www.dermnet.org.nz/pathology/porokeratosis-path.html. Updated December 2016. Accessed January 12, 2017.
- Skupsky H, Skupsky J, Goldenberg G. Disseminated superficial actinic porokeratosis: a treatment review [published online October 22, 2010]. J Dermatolog Treat. 2012;23:52-56.
- Spencer LV. Porokeratosis. UpToDate web site. https://eresources.library.mssm.edu:3285/contents/porokeratosis?source=search_result&search=porokeratosis&selectedTitle=1~22. Updated September 1, 2016. Accessed April 3, 2017.
- Sawyer R, Picou KA. Facial presentation of disseminated superficial actinic porokeratosis. Ear Nose Throat J. 1989;68:57-59.
- Schwarz T, Seiser A, Gschnait F. Disseminated superficial “actinic” porokeratosis. J Am Acad Dermatol. 1984;11(4, pt 2):724-730.
- Maubec E, Duvillard P, Margulis A, et al. Common skin cancers in porokeratosis. Br J Dermatol. 2005;152:1389-1391.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis [published online November 3, 2011]. Ann Dermatol. 2011;23:536-538.
- Kumari S, Mathur M. Disseminated superficial actinic porokeratosis. Nepal J Dermatol Venereol Leprol. 2010;9:22-24.
- Lebwohl M, Shumack S, Stein Gold L, et al. Long-term follow-up study of ingenol mebutate gel for the treatment of actinic keratosis. JAMA Dermatol. 2013;149:666-670.
- Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
Practice Points
- Disseminated superficial actinic porokeratosis (DSAP) is an uncommon skin condition consisting of multiple annular hyperkeratotic lesions on sun-exposed areas.
- Treatment of DSAP is necessary due to its potential for progression to malignancy.
- Consider ingenol mebutate gel 0.05% for the treatment of DSAP on the arms and legs.