User login
Functional medicine offers another approach to treating psychiatric illness
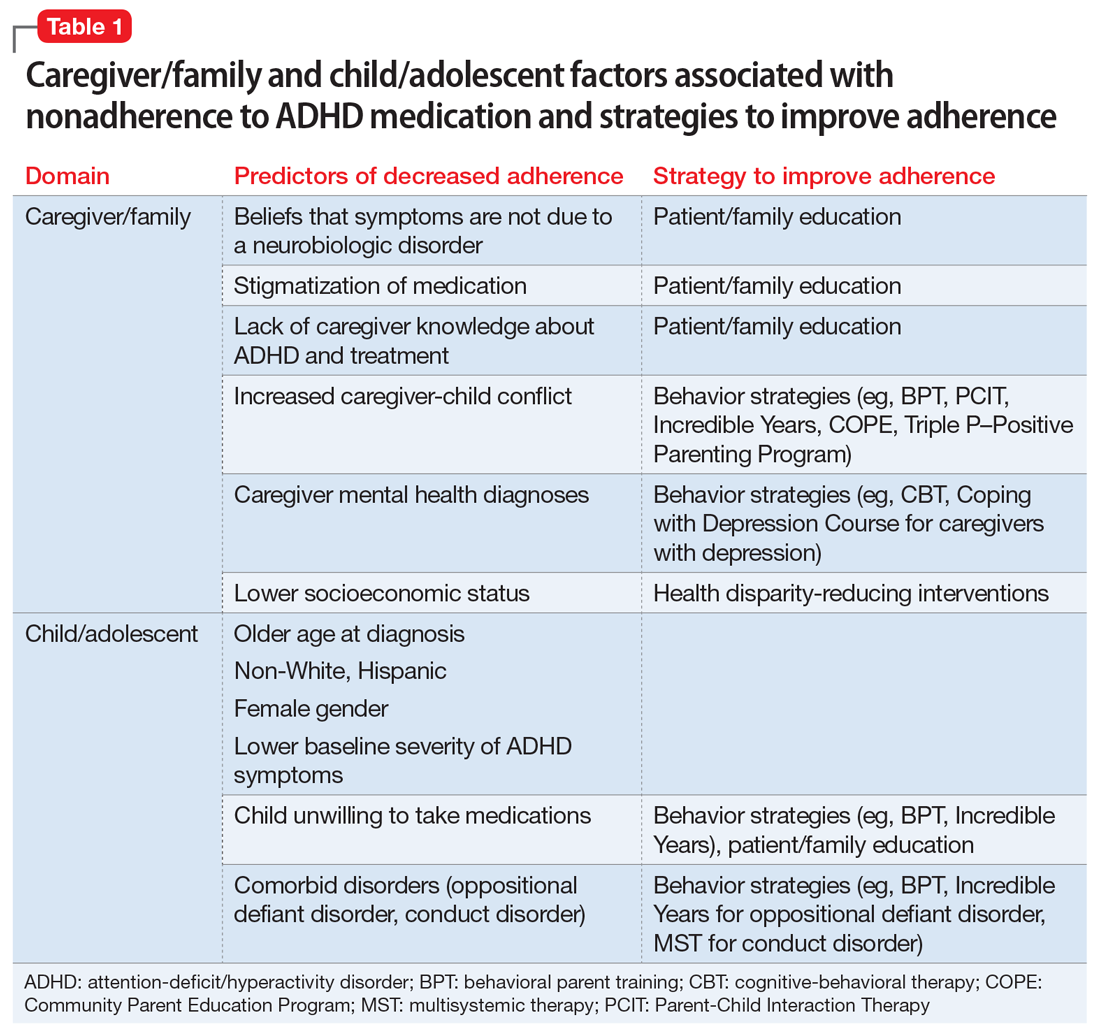
The shortage of psychiatrists, other mental health clinicians, and primary care physicians who treat patients with mental illness is a profound problem in the United States and around the world. What would happen to those trends if psychiatrists incorporated a functional medicine approach to treating patients?
In functional medicine, we look for underlying causes, physiological damage that results from those causes, clinical body system imbalances, and ultimately, symptoms that patients are experiencing. By addressing the root causes of chronic problems, treating physiological damage, and creating balance in body systems, psychiatrists and other physicians can help our patients achieve optimal health.
For example, a functional medicine approach to treating a child with ADHD might focus on encouraging behavioral changes such as improving sleep hygiene,1 increasing hydration,2 changing nutrition, or prescribing adjunctive meditation rather than medication alone. A functional medicine approach to Alzheimer’s prevention, for example, could include “prescribing” an increase in the amount of regular physical exercise.3 In other words, functional medicine uses a different lens to prevent, arrest, and in some cases, reverse certain diseases.
Medicine has long recognized the links between inflammation and chronic illness. Autoimmune conditions, asthma, heart disease, stroke, diabetes, obesity, peripheral neuropathy, thyroid problems, joint pain, and cancer all are chronic inflammatory diseases. Because inflammation affects the brain, it has been theorized and is being investigated that psychiatric disorders such as depression, schizophrenia, anxiety, panic attacks, dementia, and autism might result.4,5,6,7
Besides the brain, the GI tract is the only organ system that has its own nervous system, which is called the enteric nervous system, or ENS. The ENS functions independently from the central nervous system, and transmits important messages to and from the brain. When one feels stressed, the brain communicates to the hormonal system and floods the body with stress hormones, such as cortisol, which by themselves, can cause increased intestinal permeability. In addition, the gut produces its own neurotransmitters that affect the brain. In fact, every class of neurotransmitter found in the brain also is found in the GI tract. For example, serotonin is an important neurotransmitter for feeling happy and optimistic. Ninety-five percent of the body’s serotonin is produced in the gut. It is produced from 5-HTP, which is derived from tryptophan. However, in the presence of inflammation in the body, tryptophan is converted into kynurenate and quinolate. Both cause fatigue, and quinolate causes neurotoxicity. The subsequent depletion of serotonin produces symptoms of depression. Problems in the gut can lead to problems in the brain and the whole body.
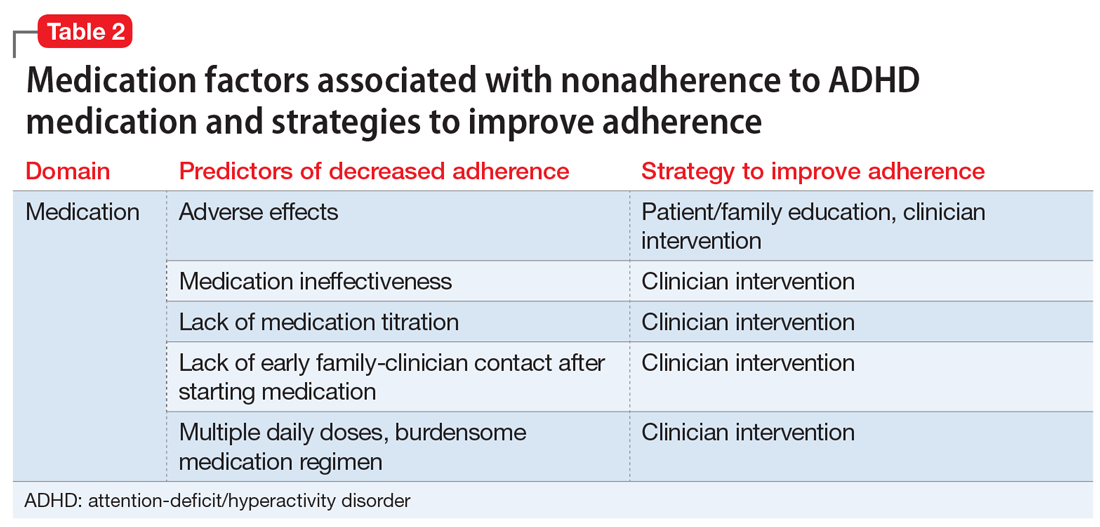
Other problems affecting patients are tied to toxins in the environment. The air we breathe, food we eat, water we drink, and clothing we wear all are sources of toxins. Toxins include biotoxins, dioxine, phthalates, PCBs, and heavy metals, such as mercury, lead, cadmium, aluminum. About 2,000 new chemicals have been introduced into our environment each year since the 1940s, and it is estimated that we are exposed to more than 80,000 chemicals on a regular basis.8
The Environmental Working Group, a nonprofit organization dedicated to educating the public about the environment, has estimated that average babies are born with 287 chemicals in their body, 217 of which are neurotoxins.9 As children grow up, their body accumulates more toxins. According to the Centers for Disease Control and Prevention, every American has hundreds of neurotoxins in their bodies right now.
As we become more aware of the many changes in our environment, functional medicine brings a new way of thinking about and looking at chronic disease. As physicians, we can continue treating symptoms, and we should. But we can look deeper and ask ourselves what has changed in our lives that has caused such a decline in human mental and physical health. I urge psychiatrists to help lead the way.
Dr. Gaitour, a physiatrist, trained at NYU Langone Medical Center in New York. She is a functional medicine practitioner.
References
1. Peppers KH et al. J Pediatr Health Care. 2016 Nov-Dec;30(6):e43-8.
2. Martin EB and PG Hammerness. ADHD, stimulant medication, and dehydration. CHADD.org. 2014 Aug.
3. Guitar NA et al. Ageing Res Rev. 2018 Nov;47:159-67.
4. Mørch RH et al. Acta Psychiatr Scand. 2017 Oct;136(4):400-8.
5. Dooley LN et al. Neurosci Biobehav Rev. 2018 Nov;94:219-37.
6. Yang L et al. Brain Behav Immun. 2016 Aug;56:352-62.
7. Doenyas C. Neuroscience. 2018 Mar 15;374:271-86.
8. PBS News Hour. 2016 Jun 22.
9. Houlihan J. Environmental Working Group. 2005 Jul 14.
The shortage of psychiatrists, other mental health clinicians, and primary care physicians who treat patients with mental illness is a profound problem in the United States and around the world. What would happen to those trends if psychiatrists incorporated a functional medicine approach to treating patients?
In functional medicine, we look for underlying causes, physiological damage that results from those causes, clinical body system imbalances, and ultimately, symptoms that patients are experiencing. By addressing the root causes of chronic problems, treating physiological damage, and creating balance in body systems, psychiatrists and other physicians can help our patients achieve optimal health.
For example, a functional medicine approach to treating a child with ADHD might focus on encouraging behavioral changes such as improving sleep hygiene,1 increasing hydration,2 changing nutrition, or prescribing adjunctive meditation rather than medication alone. A functional medicine approach to Alzheimer’s prevention, for example, could include “prescribing” an increase in the amount of regular physical exercise.3 In other words, functional medicine uses a different lens to prevent, arrest, and in some cases, reverse certain diseases.
Medicine has long recognized the links between inflammation and chronic illness. Autoimmune conditions, asthma, heart disease, stroke, diabetes, obesity, peripheral neuropathy, thyroid problems, joint pain, and cancer all are chronic inflammatory diseases. Because inflammation affects the brain, it has been theorized and is being investigated that psychiatric disorders such as depression, schizophrenia, anxiety, panic attacks, dementia, and autism might result.4,5,6,7
Besides the brain, the GI tract is the only organ system that has its own nervous system, which is called the enteric nervous system, or ENS. The ENS functions independently from the central nervous system, and transmits important messages to and from the brain. When one feels stressed, the brain communicates to the hormonal system and floods the body with stress hormones, such as cortisol, which by themselves, can cause increased intestinal permeability. In addition, the gut produces its own neurotransmitters that affect the brain. In fact, every class of neurotransmitter found in the brain also is found in the GI tract. For example, serotonin is an important neurotransmitter for feeling happy and optimistic. Ninety-five percent of the body’s serotonin is produced in the gut. It is produced from 5-HTP, which is derived from tryptophan. However, in the presence of inflammation in the body, tryptophan is converted into kynurenate and quinolate. Both cause fatigue, and quinolate causes neurotoxicity. The subsequent depletion of serotonin produces symptoms of depression. Problems in the gut can lead to problems in the brain and the whole body.
Other problems affecting patients are tied to toxins in the environment. The air we breathe, food we eat, water we drink, and clothing we wear all are sources of toxins. Toxins include biotoxins, dioxine, phthalates, PCBs, and heavy metals, such as mercury, lead, cadmium, aluminum. About 2,000 new chemicals have been introduced into our environment each year since the 1940s, and it is estimated that we are exposed to more than 80,000 chemicals on a regular basis.8
The Environmental Working Group, a nonprofit organization dedicated to educating the public about the environment, has estimated that average babies are born with 287 chemicals in their body, 217 of which are neurotoxins.9 As children grow up, their body accumulates more toxins. According to the Centers for Disease Control and Prevention, every American has hundreds of neurotoxins in their bodies right now.
As we become more aware of the many changes in our environment, functional medicine brings a new way of thinking about and looking at chronic disease. As physicians, we can continue treating symptoms, and we should. But we can look deeper and ask ourselves what has changed in our lives that has caused such a decline in human mental and physical health. I urge psychiatrists to help lead the way.
Dr. Gaitour, a physiatrist, trained at NYU Langone Medical Center in New York. She is a functional medicine practitioner.
References
1. Peppers KH et al. J Pediatr Health Care. 2016 Nov-Dec;30(6):e43-8.
2. Martin EB and PG Hammerness. ADHD, stimulant medication, and dehydration. CHADD.org. 2014 Aug.
3. Guitar NA et al. Ageing Res Rev. 2018 Nov;47:159-67.
4. Mørch RH et al. Acta Psychiatr Scand. 2017 Oct;136(4):400-8.
5. Dooley LN et al. Neurosci Biobehav Rev. 2018 Nov;94:219-37.
6. Yang L et al. Brain Behav Immun. 2016 Aug;56:352-62.
7. Doenyas C. Neuroscience. 2018 Mar 15;374:271-86.
8. PBS News Hour. 2016 Jun 22.
9. Houlihan J. Environmental Working Group. 2005 Jul 14.
The shortage of psychiatrists, other mental health clinicians, and primary care physicians who treat patients with mental illness is a profound problem in the United States and around the world. What would happen to those trends if psychiatrists incorporated a functional medicine approach to treating patients?
In functional medicine, we look for underlying causes, physiological damage that results from those causes, clinical body system imbalances, and ultimately, symptoms that patients are experiencing. By addressing the root causes of chronic problems, treating physiological damage, and creating balance in body systems, psychiatrists and other physicians can help our patients achieve optimal health.
For example, a functional medicine approach to treating a child with ADHD might focus on encouraging behavioral changes such as improving sleep hygiene,1 increasing hydration,2 changing nutrition, or prescribing adjunctive meditation rather than medication alone. A functional medicine approach to Alzheimer’s prevention, for example, could include “prescribing” an increase in the amount of regular physical exercise.3 In other words, functional medicine uses a different lens to prevent, arrest, and in some cases, reverse certain diseases.
Medicine has long recognized the links between inflammation and chronic illness. Autoimmune conditions, asthma, heart disease, stroke, diabetes, obesity, peripheral neuropathy, thyroid problems, joint pain, and cancer all are chronic inflammatory diseases. Because inflammation affects the brain, it has been theorized and is being investigated that psychiatric disorders such as depression, schizophrenia, anxiety, panic attacks, dementia, and autism might result.4,5,6,7
Besides the brain, the GI tract is the only organ system that has its own nervous system, which is called the enteric nervous system, or ENS. The ENS functions independently from the central nervous system, and transmits important messages to and from the brain. When one feels stressed, the brain communicates to the hormonal system and floods the body with stress hormones, such as cortisol, which by themselves, can cause increased intestinal permeability. In addition, the gut produces its own neurotransmitters that affect the brain. In fact, every class of neurotransmitter found in the brain also is found in the GI tract. For example, serotonin is an important neurotransmitter for feeling happy and optimistic. Ninety-five percent of the body’s serotonin is produced in the gut. It is produced from 5-HTP, which is derived from tryptophan. However, in the presence of inflammation in the body, tryptophan is converted into kynurenate and quinolate. Both cause fatigue, and quinolate causes neurotoxicity. The subsequent depletion of serotonin produces symptoms of depression. Problems in the gut can lead to problems in the brain and the whole body.
Other problems affecting patients are tied to toxins in the environment. The air we breathe, food we eat, water we drink, and clothing we wear all are sources of toxins. Toxins include biotoxins, dioxine, phthalates, PCBs, and heavy metals, such as mercury, lead, cadmium, aluminum. About 2,000 new chemicals have been introduced into our environment each year since the 1940s, and it is estimated that we are exposed to more than 80,000 chemicals on a regular basis.8
The Environmental Working Group, a nonprofit organization dedicated to educating the public about the environment, has estimated that average babies are born with 287 chemicals in their body, 217 of which are neurotoxins.9 As children grow up, their body accumulates more toxins. According to the Centers for Disease Control and Prevention, every American has hundreds of neurotoxins in their bodies right now.
As we become more aware of the many changes in our environment, functional medicine brings a new way of thinking about and looking at chronic disease. As physicians, we can continue treating symptoms, and we should. But we can look deeper and ask ourselves what has changed in our lives that has caused such a decline in human mental and physical health. I urge psychiatrists to help lead the way.
Dr. Gaitour, a physiatrist, trained at NYU Langone Medical Center in New York. She is a functional medicine practitioner.
References
1. Peppers KH et al. J Pediatr Health Care. 2016 Nov-Dec;30(6):e43-8.
2. Martin EB and PG Hammerness. ADHD, stimulant medication, and dehydration. CHADD.org. 2014 Aug.
3. Guitar NA et al. Ageing Res Rev. 2018 Nov;47:159-67.
4. Mørch RH et al. Acta Psychiatr Scand. 2017 Oct;136(4):400-8.
5. Dooley LN et al. Neurosci Biobehav Rev. 2018 Nov;94:219-37.
6. Yang L et al. Brain Behav Immun. 2016 Aug;56:352-62.
7. Doenyas C. Neuroscience. 2018 Mar 15;374:271-86.
8. PBS News Hour. 2016 Jun 22.
9. Houlihan J. Environmental Working Group. 2005 Jul 14.
Higher teen pregnancy risk in girls with ADHD
Teenage girls with ADHD may be at greater risk of pregnancy than their unaffected peers, which suggests they may benefit from targeted interventions to prevent teen pregnancy.
A Swedish nationwide cohort study published in JAMA Network Open examined data from 384,103 nulliparous women and girls who gave birth between 2007-2014, of whom, 6,410 (1.7%) had received treatment for ADHD.
While the overall rate of teenage births was 3%, the rate among women and girls with ADHD was 15.3%, which represents a greater than sixfold higher odds of giving birth below the age of 20 years (odds ratio, 6.23; 95% confidence interval, 5.80-6.68).
“Becoming a mother at such early age is associated with long-term adverse outcomes for both women and their children,” wrote Charlotte Skoglund, PhD, of the department of clinical neuroscience at the Karolinska Institute in Stockholm and coauthors. “Consequently, our findings argue for an improvement in the standard of care for women and girls with ADHD, including active efforts to prevent teenage pregnancies and address comorbid medical and psychiatric conditions.”
The study also found women and girls with ADHD were significantly more likely to be underweight (OR, 1.29; 95% CI, 1.12-1.49) or have a body mass index greater than 40 kg/m2 (OR, 2.01; 95% CI, 1.60-2.52) when compared with those without ADHD.
They were also six times more likely to smoke, were nearly seven times more likely to continue smoking into their third trimester of pregnancy, and had a 20-fold higher odds of alcohol and substance use disorder. Among individuals who had been diagnosed with ADHD, 7.6% continued to use stimulant and nonstimulant ADHD medication during pregnancy, and 16.4% used antidepressants during pregnancy.
Psychiatric comorbidities were also significantly more common among individuals with ADHD in the year preceding pregnancy, compared with those without ADHD. The authors saw a 17-fold higher odds of receiving a diagnosis of bipolar disorder, nearly 8-fold higher odds of a diagnosis of schizophrenia or other psychotic disorder, and 22-fold higher odds of being diagnosed with emotionally unstable personality disorder among women and girls with ADHD versus those without.
The authors commented that antenatal care should focus on trying to reduce such obstetric risk factors in these women, but also pointed out that ADHD in women and girls was still underdiagnosed and undertreated.
Commenting on the association between ADHD and teenage pregnancy, the authors noted that women and girls with ADHD may be less likely to receive adequate contraceptive counseling and less likely to access, respond to, and act on counseling. They may also experience more adverse effects from hormonal contraceptives.
While Swedish youth clinics enable easier and low-cost access to counseling and contraception, the authors called for greater collaboration between psychiatric care clinics and specialized youth clinics to provide adequate care for women and girls with ADHD.
Three authors declared advisory board positions, grants, personal fees, and speakers’ fees from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Skoglund C et al. JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12463
Teenage girls with ADHD may be at greater risk of pregnancy than their unaffected peers, which suggests they may benefit from targeted interventions to prevent teen pregnancy.
A Swedish nationwide cohort study published in JAMA Network Open examined data from 384,103 nulliparous women and girls who gave birth between 2007-2014, of whom, 6,410 (1.7%) had received treatment for ADHD.
While the overall rate of teenage births was 3%, the rate among women and girls with ADHD was 15.3%, which represents a greater than sixfold higher odds of giving birth below the age of 20 years (odds ratio, 6.23; 95% confidence interval, 5.80-6.68).
“Becoming a mother at such early age is associated with long-term adverse outcomes for both women and their children,” wrote Charlotte Skoglund, PhD, of the department of clinical neuroscience at the Karolinska Institute in Stockholm and coauthors. “Consequently, our findings argue for an improvement in the standard of care for women and girls with ADHD, including active efforts to prevent teenage pregnancies and address comorbid medical and psychiatric conditions.”
The study also found women and girls with ADHD were significantly more likely to be underweight (OR, 1.29; 95% CI, 1.12-1.49) or have a body mass index greater than 40 kg/m2 (OR, 2.01; 95% CI, 1.60-2.52) when compared with those without ADHD.
They were also six times more likely to smoke, were nearly seven times more likely to continue smoking into their third trimester of pregnancy, and had a 20-fold higher odds of alcohol and substance use disorder. Among individuals who had been diagnosed with ADHD, 7.6% continued to use stimulant and nonstimulant ADHD medication during pregnancy, and 16.4% used antidepressants during pregnancy.
Psychiatric comorbidities were also significantly more common among individuals with ADHD in the year preceding pregnancy, compared with those without ADHD. The authors saw a 17-fold higher odds of receiving a diagnosis of bipolar disorder, nearly 8-fold higher odds of a diagnosis of schizophrenia or other psychotic disorder, and 22-fold higher odds of being diagnosed with emotionally unstable personality disorder among women and girls with ADHD versus those without.
The authors commented that antenatal care should focus on trying to reduce such obstetric risk factors in these women, but also pointed out that ADHD in women and girls was still underdiagnosed and undertreated.
Commenting on the association between ADHD and teenage pregnancy, the authors noted that women and girls with ADHD may be less likely to receive adequate contraceptive counseling and less likely to access, respond to, and act on counseling. They may also experience more adverse effects from hormonal contraceptives.
While Swedish youth clinics enable easier and low-cost access to counseling and contraception, the authors called for greater collaboration between psychiatric care clinics and specialized youth clinics to provide adequate care for women and girls with ADHD.
Three authors declared advisory board positions, grants, personal fees, and speakers’ fees from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Skoglund C et al. JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12463
Teenage girls with ADHD may be at greater risk of pregnancy than their unaffected peers, which suggests they may benefit from targeted interventions to prevent teen pregnancy.
A Swedish nationwide cohort study published in JAMA Network Open examined data from 384,103 nulliparous women and girls who gave birth between 2007-2014, of whom, 6,410 (1.7%) had received treatment for ADHD.
While the overall rate of teenage births was 3%, the rate among women and girls with ADHD was 15.3%, which represents a greater than sixfold higher odds of giving birth below the age of 20 years (odds ratio, 6.23; 95% confidence interval, 5.80-6.68).
“Becoming a mother at such early age is associated with long-term adverse outcomes for both women and their children,” wrote Charlotte Skoglund, PhD, of the department of clinical neuroscience at the Karolinska Institute in Stockholm and coauthors. “Consequently, our findings argue for an improvement in the standard of care for women and girls with ADHD, including active efforts to prevent teenage pregnancies and address comorbid medical and psychiatric conditions.”
The study also found women and girls with ADHD were significantly more likely to be underweight (OR, 1.29; 95% CI, 1.12-1.49) or have a body mass index greater than 40 kg/m2 (OR, 2.01; 95% CI, 1.60-2.52) when compared with those without ADHD.
They were also six times more likely to smoke, were nearly seven times more likely to continue smoking into their third trimester of pregnancy, and had a 20-fold higher odds of alcohol and substance use disorder. Among individuals who had been diagnosed with ADHD, 7.6% continued to use stimulant and nonstimulant ADHD medication during pregnancy, and 16.4% used antidepressants during pregnancy.
Psychiatric comorbidities were also significantly more common among individuals with ADHD in the year preceding pregnancy, compared with those without ADHD. The authors saw a 17-fold higher odds of receiving a diagnosis of bipolar disorder, nearly 8-fold higher odds of a diagnosis of schizophrenia or other psychotic disorder, and 22-fold higher odds of being diagnosed with emotionally unstable personality disorder among women and girls with ADHD versus those without.
The authors commented that antenatal care should focus on trying to reduce such obstetric risk factors in these women, but also pointed out that ADHD in women and girls was still underdiagnosed and undertreated.
Commenting on the association between ADHD and teenage pregnancy, the authors noted that women and girls with ADHD may be less likely to receive adequate contraceptive counseling and less likely to access, respond to, and act on counseling. They may also experience more adverse effects from hormonal contraceptives.
While Swedish youth clinics enable easier and low-cost access to counseling and contraception, the authors called for greater collaboration between psychiatric care clinics and specialized youth clinics to provide adequate care for women and girls with ADHD.
Three authors declared advisory board positions, grants, personal fees, and speakers’ fees from the pharmaceutical sector. No other conflicts of interest were declared.
SOURCE: Skoglund C et al. JAMA Netw Open. 2019 Oct 2. doi: 10.1001/jamanetworkopen.2019.12463
FROM JAMA NETWORK OPEN
Premature mortality across most psychiatric disorders
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
The evidence is robust and disheartening: As if the personal suffering and societal stigma of mental illness are not bad enough, psychiatric patients also have a shorter lifespan.1 In the past, most studies have focused on early mortality and loss of potential life-years in schizophrenia,2 but many subsequent reports indicate that premature death occurs in all major psychiatric disorders.
Here is a summary of the sobering facts:
- Schizophrenia. In a study of 30,210 patients with schizophrenia, compared with >5 million individuals in the general population in Denmark (where they have an excellent registry), mortality was 16-fold higher among patients with schizophrenia if they had a single somatic illness.3 The illnesses were mostly respiratory, gastrointestinal, or cardiovascular).3 The loss of potential years of life was staggeringly high: 18.7 years for men, 16.3 years for women.4 A study conducted in 8 US states reported a loss of 2 to 3 decades of life across each of these states.5 The causes of death in patients with schizophrenia were mainly heart disease, cancer, stroke, and pulmonary diseases. A national database in Sweden found that unmedicated patients with schizophrenia had a significantly higher death rate than those receiving antipsychotics.6,7 Similar findings were reported by researchers in Finland.8 The Swedish study by Tiihonen et al6 also found that mortality was highest in patients receiving benzodiazepines along with antipsychotics, but there was no increased mortality among patients with schizophrenia receiving antidepressants.
- Bipolar disorder. A shorter life expectancy has also been reported in bipolar disorder,9 with a loss of 13.6 years for men and 12.1 years for women. Early death was caused by physical illness (even when suicide deaths were excluded), especially cardiovascular disease.10
- Major depressive disorder (MDD). A reduction of life expectancy in persons with MDD (unipolar depression) has been reported, with a loss of 14 years in men and 10 years in women.11 Although suicide contributed to the shorter lifespan, death due to accidents was 500% higher among persons with unipolar depression; the largest causes of death were physical illnesses. Further, Zubenko et al12 reported alarming findings about excess mortality among first- and second-degree relatives of persons with early-onset depression (some of whom were bipolar). The relatives died an average of 8 years earlier than the local population, and 40% died before reaching age 65. Also, there was a 5-fold increase in infant mortality (in the first year of life) among the relatives. The most common causes of death in adult relatives were heart disease, cancer, and stroke. It is obvious that MDD has a significant negative impact on health and longevity in both patients and their relatives.
- Attention-deficit/hyperactivity disorder (ADHD). A 220% increase in mortality was reported in persons with ADHD at all ages.13 Accidents were the most common cause of death. The mortality rate ratio (MRR) was 1.86 for ADHD before age 6, 1.58 for ADHD between age 6 to 17, and 4.25 for those age ≥18. The rate of early mortality was higher in girls and women (MRR = 2.85) than boys and men (MRR = 1.27).
- Obsessive-compulsive disorder (OCD). A study from Denmark of 10,155 persons with OCD followed for 10 years reported a significantly higher risk of death from both natural (MRR = 1.68) and unnatural causes (MRR = 2.61), compared with the general population.14 Patients with OCD and comorbid depression, anxiety, or substance use had a further increase in mortality risk, but the mortality risk of individuals with OCD without psychiatric comorbidity was still 200% higher than that of the general population.
- Anxiety disorders. One study found no increase in mortality among patients who have generalized anxiety, unless it was associated with depression.15 Another study reported that the presence of anxiety reduced the risk of cardiovascular mortality in persons with depression.16 The absence of increased mortality in anxiety disorders was also confirmed in a meta-analysis of 36 studies.17 However, a study of postmenopausal women with panic attacks found a 3-fold increase in coronary artery disease and stroke in that cohort,18 which confirmed the findings of an older study19 that demonstrated a 2-fold increase of mortality among 155 men with panic disorder after a 12-year follow-up. Also, a 25-year follow-up study found that suicide accounted for 20% of deaths in the anxiety group compared with 16.2% in the depression group,20 showing a significant risk of suicide in panic disorder, even exceeding that of depression.
- Oppositional defiant disorder (ODD) and conduct disorder (CD). In a 12-year follow-up study of 9,495 individuals with “disruptive behavioral disorders,” which included ODD and CD, the mortality rate was >400% higher in these patients compared with 1.92 million individuals in the general population (9.66 vs 2.22 per 10,000 person-years).21 Comorbid substance use disorder and ADHD further increased the mortality rate in this cohort.
- Posttraumatic stress disorder (PTSD). Studies show that there is a significantly increased risk of early cardiovascular mortality in PTSD,22 and that the death rate may be associated with accelerated “DNA methylation age” that leads to a 13% increased risk for all-cause mortality.23
- Borderline personality disorder (BPD). A recent longitudinal study (24 years of follow-up with evaluation every 2 years) reported a significantly higher mortality in patients with BPD compared with those with other personality disorders. The age range when the study started was 18 to 35. The rate of suicide death was Palatino LT Std>400% higher in BPD (5.9% vs 1.4%). Also, non-suicidal death was 250% higher in BPD (14% vs 5.5%). The causes of non-suicidal death included cardiovascular disease, substance-related complications, cancer, and accidents.24
- Other personality disorders. Certain personality traits have been associated with shorter leukocyte telomeres, which signal early death. These traits include neuroticism, conscientiousness, harm avoidance, and reward dependence.25 Another study found shorter telomeres in persons with high neuroticism and low agreeableness26 regardless of age or sex. Short telomeres, which reflect accelerated cellular senescence and aging, have also been reported in several major psychiatric disorders (schizophrenia, bipolar disorder, MDD, and anxiety).27-29 The cumulative evidence is unassailable; psychiatric brain disorders are not only associated with premature death due to high suicide rates, but also with multiple medical diseases that lead to early mortality and a shorter lifespan. The shortened telomeres reflect high oxidative stress and inflammation, and both those toxic processes are known to be associated with major psychiatric disorders. Compounding the dismal facts about early mortality due to mental illness are the additional grave medical consequences of alcohol and substance use, which are highly comorbid with most psychiatric disorders, further exacerbating the premature death rates among psychiatric patients.
Continue to: There is an important take-home message...
There is an important take-home message in all of this: Our patients are at high risk for potentially fatal medical conditions that require early detection, and intensive ongoing treatment by a primary care clinician (not “provider”; I abhor the widespread use of that term for physicians or nurse practitioners) is an indispensable component of psychiatric care. Thus, collaborative care is vital to protect our psychiatric patients from early mortality and a shortened lifespan. Psychiatrists and psychiatric nurse practitioners must not only win the battle against mental illness, but also diligently avoid losing the war of life and death.
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
1. Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341.
2. Laursen TM, Wahlbeck K, Hällgren J, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8(6):e67133. doi: 10.1371/journal.pone.0067133.
3. Kugathasan P, Stubbs B, Aagaard J, et al. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019. doi: 10.1111/acps.13076.
4. Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131(1-3):101-104.
5. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
6. Tiihonen J, Mittendorfer-Rutz E, Torniainen M, et al. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. 2016;173(6):600-606.
7. Torniainen M, Mittendorfer-Rutz E, Tanskanen A, et al. Antipsychotic treatment and mortality in schizophrenia. Schizophr Bull. 2015;41(3):656-663.
8. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627.
9. Wilson R, Gaughran F, Whitburn T, et al. Place of death and other factors associated with unnatural mortality in patients with serious mental disorders: population-based retrospective cohort study. BJPsych Open. 2019;5(2):e23. doi: 10.1192/bjo.2019.5.
10. Ösby U, Westman J, Hällgren J, et al. Mortality trends in cardiovascular causes in schizophrenia, bipolar and unipolar mood disorder in Sweden 1987-2010. Eur J Public Health. 2016;26(5):867-871.
11. Laursen TM, Musliner KL, Benros ME, et al. Mortality and life expectancy in persons with severe unipolar depression. J Affect Disord. 2016;193:203-207.
12. Zubenko GS, Zubenko WN, Spiker DG, et al. Malignancy of recurrent, early-onset major depression: a family study. Am J Med Genet. 2001;105(8):690-699.
13. Dalsgaard S, Østergaard SD, Leckman JF, et al. Mortality in children, adolescents, and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet. 2015;385(9983):2190-2196.
14. Meier SM, Mattheisen M, Mors O, et al. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA Psychiatry. 2016;73(3):268-274.
15. Holwerda TJ, Schoevers RA, Dekker J, et al. The relationship between generalized anxiety disorder, depression and mortality in old age. Int J Geriatr Psychiatry. 2007;22(3):241-249.
16. Ivanovs R, Kivite A, Ziedonis D, et al. Association of depression and anxiety with the 10-year risk of cardiovascular mortality in a primary care population of Latvia using the SCORE system. Front Psychiatry. 2018;9:276.
17. Miloyan B, Bulley A, Bandeen-Roche K, et al. Anxiety disorders and all-cause mortality: systematic review and meta-analysis. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1467-1475.
18. Smoller JW, Pollack MH, Wassertheil-Smoller S, et al. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psychiatry. 2007;64(10):1153-1160.
19. Coryell W, Noyes R Jr, House JD. Mortality among outpatients with anxiety disorders. Am J Psychiatry. 1986;143(4):508-510.
20. Coryell W, Noyes R, Clancy J. Excess mortality in panic disorder. A comparison with primary unipolar depression. Arch Gen Psychiatry. 1982;39(6):701-703.
21. Scott JG, Giørtz Pedersen M, Erskine HE, et al. Mortality in individuals with disruptive behavior disorders diagnosed by specialist services - a nationwide cohort study. Psychiatry Res. 2017;251:255-260.
22. Burg MM, Soufer R. Post-traumatic stress disorder and cardiovascular disease. Curr Cardiol Rep. 2016;18(10):94.
23. Wolf EJ, Logue MW, Stoop TB, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosom Med. 2017. doi: 10.1097/PSY.0000000000000506.
24. Temes CM, Frankenburg FR, Fitzmaurice MC, et al. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. 2019;80(1). doi: 10.4088/JCP.18m12436.
25. Sadahiro R, Suzuki A, Enokido M, et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur Psychiatry. 2015;30(2):291-295.
26. Schoormans D, Verhoeven JE, Denollet J, et al. Leukocyte telomere length and personality: associations with the Big Five and Type D personality traits. Psychol Med. 2018;48(6):1008-1019.
27. Muneer A, Minhas FA. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci. 2019;17(3):343-363.
28. Vakonaki E, Tsiminikaki K, Plaitis S, et al. Common mental disorders and association with telomere length. Biomed Rep. 2018;8(2):111-116.
29. Malouff
Psychotherapy for psychiatric disorders: A review of 4 studies
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4
1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4
1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
Psychotherapy is among the evidence-based treatment options for treating various psychiatric disorders. How we approach psychiatric disorders via psychotherapy has been shaped by numerous theories of personality and psychopathology, including psychodynamic, behavioral, cognitive, systems, and existential-humanistic approaches. Whether used as primary treatment or in conjunction with medication, psychotherapy has played a pivotal role in shaping psychiatric disease management and treatment. Several evidence-based therapy modalities have been used throughout the years and continue to significantly improve and impact our patients’ lives. In the armamentarium of treatment modalities, therapy takes the leading role for several conditions. Here we review 4 studies from current psychotherapy literature; these studies are summarized in the Table.1-4
1.
Panic disorder has a lifetime prevalence of 3.7% in the general population. Three treatment modalities recommended for patients with panic disorder are psychological therapy, pharmacologic therapy, and self-help. Among the psychological therapies, cognitive-behavioral therapy (CBT) is one of the most widely used.1
Cognitive-behavioral therapy for panic disorder has been proven to be an efficacious and impactful treatment. For panic disorder, CBT may consist of different combinations of several therapeutic components, such as relaxation, breathing retraining, cognitive restructuring, interoceptive exposure, and/or in vivo exposure. It is therefore important, both theoretically and clinically, to examine whether specific components of CBT or their combinations are superior to others for treating panic disorder.1
Pompoli et al1 conducted a component network meta-analysis (NMA) of 72 studies in order to determine which CBT components were the most efficacious in treating patients with panic disorder. Component NMA is an extension of standard NMA; it is used to disentangle the treatment effects of different components included in composite interventions.1
The aim of this study was to determine which specific component or combination of components was superior to others when treating panic disorder.1
Study design
- Researchers reviewed 2,526 references from Medline, EMBASE, PsycINFO, and Cochrane Central and selected 72 studies that included 4,064 patients with panic disorder.1
- The primary outcome was remission of panic disorder with or without agoraphobia in the short term (3 to 6 months). Remission was defined as achieving a score of ≤7 on the Panic Disorder Severity Scale (PDSS).1
- Secondary outcomes included response (≥40% reduction in PDSS score from baseline) and dropout for any reason in the short term.1
Continue to: Outcomes
Outcomes
- Using component NMA, researchers determined that interoceptive exposure and face-to-face setting (administration of therapeutic components in a face-to-face setting rather than through self-help means) led to better efficacy and acceptability. Muscle relaxation and virtual reality exposure corresponded to lower efficacy. Breathing retraining and in vivo exposure improved treatment acceptability, but had small effects on efficacy.1
- Based on an analysis of remission rates, the most efficacious CBT incorporated cognitive restructuring and interoceptive exposure. The least efficacious CBT incorporated breathing retraining, muscle relaxation, in vivo exposure, and virtual reality exposure.1
- Application of cognitive and behavioral therapeutic elements was superior to administration of behavioral elements alone. When administering CBT, face-to-face therapy led to better outcomes in response and remission rates. Dropout rates occurred at a lower frequency when CBT was administered face-to-face when compared with self-help groups. The placebo effect was associated with the highest dropout rate.1
Conclusion
- Findings from this meta-analysis have high practical utility. Which CBT components are used can significantly alter CBT’s efficacy and acceptability in patients with panic disorder.1
- The “most efficacious CBT” would include cognitive restructuring and interoceptive exposure delivered in a face-to-face setting. Breathing retraining, muscle relaxation, and virtual reality may have a minimal or even negative impact.1
- Limitations of this meta-analysis include the high number of studies used for the data analysis, complex statistical analysis, inability to include unpublished studies, and limited relevant studies. A future implication of this study is the consideration of formal methodology based on the clinical application of efficacious CBT components when treating patients with panic disorder.1
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
Psychotherapy is also a useful modality for treating posttraumatic stress disorder (PTSD). Sloan et al2 compared brief exposure-based treatment with cognitive processing therapy (CPT) for PTSD.
Clinical practice guidelines for the management of PTSD and acute stress disorder recommend the use of individual, trauma-focused therapies that focus on exposure and cognitive restructuring, such as prolonged exposure, CPT, and written narrative exposure.5
Continue to: One type of written narrative...
One type of written narrative exposure treatment is written exposure therapy (WET), which consists of 5 sessions during which patients write about their trauma. The first session is comprised of psychoeducation about PTSD and a review of treatment reasoning, followed by 30 minutes of writing. The therapist provides feedback and instructions. Written exposure therapy requires less therapist training and less supervision than prolonged exposure or CPT. Prior studies have suggested that WET can significantly reduce PTSD symptoms in various trauma survivors.2
Although efficacious for PTSD, WET had not been compared with CPT, which is the most commonly used first-line treatment of PTSD. The aim of this study was to determine whether WET is noninferior to CPT.2
Study design
- In this randomized noninferiority clinical trial conducted in Boston, Massachusetts from February 28, 2013 to November 6, 2016, 126 veterans and non-veteran adults were randomized to WET or CPT. Participants met DSM-5 criteria for PTSD and were taking stable doses of their medications for at least 4 weeks.2
- Participants assigned to CPT (n = 63) underwent 12 sessions, and participants assigned to WET (n = 63) received 5 sessions. Cognitive processing therapy was conducted over 60-minute weekly sessions. Written exposure therapy consisted of an initial session that was 60 minutes long and four 40-minute follow-up sessions.2
- Interviews were conducted by 4 independent evaluators at baseline and 6, 12, 24, and 36 weeks. During the WET sessions, participants wrote about a traumatic event while focusing on details, thoughts, and feelings associated with the event.2
- Cognitive processing therapy involved 12 trauma-focused therapy sessions during which participants learn how to become aware of and address problematic cognitions about the trauma as well as thoughts about themselves and others. Between sessions, participants were required to write 2 trauma accounts and complete other assignments.2
Outcomes
- The primary outcome was change in total score on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5). The CAPS-5 scores for participants in the WET group were noninferior to those for participants in the CPT group at all assessment points.2
- Participants did not significantly differ in age, education, income, or PTSD severity. Participants in the 2 groups did not differ in treatment expectations or level of satisfaction with treatment. Individuals assigned to CPT were more likely to drop out of the study: 20 participants in the CPT group dropped out in the first 5 sessions, whereas only 4 dropped out of the WET group. The dropout rate in the CPT group was 39.7%. Improvements in PTSD symptoms in the WET group were noninferior to improvements in the CPT group.2
- Written exposure therapy showed no difference compared with CPT in decreasing PTSD symptoms. Furthermore, this study demonstrated that PTSD symptoms can decrease with a smaller number of shorter therapeutic sessions.2
Conclusion
- This study demonstrated noninferiority between an established, commonly used PTSD therapy (CPT) and a version of exposure therapy that is briefer, simpler, and requires less homework and less therapist training and expertise. This “lower-dose” approach may improve access for the expanding number of patients who require treatment for PTSD, especially in the Veterans Affairs system.2
- In summary, WET is well tolerated and time-efficient. Although it requires fewer sessions, WET was noninferior to CPT.2
Continue to: Multisystemic therapy versus management as usual...
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
Multisystemic therapy (MST) is an intensive, family-based, home-based intervention for young people with serious antisocial behavior. It has been found effective for childhood conduct disorders in the United States. However, previous studies that supported its efficacy were conducted by the therapy’s developers and used noncomprehensive comparators, such as individual therapy. Fonagy et al3 assessed the effectiveness and cost-effectiveness of MST vs management as usual for treating adolescent antisocial behavior. This is the first study that was performed by independent investigators and used a comprehensive control.3
Study design
- This 18-month, multisite, pragmatic, randomized controlled superiority trial was conducted in England.3
- Participants were age 11 to 17, with moderate to severe antisocial behavior. They had at least 3 severity criteria indicating difficulties across several settings and at least one of the 5 inclusion criteria for antisocial behavior. Six hundred eighty-four families were randomly assigned to MST or management as usual, and 491 families completed the study.3
- For the MST intervention, therapists worked with the adolescent’s caregiver 3 times a week for 3 to 5 months to improve parenting skills, enhance family relationships, increase support from social networks, develop skills and resources, address communication problems, increase school attendance and achievement, and reduce the adolescent’s association with delinquent peers.3
- For the management as usual intervention, management was based on local services for young people and was designed to be in line with current community practice.3
Outcomes
- The primary outcome was the proportion of participants in out-of-home placements at 18 months. The secondary outcomes were time to first criminal offense and the total number of offenses.3
- In terms of the risk of out-of-home placement, MST had no effect: 13% of participants in the MST group had out-of-home placement at 18 months, compared with 11% in the management-as-usual group.3
- Multisystemic therapy also did not significantly delay the time to first offense (hazard ratio, 1.06; 95% confidence interval, 0.84 to 1.33). Also, at 18-month follow-up, participants in the MST group had committed more offenses than those in the management-as-usual group, although the difference was not statistically significant.3
- Parents in the MST group reported increased parental support and involvement and reduced problems at 6 months, but the adolescents’ reports of parenting behavior indicated no significant effect for MST vs management as usual at any time point.3
Conclusion
- Multisystemic therapy was not superior to management as usual in reducing out-of-home placements. Although the parents believed that MST brought about a rapid and effective change, this was not reflected in objective indicators of antisocial behavior. These results are contrary to previous studies in the United States. The substantial improvements observed in both groups reflected the effectiveness of routinely offered interventions for this group of young people, at least when observed in clinical trials.3
Continue to: Mindfulness-based cognitive therapy...
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
There is empirical support for using psychotherapy to treat attention-deficit/hyperactivity disorder (ADHD). Although medication management plays a leading role in treating ADHD, Janssen et al4 conducted a multicenter, single-blind trial comparing mindfulness-based cognitive therapy (MBCT) vs treatment as usual (TAU) for ADHD.
The aim of this study was to determine the efficacy of MBCT plus TAU vs TAU only in decreasing symptoms of adults with ADHD.4
Study design
- This multicenter, single-blind randomized controlled trial was conducted in the Netherlands. Participants (N = 120) met criteria for ADHD and were age ≥18. Patients were randomly assigned to MBCT plus TAU (n = 60) or TAU only (n = 60). Patients in the MBCT plus TAU group received weekly group therapy sessions, meditation exercises, psychoeducation, and group discussions. Patients in the TAU-only group received pharmacotherapy and psychoeducation.4
- Blinded clinicians used the Connors’ Adult ADHD Rating Scale to assess ADHD symptoms.4
- Secondary outcomes were determined by self-reported questionnaires that patients completed online.4
- All statistical analyses were performed on an intention-to-treat sample as well as the per protocol sample.4
Outcomes
- The primary outcome was ADHD symptoms rated by clinicians. Secondary outcomes included self-reported ADHD symptoms, executive functioning, mindfulness skills, positive mental health, and general functioning. Outcomes were examined at baseline and then at post treatment and 3- and 6-month follow-up.4
- Patients in the MBCT plus TAU group had a significant decrease in clinician-rated ADHD symptoms that was maintained at 6-month follow-up. More patients in the MBCT plus TAU group (27%) vs patients in the TAU group (4%) showed a ≥30% reduction in ADHD symptoms. Compared with patients in the TAU group, patients in the MBCT plus TAU group had significant improvements in ADHD symptoms, mindfulness skills, and positive mental health at post treatment and at 6-month follow-up. Compared with those receiving TAU only, patients treated with MBCT plus TAU reported no improvement in executive functioning at post treatment, but did improve at 6-month follow-up.4
Continue to: Conclusion
Conclusion
- Compared with TAU only, MBCT plus TAU is more effective in reducing ADHD symptoms, with a lasting effect at 6-month follow-up. In terms of secondary outcomes, MBCT plus TAU proved to be effective in improving mindfulness, self-compassion, positive mental health, and executive functioning. The results of this trial demonstrate that psychosocial treatments can be effective in addition to TAU in patients with ADHD, and MBCT holds promise for adult ADHD.4
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
1. Pompoli A, Furukawa TA, Efthimiou O, et al. Dismantling cognitive-behaviour therapy for panic disorder: a systematic review and component network meta-analysis. Psychol Med. 2018;48(12):1945-1953.
2. Sloan DM, Marx BP, Lee DJ, et al. A brief exposure-based treatment vs cognitive processing therapy for posttraumatic stress disorder: a randomized noninferiority clinical trial. JAMA Psychiatry. 2018;75(3):233-239.
3. Fonagy P, Butler S, Cottrell D, et al. Multisystemic therapy versus management as usual in the treatment of adolescent antisocial behaviour (START): a pragmatic, randomised controlled, superiority trial. Lancet Psychiatry. 2018;5(2):119-133.
4. Janssen L, Kan CC, Carpentier PJ, et al. Mindfulness-based cognitive therapy v. treatment as usual in adults with ADHD: a multicentre, single-blind, randomised controlled trial. Psychol Med. 2019;49(1):55-65.
5. US Department of Veterans Affairs and Department of Defense. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder . https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal082917.pdf. Published June 2017. Accessed September 8, 2019.
Four genetic variants link psychotic experiences to multiple mental disorders
Four genetic variations appear to link psychotic experiences with other psychiatric disorders, including schizophrenia, major depressive disorder, bipolar disorder, and neurodevelopmental disorders, a large genetic study has concluded.
, reported Sophie E. Legge, PhD, and colleagues. Their study was published in JAMA Psychiatry.
Although it is informative, the study is unlikely to expand the knowledge of schizophrenia-specific genetics.
“Consistent with other studies, the heritability estimate (1.71%) was low, and given that the variance explained in our polygenic risk analysis was also low, the finding suggests that understanding the genetics of psychotic experiences is unlikely to have an important effect on understanding the genetics of schizophrenia specifically,” wrote Dr. Legge, of the MRC Center for Neuropsychiatric Genetics and Genomics in the division of psychological medicine and clinical neurosciences at Cardiff (Wales) University, and colleagues.
The team conducted a genomewide association study (GWAS) using data from 127,966 individuals in the U.K. Biobank. Of these, 6,123 reported any psychotic experience, 2,143 reported distressing psychotic experiences, and 3,337 reported multiple experiences. The remainder served as controls. At the time of the biobank data collection, the subjects were a mean of 64 years of age; 56% were women.
First psychotic experience occurred at a mean of almost 32 years of age, but about a third reported that the first episode occurred before age 20, or that psychotic experiences had been happening ever since they could remember. Another third reported their first experience between ages 40 and 76 years.
The investigators conducted three GWAS studies: one for any psychotic experience, one for distressing experiences, and one for multiple experiences.
No significant genetic associations were found among those with multiple psychotic experiences, the authors said.
But they did find four variants significantly associated with the other experience categories.
Two variants were associated with any psychotic experience. Those with rs10994278, an intronic variant within Ankyrin-3 (ANK3), were 16% more likely to have a psychotic experience (odds ratio, 1.16). Those with intergenic variant rs549656827 were 39% less likely (OR, 0.61). “The ANK3 gene encodes ankyrin-G, a protein that has been shown to regulate the assembly of voltage-gated sodium channels and is essential for normal synaptic function,” the authors said. “ANK3 is one of strongest and most replicated genes for bipolar disorder, and variants within ANK3 have also been associated in the Psychiatric Genomics Consortium cross-disorder GWAS, and in a rare variant analysis of autism spectrum disorder.”
Two variants were linked to distressing psychotic experiences: rs75459873, intronic to cannabinoid receptor 2 (CNR2), decreased the risk by 34% (OR, 0.66). Intergenic variant rs3849810 increased the risk by 12% (OR, 1.12).
“CNR2 encodes for CB2, one of two well-characterized cannabinoid receptors. Several lines of evidence have implicated the endocannabinoid system in psychiatric disorders, including schizophrenia and depression. The main psychoactive agent of cannabis, tetrahydrocannabinol, can cause acute psychotic symptoms and cognitive impairment. Given that cannabis use is strongly associated with psychotic experiences, we tested, but found no evidence for, a mediating or moderating effect of cannabis use on the association of rs75459873 and distressing psychotic experiences. However, while no evidence was found in this study, a mediating effect of cannabis use cannot be ruled out given the relatively low power of such analyses and the potential measurement error.”
Also, significant genetic correlations were found between any psychotic experiences and major depressive disorder, autism spectrum disorder, ADHD, and schizophrenia. However, the polygenic risk scores for schizophrenia, major depressive disorder, bipolar disorder, ADHD, and autism spectrum disorder, were low.
“We also considered individual psychotic symptoms and found that polygenic risk scores for schizophrenia, bipolar disorder, depression, and ADHD were more strongly associated with delusions of persecution than with the other psychotic symptoms.”
Those with distressing psychotic experiences tended to have more copy number variations (CNVs) associated with schizophrenia (OR, 2.04) and neurodevelopmental disorders (OR, 1.75). The team also found significant associations between distressing experiences and major depressive disorder, ADHD, autism spectrum disorder, and schizophrenia.
“We found particular enrichment of these [polygenic risk scores] in distressing psychotic experiences and for delusions of persecution,” they noted. “ ... All schizophrenia-associated [copy number variations] are also associated with neurodevelopmental disorders such as intellectual disability and autism.”
The study’s strengths include its large sample size. Among its limitations, the researchers said, are the study’s retrospective measurement of psychotic experiences based on self-report from a questionnaire that was online. Gathering the data in that way raised the likelihood of possible error, they said.
Dr. Legge reported having no disclosures.
SOURCE: Legge SE et al. JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2508.
The genetic links uncovered in this study offer an intriguing, but incomplete look at the risks of psychotic experiences and their complicated intertwinings with other mental disorders, wrote Albert R. Powers III, MD, PhD.
“Penetrance of the genes in question likely depends at least in part on environmental influences, some of which have been studied extensively,” he wrote. “Recently, some have proposed risk stratification by exposome – a composite score of relevant exposures that may increase risk for psychosis and is analogous to the polygenic risk score used [here].
“The combination of environmental and genetic composite scores may lead to improved insight into individualized pathways toward psychotic experiences, highlighting genetic vulnerabilities to specific stressors likely to lead to phenotypic expression. Ultimately, this will require a more sophisticated mapping between phenomenology and biology than currently exists.”
One approach would be to combine deep phenotyping and behavioral analyses in a framework that could link all relevant levels from symptoms to neurophysiology.
“One such framework is predictive processing theory, which is linked closely with the free energy principle and the Bayesian brain hypothesis and attempts to explain perceptual and cognitive phenomena as manifestations of a drive to maintain as accurate an internal model of one’s surroundings as possible by minimizing prediction errors. This relatively simple scheme makes specific – and, importantly, falsifiable – assessments of the mathematical signatures of neurotypical processes and the ways they might break down to produce specific psychiatric symptoms.”
Dr. Powers is an assistant professor at the department of psychiatry at Yale University, New Haven, Conn., and serves as medical director of the PRIME Psychosis Research Clinic at Yale. His comments came in an accompanying editorial (JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2391 ).
The genetic links uncovered in this study offer an intriguing, but incomplete look at the risks of psychotic experiences and their complicated intertwinings with other mental disorders, wrote Albert R. Powers III, MD, PhD.
“Penetrance of the genes in question likely depends at least in part on environmental influences, some of which have been studied extensively,” he wrote. “Recently, some have proposed risk stratification by exposome – a composite score of relevant exposures that may increase risk for psychosis and is analogous to the polygenic risk score used [here].
“The combination of environmental and genetic composite scores may lead to improved insight into individualized pathways toward psychotic experiences, highlighting genetic vulnerabilities to specific stressors likely to lead to phenotypic expression. Ultimately, this will require a more sophisticated mapping between phenomenology and biology than currently exists.”
One approach would be to combine deep phenotyping and behavioral analyses in a framework that could link all relevant levels from symptoms to neurophysiology.
“One such framework is predictive processing theory, which is linked closely with the free energy principle and the Bayesian brain hypothesis and attempts to explain perceptual and cognitive phenomena as manifestations of a drive to maintain as accurate an internal model of one’s surroundings as possible by minimizing prediction errors. This relatively simple scheme makes specific – and, importantly, falsifiable – assessments of the mathematical signatures of neurotypical processes and the ways they might break down to produce specific psychiatric symptoms.”
Dr. Powers is an assistant professor at the department of psychiatry at Yale University, New Haven, Conn., and serves as medical director of the PRIME Psychosis Research Clinic at Yale. His comments came in an accompanying editorial (JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2391 ).
The genetic links uncovered in this study offer an intriguing, but incomplete look at the risks of psychotic experiences and their complicated intertwinings with other mental disorders, wrote Albert R. Powers III, MD, PhD.
“Penetrance of the genes in question likely depends at least in part on environmental influences, some of which have been studied extensively,” he wrote. “Recently, some have proposed risk stratification by exposome – a composite score of relevant exposures that may increase risk for psychosis and is analogous to the polygenic risk score used [here].
“The combination of environmental and genetic composite scores may lead to improved insight into individualized pathways toward psychotic experiences, highlighting genetic vulnerabilities to specific stressors likely to lead to phenotypic expression. Ultimately, this will require a more sophisticated mapping between phenomenology and biology than currently exists.”
One approach would be to combine deep phenotyping and behavioral analyses in a framework that could link all relevant levels from symptoms to neurophysiology.
“One such framework is predictive processing theory, which is linked closely with the free energy principle and the Bayesian brain hypothesis and attempts to explain perceptual and cognitive phenomena as manifestations of a drive to maintain as accurate an internal model of one’s surroundings as possible by minimizing prediction errors. This relatively simple scheme makes specific – and, importantly, falsifiable – assessments of the mathematical signatures of neurotypical processes and the ways they might break down to produce specific psychiatric symptoms.”
Dr. Powers is an assistant professor at the department of psychiatry at Yale University, New Haven, Conn., and serves as medical director of the PRIME Psychosis Research Clinic at Yale. His comments came in an accompanying editorial (JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2391 ).
Four genetic variations appear to link psychotic experiences with other psychiatric disorders, including schizophrenia, major depressive disorder, bipolar disorder, and neurodevelopmental disorders, a large genetic study has concluded.
, reported Sophie E. Legge, PhD, and colleagues. Their study was published in JAMA Psychiatry.
Although it is informative, the study is unlikely to expand the knowledge of schizophrenia-specific genetics.
“Consistent with other studies, the heritability estimate (1.71%) was low, and given that the variance explained in our polygenic risk analysis was also low, the finding suggests that understanding the genetics of psychotic experiences is unlikely to have an important effect on understanding the genetics of schizophrenia specifically,” wrote Dr. Legge, of the MRC Center for Neuropsychiatric Genetics and Genomics in the division of psychological medicine and clinical neurosciences at Cardiff (Wales) University, and colleagues.
The team conducted a genomewide association study (GWAS) using data from 127,966 individuals in the U.K. Biobank. Of these, 6,123 reported any psychotic experience, 2,143 reported distressing psychotic experiences, and 3,337 reported multiple experiences. The remainder served as controls. At the time of the biobank data collection, the subjects were a mean of 64 years of age; 56% were women.
First psychotic experience occurred at a mean of almost 32 years of age, but about a third reported that the first episode occurred before age 20, or that psychotic experiences had been happening ever since they could remember. Another third reported their first experience between ages 40 and 76 years.
The investigators conducted three GWAS studies: one for any psychotic experience, one for distressing experiences, and one for multiple experiences.
No significant genetic associations were found among those with multiple psychotic experiences, the authors said.
But they did find four variants significantly associated with the other experience categories.
Two variants were associated with any psychotic experience. Those with rs10994278, an intronic variant within Ankyrin-3 (ANK3), were 16% more likely to have a psychotic experience (odds ratio, 1.16). Those with intergenic variant rs549656827 were 39% less likely (OR, 0.61). “The ANK3 gene encodes ankyrin-G, a protein that has been shown to regulate the assembly of voltage-gated sodium channels and is essential for normal synaptic function,” the authors said. “ANK3 is one of strongest and most replicated genes for bipolar disorder, and variants within ANK3 have also been associated in the Psychiatric Genomics Consortium cross-disorder GWAS, and in a rare variant analysis of autism spectrum disorder.”
Two variants were linked to distressing psychotic experiences: rs75459873, intronic to cannabinoid receptor 2 (CNR2), decreased the risk by 34% (OR, 0.66). Intergenic variant rs3849810 increased the risk by 12% (OR, 1.12).
“CNR2 encodes for CB2, one of two well-characterized cannabinoid receptors. Several lines of evidence have implicated the endocannabinoid system in psychiatric disorders, including schizophrenia and depression. The main psychoactive agent of cannabis, tetrahydrocannabinol, can cause acute psychotic symptoms and cognitive impairment. Given that cannabis use is strongly associated with psychotic experiences, we tested, but found no evidence for, a mediating or moderating effect of cannabis use on the association of rs75459873 and distressing psychotic experiences. However, while no evidence was found in this study, a mediating effect of cannabis use cannot be ruled out given the relatively low power of such analyses and the potential measurement error.”
Also, significant genetic correlations were found between any psychotic experiences and major depressive disorder, autism spectrum disorder, ADHD, and schizophrenia. However, the polygenic risk scores for schizophrenia, major depressive disorder, bipolar disorder, ADHD, and autism spectrum disorder, were low.
“We also considered individual psychotic symptoms and found that polygenic risk scores for schizophrenia, bipolar disorder, depression, and ADHD were more strongly associated with delusions of persecution than with the other psychotic symptoms.”
Those with distressing psychotic experiences tended to have more copy number variations (CNVs) associated with schizophrenia (OR, 2.04) and neurodevelopmental disorders (OR, 1.75). The team also found significant associations between distressing experiences and major depressive disorder, ADHD, autism spectrum disorder, and schizophrenia.
“We found particular enrichment of these [polygenic risk scores] in distressing psychotic experiences and for delusions of persecution,” they noted. “ ... All schizophrenia-associated [copy number variations] are also associated with neurodevelopmental disorders such as intellectual disability and autism.”
The study’s strengths include its large sample size. Among its limitations, the researchers said, are the study’s retrospective measurement of psychotic experiences based on self-report from a questionnaire that was online. Gathering the data in that way raised the likelihood of possible error, they said.
Dr. Legge reported having no disclosures.
SOURCE: Legge SE et al. JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2508.
Four genetic variations appear to link psychotic experiences with other psychiatric disorders, including schizophrenia, major depressive disorder, bipolar disorder, and neurodevelopmental disorders, a large genetic study has concluded.
, reported Sophie E. Legge, PhD, and colleagues. Their study was published in JAMA Psychiatry.
Although it is informative, the study is unlikely to expand the knowledge of schizophrenia-specific genetics.
“Consistent with other studies, the heritability estimate (1.71%) was low, and given that the variance explained in our polygenic risk analysis was also low, the finding suggests that understanding the genetics of psychotic experiences is unlikely to have an important effect on understanding the genetics of schizophrenia specifically,” wrote Dr. Legge, of the MRC Center for Neuropsychiatric Genetics and Genomics in the division of psychological medicine and clinical neurosciences at Cardiff (Wales) University, and colleagues.
The team conducted a genomewide association study (GWAS) using data from 127,966 individuals in the U.K. Biobank. Of these, 6,123 reported any psychotic experience, 2,143 reported distressing psychotic experiences, and 3,337 reported multiple experiences. The remainder served as controls. At the time of the biobank data collection, the subjects were a mean of 64 years of age; 56% were women.
First psychotic experience occurred at a mean of almost 32 years of age, but about a third reported that the first episode occurred before age 20, or that psychotic experiences had been happening ever since they could remember. Another third reported their first experience between ages 40 and 76 years.
The investigators conducted three GWAS studies: one for any psychotic experience, one for distressing experiences, and one for multiple experiences.
No significant genetic associations were found among those with multiple psychotic experiences, the authors said.
But they did find four variants significantly associated with the other experience categories.
Two variants were associated with any psychotic experience. Those with rs10994278, an intronic variant within Ankyrin-3 (ANK3), were 16% more likely to have a psychotic experience (odds ratio, 1.16). Those with intergenic variant rs549656827 were 39% less likely (OR, 0.61). “The ANK3 gene encodes ankyrin-G, a protein that has been shown to regulate the assembly of voltage-gated sodium channels and is essential for normal synaptic function,” the authors said. “ANK3 is one of strongest and most replicated genes for bipolar disorder, and variants within ANK3 have also been associated in the Psychiatric Genomics Consortium cross-disorder GWAS, and in a rare variant analysis of autism spectrum disorder.”
Two variants were linked to distressing psychotic experiences: rs75459873, intronic to cannabinoid receptor 2 (CNR2), decreased the risk by 34% (OR, 0.66). Intergenic variant rs3849810 increased the risk by 12% (OR, 1.12).
“CNR2 encodes for CB2, one of two well-characterized cannabinoid receptors. Several lines of evidence have implicated the endocannabinoid system in psychiatric disorders, including schizophrenia and depression. The main psychoactive agent of cannabis, tetrahydrocannabinol, can cause acute psychotic symptoms and cognitive impairment. Given that cannabis use is strongly associated with psychotic experiences, we tested, but found no evidence for, a mediating or moderating effect of cannabis use on the association of rs75459873 and distressing psychotic experiences. However, while no evidence was found in this study, a mediating effect of cannabis use cannot be ruled out given the relatively low power of such analyses and the potential measurement error.”
Also, significant genetic correlations were found between any psychotic experiences and major depressive disorder, autism spectrum disorder, ADHD, and schizophrenia. However, the polygenic risk scores for schizophrenia, major depressive disorder, bipolar disorder, ADHD, and autism spectrum disorder, were low.
“We also considered individual psychotic symptoms and found that polygenic risk scores for schizophrenia, bipolar disorder, depression, and ADHD were more strongly associated with delusions of persecution than with the other psychotic symptoms.”
Those with distressing psychotic experiences tended to have more copy number variations (CNVs) associated with schizophrenia (OR, 2.04) and neurodevelopmental disorders (OR, 1.75). The team also found significant associations between distressing experiences and major depressive disorder, ADHD, autism spectrum disorder, and schizophrenia.
“We found particular enrichment of these [polygenic risk scores] in distressing psychotic experiences and for delusions of persecution,” they noted. “ ... All schizophrenia-associated [copy number variations] are also associated with neurodevelopmental disorders such as intellectual disability and autism.”
The study’s strengths include its large sample size. Among its limitations, the researchers said, are the study’s retrospective measurement of psychotic experiences based on self-report from a questionnaire that was online. Gathering the data in that way raised the likelihood of possible error, they said.
Dr. Legge reported having no disclosures.
SOURCE: Legge SE et al. JAMA Psychiatry. 2019 Sep 25. doi: 10.1001/jamapsychiatry.2019.2508.
FROM JAMA PSYCHIATRY
Strategies for improving ADHD medication adherence
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.
Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.
Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44
Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.
Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.
Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44
Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
Attention-deficit/hyperactivity disorder (ADHD) is the most common childhood neurodevelopmental disorder, affecting 8% to 12% of school-aged children in the United States1-3 with significant impairments that often persist into adulthood.4-8 Current guidelines recommend stimulant medication and/or behavioral therapies as first-line treatments for ADHD.9,10 There is a wealth of evidence on the efficacy of stimulants in ADHD, with the most significant effects noted on core ADHD symptoms.11,12 Additional evidence links stimulants to decreased long-term negative outcomes, including reduced school absences and grade retention,13 as well as modestly but significantly improved reading and math scores.14 Other studies have reported that individuals with ADHD who receive medication have decreased criminality,15,16 motor vehicle accidents,17,18 injuries,19 substance abuse,20-22 and risk for subsequent and concurrent depression.23 Therefore, the evidence suggests that consistent medication treatment helps improve outcomes for individuals with ADHD.
Adherence is defined as “the extent to which a person’s behavior (eg, taking medication) corresponds with agreed recommendations from a clinician.”24 Unfortunately, pediatric ADHD medication adherence has been found to be poor (approximately 64%).25-30 Nonadherence to ADHD medication has been linked to multiple factors, including caregiver/family and child/adolescent factors (Table 1), medication-related factors (Table 2), and health care/system factors (Table 3). Understanding and addressing these factors is essential to maximizing long-term outcomes. In this article, we review the factors associated with nonadherence to ADHD medication, and outline strategies to improve adherence.
Caregiver/family characteristics
Caregiver beliefs about ADHD and their attitudes toward treatment have been associated with the initiation of and adherence to ADHD medication. For example, caregivers who view a child’s difficulties as a medical disorder that requires a biologic intervention are more likely to accept and adhere to medication.31 Similarly, caregivers who perceive ADHD medication as safe, effective, and socially acceptable are more likely to be treatment-adherent.32-35
- increased caregiver knowledge about ADHD33
- receiving an ADHD diagnosis based on a thorough diagnostic process (ie, comprehensive psychological testing)36
- satisfaction with information about medicine
- comfort with the treatment plan.34
Socioeconomic status, family functioning, and caregiver mental health diagnoses (eg, ADHD, depression) have also been linked to ADHD medication adherence. Several studies, including the Multimodal Treatment Study of Children with ADHD,11 a landmark study of stimulant medication for children with ADHD, have found an association between low income and decreased likelihood of receiving ADHD medication.2,37-39 Further, Gau et al40 found that negative caregiver-child relationships and family dysfunction were associated with poor medication adherence in children with ADHD.9 Prior studies have also shown that mothers of children with ADHD are more likely to have depression and/or anxiety,41,42 and that caregivers with a history of mental health diagnoses are more accepting of initiating medication treatment for their children.43 However, additional studies have found that caregiver mental health diagnoses decreased the likelihood of ADHD medication adherence.40,44
Child characteristics
Child characteristics associated with decreased ADHD medication adherence include older age (eg, adolescents vs school-aged children),29,30,34,40,45-47 non-White race, Hispanic ethnicity,29,33,48-51 female gender,29,33,52 lower baseline ADHD symptom severity,30,37 and child unwillingness to take medications.34 However, prior studies have not been completely consistent about the relationship between child comorbid conditions (eg, oppositional defiant disorder [ODD], conduct disorder) and ADHD medication adherence. A few studies found that child comorbid conditions, especially ODD, mediate poor ADHD medication adherence, possibly secondary to an increased caregiver-child conflict.30,53,54 However, other studies have reported that the presence of comorbid ODD, depression, and anxiety predicted increased adherence to ADHD medications.37,46
Medication-related factors
Adverse effects of medications are the most commonly cited reason for ADHD medication nonadherence
On the other hand, increased ADHD medication effectiveness has been associated with improved medication adherence.5,34,54-56 Medication titration and dosing factors have also been shown to affect adherence. Specifically, adherence has been improved when ADHD medications are titrated in a systematic manner soon after starting treatment, and when families have an early first contact with a physician after starting medication (within 3 months).28 In addition, use of a simplified dose regimen has been linked to better adherence: patients who are prescribed long-acting stimulants are more likely to adhere to treatment compared with patients who take short-acting formulations.26,40,49,61-63 It is possible that long-acting stimulants increase adherence because they produce more even and sustained effects on ADHD symptoms throughout the day, compared with short-acting formulations.64 Furthermore, the inconvenience of taking multiple doses throughout the day, as well as the potential social stigma of mid-school day dosing, may negatively impact adherence to short-acting formulations.10
Continue to: Health care/system factors
Health care/system factors
Several studies have investigated the influence of health services factors on ADHD medication adherence. Specifically, limited transportation services and lack of mental health providers in the community have been linked to decreased ADHD medication adherence.47,65,66 Furthermore, limited insurance coverage and higher costs of ADHD medications, which lead to substantial out-of-pocket payments for families, have been associated with decreased likelihood of ADHD medication adherence.29,67
Clinician-related factors also can affect ADHD medication adherence. For example, a clinician’s lack knowledge of ADHD care can negatively impact ADHD medication adherence.68 Two studies have documented improved ADHD medication adherence when treatment is provided by specialists (eg, child psychiatrists) rather than by community primary care providers, possibly because specialists are more likely to provide close stimulant titration and monitoring (ie, ≥ 3 visits in the first 90 days) and use higher maximum doses.62,69 Furthermore, ADHD medication initiation and adherence are increased when patients have a strong working alliance with their clinician and trust the health care system,31,34,35 as well as when there is a match between the caregiver’s and clinician’s perception of the cause, course, and best treatment practices for a child’s ADHD.65
Strategies to improve medication adherence
A number of strategies to improve ADHD medication adherence can be derived from our knowledge of the factors that influence adherence.
Patient/family education. Unanswered questions about ADHD diagnosis, etiology, and medication adverse effects can negatively impact the ADHD treatment process. Therefore, patient/family education regarding ADHD and its management is necessary to improve medication adherence, because it helps families attain the knowledge, confidence, and motivation to manage their child’s condition.
Clinicians have an important role in educating patients about70:
- the medications they are taking
- why they are taking them
- what the medications look like
- the time of medication administration
- the potential adverse effects
- what to do if adverse effects occur
- what regular testing/monitoring is necessary.
Clinicians can provide appropriate psychoeducation by sharing written materials and trusted websites with families (see Related Resources).
Behavioral strategies. Behavioral interventions have been among the most effective strategies for improving medication adherence in other chronic conditions.71 Behavioral strategies are likely to be particularly important for families of children with ADHD and comorbid conditions such as ODD because these families experience considerable caregiver-child conflict.72 Moreover, parents of children with ADHD are at higher risk for having ADHD and depression themselves,73 both of which may interfere with a parent’s ability to obtain and administer medications consistently. Thus, for these families, using a combination of psychoeducation and behavioral strategies will be necessary to affect change in attitude and behavior. Behavioral strategies that can be used to improve medication adherence include:
- Technology-based interventions can reduce the impact of environmental barriers to adherence. For example, pharmacy automatic prescription renewal systems can reduce the likelihood of families failing to obtain ADHD medication refills. Pill reminder boxes, smartphone alerts, and setting various alarms can effectively prompt caregivers/patients to administer medication. In particular, these methods can be crucial in families for which multiple members have ADHD and its attendant difficulties with organization and task completion.
- Caregiver training may assist families in developing specific behavioral management skills that support adherence. This training can be as straightforward as instructing caregivers on the use of positive reinforcement when teaching their children to swallow pills. It may also encompass structured behavioral interventions aimed at training caregivers to utilize rewards and consequences in order to maximize medication adherence.74
Continue to: Clinician interventions
Clinician interventions. Clinicians can use decision aids to help inform families about treatment options, promote shared decision making, and decrease uncertainty about the treatment plan75 (see Related Resources). Early titration of ADHD medications and early first contact (within months of starting medication treatment) between caregivers and clinicians, whether via in-person visit, telephone, or email, have also been related to improved adherence.28 Furthermore, clinicians can improve adherence by prescribing a simplified medication regimen (ie, long-acting formulations that provide full-day coverage). To address the negative impact of high out-of-pocket ADHD medication costs on adherence, clinicians can also prescribe generic preparations and/or “preferred” medications options on an individual patient’s formulary.
Because clinician knowledge and expertise in ADHD care has been linked to improved patient medication adherence,68 clinicians are encouraged to use the American Academy of Pediatrics (AAP) guideline for diagnosis and treatment of ADHD, which includes a supplemental process of care algorithm (last published in 2011,10 with an updated guideline anticipated in 2019), as well as the AAP/National Institute for Children’s Health Quality (NICHQ) ADHD Toolkit,76 which includes items helpful for ADHD diagnosis and treatment. The Society for Developmental and Behavioral Pediatrics is also developing a clinical practice guideline for the diagnosis and treatment of complex ADHD (ie, ADHD complicated by coexisting mental health, developmental, and/or psychosocial conditions or issues), with publication anticipated in 2019. Primary care providers can also improve their expertise in ADHD care by pursuing additional mental health–related trainings (such as those conducted by the REACH Institute).77
Because receiving ADHD care from a specialist has been shown to improve medication initiation and adherence,62,69 other strategies to address the short supply of child psychiatrists include offering incentives to medical students to pursue a career in child psychiatry (eg, loan forgiveness). Telepsychiatry and co-location of mental health specialists and primary care providers are additional innovative ways in which ADHD specialty care can be delivered to more patients.64
Finally, providing culturally-sensitive care can strengthen the clinician-caregiver relationship and promote adherence to treatment. For example, clinicians can partner with local groups to increase their understanding of how different racial/ethnic groups perceive ADHD and its treatment.64
Peer support models. Peers are credible role models who have a valued role in facilitating the use of mental health services by empowering families and enhancing service satisfaction.78 In several communities in the United States, peer models using family advocates have been introduced.79 Family advocates are typically caregivers of children who have special needs or have been involved in the mental health system. Their perspective—as peers and first-hand consumers of the health care and/or mental health system—can make them powerful and effective coaches to families of children with ADHD. By helping families to navigate ADHD care systems successfully, family advocates can play an important role in enhancing ADHD medication adherence, although further investigation is needed. In addition, the stigma around ADHD medication use, which adversely impacts adherence, can be mitigated if caregivers participate in organized ADHD-related support groups (eg, Children and Adults with ADHD [CHADD]).
Continue to: Health disparity-reducing interventions
Health disparity-reducing interventions. Successful health disparity-reducing interventions—such as those developed to enhance care of other chronic disorders including asthma and diabetes—can be applied to improve ADHD care. These interventions, which include medical-legal partnerships (eg, between clinicians, social workers, legal advocates, and community partners) in primary care centers, have been shown to improve health insurance coverage and therefore health care access.80,81 Although some hardships linked to nonadherence (eg, low socioeconomic status) may not be amenable to health care–related interventions, screening for these hardships can identify children who are most at risk for poor adherence. This would alert clinicians to proactively identify barriers to adherence and implement mitigation strategies. This might include developing more streamlined, easier-to-follow management plans for these patients, such as those that can be delivered through pharmacist-physician collaborative programs82 and school-based therapy programs.83-85
Bottom Line
Suboptimal adherence to medications for attention-deficit/hyperactivity disorder (ADHD) can be addressed through patient/family education, behavioral strategies, clinician interventions, peer support models, and health disparity-reducing interventions. By improving ADHD treatment adherence, these interventions have the potential to maximize long-term outcomes.
Related Resources
- Cohen Children’s Medical Center Northwell Health. The ADHD Medication Guide. www.ADHDMedicationGuide.com. Revised December 31, 2017.
- Cincinnati Children’s Hospital Medical Center. Decision aids to facilitate shared decision making in practice. www.cincinnatichildrens.org/service/j/anderson-center/ evidence-based-care/decision-aids.
- CHADD. Children and Adults with attention-deficit/ hyperactivity disorder. www.chadd.org.
Drug Brand Name
Methylphenidate • Concerta, Ritalin
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
1. Froehlich TE, Lanphear BP, Epstein JN, et al. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161(9):857-864.
2. Visser SN, Lesesne CA, Perou R. National estimates and factors associated with medication treatment for childhood attention-deficit/hyperactivity disorder. Pediatrics. 2007;119 (Suppl 1):S99-S106.
3. Danielson ML, Bitsko RH, Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47(2):199-212.
4. Molina BS, Hinshaw SP, Swanson JM, et al. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):484-500.
5. Charach A, Dashti B, Carson P, et al. Attention deficit hyperactivity disorder: effectiveness of treatment in at-risk preschoolers; long-term effectiveness in all ages; and variability in prevalence, diagnosis, and treatment. Rockville, MD: Agency for Healthcare Research and Quality; 2011. http://www.ncbi.nlm.nih.gov/books/NBK82368/.
6. Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46(3):209-217.
7. Barkley RA, Fischer M, Smallish L, et al. Young adult outcome of hyperactive children: adaptive functioning in major life activities. J Am Acad Child Adolesc Psychiatry. 2006;45(2):192-202.
8. Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32(6):631-642.
9. Pliszka S, the AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(7):894-921.
10. Subcommittee on Attention-Deficit/Hyperactivity Disorder; Steering Committee on Quality Improvement and Management. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128(5):1007-1022.
11. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry. 1999;56(12):1073-1086.
12. Abikoff H, Hechtman L, Klein RG, et al. Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. J Am Acad Child Adolesc Psychiatry. 2004;43(7):802-811.
13. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term school outcomes for children with attention-deficit/hyperactivity disorder: a population-based perspective. J Dev Behav Pediatr. 2007;28(4):265-273.
14. Scheffler RM, Brown TT, Fulton BD, et al. Positive association between attention-deficit/ hyperactivity disorder medication use and academic achievement during elementary school. Pediatrics. 2009;123(5):1273-1279.
15. Dalsgaard S, Nielsen HS, Simonsen M. Five-fold increase in national prevalence rates of attention-deficit/hyperactivity disorder medications for children and adolescents with autism spectrum disorder, attention-deficit/hyperactivity disorder, and other psychiatric disorders: a Danish register-based study. J Child Adolesc Psychopharmacol. 2013;23(7):432-439.
16. Lichtenstein P, Halldner L, Zetterqvist J, et al. Medication for attention deficit-hyperactivity disorder and criminality. N Engl J Med. 2012;367(21):2006-2014.
17. Chang Z, Lichtenstein P, D’Onofrio BM, et al. Serious transport accidents in adults with attention-deficit/hyperactivity disorder and the effect of medication: a population-based study. JAMA Psychiatry. 2014;71(3):319-325.
18. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603.
19. Dalsgaard S, Leckman JF, Mortensen PB, et al. Effect of drugs on the risk of injuries in children with attention deficit hyperactivity disorder: a prospective cohort study. Lancet Psychiatry. 2015;2(8):702-709.
20. Chang Z, Lichtenstein P, Halldner L, et al. Stimulant ADHD medication and risk for substance abuse. J Child Psychol Psychiatry. 2014;55(8):878-885.
21. Fischer M, Barkley RA. Childhood stimulant treatment and risk for later substance abuse. J Clin Psychiatry. 2003;64(Suppl 11):19-23.
22. Biederman J. Pharmacotherapy for attention-deficit/hyperactivity disorder (ADHD) decreases the risk for substance abuse: findings from a longitudinal follow-up of youths with and without ADHD. J Clin Psychiatry. 2003;64(Suppl 11):3-8.
23. Chang Z, D’Onofrio BM, Quinn PD, et al. Medicationfor attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry. 2016;80(12):916-922.
24. World Health Organization. Adherence to long-term therapies: evidence for action. https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Published 2003. Accessed July 22, 2019.
25. Perwien A, Hall J, Swensen A, et al. Stimulant treatment patterns and compliance in children and adults with newly treated attention-deficit/hyperactivity disorder. J Manag Care Pharm. 2004;10(2):122-129.
26. Faraone SV, Biederman J, Zimmerman B. An analysis of patient adherence to treatment during a 1-year, open-label study of OROS methylphenidate in children with ADHD. J Atten Disord. 2007;11(2):157-166.
27. Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011;27(Suppl 2):13-22.
28. Brinkman WB, Baum R, Kelleher KJ, et al. Relationship between attention-deficit/hyperactivity disorder care and medication continuity. J Am Acad Child Adolesc Psychiatry. 2016;55(4):289-294.
29. Bokhari FAS, Heiland F, Levine P, et al. Risk factors for discontinuing drug therapy among children with ADHD. Health Services and Outcomes Research Methodology. 2008;8(3):134-158.
30. Thiruchelvam D, Charach A, Schachar RJ. Moderators and mediators of long-term adherence to stimulant treatment in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40(8):922-928.
31. DosReis S, Mychailyszyn MP, Evans-Lacko SE, et al. The meaning of attention-deficit/hyperactivity disorder medication and parents’ initiation and continuity of treatment for their child. J Child Adolesc Psychopharmacol. 2009;19(4):377-383.
32. dosReis S, Myers MA. Parental attitudes and involvement in psychopharmacological treatment for ADHD: a conceptual model. Int Rev Psychiatry. 2008;20(2):135-141.
33. Bussing R, Koro-Ljungberg M, Noguchi K, et al. Willingness to use ADHD treatments: a mixed methods study of perceptions by adolescents, parents, health professionals and teachers. Soc Sci Med. 2012;74(1):92-100.
34. Brinkman WB, Sucharew H, Majcher JH, et al. Predictors of medication continuity in children with ADHD. Pediatrics. 2018;141(6). doi: 10.1542/peds.2017-2580.
35. Coletti DJ, Pappadopulos E, Katsiotas NJ, et al. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(3):226-237.
36. Bussing R, Gary FA. Practice guidelines and parental ADHD treatment evaluations: friends or foes? Harv Rev Psychiatry. 2001;9(5):223-233.
37. Charach A, Gajaria A. Improving psychostimulant adherence in children with ADHD. Expert Rev Neurother. 2008;8(10):1563-1571.
38. Rieppi R, Greenhill LL, Ford RE, et al. Socioeconomic status as a moderator of ADHD treatment outcomes. J Am Acad Child Adolesc Psychiatry. 2002;41(3):269-277.
39. Swanson JM, Hinshaw SP, Arnold LE, et al. Secondary evaluations of MTA 36-month outcomes: propensity score and growth mixture model analyses. J Am Acad Child Adolesc Psychiatry. 2007;46(8):1003-1014.
40. Gau SS, Shen HY, Chou MC, et al. Determinants of adherence to methylphenidate and the impact of poor adherence on maternal and family measures. J Child Adolesc Psychopharmacol. 2006;16(3):286-297.
41. Barkley RA, Fischer M, Edelbrock C, et al. The adolescent outcome of hyperactive children diagnosed by research criteria--III. Mother-child interactions, family conflicts and maternal psychopathology. J Child Psychol Psychiatry. 1991;32(2):233-255.
42. Kashdan TB, Jacob RG, Pelham WE, et al. Depression and anxiety in parents of children with ADHD and varying levels of oppositional defiant behaviors: modeling relationships with family functioning. J Clin Child Adolesc Psychol. 2004;33(1):169-181.
43. Chavira DA, Stein MB, Bailey K, et al. Parental opinions regarding treatment for social anxiety disorder in youth. J Dev Behav Pediatr. 2003;24(5):315-322.
44. Leslie LK, Aarons GA, Haine RA, et al. Caregiver depression and medication use by youths with ADHD who receive services in the public sector. Psychiatr Serv. 2007;58(1):131-134.
45. Barbaresi WJ, Katusic SK, Colligan RC, et al. Long-term stimulant medication treatment of attention-deficit/hyperactivity disorder: results from a population-based study. J Dev Behav Pediatr. 2006;27(1):1-10.
46. Atzori P, Usala T, Carucci S, et al. Predictive factors for persistent use and compliance of immediate-release methylphenidate: a 36-month naturalistic study. J Child Adolesc Psychopharmacol. 2009;19(6):673-681.
47. Chen CY, Yeh HH, Chen KH, et al. Differential effects of predictors on methylphenidate initiation and discontinuation among young people with newly diagnosed attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(3):265-273.
48. Winterstein AG, Gerhard T, Shuster J, et al. Utilization of pharmacologic treatment in youths with attention deficit/hyperactivity disorder in Medicaid database. Ann Pharmacother. 2008;42(1):24-31.
49. Marcus SC, Wan GJ, Kemner JE, et al. Continuity of methylphenidate treatment for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2005;159(6):572-578.
50. Cummings JR JX, Allen L, Lally C, et al. Racial and ethnic differences in ADHD treatment quality among Medicaid-enrolled youth. Pediatrics. 2017;139(6):e2016-e2044.
51. Hudson JL, Miller GE, Kirby JB. Explaining racial and ethnic differences in children’s use of stimulant medications. Med Care. 2007;45(11):1068-1075.
52. van den Ban E, Souverein PC, Swaab H, et al. Less discontinuation of ADHD drug use since the availability of long-acting ADHD medication in children, adolescents and adults under the age of 45 years in the Netherlands. Atten Defic Hyperact Disord. 2010;2(4):213-220.
53. Charach A, Ickowicz A, Schachar R. Stimulant treatment over five years: adherence, effectiveness, and adverse effects. J Am Acad Child Adolesc Psychiatry. 2004;43(5):559-567.
54. Toomey SL, Sox CM, Rusinak D, et al. Why do children with ADHD discontinue their medication? Clin Pediatr (Phila). 2012;51(8):763-769.
55. Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescents with attention-deficit/hyperactivity disorder stop and restart taking medicine. Acad Pediatr. 2018;18(3):273-280.
56. Wehmeier PM, Dittmann RW, Banaschewski T. Treatment compliance or medication adherence in children and adolescents on ADHD medication in clinical practice: results from the COMPLY observational study. Atten Defic Hyperact Disord. 2015;7(2):165-174.
57. Frank E, Ozon C, Nair V, et al. Examining why patients with attention-deficit/hyperactivity disorder lack adherence to medication over the long term: a review and analysis. J Clin Psychiatry. 2015;76(11):e1459-e1468.
58. Pozzi M, Carnovale C, Peeters G, et al. Adverse drug events related to mood and emotion in paediatric patients treated for ADHD: a meta-analysis. J Affect Disord. 2018;238:161-178.
59. Stuckelman ZD, Mulqueen JM, Ferracioli-Oda E, et al. Risk of irritability with psychostimulant treatment in children with ADHD: a meta-analysis. J Clin Psychiatry. 2017;78(6):e648-e655.
60. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5(9):727-738.
61. Lawson KA, Johnsrud M, Hodgkins P, et al. Utilization patterns of stimulants in ADHD in the Medicaid population: a retrospective analysis of data from the Texas Medicaid program. Clin Ther. 2012;34(4):944-956 e944.
62. Olfson M, Marcus S, Wan G. Stimulant dosing for children with ADHD: a medical claims analysis. J Am Acad Child Adolesc Psychiatry. 2009;48(1):51-59.
63. Jensen PS, Arnold LE, Swanson JM, et al. 3-year follow-up of the NIMH MTA study. J Am Acad Child Adolesc Psychiatry. 2007;46(8):989-1002.
64. Van Cleave J, Leslie LK. Approaching ADHD as a chronic condition: implications for long-term adherence. Pediatr Ann. 2008;37(1):19-26.
65. Leslie LK, Plemmons D, Monn AR, et al. Investigating ADHD treatment trajectories: listening to families’ stories about medication use. J Dev Behav Pediatr. 2007;28(3):179-188.
66. Fiks AG, Mayne S, Localio AR, et al. Shared decision making and behavioral impairment: a national study among children with special health care needs. BMC Pediatr. 2012;12:153.
67. Stevens J, Harman JS, Kelleher KJ. Race/ethnicity and insurance status as factors associated with ADHD treatment patterns. J Child Adolesc Psychopharmacol. 2005;15(1):88-96.
68. Charach A, Skyba A, Cook L, et al. Using stimulant medication for children with ADHD: what do parents say? A brief report. J Can Acad Child Adolesc Psychiatry. 2006;15(2):75-83.
69. Chen CY, Gerhard T, Winterstein AG. Determinants of initial pharmacological treatment for youths with attention-deficit/hyperactivity disorder. J Child Adolescent Psychopharmacol. 2009;19(2):187-195.
70. National Council on Patient Information and Education. Enhancing prescription medication adherence: a national action plan. http://www.bemedwise.org/docs/enhancingprescriptionmedicineadherence.pdf. Published August 2007. Accessed July 22, 2019.
71. Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590-611.
72. Johnston C, Mash EJ. Families of children with attention-deficit/hyperactivity disorder: review and recommendations for future research. Clin Child Fam Psychol Rev. 2001;4(3):183-207.
73. Chronis AM, Lahey BB, Pelham WE Jr., et al. Psychopathology and substance abuse in parents of young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2003;42(12):1424-1432.
74. Chacko A, Newcorn JH, Feirsen N, et al. Improving medication adherence in chronic pediatric health conditions: a focus on ADHD in youth. Curr Pharm Des. 2010;16(22):2416-2423.
75. Brinkman WB, Hartl Majcher J, Polling LM, et al. Shared decision-making to improve attention-deficit hyperactivity disorder care. Patient Educ Couns. 2013;93(1):95-101.
76. American Academy of Pediatrics. Caring for children with ADHD: a resource toolkit for clinicians. 2nd ed. https://www.aap.org/en-us/pubserv/adhd2/Pages/default.aspx. Published 2011. Accessed July 22, 2019.
77. The REACH Institute. Course dates and registration. http://www.thereachinstitute.org/services/for-primary-care-practitioners/training-dates-and-registration. Accessed July 22, 2019.
78. Sells D, Davidson L, Jewell C, et al. The treatment relationship in peer-based and regular case management for clients with severe mental illness. Psychiatr Serv. 2006;57(8):1179-1184.
79. Hoagwood KE, Green E, Kelleher K, et al. Family advocacy, support and education in children’s mental health: results of a national survey. Adm Policy Ment Health. 2008;35(1-2):73-83.
80. Klein MD, Beck AF, Henize AW, et al. Doctors and lawyers collaborating to HeLP children—outcomes from a successful partnership between professions. J Health Care Poor Underserved. 2013;24(3):1063-1073.
81. Weintraub D, Rodgers MA, Botcheva L, et al. Pilot study of medical-legal partnership to address social and legal needs of patients. J Health Care Poor Underserved. 2010;21(Suppl 2):157-168.
82. Bradley CL, Luder HR, Beck AF, et al. Pediatric asthma medication therapy management through community pharmacy and primary care collaboration. J Am Pharm Assoc (2003). 2016;56(4):455-460.
83. Noyes K, Bajorska A, Fisher S, et al. Cost-effectiveness of the school-based asthma therapy (SBAT) program. Pediatrics. 2013;131(3):e709-e717.
84. Halterman JS, Fagnano M, Montes G, et al. The school-based preventive asthma care trial: results of a pilot study. J Pediatr. 2012;161(6):1109-1115.
85. Halterman JS, Szilagyi PG, Fisher SG, et al. Randomized controlled trial to improve care for urban children with asthma: results of the school-based asthma therapy trial. Arch Pediatr Adolesc Med. 2011;165(3):262-268.
Most preschoolers with signs of ADHD aren’t ready for primary school
Are preschoolers with signs of ADHD ready for school? A new study suggests they’re far from prepared.
A small sample of children with symptoms of moderate to severe ADHD scored markedly lower than comparable children on 8 of 10 measures of readiness for primary education in a study published in Pediatrics.
These children require early identification and intervention,” Hannah T. Perrin, MD, of Stanford University and associates wrote.
There’s sparse research into the prevalence of ADHD symptoms in preschoolers, but the Centers for Disease Control and Prevention reports that nearly half of children aged 4-5 years with the condition got no behavioral therapy from 2009 to 2010. About 25% received only medical treatment.
Dr. Perrin and colleagues recruited 93 children aged 4-6 years from the community. Their parents, who were compensated, took the Early Childhood Inventory-4 (ECI-4) questionnaire. It revealed that 80% (n = 45) of those diagnosed with ADHD had scores considered signs of moderate or severe ADHD symptom severity based on the parent ratings. Those with lower scores made up the comparison group (n = 48).
The groups were similar, about 60% male and more than 50% white; neither difference between groups was statistically significant. However, those in the comparison group were much more likely to have non-Latino/non-Hispanic ethnicity; 61% in ADHD group vs. 91% in comparison group, P = .001.
The children were tested for school readiness through several measures in two 1- to 1.5-hour sessions.
The researchers reported that 79% of children in the ADHD group were not ready for school (impaired) vs. 13% of the comparison group. (odds ratio, 21, 95% confidence interval, 5.67-77.77, P = .001).
“We found that preschool-aged children with ADHD symptoms demonstrated significantly worse performance on 8 of 10 school readiness measures,” the authors added, “and significantly greater odds of impairment in four of five domains and overall school readiness.”
Dr. Perrin and associates cautioned that the findings rely on a convenience sample, are based on parent – but not teacher – input, do not include Spanish speakers, and do not follow children over the long term.
Going forward, they wrote, “family dynamics and social-emotional functioning should be assessed for each preschool-aged child with ADHD symptoms, and appropriate therapeutic interventions and community supports should be prescribed to enhance school readiness.”
The study authors had no disclosures. Study funders include the Maternal and Child Health Bureau, the Katharine McCormick Faculty Scholar Award, Stanford Children’s Health and Child Health Research Institute Pilot Early Career Award, and the National Institutes of Health.
SOURCE: Perrin HT et al. Pediatrics. 2019 Aug. doi: 10.1542/peds.2019-0038.
Are preschoolers with signs of ADHD ready for school? A new study suggests they’re far from prepared.
A small sample of children with symptoms of moderate to severe ADHD scored markedly lower than comparable children on 8 of 10 measures of readiness for primary education in a study published in Pediatrics.
These children require early identification and intervention,” Hannah T. Perrin, MD, of Stanford University and associates wrote.
There’s sparse research into the prevalence of ADHD symptoms in preschoolers, but the Centers for Disease Control and Prevention reports that nearly half of children aged 4-5 years with the condition got no behavioral therapy from 2009 to 2010. About 25% received only medical treatment.
Dr. Perrin and colleagues recruited 93 children aged 4-6 years from the community. Their parents, who were compensated, took the Early Childhood Inventory-4 (ECI-4) questionnaire. It revealed that 80% (n = 45) of those diagnosed with ADHD had scores considered signs of moderate or severe ADHD symptom severity based on the parent ratings. Those with lower scores made up the comparison group (n = 48).
The groups were similar, about 60% male and more than 50% white; neither difference between groups was statistically significant. However, those in the comparison group were much more likely to have non-Latino/non-Hispanic ethnicity; 61% in ADHD group vs. 91% in comparison group, P = .001.
The children were tested for school readiness through several measures in two 1- to 1.5-hour sessions.
The researchers reported that 79% of children in the ADHD group were not ready for school (impaired) vs. 13% of the comparison group. (odds ratio, 21, 95% confidence interval, 5.67-77.77, P = .001).
“We found that preschool-aged children with ADHD symptoms demonstrated significantly worse performance on 8 of 10 school readiness measures,” the authors added, “and significantly greater odds of impairment in four of five domains and overall school readiness.”
Dr. Perrin and associates cautioned that the findings rely on a convenience sample, are based on parent – but not teacher – input, do not include Spanish speakers, and do not follow children over the long term.
Going forward, they wrote, “family dynamics and social-emotional functioning should be assessed for each preschool-aged child with ADHD symptoms, and appropriate therapeutic interventions and community supports should be prescribed to enhance school readiness.”
The study authors had no disclosures. Study funders include the Maternal and Child Health Bureau, the Katharine McCormick Faculty Scholar Award, Stanford Children’s Health and Child Health Research Institute Pilot Early Career Award, and the National Institutes of Health.
SOURCE: Perrin HT et al. Pediatrics. 2019 Aug. doi: 10.1542/peds.2019-0038.
Are preschoolers with signs of ADHD ready for school? A new study suggests they’re far from prepared.
A small sample of children with symptoms of moderate to severe ADHD scored markedly lower than comparable children on 8 of 10 measures of readiness for primary education in a study published in Pediatrics.
These children require early identification and intervention,” Hannah T. Perrin, MD, of Stanford University and associates wrote.
There’s sparse research into the prevalence of ADHD symptoms in preschoolers, but the Centers for Disease Control and Prevention reports that nearly half of children aged 4-5 years with the condition got no behavioral therapy from 2009 to 2010. About 25% received only medical treatment.
Dr. Perrin and colleagues recruited 93 children aged 4-6 years from the community. Their parents, who were compensated, took the Early Childhood Inventory-4 (ECI-4) questionnaire. It revealed that 80% (n = 45) of those diagnosed with ADHD had scores considered signs of moderate or severe ADHD symptom severity based on the parent ratings. Those with lower scores made up the comparison group (n = 48).
The groups were similar, about 60% male and more than 50% white; neither difference between groups was statistically significant. However, those in the comparison group were much more likely to have non-Latino/non-Hispanic ethnicity; 61% in ADHD group vs. 91% in comparison group, P = .001.
The children were tested for school readiness through several measures in two 1- to 1.5-hour sessions.
The researchers reported that 79% of children in the ADHD group were not ready for school (impaired) vs. 13% of the comparison group. (odds ratio, 21, 95% confidence interval, 5.67-77.77, P = .001).
“We found that preschool-aged children with ADHD symptoms demonstrated significantly worse performance on 8 of 10 school readiness measures,” the authors added, “and significantly greater odds of impairment in four of five domains and overall school readiness.”
Dr. Perrin and associates cautioned that the findings rely on a convenience sample, are based on parent – but not teacher – input, do not include Spanish speakers, and do not follow children over the long term.
Going forward, they wrote, “family dynamics and social-emotional functioning should be assessed for each preschool-aged child with ADHD symptoms, and appropriate therapeutic interventions and community supports should be prescribed to enhance school readiness.”
The study authors had no disclosures. Study funders include the Maternal and Child Health Bureau, the Katharine McCormick Faculty Scholar Award, Stanford Children’s Health and Child Health Research Institute Pilot Early Career Award, and the National Institutes of Health.
SOURCE: Perrin HT et al. Pediatrics. 2019 Aug. doi: 10.1542/peds.2019-0038.
FROM PEDIATRICS
About one in four youths prescribed stimulants also use the drugs nonmedically
SAN ANTONIO – Of 196 U.S. youth who reported use of at least one prescribed stimulant in their lifetimes, 25% also said they used the drugs nonmedically, based on a survey of children and adolescents aged 10-17 years.
Another 5% of the youth surveyed reported exclusively nonmedical use of stimulants. The survey participants lived in six U.S. cities and their outlying areas.
“Parents of both users and nonusers should warn their children of the dangers of using others’ stimulants and giving their own stimulants to others,” concluded Linda B. Cottler, PhD, MPH of the University of Florida, and colleagues.
“Physicians and pharmacists should make users and their families aware of the need to take medications as prescribed and not to share medications with others,” they wrote in their research poster at the annual meeting of the College on Problems of Drug Dependence. “Continuous monitoring of these medications in the community should be a priority.”
Though prevalence research has shown increasing stimulant misuse among youth, little data exist for younger children, the researchers noted. They therefore conducted a survey of 1,777 youth aged 10-17 years from September to October 2018 in six cities in California, Texas, and Florida, the most populous U.S. states.
The participants included youth from urban, rural, and suburban areas of Los Angeles, Dallas, Houston, Tampa, Orlando, and Miami. Trained graduate students and professional raters approached the respondents in entertainment venues and obtained assent but did not require parental consent. The respondents received $30 for completing the survey.
A total of 11.1% of respondents reporting having used prescription stimulants in their lifetime, and 7.6% had done so in the past 30 days. Just under a third of those who used stimulants (30.1%) did so for nonmedical purposes, defined as taking the stimulant nonorally (except for the patch Daytrana), getting the stimulant from someone else, or taking more of the drug than prescribed.
A quarter of the respondents who used stimulants reported both medical use and nonmedical use. And 5.1% of these youths reported only using stimulants nonmedically.
Among those with any lifetime stimulant use, 13.8% reported nonoral administration, including 9.7% who snorted or sniffed the drugs, 4.1% who smoked them, and 1.0% who injected them. Just over half (51.8%) of those reporting nonoral use had also used prescription stimulants orally.
The likelihood of using stimulants nonmedically increased with age (P less than .0001). The researchers found no significant associations between nonmedical use and geography or race/ethnicity. Among 10- to 12-year-olds, 3.1% reported only medical use of stimulants, and 0.7% (2 of 286 respondents in this age group) reported any nonmedical use of stimulants.
Of those aged 13-15 years, 2.1% reported any nonmedical stimulant use.
Nonmedical stimulant use was reported by twice as many boys (67.8%) as girls (32.2%), though this finding may not be surprising as the majority of nonmedical users were also medical users and stimulants are prescribed more frequently to boys than to girls (P less than .0006).
The research was funded by Arbor Pharmaceuticals. The authors noted no conflicts of interest.
SAN ANTONIO – Of 196 U.S. youth who reported use of at least one prescribed stimulant in their lifetimes, 25% also said they used the drugs nonmedically, based on a survey of children and adolescents aged 10-17 years.
Another 5% of the youth surveyed reported exclusively nonmedical use of stimulants. The survey participants lived in six U.S. cities and their outlying areas.
“Parents of both users and nonusers should warn their children of the dangers of using others’ stimulants and giving their own stimulants to others,” concluded Linda B. Cottler, PhD, MPH of the University of Florida, and colleagues.
“Physicians and pharmacists should make users and their families aware of the need to take medications as prescribed and not to share medications with others,” they wrote in their research poster at the annual meeting of the College on Problems of Drug Dependence. “Continuous monitoring of these medications in the community should be a priority.”
Though prevalence research has shown increasing stimulant misuse among youth, little data exist for younger children, the researchers noted. They therefore conducted a survey of 1,777 youth aged 10-17 years from September to October 2018 in six cities in California, Texas, and Florida, the most populous U.S. states.
The participants included youth from urban, rural, and suburban areas of Los Angeles, Dallas, Houston, Tampa, Orlando, and Miami. Trained graduate students and professional raters approached the respondents in entertainment venues and obtained assent but did not require parental consent. The respondents received $30 for completing the survey.
A total of 11.1% of respondents reporting having used prescription stimulants in their lifetime, and 7.6% had done so in the past 30 days. Just under a third of those who used stimulants (30.1%) did so for nonmedical purposes, defined as taking the stimulant nonorally (except for the patch Daytrana), getting the stimulant from someone else, or taking more of the drug than prescribed.
A quarter of the respondents who used stimulants reported both medical use and nonmedical use. And 5.1% of these youths reported only using stimulants nonmedically.
Among those with any lifetime stimulant use, 13.8% reported nonoral administration, including 9.7% who snorted or sniffed the drugs, 4.1% who smoked them, and 1.0% who injected them. Just over half (51.8%) of those reporting nonoral use had also used prescription stimulants orally.
The likelihood of using stimulants nonmedically increased with age (P less than .0001). The researchers found no significant associations between nonmedical use and geography or race/ethnicity. Among 10- to 12-year-olds, 3.1% reported only medical use of stimulants, and 0.7% (2 of 286 respondents in this age group) reported any nonmedical use of stimulants.
Of those aged 13-15 years, 2.1% reported any nonmedical stimulant use.
Nonmedical stimulant use was reported by twice as many boys (67.8%) as girls (32.2%), though this finding may not be surprising as the majority of nonmedical users were also medical users and stimulants are prescribed more frequently to boys than to girls (P less than .0006).
The research was funded by Arbor Pharmaceuticals. The authors noted no conflicts of interest.
SAN ANTONIO – Of 196 U.S. youth who reported use of at least one prescribed stimulant in their lifetimes, 25% also said they used the drugs nonmedically, based on a survey of children and adolescents aged 10-17 years.
Another 5% of the youth surveyed reported exclusively nonmedical use of stimulants. The survey participants lived in six U.S. cities and their outlying areas.
“Parents of both users and nonusers should warn their children of the dangers of using others’ stimulants and giving their own stimulants to others,” concluded Linda B. Cottler, PhD, MPH of the University of Florida, and colleagues.
“Physicians and pharmacists should make users and their families aware of the need to take medications as prescribed and not to share medications with others,” they wrote in their research poster at the annual meeting of the College on Problems of Drug Dependence. “Continuous monitoring of these medications in the community should be a priority.”
Though prevalence research has shown increasing stimulant misuse among youth, little data exist for younger children, the researchers noted. They therefore conducted a survey of 1,777 youth aged 10-17 years from September to October 2018 in six cities in California, Texas, and Florida, the most populous U.S. states.
The participants included youth from urban, rural, and suburban areas of Los Angeles, Dallas, Houston, Tampa, Orlando, and Miami. Trained graduate students and professional raters approached the respondents in entertainment venues and obtained assent but did not require parental consent. The respondents received $30 for completing the survey.
A total of 11.1% of respondents reporting having used prescription stimulants in their lifetime, and 7.6% had done so in the past 30 days. Just under a third of those who used stimulants (30.1%) did so for nonmedical purposes, defined as taking the stimulant nonorally (except for the patch Daytrana), getting the stimulant from someone else, or taking more of the drug than prescribed.
A quarter of the respondents who used stimulants reported both medical use and nonmedical use. And 5.1% of these youths reported only using stimulants nonmedically.
Among those with any lifetime stimulant use, 13.8% reported nonoral administration, including 9.7% who snorted or sniffed the drugs, 4.1% who smoked them, and 1.0% who injected them. Just over half (51.8%) of those reporting nonoral use had also used prescription stimulants orally.
The likelihood of using stimulants nonmedically increased with age (P less than .0001). The researchers found no significant associations between nonmedical use and geography or race/ethnicity. Among 10- to 12-year-olds, 3.1% reported only medical use of stimulants, and 0.7% (2 of 286 respondents in this age group) reported any nonmedical use of stimulants.
Of those aged 13-15 years, 2.1% reported any nonmedical stimulant use.
Nonmedical stimulant use was reported by twice as many boys (67.8%) as girls (32.2%), though this finding may not be surprising as the majority of nonmedical users were also medical users and stimulants are prescribed more frequently to boys than to girls (P less than .0006).
The research was funded by Arbor Pharmaceuticals. The authors noted no conflicts of interest.
REPORTING FROM CPDD 2019
Amphetamine tied to higher risk of new-onset psychosis than methylphenidate
Greater risk applies only to adolescents, young adults with ADHD treated in primary care
Adolescents and young adults with ADHD who start on amphetamine might have twice the risk of developing new-onset psychosis as do those who start on methylphenidate, a cohort study of more than 220,000 patients suggests.
“The percentage of patients who had a psychotic episode was 0.10% among patients who received methylphenidate and 0.21% among patients who received amphetamine, reported Lauren V. Moran, MD, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston and her colleagues. The study was published by the New England Journal of Medicine.
(aged 13-25 years) with ADHD between January 2004 and September 2015 who were prescribed methylphenidate or amphetamine (both 110,923 patients; 143,286 total person-years of follow-up). They looked for an ICD-9 or ICD-10 code for new-onset psychosis followed by a prescription for an antipsychotic medication the same day or within 60 days of the psychosis diagnosis. Hazard ratios were calculated by matching patients taking methylphenidate with patients taking amphetamine across both databases and calculating the incidence rate of psychosis in each group.
The researchers found 343 new cases of psychosis overall, with an incidence of 2.4 cases per 1,000 person-years. There were 106 episodes of psychosis among patients receiving methylphenidate (0.10%) and 237 new cases among patients receiving amphetamine (0.21%). There was an incidence rate of 1.78 cases per 1,000 person-years for methylphenidate patients and 2.83 cases per 1,000 person-years for amphetamine patients. Across both databases, the pooled hazard ratio for amphetamine use and new-onset psychosis, compared with matched patients, was 1.65 (95% confidence interval, 1.31-2.09).
“The attribution of the higher risk of psychosis to amphetamine use was supported by negative control outcome analyses, which showed that there was no difference in the risk of other psychiatric events between the two stimulant groups,” Dr. Moran and her colleagues reported. “The different biologic mechanisms of methylphenidate and amphetamine activity on neurotransmitters could explain our findings.”
Patients who were prescribed amphetamine by family medicine physicians, internists, and pediatricians were at a higher risk of developing psychosis. That risk, however, did not extend to patients prescribed amphetamine by psychiatrists, the researchers said.
“Psychosis may develop in these patients regardless of stimulant treatment. Alternatively, psychiatrists may prescribe amphetamine more cautiously than other providers and may screen for risk factors for psychosis,” Dr. Moran and her colleagues wrote.
The researchers said the study was limited by unmeasured confounders, such as substance or stimulant misuse; the rate of diversion for amphetamine; and lack of information on race, gender, or socioeconomic status. In addition, they noted, the results could not be generalized to patients with public insurance or no insurance, “which disproportionately applies to patients who are black or Hispanic.”
Dr. Moran reported receiving grants from National Institute of Mental Health (NIMH). The other authors reported grants, personal fees, and other relationships with several entities, including Boehringer Ingelheim, the Food and Drug Administration, the NIMH, and Takeda.
SOURCE: Moran LV et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1813751.
The findings by Moran et al. are consistent with other randomized controlled trials that suggest a better safety profile for methylphenidate over amphetamine. But the data cannot determine causality in this patient population, Samuele Cortese, MD, PhD, wrote in a related editorial.
“The findings of the current study should not be considered definitive. Observational studies such as this one can provide information on uncommon adverse events in real-world clinical practice that are challenging to assess in randomized trials performed over brief periods,” he said. “However, even sophisticated approaches, such as the ones used in this study to address possible biases, do not have the advantages of randomized trials in excluding confounding factors.”
It is still unclear why some patients developed psychosis, such as in cases of patients with stimulant use and had a “low” or “high” vulnerability to developing psychosis after exposure. The lack of association between psychosis and prescribing amphetamines among psychiatrists also might indicate that those clinicians identified risk factors in patients that predicted the development of psychosis and thus avoided prescribing amphetamines to these patients, he said.
“Currently, it is not possible to predict which patients will have psychotic episodes after stimulant treatment,” Dr. Cortese concluded. “Perhaps techniques such as machine learning applied to large data sets from randomized trials, combined with observational data, will provide predictors at the individual patient level.”
Dr. Cortese is affiliated with the Center for Innovation in Mental Health at the University of Southampton (England). These comments summarize his accompanying editorial (N Engl J Med. 2019. doi: 10.1056/NEJMe1900887 ). He reported nonfinancial relationships with the Association for Child and Adolescent Central Health and the Healthcare Convention & Exhibitors Association.
Greater risk applies only to adolescents, young adults with ADHD treated in primary care
Greater risk applies only to adolescents, young adults with ADHD treated in primary care
The findings by Moran et al. are consistent with other randomized controlled trials that suggest a better safety profile for methylphenidate over amphetamine. But the data cannot determine causality in this patient population, Samuele Cortese, MD, PhD, wrote in a related editorial.
“The findings of the current study should not be considered definitive. Observational studies such as this one can provide information on uncommon adverse events in real-world clinical practice that are challenging to assess in randomized trials performed over brief periods,” he said. “However, even sophisticated approaches, such as the ones used in this study to address possible biases, do not have the advantages of randomized trials in excluding confounding factors.”
It is still unclear why some patients developed psychosis, such as in cases of patients with stimulant use and had a “low” or “high” vulnerability to developing psychosis after exposure. The lack of association between psychosis and prescribing amphetamines among psychiatrists also might indicate that those clinicians identified risk factors in patients that predicted the development of psychosis and thus avoided prescribing amphetamines to these patients, he said.
“Currently, it is not possible to predict which patients will have psychotic episodes after stimulant treatment,” Dr. Cortese concluded. “Perhaps techniques such as machine learning applied to large data sets from randomized trials, combined with observational data, will provide predictors at the individual patient level.”
Dr. Cortese is affiliated with the Center for Innovation in Mental Health at the University of Southampton (England). These comments summarize his accompanying editorial (N Engl J Med. 2019. doi: 10.1056/NEJMe1900887 ). He reported nonfinancial relationships with the Association for Child and Adolescent Central Health and the Healthcare Convention & Exhibitors Association.
The findings by Moran et al. are consistent with other randomized controlled trials that suggest a better safety profile for methylphenidate over amphetamine. But the data cannot determine causality in this patient population, Samuele Cortese, MD, PhD, wrote in a related editorial.
“The findings of the current study should not be considered definitive. Observational studies such as this one can provide information on uncommon adverse events in real-world clinical practice that are challenging to assess in randomized trials performed over brief periods,” he said. “However, even sophisticated approaches, such as the ones used in this study to address possible biases, do not have the advantages of randomized trials in excluding confounding factors.”
It is still unclear why some patients developed psychosis, such as in cases of patients with stimulant use and had a “low” or “high” vulnerability to developing psychosis after exposure. The lack of association between psychosis and prescribing amphetamines among psychiatrists also might indicate that those clinicians identified risk factors in patients that predicted the development of psychosis and thus avoided prescribing amphetamines to these patients, he said.
“Currently, it is not possible to predict which patients will have psychotic episodes after stimulant treatment,” Dr. Cortese concluded. “Perhaps techniques such as machine learning applied to large data sets from randomized trials, combined with observational data, will provide predictors at the individual patient level.”
Dr. Cortese is affiliated with the Center for Innovation in Mental Health at the University of Southampton (England). These comments summarize his accompanying editorial (N Engl J Med. 2019. doi: 10.1056/NEJMe1900887 ). He reported nonfinancial relationships with the Association for Child and Adolescent Central Health and the Healthcare Convention & Exhibitors Association.
Adolescents and young adults with ADHD who start on amphetamine might have twice the risk of developing new-onset psychosis as do those who start on methylphenidate, a cohort study of more than 220,000 patients suggests.
“The percentage of patients who had a psychotic episode was 0.10% among patients who received methylphenidate and 0.21% among patients who received amphetamine, reported Lauren V. Moran, MD, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston and her colleagues. The study was published by the New England Journal of Medicine.
(aged 13-25 years) with ADHD between January 2004 and September 2015 who were prescribed methylphenidate or amphetamine (both 110,923 patients; 143,286 total person-years of follow-up). They looked for an ICD-9 or ICD-10 code for new-onset psychosis followed by a prescription for an antipsychotic medication the same day or within 60 days of the psychosis diagnosis. Hazard ratios were calculated by matching patients taking methylphenidate with patients taking amphetamine across both databases and calculating the incidence rate of psychosis in each group.
The researchers found 343 new cases of psychosis overall, with an incidence of 2.4 cases per 1,000 person-years. There were 106 episodes of psychosis among patients receiving methylphenidate (0.10%) and 237 new cases among patients receiving amphetamine (0.21%). There was an incidence rate of 1.78 cases per 1,000 person-years for methylphenidate patients and 2.83 cases per 1,000 person-years for amphetamine patients. Across both databases, the pooled hazard ratio for amphetamine use and new-onset psychosis, compared with matched patients, was 1.65 (95% confidence interval, 1.31-2.09).
“The attribution of the higher risk of psychosis to amphetamine use was supported by negative control outcome analyses, which showed that there was no difference in the risk of other psychiatric events between the two stimulant groups,” Dr. Moran and her colleagues reported. “The different biologic mechanisms of methylphenidate and amphetamine activity on neurotransmitters could explain our findings.”
Patients who were prescribed amphetamine by family medicine physicians, internists, and pediatricians were at a higher risk of developing psychosis. That risk, however, did not extend to patients prescribed amphetamine by psychiatrists, the researchers said.
“Psychosis may develop in these patients regardless of stimulant treatment. Alternatively, psychiatrists may prescribe amphetamine more cautiously than other providers and may screen for risk factors for psychosis,” Dr. Moran and her colleagues wrote.
The researchers said the study was limited by unmeasured confounders, such as substance or stimulant misuse; the rate of diversion for amphetamine; and lack of information on race, gender, or socioeconomic status. In addition, they noted, the results could not be generalized to patients with public insurance or no insurance, “which disproportionately applies to patients who are black or Hispanic.”
Dr. Moran reported receiving grants from National Institute of Mental Health (NIMH). The other authors reported grants, personal fees, and other relationships with several entities, including Boehringer Ingelheim, the Food and Drug Administration, the NIMH, and Takeda.
SOURCE: Moran LV et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1813751.
Adolescents and young adults with ADHD who start on amphetamine might have twice the risk of developing new-onset psychosis as do those who start on methylphenidate, a cohort study of more than 220,000 patients suggests.
“The percentage of patients who had a psychotic episode was 0.10% among patients who received methylphenidate and 0.21% among patients who received amphetamine, reported Lauren V. Moran, MD, of the division of pharmacoepidemiology and pharmacoeconomics at Brigham and Women’s Hospital in Boston and her colleagues. The study was published by the New England Journal of Medicine.
(aged 13-25 years) with ADHD between January 2004 and September 2015 who were prescribed methylphenidate or amphetamine (both 110,923 patients; 143,286 total person-years of follow-up). They looked for an ICD-9 or ICD-10 code for new-onset psychosis followed by a prescription for an antipsychotic medication the same day or within 60 days of the psychosis diagnosis. Hazard ratios were calculated by matching patients taking methylphenidate with patients taking amphetamine across both databases and calculating the incidence rate of psychosis in each group.
The researchers found 343 new cases of psychosis overall, with an incidence of 2.4 cases per 1,000 person-years. There were 106 episodes of psychosis among patients receiving methylphenidate (0.10%) and 237 new cases among patients receiving amphetamine (0.21%). There was an incidence rate of 1.78 cases per 1,000 person-years for methylphenidate patients and 2.83 cases per 1,000 person-years for amphetamine patients. Across both databases, the pooled hazard ratio for amphetamine use and new-onset psychosis, compared with matched patients, was 1.65 (95% confidence interval, 1.31-2.09).
“The attribution of the higher risk of psychosis to amphetamine use was supported by negative control outcome analyses, which showed that there was no difference in the risk of other psychiatric events between the two stimulant groups,” Dr. Moran and her colleagues reported. “The different biologic mechanisms of methylphenidate and amphetamine activity on neurotransmitters could explain our findings.”
Patients who were prescribed amphetamine by family medicine physicians, internists, and pediatricians were at a higher risk of developing psychosis. That risk, however, did not extend to patients prescribed amphetamine by psychiatrists, the researchers said.
“Psychosis may develop in these patients regardless of stimulant treatment. Alternatively, psychiatrists may prescribe amphetamine more cautiously than other providers and may screen for risk factors for psychosis,” Dr. Moran and her colleagues wrote.
The researchers said the study was limited by unmeasured confounders, such as substance or stimulant misuse; the rate of diversion for amphetamine; and lack of information on race, gender, or socioeconomic status. In addition, they noted, the results could not be generalized to patients with public insurance or no insurance, “which disproportionately applies to patients who are black or Hispanic.”
Dr. Moran reported receiving grants from National Institute of Mental Health (NIMH). The other authors reported grants, personal fees, and other relationships with several entities, including Boehringer Ingelheim, the Food and Drug Administration, the NIMH, and Takeda.
SOURCE: Moran LV et al. N Engl J Med. 2019. doi: 10.1056/NEJMoa1813751.
Eczema increases the risk of impaired mental health among children
WASHINGTON – according to an analysis described at the annual meeting of the American Academy of Dermatology. Eczema appears to influence several domains of mental health, and the association remains in the absence of other atopic illnesses.
Estimates of the prevalence of eczema in children have ranged as high as 20%. European and Japanese studies have suggested that children with eczema have greater mental health impairments overall, but researchers have not evaluated this association among U.S. children. Although it has been established that children with eczema consult health care providers more often than children without eczema, data on health care utilization among children with eczema and impaired mental health are limited.
Joy Wan, MD, a dermatologist at Children’s Hospital of Philadelphia, and her colleagues performed a cross-sectional analysis of data obtained from 2013 to 2017 by the National Health Interview Survey. The Centers for Disease Control and Prevention administers the survey to a representative sample of the U.S. population. Children in each household are randomly sampled, and adult caregivers provide detailed health information about them.
Dr. Wan and her colleagues included children aged between 4 and 17 years in their analysis. The exposure of interest was eczema. Caregivers reported eczema in response to the question, “During the past 12 months, has the child had eczema or any kind of skin allergy?” The study’s primary outcome was mental health impairment. Using the Strengths and Difficulties Questionnaire (SDQ), the investigators categorized mental health impairment as none, mild, or severe. The SDQ is a validated instrument that assesses symptoms of mental health in children in domains such as conduct, emotion, peer relationships, and attention, which the researchers chose as secondary outcomes of interest. Dr. Wan’s group also examined the utilization of mental health and other health and social services among children with eczema.
The researchers performed logistic regression analysis to obtain odds ratios for mental health impairment among children with eczema, adjusting the analysis for potential socioeconomic and demographic confounders. Furthermore, they stratified the primary model by other atopic and behavioral disorders to assess for potential effects modification by these concomitant illnesses.
Approximately 12% of the children in the sample had eczema. Children with eczema tended to be female, non-Hispanic, or black; they also were more likely to report good, fair, or poor health, compared with children without eczema. Asthma, allergic rhinitis, and ADHD were more common among children with eczema than those without.
About 27% of children with eczema had any mental health impairment, compared with approximately 18% of children without eczema. About 11% of children with eczema had severe impairment; this rate was almost twice as high as that in children without eczema, Dr. Wan said. The adjusted odds of mental health impairment were 52% per year among children with eczema, compared with those without.
When the researchers examined specific domains of mental health, they found that children with eczema were significantly less likely to be reported to be well behaved or to have good attention spans. They also were significantly more likely to worry often, be unhappy or depressed, and to get along better with adults than their peers.
When Dr. Wan and his colleagues stratified the primary model by other atopic illnesses, they found that, among children without any other atopic illness, eczema remained independently associated with mental health impairment (OR, 1.52). The effect remained similar among children with asthma alone, but was attenuated among children with allergic rhinitis alone or with asthma and allergic rhinitis.
In the absence of ADHD, the investigators found a statistically significant effect of eczema on mental health impairment (OR, 1.46). In the presence of ADHD, the effect remained significant, but was attenuated.
Finally, approximately 20% of children with mildly impaired mental health had seen a mental health professional in the past year. In addition, 54% of children with severe mental health symptoms had seen a mental health professional in the past year. Among children with severe impairment, about 80% had consulted a general practitioner in the past year; 45% of them reported emotional or behavioral issues as the reason for the visit. Use of special education and early intervention services were more prevalent among children with increasing degrees of mental health impairment.
The study’s strengths include its population-based design, the use of a validated psychometric instrument, and the adjustment of data for socioeconomic factors and other comorbid illnesses, Dr. Wan said. The study is cross sectional, however, which precludes conclusions about the directionality of the relationship between eczema and mental health. In addition, the SDQ may not capture all mental health symptoms that eczema affects.
It is imperative that clinicians and caregivers recognize how common mental health impairment is among children with eczema so that children can be appropriately screened and referred for care, Dr. Wan said. “Our study suggests that there may be a critical gap in mental health services utilization by children who have eczema and concomitant mental health impairment. Some of the future directions in this area may be to understand the potential barriers to mental health care in children with eczema, and certainly to identify potentially effective interventions to reduce the mental health burden in pediatric eczema.”
Dr. Wan reported receiving research fellowship funding from Pfizer.
WASHINGTON – according to an analysis described at the annual meeting of the American Academy of Dermatology. Eczema appears to influence several domains of mental health, and the association remains in the absence of other atopic illnesses.
Estimates of the prevalence of eczema in children have ranged as high as 20%. European and Japanese studies have suggested that children with eczema have greater mental health impairments overall, but researchers have not evaluated this association among U.S. children. Although it has been established that children with eczema consult health care providers more often than children without eczema, data on health care utilization among children with eczema and impaired mental health are limited.
Joy Wan, MD, a dermatologist at Children’s Hospital of Philadelphia, and her colleagues performed a cross-sectional analysis of data obtained from 2013 to 2017 by the National Health Interview Survey. The Centers for Disease Control and Prevention administers the survey to a representative sample of the U.S. population. Children in each household are randomly sampled, and adult caregivers provide detailed health information about them.
Dr. Wan and her colleagues included children aged between 4 and 17 years in their analysis. The exposure of interest was eczema. Caregivers reported eczema in response to the question, “During the past 12 months, has the child had eczema or any kind of skin allergy?” The study’s primary outcome was mental health impairment. Using the Strengths and Difficulties Questionnaire (SDQ), the investigators categorized mental health impairment as none, mild, or severe. The SDQ is a validated instrument that assesses symptoms of mental health in children in domains such as conduct, emotion, peer relationships, and attention, which the researchers chose as secondary outcomes of interest. Dr. Wan’s group also examined the utilization of mental health and other health and social services among children with eczema.
The researchers performed logistic regression analysis to obtain odds ratios for mental health impairment among children with eczema, adjusting the analysis for potential socioeconomic and demographic confounders. Furthermore, they stratified the primary model by other atopic and behavioral disorders to assess for potential effects modification by these concomitant illnesses.
Approximately 12% of the children in the sample had eczema. Children with eczema tended to be female, non-Hispanic, or black; they also were more likely to report good, fair, or poor health, compared with children without eczema. Asthma, allergic rhinitis, and ADHD were more common among children with eczema than those without.
About 27% of children with eczema had any mental health impairment, compared with approximately 18% of children without eczema. About 11% of children with eczema had severe impairment; this rate was almost twice as high as that in children without eczema, Dr. Wan said. The adjusted odds of mental health impairment were 52% per year among children with eczema, compared with those without.
When the researchers examined specific domains of mental health, they found that children with eczema were significantly less likely to be reported to be well behaved or to have good attention spans. They also were significantly more likely to worry often, be unhappy or depressed, and to get along better with adults than their peers.
When Dr. Wan and his colleagues stratified the primary model by other atopic illnesses, they found that, among children without any other atopic illness, eczema remained independently associated with mental health impairment (OR, 1.52). The effect remained similar among children with asthma alone, but was attenuated among children with allergic rhinitis alone or with asthma and allergic rhinitis.
In the absence of ADHD, the investigators found a statistically significant effect of eczema on mental health impairment (OR, 1.46). In the presence of ADHD, the effect remained significant, but was attenuated.
Finally, approximately 20% of children with mildly impaired mental health had seen a mental health professional in the past year. In addition, 54% of children with severe mental health symptoms had seen a mental health professional in the past year. Among children with severe impairment, about 80% had consulted a general practitioner in the past year; 45% of them reported emotional or behavioral issues as the reason for the visit. Use of special education and early intervention services were more prevalent among children with increasing degrees of mental health impairment.
The study’s strengths include its population-based design, the use of a validated psychometric instrument, and the adjustment of data for socioeconomic factors and other comorbid illnesses, Dr. Wan said. The study is cross sectional, however, which precludes conclusions about the directionality of the relationship between eczema and mental health. In addition, the SDQ may not capture all mental health symptoms that eczema affects.
It is imperative that clinicians and caregivers recognize how common mental health impairment is among children with eczema so that children can be appropriately screened and referred for care, Dr. Wan said. “Our study suggests that there may be a critical gap in mental health services utilization by children who have eczema and concomitant mental health impairment. Some of the future directions in this area may be to understand the potential barriers to mental health care in children with eczema, and certainly to identify potentially effective interventions to reduce the mental health burden in pediatric eczema.”
Dr. Wan reported receiving research fellowship funding from Pfizer.
WASHINGTON – according to an analysis described at the annual meeting of the American Academy of Dermatology. Eczema appears to influence several domains of mental health, and the association remains in the absence of other atopic illnesses.
Estimates of the prevalence of eczema in children have ranged as high as 20%. European and Japanese studies have suggested that children with eczema have greater mental health impairments overall, but researchers have not evaluated this association among U.S. children. Although it has been established that children with eczema consult health care providers more often than children without eczema, data on health care utilization among children with eczema and impaired mental health are limited.
Joy Wan, MD, a dermatologist at Children’s Hospital of Philadelphia, and her colleagues performed a cross-sectional analysis of data obtained from 2013 to 2017 by the National Health Interview Survey. The Centers for Disease Control and Prevention administers the survey to a representative sample of the U.S. population. Children in each household are randomly sampled, and adult caregivers provide detailed health information about them.
Dr. Wan and her colleagues included children aged between 4 and 17 years in their analysis. The exposure of interest was eczema. Caregivers reported eczema in response to the question, “During the past 12 months, has the child had eczema or any kind of skin allergy?” The study’s primary outcome was mental health impairment. Using the Strengths and Difficulties Questionnaire (SDQ), the investigators categorized mental health impairment as none, mild, or severe. The SDQ is a validated instrument that assesses symptoms of mental health in children in domains such as conduct, emotion, peer relationships, and attention, which the researchers chose as secondary outcomes of interest. Dr. Wan’s group also examined the utilization of mental health and other health and social services among children with eczema.
The researchers performed logistic regression analysis to obtain odds ratios for mental health impairment among children with eczema, adjusting the analysis for potential socioeconomic and demographic confounders. Furthermore, they stratified the primary model by other atopic and behavioral disorders to assess for potential effects modification by these concomitant illnesses.
Approximately 12% of the children in the sample had eczema. Children with eczema tended to be female, non-Hispanic, or black; they also were more likely to report good, fair, or poor health, compared with children without eczema. Asthma, allergic rhinitis, and ADHD were more common among children with eczema than those without.
About 27% of children with eczema had any mental health impairment, compared with approximately 18% of children without eczema. About 11% of children with eczema had severe impairment; this rate was almost twice as high as that in children without eczema, Dr. Wan said. The adjusted odds of mental health impairment were 52% per year among children with eczema, compared with those without.
When the researchers examined specific domains of mental health, they found that children with eczema were significantly less likely to be reported to be well behaved or to have good attention spans. They also were significantly more likely to worry often, be unhappy or depressed, and to get along better with adults than their peers.
When Dr. Wan and his colleagues stratified the primary model by other atopic illnesses, they found that, among children without any other atopic illness, eczema remained independently associated with mental health impairment (OR, 1.52). The effect remained similar among children with asthma alone, but was attenuated among children with allergic rhinitis alone or with asthma and allergic rhinitis.
In the absence of ADHD, the investigators found a statistically significant effect of eczema on mental health impairment (OR, 1.46). In the presence of ADHD, the effect remained significant, but was attenuated.
Finally, approximately 20% of children with mildly impaired mental health had seen a mental health professional in the past year. In addition, 54% of children with severe mental health symptoms had seen a mental health professional in the past year. Among children with severe impairment, about 80% had consulted a general practitioner in the past year; 45% of them reported emotional or behavioral issues as the reason for the visit. Use of special education and early intervention services were more prevalent among children with increasing degrees of mental health impairment.
The study’s strengths include its population-based design, the use of a validated psychometric instrument, and the adjustment of data for socioeconomic factors and other comorbid illnesses, Dr. Wan said. The study is cross sectional, however, which precludes conclusions about the directionality of the relationship between eczema and mental health. In addition, the SDQ may not capture all mental health symptoms that eczema affects.
It is imperative that clinicians and caregivers recognize how common mental health impairment is among children with eczema so that children can be appropriately screened and referred for care, Dr. Wan said. “Our study suggests that there may be a critical gap in mental health services utilization by children who have eczema and concomitant mental health impairment. Some of the future directions in this area may be to understand the potential barriers to mental health care in children with eczema, and certainly to identify potentially effective interventions to reduce the mental health burden in pediatric eczema.”
Dr. Wan reported receiving research fellowship funding from Pfizer.
REPORTING FROM AAD 2019