User login
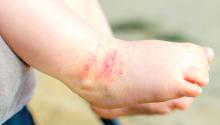
Parental atopic dermatitis, asthma linked to risk of AD in offspring
of life, an analysis of a large birth cohort found.
“The prevalence of AD in children has increased dramatically in recent years, and most studies reporting the impact of parental atopic history on AD are based on older data,” wrote the study authors, led by Cathal O’Connor, MD. “Given the recent interest in early intervention to prevent AD and other allergic diseases, enhanced early identification of infants at risk of AD is increasingly important.”
The detailed analysis of AD risk associated with parental atopy in early life “may help to risk stratify infants to optimize early interventions for prevention or early treatment of AD,” they wrote.
The study was published in Pediatric Dermatology.
For the analysis, Dr. O’Connor of the department of pediatrics and child health at University College Cork (Ireland) and colleagues conducted a secondary analysis of the Cork Babies After Scope: Evaluating the Longitudinal Impact Using Neurological and Nutritional Endpoints (BASELINE) Birth Cohort Study.
The study recruited 2,183 healthy first-born babies between August 2009 and October 2011 to examine the effects of environmental factors during pregnancy and infancy on childhood health and development. Skin barrier assessments were performed at birth, 2 months, 6 months, 12 months, and 24 months using a validated open chamber system to measure transepidermal water loss.
Parental atopy was self-reported at 2 months. Parents were asked at 2 months if the infant had an “itchy rash on the face or in the folds of the arms or legs,” as a screening question for AD. Experienced health care personnel used UK Working Party criteria to diagnose AD at 6, 12, and 24 months.
Complete data on AD status was available for 1,505 children in the cohort. Dr. O’Connor and colleagues calculated an overall AD prevalence of 18.6% at 6 months, 15.2% at 12 months, and 16.5% at 24 months.
Overall prevalence of AD was highest at 6 months. The study showed a similar or slightly higher impact of paternal atopy on offspring AD development, compared to maternal atopy.
Multivariable logistic regression analysis revealed that the odds of AD were 1.57 at 6 months and 1.66 at 12 months for maternal AD; 1.90 at 6 months and 1.85 at 24 months for paternal AD; 1.76 at 6 months and 1.75 at 12 months for maternal asthma; and 1.70 at 6 months, 1.86 at 12 months, and 1.99 at 24 months for paternal asthma.
“Parental allergic rhinitis was not associated with AD in offspring in the first 2 years, except for maternal rhinitis at 24 months [an adjusted odds ratio of 1.79],” the authors wrote. “The genetic predisposition to allergic rhinitis, given the key role of aeroallergen sensitization in its pathogenesis, may not be associated with early onset AD, but may have a greater impact in later onset or persistent AD.”
The authors acknowledged certain limitations of the study, including the fact that it was a secondary data analysis, and that parental AD, asthma, and rhinitis were self-reported, “which may reduce reliability and may contribute to the differences seen between the impact of maternal and paternal reported atopy on offspring,” they wrote. “Data on siblings were not captured, as participants in the study were first-born children. Filaggrin mutational analysis was not performed, which would have provided richer detail.”
Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, who was asked to comment on the work, said that the study confirms the well-known association between parental atopy and the risk of atopy in offspring, which has been shown in several studies dating back decades.
“The authors try to parse risk based on maternal or paternal or biparental history of AD and/or asthma and/or rhinitis, but this type of nuanced analysis when diagnosis is based solely on parental report may be an over-reach,” she said.
“Given that this data supports the association between parental atopy and risk of AD in infants at various time points, the clinically relevant immediate next question is how can we leverage this knowledge to prevent onset of AD in infants at risk?” she said. “To date, interventions such as early introduction of emollients have been evaluated with mixed results.”
A recent Cochrane analysis concluded that, based on available data, skin care interventions such as emollient use during the first year of life in otherwise healthy infants is probably not effective for preventing eczema and may increase risk of skin infection.
“Effects of skin care interventions on risk of asthma are also uncertain,” said Dr. Cordoro, who is also chief of the division of pediatric dermatology at UCSF.
“In sum, this study offers additional data in support of the link between atopy in parents and offspring,” she said. “Understanding how to mitigate risk and prevent atopy requires unraveling of the complex interplay between genetic, environmental, immunologic, microbial and other factors. For now, dermatologists are unable to make broad evidence-based recommendations for otherwise healthy (i.e., with normal skin) but at-risk infants in terms of approaches to skin care that might prevent eczema and asthma.”
of life, an analysis of a large birth cohort found.
“The prevalence of AD in children has increased dramatically in recent years, and most studies reporting the impact of parental atopic history on AD are based on older data,” wrote the study authors, led by Cathal O’Connor, MD. “Given the recent interest in early intervention to prevent AD and other allergic diseases, enhanced early identification of infants at risk of AD is increasingly important.”
The detailed analysis of AD risk associated with parental atopy in early life “may help to risk stratify infants to optimize early interventions for prevention or early treatment of AD,” they wrote.
The study was published in Pediatric Dermatology.
For the analysis, Dr. O’Connor of the department of pediatrics and child health at University College Cork (Ireland) and colleagues conducted a secondary analysis of the Cork Babies After Scope: Evaluating the Longitudinal Impact Using Neurological and Nutritional Endpoints (BASELINE) Birth Cohort Study.
The study recruited 2,183 healthy first-born babies between August 2009 and October 2011 to examine the effects of environmental factors during pregnancy and infancy on childhood health and development. Skin barrier assessments were performed at birth, 2 months, 6 months, 12 months, and 24 months using a validated open chamber system to measure transepidermal water loss.
Parental atopy was self-reported at 2 months. Parents were asked at 2 months if the infant had an “itchy rash on the face or in the folds of the arms or legs,” as a screening question for AD. Experienced health care personnel used UK Working Party criteria to diagnose AD at 6, 12, and 24 months.
Complete data on AD status was available for 1,505 children in the cohort. Dr. O’Connor and colleagues calculated an overall AD prevalence of 18.6% at 6 months, 15.2% at 12 months, and 16.5% at 24 months.
Overall prevalence of AD was highest at 6 months. The study showed a similar or slightly higher impact of paternal atopy on offspring AD development, compared to maternal atopy.
Multivariable logistic regression analysis revealed that the odds of AD were 1.57 at 6 months and 1.66 at 12 months for maternal AD; 1.90 at 6 months and 1.85 at 24 months for paternal AD; 1.76 at 6 months and 1.75 at 12 months for maternal asthma; and 1.70 at 6 months, 1.86 at 12 months, and 1.99 at 24 months for paternal asthma.
“Parental allergic rhinitis was not associated with AD in offspring in the first 2 years, except for maternal rhinitis at 24 months [an adjusted odds ratio of 1.79],” the authors wrote. “The genetic predisposition to allergic rhinitis, given the key role of aeroallergen sensitization in its pathogenesis, may not be associated with early onset AD, but may have a greater impact in later onset or persistent AD.”
The authors acknowledged certain limitations of the study, including the fact that it was a secondary data analysis, and that parental AD, asthma, and rhinitis were self-reported, “which may reduce reliability and may contribute to the differences seen between the impact of maternal and paternal reported atopy on offspring,” they wrote. “Data on siblings were not captured, as participants in the study were first-born children. Filaggrin mutational analysis was not performed, which would have provided richer detail.”
Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, who was asked to comment on the work, said that the study confirms the well-known association between parental atopy and the risk of atopy in offspring, which has been shown in several studies dating back decades.
“The authors try to parse risk based on maternal or paternal or biparental history of AD and/or asthma and/or rhinitis, but this type of nuanced analysis when diagnosis is based solely on parental report may be an over-reach,” she said.
“Given that this data supports the association between parental atopy and risk of AD in infants at various time points, the clinically relevant immediate next question is how can we leverage this knowledge to prevent onset of AD in infants at risk?” she said. “To date, interventions such as early introduction of emollients have been evaluated with mixed results.”
A recent Cochrane analysis concluded that, based on available data, skin care interventions such as emollient use during the first year of life in otherwise healthy infants is probably not effective for preventing eczema and may increase risk of skin infection.
“Effects of skin care interventions on risk of asthma are also uncertain,” said Dr. Cordoro, who is also chief of the division of pediatric dermatology at UCSF.
“In sum, this study offers additional data in support of the link between atopy in parents and offspring,” she said. “Understanding how to mitigate risk and prevent atopy requires unraveling of the complex interplay between genetic, environmental, immunologic, microbial and other factors. For now, dermatologists are unable to make broad evidence-based recommendations for otherwise healthy (i.e., with normal skin) but at-risk infants in terms of approaches to skin care that might prevent eczema and asthma.”
of life, an analysis of a large birth cohort found.
“The prevalence of AD in children has increased dramatically in recent years, and most studies reporting the impact of parental atopic history on AD are based on older data,” wrote the study authors, led by Cathal O’Connor, MD. “Given the recent interest in early intervention to prevent AD and other allergic diseases, enhanced early identification of infants at risk of AD is increasingly important.”
The detailed analysis of AD risk associated with parental atopy in early life “may help to risk stratify infants to optimize early interventions for prevention or early treatment of AD,” they wrote.
The study was published in Pediatric Dermatology.
For the analysis, Dr. O’Connor of the department of pediatrics and child health at University College Cork (Ireland) and colleagues conducted a secondary analysis of the Cork Babies After Scope: Evaluating the Longitudinal Impact Using Neurological and Nutritional Endpoints (BASELINE) Birth Cohort Study.
The study recruited 2,183 healthy first-born babies between August 2009 and October 2011 to examine the effects of environmental factors during pregnancy and infancy on childhood health and development. Skin barrier assessments were performed at birth, 2 months, 6 months, 12 months, and 24 months using a validated open chamber system to measure transepidermal water loss.
Parental atopy was self-reported at 2 months. Parents were asked at 2 months if the infant had an “itchy rash on the face or in the folds of the arms or legs,” as a screening question for AD. Experienced health care personnel used UK Working Party criteria to diagnose AD at 6, 12, and 24 months.
Complete data on AD status was available for 1,505 children in the cohort. Dr. O’Connor and colleagues calculated an overall AD prevalence of 18.6% at 6 months, 15.2% at 12 months, and 16.5% at 24 months.
Overall prevalence of AD was highest at 6 months. The study showed a similar or slightly higher impact of paternal atopy on offspring AD development, compared to maternal atopy.
Multivariable logistic regression analysis revealed that the odds of AD were 1.57 at 6 months and 1.66 at 12 months for maternal AD; 1.90 at 6 months and 1.85 at 24 months for paternal AD; 1.76 at 6 months and 1.75 at 12 months for maternal asthma; and 1.70 at 6 months, 1.86 at 12 months, and 1.99 at 24 months for paternal asthma.
“Parental allergic rhinitis was not associated with AD in offspring in the first 2 years, except for maternal rhinitis at 24 months [an adjusted odds ratio of 1.79],” the authors wrote. “The genetic predisposition to allergic rhinitis, given the key role of aeroallergen sensitization in its pathogenesis, may not be associated with early onset AD, but may have a greater impact in later onset or persistent AD.”
The authors acknowledged certain limitations of the study, including the fact that it was a secondary data analysis, and that parental AD, asthma, and rhinitis were self-reported, “which may reduce reliability and may contribute to the differences seen between the impact of maternal and paternal reported atopy on offspring,” they wrote. “Data on siblings were not captured, as participants in the study were first-born children. Filaggrin mutational analysis was not performed, which would have provided richer detail.”
Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, who was asked to comment on the work, said that the study confirms the well-known association between parental atopy and the risk of atopy in offspring, which has been shown in several studies dating back decades.
“The authors try to parse risk based on maternal or paternal or biparental history of AD and/or asthma and/or rhinitis, but this type of nuanced analysis when diagnosis is based solely on parental report may be an over-reach,” she said.
“Given that this data supports the association between parental atopy and risk of AD in infants at various time points, the clinically relevant immediate next question is how can we leverage this knowledge to prevent onset of AD in infants at risk?” she said. “To date, interventions such as early introduction of emollients have been evaluated with mixed results.”
A recent Cochrane analysis concluded that, based on available data, skin care interventions such as emollient use during the first year of life in otherwise healthy infants is probably not effective for preventing eczema and may increase risk of skin infection.
“Effects of skin care interventions on risk of asthma are also uncertain,” said Dr. Cordoro, who is also chief of the division of pediatric dermatology at UCSF.
“In sum, this study offers additional data in support of the link between atopy in parents and offspring,” she said. “Understanding how to mitigate risk and prevent atopy requires unraveling of the complex interplay between genetic, environmental, immunologic, microbial and other factors. For now, dermatologists are unable to make broad evidence-based recommendations for otherwise healthy (i.e., with normal skin) but at-risk infants in terms of approaches to skin care that might prevent eczema and asthma.”
FROM PEDIATRIC DERMATOLOGY
Pediatric emergencies associated with unnecessary testing: AAP
Children seen for these conditions in emergency settings and even in primary care offices could experience avoidable pain, exposure to harmful radiation, and other harms, according to the group.
“The emergency department has the ability to rapidly perform myriad diagnostic tests and receive results quickly,” said Paul Mullan, MD, MPH, chair of the AAP’s Section of Emergency Medicine’s Choosing Wisely task force. “However, this comes with the danger of diagnostic overtesting.”
The five recommendations are as follows:
- Radiographs should not be obtained for children with bronchiolitis, croup, asthma, or first-time wheezing.
- Laboratory tests for screening should not be undertaken in the medical clearance process of children who require inpatient psychiatric admission unless clinically indicated.
- Laboratory testing or a CT scan of the head should not be ordered for a child with an unprovoked, generalized seizure or a simple febrile seizure whose mental status has returned to baseline.
- Abdominal radiographs should not be obtained for suspected constipation.
- Comprehensive viral panel testing should not be undertaken for children who are suspected of having respiratory viral illnesses.
The AAP task force partnered with Choosing Wisely Canada to create the recommendations. The list is the first of its kind to be published jointly by two countries, according to the release.
“We hope this Choosing Wisely list will encourage clinicians to rely on their clinical skills and avoid unnecessary tests,” said Dr. Mullan, who is also a physician at Children’s Hospital of the King’s Daughters and professor of pediatrics at Eastern Virginia Medical School, Norfolk.
A version of this article first appeared on Medscape.com.
Children seen for these conditions in emergency settings and even in primary care offices could experience avoidable pain, exposure to harmful radiation, and other harms, according to the group.
“The emergency department has the ability to rapidly perform myriad diagnostic tests and receive results quickly,” said Paul Mullan, MD, MPH, chair of the AAP’s Section of Emergency Medicine’s Choosing Wisely task force. “However, this comes with the danger of diagnostic overtesting.”
The five recommendations are as follows:
- Radiographs should not be obtained for children with bronchiolitis, croup, asthma, or first-time wheezing.
- Laboratory tests for screening should not be undertaken in the medical clearance process of children who require inpatient psychiatric admission unless clinically indicated.
- Laboratory testing or a CT scan of the head should not be ordered for a child with an unprovoked, generalized seizure or a simple febrile seizure whose mental status has returned to baseline.
- Abdominal radiographs should not be obtained for suspected constipation.
- Comprehensive viral panel testing should not be undertaken for children who are suspected of having respiratory viral illnesses.
The AAP task force partnered with Choosing Wisely Canada to create the recommendations. The list is the first of its kind to be published jointly by two countries, according to the release.
“We hope this Choosing Wisely list will encourage clinicians to rely on their clinical skills and avoid unnecessary tests,” said Dr. Mullan, who is also a physician at Children’s Hospital of the King’s Daughters and professor of pediatrics at Eastern Virginia Medical School, Norfolk.
A version of this article first appeared on Medscape.com.
Children seen for these conditions in emergency settings and even in primary care offices could experience avoidable pain, exposure to harmful radiation, and other harms, according to the group.
“The emergency department has the ability to rapidly perform myriad diagnostic tests and receive results quickly,” said Paul Mullan, MD, MPH, chair of the AAP’s Section of Emergency Medicine’s Choosing Wisely task force. “However, this comes with the danger of diagnostic overtesting.”
The five recommendations are as follows:
- Radiographs should not be obtained for children with bronchiolitis, croup, asthma, or first-time wheezing.
- Laboratory tests for screening should not be undertaken in the medical clearance process of children who require inpatient psychiatric admission unless clinically indicated.
- Laboratory testing or a CT scan of the head should not be ordered for a child with an unprovoked, generalized seizure or a simple febrile seizure whose mental status has returned to baseline.
- Abdominal radiographs should not be obtained for suspected constipation.
- Comprehensive viral panel testing should not be undertaken for children who are suspected of having respiratory viral illnesses.
The AAP task force partnered with Choosing Wisely Canada to create the recommendations. The list is the first of its kind to be published jointly by two countries, according to the release.
“We hope this Choosing Wisely list will encourage clinicians to rely on their clinical skills and avoid unnecessary tests,” said Dr. Mullan, who is also a physician at Children’s Hospital of the King’s Daughters and professor of pediatrics at Eastern Virginia Medical School, Norfolk.
A version of this article first appeared on Medscape.com.
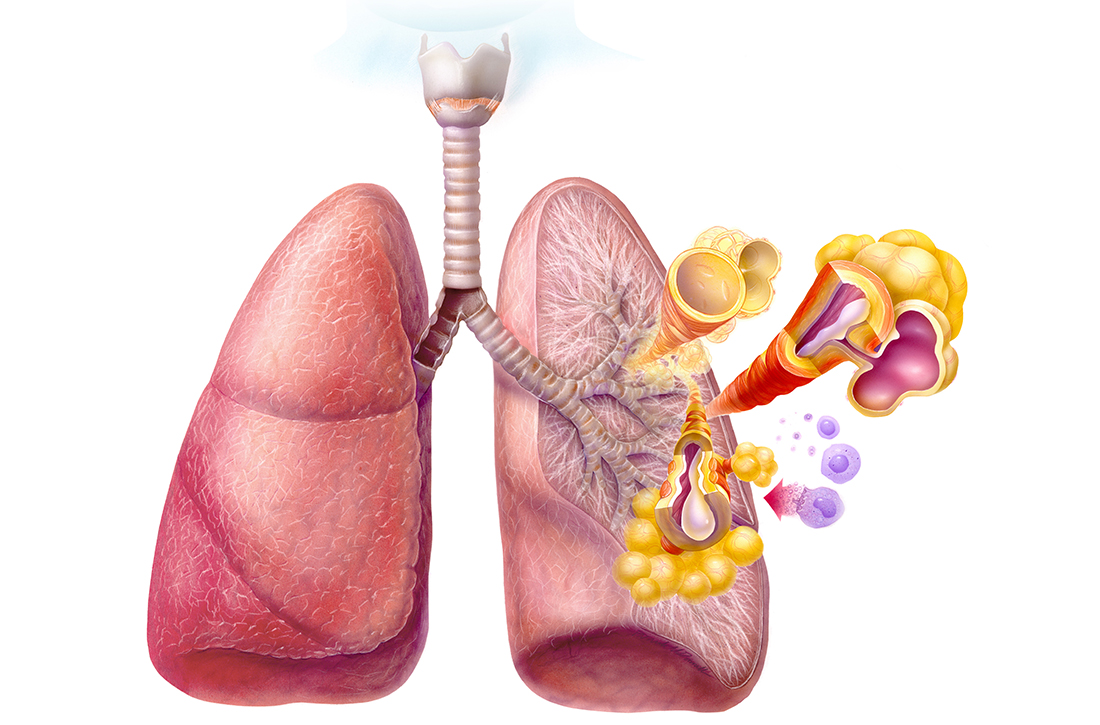
Persistent asthma linked to higher carotid plaque burden
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).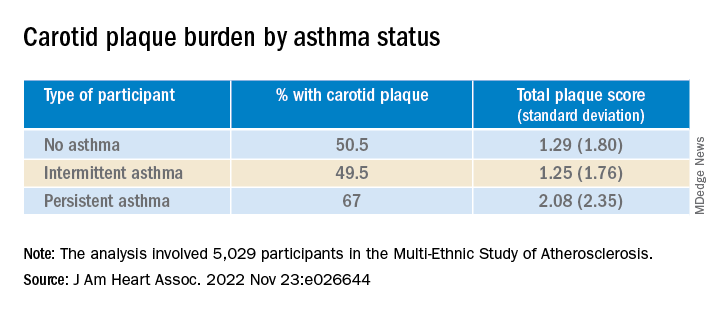
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.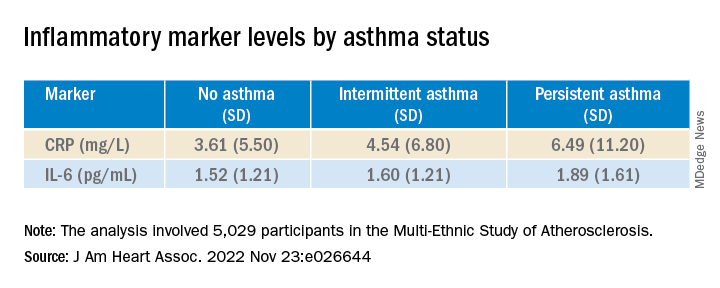
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent asthma is associated with increased carotid plaque burden and higher levels of inflammation, putting these patients at risk for atherosclerotic cardiovascular disease (ASCVD) events, new research suggests.
Using data from the MESA study, investigators analyzed more than 5,000 individuals, comparing carotid plaque and inflammatory markers in those with and without asthma.
They found that carotid plaque was present in half of participants without asthma and half of those with intermittent asthma but in close to 70% of participants with persistent asthma.
.
“The take-home message is that the current study, paired with prior studies, highlights that individuals with more significant forms of asthma may be at higher cardiovascular risk and makes it imperative to address modifiable risk factors among patients with asthma,” lead author Matthew Tattersall, DO, MS, assistant professor of cardiovascular medicine, University of Wisconsin School of Medicine and Public Health, Madison, told this news organization.
The study was published online in the Journal of the American Heart Association.
Limited data
Asthma and ASCVD are “highly prevalent inflammatory diseases,” the authors write. Carotid artery plaque detected by B-mode ultrasound “represents advanced, typically subclinical atherosclerosis that is a strong independent predictor of incident ASCVD events,” with inflammation playing a “key role” in precipitating these events, they note.
Serum inflammatory markers such as C-reactive protein (CRP) and IL-6 are associated with increased ASCVD events, and in asthma, CRP and other inflammatory biomarkers are elevated and tend to further increase during exacerbations.
Currently, there are limited data looking at the associations of asthma, asthma severity, and atherosclerotic plaque burden, they note, so the researchers turned to the MESA study – a multiethnic population of individuals free of prevalent ASCVD at baseline. They hypothesized that persistent asthma would be associated with higher carotid plaque presence and burden.
They also wanted to explore “whether these associations would be attenuated after adjustment for baseline inflammatory biomarkers.”
Dr. Tattersall said the current study “links our previous work studying the manifestations of asthma,” in which he and his colleagues demonstrated increased cardiovascular events among MESA participants with persistent asthma, as well as late-onset asthma participants in the Wisconsin Sleep Cohort. His group also showed that early arterial injury occurs in adolescents with asthma.
However, there are also few data looking at the association with carotid plaque, “a late manifestation of arterial injury and a strong predictor of future cardiovascular events and asthma,” Dr. Tattersall added.
He and his group therefore “wanted to explore the entire spectrum of arterial injury, from the initial increase in the carotid media thickness to plaque formation to cardiovascular events.”
To do so, they studied participants in MESA, a study of close to 7,000 adults that began in the year 2000 and continues to follow participants today. At the time of enrollment, all were free from CVD.
The current analysis looked at 5,029 MESA participants (mean age 61.6 years, 53% female, 26% Black, 23% Hispanic, 12% Asian), comparing those with persistent asthma, defined as “asthma requiring use of controller medications,” intermittent asthma, defined as “asthma without controller medications,” and no asthma.
Participants underwent B-mode carotid ultrasound to detect carotid plaques, with a total plaque score (TPS) ranging from 0-12. The researchers used multivariable regression modeling to evaluate the association of asthma subtype and carotid plaque burden.
Interpret cautiously
Participants with persistent asthma were more likely to be female, have higher body mass index (BMI), and higher high-density lipoprotein (HDL) cholesterol levels, compared with those without asthma.
Participants with persistent asthma had the highest burden of carotid plaque (P ≤ .003 for comparison of proportions and .002 for comparison of means).
Moreover, participants with persistent asthma also had the highest systemic inflammatory marker levels – both CRP and IL-6 – compared with those without asthma. While participants with intermittent asthma also had higher average CRP, compared with those without asthma, their IL-6 levels were comparable.
In unadjusted models, persistent asthma was associated with higher odds of carotid plaque presence (odds ratio, 1.97; 95% confidence interval, 1.32-2.95) – an association that persisted even in models that adjusted for biologic confounders (both P < .01). There also was an association between persistent asthma and higher carotid TPS (P < .001).
In further adjusted models, IL-6 was independently associated with presence of carotid plaque (P = .0001 per 1-SD increment of 1.53), as well as TPS (P < .001). CRP was “slightly associated” with carotid TPS (P = .04) but not carotid plaque presence (P = .07).
There was no attenuation after the researchers evaluated the associations of asthma subtype and carotid plaque presence or TPS and fully adjusted for baseline IL-6 or CRP (P = .02 and P = .01, respectively).
“Since this study is observational, we cannot confirm causation, but the study adds to the growing literature exploring the systemic effects of asthma,” Dr. Tattersall commented.
“Our initial hypothesis was that it was driven by inflammation, as both asthma and CVD are inflammatory conditions,” he continued. “We did adjust for inflammatory biomarkers in this analysis, but there was no change in the association.”
Nevertheless, Dr. Tattersall and colleagues are “cautious in the interpretation,” since the inflammatory biomarkers “were only collected at one point, and these measures can be dynamic, thus adjustment may not tell the whole story.”
Heightened awareness
Robert Brook, MD, professor and director of cardiovascular disease prevention, Wayne State University, Detroit, said the “main contribution of this study is the novel demonstration of a significant association between persistent (but not intermittent) asthma with carotid atherosclerosis in the MESA cohort, a large multi-ethnic population.”
These findings “support the biological plausibility of the growing epidemiological evidence that asthma independently increases the risk for cardiovascular morbidity and mortality,” added Dr. Brook, who was not involved with the study.
“The main take-home message for clinicians is that, just like in COPD (which is well-established), asthma is often a systemic condition in that the inflammation and disease process can impact the whole body,” he said.
“Health care providers should have a heightened awareness of the potentially increased cardiovascular risk of their patients with asthma and pay special attention to controlling their heart disease risk factors (for example, hyperlipidemia, hypertension),” Dr. Brook stated.
Dr. Tattersall was supported by an American Heart Association Career Development Award. The Multi-Ethnic Study of Atherosclerosis was supported by the National Heart, Lung, and Blood Institute and the National Center for Research Resources. Dr. Tattersall and co-authors and Dr. Brook declare no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Asthma management: How the guidelines compare
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.
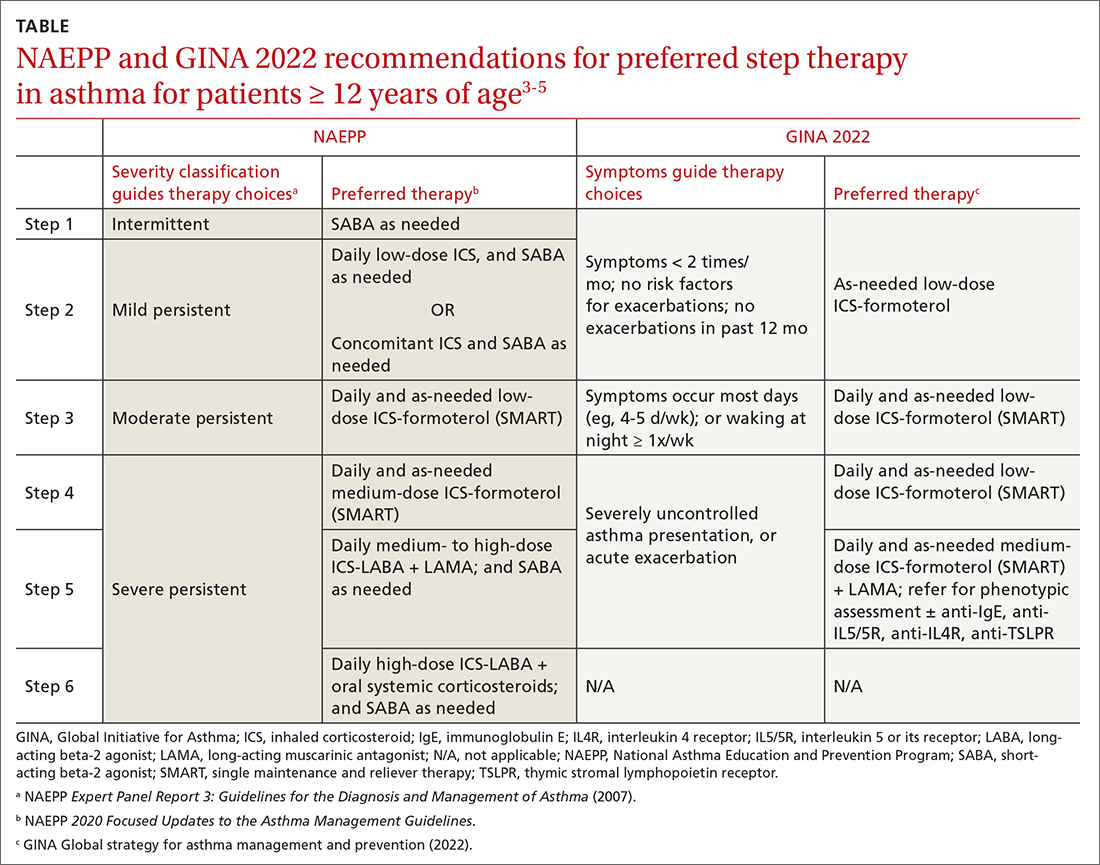
A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; nissl003@umn.edu
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; nissl003@umn.edu
CASE
Erica S*, age 22, has intermittent asthma and presents to your clinic to discuss refills of her albuterol inhaler. Two years ago, she was hospitalized for a severe asthma exacerbation because she was unable to afford medications. Since then, her asthma has generally been well controlled, and she needs to use albuterol only 1 or 2 times per month. Ms. S says she has no morning chest tightness or nocturnal coughing, but she does experience increased wheezing and shortness of breath with activity.
What would you recommend? Would your recommendation differ if she had persistent asthma?
* The patient’s name has been changed to protect her identity .
As of 2020, more than 20 million adults and 4 million children younger than 18 years of age in the United States were living with asthma.1 In 2019 alone, there were more than 1.8 million asthma-related emergency department visits for adults, and more than 790,000 asthma-related emergency department visits for children. Asthma caused more than 4000 deaths in the United States in 2020.1 Given the scale of the burden of asthma, it is not surprising that approximately 60% of all asthma visits occur in primary care settings,2 making it essential that primary care physicians stay abreast of recent developments in asthma diagnosis and management.
Since 1991, the major guidance on best practices for asthma management in the United States has been provided by the National Heart, Lung, and Blood Institute (NHLBI)’s National Asthma Education and Prevention Program (NAEPP). Its last major update on asthma was released in 2007 as the Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma (EPR-3).3 Since that time, there has been significant progress in our understanding of asthma as a complex spectrum of phenotypes, which has advanced our knowledge of pathophysiology and helped refine treatment. In contrast to the NAEPP, the Global Initiative for Asthma (GINA) has published annual updates on asthma management incorporating up-to-date information.4 In response to the continuously evolving body of knowledge on asthma, the NAEPP Coordinating Committee Expert Panel Working Group published the 2020 Focused Updates to the Asthma Management Guidelines.5
Given the vast resources available on asthma, our purpose in this article is not to provide a comprehensive review of the stepwise approach to asthma management, but instead to summarize the major points presented in the 2020 Focused Updates and how these compare and contrast with the latest guidance from GINA.
A heterogeneous disease
Asthma is a chronic respiratory disease characterized by both variable symptoms and airflow limitation that change over time, often in response to external triggers such as exercise, allergens, and viral respiratory infections. Common symptoms include wheezing, cough, chest tightness, and shortness of breath. Despite the common symptomatology, asthma is a heterogeneous disease with several recognizable phenotypes including allergic, nonallergic, and asthma with persistent airflow limitation.
Continue to: The airflow limitation...
The airflow limitation in asthma occurs through both airway hyperresponsiveness to external stimuli and chronic airway inflammation. Airway constriction is regulated by nerves to the smooth muscles of the airway. Beta-2 nerve receptors have long been the target of asthma therapy with both short-acting beta-2 agonists (SABAs) as rescue treatment and long-acting beta-2 agonists (LABAs) as maintenance therapy.3,4 However, there is increasing evidence that cholinergic nerves also have a role in airway regulation in asthma, and long-acting muscarinic antagonists (LAMAs) have recently shown benefit as add-on therapy in some types of asthma.4-6 Inhaled corticosteroids (ICSs) have long held an important role in reducing airway inflammation, especially in the setting of allergic or eosinophilic inflammation.3-5
Spirometry is essential to asthma Dx—but what about FeNO?
The mainstay of asthma diagnosis is confirming both a history of variable respiratory symptoms and variable expiratory airflow limitation exhibited by spirometry. Obstruction is defined as a reduced forced expiratory volume in 1 second (FEV1) and as a decreased ratio of FEV1 over forced vital capacity (FVC) based on predicted values. An increase of at least 12% in FEV1 post bronchodilator use indicates asthma for adolescents and adults.
More recently, studies have examined the role of fractional exhaled nitric oxide (FeNO) in the diagnosis of asthma. The 2020 Focused Updates report states that FeNO may be useful when the diagnosis of asthma is uncertain using initial history, physical exam, and spirometry findings, or when spirometry cannot be performed reliably.5 Levels of FeNO > 50 ppb make eosinophilic inflammation and treatment response to an ICS more likely. FeNO levels < 25 ppb make inflammatory asthma less likely and should prompt a search for an alternate diagnosis.5 For patients with FeNO of 25 to 50 ppb, more detailed clinical context is needed. In contrast, the 2022 GINA updates conclude that FeNO is not yet an established diagnostic tool for asthma.4
Management
When to start and adjust an ICS
ICSs continue to be the primary controller treatment for patients with asthma. However, the NAEPP and GINA have provided different guidance on how to initiate step therapy (TABLE3-5). NAEPP focuses on severity classification, while GINA recommends treatment initiation based on presenting symptoms. Since both guidelines recommend early follow-up and adjustment of therapy according to level of control, this difference becomes less apparent in ongoing care.

A more fundamental difference is seen in the recommended therapies for each step (TABLE3-5). Whereas the 2020 Focused Updates prefers a SABA as needed in step 1, GINA favors a low-dose combination of ICS-formoterol as needed. The GINA recommendation is driven by supportive evidence for early initiation of low-dose ICS in any patient with asthma for greater improvement in lung function. This also addresses concerns that overuse of as-needed SABAs may increase the risk for severe exacerbations. Evidence also indicates that the risk for asthma-related death and urgent asthma-related health care increases when a patient takes a SABA as needed as monotherapy compared with ICS therapy, even with good symptom control.7,8
Continue to: Dosing of an ICS
Dosing of an ICS is based on step therapy regardless of the guideline used and is given at a total daily amount—low, medium, and high—for each age group. When initiating an ICS, consider differences between available treatment options (eg, cost, administration technique, likely patient adherence, patient preferences) and employ shared decision-making strategies. Dosing may need to be limited depending on the commercially available product, especially when used in combination with a LABA. However, as GINA emphasizes, a low-dose ICS provides the most clinical benefit. A high-dose ICS is needed by very few patients and is associated with greater risk for local and systemic adverse effects, such as adrenal suppression. With these considerations, both guidelines recommend using the lowest effective ICS dose and stepping up and down according to the patient’s comfort level.
Give an ICS time to work. Although an ICS can begin to reduce inflammation within days of initiation, the full benefit may be evident only after 2 to 3 months.4 Once the patient’s asthma is well controlled for 3 months, stepping down the dose can be considered and approached carefully. Complete cessation of ICSs is associated with significantly higher risk for exacerbations. Therefore, a general recommendation is to step down an ICS by 50% or reduce ICS-LABA from twice-daily administration to once daily. Risk for exacerbation after step-down therapy is heightened if the patient has a history of exacerbation or an emergency department visit in the past 12 months, a low baseline FEV1, or a loss of control during a dose reduction (ie, airway hyperresponsiveness and sputum eosinophilia).
Weigh the utility of FeNO measurement. The 2020 Focused Updates also recommend considering FeNO measurement to guide treatment choice and monitoring, although this is based on overall low certainty of evidence.5 GINA affirms the mixed evidence for FeNO, stating that while a few studies have shown significantly reduced exacerbations among children, adolescents, and pregnant women with FeNO-guided treatment, other studies have shown no significant difference in exacerbations.4,9-15 At this time, the role for FeNO in asthma management remains inconclusive, and access to it is limited across primary care settings.
When assessing response to ICS therapy (and before stepping up therapy), consider patient adherence, inhaler technique, whether allergen exposure is persistent, and possible comorbidities. Inhaler technique can be especially challenging, as each inhaler varies in appearance and operation. Employ patient education strategies (eg, videos, demonstration, teach-back methods). If stepping up therapy is indicated, adding a LABA is recommended over increasing the ICS dose. Since asthma is variable, stepping up therapy can be tried and reassessed in 2 to 3 months.
SMART is preferred
Single maintenance and reliever therapy (SMART) with ICS-formoterol, used as needed, is the preferred therapy for steps 3 and 4 in both GINA recommendations and the 2020 Focused Updates (TABLE3-5). GINA also prefers SMART for step 5. The recommended SMART combination that has been studied contains budesonide (or beclomethasone, not available in combination in the United States) for the ICS and formoterol for the LABA in a single inhaler that is used both daily for control and as needed for rescue therapy.
Continue to: Other ICS-formoterol...
Other ICS-formoterol or ICS-LABA combinations can be considered for controller therapy, especially those described in the NAEPP and GINA alternative step therapy recommendations. However, SMART has been more effective than other combinations in reducing exacerbations and provides similar or better levels of control at lower average ICS doses (compared with ICS-LABA with SABA or ICS with SABA) for adolescent and adult patients.3,4 As patients use greater amounts of ICS-formoterol during episodes of increased symptoms, this additional ICS may augment the anti-inflammatory effects. SMART may also improve adherence, especially among those who confuse multiple inhalers.
SMART is also recommended for use in children. Specifically, from the 2020 Focused Updates, any patient ≥ 4 years of age with a severe exacerbation in the past year is a good SMART candidate. Also consider SMART before higher-dose ICS-LABA and SABA as needed. Additional benefits in this younger patient population are fewer medical visits or less systemic corticosteroid use with improved control and quality of life.
Caveats. Patients who have a difficult time recognizing symptoms may not be good candidates for SMART, due to the potential for taking higher or lower ICS doses than necessary.
SMART specifically refers to formoterol combinations that produce bronchodilation within 1 to 3 minutes.16 For example, the SMART strategy is not recommended for patients using ICS-salmeterol as controller therapy.
Although guideline supported, SMART options are not approved by the US Food and Drug Administration for use as reliever therapy.
Continue to: With the single combination...
With the single combination inhaler, consider the dosing limits of formoterol. The maximum daily amount of formoterol for adolescents and adults is 54 μg (12 puffs) delivered with the budesonide-formoterol metered dose inhaler. When using SMART as reliever therapy, the low-dose ICS-formoterol recommendation remains. However, depending on insurance coverage, a 1-month supply of ICS-formoterol may not be sufficient for additional reliever therapy use.
The role of LAMAs as add-on therapy
Bronchiolar smooth muscle tone is mediated by complex mechanisms that include cholinergic stimulation at muscarinic (M3) receptors.17 LAMAs, a mainstay in the management of chronic obstructive pulmonary disease (COPD), are likely to be effective in reducing asthma exacerbations and the need for oral steroids. When patients have not achieved control at step 4 of asthma therapy, both the 2020 Focused Updates and GINA now recommend considering a LAMA (eg, tiotropium) as add-on therapy for patients > 12 years of age already taking medium-dose ICS-LABA for modest improvements in lung function and reductions in severe exacerbations. GINA recommendations also now include a LAMA as add-on treatment for those ages 6 to 11 years, as some evidence supports the use in school-aged children.18 It is important to note that LAMAs should not replace a LABA for treatment, as the ICS-LABA combination is likely more effective than ICS-LAMA.
Addressing asthma-COPD overlap
Asthma and COPD are frequently and frustratingly intertwined without clear demarcation. This tends to occur as patients age and chronic lung changes appear from longstanding asthma. However, it is important to distinguish between these conditions, because there are clearly delineated treatments for each that can improve outcomes.
The priority in addressing asthma-COPD overlap (ACO) is to evaluate symptoms and determine if asthma or COPD is predominant.19 This includes establishing patient age at which symptoms began, variation and triggers of symptoms, and history of exposures to smoke/environmental respiratory toxins. Age 40 years is often used as the tipping point at which symptom onset favors a diagnosis of COPD. Serial spirometry may also be used to evaluate lung function over time and persistence of disease. If a firm diagnosis is evasive, consider a referral to a pulmonary specialist for further testing.
Choosing to use an ICS or LAMA depends on which underlying disorder is more likely. While early COPD management includes LAMA + LABA, the addition of an ICS is reserved for more severe disease. High-dose ICSs, particularly fluticasone, should be limited in COPD due to an increased risk for pneumonia. For asthma or ACO, the addition of an ICS is critical and prioritized to reduce airway inflammation and risk for exacerbations and death. While a LAMA is likely useful earlier in ACO, it is not used until step 5 of asthma therapy. Given the complexities of ACO treatment, further research is needed to provide adequate guidance.
CASE
For Ms. S, you would be wise to use an ICS-formoterol combination for as-needed symptom relief. If symptoms were more persistent, you could consider recommending the ICS-formoterol inhaler as SMART therapy, with regular doses taken twice daily and extra doses taken as needed.
CORRESPONDENCE
Tanner Nissly, DO, University of Minnesota School of Medicine, Department of Family Medicine and Community Health, 2426 West Broadway Avenue, Minneapolis, MN 55411; nissl003@umn.edu
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
1. CDC. Most recent national asthma data. Accessed October 24, 2022. www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Akinbami LJ, Santo L, Williams S, et al. Characteristics of asthma visits to physician offices in the United States: 2012–2015 National Ambulatory Medical Care Survey. Natl Health Stat Report. 2019;128:1-20.
3. NHLBI. National Asthma Education and Prevention Program expert panel report 3: guidelines for the diagnosis and management of asthma. NIH Publication 07-4051. 2007. Accessed October 24, 2022. www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf
4. Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. 2022. Accessed October 24, 2022. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-FINAL-22-07-01-WMS.pdf
5. NHLBI. 2020 Focused updates to the asthma management guidelines. Accessed October 24, 2022. www.nhlbi.nih.gov/health-topics/all-publications-and-resources/2020-focused-updates-asthma-management-guidelines
6. Lazarus SC, Krishnan JA, King TS, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009-2019. doi: 10.1056/NEJMoa1814917
7. Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332-336. doi: 10.1056/NEJM200008033430504
8. Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax. 2002;57:880-884. doi: 10.1136/thorax.57.10.880
9. Szefler SJ, Mitchell H, Sorkness CA, et al. Management of asthma based on exhaled nitric oxide in addition to guideline-based treatment for inner-city adolescents and young adults: a randomised controlled trial. Lancet. 2008;372:1065-1072. doi: 10.1016/S0140-6736(08)61448-8
10. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987-997. doi: 10.1001/2012.jama.10893
11. Garg Y, Kakria N, Katoch CDS, et al. Exhaled nitric oxide as a guiding tool for bronchial asthma: a randomised controlled trial. Med J Armed Forces India. 2020;76:17-22. doi: 10.1016/j.mjafi.2018.02.001
12. Honkoop PJ, Loijmans RJ, Termeer EH, et al. Symptom- and fraction of exhaled nitric oxide-driven strategies for asthma control: a cluster-randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682-8.e11. doi: 10.1016/j.jaci.2014.07.016
13. Peirsman EJ, Carvelli TJ, Hage PY, et al. Exhaled nitric oxide in childhood allergic asthma management: a randomised controlled trial. Pediatr Pulmonol. 2014;49:624-631. doi: 10.1002/ppul.22873
14. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011;378:983-990. doi: 10.1016/S0140-6736(11)60971-9
15. Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231-237. doi: 10.1164/rccm.200610-1427OC
16. Stam J, Souren M, Zweers P. The onset of action of formoterol, a new beta 2 adrenoceptor agonist. Int J Clin Pharmacol Ther Toxicol. 1993;31:23-26.
17. Evgenov OV, Liang Y, Jiang Y, et al. Pulmonary pharmacology and inhaled anesthetics. In: Gropper MA, Miller RD, Evgenov O, et al, eds. Miller’s Anesthesia. 8th ed. Elsevier; 2020:540-571.
18. Rodrigo GJ, Neffen H. Efficacy and safety of tiotropium in school-age children with moderate-to-severe symptomatic asthma: a systematic review. Pediatr Allergy Immunol. 2017;28:573-578. doi: 10.1111/pai.12759
19. Global Initiative for Asthma (GINA). Asthma, COPD, and asthma-COPD overlap syndrome (ACOS). 2015. Accessed October 24, 2022. https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf
PRACTICE RECOMMENDATIONS
› Consider early initiation of intermittent inhaled corticosteroid (ICS)- formoterol over a short-acting beta-2 agonist for reliever therapy. A
› Start prescribing single maintenance and reliever therapy (SMART) with ICS-formoterol to reduce exacerbation rates and simplify application. A
› Consider FeNO assessment when the diagnosis of asthma remains unclear despite history and spirometry findings. B
› Consider adding a longacting antimuscarinic agent to a medium- or high-dose ICS-LABA (long-acting beta-2 agonist) combination in uncontrolled asthma. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Add tezepelumab to SCIT to improve cat allergy symptoms?
according to results of a phase 1/2 clinical trial.
“One year of allergen immunotherapy [AIT] combined with tezepelumab was significantly more effective than SCIT alone in reducing the nasal response to allergen challenge both at the end of treatment and one year after stopping treatment,” lead study author Jonathan Corren, MD, of the University of California, Los Angeles, and his colleagues wrote in The Journal of Allergy and Clinical Immunology.
“This persistent improvement in clinical response was paralleled by reductions in nasal transcripts for multiple immunologic pathways, including mast cell activation.”
The study was cited in a news release from the National Institutes of Health that said that the approach may work in a similar way with other allergens.
The Food and Drug Administration recently approved tezepelumab for the treatment of severe asthma in people aged 12 years and older. Tezelumab, a monoclonal antibody, works by blocking the cytokine thymic stromal lymphopoietin (TSLP).
“Cells that cover the surface of organs like the skin and intestines or that line the inside of the nose and lungs rapidly secrete TSLP in response to signals of potential danger,” according to the NIH news release. “In allergic disease, TSLP helps initiate an overreactive immune response to otherwise harmless substances like cat dander, provoking airway inflammation that leads to the symptoms of allergic rhinitis.”
Testing an enhanced strategy
The double-blind CATNIP trial was conducted by Dr. Corren and colleagues at nine sites in the United States. The trial included patients aged 18-65 years who’d had moderate to severe cat-induced allergic rhinitis for at least 2 years from 2015 to 2019.
The researchers excluded patients with recurrent acute or chronic sinusitis. They excluded patients who had undergone SCIT with cat allergen within the past 10 years or seasonal or perennial allergen sensitivity during nasal challenges. They also excluded persons with a history of persistent asthma.
In the parallel-design study, 121 participants were randomly allocated into four groups: 32 patients were treated with intravenous tezepelumab plus cat SCIT, 31 received the allergy shots alone, 30 received tezepelumab alone, and 28 received placebo alone for 52 weeks, followed by 52 weeks of observation.
Participants received SCIT (10,000 bioequivalent allergy units per milliliter) or matched placebo via subcutaneous injections weekly in increasing doses for around 12 weeks, followed by monthly maintenance injections (4,000 BAU or maximum tolerated dose) until week 48.
They received tezepelumab (700 mg IV) or matched placebo 1-3 days prior to the SCIT or placebo SCIT injections once every 4 weeks through week 24, then before or on the same day as the SCIT or placebo injections through week 48.
Measures of effectiveness
Participants were also given nasal allergy challenges – one spritz of a nasal spray containing cat allergen extract in each nostril at screening, baseline, and weeks 26, 52, 78, and 104. The researchers recorded participants’ total nasal symptom score (TNSS) and peak nasal inspiratory flow at 5, 15, 30, and 60 minutes after being sprayed and hourly for up to 6 hours post challenge. Blood and nasal cell samples were also collected.
The research team performed skin prick tests using serial dilutions of cat extract and an intradermal skin test (IDST) using the concentration of allergen that produced an early response of at least 15 mm at baseline. They measured early-phase responses for the both tests at 15 minutes and late-phase response to the IDST at 6 hours.
They measured serum levels of cat dander–specific IgE, IgG4, and total IgE using fluoroenzyme immunoassay. They measured serum interleukin-5 and IL-13 using high-sensitivity single-molecule digital immunoassay and performed nasal brushing using a 3-mm cytology brush 6 hours after a nasal allergy challenge. They performed whole-genome transcriptional profiling on the extracted RNA.
Combination therapy worked better and longer
The combined therapy worked better while being administered. Although the allergy shots alone stopped working after they were discontinued, the combination continued to benefit participants 1 year after that therapy ended.
At week 52, statistically significant reductions in TNSS induced by nasal allergy challenges occurred in patients receiving tezepelumab plus SCIT compared with patients receiving SCIT alone.
At week 104, 1 year after treatment ended, the primary endpoint TNSS was not significantly different in the tezepelumab-plus-SCIT group than in the SCIT-alone group, but TNSS peak 0–1 hour was significantly lower in the combination treatment group than in the SCIT-alone group.
In analysis of gene expression from nasal epithelial samples, participants who had been treated with the combination but not with either therapy by itself showed persistent modulation of the nasal immunologic environment, including diminished mast cell function. This was explained in large part by decreased transcription of the gene TPSAB1 (tryptase). Tryptase protein in nasal fluid was also decreased in the combination group, compared with the SCIT-alone group.
Adverse and serious adverse events, including infections and infestations as well as respiratory, thoracic, mediastinal, gastrointestinal, immune system, and nervous system disorders, did not differ significantly between treatment groups.
Four independent experts welcome the results
Patricia Lynne Lugar, MD, associate professor of medicine in the division of pulmonology, allergy, and critical care medicine at Duke University, Durham, N.C., found the results, especially the 1-year posttreatment response durability, surprising.
“AIT is a very effective treatment that often provides prolonged symptom improvement and is ‘curative’ in many cases,” she said in an interview. “If further studies show that tezepelumab offers long-term results, more patients might opt for combination therapy.
“A significant strength of the study is its evaluation of responses of the combination therapy on cellular output and gene expression,” Dr. Lugar added. “The mechanism by which AIT modulates the allergic response is largely understood. Tezepelumab may augment this modulation to alter the Th2 response upon exposure to the allergens.”
Will payors cover the prohibitively costly biologic?
Scott Frank, MD, associate professor in the department of family medicine and community health at Case Western Reserve University, Cleveland, called the study well designed and rigorous.
“The practicality of the approach may be limited by the need for intravenous administration of tezepelumab in addition to the traditional allergy shot,” he noted by email, “and the cost of this therapeutic approach is not addressed.”
Christopher Brooks, MD, clinical assistant professor of allergy and immunology in the department of otolaryngology at Ohio State University Wexner Medical Center, Columbus, also pointed out the drug’s cost.
“Tezepelumab is currently an expensive biologic, so it remains to be seen whether patients and payors will be willing to pay for this add-on medication when AIT by itself still remains very effective,” he said by email.
“AIT is most effective when given for 5 years, so it also remains to be seen whether the results and conclusions of this study would still hold true if done for the typical 5-year treatment period,” he added.
Stokes Peebles, MD, professor of medicine in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., called the study “very well designed by a highly respected group of investigators using well-matched study populations.
“Tezepelumab has been shown to work in asthma, and there is no reason to think it would not work in allergic rhinitis,” he said in an interview.
“However, while the results of the combined therapy were statistically significant, their clinical significance was not clear. Patients do not care about statistical significance. They want to know whether a drug will be clinically significant,” he added.
Many people avoid cat allergy symptoms by avoiding cats and, in some cases, by avoiding people who live with cats, he said. Medical therapy, usually involving nasal corticosteroids and antihistamines, helps most people avoid cat allergy symptoms.
“Patients with bad allergies who have not done well with SCIT may consider adding tezepelumab, but it incurs a major cost. If medical therapy doesn’t work, allergy shots are available at roughly $3,000 per year. Adding tezepelumab costs around $40,000 more per year,” he explained. “Does the slight clinical benefit justify the greatly increased cost?”
The authors and uninvolved experts recommend further related research.
The research was supported by the National Institute of Allergy and Infectious Diseases. AstraZeneca and Amgen donated the drug used in the study. Dr. Corren reported financial relationships with AstraZeneca, and one coauthor reported relevant financial relationships with Amgen and other pharmaceutical companies. The remaining coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a phase 1/2 clinical trial.
“One year of allergen immunotherapy [AIT] combined with tezepelumab was significantly more effective than SCIT alone in reducing the nasal response to allergen challenge both at the end of treatment and one year after stopping treatment,” lead study author Jonathan Corren, MD, of the University of California, Los Angeles, and his colleagues wrote in The Journal of Allergy and Clinical Immunology.
“This persistent improvement in clinical response was paralleled by reductions in nasal transcripts for multiple immunologic pathways, including mast cell activation.”
The study was cited in a news release from the National Institutes of Health that said that the approach may work in a similar way with other allergens.
The Food and Drug Administration recently approved tezepelumab for the treatment of severe asthma in people aged 12 years and older. Tezelumab, a monoclonal antibody, works by blocking the cytokine thymic stromal lymphopoietin (TSLP).
“Cells that cover the surface of organs like the skin and intestines or that line the inside of the nose and lungs rapidly secrete TSLP in response to signals of potential danger,” according to the NIH news release. “In allergic disease, TSLP helps initiate an overreactive immune response to otherwise harmless substances like cat dander, provoking airway inflammation that leads to the symptoms of allergic rhinitis.”
Testing an enhanced strategy
The double-blind CATNIP trial was conducted by Dr. Corren and colleagues at nine sites in the United States. The trial included patients aged 18-65 years who’d had moderate to severe cat-induced allergic rhinitis for at least 2 years from 2015 to 2019.
The researchers excluded patients with recurrent acute or chronic sinusitis. They excluded patients who had undergone SCIT with cat allergen within the past 10 years or seasonal or perennial allergen sensitivity during nasal challenges. They also excluded persons with a history of persistent asthma.
In the parallel-design study, 121 participants were randomly allocated into four groups: 32 patients were treated with intravenous tezepelumab plus cat SCIT, 31 received the allergy shots alone, 30 received tezepelumab alone, and 28 received placebo alone for 52 weeks, followed by 52 weeks of observation.
Participants received SCIT (10,000 bioequivalent allergy units per milliliter) or matched placebo via subcutaneous injections weekly in increasing doses for around 12 weeks, followed by monthly maintenance injections (4,000 BAU or maximum tolerated dose) until week 48.
They received tezepelumab (700 mg IV) or matched placebo 1-3 days prior to the SCIT or placebo SCIT injections once every 4 weeks through week 24, then before or on the same day as the SCIT or placebo injections through week 48.
Measures of effectiveness
Participants were also given nasal allergy challenges – one spritz of a nasal spray containing cat allergen extract in each nostril at screening, baseline, and weeks 26, 52, 78, and 104. The researchers recorded participants’ total nasal symptom score (TNSS) and peak nasal inspiratory flow at 5, 15, 30, and 60 minutes after being sprayed and hourly for up to 6 hours post challenge. Blood and nasal cell samples were also collected.
The research team performed skin prick tests using serial dilutions of cat extract and an intradermal skin test (IDST) using the concentration of allergen that produced an early response of at least 15 mm at baseline. They measured early-phase responses for the both tests at 15 minutes and late-phase response to the IDST at 6 hours.
They measured serum levels of cat dander–specific IgE, IgG4, and total IgE using fluoroenzyme immunoassay. They measured serum interleukin-5 and IL-13 using high-sensitivity single-molecule digital immunoassay and performed nasal brushing using a 3-mm cytology brush 6 hours after a nasal allergy challenge. They performed whole-genome transcriptional profiling on the extracted RNA.
Combination therapy worked better and longer
The combined therapy worked better while being administered. Although the allergy shots alone stopped working after they were discontinued, the combination continued to benefit participants 1 year after that therapy ended.
At week 52, statistically significant reductions in TNSS induced by nasal allergy challenges occurred in patients receiving tezepelumab plus SCIT compared with patients receiving SCIT alone.
At week 104, 1 year after treatment ended, the primary endpoint TNSS was not significantly different in the tezepelumab-plus-SCIT group than in the SCIT-alone group, but TNSS peak 0–1 hour was significantly lower in the combination treatment group than in the SCIT-alone group.
In analysis of gene expression from nasal epithelial samples, participants who had been treated with the combination but not with either therapy by itself showed persistent modulation of the nasal immunologic environment, including diminished mast cell function. This was explained in large part by decreased transcription of the gene TPSAB1 (tryptase). Tryptase protein in nasal fluid was also decreased in the combination group, compared with the SCIT-alone group.
Adverse and serious adverse events, including infections and infestations as well as respiratory, thoracic, mediastinal, gastrointestinal, immune system, and nervous system disorders, did not differ significantly between treatment groups.
Four independent experts welcome the results
Patricia Lynne Lugar, MD, associate professor of medicine in the division of pulmonology, allergy, and critical care medicine at Duke University, Durham, N.C., found the results, especially the 1-year posttreatment response durability, surprising.
“AIT is a very effective treatment that often provides prolonged symptom improvement and is ‘curative’ in many cases,” she said in an interview. “If further studies show that tezepelumab offers long-term results, more patients might opt for combination therapy.
“A significant strength of the study is its evaluation of responses of the combination therapy on cellular output and gene expression,” Dr. Lugar added. “The mechanism by which AIT modulates the allergic response is largely understood. Tezepelumab may augment this modulation to alter the Th2 response upon exposure to the allergens.”
Will payors cover the prohibitively costly biologic?
Scott Frank, MD, associate professor in the department of family medicine and community health at Case Western Reserve University, Cleveland, called the study well designed and rigorous.
“The practicality of the approach may be limited by the need for intravenous administration of tezepelumab in addition to the traditional allergy shot,” he noted by email, “and the cost of this therapeutic approach is not addressed.”
Christopher Brooks, MD, clinical assistant professor of allergy and immunology in the department of otolaryngology at Ohio State University Wexner Medical Center, Columbus, also pointed out the drug’s cost.
“Tezepelumab is currently an expensive biologic, so it remains to be seen whether patients and payors will be willing to pay for this add-on medication when AIT by itself still remains very effective,” he said by email.
“AIT is most effective when given for 5 years, so it also remains to be seen whether the results and conclusions of this study would still hold true if done for the typical 5-year treatment period,” he added.
Stokes Peebles, MD, professor of medicine in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., called the study “very well designed by a highly respected group of investigators using well-matched study populations.
“Tezepelumab has been shown to work in asthma, and there is no reason to think it would not work in allergic rhinitis,” he said in an interview.
“However, while the results of the combined therapy were statistically significant, their clinical significance was not clear. Patients do not care about statistical significance. They want to know whether a drug will be clinically significant,” he added.
Many people avoid cat allergy symptoms by avoiding cats and, in some cases, by avoiding people who live with cats, he said. Medical therapy, usually involving nasal corticosteroids and antihistamines, helps most people avoid cat allergy symptoms.
“Patients with bad allergies who have not done well with SCIT may consider adding tezepelumab, but it incurs a major cost. If medical therapy doesn’t work, allergy shots are available at roughly $3,000 per year. Adding tezepelumab costs around $40,000 more per year,” he explained. “Does the slight clinical benefit justify the greatly increased cost?”
The authors and uninvolved experts recommend further related research.
The research was supported by the National Institute of Allergy and Infectious Diseases. AstraZeneca and Amgen donated the drug used in the study. Dr. Corren reported financial relationships with AstraZeneca, and one coauthor reported relevant financial relationships with Amgen and other pharmaceutical companies. The remaining coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a phase 1/2 clinical trial.
“One year of allergen immunotherapy [AIT] combined with tezepelumab was significantly more effective than SCIT alone in reducing the nasal response to allergen challenge both at the end of treatment and one year after stopping treatment,” lead study author Jonathan Corren, MD, of the University of California, Los Angeles, and his colleagues wrote in The Journal of Allergy and Clinical Immunology.
“This persistent improvement in clinical response was paralleled by reductions in nasal transcripts for multiple immunologic pathways, including mast cell activation.”
The study was cited in a news release from the National Institutes of Health that said that the approach may work in a similar way with other allergens.
The Food and Drug Administration recently approved tezepelumab for the treatment of severe asthma in people aged 12 years and older. Tezelumab, a monoclonal antibody, works by blocking the cytokine thymic stromal lymphopoietin (TSLP).
“Cells that cover the surface of organs like the skin and intestines or that line the inside of the nose and lungs rapidly secrete TSLP in response to signals of potential danger,” according to the NIH news release. “In allergic disease, TSLP helps initiate an overreactive immune response to otherwise harmless substances like cat dander, provoking airway inflammation that leads to the symptoms of allergic rhinitis.”
Testing an enhanced strategy
The double-blind CATNIP trial was conducted by Dr. Corren and colleagues at nine sites in the United States. The trial included patients aged 18-65 years who’d had moderate to severe cat-induced allergic rhinitis for at least 2 years from 2015 to 2019.
The researchers excluded patients with recurrent acute or chronic sinusitis. They excluded patients who had undergone SCIT with cat allergen within the past 10 years or seasonal or perennial allergen sensitivity during nasal challenges. They also excluded persons with a history of persistent asthma.
In the parallel-design study, 121 participants were randomly allocated into four groups: 32 patients were treated with intravenous tezepelumab plus cat SCIT, 31 received the allergy shots alone, 30 received tezepelumab alone, and 28 received placebo alone for 52 weeks, followed by 52 weeks of observation.
Participants received SCIT (10,000 bioequivalent allergy units per milliliter) or matched placebo via subcutaneous injections weekly in increasing doses for around 12 weeks, followed by monthly maintenance injections (4,000 BAU or maximum tolerated dose) until week 48.
They received tezepelumab (700 mg IV) or matched placebo 1-3 days prior to the SCIT or placebo SCIT injections once every 4 weeks through week 24, then before or on the same day as the SCIT or placebo injections through week 48.
Measures of effectiveness
Participants were also given nasal allergy challenges – one spritz of a nasal spray containing cat allergen extract in each nostril at screening, baseline, and weeks 26, 52, 78, and 104. The researchers recorded participants’ total nasal symptom score (TNSS) and peak nasal inspiratory flow at 5, 15, 30, and 60 minutes after being sprayed and hourly for up to 6 hours post challenge. Blood and nasal cell samples were also collected.
The research team performed skin prick tests using serial dilutions of cat extract and an intradermal skin test (IDST) using the concentration of allergen that produced an early response of at least 15 mm at baseline. They measured early-phase responses for the both tests at 15 minutes and late-phase response to the IDST at 6 hours.
They measured serum levels of cat dander–specific IgE, IgG4, and total IgE using fluoroenzyme immunoassay. They measured serum interleukin-5 and IL-13 using high-sensitivity single-molecule digital immunoassay and performed nasal brushing using a 3-mm cytology brush 6 hours after a nasal allergy challenge. They performed whole-genome transcriptional profiling on the extracted RNA.
Combination therapy worked better and longer
The combined therapy worked better while being administered. Although the allergy shots alone stopped working after they were discontinued, the combination continued to benefit participants 1 year after that therapy ended.
At week 52, statistically significant reductions in TNSS induced by nasal allergy challenges occurred in patients receiving tezepelumab plus SCIT compared with patients receiving SCIT alone.
At week 104, 1 year after treatment ended, the primary endpoint TNSS was not significantly different in the tezepelumab-plus-SCIT group than in the SCIT-alone group, but TNSS peak 0–1 hour was significantly lower in the combination treatment group than in the SCIT-alone group.
In analysis of gene expression from nasal epithelial samples, participants who had been treated with the combination but not with either therapy by itself showed persistent modulation of the nasal immunologic environment, including diminished mast cell function. This was explained in large part by decreased transcription of the gene TPSAB1 (tryptase). Tryptase protein in nasal fluid was also decreased in the combination group, compared with the SCIT-alone group.
Adverse and serious adverse events, including infections and infestations as well as respiratory, thoracic, mediastinal, gastrointestinal, immune system, and nervous system disorders, did not differ significantly between treatment groups.
Four independent experts welcome the results
Patricia Lynne Lugar, MD, associate professor of medicine in the division of pulmonology, allergy, and critical care medicine at Duke University, Durham, N.C., found the results, especially the 1-year posttreatment response durability, surprising.
“AIT is a very effective treatment that often provides prolonged symptom improvement and is ‘curative’ in many cases,” she said in an interview. “If further studies show that tezepelumab offers long-term results, more patients might opt for combination therapy.
“A significant strength of the study is its evaluation of responses of the combination therapy on cellular output and gene expression,” Dr. Lugar added. “The mechanism by which AIT modulates the allergic response is largely understood. Tezepelumab may augment this modulation to alter the Th2 response upon exposure to the allergens.”
Will payors cover the prohibitively costly biologic?
Scott Frank, MD, associate professor in the department of family medicine and community health at Case Western Reserve University, Cleveland, called the study well designed and rigorous.
“The practicality of the approach may be limited by the need for intravenous administration of tezepelumab in addition to the traditional allergy shot,” he noted by email, “and the cost of this therapeutic approach is not addressed.”
Christopher Brooks, MD, clinical assistant professor of allergy and immunology in the department of otolaryngology at Ohio State University Wexner Medical Center, Columbus, also pointed out the drug’s cost.
“Tezepelumab is currently an expensive biologic, so it remains to be seen whether patients and payors will be willing to pay for this add-on medication when AIT by itself still remains very effective,” he said by email.
“AIT is most effective when given for 5 years, so it also remains to be seen whether the results and conclusions of this study would still hold true if done for the typical 5-year treatment period,” he added.
Stokes Peebles, MD, professor of medicine in the division of allergy, pulmonary, and critical care medicine at Vanderbilt University Medical Center, Nashville, Tenn., called the study “very well designed by a highly respected group of investigators using well-matched study populations.
“Tezepelumab has been shown to work in asthma, and there is no reason to think it would not work in allergic rhinitis,” he said in an interview.
“However, while the results of the combined therapy were statistically significant, their clinical significance was not clear. Patients do not care about statistical significance. They want to know whether a drug will be clinically significant,” he added.
Many people avoid cat allergy symptoms by avoiding cats and, in some cases, by avoiding people who live with cats, he said. Medical therapy, usually involving nasal corticosteroids and antihistamines, helps most people avoid cat allergy symptoms.
“Patients with bad allergies who have not done well with SCIT may consider adding tezepelumab, but it incurs a major cost. If medical therapy doesn’t work, allergy shots are available at roughly $3,000 per year. Adding tezepelumab costs around $40,000 more per year,” he explained. “Does the slight clinical benefit justify the greatly increased cost?”
The authors and uninvolved experts recommend further related research.
The research was supported by the National Institute of Allergy and Infectious Diseases. AstraZeneca and Amgen donated the drug used in the study. Dr. Corren reported financial relationships with AstraZeneca, and one coauthor reported relevant financial relationships with Amgen and other pharmaceutical companies. The remaining coauthors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY
Best Practice Implementation and Clinical Inertia
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.
Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.

Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
From the Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Clinical inertia is defined as the failure of clinicians to initiate or escalate guideline-directed medical therapy to achieve treatment goals for well-defined clinical conditions.1,2 Evidence-based guidelines recommend optimal disease management with readily available medical therapies throughout the phases of clinical care. Unfortunately, the care provided to individual patients undergoes multiple modifications throughout the disease course, resulting in divergent pathways, significant deviations from treatment guidelines, and failure of “safeguard” checkpoints to reinstate, initiate, optimize, or stop treatments. Clinical inertia generally describes rigidity or resistance to change around implementing evidence-based guidelines. Furthermore, this term describes treatment behavior on the part of an individual clinician, not organizational inertia, which generally encompasses both internal (immediate clinical practice settings) and external factors (national and international guidelines and recommendations), eventually leading to resistance to optimizing disease treatment and therapeutic regimens. Individual clinicians’ clinical inertia in the form of resistance to guideline implementation and evidence-based principles can be one factor that drives organizational inertia. In turn, such individual behavior can be dictated by personal beliefs, knowledge, interpretation, skills, management principles, and biases. The terms therapeutic inertia or clinical inertia should not be confused with nonadherence from the patient’s standpoint when the clinician follows the best practice guidelines.3
Clinical inertia has been described in several clinical domains, including diabetes,4,5 hypertension,6,7 heart failure,8 depression,9 pulmonary medicine,10 and complex disease management.11 Clinicians can set suboptimal treatment goals due to specific beliefs and attitudes around optimal therapeutic goals. For example, when treating a patient with a chronic disease that is presently stable, a clinician could elect to initiate suboptimal treatment, as escalation of treatment might not be the priority in stable disease; they also may have concerns about overtreatment. Other factors that can contribute to clinical inertia (ie, undertreatment in the presence of indications for treatment) include those related to the patient, the clinical setting, and the organization, along with the importance of individualizing therapies in specific patients. Organizational inertia is the initial global resistance by the system to implementation, which can slow the dissemination and adaptation of best practices but eventually declines over time. Individual clinical inertia, on the other hand, will likely persist after the system-level rollout of guideline-based approaches.
The trajectory of dissemination, implementation, and adaptation of innovations and best practices is illustrated in the Figure. When the guidelines and medical societies endorse the adaptation of innovations or practice change after the benefits of such innovations/change have been established by the regulatory bodies, uptake can be hindered by both organizational and clinical inertia. Overcoming inertia to system-level changes requires addressing individual clinicians, along with practice and organizational factors, in order to ensure systematic adaptations. From the clinicians’ view, training and cognitive interventions to improve the adaptation and coping skills can improve understanding of treatment options through standardized educational and behavioral modification tools, direct and indirect feedback around performance, and decision support through a continuous improvement approach on both individual and system levels.

Addressing inertia in clinical practice requires a deep understanding of the individual and organizational elements that foster resistance to adapting best practice models. Research that explores tools and approaches to overcome inertia in managing complex diseases is a key step in advancing clinical innovation and disseminating best practices.
Corresponding author: Ebrahim Barkoudah, MD, MPH; ebarkoudah@bwh.harvard.edu
Disclosures: None reported.
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
1. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. doi:10.7326/0003-4819-135-9-200111060-00012
2. Allen JD, Curtiss FR, Fairman KA. Nonadherence, clinical inertia, or therapeutic inertia? J Manag Care Pharm. 2009;15(8):690-695. doi:10.18553/jmcp.2009.15.8.690
3. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. doi:10.1016/j.pcd.2010.07.003
4. Khunti K, Davies MJ. Clinical inertia—time to reappraise the terminology? Prim Care Diabetes. 2017;11(2):105-106. doi:10.1016/j.pcd.2017.01.007
5. O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163(22):2677-2678. doi:10.1001/archinte.163.22.2677
6. Faria C, Wenzel M, Lee KW, et al. A narrative review of clinical inertia: focus on hypertension. J Am Soc Hypertens. 2009;3(4):267-276. doi:10.1016/j.jash.2009.03.001
7. Jarjour M, Henri C, de Denus S, et al. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail. 2020;8(9):725-738. doi:10.1016/j.jchf.2020.04.019
8. Henke RM, Zaslavsky AM, McGuire TG, et al. Clinical inertia in depression treatment. Med Care. 2009;47(9):959-67. doi:10.1097/MLR.0b013e31819a5da0
9. Cooke CE, Sidel M, Belletti DA, Fuhlbrigge AL. Clinical inertia in the management of chronic obstructive pulmonary disease. COPD. 2012;9(1):73-80. doi:10.3109/15412555.2011.631957
10. Whitford DL, Al-Anjawi HA, Al-Baharna MM. Impact of clinical inertia on cardiovascular risk factors in patients with diabetes. Prim Care Diabetes. 2014;8(2):133-138. doi:10.1016/j.pcd.2013.10.007
Comorbidities, Racial Disparities, and Geographic Differences in Asthma
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
Two-year dupilumab data: Continued response for moderate to severe pediatric asthma
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – For children with uncontrolled asthma on standard therapies and meeting criteria of a type 2 (T2) inflammatory phenotype, a prospective 1-year extension from a phase 3 trial supports the biologic dupilumab as a potential treatment standard, according to the investigator who presented the findings at the annual meeting of the American College of Chest Physicians (CHEST).
said Leonard B. Bacharier, MD, section chief, division of pediatric allergy, immunology, and pulmonary medicine, Vanderbilt University Medical Center, Nashville, Tenn.
By T2 inflammatory phenotype, Dr. Bacharier specified that key features include an eosinophil count of at least 150 cells/mL and a FENO level of at least 20 ppb. If children meet these and inadequate standard-therapy response criteria, Dr. Bacharier thinks the extension data support dupilumab as a routine therapy despite the cost.
“As a pediatrician, I think it is really important that children with asthma finish their childhood with the best bone health and the lowest risk of other steroid-associated adverse events,” Dr. Bacharier said.
Over the course of the 1-year extension, called EXCURSION, there was no evidence of diminished efficacy nor of any new safety signal. In other words, patients have remained well controlled for 2 years with a well-tolerated therapy. Dr. Bacharier pointed out, however, that one of the most compelling reasons to consider this as a potential standard was the very low rates at which patients required a course of steroids.
At the end of 1 year in the extension trial, called VOYAGE, the unadjusted annualized total number of steroid courses per patient was 0.414 in the dupilumab group vs. 0.816 in the placebo group. At the end of EXCURSION, following an additional year of therapy, the rate was 0.152.
“This means that fewer than 2 patients out of 10 required prednisone in the previous year,” Dr. Bacharier said.
The EXCURSION extension study did not capture data on steroid-related adverse events, but Dr. Bacharier said that these data are reassuring for both acute and long-term risks of steroid exposure.
“We know that the adverse effects associated with oral steroids are related to cumulative exposure. The more you receive, the greater the risk of adverse effects,” he said.
In patients who were randomly assigned to placebo in the VOYAGE trial and then switched to dupilumab in the EXCURSION extension, steroid exposure was also very low, but whether evaluated as annualized total courses (0.152 vs. 0.181) or by proportion of patients with any steroid intake (10.5% vs. 13.2%), there was a numerical advantage for starting and remaining on dupilumab over the 2-year follow-up.
In VOYAGE, which was published last year in the New England Journal of Medicine, 408 children from ages 6 to 11 years were randomly assigned in a 2:1 ratio to dupilumab or matching placebo. For children weighing less than 30 kg, the dose was 200 mg. For those who weighed less, the dose was 100 mg. Both doses were administered every 2 weeks.
As previously reported, the study met the primary endpoint of annualized rate of severe asthma exacerbations, which was 0.31 in the dupilumab group vs. 0.75 in the placebo group, a relative reduction of 59.3% (P < .001). Dupilumab was also superior on several secondary endpoints, including measures of lung function and asthma control.
The EXCURSION extension study enrolled 365 of the patients who participated in VOYAGE. This included 125 of the 135 randomly assigned to placebo and 240 of the 273 randomly assigned to dupilumab. Those initially randomly assigned to placebo were transitioned to dupilumab. The same weight-based dosing was employed.
At baseline, the children enrolled in VOYAGE had an annualized rate of 2.560 severe exacerbations. At the end of VOYAGE, this rate was 0.330. At the end of EXCURSION after 2 years on dupilumab, the rate was 0.118. In the group switched from placebo to dupilumab, the rate was 0.124.
During EXCURSION, treatment-emergent adverse events occurred in 2.5% of those who remained on dupilumab and 0.8% of those switched from placebo to dupilumab. Three patients (1.3%) permanently discontinued therapy because of a treatment-related event. The most common adverse events involved upper respiratory complaints, such as nasopharyngitis, pharyngitis, upper respiratory tract infections, and rhinitis influenza, but all were reported in fewer than 10% of patients. Other reported side effects, such as injection-site reactions and diarrhea, occurred in 5% or fewer of patients.
“Over the 2 years, dupilumab was well tolerated, and there was evidence of an increased risk of adverse events for longer exposure,” Dr. Bacharier reported.
It is for this reason that Dr. Bacharier concluded that children with repeated exacerbations requiring steroids despite standard therapies should be considered for dupilumab if they also meet criteria for the T2 inflammatory phenotype. This last point is important.
“In children with low levels of eosinophil and low phenol, we are not seeing these kinds of response,” Dr. Bacharier said. Rather, in the absence of eosinophilia, “there is probably no difference between dupilumab and placebo.”
An important steroid-sparing effect is “suggested” by the data, but Sally E. Wenzel, MD, director of the University of Pittsburgh Asthma and Environmental Lung Health Institute in Pittsburgh, characterized the idea that dupilumab is emerging to be a standard in uncontrolled asthma in children with the T2 phenotype as “a bit premature.”
She challenged the conclusion that the EXCURSION data associated dupilumab with a reduction in annualized steroid courses over time. While the number was lower after 2 years of treatment than after 1, Dr. Wenzel pointed out that all patients were on dupilumab in the second year, “so we don’t know what really happens without treatment.” She said there are other potential explanations, including the possibility that aging children have less active disease.
More importantly, Dr. Wenzel said in an interview that she would also hesitate to urge biologics in every child who meets the criteria that Dr. Bacharier outlined.
“The most important concern is that we do not know how long one should continue the dupilumab and if the long-term treatment adversely or positively affects a growing immune system,” she said.
There is reason to be concerned that blockage of an entire immune pathway with a biologic could adversely affect autoimmunity as well as susceptibility to cancer, according to Dr. Wenzel. She hopes this does not prove to be the case, but she encouraged prudence until there are more data to judge.
While extension data for dupilumab “sound good,” she thinks moving toward any type of standard of care with biologics in children “has to be done with caution and constant evaluation and reevaluation.”
Dr. Bacharier disclosed relationships with AstraZeneca, GlaxoSmithKline, Regeneron and Sanofi. The two latter companies collaborated on the development and marketing of dupilumab. Dr. Wenzel disclosed relationships with AstraZeneca, GlaxoSmithKline, Knopp Pharmaceuticals, Pieris, and Sanofi-Regeneron.
A version of this article first appeared on Medscape.com.
AT CHEST 2022
Asthma ED visits predict failed housing inspections
, according to a new study presented at the annual meeting of the American College of Emergency Physicians.
While links between asthma and low-quality housing prone to harboring allergens have been well-documented, the current study takes the extra step of looking at housing down to the level of individual land parcels and suggests that asthma hospital visits can be used to identify hazardous housing earlier.
“Emergency department visits for asthma provide a leading indicator that can be used by health departments or housing authorities to direct housing inspections and remediation of poor housing conditions, track improvements in housing quality, measure housing department performance, support resident grievances, and inform funding allocation decisions,” said the study’s lead researcher, Elizabeth Samuels, MD, who is assistant professor of epidemiology and emergency medicine at Brown University, Providence, R.I.
Researchers retrospectively looked at cases of children and adults in the Greater New Haven area of Connecticut seen at the Yale New Haven Hospital ED for asthma-related problems between March 2013 and August 2017. The region has the fifth-highest prevalence of asthma in the United States, the researchers point out, due to its air quality, pollens, and quality of its housing. More than half of residences were built before 1,940, compared with about 13% nationally. Patient addresses were matched with HUD inspection records.
The review encompassed 11,429 ED visits by 6,366 individuals; 54% were insured by Medicaid, and 42% were Black. Controlling for patient and neighborhood data, researchers found that increased asthma ED visits at the parcel level were associated with decreased HUD inspection scores to a highly significant degree (P < .001).
They also found that there was a relationship in terms of timing between asthma ED visits and inspection scores: asthma ED visits increased more than 1 year before a failed HUD inspection. They also found that asthma ED visits were not elevated at housing units that passed inspection. Using asthma ED visits to predict failed housing inspections produced a specificity rate of 92.3% in an adjusted model, Dr. Samuels noted.
“This approach represents a novel method of early identification of dangerous housing conditions, which could aid in the prevention of asthma-related morbidity and mortality,” Dr. Samuels said.
The investigators noted that, of the parcels with the top three incidence rates of asthma ED visits, “all of them have been closed or demolished.”
In addition to limiting exposure of patients with asthma to the allergens of mold, mice and rats, and cockroaches, improving poor-quality housing earlier could help asthma by reducing stress, she said.
“There is also an increasing evidence base that psychosocial stress increases the risk of asthma attacks, and it’s therefore possible that living in poor housing conditions – often highly stressful situations – drives exacerbation risk via this pathway,” she said. “Synergistic effects between these pathways are also possible or even likely.”
Neeta Thakur, MD, associate professor of medicine at the University of California, San Francisco, who researches asthma, said the findings could lead to a strategy for improving poor-quality housing more quickly. As it is, inspections are too infrequent, often prompted by resident complaints.
“Once the complaints get to a certain threshold, then there might be an inspection that happens, and if there is a periodic review of the buildings, they often happen few and far between,” she said. “We could actually use some of the information that we’re already getting from something like ED visits and see if there is a pattern.”
An important follow-up would be to see whether asthma outcomes improve after housing deficiencies are addressed and whether the predictive capacity of ED visits occurs in other places.
“Would you then see a decline in the ED visit rates from individuals living in those buildings?” Dr. Thakur said. “It’s important to find a leading indicator, but you want to be sure that that leading indicator is useful as something that can be intervened upon.”
Dr. Samuels and Dr. Thakur have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the annual meeting of the American College of Emergency Physicians.
While links between asthma and low-quality housing prone to harboring allergens have been well-documented, the current study takes the extra step of looking at housing down to the level of individual land parcels and suggests that asthma hospital visits can be used to identify hazardous housing earlier.
“Emergency department visits for asthma provide a leading indicator that can be used by health departments or housing authorities to direct housing inspections and remediation of poor housing conditions, track improvements in housing quality, measure housing department performance, support resident grievances, and inform funding allocation decisions,” said the study’s lead researcher, Elizabeth Samuels, MD, who is assistant professor of epidemiology and emergency medicine at Brown University, Providence, R.I.
Researchers retrospectively looked at cases of children and adults in the Greater New Haven area of Connecticut seen at the Yale New Haven Hospital ED for asthma-related problems between March 2013 and August 2017. The region has the fifth-highest prevalence of asthma in the United States, the researchers point out, due to its air quality, pollens, and quality of its housing. More than half of residences were built before 1,940, compared with about 13% nationally. Patient addresses were matched with HUD inspection records.
The review encompassed 11,429 ED visits by 6,366 individuals; 54% were insured by Medicaid, and 42% were Black. Controlling for patient and neighborhood data, researchers found that increased asthma ED visits at the parcel level were associated with decreased HUD inspection scores to a highly significant degree (P < .001).
They also found that there was a relationship in terms of timing between asthma ED visits and inspection scores: asthma ED visits increased more than 1 year before a failed HUD inspection. They also found that asthma ED visits were not elevated at housing units that passed inspection. Using asthma ED visits to predict failed housing inspections produced a specificity rate of 92.3% in an adjusted model, Dr. Samuels noted.
“This approach represents a novel method of early identification of dangerous housing conditions, which could aid in the prevention of asthma-related morbidity and mortality,” Dr. Samuels said.
The investigators noted that, of the parcels with the top three incidence rates of asthma ED visits, “all of them have been closed or demolished.”
In addition to limiting exposure of patients with asthma to the allergens of mold, mice and rats, and cockroaches, improving poor-quality housing earlier could help asthma by reducing stress, she said.
“There is also an increasing evidence base that psychosocial stress increases the risk of asthma attacks, and it’s therefore possible that living in poor housing conditions – often highly stressful situations – drives exacerbation risk via this pathway,” she said. “Synergistic effects between these pathways are also possible or even likely.”
Neeta Thakur, MD, associate professor of medicine at the University of California, San Francisco, who researches asthma, said the findings could lead to a strategy for improving poor-quality housing more quickly. As it is, inspections are too infrequent, often prompted by resident complaints.
“Once the complaints get to a certain threshold, then there might be an inspection that happens, and if there is a periodic review of the buildings, they often happen few and far between,” she said. “We could actually use some of the information that we’re already getting from something like ED visits and see if there is a pattern.”
An important follow-up would be to see whether asthma outcomes improve after housing deficiencies are addressed and whether the predictive capacity of ED visits occurs in other places.
“Would you then see a decline in the ED visit rates from individuals living in those buildings?” Dr. Thakur said. “It’s important to find a leading indicator, but you want to be sure that that leading indicator is useful as something that can be intervened upon.”
Dr. Samuels and Dr. Thakur have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a new study presented at the annual meeting of the American College of Emergency Physicians.
While links between asthma and low-quality housing prone to harboring allergens have been well-documented, the current study takes the extra step of looking at housing down to the level of individual land parcels and suggests that asthma hospital visits can be used to identify hazardous housing earlier.
“Emergency department visits for asthma provide a leading indicator that can be used by health departments or housing authorities to direct housing inspections and remediation of poor housing conditions, track improvements in housing quality, measure housing department performance, support resident grievances, and inform funding allocation decisions,” said the study’s lead researcher, Elizabeth Samuels, MD, who is assistant professor of epidemiology and emergency medicine at Brown University, Providence, R.I.
Researchers retrospectively looked at cases of children and adults in the Greater New Haven area of Connecticut seen at the Yale New Haven Hospital ED for asthma-related problems between March 2013 and August 2017. The region has the fifth-highest prevalence of asthma in the United States, the researchers point out, due to its air quality, pollens, and quality of its housing. More than half of residences were built before 1,940, compared with about 13% nationally. Patient addresses were matched with HUD inspection records.
The review encompassed 11,429 ED visits by 6,366 individuals; 54% were insured by Medicaid, and 42% were Black. Controlling for patient and neighborhood data, researchers found that increased asthma ED visits at the parcel level were associated with decreased HUD inspection scores to a highly significant degree (P < .001).
They also found that there was a relationship in terms of timing between asthma ED visits and inspection scores: asthma ED visits increased more than 1 year before a failed HUD inspection. They also found that asthma ED visits were not elevated at housing units that passed inspection. Using asthma ED visits to predict failed housing inspections produced a specificity rate of 92.3% in an adjusted model, Dr. Samuels noted.
“This approach represents a novel method of early identification of dangerous housing conditions, which could aid in the prevention of asthma-related morbidity and mortality,” Dr. Samuels said.
The investigators noted that, of the parcels with the top three incidence rates of asthma ED visits, “all of them have been closed or demolished.”
In addition to limiting exposure of patients with asthma to the allergens of mold, mice and rats, and cockroaches, improving poor-quality housing earlier could help asthma by reducing stress, she said.
“There is also an increasing evidence base that psychosocial stress increases the risk of asthma attacks, and it’s therefore possible that living in poor housing conditions – often highly stressful situations – drives exacerbation risk via this pathway,” she said. “Synergistic effects between these pathways are also possible or even likely.”
Neeta Thakur, MD, associate professor of medicine at the University of California, San Francisco, who researches asthma, said the findings could lead to a strategy for improving poor-quality housing more quickly. As it is, inspections are too infrequent, often prompted by resident complaints.
“Once the complaints get to a certain threshold, then there might be an inspection that happens, and if there is a periodic review of the buildings, they often happen few and far between,” she said. “We could actually use some of the information that we’re already getting from something like ED visits and see if there is a pattern.”
An important follow-up would be to see whether asthma outcomes improve after housing deficiencies are addressed and whether the predictive capacity of ED visits occurs in other places.
“Would you then see a decline in the ED visit rates from individuals living in those buildings?” Dr. Thakur said. “It’s important to find a leading indicator, but you want to be sure that that leading indicator is useful as something that can be intervened upon.”
Dr. Samuels and Dr. Thakur have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
Climate change magnifies health effects of wildfire smoke in care deserts
DRESSLERVILLE, NEV. – Smoke began billowing into the skies of northwestern Nevada in September, clouding the mountains, dimming the sun – and quashing residents’ hopes that they would be spared from wildfires and the awful air quality the blazes produce.
The lung-irritating particles were blowing in from burning forests in California and settling in Douglas County, Nevada, home to nearly 50,000 people, prompting warnings that air quality had reached hazardous levels.
Those levels meant the air was very unhealthy, bad enough to raise alarms about people’s immediate health care needs and questions about whether worsening pollution could result in long-term health issues. People could increasingly face such risks as climate change makes wildfires, drought, dust storms, and floods more frequent across the United States and the world.
Some people simply feel powerless.
“There’s not much we could do about it,” said Serrell Smokey, chairman of the Washoe Tribe of Nevada and California. The tribe’s land straddles the border between California and Nevada near Lake Tahoe and extends into Douglas County, about 60 miles south of Reno.
Tribe members and other area residents are among millions of people nationwide who this year will experience poor air quality because of wildfires. In September, as smoke settled over Nevada, fire-related air quality alerts were dispatched in six other states: California, Idaho, Montana, Oregon, Washington, and Wyoming.
Yet, by one measure, people who live in Douglas County are better off than those in some other hard-hit areas. Douglas County residents must drive 30 minutes, on average, for medical care from lung specialists called pulmonologists. In other parts of the West and Upper Midwest, however, patients must drive an hour or more, according to data analyzed by GoodRx, a website that tracks prescription drug prices and conducts research.
Specifically, the research found that about 5.5 million Americans live in the 488 counties where drive times to pulmonologists are an hour or more. Much of Nevada and large parts of Montana fall into those gaps between specialists – places that have recently grappled with wildfires that fill the air with smoke and ash, which can cause lung problems or exacerbate existing ones.
Data from the Association of American Medical Colleges shows the number of pulmonary disease specialists in the United States dropped nearly 11% from 2014 to 2019. The group, which is based in Washington, D.C., and represents the academic medicine community, noted that the decline might not be as high as it appears because some physicians are opting to practice pulmonary critical care rather than just pulmonology. Many of those types of pulmonologists work in hospital intensive care units.
About 15,000 pulmonologists are practicing in the United States, according to the GoodRx report. Yet vast swaths of the country have few or none.
“New Mexico has one pulmonologist for the entire southeastern part of state, not counting Las Cruces, which is closer to El Paso,” said Victor Test, MD, a pulmonologist at Texas Tech Physicians.
Dr. Test, one of 13 pulmonologists in the Lubbock, Tex., region, said that his patients from within Texas sometimes drive 4 hours for an appointment and that other people travel from “New Mexico, Oklahoma, even far western Kansas.”
Increases in wildfires and their intensity will likely expand the need for pulmonologists.
“Climate change is going to affect lung disease,” said Nicholas Kenyon, MD, a professor of pulmonary, critical care, and sleep medicine at the University of California, Davis, where he and several other researchers are tracking the effects of wildfires. At his Sacramento practice, Dr. Kenyon said, he sees patients from far northern parts of California, including Eureka, a 5-hour drive from the state capital.
The short-term effects of breathing smoke are pretty well known. People show up in emergency rooms with asthma attacks, exacerbation of COPD, bronchitis, and even pneumonia, Dr. Kenyon said. Some have chest pain or other cardiac concerns.
“But we have very little understanding of what happens over the longer term,” he said. “If people get 2 or 3 weeks of wildfire exposure for 2 or 3 years, does that lead to worsening of asthma or COPD? We just don’t know.”
Fires release multiple pollutants, including carbon dioxide, carbon monoxide, and chemicals like benzene. All fires send particles into the air. Health researchers and air quality experts are most concerned about tiny pieces referred to as particulate matter 2.5. Far smaller than a human hair, the particles can lodge deep in the lungs and have been linked to heart and lung conditions.
Increases in those tiny particles are associated with a greater risk of death from all causes, excluding accidents, homicides, and other nonaccidental causes, for up to 4 days after a population is exposed, according to a 2020 New England Journal of Medicine overview.
The concentration of fine particulate matter is one of five gauges used to calculate the Air Quality Index, a numerical and color-coded index used to let the public know about local air pollution levels. Green denotes good air quality and is given if the total index is 50 or less. When the measurement exceeds 100, the air quality gets an orange label and may be bad for certain groups. Levels over 200 get a red label and are considered unhealthy for everyone.
Government agencies track those levels, as do people who use apps or websites to determine whether it’s safe to go outside.
When the AQI rises above 150, Farah Madhani-Lovely, MD, a pulmonologist, said, Renown Regional Medical Center in Reno shuts its outpatient pulmonary rehabilitation clinic because it doesn’t want to encourage patients to drive in. Some patients from Douglas County opt for care near home, about an hour away. “We don’t want these patients exposed outside because just 1 minute of exposure to the smoke can trigger an exacerbation of their chronic disease,” Dr. Madhani-Lovely said.
Mr. Smokey said connecting with pulmonologists can be difficult for Washoe Tribe members, particularly those who live on the California side of the reservation. “We cannot find providers for them,” he said. “We end up referring them out and sending them hundreds of miles out of their way just to get care that we should be able to provide here.”
Recruiting specialists to rural areas or smaller cities has long been difficult. For one thing, a specialist might be the only one for miles around, “so there’s a tremendous burden in terms of coverage and days off,” Dr. Test said.
Another concern is that physicians tend to train in larger cities and often want to practice in similar places. Even recruiting pulmonary physicians to Lubbock, a city of 260,000 in West Texas, is a challenge, Dr. Test said.
“I love Lubbock,” he said. “But I tell people who have never been here, I say, ‘It’s really flat.’ They don’t understand flat until they get here.”
In Nevada, on days when the air quality is bad, Washoe tribal members try to protect themselves with makeshift air purifiers created from fans, duct tape, and air filters, Mr. Smokey said.
Longer term, Mr. Smokey and other tribal leaders are pushing the Indian Health Service to establish a specialty care hospital in northern Nevada. The closest specialty care hospital for Washoe tribal members is more than 700 miles away, in Phoenix.
It’s difficult because “there’s a need we should be taking care of,” Mr. Smokey said. “But we have to fight for it. And sometimes that fight takes years, years, and years to accomplish.”
A version of this article first appeared on Medscape.com.
DRESSLERVILLE, NEV. – Smoke began billowing into the skies of northwestern Nevada in September, clouding the mountains, dimming the sun – and quashing residents’ hopes that they would be spared from wildfires and the awful air quality the blazes produce.
The lung-irritating particles were blowing in from burning forests in California and settling in Douglas County, Nevada, home to nearly 50,000 people, prompting warnings that air quality had reached hazardous levels.
Those levels meant the air was very unhealthy, bad enough to raise alarms about people’s immediate health care needs and questions about whether worsening pollution could result in long-term health issues. People could increasingly face such risks as climate change makes wildfires, drought, dust storms, and floods more frequent across the United States and the world.
Some people simply feel powerless.
“There’s not much we could do about it,” said Serrell Smokey, chairman of the Washoe Tribe of Nevada and California. The tribe’s land straddles the border between California and Nevada near Lake Tahoe and extends into Douglas County, about 60 miles south of Reno.
Tribe members and other area residents are among millions of people nationwide who this year will experience poor air quality because of wildfires. In September, as smoke settled over Nevada, fire-related air quality alerts were dispatched in six other states: California, Idaho, Montana, Oregon, Washington, and Wyoming.
Yet, by one measure, people who live in Douglas County are better off than those in some other hard-hit areas. Douglas County residents must drive 30 minutes, on average, for medical care from lung specialists called pulmonologists. In other parts of the West and Upper Midwest, however, patients must drive an hour or more, according to data analyzed by GoodRx, a website that tracks prescription drug prices and conducts research.
Specifically, the research found that about 5.5 million Americans live in the 488 counties where drive times to pulmonologists are an hour or more. Much of Nevada and large parts of Montana fall into those gaps between specialists – places that have recently grappled with wildfires that fill the air with smoke and ash, which can cause lung problems or exacerbate existing ones.
Data from the Association of American Medical Colleges shows the number of pulmonary disease specialists in the United States dropped nearly 11% from 2014 to 2019. The group, which is based in Washington, D.C., and represents the academic medicine community, noted that the decline might not be as high as it appears because some physicians are opting to practice pulmonary critical care rather than just pulmonology. Many of those types of pulmonologists work in hospital intensive care units.
About 15,000 pulmonologists are practicing in the United States, according to the GoodRx report. Yet vast swaths of the country have few or none.
“New Mexico has one pulmonologist for the entire southeastern part of state, not counting Las Cruces, which is closer to El Paso,” said Victor Test, MD, a pulmonologist at Texas Tech Physicians.
Dr. Test, one of 13 pulmonologists in the Lubbock, Tex., region, said that his patients from within Texas sometimes drive 4 hours for an appointment and that other people travel from “New Mexico, Oklahoma, even far western Kansas.”
Increases in wildfires and their intensity will likely expand the need for pulmonologists.
“Climate change is going to affect lung disease,” said Nicholas Kenyon, MD, a professor of pulmonary, critical care, and sleep medicine at the University of California, Davis, where he and several other researchers are tracking the effects of wildfires. At his Sacramento practice, Dr. Kenyon said, he sees patients from far northern parts of California, including Eureka, a 5-hour drive from the state capital.
The short-term effects of breathing smoke are pretty well known. People show up in emergency rooms with asthma attacks, exacerbation of COPD, bronchitis, and even pneumonia, Dr. Kenyon said. Some have chest pain or other cardiac concerns.
“But we have very little understanding of what happens over the longer term,” he said. “If people get 2 or 3 weeks of wildfire exposure for 2 or 3 years, does that lead to worsening of asthma or COPD? We just don’t know.”
Fires release multiple pollutants, including carbon dioxide, carbon monoxide, and chemicals like benzene. All fires send particles into the air. Health researchers and air quality experts are most concerned about tiny pieces referred to as particulate matter 2.5. Far smaller than a human hair, the particles can lodge deep in the lungs and have been linked to heart and lung conditions.
Increases in those tiny particles are associated with a greater risk of death from all causes, excluding accidents, homicides, and other nonaccidental causes, for up to 4 days after a population is exposed, according to a 2020 New England Journal of Medicine overview.
The concentration of fine particulate matter is one of five gauges used to calculate the Air Quality Index, a numerical and color-coded index used to let the public know about local air pollution levels. Green denotes good air quality and is given if the total index is 50 or less. When the measurement exceeds 100, the air quality gets an orange label and may be bad for certain groups. Levels over 200 get a red label and are considered unhealthy for everyone.
Government agencies track those levels, as do people who use apps or websites to determine whether it’s safe to go outside.
When the AQI rises above 150, Farah Madhani-Lovely, MD, a pulmonologist, said, Renown Regional Medical Center in Reno shuts its outpatient pulmonary rehabilitation clinic because it doesn’t want to encourage patients to drive in. Some patients from Douglas County opt for care near home, about an hour away. “We don’t want these patients exposed outside because just 1 minute of exposure to the smoke can trigger an exacerbation of their chronic disease,” Dr. Madhani-Lovely said.
Mr. Smokey said connecting with pulmonologists can be difficult for Washoe Tribe members, particularly those who live on the California side of the reservation. “We cannot find providers for them,” he said. “We end up referring them out and sending them hundreds of miles out of their way just to get care that we should be able to provide here.”
Recruiting specialists to rural areas or smaller cities has long been difficult. For one thing, a specialist might be the only one for miles around, “so there’s a tremendous burden in terms of coverage and days off,” Dr. Test said.
Another concern is that physicians tend to train in larger cities and often want to practice in similar places. Even recruiting pulmonary physicians to Lubbock, a city of 260,000 in West Texas, is a challenge, Dr. Test said.
“I love Lubbock,” he said. “But I tell people who have never been here, I say, ‘It’s really flat.’ They don’t understand flat until they get here.”
In Nevada, on days when the air quality is bad, Washoe tribal members try to protect themselves with makeshift air purifiers created from fans, duct tape, and air filters, Mr. Smokey said.
Longer term, Mr. Smokey and other tribal leaders are pushing the Indian Health Service to establish a specialty care hospital in northern Nevada. The closest specialty care hospital for Washoe tribal members is more than 700 miles away, in Phoenix.
It’s difficult because “there’s a need we should be taking care of,” Mr. Smokey said. “But we have to fight for it. And sometimes that fight takes years, years, and years to accomplish.”
A version of this article first appeared on Medscape.com.
DRESSLERVILLE, NEV. – Smoke began billowing into the skies of northwestern Nevada in September, clouding the mountains, dimming the sun – and quashing residents’ hopes that they would be spared from wildfires and the awful air quality the blazes produce.
The lung-irritating particles were blowing in from burning forests in California and settling in Douglas County, Nevada, home to nearly 50,000 people, prompting warnings that air quality had reached hazardous levels.
Those levels meant the air was very unhealthy, bad enough to raise alarms about people’s immediate health care needs and questions about whether worsening pollution could result in long-term health issues. People could increasingly face such risks as climate change makes wildfires, drought, dust storms, and floods more frequent across the United States and the world.
Some people simply feel powerless.
“There’s not much we could do about it,” said Serrell Smokey, chairman of the Washoe Tribe of Nevada and California. The tribe’s land straddles the border between California and Nevada near Lake Tahoe and extends into Douglas County, about 60 miles south of Reno.
Tribe members and other area residents are among millions of people nationwide who this year will experience poor air quality because of wildfires. In September, as smoke settled over Nevada, fire-related air quality alerts were dispatched in six other states: California, Idaho, Montana, Oregon, Washington, and Wyoming.
Yet, by one measure, people who live in Douglas County are better off than those in some other hard-hit areas. Douglas County residents must drive 30 minutes, on average, for medical care from lung specialists called pulmonologists. In other parts of the West and Upper Midwest, however, patients must drive an hour or more, according to data analyzed by GoodRx, a website that tracks prescription drug prices and conducts research.
Specifically, the research found that about 5.5 million Americans live in the 488 counties where drive times to pulmonologists are an hour or more. Much of Nevada and large parts of Montana fall into those gaps between specialists – places that have recently grappled with wildfires that fill the air with smoke and ash, which can cause lung problems or exacerbate existing ones.
Data from the Association of American Medical Colleges shows the number of pulmonary disease specialists in the United States dropped nearly 11% from 2014 to 2019. The group, which is based in Washington, D.C., and represents the academic medicine community, noted that the decline might not be as high as it appears because some physicians are opting to practice pulmonary critical care rather than just pulmonology. Many of those types of pulmonologists work in hospital intensive care units.
About 15,000 pulmonologists are practicing in the United States, according to the GoodRx report. Yet vast swaths of the country have few or none.
“New Mexico has one pulmonologist for the entire southeastern part of state, not counting Las Cruces, which is closer to El Paso,” said Victor Test, MD, a pulmonologist at Texas Tech Physicians.
Dr. Test, one of 13 pulmonologists in the Lubbock, Tex., region, said that his patients from within Texas sometimes drive 4 hours for an appointment and that other people travel from “New Mexico, Oklahoma, even far western Kansas.”
Increases in wildfires and their intensity will likely expand the need for pulmonologists.
“Climate change is going to affect lung disease,” said Nicholas Kenyon, MD, a professor of pulmonary, critical care, and sleep medicine at the University of California, Davis, where he and several other researchers are tracking the effects of wildfires. At his Sacramento practice, Dr. Kenyon said, he sees patients from far northern parts of California, including Eureka, a 5-hour drive from the state capital.
The short-term effects of breathing smoke are pretty well known. People show up in emergency rooms with asthma attacks, exacerbation of COPD, bronchitis, and even pneumonia, Dr. Kenyon said. Some have chest pain or other cardiac concerns.
“But we have very little understanding of what happens over the longer term,” he said. “If people get 2 or 3 weeks of wildfire exposure for 2 or 3 years, does that lead to worsening of asthma or COPD? We just don’t know.”
Fires release multiple pollutants, including carbon dioxide, carbon monoxide, and chemicals like benzene. All fires send particles into the air. Health researchers and air quality experts are most concerned about tiny pieces referred to as particulate matter 2.5. Far smaller than a human hair, the particles can lodge deep in the lungs and have been linked to heart and lung conditions.
Increases in those tiny particles are associated with a greater risk of death from all causes, excluding accidents, homicides, and other nonaccidental causes, for up to 4 days after a population is exposed, according to a 2020 New England Journal of Medicine overview.
The concentration of fine particulate matter is one of five gauges used to calculate the Air Quality Index, a numerical and color-coded index used to let the public know about local air pollution levels. Green denotes good air quality and is given if the total index is 50 or less. When the measurement exceeds 100, the air quality gets an orange label and may be bad for certain groups. Levels over 200 get a red label and are considered unhealthy for everyone.
Government agencies track those levels, as do people who use apps or websites to determine whether it’s safe to go outside.
When the AQI rises above 150, Farah Madhani-Lovely, MD, a pulmonologist, said, Renown Regional Medical Center in Reno shuts its outpatient pulmonary rehabilitation clinic because it doesn’t want to encourage patients to drive in. Some patients from Douglas County opt for care near home, about an hour away. “We don’t want these patients exposed outside because just 1 minute of exposure to the smoke can trigger an exacerbation of their chronic disease,” Dr. Madhani-Lovely said.
Mr. Smokey said connecting with pulmonologists can be difficult for Washoe Tribe members, particularly those who live on the California side of the reservation. “We cannot find providers for them,” he said. “We end up referring them out and sending them hundreds of miles out of their way just to get care that we should be able to provide here.”
Recruiting specialists to rural areas or smaller cities has long been difficult. For one thing, a specialist might be the only one for miles around, “so there’s a tremendous burden in terms of coverage and days off,” Dr. Test said.
Another concern is that physicians tend to train in larger cities and often want to practice in similar places. Even recruiting pulmonary physicians to Lubbock, a city of 260,000 in West Texas, is a challenge, Dr. Test said.
“I love Lubbock,” he said. “But I tell people who have never been here, I say, ‘It’s really flat.’ They don’t understand flat until they get here.”
In Nevada, on days when the air quality is bad, Washoe tribal members try to protect themselves with makeshift air purifiers created from fans, duct tape, and air filters, Mr. Smokey said.
Longer term, Mr. Smokey and other tribal leaders are pushing the Indian Health Service to establish a specialty care hospital in northern Nevada. The closest specialty care hospital for Washoe tribal members is more than 700 miles away, in Phoenix.
It’s difficult because “there’s a need we should be taking care of,” Mr. Smokey said. “But we have to fight for it. And sometimes that fight takes years, years, and years to accomplish.”
A version of this article first appeared on Medscape.com.