User login
New screening tool identifies asthma risk in toddlers
A symptom-based screening tool can identify 2-year-olds at increased risk of asthma, persistent symptoms of wheeze, and health care burden by the age of 5, according to researchers.
The validated CHILDhood Asthma Risk Tool (CHART) determines high, moderate, or low risk of asthma based on symptoms reported before the age of 3 years. It also recommends follow-up.
Potentially, CHART could be used “to identify children who need monitoring, timely symptom control, and introduction of preventive therapies,” said Padmaja Subbarao, MD, MSc, associate chief of clinical research at the Hospital for Sick Children, Toronto, and colleagues in JAMA Network Open.
“The implementation of CHART as a first-step screening tool in general practice could promote timely treatment control and, in turn, improve quality of life for patients and reduce the clinical and economic burden of asthma,” they wrote.
Dr. Subbarao and colleagues developed CHART using data from parent questionnaires and 3- and 5-year clinic visits in the CHILD study. Children were categorized as “high risk” when they experienced two or more episodes of wheeze annually at both 3 and 5 years of age, concurrent with ED visits, hospitalizations, asthma medication, or frequent dry cough. Children with only cough episodes or with cough episodes plus one episode of wheeze in the past 12 months were categorized as “low risk.”
“Our unique approach to classification of wheeze symptoms is important because it helps busy practitioners identify the smaller subset of children with more frequent or severe wheezing episodes who have a higher probability of continued symptoms and impaired lung function in adult life among most children with infrequent wheeze,” Dr. Sabbarao and coauthors said.
Their diagnostic study to evaluate CHART’s predictive capacity showed that the tool had the highest proportion of true-positive asthma at 5 years (sensitivity, 50.0%), compared with physicians’ diagnosis at 3 years (sensitivity, 43.5%), and positive standardized modified Asthma Predictive Index (mAPI) at 3 years (sensitivity, 24.4%).
CHART also outperformed physician assessments and mAPI for predicting persistent wheeze at 5 years and provided the highest predictive capacity for subsequent health care use at 5 years of age. The study showed that it identified 20% more children with emergency department visits or hospitalizations than the standardized mAPI (sensitivity 45.5% vs. 25.0%), and approximately 10% more at-risk children than physician diagnosis.
“These findings are especially important given that many hospitalizations are avoidable if appropriate treatment and management of asthma are implemented at primary care,” Dr. Subbarao and colleagues wrote.
CHART has been validated in two external cohorts: a general-population cohort of 2,185 children from the Raine Study in Australia at 5 years of age; and the other a high-risk cohort of 349 children from the Canadian Asthma Primary Prevention Study at 7 years of age.
“We want to highlight the importance of periodic monitoring of wheeze symptoms and simplify the identification of high-risk children for primary care providers and parents or caregivers,” said Dr. Subbarao, who is director of the CHILD study and professor of pediatrics at the University of Toronto.
The tool “does not identify the underlying biology, which could impact the efficacy of our current standard asthma treatment,” Dr. Subbarao emphasized. CHART has not been tested in low-prevalence settings or in countries in which the term “wheeze” is not commonly recognized, she added.
“CHART helps you focus your crystal ball a little bit, look into the future, and see what’s going to happen,” said Harold Farber, MD, a pediatric pulmonologist who was not involved in the study. “It’s useful even if it just confirms what I’m already doing clinically.”
Dr. Farber, who is professor of pediatrics at Baylor College of Medicine and the Texas Children’s Hospital, Houston, cautioned that the predictive value of CHART is based on the diagnosis of asthma, and that this can differ across health care communities. “Between the extremes and what’s considered borderline, there’s a lot of diagnostic variation in what we call asthma,” he explained in an interview. “The diagnosis is, to some extent, subjective.”
However, Dr. Farber agreed that two or more wheezing episodes in the past 12 months – enough to require treatment – puts a child at very high risk for future wheezing. “Kids with a bunch of wheezing problems at 3 years are likely to have wheezing problems at 5. We have to think about what we can do for a toddler today to keep him from wheezing later.”
CHART is simple to use, the investigators said. The information needed can be easily gathered through interviews and parent-reported questionnaires, then put into the electronic medical record to flag children at high risk for further investigation, and well as those at low or moderate risk for monitoring.
Parents and caregivers can also use CHART to document symptoms every 6 months in children older than 1 year of age, said Dr. Subbarao. This information can be brought to the attention of the doctor “to facilitate a deeper discussion,” she suggested.
This study was funded by the Canadian Institutes of Health Research, Allergy, Genes and Environment Network of Centers of Excellence; Don and Debbie Morrison; Women’s and Children Health Research Institute; and Canada Research Chairs. Dr Subbarao reported having no potential conflicts of interest. Coauthor Vanessa Breton, PhD, disclosed being employed by F. Hoffmann-La Roche Ltd., and coauthor Elinor Simons, MD, PhD, reported membership on the Sanofi-Genzyme Data Monitoring Board. No other conflicts of interest were reported by the study authors. Dr Farber disclosed having no potential conflicts of interest.
A symptom-based screening tool can identify 2-year-olds at increased risk of asthma, persistent symptoms of wheeze, and health care burden by the age of 5, according to researchers.
The validated CHILDhood Asthma Risk Tool (CHART) determines high, moderate, or low risk of asthma based on symptoms reported before the age of 3 years. It also recommends follow-up.
Potentially, CHART could be used “to identify children who need monitoring, timely symptom control, and introduction of preventive therapies,” said Padmaja Subbarao, MD, MSc, associate chief of clinical research at the Hospital for Sick Children, Toronto, and colleagues in JAMA Network Open.
“The implementation of CHART as a first-step screening tool in general practice could promote timely treatment control and, in turn, improve quality of life for patients and reduce the clinical and economic burden of asthma,” they wrote.
Dr. Subbarao and colleagues developed CHART using data from parent questionnaires and 3- and 5-year clinic visits in the CHILD study. Children were categorized as “high risk” when they experienced two or more episodes of wheeze annually at both 3 and 5 years of age, concurrent with ED visits, hospitalizations, asthma medication, or frequent dry cough. Children with only cough episodes or with cough episodes plus one episode of wheeze in the past 12 months were categorized as “low risk.”
“Our unique approach to classification of wheeze symptoms is important because it helps busy practitioners identify the smaller subset of children with more frequent or severe wheezing episodes who have a higher probability of continued symptoms and impaired lung function in adult life among most children with infrequent wheeze,” Dr. Sabbarao and coauthors said.
Their diagnostic study to evaluate CHART’s predictive capacity showed that the tool had the highest proportion of true-positive asthma at 5 years (sensitivity, 50.0%), compared with physicians’ diagnosis at 3 years (sensitivity, 43.5%), and positive standardized modified Asthma Predictive Index (mAPI) at 3 years (sensitivity, 24.4%).
CHART also outperformed physician assessments and mAPI for predicting persistent wheeze at 5 years and provided the highest predictive capacity for subsequent health care use at 5 years of age. The study showed that it identified 20% more children with emergency department visits or hospitalizations than the standardized mAPI (sensitivity 45.5% vs. 25.0%), and approximately 10% more at-risk children than physician diagnosis.
“These findings are especially important given that many hospitalizations are avoidable if appropriate treatment and management of asthma are implemented at primary care,” Dr. Subbarao and colleagues wrote.
CHART has been validated in two external cohorts: a general-population cohort of 2,185 children from the Raine Study in Australia at 5 years of age; and the other a high-risk cohort of 349 children from the Canadian Asthma Primary Prevention Study at 7 years of age.
“We want to highlight the importance of periodic monitoring of wheeze symptoms and simplify the identification of high-risk children for primary care providers and parents or caregivers,” said Dr. Subbarao, who is director of the CHILD study and professor of pediatrics at the University of Toronto.
The tool “does not identify the underlying biology, which could impact the efficacy of our current standard asthma treatment,” Dr. Subbarao emphasized. CHART has not been tested in low-prevalence settings or in countries in which the term “wheeze” is not commonly recognized, she added.
“CHART helps you focus your crystal ball a little bit, look into the future, and see what’s going to happen,” said Harold Farber, MD, a pediatric pulmonologist who was not involved in the study. “It’s useful even if it just confirms what I’m already doing clinically.”
Dr. Farber, who is professor of pediatrics at Baylor College of Medicine and the Texas Children’s Hospital, Houston, cautioned that the predictive value of CHART is based on the diagnosis of asthma, and that this can differ across health care communities. “Between the extremes and what’s considered borderline, there’s a lot of diagnostic variation in what we call asthma,” he explained in an interview. “The diagnosis is, to some extent, subjective.”
However, Dr. Farber agreed that two or more wheezing episodes in the past 12 months – enough to require treatment – puts a child at very high risk for future wheezing. “Kids with a bunch of wheezing problems at 3 years are likely to have wheezing problems at 5. We have to think about what we can do for a toddler today to keep him from wheezing later.”
CHART is simple to use, the investigators said. The information needed can be easily gathered through interviews and parent-reported questionnaires, then put into the electronic medical record to flag children at high risk for further investigation, and well as those at low or moderate risk for monitoring.
Parents and caregivers can also use CHART to document symptoms every 6 months in children older than 1 year of age, said Dr. Subbarao. This information can be brought to the attention of the doctor “to facilitate a deeper discussion,” she suggested.
This study was funded by the Canadian Institutes of Health Research, Allergy, Genes and Environment Network of Centers of Excellence; Don and Debbie Morrison; Women’s and Children Health Research Institute; and Canada Research Chairs. Dr Subbarao reported having no potential conflicts of interest. Coauthor Vanessa Breton, PhD, disclosed being employed by F. Hoffmann-La Roche Ltd., and coauthor Elinor Simons, MD, PhD, reported membership on the Sanofi-Genzyme Data Monitoring Board. No other conflicts of interest were reported by the study authors. Dr Farber disclosed having no potential conflicts of interest.
A symptom-based screening tool can identify 2-year-olds at increased risk of asthma, persistent symptoms of wheeze, and health care burden by the age of 5, according to researchers.
The validated CHILDhood Asthma Risk Tool (CHART) determines high, moderate, or low risk of asthma based on symptoms reported before the age of 3 years. It also recommends follow-up.
Potentially, CHART could be used “to identify children who need monitoring, timely symptom control, and introduction of preventive therapies,” said Padmaja Subbarao, MD, MSc, associate chief of clinical research at the Hospital for Sick Children, Toronto, and colleagues in JAMA Network Open.
“The implementation of CHART as a first-step screening tool in general practice could promote timely treatment control and, in turn, improve quality of life for patients and reduce the clinical and economic burden of asthma,” they wrote.
Dr. Subbarao and colleagues developed CHART using data from parent questionnaires and 3- and 5-year clinic visits in the CHILD study. Children were categorized as “high risk” when they experienced two or more episodes of wheeze annually at both 3 and 5 years of age, concurrent with ED visits, hospitalizations, asthma medication, or frequent dry cough. Children with only cough episodes or with cough episodes plus one episode of wheeze in the past 12 months were categorized as “low risk.”
“Our unique approach to classification of wheeze symptoms is important because it helps busy practitioners identify the smaller subset of children with more frequent or severe wheezing episodes who have a higher probability of continued symptoms and impaired lung function in adult life among most children with infrequent wheeze,” Dr. Sabbarao and coauthors said.
Their diagnostic study to evaluate CHART’s predictive capacity showed that the tool had the highest proportion of true-positive asthma at 5 years (sensitivity, 50.0%), compared with physicians’ diagnosis at 3 years (sensitivity, 43.5%), and positive standardized modified Asthma Predictive Index (mAPI) at 3 years (sensitivity, 24.4%).
CHART also outperformed physician assessments and mAPI for predicting persistent wheeze at 5 years and provided the highest predictive capacity for subsequent health care use at 5 years of age. The study showed that it identified 20% more children with emergency department visits or hospitalizations than the standardized mAPI (sensitivity 45.5% vs. 25.0%), and approximately 10% more at-risk children than physician diagnosis.
“These findings are especially important given that many hospitalizations are avoidable if appropriate treatment and management of asthma are implemented at primary care,” Dr. Subbarao and colleagues wrote.
CHART has been validated in two external cohorts: a general-population cohort of 2,185 children from the Raine Study in Australia at 5 years of age; and the other a high-risk cohort of 349 children from the Canadian Asthma Primary Prevention Study at 7 years of age.
“We want to highlight the importance of periodic monitoring of wheeze symptoms and simplify the identification of high-risk children for primary care providers and parents or caregivers,” said Dr. Subbarao, who is director of the CHILD study and professor of pediatrics at the University of Toronto.
The tool “does not identify the underlying biology, which could impact the efficacy of our current standard asthma treatment,” Dr. Subbarao emphasized. CHART has not been tested in low-prevalence settings or in countries in which the term “wheeze” is not commonly recognized, she added.
“CHART helps you focus your crystal ball a little bit, look into the future, and see what’s going to happen,” said Harold Farber, MD, a pediatric pulmonologist who was not involved in the study. “It’s useful even if it just confirms what I’m already doing clinically.”
Dr. Farber, who is professor of pediatrics at Baylor College of Medicine and the Texas Children’s Hospital, Houston, cautioned that the predictive value of CHART is based on the diagnosis of asthma, and that this can differ across health care communities. “Between the extremes and what’s considered borderline, there’s a lot of diagnostic variation in what we call asthma,” he explained in an interview. “The diagnosis is, to some extent, subjective.”
However, Dr. Farber agreed that two or more wheezing episodes in the past 12 months – enough to require treatment – puts a child at very high risk for future wheezing. “Kids with a bunch of wheezing problems at 3 years are likely to have wheezing problems at 5. We have to think about what we can do for a toddler today to keep him from wheezing later.”
CHART is simple to use, the investigators said. The information needed can be easily gathered through interviews and parent-reported questionnaires, then put into the electronic medical record to flag children at high risk for further investigation, and well as those at low or moderate risk for monitoring.
Parents and caregivers can also use CHART to document symptoms every 6 months in children older than 1 year of age, said Dr. Subbarao. This information can be brought to the attention of the doctor “to facilitate a deeper discussion,” she suggested.
This study was funded by the Canadian Institutes of Health Research, Allergy, Genes and Environment Network of Centers of Excellence; Don and Debbie Morrison; Women’s and Children Health Research Institute; and Canada Research Chairs. Dr Subbarao reported having no potential conflicts of interest. Coauthor Vanessa Breton, PhD, disclosed being employed by F. Hoffmann-La Roche Ltd., and coauthor Elinor Simons, MD, PhD, reported membership on the Sanofi-Genzyme Data Monitoring Board. No other conflicts of interest were reported by the study authors. Dr Farber disclosed having no potential conflicts of interest.
FROM JAMA NETWORK OPEN
Would your patient benefit from a monoclonal antibody?
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).
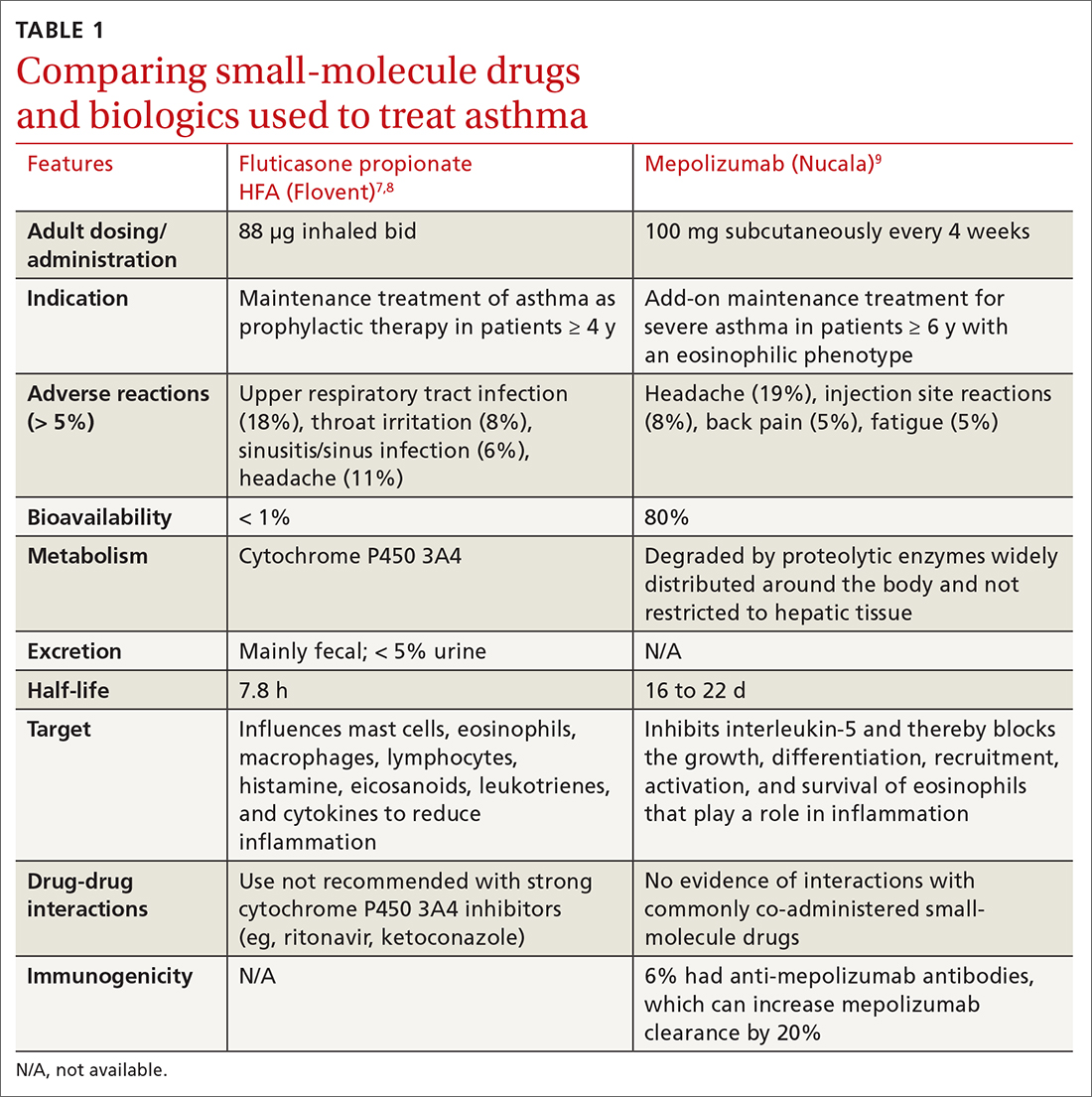
MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46
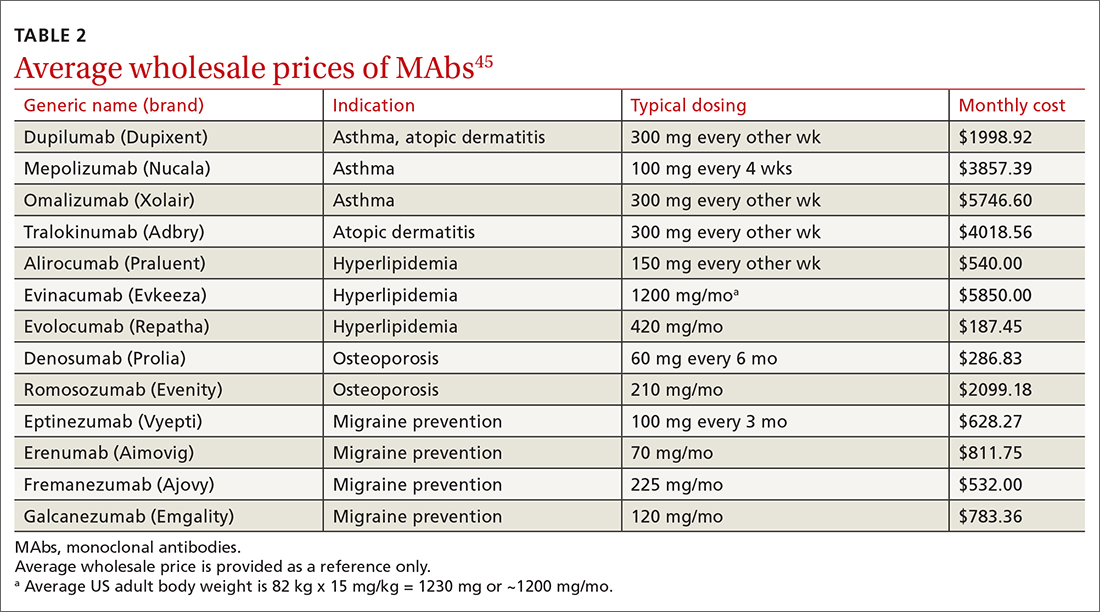
MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; evelyn.sbar@ttuhsc.edu
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; evelyn.sbar@ttuhsc.edu
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; evelyn.sbar@ttuhsc.edu
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
PRACTICE RECOMMENDATIONS
› Consider anti-immunoglobulin E, anti-interleukin 5, or anti-interleukin 4/interleukin 13 for patients with moderate-to-severe asthma and type 2 airway inflammation. B
› Consider dupilumab for patients with moderate-to-severe atopic dermatitis (with or without topical corticosteroids), or when traditional oral therapies are inadequate or contraindicated. B
› Consider proprotein convertase subtilisin/kexin type 9 inhibitors for patients with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease when maximally tolerated statins or ezetimibe have not lowered low-density lipoprotein cholesterol levels far enough. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Childhood peanut allergy linked with other legume allergies
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
French children with peanut allergy tend to have reactions to other legumes, including soy, lentil, pea, bean, lupin, and fenugreek, and those other allergies often lead to anaphylactic reactions, a retrospective study from France reports.
“Among children allergic to peanut, at least two-thirds were sensitized to one other legume, and legume allergy was diagnosed in one-quarter of the sensitized patients,” wrote senior study author Amandine Divaret-Chauveau, MD, of Centre Hospitalier Universitaire de Nancy, Vandoeuvre-les-Nancy, and colleagues. The report is in Pediatric Allergy and Immunology.
People worldwide are eating more legumes these days, the authors noted. High in protein, low in unsaturated fats, with low production costs, legumes are important components of increasingly vegetarian, healthy, sustainable diets.
Food allergens are the most common childhood triggers of allergic reactions. Among children in France, legumes cause 14.6% of food-related anaphylactic reactions, with peanut as the main allergen, they added.
Dr. Divaret-Chauveau and colleagues assessed the prevalence and relevance of sensitization to legumes among all children and adolescents aged 1-17 years who had peanut allergy and had been admitted to one academic pediatric allergy department over roughly 3 years, beginning in early 2017. For the 195 study participants, peanut allergy had been confirmed, and they had been documented to have consumed or to have sensitization to at least one non-peanut legume; 69.7% were boys.
The researchers analyzed data on consumption history, skin prick tests, specific immunoglobulin E status, prior allergic reactions, and oral food challenges for each legume. They found the following:
- Among the 195 children with peanut allergy, 98.4% had at least one other atopic disease.
- Of the 195 children with peanut allergy, 122 (63.9%) were sensitized to at least one other legume. Of these 122 children, 66.3% were sensitized to fenugreek, 42.2% to lentil, 39.9% to soy, and 34.2% to lupin.
- Allergy to one or more legumes was confirmed for 27.9% of the 122 sensitized children, including 4.9% who had multiple legume allergies. Lentil, lupin, and pea were the main allergens.
- Of the 118 children also having a non-legume food allergy, the main food allergens were egg (57.6%), cow’s milk (33.0%), cashew (39.0%), pistachio (23.7%), and hazelnut (30.5%).
- Fifty percent of allergic reactions to non-peanut legumes were severe, often showing as asthma. Atopic comorbidities, including asthma, in most participants may have contributed to the severity of allergic reactions, the authors noted.
Allergy awareness needs to grow with plant-based diets
“The high prevalence of legume sensitization reported in our study highlights the need to explore legume consumption in children with PA [peanut allergy], and the need to investigate sensitization in the absence of consumption,” they added.
Jodi A. Shroba, MSN, APRN, CPNP, coordinator for the Food Allergy Program at Children’s Mercy Kansas City, in Missouri, told this news organization that few data are available in the literature regarding allergies to legumes other than peanut.
“It was interesting that these authors found such a high legume sensitization in their peanut-allergic patients,” Ms. Shroba, who was not involved in the study, said by email. “As more people are starting to eat plant-based diets, it is important that we better understand their allergenicity and cross-reactivity so we can better help guide patient management and education.”
Deborah Albright, MD, assistant professor of pediatrics at the University of Pittsburgh, agreed.
“As plant-based protein consumption broadens worldwide, awareness of the potential for cross-reactivity and co-allergy amongst legumes will become increasingly important,” she said by email.
“However, positive allergy tests do not reliably correlate with true food allergy; therefore, the diagnosis of legume co-allergy should be confirmed by the individual patient’s history, a formal food challenge, or both,” advised Dr. Albright. She was not involved in the study.
“Cross-sensitization to other legumes in patients with a single legume allergy is common; however, true clinical reactivity is often not present,” she added. “Also, legume allergy test sensitization rates and objective reactivity on food challenge can vary by region, depending on diet and pollen aeroallergen exposure.
“Systematic exploration of tolerance versus co-allergy to other legumes should be considered in patients allergic to peanut or other legumes,” Dr. Albright said.
The authors recommend further research and registry data collection of legume anaphylaxis.
Details regarding funding for the study were not provided. The authors, Ms. Shroba, and Dr. Albright report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mindfulness eases asthma burden
Adults with asthma who received mindfulness training showed significant improvement in symptoms compared to those who did not receive such training, based on data from 73 individuals.
Although previous research shows the contribution of psychological factors to poor asthma control and exacerbations, the ability of mindfulness-based stress reduction (MBSR) to improve asthma symptoms in particular has not been well studied, wrote Estelle T. Higgins, BA, of the University of Wisconsin, Madison, and colleagues.
they wrote. The researchers hypothesized that MBSR training would reduce the effect of psychological distress on asthma control and inflammation compared to asthma patients in a waitlist control group.
In a study published in Brain, Behavior, & Immunity – Health, the researchers randomized 38 adults with asthma to a program of MBSR and 24 to a waitlist. The participants ranged in age from 18 to 65 years, with a mean of 38.1 years, and 43 were female. All patients had an asthma diagnosis for at least 6 months; airway inflammation was based on measures of fraction of exhaled nitric oxide (FeNO) ≥ 30 ppb, 138 blood eosinophil count ≥ 150 cells/mcL, or percent sputum eosinophils ≥ 2% of total leukocytes. Individuals with ongoing medical conditions other than asthma were excluded.
The MBSR group had seven clinical data collection visits at approximately 1-month intervals. MBSR training sessions occurred within classes offered to the community over a period of 8 weekly sessions and one 6-hour retreat, and included breath-focused attention, body scan, and mindful awareness in seated positions, walking, and yoga. Participants completed questionnaires about mindfulness, distress, depression, and anxiety symptoms. These were assessed at baseline, post intervention, and at study completion. Chronic stress level was determined at baseline only.
The primary outcome was asthma control based on the Asthma Control Questionnaire 6-item version (ACQ6) Minimally Important Difference.
Overall, asthma control improved significantly among those randomized to MBSR compared to waitlisted controls (P = .01) and this difference persisted at 4 months after the intervention.
Nearly one-third (32%) of the MBSR participants met the criteria for clinically significant improvement in asthma symptoms, compared to 12% of those on the wait list.
In addition, MBSR-related improvement in asthma control was significantly associated with a reduced distress (P = .043), and was especially effective for individuals with the highest levels of depressive symptoms at baseline, the researchers noted. Individuals who received MBSR also showed significantly reduced levels of exhaled nitric oxide compared to waitlist controls (P < .05).
The study findings were limited by the lack of an active control group, the researchers noted. “Though a wait-list control group was employed to control for variation in outcome measures over time, it is possible that effects reported here were driven by factors that are not specific to training in mindfulness, such as social support or expectancy effects,” they wrote. However, the results support the value of mindfulness in reducing psychological stress, FeNO, and impairments related to asthma that were sustained over time, they said. The results also support the potential of MBSR to both augment and reduce the need for pharmacological treatment in asthma, and mindfulness may be an effective way to improve overall disease control by reducing the contribution of psychological factors to asthma morbidity, they concluded.
The study was supported by the National Center for Complementary and Integrative Health. Coauthor Richard J. Davidson, MD, is the founder and president, and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. The researchers had no financial conflicts to disclose.
Adults with asthma who received mindfulness training showed significant improvement in symptoms compared to those who did not receive such training, based on data from 73 individuals.
Although previous research shows the contribution of psychological factors to poor asthma control and exacerbations, the ability of mindfulness-based stress reduction (MBSR) to improve asthma symptoms in particular has not been well studied, wrote Estelle T. Higgins, BA, of the University of Wisconsin, Madison, and colleagues.
they wrote. The researchers hypothesized that MBSR training would reduce the effect of psychological distress on asthma control and inflammation compared to asthma patients in a waitlist control group.
In a study published in Brain, Behavior, & Immunity – Health, the researchers randomized 38 adults with asthma to a program of MBSR and 24 to a waitlist. The participants ranged in age from 18 to 65 years, with a mean of 38.1 years, and 43 were female. All patients had an asthma diagnosis for at least 6 months; airway inflammation was based on measures of fraction of exhaled nitric oxide (FeNO) ≥ 30 ppb, 138 blood eosinophil count ≥ 150 cells/mcL, or percent sputum eosinophils ≥ 2% of total leukocytes. Individuals with ongoing medical conditions other than asthma were excluded.
The MBSR group had seven clinical data collection visits at approximately 1-month intervals. MBSR training sessions occurred within classes offered to the community over a period of 8 weekly sessions and one 6-hour retreat, and included breath-focused attention, body scan, and mindful awareness in seated positions, walking, and yoga. Participants completed questionnaires about mindfulness, distress, depression, and anxiety symptoms. These were assessed at baseline, post intervention, and at study completion. Chronic stress level was determined at baseline only.
The primary outcome was asthma control based on the Asthma Control Questionnaire 6-item version (ACQ6) Minimally Important Difference.
Overall, asthma control improved significantly among those randomized to MBSR compared to waitlisted controls (P = .01) and this difference persisted at 4 months after the intervention.
Nearly one-third (32%) of the MBSR participants met the criteria for clinically significant improvement in asthma symptoms, compared to 12% of those on the wait list.
In addition, MBSR-related improvement in asthma control was significantly associated with a reduced distress (P = .043), and was especially effective for individuals with the highest levels of depressive symptoms at baseline, the researchers noted. Individuals who received MBSR also showed significantly reduced levels of exhaled nitric oxide compared to waitlist controls (P < .05).
The study findings were limited by the lack of an active control group, the researchers noted. “Though a wait-list control group was employed to control for variation in outcome measures over time, it is possible that effects reported here were driven by factors that are not specific to training in mindfulness, such as social support or expectancy effects,” they wrote. However, the results support the value of mindfulness in reducing psychological stress, FeNO, and impairments related to asthma that were sustained over time, they said. The results also support the potential of MBSR to both augment and reduce the need for pharmacological treatment in asthma, and mindfulness may be an effective way to improve overall disease control by reducing the contribution of psychological factors to asthma morbidity, they concluded.
The study was supported by the National Center for Complementary and Integrative Health. Coauthor Richard J. Davidson, MD, is the founder and president, and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. The researchers had no financial conflicts to disclose.
Adults with asthma who received mindfulness training showed significant improvement in symptoms compared to those who did not receive such training, based on data from 73 individuals.
Although previous research shows the contribution of psychological factors to poor asthma control and exacerbations, the ability of mindfulness-based stress reduction (MBSR) to improve asthma symptoms in particular has not been well studied, wrote Estelle T. Higgins, BA, of the University of Wisconsin, Madison, and colleagues.
they wrote. The researchers hypothesized that MBSR training would reduce the effect of psychological distress on asthma control and inflammation compared to asthma patients in a waitlist control group.
In a study published in Brain, Behavior, & Immunity – Health, the researchers randomized 38 adults with asthma to a program of MBSR and 24 to a waitlist. The participants ranged in age from 18 to 65 years, with a mean of 38.1 years, and 43 were female. All patients had an asthma diagnosis for at least 6 months; airway inflammation was based on measures of fraction of exhaled nitric oxide (FeNO) ≥ 30 ppb, 138 blood eosinophil count ≥ 150 cells/mcL, or percent sputum eosinophils ≥ 2% of total leukocytes. Individuals with ongoing medical conditions other than asthma were excluded.
The MBSR group had seven clinical data collection visits at approximately 1-month intervals. MBSR training sessions occurred within classes offered to the community over a period of 8 weekly sessions and one 6-hour retreat, and included breath-focused attention, body scan, and mindful awareness in seated positions, walking, and yoga. Participants completed questionnaires about mindfulness, distress, depression, and anxiety symptoms. These were assessed at baseline, post intervention, and at study completion. Chronic stress level was determined at baseline only.
The primary outcome was asthma control based on the Asthma Control Questionnaire 6-item version (ACQ6) Minimally Important Difference.
Overall, asthma control improved significantly among those randomized to MBSR compared to waitlisted controls (P = .01) and this difference persisted at 4 months after the intervention.
Nearly one-third (32%) of the MBSR participants met the criteria for clinically significant improvement in asthma symptoms, compared to 12% of those on the wait list.
In addition, MBSR-related improvement in asthma control was significantly associated with a reduced distress (P = .043), and was especially effective for individuals with the highest levels of depressive symptoms at baseline, the researchers noted. Individuals who received MBSR also showed significantly reduced levels of exhaled nitric oxide compared to waitlist controls (P < .05).
The study findings were limited by the lack of an active control group, the researchers noted. “Though a wait-list control group was employed to control for variation in outcome measures over time, it is possible that effects reported here were driven by factors that are not specific to training in mindfulness, such as social support or expectancy effects,” they wrote. However, the results support the value of mindfulness in reducing psychological stress, FeNO, and impairments related to asthma that were sustained over time, they said. The results also support the potential of MBSR to both augment and reduce the need for pharmacological treatment in asthma, and mindfulness may be an effective way to improve overall disease control by reducing the contribution of psychological factors to asthma morbidity, they concluded.
The study was supported by the National Center for Complementary and Integrative Health. Coauthor Richard J. Davidson, MD, is the founder and president, and serves on the board of directors for the nonprofit organization, Healthy Minds Innovations Inc. The researchers had no financial conflicts to disclose.
FROM BRAIN, BEHAVIOR, & IMMUNITY – HEALTH
Inhaled, systemic steroids linked to changes in brain structure
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Minimal differences between biologics approved for severe asthma
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Differences in the safety and efficacy between the biologics approved for the treatment of severe eosinophilic asthma are so minimal as to not meet clinically important thresholds, a network meta-analysis shows.
“We know relatively little of the comparative effectiveness or safety of biologics approved for the treatment of asthma [but since] the opportunities to use these biologics will only continue to increase, and we need to know more about their comparative effectiveness to optimize their use,” Ayobami Akenroye, MD, MPH, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston, said in an interview.
“But the decision to use one biologic or not is complex and goes beyond comparative effectiveness, and factors such as insurance coverage, convenience of self-administration, and comorbidities all play a role in the choice of biologics,” she said, adding that all the outcomes assessed in the study contribute to or reflect a patient’s underlying asthma control.
The study was published online in the Journal of Allergy and Clinical Immunology.
Interleukin pathways
Drugs that target various interleukin signaling pathways involved in the pathogenesis of asthma include mepolizumab (Nucala), benralizumab (Fasenra), and dupilumab (Dupixent), all of which have been shown to decrease exacerbation rates, improve lung function, and enhance quality of life for patients with severe eosinophilic asthma. In a Bayesian network meta-analysis that allows for simultaneous comparisons of these three treatments, investigators analyzed eight randomized, placebo-controlled trials that compared each of the drugs with placebo. In total, the trials involved 6,461 patients; the duration of follow-up was between 24 and 56 weeks.
“In the subgroup of patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics were significantly better than placebo in reducing exacerbations,” Dr. Akenroye and colleagues reported. For example, dupilumab reduced the exacerbation risk by 68% at a risk ratio of 0.32 (95% confidence interval, 0.23-0.45), while mepolizumab reduced it by almost as much at 63% (RR, 0.37; 95% CI, 0.30-0.45).
Benralizumab was slightly less effective than the other two biologics, reducing exacerbation risk by 51% (RR, 0.49; 95% CI, 0.43-0.55). “In patients with eosinophil counts of ≥ 300 cells/mcL, all three biologics had a probability of 1 in improving the exacerbation rate by 20% or more ... in comparison to placebo,” the authors wrote.
Regarding each drug’s effect in improving forced expiratory volume in 1 second (FEV1), the mean difference in milliliters with dupilumab before and after treatment was 230 (95% CI, 160-300), while for benralizumab, the MD was 150 (95% CI, 100-220) before and after treatment. With mepolizumab, the MD in FEV1 before and after treatment was also 150. In the same subgroup of patients with eosinophil counts of at least300 cells/mcL, all three biologics again had a probability of 1 in improving FEV1 by 50 mL or more above the placebo effect. A third endpoint that was analyzed was the potential reduction in asthma control questionnaire (ACQ) scores. With mepolizumab, the MD before and after treatment was –0.65 (95% CI, –0.81 to –0.45); with dupilumab, it was –0.48 (95% CI, –0.83 to –0.14); and with dupilumab, it was –0.32 (95% CI, –0.43 to –0.21).
“Dupilumab was significantly better than benralizumab in improving exacerbations,” the authors noted (RR, 0.66; 95% CI, 0.47-0.94), while mepolizumab was also better than benralizumab (RR, 0.75; 95% CI, 0.60-0.95). On the other hand, both dupilumab and benralizumab led to greater improvements in FEV1 than mepolizumab, although the effects of dupilumab and benralizumab on ACQ scores were not significantly different for patients whose lower eosinophil counts were between 150 and 299 cells/mcL.
As for safety outcomes, both mepolizumab and benralizumab were associated with a lower risk of serious adverse events, but dupilumab was not different from placebo in terms of overall safety, according to the authors. “The ultimate choice of biologic for each patient would ... depend on multiple factors including cost considerations and timing of administration.
“[However], these results may be helpful to clinicians as they optimize patient care,” they concluded. Limitations to the analysis include the fact that indirect comparisons cannot replace randomized trials that compare the three drugs directly.
It’s estimated that 5%-10% of the 26 million individuals with asthma in the United States have severe disease.
Dr. Akenroye disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Biologics reduce exacerbations in severe asthma
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY
Mepolizumab curbed corticosteroid use for severe asthma
Use of mepolizumab significantly reduced the need for maintenance oral corticosteroids in adults with severe asthma, based on data from more than 800 individuals.
Many patients with severe asthma require bursts of systemic corticosteroids (SCS) or maintenance oral corticosteroids (mOCS) for disease control, but these strategies are associated with side effects that can increase the disease burden, wrote Charles Pilette, MD, of Cliniques Universitaires Saint-Luc, Brussels, and colleagues.
Previous studies have shown that the humanized, monoclonal anti-interleukin (IL)–5 antibody mepolizumab, which is approved for the treatment of severe asthma, reduced use of SCS and has shown effectiveness in less homogeneous populations, but robust, real-world data on the occurrence and magnitude of these effects are lacking, the researchers said.
In a study known as REALITI-A, the researchers enrolled 822 adults with asthma diagnoses from 82 centers in Europe, Canada, and the United States who initiated mepolizumab at a subcutaneous dose of 100 mg. The study endpoints included daily use of oral corticosteroids at baseline and 1 year, percentage reduction in oral corticosteroid use from baseline, patients discontinuing oral corticosteroids; the primary outcome was the rate of clinically significant exacerbations (CSEs). CSEs were defined as the need for OCS for at least 3 days/parenteral administration, and/or an emergency department or hospital admission before and after treatment. The mean age of the participants was 54 years, 63% were women, and 60% were never-smokers. The mean asthma duration was 19.7 years.
A total of 319 patients (39%), used mOCS at baseline, and dose information was available for 298.
Real-world outcomes
At 1 year, the median mOCS dose in the study population was reduced by 75%, and 64% reduced their mOCS dose by at least 50% from baseline.
In addition, the proportion of patients who discontinued daily mOCS increased from 29% during week 25-28 to 43% during week 53-56.
Overall, 80% of patients remained on mepolizumab at 1 year. Lack of efficacy and patient decision were the top two reasons for discontinuation (6% and 4%, respectively).
The primary outcome of rate of CSE decreased by a clinically significant rate ratio of 0.29 (P < .001).
“The requirement for SCS bursts was also reduced, as observed by a decreased rate of CSEs,” the researchers wrote in their discussion. The results were consistent for patients receiving lower (less than 10 mg/day) or higher (10 mg/day or more) mOCS doses at baseline, they said. No unexpected safety signals were noted during the study period.
“Furthermore, , or those requiring hospitalization or an ER visit, improved symptom control, and lower work productivity and activity impairment,” they added.
The study findings were limited by several factors including the observational design, lack of mepolizumab comparator, and open-label data capture, the researchers noted. However, the results were consistent with similar studies, and support the use of mepolizumab as part of the standard of care for clinically effective disease control in severe asthma patients, they concluded.
The study was funded by GlaxoSmithKline. Lead author Dr. Pilette disclosed fees for advisory boards, speaker meetings, and 42 research grants from GSK, AstraZeneca, Chiesi, Novartis, Teva, and ALK-Abello.
Use of mepolizumab significantly reduced the need for maintenance oral corticosteroids in adults with severe asthma, based on data from more than 800 individuals.
Many patients with severe asthma require bursts of systemic corticosteroids (SCS) or maintenance oral corticosteroids (mOCS) for disease control, but these strategies are associated with side effects that can increase the disease burden, wrote Charles Pilette, MD, of Cliniques Universitaires Saint-Luc, Brussels, and colleagues.
Previous studies have shown that the humanized, monoclonal anti-interleukin (IL)–5 antibody mepolizumab, which is approved for the treatment of severe asthma, reduced use of SCS and has shown effectiveness in less homogeneous populations, but robust, real-world data on the occurrence and magnitude of these effects are lacking, the researchers said.
In a study known as REALITI-A, the researchers enrolled 822 adults with asthma diagnoses from 82 centers in Europe, Canada, and the United States who initiated mepolizumab at a subcutaneous dose of 100 mg. The study endpoints included daily use of oral corticosteroids at baseline and 1 year, percentage reduction in oral corticosteroid use from baseline, patients discontinuing oral corticosteroids; the primary outcome was the rate of clinically significant exacerbations (CSEs). CSEs were defined as the need for OCS for at least 3 days/parenteral administration, and/or an emergency department or hospital admission before and after treatment. The mean age of the participants was 54 years, 63% were women, and 60% were never-smokers. The mean asthma duration was 19.7 years.
A total of 319 patients (39%), used mOCS at baseline, and dose information was available for 298.
Real-world outcomes
At 1 year, the median mOCS dose in the study population was reduced by 75%, and 64% reduced their mOCS dose by at least 50% from baseline.
In addition, the proportion of patients who discontinued daily mOCS increased from 29% during week 25-28 to 43% during week 53-56.
Overall, 80% of patients remained on mepolizumab at 1 year. Lack of efficacy and patient decision were the top two reasons for discontinuation (6% and 4%, respectively).
The primary outcome of rate of CSE decreased by a clinically significant rate ratio of 0.29 (P < .001).
“The requirement for SCS bursts was also reduced, as observed by a decreased rate of CSEs,” the researchers wrote in their discussion. The results were consistent for patients receiving lower (less than 10 mg/day) or higher (10 mg/day or more) mOCS doses at baseline, they said. No unexpected safety signals were noted during the study period.
“Furthermore, , or those requiring hospitalization or an ER visit, improved symptom control, and lower work productivity and activity impairment,” they added.
The study findings were limited by several factors including the observational design, lack of mepolizumab comparator, and open-label data capture, the researchers noted. However, the results were consistent with similar studies, and support the use of mepolizumab as part of the standard of care for clinically effective disease control in severe asthma patients, they concluded.
The study was funded by GlaxoSmithKline. Lead author Dr. Pilette disclosed fees for advisory boards, speaker meetings, and 42 research grants from GSK, AstraZeneca, Chiesi, Novartis, Teva, and ALK-Abello.
Use of mepolizumab significantly reduced the need for maintenance oral corticosteroids in adults with severe asthma, based on data from more than 800 individuals.
Many patients with severe asthma require bursts of systemic corticosteroids (SCS) or maintenance oral corticosteroids (mOCS) for disease control, but these strategies are associated with side effects that can increase the disease burden, wrote Charles Pilette, MD, of Cliniques Universitaires Saint-Luc, Brussels, and colleagues.
Previous studies have shown that the humanized, monoclonal anti-interleukin (IL)–5 antibody mepolizumab, which is approved for the treatment of severe asthma, reduced use of SCS and has shown effectiveness in less homogeneous populations, but robust, real-world data on the occurrence and magnitude of these effects are lacking, the researchers said.
In a study known as REALITI-A, the researchers enrolled 822 adults with asthma diagnoses from 82 centers in Europe, Canada, and the United States who initiated mepolizumab at a subcutaneous dose of 100 mg. The study endpoints included daily use of oral corticosteroids at baseline and 1 year, percentage reduction in oral corticosteroid use from baseline, patients discontinuing oral corticosteroids; the primary outcome was the rate of clinically significant exacerbations (CSEs). CSEs were defined as the need for OCS for at least 3 days/parenteral administration, and/or an emergency department or hospital admission before and after treatment. The mean age of the participants was 54 years, 63% were women, and 60% were never-smokers. The mean asthma duration was 19.7 years.
A total of 319 patients (39%), used mOCS at baseline, and dose information was available for 298.
Real-world outcomes
At 1 year, the median mOCS dose in the study population was reduced by 75%, and 64% reduced their mOCS dose by at least 50% from baseline.
In addition, the proportion of patients who discontinued daily mOCS increased from 29% during week 25-28 to 43% during week 53-56.
Overall, 80% of patients remained on mepolizumab at 1 year. Lack of efficacy and patient decision were the top two reasons for discontinuation (6% and 4%, respectively).
The primary outcome of rate of CSE decreased by a clinically significant rate ratio of 0.29 (P < .001).
“The requirement for SCS bursts was also reduced, as observed by a decreased rate of CSEs,” the researchers wrote in their discussion. The results were consistent for patients receiving lower (less than 10 mg/day) or higher (10 mg/day or more) mOCS doses at baseline, they said. No unexpected safety signals were noted during the study period.
“Furthermore, , or those requiring hospitalization or an ER visit, improved symptom control, and lower work productivity and activity impairment,” they added.
The study findings were limited by several factors including the observational design, lack of mepolizumab comparator, and open-label data capture, the researchers noted. However, the results were consistent with similar studies, and support the use of mepolizumab as part of the standard of care for clinically effective disease control in severe asthma patients, they concluded.
The study was funded by GlaxoSmithKline. Lead author Dr. Pilette disclosed fees for advisory boards, speaker meetings, and 42 research grants from GSK, AstraZeneca, Chiesi, Novartis, Teva, and ALK-Abello.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Frequent asthma deteriorations? Check for bronchiectasis
, according to the authors of a retrospective study in the Journal of Allergy and Clinical Immunology: In Practice. While bronchiectasis is known to worsen the clinical and functional outcomes in patients with asthma, data regarding the long-term effects of bronchiectasis on the clinical course of asthma have been limited, stated corresponding author Jung-Kyu Lee, MD, division of pulmonary and critical care medicine, Seoul (Republic of Korea) Metropolitan Government – Seoul National University.
Moderate to severe acute clinical deterioration risks were increased among the 251 patients (mean age 66.6 years, 77.2% men) with bronchiectasis out of 667 asthma patients included in the study. All studied patients underwent chest computed tomography and pulmonary function tests from 2013 to 2019 at two tertiary hospitals in Seoul. The primary outcome, annual incidence of moderate to severe acute exacerbations requiring additional treatment (systemic steroids, antibiotics, or both), was significantly higher in patients with bronchiectasis after a mean follow-up period of 3.96 years. Compared with patients who did not exhibit bronchiectasis, the annual rates of severe exacerbations (0.15 ± 0.43 vs. 0.08 ± 0.27; P = .010), moderate to severe (0.47 ± 0.79 vs. 0.34 ± 0.63; P = .018), and acute exacerbations during the follow-up period (49.8% vs. 39.4%; P = .009) were all significantly higher. There was no difference in the proportion of frequent exacerbators between the two groups, however. Severe acute exacerbations leading to hospitalizations, also, were more frequent in the group with bronchiectasis.
Risk factors explored
Significant factors conferring greater risk of severe and moderate to severe acute exacerbations in multivariable analysis included low body mass index, low baseline forced expiratory volume in 1 second (FEV1), high use of inhaled corticosteroids, high medication possession, and high neutrophil/lymphocyte ratios. The existence of bronchiectasis remained an independent risk factor for severe and moderate to severe acute exacerbations despite adjustment for all other factors. While bronchiectasis score showed no association with annual rate of acute exacerbation, progression of bronchiectasis confirmed on follow-up CT was associated with increased risks of severe and moderate to severe acute exacerbation.
Included patients had a diagnosis of asthma confirmed by variable expiratory airflow limitation with pulmonary function tests (that is, positive bronchodilator response, positive bronchial provocation test, or excessive variation in lung function between visits). Past histories of tuberculosis and nontuberculous mycobacterial lung disease, lower absolute and predicted values of both baseline FEV1 and forced vital capacity were more common among patients with bronchiectasis.
Dividing the study population into a group that had at least one moderate to severe acute exacerbation during the follow-up period and a group that did not, the researchers identified characteristics shared by exacerbators: a greater proportion were women, they had lower forced vital capacity and lung-diffusing capacity for carbon monoxide, higher blood FVC and blood neutrophil/lymphocyte ratio, and more medication use (inhaled corticosteroids, long-acting antimuscarinic agent, leukotriene-receptor antagonist, and methylxanthine), compared with the nonexacerbators. More bronchiectasis, more severe bronchiectasis (higher bronchiectasis score), and more progression of bronchiectasis were common among the exacerbators.
Higher acute exacerbation risks accompanied bronchiectasis, at 1.47-fold for moderate, 1.72-fold for severe, and 1.50-fold for moderate to severe exacerbations. Higher risk for severe and moderate to severe exacerbations was conferred by bronchiectasis progression, also.
The researchers pointed to contradictory effects of inhaled corticosteroid use, noting both corticosteroids’ essential role in controlling airway inflammation and hyperresponsiveness, exacerbations, and lung-function decline in asthma patients and that longer or greater inhaled corticosteroid use is associated with both clinical deterioration in asthma and bronchiectasis, and exacerbation history. For bronchiectasis, however, inhaled corticosteroid use offers no benefit while increasing susceptibility to infection and its risks through partial immunosuppression.
“Considering these contradictory effects of inhaled corticosteroid use, further research is needed regarding its risks and benefits in asthma patients with bronchiectasis, including differences in the benefit of inhaled corticosteroid use according to patient phenotype,” Dr. Kim and his colleagues concluded.
The role of corticosteroids
“One of the more important points discussed in this observational cohort study is the role of inhaled corticosteroid use in bronchiectasis,” said Mary Jo Farmer, MD, PhD, director of pulmonary hypertension services, Baystate Health, and assistant professor of medicine, University of Massachusetts – Baystate, both in Springfield, in an interview with this news organization. She cited a review finding no significant benefit versus placebo in spirometry, exacerbation rate, or sputum volume in the Cochrane Database of Systematic Reviews and another suggesting that quality of life was improved with inhaled corticosteroid use in individuals with blood eosinophils greater than 3%, compared with those not using inhaled corticosteroids or having lower eosinophil counts in the European Respiratory Journal. She cited also higher percentages (48% versus 23%) of adrenal insufficiency in bronchiectasis patients among those taking inhaled corticosteroids versus those not taking them.
Dr. Farmer added, “According to the 2018 Cochrane review of inhaled corticosteroid treatment for non–cystic fibrosis bronchiectasis, results from most randomized, placebo-controlled trials have been disappointing in terms of effects on most endpoints such as pulmonary function and exacerbation frequency. As such, the European Respiratory Society guidelines for the management of adult bronchiectasis advise against prescribing inhaled corticosteroids to patients with bronchiectasis, unless otherwise indicated by either an asthma or chronic obstructive pulmonary disease diagnosis. Also, inhaled corticosteroid treatment in asthma and COPD is associated with common side effects such as oral candidiasis, dysphonia and, in some cases, systemic corticosteroid effects. The rate of adverse events from inhaled corticosteroid treatment of bronchiectasis, however, is largely unknown.Dr. Lee and Dr. Farmer reported no relevant financial relationships. The study was independently supported.
, according to the authors of a retrospective study in the Journal of Allergy and Clinical Immunology: In Practice. While bronchiectasis is known to worsen the clinical and functional outcomes in patients with asthma, data regarding the long-term effects of bronchiectasis on the clinical course of asthma have been limited, stated corresponding author Jung-Kyu Lee, MD, division of pulmonary and critical care medicine, Seoul (Republic of Korea) Metropolitan Government – Seoul National University.
Moderate to severe acute clinical deterioration risks were increased among the 251 patients (mean age 66.6 years, 77.2% men) with bronchiectasis out of 667 asthma patients included in the study. All studied patients underwent chest computed tomography and pulmonary function tests from 2013 to 2019 at two tertiary hospitals in Seoul. The primary outcome, annual incidence of moderate to severe acute exacerbations requiring additional treatment (systemic steroids, antibiotics, or both), was significantly higher in patients with bronchiectasis after a mean follow-up period of 3.96 years. Compared with patients who did not exhibit bronchiectasis, the annual rates of severe exacerbations (0.15 ± 0.43 vs. 0.08 ± 0.27; P = .010), moderate to severe (0.47 ± 0.79 vs. 0.34 ± 0.63; P = .018), and acute exacerbations during the follow-up period (49.8% vs. 39.4%; P = .009) were all significantly higher. There was no difference in the proportion of frequent exacerbators between the two groups, however. Severe acute exacerbations leading to hospitalizations, also, were more frequent in the group with bronchiectasis.
Risk factors explored
Significant factors conferring greater risk of severe and moderate to severe acute exacerbations in multivariable analysis included low body mass index, low baseline forced expiratory volume in 1 second (FEV1), high use of inhaled corticosteroids, high medication possession, and high neutrophil/lymphocyte ratios. The existence of bronchiectasis remained an independent risk factor for severe and moderate to severe acute exacerbations despite adjustment for all other factors. While bronchiectasis score showed no association with annual rate of acute exacerbation, progression of bronchiectasis confirmed on follow-up CT was associated with increased risks of severe and moderate to severe acute exacerbation.
Included patients had a diagnosis of asthma confirmed by variable expiratory airflow limitation with pulmonary function tests (that is, positive bronchodilator response, positive bronchial provocation test, or excessive variation in lung function between visits). Past histories of tuberculosis and nontuberculous mycobacterial lung disease, lower absolute and predicted values of both baseline FEV1 and forced vital capacity were more common among patients with bronchiectasis.
Dividing the study population into a group that had at least one moderate to severe acute exacerbation during the follow-up period and a group that did not, the researchers identified characteristics shared by exacerbators: a greater proportion were women, they had lower forced vital capacity and lung-diffusing capacity for carbon monoxide, higher blood FVC and blood neutrophil/lymphocyte ratio, and more medication use (inhaled corticosteroids, long-acting antimuscarinic agent, leukotriene-receptor antagonist, and methylxanthine), compared with the nonexacerbators. More bronchiectasis, more severe bronchiectasis (higher bronchiectasis score), and more progression of bronchiectasis were common among the exacerbators.
Higher acute exacerbation risks accompanied bronchiectasis, at 1.47-fold for moderate, 1.72-fold for severe, and 1.50-fold for moderate to severe exacerbations. Higher risk for severe and moderate to severe exacerbations was conferred by bronchiectasis progression, also.
The researchers pointed to contradictory effects of inhaled corticosteroid use, noting both corticosteroids’ essential role in controlling airway inflammation and hyperresponsiveness, exacerbations, and lung-function decline in asthma patients and that longer or greater inhaled corticosteroid use is associated with both clinical deterioration in asthma and bronchiectasis, and exacerbation history. For bronchiectasis, however, inhaled corticosteroid use offers no benefit while increasing susceptibility to infection and its risks through partial immunosuppression.
“Considering these contradictory effects of inhaled corticosteroid use, further research is needed regarding its risks and benefits in asthma patients with bronchiectasis, including differences in the benefit of inhaled corticosteroid use according to patient phenotype,” Dr. Kim and his colleagues concluded.
The role of corticosteroids
“One of the more important points discussed in this observational cohort study is the role of inhaled corticosteroid use in bronchiectasis,” said Mary Jo Farmer, MD, PhD, director of pulmonary hypertension services, Baystate Health, and assistant professor of medicine, University of Massachusetts – Baystate, both in Springfield, in an interview with this news organization. She cited a review finding no significant benefit versus placebo in spirometry, exacerbation rate, or sputum volume in the Cochrane Database of Systematic Reviews and another suggesting that quality of life was improved with inhaled corticosteroid use in individuals with blood eosinophils greater than 3%, compared with those not using inhaled corticosteroids or having lower eosinophil counts in the European Respiratory Journal. She cited also higher percentages (48% versus 23%) of adrenal insufficiency in bronchiectasis patients among those taking inhaled corticosteroids versus those not taking them.
Dr. Farmer added, “According to the 2018 Cochrane review of inhaled corticosteroid treatment for non–cystic fibrosis bronchiectasis, results from most randomized, placebo-controlled trials have been disappointing in terms of effects on most endpoints such as pulmonary function and exacerbation frequency. As such, the European Respiratory Society guidelines for the management of adult bronchiectasis advise against prescribing inhaled corticosteroids to patients with bronchiectasis, unless otherwise indicated by either an asthma or chronic obstructive pulmonary disease diagnosis. Also, inhaled corticosteroid treatment in asthma and COPD is associated with common side effects such as oral candidiasis, dysphonia and, in some cases, systemic corticosteroid effects. The rate of adverse events from inhaled corticosteroid treatment of bronchiectasis, however, is largely unknown.Dr. Lee and Dr. Farmer reported no relevant financial relationships. The study was independently supported.
, according to the authors of a retrospective study in the Journal of Allergy and Clinical Immunology: In Practice. While bronchiectasis is known to worsen the clinical and functional outcomes in patients with asthma, data regarding the long-term effects of bronchiectasis on the clinical course of asthma have been limited, stated corresponding author Jung-Kyu Lee, MD, division of pulmonary and critical care medicine, Seoul (Republic of Korea) Metropolitan Government – Seoul National University.
Moderate to severe acute clinical deterioration risks were increased among the 251 patients (mean age 66.6 years, 77.2% men) with bronchiectasis out of 667 asthma patients included in the study. All studied patients underwent chest computed tomography and pulmonary function tests from 2013 to 2019 at two tertiary hospitals in Seoul. The primary outcome, annual incidence of moderate to severe acute exacerbations requiring additional treatment (systemic steroids, antibiotics, or both), was significantly higher in patients with bronchiectasis after a mean follow-up period of 3.96 years. Compared with patients who did not exhibit bronchiectasis, the annual rates of severe exacerbations (0.15 ± 0.43 vs. 0.08 ± 0.27; P = .010), moderate to severe (0.47 ± 0.79 vs. 0.34 ± 0.63; P = .018), and acute exacerbations during the follow-up period (49.8% vs. 39.4%; P = .009) were all significantly higher. There was no difference in the proportion of frequent exacerbators between the two groups, however. Severe acute exacerbations leading to hospitalizations, also, were more frequent in the group with bronchiectasis.
Risk factors explored
Significant factors conferring greater risk of severe and moderate to severe acute exacerbations in multivariable analysis included low body mass index, low baseline forced expiratory volume in 1 second (FEV1), high use of inhaled corticosteroids, high medication possession, and high neutrophil/lymphocyte ratios. The existence of bronchiectasis remained an independent risk factor for severe and moderate to severe acute exacerbations despite adjustment for all other factors. While bronchiectasis score showed no association with annual rate of acute exacerbation, progression of bronchiectasis confirmed on follow-up CT was associated with increased risks of severe and moderate to severe acute exacerbation.
Included patients had a diagnosis of asthma confirmed by variable expiratory airflow limitation with pulmonary function tests (that is, positive bronchodilator response, positive bronchial provocation test, or excessive variation in lung function between visits). Past histories of tuberculosis and nontuberculous mycobacterial lung disease, lower absolute and predicted values of both baseline FEV1 and forced vital capacity were more common among patients with bronchiectasis.
Dividing the study population into a group that had at least one moderate to severe acute exacerbation during the follow-up period and a group that did not, the researchers identified characteristics shared by exacerbators: a greater proportion were women, they had lower forced vital capacity and lung-diffusing capacity for carbon monoxide, higher blood FVC and blood neutrophil/lymphocyte ratio, and more medication use (inhaled corticosteroids, long-acting antimuscarinic agent, leukotriene-receptor antagonist, and methylxanthine), compared with the nonexacerbators. More bronchiectasis, more severe bronchiectasis (higher bronchiectasis score), and more progression of bronchiectasis were common among the exacerbators.
Higher acute exacerbation risks accompanied bronchiectasis, at 1.47-fold for moderate, 1.72-fold for severe, and 1.50-fold for moderate to severe exacerbations. Higher risk for severe and moderate to severe exacerbations was conferred by bronchiectasis progression, also.
The researchers pointed to contradictory effects of inhaled corticosteroid use, noting both corticosteroids’ essential role in controlling airway inflammation and hyperresponsiveness, exacerbations, and lung-function decline in asthma patients and that longer or greater inhaled corticosteroid use is associated with both clinical deterioration in asthma and bronchiectasis, and exacerbation history. For bronchiectasis, however, inhaled corticosteroid use offers no benefit while increasing susceptibility to infection and its risks through partial immunosuppression.
“Considering these contradictory effects of inhaled corticosteroid use, further research is needed regarding its risks and benefits in asthma patients with bronchiectasis, including differences in the benefit of inhaled corticosteroid use according to patient phenotype,” Dr. Kim and his colleagues concluded.
The role of corticosteroids
“One of the more important points discussed in this observational cohort study is the role of inhaled corticosteroid use in bronchiectasis,” said Mary Jo Farmer, MD, PhD, director of pulmonary hypertension services, Baystate Health, and assistant professor of medicine, University of Massachusetts – Baystate, both in Springfield, in an interview with this news organization. She cited a review finding no significant benefit versus placebo in spirometry, exacerbation rate, or sputum volume in the Cochrane Database of Systematic Reviews and another suggesting that quality of life was improved with inhaled corticosteroid use in individuals with blood eosinophils greater than 3%, compared with those not using inhaled corticosteroids or having lower eosinophil counts in the European Respiratory Journal. She cited also higher percentages (48% versus 23%) of adrenal insufficiency in bronchiectasis patients among those taking inhaled corticosteroids versus those not taking them.
Dr. Farmer added, “According to the 2018 Cochrane review of inhaled corticosteroid treatment for non–cystic fibrosis bronchiectasis, results from most randomized, placebo-controlled trials have been disappointing in terms of effects on most endpoints such as pulmonary function and exacerbation frequency. As such, the European Respiratory Society guidelines for the management of adult bronchiectasis advise against prescribing inhaled corticosteroids to patients with bronchiectasis, unless otherwise indicated by either an asthma or chronic obstructive pulmonary disease diagnosis. Also, inhaled corticosteroid treatment in asthma and COPD is associated with common side effects such as oral candidiasis, dysphonia and, in some cases, systemic corticosteroid effects. The rate of adverse events from inhaled corticosteroid treatment of bronchiectasis, however, is largely unknown.Dr. Lee and Dr. Farmer reported no relevant financial relationships. The study was independently supported.
FROM JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY: IN PRACTICE
Antibiotics during pregnancy may increase child’s risk for asthma and other atopic diseases
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a systematic review and meta-analysis reports.
“Antibiotic use during pregnancy is significantly associated with the development of asthma in children. Additionally prenatal antibiotic exposure is also associated with disorders present in the atopic march including atopic sensitization, dermatitis/eczema, food allergy, allergic rhinitis, and wheeze,” lead study author Alissa Cait, PhD, of Malaghan Institute of Medical Research in Wellington, New Zealand, and colleagues write in Allergy.
“Antibiotics account for 80% of prescribed medications during pregnancy, and it is estimated that 20%-25% of pregnant women receive at least one course of an antibiotic during this time period,” they add.
The researchers evaluated prenatal antibiotic exposure and the risk for childhood wheeze or asthma, as well as for diseases associated with the atopic march, by searching standard medical databases for controlled trials in English, German, French, Dutch, or Arabic involving the use of any antibiotic at any time during pregnancy and for atopic disease incidence in children with asthma or wheeze as primary outcome. They excluded reviews, preclinical data, and descriptive studies.
From the 6,060 citations the search returned, 11 prospective and 16 retrospective studies met the authors’ selection criteria. For each study, they evaluated risk of bias using the Newcastle-Ottawa Quality Assessment Scale, and they rated certainty of the evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) protocol.
The studies, published between 2002 and 2020, were conducted in Europe, North America, Asia, and South America. Exposure to antibiotics during the prenatal period was assessed through unsupervised questionnaires, interviews by medical professionals, or extraction from official medical databases.
The results showed that:
- Antibiotic use during pregnancy was linked with increased relative risk of developing wheeze (relative risk, 1.51; 95% confidence interval, 1.17-1.94) or asthma (RR, 1.28; 95% CI, 1.22-1.34) during childhood.
- Antibiotic use during pregnancy also increased a child’s risk for eczema or dermatitis (RR, 1.28; 95% CI, 1.06-1.53) and allergic rhinitis (RR, 1.13; 95% CI, 1.02-1.25).
- Food allergy increased in one study (RR, 1.81; 95% CI, 1.11-2.95).
Quality of studies
“These results have importance for antibiotic stewardship throughout the prenatal period,” the authors write. However, due to issues including high heterogeneity, publication bias, and lack of population numbers in some studies, the overall quality of the evidence presented in the studies was low. Other limitations include mainly White and European study populations, underpowered studies, and study protocol inconsistencies.
“Though there is evidence that antibiotic treatment during pregnancy is a driver of the atopic march, due to a large heterogeneity between studies more research is needed to draw firm conclusions on this matter,” the authors add. “Future studies should employ and report more direct and objective measurement methods rather than self-reported questionnaires.”
Dustin D. Flannery, DO, MSCE, a neonatologist and clinical researcher in perinatal infectious diseases and neonatal antimicrobial resistance and stewardship at Children’s Hospital of Philadelphia, said in an email that the study was well done.
He noted, though, that “although the study reports an association, it cannot prove causation. The relationship between prenatal antibiotics and childhood allergic disorders is likely multifactorial and quite complex.”
He joins the authors in recommending further related research. “Due to the variation in how exposures and outcomes were defined across the studies, more rigorous research will be needed in this area.”
Despite the study’s limitations, “given that some studies have found associations between prenatal antibiotic exposure and childhood atopic and allergic disorders, including asthma, while other studies have not, this systematic review and meta-analysis asks an important question,” Dr. Flannery, who was not involved in the study, said in an interview.
“Investigators found a strong association between prenatal antibiotic exposure and risk of childhood asthma and other disorders,” he said. “This finding supports efforts to safely reduce antibiotic use during pregnancy.”
The study was supported by the Deutsche Forschungsgemeinschaft and by the Konrad Adenauer Foundation. The authors and Dr. Flannery have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALLERGY